4
Environmental Transport and Exposure Pathways of Substances Emitted from Incineration Facilities
The main pathway for pollutants to get into the environment from a waste-incineration facility is, as for many other sources, through emission to the atmosphere. A large number of substances have been detected—most of them at very low concentrations—in the gaseous and particulate emissions from waste incineration. Among the emitted pollutants are metals and other noncombustible matter; acid gases; and products of incomplete combustion that include a large number of organic compounds as well as oxides of nitrogen, sulfur, and carbon. These pollutants are partitioned among the gas and particulate phases of the stack emissions from an incineration facility. As the pollutants disperse into the air, facility workers and people close to a facility might be exposed directly through inhalation or indirectly through consumption of food or water contaminated by deposition of the pollutants to soil and vegetation. Other people can be exposed through a different mix of environmental pathways after the pollutants travel some distance in the atmosphere; go through various chemical and physical transformations; or pass through soil, water, or food. As part of estimating the amount of incineration-released contaminants that people are exposed to and the patterns of such exposure, investigators seek to track the concentration and movement of, and changes that occur in, the contaminants as they move through the environment from the incineration facility to a point of contact with people. Such information is also helpful in determining the contribution of incineration to the mix of environmental contaminants from all sources.
This chapter provides a review of the environmental dynamics of substances emitted from waste-incineration facilities and the pathways that could result in human exposure to such contaminants. The chapter is not intended to provide a
comprehensive examination of the many aspects considered because such an examination is beyond the committee's task. To illustrate some of the important considerations with respect to environmental dynamics and exposure, particular attention is given to the main substances of concern that are discussed in Chapter 5 from a health-effects perspective. The chapter also examines approaches for estimating environmental concentrations that are used to estimate human exposures. As an illustration of how incineration facilities contribute to environmental concentrations at different geographical scales and for different agents, information is provided on particulate matter, various metals (cadmium, arsenic, mercury, and lead), dioxin-like compounds, carbon monoxide, and hydrogen chloride.
TRANSPORT PATHWAYS IN THE ENVIRONMENT
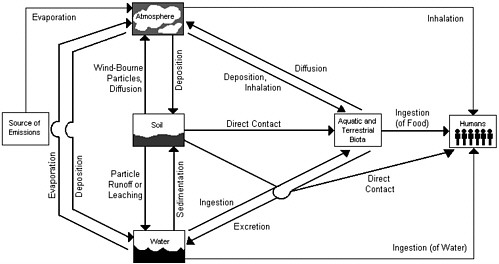
Substances released from combustion sources are ultimately dispersed among, and can at times accumulate in, various environmental compartments (e.g., soils, vegetation, indoor dusts, animals, and humans). Some contaminants that are released from incineration facilities are likely to contribute primarily to environmental compartments on a local scale (within 10 km). However, others that are more persistent in the environment, can be distributed over much greater distances— even up to a regional scale over hundreds of kilometers. Most of the substances released from incineration facilities to air do not remain in air but are deposited to soil, vegetation, or surface water and can come into contact with humans through a series of complex environmental pathways that include transport through several environmental media (see Figure 4-1).
As discussed in the great detail in Chapter 5, understanding the potential health impacts of waste incineration requires an understanding of the relative contribution of indoor, local, and regional sources of many pollutants. Therefore, an investigation must account for transport of pollutants through environmental compartments, and should examine large space- and time-scales, in addition to a combination of local environmental media over the short term. The required characterization of concentrations of contaminants in an environmental medium, such as air, involves accounting for the gains (or inputs to) and losses from that medium, and transport through it. For example, Table 4-1 lists the types of gains and losses that are considered in estimating the concentrations of contaminants in air.
In order to take account of the multimedia nature of pollutant transport, assessments usually examine multiple pathways that define the movement of a pollutant from a source, through a linear sequence of environmental compartments, to a receptor. An example of a particular pathway is a source emitting a pollutant to air (an air compartment), transport of the pollutant to the air above a field (another air compartment), deposition on vegetation (a vegetation compartment), eating of the vegetation by cows (an animal compartment), and drinking
TABLE 4-1 Modes of Gains and Losses of Contaminants in the Lower Atmosphere
|
Gains |
Losses |
|
Emission sources resulting from human activity, including incineration |
Washout by rainfall |
|
Convection to higher levels in the atmosphere |
|
|
Diffusion from soil |
|
|
Deposition on soil |
|
|
Diffusion from plants |
|
|
Resuspension of deposited soil particles |
|
|
Diffusion from surface water |
|
|
Deposition on plants |
of the cows' milk by humans (a receptor, exposed by the ingestion route). The pathway may be elaborated to almost any arbitrary degree, depending on how it is to be evaluated. For example, the deposition of a pollutant from air to vegetation may incorporate additional air compartments like a boundary layer of air around the vegetation, and a laminar flow layer of air above that, and so forth.
Multiple pathways may intersect one another in various environmental compartments, although each pathway individually usually does not self-intersect. Evaluation of each pathway individually is generally simpler than attempting to evaluate all simultaneously. The complexity of multiple connected compartments is reduced by examining pair-wise interactions between them.
The correctness of an approach for assessing the environmental transport of a specific substance depends on the linearity of the physical and chemical processes involved in pollutant transport with respect to pollutant concentrations in each compartment—fluxes between compartments usually depend linearly on the concentrations in connected compartments. If this linearity holds, a transport network through multiple compartments may be represented by the linear super-position of non-self-intersecting pathways. Where nonlinearities occur, the approach becomes less useful, and all compartments may have to be examined simultaneously, although it sometimes may be possible to contain all the nonlinearities within more-complex components of single pathways.
Persistence and Spatial Scale
Persistent air pollutants, such as dioxins, furans, and mercury, can be dispersed over large regions—well beyond the local areas and even the countries from which the sources first emanate. Many such pollutants are semi-volatile organic compounds (SVCs), with vapor pressures typically between about 10-1-10-5 Pascal at ambient temperature (Wania and Mackay 1993), but they also
include high-vapor-pressure metallic compounds (e.g., of mercury) and very low-vapor-pressure materials (e.g., most metals) incorporated in fine particles. The organic persistent SVCs tend to be lipophilic so that they readily partition into carbon and lipid tissues of plants and animals, and will often largely partition to the fine particles in ambient air. If they are resistant to physical, chemical, and biological degradation processes, they can persist for many years —such compounds have been labeled “persistent organic pollutants” (POPs) (Wania and Mackay 1993). Their small, but still significant, vapor pressure allows them to continually be re-emitted from the environmental sinks into which they partition. It has been postulated that, when released into the atmosphere, POPs tend to undergo a repeated deposition to and re-emission from soils, vegetation, and water, with transport effected in the vapor phase or adsorbed to ambient fine particles (Wania and Mackay 1993). ATSDR (1998a) reported a surprising extent of large-scale distribution and mixing for one class of POPs, the dioxins, which have been attributed to waste incineration. Similarly, efforts to assess pesticide POP use in various regions of the globe by sampling tree bark revealed a significant transport of such persistent compounds over a very large distances (Simonich and Hites 1995).
The recognition of POPs has created a need for environmental assessment and management strategies that provide an appropriate regional-scale framework for assessing the dispersion, persistence, and potential long-term impacts on human health and ecosystems. What is also needed is a process by which field data can be used to calibrate and validate models so that they can be used to inform control-strategy decisionmakers. For example, Scheringer (1996) has shown that the spatial scale needed to characterize the multimedia dispersion of organic chemicals is chemical dependent and should address the competition among reaction, atmospheric dispersion, and deposition. It should also address the impact of chemical partitioning into soil, vegetation, and surface water on the effective dispersion velocity in the air. According to Scheringer (1996), the effective dispersion velocity of a chemical is no greater than the average velocity of a parcel of air moving along the land surfaces. It is essentially equal to the velocity of the associated air parcel for high vapor-pressure compounds. However, the effective velocity is slower than the air-parcel velocity to the extent that a chemical partitions to particles, vegetation, surface water, and surface soil.
Efforts to move from an existing qualitative characterization of the large-scale disposition of POPs to a more-quantitative characterization are hindered by a number of scientific obstacles. One problem is the lack of a modeling framework that includes coupled mass exchange at boundaries of various environmental compartments and appropriately links the space- and time-scales involved in long-range transport. The low quality of many measurements of the large-scale partitioning of these chemicals between air and airborne particulate matter, between air and soils, and between air and vegetation, is another problem. For example, the measurements of vapor-particle partitioning that have been made
are known to have large artifactual biases due to the sampling methods used (Gundel et al. 1995).
Quantification of Local Air Dispersion
Substances in outdoor (or ambient) air are dispersed by atmospheric advection and diffusion. Meteorological conditions, local terrain, and facility designs have an overwhelming influence on the behavior of contaminants in the lower atmosphere. Wind (direction, speed, and turbulence) and atmospheric stability are the most important. The standard models for estimating the local time and spatial distribution of contamination in the atmosphere from point sources are the Gaussian statistical solutions of the atmospheric diffusion equation. These models are obtained from solution of the classical differential equation for time-dependent diffusion in three dimensions. Pasquill (1961) has discussed the physical basis, analytical solutions, and the use of these equations. Turner (1970) and Hanna et al. (1982) have compiled workbooks on applications of these solutions to air pollution problems, including the application of the Gaussian models to area and line sources. There are numerous computer programs available and many papers describing algorithms for assessing the dispersion of point (e.g., stack), line (e.g., roadway) and area (e.g., shopping mall) air pollution sources. The output of a standard Gaussian plume model can be expressed as the ratio of the atmospheric concentration to the source strength release rate. Typical units are µg/m3 per µg/sec, or sec/m3. This ratio is typically estimated using screening-level models such as SCREEN3 (EPA 1995a,b), or more complex, site-specific, models such as the Industrial Source Complex (ISC) models (EPA 1995a). (Such models are easily obtained from the U.S. Environmental Protection Agency at the following website address: http://www.epa.gov/scram001/.) For example, SCREEN3 provides a high-end estimate for the worst-case 1-hour average of this ratio as large as 0.05 sec/m3 for ground-level releases in urban areas, but the ratio typically decreases with the height of release. The annual average concentration-to-source ratio is likely to be about 0.08 (±0.02) times the maximum 1-hour average (EPA 1995b). The ISC models can provide specific estimates for any given location, and can also take account of simple, intermediate, and complex terrain; dry deposition; wet deposition; and plume depletion.
Simpler approaches to estimating the dispersion of substances in the atmosphere may be based on the application of a mass balance to a volume element, parcel, or box of air. This gives rise to the “box” models. In this approach, the region to be studied is divided into cells or boxes. The concentration in each box is assumed to be uniform and is a function of the box volume, the rate at which material is being imported, emission rates within the box, and the rate at which material is exported from the box. Such simplified approaches may be more appropriate than the Gaussian plume models in circumstances where dispersion
is not describable by Gaussian plumes. Also, such approaches may be sufficient to demonstrate that it is not necessary to go to the expense of employing more-complex models.
Deposition on and Accumulation in Soil
Soil is formed from the weathering action of climate on rocks and minerals and from the actions of living organisms. It is a mixture of minerals, water, air, and organic substances. The proportion of these components and the characteristics of the contaminants of concern determine, to a large extent, how such a contaminant is transported or transformed in soil. A contaminant can enter soil water, soil solids (mineral and organic phases), and soil air. Soils are characteristically heterogeneous in the vertical direction, so that a trench dug into soil typically reveals several horizontal layers that have different colors and textures.
