3
Prevention of Iron Deficiency
Fernando E. Viteri, M.D., Sc.D.
University of California at Berkeley
Iron is an essential nutrient. Iron deficiency in humans has wide-ranging negative consequences, including impaired physical growth, compromised cognitive development, short attention span and impaired learning capacity, reduced muscle function and energy utilization, decreased physical activity and lower work productivity, lowered immunity, increased infectious disease risk, impaired fat absorption (most probably including fat-soluble vitamin A), increased lead absorption with all its negative consequences, and poorer pregnancy outcomes (Alaudin, 1986; Chandra, 1990; Dallman, 1974, 1986; Enwonwu, 1989; Husaini et al., 1990; Judisch et al., 1986; Li et al., 1994; Lozoff et al., 1992; Pollitt et al., 1982; Scrimshaw and SanGiovanni, 1997; Viteri and Torun, 1974; Walter, 1992). Iron deficiency also impairs the transformation of the thyroid hormones, T4 to T3, in peripheral tissues, the production and metabolism of epinephrine and norepinephrine, and leads to difficulty in maintaining body temperatures upon exposure to cold (Beard, 1990).
Functional consequences of severe iron-deficiency anemia during pregnancy include increased rates of premature delivery, perinatal complications in mother and newborn, low birthweight, low iron stores, and indications of iron deficiency and anemia in the newborn or in later infancy. Of great concern is the finding that some of the negative effects on cognitive and affective function of iron deficiency in infancy may persist, even after ion deficiency and anemia have been corrected (Lozoff et al., 1992). The majority of studies also report adverse consequences from mild to moderate iron deficiency and anemia.
The standard WHO criteria for anemia are shown in Table 3-1 (NSS1). These criteria indicate that the iron deficiency is of sufficient severity to interfere with hemoglobin formation, but iron has many other functions that are
more sensitive to iron depletion. Approximately 73 percent of the body's iron is normally incorporated into hemoglobin and 12 percent in the storage complexes ferritin and hemosiderin. A very important 15 percent, however, is incorporated into a variety of other iron-containing compounds essential to cell function.
WHO data indicate global rates for iron deficiency anemia in developing countries of 51 percent for children 0—4 years of age, 46 percent for school-age children, 42 percent for women, and 26 percent for men (NSS1) (see Table 3-1 for the WHO diagnostic criteria for iron-deficiency anemia). Even in the United States, the NHANES II survey found an overall 7 percent prevalence of actual anemia in women 15–44 years of age, but with the highest burden in minority and poverty groups (WHO/UNICEF/UNU, in press).
Diagnosis Of Iron Deficiency And Anemia
In the absence of pathological iron losses, iron requirements are greatest during periods of growth (e.g., childhood); pregnancy; and, in women of reproductive age, because of menstruation. Documented associations between iron deficiency and ferropenic anemia include smaller babies, higher rates of stillbirth and perinatal mortality, more premature deliveries, and newborns with lower iron stores. An infant's risk of developing iron deficiency begins in utero, because premature delivery deprives the baby of the accumulation of iron near the end of pregnancy and smaller babies generally have less body iron (Widowson and Spray, 1951; Rosso, 1990). Unfortunately, the iron in breast milk cannot prevent the exhaustion of iron reserves in the first 4–6 months brought about by rapid growth. Poor weaning practices and inadequate feeding during childhood contribute further to the persistence or development of iron deficiency. When growth rates diminish, risk of iron deficiency is reduced unless there is abnormal blood loss to parasitic infection; menstruating women, however, continue to be at risk. In this group, about 20 percent have skewed menstrual blood (iron) losses in the upper ranges of the normal distribution that cannot be covered by their usual dietary intake, and over 50 percent have inadequate or depleted prepregnancy iron reserves (Cook et al., 1986; Custer et al., 1995; Franzetti et al., 1984; Hallberg and Rossander-Hulten, 1991). Because of the high iron requirements of pregnancy, iron deficiency is the rule, particularly in teenage gestations and in women with frequent pregnancies.
The stages in the development of iron deficiency are the depletion of iron stores, as indicated by low plasma ferritin; interference with biochemical processes, indicated by low transferrin saturation and elevated free erythrocyte protoporphyrin and serum transferrin receptors; and, finally, anemia, as indicated by low hemoglobin. It should be noted that although transferrin receptors appear promising as an indicator, standard cutoffs and interpretation of values from different commercial assays are yet to be developed. Up to an anemia prevalence
of 50 percent, the proportion of individuals with biochemical iron deficiency is about double those with actual anemia (WHO/UNICEF/UNU, in press). Above 50 percent, it can be assumed that nearly all of the population described is iron deficient. The significance of this finding is that these subclinical degrees of iron deficiency can interfere with cognitive, immune, and muscle function.
Pregnancy presents challenges in the diagnosis of both anemia and iron deficiency because of the normal and variable hemodilution, which lowers hemoglobin concentration to varying degrees, and the hormonal changes and the frequency of infection, both of which modify the indicators (Cook et al., 1994; Hytten, 1985; Puolakka et al., 1980; Romslo et al., 1983). Serum transferrin receptor levels appear especially useful in diagnosing iron deficiency in pregnancy (Carriaga et al., 1991). The lack of appropriate hemodilution in chronic undernutrition may mask the true level of anemia in the face of iron deficiency and decreased circulating hemoglobin mass (Rosso, 1990). Prepregnancy iron nutrition and hemoglobin level markedly influence the development of gestational anemia (Kauffer and Casanueva, 1990). There is thus a need to consider interventions that will improve prepregnancy iron reserves and provide extra amounts of iron, in addition to that in the diet, during gestation (Sloan et al., 1992; Viteri, 1994a,b, in press a,b).
After initial diagnosis of the prevalence of anemia and, ideally, the Hb distribution within a population, with emphasis on at-risk groups, the diagnosis of iron deficiency can be refined and verified by further biochemical tests. The Hb response to iron administration is best measured as part of ongoing surveillance of an adequate sample of the population. These additional steps could be implemented simultaneously. The surveillance system should be based on serial hemoglobin determinations in samples of population groups at risk (ideally also including serum ferritin) and periodic assessments at sentinel epidemiological sites.
TABLE 3-1 Cutoff Values for the Diagnosis of Anemia (WHO)
|
|
Hemoglobin < |
|
|
|
Age/Gender Group |
g/l |
mmol/l |
Hematocrit < l/l |
|
Children |
|
|
|
|
6 months–5 years |
110 |
6.83 |
0.33 |
|
5–11 years |
115 |
7.13 |
0.34 |
|
12–14 years |
120 |
7.45 |
0.36 |
|
Nonpregnant women (>15 years) |
120 |
7.45 |
0.36 |
|
Pregnant womena |
110 |
6.83 |
0.33 |
|
Men (≥ 15 years) |
130 |
8.07 |
0.39 |
|
a The CDC proposes a cutoff point of 105 g/l during the second trimester. Severe anemia in pregnancy: Hb levels < 70 g/l; very severe anemia: < 40 g/l. |
|||
The general diagnosis of anemia should lead to a causal analysis. The necessary interventions and community participation toward the common aim of controlling iron deficiency and anemia must be the objective (WHO, 1991). The higher the anemia prevalence rates in a population, the greater the proportion arising from iron deficiency. There are also many different kinds of hemoglobinopathies, however; the most frequent is Hb-C, Hb-S and the thalassemias (alpha and beta; major, intermedia and minor, based on the degree of anemia they produce). The heterozygous A-S Hb affects up to 30 percent of some African populations (8 percent in African Americans). This genotype has essentially no hematological consequences, in contrast with the Hb S-S, which produces severe hemolytic and thrombotic crises (1 in 400 African Americans) and requires specialized medical attention. Hb-C produces mild anemia and affects about 4 percent of African Americans. The S-C Hb condition is associated with more severe anemia and is easily diagnosed. It affects about 1 in 850 African Americans. These hemoglobinopathies may explain failures of response to nutritional interventions in individuals, but they should not be a cause for modifying iron fortification or supplementation programs for populations at risk.
The thalassemias are a different problem because they produce anemia brought about by a failure in Hb production and chronic hemolysis. Children affected by thalassemia major generally have Hb levels below 60 g/l; those with thalassemia intermedia have Hb levels between 60 and 95 g/l, and those with thalassemia minor have Hb levels between 95 and 135 g/l. The more severe the anemia, the greater the stimulus to absorb iron and the greater the tendency to become iron-loaded, particularly because the only therapy customarily available for thalassemia anemia is repeated transfusions (justified only in thalassemia major or in special cases of thalassemia intermedia). The thalassemias are distributed primarily in populations of Mediterranean origin and of tropical or subtropical African, Middle Eastern, and Asian origin, generally areas where malaria has been endemic. In populations seriously affected by the thalassemias the concomitant iron deficiency of dietary and pathological origin (e.g., hookworm infection), as well as the risk of iron overload, must be evaluated and the programs adjusted accordingly (Charoenlarp et al., 1988).
Box 3-1 presents the suggested minimum information needed to make a tentative diagnosis of iron deficiency, estimate its public health significance, and plan the most appropriate interventions.
Causes Of Iron Deficiency
Iron nutritional status depends on long-term iron balance. It is favored by the ingestion of sufficient iron in food (native, or added through fortification) in a bioavailable form or through iron supplementation. Regulation of iron absorption is crucial in favoring absorption in iron deficiency and in avoiding iron excess.
Balance is adversely affected by the amount of iron lost through gut mucosal turnover and skin desquamation; intestinal excretion; menstruation; the pregnancy-delivery-lactation cycle; and pathologic blood losses, mainly from excessive menstrual flow, hookworm and schistosomiasis, gastrointestinal bleeding from ulcerations, hemorrhoids, diarrhea, and other occult blood losses (Bothwell et al., 1979).
In a healthy steady state, iron losses are fairly constant and iron balance depends mainly on the regulation of iron absorption: upward in iron deficiency and downward in iron sufficiency. The greater capacity to absorb iron in iron-deficiency situations is the most important short-term factor in the body's effort to maintain iron homeostasis. The amount of bioavailable iron in food is very important in the long term (Cook, 1990; Hulten et al., 1995).
There are no effective mechanisms for excreting the excess iron. Parenterally administered iron, including repeated blood transfusions, chronically excessive medicinal iron intake, or elevated iron absorption caused by impaired downward regulation of iron absorption (people homozygous for the hemochromatosis gene, some types of thalassemia, and hemosiderosis trait in some Black populations) lead to excess iron accumulation.
Food iron is present in most diets in a proportion of 6 mg/1,000 calories and is composed of two different pools: heme and nonheme iron (Hallberg and Bjorn-Rassmussen, 1972; Layrisse et al., 1969).
The heme iron pool includes all food compounds that have iron as part of heme molecules. Dietary heme iron is provided by animal blood, flesh, and viscera; the most important is hemoglobin in blood and myoglobin in muscle. In general, heme iron absorption is not modified by most inhibitors and enhancers of iron absorption. Exceptions are dietary protein, which increases heme iron absorption, and food calcium and manganese, which inhibit it. It must be clearly understood that these interactions occur while digestion and absorption of iron are taking place (within 2 hours of meal ingestion), and that only partial inhibition is produced by dairy products and other calcium-rich foods consumed in a varied meal, reducing iron absorption by 30 percent, at most (Gleerup et al., 1995; Hallberg et al., 1993). At the same time, heme iron absorption is also regulated upward and downward, but to a lesser extent than absorption of nonheme iron. In normal individuals, heme iron absorption fluctuates between 15 and 30 percent, but can increase up to about 50 percent in iron-deficient anemic subjects and can decrease to about 5–8 percent when the amount of heme iron is around 50 mg (Cook, 1990; Layrisse et al., 1973; Viteri et al., 1978).
The nonheme iron pool is made up of all other sources of iron. Nonheme iron is often bound in seeds, to phytic acid, and in other vegetable tissues to phenolic compounds. Nonheme iron is also present in heme-iron-containing and other animal tissues and in animal products such as milk and eggs. In contrast with heme iron, nonheme iron absorption is affected by many dietary components. Heme-iron-containing proteins and ascorbic, malic, tartaric, and succinic acids and some fermentation products are enhancers of nonheme iron uptake. Meat and alcohol also enhance nonheme iron absorption by promoting gastric acid production. Inhibitors include phytic acid and other polyphosphates, fibers, calcium, manganese, polyphenols such as tannins, and other compounds present in foods and beverages, especially tea, coffee, chocolate, and herbal infusions that produce polymers and insoluble, unabsorbable iron chelates.
Nonheme iron constitutes over 90–95 percent of dietary iron, particularly in the developing world. The absorption of nonheme iron can vary from 1 to 30 percent or more, depending on the presence of enhancers or inhibitors of absorption, and especially on the iron status of the individual (Bothwell et al., 1979; Cook, 1990; Layrisse et al., 1969). The latter is the most important factor in controlling iron absorption. In general, with meals of intermediate and high bioavailability, iron absorption can be as high as 5 mg of iron daily in iron deficiency. This is reduced to about 2–3 mg/day when diets are of poor bioavailability. As iron reserves increase, iron absorption decreases. When serum ferritin reaches 50–60 µg/l, equivalent to about 500 mg of iron reserves, iron absorption from daily meals of intermediate and high iron bioavailability allows the absorption of only about 1 mg of food iron/day, which is equivalent to the replacement of average obligatory losses, not including menstruation (Hulten et al., 1995). There is no published information of this kind for daily meals with poor bioavailability, but extrapolations suggest that this amount of iron would be absorbed with iron reserves of only about 140 mg.
Cook (1990) has also summarized the importance of iron nutritional status on heme and nonheme iron absorption in a single meal containing both kinds of iron (see Table 3-2). The percentage of heme and nonheme iron absorbed increased by a multiple of 2.4 to 8.4 among iron-deficient, compared with normal, men. If the meal contained only nonheme iron, the percentage of absorption would be reduced to one-half that presented in Table 3-2.
Several important conclusions can be derived from the above:
- Dietary composition appears to be particularly important when iron reserves are low or in the presence of iron deficiency.
- Downward regulation of iron absorption is very effective, even when the diet is rich in heme iron and of a composition that favors iron absorption. Therefore, the development of iron-overload conditions from dietary iron intake in normal individuals is highly improbable.
- Poor-quality diets would not satisfy the iron needs of a large percentage of menstruating women and would not allow the accumulation of iron reserves beyond about 150 mg, which is below the ideal for women entering pregnancy.
For simplicity, diets have been classified as of high, intermediate, and low bioavailability, depending on the proportion of heme iron and the presence of inhibitors and enhancers of nonheme iron absorption. Their respective bioavailabilities have been averaged at 15, 10, and 5 percent, respectively. A woman of childbearing age with requirements of absorbed iron at the median of 1.25 mg/day, and consuming a diet of poor bioavailability, would need to ingest 25 mg of iron in order to achieve adequate intake. This would mean that she would have to ingest 4,170 calories daily of an average diet containing 6 mg of iron/1,000 cal compared with an average energy intake of 2,100 cal/day by this population. If the diet is of intermediate (10 percent) bioavailability, only 50 percent of women would be able to maintain a normal iron status and about 20 percent would develop anemia. Only a very small proportion would be able to build adequate iron reserves for pregnancy. The majority of these women would rapidly develop iron deficiency and gestational anemia during pregnancy.
Most of the iron compounds used for the fortification of foods become part of the nonheme dietary iron pool, and their absorption is similar to that of the other components of the pool and subject to inhibitors and enhancers (Bothwell et al., 1979). Exceptions to this rule are soluble iron chelates, which are 2 to 5 times more efficiently absorbed than the dietary nonheme iron pool in the presence of inhibitors, and purified bovine Hb, which becomes part of the heme-iron pool when used as a fortificant. The bioavailability of soil iron, which contaminates many staples and vegetables, is largely unknown, although it is generally considered low.
The absorption of iron compounds administered as 30–120 mg boluses for supplementation or therapeutic purposes presents a different picture. When given without food, absorption declines logarithmically with logarithmic dose increments, but it remains at about 6 to 8 percent, even after apparent repletion of iron stores, possibly because of mass action (Bothwell et al., 1979; Grebe et al., 1975; Hallberg and Sölvell, 1967; Viteri et al., 1978). Svanberg (1975), however, found only 2 percent absorption of supplemental iron in late pregnancy. This steady absorption from large doses of iron explains why, in mg of iron absorbed, higher iron intakes allow higher, but less efficient, iron absorption.
Animal studies have demonstrated that iron absorption is particularly inefficient when supplemental or therapeutic iron is administered at short intervals (several times a day, daily, or even every 2 or 3 days). This "mucosal block" to iron absorption caused by repeated iron administration has been well documented in several animal species (Fairweather-Tait et al., 1985; Hahn et al., 1943; Stewart et al., 1950; Viteri et al., 1995a; Wright and Southon, 1990).
In humans, the absorption data are not as clear, but they suggest that for less than 1 week of daily iron supplementation, or even with 2 to 4 daily doses, the blockage is minor, if it operates at all, among nonanemic and normal or mildly iron-deficient subjects (Cook and Reddy, 1995; Höglund, 1969; Norrby, 1974;
O'Neil-Cutting and Crosby, 1987; Reizenstein et al., 1975;. Rush et al., 1966; Smith and Pannacciulli, 1958; Solomons, 1995). Iron blockage in the human under different iron nutritional conditions has not been fully explored. In a detailed study by Hallberg (1970), the absorption efficiency of administering 37 or 74 mg up to 4 times a day was highly variable. On average, iron absorption was around 8–9 percent.
Finally, in considering iron regulation and metabolism with the aim of preventing iron deficiency, interactions with other nutrients are important in their effect on the absorption and utilization of iron. Copper is involved in oxidoreduction of iron in the process of absorption, transport, storage, and mobilization; folate and vitamin B12 are involved in nucleic acid synthesis of all cells and clearly in erythropoiesis, thus modifying iron utilization; vitamins B6 and B2 are specifically required in the process of heme synthesis; and amino acids are required for protein synthesis in general, and for hemoglobin synthesis in particular. Vitamin A is involved in mobilization of iron reserves, in Hb synthesis, and appears to favor iron absorption in the presence of inhibitors (Hodges, et al., 1978; Layrisse et al., 1997; Mejia et al., 1979).
Low dietary iron intakes—particularly where much of the iron is in non-heme form—combined with the increased iron needs of growth or pregnancy, and even the small chronic iron losses of mildly excessive menstrual flow, increase the risk of developing iron deficiency and anemia. These risks are often further exacerbated in developing countries by parasitic infections. Endemic malaria increases the prevalence and aggravates the severity of anemia, particularly among young children and pregnant women, and produces iron sequestration and some iron losses (Brabin, 1992). As with other hemolytic processes, folate and vitamin B12 requirements are also elevated by malaria (Fleming, 1990). Hookworm disease is a serious cause of intestinal blood loss (Layrisse and Roche, 1964; Roche and Layrisse, 1966). Infection with Schistosomia haematobium causes blood loss in the urine and can result in intestinal bleeding (Scrimshaw et al., 1968).
