8
Treatment
Substantial progress has been made in our knowledge of drug abuse treatment. Much of the treatment research was made possible by expansion of research funding by the National Institute on Drug Abuse (NIDA). Research has shown that drug abuse treatment is both effective and cost-effective in reducing not only drug consumption but also the associated health and social consequences. This chapter begins with a discussion of the need for treatment and then presents the many accomplishments in drug abuse treatment including the range of treatment options available (e.g., pharmacotherapies and psychosocial treatments), treatment effectiveness, the cost-effectiveness of treatment, and the development of tools and techniques for clinical assessment and diagnostic differentiation. The remainder of the chapter discusses opportunities for future research on medications development, treatment of HIV-infected drug abusers, matching patients to treatment options, treatment of patients with co-occurring psychiatric disorders and drug abuse, and treatment of drug abuse in special populations.
OVERVIEW OF DRUG ABUSE TREATMENT
Treatment is clearly indicated for individuals diagnosed with drug dependence, the most serious of the three levels of drug consumption—use, abuse, and dependence (see definitions in Chapter 1). Drug dependence occurs when a person has met three or more of the seven DSM-IV criteria items for dependence within the last year (see Appendix C for
DSM-IV criteria) (APA, 1994). As a consequence of compulsive drugseeking behavior and loss of control over consumption, drug dependence is usually a chronically relapsing disorder (i.e., one that may persist indefinitely and is prone to recur even after periods of remission). A diagnosis of drug abuse may also require treatment, but most clients in treatment have the more serious diagnosis of dependence.
The number of heavy drug users, using at least once a week, is difficult to determine. It has been estimated that in 1993, there were 2.1 million heavy cocaine users and 444,000-600,000 heavy heroin users (Rhodes et al., 1995). Although cocaine and heroin represent the major drugs of abuse for a large proportion of individuals who seek treatment, most patients abuse more than one drug. In addition, others seek help for abuse of marijuana, phencyclidine, benzodiazepines, other sedatives, or abuse of multiple drugs. It was estimated that in 1994, 3.6 million people in the U.S. had drug problems severe enough to need drug treatment services (ONDCP, 1996). The actual number of clients in treatment falls far short of this estimate. For example, the National Drug and Alcoholism Treatment Unit Survey (NDATUS) reported that almost 1.0 million people in 1993 were in private and public drug abuse treatment programs; approximately 20 percent of those in treatment were enrolled mainly for illicit drug abuse, 45 percent for alcohol, and 35 percent for combined alcohol and other drug dependencies (SAMHSA, 1995a). Although the figures are not comparable or definitive, the magnitude of the gap between the need for treatment and the use of treatment services is clear.
There are many reasons for the inadequate number of clients in treatment, including insufficient public funding for drug abuse treatment, cutbacks in treatment availability in the private sector, an unwillingness of many clients to seek treatment, and the deterrent effect of being placed on a waiting list for treatment (IOM, 1990b). Treatment should be available to all who request it, and long waiting lists are counterproductive (Goldstein and Kalant, 1990). That is particularly true given recent studies (cited later in this chapter and elsewhere) that demonstrate the effectiveness and cost-effectiveness of treatment.
ACCOMPLISHMENTS
Clearly, the development of varied treatment modalities and interventions discussed below are major accomplishments of drug abuse research. They include treatment options (e.g., pharmacotherapies and/or psychosocial), treatment effectiveness, cost-effectiveness, and the development of tools and techniques for clinical assessment and diagnostic differentiation.
Treatment Options
Treatment is provided in a variety of settings, and within each treatment setting a range of interventions may be available (e.g., pharmacotherapy, education, psychosocial treatment) (IOM, 1990a,b). Structured treatment programs are generally classified according to four major treatment modalities: methadone maintenance, outpatient drug-free programs, therapeutic communities, and chemical dependency programs. Methadone maintenance with counseling is the primary treatment option for opiate addiction (McLellan et al., 1993). Methadone maintenance treatment is provided in tightly regulated programs or clinics, which are almost universally located in outpatient facilities. Outpatient drug-free programs serve the largest share of patients in drug abuse treatment. The programs provide counseling as the predominant form of treatment, but there is great variation in the array and intensity of counseling services, the quality and training of treatment staff, and the composition of patients. Therapeutic communities are highly structured long-term residential programs lasting up to 18 months and tailored primarily to the hardcore user. Chemical dependency programs are short-term residential programs patterned after the 12-step model of treatment (for more detailed descriptions, see IOM, 1990b). Commonalities across all treatment settings include a combination of individual and group counseling, education, and/or pharmacotherapy. Additionally, treatment providers generally recommend that formal therapy be combined with participation in self-help groups such as Alcoholics Anonymous. Furthermore, patients are usually encouraged to continue self-help group participation after leaving formal treatment to reinforce abstinence and a healthy life-style, because relapse to dependence after periods of remission is common (Woody and Cacciola, 1994).
The following sections separate pharmacotherapeutic and psychosocial treatment options, it should be understood, however, that those approaches are combined in most clinical settings. The utility of that approach has been demonstrated, and it has been shown that methadone alone for the treatment of opiate dependence was not as effective as a combined regimen of methadone and psychosocial services as a more comprehensive approach to treatment (McLellan et al., 1993).
Pharmacotherapy
Pharmacotherapies have been developed or are being tested for the full spectrum of clinical needs: overdose, detoxification,1 dependence,
and relapse prevention. NIDA's Medications Development Division has made important contributions in the development of pharmacotherapies for drug addiction and has served as a catalyst in promoting drug development (IOM, 1995b). Medications development for the treatment of heroin and cocaine addictions is discussed more fully in a recent Institute of Medicine report (IOM, 1995b).
Two opiate agonist medications, methadone and LAAM (levo-alpha-acetylmethadol), have been approved for the treatment of opiate addiction. Agonists act by substituting at the opioid receptor site, thereby blocking the euphoria of subsequently administered opiates (via crosstolerance) and inhibiting the symptoms of acute and chronic abstinence. Methadone was approved for use in 1972, and there are currently an estimated 650 methadone maintenance programs throughout the United States (IOM, 1995a,b). The data supporting the efficacy of methadone maintenance have been reviewed extensively (e.g., Ball and Ross, 1991; Kreek, 1992). In 1993, LAAM was approved for use in treating opiate dependence; this medication has the advantage of requiring three doses per week rather than daily doses, thus freeing subjects from daily clinic attendance. Clinical guidelines for the use of each of these medications have recently been published by the Substance Abuse and Mental Health Services Administration (SAMHSA, 1993, 1995c).
Naltrexone, an orally effective and long-acting opiate antagonist, has been shown effective in preventing relapse to opiate dependence in highly motivated patients (e.g., probationers, parolees, health care providers) who are under strong external pressure to remain opiate free (Brahen et al., 1978). Naltrexone has also been found to reduce relapse to alcohol dependence (Volpicelli et al., 1992). A newer opiate antagonist, nalmafene, which is currently undergoing testing, appears to have positive effects similar to those of naltrexone (Mason et al., 1994). Opiate antagonist medications work by binding to the opioid receptor site, preventing receptor activation by the abused drug and thereby blocking the drug's euphorigenic and dependence-producing effects. This blockade represents competitive antagonism, and thus its clinical efficacy can be modified by the dose of the antagonist, the time elapsed since the antagonist was taken, and the dose of the abused drug.
Buprenorphine is a partial opiate agonist that produces less physiological dependence than methadone or LAAM and is currently in clinical trials (Bicket and Amass, 1995; Cowan and Lewis, 1995). It has been shown to be effective in maintenance therapy, in retaining patients in treatment, and in facilitating abstinence from illicit opiates Johnson et al., 1992; Kosten et al., 1993). Buprenorphine is currently being tested in combination with naloxone in a sublingual preparation to reduce its abuse liability. The eventual goal is to develop a pharmacotherapy that avoids
the strict scheduling controls that have been applied to methadone and LAAM. Fewer scheduling requirements would expand use to a wider range of settings (see IOM, 1995b).
Psychosocial Treatments
Psychosocial treatments include counseling, psychotherapy, and cognitive skills development. Counseling attempts to identify specific problems in the patient's life and to provide support, deliver concrete services, encourage abstinence, foster compliance with clinic rules, identify emergent problems, and refer the patient to more specialized services when needed (Woody et al., 1983). A series of well-designed studies has shown that drug counseling can produce substantial reductions in drug use and in the severity of problems that are associated with dependence. Those studies have been carried out in methadone programs (McLellan et al., 1982, 1988, 1993) and, more recently, in programs treating patients with cocaine and/or alcohol dependence (Rawson et al., 1993; Alterman et al., 1994, 1996; Shopshaw et al., 1995). In some instances, counseling may be provided by individuals who are recovering from drug dependencies and who have little formal education in health-related fields.
