8
Stable Isotope Tracers: Technological Tools That Have Emerged
Dennis M. Bier1
ISOTOPES IN NATURE
By and large, when one discusses biomedical applications of isotopic methods, the public, and often many scientists as well, conceptualize the notion as the use of radioactive counterparts of elements occurring in nature, which, in turn, are assumed to be nonradioactive. This ideation ignores what most everyone learned in high school chemistry (Table 8-1), namely that only a few biologically important elements are monoisotopic in nature (e.g., 31P); there are trace amounts of naturally occurring radioactive isotopes of several common elements (e.g., 14C and 40K); and most of the common naturally occurring elements occur as a mixture of two or more stable, nonradioactive isotopic forms (e.g., 16O, 17O, and 18O). Twenty-five years ago, the latter information was of limited use to the biomedical investigator since significant quantities of highly
TABLE 8-1 Natural Abundance of Elements Commonly Found in Biological Substances
enriched, minor, stable isotopic nuclides of the elements were generally unavailable, save for deuterium. This situation has changed dramatically over the last two decades. Now, except for enriched stable isotopic forms of the mineral elements, which remain in short supply and are prohibitively expensive, the stable isotopes of carbon, hydrogen, nitrogen, and oxygen and a wide variety of biochemicals containing these nuclides are commercially available in relative abundance. Similarly, analytical instrumentation for routine measurements of these isotopes is now widely available and, because of the advances in computer
hardware and software, this instrumentation is more user friendly. For these reasons, the use of nonradioactive, stable isotope tracers as probes for in vivo nutrition and metabolic studies has increased enormously over the last 20 years.
ACCESS TO INACCESSIBLE COMPARTMENTS
Why are these isotopic probes needed? The answer is best illustrated by Figure 8-1. This schematic representation of an intricate biological system shows a multicompartmental complex, in which the compartments reflect constitutive entities that are discrete but otherwise not constrained. Thus, depending on the question, the compartments might be viewed anatomically (e.g., brain, liver, and kidney), functionally (e.g., respiration, circulation, and digestion), biochemically (e.g., glucose, lactate, and alanine), or otherwise.
For human nutritional studies in vivo, there are generally only a few compartments, such as the blood or urine, that are accessible for sampling information of relevance to nutrient dynamics or action. However, the direct answers to
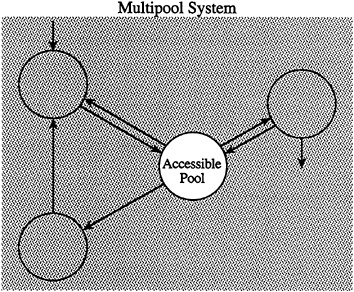
FIGURE 8-1 Schematic representation of a generic multipool system. Each pool or compartment reflects a relatively homogenous, well-mixed portion of the system. The arrows represent movement of materials between compartments. Compartments may be anatomical, functional, or biochemical. The uncovered central compartment, labeled accessible pool, is meant to reflect the fact that that portion of the entire system is available to the investigator for sampling. Thus, for an anatomical model, the accessible pool might be venous blood. The stippled, covered pools are meant to represent those hidden within the system and inaccessible for routine sampling. A tracer that is injected into the accessible pool and then traverses the system provides the necessary information to uncover the hidden structure of the system.
most biological questions lie at the site of the nutrient's metabolic effects, that is, within organs and cells. In some instances, this information can be obtained invasively by inserting sampling catheters into the site of interest. Nonetheless, this approach is generally not ethically acceptable for routine studies in otherwise healthy individuals. For this reason, alternative approaches that provide information about events within inaccessible compartments from data collected in the accessible ones must be used. An established approach to this problem is to inject an appropriate tracer into the accessible compartment and monitor the dilution of the tracer in the accessible pool by sampling the compartment repeatedly after introduction of the tracer. The shape of the tracer dilution curve is determined by the characteristics of the tracer outflow into the inaccessible compartments and the nature of the unlabeled substrate movement into the accessible compartment—in other words, by the structure of the system in question. Thus, by analyzing the attributes of the tracer dilution curve by the widely verified mathematical techniques of compartment analysis (Shipley and Clark, 1972), the previously hidden structure of the system can be uncovered. Stable isotope tracers are the optimal candidates for this analytical approach in humans, as discussed below.
