PLANT DIVERSITY IN TATRA NATIONAL PARK (SLOVAKIA)
Rudolf Soltes, Anna Soltesova, Zuzana Kyselova
Research Station of Tatra National Park
A database including hundreds of thousands of records has provided objective information on a habitat's environmental factors to help pinpoint priorities for plant conservation. Although the results are only approximate, they have integrated long-term environmental conditions and can give information presentable on a spatial projection. Unlike other time-consuming and technically-advanced methods, this method offers a prompt ecological foundation which can be expressed quantitatively. We have found that the creation of a database information system offers the most convenient method for storing data on biodiversity mapping in Tatra National Park. However, the database is only as good as the data that is fed into it. The structure of the file is as follows: species, subspecies, chassis, inventory number of the specimen, date, orographic unit, location, substratum, altitude, community, density, cover of respective layer, notes (memo field), collector, determinator, and square.
We have found that the methods recommended by Jurko (1990) are most convenient for our purpose. We have decided to use more index types because each is specific and renders information of a different type. The following selected indices will be calculated by sub-programs:
The scale diversity index Dsc (equation 1)
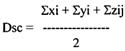
xi - values for mean number of species
yi - values for mean cover of respective layer
ziy - mean cover of sublayer
The advantage of this index is the considerable stability of the values entered and the corresponding relative constancy of the communities as well.
McNaughton's dominance index Cn (equation 2)
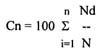
Nd - values for cover of the dominants
N - total cover
This index expresses the sum of values of dominants compared to total cover, where dominants are considered to be those species with more than 40% dominance. The function of dominants is very important, and so index Cn is especially valuable in succession studies.
Hill's diversity index (equation 3)
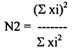
xi - the value of species significance
This index is transparent and gives a wide spectrum of values along the scale of 1 - 100. However, even highly-diversified communities do not have values higher than 50.
Shannon's index (equation 4)
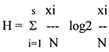
S - number of species
N - the sum of significances
Shannon's index is the function of relative cover and species significance and is logarithmically related to the number of species (xi). So, index H' is especially sensitive to the total number of species and to their coefficients of significance.
Pielouov's equitability index (equation 5)
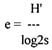
H' - Shannon's index
s - number of species
The real diversity is compared to the maximum possible, i.e., the ideal distribution of species. This index is useful in some specific investigations.
The values of these indices will be Version 2 projected on a network consisting of 90×150 m oblongs using the ARCVIEW program. The scales will be settled later and distinguished by color or pattern. To date, we have stored nearly 12,000 records, and the number is increasing constantly.
ENDANGERED SPECIES OR HABITATS OF SPECIAL IMPORTANCE
Peatland Habitats
Peatland habitats were lost in the past as a result of land reclamation, the intensification of agricultural practices, the construction of communications infrastructures, river straightening, turf cutting, etc. These habitats are suitable for many endangered and precious species and communities of vascular plants and mosses. Peatland habitats include acid bog peats, fen peats enriched by base compounds, and intermediate peats. The number and extent of peatland habitats have been greatly reduced, and protection of the remainder is thus a matter of concern. The majority already enjoy protection in protected areas covering 227 hectares. But they are still endangered by air pollution, and the extent of the harmful influence of the atmospheric deposition of acidifying agents is still unclear. Another human influence, especially in forest ecosystems, is groundwater extraction for drinking water.
The critically-endangered species Pedicularis sceptrum-carolinum is restricted to this ecotope. Despite intensive searches, this species has been confirmed at only 21 locations. The species occurs abundantly at only one location, with the others being endangered by the natural seeding of trees.
Most endangered of all is Carex chordorrhiza, which had declined in its location to one very depauperate population of some 13 sterile plants in 1993. Most likely this decline is caused by natural succession as the site is becoming dry.
Andromeda polifolia appears to be restricted in Tatra National Park to two locations. The best site, close to the major tourist resort of Strbske Pleso, is endangered by human activities, including garbage accumulation and trampling.
Ledum palustre has been confirmed at four sites, of which only one has a large enough population to survive. The others are only remnants of the previous, more extensive distribution, and two populations consist of less than five plants each.
Scheuchzeria palustris was noted in three locations, but is more abundant in only one. In the 1980's, a new species for the Tatra Mountains, Calla palustris , was found. The population is large enough to survive, and the main danger is thus the competition of other populations.
Worthy of mention are other critically-endangered vascular plants like Carex limosa, Carex lasiocarpa, Baeothryon alpinum, and Baeothryon caespitosum. Rare and endangered mosses include Paludella squarrosa, Meesia triquetra, Hypnum pratense, and Sphagnum platyphyllum. The peatland habitats represent a valuable natural heritage of Tatra National Park, and their adequate protection is an essential part of the Park's function.
Freshwater Habitats
The main features of this ecosystem are the generally low diversity and the marked vulnerability to acid rain and anthropogenic contamination.
Sparganium angustifolium is found in still water at only one site, and Ranunculus reptans is found in its splash zone. Drepanocladus trichophyllus is a submerged moss found at three locations.
Running water is the habitat of the critically-endangered species Juncus castaneus. Only three small, isolated sites are known for this species, which needs special attention to prevent its decline and disappearance from the Tatra Mountains. Some rare moss species restricted to this habitat include Racomitrium aciculare, Racomitrium aquaticum, and Fontinalis antipyretica.
Epiphytic Lichens and Mosses
Epiphytic moss and lichen species are rarely afforded any special conservation efforts because of the lack of experts. The bark of coniferous and deciduous trees is a convenient substratum for some mosses and lichens which are declining on the European scale and some species which must presently be considered extinct (the lichen Usnea longissima and the mosses Ulota rehmanii and Antitrichia curtipendula ). Some important epiphytes are restricted to deep, constantly-humid, and shaded forest stands which are protected from pollution inputs and strong winds. Such phorophytes were found on the broadleaved trees Populus tremula, Salix caprea, Alnus incana, and Betula carpatica . Important phorophytes also occur on some trees managed on parkland or trees in avenues, for example, Fraxinus excelsior, Tilia cordata, Populus tremula, Populus alba, Ulmus glabra, Betula pendula, Sorbus aucuparia, and Acer pseudoplatanus.
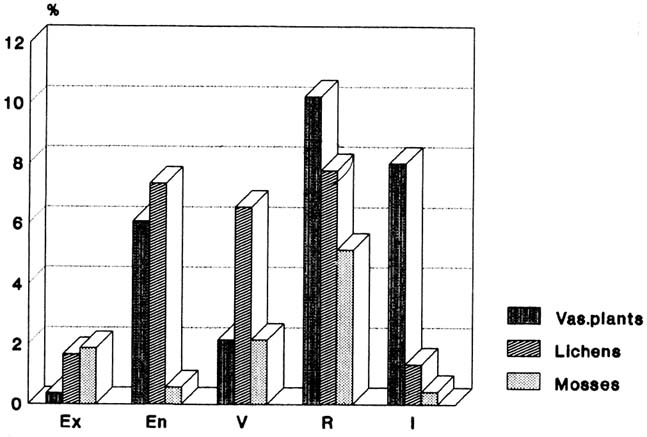
The frequency histogram of IUCN categories (Fig. 1) shows the apparently greater sensitivity of cryptogamic plants to environmental conditions (1.66% of moss species extinct and 1.86% of lichens, but only 0.6% of higher plant species).
The frequency histogram of IUCN categories (Fig. 1) shows the apparently greater sensitivity of cryptogamic plants to environmental conditions (1.66% of moss species extinct and 1.86% of lichens, but only 0.6% of higher plant species).
It can be assumed that changes in the vegetation of Tatra National Park will continue as long as habitat quality continues to be affected.
The nomenclature of mosses follows Corley et al. (1981), while that of vascular plants is after Dostal and Cervenka (1992), and that of lichens mainly after Wirth (1987) and occasionally Santensson (1984).
REFERENCES
Corley, M.F.C., A.C. Crundwell, R. Dull, M.O. Hill and A.J.E. Smith, 1981. "Mosses of Europe and the Azores; an Annotated List of Species, ith Synonyms from the Recent Literature," J. Bryol., vol. 11, pp. 609-689.
Dostal, J. and M. Cervenka, 1992. Velky kluc na urcovanie vyssich rastlin. Bratislava.
Jurko, A., 1990. Ekologicke a socioekonomicke hodnotenie vegetacie. Bratislava.
Santensson, R., 1984. The Lichens of Sweden and Norway. Stockholm and Uppsala.
Wirth, V., 1987. Die Flechten Baden-Wurtembergs. Stuttgart.
FLORISTIC DIVERSITY IN THE UKRAINIAN EASTERN CARPATHIANS AND THE UKRAINIAN PART OF THE BIOSPHERE RESERVE
Lydia Tasenkevich
State Museum of Natural History
Ukrainian Academy of Sciences
The significance of the Carpathians as a European center of floristic diversity has not yet been recognized. Only recently (particularly in the East and South) have studies begun to focus on this mountain system's influence as a floristic barrier on the one hand and a linking bridge on the other.
The Ukrainian part of the Eastern Carpathians is a typical medium-sized mountain system with mainly dome-shaped summits which are frequently united into long ranges or massifs and dissected by the deep river valleys. Only in the southeastern part of the Ukrainian Eastern Carpathians do altitudes increase, reaching 2061 m a.s.l. at Hoverla, the highest peak in the Ukrainian Carpathians. Such smoothness of relief is caused by the easily-destroyed flysch deposits that are the prevailing geological bedrocks in the Ukrainian Eastern Carpathians. Only in the southeastern part are some massifs, like the Chornohora and Marmarosh Mountains, built of the weathering-resistant sandstones and crystalline and metamorphic rocks whose relief is characterized by more severe forms. Here, peaks frequently have the form of inaccessible rocks, and the bases of the rock faces develop extensive fields of scree. Traces of former Pleistocene glacier activity (glacial cirques, valley-steps, moraines) are also particularly distinct.
