12
Diabetes Mellitus in Native Americans: The Problem and Its Implications
K.M. Venkat Narayan
Introduction
Diabetes mellitus is a group of metabolic disorders characterized by abnormally high levels of blood glucose secondary to inefficient insulin action and/or secretion. The disease often leads to significant disability, including renal failure, blindness, and limb amputation, and to premature death.
Diabetes was apparently rare among Native Americans until the middle part of the twentieth century (Joslin, 1940; West, 1974; Sievers and Fisher, 1985). However, since World War II, it has become one of the most common serious diseases among many Native American tribes (Sievers and Fisher, 1985); in 1987, there were at least 72,000 Native Americans in the United States with diagnosed diabetes (Newman et al., 1990). Diabetes occurring in Native Americans is almost exclusively the type referred to as NIDDM or non-insulin-dependent diabetes mellitus (Sievers and Fisher, 1985). The Pima Indians have the highest recorded prevalence and incidence of NIDDM in the world (Knowler et al., 1978; King and Rewers, 1991). High rates have also been observed among other Native American tribes (Sievers and Fisher, 1985; Gohdes, 1986; Young and Shah,
I wish to thank my colleagues, Drs. Maximilian de Courten, Richard Fernandes, Robert Hanson, Bill Knowler, Robert Nelson, and David Pettitt, for their help and advice. I am also grateful to the members of the Gila River Indian Community for their enormous contribution to the understanding of diabetes.
1987), as well as in many diverse societies worldwide that have recently adapted to western culture (Prior and Tasman-Jones, 1981; Cameron et al., 1986; Zimmet et al., 1990).
It is not entirely clear why the frequency has increased among Native Americans during this century, and the question is the subject of considerable research attention. While it is reasonable to conjecture a genetic predisposition to NIDDM, the role of environmental factors is of undoubted importance in explaining the dramatic increase in rates of NIDDM among many populations. Many of the known environmental determinants are potentially modifiable and offer immediate prospects for preventing or postponing NIDDM (Knowler and Narayan, 1994; Knowler et al., 1995). The complications of diabetes, which account for the increased mortality and morbidity among diabetic subjects, may also be prevented or delayed by systematic application of current knowledge (The DCCT Research Group, 1993; Weir et al., 1994). With a view to informing and influencing health policy, this paper:
- reviews the magnitude of the problem of NIDDM among Native American populations;
- summarizes current knowledge about the determinants of NIDDM;
- describes the major complications of NIDDM;
- assesses the potential for preventing or delaying NIDDM and its main complications; and
- suggests research directions that can facilitate the prevention of NIDDM and its complications in Native Americans.
Pima Indian Study
Pima Indians, living in a geographically defined part of the Gila River Indian Community of Arizona, have participated in a longitudinal study of diabetes and its complications since 1965 (Bennett et al., 1971), from which much of our current understanding of diabetes among Native Americans has been obtained. As this paper repeatedly refers to data from the Pimas, a brief description of this study and of the Pimas is presented.
Approximately every 2 years, each resident of the study area who is at least 5 years old is invited for an examination that includes a medical history; a physical examination; an oral glucose tolerance test; and measurements of height and weight, serum lipids, serum insulin, and urinary proteins. The same standardized methods are used for subjects of all ages, and DNA samples are also collected for genetic studies (Knowler et al., 1990).
The Pimas originated from a much larger group of Native Americans
who lived in an area that is now in northwestern Mexico and southern Arizona, and have lived for over 2000 years in the valleys of the Gila and Salt rives in what is now Arizona. It is believed that the Pimas derived from the Paleoindians, those Native Americans descended from the first of the three migrations across the Bering Land Bridge from Asia to America (Williams et al., 1985). Originally a desert people who subsisted on riverine agriculture supplemented by hunting and gathering, they expanded their farming system after contact with early European missionaries (Castetter and Bell, 1942). Subsequent development of the region by European settlers resulted in diversion of the Pimas' water supply and curtailment of their farming activities (Lippincott, 1980). Today much of the Pima land is leased to non-Indian farmers, and the Pimas work in sedentary government jobs or as wage laborers on or off the reservation (Pablo, 1983).
Magnitude Of The Problem Of Niddm In Native Americans
Prevalence
Some idea of the prevalence (the proportion of the population that is affected by the disease at a given point in time) of diabetes among Native Americans can be obtained from case registries held at Indian Health Service (IHS) facilities. The prevalence rates of diagnosed diabetes among Native Americans vary across tribes and are generally higher than in the U.S. population as a whole (Carter et al., 1989; Freeman et al., 1989; Acton et al., 1993b; Valway et al., 1993). In one study, the age-adjusted rate of diagnosed diabetes among all IHS patients was 6.9 percent—2.8 times the U.S. all-races rate. Of the 11 IHS areas examined in this study, all except the Alaska Area had a significantly higher prevalence rate of diagnosed diabetes than the U.S. rate (Valway et al., 1993); however, there are indications that the rates of diagnosed diabetes among Alaska Natives may also be increasing (Schraer et al., 1993).
Because nearly 50 percent of diabetes may remain undiagnosed (Harris et al., 1987), population-based studies may provide more accurate estimates of true diabetes prevalence. Data on prevalence of NIDDM from population-based studies (Hall et al., 1992; Sugarman et al., 1992; Rith-Najarian et al., 1993; Lee et al., 1995) are available for only a few Native American tribes (see Table 12-1). These data reveal that the prevalence of diabetes among Native Americans is higher for women than for men and that the rates vary among tribes. However, not all these surveys used the World Health Organization (WHO) definition of diabetes (World Health Organization, 1981), and this is likely to have led to an underestimation of
TABLE 12-1 Age-Adjusted Prevalence of Diabetes Among Native Americans from Population-based Studies
|
|
|
Prevalencea (%) |
|
|
|
Author |
Study Population |
Male |
Female |
Total |
|
Lee et al. (1995) |
Men and women aged 45-74: |
|
|
|
|
|
Pima/Maricopa/Papago, Arizona |
65 |
72 |
70 |
|
|
Apache, Caddo, Comanche, Delaware, Fort Sill Apache, Kiowa, Wichita, Oklahoma |
38 |
42 |
40 |
|
|
Oglala, Sioux, Cheyenne River Sioux, Devils Lake Sioux, Dakota |
33 |
46 |
40 |
|
Rith-Najarian et al. (1993)b |
Men and women of all ages, Red Lake Chippewa Indians |
13 |
16 |
15 |
|
Sugarman et al. (1992)b |
Men and women aged 20-74, Navajo Indians, Shiprock |
14 |
18 |
17 |
|
Hall et al. (1992)b |
Men and women aged >20 years, Navajo Indians, Many Farms-Rough Rock |
11 |
14 |
12 |
|
a Prevalence rates are standardized to the U.S. general population for the relevant ages. b These studies did not use the WHO criteria for NIDDM (World Health Organization, 1985), and therefore the prevalence rates are likely to be underestimates. |
||||
prevalence in some studies (Hall et al., 1992; Sugarman et al., 1992; Rith-Najarian et al., 1993). Overall, the prevalence of diabetes among Native Americans is higher than the rate of 6.6 percent for the U.S. population at large (Harris et al., 1987). Evidence for a higher prevalence of diabetes among Native Americans is also available from an epidemiological study that compared the Pima Indians with a predominantly white population of Rochester, Minnesota (Knowler et al., 1978). This study found that the Pimas had an age-sex standardized diabetes prevalence rate 12.7 times that of Caucasians and that, in contrast to the picture among the Pimas, diabetes prevalence in Rochester was higher for men than for women.
