3
Technologies for the Small System
Most source waters used for public drinking water supplies are not of suitable quality for consumption without some form of treatment. The U.S. Environmental Protection Agency (EPA) has ruled that all surface waters must be filtered and disinfected before consumption unless the purveyor can justify avoidance of filtration; some surface waters also need to be treated with additional processes to remove chemical contaminants before they are suitable for use as drinking water. Many ground water sources are disinfected, and many are treated to remove nuisance chemicals (such as iron and manganese) and chemical contaminants before distribution. This chapter evaluates water treatment processes that can be used by small systems and discusses their suitability under various conditions.
The fundamental responsibility of a public water system is to provide safe drinking water, as defined by the Safe Drinking Water Act (SDWA) and its amendments. Water utilities are required by the SDWA to monitor drinking water quality. When source water used by a water system does not meet quality requirements, the utility has several options. The first that should be considered is finding a cleaner, safer source water that requires less treatment than the existing source water, for this is often the most cost- and resource-efficient way to meet demand. Surface water sources tends to be turbid and typically contain higher concentrations of colloidal and microbiological material than ground water sources. Ground water sources generally have higher initial quality and tend to require less treatment than surface water sources, making ground water sources a good choice for small water systems. In fact, as shown in Table 3-1, most small systems already use ground water sources. Before installing new treatment systems, a small utility using surface water might seek a ground water source, or a
TABLE 3-1 Water Source for Community Water Systems of Various Sizes
|
|
Water Source |
|
|
Population Served |
Ground Water |
Surface Water |
|
Small systems |
||
|
Under 500 |
91% |
9% |
|
501-3,300 |
74% |
26% |
|
3,301-10,000 |
58% |
42% |
|
Large systems |
||
|
10,001-100,000 |
46% |
54% |
|
More than 100,000 |
28% |
72% |
|
SOURCE: EPA, 1994. |
||
utility using a poor ground water source might develop a new well in an alternative location or use a deeper aquifer by extending the depth of a well or drilling a deeper one. In either case, if alternative sources of high-quality raw water are not available, the utility might seek a source of treated water from a water utility that has an adequate supply of water and is located close enough to extend a transmission main at an affordable cost. If such options cannot be found, however, then the utility needs to explore adding additional treatment systems.
Treatment Technologies: Overview
Table 3-2 lists treatment processes according to the water quality problems they address. No single process can solve every water quality problem. Rather, a utility must choose from a wide range of processes that are used for different purposes. The treatment technology or combination of technologies to be used in a specific situation depends on the source water quality, the nature of the contaminant to be removed, the desired qualities of the treated water, and the size of the water system. For very small systems, treatment may not be a feasible alternative because of the high cost of having a treatment system designed and installed and the complexity of maintaining it.
Historically, the design of drinking water treatment systems has been driven by the need to remove microbial contaminants and turbidity. Microbial contaminants are the central concern because they can lead to immediate health problems. Turbidity is a concern not only because water containing particles can have an objectionable taste and appearance but also because particles of fecal matter can harbor microorganisms, and soil particles can carry sorbed contaminants such as pesticides and herbicides. Aesthetic problems such as excess hardness, which
TABLE 3-2 Treatment Technologies by Contaminant Type
|
|
Disinfectants/Oxidants |
|
Air Stripping Systems |
|||||
|
|
Free Cl2 |
NH2Cl |
CIO2 |
O3 |
Ultraviolet Radiation |
KMnO4 |
Aeration |
Membrane Aeration |
|
General water quality parameters |
|
|||||||
|
Turbidity |
|
|||||||
|
Color |
|
X |
|
X |
|
|
||
|
Disinfection byproduct precursors |
|
|||||||
|
Taste and odor |
|
X |
X |
X |
X |
X |
||
|
Biological contaminants |
|
|||||||
|
Algae |
|
|||||||
|
Protozoa |
|
X |
X |
|
||||
|
Bacteria |
X |
X |
X |
X |
X |
|
||
|
Viruses |
X |
X |
X |
X |
X |
|
||
|
Organic chemicals |
|
|||||||
|
Volatile organic compounds (VOCs) |
|
X |
X |
|||||
|
Semivolatile compounds |
|
X |
||||||
|
Pesticides |
|
|||||||
|
Biodegradable organic matter |
|
|||||||
|
|
Adsorption Systems |
|||
|
|
Powdered Activated Carbon |
Granular Activated Carbon |
Ion Exchange |
Activated Alumina |
|
General water quality parameters |
|
|||
|
Turbidity |
|
|||
|
Color |
X |
X |
|
|
|
Disinfection byproduct precursors |
X |
X |
|
|
|
Taste and odor |
X |
X |
|
|
|
Biological contaminants |
|
|
|
|
|
Algae |
|
X |
|
|
|
Protozoa |
|
X |
|
|
|
Bacteria |
|
X |
|
|
|
Viruses |
|
X |
|
|
|
Organic chemicals |
|
|||
|
VOCs |
X |
X |
|
|
|
Semivolatile compounds |
X |
X |
|
|
|
Pesticides |
X |
X |
|
|
|
Biodegradable organic matter |
X |
X |
|
|
|
|
Membrane Processes> |
||||
|
|
Microfiltration |
Ultrafiltration |
Nanofiltration |
Reverse Osmosis |
Electrodialysis/ Electrodialysis Reversal |
|
General water quality parameters |
|
||||
|
Turbidity |
X |
X |
X |
|
|
|
Color |
|
X |
X |
X |
|
|
Disinfection byproduct precursors |
|
X |
X |
X |
|
|
Taste and odor |
|
||||
|
Biological contaminants |
|
||||
|
Algae |
X |
X |
X |
|
|
|
Protozoa |
X |
X |
X |
X |
|
|
Bacteria |
|
X |
X |
X |
|
|
Viruses |
|
|
X |
X |
|
|
Organic chemicals |
|
||||
|
VOCs |
|
||||
|
Semivolatile compounds |
|
X |
|
||
|
Pesticides |
|
|
X |
X |
|
|
Biodegradable organic matter |
|
||||
|
|
Filtration Systems |
|||||||
|
|
Direct Filtration |
Conventional Filtration |
Dissolved Air Flotation |
Diatomaceous Earth Filtration |
Slow Sand Filtration |
Bag/Cartridge Filters |
Lime Softening |
|
|
General water quality parameters |
|
|||||||
|
Turbidity |
X |
X |
X |
X |
X |
|
X |
|
|
Color |
X |
X |
X |
|
||||
|
Disinfection byproduct precursors |
X |
X |
X |
|
X |
|
|
|
|
Taste and odor |
|
X |
|
|
||||
|
Biological contaminants |
|
|||||||
|
Algae |
|
X |
X |
X |
|
|
|
|
|
Protozoa |
X |
X |
X |
X |
X |
X |
X |
|
|
Bacteria |
X |
X |
X |
X |
X |
|
X |
|
|
Viruses |
X |
X |
X |
X |
X |
|
X |
|
|
Organic Chemicals |
|
|||||||
|
VOCs |
|
|||||||
|
Semivolatile compounds |
|
|||||||
|
Pesticides |
|
|||||||
|
Biodegradable organic matter |
|
Xa |
Xa |
|
Xa |
Xa |
|
|
can lead to scaling of water heaters and excess soap consumption, and objectionable tastes and odors have also played an important historical role in the development of drinking water treatment technologies. Finally, the corrosivity of the water has been a long-standing concern because of the need to protect water mains and plumbing. Drinking water treatment systems are still designed primarily with these objectives in mind rather than being based on the need to remove trace levels of synthetic chemicals to comply with requirements of the SDWA and its amendments.
Because so many regulations apply to drinking water, small systems must look at the entire spectrum of drinking water regulations before deciding on a treatment method. The system manager who considers the regulations and other water concerns on a piecemeal basis can end up using first one process and then another until finally the treatment plant becomes a costly chain of processes inefficiently tacked on to one another. Eventually the small system could find that it can no longer afford to install further treatment systems, and the whole investment might be made for naught.
A number of the treatment processes listed in Table 3-2 and described in more detail below are available to small communities as package plants. The term ''package plant" is not intended to convey the concept of a complete water treatment plant in a package. Rather, a package plant is a grouping of treatment processes, such as chemical feed, rapid mixing, flocculation, sedimentation, and filtration, in a compact, preassembled unit. To provide a complete treatment plant, other equipment, or in some cases a series of package plants, generally is required. For example, most package plants designed to provide water filtration are not also equipped with equipment for disinfection, corrosion control, or adsorption of organic contaminants by granular activated carbon (GAC).
Some manufactures prefer to call package plant "preengineered" process equipment because the process engineering for the package plant design has been done by the manufacturer. What remains for the water system's engineer to design is the specifics of the on-site application of the equipment. Because package plants do not require custom design, and because the process facilities (for example, mixing chamber, flocculation basin, sedimentation basin, and filter) are built in a factory instead of on-site, such systems have the potential to provide significant cost savings to small communities.
Table 3-3 outlines important capital considerations for common water treatment processes. Water treatment technologies change constantly. As shown in the table, at any given time they fall into one of several broad categories:
- Conventional technologies are in widespread use and familiar to practicing treatment engineers and operators.
- Accepted technologies are not as widely used as conventional technologies. Sometimes these technologies have been developed for other fields and adopted by the water community. Some process of this type have performed
- satisfactorily in water treatment, but some personnel in the field may not be familiar with them.
- Emerging technologies include those that have not been applied to water treatment in an operating system but show great promise for acceptance in the near future. These technologies are likely to be in the research or pilot plant stage.
Table 3-3 also shows the costs of different treatment processes on a relative scale. Precise cost information cannot be provided because costs change constantly. For example, advances in membrane processes are reducing the costs of membrane systems.
Table 3-4 shows operating considerations—raw water quality, operator skills, monitoring requirements, and costs—for common treatment processes. As shown in the table, different treatment processes have different requirements for source water quality. Some processes require "high-quality" or "very-high-quality" source water. Details about quality requirements are provided with the individual technology descriptions later in this chapter.
Once a treatment system has been selected and installed, it is common to believe that the major expenditure is over. This is true for relatively few technologies. Operation and maintenance costs must be considered in long-term planning and in selection of treatment processes because they vary with the technology, as shown in Table 3-4. Skill levels required of water treatment plant operators also vary with system complexity and type of technology. Table 3-4 indicates different skill levels:
- In a basis system, an operator with minimal experience in the water treatment field can perform the necessary system operation and monitoring if provided with proper instruction. The operator is capable of reading and following explicit directions but would not necessarily have water treatment as a primary career.
- In an intermediate system, the operator needs to understand the principles of water treatment and have a knowledge of the regulatory framework. The operator must be capable of making system changes in response to source water fluctuations.
- In an advanced system, the operator must possess a thorough understanding of the principles of system operation. The operator should be knowledgeable in water treatment and regulatory requirements, with water treatment being the career objective. (The operator may, however, have advanced knowledge of only the particular treatment technology.) This operator seeks information, remains informed, and reliably interprets and responds to water fluctuations and system intricacies.
Tables 3-3, 3-4, and others in this chapter are meant only to guide preliminary
TABLE 3-3 Capital Considerations for Treatment Technologies
|
Technology |
Contaminants |
State of Technology |
Relative Capital Cost |
|
|
All water sources |
|
|
|
|
|
Disinfection |
Microbiological contaminants |
|
|
|
|
Free Cl2 |
|
Conventional |
Low |
|
|
NH2Cl |
|
Conventional |
Low |
|
|
ClO2 |
|
Accepted |
Low |
|
|
O3 |
|
Accepted |
Medium |
|
|
Ultraviolet radiation |
|
Accepted |
Medium |
|
|
Corrosion control |
Prevention of system corrosion, lead, copper |
|
|
|
|
Chemical feeders |
|
Conventional |
Low |
|
|
Limestone contactor |
|
Accepted |
Medium |
|
|
Membrane filtration systems |
Turbidity, protozoa (Giardia and Cryptosporidium), algae, and the following: |
|
|
|
|
Microfiltration |
Some bacteria |
Accepted |
Medium |
|
|
Ultrafiltration |
Bacteria, some viruses, some color |
Accepted |
Medium |
|
|
Nanofiltration |
Bacteria, viruses, color, some organic chemicals, hardness |
Emerging |
Medium |
|
|
Reverse osmosis |
Bacteria, viruses, humic acids, some organic chemical, inorganic chemicals, hardness, radium, salts |
Conventional |
Medium |
|
|
Electrodialysis/electrodialysis reversal |
Inorganic chemicals (charged) |
Accepted |
High |
|
Adsorption |
|
|
|
|
Powdered activated carbon (PAC) |
Organic chemicals, tastes and odors |
Conventional |
Low/medium |
|
Granular activated carbon (GAC) |
Organic chemicals, tastes and odors, radon, microorganisms |
Accepted |
Medium/high |
|
Lime softening |
Hardness, iron, manganese, turbidity |
Accepted |
High |
|
Ground water sources |
|
|
|
|
Aeration |
|
|
|
|
Diffused air |
Volatile organic chemicals, radon, tastes and odors |
Accepted |
Low |
|
Mechanical aeration |
Volatile organic chemicals, radon, tastes and odors |
Accepted |
Low |
|
Tray aerators |
Volatile organic chemicals, radon, tastes and odors |
Conventional |
Low/medium |
|
Packed tower aeration |
Volatile organic chemicals, radon, tastes and odors |
Conventional |
Medium |
|
Membrane aeration |
Volatile and semivolatile organic chemicals radon, tastes and odors |
Emerging |
Medium |
|
Oxidation/filtration |
|
|
|
|
Permanganate |
Reduced iron and manganese, organic chemicals, radon, tastes and odors |
Conventional |
Low |
|
O3 |
Reduced iron and manganese, organic chemicals, tastes and odors |
Accepted |
High |
|
Technology |
Contaminants |
State of Technology |
Relative Capital Cost |
|
Ion exchange |
Inorganic chemicals, radium, nitrate |
Accepted |
Medium |
|
Activated alumina |
Arsenic, thallium, selenium, fluoride, other inorganic chemicals |
Accepted |
High |
|
Surface water sources |
|
|
|
|
Coagulation-filtration |
Turbidity, color, disinfection byproduct precursors, microorganisms, algae, iron, manganese, biodegradable organic matter,a ammoniaa |
|
|
|
Direct filtration |
|
Accepted |
High |
|
Conventional, with sedimentation |
|
Conventional |
High |
|
Dissolved air flotation |
|
Accepted |
High |
|
Diatomaceous earth filtration |
Turbidity, algae, Giardia, Cryptosporidium, biodegradable organic matter,a ammoniaa |
Accepted |
Medium/high |
|
Slow sand filtration |
Turbidity, microorganisms, biodegradable organic matter,a ammonia,a tastes and odors |
|
|
|
Uncovered filters |
|
Conventional in some states |
Medium |
|
Covered filters |
|
Conventional in some states |
Medium/high |
|
Bag filters cartridge filters |
Giardia cysts and Cryptosporidium oocysts |
Accepted in some states |
Low |
|
a If operated in biologically active mode. |
|||
consideration of a treatment process. Reference to the textual description of the process later in this chapter is also necessary to further assess its applicability to a given water system.
In the descriptions that follow, treatment processes are grouped according to whether they are suitable for small systems using either surface water or ground water, are best suited to ground water systems, or are used primarily for surface water systems.
Technologies for All Systems
Contamination with microorganisms is common to surface water sources and is becoming an increasing concern for ground water sources. Other water quality concerns common to both surface and ground water systems are excess corrosivity, hardness, and, increasingly, contamination with synthetic organic chemicals. The technologies described in this section address these water quality concerns, as well as some others, and are suitable for use in treating either surface water or ground water.
Disinfection
How the Process Works
Disinfection is the inactivation of pathogens in drinking water. Although not entirely effective against all pathogens, disinfection is the most cost-effective way to reduce the incidence of waterborne disease. Two common techniques are chemical disinfection and irradiation with ultraviolet (UV) light.
UV disinfection is used primarily in small systems that treat ground water. UV radiation has been demonstrated to be effective against bacteria and viruses, which are the microbiological contaminants likely to be found in ground waters for which the quality is not directly influenced by surface water. However, because it does a poor of killing Giardia and Cryptosporidium, UV radiation is not an accepted means for disinfecting surface waters, unless they have already been treated in a way that would physically remove the cysts and oocysts of the Giardia and Cryptosporidium.
The chemical disinfectants used in drinking water treatment are free chlorine, chloramine, ozone, and chlorine dioxide. Iodine has been studied as a disinfectant, but the EPA restricts its use to short-term, limited, or emergency purposes because of concerns over possible adverse health effects such as iodine hypersensitivity and thyroid problems (EPA, 1982, 1995).
Of the chemical disinfectants, free chlorine is probably used most commonly, with chloramine next in popularity. In a survey conducted in 1989 and 1990, approximately 72 percent of the nearly 280 water utilities responding reported using free chlorine (AWWA Committee, 1992). Approximately 21 percent
TABLE 3-4 Operational Considerations for Treatment Technologies
|
Technology |
Raw Water Quality Rangea |
|
All water sources |
|
|
Disinfection |
All, but better with higher quality |
|
Free Cl2 |
|
|
NH2Cl |
|
|
ClO2 |
|
|
O3 |
|
|
Ultraviolet radiation |
|
|
Corrosion control |
|
|
Chemical feeders |
All ranges |
|
Limestone contactor |
Low iron, low turbidity |
|
Membrane filtration systems |
|
|
Microfiltration |
Needs high water quality (or pretreatment) |
|
Ultrafiltration |
Needs very high water quality (or pretreatment) |
|
Nanofiltration |
Needs very high water quality (or pretreatment) |
|
Reverse osmosis |
Requires prefiltration for surface water |
|
Electrodialysis/electrodialysis reversal |
Requires prefiltration for surface water |
|
Adsorption |
|
|
Powdered activated carbon (PAC) |
All waters |
|
Granular activated carbon (GAC) |
Surface water may require prefiltration |
|
Lime softening |
All waters |
|
Ground water sources |
|
|
Air stripping |
|
|
Diffused air |
All ground waters |
|
Mechanical aeration |
All ground waters |
|
Tray aeration |
All ground waters |
|
Packed tower aeration |
All ground waters |
|
Membrane aeration |
All ground waters |
|
Oxidation/filtration |
|
|
Tray aerators |
All ground waters |
|
Permanganate |
All ground waters |
|
O3 |
All ground waters |
|
Cl2 |
All ground waters |
|
Ion exchange |
All ground waters |
|
Activated alumina |
All ground waters |
|
Surface water sources |
|
|
Coagulation-filtration |
|
|
Direct filtration |
Needs high raw water quality |
|
Conventional, with sedimentation |
Can treat wide range of water quality |
|
Dissolved air flotation |
Very high algae OK, high color OK, moderate turbidity |
|
Diatomaceous earth filtration |
Needs very high water quality |
|
Slow sand filtration |
Needs very high water quality |
|
Bag and cartridge filters |
Needs very high quality water |
|
a Refer to text for detailed description of water quality needs. |
|
|
Operator Skill Level Required |
Monitoring Requirements |
Relative Operating Cost |
|
Basic |
Low |
Low |
|
Basic |
Low |
Low |
|
Intermediate |
High |
Low |
|
Intermediate |
Low |
Medium |
|
Basic |
Low |
Low |
|
Basic |
Low |
Medium |
|
Basic |
Low |
Low |
|
Basic |
Low |
Low |
|
Basic |
Low |
Medium |
|
Basic |
Low |
Medium/high |
|
Advanced |
Medium |
High |
|
Advanced |
Medium |
High |
|
Intermediate |
Low |
Medium/high |
|
Basic |
Low/medium |
Medium/high |
|
Advanced |
High |
High |
|
Basic |
Low |
Low |
|
Basic |
Low |
Low |
|
Basic |
Low |
Low |
|
Intermediate |
Low |
Medium |
|
Intermediate |
Low |
Medium |
|
Basic |
Low |
Low |
|
Intermediate |
Medium |
Medium |
|
Intermediate |
Low |
Low |
|
Basic |
Low |
Low |
|
Intermediate |
Medium |
Medium/high |
|
Advanced |
Medium |
High |
|
Advanced |
High |
Medium/high |
|
Advanced |
High |
Medium/high |
|
Advanced |
High |
Medium/High |
|
Intermediate |
Medium |
Medium/high |
|
Basic |
Low |
Low, with good raw water |
|
Basic |
Low |
Low/high; depends on cycle length |
of the utilities reported using chloramine; approximately 5 percent used chlorine dioxide in conjunction with free chlorine or chloramine. Ozone was used by approximately 0.4 percent of the utilities.
