3
Regional Variations
The United States contains an extraordinarily diverse landscape, with tremendous variation in physical geography, climate, and ecology, as well as parallel diversity in the political and economic landscape. As a result, approaches to watershed management differ, too. This chapter describes regional variations in physical hydrology, ecology, and human impacts. These regional variations and human aspects significantly affect the functioning of watersheds, and managers must consider them when creating plans and regulations and when implementing watershed approaches. This chapter demonstrates that no single approach to watershed planning can fit the wide range of conditions present, and sets the stage for understanding why site-specific research planning will always be necessary for watershed management.
Physical Hydrology
Physical hydrology sets the limits within which the watershed operates. The physical hydrology includes precipitation, evaporation, the amount of water held in the soil, streamflow, groundwater, and water quality.
Precipitation
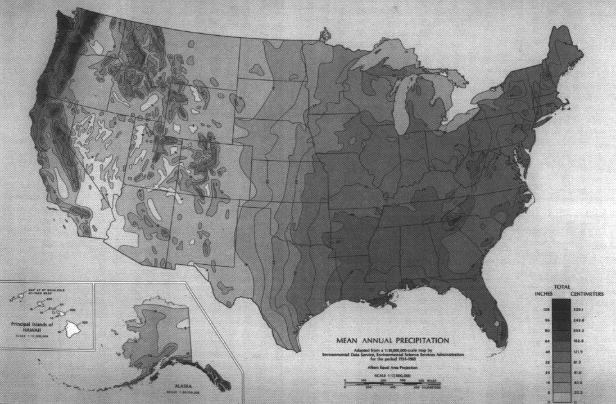
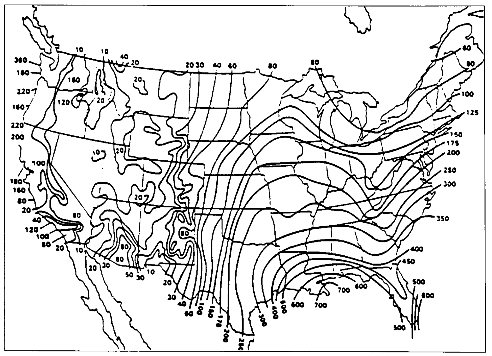
The contiguous United States receives an average of approximately 75 centimeters (30 inches) precipitation per year, but there is great spatial variability (Figure 3.1). The heavy precipitation of the Pacific Northwest is a function of cool eastward-moving wet and cool air masses, mid-latitude cyclones, and oro-
graphic lifting by coastal mountains. The same mountains, along with the dominant high pressure in the Southwest, block most Pacific moisture from the continental interiors, creating an arid effect extending eastward to mid-continent. The humid East is affected by warm, moist air from the Gulf and South Atlantic, with mid-latitude and tropical cyclones and strong convection.
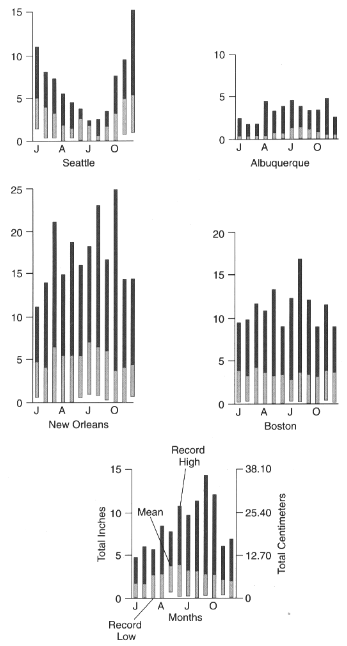
Average annual precipitation is only a crude guide to natural water supply, given its high annual and monthly variability (Figure 3.2). First, there is an annual pattern in the timing of precipitation. On the West Coast, a strong summer minimum of precipitation exists while the North-Central states and East show a summer maximum. Second, there is a great deal of long-term variance of total amounts received on a monthly and annual basis. There is also great spatial variation in the frequency of precipitation, with the Northeast and Northwest having more rainy days. While there is a general spatial correlation between amount and frequency of precipitation (Figure 3.1), the Southeast often receives great amounts of precipitation in fewer days while the Northeast has more precipitation days with smaller amounts.
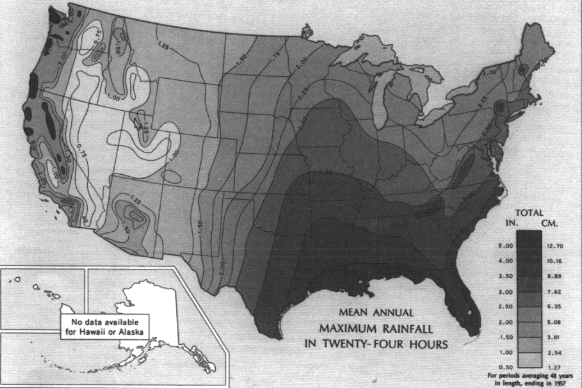
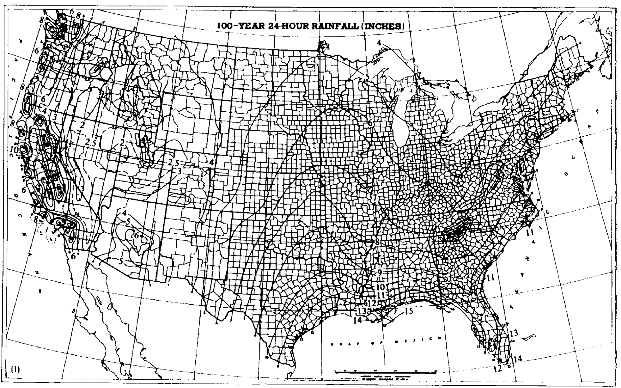
The frequency and magnitude of rainfall events have important implications for flood control aspects of watershed management. For any given return period (that is, the time between events of the same magnitude), the magnitude of those events varies with geography. Magnitudes are highest in the Southeast, followed by small areas in the mountains of the far West (Figure 3.3). Minimum values are found in the intermountain basins of the West. An example showing these distributions is the map of 100-year, 24-hour values of precipitation (Figure 3.4), which shows the maximum 24-hour rainfall amounts expected on average every 100 years. Watershed planning often uses this 24-hour maximum value. A complete set of similar maps is available showing the distribution of small events (a minor 1-year, 30-minute storm) to major 100-year, 10-day events (Miller, 1964; Hershfield, 1961). These frequency-magnitude values are on an annual basis, but monthly probabilities are also available for the eastern United States (Hershfield, 1961).
Another important characteristic of precipitation in watershed management is its erosivity, or ability to erode soil, expressed in units of 100 foot-tons per acre-year (Figure 3.5). These values, in conjunction with the other factors of the Revised Universal Soil Loss Equation (RUSLE), give a prediction of annual sheet and rill erosion in tons per acre for any location in the nation (Renard et al., 1995).
Evaporation
Evaporation is important to water supply and watershed management because it represents the natural loss of otherwise available water. One measure of the concept is the combination of potential evaporation and transpiration, known as potential evapotranspiration or PET. PET data are very sparse, but a suitable
surrogate is lake evaporation. Annual average values range from 50 centimeters (20 inches) in northern Maine to 215 centimeters (86 inches) in Southwest California (Figure 3.6). The amount of mean annual lake evaporation reflects the major physical controls of latitude, altitude, and relative humidity. Like precipitation, PET has an annual temporal pattern (systematic variance through the course of the year) as well as year-to-year variation. One important consideration in watershed management is what proportion of the year's moisture loss occurs during the growing season (May to October). Values range from a high of more than 80 percent in the northern U.S. to below 60 percent in south Florida (Kohler et al., 1959).
Soil Water Budgets
Soil water—that is, water contained in soil—is necessary for most plant and animal life. The availability of soil water is a function of both precipitation (counted as income) and evapotransporation (counted as expenditures). These budgets or balances can be expressed in diagrammatic form to show soil water surpluses, deficits, recharge, and utilization of stored soil water (Figure 3.7). Such water budgets not only give insight into a major control on natural processes, they also provide information relevant to irrigation and other water requirements. Amounts of water that exceed a soil's holding capacity move down through the soil into groundwater for aquifer recharge. Some of the groundwater provides baseflow for streams and leaves the region as runoff. Representative soil water budgets for the United States show that the magnitude of deficits and surpluses varies greatly by region and season. The greatest deficits occur in the Southwest, while the strongest surpluses occur in the Pacific Northwest and the East. Short but significant soil water deficits may occur; even in humid areas, near the end of the growing season. Given variations in precipitation and evaporation, water budgets can vary significantly from year to year. ''Drought" occurs when precipitation is far enough below the long-term average to create a soil water deficiency great enough to adversely affect economic and social systems. There are great regional differences in the United States regarding the severity of drought (USGS, 1970).
Streamflow
Streamflow plays an important role in water supply, flooding, navigation, pollution, and recreation. It is composed of two major components: baseflow and stormflow. Baseflow is the more or less continuous flow that results from groundwater and a surplus of soil water. Stormflow results from rainfall or snowmelt events. In soils with high infiltration capacities and hydraulic conductivities, most stormflow may be subsurface except where soil is absolutely saturated. Where infiltration and/or conductivity is low, as in areas affected by compaction
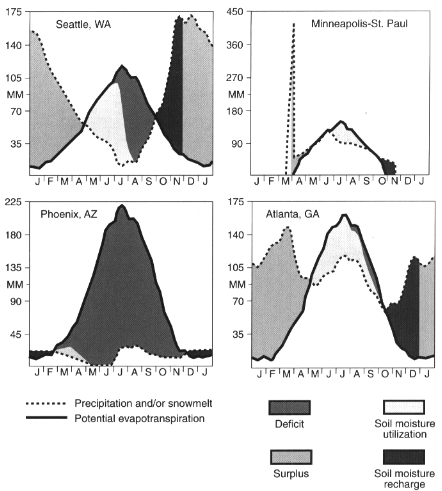
FIGURE 3.7
Soil water budgets. SOURCE: Calculated from Mather, 1978.
and/or deterioration of soil structure, rainfall from intense storms may be forced to move toward streams as overland flow, increasing the risk of soil erosion and downstream flooding. Similarly, urbanized areas are effectively "waterproofed" with roofs and pavement so that surface runoff is greatly increased.
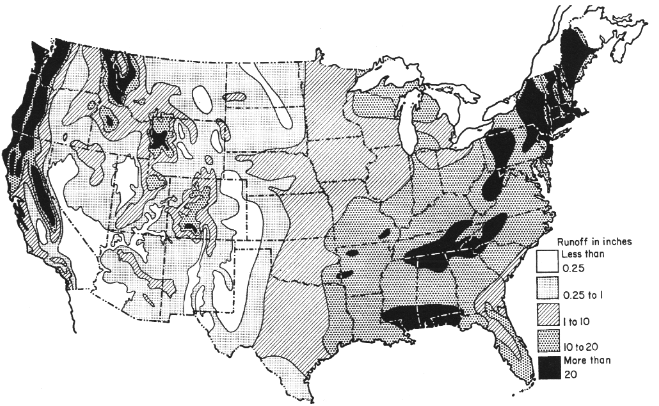
The average annual runoff for the contiguous United States is approximately 30 centimeters (12 inches), but varies greatly by region (Figure 3.8), with rates ranging from less than 0.6 centimeter (0.25 inches) in some western areas to over 50 centimeters (20 inches) in some parts of the East. The runoff rates are roughly predicted by the soil water budgets.
In addition to their geographic variability, precipitation, evaporation, and runoff usually vary considerably in monthly patterns and from year to year. Regime (or normal) monthly distribution of runoff, somewhat resembles the precipitation regimes discussed earlier. However, these are modified everywhere by evaporation regimes and by snowmelt in the mountainous West.
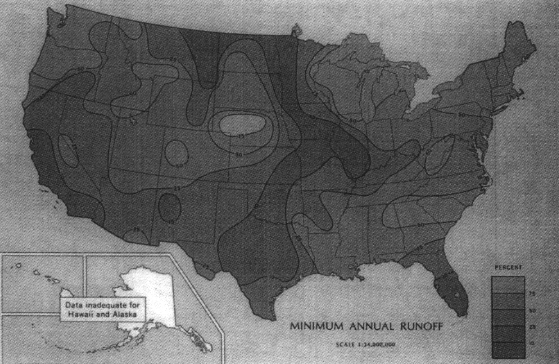
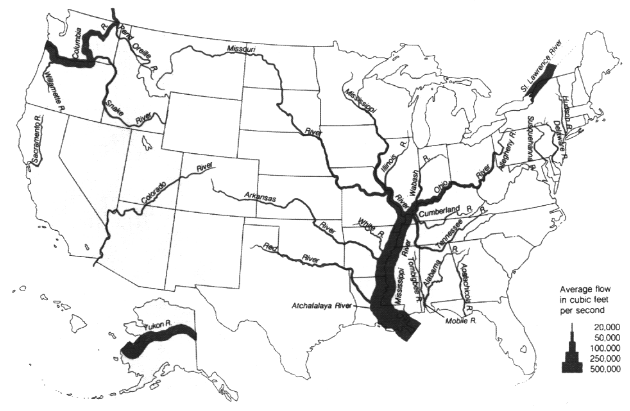
As with precipitation and evaporation, runoff can vary greatly from year to year, with one-year amounts ranging from less than 10 percent of the long-term average to over 400 percent (Figure 3.9 and Figure 3.10). Standardized to the coefficient of variation, the variance is greatest in the central U.S. and the Southwest, and least in the Northwest and Northeast (USGS, 1970). Given the greater average annual runoff (Figure 3.8), there is greater river flow in the eastern half of the country and in the Northwest than elsewhere (Figure 3.11).
