3
Incineration Processes and Environmental Releases
Waste incineration is one of many societal applications of combustion. As illustrated in Figure 3-1, the typical waste-incineration facility includes the following operations:
-
Waste storage and feed preparation.
-
Combustion in a furnace, producing hot gases and a bottom ash residue for disposal.
-
Gas temperature reduction, frequently involving heat recovery via steam generation.
-
Treatment of the cooled gas to remove air pollutants, and disposal of residuals from this treatment process.
-
Dispersion of the treated gas to the atmosphere through an induced-draft fan and stack.
There are many variations to the incineration process, but these unit operations are common to most facilities.
This chapter addresses the combustion and air-pollution control operations commonly used in municipal solid-waste, hazardous-waste, and medical-waste incineration facilities. The intent is to identify, and briefly discuss, the design features and operating parameters that have the greatest influence on emissions. Waste storage, feed preparation, and gas temperature reduction (which may involve heat-recovery operations) are addressed to a lesser extent.
This chapter also addresses the air pollutants emitted from incineration processes that are of primary concern from a health effects standpoint (see Chapter
5). Formation mechanisms and emission-reduction techniques are discussed. Information is provided on stack emission rates during normal operation vs. offnormal operating scenarios such as startup, shutdown, and process upset conditions. Fugitive emissions, residual ash, and scrubber water handling are briefly discussed.
WASTE STORAGE, FEED PREPARATION, AND FEEDING
Table 3-1 lists the common waste storage, waste staging, feed preparation and feeding practices for municipal solid-waste, hazardous-waste, and medical-waste incinerators. These practices are highly waste-and facility-specific.
Proper design and operation of these “front-end” plant operations are important for several reasons:
-
While the plant is operating, the potential for worker exposure to hazardous materials is the greatest in this part of the facility. Without appropriate engineered and administrative controls, including personnel protective equipment, operators can be exposed to hazardous dust and vapors.
-
This part of the plant is the highest potential source of fugitive dust and vapor emissions to the environment, and the greatest potential fire hazard.
-
Without proper waste preparation and feeding, the furnace combustion performance may be impaired.
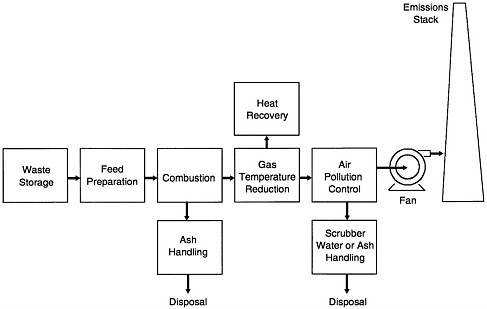
FIGURE 3-1 Typical waste-incineration facility schematic.
TABLE 3-1 Common Waste Storage, Feed Preparation, and Feeding Practices in Municipal Solid-Waste, Hazardous-Waste, and Medical-Waste Incineration Facilities
|
Waste Type |
Storage/Staging |
Feed Preparation |
Feeding to Incineratora |
|
Municipal Solid Waste |
|
|
|
|
Hazardous Waste—Solids |
|
|
|
|
Hazardous Waste—Liquids |
|
|
|
|
Medical Waste |
|
|
|
|
a There are many variations in the methods of waste feeding in small or old municipal solid-waste incineration facilities. |
|||
There are many regulations and guidelines for the design and operation of waste storage, handling, and feeding systems. Organizations that develop such regulations and guidelines include the U.S. Occupational Safety and Health Administration (OSHA), U.S. Environmental Protection Agency (EPA), and National Fire Protection Association (NFPA).
COMBUSTION PROCESSES
General Considerations
Combustion is a rapid, exothermic reaction between a fuel and oxygen (O2). In incineration applications, the fuel is predominately waste (although fossil fuels may be co-fired) and the oxygen source is air. Combustion produces many of the same stable end products, whether the material burned is natural gas, coal, wood, gasoline, municipal solid waste, hazardous waste, or medical waste. The flame zone of a well-designed incinerator is sufficiently hot to break down all organic and many inorganic molecules, allowing reactions between most volatile components of the waste and the oxygen and nitrogen (N2) in air. The predominant reactions are between carbon (C) and oxygen, producing carbon dioxide (CO2), and between hydrogen (H) and oxygen, producing water vapor (H2O). Incomplete combustion of organic compounds in the waste feedstream produces some carbon monoxide (CO) and carbon-containing particles. Hydrogen also reacts with organically-bound chlorine to produce hydrogen chloride (HCl). In addition, many other reactions occur, producing sulfur oxides (SOx) from sulfur compounds, nitrogen oxides (NOx) from nitrogen compounds (and, a little, from the nitrogen in the air), metal oxides from compounds of some metals, and metal vapors from compounds of others.
The furnace is designed to produce good mixing of the combustion air and the gases and vapors coming from the burning waste. Nevertheless, in parts of the furnace where combustion is not complete (for example, near the walls of the furnace), combustible components of organic compounds are burned off, leaving the incombustible particulate matter known as fly ash entrained in the flue gas. The incombustible portion of the waste (known as bottom ash) is left behind.
Incineration facilities incorporate a number of general methods for ensuring proper combustion and reducing emissions. A steady situation with no major fluctuations in the waste-feed supply rate, combustion-air flows, or other incineration conditions promotes efficient combustion. Inefficient combustion can result in higher levels of products of incomplete combustion. Similarly, the more often a facility is started up and shut down (for maintenance or because of inadequate or varying waste stream volume), the more uneven the combustion and the greater the potential for increased emissions.
Optimal design and operation of a furnace requires attention to incineration temperature, turbulence of the gas mixture being combusted, and gas-residence
time at the incineration temperature. To achieve efficient combustion, every part of the gas stream must reach an adequately high temperature for a sufficient period of time, and there must be adequate mixture of fuel and oxygen.
The temperature achieved is the result of heat released by the oxidation process, and has to be maintained high enough to ensure that combustion goes to completion, but not so high as to damage equipment or generate excessive nitrogen oxides. Typically, temperatures are controlled by limiting the amount of material charged to the furnace to ensure that the heat-release rate is in the desired range, and then tempering the resulting conditions by varying the amount of excess air.
Turbulence is needed to provide adequate contact between the combustible gases and oxygen across the combustion chamber (macroscale mixing) and at the molecular level (microscale mixing). Proper operation is indicated when there is sufficient oxygen present in the furnace, and the gases are highly mixed. Cool spots can occur next to the furnace's walls; where heat is first extracted from the combustion process. Such cool spots on walls are more substantial in waterwall furnaces than in refractory-lined furnaces.
A number of new design features and operating techniques have been adopted to increase temperature, extend residence time, and increase turbulence in waste incinerators in order to improve combustion efficiency and provide other benefits like improved ash quality. They include high-efficiency burner systems, waste-pretreatment practices such as shredding and blending, and oxygen enrichment in addition to the features and methods discussed below. Considerable attention has also been given to measurement and control of key process operating conditions to allow better control of the whole combustion process.
Furnace Types
Table 3-2 lists the types of furnaces used for municipal solid-waste, hazardous-waste, and medical-waste incineration. Municipal solid-waste furnace designs have evolved over the years from simple batch-fed, stationary refractory hearth designs to continuous feed, reciprocating (or other moving, air-cooled) grate designs with waterwall furnaces for energy recovery. The newer municipal solid-waste incinerators are waste-to-energy plants that produce steam for electric power generation.
The predominant hazardous-waste incinerator designs are liquid-injection furnaces and rotary kilns. Hazardous wastes are also burned in cement kilns, light-weight aggregate kilns, industrial boilers, halogen-acid recovery furnaces, and sulfuric-acid regeneration furnaces.
Medical wastes are burned in fixed-hearth incinerators, with the primary chamber operated in the starved-air mode (newer “controlled air” designs) or excess air mode (older Incinerator Institute of America (IIA) design). Both designs incorporate secondary, afterburner chambers. The smallest medical-waste incinerators are single-chamber, batch-operated devices.
TABLE 3-2 Furnace Designs for Municipal Solid-Waste, Hazardous-Waste, and Medical-Waste Incineration
|
Waste Type |
Furnace Design |
Type Application |
|
Municipal Solid Waste |
Mass burn Waterwall furnace Reciprocating or other continuous moving grate |
Most newer municipal-scale facilities |
|
Mass burn Refactory furnace lining Various grate or stationary hearth designs |
Old or small facilities |
|
|
Refuse-derived fuel Spreader-stoker/cyclone furnaces |
Few facilities in United States |
|
|
Fluidized bed |
Foreign applications |
|
|
Hazardous Waste |
Liquid injection Rotary kiln with secondary combustion chamber |
Common Common |
|
Fluidized bed |
Few in United States. More common for biosludge incinerators |
|
|
Fixed hearth with secondary chamber |
Mostly with plant trash co-feed |
|
|
Medical Waste |
Multiple chamber |
Old IIA design for older facilities |
|
Controlled-air primary chamber with afterburner |
Predominant design in United States since 1970s |
|
|
Rotary kiln with afterburner |
Few in United States |
For illustrative purposes, the following discussion focuses on the basic design and operating considerations for one type of furnace.
Furnace Design Considerations For Municipal-Waste Incinerators
The design of the furnace is critical to optimal combustion. Furnace configurations depend on what they were designed to burn. Older designs, many of which are still used, do not generally permit as efficient combustion as newer designs.