Diabetes among the Pimas is remarkably frequent at younger ages, and it is especially striking that about 50 percent of Pima adults over 35 years of age have NIDDM (Knowler et al., 1981). The prevalence of diabetes among the Pimas, defined by the oral glucose tolerance test (World Health Organization, 1985), has increased over three successive decades (Figure 12-1). Overall, the prevalence increased by 29 percent in men during 1965-1974 (17.62 percent) and 1985-1994 (22.69 percent) and by 35 percent in women during the same period (1965-1974:23.10 percent, 1985-1994:31.24 percent).
Why is the prevalence of diabetes increasing among Native Americans?
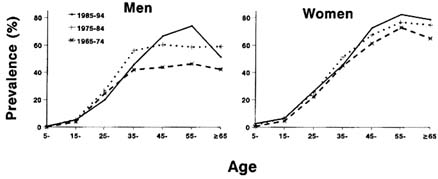
FIGURE 12-1 Age-sex specific prevalence of diabetes in Pima Indians in three time periods. Prevalence rates were estimated from data for all subjects examined in each of the 10-year periods 1965-1974, 1975-1984, and 1985-1994. SOURCE: Updated from Knowler et al. (1990).
Prevalence can increase for two reasons: improvement in survival and/or increase in the rate of development of new cases. The length of survival following the onset of diabetes may have increased over a period of time, probably as a result of better treatment or a change in the natural history of the disease. However, diabetes contributes little to mortality rates among people under the age of 55 (Pettitt et al., 1982), and an improvement in survival is thus an unlikely explanation for the increase in prevalence among younger Native Americans. This suggests that at least part of the increase in prevalence among Native Americans may be due to an increase in the incidence (the rate at which new cases develop) of the disease.
Incidence
The Pimas have the highest reported incidence of diabetes in the world—19 times the rate of diagnosed diabetes among the predominantly white population of Rochester, Minnesota (Knowler et al., 1978), and a high incidence of the disease has also been reported among other Native American tribes (Rith-Najarian et al., 1993). Figure 12-2 shows the age-sex specific incidence of diabetes among Pima Indians during three successive decades. As reported earlier (Knowler et al., 1990), the incidence rates vary by age, peaking between 35 and 44 years in men in 1965-1974 and between 45 and 54 years in men in more recent years. In women, the incidence rates peak between 45 and 54 years in 1965-1974 and 1985-1994 and between 55 and 64 years in 1975-1984. The incidence of diabetes has also increased over three successive decades at most ages and in both men
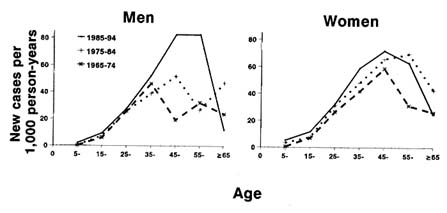
FIGURE 12-2 Age-sex specific incidence rates of diabetes among Pima Indians during three decades. Incidence rates are expressed as new cases of diabetes per 1000 person-years (PYR) of observation of nondiabetic subjects. Cases and PYRs are divided into three time periods: 1965-1974, 1975-1984, and 1985-1994. SOURCE: Updated from Knowler et al. (1990).
and women. Overall, in men, controlled for age, the incidence increased by 102 percent, from 11.79/1000 person-years (PYR) in 1965-1974 to 23.82/1000 PYR in 1985-1994. During this period, the incidence in women, controlled for age, increased by 87 percent, from 15.19/1000 PYR in 1965-1974 to 28.41/1000 PYR in 1985-1994.
Why is the incidence of diabetes increasing among Native Americans? While it is likely that there is an underlying genetic susceptibility to diabetes, the dramatic increase in incidence over a relatively short period of time emphasizes the overriding importance of environmental determinants. The discussion now turns to the determinants of diabetes, both genetic and environmental.
Determinants Of Niddm
Genetic Factors
The risk of diabetes is associated with the degree of Indian heritage (Drevets, 1965; Brousseau et al., 1979; Knowler et al., 1986). Diabetes aggregates in Native American families (Lee et al., 1985; Knowler et al., 1990), and the risk of diabetes occurring at an early age is strongly transmitted from parent to offspring, but diabetes occurring at an older age in parents has less effect on the risk of diabetes in offspring (Knowler et al., 1990). Diabetes among Pima Indians is associated with the HLA-A2 phenotype
(Williams et al., 1981), and genetic markers on chromosome 4q and 7q have been linked to insulin resistance (the underlying abnormality in NIDDM) among this population (Prochazka et al., 1993, 1995). However, knowledge concerning the genetics of NIDDM is still rudimentary, and it may be hoped that research will lead to a better understanding of the pathogenesis of the disease.
Environmental Factors
A number of potentially modifiable factors, including obesity, dietary composition, and physical inactivity, are thought to contribute to the progression from genetic susceptibility to NIDDM (Saad et al., 1988; Tuomilehto et al., 1992; Knowler et al., 1995).
Obesity
Obesity is a powerful and well-established risk factor for the development of NIDDM (Knowler et al., 1981). As shown in Figure 12-3, the age-sex adjusted incidence of diabetes among Pima Indians increases with body mass index (BMI), a measure of obesity. Compared with those with a BMI <20 kg/m2, Pima Indians with a BMI of 20-25 have a 13.6-fold higher incidence of NIDDM, and those with a BMI of 25-30 have a 21.6-fold higher incidence (Knowler et al., 1981). Furthermore, the incidence of diabetes increases with the duration of obesity (BMI (>30 kg/m2); compared with Pima Indians with less than 5 years of obesity, those with 5-10 years of obesity have 1.4 times the incidence of NIDDM, while those with at least 10 years of obesity have 2.4 times the incidence (Everhart et al., 1992). The distribution of body fat may also be important, with central obesity having been found to be related to the risk of the disease (Knowler et al., 1991; Hall et al., 1991; Warne et al., 1995).
The prevalence of obesity among Native Americans is higher than among the U.S. general population in both males and females and at all ages (Broussard et al., 1991). The reasons for higher obesity among Native Americans are not entirely clear. Broussard et al. (1991) estimated that the overall prevalence of obesity1 among Native Americans was 13.7 percent for men and 16.5 percent for women, higher than the U.S. rates of 9.1 percent and 8.2 percent, respectively. Data from the Pimas are consistent with this finding. Furthermore, the mean BMI among Pima adults has increased over time (Figure 12-4), and a secular increase in the prevalence
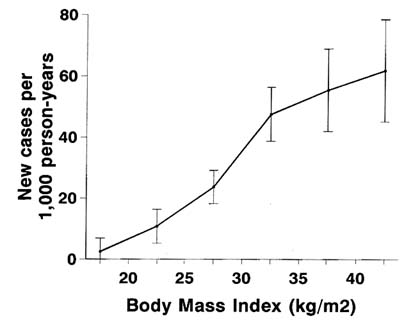
FIGURE 12-3 Age-sex adjusted incidence of diabetes by body mass index (BMI), with 95 percent confidence intervals. SOURCE: Knowler et al. (1990).
of overweight has been reported among the Navajo Indians (Hall et al., 1992). Pima children have also, on average, become heavier during this century and continue to do so (Knowler et al., 1991).
