Appendix D
ACUTE TOXICITY OF HYDROGEN CHLORIDE
BACKGROUND INFORMATION
HYDROGEN chloride (HCl) is a colorless, corrosive gas with a pungent, suffocating odor. It is highly soluble in water, forming hydrochloric acid. HCl can occur in gaseous and aerosol forms in the atmosphere, and its partitioning between the two is dependent on temperature and humidity (Sebacher et al. 1980). Lower ambient temperature and higher relative humidity in the atmosphere favor aerosol formation (Sebacher et al. 1980). The gas-liquid equilibrium is also affected by available droplet nucleation sites, which are plentiful in rocket-exhaust clouds. Inhaled HCl in contact with moisture in the upper respiratory tract is expected to rapidly disassociate because of its high water solubility.
PHYSICAL AND CHEMICAL PROPERTIES
|
CAS No.: |
7647-01-0 |
|
Molecular formula: |
HCl |
|
Molecular weight: |
36.47 |
|
Chemical name: |
Hydrogen chloride |
|
Synonyms: |
Muriatic acid, spirits of salt, chlorohydric acid, hydrochloric acid gas |
|
Physical state: |
Gas |
|
Boiling point: |
-84.9°C |
|
Melting point: |
-144.8°C |
|
Vapor density: |
1.26 (air = 1.0) |
|
Vapor pressure: |
40 mm Hg at 17.8°C |
|
Solubility: |
Highly soluble in water, forming hydrochloric acid (82.3 g/100 g of water at 0°C) |
|
Color: |
Colorless as a gas |
|
Conversion factors |
1 ppm = 1.49 mg/m3 at 25°C, 1 atm: |
|
1 mg/m3 = 0.671 ppm |
|
SOURCES AND OCCURRENCE
HCl can be produced by several methods. The majority (90%) of the HCl produced in the United States is a by-product of various chlorination processes (Hisham and Bommaraju 1995). Lesser amounts (8%) are produced directly from hydrogen (H2) and chlorine (Cl2). Combustion of chlorine-containing organic compounds results in the formation of HCl. Average HCl concentrations in combustion flue gas have been reported as high as 3,030 ppm (Sebacher et al. 1980). HCl is also found naturally in volcanic gases particularly in Mexico and South America and might have been one of the gases in the original atmosphere of the earth (Hisham and Bommaraju 1995). HCl from sea salt is the main source of tropospheric HCl from natural sources (Symonds et al. 1988). Combustion of fossil fuels (especially coal) is the most common anthropogenic source of ambient HCl concentrations, which have been measured in the range of 0.5-7.6 ppb (Kamrin 1992). In areas near sources of HCl from combustion, Kamrin (1992) estimated the maximum HCl concentrations to be in the range of 20-30 ppb.
HCl is formed during the combustion of rocket propellants containing ammonium perchlorate (NH4Cl04). The major combustion reaction producing HCl is
CnHm + NH4Cl04 → CO + CO2 + HCl + N2 + H2 + H2O.
The HCl concentration in the exit plane of a solid propellant rocket using ammonium perchlorate as an oxidizer was calculated to be 18.3119.41 grams (g)/100 g of propellant burned for three types of rockets (Bennett 1996). Pellett et al. (1983) reported that the exhaust from a space-shuttle launch using a solid rocket fuel contained 60 tons of HCl. The total range of peak HCl concentrations measured in eight Titan III
rocket altitude-stabilized exhaust clouds was 25-0.5 ppm (for 3-300 min) (Pellett et al. 1983). Partitioning studies indicated that total hydrochloric acid produced by a U.S. space-shuttle launch was predominantly an aerosol, but due to the rapid dissipation of the aerosol, the gas phase was the predominant state within several minutes of the launch (Sebacher et al. 1980). At equilibrium under unpolluted tropospheric concentrations of HCl (less than 1 ppb), the gas phase will predominate except at high (more than 90%) humidity (Sebacher et al. 1980).
PHARMACOKINETICS AND METABOLISM
Due to its high solubility, HCl rapidly forms hydrochloric acid when it comes in contact with water. HCl dissociates in water to form hydronium ions (H30+) that can interact with tissue elements, resulting in cell injury or death (Perry et al. 1994). The predominant effects of inhaled HCl are due to local tissue contact, particularly in the upper respiratory tract. Due to the high reactivity of HCl with the upper respiratory tract, significant systemic exposure to HCl is unlikely. Absorption of substantial amounts of inhaled HCl might decrease blood pH; however, such an effect has not been observed even at high exposure concentrations. Kaplan et al. (1988) exposed baboons to HCl at 500, 5,000, or 10,000 ppm and measured blood pH. Blood pH was transiently reduced at the highest exposure concentration, but the differences were not statistically significant and were most likely due to alterations in blood gas levels. Ammonia in exhaled air increased in men and women volunteers who inhaled HCl at 0.8 or 1.8 ppm (Stevens et al. 1992). The increase in ammonia might indicate a mechanism for neutralization of HCl in the upper airways.
Pharmacokinetic studies with HCl have not been conducted, but hydrogen and chloride ions are involved in normal physiological processes. Hydrochloric acid is an important normal constituent of gastric juice. Humans ingesting 50 millimoles (mmol) per day of hydrochloric acid for 4 days had reduced blood and urinary urea and an increase in excretion of ammonia (Fine et al. 1977). Intravenous infusion of 0.15 molarity hydrochloric acid into rats (50 milliliters per kilogram (mL/kg) of body weight per hour) and dogs (20 mL/kg of body weight per hour) increased urinary chloride excretion (Kotchen et al. 1980).
SUMMARY OF TOXICITY INFORMATION
EFFECTS IN HUMANS
One-Time Exposure
The National Research Council reviewed the toxicological effects of HCl in humans (NRC 1987, 1991). The reports concluded that exposure to irritating concentrations of HCl can result in coughing, pain, inflammation, edema, and desquamation in the upper respiratory tract. Acute exposure to high concentrations might produce constriction of the larynx and bronchi and closure of the glottis. Because HCl is highly irritating to the mucosal surfaces of the respiratory tract and the eyes, it has good warning properties.
Henderson and Haggard (1943) summarized information from several sources related to how long various exposure concentrations of HCl could be tolerated by healthy workers and what effects might occur (Table D-1). Matt (1889) stated in his doctoral dissertation that work is impossible when one inhales air containing HCl in concentrations of 50 to 100 ppm; work is difficult but possible when the air contains concentrations of 10 to 50 ppm; and work is undisturbed at the concentration of 10 ppm. However, the exposure protocol used by Matt (1889) included only two individuals and three exposure concentrations. Each individual was exposed once to HCl at 10 ppm (10 min), 70 ppm (15 min), and 100 ppm (15 min). When exposed at 70 ppm, the individuals left the exposure chamber once briefly during the 15-min period, and when exposed at 100 ppm, they left several times because of their acute discomfort. During exposure at the high concentrations, the individuals experienced coughing, increased breathing frequency, and strong irritation of the throat and respiratory tract. Matt (1889) included in his report a description by another investigator of an additional volunteer exposed to HCl at 50 ppm for 13 min. Heyroth (1963) indicated in an editorial note that it was his opinion that most people can detect HCl in the air at 1-5 ppm and that 5-10 ppm is a disagreeable exposure concentration. Elkins (1959) was of the opinion that exposure to HCl at 5 ppm is immediately irritating to the nose and throat, but without long lasting effects. Sayers et al. (1934) expressed an opinion that prolonged exposure to 1-5 ppm resulted in slight symptoms, exposure to 5-10 ppm for 1 hr was the maximum exposure concentration without serious effects, and 150-200 ppm was dangerous in 30-60 min.
TABLE D-1 Interpretations of Various HCl Exposure Concentrations in the Workplace
|
HCl Concentration, ppm |
Exposure Duration |
Physiological Responses |
References |
|
1,000-2,000 |
Brief |
Dangerous for even short exposures |
Henderson and Haggard 1943 |
|
50-100 |
1 hr |
Maximum tolerable concentration |
Henderson and Haggard 1943 |
|
10-50 |
A few hr |
Maximum tolerable concentration |
Henderson and Haggard 1943 |
|
35 |
Unspecified short time |
Irritation of throat |
Henderson and Haggard 1943 |
|
10 |
Prolonged |
Maximum allowable concentration |
Henderson and Haggard 1943 |
|
1-5 |
— |
Odor threshold |
Heyroth 1963 |
Accidental Exposures
One report described a spill of approximately 380,000 L of HCl onto the ground from a storage tank containing 32% HCl in solution. Fire fighters in protective equipment controlled the spill; however, several of the response personnel developed facial rashes 2-3 days later. The HCl exposure responsible for the rashes was due to shifting winds, which increased the acid exposure to the faces of the responders (Hazardous Substances Data Bank 1996).
A clinical study involving 170 fire fighters identified HCl from degradation of polyvinyl chloride as an important contributor to respiratory symptoms. One death with hemorrhage, edema, and inflammation of the lungs was reported (Dyer and Esch 1976). Specific details about the HCl exposure experienced by the fire fighters were not reported in that study. Kilburn (1996) reported that residents and a police officer became acutely ill with burning and tearing eyes, burning throats, headache, chest pains, shortness of breath, and flu-like complaints following a leak of 200 gallons of hydrochloric acid from a tanker truck. Twenty four months later, a cohort study indicated that the incidence of neurobehavioral changes and airway obstruction was higher in the exposed population than in a referent group. The lack of measurements of exposure and health effects makes interpretation of those results difficult.
