2
Workshop Report
EFFICACY AND SAFETY
The first workshop task was to review two fundamental matters: Norplant's efficacy and its safety. These issues were addressed in a series of six presentations*, each based on accumulated experience and on fresh analysis of new data:
-
the 5-year Postmarketing Surveillance of Norplant conducted by the World Health Organization's (WHO) Special Program for Research, Development, and Research Training in Human Reproduction (WHO/HRP), with the Population Council and Family Health International (FHI) (Presentation 1)
-
5-year Population Council studies of women using Norplant and women using the two-rod levonorgestrel implant system (LNG ROD) (Presentation 2)
-
analyses of putative association between silicone and systemic disease (Presentations 3, 4)
-
analyses of relevance of reports on the effect of progesterone-induced changes on viral infectivity in a monkey model (Presentations 5, 6).
Efficacy
The workshop presentations on the Postmarketing Surveillance led by WHO/HRP and on the Population Council studies confirmed what has been found in all long-term studies of Norplant since 19803—that is, that its contraceptive efficacy is very high and that return of fertility after removal of the implant is rapid.
The purpose of the Postmarketing Surveillance was to study, over a 5-year period, major short- to medium-term side effects of Norplant not identified in clinical trials. The Surveillance followed a sample of 7,977 women for 5 years (a total of 33,627 woman-years) and found a pregnancy rate of 0.23 per 100 woman-years. For purposes of comparison, the Surveillance also followed two control groups: women who had chosen the copper-bearing intrauterine device (IUD) and women who had chosen tubal ligation. The pregnancy rates for these two groups were 0.80 and 0.15 per 100 woman-years, respectively.
The Population Council studies, undertaken to gather data for Food and Drug Administration (FDA) approval of the two-rod implant and to obtain additional information for revision of Norplant's labeling, found similarly high efficacy. In studies between 1990 and 1996 of 2,798 women in seven countries, the Council found that Norplant and the LNG ROD had similar hormonal release rates and virtually indistinguishable pregnancy rates. The cumulative 5-year pregnancy rate for the samples analyzed collectively was 1.1 per 100 woman-years.
Table 2-1, provided to the subcommittee after the workshop as additional detail for purposes of comparison, presents data on first-year and 5-year pregnancy rates for Norplant and the LNG ROD from studies in 14 countries. The evidence from those studies and from the new data presented at the workshop was that both Norplant and two-rod levonorgestrel implant system are highly efficacious, with failure rates under 1 percent per year, thus providing reversible contraceptive protection essentially equal to that of permanent methods—tubal ligation and vasectomy.
Safety
Like all hormonal contraceptives, Norplant, even though it is well tolerated by many women, is associated with adverse reactions or events.4 These are described in the prescribing information for providers and in patient labeling, both of which continue to be updated as new data become available. Of greatest concern are potential medical complications that pose serious risks to the health of the user. The Postmarketing Surveillance analysis refers to these as ''major health-related events," defined as including the following: all pregnancies, all deaths, and all complications that are potentially life-threatening, require hospitalization or at least 1 month of convalescence, leave long-term sequelae, and/or require long-term medication. A second category comprises "significant health-related problems" that may affect quality of life; these were defined as virtually anything except common colds and minor injuries. Although not life-threatening, the problems in this category may range from tolerable to annoying for some women, from distressing to intolerable for others.
TABLE 2-1 First-Year and Five-Year Pregnancy Rates and Continuation Rates for Contraceptive Implants5
|
|
First-Year Rates |
Five-Year Rates |
|
|||
|
Method, Country |
Reference |
N |
Pregnancy |
Continuation |
Pregnancy |
Continuation |
|
Norplant® |
||||||
|
Bangladesh |
Akhter, 1993 |
600 |
0.0 |
93.9 |
0.0 |
41.2 |
|
Chile |
Diaz, 1982 |
101 |
0.0 |
88.0 |
0.0 |
54.0 |
|
China |
Gu, 1994 |
10,718 |
0.1 |
94.1 |
1.5 |
72.1 |
|
Dominican Republic |
Sivin, 1988 |
1,009 |
0.2 |
79.0 |
3.5 |
25.0 |
|
Egypt |
Salah, 1987 |
250 |
1.3 |
90.0 |
1.6* |
58.0 |
|
Indonesia |
Affandi, 1987 |
437 |
0.0 |
96.5 |
1.8 |
78.2 |
|
Indonesia |
Noerpramana, 1995 |
170 |
0.0 |
97.6 |
0.0 |
90.0 |
|
Nepal |
Grubb, 1995 |
1,203 |
0.5 |
89.0 |
0.8 |
56.4 |
|
Philippines |
Grubb, 1995 |
300 |
0.0 |
95.3 |
1.7 |
64.2 |
|
Scandinavia |
Sivin, 1988 |
377 |
0.0 |
76.0 |
2.7 |
33.0 |
|
Singapore |
Singh, 1992 |
100 |
0.0 |
97.0 |
0.0 |
59.7 |
|
Sri Lanka |
Grubb, 1995 |
755 |
0.4 |
95.6 |
0.6 |
45.5 |
|
Thailand |
Chompootaweep, 1996 |
308 |
0.0 |
97.6 |
4.2 |
71.0 |
|
United States |
Sivin, 1988 |
355 |
0.0 |
82.0 |
=5.2 |
=44.0 |
|
United States |
Frank, 1993 |
1,253 |
0.2 |
87.1 |
NA |
NA |
|
United States |
Crosby, 1993 |
2,358 |
0.0 |
92.6 |
NA |
NA |
|
U.S. teenagers |
Cullins, 1994 |
136 |
0.0 |
92.0 |
NA |
NA |
|
U.S. adults |
Cullins, 1994 |
542 |
0.0 |
90.0 |
NA |
NA |
|
United Kingdom |
Peers. 1996 |
2.126 |
0.0 |
85.2 |
NA |
NA |
|
LNG ROD |
||||||
|
China |
Gu. 1994 |
1.208 |
0.1 |
94.3 |
0.6 |
65.3 |
|
India |
ICMR. 1993 |
1.466 |
0.0 |
88.0 |
0.8 |
57.9 |
|
Singapore |
Singh. 1992 |
100 |
0.0 |
95.0 |
0.0 |
62.0 |
|
* Multiple decrement rate. |
||||||
Major Health-Related Events
Both the Postmarketing Surveillance of Norplant and the 1990-1996 Population Council studies of Norplant and the LNG ROD found serious adverse events to be rare among implant users over 5 years of study. The overall mortality rate for the Population Council samples at 5 years after initiating levonorgestrel implant use was 1.1 per 10,000 woman-years of observation, well below the expected rate. Hospitalization rates for these study samples were compared with two sets of control data (a 1995 U.S. hospital discharge survey and a study in the United Kingdom by Martin Vessey in 1976), and proved to be substantially below the hospitalization rates for both those data sets. The Postmarketing Surveillance found 9 deaths in 33,627 woman-years of Norplant use; there were no differences in the overall mortality rates among women using Norplant, women using the IUD, and women who had opted for sterilization.
Nor did the Surveillance identify significant long-term morbidity. Norplant users were found to be at very low risk of ectopic pregnancy, 0.03 per 100 woman-years on average, compared to a rate of 0.19 per 100 woman-years for non-contracepting women.6 The Surveillance data were also described as reassuring with respect to cardiovascular disease, stroke, gallbladder disease, neoplastic disease, and anemias. Frequencies of systemic lupus erythematosus and collagen diseases, about which questions had been raised in connection with Norplant, were far too low to permit any conclusions. Diagnosis of hypertension was somewhat higher in Norplant users compared to IUD users and sterilized women.
Significant Health-Related Problems
The Surveillance analysis included in this category mood disturbances, anxiety, and depression; migraine or other headaches; and visual disturbances. While there initially seemed to be higher incidence of visual disturbances in Norplant users, closer scrutiny revealed no causal relationships. Mood disturbances were recorded more frequently among Norplant users than among IUD users, but their incidence was similar to that generally found with other hormonal methods of contraception. In fact, the presenter commented, noting that the observation was likely to be controversial, that Norplant appears to produce patterns of adverse effects very similar to those of combined oral contraceptives (COCs).
The noteworthy exception is changes in menstrual patterns, of which the most important are prolonged or irregular menstrual flow or increased bleeding. These changes do seem to be more commonly associated with Norplant than with COCs, although firm conclusions in that regard are constrained by lack of explicitly comparative data. The Emory/Columbia/CMC study that is discussed in greater detail below reported that, in its sample of U.S. women, fewer of the women using oral contraceptives experienced menstrual side effects, although a
substantial majority of those women had at least one such experience. Women using Norplant were considerably more likely to experience longer periods, irregular cycles, and heavier bleeding than those using either the pill or Depo-Provera.
