4
Prevention of Vitamin A Deficiency
Barbara A. Underwood, Ph.D.
National Eye Institute
Major Health Consequences
Xerophthalmia and Nutritional Blindness
Vitamin A deficiency (VAD) affects ocular tissue in two ways: by slowing the regeneration of the visual pigments following exposure to bright light and by disrupting epithelial integrity. The inability to see well in dim illumination (night blindness) is a symptom recorded in ancient Egyptian, Greek, and Assyrian medical literature and, more recently, in the writings of European physicians. Epithelial defects in ocular tissue leading to blindness were described in dogs by Magendie and in humans by Budd in the early 1800s. They observed progressive deterioration from conjunctival xerosis to corneal xerosis, ulceration, and liquefaction (keratomalacia) as a consequence of restricted diets, devoid of what we now recognize as sources of vitamin A (Wolf, 1996). Manifestations of these distinct debilitating effects were thus recognized before McCollum's discovery of an essential nutrient, coined fat-soluble vitamin A, in the early 1900s (McCollum and Davies, 1913); description of tissue changes following deprivation of this nutrient (Wolbach and Howe, 1925); elucidation of its molecular role in vision (Wald, 1968); and the recent description of its role in the regulation of genetic expression (Kastner et al., 1994; Mangelsdorf et al., 1994).
The link in humans between clinically evident symptoms and signs and a faulty diet was suggested in about 1860 and subsequently confirmed in many societies (Guggenheim, 1981; Wolf, 1996). Cure was associated with certain foods—in early times with topical application or ingestion of animal and fish liver, and in later years with ingestion of plant foods containing green and yellow pigments (Wolf, 1996). McCollum and Davies (1913), followed shortly thereafter by Osborne and Mendel (1913), described the keratomalacia-preventing, growth-limiting, fat-soluble substances isolated from efficacious foods. These substances were later designated vitamin A and carotenoids.
Steenbock (1919) postulated, and later confirmed, that carotenoid from yellow maize could support growth and prevent ocular lesions by physiological conversion to biologically active vitamin A. Since Isler et al. (1947) discovered a cost-effective way to synthesize vitamin A, cure and prevention are also possible through commercially produced, synthetic vitamin A.
Childhood Morbidity and Mortality
Working at the University of Wisconsin, and later at Johns Hopkins University, McCollum pioneered the use of mice and rats in nutrition experiments. His studies of vitamin A deprived rat colonies—and those of others—were often hampered by early deaths from respiratory and diarrheal illnesses before ocular lesions occurred. These early deaths were partly attributable to loss of epithelial integrity in tissues throughout the bodies of VAD animals, and humans as well (Chytil, 1992; Hayes, 1971; Wolbach, 1937). Similar vitamin-A-deficiency-related morbidity and mortality in human populations were not clearly demonstrated, however, until the seminal community-based studies in the 1980s of Sommer and colleagues in Indonesia (summarized in Sommer and West, 1996). These studies clearly linked increased mortality risk in preschool-age children to vitamin A deficiency, a finding later confirmed among child populations in other countries in Asia and Africa where clinical eye signs occur (Beaton et al., 1993).
Where eye signs are not evident, biochemical deficiency—that is, subclinical deficiency—is also believed to contribute to mortality risk. In free-living populations, however, an unequivocal tie to the incidence of infectious morbidity has not been established. Severity once infection is acquired provides the probable link to mortality (Ghana VAST Study Team, 1993; Underwood and Arthur, 1996). This finding implies a role for vitamin A in immunocompetence, a role suggested by an extensive review of interactions of nutrition and infection published in 1968 (Scrimshaw et al., 1968). That review concluded that VAD showed synergism with almost every known infectious disease. Recent basic studies have been unraveling the complex molecular mechanisms by which vitamin A influences the immune system and alters cellular integrity (Ross and Stephensen, 1996). The combined effect on cellular integrity and immunocompetence is believed to contribute to an annual loss of approximately 1.12 to about 3 million lives of children under 5 years of age that otherwise could be salvaged by normalizing vitamin A status (Gillespie and Mason, 1994; Humphrey et al., 1992).
Other Health Consequences
Severe vitamin A deficiency in animal models is clearly linked to other adverse health effects. These include teratogenic-developmental consequences
(Armstrong et al., 1994), adverse reproductive performance (Takahashi et al., 1975), impaired growth (Anzano et al., 1979), and depressed iron utilization (Roodenburg et al., 1996). Except for an association with anemia (Suharno et al., 1992), similar consequences among free-living human populations are less clearly attributable to vitamin A status alone. This is because in community settings, confounding is likely from coexisting nutritional deficits and disease. Nonetheless, vitamin A deficiency is undoubtedly a contributor to adverse health effects similar to those confirmed in laboratory animals, although in human populations this vitamin may not be the most immediate causative nutrient.
Magnitude And Epidemiology Of The Problem
Defining Vitamin A Status
Conceptually, vitamin A status can be visualized as a continuum (see Figure 4-1) from the absent or minimal tissue stores associated with symptoms and signs of deficiency to the excess tissue deposits associated with toxic symptoms and signs (Bauernfeind, 1980; Olson, 1994). Between the extremes is a relatively large zone where status cannot be easily quantified by currently available techniques (Underwood and Olson, 1993).
In practice, the limited fetal stores provided from maternal circulation launches newborns, especially those with low birthweights (Chytil, 1992), into extrauterine life at the low end of the continuum of vitamin A status. That position may be rapidly augmented postnatally in infants fed vitamin A-rich colostrum and early breast milk (Chappell et al., 1985) or supplements (Humphrey et al., 1996). From birth onward, an infant's vitamin A status on the continuum may advance by small increments, be maintained, or deteriorate, depending on the balance between dietary intake relative to growth and development needs and to disease patterns that effect vitamin A economy. Breast-fed infants do not usually show clinical deficiency for at least 4 to 6 months after birth. They may be at a marginally adequate point on the continuum, however, if breast-fed by a malnourished, vitamin A-depleted mother (Underwood, 1994a). At the same time, if breast-fed, even from a malnourished mother whose breast milk vitamin A has been improved through direct maternal supplementation (200,000 IU of vitamin A given within 2 months postpartum [WHO/UNICEF/IVACG, in press]), adequate infant vitamin A status may be prolonged beyond 6 months (Stoltzfus et al., 1993).
Vitamin A requirements (see Figure 4-2), therefore, are greatest during periods of rapid growth—infancy and early childhood, adolescence, and pregnancy—and when the vitamin is lost from the body through normal physiologic processes, such as lactation, or through nonphysiological losses brought about by frequent disease, such as malabsorption, diarrhea, and febrile infections (FAO/WHO, 1988).
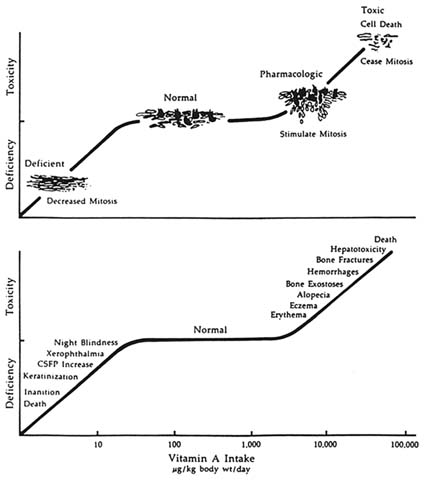
FIGURE 4-1 The logarithmic plot of vitamin A intake is depicted as a function of the biological response of man and animals in terms of deficiency, normalcy, and toxicity. The scheme at the top illustrates the response of a typical mucous epithelium, but is probably applicable to other undifferentiated blast-cell populations as well. The bottom curve indicates the clinical manifestations resulting from the altered cell function in deficiency and toxicity of vitamin A. SOURCE: Bauernfeind (1980), reproduced with permission.
Recognition of factors that influence vitamin A balance provides a foundation for understanding the epidemiology of VAD (Oomen et al., 1964; Tielsch and Sommer, 1994; Underwood, 1993).
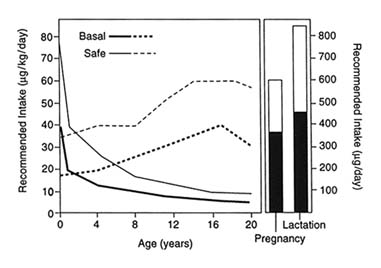
FIGURE 4-2 Recommended intake of vitamin A. SOURCE: Adapted from FAO/WHO (1988).
Extent of the Problem
Since the debilitating—and sometimes fatal—link of VAD to health is well-established, and effective and relatively inexpensive food sources and synthetic vitamin A are available for VAD prevention and control, why does a global public health problem persist? Clearly the fault lies in the application of insufficient or ineffective knowledge to the implementation of programs to rectify uneven resource distribution among and within affected populations. WHO estimated in 1995 that at least 3 million children exhibit xerophthalmia annually—they are clinically deficient and at risk of blindness. An additional 250 million children under 5 years of age are at risk of deficient vitamin A status (based on the prevalence of serum retinol distributions below 0.70 µmol/L); they are subclinically deficient, and at risk of severe morbidities and premature death (WHO, 1995a). These estimates do not include pregnant and lactating women who are in areas of endemic childhood VAD, and are thus likely to be in poor status, but for whom epidemiological data are quite limited. A high prevalence of maternal night blindness (Katz et al., 1995) and low breast milk levels of vitamin A (Newman, 1993) are reported in such areas. A lack of sensitive, survey-applicable, nonclinical indicators specific to VAD, however, has hampered population-based evaluation of status among reproductive-age women and other age and gender groups (WHO, 1996a).
Risk Factors
Age
Clinical and subclinical VAD are most prevalent in children 6 months through 5 years of age. This period is characterized by high requirements to support early rapid growth, the transition from breast-feeding to dependence on other dietary sources of the vitamin, and increased frequency of respiratory and gastrointestinal infections. Although growth rates decline sharply during infancy, decreasing the requirement for vitamin A per kilogram of body weight, the absolute quantity of the vitamin needed daily increases with growing total body mass (see Figure 4-2, based on FAO/WHO, 1988). If average dietary vitamin A intake from food progressively increases with body mass, body stores are likely to increase by small increments with advancing age. If diet is inadequate, and no vitamin A supplement is given, body reserves may only be maintained, or will decline if frequent disease, so prevalent among toddlers, tips the balance downward. How quickly the deficit can be restored depends on its magnitude and the repletion-rehabilitation program followed (see Figure 4-3).
FIGURE 4-3 Vitamin A status.
Gender
There is no consistent, clear indication in humans of a gender differential in the requirement for vitamin A during childhood. Growth rates—and presumably need for vitamin A—from birth to 10 years for boys are consistently higher than those for girls (WHO, 1995b). In the context of varied cultural and community settings, however, variations in gender-specific practices for the feeding and care of children are likely to subsume a small gender differential in the requirement to account for reported gender differentials in xerophthalmia prevalence. Pregnant and lactating women, of course, require additional vitamin A to support maternal and fetal tissue growth and lactation losses that are not endured by other postadolescent adults (NAS, FNB, IOM, 1990).
Quality of Diet
Dietary sources of biologically active vitamin A are found preformed in some animal foods or as provitamin carotenoids from plants. There is no specific human requirement for carotenoids apart from their potential conversion to biologically active retinoid. Preformed vitamin A is highly bioavailable, whereas the bioavailability of provitamin A carotenoids varies with the kind of plant source (Rodriguez-Amaya, 1997). The bioavailability of the provitamin A carotenoids from plants is greatly influenced by the nature of the embedding matrix (i.e., fibrous, dark green leafy vegetables [DGLV] or soft-fleshed yellow/orange vegetables and fruits) and the composition of the accompanying meal. Carotenoids, once released in the gastrointestinal tract from the embedding matrix, are only absorbed when fat is concurrently available. Dietary fat is needed to stimulate intestinal and pancreatic secretions. These secretions contain lipolytic enzymes for fat digestion, and phospholipids and bile salts needed for micelles to form and solubilize both preformed vitamin A (Blumhoff et al., 1991) and carotenoids (Erdman, 1988). Only micelle-solubilized carotenoids gain entrance to enterocytes where bioconversion to retinol, or intact transfer to chylomicra, occurs; that is, they become bioavailable.
Disease Occurrence
Infectious diseases contribute to vitamin A depletion. Enteric infections may alter absorptive-surface area, compete for absorption-binding sites, and increase urinary loss (Alvarez et al., 1995; Solomons and Keusch, 1981). Febrile systemic infections also increase urinary loss (Stephensen et al., 1994) and metabolic utilization rates. Disease is often associated with precipitating ocular signs in the presence of latent deficiency (Curtale et al., 1995; Feacham, 1987). Infection with the measles virus is especially devastating to vitamin A metabolism,
adversely interfering with both efficiencies of utilization and conservation (Hussey and Klein, 1990; Sommer and West, 1996). Severe protein-energy malnutrition (PEM) affects many aspects of vitamin A metabolism, and even when reserve retinyl-ester stores are adequate, it can prevent transport-protein synthesis, resulting in immobilization of existing vitamin A stores (Arroyave et al., 1967; Smith et al., 1973; Smith et al., 1975).
Seasonality
In endemic VAD areas, fluctuations in the incidence of VAD throughout the year reflect the balance between intake and need. Times of food shortage (particularly of vitamin A-rich foods), periods of peak incidence of common childhood infectious diseases (diarrheal, respiratory, and measles infections), and periodic seasonal growth spurts affect the balance.
Seasonal food availability can influence VAD prevalence in two ways. First, it directly influences access to provitamin A sources. Scarcity prevails in the hot, arid months and gluts are seen during harvest seasons—in the case of mangoes, for example (Marsh et al., 1995). Second, seasonal growth spurts in children frequently follow postharvest increases in energy and macronutrient intakes, usually from staple grains (such as rice) and tubers (light-colored yams, for example) that are not good sources of some of the micronutrients, including vitamin A, that are needed to support the growth spurt (Sinha and Bang, 1973).
Cultural Factors
Food habits and taboos often restrict consumption of potentially good food sources of vitamin A, such as mangoes and green leafy vegetables. Culture-specific practices in the feeding of children, adolescents, and pregnant and lactating women are common (Chen, 1972; Johns et al., 1992; Mele et al., 1991). Illness-related and pre- and postparturition proscription in the use of ''cold/hot" (yin/yang) foods pervade many traditional cultures (Mahadevan, 1961). Such influences alter shortand long-term food distribution within families that may only be detected by dietary intake surveys disaggregated by age and gender and/or in-depth focus group discussions (Kuhnlein and Pelto, 1997). Culture-specific information of this kind is pivotal to the design of food-based behavior change interventions.
Clustering
Epidemiological studies repeatedly report clustering of VAD, presumably because of the concurrent occurrence of several risk factors. This clustering may occur at several levels, from the national arena to neighborhoods and households
(Katz et al., 1993). Identifying the level at which clustering occurs is an important consideration in the selection, design, and targeting of VAD-control strategies.
Economic Costs Of VAD
The cost of VAD to society includes the burden of the prolonged management and care needed for such common childhood diseases as diarrhea and measles, and when deficiency is severe, provision for lifelong care of blinded victims. To illustrate the true global societal cost, Foster and Gilbert (1996) compared the cumulative disabled years in developing countries from childhood blindness with the total from unoperated cataract, the major cause of blindness after 50-60 years of age. The estimated 1.5 million blind children have a life expectancy of 50 years, equivalent to approximately 75 million years of disability. About 16 million older adults, with a much shorter life expectancy of 5 years, account for 80 million blind years. The years of economic burden to society from these two preventable causes of blindness are thus comparable, even though there is a tenfold difference in the number of individuals affected. Moreover, these costs do not account for the premature loss of life among the VAD-blinded, as well as among the subclinically VAD-deficient child population under 5 years of age. The real tragedy is that vitamin A-related childhood blindness—accounting for at least half of the total number of blinded children—can be treated or prevented (WHO, 1992), and subclinical VAD-related deaths can be substantially reduced (Beaton et al., 1993). VAD, therefore, is costly to the individual child in lost opportunity, and it has economic and social costs for the family, community, and nation as a whole.
Indicators Of VAD
Identification of Groups and Populations
A standardized classification system for xerophthalmia (clinically evident VAD) and universally accepted criteria for defining a public health problem were agreed upon in 1982 (WHO et al., 1982). These criteria (see Table 4-1) remain appropriate for identifying populations at high risk of vitamin A-related, blinding malnutrition—populations to the far left of the vitamin A status continuum (Figure 4-1). They are inadequate, however, for identifying populations with subclinical deficiency—tissue concentrations of vitamin A low enough to have adverse health consequences, even in the absence of xerophthalmia, WHO's current definition of VAD (WHO, 1996a).
TABLE 4-1 Biological Indicators of Clinical Vitamin A Deficiency: Xerophthalmiaa in Children 6–71 Months of Age
|
Indicator |
Minimum Prevalence (%) |
|
Night blindness in children 24–71 months of age (XN) |
> 1.0 |
|
Conjunctival xerosis/with Bitot's spot (X1B) |
> 0.5 |
|
Corneal xerosis/ulceration/keratomalacia (X2, X3A, X3B) |
> 0.01 |
|
Corneal scarsb (XS) |
> 0.05 |
|
NOTE: Prevalence of any one or more of the indicators indicates a public health problem. a In addition, a serum level of vitamin A (retinol) has been used with the clinical classification to provide supportive evidence of an important problem. A prevalence of > 5 percent of serum levels < 0.35 µmol/l is strong corroborative evidence of any clinical criteria met to identify an urgent public health problem. b Lack of a history of traumatic eye injury or use of topical traditional medicines increases the specificity of this VAD indicator. |
|
Unfortunately, there is no practical, single indicator of adequate specificity and sensitivity to detect subclinical deficiency under community conditions—that is, populations in the intermediate left portion of the vitamin A-status continuum (see Figure 4-1). For this reason, WHO recommends that two or more indicators be used, at least one of which is biological and below the agreed upon cutoff points provided in Table 4-2.
Where it is not possible to obtain two biological indicators, WHO suggests that one such indicator should be supported by a composite of at least four of the indirect demographic and ecological risk factors given in Tables 4-3A and 4-3B. Two of the four indirect indicators should be related to nutrition and diet (Table 4-3A). Socioeconomic indicators (Table 4-3C) are also useful qualitative indicators of the characteristics of high-risk populations. The cutoff values suggested in Table 4-3 resulted from the reflections of a WHO-sponsored consultation of experts. The group pointed out the need for additional confirmation of the utility of the values and suggested prevalence cutoffs. These ecological indicators reflect a context of dietary inadequacy and social and economic deprivation that have been associated with endemic VAD through epidemiological investigations (Sommer and West, 1996). Their usefulness is in identifying high-risk areas and populations, not individuals. Biological indicators are needed to confirm that a significant public health problem exists.
