4
Changes in the Climate System on Decade-to-Century Timescales
SUMMARY
Research on changes in the climate system on decade-to-century timescales has achieved notable successes in the past decade. The effective use of the paleoclimate record has revealed attributes of natural climate variability and has provided a context for the study of present and future global change. Findings about rapid climate change have been particularly enlightening, such as the recent recognition of decadal patterns in the atmosphere. This discovery is owed mostly to analyses of long-term, upper-air data, demonstrating the essential value of maintaining such long-term consistent records.
Recent advances in understanding climate prediction on timescales of decades to centuries include the following, among others: documentation and recognition of the scope of natural variability; documentation by calibrated satellite observations that clouds have a net global radiative cooling effect on the Earth-atmosphere system by about 15 to 20 W/m2; achievements in understanding water vapor behavior and in feedback analysis, proposed and to some degree realized, on theoretical, observational, modeling, and methodological grounds; and understanding the role of volcanic eruptions as a climate-forcing factor, as seen in measurement and assessment of the impacts of recent eruptions.
This area of research has underscored the complexities and uncertainties of detecting and projecting climate change. It has become even clearer that determining the roles of anthropogenic forcing is inseparable from understanding the natural system. Anthropogenic global change cannot be assessed without adequate understanding and documentation of natural climate variability on timescales of years to centuries —in other words, without adequate baseline understanding. This understanding encompasses solar and volcanic variability; feedbacks
resulting from the interactions of water vapor, clouds, and radiation; and the massive heat fluxes associated with the motions of the air and oceans and the exchanges between them, among other phenomena, beyond quantified understanding of anthropogenic forcing itself. To evaluate anthropogenic forcing specifically, greater knowledge is also needed of tropospheric aerosols and the carbon cycle.
The primary characteristics of the climate system must be documented through consistent long-term observations. Finally, the subtlety of slow change over long timescales, in contrast to diurnal, seasonal, and interannual variations, can disguise its potential long-term severity and thus limit society's willingness to address potential problems in advance. The problem is much exacerbated, of course, by the uncertainty in our ability to forecast such change. All these considerations further underscore the importance of achieving better understanding of climate change patterns on decade to century timescales, including their rate and range of variability, likelihood and distribution of occurrence, and the sensitivity of climate to changes in forcing (natural and anthropogenic). With such improved understanding, we ultimately hope to forecast and detect change (distinguishing natural from anthropogenic), providing a foundation on which future policy decisions and infrastructure management can be rationally based.
A number of Research Imperatives must be met to understand climate change on decadal to centennial timescales:
-
Natural climate patterns. Improve knowledge of decadal- to century-scale natural climate patterns, their distributions in time and space, optimal characterization, mechanistic controls, feedbacks, and sensitivities, including their interactions with, and responses to, anthropogenic climate change.
-
Paleorecord. Extend the climate record back through data archeology and paleoclimate records for time series long enough to provide researchers with a better database to analyze decadal- to century-scale patterns. Specifically, achieve a better understanding of the nature and range of natural variability over these timescales.
-
Long-term observational system. Ensure the existence of a long-term observing system for a more definitive observational foundation to evaluate decadal- to century-scale variability and change. Ensure that the system includes observations of key state variables as well as external forcings.
-
Climate system components. Address those issues whose resolution will most efficiently and significantly advance our understanding of decadal- to century-scale climate variability for specific components of the climate system.
-
Anthropogenic perturbations. Improve understanding of the long-term responses of the climate system to the anthropogenic addition of radiatively active constituents to the atmosphere and devise methods of detecting an-
-
thropogenic phenomena against the background of natural decadal-to century-scale climate variability.
INTRODUCTION
Climate research on decade to century (“dec-cen”) timescales is relatively new. Only recently have we obtained sufficient high-resolution paleoclimate records, and acquired faster computers and improved models allowing long-term simulations, to examine past change on these timescaies. This research has led to genuinely novel insights, most notably that the past assumption of a relatively stable climate state on dec-cen timescales since the last deglaciation is no longer a viable tenet. The paleorecords reveal considerable variability occurring over all timescales, while modeling and theoretical studies indicate modes of internal and coupled variability driving variations over dec-cen timescaies as well.
Thus, dec-cen climate research is only at the beginning of its learning curve, with dramatic findings appearing at an impressive rate. In this area even the most fundamental scientific issues are evolving rapidly. Adaptability to new directions and opportunities is therefore imperative to advance understanding of climate variability and change on these timescales.
The paradigm developed to successfully study climate change on seasonal to interannual timescales cannot be applied to the study of dec-cen climate problems. That is, we have realized considerable success studying short timescale climate problems by generating hypotheses and models that are quickly diagnosed and improved based on analysis of the amply long historical records or quickly realized future records. For dec-cen problems the paleoclimate records are still too sparse and the historical records too short. Future records will require multiple decades before even a nominal comparison to model predictions is possible. Compounding the problem, the change in atmospheric composition as a consequence of anthropogenic emissions represents a forcing whose future trends can only be estimated with considerable uncertainty. As a result, progress requires considerable dependence on improved and faster models, an expanded paleoclimate database, and imposed (rather than calculated) anthropogenic emission scenarios. Heavy reliance on these methods and assumed forcing curves, without the benefit of real-time observations for constant model validation and improvement, implies a considerable effort for model validation through alternative means, improved understanding of the limits and implications of proxy indicators constituting the paleoclimate records, and detailed monitoring of emissions to help track actual rates. As for future observations, we can only now begin collecting the data to aid future generations of scientists in understanding dec-cen climate variability and change.
Climate variability and change on decade to century timescales involves all of the elements of the U.S. Global Change Research Program: natural and anthropogenic variability and change; past, present, and future observational networks
and databases; modeling requirements; and physical, chemical, biological, and social sciences, with considerable attention to the human dimensions of climate change. The last focus is particularly important on dec-cen timescales because the magnitude of change is often, though not always, proportional to the timescale over which it varies. Consequently, climate change over these long timescales could produce much greater social, economic, and political impacts than shorter timescale variations, which are often addressed through disaster relief. On deccen timescales the impacts could be considerable, and adaptation and mitigation (of both the forcing and response) depend on policy decisions and investments in infrastructure. For example, the devastating floods that struck the Midwestern United States in 1993 and again in 1997 produced considerable hardship, loss, and destruction, requiring substantial recovery aid. However, if we knew that such floods occurred in, say, clusters of six or seven over a 20-year period, such information might dramatically reduce the negative impacts, through mitigation actions in policy and infrastructure. Perhaps we could even benefit in some ways from these events. Similar action would be possible, given advanced knowledge of the frequency or magnitude of extreme heat days for any particular region or, for that matter, knowledge of any other changes that might greatly affect agriculture, energy production and use, water resources and water quality, air quality, health, fisheries, forestry, insurance, recreation, and transportation. All of these areas are fundamental to society's well-being and would certainly be affected by any prolonged or abrupt shift in our climate system.
Unfortunately, the subtlety of slow change over long timescales, relative to diurnal, seasonal, and interannual variations, can disguise the potential severity of longer-term change and thus limit society 's willingness to address the issues in advance. This difficulty underscores the importance of better understanding of decadal- to century-scale climate change, its rate and range of variability, its likelihood and distribution of occurrence, and its sensitivity to changes in forcing (natural and anthropogenic). With such understanding we may ultimately forecast and detect change (distinguishing natural from anthropogenic), providing a foundation for more rationally based policy decisions and infrastructure management.
CASE STUDIES
The four case studies presented below all relate to issues of dec-cen climate variability. The first case reviews findings from Greenland ice cores about the natural variability of the climate system. The second illuminates human responses to climate variability in Mesopotamia, as deduced from the paleorecord. A case of modern response to climate change is then described, concerning flood control on the American River near Sacramento. The fourth and final case study discusses emerging signals of the human-influenced climate system.
Natural Variability
The prediction and modeling of future climate change and its effects on the environment and people are two of the most challenging tasks facing science today. To understand possible future changes in climate, knowledge of past climate change is essential. As explained in Chapter 6, ice cores were recovered in 1992 after a five-year drilling effort in the Summit region of Greenland by the U.S. Greenland Ice Sheet Project Two (GISP2) and from the European project GRIP (Greenland Ice Core Project, sited 30 km to the east of the GISP2 site); and they have produced an unparalleled record of climatic change for the past 110,000 years.a
The cores revealed changes in the Earth's climate system over the past 150,000 years or so, with annual resolutions over the past several thousand years. One of the most remarkable findings from these cores was that the climate during the past several thousand years—the period we would consider modern climate—has undergone considerable natural variability, including large swings or cycles of climate and, even more remarkably, abrupt changes occurring in decades or less. In addition to these findings, the long record of climate change also suggests that, relative to earlier times in the Earth's climate history, these past several thousand years have shown relatively little variability in climate change. The implication is that the impressive, and often abrupt, swings in climate recorded over the past several thousand years may, if anything, understate the potential for natural climate variability.
The Summit region has proven to be an ideal site from which to recover deep ice cores. The approximate −31°C mean annual air temperature there and the minimal occurrence of melt layers throughout the record assure the in situ preservation of a broad range of gaseous, soluble, and insoluble measurements of the paleo-environment. Similarity of the GISP2 and GRIP records is compelling evidence that the stratigraphy of the ice is reliable and unaffected by extensive folding, intrusion, or hiatuses from the surface to 2,790 m (~110,000 years ago). This agreement between the two cores strongly supports the climatic origin of even minor features of the records and suggests that investigations of subtle environmental signals (e.g., rapid climate change events with one- to two-year onset and termination) can be rigorously pursued.
|
a |
GISP2 successfully completed drilling through the base of the Greenland ice sheet and another 1.55m into bedrock in central Greenland on July 1, 1993, recovering the deepest ice core record in the northern hemisphere (3053.44m). GISP2, a component of Arctic System Science, is comprised of investigators from 22 institutions. Twenty programs with 46 types of measurements on the ice core comprise the deep drilling effort. Nine other programs provide direct information necessary for interpretation of the GISP2 ice core record. |
A Distant Past: The Younger Dryas and Other Rapid Climate Change Events Over the Past 110,000 Years
The Younger Dryas was the most important rapid climate change event that occurred during the last deglaciation of the North Atlantic region. Previous ice core studies had focused on the abrupt termination of this event because this transition marks the end of the last major climate reorganization during the deglaciation. Most recently, the Younger Dryas has been redated, using precision, subannually resolved, multivariate measurements from the GISP2 core, as an event of 1,300 +/−70 years' duration that terminated abruptly at 11,640 years before the present (BP), as evidenced by a rise in temperature of about 7°C and a twofold increase in the snow accumulation rate. The transition into the Preboreal, the Preboral/Younger Dryas transition, and the Younger Dryas/Holocene transition were all remarkably fast, each occurring over a decade or less (see Chapter 6).
The isotopic temperature records show 23 interstadial (or Dansgaard/Oeschger) events, first recognized in the GRIP record and verified in the GISP2 record, between 110,000 and 15,000 years BP. These millennial-scale events represent quite large climate deviations—probably of many degrees in temperature, twofold changes in snow accumulation, order-of-magnitude changes in wind-blown dust and sea salt loading, and roughly 100 ppb (volume) swings in atmospheric methane concentration.
In view of all these measures, the events must have been regional to global in scale. They are seen in local climatic indicators, such as snow accumulation rate and isotopic composition of snow linked to temperature; in regional climatic indicators, such as wind-blown sea salt and continental dust; and in regional to global indicators, such as atmospheric concentrations of methane, nitrate, and ammonium. Some of the events are also readily identified in the ocean-sediment record in regions critical to global ocean circulation.
Since these cores were obtained, additional investigations, involving large numbers of proxy indicators of past climate change, from all of the different climate zones on Earth, have reinforced these initial findings and more clearly driven home the vulnerability of the Earth 's climate system to natural variability. Consequently, these findings have changed our way of viewing the climate system and fundamentally undercut the notion that we live in a relatively stable climate system.
The Last 500+ Years: The Little Ice Age, Medieval Warm Period, and Fossil Fuel Era
The Little Ice Age and Medieval Warm Period environments are the most recent analogs for conditions cooler and warmer, respectively, than the present century. Each period can be characterized by interpreting the multiparameter
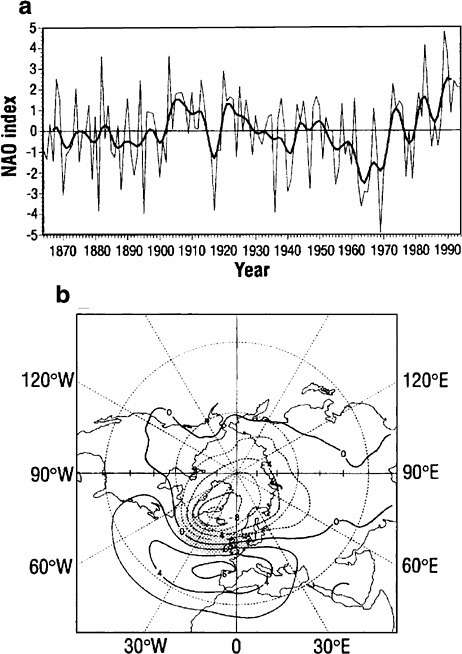
GISP2 series (e.g., CO2, stable isotopes, major ions, accumulation rate, particles). GISP2 temperature modeled from oxygen isotopes reveals a relatively subdued temperature effect at this Greenland site for the Little Ice Age. More recently, year-to-year correlations between the GISP2 isotopic record and sea surface and land temperatures over the North Atlantic, covering the period 1840 to 1970, reveal changes in atmospheric circulation patterns, such as the seesaw pattern of the North Atlantic Oscillation, demonstrating the sensitivity of the isotopic record.
Levels of continental dusts and marine sea salts increased during the Little Ice Age in response to increased meridional circulation. The Little Ice Age is one of several glaciochemically identifiable climate events in the Holocene record that correlate with other paleoclimate records. The period is characterized by the most rapid onset of any Holocene cold period.
Measurements of CO2 in air bubbles of the GISP2 core indicate that between 1530 and 1810 atmospheric CO2 levels remained relatively constant at +/−280 ppm(v). Thereafter, concentrations rose rather abruptly and smoothly connected to the atmospheric observations at Mauna Loa. Previously identified increases in sulfate and nitrate seen in south Greenland ice cores and attributed to anthropogenic activity were identified in the GISP2 core and contrasted to the preanthropogenic atmosphere. An observed increase in chloride at GISP2, as in the 1940s, is believed to be a byproduct of increased anthropogenic HNO3 and H2SO4, since these compounds are believed to aid in the volatilization of HCl from sea salt aerosol.
Human Responses to Climate Change as Deduced from the Paleorecord
Although the issue of human response to climate change is controversial, several recent studies find close correlations in timing between climate change and changes in civilization. These studies have focused on changes in temperature in relation to high-latitude societies and changes in moisture availability for mid- to low-latitude societies. In regions on the ice margins, such events as the disappearance of the Norse colonies in Greenland during the mid- to late fourteenth century appear to be chronologically correlated at some sites with the occurrence of a few extremely cold winters and at others with the general amelioration of climate produced at the onset of the Little Ice Age.1
By utilizing climate-linked paleoclimate records, it was found that periods of decreased atmospheric circulation intensity in the North Atlantic, developed from the GISP2 ice core, could be correlated with discontinuous Dead Sea level records of drying,2 which are a reasonable indicator for west Asian aridity.3; The more detailed record reveals a close correlation between major periods of drying and major social disruptions in west Asian civilization. 4 Other research5 has found that the driest period represented by a late Holocene lake sediment record from Mexico correlates closely with the collapse of the classic Mayan civilization around 750 to 900 AD.
A Modern Climate Change Dilemma: Flood Control on the American River
The significance of decadal- to centennial-scale climate variability is highlighted by a recent example of water resources planning.6 Flood control projects are designed to protect facilities from a design flood or flow. The level of protection (i.e., the risk of project failure) provided against the design flood is assessed through statistical analysis of the historical flood record. The economics of a new flood control project are determined by comparing the expected monetary benefits of reducing flood risk and the associated project cost. Flood insurance programs rely on a similar analysis. The variability of flood risk at decadal to centennial timescales and its implications for flood control are discussed here in the context of the American River near Sacramento, California.
Flood protection for Sacramento is provided by the Folsom Dam together with a system of levees. The dam was designed in the late 1940s, based in part on a flood record extending back to 1905. Since the dam's design, there have been six floods (not including the 1997 flood) on the American River larger than all previously recorded floods (see Figure 4.1). The estimated frequency of exceedance of extreme floods has correspondingly increased. It now appears that a large part of Sacramento may not even have 100-year flood protection. Should new flood mitigation projects be based on an assessment of flood risk from the
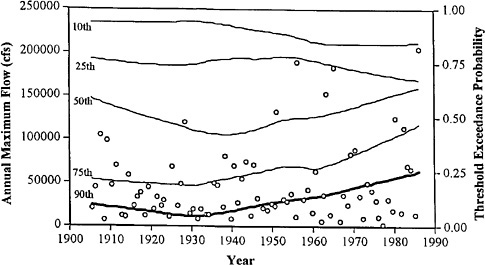
FIGURE 4.1 The time-varying probabilty of exceeding the 10th, 25th, 50th, 75th, and 90th quantiles of the full American River annual maximum flood record (shown as o), estimated by smoothing (with a 56-year span) a binary indicator (1 = exceedance, else 0) applied to the quantile. Note the trend reversal since about 1940, with an increase in the probability of exceedance of the rarer floods and a decrease for the more common floods. SOURCE: National Research Council (1995a).
