Workshop Summary
The Children's Vaccine Initiative*
A Brief History
The CVI was established to marshal the quantum advances in the science of vaccinology toward new pediatric vaccines with qualities expected to significantly enhance immunization coverage for all the world's children, with some (but not exclusive) emphasis placed on children of the developing nations.3 The issue was not that no new vaccines were being produced. On the contrary, beginning in the mid-1980s, after decades of modest growth mostly driven by each year's births, the vaccine market had entered a phase of dramatic expansion, and commercial vaccine manufacturers and biotechnology firms were busy developing innovative vaccine products.4 The target market was the industrialized world. Products exclusively for a developing-world market were viewed as unlikely to offer adequate returns under then-current market arrangements and were therefore commercially unappealing.
The CVI's mission was to alter the prevailing R&D orientation and to supply new products and models to those developing-country markets. The founders of the CVI—the Rockefeller Foundation, United Nations Children's Fund (UNICEF), United Nations Development Program (UNDP), World Bank, and World Health Organization (WHO)—realized that accomplishment of that mission would be impossible without collaboration between the public sector and industry. Although the public sector in the United States had historically conducted most of the basic research leading to development of new or improved vaccines, product-oriented R&D was undertaken almost exclusively by vaccine manufacturers and development-stage firms, with only a handful of major commercial vaccine manufacturers having the capacity to scale up and manufacture vaccines on the large scale required for global application.5 The public sector had tended to look at vaccines as a separate series of scientific problems, or as product development problems, or as delivery problems, rather than as an "end-to-end," integrated
process unfolding over a considerable period of time. The CVI would need to adopt a more comprehensive approach to the total cycle and to a range of approaches for reducing its duration in some kind of partnership with industry.
The implications of this prospective cross-sectoral strategy would prove to be far greater than anticipated, rooted as they were in distinct sectoral cultures, divergent incentive structures, and mutual perceptions that augured poorly for authentic collaboration. The public sector had historically denigrated the profit motive, correspondingly mistrusted all industrial motivation, and did not fully understand the real costs and effort involved in developing a vaccine. The private sector viewed the public sector as motivated by ideology, not economically realistic, and unpredictable, and feared the vagaries of politics and bureaucracy as essentially threatening to what it saw as its legitimate interests. A core strategy for the CVI was, necessarily, to build trust between these two traditional adversaries. Its leadership needed, therefore, to be concerned with educating both sectors, demonstrating that doing well in terms of profitability and doing good on a humanitarian level were compatible, and somehow modifying the structure of incentives for the vaccine industry—domestic and international—to produce those products defined by the public sector as priorities. The fact that, at the outset, the CVI suffered from a surfeit of bureaucracy and turf struggles would, for a while, constrain its ability to meet its own objectives.
Defining and Implementing Cross-Sectoral Collaboration
The CVI began with only a vague notion of what would be implied by "public-/private-sector collaboration," especially in a product area with so little apparent economic appeal and with so little hard cash as evidence of public-sector commitment to the Initiative. "Funds available" to the CVI Secretariat leveled off between 1993 and 1995 at about $2 to $3 million a year, including funds earmarked by donors for particular tasks. These levels are seen as insufficient for critical new activities such as communications and work with industry and, even though overall income looks as if it may grow, donor specifications will continue to limit program flexibility.
The initial CVI meetings, with government representatives at the table and industry representatives around the sides of the room, accurately reflected the CVI worldview in its early days. Another early cultural artifact was the limited presence of industry overall, confined as it was to a relatively few individuals with whom there had been some kind of historical relationship. And, because they were not suppliers of vaccine to UNICEF or to the Pan American Health Organization (PAHO), U.S. vaccine producers were not adequately included in these first encounters.
These phenomena no longer prevail. The range of industries involved with the CVI has expanded and industry's representatives are brought into dialogues sooner in a more consultative fashion, although some feel still not soon enough; and the cross-functional, cross-organizational team approach has slowly proved more
effective. The reasons for these shifts should be instructive, proceeding as they did from heightened sensitivity on the public-sector side, greater mutual understanding of sectoral motives and functioning, the CVI's growing ability to define critical areas of coincident interest, and practical implementation mechanisms.
Defining, Creating, and Stabilizing the Market
Situation Analysis
There is consensus that a most useful and in many ways groundbreaking CVI undertaking was the contracting out, to professional private-sector management consultants, of the task of analyzing the economics of the vaccine industry, thereby providing a fresh evidence base for policy determinations.6 The first situation analysis by Mercer Management Consulting calculated the size of the world vaccine market in 1993, then estimated at around $2 billion annually, and revalued it at almost $3 billion and growing rapidly; the basic pediatric vaccines accounted for one-third of that market. The report also discussed the dynamics of the world vaccine market and examined the role of large-scale purchasing by donors.7 Overall, the study provided both sectors with a common understanding of economic realities, helped the public sector feel informed and therefore able to work with industry as an equal partner, effected change in public-sector strategies, and modified industry perceptions of market potential. To date, three Mercer analyses (1994, 1995, and 1997) have been conducted, the latest of which addresses the key factors of pricing and supply as related to product life cycles.
Market Segmentation
Another CVI innovation that has enhanced private-sector views of the world vaccine market was segmentation of that market by country groupings according to ability to pay. The result has been that UNICEF no longer donates vaccine to any nation requesting it but now targets donations and shapes its strategy to fit ''bands" of countries, each band speaking with a distinct "market voice" (see Figure 1). The first two bands (A and B), comprising the poorest and smallest countries or "the CVI market," receives frank donations or highly preferential prices. The intervening mechanism is that UNICEF purchases, or subsidizes purchases, of vaccines on behalf of those countries. The third and fourth bands (C and D) contain the larger countries with higher per capita incomes, that are being strongly encouraged toward self-sufficiency either through direct procurement or local production, and who can also afford a higher price point, although they are not always pleased to do so. The fifth band (E) consists of the industrialized Western countries, the primary market for international suppliers and for newer vaccines.
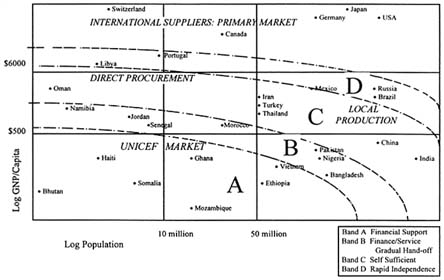
FIGURE 1. A global targeting strategy for sustainable vaccine supply, as defined by Bands A-D, and market segmentation and tiered vaccine prices according to the primary market of international suppliers, direct procurement, local production and the UNICEF market. SOURCE: A. Batson, WHO Global Program for Vaccines and Immunization. Reprinted with permission.
The decision to segment derived partly from concerns about funding sustainability and the need to target limited funds to the neediest countries.8 The premise was that industry would only provide a lowest-tier price if that price were limited to countries where market forces had failed. A reconstituted customer list would also serve as an incentive to many more countries to begin buying vaccines, in turn providing greater incentives to manufacturers, particularly U.S. manufacturers, to reassess the developing economy market.
The policy has evoked controversy and its ultimate success will be highly dependent on two lead factors: (1) the value assigned by putative purchasers to vaccines, especially to newer and more costly formulations, and on the prices those purchasers are able to negotiate with industry; and (2) agency commitment to helping countries find ways to purchase their own vaccines. Nevertheless, the segmentation approach provided a platform for such negotiation, between UNICEF and industry for the neediest countries, and between industry and countries in the higher-income bands.
Centralized Procurement
The Mercer study concluded that, in the case of vaccines, high-volume public-sector procurement does "move the market," that is, it influences manufacturers'
behavior and thus can help or hinder achievement of public-sector programmatic goals. The fact that UNICEF purchases 40 percent of the supply of traditional vaccines produced by 10–12 core suppliers, and roughly 20 percent of the total global supply of these vaccines, has been crucial to expanding demand for vaccine doses over the past eight years.9 For manufacturers with large excess production capacity, the increases in plant utilization generated by guaranteed high-volume purchases by UNICEF and PAHO have permitted them to use that capacity, thereby driving down per-dose production costs; this is believed to have been the most important economy of scale in terms of making sales to UNICEF attractive even at relatively low prices.10 In addition, the learning curve associated with greater cumulative volume is understood to be very steep and recognition of manufacturing process economies comes with corresponding speed; thus, production experience with large volumes serves to drive costs down still further and more quickly.
