4
Shipboard Treatment Options
This chapter identifies various methods of treating ballast water that are safe, practicable, technically feasible, cost-effective, and environmentally acceptable. The main focus is on treatment processes that are feasible for onboard use to control species transfer in ballast water.1 The basis for many of the candidate technologies originates in the wastewater treatment area, although the uniqueness of shipboard ballast applications was a major factor in the committee's assessment. A discussion of treatment issues and options is followed by a listing of candidate shipboard treatment technologies. The committee's methodology for data collection and technology evaluation is described, followed by an assessment of candidate systems; the most promising options are addressed in some detail. Supplementary information on some of the candidate technologies is provided in Appendix F, as appropriate.
TREATMENT ISSUES
Treating ballast water to free it from potentially harmful invasive species is a challenging problem. Many different species potentially can be transferred to areas where they become invasive and a nuisance; therefore a single preventive treatment for all such species is difficult to identify. Ships may travel great distances between ports or visit many ports of call throughout the course of one voyage. Depending on the amount of cargo carried and the weather conditions
|
1 |
For the purposes of the present discussion, changing water in a ballast tank by continuous flushing or deballasting and reballasting is not considered to be a shipboard treatment process. Ballast change is addressed in Chapter 3. |
encountered, the movement of ballast on and off a ship can be highly variable (see Chapter 2). Substantial power and space limitations on ships, together with safety concerns for the vessel and its crew, make onboard treatment options complex. Space on board any ship is at a premium, and some ships are more seriously constrained with respect to space than others.
Defining effective treatment scenarios is complicated by the variability of ships, shipping routes, and ports. Lakes and waterways typically contain large quantities of minerals but have low chloride-ion concentrations. Oceans are perhaps less complicated because of their chemical consistency worldwide when they are away from the continental shelf. The pH and salinity of seawater are kept relatively constant by a complex balance of chemicals. The unpredictable mix of ocean and fresh water in estuaries, shallow tidal bays, and inlets, where major port facilities are located, tends to create a zone rich in nutrients and resulting marine life. A short list of water parameters that must be addressed by systems for treating ballast water comprises temperature, salinity, pH, dissolved oxygen, suspended solids, and biological constituents.
Ships' ballast tanks and cargo/ballast holds represent a unique challenge for treating water due to the high flow rates, large volumes, organism diversity, and ballast water residence time. It appears that with currently available technology one overall onboard treatment scenario will not be effective for all vessels carrying ballast water in and between various regions of the world. As discussed in Chapter 3, a range of preventive measures, including onboard and shore-or port-based methods for treating ballast water, will be needed to address the problem of controlling introductions of nonindigenous aquatic nuisance species. Earlier reviews of options for treating ballast water, such as Canadian Coast Guard (1992), AQIS (1993), and Carlton et al. (1995), provide more detailed discussions of these methods.
TECHNOLOGIES FOR TREATING BALLAST WATER
Shipboard treatment methods are the most flexible for managing ballast water. For this reason, the committee determined that treating ballast water on board ships will be the ultimate solution to reducing introductions of nonindigenous aquatic species. The following sections describe candidate technologies and methods for evaluating them.
Treatment technology options can be incorporated during three different phases of ballast operations: (1) during ballasting at the cargo discharge port; (2) during the voyage, between ports; and (3) during deballasting at the cargo loading port (see Figure 3-1). Each of these scenarios has significantly different constraints with respect to treatment options. In the first and third scenarios, large quantities of water must be treated in a short period of time, while water is taken on board or discharged and flow rates through the treatment system are high. In the second scenario, water resides in the ballast tanks or cargo/ballast holds
between ports; more time is available for treatment; and flow rates through the treatment system may be lower. In addition, water can be recirculated until the number of organisms inactivated is sufficient to provide the appropriate level of protection. As noted earlier, ships can discharge ballast water at an extremely high rate (upwards of 20,000 m3/hr at flow velocities up to 3 m/s), with resulting drawbacks for inline treatment in terms of system capacity and associated space and power requirements. However, inline treatment during ballasting/deballasting is potentially more effective in inactivating large organisms throughout the entire sediment/water mixture than onboard treatment. In the latter case, the sediment fraction settles to the bottom, and at least a two-phase system (water column and sediment fraction) is present. Therefore, even treating ballast water at a lower flow rate for a longer time period may not easily achieve inactivation in the sediment fraction.
The committee identified 10 candidate technologies for shipboard treatment:
Filtration Systems. Filtration systems are widely used in municipal and industrial applications. Systems designs are determined by the size and type of particles to be removed. Filter systems require periodic cleaning, either manually or using automatic backflush systems.
Oxidizing and nonoxidizing biocides. Oxidizing biocides, notably chlorine and ozone, are widely used in waste-water treatment. Organic structures, such as cell membranes, are destroyed by the addition of strong oxidizers. Nonoxidizing biocides include a large inventory of compounds commonly used in industries for treating the growth of organisms in cooling tower water and other areas where large amounts of biological growth or sediment accumulation occur. Nonoxidizing biocides work in a manner somewhat analogous to pesticides by interfering with reproductive, neural, or metabolic functions of organisms, such as by inhibiting respiration.
Thermal techniques. High temperatures are commonly used to sterilize water in a wide variety of applications.
Electric pulse and pulse plasma techniques. The application of a pulsed electric field or an energy pulse to water can kill organisms. Electric pulse systems generate an electric field; pulse-plasma systems deliver a high energy pulse to an inwater arc mechanism and generate a plasma arc in water.
