This paper was presented at a colloquium entitled “Genetic Engineering of Viruses and of Virus Vectors,” organized by Bernard Roizman and Peter Palese (Co-chairs), held June 9–11, 1996, at the National Academy of Sciences in Irvine, CA.
Fusigenic viral liposome for gene therapy in cardiovascular diseases
VICTOR J. DZAU*, MICHAEL J. MANN*, RYUICHI MORISHITA†, AND YASUFUMI KANEDA‡
*Research Institute and Department of Medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, MA 02115; †Department of Geriatric Medicine, Osaka University School of Medicine, Osaka 565, Japan; and ‡Institute for Molecular and Cellular Biology, Osaka University, Osaka 565, Japan
ABSTRACT To improve the efficiency of liposome-mediated DNA transfer as a tool for gene therapy, we have developed a fusigenic liposome vector based on principles of viral cell fusion. The fusion proteins of hemagglutinating virus of Japan (HVJ; also Sendai virus) are complexed with liposomes that encapsulate oligodeoxynucleotide or plasmid DNA. Subsequent fusion of HVJ-liposomes with plasma membranes introduces the DNA directly into the cytoplasm. In addition, a DNA-binding nuclear protein is incorporated into the HVJ-liposome particle to enhance plasmid transgene expression. The fusigenic viral liposome vector has proven to be efficient for the intracellular introduction of oligodeoxynucleotide, as well as intact genes up to 100 kbp, both in vitro and in vivo. Many animal tissues have been found to be suitable targets for fusigenic viral liposome DNA transfer. In the cardiovascular system, we have documented successful cytostatic gene therapy in models of vascular proliferative disease using antisense oligodeoxynucleotides against cell cycle genes, double-stranded oligodeoxynucleotides as “decoys” to trap the transcription factor E2F, and expression of a transgene encoding the constitutive endothelial cell form of nitric oxide synthase. Similar strategies are also effective for the genetic engineering of vein grafts and for the treatment of a mouse model of immune-mediated glomerular disease.
Vector Development
Construction of Fusigenic Viral Liposome. Although human gene therapy trials have been initiated, the clinical efficacy of these therapies has not been clearly demonstrated (1). It has been suggested that the limited success of current gene therapy trials may result in part from inadequacies of the DNA delivery systems (1). Improvements of viral and nonviral vector systems for gene therapy are being pursued actively. The development of novel viral vectors, such as pseudotype retrovirus vector (2), adenoviral vector of low antigenicity (3), and adeno-associated virus) vector, has been reported. More recently, the lentivirus vector appears to be promising for transducing nondividing cells (4). Similarly, new lipid formulations designed to increase the efficiency of transfection are being developed (5). Other novel delivery systems include lipopolyamine-based gene delivery (6), targeted gene delivery systems (7), and devices for particle bombardment (8).
We have focused our efforts on the development of a fusigenic liposome that is a hybrid vector between viral and nonviral technologies (Fig. 1; ref. 9). Hemagglutinating virus of Japan (HVJ; also Sendai virus) is a paramyxovirus that is 300 nm in diameter and contains two distinct glycoproteins (hemagglutinating neuroaminidase and fusion protein) in its envelope, which are involved in cell fusion (10). This virus is capable of fusing with the cell membrane at neutral pH, and these fusion properties can therefore be exploited to facilitate the introduction of DNA directly into cell cytoplasm, avoiding lysosomal degradation. Hemagglutinating neuroaminidase is required for viral particle binding to receptors consisting of sialoglycoproteins or sialolipids; hemagglutinating neuroaminidase then catalyzes the removal of sugars by its neuroaminidase activity. Fusion protein interacts with the lipid bilayer of cell membranes to induce cell fusion. Fusion protein is produced in an inactive form (F0) and is activated by proteolytic cleavage to the fusion polypeptides F1 and F2, which are held together by a disulfide bridge. The hydrophobic region of F1 can interact with cholesterol to induce cell fusion. Although liposomes themselves have no receptors for the virus, a direct interaction of F1 polypeptide with lipid is likely to play an important role in the ability of HVJ particles to fuse with liposomes (11). Several attempts have been made to incorporate DNA into the HVJ envelope itself (12) or into fusion products of