UNICEF also modified its customary commodity-driven approach to evaluating potential source manufacturers and incorporated three more requirements into its tender procedures: information about the overall product portfolio a bidding company could offer to meet the needs of the developing country market, those products a company might offer that responded to UNICEF/WHO priorities in the tender period, and the company's R&D pipeline.
In addition, because the CVI, WHO, and UNICEF are interested in access to newer vaccines—Hib conjugates, hepatitis B, and, eventually, pneumococcal conjugate vaccines—they have worked together to find ways to make procurement processes more flexible and to explore with industry ways to create value other than price—for example, "bundling" vaccine orders, using supply contracts, and extending contracts beyond the typical duration of two years.11 These approaches and other efforts at true partnership have motivated some vaccine companies to donate vaccines, provide cash grants to special immunization programs and disease surveillance, lower prices, make selected new products available in some preferential fashion, and furnish R&D pipeline information for assessment by WHO/UNICEF advisory groups.
Tiered Pricing
The backbone of public-sector access to new vaccines is a strong "tiered" pricing system in which the relative ability to finance vaccines is translated into different price levels for different countries. Some countries, including industrial country governments, pay a price for a given product that covers the full costs of production as well as the costs of overhead and R&D, and provides a reasonable return. Other countries, namely the poorest countries in bands A and B, are charged a price that covers the marginal cost of producing marginal volume for these markets, plus a small contribution to overheads. This price does not cover R&D, investment in new facilities, marketing expenses, or a number of other costs not associated with supplying this market. The middle, wealthier countries (bands
C and D) pay different prices covering production and varying levels of contribution to overheads and R&D. This strategy, which has been crucial in building up national immunization programs and expanding coverage worldwide, is entirely based on marginal volume with marginal costs and resulting marginal prices, and does not increase the price to the U.S. consumer. In fact, the concept was advanced that producing solely for the United States market may make U.S. manufacturers higher-cost producers.
However, confusion about the economics underlying cost allocation has resulted in criticism in the United States and, occasionally, other countries.12 U.S. vaccine manufacturers have not bid on a UNICEF or PAHO tender since 1982, when the industry was criticized by members of the United States Congress for selling vaccines for use in developing countries at prices lower than those offered to U.S. public- or private-sector purchasers; a comparable criticism was levied in 1993.13 Also during the period 1993 to 1995 in Congress, another battle was waged over differential pricing, this time related to large-scale vaccine purchases by the U.S. government at prices substantially lower than those listed in the private sector.14
Although tiered pricing can play such a powerful role in industry decisions to invest in vaccine research, development, and production, discussions of the subject have often generated more heat than light. The mechanism could conceivably play a role in connection with emerging infections—HIV/AIDS may prove to be the most immediate example—but the public sector has yet to frame a refined and thoughtful argument to take to the Congress for more reasoned, less stereotypic discussion than has been the case. The task cannot be done by industry alone, because its motives will inevitably be perceived as suspect, particularly when the topic is a "public good" with undertones of entitlement. Drug pricing tends to be a contentious issue in and of itself, so that the components of the arguments that will need to be made are subtle and complex, requiring good evidentiary material, meticulous analysis, and careful explication, perhaps, in connection with infectious diseases, by such entities as the concerned professional societies.15
Intellectual Property and Its Protection
Intellectual property protection has been used as a policy tool for many years within the United States to promote "the right amount of research and development in the country." The mechanism rewards inventive activity with the government's promise of a certain period of exclusivity in the marketplace in exchange for full public disclosure of the invention in question, at the end of which period the invention falls into the public domain.
In the pharmaceutical industry, owing to the lengthy time required to bring a product to the market, the period during which R&D investment can be recouped may become quite brief. To remain successful, a company must have products in its pipeline in all different phases of the "product life cycle." Mature products, approaching expiration of their patents and soon to face increased competition, are
then replaced in the marketplace with new products, whose exclusivity will refresh the company's stream of earnings. Substantial interruptions in this process may threaten a company's very existence and, in fact, explain much of the contemporary explosion in industry mergers. R&D investment risks become too high to reasonably assume without adequate patent protection which becomes, as a result, a major driver of industry economics and innovation.
Patent policy has also become central in U.S. foreign trade policy. The Trade-Related Intellectual Property Rights (TRIPS) Agreement, hammered out in the General Agreement on Tariffs and Trade (GATT) negotiation process and put into effect in the United States and Europe on January 1, 1996, set minimum standards for intellectual property protection around the world. It includes protection for pharmaceutical products, a 20-year patent period as a minimum standard, and an adequate judicial enforcement system. Developing countries were given until the year 2000 to adhere to the TRIPS standards; an "IPR-resistant" group, which includes India and Argentina, was given until 2005 to adopt product protection for pharmaceuticals.
A CVI-sponsored meeting in Brazil in 1995 explored the position previously held by many in the public sector that patent protection was an obstacle to vaccine production in developing countries. At a CVI-sponsored follow-up meeting in Bellagio, Italy, in February 1997, it became clear that protection of and respect for intellectual property are now seen as rational and defensible stimuli for further innovation, a position that has been adopted as a focal activity for the CVI and for the International AIDS Vaccine Initiative (see following discussion).
Technology Transfer
The CVI began at a time when the prevailing wisdom in the international health community was that local production would be an inherently less costly, more reliable, and more affordable way to ensure vaccine supply in the developing world. With limited exceptions, that premise seems to have failed the tests of time and careful economic analysis. Even though over 53 countries worldwide now produce one or more of the basic childhood vaccines, the quality, reliability, and real costs of those vaccines have proven problematic in a number of respects and CVI strategy has been revised accordingly. CVI and WHO have shifted to proposing that each facility do a fundamental review of its long-term viability and address the financial, managerial, regulatory, and policy implications of the upgrading needed to be a reliable, quality, affordable supplier of current and future vaccines. In that connection, participants in the February 1997 CVI/Rockefeller Foundation conference in Bellagio on "The Global Supply of New Vaccines" announced agreement that, to justify public confidence in the safety and effectiveness of vaccines produced in developing countries for national immunization programs, assurance of their quality would have to be independently overseen by well-functioning national control authorities. Such assurance will be
crucial to entering into the sorts of public- and private-sector partnerships that can permit access to new technologies, including new vaccines.
Individual Product Experiences
A More Heat-Stable Polio Vaccine
Many connected with the early Polio Eradication Initiative, including the WHO/EPI Technical Advisory Group, called for research on a more heat-stable polio vaccine, believing that it would be essential to the growing commitment to eradicate the disease worldwide. Despite lack of an industrialized market for such a product, some progressive members of the business community decided to take a risk within their own companies and attempt to prove that doing well and doing good could be stretched to include something as marginal to their customary market as a more heat-stable polio vaccine. As a consequence, industrial effort and investment were deployed and the project became something of a test case in intersectoral cooperation.
Over time, however, public-sector agreement on the need for an improved vaccine, already not unanimous, became still less so with changes in CVI leadership, resistance from operational levels, and new technical and epidemiological insights, some better-founded than others. Eventually, a decision was made to abort the project. Unfortunately, the private-sector partners were not involved in the relevant processes of consultation and decision. This was perceived as a major breach of trust, yet its ramifications for future intersectoral relationships were not appreciated by the public-sector decision makers, new to working with industry and not sensitive to its understandings of investment and risk.
This history was contrasted with that of the 1976 swine influenza vaccination program, when what has been called a disaster ensued as a consequence of an agenda that did not adapt to shifts in circumstance. 16 Despite disparities between many aspects of the two events, they send a similar message: the need for a scientific and technical consensus; an explicit decision in advance as to the market for a successful R&D effort; periodic review and reevaluation that is both broad and meaningful; and, throughout, close consultation between public- and private-sector collaborators. The swine flu affair taught an additional lesson of prospective relevance to emerging infections disease: Programs to prevent such diseases are essentially insurance policies, entailing some risks almost by definition, rather than subjects for punishment when anticipated dangers fail to materialize.