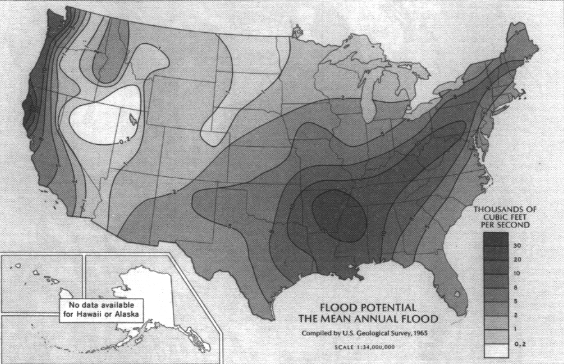
One of the most important considerations in watershed management is dealing with floods. High flood potential is caused by a combination of intense rainfall, high antecedent soil moisture, steep topography, and less permeable land conditions (Figure 3.12).
Climate Change
While year-to-year variation in precipitation and runoff can be significant, some changes occur on a scale of decades or centuries, and climatologists have only recently begun to appreciate how important these changes can be to local and regional weather patterns. The gradual warming of the earth's atmosphere over the last century has been the subject of a tremendous amount of scientific investigation, and there are models that predict how global warming will change the amount and timing of snow and rain. In the last 40 years there have been seven significant El Niño events generated by water circulation anomalies in the tropical Pacific Ocean, including very large El Niño events in 1982-1983 and 1997-1998 (Figure 3.13) that resulted in severe flooding and erosion along both the west and east coasts of North America. The frequency of occurrence of each El Niño event has apparently increased following a shift in the intensity of regional low pressure systems in the northern and southern Pacific Ocean that occurred in 1976-1977. Such events should remind us that yearly patterns of variability in precipitation and runoff are superimposed on decades-long or even centuries-long climate cycles about which much remains to be learned.
Ground Water
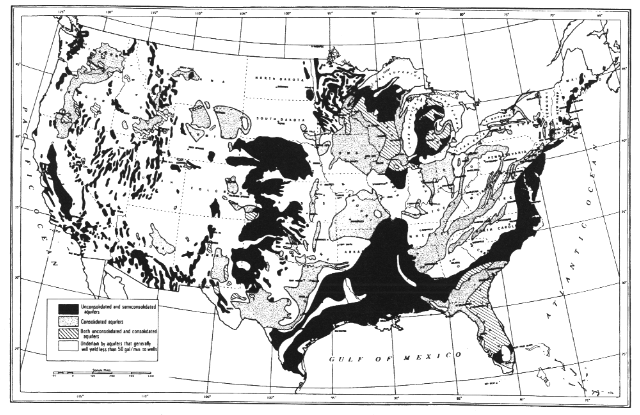
Ninety-seven percent of all fresh water on earth is subsurface; only 2 percent of the total is contained in lakes and rivers (Gleick, 1993). Scientists and water managers have studied the geohydrology of ground waters for decades, trying to define and exploit ground water supplies in aquifers for human uses (U.S. Water Atlas, 1973; Figure 3.14). Nationwide we obtain at least 23 percent of our
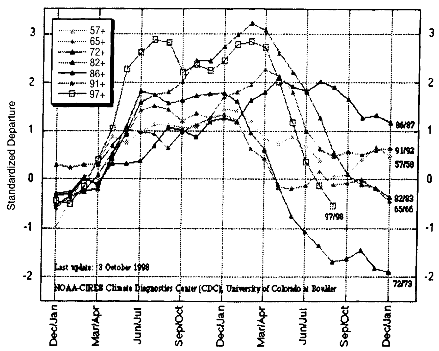
FIGURE 3.13
Comparison of a multivariate index of the strength of several El Niño events from 1957 to 1998.
SOURCE: National Oceanic and Atmospheric Administration, 1998.
domestic and industrial water supply from local and regional aquifers, many of which are restored (recharged) at rates often less than 0.2 percent per year by volume (Pimentel et al., 1997). Almost all rural homes and many cities depend heavily on ground water. As a consequence, aquifers have been thoroughly mapped and are extensively mined, especially for irrigation supplies (Figure 3.15). Throughout the western and midwestern states, large aquifers have declined substantially because rates of extraction exceed recharge rates (e.g., Ogalala Aquifer).
While ground water basins do not always coincide with watersheds, there are important interactions between surface water and ground water. In most watersheds, surface water and ground water flow paths are interconnected (Gibert et al., 1994a). The recharge areas may be wetlands or simply areas in the watershed where there are very permeable soils and sometimes a shallow water table. While the regional flow of water in aquifers generally reflects the general surface topography above, ground water resources in one watershed may also be fed by surface
sources in an adjacent watershed. Watershed-scale issues therefore must be examined above and below ground. To date, management has generally under-emphasized ground water problems and responses as a component of water resource conservation and rehabilitation (GAO, 1991). Indeed, watershed management too often focuses only on surface waters, or if ground water resources are recognized only volume and accessibility receive notice whereas quality and biologic characteristics are also important.
Research has established at least five important attributes of groundwater ecology that are essential to a holistic view of watersheds (Gibert et al., 1994b). First, there is a continuum of geohydrologic units involving surface and groundwater flow pathways. For example, in one area surface runoff may flow into caves and fissures and that ground water may then feed directly into a river via large springs. In another, water from a river may penetrate alluvial soil at the upstream end of a floodplain, flow through unconfined aquifers within the floodplain and upwell back into the channel via a network of springbrooks at the downstream end (Jaffe and Dinovo, 1987). In either case, surface water becomes ground water that then re-enters the surface systems. This simply illustrates that geohydrologic units should be viewed as a diverse, interconnected mosaic.
A second essential ecological feature of ground water is that as water and materials move from one underground unit to the next, significant biogeochemical transformations usually occur. Types and concentrations of solutes change as the geomorphology and hence flow rate changes along the geohydrological gradient. Likewise, biotic species composition and abundance may change significantly along the geohydrological gradient. Thus we recognize biophysical (e.g., flow, temperature, redox, ion concentration, biodiversity, bioproduction) gradients as water moves through the geohydrological continuum. Gradients may be very steep (i.e., conditions may change very quickly) at boundaries or ecotones between different units (Vervier, 1992).
Third, virtually all ground water has some sort of biotrophic (food) web composed of microbes (bacteria and protozoa) as well as larger, more complex organisms, except in situations where oxygen is insufficient. As in surface water, the food web relies on microbial activity that uses dissolved or particulate organic matter as the primary energy source (Stanford and Ward, 1993). Since photosynthesis cannot occur in ground water, a supply of organic matter from soils or surface waters upstream is critical. Limited supplies of this organic matter generally makes ground water much less productive than surface waters. Nonetheless, ground water can support food webs (including in some cases vertebrates) that play vital ecological roles in transforming solute concentrations (including pollutants) in waters moving through the ground.
Fourth, water's movement through ground water systems and its associated biogeochemical transformations adds complexity to our view of landscapes. For example, we now examine river ecosystems in four dimensions: upstream-down-stream (longitudinal), channel-riparian (lateral), channel-groundwater (vertical),
and temporal (time) (Ward, 1989). The penetration of river water into floodplain gravels defines the hyporheic zone, a ground water-surface water ecotone that occurs throughout river corridors of gravel-bed rivers. The hyporheic zone contains a community of organisms having both ground and surface water affinities that greatly influence local and regional biodiversity patterns. For instance, chemical transformations within the hyporheic zone and the dynamics of hyporheic discharge may actually control bioproduction within the river channel of gravel-bed rivers and the distribution and abundance of riparian vegetation throughout the river corridor (Stanford and Ward, 1993). Clearly, ground water is an interconnected component of riverine landscapes and corollaries apply to wetlands, lakes, and near-shore marine systems.
Finally, most ground water systems are naturally more biophysically constant than surface water environments. Ground water organisms are rather nonresilient in that they have evolved in conditions that, in many cases, have not changed for long periods of geologic time. Therefore, groundwater organisms are not likely to be resistant to environmental change. Yet, in many cases they have been subjected to such change, in the form of massive ground water pollution—a worldwide problem that threatens endemic ground water biota (Notenboom et al., 1994). It may be that many groundwater systems have been destroyed or severely damaged by abstraction and diversion, either for irrigation and potable use, or by disposal of wastes, before their natural integrity was recognized. If ground waters are connected components of river, lake, and marine ecosystems, vital ecosystem linkages may have been disconnected by ground water abstraction and pollution.
Watershed management must consider subsurface water resources and influences of land-use activities, especially ground water abstraction and subsurface injection of waste water and other pollutants, on these resources. Underground flow pathways provide recharge routes for aquifers and primary sites for the natural attenuation of pollution by microbes. Surface water simply cannot be effectively managed without detailed understanding of its biophysical connections to ground water.
Water Quality
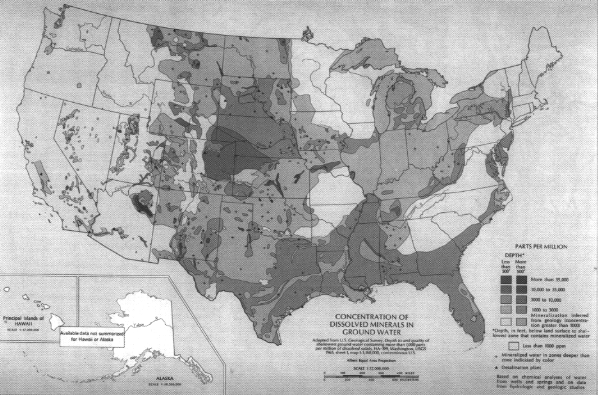
Among the most important considerations in watershed management is water quality. Several aspects of water quality follow regional trends, although a patchwork of local inputs can greatly alter the regional pattern in many locations. Water contaminants can be broadly divided into dissolved and suspended constituents, although their causes and sources are often interrelated. A key component of water quality, not always detrimental, is concentration of dissolved inorganic substances. Although many minerals may be included, the dominant constituents by mass are generally salt (NaCl) and calcium carbonate (CaCO3), the latter resulting in "hard water." For reasons described below, the highest
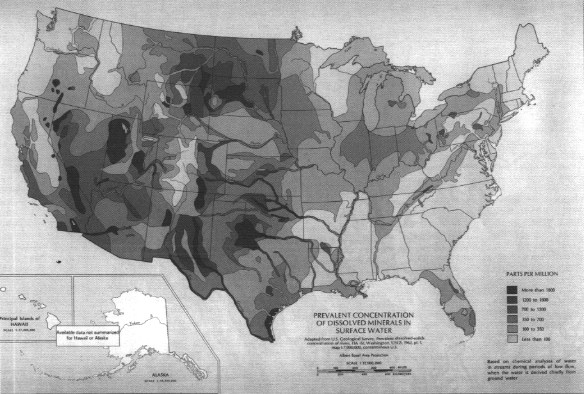
dissolved surface water mineral concentrations tend to occur in the arid West (Figure 3.16), while the highest ground water mineral concentrations tend to occur in the central and southern U.S. (Figure 3.17). This difference can occur because ground waters in the West are less diluted by rain when they enter surface streams.
Sediment is generally the most common water pollutant. Not only can it fill stream channels and harbors, but it can also degrade habitat by reducing the amount of light that reaches stream bottoms and covering spawning beds and submerged aquatic vegetation. Additionally, chemical pollutants often sorb to sediment particles and thus move through and contaminate the environment.
A broad range of factors cause marked differences in water composition and quality at various locations in the United States. First, precipitation varies in chemical composition. For instance, rain near the coasts contains a much higher concentration of sea salts than further inland. However, only in areas with no anthropogenic pollution and very slow weathering rates does this have a significant effect on stream composition. More important, acid rain created by fossil fuel use has dramatically reduced pH and acid neutralizing capacity (ANC) and increased levels of sulfate and nitrate. In areas lacking carbonate bedrock, serious acidification of surface waters can result. In the United States, acid sources are concentrated in the Ohio River Valley. Downwind from this region is a broad area underlain mainly by granitic bedrock (New York and New England) which is especially vulnerable to the atmospheric acid inputs.