Diet
Diet has been linked with the development of diabetes for over 2,500 years (Gulabkunverba, 1949). The precise role of dietary factors, which has been reviewed elsewhere (Knowler et al., 1993), remains ambiguous. However, evidence suggests that a high-fat diet may be related to the development of the disease (Eriksson and Lingärde, 1991; Marshall et al., 1994). Few data are available for Native Americans linking dietary factors with the development of NIDDM, except for one study of the Pima Indians that found a possible association with a high-calorie diet (Bennett et al., 1984). The traditional Pima diet, derived from local agricultural produce, is believed to have been high in fiber and low in fat (Knowler et al., 1990), but this diet appears to have changed during this century and is now more or less similar to the diet in the rest of the United States (Smith et al., 1996). Similar secular changes in the diet of other Native American
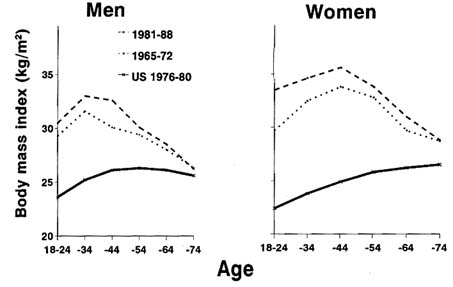
FIGURE 12-4 Mean body mass index (BMI) among Pimas for two periods and among the U.S. white population. The Pima data from each period were used for all subjects examined in each of the 8-year periods 1965-1972 and 1981-1988. The U.S. data are from the National Health and Nutrition Examination Survey (NHANES) II. SOURCE: Modified from Knowler et al. (1991).
populations are believed to have occurred; in particular, the fat content of Indian diets has increased dramatically—from 17 percent of total calories in the pre-European contact diet, to 28 percent in the reservation diet, to 38 percent in the current diet (Jackson, 1994).
Physical Activity
There is evidence that increased physical activity may have a protective effect on the development of NIDDM (Frisch et al., 1986; Schranz et al., 1991; Manson et al., 1991, 1992; Helmrich et al., 1991). As shown in Figure 12-5, the age-adjusted prevalence of NIDDM among Pima Indians aged 15-36 was lower with higher amounts of leisure physical activity in the preceding year. Among those aged 37-57, those with the lowest levels of physical activity had the highest prevalence of the disease (Kriska et al., 1993). When the exercise habits of 49 Zuni Indians of New Mexico presenting with NIDDM were compared with those of 99 nondiabetic controls (Benjamin et al., 1993), subjects with diabetes were found to be less likely to exercise frequently than those without. The hypothesis that high
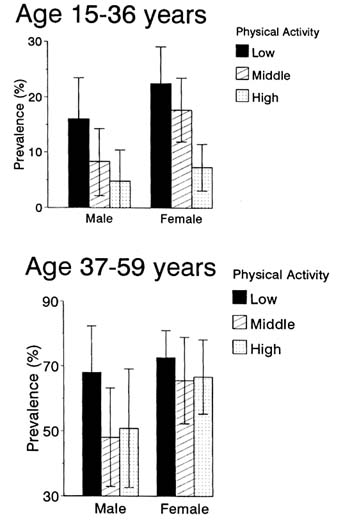
FIGURE 12-5 Age-adjusted prevalence and 95 percent confidence interval of NIDDM by tertile groups of past-year leisure physical activity among subjects aged 15-36 (upper panel) and 37-59 (lower panel). SOURCE: Modified from Kriska et al. (1993).
levels of physical activity may be protective against NIDDM is consistent with the observation that diabetes was apparently rare among Native Americans in the past, when they were a physically active agricultural and hunter-gatherer society.
Complications Of Niddm
NIDDM is associated with premature mortality and significant morbidity, including renal failure, limb amputation, blindness, ischemic heart disease, adverse outcomes of pregnancy, gum disease, neuropathy, acute glycemic complications, lipid abnormalities, and psychosocial problems. The discussion here is limited to mortality, end-stage renal disease (ESRD), and lower-extremity amputation (LEA).
Mortality
Overall, Native Americans have higher death rates than the U.S. general population (Program Statistics Branch, 1986; U.S. Department of Health and Human Services, 1985), a fact confirmed by detailed investigations in Canada (Mao et al., 1986) and among specific U.S. Native American populations (Mahoney et al., 1989; Sievers et al., 1990). The patterns of death among members of the Seneca Nation of Indians in New York State between 1955 and 1984 were compared with those of the general New York State population (Mahoney et al., 1989). As seen in Figure 12-6, compared with the New York State population, a greater-than-expected number of both male and female members of the Seneca Nation died from all causes, from infectious diseases, from diabetes, from liver cirrhosis, from accidents and injuries, and from suicides, while a lower-than-expected number died from cancers and cardiovascular diseases. A similar pattern of deaths was found among the Pima Indians (Sievers et al., 1990), and the age- and sex-adjusted average annual death rate in the Gila River Indian Community (1639/100,000) was 1.9 times the 1980 rate for the United States, all races (878/100,000). Death rates were higher among Pima men than women, and Pima men had an age-adjusted death rate 2.3 times that of U.S. men, all races. Furthermore, young Pima men aged 25-34 had 6.6 times the mortality rate of men of the same age in the U.S. general population. The age-sex adjusted death rate among the Pimas was 11.9 times the rate of the United States, all races, for diabetes, 5.9 times the rate for accidents, 6.5 times the rate for cirrhosis, and 4.3 times the rate for suicide.
Pima Indian men and women with diabetes have higher death rates than those without, and the age-sex adjusted death rate among diabetic subjects was found to be 1.7 times that among nondiabetic subjects (Sievers et al., 1992). The major cause of this higher risk of death among diabetic subjects is increased deaths from kidney disease, ischemic heart disease, and infections. A study among Oklahoma Indians (Lee et al., 1993a) also confirmed higher death rates among diabetic than nondiabetic subjects and demonstrated that on average, Oklahoma Indians developing
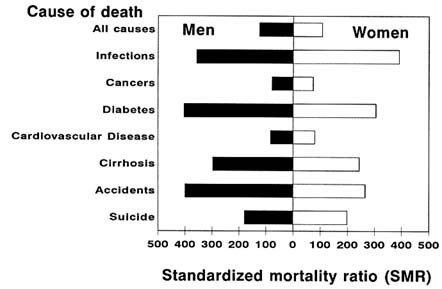
FIGURE 12-6 Standardized mortality ratios (SMR) among 1,572 male and 1,690 female members of the Seneca Nation of Indians in New York State, by cause of death, 19551984. Cause of death codes are from ICD-9, International Classification of Diseases, 9th Revision: all causes (001-999), infectious diseases (001-139), cancers (140-208), diabetes (250), cardiovascular disease (390-459), cirrhosis (571), accidents and injuries (800-949, 970-999), and suicides (950-959). SMR is the ratio (expressed as a percentage) of the number of deaths observed among male and female members of the Seneca Nation to the number that would be expected if this population had the same age-specific death rates as the New York State population. Expected number of deaths was calculated on the basis of New York State mortality rates, exclusive of New York City, for 1960 1970, and 1980. SOURCE: Adapted from Mahoney et al. (1989).
diabetes before the age of 40 lived 16.5 years less than the general population of Oklahoma.
There is considerable variation among Native American tribes in diabetes mortality rates. The IHS area-specific diabetes mortality rates for 1984-1986, without accounting for underreporting of diabetes and Native American heritage in vital statistics, ranged from 10 to 93/100,000, compared with 15/100,000 for the U.S. general population. When underreporting of diabetes and Native American heritage were accounted for, the age-adjusted mortality rate for diabetes as the underlying cause of death for Native Americans (96/100,000) was 4.3 times that for whites and twice that for blacks (Newman et al., 1993a).
