This paper was presented at a colloquium entitled “Genetic Engineering of Viruses and of Virus Vectors,” organized by Bernard Roizman and Peter Palese (Co-chairs), held June 9–11, 1996, at the National Academy of Sciences in Irvine, CA.
Alphavirus-based expression vectors: Strategies and applications
(RNA replicon/protein production/nucleic acid vaccines/gene therapy)
ILYA FROLOV, THOMAS A. HOFFMAN, BÉLA M. PRÁGAI, SERGEY A. DRYGA, HENRY V. HUANG, SONDRA SCHLESINGER, AND CHARLES M. RICE *
Department of Molecular Microbiology, Washington University School of Medicine, 660 South Euclid Avenue, St. Louis, MO 63110–1093
ABSTRACT Alphaviruses are positive-strand RNA vi-ruses that can mediate efficent cytoplasmic gene expression in insect and vertebrate cells. Through recombinant DNA technology, the alphavirus RNA replication machinery has been engineered for high-level expression of heterologous RNAs and proteins. Amplification of replication-competent alphavirus RNAs (replicons) can be initiated by RNA or DNA transfection and a variety of packaging systems have been developed for producing high titers of infectious viral particles. Although normally cytocidal for vertebrate cells, variants with adaptive mutations allowing noncytopathic replication have been isolated from persistently infected cultures or selected using a dominant selectable marker. Such mutations have been mapped and used to create new alphavirus vectors for noncytopathic gene expression in mammalian cells. These vectors allow long-term expression at moderate levels and complement previous vectors designed for short-term high-level expression. Besides their use for a growing number of basic research applications, recombinant alphavirus RNA replicons may also facilitate genetic vaccination and transient gene therapy.
Alphaviruses are enveloped positive-strand RNA viruses that have served as model systems for studies in virology and cell biology (for review, see refs. 1 and 2). Over the past 10 years, the alphavirus RNA replication and packaging machinery has been adapted for expression of heterologous RNAs and proteins in animal cells (for reviews, see refs. 3–6). As transient expression systems, alphaviruses offer several advantages. These include (i) a broad range of susceptible host cells including those of insect, avian, and mammalian origin; (ii) high levels of cytoplasmic RNA and protein expression without splicing; and (iii) the facile construction and manipulation of recombinant RNA molecules using full-length cDNA clones from which infectious RNA transcripts can be generated by in vitro transcription. Two principal strategies are being employed for expression of heterologous sequences: (i) engineering infectious recombinant RNAs that express additional subgenomic RNAs and (ii) replacement of the structural genes to produce self-replicating RNA “replicons” that can be packaged into infectious particles using defective helper RNAs or packaging cell lines. In addition, incorporation of heterologous ligands or receptors into the virion envelope may eventually allow targeting of engineered alphavirus RNAs to specific cell types. This overview briefly discusses the background, methodology, and applications of these alphavirus vector systems, which range from high-level protein production in cell culture to the induction of protective immunity in animals.
The Alphavirus Lifecycle
The alphavirus particle contains a single genomic RNA complexed with 240 molecules of a basic capsid protein (C), surrounded by a lipid bilayer containing 240 E1E2 envelope glycoprotein heterodimers. Both the nucleocapsid and the envelope are organized with T=4 icosahedral symmetry (see ref. 7). Alphaviruses can infect a variety of cell types and appear to be able to use more than one cell surface receptor (2). After entry (1), the genomic RNA initially serves as an mRNA for translation of the viral nonstructural proteins (nsPs) required for initiation of viral RNA amplification.
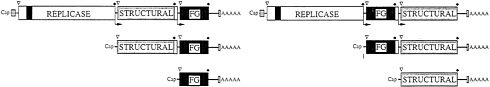
RNA replication occurs via synthesis of a full-length minus-strand intermediate that is used as the template for synthesis of additional genome-length RNAs and for transcription of a plus-strand subgenomic RNA from an internal promoter (Fig. 1). This subgenomic RNA, which can accumulate to levels approaching 106 molecules per cell, is the mRNA for translation of the structural proteins. The synthesis of minus, plus, and subgenomic RNAs is temporally regulated via proteolytic processing of nonstructural polyprotein replicase components by a virus-encoded protease residing in the C-terminal region of nsP2 (8, 9).
The structural proteins are initially translated as a polyprotein (NH2-C-E3-E2–6K-E1-COOH) that is processed coand posttranslationally to produce the mature products. Cleavage at the C-E3 site is mediated by a chymotrypsin-like protease activity residing in the C-terminal portion of the C protein. E3 and E2 are initially made as a precursor (called PE2 or P62) that is processed by a furin-like activity late during release of the virus from infected cells. Envelope glycoproteins E1 and PE2, separated by signal peptidase cleavages, form a heterodimer that migrates through the secretory pathway to the plasma membrane. In the cytoplasm, C-protein subunits complex with the genome RNA to form a nucleocapsid that matures by budding through the plasma membrane, acquiring a lipid bilayer envelope with embedded viral glycoproteins.