Since 1990, a considerable number of ozone facilities have come on line, although the percentage of utilities using ozone remains small compared to the percentages using chloramine or free chlorine. If ozone is used for disinfection of surface waters, the ozone can break down complex organic molecules into smaller organic molecules or molecular fragments that are more readily used by bacteria as a food source. Using ozone can thus increase the biological instability of the water and result in a higher level of bacterial growth in the distribution system. One remedy for biological instability is to employ biological filtration. This is done by using conventional filter media or GAC as a filter media in conjunction with a delay in the application of chlorine, chloramine, or chlorine dioxide until after the water is filtered. The growth of bacteria in the biological filter does not impair filtration efficacy, and some organic matter can be removed, improving the biological stability of the water. Any filtration plant that does not apply a disinfectant other than ozone before filtration is, in effect, practicing a form of biological filtration, so this practice would not be beyond the capability of small system operators.
EPA regulations require that a disinfectant residual be maintained in distribution systems of water utilities that treat surface water. UV radiation leaves no residual, and ozone dissipates too rapidly to leave a residual. Therefore, maintaining a distribution system residual requires using free chlorine, chloramine, or chlorine dioxide.
An emerging approach to disinfection involves the electrolytic generation of mixed disinfectants, which produces ozone, chlorine dioxide, and chlorine. Electrolytic equipment has been used in water treatment for at least 20 years in the United States to generate chlorine from a sodium chloride solution, but use of such equipment to generate a mixture of disinfectants is a new concept. However, such processes currently are not a suitable option for small systems to use in meeting the EPA's disinfection requirements because of the difficulty of measuring the concentrations of multiple disinfectants in water and lack of data for evaluating the effectiveness of mixed disinfectants. Measurement of ozone, chlorine dioxide, chlorite, chlorate, and free chlorine in a single sample probably is not possible outside of a chemistry research laboratory, if it can be done there. Furthermore, the effectiveness of disinfectants in inactivating bacteria, viruses, and protozoa is estimated based on empirical data, and insufficient data are available for disinfectant mixtures. In the absence of data on the performance of mixed disinfectants under a wide range of water quality conditions, this type of technology cannot be applied to meet the EPA's requirements for disinfection.
Monitoring Requirements
EPA regulations require systems to periodically monitor the residual concentration of disinfectant before water is served to the first customer on the distribution system. This regulatory requirement reflects the practical reality that monitoring is essential to successful disinfection because it provides evidence that a disinfectant residual has been attained. Without monitoring capability, an operator has no basis for knowing that disinfection is adequate. Test kits or spectrophotometers allow for easy monitoring of the disinfectant residual for free chlorine, total chlorine (free and combined), and ozone. Monitoring residual levels of chlorine dioxide and its degradation products, chlorate and chlorite, is more challenging and probably beyond the capability of most small systems.
The intensity or rigor of chemical disinfection provided in the treatment plant, before water is delivered to customers, is assessed in terms of CT, in which C is the residual concentration of the disinfectant in milligrams per liter and T is the time in minutes for which the water and disinfectant chemical were in contact. The product of these parameters is a measure of the effectiveness of disinfection and is used to determine compliance with drinking water standards.
The second factor in the CT value, contact time, depends on, among other things, the geometry of the vessel or basin containing the water to which disinfectant has been added and the rate of flow of the water through the contact basin. The EPA requires that the contact time be based on the time (T10) in which the first 10 percent of water entering the basin would leave, rather than on the theoretical contact time. This is a conservative approach, but it ensures that only 10 percent of the water in the contact basin has a contact time equal to or less than the time used for assessing the value of CT. Therefore, to be able to report CT values the plant operator must also know the rate of flow at the plant and the value of T10.
Because temperature influences the efficiency of disinfection, water temperature must be monitored. The values of CT required for effective disinfection increase as water temperature decreases, reflecting the experimental observation that the resistance of microorganisms to disinfectants increases by factors of 2 to 3 for each 10°C decline in temperature.
When free chlorine is used as a disinfectant, its efficacy decreases as pH increases. Therefore, monitoring the pH of the water during disinfection is important for free chlorine. The EPA's CT values for free chlorine reflect this dependence on pH.
UV disinfection devices need built-in monitors to indicate the intensity of the UV radiation. Ideally, an automatic shut-off should prevent water flow if the UV intensity is not adequate to provide the level of disinfection required.
Disinfection byproducts, harmful compounds that form when water is disinfected, will become another aspect of water quality that small water systems must monitor and manage when the EPA's proposed Disinfection/Disinfectant
Byproducts (D/DBP) Rule takes effect within a few years. While water systems serving fewer than 10,000 persons were not included in the rule that established a drinking water standard for trihalomethanes (THMs), which are common disinfection byproducts, small systems will be included in the new D/DBP Rule. The new rule will set lower limits for THMs and new standards for haloacetic acids (HAAs). Therefore, in the next century small water systems will need to use disinfection methods that are effective for killing pathogens without forming excessive disinfection byproducts. Disinfection byproduct compliance is more likely to be a problem for small water systems treating surface waters than for those treating ground waters because surface water sources tend to contain more natural organic matter that forms byproducts when mixed with disinfectants.
Formation of byproducts depends on the quality of the source water and on the disinfectant used. Free chlorine forms THMs, HAAs, and other compounds classified as disinfection byproducts. Adding ammonia to chlorinated water forms chloramine and stops formation of most byproducts. Chloramine can cause formation of cyanogen chloride, but this compound is not regulated, nor does EPA plan to regulate it in the near future. Ozone does not form chlorinated byproducts, but in some waters that contain bromide it can form bromate and brominated byproducts that will be regulated in the D/DBP Rule. Ozone also forms aldehydes, but these are not currently scheduled for regulation. Chlorine dioxide minimizes the formation of byproducts, but this disinfectant breaks down over time and forms chlorite and chlorate. The EPA plans to regulate chlorate in the future. UV radiation produces no disinfection byproducts that are of concern at the present time.
When disinfection byproducts are regulated for small water systems, systems that use a disinfectant other than UV radiation will need to monitor for these products in their distribution systems. Small systems planning to begin use of disinfection will need to evaluate byproduct formation to be sure that they will not create regulatory compliance problems from the disinfection techniques they are planning to use.
Operating Requirements
Of all operating requirements, the most critical aspect for any disinfection process is that it MUST operate whenever drinking water is produced. This is especially true for disinfection systems used in conjunction with filtration processes, such as bag filters and cartridge filters, that are not capable of removing viruses and most bacteria. Any disinfection system intended to function in the absence of a plant operator should include automatic monitoring devices that shut down the plant if disinfection becomes inadequate. Such cases require that an adequate treated water supply be on hand when the water system is shut down, or that a ''boil water" order be issued.
Routine tasks for a plant operator include monitoring disinfectant residual,
maintaining disinfectant feed equipment, and ensuring that an adequate supply of disinfectant is on hand when chlorine or chloramine are used. When chlorine dioxide is used, adequate quantities of feedstocks must be kept. Operators of systems using ozone need to maintain the ozone generation and air preparation equipment.
When chloramine is used, both chlorine and ammonia must be added to the water. This can be done with solution feeders for calcium hypochlorite or sodium hypochlorite and ammonium sulfate. Liquid chlorine in cylinders that provide chlorine gas under pressure can also be used, although its use is not favored by some systems because of transportation and storage hazards. With chloramine, chemicals must be fed accurately. If the ratio of chlorine to ammonia falls outside of the appropriate range, water quality problems can arise in the distribution system, either from production of dichloramine and nitrogen trichloride (which can cause odor problems) if chlorine is overfed or from the presence of ammonia (which can lead to biological instability of the water) if ammonia is overfed. While chloramine use provides important advantages in the distribution system, particularly with respect to minimizing disinfection byproduct formation, chloramination must be monitored and controlled carefully. In addition, chloramine is not as strong a disinfectant as chlorine, so it requires a much higher CT value.
Generation of chlorine dioxide is more complex than production of chloramine. Because of this complexity, as well as the complexity of monitoring, chlorine dioxide may not be appropriate for most small systems.
A shortcoming of many small systems, particularly those with package plants, is the small amount of disinfection contact time (T) available. To reduce capital costs, many small systems do not have the extensive storage needed to ensure the proper contact time, particularly when water temperatures are near freezing. Opferman et al. (1995), in a paper that assessed CT compliance in Ohio, reported, "In Ohio, several small operators elected to close their treatment plants and link with a larger countywide water supply system rather than invest in clearwell upgrades." Meeting the CT requirement may be a major challenge for some small systems, particularly those that use chloramine.
A second shortcoming for many water systems, both small and large, is that chemical disinfectants (mainly chlorine and chloramine, the most widely used disinfectants) are often added to water without provision for thorough and rapid mixing into the water being treated. Much greater care is used to mix coagulant chemicals in water than to mix chlorine into water, yet both can accomplish their intended functions only after they have been dispersed into all of the water. The past practice of adding chlorine to water without much forethought as to how it was mixed may reflect lax attitudes toward disinfection in the era before Giardia (i.e., to the end of the 1970s), when maintaining a free chlorine residual of 0.2 mg/liter at the end of 30 minutes (a CT of 6) was considered an adequate disinfection
practice. This practice would no longer be acceptable for surface water treatment.
Suitability for Small Systems
Some disinfection processes have already been customized for small systems. UV disinfection, in particular, is probably more appropriate for small systems that treat ground water than for large systems. UV disinfection systems require electricity to power the UV lamps in the device. If water is pumped during treatment, the UV device could be wired to operate whenever the pump runs. This sort of arrangement lends itself to operation without an operator in attendance, although some monitoring is needed to verify that the UV disinfection process is operating properly when water is being pumped.
A key factor related to the use of free chlorine in small systems is feeding the chlorine. A number of chemical solution feeders are available for feeding calcium hypochlorite or sodium hypochlorite solutions. Sodium hypochlorite is easily added to the water using a diaphragm pump, but calcium hypochlorite sometimes contains insoluble particles that can cause problems with these solution feeders. To prevent such problems, some types of equipment can add hypochlorite to the water in solid form. One such feeder discharges small hypochlorite pellets at a measured rate that can be changed by adjusting the feeder. This chlorinator typically is mounted near the top of a well casing and wired to operate whenever the well pump runs. This way, hypochlorite pellets drop into the well casing whenever water is pumped. This type of feeder is most appropriate for disinfection of ground water, but a clever operator probably could adapt it to treatment of surface waters. Another type of chlorine feeder works on the erosion feed principle. In this device, hypochlorite disks shaped like hockey pucks slowly dissolve when water flows through the feeder. This feeder has the advantage of being able to operate without electrical power, but a disadvantage is the fluctuation of chlorine dose that results from uneven rates of dissolution of the hypochlorite disks. A possible solution to the problem of uneven feed rates would be use of an equalization tank ahead of the tank or basin providing chlorine contact time. The equalization tank would be designed to dampen fluctuations in influent chlorine concentration and provide a more steady effluent chlorine concentration.
A number of manufacturers make small package ozone-generating systems. To use ozone, a utility must also provide a contractor or series of contractors. Typically these need to be 6 m (20 ft) deep to provide for efficient contact between ozone and the water being treated as the ozone bubbles added at the bottom rise to the top of the contractor. Some small systems might have flows low enough that ozone contact chambers could be made from large-diameter reinforced concrete pipes placed in the ground and aligned on a vertical axis. A
number of ozone systems use ejector or diffuser systems that do not require deep contact basins.
Within the present regulatory framework, for most small systems use of free chlorine will be the easiest disinfection process to manage because of the greater complexity associated with using the other disinfectants. If free chlorine causes formation of disinfection byproducts, a logical next step would be to use free chlorine for pathogen kill and chloramine to provide a distribution system residual. If that approach does not adequately control disinfection byproducts, use of ozone followed by chloramine would be appropriate.
Corrosion Control
How the Process Works
Many water systems include corrosion control technologies to prevent corrosion of the water distribution system and to reduce lead and copper concentrations in the water where lead and copper pipes or fittings are used. Corrosion control generally involves modifying the chemistry of the water, forming a precipitate or stabilizing compound on the surfaces of piping in contact with water, or both. Most approaches include adding chemicals that can increase the alkalinity or pH of the water or can act as corrosion inhibitors by lining pipe surfaces.
One approach to corrosion control for small systems is the use of limestone contractors to modify water chemistry. Instead of using a feeder to add chemicals that increase alkalinity and pH, low-pH, corrosive water is passed through a bed of limestone rock. The water dissolves the calcium carbonate in the limestone, increasing the alkalinity and pH. One advantage of this approach is that because the chemicals are added to the water by dissolution, they cannot be overdosed, as could happen during a malfunction of a chemical feeder. Letterman et al. (1987) have shown that this process can work for small water systems, and the application of a limestone contractor for a small water system was discussed by Benjamin et al. (1992). An approach for steady-state design of limestone contractors was described by Letterman et al. (1991).
Another approach to corrosion control is the use of orthophosphates and polyphosphates (AWWARF, 1996). Orthophosphates are effective corrosion inhibitors at concentrations of 1 to 3 mg/liter as phosphate. They will aid in the reduction of lead and copper concentrations at the tap and will also reduce the rate of iron corrosion. Polyphosphates are effective as an agent to prevent red water, an undesirable effect of iron corrosion, because they will complex the iron before it can form a reddish precipitate. They also revert to orthophosphates, and this is thought to be a major reason for their effectiveness in controlling lead and copper concentrations at the tap.
Some ground waters have high concentrations of carbon dioxide (CO2). For such waters the removal of CO2 by air stripping can raise the pH and reduce
corrosivity. Air stripping is especially useful for copper corrosion control in low-pH, high-alkalinity waters (Edwards et al., 1996).
Appropriate Water Quality and Performance Capabilities
Chemicals added through feeders can change the pH of water to virtually any desired value, depending on the type and concentration of corrosion control chemical being fed.
The range of pH and alkalinity increase that can be attained by limestone contractors is limited by the equilibrium chemistry for calcium carbonate solubility. Thus limestone contractors have a practical upper limit for the pH of treated water. If a high-pH approach to corrosion control is desired, limestone contractors will not suffice. In addition, waters containing reduced, dissolved iron could cause problems in a limestone contractor if the pH increase is sufficient to precipitate iron onto the limestone rock in the contractor. Turbidity also might foul a contractor. For these reasons, the quality of the water to be treated by a limestone contractor should be evaluated before a contractor is installed.
For orthophosphates and polyphosphates, pH control is important, because the orthophosphates work best at a pH in the range of 7.2 to 7.8 for lead and copper control.
Aeration to strip CO2 from ground water could result in oxidation of dissolved iron and thus might be inappropriate for some waters or might require use of additional treatment processes for removal of precipitated iron.
Monitoring and Operating Requirements
Distribution system and customer tap monitoring requirements for corrosion control are set forth in the EPA's Lead and Copper Rule. In addition, corrosion control process equipment should be monitored as a means of maintaining control of the treatment process. Chemical feeders require regular checking for operational status, feed rate, and amount of chemical fed during the time interval since the last check. Limestone contractors should be inspected periodically to determine the amount of limestone remaining in the contractor. (Because limestone dissolves, it must be periodically replaced.) Regular inspections to check for fouling are also wise.
Suitability for Small Systems
Chemical feeders for use in small water systems are readily available, but determining and adjusting chemical feed rates may be difficult for small systems. Water quality problems can result from both underfeeding and overfeeding pH adjustment chemicals or corrosion inhibitors. The dosages must be correct for the corrosion control chemicals to work properly, so careful monitoring is required.
In contrast, the limestone contractor concept for corrosion control was developed specifically for small systems, and if raw water quality is amenable to this treatment technique it is well suited to small systems. Use of aeration for stripping CO2 from ground water also is a manageable process for small systems, although it must be carefully controlled to prevent excessive calcium carbonate precipitation in the distribution system.
Membrane Filtration Systems
How the Process Works
Once considered a viable technology only for desalination, membranes are increasingly employed for removal of bacteria and other microorganisms, particulate material, and natural organic matter, which can impart color, tastes, and odors to the water and react with disinfectants to form disinfection byproducts. As advancements are made in membrane production and module design, capital and operating costs continue to decline.
The several membrane filtration technologies appropriate for water treatment are distinguished by their nominal pore size or nominal molecular weight cutoff (MWCO). The MWCO is an estimate of the smallest size molecule that will be retained by the membrane in a filtration process. By these guidelines, membrane filtration technologies are classified as employing microfiltration, ultrafiltration, or nanofiltration, with microfiltration using the largest pores and having the highest MWCO and nanofiltration using the smallest pores and having the lowest MWCO (see Figure 3-1). All three types use similar principles.
Pressure-driven membrane filtration systems use applied pressure to drive water from the source water side of a semipermeable membrane to the produced-water side. Impurities are retained by size separation on the membrane while the water passes through the membrane, and they concentrate in the retained concentrate stream. The membrane permeate or product water is generally of a very high quality.
Membranes are thin, porous structures produced from a variety of materials. Early membranes were commonly made of cellulose acetate, and this type of membrane remains a choice today. Membranes are also now made of polypropylene, polyethylene, aromatic polyamides, polysulfone, and other polymers. Each membrane material has relative advantages and disadvantages. Cellulose acetate membranes permit fairly high water flux but are limited to operation in fairly narrow ranges of temperature (less than 30°C) and pH (3 to 6) and are sensitive to chlorine. Polyamide membranes have a higher resistance to pH and temperature extremes but are similarly intolerant of chlorine. Polysulfone membrane materials are more resistant than either of the other types to pH extremes, temperature, and chlorine exposure but, being hydrophobic, may foul more rapidly. Reliable, durable membranes are presently available, but the science of membrane production
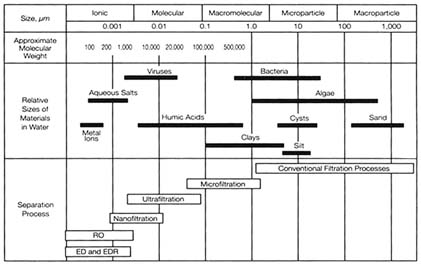
FIGURE 3-1 Sizes of molecules removed by various membrane processes in comparison to conventional filtration processes. SOURCE: Reprinted from Electrodialysis and Electrodialysis Reversal (M38), by permission. ©1995 by the American Water Works Association.
is still advancing. In addition, while all membrane materials work well under the proper conditions, choosing the most appropriate membrane for a given application still remains an art. The longevity of various membranes should be compared based on manufacturer information prior to choosing a given membrane material.
