5
Understanding Health Effects of Incineration
To understand the possible health effects attributable to waste-incineration emissions, information is needed on contributions made by incineration to human exposures to potentially harmful pollutants and the responses that might result from such exposures. As discussed in this chapter, various tools have been used in attempts to evaluate effects of incineration. Of these tools, all of which contribute to our understanding, risk assessment methods have provided the most-detailed information for regulatory decisionmakers. Although past regulatory risk assessments have suggested that the risks posed by emissions from a well-run incinerator to the local community are generally very small, the same may not be true for some older or poorly run facilities. Some of the available assessments, however, may now be considered inadequate for a complete characterization of risk, for example, due to their failure to account for changes in emissions during process upsets, or because of gaps in and limitations of the data or techniques of risk assessment available at the time. There are limitations in the data and techniques of risk assessment, for example, in considering the effect of potential synergisms between chemicals within the complex mixtures to which humans are exposed, or the possible effects of small increments of exposure on unusually susceptible people. In addition, there are important questions not typically addressed by the usual risk assessment for single facilities such as the collective effect of pollutants emitted from multiple units; regional-scale effects of persistent pollutants; and the effects on workers in the facilities themselves.
This chapter examines the tools used to evaluate the potential for health effects from incineration facilities, and discusses some of the results obtained with those tools. The two primary tools are environmental epidemiology and
risk assessment, both of which have been the subject of National Research Council reports (e.g., NRC 1991a, 1994, respectively). In addition, environmental monitoring studies provide immediately useful estimates of ambient concentrations, while biomarker studies hold some promise for future application. The first section of the chapter discusses these tools, and their strengths and limitations relative to one another.
There have been few epidemiologic studies in populations characterized as exposed to contaminants emitted by incineration facilities. Thus, there is a lack of evidence of any obvious health effects related specifically to incinerator exposure. That is, there have been few anecdotal reports that indicated any particular concern for incinerators (as opposed to air pollution in general, for example) or that generated testable hypotheses. Moreover, as discussed later in this chapter, it would be difficult to establish causality given the small populations available for study, the possible influence of factors such as variations in the susceptibility of individuals and emissions from other pollution sources, and the fact that effects might occur only infrequently or take many years to appear. The second section of the chapter summarizes what data are available, and discusses what conclusions can be drawn from those data.
The main information on potential health effects that might arise in populations potentially exposed to substances emitted by incineration facilities comes from risk assessments of individual chemicals emitted by incinerators, combined with monitoring of emissions from incinerators. Such assessments typically indicate that, of the many agents present in incinerator emissions and known to be toxic at high exposures, only a few are likely to contribute the majority of any health risks and such health risks are typically estimated to be very small. This chapter examines the toxic effects of such agents. It also illustrates ways to compare the expected ranges of environmental concentrations attributable to incineration with concentrations known to be toxic, and in the context of total exposures.
The toxic agents were selected for discussion on the basis of the current state of knowledge of the nature of emissions from incinerators and the results of various risk assessments. They are particulate matter (PM), carbon monoxide (CO), acidic gases (i.e., NOx, SO2, HCl) and acidic particles, certain metals (cadmium, lead, mercury, chromium, arsenic, and beryllium), dioxins and furans, polychlorinated biphenyls (PCBs), and polyaromatic hydrocarbons (PAHs). The emissions of most of those substances were considered in Chapter 3 and Chapter 4.
Particulate matter, CO, lead, and acidic gases and acidic particles have been under regulatory scrutiny for the longest period. Typically, there are well-defined statutory limits on their emission rates or allowable ambient concentrations or increments in ambient concentrations under federal or state statutes. In many risk assessments, such materials have been evaluated solely by comparisons with such statutorily defined limits, limits that have been designed to reduce certain risks from these pollutants below acceptable values. Although there are occupa-
tional-exposure limits for most of the other metals and organic compounds listed above, there are no well-defined ambient or emission standards under federal or some state regulations; however, in risk assessments, those materials are typically found to contribute to the majority of the estimated risk, either in contribution to lifetime cancer risks or in contribution to potential noncancer effects. Historically, risk assessments have identified the dioxins and furans as the principal contributors to estimated risks posed by most incinerators with arsenic often next. However, estimates of relative contributions of pollutants to total risk depend on incinerator emission characteristics, populations potentially exposed, potential routes of exposure, and, to some extent, the amount of information that has been collected.
In addition, this chapter discusses “at-risk” populations (populations that might be at increased risk due, at least in part, to pollutants emitted from incinerators). The chapter ends with the main conclusions on understanding health effects of waste incineration reached by the committee and presentation of research needs.
TOOLS FOR EVALUATING HEALTH EFFECTS
Whenever searching for small or subtle health effects of exposures to environmental contaminants, it is best to use a variety of approaches and to critically compare their results. The primary tools that have been used include epidemiologic studies and risk assessments. These are separately discussed in detail below, although it should be realized that there can be a good deal of overlap between the approaches. Environmental monitoring, biomarkers of exposure or effect, and life-cycle assessment are other commonly used tools that produce data which often confirm, support, or enhance the findings obtained during the conduct of epidemiologic or risk-assessment investigations. Exposure assessment plays an important role in may of those approaches.
Such approaches are used to evaluate multiple environmental media (air, surface water, soil, groundwater, sediments, and any other media that might be distinguished), multiple exposure pathways, many scenarios for exposure, multiple routes (inhalation, ingestion, and dermal), multiple chemicals, multiple population groups, and many health end points.
However, the approaches currently used to assess the effects of waste incineration are typically site-specific and facility-specific and so fail to address two important questions regarding a facility or site:
-
To what extent does an incineration facility alter the environmental concentrations of substances of concern or alter the existing magnitudes of human exposure to those substances?
-
What are the overall local and regional contributions of waste incineration to human exposures?
Epidemiologic Studies
Epidemiologic studies are conducted to test hypotheses about the occurrence (usually prevalence or incidence) of a health outcome, to measure the strengths or sizes of relationships between such outcomes and quantifiable factors (e.g., the magnitude of exposures) or qualifiable factors (e.g., exposure status), or to generate testable hypotheses about such relationships. The methodology, strengths, and weaknesses of environmental epidemiologic studies have been discussed in previous NRC reports (NRC 1991c, 1997). As discussed there, the principal strengths of epidemiologic studies are:
-
The people studied include those likely to have been exposed to the material of interest. For incinerator emissions, there is no extrapolation necessary from single chemicals to the complex mixtures to which humans are actually exposed.
-
Humans themselves are studied in actual exposure conditions—there is no extrapolation from different animal species or different conditions.
-
Individual and group variability in both exposure and sensitivity are necessarily taken into account.
The principal challenges to be addressed by epidemiologic studies in establishing causality include:
-
Identifying suitably exposed populations of sufficient size.
-
Identifying effect modifiers and/or potentially confounding factors.
-
Identifying biases (including reporting biases) in data collection (e.g., Neutra et al. (1991) present an interesting case study of this problem).
-
Measuring exposures.
-
Measuring effects that are small, might occur only infrequently, or take many years to appear.
Risk Assessments
Risk assessment is the use of procedures to estimate the probability that harm will arise from some action such as the operation of a facility. The procedures used to perform risk assessments vary widely, from a snap judgment to the use of complex analytic models. However, risk assessments of incineration or incineration facilities have become more structured and formalized, following the four-step paradigm described in previous NRC reports (NRC 1983, 1994).
In the case of a particular incinerator, the first step, hazard identification, might begin with enumeration of the chemicals present in emissions and suspected of posing health hazards (and this alone might be an expensive proposition in unusual specific cases). The emissions have to be quantified, the potential health
effects identified, and the conditions under which a chemical might cause those effects defined. The attempt to obtain emission-rate estimates might take the form of direct measurements, which are limited by the sensitivity of the measuring methods, the variability over time of emission rates, the cost of such measurements, and the inaccuracies affecting all such field work. Alternatively, similar measurements from other, comparable facilities might be used as bases to estimate emissions. The result is generally a list of chemicals with their expected average emission rates and sometimes a measure of the variability of the emission rates with time—for example, how short-term emission rates might differ from the long-term average. In many cases, there may be a list of the emission rates that are identified as maximums by the owner or operator of the facility.
After developing a list of chemicals identified as potentially of concern, a dose-response assessment is used to evaluate quantitatively the relation between exposures and toxic responses. Ideally, this assessment would consider all the particular conditions of exposure, including the complete mix of other potential contaminants from incineration, and exposures to the same and different chemicals from other sources. In practice, dose-response assessments are limited, by the regulatory milieu of most risk assessments, to the use of cancer potency-slope estimates or unit risks1 (for the evaluation of cancer risks) and reference doses2 (for the evaluation of noncancer risks) published in the Integrated Risk Information System (IRIS)3 or other regulatory documents by the Environmental Protection Agency (EPA) or the Agency for Toxic Substances and Disease Registry (ATSDR).
Most of the effort of individual risk assessments has gone into the evaluation of exposure, which is the third step in the risk-assessment paradigm. As discussed in Chapter 4, exposure assessment involves an estimation or measure-
|
1 |
Cancer potency-slope estimates or unit risks. The human cancer potency-slope is the incremental increase in lifetime cancer risk per incremental unit of lifetime average dose (generally by ingestion, occasionally by other routes of exposure). The estimates of cancer potency-slope is obtained by assuming that the dose-response curve may be linear at low doses, and extrapolating to low dose from higher experimental doses. In many cases, there is an additional extrapolation from laboratory animals to humans. The unit risk is the incremental increase in lifetime cancer risk per incremental unit of air concentration of an airborne carcinogen. It is estimated using methods similar to those used for cancer potency-slope, but with slightly different assumptions adopted for inter-species extrapolation. |
|
2 |
The reference dose is a long-term average dose rate that is expected to result in no noncancer health effects in humans. It is obtained from experimental results in humans or animals by a relatively well-defined procedure that incorporates safety factors to account for all the defined extrapolations performed. |
|
3 |
IRIS. EPA's (1992b) Integrated Risk Information System (IRIS) is a database of human health effects that might result from exposure to various substances found in the environment. IRIS is accessible via the Internet at http://www.epa.gov//iris. |
ment of the concentration of specific substances in each environmental medium, and the time individuals or populations spend in contact with the substances. The network of exposure pathways becomes more and more complex as more-remote regions are incorporated. Food contaminated near an incineration facility might be consumed by people close to the facility or far away from it. Thus, local deposition on food might result in some exposure of populations at great distances, due to transport of food to markets. However, distant populations are likely to be more exposed through long-range transport of pollutants and low-level, widespread deposition on food crops at locations remote from a source incineration facility. To be most useful, exposure assessments need careful definition of the scenarios to which the assessments apply. Within such scenarios, the distribution of individuals or populations exposed need to be accounted for, and other variabilities and uncertainties incorporated (EPA 1992c). In order to dovetail with the dose-response assessments, care must be taken in the exposure assessment so that doses can be evaluated in the correct way. Potential doses can be expressed as the average rates at which material crosses the epithelial layer of an exposed individual (by inhalation or ingestion) or enters the outer layer of skin (e.g., through dermal contact) per unit of body weight per day (EPA 1992d; DTSC 1992a,b). However, such measures do not necessarily correspond to the does-response measures (e.g., carcinogenic potency-slope, unit risk, and reference doses), which typically relate response to exposures rather than doses. In the absence of such exact correspondence, exposure-dose relationships may become crucial.
The final step of the risk-assessment paradigm, risk characterization, involves integrating the results of exposure assessment, dose-response assessment, and hazard assessment in such a way as to “develop a qualitative or quantitative estimate of the likelihood that any of the hazards associated with the agent of concern will be realized in exposed people” (NRC 1994). Risk-assessment results are generally expressed as lifetime cancer risks (calculated by taking the sum —over the pollutants of interest—of the products of lifetime average exposure to each pollutant and its potency slope) or as summary hazard indices (the sum over various chemicals of the ratio of estimated dose of each chemical to its reference dose). In the case of lead, projected blood-lead concentrations are used. A complete risk characterization should also contain a full discussion of the uncertainties associated with the estimates of risk.
Risk assessment of waste incineration facilities can involve the following aspects:
-
Measurement or estimation of emission rates from specific facilities.
-
Modeling designed for tracking the flow of substances of concern through the environment.
-
A large body of information on toxicity of many emitted substances, in particular of dose-response information.
-
Characterization of the expected effect of new incinerators, or of what might happen in the future with any incinerator.
Such risk assessments are congruent with most regulatory schemes—the principal inputs to risk assessments are also characteristics of incinerators that are usually regulated, for example, emission rates.
The lack of complete data leads to uncertainties involved and the problem of communicating such uncertainties. Those uncertainties arise from the following:
-
The lack of complete emission data, especially for nonstandard operating conditions.
-
The problem of dose-response assessment at low doses, and in particular of low-dose, cross-species, inter-route, and temporal dose-pattern extrapolation.
-
The lack of toxicity data on most products of incomplete combustion.
-
The lack of physical and chemical information on relevant characteristics of substances of concern.
-
The use of unverified models of transport of substances in the environment, due to incomplete knowledge as to how such transport occurs.
-
The variability of all aspects of the assessment, due to variations in physical conditions (e.g., topography, temperatures, rainfall, soil types, and meteorological conditions), characteristics of people (e.g., eating habits, residence times, age, and susceptibility), and so on, leading to wide ranges of exposures and risks for different people.
-
The possibility of errors and omissions in the assessment (e.g., omission of an important pathway of exposure).
Because of the variability and uncertainty, most risk assessments have not been designed to quantify actual health risks; rather they have been designed solely for regulatory purposes to yield upper-bound estimates of health risks that may be compared to regulatory criteria.
Other Tools
Environmental monitoring and biological markers of exposure or effect are two tools often used in conjunction with epidemiologic or risk assessment investigations. These tools aid in identifying or confirming pollutants that may give rise to adverse health effects. Life-cycle assessment (LCA) has been used to evaluate the resource consumption and environmental burdens associated with a product, process, package, or activity throughout its lifetime over large geographic regions. LCA can be used in conjunction with risk assessments to assess effects over a broad scale—from the time of introduction of a chemical into the environment to its destruction.
Environmental Monitoring Studies
In principle, it is desirable to measure concentrations of certain pollutants directly from the incinerator in the surrounding environment. Such monitoring is most commonly of the ambient air, but soil, water, sediments, vegetation, and foods have at times been monitored for some of the emitted pollutants.
Environmental monitoring is principally useful because it directly measures the concentrations of certain materials from a particular incinerator, in some cases in the media of immediate interest (e.g., dioxins in vegetation and cows' milk). No health effects are measured. For use in evaluating health effects, however, environmental monitoring suffers from several disadvantages, because:
-
There is usually a problem in distinguishing the contribution of the incinerator to environmental concentrations.
-
Monitoring measurements are limited both in space and in time while concentrations are often highly variable in both time and space.
For these reasons, environmental monitoring is usually most useful in confirming, calibrating, or disproving the modeling efforts used in risk-assessment methodology.
Biologic Markers (Biomarkers) of Exposure or Effect
There is now considerable interest in the use of biologic markers of exposures or effects in epidemiologic studies of the health risks posed by some occupational and environmental exposures (NRC 1989a,b, 1992a,b, 1995). Some of these studies are relevant to likely exposures to substances emitted from incinerators—for example, measurements of specific congeners of PCDDs and PCDFs in blood and adipose tissues of exposed workers (Schecter et al. 1994), analyses of chlorophenol and pyrene metabolites in blood and urine of incinerator workers (Angerer et al. 1992), analysis of selected DNA adducts in blood samples of incinerator workers and measurement of various indexes of metal exposure in workers (Malkin et al. 1992).
Such studies are likely to be generally useful for evaluating exposures to specific materials that might be present in incinerator emissions or evaluating the presence of effects that might be associated with incinerator emissions. However, no biomarker of exposure or effect associated uniquely with incinerator emissions has been identified, nor is any such biomarker likely to be identified, inasmuch as incineration emissions as a class do not (so far as is now known) have components that are peculiar to them nor that cause unique effects.
Thus, although the use of biomarkers might add substantially to the accuracy of measurement of exposures and effects in epidemiology, it is not likely to
reduce substantially other major sources of uncertainty that are entailed in the application of epidemiology to incinerator emissions.
RESULTS OF EPIDEMIOLOGIC STUDIES OF INCINERATOR-EXPOSED POPULATIONS
This section discusses the findings from epidemiologic studies of incinerator-exposed populations, including the few studies of human populations in the vicinity of incinerators and the more-detailed health studies of workers in these facilities. In general, information is rather sparse on the relationship between human exposure to pollutants released to the environment by incinerators and the occurrence of health effects.
Studies of Local Populations
In one of the earliest epidemiologic studies of populations in the vicinity of waste incinerators, Zmirou et al. (1984) obtained data on the use of medications for respiratory illnesses over a 2-year period among residents of a French village at distances of 0.2, 1, and 2 km from a refuse incinerator. Medication use was determined by examining prescription forms filed by the residents after each purchase. The purchase of respiratory medications (bronchodilators, expectorants, antitussants, and so on) decreased as the distance of the residences from the incinerator increased, and the relationship was statistically significant. However, the prevalence of other possible confounding risk factors for respiratory illness, such as socioeconomic and geographical situation, were not accounted for in this study, and no causal associations can be inferred.
