This paper was presented at a colloquium entitled “Genetics and the Origin of Species,” organized by Francisco J.Ayala (Co-chair) and Walter M.Fitch (Co-chair), held January 30–February 1, 1997, at the National Academy of Sciences Beckman Center in Irvine, CA.
Genetics and the origin of bird species
(reproductive isolation/imprinting/introgressive hybridization/ecological adaptation)
PETER R.GRANT AND B.ROSEMARY GRANT
Department of Ecology and Evolutionary Biology, Princeton University, Princeton, N.J. 08544–1003
ABSTRACT External (environmental) factors affecting the speciation of birds are better known than the internal (genetic) factors. The opposite is true for several groups of invertebrates, Drosophila being the outstanding example. Ideas about the genetics of speciation in general trace back to Dobzhansky who worked with Drosophila. These ideas are an insufficient guide for reconstructing speciation in birds for two main reasons. First, speciation in birds proceeds with the evolution of behavioral barriers to interbreeding; postmating isolation usually evolves much later, perhaps after gene exchange has all but ceased. As a consequence of the slow evolution of postmating isolating factors the scope for reinforcement of premating isolation is small, whereas the opportunity for introgressive hybridization to influence the evolution of diverging species is large. Second, premating isolation may arise from nongenetic, cultural causes; isolation may be affected partly by song, a trait that is culturally inherited through an imprinting-like process in many, but not all, groups of birds. Thus the genetic basis to the origin of bird species is to be sought in the inheritance of adult traits that are subject to natural and sexual selection. Some of the factors involved in premating isolation (plumage, morphology, and behavior) are under single-gene control, most are under polygenic control. The genetic basis of the origin of postmating isolating factors affecting the early development of embryos (viability) and reproductive physiology (sterility) is almost completely unknown. Bird speciation is facilitated by small population size, involves few genetic changes, and occurs relatively rapidly.
A major task for evolutionary biologists is to explain the origin of biodiversity. Within this broad field lies the central Darwinian problem of explaining how species are formed. When a solution is reached it will be an amalgam of information from genetics, ecology, and other disciplines, the amalgam will vary from one group of organisms to another, and genetics will be at the core. As stated most succinctly by Dobzhansky (1), “Evolution is a change in the genetic composition of populations. The study of mechanisms of evolution falls within the province of population genetics.” In this article we consider just one group of organisms, birds, and ask “How are new species produced, and what are the genetic changes involved?”
Dobzhansky’s 1937 book is a logical starting point for seeking an answer to these questions because it is at the root of current ideas about the genetic basis of speciation. However, in one respect it is disappointing: it has nothing to say about the genetics of birds. In it and the revised version in 1951, birds are used to illustrate some phenotypic and ecological patterns: geographical patterns of morphological variation within species, and changes occurring when a species enters a new area; rapid radiation in archipelagos; competition; and the evolution of reproductive isolation. The ecology of bird speciation is well known, but the genetics of speciation are the genetics of other organisms, mainly Drosophila.
In other respects the book is fascinating and rewarding because many of the issues he grappled with are still unresolved. What is a species? How are species formed? Can species form entirely sympatrically? What are the genetic mechanisms involved in the speciation process? What are the respective roles of selection and drift? What is selected, how, and why? The answers he gave to these questions have left an indelible imprint on the way we think about species and speciation. Here is a brief overview of those aspects most relevant to avian speciation.
Dobzhansky defines speciation as a process of evolutionary divergence of two or more populations. It starts with a single population and finishes with the coexistence of reproductively isolated populations: two species from one. Reproductive isolation means little or no gene exchange; it does not necessarily mean no gene exchange. It involves intrinsic, genetically conditioned, properties. “The first vestige of the isolation develops probably always in allopatric populations. Inviability of F1 hybrids, and low average adaptedness of the F2 and of backcross products, are byproducts of the genetic differentiation of allopatric populations…The hybrid inviability and breakdown provide, then, the stimulus for natural selection to build up other reproductive isolating mechanisms. Reproductive isolation diminishes the frequency of the appearance of hybrids, prevents the reproductive wastage, permits the populations of the incipient species gradually to invade each other’s territories, and finally to become partly and wholly sympatric” (1). In other words, selection enhances or reinforces (2) the differences in traits that are responsible for reproductive (behavioral) isolation when partly differentiated and previously separated populations meet, helping to complete the process of speciation. These traits constitute reproductive isolating mechanisms (1).
Thus the genetics of speciation is (a) the genetics of differentiation and (b) the genetics of reproductive isolation. Dobzhansky emphasized two other features that are relevant to our quest. The first is that speciation might be completed entirely in allopatry, but we would not know it if the populations remain geographically separated (but see ref. 3). The second is that speciation, the development of reproductive isolation, is a process that requires a long time.
These ideas may apply without qualification to some cases of bird speciation. Nevertheless we will argue that bird speciation often, perhaps usually, takes a different course and involves some different factors. Information on sexual imprinting, hybridization, and the fitness of hybrids lead us to suggest that premating isolation arises before postmating isolation; the capacity to learn through an imprinting mechanism keeps bird species apart before postmating isolating factors begin to evolve. Some of the factors involved in premating isolation are
© 1997 by The National Academy of Sciences 0027–8424/97/947768–8$2.00/0 PNAS is available online at http://www.pnas.org.
under single-gene or polygenic control (plumage and morphology), others are culturally inherited (song). The slow evolution of postmating isolation implies that the scope for reinforcement of premating isolating mechanisms is minimal. Involvement of culturally inherited traits may be partly responsible for the relatively rapid rate of speciation in bird.
Speciation in Birds
Speciation has been most thoroughly investigated, and for many years, in Darwin’s finches (Geospizinae; refs. 4–7). We therefore begin by describing a model that was devised specifically for these birds on the Galápagos Islands (8). We examine the evidence for various aspects of the model focusing on genetic factors where possible, and consider alternatives. Then we ask what needs to be added to the model to make it a comprehensive statement of speciation in birds in general.
A Model of Allopatric Speciation
Fig. 1 portrays three stages in the cycle of events leading to the division of one species into two. The choice of islands to illustrate these stages is arbitrary. In step 1, the archipelago is colonized from continental South or Central America. A breeding population becomes established, and its size increases. In step 2 some individuals disperse to another island and establish a new breeding population. Some evolutionary change takes place in the new environment through selection and drift. Step 2 may be repeated several times, giving rise to several differentiated populations of the same species. Step 3 is the contact, through dispersal of members of two populations possessing different mate signaling and recognition systems. This is the secondary sympatric phase of the cycle, and there are two types of outcomes. In one, members of the two populations do not interbreed, or if they do their offspring are inviable or infertile; the process of speciation in this case has been completed in allopatry. Alternatively the populations are only partly reproductively isolated, interbreeding occurs, and some of the hybrids survive to breed. Reinforcement of the differences between the species then may occur if the hybrids have relatively low fitness.
