9
Measurement of Energy Substrate Metabolism Using Stable Isotopes
Robert R. Wolfe1
INTRODUCTION
Atoms that are chemically identical but differ slightly in weight are called isotopes. Mass differences of isotopes are due to different numbers of neutrons. A variety of atoms have naturally occurring stable isotopes. Conveniently, hydrogen (1H and 2H, or deuterium), carbon (12C and 13C), nitrogen (14N and 15N), and oxygen (16O, 17O, and 18O) all have stable isotopes. In each of these cases, the most abundant is the one that is the lowest possible weight, and the heavier mass isotope is naturally present to a much smaller degree. Thus, the percent natural abundance of 2H, 13C, 15N, and 18O is 0.015, 1.11, 0.37, and 0.20 percent, respectively. Atoms can be enriched so that a ''mixture" is entirely a heavy stable isotope. These heavy stable isotopes can then be incorporated into molecules to create tracers.
An ideal tracer is chemically identical to the compound of interest (the tracee) but distinct in some characteristic that enables its precise detection. In the case of tracers labeled with stable isotopes, the principal characteristic of distinction is their difference in mass from the naturally occurring form. Thus, mass spectrometry is the most precise means of detecting the abundance of tracers labeled with stable isotopes. Consequently, the focus of this chapter will be on techniques relying on mass spectrometry to quantify the abundance of stable isotopes of carbon and hydrogen in order to gain insight into the regulation of substrate metabolism.
Conventionally, the methodology involves the infusion of a compound labeled in a specific position of the molecule with a stable isotope (e.g., [1-13C]-palmitic acid) in tracer doses. The infusion rate of the tracer, therefore, is trivial by comparison to the endogenous kinetics of the tracee. Blood, tissue, and/or breath samples are then obtained, and kinetic parameters are calculated using a mathematical model of varying complexity. The aim of this chapter will be to present examples of tracer methods to quantify both the plasma kinetics of glucose and free fatty acids (FFA) and also the relative contributions of the oxidation of intracellular glycogen and triglyceride to the total rate of oxidation of carbohydrate and fat, respectively. An exhaustive exposition on all possible methods of studying glucose and fat metabolism with stable isotopes is beyond the scope of this report. Because it is possible to make use of varying abundances of 13C that occur naturally, a method also will be presented in this chapter that enables the quantitation of total carbohydrate and fat oxidation using the measurement of the rate of total carbon dioxide excretion (![]() ) and the natural abundance of 13C in breath, and in the glucose, fat, and protein in the body. This method will be referred to as the "breath ratio method."
) and the natural abundance of 13C in breath, and in the glucose, fat, and protein in the body. This method will be referred to as the "breath ratio method."
BREATH 13C/12C RATIO METHOD TO MEASURE SUBSTRATE OXIDATION
Description of Methodology
The method is based on the measurement of the absolute 13C/12C ratios in expired breath and in endogenous glucose, fat, and protein (Romijn et al., 1992). Because of a small amount of fractionation of 13C in certain synthetic pathways such as photosynthesis, the natural abundance of 13C in glucose, fat, and protein varies. Whereas the macronutrients may differ in enrichment, over time in one individual, the values should be reasonably constant if the diet is stable. The differences can be further amplified by the ingestion of 13C-enriched cornstarch for a few days before the study.
