Appendix E
Abstracts of Invited Workshop and Poster Presentations
This appendix contains a collection of abstracts of individually authored background papers and posters that were presented at the May 13-15, 1996, Glass as a Waste Form and Vitrification Technology: An International Workshop, sponsored by the Board on Radioactive Waste Management of the National Research Council. These abstracts, intended solely for discussion at the workshop, have not been reviewed or approved by the National Research Council.
Contents
Invited Presentations
|
Mechanical Properties of the Waste Form Glass, |
||||
|
Radiation Effects in Glass Waste Forms, |
||||
|
Experience in Belgium, |
||||
|
Vitrification Experience in France—Development and Perspectives, |
||||
|
Experience in the United States: West Valley, New York, |
||||
|
Vitrification Experience of TVF in Japan, |
||||
|
Vitrification Experience in the UK, |
||||
|
Experiences with Vitrification HL W and Development of New Approaches in Russia, |
||||
|
Vitrification Experience in China, |
||||
|
Vitrification Experience at the Defense Waste Processing Facility (DWPF), |
Poster Presentations (Alphabetical, First Author)
|
Natural Glasses as Analogs for Nuclear Waste Glasses, |
||||
|
Alteration of Nuclear Waste Glasses Characterized by Radon Emanation Method, |
||||
|
DOE Regulatory Initiative For Vitrified Mixed Waste, |
||||
|
Stabilization of Plutonium in Hybrid Glass Materials Using a Cyclone Melter, |
||||
|
DOE Regulatory Initiative: Immobilized Mixed Debris Proposal, |
|
Reexamination of Variables Affecting the Hydration of Obsidians Using Empirical Chronological Data and Laboratory Induced Hydration Experiments, |
||||
|
A Plasma Arc-Vitreous Ceramic Process for Hazardous and Radioactive Waste Stabilization, |
||||
|
Direct Conversion of Metals, Ceramics, Amorphous Solids, Halogens, and Organics to Borosilicate Glass Using the GMODS Process, |
||||
|
Surface Layer Formation of the French SON68 Nuclear Waste Glass During Vapor Phase Alteration at 200° C, |
||||
|
Potential Application of Electron Spin Resonance to the Study of Radiation Effects in High-Level Nuclear Waste Disposal Glasses, |
||||
|
Leaching Behavior of Pu and Cm From Waste Glass Under Reducing Condition, |
||||
|
Glass and Glass-Ceramic Waste Forms Developed at the Idaho Chemical Processing Plant for Immobilizing HL W and Actinides, |
||||
|
Reproduction of Natural Corrosion by Accelerated Laboratory Testing Methods, |
||||
|
Colloid Formation During Waste Glass Corrosion, C. J. Mertz, E. C. Buck, |
||||
|
Use of DC Graphite Arc Melter Technology For Production of Stable Vitrified Waste Forms, |
||||
|
Hanford Low Level Waste Melter Tests, |
||||
|
Experimental Determination of Uranium Oxide Solubility in Hydrous Silicate Melts of Granitic Composition, |
||||
|
Mixture Models Versus Free Energy of Hydration Models for Waste Glass Durability, |
|
Microstuctures and Leach Rates of Glass-Ceramic Waste Forms for Immobilizing Plutionium and Associated Components, |
||||
|
Hot Isostatic Press (HIP) Vitrification of Radwaste Concretes, |
||||
|
Waste Glass Leaching During Open Site Tests, |
||||
|
In Situ Vitrification of Plutonium and Uranium Contaminated Buried Wastes: Microcompositional Analyses of Vitreous and Crystalline Phases and Corresponding Leach Test Results of the Vitrified Products, |
||||
|
The Interaction Between HL W Glass and Clay: Present Status and Future Programme, |
||||
|
Verification Studies on the Pamela High-Level Waste Glasses, |
||||
|
Synroc - An Alternative Waste Form, |
||||
|
Chemistry of Irradiated Solids, |
||||
|
CORAL US: In-Situ Corrosion Test on Active HLW Glass, |
Workshop on Glass as a Waste Form and Vitrification Technology
Washington, May 13-15, 1996, Washington, D. C.
Vitrification of Radioactive Waste: Past Accomplishments and Future Challenges
Werner Lutze
Center for Radioactive Waste Management (CeRaM) and Department of Chemical and Nuclear Engineering
The University of New Mexico
Albuquerque, NM 87131
Abstract
The history of vitrification of HLW is reviewed. The development of various vitrification technologies, pretreatment strategies, and the focus on two types of melters, the metallic and the ceramic melter, are explained. Various vitrification plants are in operation worldwide with France being the leader where three plants are in operation, AVM at Marcoule, R7, and T7 at La Hague. R7 and T7 are commercial plants. Another commercial vitrification plant, based on the French process, is in operation in Sellafield, England. Very recently, the first vitrification plant for defense waste was put into operation in the United States at the Department of Energy facility at Savannah River, S. C. Another vitrification plant in the U.S. will go into operation at West Valley Nuclear Services, N. Y. later this year. Experience with both melter types in hot operations (France, England and Germany) and lessons learned are highlighted. Research and development toward a new melter type, the cold crucible, is addressed and the potential advantages over existing melters are outlined. The main drivers for this effort are 1) significantly decreased melter corrosion and thus increased life time and operation safety, 2) greater flexibility in the melting temperature, and 3) greatly reduced contamination of the melter and less melter waste. For an overview of waste form development and vitrification technologies see reference 1 and for glass corrosion reference 2.
The development and selection of waste forms, glass vs. crystalline materials, is addressed and the selection criteria are discussed. Borosilicate glasses has become the most widely used waste form for high-level radioactive reprocessing waste for both defense and commercial waste. However, large quantities of high-level waste are immobilized in phosphate glass in Russia.
New glass waste forms are under development in the United States to vitrify and to dispose of surplus weapons plutonium and other waste streams high in actinides. Alternatively, a variety of ceramics is under investigation in Russia and in the United States.
Vitrified waste must meet certain waste acceptance criteria for disposal. These criteria are derived from glass properties measured in the laboratory. Glass properties are reviewed and the state of the art is presented. Glass properties are ranked in terms of significance for glass performance in the repository. Areas for further research, e.g., long-term radiation effects and long-term chemical durability and the use of natural glasses as analogs are pointed out. The dependence of glass performance on the geochemical (repository) environment is addressed.
Finally, areas of research on alternative glass processing are addressed, for example sub-liquidus processing. The wide variety of existing waste streams calls for various types of pretreatment prior to solidification. The choice of the waste form and the process to make the waste form are important considerations for the design of waste pretreatment procedures. Glass and the melting process are not always the best choice no matter how much experience has been acquired with vitrification. The limits for glass as a waste form are demonstrated using particular waste streams as examples.
References
1. Radioactive Waste Forms for the Future, W. Lutze and R. C. Ewing, eds., North Holland, Amsterdam 1988, 778p.
2. High-Level Waste Borosilicate Glass, A Compendium of Corrosion Characteristics, 3 volumes, DOE-EM-0177, 1994
OVERVIEW OF CURRENT DOE PLANS AND ACTIVITIES INVOLVING VITRIFICATION
Summary of Presentation to:
National Academy of Sciences Vitrification Workshop
by
Stephen P Cowan, U. S. Department of Energy
The Department of Energy must address the legacy of over fifty years of nuclear weapons research, development, and production. Wastes with varying levels of a broad range of radionuclides and hazardous chemical constituents must be managed to minimize: current risks to workers; future risks to the environment and potential surrounding populations; and the costs to the nation. Newly generated wastes, and wastes in storage, are the responsibility of the Office of Waste Management.
The fundamental problem the Department must address is that the wastes to be managed are not currently in the correct form or location for permanent isolation. Therefore, the waste must be treated. The question is: "What would be the ideal process for this treatment?". The ideal process would result in a reduced volume of waste in a stable, durable solid. The process should be relatively simple - suitable for remote operations in a radioactive environment, adaptable to a wide variety of feeds, and capable of destroying or immobilizing the hazardous constituents of concern as well as the radioactivity over the time period during which an unacceptable risk is posed. The vitrification process, and the glass waste form, are the best candidates the Department, and indeed other nations, have found.
A brief history: Beginning in the 1940's, the Manhattan Project scientists knew that tank storage of liquid wastes was a temporary measure.
The Atomic Energy Commission began examining immobilization and disposal options in the 1960's, and by the 1970's specific waste forms were being evaluated. Options examined included stabilizing the wastes in the tanks or making grout for underground injection beneath the sites where the wastes were stored. But the option that seemed consistently most appealing was the removal of the wastes from the tanks, separation of the high activity fraction from the low activity fraction, and immobilization of the high activity fraction in a stable, leach resistant form. Waste forms evaluated included concrete, a variety of glass formulations, and ceramics. Two criteria were of premier importance - the durability of the waste form and the simplicity of the process to enable remote operations in a radioactive environment.
Through a formal Departmental process, the Department selected borosilicate glass for high level waste immobilization. A number of vitrification processes for low level, mixed, and environmental restoration wastes are in various stages of evaluation, demonstration, or implementation.
Implementation of the vitrification waste form initially focused on high level waste (HLW) because it contains the largest inventory of curies. Of the four sites with HLW, the Savannah River Site has the most curies, followed by the waste stored at Hanford. The waste at both of these sites, as well as most of the volume at the West Valley site, is alkaline liquid stored in carbon steel tanks. The HLW at the Idaho National Engineering Laboratory is stored both as
an acidic liquid in stainless steel tanks and as calcined solids in stainless steel bins, with more than 90% of the radioactivity in the calcine. The total inventory of DOE HLW is approximately 950 million curies. Vitrification has been recognized by the Environmental Protection Agency as the "best demonstrated available technology" for HLW. Glass is also the waste form for immobilizing HLW in France, the United Kingdom, Belgium, and Japan.
The Defense Waste Processing Facility at the Savannah River Site, initiated radioactive activities on March 12, 1996; the first canister of radioactive glass is scheduled to be produced in the very near future. Initiation of radioactive activities at the West Valley Demonstration Project are imminent, with production of the first canister of radioactive glass scheduled for late June.
For the waste at the INEL, DOE is proposing a "Full Treatment Alternative" for integrating all the waste streams managed by the Office of Environmental Management. This alternative includes operation of a vitrification/ separations facility after 2017 which will process remote-handled transuranic waste, as well as the liquid HLW and the high-activity fraction of the calcine.
DOE is also planning to use vitrification technology for low level wastes at the Fernald site, Mound, and at Hanford. Wastewater treatment sludges containing both hazardous constituents and radioactivity (i.e., mixed wastes) will be vitrified for disposal at Oak Ridge, Los Alamos National Laboratory, the Rocky Flats Environmental Technology Site, and the Savannah River Site.
IDENTIFICATION AND SUMMARY CHARACTERIZATION OF MATERIALS POTENTIALLY REQUIRING VITRIFICATION1
Allen G. Croff
Oak Ridge National Laboratory2
The United States and many other nations have and continue to produce a wide variety of radioactive materials that are wastes or may otherwise be declared to be surplus and be managed as if they were wastes. In either case, it is commonplace to subject these materials to treatment processes to appropriately condition them for subsequent storage, transportation, and disposal. Less toxic wastes are generally accorded minimal treatment whereas highly toxic wastes are accorded extensive treatment. One of the more common treatment technologies applied to the more toxic end of the waste spectrum is vitrification. The purpose of this paper is to identify those materials that have some potential for vitrification and to summarize their characteristics as background for subsequent papers.
Identification of materials having some potential for vitrification begins by considering the broad categories of existing nuclear wastes or materials that may be declared surplus, which are listed in the first column of Table 1. Uranium mill tailings and enrichment plant tails are excluded because their large volume and low toxicity result in essentially no potential for vitrification.
The next three columns of Table 1 provide summary information concerning the inventory and/or production rate of the materials, the radioactivity density, and the power (radioactive heat) density. The densities can be viewed as crude measures of the toxicity of the material. The trend of these numbers reflects a longstanding paradigm for management of radioactive materials that results in the high radioactivity/power materials being concentrated in relatively small volumes and conversely for the low radioactivity/power materials. In general, as the volume of the materials increase, the practicality of applying advanced treatment technologies such as vitrification decreases. Further, as the radioactivity and power density of the material increases, the appropriateness of a process such as vitrification that yields a high-integrity product increases.
The fifth column of Table 1 provides a very brief description of the subject material. In some cases, the material is relatively uniform (e.g., LWR spent fuel, Cs/Sr capsules, surplus Pu). In other cases, the nature of the material in a waste category is extremely diverse ranging from liquids to soil to contaminated rabble to trash (e.g., transuranic waste, low-level waste).
The above considerations strongly affect the extent to which various material types or sub-types are considered to be candidates for vitrification. However, at least as important as the technical considerations given above are precedent, current Federal policies, and stakeholder views (e.g., agreements negotiated between a state and the U.S. Department of Energy). As a result, combining all of these to arrive at a conclusion of the vitrification potential of a particular material is subjective. The author's views concerning the vitrification potential of the material types and selected sub-types is given in the last column of Table 1.
TABLE 1. SUMMARY OF U.S. MATERIALS HAVING SOME POTENTIAL TO BE VITRIFIED
|
Material Type |
Volume, m3 or m3/yra |
Radioactivity Density, Ci/m3 |
Power Density, W/m3 |
Material Description |
Vitrification Possibilities |
|
1. Spent civilian nuclear fuel |
12000 |
10,000,000 |
50000 |
Light-water reactor spent fuel |
Unlikely unless required by repository |
|
2. DOE spent fuel |
1200 |
Not quantifiable; Moderate-to-high |
Not quantifiable; Moderate-to-high |
Variety of spent fuels |
Likely for Al-clad fuels, possible for others |
|
3. DOE "tank" wastes |
375000 |
1,000 - 10,000 |
5 - 50 |
Alkaline liquid, saltcake, sludge; calcine |
Highly likely for essentially all retrieved tank waste |
|
4. Capsules: Cs Sr |
3.5 1.1 |
23,000,000 21,000,000 |
115,000 140,000 |
Capsules of CsCl Capsules of SrF2 |
Likely if overpack is unacceptable |
|
5. Transuranic wastes |
|
|
|
Wide variety of materials with TRU >100 nCi/g |
Likely for only a small fraction unless WIPP-WAC change substantially |
|
Remotely handled |
2,500 + 14/yr |
1,000 |
1 - 2 |
|
|
|
Contact handled |
70,000 + 1500/yr |
25 - 50 |
0.5 - 1.5 |
|
|
|
6. Low-level radioactive waste |
|
|
|
Extremely wide variety of materials with <<100 nCi/g |
Likely for LLW from tank waste processing. Unlikely for most other LLW. |
|
DOE |
38,000/yr |
9 - 27 |
0.01 - 0.05 |
|
|
|
Commercial: Class A |
|
0.6 |
0.03 - 0.1 |
|
|
|
Commercial: Class B |
24,000/yb |
60 |
15 |
|
|
|
Commercial: Class C |
|
0.1 - 7,000 |
0.003 - 115 |
|
|
|
Commercial: > Class C |
63 + 20/yr |
>0.1 - high |
>0.003 - high |
|
|
|
7. Low-level mixed waste |
|
|
|
Extremely wide variety of materials with <100 nCi/g and hazardous chemicals |
Likely in selected applications, but extent is unpredictable |
|
Commercial |
2,100 |
Not quantifiable; low |
Not quantifiable; low |
|
|
|
DOE |
138,000 |
|
|
|
|
|
8. Surplus plutonium |
2 |
11,000,000 |
44,000 |
Plutonium metal shapes |
Either vitrification or irradiation will be used |
|
9. Environmental restoration |
78,000,000 |
Not quantifiable; low with small-volume exceptions |
Not quantifiable Low with small-volume exceptions |
Extremely wide variety of materials and contamination |
High-toxicity wastes and some in-situ are likely. Unlikely for the bulk of the waste. |
|
a Fixed values are existing volumes which are given where production has essentially ceased or where disposal rates are approximately equal to production rates. Rates are given where volumes continue to increase significantly. b Sum of annual production rates for Classes A, B, and C. |
|||||
Hanford Wastes and Glass Composition
Pavel Hrma, Pacific Northwest National Laboratory, MS P8-37, Box 999, Richland, WA 99352; PR_Hrma@PNL.GOV
Hanford Site high-level and low-level wastes (HLW and LLW) vary over a broad range of compositions. Vitrification of these wastes require the formulation of glasses that are chemically durable, processable by the melting technology available, and can achieve a minimum volume of vitrified waste (volume minimization can save $ 20 to 40 billion). This process entails producing acceptable glasses with both traditional (Na2O, Al2O3, ZrO2, Fe2O3) and unusual (P2O5, CeO2, Cr2O3, NiO) components at high concentrations that have not been attempted in commercial borosilicate systems. To achieve the minimum volume of the vitrified waste form, each step of the immobilization sequence, i.e., retrieval, pretreatment, blending, and melting, must be optimized with respect to glass formulation, which is the axial tool in the waste vitrification strategy.
Obtaining a minimum volume of waste glass translates into achieving a maximum waste loading (the mass fraction of non-volatile matter in the waste glass). The maximum waste loading is defined in terms of limiting values of key glass properties, which in turn are functions of glass composition. For glasses with maximum loading of Hanford Site wastes, the properties that limit waste loading are liquidus temperature and chemical durability. Available and demonstrated melting technology may impose a limit on melting temperature (the temperature at which glass viscosity is 5 Pa·s), but glass melting temperature does not limit waste loading per se.
The major components that limit waste loading for the Hanford Site tank wastes are Na2O, Fe2O3, Al2O3, ZrO2, P2O5, and Cr2O3. No fixed limiting concentrations of these components exist because glass properties are functions of the overall composition. When formulating the glass, its properties are adjusted by glass forming and modifying additives, namely SiO2, B2O3, Na2O, and Li2O. The task is to find an optimum mix of these additives that would enable the maximum concentration of the particular waste component that limits waste loading. Laboratory experiments have shown that waste loading as high as 80 wt% may be possible for the Hanford Site all-blend HLW (the vitrified product has a substantial fraction of crystallinity precipitated during cooling), while waste loading higher than 35 wt% is unlikely for the Hanford Site LLW.
Chemical durability is the ultimate intrinsic waste loading limitation, and, hence, the ultimate challenge to the glass formulation effort. Liquidus temperature limits waste loading in glass because most of the current continuous melters do not tolerate crystallinity; thus, this problem can be eliminated through melter development. Highly loaded HLW glass may contain a substantial fraction of crystalline phase, which does not impair durability. However, crystallization of nepheline or eucryptite during cooling weakens the glass structure by removing both aluminum and silicon from the glass network. This has a detrimental effect on glass durability and thus imposes a limit on the waste loading. Generation of other crystalline phases (e.g., spinel and zircon) or segregation of liquid phases (sulfate-phosphate-chromate) usually causes processing problems. Interestingly, though sulfate is not in sufficient concentrations m Hanford wastes to cause harm in itself, it may induce segregation of phosphates.
Formulation of Hanford waste glasses can be effectively achieved with the use of mathematical models that relate glass properties to glass composition. The model response functions are typically nonlinear because the effects of glass components on key glass properties are interactive. Therefore, the simplest form of the property-composition response functions is a second-order polynomial. Second-order empirical mixture models have been developed for viscosity, electrical conductivity, liquidus temperature, and chemical durability of Hanford
HLW glasses using statistically designed studies. These models span a broad region of composition, but are not yet fully sufficient for waste loading maximization because their original design was focused on glasses with low waste loading values (25 to 28 wt%) and low processing temperatures (1150°C).
Liquidus temperature is a property that is difficult to model because several primary crystalline phases appear in Hanford HLW glasses and each of these phases requires a special response function. This puts an extra demand on the amount of measured data required and brings a problem of determining which crystalline phase is primary for a given composition. The FACT model (Facility for the Analysis of Chemical Thermodynamics), which is semiempirical, successfully approaches these difficulties, but has not yet been developed enough to predict the primary phase and liquidus temperature with the required accuracy. Experiments are underway to improve the liquidus temperature database for more accurate model coefficients. Nepheline and eucryptite formation, which impairs durability, is even more difficult to predict because nonisothermal crystallization kinetics is involved in the process. Fortunately, promising results have been recently obtained in this area as well.
Hanford Site wastes are generally rich in sodium. The LLW contains approximately 80 wt% Na2O on the nonvolatile oxide basis, which limits waste loading to 25-40 wt%. Hanford HLW contains 20 to 30 wt% Na2O, the rest being mainly refractories. As the waste loading increases, refractory oxides precipitate forming crystalline phases while Na 2O accumulates in the residual glassy phase, which is at the same time depleted of SiO2, Al2O3, and ZrO2. Hence, both for Hanford HLW and LLW, the waste loading is ultimately limited by insufficient chemical durability due to high concentration of Na2O in glass.
Presently, Hanford HLW glass cannot be loaded to its maximum level determined by the chemical durability of the waste form because such glass may contain a substantial fraction of crystalline phase, a part of which may precipitate at a high temperature and thus may interfere with the operation of continuous melters. The rate of formation of the crystalline phase within continuous melters is strictly limited to ensure a sufficiently long melter campaign.
The major points to consider with regard to Hanford wastes and glasses are:
-
HLW glass processability is constrained by liquidus temperature, which increases with the fraction of refractory components in the glass.
-
The robustness of the glass structure deteriorates quickly when crystallization concentrates alkali oxides within the residual glass phase and removes glass stabilizing oxides, such as silica, alumina, and zirconia into crystalline phases.
-
Phase separation of a phosphate-rich liquid phase, which may or may not crystallize on cooling, constrains both glass processability and performance.
-
Glass crystallization and phase separation will be the major issue dominating HLW glass formulation in the future as long as the waste form volume minimization is targeted.
-
The main issue for the LLW glass is incorporation of a high level of sodium oxide into the glass structure without destroying its integrity.
ACCEPTANCE OF WASTE FOR DISPOSAL IN THE POTENTIAL UNTIED STATES REPOSITORY AT YUCCA MOUNTAIN, NEVADA
David Stahl
Framatome Cogema Fuels
Civilian Radioactive Waste Management System Management & Operating Contractor
Las Vegas, Nevada
INTRODUCTION
The Nuclear Waste Policy Act (NWPA) of 1982 (Public Law 97-425) established a national plan to develop a repository for the permanent disposal of high-level radioactive waste. The U.S. Department of Energy's (DOE) Office of Civilian Radioactive Waste Management (OCRWM) has the responsibility of developing the nation's first high-level waste (HLW) repository. HLW includes wastes — from defense and commercial reprocessing operations — that are encapsulated in borosilicate glass as well as spent nuclear fuel (SNF) from commercial power reactors. As described in the following paragraphs, the mix of fuel may be amended to include other DOE SNF. The U.S. Nuclear Regulatory Commission has the responsibility for promulgating the technical requirements necessary to license all phases of repository operation. The development of the repository has been delegated to the DOE's Yucca Mountain Site Characterization Project Office. Framatome Cogema Fuels (formerly B&W Fuel Company), as part of the Civilian Radioactive Waste Management System Management & Operating Contractor, is responsible for designing both the waste package and the engineered barrier system.
The goal of the DOE's Yucca Mountain Site Characterization Project is to characterize the Yucca Mountain site and design a potential geologic repository for the safe disposal of SNF and solidified HLW. Yucca Mountain is about 160 km northwest of Las Vegas, Nevada and consists mainly of compacted layers of volcanic ash flows (tuff). The repository horizon lies in the densely welded Topopah Spring member. Safe disposal of waste will rely on: the unsaturated nature of the Yucca Mountain site in which the flow of groundwater into the repository will be slow; a robust multi-barrier waste package that will remain intact for thousands of years; the slow mobilization of radionuclides from the waste forms; the retardation of radionuclides within the engineered barrier system; and the dispersion and mixing of radionuclides in the groundwater system below the repository.
WASTE ACCEPTANCE
The NWPA of 1982 limits the content of the first U.S. repository to 70 000 metric tons of heavy metal (MTHM) until a second repository is in operation. The DOE Mission Plan and Mission Plan Amendment (MPA) describe the implementation of the provisions of the NWPA for the waste management system. In the Draft 1988 MPA, the repository inventory was further broken down into about 63 000 MTHM of spent fuel and 7,000 MTHM of HLW glass. The current inventory of spent fuel located in storage at the reactor sites is about 30,000 MTHM and is expected to reach 40,000 MTHM by the year 2000. The HLW is currently anticipated to be borosilicate glass logs like those to be produced by Savannah River and West Valley. In response to a recent request from the Office of Environmental Management, OCRWM has agreed to revise its waste acceptance planning baseline to accommodate the potential substitution of DOE-owned spent fuel for some portion of the HLW glass, 7,000 MTHM defense-waste allocation, DOE-owned spent fuel proposed for geologic disposal includes types such as production reactor, research reactor, and U.S. navy.
The acceptance of waste into the waste management system is constrained by technical baseline requirements such as those delineated in the Waste Acceptance System Requirements Document (WA-SRD), DOE/RW-0351P Revision 1, March 1994. The requirements for the DOE-owned SNF have been collected in the Preliminary Requirements for the Disposition of DOE Spent Nuclear Fuel in a Deep Geologic Repository (December 1995). Future revisions to the waste acceptance requirements documentation will incorporate those requirements for the other waste forms. The WA-SRD currently picks up regulatory requirements from Title 10 of the Code of Federal Regulations. These include Part 60, Disposal of High-Level Radioactive Wastes in Geologic Repositories, Part 71, Packaging and Transportation of Radioactive Material, and Part 961, Standard Contract for Disposal of Spent Nuclear Fuel and/or High-Level Radioactive Waste. Other Title 10 regulations, DOE Executive Orders and other requirements are also included, the latter drawn from upper-tier system requirements documents or derived as a result of practice or analysis.
Particular Part 60 requirements of interest to waste acceptance include 60.135 that defines specific design criteria for the waste package and its components. These criteria include constraints on the general performance of the package, its chemical reactivity, and provisions for its handling and labeling, as well as design criteria for the waste form. Of relevance is the limitation of explosive, pyrophoric or chemically reactive materials that could compromise the ability of the waste packages to meet their containment and waste isolation requirements (identified in 60.112 and 60.113). In addition, 60.21(c)(1)(ii)(D) requires the comparative evaluation of alternative designs that would provide longer radionuclide containment and isolation. Another requirement of concern, defined in 60. 131(b)(7), is the assurance that criticality control is maintained during the period of waste isolation in the repository.
For commercial light-water reactor fuels and borosilicate glass HLW, waste form contribution to repository performance has been assessed through the use of computer models (total system performance assessment) and physical testing of spent fuel. To accommodate the acceptance and disposal of DOE-owned spent fuel, similar performance and compliance issues must be addressed through modeling and testing programs specifically oriented towards these materials.
To this end, the DOE Offices of Environmental Management and Civilian Radioactive Waste Management have established technical coordinating groups for both HLW and DOE-owned SNF which meet on a quarterly basis to monitor interface issues between these programs. The objectives if this coordination are to provide reasonable assurance that the disposal system will be able to accommodate the waste forms as designed and to craft resolution strategies for emerging technical issues.
SUMMARY
The process for the acceptance of waste into the waste management system is discussed, with detailed requirements identified from the Waste Acceptance System Requirements Document. Also described is the recently initiated issue resolution dialogue between OCRWM and the Office of Environmental Management, including the appropriate interpretation and application of regulatory and system requirements to DOE-owned spent nuclear fuel.
A Review of Status of Science of Vitrified Waste Form Development
George G. Wicks
Westinghouse Savannah River Technology Center
Aiken, SC 29802
As a result of more than three decades of study and evaluation, glass is the material of choice for incorporating and immobilizing potentially hazardous radionuclides found in high level radioactive wastes (HLW). It is also being considered for isolation of a variety of other hazardous waste types, both radioactive as well as non-radioactive. This includes vitrification of a variety of actinides resulting from clean-up operations and the legacy of the cold war as well as possible immobilization of weapons grade plutonium resulting from disarmament activities. Other types of wastes being considered for immobilization into glasses included transuranic wastes, mixed wastes, contaminated soils, asbestos, incinerator ashes, medical wastes, electronic circuitry, weapons parts, and a variety of other potential hazardous materials and components.
There are many factors which contribute to the suitability of glasses for immobilization of these wastes. In general, these considerations fall into two major technical categories. First, involves PROCESSING CONSIDERATIONS, which include the ease of being able to produce waste forms, routinely and reproducibly, even under difficult remote conditions if necessary, and second, the TECHNICAL PERFORMANCE FEATURES of the final solidified forms. Technical performance features include good waste form performance in five major areas of interest; (a) flexibility/ waste compatibility (b) mechanical integrity, (c)thermal stability, (d) radiation effects and (e) chemical durability. Chemical durability is generally considered as the most important technical property of the final waste form. There are also other important considerations of a less technical nature such as immediacy of implementation, use of existing resources, including experience, expertise and facilities, and also, the economics of the effort. Consideration of all these factors are essential in the development of high integrity, cost-effective waste forms and subsequent systems designed to manage or permanently dispose of the hazardous materials.
Using an interdisciplinary approach and building upon the experience and knowledge of classical glass science, complex borosilicate glass systems have been developed which have successfully immobilized the more than 40 elements contained within HLW as well as the large fluctuations in waste composition that can exist. This technology has now become the cornerstone of our understanding of fabrication and
performance of nuclear waste glasses as well as for vitrification of a variety of other waste systems. Significant contributions to the science of waste form development have come from many sources, including academia, industry, federal and national laboratories, and international cooperative programs. Over the past years, many hundreds of papers have been published in this field which has advanced our understanding and confidence of the waste glass systems and further defined important new areas for research. It will be the objective of this presentation to review the status and science of waste glass forms. This will be undertaken by first, providing a brief historical background into the subject, while emphasizing the U.S. HLW program and development of HLW waste glasses. Next, an overview will be given of key properties of the waste glass forms along with examples of both important and interesting data. More detailed technical discussions will be provided in each of these technical areas by experts in subsequent presentations. Finally, some key properties of waste glass systems will be related to the structure of glass and a correlation will be given which ties together the many different HLW glass systems developed world-wide.
Leach Tests and Chemical Durability
Robert H. Doremus*
Rensselaer Poly. Inst.
Troy, NY 12180
Experimental tests of the rate of reaction of water with glass allow one to judge the relative durability of different glass compositions and to predict the long term stability of encapsulants of radioactive materials. In this discussion steps in the reaction of water with glass are first described, and then methods of analyzing for the effects of this reaction are considered. Then the types of leach tests are listed and compared. Finally some results of tests of durability of different glass compositions are described.
In liquid water, there are at least three different processes taking place during reaction with silicate glasses. The mobile alkali ions in the glass exchange with hydronium (H3O+) ions from the water. The result is a surface layer depleted in alkali ions and containing H3O+ ions. When the glass is removed from the water, water molecules from decomposition of the H3O+ ions can diffuse from the glass or ''outgas.'' The exchanged alkali ions form alkali hydroxide in the liquid water. 2. The glass dissolves in the water. Silicon-oxygen bonds are broken in the glass to form SiOH groups, and finally H4SiO 4, silicic acid, dissolves in the water, as well as the other oxide constituents of the glass. 3. Surface layers form by precipitation of substances from the water, or from reaction of ions in solution with glass components at its surface. If the glass is reacting with water vapor rather than liquid water, processes 1 and 3 above are possible. The product of ion exchange is, for example, sodium hydroxide, which reacts with carbon dioxide in the atmosphere to form solid sodium carbonate, which remains on the glass surface. A variety of other compounds can also form on the glass surface in a vapor test, depending on the glass composition and temperature.