Unlike counseling, which focuses mainly on concrete, external factors, psychotherapy strives to identify and modify maladaptive interpersonal processes. There are many types of psychotherapy and they differ according to their theoretical basis and focus. For example, cognitivebehavioral psychotherapy aims to identify and change false beliefs and their associated behaviors (Beck et al., 1990). Supportive-expressive psychotherapy attempts to identify and change repetitive and problematic relationships and behaviors (Luborsky, 1984; Luborsky et al., 1995). Interpersonal psychotherapy tries to identify and change current maladaptive interpersonal problems. Motivational enhancement therapy may be more appropriate for an individual in the precontemplative stage of drug abuse (see Prochaska and DiClemente, 1983, 1986 below). Two prospective studies done in methadone programs using random assignment and a range of measures have shown that these psychotherapies can provide additional benefits to patients with moderate to high levels of psychiatric symptoms (Woody et al., 1984, 1995b).
An interesting area of research is contingency contracting, which applies behavioral methods of reinforcement to the treatment of drug abuse (Chapter 2). Contingency contracting involves the use of graduated rewards, which are given to patients when they meet specific treatment goals such as keeping appointments, seeking work, or providing drugfree urine samples. Rewards may include objects such as a lottery ticket or vouchers for the purchase of valued goods and services, methadone take-
home doses, or other socially appropriate rewards. Studies using this approach have found reductions in drug use among patients with heroin (Stitzer et al., 1992; Kidorf et al., 1994) or cocaine dependence (Higgins et al., 1993, 1994). The principles used in those studies have evolved from behavioral research, as summarized in Chapter 2. Most behavioral interventions have the advantage of being easily integrated within existing modalities.
Treatment Effectiveness
The effectiveness of treatment for drug addictions has been reviewed extensively (see Simpson and Sells, 1982, 1990; IOM, 1990b, 1995a,b; Prendergast et al., in press). Treatment gains are typically found in reduced intravenous and other drug use, reduced criminality, and enhanced health and productivity. The largest multisite studies, which are described below and cover multiple treatment modalities, provide strong evidence of long-term treatment effectiveness, usually based on comparisons between client behaviors before, during, and after treatment. The length of time in treatment consistently has been found to be an important determinant of both short- and long-term improvement. It is important to note, however, that most study results include the effects of patient self-selection in their preferred type of treatment modality. Although random assignment of patients to a treatment modality is preferred, it is difficult to achieve because of regulatory constraints, treatment facility capacities, study design, and ethical considerations.
Three comprehensive studies of drug abuse treatment effectiveness are discussed. The first study, the Drug Abuse Reporting Program (DARP), included more than 44,000 clients entering more than 50 treatment programs from 1969 to 1973 (Simpson and Sells, 1982, 1990). A subset of the cohort was studied 6 and 12 years after treatment. The second study is the Treatment Outcome Prospective Study (TOPS), which included almost 12,000 clients in 41 treatment programs (Hubbard et al., 1989). Clients were followed up to five years after treatment. The final study, which is still in progress, is the Drug Abuse Treatment Outcome Study (DATOS).
The first two studies, DARP and TOPS, both found evidence of treatment effectiveness for methadone maintenance, outpatient drug-free programs, and residential treatment (in therapeutic communities). Posttreatment outcomes were associated directly with the duration of treatment, with three months as the minimum time in treatment to observe an effect. Both DARP and TOPS found major reductions in the use of drugs and in criminal activity. TOPS also found modest improvements in productivity. In DARP, for example, the long-lasting nature of improvement was in
evidence 12 years after treatment, but most of the improvement was attained in the first 3 years after treatment. The results of these and other studies collectively indicate that 30-50 percent of patients are able to remain abstinent one year after the completion of treatment (McLellan et al., in press, a).
These gains are comparable to those seen in treatment for other chronic, relapsing disorders. Studies that compared compliance of patients in drug treatment with that of patients being treated for hypertension, adult onset diabetes, and asthma found that to remain symptomfree, each of these medical conditions requires patients to undergo major changes in life-style, often accompanied by medication (O'Brien and McLellan, 1996; McLellan et al., in press, a). Less than 30 percent of patients being treated for diabetes and hypertension were found to comply with dietary and other behavioral recommendations, and less than 30 percent of those with hypertension or asthma comply with their medication schedules.
DATOS, the final large-scale treatment outcome study begun in the early 1990s, enrolled 10,000 clients, one-third of whom were women, to determine the effectiveness of about 99 programs throughout the country. Four major modalities are under investigation: methadone maintenance, outpatient drug-free, long-term residential, and short-term inpatient programs (R. Hubbard, Research Triangle Institute, personal communication, 1995).
Treatment Cost-Benefit and Cost-Effectiveness
Drug abuse treatment is a judicious public investment and is less expensive than the alternatives (Figure 8.1). TOPS, cited above, performed a cost-benefit analysis2 by comparing the cost of treatment with the benefits (i.e., cost savings) of reduced crime and increased productivity during treatment and one year afterward. The ratio of benefits to costs for each treatment modality ranged from 4:1 to 1:1, depending on which of two complex scenarios was used to calculate societal benefits (Hubbard et al., 1989).
The economic benefits of reduced crime, enhanced productivity, and lower health care utilization were captured in a more recent study (Gerstein et al., 1994). This study, the first cost-benefit study to include the benefit of lower health care utilization, was undertaken by the State of California on 3,000 clients discharged from treatment programs in 1992.
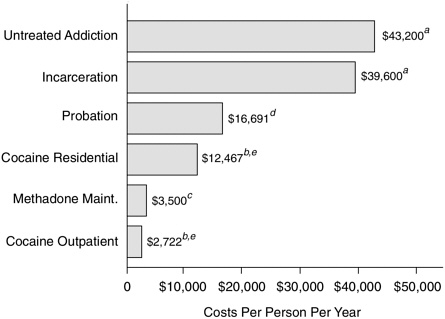
FIGURE 8.1 Treatment is less expensive than alternatives. NOTE: a1991 dollars; b1992 dollars; C1993 dollars; d1992 dollars, inflation adjusted from 1983 data; ethe average cost per admission is much lower than this figure because most patients are in treatment less than one year. SOURCES: Lewin-VHI, unpublished estimates; McLellan et al. (1994); Rydell and Everingham (1994); SAMHSA (1994a).
The study group consisted of a random sample of 150,000 clients in treatment programs throughout the state. By comparing the one-year period before treatment with the one-year period after, substantial benefits were realized relative to the cost of treatment. According to two different benefit measures, the ratio of benefits to costs was about 7:1 or 2:1 when all treatment modalities were combined. Health care costs for the sample were lowered after treatment by 23.5 percent; these savings alone offset about 55 percent of the cost of a treatment episode. Most of the economic benefits from both the TOPS and the California studies came in the form of reduced crime-related costs.
The cost-effectiveness3 of treatment has also been assessed in comparison with other drug control strategies (Everingham and Rydell, 1994; Rydell and Everingham, 1994). Investigators found treatment programs to be far more cost-effective than a range of drug control strategies in reducing cocaine use. The study analyzed the costs required by four different strategies-treatment, domestic enforcement, interdiction, and
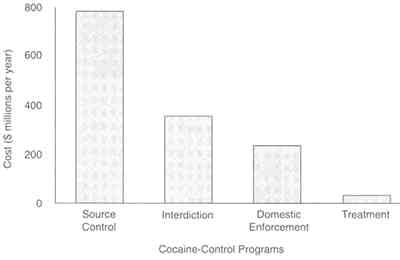
FIGURE 8.2 Effectiveness of cocaine control strategies. The RAND study compared treatment (a demand control strategy) and three supply control strategies: source country control, interdiction, and domestic enforcement. It calculated the cost required for each strategy to acheive a common measure of effectiveness-a reduction in cocaine consumption by 1 percent of current annual consumption. To meet this objective, researchers found that the additional cost of treatment would be $34 million, an amount 7.3 times less than that needed for the next most effective strategy, domestic enforcement, and 23 times less expensive than source country control. SOURCE: Rydell and Everingham (1994).
source country control-to achieve a 1 percent reduction in cocaine consumption. Treatment cost the least ($34 million) to achieve the objective, whereas other strategies cost between $250 million and $800 million (Figure 8.2). Thus, treatment was determined to be 7.3 times less costly than the least expensive alternative and more than 20 times less costly than the most expensive strategy, source country control.
It should be pointed out that all of these cost-benefit studies examined ''effectiveness" from a societal point of view and found treatment to be a wise public investment. However, studies did not address critical questions facing providers regarding the most cost-effective treatments. There are only a few studies comparing the relative cost-effectiveness of different treatments. That information gap is discussed in Chapter 9.