The enrichment of the CO2 in the breath (RB) will be the sum of the proportional contribution of the oxidation of carbohydrate (x), fat (y), and protein (z) to produce CO2, multiplied by the respective enrichments of each substrate (Rx, Ry, and Rz). Thus,
(Equation 9-1)
Only carbohydrate, fat, and protein oxidation contribute to the total CO2 production. Consequently,
(Equation 9-2)
Rx, Ry, and Rz can be determined (see below). Also, z can be calculated from urinary nitrogen excretion. Therefore, equations 9-1 and 9-2 can be solved for x and y (the proportion of CO2 from carbohydrate and fat, respectively):
(Equation 9-3)
and
(Equation 9-4)
Note that x and y can be determined without the use of indirect calorimetry. When compared to the values for proportional oxidation rates of carbohydrate and fat as determined by indirect calorimetry, a close agreement was observed (Romijn et al., 1992). It also may be of interest to quantify glucose and fat oxidation in terms of actual rates (in g/min) of oxidation, which can be accomplished with this methodology. However, the measurement of either oxygen consumption (![]() ) or CO2 production (
) or CO2 production (![]() ) also is required. The situation in which only the
) also is required. The situation in which only the ![]() is known will be considered first. It is first necessary to express total
is known will be considered first. It is first necessary to express total ![]() as follows:
as follows:
where c is the quantity of carbohydrate oxidized (in grams), f is the quantity of fat oxidized (in grams), and n is the quantity of urinary nitrogen excreted (in grams) per minute (keeping in mind that 1 g of nitrogen excretion represents the oxidation of approximately 6.25 g protein) (from Frayn, 1983). From equations 9-2 and 9-5, it can be seen that

Therefore, substituting these values into Equation 9-1, RB can be expressed as
(Equation 9-6)
Solving for c,
(Equation 9-7)
to express in terms of ˙VO2:
(Equation 9-8)
Therefore,
(Equation 9-9)
(Equation 9-10)
From these equations, both the proportion of carbohydrate and fat oxidation (Equations 9-3 and 9-4) and the total rate of each in g/min (Equations 9-7 and 9-10) can be calculated with knowledge of only the ![]() or the
or the ![]() . The reliance on the isotope ratio of expired CO2, rather than the total amount of CO2 excretion, has significant benefits in two regards. First, the isotope ratio of CO2 can be determined with much greater accuracy than can the total
. The reliance on the isotope ratio of expired CO2, rather than the total amount of CO2 excretion, has significant benefits in two regards. First, the isotope ratio of CO2 can be determined with much greater accuracy than can the total ![]() . Therefore, the precision of c and f, calculated from the isotope ratio method described above, can be much greater than when indirect calorimetry is used. Second, the isotope ratio method is not directly affected by ventilatory losses of CO2 that are not directly due to substrate oxidation, such as occurs during high-intensity exercise. In this latter circumstance, measurement of
. Therefore, the precision of c and f, calculated from the isotope ratio method described above, can be much greater than when indirect calorimetry is used. Second, the isotope ratio method is not directly affected by ventilatory losses of CO2 that are not directly due to substrate oxidation, such as occurs during high-intensity exercise. In this latter circumstance, measurement of ![]() represents much less of a problem than measurement of
represents much less of a problem than measurement of ![]() , since it does not change for reasons other than physiological changes in substrate oxidation.
, since it does not change for reasons other than physiological changes in substrate oxidation.
Performance of Breath Ratio Method
In order to apply the breath ratio method, it is not necessary to administer tracers, although pretreatment with ingested 13C-enriched cornstarch will amplify the differences among substrates and therefore improve accuracy. The enrichments of carbohydrates, fat, and protein can be obtained from a single blood sample. It has been shown that circulating lipids are a good reflection of peripheral fat stores, and, given a constant source of carbohydrate in the diet, blood glucose reflects muscle and liver tissue glycogen enrichment (Romijn et al., 1992). The procedure involves simple extraction, combustion, and isotope ratio mass spectrometry analysis. The breath 13C/12C ratio can be determined on a 15- to 20-ml sample collected through a mouthpiece and injected into a vacutainer for later analysis using an isotope ratio mass spectrometer (IRMS). ![]() must be determined if the
must be determined if the
relative contributions of fat and carbohydrate are to be converted into absolute rates. Conventionally, this is accomplished by indirect calorimetry, but portable devices are available that can accomplish this in the field. Alternatively, ![]() can be used in the calculations. For field studies,
can be used in the calculations. For field studies, ![]() may be approximated by a stable isotope technique involving a bolus injection of NaH13CO3, and collection of multiple 15-ml samples of breath for subsequent IRMS analysis (Wolfe, 1992).
may be approximated by a stable isotope technique involving a bolus injection of NaH13CO3, and collection of multiple 15-ml samples of breath for subsequent IRMS analysis (Wolfe, 1992).
Current Research Using Breath Ratio Method
Application of this method has been limited to one study of endurance-trained cyclists at rest and during steady-state exercise at 80 percent of ![]() (Romijn et al., 1992). Figure 9-1 shows the change in breath 13C/12C ratio with exercise, and Table 9-1 shows the individual values compared with values obtained from the more conventional measurement of substrate oxidation using indirect calorimetry. The results show the breath ratio method to be a reliable alternative approach to quantify substrate oxidation, both at rest and during strenuous exercise.