To analyze the results of these processes a variety of tests are possible. Measuring the weight change of the glass was the first method used to follow reaction of water with glass, and in carefully controlled tests this method gives valuable information. Chemical analysis of solution constituents as reaction progresses is the most common method of following leach tests in liquid water. Analysis of the constituents on the glass surface, and profiles of elements into the glass, are possible by a variety of modern techniques, such as electron microscopy, diffraction, nuclear analysis (Rutherford backscattering, RBS, and resonant nuclear reactions), electron microprobe, and secondary ion mass spectrometry (SIMS). The choice of methods requires care, to be certain that the information needed can be deduced from the analyses.
The way in which a leach test is carried out can have a large influence on the results. The rates of the processes described above depend on the following variables at least: temperature, solution concentrations, especially pH, glass composition, flow in the liquid, and relative humidity in a vapor test. To obtain reliable results that can be used to model the prognosis of the reaction and understand mechanisms, these variables must be controlled, as described by Dr. Bourcier. A static test in which solution conditions are allowed to wander is a poor test for comparing glass compositions and predicting long-term durability; for example, in such a test pH can increase to a maximum and then decrease. Repository conditions can vary widely, and a static test is not a model for most of them.
Ion exchange results in a hydrated surface layer on silicate glasses. In more durable glasses the surface structure remains the same as in the dry glass, with consequent low mobilities of ions and molecular water in the hydrated layer. In tests in which solution conditions are held constant, for example at pH7, there is a wide difference in rates of dissolution of different glasses. The sequence of selected
compositions is, most durable first: Obsidian < fused silica and soda-lime with 2% alumina < Pyrex borosilicate < commercial soda-lime without alumina < high soda soda-lime < binary soda-silicate. As the amount of silicic acid dissolved in the water is increased, the rate of dissolution decreases. Silicate glasses dissolve more rapidly as the pH is increased above 7.
These results show that there is a large potential for increasing the durability of glasses for encapsulating radioactive waste.
References:
1. D.E. Clark and B.K. Zoites, eds., "Corrosion of Glass, Ceramics and Ceramic Superconductors," Noyes Pub., Park Ridge, NJ, 1992.
2. R.H. Doremus, "Glass Science," 2nd ed., Wiley, New York, 1994, Chpt. 13.
3. G. Perera, R.H. Doremus, and W.A. Lanford, J. Am. Cer. Soc. 74, 1269 (1991).
4. H.H. Dunken, "Physikalische Chemie der Glasöberflache," VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, Germany, 1981.
Status of Vitrification Technologies
M. J. Plodinec
Westinghouse Savannah River Company
P. O. Box 616
Aiken SC 29802
ABSTRACT
Vitrification (here defined as any thermal technology which produces a vitreous product) is internationally accepted as the technology of choice for the immobilization of highly toxic radioactive waste. The French, British, Japanes, and Belgians are all operating vitrification plants for high level nuclear waste (HLW). In the US, the Defense Waste Processing Facility (DWPF) recently began radioactive operations on the HLW stored at Savannah River. The US Environmental Protection Agency has declared vitrification to be the "Best Demonstrated Available Technology" for the treatment of HLW.
Vitrification has been widely accepted for several reasons. The glass waste form is very tolerant of chemical variability; as a result, nearly all of the hazardous radionuclides (the most important exception is tritium) can be captured in glass. The waste form itself is robust, and has stood up well to extended testing in geologic conditions, even at elevated temperatures. In general, vitrification of toxic wastes greatly reduces the volume of material which may have to be transported, stored and placed in final disposal. Since glassmaking has been carried out for over two thousand years, the processing technology is reliable and relatively well-understood.
However, vitrification is not just a single technology, but rather a family of technologies. The main branches are differentiated by the method of heating to melt materials to form a glass. For example, bushing melters use metal screens as electrical resistance elements which heat chemicals to form a molten glass. Joule-heated melters are heated by passing a current through the glass. Plasma torch melters use torches to generate extremely high temperatures. Combustion melters burn fuels to generate heat. Graphite arc furnaces generate heat by a spark passing from a graphite electrode to either the material to be melted or another electrode. High frequency melters use either microwave energy or induction heating to form a melt.
Each of these is compatible with some types of wastes or other materials to be vitrified; none of these is compatible with all such materials. Compatibility with a particular type of waste must be judged in terms of several factors, which may include
-
Amount of characterization needed.
-
Amount of feed preparation needed.
-
Reduction of volume compared to starting material.
-
Corrosion and erosion of the melter and downstream process vessels.
-
Capability to be operated and maintained remotely.
-
Costs of facility and of operation.
-
Upstream processing.
For each application of vitrification technology, production of an acceptable waste form is
of paramount importance. This can be assured either through exhaustive characterization of materials (e.g., of waste feeds) prior to processing, or through characterization of the final product DOE has found that both paths can be followed. For HLW, extensive characterization of the waste is needed to ensure reliable processing. In this case, the processing information is sufficient to ensure that a durable glass product will be made. Conversely, the cost and risks associated with handling highly radioactive glass samples are high. While after-the-fact sampling can determine whether a given glass sample is acceptable, it would then require recycle of any unacceptable glass produced. Thus, HLW glass producers in the US are relying on feed characterization and process control to ensure production of an acceptable glass.
For LLW, or other heterogeneous materials, product sampling may be more cost-effective. Waste characterization is much more difficult than for HLW. Handling of the product samples is often almost trivial, and may provide a more representative view of the final product than feed sampling.
The probability of identifying an unacceptable product as acceptable through direct product sampling should be quite low. With a properly executed process control program, the probability of producing an unacceptable product through characterization of feed material is also quite low. As an example, during the Waste Qualification Runs in the DWPF, the feed was intentionally varied over the full range of waste types which are expected to be processed. Changes in composition were accomplished much more abruptly than is likely to occur in actual production, providing a strenuous test of the DWPF product control system. Each of the canisters of simulated waste glass produced was destructively examined, and the results compared to the specifications for the glass product. The results were outstanding — all of the glass samples tested were far below the acceptance limit, and there was greater than 99% confidence that more than 99.9% of the glass produced was acceptable.
Based on this experience, there is great confidence that vitrification technologies employed in a production setting will successfully produce glass products which will effectively immobilize hazardous species. The keys to this success will be selection of melters based on compatibility with the feed material, and application of product control programs which will ensure reliable production of acceptable products.
THE CHEMISTRY AND KINETICS OF WASTE GLASS CORROSION
John K. Bates, Chemical Technology Division, ARGONNE NATIONAL LABORATORY, Argonne, IL 60439-4837, bates@cmt.anl.gov
Under repository disposal conditions, the reaction of glass with water comprises the source term for release of radionuclides to the near-field environment. An understanding of glass reaction and the manner by which radionuclides are released is needed to design the waste package and to evaluate the total performance of the repository. The ASTM Standard C-1174-91 [1] provides a general methodology for obtaining information related to the behavior of glass. This paper reviews the application of this standard to glass reaction.
In the first step in the ASTM approach, the researcher identifies the materials and the conditions under which the long-term behavior is to be determined. Neither of these factors is easily defined, yet the successful evaluation of glass behavior requires estimates of each in order to proceed. Glass compositions have undergone a genesis over the past 15 years in response to concerns about feed streams, processing, and durability. A range of borosilicate compositions has been identified [2], but as new applications for vitrification occur, for example, immobilization of weapons plutonium and residue from plutonium processing, different compositions must be evaluated. The repository environment depends on the spatial emplacement of waste containers (glass and spent fuel) [3], and both "hot" and "cold" scenarios have been proposed for the Yucca Mountain site. Regardless of the exact configuration, the near-field hydrology is expected to be unsaturated: that is, the waste packages are contacted initially by water vapor, and ultimately by small amounts of dripping or standing water. The behavior of glass can be studied as a function of composition within the constraints the environmental conditions place on the physical parameters that affect glass reaction (temperature, radiation field, groundwater composition, etc.).
In the second step, the researcher reviews the literature and proposes a reaction pathway by which glass reacts in an unsaturated environment. This allows bounding ranges of important physical parameters to be established so that the effect of each parameter on the anticipated alteration process can be established. A reaction pathway for glass is proposed that divides glass reaction into three stages. In a thermodynamic sense, the reaction of glass with water proceeds to minimize the free energy of the system and also to minimize the chemical potential gradients between the solution and the glass. When the gradients are steep, particularly for major glass-forming components such as SiO2, the rate of reaction will be relatively high, whereas when the gradients are comparatively shallow, the rate of reaction will be relatively low. The slope of the chemical potential gradients and the energy required to extract the rate-limiting component from the surface of the glass are controlling factors in determining the rate of glass corrosion. The chemical potential gradients between the glass and water are initially large (Stage I) because the leachant is dilute, and the reaction proceeds at the forward rate. As the silicic acid concentration in solution increases, the chemical potential gradient decreases (Stage II). Glass reaction during Stages I and II usually results in the formation of a reacted layer, which may or may not be crystalline, but does not result in an increase in the glass reaction rate. However, the glass is unstable with respect to certain alteration phases that nucleate during corrosion. These phases will serve as sinks for key glass forming elements such as Si, Al, and Ca. When this occurs, the rate of glass corrosion is controlled not by the chemical potential gradient between glass and solution, but by the gradient between the glass and alteration phases. This, in turn, may accelerate the rate of glass corrosion (Stage III).
Working from the proposed reaction pathway, the researcher develops modeling and testing approaches in concert. Modeling is used to calculate the behavior of glass for long
time periods, while tests provide information regarding the mechanism of reaction and are used to validate and confirm the models.
When evaluating glass corrosion under conditions relevant to the Yucca Mountain repository, it is found that the reaction of glass in a humid environment can result in Stage III reaction because of the high ratio between the surface area of the glass and the volume of water (S/V). Tests conducted above 100°C are used to accelerate the corrosion, give a qualitative indication of the tendency of a glass to reach Stage III, and to determine the distribution of radionuclides among alteration phases. Tests done at lower temperatures confirm the application of the accelerated tests, and provide information on the rate of reaction under in-service conditions provided the duration of the tests is sufficiently long. Glass reaction in a humid environment alters the glass, but radionuclide release can occur only through thin-film diffusion.
Tests done with standing and dripping water conditions provide additional information regarding glass reaction and the distribution of radionuclides. Tests with standing water yield information about all stages. Tests performed at low S/V for short periods may be used to estimate Stage I reaction. If the S/V is increased, or the period is extended, Stage II may be observed and the reaction rate may typically decrease by about a factor of 100. performing the tests for even longer time periods or at higher S/V may result in Stage III behavior depending on the glass composition., and the reaction rate increases from the Stage II rate up to the forward rate at the solution condition. Static tests that reach Stage HI yield the same alteration phases as vapor tests when performed with the same glass. The distribution of radionuclides between the solution, glass, and other waste package components depends on the solution chemistry and whether the radionuclides are associated with colloidal phases that form as the glass reacts.
In the dripping water mode, the reaction progress depends on the rate of water contact. At an exposure of about 16 mL/d/m2 (an upper bound for water contact), as-cast glass forms a clay layer which spalls from the glass surface. As a result, the radionuclide release is dominated by a colloidal fraction, and the reaction remains between Stages I and II. If the test is done such that the dripping water contacts glass that was previously reacted in a humid environment—a likely repository scenario—the radionuclides are released initially as soluble species, which can be correlated with the large anionic content of the leachate. However, with time, the colloidal Content of the solution increases. The condition of the glass when finally contacted by water plays an important role in determining the source term for performance assessment calculations and in designing a waste package to retard radionuclide transport.
REFERENCES
1. American Society for Testing and Materials (1991) "Standard Practice for Prediction of the Long-Term Behavior of Waste Package Materials Including Waste Forms Used in the Geologic Disposal of High-Level Nuclear Waste," ASTM C-1174-91.
2. Westinghouse Savannah River Company (1993) "DWPF Waste Form Compliance Plan (U)," Savannah River report WSRC-IM-91-0, Rev. 2.
3. Buscheck, T. (1996) "Localized Dry-Out: An Approach for Managing the Thermal-Hydrological Effects of Decay Heat at Yucca Mountain," Mater. Res. Soc. Symp. Proc. Vol. 412, pp. 715-722.
This work is supported by the U.S. Department of Energy, Office of Environmental Management, under contract W-31-109-ENG-38.
Waste Glass Leaching and Long-Term Modeling
W. L. Bourcier
Lawrence Livermore National Laboratory
The important processes which take place during glass dissolution have been identified and coupled into models that are used to make predictions of glass dissolution rates. These coupled models of hydration, surface dissolution, ion exchange, and alteration mineral formation have been satisfactory in predicting glass dissolution rates over laboratory time frames. Using these models, it appears to be possible at present to place conservative bounds on long-term rates of glass corrosion and radionuclide release. However, further refinement of these models and increased accuracy of our long-term release rate predictions depend on additional work in the following areas.
We currently lack a clear mechanistic understanding of the controls on long-term glass reaction rates under near-saturation conditions, characteristic of long time periods. Our models reflect this uncertainty in their use of parameters which fix the long-term dissolution rate at some arbitrary or experimentally determined value. Because of this deficiency, the only justifiable conservative assumption is that of assuming the relatively rapid short-term rate continues over long time periods. For typical borosilicate waste glasses, such rates are generally slightly higher than the current regulation of one part in 10^5 release per year.
Few experiments have addressed this issue. Typical process control tests of glass durability are brief, complex, and difficult to interpret in terms of an analysis of rate control by multiple competing processes. Similarly, experiments that have addressed this issue indicate that long-term rates are not simple functions of silica saturation. Other factors besides or in addition to silica saturation control dissolution rates (Petit and others, 1990). There is currently a small body of empirical data on the effects of dissolved metal species on glass dissolution rates. Metals such as Mg and Pb are known to enhance glass durability, metals such as Fe and Al may degrade glass durability. In addition, glass dissolution rates are affected by solution ionic strength. Pure silica glass dissolves four times faster in 1 molal NaCl solution than in 0.01 molal NaCl solutions. Recent data on the effects of surface layers suggest they may be partially protective (Xing, Buechele, and Pegg, 1994), whereas most models assume they give rise to no slowing in corrosion rate. Many such second order effects on rates of dissolution remain to be sorted out and quantified before the existing test data can be adequately reconciled and understood.
In order to reconcile a large body of test data on glass corrosion rates, and in so doing resolve the mechanistic controls on the long-term dissolution rates, some carefully defined experimental work is needed. First, a matrix of dissolution tests of simplified glass compositions under controlled conditions are needed to separate out dissolution rate control due to saturation (affinity control), pH, ion exchange, and the effect of surface adsorption of dissolved species. It must be possible to extract from these tests the rates of each of these processes and their functional dependence on test parameters such as temperature, glass composition, and in the case of adsorbed species, the concentration of these species in
solution. Modeling is then needed to couple these mechanisms and rate dependencies to make predictions of long-term dissolution rates. A comparison of predicted versus experimentally measured rates can be used to refine or redefine the model in an iterative process. Previously identified high SA/V and vapor hydration tests provide the best indications of long-term dissolution rates.
This experimental and modeling program should be accompanied by additional work aimed at understanding glass dissolution on a molecular level. This work should identify the rate-limiting step in surface hydrolysis, the structure of the activated complex, and its dependence on surface charge (pH). This type of work is currently being performed for simple crystalline oxide and silicate phases using molecular orbital calculations of the mineral/solution interface structure. Application of this existing methodology should readily extend to oxide glasses.
There is still no glass structure model that adequately explains observed glass composition-durability relations. We cannot yet accurately relate a glass composition to its short-term durability in different types of durability tests. It is clear however that this is a difficult relationship to quantify and must follow the identification of the long-term rate controlling mechanism, as well as better information on glass structure. More fundamental work on the structures of simplified water-glass compositions using NMR and Raman and other types of spectroscopies is needed.
Finally, thermodynamically based glass leaching models are acutely dependent on the thermodynamic database used to calculate the saturation states of alteration minerals. We still lack an adequate thermodynamic database for many of the radionuclide and stable elements contained in the glass. We also lack a comprehensive database for activity coefficients for ionic species in waters having ionic strengths beyond 0.1 molal. No glass leaching model can ever be used with confidence to make long-term predictions of glass durability without this foundation of thermodynamic data, a foundation that is still lacking.
Petit, J. C., Magonthier, M. C., Dran, J. C., and Della Mea, G., 1990, Long-term dissolution rate of nuclear glasses in confined environments: does a residual chemical affinity exist? Journal of Material Sciences, v. 25, p. 3048-3052.
Xing, S.-B., Buechele, A., and Pegg, I. L., 1994, Effect of surface layers on the dissolution of nuclear waste glasses: Materials Research Society Symposium Proceedings, v. 333, p. 541-548.
Author's e-mail address: BILLB@LLNL.GOV
Waste Glass Leaching and Long-Term Durability
Etienne Y. Vernaz
Commissariat à l'Energie Atomique (CEA)
Rhône Valley Research Center
BP 171, 30207 Bagnols-sur-Cèze Cedex, France
The long-term durability of radioactive waste glass will be assessed by modeling. A strict methodology is necessary to develop a predictive model valid over long time periods, and may be summarized as follows:
-
Begin with a satisfactory material specification and characterization.
-
Identify and study the mechanisms of glass alteration by water (this requires comprehensive knowledge of the mechanisms involved in the long-term alteration of glass containment properties and their kinetics).
-
Determine the effects of various repository concepts and allow for all relevant environmental conditions (pH, Eh, flow rate, effect of container materials, host rock, groundwater composition, etc.).
-
Develop a glass alteration model applicable to a geological repository environment with the objective of modeling the alteration kinetics of the glass package in order to assess the performance of the waste form itself as the primary containment barrier.
-
Assess the release mechanisms of individual radionuclides and determine kinetic control by solubility, sorption or coprecipitation.
-
Obtain a "glass source term" for incorporation in the general codes that take into account migration and transport to the biosphere for safety purposes.
-
Validate the models by dedicated experiments (highly radioactive samples, full-scale tests, in situ tests, comparison With natural analogs, etc.).
This paper reviews the state of the art of each of these points, with a focus on long-term glass alteration mechanisms.
The French PREDIVER code integrates the principal alteration mechanisms to estimate the glass lifetime under realistic repository conditions. The code will be presented and some estimates will be given in a granitic environment. Under the disposal conditions expected in France, the lifetime of an R7T7-type reference glass package may be estimated as 107 years, and appreciably longer if the gel conserves its protective effect over the long term.
The following essential scientific issues remain to be addressed to improve the degree of confidence in long-term predictions:
What is the validity of the first-order law?
The "first-order" law ![]() where r0 is the initial glass dissolution rate in pure water and
where r0 is the initial glass dissolution rate in pure water and ![]() the affinity term, is used in PREDIVER as in most other nuclear glass alteration models. However, this law cannot account for certain experimental situations - notably with many clays - and there is not always an unequivocal relation between the silicon concentration (or the H4SiO4 concentration) and the alteration rate. For example, flowing experiments with a silicon-rich leachate show that the glass alteration rate increases with the flow rate even when the silicon
the affinity term, is used in PREDIVER as in most other nuclear glass alteration models. However, this law cannot account for certain experimental situations - notably with many clays - and there is not always an unequivocal relation between the silicon concentration (or the H4SiO4 concentration) and the alteration rate. For example, flowing experiments with a silicon-rich leachate show that the glass alteration rate increases with the flow rate even when the silicon
concentration in the leachate remains constant. This result indicates that other species than Si are involved in the kinetically limiting surface reaction. The role of aluminum has been established, but it may also be necessary to take into account other glass network-forming elements (e.g., Zr, the rare earths and perhaps Fe) in the network hydrolysis reaction. A more general rate equation would be necessary to describe nuclear waste glass alteration in any environment.
Nevertheless, we consider that the first-order law remains a justified approximation in many environments (including pure water, granite, salt or silica-saturated clay) where elements other than silicon (A1, Zr, the rare earths, etc.) quickly reach constant concentrations imposed by the low solubility of the hydroxides, clays or the surface gel.
Is there a "residual affinity"?
The first-order law predicts that under saturation conditions the alteration rate will drop to zero. This is not in contradiction with the very low alteration rates measured in the laboratory for the French R7T7 glass under "saturation conditions":
-
A "final" rate of less than one ten-thousandth of the initial rate has been observed, corresponding to better than 99.99% saturation.
-
The low "final" rates observed in pure water may generally be explained by a slight drift in the pH, increasing the apparent solubility of the glass.
-
It has been established that for a large number of glass compositions this "residual" rate is not constant, but diminishes as the product increases. This variation with the square root of time reflects the fact that interdiffusion again becomes the predominant mechanism when hydrolysis of the glass network ceases as saturation conditions are reached.
We consider the investigation of the "residual rate" to be irrelevant. The important phenomena affecting long-term glass behavior are those liable to retard (sorption) or even prevent (precipitation) saturation conditions from occurring with respect to the glass. In this regard, the choice of engineered barrier materials is decisive. The role of secondary phase precipitation on the long-term rate of glass alteration and on the retention of radionuclides must be determined for each scenario. This issue cannot be addressed in general terms: it is site-specific, and depends on the backfilling and the glass composition.
What are the long-term properties of the gel layer?
Experiments with pre-leached glass specimens have shown that the protective role of the gel depends to a greater extent on the conditions under which it was formed than on its thickness. A very thin gel formed under silica saturation conditions may provide much greater protection than a thick gel obtained in a dilute medium, at a low S/V ratio or with high flow rates.
The long-term behavior of the gel layer must be assessed by investigating natural analogs and by performing specific experiments. If it can be demonstrated that this layer conserves its transport properties over the long term, the predicted glass lifetime will be increased by about three orders of magnitude. If it can be demonstrated that this layer conserves its sorption properties over the long term for most actinides, the "glass source term" will be several orders of magnitude lower than the one predicted from glass alteration.
Corrosion Behavior of Glass: Remaining Scientific Issues
B. Grambow, Forschungszentrum Karlsruhe,
Abstract
We must address the current state of understanding of nuclear waste glass corrosion and the remaining scientific issues in the context of the needs to reliably assess the role and long-term performance of glass within the multibarrier containment system of the repository. During the last decades significant efforts and progress were made, and glass/water interactions belong now to the best studied solid/water systems. The clear result of these studies is that glass corrosion resistance is not an inherent materials property but depends on its disposal conditions. Do we know sufficiently to accurately predict its long-term behavior under realistic disposal conditions? The answer is: No, an accurate prediction is not possible. Every natural hydrogeological location is already undisturbed extremely complex and the introduction of the various engineered barrier materials multiplies potential synergetic effects and unforeseen evolution scenarios. The answer may be different if we allow for certain acceptable bounds of uncertainty. What is ,,acceptable'' will depend on the overall systems performance assessment. In most current repository performance assessments worldwide the effect of glass as a barrier is masked by the geological barrier or by other engineered barriers (bentonite, etc.). Do we now understand glass corrosion better to reassess the isolation potential of glass as a waste form? Let us see.
(1) General glass dissolution phenomena
The reaction path and rates of glass in aqueous repository environments as well as the associated radionuclide chemistry are governed by a combination of thermodynamic and kinetic factors. Today we know probably most of the main solid glass alteration products such as the initial gel phase, clay minerals, zeolites or metal oxide hydrates. Their occurrence and composition as well as the geochemical evolution (pH, etc.) of the ionic composition of the contacting groundwater can be predicted fairly realistically using geochemical codes, for saline environments as well as for granite water, tuff pore waters, etc. Some uncertainty rests with respect to the formation of borate containing phases at extremely high reaction progress (large glass/water ratios). The geochemical evolution of the near field caused by interactions with engineered barrier materials also can be predicted with some confidence and in certain bounds of uncertainty.) or the alteration products of container materials (e.g. magnetite in case of iron). However, only premature experimental and modeling experience exists for taking into account synergetic effects. Also, it is not yet possible to include radiolysis in an integrated geochemical model of glass dissolution under near field conditions. This is very critical, as the solution Eh may depend on it.
With respect to reaction rates, affinity based rate laws are now generally accepted for the initial dissolution process until ,,saturation'' has been achieved. Dissolved silica plays a key role in controlling the dissolution affinity, but Al, Fe, etc. may contribute. This ,,saturation process" is a safety relevant glass characteristics as reaction rates decrease afterwards by some orders of magnitude. Caused by silica sorption on near field materials (clay or iron corrosion products) the time to reach saturation may in some cases be quite long. Little is know on the various parameters that control this period. After saturation, the rate slows down, controlled either by resumption of water diffusion into the glass network, by the formation of secondary phases or by silica sorption on near field materials surfaces. This question is highly safety relevant and may become decisive when assessing the suitability of a given glass composition. In case of water diffusion, times for
complete glass dissolution may well exceed 107 years. On the other hand secondary phase formation has in some cases been found to reaccelerate glass corrosion. Even if we do not see any accelerating influence of secondary phases in our experiments, we cannot exclude such influence the 1st day after test termination. Hence, we may no extrapolate the square root of time diffusion rate law. We may generalize the questions: Does the hierarchy of rate controlling mechanism change when scaling up experimental data to repository relevant geometry and time frames? How to validate rate or models and how to assure their applicabilities? How to deal with uncertainties (in particular uncertainties in the conceptual model related to alternatives in interpreting experimental data) and which are the key uncertainties? There is no answer yet, as long as natural analog studies do not provide unambiguous rate laws for natural settings.
(2) Behavior of safety relevant radionuclides during glass dissolution
Depending on the geochemical environment glass corrosion may or may not be accompanied by transfer of safety relevant radionuclides to a potentially mobile aqueous phase. The relation of glass dissolution characteristics and radionuclide mobility is of key concern. Sometimes congruent dissolution of all radionuclides with the glass matrix is assumed as conservative bounding estimate. This approach is useful for the soluble nuclides (i.e. Tc under oxidizing conditions) or for those elements (Se, etc.) for which we know little on their geochemical behavior. In some cases (Mg-rich brines) such approach even gives a realistic description of experimentally observed actinide release pattern. In most cases, however, glass dissolution is incongruent and sparingly soluble phases or sorption phenomena often control release of most of those nuclides that dominate the long-term radiotoxicity of the waste, in particular for Pu and Am. This is in particular true for the actinides (Np, Pu, U) and Tc in their tetravalent oxidation states. Higher radionuclide release is observed if these Elements occur in the penta- (Np, Pu) hexa- (Pu, U) or heptavalent (Tc) states. The oxidation states strongly depend on the nuclide, on the geological formation of the repository, on radiolysis and on the presence of engineered barrier materials. For example, experiments under strongly reducing conditions, in the presence of corroding iron containers, have shown that the solution concentrations of some of the key nuclides of overall performance assessments (Np237, Tc99) almost comply to drinking water standards already at the glass/groundwater interface.
Currently there exist only premature experience in coupling of geochemical glass dissolution modeling with actinide and Tc chemistry. Remaining scientific issues deal with
-
basic unknowns of radionuclide chemistry in natural aquifers: e.g. poor understanding of the thermodynamics of tetravalent actinides and tetravalent Tc
-
temperature dependency of actinide chemistry
-
poor knowledge in the dominant redox states in particular of Pu and Np during glass dissolution
-
competition of reducing effects of container materials and host rock with oxidizing effects of radiolysis
-
stability of colloids and mechanism and limitations for colloid generation, transition from polynuclear complexes towards colloids
-
(Al)-silicate or molybdate based solid actinide phases
-
solid solution formation of radionuclides in secondary alteration products
-
sorption isotherms for radionuclides on surface gels and clay minerals
NATURAL GLASSES AND THE VERIFICATION OF THE LONG-TERM DURABILITY OF NUCLEAR WASTE GLASSES
Rodney C. Ewing
Department of Earth & Planetary Sciences
University of New Mexico
Albuquerque, New Mexico 87131
One of the unique and scientifically most difficult aspects of nuclear waste isolation is the extrapolation of short-term laboratory data (hours to years) to the long time periods (103 to 105 years) required by regulatory agencies for performance assessment and the determination of compliance. The direct verification of these extrapolations is not possible, but methods must be developed to demonstrate compliance with regulations and to satisfy the public that there is a demonstrable and reasonable basis for accepting the long-term extrapolations. Natural analogues studies, that is the study of natural systems, have been used to assess the long term behavior of components of the repository barrier system. There has been considerable effort (see selected references) devoted to the study of the alteration and dissolution of natural glasses in an effort to assess the long term behavior of nuclear waste glasses. This presentation reviews that work.
Natural glasses span a rather wide range of compositions which can be conveniently distinguished based on their silica contents: obsidians and rhyolite glasses (>70 wt. % SiO2); tektites (>65 wt. %); basalt glasses (45 wt.%); lunar glasses (45 wt.%). These natural glasses form as quenched glasses from magmas or melts generated during impact events (i.e. tektites and lunar glasses). Natural glasses show a wide variety of types and degree of alteration which are a function of the composition of the glass (in general, high silica glasses are more durable) and the geochemical environment (in the absence of water, natural glasses may show essentially no alteration). In typical terrestrial environments, the majority of the glasses are less than ten million years old, and older glasses become increasingly rare with age because of alteration. Some very old (hundreds of millions of years) glasses have been identified, but in most cases this is a result of the lack of contact with altering solutions. In addition, there have been a number of studies of anthropomorphic glasses (medieval stained glass windows, Phoenician glass containers, Th-doped borosilicate glasses, etc.) that are on the order of tens to thousands of years old.
A number of fundamental processes may be studied by the analysis of natural glasses: devitrification, hydration, hydrothermal alteration, low temperature alteration, and radiation effects; however, studies of these processes have met with varying degrees of success depending on the appropriateness of the "analogy" to the borosilicate waste form glass. This presentation will focus on the characteristics of basalt glasses, as they are the closest compositional analogue to borosilicate glasses, and they occur in a wide variety of geochemical environments. Results from experimental studies and modeled predictions of long term behavior will be compared to field observations. In most repository environments, glass may be expected to alter and corrode. The extent of release of radionuclides will depend very sensitively on the geochemical conditions (e.g., pH, flow rate, and silica content of solutions).
Details of the studies high-lighted in the presentation are found in the selected references.
Selected References
A. Abdelouas, J.L. Crovisier, W. Lutze, B. Fritz, A. Mosser and R. Müller (1994) Formation of Hydrotalcite-Like Compounds During R7T7 Nuclear Waste Glass and Basaltic Glass Alteration. Clays and Clay Minerals 41, 526-533.
T.A. Abrajano, J.K. Bates and J.J. Mazer (1989) Aqueous Corrosion of Natural and Nuclear Waste glasses. II. Mechanisms of Vapor Phase Hydration of Nuclear Waste glasses. Journal of Non-Crystalline Solids 108, 269-288.
Aa. Barkatt, M.S. Boulos, Al. Barkatt, W. Sousanpour, M.A. Boroomand, P.B. Macedo and J.A. O'Keefe (1984) The Chemical Durability of Tektites - A Laboratory Study and Correlation with Long-Term Corrosion Behavior, Geochimica et Cosmochimica Acta 48, 361-371.
J.L. Crovisier, T. Advocat, J.C. Petit and B. Fritz (1989) Alteration of Basaltic Glass in Iceland as a Natural Analogue for Nuclear Waste Glasses: Geochemical Modeling with DISSOL. Proceedings of "Scientific Basis for Nuclear Waste Management XII" vol. 127,57-64.
R.C. Ewing (1979) Natural Glasses: Analogues for Radioactive Waste Forms. MRS Proceedings of "Scientific Basis for Nuclear Waste Management I", 57-68.
R.C. Ewing and M.J. Jercinovic (1987) Natural Analogues: Their Application to the Prediction of the Long-Term Behavior of Nuclear Waste Glasses. Proceedings of "Scientific Basis for Nuclear Waste Management X", vol. 84, 67-83.
