ARTHUR CLAY COPE
June 27, 1909-June 4, 1966

BY JOHN D. ROBERTS AND JOHN C. SHEEHAN
ARTHUR CLAY COPE, an extraordinarily influential and imaginative organic chemist, was born on June 27, 1909, and died on June 4, 1966. He was the son of Everett Claire Cope and Jennie (Compton) Cope, who lived in Dunreith, Indiana, but later moved to Indianapolis to enhance their son's educational possibilities. Everett Cope was in the grain storage business and his wife worked for some time at the local YWCA office.
In 1929 Arthur received the bachelor's degree in chemistry from Butler University in Indianapolis, then, with the support of a teaching assistantship, moved to the University of Wisconsin for graduate work.
His thesis advisor at Wisconsin was S. M. McElvain, whose research program included the synthesis of organic compounds with possible pharmaceutical uses—especially local anesthetics and barbiturates. Cope's thesis work, completed in 1932, was along these lines. It led to the discovery of a useful local anesthetic and provided the major theme of his research for many years.
Cope clearly made a strong impression at Wisconsin during his graduate career. He completed his thesis work and three independent publications in three years and was recommended by the Wisconsin organic chemistry faculty (then
headed by the redoubtable Homer Adkins) for one of the highly sought-after National Research Council Fellowships at Harvard. In 1933, he moved to Harvard to work under one of the leading organic chemists of the day, E. P. Kohler.
At the end of his first year of graduate work Cope married intelligent, articulate, and forceful Bernice Mead Abbott, who had also attended Butler University and had met Cope as the teaching assistant in her freshman chemistry course. Bernice, known as ''B,'' exerted a strong influence over his early career. The Harvard period was a productive one for Cope, and the papers he published on diverse subjects reflect a general Kohler influence.
BRYN MAWR (1934-1940)
In 1934, Cope accepted his first academic position, associate in chemistry at Bryn Mawr College. Bryn Mawr was to some degree isolated from the mainstream of organic research of the period, but Cope—looking for a position in the depth of the Great Depression—could hope to follow in the footsteps of the famous Louis Frederick Fieser, who had made it in one jump from Bryn Mawr to Harvard. Bryn Mawr, in any case, had a Ph.D. program, and Cope had many friends in the field with whom he kept in contact through attending meetings and symposia. After spending a summer at the University of Illinois as an assistant professor in 1935, he was promoted to the same position at Bryn Mawr and to associate professor in 1938.
While at Bryn Mawr, Cope spent some time trying to determine the structure of Grignard reagents by precipitation procedures. Comparable approaches had been tried by a number of highly competent organic chemists with little success. Realizing the intractability of this approach, he began to concentrate on the theme of his thesis work—the synthesis of substances with potential pharmaceutical interest.
For many, this would be pedestrian chemistry—using ex-
isting procedures to synthesize easy-to-prepare compounds. But Cope directed his attention to developing new synthetic reactions for substituted barbiturates and novel aminoalcohol local anesthetics.
Bryn Mawr supplied Cope with two outstanding graduate students: Evelyn Hancock and Elizabeth Hardy, who coauthored almost half the papers arising from his stay there. He also received substantial interest and support from the Sharpe and Dohme Laboratories in Philadelphia, where he eventually accepted a consultantship.
One result of Cope's Bryn Mawr period was the development of a commercial barbiturate known as Delvinyl Sodium. More important for organic chemistry as a whole was his discovery of a facile thermal rearrangement from one carbon to another in a three-carbon system of an allyl group. The well-known "Claisen allyl-ether rearrangement" involves an analogous shift of an allyl group, but the unique carbonto-carbon feature—and the clarity with which Cope detailed the process—caused this kind of reaction to become generally known as the "Cope rearrangement." In recent years, variations on it have become extremely important, and thoroughly investigated, key steps in the synthesis of complex natural products.
In 1939, Cope's career was given a boost through his election as secretary of the Organic Division of the American Chemical Society. Though his wife, B, helped him, it was tedious and in many ways a thankless job. Nonetheless, through it he developed many contacts and friends.
