4
Priority Research Questions and Strategies
As is pointed out often in preceding chapters, values for the risk of late effects occurring in humans exposed to external radiation are based almost entirely on epidemiological studies of acute exposure of humans, primarily Japanese atomic bomb survivors, to low-LET radiation. Analysis of these data shows, for example, that there is a lifetime risk per Sievert of 8.0 × 10-2 for excess cancer mortality to an individual in the general population.1 Assessing the risk of effects from low-LET radiation thus consists simply of determining the physical dose associated with a specific scenario and then multiplying the dose by the above factor to obtain the lifetime risk of excess cancer mortality. For types of radiation with differing LET, the dose is converted to a dose equivalent in sieverts and the risk calculated as above. However, the proper conversion factors for HZE particles and the products they generate in passing through shielding are poorly known at present, as chapters above emphasize.
An estimate of the risk of adverse biological effects due to irradiation during a space mission corresponds to a determination of the relevant deterministic and stochastic biological consequences of exposure to radiation as a function of (1) dose, (2) dose rate, and (3) radiation quality as a function of radiation type and of the shielding thickness for each type of spacecraft material, and the uncertainty should be included in that risk estimate.
Below are outlined what the task group believes to be the most important of the research questions and issues that must be addressed in any endeavor to significantly reduce the risk and uncertainty of radiation hazards to the crews of interplanetary missions. The research strategy recommended for addressing each question is narrowly focused on that question and describes the minimum research model likely to provide the necessary data. The development of such a narrow set of strategies should not necessarily be taken as a recommendation to limit the scope of studies to those outlined below. If funds should become available, many of these studies could be usefully expanded to provide additional relevant information.
In accordance with current understanding of the risks and uncertainties, the research questions are separated into higher-and lower-priority groups. As more data become available some questions may shift in priority. Some strategies may be carried out independently, while others will be influenced by the outcome of the other programs and should be scheduled accordingly. The reasoning that forms the basis of these recommendations is discussed in detail in Chapters 2 and 3.
Higher-Priority Research Questions
The higher-priority research questions and issues listed below are followed by the suggested strategy for addressing each question. Research questions that were deemed important but of a lower priority are given in the next section.
- What are the carcinogenic risks following irradiation by protons and HZE particles?
- How do cell killing and induction of chromosomal aberrations vary as a function of the thickness and composition of shielding?
- Are there studies that can be conducted to increase the confidence of extrapolation from rodents to humans of radiation-induced genetic alterations that in turn could enhance similar extrapolations for cancer?
- Does exposure to heavy ions at the level that would occur during deep-space missions of long duration pose a risk to the integrity and function of the central nervous system?
- How can better error analyses be performed of all factors contributing to estimation of risk by a particular method, and what are the types and magnitude of uncertainty associated with each method? What alternate methods for calculation of risk can be used to compare with conventional predictions in order to assess absolute uncertainties? How can these analyses and calculations be used to better determine how the uncertainties in the methods affect estimates of human risks and mission costs?
- How do the selection and design of the space vehicle affect the radiation environment in which the crew has to exist?
- Can solar particle events be predicted with sufficient advance warning to allow crew members to return to the safety of a shielded storm shelter?
Question 1: What are the carcinogenic risks following irradiation by protons and HZE particles?
Answering this key question requires that two related research questions be addressed. First, can the risk due to irradiation by protons in the energy range of the space environment be predicted on the basis of the risk posed by exposure to low-LET radiation; i.e., is it appropriate to assume that the quality factor is 1, and is there evidence for repair of damage in cells following fractionated exposure to protons and HZE particles? Second, what are the appropriate quality factors for making risk calculations with respect to HZE particles?
The answers to these questions are fundamental to understanding the risk of contracting cancer as a result of travel in deep space. Without these answers, it will not be possible to improve the understanding of risk beyond the current state. These important questions can be addressed using solely ground-based studies if appropriate funding and additional radiation resources such as accelerator time are made available.
