2
AIR QUALITY, FUEL ECONOMY, AND ENGINE PERFORMANCE
A draft interagency report has been prepared that assesses the impact of winter use of motor-vehicle fuels oxygenated with compounds, such as methyl tertiary-butyl ether (MTBE), on air quality, fuel economy, engine performance, water quality, and potential health effects. This chapter is a critique of the section on air-quality benefits and the section on fuel-economy and engine-performance issues of the interagency report. It concentrates primarily on major deficiencies and omissions in those sections of the interagency report.
The committee's evaluation should not be construed as a criticism of the interagency's discussion of air quality, fuel economy, and engine performance. More precisely, this evaluation is a critical review of the quality and quantity of data available from which to draw conclusions regarding the effectiveness of oxygenated fuels in reducing ambient concentrations of carbon monoxide during winter.
Under the Clean Air Act Amendments of 1990, the U.S. Environmental Protection Agency (EPA) mandates the use of oxygenated fuels in winter in areas exceeding the National Ambient Air
Quality Standard (NAAQS) for carbon monoxide (CO). The interagency report was prompted by health effects claimed to result from use of oxygenated fuel. Most of these claims were concentrated in the colder areas, which exceeded the CO NAAQS.
Table 3 in the portion of the interagency report titled "The Potential Health Effects of Oxygenates added to Gasoline - A Review of the Current Literature" lists the areas participating in the oxygenated-fuels program in 1994-1995; these areas include cities with a broad range of average winter temperatures, e.g., Minneapolis and San Diego. For reasons explained later, there is an inverse relationship between ambient temperature and the amount of tailpipe CO emissions. Consequently, it is important that the term "winter temperature" be defined. The interagency report does not do so. A map, or similar representation, giving winter temperature ranges and mean temperature for the participating areas is essential in understanding and evaluating the data.
A major deficiency in the interagency report is that it does not give the reader information that will permit the needed assessment of the interrelationship between exhaust emissions measured during the Federal Test Procedure (FTP) and exhaust emissions produced during on-road use, especially in winter. FTP is a standard procedure for measuring vehicle exhaust emissions at 75°F (actually between 68°F and 86°F). As explained below, there are major differences between emissions at 75°F and at low temperatures.
To understand how the winter use of oxygenated fuels in vehicles can affect air quality, several factors must be taken into account in a general way:
-
The effect of oxygenated fuels on tailpipe emissions—i.e., CO, volatile organic compounds (VOCs), and oxides of nitrogen (NOx)—and emissions due to evaporation and running losses of fuel from the tank and fuel lines. It is important to account for how such effects differ among individual vehicles using different
-
emission-control techniques, having different maintenance histories, and operating at typical winter running conditions. A brief summary of the factors influencing tailpipe emissions is provided later in this chapter.
-
The inventory or population of different vehicles, including normal and high emitters in the existing fleet.
-
Particular meteorological conditions that might affect dispersion and transformation of the emitted pollutants.
It is by no means easy to integrate these factors to assess the effect of the implementation of any new regulation or technology. Ultimately, measurements of ambient-air quality provide the best assessment. Thus, any attempt to transform results from individual-vehicle operation into resulting air-quality benefits must be made with extreme caution.
Tailpipe emissions represent the difference between formation of pollutants in the engine combustion process and destruction (through oxidation or reduction) of these pollutants in the exhaust system. The most important factor influencing the formation of pollutants is air-to-fuel (A/F) ratio; the most important device used in destroying them is the catalytic converter.
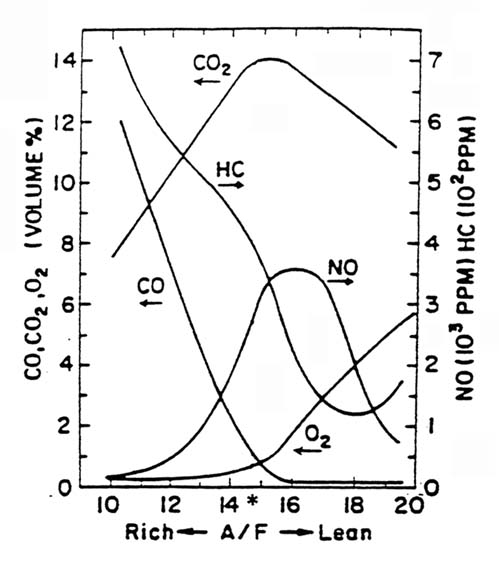
Figure 2.1 illustrates the general relationship between A/F ratio and pollutant production. Although fuel constituents other than oxygen (O2) are known to have an impact on emissions when oxygenated fuels are used, the primary factor in decreasing CO concentrations is increasing the A/F ratio through the O2 brought in with the fuel (enleanment). To illustrate this fact, Figure 2.2, for methanol, indicates that, if the same stoichiometry between normal fuel and oxygenated fuel is maintained, there is little change in CO emissions. In addition, A/F ratio has a substantial effect on power, fuel economy, cold starting, and drivability.
