5
Hexachloroethane Smoke
BACKGROUND INFORMATION
The toxicity of hexachloroethane (HCE) smoke (referred to as HC smoke)1 is attributed to the production of zinc chloride (ZnCl2). Karlsson et al. (1991) compared acute inhalation exposures of HC smoke generated with zinc oxide (ZnO) with those generated with titanium dioxide (TiO2). TiO2-HCE smoke proved to be far less toxic than ZnO-HCE smoke, and ZnO-HCE smoke was lethal, causing gross pathological pulmonary injuries and death due to pulmonary edema. These authors also compared exposures to titanium tetrachloride (TiCl4) gas with exposures to ZnCl2 aerosol. No animals died from exposure to TiCl4 at concentrations up to 2,900 mg/m3 for 10 min, whereas the LC50 for ZnCl2 was 2,000 mg/m3 for a 10-min exposure.
Most reports of accidental human exposures to HC smoke indicate symptoms consistent with exposures to the ZnCl2 component released when the smoke bomb is ignited. Therefore, the exposure-response assessments for HC smoke are probably most reliably interpreted, given present data, on the basis of the exposure-response data for ZnCl2.
Military Applications
HC smoke is used by the U.S. military in a wide variety of munitions, some of which are shown in Table 5-1. HC smoke is produced by burning a mixture containing roughly equal parts of HCE and ZnO and approximately 6% granular aluminum.
Combustion Products
The smoke mixture in a smoke bomb or grenade is initially ignited by a pyrotechnic starter mixture. The reaction is self-perpetuating and exothermic. The overall reaction was summarized by Cichowicz (1983):
Another reaction produces carbon monoxide instead of solid carbon. ZnCl2 leaves the reaction zone as a hot vapor. On cooling below the condensation point, it nucleates to form an aerosol that rapidly absorbs water from the surrounding atmosphere. Hydrated ZnCl2 particles then scatter light, thereby obscuring vision. Because of ZnCl2's affinity for water, the aerosol likely consists of the hydrated forms of ZnCl2 under most atmospheric conditions (Katz et al. 1980). A starter mixture containing silicon, potassium nitrate, charcoal, iron oxide, granular aluminum, cellulose nitrate, and acetone, which is required to initiate the reaction, might generate very small amounts of other airborne contaminants. However, the acute toxic effects of exposure to HC smoke are considered to arise primarily from inhalation of the ZnCl2 component, which comprises almost two thirds of the total mass of HC smoke (Table 5-2). All measurements of HC smoke are expressed in this chapter as milligrams of ZnCl2, unless noted otherwise.
The munitions listed in Table 5-1 all use slightly different chemical mixtures (Novak et al. 1987). An analysis of trace materials
TABLE 5-1 Characteristics of HC Smoke Munitions
|
Smoke-Pot Munitionsa |
Container Size (in.) |
Filling Weight (lb) |
Ignition Method |
Weight (lb) (approx.) with Fuse |
Delay Time (sec) |
Burning Time (min) |
|
Smoke pot, HCE, 10-lb, M1 |
9 by 5.5 diameter |
10 |
Matchhead and scratcher block or electrical |
12.5 |
10 |
5-8 |
|
Smoke pot, HCE, 30-lb, ABC-M5 |
9.5 by 8.5 diameter |
31 |
Matchhead and scratcher block or electrical |
33 |
20-30 |
2-22 |
|
Smoke pot, floating, HCE, M4A2 |
13 by 12 diameter |
27.5 |
M207A1 smoke-pot fuse |
38 |
10-20 |
10-15 |
|
Smoke grenade, HCE, M8 |
4.75 by 2.5 diameter |
1.2 |
M201A1 fuse |
1.5 |
0.7-2b |
1.7-2.5 |
|
Cartridge,c 105-mm, HCE, M84A1 |
|
12.3 |
Mechanical, time, and super-quick fuse |
13.0 |
60-90 |
3 |
|
Projectile,d 155-mm, HCE, M116A1 |
|
25.8 |
Mechanical, time, and super-quick fuse |
26.2 |
60-90 |
4 |
|
a All HC smokes are type C, which contains granular aluminum, hexachloroethane, and zinc oxide. Other types of HC smoke were used in the early years of smoke generation. b Time to functioning after release of safety lever. c No future production for the M84A1 was planned as of 1983. d M116A1 was completing its production life cycle in 1983 and would be replaced by XM 825 white phosphorus fill. Source: Cichowicz (1983). |
||||||
TABLE 5-2 Approximate Composition of HC Smokea
|
Constituent |
Estimated Mass Fraction, % |
|
Zinc chloride |
62.5 |
|
Zinc oxide |
9.6 |
|
Iron oxideb |
10.7 |
|
Aluminum oxideb |
5.4 |
|
Lead oxideb |
1.0 |
|
Total particulate phase |
89.2 |
|
Chlorinated vapors |
10.8 |
|
a The analysis does not take into account any liquid water that associates with ZnCl2. b These metals were assumed to be present as the oxide for purposes of calculating the mass fraction. Source: DeVaull et al. (1989) |
|
in HC smoke mixtures found common zinc impurities (Katz et al., 1980). Arsenic ranged from 0.13 to 5.0 microgram per gram (µg/g), mercury from 0.35 to 0.60 µg/g, cadmium from 53 to 1,523 µg/g, and lead from 50 to 858 µg/g. The cadmium and lead concentrations displayed a strong negative correlation.
Trace gas-phase products were measured in a field test of a standard M5-HCE 30-lb smoke pot (Katz et al. 1980). Table 5-3 shows the resulting gas-phase products at two distances from the pot. Laboratory tests showed that hydrogen chloride (HCl) vapor formation decreased with increasing relative humidity (Katz et al. 1980). Because the field test was performed at -2°C, humidity was probably low. Thus, HCl vapor concentrations shown in Table 5-3 could be much higher than those produced under more humid conditions. However, Katz et al. (1980) speculated that under humid conditions, HCl is absorbed from the vapor phase into ZnCl2 and water aerosol particles. Therefore, with increasing humidity, exposure of respiratory tissue to HCl might shift to lower portions of the lung, because small aerosol particles can penetrate to the lower lung and vapor can be removed readily from incoming air in the upper airways. During four field tests, estimated chlorine (Cl2) production ranged from 3 to 19 mg/g of mixture combusted.
Aerosol formation was studied in a chamber using scaled-down HC smoke pots (Katz et al. 1980). The mass-median and the count-mean diameters produced were approximately 0.4 µm and 0.3 µm, respectively, averaged over 29 experiments. The observed size distribution was log-normal at lower initial particle concentrations (83 × 106 particles per cubic centimeter) and multimodal at higher concentrations. Relative humidity had no consistent effect on the total particulate concentration or the particle size. As the aerosols aged over a 2-hr period, the mass-median and count-mean diameters nearly doubled as the particle concentration decreased by a factor of about 6.
