5
Changes in the Chemistry of the Atmosphere
SUMMARY
Within the atmospheric chemistry component of the U.S. Global Change Research Program there is a well-defined science focus with a track record of dealing with public policy implications. Development and implementation of the Montreal Protocol rested on a solid scientific foundation, realized through a strong international network of scientists and, within the United States, a multiagency effort led by the National Aeronautics and Space Administration (NASA). In fact, the model of an international, integrated, and periodically repeated assessment was largely formed from the United Nations Environment Programme/World Meteorological Organization Ozone Assessments. Moreover, this research area has a rich history of interaction with the human dimension components at fine spatial scales, as a natural consequence of air pollution studies and policies. Current developments in atmospheric chemistry are revealing the close links between chemistry, radiation, dynamics, and climate. Examples include the powerful role played by aerosol formation in both the boundary layer and the upper troposphere, chemical initiation of subvisible cirrus in the region of the tropopause, the control exerted by water vapor and temperature on the sharply nonlinear partitioning of halogen and hydrogen radicals in the lower stratosphere, and the importance of stratosphere-troposphere exchange on the composition and meteorology of the upper troposphere and lower stratosphere.
However, there are significant lessons to be remembered—lessons resulting from significant research shortcomings. Failure to recognize the Antarctic ozone hole sooner demonstrates the consequences of overreliance on models and how the selected observational strategies are so critically tied to success. This lesson
must not be forgotten in studying the complexities of climate, ecosystems, and the chemistry of the troposphere.
Today, we have far deeper knowledge about the chemistry of the atmosphere than we did just a decade ago. We also know more clearly what we do not know. These issues are also addressed in a recent National Research Council report (NRC, 1998) that is consistent with the perspective put forward in this chapter. Key challenges to atmospheric chemistry in the coming decade can be expressed in five Research Imperatives, where each Research Imperative combines one or more primary Scientific Questions with the need to know from a human dimensions perspective:
-
Stratospheric ozone and ultraviolet (UV) radiation. Define and predict secular trends in the intensity of UV exposure that the Earth receives. Document the concentrations and distributions of stratospheric ozone and the key chemical species that control its catalytic destruction and elucidate the coupling between chemistry, dynamics, and radiation in the stratosphere and upper troposphere.
-
Greenhouse gases. Determine the fluxes of greenhouse gases into and out of the Earth 's systems and the mechanisms responsible for the exchange and distribution between and within those systems. Expand global detection techniques to elucidate the processes that control the abundances and variability of atmospheric CO2, CH4, N2O, and upper-tropospheric/lowerstratospheric O3 and water vapor.
-
Photochemical oxidants. Develop the observational and computational tools and strategies that policy makers need to effectively manage ozone pollution, and elucidate the processes that control and the relationships that exist among ozone precursor species, tropospheric ozone, and the oxidizing capacity of the atmosphere.
-
Atmospheric aerosols and UV/visible radiation. Document the chemical and physical properties of atmospheric aerosols, and elucidate the chemical and physical processes that determine the size, concentration, and chemical characteristics of atmospheric aerosols.
-
Toxics and nutrients. Document the rates of chemical exchange between the atmosphere and ecosystems of critical economic and environmental import, and elucidate the extent to which interactions between the atmosphere and biosphere are influenced by changing concentrations and depositions of harmful and beneficial compounds.
INTRODUCTION
The chemistry of the Earth's atmosphere has emerged as a central theme in studies of global change. Atmospheric chemistry provides the scientific foundations to understand a number of phenomena that are part of global change. These
phenomena include (1) changes in UV dosage at the Earth's surface owing to the intrinsically chemical nature of the catalytic loss of stratospheric ozone, (2) changes in the dynamics and radiative structure of the climate system through altered thermal forcing by ozone in the upper troposphere, (3) changes in the concentration of highly oxidizing species in urban as well as remote rural regions, 1 and (4) changes in the acid levels of depositions in a variety of ecosystems. In addition, work on the chemistry of the atmosphere provides hard examples of how the scientific method can succeed in guiding public policy.
What kind of research can successfully attack global-scale problems, problems that are intrinsically complex yet require reasonably unequivocal answers for international decision making and subsequent enforcement? Addressing this question is the objective of this chapter; the issue is attacked in four steps. First, case studies are presented that illustrate the successful execution of research in which hypotheses are tested by means of observations, leading to identification of cause and effect and thus to identification of the agent of change. This case study approach, while incomplete because of length limitations, helps address a fundamental question: Why is it in the national interest that we have a global change research program to study the planet? This question deserves careful consideration. Have we learned from scientific inquiry facts that constitute a decided reordering in our thinking about how the Earth functions? Have there been notable discoveries? Are there clear links between the discoveries associated with the national program and our economic competitiveness?
Second, we identify the key unanswered scientific questions that confront the field of atmospheric chemistry today. There are three categories of such questions:
-
What are the secular and episodic trends in concentrations of environmentally important atmospheric species, on local to global scales? What mechanisms control these concentration changes?
-
How are the concentrations of these species likely to change in the future? What are the most effective and feasible policy options for managing these changes?
-
What are the societal, economic, climatic, and environmental effects of present and future trends in the concentrations of these species?
Third, we review lessons learned over the past three decades that bear directly on research strategies for the future. These lessons range from general principles about posing and testing hypotheses regarding the Earth system to more detailed points about how specific observational strategies are selected to establish cause and effect.
Fourth, we address what is needed to successfully attack the major unanswered questions confronting the field, including theoretical approaches, observational strategies, instrument and platform development, and data handling and
storage. We identify five primary research imperatives for atmospheric chemistry in global change research—the research imperatives presented in the opening paragraphs of this chapter. With these disciplinary imperatives come infrastructure initiatives, without which the research cannot be executed. Lessons extracted over the past four decades of research provide substantial guidance for such infrastructure initiatives.
CASE STUDIES
This section describes selected scientific cases that led to diagnoses central to studies of global change. In describing these developments attention is given to the ways that such transitions in our scientific thinking can be linked to specific public policy initiatives and to how such cases have been related to both environmental decisions and technological developments. Thus, we address the questions: Why is it in the national interest to pursue this research? Are there links between this research and economic competitiveness?
The Antarctic Ozone Hole
Discovery and diagnosis of the Antarctic Ozone Hole were a major surprise for both scientists and the public policy structure. In worldwide studies extending back to the 1950s, the amount of ozone over the Antarctic was tracked each year through its seasonal cycle. In the late 1970s an anomalous deficit was observed in total ozone amount in the late-winter observations. In 1985 the British Antarctic Survey reported for the first time in the scientific literature2 that dramatic losses were occurring in the ozone concentration over Halley Bay and that the degree of ozone loss was worsening as the decade progressed. Theories about the cause of this unprecedented loss blossomed. Explanations ranged from simple redistribution by atmospheric motion to chemical reactions initiated by magnetic field focusing of solar electrons and protons. Such theories were put forward by serious scientific research groups in an international effort to diagnose the cause of this unexpected development.
A number of expeditions were planned to gather more complete information. In 1986 NASA planned an airborne expedition using the ER-2 aircraft to penetrate the region of the stratosphere where ozone was disappearing. The mission, executed in August and September 1987 from Punta Arenas, Chile, and supported by concurrent laboratory and modeling studies, demonstrated unequivocally that ozone was destroyed by chlorine and bromine radicals (see Figure 5.1). The role of chlorofluorocarbons (CFCs)—the molecules that transport chlorine to the stratosphere—in the destruction of ozone over the Antarctic rests on three discoveries from the NASA mission.3 The first discovery was that the region of severe ozone depletion was isolated from the rest of the stratosphere by the polar night jet that defines the perimeter of the Antarctic vortex. This isolation creates
a continental-scale “containment vessel,” creating a sharp transition in the concentration of key chemical species associated with the destruction of ozone. The existence of this barrier preventing exchange is shown clearly by the high-resolution aircraft data in Figure 5.1. The second discovery from that mission linking CFCs to Antarctic ozone destruction is the documented evolution of the anticorrelation between O3 and ClO occurring in the stratospheric containment vessel. As Figure 5.1 shows, the initial conditions established on 23 August, as sunlight returned to the region, demonstrate that O3 had emerged from the polar night largely unaffected. Three weeks later, on 16 September, ozone had eroded sharply in the presence of high ClO concentrations in the containment vessel. The third confirming discovery emerged from laboratory studies supported by NASA that determined the rates of reactions responsible for destruction of ozone by chlorine and bromine radicals. These laboratory results allowed direct quantitative comparison of the two sets of aircraft observations: (1) observed concentrations of ClO and BrO in the containment vessel and (2) the rate of ozone disappearance in the containment vessel.
The case of Antarctic ozone depletion is particularly notable in the context of global change because of the severity of the phenomenon and the isolation afforded by the stratospheric containment vessel. The main elements of the scientific case linking CFC release to ozone destruction, as summarized here, have been extensively covered and critiqued in the international scientific literature. Taken together, the three major findings in the case provide irrefutable evidence that the dramatic reduction in stratospheric ozone over the Antarctic continent would not have occurred had CFCs not been synthesized and added to the atmosphere.
There have been additional surprises in the study of stratospheric ozone depletion as well. In 1989 and again in 1991 to 1992, NASA airborne missions staged from Stavanger, Norway, Fairbanks, Alaska, and Bangor, Maine, revealed that the containment vessel over the Arctic contained highly amplified concentrations of the same ClO radical discovered over the Antarctic.4 Because ozone destruction requires high concentrations of chlorine radicals, sunlight, and time, ozone loss over the Arctic is less severe: the slightly higher temperatures over the Arctic allow the system to recover faster, by reducing the length of time that chlorine radicals remain at high concentrations in the containment vessel. In the past five years several northern hemisphere late-winter/early-spring seasons have been marked by dramatic reductions in column ozone at high latitudes.5
High-Speed Civil Transport and Ozone Loss
The United States is the world's leader in aircraft design and the development and sale of civilian commercial aircraft. NASA has developed a research program to establish the response of ozone to injections of combustion products (NOx, H2O, particulates) from the proposed High-Speed Civil Transport (HSCT).
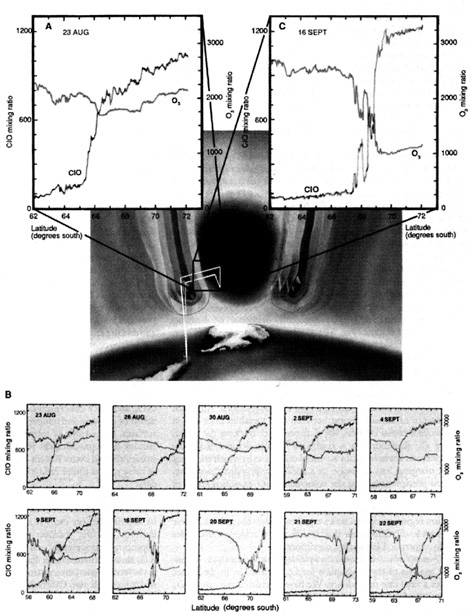
FIGURE 5.1 Rendering of the containment provided by the circumpolar jet that isolates the region of highly enhanced ClO over the Antarctic continent. Evolution of the anticorrelation between ClO and O3 across the vortex transition is traced from (A) the initial condition observed on 23 August 1987 on the southbound leg of the flight; (B) summary of the sequence over the 10-flight series; and (C) imprint on O3 resulting from three weeks of exposure to elevated levels of ClO. Data panels do not include dive segment of trajectory; ClO mixing ratios are in parts per trillion by volume; O3 mixing ratios are in parts per billion by volume. SOURCE: Anderson et al. (1991). Courtesy of the American Association for the Advancement of Science.