As with all hormonal methods, Norplant is unsuitable for some women and the contraindications involved are detailed in its labeling. The workshop presentations concurred that, in the settings where these studies were carried out, the evidence from five years of follow-up was that the method had proved to be not only highly effective but safe and well-tolerated. There was also agreement that the Postmarketing Surveillance of Norplant was valuable not only as a source of knowledge on side effects not identified in clinical trials, but as evidence that large-scale, longer-term studies using cohort methodology can now be considered feasible in developing countries.
Silicone Biocompatibility*
The possibility of association between silicone-/gel-filled breast implants and connective tissue or autoimmune disease has stimulated questions about other implants, including contraceptive implants, that employ other silicone materials.7 Two workshop presentations (3 and 4) addressed this topic from different perspectives. The first reported on studies of the biocompatibility (biological response testing) and inherent characteristics of the filled silicone elastomer or polymer known as "silicone rubber" that is a component of the Norplant implant system. The second reevaluated an earlier study (Rochester [New York] General Hospital)8 which had raised concerns about the possibility that silicone gel might act as an adjuvant that could potentiate autoimmune disease, concerns subsequently extended to a wider range of silicone implants. The reevaluation first set out to confirm, or not, that silicone gel might act as an adjuvant and, second, to determine whether silicone elastomer of the type used in Norplant might have adjuvant properties.
These analyses made the following points. First, the filler material used in the Norplant tubing is an amorphous silica, not a crystalline silica of the sort that has been associated with pathological problems, and is treated in a way that allows each silica particle to react directly with the polymer chain that holds it within the network structure. Furthermore, it is the silicone polymer, not amorphous silica, that is present at the surface of the tubing, and the surface properties of filled silicone elastomers do not include potential for abrasion.
Second, the biocompatibility studies, which looked at blood protein absorption, inflammatory response, and fibrous capsule formation, suggest that silicone rubber may actually be more biocompatible than several other major biomaterials (dacron, polyethylene, and expanded polytetrafluoroethylene) used in
|
* |
See Appendix A, Presentations 3 (Rose) and 4 (Anderson). |
other implants. Any material that is implanted, in effect, creates an injury which then produces an inflammatory response; this local foreign-body reaction is typically present at all biomaterial or medical device prosthetic interfaces with tissue; Norplant is not distinctive in this regard.
Third, the silicone gel in breast implants is not the same, chemically or biologically, as the silicone rubber used in Norplant, and there is no indication that that elastomer is implicated in immunological response. Experiments in rat and mouse models used a standard adjuvant (Freund's) as the control, and a silicone oil/gel combination combined with a foreign substance (bovine serum albumen [BSA]) that contained large and small particles of silicone elastomer of the type used in Norplant. While a marked local inflammatory response resulted, neither the large nor small particles potentiated antibody response, indicating that the presence of an inflammatory response did not entail any adjuvant activity. Therefore, were any silicone elastomer particulates to become detached from the Norplant implant, they would produce no adjuvant effect and, consequently, no risk of developing autoimmune disease associated with that biomaterial.
In sum, the Norplant implant system uses amorphous silica rather than the crystalline silica alleged to have been associated with problems, and there is no evidence that this silicone rubber is implicated in any immunologic response, even though there will be an inflammatory reaction, as to any foreign body. The presenters agreed that a matter of great concern, one that could conceivably affect the supply of biomaterial for implant contraceptives, is the current controversy and litigation over silicone products. They noted, furthermore, the much wider and negative effects of that controversy on the supply of polymers and biomaterials needed for medical devices in general, some of which—for example, cerebrospinal fluid shunt systems and pacemaker leads—are essential to life. They referred to those concerns as having precipitated the Biomaterials Access Assurance Act, which became part of the defeated Common Sense Products Liability and Legal Reform Act of 1995, which proposed to protect suppliers from lawsuits in which a company's only role is to provide the raw material.9
Progestin Effects on Vaginal HIV Transmission*
Presentation 5 reported on research at the Aaron Diamond AIDS Research Center in a rhesus macaque monkey model developed to investigate the effect of progesterone on vaginal transmission of simian immunodeficiency virus (SIV).10 Monthly subcutaneous progesterone implants had been found to facilitate the infectivity of cell-free SIV, owing to progesterone-induced changes (basically, thinning of the mucosa) in the monkey's vaginal and cervical epithelium that appeared to reduce anatomic barriers to SIV infection. Future research is planned that will look at differences in susceptibility to infection and the implications of
|
* |
See Appendix A, Presentations 5 (Marx) and 6 (Cates). |
natural changes in estrogen and progesterone in different phases of the menstrual cycle, as well as the degree of protection afforded by estrogen. More research is also needed on vaginal response to hormones, as well as other monkey studies that would include exploration into cyclic hormonal effects and the effects of progestin use in contraceptives on incidence of SIV infection.
Presentation 6 examined the extent to which these results can be extrapolated to use of Norplant or Depo-Provera in human beings, since SIV is not HIV and since the monkey vagina apparently responds differently to hormones than does the human vagina. Extension of these findings to humans has been limited so far by the fact that epidemiological data and analysis that might cast some light on the subject do not exist at an adequate level of quality or power. Lack of randomized controlled trials, design limitations in most controlled cohort studies, confounding, and small sample sizes were all said to be at issue. A recent effort to draw systematic conclusions from a group of observational studies foundered on these same difficulties and on lack of consistency in critical respects.
Given these basic and clinical research needs, a June 1996 consensus panel at the National Institute of Child Health and Human Development was reported to have concluded that until better human studies become available, the most prudent path will be to reorder clinical management priorities for counseling high-risk clients. The first priority for these clients is to ensure protection from sexually transmitted infections (i.e., through regular condom use and other risk-reduction strategies); optimal protection against conception (i.e., through implant use) becomes second priority. Workshop participants noted that human studies including vaginal biopsies are also being developed and commented that the potential for doing randomized studies in human populations will be both ethically and practically challenging.
WHO USES NORPLANT AND HOW*
User Populations
The characteristics of the women in the Postmarketing Surveillance sample differed substantially from country to country (Presentation 1). While the majority of Norplant users in that sample were aged 24-35 at time of method adoption, women in the samples in China and Egypt tended to be older at time of adoption than Norplant users in South America and "other Asia" (i.e., Bangladesh, Indonesia, Sri Lanka, and Thailand). In all countries included in the Surveillance, women opting for sterilization had less education than those opting for either an IUD or Norplant, and women opting for Norplant had less education than IUD users; the exception to the latter was South America, where IUD and Norplant users had similar educational levels.
|
* |
See Appendix A, Presentations 1 (Meirik), 7 (Darroch), 8 (Kalmuss, Davidson), and 9 (Koo). |
The most representative picture of who uses Norplant in the United States comes from data, presented at the workshop* and gathered in the 1995 National Survey of Family Growth (NSFG) in a national probability sample of 10,847 women aged 15-44.11 Of that sample, just 104 women, 1 percent of the total, were current Norplant users; women who had ever used the implant totaled 2 percent.12 These same overall percentages appeared in the 1996 Ortho Birth Control Studies, also reported in this same presentation.13 These utilization figures are lower than those found either in developing countries or in Scandinavia where, according to one participant, Norplant's market share was said to run around 3 percent.
Despite limits imposed on analysis by the small number of Norplant users, the NSFG data permit insights into who, as of 1995, was using the method and where it was being obtained. Norplant use was importantly affected by age, Medicaid coverage, parity, and geography, with age the most strongly associated factor. Most women in the NSFG sample who were currently using Norplant were under age 30. Women aged 20-24 were the largest group of users, representing 4 percent of all women using reversible contraceptive methods and a little under 4 percent of all women contracepting. Women aged 15-19 were proportionally the next largest group, followed by women aged 25-29. Women over age 30 accounted for progressively smaller proportions of Norplant users, as increasing numbers appear to opt for sterilization.