Laboratory-produced HC smoke consisted primarily of Zn+2 and Cl-1 (Katz et al. 1980). The aluminum content ranged from 0.49% to 4.06% of the Zn content, with a mean of 1.79%. The lead content ranged from 0.13 to 2.2 µg/mg of Zn, and the cadmium content ranged from 0.18 to 5.0 µg/mg of Zn. The ratios of both lead and cadmium to zinc were slightly higher than the ratios in the unburned mixture and were well correlated with them.
Physical and Chemical Properties of Zinc Chloride
|
CAS no.: |
7646-85-7 |
|
Molecular formula: |
ZnCl2 |
|
Molecular weight: |
136.29 |
|
Chemical name: |
Zinc chloride |
|
Synonyms: |
Butter of zinc, zinc butter, zinc |
|
Physical state: |
Solid |
|
Melting point: |
290°C |
|
Boiling point: |
732°C |
|
Density: |
2.907 at 25°C |
|
Vapor pressure: |
1 mm Hg at 428°C |
|
Solubility: |
4.32 × 106 mg/L at 25°C 6.15 × 106 mg/L at 100°C 1 g/1.3 mL ethyl alcohol 1 g/2 mL glycerol 1 g/0.25 mL 2% hydrochloroacetic acid |
TABLE 5-3 Chemical Analysis of Vapor Reaction Products from Field Test of 30-lb Military HC Smoke Pot
|
Distance from Mount to Pot (cm) |
CO (ppm) |
HCl (ppm) |
COCl2 (ppm) |
CCl4 (ppm) |
C2Cl4 (ppm) |
C2Cl6 (ppm) |
C6Cl6 (ppm) |
|
≈ 15 |
<1 |
1128 |
30 |
33 |
36 |
nd |
nd |
|
≈ 15 |
<1 |
1958 |
16 |
8 |
9 |
nd |
nd |
|
≈ 15 |
<1 |
5693 |
30 |
57 |
192 |
40 |
103 |
|
≈ 15 |
<1 |
6822 |
20 |
36 |
81 |
40 |
95 |
|
≈ 200 |
<1 |
1137 |
1 |
1 |
2 |
nd |
nd |
|
Abbreviation: nd, not determined. Source: Katz et al. (1980). |
|||||||
Occurrence and Use
ZnCl2 is used in preserving wood and in the manufacture and dyeing of fabrics. In addition to its use in military obscurants, ZnCl2 is also the major ingredient in smoke from smoke bombs used for crowd dispersal and in fire-fighting exercises (by both military and civilian communities) (ASTDR 1994). ZnCl2 also has uses in dental, medical, and household applications, as well as in herbicides (ATSDR 1994).
Military Exposures
Inhalation is expected to be the most important route of exposure. Undoubtedly excessive exposure has occurred in the military. Hill and Wasti (1978) summarized case reports of accidental exposures, many of which were fatal. The fatal exposures resulted from the discharge of HC smoke devices in enclosed spaces. The exposures in these reported cases generally are poorly characterized and represent only the most extreme conditions.
Very few data on HC smoke exposure are available for typical atmospheric conditions. Young et al. (1989) collected air samples during 1-hr demonstrations of M5 smoke pots and M8 smoke grenades at the U.S. Army Chemical School. Cadre members ignited these devices and students remained upwind. Personal and area samples were collected using mixed-cellulose-ester filters and high-flow personal-sampling pumps. Also, the particle-size distribution was characterized using cascade impactors. Zinc in the samples was measured by atomic absorption spectroscopy. Exposures to zinc for three cadre members were 0.0375, 0.0652, and 0.0776 mg/m3 , or 0.0781, 0.1358, and 0.1616 mg/m3 as ZnCl2. The mass-median diameters of the particles ranged from 0.4 to 2.8 µm. Thus, a large portion of the particulate mass was respirable.
Simulated combat training during a military operation on urban terrain (MOUT) exercise indicated that trainees and instructors are exposed to ZnCl2 in concentrations ranging from 0.02 to 0.98 mg/m3 during a 225-min period. The average exposure
concentration was 0.26 mg/m3 with a standard deviation of 0.26 mg/m3 (or 59 mg•min/m3 over a 225-min exposure) (Young 1992, as cited in Lundy and Eaton 1994).
The most extensive field study of HC smoke, reported by DeVaull et al. (1989), shows the average smoke composition observed over five experiments. Composition and sampling location varied from test to test. In these tests, the total weight of smoke released ranged from 218.5 to 229.3 kg for groups of 18 to 20 M5 smoke pots. The particle mass-median diameters ranged from 0.77 to 1.05 µm, with geometric standard deviations from 1.78 to 2.36. Those particle sizes generally agree with those found by Katz et al. (1980) for aged aerosol and by Young et al. (1989), confirming that the aerosol has a large respirable mass fraction. DeVaull et al. (1989) also measured four specific chlorinated organic compounds. The geometric mean ratios of tetrachloromethane, tetrachloroethylene, hexachloroethane, and hexachlorobenzene to zinc in HC smoke were found to be 0.014, 0.009, 0.010, and 0.030, respectively.
A computer simulation of exposure to smoke released from 41 M5 smoke pots was carried out for a wind speed of 6 m/sec using the HAZARD2 program (Cichowicz 1983). The roughly rectangular area with exposures expected to exceed 60,000 mg•min/m3 was 1,400 m wide in the cross-wind direction and about 1,000 m downwind of the release. Donohue et al. (1992) estimated that acute exposures in excess of 50,000 mg•min/m3 can cause death or severe injury.
Table 5-4 shows calculations of Cichowicz (1983), who estimated minimum downwind distances from an M5 smoke pot necessary to limit exposures to designated concentrations and concentration-time profiles under various atmospheric conditions. The minimum distances downwind from one M5 smoke pot are listed in column 4 of Table 5-4. Similarly, distances are shown that are calculated to yield the concentration × time (CT) products ranging from the STEL (2 mg/m3 × 15 min = 30 mg•min/m3 ) to the highest estimated exposure level of 4,800 mg•min/m3.
