Appendix C Energy Source Technologies
BATTERIES
Although batteries in general represent a very large, mature product class in commercial production, enormous improvements in specific power, specific energy, and cycle life (for rechargeable batteries) have been made in the past decade (Space Power Institute, 1990, 1992b). Much of the driving force for the technical improvements has come from the rapid growth of portable computers, cellular telephones, and other communication devices. However, very few of these improvements have been of direct benefit to communication devices used by the Army.
Battery production worldwide is approximately $40 billion (Salkind, 1996) with U.S. production at about $11 billion. Military purchases are only a small percentage of the total, and there appears to be little interest among large manufacturers in producing military batteries.
Improving the specific energy (available energy from a fixed mass) and energy density (available energy from a fixed volume) of batteries have been commercial goals. But because most commercial devices require only a few AA cells, weight reduction has been second in importance to energy capacity. As shown in Figure C-1, the capacity of AA nickel alkaline (NiCd and NiMH) batteries has risen from 0.4 Ah to 1.2 Ah in the past 20 years. Very fast recharging (in less than 1 hour) has also become available. Lithium rechargeable systems in the same size packaging have approximately the same capacity, but at much higher voltages, resulting in cells with higher specific energy. However, so far lithium rechargeable cells cannot be recharged quickly. Improvements continue to be made.
Among the Army's options for keeping pace with these rapid changes is the adaptation of commercially available cells. Current military battery systems could be replaced by systems with different voltage characteristics as long as the new system volume is the same or smaller. This should be possible with new, more efficient techniques for DC-DC conversion, which would eliminate the problem of Army communication devices being locked into using power sources with particular voltage levels.
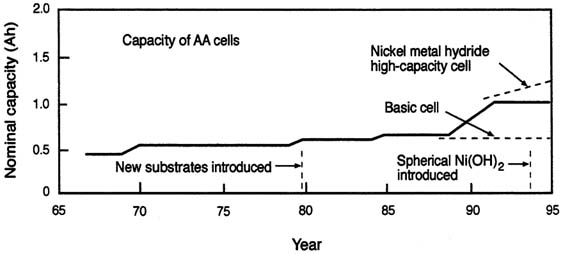
FIGURE C-1 Chronological improvements in the capacity of AA nickel batteries.
The performance characteristics and production levels of the common primary, secondary, and special battery systems considered in this report are listed in Tables C-1, C-2, and C-3.
Systems Likely to Meet the Needs of the Dismounted Soldier
Of the more than 30 rechargeable battery systems in commercial production or in advanced development, only seven or eight seem likely to meet the military goals of availability in small sealed cells with appropriate levels of safety, reliability, and low temperature and high temperature performance. These few systems are described in this section, with estimates of their present performance levels and estimates of what might be achieved in five and ten years. The research needed to achieve the listed goals is also briefly described.
Although a low temperature requirement of -40°C is still listed in some Army documents, the committee was informed that this temperature requirement was principally for storage. For operations, the committee assumed a minimum temperature requirement of -25°C but even this may be unrealistically low and may disqualify otherwise practical systems.
The systems likely to provide the desired combination of compactness, specific energy, and specific power fall into two categories: rechargeable alkaline electrolyte systems (nickel-metal hydride, nickel-zinc, MnO2-zinc) and rechargeable lithium electrode systems (lithium metal anodes, lithium intercalating anodes, lithium alloy anodes [including the tin oxide type]).
TABLE C-1 Summary of Primary Battery Data
|
|
|
|
Theoretical |
Working |
|
||||
|
Battery System |
Anode |
Cathode |
Voltage |
Ah/kg |
Wh/kg |
Voltage |
Wh/kg |
Wh/l |
Production Valuea |
|
Lechlanche (zinc-carbon) |
Zn |
MnO2 |
1.6 |
224 |
358 |
1.5 |
85 |
165 |
vl |
|
Magnesium |
Mg |
MnO2 |
2 |
271 |
758 |
1.75 |
100 |
195 |
vs |
|
Alkaline |
Zn |
MnO2 |
1.6 |
224 |
336 |
1.25 |
125 |
330 |
vl |
|
Mercury |
Zn |
HgO |
1.34 |
190 |
255 |
1.3 |
100 |
470 |
vvs |
|
Silver (silver-zinc) |
Zn |
Ag2O |
1.5 |
180 |
288 |
1.45 |
120 |
500 |
ss |
|
|
|
AgO |
1.85 |
270 |
445 |
(2 plateaus) |
140 |
650 |
|
|
Zinc-air |
Zn |
O2(air) |
1.65 |
658 |
1,066 |
1.25 |
500 |
1,050 |
1 |
|
Aluminum-air |
Al |
O2(air) |
2.7 |
2,980 |
8,046 |
1.1 |
300 |
240 |
vs |
|
Lithium Systems |
|||||||||
|
Sulfur dioxide |
Li |
SO2 |
3.1 |
379 |
1,175 |
2.8 |
260 |
415 |
1 |
|
Thionyl chloride |
Li |
SOCl2 |
3.66 |
407 |
1,489 |
3.3 |
320 |
700 |
1 |
|
Sulfuryl chloride |
Li |
SO2Cl2 |
3.9 |
360 |
1,405 |
3.7 |
450 |
900 |
vvs |
|
Manganese dioxide |
Li |
MnO2 |
3.5 |
286 |
1,001 |
2.8 |
230 |
550 |
vl |
|
Carbon monofluoride |
Li |
(CF)x |
3.1 |
703 |
2,180 |
2.5 |
250 |
600 |
l |
|
Iron disulfide |
Li |
FeS2 |
1.8 |
725 |
1,304 |
1.4 |
130 |
400 |
l |
|
a Key: vl = l = $100 million to $1 billion s = $10 million to $100 million vs =< $10 million vvs = < $2 million Improvements MnO2 cathode material improvements can increase nonlithium system capacity by as much as 15 percent. Improvements in separator material and technology can increase stability and rate of all primary cells. Air electrode improvements can increase power capability of air cathode systems. Safety for all lithium battery systems can be improved with improvements in separators. Packaging technology can increase specific energy of Li/MnO2 technology. MnO2 cathode material improvements can increase capacity and discharge rate in lithium systems. Focus Chemistries Zn, Mg/MnO2, and Li/FeS2 are commercial market driven. Zinc-air, Li/MnO2, and Li/CTx are areas of interest for the government because they have either high specific power or high specific energy or both. |
|||||||||
TABLE C-2 Summary of Rechargeable Portable Battery Data
|
|
|
|
Theoretical |
Working |
|
|
||||
|
Battery System |
Negative Electron |
Positive Electron |
Voltage |
Ah/kg |
Wh/kg |
Voltage |
Wh/kg |
Wh/l |
Production Valuea |
Estimated Life (Cycles) |
|
Lead-acid |
Pb |
PbO2 |
2.1 |
83 |
175 |
2.0 |
35–50 |
85 |
vl |
400 |
|
Nickel-iron |
Fe |
NiOOH |
1.4 |
224 |
313 |
1.2 |
35–60 |
70 |
vs |
500 |
|
Nickel-cadmium |
Cd |
NiOOH |
1.35 |
181 |
244 |
1.2 |
35–52 |
75 |
vl |
600 |
|
Nickel-zinc |
Zn |
NiOOH |
1.73 |
215 |
372 |
1.6 |
65–80 |
150 |
s |
400 |
|
Silver-zinc |
Zn |
AgO |
1.85 |
283 |
524 |
1.5 |
90–150 |
180 |
vs |
100 |
|
Nickel-hydrogen |
H2 |
NiOOH |
1.5 |
269 |
434 |
1.4 |
55–60 |
60 |
S |
600 |
|
Nickel-metal hydride |
Mhx 1.2 to 2 w/o H |
NiOOH |
1.35 |
206 |
278 |
1.2 |
55–70 |
120 |
vl |
800 |
|
Silver-cadmium |
Cd |
AgO |
1.4 |
227 |
318 |
1.2 |
60–80 |
110 |
vvs |
200 |
|
Zinc-bromineb |
Zn |
Br Complex |
1.85 |
139 |
258 |
1.55 |
70 |
60 |
vvs |
400 |
|
Alkaline manganese |
Zn |
MnO2 |
1.6 |
224 |
330 |
1.2 |
55 |
250 |
vl |
15 |
|
Zinc-air |
Zn |
O2 (air) |
1.6 |
658 |
1,085 |
1.15 |
110 |
130 |
vs |
25 |
|
Lithium Systems |
||||||||||
|
LiMn2O4 |
Li |
Mn2O4 |
4 |
143 |
510 |
3.7 |
140 |
300 |
vs |
250 |
|
LiNiO2 |
Li |
NiO2 |
4.2 |
137 |
575 |
3.6 |
155 |
325 |
res |
— |
|
LiCoO2 |
Li |
CoO2 |
4.2 |
178 |
750 |
3.7 |
95 |
235 |
vs |
250 |
|
Li/organosulfide |
Li |
R-S-S-R |
3 |
~300 |
~900 |
2 |
200 est |
300 est |
res |
300 |
|
Li/organosulfide |
Li |
(CS)x |
2 |
~400 |
~800 |
2 |
200 est |
300 est |
res |
300 |
|
|
|
|
Theoretical |
Working |
|
|
||||
|
Battery System |
Negative Electron |
Positive Electron |
Voltage |
Ah/kg |
Wh/kg |
Voltage |
Wh/kg |
Wh/l |
Production Valuea |
Estimated Life (Cycles) |
|
LiMn2O4 |
Li+C |
Mn2O4 |
4/3 |
102 |
356 |
3.7 |
70–100 |
170 |
res |
— |
|
LiNiO2 |
Li+C |
NiO2 |
4.2/3 |
100 |
360 |
3.6 |
70–100 |
170 |
res |
— |
|
LiCoO2 |
Li+C |
CoO2 |
4.2/3 |
100 |
360 |
3.7 |
70–100 |
170 |
1 |
1,000 |
|
Polymer |
Li+C |
Mn2O4 |
4/3 |
102 |
358 |
3.0 |
150 est |
300 est |
vvs |
300 |
|
Large iron sulfides |
Ll(Al) |
FeS/FeS2 |
1.33/1.73 |
285/345 |
459/514 |
1.3/1.6 |
100/180 |
200/350 |
res |
~1,000 |
|
a Key: vl = l = $100 million to $1 billion s = $10 million to $100 million vs = < $10 million vvs = < $2 million res = research b Not portable. Improvements Charger and charging methods can improve cycle life and safety of rechargeable cells. Improvements in NiOOH and separator technology can increase capacity of all nickel systems. Improvements in metal hydride anode can increase the energy by nearly 2 times (Mhx 1.2 to 2 w/o H). Material improvements can increase cycle life of rechargeable alkaline battery. Material improvements can increase cycle life of rechargeable zinc-air battery. Air cathode improvements can increase power capability and cycle life of the zinc-air system. Safety for all rechargeable lithium batteries can be improved with improvements in separators. Anode material improvements for lithium ion and lithium polymer batteries can increase the specific energy and safety. Cathode material improvements can increase specific energy of all lithium batteries. Focus Chemistries Nickel-metal/hydride, alkaline, and zinc-air are market driven; thus, unique military requirements may be overlooked. Lithium systems focus on military-unique requirements. Zn, Mg/MnO2, and Li/FeS2 market driven. |
||||||||||
TABLE C-3 Summary of Data on Reserve, Thermal, and High Temperature Rechargeable Batteries
|
Battery System |
Anode |
Cathode |
Working Voltage |
Wh/kg |
Wh/l |
Estimated Life (Cycles) |
|
Reserve |
|
|
|
|
|
|
|
Water activated |
Mg or Zn |
CuCl |
1.5–1.6 |
65 |
125 |
(Not rechargeable) |
|
|
|
MnO2 |
1.5–1.6 |
65 |
125 |
|
|
|
|
AgCl |
1.5–1.6 |
125 |
250 |
|
|
|
|
Others |
|
|
|
|
|
Spin activateda |
Pb |
PbO2 |
1.5 |
|
|
(Not rechargeable) |
|
|
Zn |
AgO |
1.4 |
|
|
|
|
|
Li |
SOCl2 |
3.5 |
|
|
|
|
|
Li |
FeS2 |
1.8 |
|
|
|
|
Electrolyte introduction-activated |
Zn |
AgO or Ag2O |
1.6 |
50 |
160 |
(Not rechargeable) |
|
|
Li |
V2O5 |
3.3 |
50 |
100 |
|
|
|
Li |
SO2 |
3 |
120 |
200 |
|
|
|
Li |
SOCl2 |
3.5 |
150 |
300 |
|
|
Thermal batteries |
Ca |
CaCrO4 |
2.4 |
30 |
40 |
(Not rechargeable) |
|
|
Mg |
V2O5 |
2.5 |
|
|
|
|
|
Li |
FeS2 |
1.8 |
40 |
100 |
|
|
High temperature rechargeable batteries |
|
|
|
|
|
|
|
Lithium-iron-sulfide |
Li |
FeS |
1.3 |
100 |
200 |
700 |
|
|
|
FeS2 |
1.6 |
180 |
350 |
1,000 |
|
Sodium-sulfur |
Na |
S |
2.1 |
170 |
250 |
100–2,000 |
|
Sodium-nickel chloride |
Na |
NiCl2 |
2.58 |
90 |
160 |
600–1,000 |
|
a These batteries are not designed to be weight or volume efficient. |
||||||
TABLE C-4 Nickel Metal Hydride Battery Systems
|
Present Advantages |
Present Disadvantages |
5 Years |
10 Years |
|
Higher specific energy than NiCd |
Lower specific power than NiCd |
Higher rate capability, 25% more capacity per volume |
40% capacity improvement per volume |
|
Rapid recharge at room temperature |
Poor charge retention, 5% per week loss at room temperature |
Charge loss reduced to 2% per week at room temperature |
— |
|
Long cycle life |
Poor thermal stability Poor overcharge recombination kinetics |
Lower vapor pressure alloys |
Lower vapor pressure alloys |
|
Maintenance free |
— |
— |
— |
Rechargeable Alkaline Electrolyte Systems
Most anode battery systems can be assembled with various cathodes and electrolytes in combinations described in the Tables C-4 through C-15. These tables present a summary of the candidates likely to meet the future power requirements of the dismounted soldier. Each table summarizes the advantages and disadvantages of each chemistry, as well as technological projections of what can be accomplished in five and ten years.