Data suggest that there has been a dramatic increase in diabetes mortality
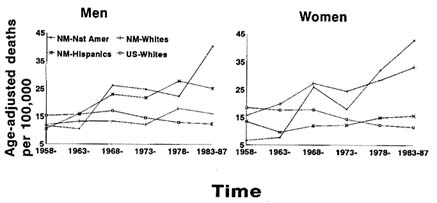
FIGURE 12-7 Age-adjusted diabetic mortality rates for men and women by six 5-year time periods, 1958-1962, 1963-1967, 1968-1972, 1973-1977, 1978-1982, and 1983-1987, comparing three ethnic groups in New Mexico: Native Americans (NM-Nat Amer), Non-Hispanic whites (NM-Whites), and Hispanic whites (NM-Hispanics). U.S. white age-adjusted rates for the same period are also presented. SOURCE: Adapted from Carter et al. (1993).
rates among Native Americans (Carter et al., 1993). Figure 12-7 shows the age-adjusted sex-specific diabetes mortality rates over six 5-year time periods during 1958-1987 for three racial groups in New Mexico, along with rates for U.S. whites for comparison. Among both men and women, Native Americans had the highest diabetes mortality rates in New Mexico starting around the beginning of the 1980s. Furthermore, age-adjusted mortality rates for Native American women, which were the lowest for any group in 1958, increased by 5.5 times over the 30-year period; during the same period, rates for Native American men increased by 2.5 times. In contrast to the increasing diabetes mortality rates among minority groups in New Mexico, the rates among both white men and women in the United States have decreased over time (Carter et al., 1993).
End-Stage Renal Disease (ESRD)
ESRD is an important public health problem for Native Americans, and nearly 60 percent of these cases occur in people with diabetes (Newman et al., 1990; Muneta et al., 1993). Between 1983 and 1987, the number of Native Americans with diabetes referred to the Medicare ESRD treatment program in the United States increased by 61 percent, and the annual incidence of diabetic ESRD increased by 47 percent, from 80.6/million to 118.2/million (Muneta et al., 1993). In 1987, the age-adjusted
incidence rate of diabetic ESRD for Native Americans was 6.8 times that for whites and 1.8 times that for blacks (Muneta et al., 1993).
A population-based study among the Pima Indians found that the age-adjusted incidence of ESRD in subjects with diabetes was 62 times the rate in those without, and that 95 percent of ESRD among the Pimas occurred in diabetic subjects (Nelson et al., 1988a). Among diabetic subjects, the incidence of ESRD was related to duration of diabetes, and at 20 years' duration, 15 percent of subjects had developed ESRD. Furthermore, the incidence rates of ESRD among diabetic Pimas were 14 times as high as the estimates for the U.S. diabetic population for those aged 45-64 and 10 times as high for those aged 65 and older.
Navajo Indians had an age-adjusted incidence of diabetic ERSD 9.6 times that among U.S. whites. The incidence of ESRD in this population increased almost 10-fold from 18 per million in 1971 to 176 per million in 1985, and much of this increase was due to diabetic kidney disease (Megill et al., 1988). During the same 15-year period, the prevalence of ESRD among the Navajos increased 26-fold, from 27 per million in 1971 to 700 per million in 1985.
Zuni Indians had an average annual incidence of ESRD of 722 per million and a prevalence of 2902 per million in 1983. However, a large proportion of ESRD among Zunis was due to other diseases, and diabetes was responsible for only 24 percent of the prevalent cases (Pasinski and Pasinski, 1987).
High rates of ESRD have also been reported among several other Native American tribes. It was found that 88 percent of ESRD among the Cherokee Indians was attributable to diabetes, and the incidence of ESRD caused by diabetes was 2.5 times the rate reported among the U.S. Native American population (Quiggins and Farrell, 1993) and 6 times the rate among the U.S. white population (Farrell et al., 1993). The age-adjusted incidence rates of ESRD among Sioux Indians were 13.5 times the rates among U.S. whites (Stahn et al., 1993). Even among Alaska Natives, who have relatively low rates of diabetes, the incidence of ESRD is higher than that in the U.S. general population (Schraer et al., 1993).
ESRD is associated with considerable morbidity and substantial loss in quality of life. The average life expectancy for ESRD patients at age 49 and 59 years is 75 to 80 percent lower than that of the U.S. general population. Furthermore, treatment of ESRD is costly. The cost was estimated at an average of $44,800 per Medicare patient per year in 1990, which did not include a number of medical and nonmedical direct cost items, such as patient travel, almost all outpatient drugs, and lost labor production in and out of home (U.S. Department of Health and Human Services, 1993).
Lower-Extremity Amputation (LEA)
There have been a number of studies of amputations using IHS hospital statistics. Subjects with diabetes are at increased risk of amputation, and over 85 percent of IHS hospitalizations for LEA occur among diabetic patients. The risk of LEA varies by age. Compared with nondiabetic subjects of a similar age, the incidence of LEA among diabetic Native Americans is 160-fold higher in subjects aged 15-44, 52-fold higher in those aged 45-64, and 19-fold higher in those over age 65. During 1982-1987, the average annual age-adjusted incidence rates of all LEAs among diabetic subjects in the Tucson (240.8/10,000), Phoenix (203.1/10,000), Oklahoma (87.3/10,000), and Navajo (74.0/10,000) IHS areas were higher than the U.S. rate (73.1/10,000) (Valway et al., 1993). A study of 10 selected Indian reservations in the Pacific Northwest in 1989 found a prevalence of LEA of 4 percent among diabetic subjects (Freeman and Hosey, 1993), but in another study, 10.3 percent of known diabetic individuals had a history of LEA (Wirth et al., 1993). In one IHS area, diabetic subjects with amputations (32.5 visits/patient/year) visited the hospital 6 times more often than patients without diabetes (5.4 visits/patient/year) and 2.3 times more often than diabetic subjects without amputation (14.1 visits/patient/year) (Wirth et al., 1993). Among Cherokee Indians, diabetic patients had a 3 times higher rate of LEA than the rate for the United States (Farrell et al., 1993), and the Sioux Indians had a 1.5 times higher rate (Stahn et al., 1993).
A population-based 12-year follow-up study among the Pima Indians found that the incidence of LEA among diabetic subjects (10.4 per 1000 PYR) was over 100 times that among nondiabetic subjects (0.1 per 1000 PYR) (Nelson et al., 1988b). LEA rates were higher among men and increased with age and duration of diabetes. Diabetic subjects with amputation had an age-adjusted death rate 1.6 times higher than the rate among nonamputees; on average, 61 percent of diabetic subjects survived 5 years after their first LEA. Furthermore, the age-sex adjusted rate of LEA among diabetic Pima Indians was 3.7 times that found in a study by the Centers for Disease Control in six U.S. states (Nelson et al., 1988b).
Diabetic Oklahoma Indians had an LEA incidence of 18.0/1000 PYR, which was higher than the rate found among the Pima Indians (Lee et al., 1993b). Similar to the findings for the Pimas, the LEA incidence among diabetic Oklahoma subjects was higher in men and increased with age and duration of diabetes. The 5-year survival rate after the first LEA was only 40.4 percent among diabetic Oklahoma Indians; the main causes of death among amputees were diabetes, cardiovascular disease, and renal disease.
Potential For Prevention
Prevention of NIDDM
There are two broad approaches available for preventing any disease: a ''high-risk strategy," which seeks to identify high-risk susceptible individuals and offer them some individual protection, and a "population strategy," which seeks to control the determinants of incidence among the population as a whole (Rose, 1985).
High-Risk Strategy
Given our current understanding of the pathogenesis of NIDDM, it is possible to identify individuals who are at high risk of developing the disease within 5 or 10 years. People with impaired glucose tolerance, defined as hyperglycemia during an oral glucose tolerance test, but not of sufficient degree for a diagnosis of NIDDM (World Health Organization, 1985), comprise an easily recognizable group at very high risk of developing NIDDM. Behavioral approaches aimed at modifying diet and exercise habits and a number of drug interventions offer potential means of delaying or preventing the progression from impaired glucose tolerance to NIDDM (see Knowler and Narayan, 1994; Knowler et al., 1995). These interventions merit testing, and a national, multicenter, randomized NIDDM prevention clinical trial in the United States is currently under way. This trial is expected to recruit about 4000 subjects from over 20 centers across the United States, and will include about 500 Native Americans with impaired glucose tolerance.
