Appendix E
ACUTE TOXICITY OF NITROGEN DIOXIDE
BACKGROUND INFORMATION
HUMAN and animal data indicate that exposure to nitrogen dioxide (NO2) can produce a variety of toxicological responses of varying degrees of severity depending on the concentration and duration of exposure and on the sensitivity of the population being exposed. Because NO2 is a gas, the primary route of exposure is via inhalation, making the lung the primary target organ; however, extrapulmonary effects also have been reported.
There have been numerous reviews on the toxicity of NO2 (NRC 1977; WHO 1977; EPA 1982, 1993). Most have focused on the health effects associated with exposures to low concentrations of NO2, such as those that might occur in the environment or in the workplace. Relatively few studies focused on toxicological responses following shortterm exposures to high concentrations of NO2.
PHYSICAL AND CHEMICAL PROPERTIES
|
CAS No.: |
10102-44-0 |
|
Molecular weight: |
46.0 |
|
Specific gravity: |
1.448 at 20° |
|
Boiling point: |
21.15°C |
|
Melting point: |
-9.3°C |
|
Freezing point: |
15°F |
|
Vapor pressure: |
720 mm Hg at 20°C |
|
Vapor density: |
1.58 (air = 1) |
|
Flash point: |
Not applicable |
|
Explosive limit: |
Not applicable |
|
Solubility: |
Soluble in concentrated nitric and sulfuric acids; decomposes in water; and nitric acid. |
|
Color: |
Reddish brown gas |
|
Conversion factor: |
1 ppm = 1.88 mg/m3 at 20°C |
|
|
1 mg/m3 = 0.53 ppm at 20°C |
SOURCES AND OCCURRENCE
In the ambient atmosphere, the major sources of NO2 are the combustion of fossil fuels and motor-vehicle emissions. Indoor sources include such appliances as gas stoves, water heaters, and kerosene space heaters. In the workplace, exposures to NO2 have been reported in such occupations as electroplating, acetylene welding, agriculture, space exploration, detonation of explosives, certain military activities, and burning of nitrogen-containing propellants (Mohsenin 1994). In such situations, exposure concentrations can be very high. For example, in armored vehicles during live-fire tests, peak concentrations of NO2 have been measured at over 2,000 parts per million (ppm). That decreases to about 500 ppm after 1 min and decreases to about 20 ppm within 5 min (Mayorga 1994).
Of concern to the Air Force is the presence of NOx/HNO3 emissions from rockets that use liquid propellants composed of nitrogen-based compounds. For normal launches, the nitrogen-associated emissions at ground level are negligible. However, in the case of a catastrophic abort, large quantities of nitrogen tetroxide can be released. That gas is rapidly converted to NO2 and HNO3, possibly resulting in production of quantities of NOx/HNO3 as high as 200,000 lb (see Appendix A).
PHARMACOKINETICS AND METABOLISM
When inhaled, NO2 reacts with the moisture in the respiratory tract, resulting in the formation of nitric acid (HNO3). The nitric acid dissoci-
ates into nitrates and nitrites. At low concentrations, NO2 reacts with moisture in the upper respiratory tract, but as the exposure concentration increases, that reaction penetrates into the lower respiratory tract. An increasing respiratory rate, such as might result from exercise, also results in higher concentrations of NO 2 and its products reaching deeper areas of the lung.
Once inhaled, NO2, or its chemical derivatives, can either remain within the lung or be transported to extrapulmonary sites via the bloodstream, where it can react with hemoglobin to form methemoglobin (MetHb). That reaction has important health implications because MetHb is an ineffective oxygen carrier. Transformation of hemoglobin to MetHb can increase health risks to vulnerable individuals who have hypoxia associated with pulmonary and cardiac disease. Increased levels of nitrates have been reported in the blood and urine following exposure to NO2, indicating that NO2 reacts to produce nitrates (EPA 1993).
SUMMARY OF TOXICITY INFORMATION
In this section, the available data on the toxicity of NO2 to humans and animals are described.
EFFECTS IN HUMANS
Data on the qualitative and quantitative toxicity of NO2 in humans come from reports of accidental exposures, clinical studies, and epidemiological studies, as described below.
Accidental Exposures
Accidental exposures to NO2 have been reported in agriculture (1502,000 ppm), mining explosions (500 ppm), space exploration (250 ppm), military activities (500 ppm), and the burning of nitrogen containing propellants (Mohsenin 1994). NO2 has an acrid, ammonia-like odor that is irritating and suffocating to heavily exposed individuals. Such accidental-exposure data, together with relevant animal studies, are most useful in establishing emergency short-term exposure limits.
NO2 can produce a variety of clinical responses, depending on the intensity and duration of the exposure (Lowry and Schuman 1956; Jones et al. 1973). For the most severely exposed, death can occur immediately or be delayed (Mohsenin 1994). Exposure above 150 ppm for 30 min to an hour results in fatal pulmonary edema or asphyxia and can result in rapid death (Lowry and Schuman 1956; NRC 1977; Mayorga 1994). Exposure to NO2 at concentrations of 150-300 ppm can result in bronchiolitis fibrosa obliterans accompanied by restrictive and obstructive ventilatory defects that might lead to death in 2 to 3 weeks (Lowry and Schuman 1956; NRC 1977; Mayorga 1994). Such exposures would likely produce permanent injury in those surviving the exposure. The LC50 (the lethal concentration for 50% of those exposed) for a 1-hr exposure for humans has been estimated to be 174 ppm (Book 1982). Four farmers who entered a freshly filled silo were exposed to high concentrations of NO2, estimated to range from 200 to 4,000 ppm. Two of the individuals died, and the others experienced immediate cough, dyspnea, and fever, which disappeared after several days but reappeared after about 3 weeks (Lowry and Schuman 1956; EPA 1982). Information on lethality from accidental exposures to NO2 is summarized in Table E-1A.
At lower exposure concentrations, a variety of nonlethal effects have been observed (see Table E-1B). From 50 to 100 ppm for a 30-min exposure, pulmonary edema and bronchiolitis with focal pneumonitis are likely to develop and last from 6 to 8 weeks; recovery is often spontaneous. Individuals exposed for 30 min at concentrations between 25 and 75 ppm might develop bronchial pneumonia, acute bronchitis, dyspnea, cyanosis, chess pain, rales, headaches, eye irritation, a dry nonproductive cough, and vomiting. Such effects usually are resolved in hours but sometimes are followed by a relapse with shortness of breath, cough, cyanosis, and fever. In addition to its effect on the lung, NO2 can transform hemoglobin to MetHb (Lowry and Schuman 1956; Grayson 1956; Stern 1968; Milne 1969; EPA 1993). Welders exposed to NO2 at 3.9 to 5.4 ppm exhibited 2.3% to 2.6% MetHb in their blood (Patty 1963).
In accidental exposures, accurate measurement of exposure concentrations are generally not available. However, during a manned space-flight, three astronauts were exposed to NO2 at concentrations reported to be 250 ppm for about 5 min; the peak was at 750 ppm (Hatton et al. 1977; Table E-1B). They reported immediate breathing difficulties that
TABLE E-1A Summary of NO2 Toxicity in Humans: Mortality from Accidental Exposures
|
Condition |
Expose Duration |
Expose Concentration ppm |
Effect Concentration, ppm |
End Points |
References |
|
Healthy |
Minutes |
200-400 |
— |
2/4 died |
Lowry and Schuman 1956 |
|
Healthy |
30 min |
>500 |
>500 |
Death in less than 2 d due to pulmonary edema |
Lowry and Schuman 1956; Grayson 1956 |
|
|
|
>300-400 |
>300 |
Fatal edema, bronchopneumonia |
Lowry and Schuman 1956; Grayson 1956 |
|
|
|
>150-200 |
>150 |
Bronchoiolitis fibrosa obliterans with death in 3-5 weeks |
Lowry and Schuman 1956; Grayson 1956 |
|
|
|
50-100 |
50 |
Bronciolitis and focal pneumonia with spontaneous recovery |
Lowry and Schuman 1956; Grayson 1956 |
|
Healthy |
1 hr |
174 |
— |
LC50 |
Book 1982 |
Table E-1B Summary of NO2 Toxicity to Humans: Nonlethal Effects from Accidental Exposure
|
Condition |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm |
End Points |
References |
|
Healthy |
5 min |
250 with peaks to 750 |
250 with peaks to 750 |
Chemical pneumonitis, 4.2 increase in MetHb |
Hatton et al. 1977 |
|
Healthy (welders) |
Daily |
3.9-5.4 |
3.9-5.4 |
2.3% and 2.6 MetH in the blood |
Patty 1963 |
|
Abbreviation: MetHb, methemoglobin |
|||||
became more severe within the first 24 hr, and chest radiographs suggested that they suffered diffuse chemical pneumonitis. The MetHb level also increased (4.2%). Several days later, the individuals became asymptomatic (Hatton et al. 1977). Because the exposure was not lethal and recovery was rapid, the exposure concentrations of NO2 might have been overestimated.