Monitoring Intervention Impact and Outcome
Appropriate indicators in the monitoring of intervention impact will vary in accordance with the intervention objective. For example, program objectives
may be to improve coverage of recipients of vitamin A supplements; to ensure that a vitamin A-fortified food meets quality-assurance standards or is selected for consumption by target groups; to cause a change in food-consumption behavior, such as the frequency of consumption of DGLV; or to increase the year-round availability of vitamin A-rich food in household or community gardens. The appropriate intervention-specific impact indicator(s) for each of these objectives will differ; in some cases process indicators will be used, in others, biological indicators are appropriate (Table 4-3). If the desired outcome of the intervention is to document a change in the vitamin A status of the recipient population, the biological indicators in Tables 4-1 and 4-2 are appropriate.
Resource availability can limit the feasibility of direct biological evaluations because these indicators are usually more costly to obtain and evaluate than indirect indicator data. In such situations, outcomes derived from metabolic and/or controlled community studies lend credence to causative inferences from similar outcomes of interventions implemented in less rigorously controlled community studies. The inability to perform biological evaluations alone should not prevent initiation of, or stop, VAD control programs when and where such programs are needed.
TABLE 4-2 Biological Indicators of Subclinical Vitamin A Deficiency in Children 6–71 Months of Age (percent)
|
Indicator (cut-off) |
Prevalence Below Cutoffs to Define a Public Health Problem and Its Level of Importance |
||
|
|
Mild |
Moderate |
Severe |
|
Functional |
|
|
|
|
Night blindness (present at 24–71 months) |
>0 to <1 |
>1 to <5 |
>5 |
|
Biochemical |
|
|
|
|
Serum retinol (£0.70 µmol/l) |
>0 to <10 |
>10 to <20 |
>20 |
|
Breast milk retinol (£1.05 µmol/l) |
<10 |
>10 to <25 |
>25 |
|
RDR ≤20%) |
<20 |
>20 to <30 |
>30 |
|
MRDR (ratio ≤ 0.06) |
<20 |
>20 to <30 |
>30 |
|
+ S30DR (≤20%) |
<20 |
>20 to <30 |
>30 |
|
Histological |
|
|
|
|
CIC/ICT (abnormal) |
<20 |
>20 to <40 |
>40 |
TABLE 4-3A Ecological Indicators of Areas and Populations at Risk of VAD: Nutrition and Diet-Related Indicators
TABLE 4-3B Illness-Related Indicators in Children 6–71 Months of Age
TABLE 4-3C Socioeconomic Indicators
|
Indicator |
|
Levels of maternal education and literacy |
|
Income/employment |
|
Water supply and level of sanitation |
|
Access to health and social services |
|
Access to land |
|
Access to agricultural services/inputs |
Critical Elements For Successful Nutrition Intervention Programs
Characteristics of successful community nutrition programs were reviewed in 1989 by the International Nutrition Planners Forum (International Nutrition Planners, 1990). Critical elements were identified within six categories: (1) political commitment; (2) community mobilization and participation; (3) human resources development, such as training, retraining, and supervision; (4) targeting; (5) monitoring, evaluation, and management information systems; and (6) replicability and sustainability. These general criteria, as well as additional factors found to be specific to the vitamin A context, were the framework used to judge the vitamin A control programs reviewed for this paper.
This paper also draws upon the 1994 report of the United Nations Subcommittee on Nutrition (SCN) of the Administrative Committee on Coordination (ACC). Impact evaluations of about 46 trials and large-scale programs to prevent VAD were summarized (Gillespie and Mason, 1994). That evaluation of some programs is updated here, and the information extended to new program evaluations. Only a few specific, successful interventions are highlighted in detail to illustrate the elements associated with success or failure in a given context. Other programs are referenced briefly, as appropriate, to corroborate elements associated with success and that transcend a specific context. No attempt is made to comprehensively review or reference all intervention programs or to provide in-depth detail. References are provided to original reports or recent reviews so readers can find the missing details they require.
Approaches To The Prevention Or Correction Of VAD
Vitamin A intervention approaches are commonly grouped into two main control strategies: (1) direct increase in vitamin A intake through dietary modification with natural or fortified foods and supplements and (2) indirect public health measures to control disease frequency. Information, education, and communication (IEC), including social marketing and specific vitamin A-oriented nutrition education, may or may not accompany each of the above interventions. Fortification is a food-based approach, but for clarity in this paper, it is considered separately from other food-based approaches. Vitamin A supplementation is also considered separately. Public health disease control measures are only briefly acknowledged because those interventions are not the primary focus of this review, except as they complement direct VAD-control strategies. Apart from controlled research projects (for example, with intervention and matched control communities), one can seldom evaluate the "success" of a single community-based intervention implemented over time. The presence
of other national and community development programs with variable coverage and impact on target populations that overlap with vitamin A-specific interventions is customary. Two examples include national economic development and community poverty alleviation schemes and increased measles immunization coverage in populations also given periodic vitamin A supplements.
Food-Based Approaches
As noted earlier, VAD as a public health problem is the result of a faulty diet that supplies inadequate bioavailable sources of vitamin A. The immediate causative factors vary among societies, but include limited availability (for economic or other access reasons); cultural taboos and/or lack of knowledge that leads to inappropriate food practices (particularly in feeding children and pregnant and lactating women); frequent illness affecting the efficiency of absorption and utilization of nutrients, as well as appetite; lack of sufficient dietary fat to facilitate absorption, especially of carotenoids; and food processing, storage, and preparation practices that cause excessive losses. To design successful food-based prevention or correction approaches, one must consider the relative importance of causal factors and the resources available—or that can be generated—to ameliorate them within the specific local context for implementation (Kuhnlein et al., 1996).
Dietary Modification: Where Food Sources of Vitamin A Are Available but Underutilized
It is ironic that VAD prevalence among preschool-age children is high in many societies where provitamin A carotenoid sources are abundant. In these circumstances, behavior modification through dietary counseling and nutrition education is clearly the logical choice. Yet the general consensus is that these approaches have been ineffective in bringing about significant, sustained, communitywide behavior changes in food consumption patterns. An extensive review of the effectiveness of strategies used to deliver nutrition education—not what the nutrition science content was—of 217 well-designed and carefully evaluated experiences in the United States concluded that nutrition education "works" when behavior change is the objective and the intervention is designed to achieve that goal, not just to transfer knowledge or change attitudes (Contento et al., 1996). Market research methodology used in the private sector to achieve consumer behavior modification—social behavior marketing or social mobilization—is now being applied in international settings in the public sector to achieve the socially desirable, health-linked behavioral goal of improved nutritional status (Parlato, et al., 1992; Seidel, 1996). Smitasiri (1994) suggests that earlier ineffectiveness in changing food behaviors in resource-poor communities
through nutrition education may relate to the lack of a systematic analysis of the local situation. Such analyses could have led to the design of appropriate interventions that are oriented toward community action, embedded in the prevailing culture, and incorporate the necessary supportive social, political, and organizational structures required for sustainability.
Thailand's Experience in Applying a Social Marketing Methodology to Increase the Utilization of Locally Available Vitamin A-Rich Foods*
Context
VAD among preschoolers in north and northeast Thailand is largely a subclinical problem, potentially controllable through locally available, inexpensive foods. The area is noted for its poor economic and environmental conditions. Although highly bioavailable animal sources of vitamin A are present, they are expensive, and fruit sources of provitamin A carotenoids (mango) are highly seasonal. Among locally available vegetables, ivy gourd (a variety of vitamin Arich DGLV) is common throughout the year, inexpensive, culturally acceptable, but underutilized because it is held in low esteem.
Design of the Intervention
A situational analysis, including formative research, was conducted with the active participation of local politicians; academicians in health, nutrition, and the social sciences; and the proposed recipient community (including representative mothers). After collectively considering the results of the analysis, a strategy was planned that incorporated active, multidisciplinary community involvement. The design agreed upon fit into the larger community development strategy. It promoted consumption of vitamin A-rich foods and the use of fat/oil in their preparation, and gave particular emphasis to increasing the production and consumption of ivy gourd through an intensive social marketing (mobilization) effort.
Qualitative and quantitative impact monitoring (process) and outcome (biological) evaluations were integral parts of the program design. The intervention plan was implemented only after systematic pretesting and appropriate adjustment to the local context. Iterative, cyclical monitoring throughout the implementation phase allowed the accumulating experience to serve as a guide in adjusting future actions. Promotional activities were embedded in the social activities and organizational structures of the community—school and community
gardens were used for production; recipes and menus appropriate for school lunch programs and family meals were developed; communitywide promotional materials and activities were planned with the active involvement of political, educational, health, and private sector leaders; and the "ivy-gourd man" (a clown clothed to represent an ivy gourd leaf) participated in local social events that attracted all ages and community groups. Regional radio and television spots and a promotional song recorded by popular Thai singers reinforced local activities and spread the message to an expanded audience. Activities related to the program were identified by a prominently displayed program logo—the ivy gourd.
Implementation and Evaluation
The intervention phase was in effect for two years before the program was evaluated. This evaluation focused on evidence of increased utilization of ivy gourd by the community, particularly among the preschoolers and mothers who had been targeted for behavior change. Measures of pre- and postintervention knowledge, attitudes, and reported food consumption behavior (KARB), in addition to 24-hour dietary recall evaluations, were obtained for both the ivy gourd and fat, particularly for women and preschool-age children. Vitamin A status was monitored through changed prevalence of ocular symptoms and low serum retinol values in children. Evaluations were done by university teams that worked independently of the project implementation team; knowledge, attitudes, and practices (KAP) evaluations were performed by the Faculty of Social Sciences and Humanities, anthropological evaluations were done by the Institute for Population and Social Research, and changes in vitamin A status were tracked by the Institute of Nutrition's Division of Community Nutrition.
Results
Statistically significant improvement in KARB occurred in the consumption of vitamin A-rich foods and the use of fat/oil in their preparation, particularly relative to ivy gourd. These changes penetrated from provincial officials through the district, subdistrict, and community levels, to reach the targeted audience of mothers and children. The dietary evaluation (and indirect indicator of vitamin A status) also showed an increased consumption of vitamin A-rich foods, including ivy gourd and fat/oil among vulnerable groups, with the exception of infants. A decreased prevalence of ocular signs of VAD (night blindness) was recorded, but no notable improvement was detected in serum retinol levels (project personnel noted that technical problems encountered in storage and delayed analysis of serum samples may have invalidated comparisons between intervention and control areas).
The following project elements were associated with success:
- A combined mass media and interpersonal education and communication approach was utilized to achieve broad and communitywide coverage, while at the same time achieving specific coverage of the targeted groups.
- Messages were prepared from a multidisciplinary perspective that accommodated local resources and culture in both message construction and dissemination.
- Image enhancement was used to broaden social acceptance and consumption of an underutilized, locally available, affordable, familiar food product.
- Behaviors were embedded in community structures—for example, schools and social activities.
- Backup problem-solving and supply-availability support was sought from local resources, including agricultural extension services, for production questions, availability of seedlings, pest control, and fertilizer.
- A sense of community ownership was created through community participation in the design and management of parts of the project.
- Participation of community political and business leaders assured a strategy that was consistent with the existing community development plan and with the larger development policy of the country (this enhanced political acceptance through the political levels, from community to national).
- Critical elements, thought to be indispensable for replicability and sustainability for the continuation of gains beyond the project's life, were built into the program (only revisiting the project area, however, will determine if successful intervention components have been replicated in other projects and desired activities and behaviors sustained).
The constraints encountered included:
- Sustained interest and participation continued as long as the program was active, but waned when the social marketing activities were less intense.
- The cost of the intensive social marketing could not be sustained by the community, although there was evidence that many of the nutrition and health concepts had been internalized and the community had continued the required behaviors beyond the project period.
- Replicability of the project as designed depends on the availability of funds to launch comparable intensive efforts in other communities or the ability to scale down the effort to a level that localities can afford.
Other Countries' Experiences
Social marketing projects in Indonesia, Bangladesh, and the Philippines were also evaluated as successful in increasing consumption of available vitamin A-rich foods. Unlike Thailand, each of these countries also has extensive programs for periodic distribution of encapsulated vitamin A supplements (VAC) because xerophthalmia is—or has been, in Indonesia—a public health problem. Large-scale social marketing projects were undertaken in each country to change attitudes and behaviors constraining consumption of increased quantities of vitamin A-rich food and capsule coverage (Favin and Griffiths, 1991; Pollard and Favin, 1996). Clinical examinations were done to detect changes in xerophthalmia prevalence, but biological evaluations, such as serum retinol, were not included.
Improvement in attitudes and behaviors concerning the consumption of vitamin A-rich foods was demonstrated in all sites. Social marketing programs in Bangladesh, however, were ineffective in increasing VAC coverage (Ali et al., 1993), but successful in Indonesia (Reis et al., 1996). The key components of success in increasing the consumption of vitamin A-rich foods were similar in all three sites and like those found in the Thailand project:
- The development of intervention and message strategies was based entirely on local consumer attitudes, practices, and behavior.
- Messages of product-image definition were used to target specific DGLVs and to reposition their image; that is, the value of DGLVs expanded from eye health to general health.
- Creative solutions were added to overcome defined and targeted local resistance points.
- A media mix of mass (to broaden coverage) and interpersonal (to reinforce sustained behavior change) communications was used, with some emphasis on application at point-of-sale (the markets where most women buy their DGLVs).
A major difference from the Thailand project was that community participation—embedding—was not emphasized. It was viewed as too time-consuming for the ''relatively short periods of donor funding." Also, multiple DGLVs were promoted, rather than a single product. The sustainability of desired change in food behavior beyond the period of donor support has yet to be documented.
Based on the lessons learned in pilot projects, Indonesia has scaled up its social marketing strategies targeted both toward improved VAC coverage and increased consumption of vitamin A-rich foods (Shaw and Green, 1996). Internally supported national, provincial, and community mass media strategies are being implemented to broaden audience coverage. National nongovernmental
organizations (NGOs) (women's groups) complement the mass media strategies to attain the interpersonal contact through home visits that is needed to reinforce desired modifications in behavior and to contact hard-to-reach and high-risk populations (such as poor urban residents and those not attending local health posts).
Other Social Marketing Experiences
Features of seven nutrition communication programs are summarized in a recent IVACG publication (IVACG, 1992). Applications in Brazil, India, Mauritania, and Nepal are added to some of the projects noted above. Vitamin A intervention programs carried out in this contextual diversity almost universally showed that applying a social marketing methodology in the development of nutrition education messages and other communication strategies can quite rapidly (in 18–24 months) modify attitudes and food behaviors of vulnerable groups to increase their vitamin A intake from familiar, available, underutilized DGLVs. The exception in most targeted social behavior modification projects was 6- to 12-month-old infants; resistance to feeding them DGLVs persisted. When focus group discussions indicate such resistance in a given population, social marketing should consider alternative, locally available vitamin A-rich foods—such as yellow fruits and orange vegetables—for this age group.
The primary constraint to replicability and sustainability of social marketing strategies in most projects is cost. Caruaru, Brazil, was a notable exception. In Caruaru, dissemination of the communication effort was confined to biannual periods when supplement distribution took place, and the communication package was developed using affordable local resources (IVACG, 1992). Indonesia also has decentralized parts of its social marketing strategy to local agencies, increasing affordability, commitment, and autonomy. In Bangladesh, a low-cost, locally developed and implemented nutrition intervention and educational and motivational project was also effective in reducing night blindness in an 18-month period (Yusuf and Islam, 1994).
Lessons Learned in Behavior Modification Where Vitamin A-Rich Foods Are Available
- A social marketing strategy using a multimedia communication mix is essential. Mass media is necessary to achieve broad audience coverage and interpersonal contact is needed to reinforce desired behavior change in targeted audiences. At least in part, the strategy needs to be decentralized—and affordable—to the lowest effective administrative unit.
- The development and delivery of media materials should be founded on local perceptions and resource availability.
- Political, public, and private sector commitment and ownership from the national to the local level is needed for sustainability. External financial and technical assistance may facilitate start-up of intervention activities, but the programs should not depend on such aid for their continuation.
- Community-level monitoring provides intermittent process feedback and the flexibility to meet changing situations. Periodic repositioning of components within the strategy will be required to ensure forward progress toward stated objectives.
Dietary Modification: Home and Community Provisioning to Increase Availability of Vitamin A-Rich Foods
Home and community gardening has been promoted for many years to control nutritional deficiencies at the household level through increased availability of nutrient-rich foods (see UNU Food and Nutrition Bulletin, 1985, for examples). This approach has special appeal in meeting family vitamin A—as well as multiple micronutrient—needs. In theory, these needs can be met by locally familiar, low-cost, provitamin A-rich vegetables and fruits that can be produced on small land areas with manageable time commitments. Needed resources can be developed (for example, seed banks and nurseries) and sustained by communities with little use of outside expertise. By its nature, this intervention necessitates a high degree of household and community involvement. An adequate water supply, however, can be a major constraint to initiating horticulture activities, render them highly seasonal, or restrict them to limited geographic areas (Brownrigg, 1985).
Until recently, few gardening projects were evaluated for biological effectiveness; of those few, changes in nutritional status were rarely demonstrated. This failure to document desired biological outcomes was attributed to the preference of farmers—even women farmers—to sell what they produced and spend only a small portion of the income earned for food (Brun et al., 1989; Florentino et al., 1993; Marsh et al. 1995). Recent large-scale homestead garden projects were designed to address barriers to achieving biologically effective programs. They have incorporated a communication and social marketing strategy designed to modify behaviors in household management of garden products. The strategy is to increase production goals to allow limited sale, as well as to encourage increased consumption. The success of these revamped projects is documented by improved health and nutritional status outcomes, as well as by measurement of the more usual production, KAP, and indicators of the impact of dietary intake programs. Examples from some recent successful projects in Bangladesh, India, and Vietnam are briefly reviewed.
Experiences in Bangladesh with Promotion of Home Gardens
Context. Xerophthalmia is highly prevalent in Bangladesh, and prevalence has not decreased substantially, even with the program of the biannual distribution of high-dose supplements that has been in place since 1973. In recent years, two large gardening projects have been undertaken, one sponsored through NGOs with the overall assistance of Helen Keller International (HKI), and one project under the sponsorship of World-view International Foundation (WIF). Very significant elements of both projects are a focus on women; the use of community agricultural extension expertise to provide low-cost gardening techniques and resources; and innovative, locally adapted IEC (Ali et al., 1993; Greiner and Mitra, 1996). In addition, both projects developed information systems for systematic monitoring and evaluation. The HKI information system was developed for continuous, interactive, community-managed monitoring.