Ultraviolet treatment. Treating water with ultraviolet energy to inactivate bacteria is a well-established technology. Ultraviolet irradiation in the fluid at wavelengths of approximately 200 nm can destroy cellular components.
Acoustic systems. Acoustic systems use transducers to apply sound energy of specified amplitude and frequency to water to be treated. The sound energy causes cavitation, and the resulting mechanical stresses disrupt cells.
Magnetic fields. Water to be treated is passed through a magnetic field of specified flux that is generated by ferromagnetic or electromagnetic devices. The biological and chemical effects of magnetic systems are not well understood, but it is thought that the organic and inorganic constituents of living organisms in the water are altered by the magnetic field.
Deoxygenation. Most potential aquatic nuisance species require oxygen to survive. When oxygen is removed from the water, many organisms (but not cysts, spores, or anaerobic bacteria) are killed. Some organisms that require oxygen can survive short periods of anoxia, but they are usually inactive under such conditions. Oxygen can be removed from water by purging with an inert gas or by binding oxygen to a chemical additive.
Biological techniques. Biological techniques to control unwanted species include the introduction of additional organisms that are predators, pathogens, or competitors of the species of concern. Such techniques have proven useful in the control of certain insect pests when the biocontrol species develops self-sustaining, reproducing populations. Biological treatment also includes the use of modern biotechnology methods to modify the genetics of the organism of concern.
Anti-fouling coatings. Anti-fouling coatings on hulls reduce biological fouling by contact toxicity, ablation or surface activity. The vast majority of coatings used today rely on toxicity or ablation, or a combination of the two. Surface-active systems are marketed as "fouling release" coatings, but their use is limited because they are expensive.
The committee's evaluations and details of the most promising technologies are discussed in the following sections.
COMMITTEE'S EVALUATION METHODOLOGY
Once the treatment problem was defined, the committee developed a list of candidate technologies, drawing in part on wastewater treatment methods. A treatment options query developed by the committee combined a list of technology requirements and capabilities with shipboard application considerations. On the basis of this questionnaire, technology vendors and research organizations were invited to provide data for typical shipboard treatment systems. These data were used by the committee in assessing candidate technologies. All technologies were required to meet safety and effectiveness criteria before being evaluated further. At the conclusion of this assessment, the candidate technologies were grouped into three categories: (1) promising options, (2) options with possible limited application, and (3) other options.
Treatment Options Query
The committee developed the ballast water treatment options query provided in Appendix G as a basis for gathering information on technologies for treating ballast water that were potentially suitable for shipboard application. The type of information needed to assess candidate technologies was determined based on the requirements and constraints discussed in Chapters 2 and 3, information gathered by the committee (see Appendix B), and insights derived from the practical shipping experience and technical knowledge and expertise of individual committee members. The major technology requirements, capabilities, and application issues are discussed below in the context of the committee's evaluation criteria. For any candidate technology, the effectiveness in destroying potential aquatic nuisance species for the volumes of water and flow rates required for shipboard ballast water treatment, and the associated operational safety for the crew and ship, are of primary importance.
The committee's query about options for treating ballast water defines two ballasting scenarios: System A—flow rate of 2,000 m3/h and tank volumes of up to 25,000 m3, with residence times as short as 24 hours; and System B—flow rate of 20,000 m3/h and tank volumes of up to 25,000 m3, with residence times as short as 24 hours.
The residence time (i.e., maximum time available to treat ballast water) for both systems is defined to be a minimum of 24 hours, reflecting practical constraints imposed by voyage times. Many voyages are likely to be of several days duration; therefore, a treatment time of 24 hours would permit operational flexibility and would be broadly applicable to diverse vessels and trade routes. Flow rates vary considerably with vessel type as discussed in Chapter 2.
Data Collection
The committee held two technology workshops in May and August 1995 in Duluth, Minnesota, and Washington, D.C., respectively, to gather data on candidate technologies for treating ballast water from equipment suppliers, technology developers, and research organizations (see Appendix B). Representatives from these bodies were invited to give presentations to the committee addressing the salient features of water-treatment technologies as applied to ships' ballast, using the committee's query on options for treating ballast water as a guide. Responses to the query were obtained through correspondence with equipment suppliers, technology developers, and research organizations. Product literature and papers from the scientific and technical literature also provided information about treatment options (see Appendices F and G).
Evaluation Criteria
The committee identified a series of parameters for rating technologies that could be used to treat ballast water onboard ships. As noted throughout this
report, safety is of overarching importance when assessing strategies for managing ballast water. Further, any such strategy is worthwhile only if it is effective in reducing the likelihood that nonindigenous aquatic nuisance species will be introduced. Therefore, the committee used safety and effectiveness as the first two gates in evaluating technologies. Only systems that were effective for the broad spectrum of organisms typically found in ballast water (see Chapter 1) and deemed safe for the ship and crew were evaluated in more detail. Because ballast water volumes, pumping rates, and engine room arrangements vary significantly from ship to ship, the type and size of a vessel influence the choice of a ballast water treatment system (AQIS, 1993). Thus, the committee made no attempt to select optimum treatment technologies for specific situations, such as for vessels operating in the Great Lakes, but rather identified promising candidates for shipboard use.