Only a very small proportion of women using Norplant had had no children and most had had more than one child. Women on Medicaid were also considerably more likely to use Norplant and younger women, especially those in their early 20s, were more likely to adopt Norplant than women of the same age band who were not on Medicaid. One-third of women using Norplant in 1995 obtained it from a clinic and better than half of those women obtained it from a publicly funded clinic, much smaller proportions than was the case for women using Depo-Provera. Overall, the picture of Norplant users that emerges from the NSFG is of predominantly young, single, minority women of lower socioeconomic status and educational levels. The samples in the two large-sample clinic-based studies presented at the workshop, while not nationally representative, had socioeconomic profiles similar to that found in the NSFG.†
Norplant use was also importantly affected by geography. Norplant users are less likely to reside in areas defined by the NSFG as rural, and use of the implant in 1995 was substantially lower in the northeastern portion of the United States than in the midwestern, southern, and western regions of the country, with the western region showing the highest utilization percentages. These regional differences were thought to be related to variations in service provision and were noted to be a matter requiring exploration.
|
* |
See Appendix A, Presentation 7 (Darroch). |
|
† |
See Appendix A, Presentations 8 and 9, hereafter "the Columbia study" and "the RTI/ Emory/CMC study," respectively. |
Workshop participants further noted that these patterns of Norplant adoption, together with the contextual data provided by the clinic-based studies, suggest that younger women are using Norplant primarily for birth spacing, while older women of higher parity, more likely to have been enrolled postpartum and less likely to want more children, are adopting Norplant as a long-term reversible alternative to tubal ligation. The observation was made that these pictures of method utilization point to two different market niches for Norplant: a larger group of younger women relatively early in their childbearing careers, attracted by the method's efficacy and convenience and using it for shorter-term spacing, and a smaller group of older women committed to wider spacing or possible termination of fertility but unsure about sterilization.
Method Continuation and Discontinuation
In addition to pregnancy rates, Table 2-1 summarized the Norplant continuation rates from 14 countries and, for three of those countries, continuation rates for the LNG ROD. First-year continuation rates were high for all countries, ranging from 76.0 in Scandinavian countries to 97.6 in Thailand. For those studies where 5-year continuation rates were available, the low was 25.0 percent in the Dominican Republic, the high Indonesia's 90.0 and 78.2 percent (in two studies), the next highest China's 72.1 percent.
What is missing from these figures is information about motivation. Until the sort of information from longitudinal, large-sample, clinic-based studies such as those reported on at the workshop became available, understanding of the causes of method continuation and discontinuation was limited. Several workshop participants commented, however, that these studies were not fully representative. For instance, clinics already experienced with Norplant had been chosen for Postmarketing Surveillance because they had the infrastructure, research experience, and managerial capabilities to conduct the necessary epidemiological follow-up at an appropriate level of quality. Good service conditions, careful counseling, and meticulous follow-up would be expected in such settings and would also be expected to contribute to high continuation rates. Similar comments were made concerning the Columbia and RTI/Emory/CMC studies, which had been located in sites with what could be deemed model programs. The response was that the sites were not selected for that reason but because their programs were large enough to provide good-size samples, as well as to enable investigators to examine issues of “steering" onto Norplant and barriers to removal, assumed to be more likely among the poor minority women who were the principal clientele of the study clinics.
Nonetheless, there was agreement that, regardless of possible bias, clinic-based studies of this type, especially those with large samples, remain valuable. They are especially useful because the NSFG and the 1996 Ortho Birth Control Study had found too few Norplant users to permit extensive analysis and because clinics are generally so important in the provision of contraceptives. According to
preliminary NSFG data reported at the workshop, of women using reversible contraceptives in 1995, 34 percent had obtained them from a clinic. Several presenters further observed that more broadly based studies, in other industrialized countries, in facilities with different client profiles, and in other delivery modalities (e.g., a sample of managed care facilities), would be highly desirable, crucial if implants of shorter terms of efficacy are to enter the market as options for a broader user population.
Table 2-2 displays continuation rates found in the studies presented at the workshop and from three additional study analyses provided for comparative purposes; it also includes information on the primary factors associated with continuation and discontinuation. The table shows that continuation rates at one year did not fall below 71 percent for any of these samples, a critical time marker since menstrual problems tend to have settled down for many women at the same time that discontinuation to start another pregnancy has not yet become a dominant factor. By the end of Norplant's approved 5-year term of use, approximately one-half of the users in these samples were continuing with the method. Although there are great differences by country and although the data for the United States are scanty (partly because of low utilization), Norplant continuation rates are high even at the 5-year mark. And, while explicitly comparative data are also scanty, continuation rates for the implant are high compared to those for other reversible methods.
Side Effects as Factors
Side effects have been generally considered major contributors to Norplant discontinuation, so that it was not surprising that the women in the Columbia and RTI/Emory/CMC studies said that what they liked least about Norplant were its side effects. As indicated above, because Norplant contains no estrogen, the most frequent side effects are changes in menstrual patterns, predominantly prolonged or irregular menstrual flow or increased bleeding. The general pattern is that the number of bleeding plus spotting days tends to be high during the first 6 to 9 months of use, stabilizing by the end of the first year at some level that becomes acceptable to a majority of continuing users. Overall, it is these effects that appear with the greatest frequency in all samples studied, including the large-sample Postmarketing Surveillance and Population Council studies discussed earlier. In the Population Council studies, menstrual problems in themselves accounted for discontinuation in the same proportions as all other side effects combined, at both 1 year and 5 years of use. Among those side effects, headache, vaginal discharge, pelvic pain, weight gain, and acne were the most frequent contributors.
The findings from the Columbia and RTI/Emory/CMC studies suggest that the role of side effects in decisions to continue or discontinue implant use may not always be clear-cut. As a general matter, there are variabilities in reported frequencies of side effects from sample to sample, from time point to time point, and in priority. There is also the question of whether a single side effect is
determining or is part of a number of effects that prove determining in the aggregate. Some of the lack of clarity also derives from differences in terminology and analytic methodologies from study to study. Although menstrual changes14 were, indeed, the most common reasons given for Norplant discontinuation at 6 months postinsertion, the Columbia study found that both "continuers" and "discontinuers" reported menstrual side effects at virtually equivalent levels. The study analysis concluded that women continuing implant use at 6 months were prepared to tolerate menstrual side effects, while women discontinuing at that time were not. In contrast, the RTI/Emory/CMC study found that women with severe menstrual side effects were more likely to discontinue use. It would seem that, absent a fuller understanding of such qualitative aspects as perceived severity and the implications of menstrual side effects for individual women, those effects are, in themselves, unreliable predictors of “early" method discontinuation.
Both the Columbia and RTI/Emory/CMC studies also found differences between continuers and discontinuers with respect to non-menstrual side effects. In the Columbia sample, women who opted for discontinuation at 6 months were more likely to have experienced headaches, hair loss, and weight gain. In the RTI/Emory/CMC sample, at 12 months, while both menstrual and non-menstrual side effects from Norplant each increased discontinuation, discontinuation attributed by women to non-menstrual side effects was higher.
The RTI/Emory/CDC study examined the frequency of Norplant-related side effects relative to other contraceptive methods and found that nearly all Norplant users reported one or more side effects, as was the case for women using Depo-Provera; considerably fewer women using oral contraceptives (OCs) reported side effects. Norplant users experienced the largest number of side effects, more Norplant users defined their side effects as "severe" and, as shown below, more were somewhat less satisfied, yet their continuation rates were considerably higher than those for the other two methods.
Table 2-2 also shows that, in those studies that were designed to compare method use, implant continuation rates tend to be high relative to those of other reversible contraceptives. For example, in the Columbia sample, 50 percent of all women discontinuing use of Depo-Provera had done so after their first injection.