Improvements in nickel metal hydride battery systems are shown in Table C-4. The anticipated improvements will require sustained research in the following areas:
-
metal hydride alloys for better thermal stability
-
cathode materials with improved volumetric efficiency (e.g., nanostructured, fibrous, and higher valence materials)
-
charge profile with optimum charging, overcharge recombination kinetics
-
better separators
Improvements in rechargeable alkaline manganese dioxide battery systems are shown in Table C-5. To achieve the projected improvements, it will be necessary to research the following areas in depth:
-
materials for better cycle life and low temperature performance (nanostructured, catalytic MnO2, improved carbons and graphites)
-
improved cellophane (or other separator) for higher rate performance
-
optimal recharging profile
TABLE C-5 Rechargeable Alkaline Manganese Dioxide (RAM) Battery Systems
|
Present Advantages |
Present Disadvantages |
5 Years |
10 Years |
|
Low cost |
Lower specific power |
Improved rate |
Improved cycle life |
|
Maintenance free |
Poor cycle life |
Improved cycle to cycle capacity |
Improved low temperature operation |
|
Good charge retention |
Decreasing capacity with cycle life and depth of discharge |
— |
— |
|
|
Poor low temperature performance |
— |
— |
Improvements in metal zinc battery systems are shown in Table C-6. To achieve the projected improvements, major research will be needed in the following areas:
-
cathode materials for improved volumetric efficiency (e.g., nanostructured, fibrous, higher valence)
-
lightweight current collectors for the nickel electrode
-
charge profile for optimal charging, overcharge recombination kinetics
-
better separators, microporous membranes, and cellulosic films
-
complex electrolytes for improved cycle life
TABLE C-6 Nickel Zinc (NiZn) Battery Systems
|
Present Advantages |
Present Disadvantages |
5 Years |
10 Years |
|
Higher specific energy than NiCd |
Poor overcharge recombination kinetics |
Higher specific power, 10% more capacity per volume |
20% specific energy improvement per volume |
|
Maintenance free |
— |
— |
— |
|
Rapid recharge |
Moderate charge retention; 2% per week at room temperature |
Charge loss reduced to 1% per week at room temperature |
— |
|
Moderate cycle life |
— |
Improved separator and electrolytes; 500–800 cycles |
Improved separator and electrolytes; 800–1000 cycles |
TABLE C-7 Lithium Batteries with Lithium Metal Anode Structures
|
Present Advantages |
Present Disadvantages |
5 Years |
10 Years |
|
Highest energy and power capability |
Safety |
Improved safety and cycle life through improved electrolytes |
— |
|
|
Poor cycle life |
|
|
|
|
No tolerance to overcharge and overdischarge |
|
|
Rechargeable Lithium Systems
Lithium systems offer the most promise in terms of specific energy (energy per unit weight). Lithium chemistry, however, raises serious safety and environmental concerns. Even though lithium systems as presently fabricated have no tolerance to overcharging or overdischarging, lithium batteries offer enormous promise as energy sources for the dismounted soldier. Lithium systems can be categorized by the type of components (anode, electrolyte, separator, cathode); each component can be used with a variety of other components to produce a complete cell. Tables C-7 through C-9 characterize lithium battery technologies in terms of their anode structure and materials.
Table C-7 shows improvements in lithium batteries with lithium metal anode structures. To achieve the projected improvements, research will be needed in the following areas:
-
Charge control in order to eliminate safety concerns
-
Electrolyte and separator development to improve charge morphology
-
Management of the film on lithiums surface for improved cycle life
Lithium intercalating anodes include carbon or graphite (LiCx); tin, aluminum, and other metals; and silicon and other nonmetals are shown in Table C-8 To achieve the projected improvements, research will be needed in the following areas:
-
Improved binders for improved stability of electrode
-
Materials research to increase rate capability and specific energy
-
Lighter weight host materials for lithium cathodes
-
Improved reversibility of positive electrode materials through new preparation methods
TABLE C-8 Lithium Batteries with Lithium Intercalated Anode Structures
|
Present Advantages |
Present Disadvantages |
5 Years |
10 Years |
|
Safer than lithium metal anodes |
Rate limiting electrode; no tolerance for overdischarge or overcharge |
— |
— |
|
Long cycle life |
Reduced power and specific energy as compared to lithium metal |
Improved power and specific energy through materials improvements |
Improved power and specific energy through materials improvements |
|
|
Reduced low-temperature performance |
Material and electrolyte improvements |
Material and electrolyte improvements |
|
|
Some voltage penalty over pure lithium |
Lightweight host materials for lithium electrode |
— |
Lithium alloy anodes include aluminum (LixAl); ternary alloys with manganese; and other lithium alloys such as silicon alloys are shown in Table C-9. To achieve the projected improvements, research will be needed in the following areas:
-
Materials research to increase rate capability and specific energy
-
Charge control in order to eliminate safety concern
-
Electrolyte and separator development to improve charge morphology
Lithium batteries can also be characterized with respect to electrolytes. Tables C-10 and C-11 project the developments and necessary research and development over the next ten years.
TABLE C-9 Lithium Batteries of Lithium Alloy Anode Structures
|
Present Advantages |
Present Disadvantages |
5 Years |
10 Years |
|
Increased power density as compared to lithium carbon |
Reduced specific energy as compared to lithium metal |
Improved specific power and specific energy through materials improvements |
Improved specific power and specific energy through materials improvements |
|
|
Voltage penalty |
Material and electrolyte improvements |
Material and electrolyte improvements |
|
|
No tolerance of overcharge and overdischarge |
— |
Increased tolerance of overcharge |
|
|
Rate limiting electrode |
— |
— |
TABLE C-10 Lithium Batteries with a Liquid Organic Electrolytes
|
Present Advantages |
Present Disadvantages |
5 Years |
10 Years |
|
Mixed organic stable at high voltages |
Volatile and flammable |
Material improvements to reduce flammability |
Material improvements to reduce flammability |
|
High conductivity |
Requires stable separator; presently microporous polyolefins |
Improved conductivity through salt research |
Improved conductivity through salt research |
|
|
Some toxicity |
Less toxic materials |
Less toxic materials |
|
|
No tolerance to overcharge and overdischarge |
— |
— |
Table C-10 shows improvements in lithium batteries using liquid organic electrolytes. To achieve the projected improvements, research will be necessary in:
-
Materials research to identify stable nonflammable electrolytes
-
Charge control in order to eliminate safety concerns
-
Electrolyte and separator development to improve charge morphology
-
Electrolyte salt investigation.
Table C-11 shows improvements in lithium batteries using liquid organic electrolytes. To achieve the projected improvements, research will be necessary in:
-
Materials research to identify higher conductivity electrolytes
-
Charge control in order to eliminate safety concerns
-
Electrolyte development to improve charge morphology
-
Electrolyte salt investigation
-
Lithium/polymer interface reactions (a rise in cell impedance on standing and/or cycling has been observed)
TABLE C-11 Lithium Batteries with Polymer Gel Electrolytes
|
Present Advantages |
Present Disadvantages |
5 Years |
10 Years |
|
Stable at high voltages |
Low conductivity |
— |
— |
|
Polymer electrolyte and separator |
— |
Material improvements improving conductivity |
Material improvements improving conductivity |
|
Encapsulates volatile and flammable electrolytes |
— |
Improved conductivity through salt research |
Improved conductivity through salt research |
|
|
No tolerance of overcharge and overdischarge |
— |
— |
Finally, lithium batteries can be categorized by cathode structures and materials. Tables C-12 through C-14 summarize improvements that can be expected over the next ten years for batteries using lithium manganese dioxide spinel, lithium nickel dioxide, and lithium cobalt dioxide cathode structures. To meet expectations, efforts must be focused on materials research that increases the rate capability and cycle life of the cathode.
Other Systems
There are a variety of battery types that the committee considered inappropriate for use in dismounted soldier applications. For completeness, Table C-15 lists these battery types and the deficiencies that make them undesirable.
TABLE C-12 Lithium Batteries with Lithium Manganese Dioxide Spinel (LixMn2O4) Cathode Structures
|
Present Advantages |
Present Disadvantages |
5 Years |
10 Years |
|
Inexpensive |
Poor cycle life |
— |
— |
|
High specific energy |
Moderate rate capability |
Improved cycle life and rate through material improvements |
Improved cycle life and rate through material improvements |
|
|
No tolerance to overcharge and overdischarge |
— |
— |
TABLE C-13 Lithium Batteries with Lithium Nickel Dioxide (LixNiO 2) Cathode Structures
|
Present Advantages |
Present Disadvantages |
5 Years |
10 Years |
|
High specific energy |
Poor cycle life |
— |
— |
|
|
Moderate rate capability |
Improved cycle life and rate through material improvements |
Improved cycle life and rate through material improvements |
|
|
No tolerance to overdischarge and overcharge |
— |
— |
TABLE C-14 Lithium Batteries with Lithium Cobalt Dioxide (LixCoO2 ) Cathode Structures
|
Present Advantages |
Present Disadvantages |
5 Years |
10 Years |
|
High specific life |
— |
— |
— |
|
Long cycle life |
Moderate specific power at 1 hour rate |
Improved cycle life and rate through material improvements |
Improved cycle life and rate through material improvements |
|
|
No tolerance of overcharge and overdischarge |
— |
— |
TABLE C-15 Battery Systems Not Appropriate for the Dismounted Soldier
|
System |
Deficiency |
|
Zinc-bromine |
Flowing system or noncompact, with poor volumetric and power characteristics |
|
Nickel-iron and most lead-acid batteries |
Nonsealed systems |
|
Sodium-sulfur and lithium-iron sulfide |
High temperature systems |
|
Nickel-cadmium and silver-cadmium |
Environmental problem |
|
Metal-air |
Poor power characteristics |
Charging, Safety, and Testing
Major developments in electronic circuitry now permit safe and rapid charging of most battery systems; and continued advances in both capability and cost are likely. Battery systems that once were relegated to the primary (nonrechargeable) category are now sometimes used as secondary systems because inexpensive chips can monitor the batteries and regulate the charging current profile (e.g., MnO2-Zinc). With some types of reverse-current pulse chips, recharging can be done in less than 30 minutes with lower cell temperatures (e.g., some nickel-cadmium cells). Many varieties of chargers now incorporate measurements of the state-of-charge in their control logics, and this information could be readily displayed as part of the charger design.
Lithium batteries especially must be charged very carefully. The effects of the charging current on cycle life include the formation of lithium deposits on lithium anodes and the possibility of lithium deposits on carbon anodes. Excessive charge voltages can degrade the electrolyte; when voltage exceeds the stability window of the electrolyte or electrode, it can cause a buildup of pressure and/or reactive products from electrolyte decomposition. If lithium batteries (even lithium ion batteries) are abused or improperly charged, fire or explosion may result.
Data comparing rechargeable batteries available in the general literature are unrealistic for Army use. Cycling, charging, and performance data needed by the Army should include schedules for rest time, the effects of temperature excursions, the effects of depth of discharge on performance, impedance at partial states of charge, and specific energy at different power levels. Lower voltage battery systems are inherently safer than lithium batteries, and the committee suggests that a nominal 8 V system be adopted as standard for future designs.
Necessary Technical Improvements
Major improvements (of more than 20 percent) in the performance characteristics of the battery and hybrid systems discussed in this appendix can be achieved by improvements in the following areas: processing technology; active material composition, and morphology; reinforcing components; electrolytes; and key cycle life and rate-limiting components, such as separators.
Aqueous Systems
Significant improvements in specific energy, specific power, and cycle life can be achieved by optimizing the structure and particle size of reactant materials. New low cost methods for actively preparing material will have to be developed. Candidates include xerojel and aerogel methods, using nanostructural materials, and optimizing heat treatments. Better separators will mean better electrolyte wicking and retention, which will yield longer cycle life. Better electrodes will act as structural materials, current collectors, and bipolar sheets. Improved seals will prevent gas leaks and allow for maintenance free cells. Advanced electrolyte systems, new compositions, and gelled electrolytes will contribute to better performance.
Rechargeable Lithium Cells
Research for rechargeable lithium cells should focus on the following areas:
-
overcharge and discharge tolerance via cell design and charge control
-
improved positive electrode materials and preparation methods for long cycle life, low cost, and environmental acceptability
-
better electrolytes with greater stability, improved conductivity (both polymer and liquid), and nonflammability
-
management of the Li/electrolyte interface and film
-
lower cost separators
Chargers and State-of-Charge Devices
These relatively inexpensive electronic components have a major effect on battery performance and safety. The Army must keep the option for incorporating improved circuit components as they become available. Advanced charging methods can provide rapid recharging, longer cycle life, and higher performance.
FUEL CELLS
Improved fuel cell systems can extend mission times for the dismounted soldier because they can be designed to carry varying amounts of fuel for short or long missions without adding weight to the power generating part of the unit. Fuel cells differ from most other fueled systems in that system efficiency improves as the power is throttled back.
Fuel cells are generally classified according to the electrolyte and the operating temperature. For example, the solid oxide electrolyte fuel cell (SOFA) operates at 1,000°C, the molten carbonate fuel cell operates at 650°C, the phosphoric acid fuel cell (PAFC) operates at about 200°C, the proton exchange membrane fuel cell (PEMFC) at 25 to 90°C, and the direct methanol fuel cells (DMFC) operates at 25 to 90°C.
The performance level of all fuel cells that operate at temperatures above 100°C is too low for use by the dismounted soldier. Even if their performance level were higher, however, they would not be attractive because they require long starting times and have distinctive thermal signatures.
Until recently, the specific powers of fuel cells were too low to be attractive for human-portable systems. Recent advances in PEMFCs, however, have greatly improved their specific powers and significantly lowered catalyst costs. Therefore, PEMFCs should be reevaluated (Rose et al., 1994).
State of the Art
State of the art PEMFCs can operate for thousands of hours with little loss of performance and can deliver about 700 mW/cm2 at 80°C, operating on pure hydrogen at 3 atmospheres pressure and oxygen or air at 5 atmospheres. Catalyst loadings have been reduced to about 0.3 mg platinum/cm2 for the cathode and less than 0.1 mg platinum/cm2 for the anode. At ambient atmospheric pressure, performance is reduced to 350 mW/cm2 of electrode area. Unfortunately, the platinum electrocatalyst of the anode is very sensitive to certain impurities in the hydrogen fuel, including carbon monoxide and sulfur compounds.
The leading supplier of PEMFC stacks is the Ballard Company of Canada. The specific power available from the 5-kW stack is about 1,000 W/kg, and the
stack is scaled down, the specific power will be reduced somewhat. Because the electrolytic conductivity of the PEMFC is a strong function of water content, the membrane must be kept in a highly hydrated state at all times. This means that heat and water management in the system are critical.
The specific power of small PEMFC stacks operating on hydrogen and air is now 50 to 100 W/kg. The rest of the system will reduce this figure significantly. For example, the Ball Aerospace ''Snorkler" fuel cell system provides 100 W of power, 5 kWh of energy, and weighs 12.24 kg, corresponding to a system specific power and energy of 8.17 W/kg and 408 Wh/kg. Recent Army fuel cell project goals for small systems have been 50 W, 200 Wh, and 2 kg with specific power and energy goals of 25 W/kg and 100 Wh/kg respectively. For larger systems, current goals are 150 W, 600 Wh, 8 2.5 kg, and specific power and energy of 60 W/kg and 240 Wh/kg. These figures are for PEMFC systems that rely on oxygen from ambient air. If bottled oxygen is used, the specific power and specific energy are substantially lower.
Figure C-2 shows the estimated weight (mass as a function of energy) of H2/PEM/air fuel cell systems, including the "Snorkler" and two future systems, one using compressed hydrogen stored at 3,000 psi and the other at 8,500 psi in an advanced wound-fiber tank. A system using hydrogen from a chemical hydride generator is also shown.
