1
Environmental Challenges
SOLVING PROBLEMS
Since environmental issues first engaged the public's interest, our nation's approach to environmental protection has been geared towards remediating specific, identified problems. These problems typically have gained attention due either to impacts on affected communities or to predictions from scientists and others of the likely effects of certain environmental stresses before these effects were widely recognized. Early examples of such problems included fish kills in contaminated surface waters, smog hazes over many American cities, the effects of DDT and other pesticides on wildlife, the effects of acid rain on ecosystems, the effects of lead on infants and children, and the effects of tributyl tin (a marine antifouling compound) on marine ecosystems.
When the public has perceived an environmental or public health threat as posing a serious risk, widespread concern has often led to the passage of legislation designed to reduce that threat. Examples of such legislation include the Clean Air Act, Clean Water Act, Safe Drinking Water Act, Toxic Substances Control Act, Resource Conservation and Recovery Act, and Comprehensive Environmental Response, Compensation, and Liability Act (also known as Superfund). The U.S. Environmental Protection Agency (EPA), created in 1970 with the broad mission of protecting human health and the environment, has the pressing and continuing task of implementing such laws: media- and substance-specific programs were created to develop appropriate regulations, and EPA's Office of Research and Development was asked to support this regulatory process by providing scientific expertise and research support.
The strategy of responding to recognized environmental issues has been effective in many cases. (Note that throughout this report we define "environmental issues" to include any issue affecting human health, ecosystems, natural resources, or the global environment.) The average level of lead in children's blood
declined by 75% between 1978 and 1995 due to bans on the use of leaded gasoline and lead-based paint; fish have returned to rivers and lakes once considered "dead"; the air in many cities is clearer than it was 30 years ago; and the production of ozone-destroying chemicals has been reduced drastically (EPA, 1995). Environmental research played an important part in these successes, as illustrated by the example of the restoration of the Kesterson National Wildlife Refuge in Box 1-1. However, a problem-oriented approach also has limitations that are increasingly apparent.
RECOGNIZING LIMITATIONS
It has become increasingly evident that environmental systems are complex and interconnected. In looking back at early environmental successes it appears that, in many cases, attempts to correct one environmental problem unwittingly created or exacerbated others. The interplay of atmospheric particulates and sulfur oxides provides one example.
Acid deposition in the northeastern United States increased substantially over the same time period in which EPA regulations reduced powerplant emissions of particles larger than 2mm by 78% (EPA, 1996). Although the removal of larger particulates from atmospheric emissions resulted in substantially clearer urban air, the bulk of those particulates were alkaline and tended to neutralize the acidic sulfur oxides. To remedy the ecosystem and health effects associated with acidic aerosols, the sulfur in atmospheric emissions needs to be removed. But the removal of sulfur seems to lead to another unanticipated effect. Very small sulfuric acid aerosols also serve as nuclei for the formation of clouds—the more nuclei, the more small cloud droplets will be present. These droplets scatter incoming solar radiation before it reaches the earth's surface, resulting in a global cooling effect that partially offsets the warming effect of greenhouse gas buildup. A decrease in sulfuric acid in the atmosphere, aimed at reducing acid deposition and its harmful effects, may affect this global heat balance with unknown consequences (NRC, 1996b).
Another example of the complexity of environmental interactions is shown in Figure 1-1. The widespread use of chlorofluorocarbons (CFCs) as apparently safe, inert refrigerants and propellants led to depletion of the earth's protective ozone layer. CFC use provided a safer home environment but led to a serious global environmental problem.