After reports of illness and neurologic symptoms in workers employed at the Caldwell Systems, Inc. hazardous-waste incinerator in western North Carolina and health complaints of nearby residents, the Agency for Toxic Substances and Disease Registry (ATSDR) performed a cross-sectional study in the surrounding community for the prevalence of self-reported respiratory, musculo-skeletal, neurologic, irritative, and other symptoms (ATSDR 1993a). A higher prevalence of self-reported respiratory symptoms, but not of respiratory or other diseases, was found in the target population than in a nearby comparison population. Prevalence data were adjusted for age, sex, and cigarette smoking. Members of the population close to the incinerator were almost nine times more likely to report recurrent wheezing or cough, and they were almost twice as likely as those living further from the site to report respiratory symptoms (after adjustment for smoking, asthma, and environmental concern). Other symptoms—including chest pain, poor coordination, dizziness, and irritative symptoms—were also statistically significantly greater in the population close to the incinerator. However, the investigators noted that neither the prevalence of physician-diagnosed diseases (as reported by subjects) nor hospital admissions
for these diseases differed between the target and comparison populations, and they pointed out that the retrospective nature of the study (the incinerator operated from 1977 to 1988, and the cross-sectional study was conducted in 1991) limited interpretation of the findings. One of the major concerns was recall bias associated, in part, with the greater than 2-year gap between the shutdown of the incinerator and the conduct of the symptom survey. Another factor was the large amount of adverse publicity that the incinerator received before shutdown. Although the investigators attempted to control for recall bias by stratifying their results according to the respondents' expression of environmental concern, they concluded that they were only partially successful, inasmuch as the higher rate of self-reported symptoms from the population close to the incinerator was not associated with any difference in physician-diagnosed disease rates or in hospital-admission rates between the two communities. The investigators also acknowledged that they had no direct measures of community exposure to incinerator-emitted pollutants, which had ceased more than two years before the study, and thus could not estimate differences in exposures among individuals within the population close to the incinerator. Thus, this study is of limited utility in evaluating the effect of incinerator exposures, but emphasizes the necessity of controlling for various types of bias.
Wang et al. (1992) tested the lung function of 86 primary-school children living in Taiwan near a wire-reclamation incinerator and compared the results with those in 92 schoolchildren in a school in a “nonpolluted city.” All children had been inhabitants of their districts since birth and had similar socioeconomic backgrounds. Air pollution in the incinerator district was considerably greater than that in the comparison city. SO2 concentrations were 18.1 and 2.1 parts per billion (ppb), respectively, and NO2 concentrations were 12.6 and 2.1 ppb. Questionnaire responses yielded no differences in the prevalence of respiratory symptoms among children in the two areas. However, the prevalence of children with abnormal forced expiratory volume in 1 second (FEV1) was statistically significantly greater in the incinerator community (17.5% vs. 3.2% with abnormal test results). Two groups of children with no reported respiratory symptoms were tested later for bronchial hyperactivity—26 children in the target population and 26 children in the comparison population. A positive methacholine-challenge test was found in 9 of the former and only 1 in the latter group. The authors concluded that “the high level of air-pollution” in the population close to the incinerator was associated with a detrimental effect on lung function in primary-school children; however, they did not obtain data that would allow them to ascribe the measured air pollution to emissions from the incinerator, nor did they characterize other sources of air pollution in the target population. Thus, this study appears to demonstrate that higher concentrations of air pollutants alter pulmonary function in children, but does not directly allow any inference about the contribution of incinerators as opposed to other pollutant sources to either environmental concentrations or health effects in particular.
Gray et al. (1994) studied the prevalence of asthma in children living in two regions of Sydney, Australia, where incinerators burned sewage sludge and in one comparison community within the same metropolitan area. They measured respiratory illness in the previous year by questionnaire, airway hyperactivity by histamine-inhalation tests, and atopy by skin tests in 713 children 8-12 years old in the two regions and in 626 children of the same age in a comparison community without an incinerator. All children attending public and parochial schools within a 5-km radius of each of the study communities were selected for the study. Measurements of SOx, NOx, H2S, O3, and particulate matter during the study period showed no differences among the three regions. The prevalence of current asthma, atopy, symptom frequency, or asthma of any category of severity was not statistically different between incinerator and comparison regions. Results of tests of baseline lung function and of airway hyperactivity also did not differ among the three groups of children. The authors pointed out that their study was not designed to measure short-term acute effects of pollutant exposures. They also noted that the prevalence of asthma symptoms and atopy in this population of Sydney children, including those from the incinerator and comparison communities, was comparable with that in four other populations of children studied in Australia, and they concluded that emissions from high-temperature sewage-sludge incinerators appeared to have no adverse effect on the prevalence or severity of childhood asthma.
Shy et al. (1995) reported on the first year of a 3-year study of three incinerator communities and three comparison communities in southwestern North Carolina. The study was designed primarily to assess the acute respiratory effects of living in the neighborhood of an incinerator. Of the incinerators, one was a biomedical-waste incinerator, one a municipal-waste incinerator, and the third an industrial furnace fueled by liquid waste. Comparison neighborhoods were pair-matched to the incinerator communities on density and quality of housing and were upwind of and at least 3 km from the incinerators. In each neighborhood, 400-500 households were surveyed by telephone for sociodemographic characteristics, including prevalence of such respiratory risk factors as smokers in the home, and the prevalence of acute and chronic respiratory symptoms. No differences in respiratory-symptom prevalence were found between the subjects living near to either biomedical-waste incinerator or municipal-waste incinerator and their comparison communities. Several chronic respiratory symptoms were reported to have a higher prevalence in the liquid-waste combustor community than in its comparison group, but this difference did not persist when the symptom prevalence in the liquid-waste combustor community was compared with the pooled prevalence of symptoms in the three comparison communities.
Concentrations of particulate matter, including PM10 and PM2.5, and of acidic gases, including SO2 and HCl, were monitored in each of the study areas and did not differ measurably between target and comparison communities, either on a daily-average or monthly-average basis. Results of baseline lung-function
tests also did not differ statistically significantly between target and comparison communities. Subjects with a history of recent wheeze or other asthma-like symptoms and nonsmoking subjects with no history of respiratory symptoms were recruited from each study community to record twice-daily peak expiratory-flow rates, acute respiratory symptoms, and (among asthmatics) use of asthma medications for 35 consecutive days during each year of study. None of the paired communities showed a difference in peak expiratory flow rates, adjusted for age, sex and height, or in the incidence of acute respiratory symptoms over the 35-day recording period during the first year of study.
A chemical mass-balance analysis of particle sources during the period of the study estimated that a maximum of 3% of the particle mass in ambient air could be attributed to emissions from the biomedical-waste incinerator on days when the prevailing wind was blowing directly from the incinerator toward the air-monitoring station less than 1 km away. On days when the prevailing wind was in other directions, the contribution of the incinerator to the particle mass measured at the monitoring station was less than 1%. Shy et al. (1995) concluded that data from the first year of study were compatible with the null hypothesis of no difference in acute or chronic respiratory symptoms or lung function between paired target and comparison communities and that particle and acid-gas emissions from the three incinerators contributed trivial quantities to the ambient-air concentrations in the adjacent neighborhoods.
Thus, the few community-based epidemiologic studies reported to date have yielded no evidence that acute or chronic respiratory symptoms are associated with incinerator emissions. However, that conclusion is based on only two community studies, that of Gray et al. (1994) in Sydney, Australia, and that of Shy et al. (1995) in North Carolina. In both measures of air quality, specifically of particles and gases, showed no difference between the incinerator and comparison communities. The lack of difference in concentrations of commonly measured air pollutants found in these studies does not rule out the possibility of differences in concentrations of unmeasured pollutants of concern (such as PCDDs and PCDFs) that may be present in incinerator emissions as well as in background pollution. Thus, such measurements do not directly show that there can be no excess of respiratory effects due to incinerators. However, the absence of differences in the prevalence of asthma among exposed children in the Sydney study and the absence of differences in the incidence of acute respiratory symptoms or in lung function in the North Carolina study are at least suggestive that unmeasured pollutants from well controlled incinerators are not causing overt short-term effects on the respiratory system.
An excess of lung-function abnormalities was found in the schoolchildren study of Wang et al. (1992) in Taiwan, in which the target population had considerably higher measured concentrations of ambient SO2 and NO2. This supports the conclusion that if incinerator emissions result in violation of air-quality standards, the adverse health effects attributable to the excesses can be expected.
After reports of a cluster of cases of cancer of the larynx near an incinerator of waste solvents and oils in Lancashire, UK, Elliott et al. (1992) analyzed the incidence of cancers of the larynx and lung in areas adjacent to all 10 licensed incinerators of waste solvents and oils in Great Britain that began operation before 1979. Exposures and cancer risks were assessed at the aggregate, or “ecological” level. No data were obtained that would allow linking of individual exposure to cancer risk. Postal-coded cancer-registration data were available for 1974-1984 in England and Wales and for 1975-1987 in Scotland. Standardized observed-to-expected incidence ratios were calculated for each postal-code area stratified by distance from the incinerator, within 3 km and 3-10 km away. Expected values were based on national rates and were stratified by region and a measure of socioeconomic status. None of the observed-to-expected incidence ratios within 3 km or 3-10 km away differed statistically significantly from unity for the two cancers. When data were further evaluated over a range of geographic circles up to 10 km away to test for trend, there was no evidence of higher risk closer to the incinerators. The authors noted that, owing to the restricted number of years available for analysis, their model assumed a lag of only 5-10 year between the beginning of incinerator operation and a potential effect on cancer incidence and that this lag is recognized to be short in light of the epidemiology of most cancers. An additional 10-year follow up of cancer incidence in these populations would be more informative, in that, as the authors note, Fingerhut et al. (1991) observed an excess cancer mortality associated with TCDD workplace exposures only after 20 years of followup. They concluded that the observed cluster of laryngeal cancer at the Lancashire site was unlikely to be attributable to residential proximity to the incinerator.
In a second, more-comprehensive study of cancer incidence in over 14 million people living near 72 municipal solid-waste incinerators in Great Britain for the years 1974-1986, Elliott et al. (1996) studied cancer incidence in relation to residential proximity to the incinerators. All postal-code areas within 7.5 km of one of the municipal incinerators in England, Wales, and Scotland—except those brought into operation after 1975—were divided into eight concentric bands on the basis of distance from the incinerator. The observed cancer incidences in all residents within the 7.5-km study area and in residents within each of the 8 bands were compared with expected numbers of cancers based on national cancer-incidence rates obtained directly from the Small Area Health Statistics Unit database and adjusted for age, sex, region, and a “deprivation score.” The deprivation score was an attempt to take into account the prevalence of unemployment, overcrowding, and social class of the head of household; this score was previously found to strongly correlate with cancer rates across Great Britain. Statistically significantly greater numbers of cancers—for all cancers combined and for cancers of the stomach, colon and rectum, liver, and lung —were observed for the entire study area; within the eight geographic bands, the excess of observed over expected numbers increased slightly closer to the incinerators.
However, on further analysis, the authors concluded that those results were likely to be largely explained by residual confounding by the deprivation score. When they compared the ratios of observed-to-expected cancers during the preincinerator period—that is before startup of a site—with postincinerator ratios and assumed a 10-year lag between year of startup and cancer incidence, the authors found that observed-to-expected ratios were somewhat larger during the preincinerator period, particularly for stomach and lung cancers. They also observed that the deprivation score was higher with increasing proximity to incinerators. A review of the histologic coding of liver-cancer cases revealed substantial disagreement between the cancer-registry and death-certificate databases. The authors concluded that the excess cancer cases in areas closest to the incinerators could be accounted for by the higher prevalence of unemployment, overcrowding, and lower social class in these areas, and that these factors were not fully controlled in the analysis but that further investigation, including histologic review of cases, should be done.
In a spatial analysis of risk as a function of distance from various sources of pollution (shipyard, iron foundry, incinerator, and city center) in Trieste, Italy, Biggeri et al. (1996) concluded that air pollution is a moderate risk factor of lung cancer. This is consistent with a study conducted in Rome, Italy (Michelozzi et al. 1998) which reported that mortality from laryngeal cancer declined with distance from the sources of pollution. In contrast, a 10-year follow up study conducted in Finland reported increased mercury exposure as the distance decreased from a hazardous-waste incinerator; however, “the increase in exposure was minimal and, on the basis of current knowledge, did not pose a health risk (Kurttio et al. 1998).”
Studies of Incinerator Workers
Motivated by findings of Pani et al. (1983) that airborne particles collected in the working areas of a municipal refuse incinerator were mutagenic, Scarlett et al. (1990) compared the frequency of urinary mutagens, measured by the Ames assay, in a sample of 104 refuse-incinerator workers in 7 incinerator plants with that in 61 water-treatment plant employees in 11 municipal facilities. When urinary-mutagen frequency was adjusted for age, cigarette-smoking, fried-meat consumption, alcohol use, and use of a wood stove in the home, the frequency of urinary mutagens in incinerator workers was found to be a factor 9.7 times as high as the comparison group of water-treatment plant workers when the assay was performed without microsomal activation and 6.3 times as high with microsomal activation. Mutagens were present in urine of workers at 4 of the 7 incinerators and only 1 of the 11 water-treatment plants.
Two years later, the investigators restudied workers at the same incinerators and water-treatment plants to evaluate the consistency of their earlier results (Ma et al. 1992). Three urine samples, collected at about 1-wk intervals, were ob-
tained from 37 incinerator workers in four facilities and from 35 water-treatment plant workers in eight facilities. When the first urine samples were compared, incinerator workers had positive mutagen assays four times more often than water-treatment workers; the difference was statistically significant. Although the frequency of mutagens was higher among incinerator workers for the second and third urine samples, the differences from frequencies in the water-treatment workers were no longer statistically significant. With microsomal activation, the proportions of incinerator workers who had positive mutagen assays declined in the three urine samples—from 21.6% to 15.2% and then 8.3%. The authors speculated that the trend might be explained in two ways. One is that incinerator workers began to take measures to reduce their exposures. The other is that exposures to mutagenic substances in incinerator plants was highly variable. The authors pointed out that the presence of mutagens in the urine does not establish that mutations are taking place in the cells of these workers, but they did recommend that measures be taken to reduce occupational exposures of incinerator workers to potential mutagens in their work environments.
Angerer et al. (1992) measured concentrations of various organic substances in the blood and urine of 53 workers at a municipal-waste incinerator in Germany and 431 men and women “who belong to different subgroups,” also in Germany. No information is provided in the report on the extent of industrial-hygiene controls in the incinerator facility. Statistically significantly higher concentrations of urinary hydroxypyrene, 2,4- and 2-5-dichlorophenol, and 2,4,5-trichlorophenol, and of plasma hexachlorobenzene (HCB) were found among incinerator workers, whereas the controls had higher concentrations of urinary 4-monochlorophenol and tetrachlorophenol. No statistically significant differences between the two groups were found for blood benzene (after stratification on cigarette-smoking), plasma polychlorinated biphenyls, or urinary 2,4,6-trichlorophenol or pentachlorophenol. Urinary hydroxypyrene was measured because it is a metabolite of pyrene and has been shown to be a good indicator of internal dose of PAHs. Plasma PCBs and HCB and urinary chlorophenols were measured because these chemicals, when combusted, are precursors of dioxins and furans, and because they are easier to measure in biological material than the dioxins and furans. The lack of consistent findings between the incinerator and comparison groups for PCBs, HCB, and chlorophenols means this study provides no conclusive evidence on the exposure, absorption, or metabolism of combustion precursors of the PCDDs and PCDFs, and so allows no inference about exposures to PCDDs and PCDFs. However, the higher concentrations of hydroxypyrene might indicate that incinerator workers had higher exposures to PAHs.
Schecter et al. (1994) measured polychlorinated dioxins and dibenzofurans in pooled samples of blood from 85 workers at a relatively old incinerator in New York City and pooled blood from 14 matched controls in the same city. Higher concentrations of several of the dioxin and furan congeners, except TCDD, were found in the blood of incinerator workers. The authors comment that the
findings document exposure and bioavailability and suggest a hazard to workers. After the findings were presented, personal protective measures were put into place for the workers at this facility. Because the samples from all workers were pooled, it was not possible to evaluate whether concentrations of congeners were related to the probable extent of occupational exposure, duration of employment, or to potentially confounding exposures; analysis of these variables could have given greater confidence that the findings were attributable to the occupational environment rather than to other sources of the organic pollutants.
In 1992, staff of the National Institute for Occupational Safety and Health (NIOSH) performed environmental sampling to investigate employee exposure to PCDDs, PCDFs, metals, and other substances at three New York City municipal-refuse incinerators (NIOSH 1995). Six area samples from working zones and five bulk fly-ash samples were collected and analyzed for PCDD and PCDF congeners, eight personal-breathing-zone samples and nine area samples were collected for metals during cleaning operations, and 10 samples were collected for respirable dust and silica. Airborne PCDD and PCDF concentrations for four of the six area samples from working zones exceeded the National Research Council guideline of 10 pg/m3 (one sample by a factor of 80); all four were collected during cleaning operations. The breathing-zone samples approached or exceeded the NIOSH and Occupational Safety and Health Administration criteria for arsenic, cadmium, lead, and nickel. Area samples collected near work locations exceeded relevant evaluation criteria for aluminum, arsenic, cadmium, cobalt, lead, manganese, and nickel. One of 10 samples exceeded the NIOSH recommended exposure limit for respirable quartz by 50%. The airborne concentrations of aluminum, arsenic, cadmium, lead, and nickel during some periods of the cleanout of the electrostatic precipitator and of PCDDs and PCDFs during cleaning of the lower chamber were high enough to exceed the protection capabilities of the air-purifying respirators worn by the workers during these operations. On the basis of this evaluation, NIOSH staff concluded that working in cleanout operations at the incinerators poses a health hazard.
Malkin et al. (1992) analyzed blood samples from 56 high-pressure plant tenders working at three New York City incinerators. The duties of these workers—involving precipitator, upper- and lower-chamber, and undercarriage cleaning—were judged to be those with the highest potential exposure to lead. Blood samples were also obtained from a control group of 25 high-pressure plant tenders working at heating plants, where maintenance of boilers was involved. Although the average blood-lead concentration (11.0 µg/dL) of the incinerator workers was not high relative to concentrations associated with clinical abnormalities, they were statistically significantly higher than the average (7.4 µg/dL) in the comparison workers. When the variation in blood lead among incinerator workers was analyzed with multiple-regression modeling (incorporating age and cigarette smoking), workers who did not always wear protective devices or who cleaned the combustion chambers more times in the last year had statistically
significantly higher blood lead. None of the known health effects of lead exposure was evaluated in this study. The results suggest that the presence of lead in combustion-chamber fly ash can increase the blood-lead concentrations of incinerator workers.