The first direct experiments on the ER-2 aircraft recast our understanding of ozone loss in the lower stratosphere. Commercial aircraft sales represent an international market measured in tens of billions of dollars annually. Development of the HSCT is a main component in the international battle for leadership in this field. A key issue for this development is that the nitrogen oxide/particulate/water vapor effluent from the proposed aircraft could trigger both enhanced ozone loss in the stratosphere and radiative changes linked, through water vapor changes and cloud formation, to climate changes. Senate hearings in the early 1970s hinged significantly on the prospect of damage to the ozone layer by large fleets of supersonic transports resulting from NOx emissions.6 Equally important, however, is recognition that, if an aircraft is detrimental to global ozone and/or climate, business decisions to build such an aircraft are compromised.
NASA is carrying out a research effort with airborne missions7 to test fundamental ideas about processes that control tropospheric and stratospheric ozone and, in particular, how the proposed HSCT and subsonic aircraft may alter those processes. The past three years have witnessed two important developments in our understanding of processes that control the catalytic destruction of ozone in the lower stratosphere. The first development emerged out of simultaneous NOx/ NOy observations8 during NASA's research and analysis airborne mission to the Arctic. The mission found that aerosols (minute liquid droplets) have a dramatic impact on the fraction of reactive nitrogen tied up in free radical form (NO and NO2). These ER-2 in situ observations clearly demonstrated that NOx was converted to NOy, thereby providing a natural “sink” for any reactive nitrogen compound added to the lower stratosphere and, in particular, for the combustion effluent from the proposed Mach 2.4 HSCT. This result constitutes the first serious challenge to the two-decades-old premise that catalytic destruction of ozone in the lower stratosphere is dominated by nitrogen radicals (NOx). It was this fundamental tenet—that ozone removal in the lower stratosphere is rate limited by NO 2 —combined with the realization that a significant fleet of supersonic transports would add appreciably to the nitrogen oxide budget of the lower stratosphere, that impugned supersonic transports in the early 1970s.9
The second key development emerged from NASA's Stratospheric Photochemistry, Aerosol, and Dynamics Expedition of May 1993 and has subsequently been confirmed in more recent airborne missions. This ER-2 mission was the first to include a new generation of solid-state laser experiments capable of detecting OH and HO2, thereby completing an ensemble of instruments capable of simultaneous in situ detection of each of the rate-limiting radicals in the dominant catalytic cycles (NO2, ClO, BrO, and HO2) and of the key coupling radicals NO and OH. These ER-2 observations 10 demonstrated the predominance of odd hydrogen HOx) and halogen free radical (ClOx and BrOx) catalysis in determining the rate of removal of ozone in the lower stratosphere. A single catalytic cycle, rate limited by HO2 + O3 → HO + O2 + O2, was found to account for nearly half the total O3 removal in the midlatitude northern hemisphere lower stratosphere. Halogen radical chemistry was found to be responsible for 30 percent of the
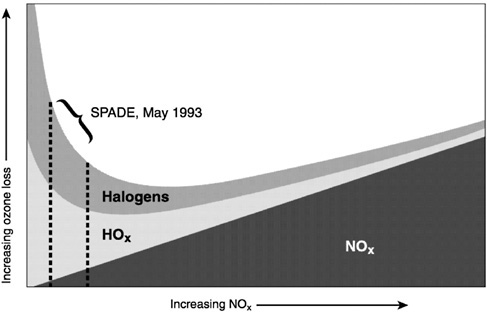
FIGURE 5.2 The O3 removal rate is shown versus NOx. Because of the coupling that exists between the radical families, the response of the total O3 removal rate to changes in NOx is highly nonlinear. At sufficiently low NOx, such as observed during the NASA mission, the removal rates are inversely correlated with NOx. SOURCE: Wennberg et al. (1994). Courtesy of the American Association for the Advancement of Science.
catalyzed loss of O3, with reactions involving bromine sustaining half of the halogen catalytic cycles. Of critical importance to the HSCT, this NASA mission demonstrated that in the region sampled by the ER-2 the rate of catalytic ozone destruction is inversely correlated with total NOx loading. The relationship between ozone loss rates and added NOx is most clearly captured in a 1994 figure displayed in Figure 5.2.
These two developments changed decidedly the scientific community 's judgment about the expected impact of the NOx component of the HSCT effluent. Specifically, if there were a region of the stratosphere where addition of NOx would actually decrease the rate of ozone catalytic destruction, it becomes plausible, contingent on the design of the aircraft and the dynamical and chemical characteristics of the stratosphere at higher altitudes, that addition of NOx to the lower stratosphere could leave the ozone column virtually unaffected. These ER-2 results decidedly rearranged our thinking on lower-stratospheric ozone photochemistry.
There are, of course, a number of other key examples, including the diagnosis by Chameides et al. (1988) showing the coupled impact of volatile organics and NOx on urban ozone production and the demonstration by Charlson et al.
(1995) of the importance to climate forcing of anthropogenic aerosols. There have also been key examples of direct sampling in supersonic aircraft exhaust in the stratosphere.11
A RESEARCH AGENDA FOR THE NEXT DECADE
The Scientific Questions facing atmospheric chemistry today are intellectually profound but also of vital social and economic importance. They relate to atmospheric constituents that are fundamentally important to our environment: stratospheric ozone, greenhouse gases, ozone and photochemical oxidants in the lower atmosphere, atmospheric aerosols or particulate matter, and toxics and nutrients. It is perhaps a measure of the strides made in recent decades that the issues of atmospheric chemistry are familiar now to the general public, policy makers, and scientists alike. Continued progress will require an ambitious and judicious commitment of financial, technological, and human resources to document the changing composition of the atmosphere and elucidate the causes and potential consequences of these changes.
Key Scientific Questions
The principal focus for atmospheric chemistry research will be on the environmentally important atmospheric species that, by virtue of their radiative and/ or chemical properties, affect climate, key ecosystems, and living organisms (including humans). From an intellectual point of view these species are interesting because they are central to the life support system of our planet. From a societal point of view they are of interest because they directly impact human health and welfare. Out of this focus emerges the challenge for atmospheric chemistry research in the coming decades: development and application of the tools and scientific infrastructure required to document and predict the concentrations and effects of environmentally important atmospheric species on local, regional, and global spatial scales and on daily to decadal timescales.
Stratospheric Ozone and UV Radiation Imperative
The stratosphere is a dynamical/radiative system12 that exports ozone from the high-altitude tropics to mid/high latitudes along downward-sloping surfaces, defined by constant mixing ratios of tracers such as N2O and CH4. The coherence of these tracer surfaces as a vertical coordinate, revealed by tight regression relationships, is a dramatic and simplifying feature of the stratosphere.13 Recognition of this fact has profound implications for research strategies in the next decade. Material enters the stratosphere primarily in the cold inner tropics in a process that desiccates the air, confines its point of entry, and establishes a “leaky chimney” that persists well into the middle stratosphere, dictating poleward motion from the tropics, as shown in Figure 5.3.
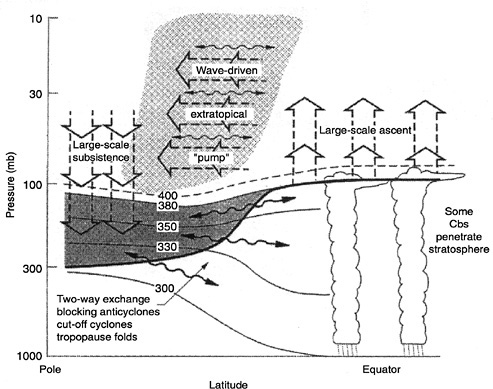
FIGURE 5.3 Dynamical aspects of stratosphere-troposphere exchange. The tropopause is shown by the thick line. Thin lines are isentropic or constant potential temperature surfaces (in degrees Kelvin). The heavily shaded region is “lowermost stratosphere,” in which isentropic surfaces span the tropopause and isentropic exchange by tropopause folding occurs. The region above the 380 K surface is the “overworld,” in which isentropes lie entirely in the stratosphere. Light shading in the overworld denotes wave-induced forcing (the extratropical “pump”). The wavy double-headed horizontal arrows denote meridional transport by eddy motions, which include tropical upper-tropospheric troughs and their cutoff cyclones, as well as their midlatitude counterparts, including folds. The broad vertical arrows show transport by the global-scale circulation, which consists of tropical upwelling and extratropical downwelling, driven nonlocally by the extratropical pump. This large-scale circulation is the primary contribution to exchange across isentropic surfaces (e.g., the ~400 K surface) that are entirely in the overworld.
Exchange between the extratropical boundary of the chimney and mid-latitudes occurs on timescales of a very few months; this meridional exchange may be highly seasonally dependent. Transfer through the confines of the chimney is largely uncharacterized. Vertical exchange in the extratropics occurs via a sequence of equatorward-vertical-poleward motions. Polar regimes are charac-
terized by rapid cooling in fall, with subsidence of many kilometers associated with the establishment of a strong polar jet that restricts exchange. This jet confines the winter polar stratosphere, most acutely in the southern hemisphere, and plays a significant role in the annual dynamical cycle of the stratosphere. The subtropical jet, the respective polar jets, and the tropopause constitute barriers to exchange; the low and high latitudes in each hemisphere are to a degree dynamically coupled in the lower stratosphere. Predicting accurately the path taken by material from a given point in the stratosphere in a given season is a central unanswered question. Our understanding of the response of the stratosphere to natural and inflicted changes is seriously compromised by this lack of understanding.
In formulating a strategy for studying the stratosphere, we have identified five basic scientific questions that we believe will motivate research on stratospheric ozone in the coming decades (see Box 5.1). The essential research activities that will be required to address these questions are outlined later in the section on research imperatives.
Atmospheric Greenhouse Gases Imperative
Observed increases in the concentrations of CO2, CH4, N2O, and CFCs provide one of the clearest manifestations of global change in the atmosphere. Historical trends in H2O and O3 have yet to be quantitatively characterized. However, limited data suggest that tropospheric ozone concentrations may have
|
BOX 5.1 Stratospheric Ozone: Key Scientific Questions
|
|
BOX 5.2 Greenhouse Gases: Key Scientific Questions
|
increased by a factor of two or more in this century, while stratospheric ozone concentrations have decreased over the past 20 years. In addition, there are preliminary indications that stratospheric H2O concentrations are currently on the rise.
The research strategy in the area of greenhouse gases is motivated by one central question: What will be the concentrations of greenhouse gases in the next century? Out of this main motivating question we have identified six critical scientific questions for investigation (see Box 5.2). These examples underscore yet again the mechanistic links between chemistry and the biosystem and between chemistry and issues of cloud/radiation feedback in the climate system.