Population Strategy
For a disease such as NIDDM that is influenced by major life-style, cultural, and societal factors, a population strategy may be the most effective way of delivering prevention, particularly in communities like those of many Native Americans, where the incidence and prevalence of the disease is very high, and the risk is spread broadly across most of the community. A population strategy requires active community involvement and coordinated action by a number of agencies and thus can be organizationally demanding. Evaluation of such a strategy may also present special challenges because of the difficulty involved in performing controlled experiments. However, given the magnitude of NIDDM among Native American populations and the nature of the major risk factors involved, it would be well worthwhile to test carefully planned population-based life-style interventions in selected communities along
the lines of some of the successful coronary heart disease prevention studies (World Health Organization, 1981; Farquhar et al., 1983; Farquhar, 1984; Lefebrve et al., 1987). Such action would be a further step forward from some preliminary efforts already evident in a few communities (Leonard et al., 1986; Heath et al., 1987; Heath et al., 1991).
Potential Benefit from Prevention
The impact of NIDDM on various adverse outcomes can be quantified by calculation of attributable risk fraction (AF) and population attributable risk fraction (PARF) (Miettinen, 1974). Suitable data for the Pima Indians were used to assess the potential impact of diabetes prevention on mortality, ESRD, and LEA, with the results shown in Table 12-2. AF and PARF were estimated as suggested by Levin (1953); the details of the computation are shown in the footnotes to Table 12-2. Among the Pimas, diabetes is associated with an excess incidence of 14.3 percent of all deaths, 92.1 percent of ischemic heart disease deaths, 94.4 percent of ESRD, and 96.6 percent of LEA.
The potential for decreasing the incidence of mortality, ESRD, and LEA by reducing the current prevalence of diabetes (27.6 percent) among
TABLE 12-2 Potential for Reduction of NIDDM Complications by Preventing Diabetes in Pima Indians
|
Complication |
RRa |
AFb(%) |
PARFc(%) |
|
Mortality (Sievers et al., 1992) |
|||
|
All causes |
1.6 |
37.5 |
14.3 |
|
All natural causes |
1.7 |
41.2 |
16.2 |
|
Ischemic heart disease |
43.4 |
97.7 |
92.1 |
|
Stroke |
1.3 |
23.1 |
7.7 |
|
Infectious diseases |
1.3 |
23.1 |
7.7 |
|
End-stage renal disease (Nelson et al., 1988a) |
62.0 |
98.4 |
94.4 |
|
Lower-extremity amputation (Nelson et al., 1988b) |
104.0 |
99.0 |
96.6 |
|
a RR = Risk ratio (ratio of incidence of complication in diabetic subjects relative to nondiabetic subjects). b AF = Attributable or etiologic fraction (the proportion of the complication that can be attributable to diabetes): RR-1/RR. c PARF = Population attributable risk fraction or prevented fraction (the proportion of incidence of the complication in the population associated with diabetes): P(RR - 1)/1 + P(RR - 1), where P is the prevalence of diabetes, which was 27.6 percent during 1984-1994. |
|||
TABLE 12-3 Estimated Percentage Reductions in the Incidence of Various Diabetes Complications If Current Prevalence of Diabetes Were Reduced to Five New Target Levels.
|
|
Expected % reduction in incidenceb if diabetes prevalence were reduced to: |
|||||
|
Complication |
PARF1a(%) |
25% |
20% |
15% |
10% |
5% |
|
All-causes mortality |
14.3 |
1.5 |
4.0 |
6.5 |
9.1 |
11.7 |
|
Natural-causes mortality |
16.2 |
1.5 |
4.5 |
7.4 |
10.4 |
13.3 |
|
IHD mortality |
92.1 |
8.1 |
24.8 |
41.9 |
58.6 |
75.4 |
|
Stroke mortality |
7.7 |
0.8 |
2.1 |
3.6 |
4.9 |
6.3 |
|
Infectious disease mortality |
7.7 |
0.8 |
2.1 |
3.6 |
4.9 |
6.3 |
|
End-stage renal disease |
94.4 |
9.7 |
26.3 |
43.4 |
60.3 |
77.3 |
|
Lower-extremity amputation |
96.6 |
8.1 |
27.7 |
44.3 |
61.4 |
79.1 |
|
a PARF1 = Population attributable risk fraction under the current diabetes prevalence of 27.6 percent. b Expected (%) reduction in incidence = {1 - (PARF1/1 - PARF2)}*100, where PARF2 is PARF at reduced prevalence of diabetes. |
||||||
the Pimas to five target levels of 25, 20, 15, 10, and 5 percent was estimated as shown in the footnotes for Table 12-3 (Ruta et al., 1993). These estimates assume that diabetes and other potential risk factors bear a constant relationship with mortality, ESRD, and LEA at all levels of diabetes prevalence. As shown in Table 12-3, even small decreases in the prevalence of diabetes can result in considerable reduction in diabetes complications, particularly ESRD and LEA. Furthermore, if the prevalence of diabetes among the Pimas could be reduced to 6.6 percent, the rate among the U.S. general population, the following reduction in the incidence of diabetes complications could result: 10.9 percent of deaths from all causes, 74.7 percent of deaths from ischemic heart disease, 71.9 percent of ESRD, and 73.9 percent of LEA.
Prevention of diabetes is therefore worth considering. However, as in most real-life healthcare situations, the question may not be simply whether diabetes is worth preventing, but rather how much diabetes is worth preventing, given resource constraints? The kind of information presented in Table 12-3 allows the estimation of marginal benefits (for example, the extra benefit from reduction in the incidence of complications if the prevalence of diabetes were reduced by an extra 5 percent). Such estimation can support policy decisions, especially if it can be combined with data on estimated costs of achieving a given reduction in the prevalence of diabetes.
Prevention of Complications
In addition to the potential reduction in complications that could result from preventing NIDDM, a number of other measures could prevent or delay the onset of complications in subjects with diabetes.
Early Detection of NIDDM
A large proportion of subjects with NIDDM remain undiagnosed (Harris et al., 1987) and might benefit from early detection and treatment. However, there are a number of questions concerning which people would benefit from such early-detection activities. As noted earlier, being a Native American is a major risk factor for developing NIDDM. According to the American Diabetes Association (1989), all individuals with at least one risk factor for NIDDM should be identified through community screening programs (defined as screening not performed under the direct and close supervision of a physician), and individuals indicating symptoms should be referred for medical evaluation.
Blood Glucose Control
Complications of NIDDM in Native Americans are related to the concentration of blood glucose (Dorf et al., 1976; Pettitt et al., 1980; Nelson et al., 1989; Lee et al., 1992). It has been shown that tight control of blood glucose can prevent or delay the onset of complications, but this evidence is based on subjects with insulin-dependent diabetes mellitus (IDDM) (The Diabetes Control and Complications Trial Research Group, 1993). However, it is believed that the beneficial effect of glucose control in forestalling complications of IDDM may also apply to NIDDM (Weir et al., 1994). What is not clear is the means of achieving blood glucose control and their relative benefits and risks.
Early Detection and Management of Complications
Structured approaches aimed at early detection and management of hypertension, dyslipidemia, retinopathy, lower extremity problems, and nephropathy are also known to be of benefit in limiting the complications of diabetes (Weir et al., 1994). Essential features of many of these activities are good coordination, active patient involvement, a multidisciplinary team approach, acceptance of standards of care, and regular evaluation. A number of such programs have been implemented successfully within IHS facilities (Acton et al., 1993a; Newman et al., 1993b).