The Hepatitis B Vaccine
The case of the hepatitis B vaccine is somewhat different, yet lack of unanimity in some public-sector quarters remains an issue. The hepatitis B vaccine was developed in the United States for a market in the Western industrialized
nations that was viewed as small but potentially lucrative. The independent International Task Force on Hepatitis B made the argument to industry that this narrow market could be expanded profitably to a much larger market in the developing world if the vaccine were sold at a substantially lower price for use in immunization programs in those nations; the lower price would be offset by high-volume sales, in other words, through a tiered pricing system and subsidized bulk procurement. Driven partly by the Task Force's argument and partly by the entry into the market of competitive products, the initial price of $30 was subsequently progressively lowered to less than $1 per pediatric dose, and, in 1992, WHO recommended that the vaccine be introduced into national immunization programs, most urgently in hyperendemic areas.
That recommendation encountered resistance in some international agencies and developing countries, resistance rooted primarily in questions about cost, whether hepatitis B is properly considered a "childhood disease," the relative merits of the two principal vaccine formulations, and concerns about delivery capacity and sustainability. The resulting mixed messages to industry, always concerned about predictability, suggest the desirability of different public-sector approaches to product introduction in connection with the new acellular pertussis vaccine, haemophilus influenzae type b (Hib) conjugates, and the measles-mumps-rubella combination (MMR).17
Other Models*
The Malaria Vaccine Development Board
Malaria is not a formal focus of the CVI but is nonetheless highly relevant to a discussion of the CVI because of the disease burden it generates for children and because of the practical and theoretical challenges it shares with the Initiative. And, of course, malaria is most relevant to Forum concerns about the special challenges of addressing those emerging and reemerging infections that predominantly threaten the developing countries. Until very recently, malaria has been the quintessential example of a pharmaceutical "orphan," partly because of the challenges the disease and its vector posed to science, partly because there has not been a prevailing view that the disease threatened the industrialized world.
The recommendation that a Malaria Vaccine Development Board be established resulted from a workshop under the aegis of a small multisectoral committee at the IOM in 1996, charged with evaluating current international malaria R&D efforts and making recommendations for implementation by the U.S. government.18 The committee concluded that even though research now indicates that it will be, in fact, technically possible to protect against malaria with
a vaccine, development of such a vaccine will be neither simple nor straightforward. The complex life cycle of the parasite will require combining multiple antigens, a novel delivery system, and the use of adjuvants to stimulate immune system response. There also may have to be two end-products: a vaccine that will provide short-term protection, primarily for the military and travelers' markets, and a vaccine that will modulate infection and reduce mortality in children in endemic areas, that is, the developing world.19
A related complication has to do with intellectual property rights. At one time, patents on malaria antigens served to stimulate research and development but, with time, they have become something of a problem. Pieces of what may be significant intellectual property are scattered around the globe. Some patents are held by Australian organizations and companies; some by Swiss, French, or U.S. companies; some by universities. There are also potentially important adjuvants that remain unlicensed and whose protection may be an issue. Aggregating all the intellectual property rights for a multicomponent, multi-antigen vaccine is a formidable dilemma that cries out for remediation.
There is also the market question. Despite the huge burden of disease produced by malaria—as many as 500 million cases a year and around 2 million deaths—the potential market for malaria vaccines has not been understood in a way that might stimulate continued investment. The fact that malaria is associated with large populations of the very poor, in regions where the delivery of health products is typically difficult, led industry to conclude that the market for a malaria vaccine would be primarily donor-dependent, with little representation from more affluent segments of developing country populations, with the limited exception of the military and travel markets. The combination of some or all of these factors, together with failure in earlier commercial development efforts, deeper understanding of the technical challenges involved, and an erratic pattern of support for public-sector malarial research, was what had led to attrition in the relatively few industrial and public-sector R&D efforts that had managed to get under way.20
The IOM committee's primary conclusions were that the dimensions of the problem demanded a commensurate commitment, and that the United States would have to take a leadership role in the search for a malaria vaccine. A secondary conclusion was that the tasks at hand—making the international case for support, attracting many more resources from all sectors worldwide, assembling the critical scatterings of intellectual property, performing a competent reassessment of developing world market realities—are necessarily multisectoral activities. The Malaria Vaccine Development Board is envisioned as a central organization that would focus communication among industry, the academic and military research communities, and public-sector donor and technical agencies, to get these tasks performed and stimulate the synergy lacking among what had become a very few parts.21
The International AIDS Vaccine Initiative (IAVI)
Like malaria, HIV/AIDS is not a formal focus of the CVI but is similarly relevant to a discussion of the Initiative. Both affect large populations, of which the majority are outside U.S. frontiers and very poor; both present daunting scientific challenges; and both must deal with failure of the market to stimulate the necessary levels of R&D investment. The IAVI and the notion of the Malaria Vaccine Development Board were included in the workshop discussion as possibly informative variations on the CVI approach to solving a "social product" problem.
The exploration that led to the establishment of the IAVI in 1996 began in 1993, when HIV vaccine research, stymied by technical difficulties, was at a nadir in comparison with the level of investment in HIV/AIDS therapies. Although the prospect of DNA vaccines has awakened greater interest in vaccinology in general, only a few companies have active programs in the area of HIV and only one HIV-DNA vaccine has progressed to human trials.22 Furthermore, these programs focus almost exclusively on subtypes found in North America and Europe.
The purpose of the IAVI is to help accelerate development of preventive HIV vaccines appropriate for use where the epidemic is spreading most rapidly, that is, the developing world; to compensate for the fact that no agency has a mandate corresponding to that purpose; and to remedy the lack of international coordination, backed with adequate resources, to accomplish what is really a global objective. The Initiative has three overarching strategies: (1) advocacy for vaccine development; (2) a "push" strategy to support targeted research and development on parallel tracks; and (3) a "pull" strategy to create a more enabling environment for vaccine development. Its scientific emphasis is on gaps in existing efforts and on accelerating applied R&D. Its first-phase scientific objectives are to focus on development of HIV-DNA vaccines and expanded safety studies of live-attenuated HIV vaccines.
The IAVI's initial backing was provided by several foundations, UNAIDS, and the World Bank. That funding base is now being broadened to include new sources. A key element in IAVI's financial strategy is to try to persuade the Bank, which has already committed over $700 million in its response to the global AIDS epidemic, to establish a $100 million line of credit for each of the 10 most disease-burdened countries. The rationale is that this would, in effect, create a $1 billion market into which research costs could be amortized as an incentive to industrial investment. The general concept is to load the front end of the R&D process using public-sector funds to drive a directed research effort.
The IAVI has also begun to invest energy in intellectual property rights issues. As vaccine development has become more complex, every stage of the R&D process is now likely to be patented. The acquisition of multiple patents was critical to the development of the hepatitis B vaccine and, as indicated above, is expected to be critical in the development of a malaria vaccine. The IAVI will attempt to determine how it can use intellectual property rights as incentives for industry to work with the Initiative in developing and distributing an HIV vaccine. Liability will be another strategic area for the IAVI; the example was cited of
recent legislation in California which provides relief from certain elements of liability to manufacturers of vaccine in that state.
An Industry Perspective On The Emerging Infections Agenda*
Periodically, the Pharmaceutical Research and Manufacturers of America (PhRMA) surveys the pharmaceutical industry to ascertain amounts of clinical research in a given disease area. These surveys are published as part of a series entitled New Medicines in Development. The last such survey on infectious disease showed that, as of mid-1996, there were 125 products in testing for infectious diseases: 38 vaccines, 27 antibiotics, 25 antivirals, 14 antifungals, 18 "other biologics," and 3 immune enhancers, for diseases ranging from travelers' diarrhea to genital warts to typhoid fever (see Figure 1).23 These totals do not include the new medicines in development for AIDS; according to a November 1996 PhRMA survey, there were 122 medicines in testing for that disease (see Figure 2). The 1996 survey showed a 33 percent increase over PhRMA's 1994 survey, reflecting greater industrial activity later in the anti-infectives pipeline but virtually no truly new compounds of the sort that will be needed to address growing problems of antimicrobial resistance.
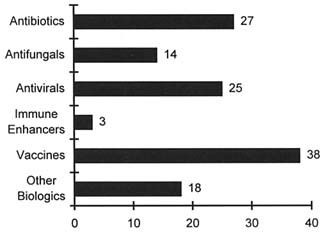
FIGURE 2. Number of medicines and vaccines in development for infectious diseases, 1996. SOURCE: J. Siegfried, Pharmaceutical Research and Manufacturers of America. Reprinted with permission.