Results. Both projects demonstrated increased consumption of several kinds of vitamin A-rich vegetables and fruits produced in the home and community gardens (Bloem, 1996; Greiner and Mitra, 1996). The prevalence of night blindness was reduced from baseline values. Benefits were directly associated with the number of varieties of vitamin A-rich foods promoted. In addition, both projects demonstrated replicability by successful expansion into new communities. Focusing efforts on women had important "gender-empowering" effects, and even though women sold part of the crops produced, their households benefited through increased food security, greater income, and healthier children (Marsh et al., 1995). Long-term sustainability remains to be demonstrated, but the critical elements needed for this to occur, including NGO backup in implementation and commitment of the national government, are thought to be present (van der Haar, 1992). HKI is now focusing efforts to implement a substantially larger project that includes the expanded involvement of local NGOs in management to reduce costs and to favor community self-sufficiency (Marsh et al., 1995).
West Bengal, India: Experience with Horticultural Interventions in a Drought-Prone and Poverty-Stricken Rural Area*
Context and Design. Committees at the state, district, block, and village levels provide guidance, coordination, and implementation. They have been extensively involved in the program from the initiation of a project to introduce home gardens and nutrition education in this socially, economically, and environmentally deprived area of India (FAO, 1996). Formative research provided
the basis for the nutrition education components. Field implementers were selected from local areas and trained in nursery development, home gardening, and food storage and preservation. One feature of the project was the involvement of academicians from the local university, who addressed the practical nutritional aspects of home gardening, such as modification in home-cooking practices for improved nutrient preservation. A variety of DGL Vs and yellow fruits were promoted and supported through local nurseries.
Results. After 18 months, project evaluation documented improved KAP, greater consumption of DGL Vs and some fruits (papaya), and a reduction of xerophthalmia, all indicators of at least short-term success. It is too soon to determine if the favorable changes demonstrated in the short follow-up period will be sustained, particularly those of attitude and practice regarding feeding DGL Vs to children. It is significant that plans for expansion are being discussed by local and state politicians, who were encouraged by the evaluation results. (This is an indication of the importance of evaluating interventions in influencing political decisions.) Key elements of success were similar to the Bangladesh experience and included the following:
- Central nurseries for high-quality seeds and saplings were established. They were controlled and managed by the community.
- The community participated at all levels and in all aspects of the project.
- Health and nutrition education was designed for relevance to the local context and disseminated by trained local change-agents.
(Note is made that similar results were achieved in the drought-prone, poverty-stricken area of Nigher, where social marketing was a strategic part of gardening promotion [Parlato and Gottert, 1996]).
Vietnam Gardening Project to Increase Production and Consumption of Vitamin A-Rich Foods*
Context and Design. The project was carried out in poor communes in four provinces with known or suspected nutrition and/or vitamin A problems. The provinces represented four distinct agroecological zones of Vietnam. There were five main components of the project: (1) nutrition education; (2) promotion for production of some specific food crops and of nursery garden development; (3) monitoring and evaluation of activities; (4) training and capacity building; and (5) upgrading food analysis capability. Project activities were implemented at the commune level through a network of trained volunteer-educators,
with backup assistance in IEC and evaluation from international experts.
Results. After 2 years of implementation, success in achieving stated objectives was documented in four of the five main project components; the least success was registered in the food analysis component. Success was documented by both process indicators, reflective of improved household food security and nutrition (FAO, 1995), and biological indicators of health and nutrition, which demonstrated decreased xerophthalmia and morbidity from acute respiratory infection and diarrheal disease (English et al., 1996).
This is one of the few gardening projects that attempted to document health benefits by monitoring morbidity outcomes. It is an important demonstration that health benefits accrue from successful gardening projects that are associated with increased micronutrient-rich food consumption. Skeptics have only had information from poorly evaluated gardening projects or from controlled DGLV-feeding projects (e.g., de Pee et al., 1995) that failed to demonstrate changes in relatively insensitive biological indicators of incremental changes in nutritional status (see discussion earlier in this paper). These indicators were sometimes used to assess changed vitamin A nutriture in populations in which mean preproject nutriture was not deficient (Brown et al., 1989; Bulux et al., 1994), or in which other potential confounding factors exist (de Pee et al., 1995; Solomons and Bulux, 1993). Nevertheless, it is impractical, expensive, and unnecessary for future large-scale gardening interventions to use morbidity indicators for project evaluation.
The success and feasibility of the Vietnam project has encouraged efforts for national expansion. The expansion design will strengthen critical elements identified in the original project and add elements that had been identified as promoting sustainability. These elements include: (1) training a cadre of indigenous trainers; (2) revolving credit and income-generating schemes; (3) locally available resources for special nutritional rehabilitation of malnourished children identified in the intervention area; (4) community-based monitoring and evaluation of the program; (5) strengthening community nursery gardens; and (6) strengthening the primary health care (PHC) role of the volunteer educators and their links with PHC centers. (It should be noted that at the expansion stage, few other horticultural projects have planned such an integrated, cross-sector-linked approach.)
Other Gardening Projects. In the Philippines and in some Central American countries, household gardening is traditional. In such contexts, it is noteworthy that social marketing strategies may still be needed to sustain interest and assure benefits to targeted land- and resource-poor families (in the Philippines, see Florentino et al., 1993) or to improve cost-efficient operations to
maximize household food-security benefits (in Honduras and Nicaragua, see Marsh, 1995).
Lessons Learned from Successful Gardening Projects
- Advocacy is needed at all levels to increase awareness of affordability, feasibility, and potential household-specific benefits from micronutrient-rich gardening.
- The availability of resource support, education, and training for low-cost gardening close to community operations is critical.
- Commitment of local NGOs and other local private and government technical resources and monetary assets are needed before expanding projects at the national level.
- A focus on women in all aspects of garden management and training in product use, including nutrition training, enhances empowerment and decisionmaking that maximize household food security and child-health benefits.
- A simple information-gathering system is needed for systematic community monitoring to resolve, in a timely manner, the ongoing and evolving problems that otherwise could constrain progress and limit gardening success.
Small Animal Husbandry and Fish Production to Increase Household and Community Availability of Preformed Vitamin A Sources
Attempts have been made to foster small-scale animal husbandry and fish production as a means of improving household dietary quality, including vitamin A nutrition. This was one of the thrusts of the applied nutrition programs of the 1960s and 1970s. These programs were short-lived in many areas, generally for reasons that pertained to a lack of resources to discourage poachers and predators (for example, to manage snake infestation in local fish ponds) and assure consumption of the products by poor, high-risk households. In Asia, fresh fish and shellfish, as well as dried whole fish, are available in local markets. When affordable, they are common recipe components (Philippines, National Nutrition Council, 1995). Fish are also to be found—even by the poor and landless—along unprotected rivers, and are available seasonally in tropical areas when monsoon rains overflow rivers and carry fish into the rice fields. Although fish flesh is not a rich source of vitamin A, fish liver is a concentrated source. In societies where fish is culturally acceptable and available, eating small fish whole can significantly contribute to vitamin A intake. Small fish can be cultured in household or community fish ponds. Again, the major constraint to consumption of homestead-produced animal products by the poor is their monetary value, which favors selling over consumption.
In Thailand, where communities are emerging into moderate affluence, promotion of household and community rearing of chickens and ducks has been successful and has the potential for expansion (Wasantwisut et al., 1995). These programs, however, should be accompanied by appropriate social marketing to facilitate household consumption of at least some of the vitamin A-rich products—liver and egg yolk, for example—by at-risk groups. Links are possible between rearing and small-scale processing efforts by local entrepreneurs for production of inexpensive, vitamin A-rich by-products, such as liver chips, that are readily accepted for child feeding. In several Asian countries, chips from many different products are traditional, inexpensive snacks that are regularly purchased by children from street vendors. These items and similar community-generated products could be linked, for example, to school feeding programs in poor villages in order to stimulate local agricultural and economic development. Such an initiative is planned by the government of Indonesia (Soekirman and Jalal, 1996).
In Central America, poultry husbandry, as well as some intermediate and large animal husbandry—when affordable, considering that they demand greater feed and health care inputs—are added to traditional gardens, providing food products for household consumption (such as eggs, meat, and milk), as well as marketable products (Marsh et al., 1995).
To summarize, successful efforts to adjust food consumption and production behaviors require social marketing methodology to strengthen behaviors favorable to good feeding practices in vulnerable groups. As noted above, modifying maternal behaviors in a baby's first year of life to feed the child DGLVs has not consistently yielded to social marketing techniques. Acceptable alternatives for this high-risk group should therefore be considered. For example, where available, small additions of red palm oil (Rukmini, 1994) or buriti (a traditional, beta-carotene-dense local crop in the Amazon region; see Mariath et al., 1989) to carbohydrate-rich paps and gruels can top off breast milk's vitamin A contribution toward meeting vitamin A needs in late infancy.
Multi-Mix Complementary and Weaning Foods
The significance of micronutrients, particularly beta-carotene, in complementary and weaning foods was recognized two decades ago (Graham et al., 1981), but it has captured the important critical attention it deserves only recently (Brown et al., 1996). Past emphasis was on adding vitamin and mineral premixes to cereal-legume mixtures. This is a viable approach when mixtures are centrally processed (Hofvander and Underwood, 1987), but few efforts to produce nutritionally adequate, safe, affordable multimixes at the community, national, or international levels have been sustained. INCAPARINA, developed and marketed in Guatemala, is one notable exception; there are a few others. Lessons learned from past failures in these ventures should provide cautious
guidance to future centralized efforts—private or public—to produce safe processed products that the poor can afford (Motarjemi et al., 1993).
Genetic Selection and Engineering to Improve Vitamin A Activity of Vegetables and Staple Crops
Breakthroughs in genetic selection and engineering have already provided high beta-carotene varieties of carrots. The potential for similar augmentation of the provitamin A activity of other native and widely cultivated food crops has not been exploited. These crops include some basic cereals such as yellow maize varieties and wheat (Graham and Welch, 1996). Bread and yellow pasta and noodles made from natural beta-carotene-enriched wheat have the potential for wide acceptance because their nonwhite color is not an issue. There is even potential for introducing beta-carotene into some varieties of rice where yellow rice is traditionally consumed (for example, saffron is added to rice dishes in many Muslim societies). Varieties of tubers and their young tender leaves—sweet potatoes and vine plants, such as yellow squash and pumpkin—also have potential for genetic selection for provitamin A activity (E-Siong et al., 1995). Constraints encountered include some changes in the texture, taste, and cooking qualities of new varieties that may limit community acceptance. In addition, these genetically selected or engineered varieties sometimes require greater resource inputs, rendering them more costly to produce, and thus decreasing their chance of adoption by poor farmers. Social marketing methodology to facilitate acceptance is needed to introduce non-traditional varieties with unfamiliar characteristics.
Genetic engineering to enhance provitamin A activity of staple food varieties is worthy of support as a potential sustainable solution to the VAD problem among low-income populations. This is a long-term strategy, however, requiring a large dollar investment for R&D. It is an approach suitable to regional agriculture development centers, but of limited potential for communities. It is not a strategy, therefore, that is expected to contribute immediately to overcoming VAD. Faster returns can be expected from genetic screening for provitamin A content of familiar vegetable and fruit varieties and from seeking out traditional, area-specific crops that contain high levels of beta-carotene but are disappearing or underutilized (NAS, 1975; NRC, 1989; Reddy and Vijayaraghavan, 1995).
Can Vitamin A Nutriture Be Improved by Feeding Plant Sources of Provitamin A?
Elimination of VAD within the next few years—a global goal to be achieved in the years that remain to the end of the decade—and sustaining adequate national vitamin A nutriture in the more than 75 countries that now have at least a moderately severe public health problem (WHO, 1996a) will depend
largely on the use of available natural plant foods that contain provitamin A. The prohibitive cost of production or purchase of animal sources for poor families and the limited feasibility of universal vitamin A fortification lead to this conclusion. DGLVs are generally the richest available sources of provitamin A, and the least expensive (Booth et al., 1992). Nevertheless, carotenoids from DGLVs, because of the fibrous, cellulose-rich embedding matrix of chloroplasts, are less bioavailable to humans than those from the chromophore-associated matrix of chromoplasts found in yellow and orange fruits and vegetables. This well-known difference was reviewed in the mid-1960s by an FAO/WHO Expert Group convened to consider dietary requirements for vitamin A. Because of the wide variation in absorption from a variety of yellow and green vegetables (1–88 percent), a middle-ground value of 33 percent, of which half was bioconvertible to retinol (based on animal studies of beta-carotene), was recommended. Recognizing all of its limitations, the carotenoid:retinol bioavailability (absorbed + converted and available for tissue utilization) ratio agreed upon for beta-carotene was 6:1; for other provitamin A carotenoids, it was 12:1 (FAO/WHO, 1967). Through the ensuing years, these ratios have continued to be recommended in evaluating the retinol equivalency (RE) of diets throughout the world (Bieri and McKenna, 1981; FAO/WHO, 1988; IOM, Food and Nutrition Board, 1989).
Infants and young children between 6 and 36 months of age have the capacity to eat sufficient green-leaf products (about 40 g minimum), based on the recommended conversion factors (Rahman et al., 1992). In practice, however, DGLVs are seldom fed to children under 3 years of age as the sole dietary RE. REs are obtained primarily from preformed breast milk retinol during early complementary feeding; they are increasingly supplied by mixtures of fruit and vegetable additions during late complementary feeding and in postweaning diets (Zeitlan et al., 1992). During complementary feeding, the milk fat and bile salt-stimulated lipase in breast milk facilitate carotenoid bioavailability (Fredrikzon et al., 1978). Absorption from fat-poor, postweaning diets of deprived children, however, may limit the capacity for the carotenoids from some plant sources to fully meet vitamin A needs (Jayarajan et al., 1980).
Questions were recently raised by a well-designed, controlled study of lactating Indonesian women as to whether carotenoids from DGLVs are sufficiently bioavailable to improve vitamin A status (de Pee et al., 1995). A between-meal supplement was given that contained DGLVs, either an enriched wafer or placebo control wafer. Worm infestation was common but remained untreated. Both positive intervention regimens contained an equivalent amount of beta-carotene (3.5 mg), and each contained fat (7.8 g with DGLVs and 4.4 g with wafers). Blood and breast milk retinol and blood beta-carotene levels were unresponsive to the DGLV intervention, but responsive to the enriched-wafer supplement. The data were interpreted to indicate that DGLV carotenoids were not bioavailable. Alternative explanations suggested by readers included parasite
loads, sufficient meal-fat content, initial vitamin A status, and the like, and were refuted by authors of the report (Reddy et al., 1995).
Only a portion of the Indonesian women studied were marginally, if at all, deficient. A subanalysis of data from the women in each of the three groups showed that all responded; the enriched-wafer group responded significantly more than the other two groups, whose responses were not significantly different (de Pee et al., 1995). Serum retinol levels of the DGLV-supplemented group, however, were twice those of the control-wafer group, although 50 percent lower than the group fed enriched wafers. This suggests that bioconversion of DGLV provitamin A carotenoids had occurred among women with the lowest serum retinol levels, although the authors attributed this to regression toward the mean. Animal studies, however, confirm a modulating role of vitamin A status on intestinal carotene dioxygenase activity (van Vliet et al., 1993; Villard and Bates, 1986); that is, efficiency of bioconversion is stimulated by deficiency. The true mark of vitamin A status is total body stores. Marginally deficient Indonesian women, therefore, may have efficiently converted DGLV carotenoids and incrementally increased their total body stores, while showing only nonsignificant increments in serum and breast milk. Among nondeficient women, bioconversion may have been less, allowing higher circulating beta-carotene to circulate in the blood while improving already sufficient stored vitamin A by a small increment that was not detectable by the indirect indicator used to signal body store change (modified relative dose response, MRDR). RDR and MRDR measurements are relatively insensitive in determining stores when they are above the critical level indicative of impaired function (Underwood, 1990a).
Thirteen of sixteen epidemiological studies in children reviewed by de Pee and West (1996) found an association, when VAD preexisted, between carotenoid intake from food sources, including DGLV, and improved vitamin A status. These studies showed positive response in clinical and/or biochemical indicators of vitamin A nutriture. Indeed, in a rehabilitation center in south India, even children with xerophthalmia (not keratomalacia) were relieved of clinical deficiency by feeding DGLV, exclusive of vitamin A supplement (Venkataswamy et al., 1976). These epidemiological studies were not as rigorously controlled as the Indonesia study in adult women, and it had design and methodological flaws as noted by de Pee and West (1996).
Nevertheless, a controlled community study equal in rigor to that of de Pee was carried out in Indonesia among 3- to 6-year-old children living in an area of Sumatra where VAD is common (Jalal et al., 1997). Meals and snacks that varied in beta-carotene levels (from DGLVs and red sweet potatoes) and in fat content were supplied at midday for 3 weeks. Some children were dewormed prior to the 3-week feeding trial. Significant improvement in serum levels of retinol followed the addition of vegetables that contained beta-carotene (750 RE/day was the highest level fed). Extra fat (highest level fed was 15 g) and deworming also caused serum levels to increase independently to a similar extent.
The three interventions—extra vegetable carotenoids, fat, and deworming—were additive. Children whose preintervention serum levels were lowest (<0.70 mmol/L) showed the greatest rise in blood retinol levels in each treatment group, and the effect of adding dietary fat was greatest when accompanied by deworming. This study supports the conclusion that vegetable-food-based interventions in vitamin A-deficient areas can successfully improve vitamin A status, particularly when dietary fat levels are also increased sufficiently and helminthic infections are controlled. The programmatic implication is that a concurrently implemented mix of provitamin A, food-based, and public health interventions are best for improving vitamin A nutriture.
Additional research in VAD-endemic areas is clearly warranted to refine our understanding of the factors associated with carotenoid bioavailability from local food sources. Precise quantitative methodologies that measure tissue stores—stable isotope dilution techniques, for example—may be needed to verify bioavailability and changed vitamin A status in some of the studies. These research activities, however, should not deter support for intervention programs to increase provitamin A consumption, including use of DGL Vs, because the weight of epidemiological evidence indicates this ''works" to improve vitamin A nutriture.