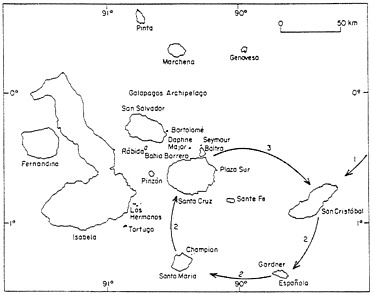
FIG. 1. Allopatric speciation of Darwin’s finches in the Galápagos archipelago (8). After an initial colonization of the archipelago (step 1), dispersal and the colonization of new islands (step 2) gives rise to allopatric populations, which diverge through selection and drift. The process is completed with the establishment of sympatry (step 3). The choice of islands to illustrate the process is arbitrary. [Reproduced with permission from ref. 7 (Copyright 1996, The Royal Society)].
Step 1 probably occurred once, or at most a few times, given the large distance separating the islands from the continent, which was greater at the time of initial colonization than at present (9). An argument from major histocompatibility complex variation suggests that there must have been a minimum of 30 individuals in the colonizing population (10). Stages 2 and 3 were repeated several times, giving rise to several species over a period of time estimated to be less than 3 million years (11). The ecological conditions would have varied from one cycle to another, but the essential features were repeated. The varying conditions include the length of the period of the allopatric phase (stage 2) before secondary contact, population sizes and hence the scope for drift, and the difference in the island environments and hence the scope for directional selection. Another important factor was the creation of new islands by volcanic activity (7, 9) and the recent periodic lowering of sea level (12). Over the last 3 million years there has been a net increase in the number of islands despite some disappearing through submergence, paralleling the increase in number of species (7). Thirteen species are recognized on the basis of morphological and biological criteria (4, 6), with as many as 10 occurring on a single island. A 14th species inhabits Cocos Island.
Evidence from Field Studies of Darwin’s Finches
We observe closely related species in sympatry and infer how they evolved from a common ancestor. Therefore we first consider how species are reproductively isolated, and then work back to their allopatric origin.
Reproductive Isolation
Species can be recognized by their morphological characteristics and songs (13, 14). With rare exceptions sympatric species pair and breed conspecifically, and as a result are reproductively isolated from each other. They choose mates on the basis of song, sung by males only, and morphological appearance, in which beak size and shape and body size play a part but plumage does not. Imprinting on adult features early in life appears to guide the choice of mates (7, 15, 16). The role of morphology in mate choice has been demonstrated experimentally with tests that show that several pairs of sympatric species of ground finches (Geospiza) discriminate between conspecific and heterospecific visual cues (17). Separately, experiments have shown that males can discriminate between conspecific and heterospecific auditory cues (18). Females were not tested in these acoustic experiments, but it would be surprising if they were not capable of making the same discriminations. The evolution of reproductive isolation in Darwin’s finches is therefore the evolution of differences in song and in morphology.
Reproductive isolation is not complete; species hybridize, rarely, and are capable of producing fertile hybrids that backcross to the parental species (12, 19, 20). The rare interbreeding of species and the mating pattern of the hybrids provide further evidence of the importance of song in mate choice. Hybridization occurs some times as a result of miscopying of song by a male; a female pairs with a heterospecific male that sings the same song as that sung by her misimprinted father (16). On Daphne Major island hybrid females bred with males that sang the same species song as their fathers (20). All G. fortis ×G. scandens F1 hybrid females whose fathers sang a G. fortis song paired with G. fortis males, whereas all those whose fathers sang a G. scandens song paired with G. scandens males. Offspring of the two hybrid groups (the backcrosses) paired within their own song groups as well. The same consistency was shown by the G. fortis ×G. fuliginosa F1 hybrid females and all their daughters, which backcrossed to G. fortis.
Thus mating of females was strictly along the lines of paternal song. The independent role of morphology in mate
choice is revealed by the rare instances where the usual association between song and morphology is disrupted. Four F1 hybrids (three females and a male) were produced by a G. fortis female that paired with a G. scandens male that sang a typical G. fortis song. The hybrids showed morphological evidence of nonrandom mating; their mates (all G. fortis) were more G. scandens-like in size and bill shape than were the potential G. fortis mates with which they did not breed. Also the one G. fortis ×G. scandens F1 hybrid male that paired with a G. fortis female, before switching to a G. scandens mate, had the most G. fortis-like beak proportions of all hybrids, male and female. Like its father, it sang a G. scandens song. Although rare, these examples give insights into the cues used in the choice of mates under natural conditions where variables (song and morphology) are normally confounded.
Inheritance of Traits that Isolate the Species
Isolation involves two sets of signals, song and morphology, and behavioral responses to them. One of the signals, song, is a culturally inherited trait in Darwin’s finches (15). Males (only) sing a single song that is sung unchanged throughout life and acquired by an imprinting-like learning process, usually from their fathers, but in a minority of cases from other conspecific males (15, 21). Beak and body size traits, on the other hand, are quantitative traits that are under polygenic control. Heritabilities of six traits in three species studied in most detail lie generally in the range of 0.5 to 0.75, and the genetic correlations among the traits are similarly high (13, 22, 23).
The possible genetic basis of the behavioral responses to song and morphological signals in the selection of a mate, and variation in the responses, are unknown, as they are for all birds (24). Crossfostering experiments are needed to dissociate the effects of parentally inherited genes from the effects of parentally influenced learning (imprinting) on mate choice. Where these have been done with other species they have shown that preferences for mates are not inherited in an inflexible way. Rather, young birds imprint on parental phenotype (25, 26), and visual and auditory stimuli they receive in early life influence their choice of mates much later in life. Thus sexual imprinting is likely to obscure any expression of genetically based variation in mate preferences that might exist. Even after effects of early experience have been experimentally manipulated by crossfostering it is still not known whether zebra finch males possess a biased (inherent) preference for conspecific females (27) or not (28). An inherent basis to female preferences is similarly not known.
Within each species there is little indication of assortative mating on the basis of beak size (20, 29) or song (13, 15, 30, 31), and little indication that mating is influenced by the measured morphological features of the parents (20). However, the interbreeding of species and the breeding of hybrids reveals evidence of imprinting. The evidence for song has been given above. With regard to morphology, there are two lines of evidence. First, G. fortis females mated with G. scandens that were morphologically similar to their mothers. Second, the group of G. fortis ×G. scandens F1 hybrids that paired with G. fortis mates showed evidence of morphologically nonrandom mating; their mates were more G. scandens-like in size and bill shape than were the potential G. fortis mates with which they did not breed. Therefore their choice of mates may have been influenced by the appearance of their G. scandens father.