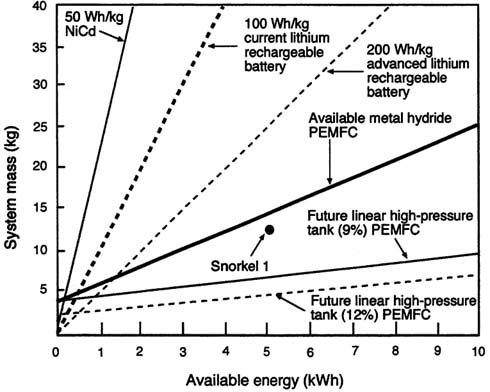
FIGURE C-2 Projected performance of 50 W hydrogen PEMFCs with a variety of fuel storage techniques.
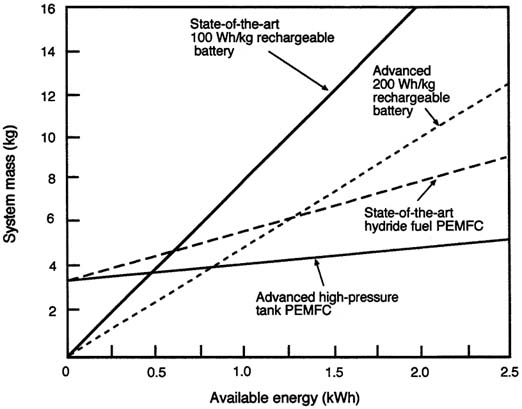
FIGURE C-3 Graph showing the crossover points for battery and fuel cell power systems asfunctions of available energy and system mass.
Figure C-3 is an expanded version of Figure C-2 that shows in more detail the crossover between the mass of battery systems and fuel cells as a function of the mission time in kWh. Fuel cells would be competitive for energy budgets greater than 1 kWh. Note that the assumed specific energy of the advanced rechargeable battery in the figure is comparable to that of current primary batteries. For advanced fuel cells, the energy storage advantage becomes apparent at approximately 0.75 kWh. For all of the figures showing system mass as a function of energy available, it is assumed that batteries can be scaled linearly to very small sizes. (In reality, off-the-shelf batteries are discrete units assembled in larger units to make up a power pack.)
On the basis of available energy, fuel cells offer a decided weight advantage when the energy demand exceeds of 1 kWh. For missions of a few hours or more, PEMFCs have an advantage over all rechargeable batteries currently available or under development. For shorter missions, the combination of relatively high specific power and reasonable specific energy make batteries more attractive.
In recent years there has been a renewed interest in DMFCs (direct methanol fuel cells) because of the possibility of avoiding the expense and technical problems involved in using hydrogen. Significant improvements in the performance of DMFCs have been made, and they now yield up to 250 mA/cm2 of electrode area at 0.5 V/cell, with a platinum/ruthenium loading of 4 mg/cm2, operating at 95°C, and 20 psi(g) oxygen, in laboratory tests. Endurance tests must still be performed. Additional improvements in DMFCs can be expected in the coming years.
Problems
The PEMFC has been improved significantly in the past few years, but some technical and economic issues have yet to be resolved. First, the cost of a PEMFC is around $1,000/m2, or $140/kW at a peak power density of 700 mW/cm2. At lower power densities, the cost is proportionately higher. Second, simultaneous heat and water management in PEMFC systems is a significant problem for small systems because the water content of the membrane must be kept high for maximum conductivity. Therefore, thermal control must be precise in order to avoid flooding or drying out the membrane. The cost of the electrocatalyst is currently about $10/kW at 700 mW/cm2, which is not an overriding issue at this point.
A more significant issue is impurities. It is desirable to use hydrogen that contains small concentrations of carbon monoxide (CO), like the hydrogen that is obtained from a reformer that produces hydrogen from hydrocarbon or alcohol fuels. But platinum electrocatalyst performs well only if the CO content of the hydrogen is not significantly more than 1 ppm. Various schemes are under development to raise the tolerance level to 100 ppm.
Bipolar plates and flow distributors (also known as flow fields) in the current cell stacks are very expensive. These bipolar plate/flow distributors must be gas impermeable and electronically conductive, as well as lightweight, thin, and corrosion resistant. Corrosion resistant metals are generally too heavy and expensive to be used as bipolar plates. Carbon-filled plastics are being developed but are not yet entirely leak free.
Hydrogen storage is also a problem. In any given storage system, the hydrogen is only a few percent of the weight of the storage device, whether it is a compressed gas tank, a chemical hydride, or a metal hydride. But until the storage problem is solved, it will not be possible to realize the promise of hydrogen as a lightweight fuel. Miniature reformers that could significantly affect the utility of hydrogen as a fuel are being investigated. Their current status, however, is unclear. Figure C-4 tabulates the advantages, disadvantages, and current research focus for hydrogen PEMFCs.
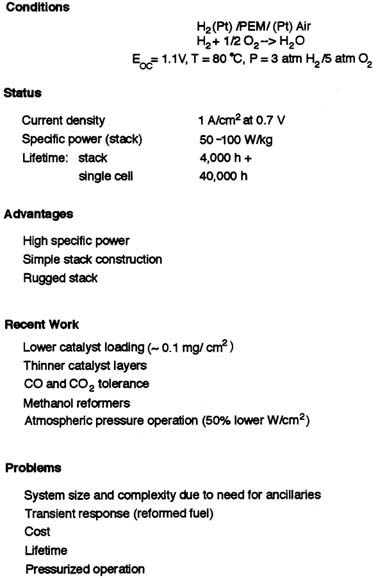
FIGURE C-4 State of the art of hydrogen PEMFCs.
The catalyst loadings for the DMFCs, are too high for practical use. The cost of DMFCs is about $500/kW. The efficiency of methanol utilization is low because methanol diffuses through the PEM at high rates and reacts directly at the cathode, reducing cathode performance and wasting fuel. Improved membranes and electrocatalysts are being investigated in a number of laboratories. Figure C-5 lists the state of the art and the research focus for DMFCs.
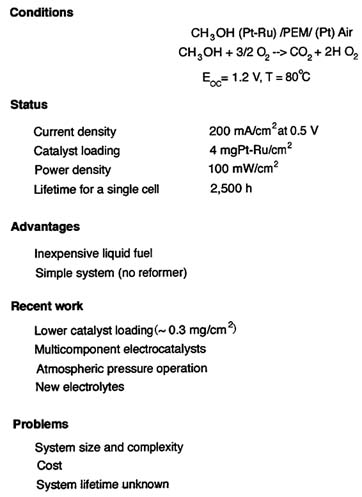
FIGURE C-5 State of the art of DMFCs.
Opportunities for Improvement
A great deal of effort has been made recently to develop PEMFCs, and significant improvements are being made every year. For small units like the ones of interest for the dismounted soldier, operating at very near atmospheric pressure (so the air feed does not need compression) will be important. Some early work in this direction looks promising. Catalyst loadings have already been reduced significantly, and several groups are working on the development of less expensive membranes and lower cost bipolar plates/flow fields. The lifetime for PEMFCs operating on pure hydrogen and air or hydrogen and oxygen are now adequate for many applications. Design modifications to meet soldier requirements will certainly yield more robust, less expensive units.
Improved electrocatalysts will be necessary for fuels other than pure hydrogen, such as methanol, for good performance with CO concentrations above 100 ppm. Improvements include more active electrocatalysts, membranes with much lower methanol permeability, and cathodes that are less sensitive to methanol. Several organizations are already working on improved membranes, but this is a difficult problem. In general, improvements in the performance and lifetimes of PEMFCs has been good, and continued progress can be expected.
In summary, the following focus areas are important to the development of fuel cells:
-
developing more efficient methods of storing and/or generating hydrogen fuel
-
reducing the operating pressures to near atmospheric pressure
-
improving the CO tolerance of systems that use reformed fuels
-
reducing the cost of bipolar plates/flow fields
-
reducing system complexity
-
improving water management
-
reducing the cost of proton exchange membranes
-
improving catalysts for DMFCs
-
reducing the rate of methanol crossover
-
improving system-specific power to levels greater than 100 W/kg for small (8100 W) systems at atmospheric pressure
HEAT ENGINES WITH ELECTROMECHANICAL ENERGY CONVERTERS
The energy requirements for extended missions or power-intensive activities often exceed the capacity of the dismounted soldier's batteries. The stored specific energy for the Army's best available battery today (BA 5590) is less than 0.2 kWh/kg, so this point is typically reached when missions require between 0.5 and 1.0 kWh of total energy (2.5 to 5.0 kg). For mission energy requirements above this level, fueled systems using either hydrogen or hydrocarbon-based fuels are the most attractive options. In addition to the extraordinarily high energy densities offered by these fuels, the cost for equivalent energy is several orders of magnitude below the cost of energy from batteries. The energy densities of hydrogen and common hydrocarbon-based fuels are given in Table C-16.
Options for converting the energy stored in fuels to electricity include fuel cells, thermoelectric and thermophotovoltaic sources, and heat engines with electromechanical energy converters. Of these options, conventional heat engines represent the most mature technology with unusually high converter operating efficiencies and power densities (Space Power Institute, 1992a). Potential problems associated with heat engines for the dismounted soldier include difficulty of starting, thermal and acoustic signatures, vibration, generation of
TABLE C-16 Specific Energies of Various Fuels
|
Fuel |
Specific Energy(kWh/kg) |
|
Hydrogen |
33.3 |
|
Gasoline |
12.2 |
|
Diesel |
11.9 |
|
Methanol |
5.5 |
|
Propane |
12.8 |
toxic or hazardous combustion products, the inability to operate in all positions or to be intermittently submerged (Army Material Command, 1992). Thus, heat engines may require an intermediate energy storage mechanism to realize their full potential. An unconventional microturbine system, which is in the very early stages of development, also looks promising (Tan et al., 1997).
A fueled power supply does not eliminate the need for a battery. It does, however, redirect the requirement to a secondary (rechargeable) battery with limited capacity that can be recharged from the fueled power supply. If battery storage is available, the heat engine can be shut off during periods of submersion, providing that reliable automatic restarting is possible.
Technical Considerations
Heat engines can be classified in a number of ways, but perhaps the distinction between internal and external combustion engines is the most appropriate discriminator for the dismounted soldier system. Internal combustion engines, such as spark-ignition and diesel engines, typically involve compressing a combustible mixture of fuel and air with a piston, igniting the mixture, which burns to produce heat, and allowing the hot gases to expand against the piston. This mechanical work can, in principle, be converted to electrical energy either by rotating or reciprocating electrical generators. In sizes appropriate for the soldier (50 to 250 W), internal combustion engines are the most mature heat engine technology (Raskovich, 1993). The impulsive nature of the thermodynamic energy conversion in internal combustion engines, however, leads to noise and vibration problems as well as difficulty in restarting.
External combustion heat engines, such as gas turbines, Stirling engines, and steam engines, are characterized by the steady-state combustion of fuel and air external to the energy conversion mechanism, which may be either rotary (turbine) or reciprocating (piston). The steady-state combustion process is generally more easily optimized, quieter, more efficient, and cleaner than the
impulsive combustion process typical of internal combustion engines. Turbines are generally smoother and quieter than reciprocating engines, and the noise they do produce is typically of a higher frequency and therefore more easily dealt with. Unfortunately, certain dominant loss mechanisms for turbines (having to do with gas leakage and heat loss) do not scale well with decreasing size. Therefore, turbines in sizes below one horsepower (746 W) are rare.
Another distinction between internal and external combustion engines is their compatibility with various fuels. Present Army doctrine calls for a single battlefield fuel, such as the standard diesel fuel, JP-8. Small spark-ignition engines (such as model airplane engines), technically the most mature, typically run on methanol. Diesel engines run well only on diesel fuel. The steady-state burners of external combustion engines are more flexible in their fuel requirements. One problem common to small spark-ignition engines is imposed by their extremely low fuel flow requirements, which means orifices and fuel metering mechanisms must be extremely small. As a result, small engines are very susceptible to dirty or contaminated fuel, which may make the use of bulk fuel difficult or impossible. This sensitivity, combined with the relatively low quantities of fuel involved and the extremely diffuse consumption of the fuel, may make prepackaged fuel preferable for the dismounted soldier. Perhaps a prepackaged fuel other than JP-8 can be justified as a battery replacement rather than as a traditional bulk fuel.
Because heat engines produce mechanical power through motion, electromechanical energy converters have been the most appropriate means of conversion to electrical power. Electromechanical energy conversion is based on the fact that an electrical conductor moving through a magnetic field generates an electrical voltage. The voltage is directly proportional to the product of the magnetic flux density, the length of conductor in the field, and the velocity of the conductor relative to the field. The magnetic field can be produced either by an electromagnet or a permanent magnet.
Generally, the electromagnet is preferable because the energy conversion process can be controlled. For dismounted soldier systems, however, efficiency, low weight, and low maintenance are critical, which means generators with permanent magnet rotors will be preferable. Ideally, the generator and engine will have a common shaft and bearings to minimize weight and volume. Given appropriate electronic controls and the presence of a battery, the permanent magnet generator can also be used as a starter motor for the engine, although this may place additional demands upon the generator design. In general, for a given power output, the size and weight of the permanent magnet generator will decrease in inverse proportion to the operating speed. For microturbines, it may be necessary to develop electrostatic generators because of the small sizes involved.
The relative merits and current state of development of various heat engines for the dismounted soldier are summarized in Table C-17. Heat engines raise a common problem for military applications—the most attractive options are the least well developed. Within the power range of interest (30 to 100 W), small spark-ignition engines represent the most mature technology by virtue of the
TABLE C-17 Internal and External Combustion Engines
|
Engine of Type |
Level of Development |
Restart |
Thermal Signature |
Acoustic Signature |
Vibration |
Weight |
Appropriate Fuels a |
|
Internal combustion |
|||||||
|
Spark-ignition |
high |
low |
high |
high |
high |
medium |
H,D,G,M,P |
|
Diesel |
medium |
high |
medium |
high |
high |
high |
D |
|
External combustion |
|||||||
|
Gas turbine |
low |
|
medium |
medium |
low |
low |
H,D,G,M,P |
|
Stirling engine |
medium |
high |
low |
low |
medium |
high |
H,D,G,M,P |
|
Vapor cycle turbine |
low |
high |
low |
low |
low |
medium |
H,D,G,M,P |
|
a Key: H = hydrogen G = gasoline D = diesel M = methanol P = propane |
|||||||
industry serving the model airplane market. However, internal combustion engines in general represent the least attractive type of engine in terms of signature, vibration, weight, operating speed, and restarting capability. The Stirling cycle engine has been the subject of substantial investigation by the Army because of its promise of multifuel operation and low signature. However present projections indicate that Stirling engines will be too heavy for the dismounted soldier system mainly because of their low effective operating pressure (Raskovich, 1993).
A current project at MIT, funded by the Army Research Office (ARO) is investigating ways to apply microfabrication technology to the development of a micro gas turbine generator (Epstein et al., 1996). Based on emerging silicon-carbide microelectronics fabrication technology, this project could lead to an economical microturboalternator with high specific power. This is a high-risk project, but it could provide extremely attractive specific power and energy figures for dismounted soldier systems. A primary disadvantage is that the first generation system is envisioned to operate on hydrogen, although plans call for the development of versions that operate on JP-8. Table C-18 gives weight estimates for the engine, generator, and fuel for intermediate-and long-term technologies capable of generating 50 W. A 50 W system today would have to be assembled from commercial off-the-shelf model airplane engines and a permanent magnet generator and would operate on methanol fuel. The engine would have to be derated for silencing and would require resilient mounting for vibration control. For the long term, the microturboalternator is much more attractive.