Y. Eyal and R.C. Ewing (1993) Impact of Alpha-Recoil Damage on Dissolution of Thoriated Glass. In Proceedings of "International Conference on Nuclear Waste Management and Environmental Remediation", Book No. 10354A, The American Society of Mechanical Engineers, pp. 191-196.
B. Grambow, M.J. Jercinovic, R.C. Ewing and C.D. Byers (1986) Weathered Basalt Glass: A Natural Analogue for the Effects of Reaction Progress on Nuclear Waste Glass Alteration. Proceedings of "Scientific Basis for Nuclear Waste Management IX", vol. 50, 263-272.
M.J. Jercinovic and R.C. Ewing (1987) Basaltic Glass from Iceland and the Deep Sea: Natural analogues to Borosilicate Nuclear Waste-Form Glass. JSS Project Tech. Rept. SKB Technical Report 88-01, 221 pages.
M.J. Jercinovic and R.C. Ewing 1991) Corrosion of Geological and Archaeological Glasses. In Corrosion of Glass. Ceramics and Ceramic Superconductors, edited by D.E. Clark and B.K. Zoitos (Noyes Publications, Park Ridge, New Jersey) pp. 330-371.
W. Lutze, G. Malow, R.C. Ewing, M.J. Jercinovic and K. Keil (1985) Alteration of Basalt Glass: Implication for Modeling Long-Term Stability of Nuclear Waste Glasses, Nature 314, 252-255.
G. Malow, W. Lutze and R.C. Ewing (1984) Alteration Effects and Leach Rates of Basaltic Glasses: Implications for the Long-Term Stability of Nuclear Waste Form Borosilicate Glasses. Journal of Non-Crystalline Solids 67, 305-321.
J.J. Mazer, J.K. Bates, C.R. Bradley and C.M. Stevenson (1992) Water Diffusion in Tektites: An Example of the use of Natural Analogues in Evaluating the Long-Term Reaction of Glass with Water. Journal of Nuclear Materials 190, 277-284.
Actinide Vitrification: Status of Savannah River Site Activities
William G. Ramsey
Technical Lead - Actinide Vitrification
Savannah River Technology Center
The Westinghouse Savannah River Company is actively investigating the vitrification of actinide materials. Large quantities of actinides were produced during the Cold War period for both national defense and basic science programs. Now, due to the end of the Cold War and the downsizing of the United States' weapon stockpile, some of the actinides (specifically plutonium) are considered to be "excess". Other materials must be transported to different locations in order to decommission older facilities. The Savannah River Site (SRS) inventory of americium and curium would fall in this latter category. This presentation will focus on the status of actinide vitrification research at SRS and the planned production of actinide glasses, scheduled to begin in 1998.
SRS maintains a large (several kilograms) inventory of rare Am and Cm isotopes. The Am and Cm are currently stored in nitric acid solution. This material is to be stabilized for shipment to the Oak Ridge National Laboratory (ORNL) where the Am and Cm will be processed through the Isotope Sales program. This requires the host form to be suitable for transportation and to also allow recovery of the Am and Cm. A glass formulation which is approximately 1000X more durable than the specification for high-level waste glass but selectively attacked by nitric acid was chosen as the optimum form. This glass can accept over 40 weight percent (oxide basis) of the Am/Cm solution components, primarily lanthanide fission products and actinides. The glass can be processed by a melter design adapted from the commercial fiber glass industry. SRS has constructed a full-scale pilot plant which is currently processing simulated Am/Cm glass. Bench-scale melting and glass property study activities are also being conducted with fully radioactive Cm doped glasses to assist the project. This vitrification of Am/Cm is scheduled to be conducted in 1998.
SRS also maintains large quantities of neptunium and plutonium in a facility scheduled for decommission. Again, ORNL has an existing programmatic need for the Np - and a high Np extraction rate is very desirable. Glass formulations are being developed to meet ORNL's requirements and Np glasses have been made which demonstrate the feasibility of processing Np through the Am/Cm melter system. Likewise, plutonium glasses have been fabricated to demonstrate that the Am/Cm melter system can successfully process the aforementioned SRS plutonium.
A national program led by Lawrence Livermore National Laboratory (LLNL) is currently underway to identify suitable disposition options for the excess fissile material no longer needed for national defense. Savannah River is actively participating with LLNL (and others) to determine the processability and chemical and physical properties of plutonium glasses. Both borosilicate and phosphate glass compositions have been examined. Borosilicate glasses with over 10% Pu and phosphate glasses with over 20% Pu (by weight) have been produced. These glasses are extremely resistant to chemical attack. The borosilicate also has the capability of accepting large quantities of neutron absorbing elements - which greatly improves the safety of both production and storage.
This presentation will center around the chemistry and properties of actinide glasses. Chemical durability and actinide solubility studies will be emphasized. Photographs or video of the Am/Cm pilot plant in operation will also be presented to familiarize the audience with the scale of SRS actinide vitrification capabilities.
Thermal Stability of Waste Form Glass
N. Jacquet-Francillon
Commissariat à l'Energie Atomique (CEA)
Rhône Valley Research Center
BP 171, 30207 Bagnols-sur-Cèze Cedex, France
Thermal stability is the inherent glass capacity for maintaining its disordered structure when submitted to extended heating. Devitrification (or crystallization) is the transformation of the vitreous material into a crystallized material under the combined effects of two phenomena: nucleation and growth by diffusion. Crystallization is a subject for concern because of its potentially detrimental effects on the technological feasibility of high-temperature melting, and on the chemical durability of the material at intermediate and low temperatures during interim storage or after disposal.
Work published during the last fifteen years in North America, Europe and the Far East was examined. Nuclear waste containment glasses are for the most part borosilicate glasses, and this critical review was therefore limited to such formulations. The published studies[1,2] primarily discussed the determination of the time-temperature-transition (TTT) curves, crystal identification and quantification techniques, and their effects on the durability of the glass matrix.
The tendency of glass to crystallize depends to a large extent on the composition of the frit and/or of the waste to be solidified. Crystalline phases varied in the heat-treated glass from 1 to 40 vol.% depending on the final glass composition. A wide range of crystals were observed: molybdates, silicates, chromites; they generally formed on contact with the noble metals (RuO2 or Rh-Pd precipitates that were not digested in the glass) which served as crystallization seeds.
For example, the thermal stability of the French "LWR" glass developed by the CEA at Marcoule in the late 1970s was investigated in various ways: specimens were maintained under isothermal conditions at 450 and 550°C for up to a year; specimens were submitted to isothermal treatment at the maximum crystallization temperature after a phase intended to favor nucleation (5 hours at 550°C + 100 hours at 780°C).
Moreover, the entire temperature range of crystalline phases was investigated systematically from 590°C to 1180°C at 30°C intervals with an isothermal residence time of 16 hours. In addition to the platinoids, which were not digested in the glass, the following crystalline phases were detected: calcium molybdate, mixed cerium and uranium oxide, chromites, and an unidentified silicate phase; all these metallic and oxide heterogeneities together accounted for less than 5 vol.% of the material. The incipient crystallization temperature was 610°C. This small crystallized fraction had no significant effect on glass alterability during Soxhlet mode tests at 100°C, nor on the mechanical properties (E, KIc).
In 1992, a 400 g radioactive "LWR" glass sample containing 13 TBq of αβγ activity was taken[3] during routine production operation in the R7 industrial vitrification facility at COGEMA's UP2-800 reprocessing plant at La Hague. After heat treatment, characterization testing fully confirmed the results obtained with the nonradioactive specimens: i.e. no induced crystallization after 30 days at 510°C and development of 2.7% crystalline phases (with platinoids) after 100 hours at 780°C, the maximum crystallization temperature.
Virtually all the published studies concur that the crystallization domain of nuclear borosilicate glass lies between 100 and 400°C above the vitreous transition temperature Tg; spinels and platinoids remain in small quantities up to the liquidus. Each glass appears to exhibit a maximum crystallization
density that cannot be exceeded even after extended heat treatment; the devitrification capacity is highly dependent on the glass composition. In reality, however, the cooling rates throughout the canister after casting determine much lower crystallization densities.
With glass formulations that tend to crystallize in significant proportions (exceeding 10 vol.%), as long as fabrication is not a problem, crystallization after cooling in the canister is acceptable, provided the following conditions are met:
-
The crystallized phases that form must not result in significant depletion of network formers in the residual glass, in order not to diminish its resistance to aqueous corrosion.
-
The differences between the thermal expansion coefficients of the crystalline phases and the glass must not be high enough to jeopardize the mechanical integrity of the glass blocks as a result of thermomechanical stresses occurring after the glass has cooled below the transition point.
-
The crystalline phases must not be subject to major structural modifications under irradiation, which could lead to dimensional changes resulting in microcracks and increasing the potentially leachable surface area.
-
The crystalline phases must not be readily leachable, particularly if they contain radionuclides.
The following recommendations may be made for future experimental investigations:
-
Mechanical behavior of the composite material constituted by the partially devitrified glass.
-
Radionuclide partitioning between the crystalline phases and the residual vitreous phase.
-
Behavior of the crystalline phases under irradiation, especially if they include radionuclides.
This implies further investigation of actual radioactive glass samples, notably those with high cumulative self-irradiation doses.
Only a few authors addressed the theoretical possibility of long-term development of crystalline phases[4,5], notably at temperatures below the vitreous transition temperature Tg[6,7]. Even when no crystallization is detected after extended heat treatment near the transition point, as is the case with the French "Light Water" glass (Tg 510°C) maintained for one year at 450 and 550°C, further investigation of the crystal nucleation and growth mechanisms is necessary to model any possible structural modification liable to result in some degree of long-term devitrification under irradiation at temperatures below the transition point.
1. S.L. Marra, M. K. Andrews and C.A. Cicero, "Time-Temperature-Transformation Diagrams for DWPF Projected Glass Compositions", Ceramic Transactions , vol. 39, pp. 283-292 (April 1993).
2. J.C. Simpson, D. Oksoy, T.C. Cleveland, L.D. Pye and V. Jain, "The Statistics of the Time-Temperature-Transformation Diagram for Oxidized and Reduced West Valley Reference 6 Glass", Ceramic Transactions , vol. 45, pp. 377-387 (April 1994).
3. P. Cheron et al., "Examination and Testing of an Active Glass Sample Produced by COGEMA", Scientific Basis for Nuclear Waste Management, Materials Research Society Symposium Proceedings, vol. 353, pp. 55-62 (1995).
4. G. Malow, "Thermal and Radiation Effects in the Range of the Glass Transition Temperature Tg", Scientific Basis for Nuclear Waste Management, Materials Research Society Symposium Proceedings, vol. 127, pp. 153-162 (1989).
5. T. Inoue, H. Yokoyama, T. Onchi and H. Koyama, "Surface Layer Crystallization of Simulated Waste Glass at Elevated Temperatures", Scientific Basis for Nuclear Waste Management, Materials Research Society Symposium Proceedings , vol. 26, pp. 535-542 (1984).
6. E.N. Boulos, R.P. Depaula, O.H. El Bayoumi, N. Lagakos, P.B. Macedo, C.T. Moynihan and S.M. Rekhson, "Crystallization of Nuclear Waste Disposal Glass", Journal of the American Ceramic Society, vol. 63 No. 9-10, pp. 496-501 (1980).
7. A.D. Stalios, R. De Batist and P. Van Iseghem, "Long-Term Crystallization Behavior of Glasses at Temperatures T < Tg", Materials Research Society Symposium Proceedings, vol. 127, pp. 163-171 (1989).
MECHANICAL PROPERTIES OF THE WASTE FORM GLASS
Hj. Matzke
European Commission, Joint Research Centre, Institute for Transuranium Elements, Postfach 2340, D-76125 Karlsruhe, Federal Republic of Germany
Abstract
The presentation covers the existing literature and the extensive work performed at the Institute for Transuranium Elements (ITU) on mechanical properties of the waste form glass, including the investigations performed in cooperation with numerous other institutions (INE, Nuclear Research Centre, Karlsruhe; Battelle-Institute, Frankfurt; PNL Richland; Univ. Padova and Trento, etc.).
At ITU, three borosilicate waste glass forms were extensively investigated but some other glasses were also examined (e.g. the alternative German product SM513 LW11). Both the simulated (hence not radioactive) forms, the simulated forms with added actinides (Pu, Am, Cm) and some fully active versions were studied. These three mainly used glass types were
|
• the German product |
GP 98/12, and precursors |
|
• the US product |
MCC 76-68 |
|
• the French products |
SON 681817 L1C2A271, and R7T7. |
The composition of these glasses and the fission products added to the simulated forms are contained in ref. [1]. The mechanical properties measured were
-
elastic or Youngs modulus, E
-
Vickers hardness, H
-
fracture toughness, KIc
-
drop tests were also performed.
KIc-values were obtained from both Vickers indentations and by short rod fractometry [2]. For the Vickers indentation technique, a detailed study of error propagation and of influences of sample preparation was performed in order to assess the effect of surface compressive stresses. The short rod method was developed further as a multi-cycle test method. In this way, a statistical analysis could be made on a single specimen and several phenomena could be studied simultaneously, e.g. crack growth during unloading. Youngs moduli were calculated from the measurements of longitudinal and shear velocity of ultrasonic waves. A hot hardness tester was used to measure H and KIc up to the softening point.
No significant differences were found at room temperature in either H, KIc or E between the different glasses studied [e.g. 1-3], in agreement with the results of other researchers. Some differences were seen in the temperature dependence.
The changes in mechanical properties due to radiation damage were also investigated. The actinide-containing samples accumulated large amounts of α-decay damage (up to 3×1025 α-decays/m3). In parallel work, ion implantation techniques were used to create radiation damage. Ion implantation was done at different temperatures.
In this way, the effects of increasing amounts of damage accumulated at different temperatures could be studied. For damage produced at room temperature, technologically positive changes were seen: the glass became softer (decrease in H by 30 %) and tougher (increase in KIc by up to 100 %) [e.g. 2-4]. The net result of these two phenomena was reduced brittleness enhancing the resistance of the glass against crack propagation. If damage was produced at ![]() , this positive effect vanished.
, this positive effect vanished.
The mechanical behavior of (simulated) waste glasses was also measured in laboratory-scale drop tests and in full-scale drop tests using European standard high level waste canisters [5]. Waste glass fracture, aerosol release and activity source term were determined for different impact energies and glass compositions. The test conditions covered those predicted for a drop from 10 m in a reloading hall and those from 600 m in a borehole of a repository (impact velocities 9 to 168 ms-1; glass temperatures 290, 470 and 570 K, different ground conditions, drops with and without canister). The size distribution of fractured glass particles (0.1 µm to 3 cm) was measured and the respirable fraction (aerosol source term, airborne particle fraction with diameters << 10 µm) was determined. Typical results are: 2×10-4 % for a reloading hall drop and ˜ 0.3 % for a borehole drop for the respirable fraction.
The large data base accumulated in these studies and contained in the published literature is presented and discussed.
References
[1] Hj. Matzke, ed.: "Indentation Fracture and Mechanical Properties of Ceramic Fuels and of Waste Ceramics and Glasses", Proc. Int. Workshop, ITU Karlsruhe, Nov. 1985, Special volume Europ. Appl. Res. Reports - Nuclear Sci. Technol 7(1987)999-1240, also European Commission Report EUR-10597 EN (1987)
[2] Hj. Matzke and E. Toscano; eds.: "Indentation Techniques", Proc. Int. Workshop Centro Ceramico, Bologna, Dec. 1988, Special volume Europ. Appl. Res. Reports - Nuclear Science and Technol. 7(1990)1355-1504, also European Commission Report EUR-12799 EN (1990)
[3] W.J. Weber, Hj. Matzke and J.L. Routbort, J. Mater. Sci. 19(1984)2533
[4] Hj. Matzke and E. Vernaz, J. Nucl. Mater. 201(1993)295
[5] H.G. Scheibel, V. Friehmelt and Hj. Matzke, Proc. 1991 Int. Waste Management Conf., Seoul, Eds. S.C. Slate et al., Korean Nucl. Soc. 2(1992)113
Radiation Effects in Glass Waste Forms
William J. Weber
Pacific Northwest National Laboratory
P.O. Box 999, Mail Stop K2-44
Richland, WA 99352 USA
A key challenge in the permanent disposal of high-level waste (HLW), plutonium residues/scraps, and excess weapons plutonium in glass waste forms is the development of predictive models of long-term performance that are based on a sound scientific understanding of relevant phenomena. Radiation effects from beta and alpha decay could potentially impact the long-term performance of glasses for HLW and Pu immobilization and disposal through the interactions of the β-particles, α-particles, recoil nuclei, and γ-rays with the atoms in the glass. These interactions fall into two broad categories: the transfer of energy to electrons (ionization) and the transfer of energy to atomic nuclei, primarily by ballistic processes involving elastic collisions. The effects of radiation on glass waste forms are complex, and there is a critical lack of fundamental understanding on the effects of radiation on glasses from the atomic to the macroscopic levels that prevents meaningful predictions of waste form performance. Because of the lack of basic understanding on radiation-damage processes, the limited existing data bases cannot be extrapolated to larger doses, lower dose rates, different temperature regimes, or different glass compositions.
Review of Radiation Effects
Recent reviews [1-3] of radiation effects in HLW forms provide excellent technical assessments. There are no data on the nature of intrinsic point defects or irradiation-induced point defects in glasses for HLW or for Pu immobilization and disposal; however, these glasses are expected to exhibit families of radiation-induced defect centers similar to those described for simple glasses. The only data available on simulated HLW glasses are of measured macroscopic changes, which are highlighted below.
Volume Changes. Radiation effects in simulated HLW glasses results in either expansion or compaction of the glass structure. For a large range of actinide-doped glasses, the volume changes normally reach an apparent plateau that is within the range of ±1.2% at a dose of 2 × 1018 α-decays/g (109 Gy). In a study of actinide-doped glass in Japan, the measured volume expansion correlated well with the size and concentration of irradiation-induced bubbles. The volume expansion and compaction of neutron-, γ-, and ion-irradiated HLW glasses are also within the bounds observed for the actinide-doped glasses; however, others have reported swelling of up to 50% when bubbles formed in simulated HLW glasses during electron irradiation. Under ion irradiation, the volume changes in complex borosilicate glasses appear to be driven by ionization processes rather than ballistic processes.
Stored energy. The interaction of radiation with glasses can result in the storage of latent energy associated with defect production. Studies on actinide-doped glasses indicate that α-decay can result in a rapid increase in stored energy that reaches saturation values of less than 150 J/g (˜0.03 eV/atom) at a dose of 0.2 × 1018 α-decays/g (108 Gy). This saturation occurs at a much lower dose than the volume changes and suggests that the defects giving rise to the stored energy precede the network rearrangements that manifest as volume changes.
Microstructural changes. Bubbles that are assumed to contain O2 were first observed in simulated HLW glasses irradiated with electrons to high doses (1010 to 1011 Gy) in 1976 as part of a study to simulate the effects of β-particles. Since then, electron-beam-induced bubble formation has been observed in a wide variety of glasses and shows a strong dependence on temperature and composition. The presence of a gas phase in the bubbles has been confirmed in several studies. Ion-beam irradiation and γ-irradiation also have been reported to produce bubbles in several simulated HLW glasses; however, the γ-irradiation results are controversial. The relevance of radiolytic bubble formation remains unresolved.
Radionuclide Release. Radiation affects the release rates of radionuclides from glasses by increasing the surface area for release (e.g., microfracturing) and by changing the dissolution rate of the glass. The dissolution rate of glass waste forms may be affected by radiation-induced defects, phase separation, and changes in chemistry, microstructure, and network bonding. Based on the limited data, it is estimated that radiation-induced increases in leach rates will be no more than a factor of 10, provided there is no radiation-induced phase separation or bubble formation. Enhanced dissolution along α-recoil tracks also occurs and can lead to preferential release of the daughter products of alpha decay.
DOE Panel Findings
A scientific panel convened recently under the auspices of the DOE Council on Materials Science to assess the current state of understanding, identify relevant scientific issues, and determine directions for future research in the area of radiation effects in glasses for high-level waste and plutonium disposal. Some of the scientific issues that were identified include: (1) the critical lack of systematic understanding of radiation effects (from both ionization and collisional processes) in glasses at the atomic, microscopic, and macroscopic levels; (2) the relative effects of ionization and elastic collision processes'' on the damage production in glasses; (3) radiation-induced phase separation and transformations in glasses as a function of dose rate and temperature; (4) radiolytic bubble formation; (5) radiation-enhance diffusion in thermal, stress, and electric-field gradients; (6) the need for accelerated irradiation techniques, which demands a thorough understanding of dose-rate effects; (7) the need for theoretical modeling and computer simulation techniques to provide theoretical validation of accelerated irradiation techniques, insights into ionization and collisional damage processes, insights into atomic migration, understanding of composition/atomic structure relationships, and guidance/interpretation for experimental studies; and (8) helium accumulation, trapping, and release in high actinide glasses. A detailed summary of this panel's findings that includes discussions of the scientific issues and recommendations for research directions is being prepared for publication.
References
1. W.J. Weber, J. Minerals, Metals and Materials Society 43, 35-39 (1991).
2. D.J. Wronkiewicz, Effects of Radionuclide Decay on Waste Glass Behavior - A Critical Review, ANL-93/45 (Argonne National Laboratory, Argonne, IL, 1993).
3. R.C. Ewing, W.J. Weber, and F.W. Clinard, Jr., Progress in Nuclear Energy 29 (2), 63-127 (1995).
4. DOE Panel on Radiation Effects in Glasses for High-Level Waste and Plutonium Disposal, chaired by W.J. Weber and R.C. Ewing, Santa Fe, New Mexico, February 25-29, 1996.
EXPERIENCE IN BELGIUM
by M. Demonie
Operational experience of the Pamela Facility
The Pamela vitrification plant, designed by the German company for the reprocessing of nuclear fuels DWK (Deutsche Gesellschaft für Wiederaufarbeitung von Kernbrennstoffen), was built at the Eurochemic site (now Belgoprocess) between 1981 and 1984. After a year of cold and hot testing with diluted HLLW, hot operation was started on October 1, 1985. Up to September 5, 1991, some 900 m3 of high level liquid waste from the reprocessing of irradiated fuel was successfully vitrified into 500 t of glass product.
Vitrification is done in a single step process using a joule-heated ceramic melter. Both the liquid waste concentrate and the glass flit are directly introduced into the melter, forming a process zone on top of the glass pool where drying, calcination and melting reactions occur. The first phase of the vitrification programme, carried out under the responsibility of DWK, demonstrated the feasibility of the process. The second phase of the programme was performed on an industrial basis under the terms of a Belgo-German cooperation agreement.
In total 1.51 E15 Bq alpha and 4.44 E17 Bq beta activity was vitrified in 2201 canisters. After six years of vitrification operations, at the end of 1991, one ceramic melter and three other large components were dismantled and conditioned, using a cement mortar as matrix. In total 34.8 t of solid waste containing 1.49 E12 Bq alpha and 4.09 E14 Bq beta were cemented in 187 200 l-drums.
Quality Assurance Programme
The success of the vitrification operations was mainly the result of the strict follow-up of a quality assurance and control programme, set up in cooperation with the Belgian National Agency for Radioactive Waste and Enriched Fissile Material (known as NIRAS). This Q/A programme consisted of 3 phases:
-
Process qualification phase.
The designer of the Pamela Plant, DWK, was in charge of the development of the vitrification process.
The selection of the basic glass was made on the basis of : technical feasibility, melting temperature, viscosity and disposal criteria.
The characteristics of laboratory glass product are compared to technical glass produced in an experimental reactor.
-
Process follow-up and production control phase.
The consecutive QA/QC steps are: control of input streams, control of process treatment steps, control of glass product and control of instruments and equipment.
All relevant data are compiled in the Conformity File.
-
Product quality verification phase.
This is carried out by a third independent laboratory :
-
the characteristics of the reference glass are verified.
-
the product from the inactive industrial demonstration is compared to the reference product.
-
the active product is compared to the product specifications as laid down in the QA/QC manual.
Recommendations for future Vitrification.
Belgoprocess and the German partner of the cooperation agreement acquired extremely valuable experience during the vitrification programmes from which the main issues are :
-
The design of the ceramic melter.
During the demonstration campaign a significant increase of 1/E ratio was noticed. This was caused by sedimentation of noble metals at the bottom of the ceramic melter. To reduce or even to eliminate such an accumulation a new ceramic melter has been designed by the Institute für Nuclear Entsorgungstechnik at FZK.
Belgoprocess, B 2480 Dessel, tel. 00.32.14.334111
-
Operations mode of the plant.
Scheduled or unscheduled interruptions of the vitrification operations led each time to an accumulation of noble metals on the melter bottom.
Continuous vitrification operations give more guarantee to successful operations. Bubbling nitrogen gas in the melter bath has a positive influence on the feedrate but very little effect on the removal of noble metals.
The off gas system proved to be very efficient. The activity release to the stack over the whole vitrification campaign of six years, was limited to less than 0.08 % of the licensed values on an annual basis.
To improve the overall efficiency of the plant, the treatment and conditioning of the solid waste generated during vitrification has to be done in parallel to the vitrification operations.
-
The lay-out of the plant.
The Pamela plant was originally conceived as a demonstration plant for the German vitrification technology. The installation proved to be suitable to vitrify HLLW to a qualitative acceptable glass product.
In total two ceramic melters have been used, each of them with a lifetime of approx. 3 years. The exchange of a ceramic melter has been performed in a period of ten weeks, using fully remotely techniques. The installation of two ceramic melters in two separate cells would considerably shorten this interruption period.
Dismantling requirements must be taken into account at the design phase of plant and equipment. The ventilation system should allow the use of plasma torches. The conditioning unit should allow the use of large drums to gain man-hours.
-
The composition of the glass frit.
In spite of the extensive R&D work which has been done, anomalies have been discovered during the vitrification programmes. These anomalies were mainly caused by the deviation of the chemical composition of the HLLW from the standard composition. A process accompanying glass laboratory service is therefore mandatory for trouble shooting.
The glass frit composition has been adapted two times during the vitrification programmes.
Key-References
1. DE, A.K.; Wiese, H.; Demonie, M. (1990). Improvement of vitrification operation by optimising of the borosilicate glassfrit at the Pamela Plant. Spectrum '90 conf. at Knoxville, TN. p 293-295
2. Wiese, H.; Demonie, M. (1992). Operational experience of the Pamela vitrification Plant. Spectrum '92 conf. at Boise, ID. p 464-467
3. De Goeyse, A.; De, A.K.; Demonie, M.; Van Iseghem, P. (1992). Qualification and characterization programmes for disposal of a glass product resulting from high level waste vitrification in the Pamela installation of Belgoprocess. International Symposium, Antwerp. p 189-200
4. Demonie, M.; Cuyvers, H. (1995). On the Feasibility of Vitrifying High-Level Liquid Waste containing high amounts of Plutonium. Nato Disarmament Technologies - Vol. 4. p 155-172
5. Demonie, M.; Luycx, P.; Snoeckx, M.; Baeten, L. (1996). Experience gained with the dismantling of large components of the Pamela vitrification Plant. Spectrum '96. Seattle, WA.
VITRIFICATION EXPERIENCE IN FRANCE - DEVELOPMENT AND PERSPECTIVES
Antoine JOUAN - Commissariat à l'Energie Atomique (CEA)
Thierry FLAMENT - Société Générale pour les Technologies Nouvelles (SGN)
Hugues BINNINGER - COGEMA - La Hague
From the early days of the development of nuclear energy, France has been concerned with the disposal of radioactive waste, and the containment of fission product produced in the fuel elements during their stay in the reactor core. Laboratory research on containment matrices began in 1957, first with crystalline materials, then with glass - which quickly proved to be more suitable for incorporating the forty-odd elements created by uranium fission (as well as additives and corrosion products resulting from fuel reprocessing) into a homogeneous matrix.
After the fabrication of highly radioactive glass blocks weighing a few hundred grams to assess their containment properties, the French research program then turned to process development: the first industrial vitrification facility, PIVER, began operating in 1969. Before it was shut down in 1972, PIVER produced 164 glass blocks, weighing a total of 12 tons, from 24 m3 of concentrated fission product solutions containing 6 × 106 Ci. The facility resumed operation a few years later to vitrify HLW solutions arising from the reprocessing of fast breeder reactor fuel, producing ten glass blocks of 90 kg each with very high specific activity. In 1989, PIVER was named a Nuclear Historic Landmark by the American Nuclear Society.
Faced with increasing demand, research was undertaken in the 1970s to develop a continuous process to obtain a final glass waste form by first evaporating and calcinating the feed solution in a rotary furnace, then melting the calcinate with glass flit in an induction-heated metal melter. The Marcoule Vitrification Facility («AVM» ) was commissioned in 1978 to vitrify fission product solutions from the French UP1 reprocessing plant. By the end of 1995, AVM had logged nearly 64,800 hours of operation and vitrified 1,920 m3 of solution containing 401 million curies, producing 857.5 tons of glass in 2,412 canisters, each containing 360 kg of glass.
The successful operating record and experience gained with AVM allowed the start-up of a commercial-scale high level waste vitrification plant in France. Two similar facilities, R7 and T7, are on line at the La Hague reprocessing plant. R7 has been commissionned in 1989 and T7 in 1992.
In short, one can easily figure out true life industrial experience obtained with French vitrification process from the following data: at the end of 1995, 3709 glass canisters have been safely produced at La Hague in accordance with specifications. More accurately, 2,434 canisters containing 400 kg of glass have been produced at R7 and 1,275 in T7. These figures are equivalent to 2,104 m3 of fission products liquid solutions received at R7 and 972 m3 at T7 (end of 1995). The total vitrified activity at R7 and T7 amounts to 1490 million curies (end of 1995). The equivalent reprocessed uranium oxide amount is 7,197 t at R7 and 2,512 t at T7; and the overall amount of fission products to be vitrified which was approximately 1200 m3 in 1989 has decreased to less than 200 m3.
Over the years, both the process and the technology have been improved. The melter lifetime for instance is now 3700 hours and the melter itself constitutes a relatively compact waste that is easy to condition.
In addition, since the active start-up of R7/T7 facilities, vitrified canisters compliance with specifications relies upon a complete quality assurance/quality control program including process control. COGEMA's Quality Control Division is regularly performing independent inspections on the La Hague R7/T7 vitrification facilities. They are mainly performed by Bureau Veritas, an independant auditing agency chosen by COGEMA's baseload customers.
And for the future, in order to get an unlimited lifetime, the CEA has developed a process in which the glass is melted by induction heating inside a water-cooled crucible. The use of a cold crucible melter (CCM) should allow glass or vitrocrystalline materials to be produced at higher temperatures and flow rates with no risk of corrosion, as the melter remains at a lower temperature. A nonradioactive prototype melter 55 cm in diameter and 70 cm high has been operating together with a calciner for several years at Marcoule. A unit of this type could advantageously replace the current melter in the future.
The CCM technique is now reaching maturity; possible applications include not only vitrifying solutions containing fission products but also incineration of combustible waste including plastics and even ion exchange resins. Recent program in implementing units with larger capacities also make the cold crucible melter a serious candidate for vitrifying low and medium level wastes. Developed primarily by the CEA in France, the CCM technique is also being investigated in Russia. The cold crucible melter was chosen: along with a high-temperature liquid fed ceramic melter (LFCM) by a committee of international experts called together by Westinghouse Hanford Company during the summer of 1995, as the design best suited for vitrifying the HLW at Hanford.
As underlined in the previous paragraphs, French vitrification process combines with great efficiency an industrial operative mode and a commercial reality. The same French technology is also used in UK (Sellafield) to produce glass since 1990. Regarding the world vitrification needs, French's process offers a wide range of opportunity for foreign countries wishing to ensure the success of their back-end policy.