Repeated Exposures
Repeated exposures to HCl gas and hydrochloric acid occur most frequently in industrial settings. The American Conference of Governmental Industrial Hygienists (ACGIH 1991) recommended a Threshold Limit Value (TLV) ceiling of 5 ppm to minimize the acute irritation associated with industrial use of HCl. The acute irritative effects of HC1 are primarily limited to the concentration, rather than to the product of concentration and time, at workplace exposure levels. Experience with HCl in the workplace provides information about the potential for long-term effects occurring from exposure to low concentrations of HCl. In general, long-term pulmonary or systemic effects have not been associated with workplace exposure at low concentrations (Perry et al. 1994). Perry et al. (1994) and IARC (1992) have reviewed the results of several studies of long-term occupational exposure. In the majority of those studies, the occupational exposures were to mixed mineral-acid vapors. Prolonged occupational exposures in which HCl concentrations repeatedly exceeded 5 ppm for 5 min or more have been associated with dental erosion and tooth decay, bleeding mucous membranes in the nose and gums, and ulceration of the nasal and oral mucosa (Remijn et al. 1982). Tarlo and Broder (1989) briefly described a case of irritant-induced asthma in a 57-year-old man exposed to hydrochloric acid and phosgene for 19 years. Exposure information was not available for that case. IARC (1992) reviewed several epidemiological studies of employees in industries associated with chronic acid exposure and concluded that the evidence was inadequate for carcinogenicity in humans exposed to HCl. Neurobehavioral and chronic obstructive lung disease have not been reported in workers chronically exposed to HCl (IARC 1992).
Potentially Sensitive Populations
Children or individuals with asthma or chronic lung disease are frequently assumed to be potentially more sensitive to irritant gases than healthy adults are; however, the data supporting that position with regard to HCl exposure are limited. Boulet (1988) described a case of rapidly progressive and severe bronchospasm in a 41-year-old asthmatic male who had been cleaning a swimming pool for almost 1 hr with a product containing hydrochloric acid. The severity of his response was enough to cause hospitalization, and 1 year after the exposure, the indi-
vidual continued to have m arked symptoms of asthma, which were triggered by exposure to low concentrations of airborne irritants. Exposure conditions are unknown for this case. Among nine pharmaceutical employees enveloped by HCl fumes for 15 sec when a small quantity of industrial-strength HCl was accidentally released, only one of the employees, who had a prior history of chronic obstructive airways disease, developed long-term airway hyper-reactivity (Boyce and Simpson 1996). Four other employees recovered, even though they developed severe symptoms following the HCl exposure.
HCl exposures (mean ± SD) at 0.8 ± 0.09 and 1.8 ± 0.21 ppm for 45 min (including two 15-min exercise periods) did not alter the pulmonary function or ease of nasal breathing of young adults with asthma (Stevens et al. 1992). In normal healthy humans, HCl did not appear to be as irritating as other acid compounds (H2SO4, SO2, or HNO3).
Fine et al. (1987) exposed eight subjects with asthma to a pH 2 aerosol of HCl over a 3-min period. The experiment was designed to deliver relatively large and uniform isotonic particles at high concentrations through a mouthpiece. The exposure concentration was not stated; however, H2SO4 was tested under the same conditions, and its exposure was estimated at 10 mg/m3. Because the predominant component of both the HCl and H2SO4 aerosols was water, the HCl concentration was most likely similar to that of H2SO4 (10 mg/m3 or 6.7 ppm). Specific airway resistance was reduced 50% in one of the eight subjects exposed to HCl, indicating that HCl was a weak stimulus to bronchi constriction. Buffering with glycine, which was intended to cause a more persistent alteration of airway pH, resulted in a 50% increase in airway resistance in all subjects, indicating that the asthmatic subjects were more sensitive to the buffered acidity.
In a comparison of H2SO4 and HCl, Kamrin (1992) estimated that populations potentially sensitive to HCl might be only fivefold more sensitive than the general population.
EFFECTS IN ANIMALS
One-Time Exposure
Pulmonary Sensory Effects A decrease in respiratory rate is considered a characteristic response to upper-respiratory-tract irritation in experimental animals and has been observed in experimental animals exposed
to HCl. Other indications of sensory effects have not been reported for experimental animals exposed to HCl.
Swiss-Webster mice (four per group) exposed to HCl at 40, 99, 245, 440, or 943 ppm for 10 min showed a dose-related decrease in respiratory rate at all concentrations (Barrow et al. 1977). At 99 ppm and above, the response began within the first minute of exposure and was at a plateau within 5-7 min. At 40 ppm, the decrease in respiration was seen during the second minute of exposure, the reduction in respiratory rate was minimal, and the return to baseline was rapid. At 245 ppm and above, the return to baseline was slow. The RD50 (concentration that decreased the respiratory rate by 50%) for HCl was calculated to be 309 ppm for a 10-min exposure.
An RD50 value of 560 ppm was calculated for Sprague-Dawley rats (three per group) exposed to HCl at 200-1,538 ppm for 30 min (Hartzell et al. 1985a). The HCl concentration calculated to cause a 50% decrease in respiratory minute volume was 605 ppm. The RD50 values indicate that the mouse (10-min RD50 at 309 ppm) is more sensitive than the rat (30-min RD50 of 560 ppm) to HCl-induced sensory irritation.
Guinea pigs also appear to be less sensitive than mice to HCl. When groups of four guinea pigs were exposed to HCl at 320,680,1,040, or 1,380 ppm for 30 min, it took 6 min for signs of sensory irritation (reduced respiratory rate) to appear at 320 ppm (Burleigh-Flayer et al. 1985). Sensory irritation at the higher concentrations was immediate. The mean exposure time to progress from sensory irritation to respiratory irritation was approximately an additional 13 min.
Malek and Alarie (1989) exposed guinea pigs to HCl at 107, 140, 162, and 586 ppm for up to 30 min while exercising on a running devise. Those exposed to 140 ppm or higher exhibited mild-to-severe irritation (coughing and gasping).
In contrast to mice and guinea pigs, baboons increased the respiratory rate of exposure to HCl (Kaplan 1987). Groups of three anesthetized (ketamine) juvenile baboons exposed to HCl at 5,000 or 10,000 ppm initially held their breath for 10-20 sec and then rapidly increased their respiratory rate. Animals exposed at 500 ppm also increased their respiratory rate but with a slight delay of 1-2 min, after which a plateau was quickly reached (approximately 1 min).
The order of most to least sensitive species to HCl inhalation was the mouse, guinea pig, rat, and baboon.
Effects on Respiratory Function and Morphology Lucia et al. (1977)
exposed Swiss-Webster mice to HCl concentrations ranging from 17 to 7,279 ppm for 10 min. Twenty-four hours later, the nasal passages were examined for evidence of histological damage. Small superficial ulcers were observed in the respiratory epithelium of mice exposed at 17 ppm. At 493 ppm, the squamous epithelium of the external nares was severely damaged. At 723 ppm, more than two-thirds of the epithelium was destroyed, and at 1,973 ppm, the entire mucosa was destroyed.
Barrow et al. (1979) also exposed Swiss-Webster mice to HCl for 10 min at concentrations ranging from 20 to 20,000 ppm and found ulceration of the nasal epithelium at concentrations of 120 ppm and higher.
Stavert et al. (1991) studied the effects of HCl at 1,300 ppm (30 min) on rats breathing through their nose or through their mouth via an endotracheal tube. The nose breathers developed severe necrotizing rhinitis, and some animals had lesions in the proximal trachea. Nasal lesions were absent in the mouth-breathing rats, but inflammatory changes were present in the trachea and lungs.
Kolesar et al. (1993) exposed rats to HCl at either 1,500 or 3,000 ppm for 1 hr by nose only. Rats were sacrificed at 24 hr, 48 hr, 7 d, or 24 d after exposure and examined for organ-weight changes and for gross pathological and histopathological changes. HCl produced damage to the surface epithelium and underlying tissues at both concentrations. By day 14, the mucosa had been restored, but scar tissue was present in animals exposed at both concentrations.
In another study, groups of four to eight guinea pigs were exposed to HCl at 320, 680, 1,040, or 1,380 ppm for 30 min (Burleigh-Flayer et al. 1985). A decrease in respiratory rate and a lengthened expiratory phase were interpreted as signs of sensory irritation; an initial increase in respiratory rate followed by a decrease due to a pause following each expiration was interpreted as a sign of respiratory irritation. Two of eight animals died during exposure at 1,380 ppm. One animal in the 1,380-ppm exposure group and two of eight in the 1,040-ppm exposure group died following exposure. Following exposures, pulmonary function was evaluated at various intervals up to 15 days by exposing the animals to room air followed by challenge with 10% CO2. The authors concluded that tidal volumes during exposure to both room air and CO2 challenge were unaffected by HCl. However, marked decreases in respiratory rates from pre-exposure baseline rates were observed in the two highest exposure groups exposed to either room air or 10% CO2. Those changes persisted throughout the 15-day observation period. No changes occurred in lung weights relative to body weights in any expo-
sure group. Histopathological examination of the lungs from the group exposed to HCl at 1,040 ppm revealed inflammatory changes, including alveolitis with congestion and hemorrhage 2 days following exposure, and inflammation, hyperplasia, and mild bronchitis 15 days following exposure. No other groups were examined.
Groups of three male baboons were exposed under ketamine anesthesia to target concentrations of HCl at 500, 5,000, or 10,000 ppm for 15 min (Kaplan et al. 1988). Analytic data indicated that actual exposures were within 20% of target concentrations in all experiments except one in which the difference was approximately 30%. Respiratory rates during exposures increased from baseline rates in a dose-related fashion: approximately 30% at 500 ppm, 50% at 5,000 ppm, and 100% at 10,000 ppm. Tidal volumes were unaffected by HCl exposure. PaO 2 (arterial blood gas) decreased approximately 40% within the 15-min exposure at the two highest concentrations, but not at the lowest, and remained lower at least 10 min following the exposures before returning to baseline rates by the time of the next analysis on day 3. Pulmonary-function tests conducted 3 days and 3 months following exposures did not reveal changes relative to baseline values. The responses of animals challenged on day 3 with 10% CO2 were no different from those challenged before HCl exposure. However, respiratory frequency seemed to increase following CO2 challenge 3 months after HCl exposure in the 5,000- and the 10,000-ppm groups but not in the 500-ppm group.