Only two morbidity or mortality studies of waste-incinerator workers have been reported. Bresnitz et al. (1992) evaluated 86 male workers among 105 active employees at a Philadelphia municipal incinerator. The workers were divided into potential high- and low-exposure groups of 45 and 41, respectively, on the basis of a worksite analysis performed by an independent industrial hygienist. Eight workers had at least one measurement in blood or urine indicating excessive exposure to heavy metals, but these elevations were unrelated to exposure category. Although 34% of the workers had evidence of hypertension, the prevalence of this condition was unrelated to exposure group. None of the biochemical measurements of blood or serum were clinically significant, and, except for hematocrit and serum creatinine, the differences between the two exposure groups were not statistically significant.
Gustavsson (1989) studied the mortality experience of 176 waste-incinerator workers in Sweden. Compared with national and local death rates standardized for age and calendar year, there was an excess of deaths from lung cancer and ischemic heart disease. Analysis of duration of exposure supported the conclusion that the excess of deaths from ischemic heart disease was attributable to occupational factors, whereas lung-cancer deaths were too few to make such an inference.
In summary, workers in the incinerator industry have not been extensively studied for morbidity and mortality risks. A Swedish study found an excess of deaths from lung cancer and ischemic heart disease among a sample of 176 incineration workers. The few available studies reviewed here yield evidence that some workers are exposed to amounts of organic compounds and metals (including dioxins, furans, and lead) that result in increased tissue concentrations. The health consequences of the exposures have not been evaluated through systematic followup of these workers.
A recent report of a retrospective mortality study of a cohort of 532 male subjects employed at two municipal-waste incineration plants in Rome, Italy (Rapiti et al. 1997) revealed an increased risk of gastric cancer. The authors concluded that these findings indicate the need to further investigate the role of cancer as a result of occupational exposure to hazardous waste.
Studies of Animal Populations
Lloyd et al. (1988) studied rates of twin births in cows (“twinning”) in an area of central Scotland surrounding two waste incinerators, one a municipal-waste incinerator and the other a chemical incinerator. The study of twin births was prompted by the anecdotal observation of a dramatic increase in twinning
among the dairy cattle in the region. The authors noted that some polychlorinated hydrocarbons have estrogenic and fertility-related properties and that endogenous or exogenous estrogens might affect the frequency of twinning. Two postal-code sectors downwind of the incinerators were considered to be areas of primary risk, and this classification was supported by finding comparatively high concentrations of polychlorinated compounds in surface soils in these sectors. Twinning rates in the upwind and more-distant postal-code sectors were 3-13 per 1000 births; the highest rates, 16 and 20 per 1,000, were observed in the two downwind sectors. The incidence of identical twins in cows is rare, but fraternal twins can occur in up to 5% of births, depending on the breed. Delay in mating or artificial insemination can contribute to twinning, as can repeated breeding and artificial insemination. The incidence of twinning is also increased once a cow has given birth to a first set of twins (Hafez 1974). The authors noted that genetic factors in twinning remain to be investigated in this population.
In a second study of the same area, Williams et al. (1992) analyzed the male-to-female ratio in calves at birth by postal-code sector and found an excess of female births downwind of an incinerator. Because of suggestions that pollution from the incinerators might have increased during later years, the data were grouped into two periods, 1975-1979 and 1980-1983. Statistically significantly lower male-to-female ratios were observed in one of the two downwind sectors during both periods, but not in the other downwind sector. By using computer mapping and smoothing techniques to analyze twinning rates in enumeration districts within each postal-code sector, the authors were able to show a persistent excess of female births, compared with other districts, along a northeast-southwest axis from the incinerators, which was consistent with the prevailing wind patterns in the area. Because many factors can alter sex ratios, and these factors were not enumerated in this study, the authors considered it premature to attribute causality to the reported associations.
RESULTS FROM RISK ASSESSMENT STUDIES
There have been hundreds of risk assessments performed on incinerators of various types in many parts of the country. These assessments have taken various forms and followed various protocols. Among the more-detailed have been the assessments for Dickerson County (Brower et al. 1990), and more recently, the Waste Technologies Incinerator (EPA 1997b), but there is no convenient listing or compilation of such assessments or their results. There is no standard way for publishing these risk assessments, and few receive peer review. Although most such assessments are in the public domain, obtaining them is difficult, and there are still many that are likely to have remained private.
Most of these risk assessments are based on methodology that was first introduced in the evaluation of nuclear power plants (NRC 1977). It should be
emphasized that these risk assessments were performed to evaluate the risks to the local population; workers's risks were generally not evaluated, nor was the regional impact considered, and not all facilities have been assessed for risk. Experience with them indicates that:
-
For modern, well-controlled incinerators, risk estimates for cancer effects even for the most-highly exposed persons (not workers), are generally small to negligible (for example, lifetime cancer risk estimates below 1 in 100,000).
-
At least some older, poorly controlled incinerators—had they continued to operate—would likely have resulted in cancer risk (above 1 in 10,000 lifetime risk).
-
The principal contributors to risk estimates tend to be dioxins and furans (through food chain routes), arsenic, HCl, mercury, lead, and particles.
-
Experience in performing such assessments is extremely important, particularly if new chemicals are inserted into models not designed for them.
Risk assessments have as one of their bases an evaluation of the health effects observed for the materials examined in risk assessment. A fundamental tenet of risk assessment is the ability to perform extrapolations, including extrapolations of dose-response results for health effects observed at different concentrations, in differing exposure circumstances, and even in different species. It is considered, however, that uncertainty is minimized by using the minimum amount of extrapolation possible. The examples in the following section were chosen to illustrate the ranges of data available for the various chemicals.
Observed Health Effects of Materials Present in Incineration Emissions
This section summarizes, for selected pollutants of concern, the adverse health effects that have been documented in humans and animals. These pollutants are known to be produced and released into the environment during the operation of various waste incinerators. The chemicals selected for discussion in this section are particulate matter, CO, acidic gases (NOx, SO2, and HCl) and acidic particles, (e.g., as H2SO4 or NH2HSO4), some metals (cadmium, lead, mercury, chromium, arsenic, and beryllium), and organic compounds—dioxins and furans and some other products of incomplete combustion (PCBs and PAHs). Human health effects have been observed for some of these agents at extremely high concentrations in various exposure circumstances; but such effects have not been observed as a direct result of exposure to emissions from a waste incinerator (as demonstrated in the following sections). PM health effects can apparently occur at concentrations previously considered acceptable. For lead, health effects occur at blood concentrations that are not far above background blood
concentrations, but these correspond to ambient air concentrations greater than current standards for lead.
Particulate Matter
Particulate Matter (PM) consists of a mixture of materials. The numbers of particles and their chemical composition can vary within specific particle-size fractions from location to location and over time, depending on the types of source emissions and atmospheric conditions. Concern about airborne particulate matter in recent years has been driven largely by epidemiologic studies that have reported relatively consistent associations between outdoor particulate-matter levels and adverse health effects. However, assessing the specific health risks resulting from exposures to airborne particulate matter, and distinguishing these effects from those produced by gaseous copollutants, involves substantial scientific uncertainty about the influence of copollutants and weather, about whether some particulate-matter fractions (size or chemical) might be more-highly associated with health risks, and about the nature of dose-response relationships between particulate matter and health (NRC 1998, 1999c).
Most available epidemiologic evidence of PM effects have employed direct or indirect metrics of PM mass, irrespective of particle composition or emission source (e.g., see Dockery and Pope 1994).
The most-clearly defined effects associated with exposure to PM have been sudden increases in the number of illnesses and deaths occurring day to day during episodes of high pollution. The most notable of those episodes occurred in the Meuse Valley in 1930, in Donora in 1948, and in London in 1952. During the December 1952 episode, 3,000-4,000 excess deaths were attributable to air pollution, with the greatest increase in death from chronic lung disease and heart disease (United Kingdom Ministry of Health 1954). The death rate increased most dramatically in those older than 45 years and among those with pre-existing respiratory illnesses (such as asthma). Collectively, studies of those and other early episodes left little doubt that airborne PM contributed to the morbidity and mortality associated with very high concentrations of urban aerosol mixtures dominated by combustion products (e.g., from burning coal) or their transformation products (such as aerosols containing sulfuric acid).
The 1982 EPA PM criteria document concluded that the available studies collectively had indicated that mortality was substantially increased when 24-hr airborne-particle concentrations exceeded 1,000 µg/m3 (as measured by the black smoke method) in conjunction with SO2 concentrations over 1,000 µg/m3 (the elderly and persons with severe pre-existing cardiovascular or respiratory disease were mainly affected).
The period since the 1982 criteria document (and its 1986 addendum) has seen many reports of time-series analyses of associations between human mortality and acute exposures to PM at or below the pre-1997 U.S. 24-hr standard
(PM10 at 150 µg/m3). As a result, EPA moved to institute a more-stringent U.S. short-term PM mass concentration limit of 65 µg/m3 for fine particles (PM2.5, the mass of particles below 2.5 µm in diameter), and an annual PM2.5 limit of 15 µg/m3. On May 14, 1999, a panel of the U.S. Court of Appeals for the District of Columbia Circuit remanded the new standards for PM2.5.
Numerous investigators have reported statistically significant positive associations between relative risk for death and various indexes of PM in many cities in the United States and other countries. The elderly (over 65), particularly those with pre-existing respiratory disease, were found to have higher risks than younger adults (Thurston 1996). Studies suggest that children are also at increased risk from the adverse health effects of air pollution. During the London fog episode, the second highest increase in mortality (after older adults) was in the neonatal age group (relative risk, (RR) = 1.93 for children less than 1 year) (United Kingdom Ministry of Health 1954). More recently, Saldiva et al. (1994) found acute exposure to air pollution in Sao Paulo, Brazil to be significantly associated with respiratory mortality in children less than 5 years of age, although the effect could not be definitively associated with a specific pollutant. Also, Bobak and Leon (1992) and Woodruff et al. (1997) both found long-term averages of air-pollution, including PM, to be associated with increased post neonatal (ages 1 to 12 months) mortality. Thus, air pollution exposure has been associated with increased mortality, with the very young and the elderly being indicated as being especially at risk.
Published summaries of PM reports have converted all results to a PM10-equivalence basis and provided quantitative comparisons (Ostro 1993; Dockery and Pope 1994; Thurston 1996). Other summaries have used total suspended particles (TSP) as the reference PM metric (Schwartz 1991, 1994a) and considered many of the same studies included in the PM10-equivalence summaries. (Other air pollutants were generally not addressed in deriving the coefficients reported by these summaries.) The results suggest about a 1% change in acute total mortality for a 10-µg/m3 change in daily PM10. Such a change represents a seemingly small increment in risk from exposure to this pollutant, but it must be remembered that peak PM 10 concentrations are commonly about 100 µg/m3 above concentrations for an average day, that large populations are affected by this ubiquitous pollutant, and that this reported RR is for total mortality (with even higher RRs being found in studies of more affected specific causes, such as respiratory disease, and for sensitive populations, such as the elderly). Also, the implied increments in lifetime risk from small increments in exposure to particles are very high compared with typical values of regulatory interest. In the reviews cited above, the highest PM10-associated relative risks for death were indicated for the elderly and for those with pre-existing respiratory conditions; both constitute populations that appear to be especially sensitive to acute exposures to air pollution.
Aggregate population-based cross-sectional studies using averages across various geopolitical units (cities, metropolitan statistical areas (MSAs), and so on) have examined the relation between mortality and long-term PM exposure. Those community-based studies sought to define the characteristics of a community that are associated with its overall average health status, in this case annual mortality. For example, Ozkaynak and Thurston (1987) analyzed 1980 total mortality in 98 MSAs, using data on PM15 and PM2.5 from the EPA inhalable-particle monitoring network for 38 of these locations. They concluded that the results suggested an effect of particles on mortality that decreased with increasing particle size.
Prospective cohort studies have considered the effect of PM exposure on the relative survival rates of individuals, as modified by age, sex, race, smoking, and other individual risk factors, finding that PM exposure can lead to substantial shortening of life in the general population. That type of analysis has a substantial advantage over aggregate population-based studies, in that the individual analysis allows stratification according to such important risk factors as smoking. Abbey et al. (1991) described a prospective cohort study of morbidity and mortality in a population of about 6,000 white, non-Hispanic, nonsmoking, long-term California residents who were followed for 6-10 years beginning in 1976. TSP and ozone were the only pollutants considered. In a followup analysis, Abbey et al. (1995) considered exposures to SO42-, PM10, and PM2.5, as well as visibility (extinction coefficient). In these analyses, no significant associations with nonspecific mortality (i.e., from all natural causes) were reported, and only high concentrations of TSP or PM10 were associated with respiratory symptoms of asthma, chronic bronchitis, or emphysema. However, a more recent analysis using an additional 5 years of follow-up on this cohort and improved PM10 exposure estimates did predict significant PM-mortality associations among men in this cohort, who reportedly spent significantly more time outdoors than women (Abbey et al. 1999). Dockery et al. (1993) analyzed the mortality experience in 8,111 adults who were first recruited in the middle 1970s in 6 cities in the eastern portion of the United States. The subjects were white and 25-74 years old at enrollment. Dockery et al. (1993) reported that “mortality was more strongly associated with the levels of fine, inhalable, and sulfate particles” than with the other pollutants. Pope et al. (1995) analyzed 7-year survival data (1982-1989) for about 550,000 adult volunteers obtained by the American Cancer Society (ACS). They took great care to control for potential confounding factors on which data were available. For example, several different measures of active smoking were considered, as was the time exposed to passive smoke. The adjusted total-mortality risk ratios for the ACS study, computed for the cities' range of the pollution exposures, were as follows: 1.15 (95% confidence interval, 1.09-1.22) for a 19.9 µg/m3 increase in sulfates and 1.17 (95% confidence interval, 1.09-1.26) for a 24.5 µg/m3 increase in PM2.5. Analysis of life-tables indicate that these effects
are associated with more than a 1-year shortening of expected lifespan for the entire population (WHO 1995).
Dockery and Pope (1994) have reviewed the effects of PM10 on both respiratory mortality and morbidity. They considered five primary health end points: mortality, hospital use, asthma attacks, respiratory symptoms, and lung function. They concluded that there was a coherence of effects across the end points, with most end points showing a 1-3% change per 10 µg/m3. A later analysis by Thurston (1996) indicated that those PM-effect estimates are reduced somewhat if the influences of copollutants are addressed.
Hospitalization data can provide an especially useful measure of the morbidity status of a community during a specified period. Hospitalization data on respiratory-illness diagnosis, or more specifically for chronic obstructive pulmonary disease (COPD) and pneumonia, give a measure of respiratory status. Both COPD and pneumonia hospitalization studies show moderate but statistically significant relative risks, in the range of 1.06-1.25, associated with an increase of 50 µg/m3 in PM10. Table 5-1 presents results of several studies of short-term exposure-response relationships of fine-particle sulfates, PM2.5, and PM10 with different health-effect indicators, as developed by the World Health Organization. The data provide quantitative estimates of the effect of PM (per unit of increment) for each outcome considered.
Acidic Gases and Acidic Aerosols
Nitrogen Oxides
Nitric oxide (NO) is the major nitrogenous pollutant emitted from incineration facilities. Although NO itself is not thought to result in any deleterious health effects at the concentrations surrounding combustion sources, it is readily oxidized in the ambient environment to nitrogen dioxide (NO2), which is the most biologically significant of the nitrogen oxides. NO2 exerts its health effects via two primary pathways. One pathway is directly through interactions with the respiratory system when breathed. The other pathway is indirectly through the photochemical formation of atmospheric ozone, a secondary pollutant with much greater respiratory effects than NO2 itself. Collectively, nitrogen oxides are often assessed as a group known as NOx.
NO2 is water-soluble and, when breathed, is efficiently absorbed in the mucous lining of the nasopharyngeal cavity and lung, where it converts to nitrous acid, HNO2, and nitric acid, HNO3, which can then react with the pulmonary and extrapulmonary tissues. NO2 has been shown in occupational settings to be rapidly fatal at extremely high concentrations (i.e., 150,000 ppb and above) because of pulmonary edema, bronchial pneumonia, or bronchiolitis fibrosa obliterans (NRC 1977, Ellenhorn and Barceloux 1988), but these exposures are 10,000 times in excess of ambient concentrations found near sources such as
TABLE 5-1 Results of Several Studies of Short-Term Exposure-Response Relationship of Sulfates, PM2.5, and PM10 with Different Health-Effect Indicators
|
Estimated Change in Daily Average Concentration Needed for Given Effect, µg/m3 |
|||
|
Health-Effect Indicator |
Sulfates |
PM2.5 |
PM10 |
|
Daily mortality |
|||
|
5% change |
8a |
29a |
50b |
|
10% change |
16 |
55 |
100 |
|
20% change |
30 |
110 |
200 |
|
Hospital admissions for respiratory conditions |
|||
|
5% change |
8c |
10d |
25e |
|
10% change |
16 |
20 |
50 |
|
20% change |
32 |
40 |
100 |
|
Bronchodilator use among asthmatics |
|||
|
5% change |
7g |
||
|
10% change |
14 |
||
|
20% change |
29 |
||
|
Symptom exacerbations among asthmatics |
|||
|
5% change |
10h |
||
|
10% change |
20 |
||
|
20% change |
40 |
||
|
Peak expiratory flow (mean population change) |
|||
|
5% change |
200i |
||
|
10% change |
400 |
||
|
20% change |
|||
|
a Based on estimates for St. Louis from Dockery et al. 1992. b Based on Pope et al. 1992; Dockery et al. 1992; Schwartz 1993; Kinney et al. 1995; Ito et al. 1995. Relative-risk estimates per 100 µg/m 3 from these studies were 1.16, 1.16, 1.11, 1.04, and 1.05, respectively; statistically significant estimates only. c Based on Thurston et al. 1994; Burnett et al. 1994. Relative-risk estimates per 10 µg/m3 from these studies were 1.11 and 1.03, respectively. d Based on Thurston et al. 1994. e Based on Schwartz 1994b,c,d. Relative-risk estimates per 100 µg/m3 from these studies were 1.40, 1.20, and 1.10. f No data. g Based on Pope et al. 1991; Roemer et al. 1993. Relative-risk estimates per 10 µg/m3 from these studies were 1.12 and 1.02, respectively. h Based on Roemer et al. 1993; Pope et al. 1992. Relative-risk estimates per 10 µg/m3 from these studies were 1.05 and 1.05, respectively. i Based on Pope et al. 1991, 1992; Roemer et al. 1993. Coefficients from these studies ranged from -0.028 to -0.041 L/min per µg/m3; mean baseline peak flow ranged from 260 to 300 L/min. Source: WHO 1995. |
|||
incinerators. Ambient concentrations of NO2 vary with motor-vehicle traffic density in most U.S. cities, and annual average concentrations range from about 4 to 34 ppb (EPA 1998b,c). Potential acute effects of concentrations above 100 ppb NO2 can include reduced pulmonary function, inflammation of the lung, and altered host defenses, especially among asthmatics (e.g., Samet and Utell 1990). The concentrations required to produce those effects can be reached indoors when unvented gas stoves or kerosene heaters are present, but are generally above the concentrations that occur in the ambient air (Klaassen et al. 1995). However, studies of healthy subjects exposed to NO2 from 75 min to 3 hr at up to 4,000 ppb have generally failed to show lung-function alterations (Bascom et al. 1996). Even in susceptible people, such as those with pre-existing respiratory disease, effects at concentrations less than 1,000 ppb are not consistently detected. Concern with respect to present-day ambient concentrations of NO 2 is focused primarily on increases in airway responsiveness of asthmatic people after short-term exposures and increased occurrence of respiratory illness among children associated with long-term exposures to NO 2 (EPA 1993).
