This paper was presented at a colloquium entitled “Genetic Engineering of Viruses and Virus Vectors,” organized by Bernard Roizman and Peter Palese (Co-chairs), held June 9–11, 1996, at the National Academy of Sciences in Irvine, CA.
Adenovirus-mediated interleukin-12 gene therapy for metastatic colon carcinoma
(gene transfer/cytokines/recombinant adenoviral vectors)
MANUEL CARUSO*†, KHIEM PHAM-NGUYEN*, YOK-LAM KWONG*, BISONG XU*, KEN-ICHIRO KOSAI‡, MILTON FINEGOLD‡, SAVIO L. C. WOO*†, AND SHU-HSIA CHEN*§
Departments of *Cell Biology and ‡Pathology, †Howard Hughes Medical Institute, Baylor College of Medicine, Houston, TX 77030
ABSTRACT Recombinant adenoviral mediated delivery of suicide and cytokine genes has been investigated as a treatment for hepatic metastases of colon carcinoma in mice. Liver tumors were established by intrahepatic implantation of a poorly immunogenic colon carcinoma cell line (MCA-26), which is syngeneic in BALB/c mice. Intratumoral transfer of the herpes simplex virus type 1 thymidine kinase (HSV-tk) and the murine interleukin (mIL)-2 genes resulted in substantial hepatic tumor regression, induced an effective systemic antitumoral immunity in the host and prolonged the median survival time of the treated animals from 22 to 35 days. The antitumoral immunity declined gradually, which led to tumor recurrence over time. A recombinant adenovirus expressing the mIL-12 gene was constructed and tested in the MCA-26 tumor model. Intratumoral administration of this cytokine vector alone increased significantly survival time of the animals with 25% of the treated animals still living over 70 days. These data indicate that local expression of IL-12 may also be an attractive treatment strategy for metastatic colon carcinoma.
Metastatic colon carcinoma is the second leading cause of death from malignancy in the United States. Eighty percent of the patients who die of colon cancer have metastases in the liver (1). Once hepatic metastases occur, surgery and chemotherapy are the only currently available treatment modalities, and the mean survival time is only 37 months (2). Therefore, the development of alternative treatments for metastases of colon cancer is needed to improve the clinical outcome of patients.
Cancer immunotherapy is an approach that has been widely investigated in different types of tumor, the goal of which is to stimulate host immune response against the cancer cells. Because of its well established immunomodulating activities in stimulating the growth and the activation of T cells as well as natural killer (NK) cells, recombinant interleukin (IL)-2 was first tested in patients for this purpose. A severe limitation of such treatment in patients is the toxicity associated with high doses of systemic recombinant IL-2 administration. Cytokine secretion in the vicinity of the tumor can potentially minimize the toxicity associated with systemic cytokine administration, which could be achieved by the transfer and expression of various cytokine genes directly in the tumor cells (3).
In the ex vivo gene therapy or “cancer vaccine” approach, cancer cells are isolated from patients, transduced with various gene vectors and expanded in vitro. After irradiation, the cells are transplanted autologously to enhance the patient’s immune response against the tumor. This strategy is not only laborious, but the treatment is also individualized as cancer cells need to be cultured and expanded from each patient for therapeutic purposes. A more attractive strategy is to deliver the cytokine genes in vivo. The retroviral vector is commonly used for the ex vivo approach, but its low titer limits its application by in vivo delivery. The recombinant adenoviral vector is characterized by high titers and is capable of efficient gene transfer into a variety of cell types in vivo. The use of recombinant adenoviral vectors in immunotherapy of metastatic colon carcinoma is reported.
MATERIALS AND METHODS
Cell Culture. MCA-26 cells, a chemically induced colon carcinoma line derived from BALB/c mouse (4), was grown and maintained in high glucose MEM/HAMF12. The 293 cells (adenoviral E1-transformed human embryonic kidney) (5) were maintained in DMEM. All cell lines were supplemented with 10% fetal calf serum (GIBCO), 2 mM glutamine, 100 unit/ml penicillin, and 100 mg/ml streptomycin.