Human Clinical Studies
Human clinical studies of NO2 exposure have been conducted using healthy subjects and volunteer patients with existing pulmonary disease. Although such controlled exposures offer the best data to directly relate cause and effect in humans, few such studies have been conducted. Because the safety of volunteer subjects is of paramount importance, high exposure concentrations cannot be used, and only a few nonevasive end points usually are measured.
Normal Subjects Some significant responses, which could be attributed to inhalation of NO2, have been reported at concentrations of more than 1.0 ppm in normal subjects, as described below.
Sensory effects. Concentrations of 13.0 ppm or more resulted in complaints of eye and nasal irritation (Stern 1968). Humans can detect the odor of NO2 at low concentrations. At 0.12 ppm, 3 of 9 subjects perceived the odor immediately, and 8 of 13 detected concentrations of 0.22 ppm (Henschler et al. 1960). At a higher concentration (0.42 ppm), 8 of 8 subjects recognized the odor (Henschler et al. 1960). Feldman (1974) reported that 26 of 28 subjects had a perception of NO2 odor at concentrations of 0.11 ppm. Bylin et al. (1985) reported an odor threshold of 0.04 ppm for healthy subjects and 0.08 ppm for asthmatic subjects. However, in another study, exposed subjects were unable to detect the odor at 0.1 ppm (Hazucha et al. 1983). A 5-min exposure to NO2 at 25 ppm caused slight-to-moderate nasal discomfort in 5 of 7 volunteers and chest pain in 3 of the 7 (Meldrum 1992). Threshold values for impairment of dark adaptation was reported to be 0.07 ppm after 5 min inhalation of NO2 by mouth or after 25 min inhalation through the nose only (4 of 4 subjects) (Shalamberidze 1967). However, Bondareva (1963) found no evidence of impairment of dark adaptation. Sensory effects in humans exposed to NO2 for a 0.5 hr or less are summarized in Table E1c.
TABLE E-1c Summary of NO2 Toxicity in Humans: Sensory Effects
|
Condition |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm |
End Points |
References |
|
Olfactory |
|||||
|
Healthy |
Minutes |
≥13 |
≥13 |
Complaints of eye and nasal irritation |
Stern 1968 |
|
Healthy |
Immediate |
0.42 |
0.42 |
Perception of odor (8/8 |
Henschler et al. 1960 |
|
|
|
0.22 |
0.22 |
Perception of odor (8/13) |
|
|
|
|
0.12 |
0.12 |
Perception of odor (3/9) |
|
|
Healthy |
Immediate |
0.11 |
0.11 |
Perception of odor (26/28) |
Feldman 1974 |
|
Healthy |
Immediate |
0.04 |
0.04 |
Odor threshold for normal and asthmatic |
Bylin et al. 1985 |
|
|
|
0.08 |
0.08 |
subjects |
|
|
Healthy |
Immediate |
0.1 |
— |
Odor not detected |
Hazucha et al. 1983 |
|
Healthy |
5 min |
25.0 |
25.0 |
Slight nasal discomfort (5/7) and chest pain (3/7) |
Meldrum 1992 |
|
Visual |
|||||
|
Healthy |
5 min, 25 min |
0.07 |
0.07 |
Impaired dark adaptation |
Shalamberidze 1967 |
Effects on lung function. von Nieding and Wagner (1977) and von Nieding et al. (1979) reported that healthy subjects exposed to NO2 for 2 hr at a concentration of 5.0 ppm exhibited increased airway resistance and impaired oxygen exchange in the lung. They also reported a decrease in carbon dioxide diffusion capacity following a 15-min exposure at 5.0 ppm. Other investigators measured increases in airway resistance following a 10-min exposure at 0.7 to 2.0 ppm or at 4 to 5 ppm (Suzuki and Ishikawa 1965; Abe 1967). However, Linn et al. (1985b) and Mohsenin (1988) failed to find any changes in airway resistance or spirometry at concentrations of 4.0 ppm for 75 min or 2.0 ppm for 2 hr. Another investigator found increases in airway resistance in some subjects after a 10- to 120-min exposure to NO2 at 7.0 ppm, but other individuals tolerated 16 ppm without any such effect (Yokoyama 1972). A 10-min exposure at 4 to 5 ppm resulted in a 40% decrease in lung compliance 30 min after exposure ended (Abe 1967). Healthy subjects exposed at 2.5 and 7.5 ppm for 2 hr had increased airway resistance, but 1.0 ppm did not elicit any such effect (Beil and Ulmer 1976).
Below 1.0 ppm, short-term exposures (2 hr or less) do not appear to cause adverse effects in healthy subjects, at least as indicated by traditional measurement of pulmonary function (Kagawa and Tsuru 1979; Kerr et al. 1979; Toyama et al. 1981; Hazucha et al. 1982; Bylin et al. 1985; Koenig et al. 1985, 1987, 1988; Kagawa 1986; Adams et al. 1987; Drechsler-Parks 1987; Mohsenin 1988; Samet and Utell 1990; Kim et al. 1991). Some investigators reported subtle effects, but those findings are rare and do not reveal any consistent pattern of response (Kulle 1982; Rehn et al. 1982; EPA 1993).
Physiological changes in airway responsiveness to NO2 have been studied using a variety of stimuli to challenge the airways. With an acetylcholine challenge, a 2-hr exposure to NO2 at 7.5 ppm, but not at 5.0, 2.5, or 1.0 ppm, resulted in an increase in airway responsiveness to NO 2 (Beil and Ulmer 1976). However, Mohsenin (1988) reported increased airway reactivity to methacholine following a 1-hr exposure to NO 2 at 2.0 ppm.
Table E-1D summarizes the data from clinical studies of human lung function following exposure to NO2 for 2 hr or less. Lung biochemical measures in BAL fluid — Markers of pulmonary effects. A number of studies examined the bronchoalveolar lavage (BAL) fluid from humans in an attempt to identify cellular and biochemical responses to NO 2 exposure. No significant changes occurred in the levels of total protein, albumin, or α-2-macroglobulin in BAL fluid taken from
healthy volunteers exposed for 3 hr at 0.05 ppm, including three 15-min peak exposures at 2.0 ppm, or exposed for 3 hr continuously at 0.6 or 1.5 ppm without peaks (Frampton et al. 1989; Utell et al. 1991). Individuals exposed to NO2 at 3 to 4 ppm for 3 hr had a decrease in activity of α-1-protease inhibitor. This inhibitor is important in protecting the lung from proteolytic damage. Mohsenin and Gee (1987) and Mohsenin (1991) suggested that such a reduction would be most significant in individuals with α-1-antitrypsin deficiency. Table E-1E summarizes the data on BAL-fluid markers of pulmonary effects in humans exposed to NO2 for 2 hr or less.
Host defense mechanisms. The BAL fluid isolated from exercising individuals exposed to NO2 at 2.0 ppm for 240 min showed an increase in polymorphonuclear neutrophils (PMNs) and a decrease in the phagocytic activity of alveolar macrophages (Devlin et al. 1992). However, Sandstroem et al. (1990) exposed exercising individuals to NO2 at 4.0 ppm for 20 min on alternate days for 12 days and found enhanced phagocytic activity, a reduction in total cell count, and a decrease in number of mast cells, T and B lymphocytes, and natural killer cells in BAL fluid 24 hr post-exposure. A simple 20-min exposure at 2.25, 4.0, and 5.5 ppm resulted in a different response. The number of mast cells in BAL fluid increased at all exposure concentrations and the number of lymphocytes increased at 4.0 and 5.5 ppm 24 hr post-exposure (Sandstroem et al. 1989). Frampton et al. (1989) exposed humans to NO2 for 3 hr at 0.6 ppm and reported a reduced ability of macrophages to inactivate the influenza virus.