Initial studies of the effects of exposure to protons should focus on cellular effects that are relevant to cancer. Research with cells would provide a more rapid resolution than would tumor induction studies with animals of whether effects of exposure to high-energy protons are similar to those arising from low-LET radiation. Theoretical models of radiation effects as well as currently available data for cellular and tumorigenic effects of exposure to protons (mostly at energies lower than those encountered in space) would argue that risks due to proton irradiation are similar to those from low-LET irradiation. To determine whether such a prediction is appropriate for higher-energy protons, the task group recommends that a series of studies be conducted in several cellular systems, including human fibroblasts and lymphocytes, to examine the effects of protons in the 1-GeV energy range on cell killing, induction of chromosomal aberrations, and induction of gene mutations. To bridge the gap between in vitro and in vivo results, chromosomal aberrations could also be studied in lymphocytes from animals irradiated in vivo. By conducting such studies with both acute and fractionated exposure regimens, it would be possible to determine whether fractionation effects (sparing of radiation response by allowing for DNA repair between fractions) similar to those for low-LET radiation exist. Animal carcinogenesis experiments with protons should be conducted only if the results of cellular studies indicate discrepancies from the predictions. If, on this basis, tumorigenesis studies are warranted, the same animal models recommended for the study of tumorigenesis following exposure to HZE particles (described below) should be employed. Facilities to conduct proton experiments are available at Brookhaven and Los Alamos national laboratories in the United States (see Appendix C). Lower-energy protons such as those at Loma Linda University Medical Center Proton Therapy Facility are somewhat useful for studies related to solar events. Although considerable data are already available for protons in this energy range, these data are not satisfactory to answer questions related to high-energy protons in the 1-GeV energy range. If animal studies are required, irradiation of sufficient numbers of animals would generally require at least 1 to 1.5 years, while conduct of the animal studies subsequent to completion of the irradiation would require 3 to 4 additional years.
To obtain reliable quality factors (and ultimately better risk coefficients) for HZE particles, a systematic series of studies of RBE-LET relationships for a select number of heavy ions with emphasis on iron particles should be conducted using well-defined animal models for tumorigenesis. Adequately defining these relationships requires that the dose-response relationship be determined for these particles in the dose range below 20 to 30 cGy, because at higher doses of high-LET radiation, the response appears to reach a maximum followed by a decrease. The model systems chosen should be those for which substantial dose-response data for other high-and low-LET radiation are already available. The task group recommends that model systems that are particularly amenable to concomitant cellular and molecular studies be given priority. Given these constraints and the fact that mice have been used more extensively than other mammalian species in studies of carcinogenesis, 2 the task group recommends the murine models for radiation-induced myelogenous leukemia and mammary gland cancer. The conduct of these studies would require considerable commitment of beam time at an appropriate facility. Under the assumption that 3 months of beam time would be available per year, the task group estimated that these studies would take approximately 6 years to complete. This time estimate assumes that irradiation of sufficient numbers of animals would require 2 years. Following irradiation, completion of the animal studies can be expected to require approximately 4 years. Under current conditions, which provide only 2 weeks of beam time each year, it would be almost impossible to complete a meaningful series of animal studies, because the period of time between the first set of animal irradiations and the last would probably be on the order of 6 years (assuming that half of the beam time could be devoted to animal irradiation). This long temporal separation of experimental groups makes comparisons more difficult even with well-defined systems. Under these conditions, completion of the carcinogenesis studies would require a minimum of 10 years after the first irradiation.
Improvements in risk estimates beyond those attained with these data would require a more complete understanding of mechanisms and of principles that will aid in the direct extrapolation of results from experimental systems to astronauts. These kinds of studies will likely require the development and exploration of new model systems and the application of developing technologies in cell and molecular biology.
Question 2: How do cell killing and induction of chromosomal aberration vary as a function of the thickness and composition of shielding?
The data obtained from the answers to question 1 are necessary background for determining the biological effects of the specific radiation qualities and fluences in a spacecraft. The quality and dosimetry of radiation produced from HZE particles traversing shielding of different thicknesses and composition would be assessed from studies that address question 6. The cellular studies should not be initiated for any particular energy (of HZE particle) and shielding until the physical characterization of that radiation is completed.
The task group recommends that at a minimum, studies using cell killing and chromosomal aberrations as end points be conducted using radiation qualities defined in dosimetry studies (question 6). For the sake of relative ease of experimentation, only the effects from acute exposures should be measured. Ground-based HZE particle sources, used with appropriate shielding to simulate in-flight conditions, should be quite suitable for such experiments. In-flight studies would be prohibitively difficult to conduct, and little gain in information would be realized.
It would be appropriate to conduct the initial studies in vitro using the same cell lines used to address question 1, i.e., both rodent and human cell lines. Subsequent cytogenetic studies would need to be conducted in vivo to develop a more appropriate database for use in risk assessment calculations. The task group recommends that bone marrow cells and peripheral lymphocytes, which are easily analyzed cytogenetically, be analyzed for chromosomal aberrations. Based on the information obtained in these cytogenetic studies, it would then be feasible to design a study to assess induction of leukemia and breast cancer in mice exposed, behind shielding, to acute doses of HZE particle radiation incident on the shielding if it appears necessary from the cellular studies. Irradiation for the in vitro studies could be accomplished in a relatively short period of time, i.e., about 2 days for each radiation type and energy. Typically, about 6 months would be required for the analysis. If six radiation types were examined in consecutive order, as might be expected for a single research team, then such a study would require on the order of 3 years. Similarly, the in vivo cytogenetic studies also require about 2 days of irradiation time for each radiation quality. Assuming that the
in vivo studies were performed in parallel with the in vitro studies, then both might reasonably be completed during the 3-year period.
