10
Modeling
INTRODUCTION
The possibility of major changes in the global environment due to human influences presents a difficult challenge to the scientific research community: to relate causes and effects and to project the course of change on a global scale and for many decades. Approaches based purely on observations are inadequate for prediction. Such rapid externally forced changes have no precedent. Moreover, the response times of many parts of the Earth system are slow, and there is a great deal of variability from place to place. Scattered observations over short time periods are unlikely to reveal clear and useful trends. Furthermore, many important processes—such as those that occur in the soil and in the interior of the ocean—cannot be measured directly or adequately over large areas. We therefore need models—numerical representations of the Earth system—to express our understanding of the many components of the system, how they interact, how they respond to perturbations, and how they feed back to provide dynamical controls on overall system behavior. It is thus evident that the study of global environmental changes—their causes, their impacts, and strategies for mitigation—inescapably requires models that encompass the mutual interactions of the principal components of the Earth system.
There is, however, a fundamental difficulty in that many environmental issues require prediction on relatively long timescales and require integration over large spatial scales. Extrapolations from models over such long time spans are prone to error as small discrepancies from reality compound; moreover, there remain open and complex issues regarding downscaling. Hence, research-quality observational datasets that span significant temporal and spatial scales are needed so that models can be refined, validated, or perhaps rejected. Such data must be
adequate in temporal and spatial coverage, in parameters measured, and in precision to permit meaningful validation or rejection of models. It is equally important that models be designed to permit confrontation with the real world through observations and that they be tested sufficiently and explored, including creating ensemble runs under differing conditions.
Over the past decade there has been remarkable progress in modeling, not only in simulating the principal individual subsystems but also in treating key linkages such as those between the ocean and atmosphere. This record of progress within the U.S. Global Change Research Program (USGCRP) makes it reasonable to expect that within the next 10 years of the USGCRP the scientific community will develop fully coupled dynamical (prognostic) models of the full Earth system (see Figure 10.11) that can be used on multidecadal timescales and at spatial scales relevant to important policy formulation and impact assessment. Such models exist in rudimentary form today. Future models will advance in completeness, sophistication, and proven predictive capability. The key will be to demonstrate some degree of prognostic skill in these future coupled models of the Earth system.
This development process will not be isolated from the needs of policy and decision making. Some of these Earth system models will be integrated into more encompassing models that link human and nonhuman processes or will be employed in various analytical or deliberative processes to inform decisions. Providing useful insights to inform decision making on global change will require dynamic representations of complex possible cause-effect-cause patterns linking human and nonhuman components of the Earth system. To develop and validate such models, observations of the Earth system must include data on human impacts from, and contributions and responses to, global change. At present, human influences generally are treated only through emission scenarios that provide external forcings to the Earth system. In future comprehensive models, human activities will interact with the dynamics of physical, chemical, and biological subsystems through a diverse set of contributing activities, impacts, feedbacks, and responses.
The focus of this chapter is on the path for realizing and evaluating a suite of such Earth system models. It should be recognized at the outset that the multi-decadal timescale places important constraints and demands on the character of such models. The most important constraint is that models must confront the ever-expanding (though still inadequate) set of time series data, both in situ and remote. The canonical example of the extraordinary value of time series information is the Keeling Record, the daily measured atmospheric concentration of carbon dioxide from Mauna Loa (see Figure 2.10 in Chapter 2).a The importance
|
a |
It is worthwhile to note that obtaining this unique record was threatened more than once by budget cuts and shortsighted federal managers. |
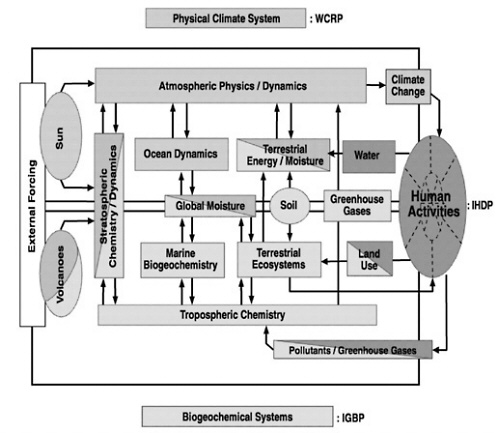
FIGURE 10.1 Conceptual model of the Earth system. SOURCE: Adapted from NASA (1986).
of this record flows from several aspects: (a) its scientific quality in terms of accuracy and precision, (b) its temporal quality in terms of resolution and duration, (c) the importance of the parameter measured (atmospheric CO2), and (d) the site (remote and well positioned for a global measure). The Keeling Record set a standard that subsequent measurements have sought to emulate. Moreover, it demonstrates the value of measurements taken to determine the state of a system rather than to test a specific hypothesis.b
The long temporal scale also demands inclusion of the biosphere and other coupling across critical interfaces. Over timescales of decades and more, the biosphere may be expected to respond dynamically to changes in many compo-
|
b |
This report emphasizes the latter, but the importance of the former must not be overlooked. See Chapter 8. |
nents of the Earth system. More broadly, if we are to understandboth the function of living ecosystems and their effects on the environment, we must have a better grasp of the controls and distribution of biological activity in the context of the overall Earth system, including the actions of humans. While necessary, observations will hardly be sufficient to understand the present and to predict the future role of ecosystems in this global context. Consequently, in this context, developing more realistic models that include successional dynamics and migration patterns of vegetation will be increasingly important in the coming decade. In sum, interactions among components over these longer timescales are likely to be as important as processes within each. Models must therefore deal with interactions between terrestrial ecosystems and the atmosphere, physical and dynamic interactions between the ocean and the atmosphere, the chemistry and physics of the atmosphere and ocean themselves, the land-ocean interface, and even the challenge of incorporating the human component. Each of these heterogeneous components and each of the diverse interfaces between them pose particular demands on research and model development.
Models of the fluid subsystems, the atmosphere and the ocean, have been developed almost in parallel with the advance of computational capacity. On the other hand, models of the terrestrial and marine biosphere have been paced by a shortage of observations at adequate time- and space scales and by the slow development of a new community of scholars willing to confront the biological system at large spatial scales. Modeling the role of humans in the Earth system has been controversial from the outset. Computational consideration of the global role of humans dates back, in part, to the provocative early system dynamics studies sponsored by the Club of Rome.3 These models were criticized for their simplistic assumptions about complex human behavior; their inadequate treatment of market forces; and their lack of explicit treatment of physical, chemical, and biological processes. However, they did awaken many to the possibility for quantitative simulation of complex systems beyond econometrics, and they contributed toward convincing the policy community of the importance of taking a system view, including explicit consideration of feedback loops and environmental constraints.
Looking more closely, we find that computer-based atmospheric models were first developed in the 1940s for weather forecasting—that is, to predict the near-term physical behavior of the atmosphere. In the subsequent development, there has been a natural branching on temporal scales: in parallel with the continuing refinement of weather forecasting models with increased skill, models that treat the longer-term dynamics inherent in climate studies have been advanced. In the process certain boundary conditions become incorporated as interactive components of the models; this is often the case where the increase in temporal scales logically forces “annexation” of what were initially external conditions (sea surface temperature is a good example). More recently, chemical processes are being included in transport codes that had their origin in weather and climate studies, so that today quite elaborate models are available to study the physical and chemical behavior of the atmosphere.
|
BOX 10.1 In the fall of 1994, the interagency Subcommittee on Global Change Research arranged for the special Forum on Global Change Modeling to provide an indication of the state of current progress in improving understanding of global change and to provide direction for future research. This forum served as a means of bringing together a representative set of scientists to develop a consensus statement on the credibility of global model estimates of future climatic change. The charge to those attending the forum and to those who submitted written comments was to develop a brief statement on the credibility of projections of climate change provided by general circulation models (GCMs) as background for potential interpretation of model results in the context of developing and considering national policy options. The focus of the forum was specifically on the climate aspects of the entire global change issue—thus not on the emission scenarios, the consequences of change to ecosystems and natural resource systems, or the socioeconomic implications and potential for responses. Still the results of the forum are of significant value to this chapter. The forum identified a number of areas where sustained or intensified research efforts would bring important gains in understanding and predictive capabilities. As an overarching statement it was noted that “while progress is clear as a result of ongoing research efforts and important steps can be taken over the coming decade that will bring new insights, significant reductions of the uncertainties in projecting changes and trends in the climate will require sustained efforts that are very likely to require a decade or more.” “Progress will require significant effort because the problems are complex, because improvements in model parameterizations will require a sustained and long-term program of research and observations, and because the records of past changes and influences require careful reconstructions to make them more complete and more useful. Although progress may be modest, there are a number of processes and feedbacks on which research must be sustained because of the large leverage to be gained from improved understanding. These processes and feedbacks include:
|
Development of models for general circulation of the ocean started slightly later but has proceeded in a manner similar to that for the atmospheric models. Rather elaborate models that deal with the physics of the oceans are now available, and, as the preceding paragraph implied, ocean models have been linked to models of the atmospheric system. Within ocean models the inclusion of geochemical and biological interactions has begun, with a focus on the carbon cycle. Since the late 1960s, the geochemical aspects of the carbon cycle have been included in low-dimensional box models.4 More recently, including the carbon-alkalinity system in general circulation models has simply been a question of allocation of computing resources. Modeling of the biological system, however, has been more challenging, and it has only been of late that primitive ecosystem models have been incorporated into global general circulation ocean models.5 Even though progress has been significant, much remains to be done. Coupling difficulties remain between the ocean and the atmosphere (though the worrisome issue of flux correction is beginning to be resolved or at least better understoodc). Fully eddy-resolving models with chemistry and biology need to be tested and validated in a transient mode. Finally, the prognostic aspects of marine ecosystems, including nutrient dynamics, need greater attention at basin and global scales.
Model development for the ocean and the atmosphere has had a fundamental theoretical advantage: it is based on the firmly established hydrodynamic equations. For example, the geostrophic constraint is particularly valuable. There is less constraint, however, on the dynamics of the global energy and water cycles, and at present there is far less theoretical basis for a “first principles” development of the dynamical behavior of the terrestrial system. We therefore need to develop a fundamental methodology to describe this very heterogeneous and complex system. For the moment it is necessary to rely quite heavily on parameterizations and empirical relationships. Such reliance is data intensive, and hence independent validation of terrestrial system models is problematical. Returning to the atmospheric models, which as noted are the most advanced dynamically, key processes like cloud formation remain too cloaked in parameterization.
Despite the difficulties that face modelers of terrestrial ecosystems, a coordinated strategy has been developed over the past five years to improve estimates of terrestrial primary productivity and respiration by means of measurement and modeling (see Box 10.1 and Chapter 2).6
For terrestrial ecosystems at the global scale, there has been a focus on the carbon cycle. This reflects demands on the science and advances in the theoretical foundation of the biogeochemical dynamics of terrestrial systems (at least under current conditions), and in this setting the strategy has begun to yield dividends. Several independent global models at mesospatial scales (roughly 50-
|
c |
This topic and others are discussed more fully in subsequent sections. |
|
BOX 10.2 Global scales, The Global Analysis, Interpretation, and Modeling (GAIM) task force of the International Geosphere-Biosphere Program (IGBP) initiated an international model intercomparison, carried out through two workshops hosted in June 1994 and July 1995 at the Potsdam Institut für Klimatologie (PIK), in Potsdam, Germany. The purpose of the Potsdam workshops was to initiate and support a series of model intercomparisons by the various modeling teams that are currently modeling the terrestrial biosphere at the global scale. More than 15 models and modeling teams have participated in the intercomparison. One, but not the only, focus in the intercomparison was NPP, which is central to models of the global carbon cycle. There are significant differences in the calculation of NPP between current global biosphere models, and a particular focus of Potsdam ‘95 was to compare model parameters and outputs using standard input datasets to determine patterns and hopefully the causes of the variability. A fundamental problem in assessing the results of terrestrial ecosystem models, which are used to provide NPP intercomparisons, is a lack of good validation data.7 Continental scales. The Vegetation/Ecosystem Modeling and Analysis Project (VEMAP) is comparing models of vegetation distribution, biogeochemistry, and biogeography for the conterminous United States under current and GCM-simulated future climates. In addition to changes in climate, the models are tested in response to changes in the chemistry of the atmosphere, in particular, to changes in the CO2 concentration and to changes in nitrogen deposition. VEMAP is also conducting factorial experiments under different forcings and thereby setting the stage to tests under multiple stresses.8 |
km grids) now exist, and others are in various stages of development. With the one-half degree gridscale, it is now possible to investigate the magnitude and geographic distribution of primary productivity on a global scale by a combination of monitoring by remote sensing and modeling of the biogeochemical aspects of terrestrial ecosystems. These models range in complexity from fairly simple regressions between key climatic variables and biological production to quasi-mechanistic models that attempt to simulate the biophysical and ecophysiological processes occurring at the plant level (including their scaling to large areas). A fundamental difficulty remaining is the evaluation and perhaps validation of such models at the global scale. The interim step of model intercomparison has been taken (see Box 10.2). Other important model intercomparison projects currently under way include the Atmospheric Model Intercomparison Project, the Coupled Model Intercomparison Project, and the Paleoclimate Model Intercomparison Project.9
During the next decade, we need to expand our efforts in domain-specific
models. In the ocean we need to improve our understanding of the controls on thermohaline circulation, of the potential changes in biological productivity, and of the overall stability of the ocean circulation system. Within terrestrial systems the question of the carbon sink-source pattern (what it is and how it might change) is central. Connected to this question is the development of dynamic vegetation models, which treat competitive processes within terrestrial ecosystems and their response to multiple stresses. For the atmosphere, a central question has been, is, and likely will continue to be the role of clouds. Further, increased efforts will be needed to link terrestrial ecosystems with the atmosphere, the ocean with the atmosphere, the chemistry of the atmosphere with the physics of the atmosphere, the land to the ocean, and finally the human system to the physical and biogeochemical subsystems.
In considering coupling atmospheric general circulation models (GCMs) to terrestrial models, where the coupling transfers not only energy and water but also important gases such as CO, CH4, and CO2, temporal and spatial scale issues again emerge. Energy, water, and CO2-O2 are actually exchanged across short timescales and exhibit a high degree of variability. Moreover, the gross fluxes are large in comparison with the net ecosystem fluxes, and hence the macro-balance of terrestrial carbon stocks, which determines the net flux of CO2, is difficult to derive by direct integration of the gross fluxes. Ecological changes, such as successional sequences of tree species, are not treated well on time steps that are appropriate for considering photon input, water exchange, or trace gas fluxes and require significant intermediate parameterizations or models. Longer time step integrations have generally been more successful for carbon dioxide. On the other hand, the flux of CH4 and other short-lived species cannot be treated by simple mass balance and crudely time-averaged responses.
The relatively simple problem of coupling land hydrology to the atmosphere remains elusive and yet is quite important. Water balances influence the exchanges of energy and many reduced gases (e.g., CH4 depends on soil moisture conditions). Modeling sensitivity studies 10 have shown that if evapotranspiration were turned off over continental-scale areas, summer precipitation would be severely reduced and temperatures would be as much as 10 degrees (K) higher than with normal fluxes. They also show that over tall vegetation the integrated resistance to transpiration implied by the stomata will have a major effect on Bowen ratios over the diurnal cycle. Since the rates of sensible heat exchange over the diurnal cycle determine the height reached by the planetary boundary layer and thus diurnal variations of precipitation in tropical and summer conditions, it is evident that the inclusion of the role of vegetation is important for simulations of the hydrological cycle. Better field data are helping to establish the parameters needed for linking plant physiology to surface evapotranspiration. Considerable further effort is needed before the appropriate submodels can be applied with confidence over a wide range of vegetation cover.
The hydrological coupling between the land and the ocean has seen signifi-
cant advances in the past 10 years, but we are still challenged to develop a more complete biogeochemical coupling. The difficulties are several: (1) there is a lack of data about the loss of important chemical compounds, such as organic carbon and nitrogen compounds, from terrestrial systems and aquatic systems; (2) we are uncertain how these exchanges might change in the face of land use change or climate change—more generally under pressure of multiple stresses on terrestrial systems; and (3) there is inadequate process-level understanding and supporting data on how these organic compounds are processed within the wide range of river systems.d
The coupling between the ocean and the atmosphere is central to the question of climate change. Atmospheric GCMs with prescribed oceans, long the mainstay of three-dimensional climate modeling, are inherently incapable of simulating the actual time-evolving response of the climate system to increasing greenhouse gases because this response involves heat uptake by the oceans. This is particularly clear when one realizes that the heat capacity of the atmosphere is roughly equivalent to that of the upper 3 m of the ocean. Fortunately, the scientific community has recognized for some time that if we are to penetrate the transient behavior of climate change we must produce credible coupled ocean-atmosphere models. Significant progress has been made in treating this demanding challenge on timescales of decades to centuries (see Box 10.3). Moreover, we have now demonstrated potential predictive skill in modeling the El Niño-Southern Oscillation (ENSO),12 where the ocean-atmosphere system responds in a coupled fashion on interannual timescales. Finally, on very long timescales, we are probing the coupled ocean-atmosphere system for which paleo-oceanographic investigations suggest that aspects of longer-term climate change are associated with changes in the ocean's thermohaline circulation.
As we seek to couple better the chemistry of the atmosphere with the physics of the atmosphere, for instance, by adding the important chemical constituents and reactions to an atmospheric GCM, the issues of scale and computational challenges become daunting for transient calculations. Many of the important chemical reactions depend on concentration and hence on grid scale. In addition, important processes often occur in the boundary layer, which generally is not adequately resolved. Adding atmospheric chemistry to a GCM thus places greater demands on the terrestrial and oceanic boundary conditions and dynamic simulations. As in most of the other areas, progress will depend in part on the availability of advanced computing facilities (and in the more distant future petaflop machines13).
Finally, global climate and environmental changes often reflect the consequences of human actions superimposed on natural variability and change. It is
|
d |
Certainly there are river systems that are well studied, and there is knowledge of general patterns of carbon and nutrient processing in rivers; however, there remain large gaps in both our observational records and in our understanding when we face the issue on continental to global scales. |
clear that humans can cause environmental change, even on a global scale. It is equally clear that environmental changes, whether human caused or not, can have impacts on humans. To understand these changes and to provide useful guidance to inform policy development and decision making will require increasingly integrated understanding of the diverse human and nonhuman components of the Earth system.e Environmental and climate change research must focus on predictions of key state variables such as rainfall, ecosystem productivity, and sea level that can be linked to estimates of economic and social impacts of possible environmental and climate change. Projections of emissions, land use, and other contributions must be related to underlying economic, technological, social, and political forces to understand linkages from causes to effects and back to causes. Uncertainties in the social side of the system, though of different character, are thus linked with the uncertainties of environmental and climate systems and are as important for understanding system behavior and informing decision making.
|
Box 10.3 Effects of Anthropogenic Carbon Dioxide Emissions on the Atmosphere-Ocean System In 1967 Syukuro Manabe and Richard Wetherald published what is now regarded as one of the first credible calculations of the possible effect of increased carbon dioxide on climate. They calculated that a doubling of atmospheric carbon dioxide would warm the Earth's surface by about 2°C. This result laid the foundation for what has become an international multidisciplinary research effort on global warming. In a recent paper, published 26 years after Manabe's pioneering one-dimensional CO2 sensitivity study, he and Ronald Stouffer used a three-dimensional coupled ocean-atmosphere model to examine possible CO2-induced climate changes over several centuries (see Figure 10.2).11 Earlier studies had focused on shorter time horizons. In their scenario, CO2 quadruples over a period of 140 years, then no longer increases. This perturbation is enough to cause the ocean's global thermohaline circulation to almost disappear in the model (though in some experiments it reappears given sufficiently long integration times). This circulation is important because in the present climate it is responsible for a large portion of the heat transport from the tropics to higher latitudes. In addition, Manabe and Stouffer's study indicates that sea level continues rising steadily for centuries after the CO2 increase is halted. From this perspective, global climate change can no longer be viewed as just a problem of our own lifetimes but as a legacy—with uncertain consequences—now being passed forward to many future generations. |
|
e |
For instance, global climate change is the subject of policy debate in most nations and of negotiations at the Conference of Parties and the Framework Convention on Climate Change. This is an obvious area that centrally requires the human component of the Earth system. |
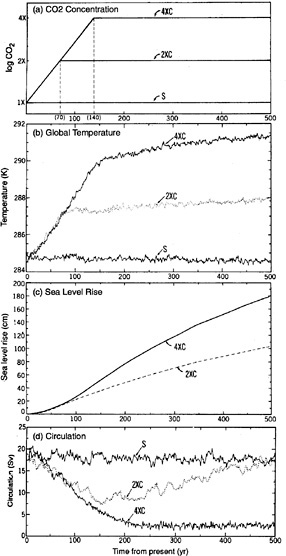
FIGURE 10.2 Impact of increasing CO2 on the Earth's climate as simulated in a Geographical Fluid Dynamics Laboratory coupled ocean-atmosphere climate model. Shown are time series of (a) prescribed CO2 concentration on a logarithmic scale in comparison to present levels; (b) global mean surface air temperature (°C); (c) global mean increase of sea level (cm) due to thermal expansion; and (d) intensity of the North Atlantic Ocean's meridional overturning circulation (10 6 m3/sec). The labels “S,” “2XC,” and “4XC” refer to separate experiments in which CO2 either remains constant (S) or increases at a rate of 1 percent per year (compounded) to double (2XC) or quadruple (4XC) the current concentration. Note that the sea level rise estimates do not include the effect of melted continental ice sheets. With this effect included, the total rise could be larger by a substantial factor. SOURCE: Manabe and Stouffer (1993). Courtesy of Macmillan Magazines Ltd.