TABLE 5-4 Downward Distances from One M5 Smoke Pot (HC) Necessary to Limit Exposure to Designated ZnCl2 Concentrations and Concentration × Time Products
|
Atmospheric Condition |
Wind Speed (m/sec) |
Plume Rise (m) |
Distance at Peak Concentration ≤ 2 mg/m3 (m) |
Distance at CT ≤ 30 mg•min/m3 (m) |
Distance at CT ≤ 2,000 mg•min/m3 (m) |
Distance at CT ≤ 4,800 mg•min/m3 (m) |
|
Night (very stable) |
1 |
0 |
3,900a |
3,700 |
190 |
100 |
|
|
3 |
0 |
2,000 |
1,720 |
80 |
40 |
|
|
1 |
18 |
3,600 |
3,200 |
– |
– |
|
Day (neutral stability) |
1 |
0 |
1,100 |
950 |
80 |
50 |
|
Abbreviation: CT, concentration × time product. Source: Adapted from Cichowicz (1983). |
||||||
The potential for exposure during HC smoke training was explored in a cancer risk-assessment study (Novak et al. 1987). On the basis of the Army's Training Ammunition Management Information System (TAMIS), 15 bases using HC smoke munitions for training were identified. Fort Irwin in California was believed to have the greatest potential for exposure of all the sites in the United States and thus was chosen for study. At that base, Army forces train by mock combat with a simulated opponent force (OPFOR). A typical scenario is that OPFOR attacks the friendly force under cover of obscurants, often a combination of HC smoke and fog oil.
Three exposure categories were identified: smoke-generator squads, OPFOR, and friendly forces (Novak et al. 1987). The smoke-generator squads must stay 50 to 75 m directly downwind of the smoke pots. These squads were generating smoke 2 weeks per month for 10 months in 1982 and 3 weeks per month for 12 months in 1986. They have the greatest consistent exposure potential. Friendly forces rotate through this training and seldom train for more than 2 to 3 months per year. OPFOR attacks one friendly force after another over the course of a year and spends much of its training time under smoke cover; the prescribed OPFOR tactics in the 1980s called for heavy use of obscurants.
Novak et al. (1987) estimated the ''worst-practical-case" long-term exposure at Fort Irwin for a soldier deploying smoke pots and standing 50 m directly downwind of the smoke pot. On the basis of usage of HC-smoke munitions in fiscal year 1982 and an assumed 2-year tour of duty, each person in the smoke-generator squad was considered exposed to emissions from 262 M5 smoke pots. Using air-dispersion modeling under very stable conditions and a wind speed of 2 m/sec, Novak et al. (1987) estimated that each member of the smoke generator squad was exposed to HC smoke at a total concentration of 9,916 mg•min/m3. Again assuming a 2-year tour of duty, exposure concentrations were estimated to range from a minimum of 52.2 to nearly 20,000
mg•min/m3 for the OPFOR, from 0.04 to 0.48 mg•min/m3 for the friendly force, and approximately 62 mg•min/m3 total HC smoke for the nearby community.
In addition to exposure to the smoke itself, workers manufacturing HC smoke munitions might be exposed to toxic materials. The most significant possible exposure is to HCE. HCE is a white crystalline solid with a vapor pressure equivalent to 770 ppm at 25°C (Eaton et al. 1994). Its camphorlike odor can be detected as low as 0.15 ppm. The HCE concentration in HC-smoke munitions production areas was found to be above the American Conference of Governmental Industrial Hygienists (ACGIH) Threshold Limit Value (TLV) of 9.7 mg/m3 (Selden et al. 1993). Workers in these areas are protected with either air-supplied hoods at fixed work stations or full-face-piece respirators with both filters and organic vapor cartridges. Nevertheless, the plasma concentrations of HCE in workers rose from 0.08 ± 0.14 µg/L to 7.30 ± 6.04 µg/L after working for more than 5 weeks in the loading and packing operations (Selden et al. 1993).
TOXICOKINETICS
The toxicokinetics of ingested Zn has been studied in humans and animals, but inhaled Zn has not been systematically investigated (Donohue et al. 1992; ATSDR 1994). Intestinal absorption of radiolabeled ZnCl2 increased up to 55% at low concentrations of Zn (90 µmol or less) in humans and declined with much higher concentrations of Zn (Payton et al. 1982). Uptake can be increased by dietary zinc deficiency (Istfan et al. 1983). Data on distribution and excretion of inhaled ZnCl2 are not available, but at least one report of unmasked humans who succumbed following acute exposure to a high concentration of HC smoke showed increased concentrations of Zn in the lung and striated muscle (Hjortso et al. 1988).
TOXICITY SUMMARY
Effects in Humans
ZnCl2 is corrosive and astringent and known to cause burning of moist body surfaces, including the respiratory and gastrointestinal tract. It has been reported to damage nerve endings in the nasal passages and to cause eye burns, damaging smell and vision. The upper respiratory tract is most affected by exposure (ATSDR 1994).
Oral Exposures
Acute oral exposures of humans to ZnCl2 are associated with numerous symptoms that include vomiting, diarrhea, lethargy, and irritation of the mouth, throat and stomach. Ingestion can produce corrosive gastritis and liver necrosis. In one case of food poisoning (83 mg of Zn per 100 g of apples), characteristic symptoms were salivation; edema of the glottis; difficulty swallowing and massive swelling of the lips; pain in the mouth, throat, and epigastrium; recurrent intense vomiting; severe abdominal pain; and bloody diarrhea. Concentrations of 225 to 450 mg are known to be emetic. Two incidents of mass food poisoning produced symptoms that included abdominal cramping and occasional nausea and vomiting (ATSDR 1994).
Inhalation Exposures
One-Time Exposures
All the effects of human exposure to HC smoke are attributed to the ZnCl2 component of the smoke.
Lethality. Death can occur with exposures to ZnCl2 and has been attributed to respiratory insufficiency due to edema of the lungs or acute respiratory distress syndrome. The lethal dose of ZnCl2 in humans has been estimated to be 50,000 mg•min/m3 (see Table 5-5), an exposure that could be achieved according to Cullumbine (1957) by one generator in a 100-ft 3 room within 2 to 3 min. Death is usually delayed by several days. For those who survive an acute exposure, recovery can be protracted.
Pulmonary Effects. Pulmonary effects include dyspnea, chest constriction, retrosternal and epigastric pain, hoarseness, cough, lacrimation, expectoration, and occasional hemoptysis. Sequelae can include cyanosis, elevated pulse, fever, and widespread edema. Exposure to low concentrations results in moderate presentation of these symptoms (Donohue et al. 1992). Cullumbine (1957) reported that concentrations of ZnCl2 at 80 mg/m3 for 2 min (160 mg•min/m3) produced slight nausea and cough, and 120 mg/m3 for 2 min (240 mg•min/m3) resulted in irritation of the nose, throat, and chest; cough; and nausea. The lower limit of detection of HC smoke by humans is reported to be approximately 40 mg/m3 (Schenker et al. 1981). In an accidental exposure case, pneumonitis was reported in teens after exposure to ZnCl2 smoke produced by a grenade at a concentration of 4,075 mg/m3 (Johnson and Stonehill 1961). The exposed individuals experienced nausea and other respiratory symptoms, such as those mentioned above. Table 5-5 summarizes the effects of inhaled ZnCl2 smoke at various combinations of concentration and time.