Incapacitation and Lethality Crane et al. (1985) studied the incapacitating potential of HCl gas (2,000-100,000 ppm) in Sprague-Dawley rats while the rats were in a cylindrical cage rotating at 6 revolutions per min (rpm). The time to incapacitation was 3 hr at 2,000 ppm and 5.5 min at 94,000 ppm. Regression equations were fit to two toxicity end points (time to incapacitation and time to death) using a nonlinear least-squares technique. The model indicated that 300 ppm was a threshold for an infinitely long exposure, and 3 min was the shortest time to death at an infinite exposure concentration. Necropsy examination indicated almost total destruction of the nares and pharynx and little damage below the trachea in incapacitated rats. The response equations for incapacitation and lethality are shown below:
ti = 3 + 336/ (HCl -0.3) for incapacitation;
td = 3 + 411/(HCl -0.4) for lethality.
Groups of two to four guinea pigs conditioned to exercise received whole-body exposure while running in air containing HCl at 107,140, 162, or 586 ppm (Malek and Alarie 1989). Exposures lasted for 30 min or until the guinea pigs were incapacitated (i.e., could no longer run and did not resume running). Animals exposed to HCl at 107 ppm completed the running protocol of 30 min; the other groups were incapacitated after an average of 16 min (140 ppm), 1.3 min (162 ppm), and 0.6 min (586 ppm). The low-exposure group exhibited signs of mild irritation, and the other groups showed signs of severe irritation and coughing and gasping before incapacitation. Respiratory frequency was decreased an average of 80% from sedentary baseline values in incapacitated animals. All animals in the highest exposure group died within an average of 3 min from the start of exposure. No deaths occurred in any other group, although the animals might have been observed only briefly following exposure, and any delayed effects would not have been detected. Gross pathological examinations revealed no indications of obstructed nostrils, hyperinflated lungs, or external lung hemorrhage. Histopathological examinations were not conducted. In the absence of pathological changes, the authors concluded that deaths might have resulted from enhanced protective respiratory reflexes due to exercise, resulting in increased toxicity of HCl compared with sedentary exposures.
Groups of 10 ICR-derived CF-1 mice and 10 Sprague-Dawley-derived CFE rats were exposed to either HCl gas or aerosol for 5 or 30 min to compare the toxicity of each form of the compound (Darmer et al. 1974). Analysis of the aerosols indicated that no droplets were larger than 5 µm in diameter. Animals were observed for 7 days following exposure. Gross pathological examination of animals that died during exposure revealed moderate-to-severe changes in the lungs and upper respiratory tract. Animals surviving 7 days following exposure showed pulmonary effects, including indications of alveolar damage. Unspecified histopathological changes were also observed. The LC50 values for exposure to HCl as an aerosol or as a gas were similar (Table D-2). For mice, the average of the two LC50 values at 5 min was 12,500 ppm and at 30 min was 2,400 ppm. For rats, the average of the two LC50 values at 5 min was 36,000 ppm and at 30 min was 5,200 ppm. Thus, the data from Darmer et al. (1974) also indicate that mice are more sensitive than rats to HCl via inhalation.
Clinical signs included brittle and discolored hair, respiratory dis-
TABLE D-2 LC50s of HCl Gas and Aerosol in Rats and Mice
|
|
5-min LC50, ppm |
30-min LC50, ppm |
||
|
Animal |
Gas |
Aerosol |
Gas |
Aerosol |
|
Rats |
41,000 |
31,000 |
4,700 |
5,600 |
|
Mice |
13,700 |
11,200 |
2,600 |
2,100 |
|
Source: Darmer et al. 1974; rounded to three significant digits. The LC50 values for rats and mice exposed to HCl gas for 5-min were reported by DiPasquale and Davis 1971, Higgins et al. 1972, and Darmer et al. 1974. |
||||
1974). Peak mortality occurred in 24 hr, but delayed deaths were seen 3-4 days later. Exposed animals exhibited pulmonary edema of varying degrees of severity, and pulmonary hemorrhage was observed at lethal concentrations. No other details were given.
Anderson and Alarie (1980) reported an LC50 value (30-min exposure, 3-hr observation) of 10,137 ppm in normal mice and 1,095 ppm in trachea-cannulated mice.
Wohlslagel et al. (1976) determined LC50 values (1-hr exposure, 14day observation) for CF-1 mice (1,108 ppm) and CFE rats (3,124 ppm). Concurrent exposure to alumina did not alter the LC50 values, and hydrogen fluoride (HF) concurrent exposure resulted only in additive effects. Necropsy of dying animals revealed pulmonary congestion and intestinal hemorrhages in rats and mice and thymic hemorrhages in rats.
Kirsch and Drabke (1982) reported a 30-min LC50 of about 2,500 ppm for guinea pigs. Guinea pigs affected by HCl had a high breathing rate, coughed, wheezed, had a frothy nasal discharge, frequently preened, and moved slowly.
Rabbits and guinea pigs exposed to HCl at 4,291 ppm for 30 min or 670 ppm for 6 hr died (Machle et al. 1942). Exposure of rabbits and guinea pigs at 3,687 ppm for 5 min was the highest concentration that produced no deaths. The longest exposure of rabbits and guinea pigs that resulted in no deaths was 6 hr per day for 5 days at 67 ppm.
Hartzell et al. (1985b) determined the 5-, 10-, 15-, 22.5-, 30-, and 60- min LC50 values for HCl gas in male Sprague-Dawley rats to be 15,900, 8,370,6,920,5,920,3,715, and 2,810 ppm, respectively. L(CT)50 (the product of concentration and time that results in death of 50% of the test animals) values for HCl (Table D-3) vary from approximately 80,000 ppm min (5-min exposure at approximately 16,000 ppm) at approxi-
TABLE D-3 L(CT)50 Values for Rats Exposed to HCl Gas
|
Exposure Duration, min |
L(CT)50 Value, ppm·min |
95% Confidence Interval, ppm·min |
|
5 |
79,500 |
57,700-109,400 |
|
10 |
83,700 |
77,700-90,100 |
|
15 |
103,800 |
50,700-133,500 |
|
22.5 |
133,200 |
77,800-228,300 |
|
30 |
111,450 |
76,200-163,000 |
|
60 |
168,600 |
|
|
Source: Hartzell et al. 1985b. |
||
mately 170,000 ppm·min (60-min exposure at approximately 2,800 ppm).
MacEwen and Vernot (1974) combined the data from Darmer et al. (1974) with their own 60-min-LC50 HCl-vapor data and calculated L(CT)50 values for rats and mice (Table D-4). They concluded that Haber's law appeared to be applicable to HCl for those exposure times.
Ocular Effects Mice exposed to HCl vapor for 10 min were studied for ocular effects (GEOMET Technologies 1981). Significant inflammatory cell infiltrations were observed at 490 ppm in the palpebral and ocular conjunctivae. Corneal necrosis and inflammatory cell infiltrates in the eyelids were observed at 1,074 ppm. Extensive damage to the eye and eyelids was seen at a concentration of 1,946 ppm and above.
Swiss-Webster mice were exposed to HCl at 20-20,000 ppm for 10 min (Barrow et al. 1979). Ocular damage indicated by polymorphonuclear leukocyte infiltration of the conjunctiva was observed in animals exposed to HCl at 480 ppm, corneal necrosis was observed at 700 ppm, and severe damage to the globes was observed at 3,000 ppm.
TABLE D-4 Comparison of L(CT)50 Values for Rats and Mice Exposed to HCl Gas
|
|
L(CT)50, ppm·min |
||
|
Animals |
5-min |
30-min |
60-min |
|
Rats |
204,945 |
141,030 |
187,440 |
|
Mice |
68,725 |
79,320 |
66,480 |
|
Source: MacEwen and Vemot 1974. |
|||
Corneal opacities were observed in all five guinea pigs exposed to HCl at 1,380 ppm, in four of six guinea pigs exposed at 1,040 ppm, and in one of four guinea pigs exposed at 680 ppm (Burleigh-Flayer et al. 1985). Corneal opacity was not observed in guinea pigs exposed at 320 ppm.
Ocular damage appears to occur at similar HCl concentrations in mice and guinea pigs. The no-effect level for ocular damage appears to be at 400-500 ppm.
Neurobehavioral Effects Single baboons were exposed to HCl at 190, 810, 890, 2,780, 11,400, 16,570, or 17,290 ppm for 5 min (Kaplan 1987). The animals had been conditioned to a relatively simple escape performance test designed to simulate human escape performance, which was begun after the 5-min exposure. An increase occurred in the number of attempts to escape after exposure compared with that before exposure, indicating an irritative response in the animals. Escape attempts increased at 190 ppm. Other signs of irritation were coughing and frothing at the mouth at 810 ppm, progressing to profuse salivation, blinking and rubbing the eyes, and head-shaking at higher concentrations. The animals exposed at 16,570 and 17,290 ppm exhibited severe dyspnea that persisted after exposure, followed by death several weeks later from bacterial infections. Histopathological examination of those animals revealed pneumonia, pulmonary edema, and tracheitis with epithelial erosion.
Kaplan et al. (1984) used an operant test (shuttle box) to evaluate escape performance in rats exposed to HCl. Rats were exposed for 5 min at concentrations ranging from 11,800 to 87,660 ppm. At concentrations up to 76,730 ppm, but not at 87,660 ppm, the rats were able to escape. Rats exposed at 11,800 and 14,410 did not die, but those exposed at 15,250 ppm and higher died after exposure and the rat exposed at the highest concentration died during exposure.
Developmental and Reproductive Toxicities
Rats received a single 1-hr exposure to HCl at 300 ppm either 12 days before mating or on day 9 of pregnancy (Pavlova 1976). Signs of severe dyspnea and cyanosis were noted and one-third of the rats died. Embryo and fetal toxicity was observed; the toxicity appeared to be secondary to severe maternal pulmonary effects.
One-Time Exposures--Combined Exposures Simulating Sensitive Populations
Higgins et al. (1972) exposed mice and rats to carbon monoxide (CO) at concentrations sufficient to produce 25% carboxyhemoglobin (1,500 ppm for mice and 2,100 ppm for rats) and determined 7-day LC50 values for HCl. The LC50 values for HCl without exposure to CO (13,700 ppm for mice and 41, 000 ppm for rats) were not significantly different from the combined HCl-CO exposure (10,700 ppm for mice and 39,000 ppm for rats). Dipasquale and Davis (1971) showed that a CO exposure sufficient to induce 25% carboxyhemoglobin (CO at 2,100 ppm in rats and 1,500 ppm in mice) had no effect on 5-min LC50 values in Wistar rats and ICR mice.