Sizing
Poor combustor design can prevent stable, optimal combustion conditions. Sizing a furnace to match the quantity of waste fed to the incinerator is important with respect to temperature, turbulence, and time. If the heat input from the waste is too low for the furnace size, the temperature in the furnace may drop to such an extent that complete combustion is not achieved, particularly in waterwall furnaces. If the furnace is too small for the quantity of waste fed, the temperature will be high and there may be difficulty in supplying sufficient oxygen for complete combustion, and the quantities of unburnt residues might be increased.
Grates
In older incinerator systems, traveling grates simply transported refuse into the combustion zone. Newer grate systems are designed to agitate the waste in various ways, causing it to be broken into smaller pieces as combustion proceeds. This process permits exposure of a larger surface area of waste to air and high temperatures, assisting complete combustion by preventing unburnt material from simply being transported through on the grate.
Air-Injection Systems
For complete combustion to occur, air must be injected into the furnace in at least two locations: under the grate that carries burning waste (primary or underfire air) and above the grate to mix additional oxygen with the combustion gases (secondary or over-fire air). Additional controls have been provided in modern municipal solid-waste incinerators to better regulate both the under-fire air at various points on the grate, depending upon burning conditions, and the over-fire air in response to temperature and heat transfer taking place in the furnace. In such advanced systems, primary air is injected into the drying, burning, and burnout zones of the grate, with a separate system for secondary air. Control may be effected by manual or automatic adjustments to dampers. The latter method is preferred, because it allows for automatic control loops with continuous monitoring devices. The temperature and oxygen needs of the furnace can be controlled by adjusting the quantity of primary and secondary air entering the furnace. In plants built before the middle 1980s, particularly those with holes in the furnace walls, the entry of primary and secondary air is not as well controlled, and the excess-air rates required for adequate combustion can be several times the amount that would be required with a more modern design. This can result in larger volumes of flue gas to be treated for contaminant removal, and reduced efficiency of utilization of the exhaust heat.
Arches and Bull Noses
To achieve complete combustion, gases produced must remain in the high-temperature zone of the furnace for a minimal residence time, usually 1-2 seconds. Achieving that residence time is usually accomplished by designing the furnace to retard the upward flow of gases, for example, by installing irregularities into the furnace walls. Modern facilities are configured to achieve improved combustion efficiency by using arches and bull noses. Arches, which are structures above the burning and burnout zones, are used to prolong the stay of combustion gases above the grate area. Bull noses are protrusions that are built into the furnace walls, usually near the point of injection of over-fire air, to upset the normal upward flow of the heated gases volatilizing from the burning waste. The induced gas redirection retards the movement of the combustion gases out of the furnace and promotes mixing with air.
Flue-Gas Recirculation
Flue-gas recirculation systems are used to recycle into the furnace relatively cool flue gas (extracted after the heat exchangers have reduced its temperature) that contains combustion products and an oxygen concentration lower than air. The process is used to lower nitrogen oxide formation by limiting the flame temperature and by slightly diluting the flame oxygen concentration. Care must be taken to ensure that not too much flue gas is recirculated, lest the combustion process be adversely affected.
Auxiliary Burners
Waste feedstock, particularly municipal solid waste, is heterogeneous, and its components, or even the whole waste stream, may vary in combustibility. That can make it difficult to maintain the minimal temperature necessary throughout a furnace. In modern combustors, maintenance of temperature can be aided by auxiliary burners that are typically set to come on automatically when the furnace temperature falls below a predetermined point; the threshold is usually set between 1,500 and 1,800°F at the location of the auxiliary burner, which is close to the chamber exit. The auxiliary burners are fed fossil fuels and are particularly intended to be used during system startup, shutdown, and upsets.
GAS-TEMPERATURE REDUCTION TECHNIQUES
The most common combustion-gas cooling techniques for incinerators are waste-heat boilers, and direct-contact water-spray quenches. Waste-heat boilers are employed on all new municipal solid waste-to-energy plants, many hazardous-waste incinerators, and some of the larger medical-waste incinerators.
Waste-to-energy plants have radiant waterwall furnaces as well as convective boiler sections. Hazardous-waste and medical-waste incinerators usually have just convective boiler sections, typically of fire-tube rather than water-tube design.
Most hazardous-waste and medical-waste incinerators, particularly the smaller units, do not have heat-recovery boilers. Combustion gases are quenched by water sprays atomized into the hot gas flow. Other, less common, gas-temperature reduction methods include air-to-gas heat exchangers and direct gas tempering with air.
Gas cooling techniques are integral to incineration system design, and can be important with respect to emissions of certain pollutants. As discussed later in this chapter, emissions of mercury and dioxins and furans can be affected by the rate of gas cooling and the air pollution control device (APCD) operating temperature. Dry APCDs, including scrubbers and particulate control devices, achieve the highest degree of reduction of mercury, dioxins and furans, and acid gases when flue-gas temperatures are lowered to about 300°F or less at the APCD inlet.
AIR-POLLUTION CONTROL TECHNIQUES
Historically, incinerator APCDs were designed to remove two classes of pollutants which are particulate matter and acid gases. More recently, some method for improving the removal of dioxins and/or mercury is considered necessary. Also, as discussed in Chapter 6, NOx emission limits have been established for some incinerators. In several instances in European plants, increasingly stringent regulations have resulted in use of more than one particulate-control device or more than one type of scrubber in a given incineration facility, and emissions have typically been reduced more than would be expected with the single device alone.
Modern municipal solid-waste incinerators in the United States are equipped for particulate, acid gas, and, in many cases, dioxin and mercury removal. These municipal solid-waste incinerators typically employ fabric filters or dry electrostatic precipitators (esp) for particulate removal. ESPs became common in the 1970s. In the 1980s, fabric filters, also known as baghouses, started to replace, or be used in tandem with, ESPs as the preferred design for particulate removal because of their improved capacity for filtering finer particles. Spray dryer absorbers and dry-lime injection systems are used for acid gas—HCl and sulfur dioxide (SO2)—removal. Dry powdered activated carbon injection systems provide dioxin and furan and mercury removal.
Many small old municipal-waste incinerators do not have effective air-pollution control systems. Some have only a particulate-control device, often a relatively ineffective one designed to meet old standards for emissions of particulate. Newer ones have both particle and acid-gas-control devices, such as wet scrubbers.
Hazardous-waste incinerators in the United States have traditionally used wet air-pollution control systems. Recently, however, there has been a trend toward fabric-filter systems (particularly in larger incineration facilities) because of their superior fine-particle-emission and metal-emission control efficiencies and their ability to produce a dry residue rather than a scrubber wastewater stream. Wet ESP devices may be favored in the future for existing wet APCDs to meet emission-control regulations.
Cement kilns and coal-fired boilers that burn waste as fuel have traditionally used either fabric filters or dry electrostatic precipitators as active control techniques. Passive controls include the neutralization of acid gases by cement materials and the recycling of cement kiln dust back into the process.
Particulate Collectors
Fine-particle control devices fall into three general categories, which are filtration collectors, including fabric filters (baghouses); electrostatic collectors, including dry and wet electrostatic precipitators (ESPs) and ionizing wet scrubbers; and wet inertial-impaction collectors, including venturi scrubbers and advanced designs that use flux-force condensation-enhancement techniques. When properly designed and operated, all of them are capable of effective fine-particle control, but they are not all equally effective.
Fabric filters are used at relatively low flue-gas temperatures (about 280-400°F). Flue gas containing particles passes through suspended filter bags. The particles suspended in the gas streams are collected on the filters and periodically removed and fed to a collection hopper.
Fabric filters are widely used today in municipal solid-waste incineration facilities, cement kilns, and lightweight-aggregate kilns because of their highly efficient collection of fine particles. They are used in a smaller number of hazardous-waste incinerators and medical-waste applications. The performance of fabric filters is relatively insensitive to particle loading, or to the size distribution and physical and chemical characteristics of the particles. They are limited to an operating temperature range between the gas dew point on the lower end and the bag-material thermal-stability limit on the upper end. A typical and practical operating-temperature for this technology in municipal solid-waste applications is about 300°F, but the best environmental performance is achieved at lower temperatures (to minimize dioxin and furan production within the APCD itself).
The primary factors affecting the performance of fabric filters are fabric type and weave, air-to-cloth ratio (gas flow rate to total bag surface area), cleaning method and frequency, bag cake formation and maintenance, and bag integrity with respect to mechanical, thermal, and chemical breakdown. The fabric type must be matched to the temperature range of the application and the chemical composition of the gas for good performance and bag longevity. Maximal
air-to-cloth ratio for good performance is also a function of fabric type and weave. The method, intensity, duration, and frequency of the bag-cleaning cycles are important to maintain mechanical integrity of the bags and good cake formation. Good cake formation (as measured by baghouse pressure differential) is required for good performance of woven and felted bags; it is less critical for laminated membrane bags, which can function using surface filtration alone.
In properly designed and operated fabric-filter systems, maintaining bag integrity is the critical determinant of day-to-day performance. Bag integrity can be monitored via pressure drop, visual stack-opacity inspections, continuous online stack-opacity monitors, or other continuous monitoring techniques that use optical sensing or triboelectric sensing.
During shutdowns, bag integrity can be checked by visual examination of the clean-gas plenum for localized dust buildup. More-sensitive techniques involve the use of fluorescent submicrometer powder and black-light examination of the plenum.
Dry ESPs are widely used today in municipal solid-waste incineration facilities and on cement kilns and coal-fired boilers that burn hazardous waste. Wet ESPs are less widely used and are primarily in hazardous-waste incineration applications. Dry ESPs operate above the dewpoint of the gas. Wet ESPs are constructed from materials that resist acid corrosion and operate under saturated-gas conditions.