Figure 2.3 illustrates the importance of catalyst temperature on destruction of CO and VOCs. If the A/F ratio is closely maintained
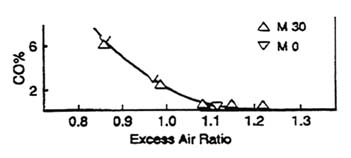
Figure 2.2 Relationship between CO emissions and excess air ratio for M30 (gasoline and methanol (30% vol)) and M0 (gasoline). Excess air ratio is the amount of O2 (from air and fuel) divided by the stoichiometric amount of O2 needed to completely combust the fuel to CO2 and water. An air-cooled single-cylinder engine was used (compression ratio 6.8, 1500 rpm, ¼ load, and spark timing-maximum best timing). Adapted from Hirao and Pefley, 1988.
around a stoichiometric level (Figure 2.1), modern catalysts will destroy CO, VOCs, and NOx very efficiently.
For good drivability, different values of A/F ratios are needed during vehicle operation. For starting the vehicle, extra fuel must be supplied because not all the fuel vaporizes under those conditions. The extra fuel needed decreases as the engine warms up. Rapid accelerations may require momentary enrichment because A/F ratio affects power. If on-road accelerations are greater than those used in the standard test cycle (Figure 2.4), actual emissions may differ from emissions measured during the FTP.
If the A/F ratio in the cylinder is too lean, or too rich, the engine may hesitate or stall, i.e., drivability will be poor. Consequently, for both emission and drivability reasons, an A/F-ratio control system is generally used. A/F-ratio control can be generally divided into two classifications: open-loop and closed-loop. Generally, in open-loop control, A/F ratios are predetermined (typically
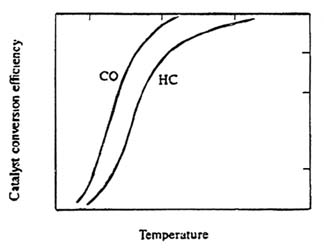
Figure 2.3. Illustrative relationship between motor-vehicle catalyst temperature and destruction efficiency of CO and hydrocarbon (HC) emissions. Adapted from Heywood, 1988.
stoichiometric or richer) but changed by ambient and operating conditions.
In closed-loop control (Figure 2.5), the A/F ratio is automatically adjusted to achieve a given goal, in this case maintaining the stoichiometric mixture needed to destroy all three pollutants. If the closed loop adapts or adjusts to changing conditions, it is called adaptive closed-loop control.
Carbureted engines use open-loop control. However, almost all current engines, using fuel injection and computer control, operate initially as open-loop but change to closed-loop as the intake manifold, O2 sensor (increasingly electrically heated), and catalytic converter warm up. While not shown in Figure 2.5, a second O2 sensor, located downstream of the catalytic converter, is increasingly being added to achieve more precise control.
Because of differences with vehicle age, model, and manufacturer, a single value for the duration of open-loop operation cannot be presented. For FTP conditions at 75°F and computer-controlled
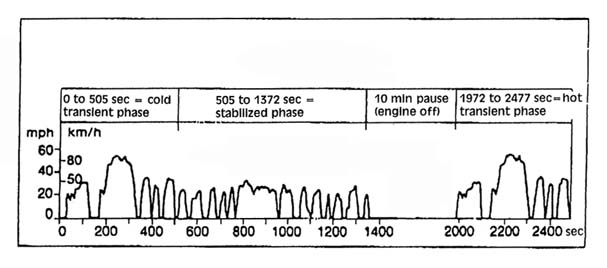
Figure 2.4. The Federal Test Procedure driving cycle. The cycle length is 11.115 miles; cycle duration is 1877 sec with a 600 sec pause; average speed is 34.1 km/hr (21.2 mile/hr); and the maximum speed is 91.2 km/hr (56.7 mile/hr). The procedure is conducted at a temperature of 75°F. Adapted from: Bosch, 1986.
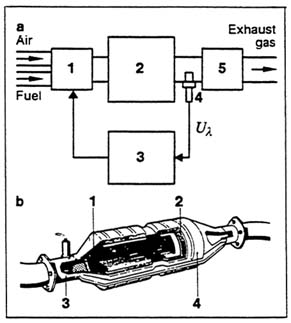
Figure 2.5 Exhaust-gas emission control with the three-way catalyst and the O2 sensor. a) Functional diagram: 1, fuel-metering system; 2, engine; 3, control unit; 4, O2 sensor; 5, three-way catalyst; U![]() , sensor voltage. b) Three-way catalyst: 1, ceramic monolith; 2, wire mesh; 3, O2 sensor; 4, housing. Adapted from Bosch, 1986.
, sensor voltage. b) Three-way catalyst: 1, ceramic monolith; 2, wire mesh; 3, O2 sensor; 4, housing. Adapted from Bosch, 1986.
operation, the open-loop time is 30-80 seconds. Depending upon the control technique, engine design, and catalytic-converter location, catalyst ''light-off" will occur 0 - 80 seconds after conversion to closed-loop control. For a cold winter startup, open-loop operation will be longer—possibly 2 or 3 times longer.