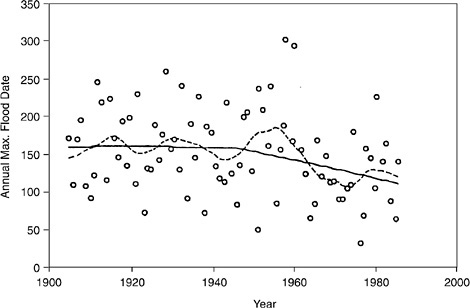
FIGURE 4.2 Date of annual maximum flood for the American River near Fair Oaks. Centennial and decadal trends are shown by the solid (56-year smooth) and the dotted lines (14-year smooth). SOURCE: National Research Council (1999).
entire flood record or from the past 50 years? A project designed to provide a 200-year level of protection based on the full flood record would provide less than 100-year protection based on the record since 1950. Project costs and potential flood damages could vary by over an order of magnitude depending on the protection level adopted. This decision-making dilemma was noted by the National Research Council Committee on Flood Control Alternatives in the American River Basin. 7
Since about 1940, the annual maximum flow on the American River has also occurred earlier in the year (see Figure 4.2), with a decadal fluctuation superposed on this trend. This pattern has implications for the types of models (e.g., rain on snow dynamics instead of rainfall runoff) needed for flood forecasting and for real-time flood control. A number of factors, including improvements in streamflow measurement technology and urbanization of the watershed, may be responsible for these changes in the flood regime. However, structured decadal to centennial climate variations are a likely cause.
Others8 argue that earlier snowmelt in California may be caused by a trend toward warmer winters there and a concurrent long-term fluctuation in winter atmospheric circulation over the North Pacific Ocean and North America. The fluctuation began to affect California in the 1940s, when the region of strongest low-frequency variation in winter circulations shifted to a part of the central
North Pacific Ocean that is strongly linked to California temperatures through the Pacific-North American (PNA) teleconnection pattern. 9 Since the late 1940s, winter wind fields have been displaced progressively southward over the central North Pacific and northward over the West Coast of North America. These shifts in atmospheric circulation are associated with concurrent shifts in both West Coast air temperatures and North Pacific sea surface temperatures and with earlier snowmelt and increased spring moisture fluxes in the American River basin.
Gridded (5° * 5°) monthly records of northern hemisphere sea level pressure (SLP) 10 and surface temperature11 for the period 1899 to 1996 have been used to reconstruct space and time patterns of quasi-oscillatory large-scale climate patterns at quasi-biennial ENSO (El Niño-Southern Oscillation), decadal, interdecadal, and secular frequency bands.12 For the analysis a 40-year moving window Multi-Taper Method/Singular Value Decomposition (MTM-SVD) was used. Simultaneous analyses of these datasets help identify dynamically consistent space- and time-coherent patterns of low-frequency climate evolution. Projections of the hemispheric low-frequency patterns of SLP and temperature at the grid point closest to the American River streamflow gauge are shown in Figure 4.3. The low-frequency SLP and temperature projections are obtained from the MTM-SVD analysis by summing over the reconstructions for the secular (>30
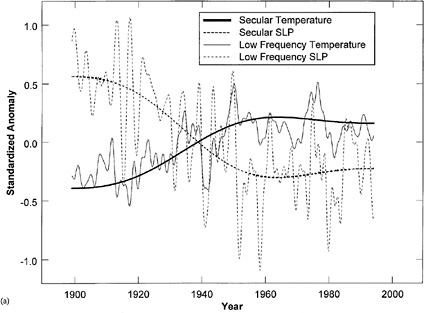
FIGURE 4.3 The secular and low-frequency components of SLP and temperature at the grid point nearest the American River from MTM-SVD. Note the secular trend toward warmer temperatures and lower pressure in the region, post-1940, coincident with the increased flood incidence and shift in flood timing. SOURCE: National Research Council (1999).
years), interdecadal (18-year period), decadal (10-year period), ENSO (3- to 6-year period), and quasi-biennial (2.2-year period) bands at the closest grid point. Note the secular trend for a shift to a lower SLP and warmer temperature at the American River region since about 1940. A remarkable connection between low-frequency climate and the high-frequency flood process is shown. Understanding and long-lead prediction of these fluctuations and their impact on regional hydrology and floods are key for dynamic flood risk assessment and better flood protection design and management. Flood insurance programs could be made much more efficient if long-term regional flood risk could be better assessed and “opposing” trends exploited.
Anthropogenic “Greenhouse” Warming
In 1896 Arrhenius pointed out that the increased concentration of CO2 in the Earth's atmosphere, introduced by the burning of fossil fuels and compounded by other societal byproducts, could enhance the Earth's natural greenhouse warming, leading to an anthropogenic warming of the climate system and affecting civilization throughout the globe. A significant amount of research has been directed toward this problem, to understand if and how such an impact could be realized (or negated by natural feedbacks) and how to detect and interpret the source of such a warming. One of the most perplexing aspects of this research has been understanding the warming that the Earth has indeed experienced over this last century (see Figure 4.4) to determine whether this warming is natural, anthropogenic, or some combination of the two.
As noted in the Intergovernmental Panel on Climate Change (IPCC) Second Assessment (1996), the focus of recent climate change and variability research has shifted from the analysis of mean global temperature to that of temperature spatial distributions. This shift reflects the expectation that climate change may manifest itself irregularly in space and time. For example, it is clear that the relatively rapid global warming experienced over the past 20 to 25 years is distinguished by enhanced warming in winter (not evident in previous decades), with a strong warming over northern hemisphere land, but some small cooling over the northern hemisphere oceans. 13 This is the so-called COWL pattern: cold oceans and warm land pattern that is readily apparent in the global surface temperature data when comparing the past 20 years to the previous 20 years (see Plate 5).
The COWL pattern is a northern hemisphere winter phenomenon. A similar geographic pattern is simulated by numerous anthropogenic modeling studies and thus considered by some to represent one component of the so-called greenhouse fingerprint14—that is, a characteristic of the changing climate that might be uniquely associated with anthropogenic warming, as opposed to natural warming. Its presence in the actual observations has therefore been accepted as additional evidence of anthropogenic warming.15
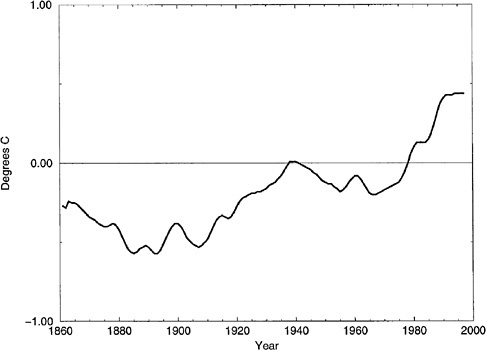
FIGURE 4.4 Annual global mean temperature for land areas from 1861 to 1997. The curve shows anomalies with respect to the mean temperature for the 30 years 1961–1990. SOURCE: Global Historical Climatology Network (GHCN); Peterson and Vose 1997. Courtesy of the National Climatic Data Center.
When the monthly averaged northern hemisphere surface temperature time series for this century is adjusted to eliminate the influence of the COWL pattern, two things become apparent:16 a large fraction of the month-to-month variability, particularly apparent in the cool-season months, is no longer seen, and a significant fraction of the accelerated hemispheric warming observed since the mid-1970s, again concentrated in the cooling-season months, is also removed, making the summer and winter trends comparable (see Figure 4.5).
Further investigation suggests that much, though not all, of the accelerated warming since the mid-1970s that is attributed to the COWL pattern, and thus much of the COWL pattern itself during this period, can be explained by the similar time-averaged polarity of two natural patterns of climate variability—the North Atlantic Oscillation (NAO) and the PNA teleconnection (explained below; Hurrell, 1996). That is, over the past 20 years, the NAO and PNA patterns (the latter as indexed by another regional pattern, the North Pacific Index) both seem to show an apparently unusually persistent tendency, on average, to occupy states that favor a warming of Europe and Northern Asia by the NAO and a warming of North America by the PNA.
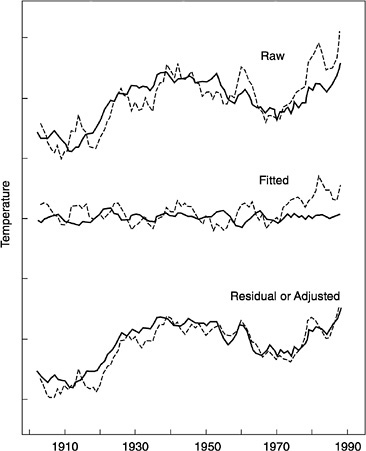
FIGURE 4.5 Smoothed monthly averaged surface temperature time series, northern hemisphere (top curves, dashed line is warm month's average, solid line is cool month's average; middle curves, same line indications but representing temperatures attributed to COWL pattern; bottom curves, same color indications but adjusted by eliminating COWL contribution shown in the middle set of curves. SOURCE: Wallace et al. (1995). Courtesy of the American Association for the Advancement of Science.
When this warming is removed, the global trends of the past two decades are similar, though still slightly larger, than the warming that occurred over several decades during the beginning of this century (e.g., from 1910 to 1940). Accordingly, several questions naturally follow: (1) Is this contribution of the NAO and PNA to the COWL warming a result of natural variability? That is, simply by chance will there likely be extended periods of time in which they display similar and relatively persistent polarity, or is this the manifestation of anthropogenic warming influencing the polarity of the natural climate modes? (2) Is the residual warming, that is, after removal of the COWL contribution, natural variability or
anthropogenic warming? (3) What are the relationships among the COWL pattern, greenhouse fingerprint, and natural climate patterns? Such issues must be addressed to advance our understanding of climate variability and change over decadal to century timescales and to evaluate natural and anthropogenic influences. These and other equally important issues are articulated further in this chapter.
A RESEARCH AGENDA FOR THE NEXT DECADE
This section examines Research Imperatives and associated Scientific Questions that should guide future research on climate variability and change on decadal to century timescales.
Issues Regarding Climate Variability
By their very meanings, climate change and climate variability implicitly refer to reference, normal, or climatological mean states. Because climate varies on all timescales,17 one mean can serve as a reference state for the study of variability on shorter timescales while itself changing on longer timescales. In practice, whatever the definition used for the mean, an anomaly is the difference between some observed state of the climate system and that mean. Climate change and variability are characterized in terms of these anomalies. Fortunately, as the study of such anomalies develops, it becomes increasingly apparent that they are not randomly distributed in space and time but often appear to be organized in relatively coherent spatial structures that tend to preserve their shape while varying their amplitude and sometimes their phasing through time. Though the precise nature and form of these structures, or patterns, vary to some extent according to the statistical methodology used in the analysis, a rather consistent set of regional characteristics is generally found to be associated with the variant patterns. In short, in studying climate variability and change, the study of patterns is a natural development.
To date, we do not have a comprehensive inventory of global patterns, nor do we understand their mechanisms, couplings, longevity, or full implications for climate prediction. However, study of the most thoroughly investigated pattern, ENSO, which dominates the tropical Pacific, led to the first-ever successful climate predictions while yielding considerable insights about the climate system, the nature of its couplings, scales of influence, and other fundamental findings. Many other patterns, while not as well documented or studied, appear to be related to regional climate, others to the frequency of hurricanes, the nature of the ocean's thermohaline circulation, agricultural yields, and regional fish inventories, among other things. These patterns vary over a broad range of space and timescales; their relative phasing can dominate global temperature variations; and they often show regional and global teleconnections, covary with other cli-
matological variables, and seem to focus different forcings and processes into single coherent responses. Because of these attributes and covarying relationships, further study of patterns may ultimately yield benefits like those obtained through the study of ENSO. Patterns thus provide one obvious avenue to pursue the search for predictive climate signals —that is, a manageable set of components into which a complex climate system can be decomposed.
Patterns in the Climate System
The literature is replete with descriptions of patterns covering a broad range of climatological variables and spatial scales. Several of these patterns have received considerable attention in recent years, and their names are now firmly established in the climatological lexicon. One goal of this chapter is to briefly describe the more widely referenced patterns that vary on decadal or longer timescales. This review thus serves as an abbreviated glossary for the remainder of the text while covering a representative selection of patterns, their characteristics, couplings, and relationships. The review also presents issues in interpreting the roles of patterns in climate variability and change over decadal to century timescales.
The North Atlantic Oscillation
The NAO is a predominantly wintertime, regional, sea level pressure (SLP) pattern whose influence extends across much of the North Atlantic and well into Europe (see Figure 4.6). A considerable part of its variance resides in a decadal-scale band.18 The NAO is often indexed by the difference in SLP in Iceland (representing the strength of the Icelandic, or Newfoundland, low) and the Azores or Lisbon (near the central ridge of the Bermuda and Azorean High). Correlation of the NAO index to surface air temperature and sea surface temperature (SST) further reveals the degree to which the pattern is shared by the North Atlantic, the northern part of Europe, and northern Asia.19 Typically, when the index is high, the Icelandic low is strong, which results in the increased influence of cold Arctic air masses on the northeastern seaboard of North America and enhanced westerlies introducing warmer, moister air masses to western Europe in winter. 20 Thus, NAO anomalies are related to wintertime temperature and precipitation downstream over Europe and across Russia and Siberia.21 They have also been linked22 to changes in the thermohaline circulation in the North Atlantic, 23 the cod stock in the northwest Atlantic,24 and the mass balance of European glaciers.25
The Pacific-North American Teleconnection
The PNA is a large-scale teleconnection between the North Pacific Ocean and North America that appears as four distinct cells in the 500 mb geopotential height field. An index of this teleconnection pattern was created26 through a
weighted average of 500-mb normalized height anomaly differences between the centers of the four cells; that is, the height anomaly differences between the North Pacific and Hawaii and between Alberta, Canada, and the southeastern United States (see Figure 4.7). However, the PNA also appears in SLP27 as well and can be depicted by the North Pacific Index.28 The North Pacific Index is expressed as the areally averaged SLP over a large area of the North Pacific Ocean near the center of the Aleutian low.
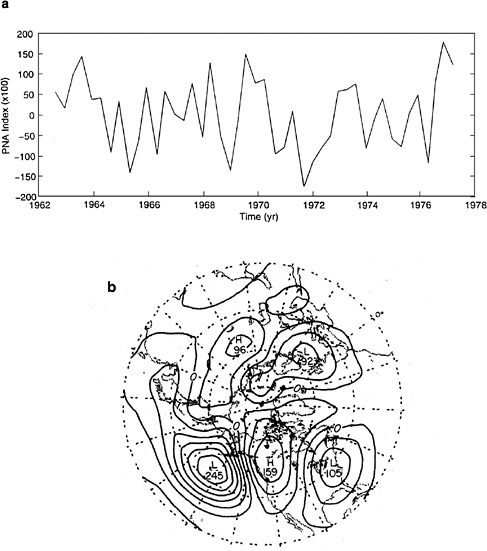
FIGURE 4.7 (a) Variation in the Pacific-North American (PNA) teleconnection index since 1962. (b) Region of PNA influence. SOURCE: Wallace and Gutzler (1981). Courtesy of the American Meteorological Society.
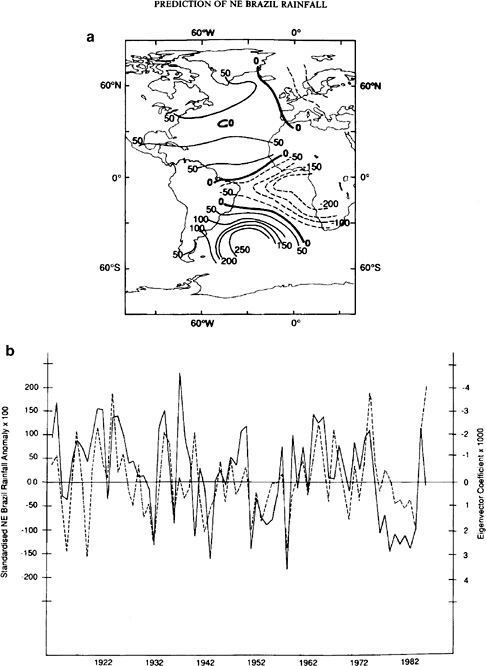
FIGURE 4.8 (a) The spatial EOF pattern of the Atlantic SST that is most associated with rainfall in Nordester, Brazil. (b) Time series (solid line) of the March-May values shown in (a) and north Nordeste Brazil rainfall anomalies (dashed line). SOURCE: Ward and Folland (1991). Courtesy of John Wiley & Sons Ltd.
The Pacific-North America pattem is highly correlated with ENSO,29 and the North Pacific Index is highly correlated with the Southern Oscillation,30 suggesting a broader extratropical influence of ENSO, though the low-frequency variability of the Pacific-North America pattern and the North Pacific Index may influence the ENSO phenomenon as well, as discussed below.31 Decadal variability in the Pacific-North America pattern is also thought to be responsible for a significant amount of the variance in the salmon inventory along the northwest Pacific coast.32
The West Pacific Oscillation, North Pacific Oscillation, and Pacific Decadal Oscillation are smaller North Pacific patterns characterized by the areally averaged SLP in the western, central, and eastern North Pacific,33 respectively. Both the West Pacific Oscillation and the North Pacific Index show a strong correlation to ENSO, although they are only weakly correlated to each other.