Infectious Alphavirus cDNA Clones
Studies on the use of alphaviruses as vectors have required the recovery of infectious replication-competent RNA transcripts from cDNA clones. Functional full-length cDNA clones from which infectious RNA transcripts can be synthesized have been reported for SIN (10), SFV (11), VEE (12), and Ross River virus (13). These clones have proven of great value for basic studies on alphavirus replication, including the definition of RNA elements important for RNA replication, subgenomic
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: C protein, capsid protein; nsP, nonstructural protein; CAT, chloramphenicol acetyltransferase; DHRNA; defective-helper RNA; PAC, puromycin acetyltransferase.
|
* |
To whom reprint requests should be addressed, e-mail: rice@borcim.wustl.edu. |

FIG. 1. Alphavirus replication cycle. Translated regions of alphavirus genomic and subgenomic RNAs are shown as boxes with the nonstructural proteins and structural proteins (STRUCTURAL) indicated as open and lightly shaded boxes, respectively. Cis-acting sequences important for replication and transcription are shown (small, checkered boxes) as is the sequence in the nonstructural region important for encapsidation (solid box). The start site for subgenomic mRNA transcription on the (−) strand genome-length RNA template is indicated by an arrow. Translation initiation (aug) and termination signals (trm) are indicated by open triangles and solid diamonds, respectively (from ref. 4).
RNA transcription, and genome RNA packaging (2) (see Fig. 1). Since the alphaviruses are positive-strand RNA viruses, virion-associated proteins are not required for initiation of the replication cycle. Capped RNA transcripts, produced by in vitro transcription with SP6 or T7 polymerase, are typically used to transfect tissue culture cells, usually a continuous hamster kidney line (BHK) or secondary chicken embryo fibroblasts. RNA transfection is facilitated by DEAE-dextran, cationic liposomes, or electroporation. In the latter method, efficiencies can approach 100% for BHK cells (11).
Although less efficient than transfection of full-length RNAs, alphavirus replication can also be initiated by transfection of plasmid DNA (14, 15). In this case, full-length 5′-capped RNAs are transcribed in the nucleus using a polymerase II promoter and transported to the cytoplasm, the site of primary translation and RNA amplification.
Replication and Packaging-Competent Vectors
Several approaches have been taken for independent expression of heterologous genes using the alphavirus RNA replication machinery. The identification of the SIN subgenomic RNA promoter element allowed the construction of RNAs with additional subgenomic RNA promoters (Fig. 2). Recombinant RNAs containing two promoters for subgenomic mRNA synthesis are referred to as double subgenomic RNA vectors (dsSIN) (16–18). Heterologous sequences, expressed via a second subgenomic mRNA, can be located either 3′ or 5′ to the structural protein genes. These vectors are both replication and packaging competent and allow the rapid recovery of high-titered infectious recombinant virus stocks usually in the range of 108–109 plaque-forming units/ml. In initial studies (16), dsSIN recombinants were engineered to express bacterial chloramphenicol acetyltransferase (CAT), a truncated form of the influenza hemagglutinin (HA), or minigenes encoding two distinct immunodominant cytotoxic T-cell (CTL) HA epitopes. Infection of murine cell lines with these recombinants resulted in the expression of 106–107 CAT polypeptides per cell and efficient sensitization of target cells for lysis by appropriate major histocompatibility complex (MHC)-restricted HA-specific CTL clones in vitro. In addition, priming of an influenza-specific T-cell response was observed after immunizing mice with dsSIN recombinants expressing either truncated HA or the immunodominant influenza CTL epitopes. This system allows the generation of high-titered recombinant virus stocks in a matter of days and has been useful for mapping and mutational analysis of class I MHC-restricted T-cell epitopes expressed via the endogenous pathway of antigen processing and presentation (19, 20). Because of packaging constraints and instability of larger inserts upon passaging, this approach is primarily useful for short (<2 kb) heterologous sequences.
In other studies, dsSIN recombinants have been used to express the Japanese encephalitis virus (21) and rubella virus (22) stuctural proteins, to deliver a single chain antibody for intracellular immunization against tick-borne encephalitis (23), to map the domain of GLUT-4, the insulin-regulatable glucose transporter, which is responsible for efficient intracellular sequestration (24), to study structure-function aspects of ras-like GTP-binding proteins involved in vesicular transport (25–29), and to probe the interplay between viral and cellular genes involved in apoptotic cell death (30, 31).
Another interesting application has been for gene expression studies in mosquito cells and mosquitoes (32). Engineered dsSIN recombinants have been used to follow virus spread in whole mosquitoes (33) and to express antisense RNAs or viral proteins that are capable of specifically inhibiting replication (34–36) and transmission (37) of heterologous viruses and may be useful for studies of normal mosquito gene function via antisense RNA-mediated inhibition.