Hartzell et al. (1985a) developed a mathematical model describing the intoxication of rats with CO. They found that HCl exposure modified the CO-induced intoxication of rats only slightly and only within a ''window" of exposure concentrations of HCl at about 400 to 1,000 ppm and CO at less than 4,000 ppm.
Tables D-5 and D-6 provide additional information on acute toxicity studies conducted with HCl.
Repeated Exposures
A group of Swiss-Webster mice was exposed to HCl at 309 ppm (the RD50) for 6 hr per day (Buckley et al. 1984). After three exposures, all the mice were dead or were moribund, and exposures were discontinued. Histopathological examination revealed severe exfoliation, erosion, ulceration, and necrosis of the nasal respiratory epithelium but only slight-to-mild changes in the squamous epithelium and olfactory epithelium. No changes were observed in the lower respiratory tract.
The NRC (1987) reviewed the data on repeated exposures of experimental animals to HCl and concluded that the primary effect was upper respiratory irritation. Rats and mice were exposed to HCl at 10, 20, or 50 ppm for 6 hr per day, 5 days per week for 90 days (Toxigenics 1984). Histopathological examination revealed minimal-to-mild rhinitis in exposed rats, and cheilitis and very mild degenerative changes in the nasal turbinates of all exposed mice. The degenerative changes were typical of those seen following exposure to many irritants and are considered reversible following cessation of exposure.
TABLE D·5 Toxicity Studies of HCl in Experimental Animals
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm1 |
End Points and Comments |
References |
|
One-Time Exposures |
|||||
|
Pulmonary sensory effects |
|||||
|
Mouse, Swiss-Webster |
10 min |
40, 99, 245, 440, 943 |
40 |
-Decreased respiratory rate at 2 min of exposure—effect minimal and rapidly reversible
|
Barrow et al. 1977 |
|
|
|
|
309 |
-Calculated RD50 (219-435 ppm 95% Cl) |
|
|
Mouse, Swiss-Webster |
10 min |
20-20,000 |
50 |
Decreased respiratory rate |
Barrow et al. 1979 |
|
Rat, Sprague-Dawley |
30 min |
200,295, 784,1,006, 1,538 |
560 |
30-min RD50 exposure-related decrease in respiratory frequency and minute volume; maximum effect within 2 min of start of exposure; 605 ppm caused a 50% decrease in respiratory minute volume |
Hartzell et al. 1985a |
|
Guinea pig, 4-8/group |
30 min. no exercise |
320, 680, 1,040, 1,380 |
320 |
-Decreased respiratory rate after 6 min of exposure |
Burleigh-Flayer et al. 1985 |
|
|
|
|
680 |
-Immediate decrease in respiratory rate |
|
|
Guinea pig, 2-4/group |
30 min, with exercise |
107, 140, 162, 586 |
140 |
Mid-to-severe irritation, cough, gasping |
Malek and Alarie 1989 |
|
Baboon, 3/group |
15 min, anesthetized |
500, 5,000, 10,000 |
500 |
-Increased respiratory rate after 1-2 min |
Kaplan 1987 |
|
|
|
|
5,000 |
-Held breath for 10-20 sec, then rapidly increased respiratory rate |
|
|
Respiratory Function and Morphology |
|||||
|
Mouse, Swiss-Webster |
10 min |
17-7,279 |
17 |
-Superficial ulcers of respiratory epithelium |
Lucia et al. 1977 |
|
|
|
|
493 |
-Severe damage to squarmous epithelium of external nares |
|
|
|
|
|
723 |
-More than two-thirds of epithelium destroyed |
|
|
|
|
|
1,973 |
-Entire mucosa destroyed |
|
|
Mouse Swiss-Webster |
10 min |
20-20,000 |
>50 |
-Decreased respiratory rate |
Barrow et al. 1979 |
|
|
|
|
120 |
-Ulceration of nasal epithelium |
|
|
|
|
|
7,000 |
-Complete destruction of nasal bone |
|
|
Rat |
30 min |
1,300 |
1,300 |
Breathed HCl through nose or endotracheal tube; nasal breathers had rhinitis and proximal thacheitis, tube breathers had tracheitis and pneumonitis (histopathological examination 24 hr later) |
Stavert et al. 1991 |
|
Rat |
1 hr |
1,500, 3,000 |
1,500 |
Damage to surface epithelium of the nasal mucosa; mucosa restored by 14 d but scar tissue present (histopathological examination at 24 hr, 48 hr 7 d, and 14 d) |
Kolesar et al. 1993 |
|
Guinea pig, 4-8/group |
30 min |
320, 680, 1,040, 1,380 |
1,040 |
Marked decrease in respiratory rate (2/8 died); lung histopatholog revealed inflammatory changes 2 and 15 d post-exposure, marked decrease in respiratory rates on challenge with CO2 exposure, but tidal volumes not affected |
Burleigh-Flayer et al. 1985 |
|
Baboon, 3/group |
15 min, anesthetized |
500, 5,000, 10,000 |
500 |
-30% increase in respiratory rate |
Kaplan et al. 1988 |
|
|
|
|
5,000 |
-50% increase in respiratory rate |
|
|
|
|
|
10,000 |
-100% increase in respiratory rate |
|
|
Species |
Exposure Duration |
Exposure Concentration ppm |
Effect Concentration |
End Points and Comments |
References |
|
Baboon, 3/group |
15 min, anesthetized |
500, 5,000, 10,000 |
= 5,000 |
PaO2 decreased 40% and possible long-term effects; respiratory frequency seemed to increase following CO2 challenge 3 mo after exposure |
Kaplan et al. 1988 |
|
Incapacitation |
|||||
|
Rat, Sprague-Dawley |
Until incapacitation |
2,000-100,000 |
2,000 |
-3 hr until incapacitation |
Crane et al. 1985 |
|
|
|
|
94,000 |
-5.5 min until incapacitation (see also Table D-6) |
|
|
Guinea pig |
Until incapacitation |
107-586 |
107 |
-All ran full 30 min |
Malek and Alarie 1989 |
|
|
|
|
140 |
-Average 16 min until incapacitation |
|
|
|
|
|
162 |
-Average 1.3 min |
|
|
|
|
|
586 |
-Average 0.6 min (all died within an average of 3 min) (see also Table D-6) |
|
|
Lethality |
|||||
|
Mouse, CF-1, 10/group (gas) |
5 min |
(see Table D-6) |
≈13,700 |
-5-min LC50 (≈10,300-18,300 ppm, 95% CI) |
Darmer et al. 1974 |
|
|
30 min |
|
≈2,600 |
-30-min LC50(≈2,300-3,100 ppm, 95% CI) |
|
|
Mouse, CF-1, 10/group (aerosol) |
5 min |
(see Table D-6) |
≈11,200 |
-5-min LC50(≈ 10,000-12,500 ppm, 95% Cl) |
Darmer et al. 1974. |
|
|
30 min |
|
≈2,100 |
-30-min LC50 (≈1,800-2,600 ppm, 95% CI) |
|
|
Rat, CFE, 10/group (gas) |
5 min |
(see Table D-6) |
≈41,000 |
-5-min LC50 (≈34,800-48,300 ppm, 95% CI) |
Darmer et al.. 1974 |
|
|
30 min |
|
≈4,700 |
-30-min LC50 (≈4,100-5,400 ppm, 95% CI) |
|
|
Rat, CFE, 10/group (aerosol) |
5 min |
(see Table D-6) |
≈31,000 |
-5-min LC50 (≈26,800-35,800 ppm, 95% Cl) |
Darmer et al. 1974 |
|
|
30 min |
|
≈5,700 |
-30-min LC50 (≈4,600-6,600 ppm, 95% CI) |
|
|
Mouse |
30 min |
range |
10,137 |
30-min LC50; 3-hr post exposure |
Anderson and Alarie 1980 |
|
Mouse |
30 min |
range |
1,095 |
30-min LC50; 3-hr post exposure; tracheal-cannulated mice |
Anderson and Alarie 1980d |
|
Mouse, female CF-1 (ICR derived) |
60 min |
(see Table D-6) |
≈1,100 |
60-min LC50; 14-d post-exposure (≈870-1,400 ppm, 95% CI) |
Wohlslagel et al. 1976 |
|
Rat, male CFE (Sprague-Dawley derived) |
60 min |
(see Table D-6) |
≈3,100 |
60-min LC50; 14-d observation (≈2,800-3,500 ppm, 95% CI) |
Wohlslagel et al. 1976 |
|
Guinea pig 8/group |
30 min |
(see Table D-6) |
≈2,500 |
30-min LC50 |
Kirsch and Drabke 1982 |
|
Rat, Sprague-Dawley, male |
5 min |
(see Table D-6) |
15,900 |
-5-min LC50 |
Hartzell et al. 1985b |
|
|
10 min |
|
8,370 |
-10-min LC50 |
|
|
|
15 min |
|
6,920 |
-15-min LC50 |
|
|
|
22.5 min |
|
5,920 |
-22.5-min LC50 |
|
|
|
30 min |
|
3,715 |
-30-min LC50 |
|
|
|
60 min |
|
2,810 |
-60-min LC50 |
|
|
Guinea pig, 4-8/group |
30 min, no exercise |
320, 680, 1,040, 1,380 |
1,040 |
-2/8 died after exposure |
Burleigh-Flayer et al. 1985 |
|
|
|
|
1,380 |
-2/8 died during exposure, 1/8 after exposure |
|
|
Baboon, 1 at each level |
5 min |
190, 810, 890, 2,780, 11,400, 16,750, 17,290 |
16,750 |
Delayed deaths |
Kaplan 1987 |
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration ppm1 |
End Points and Comments |
References |
|
Ocular Toxicity |
|||||
|
Mouse (vapor) |
10 min |
490-3,062 |
490 |
-Conjunctival PMN leukocyte infiltrates |
GEOMET Technologies 1981 |
|
|
|
|
1,074 |
-Comeal necrosis; infiltrates in lids |
|
|
|
|
|
1,946 |
-Extensive ocular damage |
|
|
|
|
|
3,062 |
-Rupture of the globe |
|
|
Mouse, Swiss-Webster |
10 min |
20-20,000 |
480 |
-Conjunctival PMN leukocyte infiltrates |
Barrow et al. 1979 |
|
|
|
|
700 |
-Comeal necrosis |
|
|
|
|
|