Hydrogen Chloride
The irritating properties of hydrogen chloride (HCl) prevent the study of more than transient voluntary exposure at concentrations that are likely to cause serious health effects, so there is a paucity of human data that can be used to evaluate the health effects of exposure to HCl at high concentrations (NRC 1991c). In humans, HCl acts primarily as an irritant of the upper respiratory tract, eyes, and mucous membranes, generally at concentrations over 5 ppm (NRC 1991c). Concentrations of 50-100 ppm are considered barely tolerable (Stokinger 1981). Bleeding of the nose and gums and ulceration of the mucous membranes have been attributed to repeated occupational exposure to HCl mist at high (unspecified) concentrations (Stokinger 1981). Etching and erosion of teeth have been reported in workers exposed to acids in battery, pickling, plating, and galvanizing operations (ten Bruggen Cate 1968); these workers were exposed to various mineral acids, including HCl (0.1 ppm), in combination with other acids, primarily sulfuric acid.
The LC50 values for HCl in rats, mice, and guinea pigs are 4,700 ppm, 2,600 ppm, and 2,500 ppm, respectively, for a 5-min exposure (Machle et al. 1942; Darmer et al. 1974). Results of studies in which mice were exposed to HCl vapors or aerosols indicate that vapors and aerosols have comparable toxicity (Darmer et al. 1974). As in humans, HCl was extremely irritating to the eyes, mucous membranes, and skin. In addition, rats and mice had scrotal ulceration and corneal erosion and clouding. Gross examination of animals that died during or shortly after exposure revealed moderate to severe emphysema, atelectasis,
and pulmonary edema. No deaths were reported in mice or rats exposed to HCl at 410 and 2,078 ppm, respectively, for 30 min (Darmer et al. 1974).
No pathologic changes were observed in experimental animals exposed to HCl at 33 ppm for 6 hr/day, 5 days/week for 4 weeks. Exposure of rats and mice at 50 ppm for 6 hr/day, 5 days/week for 90 days resulted in statistically significant decreases in body weight, whereas no change was observed in hematologic characteristics, serum chemistry, and urinalysis. Histologic examination revealed dose-related minimal to mild rhinitis at 10, 20, and 50 ppm. Exposure of rats at 10 ppm and higher for 6 hr/day, 5 day/week for life resulted in laryngeal hyperplasia in 22% of the test animals, compared with 2% of control animals, and tracheal hyperplasia in 26% of the test animals, compared with 6% of controls (Sellakumar et al. 1985).
Mortality in the progeny of rats exposed to HCl at 300 ppm on day 9 of pregnancy was 31.9 ± 9.2%, compared with 5.6 ± 3.7% in controls (p < 0.01). The progeny of rats exposed at 300 ppm either for 12 days before pregnancy and of rats exposed on day 9 of pregnancy showed disturbances in kidney function, as measured by diuresis and proteinuria (Pavlova 1976).
Baboons exhibited signs of irritation, such as coughing and frothing at the mouth, during a 5-min exposure to HCl at 810 ppm, but not at 190 ppm (Kaplan 1987). Severe irritation and dyspnea occurred at higher concentrations (16,750 and 17,290 ppm). Dyspnea persisted after exposure, followed by death several weeks later from bacterial infections. Baboons exposed at 500 ppm for 15 min also exhibited signs of irritation (increased respiratory rates) but did not develop hypoxia, did not show changes in respiratory function, and were able to perform escape tasks (Kaplan et al. 1988).
Studies have demonstrated notable differences between primates and rodents in responses to HCl exposure. Exposure of rats and mice to HCl concentrations of 560 ppm for 30 min and less than 50 ppm for 10 min, respectively, produced dose-related decreases in respiratory frequency (Barrow et al. 1979; Hartzell et al. 1985). Baboons exposed to HCl at up to 17,000 ppm for 5 min, however, exhibited increases in respiratory frequency that could be interpreted as a compensatory mechanism in response to hypoxia (Kaplan et al. 1988). Given their greater similarity to humans in the respiratory tract and its function, baboons would probably be more-appropriate animal models than rodents for extrapolation of HCl effects to humans (NRC 1991c).
It has been postulated that a toxic gas or vapor adsorbed on ambient particles of suitable size, perhaps including dust, could be carried to the bronchioles and alveoli, where more-serious damage could occur. Such an effect has been looked at to some extent by the Air Force (Wohlslagel et al. 1976) and found not to be significant in the case of hydrogen fluoride and HCl mixed with alumina particles. However, more recent studies provide evidence that strongly acidic aerosols can constitute a portion of PM that is especially associated with acute respiratory health effects in the general public (Thurston et al. 1992, 1994).
Acidic Aerosols
Most historical and present-day evidence suggests that there can be both acute and chronic effects of the strongly acidic component of PM, i.e., the hydrogen ion (H+), concentration when it is below pH 4.0 (Koutrakis et al. 1988; Speizer 1999). Increased hospital admissions for respiratory causes were documented during the London fog episode of 1952, and this association has now been observed under present-day conditions. Thurston et al. (1992, 1994) have noted associations between ambient acidic aerosols and summertime respiratory hospital admissions in both New York state and Toronto, Canada, even after controlling for potentially confounding temperature effects. In the 1994 report, statistically significant independent H+ effects remained even after the other major copollutant, in the regression model, ozone was considered. H+ effects were estimated to be largest during acid-aerosol episodes (H+ = 10 µg/m3 as sulfuric acid or H+ at ˜200 nmol/m3), which occur roughly 2 or 3 times per year in eastern North America. The studies provide evidence that present-day strongly acidic aerosols might represent a portion of PM that is contributing to the significant acute respiratory health effects noted for PM in the general public.
Results of recent symptom studies of healthy children indicate the potential for acute acidic PM effects in this population. Although the “6-city Study” of parent diaries of children's respiratory and other illness did not demonstrate H+ associations with lower respiratory symptoms except at H+ above 110 nmol/m3 (Schwartz et al. 1994), upper respiratory symptoms in two of the cities were found to be most-strongly associated with high concentrations of H2SO4 (Schwartz et al. 1991). Two recent summer-camp (and schoolchildren) studies of lung function have indicated a statistically significant association between acute exposures to acidic PM and decreases in the lung function of children, independent of those associated with O3 (Neas et al. 1995; Studnicka et al. 1995).
Reported associations between chronic H+ exposures and children's respiratory health and lung function are generally consistent with adverse effects as a result of chronic H+ exposure. Preliminary bronchitis prevalence rates reported in the “6-city Study” locales were found to be more-closely associated with average H + concentrations than with PM in general (Speizer 1989). Follow-up studies of those cities (and a seventh) that controlled for maternal smoking, education, and race suggested associations between summertime average H+ and chronic bronchitic and related symptoms (Damokosh et al. 1993). Bronchitic symptoms were observed 2.4 times more frequently (95% confidence interval, 1.9-3.2) at the highest acid concentration (H + at 58 nmol/m3) than the lowest concentration (16 nmol/m3). Furthermore, in a followup study of children in 24 United States and Canadian communities (Dockery et al. 1996) in which the analysis was adjusted for the effects of sex, age, parental asthma, parental education, and parental allergies, bronchitic symptoms were confirmed to be statisti-
cally significantly associated with strongly acidic PM (relative odds, 1.7; 95% confidence interval, 1.1-2.4). It was also found in the “24-city Study” that mean forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were lower in communities that had high concentrations of strongly acidic PM (Raizenne et al. 1996). Thus, chronic exposures to highly acidic PM have been associated with adverse effects on measures of respiratory health in children.
Asthmatic subjects appear to be more sensitive than healthy subjects to the effects of acidic aerosols on lung function, but reported effective concentrations differ widely among studies (EPA 1986b). Adolescent asthmatics might be more sensitive than adult asthmatics and might experience small decrements in lung function in response to H2SO4 at concentrations only slightly above peak ambient concentrations (for example, less than 100 µg/m3 H2SO4, or 2,000 nmol/m3) (Koenig et al. 1983, 1989). Even in studies reporting an overall absence of statistically significant effects on lung function, individual asthmatic subjects appear to demonstrate clinically important effects (Avol et al. 1990). Two studies from different laboratories have suggested that responsiveness to acidic aerosols correlates with the degree of baseline airway hyperresponsiveness (Utell et al. 1983; Hanley et al. 1992).
Studies have also examined the effects of exposure to both H2SO4 and ozone on lung function in healthy and asthmatic subjects (Frampton et al. 1995). Two recent studies found evidence that H2SO4 at 100 µg/m3 potentiates the ozone response, in contrast with previous studies. Animal studies support the hypothesis of a synergism between acidic aerosols and ozone (e.g., Last et al. 1986). Overall, acidic aerosols appear to be a contributing factor in the toxicity of PM at present-day ambient levels, either alone or in conjunction with ozone exposure. Thus, to the extent that incineration emissions increase the acidity (i.e., lowers the pH) of ambient PM, they may be expected to also increase the toxicity of those ambient aerosols.
Carbon Monoxide
Carbon monoxide (CO) is a colorless, odorless, poisonous gas formed during combustion processes as a result of carbon not being completely oxidized to carbon dioxide (CO2).
CO binds strongly to hemoglobin, with an affinity over 200 times that of oxygen. The binding of CO with hemoglobin is not readily reversible, so it reduces the oxygen-carrying capacity of the blood significantly. CO concentrations above 25 ppm might lead to carboxyhemoglobin (COHb) concentrations of 5%, which has been associated with cardiovascular and respiratory disease and can interfere with pregnancy. Major damage to brain and lung occurs at 50% COHb, and death at 70%.
The body's natural production of CO results in a normal background COHb saturation concentration of 0.4-0.7%. In the nonsmoking population, COHb
concentrations of 0.5-1.5% are typical; in those who smoke a pack of cigarettes per day, 5-6% is typical. COHb in newborns of smoking mothers is 1.1-4.3%. A blood COHb concentration of about 5% would be expected after an exposure to CO at 35 ppm for 6-8 hr (Ellenhorn and Barceloux 1988).
COHb of 2-4% has been associated with a decrease in time to myocardial ischemia and angina (Allred et al. 1989), and 2.9% has led to significant reduction in exercise tolerance and onset of angina (Kleinman et al. 1989). Furthermore, tunnel officers who were exposed to CO and who had COHb over 5% had an increased risk of dying from arteriosclerotic heart disease (Stern et al. 1988). Recently, Morris et al. (1995) reported that an increase of 10 ppm in CO in ambient air pollution was associated with a 10-37% increase in the rate of hospital admissions for congestive heart failure among those over 65.
Fetal hemoglobin has a greater affinity for CO than does adult hemoglobin; fetal COHb concentrations are typically 10-15% higher than maternal concentrations. Maternal exposure to CO at 30 ppm will lead to 5% COHb in the mother and 6% COHb in the fetus. Both the mother and the fetus are also more susceptible during pregnancy. CO has been shown to interfere with pregnancy in rats; although control rats had 100% successful pregnancy, the success rate for those exposed to CO at 30 ppm was only 69% (COHb was 4.8%), and for those exposed at 90 ppm, only 38% (Garvey and Longo 1978).
Fetuses, newborns, and pregnant women are especially susceptible to CO. Other high-risk groups include those with pre-existing heart disease and those over 65 years old (Morris et al. 1995). Hemoglobin reaches equilibrium with CO much more rapidly in people with anemia than in normal subjects; thus, a 4-hr exposure to CO at 20 ppm led to a COHb concentration of 4-5% in anemic subjects, but only 2.5% in normal subjects. Overall, CO from incinerators is not considered to be an important health factor (see discussion of “Implications to Human Health”).
Metals
Metals associated with incinerator emissions include cadmium, lead, mercury, chromium, arsenic, and beryllium. Results of human and animal studies that examined the health effects of these metals are discussed below. It should be noted that for many of the health effects of concern, exposures are uncertain or unknown and are related not to incinerators but rather to occupational studies or case reports of accidental spills or releases.
Cadmium
The various inorganic forms of cadmium investigated to date have shown similar toxic effects (ATSDR 1997a). All soluble cadmium compounds are cumulative toxicants. Inhalation studies of cadmium-containing aerosols have
shown that particle size is a major determinant of toxicity, whereas the chemical form of cadmium is relatively unimportant (Hirano et al. 1989a,b; Rusch et al. 1986). Similarly, oral-exposure studies of inorganic cadmium compounds have shown that absorption of the divalent ion (Cd2+) results from ingestion of all soluble salts and that uptake rates of free cadmium ions and those complexed with proteins are similar.
Except at very high exposures, absorbed cadmium is bound almost totally to the protein metallothionein. The cadmium-metallothionein complex is readily filtered by the glomerulus and is largely reabsorbed in the proximal tubules of the kidney (Foulkes 1978).
The toxic effects of cadmium in humans and animals are similar. The major toxic effects are acute and chronic inflammation of the respiratory tract, renal tubular effects, and lung cancer.
In general, respiratory effects occur after cadmium exposures that are usually seen only in occupational settings, and environmental exposures to cadmium are unlikely to result in acute or chronic respiratory disease. Whereas animal studies have shown that inhaled cadmium can cause lung cancer in rats (Takenaka et al. 1983; Oldiges et al. 1989), human data are less convincing. Thun et al. (1985) reported an exposure-response relationship between cumulative cadmium exposure and lung cancer. On the basis of those findings, EPA has classified cadmium as a group B1 (Probable) human carcinogen by inhalation; a unit risk4 of 1.8 × 10-3 per µg/m3 was calculated (EPA 1992b).
Human and animal data on the neurotoxicity of cadmium are sparse, but there is evidence that neurobehavioral changes appear in adults and children after exposures smaller than those causing renal effects (Marlowe et al. 1985; Struempler et al. 1985; Hart et al. 1989). Animal studies have found behavioral and structural nervous system changes after relatively small oral or parenteral cadmium exposures.
Other toxic effects include cardiovascular effects, hematologic changes, and gastrointestinal changes. These occur after very high exposures after which respiratory and renal changes are also prominent.
ATSDR has estimated inhalation cadmium exposures that pose minimal risk to humans (minimal-risk levels, MRLs) (1997a). An MRL is defined as an estimate of the greatest daily human exposure to a substance that is likely to be without an appreciable risk of noncancer adverse effects over a specified duration of exposure. On the basis of a no-observed-adverse-effects level (NOAEL) of 0.7 µg/m3 in a study of workers that reported a prevalence of proteinuria of 9% after a 30-year exposure at 23 µg/m3 (Jarup et al. 1988), ATSDR (1997a)
|
4 |
In its Integrated Risk Information System (IRIS), EPA defines inhalation “unit risk” as the upper- bound excess lifetime cancer risk estimated to result from continuous exposure to an agent at a concentration of 1 µg/m 3 in air. |
estimated an inhalation MRL at 0.2 µg/m3 and an oral MRL at 0.7 µg/kg per day. The average daily dietary intake of cadmium by adult Americans is about 0.4 µg/kg per day (Gartrell et al. 1986). The average levels of cadmium in smokers approaches the MRL (Nordberg et al. 1985), and therefore smokers are at risk of renal disease from any additional cadmium exposures, including incinerators.
The health effects of cadmium compounds in humans are summarized in Table 5-2.
Lead
Lead has been studied more thoroughly than any of the other pollutants of concern in connection with waste incineration. The public-health importance of lead is due both to its ubiquity in the environment and to the fact that it can affect virtually every organ system in humans and animals. Some effects of lead occur at intakes producing blood concentrations that are low compared with blood concentrations that were considered normal within the past 4 decades (see Table 5-3), and for some no threshold has been demonstrated. In addition, well-defined susceptible subpopulations exist, including fetuses, pre-school-age children, the elderly, smokers, alcoholics, those with nutritional disorders, those with neural or renal dysfunction, and those with genetic diseases that affect heme synthesis (ATSDR 1997b). Direct toxicity to peripheral nerves used to be common among poorly protected lead-exposed workers.
The toxicity of lead and its various inorganic and organic compounds after inhalation, ingestion, or dermal absorption depends on the total body burden and the distribution among various target organs (ATSDR 1997b). The two principal routes of exposure are ingestion and inhalation. About 50-90% of inhaled lead is absorbed by the body, whereas less than half of ingested lead is retained. Children absorb more lead through the gastrointestinal tract than do adults, about 30% compared with less than 10%, although dietary factors are important. Vitamin C, vitamin D, and calcium deficiencies might double or even triple the fraction of ingested lead that is absorbed.
Lead is stored in various body tissues, including blood, kidney, brain, and bone (ATSDR 1997b). Lead in blood has a half-life of about 35 days, in soft tissue about 40 days; and in bone about 20 years. The commonly measured blood-lead concentration is a complex function of prior exposures, showing rapid response to short-term fluctuations in lead intake; while bone lead concentration is more a measure of long-term exposure.