Repeated Exposures
Virtually no data are available at present on the effects of repeated exposure of neurological, reproductive, developmental, or immunological effects of ZnCl2 in humans.
TABLE 5-5 Effects of Inhaled ZnCl2
|
Concentration × Time (mg•min/m3) |
Effect |
|
< 160 |
Essentially no effect; some awareness of presence |
|
160-240 |
Noticeable irritation of nose, throat, and chest |
|
1,700-2,000 |
Marked irritation: Hospitalization and treatment required |
|
20,000 |
Severe irritation; chemical pneumonia: Hospitalization and treatment required |
|
50,000 |
Massive injury; fatality |
|
Sources: Stocum and Hamilton (1976) as cited in Donohue et al. (1992). |
|
Effects in Animals
Dermal and Ocular Exposures
ZnCl2 has been shown to be a skin and eye irritant in animal studies. When applied to the shaved skin of guinea pigs, weight loss was evident. In rabbits, severe erythema was noted both on abraded and unabraded skin, and eye application induced severe corneal damage, an effect manifest 4 days to 2 weeks after application (ATSDR 1994).
Oral Exposures
One-Time Exposures
Domingo et al. (1988) reported an acute oral LD50 for ZnCl2 of 528 mg/kg of body weight in rats and 605 mg/kg of body weight in mice.
Repeated Exposures
Hematological Effects. Rats fed ZnCl2 in their food ad libitum for 7 days per week for 4 weeks were reported to have a decrease in hemoglobin to 85% of control values. The lowest-observed-adverse-effect level (LOAEL) reported for such effects was calculated to be a dose of Zn at 12 mg/kg per day, equivalent to ZnCl2 at 25 mg/kg of body weight per day (Zaporowska and Wasilewski 1992).
Reproductive Effects. Rats fed 25 mg of Zn per day as ZnCl2 for 8 weeks showed an increased frequency of sperm with altered chromatin structure (Evenson et al. 1993).
Inhalation Exposures
One-Time Exposures
Lethality. The acute LCT50 (product of concentration × time lethal to 50% of the test animals) for exposure to ZnCl2 is reported to be 11,800 mg·min/m3 for mice (Cullumbine 1957).
Pulmonary Effects. Brown et al. (1990) compared the effects of acute HC smoke inhalation (11,580 mg·min/m3 as HC smoke or 4,900 mg•min/m3 as ZnCl2, assuming 20% of the total smoke is Zn and 42% is ZnCl2 ; see Marrs et al. 1989) for 60 min to instillation exposures (2.5 mg/kg) in rats. All exposed animals experienced respiratory distress. Mortality was higher in the smoke-exposed animals; respiratory distress developed more slowly after instillation but lasted longer. Both groups showed edema of the lungs, destructive alveolitis, and macrophage infiltration, followed by development of fibrosis.
Repeated Exposures
Lethality and Pulmonary Effects. Marrs et al. (1988) determined the effects of repeated exposures to freshly generated ZnO
and HC smoke in female mice, rats, and guinea pigs. Animals were exposed to three air concentrations of smoke (Zn at 0, 1.3, 12.8, or 122 mg/m3) 1 hr per day, 5 days per week, for 20 weeks, for a total of 100 1-hr exposures.
The highest exposure concentration caused excessive mortality in both guinea pigs and mice. Inflammatory changes, such as edema, emphysema, and macrophage infiltration in the lungs of rats and guinea pigs, also occurred at the highest exposure concentration. The middle-and low-exposure groups exhibited normal survival rates, and no adverse pulmonary effects were evident. The no-observed-adverse-effect level (NOAEL) was determined to be 12.8 mg of Zn per cubic meter, or 26.6 mg of ZnCl2 per cubic meter, assuming that all the Zn in the smoke is in the form of ZnCl2.
Other Systemic Effects. Marrs et al. (1988) reported no effects on other systems, including cardiovascular, gastrointestinal, hepatic, renal, immune, or reproductive systems. No effects on growth were observed at any exposure concentration.
Carcinogenic and Mutagenic Effects. The major histopathological finding of Marrs et al. (1988) was an increase in the incidence of alveologenic carcinomas in mice exposed to the highest air concentration (i.e., Zn at 122 mg/m3, or ZnCl2 at 254 mg/m3, assuming that all Zn is in the form of ZnCl2). International Agency for Research on Cancer (IARC) has not evaluated ZnCl2 for its carcinogenicity. Genotoxicity studies in bacterial and mammalian-cell culture test systems provide no evidence that ZnCl2 is mutagenic (ATSDR 1994).
Summary of Toxicity Data
Tables 5-6 and 5-7 summarize exposure-response information for the lethal and nonlethal effects, respectively, resulting from inhalation exposure to HC smoke or ZnCl2 aerosol.