Fortification
The potential for vitamin A fortification of centrally processed basic foods and/or condiments is attractive because it would require little modification of food behaviors. Remarkably rapid success has been seen with this approach in the global campaign to control iodine deficiency disorders (IDDs) through iodine fortification of salt. There are some lessons from IDD control experiences with fortification that have implications for vitamin A fortification interventions, particularly in the area of IEC and social marketing. A series of publications that review the theoretical, operational, and regulatory aspects of fortification interventions has recently become available for reference (Lotfi et al., 1996; Nathan, 1995; Nestel, 1993).
Vitamin A-Fortified Sugar in Guatemala: A Successful National Experience
Context. Xerophthalmia is not a public health problem in most of the countries of Latin America, including Guatemala, but low dietary intake of vitamin A and low serum retinol values are prevalent. Most refined sugar is processed within the country by a few producers and, except for some areas where a crude, local sugar is preferred, most of the population consumes refined sugar. There is a relatively narrow range of daily sugar intake across the age spectrum. In the 1970s, Guatemala began fortifying sugar with vitamin A, even though
there was objection from some professionals to the use of sugar as a vehicle for a public health nutrient-deficiency control program. During the start-up period, producer commitment to fortification without a price increase to consumers was mandated by the government. Extensive evaluation was built into the initial program to document biological effectiveness and process successes. This resulted in one of the most successful, best-documented national control efforts through fortification yet witnessed in a developing country (Arroyave et al., 1979).
The initial venture, however, was not sustained. Political and economic constraints, including dependency on foreign exchange to purchase vitamin A during a period of economic crisis, halted the program. During this period, VAD reappeared because other VAD control interventions had not received national attention. The fortification program was reestablished around 1990, in part because of heightened global and national political and private sector awareness—and sensitivity—to the consequences of the deficiency problem. The revitalized program was adjusted in accordance with the lessons learned in the initial experience to increase chances for sustainability. Among the measures included were the provision of cost-recovery incentives to the private producers and the creation of a sense of social responsibility among them. A social marketing effort was mounted to create and maintain consumer demand, political visibility, and social responsibility. Global commitment to eliminate VAD as a goal of the decade was made by Guatemalan political leaders who attended high-level international meetings on micronutrients, including the Summit for Children in 1990, the Conference on Hidden Hunger in 1991, and the International Congress on Nutrition in 1992. These meetings raised awareness of the political, economic, and health consequences for national and human capital development of allowing micronutrient deficiencies to persist. The endorsement of a time-bound (year 2000), international goal for eliminating VAD was a useful "lever" in Guatemala to revitalize and maintain political commitment to a national micronutrient program that had been found to be biologically effective. The technical experience with sugar fortification is codified in a series of recently available manuals (Arroyave and Dary, 1996).
Results. The most recent nationwide Guatemalan VAD survey, in 1996, revealed that the prevalence of low (< 0.70 ![]() mol/L) serum retinol levels has decreased in the 5 years since the program was revitalized (Delgado and Delrue, 1996). Guatemala is now classified by WHO criteria as having a moderate, rather than severe, VAD public health problem (WHO, 1995a, 1996a). Sugar producers are committee to continue fortification, which they now view as their social responsibility.
mol/L) serum retinol levels has decreased in the 5 years since the program was revitalized (Delgado and Delrue, 1996). Guatemala is now classified by WHO criteria as having a moderate, rather than severe, VAD public health problem (WHO, 1995a, 1996a). Sugar producers are committee to continue fortification, which they now view as their social responsibility.
Success in the Guatemalan sugar fortification program has encouraged replication in other Latin American countries—Honduras, El Salvador, Ecuador, and Bolivia, for example—and it is being pursued in some countries in other
regions where the context for sugar fortification is similarly promising. The major remaining problem is the variability in quality assurance of the fortified product at the production level. A technical solution exists and needs to be applied: upgrading machinery to ensure uniformity in bulk-mixing of the premix containing vitamin A.
Indonesian Experience with Fortified Monosodium Glutamate: A National Failure
Context. Based on successful project experience in the Philippines (Solon et al., 1979), Indonesia began a project to fortify monosodium glutamate (MSG), a condiment consumed widely every day, even by the poor, in relatively uniform and limited amounts. As in the Philippines, and in Guatemala with sugar, the selection of MSG as the vehicle to fortify with vitamin A to control a childhood nutrient deficiency was controversial. Many professionals were skeptical of the safety of consumption of MSG by young children as part of a public health program (HKI/DOH, 1986), although they acknowledged the need to provide vitamin A to poor children. Nonetheless, the pilot project, in cooperation with the somewhat cautious major producer in the private sector, moved forward with the expectation that a national program would be achieved rapidly. Concurrent with pilot field evaluations, safety questions were addressed to allay professional and political concerns (HKI/DOH, 1986).
Results. Community-based, controlled intervention trials demonstrated that the fortified product was acceptable, affordable, and biologically effective (Muhilal et al., 1988a,b). Efforts to expand program coverage stalled, however, when color changes (yellowing) occurred that manufacturers feared would jeopardize sales. Although political objections to program expansion had been overcome, additional technical development work was necessary, which prolonged implementation of a proposed national program and increased R&D costs. In spite of efforts over more than 15 years to overcome constraints, a national program was not achieved. Indonesia has turned to other, less technically bound vehicles for fortification, such as noodles and margarine. (Vitamin A-fortified noodles are also being promoted in Thailand and some other Asian countries.) In addition to the lessons learned about technical factors that contributed to the failure of the MSG-fortification program, the extended time and effort were devoted to overcoming professional concerns about a controversial vehicle; forming productive, trusting partnerships between government and private business; and generating political will (Tilden et al., 1996).
The Philippines Experience with Fortified Margarine: A Promising Government and Private-Sector Partnership
Context. A recent collaborative venture between the Philippine government and the private sector has produced a vitamin A-fortified margarine that is now widely promoted with government endorsement—an "acceptance seal." Although the program is young, indications are that it will be sustainable because government-industry alliances have been established, marketing principles followed, and consumer demand generated through a social marketing program.
Results. Biological effectiveness—an increase in serum retinol—was demonstrated in a 6-month, placebo-controlled trial in one province (Solon et al., 1996). Evaluation of biological effectiveness on a national scale is not anticipated, because this is only one of a series of national interventions to control VAD. Inferences for potential national impact are made from the controlled field trial. Nevertheless, evidence will be needed to show sustained market selection of the government-approved fortified product over the competing unfortified product by disadvantaged high-risk households when the choices are freely available.
Other Fortification Efforts
The examples cited above are only a few of a multitude of projects currently under way to seek out and fortify technically promising food and condiment vehicles with vitamin A in a country-specific context. These efforts include fortification of staple products such as vegetable oils and cereals. Rice incorporating fortified, simulated rice grains (Flores et al., 1994) and wheat including nutrient-rich premixes are being tried in some countries. Double and multiple nutrient fortification strategies are in active R&D, because there is potential for adverse nutrient interactions that will affect stability in some mineral and vitamin mixtures (vitamin A and carotenoids, for example, are readily oxidizable). These interactions can be minimized, but at increased product cost that has implications for affordability in public health programs. In countries with VAD, multinutrient product fortification—such as the addition of combined iron/vitamin A/iodine—may be difficult to achieve at prices affordable for public health programs.
Food-to-Food Fortification
Opportunities exist for household-processed, food-to-food fortification of complementary and weaning foods that take advantage of traditional home and
community preservation practices. For example, the potential for micronutrient retention in traditional sundried products (Linehan et al., 1993), such as DGLVs and yellow-colored fruits (such as mango and papaya) and vegetables (including pumpkin, squash, and carrots) can be maximized. In Haiti, a community-level solar-drying technology program focused on women was successful, popular, and became an income-generating activity. After the initial project funding terminated (Linehan, 1994), there was public demand for continuation of the program, and its popularity as an NGO enterprise for women expanded to the Dominican Republic. Other traditional household procedures that offer an opportunity for safe preservation and preparation of seasonally available and perishable vitamin A-rich products should be sought through local focus groups. Some situations may require only the availability of simple food grinders or sieves. Strengthening beneficial traditional household practices could substantially enhance the micronutrient content of the usual, nutritionally poor paps and gruels for the household complementary feeding and postweaning diets of young children. The use of such preservation practices during periods of seasonal glut, as in the cycle of mango production, could carry vitamin A-rich food sources into periods of scarcity. Attention must be given, however, to hygienic home-processing practices in the preparation of complementary foods (Motorjemi et al., 1993). Efforts are warranted to probe potential avenues for fortification at the community and household levels together with production-oriented, food-based strategies that focus on women.
Lessons Learned from Fortification Experiences
Fortification is an attractive, potentially sustainable, long-term solution to VAD in countries that have reached at least midstage in the development of their food industry and distribution system. Private sector cooperation and partnerships with government and other stakeholders are essential to initiate and sustain programs (MI/Keystone Center/PAMM et al., 1996). Lessons learned from past failures, however, should guide national decisions to undertake fortification programs. In developing countries with rudimentary food industries, careful analysis should precede any decision to use limited public resources for fortification as opposed to other potential interventions in an "either/or" choice for VAD control. In this context, several years may be needed to launch a viable national fortification program. The criteria to be used to determine start-up and maintenance costs, cost-effective coverage of vulnerable groups, and the cost of sustainable product-quality assurance are a few additional considerations. The selection of a suitable vehicle must consider political, as well as technical, issues.
Contrary to the view that behavior change is not needed in the recipient population where there is product choice, the creation of demand for fortified over unfortified products will require well-designed social marketing strategies.
Mandated national, universal fortification of a single food item, advocated by international organizations in the iodination of salt, will be difficult to enforce for vitamin A when no appropriate universal vehicle is identified. Where critical elements for sustainable programs can be assembled, fortification can benefit producers, consumers, and government through cost-effective control of VAD, but unless critical elements are in place, ventures into fortification can result in substantial losses and program delays. These decisions should be made at the national level, but only after political and social support has been created. Appropriate international assistance should be available as requested by national governments after this support has been created. External assistance may best be directed toward short-term capital investment for product-quality assurance and monitoring, rather than for ongoing operations and marketing activities that will require national financing for sustainability.
Emergency and Food-Aid Programs
For emergency and food-aid programs, foods fortified with vitamin A are critical. Lessons learned from the inappropriate use of unfortified skimmed milk to feed severely malnourished children, which precipitated xerophthalmia, were well-documented many years ago in Brazil (do Vale Pereira et al., 1966). Additional anecdotal reports of similar experiences elsewhere stimulated WHO and the World Food Program (FAO, 1977), as well as other bilateral and relief agencies, to fortify products for infant and young child feeding with vitamin A in emergency, food-aid, and supplemental feeding programs. Acute refugee and emergency-relief efforts in VAD-endemic areas require special attention to micronutrient supplementation. For vitamin A, this means a high-dose supplement for children (WHO/UNICEF/IVACG, in press), followed by fortified food rations that reach the entire unsettled population.
Supplementation
National Delivery Systems
Periodic distribution of high-dose vitamin A supplements, either to all children of a specified age range or to targeted high-risk groups, has been the most widely applied intervention with proven effectiveness for treatment, prevention, and control of VAD. Guidelines are available for the use of vitamin A supplements for these purposes (WHO/UNICEF/IVACG, in press). Experiences with periodic high-dose programs were summarized in 1987 (West and Sommer, 1987) and updated in 1994 (Gillespie and Mason, 1994). The conclusion of these reviews was that the high coverage necessary for biological effectiveness
for VAD control in the population (65 percent minimum) was not sustainable through iterative (4- to 6-month) vertical programs. Targeted supplementation was potentially more cost-effective where there is high utilization of health and/or community service. Because targeting is a passive program—deficient children are not actively sought—many at-risk children in community settings are missed (Berger et al., 1995). The cost-effectiveness for each affected child in targeted programs has thus been questioned. Nonetheless, almost all countries with VAD will continue to have some use for targeted delivery of vitamin A supplements, including treatment of xerophthalmia and severe measles, PEM, and persistent diarrhea; emergency and refugee situations; and a variety of recalcitrant settings where a shortage of foods containing vitamin A persists, such as isolated or remote, drought-prone, poverty-stricken areas.
Failure to achieve sustained high coverage through vertical universal delivery systems and the limited utilization of health/community service facilities in some targeted programs has led to alternate approaches for supplement delivery. In the Philippines (and some other countries), for example, delivery of high-dose capsules is confined to biannual "special days" that are linked to providing other deficient micronutrients as well (UNICEF-Manila/HKI, 1996). Among infants between the ages of 9 and 12 months in India, supplement distribution is encouraged during measles immunization (India, Ministry Health and Family Welfare, 1995), and every 6 months thereafter through feeding and/or growth monitoring programs. In Bangladesh, coupling low-dose supplements (25,000-50,000 IU) with immunizations scheduled throughout the first year of life, and at special-event days thereafter, is encouraged to improve coverage (Karim et al., 1996). In Indonesia, following a social marketing campaign to create increased demand, trained health volunteers were used to increase the capacity of the service delivery system to meet the demand; they were subsequently used for face-to-face education and promotion of vitamin A (Reis et al., 1996). Experiences with these alternate prophylactic approaches have substantially improved periodic supplement coverage at reduced costs (Arhin et al., 1993). Other countries are beginning to implement one or more of these modified approaches and reported similar high-coverage success. It is too early to determine if the broad coverage attained will be sustained. If national strategies are planned to concurrently begin to phase in more sustainable food-based approaches, the high coverage achieved through special event days and other specially created delivery systems should not be required beyond 2 to 5 years.
The Experience of Northeast Brazil with Community-Supported Supplement Distribution
Context. It is worth reviewing the experience of Brazil with high-dose supplementation. The program was directed toward a poverty-stricken area in
the northeast, where malnutrition, including clinical and subclinical VAD, is reported. Affordable animal sources of vitamin A are scarce in the northeast, and diets are generally low in provitamin A carotenoids, both because of limited production and seasonal availability in this water-scarce environment. In addition, there is a strong cultural aversion to the consumption of leafy green vegetables. In this context, universal vitamin A supplementation was selected as the appropriate, area-specific intervention. (Because the problem is not as serious in other, relatively affluent, parts of this large country, it has been difficult to draw national professional and political attention and resources to an area-specific problem in the less-developed regions.)
Intervention Design. In Caruaru, a mayor and city council that are conscious of the public health, with the health department and support from local academicians, determined that a communitywide, biannual distribution campaign was appropriate and affordable. The campaign had the full support, including financial assistance, of both the local political and business communities. It was organized entirely around specially trained community volunteers and was supported by the local university nutrition department with biological (serum retinol and relative dose response [RDR]) evaluation of effectiveness in representative subsamples of children before and during each of five subsequent distribution rounds.
Results. Over 90 percent coverage was sustained at each of five successive distribution rounds (over a period of nearly 3 years). Serum retinol distribution curves shifted toward the right in the left portion, and stability was achieved in the community after the third distribution. (Earlier work had demonstrated that a stable distribution curve reflected adequate vitamin A in the population; Flores et al., 1991). The program survived a change in political administrations. It was expanded into a statewide program and integrated into PHC, linked to periodic immunization initiatives (IVACG, 1992). Success was ascribed to creating consumer demand for the program through the active participation of local volunteers, business leaders, and political figures. Also, external assistance was only required for provision of the vitamin A supplement. Even the social marketing program was developed and financed locally.
The Experience of Bangladesh with an Immunization-Linked, Prophylactic Supplementation Program for Infants
Context. Bangladesh has suffered from a high prevalence of xerophthalmia in preschool children, in spite of biannual distribution of high-dose supplements since 1973 (Cohen et al., 1987; Underwood, 1990b). Breastfeeding is nearly universal, prolonged, and protective from xerophthalmia
(Mahalanabis, 1991), but because of maternal malnutrition, it is not sufficiently protective from depletion of vitamin A stores in late infancy (Underwood, 1994a). To combat this problem through prevention, the government recently adopted a program linking the distribution of vitamin A supplements to immunization contacts for infants at about 6, 10, and 14 weeks and 9 months. The rationale was that piggybacking distribution of vitamin A supplements onto expanded program on immunization (EPI) contacts would take advantage of the successful, broad coverage achieved through EPI to maintain vitamin A status throughout infancy (WHO, 1994). In theory, this approach could provide broad protection of stored vitamin A above a critical level for infants that were breast-fed by VAD mothers until they can be reached by other ongoing distribution programs (WHO/IVACG, 1992).
Results. Reports of increased prevalence of bulging fontanel at doses of both 25,000 IU (Baqui et al., 1995) and 50,000 IU (de Francisco et al., 1993), apparently linked to cumulative dosing, caused professional concern and political controversy over whether the program should continue. Follow-up revealed no evidence of lasting consequences among infants who experienced bulging (van Dillen et al., 1996). Nonetheless, program adjustments were made to dose only at 6 and 14 weeks with 25,000 IU. Consensus was then reached among scientists and politicians to continue with the modified national program. Coverage from 6 months through the preschool years is now achieved through biannual national "special days" for distribution. (A WHO-sponsored multicountry—India, Peru, Ghana—random, controlled community trial of safety and efficacy using this approach is expected to be completed in 1997/1998.) These new distribution approaches are reported to have substantially increased supplement coverage in Bangladesh (Karim et al., 1996). Follow-up surveys will be needed to substantiate sustained high coverage and to demonstrate biological effectiveness in nationwide efforts to reduce the prevalence of VAD, a goal not achieved in over two decades of supplement distribution using other strategies.
Possibilities for Low-Dose, Community Supplementation
For more than two decades, prophylactic use of vitamin A supplements focused exclusively on high doses given periodically—100,000 IU for infants 6 to 12 months old and 200,000 IU thereafter. The 200,000 IU dose given to children over 1 year of age protects longer, at least for 4–6 months, than 100,000 IU (Humphrey et al., 1994). These dosage levels, given at 4- to 6-month intervals, rarely produce significant acute toxicity symptoms beyond the ages of 6–12 months (Florentino et al., 1990; West et al., 1992), even when multiple doses are accidentally given (Rosales and Kjolhede, 1993). Among the several controlled, randomized community trials, however, the greatest impact on mortality
reduction was achieved with frequent daily intakes of low doses through fortified MSG (Muhilal et al., 1988a,b) or weekly 8,000 IU supplements (Rahmatullah et al., 1990). High-dose supplement distribution should be controlled and monitored, usually through the health infrastructure, to reduce risks of misuse (for example, to pregnant women) and multiple dosing (incident-linked disease targeting, for example). Low-dose distribution (10,000 IU and less), in contrast, could be safely managed through community systems such as trained volunteers, mothers' groups, and child-to-child programs, and could include availability through local pharmacies or community medicine kiosks. Such low doses are safe for community distribution and management, even for fertile women (WHO, 1996b). An ethnographic study recently completed among night-blind pregnant women in Nepal found that about 25 percent purchased a "goti" (low-dose, 5,000 IU preparation) from the local medicine shop or market pharmacy on their own initiative (Christian, 1996). Although the prices of these preparations were found to be quite low in Nepal (about US$0.02 for three to six tablets), they were an economic constraint for some (Christian, Johns Hopkins University, School of Public Health, 1996, personal communication). Government subsidization of low-dose community-managed programs might be necessary, although this investment would be modest compared with the high costs for the delivery of safe, large-dose supplements.
