2
Assessment of Efficacy Data
The U.S. Food and Drug Administration (FDA) originally approved the hypnotic drug Halcion (triazolam) for use at a dose of 0.5 mg for the purpose of improving the quality of sleep in 1982. In 1987, however, concerns about the drug's safety caused FDA to lower the recommended starting dose in the labeling to 0.25 mg for adults and 0.125 mg for the geriatric population (data are available demonstrating that elderly subjects clear triazolam from the blood at half the rate of younger subjects [Greenblatt et al., 1991]). This, in turn, raised questions about efficacy at the lower doses. For that reason, the Institute of Medicine (IOM) committee focused on these two lower doses, specifically examining the data for evidence of (1) improvement in certain endpoints over those from use of the placebo or the comparability of endpoints from the use of Halcion and a positive comparator drug and (2) tolerance to the drug's effects over time.1
To assess the quality and quantity of the available data regarding the efficacy of Halcion, the committee first examined the indications for the use of hypnotic drugs and the means of evaluating the efficacies of these types of drugs, including the requirements for approval by FDA. The committee then assessed the quality of the protocols and the designs of the pre- and postmarketing clinical trials investigating the efficacy of Halcion. To analyze the data from those trials, the committee reviewed several statistical methods and then reanalyzed the data across studies. Finally, the committee summarized the available literature pertinent to the efficacy of Halcion, and from all of this information, the committee drew the conclusions presented at the end of this chapter.
PURPOSE AND EVALUATION OF HYPNOTIC DRUGS
The process of assessing the efficacies of hypnotic drugs requires a statement of the purpose for the drug's use. The primary indication for use of a hypnotic agent is to improve the quality or quantity of sleep. A secondary purpose is to improve the quality of life throughout the 24-hour day. One useful distinction is-between the need for sleep improvement on a short-term (acute) versus a long-term (chronic) basis. Virtually anyone can suffer an acute sleep problem due to a variety of circumstances, including jet lag and acute situational stress. On the other hand, individuals can experience a persistent reduced quality of sleep for a variety of reasons, and this population will be quite heterogeneous. Separating people with chronic insomnia from those who suffer from acute insomnia is useful in evaluating the efficacy of a hypnotic agent.
The study populations used to study the efficacies of hypnotic drugs ideally should be heterogeneous. The exclusion criteria should be such that large segments of the population who will be treated in good clinical practice will not be eliminated. However, because impairment of sleep can be a clinical symptom associated with psychotic disorders, the standard of care is treatment of the psychotic disorder rather than prolonged use of hypnotic agents, and the exclusion of psychotic patients in clinical trials of an hypnotic agent is justified and appropriate.
Evaluating Efficacy
There are two approaches to the evaluation of sleep quality: subjective and objective. The subjective evaluation of sleep involves the use of questionnaires or interviews. The subjects indicate their evaluation of the endpoint, for example, onset of sleep, duration, awakenings, and quality. The objective approach involves the use of polysomnographic studies. In these electroencephalographic (EEG) studies the exact length of time to the onset of sleep can be measured precisely, as can sleep duration and number of awakenings. Obviously, the evaluation of the quality of the sleep is still determined by the subject. Having obtained precise sleep parameters from the polysomnograph, one can compare people with and without subjective sleep problems. Interestingly, the differences in objective sleep measurements between these two groups are relatively small. It can therefore be argued that many people who complain of sleep disturbances actually sleep quite well in an objective sense. This approach fails to recognize, however, that the experience of satisfaction is, by definition, subjective. The length of time required to fall asleep that is or is not satisfying to an individual is subjective. A statistically small reduction in sleep latency may be experienced by the subject as very valuable and desirable. Telling insomniacs that their sleep latency is actually within one standard deviation of the mean is not likely to improve their satisfaction.
In the clinical setting, the insomniac patient and the clinician are seeking increased subjective improvement in sleep. In the sleep laboratory, however, subjective measures may not coincide with objective measures. For example, tolerance to benefits from hypnotic agents often occurs in objective measures despite continued improvement with subjective questionnaires. Since the reasons for the discrepancy between subjective and objective sleep
measures are obscure, it is premature to rely solely on subjective measures in the evaluation of hypnotic agents.
Although it is important to try to develop methods that allow for a more precise analysis of sleep parameters, researchers must continue to recognize the inherent subjectivity of the evaluation of the endpoints by the individual. It follows, then, that the subjective evaluation of the endpoints is more appropriate in the sense that it is patient satisfaction that is the principal goal of the pharmacologic intervention.
The committee is not aware of studies designed specifically to compare the two methods of hypnotic evaluation. Indeed, although not a specific objective, in a study comparing the effects of nefazodone and fluexetine on mood and polysomnographic data for depressed patients complaining of insomnia, the patients and clinicians reported nearly equal improvement in subjective sleep measures for both days, whereas polysomnographic data showed a progressive deterioration of some measures for fluexetine (Gillin et al., 1997). Finally, the nature of polysomnographic studies restricts them to small numbers of subjects who are unlikely to be a representative subset of the population at large.
In assessing efficacy, the question of the duration of time between taking the hypnotic agent and actually going to bed has been raised. The point of an efficacy study is not to assess the absorption time of the compound or to have sleep onset occur during the period of absorption. Taking the active and comparator compounds at a specified interval before going to bed is an appropriate method of eliminating the measurement error incurred by adding absorption time to sleep onset.
FDA Efficacy Requirements
Approval of a New Drug Application (NDA) by FDA usually requires two well-controlled trials. The sponsor developing a new drug uses Phase I studies to determine dose and drug levels in plasma, tolerability, pharmacokinetics, and pharmacodynamic data when possible. The next step is the initiation of studies performed to determine the appropriate dose and dosing schedule to establish efficacy and safety in patients for whom the drug is intended. Using this information, the sponsor undertakes two or more clinical trials with a large enough number of subjects to provide sufficient statistical power to establish that the drug is effective for its intended use. These latter trials are referred to as the ''pivotal trials"; that is, the proof of efficacy pivots on these studies as being of vital or central importance. Supporting data from other trials are also used in the regulatory approval process. Approval of a drug for marketing, however, hinges on a determination not only of efficacy but on a risk-benefit analysis.
Available Efficacy Data on Halcion
Upjohn's NDA (NDA 17=892) for the treatment of insomnia with triazolam (Halcion) was approved in 1982 by FDA on the basis of the results of efficacy studies with a 0.5-mg dose. In response to concerns about the safety of this and a larger dose (van der Kroef, 1979;
To attempt to answer these dose-related efficacy questions and to evaluate the quality of the data on which FDA based its decision that Halcion was effective at the lower doses (Marticello, 1992), the committee undertook an evaluation and reanalysis of the available data2 using a statistical method different from that used by FDA.
From a list of all protocols in the NDA (U.S. Food and Drug Administration, 1996, Appendix C), the committee selected those that appeared by their descriptions to define well-controlled studies. These studies had adequate numbers of subjects to suggest that they constituted a sample large enough to provide adequate statistical power. The goal was to determine whether Halcion differed from the placebo in the achievement of the primary efficacy endpoints or was comparable to a positive comparator drug in achieving these endpoints. A list of the protocols examining the low dose (<0.5 mg), including a summary of some demographic data and other aspects of these protocols, is presented in Table 2-1. The committee also reviewed a final report of a study that FDA used in making its decision concerning the approval of Halcion at lower doses but that was not part of the Upjohn NDA (Lee, 1990).