TABLE 5-6 Lethality Resulting from Inhalation Exposure to HC Smoke or ZnCl2 Aerosol (expressed as milligrams of ZnCl2)
|
Species |
Exposure Frequency and Duration |
Exposure Level |
End Point and Comments |
Reference |
|
Human |
One time |
50,000 mg•min/m3 |
Some lethality |
Donohue et al. 1992 |
|
Rat |
10 min |
12,500 mg•min/m3 |
0/3 died |
Karlsson et al. 1991 |
|
|
|
19,600 |
2/3 died |
|
|
|
|
25,400 |
2/3 died |
|
|
|
|
40,600 |
3/3 died |
|
|
Mouse |
One time |
11,800 mg•min/m3 |
LCT50 |
Cullumbine 1957 |
|
Mouse |
60 min |
4,900 mg•min/m3 |
Excess mortality |
Brown et al. 1990a |
|
Mouse (female), rat, guinea pig |
1 hr/d, 5 d/wk, 20 wk |
26.6 mg/; 254 mg/m3 |
NOAEL; LOAEL |
Marrs et al. 1988b |
|
Abbreviations: hr, hour(s); d, day(s); wk, week(s); LCT50, product of concentration × time that is lethal to 50% of the test animals. a Assumes 42% of 11,580 mg•min/m3 total HC smoke is ZnCl2. b Assumes all Zn present is in form of ZnCl2; see text. |
||||
TABLE 5-7 Summary of Exposure-Response Data for Nonlethal Effects from Exposure to HC Smoke or ZnCl2 (expressed as milligrams of ZnCl2)
|
Category and Species |
Exposure Frequency and Duration |
NOAEL (mg/m3) |
LOAEL (mg/m3) |
End Point and Comments |
Reference |
|
|
Effects in Humans |
||||||
|
One-Time Inhalation Exposure |
||||||
|
Pulmonary Effects |
||||||
|
Experimental subjects |
2 min |
8160 mg•min/m3 |
160 mg•min/m3 |
2 min•80 mg/m3 : Slight nausea and cough |
Cullumbine 1957 |
|
|
Experimental subjects |
2 min |
— |
240 mg•min/m3 |
2 min•120 mg/m3 : Irritation of nose, throat, chest; cough and nausea |
Cullumbine 1957 |
|
|
Effects in Animals |
||||||
|
Repeated Oral Exposures |
||||||
|
Rat |
7 d/wk, 4 wk |
— |
25 mg/kg-d |
Hemoglobin decrease by 15% |
Zaporowska and Wasilewski 1992 |
|
|
Rat |
7 d/wk, 4 wk |
— |
52 mg/kg-d |
Increased frequency of sperm with altered chromatin |
Evenson et al. 1993 |
|
|
One-Time Inhalation Exposures |
||||||
|
Pulmonary Effects |
||||||
|
Mouse |
60 min |
— |
4,900 mg•min/m3 |
60 min•120 mg/m3 : Respiratory distress and excess mortality |
Brown et al. 1990 |
|
|
Repeated Inhalation Exposures |
|||||
|
Pulmonary Effects |
|||||
|
Mouse (female), rat, guinea pig |
1 hr/d, 5 d/wk, 20 wk (100 hr total) |
26.6 mg/m3 |
254 mg/m3 |
Inflammatory changes in the lungs; lethality |
Marrs et al. 1988 |
|
Carcinogenic Effects |
|||||
|
Mouse (female), rat, guinea pig |
1 hr/d, 5 d/wk, 20 wk (100 hr total) |
26.6 mg/m3 |
254 mg/m3 |
Alveologenic carcinomas |
Marrs et al. 1988 |
|
Abbreviations: hr, hour(s); min, minute(s); d, day(s); wk, week(s). |
|||||
Noncancer Toxicity
The target organ for inhalation exposures to HC smoke in humans and animals is the respiratory tract. Data from humans indicate that a threshold for slight nausea and irritation of the nose, throat, and chest from exposure to ZnCl2 is between 160 and 240 mg•min/m3. At concentrations of 1,700 mg•min/m3 and above, effects can be severe enough to require hospitalization and treatment. Data from animals are sparse but indicate a NOAEL for repeated 1-hr exposures to HC smoke with Zn at 12.8 mg/m3 (or ZnCl2 at 26.6 mg/m3) in rodents and a LOAEL for inflammatory changes in the lung and death with Zn at 122 mg/m3 (or ZnCl2 at 254 mg/m3), suggesting a relatively steep dose-response curve.
Example Noncancer Risk Assessment
According to reports of simulated combat training during a MOUT exercise, trainees and instructors are exposed to ZnCl2 at concentrations ranging from 0.02 to 0.98 mg/m3 for 225 min. The average exposure concentration was calculated to be 0.26 mg/m3 with a standard deviation of 0.26 (Young 1992, as cited in Lundy and Eaton 1994). In the worst-case scenario, that concentration would result in exposures as high as 225 mg•min/m3 (i.e., 1 mg/m3 × 225 min). Thus, the current MOUT exercise might engender ZnCl2 exposures that exceed the human threshold for adverse respiratory-tract effects at 160 to 240 mg•min/m3. Additionally, the values from the simulated MOUT exercise do not specify distance from the source of the smoke pot or the direction of air movement with respect to the source and exposed personnel. As indicated in Table 5-4, moderate distances (i.e., 40 to 190 m) downwind of a smoke pot might result in exposure concentrations of 2,000 mg•min/m3 or higher, which could result in the need for hospitalization and treatment (see Table 5-5).
The above example does not take into account the possibility that women of childbearing age, who might be pregnant, could be members of the exposed population. Virtually no human or animal data are available to provide any information on potential reproductive or developmental toxicity. In addition, no information is available on the effects of these exposures on nervous-system function. Combined with those caveats is the fact that the onset of toxicity tends to be delayed, and recovery, if it occurs, is slow.
Given the likely inability of the soldier to control distance and duration of exposure, the above example suggests that masks should be worn at all times during such exercises. Mask use is especially important because the irritant effects might degrade the performance of the soldiers.
Carcinogenicity
Although only one study of one species reported a positive carcinogenic response resulting from exposure to HC smoke (i.e., Marrs et al. 1988), the subcommittee decided to estimate the potential carcinogenic potency of HC smoke based on this study as a screen to determine whether further analysis is warranted. The derivation of the potential carcinogenic potency of HC smoke based on this one study is described below.
Marrs et al. (1988) exposed mice to HC smoke. The concentrations of Zn in the air were 0 (control), 1.3, 12.8, and 122 mg/m3. Because Zn represented 20% of the smoke in these experiments, the concentrations of total smoke were 5 times higher: 0, 6.5, 64, and 610 mg/m3. Assuming a 25-g mouse inhaled air at 0.0018 /hr during the 100 hr of exposure, each animal inhaled 0.18 of air containing HC smoke. The total doses of smoke for the groups were 0, 1.2 (i.e., 6.5 × 0.18), 12 (i.e., 64 × 0.18), and 110 mg (i.e., 610 × 0.18). Assuming a 2-year mouse lifetime, the average daily dose per kilogram of body weight is 0, 0.064, 0.63, and 6.0 mg/kg per day (i.e., total mg dose/body weight × 730 days).
This calculation assumes that the total dose was distributed evenly over the lifetime of the mouse and is used for estimating carcinogenic potency.
The incidences of alveologenic carcinoma at 18 months were 6 of 78 in the controls, 7 of 74 in the 1.3-mg/ group, 8 of 76 in the 12.8-mg/ group, and 15 of 50 in the 122-mg/ group (Marrs et al. 1988). Using the average daily doses calculated above and fitting a generalized multistage model to the cancer data provides an upper limit of the cancer risk (low-dose slope) of 0.036/mg/kg per day. This model was used for estimating the potency of HC smoke at low doses to provide a conservative estimate for screening purposes. The cancer potency is based on exposures expressed as milligrams per kilogram of body weight per day. Cancer potency based on scaling body weight to the 3/4 power would result in cancer risk estimates about a factor of 7 higher, or 0.25 mg/kg per day.