Many membranes are anisotropic in nature, consisting of a thin surface skin approximately 0.1 to 2 microns in thickness supported by a sturdier, more porous structure 100 to 200 microns in thickness (Cheryan, 1986). The surface skin performs the needed sieving of impurities from water. Composite membranes are also available. These consist of a highly resistant porous polymer, such as polysulfone, coated with a highly selective skin layer, such as cellulose acetate. Membranes can also be surface treated, as in a surface-sulfonated polysulfone membrane. This modified surface is more hydrophilic than the parent polymer, thus reducing fouling potential.
Membranes can be arranged in any of several types of configurations, the most common being hollow fine-fiber modules and spiral-wound modules. In either setup, the operating principle is the same. Water is pushed through the membrane by a higher upstream pressure. Contaminants are removed from the permeate water by sieving. Hollow fine-fiber membrane modules consist of thousands of hollow membrane tubes, approximately 500 to 1,000 microns in
FIGURE 3-2 Spiral-wound membrane elements.
SOURCE: Courtesy of Osmonics, Minnetonka, Minnesota.
diameter each, with the selective skin layer either on the interior or exterior surface of the tube. If the skin is on the interior surface of the tubes, pressurized source water is fed through the inside of the tubes, permeate water passes through the pores in the membrane, and the concentrate water with its impurities remains inside the fibers. The concentrate water flows out the opposite end of the membrane tubes and can be sent through a series of membrane modules for further treatment. An advantage of hollow fiber modules is the low pressure drop within a membrane module in comparison to spiral-wound modules, meaning that power requirements are lower for these units than for spiral-wound modules.
A spiral-wound module is made up of multiple sheets of flat membranes, with a mesh spacer material sandwiched between (see Figure 3-2). In order to provide a large membrane surface area within a fairly small module volume, the stack of membranes is rolled like a jelly roll, with the influent water fed to the individual membrane sets by a tube in the center of the roll, hence the term "spiral-wound." The membranes are arranged in sets of two, with the selective surfaces of the two membranes facing each other in each set. Source water passes under pressure through the interior surface of each set. Permeate water passes through the membranes and collects in the channels between membrane sets, then flows to a permeate water collection system. Concentrate water remains in the channel within the membrane sets, and, as with the hollow fine-fiber modules, this water can be further processed in a series of spiral-wound modules.
Appropriate Water Quality and Performance Capabilities
Membrane filtration is a physical rather than chemical treatment process.
Chemical characteristics of the water source do not greatly affect the process except in their potential for fouling the membrane surface. The concentration of particulate matter, such as bacteria and clays, and natural organic matter is of concern, for these substances can foul the membranes. To avoid this, water must be relatively free of particulate material prior to entering a membrane module. Surface waters may require pretreatment by a more conventional treatment process prior to polishing by membrane filtration, although membrane systems are capable of tolerating a lower quality surface water than direct filtration systems (discussed later in this chapter). Generally, coarse filtration, such as that provided by a bag or cartridge filter, will sufficiently pretreat the source water. Sometimes a coarser mode of membrane filtration is used prior to a finer filtration operation, such as pretreating a surface water with microfiltration prior to removal of disinfection byproduct precursors with a nanofiltration system.
Microfiltration is loosely defined as a membrane separation process using membranes with a pore size of approximately 0.03 to 10 microns, an MWCO of greater than 100,000 daltons, and a relatively low feed water operating pressure of approximately 100 to 400 kPa (15 to 60 psi). Representative materials removed by microfiltration include sand, silt, clays, Giardia and Cryptosporidium cysts, algae, and some bacterial species.
Ultrafiltration involves the pressure-driven separation of materials from water using a membrane pore size of approximately 0.002 to 0.1 microns, an MWCO of approximately 10,000 to 100,000 daltons, and an operating pressure of approximately 200 to 700 kPa (30 to 100 psi). Ultrafiltration will remove all species removed by microfiltration as well as some viruses and humic materials.
Nanofiltration membranes have a nominal pore size of approximately 0.001 microns and an MWCO of 1,000 to 10,000 daltons. Pushing water through these smaller membrane pores requires a higher operating pressure than either microfiltration or ultrafiltration. Operating pressures are usually near 600 kPa (90 psi) and can be as high as 1,000 kPa (150 psi). These systems can remove virtually all viruses and humic materials. They provide excellent protection from disinfection byproduct formation if the disinfectant residual is added after the membrane filtration step. Because nanofiltration membranes also remove alkalinity, the product water can be corrosive, and measures such as blending raw water and product water or adding alkalinity may be needed to reduce corrosivity.
Membrane filtration greatly reduces the need for disinfectants. Protozoa, bacteria, and even viruses can be removed in the process, which can relieve a portion of the CT disinfection requirement, if proven to the satisfaction of regulators. Nanofiltration also removes hardness from water, which accounts for nanofiltration membranes sometimes being called "softening membranes." (Hard water treated by nanofiltration will need pretreatment to avoid precipitation of hardness ions on the membrane.) Although membrane filtration is most commonly used to remove inorganic or microbiological contaminants, a pilot-scale demonstration showed that a nanofiltration system removed a variety of synthetic
organic chemicals (Duranceau et al., 1992). Removal was related to the molecular weight of the synthetic organic compound. Lower molecular weight synthetic organic compounds such as ethylene dibromide and dibromochloropropane passed through the membrane, while the slightly higher molecular weight pesticides chlordane, heptachlor, and methoxychlor were removed from the permeate. Based on the results of such studies, larger organic compounds such as natural organic matter would be removed by nanofiltration.
Membrane classification standards vary considerably from one filter supplier to the next. One supplier may sell as an ultrafiltration membrane a product similar to what another manufacturer calls a nanofiltration system. It is best to look directly at pore size, MWCO, and applied pressure needed when comparing two membrane systems.
Monitoring and Operating Requirements
Efficient operation of a membrane separation system relies as much on module design as on membrane material choice. Capital costs of membrane systems are a function of the type of system configuration and the membrane surface area: volume ratio for a given module. Operating costs are influenced by module replacement costs, pressure requirements, ease of cleaning, and cleaning solution and concentrate disposal costs. While the initial membrane purchase is a relatively minor portion of the capital cost, membrane replacement is the largest component in the cost of operation (Wiesner et al., 1994).
Prevention of fouling of microfiltration and ultrafiltration membranes requires regular backwashing of the membranes. Operation is usually automated, with backwash of contaminants from the membrane surface occurring at a prearranged time, a prescribed effluent turbidity, or a predetermined change in operating pressure. For this reason membrane plants often can be allowed to operate unattended much of the time. The principle of operation is simple and not tied directly to source water chemistry. Antiscalant chemicals may need to be added to the water when the concentrated water retained by the membrane exceeds solubility limits for salts such as calcium carbonate. This is more likely in tighter membrane systems such as those using nanofiltration.
Waste stream disposal is a significant problem in many areas. Unlike conventional treatment processes, in which approximately 5 to 10 percent of the influent water is discharged as waste, membrane processes produce waste streams amounting to as much as 15 percent of the total treated water volume. Because little or no chemical treatment is used in a membrane system, the concentrate stream usually contains only the contaminants found in the source water (although at much higher concentrations), and for this reason the concentrate can sometimes be disposed of in the source water. Other alternatives include deep well injection, dilution and spray irrigation, or disposal to the municipal sewer; these alternatives are usually necessary for nanofiltration waste, which usually
contains concentrated organic and inorganic compounds. Regardless of the type of membrane, concentrate disposal must be carefully considered in decisions about the use of membrane technology.
Suitability for Small Systems
Membrane filtration systems have very little economy of scale, so capital costs on a basis of dollars per volume of installed treatment capacity do not escalate rapidly as plant size decreases. This makes membranes quite attractive for small systems. In addition, for ground water sources not needing pretreatment, membrane technologies are relatively simple to install, and the systems require little more than a feed pump, a cleaning pump, the membrane modules, and some holding tanks. Most experts expect that membrane filtration will be used with greater frequency in small systems as the complexity of conventional treatment processes for small systems increases.
In a cost comparison of membrane filtration and conventional treatment, particle removal by ultrafiltration was estimated to be substantially less expensive than by conventional filtration technologies for small systems (Wiesner et al., 1994). As facility capacity decreased, the membrane cost advantage increased. Similarly, when nanofiltration was compared to conventional treatment with the addition of ozone and granular activated carbon to control disinfection byproduct and total organic carbon levels, the two treatment techniques produced similar water quality, but the membrane systems were substantially less costly for small system sizes (Wiesner et al., 1994).
The operation of a nanofiltration system is substantially less complicated than operation of the multiple treatment train needed to reach the same result by conventional systems. Membrane filtration should be considered for small systems that need to remove multiple contaminants. There are few limitations to the types of raw water that membrane filtration systems can treat, although pretreatment of the water to remove particles may be necessary, and testing to determine potential fouling by organic matter should be performed.
Reverse Osmosis
How the Process Works
Reverse osmosis (see Figure 3-3) is a highly efficient removal process for inorganic ions, salts, some organic compounds, and, in some designs, microbiological contaminants. Reverse osmosis resembles membrane filtration processes in that it involves the application of a high feed water pressure to force water through a semipermeable membrane. In osmotic processes, water spontaneously passes through a semipermeable membrane from a dilute solution to a concentrated solution in order to equilibrate concentrations. Reverse osmosis is produced
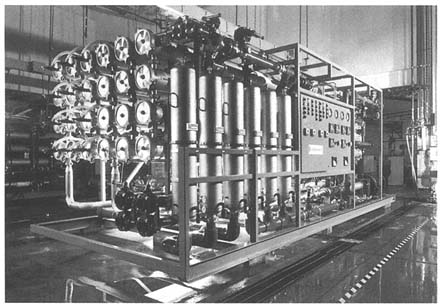
FIGURE 3-3 Skid-mounted reverse osmosis system. SOURCE: Courtesy of Osmonics, Minnetonka, Minnesota.
by exerting enough pressure on a concentrated solution to reverse this flow and push the water from the concentrated solution to the more dilute one. The result is a clear permeate water and a brackish reject concentrate.
Several differences distinguish reverse osmosis from membrane filtration. Unlike in membrane filtration, in reverse osmosis the membrane is essentially nonporous; transport of water through the membrane takes place by sequential dissolution of the water into the membrane and diffusion through the membrane to the permeate side. Any contaminants that can dissolve into and diffuse through the membrane can also pass into the permeate in this system, though such contaminants are few. The membrane rejects most solute ions and molecules, allowing water of very low mineral content to permeate; some organic contaminants can permeate reverse osmosis membranes.
Reverse osmosis produces a larger volume of reject concentrate solution than membrane filtration. The concentrate volume can be as much as 25 to 50 percent of the raw water volume. In addition, though module configurations resemble those of membrane filtration processes, operating pressures are much higher, ranging from approximately 1,400 kPa (200 psi) for water with a total dissolved solids concentration of less than 1,000 mg/liter to as high as 10,000 kPa (1,500 psi) for seawater with a total dissolved solids content of 35,000 mg/liter. The higher pressure is needed to overcome the solution osmotic pressure and
force water through the membrane from the concentrated feed side to the dilute permeate side.
The permeate from a reverse osmosis system is virtually demineralized and therefore quite corrosive. To maintain stable water in the distribution system, a predetermined fraction of the raw water is usually allowed to bypass the system and is mixed with the permeate. Posttreatment may include degasification if carbon dioxide and/or sulfide is present in the water, pH adjustment to reduce corrosiveness, and disinfection.
Appropriate Water Quality and Performance Capabilities
Removal efficiencies for inorganic ions and salts range from 85 to 99 percent. Removal of organic chemicals varies with the chemical in question. Low-molecular-weight organic compounds, as well as organic compounds with an affinity for the particular membrane material, may diffuse through the membrane. The removal efficiencies for organic compounds range from no removal to better than 99 percent removal. Humic materials, particulate matter, microorganisms, and viruses are generally removed in the process, but the bypass water will add microbiological contaminants to the treated water when the two are mixed to reduce corrosiveness. Also, leaks of concentrate water containing bacteria and viruses can occur around o-ring seals under the high operating pressures of a reverse osmosis system.
A reverse osmosis membrane can severely foul if proper pretreatment is not provided. Influent total organic carbon concentrations can be as high as 20 mg/liter, but pretreatment must be used to reduce influent turbidity and to remove any iron, manganese, and chlorine. Stabilization of the water to prevent scale formation may also be necessary, as the concentrate solution may contain inorganic contaminant concentrations so high that precipitation could occur. The water's pH may have to be adjusted to avoid reducing membrane life.
A typical membrane module lasts 3 to 5 years, after which module replacement is necessary. Membrane module replacement costs remain high and are a significant consideration in the overall cost of the treatment system.
Monitoring and Operating Requirements
Most reverse osmosis systems are set up to backwash automatically, and therefore the pressure unit itself requires little operator attention. However, pretreatment may require a skilled operator.
The membrane must be cleaned periodically to remove scale at the surface. Caution is required to avoid contamination of raw or finished water with the generally acidic cleaning solution. In addition, membranes must be flushed with product water prior to shutdown to prevent prolonged contact between the membrane and a concentrated solution; otherwise, scaling from chemical precipitation
can occur within hours of shutdown. If the plant is not operated for several days, the membranes should be filled with a disinfectant solution to prevent biological growth and possible membrane damage.
Disposal of reject water poses an even greater problem for reverse osmosis systems than for lower-pressure membrane filtration processes such as ultrafiltration or nanofiltration because they produce a larger quantity of reject water, and the contaminants are more concentrated than those produced by filtration processes. Release to evaporation ponds or the municipal sewer or injection in deep wells are current disposal strategies. However, in the future some of these strategies may no longer be permitted in some areas. Disposal needs and local regulations governing disposal must be considered in planning a reverse osmosis treatment plant.
Suitability for Small Systems
Like other modular membrane processes, reverse osmosis has little economy of scale and therefore is just as suitable for a small system as it is for a large one. Reverse osmosis is a rugged and reliable treatment process on the small scale. Air-droppable reverse osmosis units were used during the Gulf War to supply water to troops near saline water supplies. Plant expansion can be as easy as adding an additional series of membrane modules to the treatment train. Operation can be automated, allowing reverse osmosis systems to be run by part-time operators. There are 142 operating reverse osmosis drinking water plants in North America, with more than a third of them serving fewer than 3,500 people (Morin, 1994). The technology is commonly used in Florida to treat drinking water for condominiums and mobile home parks (Sorg et al., 1980).
One example of a small community that uses reverse osmosis is Wenona, Illinois. Prior to installation of a reverse osmosis system, the town's approximately 1,200 residents experienced problems with the deep-well ground water they use. The source water has high levels of dissolved solids, which imparted a salty taste to the water and damaged equipment such as water heaters and washing machines. Most residents drank bottled water. In addition, radium levels in the source water are above the drinking water standard. The reverse osmosis plant removes 99 percent of radium-226 and 95 percent of dissolved solids. Most consumers have since taken their water softeners off line and are able to use approximately half of the soap and shampoo once necessary (JAWWA, 1993).
Electrodialysis/Electrodialysis Reversal
How the Process Works
Electrodialysis (ED) and electrodialysis reversal (EDR) systems, usually employed to produce demineralized water from brackish water sources, use electrochemical
separation processes to concentrate salts from the feed water into a smaller-volume, higher-concentration solution. ED and EDR systems consist of stacks of alternating anionic and cationic selective membranes. The ionic components of dissolved salts pass through the membranes in response to an electric current applied to the water perpendicularly to the membranes. The system creates a demineralized product water stream and a brine concentrate stream.
In ED and EDR systems, the anions travel from the feed water channel toward the anode and pass through an anionic selective membrane but are rejected from transfer through the cationic selective membrane; the result is that anions are retained in the channel between the anionic and cationic membranes (see Figures 3-4 and 3-5). Simultaneously, cations from another feed channel travel toward the cathode in response to the electric current, pass through a single cationic membrane, and are concentrated in the same channel as the anions between the cationic and anionic membranes. In this matter, all the ions in a given feed channel are removed and concentrated in a concentrate channel.
In EDR, the polarity of the electrodes is reversed every 15 to 20 minutes. The change causes a reversal in ion movement. A concentrate channel at one polarity becomes a demineralized channel at the opposing polarity. Automatically operated valves tied in to the polarity change transfer incoming and outgoing flows to the proper piping. Reversing the polarity, and consequently the water flows, minimizes scale buildup by providing regular washing of the membrane surface. EDR systems can thus operate for longer periods of time between cleanings than ED systems. The majority of plants in the United States using this technology are EDR plants (Morin, 1994).
Appropriate Water Quality and Performance Capabilities
ED and EDR systems require feed water pretreatment, at a minimum with cartridge filters. Feed water turbidity should be less than 2.0 nephelometric turbidity units (NTU), the free chlorine concentration less than 0.5 mg/liter, manganese less than 0.3 mg/liter, and hydrogen sulfide less than 0.3 mg/liter (Conlon, 1990). Hydrogen sulfide is highly unlikely to be present in surface water and would generally be a concern only for ground water sources. Total dissolved solids levels of up to 4,000 mg/liter have been tolerated by EDR plants successfully producing water that meets drinking water total dissolved solids standards (Morin, 1994).
In contrast to membrane filtration processes and reverse osmosis units, the product water in ED and EDR systems does not pass through the membrane. This reduces the potential for concentration polarization and organic fouling of the membrane surface but provides no means for removing microbiological contaminants, organic compounds, or particulate or colloidal materials. SDWA requirements for these contaminants must be met through pretreatment or posttreatment of the water by other means, if necessary.
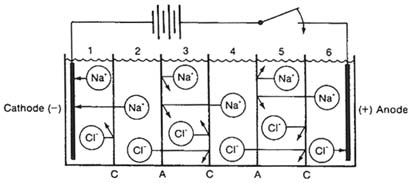
FIGURE 3-4 Removal of sodium chloride from water in an ED system. Chloride ions move toward the anode and pass through the anion selective membranes (A) but are trapped by the cation selective membranes (C); sodium ions move toward the cathode and pass through the cation selective membranes but are trapped by the anion selective membranes. The result is a demineralized water in channels 2 and 4. SOURCE: Reprinted, with permission, from Meller (1984), Electrodialysis (ED) and Electrodialysis Reversal (EDR) Technology. ©1984 by Ionics, Inc., Watertown, Massachusetts.
FIGURE 3-5 Typical membranes and membrane spacers used in ED and EDR systems. SOURCE: Courtesy of Ionics, Inc., Watertown, Massachusetts.
Both the anionic and cationic membranes used in ED and EDR systems are fairly sturdy and resistant to water quality conditions. They are 0.5 mm in thickness and resist damage from pH extremes in the range of pH 1 to 10. They can tolerate temperatures up to 46°C (115°F) (Conlon, 1990).
A disadvantage of ED and EDR systems is their high energy requirements. Pumping requirements are similar to those for an ultrafiltration system, with the costs for maintaining the direct current at least equal to the pumping costs. Energy costs other than pumping are a function of feed water salinity. The energy required to provide the current is approximately 2.0 kWh per 3,800 liters (1,000 gals.) treated per 1,000 mg/liter total dissolved solids removed (Conlon, 1990).
The recovery rate in an ED or EDR process is the percentage of feed water that becomes product water. Most EDR plants operate at a recovery rate of 70 percent or better. The remaining 30 percent is disposed of as the concentrate stream (Morin, 1994). Like water treated by reverse osmosis and nanofiltration, ED/EDR water is corrosive, so some bypassed water may be needed to stabilize the product water.