User Satisfaction
Data from the 1996 Ortho Birth Control study that were presented at the workshop in conjunction with the NSFG data showed favorable perceptions of Norplant tied with Depo-Provera at a 22 percent rating, ahead of the IUD's 15 percent but well behind tubal ligation, vasectomy, the male condom, and the pill (55, 63, 66, and 78 percent, respectively). The additional comment was made that preliminary indications from the NSFG data will also show that Depo-Provera is a less popular method than has been perceived by many providers who have tended to view it as more popular than Norplant.15
TABLE 2-2 Continuation Rates Among Implanta Users, Selected Pre-and Postmarketing Studies
|
|
Continuing Use at: |
|
||
|
Description, Sample (n/n)b |
1 Year |
Later Study Years |
Factors in Continuation |
Factors in Discontinuation |
|
Pre-introductory clinical trials in 17 countries; Population Council/FHI combined data set; 16,282 women aged 18-40, mean age 24-33, mean parity 1.85.8 births; follow-up at 1, 3, 6 mos. postinsertion, semiannually or annually thereafter; 1984-1990.c |
Highest: 97.0, Ghana, Philippines Singapore |
At 5 years, highest: 64.2 |
No data |
Younger age and higher parity at insertion were consistently associated with higher discontinuation rates after Year 1 for the whole data set. Menstrual problems were the chief reason for discontinuation in 6 countries; in 5 countries, desired pregnancy was the primary reason. Largest increases in cumulative discontinuation rates for desired pregnancy were in Years 4 and 5. Highest discontinuation rates for ''other medical” reasons were found in Latin America in Years 1,2, and 3. |
|
|
Lowest: 71.1, Brazil |
Lowest: 40.0, Bangladesh |
|
|
|
Pre-introductory trial, San Francisco; 250 women, 70% with LNG ROD; 5 years; 1986-1989.d |
No data |
at 5 years: 46.0 |
Implant chosen because of prior problems with contraception, view that easy to use, belief in long efficacy. |
Non-menstrual side-effects (mainly weight gain, acne) were the prime catalyst for discontinuation. Most users experienced at least one side-effect: menstrual changes, 82%; weight changes, 32%; headaches, 24%; mood changes, 16%; and acne, 15%. |
|
Prospective observational convenience sample; inner-city clinic; 122 largely minority women aged 13-19, parity at least 1, most already clinic clients; structured interview plus chart review follow-up; 6/91-6/93.e |
71.0 |
At 2 years: 62.0 |
Age, race, weight, parity, and school status were not predictive of retention. Past history of 1 or more induced abortions only statistically significant predictor distinguishing continuers and discontinuers. |
After 6 months, social reasons (including desire for pregnancy) were the most common reasons for discontinuation, although sample had preselected only teens intending to delay pregnancy at least 3 years. "General symptoms" (headache, fatigue, hair loss, nausea, breast symptoms, weight changes, appetite changes) were frequently reported by discontinuers in first 6 Months rarely later. Menstrual irregularities were uncommon reasons for termination, especially after first six months |
|
Clinical trial, 600 women with LNG ROD, 598 with Norplant "soft tubing" implants. randomized sample. 7 clinics. 3 years. 6/90-2/94f |
93.0 |
At 3 years: 80.0 |
Women not indicating at outset that they wanted another pregnancy were significantly more likely to continue use. Continuation rates for women whose family was completed were over 90 per 100. |
Menstrual problems (more bleeding days, irregular and/or increased bleeding) were associated with higher termination rate than were medical problems (headache, weight gain, acne), planning pregnancy. or other personal reasons. |
|
|
Continuing Use at: |
|
||
|
Description, Sample (n/n)b |
1 Year |
Later Study Years |
Factors in Continuation |
Factors in Discontinuation |
|
Postmarketing surveillance, 16,000 in eight countries, over 5 years.g |
No data |
67.0 |
Continuation at 5 years was equal to IUD (65%- 66%). Careful selection of clients well motivated to use long-term reversible method. |
>15% discontinued for medical reasons, notably bleeding irregularities (8% among IUD users). |
|
Longitudinal 5-year study 910/2,003 poor, mostly young minority women, urban hospital-based clinics (Dallas, New York, Pittsburgh); 5/93-10/96.h |
77.0 |
2-year data not ready for publication |
Continuation with Depo-Provera at 12 months postinitiation, 45%. |
Prime predictors of "early" discontinuation were partner wanting child, dissatisfaction with prior contraceptive methods, and exposure to negative media coverage. |
|
Longitudinal 4-year study 2.477 young, mostly postpartum. poor minority women: urban (Charlotte. NC. Atlanta) family planning/postpartum clinics. maternity wards. ambulatory surgery: 7/93- 10/94.i |
86.0 |
2- and 3-year data not ready for publication |
Continuation rates for both Depo-Provera and the pill at 12 months were considerably lower. |
Rates of Norplant, pill, and Depo-Provera discontinuation were highest in women with menstrual side effects subjectively defined as "severe." More Norplant users had more side effects but even for those with "severe" side effects, 1-year discontinuation rate was lowest for implants. Pattern for non-menstrual side effects was similar. |
|
aThe implant studied was Norplant® except where otherwise indicated. bn/n = number of users evaluated out of number enrolled in study. Dates refer to enrollment period. |
||||
|
cGrubb GS, D Moore, NG Anderson, et al. Pre-introductory clinical trials of Norplant implants: A comparison of 17 countries' experience. Contraception 52:287-296, 1995. All the Norplant capsules in these studies were made with the "hard tubing" which releases slightly lower levels of steroid than the currently marketed capsules. The authors believe that the great disparities in adverse events data are artifactual, probably resulting from differences in reporting practices. dDarney PD, E Atkinson, S Tanner, S MacPherson, et al. Acceptance and perceptions of Norplant® among users in San Francisco, USA. Studies in Family Planning 21(3): 152-160, 1990. eSivin I, O Viegas, I Campodinico, et al. Clinical performance of a new two-rod levonorgestrel contraceptive implant: A 3-year randomized study with Norplant® implants as controls. Contraception 55:73-80, 1997. Clinics were in Bangkok, Chile (2), Finland, New York City, and Singapore. fGlantz S, E Schaff, N Campbell-Heider, et al. Contraceptive implant use among inner-city teens. Journal of Adolescent Health 16:389-395, 1995. gWHO/HRP, Population Council, Family Health International. Data from International Collaborative Postmarketing Surveillance, presented at Workshop on Implant Contraceptives, Washington, D.C., Institute of Medicine, 7-8 April 1997. (See Presentation 1 [Meirik].) hKalmuss D, and A Davidson. Norplant Discontinuation among Low-Income Women. Supported by the National Institute of Child Health and Human Development and the Henry J. Kaiser Family Foundation. Data presented at Workshop on Implant Contraceptives, Washington, D.C., Institute of Medicine, 7-8 April 1997. (See also Presentation 8 [Kalmuss, Davidson]-Davidson A, D Kalmuss, L Cushman, S Heartwell, and M Rulin. Determinants of early implant discontinuation among 166 low-income women. Family Planning Perspectives 28(6):256-260, 1996; Davidson A, D Kalmuss, L Cushman, D Romero, S Heartwell, and M Rulin. Injectable contraceptive discontinuation and subsequent unintended pregnancy among low-income women. American Journal of Public Health 87:1532-1534, 1997.) iKoo HP, JD Griffith, ME Nennstiel, WL Graves, RA Hatcher, and S Laurent. Women's Experience with Norplant: A Comparison with Depo-Provera and Oral Contraceptives. Research Triangle Institute, Emory University, and Carolinas Medical Center. Supported by the National Institute of Child Health and Human Development and the Henry J. Kaiser Family Foundation. Data presented at Workshop on Implant Contraceptives, Washington. DC., Institute of Medicine. 7-8 April 1997. (See Presentation 9 [Koo].) |
The RTI/Emory/CMC study found that the majority16 of the women who continued Norplant use were "very satisfied" with the method, while noting that they had not found it easy to get used to. While that satisfaction level was below the satisfaction levels for Depo-Provera and OCs, nearly all Norplant continuers would recommend the method to others; not as many would do so, however, for each of the other two methods. Not surprisingly, perceptions among those discontinuing Norplant use were reported as less positive: Very few indicated that they had been very satisfied, compared to sizable minorities of those who had discontinued use of Depo-Provera and oral contraceptives. Whether they continued or discontinued Norplant use, both groups perceived that the best features of Norplant were its convenience and effectiveness; fewer Depo-Provera and OC users, whether continuers or discontinuers, cited those attributes as "their" method's best features.
The workshop presentations and discussion concluded that much remains to be understood before anyone can make broad assertions about reasons for continuing and discontinuing use of the contraceptive implant and about how those reasons differ from population to population over the reproductive cycle. Menstrual disturbances and other medical reasons are undeniably important but, overall, reasons for retaining or removing Norplant are a complex blend of personal experience of side effects, "other-directed" variables like the wishes of partners and broader social influences, the passage of time, and changes in life plans. Several participants commented that what seems to be happening is that women who stay with Norplant seem motivated to trade off side effects, even when burdensome in number or severity, for the convenience and efficacy they believe essential to greater control over their lives.
Barriers to Discontinuation
Norplant continuation rates may be high relative to other methods simply because a surgical intervention is required for discontinuation, unlike Depo-Provera and the pill which users can stop of their own accord. A related point is that women who must first surmount the mental barrier of deciding to experience another surgical procedure, albeit minor, then may face either real or anticipated barriers in the form of clinical pressure for continuation and/or financial costs associated with removal.