Because of the effects observed on the alveolar macrophages, other studies have looked for possible consequences of those macrophage changes with other end points, such as increases in respiratory infections and decreases in pulmonary clearance. However, human data examining the effects of NO2 on normal pulmonary clearance are inconclusive. Although the clearance of soot particles was significantly slower following acute exposure at 5.0 ppm (Schlipköter and Brockhaus 1963), a later study by Rehn et al. (1982) failed to find any significant changes in clearance of radiolabeled Teflon aerosols following 1 hr exposures to NO2 at 0.3 or 1.0 ppm.
Table E-1F summarizes the data on the effects of NO2 exposure for 3 hr or less on pulmonary defenses against infection in humans.
Extrapulmonary effects. Extrapulmonary effects have been reported in humans following NO2 exposure. Chaney et al. (1981) observed increases in blood glutathione levels in exercising humans exposed at
TABLE E-1D Summary of NO2 Toxicity in Healthy Humans: Pulmonary Function
|
Condition |
Exposure Duration |
Exposure Concentration ppm |
Effect Concentration, ppm |
End Points |
References |
|
Healthy |
2 hr |
5.0 |
5.0 |
Increased airway resistance; impaired oxygen exchange |
von Nieding and, Wagner 1977; vonNieding et al. 1979 |
|
Healthy |
2 hr |
1.0, 2.5, 7.5 |
≥2.5 |
Increased airway resistance |
Beil and Ulmer 1976 |
|
Healthy |
2 hr |
1.0, 2.5, 5.0, 7.5 |
7.5 |
Increased airway responsiveness to acetylcholine |
Beil and Ulmer 1976 |
|
Healthy |
75 min |
4.0 |
— |
No change in airway resistance |
Linn et al. 1985b |
|
Healthy |
2 hr |
2.0 |
— |
No change in airway resistance |
Mohsenin 1988 |
|
Healthy |
1 hr |
2.0 |
2.0 |
Increased airway responsiveness to methacholine |
Mohsenin 1988 |
|
Healthy |
10 min |
0.7-2.0 |
0.7 |
Increased airway resistance; effect increased with dose |
Suzuki and Ishikawa 1965 |
|
Healthy |
15 min |
5.0 |
5.0 |
Decreased CO diffusion capacity |
von Nieding and Wagner 1977, von Nieding et al. 1979 |
|
Healthy |
10-120 min 7.0, 16.0 |
7.0 |
7.0 |
Increased airway resistance in some subjects at 7.0 ppm, but some had no effect at 16.0 ppm |
Yokoyama 1972 |
|
Healthy |
10 min |
4-5 |
4-5 |
40% decrease in lung compliance and increase in airway resistance |
Abe 1967 |
|
Healthy |
≤2hr |
<1 |
— |
No measured Adverse effects in Healthy subjects |
Kagawa and Tsuru 1979; Kerr et al. 1979; Toyama et al. 1981; Hazucha et al. 1982; Bylin et al. 1985, 1987, 1988; Kagawa 1986, Adams et al. 1987; Drechsler-Parks 1987; Mohsenin 1988; Samet and Utell 1990, Kim et al. 1991 |
TABLE E-1E Summary of NO2 Toxicity in Humans: Lung Biochemical Measures in BAL Fluid
|
Condition |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm |
End Points |
References |
|
Healthy |
3 hr |
0.05 with three 15-min peaks at 2.0 or at 0.6 or 1.5 ppm without peaks |
— |
No change in BAL fluid composition |
Utell et al. 1991 Frampton et al. 1989 |
|
Healthy |
3 hr |
3-4 |
3-4 |
Decrease in protease inhibitor |
Mohsenin and Gee 1987; Moshenin 1991 |
|
Abbreviations: BAL, bronchoalveolar lavage; MetHb, methemoglobin. |
|||||
TABLE E-1F Summary of NO2 Toxicity in Humans: Host Defenses
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm |
End Points |
References |
|
Humans |
3 hr |
0.6 |
0.6 |
Reduced ability of macrophages to inactivate influenza virus |
Frampton et al. 1989 |
|
Humans (exercising) |
240 min |
2.0 |
2.0 |
Increase in number of PMNs in BAL; decrease in phagocytic activity of exposed macrophages |
Devlin et al. 1992 |
|
Humans |
1 hr |
5.0 |
5.0 |
Slower clearance of soot particles from lung |
Schlipköter and Brockhaus 1963 |
|
Humans |
1 hr |
0.3 |
1.0 |
No change in nasal or tracheobronchial clearance |
Rehn et al. 1982 |
|
Humans (exercising) |
20 min on alternate days for 12 d |
4.0 |
4.0 |
Total cell counts reduced; increase in macrophage phagocytic activity; decrease in number of T and B lymphocytes, mast cells, and natural killer cells 24 hr post-exposure |
Sandstroem et al. 1990 |
|
Humans (exercising) |
20 min |
2.25, 4.0, 5.5 |
2.25 |
Increase in mast cells in BAL fluid at all concentrations; increase in number of lymphocytes at 4.0 and 5.5 ppm 24 hr post-exposure |
Sandstroem et al.1989 |
|
Abbreviations: PMNs, polymorphonuclear neutrophils; BAL, bronchoalveolar lavage |
|||||
0.2 ppm for 2 hr, but because the effect was small and no other effects were observed in several other blood measurements (e.g., methemoglobin, red-blood-cell (RBC) glutathion (GSH) reductase, immunoglobulins, and complement C3), the authors thought that those responses might have been due to exercise and not to NO2 exposure. Small but significant decreases in blood pressure in exercising individuals have been reported following exposure to NO2 at 4.0 ppm for 75 min (Linn and Hackney 1983). A significant increase in plasma histamine was observed in human volunteers exposed four times at 0.3 ppm for 15 min (Kagawa 1986). Table E-1G summarizes the studies of the extrapulmonary effects of NO2 exposure on healthy humans for 2 hr or less.
Sensitive Subjects Clinical studies have examined the effects of NO2 exposure on sensitive subjects, because they might be more responsive and affected by lower concentrations of NO2, which might exacerbate existing disease. Such studies have sought to determine if NO2 increases airway responsiveness in subjects with asthma or chronic obstructive pulmonary disease (COPD).
Airway hyper-responsiveness to a wide variety of chemical and physical stimuli occurs with asthma. In clinical studies using asthmatic subjects, most significant responses have been associated with shortterm (1 to 3 hr) exposures to NO2 and low concentrations ranging from 0.2 to 0.5 ppm (EPA 1993; see Table E-1D). Those effects are not seen at higher concentrations (i.e., up to 4.0 ppm) (Orehek et al. 1976; EPA 1993). That indicates that the observed effects fail to follow a normal concentration-response relationship. Other investigators have confirmed that finding. Avol et al. (1988) reported an increase in bronchial reactivity to cold air in individuals exposed at 0.3 ppm, but not at 0.6 ppm, following a 2-hr exposure. Evidence indicates that such effects are reversible. Bauer et al.(1986) exposed asthmatic subjects to NO2 at 0.3 ppm for 20 min at rest, followed by 10 min of exercise, and found that all subjects had an increased response to cold-air bronchoprovocation, but that effect was not present 60 min post-exposure. Mohsenin (1987) found significant increases in airway responsiveness to methacholine in asthmatic subjects following a 1-hr exposure to NO2 at 0.5 ppm. Orehek et al. (1976) found increased responsiveness in asthmatic subjects to carbachol following a 1-hr exposure at 0.1 ppm. Exposure to NO2 for 1 hr at 0.4 ppm can potentiate specific airway response of patients with mild asthma to inhaled house-dust mite allergen. No significant effect was observed after similar exposure at 0.1 ppm (Tunnicliffe et al. 1994).
TABLE E-1G Summary of NO2 Toxicity in Humans: Extrapulmonary Effects
|
Condition |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm |
End Points |
References |
|
Healthy (exercising) |
2 hr |
0.2 |
0.2 |
Increase in blood glutathione levels (probably not significant) |
Chaney et al. 1981 |
|
Healthy |
75 min |
4.0 |
4.0 |
Small but significant increase in blood pressure |
Linn and Hackney 1983 |
|
Healthy |
15 min × 4 |
0.3 |
0.3 |
Increased plasma histamine levels |
Kagawa 1986 |
|
Healthy (welders) |
— |
3.9-5.4 |
3.9-5.4 |
2.3% and 2.6% MetHb in blood of welders |
Patty 1963 |
|
Abbreviations: MetHb, methemoglobin. |
|||||
Others, however, have failed to find either increases in responsiveness or any pulmonary functional changes in asthmatic subjects from exposures ranging from 0.1 to 0.6 ppm for 30 to 120 min (Kerr et al. 1979; Kulle 1982; Hazucha et al. 1982,1983; Ahmed et al. 1983a,b; Koenig et al. 1985, 1987; Roger et al. 1990; Rubenstein et al. 1990). Even at higher exposure concentrations and longer durations, subjects with mild asthma exposed at 3.0 ppm, with exercise, for 60 min or at 0.3 ppm for 3.75 hr failed to exhibit changes in pulmonary function, symptoms, or increased airway responsiveness to carbachol (Linn et al. 1986; Morrow and Utell 1989).