To summarize, the particular song a male sings, and the behavioral responses of females to song and morphological signals, are not genetically inherited in a fixed manner but are determined by learning early in life. Genes that underlie the capacity to receive, use and transmit information are the evolving properties. These genes may evolve during speciation, but this seems unlikely to us. Rather, features of the imprinting process of closely related species of Darwin’s finches (e.g., onset, duration, and cessation) are probably very similar, if not identical. Their mate recognition systems may be identical, through shared inheritance, yet usually they pair conspecifically solely as a result of sexual imprinting on their parents or similar models. Polygenic variation does underlie variation in morphological signals, however. Thus the genetics of speciation of Darwin’s finches through the evolution of reproductive isolation are very different from the genetics of Drosophila as described by Dobzhansky and elaborated by many others (32–34).
Evolution of Prezygotic Isolation: Reinforcement
Do the differences in courtship signals and responses develop entirely in allopatry, or do they arise in allopatry and continue to increase in sympatry reinforced by selection in accordance with Dobzhansky’s reasoning on the minimization of interbreeding? We answer this question by considering morphology.
The reinforcement hypothesis in its original form requires that a certain degree of genetic incompatibility has evolved in allopatry, becoming manifest in sympatry (see refs. 1, 35, and 36). This requirement is not met, and therefore the hypothesis is rejected. The evidence is as follows.
Interbreeding of allopatric birds (under controlled conditions in captivity) has not been performed, but field observations of natural hybridization have been made on the islands of Daphne Major (14, 37, 38) and Genovesa (13). These show that all six species of Darwin’s ground finches (genus Geospiza) hybridize (rarely) with at least one other congeneric species. In addition some intergeneric crosses are known among the tree finches and warbler finch, and breeding hybrids have been produced (6, 21). On Daphne Major Geospiza fortis (medium ground finch) hybridizes with G. scandens (cactus finch), another resident species, and G. fuliginosa (small ground finch), an uncommon immigrant. Contrary to expectation from the reinforcement hypothesis, hybrids formed by Geospiza fortis breeding with G. scandens and G. fuliginosa are both viable and fertile to a degree similar to that of the contemporary offspring of conspecific matings; so are the first two generations of backcrosses (12, 19).
Backcrossing negates the hypothesis of speciation occurring entirely in allopatry. The speciation process therefore continues in sympatry. But the lack of a detectable genetic fitness loss associated with hybridization shows that reinforcement of song and morphology differences in sympatry does not occur, at least not for reasons of genetic incompatibility argued by Dobzhansky. Indeed postzygotic isolation has not evolved in allopatry, either partly or wholly, to any detectable extent. To be more specific, and with reference to the summary of Dobzhansky (1) above, inviability of some F1 hybrids, and low average adaptedness of the F2 and of backcross products, are not byproducts of the genetic differentiation of allopatric populations before secondary contact. They do not cause reproductive isolation; they follow as a consequence.
A broader statement of the reinforcement hypothesis allows for reinforcement to occur when the hybrids are at a disadvantage for ecological reasons, regardless of whether there are fitness-reducing genetic incompatibilities or not. For example there are environmental circumstances under which one class of F1 hybrids (G. fortis×G. fuliginosa) do not survive well (39). Because the relative fitness of hybrids is environment-dependent it is possible that the conditions for reinforcement (low fitness) could arise episodically solely from environmental causes. However, reinforcement then would occur only if the birds that hybridized were a nonrandom, and hence selectable, subset of the population with respect to a genetically inherited trait, such as bill size. There is no evidence for this (20).
Evolution of Premating Isolating Factors in Allopatry
Despite the evidence against reinforcement, the basis of premating isolation does not evolve entirely in allopatry. If it did we would expect members of well differentiated allopatric populations or closely related species to display a high degree of discrimination against heterotypic individuals in a reproductive context if they were brought into contact. Experiments show that this does not happen; there is instead a strong potential for “reproductive confusion” between immigrants from a partly differentiated population and their resident relatives on an island to which they have dispersed.
This potential has been revealed by discrimination tests conducted experimentally with stuffed museum specimens (models) of potential mates. The experiments used a conspecific specimen and a specimen from an allopatric population of a morphologically similar species, simulating the immigration of a closely related species (40). Using a heterospecific model rather than a well differentiated conspecific model should have biased the experimental result toward a clear discrimination, but, in fact, the observed results were the opposite: a statistical lack of discrimination. The results cannot be used as a measure of the degree to which interbreeding on secondary contact would occur. Nonetheless they stand in contrast to the rarity of observed hybridization. This implies that further divergence in courtship signals and responses occurs after the establishment of sympatry; though not, as we have seen, by the Dobzhansky process of reinforcement.
A parallel set of experiments gave a similar result with song. Like beak, size or shape differences, song differences have the potential of effecting a degree of isolation between the interactants. Experiments with playback of tape-recorded song demonstrate that resident males are capable of discriminating between songs produced by members of their own population and members of a related population on a near island. Residents’ songs elicit stronger responses than do those of the potential immigrants (18). In tests of several species the discrimination was often weak, implying that song difference, by itself, would not be sufficient to prevent interbreeding.
Allopatric Divergence
The second stages in each cycle are likely to have been followed by some back-migration (dispersal) from derived to original populations, and repeated forward-migration, in view of the generally short distances separating the islands (Fig. 1). Exchange of breeding individuals between two populations tends to homogenize their gene pools. If evolutionary divergence in allopatry has been minor before the interchange, the members of the two populations are likely to treat each other as potential mates and interbreeding will ensue. How, then, do they diverge to the point of developing premating isolation? The answer is a combination of natural selection and cultural drift.
Song differences between populations of the same species, and between species, arise initially in allopatry through a random sampling of song types in the newly founded population (41), followed by change as a result of small copying errors in transmission from father to son (15, 16, 20), as well as random extinction of song types; in other words by a process of cultural change analogous to genetic drift (15). Song frequencies also could change as a result of a chance association between song and a naturally selected morphological trait in males (31); evidence for this is not strong (15). The divergence of songs in the new population away from those in the progenitor population would only be prevented if these processes were balanced by repeated immigration and subsequent breeding; song flow.
Beak and body size differences arise under the different and strong directional selection pressures affecting the way in which finches exploit the environment, principally the food supply. Islands are known to differ in the food supply available to ground finches, mainly seeds (42–44). Natural selection on beak and body size traits has been measured in a population of Geospiza fortis on the island of Daphne Major at two times of change in the seed supply (45–47). Because the morphological traits are highly heritable (22, 23), natural selection in one generation led to an evolutionary response in the next (48). The magnitude of the response was such as to probably dwarf any genetic changes occurring over the short term by drift and immigration. Unfortunately, we have no measures of these latter two changes. Selective changes in the population of G. scandens on Daphne (37, 38) and G. conirostris on the island of Genovesa (13) also have been quantified, and although evolutionary responses were not determined they probably occurred because these species also display highly heritable morphological variation (13, 23).