EXPERIENCE IN THE UNITED STATES: WEST VALLEY, NEW YORK
Victor A. DesCamp, Manager,
Vitrification Design Engineering
West Valley Nuclear Services, Co., Inc.
Introduction
One of the primary objectives of the West Valley Demonstration Project (WVDP) is the solidification of approximately 2.3 million liters of high-level radioactive waste (HLW) which resulted from nuclear fuel reprocessing. The WVDP, initiated by a Congressional Act in 1980, was directed to solidify existing liquid HLW into a safe, durable product suitable for long-term disposal. The New York State Research and Development Authority-owned site is managed by the Department of Energy (DOE). Their cooperative agreement states that the work outlined by the Act is to be accomplished using the existing facilities to the maximum extent possible. West Valley Nuclear Services, Company, Inc. (WVNS) became the management and operating contractor of the site in 1981.
High-level Waste Pretreatment and Consolidation
At the beginning of the waste processing activities at the WVDP, HLW was stored in four underground tanks. One tank contained neutralized PUREX waste. Another tank contained THOREX acidic waste from a special reprocessing run. The other two tanks were spares.
Pretreatment of the HLW began in 1988. In the PUREX HLW tank, the waste had separated into two layers: a clear liquid (supernatant) above a layer of precipitated sludge. Solidification of these separate layers required processing in two stages. The supernatant was transferred through ion-exchange columns containing zeolite that removed greater than 99.9 percent of the radioactive materials. The resulting effluent, containing salts and sulfates, was concentrated, blended with cement, and stored as low-level waste in a shielded, above-ground facility on site. Supernatant processing was completed in 1990 producing a total of about 10,000 cement drums.
Mixing pumps were then installed in the PUREX HLW tank to mobilize the sludge for washing. This included the addition of process water to the tank to dissolve the salts and sulfates. Salt/sulfate removal was necessary to reduce the number of canisters produced during the vitrification campaign. The wash water was then processed similarly to the supernatant; however, titanium-coated zeolite was alternately used as the material in the ion-exchange columns. Three sludge washes were performed and approximately 9,800 cement drums were produced.
During 1994 and 1995, radioactive constituents in all waste tanks were combined into one 2.6 million liter tank. The acidic THOREX HLW and spent zeolite from the ion-exchange process were transferred into the PUREX tank. This combined waste will be the feed for the vitrification process.
High-level Waste Vitrification Activities
From 1984 to 1989, the WVDP operated a full-scale glass production test facility. This nonradioactive Functional and Checkout Testing of Systems (FACTS Testing) determined the correct component configurations and the glass recipe to be used during radioactive operations. Conversion of this test facility for radioactive processing was completed in late 1994. Functional testing of the vitrification systems was completed in mid-1995. Several integrated operations runs were also executed to confirm the proper performance of the systems and to provide operator training. Mechanical tie-ins to the previously contaminated facilities are almost complete and radioactive feed to the Vitrification Facility is expected to be initiated in June 1996.
The feed from the HLW tank will be pumped to the Vitrification Facility through double-wall piping in a shielded concrete trench. In the Vitrification Cell, the waste will be combined with glass-forming chemicals, concentrated, and transferred to a slurry-fed ceramic melter. The joule-heated melter will heat the mixture to approximately 1150°C and the resultant molten waste/glass will be poured into stainless steel canisters. All solidification operations and transfers will be performed remotely.
All of the radioactive process components are located within the Vitrification Cell. The stainless steel-lined cell is 19.8 m long by 10.7 m wide by 13.1 m tall with 1.2 m thick concrete walls. It is equipped with two cranes, a remote robot, impact wrenches, and manipulators to enable remote operations. Lead glass windows and CCTV cameras are used for viewing. Major components within the cell include the ceramic melter, canister turntable, infrared level detection system, remote lid welder, decontamination station, and remote transfer cart.
The melter is the primary component of the vitrification process. It is a water-jacketed stainless steel box insulated by multiple types of refractory with separate cavities for glass melting and pouring. Initial heat-up of the melt cavity is started by electric heaters above the glass pool. Joule heating, using alternating current through the melter electrodes, begins at 800°C and rises to 1050°-1150°C for vitrification. The nominal operating volume of the melter is 860 liters. Feed is delivered to the melter continuously by an air displacement slurry pump at a rate of 60 to 80 liters per hour. Molten glass is produced at a rate of approximately 30 kg per hour.
The turntable is a motor-driven stainless steel structure capable of holding and rotating four, 3.05 m high by 0.61 m in diameter canisters to filling and cooling positions. Canisters are placed into and removed from the turntable by a grapple and crane. Viewing the canister fill level on the turntable is accomplished using an infrared level detection system. This system is comprised of an infrared radiometer and live thermal video feed to a remote computer in the Control Room. It provides a quantitative indication of the glass level by recognizing the temperature gradient at the glass-air interface. The target fill level is 85 percent or about 1,900 kg of radioactive glass per canister. The surface of the melter glass and the pour stream between the melter and the canister can be viewed by remote camera.
Once a canister has been filled and cooled, it is transferred to the weld station. A lid is welded onto the canister flange by a remote weld head using an automatic pulsed gas tungsten arc welding process. Tools are also available to weld a secondary lid, machine the weld area, and clean the flange.
After the lid is welded onto the canister, the canister is decontaminated using a bath of nitric acid and cerium+4. The decontamination station contains a heated titanium decontamination tank and a titanium neutralizer tank. A filled canister is placed in the decontamination tank where the chemicals react to etch about 5 microns off the canister's surface, thus removing any contamination. After decontamination, the canister is loaded onto a transfer cart for transport to an interim storage facility. The cart is remote-controlled and battery-powered with four independent drive trains, any one of which can drive the cart in either forward or reverse direction carrying a 10-ton load.
Conclusion
Construction and start-up testing of the Vitrification Facility has been completed. Integrated systems tests are in progress. Radioactive tie-ins between the Vitrification Cell, Waste Tank Farm, and canister storage cell are expected to be completed in May 1996. Production of the first radioactive glass-filled canister is expected to start in June 1996. The HLW processing campaign is anticipated to last two and one-half years and produce about 300 radioactive canisters. The canisters will be stored on site until ready for shipment to a repository.
Vitrification Experience of TVF in Japan
M. Yoshioka and H. Igarashi
Power Reactor and Nuclear Fuel Development Corporation
Ibaraki, Japan
ABSTRACT
Tokai Vitrification Facility(TVF) was constructed as the first plant in Japan to immobilize the high-level liquid waste(HLLW) to the borosilicate glass in April of 1992. The TVF finished successfully the test operations being carried out since then using both the simulated waste and HLLW transferred from the Tokai Reprocessing Plant(TRP) through December 1995.
The TVF is designed to solidify the HLLW generated from the TRP which treats the LWR spent fuels burned-up 28,000 MWD/MTU in average. The vitrification technology(1) of TVF is based on the liquid fed joule-heated ceramic melter(LFCM) process which has been developed in Power Reactor and Nuclear Fuel Development Corporation(PNC) since 1977. Many developmental works were carried out on the glass melter system, which contains 45° sloped bottom structure(2) and cold bottom mode of operation(3) to eliminate the operational difficulties caused by electro-conductive sludge in HLLW, and glass fiber additive to keep the glass melting stable without abrupt evaporation, and a freeze valve with two-zone induction heating to make a smooth start and termination of glass draining. The TVF adopts these vitrification process equipment with PNC's own improvements to attain the most suitable LFCM process for the TVF.
The vitrification process applying the technologies mentioned above is as follows. At a time receiving the HLLW from the TRP, elemental and radioactive analysis are carried out for process and product quality control. The HLLW is pretreated to adjust the composition by the addition of a sodium and by concentration using an evaporator. After the pretreatment, HLLW is transferred to a glass melter continuously using a two-stage airlift system. The HLLW fed to a melter is soaked into the glass fiber additive just before they are fed into the melter pool. The glass melted at the temperature of 1100 to 1200°C is discharged periodically through a metallic nozzle located at the bottom of the melter into a canister. During the discharge, the weight and volume of the glass in the canister are successively measured by load cells and by the gamma-ray method, respectively. The filled canister is subsequently cooled, and transferred to the welding position, and a lid is welded by a TIG welder to seal the canister. After being decontaminated by high-pressure water jet spray and wire brushing and being inspected, glass products are stored in the storage pits with the forced-air cooling system. The melter off-gas is cleaned by wet scrubbing process such as a submerged bed scrubber, a venturi scrubber, a perforated plate type water scrubber, and subsequent filtration process such as a high efficiency mist eliminator(HEME), a ruthenium adsorber(silica gel), an iodine adsorber and HEPA filters.
The TVF has two main cells, one is a vitrification cell where most of vitrification process equipment such as a receiving vessel and an evaporator treating HLLW, a glass melter, a welder, and the off-gas treatment equipment are installed. This cell incorporates a new concept of fully remote maintenance in accordance with the adoption of large cell, so that the plant availability should be increased and personnel exposure decreased. For maintenance, over head
system like the two-armed servo-manipulators, in-cell cranes, and ''rack system" which mount the process equipment on the modular frames are disposed in a vitrification cell. Another cell is a transfer cell with storage pits where the glass products are inspected and stored.
The test operations were done along step-by-step through the cold test and radioactive test since 1992. The cold tests divided into two major tests, the operation test of process equipment and the remote maintenance test, had been conducted alternately. For the process equipment, three process runs were carried out to identify the safety of process, the performance such as the glass melter, and the quality control technique of glass product by producing 83 glass products using the simulated waste. The improvements of some process equipment were carded out based on the result of cold test for getting more good operability. Each operation result shows that the TVF has enough performance for the safety and process operation, and also for the quality control of glass products. For the remote maintenance of process equipment in the vitrification cell, the remote maintenance capability by two-armed servo-manipulators and in-cell cranes had been confirmed for all remote equipment more than 1000 objects including a glass melter and the racks mounting the process equipment.
Subsequently the TVF commenced the radioactive test operations in January, 1995 after the hot preparation like a cell closing, an establishment of control area, and a connection of HLLW transfer pipe with the TRP. First of all in radioactive test, the TVF was operated to evaluate the safety performance like the cell shielding, and the release rate of radioactive material through a stuck, using actual HLLW with step-by-step dilution. After the identification of those fundamental safety performance, the TVF started the operation to produce the glass products. However, the drained glass accumulated in the coupling device between the melter and canister during the third glass draining to the canister. After carrying out improvement of the temperature control of the melter bottom glass and modification of the coupling device, the test operation was restarted to identify the performance of process equipment through the continuous production of glass products. The glass melter operation by adjusting the bottom temperature in addition to the 45° sloped bottom structure was evaluated to have enough performance to eliminate the difficulties caused by the noble metals which is contained in the same amount as the design base in HLLW. Also the performance of off-gas treatment process was identified to satisfy the design by evaluating the decontamination factors. As for a total process of TVF, it was operated stably during producing 20 glass products.
Radioactive test operation of TVF finished successfully in October, 1995 after taking the final inspection before the use. The TVF shifted from the test operation to the full operation by getting an operation license on December 1, 1995.
References
1. Yoshioka, M., et al., "Glass Melter and Process Development for PNC Tokai Vitrification Facility.", WASTE MANAGEMENT, Vol. 12, pp. 7-16, 1992.
2. Tsuboya, T., et al., "The Japanese Vitrification Program." Proc. of the symposium on waste Management at Tucson Arizona, Feb.28-Mar. 3, 1988, Vol.2 pp 181-188.
3. Yoshioka, M.; et al., "Evaluation of glass melter operation using highly simulated waste for TVF" Proc. of International Topical Meeting on the Nuclear and Hazardous Waste Management (SPECTRUM'90), Knoxville, TN, Sep.30-Oct.4, 1990.
Vitrification Experience in the UK
Graham A Fairhall and Charles R Scales
British Nuclear Fuels plc
Sellafield, Seascale, Cumbria, CA20 1PG, UK
British Nuclear Fuels plc (BNFL) has been reprocessing nuclear fuel for approximately 40 years. This results in highly active liquid waste (HALW) containing 99% of the dissolved fission products along with impurities from cladding materials, traces of unseparated U and Pu and most of the transuranics. The policy in the UK is to vitrify this waste and store the products in engineered stores for at least fifty prior to disposal or return the products to overseas customers
The vitrification process has been developed in the UK over the last 40 years with initial work carded out at Harwell in the 1950s. Process development was temporarily halted in the 1960s due to lack of an economic incentive for treating the HALW and a high degree of confidence in the highly active waste storage tanks. Work on the Harvest vitrification process was restarted in the 1970s. In 1981 it was decided to adopt the French AVH process in preference to the Harvest process.
From 1983 a full scale inactive facility (FSIF) was constructed and operated by BNFL at Sellafield to develop the vitrification process for BNFL HALW. Alongside this ran the programme to develop and fine tune the glass composition required to vitrify the waste. These programmes culminated in the construction, commissioning and active operation of the Waste Vitrification Plant (WVP) at Sellafield in 1991.
Selection criteria were established for the glass matrix aimed at a waste loading of about 25%. Early work on glasses led to the borosilicate system being chosen on a balance of factors such as durability, chemical stability, corrosiveness and melting temperature. Work also indicated that a mixed alkali system (Na and Li ) offered advantages over a single alkali system.
The borosilicate system was then further explored to give a formulation which would not only have good waste form characteristics but would exhibit good processing properties. Four glass compositions were explored and the optimum selected. This was further modified to allow some of the lithia to be added to the calcine to aid reactivity.
The glass formulations were evaluated at full scale on the FSIF in order to establish the product quality and operational envelopes for the process. A detailed evaluation of process variables was undertaken to establish their effects on the process and product. This ensured that if the plant were operated within the process envelope guaranteed acceptable products would be generated.
The waste vitrification process consists of a high active liquor storage and distribution cell, two parallel vitrification lines consisting of vitrification and pouring cells and container decontamination and monitoring/control cells. Attached to the vitrification plant is the Vitrified Product Store (VPS) with a capacity for 8000 product containers.
The process incorporates a rotary calciner through which HA liquor is fed and partially denitrated. The calcine is mixed with glass frit and fed into a elliptical inconel melter. After approximately 8 hours the glass product is fed into the container situated underneath. Following pouring the container is allowed to cool and a lid is welded on. Decontamination and checking follows before movement to the VPS.
Operation of the plant has been characterised by good performance of the glass making process with the reliable manufacture of in specification products. Due to assured control of the process it has not been necessary to carry out quality checks on the product itself.
A number of major successes have been achieved since operations began. These include the production of well over 1000 quality vitrified products to specification, very low levels of activity discharge from VPS and the minimisation of technical or secondary wastes from the plant
As would be expected in the severe operational environment involved all the main plant and equipment is enclosed within heavily shielded cells and requires remote operation, maintenance or replacement. In the early operational period this complexity of the plant resulted in product container throughput being restricted due to extended periods of downtime between production runs. This resulted from low reliability of in cell cranes and master slave manipulators (MSM), equipment which was key during maintenance and rebuild phases. Another issue was the rate at which solid waste particularly melter crucibles could be processed from the breakdown cells.
With these problems in mind, improvement projects have been undertaken in the areas of cell cranes, MSMs and breakdown cells. In addition work is being carried out on control mechanisms in order to increase the life of the melter and limit the secondary waste. These projects have allowed progressive improvements in plant performance since operations began. This coupled with the knowledge gained from plant operational experience gives confidence that these improvements in throughput can be sustained.
Reference
1 Progress with Highly active waste vitrification at BNFL Sellafield, D Millington, The Nuclear Engineer, Volume 36, No 2, 1995
Experiences with Vitrification HLW and Development of New Approaches in Russia
A. S. Aloy, RPA, V. G. Khlopin Radium Institute, St. Petersburg
V. A. Bel'tyukov, PA ''Mayak", Ozersk
A. V. Demin, VNIIPIET, St. Petersburg.
Yu. A. Revenko, Mining and Chemical Association, Krasnoyarsk.
The main R&D directions in Russian HLW Management were discussed in [1]. According to this paper the most completely studied process is that involving the production of phosphate vitreous materials in the ceramic liquid fed melter [2].
The design of the equipment units was developed and their operational suitability verified on the pilot-scale facility EP-100 with throughput of 100 l/h of simulated solutions, containing up to 400 g/l of salt. The production capacity was about 25 kg/h for phosphate glass of the following composition (wt %): Na2O 22-26; Al2O3 21-25; P2O5 47-53; Fe2O3 up to 1.5. Using the isotope (Cs, Ru, Sr) labeled solutions, it was shown that at a process temperature of up to 1100°C the losses from the melter were up to 5% for 106Ru, 0.6% for 137Cs, and 0.2-0.4 for 90Sr.
On the basis of the tested equipment and operating experience an industrial scale facility EP-500 was built for the vitrification of real HLW solutions at radiochemical plant "Mayak," S. Ural.
The basin of ceramic liquid fed melter is constructed of the aluminozirconium refractory (Bacor-30) and has electrodes from molybdenum. To suppress volatility of ruthenium tetraoxide, a reducing agent is added to the melter. earlier it was molasses, but now ethylene glycol is used.
Vitrification complex occupies two buildings connected with each other. There are pretreatment solution area as well as off-gases cleaning system in the first building, the second accommodates 2 ceramic melters, a unit for pouring glass melt into canister, a remote welding system and air cooled storage. The first melter was put in to operation in 1987 and operated for 1.5 years, including the period of "cool" runs. At this facility 998 m3 of HLW with total activity of 3.97 M Ci were vitrified and 366 canisters with total glass weight of 162 t were produced.
The first melter was stopped in 1988. because of the failure of the power connection zone due to overheating. After detail investigations of the melter failure, design changes were made and the second one was built. It was put into operation in 1991 and works upto today. At this melter 9160 m3 of HLW (230 M Ci) were vitrified and 1770 t glass was poured into 1372 containers in the middle of 1995.
The recent advances in HLW pretreatment indicate that there are a number of reasons for separation of various long-lived radionuclides from the bulk of wastes [3]. Vitrification of the obtained concentrates allows to minimize the waste volumes to be disposed. The Cs-Sr fraction separation could also simplify the waste handling operations in a geological repository and improve the geochemical compatibility between waste materials and geological environment.
The development of the process flowsheet for HLW management of the VVER-1000 spent fuel reprocessing plant at Krasnoyarsk (RT-2) is based on the achievements in the field of solvent extraction and partitioning of HLW using chlorinated cobalt dicarbollyde [4].
According to this technology three different groups of radionuclides are subject to solidification:
-
cesium and strontium reextract;
-
transuranium elements (TUE) and rare earths (RE) reextract;
-
raffinate of other elements.
Considering that cesium and strontium are recovered in the form of a pure concentrate, where salt residue is not exceed 50 g/l, detail investigations are performed now in Russia.
The aims of this new approaches are the development of a borosilicate matrix with an increased radionuclides content and a study of properties of such glasses [5]. Simultaneously, the new design of remote-dismantling ceramic liquid fed melter is been developing with production glass capacity ˜20 kg/h.
References
1. A. S. Nikiforov, A. S. Aloy, V. V. Dolgov et al. "Management with HLW after reprocessing spent fuel." Atomnaya Energia, 1987, v. 63, 5, pp. 319-323 (in Russian).
2. Design and operation of HLW vitrification and storage facilities, TRS N339, IAEA, Vienna, 1992.
3. A. S. Aloy, B. Ya. Galkin, B. S. Kuznetsov, et al. "Fractioning of Liquid High-Radioactive Waste and Incorporation of Long-lived Radionuclides into Ceramics and Vitreous Compositions." Waste Management '89, Tucson, Arizona, February 26 - March 2, v. 1, pp. 677-681, 1989.
4. Ya. A. Revenko, L. N. Lazarev, V. N. Romanovsky, "Radioactive Waste Management of the Radiochemical Plant Under construction near Krasnoyarsk," SPECTRUM '94, August 14-18, Atlanta, GA, v.3, pp. 2015-2018, 1994.
5. A. S. Aloy, A. V. Trofimenko, O. A. Iskhakova, et al. "The Development of a Glass Matrix for the Immobilization of Simulated Strontium and Cesium Concentrate after HLW Separation," SPECTRUM '94, August 11-16, Atlanta, GA, v.1, pp. 791-764, 1994.
Vitrification Experience in China
Wang Xian De
Beijing Institute of Nuclear Engineering, P.O. Box 840, Beijing 100840, China
In China, HLLW will be solidified into a stable form and disposed of in a deep geological formation. R&D Program is being conducted regarding vitrification of HLLW. At present, a non-radioactive mock-up facility with a Liquid Fed Ceramic melter (LFCM) is under construction.
R&D program of vitrification covers three phases: fundamental study, engineering study, and construction of vitrification facility on an industrial scale. The program was initiated in the mid 1970s. Research was focused on the discontinue pot process utilizing borosilicate glass as the product until 1985. Instead of the pot process, the LFCM process was adopted in 1988. Vitrification research in China has now entered the engineering study phase. The available LFCM technology and specific hardware are provided by Germany. The basic design of the mock-up facility was jointly carded out by Beijing Institute of Nuclear Engineering (BINE) and Institute für Nukleare Entsorgungstechnik (INE) in 1991. The full scale melter was constructed at INE in 1992 and delivered to China in 1994. It is planned to be put into operation in 1997.
COMPOSITION OF HLLW
The contents of sodium, iron, uranium are high in the waste. Since the sulfur solubility is limited in borosilicate glass, the waste oxide loading of glass product must be limited to 16 wt.%. Even though the content of noble metals is rather low in the waste, their presence is still given careful consideration in the melter design. Therefore, it can also be used to treat the commercial HLLW Later.
GLASS FRIT AND GLASS PRODUCT
The glass frit is used in the form of beads of 1 to 2 mm in diameter. The frit should be suitable to the following conditions of melting for LFCM process.
-
The waste oxide loading of glass product is not less than 16% in weight on conditions that no yellow phase appears and no reductive is needed.
-
The melting temperature of the glass product is kept at 1150°C to reduce the corrosion of refractory and prevent the excess volatilization of radioactive nuclides in waste and the content of frit.
-
The viscosity the of glass product should be 5-7 pas at 1150°C in order to assure the homogeneity of glass product, the escape of bubbles, and the drainage of glass product from the melter.
-
The resistivity of glass product should be equal or greater than 5.0Ω cm at 1150°C because the low resistivity causes the increase of electricity density at the surface of the electrodes and the corrosion of the electrodes.
The glass frit consists of eight metallic oxides: SiO2, B2O3, Na2O, Li2O, Al2O3, CaO, TiO2, MgO. Two kinds of glass frit named 90U/19 and 90U/20 have been developed.
VITRIFICATION PROCESS
The vitrification process is mainly divided into four parts as follows:
-
Feed preparation
-
Vitrification
-
Off Gas Treatment
-
Canister Handling
Feed Preparation
Feed preparation includes the making up of simulated solution of HLLW and the feeding of simulated solution & glass frit. The simulated solution is prepared in batches of 5 m3 and is transferred to the melter by air lifts via several tanks and vessels. The glass flit is weighed and fed to the melter in batches, controlled by computer.
Vitrification
The feed of the melter is 65 l/h of simulated solution with 5 1/h of recycled waste solution from the treatment of off gas. The maximum temperature of glass melting is limited to 1180°C, and the melter surface of 85 to 90% should be covered by the mixture of calcining and drying matters. The glass product is discontinuously poured in the stainless steel canisters with the capacity of 400 kg glass. The rate of pouring is 110 to 120 kg/h.
Off Gas Treatment
The function of off gas treatment is to keep the negative pressure of system, to remove volatile matter, dust particles, aerosol and NOx from off gas as well as to condense the aqueous vapor. Off gas is first cleaned by a dust scrubber. Further treatment follows by a condenser, a jet scrubber, a NOx adsorber, a glass fiber filter, an electrical heater, two HEPA filter, a cooler and a droplet separator. The value of DF for cleaning equipment has been obtained in the tests.
Canister Handling
The empty canister is transferred to below the pouring cell of the melter then lifted at the position of pouring; after pouring, the canister is delivered to the cooling station, where it is cooled in a heat-insulating overpack for 3 to 4 days and then sent to the welding station for lid welding.
FEATURES OF FACILITY
The facility is divided into several areas: making up of simulated solution, feeding, vitrification cell, canister handling, off gas treatment, waste solution treatment, operating and controlling, process media supply, and valve and transmitter galleries.
Melter Cell
The dimensions of the cell are 7.4 m × 5 m × 9 m (L×W×H). The cell is built with steel structure and sheet. It has two operating areas on the first and second floor. Each area also has two operating spots. The spots are equipped with two pairs of type Ms simulated manipulator and viewing window. The equipment of the cell could be maintained or replaced remotely.
Melter
The melter has a stainless steel cell and a lining consisting layers of high temperature resistant glass contact refractory, back-up bricks, and ceramic insulation material. Three pairs of Inconel 690 electrodes are installed at the wall of the melting pool opposite each other. The electrodes are cooled by air to keep them below 1050° C. The bottom of pool has the sloped walls up to 75°, and it serves as a small volume chamber for discharging glass product and collecting noble sediments. The bottom drain value with induction heating or the glass overflow system supported by airlift can be used for pouring of glass products into canisters.
TEST OF MELETER
After building the melter, two test runs were carded out at INE in 1993. The purpose of the first test run was to demonstrate the melter functions under fully representative operating conditions. Both the melter technology and particularly the processing behavior of the special type of sodium and sulfur rich HLLW simulated solution had to be investigated. The general objectives of the second test run included the confirmation of the main results of the first test run and the test of some design modifications on specific melter items for a final improvement. Further subjects were the clarification of sulfur behavior, the noble metals behavior, and the test of overflow glass pouring system, etc.
REFERENCES
1. Sun Donghni, "A Review of Research and Development of High Level Liquid Waste Vitrification in China", Spectrum '86, pp. 776-778.
2. Liu Guoming, et al. "An Overview of Vitrification for High-Level Radioactive Waste in China", Proceedings of the 1989 Joint International Waste Management Conference, Kyoto 1989, pp. 129-130.
Vitrification Experience at the Defense Waste Processing Facility (DWPF)
David B. Amerine
DWPF Program Manager
Westinghouse Savannah River Company
P.O. Box 616 (Bldg 704-S)
Aiken, SC 29802
Approximately 130 million liters of high-level radioactive waste (533 million curies) is currently stored in underground carbon steel tanks at the Savannah River Site (SRS) in Aiken, SC. This high level waste will be immobilized in stable borosilicate glass at the Defense Waste Processing Facility (DWPF). After an extensive testing program, the DWPF recently began radioactive operations with the production of its first canister of radioactive waste glass.
The radioactive waste in the SRS Tank Farms has been separated into a water soluble salt solution and saltcake, and an insoluble sludge of metal hydroxides and oxides. The salt solution and saltcake are decontaminated for disposal as low-level radioactive waste by removing the radionuclides by precipitation and sorption. The resulting slurry is filtered and the decontaminated filtrate is blended with cement, slag and flyash for disposal at SRS as a low-level radioactive waste (saltstone). The slurry of the concentrated solids is transferred to DWPF for vitrification. The sludge portion of the waste is washed to remove soluble salts prior to transfer to DWPF. Thus, the radioactive waste from the SRS Tank Farms is transferred to the DWPF for vitrification in two forms: precipitate slurry and sludge slurry.
The sludge is transferred directly into the Sludge Receipt and Adjustment Tank (SRAT) while the precipitate must first be processed in the DWPF Salt Process Cell to remove most of the organic material and produce Precipitate Hydrolysis Aqueous (PHA). The PHA contains cesium, soluble formate salts, boric acid and excess formic acid. The sludge is neutralized with nitric acid in the SRAT. The PHA is then added to the sludge. The excess formic acid in the PHA reduces the mercuric oxide in the sludge to elemental mercury. The elemental mercury is then steam stripped from the SRAT into a holding tank from which it is later pumped and decontaminated. After the PHA and sludge are blended and processed in the SRAT, this SRAT product is transferred to the Slurry Mix Evaporator (SME) where a borosilicate glass frit is added and the slurry is concentrated to produce melter feed. After the SME batch is determined to be acceptable, it is transferred to the Melter Feed Tank (MFT), for delivery to the Joule-heated, slurry-fed melter. The feed slurry is pumped from the MFT through two feed lines and fed directly on top of the molten glass surface in the melter. The DWPF melter has two pairs of electrodes. The feed slurry is introduced from the top of the melter and forms a crust, or cold cap, on the surface of the melt pool as the water is evaporated and removed via the off-gas system. The cold cap melts from the bottom and forms the borosilicate glass matrix. The nominal glass melt pool temperature is 1150°C. The glass is removed from the melter near the bottom through a riser and pour spout. A vacuum is drawn on the pour spout to pour the glass into stainless steel canisters. The canisters are sealed with an inner canister closure plug and decontaminated with a frit slurry. The inner canister closure plug is then pressed down into the canister nozzle and a weld plug is welded to the canister using upset resistance welding. The canisters are transferred to an air-cooled vault (in the Glass Waste Storage Building) for interim storage. The canistered waste forms will be held there awaiting shipment to a federal repository. The DWPF canistered waste form contains approximately 1800 kg of glass. It is 300 cm in length and 61 cm in diameter.
To ensure that the DWPF product is acceptable for final disposal the Department of Energy Office of Environmental Management has developed the Waste Acceptance Product
Specifications (WAPS) which the DWPF product (i.e. canistered waste form) must meet. The most important of the glass specifications is the product consistency specification which states that the DWPF must control its process so that the glass produced is more durable than the DWPF Environmental Assessment glass as measured by the Product Consistency Test (PCT). DWPF has developed a Glass Product Control Program to ensure that this specification is met. As part of this program the melter feed is held in the SME until it is determined that it will produce a durable as well as a processable glass. The acceptability of the melter feed is determined using glass property models and statistical algorithms which take into account analytical uncertainty. During startup testing, the DWPF demonstrated that it can comply with this durability specification as well as the other glass, canister, and canistered waste form requirements.
The DWPF Startup Test Program was modeled on the testing required for startup of a commercial nuclear reactor. The Startup Test Program demonstrated the operability of the major process systems as well as demonstrated DWPF's ability to produce an acceptable canister of borosilicate waste glass. This startup testing also provided operating experience to personnel and baselined equipment and system operating parameters. Approximately 80 canisters of simulated waste glass (approximately 310,000 lbs of glass) were produced in the DWPF prior to the introduction of radioactive melter feed material into the DWPF melter in April, 1996.
The DWPF began integrated startup testing with water in 1991 and then transitioned to cold chemical operations during which the first batch of melter feed was produced. The melter was then heated up and the first melter campaign was performed producing 16 canisters of simulated waste glass. The melter feed for this first campaign was Blend feed (i.e. a blend of all waste types in the SRS Tank Farm). During the Waste Qualification Runs portion of the test program, fifty-five canisters were filled, over four melter campaigns, with simulated waste glass. During these four melter campaigns, the feed coming into the DWPF went through abrupt changes in composition. The purpose of making such abrupt changes was to demonstrate that the DWPF process could handle such changes and that the Glass Product Control Program could control the glass product even when the feed composition is rapidly changing. This enhanced the confidence in the use of the program during normal operations, when changes in feed composition will be more modest. The glass and canistered waste forms produced during Waste Qualification Runs were extensively characterized. The results of this characterization demonstrated the DWPF's ability to produce an acceptable product.
Following the completion of the Waste Qualification Runs, a comprehensive assessment and evaluation of DWPF was performed. The acceptable completion of these reviews was required prior to the authorization to transfer radioactive waste from the SRS Tank Farms to DWPF.
The DWPF is now a fully operational radioactive facility which is operated with three continuously staffed control rooms with a five shift rotation to meet training needs. Initial operation will consist of a sludge-only flowsheet (no PHA) until the precipitate feed stream is ready for transfer to DWPF later this year. Approximately 6000 canisters of borosilicate glass will be produced over the life of the facility which is expected to be approximately 25 years.