The feasibility of sustaining frequent, low-dose supplement programs that reach vulnerable groups on a weekly or monthly basis has been questioned. The management scheme Rahmatullah et al. (1991) used achieved over 90 percent coverage with a year of weekly delivery through the use of part-time, trained community health volunteers (CHVs). Key features of this model included:
- Nominations of CHVs were community-based and selection was based on performance.
- Weekly supervision and feedback was based on problems encountered by CHVs.
- Distribution coverage responsibilities were limited to a convenient area.
- Flexibility was employed to adjust work schedules to fit the other family responsibilities of the CHVs.
- There was strict accountability for self-selected work schedules.
- Support was given for CHV-initiated referrals to the health care establishment (this created community recognition, credibility, and prominence for the CHVs).
The efficiency of community-managed, frequent low-dose vitamin A supplementation as an alternative to high doses has not been adequately tested. There is an urgent need to test this approach because increased evidence has accumulated that under supervised conditions, weekly dosing with iron is effective
in prevention and control of iron deficiency. Dual weekly supplement delivery to overlapping vulnerable groups could significantly reduce future micronutrient control costs.
Lessons Learned from Supplementation Programs
Prolonged experience in India and Bangladesh with vertical, universal supplement distribution programs convincingly shows that xerophthalmia cannot be controlled with this strategy, for operational rather than biological reasons. In contrast, the biannual ''special days" approach for nationwide distribution, especially when combined with social marketing, has dramatically improved coverage. These "special days" programs, however, have not been in effect long enough to evaluate their sustainability. Sustainable programs of this nature certainly depend on continued political commitment, which may wane as other, more visible and politically sensitive, health needs are given higher priority. As few as three years of high coverage from such distributions may be sufficient to raise vitamin A status, provided that other sustainable food-based programs are established, such as the use of fortified foods and favorable diet behaviors, to take over and maintain adequate vitamin A nutriture. Alternatives for multiple delivery channels for low-dose supplements and cost-effective delivery that is piggybacked on other broad-coverage PHC programs deserve consideration.
Public Health Interventions
As noted in the introductory paragraphs, VAD results from an imbalance between supply and need, and the need side of the equation is increased by disease frequency. Frequent disease episodes are accompanied by undulating patterns of decreased intake and absorption and increased excretion and elevated metabolism (Keusch and Scrimshaw, 1986). PHC, including breast-feeding promotion, immunization, growth monitoring, oral rehydration, family spacing, hygiene education, and environmental sanitation can be entry points for direct and indirect VAD control (Habte, 1987). Disease-control programs, however, can only contribute to VAD control; increased vitamin A intake is also necessary. High measles immunization coverage can make a particularly important contribution to VAD control. This was documented in Tanzanian children by the threefold reduction in hospital admission for corneal ulceration associated with improved measles immunization coverage (Foster and Yorston, 1992).
Respiratory infection (ARI) control does not prevent vitamin A deficiency, and providing vitamin A does not protect from pneumonia or reduce pneumonia-related deaths (WHO, 1995c), except for the severity of pneumonia associated with measles (Coutsoudis et al., 1991). Some reports of increased prevalence
of ARI (and some other disease-related symptoms) after periodic high dosing (Stansfield et al., 1993) initially caused alarm, but have other possible explanations, including a heightened disease response that is ultimately beneficial (Fawzi et al., 1995; Underwood, 1994b). Alternately, increased symptom responses may be restricted to adequately nourished children not in need of high-dose vitamin A (Dibley et al., 1996). Control of respiratory infections, however, as with other febrile infection control (Mata, 1992), improves metabolic conservation of vitamin A (Stephenson et al., 1994). When accompanied by programs that increase vitamin A intake, it contributes to at least maintaining existing vitamin A stores (Marinho et al., 1991; Rahman et al., 1996).
Diarrheal disease control contributes to VAD control by indirect effects on appetite and metabolic conservation, especially febrile episodes (Alvarez et al., 1995; Becker et al., 1991). Data are unclear regarding the effect of improved vitamin A status of deficient children on the incidence, severity, and persistence of diarrheal disease (Dibley et al., 1996; Feachem, 1987). Recent reports from closely monitored, random, controlled vitamin A supplementation field trials in deficient children clearly demonstrate a decreased severity of subsequent diarrheal morbidity (Barreto et al., 1994; Bhandari et al., 1994). High-dose supplements given during an acute attack of diarrhea are absorbed sufficiently to improve body stores (Reddy et al., 1986), but do not usually alter the course of the acute infection (Henning et al., 1992). Some trials suggest a small effect of supplementation on diarrheal disease incidence (Barreto et al., 1994; Fawzi et al., 1995). Public health measures for diarrheal disease control, therefore, can be expected to enhance the effectiveness of interventions that increase vitamin A intake from supplements or food, but will not replace them.
Evidence is unclear regarding whether deworming alone will improve the vitamin A status of deficient children unless there is also increased vitamin A intake. Ascariasis infections are not consistently reported to interfere with vitamin A absorption (Ahmed et al., 1993; Mahalanabis et al., 1979), but giardiasis infection does interfere (Mahalanabis et al., 1979). VAD Indonesian children reportedly benefited from deworming alone, and the effect was additive when vitamin A-rich foods supplemented the diet (Jalal et al., 1997). Another Indonesia-based study found deworming ineffective in improving vitamin A status without an accompanying high-dose supplement (Tanumihardjo et al., 1996). Ascariasis as well as giardiasis infections also interfere with fat absorption, which may have a particularly adverse affect on food-based carotenoid interventions (de Pee et al., 1995). Worm loads affect absorption of zinc (Marinho et al., 1991), and this micronutrient is needed for retinol-binding protein—as well as other protein—synthesis, critical to retinol transport and utilization. The complex interaction of worm load and vitamin A utilization (Solomons and Keusch, 1981), and nutritional status generally (Mata, 1992), argues strongly for concurrent deworming and improved vitamin A intake interventions to maximize immediate and sustained VAD control.
Lessons Learned from Public Health Interventions for Disease Control
Disease control is an important addition to—not a replacement for—interventions that increase the vitamin A intake of deficient populations. These measures have beneficial spin-offs in the prevention of malnutrition generally, thus increasing their cost-effectiveness in deprived populations (Keusch and Scrimshaw, 1986).
Complementarity Of Interventions
Phasing in Vitamin A Interventions
Where VAD remains a public health problem, a mix of interventions is usually needed to meet both the acute need to treat and control health-related, and often irreversible, consequences and to sustain VAD control through affordable means (Gillespie and Mason, 1994). In the short run, especially where xerophthalmia or severe subclinical deficiency is documented, vitamin A supplements will be part of any control strategy and may be distributed universally in time-limited distribution programs such as those discussed above. In Caruaru, three distribution cycles at 6-month intervals stabilized satisfactory vitamin A status in children under 6 years of age. This suggests that 18 months is a minimum time period for phased-in programs that are introduced concurrently with universal high-dose supplementation that will replace them and to sustain adequate communitywide vitamin A status in children.
Under some circumstances, a fortification program such as those operating in a number of Latin American countries is the most likely choice for a high-dose supplement-replacement intervention within about 2 years. This short time frame is possible where fortification technology is already established for a widely consumed vehicle—as was the case for the revitalized sugar fortification intervention in Guatemala. (In Venezuela, iron and vitamin A—about 228 RE/capita/day—fortification of precooked maize flour and iron in wheat flour over 2 years led to remarkable reductions in iron deficiency. Vitamin A status changes have not been reported. See Layrisse et al., 1996). In countries where vehicles are still being sought, or where technology is still in the R&D phase, a longer phase-in time for fortification interventions is expected, but the process should be started and receive increased emphasis as feasibility progresses.
Natural food-based, homestead provisioning interventions in India, Bangladesh, and Vietnam, when reinforced through social marketing to increase consumption as well as production, began to show improvement among participating communities within a 2-year period. It is unlikely that natural food-based interventions alone would control VAD in such a short period because of limited coverage and the longer time period needed to instill dietary behavior
change through education and social marketing (Contento et al., 1996). Where there is potential for increased home provisioning among deprived families, this intervention should accompany other interventions that have wider coverage (such as fortification) because they will bring additional household food security to the most needy.
Even social marketing to improve consumption of already available but underutilized DGLVs and fruits, as in Thailand, can bring about behavior change in dietary practices in 2 years, although not in the entire population. This strategy should accompany all other vitamin A interventions, especially those requiring behavior modifications in consumption. The marketing and education messages require frequent reinforcement for several years before changed behaviors can be expected to become embedded in communities.
In Tanzania, experience with expanded measles immunization coverage dramatically reduced admissions for xerophthalmia in a 3-year period. Such dramatic results from immunization-preventable and other infectious disease control measures should not be expected. These programs should be emphasized, however, because of their incremental contribution to the effectiveness of other vitamin A-specific interventions.
The examples of field experience reviewed above represent strong arguments for formulating an overall national control strategy in which interventions overlap, each receiving the degree of emphasis appropriate for the community context and the severity of the VAD problem and a comparable share of the resources available for VAD control. The aim should be to replace universal high-dose supplement use—retaining such supplements to deal with high-risk and recalcitrant situations—within a time frame ranging from 2 to 5 years at a minimum; for most countries with xerophthalmia, a more realistic estimate would be up to 15–20 years (the cases in Indonesia and Tanzania, described earlier). There are important opportunities to establish phase-in schedules for the mix of interventions identified as feasible in a given situation and to establish the degree of concurrent emphasis to be given to each one. The emphasis to be given should be established by the findings of a situation analysis so that preestablished goals can be met within a specified time frame.
Vitamin A with Other Micronutrient Initiatives
VAD seldom occurs in isolation, but within the context of deprivation (Underwood, 1990b), including multiple vitamin and mineral deficits, particularly of iron, iodine, and possibly zinc. It is attractive to conceive of dealing with all of these deficits concurrently. A careful analysis needs to be undertaken, however, to determine where program compatibility exists in the areas of awareness, assessment, analysis of causes, and resources available for solutions. Tables 4-4 through 4-6 summarize some overlapping and divergent areas for
planning consideration. Coordinated strategies are technically feasible (Trowbridge et al., 1993), but infrequently implemented.
Except for iodine, natural food-based approaches are the most logical for integrating micronutrient control programs. Interactions are avoided between potential concentrated-dose incompatibilities among supplements, such as solubility differences, susceptibility to oxidation, and competition for absorption. The situation in IDD control is different, because the deficit is not correctable by simply growing more or a different variety of food in the same iodine-depleted area. Furthermore, there is a proven cost-effective IDD control intervention—universal iodination of salt—that should receive continued support, using oral iodine supplements to control the problem in limited, unyielding situations. Nonetheless, there are areas of opportunity for cost-saving in complementary activities in assessment, program selection and design, and delivery mechanisms to vulnerable groups where micronutrient deficiencies coexist.
Costs And Benefits
Attempts to make comparative cost/benefit evaluations of vitamin A interventions require many assumptions on both sides of the equation. The World Development Report 1993 (World Bank, 1993) estimated costs in deaths averted and DALYs (disability-adjusted life years) saved by vitamin A supplement, fortification, and general food supplementation programs (Table 4-7). Irrespective of the difficulty of maintaining high coverage, distribution of vitamin A supplements was the least expensive. Recent innovations in delivery through piggybacking with other public health programs (as reviewed above) may render this approach even less expensive. Whenever food consumed by the target population can be fortified at reasonable cost, fortification can provide the same vitamin A-related benefits as changes in diet, and it is likely to be easier and occur faster. Food supplementation programs are fraught with targeting errors and the replacement of food in the normal diet, greatly increasing their relative cost-benefit ratio. The World Bank report does not attempt to apply dollar values to gardening or social marketing and education strategies.
A project conducted in Nepal attempted a cost-effective analysis of three vitamin A interventions: semiannual capsule distribution, capsule distribution piggybacked with PHC, and nutrition education activities piggybacked with PHC (Tilden et al., 1993; University of Michigan, 1993). Distribution of vitamin A supplements was the least costly, followed by PHC and nutrition education.
Phillips et al. (1994) reported on the costs and effectiveness of three vitamin A interventions in Guatemala: sugar fortification, capsule distribution, and gardening plus nutrition education. This analysis reported cost per high-risk person achieving adequate vitamin A status to be US$0.98 for fortification, US$1.86 for capsule distribution, and US$2.71 to US$4.16 for gardens.
TABLE 4-4 Etiology of Deficiencies of Iodine, Iron, and Vitamin A, Vulnerable Groups for Each Deficiency, and Appropriate Groups for Surveillance Purposes
|
Category |
Iodine Deficiency |
Iron Deficiency |
Vitamin A Deficiency |
|
Etiology |
Geographic |
Dietary/increased loss |
Dietary/increased loss |
|
Vulnerable groups |
Entire population: Women of reproductive age, infants, and young children |
Pregnant/lactating women Infants Preschool-age children Adolescent girls Women of childbearing age |
Pregnant/lactating women >6-month-old infants Preschool-age children |
|
Surveillance groups |
School-age children |
Pregnant women Preschool-age children |
Preschool-age children |
TABLE 4-5 Useful Biological Indicators in Surveys to Assess Iodine, Iron, and Vitamin A Status
|
Indicator |
Iodine Deficiency |
Iron Deficiency |
Vitamin A Deficiency |
|
Diet |
xa(±) |
x + knowledge of meal pattern |
xxx |
|
Urine |
xxx |
— |
— |
|
Blood |
xx |
xxx |
xxx |
|
Breast milk |
xx |
— |
xxx |
|
a Number of "x"s indicates level of importance as an assessment indicator. |
|||
TABLE 4-6 Intervention Strategies Applicable for Prevention and Control of Iodine, Iron, and Vitamin A Deficiencies
|
Strategy |
Iodine Deficiency |
Iron Deficiency |
Vitamin A Deficiency |
|
Food-based strategies |
Iodized irrigation/drinking water? Fortification |
Natural foods Quantitative Qualitative Fortification |
Natural foods Quantiative Qualitative Fortification |
|
Supplementationa |
Iodized oil |
Iron tablets/syrup |
High-/low-dose preparations |
|
Public health measures |
|
+++b |
++ |
|
IEC |
+++++++++ |
|
|
|
a Supplements are often necessary as a time-limited measure where the problem is severe. b Number of "+"s indicates level of importance. |
|||
TABLE 4-7 Cost-Effectiveness of Some Vitamin A Interventions
|
Intervention |
Target Group |
Approximate Cost (US$) |
|
|
|
|
Per Death Averted |
Per DALY Saved |
|
Supplementation |
Children < 5 years |
50 |
1 |
|
Fortification |
Entire population |
154 |
4 |
|
Food supplement |
Children < 5 years |
1,942 |
63 |
|
Food supplement |
Pregnant women |
733 |
24 |
|
SOURCE: Adapted from World Development Report 1993, World Bank, p. 82. |
|||
Annual fortification costs per person are estimated to range from about US$0.06–0.08 for MSG and corn/wheat flour, US$0.20 for margarine, and US$0.30–0.40 for cooking fat (Lotfi et al., 1996). Arroyave and Dary (1996) estimate the cost of sugar fortification to be about US$0.40–0.84 annually, but up to $10.53 if calculated on the basis of each recovered child—that is, recovered from inadequate to adequate vitamin A status.
The benefit/cost ratio for the pilot HKI Bangladesh gardening project, calculated only in monetary terms for target households and program costs, which underestimates true benefits since many are nonmonetary, was near 1 (0.997; a favorable ratio is considered to be anything above 1). Annual cost for each target family averaged US$39.0. When disaggregated to an individual garden level that included operating costs for seeds and seedlings, crop protection (fencing), and irrigation, US$11.7/year was spent; minus fencing and irrigation, the total was US$3.00/year. Overall, the disaggregated benefit/cost ratio—only in monetary terms—was 3.3, a very positive outcome. The national version of the project, working through Bangladeshi NGOs, has reduced costs to an estimated US$8.33/garden or US$1.50/individual (Marsh, 1995).
In summary, all the evaluations of cost-effectiveness reviewed agree that fortified foods or capsule distribution, depending on whether a fortifiable food that is widely consumed by the high-risk group is available, are potentially the least expensive interventions. Although fortified foods are likely to be the more sustainable investment, it has been difficult to identify an appropriate food to fortify in most developing countries. Capsule distribution is a proven, time-limited, cost-effective intervention if coupled with programs that have effective service delivery to target groups and there is a consistent and adequate supply. Promotion of increased consumption and/or production of food is a viable option in most contexts where water supply is not critically short, but requires application of a social marketing methodology to overcome socioeconomic and cultural barriers to behavior changes where benefits are not always obvious. There are many difficulties in quantifying nonmonetary benefits in order to realistically estimate the cost/benefit ratios associated with each intervention. Nonetheless, over the long term, interventions that provide balanced, multinutrient
improvements, such as nutrient-rich natural and/or fortified foods, are most likely to provide permanent benefit to recipients in deprived contexts.
Balancing Approaches To Country-Specific Circumstances
Countries with micronutrient deficiencies at a public health level are usually confronted with the multiple problems of underdevelopment and limited resources to deal with them. Prioritization is essential, not a choice. A series of notable political events, beginning in 1990 with the World Summit for Children (UNICEF, 1990) and the follow-up 1991 conference, Ending Hidden Hunger (WHO/UNICEF/World Bank/CID/USAID, 1991), focused the attention of the participating countries on micronutrient malnutrition. The preparatory process for the International Conference on Nutrition in 1992 (FAO/WHO, 1992) and the country-level follow-up actions have fostered planning for micronutrient deficiency control at the national level, which was virtually nonexistent in many countries before these high-profile political events. National planning is often done collaboratively with international and bilateral agencies because their financial assistance is needed for program follow-up. In spite of donor involvement, it is essential that planning be driven by nationally determined considerations, not driven by donors to achieve internationally set, time-bound goals.