The diversified geology and relief influences the richness and diversity of the vegetation cover. A number of phytogeographical units can be distinguished within the territory of the Ukrainian Eastern Carpathians. Putting aside a detailed consideration of the correctness or correspondence of the different systems of floristic and geobotanical divisions (Domin 1928, 1930; Soo 1933; Pawlowski 1948; Fodor 1960; Chopyk 1976, 1977), we accept here the system used most frequently in Ukrainian botanical literature: the one proposed by V. Chopyk (1977) and subsequently modified slightly by Tasenkevich (1986).
CURRENT STATE OF KNOWLEDGE ON THE FLORISTIC DIVERSITY OF THE UKRAINIAN EASTERN CARPATHIANS
The flora of the Ukrainian Eastern Carpathians has been studied for nearly 200 years by generations of Austrian, Polish, Ukrainian, Slovak, Czech, Hungarian, and Romanian botanists. The Flora of the Ukrainian SSR (1935 - 1965) and the vascular plant species of the Manual of the Ukrainian Carpathians (1977) may be considered unique reviews of these investigations. According to the data within them, 2012 species of vascular plants occur in the territory of the Ukrainian Carpathians (Manual 1977). They belong to 135 families and more than 600 genera. The richest families include Compositae, Rosaceae, Gramineae, Cyperaceae, Ranunculaceae and Caryophyllaceae. Also well represented are genera whose centers of diversity are the European mountains. Examples here are Astragalus, Gentiana, Potentilla, Primula, Ranunculus, and Saxifraga.
More than 150 vascular plant species are included in the second edition of the Red Data Book of Ukraine (in press). The latest data indicate that 331 species of vascular plants are rare, vulnerable, or endangered in the flora of the Ukrainian Eastern Carpathians (Tasenkevich, manuscript). It must be noted that the aforementioned Manual includes foothills as well as genuine mountain territory. It therefore lists more species than actually occur. Similarly, adequate knowledge of the native floral diversity is still lacking, with the Manual including all groups of synanthropic plants, including exotic ones.
By the time of the publication of the last volume of the Flora of Ukraine, the concept of the species as a biological system consisting of population combinations had gained a foothold in the biological world (the change from the monotypical species standard to the polytypical one had been accomplished). However, even after the completion of Flora Europaea (1968 - 1980), attempts are still being made to qualitatively and quantitatively re-estimate the flora of the Eastern Carpathians of Ukraine by the equal species standard. In contrast, the floras of our neighbors are either well advanced (Flora Slovenska, v. 1 - 4, 1966 - 1992; Flora Polski, v. 4, 5, 1985, 1987) or even completed (Beldie, 1972; Dostal, 1989).
The results of one of the first attempts at a modern approach to the floral diversity of the Ukrainian Eastern Carpathians were presented in a single, recently-published article on the endemism of vascular plants (Stoyko, Tasenkevich, 1993). On the basis of critical taxonomic and chorological study, some 95 species and subspecies were considered to be endemic to the Ukrainian Carpathians.
Various authors take up diverse positions regarding the number, taxonomy, chorology, and other aspects of endemic taxa. Thus, different results have been obtained. V. Chopyk (1976) considered only 76 species to be endemic to the Ukrainian Eastern Carpathians, while B. Pawlowski (1970) gave only 102 endemic taxa for the entire Carpathian flora.
Lists of Carpathian ''endemics" have often included what are actually subendemic taxa with wider geographical ranges. This group comprises taxa with Carpathian-Balkan distributions (for example, Rhododendron myrtifolium, Schott et Kotschy; Verbascum glabratum, Friv.; Veronica baumgartenii, Roem. et Schult.; Viola ideclinata, Waldst. et Kit.) as well as East Alpine-Carpathian-Balkan species like Cardamine opizii , Presl.; C. glanduligera, O. Schwarz; and Cirsium waldsteinii, Rouy.
All the above-mentioned facts give a striking illustration of the necessity for a critical treatment of the Ukrainian Eastern Carpathian flora on the basis of unified taxonomic and chorological foundations. A primary task in such an effort is the study of the flora of the Ukrainian Carpathians as part of the Eastern Carpathian flora linking the West and South Carpathians. The task should thus be of interest to botanists in both Ukraine and Central Europe.
Such research is expected to provide a great deal of different botanical data, and it is for the recording, storage, processing, and analysis of this information that the "Carpathians Flora" informational system was created.
This system is built on a database that contains species characteristics as follows: species name; family; genus; subspecies; occurrence in the West, East, and South Carpathians; biomorphology; ecological demands; caryotaxonomy; ecotopes; phytogeographical characteristics; economic importance; and protection status.
The foundation of the database is a species composition block, so the taxonomy and nomenclature of species from the volumes of Flora Europaea was selected as the uniting and unifying base.
THE STATE OF EXPLORATION OF THE FLORISTIC DIVERSITY OF THE UKRAINIAN PART OF THE EASTERN CARPATHIANS BIOSPHERE RESERVE
A considerable proportion of the vascular plant species grow in protected areas of several types in the Ukrainian Eastern Carpathians. Most of these protected areas have been described in general outline only (Stoyko et al. 1980). But even the main ones (Carpathian Biosphere Reserve, Carpathian National Park, and Synevir National Park) which are described in special monographs (Stoyko et al. 1982, 1993) will not be explored completely due to their recent establishment or expansion. As a result, there are no clear data on the composition and status of protected plant species in the Ukrainian Eastern Carpathians.
The most representative protected area in the Ukrainian Eastern Carpathians is Carpathian Biosphere Reserve. The main part of it was protected in 1968 as Carpathian Reserve (zapovidnyk), but it was only after numerous organizational difficulties that 32,000 ha of it was incorporated into the international network of Biosphere Reserves in 1992.
The principal part of the reserve, consisting of six separate areas, is representative of all the vegetational belts of the Ukrainian Eastern Carpathians,
from the meadows of the Transcarpathian plain to the low alpine grasses of the Marmarosh and Chornohora Mountains. The western massif in the Eastern Beskydy Mountains, situated away from the main part of the reserve, is territorially a constituent part of the Polish-Slovak Eastern Carpathians Biosphere Reserve. With the change in its legal status, the protected area on this western massif was enlarged from the original 2542 ha of Stuzhytsia Protected Forest to 14,665 ha.
Floristic and phytocenotic data from the Slovak and Polish parts of the Biosphere Reserve have been published recently (Dostal 1988, 1989; Hadac 1988, 1989, 1991; Hadac et al. 1986, 1988; Hadac and Soltan 1989; Hadac and Terray 1988; Krahulec 1987; Majovsky et al. 1987; Sojak 1959; Vazur 1988). However, no such data from Ukraine have been forthcoming because the area on the Slovak-Ukrainian border was guarded by Soviet army forces and was not accessible to scientists.
During the first growing season after the collapse of the Soviet Union, a group of Czech, Slovak, and Ukrainian botanists initiated observations of the flora and vegetation of the now-accessible Eastern Beskydy.
Interesting finds were made from the very beginning of the floristic exploration. These included Conioselinum tataricum Firch (reported previously in a single site in the Ukrainian Carpathians in the Chyvchyny Mountains by Pawlowski 1948); Aconitum anthora L., 1A. firmum Reichenb. subsp. baumgartenii (Schur) Gayer; Poa nemoralis L. subsp. carpatica Jirasek; Cotoneaster niger (Thunb.) Fries subsp. slavicus Hrab. Uhr. had not been known previously from the territory of the Ukrainian Eastern Carpathians.
The western part of Carpathian Biosphere Reserve is transected by the western delimitation boundary of Eastern-Carpathian endemics and the Central-Carpathian phytogeographical disjunction. Prolongation of this delimitation line is observed to the north in the Polish part of the Eastern Carpathians (Zemanek 1990) and to the northwest and south in the Slovakian part (Hadac 1989).
Although our collections are incomplete and fragmentary, about 560 vascular plant species were still collected in this floristic terra incognita of the Ukrainian part of the Biosphere Reserve.
Besides ensuring possibilities for much more effective protection of individual natural objects, transboundary areas may serve the important goal of transboundary integration of the efforts of scientists and nature managers. Transboundary areas in general, and the Eastern Carpathians area in particular, can be used as training grounds for the testing and unification of research methods. For example, Ukrainian botanists have an interest in the mapping methodology that is applied in the Bieszczady (Polish) part of the Biosphere Reserve. This may help to integrate Ukrainian floristics into the Europe-wide chorological programs and to form a unified database for the further floristic monitoring of the Biosphere Reserve.
The need to explore and protect floristic diversity throughout the entire territory of the Ukrainian Eastern Carpathians and in transboundary areas both protected and unprotected means that the following principal tasks must be addressed:
-
Unification of the methodological base and methods by floristic diversity is studied;
-
Elaboration, pluralization, and coordination of a database on floristic diversity;
-
Inventorying of plant species diversity in the Ukrainian Eastern Carpathians, in transboundary areas, and in protected areas in the Ukrainian Eastern Carpathians; and
-
Chorological investigation of rare, endemic, and endangered plant species and the preparation of a chorological atlas and an Endangered Species Data List for the Carpathians as a whole.
REFERENCES
Beldie, A., 1972. Flora Romaniei. Determinator ilustrat al plantelor vasculare. Edit. Academiei Republici Socialiste Romania. Bucuresti, vv. 1 - 2. (in Romanian)
Chopyk, V., 1976. The High Mountain Flora of the Ukrainian Carpathians. 267 p. Naukova Dumka Press, Kyiv (in Ukrainian)
Chopyk, V., 1977. Scheme of the Floristic Regionalization of the Ukrainian Carpathians. In: Manual of the Ukrainian Carpathians Vascular Plant Species. p. 12. Naukova Dumka Press, Kyiv (in Ukrainian).