Recombinant Adenoviral Vectors. Construction of replication-defective adenoviral vectors containing the tk and murine (m)IL-2 gene under the transcriptional control of the Rous sarcoma virus long terminal repeat (ADV/tk and ADV/mIL-2) has been reported previously. A replication-defective adenoviral vector containing the mIL-12 cDNA under the transcriptional control of the Rous sarcoma virus long terminal repeat promoter (ADV/mIL-12) was constructed and plaque purified as followed. The cDNA from both mIL-12 subunits were cloned by RT-PCR from total RNA obtained from pockweed mitogen stimulated mouse splenocytes. To ensure correct nucleotide sequence, the entire cDNA was subsequently sequenced. The two cDNA were then linked to the encephalomyocarditis virus internal ribosome entry site that was obtained from the pCITE-1 vector (Novagen). The fragment p40-internal ribosome entry site-p35 was inserted into the E1 deleted adenovirus backbone pAd.1/Rous sarcoma virus (6). The recombinant adenovirus, ADV/mIL-12, was generated by cotransfection with pBHG10 (7) into 293 cells. The viral titer [plaque-forming units (pfu)/ml] was determined by plaque assay in 293 cells (8).
Functional Analysis of ADV/mIL-12. One million MCA-26 seeded in a six-well plate were infected with an ADV/mIL-12
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: IL, interleukin; m, murine; IFN, interferon; NK, natural killer; HSV-tk, herpes simplex virus type 1 thymidine kinase; CTL, cytotoxic T-lymphocyte; m.o.i., multiplicity of infection; pfu, plaque-forming unit.
or a control adenovirus ADV/β-galactosidase at different multiplicity of infection (m.o.i.) in a total volume of 0.5 ml. After 2 hr, the viral supernatant was replaced with 2.5 ml of medium. Then, two days after, the supernatant was collected and tested for mIL-12 bioactivity. One ml of supernatant was added to 106 splenocytes from naive mice in a total volume of 2 ml. After 48 hr the supernatant was collected and analyzed for interferon (IFN)-γ release by ELISA (Endogen, Cambridge, MA).
Establishment and Treatment of Hepatic Metastasis Model of Colon Carcinoma. Metastatic colon carcinoma was induced in the liver by intrahepatic implantation of 5×104 MCA-26 cells at the tip of the left lateral liver lobe of 8- to 12-week-old syngeneic BALB/mice (Harlan-Sprague-Dawley). At day 7, various titers of recombinant adenoviral vectors were injected intratumorally in 50 μl of 10 mM Tris·HCL (pH 7.4)/1 mM MgCl2/10% (vol/vol) glycerol/Polybrene (20 μg/ml). All experiments were performed in accordance with the animal guidelines at Baylor College of Medicine.
Cytotoxic T-Lymphocyte (CTL) Assay. Viable splenocytes were isolated from various animal treatment groups at different time points after primary hepatic tumor inoculation. In vitro stimulation was performed for 5 days in 24-well plates, each well containing 6×106 splenocytes, recombinant mIL-2 (20 units/ml) and 5×105 MCA-26 cells that had received 15,000 rad (150 Gy) of radiation. Effector cells were co-incubated with Cr51 (150 μCi for 5×106) labeled target cells for 4 hr at 37°C in different effector and target cell ratios. Parental MCA-26 cells were used as target cells for the CTL assay. After incubation, the radioactivity of 100 μl of the supernatant was counted in a gamma counter. The percentage of specific cytolysis was calculated as (experimental release— spontaneous release)/(maximum release—spontaneous release)×100.
Total radioactivity present in target cells was analyzed by lysing the cells with 10% SDS. Data represent the mean of triplicate cultures and were analyzed by logistic regression.
Morphological and Histopathological Analyses of Hepatic Tumors. Fourteen days after various gene therapy treatments, the animals were sacrificed and tumor volume was calculated according to the formula V=A×B2 (A=largest diameter; B=smallest diameter). For histopathological analysis, livers from euthanized animals of various treatment groups were collected and cut in the middle at the site of the original tumor inoculation. The tissue was then fixed in 10% buffered formalin and stained with hematoxylin and eosin for histopathological analysis.