The in vitro studies would allow comparison of animal and human sensitivities to changes in shielding parameters. The in vivo experiments would provide data for in vitro and in vivo comparisons of cytogenic responses for the mouse. This parallelogram approach would provide the chromosomal aberration frequencies induced in humans in vivo (for bone marrow cells and lymphocytes). The mouse cancer studies (leukemia and breast cancer) can then be extrapolated in terms of human tumors assuming the chromosomal aberration sensitivity factors apply. This approach would seem to be particularly reasonable for leukemias, as chromosomal alterations are involved in the etiology of the cancer (see “Experimental Techniques and New Data Required” in Chapter 5).
Question 3: Are there studies that can be conducted to increase the confidence of extrapolation from rodents to humans of radiation-induced genetic alterations that in turn could enhance similar extrapolations for cancer?
The studies recommended for addressing question 2 would give relative sensitivity factors for mutations, chromosomal aberrations, and cell killing in rodent and human cells in vitro, and the in vivo cytogenetic studies would allow comparison of in vitro and in vivo responses in a single species, most likely the mouse. The sensitivity factors and other comparative data could then be used to provide an estimate of responses mentioned above in humans using the cancer induction data obtained in rodents. However, the reliability of the use of a relative sensitivity factor must first be established. Chromosomal aberration and mutation frequencies induced by exposure to radiation are influenced to a great extent by the kinetics and fidelity of DNA repair processes. Therefore, a secondary measure of relative sensitivity pertinent to cancer risk assessment would be a comparison of the features of DNA repair in human and rodent cells in vitro following acute exposure to protons and HZE particles. Techniques based on pulsed field gel electrophoresis have been developed that can measure DNA strand breaks at very low exposure levels (<10 cGy). These experiments would require a minimum of 1 day of irradiation time for each radiation quality studied. A typical analysis of chromosomes would consume about 2 months for each particle type: therefore a 12-month study could be reasonably estimated for an examination of six particles, if beam time were readily available. If these experiments had to compete with other high-priority items for beam time within the total of ~100 hr/yr currently available, they would probably extend over 3 years.
Question 4: Does exposure to heavy ions at the level that would occur during deep-space missions of long duration pose a risk to the integrity and function of the central nervous system?
A multifaceted research approach is required to answer this question so as to relate molecular changes to alterations in functions. Considering that some of the experiments could take a long time and that a few definitive answers must be obtained before final decisions about shielding and mission planning can be made, it is essential to ensure coordination of the strategy for this field of research. The studies range from induction of DNA damage, repair, and maintenance of the fidelity of DNA into old age to studies of the heavy-ion induced morphological and functional changes outlined in the Chapter 2 section titled “Central Nervous System.” The time taken would vary from about 2 years for the DNA studies to perhaps 10 or more years for studies of functional changes, depending on the species required for a definitive assessment.
The scope of the research should be agreed upon by representatives of the disciplines that should be involved, including both experimental and clinical neurologists. One essential study that could be started now is confirmation of the findings of Lett et al. on retinal cells, that late breakdown of DNA exposed to heavy ions occurs and that age at exposure is important.3 New sensitive techniques for assessing DNA damage can be applied to the problem and also to the determination of the dose-response relationships and the influence of LET.
The studies described for this question could not be performed at all with only 2 wk/yr of beam time. If 3 mo/yr were available, then experience with similar studies4 suggests a rough time estimate for the performance of all the required studies of 5 to 7 years because of the long time interval required to observe late
effects. This estimate is based on the assumption that sufficient animal facilities and staff will be available at the beam site and that rodents will be used as the animal model. For rodents, a post-irradiation period of around 3 years could be needed to observe the onset of possible late effects. If rodents are not used, then another way to complete these studies in the time frame proposed might be to repeat, confirm, and extend the work of Lett et al. A minimum of three ions with a spread of LET values would need to be examined in order to answer question 4. Iron should be one of the ions selected.
Question 5: How can better error analyses be performed of all factors contributing to estimation of risk by a particular method, and what are the types and magnitude of uncertainty associated with each method? What alternate methods for calculation of risk can be used to compare with conventional predictions in order to assess absolute uncertainties? How can these methods be used to better determine how the uncertainties in the methods affect estimates of risk to humans and mission costs?
The relative significance of uncertainties in risk assessments must be adequately established and the impact of reductions in the level of these various uncertainties must be determined.