TABLE C-18 Weight Comparison for 50-W Heat Engine Alternatives
|
|
Weight of Base Unit |
|
|
|
|
System |
Engine (g) |
Generator (g) |
Type of Fuel |
Fuel Consumption (g/Wh) |
|
Near Term |
||||
|
Spark-ignition engine w/permanent magnet generator |
450 |
100 |
methanol or propoane |
0.73 |
|
Intermediate Term |
||||
|
Steam turbine w/permanent magnet generator |
100 |
25 |
multifuel |
0.6 |
|
Long Term |
||||
|
Microturboalternator |
1 |
included |
hydrogen or |
0.28 |
|
|
|
|
JP-8 |
0.42 |
For the intermediate term, there is still great uncertainty. Without dedicated U.S. Department of Defense development programs, there is little incentive for the industry to develop 50-W size human-portable motor generators. Even if these generators are developed, reciprocating heat engines are likely to require heroic efforts to reduce noise and vibrations to acceptable levels.
The development of microturbines sponsored by ARO, however, does offer some attractive nearer-term options. The silicon carbide microfabrication requirement is driven by the high combustion temperatures associated with hydrogen fuel and the corresponding high turbine inlet temperatures necessary for efficient microturbine operation. The development program calls for turbines to be fabricated in silicon by 1998, which suggests the intermediate option of a silicon microturbine with a lower operating temperature driving a high-speed alternator based on rare-earth permanent magnet technology or electrostatic generator technology.
One way to achieve this power supply would be to operate the turbine as an open cycle steam turbine. Although this would require that a small amount of water or other working be carried, it would greatly reduce the operating temperature and speed of the. The weight of water is included in the fuel weight for the intermediate-term option in Table C-18.
A rough estimate of system weight (mass) for missions requiring various amounts of energy can be obtained from Figure C-6, which plots system mass as a function of available energy in kWh. In the figure, the data in Table C-18 was used to plot the weight of the engine, generator, and fuel required for 50 W of electric power as a function of mission energy requirements. The engine in the near-term option has been derated to allow for silencing, but the weight of silencing and vibration control equipment is not included.
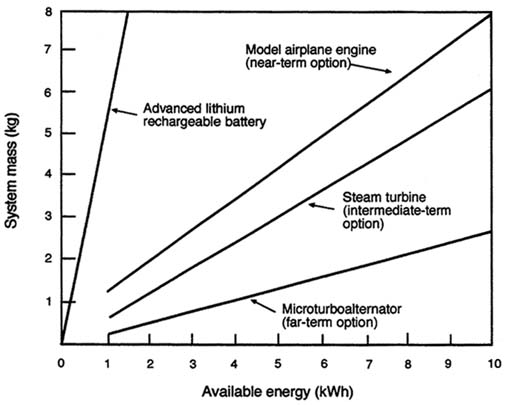
FIGURE C-6 System mass as a function of available energy.
Key Research Issues
The most promising major power systems based on rotating machinery are miniaturized turbines driven by combustion or high-pressure gases. The key research issues are:
-
liquid combustion in small systems
-
active noise canceling techniques
-
microturbine fabrication techniques miniature electrostatic generators
-
thermal signature mitigation
THERMOELECTRIC GENERATORS
The thermoelectric generator is a device that uses the Peltier effect to produce electricity from any heat source (Rowe, 1988). The efficiency of a thermoelectric generator is determined by the temperature of the heat source, the rejection temperature, and the materials that compose the thermoelectric elements. In general, thermoelectric generators are extremely reliable (they have been used for years in space), have few moving parts, and are inherently silent. They have been researched extensively for use in space.
Technical Aspects
At present, the maximum efficiency attainable from thermoelectrics is on the order of 8 to 9 percent in a laboratory device, but the efficiency is usually less than 5 percent. The best converter materials are alloys of materials like bismuth and tellurium, which are expensive and difficult to fabricate. The Defense Advanced Research Projects Agency (DARPA) has recently initiated a program to develop advanced thermoelectric materials for both power and cooling. This program may eventually enable the construction of power systems with efficiency greater than 10 percent, which would make them competitive with some of the other systems outlined in this report.
Teledyne Brown Engineering manufactures and markets large thermoelectric units for use in remote areas. The maximum power levels for these units are on the order of 100 W. The units are multifuel-capable and highly weather resistant, traits that would be of considerable interest to the military. In the 1960s, the Army experimented with thermoelectric units for battlefield use. The units were less than 5 percent efficient and very heavy. As a consequence, they did not become part of the standard inventory. More recently, the Marine Corps has funded a design study for a 500-W unit projected to weigh 20 kg and have an efficiency on the order of 9 percent (Bass et al., 1994). The specific power of this unit is on the order of 25 W/kg for the converter alone. Because this is a converter, specific power is determined by the basic weight of the assembly plus the weight of the fuel that would be needed for a mission. Continuing research at the Jet Propulsion Laboratory indicates that converters with efficiencies of greater than ten percent are possible (Halpern, 1997). Figure C-7, which shows the system mass as a function of mission energy, assumes that this device could be realized.
Key Research Issues
Thermoelectrics is a mature technology that has been used for numerous space applications for power, as well as for an enormous array of civil and military applications for cooling. Like other mature technologies, thermoelectronics tend to improve incrementally. The following are key research issues:
-
development of materials with high ''figure of merit" for power applications
-
development of low cost fabrication techniques
-
external combustion and recuperation in small systems
-
building prototype power systems
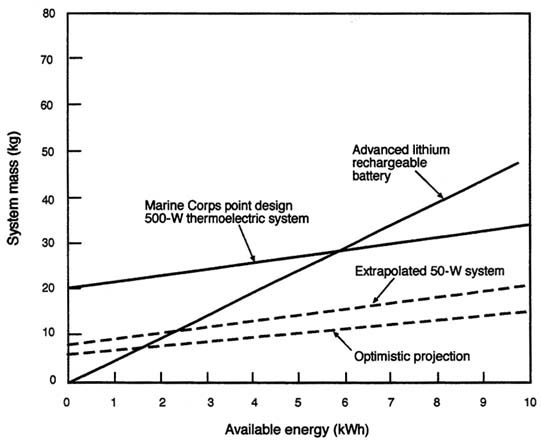
FIGURE C-7 Available energy as a function of power system mass for a thermoelectric power generator fueled by battlefield fuel.
ALKALI-METAL THERMAL-TO-ELECTRIC CONVERTER
The "sodium heat engine," or alkali-metal thermal-to-electric converter (AMTEC), is capable of converting thermal energy from any heat source to electricity with efficiency estimates as high as 35 percent (Space Power Institute, 1990). This technology has been extensively investigated in the past decade, and much progress has been made in materials technology and in understanding the basic physics of single cells. Extensive efforts have been made worldwide to reduce the technology to practice. In the civilian sector, applications such as automotive, self-powered home gas appliances, and space power have been explored. The most interest at present is in applications for deep space probes, where the heat source is nuclear. Because the fundamental physics of the converter is independent of the heat source and because modest laboratory efficiencies have been obtained, AMTEC should be a good candidate for Army applications in the 50 to 500-W range.
Technical Description
AMTEC consists of a liquid sodium loop with high and low temperature sections separated by an ion permeable membrane (Figure C-8). At the high-end, there is a pressure gradient across the membrane, which causes sodium ions to flow through the membrane but blocks sodium atoms. If electrodes are placed across the membrane, ions passing through the membrane create an electric potential that can be used to do useful work. Because the liquid associated with the converter is a metal, electromagnetic pumps or a "wick" can be used to return the liquid from the cold to the hot side, minimizing the number of moving parts. It is estimated that an AMTEC may be configured to be as high as 500 W/kg in specific power although no experimental units have demonstrated power densities approaching this value (Ivanenok and Hunt, 1994). Experimental units have been operated in a laboratory environment for thousands of hours demonstrating the potential for long life.
The basic device is adaptable to any heat source capable of maintaining a 500 to 700 K (degrees Kelvin) temperature differential across the converter section of the unit. The estimated cost per kW will be on the order of $0.30 to 0.50/W. To date, single AMTEC cells have operated in excess of 14,000 hours. There are no reported data on the operating history of cells in parallel or series arrays, which would be necessary to produce an efficient power supply. The unique construction of an AMTEC cell presents serious problems to the development of efficient series and parallel arrays.
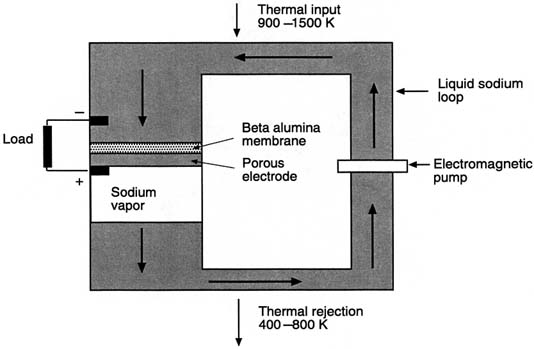
FIGURE C-8 Schematic drawing of an alkali-metal thermal-to-electrical converter (AMTEC).
Point designs, which would allow estimates of system mass, have not been built. Technology projections must, therefore, be based on analytical studies, and on the fact that AMTEC scales linearly to small sizes. Figure C-9 illustrates system mass as a function of mission duration in kWh based on published estimates of efficiency and specific power (Ivanenok et al., 1993, Ivanenok and Hunt, 1994).
AMTEC, like all fueled systems, has the problem of rejecting waste thermal energy at a relatively high temperature. This poses a serious design constraint or limits the system's utility to areas where there is no concern about thermal signature.
Key Research Issues
At the single-cell level, AMTEC converters are well understood. The primary technical and research issues to be resolved are:
-
long term materials degradation and poisoning of the alkali-metal loop
-
techniques for effectively and efficiently making parallel and series arrays that minimize heat loss
-
efficient external liquid combustion and recuperation in small systems
-
system demonstrators
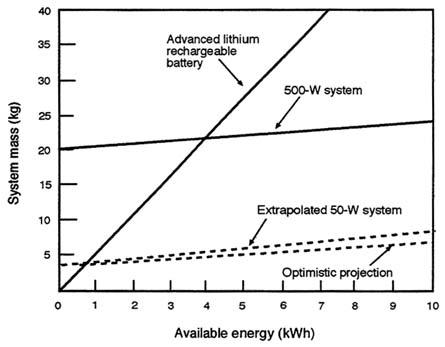
FIGURE C-9 Estimated performance of an AMTEC system.
NUCLEAR ENERGY POWER SOURCES
Power sources based on nuclear energy are capable of more than 1,000 times the specific energy of power sources based on chemical bonds (see Figure C-1) (Space Power Institute, 1992c). Releasing nuclear energy in a controlled way, however, is extremely difficult. Nuclear energy sources are included in this study for completeness, although the problems of using nuclear materials on a battlefield are formidable and could be overcome only by a concerted and expensive program. Nuclear power sources could extend the autonomy of the soldier to months and years instead of hours.
The most applicable nuclear energy sources that might be exploited by the Army are nuclear isotopes (NTSE, 1992). Isotopes have the following desirable attributes:
-
They possess enormous specific energy.
-
Systems utilizing isotopes can be made with a wide range of specific powers.
-
Isotopes suitable for power applications are by-products of nuclear reactor operations.
-
Isotope power systems are highly developed and reliable.
-
Isotope power systems offer a wide range of options for energy conversion.
The fundamental properties of isotopes will severely restrict their use, however. The most obvious limitations are:
-
Isotope power systems cannot be turned on and off. Once activated, the isotope begins to decay while still in the reactor.
-
Massive shielding is required for some isotope fuels.
-
Environmental/health issues are associated with both the manufacture and use of isotope systems.
-
Nuclear-powered systems in general have a poor public image.
-
The most desirable isotopes are expensive.
For the reasons listed above, power systems based on nuclear isotopes have been niche technologies, confined primarily to space probes, underwater power systems, and use in remote terrestrial locations. Nevertheless, the list of potential applications that could benefit by the use of isotope systems is long.
Generic Radioisotope Power Systems
Current space systems employ the general-purpose heat source-radioisotope thermal generator (GPHS-RTG) and the next generation, the
modified, or MOD-RTG. The GPHS-RTG, as flight hardware, has a nominal efficiency of 6.8 percent and a specific power of 5.18 W/kg (electric). MOD-RTG should have an efficiency of 7 to 9 percent and a specific power of 7.7 W/kg (electric). Mini-RTGs using the same technology have been designed for lunar and Martian probes with comparable efficiencies. For terrestrial use, the specific power is less important, and these units tend to be more massive, due to the use of 90Sr and 60Co. The mass increase is usually in shielding or in pressure vessels if the unit is used for deep sea submergence. The conversion efficiency is in the range of 5 to 9 percent, depending on the thermoelectric materials used. All currently operating RTGs are powered by thermoelectric converters.
Several small units designed for probes of the lunar and Martian surfaces may be of interest as terrestrial power sources. The innovative designs of these RTGs may be more mass and volume efficient because fuel does not have to be encapsulated to survive inadvertent reentry. Other innovations in insulation, such as the innovations proposed for the MOD-RTG, can also be used for mass savings. Miniature heat engines, such as Stirling engines, AMTEC, and thermophotovoltaic devices, some with efficiencies as high as 30 percent, could be coupled with RTGs with the possibility of repair and replacement. High efficiency reduces the quantity of radio isotopic materials required as the heat source.
The conversion process from nuclear to thermal energy is inherently efficient; therefore, the major advances will be in the thermal-to-electric conversion process. The most promising conversion technologies are thermoelectrics; thermodynamic cycles, such as Stirling, Brayton, and Rankine; thermionics; thermophotovoltaics; and AMTEC. All of these technologies have progressed to laboratory scale demonstrations, and some are being tested in system demonstrations. Power conditioning can be summarized as highly efficient, with power densities on the order of 2 kW/kg and efficiencies above 90 percent. With most of the low voltage, high current conversion requirements employing thermophotovoltaic, thermoelectric, and thermionic converters, such as series-parallel arrangements for small units, the output voltage and current can be tailored for a specified load. Mechanisms with rotating machinery must include alternator designs that fit specific loads; several mechanisms, such as the linear alternator, have been investigated.
Major programs are under way to develop new thermoelectric materials and improve existing ones through judicious materials engineering. The key issues are increasing thermal-to-electric conversion efficiency by selection of materials and by using dispersions to control thermal conductivity. For thermionic converters, both fuel encapsulation to prevent element swelling and high temperature emitters are vital to reliable power systems. For thermophotovoltaics, the key issues are the development of "low-bandgap" cells with acceptable efficiency; the current state of the art is approximately 10 to 20 percent. Dynamic machines and technologies, such as AMTEC, have materials problems that are not inherent in the conversion process but that require engineering of some
components for durable long lasting systems. These components include seals, membranes, bearings, and insulators.
Safety and environmental considerations are inherent in the design of all power systems. Both concerns are subject to U.S. Department of Energy procedures, as well as state and local requirements. In fact, satisfying the myriad requirements has become a major cost factor. The nuclear industry associated with small power sources has an impeccable record of addressing environmental and safety issues through extensive testing programs. Disposal is not a problem for deep space and planetary probes. But if terrestrial use increases significantly, disposal will become a critical issue that must be addressed in advance.