QUALITY OF PROTOCOLS AND STUDY DESIGN
Before undertaking its review, the committee familiarized itself with the FDA publication Guidelines for the Clinical Evaluation of Hypnotic Drugs (U.S. Department of Health, Education, and Welfare, 1977). The initial step in the review was to evaluate the quality of the protocols and the study designs. The committee was especially interested in the specificities of the primary endpoints, the method(s) used to quantify those endpoints, and the characteristics of the study population, as well as other items required for a well-controlled study. A checklist was devised and was used to evaluate each protocol. The major categories of the variables and the criteria used to evaluate the protocol review form are as follows:
-
Objective(s)
-
Inclusion and exclusion criteria
-
Study design and procedures
-
Outcome variables (endpoints)
-
Concomitant drugs
-
Statistical methods
|
2 |
The committee did not review the source data (i.e., the raw data) or the case report forms for accuracy. The committee's review was limited to the data presented in the NDA and other sources cited in the text. FDA did find some discrepancies in the data from a few investigators (U.S. Food and Drug Administration, 1996, Appendix F), but it did not use these data in its analyses, nor did this committee include these data in its review. |
TABLE 2-1 Low-Dose Premarketing Studies Reviewed by IOM Committee for Efficacy of Halcion (less than 0.5 mg)
|
|
|
|
|
|
|
|
Age (yr) |
Gender (no.) |
|||
|
Protocol Number |
Investigator |
Study Design and Focus |
Planned Duration |
Schedule |
Treatment Group (dose [mg]) |
No. of Patients |
Mean |
Min. |
Max. |
Male |
Female |
|
2401 |
Cohn and Fabre |
Controlled, DB, randomized, parallel, adjustable-dose efficacy/ safety study with outpatients with anxiety, neurosis, and insomnia |
1 wk Pbo washout; 1 wk Tx |
Triaxolam 0.25; diazepam 5; Pbo; HS dosing: after 3 days, dose could be increased to 2 capsules HS |
Triazolam (0.25) |
39 |
41 |
20 |
62 |
24 |
15 |
|
Diazepam (5) |
43 |
34 |
20 |
61 |
21 |
22 |
|||||
|
Placebo |
44 |
34 |
19 |
56 |
23 |
21 |
|||||
|
6010 |
Sunshine |
Controlled, DB, randomized, crossover efficacy/safety study with patients with insomnia |
28 days—1 wk on each treatment |
HS doing |
Triazolam (0.3) |
42 |
32 |
19 |
60 |
Data not available |
|
|
Triazolam (0.6) |
39 |
32 |
19 |
60 |
|
|
|||||
|
Flurazepam (15) |
41 |
23 |
19 |
60 |
|
|
|||||
|
Flurazepam (30) |
41 |
32 |
19 |
60 |
|
|
|||||
|
6014 IV |
Kramer |
Experiment IV: study to evaluate the EEG and hypnotic effects of triazolam with patients with insomnia; drug nights compared to Pbo baseline and withdrawal |
7 nights |
HS dosing |
Triazolam (0.25) |
6 |
21 |
18 |
24 |
6 |
0 |
|
|
|
|
|
|
|
|
Age (yr) |
Gender (no.) |
|||
|
Protocol Number |
Investigator |
Study Design and Focus |
Planned Duration |
Schedule |
Treatment Group (dose [mg]) |
No. of Patients |
Mean |
Min. |
Max. |
Male |
Female |
|
6020 |
Vogel |
Study in patients with insomnia in sleep lab to study EEG and hypnotic effects; drug nights compared to Pbo baseline and withdrawal |
7 nights |
HS dosing |
Triazolam (0.25) |
3 |
Data not available |
||||
|
Triazolam (0.5) |
6 |
|
|
|
|
|
|||||
|
Triazolam (1.0) |
3 |
|
|
|
|
|
|||||
|
6033 |
Edmondson |
Controlled, DB, randomized, crossover efficacy/safety study with patients with insomnia |
5 nights of Tx |
HS dosing |
Triazolam (0.25) |
30 |
49 |
26 |
58 |
30 |
0 |
|
Triazolam (0.5) |
31 |
49 |
26 |
58 |
31 |
0 |
|||||
|
Diazepam (10) |
31 |
49 |
26 |
58 |
31 |
0 |
|||||
|
Diazepam (5) |
31 |
49 |
26 |
58 |
31 |
0 |
|||||
|
Placebo |
31 |
49 |
26 |
58 |
31 |
0 |
|||||
|
6034 |
Knapp |
Controlled, DB, randomized, crossover efficacy/safety study with inpatients with insomnia |
5 nights; 1 night each Tx |
HS dosing |
Triazolam (0.25) |
30 |
Data not available |
||||
|
Triazolam (0.5) |
30 |
|
|
|
|
|
|||||
|
Diazepam (10) |
30 |
|
|
|
|
|
|||||
|
Diazepam (5) |
30 |
|
|
|
|
|
|||||
|
Placebo |
30 |
|
|
|
|
|
|||||
|
6035 |
Kramer |
Controlled, DB, randomized, crossover efficacy/safety study with geriatric inpatients with insomnia |
5 nights |
HS dosing |
Triazolam (0.25) |
34 |
70 |
52 |
94 |
33 |
1 |
|
Triazolam (0.5) |
32 |
70 |
52 |
94 |
31 |
1 |
|||||
|
Chloral hydrate (250) |
32 |
70 |
52 |
94 |
31 |
1 |
|||||
|
Chloral hydrate (500) |
35 |
70 |
52 |
94 |
33 |
1 |
|||||
|
Placebo |
34 |
70 |
52 |
94 |
34 |
1 |
|||||
|
6056 |
Kramer |
Controlled, DB, randomized, crossover performance study with healthy subjects |
6 weeks; 2 nights on each Tx followed by 5 night washout |
HS dosing |
Triazolam. (0.25) |
12 |
23 |
20 |
26 |
12 |
0 |
|
Triazolam (0.5) |
12 |
23 |
20 |
26 |
12 |
0 |
|||||
|
Flurazepam (15) |
12 |
23 |
20 |
26 |
12 |
0 |
|||||
|
Flurazepam (30) |
12 |
23 |
20 |
26 |
12 |
0 |
|||||
|
Secobarbital (100) |
12 |
23 |
20 |
26 |
12 |
0 |
|||||
|
Placebo |
12 |
23 |
20 |
26 |
12 |
0 |
|||||
|
|
|
|
|
|
|
|
Age (yr) |
Gender (no.) |
|||
|
Protocol Number |
Investigator |
Study Design and Focus |
Planned Duration |
Schedule |
Treatment Group (dose [mg]) |
No. of Patients |
Mean |
Min. |
Max. |
Male |
Female |
|
6060 |
Albert et al. |
Controlled, DB, randomized, crossover, preference efficacy/safety study with geriatric patients with insomnia |
2 nights |
HS dosing |
Triazolam (0.25) |
101 |
69 |
59 |
84 |
21 |
80 |
|
Placebo |
100 |
69 |
59 |
84 |
21 |
79 |
|||||
|
6060 A |
Lipani |
Controlled, DB, randomized, crossover, preference efficacy/safety study with geriatric patients with insomnia |
2 nights |
HS dosing |
Triazolam (0.125) |
42 |
76 |
62 |
90 |
7 |
35 |
|
Placebo |
41 |
76 |
62 |
90 |
7 |
34 |
|||||
|
6061 |
Cohen |
Controlled, DB, randomized, parallel efficacy/safety study with geriatric patients with insomnia |
7 nights |
HS dosing |
Triazolam (0.25) |
31 |
73 |
61 |
89 |
14 |
17 |
|
Placebo |
28 |
70 |
61 |
81 |
9 |
19 |
|||||
|
6062 |
Okawa |
Controlled, DB, randomized, parallel efficacy/safety study with geriatric patients with insomnia |
7 nights |
HS dosing |
Triazolam (0.25) |
36 |
69 |
63 |
84 |
8 |
28 |
|
Flurazepam (15) |
35 |
67 |
61 |
81 |
18 |
17 |
|||||
|
6063 |
Beber |
Controlled, DB, randomized, parallel efficacy/safety study with geriatric patients with insomnia |
14 consecutive nights |
HS dosing |
Triazolam (0.25) |
18 |
82 |
73 |
90 |
6 |
12 |
|
Placebo |
20 |
83 |
70 |
93 |
6 |
14 |
|||||
|
|
|
|
|
|
|
|
Age (yr) |
Gender (no.) |
|||
|
Protocol Number |
Investigator |
Study Design and Focus |
Planned Duration |
Schedule |
Treatment Group (dose [mg]) |
No. of Patients |
Mean |
Min. |
Max. |
Male |
Female |
|
6064 |
Cole |