The essential research activities that will be required to address these questions are outlined later. These activities are organized into three categories: (1) research related to the primary greenhouse gases that are emitted directly into the atmosphere; (2) research related to ozone, whose source is entirely photochemical; and (3) research activities related to H2O, whose sources are both surface emissions and photochemical. In general, these research categories focus first on enhancements of successful strategies that are currently under way and then progress to new strategies requiring technological developments and, in many
cases, the identification of new resources. Finally, it should be noted that many of these research activities are also relevant to other issues in atmospheric chemistry highlighted in this report. For example, investigations of the distributions and surface exchange rates of N2O, CH4, and the halogenated compounds, as well as O3, are clearly of interest in the study of stratospheric ozone and photochemical oxidants.
Photochemical Oxidants Imperative
Elevated levels of oxidants on urban and regional scales in the industrialized countries of the world are proving to be among the most intractable of air quality problems.14 To meet the information needs of society, the goals of atmospheric chemistry research in the next two decades must include more complete understanding of the processes determining the distribution and trends of photochemical oxidants and their precursors on urban, regional, and global scales. To achieve this understanding, four critical motivating scientific questions must be addressed in the coming decades (see Box 5.3).
|
BOX 5.3 Photochemical Oxidants: Key Scientific Questions
|
|
To successfully address these questions, it must be recognized that photochemical oxidants research is truly data poor and measurement limited. Significant progress in this area will require a commitment to acquire high-quality observational data that are global in coverage but with high enough spatial and
temporal resolution to reveal the important chemical and physical processes in the production, transport, and removal of photochemical oxidants. A research strategy that is both evolutionary and revolutionary will be required.
Atmospheric Aerosols and UV Radiation Imperative
Atmospheric aerosols have important impacts on human health, quality of life, and materials degradation. Another NRC (1992) report summarizes key aspects of this issue. Despite recent advances in appreciating the importance of atmospheric aerosols, our understanding of this critical class of atmospheric species is in its infancy. One notable reason for this failure is the complexity of aerosols. Compared to gases, aerosols have additional dimensions: an infinite number of sizes and a variable mixed composition. We are not able to fully comprehend the impacts of aerosols now, and we cannot make predictions about how the impacts will change in the future through human activities.
The important questions about atmospheric aerosols in the next decade concern their effects on climate, atmospheric chemistry, and human health and well-being (see Box 5.4). To answer these questions we must go far beyond our current state of knowledge of atmospheric aerosols. The essential elements of the research strategy that will be needed are outlined in the section on research imperatives later in this chapter. A more detailed discussion of many aspects of this strategy can be found in A Plan for a Research Program in Aerosol Forcing and Climate (NRC, 1992).
Toxics and Nutrients Imperative
Many of the atmosphere's naturally occurring components can have toxic and/or nutritive effects on the biosphere.16 Myriad toxic and nutritive substances in the atmosphere are significantly influenced by anthropogenic activities. While
|
BOX 5.4 Atmospheric Aerosols: Key Scientific Questions
|
|
BOX 5.5 Toxics and Nutrients: Key Scientific Questions
|
we are beginning to identify the more acute cases of atmospheric toxicity, such as benzene, vinyl chloride, PCBs, and chloroform, and overfertilization for key ecosystems, our understanding is far too limited to assess the present extent of these problems or to predict future ones.
The essential elements of a research strategy to address these questions (see Box 5.5) are outlined in the section on research imperatives. The hard lessons that have been learned over the past few decades are discussed in the next section. These lessons must be kept in mind when the research strategy is discussed.
LESSONS LEARNED
The lessons that emerged from the last four decades of the twentieth century hold the key to fundamental progress. Only by considering this experience can an effective strategy be designed to characterize the processes underlying the ozone/ climate system response to secular trends in chemical constituents, so that defensible predictions are possible. This section details both general research lessons and specific scientific lessons that should be applied.
Perhaps the most critical lesson is the realization that the stratosphere is severely undersampled, particularly from a mechanistic point of view. The Antarctic ozone hole emerged virtually unnoticed, although the removal of a major fraction of total ozone subsumed a significant fraction of our southern hemisphere. For more than two decades, from the early 1970s until the mid-1990s, nitrogen radicals were believed to dominate the destruction rate of ozone in the lower stratosphere of the Earth—a premise shown to be wrong in 1994. The central role of aerosols in the control of free stratospheric radical partitioning went unnoticed until 1992. Aerosols now constitute a dominant uncertainty in the response of the stratosphere to high-altitude aircraft, halogen emissions, volcanic eruptions, and other phenomena. While we know that ozone is eroding at midlatitudes at a rate that exceeds prediction, currently we
can only speculate about the cause. Speculation does not lead to effective public policy.
It is important to carefully distinguish between observations designed to determine long-term trends in key variables and observations designed to test specific hypotheses defining the mechanism or process that controls the system. Both are critically important to scientific progress, but they have, in a vast number of key cases, very different experimental requirements.
The character, availability, and cost of observational platforms together have been the key link between global change research and the nation's intellectual resources, related technical and scientific developments, educational opportunities, and the execution of effective public policy initiatives. For many pivotal questions the appropriate platform may be a small fast-response satellite. For others the platform must be able to pass through the atmosphere on a carefully prescribed trajectory that orthogonally traverses the meteorological fields (velocity, potential vorticity, potential temperature, etc.). The platform must be robust enough to meet takeoff and landing constraints. The integration of instruments with the platform must be straightforward. The platform must be easily deployable to remote locations. It must also be capable of reaching the required altitudes with adequate duration and payload capabilities to address the essential questions.
The verification of models by “spot checking” is another approach that can be useful, though it has limitations in effectively linking chemical and dynamical processes and dynamical and radiative processes. Models must be continuously tested, and innovative new approaches linking observations and models must be developed.
Fundamental but incorrect tenets of the field can survive for decades in the face of many observations.
The degradation of spatial resolution, poor signal-to-noise ratios, the missing of key species in a selected array of simultaneous observations, and the inability to access latitudes, altitudes, solar zenith angles, and seasons are problems that profoundly cripple datasets. The heart of this lesson is remembering the distinction between gathering data, on the one hand, and clearly answering specific questions on the other.
The large variability within and between datasets is an enemy of unambiguous interpretation unless the proper complement of observations is obtained simultaneously; then variability becomes a powerful ally for the establishment of cause and effect.
Good public policy emerges from unequivocal scientific results. Again, speculation is not an adequate foundation for public policy.
In addition to these more general lessons, we have learned several more specific and critical scientific lessons, which have emerged from major scientific transitions in the study of the chemistry of the atmosphere (see Box 5.6).
|
BOX 5.6 Key Science Lessons Learned
|
RESEARCH IMPERATIVES: PRIORITIES FOR OBSERVATIONS, MODELING, AND THEORY
We turn next to the question of defining a research strategy that addresses the primary Research Imperatives:
-
Stratospheric ozone and UV radiation. Define and predict secular trends in the intensity of UV exposure that the Earth receives. Document the concentrations and distributions of stratospheric ozone and the key chemical species that control its catalytic destruction and elucidate the coupling between chemistry, dynamics, and radiation in the stratosphere and upper troposphere.
-
Greenhouse gases. Determine the fluxes of greenhouse gases into and out of the Earth 's systems and the mechanisms responsible for the exchange and distribution between and within those systems. Expand global detection techniques to elucidate the processes that control the abundances and variability of atmospheric CO2, CH4, N2O, and upper-tropospheric/lowerstratospheric O3 and water vapor.
-
Photochemical oxidants. Develop the observational and computational tools and strategies that policy makers need to effectively manage ozone pollution and elucidate the processes that control and the relationships that exist among ozone precursor species, tropospheric ozone, and the oxidizing capacity of the atmosphere.
-
Atmospheric aerosols and UV/visible radiation. Document the chemical and physical properties of atmospheric aerosols and elucidate the chemical and physical processes that determine the size, concentration, and chemical characteristics of atmospheric aerosols.
-
Toxics and nutrients. Document the rates of chemical exchange between the atmosphere and ecosystems of critical economic and environmental import and elucidate the extent to which interactions between the atmosphere and biosphere are influenced by changing concentrations and depositions of harmful and beneficial compounds.
The research strategy recommended here addresses such questions and hypotheses, which have been selected in view of their intrinsic scientific merit, particularly regarding the Earth's ability to sustain life. The recommended research strategy is also based on a plan of attack that relies on a flexible array of experimental and theoretical analyses. The objective is to test the most important questions expeditiously and efficiently. The selected manifold of state-of-the-art techniques reflects the use of new technical developments and diagnostic calculation methods, along with recognition of the distinction between establishing secular trends and determining the fundamental processes responsible for global-scale
changes. Again, the research strategy also takes careful account of critical lessons from past scientific work.
Stratospheric Ozone and UV Radiation
Ultraviolet radiation reaching the Earth's surface is controlled first and foremost by the total overburden of ozone in the Earth's stratosphere and modified by aerosol loading in the lower troposphere. Our focus is thus primarily on ozone in the stratosphere, but we will also consider the question of aerosols in the final subsection covering this imperative. To begin, we examine secular trends in stratospheric ozone. This discussion underlines the critical role of stable long-term analyses of the altitude, latitude, longitude, and seasonal variations in ozone mixing ratios in the stratosphere. It also underlines the complementary but differing character of scientific analyses of secular trends in contrast to diagnoses of mechanisms. The latter mechanisms determine the chemical and physical changes that occur at all timescales on the global scale and are the focus of the second section. The third subject of discussion is the importance of tracers that establish the coordinate system within which all observations are interpreted. Finally, the principal contributions of fundamental laboratory studies are analyzed in the context of predicting changes in ultraviolet exposure of the Earth 's surface.
Critical considerations in measuring stratospheric ozone depletion include the uninterrupted observation of total ozone, the altitude dependence of ozone changes, and the geographic and seasonal patterns of those changes, as determined with high temporal resolution and accuracy, using a combination of intercalibrated instruments on space-based and ground-based systems as well as air-borne platforms. The fundamental nature of these research needs cannot be overemphasized in light of the problematic recent time gaps in our capability to monitor stratospheric ozone distributions from space.
Continuous cross-calibrated measurements of stratospheric ozone will document the extent of ozone loss and degree of ozone recovery if stratospheric halogen concentrations, water vapor, temperature, and other critical factors return to normal. These measurements will thus provide an essential gauge of the sufficiency of and/or world compliance with the international treaties devised to reverse the ozone depletion of the 1980s and 1990s.
Equally important, continuous measurements of stratospheric ozone will provide rapid warning of any unanticipated changes in the concentration and distribution of stratospheric ozone. The relationships between stratospheric ozone and various agents of chemical change are very complex and still poorly understood. As stratospheric chemical composition evolves under changing halogen loadings, the effects of perturbations —both natural and industrial—may vary unexpectedly. For example, we now know that volcanic eruptions into a chlorine-rich stratosphere can have a profoundly different effect on stratospheric ozone than they most likely had in the pre-CFC era. Similar phenomena may very well occur with other perturbations, such as those arising from aircraft emissions.