The availability of proven commercial treatment systems could provide an important starting point to avoid delays in applying technologies. Given the time and resources necessary to develop a technology from laboratory scale through demonstration projects to commercial status, the adaptation of existing industrial equipment to the shipping industry is a potentially attractive short cut. Although most of the equipment considered by the committee is available commercially, few systems have been used in marine applications. The reliability and maintainability of available equipment, which are extremely important for ship operators, remain to be demonstrated in a shipboard environment. Using commercially proven treatment technologies is attractive in the short term; however it will probably take many years to implement ballast water control practices on the majority of vessels. In the long term, there will be opportunities to develop new, cost-effective systems tailored to specific shipboard conditions.
Power consumption is another important consideration for shipboard applications; treatment systems require power to operate. Power on board ship is limited, and providing more power capacity to treat ballast water may not be practical.
Chemical residuals may be created when treating fresh water and sea water. These residuals can be complex organic chemicals and may or may not be regulated. For example, chlorine is an effective oxidizing biocide, but excess residual oxidant may be subject to controls in some shore locations (Christian et al., 1995). The committee determined that treatments resulting in the least environmental impact are the most attractive.
Most ballast operations involving the loading or discharging of large volumes of water are conducted when a vessel is in port. As noted above, attempts to treat large volumes of ballast water ''instantaneously" during ballasting or deballasting in port are extremely challenging. The capability to treat ballast water en-route over the period of a voyage would reduce the capacity requirements and associated costs of treatment systems. Recirculating ballast water during a voyage to increase treatment effectiveness might be practical for some techniques.
For assessment purposes, cost was divided into two components: the physical plant and crew size. Given the lack of precision in most capital cost estimates received from equipment suppliers and developers, the committee considered cost for equipment and installation on an order of magnitude basis. The cost of adapting industrial systems for marine applications is quite high in many cases. Crew size is important in the overall cost of operating a ship. Therefore, if an additional crew member is needed to operate a treatment system, this option will not be acceptable to ship operators.
Practical limitations on the size and complexity of ballast water treatment systems depend on ship type and trade. In general, the optimum system is the smallest treatment unit with the fewest auxiliary support systems. Since ships are built to carry maximum cargo, non-earning space such as engine rooms, ballast tanks, and accommodations is reduced to a minimum. In particular, engine rooms tend to have very limited space for additional equipment, although the most convenient location for a treatment facility would be in or adjacent to the engine room in which the ballast pumps are located, which is usually at the lowest level of the ship.
System maintenance must be kept to a minimum, especially during voyages. A system for treating ballast water must be simple, reliable, and easy to use. Systems that require extensive, time-consuming maintenance—possibly under difficult conditions at sea—are not suitable for treating ballast water on board ships.
Ease of monitoring relates to both the operation and evaluation of treatment effectiveness. Automated systems that combine monitoring and control of the treatment process will be necessary.
Candidate Ranking
The committee ranked candidate technologies using the evaluation criteria shown in Table 4-1. All of the technologies considered by the committee met the safety requirements for shipboard application. Although marine applications have not been demonstrated in all cases, the committee determined that there were no inherent features of the candidate technologies that would preclude safe operation of a shipboard system to treat ballast water. In some cases, special precautions will be necessary to protect ship personnel. For example, the plasma used for pulse-plasma treatment produces a very high pressure shock wave that expands and works against the surrounding water; suitable screening around the treatment system would be required. Similarly, the high voltage part of electric pulse treatment systems would need to be encapsulated to ensure that there were no high electric fields outside the volume of ballast water being treated.
The committee grouped the candidate technologies for treating ballast water into three major categories: (1) promising options, (2) options with possible limited application, and (3) others. Technologies in the first category require some
TABLE 4-1 Evaluation Matrix for Shipboard Treatment Technologies.
|
|
|
|
Commercial use |
Cost |
||||||||
|
|
Safety |
Effectiveness |
Industrial |
Marine |
Power |
Chemical residuals |
Recirculationa |
Plant |
Crew size |
Size, complexity |
Maintenance |
Ease of monitoring |
|
Oxidizing biocides |
|
|
|
|
|
|
|
|
|
|
|
|
|
halogen |
+ |
+ |
+ |
+ |
+ |
- |
+ |
+ |
+ |
+ |
+ |
+ |
|
ozone |
+ |
+ |
+ |
- |
- |
-saltwater +freshwater |
- |
- |
- |
- |
- |
+ |
|
Nonoxidizing biocides |
+ |
+ |
+ |
+ |
+ |
- |
+ |
+ |
+ |
+ |
+ |
- |
|
Filtration systems |
|
|
|
|
|
|
|
|
|
|
|
|
|
media |
+ |
++ |
+ |
- |
+ |
+ |
+ |
+ |
+ |
- |
+ |
+ |
|
screen |
+ |
++ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Thermal treatment |
+ |
+ |
+ |
- |
- |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Electric pulse and pulse plasma techniques |
+ |
+ |
- |
- |
- |
+ |
- |
-- |
- |
- |
+ |
- |
|
Ultraviolet treatment |
+ |
- |
|
|
|
|
|
|
|
|
|
|
|
Acoustic systems |
+ |
- |
|
|
|
|
|
|
|
|
|
|
|
Magnetic fields |
+ |
- |
|
|
|
|
|
|
|
|
|
|
|
Deoxygenation |
+ |
- |
|
|||||||||
|
Note: Key to ratings: + good; -poor; technologies judged to be markedly superior or inferior in a category are designated ++ or -- respectively. a + indicates that recirculation of ballast water improves the efficacy of a treatment technology. |
||||||||||||
engineering development to optimize systems for shipboard use. Appropriate demonstration projects may be helpful in providing performance data as a basis for further system improvement and assessment of different options. Technologies in the second category require further research and development (R&D) before they can be considered promising candidates for use on board ships. Such R&D would provide additional data on which to base assessments of anticipated shipboard performance. Technologies in the third category do not appear promising at present, although the results of further R&D could alter this assessment.