Alphavirus RNA Replicons
The prototype replication-competent, but packaging-defective, alphavirus RNA replicon was developed by replacing the SIN structural genes with the CAT gene (38) (Fig. 3, upper-left section). In cells transfected with this SIN recombinant RNA, CAT is expressed rapidly and up to 108 CAT polypeptides are produced per transfected cell by 16–20 h. CAT expression could be regulated by inclusion of a ts
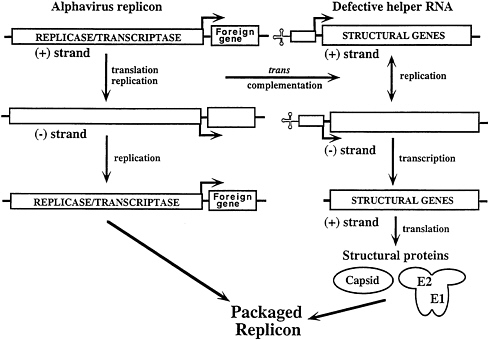
FIG. 3. Packaging of replicons by cotransfection of DHRNAs expressing the alphavirus structural proteins. See the text for details.
mutation blocking RNA synthesis. Similar RNA replicons have also been developed for SFV (39) and VEE (R.Johnston, personal communication).
Since these RNA replicons do not encode structural proteins, they are incapable of spread and the level of heterologous product synthesized in transfected cells is directly related to the transfection efficiency of the recombinant RNA. Conditions for efficient RNA transfection using either cationic liposomes or electroporation have been determined for only a few cell types, which limits the usefulness of these vectors for high-level production or experiments where expression in every cell is required.
Packaging Systems
The utility of the alphavirus replicon expression systems has been markedly improved by development of a series of defective helper RNAs that allow efficient packaging of RNA replicons (39, 40). Defective-helper RNAs (DHRNAs) are designed to contain the cis-acting sequences required for replication as well as the subgenomic RNA promoter driving expression of the structural protein genes. Packaging of SIN replicons is achieved by efficient cotransfection of BHK cells with both RNAs by electroporation (11) (Fig. 3). Replicase/ transcriptase functions supplied by the vector RNA lead not only to its own amplification but also act in trans to allow replication and transcription of the helper RNA. This results in synthesis of structural proteins that can package the replicon with >108 infectious particles per ml (5×109 infectious particles per electroporation) being produced after only 16–24 h. Such stocks can be used, without further phenotypic selection, to infect cells for expression studies or high-level protein production. Current experience suggests that it should be possible to package replicons containing at least 5 kb of heterologous sequence.
A spectrum of DHRNAs have been characterized that differ in their ability to be packaged (39–41; Fig. 4). Some DHRNAs that allow packaging of the replicon as well as themselves are useful under conditions where extensive amplification by passaging is advantageous (Fig. 4A) (41). Other DHRNAs allow efficient packaging of replicons but are packaged very poorly themselves (Fig. 4B) (40). These latter helpers are useful for applications where expression of the viral structural proteins and virus spread are not desired.
A potential problem with the helper-free “one-way” packaging strategy just described is that recombination can occur between replicon and helper RNAs to produce wild-type virus (43, 44). One approach to minimize this possibility is to use two DHRNAs, one that expresses the capsid protein and a second that expresses the viral glycoproteins (I.F., unpublished results; Fig. 5). The capsid protein, expressed independently, accumulates at high levels, but to achieve similar levels of viral glycoprotein expression retention of the 5′ terminus of the capsid protein mRNA, which acts as a translational enhancer, is required (see below). Deletions in the capsid protein gene that preserve both the 5′ terminus (the enhancer region) and the 3′ half (the sequences that code for the autoprotease activity) but eliminate the region that binds RNA produce high levels of glycoprotein expression from a second DHRNA (I.F. and S.S., unpublished results). Capsid protein genes from heterologous alphaviruses can also be used to enhance translation of the glycoproteins and should further reduce the probability of wild-type virus generation via recombination.
In addition to packaging of alphavirus RNA replicons by cotransfection with DHRNAs, continuous packaging cell lines have been developed that express a DHRNA under the control of a nuclear promoter (I.F. and S.S., unpublished results). Such cells may be useful for rescuing transfected RNA replicons, titering packaged replicons, and production of large quantities of packaged replicon stocks by low-multiplicity passage.
The Alphavirus Translational Enhancer
In the course of studying the expression of proteins by alphavirus replicons, it was noticed that the level of heterologous protein expression was much lower than that observed for the authentic C protein. This observation led to the discovery of a translational enhancer in the C-protein coding region (45, 46). A series of C-lacZ fusion constructs localized the element to the 5′ portion of the subgenomic RNA encoding the N-terminal region of the C protein (45, 46). Subsequent studies strongly suggest that an RNA element in this region of the subgenomic RNA enhances translation of the C protein in alphavirus-infected, but not uninfected, cells (45, 47). SINlacZ replicons that lack this region express ≈50 μg of β-ga-
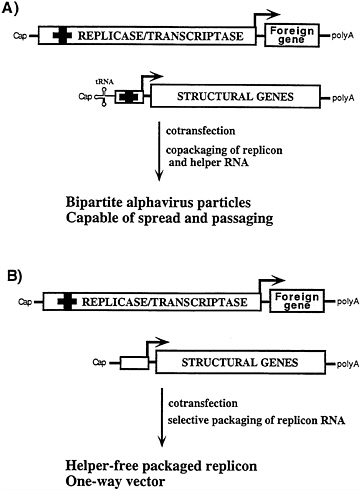
FIG. 4. Bipartite and “helper-free” packaging strategies for alphavirus replicons. (A) Cotransfection with this DHRNA containing a 5′ tRNA and cis-acting packaging signal (bold cross; ref. 42) leads to efficient packaging of both replicon and DHRNA (40). The 5′ tRNA enhances both DHRNA replication (40) and packaging (E.Frolova, I.F., and S.S., unpublished results). Such bipartite alphavirus stocks form plaques and can be amplified by multiple rounds of passaging. (B) Using DHRNAs that lack the 5′ tRNA and packaging signal, selective packaging of the replicon RNA is obtained. This method is used to produce “one way” vectors essentially free of packaged helper RNA.