Dry ESPs are less effective than fabric filters for collection of submicrometer particulate matter (0.1-1.0 µm) but are nevertheless very effective collection devices. Their performance is influenced by a variety of design characteristics and operating conditions, including the number of electric fields used, charged electrode wire (or rod) and grounded collection plate (or cylinder) geometry, specific collection area (ratio of collection surface area to gas flow rate), electrode design, operating voltage and current, spark rate, collector cleaning method (to limit buildup or re-entrainment of dust), fluctuations in gas flow rate and temperature, particulate-loading fluctuations, particle-size distribution, and particle resistivity (less important for wet ESPs). Wet ESPs have superior submicron particle collection capabilities because they do not suffer rapping re-entrainment and dust layer back-corona problems associated with dry ESPs.
In a properly designed unit, the important monitoring and process-control measures are inlet gas temperature (dry ESPs only), gas flow rate, electrical conditions (voltage, current, and spark rate), cleaning intensity and frequency, and hopper-ash level (dry ESPs only).
Wet inertial-impaction scrubbers, primarily venturi scrubbers, have historically been the particulate matter control technology of choice for most hazardous-waste and medical-waste incinerators. They are inherently less efficient for submicrometer particulate matter than fabric filters or ESPs, but nonetheless can meet regulatory requirements in many applications.
The primary performance criterion for most wet inertial-impaction scrub-
bers is the gas-pressure drop, a measure of the energy applied to atomize scrubbing liquid and create fine droplets for particle impaction. For injector venturi scrubbers, the corresponding criterion is liquid-nozzle pressure drop. Other important design and operating characteristics are the liquid-to-gas ratio, inlet gas temperature (to avoid scrubber-liquid evaporation), solid content of recirculated scrubber liquid, mist eliminator efficiency, materials of construction to avoid corrosion and erosion, particulate loading, and particulate-size distribution. In a properly designed unit, the most-important monitoring and process control measures are pressure drops, liquid and gas flow rates, and liquid blowdown rate (blowdown is used to control solids buildup).
A few designs use steam injection or scrubber-liquid subcooling to enhance flux force and condensation. For those designs, steam-nozzle pressure and scrubber-liquid temperature are additional useful monitoring measures.
Acid Gas Scrubbers
A commonly used APCD for removal of acid gases is a packed-bed absorber. A scrubbing liquid is trickled through a matrix of random or structured packings through which the gas is simultaneously passed, resulting in gas-liquid contact over a relatively large surface area. The scrubbing liquid can be water or an alkaline solution, which reacts with the acid-gas constituents to form neutral salts. The wastewater discharge from the packed-bed absorber is a salt-water brine that must be managed properly. This effluent may contain unreacted acids, trace organics, metals, and other solids removed from the gas stream.
Packed bed absorbers have been used for decades in the United States, primarily in hazardous-waste and medical-waste incineration applications. They have been used in Europe for municipal solid-waste applications. The European installations include duel-stage wet absorbers, in which the first stage is operated with an acidic scrubber liquid and the second stage is operated with an alkaline scrubber liquid. Acid gases, such as HCl, that are highly water soluble are largely collected in the first stage. Acid gases, such as SO2, that are not very water soluble are effectively collected in the second, alkaline stage.
The important design and operating criteria for wet acid-gas absorbers are gas velocity, liquid-to-gas ratio, packing mass transfer characteristics, pH of the scrubbing liquid, and materials of construction (to prevent corrosion).
In recent years, municipal solid-waste and a few larger hazardous-waste and medical-waste incineration facilities have used spray-dryer scrubbers for acid-gas control. The spray dryers use slurries of lime, sodium carbonate, or sodium bicarbonate as the alkaline reagent. The water in the atomized slurry droplets evaporates, cooling the gas, and the alkali particles react with the acid-gas constituents to form dry salts. The salts and unreacted alkali must be captured in a downstream fabric filter or electrostatic precipitator. Dry-injection scrubbers, which use an alkaline reagent without water, have also been used in recent years,
although only rarely in United States municipal-waste, hazardous-waste, and medical-waste incinerators. They are typically not as efficient as spray-dryer absorbers at removing emissions. The important design and operating criteria for spray-dryer absorbers and dry-alkali scrubbers include gas temperature in the reagent contacting zone, reagent-to-acid gas stoichiometry, reagent distribution in the gas, and reagent type.
NOxControls
NOx emissions can be reduced by combustion-furnace designs, combustion-process modifications, or add-on controls. Combustion-furnace designs that reduce thermal NOx include a variety of grate and furnace designs, bubbling and circulating fluidized-bed boilers, and boiler designs, especially those with automatic controls, that permit flue-gas recirculation. Combustion-process modifications that reduce NOx formation include controlling the amount of oxygen available during the combustion process, and operating within a specific temperature range. For minimizing NOx production in the combustion process, it is recommended that there be a lower-oxygen condition just above the grates (or in the primary chamber of a dual-chamber facility) coupled with a higher excess-oxygen condition at the location of overfire air injection (or in the secondary chamber of a dual-chamber facility). Municipal solid-waste incineration facilities tend to create the most NOx when furnace temperatures are higher than is necessary (higher than 2,000°F) to destroy products of incomplete combustion (PICs). To minimize NOx formation, and the formation of PICs, the furnace should be operated within fairly narrow ranges of temperature and excess oxygen (9-12%) with turbulent (well-mixed) conditions.
Some NOx formation is inevitable from nitrogen present in the fuel and from atmospheric nitrogen, and it may be necessary to use flue-gas controls to achieve further reduction of these emissions. Add-on NOx flue-gas control systems include selective noncatalytic reduction (SNCR), selective catalytic reduction (SCR), and wet flue-gas denitrification.
SNCR reduces NOx by injecting ammonia or urea into the furnace via jets positioned at the location where temperatures are about 1600-1800°F. In the proper temperature range, the injected ammonia or urea combines with nitrogen oxide to form water vapor and elemental nitrogen.
SCR operates at a lower flue gas temperature than SNCR, and in addition uses a catalyst. Ammonia is injected into the flue gases when they are at about 600°F, and the mixture is passed through a catalyst bed. The catalyst bed may be shaped in a variety of forms (honeycomb plates, parallel ridged plates, rings, tubes, and pellets), while the catalyst can be one of a variety of base metals (such as copper, iron, chromium, nickel, molybdenum, cobalt, or vanadium). Each combination has advantages and disadvantages with respect to catalyst-to-NOx contact, fouling of the catalyst, and pressure drop through the catalyst. The
biggest disadvantage of SCR for incineration applications is that the combustion gas must always be reheated to the required 600°F temperature range after cooling below this level to remove particulate matter. The catalyst beds required for SCR must be installed downstream of highly effective particulate removal devices to avoid fouling.
Wet scrubbers for NOx removal are comparable to wet acid gas absorbers in configuration. They use strong oxidizers in aqueous solution to convert NO to NO 2 (which is water soluble in caustic solution) or NO3-(nitrate), which is water-soluble. The exact chemistries of these systems are considered proprietary by the vendors.
Carbon Adsorption and Other Dioxin and Mercury Removal Techniques
Carbon injection refers to the injection of finely divided activated carbon particles into the flue gas stream ahead of the particulate APCD. The carbon particles adsorb pollutants on their surface, and then the carbon particles are themselves captured in the particulate APCD. Activated carbon has a large surface-area-to-volume ratio, and is extremely effective at adsorbing a wide range of vapor-phase organic-carbon compounds, and also some other vapors (like mercury) that are otherwise hard to control. Maximum effective use of the technique requires optimization of the rate of injection of activated carbon (Brown and Felsvang 1991). Studies in Europe and practical experience in the United States and elsewhere indicate that this technique can substantially reduce emission of dioxins and furans and of mercury. Also, Lerner (1993) reported that cadmium chloride is effectively removed from a flue gas stream by using activated carbon.
Dioxins and furans are removed along with mercury by injection of powdered activated carbon in a number of municipal-waste incinerators and a few hazardous-waste incinerators. This is a widely used form of emissions control in the United States and is quite effective for PCDD/PCDF removal. Removal efficiency is a complex function of carbon type, dosage, gas temperature, and gas-to-solid contact efficiency. Other add-on control technologies used outside the United States are adsorption in granular activated carbon or coke beds, catalytic oxidation in SCR units (which are also the most efficient NOx controls demonstrated commercially), and injection of an inhibitor of dioxin-formation catalyst.
For high efficiency mercury removal, many municipal solid-waste incinerators and a smaller number of hazardous-waste and medical-waste incinerators have adopted powdered activated-carbon injection upstream of dry particle collection devices, usually fabric filters. As for dioxin removal, the effectiveness of powdered activated-carbon injection is determined by the carbon type, dosage, gas temperature, and gas-contact efficiency.
Other processes for mercury removal are granular activated-carbon filtration
in fixed-bed reactors, selenium porous-media filter, gold-amalgamation filter beds, sodium sulfide injection, and wet scrubbing with mercury-reactive solutions. None of those techniques is used commercially in the United States, but fixed-bed carbon adsorbers used in Europe often produce mercury and dioxin removal efficiencies that are higher than conventional technologies used alone (e.g., scrubber/fabric filter with injection of activated carbon).
SYSTEM OPERATION
Many variables that affect incinerator operation are controlled by operators, so the combustion conditions that control emission rates may be substantially affected by operator decisions. Poor operator control either of the furnace (by permitting temperature or oxygen concentration to decrease) or of the stoking operation can cause reduced combustion efficiency. In most incinerators, mixing and charging of waste into the incinerator, grate speed, over-fire and under-fire air-injection rates, and selection of the temperature setpoint for the auxiliary burner are entirely or partially controlled by plant personnel.