Long-Term Survival Analyses. The tumor-bearing animals were treated with various recombinant vectors and kept for observation. Days of death were recorded and the results were analyzed statistically using a logrank test (9).
RESULTS
Suicide Gene Therapy for Liver Metastases of Colon Cancer. The gene encoding herpes simplex virus type 1 thymidine kinase (HSV-tk) is the most widely investigated suicide gene for cancer therapy. Unlike the mammalian thymidine kinases, HSV-tk efficiently phosphorylates nucleosidic analogs such as acyclovir or ganciclovir (10). The monophosphate form is subsequently converted into the di- and triphosphate forms by cellular kinases. The triphosphate is the toxic form of the nucleosidic analog, as it can be incorporated into elongating DNA in the dividing cells that results in cell death (11–13). This suicide gene therapy strategy for the treatment of hepatic metastases of colon cancer was investigated in rodent models. The regression of pre-established liver metastases after intratumoral injection of retrovirus-producer cells expressing HSV-tk followed by ganciclovir treatment has been reported (14). Infection of cancer cells after intratumoral injection of retroviral supernatant is low (15), although the grafting of virus producer-cells led to the transduction of up to 10% of the cells inside the tumor (14–16).
To overcome the low in vivo transduction efficiency of the retrovirus, recombinant adenoviral vectors have been used to transfer the HSV-tk gene into tumor cells. This approach, evaluated in mice, was very efficient in mediating the regression of a variety of tumors (17–20). In a mouse liver metastasis model of colon carcinoma, better than 80% of tumor regression was achieved after the suicide gene treatment (18). However, such extensive tumor destruction was not sufficient to yield significant survival benefit as compared with control vector treated mice (Fig. 1). Relapse of the hepatic tumors or the presence of disseminated metastases in other organs accounted for this lack of extended survival.
Combination Suicide and IL-2 Gene Therapy for Hepatic Metastases of Colon Carcinoma. Adenoviral mediated gene transfer of mIL-2 into the tumor was synergistical with HSV-tk and induced a systemic antitumor immunity that resulted in the further regression of the hepatic tumor as well as protection against distant site challenges of parental tumor cells (18). The antitumor immunity was attributed partly to the activation and proliferation of tumor specific CD8+ cytotoxic T lymphocytes. Animals treated with ADV/tk and ADV/mIL-2 lived significantly longer than those treated with ADV/tk or ADV/mIL-2 alone (Fig. 1). The control animals died between day 15 and day 30, while 60% of the animals were still alive at day 35 after combination treatment. The mean survival time has been increased from 22 days in the control-treated animals to 35 days in animals treated with both the HSV-tk and the mIL-2 vectors, and the results are statistically significant (P<0.03). In this model, ADV/mIL-2 treatment alone did not improve the long-term survival of the animal, as it did not cause regression of the hepatic tumors (10). After combination gene treatment, the antitumor immunity in the animals waned gradually over time (Fig. 2), resulting in the death of the animals due to tumor, recurrence in the liver and at distant sites. The antitumoral effect in the animals after combination treatment was mediated by a CTL response that could no longer be detected at day 38 (Fig. 2). To achieve long-term
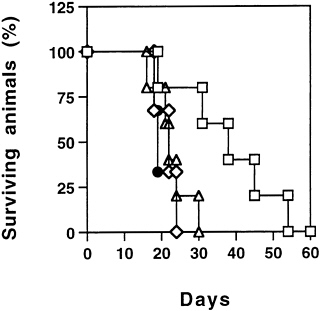
FIG. 1. Long-term survival of animals after the combination treatment. ADV/mIL-2+ADV/tk. Seven days after intrahepatic cancer cells injection, the tumor -bearing animals were divided into four treatment groups: ◇) ADV/β-galactosidase (5×108 pfu), n=4; (●) ADV/mIL-2 (3×108 pfu), n=4; (Δ) ADV/tk (5×108 pfu), n= 5; and (□) ADV/tk (5×108 pfu)+ADV/mIL-2 (3×108 pfu), n= 5. All the animals received ganciclovir treatment 12 hr after virus injection and were observed for survival over time, (logrank test, P< 0.03).