With multiple international and national agencies and NGOs working in micronutrient deficiency control, coordination is indeed difficult. Too often, this is done without adequate input from affected communities because collecting these views takes time and requires personal communication skills and knowledge of local situations that are generally not available at the national and international levels. It is in these areas that local NGOs can make major contributions. Policy and program strategies can be set nationally, but implementation flexibility is needed to take advantage of local situations, particularly in food-based interventions that require behavior change. In reviewing food-based approaches for this paper, merit was found in first developing such approaches at the community and regional level, allowing expansion to occur as success is demonstrated and elements of success are identified and adapted for application to different regional contexts.
Two National Case Studies
Indonesia
Context. Indonesia is a vast nation of multiple islands. Its 180 million people stretch over 3,000 miles across the equator. Up to the 1970s, the country
was plagued by poverty, underdevelopment, and poor health statistics; there were small-scale survey and anecdotal reports of widespread xerophthalmia. National economic resources to launch public health programs were very limited. Increased resources from oil revenues stimulated the economy in the early 1970s. In 1969, Indonesia initiated a series of five-year plans founded on the principles of economic growth, equity, and stabilization. In the 25 years that followed, Indonesia moved from among the poorest of developing nations to among the top in per capita income (Soekirman and Jalal, 1994a; Soekirman et al., 1992).
Late in the 1970s, a systematic assessment was made of the xerophthalmia problem, which was found by WHO criteria (WHO/UNICEF/USAID/HKI/IVACG, 1982) to be of public health significance in 15 of 23 provinces. Intervention strategies were planned that included nutrition education, distribution of high-dose vitamin A supplements, food fortification, and other public health disease-control measures. Emphasis was given to biannual distribution of vitamin A supplements through health service posts. This began as a pilot project in 1972–1973 and was expanded to a national program in 1974. The capsule distribution program, the backbone for national VAD control for 22 years, continues to the present with variable coverage rates across provinces. The community study reported in 1986 (Sommer et al., 1986) that documented decreased mortality risk of treated children in Aceh Province—a province identified in the nationwide prevalence survey to be highly endemic for VAD—prompted increased public and political awareness and resource commitment to vitamin A programs. Nutrition education centered on promotion of DGLV production and consumption and cooking demonstrations by health posts. These educational efforts, often managed by local women's groups (such as PKK, Family Welfare Women's Movement abbreviated to its Indonesian equivalent of PKK), were recently reinforced by a broad social marketing of vitamin A-rich foods. Fortification efforts received attention in about 1980, but it was focused exclusively on vitamin A-fortified MSG, which was found to be technically unsuccessful (reviewed earlier in this paper). No effective national fortification program currently exists.
Results. Although the national survey of 1977–1978 (23 of 27 provinces) has not been repeated, surveys in 15 individual provinces conducted in 1992 suggested that xerophthalmia was no longer of public health significance. A few provinces, however, were still affected. The prevalence of active xerophthalmia declined by 75 percent and that of active corneal disease by 95 percent (Muhilal et al., 1994). By 1994 the problem was controlled in the remaining provinces, which led Indonesia's President Suharto to declare Indonesia xerophthalmia-free (Soekirman and Jalal, 1994a). An unacceptably high prevalence of low serum vitamin A levels persists, however, indicating that subclinical vitamin A inadequacy remains.
Conclusions. In about 15 to 20 years, including 10 recent years of accelerated national effort that was further stimulated by political endorsement of international elimination goals, Indonesia has eliminated significant xerophthalmia through interventions that primarily depended on high-dose supplement distribution. Recently introduced social marketing facilitated broadened coverage and reinforced earlier nutrition education efforts. This has contributed to increased public awareness and mobilization. Noteworthy in bringing about this commendable success is the concurrent improved national economic situation, with spin-offs for health service delivery and poverty diminution. The specific reasons for the xerophthalmia decline in Indonesia cannot be ascribed to any particular intervention because of the multiple concurrent health and social changes (Muhilal et al., 1994).
Sustained high political and social awareness of the human and national development consequences of the still prominent subclinical VAD has helped retain support for intervention efforts. VAD-control emphasis is beginning to shift toward fortification programs and the social marketing of vitamin A-rich food. These programs are likely to maintain national vitamin A adequacy because natural food sources are available to vulnerable populations but underutilized, and the food-processing industry is rapidly gaining, and their processed products reaching, an expanding consumer market. Success in overcoming subclinical deficiency will require added emphasis on public health disease-control interventions and poverty alleviation among hard-to-reach, high-risk households. Indeed, it might have taken less time to reach national vitamin A adequacy if a more balanced, mixed-intervention program had been emphasized from the start. Balanced intervention efforts were envisioned in early national planning, but they were not achieved, in part because of Indonesia's stage of economic and social development at the beginning of the program.
The Indonesian experience provides important policy and program lessons, some of which are transferable to other countries that are now beginning micronutrient deficiency interventions.
Tanzanian Experience
Context. Tanzania currently accommodates about 27–30 million people on a land area of about 950,000 sq. km. (Kavishe, 1993). In spite of slow economic and social growth indicators into the early 1990s, the country ranks high on equitable education and social service accessibility. National programs are decentralized to allow decisionmaking, implementation, and management at the district level and below. In about 1982, a xerophthalmia surveillance system was established through sentinel hospitals. Data from this surveillance system, together with some small surveys and clinical trials, called attention to the serious public health problem that affects certain areas and was seen nationally in "at-risk" groups (Kavishe, 1992). Following a participatory process based on
UNICEF's triple-A approach, the government formulated a series of five-year program plans for VAD control in 1985. A mixed intervention package was designed for national coverage, with major emphasis on the dietary approach to stimulating production and consumption of affordable vitamin A-rich foods, including red palm oil; public health strategies; and targeted distribution of vitamin A supplements. The supplement program was based on 50,000 IU capsules dispensable in multiple units, as appropriate, through the health infrastructure's Essential Drugs Program, and distributed universally in a few particularly deficient areas. The 50,000 IU supplement capsule allayed the safety concerns of some professionals and allowed flexibility in accurately meeting recommended dose levels for different age groups. Fortification, not immediately feasible as a national control program, was given exploratory emphasis for possible future inclusion in the mixture of national interventions. During the intervention phase that followed, a National Vitamin A Consultative Group (NVACG) was formed under the auspices of the Tanzania Food and Nutrition Center. This multidisciplinary group had both advocacy and technical guidance roles to ensure that implementation of interventions gave balanced consideration to such varied factors as historical precedence, targeting, dietary diversification, supplementation, fortification, IEC, affordability, and issues of sustainability.
Results. An analysis of impact from 1982 to 1990, using the sentinel hospital surveillance system, indicated that the prevalence of active xerophthalmia was on the decline, although still of public health significance. In two divisions where universal distribution of supplements had occurred, an increase in the prevalence of near-adequate serum retinol levels was recorded (Kavishe, 1992). Further achievements were reported to the IVACG meeting held in Arusha in 1993. At the meeting, the NVACG group demonstrated the nationally promoted, community-constructed solar drying units that supported their food-based emphasis, and they reviewed progress in their efforts to find a suitable vehicle for vitamin A fortification (IVACG, 1993).
Conclusions. The Tanzania program provides a mixed intervention emphasis that was balanced from the beginning in identified national and community-specific needs and resources. The program provides elements that allow safe, community-based management. A food-based approach is the backbone of the program, but supplements are given targeted use. The historical tradition of decentralized participatory action—characteristic of the successful food-based projects reviewed above—suggests that in such a contextual setting, much is achievable in a decade, even in countries where national economic, industrial, and social development is slow to occur. (Comparative note is taken of the situation in Kerala, India, where the context is quite similar and where, in contrast to most other Indian states, xerophthalmia is unusual.)
Summary
Vitamin A is now recognized to regulate expression of multiple genes that influence animal health, development, and survival, hence providing the scientific underpinning for historical accounting of its essentiality in the diet of humans. Several million child lives could be saved, morbidity tempered, and sound vision preserved by ensuring adequate vitamin A nutrition among the estimated 250 million preschool-age children now subclinically deficient. Adequate vitamin A status can be achieved through food-based strategies that provide naturally occurring preformed or provitamin A food sources and fortified food products, or vitamin A supplements. These programs often work well under controlled conditions but are less effective and sustainable when scaled up to regional or national levels. A deterrent to sustainability in low-income countries with vitamin A deficiency has been lack of long-range plans that consider the local context of the problem and how to integrate supplementation, fortification and/or dietary modification, through social marketing and education, within a development framework with time-bound and measurable achievement goals. Disease control programs—e.g., breast-feeding, immunization, and deworming—should be a part of the vitamin A-deficiency treatment and control programs because parasites contribute to inefficient utilization and conservation of the vitamin.
Successful elements of all intervention strategies include social marketing and education of targeted audiences, and for sustainability, community-based involvement in the process of designing, implementing, monitoring, and evaluating programs that affect the community. Even fortification programs that, on the surface, would appear to require only passive involvement, need an informed consumer to assure choice of fortified products when there are non-fortified, often lower-cost, alternatives. High-dose, disease-targeted supplement distribution and disease control programs favor implementation through the PHC-infrastructure, whereas the low-dose supplements frequently distributed lend themselves to private sector, community control. Community-based, low-dose programs, however, have not yet been broadly tried and evaluated in low-income countries. Fortification programs in the long term are the most cost-effective and sustainable, although not immediately feasible in some affected societies.
Successful country experiences in progressing toward elimination of VAD at a public health level of significance illustrate the importance of long-term, integrated program planning within a national, as well as human, development framework. Careful context-specific integrated program planning can achieve measurable results in as little as 5–10 years, or require longer periods, depending upon the resources and infrastructure available to support and sustain these efforts. Stable political and resource commitment and flexible resource management
for program support are critical to sustaining incremental progress toward elimination of vitamin A deficiency of public health significance.
References
Ahmed, F., M. Mohiduzzaman, and A. A. Jackson. 1993. Vitamin A absorption in children with ascariasis. Br. J. Nutr. 69:817–825.
Ali, M. M., M. W. Bloem, and R. Pollard. 1993. Prevention of Vitamin A Deficiency in Bangladesh: A Social Marketing Approach. Bangladesh: Helen Keller International, Bangladesh. Dhaka, Bangladesh.
Alvarez, J. O., E. Salazar-Lindo, E. Kohatsu, P. Miranda, and C. B. Stephensen. 1995. Urinary excretion of retinol in children with acute diarrhea. Am. J. Clin. Nutr. 61:1273–1276.
Anzano, M. A., A. J. Lamb, and A. Olson. 1979. Growth, appetite, sequence of pathological signs and survival following the induction of rapid, synchronous vitamin A deficiency in the rat. J. Nutr. 109:1419–1431.
Arhin, D. C., D. A. Ross, and F. Kufour. 1993. Costs of vitamin A supplementation: the opportunity for integration with immunization in Ghana. Health Policy and Planning 8:339–348.
Armstrong, R. B., K. O. Ashenfelter, C. Eckhoff, A. A. Levin, and S. S. Shapiro. 1994. General and reproductive toxicology of retinoids. In The Retinoids: Biology, Chemistry and Medicine, 2nd ed., M. B. Sporn, A. B. Roberts, and D. S. Goodman, eds., pp. 545–572. New York: Raven.
Arroyave, G., and O. Dary. 1996. Manual for Sugar Fortification with Vitamin A, Parts 1, 2, and 3. Washington, D.C., and Guatemala: OMNI, USAID, and INCAP.
Arroyave, G., D. Wilson, J. Mendez, M. Behar, and N. S. Scrimshaw. 1967. Serum and liver vitamin A and lipids in children with severe protein malnutrition. Am. J. Clin. Nutr. 9:180–185.
Arroyave, G., J. R. Aguilar, M. Flores, and M. A. Guzmá. 1979. Evaluation of Sugar Ffortification with Vvitamin A at the Nnational Llevel. Scientific Publication 384, Pan American Health Organization, Washington, D.C.
Baqui, A. H., A. de Francisco, S. E. Arifeen, A. K. Siddique, and R. B. Sack. 1995. Bulging fontanelle after supplementation with 25,000 IU of vitamin A in infancy using immunization contacts. Acta Paediatr. 84:863–866.
Barreto, M., L. M. P. O. Santos, A. M. O. Assis, M. P. N. Araujo, G. G. Farenzena, P. A. B. Santos, and R. L. Fiaccone. 1994. Effect of vitamin A supplementation on diarrhoea and acute lower-respiratory-tract infections in young children in Brazil. Lancet 344:228–231.
Baurenfeind, J. 1980. The Safe Use of Vitamin A. Report of the International Vitamin A Consultative Group (IVACG). Washington, D.C.: The Nutrition Foundation.
Beaton, G. H., R. Martorell, K. J. Aronson, B. Edmonston, G. McCabe, A. C. Ross, and B. Harvey. 1993. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. ACC/ SCN State-of-the-Art Series, Nutrition Policy Discussion Paper No. 13, Administrative Committee on Coordination/Subcommittee on Nutrition, United Nations. Geneva, Switzerland.
Becker, S., R. E. Black, and K. H. Brown. 1991. Relative effects of diarrhea, fever, and dietary energy intake on weight gain in rural Bangladeshi children. Am. J. Clin. Nutr. 53:1–5.
Berger, R. A., P. Courtright, and J. Barrows. 1995. Vitamin A capsule supplementation in Malawi villages: missed opportunities and possible interventions. Am. J. Publ. Hlth. 85:718–719.
Bhandari, N., M. K. Bhan, and S. Sazawal. 1994. Impact of massive dose of vitamin A given to preschool children with acute diarrhoea on subsequent respiratory and diarrhoeal morbidity. Brit. Med. J. 309:1404–1407.
Bieri, J. G., and M. C. McKenna. 1981. Expressing dietary values for fat-soluble vitamins: changes in concepts and terminology. Am. J. Clin. Nutr. 34:289–295.
Bloem, M. W., N. Huq, J. Gorstein, S. Burger, T. Kahn, N. Islam, S. Baker, and F. Davidson. 1996. Production of fruits and vegetables on the homestead is an important source of vitamin A among women in rural Bangladesh. Eur. J. Clin. Nutr. 50 (Suppl 3):S62–S67.
Blumhoff, R., M. H. Green, J. B. Green, T. Berg, and K. R. Norum. 1991. Vitamin A metabolism: New perspectives on absorption, transport, and storage. Physiol. Rev. 71:951–990.
Booth, S. L., T. Johns, and H. V. Kuhnlein. 1992. Natural food sources of vitamin A and provitamin A. Food Nutr. Bull. (UNU) 14:6–19.
Brown, E. D., M. S. Micozzi, N. E. Craft, J. G. Bieri, G. Beecher, B. K. Edwards, A. Rose, P. R. Taylor, and J. C. Smith, Jr. 1989. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am. J. Clin. Nutr. 49:1258–1265.
Brown, K. H., K. G. Dewey, and L. H. Allen. 1996. Complementary Feeding of Young Children in Developing Countries: A Review of Current Scientific Knowledge. WHO/UNICEF/USAID, Nutrition Unit, WHO, Geneva.
Brownrigg, L. 1985. Home Gardening in International Development: What the Literature Shows. Washington, D.C.: The League for International Food Education.
Brun, T., J. Reynaud, and S. Chevassus-Agnes. 1989. Food and nutritional impact of one home garden project in Senegal. Ecol. Food Nutr. 23:91–108.
Bulux, J., J. Q. de Serrano, A. Giuliano, R. Perez, C. Y. Lopez, C. Rivera, N. W. Solomons, and L. M. Canfield. 1994. Plasma response of children to short-term chronic beta-carotene supplementation. Am. J. Clin. Nutr. 59:1369–1375.
Chappell, J. E., T. Francis, and M. T. Clandinin 1985. Vitamin A and E content of human milk at early stages of lactation. Early Human Develop. 11:157–167.
Chen, P. C. Y. 1972. Sociocultural influences on vitamin A deficiency in a rural Malay community. J. Trop. Med. Hyg. 75:231–236.
Christian, P. 1996. Determinants of night blindness during pregnancy in rural Nepal. DrPH thesis, School of Hygiene and Public Health, The Johns Hopkins University, Baltimore, MD.
Chytil, F. 1992. The lungs and vitamin A. Am. Physiol. Soc.: L517–L527.
Cohen, N., H. Rahman, M. Mitra, et al. 1987. Impact of massive doses of vitamin A on nutritional blindness in Bangladesh. Am. J. Clin. Nutr. 45:970–976.
Contento, I., G. I. Balch, Y. L. Bronner, L. A. Lytle, S. K. Malnowy, C. M. Olson, and S. S. Swadener. 1996. The effectiveness of nutrition education and implications for nutrition education policy, programs and research: a review of research. J. Nutr. Educ. (Special issue) 27:277–418.
Coutsoudis, A., M. Broughton, and H. M. Coovadia. 1991. Vitamin A supplementation reduces measles morbidity in young African children: a randomized placebo controlled, double-blind trial. Am. J. Clin. Nutr. 54:890–895.
Curtale, F., R. P. Pokhrel, R. L. Tilden, and G. Higashi. 1995. Intestinal helminths and xerophthalmia in Nepal. J. Trop. Pediat. 41:334–337.
de Francisco, A., J. Chakraborty, H. R. Chowdhury, M. Yunus, A. H. Baqui, A. K. Siddique, et al. 1993. Acute toxicity of vitamin A given with vaccines in infancy. Lancet 342:526–527.
Delgado, H., and Delrue, T. 1996. ''The Impact of Sugar Fortification in Guatemala." Report to the International Conference on Fortification of Sugar with vitamin A, 12–15 March 1996, Guatemala City. Institute Nutrition Central America and Panama and UNICEF.
de Pee, S., and C. E. West. 1996. Dietary carotenoids and their role in combating vitamin A deficiency: a review of the literature. Europ. J. Clin. Nutr. 50 (Suppl 3):S38–S53.
de Pee, S., C. E. West, P. Muhilal, D. Karyadi, and J. G. A. J. Hautvast. 1995. Lack of improvement in vitamin A status with increased consumption of dark-green leafy vegetables. Lancet 346:75–81.
Dibley, M. J., T. Sadjimin, C. L. Kjolhede, and L. H. Moulton. 1996. Vitamin A supplementation fails to reduce incidence of acute respiratory illness and diarrhea in pre-school-age Indonesian children. J. Nutr. 126:434–442.
do Vale Pereira, N. D., L. V. Abreu, and O. Freusberg. 1966. Observaçòes clínicas em 64 crianças portadoras de hipovitaminose A. Arquivos Catarinenses de Medicina 1, no. 1.
