Page 82
4—
Dosimetry
The risk associated with exposure to hormonally active agents (HAAs) will depend on many factors, such as developmental stage, sex, disease conditions, life-cycle stage, as well as on the potency of the compounds and on the amount of exposure or the dose. A dose can be construed in one or more ways: as the concentration of a toxicant to which organisms are exposed (the external dose); as the concentration in organs, tissues, or cells (the internal dose); or as the amount reaching and binding with a receptor or other target molecule (the biologically significant dose). The extent to which binding occurs will depend on properties of the chemical, properties of the target molecules, the duration of exposure and on the distribution of the chemical in the body. The physiology of the organism and the route to cellular or molecular targets can favor or impede binding or its consequences.
Knowing the shape of the dose-response curve for environmental contaminants is critical for understanding how such contaminants--including HAAs-act on organs and organisms. Understanding the dose-response relationship is also critical for the design of studies to test the effects of contaminants.
If an underlying monotonic dose-response function (i.e., a function where response increases as dose increases or at least does not decrease) and a dose below which there is no effect (a threshold dose) are assumed when designing a toxicologic study, there is a risk of failing to understand or properly test a contaminant that does not display a monotonic dose-response function or a threshold dose.
It is well known that some compounds produce nonlinear and even nonmonotonic dose-response functions in some organisms over certain ranges of dose. Furthermore, some compounds can produce different dose-response functions depending on the target organ and the species exposed. For those reasons, this chapter considers dose-response relationships and the processes that cancontinue
Page 83
determine whether HAAs in the environment accumulate in target organs and cells to concentrations that are biologically significant.
The toxicokinetic processes that determine the accumulation and potency of HAAs are much the same as they are for other xenobiotics, and they are described generally in textbooks (e.g., Klaassen et al. 1996). The accumulation of HAAs is determined by the rate and efficiency of several processes: absorption, or partitioning, of a bioavailable chemical between the external medium and the cell layers first exposed; transport in blood; partitioning between blood and body lipids or target cells; metabolism of the chemical; and excretion and elimination from the body. In this chapter, these processes are summarized, and examples illustrate the complexities as well as the extent of our knowledge in these areas. The processes considered include the effects of HAAs on enzymes that metabolize HAAs or endogenous hormones. In addition to influencing the elimination or persistence of HAAs, metabolism can affect their structure and biologic activity, thereby participating directly in a mechanism of action. Detailed knowledge of these processes is necessary if we are to relate external to internal doses and then to specific outcomes and mechanisms of action. Knowledge of the differences and similarities in uptake, distribution, metabolism, and elimination will determine the degree to which we can extrapolate across species for different chemicals and doses, and it will guide monitoring of humans and wildlife for relevant exposure to HAAs. Such knowledge is essential for assessing risk in species or populations exposed to HAAs in the environment, particularly where exposure concentrations are smaller than those in localized ''hot spots," where exposure concentrations are high and effects are clearly seen and reported.
Uptake, Elimination, and Accumulation
Uptake and elimination of xenobiotics by animals have been studied extensively, both under controlled conditions in the laboratory and under natural conditions in the environment. Absorption through epithelial-cell layers is the requisite first event in the uptake of xenobiotics. Electronic, steric, and hydrophobic or hydrophilic properties of the chemical will govern this process. For organic chemicals, including most HAAs, hydrophobicity is particularly important. For the more-hydrophobic (lipophilic) chemicals, uptake appears to be a passive process, involving diffusion of the chemical through the plasma membrane to the interior of the cell (DiFrancesco and Bickel 1985). Among vertebrates, there is probably little difference in the efficiency with which chemicals cross the membrane into epithelial cells (Lin 1995). Rather, the lipid content of the tissues is important. The role of lipids in accumulation of xenobiotics in many types of animals has been recognized for decades (Stegeman and Teal 1973; Cullen and McConnell 1992). Partitioning of chemicals between aqueous and nonaqueous media often is used to characterize the propensity of chemicals to accumulate in tissue. Coefficients, such as the octanol-water partition coefficient (Kow). corre-soft
Page 84
late with and predict the degree of accumulation of HAAs and other xenobiotics in organisms (Connell and Hawker 1988; Ikemoto et al. 1992). Species that possess slight capacity to metabolize the xenobiotics, such as molluscs, show equilibrium between the content of organic chemicals, including HAAs, in water and in the organism (Porte and Albaiges 1994), supporting the conclusion that uptake is largely a passive rather than an active transport process (Connell and Hawker 1988). Less hydrophobic chemicals could require facilitated transport. although the mechanisms have not been defined.
Rates of uptake and elimination, hence half-lives, differ greatly for different compounds and in different species. Examples of half-lives of selected HAAs in different organisms are given in Table 4- 1. The half-life of a chemical is influenced by the dose and the duration of exposure. The data presented in Table 4-1 are the results of experimental and environmental exposures. In the case of ambient exposure, the external dose and the duration of exposure often are unknown. Table 4-1 nevertheless indicates the large range in estimated half-lives of different HAAs. The half-lives of individual polychlorinated biphenyl (PCB) congeners in trout and in humans vary a great deal (Niimi and Oliver 1983; Ryan et al. 1993). Research conducted with fewer congeners shows that rates of elimination can vary greatly, even for closely related chemical structures. Coristine et al. (1993) reported that PCB congener 77 (3,3',4.4'-tetrachlorobiphenyl) was eliminated rapidly and congener 126 (3,3',4,4'.5-pentachlorobiphenyl) was eliminated very slowly from trout. Compounds with long half-lives could be available to act during critical windows of development or hormonal action. Compounds with short half-lives might not be present at those critical windows, depending on the timing and duration of exposure. In many environments, whether point or disseminated sources of contaminants are involved, exposure to HAAs that are slowly degraded is likely to be continuous.
For many compounds, the half-lives and the clearance from blood approximate the rate of metabolism. With chlorinated compounds, such as PCBs, polychlorinated dibenzodioxins (PCDDs), or polychlorinated dibenzodifurans (PCDFs). metabolism and elimination rates depend on the degree and the sites of chlorination (Matthews and Anderson 1975). The rates at which these compounds are metabolized decreases as the degree of chlorination increases (Chen et al. 1982). Compounds that are structural homologues can have markedly different rates of metabolism. For example, the metabolism rates of PCB congeners are strongly influenced by the presence of vicinal, unsubstituted carbons at the meta or para position (Chen et al. 1982; Matthews and Dedrick 1984). Analysis of residues in blood could suggest species patterns of metabolism (Boon et al. 1994).
Routes of Exposure
The form of the chemical will determine bioavailability (Farrington 1991 ). and the lifestyle of the free-living organism (i.e., after hatching or parturition)continue
Page 85
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Page 86
will determine which avenue of ingress—dermal, respiratory, or gastrointestinal—is most important. Hydrophobic compounds, such as dichlorodiphenyltrichloroethane (DDT). and some PCB congeners can pass through the skin of mammals, including humans and can be taken up from the water by fish (Bruggeman et al. 1981; Reifenrath et al. 1991 ). Xenoestrogens, such as nonylphenol, also can be taken up directly from water by fish (Lewis and Lech 1996). However, dietary exposure and gastrointestinal absorption are the most important routes of exposure for vertebrates (James and Kleinow 1994). Exposure of omnivores or herbivores to natural substances with estrogenic activity produced by plants (phytoestrogens; e.g., genistein) or fungi (e.g., zearalenone) is through the diet (Kuiper-Goodman et al. 1987). Ingestion of milk is the primary route of exposure for neonatal and infant humans and other mammals, because milk is the major if not the sole source of contaminants. Bovine milk also is a source of neonatal human exposure to phytochemicals that are HAAs (Bannwart et al. 1988).
Various factors affect the availability of HAAs in the diet. Thus, the uptake of chemicals in the gut can be influenced by the composition of the diet, the pH in the gut, the rate of digestion, the residence time of food, and the microflora composition in the gut (James and Kleinow 1994). In the case of some phytoestrogens, gut microflora can influence the bioavailability of the chemical for uptake (Xu et al. 1995). Gut flora also have been implicated in formation of methylsulfonyl derivatives of some PCB congeners (Brandt et al. 1982). These variables can differ substantially among species, and they need to be incorporated into pharmacokinetic models. There are, however, few data concerning how these variables apply to specific HAAs.
In mammals, maternal circulation is the source of chemicals to the developing organism. Some nonmammalian species, including live-bearing fish, have a modified placentation, and maternal exposure is also a source of contaminants that can be transported into developing embryos or fetuses (Lombardi and Wourms 1985; Hamlett et al. 1993). However, oviparous species are exposed primarily through maternal deposition of chemicals into the yolk. In either case, the transport of chemicals in the blood, discussed below, is involved in delivery of the compounds to the embryo or fetus and to the ovary and eggs. The major routes of exposure for embryos, larvae, and fetuses differ in different taxa. It also must be emphasized that models based on adult dosing might be inadequate for estimating the distribution to or the effects on target sites in embryos.
The capacity of cells at sites of entry to metabolize HAAs (first-pass metabolism) can determine the form and the amount of the chemical delivered to other parts of the body. If a compound is extensively metabolized by epithelial cells in the gut, for example, then exposure to low doses might not yield an active dose at target sites in the body. This fact could strongly influence interpretations of dose-response relationships, particularly at low doses. Such an effect of metabolism at the site of absorption was clearly demonstrated in studies by Van Veld et al.continue
Page 87
(1988) with fish fed benzo[a]pyrene (B[a]P). At low (environmentally realistic) doses of B[a]P in the diet, there was a strong induction of cytochrome P450 1A (CYP1A), which metabolizes B[a]P, in the gut. In those same animals, only the metabolites of B[a]P were passed to the liver (Van Veld et al. 1988). and there was no induction of CYP1A in the liver. At higher doses in the diet, the capacity for metabolism in the gut apparently was exceeded, and only then did induction of CYP1A begin to appear in the liver. Although B[a]P is not generally considered an HAA, the results of the study above demonstrate that first-pass metabolism can affect the systemic distribution of dietary chemicals. Metabolism in the gut wall can be important in the metabolism of steroids, such as ethinylestradiol and fungus-derived estrogens (Olsen et al. 1987; Back et al. 1990). Regulation of drug-metabolizing enzymes in mammalian small intestines show multiple forms of CYP (Kaminsky and Fasco 1991), but the significance to pharmacokinetics "has been studied to only a limited extent" (Kaminsky and Fasco 1991). The ability of dermis, lung, and gill as well as gut to metabolize xenobiotics has long been known (Wiebel et al. 1975; Steinstrasser and Merkle 1995), but the degree to which these epithelial layers in different species differ in their capacity to metabolize or transform HAAs specifically still is not well known.