EXPRESSION OF TRACER DILUTION
The similarities and differences in expressing the dilution of a radio isotope tracer and stable isotope tracer are shown in Figure 8-2 for carbon. In the context of a radiotracer experiment (B), the biological substance of interest (tracee, B1) is all unlabeled and composed of the major, naturally occurring, nonradioactive nuclide, 12C, plus its minor stable isotope, 13C. The radiolabeled tracer (B2) is the highly enriched isotope 14C. Although there is a trace amount of naturally occurring 14C in the tracee, its abundance is very low and its radioactivity negligible compared with that of the tracer. In an experiment, when the radiotracer probe is added to the tracee system and a sample taken subsequently (B3), the tracee is analyzed for its mass content by an acceptable method, and the radioactivity of the tracer is determined independently by counting the disintegrations. The resultant tracer to tracee ratio, commonly called the specific activity, represents the degree of tracer dilution.
For a stable isotope-tracer experiment (Figure 8-2, A), the situation is somewhat more complicated. The tracee (A1) remains composed of the two naturally occurring, stable isotopes of carbon, 12C and 13 C. In contrast, (A2), unlike the ''massless" radiotracer, a stable isotope "tracer" has a finite mass and, while composed principally of the enriched minor isotope 13C, it also invariably contains some 12C. When this stable tracer is injected or infused into the tracee system, a resultant sample (A3) is composed of a mixture of 12C and 13C contributed by both the tracee and the tracer. The analytical methods measure total 12C and total 13C in the sample directly. To calculate the unknown tracer-to-tracee ratio in the sample, the fractional contributions of the tracer and the tracee
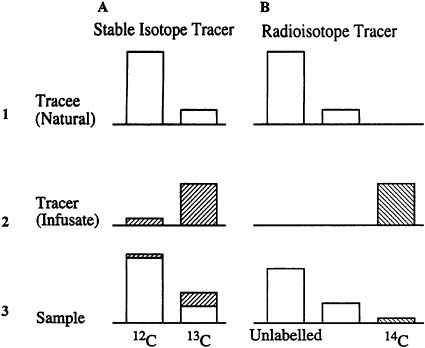
FIGURE 8-2 Generic representation of the isotopes of carbon and how they contribute to the determination of isotope content in a sample taken during a stable or radioisotope tracer experiment (see text). The clear bars represent the endogenous carbon (tracee) in the system that consists of the stable, nonradioactive nuclides of carbon, 12C and 13C. In a stable isotope experiment, these can be measured separately by mass spectrometry. In a radioisotope experiment, 12C and 13C are measured together as "unlabeled" carbon. Similarly, in a stable isotope tracer experiment, the stable carbon tracer itself (hatched bars in A) is composed primarily of 13C but also contains a small amount of residual 12C. The experimental sample consists of a mixture of these nuclides that are measured individually by mass spectrometry. In a radiotracer experiment, the 14C tracer is distinct from the unlabeled tracee and measured by an entirely different approach. In both cases, the required ratio of tracer to tracee must be derived from the individual measurements.
to each of these isotopic totals must be derived mathematically from the known isotopic characteristics of the natural or fully "unlabeled" tracee and the corresponding known isotopic composition of the "labeled" tracer. The procedures for this process are well described (Cobelli et al., 1987, 1992).
Two additional reporting conventions of stable isotope-tracer dilution are available for specialized cases. During the constant infusion of a stable isotope tracer at steady state, tracer isotopic enrichment above natural abundance can be used appropriately (Cobelli et al., 1987; Segel, 1968). Further, in experiments in which tracer and tracee abundance or enrichment differ by several orders of magnitude, their relationships are often conveniently described with respect to international reference standards according to the so-called delta (d) notation (Boutton, 1991).
ANALYTICAL CONSIDERATIONS
Although advances in magnetic resonance spectroscopy now permit limited noninvasive measurements of stable isotope tracers in vivo and a broad approach to in vitro measurements of selected stable nuclides in samples obtained during in vivo tracer experiments, mass spectrometry has been the most commonly used tool for quantifying samples containing stable, isotopically labeled substrates. This use is appropriate since mass spectrometry is the most rapid, sensitive, specific, and precise general analytical method available for this purpose. Since all biochemical substrates have mass, and since mass is the principle of mass spectrometric measurements, this approach is potentially applicable to all biochemical substrates. In practice, there are some obvious limitations, but these are relatively few for substances with molecular weights less than about 1,000 daltons. Mass spectrometry is also an extraordinarily sensitive method. Picomole analyses are commonplace, and attomole analyses can be accomplished with commercially available gas chromatography-mass spectrometry (GC-MS) instrumentation. With specialized instruments, single atoms can be detected. Analytical specificity is exceptionally high with the advent of separatory capabilities of capillary GC column inlets, as well as selective derivatization and ionization methods.