Domin, K., 1928. Introductory Remarks to the Fifth International Phytogeographic Excursion (I.P.E.) through Czechoslovakia. Acta Bot. Bohemica 6 - 7, pp. 3-76.
Domin, K., 1930. A New Division of Czechoslovakia into Natural Geobotanical Districts. Acta Bot. Bohemica, 9, pp. 55-58.
Dostal, J., 1988. Vascular Plants. In: The Eastern Carpathians. Protected Landscape Area. Priroda. Bratislava, pp. 81-90 (in Slovak)
Dostal, J., 1989. New Flora of the CSR. Academia, Praha, vv. 1 2, 1548 p. (in Czech).
Flora Europaea. 1968 - 1980. (Eds. T.G. Tutin, V.H. Heywood, N.A. Burges et al.) vv. 1 - 5, Cambridge University Press. Flora Polski. 1985 - 1987. (Ed. A. Jasiewicz) vv. 4, 5. Polskie Wydawnictwo Naukowe. Warszawa. (in Polish) Flora Slovenska. 1966 - 1992. (Ed. L. Bertova) vv. 1 - 4. Veda, Bratislava. (in Slovakian) Flora of the Ukrainian SSR. 1935 - 1965. (Ed. K. Zerov). vv. 1 -12. Academy of Sciences of the Ukrainian SSR Press, Kyiv. (in Ukrainian).
Fodor, S., 1960. Botanical and Geographical Regionalization of the Altimontane Vegetation of the Transcarpathians. In: Flora and Fauna of the Carpathians. pp. 85-96. Academy of Sciences of the USSR Press. Moscow - Leningrad (in Russian)
Hadac, E., 1988. Unforested Plant Communities. In: The Eastern Carpathians. Protected Landscape Area. Priroda, Bratislava. pp. 90-96 (in Slovak)
Hadac, E., 1989. Pflanzengeographische Bemerkungen uber die Berggruppe Bukovske vrchy in der NO - Slowakei. Flora. - VEB Gustav Fischer Verlag, Jena. pp. 481-486.
Hadac, E., 1991. Distribution of Some Vascular Plants Species in the Bukovske Vrchy Hills, NE Slovakia. Preslia. v. 63. pp. 205226.
Hadac, E., J. Andresova, J. Paukertova, V. Klescht, 1986. Four Wetland Plant Communities of the Bukovske Vrchy Hills in the NE of Slovakia. Preslia, v. 58, pp. 339-347 (in Czech)
Hadac, E., V. Hadacova, V. Potocek, 1988. Vegetation of the Bukovske Vrchy Hills on the NE of Slovakia and Soils Reaction. Preslia, v. 60. pp. 157-165 (in Czech)
Hadac, E., Z. Soltan, 1989. Plant Communities of Springs and Mountain Streamsides of the Bukovske Vrchy Hills in the NE of Slovakia. Preslia. v. 61. pp. 343-353 (in Czech)
Hadac, E., J. Terray, 1988. Notes on the Flora of Bukovske Vrchy Hills. Zpravozdania CS. Botan. Spolecn., Praha, v. 23 pp. 66-68 (in Czech).
Krahulec, F., 1987. Festuca Saxatilis - a New Species of the Czechoslovak Flora. Preslia. v. 59. pp. 273-278.
Majovsky, J., A. Murin, a kolektiv, 1987. Karyotaxonomy of Slovakian Flora. Veda, Bratislava, 436 p. (in Slovak)
Manual of the Ukrainian Carpathians Vascular Plant Species, 1977. Naukova Dumka Press, Kyiv. 434 p. (in Ukrainian).
Pawlowski, B., 1947. Characteristique Geobotanique Generale des Monts de Czywczyn. Extrait du Bulletin de l'Academie Polonaise des Sciences et des Lettres. Classe des Sciences Mathemat. et Naturel. -Ser B: Scienc. Naturelles (1) 1946 . Imprimerie de l'Universite, Cracovie, pp. 71-108.
Pawlowski, B., 1970. Remarques sur l'Endemisme dans la Flora des Alpes et des Carpates. Vegetatio, 21, pp. 181-243.
Sojak, J., 1959. Notes on the Flora of Nizke Poloniny. Preslia, v. 31, pp. 307-317.
Soo, R. Analyse der Flora des Historischen Ungarns (Elemente, Endemismen, Relikte). Arb. 1. Abt. Ungar. Biol. Forschunginst. v. 4, pp. 173 -192.
Stoyko, S., L. Milkina, et al., 1980. Nature Protection in the Ukrainian Carpathians and Adjacent Territories. Naukova Dumka Press, Kyiv, 262 p. (in Ukrainian)
Stoyko, S., L. Milkina, L. Tasenkevich, et al., 1993. Nature of the Carpathian National Park. Naukova Dumka Press, Kyiv, 212 p. (in Ukrainian)
Stoyko, S., L. Tasenkevich, 1993. Some Aspects of Endemism in the Ukrainian Carpathians. Fragmenta Floristica et Geobotanica, Suppl. 2 (1), pp. 345-353.
Stoyko, S., L. Tasenkevich, L. Milkina, et al., 1982. Flora and Vegetation of Carpathian Reserve. Naukova Dumka Press, Kyiv, 218 p. (in Ukrainian).
Tasenkevich, L., 1986. Place of the Rika-Teresva Interfluve in the Floristic Division of the Ukrainian Carpathians. Ukr. Bot. Zhurn. 43, n. 2, pp. 30-33 (in Ukrainian with English summary)
Vazur, M., 1988. Forest Plant Communities. In: The Eastern Carpathians. Protected Landscape Area. Priroda. Bratislava. pp. 97-106.
Zemanek, B., 1991. The Phytogeographical Boundary between the East and West Carpathians - past and present. Thaiszia, 1, pp. 59-67. Fig. Floristic division of the Ukrainian Carpathians (Chopyk, V., 1977, modified by Tasenkevich, 1986) I - the Eastern Beskydy Mountains, II - Gorgany, III - Krasna, IV - Svydovets, V - Chornohora, VI - Marmarosh, VII - Chyvchyny.
GRASSLANDS OF THE EAST CARPATHIAN BIOSPHERE RESERVE IN SLOVAKIA
Helena Ruzickova and Miroslav Bural
Institute of Landscape Ecology
INTRODUCTION
Since forests previously dominated the temperate zones, the grasslands are mainly of secondary origin. Nevertheless, grasslands are important sources of biodiversity in the East Carpathians Biosphere Reserve in Slovakia. Entire groups of vegetation communities, plants, and animals, which have only limited conditions for existence in forests, survive in the grasslands. Grassland ecosystems can support a stable composition of species communities, provided that there is some regularly repeated energy input and output (mowing, pasturing, fertilizing). Compensation mechanisms ensure a resilient level of stability. Both human influence over time and site conditions impact the stability. Such grasslands can be considered seminatural. However, artificial grasslands arise if the intensity of economic activity is high enough to change the site conditions, and therefore change the species composition (through reclamation, drainage, the heavy use of fertilizer, the additional sowing of grass cultivars, or large flocks). These unnatural grasslands have many non-productive functions. However, they are not subjects for nature conservation because they have no meaning as biotopes and biodiversity sources.
Transitional meadows and pastures arise through the self-seeding of former fields. This phenomenon is common in mountainous areas of Slovakia, where extreme areas and terraced fields are no longer cultivated. In the East Carpathians, these fields were managed in the double-field system of economy in which cultivated soil and meadows were alternated for periods of 10 to 12 years each. However, these fields are rarely replowed.
In a conservation program for the grasslands, one must consider the enrichment of a territory's species diversity as well as which species to include. Forests predominate the East Carpathians Reserve, while meadows comprise only about 15 percent of the area. However, meadows deserve special attention since they not only have natural, scientific significance, but they also have also cultural and historical value. They bear witness to the original and current ways of farming
in the area. The grasslands of the area can be divided into two large groups: mountain "poloniny" grasslands and grasslands of lower areas. Each group has different vegetation and different requirements for biodiversity conservation.
MOUNTAIN "POLONINY" GRASSLANDS
The mountain "poloniny" grasslands, occurring at altitudes above 1000 m, are found in the ridges of the East Carpathians. The traditional farming system, which continued until the 1940s, consisted of mowing and grazing in alternate years. Although the hay and grass were of lower quality than that grown in lower sites, the system enabled farmers to breed animals economically. Sheep, and later oxen and horses, used to graze in the meadows, but the meadows have been abandoned and left unknown since the middle of the 1960s.
The "poloniny" meadows are primarily inhabited by types of vegetation which grow in the upper boundary of the forest in the Carpathians. These meadows include the alliances Calamagrostidion arundinaceae, Nardo-Agrostion tenuis, and Vaccinion vitis-idaeae (E. Hada et al., 1988). The meadows of the alliance Polygono-Trisetion, which are typical of mountain areas in Central Europe, do not occur here. The "poloniny" are special because East Carpathian species which are often at the limits of their ranges inhabit them. Some of these species are associated only with ridge areas and are not found in lower grasslands (examples include Viola dacica, Campanula abietina, Melampyrum herbichii, Senecio papposus, Tithymalus sojakii, and Dianthus compactus).