Tropical Atlantic SST
The tropical Atlantic Ocean shows a coherent structure in SST. There, the dominant empirical orthogonal function pattern of SST often shows a warm pool in the tropical North Atlantic and a complementary cool pool in the tropical South Atlantic, or vice versa. These contrasting pools seem to vary coherently over decadal timescales, though they vary independently on shorter timescales in their regions. Consequently, the general pattern is sometimes referred to as the Atlantic Tropical Dipole, though the lack of a clear consensus on the actual dipole nature of the pattern leaves many referring to it simply as the Tropical Atlantic SST variability. This low-frequency SST phenomenon is associated with anomalies in rainfall over Brazil and northern Africa (see Figure 4.8). It has been suggested34 that the decadal changes in the SST in the subtropical North Atlantic may also be responsible for changes in the distribution and intensity of hurricanes in this region.
Decadal ENSO-like Pattern
The low-frequency covarying changes in the tropical Pacific atmosphere and ocean strongly resemble the pattern of the interannual ENSO phenomenon, including teleconnected anomalies in the midlatitude atmosphere and ocean of the North Pacific region (see Figure 4.9 a and Figure 4.9 b). The decadal ENSO-like anomalies are also teleconnected throughout the tropics, with large concurrent changes in tropical Atlantic and Indian Ocean SST,35 as well as in the North Pacific Ocean and overlying atmosphere.36
The past few decades have experienced a warm phase of this climate anomaly, which has preceded a significant reduction in the alpine glaciers throughout the tropics.37 In addition, the frequency of precipitation, streamflow, and snowpack in
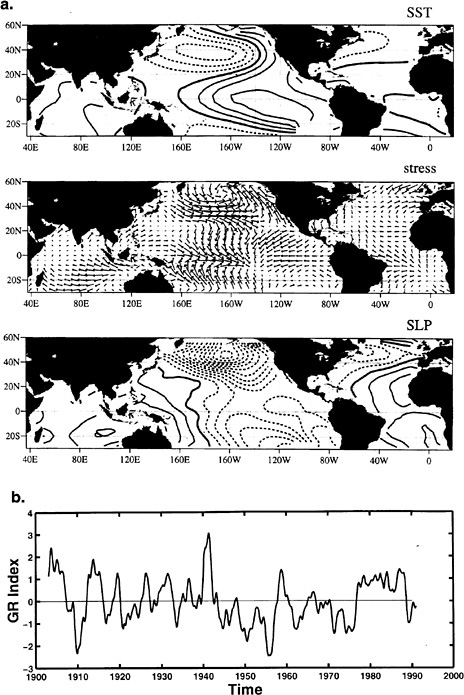
FIGURE 4.9 (a) Global residual SST pattern from which the linearly related ENSO variability has been removed. (b) Time series of the pattern displayed in (a), which indicates that the ENSO-like pattern primarily shows decadal- to century-scale variability. SOURCE: Zhang et al. (1997). Courtesy of the American Meteorological Society.
northwestern and southwestern North America38 are well correlated with this time series of the decadal ENSO-like climate phenomenon.
Other Patterns
The patterns described above, while prominent in the literature and displaying variability on decadal timescales, represent only a subset of the decadal- to century-scale patterns identified and may or may not be of any more value than patterns not discussed here. For example, a number of regional atmospheric patterns have been analyzed, such as the North Pacific Oscillation,39 West Pacific Oscillation,40 West Atlantic Pattern,41 and Pacific Decadal Oscillation.42 A completely different kind of pattern, involving sea ice, has been found in the Southern Ocean. The Antarctic Circumpolar Wave is characterized by deviations in the Antarctic sea ice extent from monthly climatological averages, although it is also apparent in surface wind, SST, and SLP anomalies along the Antarctic polar front, near the winter marginal ice zone.43 It is also highly coherent with temporal variations in ENSO44 and Indian Ocean monsoons.45 Other atmospheric patterns have been identified in the Southern Hemisphere,46 though the data are frequently too few to allow detailed analyses.
In addition, there are structures that might be considered climate patterns, although they are often related to the other patterns or presented in a similar manner. For example, the Asian monsoon, while predominantly a seasonal signal, is strongly correlated with ENSO and shows decadal variability as indexed by precipitation and wind speeds over India.47 Some investigators treat this monsoon pattern as a distinct, decadally varying pattern. Also, global thermohaline circulation has been tied to distinct changes in the ocean surface conditions and NAO in the North Atlantic Ocean. Extensive studies have shown the relationship between the NAO, ocean conditions, and thermohaline circulation, although no unique pattern has been defined.
Finally, the COWL pattern, while not a fundamental mode of climate variability, as defined by the decomposition of climatological variable fields, or a particular climate phenomenon, does appear to represent a distinct geographic distribution of near-surface temperature anomalies. Despite the apparent shortterm memory of the COWL pattern, it displays long-term variability, as discussed in the case study above of anthropogenic “greenhouse” warming. Thus, COWL does represent another pattern and is often cited as such.
Research Imperatives for Explaining Climate Patterns
The large heat capacity and slow changes of the ocean must play a considerable role in climate anomalies persisting or evolving over decade to century timescales. This role is directly realized through SST, which sets the thermal contrast between the atmosphere and ocean and thus controls (together with
shear) the heat flux. Since the heat capacity of the ocean is so much greater than that of the atmosphere, a minuscule change in ocean surface temperature corresponds to a substantial change in the overlying atmospheric column temperature. Thus, SST exerts a considerable influence on the atmospheric surface layer temperature and pressure. The decadal variability of the patterns, their covariance with SST and sea surface salinity (SSS), and their obvious geographic distributions reflecting the underlying distribution of ocean and land masses suggest that these variations and their changes on dec-cen timescales must be addressed as a coupled ocean-atmosphere problem (consistent with our current treatment of the ENSO phenomenon).
Therefore, the coupled air-sea system warrants special attention when considering longer-timescale variability of climate phenomena. In particular, several existing hypotheses predict the nature of the air-sea interaction responsible for tropical-extratropical climate links (e.g., the relationship between ENSO and PNA, or between TAO [Tropical Atmospheric-Ocean] and NAO).These hypotheses typically posit the fast propagation of an anomaly from the tropical regions (by standard atmospheric processes, or Kelvin or Rossby wave instabilities in the ocean, or western boundary current propagation) to the extratropics, followed by a slow return to the tropics via the ocean circulation system (e.g., subduction,48 gyre circulation,49 and thermocline subductive processes50), as discussed above. Because it will take so many years to test for these mechanisms through observational networks, a fundamental issue at this stage is to evaluate more fully the likelihood and signature of these different mechanisms to better focus future observing networks toward identifying the operative mechanisms.
Additionally, the coupled modes of climate variability must be clearly defined. That is, we need to define those physical modes of operation in the coupling of sea and air processes, that is, modes that would not exist without the presence of both media. Along these lines, we must determine whether all of the climate patterns fall into this classification or whether the patterns simple reflect the Hasselman 51 theory of climate change, in which the patterns and coupled modes of variability are simply the result of a white noise (weather) forcing over an alternating distribution of high-heat capacity ocean and low-heat capacity land surfaces (this can be considered the null hypothesis).
The relationship and linkages among the climate patterns, thought to reflect coupled air-sea interactions, also must be further clarified. For example, the decadal variability of ENSO and its relationship to the PNA and other North Pacific climate patterns has been noted, and ENSO's wintertime 500 mb height anomaly pattern of decadal variability is very similar to its interannual variability.52 In the other fields (SST, SLP, and wind stress) the leading empirical orthogonal function pattern of decadal and longer timescale variability looks very similar in shape to ENSO (with a slight extension into the extratropical North Pacific). Despite a number of hypotheses suggesting the reason for this pattern shape, and some knowledge of the mechanism explaining the longer-scale variation in ENSO, there is no clear under-
standing of how ENSO and other climate phenomena interact with and regulate each other, invoking processes operative on different timescales yet producing spatial distributions that are quite similar regardless of timescale. These phenomena need further attention and clarification.
While there are a number of coupled modes, or observed patterns of covariation, between atmospheric fields (e.g., wind stress, SLP) and ocean surface and subsurface property fields (e.g., SSS, SST, thermocline salinity and temperature, and water mass migrations), the causal and controlling mechanisms of these patterns are not known at this time. Because these relationships will provide direct insights into the mechanisms that communicate climate anomalies between the ocean and atmosphere, and thus preserve and propagate the anomalies in both space and time, it is imperative that we gain improved understanding of these covarying relationships.
Better description and understanding are also needed of the relationships between pattern components (e.g., SST) and regional climate anomalies (e.g., rainfall), as seen in the case of the tropical Atlantic SST and African and Brazilian rainfall. The fundamental mechanisms of these relationships also must be determined. Such relationships are among the more critical patterns to investigate, since understanding them may allow prediction of seasonal influences.
Finally, while the implications of a change in the thermohaline circulation for climate are still unclear, there seems to be a coupling between the thermohaline circulation of the North Atlantic and the thermohaline circulation in that region, as indicated by the Great Salinity Anomaly of the 1960s.54 An improved documentation of this relationship and analysis of its mechanisms and implications are required to help define the relationship and its significance.
To understand all such patterns in the climate system, the community is challenged by the following three interdependent research imperatives:
-
Natural climate patterns. Improve knowledge of decadal- to century-scale natural climate patterns, their distributions in time and space, their optimal characterizations, mechanistic controls, feedbacks, and sensitivities, including their interactions with, and responses to, anthropogenic climate change.
Meeting this goal—and the related objectives discussed in the section on climate components below—requires long-term calibrated observations. This need therefore entails two major supporting research imperatives:
-
Paleorecord. Extend the climate record back through data archeology and paleoclimate records for time series long enough to provide researchers with a better database to analyze decadal- to century-scale patterns. Specifically, achieve a better understanding of the nature and range of natural variability over these timescales.
-
Long-term observational system. Ensure the existence of a long-term
-
observing system for a more definitive observational foundation to evaluate decade-to-century-scale variability and change. Ensure that the system includes observations of key state variables as well as external forcings.
Key Scientific Questions
To address the Research Imperatives above, some difficult Scientific Questions must be answered. Despite the uncertain roles of patterns and coupled modes in global warming and climate change more generally (including those changes important for climate prediction), it is clear that these patterns and coupled modes occupy large spatial areas, describe significant climate variance, and bridge high-, mid-, and low-latitude zones, thus representing potential means through which coherent climate variations and change may be propagated globally. As noted earlier, patterns emerge naturally in the study of climate anomalies and change; moreover, their study is consistent with the IPCC Second Assessment (1996). Identification of coherent patterns with coupled modes that explain significant spatial and temporal variability offers hope that a signal may be found in what otherwise appears as conspicuous noise. The apparent persistence of such patterns, even allowing for their possible slow evolution, provides additional hope that this signal may be exploited to predict and address future change and variability. Moreover, the relationships observed between specific climate pattern dispositions and regional climate characteristics supports the notion that better understanding of these relationships may allow short-term seasonal predictions for some regions.
To realize these potentials, considerable effort must also be invested in improving our general understanding of patterns and coupled modes, their mechanisms (dynamic and thermodynamic, natural and anthropogenic), couplings, feedbacks, and sensitivities. These are truly cross-disciplinary issues, requiring a strong interdisciplinary approach. Specifically, we must address the following Scientific Questions:
-
What is the longevity of patterns and their spatial/temporal variance? Observed climate patterns offer tantalizing evidence that some part of the Earth's climate shows spatially and temporally coherent structure, with some degree of (predictable) persistence in a time-averaged sense. However, the fundamental patterns themselves may be transitory phenomena, reflecting the current configuration of a slowly changing climate. In fact, it has been suggested that, prior to the start of the twentieth century, the NAO displayed a different influence on European climate, in which case its general characteristics may have been different than today.54
-
What is the best way of characterizing the known patterns and are there additional patterns of interest? Specifically, what are the salient features of
-
the patterns, covarying components, coupled modes (including regional influences and correlations with the climate attributes of Chapter 6), sensitivities to analytical techniques, spatial distribution, and teleconnections? Likewise, robust optimal indices of these patterns should be established. Some of the indices now used, while convenient, do not capture much of the spatial and temporal complexity of the coherent atmospheric circulation patterns they represent. For example, the Bermuda-Azorean High remains relatively stable in its spatial orientation, but the Icelandic low often migrates southward to Newfoundland. Thus, the North Atlantic SST pattern tends to show a rotation around the basin55 that a simple dipole index between two fixed points, such as the NAO, cannot capture. Therefore, while the indices have proven sufficient in their ability to simplify the temporal history of complex patterns and demonstrate the patterns' broad spatial coherence and importance, additional research is required to better characterize the patterns and isolate their significant characteristics. That is, more robust indices must be developed. Additionally, what patterns and coupled modes exist in currently data-poor regions and what are their spatial and temporal characteristics?
-
Which patterns represent true dynamic modes and which are simply statistically consistent structures or geographically forced distributions? Are identified patterns fundamental modes of climate variability reflecting coupled internal and external dynamics and thermodynamics? Or are they simple reflections of the land-sea distribution in keeping with the Hasselmann (1976) theory of climate change? Or are they the consequence of simple statistics or chaos, representing attractors or random but spatially consistent distributions? This understanding is fundamental to assess their value in long-term forecasting and prediction of climate change and variability.
-
What mechanisms generate, maintain, and modify the patterns? What is the role of those mechanisms in the spatial propagation of regionally initiated variability and change? What are their critical dependencies? Certain mechanisms control the spatial and temporal evolution of the patterns and their broader influences or teleconnections. It is necessary to understand how a change in the state of a pattern in one location may dictate the regional climate in some more remote location. Such understanding will also reveal how a local disturbance may influence the dominant regional pattern, leading to the broader propagation of the anomaly, thus revealing controlling components of the climate system.
-
What is the relationship between the observed climate patterns and global warming? Is the contribution of the NAO and the PNA patterns to the COWL warming a result of natural variability? That is, by chance will there be extended periods of time in which they display similar and relatively persistent polarity or is this the manifestation of anthropogenic
-
warming through the polarity of the natural climate modes?56 That is, is the residual warming after removal of the COWL contribution attributable to natural variability or anthropogenic warming? What are the relationships between the COWL pattern, the greenhouse fingerprint, and natural climate patterns? In other words, how do the natural modes of the climate system respond to different changes in forcing, natural or anthropogenic? Are there unique characteristics or response modes? What controls the degree and nature of the spatial coalescence? How do they covary?
Components of the Climate System
While the existence of climate patterns offers hope that some part of climate variability may be related to these patterns' states, ultimately we must understand the physics controlling the evolution of the climate system in general as well as the patterns themselves. For example, understanding relationships between climate patterns and specific climate attributes may afford us statistical forecasting capabilities, but they will be limited to configurations or changes that have already been documented. Any attempt to forecast future variations or change, particularly in response to alterations in external or internal forcings, requires that we comprehend the underlying physical and biogeochemical interactions that control the responses and various feedbacks of the broader climate system of which the patterns are only the more convenient manifestations.
This section therefore looks at the component, or discipline-specific, issues that must be resolved to most efficiently advance our understanding of climate variability and change on decade to century timescales.
The atmosphere is a critical climate system component through which climate change and variability are registered. However, on dec-cen timescales, variability and change in the atmosphere, with its intrinsically short timescales, must involve considerable contributions in the form of couplings and feedbacks from the complex boundaries—the oceans, land, cryosphere, and biosphere. Thus, for convenience the climate system can be divided into four distinct components—the atmospheric circulation and three atmospheric boundary components, the oceans, cryosphere, and land and vegetation. There are also two coupled components to consider: the hydrological cycle (including the rates, paths, and storage of water through the atmosphere and at boundaries), and the chemical composition and radiative balance of the atmosphere (which includes the atmosphere and boundary coupling as well, in addition to representing the fundamental forcing of the system). The first four categories, then, are the fundamental components of the climate system (which themselves involve some dependence on their boundaries), followed by two coupled components that intimately involve the atmosphere and its boundaries. The last two are similarly critical areas
of study in understanding the forcing of change and variability in the atmosphere, and they are of particular relevance to understanding anthropogenic change.
Research Imperatives for Characterizing Climate System Components and Perturbations
To characterize the climate system sufficiently, we need to take it apart, understand how its pieces work, and then “reassemble” the system. We also need to understand how the system responds to perturbations. Two Research Imperatives can guide this process. After presenting these imperatives, we turn to specific issues and key Scientific Questions for each major type of climate system component.
-
Climate system components: Address those issues in terms of individual climate components whose resolution will most efficiently and significantly advance our understanding of dec-cen climate variability.
-
Anthropogenic perturbations: Improve understanding of the climate system's long-term response to anthropogenic additions of radiatively active constituents to the atmosphere and devise methods of detecting the anthropogenic signal over the background noise of dec-cen climate variability.
The Atmosphere
Atmospheric water content (in all three phases), distribution of radiatively active gases, and aerosol concentrations all directly force the climate system and its principal operating agent, the atmospheric circulation. Atmospheric circulation plays a key role in redistributing physical and chemical properties, such as heat, moisture, and aerosols between source and sink regions, thus determining the regional variations of climate. In doing so, atmospheric circulation also directly controls the distribution in space and time of temperature and fresh water and thus the nature and distribution of ecosystems, surface radiation (via distribution of aerosols, which influence cloud distribution and formation physics), and sea level change (via distribution of heat and moisture and ice melt and decay). Atmospheric circulation, induced by fluctuating local and remote boundary conditions, also communicates changes from one location to another. This action often manifests itself in the form of storms. In addition, atmospheric circulation influences the location and disposition of large-scale climate patterns.