In closing this introduction we state again that exciting and encouraging progress over this first decade of the USGCRP has been made in coupling parts of the major subsystems. Results from linking atmosphere and ocean GCMs reported in the literature show significantly different behavior than simulations in uncoupled models. The inclusion of biology in ocean GCMs has begun, but the biology remains rather simplistic, and we have yet to obtain results that include climatic feedback on the biology in the coupled system. Similarly, representations of linked terrestrial-atmosphere systems are in use, though the focus tends to be only on water and energyf, and the biology is still quite primitive; however, even now, revealing and unexpected teleconnections are being discovered. Finally, progress is being made toward model structures and datasets that will allow implementation of atmosphere-ocean-terrestrial models that include key biological-biogeochemical feedbacks.14 There is also encouraging early work in developing integrated assessment models that couple economic activity with associated emissions and impacts and with models of the biogeochemical and climate subsystems. This work has yielded some preliminary insights into system behavior, potential policy responses, and key policy-relevant uncertainties. 15
In the next decade we should continue and expand on this record of progress by investigating the perplexing issues framed by current coupled model experiments (e.g., the use of flux correction in coupled ocean-atmosphere models); conducting careful delimited experiments in which new linkages are explored (biogeochemical-energy-water linkages between the terrestrial system and the atmosphere); adding specific subsystems to existing linked model experiments (e.g., adding a marine biosphere model to coupled ocean-atmosphere carbon GCMs); and exploring and testing full-ensemble runs of coupled complex models. In this spirit and in recognizing the formidable tasks ahead, this chapter discusses future development of Earth system models in terms of four interface challenges: the atmosphere-terrestrial: energy, water, biogeochemical subsystem; the terrestrial-ocean: water and biogeochemical connection; the atmosphere-ocean-marine biosphere; and the physical atmosphere and the chemical atmosphere. The following sections present a brief general discussion of the current status of the currently available models at these four interfaces and of the challenges of the interface per se (see Box 10.4). The chapter concludes with a consideration of the overarching challenge: linking the biogeochemical and physical-climate subsystems with the human subsystem.
|
f |
Atmosphere-terrestial systems focused on carbon and/or trace gases tend to have only one-way coupling (atmospheric forcing) and do not yet include critical biogeochemical feedbacks. |
|
BOX 10.4 The 1994 Special Forum on Global Change Modeling found that “[i]mproving the linkages coupling the atmosphere, oceans, and land surface will reduce uncertainties in estimates of the overall climate response by improving the accuracy of the climate simulations, by eliminating the need for ad hoc adjustments to fluxes between components that are used in some models, and by allowing fuller exploration of natural climate variability over all timescales.” The basis for this is simply that “climate is a result of the complex interactions of the atmosphere, the oceans, and the land surface. The dynamics, thermodynamics, and hydrodynamics (and to an increasing extent the chemical and vegetation dynamics) must all be treated in order to provide a realistic simulation of climate. The focus has initially been on the atmosphere, then increasingly on the ocean; coupling of the atmosphere and oceans has not been completely successful due to limitations in understanding of ocean mixing and air-sea exchange mechanisms, in addition to limitations in model resolution and the full range of processes internal to each domain. Increased attention to improved representation of the coupling is starting to lead to improved representations of temperature and other climatic variables. Corresponding improvements are needed in representations of the land surface and land atmosphere interactions and fluxes. Because vegetation and chemical composition can affect radiative forcing and water vapor concentrations, these must also be treated in coupled simulations. The emerging results from the World Ocean Circulation Experiment (WOCE), the Global Energy and Water Cycle Experiment (GEWEX), and other field and analysis programs are providing the opportunity for improving the performance of coupled models.”16 |
THE TERRESTRIAL-ATMOSPHERE SUBSYSTEM
Overview
Interactions between terrestrial ecosystems and the troposphere are important components of the linkage of the land biosphere and the atmosphere. The most obvious interaction is the water-energy cycle. Water entering terrestrial ecosystems directly affects plant growth, soil water properties, recharge of groundwater pools, and discharge into river systems. Water is redirected back into the atmosphere through the processes of canopy interception, followed by evaporation, transpiration, and direct soil evaporation, all of which moderate surface temperatures and provide a mechanism to recycle water for further precipitation. Elements of the water balance also regulate the terrestrial-atmosphere exchanges of carbon and nitrogen on both continental and local scales.
The metabolic processes that are responsible for plant growth and maintenance and the microbial turnover associated with dead organic matter decomposition move carbon, nutrients, and water through plants and soil on both rapid and
intermediate timescales. Moreover, these cycles affect the energy balance and provide key controls over biogenic trace gas production. Some of the carbon fixed by photosynthesis is incorporated into plant tissue and is delayed from returning to the atmosphere until it is oxidized by decomposition or fire. This slower carbon loop through the terrestrial component of the carbon cycle, which is influenced by cycles of nutrients required by plants and decomposers, affects the rate of growth of atmospheric CO2 concentration and imposes a seasonal cycle on that trend (see Figure 2.10, Chapter 2). The structure of terrestrial ecosystems, which respond on even longer timescales, is the integrated changes to climate and to the intermediate timescale carbon-nutrient machinery. The loop is closed back to the climate system, since it is the structure of ecosystems, including species composition, that largely sets the terrestrial boundary condition in the climate system in terms of surface roughness, albedo, and latent heat exchange.
In sum, terrestrial ecosystems influence climate and biogeochemical cycles on several temporal scales that involve feedback loops that may modify the climate and biogeochemical system dynamics. Climate change will clearly drive vegetation dynamics; however, vegetation changes in amount or structure feed back to the climate system through changing water, energy, and gas exchange. Biogeochemical cycling will also change, altering the exchange of CO2, CH4, and other greenhouse gases, which further closes the loop back to the climate system.
Modeling the interactions between terrestrial and atmospheric systems requires coupling successional models to biogeochemical models to physiological models that describe the exchange of water and energy between vegetation and the atmosphere at fine timescales. There does not appear to be any obvious way to allow direct reciprocal coupling of GCM-type models of the atmosphere, which inherently run with short time steps, directly to ecosystem or successional models, which influence climate but have coarse temporal resolution, without the interposition of physiological and biogeochemical models. This coupling across timescales represents a nontrivial problem that sets the focus for the modeling strategy.
A Modeling Perspective
Intuitively, we might develop a global model of terrestrial ecosystem dynamics by combining descriptions of each of the physical, chemical, and biological processes involved in the system. In such a scheme, longer-term vegetation changes would be derived by integrating the responses of the rapidly evolving parts of the model. However, we cannot estimate productivity of whole plants, let alone entire ecosystems by simply integrating models that describe the rapid processes of CO2 diffusion, photosynthesis, fluid transport, respiration, and transpiration in cells and leaves. Carbon dioxide fluxes are strongly linked with water fluxes, and whereas we may treat water flux on fine temporal scales, the accumu-
lation aspect (i.e., growth of plants) strongly suggests that we treat carbon and water differently when spatial and temporal scales are extended. At fine spatial scales, such as the scale of a forest watershed, there is often coherence between water, energy, and carbon. Terrestrial models of water and energy exchange between the atmosphere and land surface operate at the subhourly to daily timescale, as do models of net photosynthesis.17 However, biogeochemical models capable of extrapolation over large spatial scales generally operate at weekly to monthly timescales with finer-scale dynamics being used as constraints.18 To properly model the exchange of water, energy, and important biogeochemical elements like carbon and nitrogen between the atmosphere and the land surface, it will be necessary to resolve differences in both temporal and spatial scale between linked atmospheric and biogeochemical models. In addition, the spatial averaging implied in the selection of parameters and processes to consider is difficult because of nonlinearities —that is, the choice of scale influences the calculation of averages, which can have significant and unexpected effects on results.
A nested treatment has been suggested to deal with interactions on a hierarchy of temporal scales.19 For example, the metabolic activities of terrestrial plants associated with growth and maintenance constitute the fastest interactions, on the order of seconds to days and determine latent heat, energy, water, and CO2 gas exchange through gross photosynthesis and respiration. Intermediate processes, from days to weeks, include the development of leaf area (with a characteristic carbon density), soil water balances, trace gas exchanges, and decomposition of organic soil materials. Longer-term annual time steps encompass net primary productivity, ecosystem production, and long-term changes in carbon and nutrient pools in plant tissue and soils. Similar and parallel strategies could be used for spatial scaling.
At each step toward longer timescales the climate system integrates the more fine-scaled processes and applies feedbacks onto the terrestrial biome. At the finest timescales the influence of temperature, radiation, humidity, and winds has a dramatic effect on the ability of plants to transpire. On longer timescales, integrated weather patterns regulate biological processes such as timing of leaf emergence or excision, uptake of nitrogen by autotrophs, rates of organic soil decay, and turnover of inorganic nitrogen. The effect of climate at the annual or interannual scale defines the net gain or loss of carbon by the biota, its water status for the subsequent growing season, and even its ability to survive.
As the temporal scale is extended, the development of dynamic vegetation models, which respond to both climate and human land use as well as other changes, is a central issue. These models must treat not only successional dynamics but also ecosystem redistribution. For example, following the abandonment of agricultural land, fluxes and pools of carbon, nitrogen, and phosphorus in secondary vegetation often do not attain the same levels as found in “undisturbed” natural vegetation. The recovery of natural vegetation in abandoned areas de-
pends on the intensity and length of the agricultural activity and the amount of soil organic matter on the site at the time of abandonment. To simulate the biogeochemistry of secondary vegetation, models must capture patterns of plant growth during secondary succession. These patterns depend substantially on the status of nutrient pools inherited from the previous stage. The changes in hydrology also need to be considered, since plants that experience water stress will alter the allocation of carbon (e.g., to allocate more carbon to roots). Processes such as reproduction, establishment, and light competition have been added to such models and interact with the carbon, nitrogen, and water cycles. Disturbance regimes such as fire are also incorporated into the models, and these disturbances (and potential changes in their frequency) are essential to include in order to successfully treat competitive dynamics and hence future patterns of ecosystem distribution. It should also be noted that these forcing terms themselves may be altered by the changes that result from changes in the terrestrial system. Finally, the issues of successional dynamics, which result from extending the temporal scale, also force more careful consideration of spatial scaling.
Research Priorities
Immediate challenges that confront models of the terrestrial-atmosphere system include exchanges of carbon and water between the atmosphere and land and the terrestrial sources and sinks of trace gases. An overarching grand challenge is to provide insight into the dynamics of a biosphere subjected to multiple stresses, which after all is the actual case that we confront (see Chapter 2). Hence, the development of dynamic vegetation models is, as stated, of central importance.
Carbon
In the past two decades the significant influence of the terrestrial biosphere on the global carbon balance and hence on the problem of timing and magnitude of possible climate change has been recognized. 20 Much of the remaining uncertainty in our understanding of the carbon cycle centers on the role of terrestrial ecosystems, in which at least two factors govern the level of carbon storage. First and most obvious is the anthropogenic alteration of the Earth's surface—for example, through the conversion of forest to agriculture—which can result in a net release of CO2 to the atmosphere. Second, and more subtle, are the possible changes in net ecosystem production (and hence carbon storage) resulting from changes in atmospheric CO2, other global biogeochemical cycles (particularly nitrogen), and/or the physical climate system.
The productivity of the terrestrial biosphere is primarily controlled by the radiation reaching terrestrial ecosystems, the availability of nutrients, and the climatic conditions in which they live, that is, by the conditions under which plants carry out photosynthesis and allocate photosynthates to various structural
components. Precipitation and temperature primarily govern the absorption of photosynthetically active radiation and its conversion into dry matter—that is, the net primary productivity (NPP) of the biosphere. Nitrogen and changes in its availability, as well as changes in other nutrient cycles, are the key biogeochemical controls on productivity.
At present, several rather complex models are being developed to account for the ecophysiological and biophysical processes that determine the spatial and temporal features of NPP.21 Their goal is to provide a prognostic capability. The major modeled processes are photosynthesis, growth and maintenance respiration, evapotranspiration, uptake and release of nitrogen, allocation of photosynthates to the various parts of the plant, litter production and decomposition, and phenological development. Some models focus on detailed mechanistic relationships for some processes (e.g., water and CO2 fluxes and the nitrogen cycle), while others rely on simple empirical relationships or satellite observations to derive or constrain important features (e.g., canopy characteristics and phenology).
The challenge is not simply to calculate NPP but rather to develop coherent explanations for past changes in the total carbon fluxes and/or storage, to test hypotheses about the underlying causes of these changes, and to establish the capability for estimating future changes. It is now becoming evident that models of the terrestrial carbon cycle and of terrestrial ecosystem processes in general will play an overriding role in addressing many of the issues posed by global environmental change. The question of climate change is a case in point. Describing, characterizing, and eventually understanding and predicting the spatial patterns of changes in terrestrial carbon storage and associated fluxes are essential to the assessments undertaken by the Intergovernmental Panel on Climate Change (IPCC22).23 These patterns and allied issues lie at the heart of analyzing any atmospheric CO2 stabilization policy.24 Moreover, these issues must be far better resolved if there is to be an adequate verification scheme to confirm national performance in meeting targets for CO2 emissions. From a broader perspective, the prognostic models of terrestrial carbon cycle and terrestrial ecosystem processes are central for any consideration of the effects of environmental change and analysis of mitigation strategies; moreover, these demands will become even more significant if countries begin to adopt carbon emission targets.25 Finally, while progress will be made (and is needed) on modeling terrestrial processes, more integrative studies also are needed wherein terrestrial systems are coupled to models of the physical atmosphere and eventually to the chemical atmosphere as well.26 Tying in the human component is clearly important.27
Soil Moisture
Modeling studies of extreme (theoretical) deforestation in the Amazon region have indicated a severe weakening of the water cycle attributable solely to
changes in roughness and albedo at the land surface.28 As noted above, the Earth's climate regulates the distribution of ecosystems, which in turn modifies land surface properties such as surface roughness and albedo, which then feeds back on the climate system. Elements of the water balance also regulate the carbon and nitrogen cycling on both continental and local scales.29 As such, soil moisture is a key component in the land surface schemes in GCMs, since it is closely related to evaporation and thus to the apportioning of sensible and latent heat fluxes. Accurate prediction of soil moisture is crucial for simulation of the hydrological cycle and of soil and vegetation biochemistry, including the cycling of carbon and nutrients at local, regional, continental, and global scales. It thus plays a significant role in atmospheric models, hydrological models, and ecological models.
Unfortunately, there exist large differences between models of soil moisture even for simulation runs with high-quality atmospheric forcing data in carefully chosen parameters.30 Therefore, the prediction of future soil moisture through coupled terrestrial-atmosphere models cannot be considered reliable, especially since the forcing data are necessarily inaccurate and the information required for specifying land surface parameters is crude. Moreover, current land surface schemes differ profoundly between models in terms of their structure and their treatment of various land surface processes such as evaporation, transpiration, and drainage; it appears that differences in scheme structure are of particular importance. 31
The differences among present land surface schemes32 used in models are manifested in a number of ways:
-
Different annual equilibrium when forced with the same atmospheric forcing data and the same land surface parameters.
-
Different descriptions of the seasonal cycle of soil moisture. The greatest dispersion occurs when vegetation contributes to the total evaporative flux, when there is a great atmospheric demand, and when the available soil moisture is limited.
-
Different partitioning of incoming precipitation among runoff-drainage, soil storage, and evaporation depending on timing and antecedent conditions.
Most schemes can be tuned to observations, but no single scheme predicts well all of the variables describing the land surface hydrology. Indeed, the consensus (single average) of all participating schemes generally outperforms any individual scheme. This suggests that individual schemes capture specific aspects of this complex system well but that no scheme yet captures the whole system satisfactorily and consistently. This issue is important and deserves attention.
As noted above, critical improvements in ecosystem modeling and its linkage to Earth system models will require the development of schemes for integrat-
ing together processes with very different rates of change. This implies the continued development and validation of physiological, biogeochemical, and successional/population models that are capable of representing the range of processes and communities found in ecosystems worldwide. Experiments with coupling these three levels of models are required, as are tests of the models when run interactively with atmospheric models. The different levels of models have differing data requirements, and these must guide the collection and archiving of data. This topic resurfaces in the subsection that addresses modeling perspectives at the mesoscale in the land-ocean subsystem discussion later in this chapter.
Trace Gases
The broad question is the role of terrestrial ecosystems and human activities in the regulation of atmospheric concentrations of CO 2 and other radiatively active atmospheric constituents. Understanding of these influences is still partial, but it will be essential to understanding the likely future consequences of fossil fuel burning, industrial emissions, and land use changes. Key issues include the following:
-
Developing and validating a suite of trace gas source models and coupling these models to atmospheric GCMs and atmospheric chemistry/ transport models (see the atmospheric physical-chemical subsystem discussion in this chapter) to predict atmospheric composition and its latitudinal gradients under changed climatic boundary conditions.
-
Developing a predictive model for the distribution, growth/decay, and functionality of wetlands, based on water balance, topography, and surface hydrology.
There are also three paleo challenges:
-
Documenting and explaining the time course of changes in CO2 versus CH4 during periods of rapid climate change, including the last deglaciation and early Holocene.
-
Explaining the atmospheric composition at the last glacial maximum, when concentrations of major measured greenhouse gases (CO2, CH4, N2O) were exceptionally low while concentrations of both soluble and insoluble mineral dust over the land, ocean, and ice sheets were extraordinarily high.
-
Clarifying the sources and transport of mineral dust from the terrestrial surface and its possible implications for (a) radiative forcing in the atmosphere and (b) marine primary production and subsequently the implications for glacial-interglacial changes in climate and atmospheric CO2, respectively.
In sum, we need global-scale, process-based modeling of terrestrial biogenic fluxes of CH4, CO, N2O, nonmethane hydrocarbons, and NOx and their responses to changes in climate and NPP (including effects of CO2 that may provide coupling between CO2 changes and other trace gas fluxes).
Finally, as previously stated, it is essential that we expand our ability to model multiple stresses on terrestrial ecosystems and how the effects of such multiple stresses might ripple back through other components of the Earth system. We will not understand the carbon cycle without addressing changes in the nitrogen and water cycles. The effect of climate change cannot be divorced from the ongoing human alteration of the terrestrial biosphere through land use change. Further, land use change affects the terrestrial dynamics and controls on water, carbon, and nutrient cycling. The issues of multiple stresses are described in detail in Chapter 2 (see also Chapter 7).
THE LAND-OCEAN SUBSYSTEM
Overview
The availability of water is an important regulator of plant productivity and sustainability of natural ecosystems. In turn and as previously noted, terrestrial ecosystems recycle water vapor at the land surface/atmosphere boundary, exchange numerous important trace gases with the troposphere, and transfer water and biogeochemical compounds to river systems. This section33 focuses on this latter exchange and addresses the development of models to explore the possible changes in fluxes in rivers of water, carbon, nitrogen, phosphorus, and silicon from terrestrial biomes to the world's oceans.
River systems are linked to regional and continental-scale hydrology through interactions among soil water, evapotranspiration, and runoff in terrestrial ecosystems. River systems and, more generally, the entire global water cycle control the movement of constituents over vast distances from the continental land masses to the world's oceans and, as discussed in the previous section, to the atmosphere. The system serves in part to transfer nutrients to the marine biological system and hence affects oceanic productivity. Landscape disturbance greatly increases the rate of loss from the terrestrial biosphere, g particularly with respect to nutrients and sediment. This redistribution is important to both donor (landscape) and recipient (aquatic) ecosystems. Tools must be developed to quantify these phenomena and provide prognostic insight.34
|
g |
On shorter (less than decades) timescales the effect on marine production is primarily on coastal ecosystems, whereas on longer timsescales (centuries) the effect could be on the oceanic system generally. |
The primary emphasis of this section is on modeling the fluxes and transformations of water and of biologically important constituents derived from terrestrial ecosystems, namely, carbon, nitrogen, phosphorus, and silicon. Both dissolved and particulate fractions must be considered, and attention must be paid to the physical transport of sediments. Since these materials are transported through groundwater, rivers, lakes, and wetlands, an analysis of water balances and water fluxes will be essential. Micronutrients, major cations and anions (e.g., SO4, Cl, Ca, Mg, K, Na), and weathering products such as carbonate are important in establishing overall material balances and may be crucial as missing trace nutrients (e.g., the question of iron in the surface waters of the South Pacific). However, given the complexity of the topic, these are considered secondary issues for this discussion.
The drainage basin serves as a key organizing principle in this discussion. The overarching issue is to understand and model how specific terrestrial-derived materials are mobilized, delivered to, and transformed along the full cascade of landscape-fluvial systems. Adequate consideration must be given to terrestrial ecosystem dynamics, the role of wetlands, and interactions in the river-riparian complex. The downstream boundary consists of the landward margin of the coastal zone.h Interconnections with atmospheric boundary forcing (predominantly through climatic variables), atmospheric deposition, and CO2 enrichment also are relevant, as are feedbacks to the atmosphere through CO2 and trace gas emissions from aquatic and wetlands ecosystems (both treated in the previous section). Addressing such linkages is necessary to define the integration of drainage basin dynamics into a larger Earth system context. These linkages are also particularly important in more explicit coupling of the human system (which is discussed later) with the physical, chemical, and biological subsystems.
A Modeling Perspective
From a modeling perspective, several aspects need to be addressed. First, the cycling of water between land and atmosphere can produce a “residual” or runoff. This water forms the basis of rivers and the recharge of aquifers; moreover, by definition it is tied to the coupled dynamics of the terrestrial ecosystem and the land-atmosphere water cycle. i The drainage basin “transforms” complex pat-
|
h |
In effect, consideration is then passed to the ocean-atmosphere section; however, the linkage through the coastal ocean of the inputs from the land at the land-(coastal) ocean boundary and the “open” ocean needs further consideration. |
|
i |
Note, in Figure 10.1 it might appear that the land-atmosphere hydrological system is somehow “decoupled” from terrestrial (biogeochemical) ecological systems and the terrestrial-atmosphere physical climate/energy system. Rather, the diagram should be read as showing that the hydrological component (particularly soil moisture) tightly ties the dynamics of the atmosphere and the land. |
terns of locally generated runoff into horizontal transport as rivers (see Figure 10.3 a and Figure 10.3 b). The drainage basin is the logical unit of organization; as its size is varied, the associated finite element grid varies in mesh size. Using the drainage basin as a focal unit allows a broad spectrum of fluvial systems to be considered. Although the focus is decidedly on the regional and larger domains, the legacy of research findings obtained at smaller scales provides an extraordinarily rich foundation.
The flow of water contains a variety of biogeochemical compounds (from point and nonpoint sources), and models must treat their internal processing in river systems (Figure 10.4). Thus, in addition to the transport of water and the associated loading of chemical constituents, the dynamics of the biogeochemical processes that act on constituents in the river must be treated. Finally, any global perspective on surface hydrology must explicitly recognize the impact of human intervention in the water cycle, not only through climate and land use change but also through the operation of impoundments, interbasin transfers, and consumptive use.