Bla'kov conducted the first extensive phytocoenological research on "poloniny" meadows in 1969, when farming activity was gradually ceasing (D. Bla'kov, 1991). Bla'kov revisited the areas 15 to 20 years later and recorded the successional trends following the cessation of farming. After 3 to 8 (10) years, the copses were found to be dominated by Vaccinium myrtyllus, vitis-idea, Poa chaixii, and Achillea stricta. After 15 to 20 years, the dominants were Calamagrostis arundinacea, Gentiana asclepiadea, species of the genus Rubus, and more forest species. Today, copses with Calamagrostis arundinacea occupy large areas and have relatively poor species composition.
Preserving the species diversity of mountain grasslands is a difficult problem. Usually, the primary issue is not the preservation of the grasslands with their original species diversity, but rather their re-succession. Internal conditions have completely changed in places where groups of grasses predominate which previously constituted only a small part of the original vegetation. We have seen examples of grasslands' reactions to regular long-term interference which is inevitable for the preservation or renewal of meadows. Permanent plots can be marked out within grasslands so that changes in species composition can be monitored and evaluated. Cooperation between Slovakia, Poland, and Ukraine would be valuable since the "poloniny" meadows occur primarilly on frontier ridges.
GRASSLANDS AT LOWER LEVELS
Away from the mountain ridges, usage of land as permanent meadows or pastures has affected areas whose soils were not arable. Such soils were too wet or dry, on slopes, or too shallow or acidic. These seminatural meadows and pastures often have specific species compositions. They are single- or double-mowing meadows (utilized for fodder or litter) or extensive pastures which supplemented fodder obtained from temporarily-grassed areas and meadows near houses. Today, many of them have been abandoned as a result of collectivization or the evacuation for the construction of the Starina reservoir. Farmers lost interest in mowing and grazing extensive grasslands when no livestock remained.
The group of seminatural meadows at lower elevations includes moor and peaty meadows, wet and mesophytic meadows, subxerophyll meadows and pastures, and acidic pastures. Although they are restricted to small areas around springs, the moor and peaty meadows deserve special attention. They belong to the alliances Caricion fuscae, Caricion lasiocarpae, and, in one locality, Sphagnion medii.
The moist meadows of the alliances Calthion and Molinion caerulae are located beside water courses and springs. The species composition of these meadows depends on the water regime, on the nutrient content of the soil, and on the soil reaction (pH). Meadows of the alliances Calthion and Molinion caerulae create interesting landscapes with relatively varied mixture of plant communities, and they are the biggest sources of biodiversity at lower levels. As only a few species are utilized by the local inhabitants, these meadows require directed care with a particular frequency of mowing. Without mowing, they rapidly become poor, tall herb communities of the alliance Filipenduleion.
Extensive pastures can be found in sloping, inaccessible sites with shallow soils. Like the moist meadows, these sites lost their economic importance after collectivization. Depending on the nutrient and lime content of the soil, these communities range from subxerophyllous herb communities with many species to poor pastures with Nardus stricta belonging to the alliances Cynosurion, Arrhenatherion, and Cirsio-Brachypodion. These pastures are probably among the oldest grassland types in the region and possess a large variety of animal species, especially entomofauna. To preserve selected areas, directed farming must be ensured.
The original mesophillous meadows of the alliance Arrhenatherion occur only in meadows near houses and in old fruit orchards. These are mostly secondary communities established on fields. They are often not farmed (in the area of the Starina reservoir), and the natural seeding of pioneer tree species indicates the direction of the succession. These fallows are often secondary sites with many species from the Red List of Slovak Flora, especially from the family Orchidaceae. As nature conservation alone is not able to keep grasslands open, permanent areas need to be selected so that the process of succession can be monitored and the possibilities for arresting it sought.
CONCLUSIONS
The grasslands of the East Carpathians Biosphere Reserve are vital in the preservation of biodiversity in the area. Their management should consist of the following steps:
-
The inventory of the grasslands and the evaluation of their uniqueness, species diversity, and rarity as well as natural, cultural/historical, and aesthetic value;
-
The selection of representative areas, the determination of the optimal way in which they may be utilized, and the search for ways to ensure the maintenance of the stated regime; and
-
The establishment and regulation of permanent areas.
When selecting areas, preference should be given to those with a mosaic of different kinds of grassland, along with traditionally utilized arable soil. Such biotope complexes would guarantee the preservation of species diversity and the character of the landscape.
REFERENCES
Bla'kov, D., 1991. Succession in Abandoned Mountain Meadows in the Stu'ica Nature Reserve (The Bukovsk Vrchy Mts., East Slovakia). Preslia, Praha, 63: 177-188 (in Czech).
Hada, E., Andresov, J., Klescht, V., 1988. The Vegetation of the "Poloniny" in the Bukovsk Vrchy Hills NE Slovakia.
FLORISTIC DIVERSITY IN THE LATORITSA RIVER BASIN (UKRAINIAN CARP A THIANS)
Bogdan Prots
Institute of Ecology of the Carpathians
Ukrainian Academy of Sciences
The natural and anthropogenically transformed areas of the Latoritsa river basin have long attracted the attention of botanists, and the history of their investigations is intimately linked with the historical fate of the Transcarpathians. Three periods of exploration may be distinguished. The first period, from the end of the 19th century to the beginning of the 20th century, involved the gathering of data on the distribution of separate plant species (Pax, 1895; Thasiz, 1909, 1912). The second period, from the beginning of the 20th century to the mid 1940s, saw new approaches taken in floristic research (geobotany, chorology, and nature protection). This interest took the form of a number of publications (Margittai, 1923, 1929; Nadvornik, 1929; Maloch, 1931, 1932; Zlatnik, 1934; Klastersky, 1936 and many others). The third period, from the mid 1940s to the present, is characterized by attempts at systematic and critical approaches to floristic study (Popov, 1949; Rudenko, Fodor, Riznychenko, 1956; Chopyk, 1958; Fodor, 1974; Malynovsky, 1980; and many others).
Floristic investigations in the area of the Mukachevsky Gory Mts. (west flank of the Vulcanychny Carpathy Mts.) were begun by the present author in 1988 (Zahulsky, Prots, 1991). Detailed floristic investigation began from 1991 onwards. The aim of this study was to show the floristic diversity and some aspects of the anthropogenic transformation of the flora in the Latoritsa river basin (Ukrainian Carpathians). The results of these investigations have been published in a series of papers (Kozak, Prots, 1993; Prots, 1994 and others).
The Latoritsa river basin is located in the western part of the Ukrainian Carpathians on the border of four geographic regions: the Verchovynsky Mts., the Polonynsky Mts., the Vulcanychny Mts., and the Zakarpatska Rivnyna Plain (Figure 1).
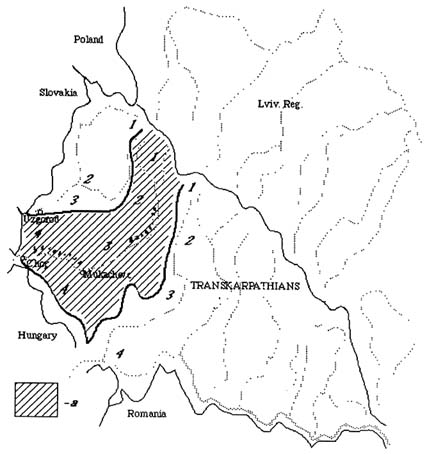
FIGURE 1 Location of the Latoritsa River Basin in the Transcarpathians (the Ukrainian Carpathians). a - extent of the Latoritsa basin, 1 - Verchovynski Mts. (Schidni Beskydy Mts.), 2 - Polonynski Mts., 3 - Vulcanychni Mts., 4 - Zakarpatska Rivnyna Plain
There are 7 vertical climatic zones which are of basic importance to the vegetation (Holubets et others, 1988). In the mountain part of the basin there are 4 altitudinal floristical belts of the Ukrainian Carpathians (Tkachyk, Prots, 1993): xerothermic - mountain, nemoral - mountain, submountain, and mountain. Two zones and 4 vegetational belts are to be found in the Latoritsa river basin (Holubets, 1978).
The Latoritsa river basin is a very heterogenic system not only in terms of its geographical, climatic, and floristic relations, but also from the hydrological, soil, and landscape points of view (Holubets et al. 1988).
The Latoritsa basin is on the border of two floristic provinces (the Central European and the Pannonian). It is included in at least three floristic districts (the Zakarpatska Rivnyna Plaine, the Vulcanychny Carpathy Mts. and the Schydny Beskydy Mts.). The basin covers about 3000 km2.
Wild (ahemerobic and oligohemerobic) areas cover about 40 percent of the basin area, while antropogenically transformed (mezohemerobic, euhemerobic, poly- and metahemerobic) areas cover about 60 percent (Blume, Sukopp, 1976). The population of the Latoritsa river basin area is 250,000, encompassing the greatest industrial centres of the Transcarpathians (such as Mukatchevo and Svaljava). The most important industries involve timber, television and mining, and many people also work in agricultural production. The Latoritsa basin is crossed by the Chop - Mukatchevo - Strij - Lviv roads and railways, though railway and road infrastructure is very weakly developed. There are many devastated areas (dust-heaps, quarries, and railway stations) which make up more than 5 percent of the basin area.
Floristic research was carried out on the basis of detailed-itinerary, half-stationary, and stationary methods (Yurtsev, Kamelin, 1987).
The flora of the Latoritsa basin includes 1146 indigenous plant species (from 137 families and 560 genera). The total number of plant species is 1502. Quantitative data on the flora of the Latoritsa Basin are given in Table 1.