Given the wide range of control techniques used and maintenance histories of vehicles, it is difficult to generalize about the effect of using oxygenated fuels on CO emissions. However, during closed-loop operation with perfect control there should be little change in CO emissions; the control sensor should maintain the same stoichiometry and therefore the same CO emissions, irrespective of fuel.
For open loop operation, the situation is more complicated. In general, rich mixtures are used during open loop operation in accordance with a pre-determined schedule of A/F, spark advance, etc, which is modified by ambient and engine conditions. Adaptive control is defined as a change in the values in this schedule made automatically in response to a change in fuel characteristics. If an oxygenated fuel is used and adaptive control is not used, enleanment will occur resulting in reductions of CO emissions. In addition, the potential for NOx increases (see Figures 2.1 and 2.2), and vehicle drivability is likely to decrease.
Use of adaptive control began approximately a decade ago. Although the first systems were rather crude in sensing when oxygenated fuels were used, current adaptive control systems adequately sense when oxygenated fuels are being used and correct the open-loop schedule for the effects of oxygen in the fuel. Thus for very recent and future vehicles operating in open loop and using oxygenated fuels, enleanment and drivability problems would be minimal. For current and future vehicles, in both open loop and closed loop operation, only small changes in CO and NOx emissions can be expected when using oxygenated fuels.
The interagency report does not provide a plausible method for
adjusting FTP data collected at the standard temperature of 75°F to represent data that would be observed at winter temperatures. Although data for temperatures below 75°F are presented, it is not clear whether the behavior of vehicles under such tests would adequately represent typical winter conditions. The interagency report should discuss this deficiency and its implications on the report's conclusions.
An alternative procedure for assessing the effect of oxygenated fuels on ambient CO is to demonstrate that ambient levels of CO have been reduced by the use of oxygenated fuel. This case is not made in the interagency report, perhaps because it is difficult to separate the reduction due to oxygenated fuels from the general reduction in CO due to reduced production by the general fleet. Figure 1.1 of the air-quality benefits section of the interagency report presents such a plot for Denver covering the period 1980 - 1995. While the annual variation in CO levels has decreased, the trend of average winter CO level shows little or no change since winter oxygenated fuels were introduced.
The interagency report states that EPA's MOBILE 5a model has predicted decreases as large as 29% in vehicle CO emissions resulting from the use of oxygenated fuels. Because 90% or more of the ambient CO in urban areas results from emissions from mobile sources, a predicted decrease of 29% in mobile-source CO emissions presumably should be observable in the ambient data. This has not been the case.
Another important deficiency of the interagency report is the failure to emphasize the fact that, from a mass-emission standpoint and consequent effect on ambient CO levels, high emitters are the major contributors to ambient CO concentrations. High emitters are vehicles whose emissions are inordinately large as a result of older control techniques, misadjustment, lack of maintenance, etc. Their emissions can be orders of magnitude larger than those from current, well-maintained vehicles. It follows that the same percentage
reduction in emissions from high emitters will produce orders of magnitude greater mass-emission reductions than will the same percentage reduction from low emitters.
In 1983, a contractor reported to the California Air Resources Board (CARB) that 12% of a fleet of vehicles recruited by CARB for its in-use surveillance program was responsible for 47% of the total CO emissions as measured by the 75°F FTP (Wayne and Horie, 1983). The contractor recommended: "Since outliers [high emitters] contribute a large fraction of the total emissions, it is more important to know their contribution accurately than to know accurately the relatively minor contributions of low-emitting vehicles." This same principle applies to any study of CO emissions from the in-use fleet. Since that report, a number of additional studies have reported the same findings—at least half the CO emissions from warm vehicles under stable operating conditions are produced by less than 10% of the fleet (Bishop and Stedman, 1990; Stephens and Cadle, 1991; Lawson, 1993; Zhang et al, 1995; Shepard et al, 1995). Some of these high-emitting vehicles produce more than 0.5 pound (about 227g) of CO per mile (Lawson et al, 1996).
Because the CO emissions of the in-use fleet are highly skewed, it is important to understand which control strategy will produce the largest reduction in CO emissions at the lowest cost to society (Beaton et al, 1995). For example, a relatively large percentage decrease in emissions from well-maintained vehicles with modern control technology will result in only a minor total reduction in ambient CO emissions.
Finally, another important issue that needs additional discussion and clarification is results obtained with EPA's MOBILE 5a model. EPA designed the MOBILE model specifically for use by states in preparing emission inventories required under the Clean Air Act. MOBILE 5a is the model version currently in use. The model is used to estimate the current fleet's tailpipe and nontailpipe emissions, the effectiveness of vehicle inspection and maintenance programs,
and the effect of oxygenated and reformulated fuels on vehicle emissions. It is also used to provide projections of the vehicle fleet's emissions many years into the future, and output from this model is used to provide emissions data that go into our nation's emission inventories. Predictions from this model are used in the interagency report but, in spite of differences between projected and real-world data, no assessment of the accuracy of this model is presented.
FTP EMISSIONS DATA FOR INDIVIDUAL VEHIClES
The available CO data in the interagency report for individual vehicles used in the study were collected largely as standard FTP 75°F cycle emissions. The report covered data from emission-control systems that ranged from early technology to recent technology. There were some data comparing high emitters and normal emitters using the standard 75°F FTP cycle. The report presents some data using the FTP cycle at 35°F and 50°F. The data for 35°F and 50°F test conditions were for relatively clean vehicles as compared to high emitters.