Studies of radioactive fallout in agricultural land-management units have revealed that, in the absence of tilling, particles deposited from the atmosphere initially accumulate in and are resuspended from a surface-soil layer that is 0.1-1 cm thick (Whicker and Kirchner 1987). Over the long term, there is mechanical transport deeper into the soil (e.g., by earthworms, ants, rabbits, anything else that burrows, and by frost heave and wetting/drying cycles). Particles in the surface layer can be transported mechanically in the horizontal direction by runoff to nearby surface waters or be blown by wind. Surface-soil contaminants can be transported (on particles) by wind erosion, by volatilization to the atmosphere, by diffusion, leaching, and mechanical movement deeper into the soil, by erosion (attached to particles) or dissolution in runoff, and may be transferred to plant surfaces by rain splash or via resuspension and deposition. They can also be transformed through photolysis by sunlight, through chemical degradation, and through degradation by microorganisms (biodegradation).
The roots of most plants are typically confined within the top 3 ft (about 90 cm) of soil. Contaminants in this root-zone soil, below the surface layers, are transported upward by vapor-and liquid-phase diffusion, root uptake, and by capillary motion of water; they are transported downward by vapor- and liquid-phase diffusion and leaching; and chemically transformed primarily by biodegradation, hydrolysis, and other liquid and solid phase chemical reactions.
Deposition on and Uptake by Plants
By mass, the dominant component of the terrestrial biota is land plants. Plants generally have contact with two environmental media —air and soil. Uptake of contaminants by plants can occur directly from air via particle deposition or by foliar uptake of contaminant vapors. Particle deposition and foliar vapor uptake can also take place from contaminated soil (itself contaminated through various pathways from air contamination), through evaporation or suspension, or
through rain splash. Uptake from soil through roots is a relatively minor pathway for many pollutants emitted from incineration facilities. Whereas many inorganic chemicals enter plants via root uptake from soil, the translocation of many organic chemicals from soil through roots appears to be a relatively minor pathway for their accumulation in plants (Fiedler et al. 1991; Trapp and Matthies 1997). For modeling purposes, there has been a reliance on simple bioconcentration factors (BCFs) that relate a soil- or air-concentration to a plant concentration, based on experimental studies that correlate these uptakes with simple chemical properties like vapor pressures, solubilities, and octanol-water partition coefficients. The earliest use of vegetation BCFs (for inorganic contaminants) was for assessing the effects of global radioactive fallout by relating concentrations of radionuclides in plants to concentrations in soil (Ng et al. 1982). Vegetation BCFs have been proposed for organic chemicals for soil and vapor-phase uptake (Briggs et al. 1983; Travis and Arms 1988; Travis and Hattemer-Frey 1988; Bacci et al. 1990, 1992; Sabljic et al. 1990; Trapp et al. 1990; Paterson and Mackay 1991; Schreiber and Schönherr 1992; Hülster and Marschner 1993; McCrady and Maggard 1993; Lorber et al. 1994; McCrady 1994; Paterson et al. 1994; Simonich and Hites 1994a,b; Tolls and McLachlan 1994; and Nakajima et al. 1995).
Surface Waters and Sediments
The behavior of chemicals in surface waters is determined, among other factors, by the rate of physical transport in the water system and chemical reactivity. Physical transport depends to a large extent on the type of water body under consideration (e.g., ocean, sea, estuary, lake, river, or wetland). Schnoor and McAvoy (1981) have summarized important issues related to surface-water transport. At low concentration, contaminants in natural waters exist in dissolved (in the water) and sorbed (to suspended particles) phases. In slow-moving surface-waters, both advection and dispersion are important. In rapidly moving water systems, advection controls mass transport, and dissolved substances move at essentially the same rate as the bulk water. Contaminants that are sorbed to suspended solids (including colloids) can also be entrained in water currents, but they might undergo additional transport processes that alter their effective residence time in surface waters; such processes include agglutination of the suspended particles, sedimentation and deposition of solids, and their scouring and resuspension. Thus, determining the transport of contaminants in surface water requires an understanding of water movement, deposition to the sediment, and resuspension from sediment.
Sediment is the porous layer of solid material and water that forms at the bottom of water bodies primarily as a result of deposition of mineral particles and organic matter. Reuber et al. (1987) note that surface-water sediments have at least two distinct layers. One layer is an active layer characterized by a high
degree of chemical and biological activity. The other layer is a deeper, inactive layer in which chemicals are relatively isolated from the water column. Deposition and resuspension of mineral and organic matter to sediments occur continuously in any water body and are an important mechanism for transferring particle-bound contaminants to the sediment layer.
Multimedia Environmental Models
For substances released from waste-incineration facilities, the ambient concentration and deposition fluxes are determined by the partitioning and transport rates of the substances between the different compartments of the environment. Evaluating how chemicals are transported between such compartments requires a model that characterizes multiple environmental media, (i.e., air, soil, vegetation, surface water, sediments, and so forth) in combination. Efforts to assess human exposure to contaminants in multiple media date back to the 1950s when the need to assess human exposure to radioactive fallout and releases led to an assessment framework that included transport both through and among air, soil, surface water, vegetation, and food chains (USNRC 1975, 1977; Hoffman et al. 1979; Moore et al. 1979; Baes et al. 1984a,b; Whicker and Kirchner 1987). Efforts to apply such a framework to nonradioactive organic and inorganic toxic chemicals have been more recent and now are becoming as sophisticated as those extant in the radionuclide field. The first widely used multimedia compartment models for organic chemicals were the “fugacity” models described by Mackay (1991).1 Fugacity models have been used extensively for modeling the transport and transformation of nonionic organic chemicals in complex environmental systems. Modified fugacity and fugacity-type models have also been used for ionic-organic and inorganic species, including metals. The advantage of the typical multimedia fugacity-type model is the simplicity with which it treats each of the compartments as being well mixed, and allowing for flows and mass transfer between all compartments, and degradation within compartments. Such treatment is clearly an oversimplification but the models, by the judicious selection of compartments to correspond to the penetration depth of the pollutants, can lead to insightful conclusions on the major pathways, reservoirs, and persistence in the environment.
More-recent multimedia models used for assessing releases from incinerators use various approaches. Air dispersion is handled by standard Gaussian plume models, with modification to incorporate wet and dry deposition of materials from the plume. The deposition models are multi-layer transport models, incorporating a well-mixed upper layer in the main plume, an intermediate shear
|
1 |
The term “fugacity” is used in thermodynamics to refer to a measure of the tendency of a substance to escape by some chemical process from the phase in which it exists. |
layer where the wind-speed increases regularly with height, and boundary-layer near the ground or vegetation surface. Transport of material deposited on the ground is handled largely by compartment models, with pathways of human exposure elaborated to varying degrees, with inter-compartmental transfer rates based on physical modeling, empirical correlations, or fugacity-type approaches. Examples of such models, with descriptions, are given in Lorber et al. (1994); Slob et al. (1993); EPA (1990, 1997b, 1998a).
ASSESSING HUMAN EXPOSURE TO ENVIRONMENTAL CONTAMINANTS
The issue of assessing human exposure to contaminants has been addressed in previous reports of the National Research Council (e.g., NRC 1991b, 1994). Exposure to a substance of concern is defined as contact at a boundary between a human and the environment at a specific concentration for a specific period (NRC 1991b). Human exposure assessment involves measuring or estimating the concentrations of specific substances in each exposure medium, and the time individuals or populations spend in contact with each such medium. Human activity patterns directly affect the magnitude of exposure to substances present in different indoor and outdoor locations. Assessing exposure to contaminants emitted as a result of waste incineration involves characterization of the rates and patterns of incineration emissions, tracking of the emitted material through the environment, and characterizing the amount of human contact with the material. In addition to incineration, other sources (for example, motor vehicles, coal-fired power plants, industrial manufacturing facilities, and some naturally-occurring sources) contribute to the total concentration of contaminants to which humans are exposed. Sexton et al. (1994) and Pirkle et al. (1995) discuss data bases that are available to help establish total exposure concentrations. Incineration facilities add some incremental amount to the total ambient concentrations in the environment for many pollutants, such as nitrogen oxides, sulfur dioxide, particulate matter, volatile organic compounds. For selected pollutants, such as dioxin, incinerators might collectively contribute major fractions of observed ambient concentrations as discussed later in this chapter. A particular incinerator, however, might be the dominant source at a particular location for concentrations of nitrogen oxides, sulfur dioxide, or particulate matter, and may, but not necessarily, be the dominant source for the dioxins.
Exposures to a substance of concern might be dominated by contacts through a single environmental pathway or they might reflect contacts through multiple pathways. Table 4-2 shows some of the pathways of exposure. All possible routes by which contaminants enter the body of an exposed person must be considered —inhalation, ingestion of food or drink, and absorption through skin because such patterns directly affect the magnitude of exposures to substances present in different indoor and outdoor environments.
TABLE 4-2 Examples of Pathways Linking Ambient Airborne Contaminants to Human Exposure
|
Exposure route |
Pathway from ambient air |
|
Inhalation |
|
|
Ingestion |
|
|
Dermal contact |
|
Exposure Pathways
Models have been developed for the multimedia transport of pollutants, and the uptake by the food chain, leading to estimates of human daily intake for various scenarios of human activity. The number of processes modeled is large and the uncertainty in the calculated results, particularly for some of the more-complex pathways, is correspondingly large. Such models might yield estimates of total exposure that can be an order of magnitude in error,2 but such an uncertainty is the norm in risk estimates (and is generally far smaller than the variability in exposures between individuals, and the uncertainty in toxicity values). The models nevertheless are extremely useful in identifying the major pathways of exposure, the major reservoirs for contaminants (e.g., PCBs in sediments), and the approximate residence time in the environment of the contaminants. Inhalation is the most direct path for exposure to pollutants emitted from incinerator stacks and dispersed into the atmosphere. For the pollutants of greatest concern (see Chapter 5), however, the combination of long-range transport, deposition, and uptake of the pollutants by the food chain appears to be the most important mode of exposure.
|
2 |
See Chapter 8 for a discussion of important sources of error. The degree of aggregation in the models, uncertainty in the input parameters, and different activity patterns contribute to uncertainty in the results. |
Application of various models suggests the major pathways of exposure. The following examples highlight the need to examine indirect pathways of exposure. For cadmium, illustrative of many metals, the major pathway of exposure is usually through the consumption of garden fruits and root vegetables, which absorb cadmium primarily through uptake via the root system. For dioxin (as modeled by the 2,3,7,8-TCDD congener and used to illustrate a highly lipophilic organic compound), the major pathway of human exposure is primarily through meats, especially beef, and dairy products. For mercury, an important exposure pathway is human consumption of fish contaminated by atmospheric deposition of the metal directly from air to surface water and by deposition from air to soil with run-off transport to surface water. Such examinations should include consideration of the pathway by which fish taken for food eventually reaches the table. Subsistence fishers and others who fish for themselves may be at higher risk than the rest of the population that consumes fish, which may have no excess risk whatever.