Controlled, DB, randomized, parallel consecutive efficacy/safety study nights with geriatric patients with insomnia |
14 consecutive nights |
HS dosing |
Triazolam (0.25) |
20 |
68 |
61 |
75 |
8 |
12 |
|
Flurazepam (15) |
23 |
70 |
61 |
91 |
8 |
15 |
|||||
|
6065 |
Reeves |
Controlled, DB, randomized, parallel efficacy/safety study with geriatric patients with insomnia |
28 nights |
HS dosing |
Triazolam (0.25) |
14 |
69 |
63 |
78 |
3 |
11 |
|
Flurazepam (15) |
13 |
70 |
61 |
83 |
5 |
8 |
|||||
|
Placebo |
14 |
71 |
61 |
81 |
6 |
8 |
|||||
|
6401 |
Wiscombe and Okawa |
Controlled, DB, randomized, parallel efficacy/safety study with patients with insomnia |
7 nights |
HS dosing |
Triazolam (0.25) |
35 |
50 |
30 |
60 |
7 |
28 |
|
Placebo |
35 |
48 |
29 |
60 |
9 |
26 |
|||||
|
6402 |
Pickering and Holvey |
Controlled, DB, randomized, parallel efficacy/safety study with outpatients with insomnia; also evaluated tolerance development potential and withdrawal effects |
28 consecutive nights, followed by 7 on Pbo |
2/3 received triazolam; 1/3 received flurazepam, HS dosing |
Triazolam (0.25) |
54 |
49 |
28 |
71 |
23 |
31 |
|
Flurazepam (30) |
27 |
46 |
24 |
68 |
12 |
15 |
|||||
Study Design
In reviewing the quality of the protocols and the study design, the committee was cognizant of the fact that the majority of the studies were designed and performed in the 1970s and were not performed with the level of detail and sophistication that is commonly expected today. For example, the protocols listed multiple objectives and endpoints without defining a priori which ones were primary and which ones were secondary. Patient selection criteria did not include body weight, a factor that might affect the levels of a drug in the blood of subjects receiving the same dose. Analyses of statistical power, which are required to determine the appropriate number of subjects to be enrolled in the study (which requires identification of the minimal detectable difference), were not recorded in the protocols. Similarly, the statistical methods and the analysis plan are not presented in the protocols, and several items were not specified at the level of detail considered appropriate today.
Other inadequacies in the study methods could lead to bias or statistical imprecision. Inadequate attention was paid to the instructions to be given to subjects. For example, although drug and alcohol abusers were excluded from participation in the studies, instructions prohibiting participants from consuming alcohol during the study were not given. Sleep latency could well be influenced by the effect of ethanol on gastric emptying (Pikaar et at., 1988), and acute ingestion of ethanol induces drowsiness, at least initially. In some protocols the amount of water to be ingested when taking the drug was not defined, but sleep latency could be influenced by the volume of fluid in the stomach. Instructions regarding other confounding factors such as caffeine ingestion and naps were also not given.
Although only cursory knowledge about the hepatic cytochrome P-450 isozymes existed when the protocols were written, it was already known that these microsomal drug-metabolizing enzymes could be inhibited or induced by other drugs. Yet, the only restrictions on the use of concomitant medication related to the use of psychoactive drugs. The committee also could find no data demonstrating that the blinding procedures did not change the bioavailabilities of the different dosage forms used in these clinical trials.
Many of these observations also relate to the few polysomnographic studies that were performed. Additional weaknesses of polysomnographic studies are their small sample sizes, and the sample is likely to be unrepresentative of the total population of people with insomnia.
The three more recent postmarketing protocols (Protocols M/2100/0366, M/2100/0235, and M/2100/0373) were more explicit and contained much more of the information and safeguards expected in a quality protocol written today. A summary of the evaluation of the protocols is presented in Table 2-2.
Endpoints
In general, the protocols listed three or more primary endpoints (e.g., sleep latency, total duration of sleep, and number of nocturnal awakenings). The protocols, however, did not define the criteria needed to establish whether efficacy required one, two, or all of the endpoints, which one(s), or how much improvement was relevant. Thus, the manner in which multiple primary endpoints should be used statistically was not specified.
TABLE 2-2 Results of IOM Committee Review of Low-Dose Protocols, Pivotal Protocols, and Postmarketing Protocols
|
|
|
|
No. of Protocols Meeting the Criterion/Total No. of Protocols |
|||
|
|
|
|
Low-Dose |
Pivotal |
Postmarketing |
|
|
Group |
Parameter |
Result |
Protocols |
Protocols |
Protocols |
Total |
|
Objectives |
Clear |
Yes |
15/20 |
2/3 |
2/3 |
19/26 |
|
|
|
No |
5/20 |
1/3 |
1/3 |
7/26 |
|
Criteria |
Psychiatric |
Yes |
18/20 |
1/3 |
1/3 |
20/26 |
|
|
|
No |
2/20 |
2/3 |
2/3 |
6/26 |
|
|
Appropriateness |
Yes |
20/20 |
1/3 |
2/3 |
23/26 |
|
|
|
No |
0/20 |
2/3 |
1/3 |
3/26 |
|
Design |
Design |
Parallel |
6/20 |
2/3 |
2/3 |
10/26 |
|
|
|
Crossover |
7/20 |
0/3 |
0/3 |
7/26 |
|
|
|
Other |
7/20 |
2/3 |
0/3 |
9/26 |
|
|
Blinding |
Yes |
20/20 |
3/3 |
3/3 |
26/26 |
|
|
Comparator |
Placebo |
8/20 |
3/3 |
3/3 |
14/26 |
|
|
|
Drug |
3/20 |
0/3 |
0/3 |
3/26 |
|
|
|
Both |
9/20 |
0/3 |
0/3 |
9/26 |
|
|
|
Neither |
0/20 |
0/3 |
0/3 |
0/26 |
|
|
Pharmacokinetics |
No |
20/20 |
3/3 |
3/3 |
26/26 |
|
|
Dose |
No |
20/20 |
3/3 |
3/3 |
26/26 |
|
|
Fluid |
Yes |
15/20 |
1/3 |
1/3 |
17/26 |
|
|
|
No |
5/20 |
2/3 |
2/3 |
9/26 |
|
|
Endpoints |
Yes |
20/20 |
3/3 |
3/3 |
26/26 |
|
|
Determination |
EEG |
4/20 |
1/3 |
2/3 |
7/26 |
|
|
|
Observer |
2/20 |
1/3 |
0/3 |
3/26 |
|
|
|
Questionnaire |
18/20 |
2/3 |
1/3 |
21/26 |
|
|
|
Other |
5/20 |
0/3 |
0/3 |
5/26 |
|
|
EEG evaluation |
Human |
4/20 |
1/3 |
2/3 |
7/26 |
|
|
|
Computer |
0/20 |
0/3 |
0/3 |
0/26 |
|
|
|
Validated |
0/20 |
0/3 |
0/3 |
0/26 |
|
|
|
Not validated |
0/20 |
0/3 |
0/3 |
0/26 |
Polysomnographic Studies
A review of the polysomnographic protocols for a 0.25-mg dose was also undertaken. Data from a very limited number of studies, each with few subjects, were available. Although the objectives and endpoints of these protocols were clear, a number of issues were not always well specified. Statistical analyses were usually appropriate, but often the small number of subjects precluded the use of statistical procedures and limited generalizability. It could not be determined whether scoring of EEG records was performed in a double-blind fashion.