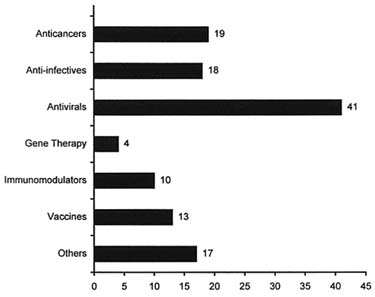
FIGURE 3. Number of AIDS medicines and vaccines in development, 1996. SOURCE: J. Siegfried, Pharmaceutical Research and Manufacturers of America. Reprinted with permission.
Although there is understandably some overlap of products, particularly for opportunistic infections occurring in AIDS sufferers and in the general population, the total of the two surveys places the number of clinical research projects currently in process for infectious disease at well over 200. In 1996, industry R&D expenditure on infectious disease of $2 billion was exceeded only by industry R&D expenditures in the categories of cardiovascular disease, cancer, and neurobiological disease.24
Many of these projects are not new anti-infectives but new dosage forms or new indications for established products. Still, modifications such as changes from multiple-dosing to once-a-day regimens, or from a poorly absorbed to a more readily absorbed product, may contribute to greater patient compliance and enhanced product usefulness. For example, several of the sexually transmitted diseases (STDs) are now treatable with a single dose of the appropriate anti-infective, and multiple-dose, long-term therapy for most urinary tract infections is now a thing of the past. Because R&D expense and time for development of a new indication or line extension may sometimes equal that of the original drug application, physicians face the dilemma of having to decide if and how to prescribe therapies that are unapproved, that is, off-label; this dilemma may be exacerbated by the fact that Food and Drug Administration (FDA) regulations do not presently permit pharmaceutical companies to disseminate scientific information concerning off-label uses to health care professionals.
If the public health agenda is to rapidly define and develop anti-infective agents for new and emerging infections, then the question is, how prepared is the pharmaceutical industry to accomplish such a task? One approach to an answer is to review the industry response to HIV/AIDS.
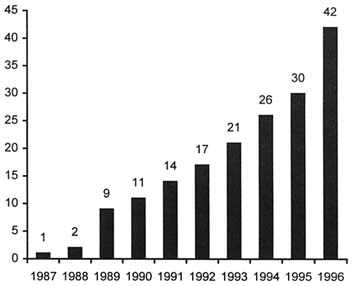
FIGURE 4. Number of approved AIDS and AIDS-related medicines, 1987–1996. SOURCE: J. Siegfried, Pharmaceutical Research and Manufacturers Association of America, annual survey: New Medicines in Development for AIDS (1996). Reproduced with permission.
Industry's Response to HIV/AIDS
The syndrome was first discovered in 1981. The etiologic agent was confirmed in 1983. The first diagnostic test was approved in 1984, the first antiretroviral in 1987. In the decade from 1987 to 1997, 42 medicines were approved for HIV/AIDS and its associated conditions. From an industry perspective, that is a remarkable achievement; from a societal, public health perspective, it is less than gratifying. Even though we now know how to prevent HIV, it still occurs in increasing numbers of individuals. Attempts to develop a preventive vaccine have been frustrating and the low efficacy of the vaccines developed so far raise large ethical and practical questions. And, although the success of a triple cocktail of a protease inhibitor plus two standard-entry antiretrovirals is much praised and offers some hope, there are still questions concerning when to start therapy, how long to continue it, the extent and duration of efficacy, and how to finance it.
The Emerging Infections Scenario and HIV/AIDS
The scenario for emerging infections is expected to be better for several reasons. First, the wealth of understanding that has come from HIV research, notably concerning the intricacies of the immune system, viral replication, and target sites of organism invasion, provides a large, specific foundation on which to build. Second, new technologies developed in response to HIV, for example, kinetic PCR assays and multiparameter flow cytometry, already lend themselves to better detection of current infectious diseases and will certainly be an asset for the future. Third, public awareness of the threat of emerging infections has grown, and movies, books, television, in fact all the media, continue to capitalize on this theme. Fourth, global surveillance for antimicrobial resistance and for emerging infectious disease is progressing. WHO's Division of Emerging and Other Communicable Diseases and Surveillance and Control has made ''WHONET" software available to laboratories in its global network for the input of antibiotic resistance data, and PhRMA's contribution in June 1996 helped make possible the extension of WHONET to Africa; the International Federation of Pharmaceutical Manufacturers Associations (IFPMA) is actively collaborating with WHO in this endeavor.
There are at least two significant lessons to be learned from HIV/AIDS that might be extrapolated to future emerging infections. One is the effectiveness of an activist community: ACT-UP, Treatment Action Group, Queer Nation, and other groups catalyzed public awareness and evoked response from the pharmaceutical industry, the FDA, and, on occasion, the Congress.
The other lesson is that cross-industry cooperation concerning a particular public health need is feasible. The Inter-Company Collaboration for AIDS Drug Development, a first-ever consortium, was established in April 1993 by 15 pharmaceutical companies who agreed to work together to facilitate early combination and comparative studies of antiviral agents and thereby accelerate development of drugs for treatment of HIV infection and AIDS. The heads of research and development in the 18 companies now members of the Collaboration share relevant preclinical and clinical data, expedite access to investigational drugs supplies, and develop standardized preclinical assays and procedures and other activities.25 Cross-industry cooperation has also occurred in less direct fashion: The AIDS Clinical Trial Group established by NIAID serves as a nexus for government and private-industry collaboration on specific clinical research issues.
Finally, the flexible and innovative character of the FDA response to AIDS provides many ideas for the future. Special mechanisms such as expanded access and parallel tracking and inclusion of activists in formal advisory bodies are just two of those (see following discussion, Legal and Regulatory Issues).
Barriers
Many of the same issues that exist today as barriers to pharmaceutical development will remain in force, without special efforts to modify them. Pharmaceutical research is expensive: In 1996, estimates for the costs of developing a drug ranged from $359 to $500 million. Of every 5,000 projects that start the journey toward the pharmacy shelf, only one completes it and, of those that do, only one in five actually returns its R&D investment. These two factors alone force the product selection environment to be much more specific and to assume even greater weight in product areas—for example, pediatrics, orphan diseases, and oncology—that may lack sufficient population size to make commercial development attractive. Still, overall investment by the pharmaceutical industry in research and development is large: The proposed research budget for the pharmaceutical sector in 1997 is approximately $19 billion, 21.2 percent of its domestic sales and exports. By way of comparison, in other industrial sectors of the U.S. economy, average R&D investment as a percentage of sales is under 4 percent.
Another compelling factor is time. The standard figure given by industry for the time required for development of a single pharmaceutical product has been 15 years, on average, though some portions of that time span are being shortened in different ways. The 1992 Prescription Drug User Fee Act (PDUFA/PL 102-571) made it possible for the FDA to reduce its review time for new chemical entities (New Drug Application [NDA]) from 32 months in 1992 to 17.8 months in 1996;26 the effect of PDUFA on development time for drugs and biologics is under study. Corporate mergers are also restructuring industry R&D strategies and structures, in an effort to cut costs, save resources, and bring products to market more quickly. The total span from bench to market remains long, nevertheless, and the Collaboration on Drug Development Improvement (CCDI), yet another agency/industry/academic collaboration, has been charged with answering the following, generic question: Where does drug development time go? The question assumes paramount importance in the context of emerging infections. The earlier example of the speedy development of the first antiretroviral is inspiring but, so far at least, singular. All things being equal, were today ground zero for a new and lethal disease, by current standards the first medicinal therapy might not be available until 2012.
Another element in industry R&D decisions is the degree of predictability across the product cycle since, like time, unpredictability has financial implications. The research itself may pose unique and unforeseeable technical problems that produce delays, if not frank failure. In areas of societal controversy such as reproductive health, drug abuse, and STDs, liability—always part of industry's profit projections—becomes weightier. In this connection, since STDs can be defined as emerging or reemerging diseases, in some instances in epidemic proportions, issues of liability need to be taken into account explicitly.
There is also the unpredictability associated with what industry perceives as undue government interference. A case in point is the Orphan Drug Act and
perceptions of the effects of the various congressional modifications to that legislation that were proposed beginning in 1990. Those modifications did not materialize but, as indicated in a more detailed discussion of the Act later in this report, just the prospect of change is said to have had a chilling effect, yet another illustration of the heavy role predictability plays as an incentive to industrial R&D investment.
