II
TRANSMISSION AMONG AND BETWEEN SPECIES
Much public discussion has centered on whether transmission of B. abortus in the wild ever can be documented. This section reviews epidemiologic evidence of transmission and associated factors, including the role of bison and elk behavior and the effects of weather on animal movement in the GYA. The National Park Service's natural-regulation policy is discussed, as is the effect of B. abortus on reproductive potential of bison.
BISON, ELK, AND CATTLE
Brucellosis was discovered in bison on first testing in 1917 (Mohler 1917), and it has existed since as a self-perpetuating disease in that species. Thus, transmission from cattle introduced by Europeans to at least one wild species must have occurred and then transmission from cattle or from the infected wild species to other wild species to account for the disease in cattle, bison, and elk (Honess and Winter 1956). Meagher and Meyer (1994) note that there were probably multiple transmissions to bison, and Thorne et al. (1991) note that recovery of B. abortus biovars 1 and 4 in Wyoming and the presence of B. abortus widely over the GYA suggest multiple exposures in elk as well. It seems likely, in view of the early free-range management of domestic stock in the West, that original transmission of the disease from livestock to bison and elk occurred during intermingling in the free-roaming state. However, at the beginning of the 20th century, restoration programs for bison (Garretson 1938) and elk (Murie 1951) resulted in capture, handling, and relocation of large numbers of both species, so the possibility of transmission in captivity cannot be ruled out.
Transmission of brucellosis from captive bison to cattle in North Dakota
was reported by Flagg (1983). The strongest evidence of transmission between free-roaming bison and elk comes from Grand Teton National Park (GTNP) and National Elk Refuge (NER) in the Jackson area of Wyoming (Williams et al. 1993, 1997). A small herd of bison was established in the wildlife park at GTNP in 1948 and in 1963 was found to be infected by B. abortus. All adults were removed, and calves were vaccinated. Brucellosis-free bison were introduced from Theodore Roosevelt National Memorial Park in 1964. This population was tested thereafter; calves were vaccinated, and all seropositive animals were removed. The last identified reactor was removed in 1967, and all adult bison tested negative in 1968. Late in 1968 and in 1969, some bison escaped from the wildlife park, and attempts were made to return them to the park. By 1970, however, nine bison were free-roaming because they could not be recaptured. The herd subsequently grew in numbers (Peterson et al. 1991b). About 1980, the animals began to winter on the NER, where they came into contact with winter-fed elk that were known to be infected with brucellosis. Cattle were not present on NER. In 1989, 11 of 16 bison collected on NER tested seropositive for brucellosis. On the basis of their modeling results, Peterson et al. (1991b) believed that the bison became infected in about 1980, and they noted that the bison herd first wintered on the NER, a potential source of B. abortus from winter-fed elk, in 1979-1980. Because the GTNP bison herd is isolated from the YNP bison herd by the continental divide, infection in GTNP bison is assumed to have derived from their contact with infected elk on the wintering grounds. Although the possibility of brucellosis having survived in the bison at the time of their escape from the wildlife park cannot be ruled out, transmission from elk seems more probable.
Two horses contracted brucellosis in the Jackson, Wyoming, area, where the only known source of the disease was elk on the winter feeding grounds (see p. 35, ''True Prevalence").
One of the most contentious issues—because it is key to determining the need for control of the disease in GYA wildlife—is the probability of transmission of brucellosis between free-roaming bison and domestic livestock. Nearly all parties to the controversy agree that the risk of transmission of brucellosis from bison to cattle in the GYA is small, but not zero. Defining small depends on whether transmission has occurred in the past and, if so, how often. That is key to determining the need to control brucellosis in bison. Advocates of no control maintain adamantly that no case of transmission of brucellosis from bison to cattle in the free-roaming state in the GYA ever has been documented. Advocates of the need to control the disease in bison to protect livestock in the surrounding areas maintain equally stoutly
that there is clear epidemiologic evidence that transmission from wildlife has occurred at least six times in the recent past, two of which might have been due to bison.
EPIDEMIOLOGIC EVIDENCE OF TRANSMISSION FROM WILDLIFE TO CATTLE
The differing interpretations of epidemiologic evidence on the two sides of the issue are the crux of the controversy. This evidence is summarized in a field report submitted to APHIS in December 1996. Between about 1961 and 1989, cattle on six ranches in the GYA became seropositive for brucellosis after testing brucellosis-free. One of the ranches was east and five were west of the continental divide in the Jackson Hole region. The evidence consisted of seropositive tests for brucellosis in cattle herds in which the disease had not previously been present, and no known source of infection occurred in cattle in the local area or in stock imported to the properties. On each of five ranches, a single outbreak occurred. On the sixth ranch, brucellosis appeared in a cattle herd in about 1961 (the exact date is not known); it was thought to have been eliminated, and the herd was found again to be seropositive when retested in 1969. One outbreak in 1988 and another in February 1989 (Cariman 1994) led to a court case in which the Parker Land and Cattle Company sued the U.S. government for damages for failing to control elk movements from the NER to private lands (Parker vs. U.S.A. and Peck vs. U.S.A. 1992). The court concluded that the brucellosis outbreak was most likely caused by contact with infected elk or bison but the plaintiffs failed to prove that the elk or bison came from the NER, GTNP, or YNP. Several elk winter feeding grounds operated by the Wyoming Game and Fish Department are between the Parker ranch and the NER. No outbreak of brucellosis in cattle in that problem area has been reported since 1989. Cattle producers in the GYA routinely vaccinate their herds for brucellosis. Vaccination is required in Idaho and strongly recommended in Montana and Wyoming.
In four of the cases, anecdotal evidence was provided that elk were adjacent to or moving onto the property; the other two cases included anecdotal evidence of elk and bison presence. Most of the elk were associated with various winter feeding grounds on which elk concentrations foster transmission of B. abortus. Free-roaming elk herds, thought at the time of the first reports not to carry brucellosis, were found on further testing to have a relatively high proportion of seropositive individuals. By 1977, brucellosis had been detected on feeding grounds (Thorne et al. 1997). The bison in
both cases would have come from the GTNP herd, in which 69% of individuals tested in 1988-1989 were seropositive for brucellosis (Peterson et al. 1991a,b; Williams et al. 1993, 1997).
Those six cases of purported transmission of brucellosis from wildlife to cattle are based on circumstantial evidence. The facts were derived from field operations of the federal-state cooperative program to eliminate brucellosis from domestic cattle in the United States. The data never were intended to meet the standard of scientific research, and inconsistent record retention resulted in further gaps in the documentation. The cases were summarized after the fact, some without supporting documents, which were discarded in the meantime. The only thing definite is that cattle in the herds tested seropositive for brucellosis. Assuming that elk and bison were in contact with cattle, there is no way to determine whether they were infective at the time and whether opportunity for transmission presented itself. Similarly, the possibility of infection from cattle is difficult to eliminate entirely, because it is always hard to prove that an event did not happen.
Some observers have noted that in states that have eliminated brucellosis from cattle in the past, occasional outbreaks are typical for some time after a state has been declared class-free by APHIS. That is because the disappearance function of the disease does not decline to zero at a constant rate but rather has a tail of gradually decreasing probability. Given the pattern of outbreaks in cattle in the GYA, with no new cases since 1989, this area might simply be mimicking the temporal pattern observed elsewhere where transmission from wildlife was not an issue. Or it could be maintained that the lack of outbreaks since 1989 is attributable to diligent cattle vaccination by ranchers. Given the ambiguity allowed by epidemiologic evidence in this situation, wildlife cannot be determined to be the source of brucellosis infection in these six cases.
BISON AND ELK BEHAVIOR AND TRANSMISSION
Considerable caution should be exercised in extrapolating results from cattle to bison beyond the consideration of a long, separate evolutionary history. There are fundamental differences between how cattle are managed and the natural behavior of free-roaming bison in the GYA. First, domestic bulls are placed with cows in lower relative numbers (typically 1:20 to 1:30) than the sex ratios of unmanipulated bison of about 1:1, or slightly skewed toward females (Meagher 1973; Van Camp and Calef 1987; Berger and Cunningham 1994). Second, domestic bulls are placed with cows only during the breeding
period, then removed; the bison sexes can intermingle throughout the year. Third, courtship is perfunctory in domestic stock because the highly skewed sex ratio largely eliminates male-male competition. Bison males compete strongly for females, and dominant bulls form close "tending bonds" with estrous females that last several days, during which the male is never more than several meters away from the female. Younger males might maintain tending bonds with females at an earlier stage; thus, females can have multiple consort males in close attendance before and leading up to breeding. The chance of nonvenereal transmission between the sexes is increased because of this protracted courtship behavior.
Still, the two most probable sources of B. abortus transmission are abortion or birth when infectious materials are in the environment. Because of long exposure of bison to B. abortus, they respond to it more like chronically infected cattle herds in which selection for genetic resistance has occurred. In about 75 years, only four cases of abortion in YNP have been recorded (Rhyan et al. 1994); of course, regular surveillance is impossible given the large numbers and scattered distribution. The real number, therefore, has to be greater. But if abortion were common, many more cases would be expected to have been reported. In two cases, abortion sites remained culture positive for B. abortus for at least 2 wk (J. Rhyan, USDA, pers. commun., 1998).
Abortion among elk on the NER and Wyoming Game and Fish Department feeding grounds has been estimated at 7% (Smith and Robbins 1994) to 12.5% (Herriges et al. 1991) of pregnancies. Given such a high abortion rate and the high concentration of animals, transmission is highly likely. Indeed, Thorne et al. (1997) suggest that any elk that lives a long life and winters on a feeding ground is likely to become infected.
Also important is the difference in probability of association between elk and bison and cattle. Elk usually move away from areas used by cattle (Skovlin et al. 1968; McCullough 1969; Oakley 1975; Long et al. 1980; Mackie 1985), and this would reduce the contact between the two species. Bison, in contrast, are behaviorally dominant over cattle and respond to them aggressively if they approach within 5 m (Van Vuren 1982). However, they tolerate them when in proximity, and in one case, Van Vuren (1982) observed a domestic cow that joined a bison social group for 7 days.
In normal birth, the probability of transmission of B. abortus to cattle is influenced by the birthing behavior of bison and elk. Wild ungulates are categorized by birthing behavior as hiders or followers (Lent 1974). Hiding and following are major strategies used by mothers to avoid predation on their offspring. Hiders use dispersion, crypsis, and concealment to prevent discovery of offspring by predators, whereas followers depend on precocial offspring
(offspring that can stand soon after birth), which can run with the mother to escape predators. Hiding is characteristic of species that have access to concealment cover in their habitat. Following is characteristic of herding species; herding is usually associated with open habitats that lack concealment cover and is itself a strategy for countering predators (McCullough 1969; Hamilton 1971).
Elk are classic hiders (Geist 1982). Females approaching labor isolate themselves from the herd (often moving several kilometers away) and seek cover in vegetation or broken terrain to give birth (Johnson 1951; McCullough 1969). After giving birth, the cow meticulously cleans the site (Livezey 1979; Clutton-Brock et al. 1982) and then moves the calf several hundred meters away to hide (Altmann 1952; Clutton-Brock et al. 1982).
The sanitation of the birth site by the mother is thorough. Females search the ground and consume small bits of birth tissue (Livezey 1979) and grass stained by fluids (Clutton-Brock et al. 1982). Bauer (1995) reported, "As we watched, the cow not only devoured the placenta and birth membranes, but also seemed to be eating the earth and grass that were saturated with birth fluids." Fraser (1968) noted that hiders eat afterbirth materials more for protection of the young than for physiologic reasons and that removal of vegetation and soil would remove any traces of scent from the site. Indeed, the entire suite of behavior of the elk cow and calf at birthing is linked to concealing the presence of the calf from predators. The calf hides alone while the cow feeds or beds in the vicinity, returning only long enough to nurse (McCullough 1969). The mother licks the calf's perineum during suckling; this stimulates voiding, after which she ingests the feces and urine (Arman 1974). The hidden calf remains motionless if approached during the first 3 or 4 days of life, running only at the last instant if hiding fails; the cow defends the calf from predators (Murie 1951; McCullough 1969). The cow and calf usually rejoin the herd in 2 or 3 wk after birth (Altmann 1952, McCullough 1969).
