2
Status
This section describes some of the important new scientific insights, novel materials, and new processing technologies that have already emerged from the interface between biology and materials science. Its purpose is to illustrate the state of the art in selected areas. It is not meant to be a complete literature survey. The examples discussed serve to establish the feasibility and underpinnings of the biomolecular materials approach.
This section is organized in order of increasing structural complexity, starting with complex fluids, whose properties, including self-assembly, mirror those of lipids. The discussion then addresses polymers, thin films, composites and templates.
Complex Fluids and Liquid Crystals
Complex fluids12 are bulk multicomponent systems that self-assemble into a variety of structural forms. Such systems include: (1) amorphous composites such as polymer solutions, emulsions, and colloidal suspensions and (2) systems in which the self-assembly is based on the amphiphilic nature of surfactants and lipids. (Amphiphilic molecules are those with a polar head and a long hydrophobic tail.) Self-assembly generally involves anisotropic molecules that are driven into ordered structures by intermolecular forces and the requirements of packing. In this sense, complex fluids are closely related to liquid crystals, which are materials that possess orientational order without crystalline translational order. All these characteristics of self-assembly are nicely demonstrated in biomembranes.
Complex fluids may exhibit various types of partial ordering, i.e., the coexistence of amorphous and highly correlated structures. This concept and the rich variety of self-assembling systems can be illustrated with a few examples:
- Colloidal suspensions are small solid particles dispersed in a carrier fluid. Interparticle interactions may lead to a long-range crystalline order, thereby producing colloidal crystals. Such materials possess the properties of crystals, including a finite shear modulus, even though the majority component is a fluid. Indeed colloidal crystals are also interesting in that they exhibit long-range order but are disordered on short length scales.
- Lyotropic liquid crystals13 exhibit a key characteristic of biomolecular self-assembly in that a local molecular feature—the amphiphilic nature of the constituent molecules—generates self-organized structures that may consist of units of mesoscopic size, which are very large compared to molecular dimensions. For example, lamellar lyotropic phases are composed of stacks of fluid lipid membranes separated by a solvent. The spacing between the membranes is well-defined and can be hundreds of nanometers even though the structure remains liquid-like at the scale of angstroms. Such systems possess quasi-long-range order but have components that are locally disordered.
As lamellar phases are increasingly diluted with solvent, a transition to the sponge phase14 may occur. This phase is characterized by a random organization of the bilayer membranes. They divide the solvent into two distinct but interpenetrating regions, forming a bicontinuous structure.
- Lyotropic nematic liquid crystals are orientationally ordered fluids composed of colloidal suspensions of anisotropic rod-like particles. Such systems can undergo phase transitions as the volume
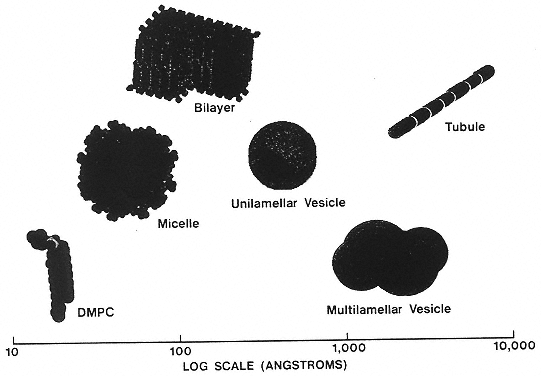
Figure 2
Schematic representation of some self-assembled lipid-based microstructures. (Reprinted, by permission, from J. Schnur, Science 262:1670 (1993). Copyright © 1993 by the American Association for the Advancement of Science.)
- fraction of the suspended anisotropic species is varied, producing prolate colloidal particles, rod-like species such as those found in tobacco mosaic virus, cylindrical micelles, or other structures.
Surfactant-based Self-Assembly
Surfactant molecules (e.g., phospholipids, soaps, detergents, and block copolymers) self-assemble15 in selective solvents (e.g., water) to form bilayer membranes and micelles of several structures (Figure 2). The membranes also organize in a variety of patterns. Such self-assembled structures may be swollen by adding an organic solvent to the system, which remains stable as a clear single phase. Such two-solvent composites are called microemulsions. Surfactant bilayers have been investigated for many years as model systems for biological membranes.