It is difficult to understand the differences in responses seen in asthmatic subjects exposed to NO2 at concentrations of a few parts per million or less. The increase in airway responsiveness to various bronchoconstrictors has been observed in some individuals in some studies, and in other studies, the effect was observed only at NO2 concentrations ranging from 0.2 to 0.5 ppm and was absent at higher concentrations (Avol et al. 1988). Such differences in response both between and within laboratories might be due to differences in the characteristics or severity of the disease from one test group to another or due to the season of the year when the studies were conducted. In many of the studies, the exposures were accompanied by exercise; exercise alone can be an important covariate in such studies.
Another potentially susceptible group that has been studied is individuals with COPD, such as emphysema and chronic bronchitis. In subjects with chronic bronchitis, exposure for 15 min to NO2 decreased blood PaO2 at 4 to 5 ppm and increased airway resistance at concentrations of 1.6 ppm or more (von Nieding et al. 1979). Exposure to NO2 for 15 min at concentrations between 1.6 and 5.0 ppm also caused significant increases in airway resistance in patients with chronic bronchitis, but below 1.5 ppm, no significant changes were observed (von Nieding et al. 1970). Linn et al. (1985a) found no changes in forced vital capacity (FVC) or forced expiratory volume at 1 sec (FEV1) at concentrations of 0.5,1.0, or 2.0 ppm in individuals with chronic bronchitis following a 1hr exposure, although there was evidence of a possible decrease in peak air flow at 2.0 ppm. Morrow and Utell (1989) examined the response of patients with COPD exposed to NO2 at 0.3 ppm for 3.75 hr and reported only small decreases in FVC and FEV1 with mild exercise. It is not obvious why some studies report that individuals with COPD experience pulmonary effects with exposure to NO2 at a few parts per million and others do not. However, COPD patients might experience some pulmo-
nary functional changes following brief high exposures to NO2. However, in the asthmatic and COPD populations, the effects from the interaction of the disease state and NO2 exposure remain questionable. Table E-1H summarizes the data from clinical studies of the responses of asthmatic and bronchitic subjects to NO2.
Epidemiological Studies
Most community studies are concerned primarily with effects resulting from low environmental exposure concentrations of NO2. Such studies have not been able to clearly document the health risks for humans exposed to ambient levels of NO2. This section will not review all of these studies, but refers the reader to EPA's (1993) Air Quality Criteria for Oxides of Nitrogen, which provides a comprehensive review of all the existing NO2 epidemiological studies.
Most epidemiological studies that have examined populations for adverse responses to NO2 have looked at either changes in normal lung function or increases in respiratory illness, especially in children. Although some studies suggest a possible association between NO2 exposure and an increase in respiratory disease in children, the concentrations of NO2 in such environments were usually very low (0.1 ppm or less) (Shy et al. 1970a,b; Pearlman et al. 1971; Melia et al. 1977; Speizer et al. 1980; Love et al. 1982). Other investigators have not been able to repeat those findings (Florey et al. 1979; Keller et al. 1979; Melia et al. 1982). The difference in findings has led to the conclusion that if an effect exists, it is subtle and difficult to distinguish from other environmental effects (EPA 1993). A similar conclusion has been drawn from those epidemiological studies examining the effects of NO2 on pulmonary function (EPA 1993). Because of the low exposure concentrations of NO2, the extended exposure durations, and the presence of other toxic chemicals in the air, evidence from epidemiological studies is of little value for establishing short-term exposure limits for accidental releases of NO2.
EFFECTS IN ANIMALS
Numerous health effects from exposure to NO2 have been confirmed in several species of animals. It is likely that such effects could occur in humans if appropriate exposures were encountered.
TABLE E-1H Summary of NO2 Toxicity in Sensitive Humans: Pulmonary Function
|
Condition |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm |
End Points |
References |
|
Asthma |
30 min (10- min 0.3 exercise) |
0.3 |
0.3 |
Significantly increased bronchial reactivity to cold air |
Bauer et al. 1986 |
|
Asthma |
1 hr |
0.5 |
0.5 |
Increased airway responsiveness to methacholine |
Mohsenin 1987 |
|
Asthma |
1 hr |
0.1 |
0.1 |
Increased airway reactivity to carbachol (13/20) |
Orehek et al. 1976 |
|
Asthma |
1 hr |
0.1, 0.4 |
0.4 |
Increased specific airway response to inhaled house-dust mite allergens |
Tunnicliffe et al. 1994 |
|
Asthma |
1 hr 3.75 hr |
3.0 0.3 |
— — |
No changes in pulmonary function; nonresponsive to carbachol |
Morrow and Utell 1989; Linn et al. 1986 |
|
Asthma |
2 hr |
0.3, 0.6 |
0.3 |
Increased bronchial reactivity to cold at 0.3 but not at 0.6 |
Avol et al. 1988 |
|
Asthma |
75 min (exercise) |
0.15, 0.30, 0.60 |
— |
No changes in pulmonary function, symptoms, or airway responsiveness to methacholine |
Roger et al. 1990 |
|
Chronic bronchitis |
15 min |
1-8 |
4-5 |
Reduced alveolar partial pressure of oxygen at 4-5 ppm (arterialized capillary blood); airway resistance increased at 1.6 ppm but not at 1.0 ppm |
von Nieding et al. 1979 |
|
Chronic bronchitis |
15 min |
0.5, 1.5, 1.6, 5.0 |
1.6 |
Significant increase in airway resistance at ≥1.6 but not at lower concentrations; decrease in earlobe blood PO2 at 5.0 ppm, probably starting at 4 ppm |
von Nieding et al. 1970 |
Mortality
The lethal concentration of NO2 varies from species to species and depends on the duration of exposure. Book (1982) reported LC50 values for a 1-hr exposure to NO2 at 99 ppm for mice, 110 ppm for rats, 91 ppm for guinea pigs, 140 ppm for rabbits, and 130 ppm for dogs. For shorter exposure times, the LC50 for rats was 415 ppm, 201 ppm, and 162 pm for a 5-, 15-, and 30-min exposure, respectively (Book 1982). The LC50 for the rabbits exposed for 15 min was 315 ppm (Carson et al. 1962). Those studies also indicate that brief high-concentration exposures to NO2 are more lethal than longer lower-concentration exposures that result in the same or higher total exposure (i.e., exposure concentration (C) multiplied by exposure duration (T)). Hine et al. (1970, as cited in EPA 1982) estimated a threshold concentration for mortality after a 1-hr exposure to be 40 to 50 ppm for a number of species of animals. Once the threshold concentration is exceeded, the death rate increases as the exposure period is lengthened. At 75 ppm, the LT50 (time at which 50% of the test animals die) ranges from 2.3 hr for mice, 2.7 hr for rabbits, 3.5 hr for rats, 4.0 hr for guinea pigs, to more than 8 hr for dogs. The NRC (1985) also cited threshold concentrations for acute mortality of 40 to 50 ppm for 1hr exposures for several species of animals.
Exercise combined with exposure at high concentrations of NO2 markedly increases the severity of edematous responses and subsequent mortality in animals. Mortality of exercising rats exposed at 1,000 ppm for 1.5 min was 80% higher than mortality in nonexercising rats exposed at 1,500 ppm for 1 min (Lehnert et al. 1994). Rats exposed for 2 min at 1,000 ppm exhibited 100% mortality following exercise (Lehnert et al. 1994). Three squirrel monkeys exposed for 2 hr at 50 ppm died following exposure (Henry et al. 1969).
Table E-2A summarizes the experimental data on the mortality of animals exposed to NO2 for 2 hr or less.