Workshop on Glass as a Waste Form and Vitrification Technology Washington, May 13-15, 1996, Washington, D.C.
Natural Glasses as Analogs for Nuclear Waste Glasses
Abdesselam ABDELOUAS and Werner LUTZE
Center for Radioactive Waste Management (CeRaM)
Department of Chemical and Nuclear Engineering
The University of New Mexico
Abstract
Numerous investigations show that natural glasses (rhyolitic and basaltic) and borosilicate nuclear waste glasses exhibit common corrosion features (e.g. Petit, 1992). Important analogies have been reported that allow us to better understand long-term corrosion processes of nuclear waste glasses. Similar, if not the same mineral associations develop on naturally altered volcanic glasses and on nuclear waste glasses if the corrosion conditions are comparable (Lutze et al. 1985). There are only a small number of distinct phases, e.g., powellite (CaMoO4), coffinite (USiO4), cerianite (CeO2) and related solid solutions, typical of the fission products or actinides contained in nuclear waste glasses (Rother et al. 1992). Corrosion rates of natural and borosilicate waste glasses are higher in pure water than in salt solutions and appear to be lowest in silica saturated solutions. There is no evidence that the glass corrosion process ever ceases in nature except for total consumption or exclusion of water (Grambow et al. 1986). Experimental studies of the corrosion mechanism of borosilicate nuclear waste glasses up to five years indicate that the glass dissolution continues at a low rate after the solution is saturated with respect silica (Lutze et al. 1988, 1989). The long-term corrosion mechanism has not yet been elucidated. Presently, extrapolations over long periods are largely speculative (Grambow et al. 1992). Here, natural glasses come in handy as they support the assumption that the matastable glass phase is ultimately replaced by thermodynamically stable crystalline phases and that the transformation is accompanied by the dissolution of some of the glass constituents. Furthermore, studies of surface features of basaltic and rhyolitic glasses, altered naturally in aqueous solutions over long periods of time, reveal the kin and composition of stable phases. There are intermediate, metastable phases such as hydrotalcite (Abdelouas et al. 1994) that are replaced by more stable phases as the corrosion process continues. The knowledge of the sequence of phases and their nature can be used to refine glass dissolution models and to complement the list of phases used in modeling efforts.
The poster presented at this meeting provides and compares the following results:
-
Phases identified on a nuclear waste glass (the French borosilicate glass R7T7), a basaltic glass, and an obsidian corroded in a Mg-rich salt solution in the laboratory (Abdelouas et al. 1994, Abdelouas, 1996).
On all glasses, the first alteration product to appear is a hydrotalcite-like compound (Mg6Al2CO3(OH)164H2O). This phase is not stable and is replaced by saponite. In addition, on the nuclear waste glass, powellite, barite (BaSO4), and cerianite formed.
Phases identified on basaltic glass experimentally and naturally altered in seawater.
-
A mineralogical association of hydrotalcite-serpentine-saponite, as on R7T7 glass, was observed for basaltic glass experimentally corroded in seawater (Crovisier et al. 1983 and Thomassin 1984). Hydrotalcite and smectite were also found on basaltic glass samples from the axial rift of the Red Sea (Ramanaidou and Noack 1987). It is supposed that hydrotalcite is the natural precursor of smectites.
-
Alteration phenomena on a rhyolitic glass naturally altered in a salt lake in Bolivia (Abdelouas, 1996).
A variety of minor phases was found in the brine-saturated sediments of the salt lake: Sr-rich barite, cerianite, celestite (SrSO4), pyrite (FeS2), alunite (KAl3(SO4)2(OH)6) and Mg-rich smectite. Though only the Mg-rich smectite was found in the sediments and on the glass surface, it is concluded that the other phases are mainly glass corrosion products. Again, analog corrosion behavior of natural and nuclear waste glasses is emphasized by common alteration phases such as Mg-rich smectite, Sr-rich barite, and cerianite. The rhyolitic glass showed substantial chemical durability in the salt brine. The corrosion rate was estimated to be 0.05 to 0.3 µm/1,000 yr.
References:
Abdelouas A. (1996) Ph.D. thesis, Univ. Strasbourg, France, 192pp.
Abdelouas et al. (1994) Clays Clay Miner., 5, 526-533.
Crovisier et al. (1983) Geochim. Cosmochim. Acta 47, 377-387.
Grambow et al. (1986) Mater. Res. Soc. Symp. Proc., 50, 263-271.
Grambow et al. (1992) Mater. Res. Soc. Symp. Proc., 257, 143-151
Lutze et al. (1985) Nature 314, 252-255.
Lutze et al. (1988) Mater. Res. Soc. Symp. Proc., 112, 575-584.
Lutze et al. (1989) Mater. Res. Soc. Symp. Proc., 127, 81-88.
Petit J. C. (1992) Appl. Geochem. Suppl., 1, 9-11.
Ramanaidou E. and Noack Y. (1987) Mineral. Mag., 51, 139-143.
Rother A. (1992) Mater. Res. Soc. Symp. Proc., 257, 57-64.
Thomassin J. H. (1984) Ph.D. thesis, Univ. Orleans, France, 168pp.
ALTERATION OF NUCLEAR WASTE GLASSES CHARACTERIZED BY RADON EMANATION METHOD
V. Balek and Z. Málek,
Nuclear Research Institute Rez plc, CZ-250 68 Rez, Czech Republic (Contact: Fax: +422 6857 567, E-mail: INTERNET balmain.nri.cz)
A. Clearfield,
Texas A & M University, Department of Chemistry, College Station, TX, U.S.A.
INTRODUCTION
At the Nuclear Research Institute Rez, Czech Republic, the Radon Emanation Method, REM [1], based on the measurement of radon release from the samples measured, has been used for the characterization of the morphology changes of the nuclear waste glass alteration and their products.
The alteration product layers formed on the glass surface may act as a silicon barrier and are rich in transition elements and heavy elements simulating the actinides. At the long time scale, the formation of the secondary minerals in the altered glass layers may enhance the glass reactivity.
PRINCIPLE OF THE RADON EMANATION METHOD (REM) AND PREPARATION OF SAMPLES
Test specimens were labeled by surface deposition of 220Rn parent nuclides, i.e., the radionuclides of 224Ra and 228Th. The sample layer (max depth 120 nm) was labeled due to the recoil of 224Ra and 220Rn atoms, whereas 228Th atoms (half life T1/2 = 1.9 years) were absorbed on the sample surface as a source of 224Ra (half life T1/2 = 3.8 days) and 220Rn (T1/2 = 55 seconds). Radon atoms formed by spontaneous alpha decay of radium in the solid, can be released by recoil and by diffusion [1,2], reflecting morphology and radon permeability of the surface layers labeled.
Ground samples of different nuclear waste glasses were examined using REM in both virgin and altered forms. The composition of the borosilicate glass samples differed, depending on the laboratories where the glass was prepared. Glass in this experiment was prepared at the NRI Rez (Czech Republic), JAERI Tokai (Japan) or Radium Institute St. Petersburg (Russian Federation). Alteration of the samples took place during storage in distilled water for 2 weeks at 25° ± 1° C; the S/V ratio was 1 000 m-1. Measurements of the radon release rate from the labeled samples (before and after alteration) were carded out during heating and subsequent cooling in air. Heating and cooling rate was 5 K/min.
RESULTS AND DISCUSSION
Characterization of Nuclear Waste Glasses and their alteration products by means of REM:
Changes in the radon release rate measured during heating of the pristine glass sample characterize following processes: annealing of surface defects (potentially the annealing of radiation defects), annealing of mechanical stresses and sintering of the glass powder, glass transition point Tg and glass softening point.
Changes in the radon release rate measured during heating of the glass alteration products characterize the following processes: dehydration and thermal decomposition of the glass alteration products, morphology changes of the dehydrated glass alteration products.
The radon release rate measured during cooling characterizes the radon permeability (diffusivity) of the heat treated samples. The thermal behavior of both virgin and altered glass surfaces as characterized by the REM curves was compared with the DSC curves and electron microphotographs. These results were used for interpretation of the REM results.
CONCLUSIONS
The temperature dependencies of radon release rate measured during heating and subsequent cooling of the virgin and altered samples of the nuclear waste glasses can be used as fingerprints characterizing morphology and radon permeability of the samples.
The systematic experimental study performed in the frame of the IAEA Coordinated Research Program have resulted in the recommendation of the Radon Emanation Method as a supplementary method for characterization of the alteration product of nuclear waste forms.
The REM has been internationally recognized as the method which give an information on differences in the morphology, porosity, crystallinity as well as on sorption properties of the glass alteration products: These properties depend on the composition of the initial glass form, on the duration and temperature at which the alteration process takes place, as well as on the S/V ratios and on the presence of various ions and colloids in the leaching liquid, etc.
The REM makes it possible to examine the kinetics of the interaction of nuclear waste glass surfaces in real time and to indicate the effects of radiation on the chemical reactivity of the glasses. Moreover, this method makes it possible to reveal effects of the formation of secondary minerals (already in the nucleation stage) on the reactivity of the glass and on the migration of the radionuclides (thorium, radium, radon) used for the sample labeling.
At present, the Radon Emanation Method has been further used in the characterization of the alteration process of nuclear waste glasses prepared and treated in different laboratories worldwide.
REFERENCES
1. V. Balek, J. Tölgyessy: Emanation thermal analysis and other radiometric emanation methods (in: Wilson and Wilson's Comprehensive Analytical Chemistry, Part XII. C) Elsevier Publishing Co., Amsterdam, 1984, 304 pp.
2. V. Balek: Thermochim. Acta, 192 (1991) 1.
ACKNOWLEDGMENTS
The work was partially performed in the frame of the IAEA Coordinated Research Program (Contract No. 6746/R3). The authors wish to express their gratitude for financial support to the IAEA and to the U.S.-Czech Science and Technology Program.
[Editor's Note: ''Rez" is our anglization of the Czech.]
DOE REGULATORY INITIATIVE FOR VITRIFIED MIXED WASTE
Sandra J. Carroll
Westinghouse Savannah River Company
Building 742A
Aiken, SC 29809
Internet: sandra.carroll@srs.gov
James E. Flaherty
SAIC
20300 Century Boulevard, Suite 200-B
Germantown, MD 20874
Internet: james_flaherty@ccmail.gmt.saic.com
Under the current Resource Conservation and Recovery Act (RCRA) regulatory scheme, wastes that are ''listed" by the Environmental Protection Agency (EPA) remain hazardous unless they are "delisted." Additionally, via application of RCRA "mixture'' and "derived-from" rules, any solid waste that is mixed with a listed hazardous waste, or that is derived-from the treatment of a listed hazardous waste, is itself, a listed hazardous waste. This is the case without regard to the concentration of hazardous constituents in the waste or resulting treatment residues.
These regulations have resulted in large volumes of low-risk wastes (with low hazardous constituent concentrations) to be managed under the rigorous RCRA regulatory regime, despite some wastes being treated using technologically advanced treatment methods (as now mandated by RCRA's Land Disposal Restriction (LDR) program). EPA recognizes that these stringent regulations may not be appropriate given the changes to the RCRA program and the level of knowledge/awareness of companies now involved in hazardous waste management.
Background
On December 21, 1995 the EPA proposed the Hazardous Waste Identification Rule (HWIR) for process wastes under RCRA. Under this proposal, generators of listed hazardous wastes that meet constituent specific exit levels for low-risk wastes would no longer be subject to the hazardous waste management system under Subtitle C of RCRA.
DOE's Regulatory, Initiative
The Department of Energy (DOE) submitted a technical data package to EPA on vitrified mixed waste forms to be considered during the HWIR rule-making process. The technical data package supports a regulatory strategy that would allow vitrified mixed waste forms treated through a permit or other environmental compliance mechanism to be granted an exemption from RCRA hazardous waste regulations based upon the inherent destruction and immobilization capabilities of the technology.
Basis for Initiative
Vitrification is a desirable treatment option for mixed waste because the vitrified waste forms will resist degradation for the thousands of years necessary to allow decay of the radioactive component while chemically binding both the radioactive and hazardous components in the glass matrix, thus, reducing the threat to human health and the environment. Due to these features, EPA has specified, under the LDR program, vitrification to be the specified (or required) treatment technology for high-level waste that has a RCRA regulated hazardous component (mixed waste).
The Department of Energy possess significant amounts of wastes that can potentially be vitrified. The Department has identified vitrification as the treatment of choice for high-level wastes. Two facilities, the West Valley Demonstration Project, and the Defense Waste Processing Facility at the Savannah River Site will begin vitrifying high-level waste this year. A number of DOE facilities will be
vitrifying low-level waste in the near term and others are planning for vitrification facilities.
Regulatory Control
Through it's technical data package, DOE is proposing that mixed waste, treated by vitrification through a regulatorily controlled process, would be exempt from RCRA Subtitle C at the time that treatment is complete. Vitrified mixed low-level waste would exit RCRA Subtitle C after treatment and, in accordance with DOE Orders, would be disposed of in a low-level waste disposal facility. Vitrified high-level waste would be exempt from RCRA Subtitle C after treatment and be disposed at a federal repository licensed by the Nuclear Regulatory Commission (NRC).
The EPA or authorized State would retain control over the vitrification process to assure, through a permit or other environmental compliance mechanism, that the process produces a glass meeting environmentally acceptable performance characteristics. It is only after the production of a vitrified waste that meets these performance characteristics that DOE proposes the waste form be exempt from RCRA Subtitle C control.
DOE's proposal recommends the establishment of a process control program describing an operations envelope that could be included in the vitrification facility's environmental permit, as a means of maintaining regulatory controls to ensure an environmentally acceptable final waste form.
Waste Form Testing Strategy
DOE's proposal for mixed vitrified waste includes suggestions for sampling and analysis of a surrogate and /or wastes depending upon the level of radioactivity. In the testing strategy, it is proposed that organics need not be analyzed in the final product since they would be destroyed by the vitrification process, or captured in the off-gas system. Additionally, DOE suggests that testing for constituent concentration exit levels should be based upon the pathway of greatest risk to the public and environment.
Summary and Conclusion
The DOE proposal to EPA on vitrified mixed waste provides that waste treated using a superior treatment technology (i.e., vitrification) would be responsibly managed under the Atomic Energy Act (AEA) while reducing overall costs. Full regulatory authority by EPA or a State would be maintained until an acceptable vitrified waste form, protective of human health and the environment, is produced.
Acknowledgments
The information contained in this paper is a brief description of a DOE proposal submitted to EPA on October 20, 1995 (Reference 1). The Department is pursuing other regulatory initiatives under the leadership of Susan Jones and Michael Kleinrock, DOE Office of Waste Management, Office of Technical Services. Additional information on DOE regulatory initiatives can be obtained by contacting Ms. Jones at (301) 903-3327 or Mr. Kleinrock at (301) 903-7149.
References
1. Vitrification Technical Data Package Supporting the Hazardous Waste Identification Rule, U.S. DOE, October 20, 1995, EPA HWIR Waste Docket No. F95 WHWP S0983.F
2. EPA Handbook "Vitrification Technologies for Treatment of Hazardous and Radioactive Waste," EPA/625/R-92-002, May, 1992.
3. Environmental Assessment Waste Form Selection for SRP High-Level Waste, DOE/EA-0179, July, 1982.
4. TCLP Testing of DWPF Projected Glass Compositions, WSRC-TR-94-025, August 12, 1994.
Stabilization of Plutonium in Hybrid Glass Materials Using a Cyclone Melter
N. V. Coppa, J. C. Simpson and J. G. Hnat
Vortec Corporation, Collegeville, PA
Extended Abstract
Introduction: In a report1 sponsored by the host institution disposition scenarios for excess weapons grade plutonium included its incorporation into mixed oxide fuel or its vitrification in a stable glass matrix. It was further suggested that such materials might be stabilized in more durable natural matrices such as zircon in the form of a plutonium zirconate.2 While both vitrification and the incorporation into natural host media are attractive, the application to both approaches encounter some performance or processing issues which may limit their economical deployment. Another approach to the immobilization of weapons plutonium using hybrid materials was suggested3 and will be fully discussed here.
Both crystalline and glass immobilization media have beneficial characteristics. Glass materials are relatively easy to make. The most cited problem with glass used as an immobilization medium is that they are unstable relative to a crystalline assembly of phases with the same overall composition. Glasses which have good environmental stability usually require higher temperatures to synthesize and suffer chemical immiscibility and often devitrifiy upon cooling. Indeed plutonium is immisible with many low temperature glass compositions. Crystalline immobilization media, e.g. the arch type synroc,4 while extremely environmentally robust suffer from densification processes which are considered problematic from a radiological perspective. The object in the design and production of hybrid glasses is to partition the immobilization and preparative properties into the different phases of the material. Therefore, hybrid materials allow for the separate and near independent development optimization of the immobilization and processing characteristics.
Hybrid glasses: The hybrid materials discussed here are defined as an intimate physical mixture of a crystalline phase and noncrystalline phase such as a, but not limited to, ceramic and glass mixture. When the non crystalline phase of the hybrid material is a glass the material is referred to as a hybrid glass. A hybrid consisting of crystalline phase and glass is not equivalent to glass-ceramics since the latter forms during the devitrification of a precursor glass. As such, the composition of resultant phases in a glass ceramics are chemically dependent while the phases of the hybrid glasses may have completely independent origins. Hybrids are equivalent to solid suspensions and the noncrystalline phase need not be limited to glasses.
Preparation: Hybrid glasses are formed by making a physical mixture of a refractory crystalline phase and a lower melting glass phase. Prior to the present time the means of preparing such a material was a complicated and problematic. Presently there exist cyclone melting technology which readily enables the formation of these hybrids. 5 A 36 ton per day vitrification plant is presently under construction at the Paducah Gaseous Diffusion Plant, Paducah, KY for the purposes of vitrifying contaminated soils and low level RCRA waste. The cyclone melting process involves mixing waste with a balance of glass formers and introducing this mixture into a direct contact suspension heater followed by a cyclone melting reactor. The cyclone melter converts the preheated mixture into a molten glass product in the form of a flowing stream or film. The glasses produced passes all state and federal regulatory tests and requirements including TCLP, PCT tests and waste acceptance performance criteria. Hybrid glass formation involves the introduction of the refractory crystalline phase
coincident with the glass formers at the top CRV section or in a lower section in a cyclonic mixer/melter. The residence time of the materials in the hot zone of the melter is brief so chemical interactions, e.g. corrosive attack of the crystalline phase by the molten glass, are kinetically unfavored. Corrosion problems can be further minimized by the proper selection of the material components or may be used advantageously to achieve specialized surface structures.
The crystalline phase is prepared using powder processing techniques. Newer low temperature and pressure, economical ones, having very high reaction rates for the production of powdered host phases exist and have been described in the literature.6 Materials produced by such processes are often superior in homogeneity and do not require sieving or milling processing steps to achieve a narrow and uniform particle size distribution. Some of these techniques, as applied to immobilization media for radioactive materials, have been criticized since hot pressing was often required to produce a fully dense product from the resultant powders. Their use in the production of hybrid glasses is natural and has many advantages especially in a mode for continuous production. Solutions of powder precursors derived from plutonium stock can be injected into a spray dryer where the crystalline phase rapidly forms. Carrier gas communicates this material directly into the CMS where the hybrid glass forms. Criticality issues are completely avoided since the CMS delivers a glass stream not a large batch pot. That stream can be directed into molds with subcritical geometry.
Partitioning of materials properties: In a hybrid glass the chemical and structural properties of the two phases are independent of each other and the chemical and physical properties of those phases can be optimized independently. The glass composition can be optimized for environmental durability and immiscibility aspects of the radioactive host phase are no longer an issue. If the decay damage is confined to the crystalline phase, then the glass phase will have greater environmental stability. The host crystalline phase can be optimized for the chemical compatibility with plutonium and its daughters, and long term radiolytic stability. Many crystalline host phases for plutonium exist which are likely to exhibit excellent host characteristics as well as have the required high melting point. The mean size and distribution of the crystalline phase should be selected so that a high percentage of the decay fragments terminate in the crystalline phase. To achieve this, the decay fragments, their energies, and the mean track length in the host material as well as the volume fraction of the crystalline phase in the hybrid is considered. One particularly interesting material to use as a host phase for plutonium in a hybrid glass is monazite. Naturally occurring uranium and thorium containing monazites remain crystalline after enduring conditions which normally render a material metamict.7
DOE REGULATORY INITIATIVE: IMMOBILIZED MIXED DEBRIS
Barbara Dubiel
Lockheed Idaho Technologies Company
12850 Middlebrook Rd. Rm 435
Germantown, MD 20874
Susan Carson
SNL
1515 Eubank, S.E.
Sandia National Laboratory
Albuquerque, NM 87123
As part of the Phase I Land Disposal Restrictions (LDR) rule, EPA promulgated the Final Rule on Hazardous Debris (Debris Rule) in August 1992. This rule allows hazardous debris treated by an extraction or destruction technology to exit the Resource Conservation and Recovery Act (RCRA) Subtitle C control provided that treated debris does not exhibit a characteristic of hazardous waste (57 FR 37222, August 18, 1992).
At the time the Debris Rule was enacted, EPA chose not to allow debris treated with an immobilization technology to exit Subtitle C control. The rationale for this decision was a lack of available data to demonstrate that, absent Subtitle C management, contaminants would not migrate from immobilized debris at levels that could pose a hazard to human health and the environment (57 FR 37240). However, EPA invited the regulated community to submit data on immobilization of debris and requested comments on whether immobilized debris should exit from Subtitle C regulations as part of the proposed Phase II LDR rulemaking (58 FR 48144, September 14, 1993).
DOE'S PROPOSAL
To effect reform for mixed wastes that present a low risk from the hazardous component and to fulfill EPA's past requests for more data on immobilized debris, DOE developed technical data supporting the position that mixed waste debris treated by immobilization, followed by disposal in a low-level waste (LLW) facility is protective of human health and the environment and should therefore be allowed to exit Subtitle C controls. These technical data were submitted to EPA in July 1995. On October 20, 1995, DOE supplemented the July 1995 report to EPA with a report entitled "Performance Evaluation for RCRA Toxic Metal Disposal in DOE Low-Level Radioactive Waste Disposal Facilities." The basis for DOE's immobilized mixed debris proposal is the combination of the integrity of the encapsulated debris waste form, coupled with the protectiveness of a LLW disposal facility, is protective of human health and the environment.
Integrity of the Final Waste Form
To ensure mixed debris treated by immobilization and placed in a low-level waste disposal facility is sufficiently protective of human health and the environment, DOE proposed that the final waste form meet or exceed established performance criteria. DOE proposed that testing of immobilized debris be conducted in two tiers. The first tier would demonstrate the treated waste form ability to prevent leaching of hazardous constituents. The tier two tests would demonstrate the integrity of the treated waste form in the disposal environment. Tier two tests would be performed after tier one tests have been performed and passed.
Proposed Encapsulants
EPA currently recognizes in the treatment standards for debris (40 CFR Section 268.45(c),
Table 1) polymeric organic materials as macro-encapsulating agents and Portland cement and lime/pozzolans as microencapsulants. Several other encapsulating agents including hydraulic cement, sulfur polymer cement, polyethylene, phosphate ceramics, epoxies, urea formaldehyde polymer, asphalt, high integrity containers, and stainless steel containers have been developed and tested. Because the performance of some of these materials is comparable or superior to the encapsulating agents listed in EPA's treatment standards for debris, DOE included these encapsulants (i.e., sulfur polymer cement, polyethylene, phosphate ceramics, specialized containers) as proposed alternate encapsulants. For the proposed encapsulating materials, DOE assembled data on waste form leachability and/or permeability, biodegradation, radiation stability, and long-term environmental stability for the proposed encapsulating materials.
Risk-Based Analysis of Low-Level Disposal Facilities
In DOE's report, "Performance Evaluation for RCRA Toxic Metal Disposal in DOE Low-Level Radioactive Waste Disposal Facilities," a risk-based analysis evaluated the environmental transport of RCRA toxic metals from six DOE LLW disposal sites. The analysis focuses on the toxic metal component of the mixed waste debris (the principal contaminants in DOE's mixed LLW debris) and the groundwater contaminant pathway, because it is the dominant transport pathway for human exposure from land disposal facilities when the waste is immobilized. The analysis estimates permissible leachate concentrations of toxic metals by using Maximum Contaminant Levels (MCL) concentration values in groundwater at a receptor point along the performance boundary (100 m from the disposal facility boundary), and attenuation factors associated with site-specific conditions. The report concludes that arid DOE LLW sites appear to provide a greater degree of protection of human health and the environment than humid DOE LLW sites based on higher attenuation and longer contaminant travel times. However, even at relatively humid sites most RCRA toxic metals (except for arsenic and selenium) are immobile in the subsurface environment.
SUMMARY
In the technical data package supporting DOE's immobilized mixed debris proposal, DOE included stabilization/immobilization performance data and related information which demonstrate debris contaminated with mixed waste and treated by immobilization is protective when disposed in a low-level waste disposal facility, and therefore, should be allowed to exit Subtitle C. Protection of human health and the environment is achieved via the combination of treatment and disposal at low-level waste disposal facilities regulated under the requirements of the Atomic Energy Act (AEA). This mixed waste management approach is protective of human health and the environment, and at the same time provides cost savings to the regulated community.
Acknowledgments
The information contained in this paper is a brief description of a DOE proposal submitted to EPA on July 21, 1995 (Reference 1). The Department is pursuing other regulatory initiatives under the leadership of Susan Jones and Michael Kleinrock, DOE Office of Waste Management, Office of Technical Services. Additional information on DOE regulatory initiatives can be obtained by contacting Ms. Mary Beth Burandt at (301) 903-7113 or Mr. Kleinrock at (301) 903-7149.
References
1. Disposal of Immobilized Mixed Waste Debris in Low-Level Waste Disposal Facilities, U.S. DOE, July 21, 1995, EPA HWIR Waste Docket No. F95 WHWP S0983.B
2. Performance Evaluation for RCRA Toxic Metal Disposal in DOE Low-Level Radioactive Waste Disposal Facilities, U.S. DOE, July 21, 1995, EPA HWIR Waste Docket No. F95 WHWP S0983.E
Abstract
Reexamination of Variables Affecting the Hydration of Obsidians Using Empirical Chronological Data and Laboratory Induced Hydration Experiments
Jonathon E. Ericson, Ph.D. and Stephen R. Lyon
University of California, Irvine
Irvine, California 92717 U.S.A.
Optically measured (filar) hydration rims of obsidian artifacts from excavated archaeological sites in California were dated by associated radiocarbon dates reported by (2). The trace element concentrations of the obsidian artifacts were analyzed by neutron activation analysis. NAA data of the samples from 20 obsidian sources in California, Oregon and Nevada were assigned membership by stepwise discriminate analysis (Ericson & Kimberlin, not published). Six models of hydration rates were calculated based on the natural hydration and associated radiocarbon data for twelve sources, herein called empirical (hydration) rates.
Eighteen source samples were hydrated in liquid phase at ambient temperatures at 150, 163, 175 and 200 °C for varying periods of 3 to 28 days. Hydration rims of the source samples were measured. The Arrhenius equation and above data were used to calculate the activation energies and diffusion rates for these sources, herein called laboratory rates. A comparison of the empirical and laboratory rates revealed that the empirical rates tended to be faster than the laboratory rate (3a).
Major elemental concentrations from 20 sources were determined by x-ray fluorescence analysis. Initial and final water contents of these samples were measured by 19F nuclear reaction profile technique (6). Density was measured by densitometer. The elemental composition, initial and final water concentrations and density were treated as independent variables of the hydration process. With empirical linear and diffusion rates and laboratory rates as activation energy and diffusion coefficients treated as dependent variables in stepwise regression analysis.
Earlier stepwise linear regression analysis (3a) of laboratory rates indicated that CaO, TiO2, Density, initial water, SiO2 and hardness accounted for 98% of the multiple R respectively were responsible for the changes in hydration rates among these sources. Reexamination of these data using current statistical techniques is warrented given the differences in variance and order of magnitude difference in the dependent and independent variables.
Statistical Analysis
The statistical analysis of the major intrinsic variables of the hydration process was carded out using stepwise regression analysis. This was applied to the empirical data shown in Table 1-11 (Rate Constants of Some Obsidian Hydration Models) and from the laboratory generated data in Table 2-6 (Coefficients for Source Specific Induced Hydration Equations). The dependent variables (Linear, T=bx, and Diffusion T=dX2) and (A,/µ2/103 year, and E, Kcal/mole) were taken from the previous tables. The independent variables, SiO2, Al2O3, CaO, Na2O, K2O, Fe2O3, MgO, TiO2, density, final water and internal water were taken from Table 3-11 (Summary Table of Chemical and Physical Properties and Calculated Factors for Obsidian Source Samples) (3b).
Stepwise Regression is a procedure for sequentially entering independent variables one at a time in a regression equation in the order that most improves the regression equation's predictive ability or removing them when doing so does not significantly degrade its predictive ability (7). The independent variables were entered both in their original form and
as Z scores. The "A" factor (experimental diffusion coefficient) was log transformed due the fact that it spanned 11 orders of magnitude. The Multiple R values of the analysis are shown in the table below:
|
Laboratory Data |
ORIGINAL DATA |
Z SCORES |
|
E (activation energy) & A (diffusion coefficient) |
Na2O(0.651) K2O(0.81) |
Na2O(0.651) K2O(0.810) |
|
E |
Na2O (0.632) |
Na2O(0.632) |
|
A |
Na2O (0.650) K2O(0.810) |
Na20(0.651) K2O(0.810) |
|
Empirical Data |
|
|
|
LINEAR RATE & DIFFUSION RATE |
MgO (0.755) |
MgO(0.755) |
|
LINEAR RATE |
MgO (0.831) |
MgO (0.831) |
|
DIFFUSION RATE |
MgO (0.755) |
MgO (0.755) |
Regression analysis of the laboratory rates indicate that Na and K are the first two significant variables related to changes in the hydration rates among the eighteen sources. These results suggest that the Doremus model of outward co-diffusion of Na(K) may be the rate limiting factor(s) of obsidian. In contrast, regression analysis of the empirical rates indicate that Mg is the first significant variable related to changes in the empirical rates. These results suggest that the Si-Mg model of hydration rate change proposed by Friedman and Trembore (1986) may have validity. The discrepancy between the analysis of the two data sets suggests that the soil moisture and low temperature of natural hydration conditions and the distilled water-high temperature conditions of laboratory-induced hydration act differentially on the structure-modifying and the silica lattice structure.
REFERENCES CITED:
1. Berger, R. and J. E. Ericson, Natural solid solutions: Obsidians and tektites, in Recent Advances in Sciences and Technology of Materials , A. Bishay, (ed), Plenum Press, New York, Vol.3,187-190 (1974).
2. Berger, R. and J. E. Ericson UCLA Radiocarbon Date List 10, Radiocarbon 25, 1,129-136 1983.
3a. Ericson, J. E. Durability of Rhyolitic Obsidian Glass Inferred from Hydration Dating Research, Scientific Basis for Nuclear Waste Management, Vol. 3, (J. G. Moore, Ed.), Plenum Press, Inc., New York, 283-290. (1981).
3b. Ericson, J. E. Exchange and Production Systems in Californian Prehistory. British Archaeological Reports, International Series, 110, 240 p. (1981).
4. Ericson, J. E. Obsidian Hydration Rate Development. Journal of Material Research Society. Vol. 123 pp. 215-224 (1988).
5. Ericson, J. E., J. D. MacKenzie and R. Berger Physics and chemistry of the hydration process in obsidians, I: Theoretical Implications, in Advances in Obsidian Glass Studies: Archaeological and Geochemical Perspectives, R. E. Taylor, (ed.) Noyes Press Park Ridge, New Jersey, 24-45, (1976).