A World Bank survey of high-level representatives from the pharmaceutical industry to ascertain industry involvement in developing products for infectious diseases identified additional barriers: (a) lack of adequate information about the basic research that is under way in universities, research councils, and biotechnology companies worldwide that could provide material for industry to screen to generate more product leads; (b) the costs and duration of clinical trials; and (c) limitations inherent in the developing country market for products that could deal with the diseases that primarily afflict those countries. The survey also elicited industry suggestions about ways to lower these barriers. These are incorporated into Table 2, which presents a categorized summary of ideas about incentives for increasing pharmaceutical research and development for priority infectious diseases.
Legal And Regulatory Issues*
Another area where disincentives to pharmaceutical research and development are said to reside is the legal and regulatory domain. Questions for the Forum have to do with the extent to which any of those disincentives applies to emerging infections, notably in connection with the widening range of threats from antimicrobial resistance and, more positively, whether there are incentives within that domain that could be brought into play.
The FDA recognizes that there are major problems with regard to resistant organisms and believes it appropriate to use all existing regulatory tools to address them. Its general strategy is to build as much as possible on mechanisms already available under existing regulations, specifically those mechanisms originally intended to expedite the development, evaluation, and marketing of new products for treating life-threatening and severely debilitating illnesses, especially where no satisfactory alternative therapy exists. The purpose of this set of regulations reflects the FDA's determination to exercise the broadest flexibility in relation to such conditions and reflects a recognition that physicians and patients are generally willing to accept greater risks or side effects from products that treat such illnesses.
Those elements of this set of regulations that might be mobilized toward the development, availability, and optimal utilization of antimicrobial products fall
under the rubric of "accelerated approval."27 Of most interest are provisions fostering more rapid product review and greater flexibility in the design of clinical studies, including early and regularized communication between the agency and industry, expansion of the allowable universe of data sources, and utilization of surrogate endpoints. Another relevant mechanism within this category is approval with restrictions on distribution so as to ensure safe use; however, while such restrictions may well be beneficial in public health terms, their meaning in market terms may act as a disincentive to industry R&D investment.
There are other legal and regulatory issues that lie beyond this special category of exception that are consequential for emerging infectious diseases. These include the labeling of existing antimicrobials; product availability; international harmonization of pharmaceutical regulations; intellectual property rights; and another category of exception, "orphan drugs."
Accelerated Approval and Development of Drugs for Serious and Life-Threatening Illnesses
Clinical Studies
FDA approval of new compounds is often constrained by difficulties inherent in clinical trials. These may relate to getting enough cases of resistant organisms in a trial cohort to be able to demonstrate efficacy, or to circumstances where full-fledged clinical trials are simply not possible, for example, military and terrorist situations. FDA regulations for life-threatening illnesses allow for considering an approval action earlier in the development process; accelerating enrollment in clinical trials; and for aggregating efficacy data from such other sources as in vitro analysis, animal models, case series and historical controls, and qualified foreign clinical trials. Which data, in which combinations, will suffice for securing approval can be defined in early consultations among FDA officials, industry representatives, and expert advisers, meetings that can take place prior to submission of an initial Investigational New Drug application and/or at the end of Phase 1.28
Surrogate Endpoints
The FDA may also grant marketing approval for a new drug for serious or life-threatening illnesses on the basis of data from controlled clinical trials establishing that the product has effect on some surrogate endpoint that is reasonably likely, based on epidemiological, therapeutic, pathophysiological, or other evidence, to predict clinical benefit, rather than solely on the basis of effect on survival or irreversible morbidity.
The purpose of surrogates is to make products available to individuals earlier, a consequence that also acts as a commercial incentive. Surrogate markers
employed in the past have included antibody levels that anticipate protective efficacy, as in the case of many vaccines; CD4, viral and plasma RNA, and branched chain DNA as measures of impact on HIV seroconversion; lowered blood cholesterol and hormone replacement levels in the case of drugs for corresponding indications; and, most recently, sputum status as a surrogate endpoint for tuberculosis therapy.
When surrogate endpoints are allowed, final product approval is typically contingent on a requirement that the applicant study the drug further to confirm and describe its clinical benefit, thereby validating the surrogate(s). Promotional material is somewhat controlled during this interim period. The duration of follow-up studies may be shortened through prior agreement between the FDA and industry about what data approaches will be acceptable. Should the studies fail to confirm the validity of the surrogates, the regulations also provide for quick removal of the product from the market, although this is difficult for the agency in practical terms and probably disappointing to industry.
Restricted Distribution and the Matter of Antimicrobial Resistance
The accelerated approval regulations also carry an option for restricted distribution of a product if there is reason to think that it should be made widely available only after confirmatory studies have been implemented. Should the FDA conclude that even a product shown to be effective can be used safely only if distribution or use is restricted, it can require postmarketing restrictions that either limit distribution to certain facilities or physicians with special training or experience, or make distribution conditional on performance of specified medical procedures.
The question was raised as to whether restricted distribution might merit consideration as a strategy for preserving the viable life span of new antimicrobial drugs for resistant isolates. The question has no simple answer. Beyond the public health importance of controlling the evolution of antimicrobial resistance, on the industrial side it is just common sense that when major investments are made in developing new products, it is reasonable to expect them to remain useful for their unique indications for some period of time. At the same time, while restricting the distribution of new antimicrobials might well serve to extend their viability, it would also constrain the size of their market and, therefore, potential returns on R&D investment. In effect, conserving the viability of new antimicrobials is at odds with the need to stimulate development of those products. Another, related dilemma is the pressure that would be applied almost inevitably by individual patients or physicians for access to new antimicrobials, versus the need to conserve their value for the benefit of the wider community.
Even were restricted distribution to be invoked as an option, it is nonetheless an authority that is limited as indicated above and one that has been used by the FDA only very rarely. The Controlled Substances Act, another regulatory effort to restrict access, applies only to psychoactive substances and steroids. This leaves
labeling as the sole current regulatory mechanism for limiting use of antimicrobials.
Options in the clinical arena are only unevenly helpful. Infection control, formulary, and peer review committees may be present in different clinical settings, but each of these responds to different guidelines and cost considerations with regard to managing antimicrobial products. The fact that resistance cannot be immediately detected in situ using available tests, together with the fact that ongoing information about local trends in resistance, beyond those at a single health care facility, are hard to come by, is another significant obstacle. Absent such information, antimicrobials are prescribed presumptively and therefore, in some cases, inappropriately. The availability of rapid, low-cost diagnostics at the point of care would reduce such misapplications and would also serve the urgent needs of the public sector and industry for ongoing data on patterns of disease and the evolution of resistance.
All this suggests three avenues of pursuit. One would explore possible regulatory pathways toward safeguarding the usefulness of new antimicrobials that would not unduly dampen industry's interest in developing new products. Another would examine the implications of other kinds of national guidelines on clinical management of antimicrobials for the pharmaceutical and managed health care industries in terms of costs, drug utilization, and industrial R&D investment decisions. For example, when vancomycin lost patent protection in the late 1980s in the United States, the price per gram dropped rapidly as the number of grams increased markedly, although since the 1996 CDC recommendations on the appropriate use of vancomycin, growth in that use has moderated. Yet another avenue would explore and define the sorts of infrastructure required for keeping the evolution of antimicrobial resistance under some kind of reasonable control.
The issues involved in restricted use are also multisectoral, given the relationships between evolving resistance and antimicrobial use in animals and, recently, trees and plants cultivated for commercial purposes. The few efforts to restrict veterinary use of antimicrobials so as to retain their value for human health needs do not seem to have occurred in a systematic, cross-sectoral way. Nor have representatives from the food production and trade sectors, or from the pharmaceutical industry, been regularly engaged in the health sector's dialogue about a problem in which all have something to gain, something to lose, and something to contribute.