The committee limited its evaluation to the generic problem of reducing the number of aquatic organisms in ballast water using a single treatment technology. The many possible technology combinations, such as filtration followed by ultraviolet or biocidal treatment, were not evaluated. Nonetheless, the committee recognizes that investigation of technology combinations would be fruitful in addressing specific ballast water control problems.
PROMISING OPTIONS
The committee considers filtration, as well as the addition of biocides (excluding ozone) and thermal treatment, to be relatively mature technologies with the potential for adaptation to treating ballast water on board ships.
Filtration Systems
The physical separation and removal of organisms above a certain size from ballast water could be achieved during ballasting operations using a shipboard filtration system. This appears to be the most promising technology for onboard treatment. Filtering ballast water as it is loaded is a potentially attractive option that would prevent or minimize the intake of unwanted organisms, thereby obviating the need for subsequent ballast change or further onboard treatment.2 Filtration systems can be designed to remove target organisms from ballast water; however, detailed engineering will be needed for adequate filtration (organism removal) that would provide the appropriate level of protection. The high flow rates and volumes associated with loading (and discharging) ballast present a particular challenge. Also, the complexity and cost of filters increase as the quantity of material removed from ballast water increases (smaller particles). Filtered organisms could be concentrated and stored on board ship and disposed of at a shore-based facility or discharged back into the water if regulations permit. The options for onboard filtration systems are either mesh strainers or deep media filtration.
Coarse strainers are routinely incorporated in shipboard machinery to prevent the intake of large objects into the shipboard systems. However, when the effective opening of screens is reduced to remove small organisms, such as those in the range of 10 µm,3 plugging or fouling rapidly occurs. Strainers have the disadvantage of being a simple layer-separation technique: once material has been deposited on the screen, the flux of water through the mesh declines rapidly. In water treatment processes where this approach is used, large areas of filtering screen are required to maintain a working flux for any period of time. Generally, backup facilities are included so that a continual flow stream can be achieved despite rapid plugging of strainers.
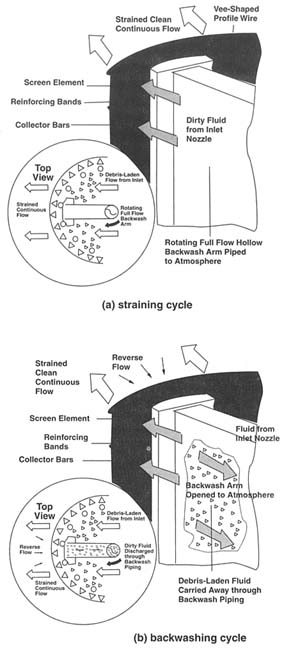
Recent advances in strainer technology have addressed the above problems, and continuously cleaned screening systems are now commercially available. For example, self-cleaning strainers with automatic control systems incorporate cleaning cycles that can be activated by differential pressure or on a time cycle. Debrisladen dirty fluid enters the large bottom chamber of the strainer and flows upward, passing through the sealed screen element. Unwanted materials are trapped inside the screen. Backwash cleaning is accomplished using the pressure differential between the in-flowing line pressure and the atmosphere. A hollow, full-flow, backwash arm that extends the full length of the screen element and is piped to the atmosphere rotates slowly inside the screen. When cleaning is required the automatic backwash valve opens the system to the atmosphere, causing a high-velocity reverse flow across the isolated section of the screen. Dirt and debris are flushed from this segment of the screen into the backwash piping. During the backwashing cycle the main flow is uninterrupted and continues to be strained in the normal manner (see Figure 4-1).
Other developments in filtration technology may offer advantages for treating ballast water on board ship. For example, flow-through centrifugation systems can separate particles prior to filtering to reduce filter clogging. New wedgewire filtration systems have high flow rates and are cleaned by scraping, rather than backwashing, which eliminates the need for storing and treating backwash water and thereby reduces the overall size of the filtration unit.
Another way to circumvent the problems of simple screening systems described above is to use deep media filters. Media filtration is one of the most common processes for treating water. Media filtration systems for shoreside application are simple to design and operate and have proven reliable for many years. When used to treat ballast water on board ship, a typical pressure filter could produce high quality water at a flux of at least 24 m3/hr per m2 (10 gpm/ft2) of filter area, depending on the media coarseness. Assuming this typical flux, a ship with a ballast flow of 5,000 m3/h would require a filter approximately 200 m2 in area by 2 m deep. Clearly, the size of such units would preclude their use on board a ship.
More efficient mixed media filters can be readily designed, and precoating of filters can be included to further enhance their effectiveness. Media can be adjusted to remove particles as small as 1 to 5 µm in diameter, if necessary. These filters would be easily cleaned using conventional backwashing mechanisms, and the backwash material could be stored on board in a separate tank prior to discharge overboard in some open ocean area. Innovative approaches such as the fuzzy filter (Tchobanoglous and Caliskaner, 1995) may permit higher flow rates per unit area, thereby reducing the size of media filtration units and rendering them feasible for shipboard use.