HVJ with red blood cell ghosts (13), but these approaches were plagued by low trapping efficiency and/or low transduction efficiency. Since DNA can be efficiently encapsulated into liposomes (14), we turned to incorporation of HVJ envelope proteins into these liposomes. We first encapsulated DNA into liposomes consisting of phosphatidylcholine and cholesterol that were prepared via vortexing or reverse-phase evaporation. The trapping efficiency of DNA into such liposomes is 10–30%, so that 400–600 molecules of plasmid DNA and more than half million copies of 20-mer oligonucleotides were enclosed into one liposome particle. We then fused the liposomes with UV-inactivated HVJ to form fusigenic viral liposomes containing DNA (400–500 nm in diameter). HVJ-liposomes can fuse with plasma membranes, and fusion is completed within 10–30 min at 37°C. This short HVJ-liposome incubation time is particularly suited for in vivo gene therapy. In contrast, gene transfer by cationic liposomes generally requires a much longer incubation time of 5–20 hr. Indeed, we have shown that exposure of rat carotid artery to HVJ-liposomes containing fluorescein isothiocianate-labeled oligodeoxynucleotides (ODNs) for 10 min results in the uptake of fluorescence by 30–50% of cells within the vessel wall. Other advantages of HVJ-liposome-mediated delivery are the introduction of molecules directly into the cytoplasm and avoidance of degradation in the endosome and lysosome. In fact, when fluorescein isothiocianate-ODN was introduced into vascular smooth muscle cells (VSMCs) using HVJ-liposomes, fluorescence was detected in cell nuclei within 5 min, and the fluorescence remained prominent in the nuclei for at least 72
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. §1734 solely to indicate this fact.
Abbreviations: HVJ, hemagglutinating virus of Japan; ODN, oligodeoxynucleotide; VSMC, vascular smooth muscle cell; AS, antisense; PCNA, proliferating cell nuclear antigen; cdc2, cell division cycle 2; ec-NOS, endothelial cell NO synthase.
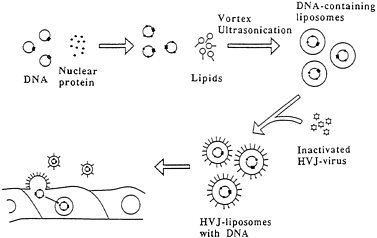
FIG. 1. Procedure of gene transfer by HVJ-liposome.
hr (15). In contrast, after direct transfer of fluorescein isothiocianate-ODN to VSMCs without the HVJ lipsome, fluorescence was observed in cytoplasmic compartments but not in the nucleus. Furthermore, the fluorescence was no longer detectable at 24 hr after direct fluorescein isothiocianateODN application.
Current gene transfer methods are also limited by the low level of expression of the transgene. We have found that cointroduction of plasmid DNA with a nuclear protein, high mobility group-1, can enhance transgene expression in animal tissues (16, 17). High mobility group-1 protein is a nonhistone DNA-binding protein of 28 kDa. It is reported that high mobility group-1 is required for the bending or looping of DNA and for enhancing transcription by specific recognition of cruciform DNA (18). An advantage of the HVJ-liposome is the capacity for such cointroduction of both DNA and proteins via their incorporation into the same particle. Indeed, we have recently cointroduced RNase H with antisense (AS) ODN for angiotensin converting enzyme in vivo into injured rat carotid artery. We observed that the AS effect was augmented 3-fold with the addition of RNase H (unpublished data). Thus, HVJ-liposomes have been useful for DNA transfer in various tissues in vivo, resulting in functional gene expression or gene suppression (Table 1).
Advantages of Fusigenic Viral Liposome. Efficient transfection of oligonucleotides, plasmid DNA, and proteins. The HVJ-liposome can encapsulate DNA up to 100 kbp. We have succeeded in transducing cosmid DNA (45 kbp) containing the thymidine kinase gene into cultured mouse cells (19). Recently, full-length cDNA of human Ducchene muscular dystrophy gene was introduced in vivo using HVJ-liposomes, resulting in its expression in skeletal muscle and diaphragm of the mdx mouse (20). When AS ODN to basic fibroblast growth factor was transfected into VSMCs using HVJ-liposomes and compared with cationic lipid transfection or direct ODN transfer without any vector, the concentration of AS basic fibroblast growth factor required to reduce cellular DNA synthesis by 75% was approximately 0.1 μM, 10 μM, and 20 μM, respectively (21). Thus, the HVJ-liposome is an effective method for ODN and plasmid DNA transfer. Ribozymes have also been efficiently introduced into cells using HVJ-liposomes, and the vector has also been useful for the introduction of recombinant proteins IgG and IgM (ref. 20 and unpublished data).