Iron Excess
Objections to the strategies for the control of iron deficiency have sometimes been raised by hematologists in developed countries. They cite the danger of possibly accelerating or inducing iron excess and overload conditions in some clinical conditions, as well as claims for its involvement in a variety of cancers and heart disease in their countries (Halliwell et al., 1992; Herbert, 1992; Lauffer, 1992; Stevens et al., 1994). These issues cannot be ignored in this paper. Nevertheless, in the face of the widespread iron deficiency and ferropenic anemia in the great majority of populations in the developing world and in groups at risk for iron deficiency everywhere, this should not be an issue (ACC/SCN, 1997; Gillespie, 1996) as long as monitoring of interventions is in place to avoid
excessive administration of iron in therapeutic and chronic supplementation programs (a minimal requirement in any nutrition intervention program). Food iron (including that included in iron-fortified food) poses no threat to these populations.
The recessive genetic disorder, hemochromatosis, is particularly prevalent in white populations of European descent, especially those of Celtic origin. Regions with particular haplotypes have been identified in central Sweden and in northeast Italy. In the U.S. Caucasian population, the homozygous state is never less than 0.1 percent and may be as much as 0.5 percent in some population groups (Lynch, 1995). Hemochromatosis exists at a possible rate of about 1 percent among African-Americans, but its etiology needs further clarification (Wurapa et al., 1996). A recent preliminary report by the Centers for Disease Control and Prevention (CDC) indicates that a prevalence among Hispanics in San Diego, California, is similar to that seen among non-Hispanic American whites (CDC, 1996). The consequences of iron excess are mainly liver cirrhosis and increased liver cancer. Heart failure from myocardial dysfunction and diabetes brought about by pancreatic disease are suggested rare consequences, but this remains highly controversial (Lynch, 1995).
The adoption of general iron fortification of foods in the developing world, where iron deficiency is highly prevalent, has been slowed further by fears of accelerating iron overload conditions in genetically prone individuals, even though this is a relatively rare clinical problem. It is not a reason to withhold the benefits of iron fortification as a public health measure from the overwhelming majority of the population (Ballott et al., 1989a). This fear has been based on concern in industrial countries, where iron deficiency is less of a problem.
Prevention Of Iron Deficiency in At-Risk Groups
Control measures for iron deficiency and anemia should not be considered in isolation, but rather as part of integrated approaches to combat micronutrient malnutrition and within the general objectives of alleviating critical poverty; achieving sustainable food security; and improving the economic, health, overall nutritional, and educational status of the population. This obvious statement is emphasized to stress that no single approach to dealing with iron deficiency and anemia will work for all populations and in all settings. The approach taken in this paper is to evaluate successful interventions for iron from a lifestyle perspective. Table 3-3 presents a summary of successful interventions that, based on experience, can be implemented in the short, medium or long term for different categories of target individuals. The implementation of medium- and long-term strategies can be accelerated under favorable circumstances. Once established, process and impact evaluations should be performed periodically within a defined surveillance system to determine if there is a need for continuation, modification, or even suspension of a given strategy.
Infancy
The first preventive measure against infant iron deficiency is assuring adequate body iron at birth by avoiding gestational iron deficiency and other conditions leading to low birthweight and premature delivery (Colomer et al., 1990; De Benaze et al., 1989; Puolakka et al., 1980; Scholl and Hediger, 1994; Scholl et al., 1992). The importance of prepregnancy iron nutrition in preventing gestational iron deficiency has not been sufficiently recognized.
Current intrauterine devices (IUD) increase menstrual flow in many women (INACG, 1981), but IUDs can be effectively used in combination with some form of iron supplementation.
Birth spacing and delaying pregnancy beyond the teen years allow the deposition or recovery of iron reserves after the pubertal growth spurt or a previous pregnancy (Beard, 1994; Bothwell et al., 1979; INACG, 1981).
A second critical measure for improving the iron stores of the newborn is delayed ligation of the umbilical cord. Ligation of the umbilical cord after it stops pulsating (about 30–60 seconds after delivery) increases the infant's blood volume about 60 ml, providing approximately 34 mg of iron, which equates to between 25 and 30 percent of the newborn's total circulating iron (Burman, 1969; Lanzkowsky, 1976). These additional 34 mg of iron are equivalent to what a healthy, exclusively breast-fed baby would absorb in 5 months. In theory, this delayed ligation will determine whether a 6-month infant is iron deficient or not.
In the first 4–6 months, breast-feeding is an important contribution to the maintenance of better iron nutrition in infants. Research has clearly shown that exclusively breast-fed infants have greater iron stores than infants who are formula-fed (Saarinen et al., 1977). The amount of iron in human milk is very small (< 0.6 mg/l), and its bioavailability, once thought to be around 50 percent (McMillan et al., 1976; Saarinen et al., 1977), has recently been shown to average 11 percent (Davidsson et al., 1994 a,b). Even though exclusively breast-fed infants generally enter into iron deficit after about 6 months, their non-breast-fed counterparts are usually iron deficient sooner. The universal promotion of exclusive breast-feeding for 4 to 6 months is thus a key element in maintaining adequate iron nutriture.
Infants beyond about 6 months of age need an additional source of iron beyond that provided by breast milk. A large body of evidence documents that iron deficiency and anemia in older infants and young children can be prevented by appropriate complementary feeding. When breast-feeding is not possible, iron-fortified milk preparations are needed (Walter et al., 1990, 1993a).
Another alternative after about 6 months of age is preventive iron supplementation. In this age group, once iron deficiency is present, anemia develops quickly and therapy with oral iron is needed to rapidly improve the infant's hematological status and avoid possible permanent developmental deficits. The
TABLE 3-3 Strategies for Improving Iron Status
|
Strategy |
Infants |
Preschool Children |
School-Age and Pubertal Children |
Pregnant and Lactating Women |
Nonpregnant Women |
|
Short-term |
Gestational iron status Exclusive breast-feeding for 4–6 months; Delayed cord ligation Preventive iron supplementation Parasite and malaria control where needed After 6 months, periodic deworming where needed |
Preventive iron supplementation Parasite and malaria control where needed Periodic deworming where needed |
Preventive iron supplementation Parasite and malaria control where needed Periodic deworming where needed General vitamin and mineral fortification in school-feeding programs |
Iron and folate supplementation Breast-feeding Parasite and malaria control where needed Deworming where needed |
Birth spacing Avoiding nonsteroidal IUDs Parasite and malaria control where needed Periodic deworming where needed Preventive iron supplementation |
|
Medium-term |
Improved sanitation and hygiene Iron-fortified milk and weaning products General iron fortification |
Improved sanitation and hygiene Targeted fortification General iron fortification |
Improved sanitation and hygiene Targeted fortification General iron fortification |
Improved sanitation and hygiene General iron fortification |
Improved sanitation and hygiene General iron fortification |
|
Long-term |
Improved sanitation and hygiene Improve complementary feeding, including heme- iron-containing foods |
Improved sanitation and hygiene Diet diversity Total food intake |
Improved sanitation and hygiene Diet diversity Total food intake |
Improved sanitation and hygiene Diet diversity Total food intake |
Improved sanitation and hygiene Income improvement Diet diversity Total food intake |
|
NOTE: Parasite and malaria control measures should continue through the medium and long terms; for specifics, consult WHO/UNICEF/UNU (in press). |
|||||
strategy of iron supplementation for this age group is often neglected by those who are unaware that the vast majority of infants live in poor households in the developing world, where the resources for preparing highly bioavailable, iron-rich foods complementary to breast-feeding or for purchasing iron-fortified foods are nonexistent. The risk associated with iron deficiency at this age is so serious that even in the United States, the American Academy of Pediatrics (1992) and the Institute of Medicine (1993) recommend that supplementary iron of 1 mg/kg/day should be provided to infants, and that the dose should be doubled for preterm infants, not to exceed 15 mg/day (a total of 105 mg/week). In preterm infants, supplementation should also start earlier, usually by 2 months of age, but very low birthweight infants should receive supplements as early as 3 weeks of age, provided that vitamin E intake is adequate (Fomon and Zlotkin, 1992).
DeMaeyer (in WHO, 1989), the ACC/SCN (1991), the World Bank (1994), and WHO/UNICEF/UNU (in press) have all emphasized the importance of making supplements available to infants where they are at risk of iron deficiency.
Preschool Children (3 to <7 Years of Age)
The rapid growth of the early years exacerbates the need for iron, and young children are particularly at risk of iron deficiency and anemia. This is true in both developed and developing countries. A number of studies have demonstrated that if anemia is mild to moderate and iron supplements are administered at proper doses, anemia correction can be achieved in a few months.
Iron therapy is intended to correct anemia quickly and uses high iron doses, while preventive iron supplementation aims at improving iron nutrition over a longer period of time and with lower doses and fewer side effects. Therefore, they differ in purpose, iron dose and frequency, and duration of intervention (Gillespie, 1996; INACG, in press). Lack of clarity in this differentiation has led to confusion and has delayed the acceptance of the prevention of iron deficiency through supplementation administered on a weekly basis, as discussed below. At present, the only short-term measures suggested by WHO for the control of iron deficiency are the treatment of those already anemic and periodic repeated administration of daily iron supplementation among populations that exhibit high prevalences of anemia, with the purpose of keeping anemia rates under control (DeMaeyer, 1989).
The alternative of continuous preventive supplementation by a weekly dose was first explored by Liu et al. (1995a,b), who studied 246 kindergarten children, ages 3 to 6, in the city of Changji in China. This investigation compared the effect of daily and weekly iron supplementation on iron nutrition by studying all of the children, whether or not they were iron deficient or anemic. In that population, 39 percent of the children were iron deficient (serum ferritin <20
µg/l) and 16 percent had very low serum ferritins (<12 µg/l); 37 percent of the children were anemic (Hb less than 110 g/l). Children were randomly assigned to 3 months of directly supervised iron supplementation. They were divided among daily, biweekly, and weekly supplementation, with three age-determined classroom groups in each regimen (nine classrooms in total). Each iron regimen provided 6 mg of elemental iron/kg per dose. This is a therapeutic level, equivalent to 120 mg of elemental iron in an adult (about 10 times the normal daily requirement). Results indicate that anemic children (Hb < 110 g/l) responded to all three supplementation regimens with an increase of 10 g Hb/l or more. There were no anemic children left at the end of any of the supplementation regimens studied. In addition, 31 percent of the nonanemic children also increased their Hb levels by at least this amount. Serum ferritin increased significantly in all three groups. Those most deficient in Hb and ferritin showed the greatest response, as would be expected. This study indicated that there was no need to administer more than 6 mg of iron/kg weekly to correct anemia characterized by Hb levels < 110 g/l and > 80 g/l and establish adequate iron stores.
The difference in side effects among the three regimens was dramatic. Among the daily dose group, 35.4 and 39.7 percent of anemics and nonanemics, respectively, reported some side effects—anorexia, nausea, some vomiting, diarrhea, constipation, and abdominal discomfort. Among the children receiving the iron twice weekly, 7.4 percent of anemics and 6.6 percent of nonanemics reported side effects. Anemic and nonanemic children receiving weekly doses presented 0 and 5.7 percent side effects, respectively. The authors conclude that the amount of iron absorbed by these children from weekly doses of 6 mg/kg was enough to prevent iron deficiency and to eliminate anemia in the course of three months. The biweekly and weekly regimens had far fewer reported side effects than the program of daily dosing.
Schultink et al. (1995), in anemic Indonesian preschool children receiving daily or twice-weekly iron at a dose of 30 mg, demonstrated that both regimes were equally effective in correcting the anemia in 8 weeks. In Bolivia, Berger et al. (1997) achieved similar results by administering 3–4 mg of iron/kg daily or weekly for 4 months to 4- to 7-year-old anemic children.
Intestinal parasitosis, and hookworm in particular, is a significant contributor to iron deficiency in older infants and preschoolers in many developing countries (Stephenson, 1987; Stolzfus et al., 1997). Chronic fecal blood loss from infection with hookworm in this age group has been recognized as an important cause of severe iron deficiency and anemia for years in many parts of the world (Bloch, 1971, 1986). Malaria and parasite control measures (including treatment of those affected) should be implemented for this age group where these conditions are endemic (Warren et al., 1993).
School-Age Children and Adolescents
As in the case of preschool children, no specific short-term measures are currently used for the continuous prevention and control of iron deficiency in these vulnerable populations. Periodic cycles of daily iron supplements and treatment of those who are already anemic, plus periodic deworming, are recommended by WHO (WHO/UNICEF/UNU, in press).
In Chile, the use of bovine hemoglobin concentrate to fortify cookies for schoolchildren met with success (Hertrampf et al., 1990; INACG, 1986; Stekel, 1984; Walter et al., 1993b). This program was extensively evaluated and proved that it could effectively prevent iron deficiency and increase iron reserves in menstruating adolescent girls attending school, reducing the proportion of girls with serum ferritin < 20 µg/l from 33 to 17 percent. Unfortunately, the cost of this fortification process is relatively high. In Guatemala, iron intake has been improved in the school population (boys and girls from 6 to 18 years of age) by the daily distribution of high-energy, high-protein cookies, fortified with a multivitamin and multimineral mix (INCAP, 1984). Unfortunately, there has not been a systematic evaluation of the effect of this intervention on iron status or anemia prevalence.
The effectiveness of weekly 60 mg iron + 0.25 mg folate doses has been demonstrated in Malaysia by Tee et al. (1995). The doses were administered by teachers for 5 months (a total of 22 tablets) to adolescent schoolgirls, anemic or not, in correcting mild to moderate anemia and improving iron reserves. In this double-blind study, a nonanemic control group that received only folate did not improve in iron nutrition.
The most comprehensive study of the impact of hookworm on anemia in schoolchildren in general, and its effect on iron status and iron-deficiency anemia, was reported recently by Stoltzfus et al. (1997). In populations in which hookworm is a public health problem, hookworm eradication could reduce anemias. Its impact is greatest among the severely infected children, who also have the most severe iron deficiency and anemia. A major challenge is to ensure the prevention of reinfection by repeated deworming, plus adequate fecal waste disposal and the wearing of closed shoes. Effective, safe, and inexpensive anti-helminthic drugs are now available and their use has accelerated the control of hookworm infection (WHO, 1995).
Women of Childbearing Age
Although in many developing countries 30–60 percent of menstruating women are victims of iron-deficiency anemia, and in some countries nearly all are iron deficient, little attention has been given to this group in planning and implementing specific control measures. These women receive attention only
when they become pregnant. Yet pregnancy is only a part of the reproductive cycle that starts prior to pregnancy and ends only at the end of lactation. It is well-established that their iron nutritional status during pregnancy will be assured only if women enter pregnancy with adequate iron reserves (<300 mg of iron reserves). Nevertheless, women are often grossly neglected as social and productive members of families and societies; they suffer all the consequences of iron deficiency and anemia, whether or not they are pregnant (e.g., Li et al., 1994).
Only recently has attention been given to menstruating women as targets for iron supplementation. In the United States, an ad hoc expert panel on iron deficiency in women of childbearing age recommended that nonpregnant women be treated with 60 to 180 mg of iron/day if they are found anemic and their serum ferritin levels < 20 µg/l (Anderson, 1991). Treatment and maintenance doses (30 mg/d) should continue until serum ferritin reaches 40 µg/l at 6- and 12-month evaluations. The panel recognized that the risk of anemia during pregnancy is greater among women who enter pregnancy with depleted iron stores and recommended universal iron supplementation during gestation as a prophylactic measure. They stress that special attention should be given to groups at greater risk: multiparous and adolescent women who have low-income, less than a high school education, are Black or Mexican Americans, and are blood donors. Similarly, Sloan et al. (1992) conclude from their analysis of multiple iron supplementation trials that "it is difficult to treat a severely iron-deficient woman and provide for increased fetal needs through iron supplementation during the relatively short period of pregnancy." They state that "as a public health approach, prolonged supplementation beginning before the woman becomes pregnant may be a better strategy to benefit the majority of the population (although some individuals will always require more aggressive treatment).''
Two published studies confirm the efficacy of weekly iron supplementation of nonpregnant women of reproductive age. In Indonesia, 273 girls with an anemia prevalence of 17.5 percent were divided into four groups (Angeles-Agdeppa et al., 1997). Group 1 received 60 mg of Fe, 750 ug retinol, 250 ug folic acid, and 60 mg. ascorbic acid. Groups 2 and 3 received 60 mg or 120 mg of Fe weekly plus 6,000 µg retinol, 500 ug folic acid, and 60 mg. ascorbic acid. Group 4 received only a placebo. After two months of supervised intake and a further three months of unsupervised intake, the increase in hemoglobin was the same in all three groups receiving iron and declined in the placebo group. With 60 mg weekly, the incidence of GI side effects was 5.7 percent, the same as in the placebo group, compared with 32.8 percent when 60 mg were given daily. The rise in ferritin at 3 months was highest in the daily supplemented group and intermediate in the weekly groups. Six months after the last supplement, however, the daily and weekly groups had the same ferritin levels, and these were in the normal range.
A 7-month, double-blind, unsupervised study was conducted in Berkeley, California, to compare 7 months of weekly with 3 months of daily iron supplementation (as recommended by WHO) in correcting iron deficiency and building iron reserves in healthy women of child-bearing age. Supplements were consumed daily in Phase I, lasting 3 months, and weekly in Phase II, lasting 4 months, by three randomized groups: Group 1 (N = 37) received daily iron + folate tablets (60 mg of iron as FeSO4 and 250 µg folic acid) during Phase I and weekly folate tablets (250 µg folic acid) during Phase II; Group 2 (N = 35) received a folate tablet 6 days and an iron + folate tablet 1 day every week in Phase I and one weekly iron + folate tablet during Phase II; Group 3 (N = 44) received only folate tablets during both phases. Overall adherence among those completing the study was over 90 percent. Side effects were given as important reasons for withdrawal from the study and were highest with daily iron. At baseline, between 11 and 16 percent of the subjects were iron deficient, and between 8 and 16 percent had low Hb levels, depending on the ferritin (<12 or <15 µg/l) and Hb (<120 or 125 g/l) "cutoffs" used. Ferritin levels during Phase I significantly increased only in Group 1 (p < .001); however, during Phase II, Group 1's ferritin levels fell back to baseline levels (p < .001), whereas Group 2 sustained a moderate increase, especially during Phase II (p < .05 vs. baseline of same group). Group 3's ferritin levels remained stable throughout the two phases. Mean Hb levels were not different among the three groups. However, the proportion of women with Hb < 125 g/l in Group 1 decreased in Phase I but returned to baseline proportion in Phase II; this proportion showed a sustained decrement in Group 2 throughout both phases and showed no specific tendency in Group 3. Iron supplementation by 30 doses administered weekly over 7 months was as effective as or even more effective than as 90 does consumed daily only during the first 3 months.