Pulmonary Injury
Inhalation of NO2 produces morphological evidence of pulmonary injury. The extent of the injury is related to the sensitivity of the target cell
TABLE E-2A Summary of NO2 Toxicity in Animals: Mortality
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm |
End Points |
References |
|
Mouse |
1 hr |
99 |
— |
LC50 |
Book 1982 |
|
Mouse |
2.3 hr |
75 |
— |
LT50 |
Hine et al. 1970 |
|
Rat |
1.5 min (exercise) |
1,000 |
— |
80% higher mortality with exercise than without exercise |
Lehnert et al. 19944 |
|
|
1.0 min (exercise) |
1,500 |
— |
|
|
|
Rat |
2.0 min (exercise) |
1000 |
1,000 |
100% mortality |
Lehnert et al. 1994 |
|
Rat |
5 min |
415 |
— |
LC50 |
Book 19822 |
|
|
15 min |
201 |
— |
LC50 |
|
|
|
30 min |
162 |
— |
LC50 |
|
|
|
1 hr |
110 |
— |
LC50 |
|
|
Rat |
3.5 hr |
75 |
— |
LT50 |
Hine et al. 19700 |
|
Guinea pig |
4.0 hr |
75 |
— |
LT500 |
|
|
Rabbit |
2.7 hr |
75 |
— |
LT500 |
|
|
Dog |
>8 hr |
75 |
— |
LT500 |
|
|
Guinea pig |
1 hr |
91 |
— |
LC50 |
Book 19822 |
|
Rabbit |
1 hr |
140 |
— |
LC50 |
|
|
Dog |
1 hr |
130 |
— |
LC500 |
|
|
Squirrel monkey(n = 3) |
2 hr |
50 |
— |
100% mortality |
Henry et al. 1969 |
|
Rabbit |
15 min |
315 |
— |
LC 50 |
Carson et al. 1962 |
|
Abbreviations: LC50, concentration at which 50% of the test animals die (for a specified exposure duration); LT50, time at which 50% of the test animals die (for a given exposure concentration). |
|||||
or tissue and the dose of NO2 delivered to the site. Once inhaled, NO2 affects mainly the bronchioles and adjacent alveolar spaces, where it can produce edema. Although a 5-min exposure of rats at 25 ppm did not produce any histopathological changes, exposure at higher concentrations (50, 75, 100 ppm) did produce mild lung injury, including type II hyperplasia and the appearance of pulmonary alveolar fibrin (Lehnert et al. 1994). Those effects progressed with increasing NO2 concentrations (150, 200, 250 ppm). As exposure time increased from 5 to 15 and 30 min, the level of type II hyperplasia correlated with increased exposure concentration, and alveolar fibrin appeared at concentrations of 25, 50,75 and 100 ppm. The fibrin response increased with increasing exposure concentration (Lehnert et al. 1994). Those studies indicate that brief exposures to high concentrations of NO2(e.g., 5 min at50 ppm) are more hazardous than longer exposures to lower concentrations (e.g., 30 min at 25 ppm) producing the same or higher C × T product. A similar C × T relationship was reported with lower concentrations and longer exposures (Gardner et al. 1979).
Histopathological examination of sheep exposed to NO2 by tracheal tube at 500 ppm for 15 to 20 min revealed patchy exudative material in all lung lobes and an increase in PMNs and mononuclear leukocytes (Januszkiewicz et al. 1992; Januszkiewicz and Mayorga 1994). When the concentration was reduced to 100 ppm for 15 min, only a modest increase in number of leukocytes was found compared with the increase found at 500 ppm (Januszkiewicz et al. 1992; Januszkiewicz and Mayorga 1994). Rats exposed to NO2 delivered through a sealed face for 15 min at 100 ppm exhibited a significant increase in edema and associated pathological lesions, whereas rats exposed at 25 or 50 ppm did not (Stavert and Lehnert 1990).
A series of studies reported the response of rats at high concentrations of NO2, ranging from 25 to 250 ppm for 1 to 30 min. A significant increase in lung wet weight (LWW) resulted from a single 5- and 15-min exposure at 150, 200, or 250 ppm but not at 25, 50, 75 or 100 ppm (Lehnert et al. 1994). A single 2-min exposure at 150 ppm also did not result in an increase in LWW (Lehnert et al. 1994). When the exposure duration was increased to 30 min, a significant increase in LWW was observed at 50 ppm (Stavert and Lehnert 1990). All rats exposed to NO2 for either 15 or 30 min at 200 and 250 ppm ultimately died. Exposure for 1 min to a high burst of NO2 at concentrations of 500, 1,000, 1,500, and 2,000 ppm all resulted in significant increases in LWW (Lehnert et al. 1994). The percentage change in LWW increased with increasing expo-
sure concentration. Exercise potentiated lung injury in the rats, resulting in further increases in LWW, extent and severity of alveolar fibrin, and red-blood-cell (RBC) extravasation post-exposure at 100 ppm for 15 min (Lehnert et al. 1994). LWWs measured in rats 25 hr post-exposure to NO2 either at 100 ppm for 15 or 30 min, at 300 ppm for 5 min, or at 1,000 ppm for 1 or 2 min significantly increased over LWWs in control rats (Lehnert et al. 1994).
Some additional damage to alveolar cell mitochondria and cell membranes at concentrations of 3 ppm or more has been reported in dogs (Dowell et al. 1971). A 4-hr exposure of rats at 0.5 ppm resulted in rupture and loss of cytoplasmic granules in mast cells (Thomas et al. 1967). When the concentration was increased to 1.0 ppm and the exposure time cut to 1 hr, there was degranulation and a decrease in number of mast cells. Those responses were reversible. Cats exposed for 3 hr at 80 ppm showed degeneration of Clara cells and loss of cilia 12 hr postexposure (Langloss et al. 1977). Clara-cell hyperplasia was evident 48 hr post-exposure. There was edema and neutrophilic emigration and extravasation of RBCs, bronchiolar congestion, increases in number of macrophages, and hyperplasia of type II cells (Langloss et al. 1977).
Table E-2B summarizes the evidence for pulmonary injury in animals following exposure to NO2 for 4 hr or less.
Effects on Lung Function
Both short-term and long-term exposures to NO2 produce changes in pulmonary function. Exposures of rats to various concentrations of NO2 for durations of 1 to 20 min produced changes in minute ventilation, tidal volumes, and breathing frequencies (Lehnert et al. 1994). Minute ventilation was reduced by 7% and 15% in rats exposed at 100 ppm for 15 and 20 min, respectively. A 10-min exposure, however, did not produce any changes. Exposures at higher concentrations and shorter dura-
TABLE E-2B Summary of NO2 Toxicity in Animals: Morphological and Pathological Effects on the Lung
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm |
End Points |
References |
|
Rat |
15 min |
200 |
— |
40% increase in LWW |
Elsayed et al. 1995 |
|
Rat |
1 min |
500, 1,000, 1,500, 2,000 |
≥500 |
Increasing LWW with increasing dose |
Lehnert et al. 1994 |
|
Rat |
15 min |
100 |
100 |
Exercise increased severity of lung injury over effects seen with no exercise |
Lehnert et al. 1994 |
|
Rat |
2 min |
150 |
— |
No increase in LWW |
Lehnert et al. 19944 |
|
Rat |
15 min |
25, 50,100 |
100 |
Pulmonary edema and pathological lesions seen only at the highest concentration |
Stavert and Lehnert 1990 |
|
Rat |
30 min |
10, 25, 50 |
≥50 |
Increase in LWW |
Stavert and Lehnert 1990 |
|
Rat |
5, 15 min |
25, 50, 75,100, 150, 200, 250 |
≥150 |
Increase in LWW post-exposure at ≥150 ppm for 5 min; at ≥100 ppm for 15 min |
Lehnert et al. 1994 |
|
Rat |
5 min |
25, 50, 75, 100, 150, 200, 250 |
≥50 |
Type II hyperplasia and appearance of alveolar fibrin, dose-response evident |
Lehnert et al. 1994 |
|
Rat |
15 min, no exercise |
25, 50, 75, 100, 150, 200, 250 |
25 |
All exposures resulted in alveolar fibrin; =50 ppm produced type II hyperplasia |
Lehnert et al. 1994 |
|
Rat |
30 min |
25, 50, 75, 100 |
≥25 |
No hyperplasia at 25 ppm, but increase in appearance of alveolar fibrin at all concentrations exercise potentiated lung injury |
Lehnert et al. 1994 |
|
Rat |
4 hr |
0.5 |
0.5 |
-Rupture and loss of cytoplasmic granules in most cells (reversible) |
Thomas et al. 1967 |
|
Rat |
1 hr |
1.0 |
1.0 |
-Degranulation and decrease in number of mast cells (reversible) |
|
|
Dog |
60 min |
3-16 |
≥3.0 |
Pulmonary edema at ≥7.0 ppm and evidence of cell membrane changes at ≥3.0 ppm |
Dowell et al. 1971 |
|
Dog |
4 hr |
37 |
37 |
Subtle changes in morphology with EM, alveolar desquamation, pulmonary edema; no gross effects with light microscopy |
Guidotti and Liebow 1977; Guidotti 1980 |
|
Sheep |
15-20 min by nasotracheal intubation |
500 |
500 |
Patchy exudative material in all lobes; increase in number of PMNs and mononuclear leukocytes |
Januszkiewicz and Mayorga 1994 |
|
Sheep |
15 min by nasotracheal intubation |
100, 500 |
100 |
Increased number of leukocytes at 100 ppm; at 500 ppm there was patchy lobular exudate and accumulation of leukocytes in alveolar sacs and interalveolar capillaries |
Januszkiewicz et al. 1992; Januszkiewicz and Mayorga 1994 |
|
Cat |
3 hr |
80 |
80 |
Degeneration and hyperplasia of Clara cells, loss of cilia, edema; increase in number of macrophages and hyperplasia of Type II cells |
Langloss et al. 1977 |
|
Squirrel monkey |
2 hr |
10-50 |
|
Concentration-related effects: |
Henry et al. 1969 |
|
|
|
|
10 |
—alveolar septa breaks and expanded alveoli; |
|
|
|
|
|
15 |
—patchy interstitial infiltration of lymphocytes; |
|
|
|
|
|
35 |
—areas of the lung collapsed and alveolar septa became basophilic; |
|
|
|
|
|
50 |
—frank edema, lungs showing extreme vesicular dilation and collapsed alveoli with lymphocyte infiltration, bronchi showing absence of cilia |
|
|
Abbreviations: LWW, lung wet weight; EM, electron microscopy; PMNs, polymorphonuclear neutrophils. |
|||||
tions (1,000 ppm for 1 and 2 min) resulted in a still greater decrease (i.e., 20% and 28%). The reduction in minute ventilation was due primarily to a reduction in tidal volume, which decreased as much as 40% at the highest concentration tested (1,000 ppm). Breathing frequencies increased only slightly during those exposures (Lehnert et al. 1994). Rats exposed to NO2 for 15 min at 200 ppm showed a decrease in inspired minute ventilation during exposure (Elsayed et al. 1995).