lactosidase per 106 BHK cells, whereas cells infected with replicons containing the enhancer element accumulate 10- to 20-fold higher levels (≈650 μg of β-galactosidase per 106 cells; refs. 45 and 46).
Such high levels of expression necessitate that the heterologous protein be expressed as a C-protein fusion. Besides the incorportion of a site for specific proteolytic cleavage in vitro, several stratagies have been tried or are envisioned to produced high-level expression of unfused product in vivo. One strategy employs the C-protein autoprotease activity that cleaves at the C-PE2 junction and requires limited downstream PE2 sequences (46) (Fig. 6). Alternatively, to produce heterologous proteins without additional N-terminal residues, a ubiquitin monomer can be inserted in-frame between between C and the heterologous product. Such constructs are cleaved efficiently in vivo by the host enzyme ubiquitin carboxyl-terminal hydrolase (see ref. 8 and citations therein).
Effects of Alphavirus Infection on Host Cell Biology
In nature, alphaviruses are transmitted to vertebrate hosts by mosquitoes. Insect vectors become chronically infected without apparent deleterious consequences. As mentioned earlier, this property has allowed SIN vectors to be used for studies requiring prolonged gene expression in whole mosquitoes. In the vertebrate host, however, the biology of virus infection is quite different. Replication is rapid to achieve titers high enough for efficient transmission before virus-specific immune reponses neutralize infectivity and clear infection. In some aspects, cell culture growth properties reflect these biological differences. In permissive vertebrate cells, virus infection results in the rapid shut off of host mRNA translation, takeover of the translational machinery by viral mRNAs, production of high titers of infectious virus, and cell death within 12–24 h. In contrast, the rate of virus replication in mosquito cells is slower, often with minimal effects on the insect cell and persistent infections are readily established.
For some applications, high expression levels and rapid shut off of host mRNA translation can be advantageous. For instance, alphavirus-expressed proteins can be metabolically labeled and analyzed directly without the need for specific antisera.
Noncytopathic Gene Expression in Mammalian Cells
For applications requiring long-term expression and minimal pertubation of vertebrate host cell biology, alphavirus-induced shut-off of host mRNA translation and cell death are undesirable. It has been possible to select for changes in the alphavirus replication machinery that allow persistent noncytopathic replication in vertebrate cells. One strategy has been to establish a persistent infection of BHK cells with SIN (72). One month later, a variant (SIN-1) was isolated by plaque purification. SIN-1 can readily establish persistent infection of naive BHK cells, suggesting that the SIN-1 genome contains appropriate adaptive mutations. These adaptive changes have recently been identified and are being incorporated into SIN vectors (S.A.D. and S.S., unpublished results).
A second strategy has been to use a dominant selectable marker, puromycin acetyltransferase (PAC; ref. 48), to select for adapted SIN replicons (I.F., T.A.H., B.M.P., M.Lippa, S.S., and C.M.R., unpublished results). This approach is outlined in Fig. 7. SIN replicons, lacking the structural genes and capable of expressing PAC, were transfected into BHK-21 cells by electroporation. After recovery, puromycin was added at sufficient levels to inhibit translation in untransfected cells. In transfected cells, SIN expression of PAC allows continued translation of viral mRNAs and replication. But SIN replication also leads to shut off of host mRNA translation and eventual cell death (see ref. 49) and the majority of the cells will die, either as a consequence of SIN replication or puromycin sensitivity. Surviving cells (10−6) must have undergone some change, either in host or viral components, that prevent cell death and allow continued expression of PAC. Given the high mutation rate of RNA viruses when compared with host DNA replication, it seemed most likely that changes in the SIN machinery will be responsible for such an adaptation. This has turned out to be the case and multiple puromycin-resistant cell lines harboring adapted replicons have been obtained and characterized. In two of these cell lines, S1 and S24, the adaptive mutation maps to the nsP2 gene. Incorporation of this change into the replicon genome produces vector RNAs that have no observable effect on host translation and are able to establish long-term replication and expression in BHK cells. Using double subgenomic RNA promoter constructs, electroporation and puromycin selection can be used to quickly establish cell populations or clonal cell lines expressing heterologous RNAs and proteins (Fig. 7) (I.F., E.Agapov, and C.M.R., unpublished results).