Penetration of the vector into tissues in vivo. Efficient in vivo transfer and expression of transgenes have been observed in cells of the tunica media of intact rabbit carotid arteries after filling the lumen with HVJ-liposomes containing the trans-
Table 1. In vivo gene transfer by HVJ-liposome
gene for 10 min under 150 mmHg (1 mmHg=133 Pa). Thus, in contrast to adenoviral vectors, this vector appears to penetrate readily the intimal layer to reach the tunica media. Our observation that the interstitium of the tunica media is stained upon incubation with liposomes without HVJ containing Evans blue dye suggests that this penetrating ability is conferred primarily by the liposome. Subsequent cell fusion and intracellular delivery of DNA is mediated by HVJ fusion proteins. Our liposome consists of the negatively charged phosphatidylserine, in addition to phosphatidylcholine and cholesterol. The presence of this negative charge may play an important role in enhancing transmigration into the vessel wall, and we are currently varying the composition of the liposome to test its effect on tissue penetration. It is our observation that negatively charged liposomes generally do not work well for DNA transfer into cultured cells in vitro. However, the converse may be true of in vivo gene transfer. Recently, we developed cationic HVJ-liposomes, and compared their gene transfer efficacy with those for anionic HVJ-liposomes and cationic lipids (Lipofectamine; GIBCO/ BRL) in vitro and in vivo. Cationic HVJ-liposomes and Lipofectamine are much more efficient than anionic HVJ-liposomes for achieving luciferase gene expression in vitro. In contrast, negatively charged HVJ-liposomes are most efficient for in vivo transfection of liver and skeletal muscle.
No apparent toxicity and low antigenicity. Thus far, using HVJ-liposomes, we have not observed significant cell damage in vitro, nor have we detected target organ dysfunction in vivo. Up to 1010–1011 HVJ lipsome particles have been injected in vivo into the portal veins of 8-week-old mice without any detectable toxicity (22). However, the fate of the HVJ proteins and the virion, as well as that of the lipids, must be analyzed more precisely before the application of HVJ-liposomes for human clinical trial. Furthermore, the effectiveness of UV light for the complete inactivation of HVJ must be documented carefully.
We have also examined the antigenicity of HVJ-liposomes in vivo. Low titers of antibodies against HVJ could be detected 1 week after injection of the HVJ-liposome into the portal vein of the rat. When HVJ-liposomes containing marker genes were injected into the portal veins of rats that had received a prior injection of empty HVJ-liposomes 7 days earlier, the marker gene expression was not attenuated, compared with rats undergoing primary HVJ-liposome transfection. Clearly, much more work has to be done to study the immunogenicity of the HVJ-liposome complex and to define the effect of repeated injections in vivo.
Improvement of current vector system. The transient nature of gene expression is a major limitation of the current HVJ-liposome system. Recently, we have succeeded in achieving longer term gene expression in vivo using the self-replicating apparatus of Epstein-Barr virus (ref. 22 and unpublished data). A plasmid containing the Ori P sequence and the EBNA-1 coding region derived from Epstein-Barr virus was constructed, and the luciferase gene, expressed under the control of chicken β-actin promotor, was cloned into this vector. Luciferase gene expression in cultured human cells (HeLa and KEK-293) increased with cell division after HVJ-liposome transfection with this vector. Southern blot analysis of episomal DNA in these cells indicated that the transgene replicated autonomously in the nucleus. However, this plasmid could not replicate autonomously in rodent cells but was retained in the nucleus. When this Epstein-Barr virus replicon vector was introduced into rat liver using HVJ-liposomes, luciferase gene expression was detected for >4 weeks, although the level gradually decreased. To enhance tissue-specific expression, the transgenes encapsulated into HVJ-liposomes have now been designed to be driven by cell type-specific promoters. We have succeeded in achieving gene expression in the liver by the use of the mouse albumin promoter or the rat pyruvate kinase promotor, and the endothelin promotor may also allow endothelial cell specific transgene expression in vivo.