In addition, the extent of emission control achieved by post-combustion APCDs depends on how the devices are operated. Suboptimal operation can be caused by poorly trained or inattentive operators, faulty procedures, and equipment failure. Operators must be attentive to the flow rate of waste into the incinerator and furnace operation so as to allow for effective function of APCDs.
Although some of the most-modern incineration equipment has been automated, there will always be a need for operators to deal with unexpected situations. In addition, automated equipment requires calibration and maintenance, and combustor parts can wear out or malfunction. Examples of what can go wrong include clogged air injection into the incineration chamber, fouled boiler tubes, a hole in the fabric filters, and a clogged scrubber nozzle.
Worker Training
Before the 1980s, there were no uniform national standards for hazardous-waste combustor maintenance or worker-training. The extent and adequacy of maintenance and worker training programs were company-specific and site-specific.
In 1986, the Occupational Safety and Health Administration 1910 regulations were promulgated, requiring worker training in hazardous-material management. Classroom training courses are now required for hazardous-waste workers at remediation sites and plant facilities. Annual refresher courses are required, as is supplemental training for supervisory personnel.
Resource Conservation and Recovery Act (RCRA) regulations impose federal requirements for inspection plans and worker-training plans for all facilities that manage hazardous waste, including combustion facilities. The inspection
plans address facility maintenance, leak inspections, and calibration schedules for monitoring equipment. The training plans are intended to address hazardous-material safety and facility operations.
The American Society of Mechanical Engineers has developed a certification guideline for hazardous waste-incinerator operators.
Monitoring and Data Collection
For the most recently completed waste incinerators, particularly hazardous-waste incinerators, environmental regulations have led to extensive monitoring of key incineration process conditions, including waste feed rates; feed rates of ash, chlorine, and toxic metals (determined by sampling and analysis of the waste stream); combustion temperatures; gas velocity (or gas residence time); facility-specific air-pollution control-system operating measures; and stack-gas concentrations of O2, CO, total hydrocarbons, HCl, NOx, and SOx, and opacity (see Chapter 6). Computerized systems collect and record process data, automatically control such process conditions as combustion temperature (by varying fuel feed and air flow rates), and automatically cut off waste feeds if operating conditions stray outside limits set by permits. For example, a low combustion temperature or high stack-gas CO concentration might initiate an automatic waste-feed cutoff.
RCRA regulations for hazardous-waste incinerators require continuous monitoring of important air-pollution control-system operating conditions, including pressure drops across venturi scrubbers, pH of acid-gas absorber scrubbing solutions, voltage or power supplied to electrostatic collectors, and fabric-filter pressure drops or triboelectric sensor readings.1 Stack-gas monitors are often used to monitor the performance of the air-pollution control system directly for such measures as HCl, SO2, NOx, and opacity.
With electronic transmission of such sensor outputs, the performance of the control and monitoring systems could be more-readily displayed and monitored. Reliable continuous emission monitors (CEMs) for dioxins and furans or for metals would be desirable, because automatic devices electronically linked to such devices (for example, to optimize the injection of alkaline and carbon reagents and water in the emissions control devices) could directly control those emissions of greatest potential health consequence. Such arrangements have been in use for continuous automatic control of acid gases for some time. CEMs for mercury have undergone in-use testing in Europe, for example see Felsvang and Helvind (1991).
|
1 |
Triboelectricity refers to an electric charge that is generated by friction. |
PROCESS EMISSIONS
The principal products of combustion are CO2, water vapor, and ash, which are respectively oxidation-reaction products of carbon, and hydrogen, and non-combustible materials in the fuel. However, when the combustion reactions do not proceed to their fullest extent, other substances, some of which are potentially harmful, can be produced. The types and concentrations of contaminants in the waste stream (flue gas) flowing from any incineration process depend on the process type, the waste being burned, and combustion conditions. Such pollutants derive from three sources: they or their precursors are present in the waste feed, they are formed in the combustion process because of incomplete oxidation, or they are created by reformation reactions in the gas cooling or APCD.
As discussed in Chapter 5, the products of primary concern, owing to their potential effects on human health and the environment, are compounds that contain sulfur, nitrogen, halogens (such as chlorine), and toxic metals. Specific compounds of concern include CO, NOx, SOx, HCl, cadmium, lead, mercury, chromium, arsenic, beryllium, dioxins and furans, PCBs, and polycyclic aromatic hydrocarbons. In addition, the total quantities of particulate matter and acid particles (which may largely be liquids condensed after emission) that escape the APCD are also considered independently. The following discussion focuses on the source and control of the following pollutants: particulate matter, acid gases, mercury (Hg), lead (Pb), and products of incomplete combustion. They are used to represent the pollutants from incineration that are of concern for possible health effects.
Particulate Matter
Particulate matter consists primarily of entrained noncombustible matter in the flue gas, and the products of incomplete combustion that exist in solid or aerosol form (and discussed separately later). Particle concentrations in the flue gas in the absence of control devices have been found to range from 180 to more than 46,000 mg per dry standard cubic meter (0.08 to more than 20 grains per dry standard cubic foot).
Particulate matter from waste combustors includes inorganic ash present in the waste and carbonaceous soot formed in the combustion process. The inorganic-ash fraction of the particulate matter consists of mineral matter and metallic species. These materials are conserved in the combustion process and leave the combustion chamber as bottom ash or fly ash. Soot is a product of incomplete combustion that consists of unburned carbon in the form of fine particles or as deposits on inorganic particles. High-molecular-weight organic compounds condense on the surface of the particles, particularly on the carbon, downstream of the combustor.
There are four general methods for limiting particulate emissions from waste combustors
-
Limiting the ash content of the waste feed via source control or selection.
-
Designing and operating the primary combustion chamber to minimize fly-ash carryover.
-
Designing and operating the combustion chamber(s) in accordance with good combustion practice to minimize soot formation.
-
Using well-designed and well-operated fine-particle APCDs.
Source control of ash-producing waste constituents is an obvious method to reduce particulate emission, but it is impractical for most waste combustors. However, some incinerators and boilers burning liquid hazardous waste are able to meet particulate matter emission limits by stringent source selection alone.
The first three methods listed above are effective in reducing particle loadings in the combustion gas but are generally not sufficient by themselves to meet current and proposed maximum-available-control-technology (MACT) emission standards for particulate matter. Add-on particulate control is expected to be needed to meet the proposed MACT standards for waste incinerators.
Fine-particle control devices are in three general categories: filtration collectors, including primary fabric filters (baghouses); electrostatic collectors, including dry and wet electrostatic precipitators (ESPs) and ionizing wet scrubbers; and wet inertial-impaction collectors, including venturi scrubbers and advanced designs that use flux-force condensation-enhancement techniques.
Acid Gases
Acid gases are flue-gas constituents that form acids when they combine with water vapor, condense, or dissolve in water. Acid gases include NOx, SOx, HCl, hydrogen bromide, hydrogen fluoride, and hydrogen iodide. HCl and SO2 are often present in uncontrolled flue-gas streams in concentrations ranging from several hundred to several thousand parts-per-million-by-volume. The concentrations of NOx, hydrogen fluoride, and sulfur trioxide are typically below several hundred parts-per-million-by-volume. Free halogens such as chlorine, bromine, and iodine can also be produced at low concentrations from combustion of wastes that contain compounds of those elements.
Emissions of SO2, HCl, and the other halogen acids can only be controlled through the use of add-on APCDs, which have been previously described in this chapter.
There are two sources of NOx from incineration (and other combustion) processes, commonly referred to as thermal NOx and fuel NOx. Thermal NOx is formed by the reaction of nitrogen and oxygen in the combustion air. Its forma-
tion is favored by high temperature (i.e., flame zone temperature), relatively large residence time at this temperature, and higher oxygen concentration.
Fuel NOx is formed by the oxidation of chemically-bound nitrogen in the waste (or fuel). Conversion of bound nitrogen to NOx is strongly influenced by the localized oxygen concentration; it is less sensitive to temperature than thermal NOx formation. Fuel NOx formation can exceed thermal NOx formation by an order of magnitude in incinerators burning wastes containing bound nitrogen.
NOx formation can be reduced, to a degree, by furnace design and combustion process changes as described earlier in the chapter. Add-on controls are required for more effective removal.
Mercury
Heavy metals in waste are not destroyed by incineration. Metallic elements with high vapor pressures, or with compounds that have high vapor pressures, can be converted to the vapor phase in the combustion chambers and tend to condense as the flue gas is cooled. They can adsorb onto fine (generally submicrometer) particles. It is likely that mercury remains in the vapor phase in the air-pollution control section of the incineration process, depending on temperature, and the same may be true for some of the more-volatile metal compounds.
Mercury emission from waste combustors is determined largely by the mercury feed rate and by whether mercury-specific APCDs are used. Virtually all mercury species found in wastes are volatile at combustion temperatures, so there is a high degree of partitioning to the gas phase, regardless of the chemical form of mercury or the combustion-system operating conditions. There is evidence that mercury is present primarily as elemental mercury vapor at incinerator combustion temperatures. The rate of cooling in the air pollution system and the HCl/Cl2 concentrations in the gas affect the conversion of elemental mercury to water-soluble mercuric chloride (Gaspar et al. 1997; Chambers et al. 1998; Gaspar 1998).
Mercury emission has been limited through operator control of waste feed rates. Conventional APCDs—such as fabric filters, ESPs, inertial-impaction scrubbers, and other wet scrubbers are at best only partially effective for mercury removal at normal operating temperatures. Traditional wet-scrubber APCDs have provided moderate (20-90%) mercury control efficiencies. The most-modern facilities use powdered activated-carbon injection into the gas stream for mercury removal. The best performances of conventional APCDs are typically those of wet scrubbers operating at saturation temperature or lower. Lower scrubber-water temperatures lead to vapor condensation, and reduced mercury vapor pressure. Soluble forms of mercury, such as HgCl2, are preferentially removed in wet scrubbing systems.