English et al. 1996. Submitted to Br. Med. J.
English, R. M., J. C. Babcock, T. Giay, T. Ngu, A-M Waters, and S. A. Bennett. In press. The effect of a nutrition improvement project on morbidity from infectious diseases in pre-school children in Vietnam. Brit. Med. J.
Erdman, J., Jr. 1988. The physiologic chemistry of carotenes in man. Clin. Nutr. 7:101–106.
E-Siong, T., G. Ah-Heng, and K. Swan-Chou. 1995. Carotenoid composition and content of legumes, tubers and starchy roots by HPLC. Malaysian J. Nutr. 1:63–64.
FAO (Food and Agriculture Organization). 1977. Enrichment of dried skim milk with special reference to vitamin A. Food Nutr. 3:2–7.
FAO. 1995. Nutrition Improvement with Special Reference to Vitamin A Deficiency Through Increased Production and Consumption of Appropriate Foods: Viet Nam Project Findings and Recommendations, Terminal Report (AG:GCP/VIE/013/AUL). Rome.
FAO. 1996. Prevention of Vitamin A Deficiency in Rural Areas of West Bengal, India. Terminal statement prepared for the Government of India by the Food and Agriculture Organization of the United Nations (ES:TCP/IND/2361). Rome.
FAO/WHO. 1967. Requirements of Vitamin A, Thiamine, Riboflavin and Niacin. Report of a Joint FAO/WHO Expert Group, WHO Technical Report Series 362. Geneva: WHO.
FAO/WHO. 1988. Requirements of Vitamin A, Iron, Folate and Vitamin B12. Report of a joint FAO/WHO Expert Consultation, FAO Food and Nutrition Series No. 23. Rome: FAO.
FAO/WHO. 1992. World Declaration and Plan of Action for Nutrition. International Conference on Nutrition, December 1992. Rome: FAO.
Favin, M., and M. Griffiths. 1991. Social Marketing of Micronutrients in Developing Countries. The World Bank, Population and Human Resources Department. Washington, D.C.
Fawzi, W. W., M. G. Herrera, W. C. Willett, P. Nestel, A. El-Amin, and D. A. Mohamed. 1995. Dietary vitamin A intake and the incidence of diarrhea and respiratory infection among Sudanese children. J. Nutr. 125:1211–1221.
Feachem, R. G. 1987. Vitamin A deficiency and diarrhoea: a review of interrelationships and their implications for the control of xerophthalmia and diarrhoea. Trop. Dis. Bull. 84: R1–R16.
Florentino, R. F., C. C. Tanchoco, A. C. Ramos, T. S. Mendoza, E. P. Natividad, J. B. M. Tangco, and A. Sommer. 1990. Tolerance of preschoolers to two dosage strengths of vitamin A preparation. Am. J. Clin. Nutr. 52:694–700.
Florentino, R. F., R. A. Pedro, L. V. Candelaria, B. D. Ungson, R. U. Zarate, Jr., A. R. M. Ramirex, and E. M. Lanot. 1993. An Evaluation of the Impact of Home Gardening on the Consumption of Vitamin A and Iron among Preschool Children. Report No. IN-17, Vitamin A Field Support Project (VITAL), Office of Nutrition, USAID, Washington, D.C.
Flores, H., M. N. A. Azevedo, F. A. C. S. Campos, M. C. Barreto-Lins, A. A. Cavalcanti, A. C. Salzano, R. M. Varela, and B. A. Underwood. 1991. Serum vitamin A distribution curve for children aged 2–6 y known to have adequate vitamin A status: a reference population. Am. J. Clin. Nutr. 54:707–711.
Flores, H., N. B. Guerra, A. C. A. Cavalcanti, F. A. C. S. Campos, M. C. N. A. Azevedo, and M. B. M. Silva. 1994. Bioavailability of vitamin A in a synthetic rice premix. J. Food Sci. 59:371–377.
Foster, A., and C. Gilbert. 1996. Childhood blindness. In Global Perspectives on the Control of Blindness: A Tribute to Mr. Alan Johns CMG OBE, G. Johnson and E. Cartwright, eds., pp. 45–56. London: International Center for Eye Health, Institute of Ophthalmology.
Foster, A., and D. Yorston. 1992. Corneal ulceration in Tanzanian children: relationship between measles and vitamin A deficiency. Trans. R. Soc. Trop. Med. Hyg. 86:454–455.
Fredrikzon, B., O. Hernell, L. Blackberg, and T. Olivecrona. 1978. Bile salt-stimulated lipase in human milk: evidence of activity in vivo and of a role in the digestion of milk retinol esters. Pediatr. Res. 12:1048–1052.
Ghana VAST Study Team. 1993. Vitamin A supplementation in northern Ghana: effects on clinic attendances, hospital admissions, and child mortality. Lancet 342:7–12.
Gillespie, S., and J. Mason. 1994. Controlling Vitamin A Deficiency. ACC/SCN State-of-the-Art Series, Nutrition Policy Discussion Paper No. 14. Geneva: ACC/SCN Secretariat.
Graham, G. G., H. M. Creed, W. C. MacLean, C. H. Kallman, J. Rabold, and E. D. Mellitis. 1981. Determinants of growth among poor children: nutrient intake-achieved growth relationships. Am. J. Clin. Nutr. 34:539–554.
Graham, R. D., and R. M. Welch. 1996. Breeding for staple food crops with high micronutrient density. Working Papers on Agricultural Strategies for Micronutrients, No. 3. Washington, D.C.: International Food Policy Research Institute.
Greiner, T., and S. N. Mitra. 1996. Evaluation of the impact of a food-based approach to solving vitamin A deficiency in Bangladesh. Food Nutr. Bull. United Nations University: 183–205. Food and Nutrition Program, Boston.
Guggenheim, K. Y. 1981. Nutrition and Nutritional Diseases, pp. 265–276. Lexington, Mass.: Collamore.
Habte, D. 1987. Control of vitamin A deficiency through primary health care. Report for IVACG. Washington, D.C.: Nutrition Foundation.
Hayes, K. C. 1971. On the pathophysiology of vitamin A deficiency. Nutr. Rev. 29:3–6.
Henning, B., K. Stewart, K. Zaman, A. N. Alam, K. H. Brown, and R. E. Black. 1992. Lack of therapeutic efficacy of vitamin A for non-cholera, watery diarrhoea in Bangladeshi children. Europ. J. Clin. Nutr. 46:437–443.
KHI/DOH (Helen Keller International and Department of Health), Indonesia. 1986. Monosodium Glutamate (MSG): What Impact on Health? Jakarta, Indonesia: Helen Keller International.
Hofvander, Y., and B. A. Underwood. 1987. Processed supplementary foods for older infants and young children, with special reference to developing countries. Food Nutr. Bull. UNU 9:1–7.
Humphrey, J. H., K. P. West, Jr., and A. Sommer. 1992. Vitamin A deficiency and attributable mortality among under-5-year-olds. Bull. WHO 70:225–232.
Humphrey, J. H., G. Natadisastra, P. Muhilal, D. S. Friedman, J. M. Tielsch, K. P. West, Jr., and A. Sommer. 1994. A 210-µmol dose of vitamin A provides more prolonged impact on vitamin A status than 105 µmol among preschool children. J. Nutr. 124:1172–1178.
Humphrey, J. H., T. Agoestina, L. Wu, et al. 1996. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J. Pediatr. 128:489–496.
Hussey, G. D., and M. Klein. 1990. A randomized, controlled trial of vitamin A in children with severe measles. N. Engl. J. Med. 323:160–164.
India, Ministry of Health and Family Welfare. 1995. Policy on Management of Vitamin A Deficiency. Government of India, pp. 7. Government of India, Ministry of Health and Family Welfare.
International Nutrition Planners Forum. 1989. Report of the Fifth International Conference of the International Nutrition Planners Forum. Crucial Elements of Successful Community Nutrition Programs. Washington, D.C.: US Agency for International Development, Bureau for Science and Technology, Office of Nutrition.
IOM (Institute of Medicine). Food and Nutrition Board, Subcommittee on Tenth Edition of the RDAs. 1989. Recommended Dietary Allowances, 10th ed. Washington, D.C.: National Academy Press.
Isler, O., W Huber, A. Ronco, and M. Kofler. 1947. Synthese des Vitamin A. Helv. Chim. Acta 30:1911–1927.
IVACG (International Vitamin A Consultative Group). 1992. Nutrition Communications in Vitamin A Programs: A Resource Book. Washington, D.C.: The Nutrition Foundation.
IVACG. 1993. Toward Comprehensive Programs to Reduce Vitamin A Deficiency. Report of the XV International Vitamin A Consultative Group Meeting, 8–12 March 1993, Arusha, Tanzania. Washington, D.C.: The Nutrition Foundation.
Jalal, F. 1991. Effects of deworming, dietary fat, and carotenoid rich diets on vitamin A status of preschool children infected with Ascaris lumbricoides in West Sumatra Province, Indonesia. Ph.D. Diss., Cornell University, 1991.
Jalal, F., M. C. Nesheim, Z. Agus, D. Sanjur, and J. P. Habicht. 1997. Serum retinol levels in children are effected by food sources of beta-carotene, fat intake, and antihelmintic
drug treatment. Division of Nutritional Sciences, Cornell University, Ithaca, New York.
Jayarajahn, P., V. Reddy, and M. Mohanram. 1980. Effect of dietary fat on absorption of β-carotene from green leafy vegetables in children. Indian J. Med. Res. 71:53–56.
Johns, T., S. L. Booth, and H. V. Kuhnlein. 1992. Factors influencing vitamin A intake and programmes to improve vitamin A status. Food Nutr. Bull. 14:20–33.
Karim, R., M. Shahjahan, S. Begum, and I. Kabir. 1996. Integration of vitamin A supplementation with EPI program in Bangladesh: an approach to increase coverage of vitamin A administration . In Virtual Elimination of Vitamin A Deficiency: Obstacles and Solutions for the Year 2000, Report of the XVII IVACG meeting, Guatemala City, Guatemala, 1996. ILSI, Human Nutrition Institute, Washington, D.C.
Kastner, P., P. Chambon, and M. Leid. 1994. Role of nuclear retinoic acid receptors in the regulation of gene expression. In Vitamin A in Health and Disease, R. Blomhoff, ed., pp. 189–238. New York: Marcel Dekker.
Katz, J., S. L. Zeger, K. P. West, J. M. Tielsch, and A. Sommer. 1993. Clustering of xerophthalmia within households and villages. Intl. J. Epidemiol. 22:709–715.
Katz, J., S. K. Khatry, K. P. West, J. H. Humphrey, S. C. Leclerq, E. K. Pradhan, R. P. Pohkrel, and A. Sommer. 1995. Night blindness is prevalent during pregnancy and lactation in rural Nepal. J. Nutr. 125:2122–2127.
Kavishe, F. P. 1992. Development of Vitamin A Control Programs: An Example from Tanzania. Publication NU 3:21–26, International Child Health Unit, University Hospital, Uppsala, Sweden.
Kavishe, F. P. 1993. Nutrition-Relevant Actions in Tanzania. UN ACC/SCN Case Study, XV Congress of the International Unity of Nutritional Sciences, Sept. 26–Oct. 1, 1993, Adelaide, Australia.
Keusch, G. T., and N. S. Scrimshaw. 1986. Selective primary health care: strategies for control of disease in the developing world. XXIII. Control of infection to reduce the prevalence of infantile and childhood malnutrition. Rev. Infectious Dis. 8:273–287.
Kuhnlein, H. V., and G. H. Pelto, eds. 1997. Culture, Environment and Food to Prevent Vitamin A Deficiency. Boston: International Nutrition Foundation for Developing Countries.
Kuhnlein, H. V., G. H. Pelto, L. S. Blum, P. J. Pelto, and members of IUNS Committee II-6 (1992–1994). 1996. Focused ethnography for community assessment of natural food sources of vitamin A. Report of XVII International Vitamin A Consultative Group Meeting: Virtual Elimination of Vitamin A Deficiency: Obstacles and Solutions for the Year 2000, Guatemala City, Guatemala, 18–22 March 1996.
Layrisse, M., J. F. Chaves, H. Mendez-Castellano, V. Bosch, E. Tropper, B. Bastardo, and E. Gonzalez. 1996. Early response to the effect of iron fortification in the Venezuelan population. Am J. Clin. Nutr. 64:903–907.
Linehan, M. 1994. Assessment of Food Preservation for Vitamin A Nutrition. VITAL, USAID Vitamin A Field Support Project. Office of Nutrition, USAID, Washington, D.C.
Linehan, M., K. Paddack, and M. Mansour. 1993. Solar Drying for Vitamin A. VITAL, USAID Vitamin A Field Support Project, Washington, D.C.
Lotfi, M., M. G. V. Mannar, R. J. H. M. Merx, and P. Naber-van den Heuvel. 1996. Micronutrient fortification of foods. Current practices, research, and opportunities. Micronutrient Initiative, Ottawa, and International Agricultural Centre, Wageningen, The Netherlands.
Mahadevan, I. 1961. Belief systems in food of the Telugu-speaking people of the Telengana region. Indian J. Social Work 21:387–396.
Mahalanabis, D. 1991. Breast feeding and vitamin A deficiency among children attending a diarrhoea treatment center in Bangladesh: a case-control study. Brit. Med. J. 303:493–496.
Mahalanabis, D., T. W. Simpson, M. L. Chakraborty, C. Ganguli, A. K. Bhattacharjee, and K. L. Mukherjee. 1979. Malabsorption of water miscible vitamin A in children with giardiasis and ascariasis. Am. J. Clin. Nutr. 32:313–318.
Mangelsdorf, D. J., K. Umesono, and R. M. Evans. 1994. The retinoid receptors. In The Retinoids, 2nd ed., M. B. Sporn, A. B. Roberts, and D. S. Goodman, eds., pp. 319–350. New York: Raven.
Mariath, J. G. R., M. C. C. Lima, and L. M. P. Santos. 1989. Vitamin A activity of buriti (Mauritia vinifera Mart) and its effectiveness in the treatment and prevention of xerophthalmia. Am. J. Clin. Nutr. 49:849–853.
Marinho, H. A., R. Shrimpton, R. Giugliano, and R. C. Burini. 1991. Influence of enteral parasites on the blood vitamin A levels in preschool children orally supplemented with retinol and/or zinc. Eur. J. Clin. Nutr. 45:539–544.
Marsh, R. R., A. Talukder, S. K. Baker, and M. W. Bloem. 1995. Improving food security through home gardening: A case study from Bangladesh. In Technology for Rural Homes: Research and Extension Experiences. Reading, U.K.: University of Reading.
Mata, L. 1992. Diarrheal disease as a cause of malnutrition. Am. J. Trop. Med. Hyg. 47(1) (Suppl):16–27.
McCollum, E. V., and M. Davies. 1913. The necessity of certain lipids during growth. J. Biol. Chem. 15:167–175.
Mele, L., K. P. West, Jr., Pandji A Kusdiano, H. Nendrawati, R. L. Tilden, I. Tarwotjo, and Aceh Study Group. 1991. Nutritional and household risk factors for xerophthalmia in Aceh, Indonesia: A Case-Control Study. Am. J. Clin. Nutr. 53:1460–1465.
MI/Keystone Center/PAMM, et al. 1996. Sharing Risk and Reward. Public–Private Collaboration to Eliminate Micronutrient Malnutrition. Report of the Forum on Food Fortification, 6–8 December, 1995, Ottawa, Canada.
Motarjemi, Y., F. Kaferstein, G. Moy, and F. Quevedo. 1993. Contaminated weaning food: a major risk factor for diarrhoea and associated malnutrition. Bull. WHO 71:79–92.
Muhilal, P., A. Murdiana, I. Azis, S. Saidin, A. B. Jahari, and D. Karyadi. 1988a. Vitamin A-fortified monosodium glutamate and vitamin A status: a controlled field trial. Am. J. Clin. Nutr. 48:1265–1270.
Muhilal, P., D. Permeisah, Y. R. Kdjradinata, Muherdivantiningsih, D. Karyadi. 1988b. Vitamin A-fortified monosodium glutamate and health, growth, and survival of children: a controlled field trial. Am. J. Clin. Nutr. 48:1271–1276.
Muhilal, P., I. Tarwotjo, B. Kodyat, S. Herman, D. Permaesih, D. Karyadi, S. Wilbur, and J. M. Tielsch. 1994. Changing prevalence of xerophthalmia in Indonesia, 1977–1992. Euro. J. Clin. Nutr. 48:708–714.
Nathan, R. 1995. Food Fortification. Legislation and Regulations Manual, 2nd ed. Program Against Micronutrient Malnutrition (PAMM), Rollins School of Public Health, Emory University, Atlanta, Georgia.
NAS (National Academy Sciences). 1975. Underexploited Tropical Plants with Promising Economic Value. Washington, D.C.: National Academy Press.
NAS, FNB, IOM. (NAS, Food and Nutrition Board, Institute of Medicine). 1990. Nutrition During Pregnancy, Part II, Nutrient Supplements. Washington, D.C.: National Academy Press.
Nestel, P. 1993. Food fortification in developing countries. VITAL/USAID, Washington, D.C.
Newman, V. 1993. Vitamin A and Breastfeeding: A Comparison of Data from Developed and Developing Countries. San Diego, CA: Wellstart International.
NRC (National Research Council). 1989. Lost Crops of the Incas: Little-Known Plants of the Andes with Promise for Worldwide Cultivation. Washington, D.C.: National Academy Press.
Olson, J. A. 1994. Vitamins: the tortuous path from needs to fantasies. J. Nutr. 124: 1771S–1776S.
Oomen, H. A. P. C., D. S. McLaren, and H. Escapini. 1964. Epidemiology and public health aspects of hypovitaminosis A. Trop. Geograph. Med. 16:271–315.
Osborne, T. B., and L. B. Mendel. 1913. The relation of growth to the chemical constituents of the diet. J. Biol. Chem. 15:311–326.
Parlato, M., and P. Gottert. 1996. Promoting vitamin A in rural Niger: strategies for adverse conditions. In Strategies for Promoting Vitamin A Production, Consumption, and Supplementation: Four Case Studies, R. E. Seidel, ed. Washington, D.C.: The Academy for Educational Development.
Parlato, M., C. Green, and C. Fishman. 1992. Communication to Improve Nutrition Behavior: The Challenge of Motivating the Audience to Act. Paper prepared for International Conference on Nutrition, Rome, 1992.