This analysis of secular trends has several key components. The first is the high accuracy analysis of long baseline trends in ozone total column measurements with spatial and temporal resolution. This tracking of decade to multidecade trends is a scientifically feasible goal, but it is not a pedestrian venture. It is also not an objective that can be attained without critical cross-checks from multiple independent techniques. The second component of analysis of secular trends is the need for high-accuracy analysis of the altitude dependence of stratospheric ozone changes. This problem is potentially much more intricate than the observation of total column ozone because of the need for high spatial resolution in the vertical, the need to tie observations to long-lived tracers, and the complications introduced by the injection of aerosols through volcanic eruptions.
Predicting Future Changes in UV Dosage: Establishing Cause and Effect in the Photochemical Cycles that Control Ozone Loss Rates
Accurately determining secular trends in the distribution of stratospheric ozone represents an essential arm of stratospheric ozone research in the coming decades. The need to understand potential changes in stratospheric chemistry in the face of changing sulfur, chlorine, bromine, N2O, methane, CO2, H2O, and other chemical loadings requires understanding the underlying mechanisms. This understanding in turn depends on mapping the distribution and variability of the species that determine the magnitude of ozone depletion and establishing a clear definition of the chemical reaction network that links these species. The total rate of stratospheric ozone destruction is governed by numerous catalytic destruction cycles, whose rates are limited by the abundances of specific free radical species, such as OH, HO2, ClO, BrO, and NO2, as well as atomic oxygen. A question fundamental to the scientific understanding of ozone depletion is: What rate-limiting steps actually dominate ozone loss as a function of altitude, latitude, and season? Of equal importance: How do the rates and relative roles of each of these loss processes change as hydrogen-, chlorine-, bromine-, and nitrogen-containing species are added to or removed from the stratosphere and with superimposed changes in reactive surface area of aerosols, water, and temperature?
Clearly, the answers to these questions require mapping the relevant radical concentrations from the troposphere to altitudes subsuming most of the ozone column. The case studies earlier in this chapter demonstrate this point. However, to define more fully the future stratospheric ozone responses to perturbations we know are in progress (e.g., sulfur from volcanic injections, NO x, aerosols and water from subsonic and supersonic aircraft, temperature changes triggered by decreases in ozone and increases in CO2 and H2O in the lower stratosphere, emissions of bromine and chlorine compounds), it is essential that we map the partial derivatives of the rate-limiting steps with respect to the variables on which the concentration depends, as well as the radical concentrations themselves. Figure 5.4 schematically illustrates the diagnostic power of this dual approach. In this case the loss rate of ozone from the catalytic cycles driven by
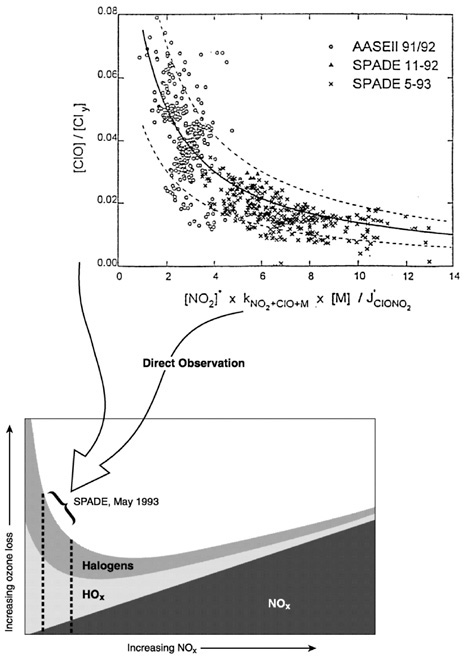
FIGURE 5.4 Schematic illustration of ozone loss rate as a function of NOx concentration. Because of the coupling that exists between the radical families, the response of the total ozone loss rate to changes in NOx is nonlinear. For example, at low NOx concentrations, ozone loss rate was found to be inversely correlated with NOx. Also shown are actual data obtained by the NASA ER-2 in the stratosphere, demonstrating that the specific slope of ozone loss is directly observable. SOURCE: Adapted from Wennberg et al. (1994). Courtesy of the American Association for the Advancement of Science.
HOx, halogen, and NOx radicals is illustrated as a function of NOx concentrations (from high-altitude aircraft or from radical repartitioning resulting from volcanic eruption), with the total ozone loss given by the sum of the individual contributions. Because of the couplings that exist between NOx and the other radical families, the response of ozone loss to a change in NOx concentrations turns out to be a complex function that depends on the individual gradients in each of the rate-limiting steps with respect to NOx. Because of the nature of these gradients, the total ozone loss is smallest at an intermediate NOx concentration. Similar effects undoubtedly are seen in the dependence of ozone destruction cycles on other radical species. The point here is that the response of stratospheric ozone to various changes is measured directly by these partial derivatives. Partial derivatives are observables of the system, and thus identification of this pattern is a significant breakthrough that redefines the relationship between measurements and models.
In general, because the interdependence of concentrations of chemical species that control ozone loss changes over various spatial and temporal scales, measurements of these species must extend to the full range of relevant conditions. Doing so will require the simultaneous deployment of critical instruments, development of new instruments, development and deployment of new measurement platforms that can cover relevant stratospheric regions, and measurements of the variations of each of the rate-limiting radicals (and its catalytic ozone loss rate) relative to each of the other critical species. In particular, current altitude limitations for piloted aircraft that are subsonic, and thus acceptable for in situ observations, are 20 km. The heart of the ozone layer, however, extends above these altitudes by a scale height. Since the required observations of the linking reactions, via the partial derivatives, demand simultaneous observations of tracers (N2O, CH4, CO2, SF6), reservoir species (HCl, ClONO2, H2O, BrONO2, HONO2), and radicals (ClO, BrO, OH, HO2, NO, NO2, O(3P)), these observations must be extended to altitudes of 25 km. Experience has shown that the required spatial resolution for these simultaneous observations must be equal to or better than 0.1 km. These requirements demand in situ observations of these species.
Predicting Future Changes in UV Dosage: The Coupling of Chemistry, Dynamics, and Radiation
The stratosphere is a coupled photochemical, dynamical, and radiative system in which ozone is exported from the high-altitude tropics to mid- and high latitudes along surfaces formed by constant mixing ratios of tracers such as N2O and CH4. The coherence of these tracer surfaces as a vertical coordinate is revealed by the tight correlations between these long-lived species and other reactive species. This regularity is both a dramatic and a simplifying feature that has led to important insights about the dynamics of the stratosphere and its role in defining the response of stratospheric ozone to chemical and physical perturbations. Air primarily enters the stratosphere in the cold inner tropics through a
process that desiccates the air and confines the upwelling flow to the middle stratosphere, as shown in Figure 5.3.
The subsequent exchange of air between this upward-moving tropical air mass and stratospheric midlatitudes apparently occurs on timescales of a few months and varies by season. However, this exchange is largely uncharacterized and is a potential source of large uncertainty in our ability to predict the response of the stratosphere to perturbations. Polar regimes are characterized by rapid cooling in the fall, with subsidence of many kilometers associated with the establishment of a strong polar jet. This action, in turn, restricts mixing with the midlatitudes, thus isolating the winter polar stratosphere from the rest of the stratosphere during the winter months, especially in the southern hemisphere. The restriction of exchange with the wintertime polar stratosphere plays a significant role in the annual dynamical cycle of the stratosphere and is an essential element in the formation of large regions of severe ozone loss over the polar regions.
Diagnosing the mechanisms that control patterns of exchange between the stratosphere and troposphere, the tropical and extratropical stratosphere, and the polar stratosphere is a prerequisite for quantifying the mechanisms for polar and midlatitude ozone loss. This circumstance gives rise to another critical research activity—to elucidate the coupling of chemistry, dynamics, and radiation in the stratosphere. Because of the tight relationships between air mass origin and tracer concentrations, clarification can best be accomplished with spatially resolved (0.1 km), highly accurate, in situ observations of N2O, CH4, SF6, CO2, CFC-11, CFC-12, O3, and NOy, and determination of the age of the air mass in which the measurements are made. Such measurements can be carried out using instruments based on the ground, piloted aircraft, and robotic aircraft and small satellites. Additionally, trajectory and three-dimensional models are required to interpret these observations and, in the process, to improve the models themselves.
Because of the sharp concentration gradients, study of the region from the upper troposphere to 30 km must rely heavily on in situ observations. At higher altitudes, where vertical and horizontal gradients begin to soften, small satellites will be critical. The altitude overlap of these two observational strategies must be enhanced, for it is essential to obtain these coupled observation sets with seasonal (~1 month) frequency for a number of annual cycles.
Predicting Future Changes in UV Dosage: Characterizing the Chemical Processes that Control Gas-Phase and Heterogeneous Reactions
Predicting changes in stratospheric ozone requires knowledge in a number of chemical physics research areas—areas of research in which we now lack sufficient understanding to predict stratospheric ozone and thus UV dosage. Understanding radical-molecule reactivity at the molecular level is essential; defining the temperature and pressure dependence of the homogeneous gas-phase cata-
lytic processes lies at the foundation of our understanding. It is also essential to understand the oxidation processes that carry reduced sulfur through to sulfuric acid, both in the open atmosphere and in the plumes from commercial aircraft. We must establish mechanistic insight into the processes that govern reaction pathways in the oxidation of organic species in the troposphere—particularly the lifetime and final product structures resulting from that oxidation sequence. We must develop molecular knowledge of which ternary sulfate, nitrate, and water mixtures form and grow particles in the atmosphere and of how this growth occurs at low temperatures and at the phase transition point, leading to frozen particles in the polar region and tropical tropopause regions.
Chemical transformations in the gas phase, or on or within condensed matter, that take place in the stratosphere ultimately determine the current and future composition of this region. The quantification and characterization of these processes through laboratory experiments (and in some cases computational techniques) provide the essential building blocks for interpreting, simulating, and predicting concentrations and changes in the stratosphere. Continued development and application of laboratory experiments and computational techniques to the study of stratospheric chemical processes are thus important components of any research strategy for the stratosphere. Especially critical in the coming decades will be investigation of heterogeneous processes, where our understanding lags far behind our knowledge of gas-phase homogeneous reactions.
Greenhouse Gases
Infrared active species absorb radiation from the Earth's surface and lower atmosphere and redirect part of this radiation back to the surface, increasing sea surface and land mass temperatures through the so-called greenhouse effect. Analysis of climate changes in response to secular trends in infrared species requires accurate knowledge of how the major infrared-active gases evolve over time and of the processes controlling the production and removal rates for those species in the atmosphere. These species include the primary infrared active molecules emitted directly into the stratosphere (e.g., CO2, CH4, N2O, CFCs) and secondary greenhouse gases produced in the atmosphere by photochemical processes (e.g., O3). Water vapor (H2O), the most important of the greenhouse gases, is unique in that it is both emitted into the atmosphere (via evapotranspiration) and produced photochemically in the stratosphere from the oxidation of CH4. Ozone is also unique in acting as both a greenhouse gas (by absorbing infrared radiation) and an important absorber of solar ultraviolet and visible radiation, as discussed earlier in the context of the stratospheric ozone and UV radiation research imperative.