Lead added to gasoline and released during combustion was the dominant source of this metal to aquatic ecosystems during most of the 20th century. The historical record of trace-metal analyses of fresh waters is so fiddled with contamination artifacts that it is impossible to know whether streams contained significant lead levels before the days of the automobile—a question further complicated by the ability of natural processes to greatly attenuate lead as it passes through soil (Wang et al., 1995). Yet substantial documentation suggests that lead from gasoline combustion significantly increased lead levels in surface waters of the world's oceans (Flegal and Patterson, 1983) and in soils throughout the United States. The elimination of lead in gasoline has caused a dramatic decline in atmospheric delivery, and surface waters now contain almost immeasurably low amounts, especially in bioavailable dissolved forms (Windom et al., 1991; Benoit, 1994).
Changes in forest ecosystems can also change water quality. For instance, gypsy moth defoliation of forested uplands in Virginia led to dramatic increases in concentrations of nitrates in stream waters. Other solute changes included increasing concentrations of calcium, magnesium, potassium, and hydrogen ions as well as decreasing concentrations of acid-neutralization capacity and SO42-. After several years, the composition of the study stream returned to predefoliation concentration levels. Short-term effects of the defoliation included an increase in the frequency and severity of episodic acidification. Long-term effects included reduction of base nutrient supplies in the catchment basin (Webb et al., 1997).
Climate can induce significant regional water-quality variations. Warmer areas undergo more rapid and complete weathering, generating large quantities of cation-depleted clays. Easily transported by running water, abundant clays contribute to the load of suspended particulate matter (SPM) and can carry contaminants. Differences in mean annual temperature thus explain indirectly (through the connection between temperature and evapotranspiration) much of the variation in extent of soil weathering and in the amount of suspended load in East Coast rivers, where SPM tends to increase as one moves south. The arid climate of the West meanwhile, enhances evapotranspiration, which can concentrate dissolved and suspended matter carried by streams and rivers.
Differences in bedrock cause most of the evident differences in regional water composition. Carbonates weather much more extensively and rapidly than silicates. Watersheds underlain mainly by silicate bedrock tend to have rivers with low ANC, pH, hardness, and total dissolved solids (TDS), and are dominated by the anions chloride and sulfate. In areas where carbonate rocks are common, hard water with high TDS occurs, and the most common anion is bicarbonate. The larger a watershed, the more likely it is to contain areas of carbonate bedrock. Thus large rivers tend to have chemistry reflecting carbonates dominating over silicates.
If evaporites (halite, gypsum) occur in a watershed, water may have high concentrations of chloride or sulfate, but pH is unaffected. Because of their very high solubility, evaporites are less common in humid regions, so they are more abundant in the West, but they underlie broad regions of the country. Finally, if reduced minerals are plentiful, their oxidation releases protons that cause stream acidification. An extreme example is found in the vicinity of mining operations, where coal and metal ores are found in association with metal sulfides. Streams in these areas can have pH as low as 3.
Land use and land cover, which influence water quality, also tend to have regional patterns. Many of these represent human-induced perturbations (e.g., agriculture and urbanization) as discussed later in this chapter. Populous regions and those where agriculture is extensive are likely to have degraded water quality. Another land cover, natural wetlands, covers a large portion of the landscape in several states (e.g., Minnesota, Maine, Florida). Waters from wetlands tend to have high levels of dissolved organic matter (humic and fulvic acids). Arid regions lack vegetation, which retains particulate matter, the combination of low vegetative cover and high evapotranspiration tend to cause high concentrations of SPM in western rivers.
Wide variations in the chemical composition of water are a natural consequence of regional differences in soils, bedrock, atmospheric inputs, and climate. In each region, aquatic ecosystems have adapted to local chemical conditions. Measurements of water quality in undeveloped headwater streams provide local benchmarks against which watershed managers can compare water quality degradations. Differences between ambient water quality and the local baseline are
usually caused by point and nonpoint sources of pollution (discussed later in this chapter). Watershed managers must decide what level of water quality is desired in relation to the inevitable costs, and design strategies to achieve those water quality goals.
Ecosystems
Plant and animal communities are interdependent and interact with their physical environment (soil, water, and air) to form distinct ecological units called ecosystems. The structures, components, processes, and even the boundaries, of ecosystems vary over time as a result of disturbances such as fires, floods, and climatic variations. However, ecosystem functions are generally resilient to the normal range of these disturbances, commonly referred to as the historic range of natural variability. In many cases, ecosystems depend on such disturbances for their regeneration and continued functioning.
The wide variation in climate and hydrology across the United States contributes to variation in its terrestrial and aquatic ecosystems. Such regional variation imposes inherent limits on attainable water quality, biotic assemblages, and trophic states that should be considered in watershed management. For example, stream-water quality in 107 watersheds across Ohio varied substantially among five ecological regions, even though all were minimally disturbed by human activity (Larsen et al., 1988). Similarly, there are distinct regional differences in water quality among Minnesota lakes, indicating that no single total phosphorus concentration should be used for setting standards across the state (Heiskary et al., 1987). Such regional differences also affect the assemblages of fish and other aquatic organisms present in lakes and streams (Larsen et al., 1986; Hughes et al., 1987; Rohm et al., 1987; Whittier et al., 1988). Watershed managers should take these regional variations into consideration in plans and regulations.
A number of maps have been developed to show ecological variation across the country using various combinations of climatic, physiographic, soils, land use, and vegetation characteristics to regionalize the landscape. Some of these maps show conditions that would exist if human influence were absent from ecosystems (Küchler, 1970). However, for most of the United States, particularly in regions of cultivated cropland, it is unrealistic to expect that water and land resources could attain the level of quality possible prior to major human settlement. Thus regionalization schemes by the U.S. Department of Agriculture (USDA, 1981) and the U.S. EPA (Omernik, 1987; USEPA, 1996) take into account the influence of human activities. Similarly the U.S. Geological Survey's ''Seasonal Land Cover Regions" (USGS, 1994) represents actual rather than potential vegetation, dividing the conterminous United States into 154 different phenological vegetation groups through analysis of multitemporal satellite imagery, elevation, and climate data.
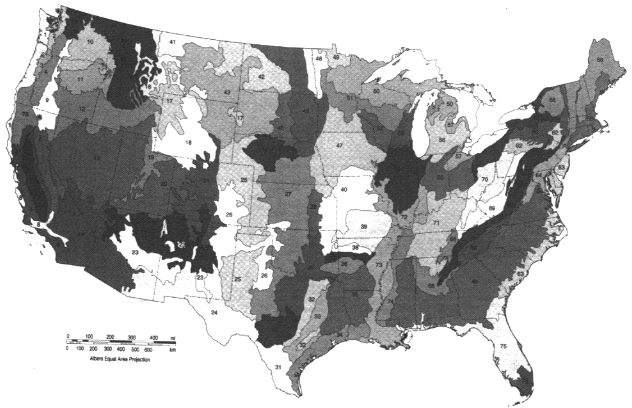
Omernik's (1987) "Ecoregions of the Conterminous United States" is notable
in that it was compiled specifically "to give managers of aquatic and terrestrial resources a better understanding of the regional patterns of attainable quality of these resources" (Omernik and Gallant, 1990). It consists of a map and accompanying tables that describe 76 regions of relative homogeneity in ecological systems based on land use, land surface form, potential natural vegetation, and soils (Figure 3.18). Tests in Arkansas, Minnesota, Ohio, and Oregon have shown this system to have practical application for water resource management (Larsen et al., 1986; Heiskary et al., 1987; Hughes et al., 1987; Omernik, 1987; Rohm et al., 1987; Hughes and Larsen, 1988; Larsen et al., 1988; Whittier et al., 1988).
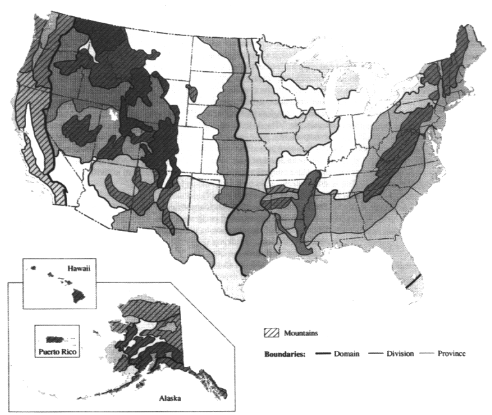
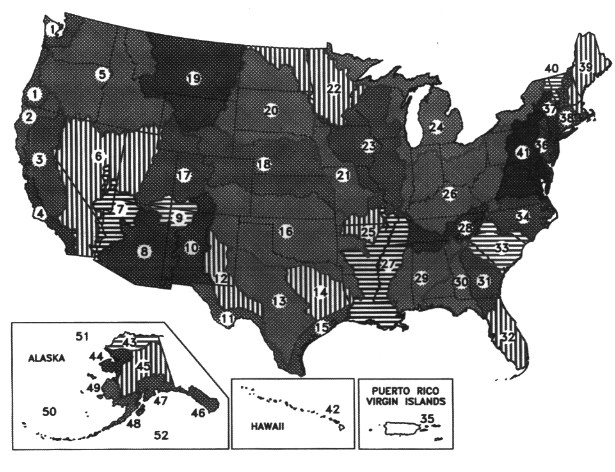
There are two initiatives to coordinate ecological regionalization schemes within North America. The first is a joint effort among Canada, the United States, and Mexico, to develop a hierarchical set of ecoregions across North America, for which draft "Level I" and "Level II" ecoregions has been developed. The second effort is a U.S. interagency effort to develop a hierarchical and common set of ecoregional boundaries that will bring greater uniformity to the different ecoregion maps the various land and water management agencies have created as they have adopted ecosystem-based approaches. For example, the U.S. Forest Service uses an ecosystem approach to forest management, considering an area's physiography, soils, and understory vegetation as indicators of the most suitable forest practices (Figure 3.19). The Service believes that maintaining or restoring ecosystems—rather than managing legislatively or administratively established land units and individual natural resources—would better address declining ecological conditions and ensure the sustainable long-term use of natural resources (GAO, 1994). The U.S. EPA has also delineated ecoregions (Omernik, 1987) and proposed their use for managing environmental resources (Hughes and Larsen, 1988; Gallant et al., 1989) (Figure 3.18). The Natural Resources Conservation Service (NRCS) (formerly the Soil Conservation Service) has divided the country into 27 Land Resource Regions based on physiography and crop potential (see color plate 1) (USDA, 1981), that form the basis for the NRCS' s regional organizational structure. In March 1994 the U.S. Fish and Wildlife Service adopted an ecosystem management approach to fish and wildlife conservation and identified 52 ecosystems (Figure 3.20).
The effort to better coordinate these ecoregion designations was created by an interagency memorandum of understanding (MOU) that established an interagency team, designated as a work group of the Federal Geographic Data Committee (FGDC), to develop a common framework of ecological regions for the nation. Achieving this common regionalization scheme required recognition of the differences in the conceptual approaches and mapping methodologies that have been used to develop the agencies' various ecoregion frameworks.
These efforts reflect the strong push toward coordinated ecosystem management called for by an Interagency Ecosystem Management Task Force formed by the White House Office on Environmental Policy in 1993. The Task Force was asked to find ways to implement an ecosystem approach to environmental man-
agement, establish overarching goals for all federal agencies, and remove barriers that frustrate more effective, efficient interagency cooperation (GAO, 1994). The Task Force's report made the case for use of ecosystem concepts of environmental management:
The ecosystem approach is a method for sustaining or restoring natural systems and their functions and values. It is goal driven, and it is based on a collaboratively developed vision of desired future conditions that integrates ecological, economic, and social factors. It is applied within a geographic framework defined primarily by ecological boundaries. The goal of the ecosystem approach is to restore and sustain the health, productivity, and biological diversity of ecosystem and the overall quality of life through a natural resource management approach that is fully integrated with social and economic goals. This is essential to maintain the air we breath, the water we drink, the food we eat, and to sustain natural resources for future populations (Interagency Ecosystem Management Task Force, 1995).
It is a common sense way for public and private managers to carry out their mandates with greater efficiency.
Demography
Patterns of human settlement have a variety of environmental impacts related to watersheds. In urban environments, watershed management issues range from the efficiency and effectiveness of high-volume water treatment to concentrated runoff from extensive impervious surfaces. In more sparsely populated areas, control of nonpoint source pollution becomes a central watershed management issue. Agricultural land use and on-site residential waste disposal pose water quality concerns. Thus while watershed management is needed across the entire landscape, specific watershed management problems vary depending on the type of land use and the pattern of population settlement within a given area.