(Romijn et al., 1992). Figure 9-1 shows the change in breath 13C/12C ratio with exercise, and Table 9-1 shows the individual values compared with values obtained from the more conventional measurement of substrate oxidation using indirect calorimetry. The results show the breath ratio method to be a reliable alternative approach to quantify substrate oxidation, both at rest and during strenuous exercise.
Scientific Advantages and Disadvantages of the Breath Ratio Methodology
The major advantage of the breath ratio method to determine the relative contributions of carbohydrate and fat oxidation to overall energy expenditure is
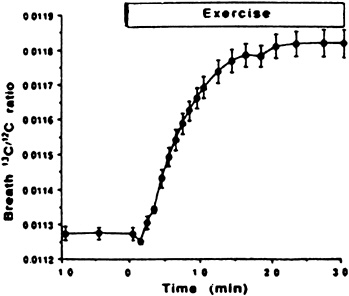
FIGURE 9-1 Absolute 13C12C molar ratios in expired air at rest and during 30 minutes of exercise at 80 to 85 percent ![]() . Plateau value in 13C/12C enrichment is reached after 15 to 20 minutes. SOURCE: American Journal of Physiology (Romijn et al., 1992), used with permission.
. Plateau value in 13C/12C enrichment is reached after 15 to 20 minutes. SOURCE: American Journal of Physiology (Romijn et al., 1992), used with permission.
TABLE 9-1 Substrate Oxidation Rates at Rest and During Exercise
the portability of the method, as no equipment is required. For quantifying rates of oxidation, however, there is less advantage because either ![]() or
or ![]() must also be determined, and this is best accomplished with indirect calorimetry. While indirect calorimetry can be performed easily to determine either
must also be determined, and this is best accomplished with indirect calorimetry. While indirect calorimetry can be performed easily to determine either ![]() or
or ![]() , the conventional use of indirect calorimetry to determine the rate of substrate oxidation requires calculation of
, the conventional use of indirect calorimetry to determine the rate of substrate oxidation requires calculation of ![]() , and thus both
, and thus both ![]() and
and ![]() must be determined with great precision. This is a particular problem during intense exercise because loss of breath CO2 occurs, not only as a result of metabolic production, but also in order to maintain acid-base balance. The
must be determined with great precision. This is a particular problem during intense exercise because loss of breath CO2 occurs, not only as a result of metabolic production, but also in order to maintain acid-base balance. The
resulting depletion of the bicarbonate pool invalidates indirect calorimetry as a tool to quantify substrate oxidation, yet this problem does not affect the validity of the breath ratio method because ![]() can still be determined accurately. The breath ratio method has an important advantage over traditional precursor-product tracer methods to measure substrate oxidation, in that isotopic exchange is not a problem. This is because no exogenous tracer is administered. Further, the true precursor enrichment for the formation of labeled CO2 from an infused tracer is extremely difficult to determine accurately, since oxidation occurs intracellularly. In contrast, by utilizing the measured 13C/12C ratios in all compounds in the body (carbohydrate, fat, and protein), all possible precursor enrichments have been taken into account. Another advantage of the new ratio method, compared with the traditional tracer methods used to quantity substrate oxidation, is that changes in bicarbonate recovery that occur during exercise (Barstow et al., 1990) need not be considered. Alterations in bicarbonate recovery have no influence on the 13C/12C ratio in the expired air, since no exogenous tracer is infused. Thus, whatever pathways of CO2 retention occur, these pathways cannot distinguish whether the CO2 has been derived from carbohydrate or fat oxidation, and therefore the enrichment (in excreted CO2) will not be affected by retention.
can still be determined accurately. The breath ratio method has an important advantage over traditional precursor-product tracer methods to measure substrate oxidation, in that isotopic exchange is not a problem. This is because no exogenous tracer is administered. Further, the true precursor enrichment for the formation of labeled CO2 from an infused tracer is extremely difficult to determine accurately, since oxidation occurs intracellularly. In contrast, by utilizing the measured 13C/12C ratios in all compounds in the body (carbohydrate, fat, and protein), all possible precursor enrichments have been taken into account. Another advantage of the new ratio method, compared with the traditional tracer methods used to quantity substrate oxidation, is that changes in bicarbonate recovery that occur during exercise (Barstow et al., 1990) need not be considered. Alterations in bicarbonate recovery have no influence on the 13C/12C ratio in the expired air, since no exogenous tracer is infused. Thus, whatever pathways of CO2 retention occur, these pathways cannot distinguish whether the CO2 has been derived from carbohydrate or fat oxidation, and therefore the enrichment (in excreted CO2) will not be affected by retention.