6. Glantz, S.A. and Slinker, B.R. in Primer of Applied Regression and Analysis of Variance. Mcgraw-Hill, Inc, New York.(1990).
7. Lee, R., D. A. Leich, T. A. Tombrello, I. I. Friedman, and J. E. Ericson Obsidian hydration profile measurements using a nuclear reaction technique, Nature, 250, 44-47, (1974).
A PLASMA ARC - VITREOUS CERAMIC PROCESS FOR HAZARDOUS AND RADIOACTIVE WASTE STABILIZATION
Xiangdong Feng and Jeffrey E. Surma,
Pacific Northwest National Laboratory, Box 999, MSIN: P8-37
Richland, Washington 99352 (E-Mail: X_Feng@PNL.gov)
Clarence G. Whitworth
MSE, Inc., DOE WETO, Butte, MT 59702
Richard C. Eschenbach
Retech of Lockheed Martin, Ukiah, CA 95482
Gary L. Leatherman
Science Applications International Corporation, Idaho Falls, ID 83402
ABSTRACT
Plasma metal-melting has been in industrial use worldwide for two decades. In the most recent decade, this technology has found new application in the stabilization of hazardous and radioactive wastes when it combines with a novel waste form - Vitreous Ceramics [1-6].
In plasma melters, plasma is generated by passing an electric current through a gas (such as helium, air, argon-oxygen, or nitrogen), which heats the gas to a very high temperature (>12,000F) and partially ionizes it. In a transfer-mode plasma torch, the torch current also passes through the material being melted, providing both radiant and resistance heating of the melt, and providing enhanced mixing by a churning action at the are impingement point. The melt normally is heated to 3000°F or higher. At such high temperature and in the presence of oxygen, waste materials are oxidized into their simple oxides and are immobilized into a durable and stable vitreous ceramic waste form. A vitreous ceramic waste form consists of crystalline phases embedded in a glassy silicate matrix. The plasma arc - Vitreous ceramic (PAVC) approach has been successfully applied to the treatment of low-level and mixed wastes, and has also been evaluated for treating spent nuclear fuels and plutonium scraps and residues [6].
The advantageous features of the PAVC process are:
-
Simplicity of operation- Wastes can be loaded without detailed characterization and pretreatment directly into the plasma furnace, and melted into the final waste form. Pretreatment such as size reduction, debris separation, and drying are unnecessary, which is especially advantageous for highly radioactive wastes, such as spent nuclear fuels;
-
Flexibility - The PAVC process can accept heterogeneous feeds and relatively large debris. Feeds can include organic, inorganic, metallic, pyrophoric, hazardous,
-
and radioactive components;
-
High waste loading - Waste loading in glass is usually limited by the solubilities of the constituent least soluble in glass. In glass melters, crystallization is unacceptable because it usually causes melt clogging and durability decrease of the product. In the PAVC approach, crystallization is a controlled and preferred process that allows higher waste loading and improved durability;
-
Superior chemical durability - Vitreous ceramics promote the formation of stable and low-solubility (in water) crystalline phases, embedded in a glass matrix. Because of crystallization of insoluble components, the glassy matrix itself becomes enriched in stabilizing network formers such as SiO2 and Al2O3. The crystals formed (e.g., zirconolite, perovskite, zircon, spinel, etc.) can incorporate large concentrations of uranium, plutonium, other fission products, and other hazardous components (Ni, Cr, Cd, etc.). The crystals are analogs of highly stable minerals which have existed in nature for billions of years. The crystals are tightly bound to the glassy matrix, resulting in good physical integrity and mechanical strength of the waste form. The glassy matrix can immobilize elements that cannot be incorporated into crystalline phases, and therefore offers greater processing flexibility than purely crystalline waste forms, as well.
As noted, the PAVC process is especially advantageous for heterogeneous and debris-laden wastes, and wastes that will not easily form glasses. For high-alkali and high-halide wastes, lower temperature, constant-throughput processes, such as joule heated melters, may be a more attractive treatment option.
REFERENCES
1. X. Feng, G. Ordaz, and P. Krumrine, "Vitreous Ceramic - A Complementary Waste Form to Homogenous Glass for the Implementation of MAWS in Treating DOE Low-level/Mixed Wastes," Proc. Spectrum '94, August 14-18, 1994, Atlanta, GA, 1275-1285 (1994).
2. K. D. Filius, C. G. Whitworth, D. M. Battleson, "Results of High Metal Content Waste Demonstration in a Plasma Centrifugal Furnace - Vol. I," MSE, Inc. Doc. No. PTP-4, October 1995, US DOE Western Environmental Technology Office, Butte, MT
3. R. C. Eschenbach, Plasma Arc Centrifugal Treatment (PACT) of Hazardous Waste," Proc. 8th Symp. Plasma Science for Materials, June 15-16, 1995
4. G. L. Leatherman, R. Geimer, J. Batdorf, G. Hassel, P. Wolf, "The Plasma Hearth Process: Process Residuals Characterization," Proc. Environ. Waste Manag. Issues in Ceramic Industry II, V. 45, 23 (1994)
5. X. Feng, W.K. Hahn, W. Gong, L. Wang, and R. C. Ewing, "Minimum Additive Waste Stabilization Using Vitreous Ceramics," September 1995, PNNL Report PNNL-10826, Richland, WA.
6. X. Feng, R.E. Einziger, R.C. Eschenbach, "A Direct, Single-Step Plsama Arc/Vitreous Ceramic Process for Stabilizing Aluminum Spent Nuclear Fuels," Pacific Northwest National Laboratory Report, April 1996.
DIRECT CONVERSION OF METALS, CERAMICS, AMORPHOUS SOLIDS, HALOGENS, AND ORGANICS TO BOROSILICATE GLASS USING THE GMODS PROCESS
Dr. Charles W. Forsberg and Dr. Edward C. Beahm
Oak Ridge National Laboratory
P.O. Box 2008, Oak Ridge, TN., USA, 37831-6495
Tel: (423) 574-6783; Email: forsbergcw@ornl.gov
INTRODUCTION
A new waste vitrification process, the Glass Material Oxidation and Dissolution System (GMODS), has been invented. This process directly converts metals, ceramics, and amorphous solids to glass; oxidizes organics with the residue converted to borosilicate glass; and converts halogens to borosilicate glass and a secondary sodium halogen stream. Laboratory work has demonstrated the conversion of cerium, uranium, Zircaloy, stainless steel, multiple oxides, and other materials to glass. However, significant work is required to develop GMODS for applications at an industrial scale.
Glass is recognized worldwide as a preferred waste form for radioactive and chemically hazardous wastes. There is however a major limitation: all existing glass processes require that the waste be in the form of oxides or oxide-like materials before vitrification. Oxide-like materials are compounds such as nitrates and carbonates that decompose to oxides at high temperatures. Conversion of wastes to oxide-like forms before vitrification is a complex and an expensive task.
GMODS allows the direct conversion of oxides, metals, ceramics, organics, halogens, and amorphous solids to glass. This allows complex waste mixtures (filters, process wastes, laboratory wastes, etc.) to be directly converted to glass without preprocessing. The alternatives are to separate the wastes into specific categories and process each category of waste and/or process (oxidize, dechlorinate, etc.) each waste before vitrification.
GMODS PROCESS DESCRIPTION
GMODS converts wastes into glass inside a glass melter. The process can operate as a batch (Fig. 1) or continuous process. For batch operation, the starting conditions are a glass melter filled with molten, lead-borate dissolution glass. Oxides dissolve in glass, but metals and organics do not. GMODS uses lead oxide (PbO) in the molten glass to oxidize (a) metals to metal oxides and (b) organics to carbon oxides. The resultant metal oxides dissolve into the glass. The carbon oxides exit the melter as gases. The lead metal reaction product separates from the glass and forms a separate layer at the bottom of the melter. The boron oxide (B2O3) in the melt assures rapid dissolution into the glass of any protective oxide layers on metal wastes.
After dissolution of the wastes, silicon oxide and other additives are added to the glass to produce a high-quality product glass. Excess PbO is removed from the glass by adding carbon, which converts the PbO to lead metal and carbon dioxide (CO2). The final glass may have some or no PbO depending upon the desired product glass. The product glass is poured from the melter into the waste packages. To generate the next batch of dissolution glass, boron oxide is added to the melter and the lead metal is oxidized to PbO with oxygen.
GMODS can convert halogen-containing materials to glass, a process which creates a separate nonradioactive sodium halogen waste stream. Halogens, such as chlorides, make poor-quality glasses; hence, they must be separated from other components. In the dissolution glass, chlorides in the waste form
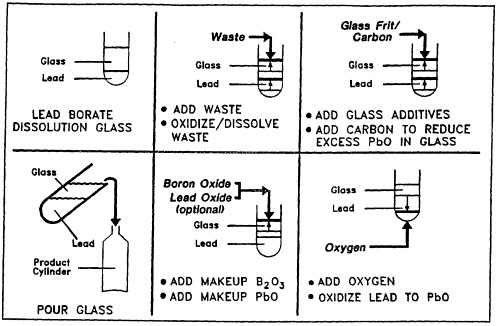
FIG. 1 GMODS batch processing of wastes to borosilicate glass.
lead chloride (PbCl2), which is volatile at glass melter temperatures and exits to the aqueous sodium hydroxide (NaOH) scrubber. In the scrubber, the PbCl2 reacts with the NaOH to yield insoluble lead hydroxide [Pb(OH)2] and soluble NaCl. The insoluble Pb(OH)2 is recycled back to the melter, where it decomposes to PbO and steam, while the aqueous NaCl stream is cleaned and discharged as a chemical waste.
Because of the corrosive characteristics of the initial dissolution glass, GMODS requires a cold-wall melter where cooling jackets in the walls produce a ''skull'' of solidified material that protects the walls from the melter's contents. The melters can be heated by fossil, induction, plasma arc, or electron-beam systems. Such systems are currently used to melt high-temperature materials (e.g., titanium and superalloys) and produce speciality glasses.
STATUS OF LABORATORY WORK
Tests demonstrated the dissolution of UO2, ZrO2, Al2O3, Ce2O3, MgO, and other oxides. Oxidation-dissolution tests demonstrated the oxidation of the following metals and alloys (followed by the dissolution of their oxides into the melt): U, Ce, Zircaloy-2, Al, stainless steel, and other metals. Oxidation-dissolution tests also demonstrated the oxidation of carbon and graphite, with production of CO2. The other process steps (adding glass frit, removing lead from the glass, and oxidizing lead back to PbO) have been investigated in the laboratory. These steps are also used in the glass, lead smelting, and lead-battery industries on very large scales and are well understood.
REFERENCES
C. W. Forsberg et al., Direct Vitrification of Plutonium-Containing Materials With The Glass Material Oxidation and Dissolution System (GMODS), ORNL-6825, Lockheed Martin Energy Systems, Inc., Oak Ridge National Laboratory, Oak Ridge, Tennessee (October 1995).
SURFACE LAYER FORMATION OF THE FRENCH SON68 NUCLEAR WASTE GLASS DURING VAPOR PHASE ALTERATION AT 200 °C
W.L. Gong, R.C. Ewing, and L.M. Wang*, E. Vernaz**, J.K. Bates and W.L. Ebert***
* Department. of Earth and Planetary Sciences, University of New Mexico, Albuquerque, NM 87131
** CEA-VALRHO, B.P. 171, 30205 Bagnols-Sur-Cèze Cedex, France
*** Chemical Technology Division, Argonne National Laboratory, Argonne, IL 60439, USA.
The SON68 inactive "R7T7" composition is the French reference glass for the LWR nuclear waste. The mechanism of aqueous corrosion of "R7T7" waste glass have been extensively investigated in the literature. 1,2 Vapor phase alteration was used to accelerate the reaction progress of glass corrosion and to develop the characteristic suite of secondary, alteration phases. Additionally, vapor phase alteration will be an important corrosion mechanism of nuclear waste glasses in a hydrologically unsaturated geological repository. The understanding of glass corrosion and radionuclide release can be enhanced by investigating secondary precipitated phases, chemical and structural evolution of surface layers. In this work, extensive solid-state characterization (AEM/SEM/HRTEM) was completed on six inactive R7T7 waste glasses that had been altered in the presence of saturated water vapor (200 °C) for 22, 91, 241, 908, 1013, and 1021 days (referred to as 22D, 91D, 241D, 908D, 1013D, and 1021D). The AEM samples were prepared using an ultramicrotomy ''slicing" technique. Then, surface layers on the reacted glasses were examined in cross-sections by AEM (lattice-fringe imaging, micro-diffraction, and quantitative thin-film EDS analysis).
The glass monoliths were invariably covered with a thin altered rind. The layer increases in thickness with increasing time of corrosion: 0.5 µm for 22 days; 4 µm for 91 days; 6 µm for 241 days; 10 µm for 908 days; 26 µm for 1013 days; and <<35 µm for 1021 days. The composite alteration layer of the SON68 samples is at least four time less thick than the layer formed on the SRL 131 glass composition under the same test condition.3
Six distinctive zones, based on phase chemistry and microstructure, were distinguished within the well-developed surface layers. Zone 1, as precipitated layer, is the outermost and is characterized by secondary phases formed by precipitation from the condensed water film. A number of crystalline phases such as analcime, tobermorite, apatite, and weeksite were identified on the surfaces of the reacted glasses as precipitates. Amorphous Ca-Si precipitates with a spherical morphology (10-30 µm in size) occurred on the surface of 1013D. The typical composition for the amorphous precipitates is: SiO2 70.91, Al2O3 3.51, Na2O 2.05, CaO 12.05, FeO 2.40, ZrO2 3.06, Ce2O3 0.70, and BaO 5.30 (wt. %). Zone 2 is the thin region consisting of well-crystallized and vertically oriented fibrous smectite crystals (nontronite-15Å). It occurred as a honeycomb-like layer occupying most of the surface. This zone was well developed on both the short- and long-term samples and increased in thickness with the increasing time of the experiments. Zone 3 is a thin fine-grained layer beneath Zone 2, with mixtures of smectite crystallites and an amorphous matrix. The concentrations of rare-earths and zirconium are extremely high as revealed by EDS analysis (˜15 wt. % rare-earth oxides and ˜13 wt. % ZrO2). In this layer, rare-earths may be incorporated into smectite
crystallites and amorphous silica-rich matrices through surface sorption or/and interlayered ion exchange.
The majority of the surface layer volume (Zones 4 and 5) is composed of two morphologically and chemically different structures: one consists of well-crystallized fibrous smectite aggregates occurring along with cavities, the A-domain; and the other consists of poorly-crystallized regions containing needle-like smectite (montmorillonite) crystallites, a silica-rich amorphous matrix, and possibly ZrO2 particles, the B-domain. Two domains are clearly identified in a HRTEM micrograph. Smectites in A-domains, identified as nontronite-15Å, contain relatively high concentrations of transition elements, such as Fe, Zn, Cr, and Ni. Zr, with a formula (Na,Ca,Nd)0.80(Al0.61,Fe3+0.52,Zn0.40,Mn,Ni) 1.84Si4O10(OH)2 on an 11-oxygen equivalent basis. B-domains have high amounts of Zr and rare-earths and low amounts of transition elements such as Fe, Zn, Cr, and Ni, as compared to those of the A-domains. In long-term samples, well-crystallized A-domains dominate the surface layer structure, while in short-term samples, poorly-crystallized B-domains dominate the structure of the surface layers. Thus, an extensive recrystallization process of earlier formed B-domains into A-domain smectites must have occurred, associated with an increased cavity volume during the continued corrosion. When early-formed B-domains recrystallized into A-domains, most of the rare-earths in B-domains may be lost into solution. However, transition elements were incorporated into thermodynamically stable A-domain smectites. The recrystallization of the B-domains into the A-domain smectites is a critical mechanism for surface layer formation and very important to understanding of the long-term behavior of rare-earths, Zr, and the transition elements. Two crystalline phases, Ag2TeO3 and (Ca,Sr)Mo3O9(OH)2, were found within the inner zones of surface layers, and they must have nucleated in situ, indicating that Ag, Te, Sr, and Mo can be retained within the surface layer. Zone 6 is the hydrated glass where smectite crystallites may have nucleated. The mechanism of surface layer formation is discussed based on the SEM/AEM results.
References
1. E. Vernaz and J.L. Dussossoy, Appl. Geochim. Suppl. Issue No. 1, 13 (1992).
2. T, Advocat, J.L. Crovisier, A. Clement, F. Gerard, and E. Vernaz, Mat. Res. Soc. Symp. Proc. 353, 31 (1995).
3. D.J. Wrokiewicz, C.R. Bradley, and J.K. Bates, Mat. Res. Soc. Symp. Proc. 333, 259 (1993).
POTENTIAL APPLICATION OF ELECTRON SPIN RESONANCE TO THE STUDY OF RADIATION EFFECTS IN HIGH-LEVEL NUCLEAR WASTE DISPOSAL GLASSES
David L. Griscom
Naval Research Laboratory, Washington, DC 20375-5338
Radiation-induced color centers in vitreous silica and silicate, borate and phosphate glasses have now been studied for forty years 1 by means of the technique of electron spin resonance (ESR). The ESR method is extremely sensitive (capable of detecting unpaired electrons in numbers as low as a few parts per billion formula units) and rich in nano-scale structural information, yielding, e.g., the chemical natures, bond lengths and bond angles of the atoms in the immediate coordination sphere of the damage site. An enormous literature (for a review, see ref.2) now enables the researcher to recognize many generic types of defect centers, even in glasses of complex or unknown compositions, from inspection of their ESR spectra. The experiment is rapidly performed, non-destructive, and non-polluting (since the preferred means of sample mounting is within hermetically sealed silica tubes). The present paper reviews this field from the perspective of its potential application to assess the long-term stability of various glass forms under consideration for immobilization and disposal of high-level nuclear wastes and excess weapons plutonium.
Neutron diffraction studies of as-melted silica glasses tell us that to an excellent approximation all silicons are bonded to four oxygens and all oxygens are bonded to two silicons.3 Such melt-quenched silicas are found4 to densify by ˜2.5% upon exposure to fast-neutron fluences exceeding ˜1020/cm2 or doses of ionizing radiation greater than ˜10 10 Gy. ESR has elucidated many important facets of this radiation-altered material. A portion of the ideal network can be symbolically represented by ![]() , where the notation "
, where the notation "![]() " denotes three bonds to other oxygens in the glass network. Structures probed by ESR are confined to those which are paramagnetic, i.e., those binding an unpaired electron (denoted by "·" in the chemical notations used below). A neutral oxygen vacancy in silica can be represented
" denotes three bonds to other oxygens in the glass network. Structures probed by ESR are confined to those which are paramagnetic, i.e., those binding an unpaired electron (denoted by "·" in the chemical notations used below). A neutral oxygen vacancy in silica can be represented ![]() . Removal of one electron from this site results in the formation of the well known E'γ center (
. Removal of one electron from this site results in the formation of the well known E'γ center (![]() ) according to
) according to
A significant conclusion of careful ESR studies by many workers is that the displaced oxygen atoms move to interstitial positions, where they readily dimerize to form molecular oxygen. Upon heating to ˜200 C, this interstitial O2 has been shown to diffuse through the network and react with E' centers according to:
Here, the entity ![]() is an ESR-active species known as the bonded peroxy radical. By ESR spin count, a radiation dose sufficient to densify the glass by 2.5% results in about one Si-Si bond per 1,000 silicons, complemented by half that many O-O bonds.
is an ESR-active species known as the bonded peroxy radical. By ESR spin count, a radiation dose sufficient to densify the glass by 2.5% results in about one Si-Si bond per 1,000 silicons, complemented by half that many O-O bonds.
The total number of Si-Si and O-O homo bonds could be 10 times higher, if the ESR-inactive ones could be counted. Thus, while the as-melted glasses are structurally disordered, the radiation-amorphized glasses are also chemically disordered.
E' center variants and bonded peroxy radicals are also noted in alkali silicate glasses subjected to γ-ray doses ˜ 106 Gy.5 More significantly from the point of view of high-level nuclear waste glasses, the additional spectra of unbonded superoxide (O2-) and ozonide (O3-) ions have been recorded in such glasses.5 The characteristic superoxide-ion ESR spectrum appears to arise from O2- ions in a disordered alkali peroxide matrix, implying that relatively low γ-ray doses have initiated a decomposition of simple silicate glasses into intimate mixtures of oxygen rich phases and chemically reduced phases. Because alkali peroxides are known6 to spontaneously disproportionate into monoxides and superoxides and the latter in turn are known to disproportionate into peroxides and O2 molecules, this multi-step process may account for observations of bubble formation in electron-, ion- or γ-irradiated nuclear waste glasses.7 This kind of decomposition is analogous to the radiation-induced growth of sodium metal colloids and evolution of chlorine in irradiated rock salt.8 In principle, at least, a radically decomposed waste glass hermetically sealed in a steel canister might be vulnerable to chemical explosion. ESR methods could be used to quickly determine which regions of glass-composition space are most susceptible to this type of decomposition.
ESR is also highly sensitive to processes which result in the precipitation of fine grained ferromagnetic phases in glasses.9 It is known that magnetite particles precipitate during cooling of terrestrial natural glasses of both volcanic and impact origins, while metallic iron particles are found in glassy phases extracted from returned lunar soil samples. Precipitated iron particles resulting from Fe ion implantation into silica substrates have been well characterized by ESR.10 What is not yet known is whether sub-solidus precipitation of ferri- or ferro-magnetic particles in iron-rich glasses can be induced by α decays of contained radionuclides. An ESR survey of ion-implanted high-iron nuclear waste glasses could reveal the existence of such phenomena occurring in parallel with or in place of the kind of radiolytic decomposition noted above.
1. R.A. Weeks, J. Appl. Phys. 27 (1956) 1376-1381.
2. D.L. Griscom, Glass: in Science and Technology Vol. 4B, D.R. Uhlmann and N.J. Kreidl, Ed. (Academic Press, Boston, 1990), pp. 151-251.
3. E. Lell, N.J. Kreidl and J.R. Hensler, in Progress in Ceramic Science Vol. 4, J. Burke, Ed. (Pergamon Press, Oxford, New York, 1966), pp. 1-93.
4. A.C. Wright, J. Non-Cryst. Solids 179 (1994) 84-115.
5. R. Cases and D.L. Griscom, Nuc. Inst. & Methods B1 (1984) 503-510.
6. N.-G. Vannerberg, in Progress in Inorganic Chemistry 4 (1962) 125-197.
7. R.C. Ewing, W.J. Weber, and F.W. Clinard, Jr., Prog. in Nucl. Energy 29 (1995) 63-127.
8. P.W. Levy, J.M. Loman, and J.A. Kierstead, Nucl. Inst. & Methods B1 (1984) 549-556.
9. D.L. Griscom, C.L. Marquardt, and E.J. Friebele, J. Geophys. Res. 80 (1975) 2935-2946.
10. D.L. Griscom, J.J. Krebs, A. Perez, and M. Treilleux, Nucl. Inst. & Methods B32 (1988) 272-278.
LEACHING BEHAVIOR OF PU AND CM FROM WASTE GLASS UNDER REDUCING CONDITION
Y.Inagaki, A.Sakai, H.Furuya, K.Idemitsu and T.Arima, Dept. of Nucl. Eng., Kyushu Univ., Fukuoka 812, JAPAN.
T.Banba, T.Maeda, S.Matsumoto and Y.Tamura, Japan Atomic Energy Research Institute, Tokai-mura, Ibaraki 319-11, JAPAN.
ABSTRACT
In recent years, some corrosion tests on actinoids-doped waste glasses have been performed, and leaching behavior of actinoids from the waste glasses has been investigated. However, most of the previous corrosion tests have been performed under oxidizing conditions, and the leaching behavior of actinoids under reducing conditions (predicted in repository environments) has not been well studied. Most of actinoids contained in the waste glasses are redox active elements, and their Oxidation states, chemical species and equilibrium solubilities are greatly influenced by redox conditions. Therefore, leaching behavior of actinoids from the waste glasses can be greatly affected by redox conditions.
The purpose of this study is to understand leaching behavior of actinoids from the waste glasses under reducing conditions. Static corrosion tests were carried out on the waste glass doped with Pu and Cm (PuO 2; 0.22wt%, CmO2; 0.09%) in deionized water at 90 °C with S/V ratio of 2500 m-1 under oxidizing and reducing conditions, respectively. The corrosion tests under oxidizing conditions were performed in air. While, the corrosion tests under reducing conditions were performed in the airtight stainless steel containers purged with mixed gas (Ar+5%H2), where Eh of the solution was maintained at -0.45 V vs.SHE. After the corrosion tests, the solution was cooled to room temperature, and the solution pH and Eh were measured immediately. The solution was filtered through a 0.45µm filter and a membrane filter of NMWL 10,000 (1.8 nm in pore size) in order to investigate the distribution of the Pu and Cm particle size fractions. The solution concentrations of Pu, Cm and other glass constituent elements were measured by α-spectrometry and ICP-AES.
Fig.1 shows the solution pH and Eh as a function of corrosion time, which shows that each redox condition was maintained sufficiently during the corrosion tests. Fig.2 and Fig.3 show the solution concentrations of Pu and Cm as a function of corrosion time under oxidizing and reducing conditions, respectively. It was observed that redox conditions have no remarkable influence on the leaching behavior of Pu and Cm, which suggests that dominant oxidation states of the Pu and Cm in the solution under reducing conditions are the same as those under oxidizing conditions.
Under both oxidizing and reducing conditions, it was observed that the Pu and Cm concentrations in the 1.8nm filtrate were one or two orders of magnitude lower than those in the 0.45µm filtrate. While, there was no difference in the Pu and Cm concentrations between non-filtered solution and the 0.45µm filtrate. The Pu and Cm concentrations in the 1.8nm filtrate were assumed to correspond to the soluble species controlled by the solubility, and the difference in Pu and Cm concentrations between the 1.8nm and 0.45µm filtrates was assumed to correspond to the insoluble suspended fractions(colloidal particles). It was suggested that
the colloidal particles with the size from 1.8nm to 0.45µm are dominant in the solution under both oxidizing and reducing conditions. The experimental results also showed that the amount of the colloidal particles of Pu and Cm was relatively large even in the early stage of glass corrosion where the concentrations of the soluble species of Pu and Cm were very low, which suggests that the colloidal particles are produced at the glass surface and released into the solution as a direct result of glass matrix dissolution under both oxidizing and reducing conditions.
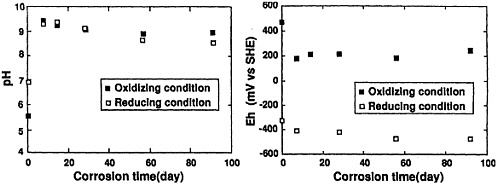
Fig.1. Solution pH and Eh as a function of corrosion time.
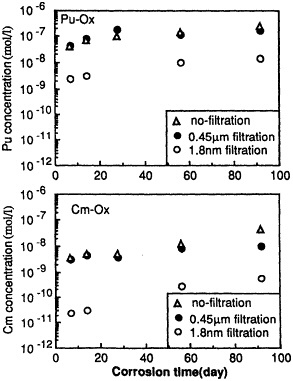
Fig.2. Solution concentrations of Pu and Cm under oxidizing conditions.
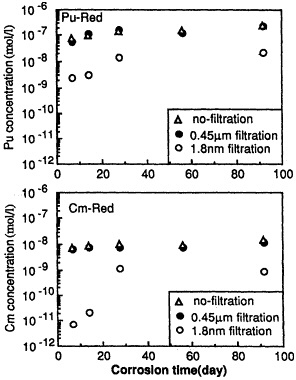
Fig.3. Solution concentrations of Pu and Cm under reducing conditions.
Glass and Glass-Ceramic Waste Forms Developed at the Idaho Chemical Processing Plant for Immobilizing HLW and Actinides
Dieter A. Knecht, Tom P. O'Holleran, Krishna Vinjamuri, Swami V. Raman, and Bruce A Staples
Lockheed Martin Idaho Technologies
P. O. Box 1625, MS 5218
Idaho Falls, ID 83415-5218
Phone - 208-526-3627, Fax - 3499, E-mail - dieter@inel.gov
The Idaho Chemical Processing Plant (ICPP), which is a part of the Idaho National Engineering Laboratory (INEL), has stored and reprocessed irradiated nuclear fuel since 1953 to recover uranium-235 and krypton-85 for the U.S. Department of Energy (DOE). The resulting acidic high-level liquid radioactive waste (HLLW) was stored in stainless-steel 1100 m3 single-shell tanks in underground concrete vaults, rather than neutralized and stored in carbon steel tanks as was the common practice at that time. A fluidized-bed solidification process was developed during the 1950s based on research at Argonne National Laboratories to form a granular calcine solid at 500 °C from the acidic HLLW with a seven-fold volume reduction. The process was successfully demonstrated in the Waste Calcining Facility (WCF), which was constructed and operated, converting 15,000 m3 of HLLW to 2,160 m3 calcine from 1963 to 1980. It was succeeded by the New Waste Calcining Facility (NWCF) in 1982, which has calcined over 13,600 m3 of HLLW to 1670 m3 of calcine. Currently there is an inventory of 3,800 m3 HLW calcine at ICPP. The calcined waste is stored in near-surface, stainless-steel bins within concrete vaults. The bin sizes are approximately 4-m diameter by 12.5 to 18.5-m high. Some of the bins are cylindrical and others are of an annular configuration.
As compared with the other DOE sites which store HLW from reprocessing, the HLW at Idaho is significantly different, mainly because the HLLW was never neutralized and that it and the resulting HLW calcine contain mainly the components of the fuel cladding and chemicals required during the processing for corrosion and criticality control. The major component (wt%) in ICPP HLW alumina calcine includes alumina (82-95), and the major components in zirconia calcines, and zirconia calcines blended with other sodium-bearing process and decontamination wastes, include fluorite (41-44), zirconia (17-19), calcia (12-13), alumina (9-14), alkali oxides (5-7), borate (2-4), and cadmium oxide (0-7). Fission product content is typically less than 1 wt%. The amount of alumina, zirconia, zirconia-Na (including fluorinel-Na) calcines is approximately 560, 1250 and 1750 m3, respectively. The remaining 240 m3 calcine inventory consists of calcines from processing other minor fuels and start-up bed material.
Several technologies have been identified to date that could immobilize calcine; these include vitrification and glass-ceramic processing. Preliminary scoping tests were run in the 1960s, and laboratory testing was started in the 1970s to develop glass formulations for ICPP calcines. A phosphate glass was prepared in the mid-1960s with the following components in wt%: 25 calcine, 40.5 P2O5, 18.6 Na2O, and 15.9 PbO using fully radioactive alumina calcine.
Leaching rates were measured using continuously circulating distilled water at 25 and 94 °C and were not changed with a devitrified sample. Other early waste forms which were studied in the late 1960s included calcine particle coating with molten aluminum and steel spray and matrix encapsulation in metal, glass, plastic or grout. In the 1970s, glass formulations were tested further. For alumina calcine, waste loadings of up to 29 wt% and 24 wt% could be obtained in a borophosphate and borosilicate glass, respectively. For zirconia calcines, waste loadings of 33 wt% were observed using a borosilicate frit. Nonradioactive laboratory- and pilot-scale and radioactive laboratory-scale tests were run using the borosilicate frit 127 (composition in wt%: SiO 2-70.3, Na2O-12.8, B2O3-8.5, Li2O-6.2, and CuO-2.1). MCC-1 and MCC-2 leach tests indicated that there did not appear to be significant differences in the responses of glasses formed using simulated zirconia calcine at laboratory or pilot scale and using radioactive zirconia calcine at laboratory scale.