The committee reviewed three protocols in which the 0.25-mg dose Was used (Protocols 6014, 6020, and N/2100/0232). In all studies the primary efficacy variables were polysomnographic sleep latency, total sleep time, and number of awakenings during the night. Subject self-evaluation questionnaires were also routinely used.
In Protocol 6014, six male subjects were studied for 14 consecutive nights and received active drug for a mean of 7 nights (range, 5 to 11 nights). Four conditions were examined: baseline, early period of drug therapy (nights 5 to 7), late period of drug therapy (nights 9 to 11), and recovery. It was found that sleep latency was affected more in the early nights of administration and that a reduction in the amount of wakefulness was primarily a function of reduced sleep latency. Analysis of the data reveals that during the early period of drug therapy, sleep latency was significantly different from the baseline sleep latency; however, sleep latency during the late period of drug therapy was not statistically different from that either at the baseline or during the early period of drug therapy. The absence of an effect in the late period of drug therapy may be due to the small sample size.
In Protocol 6020, the protocol was similar to that in Protocol 6014, with 7 nights of treatment with active drug. However, only three subjects receiving the 0.25-mg dose were studied, which precluded statistical analysis and which allowed for only a descriptive interpretation. Thus, it would appear that the effects of the 0.25-mg dose on total sleep time are similar to those of the 0.5-mg dose.
Protocol M/2100/0232 was a multicenter, randomized, double-blind, placebo-controlled trial with patients with chronic insomnia. Thirty-three women and 19 men (age range, 23 to 63 years) were enrolled in the study. These patients had (1) a complaint of chronic insomnia, that is, total sleep time of 6 hours or less or sleep latency of greater than 45 minutes on the majority of nights for the previous 2 months, and (2) confirmed objective polysomnographic insomnia on 1 of 2 screening-recording nights, that is, sleep latency of greater than 30 minutes or total sleep time of 6.5 hours or less. The patients enrolled underwent 2 nights of baseline polysomnography, followed by randomization to treatment with either Halcion (0.25 mg) or placebo nightly for 2 weeks (study days 1 to 14). A posttreatment period consisted of 2 nights of placebo substitution (all patients, single-blind, days 15 to 16). Patients were evaluated in a sleep laboratory on the 2 screening nights, on the 2 baseline nights, and on nights 1 and 2 and 13 and 16 of the study; they spent nights 3 to 12 at home.
Data for 24 patients in the placebo group and 26 patients in the Halcion group were used for the efficacy analysis, The primary endpoint variables were polysomnographic sleep latency and total sleep time. For sleep latency, Halcion was significantly more effective than placebo at both the beginning and the end of the treatment period (p = 0.015 and <0.01, respectively). For total sleep time, the changes for the Halcion group did not reach statistical
significance. The magnitudes of the sleep latency changes are considered to be clinically significant. Neither polysonmnographic sleep latency nor total sleep time was significantly different from the baseline level during the posttreatment period.
The findings on sleep latency are subject to different interpretations because of a baseline sleep latency imbalance at one of the three study sites. FDA's reanalyses of the sleep data except for those from one protocol or the creation of three strata on the basis of baseline sleep latency measurements are supportive oft he sleep latency improvement for patients receiving the active drug (Marticello, 1992). Statistical differences between the drug and the placebo groups were not achieved for the nighttime awakening measures. Finally, the results of the three polysomnographic studies reviewed (Table 2-3) suggest that tolerances did not develop under the study conditions. Despite small sample sizes and few studies, the findings are supportive of the questionnaire findings that sleep latency and total sleep time are affected by the 0.25-mg Halcion dose. There are no polysomnographic data (in the NDA) for subjects receiving Halcion at the 0.125-mg level.
TABLE 2-3 Polysomnographic Data Results for Tolerance for 0.25-mg Dose in Controlled Clinical Trials
|
|
Difference |
|
|
Protocol and Periods of Comparison |
Total Sleep Time (min)a |
Sleep Latency (min) |
|
6014 (n = 6) |
|
|
|
Baseline to early drug treatment period (5-7)b |
-19.4 |
-22.9 |
|
Baseline to late drug treatment period (9-11) |
-12.6 |
-11.8 |
|
Late drug treatment period to posttreatment period |
17.7 |
20.0 |
|
6020 (n = 3) |
|
|
|
Baseline to early drug treatment period (5-11) |
17.8 |
-4.1 |
|
Drug treatment period to posttreatment period |
-30.5 |
-0.1 |
|
M/2100/0232 (n = 26) |
|
|
|
Baseline to early drug treatment period (1-2) |
45.0 |
-23.9 |
|
Baseline to late drug treatment period (13-14) |
30.1 |
-24.7 |
|
Late drug treatment period to posttreatment period |
-43.4 |
25.4 |
|
a Total sleep time represents awake time before sleep for Protocol 6014. b Values in parentheses are days of drug treatment. |
||
REVIEW OF STATISTICAL METHODS USED BY UPJOHN AND FDA TO EVALUATE EFFICACY DATA
As stated previously, in performing an evaluation of the data analyses that were done by Upjohn and FDA, it is important to acknowledge that much of the original work was done more than 20 years ago. In the time since then, many changes and improvements have occurred—both in study design and in statistical analysis methods. Nonetheless, the statistical methods used to analyze these data were often quite limited. The analyses presented in the original reports were typically based only on data for the subjects completing the protocols, and those data may have been quite dissimilar from those for the original randomized sample. In FDA reanalyses of these data, attempts were often made to rectify such sources of bias by carrying the last observation forward (i.e., an endpoint or intent-to-treat analysis). This is a better choice, but it still ignores the majority of the available longitudinal data. Better approaches to the analysis of longitudinal data are now available and are widely used (see Gibbous et al. [1993] for a review in the context of psychiatric research).