The only significant disadvantage is that the accuracy of the breath ratio method is highly dependent on the inherent differences in the ratio of 13C/12C in carbohydrate as compared to fat and protein. For this reason, it may be desirable to add 13C-enriched carbohydrate to the diet. To use this method for an exercise study, however, it is necessary to use a glycogen-depleting exercise before starting the 13C-glucose ingestion so that the muscle glycogen pool will be labeled (Romijn et al., 1992). Use of this protocol obviously limits the flexibility of the method.
Cost/Benefit of New Technology
The major cost incurred with this methodology is that of an IRMS, which is generally in the $200,000 to $230,000 range, with service being as much as $30,000 per year. Some less versatile units can be bought for closer to $100,000. If it is necessary to increase the enrichment of the body's carbohydrate stores, about 1 g of 13C-glucose is required (Romijn et al., 1992), and this costs about $500. All other apparatus and supplies cost about $100. While the cost of the methodology may be exaggerated by these figures, it should be noted that an IRMS can be used for many other applications simultaneously.
How Practical is the Technology?
Operation of an IRMS requires a skilled technician and supervision by an experienced scientist. Training of a technician requires about 3 months. In the field, procedures are extremely simple, and all necessary procedures can be learned in
about 1 hour. Data can be computed easily, as only algebraic equations are involved. However, due to extremely small differences in enrichment, an experienced investigator must be involved to ensure validity of data.
Can the Method Be Used in the Field?
One of the major advantages of this method is that it can be used easily in the field to determine the relative contributions of fat and carbohydrate oxidation to total energy production. However, field use would be limited by the requirement for quantitative oxidation rates, as indirect calorimetry would be needed.
TRACER INFUSION MEASUREMENT OF SUBSTRATE OXIDATION
When indirect calorimetry (or the breath ratio method) is used to quantify substrate oxidation, the additional use of stable isotopes to quantify substrate kinetics (i.e., the rate of release into blood and clearance from blood can provide valuable insight into the regulation of the processes involved. Substrate kinetics can only be accomplished at the whole-body level by the infusion of tracers. The basic idea of infusing a substrate such as glucose or a fatty acid such as palmitate that is labeled with 13C to determine its rate of oxidation by the excretion of 13CO2 is elegant for its simplicity. However, whereas the use of 13C-labeled tracers to measure substrate oxidation is in fact feasible when proper correction factors are used to account for isotopic exchange, the methodology is far more complex than was thought in earlier years.
Description of Methodology
In this section, a specific application of tracers to quantify fatty acids and glucose kinetics in exercise will be discussed. With substrate oxidation quantified by indirect calorimetry, it is possible with tracers to distinguish the source (i.e., plasma vs. intracellular stores) of the substrate being oxidized and the regulation of the processes that make them available for oxidation. It should be kept in mind that the general approach to be presented is only representative of tracer methods and is by no means the only possible methodology using stable isotopes.
The rate of appearance of glucose in the plasma, and rate of tissue uptake, can be quantified by traditional tracer techniques using glucose with two deuterium atoms attached at the 6-carbon position (6, 6-D2-glucose) (Wolfe, 1992). Although widely used in a variety of circumstances, this methodology has been used infrequently in exercise. One major problem has been the complications arising from the nonsteady-state kinetics induced by exercise. The limitations in calculating glucose kinetics in the nonsteady state have been well documented (e.g., Wolfe, 1992). The problems caused by rapid changes in plasma enrichment can be overcome to a great extent, however, by appropriate changes in tracer infusion rate
(Romijn et al., 1993). By first running a pilot study to determine the magnitude of change in enrichment caused by exercise, it is possible to adjust the tracer infusion rate appropriately to maintain a relatively steady state in isotopic enrichment during exercise, even when large changes in plasma glucose concentration occur (Romijn et al., 1993). This approach enables accurate calculation of glucose appearance and uptake, even in strenuous exercise.