Monitoring and Operating Requirements
ED and EDR systems are usually fully automatic. Recordings of system operation may be taken by a computer or by an operator, if available. Membranes and, less frequently, electrodes will need to be replaced. Routine maintenance is fairly simple. Equipment such as pumps and chemical feed systems requires the usual maintenance. ED (but not EDR) systems need antiscalant chemicals.
Operation must be performed at a direct current density less than the limiting density of the system. A limiting voltage also applies in order to prevent heating the system and causing damage to the membranes and/or spacer material. These operating parameters are set at installation by the supplier.
Suitability for Small Systems
ED and EDR plants are well suited to small systems with brackish water sources. More than half of the operating ED and EDR plants in North America serve fewer than 3,500 people (Morin, 1994). Some plants serve as few as 200 people. ED and EDR plants are generally automated, allowing for part-time operation. As with reverse osmosis, energy consumption must be considered when evaluating whether to apply this technology.
Adsorption
How the Process Works
Adsorption is the physical and chemical process by which an organic contaminant
accumulates on the surface of a solid, removing the contaminant from solution in the water. Organic contaminants, including toxic synthetic organic chemicals, color-causing compounds, and taste- and odor-causing compounds, are all less polar than water and therefore have low solubility in water, which is a polar liquid. Thus, they are attracted to the nonpolar solid surface.
The most common adsorbent used to remove organic contaminants from water is activated carbon. Activated carbon is similar to charcoal. It differs from charcoal in that the base material (typically bituminous coal, lignite, petroleum coke, or bone char) has been heated in the absence of air to carbonize it and then activated by oxidation at 200°C to 1,000°C to develop a favorable pore structure. The result is a highly porous structure with a very high surface area per unit volume, which allows for significant adsorption of impurities from water. In general, the less soluble an organic compound, the better it adsorbs from solution onto the activated carbon (Lundelius, 1920; Weber, 1972).
Activated carbon is available in two common forms, powdered activated carbon (PAC) and granular activated carbon (GAC), the difference between the two being obvious from their names. PAC is generally less than 50 µm in diameter and is added to the raw water line or to a mixing basin. For effective treatment, the PAC must contact all of the incoming water. Because of its small particle size, adsorption to the surface occurs quickly. The normal contact time of mixing basins used for other elements of water treatment is sometimes sufficient for contaminant adsorption onto PAC. In such cases, no modification other than the addition of PAC dosing equipment needs to be made to an existing plant. In other cases, adsorption can require up to 8 hours of contact time. Testing prior to design is needed to determine the required contact time. Following adsorption, the carbon containing the organic compounds is settled or filtered from the water and disposed of with the plant sludge.
GAC has a grain size in the range of 0.5 to 1.5 mm, 10 to 100 times larger than PAC. It is packed into columns through which the raw water flows. Packing the carbon in columns allows more complete contact between the water and activated carbon, greater adsorption efficiency, and greater process control than is possible with PAC (Snoeyink, 1990). GAC can be removed from the column for carbon regeneration or reactivation when necessary.
In addition to being used to adsorb organic compounds, GAC systems are sometimes used as biological filters. Microbes that stabilize water quality are allowed to grow on the GAC surfaces and in particle filters.
Appropriate Water Quality and Performance Capabilities
Activated carbon adsorption historically was used primarily to remove tastes and odors from water, but its use as an adsorbent for toxic or carcinogenic organic compounds has increased steadily and is now a primary application.
PAC should be added prior to filtration in order to provide for removal of all
the powdered material. PAC can be very economical if it is only needed on a periodic basis in response to changes in influent water quality. The dose of PAC added to the system can also be adapted to deal with varying source water quality.
GAC use requires removing particulate material from the untreated water to avoid clogging the treatment column. An alternative is to use the GAC column as the filter medium, performing both filtration and adsorption in a single step. This method requires frequent backwashing of the carbon column. Backwashing mixes the carbon in the column and can cause spent carbon to be deposited near the column effluent. The spent carbon might release some of the target compound to the effluent water. If this can be tolerated, the method is simple and compact.
Competitive adsorption is an important consideration in the design of an activated carbon system for drinking water. Natural organic material in the water may compete with contaminants for adsorption sites on the carbon, increasing the amount of carbon needed to remove the target contaminant. Competing organic compounds can also displace contaminants already adsorbed to the carbon. If the competing compound is present in a high concentration, it may displace the adsorbed contaminants to such an extent that the effluent concentration of the contaminant is temporarily greater than the influent concentration. For this reason, competing chemicals must be removed from the system prior to adsorption, or the system design and carbon replacement frequency must be adequate to allow for the competition.
Monitoring and Operating Requirements
In PAC systems, care must be taken to remove all PAC from the water. This usually requires filtration. Even a small amount of PAC passing through the system can cause the water to turn gray. In addition, if PAC enters a sample vial used for determining whether the treated water meets drinking water standards, the apparent aqueous concentration of the target contaminants can exceed regulatory standards because these contaminants will be concentrated on the PAC.
Single-stage GAC systems (meaning those in which the water flows through one GAC column rather than two or more in series) must be monitored to ensure that the column is taken out of service as soon as any trace of the target compound is found in the effluent water. If an exhausted GAC column is not regenerated or replaced, no adsorption will occur, and desorption may result in the effluent having higher concentrations of some contaminants than the influent. In addition, a buildup of microorganisms in the column may clog the column or create taste and odor problems. Monitoring for organic compounds is not as simple as for some inorganic contaminants and will likely require the services of an experienced operator or outside laboratory to perform the analyses. In either case, these monitoring requirements will increase the cost of GAC implementation.
Suitability for Small Systems
GAC is quite easy to apply on a small scale; small columns can be readily obtained and installed. Virgin (rather than regenerated) carbon is often required for use in drinking water applications, which can increase the operating costs. PAC is also quite simple to employ on a small scale if the plant already uses a process train including mixing, precipitation or sedimentation, and filtration.
Lime Softening
How the Process Works
In the lime softening process, the pH of the water being treated is raised sufficiently to precipitate calcium carbonate and, if necessary, magnesium hydroxide. Calcium and magnesium ions in water cause hardness; hard water can cause scaling problems in water heaters, and soap lathers poorly in hard water. Therefore, some water utilities remove calcium and magnesium to soften the water and improve its quality for domestic use. In small systems, lime softening would typically be practiced by adding hydrated lime to raw water to raise the pH to approximately 10. This removes calcium carbonate. If magnesium removal is also required, the pH during softening would need to be closer to 11. In some waters, addition of soda ash is needed for effective hardness removal. After mixing, flocculation, sedimentation, and pH readjustment, the softened water is filtered.
Appropriate Water Quality and Performance Capabilities
Many large water systems in the midwestern United States use lime softening to treat surface waters from sources such as the Missouri and Mississippi rivers. Well-operated lime softening plants can cope with a range of quality as great as that treated by conventional treatment. However, the combination of variable source water quality and the complexity of the chemistry of lime softening may make lime softening too complex for small systems that use surface water sources. Lime softening may be more appropriate for small systems that use ground water system because of the relatively uniform quality of ground water. Once the softening chemistry for a ground water is determined, it should not change much. In comparison, chemical additions to surface waters need to be modified frequently in response to water quality changes.
In addition to removing calcium and magnesium, lime softening removes radium, which is chemically similar to calcium and magnesium. It also removes arsenic, oxidized iron, and manganese. A recent study (Logsdon et al., 1994) indicated that lime softening plants may remove Giardia cysts as effectively as conventional treatment plants.
Monitoring and Operating Requirements
Regulatory monitoring requirements for lime softening plants depend on whether the source water is surface water or ground water. Process monitoring requirements should focus on measurement of pH, hardness, and alkalinity for plants treating ground water. In addition, filtered water turbidity monitoring is needed at plants treating surface water, not merely for compliance purposes but also to manage filter operation.
One of the difficult aspects of lime softening is the operation and maintenance of lime feeders and lines carrying lime slurry to the point of application. In addition, plant operators must understand lime softening chemistry. Measurement of pH must be accurate, and the operator must know that the pH meter is properly calibrated. Failure to maintain the proper pH in softened water prior to filtration at a lime softening plant could result in precipitation of excess lime in the filter beds and formation of calcium carbonate (essentially limestone) deposits within the filters. Because of these operational difficulties, in the future, small systems that decide to soften water may seriously consider using nanofiltration or reverse osmosis for softening instead of chemical precipitation.
Suitability for Small Systems
Lime softening has been used successfully by ground water systems serving fewer than 3,000 people. Lime softening is not likely to be applied with success by small systems that treat surface waters because of the complexity of the chemistry involved. In addition, lime softening is unlikely to be suitable for treating ground water in systems serving 500 or fewer people unless those systems have some form of contract or satellite operation that would enable a trained operator to monitor the treatment process periodically. Prefabricated lime softening equipment is available for use by small systems.
Technologies for Systems with Ground Water Sources
Ground water sources generally require less treatment than surface water supplies or ground water supplies that are under the influence of surface water. Natural filtration in the subsurface reduces the concentration of many substances, including those that cause turbidity.
Ground water has generally been considered free of microbiological contamination, and throughout much of the twentieth century many ground water supplies have been distributed without treatment. Ground water has been implicated in some disease outbreaks, however (Macler, 1996). As a result, the EPA has issued a proposed Ground Water Disinfection Rule (GWDR) that would require disinfection of all ground waters except those that qualify for a variance
or meet ''natural disinfection" requirements as determined from an evaluation of criteria such as setback distance from potential contamination sources, ground water travel time, and local hydrogeologic features (Grubbs and Pontius, 1992). All systems would be required to maintain a detectable disinfectant residual in the distribution system at all times or to maintain a heterotrophic plate count level of less than 500 organisms per milliliter. Grab samples of disinfectant would be required one to four times per day under the proposed rule.
Many ground water supplies have been contaminated from improperly sealed wells, septic tank effluent, chemicals from agricultural use, leaking underground storage tanks, and leachate from waste disposal sites. In coastal areas, overpumping of ground water has led to saltwater intrusion. These newer forms of contamination have made it necessary to treat many ground water supplies prior to disinfection and distribution. As mentioned in Chapter 2, the most common chemical contaminants in ground water are nitrate, fluoride, and volatile organic chemicals.
In addition to the technologies mentioned above, which are suitable to both surface and ground water systems, some technologies are best suited to the types of contamination found in ground water. These include air stripping, oxidation/filtration, ion exchange, and activated alumina. These processes (and others) are outlined in Table 3-3 along with the contaminants they address, the state of the technology, and a relative estimate of capital costs. Table 3-4 lists operating characteristics of the technologies.
Air Stripping
How the Process Works
Air stripping, also commonly called aeration, involves continuous contact of air with water to allow aqueous contaminants to transfer from water into the air. The air is swept from the system, taking contaminants such as volatile organic chemicals, taste- and odor-causing compounds, and radon gas out of the water, or reducing the carbon dioxide concentration to raise the pH. The contaminated air is then treated if necessary and released to the atmosphere. The driving force for transfer of the contaminants is the difference between the concentration of the contaminant in the untreated water and the concentration in water that is at equilibrium with the air. An air stripping system can remove concentrations of contaminants of up to several parts per million.
Appropriate Water Quality and Performance Capabilities
Air stripping equipment must provide for a large area of contact between the air and water and for convective movement of the water or air to allow as much water as possible to contact air. This can be accomplished in several ways:
through diffused aeration, mechanical aeration, packed tower air stripping, or gas-permeable membrane air stripping.
Diffused aeration involves introducing compressed air into the bottom of a water basin through a series of diffusers. Although usually low in cost and easy to operate and maintain, this aeration mechanism does not provide for convective movement of the water and thus does not allow as much contact between the air and water as other methods. Because of its limited efficiency, it is generally used only to adapt existing plant equipment.
Mechanical aeration introduces air into the water by rapidly agitating the water surface with a mechanical mixer. Like diffused aeration, this is not a very efficient contacting system. Mechanical aerators often require large basins, long residence times, and high energy inputs. Because they are adaptable to existing basins, mechanical aerators are often installed as a system retrofit rather than as a new design.
Tray aerators (see Figure 3-6) offer an economical method of contacting air and water. As the name suggests, a tray aerator consists of a vertical series of trays down which the water flows. The water contacts air as it drips through the trays. A tray aerator can be operated with a natural draft or with a forced draft provided by an air compressor. Using a natural draft reduces operating costs but is also less efficient than using a forced draft. Slime and algae growth can pose problems with tray aerators. Though it is not particularly desirable to add additional chemicals, biological growth on the trays can be controlled by adding copper sulfate or chlorine.
Forced draft tray aerators are one step less efficient at providing contact between air and water than the next method of aeration, packed tower air stripping. In packed tower aerators, water flows down a bed of packing material such as fixed plastic grids, loose plastic rings, or loose ceramics saddles, while air flows up through the column (see Figure 3-7). The packing material breaks the water into thin sheets and droplets, creating a large and constantly changing surface area for contact between the air and water. Pretreatment for removal of microorganisms, iron, manganese, and excessive particulate matter is important for this design. Packed tower aerators have been used for decades in water and wastewater treatment. Design is currently a straightforward process for a practiced engineer.
A final method of providing contact between air and water, using gas-permeable membranes, is gaining acceptance. These systems use a membrane module made up of highly porous, small-diameter, hollow fiber membranes as a contacting device, providing an air-water contact area per equipment volume nearly an order of magnitude greater than packed tower air strippers (Semmens et al., 1989; Zander et al., 1989). Water flows through the interior of hollow membrane tubes constructed of a material that allows gases but not liquids to pass through. The large surface area for air-water contact allows for removal of semivolatile as well as volatile organic chemicals. Gas-permeable membrane systems offer the highest
FIGURE 3-7 Typical packing material used for packed tower aerators.
SOURCE: Courtesy of Delta Cooling Towers, Fairfield, New Jersey.
removal efficiencies of all contacting devices. However, the technology must be considered "emerging" because long-term performance has not been evaluated.
Monitoring and Operating Requirements
Air stripping systems are generally set for automatic operation. They usually require only daily visits to ensure proper equipment operation and to provide preventive maintenance. Remote monitoring of pumps can indicate system performance and further reduce the need for operator attention.
Pretreatment of water may be necessary in order to avoid fouling the systems with microbial growth (especially iron-oxidizing bacteria), particulate matter, calcium precipitates, or iron precipitates. Reduced iron or manganese in ground water will oxidize when exposed to air and will precipitate. High concentrations of these chemicals can completely plug an air stripping system if not removed prior to this treatment process.
Suitability for Small Systems
An aeration system can generally be installed for a fairly low-cost. The treatment process is highly adaptable to small treatment plants, often involving a simple retrofit to existing treatment basins. Cost and treatment efficiency both
increase with increasing system complexity. If contaminant concentrations are high and regulations require treatment of the air leaving the system, however, costs increase dramatically. If the water is hard and contains a high CO2 concentration, air stripping will reduce the CO2 concentration and may cause excessive precipitation of calcium carbonate. Other than the presence of reduced iron in ground waters, no particular water quality issues affect the choice of aeration technology. The specific type of aerator depends only on the degree of contaminant reduction desired.
One example of a small community that uses aeration is the Blue Mountain Subdivision near Denver, Colorado, population approximately 400. The community installed a packed tower aerator to remove radon from the ground water supply. The aerator consistently removes 96 to 99 percent of the radon. An added benefit is the simultaneous removal of CO2, which reduces corrosion problems (Tamburini and Habenicht, 1992).
Oxidation/Filtration
How the Process Works
There are multiple places in a surface water treatment train where oxidation chemicals may be used. They can be used for disinfection (discussed earlier), color removal, taste and odor control, or organic contaminant removal. However, because of the difficulty of controlling the chemistry of such reactions when water quality varies, as in surface water, it is unlikely that a small surface water system would use oxidation/filtration. The primary use of this technology by small systems is for removal of iron and manganese from ground water sources. Iron and manganese, while not primary drinking water contaminants, are responsible for many complaints in small systems. Iron or manganese spots on laundry and fixtures cause customer dissatisfaction with the water utility.
Iron and manganese are present in ground water in their reduced and very soluble forms. Before they can be removed, they must be oxidized (meaning they must lose electrons) to a state in which they can form insoluble complexes. These suspended insoluble complexes can be removed from water by filtration. Ferrous iron (Fe2+) can be oxidized to ferric iron (Fe3+), which more readily forms the insoluble iron hydroxide complex Fe(OH)3. Reduced manganese (Mn2+) can be oxidized to Mn4+, which forms insoluble MnO2. A detention time of 10 to 30 minutes following chemical addition is needed prior to filtration to allow the reaction to take place. The insoluble complexes are best removed from water using a medium with a large (>1.5 mm) effective size range in order to reduce filter head loss (Montgomery, 1985). In a similar manner, odorous sulfides (S-) are oxidized to colloidal elemental sulfur (So) and removed.
Oxidation involves the transfer of electrons from the iron, manganese, or other chemicals being treated to the oxidizing agent. The most common chemical
oxidants in water treatment are chlorine, potassium permanganate, and ozone. Chlorine has historically been the oxidant of choice, but its role in disinfection byproduct formation has led to questions about its use and a search for alternative oxidation strategies.
Chlorine is a strong oxidizing agent, and this, in addition to its ease of feeding and economy, are reasons for its long history of use. Chlorine is most effective at a pH below 7 due to its presence in water as hypochlorous acid under these conditions. Organic compounds should be removed prior to chlorine addition to reduce the formation of harmful byproducts.
Ozone is also a very strong oxidizing agent. Ozone produces fewer known byproducts than free chlorine on reaction with organic compounds, but ozone byproducts are still under study. Ozone is not greatly affected by pH levels in the water. As mentioned in the section on disinfection, ozone has a very short half-life and must be generated on-site. This is quite energy intensive and requires an experienced operator.
Potassium permanganate is a moderately strong oxidant and is easy to feed to a system. Its addition does not cause trihalomethane formation, but possible production of other byproducts is still under study. Potassium permanganate in water produces a pink solution. If it is added in excess and not fully reduced by reacting with reduced compounds in the water supply, the resulting water will remain pink. This is not pleasing to the utility customer.
A low-cost method of providing oxidation is to use the oxygen in air as the oxidizing agent in a tray aerator. Water is simply passed down a series of porous trays to provide contact between air and water. No chemical dosing is required, which allows for unattended operation. This method is not effective for water in which the iron is complexed with humic materials or other large organic molecules. Oxygen is not a strong enough oxidizing agent to break the strong complexes formed between iron and manganese and large organic molecules.
Appropriate Water Quality and Performance Capabilities
The presence in water of other oxidizable species hinders oxidation of the desired reduced compounds. Volatile organic chemicals, other organic compounds, or taste- and odor-causing compounds may result in an oxidant demand. This additional oxidant demand must be accounted for when dosing the oxidant. Other than these possible interferences, there is no strict cutoff in water quality above which oxidation followed by filtration will not work. The expense of operation derives from the chemical use in most cases and is therefore directly related to the source water quality.
Monitoring and Operating Requirements
Oxidation followed by filtration is a relatively simple process. The source
water must be monitored to determine proper oxidant dosage, and the treated water should be monitored to determine if the oxidation was successful. Filters must be backwashed. In general, manganese oxidation is more difficult than iron oxidation because the reaction rate is slower, so a longer detention is necessary prior to filtration.