Product Labeling
Another issue for development of antimicrobial products lies outside the category of regulations addressed previously—the need for regular updating of the microbiology portion of labels so as to reflect changes in patterns of resistance in a timely way and convey that information to clinicians. Time is required for an initiating company to gather the data typically required for a label modification, for submission of information to the FDA, agency review and approval, and label
printing and distribution. On the user side of the labeling equation, the primary print media such as the Physicians' Desk Reference and Morbidity and Mortality Weekly Report may not reach or be readily used by clinicians, even when available on-line.29
A fundamental problem, for industry and for public health in general, is what was described as "the woeful state of U.S. national surveillance of drug resistance in most community-acquired pathogens"—for example, drug-resistant Streptococcus pneumoniae. Implied here is the need for a comprehensive, integrated system, some sort of national clearinghouse or database that would, first, regularly collect resistance data from numerous sources, including clinical services and industry clinical trials, in the United States and offshore. Second, the system would redistribute the information to all entities concerned. The public health value of such a system would seem obvious. What may be less obvious is its great significance for industry's ability to estimate the market demand for antimicrobial products that would drive R&D processes forward correspondingly.
Nonetheless, lack of funds was described as a problem at every imaginable level of surveillance. The budget for WHO's pivotal role in bringing together the different surveillance networks worldwide is $17 million, an amount generally seen as insufficient. The CDC appropriation for FY1997 was $44 million out of the $125 million estimated as needed annually to implement its Emerging Infections Strategy, the first component of which is addressing surveillance and response needs. 30 Funding at the state level, even in states that are disposed to support infectious disease programs and that have been designated as CDC emerging infection program (EIP) sites, is inadequate. In Minnesota, for instance, 88 percent of all money for infectious disease epidemiology surveillance is soft money and there is no permanent infrastructure in place.
Product Availability
The FDA is often able to play a facilitating role in situations of drug shortage. Although little appreciated, the role is potentially important in some instances of emerging infections. An instructive case, in which both the FDA and the CDC played a crucial part, was the response to the resurgence of tuberculosis (TB) that became so painfully apparent at the beginning of the 1990s, at the same time that the supply of drugs for treating the disease had dwindled drastically. The widespread belief that TB was "cured" had acted to depress continued manufacture by large pharmaceutical companies; the overall number of suppliers, both of finished product and raw material, had dropped precipitously; and modernization of manufacturing processes had, in some companies, obliterated their capacity to make older products. Resolving each of the parts of this problem required finding and obtaining drug, domestically and offshore, from several smaller and larger companies; developing requirements for manufacture that would be flexible yet not compromise product quality; and mobilizing publicand private-sector distribution channels. The time required to replicate these processes in comparable
emergency situations in the future would vary widely, depending on availability and location of existing product; need to manufacture; the financial and organizational ability of other public-sector entities (e.g., the CDC) to collaborate; the seriousness of the disease and size of the affected population; and the risk to the wider community.
International Harmonization of Technical Requirements to Register Pharmaceuticals
As indicated earlier, the FDA has the statutory authority to utilize data generated through clinical trials conducted outside the United States.31 The acceptability of results from such trials depends on their having been well designed and well conducted by qualified investigators in accordance with ethical principles acceptable to the world community. 32 However, maybe because of misconceptions or the lack of existing infrastructure, the impact of that authority on new R&D seems to have been small.
In contrast, the International Conference on Harmonization (ICH), an initiative started in 1990, was deliberately multilateral. Its purpose was to develop, in a formal process, agreement among regulatory authorities and industry representatives from the European Community, Japan, and the United States on harmonizing technical requirements for registration or product approvals so that a pharmaceutical company could submit the same, single, core data package to the corresponding regulatory bodies. Beyond the subtle benefits from more open, more regularized communication, the guidelines produced since establishment of the ICH have proved effective in reducing duplication and the numbers of animal and human subjects involved; they have also harmonized guidance concerning complexities related to dose response, policies for some special populations, and selection of controls. Work is ongoing in harmonizing statistical principles for clinical and placebo-controlled trials; design of studies; reporting of adverse reactions; addressing ethnic and gender diversity in pharmacogenetics and metabolism; developing an international medical terminology; and establishing standards for electronic transfer of data.
The trend toward harmonization, in specific product lines and in related areas such as the development of veterinary products, should shorten product development cycles and thereby act as a positive incentive to global pharmaceutical R&D, at least in general terms. How harmonization could serve the known and possible demands of emerging infections remains unspecified.
Intellectual Property Rights Revisited
In the case of emerging infections, lack of patent protection may be acting as a major disincentive in at least one possibly important respect. Among the hundreds of potential antibiotics left undeveloped on company shelves, some compounds
might, in theory, offer new product leads. Nonetheless, because they have gone off patent and are therefore unprotected in any prospective market, there is little justification for developing them further. A debated issue, perhaps meriting future exploration, is whether the number of such compounds is significant or, when factors such as cross-resistance are taken into account, their true potential is actually quite small.
A related matter is whether, even with all things equal on the patent scene, industry would consider it worthwhile to target a single resistant mechanism, for example, methicillin-resistant Staphylococcus aureus or even vancomycin-resistant staphylococci, for which the markets are not seen as large enough to justify investment. The burgeoning trend is for large pharmaceutical companies to seek collaborations with genomics companies, with the immediate objective in mind of bringing in new targets showing no cross-resistance to compounds that already exist and that retain activity against older targets. The ultimate objective of this strategy is to develop agents that have a wider spectrum and are therefore more likely to have a larger market and longer useful life.33
The patent system is not the only source of protection for pharmaceuticals. There are provisions through the FDA, under the Patent Term Restoration Act,34 that grant a period of exclusivity for the first developer of a product, even if the product has gone off patent. Under the act, an Abbreviated New Drug Application (ANDA) process is made available to a generic competitor who is allowed to rely on the safety and efficacy data of the product innovator. For drugs containing an active ingredient not previously approved by the FDA, the innovator is protected from generic competition by the ANDA process for a period of 5 years from marketing approval. If the active ingredient has been previously approved by the FDA and new clinical studies are essential for the approval of the product, then the innovator is protected from ANDA generic competition for a period of 3 years from marketing approval. Two limitations inherent in the act reduce its value as an incentive for companies to pull existing compounds with expired patents off their shelves for application to emerging infections. One is that, in general, a 5-year or 3-year period of exclusivity is insufficient to justify the expense associated with clinical trials. Second, the act excludes antibiotic drugs such as penicillin and streptomycin that are produced by fermentation of microorganisms for which batch certification is required to insure safety and efficacy.
Orphan Drug Legislation
The Orphan Drug Act (PL99-91) was passed in 1983 to encourage development of drugs then known to be effective against rare diseases or conditions, which were defined as those affecting fewer than 200,000 people in the United States. In the case of a vaccine or drug intended for diagnosis or prevention of such a disease or condition, the persons to whom such a drug would be administered in the United States must be fewer than 200,000 per year.35 A broader underlying assumption was that there was no reasonable expectation that
the costs of drug development and marketing would be recovered in future sales of the drug in the United States. The act allows sponsors to request orphan drug designation for a previously unapproved drug or to request a new orphan indication for an already marketed drug, and also permits treatment use of investigational orphan drugs. Most importantly, it promises 7 years of exclusive marketing for the first orphan condition to receive FDA approval.
Since the act's passage, there have been 663 orphan product designations by the FDA, including antibiotics, antivirals, vaccines, and immunoglobulins. Of that number, 102 (15.4 percent) were for infectious disease designations. And, of those, 14 (14 percent, or 2 percent of the total number of designations) were for tropical diseases. An unknown number of these were "orphans" in the U.S. context but might have much broader application overseas. In an ad hoc survey by PhRMA of its member companies, the 11 companies that responded reported 7 applications pending at the FDA for orphan indications, plus 7 Investigational New Drug (IND) applications and 21 pre-IND projects intended for orphan indications. Of the 53 drugs approved by the FDA in 1996, seven (13 percent) were orphan indications.36
The FDA also issues grants under the act, the funds for which come out of its own budget and represent a direct subsidy from the agency for research on rare diseases. Since the beginning of the program, there have been about 300 such grants, with a total dollar level in 1996 of $12 million. Six of those 300 grants, or 2 percent, were for infectious disease.37 Although both grants and orphan designations require a United States consignee or agent, application for orphan status is not limited to U.S. products.