Repulsive forces between the surfactant head groups organize the bilayers on longer length scales. This process may form lamellar stacks, sponge phases, or microemulsions, depending on whether there are one or two solvents. Even more relevant to biomolecular systems is the formation of closed-film structures called vesicles (Figure 3), which are already being employed as simple containers to transport drugs within the blood system.
The self-organizing micelles may form in different shapes depending on the specific chemistry and on solvent conditions such as pH, ionic strength, and temperature. The most common form is spherical, but cylindrical micelles also occur. In fact, entropic optimization drives long cylindrical micelles into the form of flexible polymer-like chains. These polymeric micelles have many of the rheological properties of polymers with covalently bonded backbones, but their lengths are determined by equilibrium thermodynamics rather than being fixed.
An interesting example of a self-assembling structure is the tubule, a hollow phospholipid bilayer cylinder morphologically similar to a soda straw (Figure 4). The length of these ultrasmall cylinders is
|
15 |
S.A. Safran, Statistical Thermodynamics of Surfaces, Interfaces, and Membranes (Addison-Wesley, New York, 1994). |
typically tens or hundreds of microns.16 The diameter can vary from 0.1 mm or less to over 0.7 mm, with the wall thickness varying from less than 100 Å to well over 500 Å. A number of applications using both metal-clad and non-clad tubules are currently being evaluated for commercial application. A hollow tubule is essentially a “microvial” in which a solid or liquid can be encapsulated. The use of such microvials has led to controlled-release applications for marine antifouling (release over many years) and drug delivery (release over several days to months).17 These applications are now at the advanced development stage in several commercial firms both in the United States and abroad. The metal-clad structures also have interesting electromagnetic properties. Applications based on their dielectric properties (miniaturized microwave circuits) and as absorptive filters are currently under development in several universities and government laboratories. While several of the technical issues critical to successful commercialization of tubule applications have been solved, other issues (such as scale-up and a satisfactory cost/performance ratio) remain to be addressed prior to successful marketing.
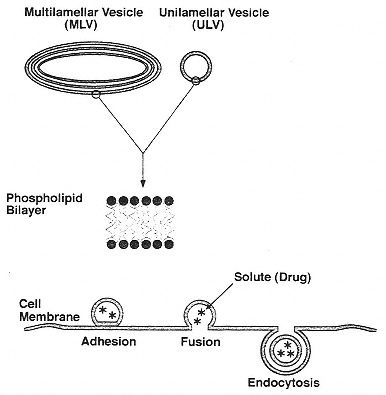
Figure 3
Vesicles: (top) sketch of multilamellar and unilamellar vesicles and the phospholipid bilayer of which they are made; (bottom) sketch of a mechanism whereby drug-containing vesicles adhere to a cell surface and present drugs for uptake by endocytosis.
Polymers.
Since much of biology is based on the special properties of macromolecules, it is widely anticipated that the area of polymeric biomolecular materials will be rich.18 Two of the tools of molecular biology, namely recombinant DNA and genetic engineering techniques, now make it possible to construct monodisperse, highly specific polymers. For example, both polyesters and polyamides (i.e., protein polymers) have been produced in this manner. A current area of investigation is to understand those features of protein polymers that confer high tensile strength, high modulus, and other advantageous properties. Once those features are understood, the tools of biotechnology will make possible entirely new paradigms for synthesis and production of materials. If they can be made economically viable, these new approaches will help reduce our dependence on petroleum and furthermore will enable making materials that are biodegradable.

Figure 4
Electron micrograph of nickel-coated tubules approximately 0.4 µm in (inner) diameter and with walls approximately 500 Å thick.
(Reprinted, by permission, from J. Schnur, Science 262:1673 (1993). Copyright © 1993 by the American Association for the Advancement of Science.)
In the last few years it has become possible to produce designer polymers comparable to those produced naturally. Use of genetic engineering techniques to obtain new polymer molecules provides the polymer scientist with model, controllable, synthetic proteins for the first time. Two aspects of this approach are biomimetic: targeted molecules are produced by biosynthesis, and they then self-assemble into desirable structures that have new or improved properties. Efforts in biosynthesis have been directed toward the preparation of precisely defined polymers of three kinds: (1) natural proteins such as silks, elastins, collagens, and marine bioadhesives, (2) modified versions of these biopolymers, such as simplified repetitive sequences of the native protein, and (3) synthetic proteins designed de novo that have no close natural analogues. Although such syntheses pose significant technical problems, these difficulties have all been successfully overcome in recent years.