The evolution of antipredator behavior in elk has resulted fortuitously in behavior that reduces the likelihood of B. abortus transmission. The dispersed birthing area and sanitation of the birth site result in a low probability that other animals will come into contact with infectious birth products.
The consensus of respondents to the National Research Council questionnaire was that B. abortus is not self-sustaining in elk herds that are not concentrated on winter feeding grounds. That is cited as the reason that the elk in the northern Yellowstone herds that are not winter-fed have a seropositive rate of only 1-2% (M. Meagher, USGS., pers. commun. as cited by Smith and Robbins 1994; Rhyan et al. 1994), whereas those using winter feeding
grounds in the southern part of the GYA have an average seropositive rate of 34%. A somewhat higher seropositive rate (5 of 126, or 4%) in northern-range elk was reported by Thorne et al. (1991), but this could reflect sampling error.
In contrast with elk, bison offspring are followers, as is consistent with the highly developed herding social structure in this species (McHugh 1958; Meagher 1973; Lott 1974). Pregnant females separate from nonpregnant females to form nursery herds (McHugh 1958; Lott and Galland 1985; Meagher 1986; Berger and Cunningham 1994). Females give birth either alone or in small subgroups and might seek cover, depending on what is available in the environment occupied by the nursery herd at the time of birth (McHugh 1958; Lott and Galland 1985). Nevertheless, birth occurs either in or close to the herd. Mean time from birth to standing by the calf is about 11 minutes and from birth to nursing about 32 minutes (Lott and Galland 1985). The mother usually consumes the afterbirth (McHugh 1958; Fraser 1968; Lott and Galland 1985; J. Berger, U. Nev., pers. commun., 1997). However, detailed observations of how thoroughly the site is cleaned are not available. In bison, consuming the afterbirth might be related mainly to hormonal and physiologic needs; the antipredator benefits of consumption would seem minimal in a species that lives in large herds in open areas and has offspring that are conspicuously different from the adults. For bison calves, the major antipredator protection is the herd. Calves are protected from predators not only by their ability to run with the herd, but also through defense by the large, formidable mothers, whose common interest—protection of young from predators—presumably is the selective advantage of forming separate nursery groups in the first place. If consumption of the afterbirth in bison is related to hormonal factors rather than predator avoidance, it might be that the birth site is not so well sanitized as by elk.
Giving birth within the herd concentrates the afterbirth in space and increases the likelihood of encounters with other herd members and roving males. That increases the probability of transmission of B. abortus associated with birth products among bison and to other species that might accidentally or purposefully encounter the nursery herd area. The dispersed distribution of birthing in elk, in conjunction with their thorough cleansing of the site, makes the probability of transmission of B. abortus among elk or from elk to other species, lower than for bison.
Abortion by B. abortus-infected females is a more serious risk factor for disease transmission than is normal birth. Abortion is spontaneous and typically occurs in the third trimester of pregnancy. That timing places most abortions in the winter when both bison and elk are concentrated, some on
artificial feeding grounds. Abortion occurs out of synchrony with the social structures of normal birth and decoupled from the usual entraining of endocrine activity that regulates normal birthing behavior. Concentration of animals on winter feeding grounds or, in bison, by the natural herd structure greatly increases the potential for contact with aborted fetuses and other afterbirth products. In addition, disruption of normal hormonal controls results in retention of placentae in bison and failure of the females to clean up the birth products. Retained placentae in bison can attract the attention of other herd members and roving bulls and extends the exposure period of B. abortus in time and space. Elk apparently do not retain the placenta after abortion, and they can reach it and remove it before it hits the ground (Thorne et al. 1978, 1997). In their study of penned elk, Thorne et al. (1978) reported that aborting females attempted to eat their fetuses but that they might have been only partially consumed. In this captive herd, other females were observed to investigate and lick the partially expelled fetuses during abortion. Intact fetuses and afterbirth remaining at the abortion site would greatly increase the probability of transmission between animals. Furthermore, at the typical time of abortion, winter temperatures and moisture would favor survival of B. abortus in the environment, as would sequestration of B. abortus in larger masses of birth tissue not consumed by the female.
TRANSMISSION BY OTHER SPECIES OF UNGULATES
Other wildlife species have the potential to contract and transmit brucellosis (see review of Remontsova 1987). Other wild ungulates in the GYA—mule deer, white-tailed deer, antelope, and bighorn sheep—have never been documented to harbor the microorganisms (McKean 1949; Steen et al. 1955; Shotts et al. 1958; Trainer and Hanson 1960; Rinehart and Fay 1981; Jones et al. 1983; Gates et al. 1991; K. Aune, Mont. Dept. Fish, Wildlife, and Parks, pers. commun., 1997). Moose are known to contract the disease, although moose living in an area where cattle were heavily infected by B. abortus tested seronegative (Hudson et al. 1980). None of several dozen moose tested in the GYA was seropositive (T. Thorne, Wyo. Game and Fish, pers. commun., 1997). Moose are considered a dead-end host for brucellosis and are not thought to be a threat to transmit the disease. They do not seem to be involved in the epidemiology of brucellosis. Moose are typically solitary, and yet the rare occurrence of brucellosis in moose, a species that does not usually carry or perpetuate the disease, illustrates the possibility of transmission of B. abortus among the species that do. Surveillance for the disease in
moose or other wildlife species that are dead-end hosts might be a way of estimating the probability of rare events of transmission among bison, elk, and cattle.
POTENTIAL ROLE OF CARNIVORES IN TRANSMISSION
Predators can become infected with B. abortus, and they are potential reservoirs for transfer to other species. The most thorough work on B. abortus in carnivores is the study done on coyotes by Davis et al. (1988). They fed macerated cattle fetal material infected with B. abortus to 40 brucella-negative coyotes, and 32 became seropositive. They also found that B. abortus can pass through the digestive tract of coyotes and remain viable in feces and urine. In each of four trials, 10 exposed coyotes were put in 1-hectare pens with six uninfected heifers. B. abortus transmission occurred in three heifers in one trial, and they aborted. No transmission occurred in the other trials; 3 of 24 heifers were infected overall. The heifers probably became infected through contact with urine or feces of coyotes (D. Davis, Texas A&M, pers. commun., 1997). Coyotes can potentially serve as a bioassay for B. abortus; a survey of two-thirds of the counties in Texas showed that seropositivity in coyotes corresponded to the known distribution of brucellosis in cattle (D. Davis, Texas A&M, pers. commun., 1997).
Transmission in the Davis et al. (1988) study occurred under confinement at artificial densities of both coyotes and cattle. Although it does verify the possibility of transmission, that cannot be translated into probabilities of transmission under natural range conditions.
Carnivores of YNP—including grizzly bears, black bears, wolves (Canis lupus), coyotes, and foxes (Vulpes fulva)—are known to contract brucellosis (Zarnke 1983; Remenëtïsova 1987; Morton 1989; Johnson 1992), presumably through consumption of infective tissues during predation and scavenging. Of 122 grizzly bears tested in Alaska, six were seropositive (Zarnke 1983). Current estimates of grizzly bear population size in the GYA are around 300 (Eberhardt and Knight 1996). There were an estimated 650 black bears in the GYA in the late 1970s (Cole 1976), but their numbers might have declined (Schullery 1992). YNP has no current estimate of black bear numbers; they are considered common in the park (Gunther 1994), but they are seldom mentioned with reference to brucella transmission. Wolves were extirpated from the GYA by the early 1930s and have been reintroduced only recently (Weaver 1978; Yellowstone Science 1995; Bangs and Fritts 1996). Consequently, the ecosystem role of wolves has been missing for many years and
is only now being re-established. The current wolf population is about 100. Coyotes are ubiquitous in the GYA.
Any debilitation due to brucellosis (Tessaro 1987; Thorne et al. 1997) would predispose adult elk and bison to predation. Grizzly bears, wolves, and coyotes scavenge and all are predators on calves. Scavenging makes them vulnerable to contact with products of birth and abortion, the likely route of acquisition of B. abortus, but it is highly unlikely that these species directly transmit the bacterium back to ungulates. They are considered dead-end hosts. Transmission of B. abortus by carnivores through transport of infective materials from birth or abortion sites to other areas, however, is a concern.
Carnivores could have positive and negative effects on the dynamics of B. abortus. On one hand, by consuming products of birth and abortion they remove the bulk of infectious materials from the site and expose remaining B. abortus on the soil and vegetation to light and desiccation, to which they are vulnerable (Mitscherlich and Marth 1984). Although it has not been quantitatively documented, known carnivore behavior makes the existence of a healthy complement of predators almost certain to be a major factor in reducing the probability of B. abortus transmission within the wildlife community and between wildlife and domestic stock. Predation and scavenging by carnivores likely biologically decontaminates the environment of infectious B. abortus with an efficiency unachievable in any other way.
On the other hand, carnivores might contribute to transmission probabilities by transporting infectious materials from one site to another. Particularly troublesome is the possibility of transporting such material between exclusive wildlife and cattle areas kept geographically separated by management. No data are available to address this question directly; the potential risk must be evaluated on the basis of what is known about the behavior of these carnivores.
Ordinarily, urine and feces from predators would be unlikely routes of direct transmission of B. abortus because the number of organisms shed is small in relation to the infective dose for cattle (Morton 1989), and cattle, bison, and elk would not be attracted to or likely to come into contact with them accidentally. However, one exceptional circumstance should be noted. B. abortus apparently can pass through the gastrointestinal tract of predators and survive in their feces (Davis et al. 1988). Under some conditions of mineral deficiency, domestic cattle show depraved appetite, or pica, in which they consume a variety of atypical objects (Church et al. 1971). Similarly, wild ruminants commonly visit mineral licks and consume soil during some times of year, usually during periods of rapid growth in the spring. Rodents and rabbits are well known to consume bones and antlers, presumably for the
minerals in them. In a mineral-deficient area in south Texas, cattle were observed to consume coyote droppings (D. Davis, Texas A&M, pers. commun., 1997), which commonly contain small mammal bones, a mineral source. Also, reindeer penned with foxes consumed fox feces (Morton 1989). If such behavior occurred in a brucellosis area, the probability of transmission of B. abortus from predators to herbivores could be substantially increased. Whether such behavior occurs in bison, elk, or cattle in the GYA is unknown.
A more important concern with predators is their transport of infected ungulate-carcass materials from a death or abortion site to other areas. Internal organs of large animals are usually consumed first, and skeletal muscle and other body parts later (E. Gese, NWRC, Ft. Collins, Colo., pers. commun., 1997). Heads, bones, and other hard materials are consumed last or not eaten at all. Coyotes and wolves sometimes transport pieces of carcasses short distances to nearby preferred microsites to complete consumption, but this would spread the bacteria only locally and not greatly increase the likelihood of transmission. Grizzly and black bears are not known for transport of carcasses or parts from the site of death; they do not usually move carcasses elsewhere to cache them, although they sometimes cover the carcass at or near a kill site (Craighead et al. 1995), which might preserve B. abortus for longer periods. They usually feed on site. Bears are followed by dependent offspring and do not provision.