The primary objective of the oxygenated-fuels program is the reduction of winter CO emissions in nonattainment areas. However, CO emissions data are lacking for the temperatures less than 20°F for both FTP and on-road conditions. Such conditions are typical of winter conditions in many regions of the United States. (The normal daily minimum temperature in Fairbanks, Alaska, in January is about -22°F.) When operating at temperatures less than 20°F, vehicles require much richer startup mixtures for much of the open-loop operation, may have reduced catalyst temperatures, and warm up slowly depending upon driving conditions, such as stop-and-go traffic, freeway driving at high speed, and congested
freeway driving. Such data are extremely critical in judging the benefits of the winter oxygenated-fuels program, especially because some of the data indicate tendencies for increased CO emissions with oxygenated fuels. High-emitting vehicles also tend to be highly variable in their measured emissions (Knepper et al., 1993). The interagency report should provide a single summary table that lists the results of all the dynamometer-based studies, the testing conditions and types of vehicles tested (normal versus high emitters), with changes in observed pollutant emissions.
ON-ROAD EMISSIONS DATA FROM VEHICLE FLEETS
Field studies of emissions from the on-road motor vehicle fleets are necessary to quantify the real-world emissions coming from the tailpipes of vehicles. There are two types of studies that can be used to infer the levels of CO emissions from in-use vehicles and the effect of oxygenated fuels in the winter. One approach uses remote sensing data and the other uses data acquired in tunnel studies, in which vehicle emissions are measured as the fleet is driven through a tunnel.
The interagency report discusses results from remote sensing studies from the Denver area and North Carolina. In the first Denver study, Bishop and Stedman (1989) reported a 6±2.5% reduction in CO attributable to the use of 1.5-wt% oxygenated fuel. In the second Denver study, Bishop and Stedman (1990) reported a 16±3% decrease in CO emissions from the use of 2.0-wt% oxygenated fuel. Another study, by PRC (1992), reported that the percentage reduction of CO emissions was nearly the same for all vehicles and that most of the CO emissions attributed to oxygenated-fuel use were from the highest-emitting vehicles.
However, two additional remote sensing studies performed in
North Carolina by Georgia Tech (Rhudy et al. 1995; Rodgers, 1996 [personal communication]) have shown no reduction in CO emissions that could be attributed to the use of oxygenated fuels. For all these studies, remote sensing devices measure the CO-to-CO2 ratio in the plume of vehicles that pass by the sensors. Because CO and CO2 emissions increase dramatically at lower-temperature winter conditions, it must be demonstrated that the ratio of these two pollutants from the in-use fleet is not adversely affected at cold temperatures for different fuel compositions in order for this type of experiment to be reliable. A decrease in measured CO-to-CO2 ratios does not necessarily represent a decrease in CO emissions; it also could result from an increase in CO2 emissions from higher fuel consumption (as explained later in this chapter). Finally, because different groups have not obtained consistent experimental results, at this time the committee cannot conclude that the remote sensing results have conclusively demonstrated a beneficial effect from oxygenated fuels.
In the second category of study, Kirchstetter et al. (1996) report a 21% reduction in the fleet's CO emissions in the Caldecott tunnel in the San Francisco area. However, the observed differences in CO emissions occurring before and during oxygenated-fuel use might not be entirely attributable to differences in fuel composition. Other factors, such as differences in typical speeds of the vehicles, might have affected the observed CO reduction. In order to obtain more conclusive results, a set of measurements should have been made after the oxygenated-fuel season ended. Also, it is important to note that measurements were taken at temperatures higher than temperatures characteristic of winter in many areas. Cold-start conditions are not measured in a tunnel; for the current fleet, such conditions provide the greatest potential emission reductions attributable to oxygenated fuels. Finally, a 21% reduction in CO would be expected to result in a marked decrease in observed ambient measurements of CO. Such changes have not been observed.
In each of these studies, a suitable experimental control group or control conditions have not been used. In the case of in-use fleet-emission experiments, precise documentation of the number of high-emitting vehicles and their actual emissions also is necessary because of their large influence on overall fleet-emission characteristics.
LOW-TEMPERATURE VEHICLE-EMISSIONS STUDIES
As ambient temperatures decrease, the amount of time that a vehicle runs in open-loop conditions increases substantially as does the catalytic converter warmup time. The length of time that vehicles are actually in open loop operation is not well documented, however. Because increases in CO emissions at low temperatures are very large, the interagency report should discuss this effect. Also, that report should compare such increases in CO emissions with reductions in CO attributable to oxygenated fuels.
The dynamometer data cited in the report suggest an extremely small reduction in CO emissions when vehicles are driven in FTP-like conditions at low temperatures (less than 35°F). Studies performed by EPA and others at low temperatures should be included in the air-quality chapter. In the low-temperature regions in the United States where oxygenated fuels are required, it is clear that additional data are needed to understand whether the use of oxygenated fuels actually decreases CO emissions. The committee recommends that the air-quality chapter of the interagency report contain a summary table which presents the results of all low-temperature dynamometer studies.