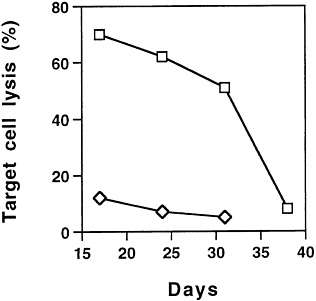
FIG. 2. Cellular immune response in animals after ADV/tk (5× 108 pfu) (◇) or ADV/tk (5×108 pfu)+ADV/mIL-2 (3×108 pfu) (□) treatments as measured by CTL assay. The splenocytes were isolated from various treatment groups at days 17, 24, 31, and 38 after tumor cell inoculation. The percentage of cell lysis is represented by the average lysis of splenocytes from four animals±SD.
protection against tumor recurrence and metastases, it is imperative for the protective immunity to be maintained. Therefore, identification of other cytokines that can enhance and prolong antitumor immunity is critically important to improve the efficacy of this therapeutic approach.
IL-12 Mediated Gene Therapy of Metastatic Colon Carcinoma. IL-12 is known to enhance the cytolytic activity of a number of immune effector cells including NK cells, lymphokine-activated killer cells, T cells and macrophages (21–24). It also stimulates the proliferation of activated NK and T cells (21, 23, 25). IL-12 is mainly produced by antigen-presenting cells (26) such as monocytes and macrophages, B cells, and dendritic cells (27) and it promotes cellular immune response by facilitating the proliferation and activation of TH1 cells (28). The antitumor activity of IL-12 is mainly mediated by IFN-γ produced by T cells and NK cells (29–31) and it has also been shown to inhibit angiogenesis through the IFN-inducible protein 10 (32, 33). IL-12 is a heterodimeric 70-kDa (p70) cytokine composed of two subunits of 40 kDa (p40) and 35 kDa (p35), and the association of both subunits is required for full biologic activity of the cytokine. To coexpress the two subunits in the same adenoviral vector, the two cDNAs were linked by the internal ribosome entry site element of the encephalomyocarditis virus and inserted into the E1 region of the adenoviral vector. Recombinant adenovirus was generated by the cotransfection into 293 cells with the plasmid harboring the bicistronic mIL-12 gene and pBHG10 (7), and individual plaques were expanded in 293 cells (8). To demonstrate the functionality of ADV/mIL-12, the colon cancer cell line MCA-26 was transduced at different m.o.i., and the supernatants were incubated with splenocytes from naive BALB/c mice to induce the release of IFN-γ. As shown by ELISA (Fig. 3), a strong IFN-γ production by the transduced cells was observed after ADV/ mIL-12 transduction, and the concentrations ranged from 7.9 ng/ml at 200 m.o.i. to 21.7 ng/ml at 1000 m.o.i. The antitumoral properties of ADV/mIL-12 were assessed in the colon carcinoma MCA-26 model. Tumors were generated in the liver as described above, and animals with tumor sizes of 4×4 to 5×5 mm2 were selected for subsequent experimentation. One animal group received intratumoral injection of ADV/mIL-12 at 5×108 pfu and another group was treated with 5×108 pfu of a control adenovirus ADV/DL312. Two weeks after viral inoculation, animals were sacrificed and liver tumors were
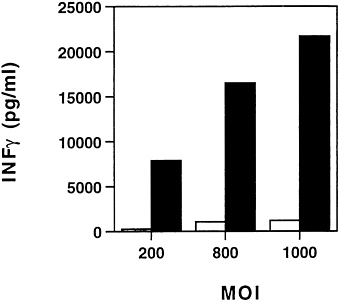
FIG. 3. In vitro characterization of ADV/mIL-12. Supernatants from ADV/mIL-12 (■) or ADV/β-galactosidase (□) MCA-26 cells infected at different m.o.i. were harvested and cocultured with splenocytes to induce the release of IFN-γ. The IFN-γ production was quantitated by ELISA.