The conventional approach for the assessment of risks is initially to calculate a dose, defined as the equivalent dose for the radiation field of interest corresponding to the dose of low-LET radiation that produces the same level of risk. The simplest method for obtaining the equivalent dose is to multiply the physical dose by a quality factor for the radiation field, but there are several other approaches, including models for normal-tissue responses, microdosimetric methods, and fluence-based techniques. In any of these cases, there is uncertainty associated with the method itself and additional uncertainty associated with each of the input quantities used to calculate that risk. In the former case, each of the quantities, such as the physical dose or quality factors, needed as input to establish the risk has a level of uncertainty associated with it. Reductions in the uncertainties in the values of the specific input quantities have differing effects on the magnitude of the uncertainty of the total risk, depending on the method chosen. For example, in the conventional approach, the squares of the fractional uncertainties in the absolute physical doses and in the quality factors will contribute additively to the total uncertainty irrespective of the absolute values of the two quantities if they are two independent quantities. In such a situation, the larger fractional contribution will dominate the total uncertainty. In contrast, for an extrapolation of effects at low doses or low dose rates with a linear-quadratic model, the squares of the absolute uncertainties, rather than the fractional uncertainties in dose and quality factor, contribute additively. Currently, the lack of knowledge concerning the uncertainties in the values of the quantities needed to assess risks is a major limitation in establishing realistic design requirements for a planetary mission.
In addition to the uncertainties in the values of the input quantities, there is an intrinsic uncertainty associated with the method used. Recognizing that use of only one method with a possible large uncertainty is at best questionable, the task group recommends that risk estimates be determined by different independent methods as a means of determining the overall uncertainty from input quantities and methods. The results of an error analysis (i.e., an analysis of the relative and absolute uncertainties) should be used to evaluate which methods will most effectively reduce the uncertainties in risk estimates and, therefore, uncertainties in cost of shielding. It would be useful if this analysis were preceded by a review of the improvements that have occurred in the physical data and theoretical methods now available compared to those available approximately 1 decade ago.
An analysis of the uncertainties in risk based on present data and methods could be achieved within about 1 year with proper support. Such analyses, however, should be updated routinely as part of a continuing effort throughout the entire project, and all investigators should be required to provide error analyses of their results.
Care should be exercised to distinguish between uncertainties in the input data owing to lack of knowledge and variability in the input data owing to fluctuations in the data themselves. For example, a lack of knowledge of cross sections for producing secondary nuclear particles in the materials used to construct a spacecraft represents a source of uncertainty that might be reduced, with a consequent potential for cost savings. However, the variability in the types and energies of the incident particles resulting from variation in the number and quality of solar events is not representative of an error in the input data used to calculate
risk. In this latter case, there is little, if anything, that can be done to reduce the variability; the best that should be anticipated is a reduction of the uncertainty in the prediction of the variability.
Question 6: How do the selection and design of the space vehicle affect the radiation environment in which the crew has to exist?
The answer to this question is based in part on an accurate knowledge of the incident radiation field, the reaction cross sections for the incident particles reacting with the vehicle materials, and the fragmentation/recoil products that such reactions produce. Current knowledge of the fragmentation products produced by HZE particles is limited to only a few particles in a few materials. For knowledgeable shielding design, the initial radiation fields, the reaction probabilities, and the secondary particles produced as a function of angle must be determined through physical measurements, at a HZE particle accelerator, of the particle types and energies resulting behind different compositions and thicknesses of shielding.
Based on the predictions of current transport codes, hydrogenous materials are preferred for shielding because they offer better shielding than other materials on a per unit mass basis (see Figure 2.5). To properly assess the accuracy of these predictions, the transport codes used to calculate the shielding efficiency have to be benchmarked against measured data for elemental (Al, Fe, etc.) and composite shields.
Complementary measurements should be made with a microdosimetric detector of the type currently being flown in space. The absorbed dose as a function of depth should likewise be measured along the axis of the beam at selected positions along the axial plane. The measured data should be compared with predictions by the Langley Research Center transport code and/or a Monte Carlo transport code. Similar measurements as a function of depth should be made for the simplest possible geometry in space and these results compared with calculations of the dose, the radiation quality, and the particle spectra.
Engineering of the storage for a spacecraft's supplies so as to form an enhanced “storm shelter” against transient high levels of radiation would be subject to the same verification of the accuracy of data and calculations. At the current level of availability of heavy ion particle accelerator time, the task group estimates (see “Knowledge Base Development” in Chapter 3) that more than 10 years will be required to collect the necessary data. With increased availability of accelerator time and other resources, data collection and analysis could be compressed into a time frame of about 4 years.
Question 7: Can solar particle events be predicted with sufficient advance warning to allow crew members to return to the safety of a shielded storm shelter?