Milk is a primary source of exposure to contaminants in nursing the young—human and animal. HAAs secreted in milk retain biologic activity. In mice, methoxychlor administered to dams was transferred via the milk to the suckling young, where it stimulated the vagina and uterine horns, indicating that the chemical excreted in milk remained biologically active in the suckling mice (Appel and Eroschenko 1992). Neonatal exposure of rats to o,p'-DDT or to the phytoestrogens genistein or coumestrol altered later pituitary responsiveness to gonadotropin-releasing hormone (GnRH) (Faber and Hughes 1991; Whitten et al. 1995). indicating that lactational exposure of neonates can affect reproductive function later in life. Similarly, lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) affects endocrine function in rats later in life (Chaffin et al. 1996).
Concentrations of HAAs and other xenobiotics have been measured in milk from humans around the globe (Skaare 1981: Wickizer et al. 1981; Slorach and Vaz 1985; Skaare and Polder 1990; Dogheim et al. 1991; Dewailly et al. 1993a, 1994; Spicer and Kereu 1993; Larsen et al. 1994; Quinsey et al. 1995; Chikuni et al. 1997). Some of the data are presented in Table 4-2.
Mothers' milk is not the only source of exposure to HAAs in human infants. Sewart and Jones (1996) calculated that PCBs in cows' milk could contribute 11% of the total daily intake of PCBs in the United Kingdom. Bovine milk is a source of HAA exposure in infants, children, and adults. Most of the data on residues in milk are for chlorinated hydrocarbon HAAs. Cows' milk can deliver phytoestrogens to infants. However, soy-based milk substitutes can deliver much greater amounts of phytoestrogens than are found in either bovine or human milk. Plasma concentrations of genistein measured in infants fed soy-based formulas averaged 684 ng/mL, a much greater amount than those found in infants fedcontinue
Page 88
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(table continued on next page)break
Page 89
(table continued from previous page)break
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Page 90
cows' milk formulas (3.2 ng/mL) or human breast milk (2.8 ng/mL) (Setchell et al. 1997). The dose of genistein in infants fed soy milk was greater than doses eliciting hormonal effects in adults (Setchell et al. 1997).
The data from most studies on the subject show that 80% or more of DDT measured in human milk is in the form of the p,p'-DDE (1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene) metabolite (Franchi and Focardi 1991: Johansen et al. 1994). Similarly, in most studies, total PCBs in milk represent input from a few congeners, primarily 153, 138, and 180 (Grandjean et al. 1995; Ramos et al. 1997a). In a study in Norway (Johansen et al. 1994), the concentrations of 23 PCB congeners and of DDT and other pesticides were examined in human milk. The researchers determined that congener 153 correlated very closely with the total of the 23 congeners, suggesting that this congener could be an indicator for total PCBs. In cows' milk also, the concentrations of total PCBs represent major input from congeners 153, 138, 118, and 180 (Sewart and Jones 1996). Although the major PCB congeners in milk are ortho-substituted, unsubstituted congeners also occur. Of these, coplanar PCB 77 was found at high concentrations in a sample from the Faroe Islands of Denmark (Grandjean et al. 1995). Johansen et al. (1994) determined that congener 126 was the major contributor to the TCDD toxic equivalents in human milk. Similarly, Ramos et al. (1997b) found that of the coplanar congeners measured, 77 and 126 were most abundant in cows' milk.
Variables that can influence the persistence of HAA residues in milk include maternal age and weight, the number of births, the duration of lactation, and the fat content of milk (Rogan et al. 1986a; Skaare and Polder 1990; Sim and McNeil 1992). In a study in Germany, Beck et al. (1994) found that TCDD concentrations in the mother declined with the number of children born and the duration of breast feeding, reflecting decreases in the content of contaminants in the maternal body. Such decreases also occur in wildlife. In some species of dolphin, it has been estimated that 60-80% of the body burden of organic xenobiotics is eliminated in maternal milk (Cockcroft et al. 1989), with serious implications for the accumulation of these compounds in calves. The shorter lactation period in fin whales is thought to result in less total transfer to the calves than in other cetaceans that have longer lactation periods (Aguilar and Borrell 1994). The milk of different species can differ greatly in lipid content. In seals and whales, milk fat content varies between 10% and 55%, depending on the species and the duration of lactation (King 1983; Trillmich and Lechner 1986; Ridgeway et al. 1995). Those percentages are much higher than those in human milk, in which the fat content typically ranges from 3.5% to 6.6% (Daly et al. 1993). Results showing close correlation between PCB content and milk fat (Schweigert and Stobo 1994 indicate that differences in fat content can be expected to influence the amounts of HAAs in milk.
Analyses of milk have shown important temporal and geographic trends in HAA content and thus in what can be delivered to nursing infants. One Swedish study demonstrated a significant decline in the content of methylsulfonyl me-soft
Page 91
tabolites of chlorobiphenyls and p,p'-DDE between 1972 and 1992 (Noren et al. 1996). A study by Johansen et al. (1994) noted a 62-79% decline in total PCBs and total DDTs between 1982 and 1991. In general, from the mid-1960s to the 1990s, there has been a consistent and rather sharp decline in concentrations of p,p'-DDE in human breast milk in Scandinavia (Ekbom et al. 1996). In a study of worldwide trends in DDT and metabolite residue concentrations in human breast milk between the 1950s and 1990s, Smith (1999) reported that population means have declined in much of the world. Declines from 5,000 to 10,000 µg DDE/kg milk fat to approximately 1,000 µg DDE/kg milk fat have been observed in many countries. Although the means differ among the various regions, the declines in the various countries correspond to their restrictions on DDE use. In regions where DDT use continues, the amounts in milk are high (Bouwman et al. 1992)—often exceeding PCB content (Chikuni et al. 1997). Factors that affect the concentration of HAAs in human and animal milk and the amounts delivered to children who are nursing or who continue to drink cows' milk demand continued close attention (Ramos et al. 1997b).
Because human milk is a biologic indicator of the body burden (the amount measured per unit body weight) of organochlorines (Furst et al. 1994), analysis of milk could indicate HAA residue concentrations in adult females. It is important to establish how HAA concentrations in milk relate to the concentrations in other parts of the body. Kanja et al. (1992) examined 41 samples of maternal blood, milk, subcutaneous fat, and umbilical-cord blood collected from mothers giving birth by Cesarean section at Kenyatta National Hospital in Nairobi, Kenya, in 1986. The mean concentrations of total DDTs (milligrams of DDT per kilogram of fat) were 5.9 in maternal subcutaneous fat, 4.8 in milk, 2.7 in maternal blood and 1.9 in umbilical-cord blood. There was a significant correlation between the concentration of total DDTs in subcutaneous fat and milk fat (r = 0.963), in subcutaneous fat and maternal serum fat (r = 0.843). and in maternal serum fat and maternal milk fat (r = 0.868). Thus, analysis of milk can reveal not only the exposure of infants but also the exposure possible in utero. However, there was no correlation between the concentration of total DDTs in adipose tissue and cord blood, in maternal blood and cord blood, and in maternal milk and cord blood.
Food-Chain Transfer and Bioaccumulation
The literature concerning food-chain transfer and accumulation of chemicals in some trophic levels is abundant. A few studies serve here as examples. Clarkson et al. (1995) reviewed the properties generally required for a contaminant to bioaccumulate in a food chain. These are a high Kow, a chemical and metabolic stability in water and in organisms in the food chain, and a toxicity that is low enough that the chain is not broken by loss of an intermediate species. PCBs, dioxins, and organochlorine pesticides, including DDTs, are among thecontinue
Page 92
organic chemicals that bioaccumulate in aquatic food chains. Top predators in short or long food chains (polar bears, cetaceans, and eagles and other raptors) accumulate higher concentrations of such compounds than do lower-trophic species (Muir et al. 1992).
Consumption of species from the top of food chains results in higher exposure to HAAs in humans. Faroe islanders who consume whale blubber and meat and Inuit who consume marine mammal tissues have unusually high concentrations of PCBs and DDTs in their bodies and breast milk (Dewailly et al. 1993a: Grandjean et al. 1995; Weihe et al. 1996). Such populations are being followed to determine whether elevated concentrations of HAAs in blood and milk are associated with any health effects.
Distribution
Blood
Blood is the central medium of distribution of chemicals to target organs and cells in the body. It is often possible to obtain blood in a minimally invasive way. making this a key tissue for monitoring. The significance of blood in monitoring chemical exposures is described in detail in a previous NRC (1991b) report. Knowledge of how the concentrations of HAAs in blood relate to the concentrations and form of the chemicals in target organs is critical for the process of inferring HAA effects. Ideally, one would like to be able to measure HAA concentrations in blood or other biopsy samples and infer the concentration in target organs. However, concentrations of HAAs generally are much greater in tissues than in blood. In one study in rats, the ratio of DDE in tissue to DDE in blood was 6:1 for liver and muscle, 35:1 for skin, and 400:1 for adipose tissue (Muhlebach et al. 1991). Similar distributions for hydrophobic compounds have been observed in several studies in rodents (Table 4-3); at equilibrium, the more-hydrophilic compounds have distribution ratios near unity (NRC 1991b). In a study of free-living herring gulls, the plasma-to-whole-body lipid partition coefficient for DDE was 0.0041 ± 0.0014 (Norstrom et al. 1986). Even before steady-state whole-body burden is reached, there generally is good correlation between blood and tissue concentrations and rates of elimination. Dragnev et al. (1994) described similar time- and dose-dependent changes in the residues of the same PCB congeners in liver, adipose tissue, and blood of rats. In all three organs at all doses, there was a rapid decrease in content of residues after exposure ended. Metabolites of HAAs also are found in blood. Bergman et al. ( 1994) reported that the PCB metabolites 4-hydroxy-2,3,5,3',4'-pentachlorobiphenyl and 4-hydroxy-2,3,5,6,2',4',5'-heptachlorobiphenyl occurred in the blood of seals and humans at concentrations that were almost the same as those of the most persistent PCB congeners. All identified compounds have a structure with the hydroxy group in a para or meta position, and they have chlorine atoms on vicinal carboncontinue
Page 93
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
atoms. The concentrations of hydroxylated PCBs in the blood were similar for seals and humans.