Permanganate can form precipitates that cause mudball formation on filters. These are difficult to remove and compromise filter performance. In addition, doses of permanganate must be controlled carefully or remaining unreacted permanganate will lead to pink coloration of the water. If not dosed carefully, ozone can oxidize reduced manganese all the way to permanganate and result in pink water formation as well. Manganese dioxide particles, also formed by oxidation of reduced manganese, must be carefully coagulated to ensure their removal.
Suitability for Small Systems
Oxidation using chlorine or potassium permanganate is frequently applied in small ground water systems. The dosing is relatively easy, requires simple equipment, and is fairly inexpensive.
Ion Exchange
How the Process Works
Ion exchange (see Figure 3-8) involves the selective removal of charged inorganic species from water using an ion-specific resin. The surface of the ion exchange resin contains charged functional groups that hold ionic species by electrostatic attraction. As water containing undesired ions passes through a column of resin beads, charged ions on the resin surface are exchanged for the undesired species in the water. The resin, when saturated with the undesired species, is regenerated with a solution of the exchangeable ion. A large variety of synthetic resins is available for specific applications.
Generally, resins can be categorized as anion exchange or cation exchange resins. Anion exchange resins selectively remove anionic species such as nitrate (NO3-), carbonate (HCO3-), dichromate (Cr2 O72-), fluoride (F-) and the selenium-containing species selenate (SeO42-) and selenite (HSeO32-). These resins are less effective at removing chromate (CrO42-). Anion exchange resins are often regenerated with sodium hydroxide or sodium chloride solutions, which replace the anions removed from the water with hydroxide (OH-) or chloride (Cl-) ions, respectively.
Cation exchange resins are used to remove undesired cations from water and exchange them for protons (H+), sodium ions (Na+), or, if sodium use is restricted, potassium ions (K+). Cation exchange is often used to soften water by exchanging the calcium (Ca2+) and magnesium (Mg2+) for another ion, usually
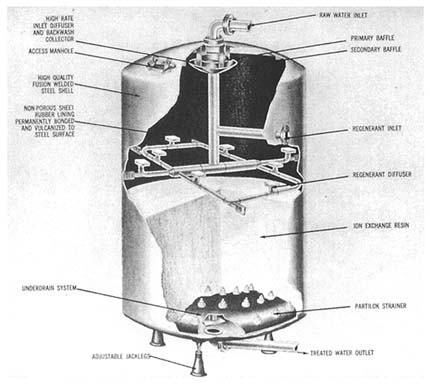
FIGURE 3-8 Cutaway diagram of a package ion exchange system.
SOURCE: The Graver Company, Graver Water Division Union, New Jersey.
sodium. At pH less than 9.3, which is typical of treated water, ammonia is present in an ionic form (NH4+) that can also be removed by cation exchange, thus reducing the possibility of microbial growth in the water distribution system. Cation exchange resins have also proven effective in removing barium (Ba2+), radium (Ra2+), cadmium (Cd2+), lead (Pb2+), and trivalent chromium (Cr3+). In many cases, cation exchange is the method of choice for radionuclide removal. It should be noted that adding sodium to water may not be desirable because of the need of some consumers to restrict their sodium intake.
Appropriate Water Quality and Performance Capabilities
Water to be treated by ion exchange must be low in solids to avoid fouling the resin. In particular, reduced iron species in ground water may become oxidized when the water is exposed to oxygen in the atmosphere and form precipitates that can damage the resin.
A resin may preferentially remove certain ions from solution. In general, it will remove highly charged ions more easily than it will monovalent ions. Calcium, magnesium, and reduced iron ions will be removed preferentially to other cations in a cation exchange system. If the target ion is other than these, the presence of such species may reduce removal efficiency.
Monitoring and Operating Requirements
An operator must monitor the system to determine the extent of resin saturation or the breakthrough of the ion to be removed. On ion breakthrough, the resin must be removed from service and regenerated. Ion exchange units can be controlled automatically, freeing the operator to make daily visits (rather than attending to the systems full time) to assure proper operation. However, determination of regeneration timing and troubleshooting requires an operator at the intermediate level of experience.
In either the cation or anion exchange process, the regeneration solution, which contains high concentrations of the undesired ions, must be carefully disposed of. Disposal can be quite costly, especially in the case of concentrated radionuclides.
Suitability for Small Systems
Ion exchange processes can be used with fluctuating plant flow rates and are readily adaptable to small units. Ion exchange is a common water treatment technology, available in point-of-use and point-of-entry devices as well as full-scale treatment plants. It is readily adaptable to small treatment plants.
An example of a small system that uses an ion exchange system is one serving approximately 400 people in Blue Mountain Subdivision, near Denver, Colorado. This system uses ion exchange for removal of uranium from ground water. Uranium levels in the raw water have been as high as 135 pCi/liter. The finished water levels are typically less than 1.5 pCi/liter, well below the drinking water standard for uranium of 30 pCi/liter (Tamburini and Habenicht, 1992). Similar beneficial results have been obtained using ion exchange for radium-226 removal from ground water near Spicewood, Texas, at the Quail Creek water system serving approximately 200 people (McKelvey et al., 1993).
Activated Alumina
How the Process Works
Activated alumina is useful for removing negatively charged ions. Activated alumina displays amphoteric properties, meaning its surface charge changes with solution pH. Alumina is not charged at a pH of 9.5, is positively charged below
this pH, and is negatively charged above it. When treated with an acid solution, alumina is strongly positively charged and will select highly for fluoride (F-), selenium species (SeO42-, HSeO32-), and arsenic (H2AsO4-). The greatest adsorption capacity for fluoride occurs at pH 5.5.
Appropriate Water Quality and Performance Characteristics
Water quality strongly influences the residence times and flow rates necessary for proper operation of activated alumina columns. In particular, the potential for preferential exchange of anions other than the target compounds in the raw water must be evaluated for each source water that will be treated with activated alumina. Therefore, pilot studies are an essential part of the design and evaluation of a full-scale activated alumina exchange system.
Monitoring and Operating Requirements
Regeneration of alumina requires a sodium hydroxide (NaOH) solution to remove the anions from the mineral surface. Following regeneration, the alumina column must be returned to the acidic state by rinsing with raw water followed by an acid solution. Anions to be removed from solution must compete with other anions, such as sulfate and hydroxide, for adsorption sites on the alumina. For this reason, sulfuric acid should not be used as the acidifying solution. In addition, the alumina dissolves slightly in sodium hydroxide. Over time the media will dissolve and require replacement.
As with ion exchange, regenerant disposal can be a problem. Some facilities discharge the regenerant brine solution to lined evaporation ponds designed for this purpose. After the water evaporates from the salts, the dried salts are disposed of in a landfill. Disposal costs can make up much of the operating cost of this technology.
Generally, the cost of an activated alumina system, including capital and operating costs, is quite high compared to other water treatment processes. In addition, operation of these systems requires advanced knowledge of water treatment principles and practice. Few systems are in operation at full-scale, possibly due to cost and operating factors.
Suitability for Small Systems
Despite its cost and operational complexity, activated alumina can adapt readily to a small system. Columns can be scaled to fit any influent flow rate. Expansion can be accommodated by adding additional columns.
Activated alumina is in use in several plants in the southwestern United States. For example, the system in Desert Center, California, serves 10,000 people and uses activated alumina to lower the fluoride level to less than 1 mg/liter
from 8 mg/liter in the source water. The plant at X-9 Ranch near Tucson, Arizona, delivers water with significantly lowered fluoride levels to its 4,500 customers (Sorg, 1978). Both plants have decades of experience with the technology.
Technologies for Systems with Surface Water Sources
Historically, the primary concerns when treating surface water have been inactivation of microbial contaminants to prevent the spread of waterborne disease and removal of turbidity to make the water more palatable and to ensure that particles that may harbor microorganisms are not conveyed to the consumer's tap. As a consequence, the EPA's Surface Water Treatment Rule (SWTR) requires disinfection of all surface waters and filtration for most surface waters before distribution to customers. The total extent of inactivation and physical removal must equal 3 logs (99.9 percent) for Giardia cysts and 4 logs (99.99 percent) for viruses. In the future, it is likely that new regulations will also require Cryptosporidium removal.
Under the SWTR, the EPA gives water utilities ''log credits" for inactivating pathogens when they use certain standard treatment processes, so that the utilities need not monitor their water for the viruses and parasites themselves. The supplementary information published with the SWTR specifies log credits to be given for both chemical disinfectants and physical filtration processes. The four filtration processes referenced in the SWTR are (1) conventional filtration, (2) direct filtration, (3) diatomaceous earth filtration, and (4) slow sand filtration. The SWTR defines conventional filtration and direct filtration to include chemical coagulation, flocculation, and, in the case of conventional filtration, sedimentation ahead of the filtration process. Several variations on treatment involving coagulation and filtration can be found in preengineered or package plants. State drinking water regulatory agencies would be responsible for deciding whether the package plants qualified for the log removal credits allotted to conventional filtration or to the reduced credit allotted to direct filtration. Filtration processes that do not function on the principles of the four defined processes listed in the SWTR are called alternative filtration processes. Examples include bag filters and cartridge filters. The logs of removal for Giardia cysts or viruses that can be allowed for alternative processes must be determined for each process. Requirements for demonstrating microbial removal vary from state to state because application of alternative filtration technology is subject to approval by the individual states.
Table 3-3 outlines the contaminants treated by various surface water filtration processes (discussed in detail below), the state of the technology, and a relative estimate of capital costs. Table 3-4 lists operating characteristics. The
tables and the following discussion group conventional and direct filtration under the heading "coagulation/filtration."
Coagulation/Filtration
How the Process Works
Coagulation/filtration processes employ chemicals such as iron salts, aluminum salts, or cationic polymers to coagulate and destabilize suspended solids in the water so they can be removed by sedimentation and filtration. The processes defined in the SWTR use some form of flocculation, meaning slow agitation of the water to promote formation of larger flocs following the addition of coagulants. A solids removal step such as sedimentation may be used. The particulate matter remaining in the water is then removed by deep-bed filtration. The filtration step works because the dose of coagulant chemical destabilizes the small particles in the water so that they attach to grains of filter material in the deep-bed. Most of the particulate matter removed in coagulation/filtration is trapped in the filter bed by surface attachment mechanisms; only a small portion is strained or screened. Deep-bed, rapid-rate filtration without the use of coagulant chemicals does not qualify as a defined filtration process under the SWTR and thus is an alternative filtration process. Coagulation/filtration treatment plants are not effective if the coagulation chemistry is incorrect. When coagulation is correct, however, these plants are effective and versatile.
Appropriate Water Quality and Performance Capabilities
The range of water quality that can be treated by coagulation and filtration depends on the process train, specifically on the extent of solids removal provided ahead of filtration. Direct filtration has the most restricted range of water quality for which it can be applied because all solids must be removed in the filter bed. As the amount of particulate matter in the flocculated water increases, run length decreases.
Some suggested guidelines can be given for direct filtration. In the hands of a small system operator, direct filtration is not appropriate for treatment of water in which the average turbidity exceeds 10 NTU or the maximum turbidity exceeds approximately 20 NTU. Source water quality should be relatively stable. If raw water turbidity can increase by a factor of 10 in one day's time, the direct filtration process may not be appropriate. Two other important raw water quality characteristics are color and algae. Color removal requires does of coagulant chemical related to the amount of color present. Thus an upper limit of approximately 40 color units would be appropriate for direct filtration (AWWA Committee, 1980). Because there are many species of algae and their effect on filtration differs according to species, no set numbers can be given for algae concentrations
that could be treated by direct filtration. Algae removal must be evaluated on a case-by-case basis. Detention times in direct filtration plants are short, so added storage may be needed for disinfectant contact time.
Removing solids prior to filtration extends the range of water quality that can be treated by coagulation and filtration. Solids removal steps that increase particulate removal but are not as versatile as sedimentation include the various upflow and downflow flocculation/filtration or "roughing filter" processes. These processes employ some type of coarse medium in which flocculation occurs as a result of the mixing caused by the twists and turns the coagulated water must make as it passes through the bed. In addition, some solids removal occurs in the coarse medium, reducing some of the load on the filter. The greatest solids removal capability in pretreatment is attained by using sedimentation, which in small package plants is usually in the form of tube settlers. These plants may be able to successfully treat water with a turbidity of 200 NTU or perhaps higher or a color of 100 to 200 color units. As with other coagulation/filtration processes, the extent of algae removal is likely to be site-specific and will depend on the type and concentration of algae present.
Coagulation/filtration has proven capable of removing turbidity, color, disinfection byproduct precursors, viruses, bacteria, and protozoa such as Giardia cysts and Cryptosporidium oocysts. Well-operated coagulation/filtration processes can produce filtered water with a turbidity of 0.10 NTU. Color removal depends on the pH during treatment and the coagulant dose employed. Removal of bacteria and protozoa can be as high as 3 to 4 logs (99.9 to 99.99 percent). Viruses are more difficult to remove, but Robeck et al. (1962) demonstrated 1-log (90 percent) to 2-log (90 to 99 percent) removal of poliovirus for direct filtration and removals exceeding 2 logs for conventional treatment.
Among the most challenging conditions for treatment by coagulation/filtration are very cold water, approximately 5°C or colder, and turbidities of approximately 10 NTU and lower. When the amount of particulate matter in the water is low, sedimentation is not very effective. Another difficult condition for water treatment is the combination of high color and moderate to high turbidity. The pH that is best for color removal may be different from the pH that is best for turbidity removal. In such a situation, identifying chemical conditions for optimum coagulation and filtration may be difficult. Finally, as mentioned previously, presence of algae in the raw water can make treatment difficult because some algae clog filters and cause very short filter runs.
Monitoring and Operating Requirements
Monitoring requirements include turbidity and pH measurement. The SWTR requires that filtered water turbidity be monitored every 4 hours, although this may be reduced to once per day for systems serving 500 or fewer persons, with state regulatory agency approval. Both streams and small reservoirs are subject
to rapid changes in water quality, especially as a result of heavy rainfall in the watershed. Therefore, turbidity monitoring frequencies of once per 4 hours or once per 24 hours are minimal and are certainly not sufficient for effective operation of coagulation and filtration when water quality is changing rapidly. Continuous monitoring of filtered water turbidity is much better because the output from the turbidimeter can be used as an aid to controlling the plant if the operator is absent. In addition to monitoring of the filtered water, raw water turbidity and the turbidity of any solids removal process ahead of filtration should be checked at periodic intervals, such as every 4 hours, when the plant is operating. Raw and treated water pH should be monitored at least once per day because of the importance of coagulation pH for turbidity and color removal.
Process equipment should be designed so that flows of raw and treated water, filter head loss, and chemical feeds can be monitored easily by the operator. In addition, sample taps should be provided so the operator can obtain samples of raw water, pretreated water, and filtered water for analysis.
When equipment is entrusted to part-time operators, the foremost operating requirement for any small system treatment process is simplicity and ease of operation. Although coagulation/filtration processes have many excellent treatment capabilities, the complexity of coagulation chemistry does not decrease with the size of the treatment plant. Therefore, small systems can face great difficulties in managing coagulation because their resources in terms of operator training and experience are in most cases limited. Equipment manufacturers have attempted to help small system operators overcome the difficulties of coagulation by providing instrumentation that can be used to control some aspect of plant operation. For example, some package use continuous turbidimeters to adjust the coagulant chemical dose upward if raw water turbidity rises to a predetermined level. Some package plants have continuous turbidimeters on the effluent, and these cause the filter to be backwashed if treated water turbidity exceeds a preset value. Other package plants use a streaming current detector to adjust the coagulant feed pump.
A second critical need for coagulation/filtration systems is continuous operation at uniform flow rates. This is the ideal mode of operation for deep-bed granular media filters. Increases in filtration rate or start-stop operation can force previously trapped floc through the filter bed and into treated water. Discharge of floc in this manner can also cause the discharge of pathogenic organisms into the treated water, with the attendant increased risk of waterborne disease. After a deep-bed filter has been shut off, it should be backwashed to clean out the floc trapped in the bed. If this is not done, floc may be discharged into the treated water when the filter is restarted. Changes in filtration rate occur at most filtration plants, but start-stop operation is probably much more common in small systems, some of which try to produce enough filtered water in a single shift to last for an entire day.
Suitability for Small Systems
Numerous variations of the coagulation/filtration treatment train are presently being marketed as package plants (see Figure 3-9). A key factor in making affordable coagulation/filtration systems is the use of high-rate sedimentation or solids removal processes and use of filtration rates on the order of 12 to 17 m/h (5 to 7 gpm/sq ft).
One approach to coagulation and filtration in package plants involves chemical addition and optional in-line mixing, followed by flocculation, sedimentation in tube settlers, and multimedia filtration. The detention time in this process train is approximately 50 minutes and is longer than the detention time in some other package plants involving coagulation and filtration.
Another approach used by a number of manufacturers involves chemical addition and optional in-line mixing followed by a "roughing filter" (a unit process given different names by different manufacturers), followed by a multimedia filter. Detention times in these units may be on the order of 10 to 20 minutes, which definitely would not be sufficient for disinfection contact time when free chlorine is the disinfectant. Because of the relatively short detention times in package plants, many small systems treating surface water with package plants may need to provide for separate treated water storage facilities at the plant site to attain adequate CT values.
The availability of package plants has encouraged application of coagulation/filtration technology to small systems. Installation and operation of a coagulation/filtration package plant was described by Brigano et al. (1994). A key aspect of this application was use of a telemetry device to relay operating data to an off-site office of a contract operator. This substantially lowered the number of hours an operator needed to spend at the treatment plant.
Coagulation/filtration process trains can cope with a wider range of surface water quality than other filtration process trains, so they would seem logical choices for treating many surface waters. However, coagulation/filtration technology requires careful monitoring and oversight, whether by an operator at the plant or by remote sensing and data transmission from another location. Poorly operated coagulation/filtration technology of any size can be ineffective for treating water. Poor operation of such plants has resulted in numerous waterborne disease outbreaks. Small water systems that employ this technology must make a commitment to sustained excellence of operation.
Dissolved Air Flotation
How the Process Works
Dissolved air flotation (DAF) is most useful for removing particulate matter
FIGURE 3-9 Example of a package filtration system. The first unit shown is a "roughing filter," used for flocculation and removal of some solids. The second unit is a multimedia filter, which contains layers of granular material to filter out particles remaining after the roughing filter. The last unit is a GAC filter (an optional add-on to this system) for removing dissolved organic compounds.
SOURCE: Culligan International Company, Northbrook, Illinois.
and flocculated material that do not readily settle. The technology is a variation of coagulation/filtration, and therefore much of what applies to conventional coagulation/filtration systems also applies to DAF.
In the DAF process, raw water is coagulated and flocculated. Flocculated water flows to a basin where the floc is floated to the water surface by a cloud of microscopic bubbles, in contrast to conventional treatment employing a sedimentation process in which the solids settle to the bottom. The solids separation step in pretreatment with DAF is as effective as the solids separation step in pretreatment with sedimentation (conventional treatment), within appropriate source water quality limits.
The flotation action in DAF is caused by injecting water containing air dissolved at high pressure into flocculated water as it enters the flotation basin. This water, called the recycle stream, constitutes approximately 5 to 10 percent of the process flow. Recycle water is withdrawn from the bottom of the flotation basin, pumped into a pressure vessel (saturator) at 350 to 500 kPa (50 to 70 psi), and then returned to the flotation basin through a valve that dissipates the pressure. After the pressure returns to atmospheric pressure, the air dissolved in the recycle water comes out of solution in the form of microscopic bubbles. The air bubbles grow and rise to the top of the flotation basin, carrying the floc up to the surface where it can be skimmed off. Thus, the DAF process is an alternative to sedimentation.