harvested for macroscopic and microscopic analysis (Fig. 4). The mean tumor volume in the control group was 910±134 mm3 (mean±SD). In the mIL-12 vector treated group, the mean tumor volume was substantially reduced to 205±107 mm3 (mean±SD). Upon histopathological examinations, the animals that were treated with ADV/DL312 had large nodules of actively growing undifferentiated carcinoma cells (Fig. 5A). Very few or no cancer cells remained in the animals treated with ADV/mIL-12 (Fig. 5B). The tumors were replaced by fibrosis associated with a strong inflammatory response. The infiltrating cells appeared to be predominantly lymphocytes with some macrophages and neutrophils.
To assess the treatment outcome, animals treated with ADV/mIL-12 were studied for their long-term survival (Fig. 6). Control animals treated with buffer or with ADV/DL312 died between day 25 and day 38 after tumor cell inoculation. The animals treated with ADV/mIL-12 survived significantly longer with 25% of the animals (3 out of 12) still alive after 70 days. The survival time of the animals was increased by mIL-12
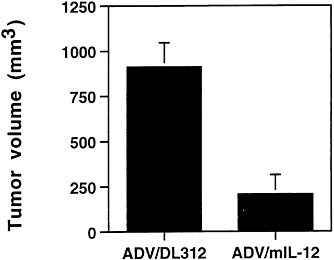
FIG. 4. Tumor volume analysis after intratumoral injection of ADV/mIL-12. Seven days after intrahepatic cancer cell injection, the tumor-bearing animals were divided into two treatment groups: ADV/DL312 (5×108 pfu); n=4 and ADV/mIL-12 (5×108 pfu); n=7. Animals were sacrificed 2 weeks after adenoviral injection for tumor volume measurement (ANOVA test, P<0.0001).
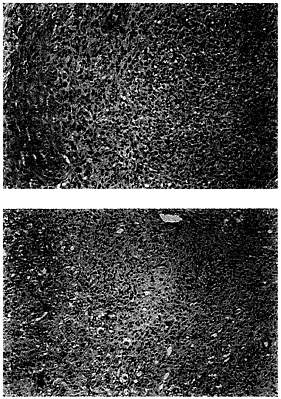
FIG. 5. Histopathological analysis of hepatic tumors after ADV/ mIL-12 treatment. (A) Section of a tumor from a control mouse injected with buffer. Undifferentiated cancer cells are dividing actively and infiltrate the liver without any inflammatory response. (B) Section of a tumor from a treated mouse injected with ADV/mIL-12 (5×108 pfu). No cancer cells remain. Instead there is a vigorous inflammatory response composed mainly by lymphocytes and macrophages. The ducts (upper right) represent a site where the tumor had adhered to pancreas and are pancreatic ductules. (×200.)
vector treatment alone, which is statistically significant (P< 0.0001).
DISCUSSION
Suicide gene therapy for the treatment of liver metastases of colon carcinoma has been evaluated in rodent models using retroviral and adenoviral vectors. In both cases, partial tumor
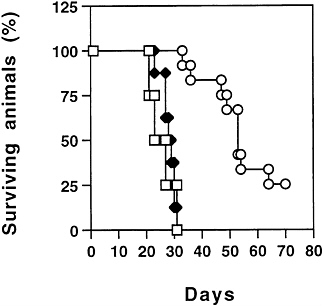
FIG. 6. Long-term survival of animals after ADV/mIL-12 treatment. Seven days after intrahepatic cancer cells injection, the tumor bearing animals were divided into three treatment groups (□) Buffer; n=4 (◆) ADV/DL312 (5×108 pfu); n=7 (○) ADV/mIL-12 (5× 108 pfu); n=12. The animals were observed for survival over time (logrank test, P<0.0001).