The richest in species are the following 10 families: Asteraceae (149), Rosaceae (69), Poaceae (65), Brassicaceae (58), Fabaceae (51), Cyperaceae (50), Scrophulariaceae (50), Lamiaceae (49), Ranunculaceae (41), and Caryophyllaceae (40). The 10 principal families accounted for 54.3 percent of the total number of species. The analogical arrangement of families in the main part of the spectrum is
TABLE 1 Taxonomic Structure of the Flora of the Latoritsa River Basin
|
TAXA |
Number of species |
% of total number of species |
Number of genera |
% of total number |
Number of families |
% of total number of families |
|
Lycopodiophyta |
5 |
0.4 |
4 |
0.7 |
4 |
2.9 |
|
Equisetophyta |
8 |
0.75 |
1 |
0.2 |
1 |
0.7 |
|
Polypodiophyta |
29 |
2.5 |
19 |
3.4 |
11 |
8.0 |
|
Pinophyta |
8 |
0.75 |
6 |
1.1 |
3 |
2.3 |
|
Magnoliophyta, |
1096 |
95.6 |
530 |
94.6 |
118 |
86.1 |
|
incl. Liliopsida |
235 |
20.5 |
103 |
18.4 |
20 |
14.6 |
|
Magnoliopsida |
861 |
75.1 |
427 |
76.2 |
98 |
71.5 |
|
Total number |
1146 |
100% |
560 |
100% |
137 |
100% |
characteristic for the Holarctic flora (Tolmachev, 1974). The predominance of the Rosaceae, Brassicaceae, and Fabaceae also attest to the influence of the ancient Mediterranean flora or formation on the present one.
Indices of the number of species in different pairs of families may be use to give a qualitative characterization of a flora. Thus the ratio of Asteraceae species to Fabaceae species, at 2.9, is characteristic of a boreal flora, while the Asteraceae Cyperaceae (ratio of 3.0), is intermediate between the boreal and Mediterranean floristic regions.
Meanwhile, three large families (Asteraceae, Poaceae and Cyperaceae) had 23 percent of the total number of species, a feature characteristic for the flora of temperate latitudes (Tolmachev, 1974). The most important genera were: Carex (36 species), Hieracium (31), Veronica (21), Rosa (17), Galium and Ranunculus (15), Euphorbia, Viola, Campanula (13), and Rumex (12). The large numbers of Rosa and Rubus species (8) are characteristic of Central European floras as opposed to East European floras.
Consequently, the flora of the Latoritsa river basin (within Ukraine) is part of the Middle European Region (Carpathian subregion) flora. This is a result of the geographical location of the region under investigation, as well as by its florogenetic connections.
Ecotypical classification is characterized by groups as follows: by degree of moisture, such as mesophytes (684 species; i.e., 59.7 percent of total number of basin flora), xerophytes (259; 22.6 percent), hydrophytes (141; 12.3 percent), and hygrophytes (62; 5.4 percent); and by soil fertility, including mesotrophs (548 species; 47.8 percent), eutrophs (528; 46.1 percent), and oligotrophs (70; 6,1 percent).
Vital forms were distinguished in the following way using the Raunkiaer system: hemicryptophytes (562 species; i.e.; 49 percent of total number of basin flora), cryptophytes (241; 21.0 percent), therophytes (216; 19.0 percent), phanerophytes (83; 7.2 percent), and chamaeophytes (44; 3.8 percent).
Nine zonal geographical elements (Malynovsky, 1980) are to be distinguished in the flora Latoritsa basin: nemoral (369 species; i.e., 32.1 percent of total number of flora's investigated territory); boreal (251; 21.9 percent); arid (222; 19.4 percent); azonal (127; 11.1 percent); mountain (87; 7.6 percent); nemoral mountain (55; 4.8 percent); boreal mountain (17; 1.5 percent); alpine (10; 0.9 percent); and arctic alpine (8; 0.7 percent).
The following endemics and subendemics may be noted: endemic to the Carpathians as a whole (6 species); endemic to the Southern and Eastern Carpathians (8); Carpathian-Balkan subendemics (2); and East Carpathian-Balkan subendemics (4).
Comparison of the numbers of plain and mountain species shows that plain species are in the majority.
The Latoritsa river basin is notable for a flora with a high degree of floristic diversity. This is due to the basin location on the boundary of several macrophytochorions.
The anthropogenic transformation (Burda, 1991) or synanthropization s.l.(Kornas, 1978; Malyshev, 1981 and others) of the flora of the Latoritsa basin is characterized by:
-
The impoverishment of the genepool of the native;
-
The invasion, expansion and naturalization of anthropophytes;
-
Disturbances to the flora (Burda, 1991);
-
Teratogenic phenomena; and
-
The appearance of floristic complexes that have no analogues in nature.
The process of impoverishment of the genepool of the native flora was investigated on the Orchidaceae, the best represented family in the Ukrainian Red Data Book. Twenty-eight species have been discovered by different investigators over the last hundred years, i.e., 75.7 percent of the orchid flora of the Transcarpathians and 70.0 percent of that of the Ukrainian Carpathians. According to the results obtained, 14 orchid species have 154 localities, and in 60 of these localities, 22 of 25 known species are found to have become extinct.
The impoverishment of the genepool of the native flora in the Latoritsa basin is leading to the isolation of populations or plant species in all areas as well as to reduced cover, loss of species, and reduced population vitality (Zahulsky, Prots, 1991, Prots, 1994).
Among the plant species which have disappeared from the Latoritsa basin are: Drosera rotundifolia L., Beckmannia eruciformis (L.) Host., Salix incubaceae L., Scheuchzeria palustris L., and Rhododendron myrtifolium Schott et Kotschy. In total, the genepool of the native species of the basin has so far been impoverished by 0.5 percent.
Geographical-historical analysis of synanthropic plant species (Thellung, 1918; Holub, Jirasek, 1968; Schroeder, 1969; Kornas, 1977) found 122 anthropophytes of 41 families and 83 genera (i.e., 23 percent of the synanthropic flora). Anthropophytes may be distinguished: by the way of migration (acolutophytes, 57 species; ergasiophygophtes, 39; and xenophytes, 26); by the time of migration (archeophytes, 47 species; and kenophytes, 75); and by the degree of naturalization (epecophytes, 73 species; ergasiophytes, 57; hemiagriophytes, 31; and holoagriophytes, 14).
Anthropophytes provide a model for microevolutionary processes (including flora perturbation). Ambrosia artemisifilia var. atropurpurea Priszter. was found in the lower part of the spruce belt (730m above sea level).
Teratogenic phenomena have been discovered among synanthropic plants. This is the result of technogenic influences on phenotypes.
TABLE 2 List of Plant Species in the Latoritsa River Basin with Abnormal Structures (terats).
|
Types of terats |
||||||
|
SPECIES |
fasciation |
prolification |
branching of floscule |
corolla turning green |
''cones" |
destruction on the leaflets |
|
Acer negundo L. |
|
|
|
|
|
# |
|
A. platanoides L. |
|
|
|
|
|
# |
|
Ambrosia artemisifolia L. |
# |
|
|
|
# |
|
|
Cardaria draba (L.) Desv. |
|
|
|
# |
|
|
|
Cichorium intybus L. |
|
|
|
# |
# |
|
|
Echium vulgare L. |
# |
|
|
|
|
|
|
Matricaria perfoliata M. |
# |
|
# |
|
|
|
|
Plantago major L. |
|
|
|
|
|
# |
|
P.lanceolata L. |
|
|
|
|
|
# |
|
Tanacetum vulgare L. |
# |
|
|
|
|
|
|
Taraxacum officinale W. |
# |
# |
|
|
|
# |
A total of 6 types of terat were found in 11 species (Table 2). Such abnormal structures as fasciation and destruction of leaflets are most frequent. Taraxacum officinale has been found to have 50 percent of the total number of terat types.
Anthropoflorocomplexes in forming aspects were considered in three groups: accidental, forming, and formed. The first group included a large number of complexes (more than 80, including dominants Vitis vinifera + Triticum aestivum L. + Papaver somniferum L.). The second group had 27 complexes (dominants: Lepidium latifolium + Urtica dioica L. + Leonurus quinquelobatus Gilib.). The third group had 12 (Quercus robur L. + Rudbeckia laciniata L. or Robinia pseudoacacia L. + Fagus sylvatica L.).
A flora's anthropogenic transformation takes place in time and in space. Hemerobicity is the result of the action of all types of anthropogenic influence on ecosystem (Blume, Sukopp, 1976). In the Latoritsa river basin, the following degrees of hemerobicity may be noted: ahemerobic (0-2 percent of total number of the hemerobic species); oligohemerobic (5-11 percent); mesohemerobic (14-22 percent); euhemerobic (26-68 percent); polyhemerobic (58-83 percent); and metahemerobic (74-100 percent). The correlation between hemerobicity and flora diversity showed unity between the processes of flora transformation and the degradation of the ecosystem as a whole. The Quantitative characteristics of biodiversity are not as important as the qualitative one. The high degree of hemerobicity points to a flora becoming increasingly cosmopolitan, xerophytic, and unified in structure. This is a basis for unstable floras. The direction of flora's transformation necessary to regulate of reconstruction of the primary communities.
Research on the processes of synanthropization on the Laritosa river basin showed a high level of degradation of plant cover, especially in the western part.
The number of protected areas must be increased and rare species safeguarded from the processes of anthropogenic transformation. We propose 16 protected areas where rare and endangered species grow in the Latositsa river basin. These include: on the Zakarpatska Rivnyna Plain, small lakes near Chop, the forest near the Dachna railway station, and the forest near the Klucharky railway station; in the Vulcanichni Carpathy Mts., hill slopes near Beregovo, the old beech forest between Synjak and Chynadijevo, the forest near Chynadijevo, the slopes of Pohar hill, and the forest near Carpathy village, Lovachka hill, and Monument hill (Mukachevski Mts.); and within Verchovynski Carpathy, the Bokjuska Polonyna Range, Hostra hill, the beech forest near the Pereval railway station, the slopes of the Velyka Hranka Range, the valley of the Pynja River near Solochyn, and the valley of the Mala Pynja River near Uklyn.