For high efficiency (>90%) mercury removal, many municipal solid-waste combustors and a smaller number of hazardous-waste and medical-waste incin-
erators have adopted powdered activated-carbon injection in tandem with alkaline reagents upstream of dry particle collection devices, usually fabric filters.
Lead
Lead (Pb) emissions from waste incinerators are influenced by the concentration of Pb in the waste feed, the chemical form of Pb, the physical matrix of the waste, the degree of ash carryover from the primary combustion chamber, thermal conditions in the primary and secondary combustion chambers that affect Pb volatilization, and the air-pollution control system efficiency for fine-particle removal from the gas. The method of feeding waste to the combustor chamber (in batches vs. continuous feeding) can have an indirect effect on Pb emissions.
The concentration of Pb in the waste is important because Pb is conserved in the combustion process; all the Pb fed to the combustor exists with the bottom ash, is collected as fly ash, or is emitted as fine particles in the stack gas.
The chemical form of Pb, the feed location and physical waste matrix, and local temperature in the combustion system are important because they affect the extent to which Pb is vaporized in the combustion process. Volatile forms of Pb, such as PbCl2, might vaporize completely in the combustion process, whereas nonvolatile species, such as PbO, tend to partition to the bottom ash in the primary combustion chamber. Pb in liquid wastes fed through burners is exposed to flame temperatures and is, thus, more likely to vaporize than Pb in solid wastes. Pb in combustible solid wastes (e.g., paper or plastics) will vaporize to a greater extent than Pb in mostly noncombustible items, such as glass. The combustion-chamber temperature profile also affects the vapor pressure and degree of volatilization of the Pb species.
The extent of Pb vaporization in the combustion process is important because it affects the distribution of Pb among the fly-ash particle-size fractions. Pb that does not vaporize during combustion either partitions to the bottom ash or carries over as fly ash with a particle-size distribution characteristic of the incoming waste material. Pb that does vaporize, however, recondenses in the cooler downstream air-pollution control environment and adsorbs to the finer particles. The finer particles are more difficult to remove from the gas. Thus, Pb-removal efficiency tends to be lower than the overall particle-removal efficiency. The behavior of Pb and other metals in the combustion environment has been extensively studied by EPA and others (Campbell et al. 1985; Barton et al. 1987, 1990, 1996; Fournier et al. 1988; Fournier and Waterland 1989; Carroll et al. 1995).
The design and operation of the primary combustion chamber as they affect ash carryover and the design and operation of the APCD also influence Pb emissions. The principles are the same as those described earlier for particulate-matter emission control.
In summary, there are four general methods for limiting Pb emissions from waste combustors:
-
Limiting the Pb content of the waste feed via source control.
-
Designing and operating the combustion process to minimize Pb vaporization.
-
Designing and operating the primary combustion chamber to minimize fly-ash carryover.
-
Using well-designed and properly operated APCDs.
From a practical standpoint, the second method is likely to be the most difficult to implement because the objective of the combustion process is to burn all the waste completely. The most-reliable methods of limiting Pb emissions are source control and good particulate APCD performance.
Products of Incomplete Combustion
Organic and inorganic substances that are broken down into free-radicals (molecular species possessing an unpaired electron) in the combustion unit sometimes do not combine with oxygen or hydroxyl radicals and instead combine among themselves to form many organic compounds. Most of these compounds can be destroyed in the postflame zone of a well-designed incineration system. Such compounds that are not combusted and released into the exhaust gas are called products of incomplete combustion (PICs). PIC emissions heavily depend on combustion conditions, which, in turn, depend on the design and operation of the combustion device. Depending on the temperature, some of the heavy organic constituents can condense onto fine particles. Examples of PICs are CO and trace organic chemicals. (The latter can also be remnants of the original feed stream.) PICs include simple compounds (e.g., methane, ethane, acetylene, and benzene), dioxins and furans, partially oxidized organic compounds (e.g., acids and aldehydes), and polycyclic aromatic hydrocarbons.
Dioxins and Furans
As discussed in Chapter 5, dioxins and furans are the most-hazardous organic PICs that have been found in the flue gas of any combustion device. (“Dioxins and furans” refers collectively to polychlorinated dibenzodioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs)). For poorly designed and poorly operated incineration facilities, the flue-gas dioxin and furan concentrations can be much higher than those generated by typical combustion devices. The polybrominated analogues have also been found in incineration emissions (see for example, Sovocool et al. 1989).
Modern incinerators produce dioxins and furans from three points in the
process: stack-gas emissions, bottom ash, and fly ash. Often, bottom ash and fly ash are mixed for waste management purposes, but they may contain different amounts of dioxins and furans. With the exception of a few older wet-scrubber units, most municipal solid-waste incineration facilities are able to achieve zero discharge with respect to aqueous waste, so there are no major contaminated waste water streams.
Three possible sources of dioxin and furan emissions are the following: (1) uncombusted components of the original fuel (dioxins and furans are present in the materials that are thermally treated, and some quantity of this material survives thermal treatment); (2) formation from precursor compounds (dioxins and furans are formed from the thermal breakdown and molecular rearrangement of particular precursor compounds); and (3) de novo synthesis (dioxins and furans are synthesized from a basic chlorine donor, a molecule that takes chlorine to the predioxin molecule, and the formation and chlorination of a precursor) (EPA 1994b).
All types of organic chemicals, including polychlorinated dioxin and furans, can be destroyed under high-temperature oxidizing conditions. Destruction can occur at around 1800°F or higher if oxygen and organic molecules are well mixed as in practical combustion devices. Destruction of polychlorinated dioxins and furans present in the waste feed stream can take place at temperatures as low as 1350°F if oxygen and organic molecules are perfectly mixed (Duvall and Rubey 1977; Dellinger et al. 1984). However, dioxins and furans are also produced within the incineration process from precursors that are not destroyed below 1,800°F. Lahl et al. (1990) suggest that, although dioxins and furans may be present in the incoming mixture, most of the dioxins and furans in the exhaust gases are the products of formation within the incinerator and not persistence of the compounds present in the waste stream.
It is known that the presence of catalytic metals (e.g., copper, nickel, zinc, iron, and aluminum and their salts) and the temperature range of 450-750°F can promote dioxin and furan formation (e.g., Stieglitz and Vogg 1987; Vogg et al. 1992). Traditionally, many APCDs are operated within that temperature range to avoid acid-corrosion problems. Other requirements for dioxin and furan formation include prolonged gas-residence time in the stated temperature range, the presence of carbon as gaseous PICs or particles, and the presence of chlorine as HCl, Cl2, or metal salt. Some types of organic compounds, such as chlorophenols and chlorobenzenes, tend to act as precursors for this type of secondary dioxin and furan formation. There is evidence that sulfur and ammonia can inhibit dioxin and furan formation.
As noted above, three sources have been proposed for the dioxins and furans found in the products of combustion. In addition, a substantial amount of research has been performed on effects of combustion conditions, facility configuration, waste stream composition, and pollution-control equipment. Siebert et al. (1988) investigated various factors associated with the operation of municipal
solid-waste combustors and found APCD outlet temperature, presence of acid-gas controls, and the startup year of the facility to be the most-important determinants of dioxin and furan formation. Fangmark et al. (1993) studied the effect of bed temperature, oxygen concentration, variations in HCl and water, and temperature and residence time in the postcombustion zone on dioxin and furan formation and concluded that postcombustion temperature was the most important. A study conducted for the American Society of Mechanical Engineers, ASME (1995), indicated that there was no statistically significant cross-incinerator correlation between chlorine content of the waste stream fed to incinerators, and the dioxin and furan concentration in the emissions of those incinerators. Numerous factors have been associated with dioxin and furan formation, including the presence of particulate carbon, metal catalysis, combustion efficiency, temperature, and presence of precursors. The only consensus at this point seems to be that good combustion efficiencies and low postcombustion temperatures reduce the secondary dioxin formation.
Dioxin and furan emissions can be controlled through good combustion practice and rapid cooling of the combustion gas to air-pollution control system temperatures (generally ranging from 285°F to 300°F). Rapid combustion-gas cooling is inherent in many wet-scrubbing system designs, except for units equipped with waste-heat boilers. A number of hazardous-waste incinerators equipped with wet scrubbers might meet regulatory standards without other addon control.
For cement kilns, analysis of the characteristics traditionally used to measure combustion efficiency (CO and total hydrocarbons) indicates that there is no substantial relationship between good combustion practice and dioxin emissions (CKRC 1995). There are two possible reasons for that. It is likely that the total hydrocarbons and CO are associated with the raw-mineral feedstock, rather than the fuel, and CO can result from nonequilibrium conditions in the kiln due to high combustion temperatures.
Dioxins and furans, as well as mercury, are removed by injection of powdered activated carbon in a number of municipal-waste incinerators and a few hazardous-waste incinerators.