Sufficient Allopatric Divergence for the Establishment of Sympatry
Differences in beak size between closely related species tend to be greater in sympatry than in allopatry (4, 6). Of the two possible reasons for this pattern, reinforcement and ecological divergence, reinforcement has been ruled out. The pattern is one of ecological, rather than reproductive, character displacement. Thus sympatry is established providing that (a) ecological conditions in the form of available food supply are favorable for the establishment of a new species, and (b) sufficient divergence in beak sizes and diets has occurred in allopatry. Further divergence occurs in sympatry (step 3 of the cycle) as a result of the morphologically most similar individuals of each species suffering the greater effects from competition for food. Supporting evidence for this interpretation includes a positive correlation between beak size differences and dietary differences that is predicted from the distribution of seed sizes on each island (42, 44, 49).
Although speciation is ecologically driven, reproductive (mating) interactions at secondary contact are far from irrelevant. Beak sizes and shapes have both mate (species) recognition and food handling functions. The outcome of immigration of individuals from a partly differentiated population could hang precariously between interbreeding and the reproductive absorption of immigrants into the population of residents, on the one hand, and establishment, divergence, and coexistence despite some degree of interbreeding, on the other hand. Because death and emigration are two other possible fates of the immigrants, establishment of a new population and completion of step 3 of the speciation cycle is a rare event.
When does a Species become Two?
In the first decade of the study on Daphne Major none of the F1 hybrids survived long enough to breed, therefore no gene exchange took place between the species, which were, in that sense, completely reproductively isolated. From 1983 onwards the hybrids backcrossed to the parental species and neither they nor their offspring experienced any apparent loss of fitness. The species were not reproductively isolated and were (and still are) moving slowly on a trajectory toward panmixia. The movement is slow because song constrains the mating of members of the backcross generations (12); none of the backcrosses have hybridized (20).
Should these be considered three species or one? Given the variety of opinions about how species should be defined and recognized (50), there is no clear answer to that question now, any more than there was when Huxley (51) wrote “we must not expect too much of the term species. In the first place, we must not expect a hard-and-fast definition, for since most evolution is a gradual process, borderline cases must occur. And in the second place, we must not expect a single or a simple basis for
definition, since species arise in many different ways.” In our view it is preferable to continue to treat the finches on Daphne as three species, expecting that environmental conditions will change back to those disfavoring the hybrids (14, 39). Elsewhere in the archipelago the three species are morphologically distinctive (52).
Avian Speciation in General
The model in Fig. 1 was designed to capture the essential features of allopatric speciation, although devised specifically for the insular speciation of Darwin’s finches. We have emphasized divergence in mate recognition and feeding traits in allopatry, song learning and imprinting, hybridization, and the absence of genetic incompatibilities. All of these features are displayed by birds elsewhere. For example, both imprinting (53) and hybridization (19, 54) are widespread in birds. Nonetheless the extent to which hybridization, imprinting, and the other features are generally involved in bird speciation is difficult to gauge because the requisite data for most bird species are lacking. The details of speciation must vary to some extent, because not all species learn song, not all species imprint on parental phenotype because not all exhibit parental care and not all hybridize.
Speciation of birds on islands elsewhere appears to follow the major paths of the model (7), with one conspicuous exception; a far greater diversity of plumage colors and patterns has evolved on other islands (7, 55, 56), presumably under sexual selection. The same applies to birds on continents. For a full understanding of avian speciation, on continents as well as on islands, we need to understand the genetic basis of plumage variation and associated behavior in courtship and mate choice, as sexual selection probably has been a major force in bird speciation (57, 58). Furthermore, to understand how plumage characters and behavior change during speciation we need to take account of demography and geography.
Genetic Changes in Plumage and Courtship Behavior
Plumage features constitute a major component of courtship signals (59). In theory, reproductive isolation between related species could be achieved by a single mutational change affecting a plumage trait that is used to identify mates as a result of the sort of imprinting process outlined for the Darwin’s finches. For example in the Bananaquit (Coereba flaveola) on the Caribbean island of Grenada a plumage polymorphism is governed by a single, diallelic, autosomal locus: melanic plumage is completely dominant to yellow plumage (60) (in other species melanism is a recessive trait; refs. 61 and 62). If the polymorphism arose through mutation in an isolated population, and the mutant allele became fixed, there would be a potential for reproductive isolation at secondary contact.
This scheme is too simple for most cases because polygenic inheritance of plumage traits is more common than single-gene inheritance (62). Polygenic inheritance is revealed by hybridization studies that show hybrids and backcrosses to possess traits intermediate between those of their parents (63–66). In these cases speciation may have involved the accumulation of frequency differences in the alleles, including novel alleles, at many loci. Yet even where speciation has involved divergent evolution of several plumage traits the changes may have been brought about by a small number of mutations of sufficient magnitude to produce appreciable effects from the beginning, with subsequent modification by genes of relatively minor effect (63). For example, a minimum of 14 loci influence the crest (color and extent) in F1 hybrids between two species of pheasants, Chrysolophus picta (Golden) and C. amherstiae (Amherst). Nevertheless alleles at a few loci have large phenotypic effects on crest morphology and display dominance and recessiveness, while others have smaller and apparently additive effects (63).
Evidence of epistasis from hybridization studies is more scarce. One plumage trait is consistently present in the F1 pheasant hybrids yet lacking in both parental species: on each side a conspicuous yellow spot extends across the breast, and the two spots nearly meet in the midline (63). The controlling factors may act epistatically, if not in an overdominant manner.
Males transmit signals in courtship through behavioral displays. In the early stages of speciation different (plumage) signals may be transmitted by the same displays. Later the displays themselves diverge. Hybridization studies show that when the parental species do differ the displays of F1 hybrids may (a) be intermediate, (b) comprise new combinations of displays found in one or both parental species, or (c) occur in neither parental species but perhaps occur in a third species (66–70). Intermediate behaviors in the F1 hybrids indicate polygenic inheritance, and different, combinations of components of the parental species repertoires in the members of the F2 generation (64) indicate a lack of linkage. As expected if behavioral features are inherited in a Mendelian fashion a broad range of variation in behaviors is shown in the F2 generation, with the all-or-none performance of some displays (64) possibly indicating dominance. Rates of bowing of some hybrid doves (70) were found to be very close to those of one of the species, suggesting dominance, and in other types of hybrids were beyond the range of both parental species, suggesting over dominance or epistasis.
Almost nothing is known from hybridization studies about the inheritance of courtship behavior of females, or of their responsiveness to particular male signals. This is particularly unfortunate in view of the importance given in theoretical models of sexual selection to genetic variation in female preferences for male traits (e.g., refs. 24, 71, and 72). Female cardueline finch hybrids solicit copulations in the same way as the parental species (67). Because the parental species in this study were not each other’s closest phylogenetic relative, the results show that copulatory behavior is evolutionary conservative in this group and does not diverge during speciation. The possible role of inherited factors in the divergence of female precopulatory behavior during speciation needs to be investigated.