In some organs, the distribution of specific lipophilic compounds can be influenced by specific proteins. In mammals, the distribution of TCDD, and possibly related planar halogenated compounds, is not fully explained by lipid partitioning. This is the result of induction of a form of CYP (CYP1A2) that can bind TCDD (Voorman and Aust 1987). CYP1A2 sequesters TCDD, and CYP1A2 induction in the liver results in hepatic accumulation that is greater than would be expected on the basis of partitioning. This effect has been confirmed in models that lack CYP1A2 (Diliberto et al. 1997). In mice, a Clara-cell secretory protein binds 4-methylsulfonyl-2,2',4',5,5'-pentachlorobiphenyl, causing accumulation in lung and kidney (Stripp et al. 1996).
The validity of inferring tissue concentration from blood concentration is supported by physiologically based pharmacokinetic (PB-PK) modeling, which is modeling the distribution of a chemical within an organism based on knowledge of exposure concentration and route, duration of exposure, partitioning between organs, as well as organ volume, blood-flow rate, binding to receptors. and rate of chemical metabolism and disposition (McKim and Nichols 1994). Parham et al. (1997) reported that the observed partitioning between blood and adipose tissue was similar to the partitioning in PB-PK models and that there was a two- to three-fold difference in the partitioning between different PCB congeners, which was consistent with the model. PB-PK models are being applied tocontinue
Page 94
partitioning of PCB congeners to different organs (Parham et al. 1997; Matthews and Dedrick 1984). That such models support extrapolation among species is indicated by comparisons of the distribution of PCDDs and PCDFs among different organs in different species, including humans (Carrier et al. 1995). but such information is lacking for many HAAs.
Persistence and organ distribution could influence extrapolations of dosage from one species to another—for example, for TCDD—but these have not been adequately determined in enough species (Whysner and Williams 1996). Although knowledge of distribution to different tissues is important to interpretations regarding toxicity, comparing the effects of some compounds on the basis of the total body burden could reveal similarities not evident when concentrations in individual tissues are used to compare responses between species. Thus, DeVito et al. (1995), in comparing the relative sensitivities of human and animal tissues to TCDD, concluded that ''it may be more appropriate to compare body burden than tissue concentration." The physiologic rationale for body-burden being predictive across species is not clear.
Plasma-Binding Proteins
Lipophilic chemicals are not free in the serum but associate with proteins, lipoproteins, or circulating cells in the blood, as do endogenous hormones. The degree to which a chemical is free or bound appears to determine the amount available for uptake by a cell. The free-hormone hypothesis posits that the biologically active fraction of total circulating steroid is the fraction that can pass from capillaries into cells and, therefore, is available to bind to intracellular steroid receptors. Only that which reaches a target can exert its activity. Serum proteins that bind hormones include those that are specific (steroid-hormone-binding globulins, SHBGs) and those that are nonspecific (such as albumins). There is evidence that xenoestrogens—including phytoestrogens, other HAAs, and endogenous estrogens such as estradiol—could bind differently to components of serum, resulting in differences in passage into tissues (Raynaud et al. 1971; Skalsky and Guthrie 1978; Sheehan and Young 1979, Akpoviroro and Fotherby 1980; Sheehan and Branham 1987; Harmon et al. 1989: Borlakoglu et al. 1990; vom Saal 1995; Nagel et al. 1998). Thus, the variables that can influence the uptake of steroids into cells include the plasma concentration of albumin and specific glycoproteins, the plasma flow rate, and the rate constant for dissociation of the steroid-protein complex (Ekins et al. 1982: Mendel 1989; Mendel et al. 1989, 1990).
Properties of specific sex-steroid-binding proteins have been described for few nonmammalian species, and not much is known about their ability to bind HAAs. Sex-steroid-binding proteins have been characterized in fish (Thomas and Laidley 1994). There also could be minor components, such as the GnRH-binding protein, that are important in endocrine responses in some species (Huangcontinue
Page 95
et al. 1991). Most vertebrate species possess serum albumins, and although there are species differences in the specific plasma glycoproteins that bind estrogen and other sex steroids with high affinity, albumin likely serves as a low-affinity, high-capacity steroid carrier in vertebrates (Siiteri 1982).
Globulin-bound steroids, such as estradiol bound to a-fetoprotein (AFP). can enter selected cells via receptor-mediated endocytosis (Toran-Allerand 1984). Dohler (1986) proposed that deglycosylation of proteins can facilitate their entry into cells. Vallette et al. (1977) demonstrated that deglycosylated AFP has a high affinity for estrogen and will preferentially enter target cells capable of internalizing AFP. However, in vitro studies showed that the general effect of AFP and SHBG is to decrease the apparent potency of estrogens and testosterone (Damassa et al. 1991), suggesting that trapping of the steroid by the binding protein is their main effect.
Once inside a target cell, a steroid presumably dissociates from the plasma protein and becomes available for binding to specific intracellular receptors because of the lower binding affinity of steroids for plasma proteins relative to intracellular receptors. Siiteri et al. (1982) suggested that binding proteins could help create an intracellular pool of bound steroid, which is protected from metabolism but in equilibrium with the pool of steroid bound to intracellular receptors. Another hypothesis is that binding proteins compete with intracellular receptors in target cells and modulate the cellular response to steroids (Sakly and Koch 1983). For example, neonatal rat testes contain AFP, so it is posited that intracellular AFP binds intracellular estradiol, which is thus rendered biologically unavailable to the receptor (Huhtaniemi 1985).
In addition to either sequestering or delivering HAAs, the binding of HAAs (including phytoestrogens) to human steroid-binding proteins can displace endogenous hormones (Martin et al. 1996), possibly affecting hormone delivery to target cells. Chemicals also associate with multiple lipoprotein fractions and might redistribute among them (Mohammed et al. 1990).
Developmental Stage and Serum Protein
Proteins that transfer chemicals or hormones to or within the fetus include AFP, uteroglobin, albumins, and transferrins. Most published information concerning concentrations of circulating steroids in pregnant females, fetuses, and animals after birth involves measurement only of the total amount of steroid present in the blood. In these studies, steroids are extracted by techniques that do not allow one to discriminate between the fraction bound to plasma proteins and the free fraction. Studies in rats (Montano et al. 1995, Nagel et al. 1998) showed the potential for misinterpreting the bioactive concentration of steroid present during fetal life that is based on information concerning total steroid concentration in blood. In fetal rats, total serum estradiol measured by radioimmunoassay was high (150 pg/mL) relative to that measured in diestrous females (15 pg/mL).continue
Page 96
However, the free fraction was much lower during development (0.3%) than adulthood (4%). Thus, the bioactive fraction of estradiol in fetuses is actually similar to that found in adult diestrous females (Montano et al. 1995; Nagel et al. 1998). Similarly, there is a 100-fold difference between human adults and fetuses in the percentage of serum estradiol that is free (Nagel et al. 1998). In humans, total serum estradiol concentration is approximately 100-fold greater in fetuses than it is in adults; the free serum estradiol concentration in human adults and fetuses is similar (Nagel et al. 1996, vom Saal et al. 1997).
The significance of protein transfer to the fetus is suggested by AFP, which might transport estradiol into selected estrogen-responsive regions of the developing brain in rats and mice (Attardi and Ohno 1976; Schachter and Toran-Allerand 1982). Recent work has shown that uteroglobin binds methylsulfone metabolites of DDT and of some PCB congeners (Hard et al. 1995). For example, 3-methlysulfonyl-DDE is transferred across the placenta and accumulates in the adrenal cortex of fetal mice (Jonsson et al. 1995). There is a high-affinity binding of a PCB methylsulfone to uteroglobin (Hard et al. 1995), which can transport these compounds also to sensitive target tissues in the fetus. More must be learned about the role of fetal and maternal serum proteins as targets or vectors of HAAs.
In nonmammalian species, the plasma proteins that can bind steroids or HAAs include SHBG or steroid-binding plasma protein (SBP), albumins, vitellogenin, and other lipoproteins. These binding proteins facilitate uptake of HAAs by eggs during oogenesis in fish and probably in other vertebrates with yolky eggs. PCBs and o,p'-DDT bind to fish vitellogenin (Ungerer and Thomas 1996a). In a follow-up study of serum lipoproteins involved in transport of xenobiotics in fish, Ungerer and Thomas ( 1996b) found that the majority of o,p'-DDT in croaker was associated with a triglyceride-rich very-low-density lipoprotein (VLDL). Uptake of the triglyceride-VLDL fraction was identified as the major route of DDT into oocytes, accounting for a majority of the accumulation in the ovary.
Target Organs
The principal target organs for HAAs are those that produce, regulate, or respond to hormones or their action: brain, gonads, liver, uterus, mammary. adrenals, prostate, placenta, and the organs in the developing embryo or fetus. Some studies have established that HAAs are distributed to all of these organs, although information on the concentrations and pharmacokinetics of HAAs in many of these organs in humans or wildlife species is limited. Studies of two key organs illustrate the complex pharmacokinetics.
Brain
In the brain, the pineal and pituitary glands and the hypothalamus are important in the control of reproductive endocrine processes. The blood-brain barriercontinue
Page 97
prevents many of the more-hydrophilic drugs from passing from the circulatory system into the brain itself. Yet, the uptake or presence of lipophilic HAAs in the brain has been measured in humans and animals (Mes et al. 1995; Corrigan et al. 1996; Jenssen et al. 1996). Moreover, parts of the brain that control reproductive function—the median eminence and other circumventricular organs—are outside the blood-brain barrier (Kandel et al. 1991), affording transfer to these structures. The action of HAAs in the brain is inferred from studies showing, for example. the effects of genistein on pituitary responsiveness to gonadotropin-releasing hormone and on brain structure in postpubertal castrated female rats (Faber and Hughes 1993).
Analysis of residues in environmentally exposed animals provides information about pharmacokinetics. In some avian species, the concentrations of lipophilic xenobiotics, which could be HAAs, are as great in the brain as they are in the liver (Burns and Teal 1979). That result appears not to be the case in some mammals. Measurable concentrations of several pesticides, including DDT (p,p'-DDE, o,p'-DDD, p,p',-DDD, o,p'-DDT, p,p'-DDT) and 22 PCB congeners, were found in samples of brain, fat, and liver of gray seal pups (Jenssen et al. 1996). The concentrations of PCBs and DDT in liver were about 75% of those in blubber. Only two PCB congeners were detected in brain tissue, where total PCB content was only about 1% of that measured in the blubber. The basis of tissue-specific accumulation of PCB congeners, with the patterns of PCB congeners in liver and brain differing from those in blood and blubber, is not known.