In terms of the analyses themselves, it was most common to compare the ordinal measures of efficacy (i.e., global ratings, onset of sleep, duration of sleep, and number of awakenings; see Box 2-1) by chi-square statistics applied to the k × 2 contingency table, where k is the number of rating categories (e.g., k = 4 for the global ratings of none, a little, quite a bit, and a lot of help). This approach ignores the ordering of rating categories and requires an additional 3 degrees of freedom for the test statistic (i.e., for the example of four categories). On the one hand, this approach limits statistical power (i.e., a 3-degree-of-freedom test versus a more powerful 1-degree-of-freedom test); on the other hand, the test may be sensitive to differences that are restricted to an intermediate rating category (e.g., helps a little), even though no difference in the more important lowest (i.e., no help) or highest (i.e., helps a lot) rating categories may be present. Other tests for comparison of ordinal response measures were available and would have been appropriate to use. In some cases the categorical nature of the response measures were simply ignored, and either analyses of variance or t-tests were used in analyses of the data, but these ignore the possible effects on inferences due to nonnormality and the discontinuous scale of measurement of these qualitative response measures.
It is important to note that probability values for tests of hypotheses (e.g., dose, duration, and differential drug effects) alone are insufficient to fully characterize the effect of a given drug. In addition, it is of critical importance to characterize the magnitude of both expected and observed effects (e.g., the size of the mean difference in overall sleep time between the Halcion and placebo groups divided by a pooled estimate of the standard deviation). Unlike probability estimates, ''effect sizes" are not dependent on sample size, and therefore, effect sizes provide a view of the absolute magnitude of the difference. The optimal approach is to design studies based on a statistical analysis with an appropriate statistical power that leads to the specification of a sample size that can reliably detect a clinically significant difference that is specified a priori by the investigator.
Statistical Reanalysis and Evaluation of Clinical Trial Efficacy Data
To determine the efficacy of low-dose Halcion (i.e., 0.25 mg in the general population and 0.125 mg in the geriatric population), data from the pivotal protocols from the randomized controlled clinical trials involving these smaller dosages and placebo controls were reanalyzed by the committee (see below). To this end, the committee compiled data from the protocols listed in Tables 2-1 and 2-4.3 These protocols were selected because (1) they were the pivotal studies conducted with the 0.5-mg dose (see Table 2-4), (2) they contained data for subjects receiving low doses for sufficient durations and had sufficient sample sizes, and (3) they provided summary data that could be compiled for the four primary endpoints obtained from questionnaires. The committee used the rating categories described in Box 2-1.
For a subset of the protocols, the global endpoint had an additional rating category of "terrific," which was combined with the "a lot" category. In addition, the same subset of protocols rated onset in terms of time to sleep, but this could not be combined with data reported in the onset item described above.
|
BOX 2-1 FOUR PRIMARY ENDPOINTS AND THEIR RESPECTIVE RATING CATEGORIES USED IN THE QUESTIONNAIRES
|
TABLE 2-4 Pivotal Premarketing Studies Reviewed by IOM Committee for Efficacy of Halcion
Random-Effect Regression Models
To provide an assessment and synthesis of the information contained in these studies, the committee performed a random-effect ordinal probit regression analysis (Gibbons et al., 1994; Hedeker and Gibbons, 1994) using the MIXOR computer program (Hedeker and Gibbons, 1996) in which the random effect was the study and the fixed effects included treatment (i.e., 0.25-mg dose versus placebo and 0.125-mg dose versus placebo in geriatric subjects), study duration, age (i.e., geriatric versus non-geriatric), and the associated interactions (i.e., treatment by age and treatment by duration). Two general analyses were performed. First, all available data for subjects receiving the 0.25-mg dose were compared with data for subjects receiving placebo. Second, data for a geriatric population receiving the 0.125-mg dose were compared with data for a geriatric population receiving placebo. The second analysis was limited to two available protocols (Protocols 6417 and 6060A), which is insufficient to estimate precisely a random effect. Therefore, analysis of these two protocols was done as a fixed-effect analysis, and the main effect of the study and the study-by-treatment interaction were combined in the general model.
In evaluating each of the endpoints separately at the 5 percent level, it should be noted that the committee did not adjust for the effects of multiple comparisons. Also, the committee did not have baseline data for individual subjects and assumed that randomization satisfactorily balanced the means of the baseline variables among the groups.4
Results of Reanalysis
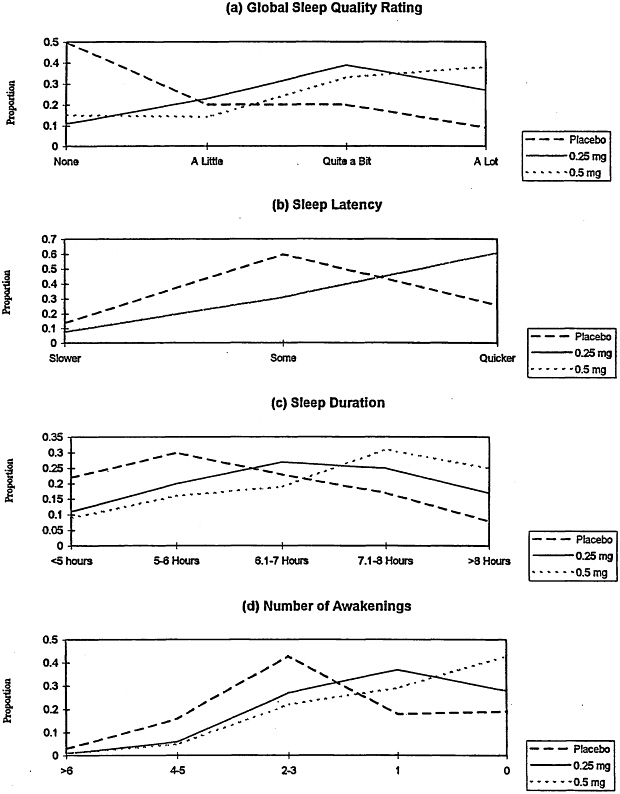
Analysis of these data revealed the following. For the 0.25-mg dose, there were no significant interactions among age, duration, and treatment. As such, the committee concludes that the relative difference between the 0.25-mg dose and placebo was comparable in geriatric and non-geriatric subjects and studies of short and long duration (i.e., a range of 2 to 43 days). In terms of treatment-related effects, the 0.25-mg dose produced significant improvement relative to that from placebo for all four endpoints (global rating, p < 0,0001; onset, p < 0.01; duration, p < 0.0001; and awakenings, p < 0.05). Table 2-5 displays the observed proportions of subjects for each endpoint category and treatment group over all studies. For example, among the subjects treated with 0.25 mg of Halcion, 30 percent reported that they received "a lot" of help from the drug, whereas only 10 percent of the subjects receiving placebo reported this level of help. By contrast, only 17 percent of the subjects receiving Halcion reported no help from the drug, whereas 51 percent of the subjects receiving placebo reported no help from the drug. For onset, 64 percent of the subjects treated with 0.25 mg of Halcion reported "quicker sleep onset," whereas only 27 percent of the subjects receiving placebo reported such an effect.