From the total rate of carbohydrate oxidation, obtained by indirect calorimetry, and the rate of tissue glucose uptake (Rd), determined by means of the tracer, the minimum contribution of muscle glycogen stores to carbohydrate oxidation can be calculated, assuming that 100 percent of plasma glucose uptake is oxidized during exercise (Romijn et al., 1993). If less than 100 percent is oxidized, the calculated value for intramuscular glycogen oxidation will be underestimated. Therefore, the following equation provides the minimal rate of muscle glycogen oxidation:
muscle glucogen oxidation = total carbohydrate oxidation-glucose Rd.
Thus, by this method, any glycogen breakdown that leads to lactate production rather than complete oxidation will not be included in the calculation of rate of oxidation. Glycogen breakdown therefore exceeds the calculated rate of glycogen oxidation by a percentage amount equal to the percent of plasma glucose converted to lactate, since the metabolic pathways of glycogen and glucose converge at glucose 6-phosphate and are the same thereafter.
Fatty acids are the predominant energy substrate in almost all circumstances in the fasting state, except during extremely strenuous exercise. Understanding the factors controlling the release of fatty acids as a result of the breakdown of stored triglyceride (TG) (lipolysis) and the oxidation of fatty acids therefore is central to understanding the metabolic response in exercise. Recent advances in stable isotope methodology now allow the quantitative aspects of many components of fatty acid kinetics and oxidation to be determined.
Because the enzyme glycerol kinase is necessary for the first step in the metabolism of glycerol, and this enzyme is found only in the liver (Dixon and Webb, 1979, 842–843), glycerol can only be metabolized in the liver. Hence, lipolysis can be quantified by means of the determination of the rate of appearance of glycerol, as determined by a stable isotope tracer of glycerol such as 2H5 or 2-13C-glycerol (Wolfe, 1992). Furthermore, since glycerol dissolves freely in water, there is no limitation to the amount of glycerol that can diffuse from the intracellular site of lipolysis into the plasma. Also, there is no endogenous metabolic production of glycerol (Wolfe and Peters, 1987). Finally, there is virtually no partial hydrolysis of triglyceride (Arner and Ostman, 1974), meaning that three fatty acids are released uniformly with every glycerol molecule that is released.
The fatty acids released into the intracellular space of the adipocyte have two potential fates—entry into the plasma or reesterification into triglycerides within the adipocyte. The process of reesterification involves the attachment of three fatty acids to a—glycerol phosphate derived from the metabolism of plasma glucose.
This may occur because of an abundance of a—glycerol phosphate, as occurs during hyperglycemia (Wolfe and Peters, 1987), or because of a low rate of blood flow through the fat. A decrease in blood flow is important because FFA are not soluble in water. They are carried in plasma bound to albumin, and availability of binding sites on albumin can be rate limiting for transport when blood flow is low.
Once released into the plasma, the fatty acids can be cleared, by tissues such as muscle, for oxidation, or they can be cleared by the liver and reesterified into TG and transported back to the periphery as very low density lipoprotein. Thus, in the general sense, TG-FFA cycling can be considered to involve the reesterification of fatty acids that have been initially released as a consequence of lipolysis.
The difference between the total release of fatty acids and the rate of appearance (Ra) of fatty acids in the plasma (FFA Ra) is equal to the rate of reesterification of fatty acids within the adipocyte. The FFA Ra can be determined by means of a labeled tracer of any individual FFA (e.g., [1-13C]-palmitate), and then converted to total FFA turnover by dividing Ra palmitate by the fraction of the total FFA concentration represented by palmitate (determined by gas chromatography). As mentioned earlier, this calculation presumes that all fatty acids released by lipolysis either enter the plasma or are reesterified. It also is possible that fatty acids might be "directly" oxidized without entering the plasma. This would cause an overestimation of the intracellular recycling of FFA. However, it seems unlikely that this pathway of oxidation occurs to a great extent at rest. Data from a large number of experiments demonstrate the ratio of FFA Ra/Ra glycerol to be about 3/1 (e.g., Miyoshi et al., 1988; Wolfe et al., 1987). Since "direct" oxidation of FFA would involve the release of glycerol into the plasma, the ratio of FFA Ra/Ra glycerol would be significantly less than 3 if there was much of this type of oxidation. This is precisely the observation in strenuous exercise, as will be discussed below.