Observed increases in concentrations of CO2, CH4, N2O, and CFCs provide one of the clearest manifestations of global change in the atmosphere. Historical trends in H2O and O3 have yet to be quantitatively characterized. However,
limited data suggest that tropospheric ozone concentrations may have increased by a factor of two or more in this century, while stratospheric ozone concentrations have decreased over the past 20 years. In addition, there are preliminary indications that stratospheric H2O concentrations are currently on the rise.
For the most part the changing concentrations in atmospheric greenhouse gases appear to be driven by human activities, in the forms of energy use, industrialization, land use changes, and agriculture. Increases result from both changes in world population and changes in use patterns as economic development expands. The fact that these drivers of global change are not likely to abate in the coming decades without significant political and economic intervention has given the scientific debate over infrared active species and their climatic impact a sense of urgency and intensity. Moreover, while significant advances in our understanding of the sources and sinks of greenhouse gases have been attained in recent years, we are far from having a reliable predictive capability for the future evolution of their concentrations. Thus, not only our ability to predict future climatic trends but also our ability to formulate effective policy options to mitigate or adapt to future climatic changes remain highly limited.
The basic research strategy for studying atmospheric greenhouse gases is motivated by one pivotal question: What will the greenhouse gas concentrations be through the course of the next century? Arising from this central concern, we have identified six critical scientific questions for investigation (see Box 5.7).
The research initiatives required to address these questions are both extensive and essential, but they can be classified into three basic categories: (1) research related to the primary infrared active species emitted directly into the atmosphere (as well as linked observations that distinguish sources such as the CO2-O2 linkage); (2) research related to ozone, whose source is entirely photochemical but whose distribution is strongly affected by atmospheric motion; and (3) research objectives related to H2O, whose sources are both surface emissions and photochemical.
In general, recommended research steps focus first on enhancements of successful strategies and then on new strategies that will require technological developments. Note that the research described here is directly relevant to other central issues in atmospheric chemistry identified in this report. For example, investigations of the distributions and surface exchange rates of N2O, CH4, and the halogenated compounds, as well as O3, are clearly relevant to the study of ultraviolet exposure and photochemical oxidants.
Primary Infrared Active Species
The primary greenhouse gases can be roughly divided into two categories: the biogenic species whose sources and sinks are closely linked to biospheric processes (i.e., CO2, CH4, N2O) and halogenated compounds (i.e., CFCs, HCFCs, SF6). For the most part, halogenated compounds come from industrial activities,
|
BOX 5.7 Critical Scientific Questions for Greenhouse Gases Research
|
providing a clear distinction between these compounds and biogenic greenhouse gases. However, there are notable exceptions: halogenated compounds such as CH3Cl and CH3 Br have important interactions with the biosphere and thus fall into both categories.
Maintaining Current Concentration Monitoring Networks
The most robust large-scale signature of the sources and sinks of greenhouse gases and their time dependence will be variations in the mixing ratios of CO2, CH4, and N2O and in their isotopic ratios. It is therefore essential that the current global monitoring networks for these parameters be maintained. For these data to be useful, high accuracy and precision will be required because the pertinent geochemical information is derived from small spatial and temporal variations.
In principle, estimates of the continental source of a “long-lived” trace gas can be obtained from measurements at a surface site that is subject to intermittent pollution events from a nearby continent. 17 For example, a pollution event on the west coast of Ireland is readily identified from trace gas concentration data; it appears as a temporal enhancement in the local concentration of a long-lived gas of industrial origin. Ratios of emissions of various halocarbons and N2O to CFCl3 were obtained from the covariance between each species and CFCl3 using this approach.18 If the magnitude of emissions of a reference gas is known and the sources are colocated, absolute source strengths of the other gases can be derived. Moreover, with a three-dimensional model that can reproduce synoptic scale pollution events, it should be possible to obtain perspective on the continental source strength of a gas without scaling to a reference gas.19
Thus, it appears that implementing a monitoring network to document changing greenhouse gas concentrations downwind of major source regions would provide critical data on the source strengths of these regions. Such information would prove highly valuable for constraining atmospheric budgets for these gases and could ultimately represent a means of verifying compliance with potential future international emissions agreements. However, the problem of defining the source strength for biogenic greenhouse gases using this method is somewhat more challenging than for CFCs, whose emissions are essentially constant through the year. Because the sources of biogenic gases vary with season, their background concentrations also vary seasonally. Thus, continuous measurements at both upwind and downwind sites will be required. The challenge will be to develop algorithms that can reliably unravel this information in a quantitative manner to define the background concentration and the excess over background caused by advection and pollutants from a given source region. An atmospheric transport model based on observed winds would play a key part in analyzing the data.
Developing a Systematic Method for Multiyear Flux Measurements and Vertical Profile Measurements over Continents
A predictive capability for infrared active species requires understanding how the surface exchange rates of these gases behave as a function of such factors as season, rainfall, and local, regional, and global climate variations. For the biogenic greenhouse gases this knowledge will require surface flux measurements, in concert with hydrological and climatic observations, over a variety of biomes and climate regimes over a multiyear period.20 These measurements will prove critical in establishing empirical relationships between climatic conditions and biospheric emission and uptakes rates, as well as the biological mechanisms responsible for these relationships.
Vertical profiles over the continents, over horizontal scales of 2,000 to 3,000 km, will be required to infer the relevant flux information for the biogenic green-
house gases. In addition, measurements from land surface sites are often difficult to interpret because of the effects of local sources and sinks. A critical advantage of profiles is that there is less sensitivity to details of vertical mixing. Profile sampling should be carried out throughout the year and at sufficient spatial density. The spatial scale is suggested by the current distribution of ecosystems and the spatial extent of major climate “anomalies,” such as the 1988 drought in the United States. For the North American continent, this would probably be several dozen sites. Consideration of the expected signal-to-noise ratio suggests that a feasible and economical strategy for the near future would be the collection of automated flask air samples aboard light aircraft at twice-weekly intervals.21 An advantage of such a system is that the samples could be analyzed for multiple species and isotopic ratios with tightly controlled calibration. Seasonal, and perhaps monthly, mass fluxes over the continent could be determined from such a system and related to variations in climate and analyzed in the context of three-dimensional climate and chemical transport models.
While multiyear flux measurements provide insight into the mechanisms responsible for gas exchange on scales of a few hundred kilometers, larger-scale studies are needed to establish the methodology and the validity of extrapolating this flux information to regional and global scales. This type of study, such as that planned for Brazil in 1998, represents a natural progression from the mesoscale studies carried out in the 1980s and early 1990s (e.g., the ABLE campaigns). It will require at least two years of ground experiments, two major aircraft campaigns, and a significant meteorology and transport modeling effort. These experiments would be greatly enhanced by the availability of advanced flux measurement technology and improved instrumentation. To address the global-scale flux/profile issue effectively, efficient use of robotic aircraft may be needed because of the combined requirement of simultaneous observations at high spatial resolution and the need for global and seasonal coverage.
Establishing a Consistent Approach to Measure the Flux of Infrared Active Molecules on the Global Scale in Remote Regions
The oceanic flux of the biogenic infrared active molecules represents a very important component of the atmospheric global budgets for these gases. Research must adequately characterize the process of air-sea gas exchange. Unfortunately, most air-sea exchange rates are not known to better than a factor of two and in some cases an order of magnitude. Direct measurements of fluxes over the oceans have not yet been successful in reducing this uncertainty because of shortcomings in current platforms and instrumentation. In principle, conventional eddy correlation methods can provide data of sufficient accuracy and precision, but this approach requires fast-response, highly precise velocity and species detection methods. For the long-lived trace gases the problem is compounded by the fact that the concentration gradients between atmosphere and ocean that must be
measured are a very small fraction of the total concentrations, and, as a result, the measured differences are often masked by much larger effects caused by the fluxes of water vapor and heat. This problem must be addressed by a new generation of instrument-platform combinations. A promising near-term approach is a variant of the eddy correlation method, namely, the conditional sampling method, which slowly accumulates samples from the upward-moving eddies in one container and samples from the downward eddies in another.22 After careful conditioning, the differences between the two containers can be measured with conventional slow-response instruments. For many of the questions about the mechanisms controlling global sources and sinks of infrared active gases, time-resolved full eddy correlation measurements are required.
Because of satellites' global coverage, they are an important element in this emerging strategy, particularly flexible small satellites that can implement new technical developments rapidly. Global databases gathered from such platforms would undoubtedly revolutionize our grasp of biogeochemical cycles, if these databases were intelligently linked to direct observations of flux—that is, strategically deployed ground-based and in situ observations of the infrared species. For public policy this approach may ultimately prove the only credible way to verify compliance with international emissions control agreements, if they were adopted. Requirements for such systems, for both accuracy (0.1 percent or better) and spatial resolution (0.5° or better with some vertically resolved information), are beyond currently available technology. High-precision remote sensing devices need to be developed and tested with in situ measurements.
Pilotless or robotic aircraft represent another emerging technology that could transform the study of greenhouse gases and be an important component in any systematic global approach to this measurement problem. Such aircraft could provide a platform for making near-continuous flux measurements over remote and inaccessible areas of the globe, such as the oceans, jungle, tundra, and icepack. To take advantage of this platform, however, lightweight instrumentation with fast response times and high accuracy must be developed. This instrumentation could be designed for in situ measurements at low altitudes within the boundary layer or for remote sensing measurements from high altitudes. These platforms will also provide an important link between broad-scale, detailed flux and concentration observations and a new generation of flexible small satellites.
Model Development
The need for model development to assess the long-term variability of biogenic greenhouse gases is severalfold. Improved global biological process models are critically needed to extrapolate from past intensive studies of limited domains in space and time to global and decadal space and timescales. The quality of such models is presently limited by the short duration of the field measurements on which they are based. Once based on longer-term field mea-
surements, such as a those described above, these global models can then be driven by such parameters as temperatures and moisture and in some cases by satellite observations of vegetation.
Improved atmospheric chemical transport models are needed to better elucidate the effects of changing surface sources and sinks on atmospheric concentrations. While atmospheric models on all scales need improvement, particular attention must be given to subgrid-scale transport processes, such as turbulent mixing of the atmospheric boundary layer, and both shallow and deep convective transport. At present, most models do not incorporate the effects of ecosystems on atmospheric dynamics, for example, via evapotranspiration. Transport models that use assimilated winds also need to be improved and used in tropospheric applications to trace gas budgets in order to incorporate the effects of interannual variability in transport; such models will be essential for regional flux studies. Ocean models are also needed to integrate sparse oceanic data, thus clarifying biogeochemical cycles in the oceans, and to provide regional estimates of trace gas exchanges between the oceans and atmosphere. Ultimately, coupled biospheric, oceanic, and atmospheric models that allow for the proper feedbacks must be developed to reach the goal of predicting trends in biogenic greenhouse gases.
Establishing a Consistent Strategy for Determining Secular Trends in Ozone as an Infrared Active Species in the Mid- to Upper Troposphere and Lower Stratosphere
Ozone has a critical role as absorber of ultraviolet radiation in the stratosphere and in the oxidant chemistry of the troposphere, but additionally its absorption feature at 9.6 µm makes it an effective greenhouse gas when present in the upper troposphere and lower stratosphere. Thus, it is essential to document trends in ozone in the upper troposphere and lower stratosphere and the causes of these trends.