3,000 |
-Severe damage to the globes |
|
|
Guinea pig, male |
30 min |
320, 680, 1,040, 1380 |
680 |
Comeal opacity; see Table D-6 for summary of exposure-response |
Burleigh-Flayer et al. 1985 |
|
Neurobehavioral Effects |
|||||
|
Baboon, 1/group |
5 min |
190, 810, 890, 2,780, 11,400, 16,570, 17,290 |
190 |
-Increased number of escape attempts |
Kaplan 1987 |
|
|
|
|
810 |
-Coughing and frothing at the mouth |
|
|
|
|
|
890 |
-Profuse salivation, blinking, head-shaking |
|
|
|
|
|
16,570 |
-Severe dyspnea, followed by death from bacterial infection |
|
|
Rat |
5 min |
11,800-87,660 |
87,660 |
Unable to escape (all rats exposed at lower concentrations performed escape task; however, all rats exposed at more than 14,410 ppm died) |
Kaplan et al. 1984 |
|
Combined Exposures Simulating Sensitive Populations |
|||||
|
Mouse, ICR |
5 min |
(see Table D-6) |
≈13,800 |
5-min LC50; 7-d post-exposure |
Higgins et al. 1972; DiPas quale and Davis 1971 |
|
Mouse, ICR |
5 min |
Unspecified range |
≈10,700 |
5-min LC50; 7-d post-exposure; combined with CO at 1,500 ppm |
Higgins et al. 1972 |
|
Rat, Wistar |
5 min |
(see Table D-6) |
≈41,000 |
5-min LC50; 7-d post-exposure. |
Higgins et al. 1972; DiPas quale and Davis 1971 |
|
Rat, Wistar |
5 min |
Unspecified range |
39,000 |
5-min LC50; 7-d post-exposure; combined with CO at 2,100 ppm |
Higgins et al. 1972 |
|
Repeated Exposures |
|||||
|
Mouse |
6 hr/d, 1-3 d |
309 |
309 |
All mice died or were moribund by third exposure; severe irritation of nasal respiratory epithelium; slight-to-moderate changes in olfactory epithelium; no changes in lower respiratory tract. |
Buckley et al. 1984 |
|
Guinea pig, rabbit, monkey |
6 hr/d, 5 d/wk, 28 d |
34 |
— |
No immediate effects and no gross pathological lesions |
Machle et al. 1942. |
|
Guinea pig |
2 hr/d, 28 d |
0.1 |
— |
No lung irritation |
Kirsch and Drabke 1982 |
|
Rat |
6 hr/d, 5 d/wk, 90 d |
0, 10, 20, 50 |
10 |
Dose-related rhinitis ranging from minimal to mild beginning at 10 ppm |
Toxigenics 1984 |
TABLE D-6 Summary of Exposure-Response Data for HCl Gas (Except as Noted)
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Level |
End Points and Comments |
References |
|
Guinea pig, male (English Smooth Hair) |
30 min |
0 320 680 1,040 1,380 |
0/4 0/4 1/4 4/6 5/5 |
Corneal opacity |
Burleigh-Flayer et al. 1985 |
|
Guinea pig, male (English Smooth Hair) |
30 min |
0 320 680 1,040 1,380 |
0/4 0/4 0/4 2/8 3/8 |
Mortality |
Burleigh-Flayer et al. 1985 |
|
Baboon, male |
15 min |
500 5,000 10,000 |
See refs. for graphs of results |
Respiratory and blood-gas measurements; baboon and rat lethality data available in Kaplan et al. 1984; also see Kaplan et al. 1987 for lack of effect of HCl in avoidance and escape behavior |
Kaplan et al. 1988 |
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Level |
End Points and Comments |
References |
|
Mouse, CF-1 (ICR derived) |
5 min |
3,200 5,060 6,145 6,410 7,525 8,065 9,276 13,655 26,485 30,000 |
1/10 1/10 2/10 0/10 6/10 2/10 5/10 6/10 13/15 13/15 |
Mortality; 5-min LC50 = 13,745 ppm (10,333-18,283, 95% Cl); clinical signs not assigned to concentrations |
Darmer et al. 1974 |
|
Mouse, CF-1 (ICR derived) |
30 min |
410 1,134 2,678 2,721 2,942 3,071 4,045 4,076 5,363 |
0/15 2/15 8/15 4/15 12/15 6/15 11/15 13/15 14/15 |
Mortality; 30-min LC50 = 2,644 ppm (2,264-3,086 ppm, 95% Cl); clinical signs not assigned to concentrations |
Darmer et al. 19744 |
|
Mouse, CF-1 (ICR derived) |
5 min |
9,058 10,059 12,104 14,913 17,000 18,773 |
3/10 3/10 5/10 9/10 9/10 10/10 |
Mortality; 5-min LC50=11,238 ppm (10,006-12,547 ppm, 95% Cl) |
Darmer et al. 1974 Note: HCl Aerosol |
|
Mouse CF-1 (ICR derived) |
30 min |
1,204 2,127 2,557 2,720 2,910 3,036 4,432 |
2/10 5/10 5/10 5/10 9/10 7/10 10/10 |
Mortality; 30-min LC50 = 2,142 ppm (1,779-2,580 ppm, 95% Cl) |
Darmer et al. 1974 Note: HCl Aerosol 4 |
|
Rat, CFE (Sprague-Dawley derived) |
5 min |
30,000 32,255 39,850 45,200 57,290 |
0/10 1/10 6/10 7/10 9/10 |
Mortality; 5-min LC50 = 40,989 ppm (34,803-48,272 ppm, 95% Cl) |
Darmer et al. 1974 |
|
Rat, CFE (Sprague-Dawley derived) |
30 min |
2,078 2,678 3,071 5,180 6,068 6,681 |
0/10 1/10 0/10 5/10 8/10 10/10 |
Mortality; 30-min LC50 = 4,701 ppm (4,129-5,352 ppm, 95% Cl) |
Darmer et al. 19744 |
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Level |
End Points and Comments |
References |
||
|
Rat, CFE (Sprague-Dawley derived) |
5 min |
6,571 19,312 25,324 29,648 38,746 40,810 62,042 |
0/10 1/10 3/10 6/10 6/10 7/10 10/10 |
Mortality; 5-min LC50 = 31,008 ppm (26,824-35,845 ppm, 95% CI); clinical signs not assigned to concentrations |
Darmer et al. 1974 Note: HCl Aerosol |
||
|
Rat, CFE (Sprague-Dawley derived) |
30 min |
2,910 4,481 6,078 6,640 |
1/10 0/10 6/8 8/10 |
Mortality; 30-min LC50 = 5,666 ppm (4,555-6,614 ppm, 95% Cl); clinical signs not assigned to concentrations |
Darmer et al. 1974 Note: HCl Aerosol |
||
|
Rat, Sprague-Dawley (n = 42-43) |
5.5 min to 3 hr |
2,000-100,000 |
See equations |
Response equations for incapacitation Ti = 3 + 336/(HCl -0.3); for lethality, Td= 3 + 441/HCl -0.4) |
Crane et al. 1985 |
||
|
Guinea pig, outbred English short hair |
|
|
|
Time to incapacitation (min) |
Distance traveled (m) |
Deaths |
Malek and Alarie 1989 |
|
|
|
107 140 162 586 |
0/3 3/3 3/3 3/3 |
>30 16.5 ± 8.6 1.3±0.9 0.65 ±0.08 |
795 437±229 47.8±7.4 17.1±2.2 |
0/3 0/3 0/3 3/3 |
|
|
Rat, Sprague-Dawley, male |
5 min |
9,200 10,785 12,584 14,307 15,459 20,300 |
0/6 3/6 2/6 0/6 3/6 6/6 |
Mortality; 5-min LC50 = 15,900 ppm (11,540-21,890 ppm, 95% Cl); 14-d observation |
Hartzell et al. 1985b |
|
Rat, Sprague-Dawley, male |
10 min |
5,444 7,629 8,114 8,425 9,170 |
0/6 1/6 5/8 1/8 6/6 |
Mortality; 10-min LC50 = 8,370 ppm (7,770-9,010 ppm, 95% Cl); 14-d observation |
Hartzell et al. 1985bb |
|
Rat, Sprague-Dawley, male |
15 min |
4,360 6,171 7,980 8,960 9,990 |
0/6 3/6 4/6 4/6 6/6 |
Mortality; 15-min LC50= 6,920 ppm (5,380-8,900 ppm, 95% Cl); 14-d observation |
Hartzell et al. 1985b |
|
Rat, Sprague-Dawley, male |
22.5 min |
4,864 6,414 7,487 8,103 8,646 10,137 |
2/6 4/6 6/6 2/6 4/6 6/6 |
Mortality; 22.5-min LC50 = 5,920 ppm (3,455-10,145, 95% Cl); 14-d observation |
Hartzell et al. 1985b |
|
Rat, Sprague-Dawley, male |
30 min |
2,610 3,713 4,090 5,776 6,470 8,280 |
2/6 4/6 1/6 8/8 4/6 6/6 |
Mortality; 30-min LC50 = 3,715 ppm (2,540-5,435 ppm, 95% Cl); |
Hartzell et al. 1985bb |
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Level |
End Points and Comments |
References |
|
Rat, Sprague-Dawley, male |
60 min |
1,793 2,281 2,600 4,277 4,460 4,854 |
0/6 3/6 1/6 7/8 6/6 6/6 |
Mortality; 60-min LC50 = 2,810 ppm(2,250-3,510 ppm, 95% Cl) |
Hartzell et al. 1985b |
|
Mouse, CF-1 (ICR derived), female |
60 min |
557 985 1,387 1,902 2,476 |
2/10 3/10 6/10 8/10 10/10 |
Mortality; 60-min LC50 = 1,108 ppm (874-1,404 ppm, 95% Cl) |
Wohlslagel et al. 1976 |
|
Rat, CFE (Sprague-Dawley derived), male |
60 min |
1,813 2,585 3,274 3,941 4,455 |
0/10 2/10 6/10 8/10 10/10 |
Mortality; 60-min LC50 = 3,124 ppm (2,829-3,450 ppm, 95% Cl) |
Wohlslagel et al. 1976 |
|
Guinea pig |
30 min |
1,309 1,538 2,125 4,082 5,708 |
0/8 1/8 3/8 7/8 8/8 |
Mortality; 30-min LC50 =2,519 ppm |
Kirsch and Drabk 1982e |
|
Mouse, IRC, 15/group |
5 min |
3,200 5,060 6,145| 6,410 7,525 8,065 9,276 13,655 26,485 30,000 |
1/15 1/15 2/15 0/15 6/15 2/15 5/15 6/15 13/15 13/15 |
Mortality; 5-min LC50 = 13,745 ppm (10,333-18,283 ppm, 95% Cl); 7-d observation |
Higgins et al. 1972 |
|
Rat, Wistar 10/group |
5 min |
30,000 32,000 39,850 45,200 57,290 |
0/10 1/10 6/10 7/10 9/10 |
Mortality; 5-min LC50 = 40,989 ppm (34,803-48,272 ppm, 95% CI); 7-d observation |
Higgins et al. 1972 |
|
Abbreviations: CI, confidence interval; LC50, concentration lethal to 50% of the test animals. |
|||||
Rabbits (three per group), guinea pigs (three per group), and a monkey exposed to HCl at 34 ppm for 6 hr per day, 5 days per week for 4 weeks showed no immediate effects and no gross pathological lesions (Machle et al. 1942).