Issues in Atmospheric Circulation
Our current understanding of atmospheric processes and large-scale circulation suggests that one critical area of research is the feedback and interactions
among moisture fields (clouds and water vapor) and motion fields. Particular issues are cloud-water vaporization feedback, cloud formation (including vertical structure, radiative, circulation, and other feedbacks), and the model representation of those processes, which often occur as subgrid-scale processes. Also important are the relationships of cloud formation and evolution and surface boundary conditions.
The relationship between atmospheric circulation variability and external radiative forcing has not been clearly resolved. Numerous studies have tried to identify periodic behavior in the atmospheric spectrum due to periodic changes in solar forcing. Because the lower atmosphere absorbs only a small part of incoming solar radiation, which varies by only 1 W/m2 over a solar cycle, it is hard to see how such a weak signal could affect climate, unless a positive feedback existed in the atmosphere. Nonetheless, the evidence suggesting such a relationship is often compelling, justifying a concerted effort to understand the potential mechanisms.
Because of the likelihood that anthropogenic change is already imprinted in records of climate variability over the past century, there is a strong need to obtain paleorecords of past atmospheric conditions for instrumental and proxy data and to increase the volume of the archives through data “archeology” (reconstruction of past climate data) and additions of new data. These efforts should proceed in parallel with establishing clear guidelines for future atmospheric observations and careful planning of observational networks, so that adequacy, continuity, and homogeneity of the records are assured. The future observations should describe both state variables (winds, pressure, temperature, humidity, and rainfall) and forcing and other related variables (solar radiation, clouds, aerosols, and chemical composition).
Key Scientific Questions About Atmospheric Circulation
How much of the dec-cen variability is unforced? For example, are dec-cen variations of the PNA, NAO, and other climate patterns driven by inherent natural climate system variations, reflecting nonlinear internal interactions, or coupled interactions (in all cases, interactions that would effectively extend the intrinsic atmospheric timescales)? Or are the variations driven predominantly by changes in radiative forcing, due to anthropogenic increases of greenhouse trace gases, natural or anthropogenic aerosols, and/or variations in solar irradiance?
How does large-scale circulation change on dec-cen timescales, and how does it interact on these scales with regional and higher-frequency changes? Better documentation is needed of large-scale circulation changes to identify how they covary with regional climate states, storm tracks, and weather systems that typically vary over shorter timescales. In other words, how do variations in the mean climate state influence the spatial and temporal distributions of the higher-
frequency variations, and how might this knowledge of the relationships help in predicting shorter-timescale climate phenomena?
What are the magnitudes, spatial and temporal patterns, and mechanisms of midlatitude atmospheric responses to both midlatitude and tropical SSTs? Numerous mechanisms have been hypothesized to explain the observed teleconnections between the tropical and extratropical latitudes, many involving the slow propagation of anomalies via ocean processes back to the equator and fast atmospheric processes away from the tropics. These and other such hypotheses must be thoroughly evaluated to identify the dominant mechanisms of anomaly persistence and communication. Also, the full spatial extent of such teleconnections has not been thoroughly documented. This information also must be pursued to determine over just what scales local and regional anomalies and influences are communicated. Moreover, links to tropical sea surface temperature and the decadal variability of ENSO were also drawn, as well as links to variations in the annual cycle of the southern hemisphere. The origin and maintenance of these phenomena and their associations must be the subject of considerable future investigations.
What are the mechanisms of interaction between the atmosphere and land surface processes on dec-cen timescales?
Through what mechanisms does the planetary boundary layer mediate between dec-cen variability of the surface boundary layer and the free atmosphere? One particular issue is how to average over short time- and space scales to study dec-cen processes, in particular boundary layer and interface processes.
What are the mechanisms of region-to-region and basin-to-basin interactions on the dec-cen timescale?
How do dec-cen changes in atmospheric trace gases and aerosols affect radiative balance and atmospheric circulation, and vice versa?
The study of decadal to centennial variability of atmospheric circulation faces many challenges. Much of our current understanding of the issue derives from the intense interest in anthropogenic climate change. This interest motivated efforts to reorganize the available instrumental and proxy data and to increase the volume of the archives through data “archeology” and additions of new data. These efforts should proceed side by side with establishing clear guidelines for future atmospheric observations and careful planning of the observational networks so that adequacy, continuity, and homogeneity of the records are assured (as discussed earlier). The observational efforts should focus on describing both state variables (winds, pressure, temperature, humidity, and rainfall) and forcing and other related variables (solar radiation, clouds, aerosols, and chemical composition).
Models of the climate system are powerful tools for the study of climate. Such models must be developed to allow the simulation of ocean, atmosphere, cryosphere, and changes in continental surface conditions. Representation of the processes controlling the evolution of all of these important components must be
improved in atmospheric circulation models to properly evaluate the dominant interactions driving long-term climate change in the atmosphere.
The Ocean
The ocean influences the climate system through surface exchange, storage, and redistribution of heat, fresh water, and carbon dioxide. Because of its large mass and heat capacity, the relatively slow moving ocean is responsible for approximately half of the global equator-to-pole meridional heat transport. Regarding climate variability, the ocean's influence becomes increasingly important as the timescales of variability increase. At seasonal to interannual timescales, the ocean influences climate primarily through its large heat capacity in the relatively thin surface layer, whereas on longer timescales the heat transport over basin and global scales predominates.
Issues in Ocean Circulation
A key issue is the oceans' role in the longevity and long-term variability of climate patterns. Oceans are intimately tied to these patterns through SST and sea surface salinity fields. These fields typically covary with atmospheric surface layer pressure (SLP) fields, and an analysis of the nature of the covariation in the North Atlantic suggests a migration of SST anomalies along the primary circulation pathways of the North Atlantic. This finding suggests that upper-ocean heat content likely plays a main role in the survival of surface anomalies and their migration from year to year.
Unfortunately, climate patterns and their relationships with upper-ocean property fields are often difficult to evaluate because the empirical orthogonal function methodology most frequently applied for analysis tends to emphasize stationary patterns, precluding the identification of spatial migrations and covariability. This situation highlights the methodological inadequacies and thus the need to apply more complex tools (e.g., complex singular value decomposition) to allow the extraction of spatially propagating covarying field anomalies.
With the appropriate analysis tools, the first tasks are to identify clearly the oceanic and air-sea coupled signatures of these patterns, including their spatial and temporal linkages, and to explore data-poor regions to identify new patterns and better define existing ones. The relationships among ocean property fields and climate patterns must be more thoroughly documented and understood as well. Specifically, we must improve our understanding of how the ocean regulates, maintains, and otherwise influences these patterns and their evolution, particularly through its couplings with the atmosphere, sea ice, and land as well as through internal ocean mechanisms.
In addition to these general issues, a number of ocean-specific issues must be addressed. They concern the internal ocean processes that influence SST, the principal property coupling the ocean to the atmosphere and climate. Such mechanisms also control the storage and redistribution of heat, fresh water, and atmo-
spherically active gases, which contribute to the atmospheric heat budget. Specifically, we require improved understanding and parameterizations of diapycnal mixing (mixing across density surfaces), surface layer processes, interbasin exchanges (including marginal seas, throughflows, and overflows), subduction and ventilation processes, and mesoscale processes to answer fundamental questions. How does subducted water (and anomalies in subducted water) mix and evolve as it flows around the subtropical gyre, and define the vertical density and circulation structure of this gyre? Can we quantify transport pathways and mixing from the time the water is subducted into the subtropical gyre to when it is re-exposed to the atmosphere at the equator?
Key Scientific Questions About Ocean Circulation
Dec-cen ocean issues involve defining patterns and mechanisms of the participation of the ocean in climate change. It is the formation and circulation of water masses that link surface forcings to the subsurface ocean. The variabilities of those water masses and forcings can alter subsurface ocean properties and circulation, and those subsurface changes can cause SST changes, locally or remotely, through advection, which feed back to alter the atmosphere.
-
What are the dec-cen patterns of ocean variability and what dynamical mechanisms govern them at dec-cen timescales? The rich literature on organized patterns of atmospheric variability is not paralleled in ocean research. Correlations of SST and associated forcing fields with these atmospheric patterns have been partly explored, but recent efforts have revealed a propagation of SST anomalies indicative of transseasonal memory of winter conditions, in particular heat content anomalies and movement of the stored anomalies by advection. Much remains to be done in documenting these anomalies and their relationships to the subsurface property and circulation changes. Regional differences need to be explored. For example, the atmospheric fields associated with NAO and PNA (NPO) seem rather similar, but the participation of the oceans beneath their action centers may differ because of the presence of deep overturning in the North Atlantic.
-
What are the processes of formation and sequestering of water masses and of their subsequent modification and eventual return to the surface? What are their dec-cen variabilities? How do anomalies of heat, fresh water, and chemical constituents translate into mixed-layer anomalies? How do the mixed-layer anomalies get into the ocean interior? How are they modified as they circulate through the interior and how are water masses re-entrained back to the mixed layer? How do freshwater fluxes (evaporation minus precipitation, sea ice, and runoff anomalies) modulate these processes through creation of salinity anomalies? Progress in
-
understanding the behavior of anomalies is inseparable from progress in understanding the processes maintaining the mean transports of heat, fresh water, and chemical constituents.
-
What are the dec-cen fluctuations of circulation structure and intensity and water mass pathways? How are they affected by surface forcing? What are the mechanisms of the fluctuations? What are the relative roles, including the interaction of wind, thermal, and haline forcing? What are the surface expressions of these fluctuations? A number of processes are thought to modulate the intensity of meridional heat transport, as effected by gyre circulations and western boundary currents. These processes include eddy-driven (subbasin- and basin-scale) recirculations and the remote influence of wind stress and buoyancy anomalies via Rossby and coastal waves. What are the relative roles of these processes in dec-cen variability of heat transport and SST? What is the role of salinity advection feedback to surface freshwater anomalies when heat transport and SST anomalies exist?
-
What feedback and coupling mechanisms maintain SST, heat, fresh water, sea ice, and chemical anomalies on dec-cen timescales? How do anomalies survive the seasonal cycle to reappear in subsequent winters, in particular, providing the observed long-lived recurrent winter SST anomalies? How do the histories of water masses evolve at higher latitudes, where sequestering is only seasonal, so that in winter there are recurrent advected heat content anomalies manifested as SST anomalies propagating downstream through the warm-to-cold water transformation pathways? What mechanisms control the strength, heave, and wobble of gyres on dec-cen timescales?
-
What are the mechanisms of region-to-region and basin-to-basin interaction on dec-cen timescales? What mechanisms control the magnitude and other characteristics of waters exchanged among the oceanic circulation gyres and thus the amplitude and property fluxes of the full ocean overturning circulation? Can the variability of this overturning circulation be measured? Decadal change evidence focuses mainly on isolated gyre or paired gyre-gyre phenomena—do more global patterns and interactions occur on interdecadal to century timescales? Are there unique patterns for Southern Ocean participation in dec-cen climate variability, reflecting the circumpolar flow and linkages provided by the Antarctic Circumpolar Current, including the effects on adjacent basins' subtropical gyres? What are the processes connecting tropical SST and the extratropical Pacific? What oceanic processes modify ENSO events on deccen timescales?
-
How is carbon partitioned in the ocean and what are the roles of physical processes in the carbon flux? What are the major processes controlling the partitioning of carbon among ocean reservoirs and between the ocean
-
and the atmosphere on dec-cen timescales? Can these fluxes be quantified? How can the capability be developed to predict the responses of oceanic biogeochemical processes to anthropogenic perturbations, as these responses relate to dec-cen climate change?
Several observational elements of a dec-cen ocean program must be directed toward elucidating the physics of key phenomena and processes to guide in their representation or parameterization in model simulations, should they not be fully resolvable in those simulations, and to provide a framework to interpret the decadal signals seen in the data and the models. Phenomena and processes need comparable concentrated efforts. For example, an examination is warranted of the special circumstance of mode water formation in the subtropics because it contributes so much of the thermocline volume and because it occurs adjacent to strong current systems, which account for most of the heat released from the ocean to the atmosphere.
Time series stations must be continued and supplemented. Discontinued stations should be reinitiated and new ones established. Sparser happenstance measurements provide limited gap filling for the interrupted stations and a background history for new sites. Improving on ship-based observations, new time series can use moored profiling conductivity-temperature-depth capabilities now coming online and subsurface floats, thereby reducing the need for ship-based support measurements. Continued satellite data are needed for global coverage of sea surface height, SST, winds, and ocean color, calibrated against in situ ocean observations.
The Cryosphere
The part of the Earth's surface that remains perennially frozen, as well as the part that is near or below the freezing point, constitutes the cryosphere ( “cryo” means cold or freezing), though our working definition of the cryosphere is all forms of frozen water on the land or sea surface, whether admixed (as in permafrost) or pure (as in snow or ice). Thus, this section addresses not only glaciers and sea ice (perennial and seasonal) but also vast areas of frozen ground and permafrost, as well as seasonal snow fields that lie beyond the limits of glaciers.
Cryosphere Issues
The cryosphere directly influences climate through enhancing the equator-to-pole thermal gradient by increasing the albedo (radiation reflected from the surface) of the polar regions through vast areas of highly reflective ice and snow fields. The cryosphere also plays a predominant role in sea level; the most vulnerable ice sheet susceptible to potentially rapid destruction is the West Antarctic ice sheet, which contains enough water to raise sea level by 18 m should it melt.
Several of the major climate patterns, for example, the PNA and NAO, extend well into the polar regions (focal points of the cryosphere). Variations in the indices describing these patterns seem to be dominated by changes in the low-pressure systems in the northern extremes of these patterns. That is, changes in the Aleutian Low influence the PNA index, and the Icelandic Low influences the NAO index. These influences reflect the fact that the subpolar low appears to be less stable than the rather statically positioned, midlatitude, high-pressure ridges that control the indices' other limits. These low-pressure cells show considerable migration and variability in strength. Since they extend well into the polar regions and show some dependence on the regional SST at their lower surfaces, we might expect that they will show some dependence on the ice and snow distributions as well. The overlap also allows the patterns to bridge the high-and midlatitude zones, representing an obvious avenue through which coherent climate variations and change may be propagated between these latitudes. Also, some preliminary model studies57 suggest that the modeled anthropogenic greenhouse distribution of surface warming differs from a natural pattern associated with the COWL distribution only with the former extending farther into the polar regions.
Previously, analyses of climate patterns neglected the polar regions, owing to the paucity of data on those locations. However, recently released historical Russian and U.S. data now allow inclusion of these regions into such analyses. It is thus now possible as well as important to identify the polar contribution and signature to these patterns. It is also essential to identify the sensitivity and dependencies of the patterns on crysophere characteristics, particularly the fairly mutable and mobile sea ice cover and snow fields. Finally, because the Antarctic Circumpolar Wave and its covariation with ENSO seem to suggest a hemispherewide teleconnection between the tropics and extratropics in the poorly examined southern hemisphere, we need to refine our understanding of the extent, nature, linkages, and controls of the Antarctic Circumpolar Wave with regard to extrapolar climate patterns (and climate in general).
In addition to issues related to climate patterns, a number of issues must be addressed to understand the nature and importance of cryosphere components in the global climate. Specifically, we need to determine parameterizations and sensitivities of the processes controlling several phenomena: (1) ice-albedo feedbacks, including spatial averaging of heterogeneous conditions; (2) snow-climate feedbacks, which are much like ice-albedo issues but for vast continental areas; (3) ice-cloud feedbacks; (4) ice-ocean feedbacks, including thermohaline circulation instabilities and interactions, surface stability influences, and ocean-ice interactions; and (5) ice sheet-ocean feedback and ice sheet instabilities.
One finding of tremendous interest in the past decade was the discovery that the ocean can generate an internal mode of variability over a variety of timescales. The nature, vigor, and characteristics of this variability are sensitive to the boundary conditions in thermohaline source regions, which are in the high latitudes and
subject to cryospheric influences. We must better understand the role of heat and salt fluxes in this deep and intermediate circulation and, similarly, the contribution of local and regional ocean-ice-atmosphere feedbacks.
Finally, given the direct influence of ice mass balance on global sea level, better knowledge is needed of ice sheet mass balance and subice shelf melting, which today are poorly understood. Specifically, the current rate of melting and sensitivity of melt rate to regional ocean-ice interaction and change need to be determined.
Key Scientific Questions About the Cryosphere
-
How do sea ice and snow fields change on dec-cen timescales and what is the relationship of these changes to atmospheric, ocean, and land surface patterns on dec-cen timescales? For example, as noted earlier, the NAO and PNA extend into the Arctic, and their indices often reflect changes originating in polar low-pressure cells. Thus, it is important to determine the covariation between changes in the ice and snow fields and these patterns. Likewise, because sea ice has been implicated in thermohaline circulation changes on a variety of timescales, how do these two phenomena covary?