In situ measurements of the vertical distribution of O3 are critical to characterizing long-term ozone trends in the upper troposphere and lower stratosphere; these measurements provide high vertical resolution and offer an independent check on remote sensing data. While there is an international ozone sonde program, the present set of ozone sonde stations does not provide a coherent or adequate program. The stations do not use the same techniques; not all maintain adequate calibration programs; and the frequency of measurements is too low at several stations. Some stations are located in sufficiently polluted locations that the quality of the tropospheric data may be compromised. Most important, the number of sites maintained under the current program is simply too few to provide a reliable global picture. A large number of sites is particularly critical in measuring upper-tropospheric O3 because of its relatively short lifetime and the spatial heterogeneity in the sources of the chemical precursors of tropospheric O3.
To make the current ozone sonde network adequate, many sites will need to
be upgraded. In addition, at least 10 new sites must be added, primarily over the tropical continents and oceans. The planned Network for Detection of Stratospheric Change will not be adequate for detection of trends in the troposphere because its measurements will be oriented toward obtaining stratospheric data and there are too few sites (about six).
Because of the labor-intensive nature of sonde observations and the difficulty of reaching remote observing sites, there is a critical need to develop a consistent pattern of observations with high accuracy and high spatial resolution, since ozone varies significantly on small spatial scales in the troposphere. These observations must also be made on wisely selected trajectories, based on detailed meteorological predictions for the vicinity of the observations. Robotic aircraft may provide a unique contribution to this problem because they aid in selected locations for sonde deployment, automation of operations, and reductions in cost.
While in situ measurements provide data with high vertical resolution, space-based measurements provide global coverage. For this reason, maintenance and enhancement of satellite measurements of the ozone vertical profile are critical. Measurements from the Stratospheric Aerosol and Gas Experiment (SAGE) II have provided valuable information on ozone trends above 17 km since 1984, with the exception of the period after the Pinatubo volcanic eruption, when data could not be obtained in the lower stratosphere. Launch of a new instrument while SAGE II is operational (e.g., SAGE III) would allow for overlap and lead to more reliably described trends in the future. Gaps in ozone observations have been a serious problem.
Unfortunately, while extremely useful for inferring ozone trends in the stratosphere, SAGE II does not yield data on ozone in the upper troposphere. Combining data from the Total Ozone Mapping Spectrometer and SAGE II has proved useful in inferring tropospheric nitrate and ozone column concentrations,23 but the lack of vertical resolution and gaps in the data record make this technique of limited use for tracking upper-tropospheric O3 trends. On the other hand, newly available remote sensing techniques could form the basis for such space-based measurements of tropospheric O3.
Mid- to Upper-Tropospheric Water Vapor: Understanding the Distribution of the Dominant Infrared Active Molecule in the Climate System
Water in its various phases constitutes the critical link between the chemical component of global change and the dynamics, radiation, and climate components. First, water in the vapor phase is the first-order source term for hydrogen radicals (augmented in the midtroposphere by hydrocarbon and peroxide photochemistry), which in turn determine the photochemical production rate for ozone in a given volume element in both the troposphere and
the stratosphere. Water in aerosol form provides the site for surface catalysis that repartitions the dominant chemical families in the troposphere and the stratosphere and alters the chemical composition (and thus the formation characteristics) of the aerosol particles themselves. As the temperature drops in the upper troposphere, particularly in the tropics and polar regions, the rate of chemical transformation increases exponentially, in some important cases by as much as three orders of magnitude over an interval of 10°C. The formation of cirrus, both visible and subvisible, is driven by the combination of water vapor density, temperature, pressure, organic aerosols, and sulfate/ nitrate loading. The role that cirrus formation plays in the climate system is a critical quantitative question but one for which little information exists. An understanding of water vapor in its phases has emerged as the main link between chemistry, radiation, dynamics, and climate. Progress in clarifying these relationships will depend on a strategic blend of in situ and remote observations that explore the links among sea surface temperature, convective drive, horizontal water vapor redistribution, and mechanisms controlling the ratio of dry subsidence regions and moist vertically ascending zones in the climate system. A consistent attack on this problem is needed, using a combination of sonde and aircraft observations with an array of chemical tracers and isotopes, together with innovative satellite observations.
For the purpose of initially determining secular trends in water, in situ measurements (using sondes with improved accuracy and more fully instrumented aircraft) offer the advantage of high vertical resolution and provide a test of existing satellite data.
Instruments such as SAGE II,24 the Halogen Occultation Experiment, and the Microwave Limb Sounder on the Upper Atmosphere Research Satellite have demonstrated that stratospheric water vapor can be measured from satellites with adequate precision to characterize temporal trends at some levels. Because of the global coverage that space-based platforms provide, continuous measurements using these techniques are critical for tracking the potential causes and effects of global climate change.
Compared to H2O concentrations typically found in the lower and midtroposphere, such concentrations in the upper troposphere are extremely small. (H2O concentrations from the surface to the tropopause typically decrease by about three orders of magnitude or more.) As a result, the technologies used for routine weather soundings do not have the accuracy or the sensitivity to reliably monitor H2 O in the upper troposphere. Moreover, current space-based platforms (e.g., SAGE II) are only able to quantify upper tropospheric H2O when aerosol loadings are low.25 For these reasons, new approaches must be developed for measuring H2O trends in the upper troposphere. Ideally, these approaches would be amenable to remote sensing from small satellite platforms, thus affording a strategy for obtaining global coverage at a reasonable cost.
Photochemical Oxidants
Global change arises as molecules, released largely at ground level, amplify or suppress oxidation patterns in the troposphere. These processes occur on urban, regional, and global scales, linked by a network of reactions. The reaction network engages homogeneous gas-phase catalysis, photolysis, heterogeneous transformations, and gas-to-particle conversion, all superimposed on a chaotic dynamical pattern. To map individual air mass motion in this pattern alone requires complex use of tracers.
Understanding of the distribution and trends in photochemical oxidants, as well as the network of reactions that dictate production and loss rates, is not yet in hand. Thus, there is relatively little ability to conduct rigorous integrated assessments of oxidant levels, health impacts, lifetimes of species released into the troposphere, and degradation pathways to terminal products—all central features of global change.
Elevated oxidants on urban and regional scales in industrialized countries are proving among the most intractable of air quality and global change problems. Related information is critical in making economic and health decisions. One goal of atmospheric chemistry research must be the better definition of mechanisms that determine the distribution and secular trends of these photochemical oxidants. This imperative has two principal objectives: (1) to understand the ability of the atmosphere to produce and destroy ozone, both now and in the decades ahead, and (2) more specifically, to understand the ability of the atmosphere to cleanse itself, via free radical oxidation, both now and in the future.
Free-Radical-Catalyzed Removal of Source Molecules: Hydroxyl Radicals and Other Oxidizers
One of nature's most important and ingenious systems for cracking the structure of a wide spectrum of molecules is via an initial oxidation step that is to some extent counterintuitive. This system depends in large part on a single chemical species—the OH radical that is present at a mole fraction of ~10-13, a tenth of a part per trillion. This mechanism places great importance on understanding the relationship between the hydroxyl concentration and its primary production and loss processes. Corresponding measurements present a serious combination of demands: detection thresholds in the parts-per-quadrillion range; high spatial resolution (0.1 km) such that establishing covariance with hypothesized source and sink molecules is possible; consistent observational methods that can cover the altitude range from the surface to the tropopause; observational ranges that can identify the major domains of tropospheric chemical character (e.g., the biomass-burning regions of the African/South American axis, the pristine marine environment of the western tropical Pacific region, the industrial regions of China,
western Europe, and the Americas); and simultaneous observations of tracers that uniquely identify the characteristic regions.
To what extent do other oxidants such as NOx, H2 O2, and halogen atoms determine oxidation rates? How do changes in ultraviolet exposure, temperature, convection drive, severe storms, aerosols, cloud cover, and rainfall patterns in the troposphere— on urban, regional, and global scales—shape oxidation patterns? Attacking this set of questions has proven both difficult and necessary. It requires a step forward in innovation —the deployment of lightweight rugged instruments at remote sites on aircraft (piloted and robotic), carried out in conjunction with strategic satellite observations and the development of innovative models.
Nitrogen Oxides and Ozone: Precursors and Oxidizers
Research on the medical impacts and biological responses of terrestrial systems to polluted air masses invariably turns to the question of ozone and the species responsible for its production. The fact that ozone is catalytically destroyed in the stratosphere by free radicals but catalytically produced in the troposphere by free radicals is both a cornerstone in our understanding and a challenge to global-scale studies. What are the predominant instigators of ozone production? What fraction of ozone precursors is emitted from natural (biogenic) sources and how do these sources change with meteorological conditions, land use, sulfates/nitrate loading, and other factors? What fraction of tropospheric ozone is imported from the stratosphere? What fraction is produced globally by lightning via NOx production? How do the specifics of urban pollution events affect the global source of tropospheric ozone? How is the NOx/NOy system partitioned in various regions of the troposphere? How does the mix of volatile organics, carbon monoxide, and nitrogen oxides affect the source strength of ozone?
Unfortunately, to date, photochemical oxidant research is truly data poor and measurement limited. Significant progress in this area will require the acquisition of high-quality observational datasets that are global in coverage as a whole but of high enough spatial and temporal resolution individually to clarify the chemical and physical processes in the production, transport, and removal of photochemical oxidants. Laboratory studies also play a central role in all aspects of atmospheric chemistry but particularly with regard to photochemical oxidants and in tropospheric chemistry. In short, a research strategy that is both evolutionary and revolutionary will be required—a strategy that is outlined below.
The key reactions relating to photochemical oxidants take place under a wide range of conditions: on salt spray in the presence of high fluxes of solar ultraviolet radiation; in biomass-burning plumes with a rich variety of organics, sulfur, aerosols, moisture, and other constituents; in clean marine regions nearly devoid of NOx but with very high levels of water vapor and ultraviolet; and in cold high-latitude regions, where heterogeneous reactions on micron-sized ice crystals cata-
lyze inorganic chlorine and bromine to free radical precursors. The intrinsic dynamic instability of the troposphere interacts with these regions on timescales of weeks, such that observational trajectories must be tactically selected to track volume elements in a Lagrangian frame. Moreover, chemical time constants linking species are typically 10 days or more. Thus, observations must be pursued in the context of local trajectory patterns defined by meteorological analysis.
As altitudes approaching the tropopause are investigated, there is increasing opportunity to use strategically designed satellite observations to simultaneously observe O3, H2O, and associated tracers such as N2 O, CH4, and CFC-11.
Instrument development and validation should aim at improving the sensitivity, specificity, and sampling rates of instruments needed to measure the compounds of interest throughout the atmosphere from the measurement platforms of choice. Development should focus on several areas:
-
simpler and more reliable instruments to be used in long-term monitoring;
-
miniaturization of instruments, to accommodate a wide array of measurements on airborne platforms;
-
continuous, fast-response instruments to be used for flux measurements and in airborne applications;
-
the use of spatially resolved long-path methods (e.g., LIDAR (light detection and ranging instrument)) that can be operated from airborne and mobile platforms to determine one- and two-dimensional distributions of compounds of interest over considerable distances from the emitter/detector units;
-
innovative aircraft platforms that can follow specific trajectories using fast-response instruments; and
-
innovative small satellites.