TABLE 2-5 Observed Proportions of Four Primary Endpoints for Subjects Who Received 0.25 mg of Halcion Versus Those for Subjects Who Received Placebo
|
|
|
Proportion for the Following Effect Category: |
|
|
||
|
Endpoint |
Treatment |
1 |
2 |
3 |
4 |
|
|
|
|
None |
A little |
Quite a bit |
A lot |
|
|
Did the medication help you sleep? |
Placebo |
0.51 |
0.21 |
0.18 |
0.10 |
|
|
(global rating) |
Halcion |
0.17 |
0.18 |
0.35 |
0.30 |
|
|
|
|
Slower |
Same |
Quicker |
|
|
|
Time to onset (sleep latency) |
Placebo |
0.14 |
0.59 |
0.27 |
|
|
|
|
Halcion |
0.07 |
0.30 |
0.64 |
|
|
|
|
|
<5 h |
5-6 h |
6.1-7 h |
7.1-8 h |
>8 h |
|
Duration (hours of sleep) |
Placebo |
0.25 |
0.29 |
0.22 |
0.15 |
0.08 |
|
|
Halcion |
0.10 |
0.17 |
0.26 |
0.31 |
0.16 |
|
|
|
>6 |
4-5 |
2-3 |
1 |
0 |
|
Number of awakenings |
Placebo |
0.05 |
0.17 |
0.41 |
0.19 |
0.17 |
|
|
Halcion |
0.02 |
0.07 |
0.29 |
0.36 |
0.26 |
For the 0.125-mg dose groups versus the placebo groups of geriatric subjects, combination of the data from the two studies revealed significant treatment-related effects for global rating (p < 0.003), onset (p < 0.002), and duration (p < 0.003) but not for the number of awakenings (p < 0.69). The main effects of the study and study-by-treatment interaction were not significant. Table 2-6 displays the observed proportions for each condition. The results are quite similar to those for the 0.25-mg dose for the total sample, with onset exhibiting the most pronounced effect and number of awakenings exhibiting the smallest effect (in this case the drug's effect on the number of awakenings was nonsignificant).
In summary, reanalysis of the efficacy data obtained by questionnaire supports the earlier findings of FDA that demonstrated the efficacy of 0.25 mg of Halcion for the general adult population and 0.125 mg of Halcion for the geriatric population.
Dose Response
To evaluate whether a dose-response relation exists for subjective efficacy ratings, data from the pivotal studies (Table 2-4) conducted with the 0.5-mg dose were included in the previous set of data from the low-dose studies (Table 2-1). The combined data set was tested for a linear dose-response relationship for each of the four endpoints by using the random-
effect probit regression model. The observed frequencies as a function of dose are presented in Figure 2-1, which reveals the visual impression of linear dose-response relations toward improving global sleep quality ratings (a), sleep duration (c), and number of awakenings (d). Sleep latency (b) is improved for subjects receiving the 0.25- and 0.5-mg doses relative to that for subjects receiving placebo; however, the observed proportions for 0.25 and 0.5 mg of Halcion are identical. The results of the statistical analysis reveal significant linear dose-response relationships for global ratings (p < 0.0001) and number of awakenings (p < 0.002), with the results for sleep duration being in the same direction but not reaching conventional levels of statistical significance (p < 0.15). The statistical test for linear dose-response relation for sleep latency was not significant given the equivalence of the effect of Halcion at the 0.25-and 0.5-mg doses.
LITERATURE REVIEW
The committee also reviewed the published literature on well-controlled clinical trials evaluating the effectiveness of Halcion or triazolam at doses of 0.25 or 0.125 mg.
TABLE 2-6 Observed Proportions of Four Primary Endpoints for Geriatric Subjects Who Received 0.125 mg of Halcion Versus Those for Subjects Who Received Placebo
|
|
|
Proportion for the Following Effect Category: |
|
|
||
|
Endpoint |
Treatment |
1 |
2 |
3 |
4 |
|
|
Did the medication help you sleep? (global rating) |
|
None |
A little |
Quite a bit |
A lot |
|
|
|
Placebo |
0.52 |
0.24 |
0.14 |
0.10 |
|
|
|
Halcion |
0.28 |
0.22 |
0.27 |
0.23 |
|
|
Time to onset (sleep latency) |
|
Slower |
Same |
Quicker |
|
|
|
|
Placebo |
0.12 |
0.62 |
0.26 |
|
|
|
|
Halcion |
0.00 |
0.43 |
0.57 |
|
|
|
Duration (hours of sleep) |
|
<5 h |
5-6 h |
6.1-7 h |
7.1-8 h |
>8 h |
|
|
Placebo |
0.26 |
0.32 |
0.26 |
0.10 |
0.06 |
|
|
Halcion |
0.09 |
0.31 |
0.23 |
0.22 |
0.14 |
|
Number of awakenings |
|
>6 |
4-5 |
2-3 |
1 |
0 |
|
|
Placebo |
0.06 |
0.20 |
0.44 |
0.23 |
0.07 |
|
|
Halcion |
0.03 |
0.15 |
0.47 |
0.27 |
0.08 |
In a study by Leppik and colleagues (1997), 335 elderly male and female subjects (ages, 60 to 85 years) with a 3-month history of subjective insomnia were studied in a double-blind, randomized, four-parallel-treatment-arm study. The dose of Halcion in that study was 0.125 mg; zolpidem (5 mg), temazepam (15 mg), and placebo were used in the other three arms. Following a 1-week period of treatment with placebo, clinical trial materials were administered daily at bedtime for 4 weeks, followed by a 4-day period of treatment with placebo to evaluate rebound insomnia. The investigators defined the primary outcome variables as a change in the subjective determination of sleep latency and total sleep time. Secondary outcome variables were items on a morning questionnaire and the subjects' global clinical assessment. Halcion improved sleep latency and the duration of sleep compared with those for subjects receiving placebo, but not to a statistically significant degree. Subjects did not categorize Halcion at this dose to be better than placebo on the global clinical assessment, whereas they did for the other two hypnotic agents.
A study by Hajak and colleagues (1994) is particularly interesting because it is the only study that the committee found in which the investigators defined responders a priori (e.g., clinically meaningful efficacy). Their definition states that a responder is someone who has a shortening of sleep latency by 15 minutes, prolongation of total sleep time by at least 20 percent, or a reduction of nocturnal awakenings to three or less and a fresh feeling in the morning as well as a lack of impairment in daytime well-being as a result of tiredness or anxiety. A total of 1,507 subjects from a group of practicing physicians was randomized to four treatment arms: zopiclone (7.5 mg), Halcion (0.25 mg), flunitrazepam (1 mg), and placebo at a ratio of 2:1:1:1, respectively. The rates of response to Halcion were most pronounced in those patients with a history of insomnia for 1 year or longer, although the number of responders in the Halcion group was not statistically different from that in the placebo group (32.2 Versus 26.8 percent).
In another study, Rosenberg and Ahlstrom (1994) compared 0.25 mg of Halcion with 10 mg of zolpidem in 178 outpatients (ages, 18 to 80 years) with at least a 1-week history of insomnia. The study involved double-blind, randomized parallel groups with patients treated nightly for 14 days. The investigators observed no difference from baseline values between the two drags in terms of improvements in duration of sleep, number of awakenings, or quality of sleep. Unfortunately, that study did not include a placebo control group.