Until the 1970s, urban population growth rates had long exceeded rural growth rates in the United States. In the 1970s, however, population growth rates in rural, nonmetropolitan areas exceeded those in metropolitan areas, demonstrating a national pattern of population dispersal. Not only did small towns grow, but there were high rates of population growth in nonurban areas, driven mostly by migration. Though the earlier pattern of higher urban growth rates reappeared in the 1980s, evidence from the early 1990s indicates that rural growth may again exceed urban rates (Fuguitt, 1985; Fuguitt et al., 1989; Johnson and Beale, 1994).
Human population is most dense in the eastern half of the nation, especially in the cities of the East Coast. A comparison of the nation's largest cities illustrates the contrast between the East and West. In 1992, the density of New York City's population was 22,900 persons per square mile, while the density of Los Angeles' population was 6,300 (U.S. Bureau of Census, 1995). These radically
|
1. North Pacific Coast |
19. Upper Missouri/Yellowstone Rivers |
36. Delaware River/Delmarva Costal Area |
|
2. Klamath/Central Pacific Coast |
20. Main Stern Missouri River |
37. Hudson River/New York Bight |
|
3. Central Valley of California/San Francisco Bay |
21. Lower Missouri |
38. Connecticut River/Long Island Sound |
|
4. South Pacific Coast |
22. Mississippi Headwaters/Tallgrass Pralrie |
39. Gulf of Maine Rivers |
|
5. Columbia River Basin |
23. Upper Mississippi River/Tallgrass Pralrie |
40. Lake Champlain |
|
6. Interior Basins |
24. Great Lakes |
41. Chesapeake Bay/Susquehanna River |
|
7. Lower Colorado River |
25. Ozark Watersheds |
42. Pacific Islands |
|
8. Galla/Salt/Verde River |
26. Ohio River Valley |
43. Arctic Alaska |
|
9. San Juan |
27. Lower Mississippi River |
44. Northwest Alaska |
|
10. Middle and Upper Rio Grande |
28. Tennessee River |
45. Interior Alaska |
|
11. Lower Rio Grande |
29. Central Gulf Watersheds |
46. Southeast Alaska |
|
12. Pecos River |
30. Florida Panhandle Watersheds |
47. South Central Alaska |
|
13. Edwards Plateau |
31. Altamsha/Sunwanee Rivers |
48. Bristol Bay/Kodiak |
|
14. East Texas |
32. Peninsular Florida |
49. Yukon - Kuskokwim Delta |
|
15. Texas Gulf Coast |
33. Savannah/Suntee/Pee Dee Rivers |
50. Bering Sea/Aleutian Islands |
|
16. Arkansas/Red Rivers |
34. Roanoka/Tar/Neusa/Cape Fear Rivers |
51. Beaufort/Chukchi Seas |
|
17. Upper Colorado River |
35. Caribbean |
52. North Pacific/Gulf of Alaska |
|
18. Platte/Kansas Rivers |
|
|
FIGURE 3.20 Ecoregions as identified by U.S. Fish and Wildlife Service. SOURCE: GAO, 1994.
different densities demand different management strategies for resources (such as watersheds) closely related to human populations.
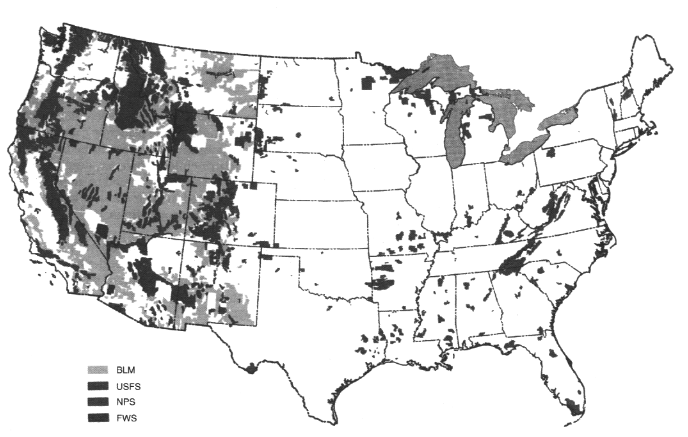
Federal lands have low population density. For example, 41 percent of the land base of six Western states (Montana, Wyoming, Colorado, Utah, New Mexico, and Arizona) is federally held land with few permanent residents (Figure 3.21).
The dispersal of population to more rural areas creates new watershed management challenges. Population growth rates from 1980 to 1990 were highest along the East, South, and West Coast. Sparsely populated areas in Texas and Nevada also had high growth rates, but this arises from population change. It is important to note that this change takes different forms, including high density urban development, rural/urban fringe, dispersed moderate density settlement, and extensive population dispersal into rural areas.
Human Effects On Watersheds and Streams
Humans affect watersheds and streams in two basic ways. First, they alter the land surface of watersheds, affecting both quantity and quality of streamflow and lakes. Second, streams and streamflow are directly affected by channel and floodplain alterations, dams, and water transfers, while water quality is affected by point sources. Many researchers have addressed these impacts (Leopold et al., 1964; Dunne and Leopold, 1978; Turner et al., 1990; Maidment, 1993; Goudie, 1994; Whitney, 1994; and Mays, 1996).
Land Use
Land use and land cover exert a powerful influence on the quantity and quality of runoff (Potter, 1991; Calder, 1993; McCutcheon et al., 1993; Malina, 1996). The primary land uses are forest, urban, agricultural, and wetlands.
A forest is usually the most hydrologically benign of land uses. First, the canopy and vegetative litter protect the soil from erosion. Second, the forest promotes permeable soils that often can accept even the most intense rains and convey the water downslope by subsurface routes. Forests thus prevent erosion, infiltrate most water, mitigate smaller floods, and yield clean streamflow. The general exception is after extensive tree harvesting when temporary erosion and nutrient export can occur; most forest erosion occurs as a result of poor harvesting practices. Although forests give many benefits, there is a cost: forests consume water (Calder, 1993) (see Box 3.1). An area of forest annually consumes a depth of water about 326 millimeters (13 inches) greater than other vegetation such as crops or grass. Thus, cutting an acre of forest will produce more than an acre-foot (1234 m3, or 326,000 gallons) of water that would otherwise be transpired by the trees. In water-short areas (where an acre-foot of water costs $200-$300) forest management for water may become common in the future (Trimble
|
Box 3.1 Watersheds of the Southern Piedmont: Forest vs. Water Supply  Water yields from watersheds in the Southern Piedmont region, which spans parts of Georgia, Alabama, and South Carolina, illustrate the interaction between forest management and water yield. With the decline of agriculture in the Southern Piedmont during this century, forests have succeeded cotton, corn, and other crops. This recovering forest has decreased erosion, but it as also significantly decreased streamflow. Between 1919 and 1967, forested areas in this region increased in size between 10 to 28 percent, bringing about average annual decreases of streamflow ranging from 4 to 21 percent. The inclusive water-yield model shows that complete afforestation can decrease annual yield by about 13 inches, while 100% deforestation can increase it by the same amount. Such changes can be significant in a region like the Piedmont where streamflows average only about 15 inches. Another factor makes the impact of trees even more significant: forest-induced water deficits become greater with less rainfall. The net effect is that natural droughts are exacerbated while the forests have little impact in very wet years. Since water supply management is often based on minimum flows, forest management for increased water yield may become significant as water-supply demands increase. This is true not only for the Piedmont, but also for much of the forested Eastern United States. SOURCE: Trimble et al., 1987. |
et al., 1987). Although forests reduce small floods, they cannot control the larger ones. The Mississippi River was in flood and 60 miles wide when a member of the expedition of the Spanish explorer De Soto first saw it in 1541 (Frankenfield, 1927).
Fire has long been perceived as the enemy of the forest and especially of its watershed values. For many years, the official policy of the U.S. Forest Service and other governmental agencies was to suppress fires. However, long experience showed that this policy allowed the accumulation of fuel so that when fire eventually occurred, the accumulated fuel created a much worse fire than had
nature been allowed to run its course. The Forest Service has therefore changed its policy to allow forest fires to burn, and even prescribes periodic fires to help prevent fuel accumulation.
Forest fires in the deciduous forests of the humid eastern United States are normally ground fires, and rarely disturb the hydrologic values of watersheds. In coniferous forests, especially in the West, complete or crown fires are more common, and these fires can significantly affect hydrologic qualities. Fire's effects are strongest in the chaparral scrub forest of the Southwest, where the waxy vegetation burns furiously and can create hydrophobic (water repellent) soil so that runoff and erosion are dramatically increased (De Bano, 1969).
The antithesis of forest is an urbanized area, where a large percentage of the surface is impermeable and pipes and sewers augment natural channels (Leopold, 1968; Loganathan et al., 1996). The runoff process in such areas is so efficient that stream discharge peaks may be several times those of comparable rural areas. Urbanization's greatest effects on floods are for smaller events; larger magnitude, lower frequency floods may be less affected. Thus urban areas and forests will produce greatly disparate 1-year and 10-year flood levels while producing the 100-year flood events or more similar magnitude.
The "waterproofing" caused by pavement in urban areas can actually "harvest" water, producing a much greater volume of surface water than unpaved areas. However, urban runoff may be of extremely poor quality because it may contain such pollutants as pet wastes, air pollution fallout, and the wastes of vehicular traffic such as antifreeze, oil, gasoline, lead, asbestos, and some exhaust products (Heaney, 1986; Makepeace et al., 1995) (see Table 3.1 and Box 3.2). Road salt is a common additional pollutant in snowy regions. Particulate matter concentrations from air pollution and street abrasion may approach levels from
TABLE 3.1 Comparison of Stormwater Quality from Various Sources for Selected Parameters
|
Study Location |
BOD (mg/l) |
Total solids (mg/l) |
Suspended solids (mg/l) |
Phosphate (mg/l) |
Chloride (mg/l) |
Fecal coliforms (MPN/100 ml)a |
|
Durham, North Carolina |
2-232 |
194-8860 |
27-7340 |
0.15-2.5 |
3-390 |
7000-86000 |
|
Cincinnati, Ohio |
1-173 |
— |
5-1200 |
0-02-7.3 |
5-705 |
500-76000 |
|
Coshocton, Ohio |
0.05-23 |
— |
5-2074 |
0-08 |
— |
2-56000 |
|
Detroit, Michigan |
96-234 |
310-914 |
— |
— |
— |
25000-930000 |
|
Seattle, Washington |
10 |
— |
— |
43 |
— |
16000 |
|
Morristown, New Jersey |
3-17 |
— |
56-550 |
0.02-4.3 |
— |
— |
|
Ann Arbor, Michigan |
28 |
— |
2080 |
0.8 |
— |
100 |
|
a MPN—Most probable number. SOURCE: Reprint, with permission, from Ellis, 1975. © 1975 by Middlesex Polytechnic Research. |
||||||
|
Box 3.2 Quinnipiac River Watershed, Connecticut: Urban Water Pollution  The Quinnipiac River watershed, draining 429 square kilometers in 15 towns in south-central Connecticut, is a typical New England coastal river basin in the densely populated eastern United States. Its case illustrates the problems associated with highly urbanized watersheds and the pollution they generate. Millions of gallons of treated municipal, domestic, and industrial wastewater are discharged into the river every day, and two U.S. Superfund sites are located on the banks of the Quinnipiac river. The Quinnipiac was once badly polluted, but regulation of point source discharges has improved water quality, and now thousands of anglers and boaters use the stream. Thousands of people rely on aquifers within the watershed for drinking water. While the health of the river is steadily improving, there are continuing threats to its water quality and habitat integrity. Nonpoint source (NPS) pollution is likely to be a major contributor to water quality degradation within the watershed and in Long Island Sound, at the river's outlet. Urban land uses near the Sound and in its major tributaries, including the Quinnipiac, contribute the greatest amount of nonpoint source pollution to the coastal waters. A study of Long Island Sound identified subbasins of the Quinnipiac watershed as of the highest priority for managing nonpoint sources of nitrogen. Priority nonpoint source problems in the watershed include water quality impacts associated with seasonally low flows, on the one hand, and high volume stormwater runoff on the other. These phenomena result in |
rural areas. Urban pollution can accumulate during dry periods and be flushed out in a highly concentrated burst during the first minutes or hours of a storm. Levels of some pollutants in urban runoff are worse than raw domestic sewage (Table 3.2). Roads, including highways, are often rural extensions of the urban phenomena, and can affect outlying areas.