Studies that directly measure HAA transfer to the brain have shown species and chemical differences in accumulation. Ingebrigtsen et al. (1990) reported different patterns of accumulation of 2,3,3',4,4'-pentachlorobiphenyl in cod and rainbow trout. In a study in rats, Ness et al. (1994) reported a difference in the accumulation of different PCB congeners. There was little difference in the regional distributions within the brain; however, 3,3',4,4'-tetrachlorobiphenyl was concentrated in the vicinity of blood vessels, whereas 2,2',4,4'-tetrachlorobiphenyl was not. That suggests a selective interaction of 3.3',4,4'-tetrachlorobiphenyl with vascular structures. The relationship between chemical structure and deposition in different regions of the brain should be studied further.
Placenta
In humans and other mammals, the placenta could constitute a barrier to the transfer of HAAs or other xenobiotics from the mother to the developing fetus. However, the rapid transfer of chemicals, including TCDD, across the placenta and the subsequent distribution of toxicants in the fetus have been conclusively demonstrated in the laboratory (Abbott et al. 1996) and are associated with effects in offspring (Gray and Ostby 1997). Transplacental toxicity of 3-methylsulfonyl-DDE in the developing adrenal cortex in mice has been reported (Jonssoncontinue
Page 98
et al. 1995). Prenatal exposure to genistein also affects sexual differentiation (Levy et al. 1995).
In mice, Darnerud et al. (1996) found that 3-4% of 3,3',4,4'-tetrachlorobiphenyl given to the mother was transferred to the fetus. These investigators also noted that the pharmacokinetics of 3,3',4,4'-tetrachlorobiphenyl differ from those of other planar PCB congeners. In one species of marine mammal, it was estimated that 4-10% of maternal PCBs could be transferred across the placenta to the fetus (Tanabe et al. 1994). Although transfer across the placenta clearly does occur, the relationship between maternal plasma and serum concentrations and the amounts that cross the placenta is not known. In one study of humans in Oslo (Johansen et al. 1994), concentrations of DDE and PCBs were found to be less in umbilical-cord blood than in maternal blood. The lower cord-blood concentration suggests that the placenta acts as a partial barrier; however, the unique physiologic state of parturition (when cord blood is collected), which is characterized by an increase in serum concentrations of cortisol, could lead to changes relative to earlier times in pregnancy. The degree to which the placenta acts to inhibit maternal-to-fetal transport is not known for most lipophilic HAAs. The placenta should be examined for toxic substances at low doses to determine whether it acts as a barrier. Studies of chemical transfer in isolated, perfused placenta, such as that by Bassily et al. (1995), could help to establish the characteristics of transfer.
Target Cells
Measuring the concentration of HAAs in target organs is one step removed from measuring the concentration that might actually reach the target cells in those organs. Few studies have been published that provide target-cell measurements. However, molecular changes, such as the induction of CYP enzymes in pituitary gonadotrophs, can indicate uptake in particular targets (Andersson et al. 1993). Cellular localization of proteins involved in HAA effects could help to identify sensitive target cells. For example, CYP19 (aromatase) is expressed in Leydig cells in humans, in the seminiferous tubule in mice, and in astrocytes of the brain in rats (Nitta et al. 1993; Inkster et al. 1995; Zwain et al. 1997), indicating that these cells are targets for aromatase-inhibiting xenobiotics.
Some cell types are overlooked as targets of HAAs and other xenobiotics, an example being those in the vasculature. Endothelial cells of blood vessels are the first cells (other then hemocytes) to encounter hormones or chemicals in the blood. Epithelial cells in the vascular system express steroid hormone receptors; estradiol affects vascular function (Rubanyi et al. 1997). The endothelium could work as a physical and metabolic barrier, intercepting molecules in the bloodstream. TCDD and PCBs strongly induce CYP1A in the endothelia of mammals and fish (Dees et al. 1982; Stegeman et al. 1989; Overby et al. 1992). Metabolism of xenobiotics bycontinue
Page 99
enzymes in the endothelium has been demonstrated (Stegeman et al. 1995). Transformation of HAAs in the endothelium has not been shown but probably occurs.
As with the sequestration of TCDD by CYP1A2 in the liver (Diliberto et al. 1997), CYP1A in the endothelium might act in some species to retain or metabolize some compounds preferentially in highly vascularized regions. For example. localization of 3,3',4,4'-tetrachlorobiphenyl near vascular structures in rat brain (Ness et al. 1994) could involve interaction with CYP induced in the vasculature.
Studies of developmental defects in fish, such as "blue sac" disease in lake trout, suggest that the endothelium or other vascular structures are targets for chemicals that produce developmental abnormalities. Blue sac disease is characterized by vascular dysfunction preceding death. It is associated with induction of CYP1A in the endothelium (Guiney et al. 1997) and with evidence of endothelial cellular damage (Cantrell et al. 1996, 1998).
Depots and Mobilization
Some fatty tissues that accumulate lipophilic chemicals, including many HAAs, can serve as reservoirs or depots for these chemicals. Fatty tissues in vertebrates include adipose tissue, liver, gonads, neural tissue, brain, and, in some fish, muscle. Adipose tissue in most vertebrates acts as a depot for organic xenobiotics. The concentration of HAAs is particularly important in assessing the exposure of some types of animals to HAAs. For example, the concentration of xenobiotics in whales is most often measured in blubber (Muir et al. 1988b; Wade et al. 1997), which can be biopsied as a marker for exposure.
A survey was conducted by the National Human Monitoring Program for the years 1970-1983 to detect and quantify the prevalence of organochlorine pesticides and PCBs in adipose tissue of the general population in the United States (Kutz et al. 1991). This survey showed that mean concentrations of DDT in adipose tissue declined from approximately 8 ppm in 1970 to about 2 ppm in 1983. Similarly, the percentage of individuals having total PCB concentrations greater than 3 ppm steadily declined, although the number of people with detectable amounts of PCBs increased. Aldrin was not detected, but its metabolite, dieldrin, was shown to decline from about 0.18 ppm to 0.06 ppm. The metabolite of chlordane, oxychlordane, was also detected, but the concentrations found in adipose tissue were relatively constant over the survey period. Endrin and toxaphene were not detected. Measurements for chlordecone (Kepone) were also taken, but the population examined was limited to the southeastern United States, because this pesticide is used to control fire ants in this region. Chlordecone was found in less than 1% of the adipose-tissue samples and ranged in concentration from 0.15 to 2.5 ppm (Kutz et al. 1991).
Adipose tissues in some organs (e.g., breast) might be important direct targets of HAA action or important internal reservoirs of HAAs. However, chemi-soft
Page 100
cals in these lipid depots can be mobilized during starvation or reproduction. Bigsby et al. (1997), for example, demonstrated that, in fasting mice, some HAAs, such as ß-HCH (hexachlorocyclohexane), are released from fat and then produce estrogenic effects, but that is not observed with o,p'-DDT, indicating that these compounds are mobilized differently from fat depots. In marine mammals, the amount of blubber can change with the season, with feeding, or with reproduction, resulting in changes in HAA content in the blood of some species (Aguilar and Borrell 1994). Fasting in female polar bears results in increased concentrations of HAAs in milk (Polischuk et al. 1995). Species that lay yolky eggs transport large quantities of egg proteins and lipids to their eggs during oogenesis (Vodicnik and Peterson 1985). In general, that process is associated with mobilization of lipids and other reserves, enhanced synthetic processes in the liver, and transfer of materials from the liver to the egg. Changes in reproductive state also produce increases in vascularization and blood flow to organs (e.g., gonads) that could be targets for the action of HAAs, resulting in transport of greater amounts of chemicals to these organs. At the same time, there is often an increase in the lipid content of the gonads. resulting in a greater degree of chemical deposition there. Similar processes can occur during lactation in mammals, enhancing the transport of chemicals to breast tissue (Ramos et al. 1997a).
Deposition of eggs or release of milk can eliminate some chemicals from the female. Studies in fish document the extensive displacement of contaminants from the maternal body to the ovary and deposition in the eggs. Thirty percent or more of maternal contaminant burden can be lost at the time of spawning, when it is shed with the eggs (Vodicnik and Peterson 1985). Likewise, release of milk can eliminate a substantial fraction of HAA from female mammals. However. that can result in the transfer of chemicals to infants, and the effects of the contaminants can appear in the developing young.
It should be noted that many of the foregoing examples of trend data might not be directly comparable because of differences in methods for detecting clinical residues. As technology advances and becomes standard, more chemicals will be detected and compared in studies (Hill et al. 1995, 1996; Needham et al. 1995; Burse et al. 1996).
Metabolism
HAAs and endogenous hormones can act though receptor-dependent and receptor-independent (i.e., nonhormonal) pathways. Inferences regarding HAA effects and dose-response relationships can be weakened by the failure to distinguish between these mechanisms. Receptor-dependent pathways are discussed in Chapter 2. This section considers enzymes that metabolize hormones and HAAs and that could be involved in receptor-independent effects.break
Page 101
Enzymes
Enzymes that metabolize steroids and HAAs include members of the CYP family (Nelson et al. 1996), catechol-O-methyltransferase, dehydrogenases such as 11ß-hydroxysteroid dehydrogenase, sulfotransferases, glutathione(s)transferases, glucuronyl transferases, and others. The CYP enzymes figure most prominently in the oxidative metabolism of HAAs and steroids. Many microsomal CYP enzymes have broad substrate specificities and hydroxylate steroids and many HAAs, often catalyzing rate limiting steps (see for reviews, Ioannides and Parke 1993; Ortiz de Montellano 1995). CYP enzymes also catalyze the synthesis and activation of steroids. The consequences of exposure to HAAs could differ, depending on the nature and extent of metabolic transformation of steroids, the metabolic transformation of the HAA, and the interplay between the two. Knowledge of the function and regulation of the enzymes is necessary to any understanding of how metabolism might contribute to the developmental, reproductive, or disease outcomes ascribed to HAAs. The complex ways in which metabolism of HAAs and hormones affects outcomes are illustrated by considering how metabolites of estradiol and HAAs can be involved in hormone-dependent carcinogenesis.