Uncertainty and sensitivity analyses performed on the calculation of the human exposure to dioxin from municipal-waste combustion indicate that the major uncertainties in the estimated exposure are due to the uncertainties in the deposition rate and ambient air concentrations, and, to a much lesser degree, in the transfer of substances through a terrestrial food chain (Cullen 1995).
ENVIRONMENTAL DYNAMICS OF AND POSSIBLE EXPOSURES TO VARIOUS SUBSTANCES
The following subsections separately discuss the environmental dynamics (transport and fate), and possible human exposures, to particulate matter, cadmium, arsenic, lead, dioxins and furans, carbon monoxide, and hydrogen chloride that are emitted from waste-incineration facilities, as well as other sources. After those subsections, studies are discussed that estimated environmental concentrations of contaminants contributed by waste-incineration facilities. In addition, Chapter 5 provides similar types of estimates for illustrations of health-effects considerations regarding particulate matter, dioxins, lead, and mercury.
Particulate Matter
The transport characteristics of particles depend on their size. Fine and coarse particles in ambient air differ in their chemical composition, solubility, acidity, sources and formation processes, atmospheric lifetime, infiltration indoors, and transport distances (See Table 4-3). Most airborne particles are quite small (less than 0.1 µm in diameter), but most of the particle volume (and mass) is found in particles with diameters greater than 0.1 µm (Whitby 1978). The size distribution of airborne particles is often multimodal. Distributions of particles measured in outdoor air in the United States are almost always bimodal with a
TABLE 4-3 Characteristics of Fine Particlesa Versus Coarse Particles
minimum between 1.0 and 3.0 µm. Fine particles are usually defined as those having an aerodynamic diameter less than 2.5 µm. Fine and coarse particles generally have distinct sources and formation mechanisms, although there may be some overlap. Fine particles are usually formed from gases in two ways: (1) nucleation (i.e., formation of new particles from low vapor-pressure substances present in vapor form, produced either from combustion or from chemical reaction of gases) and (2) condensation of gases onto existing particles. Particles formed from nucleation also coagulate to form relatively larger particles, although particles normally do not grow above 1.0 µm in aerodynamic diameter by these processes. Particles formed as a result of chemical reaction of gases are termed secondary particles because the direct emission from a source is a gas (e.g., SO2 or NO) that is subsequently converted to a low vapor-pressure substance (e.g., sulfuric acid, nitric acid) that subsequently nucleates or condenses. Examples include sulfates, some low-volatile organics, and ammonium salts. Such transformations can take place locally, during prolonged stagnations of ambient air, or during transport over long distances, and are affected by moisture, sunlight, temperature, and the presence or absence of fogs and clouds. In general, particles formed from these types of secondary processes will be more uniform in space and time than those that result from primary emissions. Particles directly emitted by sources, referred to as primary particles, are also found in the fine-particle fractions (the most common being particles less than 1.0 µm in aerodynamic diameter from combustion sources).
In contrast to fine particles, most of the coarse-particle fraction of ambient aerosol originated as particles emitted directly to the atmosphere, and some combustion-generated particles, such as fly ash and soot, might also be found in the coarse fraction.
Every particle in the atmosphere tends to settle to the ground through the effects of gravity, but the tendency to settle is opposed or abetted by other effects including electrostatic and aerodynamic forces. The net effect is that particles deposit to the ground at velocities that depend primarily on their particle diameter and density. For coarse particles, controlled primarily by gravity, the deposition velocity is proportional to the square of the particle diameter. For very fine particles, deposition is controlled more by electrostatic and other effects than by gravity, so that they deposit more rapidly than would be expected from gravity and their size alone. The result is that fine particles with aerodynamic diameters between 0.1 and 1.0 µm have the minimum deposition velocity of particles. Such fine particles will remain suspended for much longer times (on the order of days to weeks for fine particles as opposed to minutes to hours for coarse particles) and will travel much farther (i.e., hundreds to thousands of kilometers) than the coarse-particle fraction particles (i.e., kilometers to tens of kilometers) (Watson et al. 1995).
Fine particles originating outdoors infiltrate into homes and buildings to a greater degree than do coarse particles (Lioy et al. 1990). Indoor particulate matter (PM) levels are especially important because most people spend the majority of their time indoors, and thus a large amount of their exposure to PM may occur while inside. About 50-90% of the indoor fine particles are of outdoor origin (Clayton et al. 1993; Thomas et al. 1993). Spengler et al. (1981) found that for the Harvard Six City study, long-term mean infiltration of outdoor-origin PM2.5 (particulate matter of aerodynamic diameter 2.5 µm or less) was 70% for homes without air conditioning and 30% in homes with air conditioning. Koutrakis et al. (1992) using New York State data of homes without smoking or fireplaces found that 60% of the PM2.5 mass was from outdoor sources. Thus, ambient particles penetrate indoors and are available to be breathed into the lungs.
Because they can be transported long distances, penetrate indoors readily, reach deep into the lung, and are the particles most enriched in toxic compounds, it is the fine particulate matter which is of the greatest human-health concern when considering particulate matter or its precursors emitted as a result of waste incineration. The materials which are preferentially concentrated in the fine-particle fraction include volatile metals, such as cadmium and lead, and many low-volatility organic chemicals that adsorb to particle surfaces.
EPA (1998b,c) reports measured concentrations of ambient PM2.5 in the United States that range from 13.5 to 37 µg/m3 in urban areas and 3.1 to 21.6 µg/m3 in nonurban areas. Also, EPA (1998b,c) estimates that the mean PM 10 con-
centration in ambient air in 1997 was 24 µg/m3; the 10th percentile value was about 16 µg/m3 and the 90th percentile was 32 µg/m3.
Cadmium
Cadmium is released into the environment by human activities such as mining and smelting operations, fuel combustion, waste disposal and application of phosphate fertilizer or sewage sludges (Elinder 1985). Cadmium can be present in waste input to an incinerator in the form of the metal (e.g., as cadmium plating), salts, and alloys (e.g., some solders and batteries). It forms a number of salts, including cadmium chloride (CdCl2), cadmium sulfate (CdSO4), and cadmium sulfide (CdS). Cadmium and its salts can be vaporized during waste incineration and emitted to the air as chlorides, oxides, or in elemental form. Such vapors rapidly condense onto particles, either those emitted simultaneously or ambient particles. Because the condensation is to particle surfaces, the fine-particle fractions (with higher surface area per unit mass of particle) become relatively enriched in cadmium.
The sources, sinks, and distribution of cadmium in many ecosystems have yet to be fully evaluated, and cadmium transfer rates between the different compartments of the environment are only poorly known. Aspects of the global cycle of cadmium have been summarized by Nriagu (1980). The major natural sources of cadmium to the active parts of the environment are from mobilization of cadmium from the large reservoir that exists in the lithosphere. The major sink for cadmium that enters the active compartments is burial in freshwater or ocean sediments.
Behavior in the Environment, Pathways, and Exposure
In aqueous systems, water hardness and pH, determine the speciation of cadmium. In fresh water at typical environmental pH values of 6 to 8, Cd+2 is the predominant species (Bodek et al. 1988). In the presence of sulfide ions and under reducing conditions, cadmium sulfide is formed over a wide pH range. The resulting precipitation of cadmium sulfide can serve to control the effective solubility of cadmium in natural waters.
In aqueous environments, cadmium will partition between the aqueous and solid phases (e.g., between water and soil particles in soil). This partitioning is described by a distribution, or sorption, coefficient, Kd (in units of L/kg), that is the concentration ratio, at equilibrium, of a chemical species attached to solids or particles (mol/kg) to the chemical concentration in a solution, mol/L, with which the particles have contact. Several mechanisms define this partition relationship —including cation exchange, adsorption, speciation, co-precipitation, and organic complexation. Bodek et al. (1988) have reviewed and compared a number of sorption models for cadmium in soil-water and sediment-water systems. They report that, in soils, estimated Kd values range from 1 to 9,000, with a
typical value (at low water concentrations) on the order of 1,000; and that, in sediments, estimated Kd values range from 1 to 160,000, with a typical value (at low water concentrations) on the order of 6,000.
Plants are contaminated with cadmium via two routes—one is uptake of cadmium in soil through the roots and the other is deposition of cadmium from air onto leaf surfaces with translocation to other plant parts. Cadmium residues in plants are typically less than 1 mg/kg (IARC 1993).
The plant-soil partition coefficient, Kps, expresses the ratio of contaminant concentration in plant parts in mg/kg (plant fresh mass) to concentration in wet root-zone soil, in units of mg/kg. Root uptake of cadmium as Cd+2 in plants is passive and occurs though uptake by roots of cadmium dissolved in water; cadmium is highly mobile in plants and readily translocated to other plant parts (Bodek et al. 1988). Plant-soil partition coefficients have been reported in the range 0.015 to 2.1 mg/kg with a likely value on the order of 0.1 mg/kg (Bowen 1979; Friberg et al. 1979; Nriagu 1980; Baes et al. 1984a).
According to Bodek et al. (1988), airborne deposition is believed to contribute to concentrations of cadmium found in plant leaves. At low concentrations, the ratio of plant-leaf concentration to air concentration, when air and plant environments are in contact, can be estimated based on the balance of gains from wet and dry deposition versus losses by wash-off and plant decay.
Atmospheric emissions of cadmium from human sources are estimated to exceed those from natural sources by about an order of magnitude (IARC 1993). ATSDR (1997a) summarizes references indicating that the mean levels of cadmium in ambient air range from less than 0.001 µg/m3 in remote areas to 0.003 to 0.04 µg/m3 in the United States. Cadmium metal and cadmium salts exist in ambient air primarily in fine suspended particulate matter. When inhaled, some fraction of this particulate matter is deposited in the lung airways and the rest is exhaled. In urban areas, an individual who breaths 20 m3 of air will inhale about 0.2 µg/day cadmium.
Cadmium enters drinking water directly from pollution sources, deposition from air to surface water, soil runoff to surface water, or leaching from rocks and soils into ground water. The concentration of cadmium dissolved in the open ocean is less than 0.005 µg/L (Nriagu 1980; IARC 1993). The concentration of cadmium in drinking water is generally reported to be less than 1 µg/L but it may increase up to 10 µg/L as a result of industrial discharge and leaching from metal and plastic pipes (Friberg et al. 1974; ATSDR 1997a). An individual who consumes 2 L of water daily with a cadmium concentration of 1 µg/L will have an intake of 2 µg/d.
For aquatic organisms, the bioconcentration factor (BCF) provides a measure of chemical partitioning between tissue and water and has units of mol/kg (fish) per mol/L (water). Bodek et al. (1988) report both ocean- and freshwater-fish bioconcentration factors in the range 200-50,000 L/kg, with 2,000 L/kg being a typical value in this range of reported values.
Humans can be exposed to soil contaminants through soil ingestion and through dermal uptake following soil contact with skin. For metal contaminants such as cadmium, the amount of intake via these pathways is typically less significant than the amount resulting from inhalation, water intake, and food-consumption pathways (McKone and Daniels 1991). Levels of cadmium in soil vary widely. In nonpolluted areas, concentrations in top soil are about 0.25 mg/kg (ppm) (EPA 1985); whereas in polluted areas, levels of up to 800 mg/kg have been measured (IARC 1993).
Indoor dust may be contaminated by deposition of particles from the air (originating from an emission source, or from suspension of contaminated soil), or by tracking of contaminated soil from outside. Friberg et al. (1974) report that the concentration of cadmium in the dust within houses was related to cadmium concentrations on air particles more than to soil concentrations.