The characteristics of mass spectrometry approaches to stable isotope quantitation are shown in Table 8-2. This table demonstrates that while gas isotope ratio mass spectrometry has the ability to quantify the lowest tracer/tracee ratios, this advantage is at the expense of sample size. The additional disadvantage of gas isotope ratio mass spectrometry is that the sample nuclide must be converted to a purified gaseous form, a process that often adds considerably to analytical time and difficulty.
Recently, commercial GC-Combustion-Isotope ratio mass spectrometry systems have been introduced. This new alternative couples the separatory advan
TABLE 8-2 Characteristics of Quantitative Mass Spectrometry Methods
|
Characteristics |
Gas Chromatography-Mass Spectrometry |
GC-Combustion-Isotope Ratio Mass Spectrometry |
Gas Isotope Ratio Mass Spectrometry |
|
Tracer/Tracee Ratio (%) |
0.1–100 |
0.0005–0.5 |
0.00005–0.01 |
|
Sample Size |
Picogram |
Nanogram |
Microgram |
|
Preparation |
Derivatization |
Derivatization |
Purified gas |
|
Precision (%) |
±0.2 |
±0.01 |
±0.001 |
|
Matrix |
Plasma, urine |
Plasma, tissue |
Breath, urine |
tages of capillary gas chromatography to the high isotope dilution and precision characteristics of gas isotope ratio mass spectrometry and is a valuable addition to the analytical repertoire (Goodman and Brenna, 1992; Yarasheski, 1992).
The high level of precision achieved by quantitative mass spectrometric methods also is demonstrated in Table 8-2. Tables 8-3 and 8-4 demonstrate the practical implications of this precision. Presented in Table 8-3 are the individual interlaboratory coefficients of variation for the measurement of eight common substances of clinical interest by various hospital pathology laboratories surveyed by the American College of Pathologists using standard clinical laboratory protocols. The best of these (for sodium) was ±1.3 percent, while the worst was ±11.1 percent (for creatinine). Table 8-4 shows the results for a similar multiple-laboratory survey of the measurements of four stable nuclides by gas isotope ratio mass spectrometry. The most analytically challenging measurement, which is that of deuterium, had an interlaboratory coefficient of variation of ±1.02 percent, better than the best measurement of variation in the clinical laboratory. Such precise measurements are of very great value in compartmental analysis of tracer data, since they permit more precise definition of the mathematical function fitted to the isotope decay curve and, therefore, to the model of the biological compartmental system derived from this curve.
ADVANTAGES OF STABLE ISOTOPE TRACER USE
The most salient advantage of stable isotope tracers for use in human experiments is their complete safety. In the nearly half century of their use, the most significant side effect reported in humans has been transient vertigo, result
TABLE 8-3 Precision of Selected Routine Clinical Chemistry Methods
|
Analyte |
Coefficient of Variation (%) |
|
Calcium |
3.1 |
|
Chloride |
2.8 |
|
Cholesterol |
5.8 |
|
Creatinine |
11.1 |
|
Glucose |
3.9 |
|
Potassium |
2.9 |
|
Sodium |
1.3 |
|
Urea |
5.3 |
TABLE 8-4 Measurement Precision of Selected Stable Nuclides by Gas Isotope Ratio Mass Spectrometry*
|
Isotope |
Substance |
Atom % Excess |
Coefficient of Variation (%) |
|
Carbon-13 |
Glucose |
1.2143 |
0.255 |
|
Deuterium |
Water |
0.02359 |
1.021 |
|
Nitrogen-15 |
Urea |
0.45548 |
0.138 |
|
Oxygen-18 |
Water |
0.25039 |
0.292 |
|
* Different laboratories than those reporting data in Table 8-3. |
|||
ing from the acute ingestion of relatively large quantities of deuterium oxide. Other than this relatively unique event that is apparently a consequence of alterations of cochlear fluid density, the other stable nuclides have been uniformly safe in human studies. Thus, stable isotope tracers can and have been used appropriately in both children and pregnant women.