A long-term goal is to model a series of material transformations along the entire continuum of fluvial systems from the points of terrestrial mobilization to delivery and processing in the coastal zone. The transformation in and the progression through the drainage basin of constituents in various chemical states would be included in such models, paying particular attention to processes such as flocculation, settling, gaseous losses (such as denitrification), phosphate sorption and desorption, silicon uptake and release from siliceous organisms, degassing of water bodies, and so forth. The extent to which each biogeochemical process is specifically modeled would depend on the state of understanding, the availability of data, and the purpose for which the model was constructed. Multiple component models would be required dealing with terrestrial ecosystems (Chapter 2), river continuum concepts,35 nutrient cycling,36 and spiraling.37
Coupling of models between drainage basins and the near shore will also be necessary to provide a complete analysis of the interaction of terrestrial and coastal zone ecosystems. Such coupling may require coastal physical oceanographic models linked to biogeochemical process simulations of regional land-coastal margin ecosystems.38 This issue is an important research topic in itself.
The issue of scaling cuts across the entire USGCRP and is particularly challenging in this area of the land-ocean subsystem. Three spatial scales need to be considered: macroscale, mesoscale, and microscale.
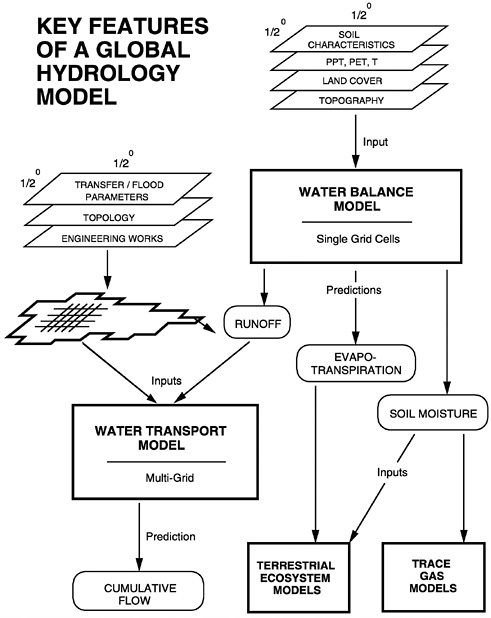
Macroscale
At the macroscale (105 to 107 km2), linked models have been successfully used to compute runoff and river flow.37 A water balance algorithm based on straightforward water budgeting procedures operating on single-grid elements provides the calculation of soil water availability and runoff for each time step from the difference between precipitation and evapotranspiration, in conjunction
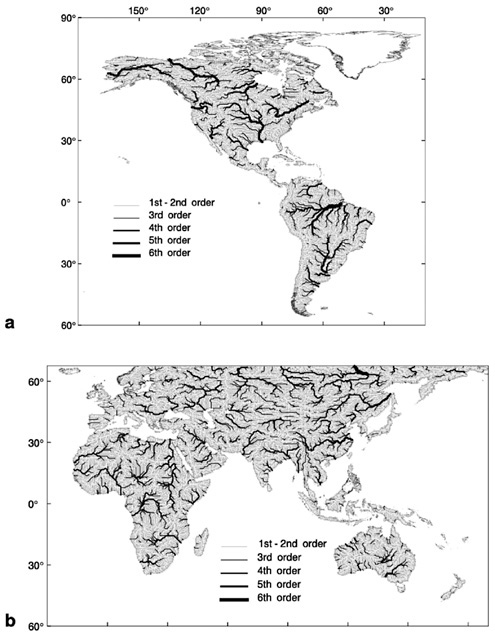
FIGURE 10.3 a and b The Simulated Topological Network (STN) for potential river systems at 30-minute (longitude × latitude) spatial resolution for (a) western and (b) eastern hemispheres. Each of 59,132 land-based grid cells is assigned a direction of flow and linked to adjoining cells. A total of 33,252 distinct river segments are topologically linked to define 6,152 individual STN-30p basins. Both exorheic (directed toward the ocean) and endorheic (internal drainage) networks are represented in this database. Order refers to individual river segments. SOURCE: Vörösmarty et al. (1998c). In review.
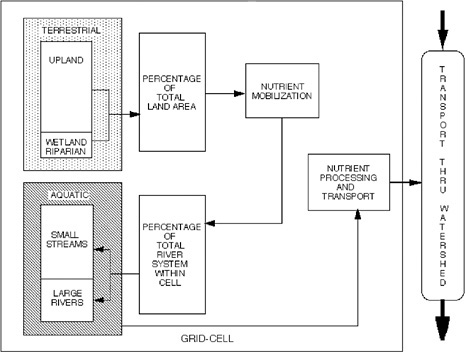
FIGURE 10.4 Conceptual terrestrial and aquatic processing model.
with a soil drying function.j These calculations have been recently enriched by the National Center for Environmental Prediction's reanalyses products, which improve estimates of vertically integrated atmospheric water vapor and wind fields. These provide estimates of time-varying atmospheric vapor content and convergence fields that, together with satellite-derived precipitation, will yield far better assessments of evapotranspiration.
Computed runoff from such single-grid elements is then routed using a set of simultaneous differential equations organized through a network topology (see again Figure 10.3 a and Figure 10.3 b). The transport algorithm is generally a quasi-linear cascade model that can be modified to accommodate wetlands inundation. This latter will be important in regions such as the Amazon, where realistic hydrographs cannot be generated without an explicit consideration of these intermediate wetlands effects, lasting typically about six months per year. In addition to meteorological forcings such as precipitation, temperature, and radiation, addi-
|
j |
We note the discussion in the previous subsection on soil moisture, which raises important modeling issues that impact these considerations on runoff. |
tional data on soil texture, land cover, topography, wetlands location and extent, and river networks are required (see Figure 10.5). The performance of each function should be judged objectively by its ability to successfully produce runoff that can be checked against verifiable discharge records.
Mesoscale
Water balances at the intermediate mesoscale (104 to 105 km2) form the crucial link between continental-scale gridded calculations and the fine scale, which is often needed for catchment-level impact assessment. In scaling up from the catchment responses (microscale; discussed below) to sequentially larger domains, investigators40 have shown that the larger-scale modeling problem can be simplified by identifying dominant process controls on the water and energy balance and that the spatial variability of these important controls could be represented in a statistical-dynamical framework. These studies identified a representative elementary area (REA), or threshold scale, for runoff and energy balance modeling. At scales greater than the REA scale (which was determined to be 1 to 2 km2), it was found that the statistical-dynamical model formulation yielded minimally biased simulation results as compared to more detailed spatially distributed simulations.
Investigators41 proposed a general mesoscale model formulation that aggregates a simplified soil-vegetation-atmosphere transfer scheme with respect to a statistical distribution of topographic and soil properties. The resulting mesoscale hydrological model may significantly advance the issue of the appropriate land surface parameterization in climate models, which was highlighted in the soil moisture discussion in the land-atmosphere subsystem. This possible advancement stems from the fact that the approach differs greatly from the current generation of land surface parameterizations since it incorporates scale spatial variability in topography and soils on scales smaller than the mesoscale grid to model downslope redistribution of soil water. In addition to providing a realistic representation of runoff processes, the redistribution of subsurface soil water feeds back through the model structure to yield subgrid variability in the surface energy fluxes. These topics are clearly important and merit increased attention.
Microscale
As mentioned above, the problem of scaling up these traditionally local studies is receiving significant attention. At the catchment scale, these studies have shown that explicit patterns of spatially variable model parameters and inputs can significantly affect hydrological response and must therefore be incorporated into models applied across these scales. Consequently, the spatially distributed grid-based water and energy balance models could be used to simulate
the runoff and energy fluxes from small watersheds and thereby treat more accurately subgrid (in macromodels)-scale information.k
Research Priorities
Two overarching themes in the research priorities for the land-ocean subsystem are (1) the important issue of data availability and (2) concerns relating to scaling, particularly with respect to spatial scaling. The first issue is being aggravated by national concerns in certain areas about releasing hydrological data, and this concern is then reflected by international agencies that hold national data. The second issue is made more difficult by the extremely varying topography and soil structure across the rivers of the world. Fortunately, techniques from geographical information systems may allow the heterogeneity to be addressed directly.
Case Studies
It is important to recognize that several projects that can potentially contribute to the issues raised in this section are already in progress or are being planned, including for the Mississippi, the Global Energy and Water-Cycle Experiment Continental-Scale International Project; 42 for the Amazon, the Large-Scale Biosphere-Atmosphere Experiment; 43 and for the Atlantic drainages,44 U.S. LMER sites.45 These case studies should be focal points for developing land-ocean interface models. They should provide for the assembly and synthesis of catchment flux data, including the biogeochemical compounds of C, N, P, and Si.46 They should also provide an assessment of the principal flux controls within the framework of both the first-order classification and the constituent budgets. These controls must explicitly consider the human component. A recent NRC report (1998), Global Energy and Water Cycle Experiment (GEWEX) Continental-Scale International Project, describes the substantial progress that has been made to characterize the variability of water and energy cycling in the Mississippi River basin and the importance of this information to improving water resource management.
Global Applications
Understanding secured through such case study work should be carried for
|
k |
Model state variables include surface temperature, canopy water storage, soil moisture in two layers, and local water table depth as a bottom boundary. Potential evapotranspiration is computed from a nonlinear energy balance equation, and actual evapotranspiration is determined as the minimum of the potential and soil-vegetation-controlled moisture limitation. Soil properties are represented parametrically and with the additional assumption that saturated hydraulic conductivity declines exponentially with depth. Vertical soil water fluxes are represented using approximate analytical solutions to water flow in the unsaturated zone. |
ward to the global scale and used to enrich this ongoing research. Fortunately, several global models of modern constituent fluxes already exist.47 To check, verify, and begin validation of the transport models will require budgets of water and constituents for large basins of the world. This requires ground-based meteorology in tandem with remotely sensed data for a series of variables, including information on precipitation, soils, land cover, surface radiation, status of the vegetative canopy, topography, floodplain extent, and inundation.48 It may even be possible to obtain hydrographics remotely. Model results can be constrained by using a database of observed discharge and constituent fluxes at key locations in the drainage basins analyzed. l These models can be coregistered to results obtained from ongoing global circulation modeling studies and thereby address the issue of climate change.
As noted above, it will be difficult to link individual processes whose respective scales encompass several orders of magnitude in space and time. This is particularly important in considering the river component of the global water cycle. The spatial resolution of current global climate models, typically 100 to 200 km, is too coarse to simulate the impact of global change on most individual river basins. Substantial efforts like the U.S. Department of Energy 's (DOE) Computer Hardware and Advanced Mathematics and Model Physics 49 program are under way to increase global model resolution. An alternative to increasing the resolution of global models is to use climatic boundary conditions to drive regional models with sufficient resolution. On the other hand, transient climatic time series and monthly discharge data for past climate over several decades at selected locations provide the opportunity for important tests of models, including appraisal of the impact of episodic events, such as El Niño, on surface water balance and river discharge in South America.
A major extension of models of runoff and riverine transport will involve development of tandem constituent transport models for the transport and processing of both dissolved and particulate material to the coastal oceans. A major initial effort could be to model the mobilization and transport of carbon and nitrogen from the terrestrial landscape into fluvial ecosystems in drainage basins that include both natural and disturbed ecosystems.
Estimates of river fluxes and chemical signatures depend on existing data resources around the globe. It will be necessary to inventory, document, and make available such datasets, to identify gaps in our knowledge and, where necessary, to collect additional data. A partial inventory of riverborne constituent data indicates that the most abundant data resources are available only for highly developed countries. Rapidly developing regions show an intermediate level of
|
l |
The World Meteorlogical Organization-sponsored Global Runoff Data Center in Koblenz, Germany, is an important partner in a global hydrologically oriented geographic information system; however, there is growing concern about the open availability of the data. |
data availability, while less developed countries are most poorly monitored. Even in the best-represented regions of the globe, however, coherent time series are available for only the past 30 years or less, constraining our ability to construct and test riverine flux models. Data quality is yet another issue limiting the usability of water quality data. Standardized protocols, both in terms of sampling frequency, spatial distribution of sampling networks, and chemical analyses are still needed to ensure the production of comparable datasets collected in disparate parts of the globe. Several upgrades of the basic monitoring system for discharge and riverborne constituents at the large scale are therefore required.
In sum, these efforts in both case studies and global applications will permit us to understand in more detail current and future patterns of landscape impoverishment as well as eutrophication of inland waters. Moreover, at both the river basin specific and the continental to global scales, the model outputs could be linked to complementary studies of coastal ocean productivity. There are, as discussed, significant issues regarding the adequacy of the needed data and the availability of the data that currently exist. All are important since all are areas of intense human habitation.
THE ATMOSPHERE-OCEAN SUBSYSTEM
Overview
Models of physical processes in the ocean and atmosphere provide much of our current basis for understanding future climate change. They incorporate the contributions of atmospheric dynamics and adiabatic thermodynamics through the methods of computational fluid dynamics. This approach was initially developed in the late 1940s and early 1950s to provide an objective numerical approach to weather prediction. It is sometimes forgotten that the early development of “supercomputers ” at that time was motivated in large part by the need to solve this problem.
The thermal/fluid dynamics approach to the weather system has tended to focus on application of the most efficient and accurate discrete representations of the Eulerian, Navier-Stokes, and thermodynamic equations for a compressible atmosphere on a rotating sphere. Meteorological observations are assimilated into initial fields consistent with the model dynamics; then the prognostic variables (e.g., horizontal winds, temperatures, surface pressure) are specified from these initial fields and integrated forward in time to generate future weather systems. Versions of these weather prediction models were developed in the 1960s to study the “general circulation” of the atmosphere—that is, the physical statistics of weather systems satisfying requirements of momentum and energy conservation. To obtain realistic simulations it was found necessary to begin to include additional energy sources and sinks, particularly by exchanges with the surface and moist atmospheric processes (i.e., moist convective adjustments and precipitation) with the attendant latent heat release and radiative heat inputs.
Incorporating the exchange of energy is an apparently simple but in practice
challenging requirement. Models incorporate many complex energy exchange processes, and it is easy to introduce spurious energy sources and sinks either through nonconservative numerical procedures or physical approximations. For example, a model may use a different treatment of latent heat release for precipitation than it does for surface melting and evapotranspiration. Because of the large number of potential sources of inconsistency, it is probably impossible to develop a model that conserves energy perfectly. However, models should be validated to conserve energy to better than 1 W/m2 and preferably have errors less that 0.1 W/m2. (The change of atmospheric radiation from doubling CO2 is about 4 W/m2.) An atmospheric model coupled to a surface with ocean temperatures prescribed from observations should have radiative imbalance at the top of the atmosphere considerably smaller than this to prevent spurious climate change when coupled to an ocean model. These conservation issues are not unrelated to concerns about numerical drift apparent in coupled atmosphere-ocean GCMs (see Chapter 3 and Chapter 4 as well as the subsequent discussion below).
The following discussion focuses attention on three specific critical areas: clouds in atmospheric models, carbon in the ocean, and the problem of linking ocean circulation models with models of atmospheric circulation. We begin with this latter topic since it is crosscutting and ever present.
A Modeling Perspective
Initialization and Coupling: One of the Challenges to Prediction
Coupled ocean-atmosphere GCMs are fundamental to the study of the climate system.50 Models, by definition, are reduced descriptions of reality and hence incomplete and with error. Missing pieces and small errors can pose difficulties, as indicated above, when models of major subsystems such as the ocean and the atmosphere are coupled. For example, inconsistencies between the submodels can lead to numerical drift when the models are coupled. Another major problem is the initialization of models so that the entire system is in a dynamical and thermodynamical balance —that is, in statistical equilibrium with respect to energy as well as the fluxes of heat, water, and momentum between the various components of the system.m Initial imbalances can also cause numerical
|
m |
There has been progress in this topic of initial conditions and strategies for setting initial conditions in coupled models that are reasonable in light of the perturbation (such as enhanced greenhouse forcing) one is investigating. For climate studies involving ocean-atmosphere-linked systems, one of the most successful initialization techniques developed to date involves integrating each submodel separately to quasi-steady state before coupling. One can then introduce artificial “flux adjustments ” to ensure that the linked system is also in quasi-equilibrium and does not drift. Another technique is to use restoring values for data fields to ensure that these fields do not drift away from observations. There remain, however, serious questions about these techniques. In sum, “the reduction and removal of flux adjustments (without significantly degrading the simulation of present-day climate) remain a high priority in the development of coupled models” (Kattenberg et al., 1996, p. 311). |
drift. The problem of determining appropriate initial conditions in which fluxes are dynamically and thermodynamically balanced throughout an ocean-atmosphere coupled system is particularly difficult because of the wide range of adjustment times ranging from days to thousands of years. To say that the ocean-atmosphere system is exceedingly stiff is not an overstatement.
As noted in the introduction, the overriding challenge to modeling (and to the USGCRP) is prediction. This challenge is particularly acute when predictive capability is sought on timescales from seasonal to decadal to centennial and where one is confronted with a coupled stiff system like the ocean-atmosphere. In the classical case of prediction—for instance, in weather prediction—one can estimate predictability by evaluating the rate of change of the system from groups of initial states that are close to each other. Relating the differences in these time-evolving states to the errors (differences) in the initial conditions gives a “measure ” or at least an insight into the predictive utility of the model. Obviously, if the rate of growth in the difference between the time-evolving states of the system is large relative to the difference in initial conditions, there is some doubt about the predictive capability of the model—or at least one should be concerned. One also has the actual weather versus the predicted weather as a test, and this is far more difficult when studying climate.
There are many variations on this theme. Some methods involve focusing on quantifying well the statistics of the initial conditions and then evaluating the time response under a distribution of initial conditions. For models of the ocean-atmosphere system this careful analysis of the statistical variability in the initial fields is problematic because of a lack of three-dimensional oceanic data fields with statistical information. This is particularly relevant to the question of understanding the physics behind the low-frequency variability of climate and the detection of anthropogenic climate change. These issues must confront longer integration periods than in calculating forecasts for tomorrow's weather or even the seasonal to interannual prediction issues (i.e., El Niño); moreover, on longer timescales (such as decadal to centennial), there is an associated inability (or at least practical difficulty) to test forecasts against what “happens,” which is such an integral part of developing and demonstrating prognostic capability. The problem is made even more difficult because of natural climate drifts and changes, whose importance has been recognized throughout this report and elsewhere.51
Observations of the ocean-atmosphere system suggest a potential for predictability in the observed broad-area patterns or modes of coherent behavior such as the tropical Pacific ENSO mode, the tropical Atlantic dipole, the North Atlantic Oscillation mode, and others that were discussed in Chapter 4 (see also Chapter 3). The existence of long-lasting, coherent, quasi-periodic modes of behavior suggests that the system may possess extended predictability. On the other hand, this could simply be oceanic damping of atmospheric white noise.52
Nevertheless, predictive skills do appear to exist for some of the ocean-
atmosphere patterns. Not all modes are simply damped white noise (see again Chapter 3 and Chapter 4). The ENSO phenomenonn is an example in which both ocean and atmosphere feed back on one another to induce a behavior that appears to be predictable. But as pointed out elsewhere, even if other coupled processes yield long-lasting, large-scale coherent patterns that may be “predictable,” there are significant practical issues in developing useful predictions. These include (1) the robustness of the mechanism, (2) the amount of variability involved, and (3) the availability of initial conditions that capture the eventual pattern that is to emerge.53 These issues and others discussed below are evidence that to use models of complex climate processes usefully one must be able to test thoroughly the coupled dynamics and use extensive ensemble runs where initial conditions are varied. Such tests are computationally intensive and require significant computing resources, but such tests are essential.
This short discussion only raises the topic and points to its importance; it is far from a full treatment. Simply stated, the issue(s) of initialization (and flux adjustment) is (are) of central importance and deserve extensive investigation.54
Clouds
Another topic that continues to haunt the modeling of the climate system is the role of clouds.55 It is generally accepted that the net effect of clouds on the radiative balance of the planet is negative56 and has an average magnitude of about 10 to 20 W/m2. This balance consists of a short-wave cooling (the albedo effect) of about 40 to 50 W/m2 and a long-wave warming (the greenhouse effect) of about 30 W/m2. Unfortunately, the size of the uncertainties in these budgets is large compared to the expected chemical greenhouse forcing. The importance of clouds is best summarized by the recent Working Group 1 report of the IPCC: “The single largest uncertainty in determining the climate sensitivity to either natural or anthropogenic changes are clouds and their effects on radiation and their role in the hydrological cycle.”57
Handling the physics and/or parameterization of clouds in climate models remains a central difficulty. Recently, it was reported (The Feedback Analysis of GCMs and In Observations [FANGIO])58 that intercomparison of climate models indicates a decreasing range of model responses to greenhouse forcing from cloud feedback; however, it was noted that this news may not be as positive when examined more closely. Much apparent reduction in uncertainty stems from simply fewer models participating or other reasons that mask the uncertainty. 59 Clouds and their impact on climate remain the key uncertainty in estimating the sensitivity of Earth 's climate to increased greenhouse gases.
|
n |
For ENSO prediction there has been a demonstration of success in the most recent event (1997). See Chapter 3. |
Cloud modeling is a particularly challenging scientific problem in the climate and global change arena because it involves processes covering a very wide range of space and timescales. For example, from cloud systems extending over thousands of kilometers to cloud droplets and aerosols of microscopic size are all important components of the climate system. The timescales of interest can range from hundreds of years (e.g., future equilibrium climates under a doubled to quadrupled CO2 concentration) to fractions of a second (e.g., droplet collisions). This is not to say that all cloud microphysics must be included in modeling cloud formation and cloud properties, but the demarcation between what must be included and what can be parameterization and still attain prognostic skill remains highly controversial.
Even the basic issue of the nature of the future cloud feedback is not clear. As the planet warms, it is likely that evaporation will increase, which could yield more clouds, but will these “added” clouds enhance the greenhouse effect or damp it? This question remains open, and it is not clear how it will be answered. As mentioned at the beginning of this subsection, today clouds appear on the basis of observations to have a negative effect on the radiative balance of the planet. Will that be as true in the future?