Food is the main source of cadmium for non-occupationally exposed individuals. The gastrointestinal uptake of cadmium from food is generally less efficient than from water or by the lungs, because cadmium binds to food constituents (IARC 1993). The average daily intake of cadmium through food varies among individuals and by geographical area. An assessment using a Total Diet Study estimates the daily dietary intake of cadmium to be almost 15 µg/day (Gunderson 1995). Chaney et al. (1999) report that when zinc is present with cadmium at a ratio that is typical of geological materials (i.e., 100:1); zinc inhibits plant uptake, transfer to edible tissues, and absorption of cadmium in the intestine. However, when cadmium is present without zinc, food-chain mobility is much greater.
Arsenic
During waste incineration, arsenic (As) can be mobilized and emitted to the air as various inorganic compounds or in elemental form. Arsenic has valance states of -3, 0, +3, or +5, and is generally found in waters as H3AsO4, H2AsO4-1, and HAsO4-2, as well as H2AsO3-1, and H2AsO4-1. The principal arsenic-bearing minerals include arsenopyrite (FeAsS), niccolite (NiAsS), cobaltite (CoAsS), tennantite (Cu12As4S13), enargite (Cu3AsS4), and native arsenic.
By 1990, 70% of U.S. consumption of arsenic became attributable to the wood preservative industry and 20% to agricultural uses (ATSDR 1998b). Arsenic is also used in glass, nonferrous alloys, and electronics. Arsenic is released into the environment by human activities including arsenical pesticide and preservative use, metal smelting, waste incineration, and coal combustion.
Behavior in the Environment, Pathways, and Exposure
The metal arsenic is insoluble in water (Weast et al. 1986). Trivalent arsenic compounds are quite soluble at ambient temperatures. Pentavalent arsenic
(AS2O5) is more soluble than trivalent. In aqueous systems, arsenic forms anions in solution, and thus it does not form complexes with simple anions such as Cl (Bodek et al. 1988). Anionic arsenic complexes behave like ligands in water. In aerobic aqueous systems, the arsenic acids H2AsO4-1 and HAsO4-2 are the dominant species in the pH range 2-11. Below pH 2, the arsenious acid, H2AsO3-1 can be the dominant species in reducing conditions and above pH 12, the arsenious acid, HAsO3-2 appears.
Bodek et al. (1988) have reviewed and compared a number of sorption measurements and sorption models for arsenic in soil-water and sediment-water systems. They report that, in soils and sediments estimated Kd values range from 15-5,500 mol/kg.
As a likely result of uptake in water, arsenic is absorbed by roots and readily translocated to other plant parts (Bodek et al. 1988). Plant-soil partition coefficients have been reported in the range 0.01-0.04 mg/kg on a dry-mass basis (Baes et al. 1984a), which corresponds to the approximate range of 0.002 to 0.008 on a fresh-mass basis.
According to Bodek et al. (1988), airborne deposition is believed to contribute to some of the concentrations of arsenic found in plant leaves. At low concentrations, the ratio of plant leaf concentration to air concentration when air and plant environments are in contact, can be estimated based on the balance of gains from wet and dry deposition versus losses by wash-off and plant decay. Bodek et al. (1988) report both ocean and freshwater fish bioconcentration factors in the range 10-500 L/kg.
Arsenic metal and arsenic compounds (with the exception of arsine gas) have low volatility and exist in air primarily incorporated as fine suspended particulate matter. When inhaled, some fraction of this particulate matter is deposited in the airways of the lung and the rest is exhaled. Mean concentrations of arsenic in ambient air are estimated to be usually in the range of less than 0.001-0.003 µg/m3 in remote areas and in the range of 0.02-0.03 µg/m3 in urban areas (ATSDR 1998b). Air concentrations of arsenic near nonferrous metal smelters were reported to reach 2.5 µg/m3 (Schroeder et al. 1987). Arsenic exposures can occur through contact with water, food, soil, and house dust in ways similar to those discussed for cadmium.
ATSDR (1998b) indicates that food is typically the greatest source of arsenic exposure for the general population. NRC (1999b) reports that one of the most comprehensive studies of arsenic in food was published in 1993 (Dabeka et al. 1993). The food groups containing the highest mean arsenic compounds were fish (1.66 µg/g), meat and poultry (0.024 µg/g), bakery goods and cereals (0.024 µg/g), and fats and oils (0.019 µg/g). The average daily dietary ingestion of total arsenic by Canadians was estimated to be 38.1 µg (48.5 µg for adults). Adams et al. (1994) estimates that food contributes 93% of total intake of arsenic in the United States, and seafood contributes 90% of that 93%.
Mercury
Mercury is a naturally occurring metal that combines with other elements, such as chlorine, sulfur, or oxygen, to form inorganic compounds. It occurs commonly in nature as the sulfide, cinnabar. Also, mercury combines with carbon to form organic compounds. Mercury is found in trace amounts in fossil fuels. Mercury is released into the environment by a number of human activities, which include incineration, fossil-fuel combustion (especially coal burning), pulp and paper manufacture, paint manufacturing and applications, preparation of amalgams for dental work, laboratory usage, battery applications, disposal of fluorescent lighting, cement manufacturing, fungicides, and medical application. In addition, soil degassing during natural fires, volcanic activity, and biogenic sources are important contributors to global emissions to the atmosphere (Nriagu 1990).
EPA (1997c) reported that U.S. annual anthropogenic emissions of mercury in 1994-1995 was 158 tons. The emissions of mercury from resource recovery facilities were found to decline with time over the period covered, 1985-1992. This is a reflection of the decline in mercury consumption in the United States, from 2,241 tons in 1980 to 793 tons in 1990, to 463 tons in 1995, with a proportionally larger decrease in consumer products which might end up in municipal solid-waste (mercury consumption for battery production in the United States decreased from 1,058 tons in 1980 to 117 tons in 1990). EPA (1997b) estimated that waste incineration contributed about 33% of the national mercury emissions in 1994-1995.
Behavior in the Environment, Pathways, and Exposure
Mercury (Hg) emissions from incinerators into the air are mostly in the form of either elemental mercury vapor or mercuric chloride (HgCl2). Mercury transport may be long range, with the distance of transport dependent on the rate of conversion of elemental mercury to the soluble mercuric ion. Mercury is washed from the atmosphere by rain (wet deposition), but also deposits (dry deposition) in both vapor and particulate form. Models of the fluxes of mercury have been developed for different watersheds (Porcella 1990; Fitzgerald and Clarkson 1991; Lindquist 1991; Hudson et al. 1994). Some of the mercury deposited in lakes or soils is converted to methylmercury by organisms in soils and water bodies. Methylmercury is of major importance because of its high lipid solubility. The bioaccumulation of mercury in fish is such that the concentration of mercury in fish is much higher than in the ambient water. A significant fraction of mercury input to water bodies might be taken up by the fish. For example, Porcella (1990) estimated that of the 1.5 g/year depositing into a seepage lake in Wisconsin, 0.2 g/year were taken up by the fish. Hall et al. (1997) reported that food
was the predominant source of mercury uptake in fish. A major path of human exposure to mercury is from eating fish from contaminated water bodies. The ability to model the pathways of mercury from the source of emissions to human uptake is constrained at present by the difficulty of calculating the interconversion of the elemental mercury and mercuric ion in the atmosphere (which determines the atmospheric lifetime of the mercury), and of determining the rate of methylation of mercury (which determines its uptake by the biota).
Because of the long range transport of mercury, regional average concentrations are uniform within a factor of two to three. The values reported for Wisconsin by Fitzgerald et al. (1991) are representative of continental values. These are a gas-phase concentration of 1.57 ng/m3 and a particulate concentration of 0.022 ng/m3. Mean values for the air over a forested watershed in Tennessee were 5.5 ng/m3 (Schroeder and Fanaki 1988), with the corresponding particle bound concentrations of 0.03 ng/m3. The particle-bound values can be seen to be less than 1% of the vapor values. However, particulate-mercury concentrations are greater in precipitation than in ambient air (ATSDR 1999).
Values are reported for concentration in rain of 10.3 ng/L, and a wet deposition rate of 6.8 µg/m2-year by Fitzgerald et al. (1991) for Wisconsin. Values reported by Glass et al. (1991) of 18 ng/L for rain and 15 µg/m2-year for Minnesota are within a factor of a little more than two of those reported in Wisconsin, and are supportive of the view that the volatile elements are uniformly distributed over wide areas.
Concentrations in freshwater fish are typically in the range of 0.1-1 µg/g fish. For example, trout from Lake Ontario had average values that declined from 0.24 µg/g in 1977 to 0.12 µg/g in 1988 (Borgmann and Whittle 1991). Fish from the Savannah River had concentrations of 0.10 to 0.72 µg/g (Winger et al. 1990). Although the concentration in fish and the water do not always correlate well because of interfering factors (such as age of the fish, pH, and the different bioavailability of various forms of mercury) the concentration in fish is of the order of a million times that in the water. The biological concentration factor of methylmercury in fish in a freshwater lake was 3 million L/kg (Porcella 1994).
Potential sources of general population exposure to mercury include inhalation, ingestion of drinking water and foodstuffs, and exposure through dental and medical treatments. Food, particularly fish consumption, is the major environmental path of exposure for mercury. Studies of the dietary intake conducted by the Food and Drug Administration show an average daily intake for adults of 0.03 µg/kg of daily weight remarkably independent of age and sex, or 2.1 µg/day for a 70-kg adult (Cramer 1994). Using a terrestrial food chain model, Travis and Blaylock (1992) estimated an average daily intake of 6.3 µg/day for adults with over 50% coming from fish intake. Other studies of dietary intake of mercury are presented in ATSDR (1999).
Lead
Waste products that contain lead include storage batteries, ammunition waste, solder, pipes, and other metal products; consumer electronic products; solid waste and tailings from lead mining; items covered with lead-based paint; and solid wastes generated by mineral ore processing, iron and steel production, and copper and zinc smelting. The general population can be exposed to lead in ambient air, foods, drinking water, soil, and dust (ATSDR 1997b). Table 4-4 presents estimates of environmental lead concentrations in remote, rural, and urban areas.
Metals processing is the major source of lead emissions to the atmosphere. The arithmetic mean concentration of lead in ambient air in 1997 is estimated by EPA to have been 0.04 µg/m3. EPA's estimate of the 95th percentile concentration is 0.12 µg/m3 and of the 5th percentile concentration is 0.01 µg/m3 (EPA 1998b,c). By comparison, the National Ambient Air Quality Standard (NAAQS) for this pollutant is 1.5 µg/m3 as an annual average. The highest ambient air concentrations of lead are found in the vicinity of ferrous and nonferrous smelters, battery manufacturers and other stationary sources of lead emissions (EPA 1998b,c). EPA estimates that less than 1% of the public water systems in the United Sates have water entering the distribution system with lead levels above 5 µg/L. Those systems are estimated to serve less than 3% of the population that receives drinking water from public systems (EPA 1991a). EPA also estimates that lead levels between 10 and 30 µg/L can be found in drinking water as a result of plumbing corrosion and subsequent leaching of lead (EPA 1989).