Integrated Field Campaigns: The Union of Chemical, Radiation, Dynamics, and Technology
Integrated field campaigns increase our understanding of fundamental atmospheric processes; elucidate the distributions, sources, and sinks of key species; and provide the data to evaluate air quality and chemical transport models. Any specific field campaign must be designed carefully with regard to the scientific questions it addresses and the uncertainties it must minimize. Atmospheric chemistry and meteorology must be integrated in the planning and deployment of air quality measurements and monitoring. The questions now before us will require multidisciplinary teams to consider chemistry, transport, and ecosystem feedbacks. Modeling tools adequate to depict or simulate these processes must be available to guide the planning of measurements as well as the interpretation of results. Moreover, an adequate fleet of research aircraft must be available to the atmospheric sciences community to make these studies feasible.
Carefully designed observations (with or without specific tracer compounds or suites of tracer compounds) can be used in conjunction with diagnostic and/or observation-based models to independently infer a number of phenomena: long-term trends and regional and seasonal variability in short-lived free radical species not amenable to continuous, spatially extensive monitoring; urban, regional, and global-scale emission inventories of ozone precursors; and the sensitivity of ozone and other photochemical oxidants to ozone precursor compounds. At the same time, the diagnostic and observation-based interpretation of field measurements will require adequate laboratory definition of the fundamental mechanisms involved in atmospheric processes.
The development and deployment of monitoring networks, along with analysis of resulting data, are needed to establish the chemical climatology of ozone, other photochemical oxidants, and their precursors. This climatology will help establish temporal and spatial trends and shorten the time required to unequivocally observe a response in ozone to changes in the concentration of its precursor compounds. These networks must include components capturing the roles that meteorology and dynamics play in the redistribution of airborne chemicals. Moreover, a comprehensive chemical climatology for photochemical oxidants must include data from the free troposphere as well as the surface. It is thus likely that these networks will require the use of balloon sondes, robotic aircraft, and spacebased platforms in conjunction with newly developed instrumentation based on small, lightweight, low-power technology.
Linking Scientific Results with Integrated Assessments
Integrated assessments draw from a wide range of scientific information and disciplines to provide more comprehensive guidance on scientific and technical matters to the decision-making community. The research strategy in atmospheric chemistry should support these assessments by providing analytical and modeling tools that can readily support these integrated assessments.
Atmospheric Aerosols and UV/Visible Radiation
Compelling evidence has emerged that aerosols play a central role in control of both the UV/visible exposure at the Earth's surface and the balance between albedo and retention of infrared radiation in the atmosphere. Aerosols demand specific attention.
Minute amounts of particulate matter in the stratosphere, along with increased levels of anthropogenic chlorine, are responsible for the Antarctic ozone hole and probably for the less dramatic but nevertheless significant global-scale ozone depletion. The atmospheric haze associated with industrial activity near major cities is now believed to partially mask the expected increase in surface temperature associated with greenhouse gas increases. Atmospheric aerosols also
|
BOX 5.8 Critical Scientific Questions for Atmospheric Aerosols
|
have important impacts on human health, quality of life, and materials degradation. Yet despite these recent advances in appreciating the importance of atmospheric aerosols, our understanding of these critical species is in its infancy. We do not comprehend the impacts of aerosols now and cannot now predict how those impacts will change in the future through human activities.
Several important questions must be addressed about the effects of atmospheric aerosols on climate, atmospheric chemistry, and human health and well-being (see Box 5.8).
To answer these questions, we must go far beyond our current state of knowledge of atmospheric aerosols. Further details of the needed research strategy can be found in A Plan for a Research Program on Aerosol Radiative Forcing and Climate Change (NRC 1996).
Stratospheric Aerosols
Limb scanning of solar extinction from satellites has been very successful in monitoring the global stratospheric sulfate layer and its spatial and temporal response to volcanic perturbations. Combined with in situ measurements of particle size distributions from balloons and stratospheric aircraft for validation, satellite multiwavelength extinction measurements have determined the surface areas of stratospheric aerosol particles with an accuracy adequate for heterogeneous chemical applications. New instruments with higher-wavelength resolution, possibly deployed on small satellites, will be the main monitoring tool for this component in the future.
Tropospheric Aerosols
The complexity of the tropospheric aerosol presents a considerably more difficult problem. Past in situ measurements focused on determining the size
distribution or chemical composition of aerosols at specific locations. Several new techniques under development are probing the chemical composition of single aerosol particles. However, these measurements are essentially point measurements, with little information about spatial and temporal variability. Moreover, methods for analyzing the composition of organic aerosols, arising, for example, from biomass burning or urban pollution, are incomplete. Clearly, new in situ instrumentation is needed to quantitatively document the complex chemical composition of tropospheric aerosols over their full size range in the various regions of the globe of interest for atmospheric chemistry.
Current remote sensing technology allows the measurement of gross tropospheric aerosol parameters over large spatial regions but not such features as composition or a complete size distribution. Technologies such as scanning polarimeters in the visible and near infrared appear able to retrieve tropospheric aerosol scattering characteristics from measurements of multispectral radiance and polarization by resolving aerosols from clouds and thus hold promise. Moreover, surface and airborne LIDAR can be used to map tropospheric aerosol backscatter and, combined with Raman measurement of scattering, can provide limited information about aerosol characteristics. Preliminary measurements with nadir-viewing LIDAR from the U.S. Space Shuttle show promise for obtaining detailed gross features of tropospheric aerosols on a global basis.
With the development of new instrumentation, monitoring networks will be needed to document the spatial and temporal trends in key aerosol characteristics. These characteristics include aerosol number, size, distribution, chemical (and toxic) composition, and radiative properties. Moreover the networks must be designed to address a variety of issues on urban, regional, and global scales. For example, on urban scales, monitoring networks are needed to uncover the characteristics of aerosols that lead to pulmonary health effects in humans. On regional scales they are needed to clarify the relationships between aerosol precursor species and visibility, and on global scales they are needed to better characterize quantitative relationships between aerosols and climatic effects.
The Strategy of Observations and Calculations
To predict how future human activities are likely to affect atmospheric aerosols and the related impacts on climate, chemistry, and human health, we must go beyond an aerosol climatology to a deeper understanding of the processes that control aerosol formation, transformation, and removal. This advance will require the design and implementation of intensive field programs that bring together chemical and physical aerosol measurements and precursor gas studies using surface, aircraft, and ship measurements.
In this regard two novel experimental strategies have emerged for resolving some of the most important questions concerning tropospheric aerosols and their effects. The first is the “closure experiment, ” in which an overdetermined set of
variables is measured. A subset of the observations and the relevant theories is then used to predict values for a “closure variable,” which is also measured independently. The result is a test of both measurements and theory and an opportunity to evaluate the quality of our understanding. With the instrumentation now available, closure experiments can be performed on aerosol number concentration (using a variety of sizing instruments), mass (based on measurements of relevant inorganic and organic species), radiative properties (using chemical composition, relative humidity, and Mie theory), and the integrated column effect of aerosols on short- and long-wave radiation. Closure experiments on aerosol mass can help answer questions about chemical composition, since missing species will make closure impossible. Theories about the impacts of aerosols on radiative climate forcing can also be tested by local and column closure experiments. Most of the aerosol experiments planned for the next decade depend heavily on this strategy because it offers a rigorous test of both measurements and the process models on which more comprehensive models depend.
The other new observational strategy is to observe the evolution of aerosols and their precursor gases in a Lagrangian reference frame. The idea of Lagrangian experiments is not new, and variations on this theme have been used occasionally. Recently, however, there has been considerable work on tagging air masses with balloons and chemical tracers, so that aircraft carrying large suites of instruments can revisit the air mass over a period of days to observe changes with time. Although these experiments cannot eliminate the effects of dispersion and vertical mixing on concentrations, with ample dynamical measurements, they make it possible to sort out the chemical and physical processes that cause changes in aerosols. These processes include gas-to-particle conversion, chemical transformations, wet and dry deposition, entrapment of air from other strata, and mixing through the sides of the “air mass” (dispersion). These experiments tend to be complex and expensive (at least one ship and one or two aircraft are required), but they offer the potential to test the aerosol models that now exist and that will be developed from future laboratory work and other process studies.
The overall strategic goal for the next two decades should be a predictive model to calculate atmospheric temperature and chemical species concentration fields and from that information to derive new aerosol particle formation rates and predict the chemical content and size distribution of the aerosol fields. Because current atmospheric models generally impose rather than predict aerosol distributions, significantly more sophistication will be needed in future models to represent precursor gas and gas/particle kinetics, nucleation and agglomeration kinetics, and vapor/particle interactions. One way to stimulate the needed improvements in aerosol modeling is to encourage the modeling community to participate directly in the planning, execution, and data analysis for the strategic field measurement programs described above.
Furthermore, predictive aerosol models will require currently unavailable quantitative mechanistic and kinetic input data describing a large number of
heterogeneous growth, nucleation, agglomeration, and accommodation/evaporation processes. These quantitative input data will have to come from a vigorous laboratory program in heterogeneous kinetics and aerosol microphysics.
Toxics and Nutrients
The atmosphere and biosphere are fundamentally coupled through the exchange of gases and aerosols. Ecological systems, including those of economic import (e.g., those dedicated to agriculture and forestry), can be profoundly affected by the wet and dry deposition of both toxic and nutritive atmospheric substances. While many of the atmosphere 's naturally occurring components can have toxic and/or nutritive effects on the biosphere, there are myriad toxic and nutritive substances in the atmosphere that are significantly shaped by industrial and agricultural activities. Moreover, while we are beginning to identify more acute cases of atmospheric toxicity and overfertilization for critical ecosystems, our understanding is far too limited to assess the extent of these problems now and to predict future ones.
In its most general form the motivating scientific question for the study of toxics and nutrients is: How are interactions between the atmosphere and biosphere influenced by the changing atmospheric concentrations of these substances and by the deposition of harmful and beneficial compounds? More specifically, from the viewpoint of atmospheric chemistry, this question can be posed as: What are the rates at which biologically important atmospheric trace species are transferred from the atmosphere to terrestrial and marine ecosystems through dry and wet deposition? The essential elements of a research strategy to address this question are outlined below.
Toxic and Nutrient Impacts: The Measurement of Deposition Fluxes
Many of the key issues for toxics and nutrients cannot be satisfactorily answered yet because we lack methods to measure deposition fluxes on appropriate spatial and temporal scales. This problem is most severe for dry deposition, where technologies for reliably measuring many of the most biologically important fluxes do not yet exist. Adequate support for technique development in this area is thus a critical need; relaxed eddy accumulation, eddy correlation, and gradient methods offer particular promise.