Guinea pigs exposed to HCl at 0.1 ppm for 2 hr per day for 4 weeks showed no effects on lung irritation (Kirsch and Drabke 1982).
Rats receiving a lifetime exposure at 10 ppm for 6 hr per day, 5 days per week developed a higher incidence of laryngeal and tracheal hyperplasia than controls but showed no evidence of HCl-induced tumors (Sellakumar et al. 1985; Albert et al. 1982).
IARC (1992) reviewed the study reported by Sellakumar et al. (1985) and single-exposure studies showing that HCl induced chromosomal aberrations and mutations in mammalian cells in vitro and concluded that the evidence was inadequate to conclude that HCl was carcinogenic in experimental animals.
ESTABLISHED INHALATION EXPOSURE LIMITS
Figure D-1 and Table D-7 list inhalation exposure limits established by various groups. ACGIH established a Threshold Limit Value (TLV) ceiling of 5 ppm in 1963. The TLV was recommended to minimize potential toxicity and acute irritation associated with occupational exposure to HCl (ACGIH 1991). Between 1946 and 1947, the ACGIH TLV for HCl was 10 ppm as a time-weighted average (TWA); in 1948, it was lowered to 5 ppm as a TWA. In 1963, ACGIH changed the 5-ppm recommendation from a TWA to a ceiling value because of reports of respiratory irritation from short-term exposures to HCl at 5 ppm and above (permissible as long as the average concentration over the 8-hr work day did not exceed 5 ppm). The Occupational Safety and Health Administration (OSHA) also established a permissible exposure limit (PEL) as a ceiling value of 5 ppm. The occupational exposure limits (OELs) recommended by ACGIH, OSHA, various other state and federal agencies, and agencies in other countries have been based more on human experience than on animal test data.
Table D-8 shows inhalation exposure guidelines for HCl recommended by previous NRC committees. The NRC (1987) derived a 10-min EEGL for HCl of 100 ppm by applying uncertainty factors of 10 for species differences and 0.64 for paucity of data to the 10-min RD50 in
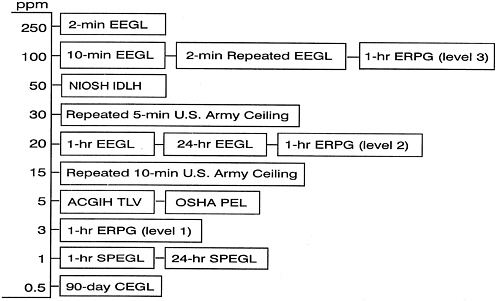
FIGURE D-1 Currently recommended exposure limits for HCl (see Table D-7 for abbreviations and sources).
TABLE D-7 Currently Recommended Exposure Limits for HCl
|
Exposure Limit |
Concentration, ppm |
Reference |
|
AIHA ERPGs |
||
|
Level 1, 1 hr |
3 |
NRC 1991 |
|
Level 2, 1 hr |
20 |
NRC 1991 |
|
Level 3, 1 hr |
100 |
NRC 1991 |
|
NRC EEGLs |
||
|
2 min |
250 |
NRC 1991 |
|
2 min repeated |
100 |
NRC 1991 |
|
10 min |
100 |
NRC 1987 |
|
1 hr |
20 |
NRC 1987 |
|
24 hr |
20 |
NRC 1987 |
|
U.S. Army Ceiling Limits |
||
|
5 min |
30 |
Cohen et al. 1982 |
|
60 min |
15 |
Cohen et al. 1982 |
|
ACGIH TLV, ceiling limit |
5 |
ACGIH 1991 |
|
OSHA PEL, ceiling limit |
5 |
U.S. Dept. of Labor 1998 |
|
NRC SPEGLs |
||
|
1 hr |
1 |
NRC 1987 |
|
24 hr |
1 |
NRC 1987 |
|
NRC 90-day CEGL |
0.5 |
NRC 1987 |
|
NIOSH IDLH |
50 |
NIOSH 1994 |
|
Abbreviations: AIHA, American Industrial Hygiene Association; ERPG, emergency response planning guidelines; NRC, National Research Council; EEGL, emergency exposure guidance level; ACGIH, American Conference of Governmental Industrial Hygienists; TLV, Threshold Limit Value; OSHA, Occupational Safety and Health Administration; PEL, permissible exposure level; SPEGL, short-term public emergency guidance level; CEGL, continuous exposure guidance level; NIOSH, National Institute for Occupational Safety and Health; IDLH, immediately dangerous to life and health. |
||
mice (309 ppm). Based largely on data in mice, it was assumed that the 100-ppm HCl concentration would cause significant irritation, that histopathological effects might be seen in mice at exposures near the RD50, and that higher exposures would result in significant persistent injuries to the respiratory tract. The NRC applied an uncertainty factor of 20 to both the 1-hr and 24-hr emergency exposure guidance level
TABLE D-8 Previously Recommended Inhalation Exposure Guidelines by the National Research Council
|
|
HCl Concentration, ppm |
||||||
|
Exposure Duration |
1965 |
1971 |
1971 |
1972 |
1977 |
1987 |
1991 |
|
2 min |
|
|
|
|
|
|
250 (EEGL) |
|
10 min |
30 (EEL) |
7 (PEL) |
4 (STPL) |
|
100 (EEL) |
100 (EEGL) |
100 (EEGL) |
|
30 min |
20 (EEL) |
3 (PEL) |
2 (STPL) |
|
50 (EEL) |
|
|
|
1 hr |
10 (EEL) |
3 (PEL) |
2 (STPL) |
5 (EEL) |
20 (EEGL) |
20 (EEGL) 1 (SPEGL) |
|
|
24 hr |
|
|
|
|
|
20 (EEGL) 1 (SPEGL) |
|
|
90 d |
|
|
|
1 (CEL) |
|
0.5 (CEGL) |
|
|
Source: Modified from NRC 1987 and 1991. Abbreviations: EEGL, emergency exposure guidance level; EEL, emergency exposure limit; PEL, permissible exposure level; STPL, short-term public limit; SPEGL, short-term public emergency guidance level; CEL, continuous exposure limit; CEGL, continuous exposure guidance level. |
|||||||
(EEGL) values to derive the 1-hr and 24-hr short-term public emergency guidance level (SPEGL) values of 1 ppm to protect sensitive populations. A 90-day continuous emergency guidance level (CEGL) was derived from subchronic inhalation studies with rats and mice that showed that 50 ppm produced minimal effects (NRC 1987). An uncertainty factor of 100 was applied to the 50-ppm exposure concentration to derive the 0.5ppm 90-day CEGL.
In 1991, the NRC recommended 2-min EEGLs for single (500 ppm) and multiple (100 ppm) exposures to HCl. These recommendations were based primarily on data showing that baboons exposed to HCl at 500 ppm for 15 min exhibited signs of irritation but did not show changes in hypoxia or pulmonary function and were able to perform escape tasks. The multiple-exposure EEGL for HCl was derived from those data by applying an uncertainty factor of 5.
EVALUATION OF TOXICITY INFORMATION
HCl is a potentially severe respiratory-tract irritant in humans. However, the irritating properties of HCl prevent more than transient voluntary exposure to concentrations that are likely to cause serious adverse effects.
As indicated in the previous section, data from rodent models have been used to establish short-term exposure guidelines for HCl and to gain a perspective on the mechanisms and effects of high-concentration short-term exposures. Following exposure to high concentrations of HCl, rodents exhibit signs of sensory and respiratory irritation. Sensory irritation is evoked by stimulation of trigeminal nerve endings in the nasal passages, whereas respiratory irritation occurs through contact of HCl with the lower respiratory tract. As HCl is inhaled, the highly soluble gas (or aerosol) readily dissolves in the mucosal lining of the nasal passages. When the ''scrubbing" mechanism of the mucosal lining is overwhelmed at high concentrations, HCl enters the lower respiratory tract. Thus, at a given concentration, a delay occurs between the onset of signs of sensory irritation and signs of respiratory irritation. In rodents, each type of irritation shows a specific threshold and dose-response curve. Each type of irritation can be detected by monitoring respiratory patterns.
In some guinea pig studies, lethality occurred following moderately high HCl exposures without obvious gross pathological causes. Protec-
tive respiratory mechanisms exacerbated by exercise in the guinea pigs might have been the proximate cause of lethality among the animals acutely exposed to moderately high HCl concentrations. That view is supported by observations that even though significant pathological effects occurred in the nasal passages of rodents following HCl exposures, no effects occurred in the lower respiratory tract except at high concentrations.