For women of childbearing age in the developing world, WHO (DeMaeyer, 1989) and a multiagency consultation (WHO/UNICEF/UNU, in press) indicate that for preventive purposes, adolescents and adult women should receive a 2- to 4-month course of 60 mg of iron daily. This course should be repeated every year, or when needed to deal with anemia. This recommendation has been largely ignored, and this regimen can now be replaced by weekly iron supplementation for as long as the women are at risk.
Pregnant Women
The regulation of iron metabolism in pregnancy is unique, because profound hormonal changes, functional adaptations, and large increments in iron requirements occur during this time. Iron absorption increases as pregnancy advances (Barrett et al., 1994; Hahn et al., 1951; Heinrich, 1970; Svanberg, 1975; Whittaker et al., 1991). The mechanism most often suggested is the increase
in red-cell mass and total blood volume, but the progressive development of iron deficiency and other metabolic adaptations during this physiologic state (e.g., increasing plasma transferrin and erythropoietin levels and high levels of placental transferrin receptors) must also play a role.
The physiologic hemodilution that leads to normal decrements in hemoglobin concentration further complicates the evaluation of iron supplementation in pregnancy. Moreover, the reported lack of anemia among chronically undernourished women because of their reduced capacity to expand their plasma volume introduces another variable that is sometimes difficult to control (Rosso and Streeter, 1979; Rosso et al., 1983). Women who enter pregnancy with iron deficiency and low Hb levels will respond to iron administration (a therapeutic effect), but their final Hb levels may still be abnormally low (e.g., Sood et al., 1975). Even in healthy, apparently well-nourished women, Hb falls progressively. Many women develop anemia from the second trimester onward. Hb concentration usually reaches its lowest concentration by 20–28 weeks gestation, and many of the women who develop anemia remain anemic after delivery (Hytten and Duncan, 1956). Iron supplementation of pregnant women has been viewed as the main global strategy for controlling iron deficiency and anemia.
The evaluation of the effectiveness of iron supplementation based on increments in Hb without controlling for initial iron status, Hb levels, and gestational period is fraught with possible errors. Moreover, pregnancy is physiologically an inopportune time to correct prepregnancy iron deficiency because of the elevated iron requirements during the last two gestational trimesters (Scholl and Hediger, 1994; Viteri, 1997a,b). A combination of Hb, plasma or serum ferritin, and transferrin receptor levels is appropriate for the evaluation of interventions during pregnancy (Carriaga et al., 1991; Skikne et al., 1990).
Because of the dual demands for growth and reproduction, pregnant teenagers are at particular risk (Osofsky, et al., 1971). Birth spacing can thus be an effective preventative measure for the control of iron deficiency in teens and adult women. Epidemiological data confirm that multiparous women are more at risk of becoming iron deficient and anemic (Andrade et al., 1991; Pilch and Senti, 1984). Multiparity is associated with larger menstrual flows, and thus with greater chronic iron losses (Andrade et al., 1991). Lactation and its accompanying amenorrhea favors a positive iron balance during the postpartum period, and hormonal contraceptives reduce menstrual flow by one-half (Cole et al., 1971).
In both developed and developing countries, iron supplementation is routinely recommended as part of antenatal care. In the United States, the Food and Nutrition Board of the National Academy of Sciences (1990) recommends the daily use of 30 mg of elemental iron after the twelfth week of pregnancy. Pregnant women with anemia, associated with low serum ferritin, should receive treatment with daily iron at doses of 60–120 mg to restore Hb to normality. When this is achieved, the woman should receive 30 mg of iron daily. The Institute of Medicine (IOM, 1990, 1993) recommended universal supplementation
with 30 mg of iron daily during the third trimester only if the pregnant woman's Hb is <110 g/l. In the second trimester, providing 30 mg of iron daily is recommended if the woman had serum ferritin levels <20 µg/l, even if she was not anemic. In the first trimester the same scheme applied, but supplementation was expanded to cover women with mild anemia (Hb 90–109 g/l).
The most recently published statement from WHO (DeMaeyer, 1989), which was directed to the developing world, recommended universal iron supplementation for pregnant women (60 mg of elemental iron and 250 µg of folic acid, once or twice daily) through the primary health care system. The twice-daily regimen was recommended where gestational anemia was common (most of the developing world). The conclusion of published studies in both the developed and developing world is that the greatest benefits to mothers and infants are achieved when iron deficiency and anemia are controlled as early as possible in pregnancy with doses that do not surpass 60 mg daily.
The review of iron nutrition during pregnancy performed by the Institute of Medicine (IOM, 1990) confirms the relative inefficiency of administering large iron doses to women in industrial countries to improve Hb levels at the 35th–40th gestational week. In effect, the maximal mean Hb concentration at term (127 g/l) was achieved by daily ingestion of either 200 mg of sustained-release iron (Puolakka et al., 1980; Svanberg, 1975) or 65 mg of iron (Taylor et al., 1982), and the maximal mean difference was only 3 g/l, compared with a ferrous fumarate supplement providing 30 mg of iron/day (Chanarin and Rothman, 1971). Moreover, Chanarin and Rothman (1971) compared the relative efficacy of administering supplements of 30, 60, and 120 mg of iron/d for 24 weeks on Hb concentration at term, and found no significant differences among the three regimens.
As demonstrated by the proportion of women who reach the end of pregnancy with Hb levels reaching the accepted cutoff of 110 g/l, short-term iron supplementation of already iron-deficient and anemic pregnant women is very inefficient, even with large daily iron doses (Charoenlarp et al., 1988; Hahn et al., 1951; Simmons et al., 1993; Sood et al., 1975; Svanberg, 1975). These studies confirm that administering high doses of iron not only results in over 90 percent of the iron remaining unabsorbed in the gut, but also produces undesirable side effects in exponential proportion to the iron dose (Hallberg et al., 1966; Sölvell, 1970). Sloan et al. (1992) conclude from their analysis of multiple iron supplementation trials that "it is difficult to treat a severely iron-deficient woman and provide for increased fetal needs through iron supplementation alone during the relatively short period of pregnancy." They suggest that "as a public health approach, prolonged supplementation beginning before the woman becomes pregnant may be a better strategy to benefit the majority of the population."
While the great majority of developing countries recommend iron supplementation during pregnancy, the programs have not been particularly effective because of logistic and compliance problems. Two recently completed studies, one in China (Liu et al., 1995a) and the other in Guatemala (Chew et al., 1996),
demonstrate that weekly iron administration to pregnant women under direct supervision is efficacious. In China the study involved 416 primiparous pregnant women older than 20 years. Anemia prevalence was 33 percent by mid-pregnancy and 20 percent at term, whether they received 60 or 120 mg of iron daily or 120 mg weekly. There were no differences by supplementation schedule and dose in either initial or final Hb and ferritin values. A negative control group had initial anemia prevalence of 20 percent, which became 47 percent at term, and serum ferritin showed a drop from 30 to 17 µg/l.
In the Guatemala study, involving 383 women, prevalence of anemia was 27 percent at about mid-pregnancy, and 16 percent, 25 percent, and 33 percent at term for supervised 60 mg daily and 180 mg weekly, and the group under usual care, respectively. In this population, which was significantly more iron deficient than the Chinese women studied, 60 mg daily supplementation resulted in a greater increase in Hb than 180 mg weekly. This is the only study to date in which the results of daily and weekly supplementation have not been the same for the effect on hemoglobin levels. Nevertheless, no women in either of the groups ended with a Hb value below 95 g/l, indicating that daily and weekly iron supplementation were both effective in reducing risks.
Current evidence indicates that the absorption of supplemental iron is lower in multivitamin and multimineral antenatal supplements than when it is administered alone. This is because of the presence of high levels of calcium and magnesium (Babior et al., 1985; Seligman et al., 1983). The administration of iron supplements with meals decreases their absorbability by about 50 percent (Layrisse et al., 1973; Reizenstein et al., 1975). In improved absorption, delayed release preparations are promising (Cook et al., 1990). The evidence is strong that a blockage of absorption of zinc and copper is a consequence of daily intake of iron in amounts that elevate the ratio of iron to zinc above 2 and that of copper above 20–40:1 (Breskin et al., 1983; Burns and Patterson, 1993; Dallman, 1990; Hambidge et al., 1987; Solomons, 1986; Solomons and Jacobs, 1981; Walsh et al., 1994). A study by Reizenstein et al. (1975) suggests that food-iron absorption is also reduced in the face of supplemental iron intake.
According to the meta-analysis performed by Mohamed and Hytten (1989), iron administration during pregnancy among well-nourished pregnant women did not have a significant beneficial effect on proteinuric hypertension, antepartum hemorrhage, maternal infection, short gestation, or low birthweight. These last results agree with those of Higgins et al. (1982) and contrast with those of Garn et al. (1981), Murphy et al. (1986), Scholl et al. (1992), and Scholl and Hediger (1994), who have clearly shown short gestation or low birthweight with anemia and iron deficiency at mid-pregnancy or before. Anemia and iron deficiency at mid-pregnancy or earlier are more critical than they will be at term in inducing preterm delivery and low birthweight. This fact may partially explain some conflicting results derived from late iron supplementation among mildly iron-deficient populations in the developed world. The controversy in demonstrating
a beneficial effect of correcting mild to moderate anemia in preventing abnormalities during pregnancy as well as in improving newborn weight and overall health in the developed world contrasts with the results in the developing world, where severe anemia is common and consistent differences in the above parameters are documented between more anemic and less anemic or normal women. A re-analysis of the data in the Indian study by Sood et al. (1975) (cited by Mohamed and Hytten, 1989) shows that iron supplementation among poorly nourished, pregnant Indian women reduced anemia prevalence and severity at term, as well as low birthweight, with an odds ratio of 0.31 (0.17–0.56). Several earlier and later papers from India (Rusia et al., 1995, 1996) have also shown functional and placental morphologic alternations and changes in newborn status among iron-deficient, anemic pregnant women. Agarwal et al. (1991) also showed a marked reduction in low birthweight babies (odds ratio: 0.54) in the supplemented compared with the control group. In this study, the percentage of low birthweight babies (weight < 2,500 g) was reduced from 37.9 among the control group to 23.1 among women supplemented with iron from the 20th to 24th week to term, and to 12.1 among those supplemented from the 16th to the 19th week to term. It is important to point out that among the 137 iron-supplemented women, only 1 had an Hb level below 101 g/l at term (0.7 percent), in contrast with the control group, where 33 percent of women had lower Hb levels.
There is also clear evidence that infants born to mothers who received iron supplements during gestation had better iron nutrition and more than twice the iron reserve at 2 months of age and beyond when compared with their counterparts whose mothers were not given iron supplements (De Benaze, 1989; Puolakka et al., 1980). Children born to iron-deficient, anemic mothers have a significantly higher risk of having anemia or iron-deficient erythropoiesis at 1 year of age, as defined by an odds ratio of 7.6, with 95 percent confidence limits of 1.9–11.2 (Colomer et al., 1990).
Nevertheless, in a meta-analysis of iron administration during pregnancy among well-nourished pregnant women by Mohamed and Hytten (1989) and in a paper by Higgins et al. (1982), it did not have a significant beneficial effect on proteinuric hypertension, antepartum hemorrhage, maternal infection, short gestation, or low birthweight. This has led to doubts about the risk associated with mildly anemic pregnant women as defined by the Hb cutoff to label a pregnant woman as anemic (110 g/l according to WHO). There is a general consensus, however, based on solid evidence, that risk is significantly elevated with Hb levels < 90 g/l (Danforth, 1982; Duthie et al., 1991; Sloan et al., 1992).
The beneficial effect of the administration of folic acid in addition to iron during pregnancy has been amply demonstrated in many parts of the world (Chanarin and Rothman, 1971; Fleming, 1990; Sood et al., 1975; Velez et al., 1966; Viteri, 1973). The effect of iron supplementation may be reduced by other nutrient deficiencies. Where vitamin A deficiency is endemic, even if not clinically
apparent, the administration of vitamin A in combination with iron and folic acid produces a greater improvement in the hematological status of pregnant women at term than iron alone (Suharno et al., 1992, 1993). Powers et al. (1985) reported an additional effect of iron supplementation with vitamin B2 in The Gambia, where the deficiency of this vitamin is endemic.
Even mild hookworm infections can be devastating to women of childbearing age, particularly to pregnant women because of their already high iron requirements. A recent WHO consultation (1995) concluded that given the safety of the new deworming drugs, "single-dose, oral anthelminthic treatment can also be given to pregnant and lactating women. However, as a general rule, no drug should be given in the first trimester." The effect of deworming on pregnant women (Atukorala et al., 1994) was clear; women receiving iron supplements and deworming medication (Mebendazole) showed better hematological responses than those receiving only supplements. The supplement tablets contained 60 mg of elemental iron and 0.25 mg of folic acid, and women were instructed to take 1, 2, or 3 tablets daily for 8–26 weeks. The number of tablets ingested, based on a questionnaire, made no difference in hematological or biochemical parameters. Duration of supplementation did: the women supplemented for > 17 weeks responded better.
Sustainable Approaches To The Elimination Of Iron Deficiency
Dietary Improvement
There is a general consensus that the most desirable, sustainable, and safest strategy for the control of iron deficiency is the sustained ingestion of bioavailable iron in food in adequate amounts and reducing iron losses throughout the life cycle. Three main approaches are recognized; a discussion is provided below.
Improving the Supply and Intake of Food Iron
It has been recognized that the availability of food iron has lagged behind that of energy and protein achieved by the enhanced production and true availability of staple foods (FAO, Food Balance Sheets, 1961–1988). The per capita availability of leguminous seeds (an important source of iron for many regions) has been declining sharply (FAO AGROSTAT/PC, 1992), and the intake of heme iron from meat, fowl, and fish in the developing world has, at best, remained stationary, at a very low level. Unfortunately, green leafy vegetables are generally poor sources of dietary iron because of its low bioavailability in these sources, although some are rich in this mineral.
There are many vegetables that are good sources of iron and vitamin C, and the identification and promotion of their use in combinations that favor nonheme
iron absorption should be undertaken by agricultural extension agencies, nutrition personnel, and community groups, particularly in the developing world and in cultures where vegetarianism is common. A balanced vegetable diet can maintain an adequate iron status and avoid anemia, even in women and children, as exemplified by studies of well-informed, Western vegetarian groups, such as the Seventh Day Adventists (Dwyer, 1988; Sanders, 1994). Nevertheless, the prevalence of iron deficiency and anemia in Asian vegetarian populations and in the populations of the developing world that are mainly vegetarian by economic necessity are significantly higher than in otherwise similar omnivore populations (Craig, 1994; Shaw et al., 1995). The inclusion of unfermented soy products in a diet reduces iron bioavailability (Cook et al., 1981).
Improving the Bioavailability of Food Iron
Extensive research, summarized by the International Nutritional Anemia Consultative Group (INACG, 1982) and Hallberg et al. (1992), identified the influence of food components and meal preparation on iron bioavailability. The key issues are reducing the ingestion of inhibitors to iron absorption and increasing the intake of enhancers in a given meal. These include germination of seeds; heat treatment of cereals; fermentation processes; higher intake of meats and of foods and beverages that contain vitamin C; and increasing the intake of acid sauces (e.g., tomato sauces). Others are improving the intake of vitamin A-rich foods, especially preformed vitamin A; cooking vegetables rich in vitamin C sparingly, and cooking cereals, seeds, and the like that are rich in phytates more intensively. The intake of inhibitors can be reduced by decreasing the consumption of high-fiber, high-phytate, and high-polyphenol foods such as tea, coffee, chocolate, and herb teas and separating the intake of high-calcium foods and supplements from iron-rich meals. Educating people about food choices, dietary combinations, cooking practices, and intrahousehold distribution strategies that improve iron supply and bioavailability in each meal is a neglected strategy.
Agricultural research into the production of new genetic varieties of cereals with reduced inhibitor (e.g., phytic acid) content, and thus more bioavailable iron, is promising (Combs et al., 1996). Most of these varieties are still in the agronomic and genetic experimental stages, but some are already the subject of studies in humans (Mendoza et al., in press). It is too early to project these results to population groups. Research on the importance of certain green leafy vegetables as a source of bioavailable iron is urgently needed, because there is currently not enough information to evaluate the effectiveness of their contribution to improving iron nutrition (de Pee et al., 1996).
Correcting other nutrient deficiencies that may alter iron absorption and metabolism through dietary changes is also a worthwhile measure. Special attention should be given to ensuring adequate vitamin A, folate, riboflavin, and vitamin B12 nutritional status. Unfortunately, the best food sources of these
nutrients, except for folate, are animal products, including milk and its products, eggs, meats, and viscera, many of which are out of reach for populations with limited resources or who live far from markets and cannot engage in the production of small livestock and poultry. The best sources of folate are organ meats, green leafy vegetables and sprouts, and some fruits (including oranges, cantaloupes, and melons). Folate can be lost by prolonged cooking in large volumes of water. It is estimated that between 50 and 90 percent of food folate is destroyed during cooking.
The favorable dietary practices previously described must be learned and utilized by food providers, preparers, and those responsible for the distribution of food in the household. Empowerment of communities is essential in achieving the desired dietary practices. Different approaches to communication and education in food and nutrition (e.g., social marketing, reflexive participation, and so forth) are only part of this process, which will be lengthy if it is to be sustainable. This is particularly important since the effects of improving iron nutrition (increasing iron reserves and/or reducing anemia prevalence and incidence) can only be perceived after many months or years and because increments in total dietary iron absorption are moderate (Hulten et al., 1995).
Food Fortification
Iron fortification of foods is a preventive measure that aims at improving and sustaining iron nutrition on a permanent basis (Bauernfeind and Lachance, 1991). It can be targeted to groups at risk of iron deficiency or to whole populations, depending on the characteristics of their diet, the epidemiology of iron deficiency and other nutritional anemias, the availability of appropriate iron compounds and food vehicles, the available industrial and logistical facilities, and the financial resources, among other considerations. Fortification of foods can include only iron (single fortification), or it can be extended to encompass two or more nutrients (multiple fortification). It can use a variety of iron compounds and vehicles. A number of recent publications address this food-based strategy in detail (Bothwell and MacPhail, 1992; Clydesdale and Wiemer, 1985; Cook and Reusser, 1983; Hurrell, 1984, 1985, 1992, in press). For fortification of any kind to be effective, three essential factors are necessary: (1) an effective and affordable iron compound must be available and acceptable; (2) a food vehicle must also be available and accessible; and (3) detailed production instructions and monitoring procedures must be in place and enforced by law.
A variety of foods have been used for iron fortification. Ideally, the food selected is consumed regularly in sufficient, stable quantities by the target populations and is centrally processed, easy to fortify, stable in storage, inelastic to price, minimally altered by the addition of the fortificant, and amenable to proper regulation and monitoring. The food that is used also needs to be priced so that an increment in cost of the fortified product, including packaging, can be
absorbed by the population, the government, the producer, or the retailer, or in some combination of these groups.