STACK EMISSION RATE INFORMATION
Normal Operation
For several types of incinerators, measured emissions have been compiled for the purposes of selecting allowable emissions under regulatory standards. EPA compiled a database (dated February 1996) containing the results of hazardous-waste combustor trial burns and facility operating and design characteristics as part of the development of the April 1996 proposed “Maximum Achievable Control Technology” (MACT) standards for hazardous-waste combustors
(Fed. Regist. 61 (April 19): 17358). The database contained information from hazardous-waste facilities in three source categories: incinerators, cement kilns, and lightweight-aggregate kilns. The database also contained data on boilers, although the last were not subject to the proposed rule. However, test data are not available for all pollutants from all of these sources. The database was updated in December 1996 to correct entries and add new test data (Fed. Regist. 62(Jan. 7, 1997):960), so that it contains information on 122 incinerators, 43 cement kilns, and 17 lightweight-aggregate kilns. Not all these facilities burn hazardous waste, so not all were used in setting MACT standards (http://www.epa.gov/epaoswer/hazwaste/combust/v1 4tsds.htm gives access to a Portable Document File version of the database; the committee believes that a version in Paradox database format is also available). This test database remains the most extensive published source of emissions data for hazardous-waste combustors in the United States. However, there are certain limitations to these data that should be noted.
All these data are the result of discrete stack-sampling events, not continuous emissions monitoring that would reflect day-to-day operation. There is no reliable representative data base of continuous emissions measurements for any of the pollutants examined here.
Many of the emissions data are from trial burns, which do not reflect typical day-to-day operation. Trial burns of hazardous-waste incinerators are intended to establish operating permit limits as well as to measure emissions performance. To meet this purpose, trial burns are usually conducted at extreme combinations of operating conditions, such as minimum combustion temperature for organics emission testing; maximum combustion temperature for metals emission testing; minimum combustion residence time and maximum gas flow rate; maximum feedrates of ash-bearing waste, halogens, and metals; and worst-case air pollution control system operating conditions. As a result, the emissions data in the database may overstate normal operating emissions. Conversely, trial burns are likely to be better controlled and more highly supervised than the day-to-day operation. As a result, upset conditions may be less prevalent during the stack-sampling events, and such events are not characterized by this EPA data base.
The database was primarily compiled to evaluate the range of stack-gas concentrations found at hazardous-waste incinerators. Although there is sufficient information to estimate total emission rates, there is no information recorded on the subsequent efficiency of dispersion of those emissions (which is facility-specific, and not usually recorded in typical emission test reports), so that it is not possible to reliably estimate resultant population-exposure concentrations.
For medical-waste incinerators, EPA has estimated emission factors based on a limited number of emission tests as reported in a memorandum (EPA 1996a). This document cites the reports for the emissions tests used, but does not list the test results In addition, the stack-gas concentration information was given only in summary form in the report, although stack flow-rates are given
for some facilities. As a result, it is impossible to estimate facility-specific total emission rates or resultant population-exposure concentrations, although an attempt was made to estimate total national dioxin emissions using these (and other) data (National Dioxin Emissions from Medical-Waste Incinerators, Item IV-A-7 in docket A-91-61 at http://www.epa.gov/ttn/uatw/129/hmiwi/rihmiwi.html).
For municipal-waste incinerators, EPA has summarized stack-concentration test data for U.S. incinerators from a total of 104 reports dated 1987-1991 in a 1993 document “Emission Factor Documentation for AP-42, Section 2.1, Refuse Combustion.” (available at http://www.epa.gov/ttn/chief/ap42c2.html) Five incinerator designs (waterwall, refuse-derived fuel, modular starved-air, mass burn-refractory wall, and mass burn-rotary waterwall) are represented, and various control technologies are separately evaluated. In connection with proposed MACT rules, EPA compiled data on U.S. municipal incinerators in Dockets A-89-08, A-90-45, and A-97-45 (see http://www.epa.gov/ttn/uatw/129/medicalwastec/rimedicalwastec.html ). The EPA presented to the committee an update to January 1995 on the stack-gas concentration information for dioxin (Compilation of MWC Dioxin Data, Office of Air Quality Planning and Standards, July 27, 1995). That update included information on the latest test reports for 122 units at 71 facilities (there were approximately 160 facilities operating at that time); although the data were obtained by telephone and so may suffer from some quality control problems; and it appears that information for some facilities was averaged across multiple units; and some units had been modified specifically to reduce dioxin formation after the date of the last available test.
Stack-gas concentrations for dioxins (total tetra through octa CDDs and CDFs—toxic equivalent (TEQ) values can be obtained approximately by dividing these values by 50) spanned approximately a 20,000-fold range in 1995. Figure 3-2 shows the distribution of stack-gas concentrations (ng/dscm at 7% oxygen) for the 122 units mentioned in the EPA update to the committee (and also shows where the proposed MACT standards would fall).
This large range in stack-gas concentrations is apparently due to dioxin formation within APCDs if the temperatures range from 450 to 750°F. The range of stack-gas concentrations would be even larger than shown were it not for some corrective actions already taken by 1995 and reflected in the test information shown in the figure, and further actions were already agreed at that time for the highest emitters. For example, at the Pulaski, MD facility, 1993 tests showed concentrations of 3,313 to 9,045 ng/dscm in the five units. Interim measures (principally modification of water sprays to reduce the gas temperature into the ESPs, together with modification of combustion conditions) reduced the concentrations to 37 to 1,500 ng/dscm (reductions of approximately 4-fold for 4 units, and 240-fold for the fifth), and then-current regulations required a reduction to less than 60 ng/dscm by 1996.
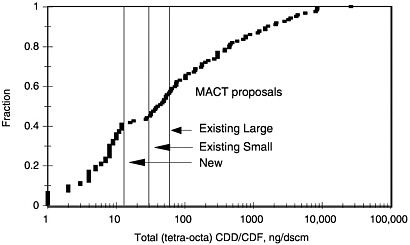
FIGURE 3-2 Distribution of stack-gas concentrations (ng/dscm at 7% oxygen) for 122 municipal solid-waste combustor units. The figure shows where the MACT standards would fall.
Similarly, initial tests at the Norfolk, VA facility showed dioxin concentrations of 21,129 to 42,995 ng/dscm before water sprays were installed to reduce temperatures to the ESPs, lowering concentrations to the range of 1,640 to 4,210 ng/dscm. One unit had already been retrofitted with a spray dryer/fabric filter APCD by March 1995, with all others to be retrofitted by 1996.
The reduction in stack-concentrations occurring after retrofit can be illustrated by the Detroit, MI facility. Initially all three units were equipped with ESPs, and a retrofit was initiated to spray dryer/fabric filters in 1987. In 1993 and 1994, the two retrofitted units showed stack concentrations in the range 2 to 10 ng/dscm. The third, nonretrofitted but otherwise similar unit, showed a stack concentration of 2,851 ng/dscm in 1993—the unit was to be retrofitted by 1996.
Webster and Connett (1998) evaluated the emissions from 81 (of about 160) municipal-waste incineration facilities over the period 1985 to 1995 using the same EPA memorandum augmented with some additional individual test reports. Their calculations confirmed the large range of total emissions from different facilities, the importance for national emission estimates of the largest emitters, and the large effect on such estimates of reducing emission rates for the large emitters. Retrofit or closing of several incinerators indicated a substantial decrease in total atmospheric emissions of dioxins from municipal-waste incinerators at the end of that period.
Off-Normal Operation
Stack-gas testing is usually performed under relatively steady-state, relatively normal conditions. For hazardous-waste incinerators, stack tests required in the permitting process are designed to be at the outer limits of normal operations, an approach that might result in higher-than-average emissions. However, there is always the option for stack testing under normal operating conditions (i.e., during normal plant operations), and this option has been used to develop emission estimates for use in evaluating average emissions for risk assessment purposes. Both types of testing are likely to miss periods of off-normal operation, including upsets, malfunctions, startups, and shutdowns. The last three terms have regulatory definitions (40 CFR 60), malfunctions specifically referring to sudden and unavoidable failures (not caused in whole or part by poor maintenance, careless operation, or other preventable upset conditions or preventable equipment breakdown).
Emissions during startup and shutdown are likely to be different in nature from those during regular burning of waste. For hazardous-waste and medical-waste incinerators, at least, startup and shutdown periods (without malfunctions) are defined in regulations to include only periods when the waste is not being burned (using auxiliary fuels to bring the facility to operational temperature, for example). However, emissions might also differ for the periods just after the beginning of feeding of waste into the incinerators, because this will induce some variations in operating conditions. Upsets may include any variation from normal operational conditions, and may or may not affect emission rates. Various attempts have been made to evaluate the effect of upset conditions on emission rates.
The effect of transient combustion upsets was tested in a Dow Chemical Company hazardous-waste incinerator in Louisiana (Trenholm and Thurnau 1987) that was burning solids (ram-fed drums every 4 minutes into the kiln, alternating types of solids), organic liquids (continuous feed to kiln and secondary chamber), and aqueous liquids (continuous feed to the kiln). It was found difficult to induce upset conditions (CO levels did not change on spiking the drums with 10 gallons of volatile hydrocarbons, or suddenly increasing the liquid waste feed). The final method was to triple the feed rate (2 gals/min to 6 gals/min) of liquid organic waste to the secondary combustion chamber for 7 seconds. The transients did not change average process conditions, but CO spikes to 700 ppm were obtained, increasing the average CO from around 0-3 ppm to 10-15 ppm, with highly variable total hydrocarbons (barely increased from a baseline 0-8 ppm in one run, increased to 60-150 ppm in two other runs). Particulate matter concentrations increased on average approximately 2-fold, while average concentrations of individual volatile organic hydrocarbons varied both up and down in a compound-specific manner.