Longer-distance transport could occur as a result of caching carcass parts and provisioning pups sequestered in dens; these behaviors are shared by coyotes, wolves, and red foxes. Parts of carcasses carried by mouth (usually pieces containing bones, which afford structural integrity) can be transported great distances. Soft tissues may be consumed and subsequently regurgitated at the den. Caching has been reported in wolves (Murie 1944; Mech 1970; Harrington 1981), coyotes (Weaver 1977), and especially red foxes (Vander Wall 1990). Caching—thought to be a way to extend the time that food is preserved, to protect it from competitors, and to hedge against difficult hunting times—seems to be most common in populations with smaller home ranges and greater population densities. In Alaska, wolves often disperse and cache chunks of caribou, burying them in soil or in creeks covered with moss (K. Taylor, Alaska Dept. of Fish and Game, pers. commun., 1997). Recently in YNP, wolves were observed to kill a pronghorn fawn and cache the carcass (F. Camenzind, Jackson Hole Conservation Alliance, pers. commun. 1997). Foxes, which have relatively small home ranges, cache frequently, coyotes less commonly, and wolves least commonly. The potential for B. abortus transmission by red foxes (Johnson 1992) should be considered more carefully, given their well-developed caching behavior (Vander Wall
1990), their common occurrence in YNP (Gese et al. 1996a), and their caching frequency (E. Gese, NWRC, Ft. Collins, Colo., pers. commun., 1997). Their small home ranges would limit long-distance transport, but their active caching would increase the local foci of B. abortus contamination.
Usually pieces cached are relatively small, weighing a few kilograms or less. Ordinarily, elk and bison calves are too large to be cached whole. But aborted fetuses are smaller. Abortion can occur at any time during gestation, but it is most frequent in the late second and early third trimesters (D. Davis, Texas A&M, pers. commun., 1997). Weights of 37 elk fetuses aborted in captive studies averaged 7.9 kg (range, 4.5 to 11.4 kg) (W. Cook, U. Wyom., pers. commun., 1997). Bison fetuses would ordinarily be even heavier. Consequently, predators are likely to reduce fetuses to smaller parts before caching, as they do with normal-birth calf carcasses.
A considerable amount of information on caching behavior in coyotes has been obtained inadvertently through radiotelemetry studies of survival of young ungulates. From radiotelemetry studies of antelope fawns, coyotes have been found to cache carcass parts commonly in Arizona (R. Ockenfels, Ariz. Game and Fish Dept., pers. commun., 1997), Kansas (E. Finck, Emporia State Univ., pers. commun., 1997), and Oregon (R. Cole, Hart Mtn. National Antelope Refuge, Ore., pers. commun., 1997). Coyotes also have been found caching black-footed ferrets in Wyoming (E. Williams, U. Wyom., pers. commun., 1997). In the GYA, Weaver (1977) and E. Gese (NWRC, Ft. Collins, Colo., pers. commun., 1997) have observed caching in the snow by coyotes. In GTNP, Weaver (1977) observed coyotes caching meat from an elk carcass. He observed 16 snow caches, ranging from 10 m to more than 400 m from the carcass, one of which contained a 78-g piece of meat. A coyote often would bed down on a cache site, perhaps to mask the site from competitors. In YNP, Gese (NWRC, Ft. Collins, Colo., pers. commun., 1997) observed coyotes caching elk and bison (less commonly because bison mortalities are far fewer than those of elk), usually less than 300 m from the carcass. Most sites were cleaned out fairly soon thereafter. Despite their potential to contract brucellosis, Gese et al. (1997) reported that 70 blood samples of coyotes from Lamar Valley in YNP were all seronegative. Caching in Lamar Valley was done most frequently by red foxes, however.
To prevent discovery by competitors, carnivores completely bury caches several inches below the soil and conceal the sites. Large herbivores are extremely unlikely to encounter these sites or, if they do, to come into contact with the buried materials. In addition, the parts that are cached are usually muscle and bone, which are less likely to harbor B. abortus than reproductive tissues are. Chance of transmission of B. abortus to cattle, bison,
or elk would seem unlikely with earthen caches. Caching in the snow during the winter, however, as has been reported for coyotes in GTNP (Weaver 1977), presents greater hazards. Furthermore, foxes steal small pieces, including soft tissues, from carcasses being fed on by coyotes and bury them in the snow as well (S. Grothe, Mont. State Univ., pers. commun., 1997). Burial in snow would favor longer survival of B. abortus; if the cache were not retrieved, buried remains would be exposed on the surface of the ground when the snow melted.
Provisioning of pups, through transporting muscle and bone by mouth and regurgitating soft parts, is a more common predator behavior than caching and therefore more likely to move B. abortus from one place to another (and between geographically separated wildlife and cattle areas). E. Gese (Fort Collins, Colo., pers. commun., 1997) observed coyotes provisioning up to several miles from a carcass. The work of Davis et al. (1988) showing that B. abortus can survive in coyote urine and feces indicates that it is likely to remain viable in the partially digested stomach contents regurgitated for pups. These contents can include organ tissues that are most likely to contain B. abortus. Both members of the territorial mated pair and associated pack members (''helpers") engage in provisioning of pups (Hatier 1995). According to research on survival of B. abortus in the environment, the bacteria likely would survive during the transport of such materials and potentially could contaminate new sites that are far removed from the initial source. However, regurgitated material is consumed by the pups immediately.
To protect pups, den sites are usually hidden in rocky, rough, timbered areas where large herbivores are less likely to go. In addition, prey species probably avoid approaching predator dens. Wolves and coyotes switch den sites regularly; if B. abortus persisted long enough, herbivores could come into contact with it around abandoned den sites in the absence of predators.
The seasonality of provisioning pups by coyotes and wolves is important in relation to the time that B. abortus—principally through abortion and calving—might be in the environment. Timing of reproductive events in bison, elk, wolves, and coyotes in YNP is shown in Figure II-1. The major period of provisioning by canids in YNP occurs after the main period of birthing in bison but overlaps with elk calving. However, there are late, aseasonal births in bison (Meagher 1973; Berger and Cunningham 1994; Roffe, unpubl. data) that are possible sources of B. abortus during the provisioning periods of wolves and coyotes. Such aseasonal births are also known in elk (Smith 1994). Similarly, B. abortus could be acquired by consumption of infected tissues from whole carcasses during predation at any season.
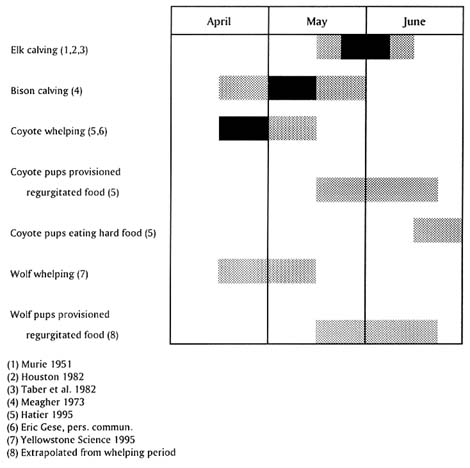
FIGURE II-1. Timing of reproduction events in bison, elk, coyotes, and wolves in YNP showing degree of overlap of Brucella exposure during normal birth in bison and elk, and provisioning periods in wolves and coyotes.
The possibilities of transfer of B. abortus between wildlife species become smaller with each ecologic complication. The wolf, coyote, or fox needs to be in contact with B. abortus via tissues from bison or elk; the B. abortus must survive being transported to a cache or den site; and it must persist at the new site long enough and in sufficient numbers to infect a susceptible bison, elk, or domestic cow that encounters the den site more or less through random movement. The chance of transfer of B. abortus among elk, bison, and cattle through the activities of predators and scavengers seems extremely small under most conditions.
Transmission of B. abortus among ungulates indirectly through predators and scavengers cannot be completely ruled out, but it seems unlikely in comparison with transmission by direct contact among bison, elk, and cattle. On balance, the positive advantage of sanitary activities of large carnivores in the GYA greatly outweighs the negative effects of their possible role in transmission of B. abortus. Consequently, the presence of carnivores in the ecosystem probably reduces the frequency of B. abortus moving between bison, elk, and cattle.
ROLE OF OTHER WILDLIFE SPECIES
A wide array of other wild vertebrates, insects, and other arthropods that occur in the GYA can harbor B. abortus (Remenëtìsova 1987), including rabbits (Peterson 1991), rodents (Thorpe et al. 1965; Moore and Schnurrenberger 1981; Rinehart and Fay 1981), and ticks. Nevertheless, these species are incidental hosts and are not important in the transmission of brucellosis.
BISON MOVEMENT OUT OF YELLOWSTONE NATIONAL PARK
There is no risk of transmission of B. abortus from bison to cattle in the northern range if bison do not leave YNP. Cattle grazing is not permitted inside YNP. For many years, bison have been reported to move out of YNP in hard winters (Meagher 1973, 1989), and a large kill of bison (1,084 animals) outside the park during the winter of 1996-1997 produced a great controversy (Peacock 1997) and reinvigorated the debate about brucellosis.
Weather and Bison
The effect of winter weather on bison movements outside YNP boundaries is a topic that is amenable to modeling, but little effort has been given to it. The first issue is the frequency of hard winters in the GYA. Farnes (1996) presented 45 years of climatic data (from the winter of 1948-1949 to the winter of 1992-1993) from several stations around the northern range, and he has furnished comparable data from 1993-1994 to 1996-1997 (P. Farnes, Snowcap Hydrology, Bozeman, Mont., pers. commun., 1997) for a total time series of 49 years. He presented the measured values and calculated severity indexes based on a range from -4 (severe) to +4 (mild). Variables included were snow water equivalent in inches (SNOW) from Lupine Creek and Crevice
Mountain snow courses (2,249 m and 2,560 m, respectively), cumulative sum of minimal daily temperatures below -18°C (TEMPERATURE) measured at Mammoth (1,890 m), and summer rainfall (RAIN) measured as the sum of June and July rainfall in the previous year at Mammoth. He also presented a combined winter severity index that included a weighted measure of SNOW (40%), TEMPERATURE (40%), and RAIN (20%). Farnes (1996) noted that early-summer rains might be related to fall animal condition and likelihood of surviving the winter, as previously reported by Meagher (1973). The objective of our analysis, however, was to examine the influence of winter weather on bison movements independently of animal condition, so SNOW and TEMPERATURE were emphasized. RAIN was included in the analysis, but was not significant, either alone or through its contributing to winter severity index, so it is not discussed further.
Examination of the frequency distribution of the two measured winter variables showed that TEMPERATURE was skewed (mean, 178.8; C.V., 0.59; median, 154.0; skewness, 0.83), with most winters being on the mild side and less-frequent severe winters, whereas SNOW was normally distributed (mean, 21.1; C.V., 0.265; median, 21.3; skewness, -0.05); that is, most years are near the mean, but for extreme years, mild and severe winters are equally likely. Autocorrelation and cross-correlation with year showed no significant pattern over time for TEMPERATURE or SNOW, although there was a weak trend toward an oscillatory pattern for Farnes's (1996) snow index. Overall, the results suggest that hard winters occur roughly randomly, and a much longer time series would be required to detect any periodicity.
The second issue is whether the measured variables or indexes correlate with the numbers of bison moving out of YNP. Bison-population estimates over time are shown in Figure II-2. (Data on bison populations are from Yellowstone National Park (1997), and data on bison removals are from Dobson and Meagher 1996 and Yellowstone Science 1997.) Three periods of different management policy give different results. An early period from 1902 to about 1930 was a time of population recovery from low numbers that survived uncontrolled market hunting. Beginning in the 1930s and extending until 1967, large removals inside YNP controlled bison numbers. The natural-regulation policy was implemented after 1967, and an increase in numbers occurred, with removals consisting of animals moving outside YNP boundaries. Virtually all the bison moving out of YNP were killed and so were lost to the population. The issue is addressed through regression analysis on data after the implementation of natural-regulation policies in 1967. The effects of individual weather variables and indices and estimated bison population size on bison moving out of YNP are examined by simple and stepwise multiple regression.
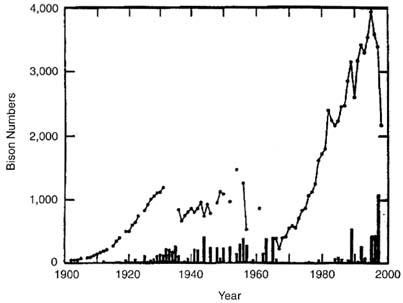
FIGURE II-2. Plot of estimated bison population (circles) and bison removals (bars) by year for YNP. Data on bison population are from Yellowstone National Park (1997: Appendix B) and bison removals from Dobson and Meagher 1996, Yellowstone Science 1997.