Virtually all HAAs are metabolized through a variety of oxidative and reductive or conjugation reactions. Metabolism can inactivate an HAA or lead to the activation of a hormonally-active metabolite from a non-hormonally active parent compound. Methoxychlor, several PCB congeners, and DDT are metabolized to products that are estrogenic, antiandrogenic, or thyroid mimics (Safe and Zacharewski 1997) (see Chapter 2). Nonestrogenic nonylphenol polyethoxylates are metabolized to the estrogenic nonylphenol in the rat (Knaak et al. 1966). CYP and conjugation enzymes also transform nonylphenol and bisphenol A (Atkinson and Roy 1995; Lewis and Lech 1996; Meldahl et al. 1996), but the reactivity of the products is not known. Methysulfonyl-DDE appears to be further metabolized to toxic derivatives in fetal mice (Jonsson et al. 1995). The metabolism of many phytoestrogens is less well known than is that of the synthetic xenobiotic HAAs. Flavonoids are hydroxylated by CYP enzymes (Silva et al. 1997). Zearalenone also appears to be metabolized to a more estrogenic compound, a-zearalenol (Kuiper-Goodman et al. 1987). In general, nonhalogenated compounds, which include many natural products, are more rapidly metabolized than are chlorinated compounds (Chen et al. 1982).
Metabolism-Modifying Factors
The capacity to metabolize steroids and HAAs in target and nontarget organs can change as a result of exposure to HAAs and to chemicals that affect enzymes that metabolize HAAs or steroids. Induction increases the rate of enzyme synthesis, but chemicals can act on gene products directly. For example, although metabolites of zearalenone can be estrogenic, the estrogenic effects of zearalenonecontinue
Page 102
could result in part from its inhibition of steroid metabolism (Pompa et al. 1986). Tributyltin is thought to cause penis growth in female molluscs by affecting steroid metabolism (Bettin et al. 1996). Methoxychlor can both induce and inhibit CYPs (Li et al. 1993, 1995). Some PCB congeners can induce CYP1As but also inhibit and inactivate them (White et al. 1997a). Interactive effects can involve one HAA affecting the metabolism of another. Thus, lower chlorinated PCBs can be metabolized to reactive products and be eliminated more rapidly than are the more highly chlorinated compounds, but the more highly chlorinated compounds can persist and act as inducers of enzymes that affect HAA and steroid metabolism. The expression and activity of these enzymes can be regulated by hormones as well. For example, the recently described CYP1B1 shows dual regulation by aryl hydrocarbon (Ah) receptor agonists and by hormones (Bhattacharyya et al. 1995). The induction of CYP1A in fish can be suppressed by hormones, ostensibly estradiol, during ovarian maturation (Gray et al. 1991).
Species differences in CYP enzymes and their role in the metabolic disposition of HAAs and hormones can introduce uncertainty in attempts to extrapolate data from one species to another, including to humans. For example, humans create a greater percentage of the more-estrogenic metabolite (a-zearalenol) of zearalenone and dispose of the compounds more slowly than do rodents (Kuiper-Goodman et al. 1987). There are few studies that directly compare rates of metabolism of HAAs in different species or of multiple HAAs in a given species (Borlakoglu and Wilkins 1993; Murk et al. 1994). A few generalizations are possible. For example, fish are much less active metabolizers of PCBs than are mammals (Murk et al. 1994; White et al. 1997b).
Steroid Metabolism and HAAs
The function or rate of action of enzymes involved in steroid synthesis, activation, or degradation can be affected by HAAs. Steroid synthesis involves numerous steps leading to the active hormones. The steroid synthetic enzymes include CYP11 (side-chain-cleavage enzyme), CYP17 (17a-hydroxylase), and CYP19 (aromatase). Functional and cloning studies of the steroidogenic enzymes in nonmammalian vertebrates, including fish (Sakai et al. 1992, Tanaka et al. 1992; Takahashi et al. 1993) and turtle (Jeyasuria et al. 1994), indicate that similar enzymes occur in mammalian and nonmammalian vertebrates. As with other CYP enzymes, the steroid synthetic enzymes are subject to inhibition by substrate analogues that can act as competitive inhibitors or mechanism-based inactivators.
An important example is aromatase, which catalyzes the conversion of testosterone to estradiol. Aromatase is expressed in many organs, where it is a factor in health and disease (Simpson et al. 1997), and it is inhibited by various chemical structures. Those include flavonoids (Ibrahim and Abul-Hajj 1990; Wang etcontinue
Page 103
al. 1994; Pelissero et al. 1996), other natural products (Blanco et al. 1997), and such compounds as fadrazole and aminoglutethimide. The mechanisms of action of those chemicals are under study (Chen et al. 1997). It is possible that exposure to aromatase inhibitors in the environment affects sex determination, as suggested by studies in fish, birds, and reptiles (Lance and Bogart 1992: Monod et al. 1993; Antonopoulou et al. 1995; Richard-Mercier et al. 1995; Abinawanto et al. 1996). There is evidence that nonsteroidal aromatase inhibitors can alter the temperature-dependent sex determination in the terrapin (Jeyasuria et al. 1994) and might be a molecular target in alligators (Crain et al. 1998).
CYP enzymes that hydroxylate steroids have been studied extensively in the liver of mammalian species (Waxman et al. 1991; Ioannides and Parke 1993). A review by Martucci and Fishman (1993) describes several of the pathways of CYP metabolism of estradiol in mammals, including humans. The same enzymes can hydroxylate estradiol (E2) and estrone (El). Many of these enzymes are involved in the hydroxylation of testosterone.
The degree of expression of the particular enzymes, their affinity for the substrate, and their catalytic efficiency will determine the rate or extent of formation of specific metabolites. Expression of multiple CYP genes in the same cell can lead to formation of multiple products in that cell. Most studies of steroid metabolism, including that of estradiol, have been done with enzyme preparations from the liver. If chemical exposure induces the expression of enzymes that hydroxylate steroids in the liver, elimination of the hormone could be accelerated, thus reducing concentrations in the body. Yet, it is not clear whether altered rates of hepatic steroid metabolism are involved in the effects of HAAs in other target organs. Reactive or protoxic metabolites of HAAs or steroids produced in the liver would need to be transported in the blood to reach other target organs. The effects of HAAs on CYP enzymes in those target organs could be more directly significant.
Extrahepatic Enzymes and HAA Effects
The complement of enzymes that metabolize HAAs can strongly influence the susceptibility of the cell to the effects of these compounds. If an HAA requires metabolism to be estrogenic, then cells lacking the requisite enzymes are less likely to be susceptible to the action of that chemical than are cells that have an active metabolism. Generally, the same enzymes will catalyze similar reactions in different organs and cells. Apart from steroidogenic enzymes in gonads and adrenals, the cellular localization of microsomal CYP enzymes in extrahepatic organs that are targets for HAAs or for hormonally related pathologies is not well known. It cannot be assumed that the steroid-metabolizing enzymes present in the liver are expressed to similar degrees in other organs. Some enzymes expressed only slightly in liver are highly expressed in other organs. Acontinue
Page 104
key example is CYP1B1, which is discussed below. Studies in three target organs (brain, mammary gland, and placenta) provide examples of the complexities in these possible sites of action.
Brain
Some CYP enzymes involved in steroidogenesis, particularly CYP19, have been identified in the brain of most vertebrate groups (Callard et al. 1988). As suggested above, altered aromatase activity has been linked to effects on sex differentiation in reptiles and fish. The brain appears to synthesize steroids, including neurosteroids, completely from cholesterol (Stapleton et al. 1995; Warner and Gustafsson 1995; Rose et al. 1997).
Other hormonally important structures, including the pituitary, are sites of action of some HAAs. Andersson et al. (1993) reported that CYP1A1 was induced in the pituitary of trout exposed to an Ah receptor agonist. Cell types in which induction was strong included gonadotrophs containing gonadotropin II; alterations in the concentrations of gonadotropin were associated with exposure. A prominent site of induction of CYP1A1 by PCBs and TCDD in the brain is the endothelium (Smolowitz et al. 1991). The prominence of induction at this site could contribute to a blood-brain barrier for CYP1A substrates. An increasing number of CYP enzymes are being found to be expressed in brain tissue in humans, other mammals, and lower vertebrates (Strobel et al. 1995; Stegeman et al. 1997). Regional expression of some brain CYPs has been found, for example, in the expression in the hippocampus of a novel CYP identified in rat brain (Stapleton et al. 1995). For most of the known brain CYPs, regional and, more important, cellular localization have yet to be determined.
Mammary Gland
Steroidogenic and hydroxylating CYP enzymes are expressed in mammary tissue. Studies of CYP enzymes in the breast are not extensive. Hellmold et al. (1995) examined the expression of CYP messenger ribonucleic acids (mRNAs) in breast tissue of rats 1, 2, 3, 6, 9, and 15 weeks after birth; 1, 2, or 3 weeks after pregnancy; 2-3 weeks postpartum; and 3 weeks after lactation. The expression of at least 10 CYP genes was detected in the breast tissues. The same CYP enzymes were detected also with antibodies, and there were distinct patterns of expression associated with age, reproductive status, and lactation. Recent studies of the mammary gland have focused on a relatively recently discovered enzyme, CYP1B1, which activates polycyclic aromatic hydrocarbon (PAH) carcinogens (Shimada et al. 1996) and forms catecholestrogens (Hayes et al. 1996). The possible significance of that enzyme in mammary carcinogenesis is considered later in this chapter.break
Page 105
Placenta
Placental CYP enzymes, especially aromatase (CYP19), have been studied for years in animals and humans. In addition to converting testosterone to estradiol, CYP19 appears to form the catecholestrogen 2-OH-E2 (Almadhidi et al. 1996), a possibly important metabolite. Developmental and cellular patterns of expression are important to the consequences of HAA metabolism. Transcripts for 10 CYP enzymes were identified by reverse transcriptase-polymerase chain reaction in at least some samples of first-trimester human placenta (Hakkola et al. 1996a). Similar studies (Hakkola et al. 1996b) of term placenta showed that the patterns of expression had changed, with fewer genes appearing to be expressed. That implies that the capacity of the placenta to metabolize HAAs, or steroids, changes during gestation. For the most part, the cellular sites of expression of these CYP enzymes have not been described. However, involvement of a CYP in arachidonic-acid metabolism in human placental vessels has been suggested (Omar et al. 1992). Also, CYP1A1, induced by and metabolizing planar PCBs and TCDD, is expressed in trophoblasts in human placenta (Slezak et al. 1997).