With regards to Polish and Ukrainian scientific collaboration, the investigation of the floristic corridor between Tarnitsa, in the Halich hills of the Polish Carpathians and Pikuj hill in the Ukrainian Carpathians are very interesting.
The results in this paper are provide the basis for continued floristic monitoring.
REFERENCES
Blume H.P., and Sukopp H., 1976. Okologische Bedeitung Anthropogener Bodenveranderungen, Schriftenr. Vegetationskunde, 10, 75-90.
Burda R.I. 1990. Flora's Antropogenic Transformation. Naukova Dumka Press, Kyjiv (in Russian).
Chopyk V.I. 1958, Flora and Vegetation of the Western Part of the Ukrainian Carpathians. An URSR Press, Kyjiv (in Ukrainian).
Fodor S.S. 1974. Flora of the Transcarpathians. State University Press, Lviv (in Ukrainian).
Holub J. and Jirasek V. 1967. Zur Vereinheitlichung der Terminologie in der Phytogeographie. Fol. Geobotan. et phytotaxon. Bochemsol. - 2, N1.
Holubets M.A. 1978. Spruce Forests of the Ukrainian Carpathians. Naukova Dumka Press, Kijev (in Russian).
Holubets M.A. and Others. 1988. The Ukrainian Carpathians Nature. Naukova Dumka Press, Kijev (in Russian).
Klasterky I. 1936. Ochranarsko-botanicke Studie na Podkarpatske Rusi. III. Penisnik karpatsky (Rhododendron Kotschyi Simk). a jeho spolecenstva. Krasa neseho Domova, 28: 49-51
Kornas J. 1977. Analiza Flor Synantropijnych. Wiadomosci botaniczne. XXI, N2-P. 385-393
Kornas J. 1978 Remarks on the Analysis of a Synanthropic flora. Acta Bot. sl.-3, -P. 385-393
Kozak I. & Prots B. The Interpenetration of Plant Species between Forest and Meadow Associations in the Ukrainian Carpathians. Ukr. Botan. Journal. Vol. 50, N1 (in Ukrainian).
Maloch M. 1931. Borzavske Poloniny v Podkarpatske Rusi. Sborn. vyzk. Ustavu zemed. 67: 1-200, Praha.
Malynovskyi K. 1980. Vegetation of High Mountain Part of the Ukrainian Carpahians. Naukova Dumka Press, Kyjiv (in Ukrainian).
Malyshev L. 1981. Flora's Change of the Earth under Anthropogenic Influence. Biol. scien. -N3.-P. 5-20 (in Russian).
Margittai A. 1923. Contributions to Flora of the Podkarpatska Rus. Kvartalnyj, Pannonia (in Russian).
Nadvornik J. 1929. Reboik Kostkovany (Fritillaria meleagris L.) Vesmir 8: 18, 19.
Pax F. 1895. Einige Neue Pflanzenarten aus den Karpathen. Oest. bot. Z. 45: 26, 27, 41-45.
Popov M. 1949. Essays on the Carpathian Vegetation and Flora. MOIP press, Moscow (in Rusiian)
Prots B. 1994. Orchidaceae Species Spreading in the Latoritsa Basin (The Transcarpathians). Materials of International conference, Rahiv (in Ukrainian).
Schroeder F.-G. 1969. Zur Klassifizierrung der Anthropochoren. Vegetatio 16 (5-6): 225-238.
Thaisz L. 1909. Adatok Beregvarmegye Florajahoz. Magyar Bot. Lapok, Budapest, N8.
Thaisz L. 1912. Syringa Josikaea Jacq. fil. Ujabb Termohelyei. Magyar Bot. Lapok, Budapest, NII.
Thellung A., 1918/19. Zur Terminologie der Adventiv- und Ruderalflora. Allg. Bot. Z. Syst. 24: 36-42.
Tolmachev A. 1974. Introduction in to the Plant Geography. Leningrad University Press, Leningrad (in Russian).
Yurtsev B. and Kamelin R. 1987. Program of the Floristical Investogations of Different Degrees of Dataility. Theoretical and metodological problems of comparative floristics. Nauka Press, Leningrad (in Russian).
Zahulskyj M. and Prots B. 1991. Some Data about Orchidaceae Species Cenopopulations in the Industrial Territories. Theses of conference, Lviv (in Ukrainian).
Zlatnik A. 1934. Studie o statnich lesich na Podkarpatske Rusi. Dil prvni. Prispevky k dejinam statnich lesu a lesnictvi na Podkarpatske Rusi. Sborn. Vyzk. Ust. Zemed. 126/8: 1-109.
ECOEDAPHIC CONDITIONS OF TRANSBOUNDARY FOREST ECOSYSTEMS AND THE IMPACTS OF AIR POLLUTION
Milan Kodrík and Eduard Bublinec
The Institute of Forest Ecology
Slovak Academy of Sciences
The stability of forest growth is threatened, particularly in the most valuable natural sectors which various countries have proclaimed their national parks. A network of monitoring areas on vertical transects within the Tatra National Park (TANAP) was established for the purpose of long-term ecoedaphic research and the monitoring of environmental factors. Research on the transects is at present aimed at investigating changes in the health condition and stability of forest ecosystems. Every monitoring area has 100 trees under investigation and evidenced in detail (by reference to biometric characteristics, tree classification, the nature of regeneration, and the diagnostics of changes in health). Also considered are bioclimatic changes and characteristics, ecoedaphic changes, changes to biocenoses and their components, etc.
This paper interprets the results of a special investigation of the soil and vegetation qualities of transects I. (Zadne Med'odoly) and III. (Skalnata dolina). The upper area of the transect I is at an altitude of 1520 m above sea-level, and it has an unsaturated brown forest carbonate soil with the Sorbi aucupariae-Piceeta association (nutritious order B) and with the mull form of surface humus. The weight of this averages 18 t.ha-1, the pH in water is 4.85, and there is limited proton loading (0.052 kmol.ha-1). The middle area of the transect has a brown gley-podzol of the average degree at an altitude 1380 m above sea-level. It has an acid association of the order A (Piceeta sorbina) with greater acidity (pH 3.45) and raw humus at 75 t.ha-1. The actual proton (H+ ion) loading is 102.5 times greater than in the preceding monitoring area (MP) and reaches a value of 5.312 kmol. The lowest area (1280 m above sea-level) has brown rendzina with an organic horizon of the rendzina moder type weighing about 5 5 t.ha-1. Acidity is least marked in this horizon (pH 5.45 in H 2O), a fact that is influenced by a soil rich in two-power
bases, which also distinguishes the group of forest types Sorbi ariaePiceeta from the transient order B/D. The actual horizon loading O is low, in accordance with the humus type, and ranges to about 0.039 kmol of hydrogen ions.
At 109 t. ha-1 the mean dry weight of surface humus in the upper area transect III (an altitude of 1430 m) is the highest observed on any of the investigated monitoring plots. A brown podzol has developed here with a typical humus of very high acidity (pH in H2O 3.36, pH in KCl 2.52). Accordingly, the group of forest types Piceeta laricina comes from the acid order A and has actual acid loading of up to 9.488 kmol. The middle area of the transect (at 1160 m) has developed surface humus with an acidity analogous to that of the upper area and an average weight of about 80 t. ha-1. The loading is otherwise lower but quite near to the upper area, reaching 8.364 kmol of H+ ions. The Piceeta pineo-laricina association again belongs to the acid order A. The lowest area on the transect (880 m above sea-level) has the average surface organic horizon weight of 73 t.ha-1. In its form it belongs to the moder type (pH in H2O 3.65). The proton loading averages 3.250 kmol and the vegetative association belongs to the transitory order A/B, with two groups of forest types (Abieti piceetaequiseti vs Abieti piceeta laricis) having moder brown semigley soil.
Further research on the soil showed the soils of this elevation transect to be extremely acidic. In the area of the Lomnicky transect, the belt of very acid cambisoils reaches to a height of approximately 1100 m a.s.l., where it joins with a belt of practically equally acid cambisoils (brown) podzols. The mean pH value in KCl of the exchangeable soil reaction of the covering humus horizons of cambiso ils amounts to about 3, and in the podzols to about 2.5. The range in the mineral soil layers (down to 50 cm) is pH 3.1 to 3.8, or 2.8 to 4.4, respectively. In comparison with earlier data for these same soil units these are evidently higher by pH 0.5 to 0.6 and are an indication of the effect of man-induced acid deposits on the soils of the TANAP.
An assessment of health conditions according to dendro-indicators permits forest growth on the Skalnata dolina transect to be assigned to the 1st and 2nd degrees of damage. Expressed as a percentage of healthy specimens, the state of health of spruce in relation to elevation is as follows: at 880 m a.s.l., 9 percent; at 1160 m a.s.l., 13 percent; and at 1430 m a.s.l., 18 percent. This yields a mean of 18 percent healthy spruce trees for the transect studied. Similarly, the greatest number of most heavily-damaged (4th degree) spruce trees was found in the uppermost area (22 percent), and then in the middle area (16 percent), with the least damage observed in the lower area (8 percent). Evidently a certain differentiation or selection takes place here in the tree population. It is necessary to underline at this point that the composition, while comparable to other ecosystems in TANAP, is not usual for the other regions of Slovakia. This is caused by the limited ability of most tree species to endure the extreme climate and ecoedaphic conditions of the Central Carpathian region and provides a potential basis for the mass destruction and dying-off of unstable forest ecosystems. The research and evaluation done to date suggests that the following associations can be considered as potentially the
most endangered by chemical and climate stresses: Piceeta sorbina, Piceeta laricina, and Piceeta pineo-laricina. The ecophysiological stability of these ecosystems is also low. Abieti piceeta-equiseti has limited resistance to the influence of mechanical stressors. From the climatic point of view, the optimum for the spruce is forest vegetation of the 5th degree. Situated in this belt are the complex of moist ecosystems of the Abieti piceeta-equiseti type only, and this truth ought to be taken into consideration in the monitoring of injurious agents. The Sorbi aucupariae-Piceeta, Sorbi ariae-Piceeta, and Abieti-Piceeta laricis associations can be indicated as relatively the most stable ones.