The selected fuel must not pose a threat to the environment or to human health. Unfortunately, the most desirable fuels are not available in quantities necessary for power applications. Hence, fuel type is also a critical issue. For terrestrial applications to date, the fuels have tended to be 60Co and 90Sr, both of which were available from reprocessed nuclear reactor fuels. Some fuel-grade materials are in storage, but they are decaying rapidly and will be of limited use in another 10 years. Because the United States does not produce suitable quantities of fuel, they must be purchased from countries that routinely reprocess nuclear materials or specialize in isotope production. The United States recently purchased 238Pu from Russia to meet NASA's projected needs. China, Canada, Japan, and France are other potential sources.
Many potential terrestrial applications for radiothermal generator technology are not being pursued, primarily because of public perception, the cost of materials, and environmental and safety issues. Most of the nuclear systems used to date have used thermoelectric converters. Conversion efficiency is expected to improve by a factor of two to five in the near future for converters on thermodynamic cycles and for technologies like AMTEC and thermophotovoltaics. But even if the use of nuclear materials were acceptable, the isotope manufacturing industry in this country is bordering on collapse. Before radiothermal generators can be widely used, the public perception of nuclear systems will have to change significantly.
Key Research Issues
The enormous specific energy of nuclear fuels makes them very attractive candidates for Army and civilian use. But the practicality of using nuclear fuels is low because of concerns about safety, environmental impact, cost, fuel infrastructure, public image, and the poor shelf-life of more powerful isotopes. Indeed, an isotope that could be used in a power system begins to generate energy the moment it is made in a reactor and cannot be turned off. Therefore, the key issues deal with system studies rather than fundamental research. The Army should:
-
Conduct system studies to characterize nuclear systems within an Army context.
-
For extremely low power requirements, explore miniature beta-voltaic nuclear powered systems that could be integrated into an electronic integrated circuit chip.
HUMAN-POWERED SYSTEMS
With sophisticated energy management and low power electronics, the energy requirements of the dismounted soldier could be reduced to a level at which the soldier could individually generate a substantial portion of the electrical energy required for a mission. It would only be necessary to convert some of the energy expended by the soldier during everyday activities to electricity.
The human body stores an enormous amount of energy. The average body is approximately 15 percent fat and represents a stored energy greater than 11,000 Wh. The average person consumes between 2,000 and 3,000 calories per day, which is, in more familiar units, approximately 2,200 Wh to 3,300 Wh. It can take 30 minutes to consume food with this energy content, so the charge rate is about 7 kW for 3,000 calories. Clearly the amount of energy consumed by an individual is sufficient to provide power for electronic devices if a suitable method can be found of converting even a small fraction of that energy to electricity.
Technical Discussion
The amount of power associated with physical activity has been estimated by Morton (1952). Table C-19 lists power levels associated with physical activity that would be of interest to the dismounted soldier.
TABLE C-19 Power Levels Required for Some Common Human Activities
|
Activity |
Power(W) |
|
Sleeping |
81 |
|
Standing at ease |
128 |
|
Walking |
163 |
|
Walking briskly |
407 |
|
Long distance running |
1,048 |
|
Sprinting |
1,630 |
TABLE C-20 Estimates of the Maximum Power Available for Conversion to Electricity from Several Body Sources
|
Source |
Maximum Power, Available(W) |
Maximum Estimated Conversion efficiency |
|
Body heat |
116 |
~3% (assuming total capture) |
|
Breath |
1.0 |
40% (based on turbine efficiency) |
|
Blood pressure |
0.9 |
about 2% |
|
Upper limb motion |
24–60 |
83% |
|
Walking (heel strike) |
67 |
piezoelectric converter~7% generator ~50% |
|
Source: Starner, 1996. |
||
In a recent article, Starner (1996) described several potential sources of energy associated with the human body that might be tapped for conversion to electricity (Table C-20).
Limb motion and the heel strike associated with walking and running are potential sources of power as long as the requirements are for levels of a few Watts. Because physical activity is inherently intermittent, a storage mechanism will be necessary. Rechargeable batteries, electrochemical capacitors, pneumatics, springs, and flywheels are candidates. Rechargeable batteries and electrochemical capacitors are discussed elsewhere in this chapter. The storage density using spring metals is on the order of 0.4 to 1.0 joules/gram, making them an attractive candidate. Conversion to electricity will require a generator of some sort. Wind-up shavers, radios, and flashlights are currently available on the civil market. Although the idea of human-powered systems is intriguing, it is impossible to estimate system performance in units like Wh/kg and W/kg at this time.
Key Research Issues
The field of human-powered systems is considered new and innovative, but human power has been used for electrical and mechanical systems for decades. The hand-cranked portable generator currently used by Army special operations forces falls into this category. It is possible to generate up to 100 W in this fashion. But devices of this type are not passive, and using them effectively immobilizes the individual while power is being generated. Another example is the small "flashlight" that is energized by squeezing a lever. For purely mechanical conversion, the Apollo astronauts took with them to the moon a rotary shaver that employed a small flywheel energy store activated by pulling a cord. Except for the few references already cited, no research on exploiting the energy associated with body motion and converting it to electricity is under way. Research in the following areas could affect human-powered electrical systems:
-
development of efficient lightweight intermediate storage units
-
analysis of the human motion in routine tasks and coupling unobtrusive converters to this motion
-
development of laboratory prototypes with small electromechanical and piezoelectric converters
PHOTOVOLTAIC TECHNOLOGY
Photovoltaics technology has been developed largely for use in space. Therefore, the following discussion relies heavily on data from that sector. All of the material for this section came from the Institute of Electrical and Electronics Engineers (IEEE) Photovoltaic Specialists conferences (IEEE, 1996). At the earth's surface, the power incident from the sun is on the order of 1 kW/m2 of surface. Conversion at modest efficiency should result in a major energy source that is "there for the taking." Successful harvesting of solar energy depends on the development of affordable photovoltaic cell technology. In general, the cost in terms of dollars per Watt have been too high for large-scale commercial exploitation, even though the U.S. Department of Energy has funded large demonstration projects capable of producing megawatts of electrical power. Solar photovoltaics is also limited because systems can produce power only during daylight hours and on clear days. Furthermore, for optimal power production, the unit must "track" the sun. The Army currently uses solar battery chargers for desert operations. These arrays can be folded and produce enough power to charge several batteries. Several thousand units were used in Operation Desert Storm.
Many domestic and foreign suppliers of photovoltaic cells have been in business for more than 30 years. They have already developed and implemented the process controls and inspections required for cells for general usage. These suppliers are reliable and they supply industry and government organizations around the world.
Currently most photovoltaic cells are used on commercial satellites, more than 400 of which are planned for the next five years. About 75 percent of production is devoted to gallium arsenide (GaAs) cells; silicon (Si) cells account for about 20 percent. A small fraction of production is devoted to higher efficiency multijunction cells and other cell types based on Group III, IV, and V elements. Three satellite classes comprise the marketplace: geostationary earth orbit communication and weather satellites, midorbit constellations (such as Iridium by Motorola, Odyssey by TRW, and Globalstar by Loral), and low earth orbit satellites (such as the space station and earth observation and earth science satellites). Divided by specific customers, the commercial marketplace is
booming. The U.S. federal government market share is small to moderate, except for the space station, whose production run is already over. Cell production capacity amounts to approximately 300 to 500 kW annually, for total sales of roughly $80 to $150 million. These specialty cells cost from $266 to $300/W, far too much for general use. Table C-21 summarizes the potential of photovoltaics as a source of energy.
Cells
Silicon
The silicon solar cell, which once was ubiquitous, is now a minor part (about 20 percent) of the market. In polycrystalline form, silicon solar cells have an efficiency of up to 15 percent and can be produced in virtually any rectangular size up to 36 to 50 cm2. In single-crystal form, their efficiency can be as high as 25 percent. Silicon solar cells come in many configurations with back surface fields (BSFs), back surface reflectors (BSRs), textured surfaces, or multilayer antireflection coatings, and in various base resistivities. For space-rated systems, the price of silicon cells is approximately $100/W, depending on the design. The more features, the higher the price. Civil sector prices for polycrystalline silicon arrays are on the order of $5/W.
TABLE C-21 Summary of Photovoltaic Technology
|
Cell Technology |
Commercial Availability |
Cost |
Power Density (W/m2) |
Efficiency (%) |
|
Amorphous silicon |
limited |
? |
50–70 |
5–10 |
|
Polycrystalline silicon |
yes |
low |
130–140 |
14–15 |
|
Single-crystal silicon |
limited |
high |
200 |
24–26 |
|
Gallium arsenide |
limited |
high |
200 |
17–18 |
|
Indium phosphide |
no |
high |
200 |
17–18 |
|
Copper indium diselinide |
no |
high |
130 |
15–17 |
|
Multibandgap |
limited |
high |
250 |
25–30 |
|
Concentrator array |
limited |
high |
250 |
30 |
Gallium Arsenide
The gallium arsenide/germanium (GaAs/Ge) solar cell has become the cell of choice in space because of its higher efficiency and its increased radiation tolerance. GaAs/Ge cells are about 17 to 18 percent efficient and are made in sizes up to about 24 cm2. They are fabricated by growing layers of GaAs onto rugged Ge substrates by chemical vapor deposition. These cells overcome the inherent fragility of GaAs, as well as the very high cost of GaAs single-crystals. The Ge substrate is inexpensive and very strong but is not part of the photovoltaic device in this design. The price of GaAs/Ge cells is approximately $300 to 400/W. There is no widespread commercial market for these cells. However, prices for terrestrial markets are much lower than for space-rated systems.
Indium Phosphide Cells
Indium phosphide (InP) solar cells are attractive for use in space because of their extreme radiation resistance. The efficiency of InP single-crystal solar cells is about the same as GaAs cells: about 17 percent, with sizes of about 4 to 8 cm2. InP cells are essentially unaffected by electron radiation and show only slight effects from proton radiation. Results of recent tests show that InP solar cells will lose about 5 percent of their power after 15 years in geostationary orbit. Furthermore, this damage can easily be annealed at temperatures of about 100°C. The main drawback of single-crystal InP solar cells is their cost, at present about $500 to $1,000/W, mostly because of the cost of the single-crystal substrates. Several researchers have tried to deposit InP solar cells onto Si substrates with modest success. Efficiencies of about 12 percent have been achieved to date. There is currently no terrestrial commercial market for these cells.
Multibandgap Solar Cells
Multibandgap (MBG) solar cells are composed of individual solar cells with appropriate bandgaps formed on top of one another for maximum utilization of the solar spectrum. Two- and three-junction devices are being studied. One example is GaAs/Ge cells, in which both the GaAs and Ge are active devices connected with tunnel junctions. Another is GaInP2/GaAs/Ge, a triple-junction cell. Most of the Group III, IV, and V elements are being investigated for use in MBG cells. Present goals of the technology are to achieve efficiencies higher than 24 percent for dual-junction cells and higher than 26 percent for triple-junction devices. So far, these values have been essentially achieved in practice (23.7 percent for GaInP2/GaAs, and 25.7 percent for GaInP2/GaAs/Ge in 4 cm2 areas), although production averages may be 1 to 2 percent lower at this time. The
cost of dual-junction MBG cells is presently $400 to 500/W. Present capacity for dual-junction cells is 100 to 200 kW per year. There is no substantial commercial market for these cells because of their high cost.
Thin-Film Cells
Thin-film solar cells have always been attractive as a low cost alternative to single-crystal cells. Unfortunately, their efficiency has always been substantially lower than conventional Si or GaAs cells. The two thin-film cells that have been proposed most frequently are amorphous Si and copper indium diselenide (CIS). Amorphous Si cells have efficiencies of about 5 to 10 percent, are deposited onto either metallic or polymeric substrates, and may have multiple junctions. They are subject to photon degradation and lose about 20 percent of their output when illuminated. Thin-film cells can be manufactured in virtually any size up to several square feet, with cells interconnected as part of the processing. CIS cells have efficiencies of about 15 to 17 percent and are not subject to photon degradation. Neither cell type is available in production quantities.
Arrays
Planar Arrays
Planar arrays, which make up more than 99 percent of the market, can be body mounted or paddle mounted. Both flexible arrays (for the space station) and rigid arrays (for communications satellites) are in regular production. Specific performance values depend on the exact application; the space station array has specific mass of about 55 to 60 W/kg with an overall efficiency of roughly 75 to 80 percent of the cell average efficiency (accounting for mismatch losses, area losses for interconnection and spacing, and wiring losses). Rigid arrays have specific powers of about 25 to 30 W/kg, with similar efficiencies. The costs of arrays are system dependent, with cell costs being up to one-third of the total. Cells are interconnected by welding or soldering metallic tabs in a series/parallel arrangement to ensure reliability. The reliability of solar arrays in space has been excellent, exceeding 99 percent.
Concentrator Arrays
A recent attempt to reduce the cost of solar arrays has been the development of the linear Fresnel-lens concentrator array. In this configuration, a
lens concentrates sunlight (about 15 times) onto a row of solar cells. The small area solar cells produce a higher efficiency because of the brighter light and smaller area—up to 25 percent for a single-junction GaAs cell or 30 percent for an MBG cell. Because the optics are less costly than for solar cells, the costs of the array are lower—as little as half the cost of a planar array. Concentrator arrays do require increased pointing accuracy (1 to 2 degrees compared to 15 degrees for planar arrays).
Key Research Issues
Photovoltaics, like many of the technologies discussed in this report, are mature in many applications and have been in the commercial domain for many years. Currently, the specialized space applications are able to afford cells that are more efficient than those widely available on a commercial basis. The cost of these specialized cells is orders of magnitude greater than the cost of cells that are commercially available generally. Photovoltaics are already being used by the Army.
Reductions in power requirements made possible by advances in low power electronics (described in Chapter 4) could make personal photovoltaic chargers in sunny climates a practical alternative to fueled systems. The following areas should be investigated:
-
bandgap-tailored photovoltaics that could function with both artificial light sources and the solar spectrum
-
manufacturing technology to reduce the cost of photovoltaic cells
-
innovative system demonstrators
THERMOPHOTOVOLTAICS
Thermophotovoltaics (TPV) is the technology for converting energy from an incandescent object (from any heat source) to electricity (Brenner et al., 1995). TPV technology shows great promise for the development of portable power sources for the dismounted soldier. Figure C-10 illustrates the concept.
Recent advances in TPV technology suggest that power systems for the dismounted soldier could provide anywhere from a few Watts of power to more than 500 W. Improvements in photovoltaics and emitters, in terms of reliability, size, weight, and energy efficiency, will translate directly into increased capability and, perhaps, lower cost. TPV is a multidisciplinary field. For example, solid-state converters must be combined with a radiant element, which is heated from a fossil-fueled combustion source. Recovering the energy remaining in combustion gases is vital for efficiency.
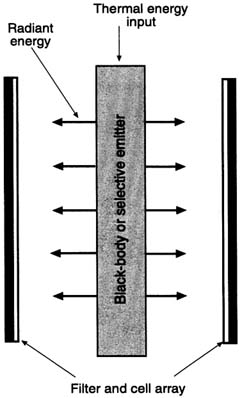
FIGURE C-10 Schematic drawing illustrating the principles of thermophotovoltaic (TPV) power systems.