-
What mechanisms underlie dec-cen patterns of interaction between sea ice and snow fields and the atmosphere, ocean, and land systems? Do regional or even local changes in surface divergence alter albedo, surface heat fluxes, and cloud formation enough to influence the Icelandic or Aleutian lows and thus the NAO and PNA? Do changes in the patterns of large-scale planetary waves or ocean circulation alter polar conditions so much that they in turn drive additional changes in other parts of the climate system? For example, could variations in NAO influence the volume of fresh water exported from the Arctic in the form of sea ice to influence thermohaline circulation significantly? Observational evidence supports this idea,58 as do model experiments.59 Also, thermohaline circulation is sensitive to surface buoyancy fluxes in the source regions. Such regions lie predominantly in the polar regions, where growth, decay, and spatial redistribution of ice play a dominant role in these fluxes and thus may play a dominant role in the formation processes. The ice in turn depends greatly on the stability of the underlying water column, a situation setting the stage for considerable feedbacks and interactions.
-
Through what mechanisms are changes in the cryosphere of the polar regions linked or teleconnected to midlatitude and tropical regions? For example, model results suggest that changes in sea ice fields alter the nature of the Hadley Cell through their influence on the equator-to-pole meridional temperature gradient. Observations suggest that the Antarctic Circumpolar Wave covaries with ENSO and Indian Ocean monsoons
-
through mechanisms not yet understood. Changes in thermohaline circulation, which may be related to the surface freshwater balance associated with growth and transport of sea ice, can alter surface volume (i.e., gyre characteristics) in subtropical regions.
-
What are the history and current global budget of land-locked ice and snow and what are the primary mechanisms controlling this budget? Given the direct impact of this budget on sea level, we must better quantify the mass balance of continental ice sheets, alpine glaciers, and permanent snow fields. In particular, the balance at the base of floating ice shelves is in considerable question, and whether the Greenland and Antarctic ice sheets are gaining or losing mass is still uncertain. These questions must be resolved. Knowledge of how the land-locked ice and snow budget has varied over time will give some indication of the range, rate, and rapidity of change experienced through natural variability. Several other phenomena are critical in this budget and also must be better understood: the melt and growth rates and dependencies at the base of floating ice sheets, as well as ice sheet drainage rate, as a function of sea level (which alters the grounding line and thus frictional retarding force to the flow), and the response of precipitation to cold-region climatic changes.
Observations critical for addressing these issues include long-term monitoring of surface salinity with SST, since salinity is the principal control on the density of seawater in high-latitude regions. Also, the sea ice fields themselves, including the motion fields and ice thickness, are required to determine the freshwater transports and buoyancy fluxes associated with the ice fields. Finally, consistent monitoring of iceberg calving and an observational system to determine basal melt or growth (e.g., temperature/salinity moorings across the floating ice shelves) must be established to measure the land-locked ice and snow budget.
Model parameterizations must be improved to better represent certain phenomena: (1) ice-albedo feedbacks, (2) snow-climate feedbacks, (3) ice-cloud feedbacks, (4) ice-ocean feedbacks, and (5) ice sheet-ocean feedbacks and ice sheet instabilities. Improved simulation of sea ice and snow distributions and related impacts also must be achieved.
Land and Vegetation
Land and vegetation influence climate through many means. Most notably, their geographic distributions relative to the oceans help define the nature of the climate pattern because their low heat capacity relative to the high heat capacity of the oceans leads to alternating cycles of surface response to the same atmospheric forcing. However, on decade to century timescales, the main influences of land and vegetation are through their influences on the carbon (and methane)
cycle and through changes in surface albedo owing to land surface and land cover changes, natural or anthropogenic.
Land- and Vegetation-Related Climate Issues
Simple model representations of vegetation in general circulation models (GCMs) suggest that the main influence of vegetation in greenhouse warming, and the main issue in comparing preindustrial (natural) vegetation to industrial (cultivated) vegetation, takes the form of albedo. Most notably, dark forests show minimal winter albedo influence in response to snow cover, whereas the loss of such forests exposes large areas that suddenly influence albedo considerably during summer and even more so during winter in the presence of snow cover. Hansen et al. (1997) show this land-use shift to be the major contribution of vegetation changes to a doubled CO2-induced warming, with vegetation changes accounting for approximately 25 percent of the CO2 direct influence. Changes in land surface characteristics can lead to similar effects, realized mostly, again, through albedo. Vegetation and land surfaces also affect climate through the hydrological cycle, as discussed in the section on the hydrological cycle below.
At the present time, our knowledge is poor regarding the effects of different vegetation covers in climate change. We must improve even our most basic understanding of these effects.
Key Scientific Questions About Land and Vegetation
-
What are the effects of human activity and climate change on ecosystem structure and function? From paleoclimatic records we know that natural vegetation responds to climatic change: individual species respond according to their climatic tolerances, and ecosystem compositions change as a result. Competitive and trophic interactions among species are thereby altered, redefining where organisms can survive and reproduce. The responses of organisms to climatic change will be greatly influenced by human land-use patterns and other anthropogenic influences. Associated with ecosystem structural changes are changes in the biogeochemical cycling of carbon and nutrients, in ways that remain difficult to anticipate. Finally, the distribution of disease-carrying organisms will change as part of ecosystem restructuring and redistribution.
-
What are the relative contributions of the different processes by which vegetation and soils store or lose carbon? Vegetation and soils store three times as much carbon as the atmosphere or upper ocean, yet large uncertainties remain about the quantitative contributions of various processes. Forest regrowth in the northern hemisphere, related to changing
-
land-use patterns, or perhaps CO2 and nitrogen fertilization or climate change, may have been an important sink over the past decades.
-
What are the expected future emissions of CH4, N2O, and volatile organic carbon compounds by soils and vegetation? CH4 production in soils depends strongly on moisture conditions. N2O production is a product of denitrification processes in soils. The emissions of volatile organic carbon compounds (ozone precursors) are strongly species dependent. Changes in these emissions depend on a combination of ecosystem and climate changes.
-
How do dec-cen changes in land use and land cover affect land surface energy balance on dec-cen timescales? The nature of land cover determines its reflectivity and is expected to change with changing climate and human activities. For example, a warmer high-latitude climate will favor the expansion of boreal forest into tundra-dominated regions, with a concomitant lowering of albedo. Desertification, which may have both human and natural components, leads to an increase in surface albedo. The thermal structure, moisture content, and dynamics of the atmosphere will be influenced by the partitioning between sensible and latent heat transferred from the surface.
-
How does vegetation influence the transfer of freshwater through the land surface on dec-cen timescales? Stomatal opening governs the rate of evapotranspiration from the land surface. Increasing CO2 will tend to reduce stomatal conductance, increasing plant water-use efficiency. More evapotranspiration results in a higher water vapor content of the atmosphere over land, more precipitation, and less runoff via rivers and groundwater flow.
-
How do changes in vegetation cover influence the loading and composition of atmospheric aerosols on dec-cen timescales? Vegetation naturally emits aerosol precursors. The nature and amount of these emitted compounds depend on the species. Thus, the distribution of aerosol precursors will change as ecosystems and species respond to climate change and human perturbations. Biomass burning generates aerosols (particularly soot) that influence the regional radiation balance. Desertification produces mineral dust that is transported in the troposphere and exerts a regional radiative forcing. The distribution of these aerosols can be expected to vary on dec-cen timescales in ways related to climatic and human influences.
Hydrological Cycle
The hydrological cycle refers to the origin and fate of water through its many phases in the atmosphere, ocean, land, and biosphere. Water evaporates as vapor from the surfaces of both land and ocean, condenses in the atmosphere, precipitates
back onto both land and ocean surfaces, and ultimately finds its way back into the atmosphere as vapor. It can sometimes reside in surface or subsurface reservoirs for hundreds or thousands of years, or longer, before completing the cycle.
Issues Regarding the Hydrological Cycle
The hydrological cycle is central to questions about the magnitude of global warming associated with an increase in atmospheric CO2. Water vapor in the atmosphere is the primary greenhouse gas, and the amount of water vapor is a function of the hydrological cycle, which controls the rate through which water is evaporated into the atmosphere and precipitated out and thus the quantity of water vapor residing in the atmosphere at any particular time.
It is generally agreed that the addition of radiatively active gases into the atmosphere will warm the surface (the degree of warming is in dispute) and significantly speed up the hydrological cycle so that total global evaporation and precipitation should both increase. This prediction of change is one of the most consistent results of models simulating anthropogenic greenhouse warming (see detailed discussion in Chapter 10). Rind et al. (1996) suggest that a doubling of atmospheric CO2 should increase precipitation and evaporation rates by approximately 10 to 15 percent, resulting in an increase of total atmospheric water vapor of 30 percent. Unfortunately, the global data do not exist to confirm or refute such predictions, and the models are likely inadequate for properly establishing the net change and distribution of water vapor in the atmosphere. Therefore, one of the primary issues for improved understanding of the hydrological cycle is achieving better knowledge and model representation of those processes controlling the rate, paths, storage, and redistribution of water vapor through the hydrological cycle.
In addition to improving model representations of this cycle, we must better document the change and distribution of water vapor in the atmosphere, particularly its vertical distribution. Considerable controversy surrounds the issue of vertical distribution, with one theory suggesting that increased moisture in lower levels of the atmosphere would be offset by decreased moisture levels in the upper troposphere, reducing surface warming (and thus its moisture increase) and cooling upper layers, thereby greatly reducing the net warming otherwise anticipated from an increase in total atmospheric water vapor.
Regarding patterns of climate variability, the hydrological cycle plays an unknown role. However, some observed covariations suggest that changes in the NAO and PNA directly influence precipitation in Europe and northwestern North America. Similarly, clear covariations are found between patterns of the tropical Atlantic SST dipole and those of rainfall and drought in the African Sahel and northern Brazil. Thus, it may be possible to make regional precipitation predictions based on knowledge of these pattern covariations. Clearly, improved documentation and understanding of these relationships are needed, including knowledge of the mechanisms that drive them and their sensitivities.
Obviously, the hydrological cycle controls much of the climate attributes of precipitation and water availability. It also directly influences sea level. If more precipitation falls as snow than is returned by evaporation (or by sublimation, the direct vaporization of snow cover), snow will accumulate, eventually forming glaciers that can account for considerable changes in sea level. In fact, aside from sea level changes associated with plate tectonic spreading rates, the largest changes in sea level result from the waxing and waning of continental ice sheets. During the last ice age, enough fresh water was removed from the oceans and stored in continental ice to lower sea level by more than 100 m. Even today, in our relatively moderate climate conditions, the return of fresh water to the oceans from melting alpine glaciers and possibly ice sheets is thought to be responsible for about one-half of the 20 cm sea level rise observed in this century. Also, runoff (and ice drift, discussed previously) can considerably influence high-latitude polar regions, where ocean surface salinity plays a predominant role in sea ice formation (and thus local albedo and freshwater transport, among other things) and deep/bottom water formation, which are natural avenues through which the hydrological cycle may influence climate over decade to century scales.
The role of vegetation in the hydrological cycle also must be vigorously investigated. Rind et al. (1997) show that, while the role of vegetation is fairly moderate in contributing to a doubled CO2 warming, the impact of the warming on the vegetation itself can be dramatic. In their GCM simulation with interactive vegetation, the impact of increased atmospheric moisture content is particularly enhanced over land, driving considerable evaporation from the vegetation (through transpiration). The vegetation attempts to limit this drying by reducing transpiration through stomata closing. While this may succeed as a short-term survival tactic against dry conditions, in the long run it also reduces productivity and eventually destroys the vegetation (particularly at lower latitudes). This result is not revealed in simple GCM studies that do not include a treatment of vegetation strain response (such as the treatment that was used in the first IPCC assessment60), though it is indicated in impact studies (such as those used in the second IPCC assessment61). Thus, we must address not only the role of vegetation in the hydrological cycle but the responses and feedbacks of the vegetation as well.
Finally, rainfall is poorly and sporadically measured, and evaporation measurements are woefully inadequate. Development of global climatologies indicates that there is disagreement between them, so it is not surprising that regional series of rainfall data of reasonable quality exist for only a few places. It is becoming more and more important that proxy reconstructions of past rainfall data be made to set the climatic context for studying decadal variability of precipitation and evaporation. Such time series are particularly important for hydrological control issues, such as water resource management; present-day levees, dams, and reservoirs are often engineered on the basis of inadequately short records of flood levels, with dramatic examples of inadequate flood protection (e.g., the Folsom Dam and Sacramento River flood controls) owing to undersized levees.
A classic but still unsolved hydrology problem is how to follow the water from rainfall back to the atmosphere and ocean. Improved understanding of this most basic process, especially, in this context, on decadal and longer timescales, is of fundamental importance; we need to know how variations in this process influence decadal- to century-scale climate variations. Where and at what rate water re-enters the ocean and how precipitation and evaporation redistribute fresh water in the ocean's surface layer play essential roles in ocean salinity, which is the driving factor in high-latitude stability, influencing thermohaline circulation and shallow and deep subduction and ventilation. In short, the role of the hydrological cycle in ocean circulation is critical to understand but is currently poorly known.
Key Scientific Questions About the Hydrological Cycle
The roles of the hydrological cycle in the climate system must be better understood, including through model representation of the processes controlling the rates, paths, storage, and redistribution of water through the cycle. The Global Energy and Water Cycle Experiment (GEWEX) program studies the detailed land surface hydrology in major drainage basins; the Mississippi basin will be a primary U.S. focus, and concurrent land experiments are proposed for other parts of the world as part of the international GEWEX program.
-
What are the patterns and mechanisms of prolonged drought on dec-cen timescales? Paleoclimatic records provide ample evidence of droughts that persisted for many decades to centuries in regions that experience more moderate conditions today. Dune fields that are now vegetated existed during the past 1,000 to 10,000 years; tree stumps are found in modern natural lakes; and long periods of slow growth are revealed in drought-sensitive tree ring records. All indicate that persistent droughts recurred throughout the past few millennia. The mechanisms that lead to the initiation and persistence of multidecade to multicentury droughts are not well known but likely involve feedbacks from vegetation and/or long-term persistent SST anomalies that influence the supply and delivery of moisture. Even the interannual to decadal droughts documented by instrumental records (such as the North American Dust Bowl of the 1930s and the killing droughts of the Sahel in more recent decades) lack a clear mechanistic explanation. A more thorough examination of the patterns of past hydrological variability and the testing of plausible mechanisms through focused observational and simulation strategies should lead to a better predictive understanding of this critical climate attribute.
-
How do the distributions of water vapor, precipitation, and clouds interact with surface boundary conditions and changes on dec-cen timescales? The hydrological cycle plays an unknown role in producing and respond-
-
ing to patterns of climate variability. However, relationships between the state of these patterns and large-scale precipitation seem to covary, and thus it may be possible to predict regional climatic precipitation based on knowledge of the pattern state. This potential underscores the need for improved documentation and understanding of these covarying relationships, refining the nature of the correlation and establishing the mechanisms responsible for driving them and their sensitivities. In addition to improving the model representations of this cycle, we must also better document the change and distribution of water vapor in the atmosphere, particularly its vertical distribution. There is considerable controversy about this subject, with one theory suggesting that increased moisture in lower levels of the atmosphere will be offset by decreased levels in the upper troposphere, reducing surface warming (and thus its moisture increase) and cooling upper layers, thereby greatly reducing the net warming otherwise anticipated from an increase in total atmospheric water vapor.62
-
By what combination of remote and in situ observations can we measure the large-scale distribution of precipitation on dec-cen timescales? Rainfall is clearly crucial for all societal and economic activities. It is also a major way in which dec-cen variability is expressed. Observed covariations suggest that changes in the NAO and PNA directly influence precipitation in Europe and northwestern North America, and there are clear covariations between the tropical Atlantic SST dipole and rainfall/drought in the Sahel of Africa and in northern Brazil, as discussed above. But rainfall is still poorly and sporadically measured. The existing global climatologies significantly disagree among themselves; not even a baseline of large-scale precipitation is in hand. The advent of satellite measurements has provided a global view of precipitation through radiative proxies, but the absolute calibration in translating proxy fields to precipitation is in doubt. In situ measurements help to quantify the proxy radiation satellite measurements and are crucial not only for determining the absolute calibration of the satellite measurements but also for providing gradient information. The in situ and satellite measurements must be carefully combined to provide the optimal estimate of large-scale fields of precipitation. For measurements on dec-cen timescales, the combined remote/in situ measurement system must be designed to assure long-term consistency and accuracy, and this implies a commitment to long-term intercalibrated measurements.
-
What are the spatial and temporal changes in land storage of water and the pathways and fluxes of land water to the oceans? The buoyancy state of the ocean (temperature and salinity) determines the water transformation properties of the ocean, the degree to which it produces deep and bottom water, and, ultimately, therefore, the transport properties of the
-
ocean and the stability of its internal oscillations. Crucial to these processes is the amount of freshwater input (directly through precipitation over the ocean and indirectly through runoff, groundwater discharge, and discharge of glaciers and other forms of land snow and ice, whether through rivers or ground discharge) and the geographic distribution of such inputs. The geographic distribution depends on the locations of rivers, the relative flows of the rivers, and the amount and distribution of groundwater discharges. These inputs are in turn determined by the relative precipitation and evaporation in the basins from which rivers and groundwater discharge into the ocean. Once fresh water reaches the ocean, it contributes to the salinity of the ocean, its sea level, its level of ions (especially from calcium carbonate) and salts, and its regional temperature. After undergoing mixing and advection, the ocean responds to salinity by altering buoyancy state and water mass transformation properties.