The ability to predict the time of occurrence and/or magnitude of solar particle events (SPEs) is currently an inexact science at best. Protecting a mission crew from SPE radiation requires improving the capability to accurately predict solar events. This effort requires that information on the status of the total solar surface be continually available. One mechanism for accomplishing this would be a series of space platform monitoring stations. Given the necessary information on the status of the sun's surface, the science and models that interpret these data must be enhanced with the goal of achieving accurate forecasts 8 hours in advance of a spacecraft encountering a SPE. Prediction of the resources and time required to reach this state of capability is beyond the expertise of the authors of this report. However, the capability to predict solar events 8 hours in advance of their occurrence is thought to be an operational requirement for a safe interplanetary mission.
Lower-Priority Research Questions
- What are the risks of reduced fertility and sterility as a result of exposure to radiation on missions of long duration in deep space?
- What are the risks of clinically significant cataracts being induced by exposure to radiation at the levels that will occur on extended spaceflights?
- Can drugs be used to protect against the acute or carcinogenic effects of exposure to radiation in space?
- Is there an assay that can provide information on an individual's sensitivity to radiation-induced mutagenicity and that can be predictive of a predisposition for susceptibility to cancer?
- Are there differences in biological response arising from exposure to particles with similar LET, but with different atomic numbers and energies?
Question 1: What are the risks of reduced fertility and sterility as a result of exposure to radiation on missions of long duration in deep space?
- Female: Studies of women receiving pelvic and abdominal radiotherapy in which there is good dosimetry could provide useful information on the effects of radiation on ovarian function. It is probable that prospective studies of women treated with cytotoxic drugs at young enough ages in whom ovarian function is compromised could provide valuable information when combined with modeling. Complementary studies of both normal and radiation-induced loss of ovarian follicles, preferably in a nonhuman primate, will be required.
- Male: An assessment of the effect of dose rate and protraction of radiation on spermatogenesis is essential. The study should be carried out on a primate, but studies previously done on other mammals could also be extended to include low dose rate or fractionated proton exposures. Sperm counts are an easy and economical assay of the effect of exposure. However, histological studies of the testes are required, especially in cases of azoospermia (total loss of sperm). The stem cells may not be the most sensitive target, because loss of the ability of the supporting tissue to enable differentiation in the spermatogenic process may determine the probability of sterility. Paracrine mechanisms, which release locally acting substances from cells directly into intracellular space, are involved in the differentiation process, but little is known about either their role or the effects of radiation on them. Studies of men receiving cyclophosphamide could provide some help in the comparison of the relative effects of acute and chronic administration of radiation doses on sperm production.
To improve understanding of the effects of radiation on fertility, pragmatic studies of the loss of ova or sperm and studies of the basic aspects of ovarian function or spermatogenesis should be carried out hand in hand. As much clinical data as can be obtained and are relevant should be collected, and priority should be given to animal experiments designed to answer the questions that cannot be answered by research on humans. Ideally, the studies should include the effects of repeated exposure to protons and heavy ions at low fluences. However, protracted exposure to gamma rays may be the most practical approach, and gamma rays should be an adequate surrogate for protons. Since there are several sources available for both gamma rays and protons, beam time is probably not a limiting factor for conducting this study. If the group conducting the study were co-located with the source, and the appropriate support staff and animal care facilities were available, then such a study might be completed in as little as 4 years. Currently, however, such a resource does not exist.
Question 2: What are the risks of clinically significant cataracts being induced by exposure to radiation at the levels that will occur on extended spaceflights?
A considerable body of data provides information about the induction of cataracts in different species by different types of radiation. There is, however, no consensus on how to collate the data and use it to estimate risk to humans. This objective, however, appears to be within reach and should be pursued. Another approach is to determine experimentally the relationship of RBE for cataractogenesis to LET, and to apply the RBE value to the data for induction of cataracts in humans by low-LET radiation. A better understanding is needed of the effects of protracted exposure at low-dose-rates for both low-and high-LET radiation, because the data currently available for humans are for high-dose-rate low-LET radiation.
As research efforts are already under way on atomic bomb survivors (who were exposed to low-LET radiation), the results of which could readily be applied to question 2, the most cost-and time-effective approach to this issue would be to ensure that current work on the of protons, the most prevalent particles in galactic cosmic rays, survivors receives continued support.5 The cataractogenic effects can be estimated
directly with reasonable confidence from data on the effects of low-LET radiation. Moreover, estimating the risk from exposure to HZE particles by any of the methods suggested so far in this report depends on the use of data obtained from humans exposed to low-LET radiation, and the major source of that data is the atomic bomb survivors. Under these circumstances a sufficient answer to the question of the magnitude of the risk for cataractogenesis owing to long-duration spaceflight might be obtained in a time frame of 4 or 5 years.