Blood lead has been the most commonly used biomarker of risk (ATSDR 1997b). Many studies have relied on blood-lead measurements as surrogates for biologically relevant lead exposures, doses, or body burdens. Such measurements may, however, be unreliable as indicators of long-term exposures during periods when exposure is changing. Some of those shortcomings can be overcome by measuring bone lead with x-ray fluorescence, which has been used in
TABLE 5-2 Cadmium Compounds: Health Effects in Humans
|
Type of Toxicity |
Route of Exposure |
Type of Effectsa |
Reference |
|
Cardiovascular |
Inhalation and ingestion |
Conflicting findings in relation to cadmium exposures and cardiovascular toxicity; cardiovascular abnormalities reported at very high exposures |
Shigematsu 1984; Kazantzis et al. 1988 |
|
Cancer |
Inhalation |
Unit risk of 1.8 × 10-3 per µg/m3 based on 1 study |
Thun et al. 1985 |
|
Gastrointestinal |
Ingestion |
Ingestion of large amounts causes acute gastric irritation and inflammation; acute doses > 0.07 mg/kg cause nausea, vomiting, and abdominal pain; death reported at 5 g |
Nordberg et al. 1973 |
|
Hematologic |
Inhalation |
Anemia reported at very high exposures |
Friberg 1950; Kagamimori et al. 1986 |
|
Neurologic |
Inhalation |
Neurobehavioral changes reported at exposures less than those causing renal effects |
Marlowe et al. 1985; Struempler et al. 1985; Hart et al. 1989 |
|
Renal |
Inhalation |
Renal tubular damage at renal cortex concentrations of 50200 µg/g wet weight; proteinuria reported at 0.023 mg/m3 |
Roels et al. 1983; Jarup et al. 1988; Bernard and Lauwerys 1989; Buchet et al. 1990 |
|
Respiratory |
Inhalation |
Acute toxicity: lung edema, tracheobronchitis, pneumonitis, and fever reported at concentrations > 0.5 mg/m3; death from acute respiratory failure at higher concentrations |
Friberg 1950; Townshend 1982; Grose et al. 1987; Greenspan et al. 1988 |
|
Chronic toxicity: impaired lung function and emphysema |
|||
|
a For many of the health effects of concern, exposures are uncertain or unknown and are related to occupational studies or case reports of accidental spills or releases. |
|||
TABLE 5-3 Lead Compounds: Health Effects in Humans
|
Type of Toxicity |
Route of Exposure |
Type of Effectsa (µg/dL blood-lead concentrations) |
Reference |
|
Cardiovascular |
Inhalation |
Cardiac lesions and ECG changes at high concentrations; increased blood pressure reported at 7 µg/dL |
Pirkle et al. 1985; Schwartz 1988; Coate and Fowles 1989; ATSDR 1997b |
|
Cancer |
Inhalation |
Inconclusive in humans |
EPA 1988a; ATSDR 1997b |
|
Developmental |
Inhalation |
No major congenital anomaly; conflicting data on reduced birthweight and gestational age at 15 µg/dL |
Bellinger et al. 1984; Needleman et al. 1984; McMichael et al. 1986; Bornschein et al. 1989; Greene and Ernhart 1991; ATSDR 1997b |
|
Gastrointestinal |
Inhalation |
Colic, abdominal pain, constipation, cramps, nausea, vomiting, anorexia, and weight loss reported at acute high concentrations |
Matte et al. 1989; ATSDR 1997b |
|
Hematologic |
Inhalation |
Inhibition of ALA-D activity reported at 12 µg/dL; increased erythrocyte protoporphyrins reported at 15 µg/dL; reduced hemoglobin synthesis reported at 40 µg/dL for children; frank anemia reported at 80 µg/dL |
Pueschel et al. 1972; Chisolm et al. 1985; Roels and Lauwerys 1987; ATSDR 1997b |
|
Hepatic |
Inhalation |
Abnormal liver-function tests and inhibition of cytochrome P-450 reported at high concentrations |
Saenger et al. 1984; ATSDR 1997b |
epidemiologic studies and found to be useful, in conjunction with blood lead, for assessing body lead burden.
Neurotoxicity is a major health concern with respect to lead exposure. Available data suggest that children are more sensitive than adults and respond to lead at lower doses (Rom 1992). Severe lead encephalopathy, occasionally fatal, occurs in adults with blood lead above 100 µ g/dL (Kehoe 1961) and in children with blood lead as low as 80 µg/dL (NRC 1993). Adults have less-severe but overt neurologic and neurobehavioral effects at blood lead as low as about 40-60 µg/dL (Baker et al. 1979, 1983; Hanninen et al. 1979), and decreased nerveconduction velocities have been reported at blood lead of 30 µg/dL (Seppäläinen et al. 1983).
Studies of neurodevelopmental effects in children have produced less-conclusive findings with respect to identifying a threshold at which effects appear. On the one hand, statistically significant relationships have been found between intelligence quotient (IQ) and blood lead in children whose individual blood-lead ranged from 6 to 46 µg/dL (Schroeder and Hawk 1987) and in groups of children whose average exposures ranged from 5.6 to 22.1 µg/dL (Fulton et al. 1987). Increasing blood lead was associated with decreasing IQ in each of those studies, and no threshold for this effect was observed. Further data on children 's IQ at higher lead concentrations suggest a deficit of about 5 points in average IQ in groups of children with mean blood lead of 50-70 µg/dL compared with a control group with mean blood lead of 21 µg/dL (Rummo 1974; Rummo et al. 1979), and a deficit of about 4 IQ points in groups of children with estimated blood lead of 30-50 µg/dL compared with a control group with mean blood lead less than 15 µg/dL (Needleman 1979). Other investigations, however, have failed to find an association between low blood lead and neurobehavioral effects or IQ deficits (Lansdown et al. 1986; Harvey et al. 1988; Cooney et al. 1989a,b; Pocock et al. 1989).
Overall, the data suggest that lead causes neurobehavioral disturbances in children at concentrations below 50 µg/dL, and possibly below 20 µg/dL. No threshold for such effects can yet be demonstrated.
Other well-documented effects of lead exposure at blood-lead concentrations above 40 µg/dL in humans are renal impairment, hematologic effects, cardiovascular effects (including high blood pressure), gastrointestinal and liver abnormalities, and reproductive and developmental effects (ATSDR 1997b). With respect to the latter, no human evidence suggests that low prenatal exposure to lead is associated with any major structural congenital anomaly (McMichael et al. 1986). Studies of prenatal exposure at low concentrations, however, have produced conflicting data with respect to low birth weight and gestational age (Bellinger et al. 1984; Needleman et al. 1984; Bornschein et al. 1989; Greene and Ernhart 1991). Some evidence suggests that lead reduces gestational age, even when maternal blood lead is below 15 µg/dL. Similarly, miscarriages and stillbirths have been reported in exposed women whose blood lead was 10 µg/dL
or higher (Baghurst et al. 1987; Hu 1991) and adverse effects on the testes of offspring of women whose blood lead was 40-50 µg/dL have been reported (Assennato et al. 1987; Rodamilans et al. 1988).
The evidence for lead as a human carcinogen is inconclusive, but lead exposures have caused renal tumors consistently in experimental animals under suitable experimental conditions (ATSDR 1997b).
Although lead toxicity has been known since antiquity, there remains considerable debate about safe exposures and about the body burden of lead below which no adverse effects might be anticipated. Several toxic effects appear to have different thresholds of exposure, and some have no clearly defined safe exposure (that is, no identifiable threshold exposure). In that regard, EPA (1986b) and ATSDR (1997b) have expressed concern about the emerging evidence of a constellation of effects that occur at low blood-lead concentrations (10-15 µg/dL, or even lower), including subtle neurologic and neurobehavioral changes, growth and blood-pressure effects, inhibition of aminolevulinic acid dehydrase and pyrimidine-5'-nucleotidase activity, reduction in serum 1,25-dihydroxyvitamin D, and increase in erythrocyte protoporphyrins. The health effects of lead toxicity in humans are summarized in Table 5-3.
Mercury
Adverse human health effects of exposure to mercury are dependent on the particular chemical species of mercury, the magnitude and route of exposure, and the degree to which the mercury is metabolized.
Mercury exists in inorganic and organic forms. Inorganic mercury occurs in 3 valence states: metallic (elemental) mercury (Hg), mercurous salts (Hg+), and mercuric salts (Hg2+). The most commonly encountered organic forms are alkyl mercury species (notably methylmercury and ethylmercury) that result largely from microbial metabolism of inorganic mercury in the environment.
Metallic mercury is poorly absorbed from the gastrointestinal tract, but inhaled metallic mercury vapor is well absorbed from the lungs (ATSDR 1994). Metallic mercury can oxidize to the mercuric state. Soluble mercuric compounds (Hg2+) are well absorbed from the intestine and are the most commonly encountered inorganic salts of mercury. Mercurous salts (Hg+) are absorbed from the intestine, but are unstable in the presence of sulfhydryl groups and convert to either metallic mercury or the mercuric state. Therefore, mercurous compounds can share the toxic characteristics of both metallic and mercuric mercury. Organomercury compounds are absorbed well from the intestine and are less readily oxidized to the mercuric state than are metallic or mercurous compounds.
In general, dermal absorption is the least-likely route of uptake of mercury, although it appears that dermal absorption can be substantial under some circumstances (ATSDR 1994). Metallic mercury is absorbed through the skin, but at
much lower rates than by inhalation. Inorganic salts might be absorbed to a greater degree, but quantitative data are lacking.
In humans, metallic mercury and organomercury compounds cross the blood-brain barrier and the placenta, and the major health effects of concern for these compounds are nervous system impairment and fetal toxicity. Inorganic salts of mercury do not cross the placenta or blood-brain barrier readily, so they are typically less toxic to the fetus and produce fewer central nervous system effects. The kidney appears to be the most-sensitive organ after ingestion of inorganic salts. The renal tract is the principal route of excretion of all forms and species of mercury. All mercury compounds have some degree of renal toxicity.
Other organs affected by mercury at higher exposures include the respiratory, cardiovascular, hematologic, gastrointestinal, and reproductive systems. Such toxic effects at high exposures might reflect the high affinity of mercuric mercury for sulfhydryl groups. Results of animal studies support a concern for the neurologic, renal, developmental and reproductive, and respiratory effects of mercury exposure in humans.
Most data concerning human health effects are related to occupational exposures, accidental spills and releases, or the major environmental contamination and consumption of fish contaminated with methylmercury in Minamata, Japan. Most reports of mercury exposures that caused serious human health effects predate 1970 and occurred in workplaces and other settings where exposures were generally high; the health effects observed under such conditions might not be directly pertinent to chronic, low-level exposures generated by waste incineration.
Several recent issues have rekindled interest in this metal. Mercury in dental amalgams has raised concerns about the slow absorption of mercury from dental fillings. Mercury arising from environmental pollution, notably from industrial pollution, waste incineration, other combustors, and natural sources has caused problems with surface water contamination in lakes and streams, and has raised concerns about human-health effects of eating fish with high tissue mercury concentrations (Amdur et al. 1991). The latter subject is particularly relevant to the present discussion. The health effects of mercury compounds in humans are summarized in Table 5-4.
Chromium
Chromium is most commonly encountered in 4 valence states: 0 (metal), II (chromous), III (chromic), and VI. Cr(VI) in the environment is almost always related to human activity (ATSDR 1993b).
Cr(III) is an essential nutrient, forming an organic complex that facilitates the interaction of insulin with cell-membrane receptors. The recommended dietary intake of Cr(III) is 50-200 µg/day (ATSDR 1993b; NRC 1989b).
In general, Cr(VI) compounds are more toxic than Cr(III) compounds, and are better absorbed after inhalation, ingestion, and dermal contact (ATSDR
TABLE 5-4 Mercury Compounds: Health Effects in Humans
|
Type of Toxicity |
Form of Mercury |
Route of Exposure |
Type of Effectsa |
Reference |
|
Cardiovascular |
Metallic |
Inhalation |
Increased blood pressure and heart rate at acute high concentrations (>0.27 mg/m3); hypertension, death from ischemic heart disease, and cerebrovascular disorders reported at chronic exposure to high concentrations |
Vroom and Greer 1972; Piikivi 1989; Aronow et al. 1990; Barregård et al. 1990; Bluhm et al. 1992; Soni et al. 1992; Taueg et al. 1992; ATSDR 1994 |
|
Mercurous and mercuric salts |
Inhalation |
Increased blood pressure and acrodynia reported in children |
Warkany and Hubbard 1953; ATSDR 1994 |
|
|
Cytogenic |
Metallic |
Inhalation |
No chromosomal damage from occupational exposures (clastogenic for mammalian germ cells) |
ATSDR 1994 |
|
Death |
Metallic and organic |
Inhalation |
Death reported at exposures of 1.0 mg/m3 |
Hill 1943; ATSDR 1994 |
|
Inorganic and organic |
Ingestion |
Death reported at exposures of 10-60 mg/kg |
Troen et al. 1951; Gleason et al. 1957; Bakir et al. 1973; Tsubaki and Takahashi 1986; ATSDR 1994 |
|
|
Inorganic |
Dermal |
Death reported at exposures of 93 mg/kg |
ATSDR 1994 |
|
|
Developmental |
Organic |
Inhalation and ingestion |
Developmental toxicity at high concentrations |
Engleson and Hermer 1952; Amin-Zaki et al. 1976; Marsh et al. 1980, 1981, 1987; ATSDR 1994; |
|
Gastrointestinal |
All forms |
Inhalation and Ingestion |
Acute gastrointestinal toxicity (nausea, diarrhea, abdominal pains, inflammation of oral mucosa, inflammation, and acute ulceration) reported at 20-30 mg/kg; similar effects seen after inhalation |
Campbell 1948; Brown 1954; Tennant et al. 1961; Sexton et al. 1978; Fagala and Wigg 1992; ATSDR 1994 |
1993b). However, after ingestion, Cr(VI) is reduced to Cr(III) in the stomach, so that the ingestion route is of lesser importance.
Occupational exposures of humans to chromium—mainly Cr(VI)—compounds have caused ulceration and perforation of the nasal septum; respiratory tract irritation; sensitization of the respiratory tract, skin, and mucous membranes; and increased risk of lung cancer. Renal damage, gastrointestinal changes, and hematologic effects have also been described. Skin problems caused by direct contact with chromium compounds include ulceration and allergic sensitization. Among chromate workers, those problems were severe in the past when skin contact was high (Lucas and Kramkowski 1975).
The health effects of chromium compounds in humans are summarized in Table 5-5.
Arsenic
Arsenic is a powerful human toxicant. Exposures to inorganic arsenic compounds—chiefly oxides and oxyacids (arsenates and arsenites)—are the mostcommon sources of exposure, although organic arsenicals (mainly methyl or phenyl arsenates) have been used widely in agriculture. Organic arsenicals are considered less toxic than the inorganic forms (ATSDR 1998b).
Inhalation exposures of humans to inorganic arsenic compounds have led to acute and chronic respiratory irritation, and to lung cancer (EPA 1988b; ATSDR 1998b). A wide variety of adverse health effects, including skin and internal cancers and cardiovascular and neurological effects, have been attributed to chronic arsenic exposure, primarily from drinking water (NRC 1999b). Direct skin contact has led to local irritant effects and hyperkeratoses.
Little information is available on the effects of organic arsenicals on humans. Results of animal studies suggest that organic compounds can have effects similar to those of inorganic forms (ATSDR 1998b). However, no studies have demonstrated that organic arsenic is carcinogenic in humans (ATSDR 1998b). Reduction in the carcinogenicity of arsenic, particularly at low exposure concentrations, has been linked to its methylation in vivo (Marcus and Rispin 1988). Because most forms of organic arsenic are already methylated, there is good reason to expect organic arsenic would be far less carcinogenic in humans than inorganic forms. ATSDR (1998b) has reviewed animal studies of organic arsenic and concluded that it may have weak carcinogenic potential.
In general, the toxicity of arsenic in all its forms is less in experimental animals than in humans, so animal data on arsenic are considered less reliable predictors of human effects than are animal data for many other substances (ATSDR 1998b). The major health effects of arsenic compounds in humans are summarized in Table 5-6.
TABLE 5-5 Chromium Compounds: Health Effects in Humans
|
Type of Toxicity |
Form of Chromium |
Route of Exposure |
Type of Effects a |
Reference |
|
Cancer |
Primarily Cr(VI) |
Inhalation |
Increased risk of lung cancer |
Mancuso and Hueper 1951; Bidstrup and Case 1956; Hayes et al. 1979; Hill and Ferguson 1979; Braver et al. 1985; Davies et al. 1991; Pastides et al. 1991; ATSDR 1993b |
|
Dermal |
Chromium compounds |
Dermal |
Ulceration, allergic sensitization reported at high concentrations |
Lucas and Kramkowski 1975; ATSDR 1993b |
|
Gastrointestinal |
Chromium salts |
Inhalation |
Stomach cramps, gastric and duodenal ulcers, and gastritis reported at high concentrations |
Mancuso 1951; Sassi 1956; ATSDR 1993b |
|
Hematologic |
Cr(III) and Cr(VI) |
Inhalation |
Leukocytosis or leukopenia, decreased hemoglobin, and prolonged bleeding times reported at high exposures |
Mancuso 1951; ATSDR 1993b |
|
Renal |
Cr(VI) |
Inhalation |
Proteinuria and renal tubular necrosis reported at high exposures |
Franchini and Mutti 1988; ATSDR 1993b |
|
Respiratory |
Primarily Cr(VI) |
Inhalation |
Ulceration and perforation of the nasal septum, respiratory tract irritation, and decreased lung function reported at 0.002 mg/m3; sensitization of respiratory tract and mucous membranes |
Mancuso 1951; Taylor 1966; Bovet et al. 1977; Keskinen et al. 1980; Lindberg and Hedenstierna 1983; Novey et al. 1983; ATSDR 1993b |
|
a For many of the health effects of concern, exposures are uncertain or unknown and are related to occupational studies or case reports of accidental spills or releases. |
||||
TABLE 5-6 Arsenic Compounds: Health Effects in Humans
|
Type of Toxicity |
Route of Exposure |
Type of Effectsa |
Reference |
|
Cancer |
Inhalation/inorganic compounds |
Lung cancer |
Axelson et al. 1978; Enterline and Marsh 1982; Lee-Feldstein 1986; Sobel et al. 1988; ATSDR 1998b; NRC 1999b |
|
Ingestion/inorganic compounds |
Skin cancer; evidence of increased risk of liver, bladder, and kidney cancers |
Zaldivar 1974; Chen et al. 1985, 1986; Wu et al. 1989; ATSDR 1998b; NRC 1999b |
|
|
Cardiovascular |
Ingestion/inorganic compounds |
Cardiac arrhythmia and ventricular tachycardia or fibrillation reported at high doses; vascular lesions leading to blackfoot disease reported at chronic low exposures |
ATSDR 1998b; NRC 1999b |
|
Ingestion/inorganic compounds |
Raynaud's disease |
ATSDR 1998b; NRC 1999b |
|
|
Dermal |
Ingestion/inorganic compounds |
Various skin disorders |
ATSDR 1998b; NRC 1999b |
|
Dermal |
Local irritation, hyperkeratoses |
ATSDR 1998b; NRC 1999b |
|
|
Gastrointestinal |
Ingestion/inhalation |
Nausea, vomiting, diarrhea, and abdominal pain reported at acute high doses or repeated lower doses |
ATSDR 1998b; NRC 1999b |
Beryllium
Appreciable human exposures to metallic beryllium or its salts occur almost exclusively in workplace settings. The burning of coal and fuel oil contributes a small inhaled burden to the general public, particularly in urban areas where the median air concentration is about 0.2 ng/m3 (ATSDR 1993c).