The legislative experience of the act has been uneven. Over time, unexpected uses of its provisions have evoked public and congressional complaints about price, lack of competition, unjustified exclusivity, and the sizes of target populations. Beginning in 1990, Congress proposed various pieces of legislation that would remove or limit sponsor incentives for orphan drug development at such time as a given orphan population increased—as in the case of HIV/AIDS—or when off-label use had the effect of making a given orphan product more lucrative. Pricing issues were at the center of the debate and price caps were among the legislative constraints considered. Although no legislation ensued, the mere prospect of price caps served to stifle designation and approval rates and rare disease research in general is said to have suffered.38
There have been attempts to replicate the Orphan Drug Act, in the case of drugs for treating drug abuse in 1990 and for pediatric indications in the 104th Congress. Neither bill was enacted. Whether the act might be somehow applicable for fostering development of products for emerging infectious diseases would depend on the purpose of such application. If, for example, there are many products in the R&D pipeline, perhaps an infectious disease equivalent to the Orphan Drug Act might spur those products to market and thus justify further consideration. If, however, the objective is to stimulate research on entirely new classes of products to diagnose and treat infectious disease, an orphan drug clone would be inappropriate and legislatively improbable. Major companies may develop drugs for an orphan indication without requesting orphan designation.
The proposition was advanced that congressional concern about emerging infections might be mobilized to address a range of possibilities, for example: (a) an enhanced basic research agenda on immune response mechanisms; (b) more comprehensive surveillance of infectious diseases, especially foodborne pathogens; (c) a major public education campaign to promote more judicious use of antibiotics by both patients and physicians; and (d) options for energizing product development for niche markets.
Public-Sector Agendas And Priorities*
The R&D agendas of key public-sector entities—their priorities, program implementation, collaborations, budget allocations, and issues for the future—were presented at the workshop in a series of thumbnail reports on each agency's activities in the area of emerging infectious diseases. These are presented in Appendix A. The institutional priorities of each public-sector entity are also mapped in Table 3.
Several aspects of public-sector activity are quite clear from these two summaries. One is that there is no pattern of priority commitments to any single disease. Overall, public-sector thinking is oriented toward foundation issues: encouraging basic science and its translation into products; understanding and strengthening systems; gathering and disseminating good data; and, throughout, seeking participation by the private sector and developing the contexts that will make that possible. By these accounts, the public sector has not yet developed a list of emerging infectious diseases that might constitute the kind of R&D ''menu" that some industry representatives have indicated could be useful. The questions of whether such a list is possible, whether such a list might serve to guide industry developmental activity or whether, in that connection, such a list might serve as the basis for a series of market analyses, were not asked at the workshop but could be worth posing.
Lessons Learned And Issues For Resolution
This workshop set out to extract from experience ideas on how best to assure availability of pharmaceutical products needed to address emerging infectious diseases, particularly those products most likely to be orphaned by the customary workings of the market. Because the CVI continues to be a learning process, it served well as a case study. Industry and other public-sector perspectives then enlarged upon lessons from the CVI, provided other case material, including useful learning from experience with HIV/AIDS, and added understandings about
options and the large dilemmas that remain. Table 2 (see the Summary) synthesizes all these workshop perspectives and might be considered as a map of core incentive areas for targeted efforts in the future.
Lessons from the Children's Vaccine Initiative
Divergent Sectoral Mandates and Notions of Risk
The overarching lessons the founders of the Initiative had to internalize were the legitimacy of economics as the driver of the industrial sector; the size of the costs of developing and producing vaccine according to good manufacturing practices; and the obligatory linkages among costs, time, and predictability in the processes of research, development, production, and market revenue growth. Another important lesson for the public sector was reinforced in the conclusions of the CVI/Rockefeller Bellagio meeting, namely, that the public-sector international organizations still have much to do to fulfill their responsibilities in the public/private partnership, including, importantly—but surely not limited to—providing earlier estimates of demand for public health products and fully engaging in well-articulated advocacy for public health needs.
An ancillary fundamental was acknowledgment of what are basic disparities between the two sectors in the understandings of risk. In public health, risk derives primarily from epidemiology. For industry, in this case the pharmaceutical industry, risk is ultimately an economic function. Industry wants as much predictability and consistency as possible when it makes its R&D investment decisions, especially when profit margins are likely to be narrow. Thus, lack of sufficient, reliable, and decisive consensus about public-sector needs, priorities, and policies may well dampen prospects for private-sector collaboration.
A next step is figuring out how to share divergent risks. This implies sharing costs and other resources, as well as acting on the recognition that both sectors typically must deal with political risks and with the risks, real or perceived, of making a mistake, both of which can lead to excessive caution. As elementary as all this may seem, none of these notions had been part of the standard catechism for some of the CVI "pioneers," whose professional lives had been spent in the public health sector.
The Product Life Cycle and the Role of Market Analysis
Research and development is a total process, a pushing and pulling between demand and supply that ultimately connects laboratory and consumer. The CVI gradually implemented its recognition of the need to understand, define, and explain the market for publicly needed products, since it is in the marketplace that the interests of the two sectors ultimately coincide. This requires well thought out, timely, and inclusive epidemiological, market, and cost-effectiveness analyses on
which to build public-sector strategies and readjust industry perceptions of market potential. It also means early interventions to make a given market more attractive to investment, in effect creating that market by limiting demand uncertainties and generating appealing economies of scale. This has involved tactics such as price tiering and attempts to enhance market exclusivity, all possibly relevant to emerging infectious diseases, some still incompletely resolved, none simple.
Authentic Collaboration
Particularly difficult was learning to implement collaboration in ways that go beyond rhetoric. The CVI experience resonates with the value of beginning partnerships very early and sustaining them with frequent, regular interactions at several institutional levels. Experience indicates that there will continue to be particular challenges in sharing information not typically shared such as information about what is well back in industry's R&D pipeline, about potential scientific leads residing in the nonprofit sector, and about private-sector pricing rationales. Another challenge relates to sharing certain types of information more speedily and more often, for example, information from clinical trials and from the ongoing processes of priority-setting in the public sector, especially when those entail crucial shifts.
There are now interesting models of "sharing" within the private sector. Industry's "Inter Company Collaboration" on HIV/AIDS therapies also suggests options for cooperation within the private sector toward public objectives, a very new approach.
The Pivotal Roles of Advocacy and Public Information
A lesson still being learned, and much informed by ongoing instruction from the HIV/AIDS campaign, is the quintessential value of advocacy and public education in promoting public health priorities and catalyzing public awareness. This is a role highly suited to the public and nonprofit sectors but rarely truly or fully utilized by the former and not easy to fund.39 Advocacy for the importance of vaccines in general and for the need to develop new vaccines for existing and prospective epidemiological requirements remains largely unimplemented.
TABLE 3 Public-Sector Priorities for Addressing Emerging Infections
|
|
State Health Depts. |
CDC |
DARPA |
FDA |
NIAID |
DOS |
VA |
World Bank |
|
Surveillance and response |
X |
X |
X |
|
|
X |
X |
|
|
Basic research |
X |
|
X |
X |
X |
|
X |
X |
|
Applied research |
X |
X |
X |
X |
X |
X |
X |
|
|
Prevention and control |
X |
X |
|
|
X |
X |
X |
|
|
Public health infrastructure |
X |
X |
|
|
X |
X |
X |
|
|
Public education |
X |
X |
|
X |
|
X |
|
|
|
Promote global preparedness |
|
X |
X |
|
|
X |
|
|
|
Product availability |
|
X |
|
X |
|
X |
|
|
|
Engagement of private sector |
|
X |
X |
X |
X |
X |
|
X |
Although many lessons from the CVI have been converted into effective action, issues inevitably remain. Much of the Initiative's energy has been dedicated recently to catalyzing developing world access to vaccines already in limited use, a focus justified by the need to solve immediate problems of vaccine introduction so as not to generate a backlog of under-used vaccines. The point was made that the original notion of creating and licensing a brand new, single-dose, oral, multivalent vaccine was somewhat deflected by the gradual recognition that vaccines for many of the major causes of morbidity and mortality in developing countries were already being developed for industrialized markets. Although a return to that early focus will require a different set of processes, it is one for which industry has all the necessary tools. The concept was advanced that what may be needed is the development of a broad portfolio of potential components at different stages of development, where a number of risks have already been resolved by the public- and nonprofit-sector R&D through early-stage trials. The latter is the future challenge the CVI faces and for which its half decade of experience has been, in effect, a practice session.
Issues for Resolution
Other issues arose in the workshop that go beyond vaccines and were identified as being of special, even profound relevance to emerging infectious diseases, especially when those diseases are likely to be commercial orphans. In their majority, these are areas of tension concerning mechanisms that could stimulate the research that is most directly necessary for addressing emerging infections, but that are somehow problematic.