Media filtration treatment would remove not only all organisms larger than 5 to 10 µm in diameter but also most of the sediment load in the ballast water. Therefore, all organisms, whether residing in the sediment or dispersed in the water column, would be effectively separated from the ballast water during the ballasting operation. Because deep media filters are highly reliable and do not plug rapidly, the ship would be able to use them on an intermittent basis during ballasting operations. The time-dependent plugging of deep media filters can be monitored easily by measuring the pressure drop or head loss across the media, and a shipboard system could be cleaned or backwashed when time permitted. Installation of deep media filters on board ships would not be a severe problem as a retrofit. Bypasses from the main ballast water system would need to be rerouted through the filter, but neither the piping system nor the ballast tanks themselves would need to be substantially modified.
A potential advantage of an inline media-filtration system is that ballast water would be treated effectively regardless of when it was loaded. Thus, discharge of treated ballast water could occur at any time in any location without increasing the risk of introducing nonindigenous aquatic nuisance species. Further investigation of this option for treating ballast water is needed to determine the relationships between achievable flow rates and selected media for effective organism removal. Deep media filtration systems could be designed for individual ships and ship classes to optimize shipboard use.
Several earlier assessments of the possible use of filtration systems for treating ballast water are available. In general, the findings and conclusions of these studies are consistent with those of the committee. An assessment for the Australian Quarantine and Inspection Service (1993) of ballast water treatment technologies concluded that media filtration is effective in removing the majority of marine organisms from ballast water, although the large footprint of existing units precludes shipboard use. Strainers were found to be relatively compact, simple to operate, and amenable to retrofit on existing vessels, but they were found to have limited effectiveness because many organisms of concern are smaller than the existing strainer slot sizes and can pass through the treatment system. The design of a filtration system for a typical large bulk carrier is discussed in the Australian study, and space requirements and estimated costs are provided for a range of strainer systems capable of handling flow rates up to 4,000 m3/h.
A study for the Canadian Coast Guard (1992) concluded that the physical removal of organisms by filtering may be an effective stand-alone treatment process or may be used in conjunction with other technologies, such as chemical oxidation or ultraviolet sterilization. Space constraints for media filtration units were identified as an issue, as were high flow rates during ballast uptake and discharge. A recirculation type treatment system was also considered in the Canadian study. The reader is referred to the original report for cost estimates and technical details of systems with different pumping rates and strainer slot sizes.
In another recent study of ways to prevent organism intake in ballast water by filtration (Carlton et al., 1995), a two-stage system using woven mesh screen filters is considered. The first filter would remove most of the larger zooplankton; the second filter would remove most of the smaller zooplankton and most of the large and medium-sized phytoplankton. The relative merits of woven mesh and wedgewire filters and discussed, as are space requirements, mesh size, and order of magnitude costs.
Biocides
Perhaps the most compelling reason to consider biocidal treatment is its ease of application. Biocides could be added to ballast water by metering concentrated solid chemicals or they could be generated electrolytically from sea water. These two methods of applying biocides are currently used on board ships, although not for treating ballast water. Alternatively, simple chemical injection pumps, feeding on line with the main ballast pumps, could routinely add a measured amount of premixed liquid biocide during any ballasting operation. The turbulence within the pumping system would ensure complete mixing of the biocide with the sediment and water column, resulting in efficient inactivation of target organisms. Chemical feed systems would require small amounts of power to pump concentrated bulk solutions to the ballast system. Electrolytic generation systems would require significant amounts of power, typically in excess of 200 kW. In addition, in-situ generation requires large and expensive equipment costing $400,000 to $800,000.
Biocides are among the most widely used industrial chemicals, and there is a large body of scientific data on their use in waste-water treatment. Effective biocide concentrations are typically in the range 1 to 5 mg/l (ppm). Dose levels and contact times need to be determined for effective treatment of aquatic nuisance species. However, if doses are similar to those used for waste-water treatment, only 5 m3 of biocide would be required for each million cubic meters of ballast water to give a 5 mg/l biocide concentration. Therefore, even large ships carrying thousands of cubic meters of ballast would be required to carry only a few cubic meters of biocide per voyage.
The effectiveness of many biocides is quite simple to monitor: others would require biological indicators to ascertain their level of effectiveness. There is no
general, automated monitoring method currently available that could be easily applied on board ship.
Biocide treatment units are relatively simple, although their size could be an issue when retrofitting existing vessels. Units can be designed to require little maintenance, and the greatest burden to the crew would be the filling and monitoring of bulk solution tanks.
Chemical addition for inactivation of micro-organisms has been reviewed recently (Carlton et al., 1995). In general this approach has been dismissed for many reasons including the following:
-
reluctance to add any compound to water that might be discharged back to the ocean
-
uncertain effectiveness of biocides in achieving inactivation of the target organism(s)
-
handling of chemicals on board ship
-
compliance with discharge regulations for such chemicals in certain areas of the world
Although these concerns are real, they can be addressed by appropriate research, development, and demonstration activities. Addition of selected biocides may be the most economic solution to achieving the desired goal of inactivating target species.