AMBIENT STUDIES
The interagency report cites studies of ambient measurements by
the state of California (Dolislager et al., 1993, 1996), Centers for Disease Control and Prevention (CDC) (Mannino and Etzel, 1996) and the University of Colorado at Denver (Anderson et al., 1993, 1994) of ambient CO data from western states and additional studies from Alaska (Heil, 1993) and North Carolina (Vogt, 1994, Vogt et al., 1994). Only one of those studies has appeared in peer-reviewed literature; the rest are only in the gray literature. The peer-reviewed paper, Mannino and Etzel (1996) however, has a number of serious shortcomings. It states that oxygenated fuels reduce ambient CO concentrations, but it also reports that "the largest [CO] decreases were at two sites that were not using oxygenated fuels…. At some monitors, however, CO levels increased slightly." The report also states that "these decrements were small in terms of absolute concentrations" The authors did not explain why these areas had increases or only small decreases in ambient CO concentrations. During the period of the CDC study, the frequency of occurrence of CO exceedances in oxygenated-fuel air-monitoring locations decreased from 66% to 28%, while the percentage of nonoxygenated-fuel air-monitoring locations with CO exceedances decreased from 52% to 11%. There was a larger decrease of CO exceedances in conventional-fuel areas than in areas using oxygenated gasolines during the CDC study period. Most important, however, the CDC report did not account for the influence of motor-vehicle inspection and maintenance (I/M) programs in their analysis. In addition to oxygenated fuels, CO nonattainment areas are required to have I/M programs in place. According to EPA's MOBILE model, CO reductions resulting from I/M programs are substantial. However, as discussed previously the accuracy of the MOBILE model is uncertain. It is possible that I/M programs were responsible for the larger CO decreases in the oxygenated-fuel regions than in the conventional-fuel (and non-I/M-program) regions. For example, California's mobile-source emissions model predicts a decrease of 15% in the fleet's CO emissions resulting
from that state's I/M program (California I/M Review Committee, 1993).
Although the calculations appear to be thorough, Dolislager (1993, 1996) did not perform an analysis of ambient concentrations and ratios in the three separate periods (early fall, winter, and early spring) for the several years before oxygenated fuels were used in California to determine whether CO, VOC, and NOx relationships were similar for those three seasons when oxygenated fuels were not used. A similar comparison for the three seasons during years when oxygenated fuels were used would allow an oxygenated-fuels effect to be adequately evaluated. Also, Dolislager calculated changes in CO-to-NOx ratios to infer changes in CO emissions due to oxygenated fuels by assuming that oxygenated fuels do not substantially increase NOx emissions. The data from the interagency report show that oxygenated fuels do increase NOx emissions by as much as 2% per weight % oxygen. Thus, the actual CO reductions resulting from the use of oxygenated fuels would be lower than the 6-10% reported. The California analysis reports a 6-10% decrease of ambient CO attributable to the use of oxygenated fuels, and it should be noted that this decrease has been observed in areas having very mild winters. As is noted in other parts of this report, low-temperature dynamometer data show extremely small (only 1.8-1.9%) decreases in CO emissions per weight % of oxygen (in some cases, CO emission increases have been reported).
Two separate reports have been written by Keislar et al. (1995, 1996) summarizing an ambient study in Provo, Utah, designed to assess the effect of oxygenated fuels on ambient CO concentrations. In the first report, the authors calculated an upper limit of 9-10% decrease in CO but at a confidence level of only 80%. In the second paper, the authors cited a benefit of 15 ± 20%.
Several additional ambient-effects studies cited in the interagency report show no observable effect on ambient CO from the use of oxygenated fuels. The committee feels that the wording in the
conclusions section of the interagency report should explicitly state that several locations where oxygenated fuels have been used have shown no effect of the fuels on reduction of ambient CO concentrations and that in some cases CO concentrations have actually increased. The committee recommends that the air-quality chapter contain a summary table that presents the ambient conditions, reported uncertainties, and results reported in all the ambient studies.
From the data cited in the interagency report, the effects of oxygenated fuel on ambient levels of CO are small at best; in some locations, increases in ambient CO have actually occurred (Mannino and Etzel, 1996). In some locations having moderate climates, (e.g., California), a 5-10% effect has been reported (Dolislager, 1993, 1996); in another moderate climate location (e.g., North Carolina), no effect was observed (Vogt, 1994; Vogt et al., 1994; Cornelius, 1995). Only one of these studies has been subjected to the peer-review process, and that report, as discussed in the committee's report, suffers deficiencies. Few or no data from low-temperature winter locations exist: only one report from Alaska. The major problem is a lack of thorough, statistically defensible analysis of ambient data where such confounding features as the lack of a control population for comparison, fleet turnover, occurrence of high emitters, I/M programs, and local economy and fleet population are properly accounted for.