None of the weather variables or indexes shows a significant correlation with bison moving out of YNP; indeed, none is even suggestive. Only estimated bison population size is significantly related to the number of bison migrating out of the park (P < 0.001). The plot of bison moving out of YNP on estimated bison population, however, shows the relationship to be highly nonlinear (Figure II-3); indeed, the abrupt transition is best described as a threshold. Above a population of 3,000, bison show the greatest probability of moving out of YNP. Log transformation of bison moving out of YNP yields a significant linear fit with bison population (R2 = 0.53; P < 0.001), but even that transformation does not adequately reflect the abruptness of the threshold.
To examine the effects of weather on populations below the threshold, the regression analysis is repeated for bison population estimates of less than 3,000. Again, no weather variable or weather index is close to significant. Cross-correlation shows that relationships are not delayed; zero lag yields the highest correlations. Again, bison population size is the most important variable, but it did not quite reach statistical significance (P = 0.06).
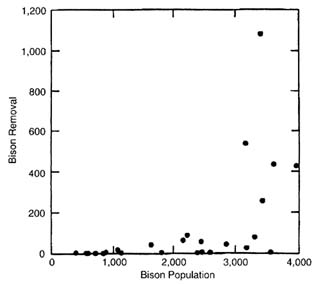
FIGURE II-3. Plot of bison removals on estimated bison population size for years under management by natural regulation (1968-1997).
The final issue addresses relationships at populations above the 3,000 threshold. One might expect the response to winter conditions to be strongest in large populations. Given bison populations of more than 3,000, does winter severity influence the number of bison moving out of the park? Regression analysis of bison populations on various indexes of winter severity in years when there were more than 3,000 bison show that SNOW and snow index are strongly related to bison moving out of YNP (Figure II-4) (R2 = 0.84 and P = 0.001, and R2 = 0.71 and P = 0.009, respectively). No other winter-severity variable is close to significance, nor does stepwise regression result in an improved fit.
Of the two measures of winter severity available, TEMPERATURE and SNOW, only SNOW proves to be important statistically in explaining bison movements. Figure II-4 shows that for populations over 3,000, the number of bison moving out of YNP increases rapidly with increasing SNOW (on average, 68 bison for each inch of SNOW). Furthermore, on the average, no bison moved outside YNP when SNOW was 17 in. or less. That average fails to capture the fact that historically some bison have moved outside the park even when the population was low (Meagher 1973). Nevertheless, 17 in. of SNOW is a useful benchmark for increased probability that bison will move
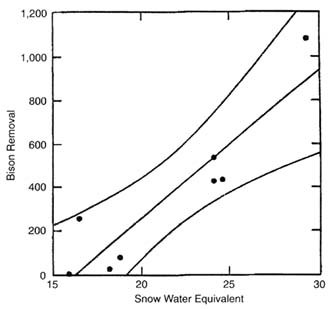
FIGURE II-4. Plot of bison removals on snow water equivalent for bison populations greater than 3,000. Lines are least-squares fit (Y = -111.16 + 68.40(X) and 95% confidence intervals.
out of YNP and, if not controlled, potentially come into contact with cattle. The two points in Figure II-4 farthest above the line (1991-1992 and 1996-1997) are identified by the statistical program (SYSTAT: SPSS, Inc. 1996) as outliers; this suggests the involvement of additional variable or variables. Many observers have noted that snow depth itself might be unimportant because bison are adept at digging craters in deep snow to forage. A freezing and thawing pattern that produces ice layers in the snow might be more important (Peacock 1997). In the winter of 1991-1992, an early snowfall was followed by a thaw, which in turn created an ice layer at ground level (Gese et al. 1996b); in the winter of 1996-1997 ice layers in the snow pack formed a physical barrier to foraging beneath the snow (Peacock 1997). Thus, relatively mild winters that have thawing followed by freezing might be more difficult for bison (and probably elk, which also dig craters in snow to forage) than severely cold winters with deep snow.
Farnes (Snowcap Hydrology, Bozeman, Mont., pers. commun., 1997) suggests that bison typically move to areas that have less than 6 in. of snow water equivalent and have available forage. He notes further that bison-snow relationships are complex, involving snow water equivalent, snow density, spatial and temporal variation, and perhaps other variables. Learned behavior and forage availability also interact with snow conditions to influence bison movements.
SNOW shows a normal distribution, and most values are expected to fall around the mean of 21.1 in. The winter carrying capacity of YNP is about 3,000 bison; this analysis suggests that above this population size, bison will move out of the park in all but the mildest winters (Figure II-4). Therefore, the regression in Figure II-4 would predict that under average conditions, about 332 bison will move out of YNP each winter, more in high-SNOW years and few or none in low-SNOW years. Experience with habitats outside of YNP might encourage bison movement in the future that is not driven solely by population size and winter severity. However, so long as bison moving out of the park are removed from the ecosystem, this behavior will be discouraged. Furthermore, the relative role of experience is not clear, for the basic tendency of bison to move in the face of adverse conditions seems to be a primary motivation, and the landscape funnels them to lower elevations outside the park. Therefore, experience might contribute to the tendency to move, but it is probably not necessary to account for the behavior.
Obviously, more years of data will be needed to refine the interaction of bison numbers, SNOW, and bison movement out of YNP. The relationship of SNOW to bison leaving the park (Figure II-4) is based on few points and has wide confidence limits. Only a few measures of weather variables are analyzed, and those only from a few sites. They might not have captured the important aspects of a hard winter from a bison's point of view. A measure of ice layering, for example, would be valuable. Current analysis of spatial data on weather variables now being done by Farnes (Snowcap Hydrology, Bozeman, Mont., pers. commun., 1997) will better elucidate the causal relationships between snow characteristics and bison movements and expand on the correlation reported here. For the present, however, the importance of this analysis is the degree to which it reveals broad patterns. Bison population size appears to be the overwhelmingly significant variable controlling movement of bison out of YNP. Still, as long as bison are artificially controlled at or near the YNP boundary, the predictions of the number of bison moving out of the park in relation to SNOW should be sufficient for management because they are not allowed to move further. If bison are not artificially controlled, however, continued, more directed research using refined
measurements of specific attributes of weather and its variance over time, space, and topography would go beyond simply predicting how many bison move out of YNP to how far they go and for how long. Refinement in recording and modeling weather measurements and more data on bison movements will refine the quality of predictions but are unlikely to alter these conclusions.
Natural Regulation in YNP Bison
As just noted, the expansion of the bison population in YNP appears to be the fundamental force pushing bison out of YNP, contributing both to increased risk of transmission of B. abortus to livestock and to the need to take action to deal with bison in unwanted places. Whether natural regulation is occurring is the underlying issue for B. abortus risk-management and bison-management policy: Is there evidence that bison are likely to reach a dynamic equilibrium with the carrying capacity in YNP?
That question can be addressed by examining the natural logarithm of bison population estimates over time (Figure II-5). If the population growth rate is constant, then logarithmic plots on time should be linear. In Figure II-5, it can be seen that the plots are approximately linear over much of the population growth record. The reduction in population growth rate toward the end of the early period (1902-1930) is accounted for largely by bison removal inside YNP (Figure II-2) and therefore does not reflect carrying capacity. Offsetting time by 51 years (open circles in Figure II-5) demonstrates that the growth trajectory of the early period is consistent with the growth trajectory of the natural regulation period (after 1967).
The reduction of population growth rate after about 1980 (Figure II-5) is more complicated. The population was reaching numbers at which SNOW becomes more influential in movements of bison out of YNP. Removal of these animals (Figure II-2) therefore accounts for at least part of the diminution in population growth. The last three winters, particularly, have been marked by the removal of many bison (Figure II-2). However, hard winters contribute to natural mortality inside YNP as well, and this is part of natural regulation. Estimates of natural mortality were not available over most of this time, so the effects of artificial removal, compared with natural mortality, are difficult to discern. For the last 3 years, the difference in population estimates between years is only partially accounted for by removals; this suggests a substantial residuum of natural mortality. Certainly, the combination of both resulted in a large reduction in bison numbers (3,400 to 2,169) over the winter of 1996-1997.
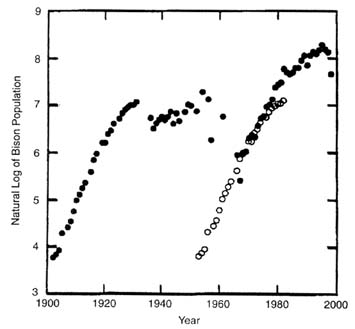
FIGURE II-5. Plot of loge of estimated bison population on year for YNP bison (filled circles). The three time periods noted in Figure 2 can be seen here. The open circles are the data points for the early period (1902 to 1930) advanced by 51 years to align the early growth period with that following implementation of the natural regulation policy in 1967.
Nevertheless, the period 1972-1994 was one of relatively few removals (Figure II-2). Furthermore, when the effects of removals are canceled by adding them back to the following year's population estimate, a remarkably good linear fit of bison numbers on time is obtained for the estimates from 1972 to 1995 (R2 = 0.987; P < 0.001) (Figure II-6, filled circles). The linear fit in Figure II-6 shows that the annual increment in the bison population was more or less constant at 145 per year. The close fit suggests that natural mortality in YNP during this period was low and roughly constant. That in turn suggests that natural mortality was minor in years other than those with hard winters and that at high populations a large portion of mortality was due to artificial removals when bison moved out of YNP.
Only the last 2 years of the time series (open circles in Figure II-6) deviate from the regression line, and even the point for 1996 is within the variance previously observed in the time series, which might be related to actual
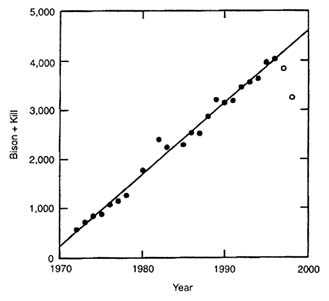
FIGURE II-6. Plot of estimated bison population plus previous year's removal on year for 1972-1997. The line represents a least-squares linear regression equation to the filled circles (1972-1995); Y = -286137.746 + 145.371(X); R2 = 0.987, P < 0.001. The open circles (1996 and 1997) show years in which annual increment was below the regression line. See text for further explanation.
changes in bison increment or to error in bison population estimation. Including all but the last year (1997) changes the relationship only slightly (R2 = 0.98; P < 0.001), with the estimate of annual increment lowered from 145 to 143 bison. Only the point for 1997 clearly deviates from the linear regression (although inclusion gave an R2 = 0.955 and P < 0.001) but lowers the annual increment to 134. The winter of 1996-1997 was a severe one that caused substantial mortality, both outside and in YNP. Even the previous two winters had above-average SNOW (24.6 and 24.1 in., compared with the average of 21.1 in.). Thus, even in the most recent years with high bison numbers, there is little evidence of natural diminution of bison population growth except that induced by the severe winter in 1996-1997.
Although the absolute annual increase is essentially constant at 145 bison, the per capita rate is declining. For example, the per capita growth rate of the population of about 530 in 1972 (at the beginning of this period) would have been 0.27, whereas that of the population of about 3,730 in 1994 (at the
end of the period), would have been 0.04. This apparent contradiction can be explained by a simple economic analogy. Assume a constant, fixed income was always put into an account. As the account grows, the income becomes a lower proportion of the capital similar to the decline in per capita growth rate in a density-dependent population response. However, the income remains constant, so the capital continues to grow despite the relatively lower proportion of the capital the income represents. So long as the income remains fixed, the capital will continue to increase, and the per capita rate of growth will never drop to zero.