Clinical Assessment and Diagnostic Differentiation
Research advances in diagnosis have made it possible to conduct
detailed assessments of clients in treatment. Among these advances has been the development of instruments that reliably assess drug abuse and dependence and co-occurring psychiatric disorders according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; APA, 1994) and the International Classification of Diseases (ICD-10; WHO, 1992). Some of the most commonly used instruments are the Composite International Diagnostic Interview (CIDI); the Substance Abuse Module of the CIDI (the CIDI-SAM); the Diagnostic Interview Schedule (DIS); and the Structured Clinical Interview for DSM-IV (SCID). Work is currently under way to modify them to improve the distinctions between primary psychiatric disorders and drug-produced psychiatric syndromes in order to further improve treatment (D. Hasin, 1995 New York State Psychiatric Institute, personal communication).
Other instruments have been developed to assess the severity of patients' problems and their need for treatment across a wider range of areas. Among these, the most widely used is the Addiction Severity Index (ASI), which was developed in the early 1980s with research funds from both NIDA and the Department of Veterans Affairs (VA). ASI measures the degree of impairment and the need for treatment in each of seven areas commonly affected by drug abuse: drug and alcohol use, medical, family or social, employment, legal, and psychiatric (McLellan et al., 1980). The ASI has been found to be reliable and applicable within a wide range of settings, provided that appropriate training has been given to those who administer it. Unlike CIDI, DIS, and SCID, ASI does not make diagnoses but rather quantifies the degree to which impairment exists (and treatment is needed) in each of the seven areas. It is often used in clinical practice for evaluation and treatment planning.
One immediate positive effect of newer assessment techniques is the development of improved descriptions of patients. A very consistent finding, from a large number of studies using one or more of these assessment measures, is that the patient population is often engaged in polydrug use (i.e., use of a variety of illicit drugs and alcohol) and has other serious current or past problems in addition to drug abuse (e.g., psychiatric, employment, family/social problems) (Rounsaville et al., 1982; McLellan et al., 1994). These findings have been useful in developing treatment matching strategies (discussed below).
Advances in diagnosis have also led to comprehensive and accurate methods for assessing outcomes. The ASI has been particularly useful because it can measure degrees of improvement when administered on repeated occasions before, during, and after treatment. In treatment outcome studies, the ASI is usually supplemented with other measures such as urinalysis, breath alcohol tests, structured interviews for assessing psy-
chiatric disorders, measures of psychiatric symptoms, arrest and employment records, and interviews with family members.
A newer assessment instrument that derives from the ASI is the Treatment Services Review (TSR). It is administered by a trained technician at periodic intervals during an episode of treatment, and it measures the services actually delivered in each of the areas assessed by ASI (McLellan et al., 1992). By using the TSR along with the ASI, treatment outcome can be measured against services actually received. Early studies using ASI and TSR show that patients generally improve if they receive the services they need and usually do not improve if services are not tailored to their needs (McLellan et al., 1994). Thus, programs may also be assessed on how effectively they have addressed the needs of patients.
RESEARCH OPPORTUNITIES
The continued research challenge will be to develop more effective and cost-effective pharmacotherapeutic and psychosocial treatments that address the specific needs of individual patients and to refine the tools and techniques for clinical assessment and diagnostic differentiation. Questions remain regarding the different outcomes among programs using the same treatment modality; studies are needed to evaluate those program characteristics that produce the most efficacious results (e.g., the degree to which programs are willing to retain patients with persistent "dirty" urines or other signs of less than optimal progress, or the degree to which difficult patients are accepted into treatment). Furthermore, while long-term methadone maintenance has proven to be effective (Ball and Ross, 1991), questions remain regarding the length of time patients remain in treatment. Studies consistently have demonstrated that as long as patients are in methadone maintenance treatment there is a reduction in drug abuse. However, relapse to prior drug use occurs when treatment is terminated (IOM, 1990b; Ball and Ross, 1991). These findings have serious implications for HIV transmission as current data show that HIV infection is more likely among those who leave treatment than those who remain in treatment (e.g., Metzger and coworkers [1993] found that 4 percent of injection drug users who remained in treatment for the first 18 months became HIV infected, as compared with 22 percent of those not in treatment).
Additionally, research in medications development, HIV/AIDS and injection drug use, treating patients with co-occurring psychiatric disorders and drug abuse, and treating special populations of drug abusers is critical to fully meet the treatment needs of this population and to reduce the associated social and health consequences to society. These issues are discussed more fully below.
Medications Development
NIDA, through its Medications Development Division, is actively supporting the development of antiaddiction medications (see IOM, 1995b). The development of medications to treat drug addictions is tied closely to advances in basic research. The behavioral sciences (discussed in Chapter 2) have provided the foundation for objectively measuring drug-taking behaviors, for understanding many of the basic biobehavioral mechanisms underlying addiction, and for evaluating the abuse liability of new drugs. Neuroscientists have identified neural circuits in the brain that mediate the acute reinforcing effects of drugs, specific changes in the brain that are associated with withdrawal and sensitization, and specific neurotransmitter receptors and receptor subtypes for mediating reinforcement actions that may provide a molecular basis for long-term changes in the brain associated with relapse and vulnerability (see Chapter 3). The wealth of scientific information and understanding of opiate addiction, ranging from the clinical to the molecular level, that has been obtained over the last several decades has led to the development of several medications. Studies of methadone, LAAM, naltrexone, and buprenorphine have demonstrated the potential effectiveness of these medications as treatment approaches.
While basic research has shown that addictive drugs reinforce voluntary drug taking in humans and laboratory animals (see Chapters 2 and 3) and that the reinforcing effects of opiates and cocaine can be reduced by medications that alter their ability to activate the brain's reward system, there has not been a medication developed to treat cocaine addiction. Although recent work has provided information on the molecular basis of acute cocaine action and on the several neurotransmitter systems, including those mediated by dopamine, serotonin, and norepinephrine, that play a role in cocaine's effect on the brain reward system, there are gaps in knowledge about cocaine addiction.
Cocaine addiction differs importantly from opiate addiction (see Chapter 3), and the complexity of cocaine's mechanism of action, coupled with gaps in knowledge, has resulted in difficulty in developing an effective pharmacotherapy. Although an effective medication to treat cocaine addiction has not been developed, a wide range of medications continues to be tested that may affect the euphoria, craving, or withdrawal associated with cocaine dependence (Kleber, 1992). Some data suggest that tricyclic antidepressants may be useful for selected patients with intranasal use of cocaine or with co-occurring depression, but further studies are needed to confirm that observation (Nunes et al., 1995).
Despite advances in slow-release formulations of many medications, there are no slow-release, depot preparations or implantable pumps for
the treatment of drug abuse. Those methods are ideal for noncompliant and difficult patient populations. The one notable exception has been the work to develop depot naltrexone for alcohol and opiate dependence, but no clinically usable product has emerged thus far. Polydrug abuse poses another challenge to medications development because many opiate and cocaine abusers also abuse alcohol, tobacco, and other illicit drugs.
A few current pharmacotherapeutic agents are useful in the treatment of drug dependence. More are useful in the treatment of co-occurring psychiatric disorders or withdrawal. Treatment providers require additional pharmacotherapies to assist them in treating the range of behavioral and physiological manifestations of drug addiction. Thus, it is critical to continue support of basic research in the behavioral and neurosciences to provide the foundation for medications development.
HIV/AIDS
Drug users with HIV infection pose many challenges for both the drug abuse and the primary care treatment systems. As discussed in Chapter 7, it now appears that injection drug use is the leading risk factor for new HIV infection in the United States (Holmberg, 1996). This section does not attempt to review the large volume of HIV/AIDS treatment research (other Institute of Medicine [IOM] and National Research Council [NRC] reports on HIV/AIDS include IOM, 1994; NRC, 1989, 1990, 1993, 1995), rather, it highlights research opportunities unique to the treatment of HIV-infected drug abusers and focuses specifically on medical complications and health care delivery issues.
Medical Complications
Although injection drug users (IDUs) display the host of typical HIV related illnesses and complications, there are some unique differences from non-drug-using HIV-infected populations. The complications listed in Table 8.1 are common adverse consequences of HIV infection, drug use, or both that seem to appear more often among IDUs with HIV infection than among non-drug users (O'Connor et al., 1994). Thus, distinctive clinical characteristics of IDUs with HIV infection include increased rates of endocarditis, cellulitis or abscess, and other infections including bacterial pneumonia, sepsis, hepatitis, and tuberculosis (O'Connor et al., 1994). Kaposi's sarcoma, although common among male homosexuals with AIDS, is uncommon among drug users (Beral et al., 1990; Des Jarlais, 1991). The differences in the medical complications of HIV infection seen in the drug-abusing population are important both as predictors of progression to AIDS and as a source of HIV-related morbidity and mortality
TABLE 8.1 Spectrum of HIV-Related Disease in Injection Drug Users
|
Bacterial infections |
|
Pneumonia |
|
Endocarditis or sepsis |
|
Tuberculosis |
|
Sexually transmitted diseases (e.g., syphilis, human papillomavirus) |
|
Hepatitis |
|
Cancer |
|
SOURCE: O'Connor et al. (1994). |
before AIDS (Selwyn et al., 1992; Selwyn and O'Connor, 1992). Preliminary data suggest that certain clinical conditions (e.g., tuberculosis) may hasten disease progression in HIV-infected drug users (Farizo et al., 1992; Mientjes et al., 1992; IOM, 1994); and it has been hypothesized that the immunosuppressive or immunostimulatory effects of psychoactive drugs may influence HIV disease progression (Des Jarlais, 1991). Further research is needed to determine the possible impact of cofactors in HIV disease progression among drug abusers.