It is probable that the southern slopes of the High Tatra (inclining into the Poprad valley) resemble those of the Alps in having 2 elevation zones of damage to forests, viz. at 850-1200 m and at 1400-1650 m a.s.l. The lower zone is related to inverted stratification of the air and damage in it is due to classical pollutants, such as SO2 and NOx. In contrast the second elevation zone is characterized by the prevalence of ozone-conditioned photooxidative stress.
Finally, we must say that the development and further deepening of this kind of research enables us to signal in time the decline of the potential resistance of TANAP-forest ecosystems and to make provisions to support their stability. The TANAP research is thus linked with the United Nations Environment Program (UNEP) and can help shed light on information which is being obtained from other monitoring categories within the so called "great representative forest investigation" in Slovakia.
REFERENCES
Bublinec E., Cicák A., Štefanèík I., and Kukla J. Soil Properties and Condition of the Spruce on a Vertical Transect in the High Tatras . Zborník prác o Tatranskom národnom parku, 32, 1992, s. 335-352.
Voško M., Kukla J., and Klubica D. The Analysis of the Ecological Factors and of the Forest Ecosystem Structure in the Monitoring Areas on the TANAP-territory. Zborník prác o Tatranskom národnom parku, 30, 1990, s. 227–276.
THE BELOW-GROUND BIOMASS OF MATURE FORESTS AND IMMISSION LOADING
Milan Kodrik
Institute of Forest Ecology
Slovak Academy of Sciences
INTRODUCTION
Roots provide a crucial link in the soil-plant-continuum. Their health depends on both the soil environment and the functioning of the tree canopy. Changes in either one of these compartments gives rise to a root response (Persson 1980), and the state of health of roots in turn determines multiple above-ground functions of plants, such as water and nutrient exchange (Chapin 1980), growth (Ingestad 1982), and hormonal root/shoot interactions (Schulze 1986). There is a strong interrelationship between the tree canopy and the root system, with the canopy supplying carbohydrates for root growth (Marshall and Waring 1985) and the roots supplying water and nutrients to the canopy. However, root growth, mineral concentrations (Bublinec 1992), and the formation of root tips are determined by such chemical and physical properties of the soil as the nitrogen supply (Meyer 1985) and degree of soil acidification.
In accordance with this knowledge, the aim of the work described here is to gain data on the division and total quantity of below-ground biomass in Norway spruce stands under varying pollution regimes.
MATERIALS AND METHODS
Four monitoring plots (MP1-MP4) were established in the area of the Moravian-Silesian Beskids. In all cases the stands involved were Norway spruce monocultures. The soil substrate consisted of an iron podzol, and all plots had northwest exposure with an inclination of 10-15 {SYMBOL 176 \f "Symbol"}. More details can be found below in Table 1 (and see also Kodrík 1992).
TABLE 1 Stand Characteristics of the Four Monitoring Plots in the Moravian-Silesian Beskids (MP1-4)
|
Plot |
Altitude [m] |
Age [years] |
Height [m] |
Diameter [cm] |
|
MP1 |
900 |
80 |
23 |
26 |
|
MP2 |
920 |
75 |
22 |
28 |
|
MP3 |
910 |
75 |
22 |
28 |
|
MP4 |
830 |
70 |
22 |
25 |
Pollution regimes were taken into consideration when the plots were chosen. The first three plots were characterized by pollution loadings of different intensities (expressed by 50-60 percent loss of needles), while the fourth was a control plot with minimum inputs of pollution. Exact values for loads in the investigated monitoring plots are given below in Table 2 (Kontrišová 1990).
All data on below-ground biomass were gained through destructive sampling of trees. To get a complete picture of the position of trees as individuals, after repeated stock-takings each tree was sorted by Kraft's classification scale (Vyskot et al. 1971). This scale takes the relative altitudinal position of the tree and the formation of the crown into account. Kraft (Vyskot et al. 1971) distinguishes the following classes: Dominant, codominant, partially codominant, undertopping, and fully-shaded trees. The selection resulted from calculated mensurational tree variables, separately for each Kraft class (Oszlányi 1975).
We processed one sample tree from of the first three classes on each research plot. The tree root system was elevated by means of the archeological method (Kodrík 1992), with the whole root system being gradually uncovered using shovels, hoes and brushes. A tractor with a winch and a powersaw were also used. The fresh weight was determined in the field on scales accurate to 0.05 kg
RESULTS
The results are shown in Table 3. It is evident from the data that the most substantial share of below-ground biomass is on MP4 - at 72.6 t ha-1 in terms of dry weight. There are no substantial differences in the structure of below-ground biomass except in the first diameter category. The most substantial share of the
TABLE 2 Pollution Inputs in the Eadca Area - Average Values in 1980-1990
|
DUST |
TRACE ELEMENTS IN DUST [mg kg-1 year-1] |
|||||
|
[g m-2 month-1] |
Cd |
Cu |
Cr |
Mn |
Ni |
Pb |
|
8.67 |
9.6 |
72.1 |
34.9 |
440.4 |
94.5 |
462.6 |
TABLE 3 Root Biomass of Norway Spruce (Picea Abies (1.) Karst.) in Different Diameter Classes in Terms of Dry Weight [kg ha-1] and Percentage Share in Every Diameter Class
|
|
Root size classes [cm] |
|||||||
|
Plot |
Total |
|||||||
|
|
<0.5 |
0.6-2.0 |
2.1-5.0 |
5.1-7.0 |
.1-10.0 |
>10.0 |
Stump |
|
|
MP1 |
740 1% |
2810 5% |
5960 11% |
3960 7% |
3880 7% |
19420 36% |
18370 33% |
55140 100% |
|
MP2 |
880 1.5% |
4500 7% |
7570 12% |
3630 5.5% |
3600 6% |
19850 31% |
23500 37% |
63530 100% |
|
MP3 |
1390 2.5% |
2340 4% |
4480 8% |
2990 5% |
2360 4.5% |
18960 35% |
22010 41% |
54530 100% |
|
MP4 |
2130 3% |
3500 5% |
6840 10% |
3910 5% |
4470 6% |
32160 44% |
19600 27% |
72610 100% |
biomass in this diameter category is on MP4, where it represents 3 percent of total below-ground biomass.
It is obvious from the biomass distribution (Table 3) that almost all diameter categories on the control plot (MP4) obtained higher estimates. Also, while the total biomass is 72.6 t DW ha-1 on MP4, the lowest total biomass on the polluted plots (on MP3) was as low as 54.5 t, or only 75 percent of the total below-ground biomass recorded from the control plot. The differences in biomass noted on the other polluted plots were not so great.
DISCUSSIONS
Nihlgãrd (1972) estimated the below-ground biomass in a 55-year-old Norway spruce stand to be 59 t DW ha-1. On the other hand, Parshevnikov (1975) estimated root biomass in a 110-year-old Norway spruce stand at 66 t DW ha-1. It may be concluded with regard to MP1-MP4 that the stand density is higher and the growth conditions different. Our data are further confirmed by results from Oszlányi (1986), who estimated the root biomass in terms of below-ground fresh weight at 115 t ha-1 in a 60 year-old Norway spruce stand.
REFERENCES
Bublinec, E., 1992. The Content of Biogenic Elements in Forest Tree Species. Lesn.Èas.-Forestry Journal 38, 365-375.
Ingestad, T., 1982:. Relative Addition Rate and External Concentration: Driving Variables Used in Plant Nutrition Research. Plant Cell. Environ. 5, 443-453.
Kodrik, M., 1992. Below-ground Biomass Distribution of Norway Spruce. In: Forest-Wood-Ecology, Internat. Sci. Conf. pp 151-157. Technical University Press, Zvolen, Slovakia.
Kontriková, O., 1990. Air Quality on the Monitoring Plots. Final Report Institute of Forest Ecology SAS, Slovakia. 27 p. (in Slovak) Slovakia.
Marshall, J.O., Waring, R.H., 1985. Predicting Fine Root Production and Turnover by Monitoring Root Starch and Soil Temperature. Plant Soil 91, 51-60.
Nihlgãrd, B., 1972. Plant Biomass, Primary Production and Distribut. of Chemical Elements in a Beech and Planted Spruce Forest in South Sweden. Oikos, 23, 69-81.
Oszlányi, J., 1979: Biomass Energetic Value of Different Biosociological Position Trees. Lesnícky èasopis 23, 177-188. (in Slovak)
Oszlányi, J., 1986: Analysis into Biomass Production and into its Energy Equivalent of the Tree Layer in Five Forest Ecosystems. Biologické práce 32, 1, Veda Bratislava, 1-157. (in Slovak)
Parshevnikov, A.L., 1975: Productivity and Turnover of Chemical Elements in Northern Phytocoenoses. Nauka, Leningrad, USSR, 128 p. (in Russian).
Persson, H., 1980: Fine Root Dynamics in a Scots Pine Stands with and without Near-Optimum Nutrient and Water Regimes. Acta Phytogeogr. Suec. 68, 101-110.
Schulze, E.D., 1986: Carbon Dioxide and Water Vapor Exchange in Response to Drought in the Atmosphere and in the Soil. Annu. Rev. Plant Physiol. 37, 247-274.
Vyskot, M. et al., 1971: Bases of Growth and Production of Forest. State Agricultural Publishing house Praha, Czechoslovakia. 440 p.