Genetic Changes During Speciation
Electrophoretically detected genetic differences between closely related species provide an indirect measure of the genetic changes taking place during or shortly after speciation. They are indirect because they are unlikely to have any bearing on plumage and courtship behavior, or ecologically important morphological traits. Genetic distances between bird species are unusually small in comparison with other vertebrates (73–76). This has given rise to the idea that few genetic changes are involved in speciation, and that their phenotypic effects are minor (77, 78): “Perhaps speciation is often a rather superficial phenomenon, involving chiefly inherited changes in behavior and plumage” (77). Consistent with this “few genes” view of avian speciation, a small number of changes in plumage-governing genes apparently are involved in speciation.
Closely related species of birds are also chromosomally similar. Based on a comparison of 177 possible congeneric pairs of species, mainly passerines, Shields (79) concludes that chromosome change has not been involved in the promotion of reproductive isolation in most cases. Other authors have reached the same conclusion (77, 80). Nonetheless chromosomal evolution may play some role in speciation as it has been five times as fast in the more rapidly speciating passerines as in the nonpasserines (79). Against a background of chromosomal similarity among congeneric finch species in four families, a few pairs of congeneric species stand out by differing in
diploid number of chromosomes and several pericentric inversions (81, 82).
A minor complication with the “few genes” view arises from hybridization. Natural hybridization in birds is widespread though usually not common (19, 54), and in several cases it occurs without a marked loss of fitness (19). Genetic distances between bird species may be unusually short because such species occasionally exchange genes. Introgressive hybridization is, on the one hand, permitted by the small number of gene differences between closely related species and, on the other hand, contributes to the smallness of the genetic differences. The important point is that closely related species are almost completely reproductively isolated behaviorally, despite having the potential of producing viable and fertile hybrid offspring as a result of their genetic similarity. They remain isolated as a result of different signal and response systems in courtship.
The Prevalence and Potential Importance of Hybridization
If postmating isolation evolves in allopatry, hybridization at the sympatric stage will be lacking, or at most extremely rare and of little consequence. This was the view expressed by Mayr (83) when he wrote that few situations are known in birds where hybridization occurs, few hybrids are known, the majority are likely to be sterile, and if hybridization occurs and genes introgress the process will be self-reinforcing, with selection eliminating disharmonious mixed gene combinations. Even where hybridization is locally relatively common, as between black grouse (Tetrao tetrix) and capercaillie (T. urogallus) in Europe, it appears to have not led to introgression (83).
If, on the other hand, postmating isolation evolves slowly after sympatry is established there will be greater opportunity for hybridization to not only occur but, through introgression, to influence the evolution of the populations that exchange genes. Modern evidence suggests this applies to birds. In the last 25 years several field studies have documented by observation the occurrence of hybridization of bird species and shown that hybrids are viable and fertile to a large degree (19, 54). For example, at least half of 29 sympatric species pairs of North American birds hybridize, and at least a quarter backcross frequently (84). Eight types of intrageneric and intergeneric hybridization have been documented among sympatric hummingbird species in North America (85), and 13 types of intergeneric hybridization have been recorded of a possible 28 in sympatric birds of paradise (86).
Introgressive hybridization is underestimated by observation because it is not easy to detect (87). For example, detailed studies show that the F1 hybrids produced by black grouse and capercaillie do, in fact, backcross to capercaillie, possibly to black grouse as well, but, in accordance with Haldane’s rule, it is only the males that do (88). With regard to speciation, the more appropriate hybridization is between the sister taxa capercaillie (Tetrao urogallus) and the black-billed capercaillie (T. urogalloides). Where the ranges overlap in eastern Siberia not only do they hybridize, the hybrids (Kirpicev’s capercaillie) are fertile and show morphological evidence of hybrid vigor (88).
Molecular data have revealed evidence of previously unsuspected or underappreciated introgression (89–91). The occurrence of hybridization in both socially monogamous and polygynous species raises the possibility that interbreeding occurs cryptically, even if rarely, outside the pair-bond of the former and away from the main mating arenas of the latter. The prevalence of intergeneric hybridization in some groups of birds (92, 93) with little or no obvious fitness loss argues for long retention of the ability to hybridize successfully (see also ref. 94), as does the hybridization of nonsister taxa within genera (95) without the hybrids experiencing a disadvantage (96).
Introgressive hybridization has the potential of leading to further evolutionary change as a result of enhancing genetic variances, in some cases lowering genetic covariances (23), introducing new alleles (97), and creating new combinations of alleles, some of which might be favored by natural selection (1, 98) or sexual selection (99). Svärdson (100) believed that introgression in coregonid fishes has replaced mutation as the major source of evolutionary novelty. Introgression and mutation are not independent; introgressive hybridization may elevate mutation rates (101).
A particularly clear example of introgressive hybridization has been described by Chapin (102, 103). Exaggerated plumage traits (crest and tail length) have entered populations of two species of paradise flycatchers (genus Tersiphone) as a result of hybridization with a third species, in both east and west Africa. These traits are likely to have been favored by sexual selection. To judge from the inheritance of crests in quail (65) and extreme tail development in pheasants (63), just a few introgressed alleles could have effected the plumage transformations. The relevance to speciation lies in the fact that regions of introgression are peripheral areas, which could become isolated from the main range of the species through a change in climate and habitat: they are potential sites of speciation.
Geography and Demography of Speciation on Continents
In the early stages of allopatric speciation on continents, two populations are derived from one either by colonization of a second area through dispersal or by the splitting of a continuous range into two (vicariance). The dispersal mode is not fundamentally different from step 2 in Fig. 1. This is illustrated by ring species, which appear to offer the closest continental parallel to the dispersalist seheme in Fig. 1. According to the classical explanation of Dobzhansky (1), through dispersal a chain of partly differentiated populations becomes converted to a ring (see also refs. 54 and 83). At the point of ring closure or overlap where two populations establish secondary contact they do not interbreed, or do so extremely rarely; e.g., herring gull and lesser black-backed gull (104). A crossfostering experiment with these gulls showed that, as in Darwin’s finches, misimprinted birds are capable of producing viable hybrids, i.e., once the premating isolating mechanism is broken (104, 105).
The vicariance mode is different. Here the problem is to understand how a mutation arising in a large population might increase from an initially extremely low frequency to fixation. Directional natural or sexual selection would have to be persistent and strong, over a range of environmental conditions, to accomplish this. Speciation on continents might be quite different from speciation on islands if vicariance is a relatively common mode and dispersal into peripheral isolates is relatively rare, as is often claimed (106–108). However, the distinction between the two is not easy to make, principally because current distributions may be much broader as a result of postglacial range changes than the ones in which the main evolutionary events took place (108). It seems to us likely that some combination of vicariance and dispersal events might be more frequent than either is alone. The ring species may be a case in point (83).