Understanding the natural history of HIV among IDUs and the increased risk of certain infections and complications is important for developing and providing effective treatment strategies for drug users. The medical complications of injection drug use may mimic, obscure, mask, or coexist with HIV-related infection and conditions, resulting in difficulties in diagnosis and more costly interventions. Research is needed to examine the effects of antiretroviral and other HIV medications on the occurrence of bacterial infections in drug users and to explore possible interactions of HIV medications with abused psychoactive drugs. Without proper knowledge of the etiological relationship between drug abuse and the course and progression of HIV disease, efforts at preventing transmission will continue to fall short of addressing the epidemic adequately.
Health Care Services
As research continues to address the best treatment modalities for HIV-infected drug abusers, studies are needed on the issues of access and utilization of treatment that are unique to that population. Research has established that drug abuse treatment is associated with a reduction in HIV transmission or related risk behaviors (see Chapter 7).
The link between the need to treat both AIDS and drug abuse has heightened awareness of drug abuse treatment-a significant step toward the integration of drug abuse treatment into mainstream medical
education and practice (National Commission on AIDS, 1991). However, of populations with HIV, drug users are the group least likely to have consistent contact with the health care system, especially primary care or preventive services. This may be due in part to fear of criminal sanctions (Chapter 10). HIV-infected drug abusers are more likely to rely on acute care through sporadic use of emergency rooms for medical complications from injecting drugs and acute HIV-related illnesses (O'Connor et al., 1992). Most of the therapies used to treat HIV and related illnesses (e.g., tuberculosis) require long-term treatment and compliance with treatment regimens. The effective treatment of this population of drug abusers is important for the patient and the general public, as in the case of tuberculosis.4 Therefore, access to and utilization of health care services for this population are critical.
When HIV-infected drug abusers enter drug abuse treatment programs, they typically are referred to medical clinics for diagnosis and treatment of HIV-related medical problems (Umbricht-Schneiter et al., 1994). Instead of referring them to other sites, however, drug abuse treatment programs may serve as ideal places for primary care (Selwyn et al., 1989a; Haverkos, 1991). Some of the primary care and preventive interventions for HIV-infected drug abusers include monitoring of immune function (e.g., CD4 count), administration of vaccines and antituberculosis therapy, skin testing for tuberculosis, and serologic testing and treatment for sexually transmitted diseases (O'Connor et al., 1994). With appropriate resources, the drug abuse treatment program could perform the following medical functions: assessment and diagnosis, referral to specialty care, coordination and monitoring, counseling, and primary care.
Several paradigms have been developed specifically targeted to the needs of drug users including on-site services in methadone programs and prisons, special hospital-based services, and outpatient programs (e.g., mobile vans linked to needle exchange programs) (Selwyn et al., 1989b; O'Connor et al., 1992). A randomized study of methadone patients found that those who received medical care on-site were far more likely to receive treatment than those whose care was referred off-site. It has been demonstrated that more than 90 percent of methadone patients received medical treatment if it was available on-site whereas only 35 percent received treatment when referred to a nearby location (UmbrichtSchneiter et al., 1994). The actual number of HIV-infected drug abusers
included in the study was small because the study was targeted to a variety of medical conditions.
There is a plethora of research opportunities to investigate the potential benefits of delivering primary care in drug abuse treatment programs. One major benefit is likely to be the frequent and long-term contact; daily methadone dosing could be combined with daily supervised treatment or prophylaxis for tuberculosis and HIV. Drug treatment programs also are likely to be familiar with some of the unique medical characteristics of HIV infection in drug abusers and with patients' psychosocial needs (Sorensen and Batki, 1992). There is also the possibility of lower costs-if patients' primary care needs are fulfilled, costly hospitalizations may be avoided. Studies demonstrating the cost-effectiveness of the delivery of primary care through drug treatment programs might encourage legislators and public health agencies to provide funding for the expansion of services, including outreach to the community to encourage drug abusers to seek treatment. It is also important for the clinician outside of the drug abuse treatment system to be familiar with the full spectrum of HIV related diseases and medical complications in this population.
Research is needed to study barriers and access to medical care among the drug-using population, given that this population has high levels of medical needs and historically poor engagement with the health care system. Further, research is needed to explore and evaluate alternative health care delivery systems for drug users who are not well served by mainstream systems of care.
Matching Patients to Treatments
Matching patients to treatments means tailoring treatments to patient needs in an effort to improve outcomes, in contrast to giving the same treatment to all patients with the same diagnosis. Matching can take place on many levels: drug-free versus pharmacotherapy, inpatient versus outpatient, treating or not treating psychiatric or medical disorders in the context of the drug-abuse program, using different types of counseling or psychotherapy, choosing various behavioral contingencies, matching the personality or background of the therapist with the patient, combining legal pressure with treatment in a therapeutic community versus a less intensive and briefer rehabilitation program, and many others. Patients will self-select a treatment modality if given the opportunity. It is critical to the implementation of patient matching strategies that costcutting not be the overriding criterion for matching a patient to a given treatment modality.
To conduct patient matching, three elements are needed: comprehensive assessment tools to identify patient problems and needs; placement
criteria to ensure placement in the appropriate level (setting), phase (detoxification, rehabilitation, etc.), and intensity of care; and a means of facilitating movement through a continuum of treatment services (SAMHSA, 1995b). Ideally, all three elements are incorporated into patient placement criteria.
There is, however, no national consensus about the most appropriate patient placement criteria, mainly because of the dearth of research, methodological limitations in its conduct, and the inconsistency of some studies (SAMHSA, 1995b). Few managed care companies publish their patient placement criteria. The American Society of Addiction Medicine (ASAM) is one of the first organizations to have developed placement criteria, and the most widely used. The few studies that have evaluated the validity of ASAM criteria have failed to demonstrate effects on patient outcome (McKay et al., 1992); another large-scale study is in progress (D. Gastfriend, Massachusetts General Hospital, personal communication, 1995). Assessing the clinical validity of existing patient placement criteria is a vital area for research attention. This task is complex because there are a range of treatment settings and other options for matching, and most phases of treatment can be delivered in more than one setting (Hayashida et al., 1989; Washton, 1989; Alterman et al., 1994; McKay et al., 1994; Mattick and Hall, 1996).
In addition to decisions about treatment phase, setting, and services for associated problems, providers are faced with other decisions about program characteristics (e.g., intensity, duration, structure, and philosophy) and therapy characteristics (e.g., therapeutic approach, group versus individual therapy, and therapist characteristics) (Hser, 1995). A particularly important element of matching is identifying how patient characteristics interact with different types of treatments and treatment programs. Patients who have psychiatric, medical, family, and/or legal problems are likely to need highly trained staff with special training in complex interventions.
Two studies of matching patients strictly with drug dependencies to treatment settings that addressed particular needs demonstrated better treatment outcomes than treatment settings that did not address specific needs (McLellan et al., 1983, in press, b). Both studies were prospective in nature, however, assignments were not random.
A newer paradigm for matching patients to treatment stems from the concept of "stages of change" (Prochaska and DiClemente, 1983, 1986). This concept was developed from clinical observations that patients with drug abuse disorders have varying levels of motivation to alter their behavior. Six stages have been described: precontemplation, contemplation, determination, action, maintenance, and relapse. Those stages are seen as making up a circle around which patients move in their attempts to stop
drug abuse. In studies of smokers, they found that most went through the six stages three to seven times before achieving sustained remission (Prochaska and DiClemente, 1983). This model implies that a therapist should develop different approaches according to the patient's stage. For example, motivational enhancement may be the only approach possible for someone who is a "precontemplator," whereas relapse prevention would be more appropriate for one who is in the action or maintenance phase. Research on how best to measure the stage of change and on developing interventions that address specific changes is currently under way and may provide a mechanism for improving treatment.