Appropriate Water Quality and Performance Capabilities
The use of DAF as a pretreatment step before filtration has advantages over gravity sedimentation for treating algae-laden waters, highly colored waters, waters with low-turbidity and low alkalinity, waters supersaturated with air, cold waters, and waters in intermittently operated treatment plants (Kollajtis, 1991).
DAF is best suited to removal of floc having low density because the floc must be floated to the surface. An example of this is floc formed by coagulation of color in low-turbidity water. DAF has been used to treat the algae-laden effluent from wastewater stabilization ponds, so it is not likely that an upper limit would apply on algae concentrations for potable water treatment.
When clay and silt are present in the source water, floc formed by coagulation is denser and not easily floated. Therefore, DAF is not an appropriate technology for treatment of turbid raw waters. An upper limit for turbidity might be in the range of 30 to 50 NTU for small systems, although Kollajtis (1991) has suggested that DAF may be applicable to waters having turbidity up to 100 NTU.
Malley and Edzwald (1991) compared DAF to conventional gravity sedimentation and found that for treatment of low-turbidity source waters, DAF was superior for removal of turbidity, and its performance for removal of total organic carbon, true color, and dissolved organic halide precursor materials equaled that of sedimentation. This reinforces the concept that a water treatment plant employing DAF for solids separation in pretreatment should be considered the equivalent of a conventional treatment plant for regulatory compliance purposes. The very short detention times in flocculation and flotation, however, mean that storage may be needed after filtration to increase the disinfectant contact time.
Hall et al. (1994) evaluated DAF for removal of Cryptosporidium oocysts. Their studies suggest that a treatment train consisting of chemical coagulation, flocculation, DAF, and filtration should be capable of removing 3 logs (99.9 percent) of the oocysts.
Monitoring and Operating Requirements
Monitoring needs for DAF are similar to those for conventional coagulation/filtration systems. Raw and filtered water turbidity and pH and filter head loss should be monitored at the same frequencies as those employed in conventional treatment plants. Process equipment should be designed so that the operator can easily monitor the flow of raw and treated water, head loss, and chemical feeds. In addition, sample taps should be provided so the operator can obtain samples of raw water, pretreated water, and filtered water for analysis.
Additional monitoring, beyond that required for conventional coagulation/filtration processes, is needed for DAF to control the air dissolution step. The key factors are saturator pressure and flow rate for the recycle stream. These must be metered to enable the operator to control the recycle step. In addition, the plant
operator should periodically observe the condition of the floc that has floated to the surface of the flotation basin, as well as the nature of the bubbles formed in the basin. Vigorous, turbulent bubbling action is a sign of problems with the DAF process, and this must be avoided because excessive turbulence can break up floated floc and cause it to sink into the flotation basin, from which it could be discharged to the filters.
Suitability for Small Systems
Preengineered package treatment plants using DAF are available and have been used for more then a decade in the United States and even longer in Europe. To provide for economical and affordable small treatment plants, DAF package plants commonly combine flotation and filtration into one process basin. This is feasible because the solids separation step carries the floc to the surface of the water, producing clarified water at the bottom of the basin, which can be used to provide space for filter media and underdrain facilities. Two treatment steps are accomplished in the same space, resulting in substantial economies. However, combining the two processes results in operation of the DAF process at an overflow rate that is the same as the filtration rate for the plant. This tends to place an upper limit of 5 to 10 m/h (2 to 4 gpm/sq ft) on the DAF overflow rates. In addition, filter backwashing would interrupt the DAF process, but because DAF can produce good treated water very quickly upon start-up, this may not be a problem. Thorough flocculation is essential in these systems because it is not possible to improve filtration by adding a filter aid.
The DAF process is more complex to operate than a conventional coagulation/filtration system because of the need to control the recycle flow stream and saturator operation. Failure of either the air saturation or flow recycle steps will cause the flotation step to fail. If this happens, all of the coagulated and flocculated solids have to be removed by the filter, in a process analogous to direct filtration. However, DAF is the best process for treating raw water with high concentrations of algae, and it is excellent for treating soft, highly colored waters with low-turbidity waters. For these reasons, it may find application in some small systems, despite its complexity.
Diatomaceous Earth Filtration
How the Process Works
Diatomaceous earth (DE) filtration is used primarily for particulate contaminant removal. Industries have long used the process for filtration of liquids. The technology was developed for potable water treatment during World War II. Because of the need for portable water treatment equipment , the U.S. Army developed DE filters that could be mounted on trucks and transported to field
locations. The portable size of these units makes them appropriate for small systems.
DE filtration works by straining particulate matter from the water. Coagulant chemicals are rarely used. Filtration is accomplished at the surface of a cake of diatomaceous earth (a fine-grade material composed of the fossil remains of diatoms) placed on filter leaves, or septa. This cake, called precoat, is established on the filter by recirculating a slurry of DE through the filter. After the precoat forms on the filter leaves, raw water containing some diatomaceous earth (body feed) is fed through the filter.
During a filter run, removal of particulate matter in the raw water, plus the accumulation of body feed diatomaceous earth material, causes the head loss to build up in the filter. When terminal head loss is reached, the flow of water into the filter is stopped and the filter is cleaned. The diatomaceous earth removed from the filter leaves is discarded.
There are two types of DE filters: (1) pressure filters, which have a pump or high-pressure water source on the influent side, and (2) vacuum filters, which have a pump on the effluent side. Vacuum filters are open to the atmosphere. Pressure filters are enclosed within pressure vessels.
Appropriate Water Quality and Performance Capabilities
Raw water quality should be excellent, with an upper limit of approximately 10 NTU (Letterman and Logsdon, 1976). Because DE filtration usually does not involve coagulation, capability for removal of dissolved constituents, such as color and inorganic contaminants, is very low. Thus, it is very important to determine in advance the quality of the raw water to be treated by DE filtration.
The size of the particles removed by DE filtration is a function of the size distribution of the diatomaceous earth particles used for the precoat and body feed. Fine grades of diatomaceous earth can remove smaller particles, such as bacteria. The grades of diatomaceous earth commonly used in potable water treatment are very effective for removal of Giardia cysts and Cryptosporidium oocysts. Schuler et al.(1988) reported removals exceeding 4 logs (99.99 percent) for both Giardia and Cryptosporidium. DE filtration is not as effective for bacteria removal, and it does not remove viruses very well unless the diatomaceous earth has been specially treated to alter its surface charge and bring about attachment of viruses to the diatomaceous earth. DE filters can remove algae to a very high degree, but the accumulation of algae cells on the surface of the filter cake can cause rapid clogging, so care must be taken to avoid excessive algae when applying DE filtration. Syrotynski and Stone (1975) reported that DE filters subjected to raw water containing microscopic total counts of 3,000 areal standard units per milliliter would experience shorter filter runs. Because DE filters have short detention times, disinfection contact time is necessary after filtration.
Monitoring and Operating Requirements
Monitoring requirements for DE filtration are simpler than those for coagulation and filtration because coagulant chemicals are hardly ever used. Raw and filtered water turbidity should be monitored, with compliance monitoring for filtered water turbidity done every 4 hours except for systems serving 500 or fewer people, which, after obtaining state approval, may monitor only once per day. If the nature of the turbidity-causing particulate matter remains stable, it may be possible to establish a ratio between the raw water turbidity and the appropriate dose of diatomaceous earth for use in the body feed. This situation might apply to treatment of lake water, for example. Filter head loss monitoring is necessary so the operator can determine when to backwash the filter. In addition, monitoring head loss can help establish the appropriate body feed. If water quality suddenly changes and the rate of head loss accumulation increases, more body feed may be needed. In addition to monitoring the flow-through the DE filter, the operator needs to monitor the flow rate for body feed addition in order to control this aspect of plant operation.
In general, DE filter plant operators need mechanical skills to operate the body feed pumps, precoat pumps, mixers, and pipes and valves. Keeping the filter leaves clean in a DE filter is of primary importance. A filter leaf that is not properly cleaned at the end of a filter run can accumulate dirt and slime on the filter cloth, and this can prevent the formation of a uniform precoat when the filter is restored to service. DE filtration equipment should be designed so that the plant operator can easily inspect the cleanliness and integrity of the filter leaves.
One problematic aspect of small DE filter plants is the tendency of many small systems to operate filters intermittently rather than on a 24-hour-per-day basis. Unless provision to continuously recirculate filtered water through the DE filter is provided, every time the filter is stopped, the filter leaves must be cleaned and the used diatomaceous earth thrown away. When DE filters are capable of having continuous runs lasting as long as 2 to 4 days, wasting the precoat and body feed diatomaceous earth at the end of a run as short as 8 hours can drive up operating costs. Used or spent diatomaceous earth must always be cleaned out of the filter, or contaminants trapped in the filter cake may pass through the filter and into the treated water in a subsequent filter run.
Suitability for Small Systems
DE filtration is well suited to small systems and has been used in the past by such systems. In a survey of direct filtration, Letterman and Logsdon (1976) reported that among the 13 DE filter plants responding to the survey, 4 served approximately 3,300 to 4,800 people, and another 4 served approximately 6,700 to 20,000 people. A key factor in the use of DE filtration for small systems is that
chemical coagulation is not necessary, so operators do not have to learn about this complex aspect of water treatment. Waters suitable for DE filtration are low in turbidity and in color or other organic matter that can form disinfection byproducts when chlorinated.
Slow Sand Filtration
How the Process Works
In slow sand filtration, biological action breaks down some organic matter, and some inert suspended particles are physically removed from the water. Slow sand filtration was the original form of water treatment used by municipalities in the nineteenth century and is now considered a low-technology approach to water treatment.
The SWTR defines slow sand filtration as ''… a process involving passage of raw water through a bed of sand at low velocity (generally less than 0.4 m/h) resulting in substantial particulate removal by physical and biological means" (EPA, 1989). In this process, uncoagulated water is applied to a bed of sand having an effective size of approximately 0.3 mm and a depth of approximately 0.6 to 1.2 m (2 to 4 ft) at a filtration rate of 0.1 to 0.4 m/h (0.04 to 0.16 gpm/sq ft). With extended use of the filter, a biological ecosystem grows in the sand bed. On the top of the filter media, a biologically active organic layer (known by the German term Schmutzdecke) builds up and assists filtration. The water then enters the top layer of sand, where more biological action occurs and particles attach to sand grain surfaces by adsorption and sedimentation in pores between sand grains.
The biological activity within the sand bed is a key factor in the effective action of slow sand filters. Biota in slow sand filters include bacteria, algae, rhizopods, ciliates, rotifers, copepods, and aquatic worms (Haarhoff and Cleasby, 1991). Fresh, clean sand is not as effective as a "ripened" sand bed that has been in service long enough for the ecosystem to become established. Depending on the available nutrients in the source water and the water temperature, establishing the ecosystem could take from a few weeks to 2 to 3 months. Operation and maintenance activities that harm or inactivate the ecosystem therefore tend to cause slow sand filter performance to deteriorate.
Providing for storage of filtered water is essential at a slow sand filter plant for two reasons. First, because of the importance of establishing biological activity, using chlorine ahead of the filter is inappropriate, and disinfectant contact time must be provided in a storage basin after filtration. Second, storage is needed for equalization of production and demand. Slow sand filters should be operated at steady rates, if possible, and flows should not be increased or decreased frequently to keep pace with system demand. In very small systems, the
need for disinfectant contact time plus equalization storage could require the provision for storage of approximately 1 day's production at the plant.
Appropriate Water Quality and Performance Capabilities
Because slow sand filters in the United States generally are used without pretreatment, the range of raw water quality appropriate for treatment by this process is rather narrow. Cleasby (1991) recommends the following guidelines for ideal source water quality for slow sand filtration without pretreatment:
|
• |
turbidity |
< 5 NTU |
|
• |
algae |
no heavy seasonal blooms; chlorophyll-a<5 µg/liter |
|
• |
iron |
< 0.3 mg/liter |
|
• |
manganese |
< 0.05 mg/liter |
Source waters with clay content may cause treatment problems. Fox et al. (1984) operated a slow sand filter to treat Ohio River water (a clay-bearing water source) and found that although the influent turbidity ranged from approximately 10 to 23 NTU for the first 50 days and then was between 10 and 0.4 NTU for the next 130 days, filtered water turbidity was progressively poorer during the filter operation and eventually exceeded 1 NTU. In addition, the length of each filter run became shorter, from 98 days for the first run to 6 days for the last, indicating progressive clogging of the sand bed with clay.
In most waters, slow sand filters can reduce turbidity sufficiently to satisfy regulatory requirements, but in others, turbidity reduction may be minimal. Turbidity removal may be impaired in waters with very low nutrient content (Bellamy et al., 1985b), as some nutrients must be present to promote growth of the biological ecosystem within the filter bed.
Algae in raw water can clog slow sand filters. Cleasby et al. (1984) found that when chlorophyll-a (an indirect measure of algal concentration) was between 8 and 138 µg/liter, four filter runs varied in length between 10 and 22 days. Filter runs were 34 to 123 days when chlorophyll-a was in the range of 1 to 4 µg/liter.
Slow sand filters are not very effective at removing disinfection byproduct precursors or color. If the biological action within a filter bed were effective for removal of organic matter of this type, biological action in lakes and rivers would have already removed the organic matter from the source water.
Slow sand filtration excels at removing microorganisms. Its effectiveness for this purpose was demonstrated in the nineteenth century by the reduction in waterborne disease in European and English cities that used slow sand filtration. Research in the twentieth century has documented the efficacy of slow sand filtration for virus removal (Poynter and Slade, 1977), Giardia cyst removal (Bellamy et al., 1985a,b; Pyper, 1985; Seelaus et al., 1986; Schuler et al. 1988),
and Cryptosporidium oocyst removal (Schuler et al., 1988). Slow sand filters are less effective at removing microorganisms from cold waters because as temperatures decrease, the biological activity within the filter bed declines. For this reason, slow sand filters that will treat water at temperatures below approximately 10°C should be conservatively designed; i.e., filtration rates should be near 0.12 to 0.17 m/h during winter operation (Pyper and Logsdon, 1991).
One modification of slow sand filtration that offers promise for removal of organics is the GAC sandwich filter. This filter uses a base sand layer of approximately 30 cm, an intermediate GAC layer of approximately 15 cm, and a top sand layer of approximately 45 cm. This modified slow sand filter has been effective for removal of pesticides, total organic carbon, and trihalomethane precursors (Bauer et al., 1996).
Monitoring and Operating Requirements
Monitoring and operation of slow sand filters is not complicated. Daily tasks include reading and recording head loss, raw and filtered water turbidity, flow rates, and disinfectant residual. If necessary, flow should be adjusted to bring water production in line with demand. In addition, with the promulgation of the SWTR, each day the operator would need to use the flow data and disinfectant residual data to calculate CT values and determine if disinfection is sufficiently rigorous. These duties may require 1 to 2 hours unless automated.
As head loss increases in the slow sand filter bed, eventually the filter will need to be cleaned. This is accomplished by draining the filter and removing 1.2 to 2.5 cm (0.5 to 1 in.) of sand from the top of the bed. In a study of slow sand filter operation and maintenance, Cullen and Letterman (1985) estimated that approximately 5 hours would be required to scrape 100 m2 of sand bed. After repeated scrapings, so much sand will have been removed that replacement of sand is necessary. This replacement, known as "resanding", is labor intensive. Cullen and Letterman (1985) estimated that resanding a depth of 15 to 30 cm would require 48 to 59 hours of labor per 100 m2 of filter bed. These values would be modified somewhat if more machinery were used.
Suitability for Small Systems
Slow sand filtration has been adapted to package plant construction. Hall and Hyde (1987) reported on a project to evaluate a slow sand filtration package plant consisting of two separate 2-m2 filters, each with a raw water inlet, two flow controllers, a chlorine feeder, a chlorine contact tank, and a service reservoir. The package slow sand filter produced filtered water turbidity averaging less than 1 formazin turbidity unit (FTU) through the study, and filtered water turbidity remained at less than 2 FTU even when raw water turbidity was as high as 94
FTU. Average coliform removal by filtration was 94 percent. Fewer than 2 days per year were required for sand cleaning.
In a recent application of slow sand filtration technology, one small water system used precast concrete boxes as filter cells for a 300-liter/min plant (Riesenberg et al., 1995). The precast filter boxes could be tested for water tightness and repaired if needed at the manufacturing facility. Such an approach could provide both labor savings and improved quality control for construction of slow sand filter plants serving approximately 500 or fewer persons.
Slow sand filtration is among the simplest and most easily used of the technologies available for small water systems because the efficacy of the process is mainly dependent on the inherent mechanisms at work in the process rather than on the actions of the plant operator. However, because few remedies are available to a plant operator if slow sand filtration is ineffective, the process must be used with caution. Only high-quality surface waters (low in turbidity, algae, and color) are suitable for application to slow sand filters without pretreatment or process modifications such as the use of a GAC layer in the filter. When used with source water of appropriate quality, however, this process may be the most suitable choice for small systems that must filter surface water.
Bag and Cartridge Filters
How the Process Works
Bag filters and cartridge filters are technologies specifically developed for small to very small systems. They are made from pressure vessels containing a woven bag or a cartridge with a wound filament filter. Water passes through the bag or the wound filament cartridge, and the filter removes particulate matter large enough to be trapped in the pores of the bag or cartridge. The filters are appropriate for removal of Giardia cysts and possibly for removal of Cryptosporidium oocysts (which are large enough to be strained in the filter pores) but not for removal of bacteria and viruses. They are designed for simple operation; no coagulant chemicals are used.
Proper selection of the pore size of cartridge and bag filters is critical. Because cysts and oocysts are biological particles without hard shells or skeletons, they are capable of deforming somewhat and squeezing through pores that might seem to be small enough to prevent their passage. In addition, wound filament filter cartridges have pores that are both larger and smaller than the nominal size indicated in the equipment literature. Therefore, these filters do not provide an absolute cutoff for particles at or slightly larger than their nominal size.
Bag filters and cartridge filters function by surface straining, so a mat or cake builds up on the filter surface. If the materials being removed are not compressible, the buildup of this cake may not hinder filtration. Conversely, removal of compressible particles such as algae or fragments of biological matter can blind
the filter. This same phenomenon can occur in DE filtration, which also involves a surface filtration mechanism. In some instances, decreasing the influent pressure on the filters can result in longer service life and greater throughput. This probably happens because the lower head loss through the filter causes less compression of the compressible particulate matter and thus reduces the tendency of compressible particles to blind the filter surface.
As water flows through a bag or cartridge filter, eventually the pressure drop within the filter builds up until it becomes necessary to terminate the filter run. When this happens the used bag or cartridge is thrown away and replaced with a clean one.
Appropriate Water Quality and Performance Capabilities
Because filtration of some types of particles can blind bag and cartridge filters, these filters are appropriate only for high-quality waters. In fact, source water quality for bag filters should be higher than the quality for slow sand filters. Source water turbidity may not be an adequate indicator of the water's suitability for treatment by bag and cartridge filters. Hard, mineral materials are not as likely to blind a filter as are biological particles such as algae and fragments of disintegrating biological matter. The number of gallons of water that can be filtered could vary by a factor of 10 or greater for water of a given turbidity, depending on the nature and concentration of particulate matter in the raw water.