The safety issues associated with handling chemicals on board a ship may be of concern. Ships routinely carry hazardous industrial compounds and lubricants because they are needed for ship operations. Personnel responsible for handling these compounds are well trained and would be able to adapt to handling other materials without serious constraints. The concentrations of chemicals needed to treat ballast water should be small, and storage requirements may be insignificant. The residual chemicals left in a ship's ballast tanks following treatment and the possibility of corrosion of piping, pumps, and structure need to be evaluated.
Oxidizing Biocides
Decades of studies have demonstrated that, in general, chlorine is an effective biocide. Its specific application for ballast water will require data on the required concentrations and contact times for different categories of organisms—ranging from bacteria and viruses to adult crustaceans and fish—and relative to water flow rates and/or tank volume and water residence time.
Electrolytic chlorination creates active chlorine by passing an electrical current through water containing chloride ions. The chloride ions in brackish water and seawater are used in this process. Chlorination units are comparatively small and used on many ships for biofouling control of seawater cooling systems. A
typical unit can treat 7.5 m3/min (450 m3/h) at a concentration of one mg/l of oxidant. The use of chlorine gas would be inappropriate for enclosed spaces such as on board ship, but calcium hypochlorite or sodium hypochlorite could be used for treating ballast water. It is well known that the addition of a powerful oxidant such as chlorine to seawater can generate toxic compounds (byproducts) due to the oxidation of halogens (Cl-, Br-, I-). This issue needs to be addressed in the context of various options for treating ballast water.
Nonoxidizing Biocides
Reviews of water treatment conducted in the past have focused on the use of oxidizing biocides. However, as noted above, there is a large inventory of nonoxidizing biocides that are used by industry for the effective control of a wide variety of organisms. An example is the group of glutaraldehyde-based chemicals used in industrial water treatment. The limited reactions of nonoxidizing biocides with compounds in the water may be a possible advantage for ballast water treatment. The residuals generated by nonoxidizing biocides generally decay fairly rapidly into nontoxic byproducts. Thus, treated water might not pose a substantial environmental hazard if it were discharged in large quantities.
Thermal Treatment
The use of waste heat from a ship's propulsion and service cooling is an attractive option for the inactivation of organisms in ballast water because (1) waste heat from ship propulsion and onboard equipment may be used to raise the temperature of water and (2) no chemical byproducts or residuals are discharged (Rigby and Taylor, 1993). Additional piping would be needed to pump ballast water through the existing heat exchangers. To destroy most organisms, ballast water would need to be heated to temperatures in the range 35°C to 45°C (95°F to 113°F) and held there for a set period of time. Investigations of the relationships between treatment temperature and time needed to kill or inactivate certain organisms are continuing (Taylor, 1995).
A number of critical factors will probably limit the practicality of thermal treatment to certain vessels on specific trade routes:
-
Length of voyage. Some voyages will be too short to permit heating the water to the required temperature or holding the temperature for the required period.
-
Volume of ballast water requiring treatment. There is a limited amount of energy available from waste heat sources. Thus, there are constraints on the volume of ballast water that can be treated.
-
Ambient water temperature. The heat input needed for thermal treatment can be reduced significantly where water temperatures are at tropical or summer levels (30°C or higher). The heat loss due to ambient water temperatures outside the hull must be considered. Due to low ambient water temperature, ballast tanks located along the hull shell may have a higher total heat input than those adjacent to cargo.
-
Specificity to target organism(s). Higher organisms, such as fish, are more easily killed by thermal treatment than are microbes. Studies will be required to determine the effectiveness of heat treatment to specific target organisms.
Further discussion of thermal treatment is provided in Appendix F.
Technology Demonstration
The promising technology options described above—filtration, the addition of biocides (excluding ozone), and thermal treatment—are currently the most attractive for shipboard demonstration. However, the selection of a technology for demonstration will require an analysis of voyage features such as trading patterns and ports of call. For example, thermal treatment is unlikely to be chosen for short voyages because of the time needed to heat and treat ballast water. The results of the committee's assessment indicate that filtration with state-of-the-art strainers is likely to be the most widely applicable option for treating ballast water on board ships. Shipboard demonstration would provide an opportunity to resolve engineering issues associated with the installation and operation of a strainer system on an existing vessel. Regardless of which technology is chosen for demonstration, a control tank will be needed to establish a baseline against which to compare the results of ballast water treatment. Ideally the treatment and control tanks should be of similar configuration. In addition, it will be necessary to incorporate appropriate sampling and monitoring procedures to determine the effects of treatment (see Chapter 5).
OPTIONS WITH POSSIBLE LIMITED APPLICATIONS
The committee determined that ozonation and electric pulse and pulse plasma technologies are potentially safe and effective methods for treating ballast water, although their current status renders them unsuitable for immediate shipboard applications. Nonetheless, limited applications may be possible in the future. The transition of ozone treatment systems from industrial to shipboard applications is a major challenge. Further development of electric pulse and pulse plasma systems for land-based applications should provide more information regarding the suitability of these technologies for shipboard use.