Inhalation is the principal route of exposure (ATSDR 1993c). Granulomatous lung disease is the most common health effect in humans, although beryllium disease is a multisystem disorder (ATSDR 1993c). Dermal contact can cause sensitization and systemic illness, but beryllium compounds are absorbed poorly through the skin (ATSDR 1993c). Absorption of beryllium from the gastrointestinal tract is also poor, and this route of exposure has rarely caused appreciable toxicity.
Epidemiologic data have suggested an increased risk of lung cancer associated with occupational exposures to beryllium. Results of a recent study that accounted for smoking habits and used an appropriate unexposed comparison group showed an increased risk of lung cancer among exposed people (Steenland and Ward 1991). Animal studies in rats and monkeys have also shown that beryllium can cause lung tumors (ATSDR 1993c).
The current workplace exposure limit for beryllium of 0.002 mg/m3 was established in 1950 to prevent nonmalignant beryllium disease and has been successful in reducing the rate of chronic lung disease (ATSDR 1993c). That limit might not be sufficient to protect against lung cancer. In this regard, EPA (1992b) has estimated the upper bounds for inhalation unit risk of 2.4 × 10-3 m3/µg, and for ingestion a potency of 4.3 kg-d/mg.
The health effects of beryllium toxicity in humans are summarized in Table 5-7.
Organic Compounds
Dioxins and Furans
Dioxins and furans have been the subject of much controversy and study (e.g., NRC 1994). Their toxic effects are summarized below.
Acute Toxicity
Case studies of acute reactions caused by exposure to TCDD have been documented. Workers exposed to TCDD in a plant explosion were examined; they had a number of acute symptoms “characterized by skin, eye and respiratory tract irritation, headache, dizziness, and nausea” (Suskind and Hertzberg 1984). The acute symptoms subsided within a week but were followed by “acneiform eruption, severe muscle pain affecting the extremities, thorax and shoulders, fatigue, nervousness and irritability, dyspnea, complaint of decreased libido and intolerance to cold” (Suskind and Hertzberg 1984).
TABLE 5-7 Beryllium Compounds: Health Effects in Humans
|
Type of Toxicity |
Form of Beryllium |
Route of Exposure |
Type of Effectsa |
Reference |
|
Cancer |
Beryllium or its salts |
Inhalation |
Lung cancer |
Steenland and Ward 1991; ATSDR 1993c |
|
Dermal/ocular |
Beryllium salts |
Dermal, ocular |
Ocular granulomas and sensitization resulting in papulovesicular dermatitis and ulceration reported at high concentrations |
ATSDR 1993c |
|
Respiratory |
Beryllium or its salts |
Inhalation |
Acute tracheobronchoalveolitis reported at high concentrations; chronic beryllium disease—multisystem granulomatous disease—principally involving lungs reported at lower concentrations |
Van Ordstrand et al. 1945; Hardy and Tabershaw 1946; Kriebel et al. 1988a,b; Rossman et al. 1988; ATSDR 1993c |
|
a For many of the health effects of concern, exposures are uncertain or unknown and are related to occupational studies or case reports of accidental spills or releases. |
||||
Dioxin is acutely toxic to experimental animals at sufficiently high doses. The lethal dose of TCDD varies extensively among species and with sex, age, and route of administration (NRC 1994). A symptom known as severe wasting syndrome has been reported in several laboratory animals. Weight loss typically manifests itself within a few days after exposure and is associated with a loss of adipose and muscle tissue (Max and Silbergeld 1987). Typically, at the lethal dose, there is a delayed toxicity, and death usually occurs several weeks after exposure (EPA 1994d).
Chronic Toxicity
Results of epidemiologic studies suggest that chloracne (an acne-like eruption due to prolonged contact with certain chlorinated compounds (NRC 1994), increased gamma-glutamyltransferase (GGT) (a hepatic enzyme that is measured in human serum to evaluate liver toxicity), increased diabetes, and altered reproductive hormone concentrations appear to be long-term, noncarcinogenic consequences of exposure to TCDD (EPA 1994d; NRC 1994). Other effects reported include eyelid cysts, hypertrichosis and hyperpigmentation, actinic keratosis (abnormal distribution of the hair), Peyronie's disease (progressive scarring of penile membrane), cirrhosis, liver enlargement, alteration of liver enzyme concentrations, porphyria (alteration of porphyrin metabolism), and renal, neurologic, and pulmonary disorders (EPA 1994d). But results of other studies suggest possible acute effects and few chronic effects other than chloracne.
A chronic-toxicity study performed by Kociba et al. (1978, 1979) on laboratory rats over 2 years showed urinary disorders in females. Alterations of the liver were found in both males and females. Other specific effects of TCDD toxicity in animals include wasting syndrome, hepatotoxicity, enzyme induction (in particular, the induction of cytochrome P-450 1A1, which is responsible for the activation and detoxification of endogenous and exogenous chemicals), endocrine alterations, decreased vitamin A storage, and decreased lipid peroxidation (NRC 1994). The most-consistent syndrome of TCDD toxicity among all animals is wasting syndrome (NRC 1994).
In humans, several studies documenting blood or adipose-tissue measurements, workplace exposure, and the occurrence of chloracne reported increased cancer rates after a relatively long latency in workers exposed to TCDD at relatively high concentrations (Zober et al. 1990; Fingerhut et al. 1991; Manz et al. 1991). Specifically, an excess of respiratory cancer was reported, as was a suggested increased risk of connective, soft tissue, and lung cancers. However, substantial uncertainties with regard to the database of epidemiologic evidence could influence risk estimates (for example, a large variety of tumor types, uncertainties as to exposure, possible confounding with such known human carcinogens as asbestos, and possible confounding with cigarette-smoking).
Several long-term studies have been performed to determine the carcinogenesis of TCDD in experimental animals. Long-term carcinogenicity bioassays of TCDD have been conducted in rats, mice, and hamsters (Van Miller et al. 1977; Kociba et al. 1978; Toth et al. 1979; NTP 1982a,b; Della Porta et al. 1987; Rao et al. 1988). Exposure has been oral, intraperitoneal, dermal, and subcutaneous. Results of the studies have been summarized in NRC 1994, Table 4-2. Increased tumor rates reportedly occurred at several sites in the body in different studies, although the liver was consistently a site of tumor formation in different studies and different species. In studies in which liver cancer occurred, other toxic changes in the liver also occurred. Other organs in which increased cancer rates were observed in animals exposed to TCDD include the thyroid, adrenals, skin, and lungs. Further animal studies indicate that PCDD and PCDF carcinogenesis may proceed through a receptor-mediated mechanism, although details are unclear (Stone 1995), and that PCDDs and PCDFs are tumor promoters in animal liver and skin assays.
Developmental Toxicity
Alterations in development due to dioxin exposure have also been reported in experimental animals (EPA 1994d), including such structural malformations as cleft palate and hydronephrosis in mice, while other species have shown postnatal functional alterations, some irreversible, including effects on the reproductive system and object-learning behavior (EPA 1994d). The resemblance between some effects observed in adult monkeys and neonatal mice exposed to TCDD and those documented in Yusho or Yu-Cheng infants (for example, sub-cutaneous edema of the face and eyelids, larger and wider fontanel, and abnormal lung sounds) suggests that particular effects reported in these infants were caused by TCDD-like PCB and chlorinated dibenzofuran (CDF) congeners in the rice oil ingested by the mothers (Harada 1976; Urabe et al. 1979; Hsu et al. 1994).
Reproductive Toxicity
Although there have been no studies concerning the reproductive effects of dioxin-related compounds in humans, the potential exists for dioxin and related compounds to cause reproductive toxicity (Kimmel 1988). A variety of animal studies have shown that TCDD and its structurally related compounds affect female reproduction (Kociba et al. 1976; Barisotti et al. 1979; Murray et al. 1979). The foremost effects seem to be decreased fertility, inability to carry to term, and, in rats, decreased litter size. There are also effects on gonads and the estrous cycle. In males, TCDD and related compounds decrease testis and accessory sex organ weights, cause abnormal testicular structure, decrease spermatogenesis, and reduce fertility.
Neurologic Effects
In 1976, an industrial accident at a chemical manufacturing plant near Seveso, Italy, released kilogram amounts of TCDD into the environment. Neurologic effects were reported to have occurred shortly after exposure to TCDD in some workers and residents of contaminated areas (ATSDR 1998a). Symptoms included headache, insomnia, nervousness, irritability, depression, anxiety, loss of libido, and encephalopathy.
Immunotoxicity
Studies in mice, rats, guinea pigs, and monkeys have indicated that TCDD suppresses the function of some components of the immune system in a doserelated manner; that is, as the dose of TCDD increases, suppression of immune function increases. TCDD suppressed the function of cells of the immune system, such as lymphocytes (affecting cell-mediated immune response) and the generation of antibodies by B cells (affecting humoral immune response). Increased susceptibility to infectious disease has been reported after TCDD administration. In addition, TCDD increased the number of tumors that formed when tumor cells were injected into mice.
The effects of TCDD on the immune system appear to vary among species, although most studies used different treatments and are not completely comparable. However, some species seem more sensitive than others to the effects of TCDD on the immune system. It is not known whether humans would be more or less sensitive than laboratory animals.
Other Products of Incomplete Combustion (PICs)
The remainder of this section will focus on two other products of incomplete combustion—PCBs and PAHs.
PCBs
Most of the data on the adverse health effects of PCBs in humans are derived from occupational studies. Dermal and ocular effects of exposure have been relatively well established in these studies (ATSDR 1998c). There are also reports of respiratory, gastrointestinal, hematologic, hepatic, musculoskeletal, developmental, and neurologic effects, but the evidence is not strong enough to establish cause-effect relationships, in part because PCB concentrations were not measured and because other compounds were present in the work environment. Occupational studies have been inconclusive regarding the association of PCB exposure and cancer risk (ATSDR 1998c).
In studies of women assumed to have consumed PCB-contaminated fish, their offspring were found to have neurobehavioral deficits at birth, some of which persisted through the follow-up period of several years from birth. However, the findings are inconclusive because of various limitations of the studies regarding exposure assessment and the comparability of exposed and nonexposed subjects. Lower birthweight and shortened gestational age were reported in infants born to mothers occupationally exposed to PCBs, but these effects did not follow an exposure gradient (ATSDR 1998c). Estimates of PCB body burdens in populations exposed at concentrations commonly found in the United States indicate that neurobehavioral effects can occur after prenatal maternal exposures (ATSDR 1998c). Evaluations of blood samples from women who miscarried or delivered prematurely showed associations between these effects and concentrations of PCBs. Because of confounding factors, including exposure to DDT and other organochlorine pesticides, the adverse developmental effects reported in these studies cannot be attributed specifically to PCB exposure.
Effects of PCBs observed in experimental animals are generally consistent with the human data. Most of the toxicity studies of PCBs have involved oral exposures, and numerous effects have been documented, including hepatic, gastrointestinal, hematologic, dermal, immunologic, neurologic, and developmental and reproductive effects (ATSDR 1998c). Other effects of oral PCB exposure include weight loss, thyroid toxicity, and liver cancer (ATSDR 1998c). Adverse effects on liver and body weight were observed in the only animal-inhalation study of PCBs.
PAHs
PAHs occur ubiquitously in the environment from both anthropic and natural sources. PAHs occur in the atmosphere most commonly as products of incomplete combustion. They are found in the exhausts from fossil fuels; combustion; industrial processes (such as coke production and refinement of crude oil); gasoline and diesel engines, oil-fired heating, and in cigarette smoke. PAHs are present in groundwater, surface water, drinking water, waste water, and sludge. They are found in foods, particularly charbroiled, broiled, or pickled food items, and at low concentrations in refined fats and oils.
Occupational studies of workers who were exposed to mixtures that contain PAHs (for example, from exposure to coke-oven emissions and roofing tars) for long periods show an excess of cancer, particularly of the lung and skin (ATSDR 1995). Several of the PAHs, including benzo[a]pyrene, the most-studied PAH, have caused tumors in laboratory animals by the inhalation, ingestion, or dermal routes (ATSDR 1995). However, many animal studies involving PAHs have been negative with respect to carcinogenicity. Noncancer adverse health effects with PAH exposure have been observed in animals but generally not in humans with the exception of adverse hematological and dermal effects. In various
animal studies, most involving oral exposures of test animals, various PAHs increased mortality, primarily because of adverse hematopoietic effects, including aplastic anemia and pancytopenia (ATSDR 1995). Benzo[a]pyrene induced reproductive toxicity in rodents, but the incidence and severity of the effects depended on the strain of animal and the method of administration (ATSDR 1990). Prenatal exposures of rats and mice to benzo[a]pyrene produced a decrease in mean pup weight during postnatal development and caused a high incidence of sterility in the F1 progeny of mice (ATSDR 1990). PAHs are a broad and complex category of compounds, with many generally co-occurring and as such, are difficult to characterize merely by evaluating individual components. Nevertheless, the occupational health effects of various mixtures of PAHs have been evaluated in some groups of workers, e.g. coke oven workers and roofers, and occupational criteria and standards for protection of workers have been developed.
POPULATIONS AT RISK
In this section, we discuss sensitive populations and worker populations, which may be at especially increased risk because of exposure to incinerator emissions.
Sensitive Populations
Although not a well-defined term, sensitive subpopulation refers to some subset of the population that might suffer much more serious adverse health effects as the result of exposure to a toxic agent than the average population. Identifying high-risk persons is a critical part of the definition of sensitive sub-populations. Variation in sensitivity is due to many factors, some more easily recognized than others. For a specific population, these factors may include variations in underlying health conditions, diet, stages of development, and age, as well as genetic differences (e.g., in metabolic rates). Classic examples include the 1952 London smog and the 1948 Donora, PA, episodes, in which the increased mortality associated with pollution most severely affected the very young and the elderly (UK Ministry of Health 1954). Fetuses exposed during organ development can be extremely sensitive to relatively low exposures to chemicals that cause little or no harm in adults; for example, thalidomide interferes with the fetal development of limbs at doses that are harmless in adults. Lead exposure in utero is linked to adverse central nervous system (CNS) development at blood levels lower than required to produce neurological effects in adults (Kimmel and Buelke-Sam 1994). Because the blood-brain barrier is less well developed in infants than in adults, it should be expected that chemicals, in general, can more readily affect the central nervous system of infants (Kimmel and Buelke-Sam 1994). It is hypothesized that DNA-repair mechanisms are not
well developed in fetuses or babies—human fetuses have only 20-50% of the DNA repair enzyme activity of adults; if this hypothesis is true, this population may be particularly sensitive to carcinogens (Kimmel and Buelke-Sam 1994). Much greater absorption of lead through the gastrointestinal tract in children (30%) than adults (6%) has been demonstrated (Ross et al. 1992).
The normal decline of many physiologic functions (such as immunologic responses) with aging might make the elderly more susceptible to various pollutants. Increased mortality due to both pulmonary and cardiovascular disease has been documented when pollution (for example, with particles and CO) has been only slightly increased, even when the levels of pollution remained within EPA guidelines (see earlier discussion in this chapter). However, there are substantial uncertainties about whether the correlations between measured pollution indicators (PM, CO, etc.) and mortality reflect cause and effect.
Some behavior patterns of children result in their receiving greater doses of pollutants than adults who experience the same environment. Running and playing outdoors lead to higher breathing rates and hence potentially greater intake of airborne pollutants; this might also affect adults who are working hard or who exercise regularly. Young children engage in a high degree of hand-to-mouth behavior; videotapes have documented about 40 hand-to-mouth actions per hour among young children (Ross et al. 1992). Thus, contaminated dirt and dust might enter children's systems to a greater degree than adults.
Sensitive populations can include those whose health is already compromised. For example, asthmatics respond to SO2 at lower concentrations than nonasthmatics (see section on Acidic Aerosols and Gases). African-Americans are more likely than whites to have hypertension and kidney disease and therefore could be more susceptible to pollutants, such as lead, which adversely affect the circulatory and renal systems (see section on Lead). Similarly, some people may be much more sensitive to the effects of some chemical exposures because of pre-existing conditions brought on by exposures to other agents (possibly including other chemicals). For example, people who have experienced a hepatitis B virus infection appear to be at greatly increased risk of cancer due to aflatoxin B exposure, compared with those who have not had hepatitis B infection.