DISCREPANCIES BETWEEN MODEL RESULTS AND OBSERVATIONS
The interagency report also suggests that there are major discrepancies between real-world observations and the results of the MOBILE 5a model. Since 1987, there have been a number of independent studies by researchers throughout the country that document
inadequacies in EPA's MOBILE model (Ingalls et al., 1989; Pierson et al., 1990). The observed CO reductions attributed to oxygenated fuels are much less than predicted by the MOBILE model. The model's predictions for CO reductions, as cited in the report, are as large as 29%. No ambient study reports reductions in CO anywhere close to this value.
It should be noted that variations in factors including meteorological conditions, monitor location, engine operating conditions, vehicle fleet composition, travel activity, and variability in measurements can make it difficult to detect statistically significant changes in ambient concentrations. However, when ambient studies experience difficulties in detecting the effects of emission control programs, it suggests that the effects are not major and that additional analysis would be needed to isolate the effect.
The interagency report should include an assessment of the utility of the MOBILE 5a model for prediction of winter emissions from oxygenated fuels and discuss possible reasons for differences between observations and modeled predictions.
COPOLLUTANT EFFECTS OF OXYGENATED FUELS
The interagency report attempts to collect and interpret the existing data on the effects of winter oxygenate addition on emissions of pollutants other than CO. The attention devoted to this aspect is found in two short sections (1.32.2 and 1.59) and Appendix 3 of the interagency report. The main issue is whether addition of oxygenates to gasoline produces unintentional increases in pollutants other than the intended target CO reduction.
Measurements are quoted primarily from the Auto/Oil AQIRP study and from a few measurements of ambient data from selected locations during the period when oxygenated fuels were in use. The authors in general refer to the AQIRP bulletins (1991) rather
than to the corresponding peer-reviewed SAE publications (SAE SP-920 and SP-1000 and additional publications) and sometimes refer to specific papers containing AQIRP data. It would be preferable to make specific reference to the peer-reviewed SAE publications.
Review of the existing data reveals a lack of measurements at conditions relevant for winter conditions (temperatures less than 35°F) likely to be prevalent when the oxygenated-fuel program is in effect.
DYNAMOMETER TESTS
NOx EMISSIONS
NOx is a pollutant of major concern for areas not in attainment of the ozone National Ambient Air Quality Standards (NAAQS) (NRC, 1991a). Exceedances of the ozone NAAQS do not occur for many areas during the winter when oxygenated fuels are used to reduce ambient levels of CO. However, for areas not in attainment of ozone NAAQS, potential increases in NOx from motor vehicles using oxygenated fuels should be given greater consideration in the interagency report.
The interagency report adequately covers the available data on the effect of oxygenates at the standard FTP conditions (75°F); data from Table 1.3 of the interagency report indicate that NOx emissions increase slightly with the addition of oxygenates (0 to 1% per weight % oxygen). Data from the AQIRP program for high emitters (high emitters were defined by the HC emissions relative to the average) indicate a more pronounced effect (up to 12% increase per weight % oxygen). However, conflicting data were provided by an EPA study (Mayotte et al., 1994a and b) showing that the effect of oxygenates on high emitters led to no or a small decrease in NOx emission levels.
The conflicting data emphasize that, even at 75°F, the effects of oxygenates on NOx emissions is complex. Figures 2.1 and 2.2 indicate that if the air-fuel ratio is rich and additional oxygen is supplied (regardless of the source) NOx emissions increase and CO emissions decrease. It follows that, if the open-loop, air-fuel-ratio schedule of motor vehicles is constructed using a nonoxygenated fuel, use of an oxygenated fuel (with all other fuel characteristics the same) will increase engine-out NOx. However, adding oxygenates may require changes in fuel composition to achieve the overall desired characteristics; these changes may affect NOx. In addition, the net amount of pollutants emitted from a motor vehicle's tail pipe is the result of engine emissions modified by the effects of the catalytic converter. Consequently, NOx emissions to the atmosphere are affected by a variety of factors including type of emission control systems, maintenance practice, and catalyst age.
More glaring is the lack of data at low temperatures. The reported data by Most (1989) in Table 1.6 of the interagency report cover the effect on CO, but no measurements are reported on NOx. Additional data by Lax (1994) are provided in a review of a different study, remarking that ''The NOx emissions were generally increased by the addition of the fuel oxygenate" (last paragraph of page 12). The discussion on NOx is not clearly separated in the current report, appearing as incidental comments in the discussion of CO effects, and again in the discussion of other emissions.
HC EMISSIONS, MTBE, AND TOXICS
The interagency report correctly cites the result of dynamometer studies: enleanment by addition of oxygenates in general decreases hydrocarbon emissions (Figure 1.1). Only one study is reported at low temperatures (Table 1.7), although presumably the report by Most (1989) in Table 1.6 also contains relevant data. The main conclusions quoted from the review by Hood and Farina (1995)
appear to indicate that the available data fail to show consistent reductions of total hydrocarbon emissions with added oxygenates at low temperatures and that only modest reductions are achieved for properly operating, closed-loop control vehicles.