Given that the current generation of global climate models represent the Earth in terms of gridpoints spaced several hundred miles apart, many observed features on smaller scales, such as individual cloud systems, are not explicitly resolved by the global models. This is at the heart of the spatial problem, but there are interesting approaches being developed that attack this issue. These include efforts to focus on models that include detailed treatment of cloud processes and atmospheric radiation and that resolve atmospheric motions and other processes on a much finer scale than the current global models; 60 to explore this detailed information from the very high resolution limited-area models; to compare these explorations with detailed satellite observations (CERES (Cloud and the Earth's Radiant Energy System) and MODIS (Moderate Resolution Imaging Spectrometer) measurements from Earth Observing System (EOS) AM-1 and EOS PM-1 will likely be particularly useful61); and then to develop improved ways to represent small-scale features in global models, despite the fact that the features themselves are not explicitly resolved in the global models.62
As previously discussed, cloud radiative feedback is the single most important effect determining the magnitude of possible climate responses to increased greenhouse forcing. DOE's Atmospheric Radiation Measurement (ARM) program63 was developed to improve predictive capability, particularly as it relates to the cloud-climate feedback. The experimental objective of the ARM program is to characterize empirically the radiative processes in the Earth's atmosphere with improved resolution and accuracy. Two issues are being addressed in ARM: (1) the radiation budget and its spectral dependence and (2) the radiative and other properties of clouds. Understanding cloud properties and how to predict them under expanded greenhouse forcing is critical because cloud properties may
very well change as climate changes. Progress here is important, since uncertainty about this important feedback will continue to constrain the utility of coupled climate models for clarifying the role of human activity as a driver of climate change.64
Ocean-Atmosphere Carbon System
The natural marine carbon cycleo plays an important role in the partitioning of carbon dioxide between the atmosphere and the ocean. The primary controls are the circulation of the ocean and two important biogeochemical processes: the solubility pump and the biological pump, both of which act to create a global mean increase of dissolved inorganic carbon (DIC) with depth and therefore to maintain atmospheric CO2 at a level considerably lower—about a factor of three—than it would otherwise be.65 The interplay between the circulation of the oceans and the biogeochemical “pumps” determines the sea surface PCO2 and hence the primary determinants (with atmospheric PCO2 and sea surface winds) of the air-sea exchange rates of carbon dioxide.
It is difficult to determine directly from observations the relative strengths of the carbon pumps and ocean circulation on the patterns of air-sea exchange of carbon dioxide and exchange and DIC distribution. Ocean carbon cycle models (see Plate 7), however, provide an attractive means for estimating the relative strengths and potential future patterns and rates of exchange.66 (See Box 10.5.)
Ocean Circulation
Improvements in ocean-atmosphere models of circulation68 are central to modeling climate, as are considerations of ocean uptake of carbon dioxide.69 Central to this improvement is achieving better spatial resolution in atmospheric and oceanic models. In the future we should expect significant improvements in the oceanic component of ocean-atmosphere models as the resolution increases, with increasing availability of computing resources, and as more and more sophisticated subgrid-scale parameterizations are developed and incorporated into these models. 70 This places significant demands not only on the scientific community but also on the available computing resources and is a major issue.
As discussed in the 1994 Special Forum on Global Change Modeling, 71 the atmospheric component of climate models is able to capture significantly better the major storm systems and circulation patterns with models having finer resolu-
|
o |
We take the natural carbon cycle to mean the state of the ocean-atmosphere carbon system prior to significant anthropogenic influence on the global carbon budget, usually considered to be the millennia leading up to the nineteenth century, when atmospheric CO2 concentrations were “steady” at about 280 ppm ± 10 ppm. |
|
BOX 10.5 The IGBP-GAIM Ocean Carbon Model Intercomparison Project (OCMIP67) was initiated in 1995 to investigate to what extent predictions from three-dimensional ocean carbon models vary and to understand why. The main goal is to help improve our understanding of the ocean as the major long-term depository of CO2. The initial effort was put into a comparison of the air-sea CO2 flux and bomb 14C inventories of four established ocean carbon models (Princeton/GFDL, USA; Max Planck/ Hamburg, Germany; Hadley Centre, United Kingdom; and Institut Pierre Simon Laplace (IPSL), France). This exploratory study found large regional discrepancies between ocean carbon models in estimating CO2 fluxes and carbon storage (see Plate 8). The issue and approach to resolving these significant regional discrepancies are important. The next and more difficult stage is to expand the set of three-dimensional ocean carbon models participating in OCMIP and to include models of the important biogeochemical processes in three-dimensional ocean carbon models to evaluate more fully the role of biological processes in the carbon cycle and hence be better positioned to employ coupled ocean-atmosphere models to investigate the potential feedbacks over the next two centuries. |
tion. In part this is a reflection of the associated improvement in the representation of major orographic features and in part because of improved ability to represent weather systems, such as the Intertropical Convergence Zone, the Hadley circulation, and other circulation features. In the oceanic component of climate models, ocean current patterns are significantly better represented in models having resolutions finer than about 0.5 to 1 degree in large part because important ocean current systems (e.g., the Gulf Stream and Kuriosho current), ocean variability (including ENSO events), and the thermohaline circulation and other vertical mixing processes can be better represented. Improved resolution in both atmosphere and ocean components of global climate models has also proven to reduce flux imbalance problems (discussed earlier) arising in the coupling of these components. With the increasing parallelism of supercomputers and the availability of massively parallel computers and eventually advanced petaflop machines, a central impediment to gains in model accuracy by improving model resolution is the commitment to make computational resources available. However, as the 1994 forum noted, a concomitant increase in efforts for process studies and diagnosis and analysis of model results also is required. In recent years we have seen a “potential” increase in the availability of computing resources via access to resources at the national laboratories; however, the degree of availability has been unclear. The necessary “concomitant ” increase in resources for process studies, diagnosis, and analysis has been even more difficult to realize.
The coming decade will be particularly important for ocean circulation in the context of global change. The World Ocean Circulation Experiment (WOCE)72 will be executing its Analysis, Modeling, and Synthesis Phase coupled with the maturing of the exciting ocean topography data that are flowing from the TOPEX-Poseidon mission. A central theme will be on the match between oceanic models and the observed data. Key questions, such as how well ocean models capture the inferred heat flux or tracer distributions (see below), are central to the use of these models in climate and other global change studies. A particularly exciting development is the potential for assimilating ocean topography data into ocean general circulation models. The intercomparison of ocean models through the efforts of the World Climate Research Program 73 and the International Geosphere-Biosphere Programme74 and the direct comparison of models with data must be a continuous theme for the future. The latter effort, comparing models with data, as the direct path for model rejection and model improvement is particularly important. It is, in a sense, the true reason for WOCE and Joint Global Ocean Flux Study (JGOFS)75; the latter has been particularly focused on the various biogeochemical pumps at work in the ocean carbon system.
Biogeochemical Pumps
The “biogeochemical pump,” which transfers CO2 in the surface ocean to other physical, chemical, and biological components, is actually three pumps: the solubility pump and the biological pump, which is itself two pumps—the organic matter pump and the calcium carbonate pump.
The solubility pump maintains a vertical DIC gradient because cold waters, which originate in high latitudes and fill up the deep ocean, can hold more DIC than warm waters at equilibrium with a fixed atmospheric pCO2, a result of the higher solubility and dissociation of CO2 (into carbonate and bicarbonate ions) in cold water. The vertical DIC gradient depends not only on the vertical temperature gradient but also on the degree to which surface waters equilibrate with the atmosphere before sinking. Unlike most gases, the equilibration of surface water CO2 with the atmosphere takes about one year, and therefore the strength of the solubility pump may depend critically on the kinetics of air-sea gas exchange. It is worthwhile to note that important uncertainty remains about the basic chemical (apparent) disassociation coefficients that must be removed.
The biological pump consists, as mentioned, of two separate pumps —that of organic matter and calcium carbonate. The organic matter pump affects the DIC (and dissolved organic carbon, DOC) distribution through the photosynthetic formation of organic carbon in surface waters and the sinking and subsequent remineralization of this organic matter deeper in the water column. The carbonate pump affects the DIC distribution though the biogenic precipitation of calcium carbonate in surface waters and the subsequent sinking and dissolution of this material deeper in the water column. These two pumps also affect the alkalinity
of seawater (and hence link to the solubility pump) through the nitrate and dissolved calcium distributions.
It has been pointed out by a number of modeling studies that if there were no biological pump the preindustrial atmospheric CO2 concentration would have been 450 ppm instead of 280 ppm. Any complete model of the natural ocean carbon cycle should therefore include the biological pump; however, most recent assessments of the oceanic uptake of anthropogenic CO2 have assumed that the biological pump would not be affected by climate change and have therefore only modeled the physical solubility pump. 76 A recent exception is a coupled ocean-atmosphere model used to show that including a simple parameterization of the biological pump significantly altered the calculations of total uptake of CO2 over timescales of 70 to 140 years.77 This result demonstrates the importance of including simple models of the biological pump in ocean carbon cycle models.
Models of the organic matter pump need to simulate the net biogenic uptake of dissolved inorganic carbon in surface waters (new production) and the net remineralization in the aphotic zone of the organic carbon. Models of varying complexity and assumptions are rapidly being developed, and the JGOFS datasets will no doubt provoke the formulation of more realistic biological models. Simulating new production is complicated by the many factors that govern the rates of photosynthesis, such as light and the availability of a variety of nutrients (nitrate, ammonia, phosphate, silicate, and possibly iron), both of which depend on the physical environment, such as mixed-layer depth and the flow field, particularly vertical velocity. A simplification is possible, however, if the organic matter pump treats phosphorus (or nitrogen) and carbon in a grossly similar fashion, differing only in the relatively constant Redfield ratios. In that event, simulation of the organic matter pump is tantamount to simulating the distributions of nitrate or phosphate. There are deviations from an invariant Redfield stoichiometry, but these deviations may at least initially be regarded as relatively minor. The topic is important and needs research. Current nutrient restoring or other empirical approaches (see below) are useful and need investigation, but for prognostic calculations a more process-oriented approach is necessary. A problem remains in defining a minimum complexity model that can represent the preindustrial pump and, by extension, the present-day organic pump. Candidate models range from those in which new biological production (and therefore the export flux to the deep ocean) is treated implicitly by relaxing surface nutrients to observed values78 to multicomponent ecosystem models79 in which the biological processes controlling the export flux are modeled explicitly.80
The principal advantage of the nutrient-restoring approach is that it provides a data-constrained and circulation-coherent estimate of an important but poorly constrained quantity on large scales: new production. Given that the new production so estimated will depend critically on the modeled ocean circulation field, it is important to have several estimates from different circulation models to pro-
duce confidence intervals for predicted global and regional new production. We note that the nutrient-restoring algorithms are relatively easy to install in circulation codes. A second advantage of this approach is that it facilitates a focus on aphotic zone remineralization. Modeling studies suggest that the aphotic zone nutrient and oxygen distributions are highly sensitive to the type of organic matter (dissolved or sinking particulate) exported from the photic zone, as well as the lifetime of that organic matter. Thus, improved estimates of aphotic zone remineralization can be coherently linked and/or compared using the nutrient-restoring method.
There are two main disadvantages of the nutrient-restoring approach. First, small mismatches between the observed and simulated ocean circulation field, particularly with regard to vertical motion, can lead to erroneous and unreasonably patchy estimates of new production. This difficulty can be minimized, as indicated above, by running the nutrient-restoring algorithm in several different circulation codes. A second disadvantage of the nutrient-restoring approach is that it is of little use for predicting future variations in the natural marine carbon cycle, such as might be expected if ocean circulation changes. The nutrient-restoring method is still a useful check and a reasonable first step in addressing the organic pump but what is needed are more process-oriented marine ecosystem models.
Beyond the check from the nutrient-restoring method, evaluation of such process-based organic pump models (i.e., predicted new production fields) becomes a crucial issue. One promising approach is to simulate dissolved oxygen, linking the source/sink terms for nutrients to those for oxygen using a Redfield ratio, and comparing the model-predicted oxygen with observations. Until recently, such a model evaluation was only possible in the deep sea, outside the depth range of seasonal variability. Now, however, seasonal surface analyses are available, 81 revealing summertime supersaturation and wintertime undersaturation (on average) patterns that largely reflect summertime new production and wintertime entrainment of deeper oxygen-depleted waters. Such models tested against in situ data take on even greater importance when complemented by ocean color observations from satellite. In sum, prognostic ecosystems are needed for the organic pump, but it will be important to cross-check these models with the nutrient-restoring method, and this cross-check can now include seasonal considerations.
Simulating the calcium carbonate pump with a process-oriented model presents another level of complexity beyond simulating the organic matter pump: the distribution of particular phytoplankton species (mainly coccolithophorids) must be simulated. Fortunately, the calcium carbonate pump contributes relatively little to the vertical DIC gradient compared to the organic matter and solubility pumps. The importance of this pump needs careful evaluation and its past (paleo) role in the carbon cycle needs to be considered. It is not yet completely clear how to treat the CaCO3 cycle in ocean carbon cycle models. One possibility is to use a restoring technique similar to the nutrient cycle. This is more difficult for
CaCO3 because the amount and quality of alkalinity observations are not as high as for nutrients. Yet another approach is to restore surface silicate to observations, determining net biogenic silica formation. In regions of low silica production, one can assume that coccolithophorids dominate production. In such regions the CaCO3 uptake can be linked to new production using a fixed ratio. Another possibility is to assume that coccolithophorids dominate in low-productivity regions. Finally, it may be best to evaluate more carefully the role of the individual pumps as one means of focusing on the most important issues.
In principle, it is possible to evaluate models of the separate pumps (even though they are quasi-linked). For instance, removing the biological pump and anthropogenic contamination from the DIC distribution can test models of the solubility pump. The organic matter pump can be removed using the apparent oxygen utilization and an estimate of the respiration quotient. Alkalinity, corrected for nitrate, can be used to eliminate the calcium carbonate pump. The anthropogenic component can be eliminated using related techniques or by incorporating transient tracers that reveal age information.82
In early IPCC assessments of the effects of increased atmospheric CO2 on climate change, the role of the marine biota was largely ignored. 83 This was based on the understanding that marine algal growth is limited by nitrate or other nutrients but not, as mentioned above, by CO2. There would therefore be no CO2 fertilization effect as has been suggested for terrestrial plants, and unless there was a large change in the nutrient supply to the upper ocean because of a climate-induced shift in circulation, no extra anthropogenic CO2 could be sequestered to the deep ocean by the biological pump.84 Since 1990, JGOFS has been studying the biological pump, and this effort and other research work have suggested a number of possible ways in which the biological pump might be affected by climate change over a 200-year timescale.85 The main conclusion was that because of the complexity of biological systems it was not yet possible to say whether some of the likely feedbacks would be positive or negative. However, it is clear that an understanding of some of the scientific issues could be greatly assisted by an integrated and focused cross-disciplinary modeling program. It is difficult to determine directly from observations the relative strengths of the carbon pumps and ocean circulation on the patterns of air-sea exchange of carbon dioxide and DIC distribution. Ocean carbon cycle models, however, provide an attractive means for estimating the relative strengths as well as potential future patterns and rates of exchange.
Ocean Tracers: A Diagnostic Tool for Ocean Carbon Cycle Models
Since ocean circulation plays a key role in the natural and anthropogenic marine carbon cycle, we need to quantify its current effect to understand better its potential future role. The biological pumps also need to be understood and evaluated in the context of the general circulation of the ocean and the ocean
carbon cycle. Tracers provide information about the types of ocean circulation relevant for the marine carbon cycle, and many give insight into aspects of the biological pump (in fact, the nutrient-restoring algorithm is a purely tracer-driven approach). Fortunately, tracers such as radiocarbon, both natural and bomb produced, and chlorofluorocarbons as well as DIC and DOC have been extensively measured in the ocean (most recently and extensively in the WOCE and JGOFS programs). The transient tracers are particularly attractive because they have atmospheric histories similar (though not identical) to anthropogenic CO2 and they have equilibration times in surface waters (~1 month for fluorocarbons and 10 years for 14C) that bracket the equilibration time for CO2 (~1 year). Natural radiocarbon is most useful for establishing the veracity of model representations of the circulation of abyssal waters. This circulation currently does not play a large role in CO2 uptake but will likely become very important over the next few hundred years. The following brief discussion focuses on carbon 14 and the fluorocarbons as example tracers.
Atoms of 14C are produced naturally when nitrogen is bombarded with cosmic radiation in the upper atmosphere. This 14C rapidly attaches to molecules of CO2, enters the ocean through air-sea gas exchange, and subsequently decays (with a half-life of 5,730 years), mostly in the deep ocean. Gradients of 14C result, both vertically and horizontally, that depend on how long waters have been isolated from the ocean's surface. Beginning with the atmospheric nuclear weapons tests during the 1950s, the 14C content of the atmosphere increased sharply. At its peak in 1963, atmospheric 14C was nearly double that of preindustrial times. Subsequently, atmospheric 14C has declined, following implementation of international treaties banning atmospheric weapons tests. Today atmospheric 14C is only about 10 percent greater than during preindustrial times. By carefully separating out the nuclear component through correlations with other ocean tracers, oceanographers have been able to use the bomb 14C signal in the ocean as a primary dataset with which to evaluate ocean circulation models.86
Many new high-precision measurements of oceanic 14C are now becoming available from samples collected during WOCE and JGOFS. Because of its much finer resolution, validations using both natural and bomb 14C will benefit from this dataset. Due to the weak flows characteristic of most of the deep ocean, it is not possible to measure directly much of the ocean's deep circulation. One can use natural 14C, however, as a type of clock for deep-ocean circulation. An additional interest for the bomb component concerns its use as an analog for anthropogenic CO2. Ocean carbon cycle model comparisons confirm that the distribution of anthropogenic CO2 resembles much more closely the bomb 14C during WOCE than during the earlier Geochemical Ocean Sections Study (GEOSECS87). Boundary conditions have changed considerably since the latter campaign, which originated only 10 years after the 1963 peak in atmospheric 14C. An important test is to compare ocean model results to both the GEOSECS and WOCE, focusing on site-specific changes in 14C (where possible). This time
increment can be used to remove uncertainties associated with estimating prenuclear 14C from the observations, and it can also provide a tracer that more closely resembles anthropogenic CO2.
An example of the utility of natural radiocarbon for evaluating the deep circulation fields of ocean models can be seen by considering Plate 8, which contains radiocarbon results from the initial phase of OCMIP. Along the Western Atlantic GEOSECS section, the observations reveal that the basin north of the equator is filled with waters penetrating from the north, which are at most 500 years old, a signature of North Atlantic deep water (NADW). In the Geophysical Fluid Dynamics Laboratory model, no younger waters from the north penetrate deeper than 2,500 m. In the Hadley Center model, younger waters fill the basin, but the structure of the14C distribution is rather different from observations; apparently, vertical infiltration of14C is excessive. In the Max Planck Institute model, young waters also fill the North Atlantic basin, although here the structure of the north-south 14C gradient is closer to that observed, despite evidence of somewhat inadequate infiltration of older waters from the south along the bottom. In the Institut Pierre Simon Laplace model, young 14C waters fill the deep northern basin, but southward penetration of the youngest waters (north of 30°N) is not deep enough. On the other hand, this model seems to most closely match observations, which show a tongue of young 14C water extending from the north at around 2,500 m (the NADW) and overlying intermediate waters that move northward from the south. Analogous natural 14C sections for the Pacific reveal that all four models predict rates for the northward penetration of bottom waters, which are consistently too slow.88
These penetration issues are important to past, present, and future rates of uptake of carbon dioxide and are tied to the ocean role in longer-term climate change. They need to be better understood.
In addition to the challenge of matching 14C, models need to be compared to direct anthropogenic CO2 data that are based on measured CO2 system variables and tracer ages along isopycnal surfaces. More generally, full natural carbon cycle simulations should be validated with available datasets for dissolved inorganic carbon, oxygen, and alkalinity. Gridded seasonal maps of Delta-pCO2 (pCO2(ocean) - pCO2(atmosphere)) have recently become available for the North Atlantic 89 and will be used to constrain CO2 air-sea fluxes. New U.S. efforts are beginning to provide maps for the entire Atlantic Ocean and the North Pacific, and international efforts have begun to construct a global Delta-pCO2 dataset. Finally, chlorofluorocarbons (CFCs) are excellent tracers of ocean circulation for several reasons. First, they readily equilibrate in surface waters, so that the “pre-formed” CFC concentration is easily estimated from the well-known atmospheric CFC concentrations and the CFC solubility in seawater. Second, CFCs are entirely anthropogenic, having no natural “contamination,” and are essentially inert in seawater. Third, the ratio of CFC-11 to CFC-12 in the atmosphere has, until recently, varied coherently, so that this ratio in seawater can be used in many
cases to estimate the age of water parcels; moreover, accurate CFC measurements can now be made relatively easily, and as a result nearly 100,000 measurements for CFCs now exist. Most importantly, the distributions of CFCs have yielded insight into the many circulation processes relevant to the uptake of anthropogenic tracers, such as ventilation of the thermocline, intermediate and deep-water formation, ventilation of the interior deep ocean through exchange with deep western boundary currents, and evaluation of oceanic general circulation models in general.90
Despite some uncertainty associated with this tracer-based approach, continued efforts with newly released WOCE-JGOFS data will eventually provide an excellent global database with which to evaluate three-dimensional ocean carbon cycle models.
Research Priorities
First and foremost, long-term consistent data are needed to support modeling investigations.p The various reanalysis projects are immensely valuable and should be extended to include coherent boundary forcing fields such as surface temperatures, wind stress, and sea-ice extent as well as independent estimates of precipitation and evaporation. As mentioned earlier, a central activity for the coming decade is to compare models with data; for the oceans this means careful comparison of models with WOCE and JGOFS ocean data fields.
Along with data from the present and recent past (the past 100 years), it is important to establish highly credible global climate-relevant data fields for the past 1,000 years along with lower-frequency data for the past 6,000 years. Given the scarcity of geographically specific detailed climate data for the past 1,000 years and the importance of climate variability over timescales of a millennium, it is important that extensive climate system models be carefully intercompared. This intercomparison should take place in models not restricted to the ocean-atmosphere system but should also include the terrestrial biosphere, although perhaps initially in a somewhat passive mode.