In a series of tests on the Marion County WTE, the EPA evaluated the effect
of running at various operating conditions including low and high total air, low and high overfire air, and combinations of low load and high or low total or overfire air. Excess air varied approximately 2-fold from the baseline of about 72%, and CO stack concentration varied approximately 5-fold down and 1.15-fold up from the baseline of about 11 ppmv. Measured particulate stack concentrations were reduced around 25% under the off-normal conditions tested. Emissions testing was also performed on this facility during startup (beginning measurement when the waste ignited, and continuing for 4 hours) and shutdown (beginning 5 minutes before cessation of waste feed, and continuing for 3 hours, just after the forced draft fan was shut off). Baseline stack-gas total concentration of CDD/CDF was 2.2 ng/dscm (at 12% oxygen), with total concentrations of 3.47 ng/dscm during shutdown and 11.7 ng/dscm during startup. There was a considerable shift in congener distribution during these periods, with the corresponding 2,3,7,8-TEQs (I-TEF/87) being 0.063, 0.008, and 0.013, respectively.
Various tests have been performed on incinerators in addition to the empirical results found during the interim corrective measures described above. Most of these have been to evaluate the effect of process variations, rather than process upsets, but the results have implications for upsets. Six examples of such tests are summarized here.
During testing of the Prince Edward Island facility (Environment Canada 1985), low combustion temperatures were associated with increased dioxin emissions (see Appendix B, Box B-1). The Pittsfield, MA facility (NYSERDA 1987) was tested under variable conditions (see Appendix B, Box B-2). The results showed dioxin increasing with both too much and too little excess oxygen, that low primary combustion temperature substantially increased dioxin emission rates, and that high CO concentrations usefully indicated combustion conditions that also correlated with high dioxin concentrations. During testing at the Peekskill incinerator in Westchester County (NYSERDA 1989), two approximately hour long tests were performed during cold-start conditions (see Appendix B, Box B-3). Total CDD/CDF concentrations were 18-51 times baseline (normal operation) values at the ESP inlet, and 40-96 times normal values at the ESP outlet. CO was also elevated (5-57 ppmv normal, cold starts 114 and 180 ppmv at the superheater exit). A comparative report of these three early tests (Visalli 1987) stated that “test results indicate that levels of dioxin and furan in the flue gas entering a pollution control device are affected by different plant operating conditions if the conditions deviate sufficiently from normal operations, ” that furnace temperature can be used as a gross indicator of total dioxin and furan emissions, and that operating an incinerator at excess oxygen levels below about 5% may cause an increase in dioxin and furan emissions.
The Quebec City mass burn incinerator (Finkelstein et al. 1987) was tested under various operating conditions, some characterized as very poor (primary/secondary air ratio 90/10, high excess air of 115%), and poor (furnace temperature 850°C, 130% excess air, and primary/secondary air ratio 60/40) (see Appen-
dix B, Box B-4). Three other operating condition combinations, under low, design, and high load, were designated as good. Dioxin and furan emissions “become exponential” and were correlated with over 125% excess air, and also with departure from full operating load.
The Oswego mass-burn facility was tested in groups of three runs (NYSERDA 1990) to evaluate the effect of a clean combustion chamber (right after startup) versus end-of-campaign (just prior to maintenance shutdown), and (two groups of runs) the effect of secondary chamber temperature (see Appendix B, Box B-5). Low furnace temperatures were correlated with high dioxin and furan concentrations (5- to 6-fold increase) at the secondary chamber outlet and ESP inlet. The dioxin and furan concentrations were also highly correlated with high CO levels, particularly with upper percentiles of distributions of CO levels from a continuous emission monitor.
The Hartford refuse-derived fuel facility was tested to determine generation of trace organics and metals in the furnace under different process operating conditions (EPA 1994c) (see Appendix B, Box B-6). Steam flow rate (an indicator of load) and combustion air flow rates/distributions were the primary independent variables defining operating conditions as “good,” “poor,” and “very poor.” Dioxins, furans, CO, total hydrocarbons, PCBs, chlorobenzenes, chlorophenols, and PAHs were measured. Multiple-regression models were developed to evaluate the effect of various continuously monitored emission and process parameters on dioxin emissions (prediction models) and the effect of various combustion control measures on dioxin emissions (control models). The best prediction model showed that CO, NOx, moisture in the flue gas at the spray-dryer inlet, and furnace temperature explained 93% of the variation in uncontrolled dioxin emissions, with CO explaining 79% by itself. The best control model showed that refuse-derived fuel moisture, rear wall overfire air, underfire air flow, and total air explained 67% of the variation in uncontrolled dioxin emissions. Since CO was found to be such a strong predictor of dioxin emissions, the relationship was explored further. It was found that the fraction of time the CO level was over 400 ppm was quite strongly correlated to the amount of uncontrolled dioxins generated, particularly when examining only those runs where there was poor combustion.
In summary, these test results and empirical demonstrations, together with other lines of evidence (including other tests and laboratory demonstrations), show that dioxin and furan concentrations exiting the furnace are controlled by combustion conditions. Subsequently, dioxins and furans may be produced by reactions on surfaces in the flue-gas duct or in APCDs, with production rates increasing substantially above a certain temperature. Production of dioxins and furans during upset conditions are thus expected to rapidly increase outside a window of good-combustion conditions. Various monitors of these conditions (including CO emissions and temperatures throughout the flue-gas train) should thus correlate with dioxin and furan emissions, even during upset conditions.
FUGITIVE EMISSIONS
In most state-of-the-art municipal-waste incinerators, fugitive emissions, consisting of vapors or particles from waste tipping, waste feeding, incineration, and ash handling are mitigated by designing buildings to be under negative pressure. Air is drawn from the waste-handling areas into the combustion chamber, where it is mixed with the combustion gases. Potential fugitive emissions collected in this manner and drawn through the combustion chamber and emission-control devices leave the plant with odors virtually destroyed and dust removed by the particle-control devices.
Fugitive dusts can also be created in the bottom-ash pits and the fly-ash hoppers. Enclosed ash-handling areas are part of state-of-the-art municipal-waste incinerator designs, but older incinerators may not have such advanced enclosed ash handling. In the modern systems, emissions created in the ash-handling areas (bottom ash and fly ash) are drawn through the emission control devices so that workers are not unnecessarily exposed to dust from the ash. Such dusts, particularly fly-ash dusts from particulate APCDs, may be enriched in toxic metals and contain condensed organic matter.
At hazardous-waste incineration facilities, the most common fugitive emissions are (from liquid wastes) vapors from tank vents, pump seals, and valves; and (from solid wastes) dust from solid-material handling, together with possible fugitives from particulate APCDs. The magnitude of those emissions and their control mechanisms are similar to those in other process industries that handle hazardous materials and are therefore regulated under RCRA subpart BB. However, the high-temperature seals on rotary-kiln incinerators are a potential source of vapor and dust emissions peculiar to such incineration facilities; these emissions are controlled by maintaining a negative pressure in the kiln.
ASH AND OTHER RESIDUES
Types of Ash and Other Residues
Residues generated by incinerators include bottom ash, fly ash, scrubber water, and various miscellaneous waste streams.
Bottom ash is the remains of the solid waste that is not burned on the grate during the combustion process and consists of unburned organic material (char), large pieces of metal, glass, ceramics, and inorganic fine particles. Bottom ash is collected in a quench pit beneath the burnout section of the grate.
Fly ash is the solid and condensable vapor-phase matter that leaves the furnace chamber suspended in combustion gases and is later collected in APCDs. The APCDs in use since the middle 1980s capture a high percentage of the contaminants in the flue-gas stream. Fly ash is a mixture of fine particles with volatile metals and metal compounds, organic chemicals, and acids condensed
onto particle surfaces. It can also contain residues from reagents, such as lime and activated carbon, themselves with condensed or absorbed contaminants. Fly ash is collected in hoppers beneath the APCDs.
Scrubber water is a slurry that results from the operation of wet scrubbers and contains salts, excess caustic or lime, and contaminants (particles and condensed organic vapors) scrubbed from the flue gas.
In addition, there are various other waste streams that may be generated by the incinerator. For example, waste-to-energy plants produce blow-down water from the heat recovery boilers; some municipal solid-waste incinerators recover small quantities of condensed metals (e.g., lead alloys) from parts of their flue gas system. The initial sorting of municipal-solid waste produces a stream of large items unsuitable for burning (such as whole refrigerators, gas stoves, and auto batteries).
In 1995, the International Ash Working Group reviewed the available scientific data and developed a treatise on municipal solid-waste incinerator-residue characterization, disposal, treatment, and use (IAWG 1995). It found that the different temperature regimes in a municipal solid-waste incineration facility impart different characteristics to the residues collected from the various operational steps in a facility. Its report concluded that the development of management strategies for municipal solid-waste incinerator residues requires knowledge of the intrinsic properties of the material, including the physical, chemical, and leaching properties.
Cement kilns burning hazardous waste are in a class by themselves. All cement kilns are major sources of particulate emissions and are regulated as such by EPA and the states. Kiln-exhaust gases contain large amounts of entrained particulate matter known as cement-kiln dust, a large fraction of which is collected in APCDs. The kiln dust so collected is generally recycled to the kiln feed. Under the current BIF regulation, residue generated primarily by the combustion of fossil fuels may be exempted as RCRA hazardous waste provided that the facility operator can demonstrate that such wastes are no different from normal process residues or that any change caused by the combustion of hazardous waste as supplemental material in the fuel will not cause harm to human health or the environment. Cement-kiln dust is in that category.
Ash Handling
Two concerns of on-site ash management at incineration facilities are the safety of workers and the possibility that fugitive ash will escape into the environment during handling or removal of the ash for disposal. Both concerns require that the ash be contained at all times both inside and outside the facility, as described above. In the facility, water is used to quench the ash, simultaneously reducing dust generation and minimizing the possibility of ash-dust inhalation or ingestion by workers. In modern systems, a closed system of con-
veyors to transport the ash from the furnace to trucks helps to minimize worker exposure. Although some facilities have partially closed ash-removal systems, few have completely enclosed ash-handling systems throughout the plant.