The effect of oxygenates on toxic emissions is mentioned only briefly. Clearly, very few data are available on the emissions of MTBE and aldehydes at low temperatures, even though a general decrease in total hydrocarbons is expected with the addition of oxygenated fuels, as well as a decrease (by dilution) in the emissions of benzene and 1,3-butadiene. FTP studies at 75°F indicate a 26% increase in emissions of formaldehyde (Gorse et al., 1991), and 5% and 8% decreases in benzene and 1,3-butadiene emissions with the addition of 15% MTBE to the gasoline. Ethanol addition leads to an increase in acetaldehyde emissions. These results are adequately reported in the conclusions to the emissions studies (p. 19) but are not carefully discussed in the main text. The authors should add a table summarizing the existing data on the effects of toxic compounds and total MTBE emissions. In addition, a link with the potential health effects of the different toxics relative to the different cancer potencies (e.g., a reduction in benzene but an increase in formaldehyde) in the health-effects section should be made.
FIELD MEASUREMENTS
There is clearly a gap in the available field data on emissions other than CO associated with oxygenate additions. No remotesensing data on hydrocarbons or other emissions are apparently available. The authors of the report quote measurements by Anderson et al. (1993, 1994) indicating the increase in aldehyde and MTBE concentrations in ambient-air measurements in the Denver area after the introduction of the oxygenated-fuels program. The
latter are not peer-reviewed publications and were not available to the committee. Another part of the report (HEI, 1996) indicates that measurements of MTBE, aldehydes, and other compounds are available for other areas of the country where oxygenated fuels have been in use. It would be useful to attempt to cross-reference or consolidate the few available air-quality measurements in one single section on ambient air quality-effects. Whereas most of the interagency report appears to be concerned with MTBE proper as a potential health hazard, it might be advisable to consider the combined effects of introduction of MTBE with the potential increase in aldehyde emissions and production of byproducts in the environment. Furthermore, the committee recommends that an assessment of the toxic emissions associated with the introduction of oxygenated fuels during the winter (or associated dynamometer tests at low temperatures) be made.
ATMOSPHERIC CHEMISTRY
The interagency report contains an appendix on the "Explanation of [OH] Trace Gas Life Time Estimates." The importance of secondary effects related to emissions of copollutants associated with oxygenated fuels deserves a discussion incorporated in the main report. In particular, a clearer understanding is needed of the atmospheric chemistry, transport, and fate of MTBE and byproducts including transport into waterways and production of secondary photochemical products. The discussion in Appendix 3 of the interagency report is a very good introduction to the subject and covers the existing literature, but the relatively long lifetime of oxygenated compounds raises some concern regarding the fate of the compounds.
FUEL ECONOMY, ENGINE PERFORMANCE, AND PROGRAM COSTS
There have been complaints that the addition of oxygenates to gasoline reduces fuel economy (measured in miles per gallon) and leads to poor engine performance. The interagency report addresses both of these issues.
After new oxygenated-gasoline programs were implemented in the winter of 1992-1993 to reduce CO emissions, consumers in some areas of the country expressed concerns that these fuels had led to large reductions in fuel economy and deterioration of engine performance. Losses in fuel economy in excess of 20% were claimed by some consumers. In addition, a variety of engine-performance problems, including rough engine operation and difficulty in starting, were reported. Therefore, it is important to establish the extent to which the addition of oxygenates to gasoline brought about these problems.
FUEL ECONOMY
The interagency report reviews a number of credible sources of data on the effect of addition of different oxygenates (MTBE, ETBE, and ethanol) on fuel economy. In general the studies cited1 compare the fuel economy obtained with conventional fuels and that obtained with fuels containing oxygenates.
The report concludes that the reduction in fuel economy expected based on theoretical considerations is 2%-3% and that real-world changes correspond to these theoretical predictions. While the committee agrees in general with the conclusion, it recommends stating explicitly that fuel-economy changes are reliably predicted
by the change in energy content per gallon of fuel brought about by a given change in composition2. There is agreement based on data from a wide variety of sources that if a given level of an oxygenate reduces the energy content per gallon of a formulated gasoline by 1.6%, for example, the expected reduction in fuel economy is also 1.6%.
ENGINE PERFORMANCE
Fuel-related sources of engine-performance problems for all types of gasoline include excessively high or low volatility, water absorption, improper storage and handling, enleanment (higher oxygen-to-fuel ratios), reduced motor octane, and materials compatibility. These sources can lead to rough engine operation, overheating, damaged pistons, vapor lock, starting difficulties, plugged fuel filters, fouled spark plugs, fuel leaks, hesitation during acceleration, flooding, stalling, and engine fires.
The interagency report deals with all these issues and concludes that, except for possible drivability problems due to enleanment, performance problems due solely to the presence of oxygenates in gasoline are not expected. The committee agrees with this conclusion. As the report states, there are a number of factors other than gasoline quality and composition that contribute to engine-performance problems that may be corrected by relatively simple consumer actions.
Program Costs
The draft executive summary in the interagency report states that
the report identifies "areas where the data are too limited to make definitive conclusions about the costs, benefits, and risks of using oxygenated gasoline." The committee believes that reasonably reliable costs of oxygenated-fuel production, fuel use, and program administration can and should be calculated to help guide the program. After calculating those costs, the interagency report might compare, at least at a broad level, the expected amount of ambient CO reduction using oxygenated fuels with the reduction that would be expected from alternative approaches, such as the repair or elimination of high-emitting vehicles for the same expenditure of funds.