Variability in diet can be fairly extreme (e.g., vegan diets, which excludes all animal products, versus average American fare), resulting in substantial differences in the intakes of some pollutants (NRC 1993). Vegans, for example, should have substantially lower exposure to PCDDs and PCDFs, since the majority of the intake of these materials in the average American diet comes from their presence in animal fats. In addition, dietary deficiencies may also play a role in increasing the variability of uptake of certain pollutants. For example, iron deficiency can result in higher uptake of lead in the diet, while calcium deficiency might affect lead excretion (as observed in animal models) (ATSDR 1997b).
It has been observed that at high enough exposures, some chemicals or exposure situations alter the toxic effects of other chemicals or exposure situa-
tions. For example, the effects of high exposure to asbestos are compounded by cigarette smoking, so that the relative risk for lung cancer from the combined exposures is substantially higher than the sum of the relative risks due each separately (ATSDR 1993a). Similar interactions (some of them showing protective effects) have been seen in humans for other combinations of high exposures in occupational settings (with smoking generally being one of the exposures), and in medical situations (drug interactions), and at high exposures in animal models. Nevertheless, people are exposed to mixtures of chemicals for which interactions have not been studied (NRC 1988a, 1996).
Xenobiotic materials can be metabolized by various tissues, particularly the liver, and the rate of metabolism can be altered by exposure to various exogenous chemicals or drugs such as alcohol and tobacco (Petruzzelli et al. 1988). The metabolites produced may be more or less toxic than the parent chemical, and may be more or less easy for the body to further metabolize or excrete, so that the effective toxicity of any material may depend on such factors as the rate of metabolism and excretion. These factors can be highly variable within the human population. The genetic variability of AAH mediated metabolic rates in the liver, for example, is in a range of a factor of several thousand, possibly leading to variability in sensitivity to toxic effects from PAH exposure. Such variability is one possible explanation for the 7.3-fold odds-ratio for a particular genetic difference in PAH metabolism observed for squamous-cell lung cancer cases among Japanese light smokers (Nakachi et al. 1991). Similarly, another genetic factor, extensive-hydroxylator phenotype, has been associated with an increased risk of lung, liver, and bladder cancer in Americans, and in British workers exposed to asbestos or PAHs at high concentrations (Caporaso et al. 1989).
Overall, laboratory animals of different strains have exhibited a difference of a factor of 40 in tumorigenesis in response to carcinogens. NRC (1994) has estimated that the range of susceptibilities of humans to carcinogens is quite large: 1% of the population might be 100 times more susceptible than the average person, and 1% might be only one-hundredth as susceptible.
Worker Populations
Incinerator operators and maintenance workers, and those involved in the collection, transport, and disposal of fly ash and emission control equipment residues, have the potential to be most exposed to toxic substances associated with incineration.
As is true in many other industries, maintenance and cleaning often present the greatest opportunities for exposure to hazardous materials. The residual wastes after incineration can contain high concentrations of metals and dioxins, and firebrick can add crystalline silica to these hazards (Steenland and Stayner 1997). Air-pollution control equipment collects and concentrates certain toxic
chemicals, so workers who maintain and clean these devices may be particularly at risk. Two recent studies of four municipal incinerators have documented very high exposures of workers to hazardous waste during the routine cleaning of the incinerator chambers and the electrostatic precipitators (NIOSH 1995; Richey 1995). According to those studies, the incinerators were periodically shut down (monthly to quarterly) to remove accumulated slag from the walls of the burn chamber and to clean fly ash out of the burn chambers and the electrostatic precipitators. To move the waste material to a point where it can be vacuumed out, the slag and fly ash were swept and shoveled, two operations that generated high airborne concentrations of particles containing heavy metals and dioxins at relatively high concentrations.
Dioxin concentrations were measured by NIOSH (1995) during incinerator cleanout operations, albeit with only single samples in various locations. PCDD and PCDF concentrations (measured as TEQs) ranged from 9 to 800 pg/m3, uniformly high compared with the NRC (1988b) guideline of 10 pg/m 3 (established after a transformer fire). All samples collected during these maintenance operations indicated that workers were exposed at or above the NRC exposure guideline.
Analysis of bulk samples of fly ash from the first incinerator indicated that the dioxin content increased as one moved from the burn and upper (expansion) chamber (TEQ, 3 parts per trillion, or 3 ppt) to the lower (cooling) chamber (TEQ, 7 ppt) to the electrostatic precipitator (TEQ, 900 ppt). The second incinerator had even more dioxin in the one composite sample from the upper and lower chamber—TEQ, 50 ppt. The NIOSH health hazard evaluation was undertaken after a report of increased PCDD and PCDF concentrations in a pooled blood sample from 56 municipal incinerator workers, indicating that municipal incinerator workers suffer higher exposures on the job than the general population (Schecter et al. 1991).
The NIOSH investigation also indicated that exposures to arsenic, cadmium, lead, and aluminum substantially exceeded occupational exposure limits during the clean-out operations.
Richey (1995) reported very high exposures during cleaning operations at two municipal incinerators over a 2-year period. Personal air samples were collected for 39 workers before a vacuum system was introduced to reduce exposures, and for 22 workers while the vacuum system was in use. During normal maintenance operations, without the vacuum, half the 24 samples from those cleaning the incinerator chamber were above the PEL for lead (50 µg/m3). In addition, the samples collected from two of seven workers drilling boiler tubes and seven of eight workers cleaning out the electrostatic precipitators were above the PEL for lead. The geometric mean exposures were 36 µg/m3 and 38 µg/m3 for the incinerator-chamber cleaning and boiler-tube drilling, respectively (dropping to 5.1 and 3.6 µg/m3 with the use of vacuum), and cleaning the electrostatic precipitator resulted in a geometric mean exposure of 1,300 µg/m3, dropping to
320 µg/m3 with vacuum use—still over 6 times the PEL. The same proportion of samples were above the PEL for cadmium. The geometric mean concentrations without and with vacuum use were 1.8 and 0.4 µg/m3 during cleaning of the incinerator chamber, 2.5 and 0.2 µg/m3 during boiler-tube drilling, and 64.1 and 18.9 µg/m3 during cleanout of the electrostatic precipitator. The fact that a separate study of 56 incinerator workers found them to have substantially higher blood lead concentrations than a comparison group of high-pressure plant tenders working at heating plants (Malkin et al. 1992) is consistent with the high lead exposures observed and suggests that incinerator workers in general are at risk of measurably increased lead absorption.
THE COMMITTEE'S CONSENSUS JUDGMENTS ABOUT WASTE INCINERATION AND PUBLIC HEALTH
After considering information on incineration operations and emission characteristics (Chapter 3), environmental behavior of pollutants of concern and contributions of incineration to environmental media (Chapter 4), and health-effects information summarized earlier in this chapter, the committee reached consensus judgments on various degrees of concern about incineration and public health on the basis of what is known, and in view of the lack of important information, as described in this report. The lack of such information has contributed to the substantial concern among many communities about possible adverse health effects resulting from incinerators. It is important to note that uncertainty also exists around current estimates of exposures and health effects with respect to other waste management practices.
In developing its consensus judgments about the various degrees of concern, the committee used an approach similar to a preliminary screening assessment intended to err on the side of caution in the face of substantial uncertainty. But in expressing its degrees of concern, the committee is not attempting to judge whether health effects are occurring. That would take a full-scale evaluation and would require much more information than was available to the committee. After additional information is obtained, it is possible that a degree of concern for a particular pollutant might change.
Table 5-8 shows the committee's qualitative consensus judgments of the relative degrees of concern for potential health consequences generally posed by waste incineration facilities. The following three populations are considered in this table: persons who work at the facilities, persons who live in close proximity to the facilities, and those individuals residing farther away who may be exposed to pollutants from multiple distant incinerator facilities. Each population is expected to experience quite different exposures because of different time-activity patterns, distances from the emission sources, or the chemical-specific nature of the pathways through which they may be exposed.
TABLE 5-8 Degrees of Concern for Potential Health Effects of Waste Incineration as Judged by the Committeea
|
Before MACT Complianceb |
After MACT Complianceb |
|||||
|
Substance |
Potential Effects on Workers at a Facilityc |
Potential Effects from a Single Facility on a Local Populationd |
Potential Effects from Multiple Facilities on a Broader Population e |
Potential Effects on Workers at a Facilityc |
Potential Effects from a Single Facility on a Local Populationd |
Potential Effects from Multiple Facilities on a Broader Population e |
|
Particulate matter |
Substantial |
Substantial |
Minimal |
Substantial |
Minimal |
Minimal |
|
Dioxinf |
Substantial |
Minimal |
Substantial |
Substantial |
Minimal |
Substantial |
|
Lead |
Substantial |
Substantial |
Moderate |
Substantial |
Minimal |
Moderate |
|
Mercury |
Substantial |
Moderate |
Moderate |
Substantial |
Minimal |
Moderate |
|
Other metalsg |
Substantial |
Moderate |
Moderate |
Substantial |
Minimal |
Moderate |
|
Acidic gasesh |
Moderate |
Minimal |
Negligible |
Moderate |
Negligible |
Negligible |
|
Acidic aerosolsi |
Moderate |
Moderate |
Moderate |
Moderate |
Minimal |
Minimal |
|
a The four degrees of concern (substantial, moderate, minimal, negligible) were chosen based on general estimates of the following aspects: incineration emissions; persistence of emitted substances in the environment; mobility through air, soil, water, and food; potential total exposure through routes of inhalation, ingestion, and dermal absorption; and relative toxicity of the individual substances inferred from studies not involving incineration. The degrees are intended to represent the committee's qualitative assessment and consensus judgment of the possibility of health effects to workers and segments of the general public from incineration emissions. The degrees of concern are not derived from direct evidence of adverse health effects observed from incineration use. Also, a particular degree of concern is not intended to imply the extent to which the committee believes health effects are actually occurring. In addition, the selection of a particular degree resulted only from a preliminary screening effort in the absence of sufficient information to characterize all the important parameters accurately. For example, in this preliminary assessment, the committee did not attempt to assign different degrees of concern according to types of waste incinerated, design and operation of the facility, emission controls, or extent of worker protection. It is possible that a degree of concern for a particular pollutant and category might change after more specific information is obtained. The term “substantial” is used to express the committee's highest degree of concern about possible exposures that could lead to health effects among workers, a local population, or a broader population. Lower degrees of concern correspond to less possibility that the specific groups are exposed to concentrations associated with adverse health effects. |
||||||
Selection of Pollutants
Table 5-8 reflects pollutants emitted by incinerators that currently appear to have the potential to cause the largest health effects due to their toxicity and the potential for exposures to occur. Also, pollutants are included that have the potential to be widely distributed in the environment, as well as those that do not have such a potential. Thus, some pollutants might be important locally and some might be more important when considered on a broader scale. Pollutants were also identified for which typical environmental concentrations are near levels at which health effects are expected. In areas where the ambient concentrations are already close to or above environmental guidelines or standards, even relatively small increments of substances can be important.
Workers at a Facility
The committee considered information presented earlier in this chapter on studies of incineration workers and other types of workers who had been exposed to high concentrations of pollutants listed in the table. Studies at municipal solid-waste incinerators show that workers are at much higher risk for adverse health effects than individual residents in the surrounding area. In the past, incinerator workers have been exposed to high concentrations of dioxins and toxic metals, particularly lead, cadmium, and mercury. Workers may be particularly at risk, not only because of emissions from the facility, but even more so if their work involves maintaining and cleaning the air-pollution control devices without proper safeguards. The electrostatic precipitators and bag houses, where potential emissions are captured, present risks to workers handling the concentrated pollutants.
A Single Facility and a Local Population
As discussed in Chapter 4, results of environmental monitoring studies around individual incineration facilities have indicated that the specific facilities studied were not likely to be major contributors to local ambient concentrations of the substances of concern, although there are exceptions. However, methodological limitations of those studies do not permit general conclusions to be drawn about the overall contributions of waste incineration to environmental concentrations of those contaminants. Particulate matter emitted by incinerators is especially important for local populations living in areas with high ambient concentrations of airborne particles.
Multiple Facilities and a Broader Population
The potential effects of metals and other pollutants that are very persistent in the environment may extend well beyond the area close to the incinerator. Persistent pollutants can be carried long distances from their emission sources, go through various chemical and physical transformations, and pass numerous times through soil, water, or food. Dioxins, furans, and mercury are examples of persistent pollutants for which incinerators have contributed a substantial portion of the total national emissions. Whereas one incinerator might contribute only a small fraction of the total environmental concentrations of these chemicals, the sum of the emissions of all the incineration facilities in a region can be considerable. The primary pathway of exposure to dioxins is consumption of contaminated food, which can expose a very broad population. In such a case, the incremental burden from all incinerators deserves serious consideration beyond a local level.
Before MACT Compliance
The committee is aware that incinerator emissions are expected to decrease as a consequence of improved design and operations, modifications of the waste stream, improved emission control devices, and changing waste management practices. In reviewing incineration practices and emissions data, the committee found that the data typically have been collected from incineration facilities during only a small fraction of the total number of incinerator operating hours. Generally, data are not collected during startup, shutdown, and upset conditions —when the greatest emissions are expected to occur. Furthermore, such data are typically based on a few stack samples for each pollutant. Thus, the adequacy of such emissions data to characterize fully the contribution of incineration to ambient pollutant concentrations for health-effects assessments is uncertain.
After MACT Compliance
Implementation of EPA's regulatory requirements for MACT for incineration facilities is expected substantially to reduce emissions from the highestemitting facilities. For such facilities, MACT would reduce the degree of concern indicated for potential health effects from exposures within local areas. However, on a broader scale, considering multiple facilities and broader populations, implementation of MACT is unlikely to alter the committee's relative degree of concern for the potential health effects due to pollutants such as dioxin and some metals, and the concerns would remain because these pollutants are persistent, widespread, and potent. Furthermore, there would be no change in
the committee's degree of concern for potential worker exposures, because MACT alone would be unlikely to change their exposures.
Various Degrees of Concern
The four degrees of concern (substantial, moderate, minimal, negligible) shown in Table 5-8 are intended to convey the committee's qualitative assessment and consensus judgment of the possibility of health effects to workers and the general public from incineration emissions. A degree was chosen for each specific pollutant and category based on general information on incineration emissions; persistence of the pollutant in the environment; mobility through air, soil, water, and food; potential total exposure through routes of inhalation, ingestion, and dermal absorption; and relative toxicity. The term “substantial” is used to express the committee's highest degree of concern about possible exposures that might lead to health effects among workers, a local population, or a broader population. Lower degrees of concern correspond to less possibility that the specific groups are exposed to concentrations associated with adverse health effects. The following sections provide additional discussion about the levels of concern for specific pollutants.
Particulate Matter
Given the possible health effects of typical environmental concentrations of PM and despite considerable scientific uncertainty, the committee has a substantial degree of concern for potential effects on local populations from exposure to PM contributed by high emitting (principally older) facilities. With modern PM control in a well-run facility, the emissions are so much lower that their contributions to local exposures are very low. Even in the most modern facilities, however, there is continued high concern by the committee for potential health effects from exposure to workers without proper safeguards. The handling of additional emission-control residues by workers might even add to their PM exposures and health risks after MACT implementation. On a broader geographical scale, the collective contribution of incineration facilities is comparatively small, and only minimal concern is associated with incineration on this scale, both before and after MACT compliance.
As seen in Figure 5-1, most U.S. metropolitan areas experience PM air pollution in the range at which adverse effects, including immediately increased mortality, have been associated with PM pollution. Any increases in PM concentrations —and especially in the fine particles emitted by combustion facilities, such as incinerators—can be expected to add to any existing PM health-effects burden. Increases in concentrations will be proportional to the PM emission rate by the facility and can be crudely estimated on the basis of “typical” ambient concentration estimates provided for various incinerator types shown in Tables 4-8,
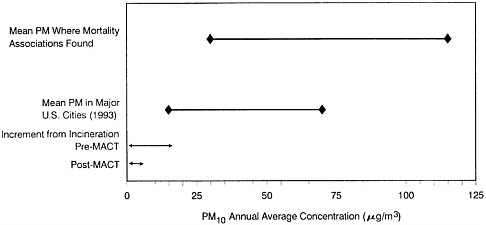
FIGURE 5-1 Comparison of range and mean PM10 concentrations in cities in which PM-death associations have been reported, range of mean PM10 in U.S. cities in 1993, and range of increment from incineration.
Table 4-9, Table 4-10 through Table 4-11 (see Chapter 4) and the health-effect information presented in Table 5-1. On the basis of these tables, it is seen that the highest PM effect of the uncontrolled incinerators, and especially cement kilns incinerating waste (potentially reaching 30 µg/m3 total PM, or about 20 µg/m3 PM10), might be projected to produce increases in health effects on the worst days in the highesteffect locations (potentially about a 2% increase in daily mortality and a 4% increase in respiratory hospital admissions on the maximum day in the case of the pre-MACT cement kiln). However, after MACT controls are applied to these plants, such projected air-pollution effects should be reduced by almost a factor of 10. As a result, the local effects of individual post-MACT plants (though still non-zero) would be so small that such projections would represent much less than a 1% increase in risk of acute morbidity or death, even at the most affected receptor on the worst-case day, and it is highly unlikely that such potential effects could be detected by even the most carefully designed epidemiologic study.
Dioxins
The committee has a substantial degree of concern for the potential health effects from exposures of plant workers to highly potent pollutants such as dioxin. There is uncertainty as to whether there is any adequate margin of safety between typical background exposures to dioxins and those with measurable responses that might be related to health. Implementation of MACT controls are unlikely to alter the committee 's degree of concern, because MACT is not designed to reduce worker exposures.
On a wider scale, it appears that a portion of dioxins in the environment has been produced by waste incineration and that a portion of the current input into the environment is produced by incineration, but how much is not known. There is substantial evidence that the average concentrations in the biosphere are now decreasing despite past increases in incineration, and it is not clear what effect MACT will have on these average concentrations. The wide dissemination of dioxins throughout the environment including the food supply, results in widespread exposures. Exposure indicators (such as blood and fat concentrations) arising from such exposures are close to the levels that, in some experimental systems, give rise to measurable biologic responses that might be related to adverse health outcomes. Thus, the committee has a substantial degree of concern for the incremental contribution to dioxins emissions from all incinerators on a regional level and beyond. Because the major route of exposure to dioxin is the food chain, the exposure of the local population is not expected to be affected much more by a local incinerator than by one located in another state. The local population shares the widespread increase in dioxin exposure from each incinerator, but experiences minimal additional risk. However, there may be specific individuals who have higher exposures because of their location and activity patterns.