Still another example of the interconnectedness of environmental problems is the rapid invasion of the non-native zebra mussel into Lake Erie, which has caused fouling of structures such as water intakes, outfall pipes, and piers. Engineers have experienced some success in designing materials that resist mussel colonization. However, this solution will not help remedy another, more surprising problem: it appears that the mussels are increasing the concentration of chemical contaminants throughout the aquatic food web in the lake and adjacent rivers.
|
BOX 1-1 Selenium Contamination at Kesterson National Wildlife Refuge: Research Helps Solve Identified Environmental Problem Selenium contamination at Kesterson National Wildlife Refuge in California's San Joaquin Valley drew national attention in the early 1980s. In 1982 scientists began to notice biological changes—dying cattails, algal blooms, declining use by waterfowl—in reservoir ponds, which in addition to providing wetland habitat served as irrigation drainage ponds. Further investigation revealed high levels of selenium in mosquito fish and dead and deformed bird embryos. While the California Department of Public Health issued notices limiting waterfowl consumption from the Kesterson area and the U.S. Fish and Wildlife Service closed the ponds to public access, it was broad, ongoing research on the underlying geochemical, material transport, and hydrologic processes in the area that revealed the source and potential scope of the problem and provided managers with the necessary information to begin to look for solutions (NRC, 1989). Investigations found that the soils on the west side of the San Joaquin Valley are derived primarily from marine sedimentary rock containing high levels of soluble salts and pyritic materials. Selenium and seleniferous salts are commonly associated with pyritic material. Thus, as irrigation water, applied to cropland, percolated through the soil, it leached out the selenium. This selenium-laden water was then transported though a system of drainage canals to the closed basin ponds at Kesterson. With no outlet, the selenium was concentrated by evaporation to toxic levels and accumulated in the biota. Research also found that the pH and redox conditions of the system can affect selenium toxicity. While the Bureau of Reclamation, which is responsible for federal irrigation projects in the West, anticipated problems associated with the management of saline soils and drainage water in the San Joaquin Valley and had made plans to mitigate them, it did not anticipate selenium contamination. As a result of breakthroughs in selenium research, the Bureau has since been able to assess the potential for selenium contamination at other federal irrigation projects. Research on selenium speciation continues, creating the knowledge that will be needed to continue to address selenium contamination problems. |
Due to past industrial practices, significant amounts of persistent, toxic compounds are buried in sediments at the bottom of Lake Erie. The mussels consume and recirculate these contaminated sediments, re-introducing them into the rest of the food chain. Thus, one environmental problem, the physical manifestation of this introduced species, turns out to be linked to a seemingly unrelated environmental
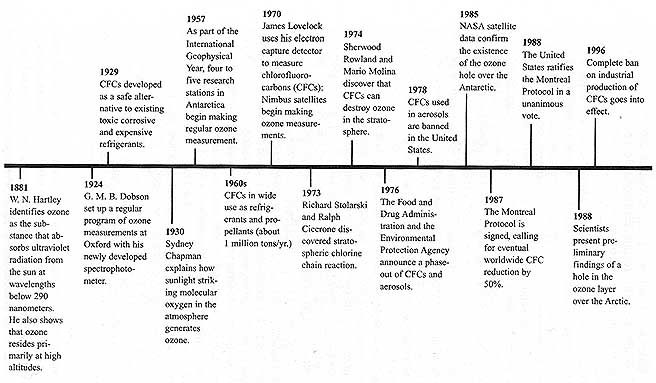
FIGURE 1-1 Chlorofluorocarbons (CFCs) and Ozone Depletion: Advances in science and policy through 1996. A growing body of chemical and atmospheric research led to the prediction of a potential problem that was then detected at an early stage. If the problem had not been recognized and addressed until the effects of increased UVB (such as skin cancer, ecosystem damage, etc.) were discernable, ecosystem and human health impacts would have been more severe, and effective mitigation would have been extremely difficult. SOURCE: Adapted from The Ozone Depletion Phenomenon (NAS, 1996).
problem, chemical contamination of aquatic ecosystems. Many actions that would be adequate to solve the first problem could be ineffective in solving the second.