Table 8-5 demonstrates several additional but perhaps less well-appreciated advantages of using a stable isotope tracer. In addition to the analytical and ethical advantages, there is the very practical advantage of not having the isotope disposal costs that are associated with the use of radiotracers. Depending on the magnitude of activity in a research laboratory, this may represent a significant savings. Additionally, with mass spectrometry, the position of the isotopically labeled nuclide within a molecule usually can be determined with relative ease. This approach, called mass isotopomer analysis because mass isotopomers are chemically identical molecules that differ in mass by virtue of the number and position of their isotopic constituents, is a valuable tool for dissecting precursor
TABLE 8-5 Selected Advantages of Stable Isotope Tracer Methods
|
1. |
No practical radiotracer exists for certain elements. |
|
2. |
There are no isotope disposal costs. |
|
3. |
Some studies are not practical to perform using radiotracers. |
|
4. |
Isotope effects are less than with corresponding radiotracer. |
|
5. |
Substrate content and isotope enrichment are measured simultaneously. |
|
6. |
Confidence in assay specificity is very high. |
|
7. |
Intramolecular location of label(s) is determined easily. |
|
8. |
Simultaneous and repeated use of several tracers is possible in the same subject. |
product relationships in biological systems, as described more fully below. Lastly, because of their safety, several stable isotope tracers can be used simultaneously and repeatedly in the same subject, a luxury not available with radiotracers because of acceptable exposure limits. This advantage of using stable isotope tracers is extraordinarily valuable in human experiments since the use of multiple tracers maximizes the information content of each human study. Further, the ability to study the same individual repeatedly allows analysis of the results of various test interventions to be compared on the basis of paired statistics, with the subject serving as his own control. The combined use of several isotopes and paired analysis allows a reduction in the number of research subjects needed to accomplish multiple objectives, without sacrificing statistical power.
SELECTED RECENT EXAMPLES OF STABLE ISOTOPE USE
The stable isotope literature is now sufficiently large that innumerable examples of human kinetic studies that are of direct basic or applied nutritional importance are available (Bier, 1987). Further, the technology has been used successfully in military field studies (Hoyt et al., 1994). Most recently, however, the realization that the use of the full isotopic information content inherent within substrate mass isotopomers has opened up a highly productive new approach to stable isotope tracer research, called mass isotopomer distribution analysis (MIDA) (Hellerstein and Neese, 1992). MIDA is the process of quantifying precursor-product events from the distribution of mass isotopomer patterns in the labeled precursor and product. A simple example, depicted by the data in Figure 8-3, will illustrate this concept. Berthold and coworkers (1991) incorporated uniformly 13C-labeled Spirulina platenis algal hydrolysate into the diet of a laying hen that was fed this mixture for 27 days. During that time, they measured the isotopic enrichment of each carbon atom of egg white protein phenylalanine and glutamine plus glutamate (GLX). Figure 8-3A shows that there was a gradual decline in the fraction of egg protein phenylalanine that was unlabeled (M) over the period of feeding with a corresponding fractional increment in phenylalanine that was fully labeled with carbon-13 (M+9). No egg white protein phenylalanine isotopomers were found with fewer labeled carbon atoms (M+1 to M+8). These data show convincingly that dietary algal [U-13C]phenylalanine was incorporated fully intact into the egg protein; in other words, all egg protein was derived from the diet, confirming that phenylalanine is an essential amino acid with no evidence of endogenous synthesis. Figure 8-3B shows that the situation for glutamine plus glutamate is quite different. Thus, while the decline in unlabeled GLX(M) occurs as expected, carbon-13 labeling of egg protein GLX is distributed throughout the isotopomers having one to five labeled carbons (M+1 to M+5). These data show that dietary algal [U-13C]GLX is metabolized extensively and there is de novo resynthesis before synthesis of egg white protein, confirming the nonessentiality of the amino acids
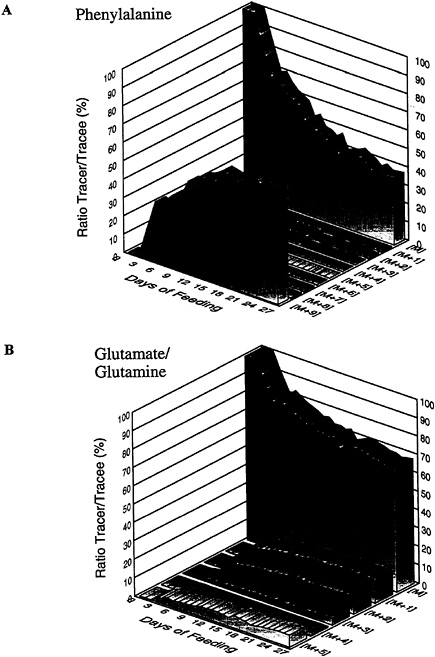
FIGURE 8-3 Mass isotopomer distribution patterns in egg white protein phenylalanine (A) and glutamine plus glutamate (B) obtained from eggs laid during a 27-d period when a chicken was fed [U-13C]-labeled algal biomass. M represents the completely unlabeled isotopomer (i.e., all 12C). The isotopomers at M+1 to M+n represent those molecules labeled with a single carbon-13 atom (i.e., 13C1) to molecules fully labeled with carbon-13 (i.e., 13Cn), respectively. The vertical axes represent the percent label at the individual isotopomers.