Ozonation
Ozone is an oxidizing biocide used for treatment of potable and industrial process waters. Environmental concerns regarding the use of chlorine have resulted in increased use of ozone as a biocide. Since ozone is an unstable gas that quickly decomposes to oxygen, it must be generated as needed by passing air or oxygen between a high-voltage discharge gap. Large capacity ozone generation units are not currently used for marine applications. Industrial units are relatively complex and bulky and require compressed air sources for the oxygen separator systems and cooling water for the ozone generator. Ozone generators have demonstrated good reliability in industrial applications, requiring about 10 hours of maintenance per month. However, the successful operation of these generators depends on the quality of the feed gas supply, adequate cooling, and a constant, relatively clean electrical power source. In addition, ozone systems have some serious materials problems, including increased corrosion rates of some alloys and deterioration of seals. Given the uncertainties associated with shipboard operation, the committee assigned negative ratings to ozonation for maintenance and crew impact. In salt water (brackish and sea water), ozonation produces the same residuals as chlorination.
Electric Pulse and Pulse Plasma Techniques
Electric pulse and pulse-plasma treatments have been grouped together since both inactivate organisms in a similar manner. Both methods have been successfully demonstrated in the laboratory, and prototype demonstrations are planned. Applied electrical voltages for these systems are in the 15 to 45 kV range, with pulse durations on the order of 1 µs. Although the power requirements for prototype systems are relatively modest (10 to 50 kW, large energy sources would probably be needed for systems capable of treating large volumes of ballast water. Neither the electric pulse nor the pulse plasma process produces toxic chemical residuals, but a pulse power system would generate gaseous decomposition products, notably carbon dioxide. Theoretically, pulses of electricity through seawater could generate chlorine, although none has been detected during laboratory tests of electric pulse systems.
Given the relatively immature status of both the electric pulse and pulse-plasma technologies for treating water, the costs of developing systems suitable for shipboard application are likely to be high, with resulting high equipment acquisition costs. Because both systems are automated and do not require attended operation, their technical complexity should not be a serious disadvantage if the mean time between equipment failures is long and repair can be achieved by replacement. Electric pulse and pulse-plasma systems are currently being designed for long lifetime and low maintenance. However, in the absence of supporting
performance data from commercial equipment, there is considerable uncertainty about the practical implications of operating and maintaining these systems. For this reason, the ratings for complexity, ease of monitoring, and crew impact given in Table 4-1 are negative. Further discussion of electric pulse and pulse plasma systems is provided in Appendix F.
OTHER OPTIONS
The committee judged four candidate treatments—ultraviolet, acoustic, magnetic, and deoxygenation—to be inappropriate for shipboard treatment of ballast water since these methods have not been proven effective for a broad range of freshwater and marine organisms. Two other techniques, biological treatment and antifouling coatings, were not evaluated in any detail by the committee because their disadvantages clearly outweighed their advantages.
Ultraviolet Treatment
Ultraviolet treatment is effective in destroying micro-organisms, but not in removing or inactivating higher organisms and cyst or spore stages of protozoa, fungi, microalgae (including dinoflagellates), and macroalgae (AQIS, 1993). In addition, the effectiveness of ultraviolet disinfection is greatly reduced in water containing suspended matter due to absorption and screening effects.
Acoustic Systems
The use of underwater acoustic energy sources (including ultrasonics) to destroy or deter various aquatic species has been demonstrated on a laboratory scale, but the effects appear to be dependent on frequency and species, with no general effectiveness over the wide range of organisms likely to be found in ships' ballast tanks. Ultrasonic frequencies around 20 kHz kill or inactivate bacteria and fungi but not higher organisms. Lower frequency acoustic signals deter fish, although the deterrent frequency is species dependent. High-intensity acoustic sources can shatter the shells of juvenile zebra mussels and lead to the lethal disintegration of veligers. Further details of investigations of acoustic treatment are given in Appendix F.
Magnetic Fields
Magnetic treatment of water on a laboratory scale has been effective against calcareous shell-forming invertebrates, notably zebra mussels, and has undergone significant development for this application. However, the treatment mechanism is not understood and the effect of magnetic treatment on marine
micro-organisms and vertebrates is unknown. The method has not been used to treat sea water.
Deoxygenation
Oxygen deprivation (or deoxygenation) is toxic to a range of fish, invertebrate larvae, and aerobic bacteria but is ineffective against anaerobic bacteria and cyst and spore stages, including dinoflagellate cysts, and would provide only a partial solution to killing the range of aquatic species likely to be found in ballast water.4
Biological Techniques
Whether biological techniques are effective in removing, reducing or altering living organisms in ballast water and sediment remains theoretical. The addition of new species is unlikely to act as a successful control agent against the wide diversity of microbial animal and plant life that may be present in ballast water. The issue of the transport and release of the added species must also be addressed. Using genetically engineered organisms is also unlikely to provide a solution for the ballast water problem in the near future. The committee did not evaluate biological remediation in detail.
Anti-fouling Coatings
The committee did not consider anti-fouling coatings to be suitable for controlling introductions of nonindigenous species because the surfaces of ballast tanks and the organisms that settle on these surfaces represent only a small fraction of the total problem. Ballast water is a suspension of organisms, and the bulk fluid must be treated to prevent introductions of nonindigenous species by discharged ballast. A coating could be formulated that was highly loaded with toxic material and would release lethal concentrations of biocide into the water. However, this coating would have a short lifetime, and the treated ballast water would probably be toxic and require treatment prior to discharge.
New types of surface-active anti-fouling coatings are being introduced into the market in response to environmental concerns over paints containing copper and tin. The surface-active systems are nontoxic and rely on flow velocity to remove loosely attached fouling. These paints do not prevent fouling and are not appropriate for ballast tanks.