However, there has been limited independent research on the costs and benefits of patient matching, and managed care providers and others are matching patients to treatments without empirical evidence that the matching they perform yields the most cost-effective outcomes (Hser, 1995). Further, there is limited research on the optimal, most cost-effective configuration of services for different groups of patients. Yet some managed care contracts place severe limits on the addiction-focused services (e.g., not allowing more than three days for detoxification), whereas others "carve out" drug abuse-focused treatments from treatment services that are related to the psychiatric, medical, and other problems associated with abuse and dependence (see Chapter 9). In some cases, state guidelines require separate licensing for drug abuse and mental health services and separate administration of drug abuse treatment from psychiatric, medical, family, and other related services. The result of these practices can be less service delivery (McLellan et al., in press, a) and poorer outcomes (T. McLellan, University of Pennsylvania, personal communication, 1995) and may defeat the principle of matching patients to the most effective treatments.
Treatment of Patients with Co-Occurring Psychiatric Disorders and Drug Abuse
A related issue is treatment of patients with co-occurring psychiatric disorders and drug abuse.5 About 64 percent of those seeking treatment for drug abuse have one or more co-occurring psychiatric disorders. The prevalence of co-occurring disorders is much higher in this population than in the general population (Regier et al., 1990; Kessler et al., 1994). These patients are an important population because they are more expensive to treat, they usually require more complex and costly interventions, and they relapse more frequently (Garnick et al., in press).
Assessment and Diagnosis
A long-standing problem has been the difficulty in correctly identifying psychiatric disorders that occur in drug abusers. Drug-produced psychiatric disorders can result from acute drug effects, chronic intoxication, drug withdrawal, or effects that persist for months or years after detoxification has been completed (Woody et al., 1995a). Examples of psychopathology resulting from acute drug effects are alcohol-induced depression, stimulant-induced psychoses, or withdrawal-induced depression and anxiety. Examples of persistent drug effects are dementia associated with chronic alcohol, sedative, or inhalant dependence, or ''flashbacks" from hallucinogens such as lysergic acid diethylamide (LSD) (APA, 1994).
Research has clearly demonstrated that drug-produced psychiatric disorders can appear identical to primary, independent psychiatric disorders. Differentiation on the basis of presenting signs and symptoms alone is often impossible (APA, 1994). Correct identification is critical because it has important treatment implications. Primary psychiatric disorders tend to run a long-term course and require extended treatment. Drug-produced psychiatric disorders tend to follow the course of the drug abuse; usually resolve when the drug abuse remits; and often need only observation, supportive counseling, or short-term pharmacotherapy. Additional study of persistent drug-produced psychiatric disorders (e.g., potential long-term effects of dependence on stimulants such as cocaine) is important both in prevention and for the design and implementation of treatment programs.
Pharmacotherapy
Among persons with drug abuse or dependence, the rates of depression reach 26 percent, a higher rate than that found in the general population (Regier et al., 1990; Kessler et al., 1994). Progress has been made in the treatment of depression in clients with opioid or alcohol dependence. Some of this depression may be drug induced and resolve with sustained abstinence (Schuckit, 1994). Several well-controlled studies have shown that antidepressant pharmacotherapies, such as desipramine or imipramine, can be very helpful with carefully selected depressed patients who are drug dependent (Nunes et al., 1995; Mason et al., 1996). However, due to the possible contribution of the drug abuse to the development of depression, there has been some controversy about the conditions under which antidepressants and other pharmacotherapies should be used in this population.
Few studies, however, have been performed on the use of pharmacotherapies and psychotherapies for other co-occurring psychiatric disor-
ders. One controversial group of pharmacotherapies for anxiety disorders among patients with drug abuse has been benzodiazepines. They are generally considered safe and effective for a range of anxiety disorders and are among the most widely used pharmacotherapies (Tyrer, 1984). However, there is clear evidence that some benzodiazepines have a significant abuse liability (Sellers et al., 1993). Consequently, the use of benzodiazepines in patients with a history of drug abuse or dependence is often judged to be contraindicated. However, not all benzodiazepines are equally prone to abuse. Medications such as oxazepam, clorazepate, or others with longer duration to onset of peak effect not only might be useful in treating patients with co-occurring anxiety, but may have little risk of abuse when taken orally. Studies to determine the usefulness of benzodiazepines with slow onset to peak effects for patients with drug abuse and anxiety disorders would be helpful in providing data on this issue.
Additionally, since U.S. drug abusers often abuse multiple drugs, studies are needed to determine the interactions among drugs of abuse, medications used to treat drug dependence, and medications used to treat comorbid psychiatric and medical disorders. For example, a medication intended to prevent relapse to cocaine dependence should be tested for adverse consequences when used in combination with alcohol or opiates since these drugs are commonly used together. This is an area in which the behavioral models discussed in Chapter 2 could make significant contributions.
Further, pharmacokinetic studies of medications are typically carried out in healthy individuals, whereas many drug abusers have multiple health problems. Thus, the interactions among abused drugs, medications for drug abuse or dependence, and medications for psychiatric and medical problems may be altered in drug abusers. To optimize treatment strategies and to prevent adverse health outcomes, pharmacokinetic studies are needed to determine these possible interactions. Additionally, comparative studies of pharmacotherapies and psychotherapies for specific, well-defined depressive or anxiety disorders with this population (drug abuse and psychiatric disorders) could provide important data about the most appropriate therapies.
Special Populations
There is a dearth of research on drug-abusing women, prisoners, and adolescents. For reasons discussed below, it is extremely important for those populations to gain access to, enter, and remain in treatment. Most of the research opportunities center on treatment access, retention, and
effectiveness. There are large gaps in our knowledge of these areas, much of which is attributable to methodological difficulties.
Women
The health consequences of drug abuse can be more serious for women than for men, in spite of the fact that fewer women abuse drugs. Women can contract HIV through injection drug use or prostitution to purchase drugs. Maternal drug use can result in transmitting the disease to their fetus, premature delivery with serious complications, and impairments in parenting. Yet research has documented more barriers to treatment entry for female than for male alcoholics (Weisner and Schmidt, 1992; Schmidt and Weisner, 1995). Some of the obstacles for women are the cost of treatment, the possible loss of custody of their children, and the lack of child care (Beckman, 1994). Similar barriers to treatment may be operating for male drug abusers, but the extent of the problem is unknown.
A recent study of more than 12,000 clients in treatment found that women tended to drop out of treatment at higher rates than men (Mammo and Weinbaum, 1993). It is possible that women have difficulty making child care arrangements, fear retribution, or feel uncomfortable talking about their problems when being treated in programs that are predominantly male. Some programs, in an attempt to overcome these barriers, have experimented with women-only groups and with on-site facilities for child care.
Studies have also shown that women with drug abuse disorders typically have more psychiatric disorders (including depression and anxiety) than males (Blume, 1992). Many drug-dependent women have been sexually abused as children, suffer from posttraumatic stress disorder, and have significant problems forming healthy relationships with males (SAMHSA, 1994b). Abusive relationships with drug-abusing males are common, sometimes characterized by situations in which the male exerts control by providing drugs. These complex issues indicate that psychiatric assessment and treatment constitute a particularly important aspect of drug abuse treatment for women. Few studies have been done to examine the effect of integrating psychiatric treatment into the ongoing services of programs that treat drug-abusing women.
In spite of those problems, research shows that when women remain in treatment, they benefit just as much as men do (Sanchez-Craig et al., 1989; Ball and Ross, 1991; Finnegan, 1991). Methadone maintenance programs for pregnant women are the best studied, but outcomes in many other settings indicate that women benefit at least as much as men from the range of treatments that are currently available (Hubbard et al., 1989;
IOM, 1990a). There is a dearth of studies on programs that deliver services tailored to women's needs.
Problems continue to be greatest for pregnant women. In the past, many treatment programs automatically excluded pregnant women because of liability concerns or concerns about lack of expertise with medical complications of pregnancy. Some areas of the country have enacted laws that classify drug abuse during pregnancy as a form of child abuse, which would lead to the placement of children in foster care. These laws do not seem to reduce drug abuse, but they may have the negative effect of discouraging pregnant drug users from seeking treatment (see Chapter 10). Exclusion of pregnant women from treatment programs is beginning to diminish, however. A recent survey of 294 drug treatment programs in five cities revealed that the majority of programs (70-83 percent) accepted pregnant women. Fewer programs, however, accepted women who were Medicaid recipients, and even fewer programs provided child care (Breitbart et al., 1994).
When pregnant women succeed in gaining access to treatment, they face yet another hurdle—the lack of pharmacotherapies specifically approved for use in pregnancy (IOM, 1995b). This problem is true for medications of all kinds, not just for those used in drug abuse treatment. Pharmaceutical firms rarely, if ever, seek Food and Drug Administration (FDA) approval for use of their products in pregnancy, mostly because of liability concerns. When pregnant heroin drug users, for example, need treatment to reduce drug use and the risk of HIV transmission to themselves and their unborn, their doctors are strongly discouraged by federal treatment regulations and by the manufacturer from prescribing LAAM. According to federal treatment regulations, pregnant women are offered methadone, which is not formally approved by the FDA for use in pregnancy. FDA has drafted guidelines recommending that future studies of antiaddiction medications include women, but the guidelines do not provide advice for a mechanism dealing with increased risk for product liability (Woody et al., 1996).