Initialization of coupled models for use in investigating climate variability and anthropogenic change has been and remains an important research topic.
|
p |
“It is important to understand how natural climate variations interact with human-induced changes. There are several issues here. First, as already noted, there needs to be a continuing high quality global climate monitoring system to better establish the changing state of the climate. Then we must know how the climate system varies in the absence of anthropogenic forcing. For example, a better understanding of El Niño should include factors that determine its intensity and frequency. El Niño may be affected by anthropogenic climate change. Through better observations, paleoreconstrutions and improved knowledge and understanding of natural variability, it will be possible to detect the anthropogenic climate signal with greater confidence” (McBean et al.,1995, p. 527). |
Success in initialization will be essential if geographical resolution is to be improved. Initialization focuses attention on the evaluation of model results and the intercomparison of models. As we resolve finer-scale issues, such as ocean eddies, these accomplishments will raise afresh the issues of coupling shock and model initialization. In such investigations, hybrid schemes should be considered in which low-resolution atmospheric models might be used in the initial stage of coupling. The questions of coupling and initialization are so closely connected with the long-term prediction problem of climate and of CO2 uptake by the oceans (and the terrestrial biosphere) that they must be central in future modeling work.
There are large differences in GCMs with respect to the scales of cloud microphysics and the treatment of these processes. There also tends to be a very real difference in focus between those models addressing the climate system at the large scale and those focused on small-scale processes as they occur in reality. To clarify the role of clouds in models, a hierarchy of models and observations needs to be used, and there should be greater emphasis on studies to isolate specific cloud processes. Finally, it would be useful to have a benchmark set of cloud and radiation diagnostics to be used to analyze feedbacks and compare models with observations.
The oceans are very energetic on spatial scales of 10 to 100 km, yet these motions are not resolved in the current generation of global climate models. A major unsolved problem in oceanography is to determine the effects of these unresolved mesoscale ocean eddies on large-scale circulation and climate.
The WOCE and JGOFS datasets of carbon and CFCs in combination with the ocean topography from the Ocean Topography Experiment (TOPEX/ Poseidon)91 and the current and next generation of satellite ocean color products, 92 as well as a number of existing seasonal, global-scale syntheses of nutrients, dissolved oxygen, surface carbon dioxide, and chlorophyll, present an unprecedented opportunity for evaluating models of the marine carbon cycle and extending our knowledge of its current state and potential future states. This is, as stated repeatedly, a central effort for the next decade.
THE ATMOSPHERIC PHYSICAL-CHEMICAL SUBSYSTEM
Overview
As we have noted throughout this report, important future changes of the Earth system will probably result, in part, from increasing atmospheric concentrations of greenhouse gases such as carbon dioxide, CFCs, methane, and nitrous oxide. These substances have biological, industrial, and other anthropogenic sources. In addition to their direct radiative effect, some of these gases undergo atmospheric chemical and photochemical transformations that alter the natural balance of other atmospheric gases. An important example is ozone, whose
changes in both the stratosphere and the troposphere are a source of serious global concern (see Chapter 5).
In reflecting about challenges in modeling the interaction of the physics and the chemistry of the atmosphere it is important to review the extraordinary successes that have been achieved and to recall the scientific and political challenges that faced the planet just a few decades ago.
The Earth's stratosphere contains a thin but crucial layer of ozone that filters out many damaging forms of solar radiation and makes life as we know it possible on the Earth's surface. Beginning in the 1970s, scientists became concerned that certain human-produced chemicals, known as CFCs, could diminish the stratospheric ozone layer.
In the 1980s atmospheric concentrations of CFCs continued to increase, and decreases in stratospheric ozone began to be detected. Stratospheric ozone concentrations over Antarctica plummeted at a remarkable rate during the Antarctic springtime—a phenomenon now referred to as the Antarctic ozone hole. Meanwhile, atmospheric scientists continued working to refine their models of ozone depletion, which had not predicted losses nearly as large as those observed over Antarctica. As a result of a crosscutting scientific effort, including theory, modeling, and observations, a sound scientific basis for the protection of stratospheric ozone was established. In response to the scientific findings, an international agreement was reached to halt production of the most destructive ozone-depleting chemicals. The decisive response of the world community to the stratospheric ozone threat was a tribute to a combined international scientific and policy-making effort.
Advanced three-dimensional atmospheric models were developed to study the interaction of chemistry, dynamics, and radiation in the stratosphere. These extensive calculations were necessary for evaluating the simpler models used in the policy assessment studies as well as for understanding the climatic impact of the Antarctic ozone hole.
Many questions are still unanswered about the future of stratospheric ozone. Will an ozone hole like the one over Antarctica develop over the northern hemisphere in the coming years? How will greenhouse gas-induced climate change interact with stratospheric ozone chemistry? These and other problems, such as the emergence of a global tropospheric ozone problem (see Chapter 5), involving the physics and chemistry of the atmosphere will challenge the scientific community for decades into the future.93
A Modeling Perspective
The goal is a completely interactive simulation of the dynamical, radiative, and chemical processes in the atmosphere. Such a model will be essential in future studies of tropospheric trace constituents such as nitrogen oxides, ozone, and sulfate aerosols. Nitrogen oxides are believed to control the production and
destruction of tropospheric ozone, which controls the chemical reactivity of the lower atmosphere and is itself a significant greenhouse gas. Tropospheric sulfate aerosols, on the other hand, are believed to significantly affect the Earth's radiation budget by scattering solar radiation.
Models that incorporate atmospheric chemical processes provide the basis for much of our current understanding in such critical problem areas as acid rain, photochemical smog production in the troposphere, and depletion of the ozone layer in the stratosphere.94 These formidable problems require that models include chemical, dynamical, and radiative processes, which through their mutual interactions determine the circulation, thermal structure, and distribution of constituents in the atmosphere. That is, the problems require a coupling of the physics and chemistry of the atmosphere. Furthermore, the models must be applicable on a variety of spatial (regional to global) and temporal (days to decades) scales.95 Fortunately, there have been advances in three-dimensional modeling of the chemistry of both the stratosphere and the troposphere, including modeling the tropospheric distribution of aerosols.96
Until relatively recently, atmospheric chemistry studies have often relied on two-dimensional (latitude and altitude) models.97 These models solve the zonally averaged momentum, thermodynamic, and mass continuity equations and include a detailed treatment of chemistry and radiative processes. Because of the demanding computational requirements, many two-dimensional models group related constituents into “families” to avoid explicit integration of a mass continuity equation for each individual chemical species (not unlike the grouping that occurs in ecosystem models). A major problem with two-dimensional models has been the necessity to include the effects of horizontal transport by zonally asymmetric motions (waves or eddies) by means of eddy diffusion terms analogous to the approach adopted for vertical transport in the one-dimensional models. As a consequence, these models do not correctly represent the interactive behavior of the chemical, radiative, and dynamical processes. Despite their shortcomings, the models have provided significant insight into atmospheric chemical processes through incorporation of horizontal motions. They will also continue to provide the basis for ozone assessment studies well into the next decade, until significant progress is made in developing three-dimensional models and acquiring and making available the essential, more powerful computing resources, since it is not just the computational cost of the fluid dynamic equations but the chemistry equations as well (which are often the most computationally expensive step).
Most effort in three-dimensional atmospheric chemistry models over the past decade has been in the use of transport models in the analysis of certain chemically active species (e.g., long-lived gases such as N2O or the CFCs). In part, the purpose of these studies was not to improve our understanding of the chemistry of the atmosphere but rather to improve the transport formulation associated with GCMs and, in association with this improvement, for understanding sources and sinks of carbon dioxide.98 More recently, attempts have been made to develop
more chemically intensive three-dimensional global models. Although efforts to include chemistry in a three-dimensional model date back at least two decades, progress has been relatively slow due to the enormous computational requirements for treating the fluid dynamic equations alone. The additional burden imposed by incorporating detailed chemistry into a comprehensive GCM has made long-term simulations and transient experiments with existing computing resources impractical. Current three-dimensional atmospheric chemistry models that focus on the stratosphere seek a compromise solution by combinations of expedients: using coarse resolution (both vertical and horizontal dimensions); incorporating constituents by families (similar to the practice used in most two-dimensional models); omitting or simplifying parameterizations for tropospheric physical processes; or conducting “off-line” transport simulations in which previously calculated wind and temperature fields are used as known input to a series of mass continuity equations including chemical source/sink terms. This last approach renders the problem tractable and has produced much progress toward understanding the transport of chemically reacting species in the atmosphere. The corresponding disadvantage is the lack of interactive feedback between the evolving species, distributions, and the atmospheric circulation.
As attention is turned toward the troposphere, the experimental strategy simply cannot adopt the stratospheric simplifications. The uneven distribution of emission sources at the surface of the Earth and the role of meteorological processes at various scales must be addressed directly. Fine-scaled three-dimensional models of chemically active trace gases in the troposphere are needed, which should resolve transport processes at the highest-possible resolution. These models should be designed to simulate the chemistry and transport of atmospheric tracers on global and regional scales with accurate parameterizations of subscale processes that affect the chemical composition of the troposphere. It is therefore necessary to pursue an ambitious long-term perspective to develop comprehensive models of the troposphere system, including chemical, dynamical, radiative, and eventually biological components. The development of such models and their integration in even more complex Earth system models will require stable long-term support for interdisciplinary research teams to clarify processes and develop the needed in situ datasets, including improved estimates of past and present trace gas emissions. A large effort will have to be devoted to studies of various individual processes affecting the interplay of atmospheric chemistry and physics on global and regional scales. These models will require significantly advanced computing machinery that is currently simply not available to the USGCRP. There are no shortcuts with respect to the needed process studies, the required in situ data, the essential computing power, and organized support for model development and evaluation.
Efforts to develop a fully coupled atmosphere-ocean GCM with linked atmospheric chemistry and physics are still in their infancy. Fortunately, there has been exciting progress in the past decade. Two examples bear special mention:
the National Center for Atmospheric Research (NCAR) IMAGES model and the chemical transport models coupled to the NCAR CCM2.99
NCAR IMAGES Model
The Intermediate Model for Global and Annual Evolution of Species (IMAGES) has been developed to reproduce the three-dimensional distribution of chemically active trace gases in the troposphere and to study the relative contributions of chemistry, advective and convective transport, surface emission, and deposition in the global budget of these species. The model extends from the surface to the 50-mbar pressure level, including 24 unequally spaced levels in the vertical and a horizontal resolution of 5° in latitude and longitude. It deals with species belonging to the oxygen family (ozone and oxygen atoms), the nitrogen family (NO, NO2, NO3, N2O5, HNO3, peroxyacetylnitrate), the hydrogen family (OH, HO2, H2O2), carbon oxides (CO, CO2), hydrocarbons (CH4, isoprene), and several intermediate products of hydrocarbon degradation. Chemical and photochemical reactions required to simulate the reactions affecting these species are taken into account. To ensure computing efficiency, the photodissociation coefficients are provided from a look-up table as a function of altitude, ozone column abundance, solar zenith angle, and surface albedo. Diurnal variations of photochemical processes are either explicitly simulated or parameterized through a correction in the reaction rates.
The transport of trace constituents is formulated by the semi-Lagrangian transport model. What makes the model “intermediate” is that the transport is driven by observed monthly mean winds (as opposed to winds provided every few hours). The effect of wind variability over a month is expressed through an eddy diffusion formulation with the diffusivity derived from the observed wind variance. Convection is assumed to take place in cumulonimbus-type clouds whose spatial and temporal distribution is provided by climatology.
In the present version of the model the geographical distributions of trace gas surface emission and deposition on the global scale are taken from literature estimates. Biogenic sources, including emissions from biomass burning, foliage, and soil, are established for each month of the year on the basis of a world ecosystem database. Anthropogenic sources, including fossil fuel burning, industrial processes, and waste disposal, are established on the basis of economic statistics.
Chemical Transport Models Coupled to the NCAR CCM2
The chemical transport model coupled to the NCAR Community Climate Model (CCM2) is very similar to IMAGES for the surface sources of trace constituents, chemical reactions, and surface deposition. Transport is also expressed through a semi-Lagrangian formulation. There are, however, important
differences from IMAGES. First, the model extends up to the pressure level of 2 mb and therefore includes a large part of the stratosphere. Second, the horizontal resolution corresponds to a spectral model with 42 waves in the meridional and zonal directions, respectively, with triangular truncation (T42). Versions of the model at lower resolution are also available. Third, the winds are provided by CCM2 at relatively short intervals (e.g., six hours). The model can be run either in an online mode (synchronously with the dynamical model) or an offline mode (using the winds previously calculated and stored). Tests are currently performed to validate the offline approach and determine the most appropriate way by which dynamical variables (including the information on vertical convection) should be transferred from the GCM to the transport model. The model will also be run with analyzed winds (e.g., from the European Centre for Medium-Range Weather Forecasts), especially when model results will be compared to observations at specific sites.
One of the major challenges is incorporating heterogeneous reactions into models. As discussed in Chapter 5, we now know that during Antarctic springtime reactions on the surface of polar stratospheric cloud particles are instrumental in the destruction of polar ozone. Similar heterogeneous reactions on sulfate aerosol particles at middle latitudes are also possible. There is also concern about enhancements of the stratospheric aerosol burden by large volcanic injection events (e.g., El Chichon) and the release of aerosols through industrial activity and their diffusion into the stratosphere. Although a substantial database on aerosols exists (both satellite and ground-based data), global atmospheric chemistry models typically do not include aerosol effects.
In addition to chemistry issues, a number of shortcomings in current models are related to dynamical processes and thereby affect the ability of the models to predict the distribution of chemically reactive species. For example, global models typically do not simulate those equatorial wave modes (Kelvin and Rossby gravity waves) that are thought to force the semiannual and quasi-biennial oscillations in the stratosphere. This inadequacy of the models is either a result of insufficient resolution or failure to include tropospheric convective processes believed to be the source of these waves. Some atmospheric chemistry models (notably two-dimensional models) have attempted to include these effects by ad hoc methods.
Perhaps an even more important deficiency in models used to study atmospheric chemistry is the failure to include, or to treat adequately, cloud processes and the hydrological cycle. This fault results from both inadequacy of computational resources and incomplete understanding of the hydrological cycle. The consequence of this deficiency is typically a poor simulation of the observed water distribution, and this implies an inadequate treatment of gas sources and sinks, particularly in terrestrial systems.
In sum, there is an emerging consensus that both two- and three-dimensional
atmospheric chemistry models will require significantly higher resolution than is now common. Furthermore, there is a need for long-term simulations (tens of years) to examine the interannual variability exhibited by the models and the degree to which that variability is consistent with observed statistics. These considerations pose significant computing resource demands if progress is to be achieved. In addition, there are many gaps in our fundamental understanding of chemical and dynamical processes (and radiative processes to a lesser extent), which inhibit progress in modeling atmospheric chemistry.
Research Priorities
Further progress in modeling the interplay between the physics and chemistry of the atmosphere requires a better knowledge in five key areas:
-
Surface sources and sinks of trace gases, in particular exchanges of terrestrial ecosystems with the atmosphere and exchanges between the surface ocean and the atmosphere. Understanding will be advanced partly by systematic observations of different terrestrial ecosystems and surface marine ecosystems under variable meteorological conditions and by the development of ecosystem and surface models that will provide parameterizations of these exchanges.
-
Global empirical models of surface emissions are necessary to extrapolate and interpolate individual measurements provided in different environments under different conditions. These models will be based on empirical relationships accounting for the variation in emissions with climate parameters such as temperature, solar radiation, and soil moisture. In addition, there is a need to improve our understanding of historical as well as present emissions.
-
Models of detailed biological mechanisms in terrestrial and oceanic systems associated with trace gas emissions in soil, oceans, etc., need to be developed. The processes to be considered will range from the leaf of a tree to an entire ecosystem.
-
Models and associated in situ data of physical processes, surface exchanges, and transport in the boundary and surface layers that describe the transfer of key chemical species between the ocean or land surfaces and the atmosphere.
-
An extensive and long-term system of in situ atmospheric observations of key chemical species (e.g., carbon dioxide) from a suite of towers (e.g., AmeriFlux,100 EUROFLUX,101 and JapanNet102) is needed, together with long-duration aircraft and oceanic buoy arrays.
-
-
Chemical models with a detailed set of reactions, in which transport is ignored, need to be developed. These models will describe the complex relationship between hydrogen, nitrogen, and oxygen species as well as
-
hydrocarbons and other organic species and will be used to establish simplified chemical schemes that will be implemented in chemical/transport models.
-
Transport models coupled to GCMs, with detailed representation of physical processes, including cloud formation and boundary layer transport, are required to simulate how advection, turbulence, and convection affect the chemical composition of the atmosphere. Several approaches can be used, including Eulerian and Lagrangian formulations. These models will be used with minimum chemistry to simulate the global distribution and variability of long-lived species (e.g., greenhouse gases) and with more detailed chemistry to explore the role of meteorological processes in determining the spatial distribution and temporal variability of short-lived species. In this second case the importance of continental pollution on the remote troposphere and on the oxidizing capacity of the atmosphere needs further study.
-
Hydrological processes and energy exchange, especially processes involving clouds, surface exchanges, and their interactions with radiation, are crucially important research problems. Determining feedbacks between the land surface and other elements of the climate system will require careful attention to the treatments of evapotranspiration, soil moisture storage, and runoff. These topics have arisen several times in this chapter. All of these occur on spatial scales that are small compared to the model meshes, so the question of scaling must be addressed. These improvements must be paralleled by the acquisition of global datasets for validation of these treatments. Validation of models against global and regional requirements for energy conservation is especially important in this regard.
In a similar vein, to simulate the effects of clouds on chemical constituents and to investigate the mechanisms involved in wet removal of atmospheric constituents, models should be developed to account for the following processes: cloud convection, which provides an efficient mechanism for vertical transport in the troposphere; meteorological transport through the boundary and surface mixed layer; aqueous transformations of species in clouds; and precipitation of trace gases and wet deposition. This may require the development of subgrid-scale convection routines in the models, including the planetary boundary layer, with moist processes and treatment of cloud and precipitation physics to provide radiatively important parameters, such as cloud liquid water and drop size distributions.
Finally, improvements are needed in models of aerosols and how they affect homogeneous chemistry and the hydrological cycle. This coupling of aerosols with both the energy and the water cycles as well as with the chemistry components of the system is of increasing importance. In
-
addition, advances are needed in models to examine the effects of chemical changes on climate processes such as convection changes through heating the midtroposphere.
-
Models of the middle atmosphere are used in relation with the ozone budget. Many studies are based on two-dimensional models that include a relatively detailed chemical scheme as well as a radiative code that takes into account the important coupling between radiative (thermodynamic) and chemical processes. Three-dimensional models need to be improved and applied to the middle atmosphere. These models will be able to reproduce explicitly the propagation of planetary waves and simulate their effects on the meridional transport of trace gases such as ozone. These models are also needed to study the dynamical and chemical mechanisms involved in the formation and dissipation of the ozone hole over Antarctica. Models of the stratosphere should include the effects of aerosols and polar stratospheric clouds on chemical budgets.
THE HUMAN LINKAGE TO THE EARTH SYSTEM
Human processes are critically linked to the Earth system as contributing causes of global change, as determinants of impacts, and through responses. Representing these linkages poses perhaps the greatest challenge in modeling the Earth system. But understanding them is essential to understanding the behavior of the whole system and to providing useful advice to inform policy and response. Significant progress has been made, but formidable challenges remain (see Chapter 7).103
Human activities have altered the Earth system at all spatial scales, and many such influences are accelerating. Fossil fuels and chemical fertilizers are major influences as is the human transformation of much of the Earth's surface over the past 300 years. Land use change illustrates the potential complexity of linkages between human activity and major nonhuman subsystems of the Earth system. The terrestrial biosphere is fundamentally modified by land clearing for agriculture, industrialization, and urbanization and by forest and rangeland management practices. These changes affect the atmosphere through altered physical properties such as albedo and roughness and consequently an altered energy balance over the more intensively managed parts of the land surface, as well as through changed fluxes of H2O, CO2, CH4, and other trace gases between soils, vegetation, and the atmosphere. Changed land use also greatly alters the fluxes of carbon, nutrients, and inorganic sediments into river systems and consequently into many oceanic coastal zones.
The response of the total Earth system to these changes in anthropogenic forcing is currently not known. Sensitivity studies with altered land cover distributions in GCMs have shown that unrealistically drastic changes, such as total deforestation of all tropical or boreal forests, may lead to feedbacks in atmo-
spheric circulation and a changed climate that would not support the original vegetation.104 As pure sensitivity studies of the atmospheric circulation, however, these global experiments do not attempt to mimic the land use changes that have actually occurred—they only indicate that such feedbacks may indeed be critical for the stability of the overall system. Regional climate simulations, on the other hand, have shown that at the continental scale important teleconnections may exist through which tropical forest clearing may cause a change in climate conditions in much less disturbed areas.105
Human land use change will likely continue and accelerate over large areas due to increasing demands for food and fiber, changes in forest and water management practices, and possibly large-scale projects to sequester carbon in forests or to produce biomass fuels. Predicting the future response of the Earth system to changes in land use and land cover will require projections of trends in the human contributions to these global changes, and this sort of modeling presents difficult challenges because of the multiple factors operating at local, regional, national, and global levels to influence local land use decisions. 106 The complex linkages between human activity and global change are equally important for activities other than land use. In particular, anthropogenic changes in material and energy fluxes, resulting from such activities as fossil fuel combustion and chemical fertilizer use, are expected to increase in the coming decades. Predictions of changes in the carbon and nitrogen cycles are sensitive to estimates of human activity, and predictions of the impacts of these global changes must take into account human vulnerability, adaptation, and response.
Representing human processes is also essential for understanding impacts of global change. Social, political, and economic mechanisms of adaptation and response determine vulnerabilities and mediate the impact of changes in biophysical systems. Responses to perceived or anticipated environmental changes by individuals, organizations, communities, markets, and governments may modify the behaviors that contribute to global change and so create feedbacks between the human and nonhuman systems.