That result is unexpected because with increased population size, density dependence in ungulates is usually expressed by declines to zero in both rate of increase and absolute increase. There is no established model for this population behavior in ungulates, although it was anticipated by McCullough (1990). This kind of population behavior is known for other taxa. In territorial species, such as most passerine birds, and carnivores, such as wolves and coyotes, territorial holders are the only individuals that can effectively breed and only so many territories can be fit into the habitat. Thus, reproductive output tends to be relatively stable over broad ranges of total population size. We do not know what mechanism might be operating to stabilize the annual increment in YNP bison.
That the rate of increase declines, but not to zero because of a virtually constant absolute annual increment, suggests that some variable other than scramble competition (each individual doing the best it can to obtain resources) is modifying the density-dependent process. Because adult bison mortality seemed to be relatively low during the years included in this analysis (1972-1994), calf recruitment (birth and survival to yearling age) is the likely source of the constant absolute annual increment.
Estimates of pregnancy rates have shown that a relatively high proportion of adult cows become pregnant. From 1935 to 1950, up to 90% of adult females were pregnant (Coburn 1948; Rogers 1950). In 1988-1989, 74 of 102 (73%) of mature females were pregnant (Pac and Frey 1991); in 1990-1991, 54 of 68 (79%) were pregnant (Meyer and Meagher 1995). Aune (Mont. Fish, Wildlife, and Parks, pers. commun., 1997) reported that 90% of radiocollared bison cows calved in 1995-1996. Kirkpatrick et al. (1996), however, reported a 3-year (1990-1992) mean calving rate for the YNP northern-range herd of 52.6%. T. Roffe (USGS, pers. commun., 1997) found that 90% of a sample of 52 bison cows killed in the winter of 1996-1997 were pregnant. High pregnancy rates point to survivorship between midgestation and 1 year of age, rather than failure to conceive, as the critical variable in calf recruitment. Meyer and Meagher (1995) reported that about 400-600 calves were born
each year before 1995. An annual population increment of only 145 would require that a large proportion of calves died, because adult mortality was low. The most likely explanation for the constant increment is that either dominance in females is determining success in recruiting calves so that some cows consistently produce while others consistently fail or there are few good habitats in which females succeed in recruiting calves, whereas elsewhere females fail. These two possibilities are not mutually exclusive—dominant females are likely to displace subordinates from the best habitat—so both might be interacting to result in constant annual recruitment.
YNP bison population behavior contrasts with the northern-range elk population behavior, in which the dynamic equilibrium is expressed by year-to-year changes. The lack of stabilization of bison population growth over time since the natural-regulation policy was adopted suggests that bison have expanded like a wave front across suitable habitat in YNP with little diminution until now they are pressing against the borders of YNP in winter. The prospect, therefore, is for the bison population to increase over some years until the coincidence of a high population and a hard winter results in the population being reduced once again (as happened in 1996-1997). Given the lack of a dynamic equilibrium, the bison numbers are expected to start building again. It will be instructive to determine whether the constant increment persists and the absolute value remains the same during recovery as during the 1972-1994 period.
McCullough (1990, 1992) proposed that ungulates that feed on homogeneous, relatively coarse, low-quality food (bulk feeds), as bison do, with diets 95% or more grasses and sedges (McCullough 1980; Van Vuren 1982; Singer and Norland 1996), might show roughly constant population growth until carrying capacity is reached, at which point growth drops abruptly to zero (a plateau with a cliff edge). That contrasts with animals such as elk (Kufeld 1973; Hobbs et al. 1979; Marcum 1979; McCullough 1980), in which qualitative aspects of the forage result in a declining (ramp) adjustment of population growth before carrying capacity is reached at zero growth. Environments that present considerable amounts of unoccupied habitat to which the population can expand into with continuing growth also contribute to relatively constant population growth rates (McCullough 1990).
Bison in YNP seem to follow the cliff-edge model1 (modified appropriately
for constant recruitment instead of constant growth rate) but do not actually reach the cliff edge in that spatial limits of YNP were exceeded before carrying capacity was reached. It could be argued that the decline in annual increment in 1994-1995 and 1995-1996 (Figure II-6) reflected such a cliff edge. However, the decline was so clearly associated with hard winters, particularly in 1996-1997, that it seems more like a catastrophe than a reflection of exceeding carrying capacity on the basis of resource limitation. No equilibrium point is likely in a system in which the average annual population increment is 145 head, whereas once the population exceeds 3,000, the average SNOW condition results in an artificial removal of 332. That inequality is exacerbated by the unpredictable occurrence of mild and hard winters. It is to be expected that the population will build up until an inevitable winter reduction, only to repeat the process—much like kangaroo populations in Australia confronting periodic drought (Caughley 1987).
Meagher (1993) has noted that bison could be affecting their habitat in ways that will lower carrying capacity. YNP, however, states that no resource damage has been documented (Montana Department of Fish, Wildlife, and Parks et al. 1990). Taylor (1992) noted that bison killed outside the park in the hard winter of 1991-1992 were in excellent body condition, with more than adequate body-fat stores, and the same was true of animals removed in the hard winter of 1996-1997, which had substantial layers of brisket fat, particularly early in the winter (P. Gogan, USGS, pers. commun., 1997). Late-winter samples of brisket fat reflect access to forage rather than adequacy; therefore, early-winter values are more instructive than habitat quality. For the 1991-1992 kill, Zaugg et al. (1993) reported normal blood values and moderate parasite loads. Thus, there is little evidence of inadequate forage or quality available to YNP bison. Effects on habitat are a natural consequence of building populations, and no diminution of absolute population growth is apparent. Whether diminution of population growth will occur in the future can be determined only with time. Furthermore, periodic reductions of high populations by severe winters will reduce bison numbers and probably allow periodic recovery of the habitat. Given those uncertainties and the occurrence of a ''natural experiment" because of the decline in the 1996-1997 winter, it is imperative that research be pursued.
Another issue is the effect of the 1988 fire that burned 42% of YNP. Boyce and Merrill (1996) modeled the expected ungulate-population response to the fire, and the model prediction was that bison would show a population increase. At least through 1994, no such response was reflected in the constant recruitment (Figure II-6).
Influences of Plowing and Grooming Snow
Meagher (1989, 1993) has described bison movement and suggested that learning of the landscape has gradually re-established behavior that was lost during periods when artificial control inside YNP confined bison to low numbers in a few areas of the park. Plowing and grooming of YNP roads in the winter for snowmobiles might have facilitated bison movements (Meagher 1989), and Meagher proposed that such pathways are energetically efficient to use, although there are no data to test that proposal. Limited plowing began in the late 1940s, but other than a few males, bison did not use the plowed roads until 1975-1976 (Meagher 1989). Grooming for snowmobiles began in 1970, but according to Meagher (1993) the first bison use of groomed roads began in the winter of 1980-1981.
The fact that groomed roads were not used when first available (Meagher 1993) raises the question of why, if the groomed roads were valuable energetically and opened up valuable new habitat, bison did not quickly take advantage of the opportunity. The delay might be attributable to behavioral inertia. Or it might have been that bison had not yet reached numbers that forced expansion movement. Attributing bison population increase to road grooming instead of attributing use of groomed roads to population increase might therefore reverse cause and effect.
Still, the important issue is to separate behavior (proximate effect) from demographic consequences (ultimate effect). Expansion of bison into previously unused habitat and movement through greater snow barriers would be expected simply because of the increase in the population (McCullough 1985). If grooming of roads led to substantial gains in winter energy savings (with presumed greater winter survival) and the opening of new habitat (with a presumed increase in carrying capacity), increased population growth would be expected. That proposition can be examined by looking at bison population growth before and after bison use of groomed trails began in the winter of 1980-1981. Absolute population growth rate was essentially constant before and after bison began to use groomed roads (Figure II-6); this finding suggests no substantial influence of snow grooming on demographic performance. There seems to be little supporting evidence of an ultimate effect of road grooming on bison population dynamics.
Bison were known to move along natural topographic routes before grooming began (Meagher 1973), and they cross barriers where roads do not occur at all (Meagher 1993; R. Garrott, Mont. State Univ., pers. commun., 1997). Furthermore, tallies of observations from Meagher (1993) showed 50 observations of bison using roads and 46 of bison traveling cross-country.
Those observations might have been biased in accordance with the observational effort in the two categories, but they show that cross-country travel by bison is common. Failure to prevent bison movements by hazing, herding, and fencing (Meagher 1989; Thorne et al. 1997) suggests that it will be difficult to prevent bison from moving where they please. Bison evolved in open plains largely as a nomadic species (Roe 1951; Meagher 1973). Just as other behavior—formation of large herds and other social behaviors—re-emerged with the increase in numbers after the bottleneck at the beginning of the 20th century, a nomadic tendency might be manifested in behavior of bison in YNP. The nomadic tendency is fostered by a large aggregate social structure in which individuals shift repeatedly between groups and the mother-calf relationship is the only consistent social bond (Lott and Minta 1983; Van Vuren 1983). Bison appear to behave as though continuous habitat were to be found down the valley or over the next hill, and that might account for their fluidity of movements when local conditions worsen. Whatever the case, now that locations of other habitat areas are known to the herd, it is unlikely that discontinuance of snow grooming will prevent their movements.
The suggestion that discontinuing winter road grooming will contain bison better within YNP and that starvation and other natural factors will relieve the need for artificial control outside the park appears optimistic. Certainly, periodic starvation of some bison in YNP during hard winters has occurred over many years (Meagher 1973). But many YNP bison in recent years have moved in search of better conditions elsewhere rather than attempt to survive winter in their traditional locale within park boundaries. The number of losses associated with movement out of YNP and being killed and the number of losses in the park can be examined. Of 1,805 total deaths listed by Meagher (1993) from 1975 to 1993, there were 1,127 outside and 678 (38%) inside YNP. In addition, natural mortality in the exceptionally hard winter of 1996-1997 can be estimated by subtracting the known kill in the winter of 1996-1997 and the 1997 summer count exclusive of new calves from the 1996 summer count. The summer count in 1996 was 3,436, of which 1,141 were killed or removed in the winter of 1996-1997, leaving 2,295 animals. If there were no natural mortality, 2, 295 adults should have been in the population in 1997; however, the highest summer count of adults was 1,921. The difference—374 animals (25% of the total mortality)—is the apparent natural mortality in YNP.
Gunther et al. (1997) reported bison and elk carcasses counted on systematic hiked, snowshoed, or skied routes (131.5 km) in various parts of YNP for 1992-1993 through 1996-1997. Mortality of bison varied from 5 to 22 from
1992-1993 to 1995-1996 and form a cluster around 20 per year, except for the low of 5 in 1993-1994, a low-SNOW (15.9 in.) winter. A total of 69 carcasses was found in the hard winter of 1996-1997, only one of which was in the low-elevation northern range. Bison carcasses are nearly significantly correlated with SNOW (P = 0.07), but that is entirely due to the leverage of the 1996-1997 point and is invalid because of violation of assumptions of the regression model. In addition, the current study of the Madison-Firehole bison showed that most of the deaths in the 1996-1997 winter were of calves, not adults, relatively few carcasses of which were found (R. Garrott, Mont. State Univ., pers. commun., 1997).
Those demographic analyses are subject to caveats. First, bison numbers are estimates and subject to all the errors associated with the problems of censusing wild animals in a heterogeneous habitat. But the population estimates given in Figures II-5 and II-6 show consistency over time that would not be expected if the error were large. The estimate is not likely to be so inaccurate as to invalidate the conclusion that most mortality occurred outside the park. Second, measurement error applies to weather variables as well, although these errors are more likely insignificant for the purposes of the analysis.