Fortification Compounds
The iron preparations used in the fortification of a variety of food products can be categorized in four main groups (Hurrell et al., 1989):
- Water-soluble compounds, such as FeSO4 and ferrous gluconate, are inexpensive but have a metallic taste and are reactive, leading over time to oxidation products and off-flavors and colors. Nevertheless, for products with rapid turnover, they are usually the fortificants of choice. Microencapsulation can extend shelf life, but increases price.
- Poorly water-soluble compounds, soluble in dilute acid solutions, can be used. Ferrous fumarate, succinate, and saccharate belong to this category. Because they are less reactive, they are preferred for fortifying semisolid or solid foods, such as infant cereals and food powders, but they are more expensive.
- Water-insoluble compounds, poorly soluble in acid solutions, including the phosphate salts (ferric orthophosphate, pyrophosphate, and ferric-ammonium orthophosphate) and forms of elemental iron (reduced, carbonyl, and electrolytic irons), belong in this group. These compounds are stable, poorly reactive, and essentially tasteless, but they are generally poorly and unpredictably absorbed, depending on the manufacturing processes. Heating of the fortified product can improve the bioavailability of these compounds. Ferric-ammonium orthophosphate has a significantly better absorbability.
- Chelated iron compounds, such as bovine hemoglobin concentrate, FeNaEDTA, and amino-acid chelates are soluble in water. FeNaEDTA has the advantage of being stable and without flavor. An important characteristic of iron chelated with EDTA is that it enhances the absorption of the whole nonhemeiron pool (INACG, 1993; Viteri et al., 1978). EDTA itself (for example, FeNaEDTA, CaNaEDTA, or NA2EDTA), at a molar ratio to nonheme iron ≥ 1, also enhances iron absorption by a factor of approximately 3 or 4 (MacPhail et al., 1994). Bovine Hb concentrate has a definite taste, requires a strong sanitary infrastructure, and is more reactive than FeNaEDTA. It is very effective as a fortificant, however, and also improves the absorption of dietary nonheme iron (Stekel, 1984; Walter et al., 1993b). Its higher cost is partly compensated by its much higher absorption.
The iron content, relative bioavailability in the rat and the human, enhanced absorption achieved by addition of vitamin C to fortified cereals, and approximate relative cost of the most common iron sources for food fortification are presented in Table 3-4.
Fortification Strategies
Many industrial countries and some developing countries fortify a cereal product. Fish sauce, sugar, and curry powder have also been successfully used as vehicles for iron fortification with NaFeEDTA in Thailand, Guatemala, and South Africa, respectively (Ballot et al., 1989a,b; Garby and Areekul, 1974; Viteri et al., 1983; 1995b). Amino-acid chelates are being tested for their effect on anemia prevalence and ferritin levels in feeding trials in Brazil and other countries. These include cheeses and wheat and maize flours fortified with low levels of iron (to provide about 2 mg/d). Results appear very promising (Bovell-Benjamin et al., 1997; Olivares et al., 1997; Pineda, 1996).
Many industrial countries have fortified milk and soy-based formulas with different iron compounds (ferrous sulfate, ferric ammonium citrate, and ferric or sodium iron pyrophosphates) for consumption by infants and young children (Bothwell and MacPhail, 1992; Fomon, 1987; Theuer, 1985). Most formulas are currently fortified with ferrous sulfate (12 mg of iron/l), with the addition of 55 mg of vitamin C. Several studies in Chile have also demonstrated the effectiveness of iron fortification of cow's milk with ferrous sulfate alone, or with ascorbic acid (INACG, 1986; Stekel, 1984).
Cereal-milk infant and weaning foods have also been fortified with about 100–120 mg of insoluble iron compounds/kg for many years, but the bioavailability of such iron compounds is generally poor (Rios et al., 1975). Nevertheless, the consumption of 16 mg of iron/day from a rice cereal fortified with 550 mg of electrolytic iron /kg was effective in preventing iron deficiency and anemia in Chilean infants (Walter et al., 1993a). These results are encouraging industry to add water-soluble or poorly water-soluble/acid-soluble iron compounds together with ascorbic acid to prepared infant foods (Cook and Reusser, 1983; Hurrell et al., 1989). Formulated protein mixtures intended for infants and preschool children—such as INCAPARINA, COLOMBIARINA, BIENESTARINA, and many others—including rations for refugee populations, are also fortified with iron, mostly as ferrous sulfate. Rice-based weaning foods have been successfully fortified with bovine hemoglobin concentrate (Calvo et al., 1989).
Complementary foods and a variety of cookies used in school feeding programs are being fortified with iron and other nutrients in different countries. The short shelf life of these products makes it possible to use the relatively inexpensive ferrous sulfate. In Chile, the use of bovine hemoglobin concentrate to fortify cookies for schoolchildren has been successful (Hertrampf et al., 1990; INACG, 1986; Walter et al., 1993b). This program effectively prevented iron deficiency and increased iron reserves in menstruating adolescent girls attending school, reducing the proportion of girls with serum ferritin < 20 µg/l from 33 to 17 percent.
TABLE 3-4 Characteristics of Iron Sources Commonly Used to Fortify Foods
|
Iron Source |
Approximate Iron Content |
Average Relative Bioavailability |
Approximate Relative Cost |
|||
|
|
|
Rata |
Mana |
+ Vitamin Cb |
Ac |
Bd |
|
Freely water-soluble |
|
|
|
|
|
|
|
Ferrous sulfate 7H2O |
20 |
100 |
100 |
×3.7 |
1.0 |
1.0 |
|
Dried ferrous sulfate |
33 |
100 |
100 |
— |
0.7 |
0.7 |
|
Ferrous gluconate |
12 |
97 |
89 |
— |
5.1 |
5.7 |
|
Ferrous lactate |
19 |
— |
106 |
— |
4.1 |
3.9 |
|
Ferric ammonium citrate |
18 |
107 |
— |
×12.9 |
2.1 |
— |
|
Poorly water-soluble/soluble in dilute acid |
|
|
|
|
|
|
|
Ferrous fumarate |
33 |
95 |
100 |
— |
1.3 |
1.3 |
|
Ferrous succinate |
35 |
119 |
92 |
— |
4.1 |
4.4 |
|
Ferric saccharate |
10 |
92 |
74 |
— |
5.2 |
7.0 |
|
Water-insoluble/poorly soluble in dilute acid |
|
|
|
|
|
|
|
Ferric orthophosphate |
28 |
6–46 |
25–32 |
×4.0 |
4.1 |
16.4–12.8 |
|
Ferric ammonium orthophosphate (EKA Nobel, Sweden) |
19 |
— |
45–58 |
— |
2.3 |
5.1–4.0 |
|
Ferric pyrophosphate |
25 |
45–58 |
21–74 |
— |
2.3 |
11.0–3.1 |
|
Elemental iron powders |
|
|
|
|
|
|
|
Electrolytic |
98 |
44–48 |
5–100 |
×2.4 |
0.5 |
10.0–0.5 |
|
Carbonyl |
98 |
39–66 |
5–20 |
— |
1.0 |
20.0–5.0 |
|
Reduced |
97 |
24–54 |
13–148 |
— |
0.2 |
1.5–0.14 |
|
Chelated compounds Gastric delivery system (Ferrous sulfate) |
|
|
|
|
|
|
|
NaFeEDTA - 3 H2O |
14 |
— |
100–416e |
×2.1 |
6.0 |
6.0–1.4f |
|
Amino-chelated iron (Albion) |
26 |
— |
100–500g |
— |
— |
— |
|
Concentrated bovine hemoglobin |
0.34 |
— |
100–700 |
— |
30.0 |
6.0f |
|
a Relative to FeSO4-7H2O = 100, at the same level of total iron. b Relative increase in absorption when vitamin C is added to cereal products. c Relative to FeSO4-7H2O = 1/mg Fe. d Relative to FeSO4-7H2O iron actually absorbed = 1/mg Fe. e At proper doses (1 molar ratio EDTA/Fe ≥ 1). f Cost per mg total Fe absorbed may be even lower because of higher total nonheme dietary iron absorption. g Preliminary. SOURCE: Bothwell and MacPhail, 1992; Bovell-Benjamin et al., 1997; Cook and Reusser, 1983; Forbes et al., 1989; Hurrell, 1985, 1992, 1997; Hurrell et al., 1989; Olivares et al., 1997; Viteri et al., 1978; Walter et al., 1993. |
||||||
Effectiveness of Iron Fortification
Many studies involving large groups of subjects have been carried out to evaluate the impact of a given iron fortification strategy on anemia and iron nutrition (Cook and Reusser, 1983; Dutra de Oliveira et al., 1996; Fomon, 1987; Hurrell, 1997; Indian Council of Medical Research, 1989; Viteri et al., 1995b). Despite the long history of cereal fortification in industrial countries, there are almost no studies confirming its effectiveness for reducing iron deficiency. The majority of studies have involved infants and children, where the impact of iron-fortified formulas, milk and soy preparations, and infant foods has been amply documented. The experience of these trials can be summarized by indicating that iron deficiency and anemia in older infants (8 months and older) and young preschool-age children can be essentially eradicated by the consumption of iron-fortified milk preparations after 4 to 6 months of exclusive breast-feeding, by the consumption of iron-fortified infant foods, or both. Even when breast-feeding is not possible, the consumption of fortified formula is efficacious. Vitamin C is an important additive in these preparations (Dallman, 1986; Filer, 1989; INACG, 1986).
There are five large studies in developing countries that address the effectiveness of general iron fortification, one each in Thailand, India, South Africa, Guatemala, and Venezuela (Ballot et al., 1989a; Garby and Areekul, 1974; Layrisse et al., 1996; Viteri et al., 1983, 1995b; Working Group on Fortification of Salt with Iron, 1982) (see the Appendix, p. 83). These five studies provide reassuring examples of the effectiveness of general iron fortification of foods when it is based on careful planning and well-established guidelines. A study of a province in Argentina (Tierra del Fuego) is in progress involving iron-fortified milk with microencapsulated ferrous sulfate, recommended especially for consumption by children and pregnant and lactating women (A. O'Donnel and E. Carmuega, personal communication, 1996), and two large projects involving fortification of corn tortillas and sugar with multiple nutrients is ongoing in Mexico (A. Chavez, International Life Sciences Institute (ILSI) ILSI News, December, 1996).
These studies also demonstrate that:
- Many different sources of iron and the vehicles employed can be effective in raising the iron intake of populations to levels that could not be achieved by diet alone, unless dramatic changes took place in food patterns. For example, it would be almost impossible to raise the iron intake of the average Indian citizen by 10 to 15 mg/d. It would require doubling or tripling the current dietary intake.
- Significantly raising the intake of nonheme iron, even though its absorption is low, can markedly improve the status of iron-deficient populations.
- Significant changes, compared with groups with no intervention, occur rather quickly in such populations, but slow down as iron nutrition improves and iron stores increase. Therefore, the full effect of fortification requires a long time.
- With rare exceptions, iron fortification of foods does not affect the iron status of populations that are well nourished in iron. This implies that the risk of iron overload from iron fortification in normal individuals is essentially nil, and that probably only in the long term will the iron reserves of menstruating women reach the levels needed to reduce the risk of iron deficiency in pregnancy.
- The use of chelated iron as NaFeEDTA has demonstrated advantages: it is well absorbed, it promotes dietary nonheme iron absorption in the face of inhibitors, it improves zinc absorption and nutrition, and it does not appear to affect the absorption of calcium or copper. The use of amino-acid-chelated iron appears promising.
- Double fortification of sugar with microencapsulated retinyl palmitate and NaFeEDTA is possible and effective. Equally possible is the double fortification of salt with iron and iodine, now done in some Indian states, but problems with the stability of iodine in salt still prevail. Multiple fortification of flours and specially prepared foods can also be effective (for example, maize and wheat flours fortified with ferrous fumarate and cookies fortified with Hb or multivitamin and multimineral mixes).
Iron Supplementation
During Pregnancy
A synthesis of iron supplementation trials prior to 1956 and up to 1983 (Hytten and Duncan, 1956; Mohamed and Hytten, 1989) and an exhaustive review of studies from 1966 to 1989 (Sloan et al., 1992) indicate that iron administration during pregnancy drastically reduced the prevalence of Hb values < 100–105 g/l (odds ratio 0.12, 95 percent confidence interval 0.07–0.20). The mean Hb change in the combined studies was positively related to duration of supplementation, and the lower the Hb before supplementation, the greater its positive change, as would be expected. In the absence of iron supplementation, pregnancy was generally associated with declines in hemoglobin. Iron administration during pregnancy also reduced the prevalence of serum ferritin values <10 µ/l at 36–40 weeks gestation in relation to unsupplemented women (odds ratio 0.05, 95 percent confidence interval 0.02–0.11).
Given the clear benefit of iron supplementation during pregnancy to both mother and child among populations where iron deficiency is a serious public health problem, why has this intervention not had more success in reaching the
target audience? There are a number of reasons: lack of political will and program support; inadequate awareness of the seriousness and magnitude of the problem; lack of clarity in differentiating iron deficiency and anemia; poor compliance with taking the iron supplements—in part, because of the side effects—and low coverage of at-risk populations.
The lack of political will and program support in Latin America are clearly evident from a survey of programs in 38 countries by Viteri (1996) and the reports of two subregional workshops covering all Latin American countries (Gueri and Viteri, 1996; Viteri et al., 1996b). Opinions on the relationship between noncompliance with iron supplementation and reported side effects vary. Studies conducted in Africa, Asia, the English-speaking Caribbean, Latin America, and the western Pacific vary in the level of side effects reported (ACC/SCN, 1991; Charoenlarp et al., 1988; Chew et al., 1996; Ekstrom et. al., 1996; Liu et al., 1995a; Ridwan et. al., 1996; Simmons et. al., 1993; Viteri, 1996). Adherence to supplemental programs can be improved by information, education, and motivation at the community level, including explanations of the possible temporary discomforts secondary to iron intake. Flexibility in dose to reduce side effects; in the rigidity of daily intake; in the time the supplement is taken (with meals or at bedtime, rather than between meals during the day); and other modifications according to culture, knowledge, practices, and attitudes also improve adherence (ACC/SCN, 1991). With weekly dosage, side effects were lessened in infants (Husaini, 1996), preschool children (Liu et al., 1995b), and pregnant women (Chew et al., 1996; Liu et al., 1995a).
Lack of adequate coverage of the target populations has been an equally important reason for the poor success of iron supplementation interventions. Current practices of iron supplementation rely almost exclusively on the health care system. Lack of commitment on the part of medical and other health personnel, inadequate operation of the health system, insufficient and inconsistent supplies of the needed supplements, and difficulty gaining access to medical facilities are all reasons that have been given for low coverage rates of pregnant women. Other reasons, frequently ignored, are that people simply forget to take the supplement, particularly if no clear benefit is evident. There are also misconceptions about the effect of supplements (e.g., sterilization, big babies with more difficult labor, and the like).
As with many other public health measures, the key to success is involvement of all levels of the community from the inception of the program and active community participation in its operation, evaluation, and in implementing the modifications that are necessary based on rapid-evaluation feedback. This requires the informed delegation of authority from the health system to the community. The former should establish efficient information and monitoring systems to ensure the efficiency and safety of the programs and to actively seek integration of efforts both within and outside the health system, including the cooperation of community resources (for example, schoolteachers, religious and
civic leaders, industrial and commercial organizations, mother and infant clubs, AA, and the like).
Prepregnancy Supplementation
If a woman enters pregnancy with adequate iron stores, she has a higher probability of going through pregnancy and the full reproductive cycle without developing iron deficiency. Preventive supplementation of women of childbearing age before and after pregnancy, through repeated reproductive cycles, offers the best potential for reducing iron deficiency.
The historical development of iron supplementation programs and general efforts to control iron deficiency have suffered from several factors:
- Iron deficiency has no evident and dramatic health effects until it is extremely severe, although its hidden functional and health effects may be more widespread and equally important in their detrimental effects on individual, social, and economic development and well-being. Even the 40 percent of maternal deaths that are associated with severe anemia (hematocrit < 14 percent in young teenagers) have been often ignored, except for acute emergency treatment (Harrison and Rossiter, 1985). Overall association of severe anemia and maternal deaths was about 20 percent in India (Menon, 1968) and as high as 50 percent in severely anemic Nigerian women (Fullerton and Turner, 1962).
- The factors that influence adherence and rejection of current iron supplementation programs have been given inadequate analysis and attention. Failure of programs has been attributed mainly to attributes of the recipients and the characteristics of the medication; little attention has been given to the operational difficulties of daily antenatal supplementation.
- Prepregnancy iron reserves have not received adequate attention, and their importance has often been ignored, even among the leaders in the field of iron nutrition. Initial Hb level is the strongest predictor of Hb level at term, and prepregnancy serum ferritin is an important predictor. The value of entering pregnancy with iron reserves has been well demonstrated (Kauffer and Casanueva, 1990), can be easily understood, and is clearly recommended by two papers (Sloan et al., 1992; WHO/UNICEF/UNU, in press), but it is not mentioned in many major declarations regarding iron supplementation in pregnancy or in important reviews of the subject.
- There has been confusion about the meaning of ''physiologic anemia of pregnancy" in both well-nourished populations in industrial countries and in poorly nourished populations in developing countries. In the industrial world, Hb levels at term at the lower limit of normality or in the upper end of the "anemia" range (100g/l) are of no consequence; they may even appear beneficial in many cases (Mohamed and Hytten, 1989). In the developing world, many chronically undernourished women are incapable of fully expanding
- plasma volume (Rosso, 1990). Consequently, the true deficit in Hb mass from anemia is masked, and even mild anemia is pathological under these circumstances.
- Limiting the implementation period for measures to correct iron deficiency and anemia to the last half of pregnancy is far from ideal, particularly if large doses of daily iron are recommended, because of the unpleasant side effects produced in a significant proportion of women. In many places there has been no other choice, because iron supplementation has depended exclusively on the health system and pregnancy has been the only time when health centers have contact with women.
- The "medicinal," high-iron-dose approach has faced many problems, including increased dose-related side effects, having to ingest several tablets a day, and logistic and motivational problems among providers (ACC/SCN, 1991; Galloway and McGuire, 1994). Furthermore, it has tended to limit the practice of iron supplementation to the health system. Unfortunately, this system works very poorly in many countries, and it is essentially unavailable to over half of the rural population in the developing world (UNDP, 1991).