Agendas and Priorities
Although each public-sector institution is sui generis and driven by different basic mandates, there seemed to be general agreement among the public-sector participants in the workshop about the broad areas of programmatic importance for dealing with emerging infections. What did not emerge as anticipated was a list in response to industry's expressed interest in clear portrayals of specific disease priorities, although HIV/AIDS, tuberculosis, and malaria would now seem to be obvious enough. Given the difficulty inherent in predicting the arrival and future significance of emerging infections, any such list is necessarily limited to "arrived" diseases about which there is already justification for concern. The rest, as the public-sector agendas summarized in Appendix A suggest, is being categorically prepared.
Two alternative responses to industry's need to know were advanced.
- Well-articulated agendas for development of generic requirements, for example, diagnostics that could not only identify pathogens but assess development of resistance, or multiagent therapeutics;
- Surveillance systems integrated and configured so as to be, continuously and formally, accessible to all interested parties. The value added to the second alternative would be the base it would provide for more rapid and efficient updating of labeling for antimicrobials, another highly important problem that remains unresolved.
Funding
Inadequate funding appears to be an issue at every level of infectious disease surveillance, as evidenced in insufficient infrastructure, inability to support recurrent costs, or both. These problems are not exclusive to the developing world; the surveillance capability of the United States is similarly threatened. The subject of emerging infectious diseases has commanded interest in the U.S. Congress that has resulted in additional allocations for addressing the issue. However, today's pressures for public-sector austerity augur poorly for future increases, particularly with regard to global requirements. Two major areas of U.S. contribution to global health are problematic: (1) official development assistance (ODA) and (2) investment in research and development for those diseases that dominate the needs of developing countries. Beyond the matter of inadequate absolute amounts, funding instability is a critical problem, especially for research and development, characteristically a long-term endeavor.40 Erratic funding also hampers maintenance of infectious disease epidemiology surveillance of the quality needed to properly inform both the public health and industrial agendas.
Multi-Tiered Pricing
Price tiering, in conjunction with new approaches to market segmentation and high-volume commodity procurement, could serve the needs dictated by certain infectious diseases. At the same time, despite its potential leveraging role, differential pricing carries considerable political freight. The public sector has yet to refine arguments that might be made usefully to policy makers, not just in the United States but in other countries that question the appropriateness of the mechanism.
Restricted Distribution
Any imposition of restrictions on the distribution of products that acts to constrain their market share for a significant period of time is an obvious economic
disincentive to industrial investment in developing new products. This disincentive is unlikely to be overcome by imposition of higher prices to compensate for lost volume. Whether the longer useful life that can be achieved by limiting utilization of an antimicrobial product would act as adequate compensation does not seem to have been analyzed. The tension between responsible marketing and reasonable profits is high; the importance of getting a handle on this topic was agreed to be similarly high.
Surrogate Endpoints
The identification and use of surrogate endpoints has been critical for developing new products to address the AIDS pandemic and is part of the FDA strategy for accelerated approval of certain product classes. The development of generic categories of endpoints that might be used in connection with a range of infectious disease endpoints is an issue awaiting consideration, as is the development of alternatives to correlates of protection for vaccines against diseases for which clinical trials would be difficult or even impossible, for example, the case of Ebola virus or pathogens used in biowarfare or bioterrorism.
Patent Extensions
Lack of patent protection has been identified as a disincentive to developing new antimicrobials using unexplored compounds that have been abandoned by pharmaceutical companies. Because these are now off-patent and are therefore insufficiently protected from competitive market forces, there is little reason to pursue them further. Existing protections are viewed as insufficient, given R&D costs.
Extension of patent protection in these circumstances could conceivably be motivating for patent holders. On the other hand, would-be generic competitors might be expected to oppose such extensions. Thus, as a minimum, a delicate balancing of interests would be required. However, both the general notion of patent extension for abandoned compounds and its specific implications remain unexamined as possible incentives for development of infectious disease products.
Technology Transfer and Local Production
As a way for developing country markets to acquire better access to good quality health products, technology transfer for local production of those products has often disappointed, largely because of the unreliability of local infrastructures on which advanced technologies are highly dependent. On the other hand and somewhat ironically, the success of technology transfer may prove to be a problem, since it may cut into market share for imported products. In the case of
infectious disease products where a key technology is not readily transferable, this is not an issue; however, for older, lower-cost technologies, local production does become a factor in diminishing market appeal. The question of how big an issue this may be for emerging infectious diseases has not been asked and may be, with the possible exception of drugs for tuberculosis, largely premature.
Orphan Drug Designation
Work remains on the subject of the potential of orphan drug designation for at least some emerging infectious diseases. As the legislation is written, there would seem to be elasticity in terms of diseases affecting very small U.S. populations, at the same time that non-U.S. populations, specifically developing world populations suffering from those diseases, might be quite large. Furthermore, even though congressional receptivity to utilization of orphan drug legislation for generic public health needs has been limited, prospects for orphan drug designation seem to be more likely when the objective is to spur products already in company pipelines to market. The challenge is then to define what products are needed for what emerging infectious diseases and to seek to make a match with what industry may have in relevant pipelines. This is obviously a topic for continuing intersectoral conversations.
Other Topics
Other topics for such conversations might well build on reported congressional interest in an enhanced basic research agenda on immune response mechanisms; more comprehensive surveillance of infectious diseases, especially foodborne pathogens; a major public education campaign to promote more judicious use of antibiotics by both patients and physicians; and options for energizing product development for niche markets. Each of these, like the potential for orphan drug designation, might well reward more precise focus and articulation than has been the case so far.
A Final Observation
The scientific quandaries posed by complex diseases like malaria, STDs, and HIV, as well as the entire matter of antimicrobial resistance, are daunting. In addition, while the market system has served well, it engenders a sometimes fierce adversarial environment, in which the interests of each party—not to mention the overall public good—are often submerged in the service of emotion-laden stereotypes and external forces. Each of the parties—labeled generally as industry, government, and the consuming public—still has a way to go in articulating its vision of the optimum outcome as the elements of a rational policy confrontation.
Still, the strategies and learning from the CVI, from HIV/AIDS, and from the many mechanisms described in this report, offer plentiful options for stimulating research and development on products for emerging infectious diseases, at least some of which will be, for one reason or another, orphans that will need to be adopted creatively.
Notes
-
1.
Institute of Medicine. Contraceptive Research and Development: Looking to the Future. PF Harrison, A Rosenfield, eds. Washington, D.C.: National Academy Press, 1996.
-
2.
R Twombly. The future face of disease. Environmental Health Perspectives 105(2):184–186, February 1997.
-
8.
As of this writing, UNICEF has not found the funds it needs to purchase Hib vaccine.
-
11.
Contract extensions, in a manner not dissimilar to patent extensions, can have the effect of reducing market competition, a dynamic perhaps worth noting in future strategy discussions.
-
15.
The transcript will show that Forum members representing industry recused themselves from any discussion of price.
-
Program. Washington, D.C.: Pharmaceutical Research and Manufacturers Association, 1997.)
-
27.
Food and Drug Administration. Drugs Intended to Treat Life-Threatening and Severely Debilitating Illnesses. 21 CFR Ch. 1 (4-1-96 Edition): Part 312, Subpart E, §312; Part 314, Subpart H, §314.
-
29.
The Morbidity and Mortality Weekly Report is already available on-line and the Physician's Desk Reference is supposed to be on-line by the end of 1997.
-
32.
Food and Drug Administration. Miscellaneous. 21 CFR Ch. 1 (4-1-96 Edition): Part 312, Subpart F, §312.120.
-
FDA to review the new drug application plus one-half of the clinical testing time; it does not allow extension beyond 14 years of effective patent life.
-
36.
Government Reform and Oversight Committee, Subcommittee on Human Resources, United States House of Representatives.
-
38.
In this connection, FDA approval must be sought for each indication for which a company would like to market a given drug, a condition that some analysts define as yet another barrier (OTA 1993).
-
39.
Though what has happened in advocacy for breast cancer research was not discussed at the workshop, it is very much a model. With several other examples of the role of advocacy, it is represented in the inventory in Appendix B.






