These issues were examined in both the Columbia and RTI/Emory/CMC studies, which looked at the two primary points at which pressure might be placed on women at the provider level: the point of initial method election and the point of deciding to discontinue method use—that is, removal. In terms of the first potential pressure point, the Columbia study found that of the 2,000 women interviewed, only three reported feeling pressure from a health care provider to use Norplant. Choice had instead been predicated on perceived convenience, effectiveness, and duration; in fact, Norplant electors found the method more difficult to obtain than oral contraceptives.
The second potential pressure point examined was whether provider-or cost-based barriers impeded access to Norplant removal. Here, presenters noted a mixed picture. The finding in the RTI/Emory/CMC study was that slightly over 15 percent of the women in that sample who planned, considered, or actually proceeded to remove Norplant had perceived provider pressure not to do so; in addition, those women had to pay more visits to the clinic to obtain removal and expressed considerably less satisfaction with these clinic, visits. Preliminary evidence from the Columbia study indicated that for some women, cost factors did act as an impediment to Norplant removal. While the great majority of women did not perceive that cost factors would make it more difficult to obtain implant removal, those who did were significantly less likely to discontinue Norplant use.
In summary, although the great preponderance of women, at least in these clinic settings, did not encounter barriers to removal, workshop participants agreed that the fact problems were encountered points to the need for improvements in clinic policy and in provider education in several areas. The most critical of those are method costs and financing, assurance of removal, and how to communicate this information to women clearly and effectively—needs pertinent, in fact, to any long-acting, provider—dependent contraceptive method. None of these are small challenges, participants observed. The price of Norplant remains an issue and providers have a delicate balance to strike between helping women tolerate side effects without conveying the sense that removal is being resisted for any reason.
Cost and Cost-Effectiveness
In its Contraceptive Research and Development report, the 1996 committee argued that contraception was highly cost-effective and recommended continued and sufficient government support of contraceptive services and the inclusion by third-party payers of contraception as a covered service, particularly for low-income individuals and in developing countries.17 In this connection, the committee believed that examination of experience with Norplant would usefully include consideration of its cost-effectiveness.
Presentation 10* began with the premise that at the core of contraceptive cost and effectiveness is the relationship among initial method cost, duration of use, and pregnancies averted. A "savings" model was developed for the United States that calculated a total cost for each of 15 categories of reversible and irreversible contraceptives and the costs of pregnancies resulting from contraceptive failure based on the four possible unintended pregnancy outcomes-spontaneous abortion, ectopic pregnancy, induced abortion, or birth-each in the proportion expected nationally in the United States. All costs were derived from a state Medicaid schedule of benefits and compared to a private payer database. The model also incorporated assumptions about time horizons, since some contraceptive methods require a greater one-time (e.g., sterilization) or initial (e.g.,
|
* |
See Appendix A, Presentation 10 (Stewart). |
implant) investment which would bias a 1-year time frame considerably. Periods of use of I through 5 years were calculated for all methods, together with their cumulative costs over 5 years, to locate the point at which investment in a given method would become cost-effective compared to use of no method or compared to all other methods.
The analysis made it clear that all forms of contraception, including dual-method use, are far less costly in the United States than an unintended pregnancy. In the array of individual method costs and associated savings, whose rank order is driven largely by their relative failure rates, by their consequences in the form of unintended pregnancy, and by the high price of pregnancy, the implant ranks very high in cost-effectiveness relative to other contraceptive methods, saving $13,813 over a 5-year period of use. This is the case even for younger users, for whom a pregnancy prevented is typically delayed rather than averted (in which instance savings are reduced), and even though the method entails substantial initial costs.18
However, while amortization of costs over time may make sense for planners, the subcommittee's analysis was that it is far less meaningful for the user who needs to find the total funds up front, as well as for providers subsidizing that up-front payment. Because it has not been possible to negotiate a public-sector price for Norplant, its cost was an issue for U.S. public-sector (Title X) clinics which are generally affected by repeated struggles for reasonable levels of program funding. In the case of Norplant, some of those clinics found themselves dependent on financial help from foundations for keeping adequate quantities of the method on their shelves. Ironically, such clinics, as a group, may have inserted more implants and, some participants commented, done the best screening and counseling in the nation.
Participants also raised the question of what happens to amortization of costs when users discontinue use of a long-term contraceptive method prior to termination of its full term of approved use, 5 years in the case of Norplant. They noted the tension this establishes—citing examples from publicly funded or managed care contexts, in the United States and in at least one developing country (Indonesia)—in terms of provider and client concerns about the size of the initial investment in Norplant insertion and the corresponding loss when use is discontinued. To these may be added concerns about additional costs—either to provider or client-associated with removal earlier than might have been hoped or expected. In some settings, these concerns have been expressed as pressure (anticipated or expressed) on women not to have the implant removed. Yet another source of tension in some program settings is the fact that while implant insertion is free of cost to the client, removal is not.
Furthermore, because Norplant's approved term of efficacy is 5 years, there has been a tendency to view it as a method intended for a 5-year period of use, not as a method that can provide effective contraception for up to 5 years.19 This perception has prompted such concepts as "early" or "premature" removal, and an emphasis on 5-year continuation rates as critical indicators of the acceptability of and satisfaction with the method. Thus, method "switching" may be affected by concerns among some users and providers about costs. This fits poorly with the
fact that freedom to change methods is generally considered a good thing in family planning, as well as with the fact that many women may want to use Norplant to space births at intervals considerably shorter than 5 years.
Data from the RTI/Emory/CMC studies suggest that women who discontinue Norplant use seem much less likely than women who discontinue use of the pill or Depo-Provera to switch to exposed non-use, and much more likely to switch to an effective contraceptive method. In that case, the costs of unintended pregnancy that act as the main driver in the savings model would be attenuated. These conclusions were based on a small sample and may be too subtle for a standard cost-effectiveness analysis; they do point to the vulnerability of cost-effectiveness arguments to circumstances and to the vagaries of human behavior.
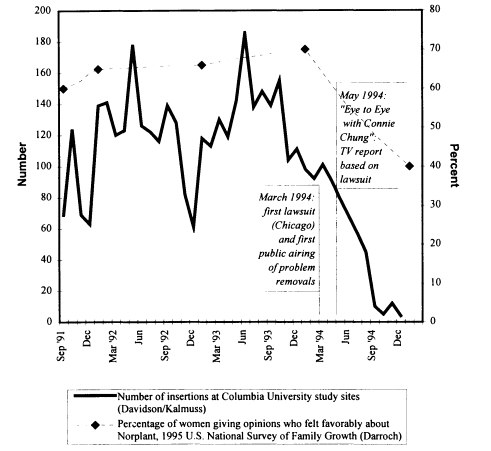
Effects of Media Coverage and Litigation on Norplant Use
A point of debate at the workshop had to do with what constituted the precipitating event in the sudden decline in Norplant utilization in the United States that began in early 1994.* Some participants thought that communication among individual users about their negative experiences with the method had been the primary stimulus; others thought that it had been media coverage; others thought it was the litigation itself. Figure 2-1 is an attempt to sort out these differing perceptions by tracking patterns of Norplant insertions, women's opinions about the method, events in the courts, and media coverage.
The figure shows coincidence among decline in method adoption, negative attitudes, legal actions, and media coverage of those actions. While previous years had shown periods of decline in Norplant adoption, there had been a regular rhythm to those declines at the end of each calendar year that was interpreted as a general seasonal falloff in family planning clinic attendance. The pattern for 1994 was, however, distinctive. The sequence displayed in the figure would suggest that the catalytic event was arguably the March 1994 suit filed by Chicago lawyer Jewel Klein, followed by the first broad public airing in May 1994 of the fact that some women were undergoing difficult implant removals. This does not exclude—and may even imply—an effect from communication among individual users; however, that dynamic might have remained a largely local phenomenon had conversations about Norplant not become a matter for the media. Media coverage of method problems expanded from that point, negative attitudes toward Norplant mounted, and the number of adoptions fell in a straight-line pattern unlike the patterns of the preceding 2 years.
The Columbia study, which had begun to gather data in spring 1993, was expanded in August 1994 to incorporate questions concerning the effect of these dynamics on women's decisions about method adoption and continuation. Preliminary analysis of those data indicates that the large majority of the total study sample reported exposure to media coverage of Norplant-related events; of these, a similarly large majority had heard negative coverage, and a significant
|
* |
The reader is also referred to the chronological material in Appendix B. |
minority of the "media-exposed" women had been motivated to seek removal by what they had gathered from various media sources. The RTI/Emory/CMC study, which began in July 1993, encountered a sharp decline in the proportion of women choosing Norplant in the third quarter (July-September) of 1994. This decline was attributed to a decrease in that quarter in the proportion of women who thought that the method was "very effective" or "very convenient," or who were favorably disposed toward using it.