In the 1980s, glass ceramic formulations were tested using a hot isostatic press (HIP) for high temperature sintering to produce a 70 wt% waste loading form with a 2.6-fold lower volume than the equivalent glass waste forms. Durable glass-ceramic forms could be made for zirconia calcine with additives including silica, alkali, and yttria. Detailed microstructure analysis of these waste forms revealed the presence of fluorite, monoclinic zirconia, stabilized cubic zirconia, zircon, zirconolite, perovskite and amorphous aluminosilicate phases. Normalized MCC-1 release rates at 90 °C of all major elements were less than 1 g/m2 day. More recently developed glass-ceramic formulations for alumina, zirconia and zirconium-sodium blended calcines used borosilicate flit and other additives such as titania and metallic Ti and Al powders. Major crystalline phases included fluorite, zirconia, and zircon. Sphene and other titanates were formed with added titanium, and calcium-aluminum silicates were formed with the added aluminum. The higher amounts of metallic powder increased the amount of zirconia at the expense of zircon. MCC-1 leach rates decreased with decreased borate concentration, and the lowest normalized elemental leach rates of <<1 g/m2d were found in the formulations containing 4-6 wt% Ti and 2-3 wt% Al. The amounts in wt% of crystalline phases were identified by X-ray diffraction as fluorite-37, zirconia-17, zircon-14, and sphene-16. The major components in wt% of the glass phase were identified by SEM as silica-62.1, alumina- 18.8, and calcia- 19.1. Additional studies with similar calcine and frit composition were run to determine the effect of process soak time and added metal Si and Al powders on the microstructure and durability The lowest leach rates were found for the Al concentration at 2 wt% and were found to increase with increasing Si concentration. Constant leach rates were found for soak times of 4, 8, 16 and 24 hr. Major crystalline phases were identified as fluorite, zirconia, and zircon. Minor phases of albite and anorthite were also identified.
This paper will review the compositions and properties of the waste forms developed at the INEL which are applicable to the immobilization of HLW and actinides in a durable glass or glass-ceramic. The results of new tests will be presented, including amounts of zirconolite and other minerals with high actinide retention capability formed at different conditions, partitioning of lanthanides between crystals and glass phase, and the leaching characteristics of the resulting waste forms.
REPRODUCTION OF NATURAL CORROSION BY ACCELERATED LABORATORY TESTING METHODS
J. S. Luo,* J. J. Mazer, D. J. Wronkiewicz, and J. K. Bates
Chemical Technology Division, Argonne National Laboratory, Argonne IL 60439-4837, Luo@cmt.anl.gov IL 60439-4837, Luo@cmt.anl.gov
Various laboratory corrosion tests have been developed to study the behavior of glass waste forms under conditions similar to those expected in an engineered repository. The data generated by laboratory experiments are useful for understanding corrosion mechanisms and for developing chemical models to predict the long-term behavior of glass. However, it is challenging to demonstrate that these test methods produce results that can be directly related to projecting the behavior of glass waste forms over time periods of thousands of years. One method to build confidence in the applicability of the test methods is to study the natural processes that have been taking place over very long periods in environments similar to those of the repository [1].
In this paper, we discuss whether accelerated testing methods alter the fundamental mechanisms of glass corrosion by comparing the alteration patterns that occur in naturally altered glasses with those that occur in accelerated laboratory environments. This comparison is done by (1) describing the alteration of glasses reacted in nature over long periods of time and in accelerated laboratory environments and (2) establishing the reaction kinetics of naturally altered glass and laboratory reacted glass waste forms.
Vapor Hydration Tests on Tektite and Obsidian Glasses
We have performed vapor hydration tests on tektite and obsidian glasses between 75 and 230°C for up to 400 days. The mechanisms of glass corrosion are generally complex and strongly depend on reaction conditions [2]. In laboratory vapor hydration tests, however, reaction of water vapor with tektite and obsidian glasses is found to produce birefringent alteration layers, for which the growth kinetics can be simply expressed as a function of the square root of time for a given glass at a fixed temperature. These findings seem in agreement with findings from previous studies, in particular, those for naturally altered obsidians, for which a dating method has been developed based on measurements of the birefringent thickness in natural samples [3]. We also investigated various parameters that affect alteration of these glasses, and we find a strong correlation between the total water content of obsidian and the hydration rate and the activation energy of the reaction. Our studies indicate that the natural hydration of glasses can be quantitatively reproduced under accelerated laboratory conditions, when the reaction is dominated by a relatively simple process such as the molecular water diffusion.
Nine-Year Results from Testing Basaltic and Simulated Waste Glasses
We have performed vapor hydration tests on synthetic basaltic and simulated nuclear waste glasses at temperatures ranging from 70 to 250°C for periods of up to 9 years. Basaltic and waste glasses were found to react by similar processes. At temperatures higher than 150°C, glasses were altered progressively to various secondary phases within months, following this paragenetic sequence: unaltered glass —> smectite —> Na-chabazite —> analcime + phillipsite —> K-feldspar—> illite —> albite + tobermorite. This trend is found to be similar to both the horizontal mineral zonation formed during the surficial-temperature alteration of volcanic glass in saline-alkali lakes and the vertical stratification resulting from the percolation of water through hot volcanic materials [4].
Tests were also performed at temperatures below 100°C with synthetic basalt and simulated waste glasses. After 9 years of exposure to a saturated vapor environment, both glasses formed an amorphous hydrated gel plus small amounts of clay. This is similar to the initial reaction of these glasses at higher temperatures in short time periods. For comparison, we also conducted detailed microscopy analyses of natural basaltic glasses that had been subaerially weathered in Hawaii for the past 500 to 700 years at ambient temperature. We discovered that alteration layers formed in Hawaiian basalts (palagonite) were nearly identical to those formed in laboratory-reacted glasses in both microstructure and chemical composition.
Although the above studies seem to indicate the corrosion reaction in nature can be reproduced reasonably well under accelerated laboratory conditions without changing the underlying reaction mechanisms, it is still difficult to use data from natural samples to verify a kinetic expression developed based on laboratory experiments. This difficulty arises because the alteration layers formed on the natural glass surfaces were frequently found to have spalled from the base glass, and, as a consequence, the reaction rate, which is calculated from measurements of layer thickness could not be adequately established. There is also evidence that the corrosion of natural glasses may have been greatly affected by the local microenvironment (e.g., temperature, humidity, pH, etc.) of each individual glass. This result implies that the quantitative correlation between natural analogue and short-term laboratory results could be further hindered by a limited knowledge of the reaction conditions of natural samples.
REFERENCES
1. Ewing, R.C. (1979) ''Natural analogues: analogues for radioactive waste forms," Scientific Basis for Nuclear Waste Management, edited by G.J. McCarthy, Plenum Press, New York, p. 57.
2. McGrail, B.P., L.R. Pederson, D.M. Strachan, R.C. Ewing, and L.S. Cordell (1988) "Obsidian hydration dating - field, laboratory and modeling results," Mater. Res. Soc. Symp. Proc. Vol. 125, p. 393.
3. Friedman, I., and F.W. Trembour (1978) "Obsidian: The dating stone," American Scientist, Vol. 66, p. 44.
4. Iijima, A. (1978) "Geological occurrences of zeolite in marine environments," Natural Zeolites, edited by L.B. Sand and F.A. Mumpton, Pergamon Press, New York, p. 175.
COLLOID FORMATION DURING WASTE GLASS CORROSION
C. J. Mertz, E. C. Buck, J. A. Fortner, and J. K. Bates
Chemical Technology Division, Argonne National Laboratory, Argonne, IL 60439-4837, mertz@cmt.anl.gov
The long-term behavior of nuclear waste glass in a geologic repository may require a technical consideration of the role of colloids in the release and transport of radionuclides. The neglect of colloidal properties in assessing the near- and far-field migration behavior of actinides may lead to significant underestimates and poor predictions of biosphere exposure from high-level waste (HLW) disposal. Existing data on colloid-facilitated transport suggests that radionuclide migration may be enhanced, but the importance of colloids is not adequately assessed [1]. Indeed, the occurrence of radionuclide transport, attributed to colloidal species, has been reported at Mortandad Canyon, Los Alamos and at the Nevada Test Site [2]; both unsaturated regions are similar to the proposed HLW repository at Yucca Mountain. Although some developments have been made on understanding the transport characteristics of colloids [3], the characterization of colloids-generated from the corrosion of the waste form has been limited [4]. Colloids are known to incorporate radionuclides either from hydrolysis of dissolved species (real colloids) or from adsorption of dissolved species onto existing groundwater colloids (pseudocolloids) [5]; however, these colloids may be considered secondary and solubility limited when compared to the colloids generated during glass alteration [6].
Glass alteration results from complex interactions between waste form and groundwater or between waste form and humid air and is governed by the near-field chemistry (glass composition, groundwater composition, engineered barrier systems, time of barrier breachment, etc.). As the glass is altered under repository conditions, new phases are formed which are waste form dependent. Under certain conditions, these altered glass phases are a source of colloidal material, whereby colloidal-sized particles may detach from the reacted surface and become solution-borne colloids [7-9]. The actinides can thereby be present in solution at higher concentrations as an insoluble glass alteration phase than as real or pseudocolloids.
The formation of actinide-bearing colloids is best understood from the investigation and characterization of colloids in the near-field (waste form colloids) [6,7]. Long-term drip tests designed to simulate repository conditions have resulted in the formation of clay colloids and brockite, a thorium orthophosphate, in the leachant [7]. Brockite is known to incorporate rare earths, uranium, americium, and possibly plutonium into its crystal structure. Initial release rates of actinides from the glass were low, but increased rates were observed when the reaction progress had incorporated spalling of the reaction layer [7].
The reactivity of high Pu loaded glasses has exhibited further evidence of waste form reaction phases spalling actinide-bearing colloids [8]. Glasses doped with various concentrations of Pu were reacted under accelerated conditions [8]. Plutonium was found associated with colloidal material suspended in solution as a plutonium oxide particle attached to an Fe-rich clay. Some clay was also found to have incorporated substantial amounts of Pu. While the incorporation of actinides in alteration phases may retard the initial release of actinides; spalling of the reacted layer has been shown to release these materials as colloids [6-9].
The structure and composition of colloidal particles are commonly determined with analytical transmission electron microscopy and ultrafiltration for glass reaction tests [6-9]. These techniques have been combined with small particle handling techniques and autoradiography to determine the sites for actinide adsorption on waste glass derived colloids
[6]. Americium and plutonium were identified in the brockite inclusions rather than the clay colloid matrix [6]. Techniques used to examine colloids often have a deleterious effect on the colloidal system and it is a challenge to find a technique which can extract representative information on the nature of colloidal particles. Presently, dynamic light scattering and electrophoretic mobility measurements are nonintrusive methods that can be used to characterize physical characteristics of actinide-bearing colloids [10, 11].
REFERENCES
1. J. F. McCarthy and J. M. Zachara, Environ. Sci and Technol. 23 , 497-502 (1989).
2. I. Triay et al., Colloid-Facilitated Radionuclide Transport at Yucca Mountain, Los Alamos National Laboratory Report. LA-12779-MS (1995).
3. H. E. Nuttall and R. L. Long, Radio. Waste Mgmt. Nucl. Fuel Cycle, 17, pp. 237-251 (1993).
4. J.-C. Petit, Radiochim. Acta 51, 181-188 (1990).
5. J. I. Kim, The Scientific Basis for Nuclear Waste Management XIX, MRS Symposium Proc. 294, 3-21 (1993).
6. J. K. Bates et al., Science 256, 649-651 (1992).
7. J. A. Fortner and J. K. Bates, The Scientific Basis for Nuclear Waste Management XIX, MRS Symposium Proc. 412, pp. 205-211 (1996).
8. J. K. Bates et al., High Level Radioactive Waste Management 1995, Proc. of the 6th Ann. Intern. Conf., pp. 588-593 (1995).
9. E. C. Buck et al., The Scientific Basis for Nuclear Waste Management XIX, MRS Symposium Proc. 294, pp. 199-206 (1993).
10. I. R. Triay et al., Radiochim. Acta 52/53, 127-131 (1991).
11. J. D. F. Ramsay et al., Radiochim. Acta 44/45, 119-124 (1988).
ABSTRACT
National Academy of Sciences
Glass as a Waste Form and Vitrification Technology
May 13-15, 1996
Use Of DC Graphite Arc Melter Technology For Production Of Stable Vitrified Waste Forms
T. J. Overcamp, D.L. Erich/ Clemson University
J.K. Wittle, R.A. Hamilton/ Electro-Pyrolysis, Inc.
P. J. Wilver/ Svedala Industries, Inc.
The science of glass production, as a method for the stabilization of heavy metals and radionuclides, has a long history. The technical literature is extensive and demonstrates the viability for projecting the long term stability of the glasses over the periods of time necessary to minimize release of radioactivity into the environment. These glasses in the majority of the studies have been based on conventional glass chemistry in which the radioactive waste is added to a glass of known composition and the waste dissolved into the glass matrix.
The use of the higher temperature capability of the DC Arc Melter offers an alternative approach in which the waste is melted in the melter and the composition of the melt modified with only enough additives to form a glass/ceramic material.
A batch type, single graphite electrode melter system is currently being used at the Clemson University Vitrification Laboratory to demonstrate the flexibility of this approach.
A series of extensive tests at MIT and now at Clemson University under private and DOE sponsorship demonstrated that the DC Melter approach is capable of handling a number of wastes not conducive to treatment using conventional glass/joule melting technology. These tests produced vitrified products which passed the conventional leachate tests.
An added benefit of this technology is not only the production of stable vitrified product but also the recovery of metals from the waste. Initial studies indicate that the metals recovery does not preclude the incorporation of the radionuclides into the vitrified product.
Glass melter technology normally utilizes the conventional joule melter technology in which electrodes are permanently incorporated into the melter system and located under the glass surface. This limits the type of material which can be processed in the melter. Metals may adversely react with the electrodes and/or collect on the bottom of the melter shorting out the system.
In the D C Graphite Arc Melter system, the electrical path is made from a graphite electrode, whose height can be adjusted, through the ionized plasma (the arc) to the molten conductive slag and metal, and to the hearth to complete the circuit. Energy is transferred to the bath in two ways: by radiation and resistance heating. Resistance heating (joule heating) results from the completion of the electrical circuit through the bath. The arc transfers to the conducting molten slag and metal of the bath taking the
shortest electrical path to the carbon hearth. The amount of resistance heating varies with the conductivity of the melt.
The radiant energy transfer comes from the arc itself. When the arc is submerged in the slag, the energy goes into the slag bath. This has the additional benefit of keeping the refractory in the melter walls from being exposed to the arc, adding to refractory life. The electrode can be raised or lowered to change the length of the arc. The longer the arc, the more of the arc energy is diverted to the walls of the melter by radiation. The DC Melter can be operated more efficiently with a short arc which concentrates the radiant arc energy in the molten bath as well as joule heating the bath, thus reducing the amount and cost of electrical energy.
The types of waste simulants run to date in the melter are summarized as follows:
|
Soil |
INEL Soil-unsubmerged arc |
|
Soil |
INEL Soil-submerged arc |
|
Soil and Metal |
INEL Soil and 25% metal Mix |
|
Soil and Metals |
INEL Soil and 50% metal Mix |
|
Soil and Combustibles |
INEL Soil and wood, paper, plastic, cloth and concrete |
|
Soil and Combustibles |
Hospital Ash |
|
Soil and RFP Sludge |
INEL Soil and RFP 745 sludge, Sodium nitrate and Potassium nitrate sludge with Portland Cement |
|
Soil and RFP Sludge |
INEL Soil and RFP 741/742 sludge, Metal hydroxide sludges. |
|
Soil and RFP Sludge |
INEL Soil and RFP 743 sludge, Regal Oil and Microcell-E |
|
Soil and Volatile Metals |
INEL Soil and (CsNO3 and Ce(NO3)3)-submerged |
|
Soil and Volatile Metals |
INEL Soil and (CsNO3 and Ce(NO3)3)-unsubmerged |
|
Soil and additives |
South Carolina high Silica soil with fluxing agent Ca(OH)2 |
|
Soil and additives |
South Carolina high Silica soil with fluxing agent Li2(CO3) |
|
Soil and additives |
South Carolina high Silica soil with fluxing agent Ca(OH)2 |
|
Soil and additives |
South Carolina high Silica soil with fluxing agent Na(HCO3) |
|
Demolition debris |
Cement Blocks |
|
Demolition debris |
Refractory Brick |
|
Demolition debris |
Sand |
|
Mixed Waste Simulant |
Wood, PVC, and metal The results of these tests indicated: |
-
The DC Arc Melter system was capable of treating a heterogeneous mixture of materials.
-
The system can handle material containing a high metals content.
-
The metal in the final product was easily separated from the slag.
-
The iron in the soil was reduced by a carbothermic reaction in the melt.
J. Kenneth Wittle, Ph.D. (610) 687-9070, e-mail kwittle@aol.com.
Hanford Low Level Waste Melter Tests
Dr. Ian Pegg, Dr. Pedro Macedo, Dr. Keith Matlack, Dr. Harold Hojaji, Dr. Shi-Ben Xing Catholic University of America
Jacqueline Ruller, William Greenman
GTS Duratek
Approximately 230,000 m3 of defense nuclear wastes is stored in underground tanks at the US Department of Energy site in Hanford, Washington. Retrieval and pretreatment will lead to a low level waste stream that contains sodium nitrate and nitrite salts in a highly alkaline liquid slurry. Westinghouse Hanford has been evaluating alternative vitrification technologies for treating this low level waste stream. GTS Duratek and the Vitreous State Laboratory of the Catholic University of America demonstrated low temperature vitrification (1150°C) on the DuraMelter™ 100 and 1000 joule-heated vitrification systems. The Hanford LLW simulant was successfully vitrified at sustained feed rates that were twice the nominal capacity of the melters. Approximately 610 kg and 10,700 kg of glass was produced in the DuraMelter™ 100 and 1000 tests, respectfully. All glasses produced far exceeded stated leach resistance requirements. The off-gas system performed effectively with reduced nitrogen oxide emissions, and final particulate and metal emissions from the process were all below measurable and regulatory limits.
Experimental Determination of Uranium Oxide Solubility in Hydrous Silicate Melts of Granitic Composition
Peiffert, Chantal and Cuney, Michel
CREGU and GdR
CNRS-CREGU, 77, B.P. 23, 54 501
Vandœuvre-Lès-Nancy Cedex, France
e-mail : cuney@cregu.cnrs-nancy.fr
The solubility of uranium oxide (UO2+x) in silicate melts of granitic composition was determined for variable melt composition under three oxygen fugacity conditions corresponding to Ni-NiO, Fe3O4-Fe2O3 and Cu2O-CuO oxygen buffers at 780°C and 2 kbar (respectively 10-15, 10-10 and 10-4.5 bar) and water saturation of the system. Samples were prepared using a quadruple capsule technique. 50 mg of stoichiometric UO2.000 and 200 mg of gel, with simplified granite composition (SiO 2, Al2O3, Na2O, K2O), were loaded into separate platinum capsules. These two platinum capsules were only crimped. 100 mg of one of the three assemblages (Ni-NiO, Fe3O4-Fe2O3 or Cu2O-CuO) and 100 mg of H2O were loaded in the third platinum capsule. This capsule was welded shut. The three platinum capsules containing UO2, gel, oxygen buffer and 200 mg of aqueous solution with variable concentrations of carbonate, fluoride, chloride and phosphate were loaded into a gold capsule. The gold capsule assembly was welded shut and loaded in a cold-seal pressure vessel. The end containing the UO2 capsule was placed in the bottom i.e. the warmest part of the vessel. The capsule with the gel was systematically placed immediately after the UO2 capsule. This technique has two major advantages : (1) UO2, loaded in excess, represents an inexhaustible source of uranium and thus saturates the system with this element; and (2) the separation of UO2 and gel before the experiments prevents UO2 particles to enter the silicate melt and aqueous solution and thus, preserves pure solid and aqueous phases after run. The experiments were carried out using rapid quench cold-seal pressure vessels.
After run, the silicate glasses were analyzed using different techniques. Si, Al, Na, K, F, Cl, P, and U were determined by electron microprobe (Cameca SX 50). Low uranium concentrations were calibrated against NBS standard (461.5 ppm). For glass samples containing uranium less than 1000 ppm, global analysis using ICP-MS technique and fission track method were used. The amount of water in glasses were measured by Karl Fisher method.
The parameters governing uranium solubility in silicate melts were (i) melt agpaicity (Na+K/Al ratio), (ii) concentration of anions such as fluoride and phosphate in the silicate melt, (iii) oxygen fugacity.
-
For the experiments in which the modifiers of silicate network such as carbonate and chloride which are weekly dissolved in the silicate melt (in the range of 0.01 to 0.1 wt.%) the melt agpaicity appear to be by far the main parameter controlling uranium solubility. For an agpaitic index varying from 0.7 to 1.5, uranium solubility continuously increases of nearly 4 orders of magnitude (from 10 to 3×10+4 ppm U). Thus, increasing depolymerisation of the silicate
-
melt strongly favor uranium dissolution in the melt. However for an agpaitic index higher than 1.5, uranium solubility reaches a saturation plateau.
-
Increasing concentrations of fluorine and phosphorus (0 to 4 wt.%) in the silicate melts also have a strong effect on uranium solubility. These anions act like excess alkalis for the depolymerisation of the silicate melts. Below 1 wt.% fluorine uranium solubility in the silicate melt increases proportionally with fluorine content. In oxidizing condition (Cu2O-CuO buffer) the atomic ratio F/U was close to 4. This relation results suggests the occurrence of UO2F4 22- complex in the silicate melt. For higher fluorine contents a solubility plateau (2×10+4 ppm U) similar to that obtained for high melt agpaicity is reached. The same behavior is observed for phosphorus-bearing silicate melts.
-
An increase of fO2 from 10-15 to 10-4.5 bar increases up 4 times the solubility of uranium. The silicate glasses obtained in oxidizing condition (fO2 = 10-4.5 bar) were translucent yellow colored which is characteristic of the presence of uranyl ion in the glasses. The glasses obtained in more reducing conditions (fO2 = 10-4.5 -10 -10 bar) were colorless and thus characteristic of U (+V) and U(+IV) in silicate glasses. The presence of the different oxidation degrees of uranium was shown by UV-visible-near infrared absorption spectroscopy with the different bands: U (+IV) (1890 nm) and U (+V) (1415 nm) and UO22+ (410 nm). The effect of fO2 on uranium solubility decreases with increasing agpaicity and increasing fluoride and phosphate concentrations of the melt.
The stoichiometry of the uranium oxide in equilibrium with the silicate melt also gives a direct information about the variation of the oxidation state of uranium under variable fO2 conditions. Results obtained from quantitative analysis of uranium solid phases using x-ray diffraction patterns indicated that only well crystallized and cubic uranium oxides were in equilibrium with fluid and melt. Three values of cell parameters ao (Å) were determined: (5.470±10.02), (5.459±0.02), and (5.442±0.02) Å corresponding respectively to the three fO2 fixed by Ni-NiO, Fe3O4-Fe2O3, and Cu2O-CuO buffers. From the values of ao (Å) three compositions can be calculated : UO(2.01±0.01); UO(2.10±0.02) ; and UO(2.254±0.02).
Mixture Models Versus Free Energy of Hydration Models for Waste Glass Durability
Greg Piepel and Trish Redgate
Pacific Northwest National Laboratory(a)
P.O. Box 999, Mail Stop K5-12
Richland, Washington 99352
voice: 509-375-6911, email: gf_piepel@pnl.gov
Abstract
Two approaches for modeling high-level waste glass durability as a function of glass composition are compared for several simulated waste glass composition/durability data sets. The mixture approach uses least squares regression to fit to composition/durability data any one of a large number of approximating functions discussed in the mixture experiment literature. However, only first- or second-order mixture polynomials in composition are considered in this work. The free energy of hydration (FEH) approach assumes durability is linearly related to the FEH of glass, with the line fitted to data by least squares regression. The FEH of a glass is calculated as a composition-weighted linear combination of free energies of hydration of the glass components. The FEH approach is shown to be a restricted version of the first-order mixture (FOM) approach.
The mixture and FEH approaches are compared in terms of their ability to model Product Consistency Test (PCT) normalized boron releases as a function of glass composition for several simulated waste glass data sets, including ones from Savannah River, West Valley, and Hanford. Least squares regression was used to fit FEH and FOM models to each data set. Goodness-of-fit statistics show that the FOM model fits/predicts PCT boron release in each data set better (sometimes much better) than the FEH model. The model R2 statistics (proportion of variation in PCT boron releases accounted for by a model) summarized in Table 1 illustrate this.
Considerable differences also exist between some FEH and FOM model component coefficients for each of the data sets. Comparing FOM coefficients across the data sets shows that the effect of a glass component on PCT normalized boron release can depend on the level and range of the component and on the levels of other glass components. The FEH approach has a limited ability to represent such behavior for different glass composition regions, due to its reliance on assumed constant effects of each component. The mixture approach, on the
other hand, determines the effects of glass components on durability from a given data set. It can also account for higher-order (e.g., curvilinear or interactive) effects of components. Second-order mixture (SOM) models were developed for three of the data sets, and are shown to improve on the corresponding FOM models (see R2 statistics in Table 1).
It is concluded that the mixture approach is more flexible and performs better than the FEH approach for approximating the relationship between glass composition and durability for various glass composition regions.
Table 1. R2 Statistics(a) for FEH, FOM, and SOM Models Fitted to Several Simulated Waste Glass Composition/Durability Data Sets Data Set(b)
MICROSTRUCTURES AND LEACH RATES OF GLASS-CERAMIC WASTE FORMS FOR IMMOBILIZING PLUTONIUM AND ASSOCIATED COMPONENTS
Swami V. Raman
Idaho National Engineering Laboratory
Lockheed Martin Idaho Technologies
Idaho Falls, Id.
208-526-3606
Introduction
There are six different types of wastes at Idaho Chemical Processing Plant (ICPP). Five of those are in the calcined form. The sixth waste is currently a liquid solvent and may be calcined in the near future. This waste will be sodium enriched once water and nitrates are degassed during calcination. All of these wastes are a by-product of the recovery of uranium-235 and krypton-85 from irradiated defense nuclear fuels by acidic dissolution. They contain transuranics, radioactive isotopes of strontium and cesium, transition elements, chlorine and sulphur in minor to trace amounts. They abundantly vary in alumina, zirconia, fluorite, boria, soda and calcia contents. The main task in their immobilization is one of durably accommodating the major components in a variety of glass and crystalline compositions that are also acceptors of the radionuclides and transition elements.
The experimental methods of hot isostatic pressing (HIP) and ambient glass melting were attempted to vitrify the waste with glass forming and modifying additives. In the course of these experiments the glass was partly deprived of its vitreous characteristics. Since, devitrification is a spontaneous phenomenon resulting from the presence of nucleating agents and undercooling, much attention was given for forming desirable mineral analogues in the glass matrix. The compositional stability regimes for homogeneous glass, glass-ceramic and ceramic are a function of waste loading at a specified temperature. The microstructural characteristics and leach rates of these regimes are discussed in this paper.
Vitrification Under Pressure
Since, the calcines contain volatile species like chlorine, sulphur, fluorine, sodium and cesium, the waste+additive batch was melted at 20,000 PSI and 1000°C, and cooled in the hot isostatic press. The relative proportions of glass, crystalline matter and calcine relics form as a function of waste loading at the expense of silica, magnesia and metal reductant additives. At 80 weight % calcine loading, the microstructure is fine grained and is similar to natural rhyolite, although compositionally the two are different. As the loading is reduced to 60 weight% the glass content increases with contemporaneous crystal growth. The overall microstructure is similar to basalt, albeit compositional differences. The predominant natural mineral analogues of this waste form are fluorite (CaF2), baddeyelite (ZrO 2), zircon (ZrSiO4),
apatite (Ca5(PO4)3F), and greenockite (CdS). The crystal growth is particularly noticeable with increase in the MgO additive. In addition, anomalously high MgO contents lead to the formation of biotite mica and dendritic nepheline. The most leachable elements are boron, cesium and sodium. Their MCC-1 leach rates are below 1g/m2-day in microstructures that are devoid of dendrites and micaceous layers. The most leach resistant species are zirconium, phosphorous and cerium. They are more enriched in the crystalline apatite and zircon than glass. These element sites are hosts of transuranics and lanthanides in natural minerals. Analogues of these minerals are considered in the present work as potential hosts for plutonium. Given the appropriate chemical composition, their formation appears to increase with decrease in melt viscosity.
Vitrification In Ambient Atmosphere
In the HIP experiments a very low solubility in glass was noted for the important transuranic bearing phase zircon. A separate vitrification experiment was conducted to determine the solubility of zirconium in glass and also delineate the important ceramic formation regimes. A boroaluminosilicate glass with varying soda, magnesia and zirconia contents was melted at 1200°C and quenched by pouring into a mold. The results suggest that zirconium solubility in glass occurs with substitution for sodium and magnesium. Equilibrated zircon and forsterite phases compose the glass-ceramic regime. Homogeneous glass forms in the neighborhood of soda. The glass-ceramic transforms to liquid phase ceramic with further decreases in soda. Boron and sodium are the most leachable species. However, their leach rates fluctuate with magnesia/zirconia substitutions at a constant soda content. Further, the results also show decreases in the leach rates with increases in soda content. These leach rate variations are corroborated by the visible Raman spectra. The variations in composition and leachability are manifested in these spectra in the form of Raman frequency shifts and background fluorescence. The frequency shifts are observed in the bridging and non-bridging structural domains in response to changes in composition and leaching. In glasses with pronounced leaching of boron and sodium, the Raman spectrum is marked by intense fluorescence in the background. Possibly, the fluorescent effects arise from the development of altered layers on the leached surfaces, following glass corrosion reactions. The leaching characteristics of boron were additionally examined in a related liquid phase sintering experiment. The results suggest intense boron leaching due to the formation of metastable borate phases. The present Vitrification experiment is also significant considering the current focus at ICPP to separate the wastes into low activity and high activity enrichments. Invariably, the transuranics inclusive of plutonium would be a part of the high activity wastes for which zircon is the potential host in a neutron absorbing borosilicate matrix.
HOT ISOSTATIC PRESS (HIP) VITRIFICATION OF RADWASTE CONCRETES
Darryl Siemer: 12N 3167 E, Idaho Falls, ID, (208) 526-1373, FAX (208) 526-3499
Barry Scheetz:
MRL, Pennsylvania State University, University Park, PA, (814) 865-3529, FAX (814) 865-2326
Cliff Orcutt: A.I.P. (American Isostatic Press, Inc.), Columbus, OH, (614)228-5709, FAX (614) 445-9233
For the past two decades US decision-makers have tacitly assumed that irrespective of how radioactive it might be, any defense-type reprocessing waste that ''contains'' first-cycle raffinates must be vitrified prior to disposal. The cost of safely implementing this paradigm on the required scale has contributed to the persistent lack of tangible progress at most DOE sites. On the other hand, because it chose to apply cementitious solidification technologies instead, Great Britain is now rapidly converting its accumulation of equally-radioactive historic radwastes to competent waste forms. This paper discusses how cementitious waste forms made from US defense-type radwaste (e.g., ICPP calcine) now could still be "vitrified" at some future time. The first stage of the process, cementitious solidification, would involve (1) formulating grouts so that the ratios of network-forming elements (silicon, aluminum, boron) to glass-modifying elements (alkali and alkaline earths) are similar to those of natural aluminosilicate minerals, and (2) the use of stainless steel canisters (forms) designed so that they could subsequently serve as HIP cans. Cementitious additives include granulated blast furnace slag cement, additional silica, vermiculite (to improve 137Cs retention), and an activator consisting of sodium (or potassium) hydroxide dissolved in the mix water. After proper curing, these grouts become hydroceramic materials (concretes) strong enough to meet IAEA & US transport requirements (compressive strengths are typically 2000 - 6000 psi) and chemically durable enough to satisfy the usual radwaste leach test criteria (TCLP, ANSI-16.1, and MCC-1) for toxic & radioactive elements. Hydroceramic materials would be closer to thermodynamic equilibrium in likely repository settings than glasses. If deemed necessary, the second stage, "vitrification", could be accomplished as follows: vent the canisters & bake out most of the water by heating them to ˜800-C, reseal them, & then HIP for 1-3 hours (typically @ 1000_ C & 5 kpsi). The resulting glass-ceramic materials consist of an assemblage of discrete mineral phases (with ICPP radwastes, primarily fluorite and zirconia) embedded in a durable aluminosilicate glass. Because cementitious processing effects molecular-scale mixing of those elements that form the subsequent glass phase, properly formulated concretes can be HIP-densified at relatively moderate temperatures and pressures. HIPed-concrete glass-ceramics are typically about twice as dense as are the original hydroceramic materials, 10-30 times stronger, and they usually perform somewhat better in the usual leach tests (most notably for innocuous components such as sodium, potassium, boron, & silicon). Waste loadings are generally in the range of 30-45 %.