Application of Fusigenic Virus Liposome to Gene Therapy of Cardiovascular Diseases
Vascular Proliferative Disease (e.g., Restenosis). AS strategy. Balloon angioplasty is one of the major therapeutic approaches to coronary artery stenosis. Restenosis, however, occurs in 30–40% of patients after angioplasty. A major component of restenosis is neointimal hyperplasia, which is characterized primarily by abnormal growth and migration of VSMCs. Multiple growth factors are involved in the stimulation of VSMC growth. Cell cycle progression to cell division is ultimately regulated by cell cycle regulatory genes. We have therefore developed a strategy to inhibit abnormal growth of VSMC in vivo by suppressing the expression of cell cycle regulatory proteins. Indeed, we reported that the combination of AS ODN against proliferating cell nuclear antigen (PCNA) and cell division cycle 2 (cdc2) kinase inhibited serumstimulated VSMC proliferation in vitro (23, 24). Similarly, the combinations of AS cdc2 kinase/AS cyclin B1 and AS cdc2 kinase/AS cyclin-dependent kinase 2 completely inhibited serum-stimulated DNA synthesis. Since neointima formation is initiated by an acute phase of medial smooth muscle cell replication, we transfected AS ODN to PCNA and cdc2 kinase via HVJ-liposomes into balloon-injured rat carotid arteries in vivo. As shown in Fig. 2, neointima formation was completely inhibited for 2 weeks after AS ODN transfer, and the inhibitory effect was sustained up to 8 weeks after a single transfection. However, no inhibitory effect was observed after transfection with control sense ODN. Combinations of AS ODN with cdc2 kinase/cyclin B1 and AS with cdc2 kinase/ cyclin-dependent kinase 2 also resulted in suppression of neointimal hyperplasia in this experimental model of vascular proliferative disease.
Transcriptional factor decoy strategy. The transcriptions of PCNA, cdc2 kinase, and c-myc and c-myb protooncogenes are activated by a common transcriptional factor, E2F. In quiescent VSMCs, E2F forms a protein complex with retinoblastoma gene product, RB. Upon growth stimulation, the RB protein is phosphorylated, and E2F is subsequently released
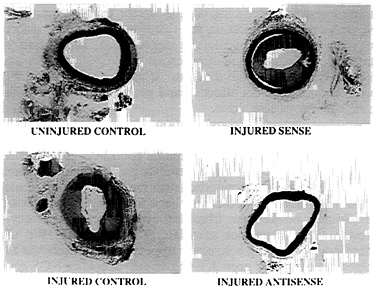
FIG. 2. Long-term suppression of neointima formation by AS-cdc2 kinase and AS-PCNA. Uninjured rat carotid artery (Upper Left), injured rat carotid artery without protein-liposome (Lower Left), injured rat carotid artery treated with protein-liposome containing 15 μM sense ODNs for both molecules (Upper Right), and injured rat carotid artery treated with protein-liposome containing 15 μM AS ODNs (Lower Right) were shown. At 2 weeks after transfection, rats were killed and vessels were fixed with 4% paraformalehyde.
from the complex. E2F then binds to the promoter region of the above cell cycle genes and activates their transcription. The consensus sequence TTTCGCGC is the binding site for E2F. Our strategy for inhibition of cell proliferation is the intracellular delivery of double-stranded ODN containing the TTTCGCGC sequence to act as a decoy to trap the released E2F (25). We synthesized a 14-mer as well as a 30-mer double-stranded ODN containing the consensus sequence and demonstrated that both are effective E2F based on competitive gel-shift assay. Using HVJ-liposomes, E2F decoy ODN was then introduced into cultured VSMCs, and it completely inhibited serum-stimulated growth. This growth inhibition was accompanied by reductions in PCNA and cdc2 kinase levels in these VSMCs. In contrast, mismatched decoy showed no inhibitory effect.
Based on these in vitro results, we examined the effect of E2F decoy on the prevention of neointimal hyperplasia in vivo. E2F decoy was transduced into balloon-injured rat carotid arteries using HVJ-liposomes. Our results demonstrated a marked suppression of neointimal formation at 2 weeks after balloon injury. In contrast, mismatched, scrambled, or progesterone responsive element decoy had no effect on neointimal development. Interestingly, we observed that a single administration of E2F decoy resulted in a sustained inhibition of neointimal formation up to 8 weeks after the treatment.
Gene transfer approach. Using HVJ-liposomes, we also attempted to inhibit neointimal formation by plasmid DNA gene transfer (26). Several studies had suggested NO could inhibit neointimal formation. For example, NO inhibited VSMC growth and migration in vitro. Systemic administration of a NO synthase inhibitor accelerated atherosclerotic lesion formation and impaired vascular reactivity. We therefore postulated that overexpression of endothelial cell NO synthase (ec-NOS) is an effective gene therapeutic strategy. Accordingly, we transfected balloon-injured rat carotid arteries with an expression vector containing the ec-NOS gene.