The ultimate utility of TPVs for the Army may depend less on the fundamentals of the devices themselves than on other factors, such as whether a device can be mass produced from affordable materials, whether a device can be made robust and reliable enough to function in a hostile environment, whether it can be engineered into a package with minimal signature, and whether it will enhance the capability of soldiers in the field.
State of the Art
Many organizations have become interested in TPV technology, and a wide range of individual components have been demonstrated on a laboratory scale. These components appear to be ready for rapid development once applications have been identified. Examples include photovoltaic cells with conversion efficiency of greater than 20 percent, black-body-like emitters, selective emitters that emit greater than 50 percent of the energy in a narrow band, burners with combustion efficiency greater than 90 percent, cavities with losses just now being defined, filters with efficiency greater than 80 percent, coolant schemes that are readily adaptable to cooling photovoltaic cells, and designs for
high temperature recuperators (Space Power Institute, 1996). So far, only a few of these components have been assembled into laboratory systems that indicate feasibility but provide only inefficient demonstrators. The most prominent example is the Midnight Sun™ device from JX Crystals.
Several programs have been funded to increase power output to more than 500 W. Most of these are funded by the DARPA, ARO, and the Army Research Laboratory; laboratory demonstrations are expected within one year. The emphasis to date has been on demonstrating capability, materials, and processes for laboratory devices. Packaging is only now being addressed and should clarify many of the obstacles to fielding devices. Very little attention has been paid to demonstrating full systems or to establishing engineering parameters, such as figures-of-merit, performance specifications for each component, or the range of parameters for each component, although an infrastructure is emerging. Major potential applications in the military are auxiliary power units (APUs), battery chargers, and direct battery replacement.
TPV can potentially compete in the civilian markets in cogeneration schemes with gas furnaces, gas water heaters, and as stand-alone auxiliary power for pleasure craft. The technology to build systems with efficiencies greater than 10 percent is already available. The most optimistic projections for efficiency are on the order of 30 percent. Until more emphasis is placed on recuperation, it will not be possible to determine the specific power; however, specific powers greater than 100 W/kg do appear to be reasonable. Useful devices can be built with existing technology, but research to optimize and improve performance can be pursued in two areas, materials technologies, and manufacturing and packaging technologies.
A host of materials are used in TPV technology, especially in the radiating element. Both black-body and selective radiators are possible. In general, the choice of material has been at the discretion of the particular investigator. Little is known about the degradation (if any) that will occur when radiators are operated at high temperature for long periods of time. Strength, chemical composition, and vapor pressures at the operating temperature must still be studied.
Efficient reflective filters are also necessary for efficient TPV systems. Optical recuperation and thermal recuperation are necessary. Placement of the filter is critical for efficient optical recuperation; filters will be subjected to the total radiant thermal flux and must be able to withstand high temperatures. Highly efficient, cost effective filters are vital and should be researched in depth.
For TPV power systems to be affordable, the cost of photovoltaic cells must be reduced by orders of magnitude. The requisite manufacturing technology will be put in place only if there is an adequate market. To date, the best cell technology has not been identified; GaSb, In GaAs, and Si are all contenders. The difficulty with Si is the large bandgap, which necessitates high temperatures in the TPV unit. These temperatures place unique demands on recuperators. Cells based on other materials should be researched to provide the data for cost / efficiency trade-off studies for specific applications.
Recuperators are reasonably well established for temperatures compatible with high temperature metals and alloys. Considerable work needs to be done to establish the technologies for temperatures greater than 1,700 K. Because there are no complete systems, little is known about subsystem interactions. Models that can accurately predict system performance and subsystem interactions will have to be developed. Issues like service life can only be discussed in the framework of a specific application and system concept. At this stage, there are no specific devices that can be evaluated in the context of the battlefield environment.
For a given illumination intensity, the power output scales linearly with the area of the photovoltaic array. Energy scaling is related solely to system efficiency. Simple scaling estimates can be derived from the response of the photovoltaic cells and assumptions about efficiency. The total mass of the system is quickly dominated by the fuel mass. The minimal mass of the system is determined by items such as fuel tank, recuperator, cell array and structure, coolant scheme, and controls necessary to make the device user friendly. It is impossible to determine how these components effect scaling until some complete systems are built. Once fuel mass becomes dominant, scaling is linear in fuel mass. Size and capability are only meaningful in the framework of an application. As systems emerge, detailed scaling can be established. Figure C-11 illustrates the performance of TPV as extrapolated from laboratory data and goals from funded programs.
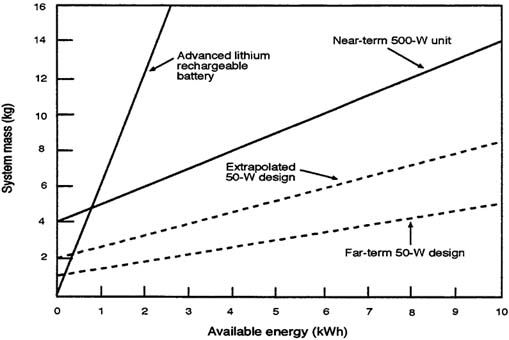
FIGURE C-11 Estimated thermophotovoltaic (TPV) system mass as a function of mission energy for point designs currently funded by DARPA.
Key Research Issues
The potential for TPV power systems has already been demonstrated. The most promising approach for the Army at present is to build prototype systems for evaluation with the research process keyed to correcting system flaws and inadequacies. Several topics must be thoroughly investigated before optimized energy systems can be developed. The most important are:
-
liquid fuel combustion in small systems
-
bandgap tailoring in photovoltaic materials and devices
-
design of cavity structures, including emitter, filter, cell array, and coolant/recuperator schemes
-
high temperature recuperators
ELECTROCHEMICAL CAPACITORS
Traditional capacitors are, in general, highly power dense but are incapable of high energy densities. Unlike capacitors, batteries have high specific energies but are incapable of extremely high specific powers. In many situations, the power source must have the best attributes of both, that is, high specific energy and high specific power. In recent years, a class of devices called ''electrochemical capacitors" has emerged that have some of the attributes of both batteries and capacitors (Florida Educational Seminars, 1996).
In terms of both specific power and specific energy, electrochemical capacitors are intermediate between classical batteries and capacitors. They have specific energies on the order of 10 to 20 percent of batteries and specific powers at least an order of magnitude better than a conventional batteries. Compared with conventional capacitors, they have specific power an order of magnitude less but specific energy an order of magnitude larger. The Army needs a device with the energy storage capability of a good battery and the power capability of a good capacitor. Electrochemical capacitors are a step in the right direction, and this technology is beginning to emerge in the marketplace. The new capacitors should have an enormous number of applications, including meeting the power needs of the dismounted soldier.
In 1887, Helmholtz discovered that the interface between a conducting material and an electrolyte was capable of storing an electrical charge. The restrictions on the potential storage are associated with the dissociation potential of the electrolyte. If aqueous electrolytes are used, the dissociation potential is the potential necessary to dissociate water through the process of electrolysis, i.e., on the order of 1.20 V. The interface thickness is on the order of 10-9 meters for highly conducting electrolytes.
Activated carbon has a surface area greater than 1,000 m2/g. A capacitor that uses 1 g of material should have a capacitance greater than 1 F and should operate at a voltage on the order of 1 V. Note that the electric field in the interface
is on the order of 1 V divided by 10-9 m. This corresponds to an electric field of 1,000 MV per meter, an enormous electric field. It is possible to engineer such devices. In 1969, Standard Oil of Ohio (SOHIO) successfully engineered and patented a practical device with these characteristics. SOHIO did not develop the technology but did license it to a Japanese firm. Since that time, firms such as NEC, Isuzu, Panasonic, Matsushita, Murata, and Elna, have continued to develop electrochemical capacitor technology with both organic and inorganic (sulfuric acid) electrolytes.
In the 1970s, B.E. Conway, at the University of Ottawa, discovered that an extremely fast oxidation-reduction (redox) reaction was possible at the surface of some low resistivity oxides. This discovery led to the development of an electrochemical capacitor based on the intercalation of hydrogen ions (protons) into a surface to cause charge separation. This concept has been developed by several firms, such as Continental Group (now disbanded), Pinnacle Research, and Giner, Inc. Obviously, for this technique to be successful, there must be a ready source of hydrogen ions in the electrolyte solution. For best performance, acid or hydroxide electrolyte solutions are used, the most common being sulfuric acid. However, other electrolytes, including some solids, will also work.
Although many oxides will perform satisfactorily, the most common oxide is ruthenium. Unfortunately, in its present format, ruthenium presents considerable problems in scaling-up from laboratory prototypes because the oxide is deposited in a thin-film on a metallic electrode. The thickness of the film is critical, and in addition to the manufacturing technology for producing the thin-film, poor packing fraction results when the film thickness approaches the electrode thickness. Recently, the Army Research Laboratory has developed, a version of the ruthenium technology that promises the manufacturing ease of carbon powder technology while doubling the specific energy associated with ruthenium oxide-based electrochemical capacitors. The specific energy is greater than carbon technology by a factor of three or four.
Because the resistance of any finished device must be minimized, the most desirable materials have low resistivity and maximum surface area. For most practical uses, both energy and power density should be maximized. Although the charge storage mechanisms for different classes of electrochemical capacitors vary, they all need electrolytes that can effectively cover and wet the large surface areas characteristic of electrochemical capacitors. The electrolyte acts as a distributed connection between active storage areas within the capacitor. All practical versions of electrochemical capacitors are composed of two capacitors in series, with the electrolyte forming the interconnecting current path. The electrode can be a porous solid, in which case there is no need for a true separator; it can be a porous compact made up of extremely fine particles, with or without binders; or it can be a surface film. To produce a distributed contact, the two halves of the capacitor must be physically separated by a material that allows conduction by ions but not by electrons. This material is usually a plastic, such as Celgard™, which can have a pore volume of 50 percent or greater. The primary
conduction mechanism within the material comprising the energy storage medium is electronic and, in the absence of a separator, would create a short circuit between the halves of the capacitor if they touched.
Particle Bed Chemical Double Layer Capacitor
Helmholtz and others developed the idea that the interface between an ionic conducting electrolyte and an electronic conducting material, such as a metal, could store an electrical charge. Figure C-12 is an illustration of a "particle bed" chemical double layer (CDL) capacitor that is a practical embodiment of this concept (Rose et al., 1994).
At the positive current collector, carbon particles are in physical contact with each other and come into physical contact with the collector to form one continuous physical contact for the capacitor. The electrolyte wets the large surface area carbons, as well as the surface of the collector, forming a distributed contact that acts as a second physical contact for one of the two capacitors in series within an individual cell. The membrane conducts ironically, but not electronically, and physically separates the two sides of the cell. The electrolyte wets the large surface area carbon in the second half, acting again as a distributed contact. Contact between individual carbon particles and the negative current collector form the remainder of the cell. In other words, the cell consists of two capacitors (mirror image around the membrane) in series.
Organic electrolyte CDL capacitors are similar to capacitors with aqueous electrolytes. Because organic electrolyte CDLs can operate at higher voltage, their specific energy should be greater by the square of the ratio of the operating voltage in the organic electrolyte. In practice, however, the specific energy is less because organic electrolytes cannot wet and form double layers in small pores the
FIGURE C-12 Schematic representation of a particle bed CDL.
way aqueous electrolytes can. Aluminum foils are usually used as current collectors. Because an oxide coating forms on the aluminum, it is particularly stable in organic electrolytes and can be safely operated at voltages as high as 3 V.
In the manufacturing process, the carbon powder is mixed with a suitable binder, such as Teflon™, and processed into a tape. The tape is pressed to a current collector in the form of an aluminum foil or screen, a separator is added, and the ensemble is rolled on a suitable mandrel. The unit is then impregnated with electrolyte and packaged. Because of the high resistivity of organic electrolytes, the planar area of the tape must be high to achieve a low equivalent series resistance (ESR).
This process is suitable for a single cell, but it does not lend itself readily to bipolar stacking, and the units on the market are single-cell, cylindrical packages. This places a burden on high voltage, high energy units because individual cylinders must be packaged and a penalty paid for the packing fraction in addition to the penalty for packaging of the individual cell.
Pseudocapacitors
Pseudocapacitors store energy electrochemically, rather than electrostatically, much like a very fast battery. The only criterion that must be met is that the reaction at the electrode surface be reversible and, of course, rapid:
Ox + e ⇔ Red
The device must have a species that can be oxidized and reduced reversibly. It is easy to show that:
C / Q = d { Ox/ Q } / dV
where Ox is the fractional charge associated with the oxidation, and Q is the total charge. The capacitance, C, in the above expression is not a true electrostatic capacitance but is the result of the reversible reaction. Putting optimal values in the equation yields a limiting capacitance from this mechanism on the order of 4000 F/cm 3, which is about an order of magnitude more than the CDL technologies. It is worth noting that real devices do not attain this value, however, and the equation (in effect) is Q = CV. That is to say, the adsorption of a charge-bearing species on a surface is a function of the driving point potential, which defines the uniqueness of pseudocapacitors and differentiates them from batteries. Pseudocapacitors have additional internal resistance because the process by which the charge is stored is faradaic (like a battery) and depends on the potential driving the process.
Numerous oxides have been investigated for pseudocapacitance. The most prominent are oxides of ruthenium, iridium, vanadium, nickel, tungsten, cobalt, molybdenum, and some plastics. The mechanism in aqueous electrolytes is the
same with respect to storage. In the interest of space, only devices based on ruthenium oxides are discussed in any detail here, although devices based on other oxides are included in the tabular data.
Ruthenium oxide has been found to be an excellent material for pseudocapacitors. The oxide can be anodically formed on ruthenium metal or can be prepared by chemical means using ruthenium chloride as the precursor. Successive redox processes take place reversibly over the range 0.00 to 1.40 V in aqueous sulfuric acid solutions. The states involved over this range are Ru(II), Ru(III), and Ru(IV). Above this potential, hydrogen and oxygen are evolved from the water. The reversibility of the reaction has been established up to approximately 100,000 cycles. The reaction is:
RuO2 + dH+ + de- = RuO2-d (OH)d 0 = d = 2
The highest specific capacitance reported in the literature is on the order of 380 F/g. This material had a specific area on the order of 120 m2/g. The maximum number of electrons that can be transferred is two for the above reaction. If every ruthenium atom were involved in the transfer process, the specific capacitance would be on the order of 1,000 F/g for a voltage of 1.4 V. The 380 F/g cited above indicates that only about 40 percent of the ruthenium atoms are exposed to the electrolyte solution.
Researchers at the Army Research Laboratory recently discovered that a hydrated amorphous form of ruthenium oxide performed much better than the crystalline version (Zheng and Jow, 1996). The hydrated material is formed by a sol-gel process with precursors of RuCl3 · xH2O in a NaOH aqueous solution. At the appropriate pH, RuO2 · xH2O precipates in extremely small particulate form. The resistivity of a pressed pellet of the material is on the order of 10-3 ohm-cm, a value that is excellent for use in capacitors. The resistivity is determined by the contact area between individual particles in the compact.