Given the rate of total lipolysis and total FFA release into (and uptake from) plasma and the rate of total fat oxidation, it is possible to distinguish quantitatively between lipolysis in adipose tissue (peripheral lipolysis) and intramuscular lipolysis. If all FFA taken up from the plasma during exercise can be assumed to be oxidized (Romijn et al., 1993), then the following equation provides a minimal rate of intramuscular fatty acid oxidation:
where FFA Rd is the rate of tissue uptake of FFA. For every three fatty acids released from the intramuscular triglyceride pool, one glycerol will be released into plasma. Consequently, the minimum rate of release of glycerol from the intramuscular TG pool (intramuscular lipolysis) will be calculated as follows:
The total rate of glycerol release is equal to the glycerol released from peripheral adipocytes plus the glycerol released from the intramuscular pool. Consequently, it is possible to calculate the rate of adipocyte (peripheral) lipolysis as follows:
Review of Application of Methodology
Tracer infusion has been used to assess substrate metabolism in highly trained endurance cyclists at three different exercise intensities (25, 65, and 80% ˙VO2max). The subjects were studied in the postabsorptive state on 3 consecutive days. On each day, a different exercise intensity was performed; the order of the intensities was randomized. On each occasion, stable isotopes were infused, and indirect calorimetry was used to determine substrate oxidation. The following tracers were infused: 6, 6-D2-glucose; 2H5-glycerol; and 2H2-palmitate. Changes in tracer infusion rates maintained plasma enrichments relatively constant.
The contribution to energy expenditure derived from glucose and FFA taken up from blood and from muscle glycogen and triglyceride are shown in Figure 9-2. The total amount of calories available from plasma does not change in relation to exercise intensity. At higher exercise intensities, muscle stores of glycogen and triglyceride become more important as energy substrates.
Scientific Advantages and Disadvantages
Alternative methodologies utilizing stable isotope tracers include the tissue or organ balance technique, biopsy analysis, and the use of radioactive tracers.
The balance technique, in which the arteriovenous difference of a substrate is multiplied by the rate of blood flow, can provide useful information about regional metabolism, but the balance approach cannot be used to quantify glucose production or the whole-body rate of lipolysis. The leg balance technique can give information about muscle, but strict interpretation of the data is made difficult by the inclusion of blood from skin and fat in the femoral vein. Finally, no information about the metabolism of the intramuscular stores of carbohydrate (i.e., glycogen) or TG can be obtained with the balance technique.
Considerable information about muscle glycogen metabolism has been obtained from biopsy data, but this approach has been far less successful in analyzing intramuscular TG metabolism. Limitations of the biopsy approach are its invasiveness and the unquantitative nature of the data as related to whole-body substrate metabolism.
The use of tracer methodology as described in this paper is relatively noninvasive, in that only peripheral venous catheters are required (for infusion of tracer and removal of samples). Further, a continuum of data can be obtained, as opposed
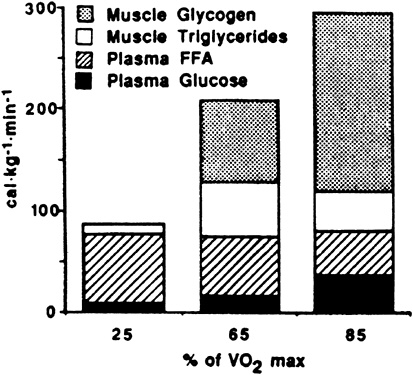
FIGURE 9-2 Maximal contribution to energy expenditure derived from glucose and free fatty acids (FFA) taken up from blood and minimal contribution of muscle triglyceride and glycogen stores after 30 minutes of exercise, expressed as function of exercise intensity. Total energy (cal) available from plasma does not change in relation to exercise intensity.