To provide useful guidance to inform policy requires insights into all of these processes, which in turn requires observation and description of human contributions, impacts, and responses, as well as modeling and theoretical studies of the underlying social processes that shape them. Active research is under way to address these questions, and progress is being made (see Chapter 7). Significant examples of recent progress include studies of the multiple linked determinants and consequences of land use change, models of agricultural impacts of climate change that incorporate adaptive behavior, and models of human health stresses and agricultural impacts from potential climate-induced shifts in disease vector and pest populations.107
Causal models of social processes have large uncertainties and pose deep problems, which may be of a qualitatively different character than those associated with modeling nonhuman components of the Earth system. The diversity of
societies, cultures, and political and institutional contexts may frustrate attempts to develop predictive or causal rules of human behavior that can be generalized globally. It also highlights the importance of global observations and comparative studies. Representation of human behavior at the micro- (individual) and macro- (collective) scales may require fundamentally different approaches to explanation, and linking between micro- and macroscales presents additional modeling challenges.108 Moreover, predictive models directed to decision makers may alter the behavior they seek to explain and predict—indeed, such models may be used explicitly with that purpose.
While difficulties such as these may intrinsically limit the predictive power that can be ascribed to models of social processes, such modeling may still provide various forms of useful insights to inform policy deliberations or other decision making. There has been a rapid increase in attempts to integrate representations of human causes, impacts, and responses in models with explicit formal linkages to other components of the Earth system. Such integrated assessment models have offered preliminary characterizations of human-climate linkages, particularly through models of multiple linked human and climatic stresses on land cover; have provided preliminary characterization of broad classes of policy responses; and have been used to characterize and prioritize key policy-relevant uncertainties.109
This early progress gives ground for optimism, although serious difficulties remain. Studying human linkages with other components of the Earth system may be the most difficult challenge in modeling global change and the most important. Understanding human impacts and potential responses is a central purpose of the endeavor of global change research. Both the uncertainties in human processes and the sensitivity of other Earth system components to human perturbations are large. Indeed, early integrated assessment results suggest that the contribution of social and economic uncertainties to uncertainty in future impacts, and to preferred responses, are likely to exceed that of biophysical uncertainties.
SUMMARY
As the USGCRP is preparing to enter its second decade, the integrative phase, the strategy for the coming decade must establish techniques for coupling and integration of physical, biogeochemical, and the human dimension subsystem models in preparation for the construction of integrated prognostic Earth system models. The strategy should include four aspects, each of which will contribute to an overall objective of developing the prognostic modeling capacity essential to the needs of the USGCRP. These aspects are at different levels of organization: the first is at the component level where work, though advanced, is still required. The second level is at the subsystem level and focuses on the issues of boundary compatibility across key interfaces highlighted in this chapter. This will require
modeling workshops involving intercomparisons of like subsystem models and intercomparisons involving coupling between adjacent subsystem models (which must match boundary conditions and fluxes). Issues of the adequacy of data for testing and rejection will be central. The third and fourth segments will be at the system level. The third level focuses on simple Earth system models, wherein models are compared to highlight differences in coupling techniques, interelement fluxes across key boundaries, and sensitivity studies to reveal the differences between models and the relative importance of individual system parameters. It sets the stage for the fourth level, which will be at the Earth system science level with richly developed components. Here the challenges will be significant.
This chapter focuses on level two: subsystem integration and linkage. Subsystem integration involves at least four key linkages: land-atmosphere, land-ocean, ocean-atmosphere, and atmospheric physics with atmospheric chemistry. In addition to this complexity, there is the essential component of the human dimension to global environmental change.
The Terrestrial-Atmosphere Subsystem
Immediate challenges that confront models of the terrestrial-atmosphere subsystem include exchanges of carbon and water between the atmosphere and land and the terrestrial sources and sinks of trace gases. An overarching grand challenge is to provide insight into the dynamics of a biosphere subjected to multiple stresses, which after all is the actual case we confront (see Chapter 2 and Box 10.6).
A central challenge is to develop coherent explanations for past changes in the total carbon fluxes and/or storage, to test hypotheses about the underlying causes of these changes, and to establish the capability for estimating future changes. It is now becoming evident that models of the terrestrial carbon cycle and of terrestrial ecosystem processes in general are going to play an overriding role in addressing many of the issues posed by global environmental change. The question of climate change is a case in point. Describing, characterizing, and eventually understanding and predicting the spatial patterns of changes in terrestrial carbon storage and associated fluxes are critical for understanding and coping with global environmental change. Understanding the carbon cycle is directly linked to understanding nutrient cycles, particularly nitrogen, and the water cycle, particularly soil moisture.
There is a similar challenge in understanding the hydrological cycle. For terrestrial systems themselves and their interaction with climate and the chemistry of the atmosphere, soil moisture is central. It is a key component in the land surface schemes in GCMs, since it is closely related to evaporation and thus to the apportioning of sensible and latent heat fluxes, and accurate prediction of soil moisture is crucial for simulation of primary production and of soil and vegetation biochemistry, including trace gas exchanges.
|
BOX 10.6 In the 1995 IPCC Summary for Policy Makers: The Science of Climate Change, the concluding section says that “there are still many uncertainties.” This section observes that “[m]any factors currently limit our ability to project and detect future climate change. In particular, to reduce uncertainties further work is needed on the following priority topics:
The IPCC further notes that “[f]uture unexpected, large and rapid climate system changes (as have occurred in the past) are, by their nature, difficult to predict. This implies that future climate changes may also involve “surprises. ” In particular, these arise from the non-linear nature of the climate system. When rapidly forced, non-linear systems are especially subject to unexpected behaviour. Progress can be made by investigating non-linear processes and sub-components of the climatic system.”110 |
Dynamic vegetation models in which land use and land cover changes are interrelated in terms of processes and feedbacks offer an advanced approach to coupling the human driving variables with ecosystem response functions, hydrological dynamics, atmospheric conditions, and edaphic (fire-related) factors. Such models account for the role of transient states of secondary succession following disturbance. These developing models can simulate ecosystem responses with particular emphasis on vegetation dynamics on timescales from decades to centuries and provide a means of investigating responses to disturbances such as deforestation. However, a fundamental problem in assessing the results of terrestrial ecosystem models is a lack of good validation data.
Finally, since agricultural and forestry production provides the essential food, fuel, and economic resources for the world, monitoring and modeling of biospheric primary production are important to support global economic and political policy making. Fortunately, during the past decade of the USGCRP, it has become possible to investigate the magnitude and geographical distribution of
these processes on a global scale by a combination of ecosystem process modeling and monitoring by remote sensing. While progress will be made (and is needed) on modeling terrestrial processes, more integrative studies are also needed wherein terrestrial systems are coupled to models of the physical atmosphere and eventually to the chemical atmosphere as well. Tying in the human component is clearly an important and needed future crucial step.
The Terrestrial-Ocean Subsystem
The cycling of water between land and atmosphere often produces a “residual” or runoff, and this water forms the basis of rivers and the recharge of aquifers. These flows are the focus of water transport models, which are tied to the coupled dynamics of the terrestrial ecosystem and the land-water cycle. The drainage basin, then, becomes the logical unit of organization; as its size is varied, the associated finite element grid varies in mesh size. Using the drainage basin as a focal unit allows a broad spectrum of fluvial systems to be considered.
Coupling of models between drainage basins and the nearshore will also be necessary to provide a complete analysis of the interaction of terrestrial and coastal zone ecosystems. Such coupling may require coastal physical oceanographic models linked to biogeochemical process simulations of regional land-coastal margin ecosystems. This issue is an important research topic in itself. A long-term goal is to model a series of material transformations along the entire continuum of fluvial systems from the points of terrestrial mobilization to delivery and processing in the coastal zone. The fluxes of constituents in various chemical and physical states would be included in such models.
The Ocean-Atmosphere Subsystem
This, with the terrestrial-atmosphere coupling, is a central aspect in the building and coupling of Earth system models. The ocean and the atmosphere have significantly different space and timescales (which themselves depend on what is being tracked), and they are often quite stiff as linked systems and therefore present major difficulties in perturbation experiments. There are also greatly differing degrees of parameterization with insufficient understanding as to their effects.
For this subsystem attention is focused on three specific areas: clouds in atmospheric models, carbon in the ocean, and the problem of linking ocean circulation models with models of atmospheric circulation.
Clouds
Handling the physics and/or parameterization of clouds in climate models remains a central difficulty. Clouds and their impact on climate remain the key
uncertainty in estimating the sensitivity of the Earth's climate to increased greenhouse gases. Cloud modeling is a particularly challenging scientific problem in the climate and global change arena because it involves processes covering a very wide range of space and timescales.
Even the basic issue of the nature of the future cloud feedback is not clear. As the planet warms, it is likely that evaporation will increase, which could yield more clouds, but will these additional clouds enhance the greenhouse effect or damp it? This question remains open, and it is not clear how it will be answered. There are large differences in GCMs with respect to the scales of cloud micro-physics and the treatment of these processes. To clarify the role of clouds in models, a hierarchy of models and observations needs to be used, and there should be greater emphasis on studies to isolate specific cloud processes. Finally, it would be useful to have a benchmark set of cloud and radiation diagnostics to be used to analyze feedbacks and compare models with observations.
Ocean Carbon
The ocean's critical role in the global carbon cycle and thus climate change is illustrated by its immense carbon reservoir and ability to continue to absorb and retain substantial quantities of excess CO2 as atmospheric levels continue to rise. Modeling the ocean's carbon cycle is essential in part because data coverage is limited, both spatially and temporally. Realistic three-dimensional models can be used for interpolation and extrapolation. Furthermore, ocean models can be used to estimate the anthropogenic component of CO 2 in the ocean, which is difficult to estimate by direct measurement. Ocean models are far from perfect, however, and much work is required if reliable predictions of future oceanic CO2 uptake are ever to become feasible.
There is a fundamental need to compare ocean carbon cycle models and thereby to clarify key physical and biogeochemical processes. Fortunately, this process has begun by comparing simulations of bomb and natural 14C. The latter offers a powerful test of an ocean model's deep-ocean circulation, whereas the former is considered a reasonable analog for anthropogenic CO2. Of particular value in this regard are the new high-precision measurements of oceanic carbon and 14C that are now becoming available from samples collected during the WOCE and the JGOFS.
More generally, the WOCE and JGOFS datasets of carbon and CFCs in combination with the ocean topography from TOPEX/Poseidon and the next generation of satellite ocean color products, as well as a number of existing seasonal global-scale syntheses of nutrients, dissolved oxygen, surface carbon dioxide, and chlorophyll, present an unprecedented opportunity for evaluating models of the marine carbon cycle and extending our knowledge of its current state and potential future states. This is a central effort for the next decade. These data form the anvil on which to shape the next generation of ocean-atmosphere
carbon cycle models, but as these data are consumed, meeting the challenge of model validation must not be postponed; this must be confronted.
Coupling and Initialization
Coupled ocean-atmosphere GCMs are fundamental to the study of the climate system. Models, by definition, are reduced descriptions of reality and hence incomplete and with error. Missing pieces and small errors can pose difficulties, as indicated above, when models of major subsystems such as the ocean and the atmosphere are coupled. Inconsistencies with processes or data between the submodels and/or incompleteness can lead to numerical drift when the models are coupled. The longer-term transient integrations needed in decadal to centennial global change challenges highlight these difficulties. The overriding challenge to modeling is prediction. This challenge is particularly acute when predictive capability is sought on timescales from seasonal to decadal to centennial and where one is confronted with a coupled stiff system like the ocean-atmosphere. It is a challenge that must be met in the coming decade.
The Atmospheric Physical-Chemical Subsystem
Models that incorporate atmospheric chemical processes provide the basis for much of our current understanding in such critical problem areas as acid rain, photochemical smog production in the troposphere, and depletion of the ozone layer in the stratosphere. These formidable problems require that models include chemical, dynamical, and radiative processes, which through their mutual interactions determine the circulation, thermal structure, and distribution of constituents in the atmosphere. That is, the problems require a coupling of the physics and chemistry of the atmosphere. Furthermore, the models must be applicable on a variety of spatial (regional to global) and temporal (days to decades) scales.
Further progress in modeling the interplay between the physics and chemistry of the atmosphere requires better knowledge in five key areas:
-
Surface sources and sinks of trace gases, in particular exchanges of terrestrial ecosystems with the atmosphere and exchanges between the surface ocean and the atmosphere.
-
Chemical models with a detailed set of reactions, in which transport is ignored, need to be developed.
-
Transport models coupled to GCMs, with detailed representation of physical processes, including cloud formation and boundary layer transport, are required to simulate how advection, turbulence, and convection affect the chemical composition of the atmosphere.
-
Hydrological processes and energy exchange, especially processes involving clouds, surface exchanges, and their interactions with radiation,
-
are crucially important research problems. Improvements are needed in models of aerosols and how they affect homogeneous chemistry and the hydrological cycle.
-
Three-dimensional models of the middle atmosphere, which are used in relation with the ozone budget, need to be further developed.
The Human Linkage to the Earth System
Finally, human processes are linked to the Earth system as contributing causes of global change, as determinants of impacts, and through responses. Studying human linkages with other components of the Earth system may be the most difficult challenge in modeling global change and the most important. Understanding human impacts and potential responses is a central purpose of the endeavor of global change research, but both the uncertainties in human processes and the sensitivity of other Earth system components to human perturbations are large. Hence, representing the linkages between humans and other components of the Earth system poses a challenge in modeling the Earth system, and hence understanding them is essential to understanding the behavior of the whole system and to providing useful advice to inform policy and response.
Causal models of social processes have large uncertainties and pose deep problems, which may be of qualitatively different character than those associated with modeling nonhuman components of the Earth system. The diversity of societies, cultures, and political and institutional contexts may frustrate attempts to develop predictive or causal rules of human behavior that can be generalized globally. Moreover, predictive models directed to decision makers may alter the behavior they seek to explain and predict—indeed, such models may be used explicitly with that purpose in mind.
The challenges in modeling the Earth system, including the human component, are daunting, but the need for integrative insights, which models can produce, is ever more important. The challenges simply must be met.
NOTES
1. NASA (1986).
2. See http://www.gcrio.org/ipcc/cover.html.
3. Meadows et al. (1972) and Mesarovic and Pestel (1974). See also Legasto et al. (1980).
4. For a general account of models of this period, see Bolin et al. (1979) and Bolin (1981). See also Bolin et al. (1986).
5. For instance, see Fasham (1995) and Fasham et al. (1993).
6. This strategy has been devised mainly through the activities of the International Geosphere-Biosphere Programme (IGBP), primarily through its task force on Global Analysis, Interpretation, and Modeling (GAIM) and its core project on Global Change and Terrestrial Ecosystems (GCTE). See the subsequent cites to the Potsdam '95 and VEMAP intercomparison efforts. See also Heimann et al. (1997) and Kicklighter et al. (1997).
7. See Cramer et al. (in press) and Bondeau et al. (in press). Also see Churkina et al. (in press),
Kicklighter et al. (in press), Nemry et al. (in press), Ruimy et al. (in press), Schloss et al. (in press), and Hibbard and Sahagian (1998).
8. VEMAP Participants (1995), and Schimel et al. (1997a). Also Pan et al. (1998), Field et al. (1996).
9. Under the auspices of the World Climate Research Programme (WCRP), the Atmospheric Model Intercomparison Project (AMIP) was established in which the 10-year period 1979 to 1988 has been simulated by 30 different atmospheric models under specified conditions (see http://www.wmo.ch/); the Coupled Model Intercomparison Project (CMIP) is being organized in a similar manner; the Paleoclimate Model Intercomparison Project (PMIP) is comparing the response of 17 climate models to identical orbital forcing for 6,000 years.
10. For instance, see Dickinson and Henderson-Sellers (1988), Lean and Warrilow (1989), Shukla et al. (1990), and Henderson-Sellers et al. (1993).
11. Manabe and Stouffer (1994). See also Manabe and Stouffer (1993).
12. See Chapter 3 and Chapter 4 of this report; see also Chang and Battisti (1998) and Glantz (1996). Furthermore, although there appears to be an improvement in our ability to predict an El Niño, the post-El Niño dynamics remain very troublesome.
13. “Among the first technologies to be encountered on the frontiers of computer technology is the petaFLOPS architecture of computing. The ‘FLOPS' in the curious name; ‘petaFLOPS' comes from ‘Floating Point Operations per Second,' and refers to the rate at which a computer can process instructions. A petaFLOPS computer theoretically can perform a million billion operations per second. That's equivalent to nearly 15 times all the networked computing capability in the US today. It would be roughly 10,000 times faster than the largest available massive parallel computer. ” From http://www.aminsights.com/peta.htm.
14. There is an exciting new national effort on Earth system models being advanced by Japan. See Normile (1997). See also http://www.sta.go.jp/umi/e-umi.html.
15. For instance, the work at the MIT Joint Program on the Science and Policy of Global Change (http://web.mit.edu/globalchange/www/) and at the Center for Integrated Study of the Human Dimensions of Global Change (http://hdgc.epp.cmu.edu/main.html) of the Department of Engineering and Public Policy (http://www.epp.cmu.edu/) at Carnegie Mellon University.
16. See http://www.gcrio.org/ipcc/cover.html.
17. E.g., Sellers et al. (1996) and Myneni et al. (1997). Also Henderson-Sellers (1993a, 1993b) and Henderson-Sellers and McGuffie (1995).
18. See previous cites to NPP intercomparasion efforts; see also Malmstrom et al. (1997) and McGuire et al. (1992, 1993, 1997). See also Melillo et al. (1993), Potter et al. (1993), Raich et al. (1991), and Schimel et al. (1994, 1997b, 1997c).
19. National Research Council (1990), page 30.
20. E.g., Melillo et al. (1996), Schimel et al. (1996), Schimel (1995).
21. E.g., see cites for the NPP intercomparasion efforts; see also Hibbard and Sahagian (1998).
22. See Houghton et al. (1996).
23. Schimel et al. (1996). Also Melillo et al. (1996).
24. See Section 2.1.3 in Schimel et al. (1996). See also Moore and Braswell (1994), Wigley (1993, 1997), and Wigley et al. (1996).
25.For an important and overall view of the terrestrial carbon cycle and the policy issues, see the recent article of the IGBP Terrestrial Carbon Working Group, “The terrestrial carbon cycle: Implications for the Kyoto protocol,” Science 280:1393-1394, 1998.
26. Regarding the coupling of the carbon and climate system, see Section 6.7.2 (and Section 6.7.3 for the coupling of the chemistry of the atmosphere) in Kattenberg et al. (1996).
27. See Weyant et al. (1996).
28. See Dickinson and Henderson-Sellers (1988), Lean and Warrilow (1989), Shukla et al. (1990), Henderson-Sellers et al. (1993a, 1993b), Henderson-Sellers (1994), and Ciret and Henderson-Sellers (1997).
29. Raich and Nadelhoffer (1989), McGuire et al. (1996, 1997), Schimel et al. (1994, 1997b, 1997c).
30. E.g., Henderson-Sellers (1996a, b), Henderson-Sellers et al. (1995a, b), Henderson-Sellers and Verner (1995).
31. National Research Council (1998).
32. Ibid. See particularly Henderson-Sellers (1996a) and Vörösmarty et al. (1998a).
33. The IGBP report by Vörösmarty et al. (1997b) was particularly useful in developing this section of the chapter.
34. See Correll (1986). Also see van de Ven et al. (1991), Vörösmarty et al. (1989, 1997a, 1998b), Vörösmarty and Moore (1991), Walling and Probst (1997) and Wilkinson (1993).
35. E.g., Vannote et al. (1980), Sedell et al. (1989).
36. E.g., Billen et al. (1994), Gildea et al. (1986), and Vörösmarty et al. (1986).
37. E.g., Elwood et al. (1983) and Newbold (1992).
38. E.g., Hofmann (1991) and Ver et al. (1994).
39. E.g., Vörösmarty and Moore (1991) and Vörösmarty et al. (1989).
40. E.g., Famiglietti and Wood (1994a, 1994b).
41. Ibid.
42. http://www.ogp.noaa.gov/gcip/gcipover.html.
43. http://www.cptec.inpe.br/lba/index.html.
44. http://www.orstom.fr/pgardes/page_presentation_ang.html, L'Institut français de recherche scientifique pour le développement en coopération.
45. See National Research Council (1998); http://www.mbl.edu/html/ecosystems/lmer/lmer.html.
46. More broadly, an extraordinary set of data is found in Degens et al. (1985).
47. E.g., Mackenzie et al. (1993). See also Vörösmarty et al. (1991, 1989).
48. See Kalma and Calder (1995).
49. http://www.epm.ornl.gov/chammp/chammp.html.
50. Gates et al. (1996), Kattenberg et al. (1996).
51. See CLIVAR (1995). See also www.dkrz.de/clivar/hp.html.
52. See Hasselmann (1976).
53. E.g., Boer (1998).
54. Key discussions are found in Section 5.2.2 in Gates et al. (1996) and Sections 6.2.3 and 6.2.4 in Kattenberg et al. (1996).
55. See Section 4.2 in Dickinson et al. (1996). See also Section 8.2.3 in Santer et al. (1996).
56. Ramanathan (1995), Cess et al. (1995), Sherwood et al. (1994), Ramanathan and Collins (1991), Ramaswamy and Ramanathan (1989).
57. Kattenberg et al. (1996).
58. FANGIO Workshop (1993).
59. See J. F. B. Mitchell's working paper for the JSC/CLIVAR Working Group on Coupled Models, September 1997. See also Section 4.2.5 (particularly p. 206) in Dickinson et al. (1996) and Cess et al. (1996).
60. See (particularly Section 11.6) McBean et al. (1995). See also Section 6.7 (particularly Section 6.7.1) in Kattenberg et al. (1996).
61. See Wielicki et al. (1995).
62. There is important work in GEWEX that holds promise for the future, but more effort needs to be made on attacking this important and vexing research issue. See Chahine (1992).
63. http://www.arm.gov.
64. The overarching issue of human-induced climate change raises the key issue of detecting this change. See Santer et al. (1996).
65. E.g., Najjar et al. (1992).
66. See Sarmiento et al. (1995) and Sarmiento and Le Quéré (1996).
68. For an excellent overview of the modern history and the issues confronting the modeling of ocean circulation, see Semtner (1995).
69. See Section 5.3.3 in Gates et al. (1996). For a particularly provocative article, see Broecker (1997).
70. Kattenberg et al. (1996, p. 346).
71. See http://www.gcrio.org/ipcc/cover.html.
72. http://topex-www.jpl.nasa.gov/index.html.
73. http://www.soc.soton.ac.uk/OTHERS/woceipo/ipo.html.