Host Range and Targeting Infection
While alphaviruses replicate in a variety of tissues, there are substantial differences in tissue tropism for a particular alphavirus or among alphaviruses (50). Unfortunately, little is known about the viral determinants, cognate cell surface receptors, and intracellular environments that modulate entry and replication. For example, SIN replicates efficiently in
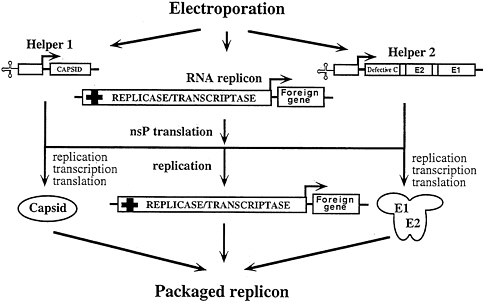
FIG. 5. Cotransfection of two DHRNAs to diminish production of wild-type virus via recombination. Packaging is accomplished by cotransfection of two DHRNAs with the expression replicon. One DHRNA (Helper 1) expresses the C protein and a second DHRNA (Helper 2) is designed for high-level expression of the virion glycoproteins. Helper 2 uses a deleted version of the C-protein coding region that is still able to function as a translational enhancer and an autoprotease but is defective for packaging.
fibroblasts (20) and muscle but poorly in lymphoid cells (B.D. Lindenbach and C.M.R., unpublished results). In contrast, VEE readily infects lymphoid cells and engineered VEE vaccine vectors can elicit protective mucosal immune reponses (51). Most alphaviruses replicate in neurons, although the efficiency of replication and cytotoxicity differ depending upon the virus strain and the differentiated state of the neuron (52, 53). In terms of choosing a particular alphavirus expression system, these differences are worth noting but the optimal vector for a given application is best determined empirically.
For transient gene therapy in vivo, it would be advantageous to target engineered alphavirus RNAs to specific cell types. One approach is to modify the viral envelope. Such modified viruses must be competent for assembly and release from transfected cells but unable to bind and enter the normally wide range of host cells that the virus usually infects. A functional heterologous ligand or binding domain displayed on the virion surface would allow selective virus binding to target cells expressing the cognate binding partner. Once bound, the
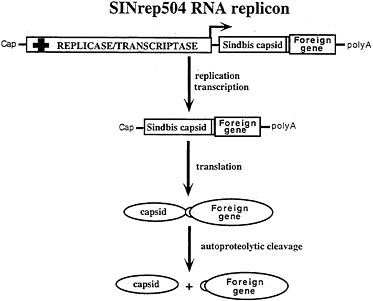
FIG. 6. Alphavirus vectors for high-level protein expression. In SINrep504 (I.F. and A.A.Kolykhalov, unpublished results) replicons, heterologous protein coding regions are fused, in-frame, downstream of the C-PE2 cleavage site. Rapid autoproteolytic cleavage by the chymotrypsin-like C protease releases the foreign gene product.
engineered virus must still be able to efficiently enter the cell and initiate replication.
Experiments along these lines have been undertaken using SIN (54, 55). Since a high-resolution structure is not available for the SIN virion or the E1E2 heterodimer, a random mutagenesis strategy was used to identify sites in the viral glycoproteins permissive for insertion of an 11-amino acid protective epitope (called “4D4”) from Rift Valley fever virus. Random insertion libraries were derived by treating plasmid DNA with DNase I or methidiumpropyl-EDTA-Fe(II) and permissive insertion sites were mapped in the E3, E2, 6K, and E1 proteins (ref. 54 and unpublished data). Insertions near the N terminus of the E2 glycoprotein or in an internal region of E2 resulted in 4D4 epitope expression on the virion surface and elicited a partially protective immune response against lethal Rift Valley fever virus challenge (54).
These full-length random insertion libraries are now being used to identify permissive insertion sites for other peptides and larger functional domains that are compatible with recovery of infectious virus. In the case of the Rift Valley fever virus epitope library, replacement of the 4D4-encoding oligonucleotide in the full-length random insertion library with another oligonucleotide can be accomplished in a single step and was used to identify a cluster of sites in the E3 protein permissive for insertion of an 81-residue heterologous peptide (S. London and C.M.R., unpublished results). Similar experiments are in progress for the gp120-binding domain of CD4, protein G, the measles virus receptor (CD46), and single-chain antibodies (S.A.D., E.Mendez, C.M.R., and S.S., unpublished results).
Besides modifying the alphavirus packaging machinery, heterologous packaging systems may also prove useful for delivering engineered alphavirus RNAs. Pseudotypes are readily produced during encapsidation of retrovirus RNAs and it may be possible to modify the retrovirus packaging machinery to allow selective packaging and targeting (see ref. 56) of heterologous RNAs.
Other strategies may be useful for extending the range of susceptible host cells. For vesicular stomatitis virus, the G glycoprotein mediates attachment and infection of many cell types. Remarkably, expression of the G glycoprotein by an SFV replicon leads to the production of low titers of infectious virus-like particles (57, 58).