In 1940-41, Cope received a Guggenheim Fellowship but was not able to visit Europe because of the war. He spent part of the year doing research at Bryn Mawr and the rest visiting organic chemistry research groups at universities throughout the United States.
In 1941 he moved to Columbia University as associate professor, and when World War II started, joined the Office
of Scientific Research and Development as technical aide and section chief of Division 9 of the National Research Council. He was responsible for projects ranging from chemical warfare agents and insect repellents to antimalarial drugs and, in 1946, received the Certificate of Merit for his contributions to the war effort.
MASSACHUSETTS INSTITUTE OF TECHNOLOGY (1945-1966)
Cope's stay at Columbia was relatively brief. The Massachusetts Institute of Technology had been an early leader in American chemistry but had become heavily inbred. It needed a fresh approach, particularly in organic chemistry, and Karl Compton, then president of MIT, followed the strong recommendation of the University of Illinois' Roger Adams and offered Arthur Cope the job. In 1945 he came to MIT to head the Department of Chemistry.
By that time Cope had an outstanding research record and in 1944 had received the coveted American Chemical Society Award in Pure Chemistry for his discovery of the "Cope rearrangement." He was elected to the National Academy of Sciences in 1947.
Although eager to continue on into new fields of research, he was thoroughly cognizant of the need to revitalize the department at MIT, world-class at that time only in physical chemistry. As head of the department, he had substantial powers, particularly in the area of new appointments, and some felt he used those powers ruthlessly.
When Cope later became president of the American Chemical Society, he was described as "mild-mannered"; "outwardly mild-mannered" would have been more accurate. He was easy to underestimate: of average height, long-faced, usually with a pallor bespeaking an indoor life (although he was an avid skier at one point and—with Adkins, Marvel, and a few others—participated in an annual muskellunge fishing
expedition), he was outwardly courteous and almost effetely affable. Yet this even façade covered a strong and hot-blooded temperament. In meetings where things were not going his way, he tended to speak more softly and slump deeper into his chair, and those who knew him soon learned these danger signals. His graduate students called him the "iron fist in the velvet glove."
Cope was given strong support by the MIT administration during the early years. The teaching laboratories were completely rebuilt to a very high standard, and the research facilities for organic, inorganic, and analytical chemistry became as good as, or better than, any other in the world. Among the new professorial faculty in 1945-46 were Charles D. Coryell from Caltech via UCLA and Oak Ridge, John C. Sheehan from Michigan via Merck, John D. Roberts from UCLA via Harvard, Gardner C. Swain from Harvard via Caltech, David N. Hume from Minnesota via Kansas and Oak Ridge, Lockhart B. Rogers from Princeton via Stanford, Richard C. Lord from Johns Hopkins, and David Shoemaker from Caltech.
This influx of first-rank new professors produced almost inevitable and long-lasting strains for many of the preexisting faculty in the MIT Chemistry Department. But—along with new facilities and Cope's untiring efforts to get research support and the highest quality of graduate students and postdoctoral fellows—the effect on the Department's research productivity was both immediate and awe-inspiring.
SCIENTIFIC WORK
Cycloöctatetraene
During his war work, Cope had encountered an intriguing intelligence report from Germany regarding the use of cycloöctatetraene to treat mustard-gas poisoning. In 1911,
the German chemist Richard Willstätter had published an arduous, multistep synthesis for this highly interesting cyclic polyolefin derived from a rare alkaloid occurring in small amounts in the bark of the pomegranate tree. Though the structure of cycloöctatetraene was seemingly well-documented, the resemblance of one of its transformation products to a derivative of styrene, the well-known isomeric substance, brought it into question in later years.
With fragmentary reports trickling in from Germany on the extraordinary properties of cycloöctatetraene (which Walter Reppe's group had prepared in one step by the tetramerization of acetylene), Cope determined to repeat the Willstätter synthesis. Having very little pomegranate bark, he developed an efficient synthesis of the alkaloid it contains, then proceeded to substantiate Willstätter's synthesis in all respects. Extending the Reppe process with mixtures of substituted acetylenes, he also devised alternative syntheses of both cycloöctatetraene and its derivatives. This massive effort led Cope and his coworkers into studies of the chemistry of medium-sized ring compounds.