Prisoners
Treatment programs have recently become more prominent in some correctional settings, with therapeutic communities among the most common modalities. The therapeutic community provides a total treatment environment isolated from the rest of the prison population—separated from the drugs, the violence, and the norms and values that mitigate against treatment, habilitation, and rehabilitation. Treatment programs based in correctional settings sometimes include aftercare in the community after release from prison. Although therapeutic communities appear
to be the most visible drug abuse treatment programs in correctional settings, there are numerous other modalities, many of which are grounded in individual and group counseling and 12-step approaches. However, there is limited information about these programs in the drug abuse literature. There are virtually no methadone maintenance programs offered in correctional settings, which is most likely a result of policies to eliminate the availability of a medication that is itself a controlled drug.
Most treatment of drug-involved offenders takes place in community-based settings as a condition of parole or probation or in lieu of prison. Treatment in the community is made possible through programs that link the criminal justice system with specialty drug abuse treatment programs. The most prominent example is Treatment Alternatives to Street Crime (TASC), whose programs are found in more than 25 states (Inciardi and McBride, 1991). Evaluation data indicate that TASC-referred clients remain in treatment longer than non-TASC clients (court referrals to treatment without TASC services). Other programs linking treatment to parole and probation have experienced favorable results (Chavaria, 1992; Van Stelle et al., 1994).
Although there are extensive studies of drug-involved offenders who are treated effectively in community settings, there is a dearth of information about drug treatment programs in prisons or about the best means of treating drug abusers in these settings. What is known is that for the few prisoners who succeed in gaining access to a limited number of prisonbased therapeutic communities, treatment is effective. Many in the drug treatment community believe that prisoners have the most profound treatment system needs in light of the pervasive violence and widespread availability of illicit drugs within the prison system. The co-occurrence of addictive and severe psychiatric disorders is also highest in the prison population (Regier et al., 1990).
Adolescents
Adolescents are also vulnerable to the consequences of drug abuse, including health effects, accidents and injuries, involvement with violence resulting from illegal activities, and the transmission of HIV (Czechowicz, 1991). Adolescent drug abusers differ from adult drug abusers in several ways that are significant for treatment. The majority of adolescent drug abusers have a shorter history of drug abuse; have less severe symptoms of tolerance, craving, and withdrawal; and usually do not have the long-term physical effects of drug abuse (Kaminer, 1994). However, they are at the greatest risk for developing lifelong patterns of drug abuse (Dusenbury et al., 1992).
Adolescents accounted for about 11.1 percent of all patients in spe-
cialty drug abuse treatment programs in 1993 (SAMHSA, 1995a), down from 16.9 percent in 1987, although their proportion appears to be rising again (SAMHSA, 1995a). There is increasing recognition of the need to implement and evaluate treatment programs designed specifically for adolescents (IOM, 1990b). A new study of treatment effectiveness for 3,000 adolescents enrolled in standard treatment programs is under way, with findings to be reported in 1997 (R. Hubbard, Research Triangle Institute, personal communication, 1995). There are additional opportunities to design and evaluate the effectiveness of special programs with services tailored to adolescents. Results from such studies will enable the development of targeted treatment and prevention programs.
RECOMMENDATION
Substantial progress has been made during the past 20 years in our knowledge of drug abuse treatment. Research has shown that drug abuse treatment is both effective and cost-effective in reducing not only drug consumption but also the associated health and social consequences. Continued research on drug abuse treatment is needed in many priority areas.
The committee recommends that the appropriate federal and private agencies continue to support research to improve and evaluate the effectiveness of drug abuse treatment. This includes studies on optimal strategies for matching patients to the most appropriate treatment modalities; development of medications for the treatment of drug abuse and dependence; the efficacy of pharmacotherapies and psychosocial therapies to treat individuals with co-occurring psychiatric disorders and drug abuse; the natural history of HIV infection among drug users and effective models of health care delivery for HIV-infected drug abusers; and the efficacy of treatment programs designed toward addressing the needs of special populations (i.e., women, adolescents, and prisoners).
REFERENCES
Alterman Al, O'Brien CP, McLellan AT, August DS, Snider EC, Droba M, Cornish JW, Hall CP, Raphaelson AH, Schrade FX. 1994. Effectiveness and costs of inpatient versus day hospital cocaine rehabilitation. Journal of Nervous and Mental Disease 182:157-163.
Alterman Al, Snider EC, Cacciola JS, May DJ, Parikh G, Maany I, Rosenbaum PR. 1996. Treatments for cocaine dependence. Journal of Nervous and Mental Disease 184(1):54-56.
APA (American Psychiatric Association). 1994. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: APA.
Ball JC, Ross A. 1991. The Effectiveness of Methadone Maintenance Treatmnent. New York: Springer-Verlag.
Bayer R, Wilkinson D. 1995. Directly observed therapy for tuberculosis: History of an idea. Lancet 345(8964):1545-1548.
Beck AT, Wright FD, Newman CF. 1990. Cognitive Therapy of Cocaine Abuse. Philadelphia: Center for Cognitive Therapy.
Beckman LJ. 1994. Treatment needs of women with alcohol problems. Alcohol Health and Research World 18(3):206-211.
Beral V, Peterman TA, Berkelman RL, Jaffe HW. 1990. Kaposi's sarcoma among persons with AIDS: A sexually transmitted infection? Lancet 335(8682):123-128.
Bicket WK, Amass L. 1995. Buprenorphine treatment of opioid dependence: A review. Experimental and Clinical Psychopharmacology 3:477-489.
Blume SB. 1992. Alcohol and other drug problems in women. In: Lowinson JH, Ruiz P, Millman RB, eds. Substance Abuse: A Comprehensive Textbook. Baltimore: Williams & Wilkins. Pp. 794-807.
Brahen LS, Capone T, Bloom S, et al. 1978. An alternative to methadone for probationer addicts: Narcotic antagonist treatment. Contemporary Drug Issues 13:117-132.
Breitbart V, Chavkin W, Wise PH. 1994. The accessibility of drug treatment for pregnant women: A survey of programs in five cities. American Journal of Public Health 84(10):1658-1661.
Center of Alcohol Studies, Rutgers University. 1993. Socioeconomic Evaluations of Addictions Treatment. Prepared for the President's Commission on Model State Drug Laws by the Center of Alcohol Studies, Rutgers University, Piscataway, NJ.
Chaulk CP, Moore-Rice K, Rizzo R, Chaisson RE. 1995. Eleven years of community-based directly observed therapy for tuberculosis. Journal of the American Medical Association 274(12):945-951.
Chavaria FR. 1992. Successful drug treatment in a criminal justice setting: A case study. Federal Probation 56:48-52.
Cowan A, Lewis J, eds. 1995. Buprenorphine: Combatting Drug Abuse with a Unique Opioid. New York: John Wiley & Sons.
Czechowicz D. 1991. Adolescent alcohol and drug addiction and its consequences: An overview. In: Miller NS, ed. Comprehensive Handbook of Drug and Alcohol Addiction. New York: Marcel Dekker. Pp. 205-210.
Des Jarlais DC. 1991. Potential cofactors in the outcomes of HIV infection in intravenous drug users. NIDA Research Monograph 109:115-124.
Dusenbury L, Khuri E, Millman RB. 1992. Adolescent substance abuse: A sociodevelopmental perspective. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, eds. Substance Abuse: A Comprehensive Textbook. Baltimore: Williams & Wilkins. Pp. 832-842.
Everingham S, Rydell C. 1994. Modeling the Demand for Cocaine. MR-332-ONDCP/A/DPRC. Santa Monica, CA: RAND Drug Policy Research Center.
Farizo K, Buehler J, Chamberland M, Whyte BM, Froelicher ES, Hopkins SG, Reed CM, Mokotoff ED, Cohn DL, Troxler S, et al. 1992. Spectrum of disease in persons with human immunodeficiency virus infection in the United States. Journal of the American Medical Association 267:1798-1805.
Finnegan LP. 1991. Treatment issues for opioid-dependent women during the perinatal period. Journal of Psychoactive Drugs 23:191-199.
Garnick DW, Hendricks AM, Drainoni ML, Horgan CM, Comstock C. In press. Private sector coverage of people with dual diagnoses. Journal of Mental Health Administration.
Gerstein DR, Johnson RA, Harwood HJ, Fountain D, Suter N, Malloy K. 1994. Evaluating Recovery Services: The California Drug and Alcohol Treatment Assessment (CALDATA). Sacramento, CA: California Department of Alcohol and Drug Programs.