Other Applications
Besides reporters such as β-galactosidase and CAT, alphavirus replicons have been used to express a variety of RNA and
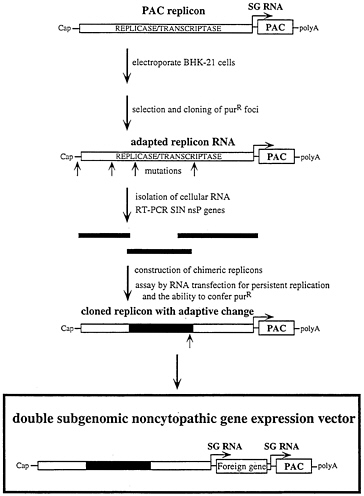
FIG. 7. Strategy for selecting adapted replicons for noncytopathic gene expression in mammalian cells. A SIN replicon expressing the PAC gene is used to transfect BHK cells followed by puromycin selection. At low frequency, surviving puromycin-resistant (purR) foci appear and are cloned. RNA from these cell lines is screened for adaptative mutations in the SIN replicon by transformation of naive BHK cells to puromycin resistance at high frequency. Adaptive mutations are then cloned and mapped by making chimeras with the parental replicon. Modified dsSIN vectors, which include the adaptive mutations and the PAC gene, allow rapid production of BHK cell lines expressing heterologous genes without deleterious effects on the host cell.
protein products in cell culture (3–6). Some published examples include the hepatitis B virus pregenome RNA (59), the papillomavirus 16 capsid protein (60), the neurokinin receptor (61), the human immunodeficiency virus glycoproteins (62), and the hepatitis C virus glycoproteins (63, 64).
Given their efficient production of heterologous antigens, engineered alphavirus RNAs also have significant potential for in vivo applications (16, 51, 54, 65, 66) including vaccination against primate immunodeficiency viruses (67). Various delivery strategies are just beginning to be explored. As described above, infectious particles containing either double subgenomic RNAs or packaged RNA replicons could be used. In the case of constructs expressing alphavirus structural proteins, which have the potential to spread in vivo, safety issues related to alphavirus pathogenicity remain a major concern. Even using the best “helper-free” packaging system, packaged replicons are likely to include low levels of packaged helper RNA or recombinant wild-type virus (43, 44). Additional safeguards, such as mutations in the spike glycoproteins that require activation by in vitro proteolysis (68) or the use of packaging machinery from highly attenuated alphaviruses (51, 69, 70), may help to diminish the possibility of pathogenic consequences.
Alternatively, genetic immunization or transient gene therapy could be accomplished using DNA or RNA constructs lacking the structural proteins. In the case of DNA, a nuclear promoter can be used to drive expression of replication-competent SIN RNA replicons after transfection with DNA (14, 15). Although less stable than DNA, RNA delivery should also be considered since this would result in only transient exposure to the nucleic acid minimizing the possibility of integration and undesirable mutagenic consequences (65, 71). In addition, replicons can be engineered to express multiple subgenomic RNAs allowing coexpression of several protective antigens along with cytokines or other immunomodulators to enhance the generation of desired immune responses.
We thank our collaborators and colleagues, past and present, who have contributed to the development of Sindbis virus-based alphavirus vectors. Special thanks also go to Kaveh Ashrafi, Peter J.Bredenbeek, Joel M.Dalrymple, Jean Dubuisson, Arash Grakoui, Teryl K.Frey, Ute Geigenmuller-Gnirke, Chang S.Hahn, Young S.Hahn, Alexander A.Kolykhalov, Robin Levis, Guangpu Li, Brett D.Lindenbach, Steven D.London, Alan L.Schmaljohn, Barabara Weiss, and Cheng Xiong. Work from our laboratories has been supported by grants from the Public Health Service (AI24134, All 1377, and AI26763), the Monsanto/Washington University Biomedical Research Contract, and the Pew Memorial Trust. B.M.P. is a Visiting Professor on leave from Albert Szent-Györgyi Medical University, Department of Microbiology, Szeged, Hungary.
1. Garoff, H., Wilschut, J., Liljeström, P., Wahlberg, J.M., Bron, R., Suomalainen, M., Smyth, J., Salminen, A., Barth, B.U., Zhao, H., Forsell, K. & Ekström, M. (1994) Arch. Virol. Suppl. 9, 329–338.