A biphasic response consisting of an immediate and delayed reaction was reported in sheep exposed to NO2 by intubation (nasal passages anesthetized by Xylocaine), at 500 ppm for 15 min (Januszkiewicz and Mayorga 1994). The immediate effect is manifested by an increase in minute-ventilation rate (+64%), an increase in respiratory rate (+129%), and a nonsignificant reduction in tidal volume (-20%). About 6 hr later, airway resistance and hypoxemia increased and lung compliance decreased (Januszkiewicz and Mayorga 1994).
When sheep were exposed to NO2 for 15 min at 100 ppm by intubation, only a modest increase in minute ventilation was found, compared with the increase seen following a similar exposure at 500 ppm (Januszkiewicz et al. 1992). There were no changes in pulmonary resistance and no demonstrable pathological lesions (Januszkiewcz et al. 1992). When NO2 was delivered to the sheep by face mask, the response was less severe than when delivered directly to the lung, indicating some protective effect afforded by the nasal passage and upper airway (Januszkiewicz and Mayorga 1994).
Abraham et al. (1980) reported that sheep exposed to NO2 at 7.5 or 15 ppm for 2 or 4 hr showed effects at only the highest concentration and exposure duration (15 ppm for 4 hr); the effects were increases in pulmonary resistance immediately following the exposure. Other species have been tested for pulmonary functional effects.
Guinea pigs exposed for 1 hr at NO2 concentrations ranging from 7 to 146 ppm showed concentration-related increases in respiratory rate and decreases in tidal volume, as well as an increase in sensitivity to inhaled histamine aerosols (Silbaugh et al. 1981). Exercising dogs exposed for 2 hr at 5 ppm had a statistically significant decrease in the ventilation equivalent for O2 (Kleinman and Mautz 1991). When squirrel monkeys were exposed for 2 hr at 10 or 14 ppm and challenged after exposure with a bacterial aerosol, tidal volume decreased but returned to normal or increased within 24 hr post-exposure (Henry et al. 1969).
Table E-2c summarizes the morphological and pathological effects on the lungs of animals following exposure to NO2 for 4 hr or less.
TABLE E-2C Summary of NO2 Toxicity in Animals: Pulmonary Function
|
Species |
Exposure Duration |
Exposure Concentration ppm |
Effect Concentration ppm |
End Points |
References |
|
Rat |
1, 2 min |
1,000 |
1,000 |
—Minute ventilation and tidal volume reduced by 20% at1 min and 28% at 2 min |
Lehnert et al. 1994 |
|
Rat |
10, 15, 20 min |
100 |
100 |
—Minute ventilation reduced by 7% at 15 min and 15% at 20 min |
|
|
Rat |
15 min |
200 |
200 |
Decrease in inspired minute-ventilation rate |
Elsayed et al. 1995 |
|
Guinea pig |
1 hr |
7-146 |
|
Concentration-related increases in respiratory rate; decrease in tidal volume; increase in sensitivity to histamine |
Silbaugh et al. 1981 |
|
Dog |
2 hr (exercise) |
5.0 |
5.0 |
Decrease in ventilation equivalent for O2 |
Kleinman and Mautz 1991 |
|
Sheep |
15 min by intubation |
100 |
100 |
Modest increase in minute ventilation |
Januszkiewicz et al. 1992 |
|
Sheep |
15 min by intubation |
500 |
500 |
Increase in minute ventilation; increase in respiration rate; increase in airway resistance; hypoxemia; decrease in compliance |
Januszkiewicz and Mayorga 1994 |
|
Sheep |
2 hr 4 hr |
7.5 15 |
15 (4 hr) |
Increase in pulmonary resistance only at 15 ppm for 4 hr |
Abraham et al. 1980 |
|
Squirrel monkey |
2 hr |
10, 14 before bacterial aerosol |
10 |
Decrease in tidal volume (reversible) |
Henry et al. 1969 |
Effects on Lung Biochemistry
Biochemical studies of lung lavage fluid recovered from exposed animals have focused on either the mechanism of toxicity or the detection of indicators of NO2-induced tissue and cell injury. Following exposure of rats to NO2 at 100 ppm for 15 min, isolated BAL fluid indicated a significant increase in the number of PMNs and lavageable protein but no increase in the number of macrophages (Lehnert et al. 1994). Those effects became evident 8 hr post-exposure and subsided after several hours in clean air. At still higher concentrations (200 ppm), Elsayed et al. (1995) found that a 15-min exposure of rats resulted in a decrease in the number of macrophages, increases in LWW, and an increase in the number of epithelial cells in BAL fluid. In addition, those authors reported a raised lipid peroxidation rate measured as fluorescent materials in isolated lipid extracts.
When sheep were exposed (lung only, nasal intubation under local anaesthesia) at 500 ppm for 15 to 20 min, they had significant increases in protein, albumin, and number of epithelial cells in BAL fluid and a significant decrease in the number of alveolar macrophages in BAL fluid (Januszkiewicz and Mayorga 1994; Mayorga et al. 1995). A similar response has been reported in dogs exposed at 200 ppm for 1 hr (Man et al. 1990).
A 3-hr exposure of guinea pigs at 5.0 ppm resulted in an increase in lipid content of the lavage fluid if the animals were vitamin-C depleted; no increase occurred if they were not vitamin-C depleted (Selgrade et al. 1981).
Total lecithin was reduced in BAL fluid in dogs exposed for 1 hr at 7 to 16 ppm but not at 3 ppm. There was a decrease in total phospholipids in BAL fluid from animals with intra-alveolar edema (Dowell et al. 1971). There was also an increase in the amount of unsaturated fatty acids in the phospholipids. Those changes were not noted in animals exposed at 3 ppm (Dowell et al. 1971).
Products of arachidonic acid metabolism in the lungs are also affected by NO2. The concentration of thromboxane B2 was higher in BAL fluid in rabbits exposed at 1.0 ppm for 2 hr (Schlesinger et al. 1990). When the NO2 concentration was increased to 3 ppm or 10 ppm, the concentration of thromboxane B2 in BAL fluid was depressed; at 10 ppm, the 6-keto-prostaglandin F1a concentrations in BAL fluid also were depressed.
Table E-2D summarizes the animal studies of biochemical changes in BAL fluid in response to exposure to NO2 for 3 hr or less.