75. http://ads.smr.uib.no/jgofs/jgofs.htm.
76. For a good discussion of these issues, see (particularly Section 10.3.2) Denman et al. (1996).
77. Sarmiento and Le Quéré (1996).
78. E.g., Najjar et al. (1992).
79. For instance see Fasham (1995) and Fasham et al. (1993). Also, Sarmiento et al. (1993) and Hurtt and Armstrong (1996), Flynn and Fasham (1997), Popova et al. (1997), Ryabchenko et al. (1997).
80. For a slightly broader set of modeling issues, see Section 10.4 in Denman et al. (1996).
81. Until recently this “nutrient-restoring” approach was limited to annual mean models (Najjar et al., 1992) due to the lack of seasonally resolved surface nutrient observations. An intensive data archeology program at the National Oceanographic Data Center has resulted in a much larger global database of nutrients (see Levitus et al., 1993, and Levitus and Boyer, 1994), so that seasonal analyses are now possible (e.g., Anderson and Sarmiento, 1994, 1995; Sarmiento and Le Quéré, 1996; Najjar and Keeling, 1997).
82. E.g., Gruber et al. (1996).
83. The role of the biological pump in the ocean carbon cycle, discussed only briefly in Houghton et al. (1996); Sections 1.3.3.3 and 1.4.3 are expanded and updated here. From Section 10.3.2.1 in Denman et al. (1996).
84. http://ads.smr.uib.no/jgofs/jgofs.htm.
85. See again Denman et al. (1996).
86. E.g., Broecker et al. (1985, 1995).
87. http://ingrid.ldgo.columbia.edu/sources/.geosecs.
88. See OCMIP discussion on http://www.ipsl.jussieu.fr/OCMIP.
89. E.g., Lefevre et al. (1996, 1998), Takahashi and Sutherland (1995), Takahashi et al. (1997).
90. E.g., Warner and Weiss (1992), England et al. (1994), and England (1993, 1995), Dixon et al. (1996), and Warner et al. (1996). See also Rhein (1994) and Smethie and Pickart (1993).
91. http://topex-www.jpl.nasa.gov/index.html.
92. E.g., Yoder et al. (1993) and Banse and English (1994).
93. For an important discussion of the radiative forcing implications associated with the chemistry of the atmosphere, see Schimel et al. (1996).
94. For instance, see Langner and Rodhe (1991), Kanakidou and Crutzen (1993), Chuang et al. (1994), Cooke et al. (1996), Klonecki and Levy (1997), Berntsen et al. (1996, 1997), and Berntsen and Isaksen (1997).
95. For an example array of atmospheric chemistry models see http://www-pcmdi.llnl.gov/, http://www.gfdl.gov/gfdl research.html, and http://www-as.harvard.edu/chemistry/trop/index.html.
96. See Sections 2.2.1 and 2.3.2 in Schimel et al. (1996).
97. E.g., Hough (1991) and Wang et al. (1998).
98. The importance of this associated effort can be seen in Ciais et al. (1997a, 1997b) and Bousquet et al. (1996).
99. We note that this is not an exhaustive review of chemistry-climate models and that the selection of the two models from the NCAR is simply for exposition. There are advanced chemistry-climate (or circulation) models in Europe (e.g., Max Planck) and in the United States
(again for an example array of atmospheric chemistry models in the United States see http://www-pcmdi.llnl.gov/, http://www.gfdl.gov/gfdl_research.html, and http://www-as.harvard.edu/chemistry/trop/index.html.) Finally, in the context of climate modeling see also Section 6.7.3 in Kattenberg et al. (1996).
100. See http://www.esd.ornl.gov/programs/NIGEC/; see also http://cdiac.esd.ornl.gov/programs/NIGEC/fluxnet/index.html.
101. See http://www.unitus.it/eflux/euro.html.
102. See http://cdiac.esd.ornl.gov/programs/NIGEC/fluxnet/japan.txt.
103. An important summary of issues is provided by Bruce et al. (1996).
104. E.g., Claussen (1996) and Kutzbach et al. (1996).
105. E.g., Dickinson and Henderson-Sellers (1988), Salati (1986), Lean and Warrilow (1989), and Shukla et al. (1990).
106. A good introduction to this important literature can be found through the IGBP-IHNP Core Project: Land Use Cover Change (LUCC). See http://www.icc.es/lucc.
107. See again Bruce et al. (1996).
108. The important issue of scaling is addressed more fully in Gibson et al. (1998). See also http://www.uni-bonn.de/ihdp.
109. An important summary of progress on integrated assessment modeling can be found in Nakicenovic et al. (1994). See also the related publications Kaya et al. (1993) and Nakicenovic et al. (1994a, 1994b).
110. Houghton et al. (1996).
REFERENCES AND BIBLIOGRAPHY
Anderson, L., and J. Sarmiento. 1994. Redfield ratios of remineralization determined by nutrient data analysis . Global Biogeochemical Cycles 8:65-80.
Anderson, L., and J. Sarmiento. 1995. Global ocean phosphate and oxygen simulations. Global Biogeochemical Cycles 9:621-636.
Banse, K., and D.C. English. 1994. Seasonality of coastal zone color scanner phytoplankton pigment in the offshore oceans. Journal of Geophysical Research—Oceans 99:7323-7345.
Berntsen, T., I.S.A. Isaksen, W.C. Wang, and X.Z. Liang. 1996. Impacts of increased anthropogenic emissions in Asia on tropospheric ozone and climate—a global 3-D model study. Tellus Series B—Chemical and Physical Meteorology 48(1):13-32.
Berntsen, T.K., and I.S.A. Isaksen. 1997. A global three-dimensional chemical transport model for the troposphere model description and CO and ozone results. Journal of Geophysical Research—Atmospheres 102(ND17):21,239-21,280.
Berntsen, T.K., I.S.A. Isaksen, G. Myhre, J.S. Fuglestvedt, F. Stordal, T.A. Larsen, R.S. Freckleton, and K.P. Shine. 1997. Effects of anthropogenic emissions on tropospheric ozone and its radiative forcing. Journal of Geophysical Research—Atmospheres 102(ND23):28,101-28,126.
Billen, G., A. Eisenhauer, R.F. Spielhagen, M. Frank, and G. Hentzschel. 1994. BE-10 records of sediment cores from high northern latitudes—implications for environmental and climatic changes. Earth and Planetary Science Letters 124:171-184.
Boer, G.J. 1998. A study of atmophere-ocean predictability on long time-scales. Submitted to Climate Dynamics.
Bolin, B.B., ed. 1981. Carbon Cycle Modelling, SCOPE 16. Bolin, B. John Wiley & Sons Ltd., Chichester, U.K.
Bolin, B.B., E.T. Deegens, S. Kempe, and P. Ketner. 1979. The Global Carbon Cycle, SCOPE 13. John Wiley & Sons, New York.
Bolin, B.B., B.R. Doos, J. Jager, and R. Warrick. 1986. The Greenhouse Effect, Climate Change, and Ecosystems. John Wiley & Sons, Chichester, U.K.
Bondeau, A., J. Kaduk, D. Kicklighter, and the participants of Potsdam ‘95. In press. Comparing global models of terrestrial net primary productivity (NPP): Overview and key results. Global Change Biology (Special Issue).
Bousquet, P., P. Ciais, P. Monfray, Y. Balkanski, M. Ramonet, and P. Tans. 1996. Influence of two atmospheric transport models on inferring sources and sinks of atmospheric CO2. Tellus Series B—Chemical and Physical Meteorology 48(4):568-582.
Broecker, W.S. 1997. Thermohaline circulation, the Achilles heel of our climate system: Will manmade CO2 upset the current balance? Science 278(5343):1582-1588.
Broecker, W.S., T. Peng, G. Ostlund, and M. Stuiver. 1985. The distribution of bomb radiocarbon in the ocean. Journal of Geophysical Research 90:6953-6970.
Broecker, W.S., S. Sutherland, W. Smethis, T-H. Peng, and G. Ostlund. 1995. Oceanic radiocarbon: Separation of the natural and bomb components . Global Biogeochemical Cycles 263-288.
Bruce, J.P., H. Lee, and E.F. Haites. 1996. Climate change 1995: Economic and Social Dimensions of Climate Change. Cambridge University Press, New York.
Cess, R.D., M. Zhang, P. Minnis, L. Corsetti, E.G. Dutton, B.W. Forgan, D.P. Garber, W.L. Gates, J.J. Hack, E.F. Harrison, X. Jing, J.T. Kiehl, C.N. Long, J.J. Morcrette, G.L. Potter, V. Ramanathan, B. Subasilar, C.H. Whitlock, D.F. Young, and Y. Zhou. 1995. Absorption of solar radiation by clouds—observations versus models. Science 267(5197):496-499.
Cess, R.D., M.H. Zhang, W.J. Ingram, G.L. Potter, V. Alexseev, H.W. Barker, E. Cohen-Solal, R.A. Colman, D.A. Dazlich, A.D.D. Genio, M.R. Dix, V. Dymnikov, M. Esch, L.D. Fowler, J.R. Fraser, V. Galin, W.L. Gates, J.J. Hack, J.T. Kiehl, H.L. Treut, K.W. Lo, B.J. McAvaney, V.P. Meleshko, J-J. Morcrette, D.A. Randall, E. Roeckner, J-F. Royer, M.E. Schlesinger, P.V. Sporyshev, B. Timbal, E.M. Volodin, K.E. Taylor, W. Wang, and R.T. Wetherald. 1996. Cloud feedback in atmospheric general circulation models—An update. Journal of Geophysical Research 101:12,791-12,794.
Chahine, M.T. 1992. The hydrological cycle and its influence on climate. Nature 359:373-380.
Chang, P., and E. Battisti. 1998. The physics of El Niño. Physics World 11(8):41-47.
Chuang, C.C., J.E. Penner, K.E. Taylor, and J.J. Walton. 1994. Climatic effects of anthropogenic sulfate: Simulation from a coupled chemistry, climate model. Preprints from the Conference on Atmospheric Chemistry, Nashville, Tenn., Jan. 1994, American Meteorological Society, Boston.
Churkina, G., S. Running, A. Schloss, and the participants of Potsdam '95. In press. Comparing global models of terrestrial net primary productivity (NPP): Analysis of the seasonal behaviour of NPP, LAI, FPAR along climatic gradients across ecotones.Global Change Biology (Special Issue).
Ciais, P.H., A.S. Denning, P.P. Tans, J.A. Berry, D.A. Randall, J.J.G. Collatz, P.J. Sellers, J.W.C. White, M. Trolier, H.J. Meijer, R.J. Francey, P. Monfray, and M. Heimann. 1997a. A three dimensional synthesis study of D18O in atmospheric CO2. Part I: Surface fluxes. Journal of Geophysical Research 102:5857-5872.
Ciais, P.H., P.P. Tans, A.S. Denning, R.J. Francey, M. Trolier, H.J. Meijer, J.W.C. White, J.A. Berry, D.A. Randall, J.J.G. Collatz, P.J. Sellers, P. Monfray, and M. Heimann. 1997b. A three dimensional synthesis studyof D18O in atmospheric CO2. Part II: Simulations with the TM-2 transport model. Journal of Geophysical Research 102:5873-5883.
Ciret, C., and A. Henderson-Sellers. 1997. Sensitivity of global vegetation models to present-day climate simulated by global climate models. Global Biogeochemical Cycle 11(3):415-434.
Claussen, M. 1996. Variability of global biome patterns as a function of initial and boundary conditions in a climate model. Climate Dynamics 12:371-379.
CLIVAR. 1995. A Study of Climate Variability and Predictability: Initial Science Plan. World Climate Research Programme, EMO, Geneva.
Cooke, W.F., B. Koffi, and J.M. Gregoire. 1996. Seasonality of vegetation fires in Africa from remote sensing data and application to a global chemistry model. Journal of Geophysical Research—Atmospheres 101(15):21,051-21,065.
Correll, D. ed. 1986. Watershed Research Perspectives. Smithsonian Institution Press, Washington, D.C.
Cramer, W., D. Kicklighter, A. Bondeau, B. Moore III, G. Churkina, A. Ruimy, A. Schloss, and the Participants of “Potsdam ‘95.” In press. Comparing global models of terrestrial net primary productivity (NPP): Overview and key results. Global Change Biology (Special Issue).
Degens, E.T., S. Kempe, and R. Herrera. 1985. Transport of Carbon and Minerals in Major Rivers: Part 3. SCOPE/UNEP. Geologisch-Paläontologischen Institut der Universität Hamburg, Hamburg, Germany.
Delgenio, A.D., V. Alekseev, V. Dymnikov, V. Galin, and E.M. Volodin. 1996. Cloud feedback in atmospheric general circulation models—An update. Journal of Geophysical Research 101:12,791-12,794.
Denman, E., H. Hofmann, and Marchant. 1996. Marine biotic responses to environmental change and feedbacks to climate. Pp. 482-516 in Climate Change 1995: The Science of Climate Change, J.T. Houghton et al., eds. Contribution of Working Group 1 to the Second Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York.
Dickinson, R.E., and A. Henderson-Sellers. 1988. Modelling tropical deforestation: A study of GCM land-surface parameterizations . Quarterly Journal of the Royal Meteorological Society 114(B):439-462.
Dickinson R., V. Meleshko, D. Randall, E. Sarachik, P. Silva-Dias, and A. Slingo. 1996. Climate processes. Pp. 193-228 in Climate Change 1995: The Science of Climate Change. Contribution of Working Group 1 to the Second Assessment Report of the Intergovernmental Panel on Climate Change, J.T. Houghton, ed. Cambridge University Press, New York.
Dixon, K.W., J.L. Bullister, R.H. Gammon, and R.J. Stouffer. 1996. Examining a coupled climate model using CFC-11 as an ocean tracer . Geophysical Research Letters 23(15):1957-1960.
Elwood, J., J. Newbold, R. O'Neill, and W.V. Winkle. 1983. Resource spiralling: An operational paradigm for analyzing aquatic ecosystems. In Dynamics of Aquatic Ecosystems, T.D. Fontaine III and S.M. Bartell, eds. Ann Arbor Science, Ann Arbor, Mich.
England, M.H. 1993. Representing the global-scale water masses in ocean general circulation models. Journal of Physical Oceanography 23(7):1523-1552.
England, M.H. 1995. Using chlorofluorocarbons to assess ocean climate models. Geophysical Research Letters 22:3051-3054.
England, M.H., V. Garcon, and J.F. Minster. 1994. Chlorofluorocarbon uptake in a world ocean model. Sensitivity to the surface gas forcing. Journal of Geophysical Research—Oceans 99(C12):25,215-25,233.
Famiglietti, J.S., and E.F. Wood. 1994a. Application of multiscale water and energy balance models on a tallgrass prairie. Water Resources Research 30(11):3079-3093.
Famiglietti, J.S., and E.F. Wood. 1994b. Multiscale modeling of spatially variable water and energy balance processes. Water Resources Research 30(11):3061-3078.
FANGIO Workshop. 1993. Meeting report from the Bologna workshop of FANGIO: The Feedback Analysis for GCM Intercomparison and Observation Project, Bologna, Italy, 10-12 May 1993.
Fasham, M. 1995. Variations in the seasonal cycle of biological production in subarctic oceans—a model sensitivity analysis. Deep-Sea Research Part I—Oceanographic Research Papers 42(7):1111-1149.
Fasham, M., J. Sarmiento, R. Slater, H. Ducklow, and R. Willians. 1993. Ecosystem behavior at Bermuda Station-S and Ocean Weather Station India—a general circulation model and observational analysis. Global Biogeochemical Cycles 7(2):379-415.
Fasham, J.R., A.V. Osipov, and V.A. Ryabchenko. 1997. Chaotic behaviour of an ocean ecosystem model under seasonal external forcing. Journal of Plankton Research 19(10):1495-1515.
Field, C.B., A. Ruimy, Y. Luo, C.M. Malmstrom, J.T. Randerson, and M.V. Thompson. 1996. VEMAP—model shootout at the sub-continental corral. Trends in Ecology & Evolution 11(8):313-314.
Flynn, K.J., and M.J.R. Fasham. 1997. A short version of the ammonium-nitrate interaction model. Journal of Plankton Research 19(12):1881-1897.
Fowler, A.C., V. Alekseev, V. Dymnikov, V. Galin, and E.M. Volodin. 1996. Cloud feedback in atmospheric general circulation models—an update. Journal of Geophysical Research 101:12791-12794.
Gates, W.L., A. Henderson-Sellers, G.J. Boer, C. Folland, A. Kitoh, B.J. McAvaney, F. Semazzi, N. Smith, A. Webster, and Q-C. Zeng. 1996. Climate models—evaluation. Pp. 228-284 in Climate Change 1995: The Science of Climate Change, J.T. Houghton et al., eds. Contribution of Working Group 1 to the Second Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York.
Glantz, M.H. 1996. Currents of Change: El Niño Impact on Climate and Society. Cambridge University Press, Cambridge, U.K.
Gibson, C., E. Ostrom, and T.K. Ahn. 1998. Scaling Issues in the Social Sciences. IHDP Working Paper No. 1. International Human Dimensions Programme on Global Environmental Change, Bonn, Germany.
Gildea, M.P., B. Moore, C.J. Vörösmarty, B. Berquist, J.M. Melillo, K. Nadelhoffer, and B.J. Peterson. 1986. A global model of nutrient cycling: I. Introduction, model structure and terrestrial mobilization of nutrients. In Watershed Research Perspectives, D. Corrrell, ed. Smithsonian Institution Press, Washington, D.C.
Glanz, M.H. 1996. Currents of Change: El Niño Impact on Climate and Society. Cambridge University Press, Cambridge, U.K.
Gruber, N., J.L. Sarmiento, and T.F. Stocker. 1996. An improved method for detecting anthropogenic CO2 in the ocean. Global Biogeochemical Cycles 10:809-837.
Hasselmann, K. 1976. Stochastic climate models. Part I: Theory. Tellus 28:473-485.
Heimann, M., G. Esser, A. Haxeltine, J. Kaduk, D.W. Kicklighter, W. Knorr, G.H. Kohlmaier, A.D. McGuire, J. Melillo, B. Moore III, R.D. Otto, I.C. Prentice, W. Sauf, A. Schloss, S. Sitch, U. Wittenberg, and G. Wurth. 1997. Evaluation of terrestrial carbon cycle models through simulations of the seasonal cycle of atmospheric CO2: First results of a model intercomparison study. Global Biogeochemical Cycles.
Henderson-Sellers, A. 1993a. Continental vegetation as a dynamic component of a global climate model—a preliminary assessment. Climatic Change 23(4):337-377.
Henderson-Sellers, A. 1993b. A factorial assessment of the sensitivity of the BATS land-surface parameterization scheme. Journal of Climate 6(2):227-247.
Henderson-Sellers, A. 1994. Land-use change and climate. Land Degradation and Rehabilitation 5(2):107-126.
Henderson-Sellers, A. 1995. Global terrestrial vegetation prediction—the use and abuse of climate and application models. Progress in Physical Geography 18(2):209-246.
Henderson-Sellers, A. 1996a. Soil moisture simulation: Achievements of the RICE and PILPS intercomparison workshop and future directions. Global Planetary Change 13:99-116.
Henderson-Sellers, A. 1996b. Soil moisture: A critical focus for global change studies. Global Planetary Change 13:3-9.
Henderson-Sellers, A., and K. McGuffie. 1995. Global climate models and dynamic vegetation changes. Global Change Biology 1(1):63-75.
Henderson-Sellers, A., and J. Verner. 1995. Project for Intercomparison of Landsurface Parameterization Schemes —Application of some structural complexity metrics. Mathematics and Comp. Modelling 21:55-59.
Henderson-Sellers, A., R.E. Dickinson, T.B. Durbidge, P.J. Kennedy, K. Mcguffie, and A.J. Pitman. 1993. Tropical deforestation—modeling local-scale to regional-scale climate change. Journal of Geophysical Research—Atmospheres 98(4):7289-7315.
Henderson-Sellers, A., K. McGuffie, L. Zhang, and C. Gross. 1995a. Sensitivity of global climate model simulations to increased stomatal resistance and CO2 increases. Journal of Climate 8:1738-1756.
Henderson-Sellers, A., A.J. Pitman, P.K. Love, P. Irannejad, and T.H. Chen. 1995b. The Project for Intercomparison of Land Surface Parameterization Schemes (PILPS)—Phases 2 and 3. Bulletin of the American Meteorological Society 76:489-503.
Hibbard, K., and D. Sahagian, eds. 1998. Net Primary Productivity Model Intercomparison Activity (NPP). GAIM Report #5. IGBP/GAIM, Durham, N.H.
Hofmann, E. 1991. How do we generalise coastal models to global scale? Pp. 401-417 in Ocean Margin Process in Global Change, in R. Mantoura, J. Martin, and R. Wollast, eds. John Wiley & Sons, New York.
Hough, A.M. 1991. Development of a 2-dimensional global tropospheric model-model chemistry . Journal of Geophysical Research—Atmospheres 96(4):7325-7362.
Houghton, J.T., L.G.M. Filho, B.A. Callandar, N. Harris, A. Kattenberg, and K. Maskell, eds. 1996. Climate Change 1995: The Science of Climate Change. Contribution of Working Group 1 to the Second Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York.
Hurtt, G.C., and R.A. Armstrong. 1996. A pelagic ecosystem model calibrated with BATS data. Deep-Sea Research Part II—Topical Studies in Oceanography 43(2-3):653-683.
International Geosphere Biosphere Program (IGBP), Terrestrial Carbon Working Group. 1998. The terrestrial carbon cycle: Implications for the Kyoto Protocol . Science 280:1393-1394.
Kalma, J.D., and I.R. Calder, eds. 1995. Land Surface Processes in Large-Scale Hydrology. Report to the Commission for Hydrology of the World Meteorological Organization, Operational Hydrology Report No. 40. WMO, Geneva.
Kanakidou, M., and P. J. Crutzen. 1993. Scale problems in global tropospheric chemistry modeling—comparison of results obtained with a 3-dimensional model, adopting longitudinally uniform and varying emissions of NOx and NMHC. Chemosphere 26(1-4):787-801.