The subcommittee also estimated carcinogenic potency expressed in terms of ZnCl2 instead of total HC smoke. Given that Zn comprised 20% of the HC smoke in the experiments of Marrs et al. (1988), ZnCl2 should have comprised approximately 42% of the total smoke used in those experiments. If one assumes that essentially all the Zn present is in ZnCl2 (although some will be present in an oxide form), the resulting upper 95% confidence limit of the cancer risk (low-dose slope) would be 0.086 mg/kg of body weight per day (i.e., the potency of total HC smoke of 0.036 mg/kg per day divided by 0.42). Cancer potency based on scaling body weight to the 3/4 power would be about a factor of 7 higher, or 0.6 mg/kg per day.
Lifetime cancer risks are generally based on the tumor incidence from a 2-year rodent bioassay. Studies of shorter duration might underestimate the true risk, because cancer incidence generally increases rapidly with age. On the other hand, animals were exposed to HC smoke during the first 20 weeks of the study. Because young animals might be more sensitive to HC smoke and have a longer time for tumors to develop from early-life exposure, the observed tumor incidence might be higher than would be observed
if the same total dose were administered over 2 years. Because the tumor incidence could be adjusted upward and downward, no adjustment will be made based on the less-than-lifetime exposure duration.
Example Cancer Risk Assessment
Estimated Exposure
Novak et al. (1987) estimated exposures to HC smoke for smoke-generator operators. An operator was assumed to be 50 m downwind, and 100% of the available respirable dose was assumed to be absorbed. Under those conditions, the Army calculated the exposure concentration from a single smoke pot to be 1,120 mg•min/m3 for an atmospheric stability condition defined by the Army as ''neutral." Using an inhalation rate of 0.03 /min, the dose from a single smoke pot is 1,120 × 0.03 or 33.6 mg of HC smoke. An operator was assumed to be exposed to 262 releases during a 2-year tour of duty, resulting in 8,800 mg of total HC smoke inhaled (i.e., 262 × 33.6 mg). Averaged over a 70-year lifetime for a 70-kg person, the average daily dose is
Under "very stable" atmospheric conditions, the exposure concentrations from a single smoke pot is 9,900 mg•min/m3, resulting in an average daily lifetime dose of 0.044 mg/kg per day for a smoke-pot operator. Note, however, that very stable and neutral atmospheric conditions are worst-case estimates (i.e., little or no wind to disperse the smoke plume by convection) and would not apply most of the time. Hence, assuming these conditions will overestimate risk. Average atmospheric stability conditions could be used instead to estimate risks for a particular facility.
In the community of Baker nearby Fort Irwin, Novak et al. (1987) estimated total lifetime exposure to be 62.3 mg•min/m3.
Assuming an inhalation rate of 0.03 /min and 100% absorption, the total absorbed dose is 1.86 mg of HC smoke (i.e., 62.3 × 0.03). The average daily dose over a 70-year lifetime for a 70-kg person is
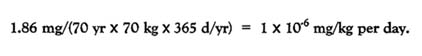
This dose is quite small compared with the dose estimated for smoke-pot operators.
Estimated Cancer Risk
Using the information above, the subcommittee estimated a lifetime cancer risk by multiplying the upper limit of the cancer potency of HC smoke (0.036/mg/kg per day) by the dose averaged over a lifetime. For smoke-pot operators on a 2-year tour of duty, the lifetime cancer risk for an average daily dose of 0.0049 mg/kg per day was estimated to be
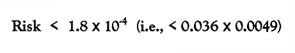
for a neutral atmospheric condition. For a very stable atmospheric condition, the average daily lifetime dose was estimated to be 0.0436 mg/kg per day, giving a lifetime cancer risk of
If average atmospheric conditions are assumed, the estimated risks for smoke-pot operators would be lower.
In the community of Baker, even using the unrealistic worst-case atmospheric conditions, the average daily lifetime dose was estimated to be 1 × 10-6 mg/kg per day, resulting in a lifetime cancer risk of less than 3.6 × 10-8 (i.e., 0.036 × 10-6) throughout the lifetime of Baker residents.
EXISTING RECOMMENDED EXPOSURE LIMITS
The ACGIH (1991) proposed a TLV time-weighted average (TWA) of 1 mg/m3 for 8 hr per day, 5 days per week (or 40 hr per week) and a 15-min TLV short-term exposure limit (STEL) of 2 mg/m3 for ZnCl2.
SUBCOMMITTEE EVALUATION AND RECOMMENDATIONS
On the basis of the toxicity information presented for ZnCl2, the subcommittee developed exposure guidance levels for military personnel exposed during an emergency release or during regular-training exercises and for nearby communities to protect them from emergency or repeated releases of HC smoke.
Military Exposures
Emergency Exposure Guidance Level (EEGL)2
To define an EEGL for ZnCl2, several factors must be taken into consideration. Based on reports from acute human inhalation exposures, CT products of 160 mg•min/m3 produced slight nausea and cough, and 240 mg•min/m3 was associated with irritation of the nose, throat, and chest and with nausea and cough (Cullumbine 1957). Donohue et al. (1992) summarized these data by suggesting that the noticeable irritation range for nose, throat, and chest is 160 to 240 mg•min/m3.
The NOAEL of 160 mg•min/m3 for ZnCl2 is based on short-term exposures of humans and therefore can be used for the EEGL
without adjustment to account for extrapolation uncertainties (e.g., animal to human, LOAEL to NOAEL). Thus, for a 60-min exposure period, the EEGL would be 2.7 mg/m3, rounded off to 3.0 mg/m3. Similarly, for a 15-min exposure period, the EEGL would be 10 mg/m3, and for a 6-hr exposure period, the EEGL would be 0.4 mg/m3. The 15- and 60-min EEGLs exceed the current STEL value set by ACGIH (2 mg/m3) but can be justified by the fact that the EEGL is set for a one-time emergency exposure situation which is not expected to reoccur.
Permissible Exposure Guidance Levels (PEGL)3
A PEGL is required because of the chronic intermittent exposures to smokes that are experienced by soldiers, which would approximate 50 8-hr exposures during a 2-year tour of duty. Virtually no human data are available upon which to base a PEGL. One repeated inhalation toxicity exposure study carried out by Marrs et al. (1988) identified a NOAEL for rodents as ZnCl2 at 26.6 mg/m3 for 1 hr per day, 5 days per week, for 20 weeks. Using that NOAEL, a divisor of 10 for uncertainties associated with sparse animal data and with the shorter daily duration of exposure (1-hr exposures for the rodent compared with 8-hr exposures for military personnel), and another divisor of 10 to extrapolate from animals to humans, the subcommittee recommends a PEGL for ZnCl2 of 0.2 mg/m3.