SOURCE: American Journal of Physiology (Romijn et al., 1993), used with permission.
to the spot samples obtained by biopsy. Although assumptions are required to quantify intramuscular glycogen and TG metabolism, these assumptions are reasonable, and this is the only way to obtain such estimates, as well as the only method to quantify plasma fatty acid and glucose kinetics. In theory, the experiments described in this paper could be done with radioactive tracers. Advantages provided by stable isotopes are that they pose no health risk, and the isolation of compounds by gas chromatography prior to mass spectrometry not only provides a high degree of precision in analysis but also enables the concurrent use of multiple tracers. In the example provided in which glucose, glycerol, and FFA kinetics were determined during exercise, three different tracers labeled with 2H were used. It would be extremely difficult (or impossible) with routine analytical procedures to use simultaneously three tracers labeled with the radioactive label 3H and sort out the relative contributions of the three compounds to the observed radioactivity.
The only potential disadvantage of stable isotope methodology is that a larger amount of stable isotope tracer must be infused then when a radioactive tracer is
used. This limits the experimental protocols so that the experimental procedure does not affect the endogenous kinetics of the substrate being traced.
AUTHOR'S CONCLUSIONS AND RECOMMENDATIONS
The use of tracers labeled with stable isotopes is the most effective means by which to quantify plasma substrate kinetics. The technology is readily available, and gas chromatography-mass spectrometers (GC-MS) are being produced at progressively lower prices as computers become available. An adequate GC-MS for the procedures described in this paper can be purchased for about $75,000. The cost of stable isotopes varies according to the tracer, but an individual experiment will cost about $200. The use of stable isotopes to quantify substrate oxidation is complicated by the need to make appropriate corrections for isotopic exchange, and in many circumstances, it can be more easily accomplished by classical indirect calorimetry. However, for specific applications, the use of tracers is valuable and can be accomplished with precision and accuracy.
Support of further development of the methodology is probably not required. The limitation of the methodology to field studies is significant because infusion pumps and intravenous infusions are required for optimal performance of the techniques. As currently used, this methodology is limited to a lab setting. Modifications to enable field studies are possible but not yet developed.
REFERENCES
Arner, P., and J. Ostman 1974 Mono and di-acyl glycerols in human adipose tissue. Biochem. Biophys. Acta 369:209–221.
Barstow, T.J., D.M. Cooper, E.M. Sobel, E.M. Landau, and S. Epstein 1990 Influence of increased metabolic rate on 13C-bicarbonate washout kinetics. Am. J. Physiol. 259:R163–R171.
Dixon, M., and E.L. Webb 1979 Enzymes. New York: Academic.
Frayn, K.N. 1983 Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 55:628–634.
Miyoshi, H., G.I. Schulman, E.J. Peters, M.H. Wolfe, D. Elahi, and R.R. Wolfe 1988 Hormonal control of substrate cycling in humans. J. Clin. Invest. 81:1545–1555.
Romijn, J.A., E.F. Coyle, J. Hibbert, and R.R. Wolfe 1992 Comparison of indirect calorimetry and a new breath 13C/12C ratio method during strenuous exercise. Am. J. Physiol. 263(28):E64–E71.
Romijn, J.A., E.F. Coyle, L.S. Sidossis, A. Gastaldelli, J.F. Horowitz, E. Endert, and R.R. Wolfe 1993 Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 265 (28):E380–E391.
Wolfe, R.R. 1992 Radioactive and Stable Isotope Tracers In Biomedicine: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss.
Wolfe, R.R., and E.J. Peters 1987 Lipolytic response to glucose infusion in human subjects. Am. J. Physiol. 252(15):E189–E196.
Wolfe, R.R., E.J. Peters, S. Klein, O.B. Holland, J. Rosenblatt, and H. Gary, Jr. 1987 Effect of short-term fasting on the lipolytic responsiveness in normal and obese human subjects. Am. J. Physiol. 252:E189–E196.
DISCUSSION
VERNON YOUNG: Bob, would you just remind me what the protocol is with respect to the slide about three slides back where you brought the oxidative contribution of plasma and intracellular fats, what is the actual protocol?
ROBERT WOLFE: The subjects are infused with 13C-oleate and 14C-octanoate.
VERNON YOUNG: No, I do not mean the oleate, but the earlier one which you indicated was totally noninvasive.
ROBERT WOLFE: In that protocol, the subjects are infused with 13C-palmitate and deuterated glucose. Then the substrate oxidation is measured by indirect calorimetry.
In the calculation of the data, the assumption was made that during exercise, 100 percent of plasma glucose uptake is oxidized, so the calculation gives you a minimal value for intramuscular glycogen.