Atmospheric deposition is an important source of lead found in soils. The strong absorption of lead to organic matter in soil tends to limit the bioavailability of lead and thus it tends not to bioaccumulate in aquatic and terrestrial food chains. Lead can be added to food crops through uptake from soil, direct deposition onto crop surfaces from the atmosphere, during transport to market, food processing, and kitchen preparation.
ATSDR (1997b) reports that data from Phase 2 of the National Health and Nutrition Examination Surveys (NHANES) III (conducted during October 1991 to September 1994) indicate that the overall geometric-mean blood-lead level of the population aged 1 year or younger was 0.11 µmol/L (2.3 µg/dL). Among those aged 1-5 years, approximately 4.4% had blood-lead levels of 10 µg/dL, representing an estimated 930,000 children with levels high enough to be of concern.
Dioxins and Furans
Dioxins and furans refers collectively to polychlorinated dibenzodioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs). Those chemical compounds are generally classified as halogenated aromatic hydrocarbons (HAHs).
TABLE 4-4 Environmental Lead Concentrations in Remote and Rural Areas and Urban Areasa
|
Remote and Rural Lead Concentration, µg/gb |
Reference |
Urban Lead Concentration, µg/gb |
References |
|
|
Air |
0.05 |
Lindberg and Harriss 1981 |
0.3 |
Facchetti and Geiss 1982; Galloway et al. 1982 |
|
Fresh water |
1.7 × 10-5 |
Elias et al. 1982 |
0.005-0.030 |
EPA 1986a, Vol. II |
|
Soil |
10-30 |
EPA 1986a, Vol. II |
150-300 |
EPA 1986a, Vol. II |
|
Plants |
0.18c |
Elias et al. 1982 |
950d |
Graham and Kalman 1974 |
|
Herbivores (bone) |
2.0d |
Elias et al. 1982 |
38d |
Chmiel and Harrison 1981 |
|
Omnivores (bone) |
1.3d |
Elias et al. 1982 |
67d |
Chmiel and Harrison 1981 |
|
Carnivores (bone) |
1.4d |
Elias et al. 1982 |
193d |
Chmiel and Harrison 1981 |
|
a Values can be highly variable, depending on organism and habitat location b Except µg/m3 in air c Fresh weight d Dry weight Source: NRC 1993. |
||||
Chlorinated and brominated dibenzodioxins and dibenzofurans are tricyclic aromatic compounds with similar chemical and physical properties. There are 75 congeners of chlorinated dibenzo-p-dioxins. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is the most widely studied of these compounds. TCDD and chemically similar compounds are collectively called dioxins; TCDD serves as the reference compound for this class of compounds (EPA 1994b,d), but it represents a small portion of incineration emissions of PCDDs and PCDFs.
Sources
Historical records of the concentrations in sediment cores show that in the Great Lakes area the levels of dioxins started to rise greatly in the mid 1930s (Czuczwa and Hites 1986), a time corresponding to the growth in the production of chlorinated organic chemicals. The levels in the sediments began to decline in the 1970s, as particulate emission controls began to be imposed. Although such observations are consistent with combustion sources being a major source (particularly incinerators without heat recovery which were phased out in the 1970s), unidentified sources still might be dominant, because attempts at mass balances suggest that the observed deposition rates are greater than can be accounted for by known sources (Brzuzy and Hites 1996).
In addition, there are substantial differences between the homologue distribution of dioxins found in the environment and those emitted by incinerators—differences that cannot be explained by models of the environmental fate of dioxins from combustion sources. Other sources which may be important include the burning of wood treated with pentachlorophenols, secondary copper smelting, fireplaces, and motor vehicles.
Behavior in the Environment, Pathways, and Exposures
The 2,3,7,8 chlorinated dioxin and furan congeners appear to be resistant to natural degradation, bioaccumulate in many organisms and, possibly, biomagnify to the highest levels of the food chain (ATSDR 1998a). The dominant transformation processes affecting their fate are surface photolysis and gas-phase diffusion or volatilization with subsequent photolysis (Yanders et al. 1989). Models (ATSDR 1998a) of the behavior of TCDD show that it is transported primarily through the air and distributed regionally, and that it accumulates primarily in soils. The decomposition rates, both photochemical and bacterial, decrease with extent of chlorination so that the higher chlorinated PCDDs persist longer.
ATSDR (1998a) reports that most of the measurements of CDDs in air tend to be very close to current detection limits. CDDs are found at the greatest concentrations in urban air with octachlorinated dioxin (OCDD) being the most prevalent congener (up to 0.100 ppq), heptachlorinated dioxins (HpCDDs) being
the next most common congener, and 2,3,7,8-TCDD being the least common congener (0.014 ppq). Concentrations of all CDDs are highest in the air near industrial areas. Rural areas usually have very low or undetectable levels of all CDDs. In urban and suburban areas, concentrations of CDDs may be greater during colder months of the year when furnaces and wood stoves are used for home heating. Furst et al. (1990), Gilman et al. (1991), Theelen (1991), and Schaum et al. (1994) estimate that about 90% of human exposure to dioxin occurs via contaminated food, including human milk. Hattemer-Frey and Travis (1989) estimated that meat and dairy products accounted for 98% of the total intake of TCDD. The primary mechanism by which dioxin-like compounds enter the terrestrial food chain is suspected to be via atmospheric deposition. Support for this hypothesis includes studies that have measured dioxin compounds even in the most remote areas throughout the world, where atmospheric transport and deposition is the only plausible mechanism (ATSDR 1998a). Deposition can occur directly onto plant surfaces or onto soil. Soil deposits can enter the food chain via direct ingestion (especially applicable to children) or soil ingestion by food animals (cows, for example). Dioxin compounds become available to plants by volatilization and vapor absorption, root uptake or particle resuspension, and adherence to plant surfaces.
The multi-compartment fugacity model of Mackay, combined with the terrestrial food chain model, has been used to calculate the exposure to dioxins in Southern Ontario, Canada (Paterson et al. 1990). The tetra chlorinated, hexachlorinated, and octachlorinated dioxins were treated separately because of their different physico-chemical properties. Air, soil, water, and sediment were considered in the analysis. One major conclusion drawn from the calculations is that the soil compartment is the principal reservoir for the dioxins. From the concentrations and fluxes in the different compartments, the human exposure models are developed using the correlation of Travis and Hattemer-Frey (1988) on the assimilation of the dioxins in dairy products, beef, and vegetation. The results for the tetrachlorinated dioxins are shown in Figure 4-2. For the system modeled, fish consumption is shown to be the major pathway of exposure. The results obtained from the model are compared with experimental observations in Table 4-5, which show that the calculated concentrations in the different compartments are generally within one to two orders of magnitude of the measured values, with the calculated values being low. The authors indicated that these discrepancies could be a result of either the measurements being reported for more polluted areas, and therefore being high, or due to not accounting for all dioxin sources in the model input parameters. Despite their uncertainty, the models provide valuable insight as to which pathways to exposure are important and in which compartments in the environment dioxins accumulate.
The correlation between total exposure and local emissions is expected to be low because the pollutants are dispersed over wide areas, and the foods that are implicated as the major path of exposure will contain a fairly high portion of
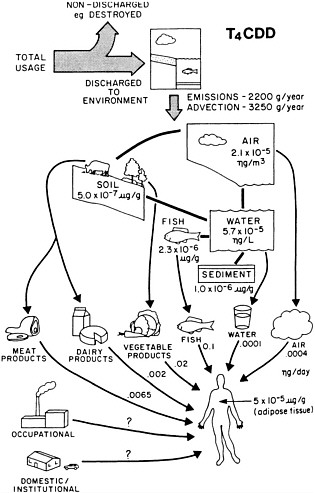
FIGURE 4-2 Estimated environmental compartment concentrations of and human exposures to T4CDD by various pathways in Southern Ontario. Emission and advection rates are provided at the top of the figure. Transfer rates are indicated along the arrows. Source: Paterson et al. 1990. Reprinted with permission from Emissions from Combustion Processes: Origin, Measurement, Control; copyright 1990, CRC Press, Boca Raton, Fla.
TABLE 4-5 Comparison of Predicted and Measured PCDDs for Southern Ontario
|
Medium |
T4CDDg |
H6CDDg |
O8CDDg |
|
Air (ng/m3) |
|||
|
Estimated |
2 × 10-5 |
2.2 × 10-5 |
3.8 × 10-5 |
|
Reporteda |
1 × 10-5 |
6 × 10-4 |
5 × 10-4 |
|
Water (ng/l) |
|||
|
Estimated |
5.7 × 10-6 |
2.7 × 10-4 |
3.8 × 10-4 |
|
Soil (mg/kg) |
|||
|
Estimated |
4.8 × 10-8 |
1.6 × 10-7 |
7.9 × 10-7 |
|
(<1-9) × 10-3 |
1.7 × 10-4 |
3.5 × 10-3 |
|
|
Sediment (mg/kg) |
|||
|
Estimated |
1 × 10-6 |
8.7 × 10-7 |
1.4 × 10-5 |
|
Reportedd |
2.6 × 10-5 |
1 × 10-5 |
5.6 × 10-4 |
|
Vegetation (mg/kg) |
|||
|
Estimated |
4.8 × 10-8 |
1.6 × 10-7 |
7.9 × 10-7 |
|
4 × 10-7 |
<1 × 10-8 |
<1 × 10-6 |
|
|
Reportedf |
<2 × 10-8 |
<3 × 10-7 |
1 × 10-6 |
|
Meat (mg/kg) |
|||
|
Estimated |
2,2 × 10-8 |
7.4 × 10-8 |
3.5 × 10-7 |
|
<1 × 10-6 |
<1 × 10-6 |
1 × 10-6 |
|
|
Reportedf |
<2 × 10-6 |
<1 × 10-6 |
(3-24) × 10-6 |
|
Dairy (mg/kg) |
|||
|
Estimated |
7.7 × 10-9 |
2.6 × 10-8 |
1.3 × 10-7 |
|
Reportede |
2.5 × 10-6 |
<1 × 10-6 |
3.6 × 10-5 |
|
Reportedf |
<1 × 10-7 |
<1 × 10-7 |
1 × 10-6 |
|
Fish (mg/kg) |
|||
|
Estimated |
2.3 × 10-6 |
1.3 × 10-5 |
1.8 × 10-5 |
|
Reportedb |
<1 × 10-6 |
||
|
Human Adipose Tissue (mg/kg) |
|||
|
Estimated |
4.8 × 10-5 |
5.3 × 10-5 |
3.9 × 10-5 |
|
Reportedc |
5 × 10-6 |
7.2 × 10-5 |
5.6 × 10-4 |
|
a Cantox Inc “Health Hazard Evaluation of Seicfic PCDDS, PCDFS, and PAH in Emissions from the Proposed Petrous/S.C. Resource Recovery Incineration and From Ambient Background Sources”, Petrous/S.C. Operations Ltd., Oakville, Ontario (February, 1988) b Travis, C. C., and H. A. Hattemer-Frey, “Human Exposure to 2,3,7,8-TCDD”, Chemosphere, 16:2331-2342 (1987) c Stanley, J. S., Boggess, K. E., Onstot, J., Sack, T. M., Remmers, J. C., Breen, J., Kutz, F. W., Carra, J., Robinson, P., and Mack, G. A., “PCDDs and PCDFs in Human Adipose Tissue from the EPA FY82 NHATS Repository”, Chemosphere, 15:1605-1612 (1986) d Astle, J. W., Gobas, F. A. P. C., Shiu, W. J., and Mackay, D., “Lake Sedimentation in Historic Records of Atmospheric Contaminants by Organic Chemicals”, pp. 57-77 in Sources and Fates of Aquatic Pollutants, R. A. Hites and S. J. Eisenreich, Eds., American Chemical Society, Washington, D. C., 1987. e Davies, K. “Concentration and Dietary Intake of Selected Organochlorines, including PCBs, PCDDs, PCDFs in Fresh Food Composites Grown in Ontario, Canada ”, Chemosphere, 17:263-276 (1989) f Ontario Ministry of Agriculture and Food, Ontario Ministry of the Environment, Toxics in Food Steering Committee. Polychlorinated Dibenoz-p-dioxins and Polychlorinated Dibenzofurans and other Organochlorine Contaminants in Food, Toronto. (1988). g “T4CDD” refers to tetra-chlorinated dioxin, “H6CDD” refers to hexa-chlorinated dioxin, and “O8CDD” refers to octa-chlorinated dioxin. Source: Paterson et al. 1990. Reprinted with permission from Emissions from Combustion Processes: Origin, Measurement, Control;copyright 1990, CRC Press, Boca Raton, Fla. |
|||
products imported from other regions. Hattemer-Frey and Travis (1989) concluded that emissions from municipal solid-waste incinerators do not substantially increase human exposure to CDDs and CDFs above normal background levels.