Comparison with Other Exposure Guidance Levels
The ACGIH 8-hr TLV-TWA for ZnCl2 of 1.0 mg/m3 is higher than both the 6-hr EEGL of 0.4 and the PEGL of 0.2 mg/m3 recommended above. The ACGIH TLV-TWA is based on
unpublished reports that a 30-min exposure at 4.8 mg/m3 caused mild, transient respiratory irritation and that 0.4 mg/m3 (duration of exposure not specified) was not considered irritating. ACGIH did not indicate why Haber's law was not applied to the 30-min exposure level to extrapolate to longer exposure periods. Without evidence to the contrary, the subcommittee assumes that Haber's law is applicable and therefore recommends the lower values for the 6-hr EEGL and PEGL rather than the ACGIH recommendation for its 8-hr TLV-TWA.
The actual exposures that can be experienced if cancer risks are not to exceed 1 × 10-4, for example, would require that a total dose of no more than 5,000 mg of HC smoke be experienced (i.e., the 8,800-mg HC smoke associated with a 1.8 × 10-4 cancer risk divided by 1.8 to yield a 1 × 10-4 cancer risk). Assuming 262 exposures during a 2-year tour of duty, the total dose from a single smoke pot could not exceed 19 mg (5,000 mg of HC smoke divided by 262 exposures). Assuming further an inhalation rate of 0.03/min, the exposure concentration generated by the smoke pot could not exceed 630 mg•min/m3 (i.e., 19 mg divided by 0.03 /min) or approximately 10 mg•hr/m3 (i.e., 630 mg•min/m3 divided by 60 min/hr) for a total of 262 such exposures. In the case of the training staff who endure a greater number and duration of such exposures, the values would have to be altered accordingly.
Public Exposures
Short-Term Public Emergency Guidance Level (SPEGL)4
In calculating the SPEGL, the EEGL is divided by an uncertainty factor of 10 to account for the susceptible subpopulations of the general public (e.g., the elderly, chronically ill, and children) that could conceivably be exposed. This calculation results in a
SPEGL, expressed as a CT product, of 16 mg•min/m3. The corresponding 15-min, 60-min, and 6-hr SPEGLs are 1, 0.3, and 0.04 mg/m3, respectively.
Permissible Public Exposure Guidance Level (PPEGL)5
The general public living or working near military-training facilities could experience chronic intermittent exposures. For the PPEGL, the PEGL is divided by an uncertainty factor of 10 to account for sensitive populations (e.g., the elderly, chronically ill, and children). A PPEGL of 0.01 mg/m3 is recommended.
Comparing the outcome of the example of cancer-risk estimates with the PEGL suggests that, because the cancer risks for the nearby community, as computed above, are well below the usual level of concern, the PPEGL guidelines should be used.
Comparison of Recommendations with Conservative Screening Cancer-Risk Estimates
Given the one finding of cancer in mice exposed to HC smoke (Marrs et al. 1988), the subcommittee estimated a conservative cancer potency factor for HC smoke (see Carcinogenic Effects) and estimated the corresponding cancer risk associated with the recommended exposure guidance levels for military personnel and the public.
For a 60-min EEGL of 3 mg/m3 for ZnCl2, the total exposure of 3 mg averaged over a 75-hr lifetime for a 70-kg adult is 3 mg/m3 (75 yr × 365 days × 70 kg) = 1.6 × 10-6 mg/kg per day. The cancer risk is estimated to be below 0.086 × (1.6 × 10-6) = 1 × 10-7.
For 400 hr of exposure of military personnel during a tour of duty at a PEGL of 0.1 mg/m3, the total exposure would be 40 mg
of ZnCl2. For this exposure, the cancer risk is estimated to be less than 2 × 10-6.
For the SPEGL, the possible lifetime exposure would be 0.3 mg of ZnCl2, which would correspond to a cancer risk of less than 2 × 10-8.
For 30 hr of exposure per year at a PPEGL of 0.01 mg/m3 for ZnCl2, the total annual dose would be 0.3 mg of ZnCl2, corresponding to an annual cancer-risk estimate of less than 2 × 10-8. For a 30-year period of residence in the community, the corresponding risk estimate would be less than 6 × 10-7.
Thus, the subcommittee concludes that the exposure guidance levels recommended for military personnel and the public developed on the basis of noncancer end points are sufficiently low to represent a negligible (i.e., approximately 1 × 10-6 or less) cancer risk if the substance is a human carcinogen. The data available to date, however, are insufficient to conclude that ZnCl2 is a human carcinogen.
Summary of Subcommittee Recommendations
Table 5-8 summarizes the subcommittee's recommendations for EEGLs and the PEGL for military personnel exposed to HC smoke. Table 5-9 summarizes the subcommittee's recommendations for SPEGLs and the PPEGL for military-training facilities to ensure that nearby communities are not exposed at concentrations that might cause adverse effects.
TABLE 5-8 EEGLs and PEGL for HC Smoke for Military Personnel
|
Exposure Guideline |
Exposure Duration |
Guidance Level (mg/m3)a |
|
EEGL |
15 min |
10 |
|
|
1 hr |
3 |
|
|
6 hr |
0.4 |
|
PEGL |
8 hr/d |
0.2 |
|
a Expressed in milligrams of ZnCl2 per cubic meter. |
||
TABLE 5-9 SPEGLs and PPEGL for HC Smoke at the Boundaries of Military Training Facilities
|
Exposure Guideline |
Exposure Duration |
Guidance Level (mg/m3)a |
|
SPEGL |
15 min |
1 |
|
|
1 hr |
0.3 |
|
|
6 hr |
0.04 |
|
PPEGL |
8 hr/d |
0.02 |
|
a Expressed in milligrams of ZnCl2 per cubic meter. |
||
RESEARCH NEED
It is clear from the data reviewed in this chapter that insufficient information is available to evaluate potential long-term toxicity of ZnCl2. As noted above, almost all studies have focused on pulmonary effects, and little information exists on potential toxicity to other systems, such as the developmental or reproductive systems or nervous system. In addition, little information exists on effects resulting from repeated exposures and on the reversibility of observed effects. If use of HC smoke devices based on reactions of HCE with ZnO continues, then additional information with respect to effects on other organs and systems is required to ensure the health of military personnel and to prevent releases beyond military facilities that might pose risks to the general public.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 1991. Documentation of the Threshold Limit Values and Biological Exposure Indices, 6th Ed. American Conference of Governmental Industrial Hygienists, Cincinnati, Ohio.
ATSDR (Agency for Toxic Substances and Disease Registry). 1994. Toxicological Profile for Zinc (Update). TR-93/15. Agency for Toxic Substances and Disease Registry, Public Health Service, U.S. Department of Health and Human Services, Atlanta, Ga.