- Even though every study shows that unsupplemented women have lower Hb levels and display clear indications of iron depletion at term when compared with supplemented women, confusion arises because supplemental iron intake should be planned primarily to prevent iron deficiency and an abnormal fall in Hb. Increments in Hb are to be expected only among women who are already anemic on first evaluation, but initial Hb level is the strongest predictor of Hb level at term (Baker and DeMaeyer, 1979; Charoenlarp et al., 1988; Chew et al., 1996; Liu et al., 1995a; Ridwan et al., 1996; Sood et al., 1975).
- Hb concentration at term is also subject to the level of plasma expansion. In the therapy of ferropenic anemia, the rate and the magnitude of the Hb response to iron intake is proportional to the initial Hb deficit and is, up to a point, modified by the supply of iron, among several factors. It appears that duration of supplementation is more important than dose; the earlier iron supplementation starts, the more effective it is (Atukorala et al., 1994; Charoenlarp et al., 1988; Sloan et al., 1992).
As a response to these issues, "preventive supplementation" based on a community-centered regimen of weekly iron doses to populations or groups within populations at risk of developing iron deficiency has been proposed (Viteri 1993, 1994b, 1995). This proposal originated from animal studies showing that synchronizing the administration of iron supplements with the turnover of the intestinal mucosa improved the efficiency of iron absorption by a factor of more than 2.5 (Viteri et al., 1995a) and by the results of a pilot study in preschool children in China by Liu et al. (1995b). The latter demonstrated equally satisfactory results in improving hemoglobin status after 3 months of supplying 6 mg of iron/kg in daily, twice-weekly, and weekly iron supplementation.
The theoretical basis for the weekly schedule in the human is that intestinal mucosal turnover occurs every 5 to 6 days.
The International Iron Nutrition Project (IINP) of the United Nations University (UNU), in cooperation with WHO and UNICEF, and with the financial support of various donor agencies—Micronutrient Initiative (MI), International Development Research Centres (IDRC), IMPACT/AID—initiated comparative studies of the efficacy of weekly iron in field studies covering a number of population groups, from infants to pregnant and childbearing-age women. Stimulated by the protocol, a number of independent studies have also been completed. Ten studies in which consumption of each supplements is supervised have now been completed in women and children in Bolivia, China, Guatemala, Indonesia, Malaysia, and the United States (ACC/SCN, 1997). The results published to date are given in the section on Prevention of Iron Deficiency in At-Risk Groups. They indicate that at an appropriate dosage, and with assured compliance, hemoglobin levels are the same after 2 to 3 months with both daily and weekly regimens unless there are complicating factors such as malaria. The potential logistic and financial advantages of a weekly supplement compared with daily dosage are obvious. The challenge is to demonstrate the practicality of large-scale weekly programs with sustainable mechanisms to ensure adequate compliance.
Based on these results, women of childbearing age with low iron reserves (at risk of iron deficiency and anemia) can take a tablet containing 60 mg of iron + 3.5 mg of folic acid on a weekly basis, before going to bed, as an established routine supported by community organizations under the direction and monitoring of the health sector. Several studies now indicate the safety and efficacy of this dosage. An average of 8 percent of that iron is expected to be absorbed in iron replete subjects (Cook and Reddy, 1995), providing the equivalent of 0.7 mg of iron daily. Absorption may be as high as 40 percent or more in iron-deficient anemic subjects, providing the equivalent of 3.8 mg/day. The long-term effect would be to correct iron deficiency and increase iron reserves to desired levels (currently found in only 50 percent of women of childbearing age in the United States—Cook et al., 1986—and few women in most developing countries). Women should be encouraged to double the dose as soon as they are found to be pregnant. After delivery they could go back to the single, weekly dose.
Benefits And Costs Of Preventing Iron Deficiency
Table 3-5, modified from WHO/UNICEF/UNU (in press), presents the estimates of relative effectiveness and cost per Disability Adjusted Life Year (DALY) of different supplementation strategies and offers a comparison with iron fortification computed by various models (Murray and Lopez, 1994).
Community-based preventive supplementation through widespread weekly iron supplements that cover all subjects at risk of iron deficiency and anemia is estimated to have a relative cost 1.5 times the cost per DALY in the case of universal iron fortification plus weekly residual prenatal iron supplementation. Current practice of only daily prenatal iron supplementation is estimated to be 6.25 times more expensive per DALY than the estimate for community-based, preventive weekly supplementation. In addition, the number of DALYs achieved in the short-term with either widespread preventive iron supplementation or universal fortification multiplied by the average yearly income of the population that benefits from such programs, divided by the estimated cost of the intervention ($24,00 or $16,000), represents the respective benefit/cost ratio. Based on UNDP average yearly income figures (US$241.5), adjusted by the proportion of the work force that stands to benefit from the control of iron deficiency and anemia, benefit/cost ratios are 46.9 and 81.4, respectively. This ratio increases further when the long-term benefits are considered and the medium- to long-term reduction in costs of treatment of anemia are taken into account.
TABLE 3-5 Cost and Benefit/Cost Ratios of Iron Supplementation Schemes and General Iron Fortification Programs
|
Intervention |
Benefit/Cost Intervention |
Number of DALYsa Achieved |
Cost per DALY (US$) |
|
Short-term (daily, weekly) benefits and costs of iron supplementation programs |
|
|
|
|
Prenatal supplementation only |
511 |
100 |
51 |
|
Widespread supplementation to all iron-deficient and anemic subjects and at-risk groups |
4,665 |
88 |
24 |
|
Universal fortification |
5,038 |
16 |
11 |
|
Plus residual prenatal supplementb |
5,394 |
39 |
16 |
|
Long-term benefits and costs of iron supplementation programs |
|
|
|
|
Preventive supplementation |
2,679 |
37 |
17 |
|
Fortification |
3,332 |
9 |
|
|
a Per 100,000 population, considering global birth rates, fixed and other operational costs, and current individual expenditures in purchasing iron-containing preparations (based on information from Guatemala). b Considering that in spite of iron fortification and adequate prepregnancy iron reserves, prophylactic iron supplementation will still be recommended during pregnancy. |
|||
Prevention of Iron Loss Due to Parasites
Many recent reviews have been written on the problem of intestinal parasitosis, hookworm in particular (Stephenson, 1987; Stolzfus et al., 1997a,b; Warren et al., 1993; WHO, 1995). The chronic fecal blood loss from infection with hookworm has been recognized as a cause of iron deficiency and anemia for many years (Layrisse and Roche, 1964; Roche and Layrisse, 1966; Scrimshaw et al., 1968). Eliminating hookworm infection by adequate fecal waste disposal and the wearing of shoes is the best solution. As an immediate intervention, deworming, especially the effective prevention of reinfection, is an important intervention in the control of iron deficiency and anemia among male tropical agricultural workers.
A significant advance in the availability of effective, inexpensive, essentially unabsorbable and safe anthelmintic drugs in recent years has allowed the acceleration of the control of hookworm infection by repeated anthelmintic medication. Costs of deworming are difficult to calculate, because most estimates of cost are based on health system interventions, which are expensive, and costs to prevent reinfection are not considered. For example, a school nurse who rotates among schools could direct and supervise interventions at the schools at a cost of about US$2.00 per pupil yearly. One dose of albendazole costs about US$0.15. Given the safety and efficacy against hookworms of the drugs being used now, community-based programs should be implemented. This would reduce the costs. Deworming has many other advantages in addition to decreasing iron losses, so that benefit/cost ratios are favorable. Data on costs and benefit/cost ratios are urgently needed. Even World Bank documents (Warren et al., 1993) do not have specific data on the costs of treating hookworm infection.
Suggested National Goals
There is no reason to delay actions to control iron deficiency among populations at risk, particularly those in the developing world. The evidence is over-whelming that iron deficiency and anemia have serious negative effects.
Short-Term
- Each country should draw up a plan of action that will lead to the following medium- and long-term goals:
-
- The 5-year, medium-term goal: Reduce to one-third the current prevalence estimates of anemia in pregnant women and children from 9 months
- of age to school-age and improve prepregnancy iron nutrition and antenatal supplementation practices.
- The 10-year, long-term goal: Eradicate nutritional anemia and iron deficiency as a public health problem, defined as not more than 5 percent ferropenic anemia in each age and gender group at risk of iron deficiency. This would mean not more than 10 percent iron deficiency in each age and gender group at risk.
- Each country should determine which of the possible interventions available to prevent and correct iron deficiency and anemia, and their combinations, are most suitable to implement and/or improve immediately. These should include the adoption of the concept that pregnancy is a part of the life of mature women, and that it should not be considered in isolation. The chosen plan of action, including the suitable interventions, should be coordinated with efforts to control vitamin A deficiency and other nutritional and relevant health problems that impinge on the iron nutritional situation of the population, with emphasis on the pregnant and lactating woman, early childhood, and women of childbearing age.
- Each country and the international community should implement mechanisms for national and international decisionmakers to become aware of the extent and severity of the problem; to be informed of the existing options for the control of iron deficiency and anemia, including benefit/cost ratios; and to be sensitized to take action. International and bilateral agencies, nongovernmental organizations, and other donor agencies must be prepared to assist governments that are willing to act.
- Each country should establish national and local groups to promote the programs. The group will include technical members in government and academic institutions, as well as leaders from both within and outside the community who will develop a sense of ownership of the campaign to control iron deficiency and the sustainability of the program.
- With full participation of the citizens of the country or of a specific community, clear strategies and well-structured programs should be designed, with precise goals and targets to be reached at specified times. This includes the development of a functional nutritional anemia and iron deficiency surveillance system, integrated with the national health, food, and nutrition surveillance system.
- Action-oriented research should be carried out by local institutions and will receive the necessary support.
Long-Term
- The development of food-based approaches should be promoted to ensure adequate intake of bioavailable iron by the population.
- All conditions that result in chronic blood loss should be reduced; if possible, they will be eliminated.
- The necessary mechanisms, based on periodic evaluations of the situation, should be established to ensure the flexibility of existing programs and the sustainability of actions that ensure the adequacy of iron nutrition of all the population.
The author thanks Mr. Mitchell Knutson for his editorial suggestions and revisions of various drafts and sections of this paper and for several technical discussions.
Appendix
Five Studies in Developing Countries Addressing the Effectiveness of Iron Fortification
The Thai Study
Garby and Areekul (1974) evaluated fish sauce fortified with NaFeEDTA to a concentration of 0.5 to 1 mg of iron/ml. The average per capita consumption was estimated at between 10 and 15 ml/day. The study involved stability and blind acceptability trials of fortified fish sauce added to three typical dishes. There was no statistical preference for fortified or unfortified fish sauce in any of the three dishes. Based on bioavailability studies, the fortified fish sauce in each meal would provide close to 0.4 mg of absorbable iron.
The field study, which involved 880 persons at the start, was conducted for 1 year in two villages. One village received the fortified product and the other did not. Hookworm infection was highly prevalent and was not treated. The children, men, and women in the village consuming the fortified fish sauce increased their hematocrit by an average of about 1.5 percent above the changes observed in the control village; the differences in response were greater among the subjects with initially low hematocrits, as expected. It was concluded that the fortified fish sauce was effective in correcting iron deficiency and anemia and that the hematocrit changes would probably be greater with a longer follow-up. Consuming fortified fish sauce would cost between 0.1 and 0.3 percent of monthly salary because of a 20 to 30 percent increase in the cost of the fish sauce. Unfortunately, fish sauce is not used throughout all of Thailand.
The India Study
The Working Group on Fortification of Salt with Iron (1982) undertook a large study that followed a small pilot study in schoolchildren that proved satisfactory in stability of the product, tolerance, and efficacy in increasing Hb levels (Nadiger et al., 1980). Iron added to salt by various formulations had been proven bioavailable (Narasinga-Rao and Vijayasarathy, 1975, 1978).
This study evaluated the effect of iron-fortified salt on Hb levels in three lower-middle-income or low-income groups in rural areas (near Calcutta, Hyderabad, and New Delhi) and in an urban area (Madras City). The experimental groups, which totaled over 7,000 persons, were compared to a similar number of subjects in control groups, who consumed ordinary salt. The study lasted up to 2 years. Fortified salt was sold in village shops in one rural site and freely distributed to the households of the other three sites. Minimal medical attention was provided to all the sites, and all persons with Hb levels < 60 g/l were treated and excluded from analysis.
Anemia prevalences in young children in the rural areas ranged from 98 to 53 percent, and 23 to 9 percent in older children. In Calcutta, all age and gender groups were affected (including adult men), with anemia prevalences above 90 percent. In Madras, anemia prevalence in women was around 30 percent and in men was around 30 and < 7 percent. Prevalences among adults in the other rural areas ranged from 77 to 32 percent. In summary, rural experimental communities exhibited progressive increments in Hb, reaching an average of near 30 g/l after one year in Calcutta and near 10 g/l in Hyderabad (the rise was inversely proportional to the initial Hb deficit, as expected). In the urban center, close to a 5 g/l increment was observed at the 6-month evaluation; essentially no further increment was observed at the 12-month evaluation. The control groups showed only marginal changes. Deworming improved the response to fortified salt by about 4 g/l, from about 10 to about 14 g/l at the 6-month evaluation.
The stability of iodine in iron-fortified salt has been a point of continuous debate, and it has led to new, ongoing research with microencapsulation of the iodine with dextrin, sodium chloride, or sodium hexametaphosphate.
The cost estimate of producing and packaging the fortified salt is about US$0.01 per kilo of salt and would raise the price from 5 cents to 6 cents/kg. This means that the annual cost per capita would be between US$0.036 and US$0.072.
The production of iron-fortified salt on a commercial scale was approved by the government of India, and two private companies, in Hyderabad and Madras, are producing it. In Tamil Nadu and Rajasthan, the government has installed two large, commercial-scale fortification plants. Consideration of the impact on iron nutrition in these populations is in progress (India, National Institute of Nutrition, 1992).
The South Africa Study
Ballot et al. (1989a) studied, in a double-blind fashion, the effect of curry powder (masala) fortified with NaFe(III)EDTA at a concentration of 1.4 mg of iron/g (total mean daily intake 7.7 mg of iron). Iron nutritional status was evaluated in children > 10 years old and adult male and female members of 135 families in a South African community. A similar number of members of other families that used unfortified masala served as controls. Subjects with Hb levels
< 90 g/l were treated and excluded from the study. A total of 608 persons (335 women and 273 men) completed the study, which lasted two years. Iron nutrition was evaluated by determinations of Hb, transferrin saturation and serum ferritin, and iron stores, which were calculated with a modified algorithm based on Cook et al. (1986). The impact of fortification was determined by the change in these variables at 1 and 2 years after initiation of the program, compared with the changes observed in the unfortified subjects.
The populations studied were previously characterized by iron nutritional status, masala consumption, organoleptic characteristics, and acceptability of the fortified product (Ballot et al., 1989b), and iron absorption from the added NaFeEDTA (Lamparelli et al., 1987).
Significant and progressive improvements were seen in Hb, ferritin, and body iron stores (Figure 3-1). In men, the increments in body iron stores failed to reach statistical significance. The greatest change in all variables studied occurred in the first year, and the change was larger among those with initially low values. Transferrin saturation did not change. Estimates of total iron absorption, based on changes in iron stores, fell within the range expected from previous iron absorption studies.
The Guatemala Study
This was a 32-month-long, double-blind field study involving one highland control community (C) that received only vitamin A-fortified sugar containing 15 mg retinol as retinyl palmitate/kg as part of the ongoing national program of vitamin A fortification, and three communities that received sugar fortified with vitamin A and NaFeEDTA (130 mg of iron [1 g NaFeEDTA)/kg sugar]) (Viteri et al., 1983, 1995b). Two of the three communities (T1 and T2) were located in the lowlands, and one (No. 16), paired with the control community, was located in the highlands. Each community had between 1,200 and 1,700 inhabitants. Hookworm was endemic in the lowland communities; its prevalence and degree of infestation were particularly severe in community T2, which presented severe iron deficiency.
Basic medical and antenatal care were provided in health posts that supplemented all pregnant women and treated all anemic individuals (hematocrit below 28 and 30 percent in the lowlands and the highlands, respectively). These subjects were excluded from the final analysis of the data. No treatment for hookworm was given. Analysis of the impact of iron fortification on iron nutrition was measured in a subsample through determinations of Hb, transferrin saturation, free erythrocyte protoporphyrins, and serum ferritin. Iron stores were estimated by means of the algorithm proposed by Cook et al. (1986), adapted for the Guatemalan populations and to include children above 1 year of age. Impact was measured by changes from baseline values in all of the above parameters, performed at 8, 20, and 32 months after the start of the intervention. Zinc and copper nutritional status were evaluated through plasma and urinary levels at baseline and at the 32-month evaluation. Iron urinary levels were also measured.
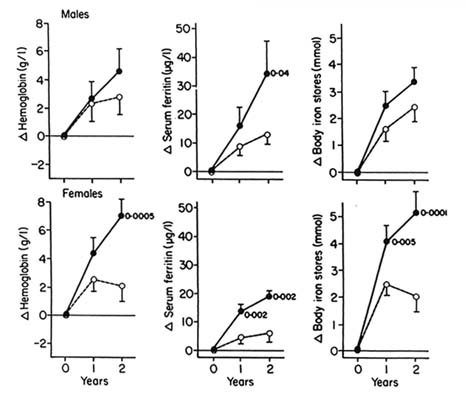
FIGURE 3-1 Changes (Δ) in estimates of iron status after 1 and 2 years of iron fortification in fortified (•) and control (○) groups of males and females in South Africa (mean and standard deviation from the mean). Probabilities that the fortified populations were different from the the controls are also shown. SOURCE: Ballot et al. (1989b); reproduced with permission.
The field trial was based on previous, extensive hematological and dietary characterization of the Guatemalan population, including per capita sugar consumption and its variation by age and gender groups, locations, and other population characteristics (INCAP/ICCND, 1972; Viteri, 1973). Previous research had also measured the response to iron and iron plus folate supplementation of similar populations, indicating that anemia was almost exclusively the product of iron deficiency. Clinical trials of small-scale fortification had also been conducted satisfactorily, and the absorption of NaFeEDTA-iron and nonheme iron in Guatemalan diets had been measured previously (Viteri et al., 1978). Experimental studies had also been performed on the stability, acceptability, organoleptic characteristics, and availability of iron and vitamin A in the doubly fortified sugar and on the effect of NaFeEDTA on zinc absorption and turnover in rats. Sugar was sold in the village stores at the usual prices. Sugar consumption
was measured by family purchase records and periodic dietary evaluations. Adherence to the consumption of iron-fortified sugar was further corroborated by detection of iron in household sugar.
The results on the impact of sugar fortification on iron nutrition are best summarized by the evolution of iron stores in various age and gender groups in the communities (Figures 3-2 and 3-3). In these figures, negative iron stores reflect the severity of the iron deficit and iron reserves are expressed as values above 0, both in absolute terms and as a percentage of norms for body iron. With few exceptions, iron stores did not change significantly in the control community, but increased progressively in all age and gender groups in the fortified communities. Estimates of total iron absorption based on changes in iron stores fall within those expected from previous iron absorption studies.