Placental CYP enzymes might constitute a metabolic barrier, intercepting xenobiotics before they can reach the fetus. That possibility is suggested by studies of placental CYP2E1, which metabolizes alcohol, and fetal alcohol syndrome (Rasheed et al. 1997). Fetal alcohol syndrome might result from acetaldehyde produced from alcohol in the fetus (Holownia et al. 1996). CYP1A, which can metabolize and activate many procarcinogens, was found to be elevated in the placenta of women who were accidentally exposed to high concentrations of PCBs (Lucier et al. 1987) and who smoked during pregnancy (Gallagher et al. 1994; Slezak et al. 1997). In addition to possible involvement of CYP1A, smoking appears to decrease placental aromatase activity (Kitawaki et al. 1993). The significance of placental xenobiotic enzymes to HAA effects in the fetus are not well documented.
Developmental Stage and CYP Enzymes
The expression of CYP enzymes during embryonic, larval, or fetal development might be crucial to the developmental effects of exposure to HAAs or other xenobiotics. Stromstedt et al. (1996) reported that steroidogenic enzymes were not expressed in mouse preimplantation blastocyst, although aromatase is expressed in preimplantation blastocyst of other species (Choi et al. 1997). Toda et al. (1994) documented the presence of steroidogenic CYP enzymes in mammalian fetal tissue. CYP19 is expressed in fetal gonads and brain (Hutchison et al. 1995). Keeney et al. (1995) reported spatial and temporal differences in the patterns of expression of CYP17, aldosterone synthase, and cholesterol sidechain cleavage CYP mRNAs, and they suggested that several factors were required to program cell type and species-specific expression of steroid hydroxylasescontinue
Page 106
during embryonic development. The development of enzymes involved in conjugation and deconjugation of steroids was examined in a study of rats from birth to 50 d of age (Fisher and Weissinger 1972). The clearance time of [14C]diethylstilbestrol (DES) decreased 10-fold between birth and 25 d of age. Intestinal hydrolysis of DES conjugates was minimal at birth (because of the lack of intestinal bacteria) but fully developed by 25 d of age, and there was a deficiency in liver enzymes required for conjugation in newborn rats. Not much is known about such patterns across species.
Whether mammalian blastocysts express xenobiotic-metabolizing CYP enzymes is not clear. However, xenobiotic-metabolizing enzymes detected at the level of mRNA in human fetal liver include CYPs 2C8, 2D6, 3A3/3A4, and 3A7 (Hakkola et al. 1994). The expression of CYP1A1 has been detected during organogenesis in animals (Dey et al. 1989; Yang et al. 1991) and in human fetal liver (Murray et al. 1992). Morse et al. (1995), however, reported that fetal rats had little inducible capacity to metabolize 3,3',4,4'-tetrachlorobiphenyl, although other CYP1A activity was induced (Sinjari et al. 1993). There might be development-specific CYP enzymes. In mice and humans, there appears to be a fetus-specific member of the CYP3A subfamily (Itoh et al. 1994). CYP3A enxymes are active steroid 6ß-hydroxylases, and they are catalysts for most of the drugs metabolized in adult human liver.
Still less is known about the expression of CYP enzymes at different stages of nonmammalian developmental stages. Few homologous CYP enzymes have been identified in many species, and only CYP1A has been well studied. CYP1A1 is induced by TCDD and other Ah receptor agonists at various developmental stages in birds and fish. In fish, that induction is prominent in the endothelium, where it has been associated with developmental toxicity (Guiney et al. 1997; Cantrell et al. 1998). Determination of the forms and functions of CYP enzymes expressed at different stages is an important research need.
Biologically Active Metabolites of Estradiol
As pointed out by Wolff and Toniolo (1995), ''different groups of polychlorinated biphenyl . . . congeners evoke different biological responses, and a wide divergence of estrogenic response, CYP enzyme activity, and biological half-life exists within these groups. . . . "A consideration of multiple mechanisms by which HAAs and natural estrogens might act is crucial to evaluating the risk associated with exposure to these compounds. One such consideration is how estradiol metabolism might be related to breast cancer and how HAAs might contribute to the process through multiple mechanisms.
A role for estradiol metabolism in breast cancer has been suggested repeatedly (Yager and Liehr 1996). The formation of 16a-hydroxy-E2, a metabolite of estradiol, has been suggested by some investigators to be a risk factor in human breast cancer (Bradlow et al. 1995). The high estrogenic activity of 16a-OH-E2continue
Page 107
and partial antiestrogenic activity of 2-OH-E2 has recently been confirmed (Gupta et al. 1998) and is consistent with the Bradlow hypothesis. However, other data suggest that catecholestrogen formation is a risk factor in nonfamilial breast cancer (Lemon et al. 1992; Adlercruetz et al. 1994) and that catecholestrogen might be causally involved.
Catecholestrogens, both 4-OH-E2 and 2-OH-E2, are estrogen receptor agonists (Schutze et al. 1993). However, data suggest that the action of these products involves generation of reactive oxygen species through redox cycling. Nutter et al. (1993) established that quinone derivatives of catecholestrogens can generate reactive species when incubated with breast-cancer preparations. In particular, 4-OH-E2 has been implicated in such effects (Han and Liehr 1995: Liehr et al. 1995; Liehr and Ricci 1996). Han and Liehr (1995) examined the ability of 4OH-E2 and 2-OH-E2 to cause oxidative damage of DNA and reported that 4-OHE2 caused greater damage than did 2-OH-E2, consistent with a more-rapid redox cycling of the 4-OH-E2. Reactive oxygen species can damage DNA, but they also can alter various signal transduction pathways involving cell proliferation, notably in early development (Ozolins and Hales 1997). 2-OH-E2 is more active as an antioxidant (Ruiz-Larrea et al. 1994) and might indeed have some benefit (Bradlow et al. 1996). There is evidence that oxidative damage of DNA is associated with breast cancer (Malins and Haimanot 1991; Malins et al. 1993, 1996), and the evidence is quite consistent with the evidence suggesting involvement of radical formation that could come from redox cycling involving catecholestrogens.
4-OH-E2 has been identified as a risk factor in mammary carcinogenesis (Cavalieri et al. 1997); thus, it is important to discern the regulation of enzymes that form this product. In rodents and humans, CYP1B1 has been established as a primary catalyst responsible for 4-hydroxylation of E2 (Spink et al. 1994). Cloning and expression research shows that human CYP1B1 catalyzes E2-2OHase activity, but that it is primarily an E2-4-OHase (Hayes et al. 1996). However, in some organs, 4-OHase and 2-OHase appear to be equally important. CYP1B1 also is expressed in breast and breast-cancer tissue (Christou et al. 1995; McKay et al. 1995). CYP1B1 or related proteins are responsible for 4-OH-E2 formation in the uterus, where they might participate in carcinogenesis (Liehr et al. 1995). Other organs where 4-OHase activity is high are targets for estrogen-dependent tumorigenesis.
If the postulated role of catecholestrogens in estradiol-induced carcinogenesis is correct, then exposure to xenobiotic HAAs could influence that process through effects on the formation or persistence of catecholestrogens. For example, TCDD and other Ah receptor agonists, including PCBs, can induce the expression of CYP1B1 (Brake and Jefcoate 1995). The flavonoid quercitin inhibits catechol-O-methyltransferase, an enzyme that can inactivate the catecholestrogens. That inhibition can result in accumulation of the catecholestrogens, thereby increasing their availability for redox cycling (Zhu and Liehr 1996). It follows that HAAs contrib-soft
Page 108
ute to detrimental effects through their own metabolic generation of reactive oxygen species. Thus, some PCB metabolites form quinones that can undergo redox cycling and damage DNA (Oakley et al. 1996), but those congeners demonstrated to form quinones are not found in the environment.
An additional complexity in carcinogenic effects involves angiogenesis. Growth of vessels to the tumor is essential for the growth of tumors. Notably. 2-methoxyestradiol is an angiogenesis inhibitor that can suppress tumor growth (Fotsis et al. 1994). Inhibition of catechol-O-methyltransferase therefore might not only promote accumulation of catecholestrogens but also decrease the formation of a product that can impede tumor growth. Understanding of how estrogen metabolism is affected by HAAs is incomplete. Whether rates of angiogenesis are prognostic indicators for breast cancer is not clear (Hall et al. 1992). The proposed mechanism by which catecholestrogens act in carcinogenesis, involving redox cycling and the generation of free radicals, would not necessarily involve the estrogen receptor but would involve the enzymes of E2 metabolism. It will be necessary to determine whether E2 action on cell proliferation is an independent process that contributes to carcinogenesis in the breast or is related to the radical pathways to carcinogenesis. The balance of these different pathways is not understood. Moreover, estradiol might act as an antioxidant. The diversity of responses could be disclosed when the regulation of the catalysts for estradiol and HAAs is understood.
As with estradiol, HAAs also might act through multiple mechanisms, with detrimental and beneficial effects. Genistein is an excellent example. Genistein is an estrogen receptor agonist, but it inhibits 17ß-hydroxysteroid oxidoreductase and thus can limit the conversion of estrone to estradiol (Mäkelä et al. 1995b). Genistein also possesses antioxidant properties and can enhance antioxidant enzymes (Cai and Wei 1996), which can counter the oxidative damage ascribed to catecholestrogens. The compound also inhibits the p-glycoproteins (Castro and Altenberg 1997), which transport drugs and some steroids out of the cell. Genistein is an inhibitor of angiogenesis (Fotsis et al. 1993); the mechanism of that effect is not known, but it might confer protection against tumorigenesis, as vessel growth is essential to tumor growth and to metastasis. Inhibition of angiogenesis also can affect fetal development. Moreover, genistein is a potent inhibitor of membrane tyrosine kinases (Spinozzi et al. 1994), which might be involved in effects on cell proliferation independent of effects on estrogen metabolism. Whether different doses of genistein act through different modes is not clear. Prenatal exposure to genistein affects later sexual differentiation (Levy et al. 1995), but it is not clear which mechanisms are involved.
Species Differences in Metabolism
Species differences in response to xenobiotics, including HAAs, can be enormous (Kennedy et al. 1996). Whether an effect of HAAs observed in one speciescontinue
Page 109
occurs in others is the subject of concern when researchers try to extrapolate data for a particular disease, such as breast cancer, from animal models to humans. For procarcinogens, or HAAs that are metabolically activated, the presence of enzymes that activate the chemical in question could be a determining factor. The enzymes involved in the metabolism of hormones in vertebrates are similar in function, but the substrate specificity of xenobiotic-metabolizing and steroid-hydroxylating enzymes can differ substantially for homologous enzymes in different species. There is little information available about CYP enzymes in nonmammals (Hahn and Stegeman 1994). Only now are CYP enzymes that might metabolize HAAs being described in reptiles, including alligators (Ertl and Winston 1997).