Figure 5 shows the key steps in the in vivo synthesis of protein-like polymers under direct genetic control in bacteria. The first requirement is that the amino acid sequence of interest must be encoded into a complementary sequence of DNA. For natural proteins, the requisite DNA sequence is obtained by isolating the appropriate gene from the natural host organism. De novo design and synthesis require chemical synthesis of an artificial coding sequence. In either case, the isolated DNA fragment is ligated into a loop of DNA (a plasmid) that can be replicated in a microbial host and that includes the signals needed for controlled transcription and translation. The resulting recombinant DNA is then introduced into the host cell population, cells are grown in large numbers, and protein production is induced. To date, this approach has been used successfully to produce a variety of natural proteins, as well as dozens of wholly artificial protein-like polymers. 19
It is now relatively routine to use bacterial hosts to produce multigram quantities of polymers with degrees of polymerization up to 1000 and molecular weights up to 100,000. The first commercial product based on this technology, an artificial cell attachment protein that can be used to coat polystyrene culture dishes, has already appeared. Although the anticipated structural regularity has been thoroughly demonstrated in several instances, mutations have also occurred that have led to an altered chain sequence. There have also been examples of enzymatic degradation, which leads to chain length heterogeneity. Such problems have as yet unknown consequences for targeted secondary and tertiary structures.
Biological synthesis is also being used to prepare important classes of polymers other than proteins. Bacterial polyesters have attracted particular attention because of their biodegradability.20 They are
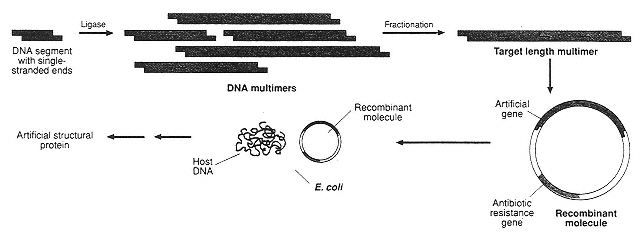
Figure 5
Key steps in the in vivo synthesis of protein-like polymers under direct genetic control in bacteria. First a DNA sequence is produced that codes for the desired polymer. This DNA is then inserted into a plasmid. When the plasmid is inserted into E. coli bacteria, the bacteria produce the polymer. (Reprinted with permission from J.G. Tirrell, M.J. Fournier, T.L. Mason, and D.A. Tirrell, Chemical & Engineering News 72(51:December 19):43 (1994). Copyright © 1994 by the American Chemical Society.)
already finding commercial application in specialty packaging uses. Costs are still high in comparison to those for commodity polymers, but increasing volume seems likely to bring production costs down to levels that are economically competitive in a wider range of applications. Furthermore, it has recently been demonstrated that the production of bacterial polyesters can be transferred to plants, opening the way for their production from biomass. Natural polysaccharides are under development for biodegradable packaging applications. Several companies have recently commercialized starch-based polymer blends that can be processed using existing thermoplastic methods.
Biology often combines very different materials in closely interwoven patterns to develop unique properties that stem from the co-continuous nature of the assembly. The liquid-crystalline state in polymers, first viewed as unusual, is now becoming accepted as a normal state occurring between the crystalline and isotropic liquid states. High-performance fibers based on solution processing of lyotropic liquid-crystalline polymer solutions (e.g., Kevlar) have contributed to a host of high-technology composites developed for their light weight, high strength and stiffness, and high temperature stability (service temperatures of up to 500°C). Certain insects, including spiders, take advantage of the low viscosity in the liquid crystalline regime. Spider dragline silk is a versatile engineering material that performs several demanding functions. The mechanical properties of dragline silk exceed those of many synthetic fibers. Moreover, dragline silk exhibits the unusual behavior that the strain required to cause failure actually increases with increasing deformation.21 This property is particularly advantageous because webs need excellent energy-absorbing capability to capture flying prey. Spiders extrude an aqueous solution of silk protein to spin the molecules into oriented fibers. The female garden cross spider can use seven different glands, each containing silk with a unique amino acid sequence, to
produce fibers with different properties. Figure 6 illustrates a proposed model for the structure of spider silk.