After suitable heat treatment, the measured capacitance was 768 F/g, representing more than two-thirds of the theoretical value. The specific capacitance, and hence the specific energy, is a factor of two better than for crystalline ruthenium oxide. This translates into half the cost per joule stored, all other factors being equal. A finished device, a mixture of the particulate and carbon powder, was made using the same technique described above for carbon powder bed capacitors. The current collectors were made from graphitized rubber. Several capacitors were constructed and characterized, and the highest specific energy reported was 8.6 Wh/kg, a phenomenally high value for a small device. The significance of this work is:
-
The specific energy is double the density of crystalline ruthenium oxide and three to four times that of carbon technologies.
-
The fabrication technique is the same as the well established carbon powder technique.
-
The internal resistance is somewhat less than for carbon powder technology but comparable to carbon composite technology.
-
Packing fraction is excellent compared with crystalline ruthenium.
The equivalent series resistance (ESR) is typical of aqueous systems and has the disadvantage of all devices that use powder. The ESR depends on the interparticle contact area. High pressures must be applied to the finished compact to achieve the minimum value. This problem is not serious in small devices because crimp sealing of a metal can under pressure is sufficient to produce a minimum ESR.
Equivalent Parallel Resistance
In the charged state, electrochemical capacitors, like batteries, are in a state of high energy relative to the state of minimal energy associated with discharge. As a result, there is a tendency for nonequilibrious states to lower their energy if there is a suitable mechanism for the process to proceed. An ideal capacitor based on polarization would retain a charge indefinitely, but all practical devices tend to self-discharge through a variety of mechanisms. This self-discharge time can be from months (NiCd) to years for some of the solid alkaline batteries available on the consumer market.
The self-discharge time for capacitors will be critical, especially for some of the applications contemplated by the Army. If the power train is a battery-capacitor hybrid, as is envisioned for digital cellular telephones, the self-discharge characteristics of the capacitor must be added to those of the battery. If the self-discharge characteristics of the capacitor are poor, it will require a continual drain on the battery to maintain full charge, which will limit battery life, require more frequent recharging, and reduce the attractiveness of a battery-capacitor hybrid. An isolating switch can be placed between the battery and the capacitor to limit this loss. The battery will still have to recharge the capacitor for each use, but the losses may be less severe than for a capacitor continuously connected to the battery.
Some problems can arise with electrochemical capacitors. If the capacitor is overcharged, the potential will simply drop until it is lower than the dissociation potential. The effect is to reduce the total energy stored. If the capacitance per cell is nonuniform in bipolar stacks, this could result in a substantial reduction of stored energy. The second potential problem is redox reactions caused by impurities in the electrode materials or in the electrolyte. The theoretical treatments for this are complex (Florida Educational Seminars, 1996). From a practical perspective, it is necessary to minimize both the amount and types of impurities that could cause redox reactions. For carbon technology, there are numerous examples of 1 V single-cell devices built with an equivalent parallel
resistance (EPR) of several thousand ohms. Table C-22 illustrates the state of the art in electrochemical capacitor technology based on data from technical and manufacturers' literature.
Key Research Issues
Although commercial electrochemical capacitors are available commercially, none has the internal parameters that would allow them to be used in the communications systems envisioned in this report. With further development, this technology could have a favorable impact on the power problem. Pulsed digital communications can reduce the demand for energy while increasing the life of a primary battery or the time between recharges for a secondary battery. Some laboratory prototypes can satisfy many criteria for battlefield use, but they are handmade, and the technology to mass produce devices with acceptable, reproducible results has not been established. The key areas for research are:
-
physical phenomena that limit specific energy, specific power, internal series resistance, internal parallel resistance, degradation mechanisms, temperature dependent phenomena, and useful life of electrochemical capacitors
-
development of a series of laboratory prototypes for evaluation in hybrid power systems
-
development of high voltage electrolytes
-
development of low cost materials for use in both the chemical double layer and pseudocapacitors
HYBRID SYSTEMS
Hybrid systems offer an alternative approach to providing portable power and energy. Hybrid systems combine the advantages of a very high specific energy source capable of maintaining the base load with a very high specific power source capable of providing pulse power. This configuration will greatly enhance the power and energy capabilities in small, portable packages. Hybrid systems can also be used as battery chargers and field generators.
Digitization will alter the demand cycle for military communications. Digital transmission is most effective if the transmission is pulsed, which requires high peak power. An analysis of the power requirements for the dismounted soldier indicates that the demand for power will be cyclic, with peaks several times the average. Furthermore, for long periods of time, the demand for power will be almost zero.
TABLE C-22 Summary of Electrochemical Capacitor Technology
|
Construction |
Performance |
Status |
||||||||||
|
Name |
Electrode Configuration |
Electrolyte |
Energy Density (kJ/kg) |
Energy Density (kJ/I) |
Resistance (ohms/cm2) |
Maximum Power (W/kg) |
Cost |
Voltage |
Typical Capacitance(F) |
Largest Unit(J) |
Basis for Projection |
Readiness Level |
|
NEC Supercap |
bipolar carbon/carbon composite |
sulfuric acid |
4.7 |
6.8 |
0.16 |
4 |
low |
15 |
470 |
55k |
manufacturer specification |
commercially available |
|
NEC FY |
bipolar carbon |
sulfuric acid |
1.2 |
1.98 |
45 |
— |
low |
5 |
2.2 |
— |
manufacturer specification |
commercially available |
|
NEC FE |
bipolar carbon |
sulfuric acid |
0.036 |
0.65 |
1.9 |
— |
low |
5 |
1.5 |
— |
manufacturer specification |
commercially available |
|
Panasonic |
spiral wound, single-cell carbon |
organic |
7.9 |
10.4 |
7 |
2.7 |
low |
3 |
470 1,500 |
6.7k |
commercial device |
commercially available |
|
Evans |
prismatic carbon |
sulfuric acid |
0.72 |
1.8 |
1 |
— |
low |
11 |
— |
40k |
manufacturer specification |
commercially available |
|
Seiko Instruments |
polyacene polymer, button cell |
organic |
6.84 |
17.6 |
12 |
— |
— |
5 |
2.5 |
— |
manufacturer specification |
commercially available |
|
Pinnacle Research Institute |
bipolar pseudocap using mixed oxides (Ru, Ta) |
sulfuric acid |
18 |
50.4 |
102 |
2 |
high |
100 |
0.01 |
15k |
manufacturer test data |
custom order |
|
|
|
46.8 |
144 |
<102 |
|
med |
|
|
|
|
theoretical lab projections |
|
|
Maxwell/ |
bipolar carbon/ |
KOH |
4.32 |
7.2 |
0.1–0.2 |
1.7 |
med |
28 |
12 |
6k |
engineering |
custom order |
|
Auburn |
metal composite |
organic |
22 |
32.4 |
1.5 |
3 |
med |
3 |
2,700 |
12.5k |
prototypes |
|
|
SAFT |
bipolar carbon |
organic |
10.4 |
15.8 |
15 |
1.2 |
low |
3 |
175 |
— |
engineering prototype |
custom order |
|
ARL |
bipolar hydrous RuO2 |
sulfuric acid |
96 (active material only) |
18.7 (active material only) |
— |
10 |
high |
5 |
2.72 |
34 |
lab cell |
— |
|
Livermore National Laboratory |
bipolar aerogel carbon particulate |
KOH |
3.6 |
5.4 |
— |
— |
med |
1 |
35 |
— |
lab cells |
— |
|
Sandia National Laboratory |
bipolar synthetic, activated carbon |
aqueous |
5.0 |
6.1 |
0.35 |
1 |
med |
1 |
3.5 |
— |
lab cells |
— |
|
Los Alamos National Laboratory |
bipolar conducting polymer on carbon |
solid organic |
36–72 |
— |
— |
— |
low |
— |
— |
— |
theoretical lab projections |
— |
|
Technautics Hypercap |
bipolar pseudocap, Ag-anode, C-cathode |
Solid RbAg4I5 |
1.98 |
12.6 |
>1 |
— |
— |
0.6 |
— |
— |
manufacturer test data |
custom order |
TABLE C-23 Most Promising Component Technologies for Hybrid Systems
|
Prime Source |
Intermediate Storage Unit |
|
Fueled system |
High power density rechargeable battery |
|
Battery |
Electrochemical capacitor |
|
Solar photovoltaic |
Regenerative fuel cell |
|
Nuclear |
Flywheel |
|
Metal-air battery |
Superconducting inductor |
The demand for electrical power in any system is rarely constant. Typically, the demand is cyclic, with the peak demand far exceeding the average power requirements. Because power sources rarely have both high specific energy and high specific power simultaneously, designers have typically designed power systems to meet the maximum demand to ensure adequate energy for the worst case. Thus, systems may be heavier than necessary, or planners may be forced to plan shorter missions or to resupply the primary energy sources. If the differences between the peak and average demands are large, it is advantageous to combine a high specific energy, low-specific-power source with a low-specific-energy, high specific power intermediate store to provide load leveling, which would meet the demand with substantial mass savings or with longer operational times for the same mass.
Many combinations of energy sources and intermediate storage are in use today, such as portable x-ray machines, photoflash units, electric cars, and portable cardiac defibrillators. Usually these are battery-capacitor systems. However, the principle could be applied equally well to a number of prime source-intermediate storage technologies. The most promising component technologies for the dismounted soldier, are listed in Table C-23.
Any combination of a primary power source and intermediate storage unit is capable of producing a power train suited to pulsed operation. A limited number of combinations are described here. The Army is already investing in solar photovoltaic-battery systems, which have been proven in combat.
Fueled System and Battery Hybrid
All fueled systems will probably be hybrids of one kind or another. Any fueled system operated in the battlefield environment will be subject to conditions under which it will be difficult or impossible to operate. Examples are submersion, extreme dust, and closed or confined spaces where exhaust fumes would be harmful to humans. Even in less extreme situations, a battery may still have to provide initial start-up for the fueled system. Depending on the climate and type of system, the battery may have to provide power for preheating the fuel or system, for initial pump power, or for control power.
TABLE C-24 High Specific Power Batteries for Hybrid Systems
|
Chemistry |
Current Status |
Future |
|
|
||
|
Specific Power (W/kg) |
Specific Energy (Wh/kg) |
Specific Power (W/kg) |
Specific Energy (Wh/kg) |
Present Cycle Life (Cycles) |
State of Development |
|
|
Nickel |
||||||
|
Aqueous |
100–200 |
40–52 |
150–250 |
52–70 |
500–1,000 |
available |
|
Aqueous (future) |
200–500 |
25–36 |
250–1,000 |
30–40 |
400–800 |
possible |
|
Bipolar |
— |
— |
200–400 |
60–80 |
— |
under development |
|
Pb-acid |
||||||
|
Bipolar |
200 |
25–45 |
— |
— |
300 |
available |
|
Bipolar (future) |
— |
— |
300 |
45–60 |
300 |
possible |
|
Thin foil |
1,000 |
5 |
— |
— |
300 |
under development |
Data described in the section on fueled systems indicate that they are five to ten times more energy dense than batteries for the same mission profile. Therefore, the fueled system, not the battery, provides practically all of the overall mission energy requirement.
For the fueled system-battery combination, there are at least three battery chemistries that warrant further consideration: nickel-cadmium; lithium; and lead-acid. Tables C-24, C-25, and C-26 show the specific energy, specific power, and
TABLE C-25 Commercial and Developmental High Specific Energy Batteries as Energy Sources in Hybrid Systems
|
Chemistry |
Current Status |
Future |
|
|
|
||
|
Specific Power (W/kg) |
Specific Energy (Wh/kg) |
Specific Power (W/kg) |
Specific Energy (Wh/kg) |
Present Cycle Life (Cycles) |
Future Cycle Life (Cycles) |
State of Development |
|
|
Li-ion/CoO2 |
100 |
100 |
150 |
150 |
1,000 |
2,000 |
commercially available |
|
Li-ion/Mn2O4 |
70–100 |
70–100 |
150 |
150 |
300 |
600+ |
available soon |
|
Li(c)/polymer/Mn2O4 |
150 |
150 (est) |
200+ |
200 |
300 |
600+ |
prototype soon |
|
Li(c)/polymer/(CS)x |
200a |
200a |
400 |
300 |
300 |
600+ |
prototype soon |
|
Li(c)/polymer/S |
200b |
400b |
400 |
600 |
research |
research |
research phase |
|
a Prototype b Laboratory cells |
|||||||
TABLE C-26 Potential Fueled Systems for Hybrid Power Systems
|
System |
Specific Power (W/kg)a |
Specific Energy (Wh/kg) |
Status |
|
PEM fuel cell |
28 |
571 |
prototype |
|
Thermophotovoltaic |
25 |
520 |
research phase |
|
Alkali-metal thermal-to-electrical converter (AMTEC) |
16 |
1,040 |
research phase |
|
a Calculated for 2 kg of fuel. |
|||
state of development of battery technologies that could be intermediate stores for a fueled-system battery hybrid (Arthur D. Little, 1996). As shown in the tables, a system consisting of an AMTEC and a 0.5 kg lithium-polymer battery would provide 5.3 kWh of energy, with peaks of 100 W, for a total mass of 5 kg. The battery pack could provide 100 Wh of energy without recharging. In some scenarios, this might correspond to an hour or more of operating capability. A lithium-polymer battery pack that could provide the total energy would have a mass of 26 kg. It is impossible to estimate the weight of the associated electronics and packaging that would be necessary to use this technology in a practical scenario. There is, however, almost a factor of five difference in mass (5 kg to 26 kg) for the same available energy.
An AMTEC-NiCd system designed to perform the same functions would have a similar mass. The weight of a NiCd system for the total energy requirement alone would be on the order of 170 kg. The NiCd battery could meet the total energy demand for about 20 minutes, but the NiCd battery would have to be recharged more often than the lithium—polymer battery. In any case, the total energy available would be dominated by the energy in the fuel. It is assumed that the AMTEC is 20 percent efficient in converting the heat of combustion of JP-8 to usable electricity. For this system, the pulse time would be on the order of hours depending on the scenario. In general, a status monitor for the battery would determine its state-of-charge and command the fueled system to maintain an acceptable level automatically. The individual soldier would have override capability. In most scenarios, the fueled system could maintain an intermediate store at 90 percent or more most of the time.
Battery and Electrochemical Capacitor
A battery-capacitor combination for an energy storage system would exploit the high specific power of a capacitor and the high specific energy of a battery. For this system, the time scale of the peak power delivery intervals would
be shifted from the tens of minutes or hours required for the fueled-battery system to minutes or less for the battery-capacitor system. This combination offers a better system for brief pulses of high power, the kind anticipated for portable digital telephones. Meeting this requirement with a battery alone would require a battery that could provide high power pulses at 8 to 10 times normal capacity and would still have maximum life and adequate operational time between charges. Using a capacitor to meet the peak power requirement would provide better operating performance, longer battery life, and better low temperature operation while lowering life cycle costs and a smaller, lighter weight package. Figure C-13 shows a generic power-time profile for a pulsed digital communications system.
In a recent paper, J.R. Miller (1996) developed a simple simulation of a 1 Ah lithium battery in parallel with an experimental electrochemical capacitor. The battery had an open circuit voltage of 4.1 V and an internal resistance of 0.1 ohms. The parameters assumed for the electrochemical capacitor were a capacitance of 1.28 F and an internal resistance of 0.069 ohms. For a repetitive pulse train of 8.3 ms at 10 A spaced by 90 ms, the battery alone was able to provide 12 minutes of operation. The battery-capacitor combination was able to power the system for 61 minutes, an improvement of roughly a factor of five. Simple circuit models were developed that can be used to predict the performance
FIGURE C-13 Typical power-time profile for pulsed digital communications devices.