BISON IN GRAND TETON NATIONAL PARK AND THE NATIONAL ELK REFUGE
Bison summer in GTNP and migrate to winter in the NER (Meagher et al. 1997). The history of this herd is given by Smith and Robbins (1994) and Williams et al. (1993). Twenty bison were reintroduced into Jackson Hole from YNP in 1948 and confined in the Jackson Hole Wildlife Park, a 1,500-acre enclosure for displaying prominent indigenous wildlife. A population of 15-30 bison was maintained in the park until 1963, when brucellosis was discovered in the herd. Several months later, all 13 adults in the population were destroyed, and four yearlings that had been vaccinated as calves and five newly vaccinated calves were retained. In 1964, 12 brucellosis-free adult bison (six of each sex) were introduced from Theodore Roosevelt National Park. Over the years, the enclosure's fence deteriorated, making it increasingly difficult to contain the bison. In 1969, when the remaining 16 captive bison in the herd were determined to be brucellosis-free, the herd was released to range freely. That event marked the beginning of the free-ranging Jackson bison herd. The current number is about 380 (S. Cain, GTNP, pers. commun., 1997), which shows that the population is continuing to grow. The
herd is infected by brucellosis (76% seropositive, Smith and Robbins 1994; 36% culture-positive, Williams et al. 1993) and is in contact with infected elk on the winter feeding grounds in the NER, which are not being vaccinated. It is presumed that the brucellosis-free bison stock was originally infected on the NER feeding grounds through contact with aborted elk fetuses in about 1980 (Peterson et al. 1991b).
Bison and cattle have no contact on the winter range, because cattle are excluded from the NER. Bison are in contact with cattle as they cross private lands during migration, and cattle trail driveways in spring and fall and on grazing allotments on GTNP and Forest Service lands in summer (Smith and Robbins 1994). Cattle in this region of the GYA are invariably vaccinated because of the perceived risk of transmission of B. abortus from elk and bison to cattle. Smith and Robbins (1994) maintain that only one case of possible transmission of B. abortus from elk or bison to cattle has occurred in the GTNP-NER area since 1951, and it might have been due to incorrect vaccination rather than contact with wildlife.
ELK IN THE GREATER YELLOWSTONE AREA
As did bison, elk have consistently increased from low numbers since the beginning of the 20th century (see Figure 2). However, elk population size is substantially greater than that of bison. The current estimate of the number of elk in the northern range is about 17,000 (Figure II-7), and the total elk population in the GYA is around 120,000 (Toman et al. 1997). Traditionally, elk migrated out of YNP and GTNP to lower elevations, where, as their numbers increased, they became subject to public hunting under the authority of the wildlife agencies of the surrounding states. Elk are also extremely important to the socioeconomics of the GYA as a tourist attraction and game animal. Substantial hunter take of elk has generated less controversy than bison removals, presumably because of the much larger numbers of elk, their dispersion over a greater area, and more favorable perceptions of fair chase, and perhaps because the elk is less likely to be perceived as a national icon. Policy issues related to elk management have generated controversy, but more within the scientific and resource-management community than in the public arena. Because the debates over elk management are long standing, considerably more research has been done on elk than on bison. Herd units are recognized, and their population estimates are reported by Toman et al. (1997).
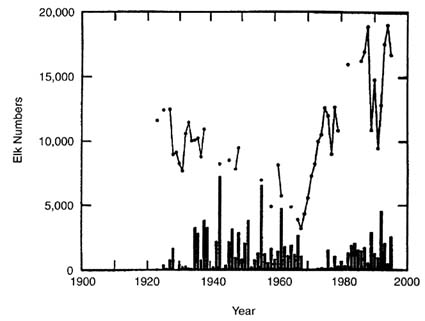
FIGURE II-7. Elk population estimates (circles) and hunting removals (bars) plotted on year for the northern YNP elk herd (data from Yellowstone National Park 1997).
Northern Elk Herd Movements Out of Yellowstone National Park
In contrast with bison, there is substantial evidence that elk of the YNP northern range show density-dependent demography and are fluctuating about a dynamic equilibrium in response to resource carrying capacity, as well as being influenced by density-independent winter stress conditions (Houston 1982; Merrill and Boyce 1991; Coughenour and Singer 1996). The leveling of population growth since about 1980 is apparent from the plot of northern-herd numbers over time (Figure II-7). This herd was controlled by shooting in YNP until 1968; shooting in the park ended with the adoption of the natural-regulation policy. Elk kills thereafter (Figure II-7) were made during public hunting seasons on lands outside YNP under the control of Montana Department of Fish, Wildlife, and Parks.
The conclusion of other researchers about density-dependent control of the northern elk herd since the beginning of the natural-regulation policy in 1969 was rechecked with a somewhat different approach, including more-recent data. Change in elk population size between years (difference) was regressed against the elk population size in the first year, weather variables and indexes for the winter between the 2 years, and the elk kill in the fall and winter between the 2 years. Elk population size is the only variable that relates significantly to the difference in population size between years (R2 = 0.33; P = 0.03) (Figure II-8). Regressions such as this have a small negative bias (Eberhardt 1970) because the Y-axis variable is not measured independently of the X-axis variable. Appropriate tests of density dependence have been much debated in the literature (White and Bartmann 1997), and no universally accepted method of analysis of non-independent data is available. Independent data, unfortunately, were not available to us. Still, the cessation of growth after 1987-1988 (apparent to the eye in Figure II-7), the relatively strong negative relationship in Figure II-8, and that various methods of analysis applied by others have led to the same conclusion of density dependence, are reason to accept tentatively that density dependence is more likely descriptive of the northern YNP elk population than density independence.
Including elk kill in more-complex models lowers the fit. Because the kills did not contribute to the prediction of the next year's elk population, either elk kills were swamped by density-dependent population changes, the kill was compensatory with natural mortality, or a combination of the two.
Although the dynamic equilibrium shown in Figure II-8 is characterized by high variance, the population size where difference = zero gives an estimate of mean carrying capacity. Note that if there is a negative bias (induced correlation) due to the analytical method, the mean will somewhat underestimate the true value. The negative bias is small, however, so this difference is probably negligible compared with the variance of the relationship. The dynamic equilibrium estimated elk population mean of about 11,300 includes the effects of elk removal by hunting. If kill is added back, the equilibrium population is about 17,400. Furthermore, the population estimates are corrected for sightability after 1986, and that results in higher estimates for 1986-1995 (Yellowstone National Park 1997). There is a high correlation between the estimated and corrected elk population size (R2 = 0.97; P < 0.001). When elk difference plus kill is plotted against corrected elk populations (Figure II-9), an estimate of 17,812 is obtained, although the fit is not significant (R2 = 0.16; P = 0.20). Despite the poor fit, this is a more realistic estimate of mean equilibrium and is in the range of values derived by other workers (17,058 by Houston 1982; 14,522-17,819 by Merrill and Boyce 1991;
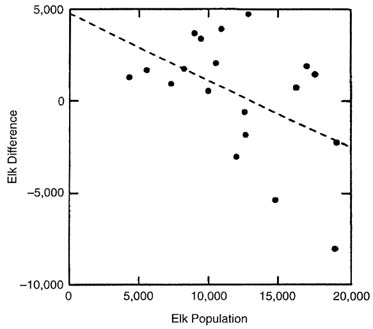
FIGURE II-8. Change in estimated elk population size between years plotted against elk population size in the first year for the years after 1969 when the natural regulation policy was implemented. The least-squares regression equation was Y = 4731.951 - 0.362(X); R2 = 0.24, P = 0.04.
18,010 by M. Taper (Mont. State Univ., and P. Gogan, USGS, pers. commun., 1997).
Natural regulation of elk population size occurs in the northern range of YNP (albeit with considerable amplitude in the dynamic equilibrium), in marked contrast with that of bison. Elk are mixed-diet feeders (Kufeld 1973; McCullough 1980; Singer and Norland 1996) and thus have a much higher amplitude in quality of diet. Taper (Mont. State Univ.) and Gogan (USGS, pers. commun., 1997) have shown that elk in the northern range follow a plateau and ramp model (McCullough 1990, 1992). That the effects of SNOW, hunter kill, and other variables seem to be dampened or compensatory with natural mortality suggests the risk of B. abortus transmission from elk to cattle is roughly stable in the northern Yellowstone range. And, of course, the seropositive rate in this elk herd is very low.
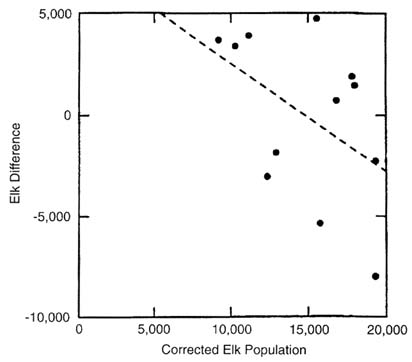
FIGURE II-9. As for Figure II-8 except using corrected elk population size. The least-squares regression equation was Y = 9333.435 - 0.524(X); R2 = 0.16, P = 0.20.
Addressing issues of potential contact between elk and cattle requires identification of variables that influence the number of elk outside the park. Can a predictive model be found that explains elk movement, such as SNOW predicts bison movement at populations exceeding 3,000? The Montana Department of Fish, Wildlife, and Parks (unpublished data) conducted aerial counts from 1989 to 1997 to assess the number of elk leaving YNP in the northern range. Regression of these data on SNOW yields a significant relationship between snow water equivalent and elk migration out of YNP (Y = -3452.6 + 414.9(X); R2 = 0.44; P = 0.05). Zero elk migration (on average) is at about 8 in. of SNOW; this suggests that elk are more easily moved by snow than are bison (17 in.). Furthermore, the kill of elk outside YNP in the Gardiner late hunt correlates with elk migrating out of YNP (R2 = 0.67, P = 0.007). Carcass counts of elk in YNP (Gunther et al. 1997) correlate with snow
(R2 = 0.83; P = 0.01), and this also points to greater susceptibility to snowfall for elk than for bison. The equation predicts that no mortality will occur if SNOW is 15 in. or less.
Other Elk Herds in the GYA
Because of the controversy over the National Park Service's natural-regulation policy, the most attention paid to elk is paid to the northern-range herd in YNP, but that herd constitutes less than 20% of the elk in the GYA. One herd, the Madison-Firehole herd, is mainly (75%) nonmigratory and winters in thermal areas in YNP (Craighead et al. 1972). Six other recognized herds are scattered about the perimeter of the park; they typically summer within the boundaries of the park and winter outside it in three states—Montana, Idaho, and Wyoming. Each state has its own management program. Estimates of the size of these herds vary, but the total is around 120,000 elk. These populations are subjected to hunting outside YNP, and in a number of cases hunting mortality is great enough to limit the numbers. Migratory movements were curtailed historically by establishment of private ranches, and to maintain numbers and attract elk from private lands, winter feeding is common. In addition to the NER, the Wyoming Game and Fish Department (WGFD) maintains 22 feeding grounds (see Figure 2 for a map of wintering areas). Feeding in Wyoming usually occurs from January to April. Fencing and hazing are used to keep wintering elk confined to feeding grounds and separated from livestock. Idaho feeds elk on one area in years when it is necessary to keep elk off of developed lands (Smith et al. 1997). Montana does not provide prepared feed (alfalfa pellets) for elk but has made an alfalfa planting available to wintering elk at Dome Mountain (see numbers using this area in Coughenour and Singer 1996).
In addition to the roughly 23,000 elk on feeding grounds (GTNP 1993), about 25% of the Wyoming elk in the GYA winter on natural range away from livestock operations. The WGFD has embarked on a program to improve habitat in natural wintering areas to reduce the density of elk on the feeding grounds and disperse elk over the landscape (S. Smith, WGFD, pers. commun., 1997). Elk have been found to use areas improved by controlled burning. However, it is not yet clear whether there is sufficient opportunity to spread the population adequately or to reduce substantially the numbers of animals using the feeding grounds. Oldemeyer et al. (1990) have shown that weight loss in elk on the NER is related to the amount of feed given and that elk use natural feed if it is available. Thus, density on feeding grounds
potentially could be reduced by a combination of greater hunter harvests, manipulating the amount of feed offered and its placement, and making natural feed more available. Many Wyoming elk herds are above herd objectives (Toman et al. 1997), and increased hunter harvests are desired to bring numbers into compliance with management goals. This would reduce the need for supplemental feeding and the consequent crowding of elk where transmission of B. abortus is most probable.