Other examples can be cited where efforts to solve a specific problem must be considered within a broader context. This is particularly true of the growing number of regional- and global-scale problems associated with population growth, industrial development, and the corresponding pressure on limited natural resources. Indeed, the very nature of what are recognized as "environmental" problems is changing. Recently recognized problems such as global climate change, stratospheric ozone depletion, the loss of biological diversity, long-range transport of pollutants in air or water, global pressures on ocean resources, including fish, and regional water scarcity are broader, more complex environmental problems than those that received major attention several decades ago. One of the "greenhouse gases" associated with possible climate change is carbon dioxide, once considered a harmless product of combustion. Addressing such problems in politically, socially, and economically acceptable ways will require a much greater understanding of environmental systems, including the impact of human behavior on the environment, than is currently available.
COMPLEXITY, UNPREDICTABILITY, AND SURPRISE
Our society is frequently dealing with environmental problems that have not been experience before this century. Why have complete solutions to these problems proven elusive? A hint can be found by considering three features common to environmental problems: (1) they are systems problems, with multiple causes and effects; (2) they are characterized by nonlinear relationships, including complex feedback loops; (3) they often result when incremental changes, occurring over years or decades, accumulate, causing precipitous changes in environmental variables that "directly affect the health of people, productivity of renewable resources, and vitality of societies" (Holling, 1995). Based on his analysis, Holling concludes that environmental problems are "not amenable to solutions based on knowledge of small parts of the whole or on assumptions of constancy or stability of fundamental relationships."
Because of the complexity of natural systems, the inherent uncertainty in measurements, as well as the synergies and amplification effects that can occur, environmental problems will often include a strong element of surprise. At least three types of environmental surprises can be distinguished: (1) events with predictable consequences whose timing and magnitude are unexpected, such as the Exxon Valdez oil spill or the Chernobyl nuclear accident; (2) discontinuities in seemingly smooth trends, such as the sudden drop in pH that occurs when the buffering capacity of a water body is exceeded; and (3) unanticipated consequences of deliberate actions, such as the effects of using leaded gasoline on health or the relationship between widespread CFC use and stratospheric ozone
depletion. As Kates and Clark (1996) conclude, "The next 25 years will surely bring more surprises. The occurrence of such surprises, of course, will not be new. What will be new is the rapidity with which they emerge, the complexity of their sources and consequences, and the difficulty we will experience in devising appropriate institutional responses."
The seeds of future surprises often lie in our response to current surprises. For example, in response to the now-recognized deleterious effect of CFCs on stratospheric ozone, industries are seeking to replace them with safer substitutes. Trifluoroacetic acid (TFA) is a breakdown product of several CFC replacements. Although TFA appears to be harmless in the atmosphere, its potential for accumulation in terrestrial and aquatic ecosystems is only now being assessed. If TFA does accumulate, it may contain the makings of a future surprise.
Unanticipated environmental problems, "surprises," are an inevitable consequence of the complexity of environmental systems and expanding human activity. By expanding our understanding of environmental systems, we will be more likely to understand the consequences of particular actions, thus avoiding some surprises, and be better prepared to respond to the inevitable surprises that will arise.
EPA'S RESEARCH CHALLENGE
Because EPA is a regulatory agency, questions have long arisen about the role EPA should play in sponsoring and conducting environmental research. Yet, to be an effective regulator, EPA must understand underlying environmental processes. To paraphrase Vannevar Bush (1945), an agency that depends exclusively on others for its basic scientific and technical knowledge will be slow in its progress and weak in its attempts to protect human health and the environment.
EPA's research program has been expected to support the immense and widely varied array of regulatory and enforcement activities within the agency, providing the scientific and technological bases for decisions. Not surprisingly, the problem-by-problem approach to environmental protection has also influenced the nature of environmental research at EPA.
Clearly, EPA cannot conduct or sponsor research on every issue of concern to the public, the Congress, or even its own program offices. At just over $500 million a year, the environmental research budget at EPA is small compared to the breadth and complexity of the issues it confronts and small even as a fraction of our nation's environmental research portfolio. In 1995, EPA's research budget accounted for only 8% of total annual environmental research expenditures by federal agencies (Teich, 1996). Hence, environmental research topics at EPA must be chosen carefully, in the context of broader national and international efforts, and efforts must be made to link EPA to these other sources of information.