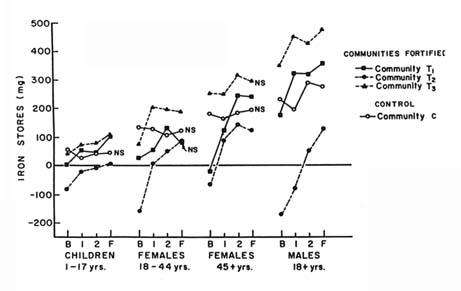
FIGURE 3-2 Mean iron stores in the four hematological and biochemical evaluation points in communities that consumed sugar fortified with NaFeEDTA (Communities T1, T2, and T3) and a control community consuming regular sugar (Community C) for 32 months in Guatemala. Negative iron reserves reflect iron deficits. Communities T1 and T2 were in the tropical lowlands, in which hookworm is endemic. SOURCE: Viteri et al. (1995b); reproduced with permission.
Of special significance is that the members of the community most severely infected with hookworm (T2) and the most iron deficient at the start of the study significantly increased their iron reserves with iron fortification in spite of nontreatment for parasites. Increments in iron stores decreased progressively with time of fortification, tending to reach an acceptable plateau. This indicates
that downward regulation of iron absorption was taking place. There were no individuals with biochemical indicators of iron overload.
Mean plasma zinc levels increased significantly in all communities, including the control community. Mean urinary zinc, expressed as mg/l or as mg/g creatinine, increased in all communities and reached statistical significance in communities 14 and 16. Between 16 and 20 percent of subjects in the fortified communities presented plasma zinc levels compatible with zinc deficiency at the start, and none at the end, of the study. In the control community, 16 percent persisted with plasma zinc levels compatible with zinc deficiency at the end of the study. Plasma and urinary copper levels were erratic and highly variable.
In summary, there is no evidence that zinc or copper nutritional status were compromised in any of the fortified communities. Moreover, it appears that zinc nutrition actually improved in most of the fortified communities in spite of increased urinary losses. These findings would agree with experimental evidence (Hurrell et al., 1994; Oberleas et al., 1966) and with human clinical (Davidsson et al., 1994a) and field studies (Ballot et al., 1989a,b).
Urinary iron also increased in all communities, but the increase was not significant in the control community. The highest increment occurred in community T2, the most iron-deficient community; the population absorbed more iron, on the basis of increments in iron stores.
Annual cost estimates for sugar fortification with 1g NaFeEDTA/kilo are US$0.08 per capita (Levin et al., 1993; Viteri, 1992).
The Venezuelan Study
For many years, Venezuelan scientists, frequently under the leadership of Dr. M. Layrisse, have been studying the many aspects of nutritional anemias and iron deficiency and seeking their solution. The latest paper (Layrisse et al., 1996) is the continuation of a series of studies leading to the selection of precooked maize flour as a vehicle for iron fortification and to the use of ferrous gluconate as an iron source (Martinez-Torres et al., 1991).
The study consisted of an evaluation of the changes in the prevalence of anemia, iron deficiency, and serum ferritin levels that have taken place in cross-sectional samples of schoolchildren of low socioeconomic status at the ages of 7, 11, and 15 years between the years 1989 and 1990, and in 1992. In 1994, additional data were obtained from a sample of children in the capital city of Caracas, about 1 year after fortification of both precooked maize flour and white wheat flour was introduced in the country. The 1989–1990 and 1992 sample consisted of at least 100 children of each gender in each age bracket, representing 80 percent of the regions in Venezuela. The 1994 sample was randomly chosen from 392 public schools in Caracas.
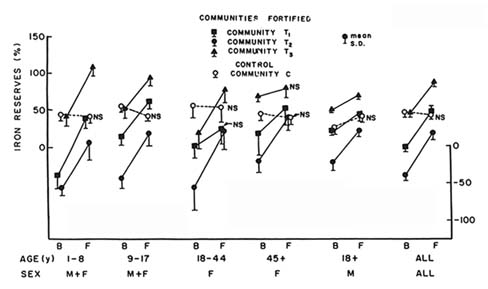
FIGURE 3-3 Iron reserves and iron deficit as ''percent of norm" in the basal and final evaluations in communities that consumed sugar fortified with NaFeEDTA (Communities T1, T2, and T3) and a control community consuming regular sugar (Community C) for 32 months in Guatemala. Negative iron reserves reflect iron deficits. Communities T1 and T2 were in the tropical lowlands, in which hookworm is endemic. SOURCE: Viteri et al. (1995b); reproduced with permission.
This study was prompted by two considerations. First, there had been a clear deterioration in the economic situation of the country from 1983 onward. This situation resulted in a decline in the quality and quantity of food available to those in the lower socioeconomic strata, which resulted in an increase in the prevalence of iron deficiency and anemia in the country. In 1989–1990, the prevalences of iron deficiency and anemia were 13.5 percent and 5.6 percent, respectively. By 1992, these prevalences had increased to 30.5 and 13.2 percent. The second consideration was the establishment of the national program for fortification of precooked maize flour with iron plus vitamins (A, B1, B2, and niacin), which was started in February of 1993 and was followed in August of the same year by fortification of wheat flour. The iron fortificant was ferrous fumarate and provided 50 mg of elemental iron /kg of precooked maize flour and 20 mg/kg of white wheat flour. Mean estimates of per capita consumption of precooked maize and wheat flour were 80 g/d and 74 g/d.
The 1994 evaluation of the Caracas public school population, compared with those performed in 1989–1990 and 1992, should reflect the effect of the iron fortification program in the schoolchildren of the city's lower socioeconomic strata. As in the national sample, iron deficiency in the Caracas children
showed an increase in prevalence from 14.1 to 36.6 percent between the years 1989–1990 and 1992, only to drop again to 15.8 percent in the 1994 survey. Low serum ferritin levels did not show a significant rise in prevalence between the years 1989–1990 and 1992, nor a fall in 1994. Anemia prevalence did show changes with time: it rose from 3.6 percent to 19.0 percent, and then fell to 9.3 percent. The interpretation of these findings is that iron fortification of precooked maize flour and white wheat flour is effectively reducing iron deficiency within 12 to 18 months of the establishment of the national programs.
References
ACC/SCN. 1991. Controlling Iron Deficiency. A report based on an ACC/SCN workshop. S. Gillespie, J. Kevany, and J. Mason, eds. ACC/SCN State of the Art Series, Nutrition Policy Discussion Paper No. 9. Geneva: WHO.
ACC/SCN. 1996. How Nutrition Improves. Geneva: WHO.
ACC/SCN. 1997. Major Issues in Developing Effective Approaches for the Prevention and Control of Iron Deficiency. New York: UNICEF.
Agarwal, K. N., D. K. Agarwal, and K. P. Mishra. 1991. Impact of anaemia prophylaxis in pregnancy on maternal haemoglobin, serum ferritin and birth weight. Indian J. Med. Res. 94:277–280.
Alaudin, M. 1986. Maternal mortality in Bangladesh: the Tangail district. Studies Fam. Plan. 17:13–17.
American Academy of Pediatrics. 1976. Iron supplementation for infants. Pediatrics 58:765–768.
American Academy of Pediatrics. 1992. The use of whole cow's milk in infancy. AAP Committee on Nutrition. AAP News 8:18–19.
Anderson, S. A., ed. 1991. Guidelines for the Assessment and Management of Iron Deficiency in Women of Childbearing Age. Bethesda, MD: Federation of American Societies for Experimental Biology.
Andrade, A. T., J. P. Souza, S. T. Shaw, Jr., E. M. Belsey, and P. J. Rowe. 1991. Menstrual blood loss and body iron stores in Brazilian women. Contraception 42:241–149.
Angeles-Agdeppa, I., W. Schultink, S. Sastroamidojo, R. Gross, and D. Karyadi. 1997. Weekly micronutrient supplementation to build iron stores in female Indonesian adolescents. Am. J. Clin. Nutr. 66:177–183.
Atukorala, T. M., D. R. de Silva, W. H. J. C. Dechering, T. S. de C. Dassenaike, and R. S. Perera. 1994. Evaluation of effectiveness of iron-folate supplementation and anthelmintic therapy against anemia in pregnancy—A study in the plantation sector of Sri Lanka. Am. J. Clin. Nutr. 60:286–292.
Babior, B. M., W. A. Peters, P. M. Briden, and C. L. Cetrulo. 1985. Pregnant women's absorption of iron from prenatal supplements. J. Reproduct. Med. 30:355–357.
Baker, S. J., and E. M. DeMaeyer. 1979. Nutritional anemia: its understanding and control with special reference to the work of the World Health Organization. Am. J. Clin. Nutr. 32:368–417.
Ballot, D. E., A. P. McPhail, T. H. Bothwell, M. Gillooly, and F. G. Mayet. 1989a. Fortification of curry powder with NaFe(III)EDTA in an iron deficient population: initial survey of iron status. Am. J. Clin. Nutr. 49:156–161.
Ballot, D. E., A. P. McPhail, T. H. Bothwell, M. Gillooly, and F. G. Mayet. 1989b. Fortification of curry powder with NaFe(III)EDTA: report of a controlled iron fortification trial. Am. J. Clin. Nutr. 49:162–169.
Barrett, J. F., P. G. Whittaker, J. G. Williams, and T. Lind. 1994. Absorption of nonheme iron from food during normal pregnancy. Brit. Med. J. 309:79–82.
Bauernfeind, J. C., and P. A. Lachance. 1991. Nutrient Additions to Food. Nutritional, Technological and Regulatory Aspects. Trumbull, Conn.: Food and Nutrition Press.
Beard, J. L. 1990. Neuro-endocrine alterations in iron deficiency. Prog. Food Nutr. Sci. 14:45–82.
Beard, J. 1994. Iron deficiency: Assessment during pregnancy and its importance in pregnant adolescents. Am. J. Clin. Nutr. 59 (2 Suppl.):502S–510S.
Berger, J. V., M. Aguayo, S. San-Miguel, V. Tellez, C, Lujan, and P. Traissac. In press. Weekly iron supplementation is as effective as 5 per-day iron supplementation in Bolivian schoolchildren living at high altitude. Eur. J. Clin. Nutr.
Bloch, M. 1971. La anemia uncinariasica como causa de muerte. Sangre 16:39–44.
Bloch, M. 1986. Hookworm infection. Rev. Infect. Dis. 8:306–307.
Bothwell, T., and P. MacPhail. 1992. Prevention of iron deficiency by food fortification. In Nutritional Anemias, S. J. Fomon, and S. Zlotkin, eds. New York: Vevey-Raven.
Bothwell, T. H., R. W. Charlton, J. D. Cook, and C. A. Finch. 1979. Iron Metabolism in Man. Oxford, U. K.: Blackwell.
Bovell-Benjamin, A. C., L. Allen, and F. Viteri. 1997. Iron is well absorbed from ferrous bisglycinate (Ferrochel) added to high phytate whole-maize meal. FASEB J. 11:A3500.
Brabin, B. J. 1992. The role of malaria in nutritional anemias. In Nutritional Anemias, S. J. Fomon, and S. Zlotkin, eds. Nestlé Nutrition Workshop Series, vol. 30. New York: Vevey-Raven.
Breskin, M. W., B. S. Worthington-Roberts, R. H. Knoop, et al. 1983. First trimester serum zinc concentrations in human pregnancy. Am. J. Clin. Nutr. 38:943–953.
Burman, D. 1969. Iron requirements in infancy. Brit. J. Haemat. 20:243–247.
Burns, J., and C. R. Patterson. 1993. Effect of iron-folate supplementation on serum copper concentration in late pregnancy. Acta Obstet. Gynecol. Scand. 72:616–618.
Calvo, E., E. Hertrampf, S. de Pablo, M. Amar, and A. Stekel. 1989. Haemoglobin fortified cereal: an alternative weaning food with high iron bioavailability. Eur. J. Clin. Nutr. 43:237–243.
Carriaga, M. T., B. S. Skikne, B. Finley, B. Cutler, and J. D. Cook. 1991. Serum transferrin receptors for the detection of iron deficiency in pregnancy. Am. J. Clin. Nutr. 54:1077–1081.
CDC (Centers for Disease Control and Prevention). 1996. Iron overload disorders among Hispanics—San Diego California, 1995. MMWR 45:991–993.
CDC. Nutrition Section. 1978. CDC analysis of nutritional indices for selected WIC participants. Atlanta, Ga.: CDC.
Chanarin, I., and D. Rothman. 1971. Further observations of the relation between iron and folate status in pregnancy. Br. Med. J. 2:81–84.
Chandra, R. K. 1990. Influence on immunity and risk of infection by iron and folacin. In Recent Knowledge on Iron and Folate Deficiencies in the World, S. Hercberg, P. Galan, and H. Dupin, eds. Colloque INSERM 197:499–503.
Charoenlarp, P., S. Dhanamitta, R. Kaewvichit, et al. 1988. A WHO collaborative study on iron supplementation in Burma and in Thailand. Am. J. Clin. Nutr. 47:280–297.
Chew, F., B. Torun, and F. E. Viteri. 1996. Comparison of weekly and daily iron supplementation to pregnant women in Guatemala (supervised and unsupervised). FASEB J. 10:A4221.
Clydesdale, F. M. and K. L. Wiemer, eds. 1985. Iron Fortification of Foods. New York: Academic Press.
Cole, S. K., W. Z. Billewicz, and A. M. Thomson. 1971. Sources of variation in menstrual blood loss. J. Obstet. Gynaecol. Brit. Commwlth. 78:933–938.
Colomer, J., C. Colomer, D. Gutierrez, et al. 1990. Anemia during pregnancy as a risk factor for infant iron deficiency. Report from the Valencia infant anemia cohort. In Recent Knowledge on Iron and Folate Deficiencies in the World , S. Hercberg, P. Galan, and H. Dupin, eds. Colloque INSERM 197:577–582.
Combs, G. F., R. M. Welch, J. M. Duxbury, N. T. Uphoff, and M. C. Nesheim. 1996. Food-Based Approaches to Preventing Micronutrient Malnutrition: An International Research Agenda. Cornell University, Ithaca, New York.
Cook, J. D. 1990. Adaptation in iron metabolism. Am. J. Clin. Nutr. 51:301–308.
Cook, J. D., and E. R. Monsen. 1976. Food iron absorption in human subjects. III. Comparison of the effects of animal protein on nonheme iron absorption. Am. J. Clin. Nutr. 29:859–864.
Cook, J. D., and M. B. Reddy. 1995. Efficacy of weekly compared with daily iron supplementation. Am. J. Clin. Nutr. 62:117–120.
Cook, J. D., and M. E. Reusser. 1983. Iron fortification: an update. Am. J. Clin. Nutr. 38:648–659.
Cook, J. D., T. A. Morck, and S. R. Lynch. 1981. The inhibitory effect of soy products on nonheme iron absorption in man. Am. J. Clin. Nutr. 34:2622–2629.
Cook, J. D., B. S. Skikne, S. R. Lynch, and M. E. Reusser. 1986. Estimates of iron sufficiency of the U.S. population. Blood 68:726–731.
Cook, J. D., M. Carriaga, S. G. Kahn, W. Schalch, and B. S. Skikne. 1990. Gastric delivery system for iron supplementation. Lancet 335:1136–1139.
Cook, J. D., R. D. Baynes, and B. S. Skikne. 1994. The physiological significance of circulating transferrin receptors. Adv. Exper. Med. Biol. 352:120–126.
Craig, W. T. 1994. Iron status of vegetarians. Am. J. Clin. Nutr. 59:1233S–1237S.
Crompton, D. W. T., and R. R. Whitehead. 1993. Hookworm infections and human iron metabolism. Parasitology 107:S137–S145.
Custer, E. M., C. A. Finch, R. E. Sobel, and A. Zettner. 1995. Population norms for serum ferritin. J. Lab. Clin. Med. 126:88–94.
Dallman, P. R. 1974. Tissue effects of iron deficiency. In Iron in Biochemistry and Medicine, E. Jacobs and M. Worwood, eds., pp. 437–475. London: Academic Press.
Dallman, P. R. 1986. Iron deficiency in the weanling: a nutritional problem on the way to resolution. Acta Paediatr. 323:59–67.
Dallman, P. R. 1990. Iron. Present Knowledge of Nutrition, 6th ed., pp. 241–250.
Danforth, D. N. 1982. Other complications and disorders due to pregnancy. In Obstetrics and Gynecology, 4th ed., D. N. Danforth, ed. Philadelphia, Pa.: Harper and Row.
Davidsson, L., P. Kastenmayer, and R. F. Hurrell. 1994a. Sodium iron EDTA (NaFe(III)EDTA) as a food fortificant. The effect on the absorption of zinc and calcium in women. Am. J. Clin. Nutr. 60:231–237.
Davidsson, L., P. Kastenmayer, M. Yuen, B. Lonnerdal, and R. F. Hurrell. 1994b. Influence of lactoferrin on iron absorption from human milk in infants. Pediatric Res. 35:117–124.
De Benaze, C., P. Galan, R. Wainer, and S. Hercberg, 1989. Prévention de l'anémie ferriprive au cours de la grossesse par une supplementation martial precoce: un essai controle. Rev. Epidem. et de Sante Publ. 37:109–114.
DeMaeyer, E. M., ed. 1989. Preventing and Controlling Iron Deficiency Anaemia through Primary Health Care. Geneva: WHO.
de Pee, S., C. West, Muhilal, D. Karyadi, and J. Hautvast. 1996. Can increased vegetable consumption improve iron status? Food Nutr. Bull. 17:34–37.
Duthie, S. J., D. Ven, P. A. King, W. K. To, A. Lopes, and H. K. Ma. 1991. A case controlled study of pregnancy complicated by severe maternal anaemia. Aust. N.Z. J. Obstet. Gynaecol. 31:125–127.
Dutra de Oliveira, J. E., J. B. Ferreira, V. P. Vasconcellos, and J. B. Marchini. 1994. Drinking water as an iron carrier to control anemia in preschool children in a day-care center . J. Am. Coll. Nutr. 13:198–202.
Dutra de Oliveira, J. E., J. S. Marchini, and I. Desai. 1996. Fortification of drinking water with iron: a new strategy for combating iron deficiency in Brazil. Am. J. Clin., Nutr. 63:612–614.
Dwyer, J. T. 1988. Health aspects of vegetarian diets. Am. J. Clin. Nutr. 48:712–738.
Ekström, E-C., F. P. Kavishe, J. P. Habicht, E. A. Frongillo, K. Rasmussen, and L. Hemed. 1996. Adherence to iron supplementation during pregnancy in Tanzania: determinants and hematological consequences. Am. J. Clin. Nutr. 64:368–374.
Fairweather-Tait, S. J., T. S. Swindell, and A. J. A. Wright. 1985. Further studies in rats on the influence of previous intake on the estimation of bioavailability of iron. Brit. J. Nutr. 54:79–86.