Conditions for fixation to occur are much less stringent in a subdivided population, least stringent of all when the subdivisions occupy small habitat islands in a fragmented landscape: in effect an archipelago of insular populations within a continent. For this reason refuges at high (98, 109) and at low (110, 111) latitudes often have been invoked to account for the evolution of distinctive traits in closely related species. Hall and Moreau (112) have argued that “for continental [African] birds the period of most active speciation is a time of unfavourable climate, such as will break its natural habitat into small blocks or ‘islands’…” Quaternary fluctuations in climate have produced these conditions repeatedly (113, 114); humans are doing the same now (102). Each time the “islands” were probably reconstituted differently as a result of semi-
independent (individualistic) responses by components of the biota to climatic change (115).
If continental speciation is promoted by temporarily insular conditions the demography and genetics of founding populations may be of importance. It frequently has been suggested that on small islands or in fragmented habitats major genetic changes can take place in the early history of the founding of a new population by a few individuals, or during the bottlenecks caused by subsequent population crashes (116–119). The changes are believed to involve a few major genes that comprise a strongly epistatic polygenic system. Certainly changes can be rapid under these demographic circumstances. Selection is likely to be effective during the period of rapid growth from low population numbers, particularly for low-frequency alleles (120). High speciation rates (77), a greater genetic gap between similar species than between the most different conspecific populations (76), and the fact that populations are large yet genetic differences are small (74, 79), have all been interpreted as evidence for founder effects arising in subdivided populations and contributing to bird speciation.
The theory of founder speciation was developed when speciation was viewed as the evolution of postmating isolation largely, if not entirely, in geographical isolation (116). Derivatives of it are useful to account for some speciation phenomena in Drosophila (118–121) and similar organisms, but for birds it needs to be reappraised in light of a refocus on the origin of premating isolation (7). The idea that closely related species of birds differ in coadapted syndromes of mating behavior with an extensive genetic basis (122) is not supported by modern studies of hybridization and imprinting. The theory of founder effects does not explain how novel features like plumage traits arise. Founder effects may have contributed to bird speciation in the more limited way of altering the frequencies of alleles already in existence at the time of founding of a new population and initiating the evolution of premating isolating mechanisms.
Completion of Speciation
The process of speciation is completed with the cessation of genetic exchange. In broad outline the last stages of speciation are known, but the genetic basis to the physiological details of postmating isolation are not. As species diverge they accumulate different mutations that contribute to lowered viability and fertility of hybrids, possibly also to prezygotic incompatibilities in the female reproductive tract. For example, hybrids of Agapornis parrots develop excessive fat, lipomas, defeathering (males), and gout (uric acid crystals in joints), and females are sterile (69). In accordance with Haldane’s rule, genetic problems first arise in the heterogametic females (123, 124). Premating isolation increases as these forms of postzygotic isolation develop. The sexual display activity and vocalizations of hybrids becomes reduced (e.g., see refs. 64 and 69) or disrupted at points in a courtship sequence corresponding to differences between the parental species (69, 125). The disruptions are caused by conflicts between incompatible units of behavior, missing parts of the repertoire of one or both parental species, or unusual behaviors not expressed by either parental species. Sexual behavior breaks down altogether and is not exhibited in hybrids produced in captivity between very disparate parental species, and cannot be induced by a large dose of sex hormones (67). Such species do not interbreed in nature.
Genetic incompatibilities that conform to Haldane’s rule do not always arise in the simple speciation process of one species splitting into two. Sometimes they arise between two species so formed only after each of them has split farther into yet more species. The frequency of this is unknown.
General Trends: Six Rules of Avian Speciation
As a means of summarizing the preceding discussion and survey of the literature we suggest there are six rules of speciation in birds:
-
Speciation is initiated in allopatry.
-
The sympatric phase of the speciation process is established after an allopatric period of ecological divergence.
-
Allopatric evolution of premating isolating mechanisms precedes the evolution of postmating mechanisms in allopatry or sympatry.
-
Premating mechanisms are governed mainly by additive effects of polygenes, postmating mechanisms are due mainly to nonadditive genetic effects (dominance and epistasis).
-
Premating mechanisms include effects of the cultural process of sexual imprinting.
-
Postzygotic incompatibilities arise first in females. This is Haldane’s rule applied to birds in which females are the heterogametic sex.
All rules have exceptions, otherwise they would be laws, and it is doubtful if there are any speciation laws. Birds provide overwhelming support for Haldane’s rule (126), with a few exceptions affecting fertility (e.g., see refs. 32 and 65) but apparently none affecting viability in captive birds (ref. 32; but see ref. 127). Rule 5 would seem to have the greatest number of exceptions, particularly among those species lacking paternal care (128). We include it on the strength of a literature survey that concluded that sexual imprinting “seems to have been found wherever it has been looked for, and should be considered the rule rather than exceptional for the development of mate preferences in birds” (53). The rules should apply to other nonavian taxa, to varying degrees.
We conclude with a caveat. Almost 10,000 bird species are recognized under the biological species concept (129). Interpretations of speciation have been applied to perhaps 500 of them. The genetic basis of variation in premating isolating traits believed to be involved in speciation is known (incompletely) for less than 100 species, and the genetic basis of postmating isolation is virtually unknown for all of them. The knowledge base from which to generalize about the genetics of bird speciation is precariously thin. Recognition of this should be a stimulus for future research. The ease with which closely related species can be induced to hybridize in captivity suggests that a program of experimental hybridization has much to teach us about the genetics of bird speciation.
We are grateful to the A. von Humboldt Foundation and Peter Berthold of the Max-Planck-Institut at Radolfzell for support while this paper was written, and to T.D.Price, D.S.Woodruff, and an anonymous referee for helpful comments. Research on Darwin’s finches has been supported by the National Science and Engineering Research Council (Canada) and the National Science Foundation (USA).
1. Dobzhansky, T. (1937) Genetics and the Origin of Species (Columbia Univ. Press, New York), revised 1951.
2. Blair, W.F. (1955) Evolution 9, 469–480.
3. Wittmann, U., Heidrich, P., Wink, M. & Gwinner, E. (1995) J. Zool. Syst. Evol. Res. 33, 116–122.
4. Lack, D. (1947) Darwin’s Finches (Cambridge Univ. Press, Cambridge).
5. Bowman, R.I. (1961) Univ. Calif. Publ. Zool. 58, 1–302.
6. Grant, P.R. (1986) Ecology and Evolution of Darwin’s Finches (Princeton Univ. Press, Princeton, N.J.).
7. Grant, P.R. & Grant, B.R. (1996) Philos. Trans. R. Soc. London Ser. B 351, 765–772.
8. Grant, P.R. (1981) Am. Sci. 69, 653–663.
9. Christie, D.M., Duncan, R.A., McBirney, A.R., Richards, M.A., White, W.M., Harpp, K.S. & Fox, C.G. (1992) Nature (London) 355, 246–248.