Acting on recommendations from a variety of expert review committees (Carnegie Commission, 1992; EPA/SAB, 1988, 1990, 1994; EPA, 1992; MITRE,
1994; NAPA, 1994, 1995; NRC, 1993a, 1995b), EPA's Office of Research and Development (ORD) set out in 1995 to create a new mission statement, strategic plan, and organizational structure to help focus and prioritize the many demands on its limited resources. The strategic plan (EPA, 1996) is based on risk assessment concepts and calls for EPA to:
-
focus research and development on the greatest risks to people and the environment, taking into account their potential severity, magnitude, and uncertainty; and
-
focus research on reducing uncertainty in risk assessment and on cost-effective approaches for preventing and managing risks (EPA, 1996).
As concluded in the interim report of this committee (Appendix 1), risk assessment is one useful tool for organizing research, but it has limitations. Not all environmental issues can be assessed and ranked within the risk paradigm. The more complex and global the problem, the more difficult the task of risk assessment will be. While the risk-based research strategy is sound, it must be augmented and adapted to encompass potential and emerging risks as well as current ones. (A more detailed discussion of the appropriate role for, and limitations of, risk assessment is presented in Chapters 2 and 3.)
ORD's strategic plan also sets a goal of working with others to identify, characterize, and resolve "emerging" environmental issues. The work of this committee is intended to help EPA in this ongoing effort to define its research agenda.
HISTORY AND PURPOSE OF THIS STUDY
In the fall of 1995, EPA's assistant administrator for research and development, Dr. Robert Huggett, asked the National Research Council (NRC) to undertake a study of research opportunities and priorities for EPA. In response, the NRC, through its Board on Environmental Studies and Toxicology and its Water Science and Technology Board, established a multidisciplinary committee (see Appendix 3 for biographical sketches of committee members). The committee was charged with the following tasks:
-
provide an overview of significant emerging environmental issues;
-
identify and prioritize research themes and projects that are most relevant to understanding and resolving these issues; and
-
consider the role of EPA's research program in addressing these issues, in the context of research being conducted or sponsored by other organizations.
The charge did not include a review of existing research programs at EPA or elsewhere, nor an assessment of issues related to organization and management of EPA's research program. (A companion NRC study, initiated shortly before this one, is currently examining research management practices at EPA. The
report of that study, Improving Research Management and Peer Review Practices in the U.S. Environmental Protection Agency, will be available by the fall of 1997.) An interim report from this committee (Appendix 1) assessed the ORD draft strategic plan. This final report is based on collection of information related to environmental research programs and deliberations, including four committee meetings, over a period of approximately nine months.
SCOPE OF THIS REPORT
This report includes a broad overview of current and emerging environmental issues, as compiled from a review of two dozen recent reports on environmental research augmented by committee input. After much discussion, the committee decided that, rather than simply turn this rather long list into a shorter list of problems that appear important at this moment in time, it would be more useful to EPA to describe a lasting framework for environmental research and encourage the agency to build and nurture its own internal capacity for identifying and selecting future research areas. The report also describes major research themes and programs of relevance to EPA; suggests criteria that can be used to identify and prioritize among important research areas; recommends actions EPA should take to build its scientific capacity; and provides illustrations of the kinds of research projects that EPA should consider.
This report is not intended to be highly technical. The advice it contains is targeted primarily to an audience of environmental policymakers and managers, as well as anyone with a broad interest in the conduct of environmental research. The report explains how environmental research can play a critical role in achieving the dual goals of finding workable solutions to current environmental problems while developing the scientific capacity to recognize and better respond to future problems. As stated in the report Safeguarding the Future: Credible Science, Credible Decisions (EPA, 1992):
[S]cience is one of the soundest investments the nation can make for the future. Strong science provides the foundation for credible environmental decision making. With a better understanding of environmental risks to people and ecosystems, EPA can target the hazards that pose the greatest risks, anticipate environmental problems before they reach a critical level, and develop strategies that use the nation's, and the world's, environmental protection dollars wisely.