In their effects on the quantity and quality of runoff, agricultural fields usually lie somewhere between forest and urban land (Calder, 1993). The variance depends on soil condition, type of crop or vegetation, and level of management. Cropland, ranging from close-grown grasses to row crops with much bare
|
water pollution, habitat impairment, severe erosion, and sedimentation. However, many aspects of the degradation process are poorly understood. For example, salt- and fresh-water wetlands may play a critical role in mitigating the effects of NPS pollution by removing excess nitrogen, heavy metals, and suspended solids from the river before they reach the Sound. On the other hand, badly degraded marshes may themselves become sources of contamination. With funding from a Nonpoint Source Pollution Grant under Section 319 of the Clean Water Act, a local consortium has organized to resolve NPS problems in the Quinnipiac River Watershed Association, and the Peabody Museum of Natural History joined forces with the Connecticut Department of Environmental Protection, U.S. Environmental Protection Agency, The Regional Water Authority, Natural Resources Conservation Service, municipal personnel, and concerned citizens. The consortium has initiated data collection, technical advisory activities, planning processes, and the development of a GIS database. The consortium also strives to raise community awareness of the issues because the majority of the population is unaware of the river, the resources it provides, and the threats to its health. The next major step is a proposed broad, multi-objective planning and management initiative under the Connecticut Multiple Use Rivers Act. The approach will integrate solutions to a wide range of issues, including water quality, water supply, habitat, and land use, all focused on the watershed. The nonpoint source pollution project, with its assessment, prioritization, and implementation of abatement measures, will be the model for resolution of other problems. The Quinnipiac experience shows how a variety of interested groups can organize around the watershed concept, and how the solution of one problem leads to confidence in dealing with a broad array of issues. |
soil exposed, usually shows the greatest variance in the quantity of runoff. Crop-land receives the bulk of agricultural chemicals such as fertilizers (especially nitrates), herbicides, and pesticides. The movement of these chemicals into streams is enhanced because they often adsorb onto soil particles, and soil erosion is usually greatest from cropland. The need to reduce erosion from cropland has led to greatly improved management techniques, including minimum- and no-till farming.
Irrigation of cropland, especially in the Southwest, leads to great water losses from infiltration and evaporation. Without careful management, soils can become
TABLE 3.2 Comparison of Contaminant Profiles for Urban Surface Runoff and Raw Domestic Sewage, Based on Surveys Throughout the United States
|
Constituenta |
Urban surface runoff |
Raw domestic sewage |
|
Suspended solids |
250-300 |
150-250 |
|
BODb |
10-250 |
300-350 |
|
Nutrients |
|
|
|
(a) Total nitrogen |
0.5-5.0 |
25-85 |
|
(b) Total phosphorus |
0.5-5.0 |
2-15 |
|
Coliform bacteria (MPN/100ml)c |
104-106 |
106 or greater |
|
Chlorides |
20-200 |
15-75 |
|
Miscellaneous substances |
|
|
|
(a) Oil and grease |
Present |
Present |
|
(b) Heavy metals |
(10-100) times sewage cone. |
Traces |
|
(c) Pesticides |
Yes |
Seldom |
|
(d) Other toxins |
Potential exists |
Seldom |
|
a All concentrations are expressed in mg 1-1 unless stated otherwise. b Biochemical oxygen demand. c MPN—Most probable number. SOURCE: Reprint, with permission, from Burke, 1972. © by Academic Press. |
||
salinized. Excess water from irrigated fields (return flow) often contains excess salts, nutrients, and other chemicals that can pollute streams and groundwater.
Because it is vegetated and usually untilled, pasture may be a relatively benign use of land. Heavy grazing with soil compaction and vegetative damage, however, can transform the hydrologic characteristics of pastures into something near those of a parking lot. Such poorly managed pasture produces more overland flow, which causes soil erosion and allows animal wastes to be washed into streams. Because of sparse vegetation and poorly developed soil, semi-add rangeland is even more fragile than pasture, and has been heavily abused and eroded in the past (Cooke and Reeves, 1976; Branson et al., 1981).
Aside from their value as wildlife habitat, wetlands are also generally valuable as sinks for sediment and nutrients (Johnston et al., 1984, 1990; Johnston 1991, 1993; NRC, 1995; Richardson, 1996). Both empirical and modeling studies have also demonstrated the value of wetlands for reducing flood peaks (Novitzki, 1979; Jacques and Lorenz, 1988), although the location of wetlands in relation to stream order influences how far downstream their flood peak reduction effects are observed (Ogawa and Male, 1986).
Large areas of wetlands have been drained for urban development and agricultural purposes, especially in the Midwest. The purpose of wetland drainage is
to lower the water table and move water faster off the wetland surface. This reduces or eliminates their wildlife habitat, nutrient retention, and flood peak reduction capability. Lowering the water table may mitigate runoff from small rainfall events by increasing water storage in the soil (Goudie, 1994), but this mechanism ceases to be effective once the soil becomes saturated. Drainage also increases oxidation and subsidence of organic soils.
Strong state and federal efforts have been made in the last two decades to save the remaining American wetlands. Additionally, large areas of wetlands have been created in the eastern United States from the effects of historical soil erosion and associated sedimentation.
Changes of Streams and Streamflow
Many United States streams have undergone significant changes of morphology and/or streamflow during the past two centuries (Schumm, 1977; Goudie, 1994; Chapra, 1996). Some of these changes were deliberate, some unintentional. Land use, channelization, changes in riparian vegetation, dams and reservoirs, water transfers, and changes in groundwater depths all helped create changes in streams and streamflow.
Some of the greatest impacts on streams have come from the land-use changes discussed earlier. Generally, the greatest impact on runoff and floods is from urban land use (Leopold, 1968) (see Box 3.3). Greatly enhanced floods can cause severe channel erosion, while decreased soil-infiltration rates leave little base flow between storms so that water often collects in stagnant pools (Wolman, 1967; Graf, 1975, 1977; NRC, 1997).
On agricultural land, accelerated soil erosion over the years has streams and valleys to aggrade (fill with eroded sediment and other detritus) in some cases over at depths over 5 meters. Frequently, streams were aggraded more rapidly than floodplains, creating wetlands. Such large amounts of sediment take centuries or millennia to move through watersheds (Happ et al., 1940; Meade, 1982) (Figure 3.22). The management of migrating sediment can be an important aspect of watershed management (Trimble, 1993). In other cases, runoff has increased disproportionately to sediment, causing tributary stream channels to erode and enlarge and sending sediment downstream to be deposited in larger valleys with lower gradients (Happ et al., 1940; Meade, 1982). With the implementation of soil conservation measures since the 1930s, both runoff and erosion have been reduced (Trimble and Lund, 1982; Potter, 1991). As a result, formerly deposited sediment has been eroded and transported farther downstream and redeposited (Figure 3.22). Stream sediment loads therefore do not necessarily indicate current upland erosional processes—which makes sediment management more complex than just managing upland land use.
Channelization with a combination of open surface ditches and subsurface feeder drains is a common means of wetland drainage that has been employed to
|
Box 3.3 San Diego Creek, California: Urban Channel Erosion  While stream-channel erosion in urbanizing watersheds has been documented in humid regions, few studies have examined such erosion in arid or semi-arid locales (Graf, 1988). San Diego Creek came to the attention of the public in the late 1970s when it was realized that sediment from the watershed was filling Newport Bay, a large and important estuarine habitat. An extensive and expensive 208 study attributed the problem to upland erosion (Boyle Engineering, 1982). Research since then, however, has shown that stream channel erosion has been responsible for much of the sediment moving toward Newport Bay (Trimble, 1997a). Most channel erosion is in tributaries while the broad, downstream trunk channels are usually sediment sinks. Both situations present controversial management problems. Despite attempts to control channel erosion, many tributaries have had their cross-sectional area increased by a factor two to ten during the past one to seven decades. This erosion not only sends vast amounts of sediment to Newport Bay, but can also remove significant areas of expensive real estate as channels widen. Undermined trees and other debris fall into the channel, sometimes blocking the channel and increasing flood damage. These processes lead to a major management question: Should such channels be left in the earthen condition to presumably erode at high rates, or should the channels be paved or armored with rip-rap? Paved channels prevent erosion and convey floodwaters more efficiently, but many people oppose them on both cost and aesthetic grounds. Downstream trunk channels have been vastly enlarged to transport the expected 500-year flood. However, these broad channels have become sediment traps because they do not efficiently transport the smaller, sediment-laden flows. The resulting sediment deposits are rapidly colonized by trees and brush which, in turn, quickly become wildlife habitat. These deposits, along with their trees and brush, reduce the flood conveyance capacity of the trunk channels. Additionally, large floods could remobilize these materials, sweeping them downstream where the vegetation can snag on bridge piers, thus decreasing openings and potentially increasing flooding. Regular maintenance would remove these deposits, but local pressure has been brought to protect the incipient wildlife habitat. Thus, the management question in the lower reaches becomes wildlife habitat versus public safety. |
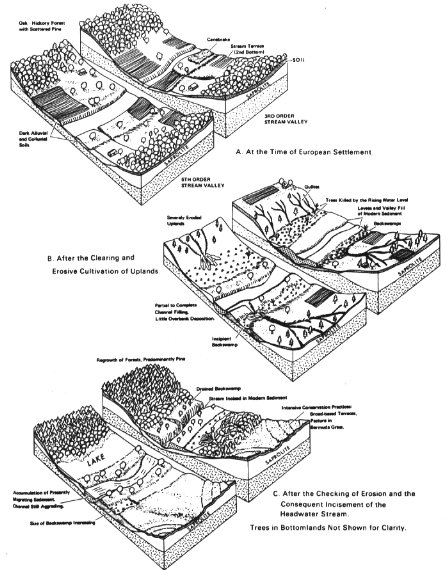
FIGURE 3.22
Severe erosion and sedimentation from poor agricultural land use in the Southern Piedmont, 1700-1970. Note the aggradation of streams, the transformation of floodplain into swamps, and the transfer of sediment downstream and its relation to land use. SOURCE: Reprint, with permission, from Trimble, 1974. © by Soil Conservation Society of America.
drain large wetland areas stream valleys that had been swamped by historical sedimentation. Such channels were usually deep, straight, and inherently unstable (Keller, 1976; Brookes, 1985). Some channels have eroded laterally or vertically, or both, with the sediment being redeposited further downstream (Schumm et al., 1984). In cases where hillside and upstream erosion have not been curtailed, the enlarged channels have simply filled with sediment. Ditching these mainstream channels lowers the base level of tributaries, thus destabilizing them and causing channel erosion which helped fill the trunk channel (Happ et al., 1940). Such complications make management of such channels a growing art (Shields et al., 1995; Wang et al., 1997).
Managers also channelize streams as a flood protection measure. This is especially true in urban areas where rapidly eroding channels are straightened to increase a stream's slope, reshaped into a hydraulically efficient cross section with a uniform grade, and paved. Although not aesthetically pleasing, paving is important to (a) reduce hydraulic friction and thereby lower flood stages, (b) prevent channel erosion, and (c) maintain a uniform grade (Loganathan et al., 1996). One problem with paved channels in arid regions is that recharge of groundwater through the channel floor is prevented. Thus, such areas need recharge basins in or next to urban channels.
Where streams are too large for channelization by excavation, some flood control may be gained by building levees. In addition to being very expensive to build and maintain, levees lead to two additional problems. First, they keep floods out of the storage zones on floodplains, thus increasing the floodwave downstream. Second, by reducing the flood-channel width by restricting it between the levees, levees cause flood stages to be higher. If levees fail or are overtopped, the resulting flood is locally much higher than it would have been without the levees. Both effects were apparent in the 1993 Midwest flood (Interagency Floodplain Management Review Committee, 1994).
The most striking historic changes in streamflow and stream channels have occurred in the Southwest, where exotic rooted plants, especially salt cedar (Tamarix chinesis), have colonized stream channels, transpiring huge amounts of water and causing streams to aggrade and braid (Graf, 1978). In coastal streams, giant reed (Arundo donax) causes similar problems. Channelization measures that remove all vegetation along streams without stabilizing them somehow have left many streambanks especially vulnerable to erosion, creating a highly unstable stream environment (Keller, 1976; Schumm et al., 1984; Gregory, 1985; Madej et al., 1994).
Stream morphology and the quality of habitat for aquatic organisms are strongly influenced by the presence of large woody debris (Harmon et al., 1986; Sedell et al., 1988). As riparian trees grow and die, they fall into channels or onto stream banks and the floodplain. Large woody debris also results from environmental disturbances such as floods, windstorms, wildfires, and landslides (Keller and Swanson, 1979; Benda, 1990). Large woody debris deflects streamflow,
causing scour of the bed and banks and creating pools that are used by many species of fish and other animals (Bisson et al., 1987). In addition to creating pools, large woody debris in small streams may store sediment and organic matter, regulating the rate of movement of these materials downstream (Naiman and Sedell, 1980; Bilby, 1981; Megahan, 1982; Triska and Cromack, 1982). The structural and ecological diversity created by large woody debris in many streams is critical to the support of rich and diverse communities of plants and animals (Gregory et al., 1991; Reice, 1994).