Carbon Monoxide
Outdoor sources contributing most carbon monoxide (CO) emissions are motor vehicle exhaust, industrial processes, nontransportation fuel combustion, and natural sources such as wildfires. Wood stoves, cooking, tobacco smoking, and space heating are major sources of indoor CO emissions (EPA 1998b,c).
In the atmosphere, CO is transported, by diffusion and eddy currents to the troposphere and stratosphere, where it is oxidized to carbon dioxide by hydroxyl radicals. In soil, microorganisms oxidize it to carbon dioxide. The oceans are reservoirs for CO.
Background levels of CO are quite low, under 1 ppm. Although annual average CO levels are usually below 9 ppm (the 8-hour National Ambient Air Quality Standard), there is great variability in urbanized areas, especially through the day, as a result of traffic patterns. It is unlikely that waste combustion is a major source of CO exposure to the general public.
Hydrogen Chloride
Hydrogen chloride exists as an aqueous acid aerosol in the environment. Anthropogenic and natural chlorine compounds have the same dispersion characteristics, and transport and mixing are similar. Dissociation of hydrogen chloride to form free chlorine atoms can occur by photochemical reaction or by reaction of hydrogen chloride with OH. These free chlorine atoms react by well-established pathways to destroy ozone in the stratosphere.
Gaseous chlorine compounds are removed by rainfall and adsorption onto particles. Chlorine is probably removed from the atmosphere by interaction with particles or water cloud processes. Background levels of particle phase chlorine are primarily due to ocean spray, while volcanic gases account for most gaseous hydrogen chloride. Ambient levels of particle phase chlorine compounds are about 3 µg/m3 in U.S. cities, while the total chlorine are about 10-100 µg/m3.
ENVIRONMENTAL POLLUTION CONCENTRATIONS ASSOCIATED WITH WASTE INCINERATION
At large distances, the only emissions from incineration facilities which need to be considered are those which contribute significantly to the total regional emissions. At short distances, a site-specific evaluation of the contribution of waste incineration to the local concentration of individual pollutants is needed.
TABLE 4-6 Qualitative Contribution to Air Emissions from All Sources, Dispersion Scale, and Routes of Exposure of Waste Incineration Pollutants
|
Pollutant |
Contribution to U.S. Emissions by Incinerators |
Geographic Scale of Dispersion |
Major Pathways of Exposure |
|
CO |
Minoa |
Local |
Inhalation |
|
HCl |
Minor |
Local |
Inhalation |
|
Dioxins |
Major |
Regional |
Food (especially meat, dairy products, and fish) |
|
PAH |
Minor |
Regional |
Food |
|
PCBs |
Minor |
Regional |
Food |
|
Mercury |
Major |
Regional |
Food (especially fish, meat, and dairy products) |
|
Cadmium |
Intermediate |
Local, Regional |
Food |
|
Lead |
Intermediate |
Local |
Variable, mainly food, such as cereals, vegetables |
|
Particles, fine |
Minor |
Regional |
Inhalation |
|
Particles, coarse |
Minor |
Local |
Inhalation |
|
a Major refers to >20%, Minor refers to < 1%. Source: Adapted from Koshland 1997. |
|||
The fraction of any pollutant transported over long distances depends on its environmental residence times in various environmental media. For organic compounds, the controlling factor is often an atmospheric residence time governed by a reaction rate with OH. Values of these for many of the air toxics in combustor effluents are provided by Koshland (1997). For inorganic elements which occur in the condensed phase or as soluble vapors, the atmospheric residence time is determined by the deposition velocity and rainfall events. Table 4-6 summarizes the scale of dispersion and the exposure routes for the pollutants for which the possible health impacts of waste incineration are presented in Chapter 5.
Estimates of Ambient Air Concentrations of Various Pollutants from Waste Incineration
Estimates of air pollutant concentrations associated with hazardous-waste incinerators and cement kilns that burn hazardous waste are provided here to give some idea of the contribution of an incineration facility to air quality. The estimates are not intended to represent the full range of air concentrations that might be associated with incineration facilities currently operating. These estimates of atmospheric concentrations were developed from the databases used by
EPA to support the Maximum Achievable Control Technology (MACT) standards as proposed in April, 1996 for hazardous-waste incinerators and cement kilns using hazardous waste as fuel. An approximate estimate of the impact of the proposed MACT on ambient air quality is also presented for illustrative purposes only. The emission rates were all summarized and analyzed for the shape of their statistical distributions. In all cases, there was a reasonable fit to a lognormal model. These distributions were multiplied together along with the probability distribution for unit air concentrations from Cullen (1995) using a Monte Carlo routine. The atmospheric concentrations were calculated by using 5,000 trials for each chemical. Results (rounded to two significant figures) are summarized in Table 4-7, Table 4-8, Table 4-9 through Table 4-10.
The primary uncertainty associated with this analysis is in Cullen 's unit air concentration distribution. This was apparently developed for a municipal solid-waste incinerator in Bridgeport, CT, for a radial area located between 0.4 km and 30 km from the stack—a distance that included the point of maximum ambient concentration. Thus, these results will reflect ambient air concentrations expected from the incinerators and cement kilns considered in this exercise only to the extent that the emission characteristics and the local meteorology of the Bridgeport, CT, incinerator are similar to others. It must be emphasized that a person could be exposed to higher or lower concentrations depending on where he or she was located with respect to any particular combustor, and depending on characteristics of that particular combustor.
In these calculations, the particulate-matter concentrations reflect total rather than respirable particles. It is not possible to generalize, with any certainty, regarding respirable particulate-matter concentration, because there is a lack of data concerning particle-size distributions in emissions. EPA (1992a) states that approximately 62% of the mass of the particles escaping a fabric filter for a municipal solid-waste incinerator are less than or equal to 12 µm in diameter and approximately 54% of the particles are less than 2.3 µm in diameter. These factors could be applied to the concentrations given for total particulate to yield an estimate of respirable particulate.
This analysis should be viewed as illustrative rather than comprehensive. One primary source of uncertainty is the assumption that one single probability distribution of dispersion coefficients applies to all incinerators in the database. The committee was unable to be more comprehensive because of the lack of any database that linked emissions estimates with dispersion estimates for individual facilities.
Post-MACT data were taken from information presented at the U.S. EPA Boiler and Industrial Furnace and Hazardous Waste Incinerator Technical Meeting, Kansas City, KS., March 7, 1996. The database presented at this meeting included mass flow (in pounds per year) in addition to gas flow in dry standard cubic feet per minute (dscfm) for 20 commercial hazardous-waste incineration units and 30 cement kilns using hazardous waste as fuel. It should be kept in
TABLE 4-7 Estimated Ambient Air Concentrations of Various Pollutants Associated with Hazardous-Waste Incinerator Emissions Prior to Compliance with MACT (All concentrations are annual averages in units of micrograms per cubic meter.)
|
Pollutant |
Number of Samples |
Mean |
Standard Deviation |
Median |
Range |
|
Mercury |
718 |
5.5 × 10-4 |
7.2 × 10-3 |
7.2 × 10-6 |
1.9 × 10-10 to 0.4 |
|
Lead |
789 |
2.2 × 10-2 |
0.2 |
2 × 10-4 |
2 × 10-8 to 7.2 |
|
PCDDS/F TEQa |
182 |
4.1 × 10-9 |
9.4 × 10-9 |
5.6 × 10-10 |
7.8 × 10-13 to 8.8 × 10-8 |
|
Particulate Matter |
633 |
8.9 × 10-2 |
0.31 |
1.5 × 10-2 |
8.6 × 10-6 to 7.7 |
|
a PCDD/F is the toxic equivalent of the sum of the polycholorinated dibenzodioxins and polychlorinated dibenzofurans. |
|||||
TABLE 4-8 Estimated Ambient Air Concentrations of Various Pollutants that may be Associated with Hazardous-Waste Incinerator Emissions After Compliance with MACT (All concentrations are annual averages in units of micrograms per cubic meter.)a
|
Pollutant |
Mean |
Standard Deviation |
Median |
Range |
|
Mercury |
8.7 × 10-5 |
4.6 × 10-5 |
7.9 × 10-5 |
9.3 × 10-6 to 4.6 × 10-4 |
|
PCDDS/F TEQb |
5.8 × 10-10 |
3.0 × 10-10 |
5.2 × 10-10 |
5.3 × 10-11 to 2.5 × 10-9 |
|
Particulate matterb |
0.20 |
0.10 |
0.18 |
2.2 × 10-2 to 0.96 |
|
a Based on the MACT emission limits that were proposed in April 1996. b PCDD/F is the toxic equivalent of the sum of the polycholorinated dibenzodioxins and polychlorinated dibenzofurans. |
||||
mind that an individual facility might have more than one unit. Data are presented in Table 4-7, Table 4-8, Table 4-9 through Table 4-10 for mercury, PCDDS/F TEQs, and particulate matter. Lead is not presented in Table 4-8 and Table 4-10 because the proposed MACT standards did not include an explicit standard for lead.
Due to the uncertainties associated with the air modeling as mentioned above, the results shown should not be used in an absolute sense; however, they might be useful in a relative sense to detect trends. In this calculation, the air concentrations attributable to cement kilns are somewhat higher than those for hazardous-waste incinerators, even after imposition of MACT. This is likely due to a combination of factors. The main factor appears to be that the MACT standard is on a mass per volume basis (e.g., 0.2 µg PCDDS/F TEQ/dscm) and the cement kilns have a much greater air flow rate and, consequently, volume than hazardous-waste incinerators. (The average air flow rate for cement kilns is about 3,000 dscm/min. The average air flow rate for hazardous-waste incinerators is about 900 dscm/min.) A higher flow rate indicates that the typical mass emission of pollutants from cement kilns would be higher than hazardous-waste incinerators when a common concentration-based emission limit is used. The average difference in estimated ambient concentrations may be an artifact of using a single dispersion distribution for all facilities, however, the emission characteristics of cement kilns may on average be sufficiently different from hazardous-waste incinerators to cancel the effect. The committee cautions that comparisons between facilities based on these calculations is not a valid exercise.