Ash and Scrubber-Waste Disposal
Fly ash from municipal-waste incineration is characteristically more likely than bottom ash to exhibit the toxicity characteristic as defined by the RCRA leaching test as a result of high concentrations of lead or cadmium. Since 1994, it has been required that municipal-incinerator ash be tested to determine whether it is hazardous. If it is hazardous according to RCRA definitions, it must be disposed of as hazardous waste.
All residues generated by hazardous-waste incineration, except waste burned for metal recovery, are considered hazardous waste. That stems from the “derived-from” rule, which states that residues generated by the treatment of hazardous waste remain hazardous until delisted. Ash from hazardous-waste combustion must be handled and disposed in a secure hazardous-waste landfill that is designed to ensure that there will be no groundwater pollution. Under some circumstances, the ash can be classified as nonhazardous after a comprehensive test procedure, as provided under RCRA regulations.
The most common management method for ash generated by municipal solid-waste incineration is landfill disposal, either commingled with municipal solid waste or alone in an ash monofill, although some ash is used in production on construction materials, roadbeds, or experimental reefs.
Dry and spray-dry scrubber waste is incorporated in the fly ash, because the APCD is where the injected material is collected. Wet-scrubber wastewater is discharged to on-site wastewater-treatment systems, or discharged to the municipal sewer, after whatever pretreatment is required by local regulations.
SUMMARY
The pollutants of concern including dioxins and furans, heavy metals (in particular, cadmium, mercury, and lead), acid gases, and particulate matter, either are formed during waste incineration or are present in the waste stream fed to the incineration facility.
Emissions of dioxins and furans result, in part, by the processes in the combustion chamber that lead to the escape of products of incomplete combustion (PICs) that react in the flue gas to form the dioxins. PICs are formed when combustion reactions are quenched or incompletely mixed. The combustion chamber for incineration must therefore be designed to provide complete mixing of the gases evolved from burning of wastes in the presence of air and to provide adequate residence time of the gases at high temperatures to ensure complete reactions.
The operation of the combustion chamber also affects the emission of pollutants, such as heavy metals, that are present in the waste feed stream. Such compounds are conserved during combustion and are partitioned among the bottom ash, fly ash, and gases in proportions that depend on the compounds' volatility and the combustion conditions. Mercury and its salts, for example, are volatile, so most of the mercury in the waste feed is vaporized in the combustion chamber. In the cases of lead and cadmium, the partitioning between the bottom ash and fly ash will depend on operating conditions. More of the metals appear in the fly ash as the combustion-chamber temperature is increased. In general, there is a need for the combustion conditions to maximize the destruction of PICs and to minimize the vaporization of heavy metals. It is also important to minimize the formation of NOx (which is favored by high temperatures or the presence of nitrogen-containing fuels).
In addition to the composition of the waste feed stream and the design and operation of the combustion chamber, a major influence on the emissions from waste-incineration facilities is their air-pollution control devices. Particulate matter can be controlled with electrostatic precipitators, fabric filters, or wet inertial scrubbers. Hydrochloric acid (HCl) and sulfur dioxide (SO2) can be controlled with wet scrubbers, spray dryer absorbers, or (to a lesser extent) dry-sorbent injection and downstream bag filters. NOx can be controlled, in part, with combustion-process modification and with ammonia or urea injection through selective or nonselective catalytic reduction. Concentrations of dioxins and mercury can be reduced substantially by injecting activated carbon into the flue gas, or by passing the flue gas through a carbon sorbent bed, which adsorbs the trace gaseous constituents and mercury.
The application of improved combustor designs, operating practices, and air-pollution control equipment and changes in waste feed stream composition have resulted in a dramatic decrease in the emissions that used to characterize uncontrolled incineration facilities. For example, emission of dioxins from uncontrolled incinerators exceeded 200 nanograms/TEQ per dry standard cubic meter (200 ng/TEQ-dscm) in a number of commercial units. It has been reduced to below 0.1 ng/TEQ-dscm in many modern units. Rates of emission of mercury have decreased, at least in part, as a consequence of changes in the waste feed streams resulting from the elimination of mercury in some waste stream components, such as alkaline batteries.
To maximize combustion efficiency, it is necessary to maintain the appropriate temperature, residence time, and turbulence in the incineration process. Optimal combustion conditions in a furnace ideally are maintained in such a manner that the gases rising from the grate mix thoroughly and continuously with injected air; the optimal temperature range is maintained by burning of auxiliary fuel in an auxiliary burner during startup, shutdown, and upsets; and the furnace is designed for adequate turbulence and residence time for the combustion gases at these conditions. The combustion efficiency of an incinerator
|
BOX 3-1 Best Practices for Reducing Incineration Emissions a
a Optimization with respect to cost was not considered because it was not within the committee's scope of work. |
thus depends both on the design of the furnace and on operating practices. Furthermore, adequate operator training and certification is needed with monitoring of performance conditions to ensure that emission targets are met.
The committee has identified specific best practices for reducing incineration emissions primarily from municipal solid-waste incineration; see Box 3-1.
CONCLUSIONS
-
Waste-incineration technology and practice can be implemented under conditions that meet currently applicable and proposed emissions limits and other environmental regulatory constraints.
-
Emission data needed to fully characterize environmental concentrations for health-effects assessments are not readily available for most incineration facilities. Such information is lacking especially for dioxins and furans, heavy metals (such as lead, mercury, and cadmium), and particulate matter. The variation of these emissions over short and long time frames needs to be taken into account to characterize environmental concentrations fully, but the data are not available. Variations over short periods can result from process upset conditions; variations over long periods can result from replacement of less-efficient incineration facilities with modern low-polluting units.
-
The characteristics of incineration emissions and residual ash are affected by the wastes fed to an incineration facility, its combustion efficiency, and the degree of emission control of that facility.
-
Improving the combustion efficiency of an incineration process by optimizing combustor operations will reduce the quantity of soot produced and will lessen the formation of PICs, such as dioxins and furans. However, one must take into account the potential to increase the heavy-metal content in the emissions due to volatilization resulting from the higher combustion temperatures needed to improve combustion efficiency.
-
Emissions from incineration facilities are reduced by modifying operating characteristics—such as furnace temperature, air-injection rate, flue-gas temperature, reagent type, and injection rate, and by selecting optimal combustor designs and emission-control technologies.
-
Use and continued calibration and maintenance of continuous monitors of emissions and process characteristics provide real-time feedback and facilitate maintenance of optimal operating conditions at all times by incineration operators.
-
Continuous emission monitors (CEMs) for CO, O2, SOx, NOx, and HCl are available and have been certified by jurisdictions in this country and in other countries. CEMs for particulate matter and total mercury are under development and are in the process of being certified.
-
Emissions from incineration facilities can be reduced by choosing advanced combustion designs and emission-control technologies for the pollutant of concern and by having well-trained and certified employees who can help to ensure that the combustor is operated to maximize combustion efficiency and that the emission control devices are operated to optimize conditions for pollutant capture or neutralization.
-
If emission rates are desired that are lower than current and proposed regulations require, incineration and emission-control technologies and
-
operating practices exist to reduce emissions further. Some modifications involve purchase of equipment, and others require greater use of reagent or changes in other process conditions. Others simply require vigilant monitoring and adjustment of process conditions.
RECOMMENDATIONS
-
Government agencies should conduct studies of incineration facilities to characterize emissions and process conditions during startup, shutdown, and other upset conditions. Studies should consider variations in the waste's heating value over normal operating range, and variability over winter and summer conditions.
-
Emissions from small, as well as larger, incineration facilities need to be evaluated. In conducting post-MACT assessments, EPA should evaluate all types of facilities, even those exempt from the regulations.
-
Government agencies should encourage the development and adoption of continuous-emission-monitoring technology. These data should be made easily available to the public routinely. Experiences of other countries should be considered. Continuous emission monitoring of particulate matter and other pollutants of concern from incineration processes, such as mercury, should be implemented when practical and reliable techniques are available.
-
Consideration should be given to establishing a certification procedure for municipal solid-waste incinerator control-room operators. Certification standards have been developed as part of the American Society of Mechanical Engineers, standards for qualification of resource recovery operators, medical-waste incinerator operators, and hazardous-waste incinerator operators. Renewal of certification should include retesting on new techniques, practices, and regulations.
-
Government agencies should gather and disseminate information on the effects on emissions and ash as a result of various operating conditions, such as furnace and downstream flue-gas temperatures, reagent types and injection rates, and air-injection adjustments. Such guidance should show how specific emissions and ash characteristics are affected by modifying these process conditions. Government agencies should also compile and disseminate to the public information regarding new combustor designs, continuous emission monitors, emission-control technologies, operating practices, and source reduction/fuel cleaning/preparation techniques, including records of environmental performance and effect on emissions and ash as shown in pilot and full-scale tests.
-
Emissions and facility-specific data should be linked to better characterize the contributions to environmental concentrations for health-effects assessments. Existing databases should be linked to provide easy access
-
to specific operating conditions of an incinerator, including temperature at inlet of air-pollution control device, exit-gas temperature, furnace temperature, stack height, stack diameter, flow rate, and temperature of the gases leaving the stack, injection rate of all reagents, local meteorological conditions, air-dispersion coefficients as a function of distance from the facility, and precise geographical location of the emission point (to within 10 meters). When it is appropriate, data should be standardized to 7% oxygen and measurements to units of dry standard cubic meters. All data collected should be easily accessible by the public.





