Kattenberg, A., F. Giorgi, H. Grassl, G.A. Meehl, J.F.B. Mitchell, R.J. Stouffer, T. Tokioka, A.J. Weaver, and T.M.L. Wigley. 1996. Climate models—projections of future climate. Pp. 285-357 in Climate Change 1995: The Science of Climate Change. J.T. Houghton et al., eds. Contribution of Working Group 1 to the Second Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York.
Kaya, Y., N. Nakicenovic, W.D. Nordhaus, and F.L. Toth, eds. 1993. Costs, Impacts, and Benefits of CO2 Mitigation. International Institute for Applied Systems Analysis, Laxenburg, Austria.
Kicklighter, D.W., M. Bruno, S. Donges, G. Esser, M. Heimann, J. Helfrich, F. Ift, F. Joos, J. Kaduk, G. Kohlmaier, A.D. McGuire, J.M. Melillo, R. Meyer, B. Moore III, A. Nadler, I.C. Prentice, W. Sauf, A.L. Schloss, S. Sitch, U. Wittenberg, and G. Wurth. 1997. A first order analysis of the potential of CO2 fertilization to affect the global carbon budget: A comparison of four terrestrial biosphere models. Global Biogeochemical Cycles.
Kicklighter, D.W., A. Bondeau, A. Schloss, J. Kaduk, A. McGuire, and the participants of Potsdam ‘95. In press. Comparing global models of terrestrial net primary productivity (NPP): The importance of water availability to primary productivity. Global Change Biology (Special Issue).
Klonecki, A., and H. Levy. 1997. Tropospheric chemical ozone tendencies in CO-CH4-NOy-H2O system: Their sensitivity to variations in environmental parameters and their application to a global chemistry transport model study . Journal of Geophysical Research—Atmospheres 102(17): 21,221-21,237.
Kutzbach, J., G. Bonan, J. Foley, and S. Harrison. 1996. Vegetation and soil feedbacks on the response of the African monsoon to the orbital forcing in the early to middle Holocene. Nature 381:503-505.
Langner, J., and H. Rodhe. 1991. A global three-dimensional model of the global sulfur cycle. Journal of Atmospheric Chemistry 13:255-263.
Larichev, V.D., and I.M. Held. 1995. Eddy amplitudes and fluxes in a homogeneous model of fully developed baroclinic instability. Journal of Physical Oceanography 25(10):2285-2297.
Lean, J., and D.A. Warrilow. 1989. Simulation of the regional climatic impact of Amazon deforestation . Nature 342(6248):411-413.
Lefevre, N., C. Andrie, Y. Dandonneau, G. Reverdin, and M. Rodier. 1994. PCO2, chemical properties, and estimated new production in the Equatorial Pacific in January-March 1991. Journal of Geophysical Research—Oceans99(C6):12,639-12,654.
Lefevre, N., G. Moore, J. Aiken, A. Watson, D. Cooper, and R. Ling. 1998. Variability of pCO2 in the tropical Atlantic in 1995. Journal of Geophysical Research—Oceans 103(C3):5623-5634.
Legasto, A., J.W. Forrester, and J.M. Lyneis. 1980. System Dynamics. North-Holland Publishing Co., New York.
Levitus, S., and T.P. Boyer. 1994. World Ocean Atlas 1994. NOAA Atlas NESDID 4. NODC, Washington, D.C.
Levitus, S., M.E. Conkright, J.I. Reid, R.G. Najjar, and A. Mantyla. 1993. Distribution of nitrate, phosphate and silicate in the world oceans . Progress in Oceanography 31(3):245-273.
Mackenzie, F., L. Ver, C. Sabine, M. Lane, and A. Lerman. 1993. C, N, P, S Global Biogeochemical Cycles and Modeling of Global Change. NATO ASI Series 14.
Mahlman, J.D., J.P. Pinto, and L.J. Umscheid. 1994. Transport, radiative, and dynamical effects of the Antarctic ozone hole: A GFDL “SKYHI” model experiment. Journal of the Atmospheric Sciences 51(4):489-508.
Malmstrom, C.M., M.V. Thompson, G.P. Juday, S.O. Los, and others. 1997. Interannual variation in global-scale net primary production: Testing model estimates. Global Biogeochemical Cycles. 11(3):367-392.
Manabe, S., and R. Stouffer. 1993. Century-scale effects of increased atmospheriec CO2 on the ocean-atmosphere system. Nature 364(6434):215-218.
Manabe, S., and R.J. Stouffer. 1994. Multiple-century response of a coupled ocean-atmosphere model to an increase of atmospheric carbon dioxide. Journal of Climate 7:5-23.
McBean, G.A., P.S. Liss, and S.H. Schneider. 1995. In Climate Change 1995: The Science of Climate Change, J.T. Houghton et al., eds. Contribution of Working Group 1 to the Second Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York.
McGuire, A.D., J.M. Melillo, L.A. Joyce, D.W. Kicklighter, A.L. Grace, B. Moore, and C.J. Vörösmarty. 1992. Interactions between carbon and nitrogen dynamics in estimating net primary productivity for potential vegetation in North America. Global Biogeochemical Cycles 6:101-124.
McGuire, A.D., L.A. Joyce, D.W. Kicklighter, J.M. Melillo, G. Esser, and C.J. Vörösmarty. 1993. Productivity response of climax temperate forests to elevated temperature and carbon dioxide: A North American comparison between two global models. Climatic Change 24:287-310.
McGuire, A.D., D.W. Kicklighter, and J.M. Melillo. 1996. Global climate change and carbon cycling in grasslands and conifer forests. Pp. 389-411 in Global Change: Effects on Coniferous Forests and Grasslands, SCOPE Volume 56, A.I. Breymeyer et al., eds. John Wiley & Sons, Chichester, U.K.
McGuire, A.D., J.M. Melillo, D.W. Kicklighter, Y. Pan, X. Xiao, J. Helfirch, B. Moore III, C.J. Vörösmarty, and A.L. Schloss. 1997. Equilibrium responses of global net primary production and carbon storage to doubled atmospheric carbon dioxide: Sensitivity to changes in vegetation nitrogen concentration. Global Biogeochemical Cycles 11:173-189.
Meadows, D.H., J. Randers, and W.W. Behrens III. 1972. The Limits to Growth: A Report for the Club of Rome's Project on the Predicament of Mankind. Universe Books, New York.
Mesarovic, M.D., and E.C. Pestel. 1974. Mankind at the Turning Point: The Second Report to the Club of Rome. Dutton, New York.
Melillo, J.M., A.D. McGuire, D.W. Kicklighter, B. Moore, C.J. Vörösmarty, and A.L. Schloss. 1993. Global climate change and terrestrial net primary production. Nature 363:234-240.
Melillo, J., I. Prentice, G. Farquhar, E. Schulze, and O. Sala. 1996. Terrestrial biotic responses to environmental change and feedbacks to climate. Pp. 445-482 in Climate Change 1995: The Science of Climate Change, J.T. Houghton et al., eds. Contribution of Working Group 1 to the Second Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York.
Moore, B., and B.H. Braswell. 1994. The lifetime of excess atmospheric carbon dioxide. Global Biogeochemical Cycles 8(1):23-38.
Myneni, R.B., R.R. Nemani, and S.W. Running. 1997. Estimation of global leaf area index and absorbed par using radiative transfer models. IEEE Transactions on Geoscience and Remote Sensing 35(6):1380-1393.
Najjar, R.G., and R.F. Keeling. 1997. Analysis of the mean annual cycle of the dissolved oxygen anomaly in the World Ocean. Journal of Marine Research 55:117-151.
Najjar, R.G., J.L. Sarmiento, and J.R. Toggweiler. 1992. Downward transport and fate of organic matter in the ocean: Simulations with a general ocean circulation model. Global Biogeochemical Cycles 6:45-76.
Nakicenovic, N., W.D. Nordhaus, R. Richaels, and F.L. Toth, eds. 1994a. Climate Change: Integrating Science, Economics, and Policy. International Institute for Applied Systems Analysis, Laxenburg, Austria.
Nakicenovic, N., W.D. Nordhaus, R. Richaels, and F.L. Toth, eds. 1994b. Integrative Assessment of Mitigation, Impacts, and Adaptation to Climate Change. International Institute for Applied Systems Analysis, Laxenburg, Austria.
National Aeronautics and Space Administration (NASA). 1986. Earth System Science Overview. Earth System Sciences Committee, NASA Advisory Council, Washington, D.C.
National Research Council. 1990. Research Strategies for the U.S. Global Change Research Program. National Academy Press, Washington, D.C.
National Research Council. 1998. Global Energy and Water Cycle Experiment (GEWEX) Continental-Scale International Project. National Academy Press, Washington, D.C.
Nemry, B., L. Francois, A. Bondeau, M. Heimann, and the participants of Potsdam ‘95. In press. Comparing global models of terrestrial net primary productivity (NPP): Analysis of the seasonal atmospheric CO2 signal. Global Change Biology (Special Issue).
Newbold, J. 1992. Cycles and spirals of nutrients. Pp. 379-408 in The Rivers Handbook: Hydrological and Ecological Principles, P. Calow and G. Petts, eds. Blackwell, Oxford.
Normile, D. 1997. Japan starts to carve out its place in the world. Science 276:1025-1026.
Pan, Y.D., J.M. Melillo, and D.S. Schimel. 1998. Vegetation/Ecosystem Modeling and Analysis Project (VEMAP). Modeled responses of terrestrial ecosystems to elevated atmospheric CO2: A comparison of simulations by the biogeochemistry models of the Vegetation/Ecosystem Modeling and Analysis Project (VEMAP). OECOLOGIA 114(3):389-404.
Popova, E.E., M.J.R. Fasham, A.V. Osipov, and V.A. Ryabchenko. 1997. Chaotic behaviour of an ocean ecosystem model under seasonal external forcing. Journal of Plankton Research 19(10): 1495-1515.
Potter C.S., J.T. Randerson, C.B. Field, P.A. Matson, P.M. Vitousek, H.A. Mooney, and S.A. Klooster. 1993. Terrestrial ecosystem production—a process model based on global satellite and surface data. Global Biogeochemical Cycles 7(4):811-841.
Raich, J.W., and K.J. Nadelhoffer. 1989. Belowground carbon allocation in forest ecosystems—global trends. Ecology 70(5):1346-1354.
Raich, J., E. Rastetter, J. Melillo, B.J. Peterson, D. Kicklighter, P.A. Steudler, B. Moore, C. Vörösmarty, and A. Grace. 1991. Potential net primary productivity in South America: Application of a global model. Ecological Applications 1:399-429.
Ramanathan, V. 1995. Clouds and climate. Proceedings of the Koninklijke Nederlandse Akademie Van Wetenschappen (Biological Chemical Geological Physical and Medical Sciences) 98(4): 361-383.
Ramanathan, V., and W. Collins. 1991. Thermodynamic regulation of ocean warming by cirrus clouds deduced from observations of the 1987 El Niño. Nature 351(6321):27-32.
Ramaswamy, V., and V. Ramanathan. 1989. Solar absorption by cirrus clouds and the maintenance of the tropical upper troposphere thermal structure. Journal of the Atmospheric Sciences 46(14): 2293-2310.
Rhein, M. 1994. The deep western boundary—tracers and velocities. Deep-Sea Research Part I—Oceanograpic Research Papers 41:263-281.
Ruimy, A., L. Kergoat, A. Bondeau, and the participants of “Potsdam ‘95.” In press. Comparing global models of terrestrial net primary productivity (NPP): Analysis of differences in light absorption, light-use efficiency, and whole respiration cost. Global Change Biology (Special Issue).
Ryabchenko, V.A., M.J.R. Fasham, B.A. Kagan, and E.E. Popova. 1997. What causes short-term oscillations in ecosystem models of the ocean mixed layer? Journal of Marine Systems 13(14): 33-50.
Salati, E. 1986. Amazon: Forest and hydrological cycle. Pp. 110-112 in Climate-Vegetation Interactions, C. Rosenzweig and R.E. Dickinson eds. University Corporation for Atmospheric Research, Boulder.
Santer, B.D., T.M.L. Wigley, T.P. Barnett, and E. Anyamba. 1996. Detection of climate change and attribution of causes. Pp. 406-443 in Climate Change 1995: The Science of Climate Change, J.T. Houghton et al., eds. Contribution of Working Group 1 to the Second Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York.
Sarmiento, J.L., and C. Le Quéré 1996. Oceanic carbon dioxide uptake in a model of century-scale global warming. Science 274(5291):1346-1350.
Sarmiento, J.L., R.D. Slater, M.J.R. Fasham, HW. Ducklow, J.R. Toggweiler, and G.T. Evans. 1993. A seasonal 3-dimensional ecosystem model of nitrogen cycling in the North Atlantic euphotic zone. Global Biogeochemical Cycles 7(2):417-450.
Sarmiento, J.L., C. Le Quéré, and S.W. Pacala. 1995. Limiting future atmospheric carbon dioxide. Global Biogeochemical Cycles 9(1):121-137.
Schimel, D.S. 1995. Terrestrial biogeochemical cycles: Global estimates with remote sensing . Remote Sensing Environment 51:49-56.
Schimel, D.S., B.H. Braswell, E.A. Holland, R. McKeown, D.S. Ojima, T.H. Painter, W.J. Parton, and A.R. Townsend. 1994. Climatic, edaphic, and biotic controls over storage and turnover of carbon in soils. Global Biogeochemical Cycles 8(3):279-293.
Schimel, D., I. Enting, M. Heimann, T. Wigley, D. Raynaud, D. Alves, and U. Siegenthaler. 1996. Radiative forcing of climate change. Pp. 65-331 in Climate Change 1995: The Science of Climate Change, J.T. Houghton et al., eds. Contribution of Working Group 1 to the Second Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, New York.
Schimel, D.S., VEMAP Participants, and B.H. Brasswell. 1997a. Continental scale variability in ecosystem processes: Models, data and the role of disturbance. Ecological Monographs 67:251-271.
Schimel, D.S., B.H. Braswell, and W.J. Parton. 1997b. Equilibration dynamics of the terrestrial water, nitrogen, and carbon cycles: Conclusions from a preindustrial simulation. Proceedings of the National Academy of Sciences 94:8280-8283.
Schimel, D.S., B.H. Braswell, R. McKeown, D.S. Ojima, W.J. Parton, and W. Pulliam. 1997c. Climate and nitrogen controls on the geography and timescales of terrestrial biogeochemical cycling. Global Biogeochemical Cycles 10:677-692.
Schloss, A., U. Wittenberg, D. Kicklighter, J. Kaduk, and the participants of Potsdam ‘95. In press. Comparing global models of terrestrial net primary productivity (NPP): Comparison of annual NPP to spatial climatic drivers and the normalized difference vegetation index. Global Change Biology (Special Issue).
Sedell, J.R., J.E. Richey, and F.J. Swanson. 1989. The river continuum concept: A basis for the expected ecosystems behavior of very large rivers. Pp. 49-55 in Can. Sp. Public. Fish. Aquatic Science, Proc. of the International Large River Symposium, D.P. Dodge, ed.
Sellers, P.J., D.A. Randall, G.J. Collatz, J.A. Berry, C.B. Field, D.A. Dazlich, C. Zhang, G.D. Collelo, and L. Bounoua. 1996. A revised land surface parameterization (SiB2) for atmospheric GCMs: 1. Model formulation. Journal of Climate 9(4):676-705.
Semtner, A.J. 1995. Modeling ocean circulation. Science 269:1379-1385.
Sherwood, S., V. Ramanathan, T. Barnett, M. Tyree, and E. Roeckner. 1994. Response of an atmospheric general circulation model to radiative forcing of tropical clouds. Journal of Geophysical Research—Atmospheres 99(10):20,829-20,845.
Shukla, J., C. Nobre, and P. Sellers. 1990. Amazon deforestation and climate change. Science 247:1322-1324.
Smethie, W.M., and R.S. Pickart 1993. How does the deep western boundary current cross the gulf stream? Journal of Physical Oceanography 23:2602-2616.
Takahashi, T., and S. Sutherland. 1995. An assessment of the role of the North Atlantic as a CO2 sink. Phil. Trans. R. Soc. Lond. B348:143-152.
Takahashi, T., R.A. Feely, R.F. Weiss, R.H. Wanninkhof, D.W. Chipman, S.C. Sutherland, and T.T. Takahashi. 1997. Global air-sea flux of CO2: An estimate based on measurements of sea-air pCO(2) difference. Proceedings of the National Academy of Sciences of the United States of America 94:8292-8299.
van de Ven, F.H.M., D. Gutknecht, D.P. Loucks, and K.A. Salewicz, eds. 1991. Hydrology for the Water Management of Large River Basins. IAHS Publication #201. Wallingford, U.K.
Vannote, R.L., G.W. Minshall, K.W. Cummins, J.R. Sedell, and C.E. Cushing. 1980. The river continuum concept. Can. J. Fish Aquat. Science 37:130-137.
VEMAP Participants. 1995. Vegetation/ecosystem modelling and analysis project: Comparing biogeography and biogeochemistry models in a continental-scale study of terrestrial ecosystem responses to climate change and CO2 doubling. Global Biogeochemical Cycles 9(4):407-437.
VEMAP Participants and B.H. Brasswell. 1997. Continental scale variability in ecosystem processes: Models, data, and the role of disturbance. Ecological Monographs 67:251-271.
Ver, L.M.B., F.T. Mackenzie, and A. Lerman. 1994. Modeling pre-industrial C-N-P-S biogeochemical cycling in the land coastal margin system. Chemosphere 29:855-887.
Vörösmarty, C.J., and B. Moore III. 1991. Modeling basin-scale hydrology in support of physical climate and global biogeochemical studies: An example using the Zambezi River . Studies in Geophysics 12:271-311.
Vörösmarty, C.J., M.P. Gildea, B. Moore, B.J. Peterson, B. Berquist, and J.M. Melillo. 1986. A global model of nutrient cycling: II. Aquatic processing, retention, and distribution of nutrients in large drainage basins. In Watershed Research Perspectives, D. Correll, ed. Smithsonian Institution Press, Washington, D.C.
Vörösmarty, C.J., B. Moore, M.P. Gildea, B. Peterson, J. Melillo, D. Kicklighter, J. Raich, E. Rastetter, and P. Steudler. 1989. A continental-scale model of water balance and fluvial transport: Application to South America. Global Biogeochemical Cycles 3:241-65.
Vörösmarty, C.A., B.Grace, B. Moore, B. Choudhury, and C. Willmott. 1991. A strategy to study regional hydrology and terrestrial ecosystem processes using satellite remote sensing, ground-based data and computer modeling. Acta Astronautica 25:785-792.
Vörösmarty, C.J., K. Sharma, B. Fekete, A.H. Copeland, J. Holden, J. Marble, and J.A. Lough. 1997a. The storage and aging of continental runoff in large reservoir systems of the world. Ambio 26: 210-19.
Vörösmarty, C.A., R. Wasson, and J.E. Richey, eds. 1997b. Modeling the Transport and Transformation of Terrestrial Materials to Freshwater and Coastal Ecosystems: Workshop Report and Recommendations for IGBP Inter-Core Project Collaboration. IGBP Secretariat, Stockholm.
Vörösmarty, C.J., C.A. Federer, and A. Schloss. 1998a. Potential evaporation functions compared on U.S. watersheds: Implications for global-scale water balance and terrestrial ecosystem modeling . Journal of Hydrology 207:147-169.
Vörösmarty, C.J., C. Li, J. Sun, and Z. Dai. 1998b. Emerging impacts of anthropogenic change on global river systems: The Chinese example. In Asian Change in the Context of Global Change: Impacts of Natural and Anthropogenic Changes in Asia on Global Biogeochemical Cycles, J. Galloway and J. Melillo, eds. Cambridge University Press, Cambridge, U.K.
Vörösmarty, C.J., B.M. Fekete, M. Meybeck, and R.B. Lammers. 1998c. The global system of rivers: Its role in organizing continental land mass and defining land-to-ocean linkages. Submitted to Global Biogeochemical Cycles.
Walling, D., and J-L. Probst, eds. 1997. Human Impact on Sedimentation. IAHS Press, Wallingford, U.K.
Wang, C., R.G. Prinn, and A. Sokolov. 1998. A global interactive chemistry and climate model: Formulation and testing. Journal of Geophysical Research—Atmospheres 103(3):3399-3417.
Warner, M.J., and R.F. Weiss. 1992. Chlorofluoromethanes in South Atlantic Antarctic intermediate water . Deep-Sea Research Part A—Oceanographic Research Papers 39:2053-2075.
Warner, M.J., J.I. Bullister, D.P. Wisegarver, R.H. Gammon, and R.F. Weiss. 1996. Basin-wide distributions of chlorofluorocarbons CFC-11 and CFC-12 in the North Pacific—1985-1989. Journal of Geophysical Research—Oceans 101(9):20,525-20,542.
Weyant, J., O. Davidson, H. Dowlatabadi, J. Edmonds, M. Grubb, E.A. Parson, R. Richels, J. Rotmans, P.R. Shukla, R.S.J.Tol, W. Cline, and S. Fankhauser. 1996. Integrated assessment of climate change: An overview and comparison of approaches and results. In Climate Change 1995: Economic and Social Dimensions of Climate Change, J.P. Bruce, H. Lee, and E.F. Haites, eds. Cambridge University Press, New York.
Wielicki, B.A., R.D. Cess, M.D. King, D.A. Randall, and E.F. Harrison. 1995. Mission to Planet Earth: Role of clouds and radiation in climate. Bulletin of the American Meteorological Society 76(11):2125-2153.
Wigley, T.M.L. 1993. Balancing the carbon budget: Implications for projections of future carbon dioxide concentrations. Tellus 45B:409-425.
Wigley, T.M.L. 1997. Implications of recent CO2 emission-limitation proposals for stabilization of atmospheric concentrations . Nature 390:267-270.
Wigley, T.M.L., R. Richels, and J.A. Edmonds. 1996. Economic and environmental choices in the stabilization of atmospheric CO2concentrations. Nature 379:240-243.
Wilkinson, W.B., ed. 1993. Macro-scale Modeling of the Hydrosphere. IAHS Publ. #214. IAHS, Wallingford, U.K.
Yoder, J.A., C.R. McClain, G.C. Feldman, and W.E. Esaias. 1993. Annual cycles of phytoplankton chlorophyll concentrations in the global ocean—A satellite view. Global Biogeochemical Cycles 7:181-193.