The advantages of "staging" the solidification of defense-type radwaste include:
-
staging could end today's paralysis because it would no longer be necessary to commit to vitrification before doing anything with radwaste (formal performance assessments of several different disposal systems indicate that vitrification is not necessary to effect competent disposal)
-
the processing temperature required to convert radwaste to a glass-ceramic in this manner is much lower than that required to turn it into a similar volume of equally-durable glass... this plus the fact that "vitrification" is performed within a hermetically-sealed can means that volatility losses (contamination) are apt to be relatively low
-
because the "melt" does not contact the walls of the processing equipment, the corrosion problems often encountered in the manufacture of radwaste-type glasses are obviated
-
relative to conventional dry-powder HIP processing of the same materials, the application of cementitious technologies would eliminate most of the practical difficulties anticipated in attempting to remote the frit-mixing and canister-filling operations
This presentation will compare the products produced at each stage both with each other and with typical radwaste glasses. It will also address the practical aspects of HIPing large canisters of concrete.
Reference:
D.C. Seimer, B.E. Scheetz, M.L Gougar, Hot Isostatic Press (HIP) Vitrification of Radwaste Concretes, In Scientific Basis for Nuclear Waste Management, XIX, eds. W.M. Murphy and D.A. Knecht (Mater. Res. Soc. Proc. Vol 412, Pittsburgh, PA, 1995, pp 403-410.
Waste Glass Leaching During Open Site Tests
Igor A. Sobolev, Alexander S. Barinov, Michael I. Ojovan, Natalya V. Ojovan
Scientific and Industrial Association "Radon"
The 7-th Rostovsky Lane, 2/14, Moscow, 119121, Russia,
fax (095)248 1941, E-mail oj@nporadon.msk.ru
Waste glass, that contain real intermediate level radioactive waste from nuclear power plants, was obtained at experimental vitrification plant at SIA "Radon". Glass blocks were disposed for long term tests 8-9 years ago in a shallow ground repository as well as on an open testing site. In the last case the behaviour of waste glass was investigated related to possible impact of vitrified waste into environment. It was found out that radionuclide leaching of waste glass differs significantly in open area conditions in contrast to laboratory behaviour. There are many fluctuations - as when the leach rate grows by an order of magnitude - after that it remains almost smooth and slowly decreases as in laboratory conditions. By processing the obtained data average leaching factors were obtained. These factors can be used for long term predictions since they take into account the real character of leaching. Therefore in is possible to foresee the leaching behaviour of waste glass in the conditions of open site tests.
INTRODUCTION
Glass now is one of the most useful and utilized material. Historical experience of application of glass gives evidence of high stability of this material and good environmental compatibility. Most of radioactive waste components can be included into the structure of glass. Glass incompatible components can be also included into the glass matrix in the form of disperse phase. High physical and chemical durability of glass provides long term retention of radionuclides. Although glass was initially proposed for high level radioactive waste treatment, now vitrification is considered as possible process for intermediate level radioactive waste immobilization [1]. Equipment to provide waste vitrification in this case is much simpler. Requirements for the glass product are not as stringent as in the case of high level waste. On the other hand, durability of glass provides simplification of disposal facilities, shallow ground disposal being the most suitable.
Long-term laboratory tests as well as long term in-situ tests of waste glass have great importance for the assessment of vitrified waste behaviour. Now there are many well established data on the behaviour of waste glass in the case of high level vitrified waste [2]. Most of these data can be used to understand the nature of glass behaviour of vitrified intermediate level waste. Nevertheless some peculiarities of glass products and storage conditions have to be considered in this case. Natural tests give the most reliable data on the real behaviour of materials under conditions close to those of real disposal. The aim of this paper is to review the results of long term observation on the behaviour of vitrified intermediate level waste under conditions of open site tests.
EXPERIMENTS AND RESULTS
SIA °Radon began experimental vitrification of radioactive waste in the early 1970's. Borosilicate glass was selected as a matrix material for the immobilization of waste components. A ceramic melter with direct Joule heating and capacity up to 50 kg/h for glass mass was used for vitrification (high frequency induction melting in cold crucibles now are used at vitrification plant [1]). Electrical power supply of the system was 150 kW, temperature in melter being 1250°C. Many types of wastes with specific activity up to 37 MBq/l and among them wastes from atomic power
stations with reactors WWER and RBMK were vitrified. The initial liquid radioactive waste consists of aqueous sludges. About 30-40% of waste salts were incorporated into glass. The main radionuclides m waste were 137Cs(63.2- 82%), 134Cs(17- 35.1%), 60Co(1 - 1.6%), 239Pu and 90Sr (less than 0.1%). Volume reduction factors for vitrification are 4.2 - 4.5. Total amount of glass produced by ceramic melted constitutes more than 10 tons.
Glass blocks were disposed for long term tests during 1987-1989. Glass blocks were placed on stainless steel trays (52 × 52 cm) at 60 cm height from the surface of the ground. They were able to collect all water which contacted the glass. Atmospheric sediments that contacted waste glass were sampled for chemical and radiometric analysis. Usually water sampling was performed twice per month. In the following table one can see results of long term tests of two specimens for 1 year testing time and 8 years.
|
Specimen |
leaching rate after 1 year, g/cm2 day |
leaching rate after 8 years, g/cm2 day |
leaching factor, testing time 8 years, cm2/day |
|
BS-10 |
3.1 × 10-6 |
1.2 × 10-6 |
1.9 × 10-6 |
|
BS-11 |
9.8 × 10-6 |
2.7 × 10-6 |
3.7 × 10-6 |
The leaching process under natural conditions has some peculiarities when compared to laboratory testing results: the leaching process is not monotonic [3]. There are many fluctuations when the leach rate grows by an order of magnitude- after that it remains almost constant and slowly decreases like in laboratory conditions. It is supposed that changes in the leaching processes are caused by the generation of new surface regions that contact water. Actually, many small cracks were found on the glass surface after prolonged tests in an open site. In time, they form an entire network over the surface of the glass. Nevertheless one should mention that basically the glass status after prolonged tests remains satisfactory and radionuclide retention is reliable (see table). Since the leaching factor takes into account the real character of leaching including discontinuities in the leaching rate they can be used for long term predictions of radionuclide losses. The specific radioactivity of water which contacted vitrified radioactive waste was within 40-110 Bq/l for 1 year testing time, and within 10-20 Bq/l for the 8th year. Only 137Cs was detected in the exposed water.
CONCLUSIONS
Long-term test of vitrified radioactive waste on the open site has given appropriate data on the leaching process. The leaching is discontinuous, however, radionuclide retention by glass matrix remains reliable. Accurate prediction of radionuclide losses can be done by using leaching factors obtained by processing experimental data.
REFERENCES
1. S.A. Dmitriev, F.A. Lifanov, S.V. Stefanovsky et al., "Vitrification of intermediate, low-level radioactive and toxic wastes with a cold crucible," International Symposia Waste Management '96, February 25-29, 1996, Tucson, Arizona, Proceedings on CD-ROM.
2. D.E. Clark, R.L. Schulz, G.G. Wicks, A.R. Lodding, "Waste glass alteration processes, surface layer evolution and rate limiting steps," Mat. Res. Soc. Syrup. Proc. Vol. 333, p. 107-122 (1994).
3. A.S. Barinov, M.I. Ojovan, N.V. Ojovan, "7-years leaching tests of NPP vitrified radioactive waste," Mat. Res. Soc. Syrup. Proc. Vol. 412, to be published, (1996).
In Situ Vitrification of Plutonium and Uranium Contaminated Buried Wastes: Microcompositional Analyses of Vitreous and Crystalline Phases and Corresponding Leach Test Results of the Vitrified Products
Leo E. Thompson; Dale M. Timmons, R.G.; and Jack L. McElroy
Geosafe Corporation
ISV Process
In Situ Vitrification (ISV) is a waste treatment technology which uses electrical power to melt in situ contaminated earthen media such as soil, sediment, and mine tailings. The process permanently destroys, removes and/or immobilizes hazardous and radioactive contaminants contained in the media. ISV was invented by Battelle Pacific Northwest Laboratories in 1980 for the U.S. Department of Energy (DOE). Geosafe Corporation provides full-scale commercial ISV remediation services in the U.S. and has established operations in both Japan and Australia.
The ISV process is initiated by forming a pool of molten soil at the surface of a treatment zone between four electrodes. The molten soil serves as the heating element for the process, wherein electrical energy is directly converted via joule heating as it passes between the electrodes. As power continues to be applied, the molten mass grows outward and downward creating individual batch melts up to 1000 tons in size. Melt temperatures typically reach 1500-2000°C. Off-gases are collected in a steel containment hood and directed to an off-gas treatment system. When the desired treatment volume has been processed, power is terminated and the molten mass solidifies into a glass and crystalline mass. The standard ISV treatment approach involves the in-place treatment of contaminated soil and debris. However, that approach is being supplemented with staged approaches wherein stored wastes or wastes from other on-site locations are positioned in cells for treatment.
The ISV process produces vitrified products that exhibit a variety of appearances and textures when performed on different soil and waste matrices. These differences can be attributed to the bulk chemistry of the waste and the cooling history of the molten material after treatment. Often, these differences are the results of partial crystallization of the molten mass upon cooling. ISV does not have melt temperature limitations on equipment because the process container is the earth itself. Thus, fluxing materials are not generally required and, consequently, the composition of the melts are typically high in silica (60-80%) and very low in alkali (<<5%) resulting in superior chemical durability.
The ISV process has been demonstrated at full-scale on a wide range of radioactive contaminants. A full-scale ISV demonstration involving three melts is currently underway at the Oak Ridge site on a liquid waste seepage trench contaminated with cesium and strontium. The ISV process was also successfully applied to remediate 15,000 tons of contaminated soft at three Superfund Sites involving VOCs, SVOCs, and metals. All three commercial sites required the treatment of substantial amounts of debris including wood, plastic, cardboard, protective clothing, HEPA filters, drums, concrete, asphalt, tires, and scrap metal.
Demonstrations on Plutonium and Uranium Contaminated Buried Wastes
Two multi-ton intermediate-scale ISV demonstrations were recently completed for the Australian Government at the Maralinga Nuclear Test Range in South Australia. The Maralinga site is a former British nuclear weapons test site. At Taranaki, Maralinga's most heavily contaminated area, a series of minor trials involving the explosive dispersal of plutonium and uranium resulted in extensive contamination of surface soil and generated massive quantifies of contaminated debris. The heavily contaminated debris from the trials was buried in a series of shallow pits at Taranaki. The Australian Department of Primary Industries and Energy, with the assistance of a scientific advisory committee, has selected the ISV process to remediate the Taranaki Pits.
The ISV demonstrations at Maralinga involved preparing test pits containing 37 wt% steel, and other debris including lead, barite shielding bricks and organic-based materials. Actual blast debris contaminated with ˜0.5 g of 239Pu was used in one demonstration and each demonstration involved the vitrification of one kg of U3O8. Results indicate that all demonstration objectives were met and that 99.99997% of the plutonium was retained in the melt based on isokinetic off-gas sampling. Plutonium and uranium activity on the inside the off-gas containment hood and off-gas piping was negligible; decontamination of the equipment was not required. Plutonium or uranium was not detected in other phases, such as the reduced metal phase at the base of the melt created by the melting of the steel debris.
Samples of the vitrified product were subjected to analyses by the Australian Nuclear Science and Technology Organization (ANSTO). Analyses included gamma and alpha spectroscopy, X-ray fluorescence, scanning electron microscopy, and inductively couple plasma emissions spectroscopy. Geosafe also conducted electron microprobe analysis with elemental X-ray dispersion and wavelength scans. A summary of the resulting data is as follows:
-
The two melts were extremely well mixed. XRF data indicate that 20 of 21 vitrified product samples from different locations of the vitrified mass involving U and Pu had U3O8 concentrations of 0.037 to 0.041 wt%. The remaining sample collected from the boundary of the vitrified monolith had a U3O8 concentration of 0.027 wt%. A wider variation in the concentration of plutonium was indicated by alpha spectroscopy. The average 239Pu concentration in six samples was 328 kBq/kg in a range of 292 to 419 kBq/kg. Alpha track etch imaging was used to provide a visual image of the distribution of plutonium in thin slices of the product by using a plastic film sensitive to alpha radiation.
-
Since the sod types varied significantly at Taranaki, the demonstration melts involved sod combinations, including stratified silica sand (i.e., 70-90% SiO2) and carbonate rocks. The resulting vitrified products included silica rich glass phases intermixed with crystalline phases including wollastonite, diopside and cristobalite. The localized compositions around the crystalline growths were evaluated by electron microprobe analyses. Depending on the type of crystalline growth, the relative silica content in the glass phases surrounding the crystal growths were either slightly increased (around wollastonite or diopside) or slightly decreased (around cristobalite). However, the bulk silica concentration in the melt was sufficiently high that slight changes in the silica concentration in the altered phases were insignificant (i.e., the silica concentration was still very high at >60%). Thus, all portions of the vitrified product have high chemical durability despite crystallization.
-
Leach tests were conducted including Product Consistency Tests (PCT) and other MCC-type tests involving both monolithic and pulverized vitrified product samples. The leach tests were conducted at temperatures of 26°C and 90°C for periods of 7 and 28 days. Some of the leach tests were conducted at the ambient conditions expected to prevail at the Taranaki site. Leachants included deionized water and buffers at pH levels of 7 and 10. Both the leachates and an acid strip of the vessel walls were used to determine the total release of each element from the vitrified product. For all samples under PC conditions, the critical releases of Na and Si from the vitrified products were less than 0.5 and 0.1 g·m-2 respectively for 0 to 7 days. Results also indicate that the releases of Ba, Ca, and Si increased over the temperate range from 26° to 90°C by a factor of between 2.2 to 3.2, while K increased by a factor of 10 over the temperature range. Conversely, Al, Mg, and Na had lower releases at 90°C possibly due to matrix saturation or inverse solubility of phases with temperature. Increasing the leach durations from 7 to 28 days only increased the releases of the highly soluble ions (Na and K) with all other release rates decreasing. PCT leach tests were also carried out using a pH 10 carbonate buffer. Under these conditions, only releases of U increased significantly, by a factor of 10, however, the releases were still significantly less than 1 g·m-2. Results for Pu indicate that as the leach durations are increased, the Pu release rates decrease.
THE INTERACTION BETWEEN HLW GLASS AND CLAY: PRESENT STATUS AND FUTURE PROGRAMME.
Pierre Van Iseghem, Karel Lemmens, Marc Aertsens, Philippe Lolivier, Wei Jiang, Pierre De Cannière
Waste and Disposal, SCK·CEN, Boeretang 200, 2400 Mol, Belgium
We have completed a large parametric study to identify the role of different engineered (container corrosion products, backfill materials) and natural (Boom clay) barrier materials on the dissolution behaviour of high-level waste glass. We considered the Cogéma R7T7 and the DWK/ Pamela glasses. This way we obtained specific information on the influence of the Al2O3 concentration in the glass. Other parameters were the temperature and the presence of a gamma radiation field. Different SA/V (surface area to solution volume) were applied as a means to accelerate the glass dissolution. By combining high SA/V conditions and interacting durations up to 2 years we reached high reaction progress values (up to 2200 y/m). The leaching behaviour of the long-living radionuclides was studied, through glasses doped with the radionuclides of interest (Pu-239, Am-241, Np-237, Tc-99), or fully active glass samples. In-situ tests were carried out to verify the results obtained in the laboratory.
As a basic conclusion, we identified some main dissolution mechanisms:
-
congruent glass dissolution in (mainly) clay media; we anticipate that the congruent dissolution is not permanent.
-
ion exchange controlled dissolution at high reaction progress; the ion exchange between H3O+ / H+ and Na+/Li+ was proposed.
Besides, discontinuous excursions in the glass dissolution due to secondary phase formation were observed in a number of situations, after the saturation concentration of certain elements was reached.
We also obtained qualitative information on the influence of the presence of iron corrosion products and of bentonite or cement backfill.
As a consequence of our new insight on the basic dissolution mechanisms we decided to develop a mathematical model for the glass dissolution, accounting for the ion exchange controlled dissolution. The model also considers the transport properties of SiO2 through the pore water in clay. Special migration tests were designed using Si-32 tracer.
The in-situ tests were performed in the underground laboratory in clay beneath the SCK·CEN laboratory. We obtained data at temperatures of 16°C (rock temperature), 90°C and 170°C, for maximum durations of 7.5 years. At higher temperature the corrosion data were similar to the data from the laboratory tests. We evidence the yet important role of the glass composition: the current borosilicate glasses such as the R7T7 one corrode really congruently in Boom clay, whereas the high Al2O3 Pamela glass corrodes by selective dissolution. We have confirmed the fundamental difference in glass dissolution by profile analysis using SIMS (secondary ion mass spectroscopy). On the other hand, the glass dissolution is extremely small at ambient rock temperature of 16°C, and remains below 0.1 µm/y. This result together with the results from the laboratory tests provide large confidence in the quality of the HLW glass as an engineered barrier.
We obtained a large data base on the leaching behaviour of Tc, Pu, Am and Np in claywater or clay slurries, over extended reaction progress. We measured both the radionuclide concentration in the solution (which is able to migrate through the clay rock) and the radionuclide inventory sorbed on the clay. The average mobile radionuclide inventories released are, in M, 10-8 (Pu), 10-6 (Np), 10-11 (Am), 10-6 (Tc). These data for Np, Tc and Am are lower than the resp. solubilities assumed in the Belgian performance assessment studies, and therefore add to the safety of the disposal concept. When bentonite substitutes part of the Boom clay, these concentrations decrease by as much as 100 times.
The upcoming programme 1996- 1999 is focussed on three main areas:
-
The dissolution behaviour of the glasses will be further modelled, by using mathematical and geochemical codes. Specific laboratory tests will be performed to determine specific parameters required by the model, such as the diffusion coefficient of Si through the glass surface layer and through the clay, the porosity of the surface layer.
-
The identification of the complexes including Tc and Np in the interacting media. This study will consider as important parameter the presence of humic acids in the solution. The complexes will be characterized by various techniques, such as laser spectroscopy. The experimental data will be correlated with theoretical calculations of solubility.
-
The demonstration by in-situ testing of the performance of an alpha active glass in disposal environments. This test is called "Coralus", and will consider the presence of a gamma irradiation field, and different interacting materials (Boom clay, bentonite backfill). This project is discussed in a separate poster.
The presentation will summarize the actual state of knowledge, and present the main actions in the future programme.
This programme is partly sponsored by NIRAS/ONDRAF and the European Commission.
VERIFICATION STUDIES ON THE PAMELA HIGH-LEVEL WASTE GLASSES
P. Van Iseghem, E. Hoskens, G. Smeyers, D. Huys, L. Sannen, L. Vandevelde
SCK·CEN, 2400 Mol, Belgium
As part of the Quality Assurance/Quality Control programme implemented by the national nuclear waste management authority (NIRAS/ONDRAF), various material properties are being verified. This is done through measurements on three types of samples :(1) laboratory made samples (MT1), (2) demonstration 1/1 scale drums (MT2) and (3) fully active samples (MT3).
This paper deals with the Verification programme for the high-level waste glasses produced in the PAMEAL vitrification furnace, operated by DWK/Belgoprocess. In this plant, about 800 m3 high-level waste were vitrified so far. In 1988, a programme was launched by NIRAS/ONDRAF to measure a number of properties on the various material types, including MT1 samples, 4 MT2 drums, and 30 active MT3 samples.
Three glass compositions were produced: SM513 and SM527/SM539, resp. for the low and high enriched waste concentrates.
The investigations were carried out by the SCK·CEN. The following properties were checked:
-
chemical and radiochemical analysis, to compare with the nominal composition (MT1, MT2, MT3 samples);
-
homogeneity, on a microscale, or between various positions inside a container, or between containers. This is done through microscopical techniques (elemental X-ray mapping, SEM analysis, radiography), chemical or radiochemical analysis (MT1, MT2, MT3 samples);
-
chemical stability, based on standard corrosion tests (the Soxhlet MCC5 flow test, and the static MCC1 test), on MT1, MT2 and MT3 samples;
-
thermal stability, in terms of devitrification and phase separation behaviour (MT1 samples).
Some of the main observations are:
-
the chemical stability of the MT1 samples is comparable with other, actual or procursor HLW glasses;
-
transmission electron microscopy analysis on the HEWC glass SM527 reveals some glass-in-glass phase separation. The size of the droplets is between 300 and 500 nm;
-
some secondary phases, consisting of noble metals (Ru, Rh) or transition metals (Fe, Ni, Cr) are present in the active samples. The α and βγ active elements are homogeneously incorporated;
-
for both MT2 and MT3 samples the glass matrix (based on Si analysis) is quite homogeneous; whereas minor components, e.g. rare earths are less homogeneous. Differences may be as large as 10%.
-
the chemical as well as radiochemical (atot,241Am, ßtot,90Sr,137 Cs) composition of the active samples seems to quite reproducible: standard deviations are fairly small.
The paper will review all data generated on two glass compositions (SM513 and SM527), with attention to the experimental procedures and the reliability of the data. Interpretation will be done with respect to the materials properties selected, and the various types of samples.
This work was carried out under contract 758048 and 058068A2 with NIRAS/ONDRAF.
Synroc - an Alternative Waste Form
E. R. Vance, K. P. Hart and A. Jostsons,
Australian Nuclear Science and Technology Organization
It has been noted that given the compositional diversity of nuclear waste and the variations in potential repository geologies in different countries, it is important to have a ''menu'' of waste forms so that one is able to choose the waste form that best suits the type of waste and the geology of the repository (Lutze and Ewing, 1988). This paper summarises the work that has been carried out on Synroc to establish its viability as an alternative waste form for the safe disposal of nuclear waste.
The feasibility of production of good quality Synroc on a commercial scale has been demonstrated in the Synroc demonstration plant at ANSTO on a non-radioactive basis. This plant has already produced more than 6 tones of Synroc and is used to optimise process steps and to provide information for the conceptual design of a radioactive plant. The plant has a capacity of 10 kg/hr and produces Synroc of a similar quality to that produced in the laboratory.
Chemical durability studies of Synroc have been carried out on non-radioactive samples and Synroc doped with actinides and fission products. Leach tests initially concentrated on predicting the durability of Synroc under standard conditions but lately have been extended to include studies based on different repository geology and ground water compositions. Data will be presented for the release of actinides and fission products measured under standard and simulated repository conditions and also from in-situ studies carried out in the Mol underground facility. In general, the data show that long term release rates are low, << 10-5/g/m-2/d, and continue to decrease, albeit slowly, with time.
Studies of radiation damage have been carried out by doping Synroc with 238Pu or 244Cm to simulate damage corresponding to about 105 years of storage in about 2 years. These studies have shown that the volume expansion of Synroc saturates at about 4 to 7% at doses of around 5 to 8 × 1018 a-decays/g, under ambient conditions. Storage of the specimens at 200°C reduces the rate of density change by about 30%. Samples that have accumulated damage equivalent to 13,000 years of storage have leach rates for Mo, Sr, Ca, and Ba which are less than a factor of 10 higher than those of undamaged Synroc. Cs leach rates increase by about a factor of 50 over this storage period.
The design of Synroc was based on natural minerals which were known to be stable in the earth's crust for 106 to greater than 109 years. Samples of naturally-occurring zirconolite containing U and Th in trace to major proportions, resulting in a-decay doses from less than 1017 to greater than 1020 a/g allow the study of the effect of radiation damage incurred slowly under geological conditions to be studied. Studies of these samples have shown that
the crystal chemistry of the natural samples is close to that of the zirconolite phase in Synroc containing 10 and 20 wt% of simulated PW-4b waste and that the structure of these phases is rendered aperiodic at doses similar to those for the actinide-doped samples. In addition, the natural samples have remained as closed systems for actinide elements regardless of the amorphisation of their structure or contact with ground waters.
The work carried out thus far has established that Synroc is a viable alternative for disposal of nuclear waste. Additional work is on-going, however, to further develop the Synroc concept and applicability of this waste form to some special waste streams including excess military Pu disposition. This work will also be detailed within the paper.
CHEMISTRY OF IRRADIATED SOLIDS
D. W. Werst and A. D. Trifunac
Argonne National Laboratory, 9700 Cass Av, Argonne, IL 60439
werst@anlchm.chm.anl.gov and trifunac@anlchm.chm.anl.gov
Studies of chemical mechanisms of radiation effects in solids address the need to provide a scientific basis for predicting the performance of solid radioactive waste forms. In the Radiation and Photochemistry Group at Argonne National Laboratory many state-of-the-art capabilities have been developed to identify reactive intermediates and elucidate radiolysis mechanisms in solids. Radiolytic H atoms in silica and ice have been studied by time-resolved electron paramagnetic resonance (EPR), with information gained about the activation energy for diffusion, diffusion coefficient and likely reaction partners and their properties. Radiolytic yields and annealing behavior of trapped H atoms have been measured by low-temperature EPR in microporous solids (sol-gel silica, porous vycor glass) and crystalline materials (zeolites). Charge transfer has been studied in sol-gel and zeolite systems by using variable-temperature EPR. Charge recombination reactions in frozen hydrocarbons have been probed by time-resolved fluorescence-detected magnetic resonance (FDMR). Measurements of hydrogen gas evolution from radiolyzed cement grout as a function of water content revealed higher than statistical energy absorption by water, leading to O-H bond rupture. A presentation of these experimental results will accompany discussion of proposed applications of modem radiation chemical techniques to understand fundamental chemistry of irradiated solids by using the unique facilities for pulse radiolysis and detection of reactive intermediates at Argonne.
CORALUS: IN - SITU CORROSION TEST ON ACTIVE HLW GLASS
P. Van Iseghem*, E. Vernaz**, N. Jockwer***
* SCK·CEN (B), ** CEA Valrhô (F), *** GRS Braunschweig (G)
1. Objectives
The performance of waste forms in repository conditions must be known, to validate models and conclusions from laboratory tests, and to confirm data on the source term. The various interactions between the engineered and natural barriers must be evaluated, to determine and validate the parameters used in the safety assessment.
In this proposal a new kind of in-situ test is proposed, which contributes to answer the problems mentioned above, and which is based on the experience gained in different in-situ tests developed in the past ten years:
-
the Belgian in-situ corrosion tests in clay,
-
the Belgian "CERBERUS" in-situ test, using an active 60Co source,
-
the Swedish tests in the STRIPA mine in granite,
-
the USA "MIIT" tests in salt.
So far, in-situ tests on waste forms were restricted to small, inactive or doped samples. This proposal includes glass samples doped with large amounts of alpha emitters. This is consistent with the recommendations of an International Workshop on in-situ testing of nuclear waste glasses [1], stating
"Future Tests With Waste Glasses Containing Radioactive Tracers are Recommended "
The objectives of the proposed in-situ test are:
-
to determine the dissolution of the glass in simulated disposal conditions. Both the global dissolution and the specific release of radionuclides will be measured. Because we will use coupon glass specimens, we will be able to compare these results with previous lab and in-situ tests, and interpret with the dissolution models based on laboratory tests. Surface and bulk studies of the reacted glasses will help elucidate corrosion mechanisms.
-
to evaluate the migration of the radionuclides through the interacting media in a radiation field. We will investigate two reference situations, the first including both a gamma radiation field and the alpha activity in the glass. In the second situation no gamma radiation sources will be used.
-
in both former objectives, also inactive simulants of other long-living nuclides of interest, such as 99Tc, 135Cs, 79Se, 93Zr, 107Pd, can be investigated in terms of release and migration;
-
to measure various parameters in the contacting media ( dose rate, pH, Eh, gas generation, gas release, the petrophysical properties of the backfill) and their effect on waste form behaviour;
-
to gain experience with techniques for in-situ testing and monitoring in more realistic repository-relevant conditions;
-
to encourage further international cooperation in the Waste Management field.
We will consider three interacting media: Boom clay (the Belgian candidate host rock), and two bentonite based mixtures, studied as backfill material within the EU.
2. Work Content
High-level waste glass doped with the real actinide concentration, and with the insertion of a gamma radiation source will be exposed to different surroundings in the underground laboratory in clay under the SCK·CEN site. As surroundings we select Boom clay, the Belgian candidate host rock, and two bentonite based mixtures, which are being proposed by the partners as backfill material in the repository concept. Modules corresponding to one kind of interacting environment (Boom clay, bentonite backfills) such as shown in Figure 1 will be assembled into tubes, and three of them will be introduced into openings in the underground laboratory in clay. Each module contains eight glass coupons, consisting of samples doped with one alpha emitter (3 samples total), an inactive sample, and duplicate samples. They will be exposed to media consisting of either the Boom clay or candidate backfill materials. The chemistry of the interaction environment will be carefully monitored during the test, together with the dose rate. The generation and release of gases formed (e.g., H2, CH4, H2S, HCl) will be monitored, and coupled with laboratory investigations of the gas release and of the petrophysical properties of the backfill materials. At well specified times the tubes will be retrieved by overcoring, and the interacting materials and the reacted glass will be analyzed by various radiochemical, chemical and surface analytical techniques. A view of a corrosion tube is shown in Figure 1.
Glass samples of the Cogéma R7T7 composition, doped with about 0.85 wt% of NpO2, PuO2 or Am2O3 (the industrial R7T7 glass has a total actinide content of 0.85 wt%), as well as the inactive composition will be made available by CEA Valrhô (F). Because of the different specific activities of these actinides, we will be able to obtain different levels of alpha radiolysis in the interacting material.
To demonstrate the feasibility of the assembly, operation, retrieval, dismantling and clay sampling of the active tubes, we will first assemble an inactive tube, loaded with inactive glass samples, without gamma sources. We foresee an operation period of about one year, after which the active tubes will be introduced in the underground laboratory. This test will identify and potentially remediate problems with the active set-ups. The test will also enable GRS (Germany) to demonstrate the gas sampling and analysis system.
The work content in the present proposal covers a three year period. This will include the design, preparation and installation of the different tubes. Following our present planning the active tubes will be installed in the underground laboratory in the second half of 1999. Their scheduled operation time will be one and five years. Therefore the retrieval and analyses of the active tubes will must be carried out within a following contract.
The paper will present the major conclusions form previous in-situ tests in the Mol underground laboratory, and the outline of the new in-situ test.
Reference
[1] T.McMenamin (editor), Conclusions of the Int. Workshop "In-situ testing of radioactive waste forms and engineered barriers", EUR 15629 (pre-print), 1994