Climate models suffer from a lack of precipitation data through which they could be evaluated. In fact, precipitation on both large and small scales and the fate of hydrometers ejected from clouds in the upper atmosphere (which evaporate to become vapor rather than fall as precipitation) are crucial processes for the hydrological cycle in climate. Additional understanding is needed of evapotranspiration, the dynamics of vegetative cover, the dynamics of soil moisture, and all their parameterizations. The correct simulation of land pathways and water storage is a difficult modeling problem, inasmuch as water pathways depend on conditions far more local than climate models can resolve now or in the foreseeable future. The detailed paths of water on land and the modeling of these pathways on the catchment level are major themes of the GEWEX program.
It is currently believed that discharges from the melting ice pack that covered the United States during the last glacial maximum had significant influence on the thermohaline circulation of the North Atlantic and therefore the temperature of the North Atlantic. Additionally, modulations by iceberg discharges (Heinrich events) were likely significant punctuations in the region's climate record throughout the glacial period.63
-
What are the patterns and mechanisms of the dec-cen droughts? A gridded 300-year reconstruction of the Palmer Drought Severity Index has been developed from 388 precisely dated tree ring records 64 to indicate periods of regional drought. Although the 1930s proved to be the most extensive drought in this record, more localized records 65 indicate that droughts more intense and prolonged than the Dust Bowl have been present in the record. As recently as 1,000 years ago in the Great Plains, ancient dune fields, now covered by vegetation, were active, suggesting that the ability of this region to maintain vegetative cover without human intervention is marginal.66 California also provides evidence of simultaneous droughts (to within unavoidable error of roughly 100 years) with
-
those in the Great Plains.67 Similar evidence supports extreme droughts in Patagonia during these common periods, suggesting a common global cause for these droughts. Even longer tree ring records from California, spanning several millennia, indicate periods of below-average rainfall lasting on the order of a millennium.68 This record also indicates a recurrence of short-term droughts of greater magnitude and duration than any in the instrumental record of the past 100 years. Were such droughts to recur during present times in California, drastic consequences would ensue for a vulnerable and populous region.
Atmospheric Composition and Radiation Budget
While the atmospheric boundary conditions are essential for extending the timescales of climate variability, changes in the atmospheric composition and radiation budget, owing to internal, coupled, or external mechanisms, are directly responsible for determining the atmospheric temperature distributions in space and time. Consequently, variations in composition and radiation budget, and the direct and indirect consequences of these variations, are of critical importance.
Atmospheric Composition and Radiation Budget in the Climate System: The Issues
Atmospheric composition can change because of many factors. Among those of considerable current interest are changes due to the influence of humans, most particularly through the anthropogenic increase in atmospheric carbon dioxide, chlorofluorocarbons (CFCs) and other halocarbons, methane, nitrogen oxides (NOx), and aerosols. Some of these gases, such as CFCs and halocarbons, have no known natural sources, so their influences are the direct result of anthropogenic change.
Each of these compounds plays a direct role in the atmospheric equilibrium temperature, and this direct influence is fairly predictable given any particular emissions scenario. However, more importantly, the indirect influence of increased gas and aerosol concentrations is not well known. For example, as discussed above, an increased direct warming is thought to drive an increase in the rate of the hydrological cycle, an influence that will have an uncertain net effect on the amount and vertical distribution of water vapor in the atmosphere. Since water vapor is the primary greenhouse gas, being able to predict its response to a direct forcing is essential.
In addition to the direct anthropogenic forcing, there are possible feedbacks in the atmosphere involving the natural system that must be understood. Most notable is the response of continental biomass, which can alter the rate and storage times of carbon in vegetation and thus either exacerbate CO2, methane, and NOx emissions or provide them an enhanced sink. Similarly, direct changes
may alter ocean circulation and productivity, which can drive additional feedbacks. The direct influence of changes in the ocean productivity on atmospheric composition have been shown to be fairly small, but changes in the ocean circulation can introduce changes in the ventilation of deep waters, which contain approximately 50 times more carbon dioxide than the atmosphere, thus driving potentially larger feedbacks, though presumably on longer timescales. This kind of response implies a more gradual feedback process but a more persistent one as a result. That is, this influence may not be reversed quickly, regardless of short-term changes in direct forcing (e.g., in the emissions).
Changes in atmospheric composition, particularly additions of CFCs, also influence climate through their destruction of atmospheric ozone. This effect is particularly important to the vertical temperature distribution in the lower stratosphere. Such destruction also alters surface ultraviolet (UV)B radiation, posing a climatic change that has direct societal consequences as well (e.g., increased risk of skin cancer and alteration of at least some components of ecosystems such as the ocean plankton). While the chemistry of ozone response to a given concentration of chlorine and bromine is fairly well known, within our envelope of experience, one of the largest issues facing ozone destruction and recovery is the potential deviation of effects from the pattern of our current understanding—if there are continued large increases in CH4 and H2O, which may move us into chemical and physical regimes that are not clearly understood.
Changes in tropospheric ozone directly influence the Earth's radiation budget and thus directly influence climate change and variability. Of primary importance in this area is improved understanding of tropospheric chemistry models and improved representation of cloud-chemistry interactions.
Although carbon dioxide cycles quickly between the mobile reservoirs, it leaves that system only very slowly, through the burial of organic matter. Dissolution of calcite, also a very slow process, adds carbon to the mobile reservoirs but increases the carbon-holding capacity of the oceans even more. Therefore, future levels of atmospheric CO2 depend on how the additional carbon from fossil fuel burning is partitioned between the mobile reservoirs, which can be considered an almost-closed system. Important factors are, foremost, the rate of fossil fuel consumption, followed by circulation of the ocean and its interaction with marine biological productivity (including the potential sensitivity of the biological pump at high latitudes), management of the land (including deforestation and the relationship between the ecosystem and CO2 changes), and carbon storage by ecosystems, possibly stimulated by increased CO2 and deposition of nitrogen. Climate change will affect all of the natural processes cited above, and considerable effort must be extended to understand these processes further, including their feedbacks and sensitivities.
Current representation of the processes responsible for partitioning of carbon among the mobile reservoirs is very crude and sometimes speculative and involves many assumptions. These models are best viewed as extrapolation tools,
and their predictive powers beyond the next few decades are tenuous. Progress in understanding the driving forces of carbon storage on large spatial scales is especially hampered by the current inability to verify through observations how local processes and fluxes are extrapolated to large scales. For greenhouse gas management the highest priority is to learn more about the behavior and model representation of the terrestrial biosphere, since we exert considerable influence on it. A long sustained effort of observation and modeling, developed together, will be necessary. Uptake by the oceans needs to be closely monitored via repeated transects every 5 to 10 years and via measurements of the difference in CO2 partial pressure (pCO2) and isotopic ratio between the atmosphere and ocean surface. With respect to the latter, actual measurements of air-sea exchange flux over the open ocean would lead to a much better parameterization of the exchange process.
The injection of volcanic aerosols affects climate through stratospheric cooling (but warming in the tropics), lowering planetary albedo, and increasing surface area for chemical reactions that influence ozone levels, among other things. In fact, with respect to the latter, it has been demonstrated that changes in ozone cannot be properly interpreted without consideration of stratospheric aerosol loading. Volcanic eruptions and their stratospheric effects are necessarily unpredictable in occurrence and magnitude. Since the role of volcanic aerosols in climate and particularly their role in ozone trends is complex and perhaps longer lived than is generally understood, it is crucial to maintain the capability to depict global stratospheric aerosol loading (especially in terms of surface area density) with high vertical resolution.
Finally, the Sun is the driving force of climate, and even small variations in the amount of energy that the Earth receives can have significant impact. In general, a doubling of CO2 generates a radiative forcing that is equivalent to a 2 percent increase in solar irradiance. The changes in climate over the past several centuries are much smaller than expected from such a change, and we expect decade-to-century changes of about 0.25 percent. The relationship between solar wind and solar irradiance has been calibrated for the last solar cycles. Extrapolation for conditions outside this range, if applicable, would imply a decadal to century variability in solar irradiance with periods of lower irradiance by as much as 0.25 percent. The major difficulty in hindcasting solar irradiance over the past several centuries is the use of solar activity indices as surrogates for solar irradiance. Better understanding of solar physics and modeling of the solar cycle and the Earth's climate would allow greater confidence in this connection.
Key Scientific Questions About Atmospheric Composition and Radiation Budget
Greenhouse Gases
-
What are the changes in the spatial distribution of carbon storage and flux on dec-cen timescales? Of the carbon emitted by anthropogenic
-
activities in the past two centuries, just over half of that has remained in the atmosphere. Nearly half has been taken up by other mobile reservoirs, the biosphere, and surface ocean. Although initial estimates of carbon sinks failed to account for a large fraction of anthropogenic CO2 emissions, recent calculations suggest that a growing terrestrial biosphere may account for this “missing sink.” Uncertainties in estimates of biospheric activity, together with poor knowledge of the surface ocean's uptake, suggest that our knowledge of the fate of anthropogenic CO2 may not be inaccurate. Atmospheric CO2 has been increasing steadily since the mid-1800s, but interannual to decadal fluctuations in the rate of rise also are seen in the atmospheric record and may be due to biospheric or oceanic variability. Predictive knowledge of atmospheric greenhouse gas concentrations requires that we narrow the current uncertainties in evaluations of carbon sources, sinks, and fluxes.
-
How do mixed-layer water replacement rates interact with biological processes to produce changes in ocean carbon storage? The biological pump—that is, the sequestration of inorganic carbon by primary productivity in the surface ocean—virtually determines the partitioning of carbon between ocean and atmosphere. Without the biological pump, atmospheric concentrations of CO2 would be three to four times higher than they are today. The biological pump delivers carbon to the deep ocean, which exchanges with the atmosphere on timescales of centuries and longer. The pump's level of activity depends on the extent of nutrient utilization, which can change as a consequence of changing vertical mixing rates. The latter rates can alter both nutrient supply and the time available to surface phytoplankton to utilize these nutrients.
-
What are the uptake, pathways, and fate of anthropogenic carbon in the ocean on dec-cen timescales? In addition to the “biological pump” of carbon described above, the surface ocean takes up CO2 passively through gas exchange. The exchange of CO2 between atmosphere and surface ocean occurs partly as a function of the atmosphere-ocean concentration gradient; thus, as atmospheric CO2 rises from anthropogenic inputs, the ocean gas-exchange sink strengthens. Current best estimates of the oceanic uptake of anthropogenic CO 2 derive from numerical models. The paucity of accurate observations is still a significant obstacle to the quantification of oceanic carbon uptake and its pathways.
-
What are the contributions of various sources and sinks to the recent increase in methane? Although observations document that atmospheric CH4, a potent greenhouse gas, has increased steadily since the nineteenth century, the causes of this rise are not sufficiently quantified. Likely candidates for the new sources include expanding agricultural wetlands and livestock herds, biomass burning, fossil-fuel-related industry, and landfills. The total quantity of methane added to the atmosphere is well
-
known from the atmospheric rise, but the contributions of individual source components are not. Surprisingly, the main sink for methane, oxidation by tropospheric OH, appears to be stable over at least the most recent decade.
-
How does the photochemical breakdown of methane contribute to other chemical and radiative processes in the atmosphere on dec-cen timescales? The photochemical breakdown of CH4 consumes OH and produces water throughout the atmosphere; in the stratosphere even small amounts of water vapor are extremely efficient contributors to the global greenhouse effect. Thus, dec-cen changes in methane can be expected to alter the vertical distribution of water vapor in the higher troposphere and lower stratosphere, thereby changing radiative forcing. The ice particles that form at these altitudes also provide sites for heterogeneous chemistry. The feedbacks among these chemical processes and atmospheric dynamics are largely unknown.
-
Why is N2O increasing on dec-cen timescales? Records of atmospheric N2O show a steady rise since the nineteenth century, but a quantitative budget of N2O sources and sinks that explains this rise cannot be developed from the existing scarce observations and limited understanding of processes. The global nitrogen cycle has been heavily altered by human activities, particularly by the widespread application of high-nitrate fertilizers. Processes related to soils have likely been more disrupted by recent human activity than oceanic denitrification/nitrification rates. Industrial N2O production also is a likely cause of the recent increase.
-
Why has tropospheric ozone increased since the nineteenth century and are further increases likely? What are the controls on the abundance of tropospheric ozone? To what degree are precursors, photochemistry, transport, and dilution important? Although trends over recent decades are unclear, tropospheric ozone has almost certainly increased substantially since the last century. Because ozone is a pollutant with documented health and ecosystem impacts and a greenhouse gas, we need to be able to predict its concentrations. Knowing the interactions among precursors, transport, and photochemistry also is critical.
Stratospheric Ozone
-
How does the coupling between chemistry, dynamics, and radiation in the lower stratosphere and upper troposphere operate on dec-cen timescales? For example, what do we need to know to predict the timing of ozone recovery accurately? How do changes in the stratosphere affect atmospheric circulation and the surface radiation balance? Ozone distribution influences the vertical temperature structure and dynamics of the lower stratosphere because of its absorption of UV radiation. These dynamics in turn determine the distribution of other atmospheric con-
-
stituents, including ozone and their chemical interactions, in the upper troposphere as well as the lower stratosphere. Human impacts on these coupled processes include a potential future fleet of high-flying aircraft and changes in the sources of ozone-destroying halogenated compounds. The feedbacks among human influences, chemical interactions, and atmospheric dynamics are largely unknown. The most serious uncertainties for prediction of ozone recovery during the next century may be potential changes in the chemistry and circulation of the stratosphere that put us well outside current experience. Such changes might include continued large increases in CH4 and stratospheric H2O or stratospheric circulation changes associated with global warming and greenhouse gases. The predictive models are based in part on first-principle physics and chemistry and should correctly account for these changes, but we must recognize that observations of the recent past are used to test and calibrate models.
Aerosols
-
How do the spatial distribution, chemical composition, and physical properties of aerosols vary on dec-cen timescales and how do they interact with climate variability? How do the composition and properties of aerosols determine their radiative effects? What are the regionally varying impacts of aerosols on the Earth's radiation budget? How do aerosols contribute to cloud formation, precipitation, and radiative interaction? While aerosols appear to present a critical radiative forcing on dec-cen timescales, the mechanisms and processes of this forcing remain poorly characterized. For example, the composition and properties of aerosols determine radiative effects in ways not yet well documented. The direct (reflective) effects of aerosols may change or reverse over high-albedo surfaces, where radiation absorption can contribute to overall atmospheric heating rather than cooling. The indirect effects of aerosols, related to cloud formation and radiative interactions, remain a major uncertainty but in fact may represent aerosols' primary impact on climate. Predicting future aerosol impacts requires understanding regional sources and transports and how these may vary with future climate scenarios. Human-initiated changes (e.g., through industrial emissions, biomass burning, and land use) are a key component in anticipating future aerosol variations; natural sources may be even less predictable (e.g., volcanic eruptions and natural vegetation emissions).
Solar Radiation
-
How do proxies for solar activity (e.g., sunspots, cosmogenic nuclides) relate to total solar irradiance on dec-cen timescales? Direct measurements of the Sun's radiative output span only the last solar cycle. Solar radiation appears to correlate well, however, with solar activity as repre-
-
sented by sunspot variations, solar wind intensity, and charged particle fluxes. Reconstructions of solar activity that span nearly the past 400 years can be derived from records of cosmogenic nuclides (e.g., 10Be) and historical sunspot observations. Extrapolated from measured relationships over the past solar cycle, these reconstructions indicate variability in solar radiation of 0.25 percent over decade to century timescales. The sensitivity of climate to these variations has been explored using observational data and climate models, but it is still not fully known how well the solar proxies represent irradiance. For example, while sunspot observations failed to indicate activity during the Maunder minimum (1600 to 1640 AD), 10Be records continued to show relative changes. A better understanding of the Sun's influence on dec-cen climate variability requires improving reconstructed solar irradiance.
-
What feedbacks govern climate and ecosystem responses to spectral changes in solar irradiance on dec-cen timescales? Although the part of the solar spectrum likely to have greatest climatic influence lies in the visible range, the decadal solar variability observed over the last solar cycle occurs primarily in the UV range. Variations in UV have demonstrable effects on stratospheric O3 levels and on atmospheric electricity, the last of which may influence cloud droplet nucleation. UV variability may also influence temperature patterns in the middle atmosphere, which affect the surface by altering how planetary waves propagate energy. UV changes can also impact ecosystems, particularly primary producers in both marine and terrestrial realms. The influence of UV on climate and ecosystems is poorly understood but must be considered in any evaluation of the Sun's influence on the Earth system over dec-cen timescales.
-
To what extent are dec-cen climate changes, as observed in instrumental and paleoclimate records, related to changes in the Sun's output and what mechanisms are involved in the response of climate to changes in solar radiation? Decadal variations occur in most records of climate that have sufficient length and resolution, but the degree to which these fluctuations can be attributed to solar variability has prompted significant debate. Even studies that point to apparently distinct influences of solar variability on climate sometimes indicate highly variable sensitivities for a given irradiance change. Feedbacks within the climate system, particularly in the atmosphere, may enhance or dampen the climatic response to solar forcing. Understanding the sensitivity of Earth's climate to past changes in solar activity will enable better predictions of future changes in the face of decadally varying solar irradiance superimposed on other radiative forcing trends.