Other pertinent data come from the Ortho Birth Control Survey, data that could not, for mechanical reasons, be well reflected in Figure 2-1. In 1994, of the Ortho sample of women aged 15-50, 34 percent had had a favorable opinion of Norplant, with 16 percent viewing it unfavorably. By 1995, these percentages had shifted to 22 percent and 38 percent, respectively, with the better than doubling in unfavorable views coming from those women in the previous year's sample who had had no opinion.
INSERTION AND REMOVAL: EXPERIENCE AND IMPLICATIONS
This series of presentations addressed the technical elements of implant insertion and, most particularly, removal,* and the demands those placed on the introduction of Norplant into the United Kingdom,† the United States,‡ and Indonesia.§
Technical Aspects
A distinguishing feature of Norplant is the requirement that a provider insert and remove it. Though both insertion and removal may seem simple procedures, for virtually all providers there are two learning curves: one associated with putting the device in, the other with taking it out. Done correctly, a proper insertion allows a provider to feel the capsules in a fan-like arrangement beneath the skin. These are the easiest removals. However, when the insertion is poorly done, the capsules may be in uneven relationships with one another. This is generally not a problem while the implant remains in situ but it may well produce complications when a provider, often not the same individual who inserted it, attempts removal.
Three removal methods currently predominate: (1) the "standard" method, used in Norplant introductory training in the United States; (2) the "pop-out" method, requiring more precision and, some said, more time; and, (3) the "U" technique, which removes the implanted capsules in a U-shaped fashion. For all methods, removal proceeds following injection with a local anesthetic near the base of the fan of capsules. The U technique, developed and evaluated in Indonesia as a possible alternative method, was described as requiring less manual dexterity than the other two methods, as well as less training time for acquisition of sustained competency; it also was reported to produce fewer removal problems and shorter removal times.
Case Experiences
Introduction of Norplant into the United Kingdom was based on pre-introductory market research among providers and consumers by the method's distributor, Hoechst Marion Roussel, research that provided early understanding about what was most likely to make Norplant's introduction as problem-free as possible. The findings were that success would depend on high awareness about the implant among clinicians (known to be unenthusiastic about progestin-only
|
* |
See Appendix A, Presentation 11 (Archer). |
|
† |
See Appendix A, Presentation 12 (Davey and Gaffikin). |
|
‡ |
See Appendix A, Presentation 13 (Blumenthal). |
|
§ |
See Appendix A, Presentation 14 (Simmons). |
methods), on documented competency, on appropriate client selection and fully informative counseling, and on generous training support and follow-up. Another and very crucial element of success would be training not only for insertion but for removal because, beyond the progestin-related side effects of the implant's contents, the program designers anticipated that implant removal would be a significant issue. The program first trained a small core group of senior trainers on site in Indonesia and then used them to precipitate a ''cascade" of training and one-on-one supervised clinical practice for selected providers in 35 training centers nationwide. A checklist was developed to standardize the stages to competency in training for both insertions and removals.
In the United States, the training process was different. Before putting Norplant on the U.S. market, its distributor, Wyeth-Ayerst, provided support for a national hands-on training program in Norplant insertion for physicians, nurse practitioners, and physicians' assistants, using master trainers in 37 hospital- and clinic-based locations. However, the master trainers did not constitute a standing corps as was the case in the United Kingdom, making consistency a problem. Furthermore, for the most part, it had to be left to individual practitioners to present themselves for training, and this did not always happen. A number of participants expressed the opinion that the apparent simplicity of the procedure and general optimism about the technology blocked recognition that insertion and removal each required different learning curves and that successful removals would depend greatly on proper insertion. The observation was also made that there had been little anticipation that removals would, in fact, be a major issue, at least in the foreseeable future.
In Indonesia,20 the major issues in training, especially for removal, emerged when the program went from field trials in three provinces to full-scale nationwide introduction. Implant removal on demand had been assured during trials and program managers had welcomed the need for training in removal techniques. The new method was initially popular and had potential for expanding a range of contraceptive choice that was limited by cultural sanctions on sterilization. However, when the method went to scale, provider attitudes described by evaluators as "authoritarian," lack of time for adequate counseling, and community pressures meant that women often got the message that they were making a 5-year commitment; later, those who sought removal before 5 years encountered resistance. And, though program managers recognized that not enough providers had been appropriately trained in removal skills, they felt that they had 5 years to catch up in this regard. However, at the end of that period, the volume of need, time pressures, logistical problems, and lack of resources highlighted the reality that catch-up had not, in fact, occurred and that many providers had been inadequately prepared in both insertion and removal techniques. The result was a backlog of from 350,000 to 500,000 implants awaiting removal. The weight of the lessons learned from this experience was sufficient to prompt WHO/HRP to seek fresh strategies for introducing new contraceptives, described below in the section on "New Approaches."
Rates of Complicated Removals
The rate of removal difficulties reported from Postmarketing Surveillance was 1 percent. Of 7,977 Norplant insertions, 4 were problematic and, of the 7,827 removals that had occurred by 5 years of use, 79 had been difficult, 46 of those in the same two clinics. The rate in the Population Council studies was 2.6 percent for both Norplant and the LNG ROD. Both rates are low compared to the removal complication rate of 6.2 percent in clinical trials that appears in the product labeling.21 As some workshop participants noted, the Surveillance studies and the non-randomized Population Council studies were conducted in family planning clinics chosen for their good quality, all of which had had experience with Norplant and were familiar with both insertion and removal procedures.
The workshop accounts and ancillary material provided by presenters indicated that approximately 95 percent of removals were successful without significant problems. At the same time, some workshop participants thought that the fact that removal problems were lower in clinics chosen as research sites suggested that the 5 to 7 percent of removals that were problematic were largely avoidable and could be preemptively addressed in the future; others thought that these were probably optimal situations.
Implications
As noted in the preceding section, implant removal was the catalyst for what quickly became a critical mass of opinion and events that led to the decline in Norplant use not just in the United States but, as the presenter from the United Kingdom observed, in that country as well. The core issues were provider training and competency in implant insertion and removal, real or imputed coercion of users not to remove the implant, and the character of counseling about method use in general. Workshop participants concurred that deficits in each of these had contributed, in different degrees, to litigation, negative media coverage, and loss of confidence in the method among providers and consumers.
The subcommittee diagnosis of the complexities of training for Norplant had several parts. The first had to do with the fact that introduction of new medical technologies typically requires education in their use. While many new medical devices and surgical techniques are introduced gradually, often through academic medical centers, this was not so with Norplant. The implant system was introduced countrywide and its initial market penetration grew so rapidly that the base of deliverers, though broad, was not deep; this was the case in the United States as well as in the very large Indonesian program. The combination of speed and lack of depth proved to be especially problematic when removals subsequently became an issue.
Discussion during the workshop and the subcommittee's executive session advanced the notion that medical culture and attitudes had also contributed to training problems. Many physicians apparently felt that this new, simple
technology did not require special training and they therefore were not motivated to find time in inevitably demanding schedules for training. An analysis of the training experience presented at the workshop made the point that medical training, undergraduate or postgraduate, is often provided in circumstances where competency is not demonstrated, documented, or required before use.22 The presentation noted that in one limited assessment, a physician's assistant who had undergone formal training in implant removal and for whom it became a regular procedure demonstrated faster removal times and fewer problems than a physician who was largely self-taught.23
The second explanation considered by the subcommittee had to do with another general question in medicine: Who is the first patient for any invasive procedure? When the implant was being introduced in the United States, there were relatively small numbers of users and therefore an even smaller number of women requesting removal. As a consequence, training was predominantly limited to a model arm. Recent analysis (not reported at the workshop) indicates that even with ideal placement, practitioners may require at least five trials to become proficient in removal.24 Thousands of women thus became "first patients" for Norplant insertions and, later, removals, and there were reports to the effect that women did not always understand that they were, in effect, participating in a training program, particularly in situations where language and social distance were issues. The subcommittee marked this as a chronic dilemma in medical practice, partly resolved by the manual dexterity the clinician has already accumulated that could be applied to new procedures, partly by informing patients that trainees are involved in performing that procedure, and partly by good supervision.