2. Strauss, J.H. & Strauss, E.G. (1994) Microbiol. Rev. 58, 491–562.
3. Berglund, P., Tubulekas, I. & Liljeström, P. (1996) Trends in Biotech. 14, 130–134.
4. Bredenbeek, P.J. & Rice, C.M. (1992) Semin. Virol. 3, 297–310.
5. Olkkonen, V.M., Dupree, P., Simons, K., Liljeström, P. & Garoff, H. (1994) Methods Cell Biol. 43, 43–53.
6. Piper, R.C., Slot, J.W., Li, G., Stahl, P.D. & James, D.E. (1994) Methods Cell Biol. 43, 55–78.
7. Cheng, R.H., Kuhn, R.J., Olson, N.H., Rossmann, M.G., Choi, H.K. , Smith, T.J. & Baker, T.S. (1995) Cell 80, 621–630.
8. Lemm, J.A., Rümenapf, T., Strauss, E.G., Strauss, J.H. & Rice, C.M. (1994) EMBO J. 13, 2925–2934.
9. Shirako, Y. & Strauss, J.H. (1994) J. Virol. 185, 1874–1885.
10. Rice, C.M., Levis, R., Strauss, J.H. & Huang, H.V. (1987) J. Virol. 61, 3809–3819.
11. Liljeström, P., Lusa, S., Huylebroeck, D. & Garoff, H. (1991) J. Virol. 65, 4107–4113.
12. Davis, N.L., Willis, L.V., Smith, J.F. & Johnston, R.E. (1989) Virology 171, 189–204.
13. Kuhn, R.J., Niesters, H.G.M., Hong, Z. & Strauss, J.H. (1991) Virology 182, 430–441.
14. Dubensky, T.W., Jr., Driver, D.A., Polo, J.M., Belli, B.A., Latham, E.M., Ibanez, C.E., Chada, S., Brumm, D., Banks, T.A., Mento, S.J., Jolly, D.J. & Chang, S.M. (1996) J. Virol. 70, 508–519.
15. Herweijer, H., Latendresse, J.S., Williams, P., Zhang, G., Danko, I., Schlesinger, S. & Wolff, J.A. (1995) Hum. Gene Ther. 6, 1161–1167.
16. Hahn, C.S., Hahn, Y.S., Braciale, T.J. & Rice, C.M. (1992) Proc. Natl. Acad. Sci. USA 89, 2679–2683.
17. Hertz, J.M. & Huang, H.V. (1992) J. Virol. 66, 857–864.
18. Raju, R. & Huang, H.V. (1991) J. Virol. 65, 2501–2510.
19. Hahn, Y.S., Hahn, C.S., Braciale, V.L., Braciale, T.J. & Rice, C.M. (1992) J. Exp. Med. 176, 1335–1341.
20. Lovett, A.E., Hahn, C.S., Rice, C.M., Frey, T.K. & Wolinsky, J.S. (1993) J. Virol. 67, 5849–5858.
21. Pugachev, K.V., Mason, P.W., Shope, R.E. & Frey, T.K. (1995) Virology 212, 587–594.
22. Chen, J.P., Miller, D., Katow, S. & Frey, T.K. (1995) Arch. Virol. 140, 2075–2084.
23. Jiang, W., Venugopal, K. & Gould, E.A. (1995) J. Virol. 69, 1044–1049.
24. Piper, R.C., Tai, C., Slot, J.W., Hahn, C.S., Rice, C.M., Huang, H.V. & James, D.E. (1992) J. Cell Biol. 117, 729–743.
25. Li, G. & Stahl, P.D. (1993) Arch. Biochem. Biophys. 304, 471–478.
26. Li, G. & Stahl, P.D. (1993) J. Biol. Chem. 268, 24475–24480.
27. Li, G., Barbieri, M.A., Colombo, M.I. & Stahl, P.D. (1994) J. Biol. Chem 269, 14631–14635.
28. D’Souza-Schorey, C., Li, G., Colombo, M.I. & Stahl, P.D. (1995) Science 267, 1175–1178.
29. Li, G., Barbieri, M.A. & Stahl, P.D. (1995) Arch. Biochem. Biophys. 316, 529–534.
30. Cheng, E.H.Y., Levine, B., Boise, L.H., Thompson, C.B. & Hardwick, J.M. (1996) Nature (London) 379, 554–556.
31. Levine, B., Goldman, J.E., Jiang, H.H., Griffin, D.E. & Hardwick, J.M. (1996) Proc. Natl. Acad. Sci. USA 93, 4810–4815.
32. Higgs, S., Powers, A.M. & Olson, K.E. (1993) Parasitol. Today 9, 444–452.
33. Olson, K.E., Higgs, S., Rice, C.M., Carlson, J.O. & Beaty, B.J. (1994) Insect Biochem. Molec. Biol. 24, 39–48.
34. Gaines, P.J., Olson, K.E., Higgs, S., Powers, A.M., Beaty, B.J. & Blair, C.D. (1996) J. Virol. 70, 2132–2137.
35. Powers, A.M., Olson, K.E., Higgs, S., Carlson, J.O. & Beaty, B.J. (1994) Virus Res. 32, 57–67.
36. Powers, A.M., Kamrud, K.I., Olson, K.E., Higgs, S., Carlson, J.O. & Beaty, B.J. (1996) Proc. Natl. Acad. Sci. USA 93, 4187–4191.
37. Olson, K.E., Higgs, S., Gaines, P.J., Powers, A.M., Davis, B.S., Kamrud, K.I., Carlson, J.O., Blair, C.D. & Beaty, B.J. (1996) Science 272, 884–886.