regression has been observed after ganciclovir treatment. However, the animals did not live significantly longer due to the recurrence of hepatic tumors or disseminated metastases in other organs. Addition of the ADV/mIL-2 vector in the treatment protocol induced an effective systemic antitumoral immunity in the host that significantly prolonged the survival of the tumor-bearing animals. This antitumoral immunity waned with time and eventually led to tumor recurrence and animal death. IL-12, a cytokine mainly produced by antigen-presenting cells, has shown powerful antitumoral activity against various tumors in subcutaneous models (24, 26, 36–39). Renal adenocarcinoma (RENCA), melanoma (B16), reticulum cell sarcoma (M5076), sarcoma (MCA-105 and MCA-207), and Lewis lung carcinoma and colon carcinoma (MC-38 and CC-26) respond to recombinant IL-12 administration, leading to long-term animal survival in some cases. Several clinical trials for cancer treatment have already been started, and preliminary results in one trial showed severe toxicity: 15 out of 17 patients experienced serious adverse events affecting multiple organ systems (gastrointestinal tract bleeding, asthenia, and hepatotoxicity), and two patients died (37). In vivo gene therapy is one strategy to avoid the toxicity associated with systemic delivery of recombinant IL-12. Intratumoral injection of ADV/mIL-12 can lead to cytokine expression in the vicinity of the tumor and enhance the antitumoral immunity in the host.
We constructed a recombinant adenoviral vector in which mIL-12 was cloned in the E1 deleted region. The cDNA from the two subunits, linked with an internal ribosome entry site, was efficiently transcribed from the Rous sarcoma virus promoter and produced bioactive mIL-12. Intratumoral injection of ADV/mIL-12 alone yielded substantial or complete hepatic tumor regression of the poorly immunogenic MCA-26 colon carcinoma. This treatment was able to significantly prolong the survival of tumor-bearing animals, with 25% of the treated mice still living after 70 days. This tumoricidal effect appeared to be even more potent than the combination treatment with ADV/tk and ADV/mIL-2.
NK cells are an important type of effector cells that are presumed to play a role in the surveillance of cancer and in the control of metastases. Mouse liver contains a large proportion of NK cells that can be potent cytotoxic effector cells against tumors after stimulation with mIL-12 (38, 39). These cells can lyse NK-sensitive and NK-resistant tumor targets as shown by in vitro cytotoxicity assay (38, 39). After intraperitoneal injection of an adenovirus expressing mIL-12, Bramson et al. (40) detected some NK activity in the splenocyte and in the lung effector cell population against the NK-sensitive cell line YAC-1 at day 2 after adenoviral injection. These cells act for a short period of time, and they are subsequently replaced by tumor-specific CTL (41). IL-12 is a strong activator of both NK and CTL, which could be contributing factors to the tumoricidal activities in our model.
One way to increase the CTL activity, which we are currently investigating, is to combine the ADV/mIL-12 treatment with ADV/mIL-2 and ADV/tk. Indeed, several other groups have reported the synergistic effect of IL-12 and IL-2 to generate cytotoxic activity in T cells and in NK cells (42–45). This additional treatment can potentially enhance the antitumoral efficacy of IL-12 and induce a long-lasting systemic antitumoral immunity. The results obtained with a poorly immunogenic tumor are encouraging for the further development of in vivo gene therapy as a treatment modality for metastases from colon cancer in humans.
We thank Doberta Bell for the preparation of this manuscript. The work was supported in part by National Cancer Institute Grant CA-70337–01 (to S.H.C.). M.C. is an Associate and S.L.C.W. is an Investigator of the Howard Hughes Medical Institute.
1. Dreben, J.A. & Niederhuber, J.E. (1993) in Current Therapy in Oncology, ed. Niederhuber, J.E. (Decker, St. Louis), pp. 426– 431.
2. Dreben, J.A. & Niederhuber, J.E. (1993) in Current Therapy in Oncology, ed. Niederhuber, J.E. (Decker, St. Louis), pp. 389– 395.