Formation of catecholestrogens by hepatic CYP enzymes has been shown in fish (Snowberger and Stegeman 1987) and in adult and fetal marine mammals (Goksoyr et al. 1988; R. White et al. 1994). Fish-liver microsomes also form 6a-OH-E2, 16a-OH-E2 (estriol), and estrone (Snowberger and Stegeman 1987). The major hydroxylated product appears to be 2-OH-E2. Unlike the case in some mammals, CYP1A enzymes in fish appear to have little involvement in forming 2-OH-E2, indicating that HAAs, such as TCDD, might not act through this pathway in fish. There is little evidence for hepatic formation of 4-OH-E2 in fish. Whether fish form this catecholestrogen in endocrine organs or express homologues of CYP1B enzymes has not been established. Studies of homologous CYP enzymes in different groups (e.g., the androgen-hydroxylating CYP3As (Celander and Stegeman 1997)) could show the extent of similarities and differences attendant to HAA exposure in different species. Defining structure-activity relationships for substrate binding to homologous enzymes in different species will help to achieve generalizations.
Factors Influencing Dose-Response Assessment
A central issue in evaluating possible consequences of HAA exposure is whether the concentrations accumulating in animals and humans are sufficient to elicit changes in target cells and organs of the adult or the developing organism. Accurately predicting a biologic effect solely on the basis of the chemical concentrations in tissues requires that the dose-response relationship for a particular biologic change be known. To infer an effect from environmental exposure requires extrapolation across dose; that requires a knowledge of the shape of the dose-response curve. Typically high doses used in experimental studies are extrapolated to potentially much lower doses resulting from exposure to concentrations ordinarily found in the environment in contaminated food, water, air, or soil.
Inference also requires extrapolation across species, because with some species (e.g., humans), the possibility for experimental studies is impossible. Extrapolation across species is problematic, because species can differ greatly in their sensitivity to the effects of chemicals. For example, the acute toxicity ofcontinue
Page 110
TCDD varies by orders of magnitude between the most- and the least-sensitive vertebrate species studied to date (Peterson et al. 1993; Kennedy et al. 1996). However, at the receptor level, there is little evidence for significant differences among vertebrate species in the affinity of estradiol for estrogen receptors or the protein sequence of the receptors (Katzenellenbogen et al. 1979). Therefore, if estradiol (and potentially xenoestrogens) reaches estrogen receptors in target cells, the capacity to generate responses might not be very different among vertebrate species, although it should be pointed out that the expression of the receptor and its affinity for ligand (e.g., TCDD) binding is not necessarily predictive of responsiveness. Data on the resistant Han/Wistar rat compared with other rats more responsive to Ah clearly illustrate that point in that Ah responsiveness anti Ah receptor levels are similar in both responsive and less-responsive rat strains (Pohjanvirta et al. 1994).
Dose-response assessment of HAAs presents a challenge, because HAAs might cause effects not only by acting as reactive molecules that attack biologic macromolecules, but also by acting as stable molecules interacting with the body's natural signaling systems (Barton and Andersen 1997). Based on a small number of in vivo investigations conducted to date involving a relatively small sample of natural hormones and HAAs, data suggest that in some situations a nonmonotonic dose-response relationship can occur (Kociba et al. 1978: Gray et al. 1989; Halling and Forsberg 1993; vom Saal et al. 1995, 1997; Fan et al. 1996; Calabrese 1997). The issue of the shape of the dose-response curve for HAAs could have implications for toxicity-testing protocols and dose-response assessment methods.
In attempting to establish dose-response relationships, the application of molecular probes as biomarkers for changes in cell processes might make it possible to identify changes linked to contamination, even in populations with relatively low concentrations of the contaminants (see discussion in greater detail in Chapter 11).
Dose-Response Functions and HAAs
For the body of experimental studies on estrogens or other biologically active regulators, a typical monotonic dose response or binding curve is observed: as dose increases, response increases or stays the same (Klaassen et al. 1996).
However, there are numerous examples of nonmonotonic inverted U-shaped dose-response curves from in vitro studies. These studies involve a variety of natural and anthropogenic estrogens (e.g., estradiol, estriol, nonylphenol, and DES), end points (e.g., cell proliferation, prolactin synthesis, and induction of specific mRNAs), and cell lines (e.g., Jordan et al. 1985; Soto et al. 1991; Bigazzi et al. 1992; Pilat et al. 1993; Truss and Beato 1993; Tzukerman et al. 1994: Olea et al. 1996). Sonnenschein et al. (1989) also observed a nonmonotonic response curve for androgen-induced cell proliferation in LNCAP cells by using a diverse group of steroidal and nonsteroidal compounds.break
Page 111
Both U-shaped and inverted U-shaped dose-response curves have been reported in in vivo studies of HAAs. For example, an inverted U-shaped dose-response relationship has been reported to occur with respect to prostate size when mouse fetuses are exposed to either estradiol or DES; prostate weight first increases and then decreases with dose (vom Saal et al. 1997). These investigators previously reported an inverted U-shaped dose-response relationship between maternal dose of DES and territorial marking in male offspring (vom Saal et al. 1995). In the former study, pregnant CF-1 female mice were either implanted with Silastic tubing containing increasing doses of estradiol or fed DES once per day from gestation d 11 to 17. A 50% increase in free serum estradiol in male mouse fetuses induced a 40% increase in the number of developing glandular epithelial ducts emanating from the urethra (these form the glandular ducts within the prostate); subsequently in adulthood, the number of developing prostatic androgen receptors per cell was permanently increased by two-fold, and the prostate was enlarged by 30% (due to hyperplasia) relative to unexposed males. However, when free serum estradiol concentration was increased from two- to eight-fold, adult prostate weight decreased relative to males exposed to the 50% increase in estradiol. Maternal ingestion of DES at 0.02, 0.2, or 2 ng/g of body weight significantly increased adult prostate weight in male offspring, and DES at 200 ng/g of body weight (200 ppb) significantly decreased adult prostate weight (see Figure 4-1). However, in another study to address the low-dose response, a single dose DES treatment (0.2 ng/g of body weight) in the same strain of mice failed to cause an increase in prostate weight (Cagen et al. 1999). The decrease in prostate weight reported by vom Saal et al. (1997) is consistent with numerous prior findings that exposure to a high dose of DES during development results in an abnormally small prostate in adulthood as well as a decrease in prostatic androgen receptors (Prins 1992; Newbold et al. 1994; Santti et al. 1994).
In another study of methoxychlor-induced alterations of reproductive development and function in the rat, a U-shaped dose-response curve was reported (Gray et al. 1989). In this study, rats were dosed from weaning, through puberty and gestation, to d 15 of lactation with methoxychlor at 25. 50, 100, or 200 mg/ kg/d. The onset of cyclicity in female offspring was accelerated in the low-dose group, normal in the two mid-dose groups, and delayed in the high dose group. Other investigators reported that uterine weight as a fraction of body weight following neonatal dosing displayed a nonmonotonic (inverted-U) dose response with DES but not estradiol (Halling and Forsberg 1993). In addition, there are several examples of inverted U-shaped curves showing protective effects at low doses (Calabrese 1997). A recent report by Fan et al. (1996) showed that TCDD induced an inverted U-shaped dose-response curve for its effects on cell-mediated immunity in the rat; at low doses, TCDD enhanced and at high doses TCDD suppressed the delayed-type hypersensitivity (DTH) reaction. Kociba et al. ( 1978) demonstrated that low doses of TCDD at 1 ng/kg/d decreased the rate of sponta-soft
Page 112
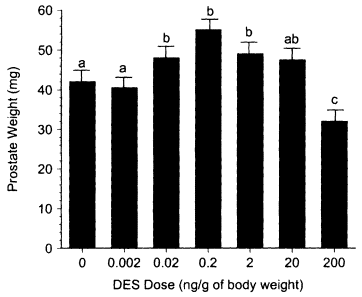
FIGURE 4-1
Mean (+SEM) prostate weight (mg) in 8-mo-old CF-1 male mice
produced by females fed different doses of DES from d 11 to d 17
of pregnancy. Group means that differed significantly are indicated
by different letters, and group means with the same letter did
not differ significantly. Prostate weights presented here were
adjusted for significant effects of DES on body weight by
analysis of covariance (ANCOVA) using the statistical
analysis system (SAS). Source: vom Saal et al. 1997.
neous tumor formation; the rate is then increased at high doses of TCDD (e.g., pituitary adenomas, males; hyperplastic liver nodules, females; and thyroid ad-enomas, females).
At this time, it is not clear how prevalent or important nonmonotonic dose-response relationships are for different types of end points (e.g., malformations vs. a permanent change in enzyme activity) or what the mechanisms might be. As in any cell assay, high doses might cause toxicity and reduce response rates (Mayr et al. 1992). In vivo nonmonotonic dose-response curves can occur as a result of underlying physiologic and toxicologic processes. Feedback processes typically respond to both excesses and deficits of signals. Thus, untoward responses might occur over a broad dose range, covering interference with natural function at low doses and excess stimulation at high doses (Barton and Andersen 1997). Such factors as down-regulation of receptors in the presence of high doses of a hormone might also partially account for this phenomenon (Gorski and Gannon 1976). Other possibilities might involve the following: stimulation ofcontinue
Page 113
response systems that are antagonistic to the initial response as saturation of receptors occurs (Amara and Dannies 1983), induction of metabolism, cross-talk between estrogen and receptors for other steroids as total estrogenic activity in blood exceeds the physiologic range, or a low-dose effect on DNA synthesis increasing cell proliferation, with a secondary effect to increase apoptosis.
In considering the question of U-shaped dose-response curves, it is important to distinguish between nonmonotonic dose-response relationships arising from underlying modes of action and those resulting from difficulties observing an end point at high doses (Barton and Andersen 1997). The latter is particularly important in reproductive and developmental studies (Selevan and Lemasters 1987). For example, developmental toxicity studies often show increases followed by decreases in developmental abnormalities with increasing dose (Lamb 1997). That occurs because fetal mortality increases and masks the developmental effect. Monotonic behavior can also be observed if the time course of the response is altered with dose. Testing over a wider range of doses and using lower doses could address the issue (Martin and Claringbold 1960).