Presumably, reduction of density on the feeding grounds would reduce the likelihood that elk would come into contact with infective products of abortion due to brucellosis and would reduce the rate of transmission of B. abortus. Whether those measures will be sufficient to reduce the incidence of brucellosis in elk remains to be seen. It seems likely that if females abort away from the feeding grounds, the rate of transmission will be reduced, leading to a reduction in the overall herd infection rate. Nevertheless, it will be difficult to reduce elk density on the feeding grounds enough to prevent transmission from abortions and avoid maintaining a problematic level of infection.
There is a small interchange of individuals between these herd units (Anderson 1958; Craighead et al. 1972; Boyce 1989), but it is only a few percent. As noted earlier, the cause of seropositivity in the northern herd is an issue. Although the exchange of individuals between herd areas, including exchange between Jackson Hole and the northern range (Craighead et al. 1972), shows that the seropositive elk could have come from southern herds to winter with the northern herd, it does not show that they did. Yet, interpretation of the presence of seropositive elk in the northern range depends entirely on whether these are the same animals that came from herd areas with high seropositive rates, rather than animals that came into contact with B. abortus through contact with other elk or bison.
The best-studied population other than the northern herd is that wintering on the NER and vicinity (Anderson 1958; Boyce 1989; Smith and Robbins 1994). In his extensive population analysis, Boyce (1989) concluded that NER elk show density dependence despite winter feeding and that they are regulated by winter severity, recruitment, and hunter harvest. Hunter harvest appears to have a larger effect on NER elk than on the northern YNP elk, but the harvest rate is also higher. Boyce (1989) reported an annual kill over many years of around 3,000-4,000 in a population of about 10,000-12,000 (25-30%). He estimated maximal sustainable yield to be about 30%, which suggests that this population has been harvested fairly near its maximal rate. Some agency biologists suggest that this estimate of maximum sustainable yield might be high due to underestimation of the herd size and inclusion of
harvest from other herd units. From feedground classification counts, they suggest that maximum sustainable yield is nearer 20%. In either case, the harvest rate on this population is relatively high. In the northern herd, by comparison, the annual kill in recent years has been about 1,800 in a population of 16,000 (11%) (Yellowstone National Park 1997).
It is clear that in contrast with the northern herd, which is limited mainly by natural phenomena, the other herds using the GYA are limited mainly by human harvest. Thus, they are more stable from year to year in their likelihood of contact with cattle and with the consequent possibility of transmission of B. abortus. Essentially, they come out of the YNP area, pass through a hunting zone, and are intercepted by winter feeding areas. To the extent that feeding areas do not stop movement, elk are hazed to return to them from private lands. Although the other herds are more predictable from year to year, the sheer numbers of elk, their proximity to grazing allotments, cattle trailing areas, and private ranches, and their relatively higher seropositive rates means that the relative risk of transmission of B. abortus from elk to cattle is greater than for the northern herd elk.
Effects on Reproductive Potential
Two questions arise when considering whether B. abortus affects the reproductive potential of bison generally, and specifically bison in the GYA. The first question is whether B. abortus lowers the reproductive rate. That question would be consistent with the traditional use of the term potential in wildlife management, in which it is viewed as the maximal possible rate of reproduction (Leopold 1933). By that definition, the answer to the question is yes because any abortion or lowering of the probability of survival of offspring—the usual manifestation of brucellosis in bison, as in cattle and elk—would reduce the maximum. However, such a strict definition probably is not the most relevant in the context of brucellosis in bison in the GYA.
The second question deals with whether brucellosis affects the population dynamics of bison, and this is more relevant to the current issues in GYA. The question is whether brucellosis lowers reproductive performance sufficiently to constitute an important factor in population dynamics in bison and thereby alters the population trend over time.
Controlled research on the magnitude of brucellosis effects is lacking, but it can be estimated from the modeling results of Peterson et al. (1991b). They modeled bison populations (females only) under brucellosis-free and brucellosis-infected states. Their projections for a brucellosis-free population
can be used to estimate the impact of brucellosis on the growth rate of the GTNP bison population (69% seropositive for B. abortus) from the 1970 escape of five female founders to the total 1989 female population. This result can be derived from their Figure 7 (panel B). The annual growth rate projected by their model was 15.48%, whereas the realized rate was 14.45%, 1.03 percentage points lower. Simple models that assume infection rates between about 10% (GYA culture-positive rate) and about 50% (GYA seropositive rate) and loss of the first calf after infection show that reduction of population growth rate because of brucellosis would be only a few percent unless the survivorship of reproducing females were extremely low, an unlikely possibility for the hardy, long-lived bison.
Empirical results bear out that conclusion. Bison populations in YNP and GTNP (and herds elsewhere) have continued to increase despite being infected with B. abortus (Figure II-2) unless artificially controlled or reduced by severe winter conditions (Dobson and Meagher 1996). In YNP, artificial removal has been important in holding bison population growth to near zero at times, particularly from 1935 to 1965, when the herd was managed to number around 400, and in the past few years (Figure II-2). Among natural variables, winter mortality is clearly the most important, but production of forage in summer also might contribute to the dynamics of the herd (Meagher 1973). Only for a bison population in a marginal habitat where it would be barely capable of holding its own would brucellosis be the deciding factor in survival of the herd. YNP is not marginal habitat.
Elk, like bison, will suffer decline in potential elk population growth due to abortion. Although the data for elk show greater variance than those for bison, the persistent increase in numbers of elk after declines have resulted in brucellosis being considered unimportant as practical matter.
RISK OF TRANSMISSION
The risk of transmission is determined largely by the number of abortions that occur, the presence and survival of B. abortus in placental exudates, and the exposure of a susceptible host through an appropriate tissue barrier. Aborted placentae might contain as many as 1013B. abortus per gram of tissue (Davis et al. 1995). Direct evidence of transmission from various wildlife species to cattle has been difficult to establish. Despite circumstantial and epidemiologic evidence of transmission, many still believe that bovine brucellosis never has been proved to be linked to free-ranging elk or bison.
The detection of transmission of B. abortus from an infected animal to a
susceptible domestic cow is complicated by lack of clinical signs in infected cattle, geography, predation of the placenta and fetus, and birthing characteristics of the infected animal. Those and other factors complicate determination of risk of transmission. The perception of, for example, some members of animal-welfare groups, is that transmission is extremely rare and might never occur. The perception of others, such as some ranchers in or near the GYA, is that seroreactive cattle do appear in their herds and that those cattle have been infected with B. abortus from either elk or bison.
Bison to Cattle
Under natural conditions, the risk of transmission from bison to cattle is very low, but the appropriate quantitative risk assessments have not been done; one, by a multiagency group, is under way (E. Williams, U. Wyom., pers. commun., 1997). Free-ranging bison or elk might have served as the source of B. abortus infection in six cattle herds in the GYA (GYIBC 1997), but as noted earlier, the evidence is ambigous. Transmission of brucellosis from naturally infected captive bison to cattle has been reported; captive bison under range conditions in North Dakota were in contact with beef cattle during the winter (Flagg 1983). Bison-to-cattle transmission in Arkansas has also been reported.
The risk of transmission of B. abortus from infected bison to cattle is a major part of this study. Brucellosis has been transmitted from bison to cattle under experimental conditions, and brucellae were transmitted from infected bison to seronegative cattle when the animals were confined together in pens (Davis et al. 1990).
YNP bison herds have had little or no contact with outside bison since the early 1900s. Serologic surveys show seroprevalence rates of 20-73% (Rush 1932; Tunnicliff and Marsh 1935; Clark and Kopec 1985; Pac and Frey 1991; Aune and Schladweiler 1992; Aune et al. 1997). The number of abortions or fetal deaths per 100,000 bison births since brucellosis was first detected in 1917 is not known, and individual cases of transmission, especially in early periods, will likely never be determined. In the past decade, two cases of abortions due to B. abortus have been established (Rhyan et al. 1994).
Isolates of B. abortus obtained from bison have been shown to be pathogenic in cattle; for example, biovar 1 isolates from a Wood Buffalo National Park bison in Canada were virulent when inoculated into cattle (Forbes et al. 1996), even though the bison had been segregated from cattle for more than 60 years.
The current risk of transmission from YNP bison to cattle is low. Furthermore,
domestic cattle adjacent to the park are vaccinated, cattle are monitored by federal agencies, and ranchers are vigilant.
Elk to Cattle
Transmission of B. abortus from elk to cattle is unlikely in a natural setting. The ability of brucellae to be transmitted from elk to cattle under experimental conditions has been proved (Thorne et al. 1979), however, and cattle mingling with aborting elk on feeding grounds would be at high risk for infection. Elk densities in YNP reach those of the winter feeding grounds (p. 76, ''Other Elk Herds in the GYA") for short periods during some times of the year; although the incidence of brucellosis in these elk is very low, that might present another risk factor. Data on the incidence of elk-to-cattle transmissions might be skewed if ranchers are not forthright in admitting when cattle might have been exposed by commingling with infected elk.
Elk to Bison
Elk can transmit B. abortus to bison. Transmission is probably limited to aborting and parturition of infected elk with release of fetal membranes and genital exudates that contain large numbers of B. abortus . That has occurred during mixing of bison with infected elk on feeding grounds of the National Elk Refuge. M. Meyer (U. Calif., pers. commun., 1997) claims that the Jackson (GTNP) bison herd was brucellosis-free until it discovered the elk feed lines. The Jackson herd, which for 20 years was confined in a wildlife park and allegedly was brucellosis-free, escaped in 1968 and commingled with infected feeding-ground elk around 1980. The herd became infected (the seroprevalence in 35 bison collected in 1989-1990 was 77%) either by elk on the National Elk Refuge (NER) or by bison that were infected (although seronegative) when they escaped.
Transmission from elk to bison might have occurred under natural conditions in the GYA (Williams et al. 1993). If low infection rates are attained through management of bison, the population of bison will remain uninfected for quite some time before a low-probability elk-bison or bison-elk transmission would occur. During the brucellosis-eradication program in Custer State Park in South Dakota, elk, deer, and antelope mingled with infected bison; there is no evidence that bison from which brucellosis was eliminated were reinfected by B. abortus from elk (Gilsdorf 1997).
Bison to Elk
Whether and at what rate B. abortus is transmitted from bison to elk are unknown (S. Olsen, USDA, pers. commun., 1997). One group states that "although controlled or field studies have not been done to establish transmission between bison and elk, it certainly is possible" (T. Kreeger, Wyo. Game and Fish, pers. commun., 1997). Evidence of transmission of brucellosis among wildlife species comes from Elk Island, a fenced national park in Alberta, Canada, where bison were believed to have been the source of B. abortus infection in elk and probably moose (Corner and Connell 1958).
Elk as a Reinfection Pathway for Bison
Bison can contract B. abortus from elk, as demonstrated by the case cited above in which a clean herd of bison was introduced in 1970 to GTNP, later wintered on the elk feeding grounds of the NER, and tested positive for B. abortus in 1989. The risk of transmission to bison will depend on the success of efforts to reduce the infection rate in elk by vaccinating elk on feeding grounds and dispersing them over a larger wintering area in the southern GYA. If infection rates are not substantially reduced in elk, it seems inevitable that reinfection of bison will occur, just as bison are a continuing reinfection source for elk (Thorne et al. 1997). It must be remembered that low-probability events multiplied by large-enough animal contacts over a long-enough time become inevitable events. Apparent multiple transmissions between some combination of cattle, bison, and elk with the arrival of B. abortus in the GYA (Meagher and Meyer 1994; Thorne et al. 1997) should be a cautionary note, as should the occurrence of a case of undulant fever in an elk hunter in the northern range (where seropositive rates of elk are low), whereas no cases in hunters in the southern range (where seropositive rates are high) are known.