3. Pardoll, D.M. (1995) Annu. Rev. Immunol. 13, 399–415.
4. Corbett, T.H., Griswold, D.P., Roberts, B.J., Peckham, J.C. & Schabel, F.M., Jr. (1975) Cancer Res. 35, 2434–2439.
5. Graham, F.L., Smiley, J., Russell, W.C. & Nairn, R. (1977) J. Gen. Virol. 36, 59–72.
6. Fang, B., Eisensmith, R.C., Li, X.H.C., Finegold, M.J., Shedlovsky, A., Dove, W. & Woo, S.L.C. (1994) Gene Ther. 1, 247–254.
7. Bett, A.J., Haddara, W., Prevec, L. & Graham, F.L. (1994) Proc. Natl. Acad. Sci. USA 91, 8802–8806.
8. Graham, F.L. & Prevec, L. (1991) in Methods in Molecular Biology: Gene Transfer and Expression Protocols, ed. Murray, E.J. (Human Press, Clifton, NJ), Vol. 7, pp. 109–128.
9. Cox, D.R. & Oakes, D. (1984) in Analysis of Survival Data, eds. Cox, D.R. & Hinkley, D.V. (Chapman & Hall, New York), pp. 91–139.
10. Elion, G.B. (1983) J. Antimicrob. Chemother. 12, 9–17.
11. Nishiyama, Y. & Rapp, F. (1979) J. Gen. Virol. 45, 227–230.
12. Furman, P.A., McGuirt, P.V., Keller, P.M., Fyfe, J.A. & Elion, G.B. (1980) Virology 102, 420–430.
13. Davidson, R.L., Kaufman, E.R., Crumpacker, C.S. & Schnipper, L.E. (1981) Virology 113, 9–19.
14. Caruso, M., Panis, Y., Gagandeep, S., Houssin, D., Salzmann, J.-L. & Klatzmann, D. (1993) Proc. Natl. Acad. Sci. USA 90, 7024–7028.
15. Short, M.P., Choi, B.C., Lee, J.K., Malick, A., Breakefield, X.O. & Martuza, R.L. (1990) J. Neurosci. Res. 27, 427–433.
16. Ram, Z., Culver, K., Walbridge, K.W., Blaese, R.M., & Oldfield, E.H. (1993) Cancer Res. 53, 83–88.
17. Chen, S.-H., Shine, H.D., Goodman, J.C., Grossman, R.G. & Woo, S.L.C. (1994) Proc. Natl. Acad. Sci. USA 91, 3054–3057.
18. Chen, S.-H., Li Chen, X.H., Wang, Y., Kosai, K.-I., Finegold, M.J., Rich, S.S. & Woo, S.L.C. (1995) Proc. Natl. Acad. Sci. USA 92, 2577–2581.
19. O’Malley, B.W., Chen, S.-H., Schwartz, M.R. & Woo, S.L.C. (1995) Cancer Res. 55, 1080–1085.
20. Eastham, J., Chen, S.-H., Sehgal, I., Timme, T., Hall, S., Woo, S.L.C. & Tompson, T. (1996) Hum. Gene Ther. 7, 515–523.
21. Kobayashi, M., Fitz, L., Ryan, M., Hewick, M., Clark, S.C., Chan, S., Loudon, R., Sherman, F., Perussia, B. & Trinchieri, G. (1989) J. Exp. Med. 170, 827–845.
22. Naume, B., Gately, M. & Espevik, T. (1992) J. Immunol. 148, 2429–2436.
23. Robertson, M.J., Soiffer, R.J., Wolf, S.F., Manley, T.J., Donahue, C., Young, D., Herrmann, S.H. & Ritz, J. (1992) J. Exp. Med. 175, 779–788.
24. Wolf, S.A., Temple, P.A., Kobayashi, M., Young, D., Dicig, M., Lowe, L., Dzialo, R., Fitz, L., Ferenz, D., Hewick, R.M., Kelleher, K., Herrmann, S.H., Clark, S.C., Azzoni, L., Chan, S.H., Trinchieri, G. & Perussia, B. (1991) J. Immunol. 146, 3074–3081.