A third set of issues related to the fact that, despite earnest efforts, training in the insertion and removal of Norplant was uneven, most acutely with respect to removal, an unevenness deriving largely from the health care system structures into which the method was introduced. The presentation on the experience in the United Kingdom made the point that the organization of the national health system made it possible to develop and implement a standardized training program tailored to the needs of family practitioners, who provide 80 percent of contraception as a regular part of the comprehensive care of their patients. In contrast, the U.S. system did not lend itself easily or rapidly to uniformity of medical education or practice. As observed earlier, while one-third of the Norplant users identified by the NSFG obtained the method through family planning clinics, two-thirds obtained it from a miscellany of sources. In neither country is there a legal way to prevent any physician who wishes to do so from inserting or removing the implant.
Where training environments were created in which practitioners had to prove competency before training in the patient setting, and where standardized checklists were developed for proficiency and prediction of difficult removals, procedure times fell and patient satisfaction rose. This, with the experience in the United Kingdom, was seen by workshop participants as evidence that client attitudes about Norplant can be altered by provision of safe and expedient
removal. Participants commented that new removal techniques and the greater simplicity of the LNG ROD described by workshop presenters should be helpful, especially if those techniques are somehow standardized. However, they added, the need for appropriate training-in insertion, removal, and communication with clients-persists.
CONSUMER PERSPECTIVES*
This section presents the main themes that emerged from a dialogue led by a panel that assembled perspectives from women's health advocacy, reproductive ethics, and the clinic. While no such panel could be fully representative, in combination with the other constituencies represented at the workshop it encompassed a fair range of experience and opinion. The intent was to capture aspects of the Norplant experience that evade statistics and to further a dialogue that, some participants noted, should have begun years ago. The observation was made that the power of perceptions in the history of contraceptive technology has been great, even when dismissed as unsubstantial or too anecdotal to be considered "real" data.
The issues raised in what became extensive discussion fall into three related categories of concern: (1) communication and quality of care, (2) informed decision-making, and, (3) consumer involvement. A fourth category—cultural, sociopolitical, and socioeconomic factors—was woven through the others.
Communication and Quality of Care
The importance of information, education, and communication—"I, E, and C"—has been a theme in family planning for years, so it was not surprising to hear it cited as critical in Norplant's history. Taking the material presented at the workshop, as well as their own experiences, as their point of departure, the panelists constructed a picture of what counseling and communication should be, with respect to Norplant and to long-acting contraceptives in general. The panelists described a continuum that would span
-
appropriate and intelligible product labeling, and
-
provider training, including
-
collaboration in initial method choice,
-
support for clients in dealing with side effects, possible discontinuation, and removal on demand or as approved efficacy ends and a new contraceptive choice must be made.
Counseling would optimally occur as truly two-way dialogue in which:
-
information exchanged and necessary understandings are complete and unconstrained by time,
-
client participation in contraceptive choice is truly voluntary, and
-
hierarchical distinctions between provider and client are muted to the extent necessary for all this to happen.
Quality of Care
The panelists described what the policy and institutional support for this communication continuum would look like in the case of contraceptive implants. That support would include
-
assurance of choice,
-
removal on demand,
-
final removal on time, and
-
capacity for following clients through each of these segments of need.
They added that the medical system's role in sustaining contraception-that is, helping women stay on a method yet change freely as appropriate-would, ideally, integrate contraceptive counseling into reproductive health care, including sexual health, and more broadly into comprehensive medical care. For both Norplant and the LNG ROD, this entails special attention during the first 6 to 9 months when bleeding patterns are most irregular, as well as thoughtful, open response if removal becomes necessary. The panelists concurred in the impression that where these characteristics had prevailed, Norplant continuation was high; where they did not, continuation rates were lower and, in some cases, problems ensued. One participant remarked that as the most complex contraceptive ever introduced onto the market, Norplant had placed sizable demands on even the best clinical situations.
Informed Decision-Making
Presenters and participants returned throughout the workshop to the following issues: (1) the importance of informed decision-making; (2) the nature and adequacy of the content of the information provided in clinical settings; (3) the special challenges of long-acting contraceptives; and, (4) community involvement
in preparing for introduction of new contraceptive methods, especially methods that history suggests might lend themselves to abuse.
Importance
Use of the terms "informed decision-making" and "informed choice" are increasingly recognized as desirable since they clearly convey the importance of informing for purposes of dissent as well as consent. These terms and the issues around them were flagged as especially crucial for groups of lower socioeconomic status, in developing and developed countries.
Content
For Norplant, an informed decision-making process would entail discussion of:
-
the removal option and cost implications;
-
other method options;
-
relative risks, benefits, and discomforts associated with each option; and
-
the need for additional protection from sexually transmitted disease.
Participants also noted the need for ongoing review-by manufacturers, providers, and system managers-to ensure that information critical to method choice is provided in appropriate formats. The discussion centered on how to adapt such information to account for differences in educational levels, language, socioeconomic status, and stage of the reproductive life span, and on the need for innovative methodologies such as modern commercial marketing techniques and focus-group approaches to accomplish this.
Special Challenges
The crux of the discussion of informed decision-making was that long-acting contraceptives that depend on a clinical provider for discontinuation (implants, IUD) or require users to wait until contraceptive effect wears off (Depo-Provera) also require management strategies different from reversible methods that can be stopped at the will of the user. The sense of some advocacy representatives is that provider dependence requires a routine policy that adopters record their understanding of, and acquiescence to, the necessary procedures through formal mechanisms usually referred to as "informed consent" documents. The rationale is that even if such instruments dissuade some prospective users, fewer "happier" users are—and, they argued, would have been in the case of Norplant—a net good. Some participants called attention to the constraints imposed by such procedures
on access to contraceptives, and to such generic dilemmas as intelligibility and differences in the nature of consent in clinical research and in actual product use. Still, there was a strong sense among the panelists and most participants that in the case of contraceptives, more information is preferable to less; again, fewer more but well-informed contraceptive users were viewed as preferable to more but poorly informed ones.
Potential for Abuse
There was much discussion of legislative and judicial attempts in the United States beginning in 1991 to utilize Norplant coercively: by mandating its use as a condition of probation in cases of child abuse, making it a precondition for access to welfare payments, or offering financial incentives to welfare recipients to adopt it.25 None of these efforts succeeded. However, because they had focused primarily on poor, single women, often black or Hispanic, they had evoked memories of other, previous attempts to restrict reproductive freedom26 that had disproportionately affected minority women. This added to an existing residue of suspicion in some quarters,27 affected objective assessment of the method itself, and highlighted the need for special regard to ensure reproductive choice in the provision of long-acting, provider-dependent contraceptive methods as a general matter. Reports indicated that lack of free choice, in method election and continuation, had on occasion been an issue in some developing countries.
During these exchanges, several avenues of exploration were suggested for improving informed decision-making processes:
-
development of core guidelines for introducing long-acting contraceptives and support for modifying those, as desired and suitable, in communities where there have been attempts to use long-acting contraceptives inappropriately or where educational level, language, culture, or socioeconomic status may act as barriers to informed decision-making;
-
participation of representatives from relevant groups in such communities in, first, developing procedures for informed decision-making that are understandable and truly informative and, second, crafting innovative communication modalities for achieving that goal; and
-
more concrete, systematic training of providers in informed decision-making as an ongoing process that would include initial discussion about removal and ongoing discussion as needed, including help with the delicate distinctions between the provider's professional tendency to recommend the technological best for a patient and what might be interpreted as pressure.
The participants as a group recognized the complexities, subtleties, and practical challenges of pursuing these suggestions and acknowledged that doing so would require creativity, diligence, time, and expense, all of which challenge any clinical environment. They noted the additional difficulties when provider
incentives run explicitly counter to free exercise of contraceptive choice. For example, when the Indonesian Norplant program went to scale, field staff were rewarded for numbers of adopters recruited, which fostered pressures on women to adopt. In the United Kingdom, the incentives were, and continue to be, in the other direction: Providers get a fee for IUD insertion but not for insertion of Norplant.
Consumer Involvement
In some countries, notably including the United States, opinions of policy makers and women's groups have diverged on a number of critical issues related to contraceptive research and development. These differences are significant because they have provoked domestic and international debates and controversies that have contributed to the persistent volatility in the contraceptives market.
The subcommittee's perception is that this has been changing for several reasons. One was the sponsorship beginning in 1991 by the WHO/HRP of meetings whose purpose was to bring women's health advocates and scientists to some kind of common ground. Another was the process of articulating the Program of Action of the 1994 United Nations International Conference on Population and Development (ICPD), which gave voice to a constituency for new types of partnerships between the public and private sectors, including women's and consumer groups, that would mobilize the experience and resources of industry while protecting the public interest. Yet another was the awareness, growing out of cumulative experience with the introduction of new contraceptives, that more care and creativity are needed. The revised perspective is that consumer involvement from the very outset should be integral to assessment of product need, iterative throughout all stages of product trial, and pivotal in product introduction and postmarketing surveillance.