Early work with vaccination of elk at Greys River winter feeding ground resulted in promising reductions in seropositive rates (67% to 12%). In the winter of 1996-1997, however, the rate rebounded to 26%. The cause of the increase is unknown, but it could be related to the hard winter of 1996-1997, which would indicate that environmental stress, as well as pregnancy stress, can contribute to B. abortus infection rates. The reduction from 67% (1976) to 12% (1996) is significant, but inclusion of the 1996-1997 point results in lack of significance (P = 0.11). It is problematic that the first year in the time series (1976) was 17 years before the first of the consecutive data points
(1993-1997). Also, Smith and Roffe (1997) have questioned the validity of conclusions from the vaccination experiments on which the program is based. Alternatively, the field-vaccination program might be reaching the limits of its efficiency. For example, modeling of vaccination in bison (Peterson et al. 1991a) and cattle shows that reduction in seropositive rates amount to only 60-80% of baseline prevalence. That would predict that elk vaccinated on the feeding grounds would show a reduction in seroprevalence from 67% to about 13-27%, the approximate range observed in recent years. Further work will be necessary to evaluate the success of the program.
The source of the 1-2% seropositive rate in elk in the YNP northern range is potentially important. That seroprevalence might be, as has been proposed, the result of movement of elk from southern to northern ranges. But alternative explanations need to be considered, such as the infection of northern-range elk by contact with infected southern-range elk or YNP bison. The calving areas of southern-range elk, where birth and abortion increase the likelihood of transmission, are well south of YNP (Boyce 1989), and this casts doubt on that source of infection. If movement of southern-range elk to the northern range is not responsible for the seropositive rate in northern elk and if northern-range elk are not in close contact with southern-range elk, then the rate would seem to be natural infection due to contact of elk with infected bison. Because the potential of such transmission—either between bison and elk or between elk away from the winter feeding grounds, a key issue in sustainability of B. abortus in non-feeding-ground elk—is of particular interest, it is important to determine whether the seropositive elk in the northern range have moved from the southern range by marking them on the feeding grounds through feed or vaccination.
Several factors contribute to the likelihood of potentially infective contact of bison with elk. First, the distributions of the two species overlap broadly in the GYA on the summer range, where they are more dispersed, and on the winter range, where they are concentrated (Meagher 1973). Bison and elk are often seen near each other.
Second, their habitat requirements overlap broadly. In YNP, Singer and Norland (1996) found overlap of diet (1 = complete overlap, 0 = no overlap) to be 0.47 and 0.63, and use of vegetation 0.43 and 0.75, slope/aspect 0.45 and 0.57, and snow 0.59 and 0.89 for early and late periods. Those overlap values are high, and that they invariably were higher in the later years suggests that increases in density in both species are increasing the overlap of their use of the environment. Similar overlap between bison and elk was reported by McCullough (1980) for the National Bison Range in northwestern Montana and by Telfer and Cairns (1979) for Elk Island National Park, Alberta.
Thus, increases in potential contacts are not a simple function of numbers, but a function of the increased forcing of overlap of niche space as well.
Third, the probability of transmission of B. abortus needs to take into account a behavioral component. The movements of the two species are essentially independent. If encountering a site (such as a birth or abortion site) at which B. abortus might occur in the environment were random, the probability of transmission would be low by chance. However, birth and abortion sites are likely to attract both species. Bison and elk are highly olfactorily oriented. The observation of W. Cook (U. Wyoming, pers. commun., 1997) of attraction of elk and bison to noninfective bovine fetuses placed in the environment illustrates the point. Such attraction might occur across species as well. Reproductive fitness is a major component of natural selection (Fisher 1930), so it behooves individuals to be cognizant of each other's reproductive state. Bison—especially males—show substantial interest in matters associated with reproduction. Berger and Cunningham (1994) discuss these aspects in detail for bison. Elk and bison engage in flehman , a behavior most commonly associated with males testing the estrus state of females by licking the vulva or urine and exposing the molecules therein to the vomeronasal organ in the palate. Bison males have been reported as displaying "very aggressive behavior towards cows in estrus, or any blood discharge, death or injury" (S. Holland, as cited by Kearley 1996). They are especially animated by the occurrence of aseasonal estrus (S. Holland, state vet., S.D., pers. commun., 1997) or by blood at any time (J. Rhyan, APHIS, pers. commun., 1997), and the aseasonality of abortion might evoke similar interest.
Behavioral attraction to sites of abortion or birth, therefore, is likely to bring individuals into contact with potentially infective materials at a rate far greater than expected from random movements. That behavior is most prevalent in bulls, but it occurs to some extent in cows. That would increase the probability that bison, especially males with greater movements and sexual curiosity, would be infected by B. abortus shed by elk. In fact, in the bison herd observed by Holland, bulls were more likely than cows to become infected by cows. That also is true of the GYA. Bulls tested in the winterkilled sample leaving YNP had a 57% culture-positive rate compared with 24% for cows; for GTNP, bulls had an 84% seropositive rate compared with 69% for cows. Furthermore, the difference is already present in subadult males (Meyer and Meagher 1997).
The higher prevalence of brucellosis in bison bulls than cows is puzzling. The difference might arise from differential survival of offspring by sex. If birth to infected mothers or acquisition of B. abortus through the milk (see p.
23, "Shedding in Mammary Glands and Milk") were the source of infection, calves of both sexes would have similar infection rates unless there were differential abortion or calf mortality by sex. We assume that mortality is more likely among infected fetuses or calves than among uninfected ones. Abortion would have to be more prevalent for female than male fetuses to account for the differences. Ordinarily the reverse would be expected—the male is larger and places greater stress on the mother. Furthermore, abortion is thought to be relatively uncommon in YNP bison, so differential abortion by sex is not an expected source of the different infection rates. Differential mortality of male calves is not likely the cause, in that uninfected calves would have to have a higher mortality. The difference could be due to differential mortality of infected female calves, but this would invert the logic in explaining why female infective rates were low rather than why male rates were high. Although possible, this explanation seems at odds with the typical higher male calf mortality that results in a prevalence of females among adult bison. Higher seropositive rates in bison males are unlikely to be due to differential mortality by sex in calves.
A more likely possibility is that higher infection rates in males arise from differential behavior of males later in life. Male behavior that might contribute to infection includes naso-oral contact with genital exudates and urine during flehman to ascertain estrus, greater inclination to smell or lick afterbirth or aborted materials (even subadult males would be subject to this route of infection), and venereal transmission during coitus or contact in tending bonds, which because of the polygynous breeding behavior of bison brings each male into contact with multiple females.
It is notable that elk show the reverse condition: females have higher seropositive rates than males. Ordinarily, elk bulls are spatially segregated from females except during rut (McCullough 1969; Geist 1982). Like bison, elk bulls perform flehman during rut and mate with multiple females. They do not form tending bonds but instead guard harems; this lessens the period of close contact and reduces the number of males involved in copulation. For example, McCullough (1969) found that only 12% of male elk were important contributors to reproduction, whereas in Badlands National Park, 51% of 37 bison males 4-9 years old mated (J. Berger, U. Nev., pers. commun., 1997). In general, elk seem less alert to strange odors outside of rut than do bison. That means that male elk on the winter feeding grounds are less likely to be curious about abortions in the winter time than are females. All those factors suggest that bison bulls are more instrumental in transmission of B. abortus than are elk bulls. As noted earlier, the role of bison bulls in transmission of B. abortus presents an important gap in our knowledge.
Other GYA Wildlife to Cattle
Infection with B. abortus is self-limiting in many wild mammals. Brucellosis occurs rarely in deer, pronghorn antelope, and mountain sheep. Brucellosis has not been documented in those species in the GYA, and any infection in them would be inconsequential for the control of brucellosis in bison and elk populations. Natural infection with B. abortus in avian species has been reported (Angus et al. 1971) but plays no role in transmission to mammals.
Transmission to Humans in the GYA
Human infection with B. abortus in the GYA has been reported, and a woman elsewhere was reported as having aborted due to Brucella spp. Hunters consume bison meat from areas outside YNP, and some bison meat is given to tribal peoples and soup kitchens for needy people. Meyer reports that "in December 1991-February 1992 over 500 bison were shot and carcasses were eviscerated, largely by Indians who literally just mucked through the guts" (M. Meyer, pers. commun., 1997). However, no evidence that B. abortus infected those Indian populations has been reported.
The U.S. Centers for Disease Control and Prevention no longer requires reporting of undulant fever. The World Health Organization Laboratory Biosafety Manual places B. abortus in risk group III, indicating a high risk to persons involved in handling infected animals or tissues. All personnel involved in sampling should be formally advised of the risk of infection and trained in the handling of infectious tissue and the use of masks and equipment. Face masks, gloves, and protective clothing should be used in high-risk situations that involve female bison that have placental lesions of brucellosis or that have aborted.
The greatest risk of human infection lies in body contact with infectious material and transmission of microorganisms from hands to body orifices. B. abortus is typically present in low numbers in blood and lymphoid tissues of animals. Although most genital tissues in males and nonpregnant females have only low numbers of organisms, the infected placenta and its fluids are extremely hazardous and can contain up to 1013 bacteria per gram, a concentration that makes aerosol transmission possible. Blood and milk are hazardous, but infection from them is unlikely if reasonable means are used to prevent contamination of hands and face and thereby controlling the potential to spread microorganisms to body orifices. Pasteurization of milk eliminates the risk of infection from milk consumption.
Human brucellosis caused by B. suis has been well documented in people
who eat rangiferine animals (reindeer and caribou) in North America (Ferguson 1997), but no systematic study of brucellosis in American Indians has been done. Brucellosis was identified in Eskimos in Canada (Toshach 1963). In Alaska, 49 cases have been reported. It was suggested that rangiferine brucellosis is widely underreported because mild cases are not brought to medical attention and that chronic human cases might be undetected by small medical clinics.
Treatment of human brucellosis involves 4-6 weeks of antibiotic therapy, which carries the possibility of toxicity in some patients. Cure is not ensured, especially in chronic disease, which can be lifelong.
OTHER SPECIES OF BRUCELLA AND BRUCELLOSIS IN WILDLIFE
Species of Brucella other than B. abortus are associated with brucellosis in wildlife (Table II-1). Rangiferine brucellosis in commercial herds of reindeer
TABLE II-1. Species of Brucella
|
Bacterium |
Primary Hosts |
Wildlife Hosts |
Pathogenicity in Humans |
|
B. abortus |
Cow |
Bison, elk, wolf, coyote |
++ |
|
B. melitensis |
Goat, sheep |
Camel, wild ruminants |
+++ |
|
B. suis |
Biogroups 1 and 3: Pig; Biogroup 2: Pig and hare; Biogroup 4: Reindeer and caribou; Biogroup 5: Rodents |
Reindeer, pig, caribou |
+++ |
|
B. ovis |
Sheep |
Mountain goat |
– |
|
B. canis |
Dog |
? |
+ |
|
B. neotomae |
Woodrat |
Desert woodrat |
– |
|
B. sp (unnamed) |
Dolphin, seal |
Many marine mammals |
+ |
and in caribou (Rangifer tarandus) throughout North America is caused by B. suis biovar 4. B. suis in these animals has been shown to infect cattle. In one study, four of eight cattle penned with 14 naturally infected reindeer became infected with B. suis and were seropositive (Forbes and Tesaro 1993). Although it has not been reported, reindeer likely can transmit brucellosis to other wild mammals, and this could cause confusing serologic responses in bison (Forbes and Tessaro 1993).
A variety of rodents have been reported to be susceptible to infection and to develop disease (Moore and Schnurrenberger 1981), but rodents have not been implicated in the spread of brucellosis in the GYA, perhaps because of inadequate investigation.















