FAO AGROSTAT/PC. 1992. Food Balance Sheets. Rome: FAO.
FAO/WHO. 1988. Requirements of Vitamin A, Iron, Folate and Vitamin B12. Report of a Joint FAO/WHO Expert Consultation. Rome: FAO.
FAO/WHO/UNU. 1985. Energy and Protein Requirements. Report of a Joint FAO/WHO/UNU Expert Consultation. Technical Report Series No. 724. Geneva: WHO.
Filer, L. J., Jr. 1989. Dietary Iron: Birth to Two Years. New York: Raven.
Fleming, A. F. 1990. Anaemia in pregnancy in Ndola, Zambia: frequency and aetiology. In Recent Knowledge on Iron and Folate Deficiencies in the World, S. Hercberg, P. Galan, and H. Dupin, eds. Colloque INSERM 197:75–77.
Fomon, S. J. 1987. Reflections on infant feeding in the 1970s and 1980s. Am J. Clin. Nutr. 46:171–182.
Fomon, S. J., and S. Zlotkin, eds. 1992. Nutritional Anemias. Nestlé Nutrition Workshop Series, vol. 30, pp. 53–64. New York: Vevey-Raven.
Fomon, S. J., E. E. Ziegler, and S. E. Nelson. 1993. Erythrocyte incorporation of ingested 58Fe by 56-day-old breast-fed and formula-fed infants. Pediat. Res. 33:573–576.
Food and Nutrition Board, National Academy of Sciences-National Research Council . 1989. Recommended Dietary Allowances, 10th ed. Washington, D.C.: National Academy Press.
Food and Nutrtion Board, National Academy of Sciences-National Research Council. 1990. Nutrition during Pregnancy. Part II: Nutrient Supplements. Washington, D.C.: National Academy Press.
Forbes, A. L., C. E. Adams, M. J. Arnaud, C. O. Chichester, J. D. Cook, B. N. Harrison, R. F. Hurrell, S. G. Kahn, E. R. Morris, J. T. Tanner, and P. Whittaker. 1989. Comparison of in vitro, animal and clinical determinations of iron bioavailability: INACG task force report on iron bioavailability. Am. J. Clin. Nutr. 49:225–238.
Franzetti, S., L. A. Mejia, F. E. Viteri, and E. Alvarez. 1984. Body iron reserves in rural and urban Guatemalan women of reproductive age. Arch. Latinoam. Nutr. 34:69–82.
Fullerton, W. T., and A. G. Turner. 1962. Exchange blood transfusions in treatment of severe anemia in pregnancy. Lancet i:75–78.
Galloway, R., and J. McGuire. 1994. Determinants of compliance with iron supplementation: supplies, side effects or psychology? Soc. Sci. Med. 39:381–390.
Garby, L., and S. Areekul. 1974. Iron supplementation in Thai fish sauce. Ann. Trop. Med. Parasitol. 68:467–476.
Garn, S. M., M. T. Keating, and F. Falkner. 1981. Hematological status and pregnancy outcomes. Am. J. Clin. Nutr. 34:115–117.
Gillespie, S., 1996. Major Issues in Developing Effective Approaches for the Prevention and Control of Iron Deficiency. New York: UNICEF.
Gleerup, A., L. Rossander-Hulthen, E. Gramatkowski, and L. Hallberg. 1995. Iron absorption from the whole diet in relation to calcium intake. A comparison of the effect of two different distributions of the daily intake of calcium. Am. J. Clin. Nutr. 61:97–104.
Grebe, G., C. Martinez-Torres, and M. Layrisse. 1975. Effect of meals and ascorbic acid on the absorption of a therapeutic dose of iron as ferrous and ferric salts. Curr. Therap. Res. 17:382–397.
Gueri, M., and F. E. Viteri, eds. 1996. Report of the II Subregional Workshop on the Control of Nutritional Anemias and Iron Deficiency. (UNU, PAHO/WHO, and Fundación CAVENDES). Washington, D.C.: PAHO.
Hahn, P. F., W. F. Bale, J. F. Ross, W. M. Balfour, and G. H. Whipple. 1943. Radioactive iron absorption by the gastrointestinal tract: influence of anemia, anoxia, and antecedent feeding distribution in growing dogs. J. Exp. Med. 78:169–188.
Hahn, P. F., E. I. Carothers, W. J. Darby, M. Martin, C. W. Sheppard, R. O. Cannon, A. S. Beam, P. M. Densen, J. C. Peterson, and G. S. McClellan. 1951. Iron metabolism in early pregnancy as studied with the radioactive isotope 59Fe. Am. J. Obstet. Gynecol. 61:477–486.
Hallberg, L. 1970. Oral iron therapy. Factors affecting the absorption. In Iron Deficiency: Pathogenesis, Clinical Aspects, Therapy, L. Hallberg, H.-G. Harwerth, and A. Vannotti, eds., pp. 551–561. New York: Academic Press.
Hallberg, L., and E. Bjorn-Rassmussen. 1972. Determination of iron absorption from whole diet. A new two pool model using two radioiron isotopes given as haem and non-haem iron. Scand. J. haematol. 9:193–197.
Hallberg, L., and L. Rossander-Hultén. 1991. Iron requirements in menstruating women. Am. J. Clin. Nutr. 54:1047–1058.
Hallberg, L., and L. Sölvell. 1967. Absorption of haemoglobin iron in man. Acta Med. Scand. 181:335–354.
Hallberg, L., L. Rossander-Hultén, and M. Brune. 1992. Prevention of iron deficiency by diet. In Nutritional Anemias, S. J. Fomon, and S. Zlotkin, eds. New York: Vevey-Raven.
Hallberg, L., L. Rossander-Hulthen, M. Brune, and A. Gleerup, A. 1993. Inhibition of haem-iron absorption in man by calcium. Brit. J. Nutr. 69:533–540.
Halliwell, B., J. M. Gutteridge, and C. E. Cross. 1992. Free radicals, antioxidants and human disease: Where are we now? J. Lab. Clin. Med. 119:598–620.
Hambidge, K. M., N. F. Krebs, L. Sibley, and J. English. 1987. Acute effects of iron therapy on zinc status during pregnancy. Obstet. Gynecol. 70:593–596.
Heinrich, H. C. 1970. Intestinal iron absorption in man. Pathogenesis, clinical aspects, therapy. In Iron Deficiency, L. Hallberg, H.-G. Harwerth, and A. Vannotti, eds., pp. 213–296. New York: Academic Press.
Herbert, V. 1988. Vitamin B12: plant sources, requirements and assay. Am. J. Clin. Nutr. 48:852–858.
Herbert, V. 1992. Everyone should be tested for iron disorders. J. Am. Diet. Assoc. 92:1502–1509.
Hertrampf, E., M. Olivares, F. Pizarro, T. Walter, M. Cayazzo, G. Heresi, S. Llanguno, P. Chadud, and A. Stekel. 1990. Haemoglobin fortified cereal: A source of available iron in breast-fed infants. Eur. J. Clin. Nutr. 44:793–798.
Higgins, A. C., P. B. Penchartz, J. E. Strawbridge, G. B. Maughan, and J. E. Moxley. 1982. Maternal haemoglobin changes and their relationship to infant birth weight in mothers receiving a program of nutritional assessment and rehabilitation. Nutr. Res. 2:641–649.
Hodges, R., H. Sauberlich, J. Ganham, D. Wallace, R. Rucker, L. Mejia, and M. Mohamran. 1978. Hematopoyetic studies in vitamin A deficiency. Am. J. Clin. Nutr. 31:876–885.
Höglund, S. 1969. Studies on iron absorption. VI. Transitory effect of oral administration of iron on iron absorption. Blood 34:505–510.
Hughes, A. 1991. Anaemia in Pregnancy. Maternal Health and Safe Motherhood, Division of Family Health. Geneva: WHO.
Hulten, L., E. Gramatkovski, A. Gleerup, and L. Hallberg. 1995. Iron absorption from the whole diet. Relation to meal composition, iron requirements and iron stores. Europ. J. Clin. Nutr. 49:794–808.
Hunnell, J. W., K. Yasumatsu, and S. Moritaka. 1985. Iron enrichment of rice. In Iron Fortification of Foods, F. M. Clydesdale and K. Wiemer, eds. New York: Academic Press.
Hurrell, R. F. 1984. Bioavailability of different iron compounds used to fortify formula and cereals. Technological problems. In Iron Nutrition in Infancy and Childhood, A. Steckel, ed., pp. 147–178. New York: Raven.
Hurrell, R. F. 1985. Types of iron fortificants. Non elemental sources. In Iron Fortification of Foods, F. M. Clydesdale and K. L. Wiemer, eds., pp. 39–53. New York: Academic Press.
Hurrell, R. F. 1992. Prospects for improving the iron fortification of foods. In Nutritional Anemias, S. J. Fomon, and S. Zlotkin, eds. New York: Vevey-Raven.
Hurrell, R. F. 1997. Strategies for the prevention of iron deficiency: food iron fortification. In Desnutricion Oculta en Latinoamerica: Deficiencia de Hierro (Occult Malnutrition in Latin America: Iron Deficiency), A. O'Donnell, F. E. Viteri, and E. Carmuega, eds. Buenos Aires: CESNI.
Hurrell, R. F., D. E. Furniss, J. Burri, P. Whittaker, S. R. Lynch, and J. D. Cook. 1989. Iron fortification of infant cereals: a proposal for the use of ferrous fumarate or ferrous succinate. Am. J. Clin. Nutr. 49:1274–1282.
Hurrell, R. F., Ribas, S. and Davidsson, L. 1994. NaFe3+EDTA as a food fortificant: influence on zinc, calcium and copper metabolism in the rat. Brit. J. Nutr. 71:85–93.
Husaini, M. A. 1996. Population Study of Relative Effectiveness of Weekly and Daily Iron Supplementation in Infants and Toddlers. Bogor, Indonesia: The Nutrition Research and Development Center.
Husaini, M. A., Suhardjo, and N. S. Scrimshaw. 1990. Field studies on work productivity in iron deficient subjects in West Java, Indonesia. In Recent Knowledge on Iron
and Folate Deficiencies in the World, S. Hercberg, P. Galan, and H. Dupin, eds. Colloque INSERM 197:515–521.
Hytten, F. 1985. Blood volume changes in normal pregnancy. Clin. Haematol. 14:609–617.
Hytten, F. E., and D. L. Duncan. 1956. Iron deficiency anaemia in the pregnant woman and its relation to normal physiological changes. Nutr. Abstr. Rev. 26:855–868.
INCAP. 1984. Annual Report.
INCAP/ICCND. 1972. Nutritional Evaluation of the Population of Central America and Panama, 1965–1967. DHEW Publication No (HSM) 72-8120.
India, National Institute of Nutrition. 1992. Annual Report. Hyderabad.
Indian Council of Medical Research. 1989. Evaluation of the National Nutritional Anaemia Prophylaxis Program. New Delhi: Indian Council of Medical Research.
IOM (Institute of Medicine). 1990. Iron Nutrition during Pregnancy. Washington, D.C.: National Academy Press.
IOM. 1993. Iron-Deficiency Anemia: Recommended Guidelines for the Prevention, Detection and Management Among U.S. Children and Women of Childbearing Age. Washington, D.C.: National Academy Press.
INACG (International Nutritional Anemia Consultative Group). 1977. Guidelines for the Eradication of Iron-Deficiency Anemia. Washington, D.C.: The Nutrition Foundation.
INACG. 1981. Iron deficiency in Women. Washington, D. C.: The Nutrition Foundation.
INACG. 1982. The Effects of Cereals and Legumes on Iron Availability. Washington, D.C.: Nutrition Foundation.
INACG. 1985. Measurement of Iron Status (Laboratory manual). Washington, D.C.: The Nutrition Foundation.
INACG. 1986. Combating Iron Deficiency in Chile: A Case Study. Washington, D.C.: The Nutrition Foundation.
INACG. 1993. Iron EDTA for Food Fortification. Washington, D.C.: The Nutrition Foundation/ILSI.
INACG. In press. Guidelines for the Use of Iron Supplements to Prevent and Treat Iron-Deficiency Anemia. Washington, D.C.: The Nutrition Foundation/ILSI.
Judisch, J. M., J. L. Naian, and F. A. Oski. 1986. The fallacy of the fat, iron deficient child. Pediatrics 37:987–992.
Kauffer, M., and E. Casanueva. 1990. Relation of prepregnancy ferritin levels to hemoglobin levels throughout pregnancy. Eur. J. Clin. Nutr. 44:709–715.
Lamparelli, R. D., A. P. MacPhail, T. H. Bothwell, et al. 1987. Curry powder as a vehicle for iron fortification: effects on iron absorption. Am. J. Clin. Nutr. 46:335–340.
Lanzkowsky, P. 1976. Iron metabolism in the newborn infant. Clin. Endocrin. Met. 5:149–173.
Lauffer, R. B. 1992. Iron and Human Disease. Boca Raton, Fl.: CRC.
Layrisse, M., and M. Roche. 1964. The relationship between anemia and hookworm infection: results of surveys of rural Venezuelan population. Am. J. Hyg. 79:279–301.
Layrisse, M., J. D. Cook, C. Martinez, M. Roche, I. N. Kuhn, R. Walker, and C. E. Finch. 1969. Food iron absorption: a comparison of vegetable and animal foods. Blood 33:430–443.
Layrisse, M., C. Martinez-Torres, J. D. Cook, R. Walker, and C. A. Finch. 1973. Iron fortification of food: its measurement by the extrinsic tag method. Blood 41:333–352.
Layrisse, M., C. Martinez-Torres, H. Mendez-Castellanos, et al. 1990. Relationship between iron bioavailability from diets and the prevalence of iron deficiency. Food Nutr. Bull. 12:301–309.
Layrisse, M., J. F. Chaves, H. Mendez-Castellanos, V. Bosch, E. Tropper, B. Bastardo, and E. Gonzalez. 1996. Early response to the effect of iron fortification in the Venezuelan population. Am. J. Clin. Nutr. 64:903–907.
Layrisse, M., M. N. Garcia-Casal, L. Solano, M. A. Baron, F. Arguello, D. Llovera, J. Ramirez, I. Leets, and E. Tropper. 1997. The role of vitamin A on the inhibitors of nonheme iron absorption. Preliminary results. J. Nutr. Biochem. 8:61–77.
Levin, H. M., E. Pollitt, R. Galloway, and J. McGuire. 1993. Micronutrient deficiency disorders. In Disease Control Priorities in Developing Countries, J. D. Jamison, W. H. Mosley, A. R. Measham, and J. L. Bobadilla, eds. New York. Oxford University Press.
Li, R., X. Chen, H. Yan, et al. 1994. Functional consequences of iron supplementation in iron-deficient female cotton mill workers in Beijing, China. Am. J. Clin. Nutr. 59:908–913.
Liu, X-N, and P-Y Liu. 1996. The effectiveness of weekly iron supplementation regimen in improving the iron status of Chinese children and pregnant women. Biomed. Environ. Sci. 9:341–347.
Liu, X-N, W. Yang, J. Zhang, H. Ying, Y. Gen, J. Xie, and F. E. Viteri. 1995a. Weekly iron supplementation is effective and safe in pregnant women. FASEB J. 9:A5658.
Liu, X-N, J. Kang, L. Zhao, and F. E. Viteri. 1995b. Intermittent iron supplementation is efficient and safe in controlling iron deficiency and anemia in preschool children. Food Nutr. Bull. 16:139–146.
Lozoff, B., E. Jimenez, and A. W. Wolf. 1992. Long-term developmental outcome of infants with iron deficiency. N. Engl. J. Med. 325:687–694.
Lynch, S. R. 1995. Iron overload: Prevalence and impact on health. Nutr. Rev. 53:255–260.
MacPhail, A. P., T. H. Bothwell, J. D. Torrance, D. P. Deyman, W. R. Bezwoda, and R. Charlton. 1981. Factors affecting the absorption of iron from Fe(III)EDTA. Brit. J. Nutr. 45:215–227.
Martinez-Torres, C., E. C. Racca, F. Rivero, M. Cano, I. Leets, E. Tropper, M. N. Garcia, J. Ramirez, and M. Layrisse, M. 1991. Iron fortification of pre-cooked maize flour. Interciencia 16:254–260.
McMillan, J. A., S. A. Landaw, and F. A. Oski. 1976. Iron sufficiency in breast-fed infants and the availability of iron from human milk. Pediatrics 58:686–691.
Mejia, L., E. Hodges, and R. B. Rucker. 1979. Role of vitamin A in the absorption, retention and distribution of iron in the rat. J. Nutr. 109:129–137.
Mendoza, C., F. Viteri, B. Lönnerdal, V. Raboy, K. Young, and K. H. Brown. 1997. Effect of genetically modified, low-phytate maize on iron absorption from tortillas. FASEB J. 11:A3504.
Menon, M. K. K. 1968. A letter from India. Obstet. Gynecol. Surv. 23:401–420.
Mohamed, K., and F. Hytten. 1989. Iron and folate supplementation in pregnancy. In Effective Care in Pregnancy and Childbirth, I. Chalmers, ed., pp. 301–317. London: Oxford University Press.
Murphy, J. F., J. O'Riordan, R. G. Newcombe, E. C. Coles, and J. F. Pearson. 1986. Relation of haemoglobin levels in first and second trimesters to outcome of pregnancy. Lancet i:992–995.
Murray, C. J. L., and A. D. Lopez. 1994. Global Comparable Assessments in the Health Sector. Geneva: WHO.
Nadiger, H. A., K. A. V. R. Krishnamachari, A. Nadaminu Naidu, B. S. Narasinga-Rao, and S. G. Srikantia. 1980. The use of common salt (sodium chloride) fortified with iron to control anaemia: results of a preliminary study. Brit. J. Nutr. 43:45–51.
Name, J. J. 1996. Food fortification with amino acid chelated minerals. In International Nutritional Anemia Consultative Group (INACG), Technical Review Meeting on Amino Acid Chelated Iron. Washington, D.C.: ILSI.
Narasinga-Rao, B. S., and C. Vijayasarathy. 1975. Fortification of common salt with iron: effect of chemical additives on stability and bioavailability. Am. J. Clin. Nutr. 28:1395–1401.
Narasinga-Rao, B. S., and C. Vijayasarathy. 1978. An alternate formula for the fortification of common salt with iron. Am. J. Clin. Nutr. 31:1112–1114.
Norrby, A. 1974. Iron absorption studies in iron deficiency. Scand. J. Haematol. (Suppl.) 20.
Oberleas, D., M. E. Muhrer, and B. L. O'Dell. 1966. Dietary metal complexing agents and zinc availability in the rat. J. Nutr. 90:56–62.
Olivares, M., F. Pizarro, O. Pineda, J. J. Name, E. Hertrampf, and T. Walker. 1997. Milk inhibits and vitamin C favors ferrous bisglycinate chelate bioavailability in humans. J. Nutr. 127:1407–1411.