Research To Facilitate Prevention Of Niddm And Its Complications
Understanding of the genetics of NIDDM among Native Americans is important and may lead to better identification of high-risk subjects and possibly to the development of newer treatments. Similarly, a well-designed clinical trial evaluating the efficacy and effectiveness of interventions aimed at preventing or delaying the progression from impaired glucose tolerance to NIDDM in Native Americans is also a priority. In addition to these topics, which are already receiving substantial attention, three key research areas can be emphasized.
Population-based Primary Prevention
Given the high prevalence of diabetes in Native American communities and the nature of the risk factors involved, much can be gained from implementing and evaluating a well-designed population-based life-style intervention study in selected communities. Such approaches are likely to complement clinical trials among high-risk individuals.
Blood Glucose Control
Multicenter studies in Native American communities aimed at understanding optimal ways of achieving blood glucose control in NIDDM subjects would facilitate the prevention of diabetic complications.
Economic Appraisal
Economic appraisal can assist in the optimal allocation of resources aimed at improving health. The total economic and social costs of diabetes and its complications among Native Americans should be estimated, and detailed assessments of the marginal costs and marginal benefits of various interventions should be undertaken.
References
Acton, K., S. Valway, S. Helgerson, J.D. Huy, K. Smith. V. Chapman, and D. Gohdes 1993a Improved diabetes care for American Indians. Diabetes Care 16 (suppl. 1):372-375.
Acton, K., B. Rogers, G. Campbell, C. Johnson, and D. Gohdes 1993b Prevalence of diagnosed diabetes and selected related conditions six reservations in Montana and Wyoming. Diabetes Care 16 (suppl. 1):263-266.
American Diabetes Association 1989 Screening for diabetes: Position statement. Diabetes Care 12(8):588-590.
Benjamin, E., J. Mayfield, and D. Gohdes 1993 Exercise and incidence of NIDDM among Zuni Indians (abstract). Diabetes 42 (suppl. 1):203A.
Bennett, P.H., T.A. Burch, and M. Miller 1971 Diabetes mellitus in American (Pima) Indians. Lancet ii:125-128.
Bennett, P.H., W.C. Knowler, H.R. Baird, W.J. Butler, D.J. Pettitt, and J.M. Reid 1984 Diet and development of non-insulin-dependent diabetes mellitus: an epidemiological perspective. Pp. 109-119 in G. Pozza, P. Micossi, A.L. Catapano, and R. Paoletti, eds., Diet, Diabetes and Atherosclerosis . New York: Raven Press.
Broussard, B.A., A. Johnson, J.H. Hines, M. Story, R. Fichtner, F. Hanck, K. Bachman-Carter, J. Hayes, K. Frohlich, N. Gray, S. Valway, and D. Gohdes 1991 Prevalence of obesity in American Indians and Alaska Natives. American Journal of Clinical Nutrition 53:1535-42S.
Brousseau, J.D., R.C. Eelkema, A.C. Crawford, and T.A. Abe 1979 Diabetes among three affiliated tribes: Correlation with degree of Indian inheritance. American Journal of Public Health 69:1277-1278.
Cameron, W.I., P. Moffit, and D.R.R. Williams 1986 Diabetes mellitus in the Australian Aborigines of Bourke, New South Wales. Diabetes Research and Clinical Practice 2:307-314.
Carter, J., R. Horowitz, and R. Wilson 1989 Tribal differences in diabetes: Prevalence among American Indians in New Mexico. Public Health Reports 104:665-669.
Carter, J.S., C.L. Wiggins, T.M. Becker, C.R. Key, and J.M. Samet 1993 Diabetes mortality among New Mexico's American Indian, Hispanic, and non-Hispanic White populations, 1958-1987. Diabetes Care 16 (suppl. 1):306-309.
Castetter, E.F., and W.H. Bell 1942 Pima and Papago Indian Agriculture. Albuquerque, NM: University of New Mexico Press. Reprinted 1980.
The Diabetes Control and Complications Trial Research Group 1993 The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The New England Journal of Medicine 329(14):977-986.
Dorf, A., E.J. Ballintine, P.H. Bennett, and M. Miller 1976 Retinopathy in Pima Indians: Relationship to glucose level, duration of diabetes, and age at examination in a population with a high prevalence of diabetes mellitus. Diabetes 25(7):554-560.
Drevets, C.C. 1965 Diabetes mellitus in Choctaw Indians. Journal of Oklahoma State Medical Association 58:322-329.
Eriksson, K.F., and F. Lindgärde 1991 Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise: The 6-year Malmö feasibility study. Diabetologia 34:891-898.
Everhart, J.E., D.J. Pettitt, P.H. Bennett, and W.C. Knowler 1992 Duration of obesity increases the incidence of NIDDM. Diabetes 41:235-240.
Farrell, M.A., P.A. Quiggins, J.D. Eller, P.A. Owle, K.M. Miner, and E.S. Walkingtick 1993 Prevalence of diabetes and its complications in the Eastern band of Cherokee Indians. Diabetes Care 16 (suppl. 1):253-256.
Farquhar, J.W., S.P. Fortmann, P.D. Wood, and W.L. Haskell 1993 Community studies of cardiovascular disease prevention. Pp. 170-181 in N.M. Kaplan, ed., Prevention of Coronary Heart Disease: Practical Management of Risk Factors. Philadelphia: W.B. Saunders.
Farquhar, J.W. 1984 The Stanford Five City Project: An overview. Pp. 1154-1164 in J.D. Matarazzo, ed., Behavioral Health: A Handbook of Health Enhancement and Disease Prevention. New York: John Wiley.
Freeman, W.L., G.M.H. Hosey, P. Diehr, and D. Gohdes 1989 Diabetes in American Indians of Washington, Oregon, and Idaho. Diabetes Care 12(4):282-669.
Freeman, W.L., and G.M. Hosey 1993 Diabetic complications among American Indians of Washington, Oregon, and Idaho. Diabetes Care 16(suppl. 1):357-360.
Frisch, R.E., G. Wyshak, T.E. Albright, N.L. Albright, and I. Schiff 1986 Lower prevalence of diabetes in female former athletes compared with nonathletes. Diabetes 35:1101-1105.
Gohdes, D.M. 1986 Diabetes in American Indians: A growing problem. Diabetes Care 9(6):609-6130.
Gulabkunverba, ed. 1949 Charaka Samhita (600 B.C.). Jamnagar, India: Ayurvedic Society.
Hall, T.R., M.E. Hickey, and T.B. Young 1991 The relationship of body fact distribution to non-insulin-dependent diabetes in a Navajo Community. American Journal of Human Biology 3:119-126.
1992 Evidence for recent increases in obesity and non-insulin-dependent diabetes mellitus in a Navajo Community. American Journal of Human Biology 4:547-553.
Harris, M.I., W.C. Hadden, W.C. Knowler, and P.H. Bennett 1987 Prevalence of diabetes and impaired glucose tolerance and plasma glucose level in U.S. population aged 20-74 yr. Diabetes 36:523-534.
Heath, G.W., B.E. Leonard, R.H. Wilson, J.S. Kendrick, and K.E. Powell 1987 Community-based exercise intervention: Zuni diabetes project. Diabetes Care 10:579-583.
Heath, G.W., R.H. Wilson, J. Smith, and B.E. Leonard 1991 Community-based exercise and weight control: Diabetes risk reduction and glycemic control in Zuni Indians. American Journal of Clinical Nutrition (suppl. 1):1642S-1646S.
Helmrich, S.P., D.R. Ragland, R.W. Leung, and F.S. Paffenbarder 1991 Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. New England Journal of Medicine 325:147-152.
Jackson, M.Y. 1994 Diet, culture, and diabetes. Pp. 381-406 in J.R. Joe and R.S. Young, eds. Diabetes As a Disease of Civilization: The Impact of Culture Change on Indigenous Peoples. New York: Mouton de Gruyter.
Joslin, E.P. 1940 The university of diabetes. Journal of the American Medical Association 115:2033-2038.