There is renewed interest in the structure-property relationships of such structural biological materials because the fibers have outstanding tensile as well as compressive properties. Work is under way to fully characterize the molecular weight and sequence distribution; the nature of the in vivo solution (speculated by some to be liquid crystalline); the structure, size, and orientation of the crystalline regions; and their interconnection to the amorphous regions. The consensus crystalline repeat in two silk proteins has recently been identified. 22 Cloning and expression of the gene for spider dragline silk to facilitate production of large quantities of synthetic silk are now under investigation.23
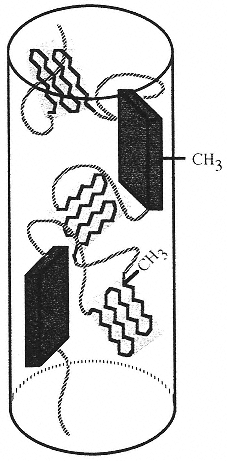
Figure 6
Diagram of the proposed model for the molecular arrangement of alanine residues in a fiber of spider dragline silk. Highly oriented alanine-rich crystals of β-sheets (rectangles) and weakly oriented yet crystalline unaggregated sheets (canted sheet-like structures) are depicted in an amorphous glycine-rich matrix (curved lines). In reality, the glycine-rich matrix composes about 70 percent of the fiber; in this drawing it has been largely suppressed for clarity. (Reprinted, by permission, from A.H. Simmons, C.A. Michal, and L. W. Jelinski, Science 271:84–87 (1996). Copyright © 1996 by the American Association for the Advancement of Science.)
Another important use of polymers is adding them to hydrocarbon-and water-based fluids to control rheological properties.24 Advances in functionalization chemistry have enabled the engineering of synthetic polymers with associating groups. Ionomers, oil-soluble polymers containing ionic functionality such as sulfonate or carboxylate groups, have been synthesized in a variety of architectures including random, block, and telechelic structures. In hydrocarbon solvents, the polar ionic groups interact to create self-assembled or aggregated polymer chains, which appear to have much higher molecular weights than the non-functionalized polymers. Conversely, hydrophobically associating polymers are water-soluble but contain a small amount of oil-soluble or hydrophobic functionality. These two classes of polymers have been called associative thickeners. The association of the functional groups in fluids enables these synthetic polymers to mimic the secondary and tertiary interactions and structures found in biomolecules such as proteins and polysaccharides. The inter-and intra-molecular interactions of the polymers define their conformation and assembly in solution, and in turn, the solution's rheological properties. Unique and useful rheological properties such as dilatancy or shear thickening, enhanced viscosification, and compatibility with other fluid components can be designed into fluids using associating polymers.
Designer fluids with controlled rheological and interfacial properties, with potential applications for processing composites, can be prepared by combining polymers and microemulsions.25 Water-soluble synthetic polymers such as polyacrylamides or polyethylene oxides and biopolymers such as xanthan or scleroglucan have both been studied in water continuous microemulsions. Polymers and surfactants may not be compatible in such complex fluid mixtures, and separation into polymer-rich and surfactant-rich phases may therefore occur. This phenomenon is similar to the coacervation observed in mixtures of two polymers in a common solvent. The compatibility between the polymer and the surfactant or microemulsion phase may be enhanced by replacing the water-soluble polymer with an associating polymer that contains hydrophobic groups. Single-phase fluids with controlled rheological and interfacial properties can thus be prepared.
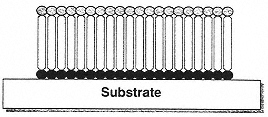
Figure 7
Schematic illustration of the molecular structure of a self-assembled monolayer (SAM) on a substrate surface. The properties the SAM are determined both by the physical properties of the molecules from which they are formed and by the nature of their packing interactions.
Molecular Thin Films
The study of molecular thin films obtained via the self-assembly of small molecules on surfaces (Figure 7) has witnessed enormous growth. Most recent work has been directed at a series of model phases, for which structural characterizations have been made in considerable depth. Monolayer phases supported on a variety of substrates have been explored. Notable among these are alkane thiols supported on coinage metal surfaces (gold, silver, and copper), alkyl silanes bound to a variety of metal oxide surfaces (especially SiO2), and carboxylic acids adsorbed on basic oxides (e.g., Al2O3). Polymer brushes formed by amphiphilic block copolymers make up a conceptually related but structurally distinct set of phases. The structures of monolayers at the air-water interface have also been very extensively studied. There is a clear relationship between the behavior of these systems and the unique phase behaviors of very thin films of liquid crystals.