In the case of wet deposition, reliable techniques have been developed in principle, but serious questions remain about sampling representativeness and contamination. The problem is most acute for measuring wet deposition fluxes over the ocean, where it is virtually impossible to collect uncontaminated rain samples from a buoy in midocean, while samples from shipboard platforms are necessarily intermittent. Present marine deposition estimates, often the result of comparing model calculations with a very small set of shipboard and island
observations, are typically subject to uncertainties of a factor of three or more. The development of new techniques for more representative determination of wet as well as dry deposition fluxes, perhaps from a low-flying airborne platform, must therefore also be considered a high priority.
In some instances, such as in high-altitude forests and foggy regions, the deposition of cloud droplets may be the primary avenue by which toxics and nutrients are delivered to the Earth's surface.26 It is extremely difficult to measure such fluxes because the droplets are so transient that their flux is easily altered by the presence of measuring devices. Thus, new methodologies need to be developed to assess the importance of droplet deposition and to provide reliable flux measurements.
In the recent past, deposition monitoring networks have proven useful in determining the ecological impacts of atmospheric deposition (e.g., the National Acid Deposition Program/National Trends Network). However, these networks have mostly been limited to monitoring the deposition of a specific chemical or class of compounds (e.g., acid deposition, ozone). For this reason these networks have provided very limited information on the stresses and benefits experienced by an ecosystem from atmospheric deposition and thus on the long-term effects of this deposition as well. With the development of new deposition measurement techniques, it should be possible to design more comprehensive atmospheric deposition/exposure monitoring networks. Implementation of these networks for key ecosystems would provide a long-term record of atmospheric deposition; with colocated ecological monitoring, this record would no doubt prove useful in establishing causal relationships between atmospheric deposition and ecosystems' vitality and succession.
Toxics and Nutrients: Processes and Mechanisms Leading to Deposition
Even with reliable and fully evaluated deposition measurement techniques, it will never be possible to measure dry and wet fluxes for all species of interest over all ecosystems of interest, over all time. Consequently, process-oriented field studies, which make observations of fluxes under carefully selected ranges of conditions, must be undertaken to identify the factors that control fluxes. With these factors identified, algorithms and parameterizations describing deposition fluxes could be developed, tested by further observations, and incorporated into regional and global atmospheric chemistry models, as well as integrated atmospheric/biospheric response models.
NOTES
1. NRC (1992).
2. Farman et al. (1985).
3. Anderson et al. (1991).
4. Brune et al. (1991).
5. Weinheimer et al. (1998).
6. Johnson (1971).
7. Climate Impact Assessment Program (1975).
8. Fahey et al. (1995).
9. Johnson (1971).
10. Wennberg et al. (1994).
11. Fahey et al. (1995), Hanisco et al. (1997).
12. See review by Holton et al. (1995).
13. Plumb and Ko (1992).
14. NRC (1992).
15. Ibid.
16. Seinfeld and Pandis (1998).
17. Prather (1985, 1986).
18. Ibid.
19. Prather et al. (1987).
20. E.g., Baldocci et al. (1996).
21. Tans et al. (1996).
22. Businger and Oncley (1990).
23. Fishman et al. (1990).
24. McCormick (1993).
25. Ibid., Rind et al. (1993).
26. Vong et al. (1991).
REFERENCES AND BIBLIOGRAPHY
Anderson, J.G., D.W. Toohey, and W.H. Brune. 1991. Free radicals within the Antarctic vortex: The role of CFCs in Antarctic ozone loss. Science 251:39-46.
Baldocchi, D., R. Valentini, S. Running, W. Oechel, and R. Dahlman. 1996. Strategies for measuring and modeling carbon-dioxide and water-vapor fluxes over terrestrial ecosystems. Global Change Biology 2(2):159-168.
Baughcum, S.L., S.C. Henderson, and D.J. Sutkus, 1998. Scheduled civil aircraft emission inventories projected for 2015: Database development and aerosols. NASA CR-1998-207638. National Aeronautics and Space Administration, Washington, D.C.
Brune, W.H., J.G. Anderson, D.W. Toohey, D.W. Fahey, S.R. Kawa, R.L. Jones, D.S. McKenna, and L.R. Poole. 1991. The potential of ozone depletion in the Arctic polar stratosphere Science 252:1260.
Businger, J.A., and S.P. Oncley. 1990. Flux measurement with conditional sampling. Journal of Atmospheric and Oceanic Technology 7(2):349-352.
California Air Resources Board. 1989. Information on substances for review as toxic air contaminants. Report No. ARB/SSD/89-01. California Air Resources Board, Sacramento.
Chameides, W.L., R.W. Lindsay, J. Richardson, and C.S. Kiang. 1988. The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science 241:1473.
Charlson, R.J., and J. Heintzenberg, eds. 1995. Aerosol forcing of climate. In Report of the Dahlem Workshop on Aerosol Forcing, Berlin, April 24-29. Wiley, Chichester, U.K.
Climate Impact Assessment Program (CIAP). 1975. DOT-TST-75-51. U.S. Department of Transportation, Washington, D.C.
Crutzen, P.J. 1995. Ozone in the troposphere. In Composition, Chemistry, and Climate of the Atmosphere, H.B. Singh, ed. Van Nostrand Reinhold, New York
Fahey, D.W., E.R. Keim, K.A. Boering, C.A. Brook, J.C. Wilson, H.H. Jonsson, S. Anthony, T.F. Hanisco, P.O. Wennberg, R.C. Miakelye, R.J. Salawitch, N. Louisnard, E.L. Woodbridge, R.S. Gao, S.G. Donnelly, R.C. Wamsley, L.A. Delnegro, S. Solomon, B.C. Daube, S.C. Wofsy, C.R. Webster, R.D. May, K.K. Kelly, M. Loewenstein, J.R. Podolske, and K.R. Chan. 1995. Emission Measurements of the Concorde supersonic aircraft in the lower stratosphere. Science 270:(5233)70-74.
Farman, J.C., B.G. Galdiner, and J.D. Shanklin. 1985. Large losses of total ozone in Antarctica reveal seasonal C1Ox/NOx interaction. Nature 315:207.
Fishman, J., C.E. Watson, J.C. Larsen, and J.A. Logan. 1990. Distribution of tropospheric ozone determined from satellite data Journal of Geophysical Research 95:3599-3617.
Friedl, R.R. ed. 1997. Atmospheric effects of subsonic aircraft: Interim assessment report of the advanced subsonic technology program. NASA-RP-1400. National Aeronautics and Space Administration, Washington, D.C.
Hanisco, T.F., P.O. Wennberg, R.C. Cohen, J.G. Anderson, D.W. Fahey, E.R. Keim, R.S. Gao, R.C. Wamsley, S.G. Donnelly, L.A. Del Negro, R.J. Salawitch, K. Kelly, and M.H. Proffitt. 1997. The role of HOx in super- and subsonic aircraft exhaust plumes. Geophysical Research Letters 24(1):65-68.
Holton, J.R., P.N. Haynes, M.E. McIntyre, A.R. Douglass, R.B. Rood, and L. Pfister. 1995. Stratosphere-troposphere exchange. Reviews in Geophysics 33:403.
Johnson, H.S. 1971. Reduction of stratospheric ozone by nitrogen oxide catalysts from supersonic transport exhaust. Science 173:517.
Keeling, C.D. 1995. Halogens in the atmospheric environment. In Composition, Chemistry, and Climate of the Atmosphere, H.B. Singh, ed. Van Nostrand Reinhold, New York.
Logan, J.A. 1994. Trends in the vertical distribution of ozone: An analysis of ozonesonde data. Journal of Geophysical Research 99:25,553-25,585.
McCormick, M.P. 1993. Annual variations of water vapor in the stratosphere and upper troposphere observed by the Stratospheric Aerosol and Gas Experiment II. Journal of Geophysical Research 98:4867-4874.
National Research Council. 1992. Rethinking the Ozone Problem in Urban and Regional Air Pollution. National Academy Press, Washington, D.C.
National Research Council. 1996. A Plan for a Research Program on Aerosol Radiative Forcing and Climate Change. National Academy Press, Washington, D.C.
National Research Council. 1998. Decade- to Century-Scale Climate Variability and Change: A Science Strategy. National Academy Press, Washington, D.C.
Plumb, R.A. 1996. A “tropical pipe” model of stratospheric transport. Journal of Geophysical Research 101:39, 57.
Plumb, R.A., and M.K.W. Ko. 1992. Interrelationships between mixing ratios of long-lived stratospheric constituents. Journal of Geophysical Research 97:10, 145.
Prather, M.J. 1985. Continental sources of halocarbons and nitrous oxide. Nature 317: 221-225.
Prather, M.J. 1986. European sources of halocarbons and nitrous oxide: Update. Journal of Atmospheric Chemistry 6:375-406.
Rind, D., E-W. Chiou, W. Chu, J. Larsen, S. Oltmans, J. Lerner, M.P. McCormick, and L. McMasters. 1993. Overview of the SAGE II water vapor observations: Method, validation and data characteristics. Journal of Geophysical Research 98:4835-4856.
Prather, M., M. McElroy, S. Wofsy, G. Russell, and D. Rind. 1987. Chemistry of the global troposphere: Fluorocarbons as tracers of air motion. Journal of Geophysical Research 92:6579-6613.
Seinfeld, J.H., and S.N. Pandis. 1998. Atmospheric Chemistry and Physics. John Wiley & Sons, New York.
Tans, P.P., P.S. Bakwin, and D.W. Guenther. 1996. A feasible global carbon cycle observing system: A plan to decipher oday's carbon cycle based on observations. Global Change Biology 2:309-318.
Vong, R.J, J.T. Sigmon, and S.F. Mueller. 1991. Cloud water deposition to Appalachian forests. Environmental Science and Technology 26:1014-1021.
Weinheimer, A.J., D.D. Montzka, T.L. Campos, J.G. Walega, B.A. Ridley, S.G. Donnelly, E.R. Keim, L.A. Del Negro, M.H. Proffitt, J.J. Margitan, K.A. Boering, A.E. Anderws, B.C. Daube, S.C. Wofsy, B.E. Anderson, J.E. Collins, G.W. Sachse, S.A. Vay, J.W. Elkins, P.R. Wamsley, E.L. Atlas, F. Flcke, S. Schauffler, C.R. Webster, R.E. May, M. Loewenstein, J.R. Podolske, T.P. Bui, K.R. Chan, S.W. Bowen, M.R. Schoeberl, L.R. Lait, and P.A. Newman. 1998. Comparison between DC-8 and ER-2 species measurements in the tropical middle troposphere: NOy, O3, CO2, CH4, and N2O. Journal of Geophysical Research—Atmospheres 103:22,807-22,096.
Wennberg, P.O., R.C. Cohen, R.M. Stimpfle, J.P. Koplow, J.G. Anderson, R.J. Salawitch, D.W. Fahey, E.L.Woodbridge, E.R. Keim, R.S. Gao, C.R. Webster, R.D. May, D.W. Toohey, L.M. Avallone, M.H. Proffitt, M. Loewenstein, J.R. Podolske, K.R. Chan, and S.C. Wofsy. 1994. Removal of stratospheric O3 by radicals: In situ measurements of OH, HO2, NO, NO2, CIO, and BrO. Science 266:398-404.














