Host Defense Mechanisms
The host defense system of the lung is one of the systems whose function has been shown to be altered by NO2 exposure in several species of animals, possibly because NO2 interferes with the efficiency of clearing unwanted substances from the lung. NO2 can disrupt the effectiveness of the mucociliary-clearance system. Exposure of sheep at 15 ppm for 2 hr resulted in a slowing of mucus transport (Abraham et al. 1980). No effect was seen at 7.5 ppm. Another study with rabbits exposed at 10 ppm for 2 hr found no effects on bronchial clearance (Schlesinger et al. 1988). However, Vollmuth et al. (1986) reported a concentration-related acceleration in clearance of particles from the lungs of rabbits following a 2-hr exposure at concentrations of 0.3, 1.0, 3.0 and 10 ppm, and the greatest increase occurred at the two lowest concentrations. That effect could possibly be considered an adaptive response to the exposure.
Pulmonary bactericidal activity decreased progressively with increasing concentrations of NO2. The effect was present in mice exposed for 4 hr at 7.0, 9.2, and 15 ppm, but not at 1.9 or 3.8 ppm (Goldstein et al. 1973). At the highest concentration tested, the bactericidal activity was reduced by 50% (Goldstein et al. 1973). With a 17-hr exposure, that dysfunction occurred at concentrations as low as 2.3 ppm (Goldstein et al. 1973). Exposure of mice for 4 hr at 5 or 10 ppm reduced the lung's bactericidal activity and increased the severity of Mycoplasma infection (Parker et al. 1989). Exposure of mice to NO2 at 5.0 ppm, but not at lower concentrations (0.5, 1.0, and 2.0 ppm), affected lung bactericidal activity against Mycoplasma (Davis et al. 1992). A 4-hr exposure of mice at concentrations of 4 ppm or more resulted in a concentration-related decrease in bactericidal activity, but no effect occurred at 2.5 ppm (Jakab 1988). A 2-hr exposure at 10,15, or 35 ppm resulted in delayed clearance of inhaled bacteria from the lungs of squirrel monkeys (Henry et al. 1969). Those reports are consistent with studies showing that macrophages obtained from rabbits exposed at 10 ppm for 3 hr had a 50% reduction in phagocytic activity (Gardner et al. 1969). Also, a 2-hr exposure at 8 ppm increased the number of PMNs in the BAL fluid of those animals compared with controls (Gardner et al. 1969).
TABLE E-2D Summary of NO2 Toxicity in Animals: Lung Biochemical Measures in BAL Fluid
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm |
End Points |
References |
|
Rat |
15 min |
100 |
100 |
Increase in protein concentrations and PMNs in BAL fluid (reversible) |
Lehnert et al. 1994 |
|
Rat |
15 min |
200 |
200 |
Evidence of increased rate of lipid peroxidation; decrease in number of macrophages, but increase in number of epithelial cells in BAL |
Elsayed et al. 1995 |
|
Guinea pig (vitamin-C depleted) |
3 hr |
5.0 |
5.0 |
Increase in BAL lipid concentrations |
Selgrade et al. 1981 |
|
Rabbit |
2 hr |
1, 3, 10 |
≥1.0 |
Increased concentrations of thromboxane B2 in BAL at 1.0 ppm, but decrease at other exposure concentrations; at 10 ppm, depression of 6-keto-prostaglandin F1α concentrations in BAL |
Schlesinger et al. 1990 |
|
Dog |
1 hr |
3, 7, 16 |
≥7 |
Decrease in total phospholipids, increase in amount of unsaturated fatty acids, no effect at 3.0 |
Dowell et al. 1971 |
|
Dogs |
1 hr |
200 |
200 |
Increase in protein, albumen, and number of epithelial cells in BAL fluid, but decrease in macrophage number |
Man et al. 1990 |
|
Sheep |
15-20 min by tracheal intubation |
500 |
500 |
Increase in protein, albumin, and number of epithelial cells in BAL, but decrease in macrophage number |
Januszkiewicz and Mayorga 1994; Mayorga et al. 1995 |
|
Abbreviations: PMNs, polymorphonuclear neutrophils; BAL, bronchoalveolar lavage. |
|||||
Other studies have shown that macrophages isolated from rabbits exposed for 3 hr at 25 ppm reduced production of interferon (Valand et al. 1970). A similar exposure at 15 ppm showed that macrophages had a lower capacity to phagocytize and to develop virus-induced resistance (Acton and Myrvik 1972).
Different experimental approaches have been used to determine the functional efficiency of the host's pulmonary defenses following NO2 exposure. Many studies measured the host's efficiency by challenging the NO2-exposed animals with a pulmonary infection. A linear exposure-duration-versus-response function was observed in mice exposed at 0.5 to 28 ppm, indicating that mortality increases with length of exposure (Gardner et al. 1979). Mortality also increased with increasing concentrations of NO2, as indicated by the steeper slopes of the exposure-duration-versus-response function at higher concentrations (Gardner et al. 1979). In general, exposure to high concentrations of NO2 for brief periods resulted in more severe pulmonary infections and greater mortality than exposure to lower concentrations for longer periods that produced similar C × T products. For example, when the C × T was held constant, exposure for 1.5 hr at 14 ppm (C × T = 21 ppm•min) increased mortality of mice by nearly 60% (Gardner et al. 1977). A 14-hr exposure at 1.5 ppm (C × T = 21 ppm•min) resulted in only a 12% increase in incidence of infection (Gardner et al. 1977). A 3hr exposure of mice at concentrations of 2 ppm or more, but not at concentrations of 1.5 ppm, resulted in increased infection from the Streptococcus organism (Ehrlich 1980). When a Klebsiella organism was used, the NO2 concentrations required to produce an increase in infections following a 2-hr exposure was 3.5 ppm or more, and no effect was seen at 1.5 or 2.5 ppm (Ehrlich 1980). Even when the bacterial infection was given 27 hr following an acute 2-hr exposure at 14 ppm, a significant increase in mortality was still evident (Ehrlich 1980). An increase in pulmonary infections was seen in hamsters and squirrel monkeys following a 2-hr exposure at 35 and 50 ppm, respectively (Ehrlich 1975, 1980).
Table E-2E summarizes the data on the effects of exposures to NO2 on the pulmonary defenses against infection in animals for 4 hr or less.
Extrapulmonary Effects
Evidence suggests that NO2 or its reaction products penetrate the lung
TABLE E-2E Summary of NO2 Toxicity in Animals: Host Defenses
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm |
End Points |
References |
|
Mouse |
1.5 hr |
14 |
14 |
60% increase in induced pulmonary infection |
Gardner et al. 1977 |
|
Mouse |
2 hr |
1.5, 2.5, 3.5, 5, 10, 15 |
>3.5 |
Concentration-related increase in induced pulmonary infection with Klebsiella |
Ehrlich 1980 |
|
Mouse |
3 hr |
1.5, 2.0, 5.0 |
>2.0 |
Concentration-related increase in induced infection with Ehrlich 1980 Streptococcus |
Ehrlic 1980 |
|
Mouse |
6 min to 12 mo |
1.5 to 28 |
Depends on C and T |
Increased induced infectious disease with increased T and C, but concentration more important than T |
Gardner et al. 1979 |
|
Mouse |
4 hr |
1.9, 3.8, 7.0, 9.2, 15.0 |
>7.0 |
Bactericidal activity decreased by 7%, 14%, and 50% at Goldstein et al. 1973 three highest concentrations, respectively |
Goldstein et al. 1973 |
|
Mouse |
4 hr |
5, 10 |
5 |
Reduced bactericidal activity; increased severity of induced Mycoplasma infection |
Parker et al. 1973 |
|
Mouse |
4 hr |
0.5, 1.0, 2.0, 5.0 |
5.0 |
Reduced bactericidal activity |
Davis et al. 1992 |
|
Mouse |
4 hr |
2.5, 4.0, 5.0, 10, 15 |
>4.0 |
Concentration-related decrease in bactericidal activity |
Jakab 1988 |
|
Hamster |
2 hr |
5.0, 25.0, 35.0 |
35 |
Increased incidence of induced pulmonary infection; no effect |
Ehrlich 1975, 1980 25 ppm |
|
Rabbit |
2 hr |
0.3, 1.0, 3.0, 10.0 |
>0.3 |
Concentration-related acceleration of pulmonary clearance of particles; greatest effect at lowest concentration |
Vollmuth et al. 1986 |
|
Rabbit |
2 hr |
8.0 |
8.0 |
Increase in number of PMNs in BAL |
Gardner et al. 1969 |
|
Rabbit |
2 hr |
10.0 |
— |
No effect on bronchial-clearance rate Schlesinger et al. 1988 |
Schlesinger et al. 1988 |
|
Rabbit |
3 hr |
25.0 |
25.0 |
Reduction in macrophages' ability to produce interferon |
Valand et al. 1970 |
|
Rabbit |
3 hr |
15.0 |
15.0 |
Reduced phagocytic activity of macrophages and reduced ability to develop virus-induced resistance |
Acton and Myrvik 1972 |
|
Rabbit |
3 hr |
10.0 |
10.0 |
50% reduction in macrophages, phagocytic activity |
Gardner et al. 1969 |
|
Sheep |
2 hr |
7.5, 15.0 |
15.0 |
Effects on mucociliary clearance, slowing of mucus transport |
Abraham et al. 1980 |
|
Squirrel monkey |
2 hr |
10, 15, 35 |
>10 |
Delayed clearance of inhaled micro-organisms |
Henry et al. 1969 |
|
Squirrel monkey |
2 hr |
50 |
50 |
Increase in pulmonary-infection incidence |
Ehrlich 1975 |
|
Abbreviations: C, concentration; T, time (exposure duration). |
|||||
and enter the bloodstream, producing a wide array of health effects beyond the lung. Rats exposed for 15 min at 200 ppm had an increase in carboxyhemoglobin (COHb) (Elsayed et al. 1995). Investigators found that when sheep were exposed at 500 ppm (lung only, nasal intubation under local anaesthesia) for 15 to 20 min, both pulmonary arterial pressure and pulmonary artery wedge pressure were significantly higher 24 hr post-exposure, and a small, but significant, increase in blood MetHb was reported (Januszkiewicz and Mayorga 1994; Mayorga et al. 1995). However, when mice were exposed for 1 hr at 5 to 40 ppm, there was no evidence of MetHb, yet increased nitrite and nitrate concentrations were found in the blood (Oda et al. 1981). In contrast, Case et al. (1979) found concentration-related increases in MetHb in mice exposed at 1 to 30 ppm for 18 hr.