Question 3: Can drugs be used to protect against the acute or carcinogenic effects of exposure to radiation in space?
A program to develop drugs capable of protecting humans against the acute toxic effects of radiation has been conducted for many years under the auspices of the Department of Defense. These efforts have yielded a number of drugs that are moderately protective against the effects of low-LET radiation because they are free-radical scavengers. Such scavengers are relatively less effective against high-LET radiation because ionizations are produced more frequently as a result of direct effects rather than indirectly through the products of water radiolysis. At the present time, the effectiveness of such agents against acute high-dose exposure to protons, such as might be experienced during an SPE, is not known. Studies should be conducted in animal models to determine the efficacy of single doses of such drugs in protecting against the damaging effects of protons, similar to those associated with an SPE, on blood-forming cells.
More recently, studies have suggested that agents related to the compound WR-2721 may be efficacious at relatively low doses in protecting against the mutagenic and carcinogenic effects of radiation through a mechanism independent of the drug's activity as a free-radical scavenger. The task group recommends that studies be pursued to determine whether such protective effects can be obtained after exposure to HZE particles. Such studies could concentrate on radiation-induced somatic cell mutagenesis, since these effects are likely to be reasonably predictive of protective effects observed for carcinogenesis in animals. Additional mechanistic studies would allow the possibility of the development of more effective and less toxic agents that might be useful for protection against late effects associated with doses resulting from SPEs. It is unlikely that a strategy of daily doses of such agents is warranted as a means of modifying the risks from daily exposure to cosmic radiation, given the relatively low risks associated with exposure at these levels.
These studies would require access to appropriate facilities for irradiation with HZE particles. Under current conditions (2 weeks' available beam time per year), it is estimated that such studies would require approximately 4 years to complete, assuming that cells for these studies could be “piggybacked” with other cellular studies. Under more ideal conditions, with 3 months of beam time available each year, these studies would require approximately 2 years to complete.
Question 4: Is there an assay that can provide information on an individual's sensitivity to radiation-induced mutagenicity and that can be predictive of a predisposition for susceptibility to cancer?
For at least 10 years, Sanford and colleagues have reported on the use of a G2 chromatid aberration assay for detecting individuals with a predisposition for cancer.6–8 In this assay, human lymphocytes or cultured skin fibroblasts are irradiated with x rays and metaphase cells arrested with colcemid between 0.5 and 1.5 hours after exposure. The analysis of chromatid aberrations gives a measure of chromosomal damage that was induced in G2; a comparison of this aberration frequency with that for metaphase cells collected in the first 0.5 hours after exposure provides an estimate of DNA repair capacity. It has been reported that individuals designated as cancer prone, irrespective of the tumor type, show an enhanced frequency of aberrations and a reduced repair capacity. Attempts to duplicate the assay in other laboratories have proven to be unsuccessful.9 Scott et al., for example, found no difference in sensitivity between controls and lymphocytes from individuals who were homozygous or heterozygous for xeroderma pigmentosum (which is a DNA repair deficiency disease), who had familial adenomatous polyposis, or who had the syndromes Li-Fraumeni, basal cell nevus, Down, or Fanconi.10 They were able to show an enhancement with ataxia telangiectasia homozygotes, a very predictable result. They report that preliminary studies using their own G2 sensitivity assay are giving promising results.11 In this assay, the x-ray dose is 0.5 Gy (vs. 1.4 Gy, used by Sanford et al.), cells are not centrifuged prior to irradiation, and cells are harvested at ice temperature. It
remains to be determined if this modified assay can detect all individuals with a cancer predisposition, or at least a predisposition in specific cases. To validate the assay, it is also of considerable importance that it be conducted in several different laboratories, and that an extensive sample from the general population be assessed in order to obtain an estimate of the range of sensitivities. Since the assay can be validated with low-LET x or gamma rays, no beam time is required. The analysis of at least 100 individuals in the general population and at least 10 cancer-prone families (in several laboratories simultaneously) would take approximately 2 years to complete.
Question 5: Are there differences in biological response arising from exposure to particles with similar LET, but with different atomic numbers and energies?
There is experimental evidence to suggest that differences in both the energy and track structure of particles may lead to differing biological effects of exposure to radiation that are independent of LET.12–14 The differences in observed RBE generally have been in the range of 2 to 3. However, the available data are derived from various sources that utilize different models and experimental conditions, thus making comparison among them difficult. Carefully designed experiments should be carried out under controlled dosimetric conditions such that the effect of factors such as atomic number, track structure, and energy can be specifically compared in the same system. It would seem reasonable to employ well-defined experimental systems such as those proposed to address higher-priority questions 1 and 2, for which substantial data for various types of radiation are already available. These would include cellular systems to examine effects on cell killing, mutagenesis, and chromosomal aberrations. If the differences observed are restricted to a factor of 2 to 3 or less, as predicted from the currently available data, conducting additional experiments in animal models for tumorigenesis would not be warranted.