Philippines, National Nutrition Council. 1995. Vitamin A rich foods, recipes and their promotion in the Philippines. In Empowering Vitamin A Foods: A Food-Based Process for Asia and the Pacific Region, E. Wasantwisut and G. Attig, eds., pp. 91–116. Salaya, Thailand: Institute of Nutrition, Mahidol University.
Phillips, M., T. Sanghvi, R. Suárez, J. McKigney, V. Vargas, and C. Wickham. 1994. The Costs and Effectiveness of Three Vitamin A Interventions in Guatemala, Final Report. Working Paper No. 2, Nutrition Cost-Effectiveness Studies, USAID, Washington, D.C.
Pollard, R., and M. Favin. 1996. Social Marketing of Vitamin A in Three Asian Countries. The Manoff Group, Washington, D.C.
Rahman, M. M., D. Mahalanabis, M. A. Islam, and E. Biswas. 1992. Can infants and young children eat enough green leafy vegetables from a single traditional meal to meet their daily vitamin A requirements? Europ. J. Clin. Nutr. 47:68–72.
Rahman, M. M., D. Mahalanabis, J. O. Alvarez, M. A. Wahed, M. A. Islam, D. Habte, and M. A. Khaled. 1996. Acute respiratory infections prevent improvement of vitamin A status in young infants supplemented with vitamin A. J. Nutr. 126:628–633.
Rahmathullah, L., B. A. Underwood, R. D. Thulasiraj, R. C. Milton, K. Ramaswamy, R. Rahmathullah, and G. Babu. 1990. Reduced mortality among children in southern India receiving a small weekly dose of vitamin A. N. Engl. J. Med. 323:929–935.
Rahmathullah, L., B. A. Underwood, R. D. Thulasiraj, and R. C. Milton. 1991. Diarrhea, respiratory infections, and growth are not affected by a weekly low-dose vitamin A
supplement: a masked controlled field trial in children in southern India. Am. J. Clin. Nutr. 54:568–577.
Reddy, V., and K. Vijayaraghavan. 1995. Carotene-Rich Foods for Combating Vitamin A Deficiency. National Institute of Nutrition, Hyderabad, India.
Reddy, V., N. Raghuramullu, Arunjyoti, M. Shivaprakash, and B. Underwood. 1986. Absorption of vitamin A by children with diarrhoea during treatment with oral rehydration salt solution. Bull. WHO 64:721–724.
Reddy, V., B. Underwood, S. de Pee, C. E. West, P. Muhilal, D. Karyadi, and J. G. A. J. Hautvast. 1995. Vitamin A status and dark green leafy vegetables. Lancet 346:1634–1636.
Reis, T. K., R. E. Seidel, S. Sudaryono, and A. Palmer. 1996. The use of integrated media for promotion of vitamin A capsule consumption in central Java, Indonesia. In Strategies for Promoting Vitamin A Production, Consumption, and Supplementation: Four Case Studies, R. E. Seidel, ed. Washington, D.C.: The Academy for Educational Development.
Rodriguez-Amaya, D. B. 1997. Carotenoids and Food Preparation: The Retention of ProVitamin A Carotenoids in Prepared, Processed, and Shared Foods. John Snow Inc./OMNI, Washington, D.C.
Roodenburg, A. J. C., C. E. West, and A. C. Beynen. 1996. Iron status in female rats with different stable plasma retinol concentrations. Nutr. Res. 16:1199–1209.
Rosales, F. J., and C. L. Kjolhede. 1993. Multiple high dose vitamin A supplementation. A report on five cases. Trop. Geogr. Med. 45:41–43.
Ross, A. C., and C. B. Stephensen. 1996. Vitamin A and retinoids in antiviral responses. FASEB J. 10:979–985.
Rukmini, C. 1994. Red palm oil to combat vitamin A deficiency in developing countries. Food Nutr. Bull. UNU 15:126–129.
Scrimshaw, N. S., C. E. Taylor, and J. E. Gordon. 1968. Interactions of Nutrition and Infection. Geneva: World Health Organization.
Seidel, R. E., ed. 1996. Strategies for Promoting Vitamin A Production, Consumption and Supplementation: Four Case Studies. Washington, D.C.: The Academy for Educational Development.
Shaw, W. D., and C. P. Green. 1996. Vitamin A promotion in Indonesia: scaling up and targeting special needs. In Strategies for Promoting Vitamin A Production, Consumption and Supplementation: Four Case Studies, R. E. Seidel, ed. Washington, D.C.: The Academy for Educational Development.
Sinha, D. P., and F. B. Bang. 1973. Seasonal variation in signs of vitamin-A deficiency in rural West Bengal children. Lancet ii: 228–231.
Smitasiri, S. 1994. Nutri-Action Analysis. Going Beyond Good People and Adequate Resources. Salaya, Thailand: Institute of Nutrition, Mahidol University.
Smitasiri, S., G. A. Attig, and S. Dhanamitta. 1992. Participatory action for nutrition education: social marketing vitamin A-rich foods in Thailand. Ecol. Food Nutr. 28:199–210.
Smith, R. S., D. S. Goodman, M. S. Zaklama, M. K. Gabr, S. El Maraghy, and V. N. Patwardhan. 1973. Serum vitamin A, retinol-binding protein, and prealbumin concentrations in protein-calorie malnutrition. 1. A functional defect in hepatic retinol release. Am. J. Clin. Nutr. 28:973–981.
Smith, R. F., R. Suskind, O. Thanangkul, C. Leitzmann, D. S. Goodman, and R. E. Olson. 1975. Plasma vitamin A, retinol-binding protein and prealbumin concentrations
in protein-calorie malnutrition. III. Response to varying dietary treatments. Am. J. Clin. Nutr. 28:732–738.
Soekirman and F. Jalal. 1994a. Priorities in dealing with micronutrient problems in Indonesia. Proceedings of Ending Hidden Hunger (A policy conference on micronutrient malnutrition), Montreal, Quebec, October 10–12, 1991, p. 88.
Soekirman and F. Jalal. 1994b. Eradicating xerophthalmia: Indonesian experience. Presentation XVI IVACG meeting, 24–28 October, Chaing Rai, Thailand, 1994.
Soekirman and F. Jalal. 1996. Outline of school feeding program in poor villages in Indonesia, National Development Planning Agency (Bappenas), IOM committee meeting, 4–6 December 1996.
Soekirman, Tarwotjo I., I. Jus'at, G. Sumodiningrat, and F. Jalal. 1992. Economic growth, equity and nutritional improvement in Indonesia. UN ACC/SCN country case study for XV Congress of the International Union of Nutritional Sciences, September 26–October 1, 1993 , Adelaide, Australia.
Solomons, N. W., and J. Bulux. 1993. Plant sources of provitamin A and human nutriture. Nutr. Rev. 51:199–204.
Solomons, N. W., and G. T. Keusch. 1981. Nutritional implications of parasitic infections. Nutr. Rev. 39:149–161.
Solon, F., T. L. Fernandez, M. C. Latham, and B. M. Popkin. 1979. An evaluation of strategies to control vitamin A deficiency in the Philippines. Am. J. Clin. Nutr. 32:1443–1453.
Solon F. S., M. S. Solon, H. Mehansho, et al. 1996. Evaluation of the effect of vitamin A-fortified margarine on the vitamin A status of preschool Filipino children. Europ. J. Clin. Nutr. 50:720–723.
Sommer, A., and K. P. West, Jr. 1996. Infectious morbidity. In Vitamin A Deficiency, Health, Survival, and Vision, pp. 19–98. New York: Oxford University Press.
Sommer, A., I. Tarwotjo, E. Djunaedi, K. P. West, A. A. Loeden, R. Tilden, L. Mele, and the Aceh Study Group. 1986. Impact of vitamin A supplementation on childhood mortality. A randomized controlled community trial. Lancet i:1169–1173.
Stansfield, S. K., M. Pierre-Louis, G. Lerebours, and A. Augustin. 1993. Vitamin A supplementation and increased prevalence of childhood diarrhoea and acute respiratory infections. Lancet 341:578–582.
Steenbock, H.
1919. White corn vs. yellow corn and a probable relation between the fat-soluble vitamin and yellow plant pigments. Science 50:352–353.Stephensen, C. B., J. O. Alvarez, J. Kohatsu, R. Hardmeier, J. I. Kennedy, Jr., and R. R. Gammon, Jr. 1994. Vitamin A is excreted in the urine during acute infection. Am. J. Clin. Nutr. 60:388–392.
Stoltzfus, R. J., M. Hakimi, K. W. Miller, K. M. Rasmussen, S. Dawiesah, J-P Habicht, and M. J. Dibley. 1993. High dose vitamin A supplementation of breast-feeding Indonesian mothers: effects on the vitamin A status of mother and infant. J. Nutr. 123:666–675.
Suharno, D., C. E. West, Muhjilal, M. H. G. M. Logman, F. G. de Waart, D. Karyadi, and J. G. A. J. Hautvast. 1992. Cross-sectional study on the iron and vitamin A status of pregnant women in West Java, Indonesia. Am. J. Clin. Nutr. 56:988–993.
Takahashi, Y. I., J. E. Smith, M. Winick, and D. S. Goodman. 1975. Vitamin A deficiency and fetal growth and development in the rat. J. Nutr. 105:1299–1310.
Tanumihardjo, S. A., D. Permaesih, Muherdiyantiningsih, E. Rustan, K. Rusmil, A. C. Fatah, S. Wilbur, P. Muhilal, D. Karyadi, and J. A. Olson. 1996. Vitamin A status
of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. J. Nutr. 126:451–457.
Tielsch, J. M., and A. I. Sommer. 1994. The epidemiology of Vitamin A deficiency and xerophthalmia. Annual Review of Nutrition 4:183–205.
Tilden, R. L., F. Curtale, R. P. Pokhrel, P. Muhilal, C. R. Pant, S. Pak, J. Gorstein, G. P. Pokhrel, Atmarita, J. Lepkowski, R. N. Grosse, and the Vitamin A Child Survival Project Team. 1993. Cost, Coverage, and Changes of Several Measures of Health Status Associated with Alternative Approaches to the Control of Vitamin A Deficiency in Nepal. Paper presented at the XV IVACG meeting, March 1993, Katmandu.
Tilden, R. L., B. Kodyat, and P. Muhilal. 1996. Lessons Learned in the Development of the MSG Vitamin A Fortification Project. Report of the XVII IVACG meeting, 18–22 March 1996, Guatemala City, Guatemala.
Trowbridge, F. L., S. S. Harris, J. Cook, J. T. Dunn, R. F. Florentino, B. A. Kodyat, M. G. V. Mannar, V. Reddy, K. Tontisirin, B. A. Underwood, and R. Yip. 1993. Coordinated strategies for controlling micronutrient malnutrition: A technical workshop. J. Nutr. 123:775–787.
Underwood, B. A. 1990a. Methods for assessment of vitamin A status. J. Nutr. 120:1459–1463.
Underwood, B. A. 1990b. Vitamin A prophylaxis programs in developing countries: past experiences and future prospects . Nutr. Rev. 48:265–274.
Underwood, B. A. 1993. The epidemiology of vitamin A deficiency and depletion (hypovitaminosis A) as a public health problem. In Retinoids. Progress in Research and Clinical Applications, M. A. Livrea and L. Packer, eds., pp. 171–184. New York: Marcel Dekker.
Underwood, B. A. 1994a. The role of vitamin A in child growth, development and survival. In Nutrient Regulation during Pregnancy, Lactation and Infant Growth, L. Allen, J. King, and B. Lonnerdahl, eds., pp. 201–208. New York: Plenum.
Underwood, B. A. 1994b. Was the "anti-infective" vitamin misnamed? Nutr. Rev. 52:140–143.
Underwood, B. A., and P. Arthur. 1996. The contribution of vitamin A to public health. FASEB J. 10:1040–1048.
Underwood, B. A., and J. A. Olson, eds. 1993. A Brief Guide to Current Methods of Assessing Vitamin A Status. International Vitamin A Consultative Group (IVACG), The Nutrition Foundation, Washington, D.C.
UNICEF (United Nations Children's Fund). 1990. First Call for Children. New York.
UNICEF-Manila and Helen Keller International, Philippines. 1996. Sangkap Pinoy. The Philippine Experience in Massive Micronutrient Iintervention. Manila: UNICEF.
University of Michigan, Department of Population Planning and International Health, School of Public Health. 1993. The Influence of Alternative Vitamin A Deficiency Control Strategies on Xerophthalmia Risk and Nutritional Status among Nepalese Children, 1988–1992. Final Report Vitamin A Child Survival Project. Ann Arbor, Michigan.
United Nations University Food and Nutrition Bulletin. 1985. Vol. 27:1–76. Boston, Mass.
van der Haar, F. 1992. Report of a Consultancy Mission to NOVIB-Sponsored Home Gardening Projects in Bangladesh. International Agricultural Centre, Wageningen, The Netherlands.
van Dillen, J., A. de Francisco, and W. C. G. Overweg-Plandsoen. 1996. Long-term effect of vitamin A with vaccines. Lancet 347:1705.
van Vliet, T., F. V. van Schaik, H. van den Berg, and W. H. P. Schreurs. 1993. Effect of vitamin A and beta-carotene intake on dioxygenase activity in rat intestine. Ann. N.Y. Acad. Sci. 691:220–222.
Venkataswamy, G., K. A. Krishnamurthy, P. Centra, S. A. Kabir, and A. Pirie. 1976. A nutrition rehabilitation centre for children with xerophthalmia . Lancet I:1120–1122.
Villard, L., and C. J. Bates. 1986. Carotene dioxygenase (EC 1.13.11.21) activity in rat intestine: effect of vitamin A deficiency and of pregnancy. Brit. J. Nutr. 56:115–122.
Wald, G. 1968. Molecular basis of visual excitation. Science 162:230–239.
Wasantwisut, E., P. Sungpuag, V. Chavasit, U. Chittchang, S. Jittinandana, and T. Viriyapanich. 1995. Identifying and recommending vitamin A rich foods in Northeast Thailand. In Empowering Vitamin A Foods: A Food-Based Process for Asia and the Pacific Region, E. Wasantwisut and G. Attig, eds., pp. 69–89. Salaya, Thailand: Institute of Nutrition, Mahidol University.
West, K. P., Jr., and A. Sommer. 1987. Delivery of Oral Doses of Vitamin A to Prevent Vitamin A Deficiency and Nutritional Blindness: A State-of-the-Art Review. ACC/SCN State-of-the-Art Series, Nutrition Policy Discussion Paper No. 2, Administrative Committee on Coordination–Subcommittee on Nutrition of the United Nations, Geneva.
West, K. P., S. K. Khatry, S. C. LeClerq, R. Adhikari, L. See, J. Katz, S. R. Shrestha, E. K. Pradhan, R. P. Pokhrel, and A. Sommer. 1992. Tolerance of young infants to a single, large dose of vitamin A: a randomized community trial in Nepal. Bull. WHO 70:733–739.
Wolbach, S. B., and P. R. Howe. 1925. Tissue changes following deprivation of fat-soluble A vitamin. J. Exp. Med. 42:753–780.
WHO (World Health Organization). 1992. Prevention of Childhood Blindness. Geneva.
WHO 1994. Using immunization contacts as the gateway to eliminating vitamin A deficiency: a policy document. WHO/EPI/GEN/94.9. Geneva.
WHO. 1995a. Global prevalence of vitamin A deficiency. MDIS Working Paper #2. Geneva.
WHO. 1995b. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee, WHO Technical Report Series 854. Geneva.
WHO, Vitamin A and Pneumonia Working Group. 1995c. Potential interventions for the prevention of childhood pneumonia in developing countries: a meta-analysis of data from field trials to assess the impact of vitamin A supplementation on pneumonia morbidity and mortality. Bull. WHO 73:609–619.
WHO. 1996a. Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluating Intervention Programmes. Document WHO/NUT/96.10, World Health Organization, Geneva.
WHO. 1996b. Safe Vitamin A-Dosage During Pregnancy and the First Six Months Postpartum. Report of a consultation 19–21 June 1996, World Health Organization, Geneva.
WHO. 1996c. Indicators for Assessing Vitamin A Deficiency and Their Application in Monitoring and Evaluating Intervention Programs, WHO/NUT/96.10, World Health Organization, Geneva.
WHO/IVACG (International Vitamin Consultative Group). 1992. Using Immunization Contacts to Combat Vitamin A Deficiency. Report of a consultation, WHO Geneva, 30 June–1 July 1992. Nutrition Unit, World Health Organization, Geneva.
WHO/UNICEF/ICCIDD. 1994. Indicators for Assessing Iodine Deficiency Disorders and Their Control Through Salt Iodization, WHO/NUT/94.6 Geneva: WHO.
WHO/UNICEF/World Bank/Canadian International Development Agency/USAID/FAO/UNDP. 1991. Ending Hidden Hunger: A Policy Conference on Micronutrient Malnutrition. Montréal, Québec. Canada. October 10–12, 1991. Task Force for Child Survival and Development, Atlanta, Georgia.
WHO/UNICEF/IVACG. In press. Vitamin A Supplements: A Guide to Their Use in the Treatment and Prevention of Vitamin A Deficiency and Xerophthalmia. World Health Organization: Geneva.
WHO/UNICEF/UNU. 1997. Indicators for Assessing, and Strategies for Preventing, Iron Deficiency (WHO/NUT/96.12) Geneva: WHO.
WHO/UNICEF/USAID/Helen Keller International/IVACG. 1982. Report of a Joint Meeting, Control of Vitamin A Deficiency and Xerophthalmia. Technical Report Series 672, World Health Organization, Geneva.
Wolbach, S. B. 1937. The pathologic changes resulting from vitamin deficiency. J. Am. Med. Assoc. 108:7–13.
Wolbach, S. B., and P. R. Howe. 1925. Tissue changes following deprivation of fatsoluble A vitamin. J. Expt. Med. 42:753–780.
Wolf, G. 1996. A history of vitamin A and retinoids. FASEB J 10:1102–1107.
World Bank 1993. World Development Report 1993: Investing in Health. Washington, D.C.: Oxford University Press for the World Bank.
Yusuf, H. K. M., and M. N. Islam. 1994. Improvement of night blindness situation in children through simple nutrition education intervention with the parents. Ecol. Food Nutr. 31:247–256.
Zeitlin, M. F., R. Megawangi, E. M. Kramer, and H. C. Armstrong. 1992. Mothers and children's intakes of vitamin A in rural Bangladesh. Am. J. Clin. Nutr. 56:136–147.
| This page in the original is blank. |
































