In a study by Roger et al. (1993), 5 or 10 mg of zolpidem was compared with 0.25 mg of Halcion in a 3-week trial with 221 hospitalized elderly patients (ages, 60 to 90 years) with insomnia requiring medication for at least 3 weeks. A 3-day washout period to eliminate previous hypnotic medication preceded the administration of active drug for 3 weeks and was followed by a 7-day period of treatment with placebo to evaluate rebound insomnia. The efficacy variables were responses to a questionnaire regarding ease of sleep onset; estimated duration of sleep; and number, time, and duration of nocturnal awakenings. Visual analog scales were used to evaluate sleep quality and quality of awakenings. A clinical global impressions rating scale and use of rescue hypnotic medication were considered secondary endpoints. All measures of efficacy improved significantly for each parameter on the questionnaire, visual analog scales, and clinical global impression scale for all three groups. On day 31 all measures of efficacy had declined, although they still remained improved over the baseline measurements.
The hypnotic effects of zolpidem (10 mg), Halcion (0.25 mg), and placebo on hospitalized patients the night before surgery were studied in a well-controlled trial in six Canadian hospitals (Morgan et al., 1997). Three hundred fifty-seven patients (ages, 19 to 71 years) were administered a drug or placebo at bedtime and were allowed to sleep for 8 hours. Analysis of subjective outcome measures provided evidence that the results for groups receiving active drug were significantly different (p < 0.001) from those for the group receiving placebo; that is, sleep latency was shorter, total sleep time was longer, patients fell asleep more easily, and the number of patients awake 2 hours after drug administration was lower. Among none of the groups were there differences in somnolence or ability to concentrate the next morning. Both active drugs were well tolerated, with adverse event incidence rates for the groups receiving active drag being nearly identical to those for the group receiving placebo.
Thus, two of the three studies provide support for the efficacy of Halcion at 0.25 mg in the general population, including elderly subjects. Halcion was not statistically better than a placebo in the third study (at 0.25 mg), which defined responders. The one study with elderly patients with a 0.125-mg dose (Leppik et al., 1997) does not support efficacy in that population.
Polysomnographic Studies of Halcion in the Published Literature
Polysomnographic studies provide the most detailed and quantitative measures of physiologic sleep. These measures include sleep latency, total sleep time, sleep efficiency, number of awakenings, wake time after sleep onset (WASO), and mount of time spent in each of the stages of non-REM and REM sleep. These so-called objective sleep measures do not always coincide with the so-called subjective measures of sleep, in which the patient estimates sleep latency, total sleep time, and so forth, with questionnaires. When comparing reported and recorded sleep, unmedicated patients with chronic insomnia typically overestimate sleep latency and WASO, and underestimate total sleep time and sleep efficiency. Since publication of the 1977 FDA guidelines (U.S. Department of Health, Education, and Welfare, 1977), both subjective and polysomnographic sleep studies have been suggested in the evaluation of hypnotic drugs.
The committee reviewed data from 15 polysomnographic sleep laboratory studies in patients with insomnia: 5 studies of Halcion at 0.5 mg given to adults (duration of treatment, 5 to 35 nights), 5 studies of Halcion at 0.25 mg in adults (5 to 28 nights), 2 studies of Halcion at 0.25 mg given to geriatric subjects (3 to 15 nights), 1 study of Halcion given at 0.125 mg to general adults (14 nights), and 2 studies of Halcion at 0.125 mg given to geriatric subjects (3 nights to 12 weeks) (Table 2-7). In addition, the committee reviewed two polysomnographic studies in which normal subjects underwent a phase shift of the sleep-wake cycle. In most studies conducted with insomniacs, subjects had chronic insomnia that was rated as severe by both subjective and objective (polysomnographic) measures. In all studies, a placebo was administered before and after the active treatment phase. Additionally, 5 of these 15 studies with insomnia subjects also included a parallel group that received placebo for the active study period. With three exceptions (Salem et al., 1994; Mendelson, 1995; Ware et al., 1997), the number of subjects in each limb of these polysomnographic studies was small (ten or fewer) for studies lasting more than 1 week.
In all but one study of subjects with insomnia, the three doses of Halcion (0.125, 0.25, and 0.5 mg) significantly improved various objective parameters of sleep on the first 1 to 3 nights compared with those for the baseline, placebo-controlled nights. In the study by Kales and colleagues (1986), which did not report improvement, the baseline values for Halcion and placebo were markedly different for latency, raising a question regarding the validity of these results. For the 0.5-mg dose in general adults, statistically significant efficacy for one or more objective measures of sleep was. maintained through 2 weeks of treatment compared with the baseline condition in the studies with data at the end of the 2 weeks (Mitler et al., 1984; Monti et al., 1994; Ware et al., 1997). With increasing duration of treatment, however, several but not all studies indicated that statistically significant benefits for these measures were lost. Interestingly, in the three studies using a parallel placebo group, statistically significant differences between Halcion (0.5 mg) and placebo disappeared by 3 to 4 weeks of treatment, due in part to the sleep improvement of patients receiving placebo compared with baseline, and in part to the less pronounced sleep improvement over time of patients on Halcion as compared with the baseline condition (Mitler et a1., 1984; Monti et al., 1994; Ware et al., 1997).
In the adult subjects with insomnia who initially benefited with Halcion at 0.25 mg, evidence for clinical efficacy was mixed starting at the end of the first week. Although no tolerance was reported in a study measuring efficacy at 14 nights (Scharf et al., 1990), in one of the two studies lasting 28 nights, return to baseline measures was evident at the end of the study, but was not measured at 2 weeks (Salem et al., 1994). In the other study, objective improvement was noted throughout each of the 4 weeks of treatment (Mendelson et al., 1995.) Interestingly, subjective improvements showed tolerance for sleep latency and sleep quality.
In two studies with elderly subjects using Halcion at 0.25 mg for 2 weeks, the sleep of subjects given Halcion showed statistical improvement over baseline when measured at the end of the study and compared to the sleep of subjects receiving placebo (Mouret et al., 1990; Scharf et al., 1990).
The committee found only two polysomnographic studies that used Halcion at 0.125 mg for more than a few days. In a study with adult subjects with insomnia, tolerance to 0.125 mg—but not 0.25 mg—developed at 2 weeks compared with initial benefits or temazepam administered in a parallel group (Scharf et al., 1990). In a study in elderly patients with chronic insomnia associated with periodic limb movements of sleep (PLMS), Halcion at 0.125 mg was statistically significantly better at 12 weeks than at baseline (Bonnet and Arandt, 1991). The committee was unable to locate any polysomnographic sleep studies in with more typical elderly insomniac patients treated with triazolam at 0.125 mg for more than a few consecutive nights.