Mice appear to be much more sensitive to the lethal effects of HCl than rats, guinea pigs, rabbits, or baboons. To some extent, this increased sensitivity might be due to a less effective scrubbing mechanism that would remove HCl in the upper respiratory tract. The data suggest that mice might not be an appropriate model for extrapolation to humans; however, rodent studies often provide the only data sets large enough for statistical model development.
Recent studies have demonstrated significant differences between primates and rodents in their responses to HCl exposure. Exposure of rodents to HCl produces dose-related decreases in respiratory frequency and increases in pauses between breaths, changes that are interpreted as protective mechanisms. Baboons exposed at concentrations of up to 17,000 ppm for 5 min, however, exhibited increases in respiratory frequency that could be interpreted as a compensatory mechanism in response to hypoxia. Moreover, conditioned baboons were able to perform tasks at those high concentrations. Although concentrations above 11,000 ppm produced delayed death, concentrations up to 500 ppm did not produce permanent respiratory-function impairment.
Although some human subpopulations are presumed to have a greater sensitivity to respiratory irritants such as HCl (Chapter 3), no data are available for HCl that directly support this hypothesis. Data for adults with mild asthma do not indicate that this population has an unusual sensitivity to HCl; however, data for moderate-to-high exposure concentrations and for more severely affected individuals are not available. Animal models using concurrent exposure to CO to induce levels of 25% carboxyhemoglobin in the blood do not support the hypothesis that hypoxia is an additional risk factor of concern for HCl exposure.
There are mechanistic considerations that suggest that asthmatic individuals could be more sensitive to acidic respiratory irritants (Spengler et al. 1990). Although the pH of tracheobronchial mucus is nearly neutral in healthy people, mucus pH is more acidic in those with asthma. As mucus becomes more acidic, its viscosity increases, resulting
in increased airway resistance and decreased mucociliary clearance. Ciliary motion stops and epithelial cells die as mucus pH approaches 4.9. Because the mucociliary apparatus in asthmatic individuals is typically impaired or severely degraded, small changes in mucus pH might have significant consequences. Some of these responses have been observed in experimental animals exposed to H2SO4, but so far, similar effects due to HCl have not been reported.
The studies by Stevens et al. (1992) and Fine et al. (1987) suggest that the sensitivity of asthmatic individuals to HCl might not be as great as previously thought. The reasons for that are not clear, but an important protective mechanism might be the buffering capacity of the oral and nasopharyngeal regions. If HCl is adequately buffered in the upper airways, substantial exposure of the more sensitive lower airways of asthmatic individuals might not occur. Thus, although it might be prudent to apply a "safety factor" to data from healthy individuals to estimate a no-effect level for sensitive subpopulations, the available data suggest that the magnitude of the safety factor could be relatively small, a factor of two or three at most, as described below.
Data presented in this chapter suggest that 2 and 5 ppm might represent no-observed-effect levels (NOELs) for sensitive and healthy populations, respectively. On the basis of the work of Stevens et al. (1992), who showed that a 1.8-ppm HCl exposure to young asthmatic adults for 45 min, including 15 min of exercise, was without effect, 2 ppm can represent a NOEL for sensitive individuals for a 45-min exposure. Opinions based on general occupational experiences suggest that slight symptoms in adult workers might occur at exposure levels around 5 ppm. Heyroth (1963) and Sayers et al. (1934) suggested that HCl exposures at 1-5 ppm might be detected or cause slight symptoms; Heyroth (1963) and Elkins (1959) indicated that 5-10 ppm is immediately irritating and disagreeable.
Table D-9 outlines mild, moderate, and severe effects that might be anticipated following HCl exposure, according to the descriptors established in Chapter 4.1 The concentrations shown in Table D-9 at which
TABLE D-9 Anticipated Health Effects Following HCl Exposure in Healthy Humans for Up to 1 Hra
|
Severity |
Concentration, ppm |
Effects |
|
Mild effects |
≥5 |
Eye and nasal irritation, irritation of the throat, slight cough, headache; no significant pathological changes anticipated |
|
Moderate effects |
≥20 |
Frequent coughs, eye and nasal burning, shortness of breath, flulike symptoms; mild inflammatory changes might occur in upper respiratory tract |
|
Severe effects |
≥100 |
Difficulty breathing, chest pain, burning throat, disorientation, corneal opacity; significant ulceration, erosion, and inflammatory changes might occur in upper respiratory tract; inflammatory changes including pulmonary edema might occur in the lower respiratory tract. |
|
a The concentrations listed are the lowest at which effects in the specified severity category might occur in some members of a healthy population. As exposure concentrations increase, more individuals are expected to be affected and the severity of response within an individual is expected to increase. For exposures of less than 1 hr, the concentrations at which mild, moderate, and severe effects might begin to occur in a healthy population could be somewhat higher than those listed. How much higher depends on the relationship between exposure duration and concentration to the effects seen. The concentrations shown in the table are based on available human data. Because of the limitations associated with these data, Haber's law should not be used for extrapolations, especially for those at the higher concentrations shown in the table. That relationship is described in Chapter 6. |
||
mild, moderate and severe effects might begin to occur in some members of a healthy population are derived from industrial hygienic experience and human-response information published by Matt (1889), Henderson and Haggard(1943), Heyroth (1963), Fine et al. (1987), Stevens et. al. (1992) and ACGIH (1991). The majority of those date were collected from healthy working individuals. The effects column in Table D-9 includes signs and symptoms reported following human exposures or those reasonably anticipated to occur in humans on the basis of animal studies. The database on human exposures to HCl is weak because much of it depends on opinion rather than documented exposure-re
Persons experiencing moderate effects are likely to seek medical attention. Severe effects are irreversible effects that alter organ function or interfere with normal activities. Severe effects usually require medical attention
sponse studies. While that type of experiential information is valuable and has been used in establishing OELs, it is difficult to extrapolate to nonoccupational settings and to the general public. The paucity of quantitative human data makes it difficult to evaluate the health effects of exposure to high concentrations of HCl or to develop guidelines for short-term exposure limits based strictly on data derived from human exposures to HCl. Although the effects listed in Table D-9 are classified according to expected severity, it must be emphasized that predicting such severities is based on the subcommittee's best judgment given available data. The subcommittee emphasizes that individuals can vary greatly in their sensitivity to any chemical, such as HCl, particularly at high exposure concentrations.
RESEARCH NEEDS
The data base for assessing the effects of HCl on the respiratory tract at low concentrations is small and much of it is not well documented. Although more modern inhalation toxicity data using animal models would be useful, the critical data that are needed can be provided only by carefully controlled human exposures at low HCl exposure concentrations, particularly between 2 and 20 ppm. Inclusion of asthmatic individuals in the study population is critical if the responses of sensitive individuals are to be understood well.
REFERENCES
ACGIH (American Conference of Governmental Industrial Hygienists). 1991. Hydrogen chloride. Pp. 773-774 in Documentation of the Threshold Limit Values and Biological Exposure Indices, Vol. 2,6th Ed. American Conference of Governmental Industrial Hygienists, Cincinnati, Ohio.
Albert, R.E., A.R. Sellakumar, S. Laskin, M. Kuschner, N. Nelson, and C. Sayder. 1982. Gaseous formaldehyde and hydrogen chloride induction of nasal cancer in rats. J. Natl. Cancer Inst. 68:597-601.
Anderson, R.C. and Y. Alarie. 1980. Acute lethal effects of polyvinylchloride thermal decomposition products in normal and canulated mice. Toxicologist, p. A3.
Barrow, C.S., Y. Alarie, J.C. Warrick, and M.F. Stock. 1977. Comparison of the sensory irritation response in mice to chlorine and hydrogen chloride. Arch. Environ. Health 32:68-76.
Barrow, C.S., H. Lucia, and Y.C. Alarie. 1979. A comparison of the acute inhalation toxicity of hydrogen chloride versus the thermal decomposition products of polyvinylchloride. J. Combust. Toxicol. 6:3-12.
Bennett, R.R. 1996. Toxicity of HCl and NOx and Their Concentrations in Launch Ground Clouds. Paper presented to the National Research Council Subcommittee on Rocket Emission Toxicants, Oct. 8,1996, Irvine, Calif.
Boulet, L.P. 1988. Increases in airway responsiveness following acute exposure to respiratory irritants. Chest 94:476-481.
Boyce, S.H. and K.A. Simpson. 1996. Hydrochloric acid inhalation: Who needs admission? J. Accid. Emerg. Med. 13:422-424.
Buckley, L.A., X.Z. Jiang, R.A. James, K.T. Morgan, and C.S. Barrow. 1984. Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicol. Appl. Pharmacol. 74:417-429.
Burleigh-Flayer, H., K.L. Wong, and Y. Alarie. 1985. Evaluation of the pulmonary effects of HCl using CO2 challenges in guinea pigs. Fundam. Appl. Toxicol. 5:978-985.
Cohen, M., J.R. Strange, and T. Rothman. 1982. Short-Term Intermittent Exposure to Hydrogen Chloride (Gas and Mist). Final Report. AD-A165 079. Prepared by Enviro Control Division, Dynamic Corp., Rockville, Md., Contract No. DAMD17-79-C-9125, Subtask 10, for the U.S. Army Medical Research and Development Command, Fort Detrick, Frederick, Md.
Crane, C.R., D.C. Sanders, B.R. Endecott, and J.K. Abbott. 1985. Inhalation Toxicology: IV. Times to Incapacitation and Death for Rats Exposed Continuously to Atmospheric Hydrogen Chloride Gas. FAA Rep. FAA-AM85-4. Federal Aviation Administration, Washington, D.C.
Darmer, K.I., Jr., E.R. Kinkead, and L.C. DiPasquale. 1974. Acute toxicity in rats and mice exposed to hydrogen chloride gas and aerosols. Am. Ind. Hyg. Assoc. J. 35:623-631.
DiPasquale, L.C. and H.V. Davis. 1971. The acute toxicity of brief exposures to hydrogen fluoride, hydrogen chloride, nitrogen dioxide, and hydrogen cyanide singly and in combination with carbon monoxide. Pp. 279-289 in Proceedings of the Second Annual Conference on Environmental Toxicity. AMRL-TR-71-120. Aerospace Medical Research Laboratory, Wright-Patterson Air Force Base, Dayton, Ohio.