Goldstein A, Kalant H. 1990. Drug policy: Striking the right balance. Science 249:1513-1521.
Haverkos H. 1991. Infectious diseases and drug abuse. Journal of Substance Abuse Treatment 8:269-275.
Hayashida M, Alterman AI, O'Brien CP, McLellan AT. 1989. Comparative effectiveness of inpatient and outpatient detoxification of patients with mild-to-moderate alcohol withdrawal syndrome. New England Journal of Medicine 320:358-365.
Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F, Badger G. 1993. Achieving cocaine abstinence with a behavioral approach. American Journal of Psychiatry 150:763-769.
Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. 1994. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry 51:568-576.
Holmberg SD. 1996. The estimated prevalence and incidence of HIV in 96 large U.S. metropolitan areas. American Journal of Public Health 86(5):642-654.
Hser Y. 1995. A referral system that matches drug users to treatment programs: Existing research and relevant Issues. Journal of Drug Issues 25(1):209-224.
Hubbard RL, Marsden ME, Rachal JV, Harwood HJ, Cavanaugh ER, Ginzburg HM. 1989. Drug Abuse Treatment: A National Study of Effectiveness. Chapel Hill, NC: University of North Carolina Press.
Inciardi JA, McBride DC. 1991. Treatment Alternatives to Street Crime (TASC): History, Experiences, and Issues. Rockville, MD: NIDA.
IOM (Institute of Medicine). 1990a. Broadening the Base for Alcohol Problems. Washington, DC: National Academy Press.
IOM (Institute of Medicine). 1990b. Treating Drug Problems. Washington, DC: National Academy Press.
IOM (Institute of Medicine) 1994. AIDS and Behavior: An Integrated Approach. Washington, DC: National Academy Press.
IOM (Institute of Medicine). 1995a. Federal Regulation of Methadone Treatment. Washington, DC: National Academy Press.
IOM (Institute of Medicine). 1995b. The Development of Medications for the Treatment of Opiate and Cocaine Addictions. Washington, DC: National Academy Press.
Johnson RE, Jaffe JH, Fudala PJ. 1992. A controlled trial of buprenorphine treatment for opioid dependence. Journal of the American Medical Association 267(20):2750-2755.
Kaminer Y. 1994. Adolescent substance abuse. In: Galanter M, Kleber HD, eds. The American Psychiatric Press Textbook of Substance Abuse Treatment. Washington, DC: American Psychiatric Press.
Kessler R, McGonagle K, Zhao S, Nelson CB, Hughes M, Eshleman S , Wittchen HU, Kendler KS. 1994. Lifetime and 12-month prevalence of DSM-IIIR psychiatric disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry 51:8-19.
Kidorf M, Stitzer ML, Brooner RK, Goldberg J. 1994. Contingent methadone take-home doses reinforce adjunct therapy attendance of methadone maintenance patients. Drug and Alcohol Dependence 36:221-226.
Kleber HD. 1992. Treatment of cocaine abuse: Pharmacotherapy. CIBA Foundation Symposium 166:195-206.
Kosten TR, Schottenfeld R, Ziedonis D, Falcioni J. 1993. Buprenorphine versus methadone maintenance for opioid dependence. Journal of Nervous and Mental Disease 181(6):358-364.
Kreek MJ. 1992. Rationale for maintenance pharmacotherapy of opiate dependence. In: O'Brien CP, Jaffe JH, eds. Addictive States. New York: Raven Press. Pp. 205-230.
Luborsky L. 1984. Principles of Psychoanalytic Psychotherapy: A Manual for Supportive-Expressive Treatment. New York: Basic Books.
Luborsky L, Woody GE, Hole A, Velleco A. 1995. Supportive-expressive dynamic psychotherapy for treatment of opiate drug dependence. In: Barber JP, Crits-Christoph P, eds. Dynamic Therapies for Psychiatric Disorders (Axis I). New York: Basic Books. Pp. 131-160.
Mammo A, Weinbaum DF. 1993. Some factors that influence dropping out from outpatient treatment facilities. Journal of Studies on Alcohol 54:92-101.
Mason BJ, Ritvo EC, Morgan RO, Salvato FR, Goldberg G, Welch B, Mantero-Atienza E. 1994. A double-blind, placebo-controlled pilot study to evaluate the efficacy and safety of oral nalmefene HCI for alcohol dependence. Alcoholism: Clinical and Experimental Research 18:1162-1167.
Mason BJ, Kocsis JH, Ritvo EC, Cutler RB. 1996. A double-blind, placebo-controlled trial of desipramine for primary alcohol dependence stratified on the presence or absence of major depression. Journal of the American Medical Association 275(10):761-767.
Mattick RP, Hall W. 1996. Are detoxification programmes effective? Lancet 347:97-100.
McKay JR, McLellan AT, Alterman Al. 1992. An evaluation of the Cleveland Criteria for inpatient substance abuse treatment. American Journal of Psychiatry 149:1212-1218.
McKay JR, Alterman Al, McLellan AT, Snider EC. 1994. Treatment goals, continuity of care, and outcome in a day hospital substance abuse rehabilitation program. American Journal of Psychiatry 151:254-259.
McLellan AT, Luborsky L, O'Brien CP, Woody GE. 1980. An improved evaluation instrument for substance abuse patients: The Addiction Severity Index. Journal of Nervous and Mental Disease 168:26-33.
McLellan AT, Luborsky L, O'Brien CP, Woody GE, Druley KA. 1982. Is substance abuse treatment effective? Journal of the American Medical Association 247:1423-1428.
McLellan AT, Woody G, Luborsky L, O'Brien C, Druley K. 1983. Increased effectiveness of substance abuse treatment: A prospective study of patient treatment "matching." Journal of Nervous and Mental Disease 171:597-605.
McLellan AT, Woody GE, Luborsky L, Goehl L. 1988. Is the counsellor an "active ingredient" in methadone treatment? An examination of treatment success among four counselors. Journal of Nervous and Mental Disease 176:423-430.
McLellan AT, Alterman Al, Woody GE, Metzger D. 1992. A quantitative measure of substance abuse treatments: The Treatment Services Review. Journal of Nervous and Mental Disease 180:101-110.
McLellan AT, Arndt IO, Metzger DS, Woody GE, O'Brien CP. 1993. The effects of psychosocial services in substance abuse treatment. Journal of the American Medical Association 269(15):1953-1959.
McLellan AT, Alterman Al, Metzger DS, Grissom GR, Woody GE, Luborsky L, O'Brien CP. 1994. Similarity of outcome predictors across opiate, cocaine, and alcohol treatments: Role of treatment services. Journal of Consulting and Clinical Psychology 62:1141-1158.
McLellan AT, Metzger DS, Alterman Al, Woody GE, Durell J, O'Brien CP. In press, a. Is addiction treatment "worth it"? Public health expectations, policy-based comparisons. Milbank Quarterly.
McLellan AT, Grissom GR, Zanis D, Randall M, Brill P, O'Brien CP. In press, b. Improved outcomes from problem-service "matching" in substance abuse patients: A controlled study in a four-program, EAP network. Archives of General Psychiatry.
Metzger DS, Woody GE, McLellan T, O'Brien CP, Druley P, Navaline H, DePhilippis D, Stolley P, Abrutyn E. 1993. Human immunodeficiency virus seroconversion among in and out-of-treatment intravenous drug users: An 18-month prospective follow-up. Journal of Acquired Immune Deficiency Syndromes 6:1049-1056.
Mientjes G, van Ameijden E, van den Hoeck A, Coutinho RA. 1992. Increasing morbidity without rise in non-AIDS mortality among HIV-infected intravenous drug users in Amsterdam. AIDS 6:207-212.
National Commission on AIDS. 1991. The Twin Epidemics of Substance Use and HIV. Washington, DC: U.S. Government Printing Office.
NRC (National Research Council). 1989. AIDS, Sexual Behavior and Intravenous Drug Abuse. Washington, DC: National Academy Press.
NRC (National Research Council). 1990. AIDS: The Second Decade. Washington, DC: National Academy Press.
NRC (National Research Council). 1993. The Social Impact of AIDS in the United States. Washington, DC: National Academy Press.
NRC (National Research Council). 1995. Preventing HIV Transmission: The Role of Sterile Needles and Bleach. Washington, DC: National Academy Press.
Nunes EV, McGrath PJ, Quitkin FM, Ocepek-Welikson K, Stewart JW, Koenig T, Wager S, Klein DF. 1995. Imipramine treatment of cocaine abuse: Possible boundaries of efficacy. Drug and Alcohol Dependence 39:185-195.
O'Brien CP, McLellan AT. 1996. Myths about the treatment of addiction. Lancet 347:237-240.
O'Connor P, Molde S, Henry S, Shockcor WT, Schottenfeld RS. 1992. Human immunodeficiency virus infection in intravenous drug users: A model for primary care. American Journal of Medicine 93:382-386.