25. Gately, M.K., Wolitzky, A.G., Quinn, P.M. & Chizzonite, R. (1992) Cell. Immunol. 143, 127–142.
26. D’Andrea, A., Rengaraju, M., Valiante, N.M., Chehimi, J., Kubin, M., Aste, M., Chan, S.H., Kobayashi, M., Young, D., Nickbarg, E., Chizzonite, R., Wolf, S.F. & Trinchieri, G. (1992) J. Exp. Med, 176, 1387–1398.
27. Heufler, C., Koch, F., Stanzl, U., Topar, G., Wysocka, M., Trinchieri, G., Enk, A., Steinman, R.M., Romani, N. & Schuler, G. (1996) Eur. J. Immunol. 26, 659–668.
28. Trinchieri, G. (1993) Immunol. Today 14, 335–338.
29. Brunda, M.J., Luistro, L., Warrier, R.R., Wright, R.B., Hubbard, B.R., Murphy, M., Wolf, S.F. & Gately, M.K. (1993) J. Exp. Med. 178, 1223–1230.
30. Brunda, M.J., Luistro, L., Hendrzak, J.A., Fountoulakis, M., Garotta, G. & Gately, M.K. (1995) J. Immunother. 17, 71–77.
31. Nastala, C.L., Edington, H.D., McKinney, T.G., Tahara, H., Nalesnik, M.A., Brunda, M.J., Gately, M.K., Wolf, S.F., Schreiber, R.D., Storkus, W.J. & Lotze, M.T. (1994) J. Immunol. 153, 1697–1706.
32. Voest, E.E., Kenyon, B.M., O’Reilly, M.S., Truitt, G., D’Amato, R.J. & Folkman, J. (1995) J. Natl. Cancer Inst. 87, 581–586.
33. Sgadari, C., Angiolillo, A.L. & Tosato, G. (1996) Blood 87, 3877–3882.
34. Tahara, H., Zeh, H.J., Storkus, W.J., Pappo, I., Watkins, S.C., Gubler, U., Wolf, S.F., Robbins, P.D. & Lotze, M.T. (1994) Cancer Res. 54, 182–189.
35. Zitvogel, L., Tahara, H., Robbins, P.D., Storkus, W.J., Clarke, M.R., Nalesnik, M.A. & Lotze, M.T. (1995) J. Immunol. 155, 1393–1403.
36. Martinotti, A., Stoppacciaro, A., Vagliani, M., Melani, C., Spreafico, F., Wysocka, M., Parmiani, G., Trinchieri, G. & Colombo, M.P. (1995) Eur. J. Immunol. 25, 137–146.
37. Lamont, A.G. & Adorini, L. (1996) Immunol. Today 17, 214– 217.
38. Hashimoto, W., Takeda, K., Anzai, R., Ogasawara, K., Sakihara, H., Sugiura, K., Seki, S. & Kumagai, K. (1995) J. Immunol. 154, 4333–4340.
39. Takeda, K., Seki, S., Ogasawara, K., Anzai, R., Hashimoto, W., Sugiura, K., Takahashi, M., Masayuki, S. & Kumagai, K. (1996) J. Immunol. 156, 3366–3373.
40. Bramson, J., Hitt, M., Gallichan, W.S., Rosenthal, K.L., Gauldie, J. & Graham, F.L. (1996) Hum. Gene Ther. 7, 333–342.
41. Kos, F.J. & Engleman, E.G. (996) Immunol. Today 17, 174–176.
42. Gately, M.K., Warrier, R.R., Honasoge, S., Carvajal, D.M., Faherty, D.A., Connaughton, S.E., Anderson, T.D., Sarmiento, U., Hubbard, B.R. & Murphy, M. (1994) Int. Immunol. 6, 157–167.
43. Soiffer, R.J., Robertson, M.J., Murray, C., Cochran, K. & Ritz, J. (1993) Blood 82, 2790–2796.
44. Rossi, A.R., Pericle, F., Rashleigh, S., Janiec, J. & Djeu, J.Y. (1994) Blood 83, 1323–1328.
45. Vagliani, M., Rodolfo, M., Cavallo, F., Parenza, M., Melani, C., Parmiani, G., Forni, G. & Colombo, M.P. (1996) Cancer Res. 56, 467–470.