Four days after HVJ-liposome-mediated ec-NOS gene transfer into injured rat carotid arteries, significant levels of ec-NOS protein expression were detected. Consequently, NO production in the injured artery was enhanced by ec-NOS gene transfer. Two weeks after ec-NOS gene transfer, histological analysis revealed a 70% reduction in neointimal area as compared with the nontransfected injured artery (26). In contrast, no inhibition of neointima formation was observed in injured vessels undergoing control vector transfection. Since NO has multiple effects on the vessel wall, including vasorelaxation, inhibition of platelet aggregation, prevention of leukocyte adhesion, and suppression of VSMC growth and migration, we propose that our strategy to augment NO production may be an effective and practical approach to the gene therapy of restenosis.
Another important consideration for the therapy of restenosis is reendothelialization of the injured artery. Although several factors are known to stimulate endothelial cell growth, we have recently found that hepatocyte growth factor is a more potent accelerator of endothelialization than either vascular endothelial cell growth factor or basic fibroblast growth factor. In addition, unlike basic fibroblast growth factor, hepatocyte growth factor does not stimulate VSMC growth. We are therefore developing a strategy to prevent restenosis via the inhibition of VSMC growth using an AS, decoy, or NOS gene transfer approach in combination with the stimulation of endothelial cell growth by hepatocyte growth factor gene transfer.
Genetic engineering of vein grafts resistant to atherosclerosis. Saphenous vein grafts are the most commonly used bypass conduits for the treatment of occlusive vascular disease. However, up to 50% of vein grafts fail within a period of 10 years, primarily as a result of accelerated graft atherosclerosis. When grafted into arteries, veins are subjected to increased intraluminal pressure and undergo adaptive wall thickening. This thickening, however, involves neointimal hyperplasia, and this neointimal layer is believed to form the substrate for the aggressive atherosclerotic disease that eventually causes graft failure. We therefore hypothesized that a cytostatic strategy to prevent the hyperplastic response to the acute injury of grafting would redirect the biology of vein graft adaptation away from neointimal hyperplasia and toward medial hypertrophy (27). Rabbit jugular vein was isolated and transfected with AS ODN against PCNA and cdc2 kinase using HVJ-liposomes. The transfected vein was then grafted into the carotid artery. Neointima formation inhibited in the AS ODN-treated vein grafts for up to 10 weeks after surgery. In response to cell-cycle arrest with AS ODN, the genetically engineered vein grafts developed hypertrophy of the medial layer. When the rabbits were fed a high-cholesterol diet, accelerated atherosclerotic changes, characterized by plaque formation and macrophage infiltration, developed in the untreated and control ODN-treated grafts. In contrast, neither plaque formation nor significant macrophage infiltration was observed in any of the AS ODN-treated grafts, despite cholesterol feeding. These results establish the feasibility of developing genetically engineered bioprostheses that are resistant to failure and better suited to the long-term treatment of occlusive vascular diseases.
Treatment of glomerulosclerosis. We have also used E2F decoy oligonucleotide to ameliorate the changes seen in an animal model of mesangial proliferative nephritis. Injection of anti-Thy-1 antibody, which specifically injures glomerular mesangial cells, results in a proliferative glomerular lesion. We demonstrated that intrarenal arterial perfusion of HVJ-liposome complexes containing 14-mer E2F double-stranded decoy ODN inhibited anti-Thy-1-induced mesangial cells proliferation, as documented by BrdUrd incorporation and total glomerular cell counts. Furthermore, this decoy treatment prevented histopathologic changes in the glomeruli that closely mimic the mesangioproliferative nephritis seen in IgA nephropathy and in some forms of focal glomerular sclerosis.
Future Direction
The fusigenic viral liposome appears to be an effective tool for gene transfer and therapy. Our current system is an HVJ-liposome complex, but other viral fusion proteins may be applicable. In addition, in forming fusigenic liposome complexes, purified or recombinant fusion polypeptides may be used instead of the entire viral envelope. Since the system is a hybrid between viral and nonviral vectors, safety issues must be considered. It will be necessary to test the safety of UV-inactivated HVJ itself, as well as the safety of the liposome and the immunogenicity of the HVJ-liposome complex. HVJ-liposomes may be useful for short-term and local gene therapy. Modifications of this system will be necessary to permit high levels of stable expression of the transgene for clinical therapy.