O'Connor PG, Selwyn PA, Schottenfeld RS. 1994. Medical care for injection-drug users with human immunodeficiency virus infection. New England Journal of Medicine 331(7):450-459.
ONDCP (Office of National Drug Control Policy). 1996. National Drug Control Strategy, 1996. Washington, DC: ONDCP.
Prendergast ML, Anglin MD, Maugh TH, Hser Y. In press. The Effectiveness of Treatment for Drug Abuse. Draft manuscript prepared April 7, 1994 for NIDA Treatment Services Research Branch. Los Angeles: UCLA Drug Abuse Research Center.
Prochaska JO, DiClemente CC. 1983. Stages and processes of self-change: Toward an integrative model of change. Journal of Consulting and Clinical Psychology 51:390-395.
Prochaska JO, DiClemente CC. 1986. Toward a comprehensive model of change. In: Miller WR, Heather N, eds. Treating Addictive Behaviors: Process of Changes. New York: Plenum Press.
Rawson RA, Obert JL, McCann MJ, Marinelli-Casey P. 1993. Relapse prevention strategies in outpatient substance abuse treatment. Psychology of Addictive Behaviors 7:85-95.
Regier D, Farmer M, Rae D, Locke BZ, Keith SJ, Judd LL, Goodwin FK. 1990. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the Epidemiologic Catchment Area (ECA) Study. Journal of the American Medical Association 264:2511-2518.
Rhodes W, Scheiman P, Pittayathikhun T, Collins L, Tsarfaty V. 1995. What America's Users Spend on Illegal Drugs, 1988-1993. Prepared for the Office of National Drug Control Policy, Washington, DC.
Rounsaville BJ, Weissman MM, Kleber HD. 1982. Heterogeneity of psychiatric diagnoses in treated opiate addicts. Archives of General Psychiatry 39:161-166.
Rydell C, Everingham S. 1994. Controlling Cocaine: Supply Versus Demand Programs. MR3331-ONDCP/A/DPRC. Santa Monica, CA: RAND Drug Policy Research Center.
SAMHSA (Substance Abuse and Mental Health Services Administration). 1993. State Methadone Treatment Guidelines. Treatment Improvement Protocol (TIP) Series 1. Rockville, MD: SAMHSA.
SAMHSA (Substance Abuse and Mental Health Services Administration). 1994a. Client Data System FY 1992: Opiate and Cocaine/Crack Admissions to Treatment. Prepared under contract for the Office of Applied Studies.
SAMHSA (Substance Abuse and Mental Health Services Administration). 1994b. Practical Approaches in the Treatment of Women Who Abuse Alcohol and Other Drugs. DHHS Publication No. (SMA)94-3006. Rockville, MD: SAMHSA.
SAMHSA (Substance Abuse and Mental Health Services Administration). 1995a. Overview of the FY94 National Drug and Alcoholism Treatment Unit Survey (NDATUS): Data from 1993 and 1980-1993. Advance Report Number 9A, August 1995. Rockville, MD: SAMHSA.
SAMHSA (Substance Abuse and Mental Health Services Administration). 1995b. The Role and Current Status of Patient Placement Criteria ill the Treatment of Substance Use Disorders. Treatment Improvement Protocol (TIP) Series 13. DHHS Publication No. (SMA) 95-3021. Rockville, MD: SAMHSA.
SAMHSA (Substance Abuse and Mental Health Services Administration). 1995c. LAAM and the Treatment of Opiate Addiction. Treatment Improvement Protocol (TIP) Series 22. Rockville, MD: SAMHSA.
Sanchez-Craig M, Leigh G, Spivak K, Lei H. 1989. Superior outcome of females over males after brief treatment for the reduction of heavy drinking. British Journal of Addiction 84:395-404.
Schmidt L, Weisner C. 1995. The emergence of problem-drinking women as a special population in need of treatment. Recent Developments in Alcoholism 12:309-334.
Schuckit MA. 1994. The relationship between alcohol problems, substance abuse, and psychiatric syndromes. In: Widiger TA, Frances AJ, Pincus HA, eds. DSM-IV Sourcebook, Vol. 1. Washington, DC: American Psychiatric Association Press. Pp. 45-66.
Sellers EM, Ciraulo DA, DuPont Rl, Griffiths RR, Kosten TR, Romach MK, Woody GE. 1993. Alprazolam and benzodiazepine dependence. Journal of Clinical Psychiatry 54(10 Suppl.):64-77.
Selwyn PA, O'Connor PG. 1992. Diagnosis and treatment of substance users with HIV infection. Primary Care 19(1):119-156.
Selwyn PA, Feingold AR, lezza A, Satyadeo M, Colley J, Torres R , Shaw JF. 1989a. Primary care for patients with human immunodeficiency virus (HIV) infection in a methadone maintenance treatment program. Annals of Internal Medicine 111:761-763.
Selwyn PA, Hartel D, Wasserman W, Drucker E. 1989b. Impact of the AIDS epidemic on morbidity and mortality among intravenous drug users in a New York City methadone maintenance program. American Journal of Public Health 79(10):1358-1362.
Selwyn PA, Alcabes P, Hartel D, Buono D, Schoenbaum EE, Klein RS, Davenny K, Friedland GH. 1992. Clinical manifestations and predictors of disease progression in drug users with human immunodeficiency virus infection. New England Journal of Medicine 327:1697-1703.
Shopshaw S, Frosch D, Rawson R, Ling W. 1995. Reductions in Risky Behavior Associated with Treatment for Cocaine Dependence. Presented at the Third Science Symposium on HIV Prevention: Current Status and Future Directions. Northern Arizona University, Flagstaff, AZ.
Simpson DD, Sells SB. 1982. Effectiveness of treatment for drug abuse: An overview of the DARP Research Program. Advances in Alcohol and Substance Abuse 2:7-29.
Simpson DD, Sells SB, eds. 1990. Opioid Addiction and Treatment: A 12-Year Follow-Up. Malabar, FL: Krieger Publishing.
Sorensen JL, Batki SL. 1992. Management of the psychosocial sequelae of HIV infection among drug abusers. In: Lowinson JH, Ruiz P, Millman RB, eds. Substance Abuse: A Comprehensive Textbook. Baltimore: Williams & Wilkins. Pp. 788-793.
Stitzer ML, Iguchi MY, Felch LJ. 1992. Contingent take-home incentive: Effects on drug use of methadone patients. Journal of Consulting and Clinical Psychology 60:927-934.
Tyrer P. 1984. Benzodiazepines on trial. British Medical Journal 288:1101-1102.
Umbricht-Schneiter A, Ginn DH, Pabst KM, Bigelow GE. 1994. Providing medical care to methadone clinic patients: Referral vs. on-site care. American Journal of Public Health 84(2):207-210.
Van Stelle KR, Mauser E, Moberg DP. 1994. Recidivism to the criminal justice system of substance-abusing offenders diverted into treatment. Crime and Delinquency 40:175-196.
Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. 1992. Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry 46:876-880.
Washton AM. 1989. Cocaine Addiction: Treatment, Recovery and Relapse Prevention. New York: W. W. Norton.
Weisner C, Schmidt L. 1992. Gender disparities in treatment for alcohol problems. Journal of the American Medical Association 268(14):1872-1876.
WHO (World Health Organization). 1992. International Statistical Classification of Diseases and Related Health Problems. 10th Revision. Geneva: WHO.
Woody G, Cacciola J. 1994. Review of remission criteria. In: Widiger T, Frances A, Pincus H, First M, Ross R, Davis W, eds. DSM-IV Sourcebook, Vol. 1. Washington, DC: American Psychiatric Association Press. Pp. 81-92.
Woody GE, Luborsky L, McLellan AT, O'Brien CP, Beck AT, Blaine J, Herman I, Hole A. 1983. Psychotherapy for opiate addicts: Does it help? Archives of General Psychiatry 40:639-645.
Woody GE, McLellan AT, Luborsky L, O'Brien CP, Blaine J, Fox S, Herman I, Beck AT. 1984. Psychiatric severity as a predictor of benefits from psychotherapy: The Penn-VA study. American Journal of Psychiatry 141:1172-1177.
Woody GE, McLellan AT, Bedrick J. 1995a. Dual diagnosis. Annual Review of Psychiatry 14:83-104.
Woody GE, McLellan AT, Luborsky L, O'Brien CP. 1995b. Psychotherapy in community methadone programs: A validation study. American Journal of Psychiatry 159:1302-1308.
Woody GE, McNicholas LF, Vocci F, eds. 1996. Guidelines for Research Involving Medications for the Treatment of Drug Addiction. Accepted by the Pilot Drug Evaluation Program and under review by the Food and Drug Administration.






