DISTRIBUTION OF CRUSTACEA IN SLOVAKIA'S EASTERN CARPATHIANS AND PROBLEMS OF PRESERVATION
Igor Hudec and Dusan Barabas
Institute of Zoology and Ecosozology
Slovak Academy of Sciences
Juraj Platko
Management in Protected Area of Eastern Carpathians
INTRODUCTION
Knowledge about amphipods in Slovakia's Eastern Carpathians is not satisfactory, but the amphipods are nearly the same in all Slovakia (STRASKRABA, 1953, 1959, 1962). Three amphipod species of the genus Gammarus are noted commonly from Slovakia (G. balcanicus tatrensis, G. fossarum and G. roeselli). Only one record of G. kishineffensis was reported by Straskraba (1962) from Zbojsky Brook. The following surface amphipods have been found to date in southern parts of Slovakia: Sinurella ambulans MULLER, Niphargus valachicus DOBR.& MAN. Other species are restricted to the Danube (Brtek, Rothschein, 1964). The crayfish has not been researched in the Slovakian Eastern Carpathians yet and only two records of A. astacus have been reported in Zbojsky Brook (J. Brtek's personal information). We did not find Asellus aquaticus L. (Isopoda) in the protected area itself but the species was recorded in the Laborec River near Krzl'ov Brod (below Medzilaborce).
CHARACTERISTICS OF THE WATERSHED
The relatively limited permeability of the East Carpathian flysch ensures a shallow circulation of ground water. The accumulation ability of watersheds is very low in spite of the high percentage forestation of the region (Kupco, 1988).
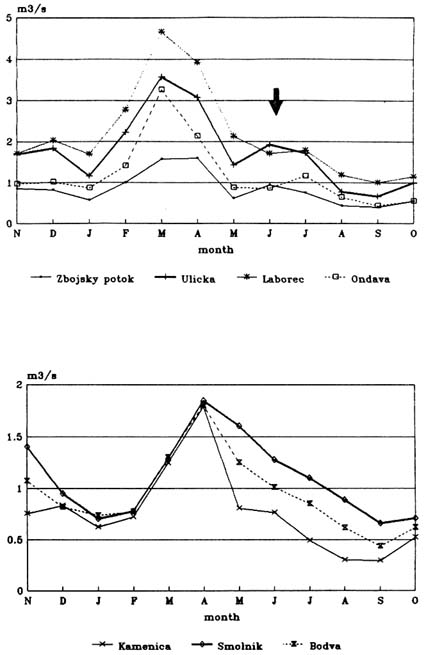
The hydrological regime has features characteristic of flysh (Figure 1, upper part):
-
Maximal mean monthly flows are in March and April, because of snow melt and spring rains;
-
There is later a rapid decrease (May); and
-
The next rise (June - July) in mean monthly flows is the result of summer rains.
The hydrological regime in crystalline complexes and neovulcanits of Slovakia (Figure 1, low part) decrease more slowly in its mean monthly flows after spring maxima, so that the summer rains do not have so strong an influence on mean monthly flows as flysch in the East Carparthians. Some characteristics from comparable large watersheds are in Table 1.
Relatively high and fast drainage of rain is also typical for the East Carpathian flysh. Fifty percent runoff is characteristic of the mean annual flow (Table 1) and up to 80 percent runoff is commonly measured during floods. Floods in such an extreme water regime are designated hydrologically as flood waves.
Both maximal and minimal flows are considered to be the extremes for the whole of the East Carpathian flysh on East Slovakian territory.
Water management is very important for many streams because of strong erosion.
MATERIAL AND METHODS
We researched the Crustacean fauna of surface waters during 1993, but older records were also included. Consideration here is restricted to flowing water habitats. We used Carausu et al (1955) and Jadzewski (1975) to determine identity of Amphipoda and Hennig (1982) for crayfish.
TABLE 1 Hydrological Parameters of Comparable Watersheds on Different Background
|
|
|
|
Mean Annual Value |
|
|
|
Brooks (background) |
Localization of profile |
Watershed area (km2) |
Precipitation (mm) |
Runoff (mm) |
Specific runoff (l.s-1.km-2) |
|
Zbojsky potok (flysh) |
Nova Sedlica |
34.5 |
1026 |
768 |
24.33 |
|
Kamenica (neovulcanits) |
Kamenienka |
39.2 |
1003 |
612 |
19.39 |
|
Ulicka (flysh) |
Ulic |
96.7 |
967 |
571 |
18.09 |
|
Bodva (crystallinic) |
Nizny Medzev |
90.2 |
890 |
329 |
10.43 |
|
Ondava (flysh) |
Vysny Orlik |
108.6 |
825 |
343 |
10.87 |
|
Smolnik (crystallinic) |
mouth |
99.2 |
937 |
350 |
11.09 |
|
Laborec (flysh) |
Krasny Brod |
158.3 |
953 |
427 |
13.52 |
RESULTS
Amphipoda
Gammarus kishineffensis SHELLENBERGER is an East Carpathian element of the Slovak fauna for it has only been found in the main flow of the Stuzica, the Zbojky, the Ulicka (near Runina), and the Ublianka (up to Klenovz). The species was not recorded from small streams in the watershed, and it was also absent from Ulicka below the town of Ulic as a consequence of urban pollution. We suggest simultaneously that as an East-Carpathian element of the Slovakian fauna, G. kishineffensis probably has its westernmost distribution in this area.
Gammarus fossau KOCH was recorded in the Cirocha River both before and during the construction of the Starina Reservoir. However the present study did not locate it there (only G. balcanicus tatrensis was found). G. fossarus is a common species in the rest of Slovak territory, occurring in the main flows of brooks, streams, and springs. The species was found by the authors below Starina Reservoir and in the lower part of the Laborec River.
Gammarus balcanicus tatensis KARAMAN is the only species in the upper and midparts of the Udava River and in all of the area above Starina Reservoir, including all researched springs. It also inhabits only the upper parts of Ulicka and Zbojsky Brooks, including the springs.
Decapoda
Only Astacus astacus L. was recorded from this region, with small populations limited to only some of the brooks and streams of the Udava, Cirocha, Ulicka watersheds. More abundant populations were only noted in and around secondary habitats (stream-ponds) in the western part of East Carpathian region. A surprisingly strong crayfish population was found in the Starina Reservoir when it is recalled that the reservoir was filled only six years ago.
Some individuals were infected with epiparasitic Annelida (Clitellata, Branchiobdellida), mostly Brachiobdella pentadonta WHITMAN, 1882, and less so B. parasitica HENLE, 1835.
CONSERVATION ISSUES
Only Astacus astacus L. needs special efforts in conservation, as according to the ''Red book" (Brtek, 1993) it is a vulnerable member of the Slovak fauna. It is recommended that the preservation of this species be linked with fish management, since both groups have very similar problems where the preservation of habitat is concerned.
The greatest problem in the conservation of crayfish is the water regime on the East Carpathian flysh (Figure 2 and Table 1). Heavy erosion destroys natural
riverine habitats, especially during floods, and this is probably the main reason for the low populations of crayfish, especially in the eastern part of area.
Some time ago a cascade of weirs was constructed on the upper part of the Zbojsky Brook. It would seems that these constructions were inadequate for the hydrological regime because they are almost destroyed now. The authors recommend than an adequate cascade of weirs be constructed in the upper part of the Zbojsky and Ulicka Brooks.
On the basis of the research presented here, the small brook-ponds would also seem to be one possible and ideal way by which to strengthen crayfish populations.
To date, industrial and urban pollution has not been one of the greater problems in the area. Pollution affects only short sections of rivers (e.g., those below the towns of Medzilaborce, Ubl'a and Ulic). It would seem that a greater problem for riverine habitats will result from the contemporary deforestration of large parts of the area (e.g., the Runina district).
Acknowledgment
We thank the Management of the Protected Area of the Eastern Carpathians (Humenne) for partial sponsoring of the research in 1993.
REFERENCES
Brtek J., 1992. Crustaceans (Crustacea), p.54-59. In: L. Skapec (Ed.). Red Book of Endangered and Rare Species of Plants and Animals in CSFR, 3 Invertebrata. PrZroda, Bratislava (in Slovak)
Brtek, J. & Rothschein, 1964. Ein Beitrag zur Kenntnis der Hydrofauna und des Reinheitzustandes des tschechoslowakishen Abschnittes der Donau. Biol. Przce, Bratislava 10, 5: 62 pp.
Carausu S., E. Dobreanu, and C. Monolache 1955. Amphipoda forme salmastre si de apa dulce. Fauna Rep. pop. Romine, Crustacea 4.4: 409 pp.
Hennig A., 1982: Das System der europaischen Fluákrebse (Decapoda, Astacidae): Vorschlag und Begrundung . Mitt. hamb. zool. Mus. Inst. 79: 187-210
Jadzewski K., 1975. Morfologia, taksonomia i wystepowanie w Polsce kieldzy z rodzajow Gammar dem Sammlungen von Prof. Hrab I. V st. eskoslov. spol. zool. 26, 2: 117-145
Kupco M., 1988. Hydrology: 41-47 p. In: I. Voloscuk (Ed.): The East Carpathian protected area. Priroda, Bratislava.
Straskraba, M., 1953. Preliminary report on distribution of the genus Gammarus in Czechoslovakia. Vest. Ceskoslov. spol. zool. 17, 3: 212-227 (in Czech)
Straskraba M., 1959, Contribution to the knowledge of the amphipod fauna of Slovakia. Biologia. Bratislava 14, 3: 161-172 (in Czech)
Straskraba M., 1962, Amphipoden der Tschechoslowakei nach dem Sammlungen von Prof. Hrabe I. Vest. Ceskoslov. spol. Zool. 26, 2: 117-145