10. Vincek, V., O’Huigin, C., Satta, Y., Takahata, N., Boag, P.T., Grant, P.R., Grant, B.R. & Klein, J. (1996) Proc. R. Soc. London Ser. B 265, 111–118.
11. Grant, P.R. (1994) Evol. Ecol. 8, 598–617.
12. Grant, B.R. & Grant, P.R. (1997) in Endless Forms: Species and Speciation, eds. Howard, D.J. & Berlocher, S.H. (Oxford Univ. Press, Oxford), in press.
13. Grant, B.R. & Grant, P.R. (1989) Evolutionary Dynamics of a Natural Population: The Large Cactus Finch of the Galapagos (Univ. Chicago Press, Chicago).
14. Grant, P.R. (1993) Philos. Trans. R. Soc. London Ser. B 340, 127–139.
15. Grant, B.R. & Grant, P.R. (1996) Evolution 50, 2471–2487.
16. Grant, P.R. & Grant, B.R. (1997) Am. Nat. 149, 1–28.
17. Ratcliffe, L.M. & Grant, P.R. (1983) Anim. Behav. 31, 1139–1153.
18. Ratcliffe, L.M. & Grant, P.R. (1985) Anim. Behav. 33, 290–307.
19. Grant, P.R. & Grant, B.R. (1992) Science 256, 195–197.
20. Grant, P.R. & Grant, B.R. (1997) Biol. J. Linn. Soc. 60, 317–343.
21. Bowman, R.I. (1983) in Patterns of Evolution in Galápagos Organisms, eds. Bowman, R.I., Berson, M. & Leviton, A.E. (American Association for the Advancement of Science, San Francisco), pp. 237–537.
22. Boag, P.T. (1983) Evolution 37, 877–894.
23. Grant, P.R. & Grant, B.R. (1994) Evolution 48, 297–316.
24. Bakker, T.C.M. & Pomiankowski, A. (1995) J. Evol. Biol. 8, 129–171.
25. Immelmann, K. (1975) Annu. Rev. Ecol. Syst. 6, 15–37.
26. Cooke, F., Finney, G.H. & Rockwell, R.F. (1976) Behav. Genet. 6, 127–140.
27. Bischoff, H.-J. & Clayton, N. (1991) Behavior 118, 144–155.
28. Kruijt, J.P. & Meeuwissen, G.B. (1993) Neth. J. Zool. 43, 68–79.
29. Price, T.D. (1984) Evolution 38, 327–341.
30. Millington, S. & Price, T. (1985) Auk 102, 342–346.
31. Gibbs, H.L. (1990) Anim. Behav. 39, 253–263.
32. Coyne, J.A. (1992) Nature (London) 355, 511–515.
33. Coyne, J.A., Crittenden, A.P. & Mah, K. (1994) Science 265, 1461–1464.
34. Wu, C.-I. & Palopoli, M. (1994) Annu. Rev. Genet. 28, 283–308.
35. Howard, D.J. (1993) in Hybrid Zones and the Evolutionary Process, ed. Harrison, R.G. (Oxford Univ. Press, Oxford), pp. 46–69.
36. Liou, L.W. & Price, T.D. (1994) Evolution 48, 1451–1459.
37. Grant, P.R. & Price, T.D. (1981) Am. Zool. 21, 795–811.
38. Boag, P.T. & Grant, P.R. (1984) Biol. J. Linn. Soc. 22, 243–287.
39. Grant, B.R. & Grant, P.R. (1993) Proc. R. Soc. London Ser. B 251, 111–117.
40. Ratcliffe, L.M. & Grant, P.R. (1983) Anim. Behav. 31, 1154–1165.
41. Grant, P.R. & Grant, B.R. (1995) Evolution 49, 229–240.
42. Abbott, I.J., Abbott, L.K. & Grant, P.R. (1977) Ecol. Monogr. 47, 151–184.
43. Smith, J.N.M., Grant, P.R., Grant, B.R., Abbott, I. & Abbott, L.K. (1978) Ecology 59, 1137–1150.
44. Schluter, D. & Grant, P.R. (1984) Am. Nat. 123, 175–196.
45. Boag, P.T. & Grant, P.R. (1981) Science 214, 82–85.
46. Price, T.D., Grant, P.R., Gibbs, H.L. & Boag, P.T. (1984) Nature (London) 309, 787–789.
47. Gibbs, H.L. & Grant, P.R. (1987) Nature (London) 327, 511–513.
48. Grant, P.R. & Grant, B.R. (1995) Evolution 49, 241–251.
49. Schluter, D., Price, T.D. & Grant, P.R. (1985) Science 227, 1056–1059.
50. Endler, J.A. (1989) in Speciation and its Consequences, eds. Otte, D. & Endler, J.A. (Sinauer, Sunderland, MA), pp, 625–648.
51. Huxley, J. (1942) Evolution: The Modern Synthesis (Allen & Unwin, London).
52. Grant, P.R., Abbott, I., Schluter, D., Curry, R.L. & Abbott, L.K. (1985) Biol. J. Linn. Soc. 25, 1–39.
53. Ten Cate, C., Vos, D.R. & Mann, N. (1993) Neth. J. Zool. 43, 34–45.
54. Panov, E. (1989) Natural Hybridisation and Ethological Isolation in Birds (Nauka, Moscow).
55. Amadon, D. (1950) Bull. Am. Mus. Nat. Hist. 95, 151–262.
56. Mayr, E. (1972) Biol. J. Linn. Soc. 1, 1–17.
57. West-Eberhard, M.J. (1983) Quart. Rev. Biol. 58, 155–183.
58. Barrowclough, T.G., Harvey, P.H. & Nee, S. (1995) Proc. R. Soc. London Ser. B 259, 211–215.
59. Baker, M.C. & Baker, A.E.M. (1990) Evolution 44, 332–338.
60. Wunderle, J. (1981) Evolution 35, 333–344.
61. Berthold, P. (1997) Naturwissenschaften 83, 568–570.
62. Buckley, P.A. (1987) in Avian Genetics: A Population and Ecological Approach, eds. Cooke, F. & Buckley, P.A. (Academic, London), pp. 1–44.
63. Danforth, C.H. (1950) Evolution 4, 301–315.
64. Sharpe, R.S. & Johnsgard, P.A. (1966) Behavior 27, 259–272.
65. Johnsgard, P.A. (1973) Grouse and Quails of North America (Univ. Nebraska Press, Lincoln).
66. Seherer, S. & Hilsberg T. (1982) J. f. Orn. 123, 357–380.
67. Hinde, R.A. (1956) Behavior 9, 202–213.
68. Lorenz, K. (1958) Sci. Amer. 199, 67–78.
69. Buckley, P.A. (1969) Z. Tierpsychol. 26, 737–747.
70. Davies, S.J.J.F. (1970) Behavior 36, 187–214.
71. Price, T.D., Schluter D. & Heckman, N.E. (1993) Biol. J. Linn. Soc. 48, 187–211.
72. Schluter, D. & Price, T. (1993) Proc. R. Soc. London Ser. B 253, 117–122.
73. Avise, J.C. (1983) in Perspectives in Ornithology, eds. Brush, A.H. & Clark, G.A., Jr. (Cambridge Univ. Press, Cambridge), pp. 262–270.