Multilayer assemblies have not been as extensively studied. Perhaps the most significant examples of current research on multilayer assemblies are systems based on zirconium phosphonates. Polymeric adsorbates, i.e., polymer films that are sufficiently thin as to be completely dominated by the structural perturbations of an interface, remain very poorly understood for the most part, although very recent work has explored ordering near solid surfaces of block copolymer films whose thicknesses are on the order of several radii of gyration or more.
Some significant progress has also been made in building function into these thin film phases, albeit primitive function by the standards of biology. Electroactive centers have been incorporated into ordered thin films. So has photochromic functionality with vectorial optical response. There have also been several reports26 of the construction of molecular thin films with significant abilities for molecular recognition, though again of a fairly rudimentary sort.
Considerable attention has been given to the influence of thin boundary layer films on the properties of interfaces. Self-assembled monolayers (SAMs) make it possible to functionalize surfaces in well-defined ways, and it is only natural that they have found considerable application in research on friction, lubrication, and wetting. It is now understood, as a direct result of the study of SAMs, that wetting phenomena on molecular solids depend sensitively on near-surface structure and composition. An understanding is now emerging from this work of how molecular structure drives the macroscopic properties of surfaces. This understanding is enabling the development of surface modification schemes for technological applications that are based on reason rather than empirical experience alone.
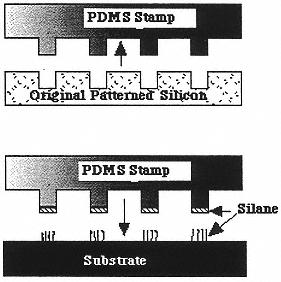
Figure 8
Chemical patterning of surfaces: (top) patterned stamps are prepared from lithographically patterned silicon; (bottom) silane-based self-assembled monolayers are then “stamped” onto a surface. PDMS is poly(dimethylsiloxane). (Reprinted with permission from P.T. Hammond and G.M. Whitesides, Macromolecules 28:17569 (1995). Copyright © 1995 by the American Chemical Society.)
SAMs are also beginning to find applications in the biological sciences. There has long been an interest in the interaction of cells with surfaces. SAMs have allowed the tailoring of surface properties in ways that allow considerable control over the ability of cells to bind to a solid. Several recent papers27 have demonstrated that spatially defined SAMs can be used to pattern cell attachment to a substrate. Neural synaptic integration in planar neural arrays can also be mediated by SAMs, and the pattern of their interconnections can be controlled by the modification of surface properties. The affinities of surfaces for protein adsorption are also easily modifiable using SAMs.
There is considerable interest in the applications of SAMs in the area of sensors. Their potential utility is being demonstrated very powerfully in electrochemical applications. They have been used to control the redox tunneling properties of gold surfaces by allowing the homogeneous placement of electroactive materials at known densities and distances from the surface. More recently, photoexcited vectorial electron transport has been demonstrated in both monolayer and multilayer assemblies. Several general sensor designs have also been described that involve the use of SAM-modified microelectrode grids.
SAMs have also been shown28 to act as effective masks for the lithographic patterning of metals (Figure 8). These studies have demonstrated that microstructures can easily be fabricated in shapes and architectures that are hard to implement flexibly with conventional multistep lithographic patterning. An exciting application has been demonstrated in the growth of thin films by chemical vapor deposition:
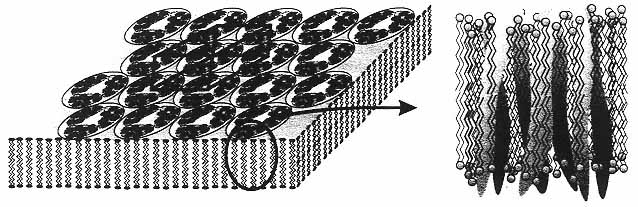
Figure 9
Schematic view of a bacterial membrane with a hexagonal lattice of bacteriorhodopsin trimers. The expanded view on the right shows one bacteriorhodopsin molecule, illustrating the seven α-helices that span the membrane. (Reprinted, by permission, from Y. Shen, C.R. Safinya, K.S. Liang, A.F. Ruppert, and K.J. Rothschild, Nature 366(6450):48 (1993). Copyright © 1993 by MacMillan Magazines Limited.)