LESSONS LEARNED
The Extent of Natural Climate Variability
Findings of the paleoclimate community have shown the large degree of natural climate variability present in the climate record on many timescales. The impressive and often abrupt swings in climate recorded over the past several thousand years, such as the Little Ice Age, may if anything understate the potential for natural climate variability. We have learned that the climate of the Holocene has been relatively tranquil by the standards of Earth history. Our heretofore implicitly accepted tenet that we live in a relatively stable climate system has been completely undercut. We cannot base future climate-related policies (e.g., water resources management) on present-day climate conditions. With or without anthropogenic greenhouse warming, we must recognize the potential for the Earth's climate system to change, over a human lifetime, in ways that may have direct and important consequences on society and people's quality of life.
The Human Capability to Change the Global Environment
The past decade has demonstrated unambiguously that the global environment has been altered by human activities. The increase in atmospheric CO2, the changes in tropospheric and stratospheric ozone, and the secular rise in sulfate aerosols in the troposphere all have been clearly tied to human activities. Detection and attribution of an anthropogenic signature in global climate have proven to be more difficult issues. While the balance of evidence points to an anthropogenic influence on climate,69 the unique and unambiguous detection of this signature remains an area of active research.
The Unique Paradigm of Decadal to Centennial Climate Research
The growing experience with decadal to centennial climate studies has shown that, while much can be learned from the seasonal to interannual experience, there are fundamental differences in how the two sets of problems must be approached. Decadal- to centennial-scale studies, unlike efforts addressing the shorter term, such as weather forecasting and ENSO prediction, suffer from the absence of any easily realized iterative cycle of prediction and observation. This drawback has emphasized the importance of using paleoclimate records to hindcast and of calibrating instrumental records to detect potentially subtle longterm changes. The use of anomaly models that assume a constant background mean state, so productive in ENSO studies, is inappropriate for longer model runs, where change of the background state is the result of interest. Finally, a far larger range of processes is important in dec-cen studies. Radiative forcing, cloud interactions, involvement of the deep ocean, and sea ice dynamics are but a few of the processes that will be major influences on the long-term evolution of
climate. Process studies are thus of particular importance for prediction. The influence of processes must be modeled correctly for there will be no opportunities to test them through direct short-term observations of today's climate system.
RESEARCH IMPERATIVES: PRIORITIES FOR OBSERVATIONS, MODELING, AND THEORY
As elaborated in the discussion above, the following Research Imperatives are required to advance most efficiently our understanding of dec-cen climate variability and change:
-
Natural climate patterns. Improve knowledge of decadal- to century-scale natural climate patterns, their distributions in time and space, optimal characterization, mechanistic controls, feedbacks, and sensitivities, including their interactions with, and responses to, anthropogenic climate change.
-
Paleorecord. Extend the climate record back through data archeology and paleoclimate records for time series long enough to provide researchers with a better database to analyze decadal- to century-scale patterns. Specifically, achieve a better understanding of the nature and range of natural variability over these timescales.
-
Long-term observational system. Ensure the existence of a long-term observing system for a more definitive observational foundation to evaluate decadal- to century-scale variability and change. Ensure the system includes observations of key state variables as well as external forcings.
-
Climate system components. Address those issues whose resolution will most efficiently and significantly advance our understanding of decadal- to century-scale climate variability for specific components of the climate system.
-
Anthropogenic perturbation. Improve understanding of the long-term responses of the climate system to the anthropogenic addition of radiatively active constituents to the atmosphere and devise methods of detecting anthropogenic phenomena against the background of natural decadal- to century-scale climate variability.
The foundation for recent progress in ENSO research was laid by Rasmussen and Carpenter's (1982) careful diagnosis of ENSO pattern variability. Similarly, understanding and predicting decadal to centennial variability depends, to a large extent, on knowledge of climate patterns on these longer timescales. Logically, we might expect that the response of the Earth system to anthropogenic forcing could be manifested in and/or obscured by these patterns. Thus, one particularly important concern is the interactions between natural variability and anthropogenic change.
For greater predictive capability it is essential to understand those processes operating in the various components of the climate system that are relevant to dec-cen variability. Because of the difficulty of directly observing phenomena of interest in dec-cen studies, in contrast to weather or seasonal to interannual studies, the importance of component process understanding is magnified.
Regarding anthropogenic perturbation, it is particularly important that we closely monitor the rate and distribution of source functions of the radiatively active gases being added to the atmosphere. These external forcings, which cannot be predicted prognostically, can then be properly introduced and diagnosed in the predictive model studies. Such models are our primary means of forecasting anthropogenic change and of guiding diagnostic and attribution studies and sampling efforts. It is therefore critical to adopt an incremental long-term observing system whose characteristics and targeted variables can evolve in parallel with our rapidly improving understanding.
Many of the issues defined here require observing systems that do not yet exist or to which no long-term commitment has yet been made. An example of this need is the monitoring of solar irradiance: current data have come from relatively short-term satellite missions that have no operational (long-term) mandate (see case study in Chapter 8). Measurements from different missions are significantly offset. Addressing decadal to centennial solar variability, as discussed above, requires a plan for long-term calibrated solar irradiance measurements across the solar spectrum.
Finally, as previously indicated, dec-cen research is in its early stages, with new insights, findings, and directions arising at an impressive rate. Likewise, the long-term sampling strategy and optimal measurement set are evolving with these advances as well. At this stage, then, it is essential that we begin (or in a few cases continue) consistent monitoring of the most fundamental state variables (e.g., atmospheric temperature and moisture profiles, ocean surface temperature and salinity values), and monitoring of those variables specifically relevant to climate system components to initialize (including via assimilation), force, and diagnose model components and variables.
Atmospheric Observations
The Physical System
The physical state of the atmosphere, regardless of the mechanisms influencing this state, is at the very core of what we call climate. Atmospheric temperature and moisture content, pressure, winds, and cloud cover (the main factor controlling surface radiation balance) must all be monitored. The spatial distribution of this monitoring can be improved with time to span the globe eventually at the relevant spatial scales, but initially a concerted effort must be made to monitor these variables at current weather station locations.
As the concentration of greenhouse gases increases in the atmosphere, the atmosphere clearly must respond in some manner to accommodate the change in radiative forcing. The atmosphere may respond by warming to some degree, it may change its vertical distribution of moisture and cloud cover, or any combination of these may occur. Each of the state variables must be monitored, including their vertical distributions through the troposphere and lower stratosphere, to evaluate the nature of anthropogenic and natural changes. One of the most hotly debated topics in modern climatology is how atmospheric moisture distribution will change in response to the addition of greenhouse gases and therefore whether, or by how much, this moisture response will moderate the temperature response. Thus, it is not enough to measure temperature, simply because temperature has been the initial focus of the greenhouse debate.
Atmospheric observations must be colocated with those stations established to monitor surface conditions. This need directly follows from the earlier point that most, if not all, dec-cen atmospheric variability and change are in response to changes in slower components of the climate system, such as land, ice, and ocean. These components represent the lower boundary of the atmosphere. In many cases, as noted above, atmospheric changes strongly covary with changes at the surface. To evaluate, diagnose, and attribute dec-cen change, such covariation must be captured in a manner that facilitates analysis and evaluation of hypotheses that describe the coupled mechanisms driving and modulating long-term variability.
Process studies and related field efforts must be directed to improving our understanding and parameterization of surface-atmosphere interaction. Obviously, it is through this boundary interaction that slower-scale components communicate their influences to the atmosphere. Thus, appropriate parameterization of these phenomena are essential, since modeling efforts are the primary tool we have for forecasting future change. We also need better parameterization of clouds, including distribution and feedback processes, since their treatment in models may prove crucial in predicting long-term climate responses to changes in radiative forcing, as well as other feedback influences associated with variability and change. These parameterizations are currently a primary limitation in existing models.
The Chemical System
The radiative effects of aerosols, direct and indirect, are poorly constrained. Cloud processes, although they occur on far shorter than decadal timescales, are a major uncertainty in predicting future radiation balances. Parameterizations need to be improved.
Carbon cycle questions require a CO2 measurement strategy that accounts for the hierarchy of scales, both temporal and spatial, inherent in ecosystem processes and their controls. Atmospheric concentration data must allow the identification and quantification of regional sources and sinks and their responses
to climate fluctuations and human perturbations. This information will permit integration over regional scales of fluxes and feedback processes that can be measured, understood, and modeled on smaller spatial and temporal scales. Isotopic data allow distinguishing between oceanic and biospheric sinks on regional scales and have provided significant insight into the regional carbon balance. Ratios of O 2 to N2 in the global atmosphere provide an independent constraint on the balance between net terrestrial and oceanic sinks. The same scaling and measurement issues are almost identical for N2O and CH4, and their biogeochemical budgets can be tackled together with a measurement program suitable for CO2.
Enormous progress in assessing trace gas budgets could be achieved if a method could be developed or refined to directly measure air-sea gas exchange rates. Promising methods are air measurements with eddy correlation and/or eddy accumulation. Such measurements would eventually lead to a realistic understanding of the processes controlling the rate of gas exchange and therefore to a parameterization that could be applied with confidence worldwide. Existing climatologies of the partial pressure differences between the air and the water for many gases could then be turned into maps of gas exchange, making oceanic data into a much more compelling constraint on the atmospheric budget and closing the open boundary of surface oceanic gas budgets.
Ocean Observations
Various types of ocean observations are needed to study the dec-cen variability associated with the primary known patterns of atmospheric climate variability: periodic (decadal) temperature, salinity, oxygen, and tracer sections; velocity profile surveys and repeat sections (starting with World Ocean Circulation Experiment sections); and higher-frequency time series stations (starting with past and present weather ship stations). These measurements will allow better quantitative description of the ocean's participation in that dec-cen variability, especially in light of the slowly propagating SST and subsurface anomalies that have revealed the ocean's dec-cen variability as more than stationary patterns. We must extend these surveys into southern hemisphere regions as the nature of the dec-cen variability begins to be revealed.
These sections and time series stations provide the baseline against which the long-term response and change of the ocean can be measured and the basic observational set from which serendipitous discoveries about the ocean's role in climate change have been realized. In addition, the time series data have been invaluable in studying the ocean' s response to atmospheric forcing and its feedback to the atmosphere. These findings are of particular importance because surface layer interaction and response dictate the volume of water in direct communication with the atmosphere. Even a small change in this volume can lead to a significant change in SST, given the same magnitude of surface forcing. The
time series stations are the only series available that allow appropriate development, diagnosis, and improvement of these parameterizations.
Continued satellite data are needed for global coverage of sea surface height, SST, winds, and ocean color, but for these data to be useful, corresponding ground-truth ocean observations also are needed. Particular data of interest concern the heat budget. A concerted effort is required to improve estimates of heat flux divergence and heat storage and their variabilities from subsurface ocean data, eliminating disparities between those estimates and air-sea heat exchange estimates. Various subsurface floats and moorings are particularly helpful to supplement shipboard measurements for this study.
Sea level change is another important observational challenge. The IPCC (1996) estimates that in the year 2000 sea level will be 46 to 72 cm higher than today (36 to 53 cm, when the effects of sulfate aerosols are included). A range is given because each projection presumes a specific scenario for increase in greenhouse gasses. To validate these predictions, better monitoring of global sea level change and its components will be needed. The prospects for sea level monitoring are good. A global network of sea level stations (Global Sea Level Observing System) is being implemented. Land movements will be measured at some of these stations with satellite geodesy and gravimetric techniques. Satellite altimetry is another important tool coming into use to measure global sea level rise.
Cryosphere Observations
Critical cryosphere-related observations for climate patterns on decadal to centennial timescales include long-term monitoring of surface salinity along with SST, since salinity represents the dominant control on the density of seawater in high-latitude regions. Also, measurements of the sea ice fields themselves, including motion fields and ice thickness, are required to determine the freshwater transports and buoyancy fluxes associated with the ice fields. This freshwater transport has been implicated in driving major changes, even mode shifts in the global thermohaline circulation. Finally, consistent monitoring of iceberg calving and an observational system for determining ice basal melt or growth (e.g., through temperature/salinity moorings across the floating ice shelves) must be established to better determine the freshwater budget. Both field and satellite studies are needed to refine the mass budgets of the Greenland and Antarctic ice sheets. Onsite studies that focus on ice flow, melting, and calving should be continued and extended. Water vapor flux divergence observations will help pin down the source of the ice sheets' mass. A laser altimeter on a polar-orbiting satellite is needed to augment existing radar altimetry. These satellite data will provide accurate estimates of ice sheet volume and give early warning of possible ice sheet collapse. As in the case of ice, the distribution of snow fields, including thickness and spatial extent must be monitored. The response of snow distribu-
tion to climate change has been hypothesized as being important in surface-climate feedbacks as well as in climate change diagnostics.
Finally, the ocean-atmosphere-ice interaction, particularly the ice or snow surface energy balance (including surface albedo and ocean-ice, ice-cloud, and snow-cloud feedbacks), must be addressed through detailed process studies to improve parameterizations of these processes in climate models.
Land and Vegetation Observations
As explained in sections above, it is also essential to monitor changes in land surface characteristics, including surface vegetation. These changes alter not only the distribution of surface reservoirs and the surface-atmosphere exchange of radiatively active gases but also albedo and even surface stress and evapotranspiration efficiency —and the last two both influence the hydrological cycle. This serves as an external forcing to the planet that cannot be predicted and must be introduced into the models as they occur to properly maintain the models' surface forcing conditions.
Long-term monitoring of near-surface aerosol distributions also is needed. These distributions may induce stationary changes in the surface radiation balance, which may lead to large-scale circulation moderation through stable gradient perturbations.
Hydrological Observations
Precipitation is the key hydrological variable. For most studies of dec-cen variability and its effects, global fields of precipitation over timescaies of 10 to 100 years are essential. We have no such global instrumental records currently. The National Aeronautics and Space Administration's Tropical Rainfall Monitoring Mission is an important first step, but global data are needed. To relate precipitation to global boundary conditions, SST, vegetative ground cover and soil moisture, and sea and land ice and snow must be simultaneously measured. Nearly every theory of anthropogenic warming finds an increased rate of the hydrological cycle and possible alteration of atmospheric distributions of moisture and of the frequency, intensity, and distribution of rainfall (including severe rainfall events). Thus, monitoring of the surface distribution of precipitation and evaporation must begin. This monitoring includes that over the oceans, where changes in the precipitation minus evaporation balance alter the surface salinity budget, which in high latitudes has been implicated in altering the thermohaline circulation (and driving internal oscillations on dec-cen timescaies in ocean models).
CONCLUSIONS
Above we observed that the dec-cen paradigm must differ from that used to study shorter-timescale variability. Moreover, even the nature of the observations collected for dec-cen studies must differ. While, for example, atmospheric state variables must be monitored in both cases, because the diurnal and seasonal cycles in these variables are often so large, they virtually swamp any longer-term, more slowly evolving timescale changes as they are taking place. Thus, shortterm climate change can often be identified using relatively coarse sampling resolution (and accompanying precision and accuracy); if longer-term change is to be detected using relatively short time series, these measures require considerably higher resolution (and precision and accuracy). How much higher changes with the variables measured and the rate at which the dec-cen change occurs. However, in any case, care must be taken to provide measurements of sufficient resolution and precision to allow extraction of the dec-cen signal at the earliest possible moment to make the most efficient use of the data. This consideration cannot be overlooked when designing joint monitoring sites geared toward weather and interannual and dec-cen studies.
In an analogous manner, the reliance of dec-cen studies on modeling demands considerable computing resources, since the models used in these efforts are often subject to long-term numerical drift in analyzing long timescales. This inadequacy simply reflects an inadequate treatment of the higher-order physics that often serve as the feedback mechanisms required to eliminate such drift. However, such higher-order physics typically involve more detailed regional or local-scale boundary interactions, which again require higher resolution, either in the vertical or horizontal dimension, or both. Over longer timescales the slower components of the system have an opportunity to become more intimately involved in climate evolution, so better treatment of additional components is also required. Further, the simulations themselves must involve much longer simulation times to resolve long timescales adequately. Obviously, the computer resources demanded by such models are extensive. Therefore, there must be a concerted effort to make the fastest computers readily available, so as to facilitate widespread access by a very broad and diverse modeling community. Sufficient resources are also needed so that the simulations required can be made as quickly and as often as needed.
Dec-cen climate studies are in their infancy, but advances and understanding are coming quickly. Because of the potential that climate change has to influence society dramatically over the timescale of a human life, we must make serious efforts to foster this research and build understanding to provide a sound scientific basis for national policy. Only then can policy makers take the necessary steps to ensure our long-term well-being—regardless of whether future climate changes are driven by natural or anthropogenic means.