TABLE C-27 Energy Storage Media That Could Be Used in Hybrid Systems
|
Storage Media |
State of Art |
Energy Density (Wh/kg) |
Practical Limit to Specific Power (Wh/kg) |
Key Issues |
Scaling Laws |
Impact |
Storage Time |
|
Batteries |
highly developed |
180–360 |
~400 |
electrodes; electrolytes; seals; safety; corrosion |
known |
major/ enabling |
years |
|
Capacitor |
highly developed |
0.25–1.00 |
~8.00 |
molecular engineering of film; manufacturing technology; thermal stability; electrical breakdown |
known |
enabling for some systems concepts |
minutes |
|
Film Foil |
|||||||
|
Paper Foil |
|||||||
|
Ceramic |
highly developed |
~0.30 |
> 3.00 |
large area samples; electrical breakdown; manufacturing technology |
known |
enabling for some systems concepts |
moderate |
|
Electrolytic |
highly developed |
< 0.5 |
>0.75 |
large surface area material; suitable oxides; electrolytes |
known |
minimal |
minutes |
|
Chemical double layer |
developing |
~7.00 |
>12.00 |
large surface area materials; electrolytes; equivalent series resistance/equivalent parallel resistance; seals |
known |
major |
minutes |
|
Magnetic |
advanced |
> 15.00 |
strength of materials limited |
advanced composites; low resistivity materials |
known |
minimal |
milliseconds |
|
Inertial |
highly developed for some applications |
100.00 |
> 300.00 |
high strength materials; gyroscopic effects; safety |
known |
minimal |
hours/days |
|
Thermal |
evolving |
sensible heat depends on dT |
absolute temperature dominated >5000 |
materials compatibility; high strength materials; high specific heat |
known |
uncertain |
days/weeks |
of battery-capacitor combinations accurately. In a similar experimental study, Merryman and Hall (1996) showed that the power train mass for an electrically actuated thrust vector control system for the space shuttle could be reduced by 59 percent when a battery-capacitor combination was used.
Table C-27 is a compilation of the characteristics of energy storage media that could possibly be used in hybrid systems. (For completeness, the table includes some media not covered in the text.)
Key Research Issues
Pulsed power techniques have been used extensively in the high-power regimes. Numerous laboratory demonstrations of hybrid systems, typical of systems appropriate for the dismounted soldier, have been performed. To date, there have been no field tests to determine the utility of using hybrid systems for human-portable power. To optimize the design, information on the power demand time history for a variety of mission profiles will be necessary. Given this data, a hybrid system can be designed for the worst case scenario that maximizes the available energy. The key issues are developmental and consist of:
-
development of computer models for predicting performance as a function of mission profile
-
development of laboratory prototypes
-
obtaining reliable field data for the development of energy utilization profiles of the various soldier subsystems
SUMMARY
Table C-28 summarizes the energy and power systems discussed in this appendix. The development of hybrid systems with a fueled primary store would be revolutionary. However, each of the technologies described in Table C-28 has drawbacks. Primary batteries cannot provide the requisite energy for the projected energy budgets of dismounted soldier systems without becoming unstable and creating a significant safety hazard. Primary batteries also pose a significant environmental hazard that will probably increase as new chemistries become available. The primary hazards of batteries are explosive rupture, toxic and corrosive electrolytes, and environmental pollution if they are not recovered. Inevitably, trade-offs among safety, energy, and power considerations will have to be carefully assessed for any system or mission. A secondary battery with the specific energy and specific power of primary batteries would be highly desirable. If this technology were available, the environmental restrictions would be lessened because less frequent recycling would be required. Even a high specific energy rechargeable battery with limited life (say, 50 charge/discharge cycles) would greatly lessen the current problems of supply and disposal.
Any system energetic enough to be considered a major advance for the Army will undoubtedly also be dangerous. Batteries are both energy storage systems and converters in the same unit, and battery safety is closely related to the oxidants and reductants. Consequently, if batteries are designed toward the margin, they have a tendency to explode.
TABLE C-28 Technology Summary of Energy Systems
|
Power System |
State of the Art |
Potential for Improvement |
Key Issues |
Scaling Laws |
Impact on Dismount Soldier |
Hostile Signature |
Suppression Potential |
Fuel Required |
Autonomy Time |
|
Primary battery |
Mature |
Moderate |
Energy density Safety Power density Environmental impact |
Known |
Longer mission Less weight Disposability |
Minimal |
Excellent |
None |
Hours/days |
|
Secondary battery |
Mature |
Moderate |
Energy density Cycle life Power density |
Known |
New capability Cost savings Less weight |
Minimal |
Excellent |
None |
Hours |
|
Thermophotovoltaics |
Emerging |
Excellent |
Requires cooling Efficiency Lifetime Ruggedization |
Uncertain |
New capability Cost savings Longer mission |
Thermal |
Moderate |
Multifuel |
Days/weeks |
|
Fuel cells (hydrogen) |
Exploratory development |
Excellent |
Fuel Water management Safety |
Known |
New capability Less weight Cost savings |
Thermal |
Excellent |
Hydrogen |
Days/weeks |
|
Fuel cells (methanol) |
Emerging |
Excellent |
Fuel and fuel crossover Catalyst |
Uncertain |
New capability Cost savings Less weight |
Thermal |
Excellent |
Methanol |
Days/weeks |
|
Alkali-metal thermal-to electrical converters |
Emerging |
Excellent |
Liquid metal Membranes Pumps/wicks Ruggedization |
Uncertain |
New capability Less weight Cost Savings |
Thermal |
Moderate |
Multifuel |
Days/weeks |
|
Nuclear isotope |
Limited |
Excellent |
Safety Environmental impact Cost Public acceptance |
Known |
New capability Autonomy |
Thermal Nuclear |
Moderate |
Special |
Month/years |
|
Internal combustion |
Some versions mature |
Moderate to excellent |
Fuels Vibration Life |
Uncertain |
Cost savings Less weight |
Thermal Acoustic |
Moderate |
Multifuel (Some Special) |
Days/weeks |
|
Microturbine |
Emerging |
Excellent |
Safety |
Uncertain |
New capability |
Acoustic |
Difficult |
|
Days/weeks |
|
Thermoelectric |
Some versions mature |
Moderate to excellent |
Efficiency Materials Coupling |
Known |
New capability Less weight |
Thermal |
Moderate |
Multifuel |
Days/weeks |
|
Human-powered |
Nonexistent |
Excellent |
Conversion mechanisms |
Unknown |
New capability Cost saving Autonomy |
Minimal |
Excellent |
Food |
Weeks |
In fueled systems, the energy dense fuel is in a separate enclosure and is slowly exposed to the oxidant so that only the fuel that is in the converter at any given time is subject to inadvertent catastrophic failure. With the exception of hydrogen, all of the other fuels are rather involatile, that is, they can burn rapidly but will probably not explode. Fuels are housed in external tanks, which would be subject to penetration and burning if the penetration were energetic enough to ignite them.
Primary batteries will be used in military systems for the foreseeable future. There will, however, continue to be problems associated with their disposal, inventory, safety, and availability, and wherever possible, they should be replaced. The logical evolution of the Army power system for the dismounted soldier is toward a rechargeable battery with improved specific power and energy that would meet or exceed the power available with current primary batteries coupled with a ''personal" charger that contains the primary store of energy for the mission. For many missions, the rechargeable battery alone would have enough energy. In those cases, the battery would be returned to the inventory after being recharged. For longer missions, the primary store would be fueled by a standard battlefield fuel. All of the fueled systems described in this appendix offer the possibility of long life with thousands of refuelings, and all of them are at a stage at which advanced development is possible. Coupled with a suitable rechargeable battery with similar cycle capability, these systems would dramatically reduce the inventory necessary to maintain combat readiness. The primary logistic consideration would be—as it is now—fuel supplies. Because batteries could be recharged many times, recycling after each mission would not be necessary, which would greatly reduce their adverse environmental impact.
High specific energy rechargeable batteries are becoming increasingly important in the commercial sector, which could provide the Army with a secure, high volume, guaranteed source of batteries. "Smart" chargers and power management circuitry will also be forthcoming from the commercial sector.
REFERENCES
Army Materiel Command. 1992. Soldier as a System Symposium/Exposition. Proceedings of a symposium sponsored by the Army Material Command, June 30–July 1, 1992, Arlington, Virginia.
Arthur D. Little, Inc. 1996. Proceedings of the Fourth International Conference on Power Requirements for Mobile Computing and Wireless Communications, Santa Clara, California, October 1996. Available from Giga Information Group, One Long water Circle, Norwell, Mass. 02061.
Bass, J.C., N.B. Elsner, and F.A. Levath. 1994. The preliminary design of a 500 W thermoelectric generator. Pp. 586–591 in Proceedings of the 29th Intersociety Energy Conversion and Engineering Conference. AIAA-94-4197-CP. Reston, Virginia.: American Institute of Aeronautics and Astronautics.
Benner, J.P., T.J. Coutts, and D.S. Ginley, eds. 1995. Proceedings of the Second NREL Conferences on the Thermophotovoltaic Generation of Electricity. AIP Conference Proceedings 358. Woodbury, N.Y.: AIP Press.
Florida Educational Seminar. 1996. Proceedings of the Sixth International Seminar on Double Layer Capacitors and Similar Energy Storage Devices, Boca Raton, Florida. Boca Raton: Paumanok Publications, Inc.
Halpern, G. 1997. Personal communication from Gerald Halpern, NASA Jet Propulsion Laboratory, with M.F. Rose, member of the Committee on Electric Power for the Dismounted Soldier, January.
IEEE (Institute of Electrical and Electronics Engineers). 1996. Proceedings of the 20th–25th Photovoltaics Specialists Conferences. Piscataway, N.J.: Institute of Electrical and Electronics Engineers.
Ivanenok, J.F., R.K. Sievers, and T. Hunt. 1993. High Power Density AMTEC . Pp. 861–865 in Proceedings of the 28th Intersociety of Energy Conversion and Engineering Conference, Atlanta, Georgia. Reston, Virginia: American Institute of Aeronautics and Astronautics.
Ivanenok, J.F., and T.H. Hunt. 1994. High voltage terrestrial AMTEC. Pp. 900–909 in Proceedings of the 29th Intersociety Energy Conversion and Engineering Conference, Monterey, Calif.. Paper no. AIAA-94-3903-CP. Reston, Virginia: American Institute of Aeronautics and Astronautics.
Merryman, S.A., and D.K. Hall. 1996. Chemical double layer power source for electromechanical thrust vector control actuator. Journal of Propulsion and Power 12(1): 89–94.
Miller, J.R. 1996. Battery-capacitor power source for digital communication applications: simulations using advanced electrochemical capacitors. Pp. 246–255 in Proceedings of the Symposium on Electrochemical Capacitors. F.M. Delnick and M. Tomkiewicz, eds. Proceedings Volume 95-29. Pennington, N.J.: Electrochemical Society.
Morton, D. 1952. Human Locomotion and Body Form. Baltimore: The Williams and Wilkins Co.
NTSE. 1992. Nuclear Technologies for Space Exploration. (NTSE '92). Proceedings of a conference held in Jackson Hole, Wyoming, August 16–19. 3 vols. Grange Park, Illinois: American Nuclear Society, Idaho Section.
Raskovich, E., ed. 1993. Front End Analysis of Soldier Individual Power Systems. USA-BRDEC-TR//2541. Ft. Belvoir, Va.: Belvoir Research, Development and Engineering Center. May.
Rose, M.F., C. Johnson, E. Owens, and B. Stevens. 1993. Limiting factors for carbon-based chemical double layer capacitors. Journal of Power Sources 47: 303–313.
Rowe, D.M., ed. 1988. Proceedings of the First European Conference on Thermoelectrics. Stevengate, Hertsfordshire, U.K.: Peter Peregrinus, Ltd.
Salkind, A.J. 1996. Estimate by A.J. Salkind, presented to the Battery Division of the Electrochemical Society at its annual meeting, San Antonio, Texas, October 6.
Schuller, M. 1997. Personal communication from Dr. Michael Schuller, U.S. Air Force, Phillips Laboratory, to M.F. Rose, member of the Committee on Electric Power for the Dismounted Soldier.
Space Power Institute. 1990. Mobile Battlefield Power Workshop. M.F. Rose, ed. Results of a workshop held in Durham, North Carolina, October 30–November 1, 1990. 2 vols. Sponsored by the Army Research Office, Contract No. DAAL03-86-D-001. Auburn, Alabama: Space Power Institute.
Space Power Institute. 1992a. RTG Power Applications Workshop. M.F. Rose, ed. Results of a workshop held in Park City, Utah, March 22–25, 1992. Sponsored by the Army Research Office, the U.S. Department of Energy, and the Jet Propulsion Laboratory. Auburn, Alabama: Space Power Institute.
Space Power Institute. 1992b. Prospector III: High Energy Density—High Power Density Power Sources RD Workshop. M.F. Rose, ed. Results of a workshop held in Auburn, Alabama, May 26–28, 1992. Sponsored by the Army Research Office. Auburn, Alabama: Space Power Institute.
Space Power Institute. 1992c. Prospector IV: Small Engines and Their Applicability to the Soldier System Workshop. M.F. Rose, ed. Results of a workshop held in Durham, North Carolina, November 10–12, 1992. Sponsored by the Army Research Office. Auburn, Alabama: Space Power Institute.
Space Power Institute. 1994. Prospector VII: Small Fuel Cells for Portable Power Workshop. C.R. Johnson and M.F. Rose, eds. Results of a workshop held in Durham, North Carolina, October 31–November 1, 1994. Sponsored by the Space Power Institute and the Army Research Office. Auburn, Alabama: Space Power Institute.
Space Power Institute. 1996. Prospector VIII: Thermophotovoltaics—An Update on DoD, Academic, and Commercial Research. C.R. Johnson and M.F. Rose, eds. Results of a workshop sponsored by the Army Research Office, Durham, North Carolina, July 14–17, 1996. Auburn, Alabama: Space Power Institute.
Starner, T. 1996. Human-powered wearable computing. IBM Systems Journal, 35(384): 618–629.
Tan, C., Y. Tzeng, I. Waitz, R. Walker, D.J. Orr, S. Senturia, A. Ayon, J. Mur Miranda, E. Piekos, C. Lin, A. Epstein, M. Spearing, G. Anathasuresh, K. Breuer, K.S. Chen, F. Ehrich, E. Esteve, G. Gauba, S. Jacobson, J. Lang, A. Mehta, S. Nagle, and M. Schmidt. 1997. Micro Gas Turbine Generators. Interim technical progress report for Grant DAAH 04-95-1-0093, Army Research Office, Research Triangle Park, North Carolina.
Zheng, J.P., and T.R. Jow. 1996. High energy and high power density electrochemical capacitors. Journal of Power Sources, 62: 155–159.





























