King, H., and M. Rewers 1991 Diabetes in adults is now a Third World problem. Bulletin of the World Health Organization 69(6):643-648.
Knowler, W.C., P.H. Bennett, R.F. Hammam, and M. Miller 1978 Diabetes incidence and prevalence in Pima Indians: a 19-fold greater incidence than in Rochester, Minn. American Journal of Epidemiology 108:497-504.
Knowler, W.C., D.J. Pettitt, P.J. Savage, and P.H. Bennett 1981 Diabetes incidence in Pima Indians. Contributions of obesity and parental diabetes. American Journal of Epidemiology 113:144-156.
Knowler, W.C., R.C. Williams, D.J. Pettitt, A.G. Steinberg 1986 GM3;5,13,14 and type 2 diabetes mellitus: An association in American Indians with genetic admixture. American Journal of Human Genetics 39:409-413.
Knowler, W.C., D.J. Pettitt, M.F. Saad, and P.H. Bennett 1990 Diabetes mellitus in the Pima Indians: Incidence, risk factors and pathogenesis. Diabetes/Metabolism Reviews 6(1):1-27.
Knowler, W.C., D.J. Pettitt, M.F. Saad, M.A. Charles, R.G. Nelson, B.V. Howard, C. Bogardus, and P.H. Bennett 1991 Obesity in the Pima Indians: Its magnitude and relationship with diabetes. American Journal of Clinical Nutrition 53:1543-51S.
Knowler, W.C., D.R. McCance, D.K. Nagi, and D.J. Pettitt 1993 Epidemiologic studies of the causes of non-insulin-dependent diabetes mellitus. Pp. 187-218 in R.D.G. Leslie, ed., Causes of Diabetes . Sussex, England: John Wiley and Sons.
Knowler, W.C., and K.M.V. Narayan 1994 Prevention of non-insulin-dependent diabetes mellitus. Preventive Medicine 23(5):701-703.
Knowler, W.C., K.M.V. Narayan, R.L. Hanson, R.G. Nelson, P.H. Bennett, J. Tuomilheto, B. Scherstèn, and D.J. Pettitt 1995 Preventing non-insulin-dependent diabetes mellitus. Diabetes 44:483-488.
Kriska, A.M., R.E. LaPorte, D.J. Pettitt, M.A. Charles, R.G. Nelson, L.H. Kuller, P.H. Bennett, and W.C. Knowler 1993 The association of physical activity with obesity, fat distribution and glucose intolerance in Pima Indians. Diabetologia 36:863-869.
Lee, E.T., P.S. Anderson, J. Bryan, C. Bahr, T. Coniglione, and M. Cleves 1985 Diabetes, parental diabetes, and obesity in Oklahoma Indians. Diabetes Care 8:107-113.
Lee, E.T., V.S. Lee, R.M. Kingsley, M. Lu, D. Russell, N.R. Asal, C.P. Wilkinson, and R.H. Bradford 1992 Diabetic retinopathy in Oklahoma Indians with NIDDM: Incidence and risk factors. Diabetes Care 15(11):1620-1627.
Lee, E.T., D. Russell, N. Jorge, S. Kenny, and M. Yu 1993a A follow-up study of diabetic Oklahoma Indians: Mortality and causes of death. Diabetes Care 16(suppl. 1):300-305.
Lee, J.S., M. Lu, V.S. Lee, D. Russell, C. Bahr, and E.T. Lee 1993b Lower-extremity amputation: Incidence, risk factors, and mortality in the Oklahoma Indian diabetes study. Diabetes 42:876-882.
Lee, E.T., B.V. Howard, P.J. Savage, L.D. Cowan, R.R. Fabsitz, A.J. Oopik, J. Yeh, O. Go, D.C. Robbins, and T.K. Welty 1995 Diabetes and impaired glucose tolerance in three American Indian Populations aged 45-74 years: The Strong Heart Study. Diabetes Care 18(5):599-609.
Lefebvre, R.C., R.M. Lasater, R.A. Carleton, and G. Paterson 1987 Theory and delivery of health programming in the community. Preventive Medicine 16:80-95.
Leonard, B.E., C. Leonard, and R.H. Wilson 1986 Zuni diabetes project. Public Health Reports 101:282-288.
Levin, M.L. 1953 The occurrence of lung cancer in man. Acta Unio Internationalis Contra Cancrum 9:531-541.
Lippincott, J.B. 1980 Storage of Water on Gila River, Arizona. Water Supply Paper No. 33. Washington, DC: U.S. Geological Survey.
Mahoney, M.C., A.M. Michalek, K.M. Cummings, P.C. Nasca, and L.J. Emrich 1989 Mortality in a Northeastern Native American Cohort, 1955-1984. American Journal of Epidemiology 129(4):816-825.
Manson, J.E., E.B. Rimm, M.J. Stampfer, G.A. Colditz, W.C. Willett, A.S. Krolewski, B. Rosner, C.H. Hennekens, and F.E. Speizer 1991 Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 338:774-778.
Manson, J.E., D.M. Nathan, A.S. Krolewski, M.J. Stampler, W.C. Willet, and C.H. Hennekens 1992 A prospective study of exercise and incidence of diabetes among U.S. male physicians. Journal of the American Medical Association 268:63-67.
Mao, Y., H. Morrison, R. Semenciw, and D. Wigle 1986 Mortality on Canadian Indian Reserves, 1977-1982. Canadian Journal of Public Health 77:263-268.
Marshall, J.A., S. Hoag, S. Shetterly, and R.F. Hamman 1994 Dietary fat predicts conversion from impaired glucose tolerance to NIDDM. Diabetes Care 17:50-56.
Megill, d.M., W.E. Hoy, and S.D. Woodruff 1988 Rates and causes of end-stage renal disease in Navajo Indians, 1971-1985. Western Journal of Medicine 149:178-182.
Miettinen, O.S. 1974 Proportion of disease caused or prevented by a given exposure, trait or intervention. American Journal of Epidemiology 99:325-332.
Muneta, B., J. Newman, J. Stevenson, and P. Eggers 1993 Diabetic end-stage renal disease among Native Americans. Diabetes Care 16 (suppl. 1):346-348.
Nelson, R.G., J.M. Newman, W.C. Knowler, M.L. Sievers, C.L. Kunzelman, D.J. Pettitt, and C.D. Moffett 1988a Incidence of end-stage renal disease in Type 2 (non-insulin-dependent) diabetes mellitus in the Pima Indians. Diabetologia 31:730-736.
Nelson, R.G., D.M. Gohdes, J.E. Everhart, J.A. Hartner, F.L. Zwemer, D.J. Pettitt, and W.C. Knowler 1988b Lower-extremity amputations in NIDDM: 12-year follow-up study in Pima Indians. Diabetes Care 11(1):8-16.
Nelson, R.G., J.A. Wolfe, M.B. Horton, D.J. Pettitt, P.H. Bennett, and W.C. Knowler 1989 Proliferative retinopathy in NIDDM: Incidence and risk factors in Pima Indians. Diabetes 38:435-440
Newman, J.M., A.A. Marfin, P.W. Eggers, and S.D. Helgerson 1990 End State Renal Disease among Native Americans, 1983-86. American Journal of Public Health 80(3):318-319.
Newman, J.M., F. DeStefano, S.E. Valway, R.R. German, and B. Muneta 1993a Diabetes-associated mortality in Native Americans. Diabetes Care 16 (suppl. 1):297-299.
Newman, W.P., J.J. Hollevoet, and K.L. Frohlich 1993b The diabetes project at Fort Totten, North Dakota, 1984-1988. Diabetes Care 16 (suppl. 1):361-363.