O'Neil-Cutting, M. A. and W. H. Crosby. 1987. Blocking of iron absorption by a preliminary oral dose of iron. Arch. Int. Med. 147:489–491.
Osofsky, H. J., P. T. Rizk, M. Fox, and J. Mondanaro. 1971. Nutritional status of low-income pregnant teen-agers. J. Reproduct. Med. 6:29–33.
Pilch, S. M., and F. R. Senti. 1984. Assessment of the Iron Nutrition Status of the US Population Based on Data Collected in the Second National Health and Nutrition Examination Survey, 1976–1980. Bethesda, MD: Life Science Research Office, Federation of American Societies for Experimental Biology.
Pineda, O. 1996. Clinical studies using iron amino acid chelate. In International Nutritional Anemia Consultative Group (INACG), Technical Review Meeting on Amino Acid Chelated Iron. Washington, D.C.: ILSI.
Pollitt E, Viteri F, Saco-Pollitt B, Leibel, RL. 1982. Behavioral effects of iron-deficiency anemia in children. In, Pollitt E, Leibel RL, eds. Iron Deficiency: Brain Biochemistry and Behavior. New York: Raven.
Powers, H. J., C. J. Bates, and W. H. Lamb. 1985. Haematological response to supplements of iron and riboflavin to pregnant and lactating women in rural Gambia. Human Nutr. Clin Nutr. 39:117–129.
Puolakka, J. 1980. Serum ferritin as a measure of iron stores during pregnancy. Acta Obstet. Gynecol. Scand. (Suppl.) 95:1–63.
Puolakka, J., O. Janne, A. Pakarinen, P. A. Järvinen, and R. Vihko. 1980. Serum ferritin as a measure of iron stores during and after normal pregnancy with and without iron supplements . Acta Obstet. Gynecol. Scand. (Suppl.) 95:43–51.
Reddy, S., and T. Sanders. 1990. Haematological studies in pre-menopausal Indian and Caucasian vegetarians compared with Caucasian omnivores. Brit. J. Nutr. 64:331–338.
Reizenstein, P., L. Ehn, K. V. Forsberg, A. Kuppevelt, and G. Liedén. 1975. Prevention of iron deficiency with ferrous iron and haemoglobin iron in subjects with controlled blood loss. In Iron Metabolism and Its Disorders, H. Kief, ed., pp. 179–189. Amsterdam: Excerpta Medica.
Ridwan, E., W. Schultink, D. Dillon, and R. Gross. 1996. Effects of weekly iron supplementation on pregnant Indonesian women are similar to those of daily supplementation. Am. J. Clin. Nutr. 63:884–890.
Rios, E., R. E. Hunter, J. D. Cook, N. J. Smith, and C. A. Finch. 1975. The absorption of iron as supplements in infant cereals and infant formulas. Pediatrics 55:686–693.
Roche, M., and M. Layrisse. 1966. The nature and causes of ''hookworm anemia." Am. J. Trop. Med. Hyg. 15:1031–1100.
Romslo, I., K. Haram, S. Norvald, and K. Augensen. 1983. Iron requirement in normal pregnancy as assessed by serum ferritin, serum transferrin saturation and erythrocyte protoporphyrin determinations. Brit. J. Obstet. Gynaecol. 90:101–107.
Rosso, P. 1990. Nutrition and Metabolism in Pregnancy. New York: Oxford University Press.
Rosso, P., and M. R. Streeter. 1979. Effects of food and protein restriction on plasma volume expansion in pregnant rats. J. Nutr. 109:1887–1892.
Rosso, P., A. Arteaga, A. Foradori, G. Grebe, P. Lira, J. Torres, and P. Vela. 1983. Physiological adjustments and pregnancy outcome in low-income Chilean women. Fed. Proc. 17:138A.
Rush, B., M. A. Figallo, and E. B. Brown. 1966. Effect of a low iron diet on iron absorption. Am. J. Clin. Nutr. 19:132–136.
Rusia, U., N. Madan, N. Agarwal, M. Sikka, and S. E. Sood. 1995. Effect of maternal iron deficiency anaemia on foetal outcome. Ind. J. Pathol. Microbiol. 38:273–279.
Rusia, U., C. Flowers, N. Madan, N. Agarwal, S. K. Sood, and M. Sikka. 1996. Serum transferrin receptor levels in the evaluation of iron deficiency in the neonate. Acta Paediat. Japan 38:455–459.
Saarinen, U. M., M. A. Siimes, and P. R. Dallman. 1977. Iron absorption in infants: high bioavailability of breast milk iron as indicated by the extrinsic tag method of iron absorption and by the concentration of serum ferritin. J. Pediatr. 91:36–39.
Sanders, T. A. B. 1994. Nutritional aspects of a meatless diet. Proc. Nutr. Soc. 53:297–307.
Scholl, T. O., and M. L. Hediger. 1994. Anemia and iron-deficiency anemia: compilation of data on pregnancy outcome. Am. J. Clin. Nutr. 59 (2 Suppl.):492S–500S. (Discussion 500S–501S.)
Scholl T. O., M. L. Hediger, R. L. Fischer and J. W. Shearer. 1992. Anemia vs. iron deficiency: increased risk of preterm delivery in a prospective study. Am. J. Clin. Nutr. 55:985–988.
Schultink, W., R. Gross, M. Gliutzki, D. Kariadi, and P. Matulessi. 1995. Effect of daily vs. biweekly iron supplementation in Indonesian preschool children with low iron status. Am. J. Clin. Nutr. 61:111–115.
Scrimshaw, N. S., and J.P. SanGiovanni. 1997. Synergism of nutrition, infection, and immunity: an overview. Am. J. Clin. Nutr. 66 (2 Suppl.):464S–477S.
Scrimshaw, N. S., C. E. Taylor, and J. E. Gordon. 1968. Interactions of Nutrition and Infection. Geneva: World Health Organization.
Seligman, P. A., J. H. Caskey, J. L. Frazier, R. M. Zucker, E. R. Podell, and R. H. Allen. 1983. Measurement of iron absorption from prenatal multivitamin-vitamin supplements. Obstet. Gynecol. 61:356–362.
Shaw, N-S, C-J Chin, and W-H Pan. 1995. A vegetarian diet rich in soybean products compromises iron status in young students. J. Nutr. 125:212–219.
Siimes, M. A., E. Vuori, and P. Kuitunen. 1979. Breast milk iron—a declining concentration during the course of lactation. Acta Paediatr. Scand. 68:29–31.
Simmons, K. W., J. D. Cook, K. C. Bingham, M. Thomas, J. Jackson, M. Jackson, N. Ahluwalia, S. G. Kahn, and A. W. Patterson. 1993. Evaluation of a gastric delivery system for iron supplementation in pregnancy. Am. J. Clin. Nutr. 58:622–626.
Skikne B. S., and J. D. Cook. 1992. Effect of enhanced erythropoiesis on iron absorption. J. Lab. Clin. Med. 120:746–751.
Skikne, B. S., C. H. Flowers, and J. D. Cook. 1990. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood 75:1870–1876.
Sloan, N. L., E. A. Jordan, and B. Winikoff. 1992. Does iron supplementation make a difference? Mother Care Project, Working Paper 15. Arlington, Va.
Smith, M. D., and I. M. Pannacciulli. 1958. Absorption of inorganic iron from graded doses: its significance in relation to iron absorption tests and the "mucosal block" theory. Brit. J. Haematol. 4:428–434.
Solomons, N. W. 1986. Competitive interaction of iron and zinc in the diet: consequences for human nutrition. J. Nutr. 116:927–935.
Solomons, N. W. 1995. Weekly versus daily oral iron administration: are we asking the right questions? Nutr. Rev. 53:326–327.
Solomons, N. W., and R. A. Jacobs. 1981. Studies on the bioavailability of zinc in man. IV. Effect of heme and non-heme iron on absorption of zinc. Am. J. Clin. Nutr. 34:475–482.
Sölvell, L. 1970. Oral iron therapy—side effects. In Iron Deficiency. Pathogenesis, Clinical Aspects, Therapy, L. Hallberg, H.-G. Harwerth, and A. Vannotti, eds., pp. 573–583. New York: Academic Press.
Sood, S. K., K. Ramachandran, M. Mathur, K. Gupta, V. Ramalingaswami, C. Swarnabai, J. Ponniah, V. I. Mathan, and S. J. Baker. 1975. WHO sponsored collaborative studies on nutritional anaemia in India. I. The effects of supplemental oral iron administration to pregnant women . Q. J. Med. 174:241–258.
Stekel, A., ed. 1984. Iron Nutrition in Infancy and Childhood. New York: Raven.
Stephenson, L. S. 1987. Impact of Helminth Infections on Human Nutrition. New York: Taylor and Francis.
Stevens, R. G., B. I. Grauberd, M. S. Micozzi, K. Neriishi, and B. S. Blumberg. 1994. Moderate elevation of body iron level and increased risk of cancer occurrence and death. Intl. J. Cancer 56:364–369.
Stewart, W. B., C. L. Yuile, H. A. Claiborne, et al. 1950. Radioiron absorption in anemic dogs. Fluctuations in the mucosal block and evidence for gradient of absorption in the gastrointestinal tract. J. Exper. Med. 92:375–384.
Stolzfus, R. J., H. M. Chwaya, J. M. Tielsch, K. J. Schulze, M. Albonico, and L. Savioli. 1997a. Epidemiology of iron deficiency anemia in Zanzibari schoolchildren: the importance of hookworms. Am. J. Clin. Nutr. 65:153–159.
Stolzfus, R. J., Dreyfuss, M. L., Jorgenson, T., Cwaya, H. M. and Albonico, M. 1997b. Hookworm control as a strategy to prevent iron deficiency. In Desnutricion oculta en Latinoamerica: Deficiencia de Hierro (Occult Malnutrition in Latin America: Iron Deficiency), A. O'Donnell, F. E. Viteri, and E. Carmuega, eds. Buenos Aires: CESNI.
Suharno, D., C. E. West, Mujilal, et al. 1992. Cross-sectional study on the iron and vitamin A status of pregnant women in West Java, Indonesia. Am. J. Clin. Nutr. 56:988–993.
Suharno, D., C. E. West, Mujilal, D. Karyadi, and J. G. A. J. Hautvast. 1993. Supplementation with vitamin A and iron for nutritional anemia in pregnant women in West Java, Indonesia. Lancet 342:3425–3428.
Svanberg, B. 1975. Absorption of iron in pregnancy. Acta Obstet. Gynecol. Scand. (Suppl.) 48.
Taylor, D. J., C. Mallen, C. McDougall, and T. Lind. 1982. Effect of iron supplementation on serum ferritin levels during and after pregnancy. Brit. J. Obstet. Gynaecol. 89:1011–1017.
Tee, E. S., M. Kandiah, N. Awin, S. M. Chong, N. Satgunasingam, L. Kamarudin, S. Milani, A. E. Duagdale, and F. E. Viteri. 1995. School-Administered Weekly Iron-Folate Supplements Improve Iron Nutrition of Malaysian Adolescent Girls: Feasibility, Safety, and Effectiveness. Seventh Asian Congress of Nutrition.
Theuer, R. C. 1985. Fortification of infant formula. In Iron Fortification of Foods, F. M. Clydesdale and K. L. Wiemer, eds. New York: Academic Press.
UNDP (United Nations Development Program). 1991. Human Development Report 1991. Oxford, U.K.: UNDP/Oxford University Press.
Velez, H., A. Restrepo, J. J. Vitale, and E. E. Hellerstein. 1966. Folic acid deficiency secondary to iron deficiency in man. Remission with iron therapy and a diet low in folic acid. Am. J. Clin. Nutr. 19:27–36.
Viteri, F. E. 1973. Hematological Status of the Central American Population: Iron and Folate Deficiencies. Pan American Health Organization (PAHO/WHO), Advisory Committee on Medical Research. Washington, D.C.
Viteri, F. E. 1992. Iron. global perspective. In Ending Hidden Hunger. A Policy Conference on Micronutrient Malnutrition, pp. 139–177. Atlanta, Ga: The Task for Child Survival and Development.
Viteri, F. E. 1993. Global Strategy for the Control of Iron Deficiency. World Health Organization, Nutrition Unit. Geneva.
Viteri, F. E. 1994a. Consequences of iron nutrition and anemia in pregnancy and lactation. Adv. Exper. Med. Biol. 352:127–139.
Viteri, F. E. 1994b. The consequences of iron deficiency and anaemia in pregnancy on maternal health, the foetus and the infant. SCN News 11:14–18.
Viteri, F. E. 1995. Iron deficiency in children: new possibilities for its control. Intl. Child Hlth. 6:49–62.
Viteri, F. E. 1996. Summary results of a survey on nutritional anemias, iron deficiency, and their control. In Report of the I Subregional Workshop on the Control of Nutritional Anemias and Iron Deficiency (UNU, PAHO/WHO/ CESNI), F. E. Viteri, M. Gueri, and E. Calvo, eds., pp. 132–177. INCAP.
Viteri, F. E. 1997a. Effective iron supplementation does not happen in isolation. Am J. Clin. Nutr. 65:889–890.
Viteri, F. E. 1997b. Iron supplementation. In Desnutricion Oculta en Latinoamerica: Deficiencia de Hierro (Occult Malnutrition in Latin America: Iron Deficiency), A. O'Donnell, F. E. Viteri, and E. Carmuega, eds. Buenos Aires: CESNI, pp. 231–258.
Viteri, F. E., and B. Torun. 1974. Anemia and physical work capacity. Clin. Haematol. 3:609–626.
Viteri, F. E., R. Garcia-Ibañez, and B. Torun. 1978. Sodium Iron NaFeEDTA as an iron fortification compound in Central America. Absorption studies. Am. J. Clin. Nutr. 31:961–971.
Viteri, F. E., E. Alvarez, and B. Torun. 1983. Prevention of iron deficiency by means of iron fortification of sugar. In Nutrition Intervention Strategies in National Development. B. Underwood, ed., pp. 287–314. New York: Academic Press.
Viteri, F. E., X-N Liu, A. Martin, and K. Tolomei. 1995a. True absorption and retention of supplemental iron is more efficient when administered every three days rather than daily to iron-normal and iron-deficient rats. J. Nutr. 125:82–91.
Viteri, F. E., E. Alvarez, R. Batres, B. Torún, O. Pineda, L. Mejía, and J. Sylvi. 1995b. Fortification of sugar with NaFeEDTA improves iron status in semi-rural populations in Guatemala. Am. J. Clin. Nutr. 61:1153–1163.
Viteri, F. E., F. Ali, and J. Tujague. 1996a. Weekly iron supplementation of fertile-age women achieves a progressive increment in serum ferritin. FASEB J. 10:A3369.
Viteri, F. E., M. Gueri, and E. Calvo, eds. 1996b. Report of the I Subregional Workshop on the Control of Nutritional Anemias and Iron Deficiency (UNU, PAHO/WHO, and CESNI). INCAP.
Walsh, C. T., H. H. Sandstead, A. S. Prasad, et al. 1994. Zinc: health effects and research priorities for the 1990s. Environ. Hlth Perspect. 102 (Suppl 2):5–46.
Walter, T. 1992. Impact of iron deficiency on cognition in infancy and childhood. In Nutritional Anemias, S. J. Fomon, and S. Zlotkin, eds., pp. 307–316. Nestlé Nutrition Workshop Series, vol. 30. New York: Vevey-Raven.
Walter, T., M. Olivares, and E. Hertrampf. 1990. Field trials of food fortification with iron: The experience of Chile. In Iron Metabolism in Infants, B. Lonnerdal, ed., pp. 127–155. Boca Raton, FL: CRC.
Walter, T., P. R. Dallman, F. Pizarro, L. Velozo, S. J. Bartholomey, E. Hertrampf, M. Olivares, A. Letelier, and M. Arredondo. 1993a. Effectiveness of iron-fortified cereal in prevention of iron deficiency anemia. Pediatrics 91:976–982.
Walter, T., E. Hertrampf, F. Pizarro, M. Olivares, S. Llanguno, A. Letelier, V. Vega, and A. Stekel. 1993b. Effect of bovine-hemoglobin-fortified cookies on iron status of school children: a nationwide program in Chile. Am. J. Clin. Nutr. 57:190–194.
Warren, K. S., D. A. P. Bundy, R. M. Anderson, A. R. Davis, D. A. Henderson, D. T. Jamison, N. Prescott, and A. Senft. 1993. Helminth infection. In Disease Control Priorities in Developing Countries, D. T. Jamison, W. H. Mosley, A. R. Measham, and J. L. Bobadilla, eds., pp. 131–160. New York: Oxford University Press.
Whittaker, P. G., T. Lind, and J. G. Williams. 1991. Iron absorption during normal human pregnancy: a study using stable isotopes. Brit. J. Nutr. 65:457–463.
Widdowson, E. M., and C. M. Spray. 1951. Chemical development in utero. Arch. Dis. Child. 26:205–214.
Working Group on Fortification of Salt with Iron. 1982. Use of common salt fortified with iron in the control and prevention of anemia: a collaborative study. Am. J. Clin. Nutr. 35:1142–1151.
World Bank. 1994. Enriching Lives: Overcoming Vitamin and Mineral Malnutrition in Developing Countries. Washington, D.C.: World Bank.
WHO (World Health Organization) 1972. Report of a WHO Group of Experts on Nutritional Anaemias. Technical report series No. 503. Geneva: WHO.
WHO. 1989. Report of the African Regional Consultation on Control of Anaemia in Pregnancy. Regional Office for Africa. Brazzaville: WHO/AFRO.
WHO. 1991. National Strategies for Overcoming Micronutrient Malnutrition. Geneva: WHO.
WHO. 1995. Report of the WHO Informal Consultation on Hookworm Infection and Anemia in Girls and Women. Geneva: WHO.
WHO/UNICEF/UNU. In press. Consultation on Iron Deficiency: Indicators and Strategies for Iron Deficiency Control Programs. Geneva: WHO.
Wright, A. J. A., and S. Southon. 1990. The effectiveness of various iron-supplementation regimens in improving the Fe status of anemic rats. Brit. J. Nutr. 63:579–585.
Wurapa, R. K., V. R. Gordeuk, G. M. Brittenham, A. Khiyami, G. P. Schechter, and C. Q. Edwards. 1996. Primary iron overload in African Americans. Am. J. Med. 101:9–18.
Ziegler, E. E., S. J. Fomon, S. E. Nelson, C. J. Rebouche, B. B. Edwards, R. R. Rogers, and L. J. Lehman. 1990. Cow milk feeding in infancy: further observations on blood loss from the gastrointestinal tract. J. Pediatr. 116:11–18.


























