38. Xiong, C., Levis, R., Shen, P., Schlesinger, S., Rice, C. & Huang, H.V. (1989) Science 243, 1188–1191.
39. Liljestrom, P. & Garoff, H. (1991) Bio/Technology 9, 1356–1361.
40. Bredenbeek, P.J., Frolov, I., Rice, C.M. & Schlesinger, S. (1993) J. Virol. 67, 6439–6446.
41. Geigenmuller-Gnirke, U., Weiss, B., Wright, R. & Schlesinger, S. (1991) Proc. Natl. Acad. Sci. USA 88, 3253–3257.
42. Weiss, B., Nitschko, H., Ghattas, I., Wright, R. & Schlesinger, S. (1989) J. Virol. 63, 5310–5318.
43. Raju, R., Subramaniam, S.V. & Hajjou, M. (1995) J. Virol. 69, 7391–7401.
44. Weiss, B.G. & Schlesinger, S. (1991) J. Virol. 65, 4017–4025.
45. Frolov, I. & Schlesinger, S. (1994) J. Virol. 68, 8111–8117.
46. Sjoberg, E.M., Suomalainen, M. & Garoff, H. (1994) Bio/ Techology 12, 1127–1131.
47. Sjoberg, E.M. & Garoff, H. (1996) J. Gen. Virol. 77, 1323–1327.
48. de la Luna, S., Soria, I., Pulido, D., Ortin, J. & Jiménez, A. (1988) Gene 62, 121–126.
49. Frolov, I. & Schlesinger, S. (1994) J. Virol. 68, 1721–1727.
50. Johnston, R.E. & Peters, C.J. (1996) in Alphaviruses, eds. Fields, B.N., Knipe, D.M. & Howley, P.M. (Raven, New York), pp. 843–898.
51. Davis, N.L., Brown, K.W. & Johnston, R.E. (1996) J. Virol. 70, 3781–3787.
52. Griffin, D.E., Levine, B., Tyor, W.R., Tucker, P.C. & Hardwick, J.M. (1994) Arch. Virol. Suppl. 9, 31–39.
53. Griffin, D.E., Levine, B., Ubol, S. & Hardwick, J.M. (1994) Ann. Neurol. 35, S23–S27.
54. London, S.D., Schmaljohn, A.L., Dalrymple, J.M. & Rice, C.M. (1992) Proc. Natl. Acad. Sci. USA 89, 207–211.
55. Dubuisson, J. & Rice, C.M. (1993) J. Virol. 67, 3363–3374.
56. Young, J.A.T., Bates, P., Willert, K. & Varmus, H.E. (1990) Science 250, 1421–1423.
57. Rolls, M.M., Webster, P., Balba, N.H. & Rose, J.K. (1994) Cell 79, 497–506.
58. Rolls, M.M., Haglund, K. & Rose, J.K. (1996) Virology 218, 406–411.
59. Huang, M. & Summers, J. (1991) J. Virol. 65, 5435–5439.
60. Heino, P., Dillner, J. & Schwartz, S. (1995) Virology 214, 349–359.
61. Lundstrom, K., Mills, A., Buell, G., Allet, E., Adami, N. & Liljeström, P. (1994) Eur. J. Biochem. 224, 917–921.
62. Paul, N.L., Marsh, M., McKeating, J.A., Schulz, T.F., Liljestrom, P., Garoff, H. & Weiss, R.A. (1993) AIDS Res. Hum. Retroviruses 9, 963–970.
63. Dubuisson, J., Hsu, H.H., Cheung, R.C., Greenberg, H., Russell, D.R. & Rice, C.M. (1994) J. Virol. 68, 6147–6160.
64. Lin, C., Lindenbach, B.D., Prágai, B., McCourt, D.W. & Rice, C.M. (1994) J. Virol. 68, 5063–5073.
65. Zhou, X.Z., Berglund, P., Rhodes, G., Parker, S.E., Jondal, M. & Liljeström, P. (1994) Vaccine 12, 1510–1514.
66. Zhou, X.Z., Berglund, P., Zhao, H.X., Liljestrom, P. & Jondal, M. (1995) Proc. Natl. Acad. Sci. USA 92, 3009–3013.
67. Mossman, S.P., Bex, F., Berglund, P., Arthos, J., Oneil, S.P., Riley, D., Maul, D.H., Bruck, C., Momin, P., Burny, A., Fultz, P.N., Mullins, J.I., Liljestrom, P. & Hoover, E.A. (1996) J. Virol. 70, 1953–1960.
68. Salminen, A., Wahlberg, J.M., Lobigs, M., Liljeström, P. & Garoff, H. (1992) J. Cell Biol. 116, 349–357.
69. Davis, N.L., Powell, N., Greenwald, G.F., Willis, L.V., Johnson, B.J.B., Smith, J.F. & Johnston, R.E. (1991) Virology 183, 20–31.
70. Davis, N.L., Brown, K.W., Greenwald, G.F., Zajac, A.J., Zacny, V.L., Smith, J.F. & Johnston, R.E. (1995) Virology 212, 102–110.
71. Johanning, F.W., Conry, R.M., LoBuglio, A.F., Wright, M., Sumerel, L.A., Pike, M.J. & Curiel, D.T. (1995) Nucleic Acids Res. 23, 1495–1501.
72. Weiss, B., Rosenthal, R. & Schlesinger, S. (1980) J. Virol. 33, 463–474.