Large woody debris has historically been removed from streams to aid river navigation (including log drives) and to facilitate upstream fish migrations (Bisson et al., 1987; Hicks et al., 1991). As a result, many streams across the nation now hold much less large woody debris than existed a century ago (Sedell and Luchessa, 1982; Sedell and Beschta, 1991) and a considerable amount of habitat diversity has been lost (Gregory et al., 1991; Bisson et al., 1992; Bayley, 1995).
Large woody debris also can be problematic. Accumulations can cause streambank erosion and damage to structures such as bridges, fences, and buildings. Large woody debris from forested floodplains may destabilize streams causing channel widening and enlargement with a loss of sediment to downstream reaches. Conversely, grassy floodplains can cause storage of sediment resulting in smaller and narrower channels (Davis-Colley, 1997; Montgomery, 1997; Trimble, 1997b).
Dams and reservoirs also can have profound influences on streams and streamflow (Baker, 1996; Stanford, 1997). Perhaps their foremost effect is to make streamflow downstream of the dam more regular by increasing low-flow discharges and decreasing the magnitudes of most floods. This downstream effect is beneficial in many ways, but there are areas in the West where coarse sediments from tributaries accumulate downstream from large dams because flows are inadequate to transport them away (Graf, 1980).
Additionally, because periodic high flows are necessary for the ecological integrity of rivers (Stanford et al., 1996), a certain degree of flooding may be desirable. Another problem, common in more populated areas of the humid East, is that although reservoirs may control smaller floods, they rarely can control the larger ones. Thus, people are lulled into economic activity on floodplains, only to be periodically flooded out with grave economic consequences.
Another characteristic of reservoirs is that they trap much of the entering sediment (Dendy and Champion, 1978; Trimble and Bube, 1990; Baker, 1996). Such sediment can displace part of the usable reservoir volume. In addition much of the sediment is usually deposited at the head of the reservoir, creating a rise in base level that sometimes causes the river to aggrade for several miles upstream. Furthermore, while the trapping of sediment by a reservoir may improve downstream water quality, the sediment-starved water may degrade channels for many miles downstream (Williams and Wolman, 1984). Wildlife habitat may be
changed significantly upstream and downstream from a reservoir (Pitlick and Van Steeter, 1994; Ligon et al., 1995).
Large reservoirs are vulnerable to evaporative losses. In the humid East, these losses are often not much larger than otherwise would have been lost to vegetative transpiration. In the add West, however, gross evaporative losses are net losses and can amount to as much as 7 feet (2 m) per year from an entire reservoir area. Evaporation also concentrates salt and other minerals in the remaining reservoir waters.
Reservoirs can affect water temperature. Shallow reservoirs allow warming during the summer, while deep reservoirs may become so cold at the greatest depths that released water may be unsuitable for irrigation and contain less dissolved oxygen level, impacting aquatic life. Stratification of deep reservoirs can also cause undesirable changes in water quality, such as when bottom water isolated from the atmosphere suffers oxygen depletion.
Whether caused by dams, water transfer, or land use alteration, changes of stream flow regimes can have significant effects on aquatic and terrestrial ecosystems. Evaluating such changes can sometimes make mitigation measures possible (Richter et al., 1996).
Because significant distances often separate areas of high water demand from those areas of water surplus, water transfers play an important role in the United States (NRC, 1992). One example is the Colorado River. Fed from the mountains, the Colorado once flowed through the deserts of the Southwest and emptied into the Gulf of California. By compact, the entire flow was apportioned to seven states including California, Nevada, and Arizona, and the river's water has been transported to regions outside the river's watershed. Indeed because of erroneous long-term streamflow measurements, more water was apportioned than would normally be available! A series of reservoirs made the water readily available for consumption so that little flow now reaches the ocean (Graf, 1985). Between 1935 and 1965, water storage in western U.S. reservoirs increased from about 5 million acre-feet to 270 million acre-feet (Graf et al., 1997).
Southern California is the nation's largest importer of water; its imported water is used extravagantly for both urban and agricultural uses, especially irrigation (Hundley, 1992). This water is brought not only from the Colorado River, but also from Northern California and the Owens River Valley on the east side of the Sierra Nevada Mountains.
Significant ground water extraction, another form of water transfer, may have any of several impacts (Graham et al., 1996). First, as the water table drops, pumping of ground water becomes increasingly difficult and expensive. Second, declining ground water can often cause depleted streamflow and local surface-water supply. Finally, excessive ground water extraction can cause the ground to subside. Subsidence of more than 9 meters has been recorded in Southern California (Figure 3.23), and some formerly inhabited areas along the Gulf Coast are now inundated.
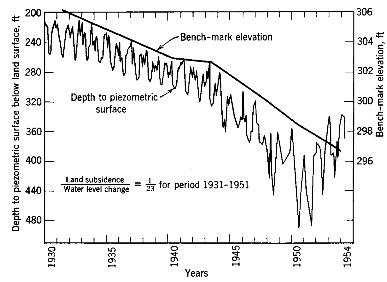
FIGURE 3.23
Changes in ground water surface and piezometric surface (ground water surface from confined aquifer) north of Bakersfield, California. SOURCE: Reprint, with permission, from Todd, 1959. © 1959 by John Wiley and Sons, Inc.
Because of heavy irrigation, some areas have too much ground water recharge. As water tables rise close to the surface, the slightly saline ground water can move to the surface by capillary action and evaporate. This concentrate salt and other minerals at the surface, often damaging the soil and polluting local ground water.
Point Sources of Pollution
Unlike the nonpoint sources considered earlier in this section, point sources of water pollution are released, often deliberately, into a stream at an identifiable place. Thus, prerelease treatment usually becomes more practicable for point sources than nonpoint sources of pollution (Malina, 1996; McCutcheon et al., 1993). The main point-source dischargers are wastewater treatment systems, industrial plants, feedlots, and mining operations.
Wastewater treatment plants put sewage from residential, commercial, and industrial areas through primary, secondary, and in some cases tertiary treatment processes that remove organic material, nitrogen, phosphorus, and pathogens. With present technology, increased public awareness, and increasingly stringent discharge permit requirements, wastewater can be treated to better-than-ambient condition and the effluent released into rivers, lakes, and oceans, or recharged
|
Box 3.4 Santa Ana River Watershed, California: An Effluent-Dominated Stream 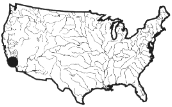 The Santa Ana River of Southern California exemplifies effluent-dominated streams and their watershed management problems. The 2,800 square-mile watershed is home to 4.5 million people, and spans ecological zones with little rainfall, ranging from arid lowlands and coastal areas to pine forests in the San Bernardino Mountains. Water users consume twice as much water as is available naturally, with the deficit made up by water imported from northern California and the Colorado River. Land uses include residential, commercial, industrial, military bases, airports, agriculture (crops, orchards, and high-density dairy farms), open spaces and parks (including Disneyland and Knott's Berry Farm), tourism and recreation (skiing, sailing, swimming, boating, marinas, hunting, and hiking), wildlife habitats for rare and endangered species, water reclamation, groundwater recharge, and major flood control facilities. The Santa Ana River is an intermittent stream, and for most of the year, 45 wastewater treatment plants contribute 85 to 90 percent of its surface flows. Many sections of the river are concrete lined and the river serves as a dry flood control channel. Because of discharges from National Pollution Discharge Elimination System (NPDES) permitted point sources, the Santa Ana River was listed by EPA on the 304 (l) ''toxic hot spots" list of impaired waterways. Water quality problems would continue to exist even if all permitted dischargers met all their discharge requirements. Nitrogen and total dissolved solids exceed water quality objectives mostly due to nonpoint source discharges from agricultural and dairy practices. Polluted urban runoff, which is growing from increasing urbanization, exacerbates the problem. This watershed illustrates the failure of the "one-size-fits-all" water quality criteria developed at the national and state governmental levels, for those criteria do not fit the unique conditions of the Santa Ana River watershed. If federally mandated "Individual Control Strategies" are added to the NPDES permits of |
|
the dischargers to the Santa Ana River, as required under the 304(l) listing, the cost (an estimated $6 billion) would have a substantial adverse effect on the economy of the region. The Santa Ana Watershed Project Authority, a joint powers agency made up of the five major water districts in the watershed that coordinate and implement projects to improve water quality in the region, and the Santa Ana River Dischargers Association, an organization of the upstream dischargers into the Santa Ana River, initiated a "Use-Attainability Analysis" for the basin. Their objective was to evaluate the physical, biological, chemical, and hydrological conditions of the Santa Ana River, and to determine what specific beneficial uses the river could support. The Santa Ana Watershed Planning Advisory Committee was also formed. It was made up of agricultural and dairy interests, city and county governments, wastewater and water supply agencies, coastal and environmental interests, stormwater and flood control interests, water quality regulators, and state and numerous federal government resource agencies. The study developed and made scientifically grounded recommendations on establishing a new beneficial use designation based on the effluent-dependent, concrete-lined conditions of the river. The Californian Regional Water Quality Control Board's Basin Plan for the watershed designated portions of the river as "Limited Warm Fresh Water Habitat," with new water quality criteria based on the site-specific characteristics of the Santa Ana River. However, the new beneficial use and water quality criteria were rejected by EPA. The use-attainability analysis was a technical success, an excellent example of a thorough, scientifically based study of the physical, biological, chemical, and hydrological conditions of a watershed. It made a strong a case for rejecting the "one-size-fits-all" regulations and instead developed a beneficial use designation designed for the site-specific characteristics of effluent-dominated streams in arid areas. However, the study was a bureaucratic failure. It was accepted at the regional and state levels, but rejected by the EPA regional and national offices. It is a prime example of the jurisdictional disputes that arise among local, state, and federal agencies over the authority to establish water quality control standards for a river (Anderson, 1996, personal communication). SOURCE: O'Connor, 1995. |
into aquifers. In some parts of the Southwest, sewage effluent may constitute the major portion of streamflow (Stanford, 1997), making the management of such streams problematic (see Box 3.4). In most parts of the country, the effluent is discharged into reservoirs and rivers that serve as drinking water sources for people living downstream. The effluent may also be used for irrigation and industrial purposes.
In non-seaward urban and rural areas, wastewater is treated on-site septic systems. Increasingly stringent public health codes and inspections are reducing the ground water and surface water pollution caused by these systems. However, in many places, septic systems are still placed too close to the ground water table, leading to ground water pollution, or in soils that are too thin, allowing the effluent to move along bedrock into springs and streams.
Industrial wastes (heat, chemical, infectious agents, and radiation) are treated prior to release into the waters of the United States. Some industries pretreat their wastewater and then release it into the wastewater treatment system. Other industries treat the waste themselves to National Pollution Discharge Elimination System (NPDES) permit standards prior to releasing it into streams and rivers. The 1995 Toxic Release Inventory Report (TRI) shows that 630,000 tons of toxins were released into the waters of the United States and 120,000 tons transferred to wastewater treatment plants (USEPA, 1997).
Air emissions from industrial sources carry many pollutants. These airborne pollutants fall directly into the rivers and lakes ("dry fall") or are collected by precipitation and brought to earth. Sulfur oxides from burning of coals have been implicated in acid precipitation, which has heavily affected both terrestrial and aquatic ecosystems. Many streams of the Northeast, where poorly buffered acidic soils predominate, have been rendered sterile. Air currents have carried pollutants, including DDT, dioxins, and other carcinogens, into all parts of the globe, so that there are no places that do not have measurable concentrations of these and other toxic chemicals. The TRI data for 1995 show a release of approximately 631,000 tons of toxic chemicals into the air (USEPA, 1997). Some of these chemicals eventually get into the water, although many are retained in the soils.
Feedlots are important point sources of pollution from agriculture. They may range from isolated barnlots for small herds to very large feeding areas where thousands of animals are kept in relatively small areas. The runoff from feedlots is toxic, high in biochemical oxygen demand (BOD), looks and smells bad, and carries a high load of nitrogen and phosphorus. Confined feeding operations must now treat their wastewater, but problems remain.
Mining can create both sediment and chemical pollution. Environmental laws have greatly curtailed impacts from present-day mining, but past mining activity has left a harsh legacy. In areas of high relief, spoil from mining often was sufficient to aggrade streams and floodplains, while clearing of forests for ore smelting caused stream erosion elsewhere (Graf, 1979). Perhaps the most dramatic example of mining impacts was from hydraulic mining for gold in
California's Sierra Nevada, where mountain valleys were sometimes buried to depths of 25 meters (Gilbert, 1917). Even though hydraulic mining was outlawed in the mid-19th century, sediment has continued to move down the rivers toward San Francisco Bay. A continuing problem is the mining of sand and gravel from streams.