SOURCE: Berthold et al. (1991), used with permission.
glutamine and glutamate. Thus, this experiment illustrates the fact that careful consideration of the full isotopic information content of a synthesized product can contain significant information about the metabolism of its precursor.
Over the last several years, this principle has been applied successfully to the study of glycogenolysis and gluconeogenesis (Des Rosiers et al., 1995; Katz and Lee, 1991; Landau et al., 1995; Neese et al., 1995), lipogenesis (Hellerstein et al., 1996), contribution of de novo fatty-acid synthesis to very-low density lipoprotein triglyceride fatty acids (Leitch and Jones, 1993; Yang et al., 1996), and incorporation of dietary pyridimidine nucleosides into hepatic DNA (Berthold et al., 1995).
SIGNIFICANCE TO THE MILITARY
The basic mechanistic questions of nutritional relevance to military personnel cannot be answered convincingly or conclusively by indirect methods. Specifically, the quantitative aspects of interorgan metabolic fuel transport and the pathophysiological basis for alterations in fuel and energy balance because of physical activity, restricted rations, environmental and psychological stress, and injury cannot be answered by static substrate measurements or balance studies. These answers require quantitative assessments of fuel dynamics. In the context of safe, relatively noninvasive approaches to these issues in normal volunteers, stable isotope tracer studies are the only practical alternative. The results of such kinetic investigations will enhance significantly the functional data available for subsequent implementation of decisions important to the health of U.S. service members under both training and battle conditions.
Given this, it is impossible to escape the conclusion that this technology should be supported by Department of Defense funds. It is unclear what the precise role of the Small Business Innovative Research program might be in the funding process other than design of specialized analytical instrumentation useful in field circumstances or the development of new synthetic routes toward cost-effective production of stable, isotopically labeled substrates of interest to the military but not commercially available. The benefit/cost ratio is extremely high once the initial, one-time capital outlay for mass spectrometry equipment is satisfied.
Since NASA sent a mass spectrometer to Mars where it collected data during the Voyager mission, the technology is clearly applicable to any terrestrial "field" study. Further, many currently available mass spectrometers are now "bench top" size and could easily be operated in the field, given the appropriate power supply and environmental protection. Nonetheless, it is far more likely that samples collected in the field would be measured at a central core facility elsewhere. This approach has been utilized already in many stable isotope studies conducted in both industrialized and developing countries.
With modern instrumentation and commercial isotope availability, the technology is very practical to apply to human studies of all kinds. This has been
amply demonstrated by the large and ever-increasing number of such studies over the last two decades. This technique is now a widely accepted, basic method for human investigation applied in laboratories worldwide by individuals of vastly different types and degrees of basic education, training, and accomplishment. Current software and data reduction routines facilitate sample analysis by mass spectrometers, the practical operation of which cannot be more difficult than many other kinds of high-tech digital equipment already utilized by appropriately trained service personnel. However, the scientific questions forming the basis for the subsequent sample analysis must be asked by highly competent military scientists. These questions and the studies designed therefrom are, in fact, the rate-limiting step to productive application of the technologies described.
REFERENCES
Berthold, H.K., D.L. Hachey, P.J. Reeds, O.P. Thomas, S. Hoeksema, and P.D. Klein 1991 Uniformly 13C-labeled algal protein used to determine amino acid essentiality in vivo. Proc. Natl. Acad. Sci. USA 88:8091–8095.