1. Marshall, E. (1995) Science 265, 1050–1055.
2. Hopkins, N. (1993) Proc. Natl. Acad. Sci. USA 90, 8759–8760.
3. Yang, Y., Nunes, F.A., Berencsi, K., Furth, E.E., Gonczol, E. & Wilson, J.M. (1994) Proc. Natl. Acad. Sci. USA 91, 4407–4411.
4. Naldini, L., Blomer, U., Gallay, P., Ory, D., Mulligan, R., Gage, F.H., Verma, I.M. & Trono, D. (1996) Science 272, 263–267.
5. Goyal, K. & Huang, L. (1995) J. Liposome Res. 5, 49–60.
6. Remy, J.-S., Kickler, A., Mordvinov, V., Shuber, F. & Behr, J.-P. (1995) Proc. Natl. Acad. Sci. USA 92, 1744–1748.
7. Wagner, E., Plank, C., Zatloukal, K., Gotten, M. & Birnstiel, M.L. (1992) Proc. Natl. Acad. Sci. USA 89, 6099–6102.
8. Cheng, L., Ziegelhoffer, P.R. & Yang, N.-S. (1993) Proc. Natl. Acad. Sci. USA 90, 4455–4459.
9. Kaneda, Y. (1994) in Cell Biolab: A Laboratory Handbook, ed. Celis, J.E. (Academic, New York) Vol. 3, pp. 50–57.
10. Okada, Y. (1993) Methods Enzymol. 221, 18–41.
11. Nakanishi, M., Uchida, T., Sugawa, H., Ishiura, M. & Okada, Y. (1985) Exp. Cell Res. 159, 399–409.
12. Wu, P., de Fiebre, C.M., Millard, V.J., Elmstrom, K., Gao, Y. & Meyer, E.M. (1995) Neurosci. Lett. 190, 73–76.
13. Sugawa, H., Uchida, T., Yoneda, Y., Ishiura, M. & Okada, Y. (1985) Exp. Cell Res. 159, 410–418.
14. Schaeffer-Ridder, M., Wong, Y. & Hoffschneider, P.H. (1982) Science 215, 215–217.
15. Kaneda, Y., Morishita, R. & Tomita, N. (1995) J. Mol. Med. 73, 289–297.
16. Kaneda, Y., Iwai, K. & Uchida, T. (1989) Science 243, 375–378.
17. Kaneda, Y., Iwai, K. & Uchida, T. (1989) J. Biol. Chem. 264, 12126–12129.
18. Lilley, J.M. (1992) Nature (London) 357, 282–283.
19. Kaneda, Y., Uchida, T., Kim, J., Ishiura, M. & Okada, Y. (1987) Exp. Cell Res. 173, 56–69.
20. Yanagihara, I., Inui, K., Dickson, G., Piper, T., Kaneda, Y. & Okada, S. (1996) Gene Ther. 3549–3553.
21. Kaneda, Y., Morishita, R. & Dzau, V.J. (1996) Ann. N.Y. Acad. Sci. in press.
22. Sugden, B., Marsh, K. & Yates, B. (1995) Mol. Cell Biol. 5, 410–413.
23. Morishita, R., Gibbons, G.H., Ellison, K.E., Nakajima, M., Zhang, L., Kaneda, Y., Ogihara, T. & Dzau, V.J. (1993) Proc. Natl. Acad. Sci. USA 90, 8474–8478.
24. Morishita, R., Gibbons, G.H., Ellison, K.E., Nakajima, M., von der Leyen, H., Zhan, L., Kaneda, Y., Ogihara, T. & Dzau, V.J. (1994) J. Clin. Invest. 93, 1458–1464.
25. Morishita, R., Gibbons, G.H., Horiuchi, M., Willison, K.E., Nakajima, M., Zhang, L., Kaneda, Y., Ogihara, T. & Dzau, V.J. (1995) Proc. Natl. Acad. Sci. USA 92, 5855–5859.
26. Von der Leyen, H., Gibbons, G.H., Morishita, R., Lewis, N.P., Zhang, L., Nakajima, M., Kaneda, Y., Cooke, J.P. & Dzau, V.J. (1995) Proc. Natl. Acad. Sci. USA 92, 1137–1141.
27. Mann, M., Gibbons, G.H., Kernoff, R.S., Diet, F.P., Tsao, P.S., Cooke, J.P., Kaneda, Y. & Dzau, V.J. (1995) Proc. Natl. Acad. Sci. USA 92, 4502–4506.