The mechanism of dioxin toxicity is known to be complex. Several acute toxic effects are mediated almost solely (at least in the mouse) by the arylhydrocarbon receptor (Fernandez-Salguero et al. 1996), but there are other mechanisms. Studies attempting to elucidate precise mechanisms of action continue, and such studies show detectable effects of dioxin-like materials at concentrations similar to those encountered in the environment although it is unclear to what extent such effects might affect health. Figure 5-2 summarizes some of the dioxin TEQs exposures that are associated with overt toxic effects. Four scales of exposure are shown because no single exposure or dose measure is known to correlate with all toxic effects, and various measures have been used in human and animal studies. The four scales are ambient air concentration, long-term average intake, adipose-tissue concentration, and serum concentration. The scales have been aligned roughly so that the background concentrations —those found in typical U.S. populations—are level (horizontal dotted line), and the range of variation of these typical concentrations is indicated (a question mark indicates little information on the range of variation). On the ambient-air scale are marked the estimated maximal concentrations (worst-case locations) around the worst-case hazardous-waste incinerator and cement kiln, as discussed and depicted in Chapter 4, Table 4-8, Table 4-9, Table 4-10 through Table 4-11.
The average-intake scale indicates average human intakes and the intakes associated with overt toxic effects in animals, and the long-term average intakes found to cause cancer in more than about 10% of laboratory animals.
Adipose-tissue concentrations that correspond in laboratory animals to no overt effects and the tissue concentration roughly corresponding to the concen-
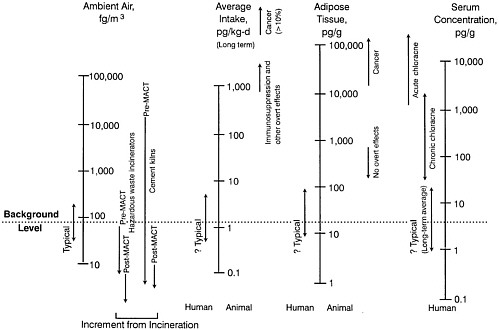
FIGURE 5-2 Dioxin TEQs associated with overt toxic effects and concentrations found in the environment. The typical range of background concentrations is shown by the double ended arrows about the “Background level” starred line, with “?” indicating uncertainty about the range. For the concentration scale on the left, the arrows show the ranges of increments in concentration potentially (as the worst-case location) associated with the labeled incineration sources and conditions. For the three scales on the right, arrows show approximate concentrations associated with the labeled end-points, in animals (to the right of each scale) and humans (to the left of each scale).
trations causing cancer in more than 10% of animals are shown. The ratios between concentrations required to cause cancer in animals and typical background concentrations in humans are different for average intake and for adipose-tissue concentrations, possibly because of differences in the pharmacokinetics of dioxin in animals and humans.
Finally, to indicate the effects of relatively short-term exposure, the serum concentrations in people who have exhibited dioxin-associated chloracne (one effect definitely associated in humans with dioxin exposure) are shown for both very-short-term exposure (e.g., Seveso children) and chronic occupational exposure.
Other Products of Incomplete Combustion
Products of incomplete combustion (PICs) have been defined as organic compounds not originally detected in the waste stream entering the incinerator, but found in incinerator stack-gas emissions (Travis and Cook 1989). PICs can arise as new organic compounds formed during the incineration process itself, might have been present in the original waste stream (but at concentrations below the cut-off level used in analyzing the waste feed), or might have been brought into the incineration system from noncombustion sources (e.g., auxiliary fuel feed, or ambient air introduced into the system). It is hypothesized that most PICs are formed from recombination of molecular fragments outside the combustion zone (Trenholm et al. 1984). Because they are widespread, persistent, and potent, the major PICs of concern are dioxins and furans, which are discussed separately in this section.
Other PICs of potential health concern are PCBs and PAHs. Incinerators are not major emission sources of these on a local or regional scale. Furthermore, in comparison with dioxins and furans, other PICs emitted by incinerators are estimated to have relatively little effect on health, or little is known about their toxicity at the relatively low concentrations emitted.
Lead
Lead at low concentrations can have adverse health effects especially infants and children. Therefore, at the local population level, the committee has substantial concerns regarding contributions to total lead exposure by incinerators operating prior to implementation of MACT controls. Incinerators operated under MACT are expected to emit only a negligible amount of lead locally, so the potential health effects in local populations from lead after the implementation of MACT are seen as minimal. Due to its toxic potential, exposures of incinerator workers to lead is of substantial concern to the committee. Implementation of MACT controls are unlikely to alter the committee 's level of concern because MACT is not designed to reduce worker exposures.
Figure 5-3 shows reported effects of lead at various concentrations in the blood. Effects that have been clearly established and are well accepted by the scientific community are indicated by solid lines, effects with less certainty are indicated by dashed lines, and more controversial effects are indicated by dotted lines. For example, frank anemia occurs at blood concentrations of 80 µg/dL or above; reduced hemoglobin synthesis occurs in adults at 50 µg/dL and above, although this effect might occur in children at lower concentrations; loss of hearing acuity occurs above 30 µg/dL, but hearing loss has been measured down to 10 µg/dL; and while the effect of lead on diastolic blood pressure is clear above 50 µg/dL, some studies indicate effects on systolic blood pressure above 30 µg/dL, and effects below 10 µg/dL are seen in some studies. Several effects have no apparent threshold (for example, the effects on children's cognitive function, on blood pressure, and on heme synthesis), and other effects might not demonstrably affect health.
The bottom of Figure 5-3 presents the most recent information on the distribution of blood lead concentrations in the United States, from NHANES III, phase I, 1988-1991 (JAMA 1994). There has been a remarkable reduction in blood lead concentrations in the United States over the last 15 years. There has been a 78% drop in the average, from 12.8 to 2.8 µg/dL, primarily it is believed, because of the removal of lead from gasoline. But a distribution of blood lead exists in the population, and the data indicate that a small portion of the population has blood lead over 10 µg/dL, as do 9% of children aged 1-5; and 0.2% of the population (over 0.5 million people) have blood lead over 30 µg/dL. Any added lead in the environment might make those people more likely to experience the adverse effects of lead.
The lead emissions of incinerators are highly variable (see Chapter 4, Table 4-8 and Table 4-10, and this is reflected in the facts that the mean value of lead emissions from hazardous-waste incinerators is 100 times the median value and that the estimated range of air concentrations due to emissions varies by more than 8 orders of magnitude (from 2.0 × 10-8 to 7 µg/m3). Although maximal lead air concentrations due to emissions is 7 µg/m3, which exceeds the ambient-air standards of the EPA, over 95% of the incinerators were estimated to produce ambient concentration increments everywhere less than 0.5 µg/m3; similarly, maximal lead air concentrations due to emissions from cement kilns was 7 µg/m3, but 95% would be less than 1.2 µg/m3. Translating airborne lead to blood lead is complex but has been well studied: for young children and accounting for both the direct route (inhalation) and the indirect route (ingestion of soil, dust, and food contaminated by airborne lead) of exposure, each microgram of airborne lead per cubic meter could increase blood lead by about 4 µg/dL (EPA 1989; CalEPA 1996).
Although the average hazardous-waste incinerator and the average cement kiln would contribute less than 1 µg/dL to the blood lead burden of children around the facilities, there is the potential for the worst-case emitters to add about 20 µg/dL to the lead burden of nearby children. Thus, while the effect of
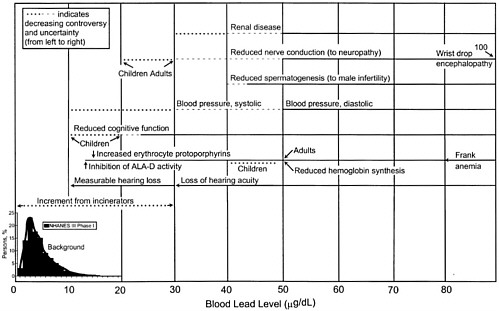
FIGURE 5-3 Blood lead concentrations: background, increment from incineration, and concentrations that have health effects. - -- - - - - - - - - - - - - - - - indicates decreasing controversy and uncertainty (from left to right). Arrows are used to indicate the blood lead level at which an effect is known to occur or the range in which an effect is known to occur.
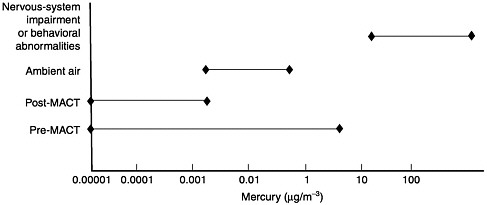
FIGURE 5-4 Mercury concentrations: Background (gas-phase and particle-bound concentrations), increment from incineration, and concentrations at which adverse effects occur for the most-sensitive end points of toxicity.
the average incinerator would be minimal, that of the highest-polluting facilities would be of some concern, and the maximally polluting facilities could add substantially to the lead burden in the local population and raise young children's blood lead to the point where multiple adverse health effects have been reported.
Mercury
Because low concentrations of mercury can have toxic effects, exposure of workers to mercury is of substantial concern to the committee. MACT controls are unlikely to alter the committee's degree of concern, because MACT is not designed to reduce worker exposures. The degree of concern about exposures of the population to mercury is expected to be reduced somewhat under MACT, but, in general, no change is expected regarding the regional level due to the environmental persistence of mercury.
Figure 5-4 compares mercury concentrations that are associated with nervous system impairment and behavioral abnormalities with concentrations found in the environment. Other human health effects associated with exposure to inorganic and organic forms of mercury, as displayed in Table 5-4, were not plotted here, because little human exposure information related to these health effects is available or exposures are uncertain or unknown. However, available data indicate that the major health effect of concern for mercury compounds is nervous system impairment. Other organ-system toxicity produced by mercury is reported to occur only after much-higher exposures. As shown in Figure 5-4,
the potential effect of the average incinerator is expected to be minimal; however, a maximally polluting facility could add substantially to the mercury burden in the community. The implementation of MACT technology is expected to reduce exposures to mercury at the local level. Air concentration estimates related to incineration (Pre-MACT and Post-MACT) are based on Table 4-8, Table 4-9, Table 4-10 through Table 4-11 in Chapter 4.
Acidic Gases and Acidic Aerosols
Incinerators directly release both acidic aerosols and gases, as well as acidic aerosol precursors that can be transformed into acid particles in the atmosphere. The acidic gases and vapors released from incinerators are generally of less concern than acids released or formed as aerosols (such as H2SO4). Thus, water-soluble acidic gases and vapors (such as SO2, HCl, and HNO3), are of low concern because, at ambient concentrations, these are efficiently “scrubbed out” in the trachea before reaching the lung. Particularly strong acidic aerosols, such as those containing H2SO4, however, more readily reach into the deepest recesses of the lung and are of greater health concern at ambient concentrations.
Acids released from incinerators therefore warrant a varied degree of concern depending on the form of the acid (particulate or gaseous) and the extent of emission (pre or post compliance with MACT). Acidic gases are of minimal health concern to the local population and of negligible concern at the regional level but represent a moderate concern to workers, given that exposures have the potential to become high. Compliance with MACT regulations further diminishes the concern regarding acidic gases at the local and regional levels, but not in the worker environment.
Acidic aerosols are associated with a somewhat higher degree of concern because of their particulate form and because MACT regulations are not directly aimed at reducing them. However, the acidity concern is reduced after MACT implementation because some MACT controls (such as SO2 limitations) can be expected indirectly to lower strongly acidic aerosols resulting from such plants.
Carbon Monoxide
Because only about 1% of all CO emissions are attributable to incineration (EPA 1998b,c), the incremental exposure to CO from incinerators is not considered to represent an important increment at either the local or regional level. Although it is possible for workers to be exposed to high levels of CO from incomplete combustion, no data are available to indicate that this has occurred.
CONCLUSIONS AND RESEARCH NEEDS
Conclusions
-
Estimates of large increments in ambient concentrations of various pollutants attributable to existing incinerators, particularly heavy metals and dioxins and furans, led to legitimate concerns about potential health effects.
-
Pollutants produced and emitted by incinerators that currently appear to have the potential to cause the largest health effects are particulate matter, lead, mercury, and dioxins and furans.
-
On the basis of available data, a well-designed and properly operated incineration facility emits relatively small amounts of those pollutants, contributes little to ambient concentrations, and so is not expected to pose a substantial health risk. However, such assessments of risk under normal operating conditions may inadequately characterize the risks or lack of risks because of gaps in and limitations of existing data or techniques used to assess risk, the collective effects of multiple facilities not considered in plant-by-plant risk assessments, potential synergisms in the combined effects of the chemicals to which people are exposed, the possible effect of small increments in exposure on unusually susceptible people, and the potential effects of short-term emission increases due to off-normal operations.
-
Reductions in emissions will certainly reduce public health risks from direct and indirect exposure to those emissions. Whether there is a minimal emission rate below which there is no further reduction in health risk has not been established, and the indirect effects of emission reductions (for example, health risks associated with efforts to reduce emissions, as through substitution of other processes or materials, the use of more energy or materials for control equipment, and the manufacture of control equipment) have not yet been evaluated.
-
Epidemiologic studies assessing whether adverse effects actually occurred at individual incinerators have been few and were mostly unable to detect any effects. That result is not surprising, given the small populations available to study; the presence of effect modifiers and potentially confounding factors (such as other exposures and risks in the same communities); the long periods that might be necessary for health effects to be manifested; and the low concentrations (and small increments in background concentrations) of the pollutants of concern. Although such results could mean that adverse health effects are not present, they could also mean that the effects may not be detectable using feasible methods and available data sources.
-
The potential health effects of particulate matter emitted by incinerators may not have received appropriate attention in traditional risk assess-
-
ments. In particular, in well-characterized situations (with well-measured emissions) where the contribution of particulate matter to the total ambient particle load is small (around 1%), the acute health effect of emitted particulate matter might be as large as or larger than that of other incinerator-related pollutants. Some past studies have shown the overall urban background of particulate matter already appear to be causing excess mortality and morbidity in the U.S. population, and the particulatematter increment from all incinerators adds to the existing burden.
-
The committee's evaluation was performed based only on emissions under normal operating conditions. Data are not available for levels during off-normal conditions, or the frequency of such conditions. Such information is needed to address whether emissions resulting from off-normal conditions are a concern with respect to possible health effects.
-
There is a need to focus health research on the greatest potential for exposure. Based on studies of municipal solid-waste incinerators, workers at these facilities are at much higher risk for adverse health effects from exposure to this technology than local residents. There is evidence that incinerator workers have been exposed to high concentrations of dioxins and toxic metals—particularly lead, cadmium, and mercury —in the past.
-
The committee's evaluation of waste incineration and public health has been substantially impaired by the lack of an adequate compilation of the associated ambient concentrations resulting from incinerator emissions. The evaluation was also impaired by the inadequate understanding of the overall contribution of incinerators to pollutants in the total environment, and large variabilities and uncertainties associated with risk-assessment predictions, which, in some cases, limit the ability to define risks posed by incinerators.
-
EPA is proceeding to regulate emissions from incinerators by requiring that incinerators reduce emissions to values achieved by the best controlled 12% of the current incinerators, a standard known as maximal achievable control technology, or MACT. Those regulations will affect emissions of the most-important pollutants unevenly; even under MACT, concerns over the widespread effects of persistent pollutants, such as dioxins, lead, and mercury will not be adequately addressed. Other potential effects can be shown to be negligibly small for some facilities on which well-measured emission data are available. However, for some individual facilities with well-measured emissions, health risks are not negligible. Collective potential effects of incinerators on a regional scale and beyond are unknown.
-
New or modified facilities that meet the proposed MACT requirements are expected to have substantially lower emissions than previous facilities.
-
The reduction in emissions will lower the potential exposures and risk to populations surrounding incinerators in the environment in general.
-
Based on a consideration of normal operating conditions, implementation of MACT standards is expected to substantially reduce the overall health risks from local impacts of particulate matter, lead, and mercury associated with incineration.
-
It is unlikely whether implementation of MACT will substantially reduce the risks at the regional level posed by the persistent environmental pollutants dioxin, lead, and mercury.
-
MACT was not designed to protect workers, and MACT regulations are unlikely to reduce worker exposures.
Recommendations
-
To increase the power of epidemiologic studies to assess the health effects of incinerators, future multi-site studies should be designed to evaluate combined data from all facilities in a local area as well as multiple localities that contain similar incinerators and incinerator workers, rather than examining health issues site by site.
-
In addition to using other exposure assessment techniques, worker exposures should be evaluated comprehensively through biological monitoring, particularly in combination with efforts to reduce exposures of workers during maintenance operations.
-
Assessments of health risks that are attributable to waste incineration should pay special attention to the risks that might be posed by particulate matter, lead, mercury, and the dioxin and furans, due to their toxicity and environmental prevalence.
-
Health risks attributable to emissions resulting form incinerator upset conditions need to be evaluated. Data are needed on the levels of emissions during process upsets as well as the frequency, severity, and causes of accidents and other off-specification performance to enable adequate risk assessments related to these factors. Such information is needed to address whether or not off-normal emissions are important with respect to possible health effects.
-
Database compilers should strive to accumulate data not only on emissions from individual facilities (as in the Hazardous Waste Combustor database), but also the resulting estimates of ambient concentrations. Facilities that have performed emissions testing have also often performed site-specific air dispersion modeling, so that little extra effort would typically be required. Moreover, the overall contribution of incinerators to pollutants in the total environment would be easier to assess if any known site-specific measurements of background concentrations of incineratorrelated pollutants were also compiled on a plant-by-plant basis.






































