Alternatively, if 13C-glucose was infused, plasma glucose oxidation could be measured directly by collecting 13CO2. However, previous data indicate that most glucose uptake is oxidized in exercise, and in any case, plasma glucose makes a small contribution to the total rate of oxidation. Therefore, the assumption is reasonable.
JOHANNA DWYER: Is that published somewhere?
ROBERT WOLFE: Yes, that experiment is in The American Journal of Physiology (Romijn et al., 1993) about a year or two ago.
WENDY KOHRT: Does your model take into account the possibility that fatty acids hydrolyzed from intramuscular triglycerides could be released into the plasma?
ROBERT WOLFE: I kind of skipped over some of the details. To the extent to which free fatty acids [FFA] are released into the plasma, then they become part of the plasma pool, so they are included in the measurement of the plasma FFA enrichment. The evidence would indicate that this process does not occur to any significant extent, and the reason is that in situations in which significant intramuscular triglyceride hydrolysis occurs, the rate of release of glycerol into blood increases dramatically in relation to free fatty acid release. There are three fatty acids relative to one glycerol normally released as a consequence of the hydrolysis of triglyceride, but the muscle has a very limited ability to use glycerol.
As the exercise intensity increases, the rate of whole-body glycerol/FFA Ra drops from approximately 3:1 to as low as 1:1, indicating almost all the fatty acid that is released from muscle triglyceride is directly oxidized in the muscle and the glycerol released into the plasma.
DONALD McCORMICK: It bothered me that this technique could not be made to do good kinetic work. You gave examples of releases of cellular drug in the plasma. Unless another very significant correction is made, every time one passes a membrane, one has the problem of diffusion. So every time you are going from wherever the site of origin is, whether oral or injected, to a series of membranes, mitochondria and otherwise, you are discriminating between 12C and 13C or 12C and 14C, and that is a multiple effect. You are not getting a reflection of the real kinetics of the predominant 13C pool compound. The smaller the molecule, the greater the discrimination.
What I am really after is obviously the question of whether you can do a 13C mass spectrometric analysis and a 14C radiotracer and get at what the effects are of multiple membranes, say, in fatty acid oxidation, whether you can get the time factor. When you are doing steady state or whole body, it does not matter. But do you or others have those factors in the kinetic sense?
ROBERT WOLFE: The extent to which 13C is discriminated as opposed to 12C, I think, is of minimal concern, and the reason I say that is that if you look at the data on which we based the total oxidation of carbohydrate, protein, and fat, using the 13C/12C breath ratio method, the values come out almost exactly the same as with indirect calorimetry, which means the same rates are obtained without use of isotopes. Furthermore, kinetic studies with radio-labeled tracers and stable isotope tracers yield comparable data.
DONALD McCORMICK: Those are long lump times, though.
ROBERT WOLFE: I presented values derived almost entirely in steady state conditions. The kinetic modeling in nonsteady state with a stable isotope is more complex, but I would say it can be done. But I think that in terms of nutritional aspects and total substrate oxidation I would definitely recommend staying away from that. The problems, however, are more with modeling than isotope discrimination.
DENNIS BIER: With regard to the isotope effects, there are rather sizable isotope effects if you are talking about deuterium and tritium. If you look at the mass differences that are smaller, I mean, people have investigated 13C and 12C isotope effects on the molecular level, on the biochemical level, on the cellular level, and even with totally 13C-labeled enzymes, for example, compared to their substrates, the differences are about 1 to 2 percent. I mean, they are small enough that we cannot measure them.
Now, we can tell those differences by isotope ratio mass spectrometry because that can measures parts per 100,000. That is exactly the basis of isotope fractionation in breath, the kind of thing Bob talked about with substrate oxidation, but it is below the limits of any detection that you can do.
DONALD McCORMICK: At steady state.
DENNIS BIER: Right. There is at least one set of experiments that I know of where people tried to look at transport in an organ with different isotope-labeled materials, specifically glucose, and that was done by Riccardo Bonnadonna, Ralph DeFronzo, Clyde Epidelli, myself, and others, where we gave 13C with different labels and modeled the differences between those isotopes as they appeared inside the cell and they appeared in metabolites, et cetera, to discriminate transport, and in fact, we think we were successful.
