MODERN TECHNOLOGY
In addition to increasingly precise adaptive control, a number of changes taking place are likely to change the outlook for CO emissions from gasoline-powered vehicles. The committee believes that the interagency group should consider these changes in its analysis.
Perhaps the most important factor is the change to 100,000-mile certification. Under this provision, auto manufacturers will be required to certify that vehicles will maintain emissions standards for 100,000 miles instead of the current 50,000 miles. In addition, vehicles will include onboard diagnostics that will detect malfunctioning emissions systems, so that consumers will know that repairs or adjustments are needed. These changes combined will result in better continued performance of these vehicles as they age in the population and should contribute to reductions in ambient CO concentration.
The sensors being used today in new cars have faster response during warm up. This reduces the time with open-loop operation; as a result, the time required to heat the catalyst becomes increasingly important in achieving a high level of CO control.
In addition, EPA is considering changes to the Federal Test Procedure used to specify the emissions from new vehicles that would take into account performance at low ambient temperatures. The current procedure measures emissions at an ambient temperature of 75°F, and these measures do not give a direct measure of CO emissions at lower starting temperatures, representative of winter operation in many areas. Modifying the FTP to include performance at winter ambient temperature would ensure that new vehicles would control CO emissions to specified levels under winter conditions, and this too would lower ambient CO concentration. Currently, motor vehicle manufacturers are not required to meet CO emission standards at temperatures less than 20°F.
The apparent advantage of a strategy to use oxygenated fuels is expected to dissipate with time as vehicles with advanced emission-control systems replace older vehicles. Closed-loop, adaptive-learning, and oxygen-sensor systems in advanced-technology vehicles control the fuel-combustion process at the same stoichiometry irrespective of whether the O2 in the engine's cylinders comes from fuel or air. In such a case, oxygenation of fuel is expected to result in small changes in CO emissions.
OVERALL CONCLUSIONS ON AIR QUALITY, FUEL ECONOMY, AND ENGINE PERFORMANCE
The interagency report concludes that there have been substantial reductions in ambient CO levels in the last 20 years and that vehicle emission controls have been a major factor in this reduction. However, the federal report should better characterize the uncertainty about the extent to which oxygenated fuels have contributed to this reduction. The committee believes that it has not been established that oxygenated fuels have been a major factor in this reduction.
The committee agrees with the conclusion in the interagency report that, under many fuel-control systems, oxygenated fuels decrease CO emissions under FTP (at 75°F) conditions. However, the data presented do not establish the existence of this benefit under winter driving conditions.
The interagency report does not clearly address the effects of fleet composition, particularly high-emitting vehicles, on total CO emissions.
The interagency report highlights some differences between EPA's MOBILE 5a model results and observed vehicle emissions and ambient concentrations, but it does not provide an assessment of why those differences exist. It should do so and should also emphasize the fact that the model apparently overpredicts the oxygenated-fuel effect by at least a factor of 2 according to comparisons of model predictions of CO emission reductions with observed data.
The interagency report provides a good summary of previous studies that have assessed the impacts of oxygenated fuels on winter CO concentrations; however, the report should state clearly that winter ambient CO reductions have been as low as zero and as high as about 10%.
The enleanment effect of oxygenated fuels presents the potential for increased NOx emissions from motor vehicles. Furthermore, much of the available data suggests that such an increase does occur. Any increase in NOx emissions could be detrimental in ozone nonattainment areas where exceedances have occurred during the period of the oxygenated fuels program.
The interagency report concludes that the fuel-economy penalty associated with the use of oxygenated fuels is approximately 2% to 3% and is related to changes in energy content per gallon. The committee agrees with these conclusions. The committee also agrees with the report's conclusion that engine performance is typically not adversely affected by their use.
OVERALL RECOMMENDATIONS ON AIR QUALITY, FUEL ECONOMY, AND ENGINE PERFORMANCE
The implications of the lack of emission data collected at low temperatures in evaluating the effectiveness of the oxygenated-fuels program in reducing CO emissions should be fully discussed in the interagency report.
A defensible field study with adequate statistical power should be performed at low temperature to assess whether oxygenated fuels reduce ambient CO concentrations under such conditions. If a beneficial effect of reduction in CO is demonstrated in that field study, a carefully designed study should then investigate the effects of oxygenated fuels on emissions of NOx, VOCs, and toxic air pollutants on winter air quality, using appropriate controls and accounting for differences in such factors as fleet population, high-emitting vehicles, I/M programs, and local fleet characteristics. The introduction of toxic organic compounds into the air, as well as a fuel-economy penalty, should also be addressed in such a study. Also, it is equally important desirable to perform such a study under cold temperature conditions in an area where no oxygenated fuels are used. Results for an area using oxygenated fuels can be compared with areas not using oxygenated fuels.
Despite uncertainties in estimating costs and even greater uncertainties in estimating benefits, full evaluation of the oxygenated-fuel program requires that the interagency report address and document program costs and benefits at least at a broad level.