NEW APPROACHES
The last set of workshop presentations described new approaches in key areas where answers are being sought to some of the problems that have contributed to the deficits in the current array of contraceptive options. Each approach takes on a different area of concern and each is described in the next section of this report. A very new activity, the "Boom and Bust Initiative." addresses the historically inadequate incorporation of consumer interests and concerns in all phases of the development, introduction, and use of contraceptive technologies. The WHO strategy approaches the full range of health system constraints to appropriate introduction and delivery of contraceptives. The Government Standards Defense offers a possible mechanism for dealing with the pressures of product liability on innovation in contraceptive research and development.
Reproductive Health Technologies Project's "Boom and Bust Initiative"
This presentation first described the Reproductive Health Technologies Project, the institutional home for this very new initiative. The Project, located in Washington, D.C., was founded in 1988 as a working group to provide public education in the United States about RU 486 and other antiprogestins. It subsequently expanded its scope and was established as a non-profit organization in 1992. Today the Project brings together leaders from a wide range of constituencies and disciplines for dialogue, debate, and consensus-building on issues of reproductive health and technology, especially on highly charged issues where science, politics, and the interests of women converge and often clash.
The "Boom and Bust Initiative" was conceived as a response to concerns about what is now seen as a pattern of repeated difficulties when new contraceptive products are developed and introduced to consumers. The pattern described in this presentation is cyclical: high sales, demand, and expectations upon introduction of a new contraceptive method, followed by market collapse when the product does not fulfill all expectations or its use is inappropriate. Both parts of the cycle have obstructed realistic, nuanced, data-based understanding of product strengths and limitations, and the costs are high: diminished contraceptive choices; health problems for women using products inappropriately; negative impact on the financial well-being of companies; discouraged investment in contraceptive research and development; and a climate of mistrust and lack of confidence about the introduction and delivery of new contraceptive products which affects researchers, providers, and consumers.
The Initiative's goal is to engage key players—sometimes adversaries—in this cycle, in collaborative efforts to interrupt it.28 The basic belief is that shared interest in meeting the contraceptive and reproductive health needs of consumers can motivate a diverse group to action, despite divergent views. The methodology will be a series of explorations of different perceptions of the cycle through case studies and carefully facilitated discussions. Possible outcomes include better working relationships among constituency groups, a "peace accord" outlining areas of agreement and future actions to which participants commit so as to avoid or disrupt the "Boom and Bust" cycle, and a monograph or documentary film chronicling the process and recounting points of consensus. The Initiative's objectives are not to achieve perfect consensus or collective will. Rather, they are, first, to understand past cycles so as to have the earliest possible input into the evolution of new contraceptives and, second, to constructively anticipate "break points" in their introduction.
The WHO Strategic Approach to Contraceptive Introduction
This presentation described a new endeavor on the part of WHO's Human Reproduction Program, based on analysis of experience with the introduction of new contraceptives in developing countries. Scrutiny of that experience revealed
that simply making new contraceptives available does not necessarily expand either choice or utilization when basic constraints on delivering adequate services and responding to technical needs have not been addressed. Case studies have made it clear that failure has come from neglect of social and operational contexts. The elements of those contexts that were found to matter most were: inadequate preparation for introduction overall and, more specifically, inappropriate technical competence and counseling and inadequate logistics and supplies, support for the experience of side effects, assurance of method choice, provision of discontinuation on demand, and ability and willingness to support the additional program costs required to remedy all the above.
Recognition of these realities led WHO to broaden its framework for introduction from what was termed a "decontextualized" focus on single technologies to a focus that, in contrast, would take context very much into account. This would include what needed to be known about services and users in order to better inform policy decisions on method selection. The new approach emphasizes:
-
method mix and reproductive choice;
-
user perspectives and needs;
-
service capacity for assuring voluntarism, quality of care, and affordable cost; and
-
a participatory, multidisciplinary, "country-owned" process that promotes collaboration among governments, women's health and community groups, non-governmental providers, researchers, international donors, and technical assistance agencies.
The strategy proceeds in three stages:
-
assessment of need for contraceptive introduction,
-
research to inform decision-making on technology introduction and reproductive choice, and
-
utilization of research for policy and planning.
Implementation is in different early stages in eight countries in four geographic regions.29 All needs assessments have discovered major philosophical, structural, and managerial barriers to quality of care in reproductive health and contraceptive services.
The new program is reported to have already had an impact. Linking introduction of new methods to quality of care has precipitated service improvements in some sites, and participatory approaches have established broad-based, ongoing dialogue among stakeholders. In the latter respect, the objectives of the program are similar to those described for Reproductive Health Technologies' "Boom and Bust Initiative." While the presentation did not propose that this new strategy would be a panacea, it did conclude that the approach has so far proven
fresh and valid. Presenter and participants agreed that the main challenges to expanding and replicating the program would be money, time, and sustainability.
A ''Government Standards Defense"*
In its 1996 report,30 the IOM committee reiterated the recommendation of the 1990 National Research Council/IOM contraceptive research and development committee that the U.S. Congress enact a federal product liability statute that would make FDA approval of contraceptive drugs and devices available to contraceptive manufacturers as a defense against punitive damages, assuming proper compliance with FDA regulatory requirements. Both committees contended that, for controversial products that contribute importantly to the public health yet produce only modest profit margins, limits on liability could act as an incentive for research and development or at least could reduce the amount of disincentive. The 1990 committee argued that pharmaceuticals and medical devices are unique among products in the United States in the degree to which quality is regulated before they are released in the market, so that the need for liability as a quality control mechanism is greatly reduced.
As conceptualized by that committee, with such a statute—variously referred to as a federal, government, or regulatory standards defense; regulatory approval or compliance defense; or simply as an "FDA defense"—companies would not be held liable for punitive damages in a lawsuit under the following assumptions: if the drug or medical device involved had received approval from the FDA and if that company had fully complied with all of the agency's requirements for premarketing testing and postmarketing surveillance. The defense would not, however, bar plaintiffs from obtaining full compensatory and non-economic damages. Nor would it be available to a manufacturer found to have withheld from the FDA either information gathered for purposes of premarketing approval, or information developed after approval for review so as to determine whether the product in question, its marketing, or its labeling should be modified. In other words, a consumer injured by a hazard that otherwise would have been discovered would not be barred from suit should a company have failed to comply with FDA requirements.
The presenter's response to the query posed by the subcommittee as to whether some kind of federal standards defense might have "made a difference" in Norplant's legal and market experience was that it might well have constituted a substantial deterrent. The contingency is that the claims related to Norplant have been for relatively modest injuries, that is, modest compared to such serious injuries as birth defects, which would have been likely to generate suits in any event.
|
* |
See Appendix A, Presentation 15 (Green). |
The principal issues proposed in this presentation for advancing thought about the potential and feasibility of some sort of regulatory approval defense in connection with contraceptives are: FDA capacity for serving as the anchor for such a defense, the related matter of postmarketing surveillance, the question of whether such a defense would actually have the stimulating effect on industry research and development that has been widely hypothesized, the instruction that can be had in this connection from the experience of those U.S. states that have enacted product liability legislation, and the most politically feasible scope of a government standards defense.
FDA's capabilities for fulfilling its responsibilities during the premarketing period are substantial, but the postmarketing period is more problematic. The 1990 IOM committee spoke frankly on the inadequacy of existing postmarketing surveillance systems for contraceptive products and on the ethical, practical, and economic obstacles to successful postmarketing surveillance, and recommended establishment of a comprehensive system to provide systematic and timely feedback about both the positive and negative health effects of contraceptive products. Both IOM committees noted that because a regulatory standards defense would necessarily interact with postmarketing surveillance efforts, any recommendation for such a statute would be more compelling were formal postmarketing surveillance studies to be an integral and general requirement.
As for the general wisdom that liability relief would be an incentive to contraceptive research and development, the proof that it has been a disincentive cannot be gotten through any retrospective study that could be called scientific, so that it must be tested prospectively in the doing. There is, however, useful instruction to be had from the experience with state-level legislation and the relevant, though imperfectly applicable, attempts in the fields of aviation and vaccines to test hypotheses about the causal relationship between product liability and industrial research and development.
Finally, and related to all of the above, is the scope of a defense that would be politically imaginable, critical to which would be a purposive and meticulous analysis of legislative experience in this area to date.