Transannular Hydrogen Migration and Valence Tautomerism
One hallmark of Cope's research was meticulousness born of an almost morbid fear that some erroneous experimental result would be published under his name. At least once, this fear cost him priority for an important scientific discovery.
Cope and his students detected a most unusual rearrangement reaction, wherein a hydrogen on one side of a cycloöctane ring migrated directly across the ring to the other side through a carbocationic intermediate. Because one of his physical-organic colleagues was skeptical of the evidence for the initial observation, Cope spent another year verifying every detail while others, having heard of the results, exploited them in related systems. Investigation of transannu-
lar hydrogen migration, in fact, became an important theme in Cope's later research.
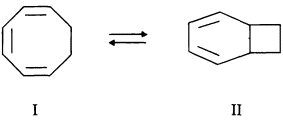
Much of Cope's work lapped over into physical organic chemistry, in which he repeatedly disavowed interest or competence while, at the same time, publishing perceptive observations that led to whole new areas of physical organic endeavor. One significant example is "valence tautomerism," where a compound isomerizes reversibly without intervention of external agents (except heat) by processes in which bonds are broken and made, usually simultaneously. What Cope and his coworkers discovered was the change of 1,3,5-cycloöctatriene (I) into bicyclo[4.2.0]-2,4-octadiene (II) at temperatures of 80-100°C.
Many chemists working intensively on processes of this type led eventually to the discovery and understanding of many other extraordinary examples. In 1981, R. Hoffmann received the Nobel Prize for the "Woodward-Hoffmann rules" predicting the ease and stereochemical consequences of such rearrangements. Valence tautomerism turned out to be a most important concept in understanding the chemistry of cycloöctatetraene and its derivatives.
Optical Isomers
One of the last important achievements of Cope's research program was his ingenious resolution of the optical isomers of trans-cycloöctene and trans-cyclononene. Using trans-cyclo-
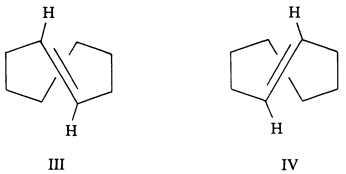
öctene as an example, there are two chiral forms (III and IV) that are nonidentical, mirror-image isomers. It turns out that if the cycloöctene double bond can turn over with its attached hydrogens through the loop of connected carbon atoms, then III will be converted into IV. The loop of carbons, however, is too tight for this to happen easily with III and IV. With the larger cyclononene ring, the interconversion is more easily possible, and the forms that correspond to III and IV are not very stable. With the still larger ring of trans-cyclodecene, interconversion is very easy and resolution extremely difficult, even at low temperatures. The possibility of stable chiral forms such as III and IV had been recognized long before, but it took imagination and enormous skill to demonstrate their existence.
SERVICE TO THE SCIENTIFIC COMMUNITY
After the war, Cope began to expand his service to chemistry on the national scene. In 1945, he was appointed to the editorial board of Organic Syntheses, a highly influential annual publication of tested laboratory procedures for the preparation of important organic compounds not yet available from commercial sources.
Through this group Cope became closely associated with Roger Adams, then the "Pope" of American organic chem-
istry. For many years head of chemistry at the University of Illinois and a consultant to the Du Pont Company, Adams had founded Organic Syntheses. He also had strong ties to the American Chemical Society, of which he would become president and chairman of the Board. In Cope, Adams recognized a comer, and Cope was in many ways Adams' successor. In 1947, Cope, also a consultant to Du Pont, joined the group that Adams had initiated to edit reviews in the influential series, Organic Reactions. He was the series' editor-in-chief from 1960 to 1966.
