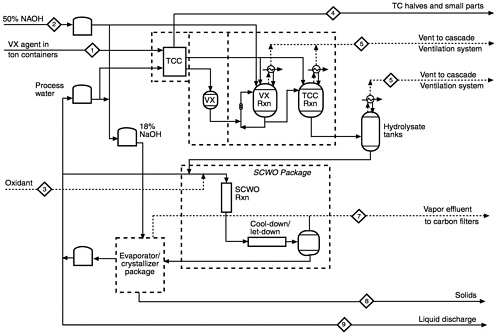
TABLE B-1 NECDF Inputs for Process Mass Balance (stream numbers correspond to streams identified in Figure B-1 )
|
Process Inputs |
||||||
|
Description |
Agent in Ton Containers |
50% NaOH Solution |
Oxidant to SCWO |
Nitrogen to Neutralization Vessels |
Process Air to TCC |
Total Inputs |
|
Stream Number |
1 |
2 |
3 |
N/A |
N/A |
N/A |
|
Total Flow (kg/1,000 kg agent) |
1,000 |
1,195 |
3,500 |
7 |
5,667 |
11,369 |
|
Physical State |
liquid |
liquid |
vapor |
vapor |
vapor |
|
|
temperature (°F) |
70 |
80 |
70 |
70 |
70 |
|
|
pressure (psig) |
14.7 |
14.7 |
3,500 |
17.7 |
100 |
|
|
Vapor Components |
||||||
|
oxygen (kg/1,000 kg agent) |
3,500 |
1,190 |
4,690 |
|||
|
nitrogen (kg/1,000 kg agent) |
7 |
4,477 |
4,484 |
|||
|
Liquid Components |
||||||
|
VX (kg/1,000 kg agent) |
957 |
957 |
||||
|
agent impurities (kg/1,000 kg agent) |
44 |
44 |
||||
|
NaOH (kg/1,000 kg agent) |
598 |
598 |
||||
|
Water (kg/1,000 kg agent) |
598 |
598 |
||||
|
Solid Components |
||||||
|
TC shells (no./1,000 kg agent) |
1.5 |
1.5 |
||||
|
TC valves (no./1,000 kg agent) |
2.9 |
2.9 |
||||
|
TC plugs (no./1,000 kg agent) |
8.8 |
8.8 |
||||
|
Source: Adapted from Stone and Webster, 1997b. |
||||||
TABLE B-2 NECDF Outputs for Process Mass Balance (stream numbers correspond to streams identified in Figure B-1)
|
Process Outputs |
||||||||
|
Description |
Ton Container Parts |
TCC Air Dry Vent |
Neutralization Reactors Vent |
Hydrolysate Tanks Vent |
SCWO Vapor Effluent |
Salt Solids to Disposal |
Discharge to STP |
Total Outputs |
|
Stream Number |
4 |
N/A |
5 |
6 |
7 |
8 |
9 |
|
|
Total Flow (kg/1,000 kg agent) |
5,667 |
6 |
1 |
3,121 |
1,531 |
1,043 |
11,369 |
|
|
Physical State |
solid |
vapor |
vapor |
vapor |
vapor |
solid |
liquid |
|
|
temperature (°F) |
46 |
40 |
80 |
95 |
100 |
|||
|
pressure (psig) |
18 |
21 |
15 |
15 |
15 |
|||
|
Vapor Components |
||||||||
|
oxygen (kg/1,000 kg agent) |
1,190 |
1,803 |
2,993 |
|||||
|
nitrogen (kg/1,000 kg agent) |
4,477 |
6 |
0.3 |
4,483.3 |
||||
|
VOCs (kg/1,000 kg agent) |
0.4 |
1,165 |
1,165.4 |
|||||
|
carbon dioxide (kg/1,000 kg agent) |
82 |
82 |
||||||
|
nitrous oxide (kg/1,000 kg agent) |
0.1 |
0.1 |
||||||
|
inorganic impurities (kg/1,000 kg agent) |
10 |
10 |
||||||
|
Solid/Liquid Components |
||||||||
|
Na2SO4 (kg/1,000 kg agent) |
70 |
451 |
1,043 |
1,574 |
||||
|
Na2HPO4 water (kg/1,000 kg agent) |
530 |
530 |
||||||
|
TOC (kg/1,000 kg agent) |
0.1 |
0.1 |
||||||
|
inorganic impurities (kg/1,000 kg agent) |
10 |
10 |
||||||
|
TC shells (no./1,000 kg agent) |
1.5 |
1.5 |
||||||
|
TC valves (no./1,000 kg agent) |
2.9 |
2.9 |
||||||
|
TC plugs (no./1,000 kg agent) |
8.8 |
8.8 |
||||||
|
Source: Adapted from Stone and Webster, 1997b. |
||||||||
TABLE B-3 Water Formation Resulting from the Neutralization of VX and Oxidation of the Hydrolysate during the NECDF Process
|
Water is formed in the neutralization supercritical water oxidation (SCWO), and pre-evaporation pH adjustment processes. The following illustrates this water formation by showing that elemental hydrogen enters the process in water and other compounds but exits the process mostly as water. |
||
|
The elemental hydrogen inputs to process are for 1,000 kg of agents. |
||
|
Stream |
Constituents |
Hydrogen Content |
|
Agent |
1,000 kg VX |
98 kg |
|
50% NaOH solution |
598 kg NaOH 598 kg H2O |
15 kg 67 kg |
|
Total |
2,195 kg |
180 kg |
|
Notes: For simplification, the agent is assumed to be pure VX, with a molecular weight of 267 and a molecular formula of C11H26O2NPS. The 50% NaOH solution flow shown here includes all caustic to the process, both for neutralization and for post-treatment. |
||
The only product other than water that contains hydrogen is the phosphate salt in stream number 8, Figure B-1, salt solids to disposal, which contains
530 kg Na2HPO4/1,000 kg of agent = 4 kg H/1,000 kg of agent
The balance of hydrogen:
180 - 4 = 176 kg H/1,000 kg of agent
is reacted with oxygen present throughout the process to form water.
The resulting water is found in the following output streams (per 1,000 kg of agent).
|
Stream |
Water Content |
Hydrogen Content |
|
SCWO vapor effluent |
70 kg H2O |
8 kg |
|
Salt solids to disposal |
461 kg H2O |
51 kg |
|
Discharge to STP |
1,043 kg H2O |
116 kg |
|
Total |
1,574 kg H2O |
175 kg |
|
The total amount of water formed in the process is water out minus water in. |
||
|
1,574 - 598 976 kg H2O/1,000 kg of agent |
||
|
Source: Stone and Webster, 1997b |
||