TABLE 2-7 Selected Polysomnographic Sleep Studios Evaluating Triazolam (Halcion) for Insomnia
|
Investigators |
Study Design |
Planned Treatment Duration |
Treatment Groups |
Results and Comments |
|
Fernandez-Guardiola and Jurado (1981) |
Double-blind crossover, placebo control; subjective insomnia; ages 24-36 (mean age, 30) |
5 nights |
Halcion at 0.25 mg and 0.5 mg (n = 8) |
At night 5, Halcion at 0.5 mg increased TST; Halcion at 0.25 mg decreased number of awakenings |
|
Mitler et al. (1984) |
Double-blind, randomized, parallel placebo group S + O; ages 38-45 for different groups |
35 nights |
Halcion at 0.5 mg; flurazepam at 30 mg; placebo (n = 7 in each group) |
Tolerance developed within and between groups at end of 2 weeks for Halcion group and 3 weeks for flurazepam group; rebound for Halcion group |
|
Mamelak et al. (1985) |
Double-blind, randomized, placebo controlled S + O; ages 32-56 (mean age, 45) |
14 nights |
Quazepam at 30 mg; Halcion at 0.5 mg (n = 6 in each group) |
Both drugs effective with no tolerance; rebound insomnia for Halcion group |
|
Kales et al. (1986) |
Double-blind, randomized placebo control; S + O; ages 19-65 (mean age, 41 ± 5) |
14 nights |
Halcion at 0.25 mg, quazepam at 15 mg (n = 6 in each group) |
Halcion: no major effect, tolerance, and withdrawal insomnia; Quazepam: persistent improvement |
|
Seidel et al. (1986) |
Double-blind, parallel placebo group; normal controls (180° shift of sleep period); ages 24-26 |
3-day shifted sleep periods (1200-2000 h) |
Experiment I: Halcion at 0.5 mg, flurazepam at 30 mg, and placebo Experiment 11: Halcion at 0.25 mg, flurazepam at 15 mg and placebo |
Halcion at 0.25 mg no better than placebo on sleep; Halcion at 0.5 mg and flurazepam better than placebo; hangover effects greater with flurazepam |
|
Bonnet and Arand (1990) |
Double-blind crossover placebo control; S + O associated with PLMS; mean age, 65 years |
3 nights |
Halcion at 0.125 and 0.25 me, placebo (n = 15 |
Halcion better than placebo for TST plus percent SE |
|
Mouret et al. (1990) |
Double-blind, randomized placebo control; elderly subjects |
15 nights |
Halcion at 0.25 mg, zopiclone at 75 mg (n = 10) |
Both drugs improved sleep |
|
Scharf et al. (1990) |
Double-blind, randomized placebo control; S + O; ages, 21-55 (mean age, 34 ± 9) |
14 nights. |
Halcion at 0.125 mg (n = 7), Halcion at 0.25 mg (n = 7), temazepam at 15 and 30 mg (n = 9) |
Halcion at 0.25 mg generally more effective than Halcion at 0.125 mg; subjects receiving Halcion at 0.125 mg showed tolerance from nights 1-3 to nights 13-14; groups receiving both doses showed greater rebound Insomnia than the group receiving flurazepam |
In contrast to Halcion, many of the other hypnotic agents that have been compared in parallel research designs have maintained efficacy for longer periods of treatment. These include flurazepam (Mitler et al., 1984), quazepam (Saletu et al., 1994), and zolpidem (Monti et al., 1997; Ware et al., 1997). Finally, the majority of laboratory sleep studies indicate that rebound insomnia develops after the administration of Halcion (clearly with the 0.5 mg dose and probably with the 0.25 mg dose, depending on the duration of treatment and other factors).
The committee's review and analysis of the questionnaire data generally supported the efficacy of the 0.25-mg dose in the non-geriatric population for 7-10 days, which is the current FDA recommendation. In addition, the polysomnographic laboratory sleep studies in the published literature generally justify the current recommended guidelines for Halcion at the 0.25 mg dose for the non-geriatric population. In addition, the committee found the questionnaire data for the 0.125-mg dose in the geriatric population to be supportive but weak. Except for the study with elderly patients with PLMS, the committee found no polysomnographic data for the 0.125-mg dose of the geriatric population for 7 to 10 nights of treatment.
CONCLUSIONS AND RECOMMENDATIONS
In summary, the IOM committee reviewed the protocols and study designs of clinical trials used to evaluate the efficacy of Halcion.5 The postmarketing trials met current standards for a well-controlled clinical trial; the premarketing trials were adequate for the time and were sufficient to provide data of adequate quality to judge the effects of the drug. A statistical reanalysis of the data from trials using questionnaires to evaluate the subjects' sleep clearly supports the previous analyses that Halcion positively affects the quality of sleep. Polysomnographic data did not exhibit evidence of tolerance over time. Additionally, the committee found that a dose-response relationship does exist, and the literature generally supports the claim that the drug is efficacious.
Data Adequacy
Based on review of the original studies, FDA's reanalysis, and the IOM committee's own reanalysis of 20 studies, the questionnaire and polysomnographic data are adequate to support the conclusion that Halcion is effective in achieving the defined endpoints in the general adult population with insomnia when used as directed (in the current labeling) at doses of 0.25 mg for up to 7-10 days. In addition, polysomnographic data from clinical trials
support the efficacy of Halcion at 0.25 mg in non-geriatric adults for 2 weeks or more.
The questionnaire data are limited but adequate to support the conclusion that Halcion is effective in achieving the defined endpoints at the 0.125-mg dose in the geriatric population. Two studies (one for 2 days' duration; one for 7 days' duration) support this conclusion; one study in the literature did not. Although there are no polysomnographic clinical trials in the New Drug Application for the 0.125-mg dose in geriatric subjects, nor in the postmarketing clinical trials or published literature for this dose in geriatric subjects with insomnia beyond 3 days of treatment, the committee's reanalysis of the combined data clearly shows statistically significant drug-related effects at the 0.125-mg dose in the geriatric population.
Although analysis of the questionnaire data supports the efficacy of Halcion at a dose of 0.125 mg in the geriatric population, inadequate data are available to establish the effect of this dose on sleep architecture in the elderly insomniac.
Recommendation 1: Improve Confidence in Lowest Dose. Definitive short-, intermediate-, and long-term polysomnographic studies are needed in a geriatric population to determine the sleep architecture of elderly insomniacs using the 0.125-mg dose.
Clinical Trial Design
The study designs and quantitative endpoints (i.e., sleep latency, duration, awakenings, and global assessment) used in the major clinical trials of Halcion in the past are of sufficient quality to yield adequate and reliable data for the determination of efficacy. The modem standards for the conduct of clinical trials have become more rigorous.
Recommendation 2: Update Guidelines. FDA should revise and update its Guidelines for the Clinical Evaluation of Hypnotic Drugs (U.S. Department of Health, Education, and Welfare, 1977) to include clinical trials on the intermediate-and long-term efficacies of hypnotic drugs. Future studies comparing Halcion with other drugs should use multiple doses of both Halcion and the comparator drugs to permit the determination of relative clinical potency.
Recommendation 3: Improve Outcomes Measures. Research is needed to identify the most valid and reliable endpoints for determination of the clinical efficacies of hypnotic agents. Most importantly, this should include endpoints that are nested in a 24-hour day-night cycle (e.g., to evaluate amnesia and daytime sedation). This should also include better integration of the subjective and objective (polysomnographic) response measures.
Tolerance
The committee's analysis of questionnaire data from studies of the efficacy of Halcion
taken for up to 43 days indicates that there is no evidence to support the development of tolerance to the hypnotic effects of Halcion; that is, the difference in the effects between drag versus placebo was consistent over time (0.5 mg for 43 days, 0.25 mg for 28 days, 0.125 mg for 8 days, and 0.25 mg for 16 weeks). In addition, polysomnographic data from clinical trials do not provide evidence of tolerance, but the polysomnographic literature suggests that tolerance may develop.
Available data suggest, however, that tens of thousands of prescriptions for much longer periods of time are being obtained by patients for much longer periods of time (e.g., the Evaluation of Medications for Insomnia in Canada study reports a mean duration of 1.7 years of use in Canada [Mariano and Gardner, 1988]; see Chapter 3). No data indicating the efficacy (or safety) of long-term use of Halcion for chronic insomnia exist.
Recommendation 4: Determine Tolerance. Controlled clinical trials of a duration of Halcion use beyond that recommended in the current labeling would be needed to determine whether tolerance to Halcion develops with long-term use.