Bag and cartridge filters merely strain particulate contaminants out of water, so they are not appropriate for removal of true color or other dissolved contaminants. Because they remove larger microbial contaminants such as protozoan cysts and oocysts, but are not particularly effective for removing bacteria and viruses, bag and cartridge filters are appropriate only for application to relatively pure surface waters, in which the concentration of bacteria and viruses that needs to be inactivated by disinfection is low. Bag and cartridge filters are not appropriate for treating source waters having elevated turbidity. They can remove approximately half of the turbidity in some raw waters, and in such cases if the raw water turbidity were greater than approximately 2 to 3 NTU, the filtered water turbidity would exceed 1 NTU.
Monitoring and Operating Requirements
Bag filters and cartridge filters are simple devices, and their monitoring requirements reflect this. Because of the SWTR requirements for turbidity monitoring, filtered water turbidity should be checked daily. Also, the operator should monitor head loss through the filter and total gallons of water filtered in order to estimate when the existing bag or cartridge will need replacement.
Operators should exercise care when changing filter bags or cartridges. The
manufacturer's instructions on these procedures should be followed so that the clean cartridges or bags are not damaged on installation.
Disposal of bags or cartridges is simple because the filters do not remove toxic substances, so the spent bags or cartridges should be suitable for disposal to a landfill after they have dried. This is not expected to be a problem even if Giardia or Cryptosporidium is being removed, as the contents of a bag or cartridge filter should be no more microbiologically hazardous than the contents of a disposable diaper from an infant with giardiasis or cryptosporidiosis.
Because the requirements for virus removal and inactivation must be met entirely by disinfection in a treatment train involving a bag or cartridge filter, extra care is needed for this process. With the use of free chlorine, this is not difficult in most situations. The very short residence times in the filters, however, mean that disinfectant contact time would be needed in storage after filtration if sufficient contact time had not been attained before filtration.
Suitability for Small Systems
Bag filters and cartridge filters were developed specifically for small systems. The treatment capability of bag filters and cartridge filters is limited to removal of particles from water. They are capable of removing Giardia cysts and perhaps Cryptosporidium oocysts. Viruses not attached to other particulate matter would pass through these filters. In addition, these filters will not remove chemical contaminants present in solution. Bag filters and cartridge filters are most appropriate for treatment of very-high-quality source waters for removal of protozoan cysts and are best suited for very small systems, such as those serving fewer than 500 people.
Centralized Operation Through Automation and Remote Monitoring and Control
Some water treatment technologies respond well to automated operation. A major advantage of remote monitoring and control is the potential to share resources among several small systems, so that a single operator can monitor and operate several small plants in a given area. The operator can work from a centralized location and receive and respond to information from each plant. Perhaps more important, an operator with more training in water treatment can be employed because the group of small systems can share the higher salary requirements of an experienced operator. In addition, the automatic control of chemical feeders often lowers chemical costs and improves water quality.
Several levels of remote monitoring and control are available. The complexity will depend on the complexity of the treatment option and the availability of the operator. A basic monitoring system might include a simple auto-telephone dialer to alert the remote operator of such problems as power outages, pressure
drops, unauthorized building intrusions, high sump levels (possible flooding), or any other condition that can be monitored by a simple on-off alarm. In their simplest form, such alarms are presented as a common alarm announcement, which then requires the operator to visit the site to determine the exact cause and nature of the alarm. Equipment operation is often performed by local hard-wired relay systems or individual control packages provided by the equipment supplier. There is no control system integration in this lowest level of monitoring and alarm.
A higher-level system would include an integrated approach, tying together the operation of the system and alarms. Different operational programs can address differing conditions. The operator can access the system remotely by computer modem to determine system status or to check the condition of an alarm; many systems also allow for the operator to remotely control system operation using the same computer modem.
The highest-level system of remote monitoring and control involves one (or more) master locations in constant communication with a number of remote unattended locations. The master location is often staffed on a full-time basis. This highest level of monitoring and control is sometimes referred to as supervisory control and automatic data acquisition (SCADA). The master operator can constantly monitor each remote system, adjusting operations at the master supervisory control console or dispatching personnel to the remote location as needed. A system of this sophistication can also allow data to be sent by telemetry to the central location for centralized performance of administrative tasks such as regulatory and management report preparation. Similarly, individual customer meter readings can be obtained for billing purposes.
Small systems may also operate without an attending operator in either of two automatic control options. In the simplest level of automatic control, sensors, instrumentation, and control devices operate on simple rules. An example is a chemical feed flow rate controller tied to the raw water influent flow rate. The feed controller sets a new chemical feed rate as the influent flow rate changes. Advanced automatic control relies on sophisticated computer models or artificial intelligence to make more advanced and/or precise corrections to system operation in response to changing water conditions. This type of advanced automated control is in its infancy.
The types of water treatment problems addressed at a given site will determine to a large extent the level of remote monitoring and control desired. If a short-term disfunction in a system could result in a high risk of an acute health effect such as breakthrough of Giardia or Cryptosporidium, or nitrate levels high enough to cause methemoglobinemia in infants, a high level of remote monitoring and control is advisable. A treatment system designed to protect the customer from a secondary, aesthetic water quality problem, such as colored water or excess iron or manganese, may need only a medium level of monitoring. In the
extreme, a ground water well not influenced by surface water and located in a residential area may only need a light on the pumphouse to indicate pump failure.
The type of system management also affects the appropriate level of remote monitoring. A local operator responsible for a single location on a part-time basis may need only the simplest set of remote monitoring and alarm tools to respond effectively to system problems 24 hours a day. A centralized regional operation may require a higher level than normally needed at each remote location in order to reduce the number of required personnel. In either case, customer satisfaction is likely to be high. If the system is properly designed and operated, the operator will normally recognize and solve a problem before customers note a water quality deficiency.
A couple of caveats apply here. First, remote monitoring and/or remote operational control does not eliminate the need for maintenance. In fact, the increased reliance on sensors inherent in remote monitoring results in an increased need for sensor maintenance and calibration. Also, as these systems are developed, it is important that they conform to a standardized communication format. The electric industry has a utility communications architecture that is being integrated into an industry-government standard system for communication (Schlenger et al., 1994). Water industry control systems should begin to adopt these standards or develop another standard communication system in order to standardize data acquisition and reporting.
Options Other than Centralized Treatment
When a centralized treatment facility is not feasible and obtaining water from some other source is not possible, small systems may need to consider installing point-of-entry (POE) or point-of-use (POU) water treatment devices in their customers' homes or distributing bottled water. These alternatives generally are appropriate for system-wide use only for very small systems, particularly those serving 500 or fewer people.
Numerous households in the United States use POE and POU devices and bottled water, primarily to deal with aesthetic concerns. This report, however, discusses these options for purposes of providing water that meets the quality requirements of the SDWA. In such a situation, adoption of POE, POU, or bottled water as the means of providing drinking water is not an individual household's choice but the choice of the water system in cooperation with regulatory authorities. Therefore, circumstances surrounding use of POE, POU, or bottled water are much different than those related to the voluntary use of these options.
POE treatment devices are used to treat all water used in a household or building and result in water from any tap being suitable for drinking when treatment is effective. POU devices are used to treat the water at a single tap or faucet, and as a consequence, only that tap or faucet has potable water (see Figure 3-10).
If a POU device is placed under the kitchen sink to treat cold water for the kitchen faucet, only that water is potable; water from a faucet in a bathroom, a likely location for brushing teeth, would not be potable. This aspect of POU treatment has been a source of objection to its use.
POU and POE Treatment
Description
POE and POU systems often use the same technology concepts employed in centralized treatment, but the technology is applied at a much smaller scale and sometimes is modified for application to treatment of small flows. Most of the processes used in POE and POU units are discussed at length in previous sections of this chapter. The following are aspects of treatment technologies that are specific to application in POE and POU systems:
- Activated alumina and granular activated carbon typically are used in cartridges or pressure vessels for POE and POU treatment devices. Activated alumina treatment is most often used for fluoride removal. When the exchange/adsorption capacity of the activated alumina has been reached, the spent cartridge must be replaced. GAC systems are used for taste and odor concerns and for removal of regulated organic compounds. The performance and life of GAC systems depend on the amount of GAC used in the device, the contact time between the GAC and the water, and the contaminants being removed. When the GAC-treated water reaches a predetermined performance concentration for the contaminant being removed, the GAC must be replaced. This is done by removing the cartridge and installing a new one.
- Reverse osmosis devices for POE and POU need to be provided with a means of discharging reject water to a drain. The discharge line should be installed with an air gap so a cross-connection between wastewater and drinking water will not occur. Reverse osmosis and other membrane technologies are among the fastest-developing types of technology with possible applications for POE systems.
- When ion exchange technology is used in homes to soften potable drinking water, all of the household water generally is softened, and outside faucets used for lawn and garden watering might be unsoftened. Radium removal would be a possible POE application for this technology.
- Air stripping has been used in POE systems to remove volatile organic compounds and radon from ground water. For these applications, it is important to vent the off-gases adequately to avoid creating an air pollution hazard inside the home. Generally, this is achieved by designing the ventilation system such that the air duct for the vent disperses the stripped contaminants above the air envelope for the structure.
Because control of acute disease should be accomplished with the highest feasible degree of competence, use of POE and POU treatment for disinfection of surface water is not generally viewed as appropriate. In the future, however, disinfection may be required for ground water sources that currently are not disinfected. The safest, most effective, and most readily manageable disinfection method of POU/POE application for inactivation of bacteria and viruses is UV light. UV light as it is typically employed is not effective for Giardia cysts or Cryptosporidium oocysts, so it is applicable only to ground water. UV is currently the most popular disinfection method for POE and POU systems because it does not involve the addition of a chemical and therefore imparts no tastes, odors, or chemical byproducts (Lykins et al., 1992). UV systems range in capacity from 2 liters/min to approximately 2,000 liters/min; manufacturers claim effective life spans of 6,000 hours to 12,000 hours for the lamps used to produce the UV light.
Appropriate Water Quality
Although POE and POU systems may in some instances be used to treat surface waters, in a regulatory setting they would be appropriate only for ground water because of the frequency of monitoring (daily) necessary with surface water treatment and because of the necessity of ensuring thorough disinfection of surface water. The uniformity of ground water quality from a given well means less emphasis needs to be placed on monitoring because quality-related changes in treatment efficacy would not be as severe for ground water as they would be for most surface waters.
Selecting POE and POU equipment does not eliminate the need for evaluation of treatment efficacy before installing the treatment equipment. Before funds are expended to treat water for regulatory compliance purposes, verification of the efficacy of the proposed treatment technique is essential. For devices that employ cartridges (e.g., GAC columns or activated alumina), pilot testing of the source water may be necessary to develop valid estimates of the service life of the unit before replacement is required. Reverse osmosis testing would be done primarily to determine whether the water being treated will foul the membranes, as contaminant removal capabilities of a membrane do not vary from water to water. Ion exchange units for radium removal could be regenerated based on exhaustion of hardness removal capability, as radium is still removed after calcium and magnesium begin to appear in the product water. Before UV disinfection is used, testing for possible interferences to the transmission of UV light through the water would be advisable.
Monitoring and Operating Requirements
Effective operation, maintenance, and monitoring programs are essential to the overall performance of any water treatment system and are especially significant
for POE and POU systems. Many homeowners assume their systems will perform properly once installed and do not understand the level of effort required to ensure proper operation. For this reason, when POE or POU systems are installed for regulatory purposes, programs for long-term operation, maintenance, and monitoring must be provided by water utilities or regulatory agencies.
Proper installation is the first step in effective long-term operation and maintenance of POE and POU systems. Installation must be done only by experienced contractors or installers whose products conform with applicable plumbing codes. Qualified installers carry liability insurance for property damage during installation, are accessible for service calls, accept responsibility for minor adjustments after installation, and give a valid estimate of the cost of installation.
After installation, POE and POU systems need a well-defined program of operation and maintenance for continued production of drinking water of acceptable quality. The equipment manufacturer's recommended operation and maintenance requirements can serve as the bases for the operation and maintenance program. Equipment dealers may provide maintenance for a limited time period as part of an installation warranty. A long-term maintenance program may be carried out by a local plumbing contractor, a POE or POU service representative or equipment dealer, a water service company, the local water utility, or a circuit rider (an individual under contract with several water systems to perform operation, maintenance, and monitoring activities) (Bellen et al., 1985). It is essential that maintenance be performed by personnel responsible to the small water system rather than to the homeowner because water system personnel will understand the need for a continuing operation and maintenance program, whereas some homeowners will not.
One way to ensure the production of water that meets regulatory requirements is to define a replacement schedule for media, cartridges, filters, and/or modules associated with POE and POU systems. Replacement schedules can be defined either by time (e.g., every 6 months) or by flow (e.g., every 30,000 liters). The advantage of using time is the avoidance of having to monitor flow. However, replacement based on time may result in equipment being replaced too early or too late. The former case would waste resources, while replacing equipment too late could result in the consumption of drinking water that exceeds one or more of the drinking water standards. Replacement based on flow requires that water meters be used as a part of the monitoring program. Although this approach requires a bit more hands-on involvement, it results in a better balance between maximizing equipment life and producing water that meets regulatory requirements.
Monitoring programs need to be site-specific and reflect the contaminant or contaminants being removed, the equipment used, the number of POE or POU units in service, and the logistics of the service area. Minimum sampling frequencies and types of analyses should be established in cooperation with the local health department, the state regulatory agency, and the small system.
Monitoring programs generally include raw and treated water sample collection, meter reading, field analyses (measuring pH, dissolved oxygen concentration, and other parameters) as appropriate, shipment of samples to a laboratory, and recordkeeping. The use of state-approved sampling methods and certified laboratories is a requirement for regulatory compliance. Lykins et al. (1992) recommend that monitoring programs provide some way to respond to water quality questions from residents both with and without POE or POU systems and to assess raw water quality trends.
In addition to having samples collected by an employee of the small water system, options for sample collection include contracting with a POE or POU service representative, an independent laboratory, a local health department, a circuit rider operator, or a trained community resident. An advantage of using a community resident or local representative is that these persons are familiar with the residents of the community and are likely to be better able to coordinate relatively convenient sample collection times. A disadvantage of using such a person is that community residents are likely to know the least about proper sample collection and preservation procedures, water quality tests, methods for recordkeeping, water meter reading, and proper procedures for transport or shipment of samples to an analytical laboratory. Training is necessary to enable a community resident to be an effective sample collector. Concepts related to training for sample collectors were presented by Bellen et al. (1985).
To avoid duplication of travel to homes and buildings equipped with POE or POU devices, the sample collector needs to be familiar with the treatment equipment used and the treatment objectives. An ability to conduct basic troubleshooting and to service equipment is also helpful, in case problems are brought to the attention of the sample collector during sampling rounds.
Monitoring of POE and POU treatment devices is problematic. When water is treated to meet MCLs or to satisfy treatment technique requirements, monitoring has to be done to verify that the water quality or treatment approach is satisfactory. From a regulatory agency perspective, monitoring of POE and POU devices is a major obstacle to acceptance. For a community consisting of 50 homes and served by a central treatment facility, regulatory compliance monitoring for most of the regulated contaminants could be done at the discharge point from the treatment plant or at the point-of-entry to the distribution system. If POE or POU devices were used instead of central treatment, the community of 50 homes would have 50 water treatment devices, any one of which might possibly malfunction or reach its capacity for effective treatment at some time. The oversight effort, both for the small water system and for the regulatory agency, is multiplied several fold in such a circumstance. The cost of monitoring every POE or POU device could be a burden on small water system customers.
One approach to lowering the cost of monitoring is to sample representative households that reflect typical POE or POU installations and levels of contamination rather than sampling all households with installed systems. The costs of
monitoring would decrease as a smaller percentage of the devices was monitored in a year's time, but the risks of noncompliance with an MCL would increase. Striking a balance between the risks to persons consuming water exceeding MCLs because of insufficient monitoring and the cost of analyzing numerous monitoring samples will be a challenging task for small water systems using POU or POE devices and for the regulatory agencies overseeing such systems.
Regulatory Approach to POE and POU Systems
The EPA (1985) has established the following conditions that must be met to ensure protection of public health when POE or POU systems are used for compliance purposes:
- Central control: Regardless of who owns the POE or POU system, a public water system must be responsible for operating and maintaining it.
- Effective monitoring: A monitoring program must be developed and approved by the state regulatory agency before POE or POU systems are installed. Such a monitoring program must ensure that the systems provide health protection equivalent to that which would be provided by central water treatment meeting all primary and secondary standards. Also, information regarding total flow treated and the physical conditions of the equipment must be documented.
- Effective technology: The state must require adequate certification of performance and field testing as well as design review of each type of device used. Either the state or a third party acceptable to the state can conduct the certification program.
- Microbiological safety: To maintain the microbiological safety of water treated with POE or POU devices, the EPA suggests that control techniques such as backwashing, disinfection, and monitoring for microbial safety be implemented. The EPA considers this an important condition because disinfection is not normally provided after POE systems.
- Consumer protection: Every building connected to the public water system must install POE or POU treatment and adequately maintain and monitor it.
Although several states have developed regulations for the certification of POE and POU devices, California has the most extensive program for regulating the use of POE and POU systems in place of central treatment. The California action may be indicative of the approach other states will take in the future. The California Department of Health Services (DHS) does not allow the installation of POE or POU devices by community water systems unless all other available alternatives have been evaluated and found to be infeasible. The evaluation submitted to regulators must document the water quality problem or problems, alternatives pursued to correct the problem, potential for connection with an adjacent utility, comparison of POU or POE treatment versus central treatment,
potential for development of new ground water sources, and potential for developing and treating a surface water source. In addition, the California DHS specifies a list of conditions that must be considered in the approval process for POE and POU devices. These conditions include utility responsibility for POE or POU ownership and maintenance, and for ongoing monitoring of contaminants, including monthly bacteriological samples. In addition, California regulations require that the POU and POE devices be either pilot tested at each individual site or that the performance of the equipment be certified in a formal testing program. Testing for certification must be conducted by a recognized testing organization and must be performed in an independent laboratory meeting laboratory accreditation requirements set forth by the California DHS. The testing must be carried out according to specified protocols accepted by the California DHS. If the equipment manufacturer makes health or safety claims regarding the ability of the device to remove specific contaminants, these claims must be verified. In addition, testing must demonstrate that the equipment will not add toxic substances to the treated water, such as by leaching from system components.
The California regulations for certification of POU and POE devices draw on standards for the testing of this equipment established by the National Sanitation Foundation (NSF) International. NSF International has issued seven standards related to the testing of POE and POU devices:
- standard 42, which covers the ability of GAC and mechanical filtration to improve the aesthetic qualities of drinking water;
- standard 44, which specifies testing protocols for cation exchange units;
- standard 54, which provides protocols for testing the ability of GAC and mechanical filtration systems to remove contaminants posing a health hazard;
- standard 55, which specifies how to test UV disinfection systems;
- standard 58, which outlines testing requirements for reverse osmosis systems;
- standard 61, which details how to test for the possibility that chemicals will leach from system components into the water; and
- standard 62, which sets forth testing protocols for distillation systems.
NSF International has a certification laboratory that can conduct a full range of physical, microbiological, radiological, inorganic, and organic analyses.
The Water Quality Association (WQA) also has a certification program for POE and POU devices. However, the WQA is a trade association for POE and POU equipment manufacturers and therefore cannot provide the type of independent analysis available from NSF International (Lykins et al., 1992). Local planners considering the purchase of POE and POU devices need to be aware of this distinction when purchasing POE and POU equipment and interpret the WQA certification accordingly.
Circumstances for Use of POU or POE Systems
The drinking water industry and state regulatory agencies have often opposed the installation of POE or POU systems as the choice of technology to treat water and comply with drinking water regulations. Regulatory objections to these devices include the following:
- POU devices do not treat all the water taps in a house, posing the potential health risk of household residents drinking untreated water.