Based on the assumptions that the appropriate heavy ions are available, that a dedicated facility is used to minimize tuning time, and that 2 wk/yr of beam time are set aside for this strategy, the in vitro experiments could be completed in 2 years, particularly if they were carried out in parallel with those intended to address higher-priority question 2. This estimate is based on the use of three biological end points and three different LET ranges, with three particles in each LET range. Since it is doubtful, however, that the necessary level of resources would be reserved for lower-priority projects such as this, an estimate of 3 years is probably more realistic at current levels of availability for HZE particle accelerator time.
If the annual available beam time were increased to 3 months, then it should still be possible to carry out this strategy in 2 years.
Time Scale of Research.
In order to carry out the research necessary to reduce the physical and biological uncertainties inherent in estimating risk and to design shielding to protect against a credible maximum risk, approximately 3,000 hours of beam time are required for experiments with HZE particles and energetic protons. At the present utilization rate of approximately 100 hr/yr at the Brookhaven Alternating Gradient Synchrotron, the research could take over 20 years—an unacceptably long time.
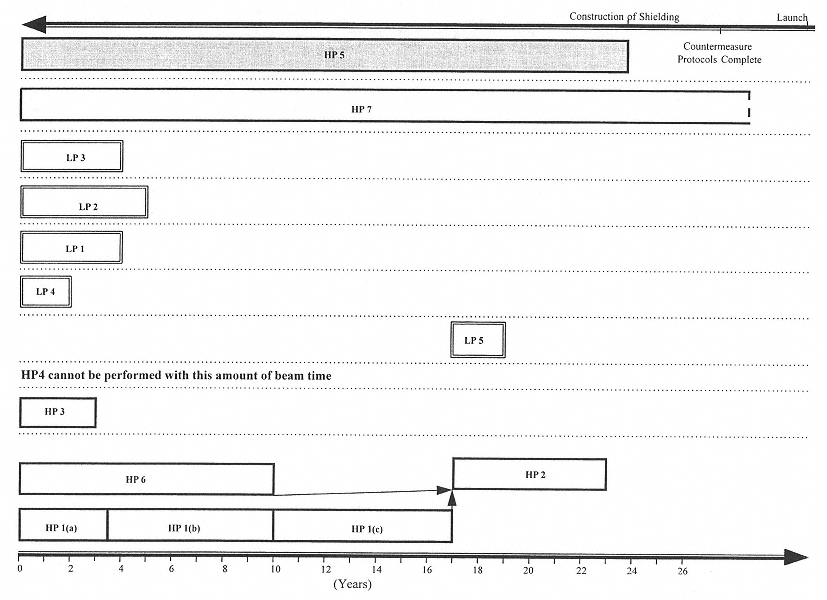
Figures 4.1 and 4.2 show potential research time lines based on the assumption that the currently available beam time at a heavy ion accelerator of about 2 wk/yr (Figure 4.1) remains unchanged or that 3 mo/yr becomes available (Figure 4.2).
The task group recommends that, if the goal of safe interplanetary missions with human crews is sought, NASA explore various possibilities, including the construction of new facilities, to increase the research time available for experiments with HZE particles.
FIGURES 4.1 and 4.2 (pp. 52–53) HP indicates strategies to address higher-priority research questions 1 through 7 and LP indicates strategies to address lower-priority research questions 1 through 5, given 2 weeks of beam time per year (Figure 4.1) and 3 months of beam time per year (Figure 4.2), respectively. In both figures, the bottom axis indicates the estimated amount of time required to carry out the various strategies; the top axis indicates the general dependence of the mission time line on the research. Strategies that can be carried out independently are separated by dotted lines. The length of the time bar associated with each strategy is based on the approximate amount of time estimated for the required research except in the case of HP 5 and HP 7. HP 5 (the shaded box) is not a laboratory research strategy, but rather a computational methodology, and the amount of time reserved for it may be flexible. It is therefore set to end when the construction of shielding begins. The amount of time devoted to HP 7 will depend on the time available between the initiation of research and flight. Since HP 1(b) utilizes protons and therefore does not require a heavy ion accelerator, it is not affected by the given restrictions on beam time.
KEY: 1(a), cell studies; 1(b), animal carcinogenesis studies with protons; and 1(c), RBE-LET relationship studies.
What Will Still Remain Unknown, and What Risk Does This Represent?