Thresholds
A basic assumption with regard to the dose-response assessment of systemic toxicants is that there is a threshold concentration below which no adverse effect will occur. This default assumption has been challenged for HAAs (vom Saal and Sheehan 1998), because data from the mouse and rat indicate that concentrations of free serum estradiol in fetuses are above threshold for producing responses in estrogen-responsive cells (Nonneman et al. 1992; Montano et al. 1995: vom Saal et al. 1997). In addition, manipulating estrogen (by administering supplemental estrogen, blocking estrogen biosynthesis via aromatase inhibitors, or blocking the binding of estrogen to receptors by antiestrogens) altered fetal development in rodents and birds (Clemens and Gladue 1978; Gladue and Clemens 1980; Adkins-Regan 1983; Takahashi et al. 1989; Lephart et al. 1992; vom Saal et al. 1997). A recent study of sex determination in red-eared slider turtles (Sheehan et al. 1999) provided evidence that regardless of whether a threshold exists for endogenous estrogen, no threshold exists for exogenous estrogen, because endogenous estrogens are at a sufficiently high concentration to exceed the threshold for sex determination. The investigators concluded that "these results provide a simple biologically based dose-response model and suggest that chemicals which act mechanistically like [endogenous estradiol] may also show no threshold dose." Whether the estrogen-response system is already operating above background in all developing animal fetuses and humans is clearly a controversial issue, which has not been unequivocally resolved.
If the threshold for response to estrogen has already been exceeded before exposure to an environmental estrogen, the additional load of an environmental estrogen might cause a significant increase in the occupied receptors required forcontinue
Page 114
the response. For that requirement to occur, one must assume that the exogenous HAA is acting just like the endogenous ligand and, therefore, would be additive to the background effect, and the background dose of an endogenous hormone can be responsible for physiologic and toxicologic responses. However, some compounds have been shown to interact with estrogen receptors differently than estradiol, leading to unique responses (Webb et al. 1995; Yang et al. 1996; Barton and Andersen 1997). The possibility that estrogenic HAAs will show tissue- and life-stage-specific effects that differ from those of natural estrogens within a species will require extensive investigation before this issue is resolved.
Tissue Sensitivity
The issue of sensitivity of tissues to estrogen or other hormones that bind to receptors belonging to the superfamily of intracellular receptors is complex. Tissue sensitivity is influenced by multiple interactions, which include the interaction between the following: the hormone and receptor, the receptor and specific DNA sequences with which it interacts in a tissue (the hormone-responsive elements), the receptor and associated regulatory proteins that modulate responses, and the associated regulatory proteins and genes. It is likely that there are differences among species in each of these interactions that would influence sensitivity to HAAs. Within a species, variability between tissues occurs, and within individual cells, different responses occur at different doses. Finally, the importance of life stage cannot be ignored, particularly with regard to the hypothesis that developing animals show responses to hormones (and thus possibly also to xenobiotics) that are relatively inactive at later times in life (Hajek et al. 1997).
In vitro studies using estrogen-responsive MCF7 breast-cancer cells have investigated the relationship between estradiol concentrations, receptor occupancy, and biologic response (Table 4-4). Estradiol-induced proliferation of MCF7 cells reached half the maximal growth response at approximately 0.54 pg/ mL of medium (Welshons and Jordan 1987). Scatchard plots of binding in intact cells show little deviation from linearity, suggesting that significant cooperativity. which has been extensively described in extracted receptors, is not generally detectable. Consistent with these findings, there was no evidence for cooperativity in the experiments that provided the data in Table 4-4.
At the tissue level, it has been shown that the potency of a particular xenoestrogen can vary for different responses in the same tissues (Hyder et al. 1997). An example in the rat uterus is the inhibition of gland genesis and down-regulation of estrogen receptors, which are responses up to an order of magnitude more sensitive to estrogenic chemicals, such as DES, than is uterine weight gain (Branham et al. 1996). Some anthropogenic estrogens, such as DES, have been shown to have increased potency (up to 100-fold) relative to estradiol in the embryo and fetus (Sheehan and Branham 1987) because of their higher availability due to the higher affinity of estradiol for a-fetoprotein as contrasted to DES.continue
Page 115
| ||||||||||||||||||||||||||||||||||||||||||||||||
The developing rat is unusual in having a serum glycoprotein present that has a high affinity for estradiol and not for other steroids.
Some compounds have been shown to interact with estrogen receptors differently than estradiol, resulting in different responses (Webb et al. 1995; Yang et al. 1996). One of the best-studied examples of tissue specificity of a chemical that interacts with estrogen receptors is tamoxifen, which is an agonist in the mouse uterus, a partial agonist and antagonist in the rat uterus, and an antagonist in the chicken oviduct (Welshons and Jordan 1987; McDonnell et al. 1995). Additionally, a new estrogen receptor (ERß) was recently discovered that has a different tissue distribution and ligand selectivity from the well-described ERa (Kuiper et al. 1996). The estrogen receptors are highly expressed in the prostate and, therefore, might play a role in mediating responses to estrogenic compounds as suggested above.
There are potential explanations for the observations described above other than the type and mixture of ERs in tissues, including differences in transcription factors and estrogen-response elements (Truss and Beato 1993; Starr et al. 1996; Uht et al. 1997). Taken together, these findings suggest that the use of a single end point is inadequate to characterize sensitivity of tissues to HAAs. Rather, the most sensitive and relevant responses and adverse effects at different life stages, in different tissues, and in different species need to be defined, recognizing that for wildlife a relevant end point might be population change.
Summary and Conclusions
The risks associated with exposure to an HAA depend upon many factors related to pharmacokinetics and pharmacodynamics. The chemical and physicalcontinue
Page 116
properties of the HAA and the nature of the species exposed determine whether a chemical will accumulate to a biologically significant dose and cause harm. In the case of HAAs, the hydrophobicity of the compound and the lipid concentration of the tissues are of particular importance. Rates of uptake and elimination, and thus half-lives, vary greatly for different HAAs in different species, and are influenced by the dose and duration of exposure. It is also important to consider the time that exposure occurs during an organism's life span. There are critical periods, such as fetal development, when organisms are more sensitive to xenobiotics.
Organisms can be exposed to environmental HAAs in a variety of ways. usually via the gut, skin, or respiratory surfaces. Contaminant concentrations increase up the levels of the food chain, and longer-lived animals accumulate greater concentrations of contaminants in their tissues. Of particular concern is the developing fetus, which can be particularly sensitive to toxic chemicals transferred transplacentally by the mother. Environmental contaminants have been found in human breast milk and in bovine milk, so these media are also possible routes of exposure.
Blood is the central medium of distribution of chemicals to target organs and cells in the body. Although distribution to different tissues is important for interpreting toxicity, comparing the effects of some compounds on the basis of the total body burden is also important, because it could reveal similarities not evident when concentrations in individual tissues are used to compare responses among species. The bioavailability of an HAA is influenced by its association with plasma-binding proteins, which affect its passage into tissues. Once inside a target cell, a steroid presumably becomes available for binding to specific intracellular receptors. Additionally, binding of HAAs to human steroid-binding proteins can displace endogenous hormones, possibly affecting hormone delivery to target cells. The proteins that transfer chemicals or hormones to or within the fetus are particularly important, because studies have shown that the biologically active concentration of steroid present during fetal life can be misinterpreted if based upon total steroid concentration in the blood.
The principal target organs for HAAs are those that produce, regulate, or respond to hormones or their action. However, there is inadequate information on the concentrations and pharmacokinetics of HAAs in many of these organs In humans and wildlife. There is evidence that biologic barriers, such as the blood-brain barrier and the placenta, which normally protect the brain and developing fetus, respectively, from hydrophilic compounds, do not inhibit the transport of lipophilic HAAs.
There are few studies with target-cell measurements. Molecular changes can indicate uptake in particular targets, and cellular localization of proteins involved in HAA effects could help to identify sensitive target cells. Some fatty tissues accumulate HAAs and can serve as depots for these chemicals. Deposition into eggs or breast milk can occur, thus exposing the fetus or newborn to greater concentrations of HAAs.break
Page 117
Virtually all HAAs are metabolized through a variety of oxidative and reductive or conjugation reactions. The processes involved in metabolism are complex, and a consideration of multiple mechanisms by which HAAs and hormones might act is crucial to evaluating the risk associated with exposure to these compounds. Metabolism can inactivate an HAA or lead to the activation of a hormonally-active metabolite from a non-hormonally active parent compound. The capacity of the enzymes involved in steroid metabolism, such as the cytochrome P450 enzymes, can be modified as a result of exposure to HAAs. The complement of enzymes that metabolize HAAs might strongly influence the susceptibility of the cell to the effects of these compounds. For example, if an HAA requires metabolic action to be estrogenic, then the cells lacking the requisite enzymes are less likely to be susceptible to the estrogenic action of that chemical than are cells that are metabolically active. The expression of cytochrome P450 enzymes during embryonic, larval, or fetal development might be crucial to the developmental effects of exposure to HAAs. However, at this time, there are few data on what forms of these enzymes are expressed at different developmental stages or what their functions are.
There are significant species differences in response to exposure to HAAs, and at this time, it is difficult to extrapolate data from one species to another. Defining structure-activity relationships for substrate binding to homologous enzymes in different species will help to achieve generalization.
Dose-response assessment for HAAs presents a challenge, because HAAs might cause effects not only by acting as reactive molecules that attack biologic macromolecules but also by acting as stable molecules interacting with the body's natural signaling systems. On the basis of a few in vivo investigations conducted to date involving a small number of HAAs, data suggest that in some situations a nonmonotonic dose-response relationship can occur. The issue of the shape of the dose-response curve for HAAs could have implications for toxicity-testing protocols and dose-response assessment methods.
Recommendations
On the basis of its evaluation on the available data on dosimetry, the committee recommends the following:
· For chemicals having certain detrimental effects, studies should be designed to distinguish between direct and indirect effects and between primary and secondary effects of HAAs, and their underlying mechanisms of action should be investigated using both in vitro and in vivo assays that could detect diverse responses.
· Species- and tissue-specific effects of exposure to environmental HAAs need to be investigated further to establish the validity of extrapolation between species.break
Page 118
· In vivo test systems for HAAs should be used to assess the consequences of prenatal and postnatal exposure on developmentally critical or sensitive processes at concentrations commonly found in the environment.
· Dose-response characteristics of recognized actions of various HAAs should he further investigated in in vitro and in vivo studies at concentrations commonly found in the environment.break





