Berthold, H.K., P.F. Crain, I. Gouni, P.J. Reeds, and P.D. Klein 1995 Evidence for incorporation of intact dietary pyrimidine (but not purine) nucleosides into hepatic RNA. Proc. Natl. Acad. Sci. USA 92(2):10123–10127.
Bier, D.M. 1987 The use of stable isotopes in metabolic investigation. Bailliere's Clin. Endocrinol. Metab. 1(4):817–836.
Boutton, T.W. 1991 Stable carbon isotope ratios of natural materials: I. Sample preparation and mass spectrometric analysis. Pp. 155–171 in Carbon Isotope Techniques, D.C. Coleman and D.M. Jones, eds. San Diego: Academic Press, Inc.
Cobelli, C., G. Toffolo, D.M. Bier, and R. Nosadini 1987 Models to interpret kinetic data in stable isotope tracer studies. Am. J. Physiol. 253:E551–E564.
Cobelli, C., G. Toffolo, and D.M. Foster 1992 Tracer-to-tracee ratio for analysis of stable isotope tracer data: Link with radioactive kinetic formalism. Am. J. Physiol. 262:E968–E975.
Des Rosiers, C., L. Di Donato, B. Comte, A. Laplante, C. Marcoux, F. David, C.A. Fernandez, and H. Brunengraber 1995 Isotopomer analysis of citric acid cycle and gluconeogenesis in rat liver. Reversibility of isocitrate dehydrogenase and involvement of ATP-citrate lyase in gluconeogenesis. J. Biol. Chem. 270:10027–10036.
Goodman, K.J., and J.T. Brenna 1992 High sensitivity tracer detection using high-precision gas chromatography-combustion isotope ratio mass spectrometry and highly enriched [U-13C]-labeled precursors. Anal. Chem. 64:1088–1095.
Hellerstein, M.K., and R.A. Neese 1992 Mass isotopomer distribution analysis: A technique for measuring biosynthesis and turnover of polymers. Am. J. Physiol. 263:E998–E1001.
Hellerstein, M.K. J-M. Schwarz, and R. Neese 1996 Regulation of hepatic de novo lipogenesis in humans. Ann. Rev. Nutr. 16:523–527.
Hoyt, R.W., T.E. Jones, C.J. Baker-Fulco, D.A. Schoeller, R.B. Schoene, R.S. Schwartz, E.W. Askew, and A. Cymerman 1994 Doubly labeled water measurement of human energy expenditure during exercise at high altitude. Am. J. Physiol. 266:R966–R971.
Katz, J., and W-NP. Lee 1991 Application of mass isotopomer analysis for determination of pathways of glycogen synthesis. Am. J. Physiol. 261:E332–E336.
Landau, B.R., V. Chandramouli, W.C. Schumann, K. Ekberg, K. Kumaran, S.C. Kalhan, and J. Wahren 1995 Estimates of Krebs cycle activity and contributions of gluconeogenesis to hepatic glucose production in fasting healthy subjects and IDDM patients. Diabetologia 38:831–838.
Leitch, C.A., and P.J.H. Jones 1993 Measurement of human lipogenesis using deuterium incorporation. J. Lipid Res. 34:157–163.
Neese, R.A., J-M. Schwartz, D. Faix, S. Turner, A. Letscher, D. Vu, and M.K. Hellerstein 1995 Gluconeogenesis and intrahepatic triose phosphate flux in response to fasting or substrate loads. Application of the mass isotopomer distribution analysis technique with testing of assumptions and potential problems . J. Biol. Chem. 270:14452–14463.
Segel, I.H. 1968 Biochemical Calculations. How to Solve Mathematical Problems in General Biochemistry. New York: John Wiley & Sons.
Shipley, R.A., and R.E. Clark 1972 Tracer Methods for In Vivo Kinetics. Theory and Applications. New York and London: Academic Press.
Yang, L-Y., A. Kuksis, J.J. Myher, and G. Steiner 1996 Contributions of de novo fatty acid synthesis to very low density lipoprotein triacylglycerols: Evidence from mass isotopomer distribution analysis of fatty acids synthesized from [2H6]ethanol. J. Lipid Res. 37:262–274.
Yarasheski, K.E., K. Smith, M.J. Rennie, and D.M. Bier 1992 Measurement of muscle protein fractional synthetic rate by capillary gas chromatography-combustion-isotope ratio mass spectrometry. Biol. Mass Spectrom. 21:486–490.