The benefits gained from pursuing these strategies will be not only a reduction by a factor of 2 or more in the uncertainty in estimates of the risk of late effects for crew members exposed to radiation in space, but also greater understanding of CNS and other effects about which little is currently known. These benefits will result in a narrowing of the scope of the types and designs of shielding that need to be considered for crew protection, and thus should result in a significant cost savings. The liability of following these strategies is that the time required to complete them may delay a decision on shielding design and consideration of any near-term (within 25 years) launch dates if suitable resources are not made available to complete the research expeditiously.
Since these research strategies are narrowly focused and based entirely on current understanding of space radiation issues, there is also no guarantee that this approach will necessarily address all of the significant radiation hazards for crews of deep-space missions. Utilizing a wider range of radiation and biological models could lead to recognition of previously unappreciated hazards for those crews and reveal useful new avenues of research.
References
1. National Council on Radiation Protection and Measurements (NCRP). 1989. Guidance on Radiation Received in Space Activities. Recommendations of the National Council on Radiation Protection and Measurements. NCRP Report No. 98. National Council on Radiation Protection and Measurements, Bethesda, Md. See also Board on Radiation Effects Research, National Research Council. 1990. Health Effects of Exposure to Low Levels of Ionizing Radiation: BIER V. National Academy Press, Washington, D.C.
2. Fry, R.J.M. 1981. Experimental radiation carcinogenesis: What have we learned? Radiat. Res. 87:224–239.
3. Lett, J. T., Keng, P.C., Bergtold, D.S., and Howard, J. 1987. Effects of heavy ions on rabbit tissues: Induction of DNA strand breaks in retinal photoreceptor cells by high doses of radiation. Radiat. Environ. Biophys. 26: 23–36.
4. Lett, J.T., Cox, A.B., Keng, P.C., Lee, A.C., Su, C.M., and Bergtold, D.S. 1980. Late degeneration in rabbit tissues after irradiation by heavy ions. Pp. 131–142 in: Life Sciences and Space Research, Volume XVIII (R. Holmquist, ed.). Pergamon Press, Oxford. See also Lett et al., 1987, Effects of heavy ions on rabbit tissues; and Williams, G.R., and Lett, J.T. 1995. Damage to the photoreceptor cells of the rabbit retina from 56Fe ions: Effect of age at exposure. Adv. Space Res. 18: 55–58.
5. Wu, B., Medvedovsky, C., and Worgul, B.V. 1994. Non-subjective cataract analysis and its application in space radiation risk assessment. Adv. Space Res. 14: 493–500.
6. Parshad, R., Sanford, K.K., and Jones, G.M. 1983. Chromatid damage after G2 phase X-irradiation of cells from cancer-prone individuals implicates deficiency in DNA repair. Proc. Natl. Acad. Sci. U.S.A. 80: 5612–5616.
7. Sanford, K.K., Parshad, R., Gantt, R., Tarone, R.E., Jones, G.M., and Price, F.M. 1989. Factors affecting and significance of G2 chromatin radiosensitivity in predisposition to cancer. Int. J. Radiat. Biol. 55: 963–981.
8. Parshad, R., Price, F.M., Pirollo, K.F., Chang, E.H., and Sanford, K.K. 1993. Cytogenetic response to G2-phase X-irradiation in relation to DNA repair and radiosensitivity in a cancer-prone family with Li-Fraumeni syndrome. Radiat. Res. 136: 236–240.
9. Bender, M.A., Viola, M.V., Riore, J., Thompson, M.H., and Leonard, R.C. 1988. Normal G2 chromosomal radiosensitivity and cell survival in the cancer family syndrome. Cancer Res. 48: 2579–2584.
10. Scott, D., Spreadborough, A.R., Jones, L.A., Robert, S.A., and Moor, C.J. 1996. Chromosomal radiosensitivity in G2-phase lymphocytes as an indicator of cancer predisposition. Radiat. Res. 145:3–16.
11. Scott, D., Spreadborough, A., Levine, E., and Roberts, S.A. 1994. Genetic predisposition in breast cancer. Lancet 344: 1444.
12. Kranert, T., Schneider, E., and Kiefer, J. 1990. Mutation induction in V79 Chinese hamster cells by very heavy ions. Int. J. Radiat. Biol. 58: 975–987.
13. Belli, M., et al. 1993. Inactivation and mutation induction in V79 cells by low energy protons: Re-evaluation of the results at the LNL facility. Int. J. Radiat. Biol. 63: 331–337.
14. Stoll, U., Schmidt, A., Schneider, E., and Kiefer, J. 1995. Killing and mutation of Chinese hamster V79 cells exposed to accelerated oxygen and neon ions. Radiat. Res. 142: 288–294.