The mining of minerals and fuels, especially of coal, expose such compounds and minerals as iron sulfides, pyrite, and marcasite (ferrous sulphide). The result is often acidification with high sulfate and iron concentrations that may not only be toxic, but also unsightly. In some cases, mining may release toxic metals into the environment. In the past, air pollution from ore processing has affected vegetation and soils over a large area. Wastes from the mining and processing of uranium and thorium may pollute the water and sediments of rivers for miles downstream (Graf, 1994).
Conclusions
The physical and hydrologic components of our environmental systems are tremendously variable from one place to another and from one time to another. The ecological systems that depend on that hydrology are also variable, giving rise to great diversity in the life forms and processes across the continent. Our human population is part of this vast and changeable ecosystem, and it too shows great variability, especially with respect to the density of settlement. Humans affect the physical behavior of hydrologic systems through engineering works, and their chemical characteristics through pollution. Watershed managers need to be aware of the regional variation of environmental systems and take it into account as they plan activities.
The variability in natural systems is matched by variability of the institutional landscape created to manage our water and watershed resources, and this diversity is described in Chapters 6 and 7. Because of the great variability in natural resources and institutional structures, it is unlikely that a standard solution for watershed problems imposed from the national level will be workable in all localities. Rather, it appears that partnerships involving a range of governmental levels, citizens, businesses, and nongovernmental organizations are necessary to accommodate the variability.
References
Baker, L. 1996. Lakes and reservoirs. Chap. 9 In Mays, L. (ed.) Water Resources Handbook. New York: McGraw-Hill.
Bayley, P.B. 1995. Understanding large river-floodplain ecosystems. BioScience 45(3):153-158.
Benda, L. E. 1990. The influence of debris flows on channels and valley floors in the Oregon Coast Range, USA. Earth Surface Processes and Landforms 15:457-466.
Benoit, G. 1994. Clean technique measurement of Pb, Ag, and Cd in fresh water: A redefinition of metal pollution. Environ. Sci. Technol. 28:1987-1991.
Bilby, R. E. 1981. Role of organic debris dams in regulating the export of dissolved and particulate matter from a forested watershed. Ecology 62:1234-1243.
Bisson, P. A., R. E. Bilby, M.D. Bryant, C. A. Dolloff, G. B. Grette, R. A. House, M. L. Murphy, K. V. Koski, and J. R. Sedell. 1987. Large woody debris in forested streams in the Pacific Northwest: past, present, and future. Pp. 143-190 in E. O. Salo and T. W. Cundy (eds.) Streamside Management: Forestry and Fishery Interactions. Contribution Number 57, Institute of Forest Resources, University of Washington, Seattle, Washington.
Bisson, P. A., T. P. Quinn, G. H. Reeves, and S. V. Gregory. 1992. Best management practices, cumulative effects, and long-term trends in fish abundance in Pacific Northwest river systems. Pages 189-232 In R. B. Naiman (ed.) Watershed Management: Balancing Sustainability and Environmental Change. Springer-Verlag, New York, N.Y.
Boyle Engineering Co. 1982. Sediment source analysis and sediment delivery analysis, Newport Bay Watershed - San Diego Creek comprehensive stormwater sedimentation control plan. San Diego, CA.
Branson, F., G. Gifford, K. Renard, and R. Hadley, 1981. Rangeland Hydrology, 2nd Ed. Dubuque, Iowa: Kendall-Hunt.
Brookes, A. 1985. River channelization: traditional engineering methods, physical consequences and alternative practices. Prog. in Phys. Geog. 9: 44-73.
Burke, R. 1972. Stormwater runoff. Pp. 727-733 in R. T. Oglesby, C. A. Carlson, and J. A. McCann (eds.) River Ecology and Man. New York: Academic Press.
Calder, I. 1993. Hydrologic effects of land use change. Chap. 13 In Maidment, D. (ed.) Handbook of Hydrology. New York: McGraw-Hill.
Chapra, S. 1996. Rivers and streams. Chap. 10 In Mays, L. (ed.)Water Resources Handbook . New York: McGraw-Hill.
Cooke, R. U., and R. Reeves. 1976. Arroyos and Environmental Change in the American Southwest. Oxford: Oxford Univ. Press.
Council on Environmental Quality. 1981. Environmental Trends. Washington, D.C.: U.S. Government Printing Office.
Davies-Colley, R. J. 1997. Stream channels axe narrower in pasture than in forest. New Zealand Journal of Marine and Freshwater Research 31:599-608.
Dendy, F. E., and W. A. Champion. 1978. Sediment deposition in U.S. reservoirs: Summary of data reported through 1975. U.S. Dept. Agr. Misc. Pub. 1362.
Dunne, T., and L. B. Leopold. 1978. Water in Environmental Planning. San Francisco, Calif.: Freeman.
Ellis, J. B. 1975. Urban Stormwater Pollution. Middlesex Polytechnic Research Report 1.
Flegal, A. R., and C. C. Patterson. 1983. Vertical concentration profiles of lead in the central Pacific at 15N and 20S. Earth Planet. Sci. Lett. 64:19-32.
Frankenfield, H. C. 1927. The floods of 1927 in the Mississippi Basin. Monthly Weather Review Supp. 29:10.
Freeze, R. A., and J. A. Cherry. 1979. Groundwater. Englewood Cliffs: Prentice-Hall.
Fuguitt, G. V. 1985. The Nonmetropolitan population turnaround. Annual Review of Sociology 11:259-280.
Fuguitt, G. V., D. L. Brown, and C. L. Beale. 1989. Rural and Small Town America. New York: Russell Sage Foundation.
Gallant, A. L., T. R. Whittier, D. P. Larsen, J. M. Omernik, and R. M. Hughes. 1989. Regionalization as a Tool for Managing Environmental Resources. EPA Research and Development Report EPA/600/3-89/060. Corvallis, Ore.: U.S. EPA Environmental Research Laboratory.
GAO. 1991. Water Pollution: More emphasis needed on prevention in EPA's efforts to protect groundwater. Washington, D.C.: U.S. General Accounting Office.
GAO. 1994. Ecosystem Management: Additional actions needed to adequately test a promising approach. GAO/RCED-94-111. Washington, D.C.: U.S. General Accounting Office.
Gibert, J., D. L. Danielopol, and J. A. Stanford (eds.). 1994a. Groundwater Ecology. San Diego, Calif.: Academic Press, Inc.
Gibert, J., J. A. Stanford, M. J. Dole-Oliver, and J. V. Ward. 1994b. Basic attributes of groundwater ecosystems and prospects for research. Pp. 7-40 in Groundwater Ecology. San Diego, Calif.: Academic Press, Inc.
Gilbert, G. K. 1917. Hydraulic Mining Debris in the Sierra Nevada. U.S. Geological Survey Professional Paper 105.
Gleick, Ph.H. (ed.). 1993. Water in Crisis: A Guide to the World's Fresh Water Resources. Oxford: Oxford University Press.
Goudie, A. 1994. The Human Impact on the Natural Environment. Cambridge: MIT Press.
Graf, W. L. 1975. The impact of suburbanization on fluvial geomorphology. Water Resources Research 11:690-692.
Graf, W. L. 1977. Network characteristics in suburbanizing streams. Water Resources Research 13: 459-63.
Graf, W. L. 1978. Fluvial adjustments to the spread of Tamarisk in the Colorado Plateau Region. Geol. Soc. Am. Bull. 89: 1491-1501.
Graf, W. L. 1979. Mining and channel response. Ann. Assoc. Am. Geogr. 69: 262-275.
Graf, W.L. 1980. The effect of dam closure on downstream rapids. Water Resources Research 16: 129-136.
Graf, W. L. 1985. The Colorado River: Instability and Basin Management. Washington, D.C.: Association of American Geographers.
Graf, W. L. 1994. Seasonal Land Cover Regions (map, scale 1:11,000,000). Sioux Falls, SD: USGS EROS Data Center.
Graf, W. L., K. K. Hirschbock, R. A. Marston, J. Pitlick, and J. C. Schmidt. 1997. Sustainability and changing physical landscapes. Pp. 1-13 In McKindley, W.L. (ed.) Aquatic Ecosystem Symposium in Western Water Policy. Tempe, Ariz.: Arizona State University.
Graham, M., J. Thomas, and F. Metting. 1996. Groundwater. Chap. 11 In Mays, L. (ed.) Water Resources Handbook. New York: McGraw-Hill.
Gregory, K. J. 1985. The impact of river channelization. Geogr. 151: 53-74.
Gregory, S. V., F. J. Swanson, and W. A. McKee. 1991. An ecosystem perspective of riparian zones. BioScience 40:540-551.
Happ, S. C., G. Rittenhouse, and G. Dobson. 1940. Some Principles of Accelerated Stream and Valley Sedimentation. U.S. Dep. of Ag. Tech. Bull. 695.
Harmon, M. E., J. F. Franklin, F. J. Swanson, P. Sollins, S. V. Gregory, J. D. Lattin, N. H. Anderson, S. P. Cline, N. G. Aumen, J. R. Sedell, G. W. Lienkaemper, K. Cromack Jr., and K. W. Cummins. 1986. Ecology of coarse woody debris in temperate ecosystems. Advances in Ecological Research 15:133-302.
Heaney, J. P. 1986. Research needs in urban-storm water pollution. Journal of Water Resources Planning and Management 112:36-47.
Heiskary, S. A., C. B. Wilson, and D. P. Larsen. 1987. Analysis of regional patterns in lake water quality: Using ecoregions for lake management in Minnesota. Lake and Reservoir Management 3:337-344.
Hershfield, D. M. 1961. Rainfall frequency atlas of the United States. U.S. Weather Bureau Technical Paper.
Hicks, B. J., J. D. Hall, P. A. Bisson, and J. R. Sedell. 1991. Response of salmonids to habitat changes. American Fisheries Society Special Publication 19:483-518.
Hughes, R. M., and D. P. Larsen. 1988. Ecoregions: an approach to surface water protection. J. Water Pollut. Control Fed. 60:486-493.
Hughes, R. M., E. Rextad, and C. E. Bond. 1987. The relationships of aquatic ecoregions, river basins, and physiographic provinces to the ichthyogeographic regions of Oregon. Copeia 2:423-432.
Hundley, N. 1992. The Great Thirst. Berkeley, Calif.: University of California Press.
Interagency Ecosystem Management Task Force. 1955. The Ecosystem Approach: Healthy ecosystems and sustainable economies, Volume 1-Overview. Washington, D.C.: Council on Environmental Quality.
Interagency Floodplain Management Review Committee. 1994. Sharing the Challenge: Floodplain Management into the 21st Century. Report to the Administration Floodplain Management Task Force. Washington, D.C.: U.S. Government Printing Office.
Jacques, J. E., and D. L. Lorenz. 1988. Techniques for estimating the magnitude and frequency of floods of ungaged streams in Minnesota. U.S. Geological Survey Water Resources Inv. Rep 87-4710.
Jaffe, M., and F. Dinovo. 1987. Local Groundwater Protection. Chicago, Ill: American Planning Association.
Johnson, K. M., and C. L Beale. 1993. The recent revival of widespread population growth in nonmetropolitan areas of the United States. Rural Sociology 59:655-667.
Johnston, C. A. 1991. Sediment and nutrient retention by fresh-water wetlands: Effects on surface and quantity. Critical Reviews in Environmental Control 21:491-565.
Johnston, C. A., G. D. Bubenzer, G. B, Lee, F. W. Madison, and J. R. McHenry. 1984. Nutrient trapping by sediment deposition in seasonally flooded lakeside wetlands, Journal of Environmental Quality 13:283-290.
Johnston, L. A., N. E. Detenbeck and G. J Niemi, 1990. The cumulative effect of wetlands on stream quality and quantity: a landscape approach. Biochemistry 10:105-147.
Johnston, C. A., 1991. Sediment and nutrient retention by fresh-water wetlands: Effects on surface and quantity . Critical Reviews in Environmental Control 21:491-565.
Keller, E. A. 1976. Channelization: Environmental, geomorphic and engineering aspects. In Coates, D. R. (ed.) Geomorphology and Engineering. Stroudsburg, Penn.: Dowden, Hutchinson and Ross.
Keller, E.A., and F.J. Swanson. 1979. Effects of large organic material on channel form and fluvial processes. Earth Surface Processes 4: 361-380.
Kohler, M. A., T. J. Nordenson, and D. R. Baker. 1959. Evaporation maps for the United States. U.S. Weather Bureau Technical Paper 37.
Küchler, A. W. 1970. Potential Natural Vegetation (map, scale 1:7,500,000). Pp. 89-91. In the National Atlas of the United States of America. Washington, D.C.: U.S. Geological Survey.
LeGrand, H. E., and V. T. Stringfield. 1973. Concepts of karst development in relation to interpretation of surface runoff. J. of Research of the U.S. Geological Survey 1(3):351-360.