TABLE 4-9 Estimated Ambient Air Concentrations of Various Pollutants Associated with Emissions from Cement Kilns that Burn Hazardous Waste Prior to Compliance with MACT (All concentrations are annual averages in units of micrograms per cubic meter.)
|
Pollutant |
Number of Samples |
Mean |
Standard Deviation |
Median |
Range |
|
Mercury |
790 |
3.2 × 10-3 |
2.1 × 10-2 |
1.2 × 10-4 |
5.6 × 10-9 to 5.2 × 10-1 |
|
Lead |
957 |
1.6 × 10-1 |
5.0 × 10-1 |
1 × 10-2 |
7.7 × 10-7 to 6.9 |
|
PCDDS/F TEQa |
135 |
7.6 × 10-8 |
5.2 × 10-7 |
4 × 10-9 |
1.5 × 10-11 to 1.6 × 10-5 |
|
Particulate Matter |
217 |
0.73 |
1.6 |
0.28 |
4.5 × 10-3 to 30 |
|
a PCDD/F is the toxic equivalent of the sum of the polycholorinated dibenzodioxins and polychlorinated dibenzofurans. |
|||||
TABLE 4-10 Estimated Ambient Air Concentrations of Various Pollutants That Might Be Associated with Emissions from Cement Kilns That Burn Hazardous Waste after Compliance with MACT (All concentrations are annual averages in units of micrograms per cubic meter.)a
|
Pollutant |
Mean |
Standard Deviation |
Median |
Range |
|
Mercury |
2.5 × 10-4 |
1.9 × 10-4 |
2.0 × 10-4 |
1.3 × 10-5 to 2.2 × 10-3 |
|
PCDDS/F TEQb |
1.6 × 10-9 |
1.3 × 10-9 |
1.6 × 10-9 |
9.2 × 10-11 to 1.2 × 10-8 |
|
Particulate matter |
0.6 |
0.45 |
0.47 |
3.2 × 10-2 to 4.8 |
|
a Based on the MACT emission limits that were proposed in April 1996. b PCDD/F is the toxic equivalent of the sum of the polycholorinated dibenzodioxins and polychlorinated dibenzofurans. |
||||
Results from Environmental Monitoring Studies Around Incineration Facilities
Mathematical models and calculations have utility as tools for prediction and correlation of measurements in the environmental sciences. But uncertainties often remain after modeling because of the complexity of the environmental problem being modeled and the necessity to make assumptions throughout the modeling process.
Monitoring is an alternative to modeling environmental pollutants in the vicinity of incinerators. Also it provides a means of assessing a model's reliability. Monitoring can be used to assess local concentrations directly and, thus, avoid some uncertainty. Although environmental monitoring studies have been conducted around waste incinerators, some of the toxicants released by incinerators persist on a regional scale, rather than only on a local scale. Modeling and monitoring complement each other. Monitoring is useful for calibrating and validating models. Models are useful for interpolating and extrapolating monitoring data over space and time.
Ambient air is the most-common environmental medium that is monitored. For example, EPA (1991b) carried out a detailed study of ambient air quality in the vicinity of a municipal solid-waste (MSW) combustor in Rutland, VT. This facility burned 240 tons of waste a day; an electrostatic precipitator and wet scrubber were used to control particulate emissions and acid-gas emissions, respectively. In the investigation, air-dispersion modeling was conducted to determine locations for ambient monitoring and environmental sampling analyses for
metals, polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), and total particulate matter. EPA (1991b) found no correlation between the amount of waste burned and ambient-air particle concentrations. It also found that the proportions of the different compounds in ambient air did not resemble those in the stack gas. It was concluded that the incinerator was not the primary source of PCDDs and PCDFs in the ambient air surrounding the facility.
Other studies have reported similar findings. Hunt et al. (1991) performed ambient-air monitoring for particulate- and vapor-phase PCDDs and PCDFs in the vicinity of the Bridgeport, CT, waste-to-energy facility. Measurements were taken before and after the plant became operational. The results showed little difference in ambient concentrations of chlorinated dibenzodioxins and furans between the preoperational phase (0.097 pg/m3) and the postoperational phase (0.088 pg/m3). Stubbs (1993) examined trace metals and air-quality measures in the vicinity of the greater Vancouver, British Columbia, municipal incinerator and concluded that startup and operation of the plant had no measurable effect on air quality. A detailed study of ambient air in the vicinity of a greater Detroit plant that burned refuse-derived fuel was undertaken. The study evaluated many potential chemicals of concern (PCDDs and PCDFs, respirable particles, metals, polycyclic aromatic hydrocarbons [PAHs], polychlorinated biphenyls [PCBs], chlorobenzenes, chlorophenols, and inorganic acids) at four monitoring sites over a period of 2.5 years. One of the monitors was installed at the expected point of maximal effect, as predicted by air-dispersion modeling. The results were analyzed with two-sample tests on means, multiple regressions, and principal-components analysis. All statistical procedures showed that there was no observable effect of the facility on the measured concentrations of any of the chemicals studied.
Single environmental media other than air have also been evaluated. Eitzer (1995) analyzed bovine-milk samples for chlorinated dibenzodioxins and furans from farms near a municipal solid-waste resource-recovery incinerator in Connecticut, and found no statistically significant differences between preoperational and postoperational concentrations. The facility was designed to incinerate up to 620 tons per day of municipal solid waste and was equipped with a spray dryer and fabric filter for emission controls. The preoperational phase consisted of 17 samples from 5 farms. The postoperational phase included 12 samples from the same farms. Student's T-tests showed no statistically significant differences at a 95% level of confidence between preoperational and postoperational results for any individual congener (the mean and standard deviation presented in Table 4-11 show that estimates have high uncertainty). Similar results were obtained for furans.
Ramos et al. (1997) analyzed bovine milk samples from 12 dairy farms in Spain and 23 samples of pasteurized bovine milk for PCDDs and PCDFs. They found that the levels of dioxins in the milk samples from farms located in rural
TABLE 4-11 Dioxins and Furans in Connecticut Cow's Milk
|
Congener |
Preoperational (fg/g) Mean ± SD (n=17) |
Postoperational (fg/g) Mean ± SD (n=12) |
|
2,3,7,8-TCDD |
17 ± 24 |
15 ± 27 |
|
1,2,3,7,8-PeCDD |
6.4 ± 9.0 |
6.8 ± 12 |
|
1,2,3,4,7,8-HxCDD |
31 ± 43 |
26 ± 35 |
|
1,2,3,6,7,8-HxCDD |
32 ± 26 |
23 ± 14 |
|
1,2,3,7,8,9-HxCDD |
15 ± 20 |
9.3 ± 15 |
|
1,2,3,4,6,7,8-HpCDD |
94 ± 120 |
120 ± 140 |
|
OCDD |
770 ± 1,500 |
1,700 ± 3,400 |
|
Source: Adapted from Eitzer 1995. |
||
areas without specific dioxin sources (background levels) were slightly lower than those found in milk from the vicinity of potential dioxin emission sources (a waste incinerator, and chemical and metallurgical facilities) and similar to milk near to a paper production facility. In contrast to the conclusions of studies presented above, the authors concluded that the waste incinerator seems to be the emission source with the highest influence on the bovine milk gathered in its vicinity. The average dioxin concentrations found in pasteurized commercial milk were lower than those found in raw milk and were comparable to those found in retail milk from other countries.
McLaughlin et al. (1989) measured dioxins and furans in the soil near a municipal solid-waste incinerator in Hamilton, Ontario. This program was initiated because airborne emissions exceeded the Provincial guidelines for these chemicals. Fourteen soil samples including three control sites and the calculated point of maximum effect were analyzed. The incinerator had been operating for 10 years when the samples were obtained. All samples contained some dioxin and furan congeners. OCDD was the congener most-frequently detected. The range of OCDD was from less than 1.3-3,500 parts-per-trillion (ppt) for the study area and 810-3,200 ppt for the controls. There was no concentration gradient or deposition pattern that was consistent with the direction of prevailing winds or the location of the maximum ground-level concentration. On the basis of these data, the authors concluded that stack emissions from the incinerator have not accumulated in surface soil in the vicinity of the plant.
Schuhmacher et al. (1998) determined concentrations of PCCDs and PCDFs in 24 soil samples collected near a municipal solid-waste incinerator (Tarragona, Catalonia, Spain). Principal Component Analysis and hierarchical cluster analysis were used to compare these soil samples with a set of 10 additional samples collected outside the influence of the plant. The authors concluded that no remarkable PCDD or PCDF contamination was found, and soils in the vicinity of the incinerator provide patterns of PCDDS/Fs quite similar to those obtained in soils collected far from the influence of that facility.
Deml et al. (1996) measured dioxins and furans in blood and human milk of persons living in the vicinity of a municipal solid-waste incinerator in Germany. The facility had been in operation for 13 years and combusted 350,000 tons of waste per year. Blood samples were obtained for 43 persons who had been living in the study area for at least 10 years and 3 persons who had lived there for 8 years. The dioxin and furan concentrations in blood for the study group ranged from 3-19 picograms/gram (TEQ), compared to a control-group concentration of 10-48 pg/g (TEQ). Similar results were found for dioxins and furans in mother's milk. These authors concluded that living in the vicinity of this facility does not result in a higher body burden for dioxins and furans. Based on previous discussions in this report about the emissions of individual incinerators, such a finding is not surprising.
Kurttio et al. (1998) studied concentrations of mercury in hair of people in the proximity of a hazardous-waste-treatment plant that contains an incinerator. A baseline survey of the surrounding population and environment was conducted prior to the plant's operation in 1984; ten years later, investigators studied the same subjects. In 1984 and 1994, the median hair mercury concentrations were 0.5 mg/kg and 0.8 mg/kg, respectively. The researchers concluded that mercury exposure increased as distance from the facility decreased; however, the increase in exposure was minimal and, on the basis of current knowledge, did not pose a health risk.
Bache et al. (1991) analyzed metals and PCBs in vegetation around an incinerator operating without air-pollution controls. The results showed that, of six metals and PCBs considered, only lead was statistically significantly higher than background. The mean of 9 upwind samples for lead was 2.1 mg/kg of vegetation with a standard deviation of 1.2 mg/kg. The downwind sample values depended on distance from the stack. The closest sample was 30 mg/kg. Samples declined to a value that was within the 95% confidence interval of the background data at a distance of 650 meters from the stack. However, Carpi et al. (1994) found increased concentrations of mercury (206 parts-per-billion (ppb), compared with a control value of 126 ppb) in sphagnum moss within 1.6 km of a municipal solid-waste incinerator in New Jersey.
Collett et al. (1998) analyzed levels of cadmium and lead in air and surface-soil samples collected in an area around the Baldovie municipal-waste incinerator in Scotland. They reported that the spatial distribution of lead levels in soils showed a marked variation downwind from the Baldovie incinerator in comparison with the background level for the area. However, the lead levels remained well within the typical range of lead in rural, unpolluted, British soils. The authors compared the observed levels of lead in local soils with the predicted downwind long-term ground-level lead distribution in air and found that atmospheric emissions of lead originating from the Baldovie incinerator directly determine concentrations of lead in soils within a radius of 5 km of the incinerator. However, in the case of cadmium, the authors found neither a marked nor exten-
sive contamination of the sampled area around the incinerator; the levels were within the typical range of cadmium levels in rural, unpolluted, British soils.