Cope served the American Chemical Society with great distinction. The posts he held after the war included chairman of the Division of Organic Chemistry (1946-1947); councillor (1950-1951); Northeastern Section chairman (1955-1956); Board of Directors (1951-1966); president-elect and president (1960-1961); chairman of the Board (1959-1960, 1962-1966); and a plethora of Board committees, including nine years on the important Committee on Professional Training.
Among other accomplishments as an American Chemical Society leader, Arthur Cope had much to do with averting collapse of Chemical Abstracts around 1960, a time when chemical abstracting services were failing. He served, in addition, as chairman of the National Academy of Sciences' Chemistry Section and was a member of the Academy's Committee on Science and Public Policy. A colleague described him during this period as the "busiest organic chemist in the world."
The cumulative strain of these widespread activities proved too much for Cope's marriage, and he and B (no children) were divorced in 1963. He later married Harriet Thomas Packard, née Osgood, who had been his secretary at MIT, and in the process acquired a stepson, Gregory.
Single-minded about the importance of chemistry education, Arthur Cope was not one to compromise his prin-
ciples concerning thoroughness and breadth—principles only reinforced by his service on the ACS Committee on Professional Training. He resisted strongly MIT's decision to reduce the chemistry requirement in the undergraduate curriculum. This, along with a certain perception among the faculty that many of his decisions were arbitrary, led to the end of his almost twenty-year tenure as head of the Chemistry Department, but he remained active in research and in service to the American Chemical Society.
HONORS AND AWARDS
Arthur Cope received many honors and awards for his achievements in research. In addition to those already mentioned, he was elected to the American Academy of Arts and Sciences and the American Philosophical Society. He received the Chandler Medal of Columbia University, the W. H. Nichols Medal of the New York Section of the American Chemical Society, the Roger Adams Medal and Award of the American Chemical Society, and an honorary Sc.D. from his alma mater, Butler University.
With royalties from his pharmaceutical patents augmented by successful investments, Cope died a relatively wealthy man. He left half of his estate to the American Chemical Society to stimulate research in organic chemistry through the Cope Awards. The fund's current income of approximately $200,000 supports one major award and ten smaller Cope Scholar Awards. The principal Cope Award carries with it a medal, a substantial cash prize for the recipient, and a larger cash award in support of research at an institution of the recipient's choice.
The epitome of the workaholic, Arthur Cope was passionately and selflessly devoted to the public service of chemistry. In his own work, he was rigidly self-critical, adhering to the highest standards for scientific integrity. It is chemistry's
tragic loss that he died suddenly at the age of fifty-six in Washington, D.C., where he had gone for American Chemical Society and National Academy of Sciences business. One could say of Arthur Cope what used to be said of Dodge automobiles—"They don't make 'em like that anymore."
WE WISH TO THANK Dr. Robert M. Joyce, Dr. Blaine C. McKusick, and Mrs. B. Abbott Cope, who provided valuable information and help with this memoir.
SELECTED BIBLIOGRAPHY1
1931 With S. M. McElvain. N-methyl-N-phenylalkyl-amino-alkyl benzoates and para-aminobenzoates. J. Am. Chem. Soc. 53: 1587-94.
1932 The cleavage of disubstituted malonic esters by sodium ethoxide. J. Am. Chem. Soc. 54: 4319-25.
1934 The mechanism of the reaction of dimethyl sulfate with arylmagnesium halides. J. Am. Chem. Soc. 56: 1578-81.
1937 Condensation reactions. I. The condensation of ketones with cyanoacetic esters and the mechanism of the Knoevenagel reaction J. Am. Chem. Soc. 59: 2327-30.
1938 The precipitation of phenylmagnesium bromide by pyridine and by dioxane. J. Am. Chem. Soc. 60: 2215-17.
With E. M. Hancock. The introduction of substituted vinyl groups. I. Isopropenyl alkyl malonic esters. J. Am. Chem. Soc. 60: 2644-47.