Dyer, R.F., and V.H. Esch. 1976. Polyvinyl chloride toxicity in fires: Hydrogen chloride toxicity in fire fighters. JAMA 235:393-397.
Elkins, H.B. 1959. P. 79 in The Chemistry of Industrial Toxicology. New York: John Wiley & Sons.
Fine, A., J.E. Carlyle, and E. Bourke. 1977. The effects of administrations of HCl, NH4Cl, and NH4HCO3 on the excretion of urea and ammonium in man. Eur. J. Clin. Invest. 7:587-589.
Fine, J.M., T. Gordon, J.E. Thompson, and D. Sheppard. 1987. The role of titratable acidity in acid aerosol induced bronchoconstriction. Am. Rev.
Respir. Dis. 135:826-830.
GEOMET Technologies. 1981. Hydrogen Chloride: Report 4, Occupational Hazard Assessment. NIOSH Contract 210-79-0001. U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health, Cincinnati, Ohio. Available from NTIS, Springfield, Va., Doc. No. PB83-105296.
Hartzell, G.E., H.W. Stacy, W.G. Switzer, D.N. Priest, and S.C. Packham. 1985a. Modeling of toxicological effects of fire gases: IV. Intoxication of rats by carbon monoxide in the presence of an irritant. J. Fire Sci. 3:263-279.
Hartzell, G.E., S.C. Packham, A.F. Grand, and W.G. Switzer. 1985b. Modeling of toxicological effects of fire gases: III. Quantification of post-exposure lethality of rats from exposure to HCl atmospheres. J. Fire Sci. 3:195-207.
Hazardous Substances Data Bank. 1996. Hydrochloric Acid. Update 6/8/94. HSBD No. 545. National Library of Medicine, Toxicology Information Program, Bethesda, Md.
Henderson, Y., and H.W. Haggard. 1943. Characteristics of irritant gases. Pp. 126-127 in Noxious Gases and the Principles of Respiration Influencing Their Action. 2nd Rev. Ed. New York: Van Nostrand Reinhold.
Heyroth, F.F. 1963. Halogens. Pp. 831-857 in Toxicology, D.W. Fassett and D.D. Irish, eds., Vol. 2 of Industrial Hygiene and Toxicology, 2nd Ed., F.A. Patty, ed. New York: Interscience.
Higgins, E.A., V. Fiorca, A.A. Thomas, and H.V. Davis. 1972. Acute toxicity of brief exposures to HF, HCl, NO2, and HCN with and without CO . Fire Technol. 8:120-130.
Hisham, M.W.M., and T.V. Bommaraju. 1995. Hydrogen chloride. Pp. 894925 in Kirk-Othmer Encyclopedia of Chemical Technology, Vol. 13, 4th Ed., J.I. Kroschwitz and M. Howe-Grant, eds. New York: John Wiley & Sons.
IARC (International Agency for Research on Cancer). 1992. Occupational Exposures to Mists and Vapours from Strong Inorganic Acids; and Other Industrial Chemicals. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 54. Lyon, France: International Agency for Research on Cancer.
Kamrin, M.A. 1992. Workshop on the health effects of HCl in ambient air. Regul. Toxicol. Pharmacol. 15:73-82.
Kaplan, H.L. 1987. Effects of irritant gases on the avoidance/escape performance and respiratory response of the baboon. Toxicology 47:165-179.
Kaplan, H.L., A. Grand, W.R. Rogers, W.G. Switzer, and G.E. Hartzell. 1984. A Research Study of the Assessment of Escape Impairment by Irritant Combustion Gases in Postcrash Aircraft Fires. AD-A146-484. Prepared by Southwest Research Institute, San Antonio, Tex. for the Federal Aviation Administration, Atlantic City Airport, N.J.
Kaplan, H.L., A. Anzueto, W.G. Switzer, and R.K. Hinderer. 1988. Effects of
hydrogen chloride on respiratory response and pulmonary function of the baboon. J. Toxicol. Environ. Health 23:473-493.
Kilburn, K. 1996. Effects of a hydrochloric acid spill on neurobehavioral and pulmonary function. J. Environ. Med. 38:1018-1025.
Kirsch, H. and P. Drabke. 1982. Assessing the biological effects of hydrogen chloride [in German]. Z. Gesamte Hyg. 28:107-109.
Kolesar, G.B., S.D. Crofoot, G.J. Sibert, and W.H. Siddigui. 1993. A comparison of the acute inhalation toxicity of methyltrichlorosilane and gaseous hydrogen chloride in the rat [abstract 520]. Toxicologist 13:151.
Kotchen, T.A., K.E. Krzyzaniak, J.E. Anderson, C.B. Ernst, J.H. Galla, and R.G. Luke. 1980. Inhibition of renin secretion by HCl is related to chloride in both dog and rat. Am. J. Physiol. 239:F44-F49.
Lucia, H.L., C.S. Barrow, M.F. Stock, and Y. Alarie. 1977. A semi-quantitative method for assessing anatomic damage sustained by the upper respiratory tract of the laboratory mouse, Mus musculis . J. Combust. Toxicol. 4:472-486.
MacEwen, J.D., and E.H. Vernot. 1974. The determination of a 60-minute LC50 for hydrogen chloride in rodents. Pp. 124-128 in Toxic Hazards Research Unit Annual Technical Report 1974. AMRL-TR-74-78. Aerospace Medical Research Laboratory, Wright-Patterson Air Force Base, Dayton, Ohio.
Machle, W., K.V. Kitzmiller, E.W. Scott, and J.F. Treon. 1942. The effect of inhalation of hydrogen chloride. J. Ind. Hyg. Toxicol. 24:222-225.
Malek, D.E., and Y. Alarie. 1989. Ergometer within a whole-body plethysmograph to evaluate performance of guinea pigs under toxic atmospheres. Toxicol. Appl. Pharmacol. 101:340-355.
Matt, L. 1889. Doctoral dissertation [in German]. Julius Maximilians University, Wurzburg, Germany.
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs). U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health, Division of Standards Development and Technology Transfer, Cincinnati, Ohio. Available from NTIS, Springfield, Va., Doc. No. PB94-195047.
NRC (National Research Council). 1987. Emergency and Continuous Exposure Guidance Levels for Selected Airborne Contaminants. Ammonia, Hydrogen Chloride, Lithium Bromide, and Toluene, Vol. 7. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1991. Permissible Exposure Levels and Emergency Exposure Guidance Levels for Selected Airborne Contaminants. Washington, D.C.: National Academy Press.
Pavlova, T.E. 1976. Disturbance in the development of the progeny of rats exposed to hydrogen chloride [in Russian]. Biull. Eksp. Biol. Med. 82:866-868.
Pellett, G.L., D.I. Sebacher, R.J. Bendura, and D.E. Wornom. 1983. HCl in rocket exhaust clouds: Atmospheric dispersion, acid aerosol characteristics, and acid rain deposition. J. Air Pollut. Control Assoc. 33:304-311.
Perry, W.G., F.A. Smith, and M.B. Kent. 1994. The halogens. Pp. 4487-4490 in Patty's Industrial Hygiene and Toxicology, Vol. 2, Part F, 4th Rev. Ed., G.D. Clayton and F.E. Clayton, eds. New York: John Wiley & Sons.
Remijn, B., P. Koster, D. Houthuijs, J. Boleij, H. Willems, B. Brunekreef, K. Biersteker, C. van Loveren. 1982. Zinc chloride, zinc oxide, hydrochloric acid exposure and dental erosion in a zinc galvanizing plant in the Netherlands. Ann. Occup. Hyg. 25:299-307.
Sayers, R.R., J.M. Dalla Valle, and W.P. Yant. 1934. Industrial hygiene and sanitation surveys in chemical establishments. Ind. Eng. Chem. 26:1251-1255.
Sebacher, D.I., R.J. Bendura, and D.E. Wornom. 1980. Hydrochloric acid aerosol and gaseous hydrogen chloride partitioning in a cloud contaminated by solid rocket exhaust. Atmos. Environ. 14:543-547.
Sellakumar, A.R., C.A. Snyder, J.J. Solomon, and R.E. Albert. 1985. Carcinogenicity of formaldehyde and hydrogen chloride in rats. Toxicol. Appl. Pharmacol. 81:401-406.
Spengler, J.D., M. Brauer, and P. Koutrakis. 1990. Acid air and health. Environ. Sci. Technol. 24:946-956.
Stavert, D.M., D.C. Archuleta, M.J. Behr, and B.E. Lehnert. 1991. Relative acute toxicities of hydrogen fluoride, hydrogen chloride, and hydrogen bromide in nose-and pseudo-mouth-breathing rats. Fundam. Appl. Toxicol. 16:636-655.
Stevens, B., J.Q. Koenig, V. Rebolledo, Q.S. Hanley, and D.S. Covert. 1992. Respiratory effects from the inhalation of hydrogen chloride in young adult asthmatics. J. Occup. Med. 34:923-929.
Symonds, R.B., W.I. Rose, and M.H. Reed. 1988. Contribution of Cl-and Fl-bearing gases to the atmosphere by volcanoes. Nature 334:415-148.
Tarlo, S.M. and I. Broder. 1989. Irritant-induced occupational asthma. Chest 96:297-300.
Toxigenics. 1984. 90-Day Inhalation Toxicity Study of Hydrogen Chloride Gas in B6C3F1 Mice, Sprague-Dawley Rats, and Fischer-344 Rats. Toxigenics, Decatur, Ill.
U.S. Department of Labor. 1998. Occupational Safety and Health Standards. Air Contaminants. Title 29, Code of Federal Regulations, Part 1910, Section 1910.1000. Washington, D.C.: U.S. Government Printing Office.
Wohlslagel, J., L. DiPasquale, and E. Vernot. 1976. Toxicity of solid rocket motor exhaust: Effects of HCl, HF, and alumina on rodents. J. Combust. Toxicol. 3:61-69.










