SAMs were very effective in improving the adhesion of the deposited film, and in several systems it has been demonstrated that they can be used to influence the activated nucleation of thin-film growth. 29
Membrane-associated Proteins
In contrast to phospholipid molecules, which form the passive permeability barrier in biomembranes, membrane-associated proteins serve as active components to facilitate important cellular processes such as nerve conduction, energy conversion, active-ion and molecular transport, and cell-cell adhesion. Integral membrane proteins are intercalated and firmly tethered to the two-dimensional host lipid membrane plane, in which they are free to diffuse. Research has begun to develop new types of materials that incorporate the functional activity of such proteins into chemical and biological sensors, materials with controlled interfacial properties, and optoelectronic materials.
A notable example of a membrane-associated protein is bacteriorhodopsin (bR),30 which embodies two functions of membrane proteins, energy transduction and active ion transport.31 This protein, which acts as a photon-induced proton pump, self-assembles into a two-dimensional crystalline patch in the lipid bilayer plane of the plasma membrane of the bacterium Halobacterium halobium. The structure of bR has been determined to near-atomic resolution of 3.5 Å parallel to the membrane and 10 Å perpendicular to the membrane; each bR molecule consists of a polypeptide chain that traverses the membrane, forming seven folded a-helices as shown in Figure 9.
The detailed mechanism by which bR absorbs light energy and transports protons against an electrochemical gradient is becoming clear. Several features make bR attractive from the standpoint of biomolecular materials development. At low temperatures, bR can function as an optically driven bistable switch. For example, when the light-adapted form of bR, which absorbs at 570 nm, is irradiated with green light at 77 K, it is converted to the stable K form, which absorbs near 630 nm. Recent studies32 using genetic engineering techniques suggest that mutant forms of the protein can be produced that exhibit optical bistability at room temperature.
Laminates and Templates
Biomineralization33 is precisely controlled by complex templating relationships and coordinated secretory processes that are ultimately encoded in genes. During formation of abalone shell, for example, secreted proteins self-assemble into two-dimensional polyanionic b-pleated sheets that serve as templates for the nucleation and epitaxial growth of calcium carbonate crystalline domains (Figure 10). A microlaminate composite is formed that has exceptional regularity, strength, and crystalline ordering. The organizing organic polymers typically contribute less than 1% of the composite material by weight, yet the material's strength and fracture resistance far exceed those of the crystals themselves. The unique properties of biosynthetic microlaminates are due to the organic matrix layers' capacity for flexible deformation and to the retardation of crack propagation at each mineral-organic interface.
The biological templating process that controls the structure of molluscan shell has been mimicked in the formation of sub-micron structures built up on tubules. The cylindrical structure of a tubule is intrinsically interesting as a template. It leads directly to a large degree of shape anisotropy, which can be tuned by adjusting the dimensions of the tubules either by manipulating the molecular structure of the protein or other molecule attached to the substrate or by changing the processing conditions under which the tubules form.
A beautiful example of the use of spontaneously self-assembled mesoscopic ordering to produce new materials is the use of the lyotropic hexagonal phase as a template to make mesoporous solids. These materials have periodic arrays of nanometer-sized pores of unprecedented regularity and density. Mesoporous metallic surface films have also been generated via molecular templating, by employing the self-assembled two-dimensional crystalline protein arrays of bacterial cell walls as patterning elements.
Biomotors
Much of the molecular transport in biological systems occurs not by diffusion but by active transport by biomotors.34 Such systems do not conform to our definition of self-assembly because the biomotors are driven by chemical energy input
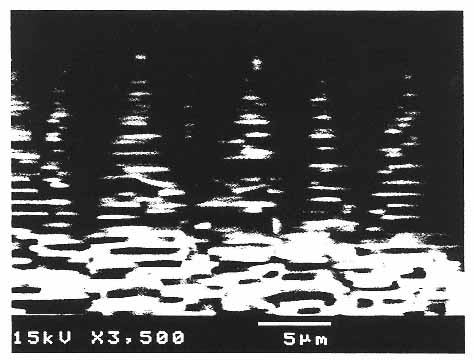
Figure 10
Scanning electron micrograph of the growth front of abalone shell. The aragonite (CaCO3) crystals in the shell form very regular stacking and interdigitating plates. A combination of polyanionic proteins and matrix proteins produces this hierarchical structure, which gives the shell its unique optical properties and its increased strength. (Courtesy of A. Belcher, D. Morse, and G. Stucky, University of California, Santa Barbara.)