Brown, R.F.R., T.C. Marrs, P. Rice, and L.C. Masek. 1990. The histopathology of rat lung following exposure to zinc oxide/hexachloroethane smoke or instillation with zinc chloride followed by treatment with 70% oxygen. Environ. Health Perspect. 85:81-87.
Cichowicz, J.J. 1983. Environmental Assessment, Programmatic Life Cycle Environmental Assessment for Smoke/Obscurants. HC Smoke, Vol. 4. ARCSL-EA-83007. Chemical Research and Development Center, U.S. Army Armament, Munitions and Chemical Command, U.S. Army Aberdeen Proving Ground, Edgewood, Md.
Cullumbine, H. 1957. The toxicity of screening smokes. J. R. Army Med. Corps 103:119-122.
DeVaull, G.E., W.E. Dunn, J.C. Liljegren, and A.J. Policastro. 1989. Analysis Methods and Results of Hexachloroethane Smoke Dispersion Experiments Conducted as Part of Atterbury-87 Field Studies. AD-A216048. Prepared by Argonne National Laboratory, Argonne, Ill., for the U.S. Army Medical Research and Development Command, Fort Detrick, Frederick, Md.
Domingo, J.L., J.M. Llobet, J.L. Paternain, and J. Corbella. 1988. Acute zinc intoxication: Comparison of the antidotal efficacy of several chelating agents. Vet. Hum. Toxicol. 30:224-228.
Donohue, J.M., L. Gordon, C. Kirman, and W.C. Roberts. 1992. Zinc Chloride Health Advisory. Interagency Agreement (IAG) 85PP5869. Office of Water, U.S. Environmental Protection Agency, Washington, D.C., and the U.S. Army Medical Research and Development Command, Fort Detrick, Frederick, Md.
Eaton, J.C., R.J. LoPinto, and W.G. Palmer. 1994. Health Effects of Hexachloroethane (HC) Smoke. USABRDL-TR-9402. AD-A277 838. U.S. Army Biomedical Research and Development Laboratory, Fort Detrick, Frederick, Md.
Evenson, D.P., R.J. Emerick, L.K. Jost, H. Kayongo, and S.R. Stewart. 1993. Zinc-silicon interactions influencing sperm chromatin integrity and testicular cell development in the rat as measured by flow cytometry. J. Anim. Sci. 71:955-962.
Hill, H.G., and K. Wasti. 1978. A Literature Review–Problem Definition Studies on Selected Toxic Chemicals. Occupational Health and Safety and Environmental Aspects of Zinc Chloride, Vol. 5, Final Report. AD A056020. Franklin Institute Research Laboratories, Philadelphia.
Hjortso, E., J. Qvist, M.I. Bud, J.L. Thomsen, J.B. Andersen, F. Wiberg-Jorgensen, N.K. Jensen, R. Jones, L.M. Reid, and W.M. Zapol.
1988. ARDS after accidental inhalation of zinc chloride. Intensive Care Med. 14:17-24.
Istfan, N.W., M. Janghorbani, and V.R. Young. 1983. Absorption of stable 70Zn in healthy young men in relation to zinc intake. Am. J. Clin. Nutr. 38:187-194.
Johnson, F.A., and R.B. Stonehill. 1961. Chemical pneumonitis from inhalation of zinc chloride. Dis. Chest 40:619-623.
Karlsson, N., I. Fangmark, I. Haggqvist, B. Karlsson, L. Rittfeldt, and H. Marchner. 1991. Mutagenicity testing of condensates of smoke from titanium dioxide/hexachloroethane and zinc/hexachloroethane pyrotechnic mixtures. Mutat. Res. 260:39-46.
Katz, S., A. Snelson, R. Farlow, R. Welker, and S. Mainer. 1980. Physical and Chemical Characterization of Fog Oil Smoke and Hexachloroethane Smoke. DAMD17-78-C-8085. AD-A080 936. IIT Research Institute, Chicago.
Lundy, D., and J. Eaton. 1994. Occutational Health Hazards Posed by Inventory U.S. Army Smoke/Obscurant Munitions (Review Update). U.S. Army Medical Research Detachment, Wright-Patterson Air Force Base, Ohio.
Marrs, T.C., H.F. Colgrave, J.A.G. Edginton, R.F.R. Brown, and N.L. Cross. 1988. The repeated dose toxicity of a zinc oxide/hexachloroethane smoke. Arch. Toxicol. 62:123-132.
Novak, E.W., L.B. Lave, J.J. Stukel, and D.J. Schaeffer. 1987. A Revised Health Risk Assessment for the Use of Hexachloroethane Smoke on an Army Training Area. USA-CERL Tech. Rep. N-87/26. Construction Engineering Research Laboratory, U.S. Army Corps of Engineers, Champaign, Ill.
Payton, K.B., P.R. Flanagan, E.A. Stinson, D.P. Chodirker, M.J. Chamberlain, and L.S. Valberg. 1982. Technique for determination of human zinc absorption from measurement of radioactivity in a fecal sample or the body. Gastroenterology 83:1264-1270.
Schenker, M.B., F.E. Speizer, and J.O. Taylor. 1981. Acute upper respiratory symptoms resulting from exposure to zinc chloride aerosol. Environ. Res. 25:317-324.
Seldén, A., M. Nygren, A. Kvamlöf, K. Sundell, and O. Spångberg. 1993. Biological monitoring of hexachloroethane. Int. Arch. Environ. Health 65:S111-S114.
Stocum, W.E., and R.G. Hamilton. 1976. A Risk Analysis of Exposure to High Concentrations of Zinc Chloride Smoke. SAND76-0386. Sandia Laboratories, Albuquerque, N.Mex.
Young, J.Y., D.A. Smart, J.T. Allen, D.L. Parmer, A.B. Rosencrance, E.E. Brueggeman, and F.H. Broski. 1989. Field Exposure of Chemical School Students and Cadre to Fog Oil and Hexachloroethane (HC) Smokes. Tech. Rep. 8908. U.S. Army Biomedical Research and Development Laboratory, Fort Detrick, Frederick, Md.
Young, J.Y. 1992. Field Exposure of Infantry Soldiers to Hexachloroethane and Colored Smoke During a ''Military Operation-Urban Terrain" Training. U.S. Army Biomedical Research and Development Laboratory, Fort Detrick, Frederick, Md.
Zaporowska, H., and E. Wasilewski. 1992. Combined effect of vanadium and zinc on certain selected hematological indices in rats. Comp. Biochem. Physiol. C 103:143-147.


