There are two dominant mechanisms through which plants can accumulate metals from the atmosphere. Materials in the vapor phase may be directly taken up into plants. Both vapor and particles may be washed out of the atmosphere by precipitation (wet deposition), and dry deposited directly on leaves. The increased concentration of mercury in the Carpi et al. (1994) study and the increased lead in the Bache et al. (1991) study were probably a consequence of wet deposition rather than vapor uptake. The significance of this conclusion is that wet deposition typically occurs in the immediate vicinity of a source, whereas vapor-phase uptake can occur on a regional basis. Yasuhara et al. (1987) showed that incinerators were not important contributors of dioxins and furans to local soil or sediment samples.
Thus, single-medium studies indicate that important dioxin and furan concentrations could not be detected in bovine milk, soil, or vegetation, but increases in lead could be found in soil and vegetation and increases in mercury could be found in moss and human hair samples collected near incinerators.
In addition to the single-medium studies, there have been several multimedia studies around incinerators. Laidlaw Environmental Services, Ltd. (LESL) has operated a hazardous-waste treatment, storage, and disposal facility in Sarnia, Ontario, for over 25 years. A component of this facility is a liquid-injection hazardous-waste incinerator that treats 120 × 106 L of waste per year. Emissions are controlled with a secondary combustion chamber, spray dryer, and fabric filter. Both LESL (Ecologistics 1993a,b) and the Ontario Ministry of Environment and Energy have conducted multimedia monitoring in soil and vegetation around the facility, including locations determined by air modeling to have the greatest potential concentration. Most organic chemicals were not detected in either study area or control locations. For example, in 1992, OCDD, typically the most-common congener, was not detected in soils at detection limits ranging from 0.0052-0.050 nanograms/gram (ng/g). The results obtained in those studies were evaluated by comparison of samples obtained from the study area to those obtained from a control area, and by comparison of concentrations in soil to those considered to be typical by the MOEE (1989). A comparison of the metals of the greatest potential toxicological significance to the “upper limits of normal” developed by MOEE is given in Table 4-12. On the basis of these studies, it was concluded that the facility was not a major source of metals or PCDDs and PCDFs in the environment.
Stubbs and Knizek (1993) analyzed vegetation in the vicinity of the greater Vancouver refuse incinerator and concluded that trends in soil and vegetation trace elements in the study area in the study period indicated little or no change due to the startup and operation of the facility. The results are given in Table 4-13. They also concluded that the facility had no measurable effect on trace-element or PAH concentrations in soil, vegetation, or vegetative growth in the
TABLE 4-12 Metals in Soil Near a Hazardous-Waste Incinerator Compared with Background
|
Element |
Upper Limit of Background (mg/kg) |
Number of Samples |
Range of Concentrations (mg/kg) |
|
Arsenic |
10 |
21 |
3.6-6.2 |
|
Cadmium |
3 |
21 |
0.3-2.3 |
|
Chromium |
50 |
21 |
15-39 |
|
Lead |
150 |
21 |
16-28 |
|
Mercury |
0.15 |
5 |
0.03-0.10 |
|
Sources: Adapted from MOEE 1989; Ecologistics 1994. |
|||
vicinity of the facility. Fruin et al. (1994) presented the results of a multimedia monitoring study (ambient air, soils, and sediment) in the vicinity around a hazardous-waste incinerator operated by 3M (Minnesota Mining and Manufacturing) in Minnesota. The incinerator is a rotary kiln with a secondary combustion chamber, heat-recovery equipment, and five pollution-control devices. The study focused on particulate matter in ambient air and metals in soils. The study found that the incinerator contributed less than 1% of the total suspended particulates and the respirable particulates (PM10) to the total concentration in the ambient environment. A total of 180 soil samples in the potential impact zone of the incinerator were also analyzed for 21 metals. The results for the metals of greatest potential toxicological significance in nearby agricultural land, along with ranges for background presented by the authors, are included in Table 4-14. On the basis of the particulate and metals data, the authors concluded that there were negligible contributions of those combustion products from the incinerator to local concentrations.
In one of the largest studies of its kind, the Texas Natural Resource Conservation Commission (TNRCC 1995) evaluated environmental media (air, soil,
TABLE 4-13 Metals in Soil Near the Greater Vancouver Incinerator
|
Element |
Preoperational (ppm) |
Postoperational (ppm) |
|
Arsenic |
0.11 |
0.09-0.12 |
|
Cadmium |
1.48 |
0.67-1.17 |
|
Nickel |
2.47 |
1.14-1.57 |
|
Lead |
1.79 |
1.74-3.13 |
|
Selenium |
0.08 |
0.05 |
|
Fluorine |
3.58 |
2.52-4.67 |
|
Sulfur |
0.19 |
0.16-0.21 |
|
Source: Adapted from Stubbs and Knizek 1993. |
||
TABLE 4-14 Metals in Soil Near the 3M Incinerator
|
Element |
Background Range (ppm) |
Facility Range (ppm) |
|
Arsenic |
(not given) |
< 10-13 |
|
Cadmium |
Trace-1 |
< 0.07-1.0 |
|
Chromium |
1-1,500 |
5.2-12 |
|
Lead |
< 5-700 |
8.1-29 |
|
Mercury |
10-3,400 |
7.0-18 |
|
Source: Adapted from Fruin et al. 1994. |
||
and vegetation) in a community that contains two cement kilns that use hazardous waste as fuel as well as a cement kiln and secondary steel mill that use conventional fuel. Over 940 ambient-air samples were analyzed for suspended particles, 188 for respirable metals, and 135 for volatile organic compounds, and 175 soil samples were analyzed for various potential pollutants. Other analyses were also conducted of acidic gases and chemicals on vegetation. As an example of their results, the dioxin and furan concentrations in soils potentially affected by the facilities ranged from 0.3 to 17.9 ppt (TEQ) with a median of 1.4 ppt (TEQ). This may be compared to the range for unaffected background of 0.8-3.2 ppt (TEQ) with a median of 1.8 ppt (TEQ).
Lorber et al. (1998) examined PCDD/PCDFs in ash from, and soil and air around the Columbus, Ohio, municipal waste-to-energy facility. This facility was estimated to emit as much as 1,000 g of TEQ per year for its approximate 11 years of operation (compared with a 1994 estimate of total emissions of 9,300 g of TEQ per year from all sources in the United States). The effect of the incinerator was detected in air and soil samples. Two air samples known to be downwind of the operating facility had TEQ concentrations approximately 5-fold higher than background. Approximately 2% of the total emitted dioxins were estimated to be present in the soil within about 3 km of the incinerator. However, the authors concluded that despite the magnitude of the emissions, soil and air concentrations in the urban area of Columbus did not exceed urban air and soil concentrations of dioxins found around the world.
Limitations of the studies cited above include reflection of a nonrandom set of facilities; inconsistency of methods, and problems with sampling and analytical techniques, detection limits, number and location of samples, duration of studies, contaminant contribution from other emission sources, and quality assurance and quality control. Therefore, no definitive conclusions can be drawn about waste incineration in general. However, taken as a whole, the weight of the evidence contained in those studies suggests the following. First, in principle, measurement of contributions of substances within various environmental media is a feasible method for assessing environmental emissions from incinerators. Second, the results are consistent with the hypothesis that emissions of
dioxins and furans are more important on a regional than a local scale, whereas the emissions of some metals, such as mercury, are important close to the stack and on a regional scale. Third, it appears that incinerators with similar waste streams, operating conditions, and emission controls to those studied are unlikely to now be a major contributor to local ambient concentrations of the chemicals of concern noted in this report.
CONCLUSIONS
-
Although releases to the environment from incineration facilities occur mainly by air emissions, multiple potential pathways in the environment to humans exist. Variations in the physical properties of substances of concern and the extent to which they persist in the environment result in differences in the types of pathways. Results of environmental monitoring studies around incineration facilities indicate that the specific facilities studied are unlikely to be major contributors to local ambient concentrations of the substances of concern noted in this report. However, methodological limitations of the studies do not allow for general conclusions to be made about waste incineration's contributions to environmental concentrations of those contaminants. They also do not allow for characterization of total human exposure.
-
The air concentration of gases (including carbon monoxide and acid gases) and fine particles can be estimated by dispersion models to data on stack emissions, stack height, and local meteorological conditions. For the case of heavy metals carried by the particles, the deposition fluxes of the particles and the transfer from the soils into the food chain through vegetable produce and animal fodder needs to be taken into account. Models for the dispersion and the uptake into the food chain are available for use in risk assessments.
-
Mercury and dioxins and furans are classes of pollutants where historically incinerators are estimated to have contributed a significant portion of the total national emissions. These classes of pollutants are characterized by their long-range air transport, persistence, and relative uniformity of deposition fluxes on regional bases. Whereas one incinerator might only contribute a small fraction of the total environmental concentration of these compounds, the sum of the emissions of all the incineration facilities in a region could be considerable. Because a number of older incinerators had been closed down and replaced by modern low-emitting units, it is now uncertain how much incineration contributes to the environmental concentrations of these compounds.
-
Computational models for the environmental fate and transport of mercury and the dioxins and furans provide useful information for assessing the major exposure pathways for humans, but are unable to provide overall
-
estimates of environmental contributions from an individual facility to better than within a factor of 10. The models suggest that fish consumption is potentially the major pathway of human exposure to mercury and that meats, dairy products, and fish are potentially the major pathways of exposure to dioxins and furans.
-
Because the food chain is potentially the primary path for exposure to dioxins and furans and toxic metals such as mercury, the correlation between total exposure and local emissions is expected to be low. Because of the persistence of these pollutants and their long range transport, not all relevant sources contributing to exposure pathways are local. In addition, the foods that are implicated as the major pathway of exposure could include a fairly high portion of products imported from other regions.
-
Detailed information is required on the distribution of contaminants in the environment once they are released from waste-incineration facilities. Better assessments are needed of transport, accumulation, and physical and chemical transformations of contaminants through all potentially important exposure pathways, including air, food, soil, and water.
-
Exposures to dioxins and furans, mercury, and other heavy metals are best assessed by monitoring food consumption. Drinking water may be an important pathway in some circumstances. Models can be used to establish the chemicals' pathways to humans, regional distribution, and persistence in the environment.
RECOMMENDATIONS
-
Environmental assessment and management strategies for emissions from individual incineration facilities should include an appropriate regional-scale framework for assessing the collective dispersion, persistence, and potential long-term impacts of incinerator emissions on human health.
-
Better material balance information—including measurements of source emissions to air and deposition rates to soil, water, and vegetation—are needed to determine the contribution of waste-incineration facilities to environmental concentrations of persistent chemicals. The variation of these emissions over time needs to be taken into account for the short-term to determine if any important emission increases occur at an incineration facility, and for the long-term to measure changes due to replacement of less-efficient incinerators with modern, lower-emitting units.
-
In characterizing potential health effects of waste-incineration emissions, all environmental media should be assessed. Also, all possible exposure routes by which contaminants enter the body of a person should be considered, including inhalation, ingestion of food or drink, and absorption through skin. Such an approach is consistent with EPA's guidelines for health risk assessments.