Larsen, D. P., R. M. Hughes, J. M. Omernik, D. R. Dudley, C. M. Rohm, T. R. Whittier, A. J. Kenney, and A. Gallant. 1986. The correspondence between spatial patterns in fish assemblages in Ohio streams and aquatic ecoregions . Environmental Management 10:815-828.
Larsen, D. P., D. R. Dudley, and R. M. Hughes. 1988. A regional approach to assess attainable water quality: an Ohio case study. J. Soil Water Conservation 43:171-176.
LeGrand and Stringfield. 1973. Concepts of Karst development in relation to interpretation of surface runoff. J. of Research of the U.S. Geological Survey 1(3):351-360.
Leopold, L. B., M. G. Wolman, and J. G. Miller. 1964. Fluvial Processes in Geomorphology. San Francisco: W.H. Freeman and Co.
Ligon, F. K., W. E. Dicterom, and W. J. Thrus. 1995. Downstream ecological effects at dams: a geomorphic perspective. BioScience 45:183-192.
Loganathan, D., D. Kibler, and T. Grizzard, 1996. Urban stormwater management. Chap. 26 in L. Mays (ed.) Water Resources Handbook. New York: McGraw-Hill.
Madej, M. A., W. E. Weaver, D. K. Hogans. 1994. Analysis of bank erosion on the Merced River, Yosemite Valley, Yosemite National Park, U.S.A. Environmental Management 18:235-250.
Maidment, D. (ed.) 1993. Handbook of Hydrology. McGraw-Hill, New York.
Makepeace, D. K., D. W. Smith, S. J. Stanley. 1995. Urban stormwater quality: summary of contaminant data. Critical Reviews in Environmental Science and Technology 25:93-139.
Malina, J. 1996. Water quality. Chap. 8 in L. Mays (ed.) Water Resources Handbook. New York: McGraw-Hill.
Mather, J. R. 1978. The Climatic Water Budget in Environmental Analysis. Lexington, Mass: Lexington Books.
Mays, L. M. ed. 1996. Water Resources Handbook. New York: McGraw-Hill.
McCutcheon, S., J. Martin, and T. Barnwell. 1993. Water quality. Chap. 11 In Maidment, D. (ed.) Handbook of Hydrology. New York: McGraw-Hill.
Meade, R. H. 1982. Sources, sinks, and storage of river sediment in the Atlantic drainage of the United States. J. Geol. 90:235-252.
Megahan, W.F. 1982. Channel sediment storage behind obstructions in forested drainage basins draining the granitic bedrock of the Idaho Batholith. Pp. 114-121 in F.J. Swanson, R.J. Janda, T. Dunne, and D.N. Swanston, editors. Sediment budgets and routing in forested drainage basins. United States Forest Service, Research Paper PNW-141, Pacific Northwest Forest and Range Experiment Station, Portland, Oregon, USA.
Miller, J. F. 1964. Two-to-ten-day precipitation for return periods of 2 to 100 years in the contiguous United States. U.S. Weather Bureau Technical Paper 49.
Montgomery, D. 1997. What's best on banks? Nature 338:328-329.
Naiman, R. J., and J. R. Sedell. 1980. Relationships between metabolic parameters and stream order in Oregon. Canadian Journal of Fisheries and Aquatic Science 37:834-847.
National Oceanic and Atmospheric Administration. 1998. CIRES Climate Diagnostics Center. http://www.cdc.noaa.gov/ENSO/enso.current.html).
National Research Council (NRC). 1995. Wetlands: Characterization and boundaries. Washington D.C.: National Academy Press.
National Research Council (NRC). 1997. Watershed Research in the U.S. Geological Survey. Washington, D.C.: National Academy Press.
Notenboom, J., S. Plenet, and M.J. Turquin. 1994. Groundwater contamination and its impact on groundwater animals and ecosystems. Pp. 477-504 in J. Gibert, D. L. Danielopol, and J. A. Stanford (ed.) Groundwater Ecology. San Diego, Calif.: Academic Press.
Novitzki. R. P. 1979. Hydrologic characteristics of Wisconsin: Wetlands and their influence on floods, stream flow, and sediment. Pp. 377-388 in R. E. Greeson, J. R. Clark, and J. E. Clark (eds.) Wetland Functions and Values: The state of our understanding. Minneapolis, Minn.: American Water Resources Association.
O'Connor, K. A. 1995. Watershed Management Planning: Bringing the Pieces Together. M.S. Thesis, California State Polytechnic University, Pomona. 166 pp.
Ogawa, H., and Male, J. W. 1986. Simulating the flood mitigation role of wetlands. Journal of Water Resources, Planning, and Management 112:114-128.
Omernik, J. M. 1987. Ecoregions of the coterminous United States. Ann. Assoc. Am. Geogr. 771:118-125.
Omernik, J. M., and A. L. Gallant. 1990. Defining regions for evaluating environmental resources. Pp. 936-947 in Global Natural Resource Monitoring and Assessments: Preparing for the 21st Century, Vol. 2. Bethesda, Md.: American Society for Photogrammetry and Remote Sensing.
Pimentel, D., J. Houser, E. Preiss, O. White, H. Fang, L. Mesnick, T. Barsky, S. Tariche, J. Schreck. and S. Alpert. 1997. Water resources: agriculture, the environment, and society. BioScience 47:97-106.
Pitlick, J., and M. Van Streeter. 1994. Changes in morphology and endangered fish habitat, the Colorado River. Colorado Water Resources Institute Compliance Report 144. Fort Collins, Colo.: Colorado Water Resources Institute.
Potter, K. W. 1991. Hydrological impacts of changing land management practices in a moderate sized agricultural catchment. Water Resources Research 27:845-855.
Reice, S. R. 1994. Nonequilibrium determinants of biological community structure. American Scientist 82(5):424-435.
Renard, K. G., G. R. Foster, and G. A. Weesies. 1991. Predicting soil erosion by water: A guide to conservation planning with the Revised Universal Soil Loss Equation. U.S. Department of Agriculture, Agricultural Handbook 703.
Richardson, C. 1996. Wetlands. Chap. 13 in Mays, L. (ed.) Water Resources Handbook. New York: McGraw-Hill.
Richter, B. D., J. V. Baumgartner, J. Powell, and D. Braun. 1996. A method for assessing hydrologic alteration within ecosystems. Conservation Biology 10:1163-1174.
Rohm, C. M., J. W. Giese, and C. C. Bennett. 1987. Evaluation of an aquatic ecoregion classification of streams in Arkansas. J. Freshwater Ecology 4:127-140.
Schumm, S. 1977. The Fluvial System. New York: John Wiley.
Schumm, S., M. D. Harvey, and C. C. Watron. 1984. Incised Channels: Morphology, Dynamics, and Control. Littleton, Colo.: Water Resources Publications.
Sedell, J.R., and K.J. Luchessa. 1982. Using the historical record as an aid to salmonid habitat enhancement. Pp. 210-223 in N.B. Annantrout (ed.) Acquisition and utilization of aquatic habitat inventory information. Proceedings of a symposium held October 28-30, 1981, Portland, Oregon. The Hague Publishing, Billings, Montana, USA.
Sedell, J.R., P.A. Bisson, F.J. Swanson, and S.V. Gregory. 1988. What we know about large trees that fall into streams and rivers. Pp. 47-81 in C. Maser, R. F. Tarrant, J. M. Trappe, and J. F. Franklin (eds.) From the forest to the sea: a story of fallen trees. United States Forest Service, Pacific Northwest Research Station, General Technical Report PNW-GTR-229, Portland, Oregon, USA.
Sedell, J.R., and R.L. Beschta. 1991. Bringing back the "bio" in bioengineering. American Fisheries Society Symposium 10:160-175.
Shields, F. D., S. S. Knight and C. M. Cooper. 1995. Rehabilitation of watersheds with incising channels. Water Resources Bulletin 31:971-982.
Stanford, J. A. 1997. Toward a robust water policy for the western USA: Synthesis of the Science in Aquatic Ecosystem Symposium: A report to Western Water Policy Review Advisory Commission. Tempe: Arizona State University.
Stanford, J. A., and J. V. Ward. 1993. An ecosystem perspective of alluvial rivers: Connectivity and the hyporheic corridor . Journal of the North American Benthological Society 12:48-68.
Stanford, J. A., J. V. Ward, W. J. Liss, C. A. Frissel, R. N. Williams, J. A. Lichatowich, and C. C. Contant. 1996. A general protocol for restoration of regulated rivers. Regulated Rivers, Research and Management 12:391-413.
Todd, D. K. 1959. Groundwater Hydrology. New York: John Wiley.
Trimble, S. W. 1974. Man-Induced Soil Erosion on the Southern Piedmont. 1700-1970. Ankeny, Iowa: Soil Conservation Society of America.
Trimble, S. W. F. H. Weirich, and B. L. Hoag. 1987. Reforestation and the reduction of water yield on the Southern Piedmont since circa 1940. Water Resources Research 23: 425-37.
Trimble, S. W., and K. P. Bube. 1990. Improved reservoir trap efficiency prediction. The Environmental Professional 12: 255-272.
Trimble, S. W., and S. W. Lund. 1982. Soil Conservation and the Reduction of Erosion and Sedimentation in the Coon Creek Basin. Wisconsin. U.S. Geological Survey Professional Paper 1234.
Trimble, S. W. 1993. The distributed sediment budget model and watershed management in the Paleozoic Plateau of the upper Midwestern United States. Physical Geography 14:285-303.
Trimble, S. W. 1997a. Contribution of stream channel erosion to sediment yield from an urbanizing watershed. Science 278:1442-1444.
Trimble, S. W. 1997b. Stream channel erosion and change resulting from riparian forests. Geology 25:467-469.
Triska, F. J., and K. Cromack. 1982. The role of wood debris in forests and streams. Pp. 171-190 in R. H. Waring (ed.) Forests: fresh perspectives from ecosystem analysis. Proceedings of the 40th Biology Colloquium, 1979, Oregon State University, Corvallis, USA.
Turner, B. L., W. C. Clark, R. W. Kates, J. F. Richards, J. T. Matthews, and B. Mayer. 1990. The Earth as Transformed by Human Actions. Cambridge, Mass.: Cambridge University Press.
U.S. Environmental Protection Agency (EPA). 1996. Level III ecoregions of the continental United States (revision of Omernik, 1987) map M-1 (various scales). Corvallis, Ore.: US EPA, National Health and Environmental Health Effects Research Laboratory .
U.S. Environmental Protection Agency (EPA). 1997. Environmental Monitoring and Assessment, U.S. Environmental Protection Agency, Program (EMAP) Draft Research Plan. Washington, D.C.: US EPA, Office of Research and Development.
U.S. Geological Survey. 1970. The National Arias of the United States of America.
Vervier, P. 1992. A perspective on the permeability of the surface freshwater-groundwater ecotone. Journal of the North American Benthological Society 11(1):93-102.
Wang, E. X., F. H. Bormann, and G. Benoit. 1995. Evidence of complete scavenging of atmospheric lead in the soils of northern hardwood forest ecosystems. Environ. Sci. Technol. 29:735-739.
Wang, S. Y., E. Langendoen, and F. D. Shields, eds. 1997. Management of Landscapes Disturbed by Channel Incision; Stabilization, Rehabilitation, and Restoration. University of Mississippi: Center for Computational Hydroscience and Engineering.
Ward, J. V. 1989. The four-dimensional nature of lotic ecosystems. Journal of the North American Benthological Society 8:2-8.
Water Information Center. 1973. Water Arias of the United States. Port Washington, New York: Water Information Center, Inc.
Webb, J. R., B. J Cosby, F. A. Deviney, J. N. Galloway, M. E. Mitch, D. M. Downey, and K. N. Eshleman. 1997. Release of NO3 to Surface Waters Following Forest Defoliation by the Gypsy Moth. Preprint from Dept. of Environmental Sciences, University of Virginia. Charlottesville, Va.: University of Virginia.
Whitney, G. G. 1994. From Coastal Wilderness to Fruited Plain: A History of Environmental Changes in Temperate America from 1500 to the Present. Cambridge, Mass.: Cambridge University Press.
Whittier, T. M., R. M. Hughes, and D. P. Larsen. 1988. The correspondence between ecoregions and spatial patterns in stream ecosystems in Oregon. Canadian Journal of Fisheries and Aquatic Science 45:1264-1278.
Williams, G. P., and M. G. Wolman. 1984. Downstream effects of dams on alluvial rivers. U.S. Geol. Surv. Prof. Paper 1286.
Windom, H. L., J. T. Byrd, J. R. G. Smith, and F. Huan. 1991. Inadequacy of NASQAN data for assessing metal trends in the nation's rivers. Environ. Sci. Technol. 25:1137-1142.
Wolman, M. G. 1967. A cycle of sedimentation and erosion in urban river channels. Geografiska Ann. 49A: 385-395.