NOTES
1. Buckland et al. (1995).
2. Mayewski and Weiss (In review).
3. Klein (1986), Frumkin et al. (1991).
4. Weiss et al. (1993), Mayewski and Weiss (1998).
5. Hodell et al. (1995).
6. Lall et al. (1997).
7. National Research Council (1995a).
8. Dettinger and Cayan (1995).
9. Leathers et al. (1991).
10. Trenberth and Paulino (1980).
11. Jones and Briffa (1992), Jones (1994).
12. Mann and Park (1994, 1996).
13. Wallace et al. (1995).
14. Wigley and Barnett (1990), Santer et al. (1996).
15. IPCC (1996).
16. Wallace et al. (1995).
17. E.g., Mitchell (1976), NRC (1995b).
18. Wallace et al. (1992).
19. Hurrell (1995), Hurrell and van Loon (1997).
20. Hurrell (1995).
21. Ibid., Hurrell and van Loon (1997).
22. E.g., Deser and Blackmon (1993).
23. Lazier (1988).
24. Dickson et al. (1988).
25. Pohjola and Rogers (1997).
26. Wallace and Gutzler (1981).
27. Rogers (1990).
28. Trenberth (1990).
29. Horel and Wallace (1981).
30. Hurrell (1996).
31. Zhang et al. (1997), Latif and Barnett (1994), Gu and Philander (1997).
32. Mantua et al. (1997).
33. Wallace and Gutzler (1981).
34. Gray et al. (1992).
35. Zhang et al. (1997).
36. E.g., Kumar et al. (1994).
37. Thompson et al. (1995).
38. Cayan and Peterson (1989), Cayan (1996), Miller et al. (1997).
39. Walker and Bliss (1932), Rogers (1981).
40. Overland et al. (1997).
41. Wallace and Gutzler (1981).
42. Mantua et al. (1997).
43. White and Peterson (1996).
44. Ibid.
45. Yuan et al. (1996).
46. Trenberth (1996), Karoly (1989).
47. Mehta and Lau (1997).
48. Deser and Blackmon (1993).
49. Latif and Barnett (1994).
50. Gu and Philander (1997).
51. Hasselman (1976).
52. Tanimoto et al. (1993), Zhang et al. (1997).
53. Dickson et al. (1988).
54. White and Peterson (1996).
55. E.g., Hansen and Bezdek (1996).
56. See discussions by Wallace et al. (1995), Trenberth (1996), Hurrell (1996).
57. Broccoli ( ).
58. Dickson et al. (1996)
59. Tremblay (1997).
60. IPCC (1990).
61. IPCC (1996).
62. E.g., Lindzen (1996).
63. E.g., Broecker (1994).
64. Cook et al. (1996).
65. E.g., Laird et al. (1996), Madole (1995).
66. Forman et al. (1992), Madole (1994), Muhs et al. (1996).
67. Stine (1994).
68. Hughes and Graumlich (1996).
69. IPCC (1996).
REFERENCES AND BIBLIOGRAPHY
Arrhenius, S. 1896. On the influence of carbonic acid in the air upon the temperature of the ground. Philosophy Magazine 41:237.
Broecker, W. 1994. Massive iceberg discharges as triggers for global climate change. Nature 372:421-424.
Buckland, P.C., T. Amorosi, L.K. Barlow, A.J. Dugmore, P.A. Mayewski, T.H. McGovern, A.E.J. Ogilvie, J.P. Sadler, and P. Skidmore. 1995. Bioarchaeological evidence and climatological evidence for the fate of Norse farmers in medieval Greenland. Antiquity 70:88-96.
Cayan, D.R. 1996. Interannual climate variability and snowpack in the western United States. Journal of Climatology 9:928-948.
Cayan, D.R., and D.H. Peterson. 1989. The influence of the North Pacific atmospheric circulation and streamflow in the West. Pp. 375-397 in Aspects of Climate Variability in the Western Americas, D.H. Peterson, ed. Geophysics Monograph 55. American Geophysical Union, Washington, D.C.
Cook, E.R., D.M. Meko, D.W. Stahle, and M.K. Cleaveland. 1996. Tree-ring reconstructions of past drought across the coterminous United States: Tests of a regression method and calibration/verification results. Pp. 155-169 in J.S. Dean, D.M. Meko, and T.W. Swetnam, eds., Tree Rings, Environment, and Humanity, Tucson.
Deser, C., and M.L. Blackmon. 1993. Surface climate variations over the North Atlantic Ocean during winter: 1900-1989. Journal of Climate 6:1743-1753.
Dettinger, M.D., and D.R. Cayan. 1995. Large-scale atmospheric forcing of recent trends toward early snowmelt runoff in California. Journal of Climate 8(3):606-623.
Dickson, R.R., J. Meincke, S-A. Malmberg, and A.J. Lee. 1988. The “great salinity anomaly” in the northern North Atlantic, 1968-1982. Progress in Oceanography 20:103-151.
Dickson, R.R., J.R.N. Lazier, J. Meincke, and P.B. Rhines. 1996. Long-term coordinated changes in the convective activity of the North Atlantic. In D. Anderson and J. Willebrand, eds., Decadal Climate Variability: Dynamics and Predictability, NATO ASI Series Vol. 44. Springer-Verlag, Berlin.
Folland, C.K., T.N. Palmer, and D.E. Parker. 1986. Sahel rainfall and worldwide sea temperatures, 1901-85. Nature 320:602-607.
Forman, S.L., A.F.H. Goetz, and R.H. Yuhas. 1992. Large-scale stabilized dunes on the high plains of Colorado: Understanding the landscape response to Holocene climates with the aid of images from space. Geology 20:145-148.
Frumkin, A., M. Magaritz, I. Carmi, and I. Zak. 1991. The Holocene climatic record of the salt caves of Mount Sedom, Israel The Holocene 1(3):191-200.
Gray, W.M., C.W. Landsea, P.W. Mielke, and K.J. Berry. 1992. Predicting Atlantic seasonal hurricane activity 6-11 months in advance Weather Forecasting 7:440-455.
Gu, D., and S.G.H. Philander. 1997. Interdecadal climate fluctuations that depend on exchanges between the tropics and extratropics. Science 275:805-807.
Hansen, D.V., and H.F. Bezdek. 1996. On the nature of decadal anomalies in North Atlantic sea surface temperature. Journal of Geophysical Research 101:9749-9758.
Hansen, J., M. Sato, R. Ruedy, A. Lacis, K. Asamoah, K. Beckford, S. Borenstein, E. Brown, B.B. Cairns, B. Carlson, B. Curran, S. De Castro, L. Druyan, P. Etwarrow, T. Ferede, M. Fox, D. Gaffen, J. Glascoe, H. Gordon, S. Hollandsworth, X. Jiang, C. Johnson, N. Lawrence, J. Lean, J. Lerner, K. Lo, J. Logan, A. Luckett, M.P. McCormick, R. McPeters, R. Miller, P. Minnis, I. Ramberran, G. Russell, P. Russell, P. Stone, I. Tegen, S. Thomas, L.Thomason, A. Thompson, J. Wilder, R. Willson, and J. Zawodny. 1997. Forcings and chaos in interannual to decadal climate change. Journal of Geophysical Research 102(D22):25,679-25,720.
Hasselmann, K. 1976. Stochastic climate models. I. Theory. Tellus 28:473-485.
Hodell, D.A., J.H. Curtis, and M. Brenner, 1995. Possible role of climate in the collapse of classic Maya civilization Nature 375:391-394.
Horel, J.D., and J.M. Wallace. 1981. Planetary-scale atmospheric phenomena associated with the Southern Oscillation. Monthly Weather Review 109.
Hughes, M.K., and L.J. Graumlich. 1996. Mulimillennial dendroclimatic studies from the western United States Pp. 109-123 in Climate Variations and Forcing Mechanisms of the Last 2000 Years, P. Jones et al., eds. Springer-Verlag, Berlin.
Hurrell, J.W. 1995. Decadal trends in the North Atlantic Oscillation: Regional temperature and precipitation. Science 269:676-679.
Hurrell, J.W. 1996. Influence of variations in extratropical wintertime teleconnections on northern hemisphere temperatures. Geophysical Research Letters 23:665-668.
Hurrell, J.W., and H. van Loon. 1994. A modulation of the atmospheric annual cycle in the southern hemisphere Tellus 46A:325-338.
Hurrell, J.W., and H. van Loon. 1997. Decadal variations in climate associated with the North Atlantic Oscillation: Climatic change at high elevation sites. Climatic Change 36(3-4):301-326.
IPCC. 1990. Climate Change: The IPCC Scientific Assessment, J.T. Houghton, G.J. Jenkins, and J.J. Ephraums, eds. Cambridge University Press, Cambridge, U.K.
IPCC. 1996. Climate Change 1995: The Science of Climate Change. Contribution of Working Group 1 to the Second Assessment Report of the Intergovernmental Panel on Climate Change, J.T. Houghton et al., eds. Cambridge University Press, Cambridge, U.K.
Jones, P.D. 1994. Recent warming in global temperature series. Geophysical Research Letters 21(12):1149-1152.
Jones, P.D., and K.R. Briffa. 1992. Global surface air temperature variations during the twentieth century Holocene 2:165-179.
Karoly, D.J. 1989. Southern hemisphere circulation features associated with El Niño-Southern Oscillation events, Journal of Climatology 2:1239-1252.
Klein, C. 1986. Fluctuations of the level of the Dead Sea and climatic fluctuations in Israel during historical times. Ph.D. thesis, Hebrew University, Jerusalem.
Kumar, A., A. Leetmaa, and J. Ming. 1994. Simulations of atmospheric variability induced by sea surface temperatures and implications for global warming. Science 266(5185):632-634.
Laird, K.R., S.C. Fritz, K.A. Maasch, and B.F. Cumming. 1996. Greater drought intensity and frequency before AD 1200 in the northern Great Plains, USA. Nature 384:552-554.
Lall, U., B. Rajagopalan, A. Bradley, and K. Potter. 1997. Can interannual, decadal and centennial climate variability explain the changes in the frequency of American River floods? UWRL Working Paper No. 97/UL05. Utah Water Research Laboratory, Utah State University, Logan.
Latif, M., A. Grozner, M. Munnich, E. Maier-Reimer, S. Venzke, and T.P. Barnett. 1996. A mechanism for decadal climate variability. Pp. 263-292 in Decadal Climate Variability, D.L.T. Anderson and J. Willebrand, eds. NATO ASI Series Vol. 44. Springer-Verlag, Berlin.
Latif, M., and T.P. Barnett. 1994. Causes of decadal climate variabilty over the North Pacific/North American secror. Science 266-634-637.
Lazier, J.R. 1988. Temperature and salinity changes in the deep Labrador Sea, 1962-1986 Deep-Sea Research 18:1247-1253.
Leathers, D.J., B. Yarnal, and M.A. Palecki. 1991. The Pacific/North American teleconnection pattern and United States climate. Part I: Regional temperature and precipitation associations Journal of Climatology 4:517-528.
Lindzen, R.S. 1996. The importance and nature of the water vapor budget in nature and models. Pp. 51-66 in Climate Sensitivity to Radiative Perturbations, H. Le Treut, ed. NATO ASI Vol. 34. Springer-Verlag, Berlin.
Madole, R.F. 1994. Stratigraphic evidence of desertification in the west-central Great Plains within the past 1000 years. Geology 22:483-486.
Madole, R.F. 1995. Spatial and temporal patterns of late Quaternary eolian deposition, eastern Colorado, U.S.A. Quaternary Science Review 14:155-177.
Mann, M.E., and J. Park. 1994. Global scale modes of surface temperature variability on interannual to century timescales. Journal of Geophysical Research 99:25,819-25,833.
Mann, M.E., and J. Park. 1996. Joint spatio-temporal modes of surface temperature and sea level pressure variability in the northern hemisphere during the last century Journal of Climate 9:2137-2162.
Mantua, N.J., S.R. Hare, Y. Zhang, J.M. Wallace, and R.C. Francis. 1997. A Pacific interdecadal climate oscillation with impacts on salmon production. Bulletin of the American Meteorological Society 78:1069-1079.
Mayewski, P.A., and H. Weiss. In review. West Asian responses to Holocene abrupt climate change events.
Mehta, V.M., and K.M. Lau. 1997. Influence of solar irradiance on the Indian monsoon-ENSO relationship at decadal-multidecadal timescales. Geophysical Research Letters 24(2):159-162.
Miller, A.J, W.B. White, and D.R. Cayan. 1997. North Pacific thermocline variations on ENSO timescales. Journal of Physical Oceanography 27(9):2023-2039.
Mitchell, J.M. 1976. An overview of climate variability and its causal mechanisms. Quaternary Research 6:481-493.
Muhs, D.R., T.W. Stafford, S.D. Cowherd, S.A., Mahan, R. Kihl, P.B. Matt, C.A. Bush, J. Nehring. 1996. Origin of the late Quaternary dune fields of northeastern Colorado Geomorphology 17:129-149.
National Research Council, Committee on Flood Control Alternatives in the American River Basin 1995a. Flood Risk Management and the American River Basin. National Academy Press, Washington, D.C.
National Research Council. 1995b. Natural Climate Variabilty on Decadal-to-Century Time Scales. National Academy Press, Washington, D.C.
National Research Council. 1998. Decade- to Century-Scale Climate Variability and Change: A Science Strategy, National Academy Press, Washington, D.C.
National Research Council, Committee on American River Flood Frequencies. 1999. Improving American River Flood Frequency Analyses. National Academy Press, Washington, D.C.
Overland, J.E., J.M. Adams, and N.A. Bond. 1997. Regional variation of winter temperatures in the Arctic. Journal of Climate 10(5):821-837.
Peterson, T.C., and R.S. Vose. 1997. An overview of the Global Historical Climatology Network temperature data base. Bulletin of the American Meteorological Society 78:2837-2849.
Pohjola, V.A., and J.C. Rogers. 1997. Atmospheric circulation and variations in the Scandinavian glacier mass balance. Quaternary Research 47:29-36.
Rasmussen, E.M., and T.H. Carpenter. 1982. Variations in tropical sea surface temperature and surface wind fields associated with the Southern Oscillation/El Niño. Monthly Weather Review 1103:54-384.
Rind, D., and J.T. Overpeck. 1993. Hypothesized causes of decade-to-century-scale climate variability: Climate model results. Quaternary Science Reviews 12:357-374.
Rind, D., P. Lonergan, and K. Shah. 1996. Climatic effect of water vapor release in the upper troposphere. Journal of Geophysical Research 101:29,395-29,405.
Rind, D., C. Rosenzweig, and M. Stieglitz. 1997. The role of moisture transport between ground and atmosphere in global change. Annual Review Energy Environment.
Rogers, J.C. 1981. The North Pacific Oscillation. Journal of Climatology 1(1):39-57.
Rogers, J.C. 1990. Patterns of low-frequency monthly sea level pressure variability (1899-1986) and associated wave cyclone frequencies. Journal of Climatology 3:1364-1379.
Santer, B.D., K.E. Taylor, T.M.L. Wigley, T.C. Johns, P.D. Jones, D.J. Karoly, J.F.B. Mitchell, A.H. Oort, J.E. Penner, V. Ramaswamy, M.D. Schwarzkopf, and S. Tett. 1996. A search for human influences on the thermal structure of the atmosphere Nature 382:39-46.
Stine, S. 1994. Extreme and persistent drought in California and Patagonia during medieval time. Nature 369:546-549.
Tanimoto, Y., N. Iwasaka, K. Hanawa, and Y. Toba. 1993. Characteristic variations of sea surface temperature with multiple timescales in the North Pacific. Journal of Climatology 6:1153-1160.
Thompson, L.G., E. Mosley-Thompson, M.E. Davis, P. Lin, K.A. Henderson, J. Cole-Dai, J.F. Bolzan, and K. Liu. 1995. Late glacial stage and Holocene tropical ice core records from Huascaran Peru. Science 269:46-50.
Tremblay, L.B., L.A. Mysak, and A.S. Dyke. 1997. Evidence from driftwood records for century-to-millennial scale variations of the high latitude atmospheric circulation during the Holocene Geophysical Research Letters 24(16):2027-2030.
Trenberth, K.E. 1990. Recent observed interdecadal climate changes in the northern hemisphere Bulletin of the American Meteorological Society 71:988-993
Trenberth, K.E. 1996. Atmospheric circulation climate changes. Climate Change 21:427-453
Trenberth, K.E., and D.A. Paulino. 1980. The northern hemisphere sea-level pressure data set: Trends, errors and discontinuities. Monthly Weather Review 108:855-872
Walker and Bliss. 1932. World weather. Memoirs of the Royal Meteorological Society 4(36):53-84.
Wallace, J.M., and D.S. Gutzler. 1981. Teleconnections in the geopotential height field during the northern hemisphere winter. Monthly Weather Review 109:784-812.
Wallace, J.M., C. Smith, and C.S. Bretherton. 1992. Singular value decomposition of wintertime sea surface temperature and 500-mb height anomalies. Journal of Climatology 5:784-812:561-576.
Wallace, J.M., Y. Zhang, and J. Renwick. 1995. Dynamical contribution to hemispheric temperature trends. Science 270:780-783.
Ward, M.N., and C.K. Folland. 1991. Prediction of seasonal rainfall in the north Nordeste of Brazil using eigenvectors of sea surface temperature. International Journal of Climatology 11:711-743.
Weiss, H., M.A. Courty, W. Wetterstrom, L. Senior, R. Meadow, F. Guichard, and A. Curnow. 1993. The genesis and collapse of third millennium North Mesopotamian civilization Science 261:995-1004.
White, W.B., and R. Peterson. 1996. An Antarctic circumpolar wave in surface pressure, wind, temperature, and sea ice extent. Nature 380:699-702.
Wigley, T.M.L., and T.P. Barnett. 1990. Detection of the greenhouse effect in the observations. Pp. 239-255 in J.T. Houghton, G.J. Jenkins, and J.J. Ephraums, eds., Climate Change: The IPCC Scientific Assessment. New York: Cambridge University Press.
Yuan, X., M.A. Cane, and D.G. Martinson. 1996. Climate variations: Cycling around the south pole. Nature 380:673-674.
Zhang, Y., J.M. Wallace, and D.S. Battisti. 1997. ENSO-like decade-to-century scale variability: 1900-93. Journal of Climatology 10:1004-1020.