- Control of treatment, water quality monitoring, routine operation and maintenance, and regulatory oversight is complex because treatment is not centralized.
- Unless monitoring requirements are decreased from those stipulated for centralized treatment, monitoring is more costly than for centralized treatment because of the numerous individual home treatment devices that must be checked.
- Ensuring regulatory compliance is more difficult than with centralized treatment.
- Service life and efficiency of treatment units depend on source water quality, so performance can vary from household to household.
- Community water systems are concerned about the liability associated with entering a customer's home to monitor or service the units.
Despite these concerns, a driving for the use of POU and POE treatment devices has been the cost differential. When POU devices are used, only water that is used for potable purposes is treated. If a source water is acceptable for drinking except for exceeding the standard for nitrate or fluoride, for example, treating the small number of liters per day needed for drinking and cooking might be less costly than installing a centralized treatment system that could remove nitrate or fluoride from all water used by the community. Water used to wash cars, water lawns, flush toilets, or launder clothing would not need to have nitrate or fluoride removed. Similarly, POE devices can save the cost of installing expensive new equipment in a central water treatment facility. They can also save the considerable costs of installing and maintaining water distribution mains when they are used in communities where homeowners have individual wells.
As the population served by a small system increases, the monitoring, operation, and maintenance costs associated with POU and POE devices increase in direct proportion to the population. Table 3-5 shows a cost comparison for using POE versus adding a GAC treatment system to the water treatment plant for a community with between 10 and 50 households (Goodrich et al., 1992). As the table shows, when 20 or more households are involved for this example, modifying the central treatment plant is less costly than installing and maintaining POE devices in individual homes. Figures 3-11 compares the cost of installing POE systems with that of connecting homes to a central water treatment plant. As
TABLE 3-5 Cost of POE versus Central Treatment for Removal of Organic Chemicals by Granular Activated Carbon
shown in this figure, if 20 homes are involved and the length of distribution pipe required is less than 4,000 ft. (1,200 ms), then connecting to a central treatment plant is more cost-effective than using POE devices.
Use of POE and POU treatment devices to satisfy drinking water regulatory requirements may be appropriate in some instances, especially for very small systems. In some cases, POE might be the only affordable solution for a very small community with limited financial resources. However, the objections to using POE and POU treatment devices are substantial and have merit, particularly as the system size increases and the complexity of monitoring and servicing the devices increases. Using centralized water treatment should be the preferred option for very small systems, and POE or POU treatment should be considered only if centralized treatment is not possible.
Bottled Water Distribution
Bottled water use in the United States has increased at a rate of approximately 15 to 20 percent per year over the past 20 years (Richardson, 1991). This
FIGURE 3-11 Cost of POE versus connecting to a central system. The POE device in this example is like that described in Table 3-5. The central treatment alternative assumes that a 6 x 103 m3/d (1.6 mgd) conventional plant serving 10,000 people exists nearby and can deliver water at $1.70 per 3,800 liters (1,000 gal). The example assumes that the conventional plant does not need any process modifications. The additional distribution system required is assumed to be a combination of 15- and 20-m (6- and 8-in.) ductile-iron pipes, fittings, and valves. SOURCE: Reprinted, with permission, from Goodrich et al. (1992). ©1992 by the Journal of the American Water Works Association.
increase has occurred despite the high costs of bottled water: the U.S. General Accounting Office found that ''consumers may be paying as much as 300 to 1,200 times more per gallon for bottled water than for tap water because they believe it tastes better, is safe and healthy, or is free of contaminants" (Community Nutrition Institute, 1991). The majority of bottled water is purchased for aesthetic reasons rather than for quality reasons related to drinking water regulations.
Some bottled water is used by necessity rather than because of personal preferences. Examples of necessary uses include water used in areas that have experienced floods, earthquakes, or hurricanes. Bottled water is commonly provided to those who cannot boil water, such as motel and hotel patrons, when a community experiences a waterborne disease outbreak. Bottled water is now recognized as an alternative water supply for emergency purposes by the Department of Interior's Emergency Water Supply Plan, the U.S. Army Corps of Engineers' Emergency Water Plan, and the EPA's National Contingency Plan under the Superfund act. In addition, the EPA rules specify that bottled water, like POU devices, may be used on a temporary basis to avoid anunreasonable risk to health or as a condition of a variance or exemption to drinking water regulations.
Bottled water comes from a variety of sources, including springs, artesian wells, and even public water systems. Bottled water derived from municipal water systems may be treated with ozone and GAC to enhance its taste and odor properties before it is bottled. The Food and Drug Administration (FDA) regulates bottled water. However, the FDA regulates fewer contaminants than does the EPA under the SDWA. If bottled water were to be provided to customers of a small water system as a means of meeting EPA regulations, bottlers who use public water supplies as their sources would probably be appropriate choices to consider, as the status of compliance with EPA regulations for the source of the bottled water would be known or readily available.
Distribution of bottled water is an important issue to resolve if a small system uses bottled water to comply with EPA regulations. One approach would be to have a supply available at the town hall or the water system office for water system customers to take home at no charge. Another approach would be to deliver a supply of bottled water to each household on a regular basis. In a recent American Water Works Association (AWWA) Research Foundation project, a supply of bottled water was delivered once every 2 weeks to each family participating in a study involving bottled water (R. Karlin, AWWA Research Foundation, personal communication, 1996). If more water was needed before the end of the 2 weeks, study participants called and more water was provided. Because of the logistics of providing bottled water, it is appropriate only for intermittent or short-term purposes, rather than for continuous, long-term needs.
Conclusions
The complexity of choosing, financing, operating, and maintaining a small water supply system cannot be overstated. Technology applications differ in their suitability for different water sources and water system sizes. Important factors in choosing a treatment technology for the small water supply system include regulatory compliance; source water quality; capital, operational, and maintenance expenses; and expertise required to operate the system.
In selecting drinking water treatment technologies, small communities should keep the following considerations in mind:
- Small systems should apply technologies to meet requirements of the Safe Drinking Water Act only after exhausting all other possible options. Other routes to compliance include finding an alternative water source, linking with another water system, or purchasing treated water from another system.
- No single water treatment process can solve all water quality problems. Water systems may need to apply a sequence of technologies to meet all regulatory requirements and customer preferences.
- The most cost-effective way to reduce the incidence of most types of waterborne disease caused by microbial pathogens is to disinfect the water. Free chlorine is the easiest type of disinfectant for small systems to apply to meet requirements of the SDWA. However, other strategies, such as use of ozone prior to treatment followed by use of chloramine in the water distribution system, may be needed to minimize the formation of disinfection byproducts that are already or will soon be regulated.
- For small systems using ground water sources, the most commonly reported chemical contaminants influencing the selection of water treatment systems are nitrate, fluoride, and volatile organic compounds. Elevated nitrate and fluoride levels can be reduced with ion exchange, electrodialysis reversal, or reverse osmosis systems. Volatile organic compounds can be stripped from the water by aeration. Other types of synthetic organic compounds can be treated by adsorption on granular or powdered activated carbon.
- For small systems using surface water sources, treatment requirements are driven by the Surface Water Treatment Rule, which requires filtration and disinfection of the water. Membrane filtration systems may best address the variety of problems in surface water because they simultaneously remove microbial contaminants (although disinfection is still required), organic matter that can form disinfection byproducts, and, in the case of reverse osmosis, inorganic chemicals. Slow sand filtration is an appropriate treatment process for surface waters of high quality.
- Automated devices for monitoring small water systems can allow several small systems to share an operator, who can be better trained than a part-time operator. However, remote monitoring does not eliminate the need for routine maintenance checks.
- Very small water systems (those serving fewer than 500 people) may consider using point-of-use or point-of-entry treatment devices in individual homes as an alternative to centralized treatment if all other options are too costly. However, maintenance and compliance responsibilities must remain with the water supplier rather than with the individual homeowner. Developing institutional arrangements for managing these systems may be a greater challenge than finding technology that is effective for removing the contaminants of concern
- and may elevate the costs of these units above the costs of central treatment. In the case of POU devices, the need to enter customers' homes to service the equipment, plus the fact that these devices treat water at only one tap, may preclude their use as a long-term solution to water quality problems.
- Bottled water can be an acceptable short-term solution for providing drinking water of acceptable quality. However, because of the difficulties associated with distributing it and making sure consumers do not ingest the tap water, it is not an appropriate long-term solution.
References
AWWA (American Water Works Association). 1980. The Status of Direct Filtration. Journal of the American Water Works Association 72(7):405–411.
AWWA Committee. 1992. Survey of water utility disinfection practices. Journal of the American Water Works Association 84(9):121–128.
AWWA. 1995. Electrodialysis and Electrodialysis Reversal, First Edition. Denver: AWWA.
AWWA Research Foundation. 1996. Internal Corrosion of Water Distribution Systems, Second Edition. Denver: AWWARF.
Bauer, M. J., J. S. Colbourne, D. M. Foster, N. V. Goodman, and A. J. Rachwal. 1996. GAC enhanced slow sand filtration. Pp. 223–232 in Advances in Slow Sand Filtration and Alternative Biological Filtration, N. Graham and R. Collins, eds. New York: John Wiley & Sons.
Bellamy, W. D., D. W. Hendricks, and G. S. Logsdon. 1985a. Slow sand filtration: Influences of selected process variables. Journal of the American Water Works Association 77(12):62–66.
Bellamy, W. D., G. P. Silverman, D. W. Hendricks, and G. S. Logsdon. 1985b. Removing Giardia cysts with slow sand filtration. Journal of the American Water Works Association 77(2):52–60.
Bellen, G., M. Anderson, and R. Gottler. 1985. Management of Point-of-Use Drinking Water Treatment Systems. Ann Arbor, Mich.: National Sanitation Foundation.
Benjamin, L., R. W. Green, A. Smith, and S. Summerer. 1992. Pilot testing a limestone contractor in British Columbia. Journal of the American Water Works Association 84(5):70–79.
BETZ. 1980. BETZ Handbook of Industrial Water Conditioning. Trevose, Pa.: BETZ Laboratories, Inc.
Brigano, F. A., J. P. McFarland, P. E. Shanaghan, and P. Burton. 1994. Dual-stage filtration proves cost-effective. Journal of the American Water Works Association 86(5):75–88.
Cheryan, M. 1986. Ultrafiltration Handbook. Lancaster, Pa.: Technomic Publishing.
Cleasby, J. L. 1991. Source water quality and pretreatment options for slow sand filters. Chapter 3 in Slow Sand Filtration, G. S. Logsdon, ed. New York: American Society of Civil Engineers.
Cleasby, J. L., D. J. Hilmoe, and C. J. Dimitracopoulos. 1984. Slow sand and direct in-line filtration of a surface water. Journal of the American Water Works Association 76(12):44–45.
Community Nutrition Institute. 1991. FDA not enforcing rules on bottled water: GAO. Nutrition Week (April):6.
Conlon, W. J. 1990. Membrane processes. Chapter 11 in Water Quality and Treatment: A Handbook of Community Water Supplies , Fourth Edition. Denver: American Water Works Association.
Cullen, T. R., and R. D. Letterman. 1985. The effect of slow sand filter maintenance on water quality. Journal of the American Water Works Association 77(12):48–55.
Duranceau, S. J., J. S. Taylor, and L. A. Mulford. 1992. SOC removal in a membrane softening process. Journal of the American Water Works Association 84(1):68–78.
Edwards, M., M. R. Schock, and T. B. Meyer. 1996. Alkalinity, pH, and copper corrosion by-product release. Journal of the American Water Works Association 88(3):81–94.
EPA (Environmental Protection Agency). 1982. Memorandum from Joseph A. Cotruvo, Director, Criteria and Standards Division, ODW, to Greene A. Jones, March 3.
EPA. 1985. Federal Register 50(219):46880.
EPA. 1989. Federal Register 40 CFR Parts 141 and 142, Thursday, June 29.
EPA. 1994. The National Public Water System Supervision Program: FY 1993 Compliance Report. EPA 812-R-94-001. Washington, D.C.: Office of Water .
EPA. 1995. Letter from Jennifer Orme Zavaleta, Acting Chief, Human Risk Assessment Branch, Offices of Sciences and Technology, to S.K. Verma, February 14.
Fox, K. R., R. J. Miltner, G. S. Logsdon, D. L. Dicks, and L. F. Drolet. 1984. Pilot plant studies of slow-rate filtration. Journal of the American Water Works Association 76(12):62–68.
Goodrich, J. A., J. Q. Adams, B. W. Lykins, and R. M. Clark. 1992. Safe drinking water from small systems: treatment options. Journal of the American Water Works Association 84(5):49—55.
Grubbs, T. R., and F. W. Pontius. 1992. U.S. EPA releases draft ground water disinfection rule. Journal of the American Water Works Association 84(9):25–31.
Haarhoff, J., and J. L. Cleasby. 1991. Biological and physical mechanisms in slow sand filtration. Chapter 2 in Slow Sand Filtration, G. S. Logsdon, ed. New York: American Society of Civil Engineers.
Hall, T., and R. A. Hyde. 1987. Evaluation of Slow Sand Filtration for Small Supplies: Package Plant Evaluation at Stalling Busk. Final Report. Stevenage, England: Water Research Centre.
Hall, T., J. Pressdee, R. Gregory, and K. Murray. 1994. Cryptosporidium removal during water treatment using dissolved air flotation. Pp. 110–114 in Flotation Processes in Water and Sludge Treatment, Proceedings of International Association of Water Quality—International Water Supply Association—American Water Works Association Joint Specialized Conference. Orlando, Fla.: Chameleon Press Ltd.
JAWWA (Journal of the American Water Works Association). 1993. Reverse osmosis solves radium problem. Journal of the American Water Works Association 85(6):111–112.
Kollajtis, J. A. 1991. Dissolved air flotation applied in drinking water clarification. Pp. 443–448 in Proceedings 1991 AWWA Annual Conference. Denver: American Water Works Association.
Letterman, R. D., and G. S. Logsdon. 1976. Survey of direct filtration practice—Preliminary report. Paper Presented at AWWA Annual Conference, New Orleans, La., June 20–25, 1976.
Letterman, R. D., C. T. Driscoll, M. Haddad, and H. A. Hsu. 1987. Limestone Bed Contractors for Control of Corrosion at Small Water Utilities. EPA/600/2-86/009. Cincinnati: Environmental Protection Agency.
Letterman, R. D., M. Haddad, and C. T. Driscoll. 1991. Limestone contractors: Steady-state design relationships. Journal of Environmental Engineering 117(3):339–358.
Logsdon, G. S., M. M. Frey, T. D. Stefanich, S. L. Johnson, D. E. Feely, J. B. Rose, and M. Sobsey. 1994. The Removal and Disinfection Efficiency of Lime Softening Processes for Giardia and Viruses. Denver: American Water Works Association Research Foundation.
Lundelius, E. F. 1920. Adsorption and solubility. Kolloid-Zeitschrift 26:145–151.
Lykins, W., Jr., R. M. Clark, and J. A. Goodrich. 1992. Point-of-Use/Point-of-Entry for Drinking Water Treatment. Boca Raton, Fla.: Lewis Publishers, Inc.
Macler, B. A. 1996. Developing the ground water disinfection rule. Journal of the American Water Works Association 88(3):47–55.
Malley, J. P., Jr., and J. K. Edzwald. 1991. Laboratory comparison of DAF with conventional treatment. Journal of the American Water Works Association 83(9):56–61.
McKelvey, G. A., M. A. Thompson, and M. J. Parrotta. 1993. Ion exchange: A cost-effective alternative for reducing radium. Journal of the American Water Works Association 85(6):61–66.
Meller, F. H. 1984. Electrodialysis (ED) and Electrodialysis Reversal (EDR) Technology. Watertown, Mass.: Ionics, Inc.
Montgomery, J. M., Consulting Engineers. 1985. Water Treatment Principles and Design. New York: John Wiley and Sons.
Morin, O. J. 1994. Membrane plants in North America. Journal of the American Water Works Association 86(12):42–54.
Opferman, D. J., S. G. Buchberger, and D. J. Arduini. 1995. Complying with the SWTR: Ohio's experience. Journal of the American Water Works Association 87(2):59–67.
Poynter, S. F. B., and J. S. Slade. 1977. The removal of viruses by slow and filtration. Progress in Water Technology 9(1):75–88.
Pyper, G. R. 1985. Slow Sand Filter and Package Treatment Plant Evaluation: Operating Costs and Removal of Bacteria, Giardia, and Trihalomethanes. EPA/600/2-85/052. Cincinnati: Environmental Protection Agency.
Pyper, G. R., and G. S. Logsdon. 1991. Slow sand filter design. Chapter 5 in Slow Sand Filtration, G. S. Logsdon, ed. New York: American Society of Civil Engineers.
Richardson, S. E. 1991. Reporting to Congress on California's bottled water program. Journal of the Association of Food and Drug Officials 55(2):45–49.
Riesenberg, F., B. B. Walters, A. Steele, and R. A. Ryders. 1995. Slow sand filters for a small water system. Journal of the American Water Works Association 87(11):48–56.
Robeck, G. G., N. A. Clarke, and K. A. Dostal. 1962. Effectiveness of water treatment processes for virus removal. Journal of the American Water Works Association 54(10):1275–1290.
Schlenger, D. L., W. F. Riddle, B. K. Luck, and M. H. Winter. 1994. Automation Management Strategies For Water Treatment Facilities. Denver: American Water Works Association Research Foundation and American Water Works Association.
Schuler, P. F., M. M. Ghosh, and S. N. Boutros. 1988. Comparing the removal of Giardia and Cryptosporidium using slow sand and diatomaceous earth filtration, Pp. 789–805 in Proceedings 1988 AWWA Annual Conference. Denver: American Water Works Association.
Seelaus, T. J., D. W. Hendricks, and B. A. Janonis. 1986. Design and operation of a slow sand filter. Journal of the American Water Works Association 78(12):35–41.
Semmens, M. J., R. Qin, and A. K. Zander. 1989. Using a microporous hollow-fiber membrane to separate VOCs from water. Journal of the American Water Works Association 81(4):162–167.
Snoeyink, V. L. 1990. Adsorption of organic compounds. Chapter 13 in Water Quality and Treatment: A Handbook of Community Water Supplies, Fourth Edition. Denver: American Water Works Association.
Sorg, T. J. 1978. Treatment technology to meet the interim primary drinking water regulations for inorganics. Journal of the American Water Works Association 70(2):105–112.
Sorg, T. J., R. W. Forbes, and D. S. Chambers. 1980. Removal of Radium-226 from Sarasota County, Fla. drinking water by reverse osmosis. Journal of the American Water Works Association 72(4):230–237.
Syrotynski, S., and D. Stone. 1975. Microscreening and diatomite filtration. Journal of the American Water Works Association 67(10):545–548.
Tamburini, J. U., and W. L. Habenicht. 1992. Volunteers integral to small system's success. Journal of the American Water Works Association 84(5):56–61.
Weber, W. J., Jr. 1972. Physicochemical Processes for Water Quality Control. New York: Wiley-Interscience.
Wiesner, M. R., J. Hackney, S. Sethi, J. G. Jacangelo, and J. M. Laine. 1994. Cost estimates for membrane filtration and conventional treatment. Journal of the American Water Works Association 86(12):33–41.
Zander, A. K., M. J. Semmens, and R. M. Narbaitz. 1989. Removing VOCs by membrane stripping. Journal of the American Water Works Association 81(11):76–81.