74. Barrowclough, G.F. (1983) in Perspectives in Ornithology, eds. Brush, A.H. & Clark, G.A., Jr. (Cambridge Univ. Press, Cambridge), pp. 223–261.
75. Johnson, N.K. & Zink, R.M. (1983) Auk 100, 871–884.
76. Corbin, K.W. (1987) in Avian Genetics: A Population and Ecological Approach, eds. Cooke, F. & Buckley, P.A. (Academic, London), pp. 321–353.
77. Prager, E.M. & Wilson, A.C. (1980) in Proc. XVII International Ornithological Congress, Berlin (Verlag der Deutsche Ornithologen-Gesellschaft), pp. 1209–1214.
78. Snell, R.R. (1991) Auk 108, 319–328.
79. Shields, G.F. (1987) in Avian Genetics: A Population and Ecological Approach, eds. Cooke, F. & Buckley P.A. (Academic, London), pp. 79–104.
80. Sandnes, G.C. (1954) Evolution 8, 359–364.
81. Christidis, L. (1986) Can. J. Genet. Cytol. 28, 762–769.
82. Christidis, L. (1990) in Chromosomes Today, eds. Fredga, K., Kihlman, B.A. & Bennett, M.D. (Unwin Hyman, London), Vol. 10, pp. 279–294.
83. Mayr, E. (1963) Animal Species and Evolution (Belknap, Harvard, MA).
84. Anderson, R.V.V. (1977) Am. Nat. 111, 939–949.
85. Johnsgard, P.A. (1983) The Hummingbirds of North America (Smithsonian Inst. Press, Washington).
86. Christidis, L. & Schodde, R. (1993) Bull. Brit. Orn. Club 113, 169–172.
87. Nagle, J.J. & Mettler, L.E. (1969) Evolution 23, 519–524.
88. Klans, S., Andreev, A.V., Bergmann, H.-H., Müller, F., Porkert, J. & Wiesner, J. (1989) Die Auerhühner: Tetrao urogallus und T. urogalloides (A. Ziemsen Verlag, Wittenberg Lutherstadt).
89. Avise, J.C. & Ball, R.M. (1990) Oxf. Surv. Evol. Biol. 7, 45–67.
90. Gill, F.B., Mostrom, A.M. & Mack, A.L. (1993) Evolution 47, 195–212.
91. Degnan, S. (1993) Mol. Ecol. 2, 219–225.
92. Meise, W. (1975) Abh. Verh. Naturwiss. Ver. Hamburg 18/19, 187–254.
93. Banks, R.C. & Johnson, N.K. (1961) Condor 63, 3–28.
94. Prager, E.M. & Wilson, A.C. (1975) Proc. Natl. Acad. Sci. USA 72, 200–204.
95. Freeman, S. & Zink, R.M. (1995) Syst. Biol. 44, 409–420.
96. Corbin, K.W., Sibley, C.G. & Ferguson, A. (1979) Evolution 33, 624–633.
97. Anderson, E. & Stebbins, G.L., Jr. (1954) Evolution 8, 378–388.
98. Miller, A.H. (1956) Evolution 10, 262–277.
99. Parsons, T.J., Olson, S.L. & Braun, M.J. (1993) Science 260, 1643–1646.
100. Svärdson, G. (1970) in Biology of Coregonid Fishes, eds. Lindsey, C.C. & Woods, C.S. (Univ. Manitoba Press, Winnipeg), pp. 33–59.
101. Woodruff, D.S. (1989) Biol. J. Linn. Soc. 36, 281–294.
102. Chapin, J.P. (1948) Evolution 2, 111–126.
103. Chapin, J.P. (1963) Ibis 105, 198–202.
104. Harris, M.P. (1970) Ibis 112, 488–498.
105. Harris, M.P., Morley, C. & Green, G.H. (1978) Bird Study 25, 161–166.
106. Cracraft, J. (1982) Amer. Zool. 22, 411–424.
107. Cracraft, J. & Prum, R.O. (1988) Evolution 42, 603–620.
108. Chesser, R.T. & Zink, R.M. (1994) Evolution 48, 490–497.
109. Zink, R.M. & Dittmann D.L. (1993) Evolution 47, 717–729.
110. Haffer, J. (1982) in Biological Diversification in the Tropics, ed. Prance, G.T. (Columbia Univ. Press, New York), pp. 6–24.
111. Brumfield, R.T. & Capparella, A.P. (1996) Evolution 50, 1607–1624.
112. Hall, B.P. & Moreau, R.E. (1970) An Atlas of Speciation in African Passerine Birds (British Museum, Natural History, London).
113. deMenocal, P. (1995) Science 270, 53–59.
114. Keast, A. (1961) Bull. Mus. Comp. Zool. Harvard 123, 303–495.
115. Jablonski, D. & Sepkoski, J.J., Jr. (1996) Ecology 77, 1367–1378.
116. Mayr, E. (1954) in Evolution as a Process, eds. Huxley, J., Hardy, A.C. & Ford, E.B. (Allen & Unwin, London), pp. 157–180.
117. Mayr, E. (1992) Oxford Surv. Evol. Biol. 8, 1–34.
118. Carson, H. (1975) Am. Nat. 109, 83–92.
119. Templeton, A.R. (1980) Genetics 91, 1011–1038.
120. Slatkin, M. (1996) Am. Nat. 147, 493–505.
121. Hollocher, H. (1996) Philos. Trans. R. Soc. London Ser. B 351, 735–743.
122. Carson, H.L. (1978) in Ecological Genetics: The Interface, ed. Brussard, P.F. (Springer, New York), pp. 93–107.
123. Tegelström, H. & Gelter, H.P. (1990) Evolution 44, 2012–2021.
124. Golter, H.P., Tegelström, H. & Gustafsson, L. (1992) Ibis 134, 62–68.
125. Dilger, W.C. (1962) Sci. Am. 206, 88–98.
126. Coyne, J.A. & Orr, H.A. (1989) in Speciation and Its Consequences, eds. Otte, D. & Endler, J.A. (Sinauer, Sunderland, MA), pp. 180–207.
127. Wu, C.-I. & Davis, A.W. (1993) Am. Nat. 145, 187–212.
128. Schutz, V.F. (1965) Z. Tierpsychol. 22, 50–103.
129. Sibley, C.G. & Ahlquist, J.E. (1990) Phylogeny and Classification of Birds: A Study in Molecular Evolution (Yale Univ. Press, New Haven).