DISCHARGE TO THE ENVIRONMENT
Any treatment process used to render ballast water safe for discharge creates some form of residual. If chemical biocides are used, either chemical residuals or reaction byproducts will be generated. If a physical separation process, such as filtration, is used, the material collected from suspension must be discharged. If waste engine heat is used to heat ballast water, the temperature of the discharged ballast water will be substantially higher than the ambient water. All of these factors must be considered when developing a treatment plan.
Treatment with biocides may be of concern because the residual biocide in the water that is discharged into a harbor could be toxic to local organisms. Although residual biocides could have lethal effects if present in high concentrations in the immediate vicinity of a ship using biocidal ballast water treatment, they also could have deleterious sublethal effects on organisms further away. In addition to concerns about the biocide itself, treating water that contains organic materials can result in the formation of a variety of organohalogen molecules. Some of these, such as trihalomethanes, can be mutagenic and carcinogenic, as well as persistent in the environment; others with low water solubility are likely to accumulate in bottom sediment and could become a source for bioaccumulation of toxic chemicals in the local aquatic food web.
Release of heated water from ballast tanks may be of environmental concern because the hot water would be lethal to organisms living immediately near the vessel. Organisms located further away would not encounter lethal temperatures but would, nevertheless, be subjected to additional stress and elevated oxygen requirements; at the same time, available oxygen would be reduced because warm water holds less oxygen than cold water. Thermal releases from ships are not currently regulated, although other sources are controlled. Legislation related to thermal releases is directed primarily at power plants that use local water to cool their machinery and continually release heated water back into a river or estuary. The continuous releases from power plants are a much greater source of heated water than sporadic discharges from ships using thermal ballast water treatment. Furthermore, if ships heated their ballast water during the initial days of a voyage, the water would probably have cooled by the time it was discharged; therefore it would have even less environmental impact.
Perhaps the most environmentally benign discharge from a system for treating ballast water consists of suspended solids washed from filters. Material released from periodically backflushing a media filter or continuously washing a membrane filter is a more concentrated version of what was originally in the local water (planktonic organisms and suspended sediment particles). The nature and concentration of materials in the backflush are predictable and would need to be evaluated only in areas of known contamination. The return of these constituents to the water at their site of origin should be of environmental concern only when toxicity has been defined.
The effectiveness of options for treating ballast water must be monitored to ensure that the risk of introductions is being reduced and to verify that operators are complying with regulations. Monitoring is essential from both regulatory and biological perspectives. Many of the candidate treatment technologies reviewed by the committee have been used to treat waste water, and proven monitoring techniques are available. Automated systems are preferred for shipboard use because of demands already placed on crews and operators. These issues are discussed in the following chapter.
SUMMARY
Based on the committee's evaluation of a wide variety of technologies for treating ballast water, the committee determined that there are no off-the-shelf technologies currently available suitable for use on board ship without some redesign and modification. Filtration, the addition of biocides (excluding ozone), and thermal treatment are currently the most promising options for shipboard application. Filtration using state-of-the-art strainers will probably be the most widely applicable approach. However, many detailed engineering issues associated with the installation and operation of a strainer system on a vessel must still be addressed. The committee's finding that there is no universally applicable option currently available for shipboard treatment of ballast water further highlights the need for diverse approaches to managing ballast water, as was noted in Chapter 3.
REFERENCES
AQIS. 1993. Ballast Water Treatment for the Removal of Marine Organisms. Report No. 1 in Ballast Water Research Series. Canberra, Australia: Gutheridge Haskins and Davey Pty Ltd. for the Australian Quarantine and Inspection Service.
Canadian Coast Guard. 1992. A Review and Evaluation of Ballast Water Management and Treatment Options to Reduce the Potential for the Introduction of Non-native Species to the Great Lakes. Ottawa, Canada: Pollutech Environmental Limited for the Canadian Coast Guard, Ship Safety Branch.
Carlton, J.T., D.M. Reid, and H. van Leeuwen1995. The Role of Shipping in the Introduction of Nonindigenous Aquatic Organisms to the Coastal Waters of the United States (other than the Great Lakes) and an Analysis Of Control Options. Washington, D.C.: U.S. Coast Guard and U.S. Department of Transportation, National Sea Grant College Program/Connecticut Sea Grant. USCG Report Number CG-D-11-95. NTIS Report Number AD-A294809.
Christian, D.K., J.O. Bergh, and E.D. Thomas. 1995. Dechlorination equipment for shipboard pollution prevention. Sea Technology36(10):51–56.
Hayward Industrial Products, Inc. 1995. Catalogue of Hayward Industrial Strainer Products. Elizabeth, New Jersey: Hayward Industries Products, Inc.
Matthews, M.A., and R.F. McMahon. 1995. The Survival of Zebra Mussels (Dreissena polymorpha) and Asian Clams (Corbicula fluminea) under Extreme Hypoxia. Vicksburg, Mississippi: U.S. Army Waterways Experiment Station.
Rigby, G., and A. Taylor. 1993. Shipping Ballast Water—Heating as a Means of Destroying Potentially Harmful Marine Organisms. Technical Note BHPR/ENV/TN/93/005. Melbourne, Australia: BHP Research Laboratories.
Taylor, A.1995. Personal communication to the Committee on Ships' Ballast Operations, Washington, D.C., October 3, 1995.
Tchobanoglous, G., and O. Caliskaner. 1995. The importance of filtration in wastewater reclamation. Water/Engineering Management Magazine, October 1995.