1939 With E. M. Hancock. Substituted vinyl barbituric acids. I. Isopropenyl derivatives. J. Am. Chem. Soc. 61: 96-98.
1940 With E. M. Hardy. The introduction of substituted vinyl groups. V. A rearrangement involving the migration of an allyl group in a three-carbon system. J. Am. Chem. Soc. 62: 441-44.
1941 With K. E. Hoyle and D. Heyl. The rearrangement of allyl groups in three-carbon systems. I. J. Am. Chem. Soc. 63: 1843-52.
With C. M. Hoffman and E. M. Hardy. The rearrangement of allyl groups in three-carbon systems. II. J. Am. Chem. Soc. 63: 1852-57.
1944 With E. M. Hancock. Benzoates, p-aminobenzoates and phenylurethans of 2-alkylaminoethanols. J. Am. Chem. Soc. 66: 1448-53.
With R. Kleinschmidt. Rearrangement of allyl groups in dyad systems. Amine oxides. J. Am. Chem. Soc. 66: 1929-33.
1948 With C. G. Overberger. Cyclic polyolefins. I. Synthesis of cycloöctatetraene from pseudopelletierine. J. Am. Chem. Soc. 70: 1433-37.
With W. J. Bailey. Cyclic polyolefins. II. Synthesis of cycloöctatetraene from chloroprene. J. Am. Chem. Soc. 70: 2305-9.
1950 With M. R. Kinter. Substituted cycloöctatetraenes. J. Am. Chem. Soc. 72: 630-31.
1952 With M. Burg. Cyclic polyolefins. XIX. Chloro- and bromo-cycloöctatetraenes. J. Am. Chem. Soc. 74: 168-72.
With A. C. Haven, Jr., F. L. Ramp, and E. R. Trumbull. Cyclic polyolefins. XXIII. Valence tautomerism of 1,3,5-cycloöctatriene and bicyclo[4.2.0]octa-2, 4-diene. J. Am. Chem. Soc. 74: 4867-71.
With S. W. Fenton and C. F. Spencer. Cyclic polyolefins. XXV. Cycloöctanediols. Molecular rearrangement of cycloöctene oxide on solvolysis. J. Am. Chem. Soc. 74: 5884-88.
1957 With C. L. Baumgardner. Amine oxides. I. 1,4-pentadiene, 3-phenylpropene and 3-phenylcyclohexene by amine oxide pyrolysis. J. Am. Chem. Soc. 79: 960-64.
With A. Fournier, Jr., and H. E. Simmons, Jr. Proximity effects. IX. Solvolysis of trans-cycloöctene oxide. J. Am. Chem. Soc. 79: 3905-9.
1960 With G. A. Berchtold, P. E. Peterson, and S. H. Sharman. Proximity effects. XXI. Establishment of 1,3- and 1,5-hydride shifts in the solvolysis of cis-cycloöctene oxide. J Am. Chem. Soc. 82: 6366-69.
1963 With C. R. Ganellin, H. W. Johnson, Jr., T. V. Van Auken, and H. J. S. Winkler. Molecular asymmetry of olefins. I. Resolution of trans-cycloöctene. J. Am. Chem. Soc. 85: 3276-79.
With D. M. Gale. Proximity effects. XXXVI. Solvolysis of deuterium-labeled cycloöctyl brosylate. J. Am. Chem. Soc. 85: 3747-52.
1965 With K. Banholzer, H. Keller, B. A. Pauson, J. J. Whang and H. J. S. Winkler. Molecular asymmetry of olefins. III. Optical stability of trans-cyclononene and trans-cyclodecene. J. Am. Chem. Soc. 87: 3644-49.
With B. A. Pauson. Molecular asymmetry of olefins. IV. Kinetics of racemization of (+ or -)-trans-cycloöctene. J. Am. Chem. Soc. 87: 3649-51.
1966 With M. M. Martin and M. A. McKervey. Transannular reactions in medium-sized rings. Q. Rev. 20: 119-52.
1968 With G. M. Whitesides and B. A. Pauson. Hindered rotation in substituted paracyclophenanes. J. Am. Chem. Soc. 90: 639-44.
