Pablo, S.G. 1983 Contemporary Pima. Pp. 212-216 in A. Ortiz, ed., Handbook of North American Indians. Vol 10. Washington, D.C.: Smithsonian Institute.
Pasinski, R., and M. Pasinski 1987 End-stage renal disease among Zuni Indians: 1973-1983. Archives of Internal Medicine 147:1093-1096.
Pettitt, D.J., J.R. Lisse, W.C. Knowler, and P.H. Bennett 1980 Development of retinopathy and proteinuria in relation to plasma-glucose concentrations in Pima Indians. Lancet ii:1050-1052.
1982 Mortality as a function of obesity and diabetes mellitus. American Journal of Epidemiology 115:359-366.
Prior, I.A.M., and Tasman-Jones 1981 New Zealand Maori and Pacific Polynesians. Pp. 227-267 in H.C. Trowell, and D.D. Bunkitt, eds., Western Diseases: Their Emergence and Prevention. London: Edward Arnold Publishers.
Prochazka, M., S. Lillioja, J.F. Tait, W.C. Knowler, D.M. Mott, M. Spraul, P.H. Bennett, and C. Bogardus 1993 Linkage of chromosomal markers on 4q with a putative gene determining maximal insulin action in Pima Indians. Diabetes 42:514-519.
Prochazka, M., B. Thompson, S. Scherer, L. Tsui, W. Knowler, P. Bennett, and C. Bogardus. 1995 Linkage and association of markers at 7q21.3q22.1 with insulin resistance and NIDDM in the Pima Indians (abstract). Diabetes 44 (suppl. 1):42A
Program Statistics Branch 1986 Indian Health Service Chart Series Book. Rockville, MD: DHHS, PHS, Indian Health Service.
Quiggins, P.A., and M.A. Farrell 1993 Renal disease among the Eastern band of Cherokee Indians. Diabetes Care 16 (suppl. 1):342-345.
Rith-Najarian, S.J., S.E. Valway, and D.M. Gohdes 1993 Diabetes in a Northern Minnesota Chippewa tribe. Diabetes Care 16 (suppl. 1):266-270.
Rose, G. 1985 Sick individuals and sick populations. International Journal of Epidemiology 14:32-38.
Ruta, D., T. Beattie, and V. Narayan 1993 A prospective study of non-fatal childhood road traffic accidents: What can seat restraint achieve? Journal of Public Health Medicine 15 (1):88-92.
Saad, M.F., W.C. Knowler, D.J. Pettitt, R.G. Nelson, D.M. Mott, and P.H. Bennett 1988 The natural history of impaired glucose tolerance in the Pima Indians. New England Journal of Medicine 319:1500-06.
Schraer, C.D., L.R. Bulkow, N.J. Murphy, and A.P. Lanier 1993 Diabetes prevalence, incidence and complications among Alaska Natives, 1987. Diabetes Care 16 (suppl. 1):257-259.
Schranz, A., J. Tuomilehto, B. Marti, R.J. Jarrett, V. Grabanskas, and A. Vassallo 1991 Low physical activity and worsening of glucose tolerance: Results from a 2-year follow-up of a population sample in Malta. Diabetes Research and Clinical Practice 11:127-136.
Sievers, M.L., and J.R. Fisher 1985 Diabetes in North American Indians. Pp. XI. 1-20 in Diabetes in America. NIH Publication No. 85-1468. Bethesda, MD: U.S. Department of Health and Human Services.
Sievers, M.L., R.G. Nelson, and P.H. Bennett 1990 Adverse mortality experience of a Southwestern American Indian Community: Overall death rates and underlying causes of death in Pima Indians. Journal of Clinical Epidemiology 43(11):1231-1242.
Sievers, M.L., R.G. Nelson, W.C. Knowler, and P.H. Bennett 1992 Impact of NIDDM on mortality and causes of death in Pima Indians. Diabetes Care 15(11):1541-1549.
Smith, C.J., R.G. Nelson, S.A. Hardy, E.A. Manahan, P.H. Bennett, and W.C. Knowler 1996 A survey of the dietary intake of the Pima Indians. Journal of American Dietetic Association (In Press)
Stahn, R.M., D. Gohdes, and S.E. Valway 1993 Diabetes and its complications among selected tribes in North Dakota, South Dakota, and Nebraska. Diabetes Care 16 (suppl. 1):244-247.
Sugarman, J.R., T.J. Gilbert, and N.S. Weiss 1992 Prevalence of diabetes and impaired glucose tolerance among Navajo Indians. Diabetes Care 15(1):114-120.
Tuomilehto, J., W.C. Knowler, and P. Zimmet 1992 Primary prevention of non-insulin-dependent diabetes mellitus. Diabetes/Metabolism Reviews 8:339-353.
U.S. Department of Health and Human Services 1985 Report of the Secretary's Task Force on Black and Minority Health, Volumes I-VII. Washington, D.C.: U.S. Government Printing Office. 1985: Vols I, II:1986:Vols III-VII.
1993 Prevalence and cost of ESRD therapy. United States Renal Data System: 1993 Annual Data Report. Bethesda, MD: The National Institutes of Health, NIDDK.
Valway, S., W. Freeman, and S. Kaufman 1993 Prevalence of diagnosed diabetes among American Indians and Alaska Natives, 1987. Diabetes Care 16 (suppl. 1):271-276
Valway, S.E., R.W. Linkins, and D.M. Gohdes 1993 Epidemiology of lower-extremity amputations in the Indian Health Service, 1982-1987. Diabetes Care 16 (suppl. 1):349-353.
Warne, D.K., M.A. Charles, R.L. Hanson, L.T.H. Jacobson, D.R. McCance, W.C. Knowler, and D.J. Pettitt 1995 Comparison of body size measurements as predictors of NIDDM in Pima Indians. Diabetes Care 18(4):435-439.
Weir, G.C., D.M. Nathan, and D.E. Singer 1994 Standards of care. Diabetes Care 17(12):1514-1522.
West, K.M. 1974 Diabetes in American Indians and other native populations of the New World. Diabetes 23:841-55.
Williams, R.C., W.C. Knowler, W.J. Butler, D.J. Pettitt, J.R. Lisse, P.H. Bennett, D.L. Mann, A.H. Johnson, P.I. Terasaki 1981 HLA-A2 and type 2 diabetes mellitus in Pima Indians: An association of allele frequency with age. Diabetologia 21:460-463.
Williams, R.C., A.G. Steinberg, H. Gershowitz, P.H. Bennett, W.C. Knowler, D.J. Pettitt, W. Butler, R. Baird, L. Dowda-Rea, and T.A. Burch 1985 GM allotypes in Native Americans: Evidence for three distinct migrations across the Bering Land bridge. American Journal of Physical Anthropology 66:1-19.
Wirth, R.B., A.A. Marfin, D.W. Grau, and S.D. Helgerson 1993 Prevalence and risk factors for diabetes and diabetes-related amputations in American Indians in Southern Arizona. Diabetes Care 16 (suppl. 1):354-356.
World Health Organization 1981 Community Control of Cardiovascular Diseases: The North Karelia Project. Copenhagen, Denmark: World Health Organization.
1985 Diabetes Mellitus: Report of a WHO study group. WHO Tech Rep Ser 727. Geneva, Switzerland: World Health Organization.
Young, T.K., and C. Shah 1987 Extent and magnitude of the problem. Pp. 11-25 in T.K. Young, ed. Diabetes in the Canadian Native Population: Bicultural Perspectives . Toronto: Canadian Diabetes Association.
Zimmet, P., G. Dowse, C. Finch, S. Serjeantson, and H. King. 1990 The epidemiology and natural history of NIDDM: Lessons from the South Pacific. Diabetes/Metabolism Reviews 6(2):91-124.



