Several studies have identified direct effects on the kidney and liver from exposures to NO2, but the exposures used in those studies usually lasted several days or weeks (EPA 1993). However, Miller et al. (1980) found that exposure of mice at 0.25 ppm for 3 hr resulted in a significant increase in pentobarbital-induced sleeping time. Such a response implies a potential effect on some aspects of liver metabolism. Also, a single 2-hr exposure at 0.3 to 4.0 ppm produced increased levels of ascorbic acid in the liver of mice (Veninga and Lemstra 1975). Information on the effects of NO2 on the central nervous system (CNS), behavior, and reproduction and developmental effects is limited to a few studies of longer exposure durations; the relation of those effects to humans is uncertain (EPA 1993).
Although exercise has been shown to increase the severity of several of the pulmonary responses, a 15-min exposure to NO2 at 25,50, and 100 ppm also reduced the rat's ability to perform maximal exercise as assessed by measuring maximum oxygen consumption (Mayorga et al. 1995). A 1-min exposure at 100 ppm also significantly reduced exercise performance (Mayorga et al. 1995).
Table E-2F summarizes the studies of the extrapulmonary effects of exposures to NO2 in animals for durations of 2 hr or less.
ESTABLISHED INHALATION EXPOSURE LIMITS
Table E-3 lists existing exposure limits for civilian and military populations. Table E-4 lists Army incapacitation criteria for NO2. Figure E-1 shows the relation among the civilian exposure limits listed in Table E-3.
TABLE E-2F Summary of NO2 Toxicity in Animals: Extrapulmonary Effects
|
Species |
Exposure Duration |
Exposure Concentration, ppm |
Effect Concentration, ppm |
End Points |
References |
|||
|
Mouse |
1 hr |
5-40 |
— |
No evidence of MetHb, but increased levels of nitrite and nitrate in blood |
Oda et al. 1981 |
|||
|
Mouse |
18 hr |
1-30 |
— |
Concentration-related increases in MetHb and decrease 1979 in catalase and iron transferrin activity in the blood |
Case et al. |
|||
|
Rat |
15 min |
200 |
200 |
Increased COHb |
Elsayed et al. 1995 |
|||
|
Sheep |
15-20 min |
500 |
500 |
Increase in pulmonary artery and pulmonary artery wedge pressure; small increase in MetHb |
Mayorga et al. 1995; Januszkiewicz and Mayorga 1994 |
|||
|
Mouse |
3 hr |
0.25 |
0.25 |
Significant increase in pentobarbital-induced sleeping time |
Miller et al. 1980 |
|||
|
Mouse |
2 hr |
0.3-4.0 |
>0.3 |
Increased levels of ascorbic acid in liver |
Veninga and Lemstra 1975 |
|||
|
Rat |
15 min |
25, 50, 100 |
>25 |
Concentration-related decrease in maximal exercise |
Mayorga et al. 1995 |
|||
|
Rat |
1 min |
25, 50, 100 |
100 |
Significant reduction in exercise performance |
Mayorga et al. 1995 |
|||
|
Abbreviations: COHb, carboxyhemoglobin; MetHb, methemoglobin |
||||||||
TABLE E-3 Currently Recommended Civilian and Military Human Exposure Limits for NO2
|
Exposure Limit |
Concentration, ppm |
Reference |
|||
|
EPA |
|
|
|||
|
NAAQS |
0.053 |
EPA 1971 |
|||
|
LOC |
2 |
EPA 1987 |
|||
|
ACGIH |
|
|
|||
|
TLV-TWA |
3 |
ACGIH 1991 |
|||
|
TLV-STEL |
5 |
ACGIH 1991 |
|||
|
NIOSH |
|
|
|||
|
IDLH |
20 |
NIOSH 1994 |
|||
|
REL |
1 (15-min STEL) |
NIOSH 1994 |
|||
|
OSHA PEL |
5 (ceiling) |
U.S. Dept. of Labor 1998 |
|||
|
NRC SPEGLs |
|
|
|||
|
1 hr |
1.0 |
NRC 1985 |
|||
|
2 hr |
0.5 |
NRC 1985 |
|||
|
4 hr |
0.25 |
NRC 1985 |
|||
|
8 hr |
0.12 |
NRC 1985 |
|||
|
16 hr |
0.06 |
NRC 1985 |
|||
|
24 hr |
0.04 |
NRC 1985 |
|||
|
AIHA EELs |
|
|
|||
|
5 min |
35 |
AIHA 1964 |
|||
|
15 min |
25 |
AIHA 1964 |
|||
|
30 min |
20 |
AIHA 1964 |
|||
|
60 min |
10 |
AIHA 1964 |
|||
|
U.S. Army Acceptable Human Criteria in Armored Vehicle During Live Fire Testing |
|||||
|
15 min |
50 |
Mayorga 1994 |
|||
|
5 min |
100 |
Mayorga 1994 |
|||
|
Abbreviations: EPA, U.S. Environmental Protection Agency; NAAQS, National Ambient Air Quality Standard; LOC, level of concern; ACGIH, American Conference of Governmental Industrial Hygienists; TLV, Threshold Limit Value; TWA, time-weighted average; STEL, short-term exposure limit; NIOSH, National Institute for Occupational Safety and Health; IDLH, immediately dangerous to life and health; REL, recommended exposure limit; OSHA, Occupational Safety and Health Administration; PEL, permissible exposure level; NRC, National Research Council; SPEGL, short-term public emergency guidance level; AIHA, American Industrial Hygiene Association; EEL, emergency exposure limits. |
|||||
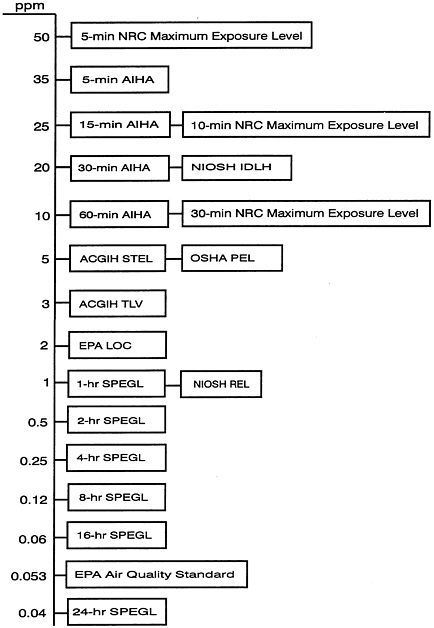
FIGURE E-1 Currently recommended exposure limits for human exposure to NO2 (see Table E-3 for sources).
TABLE E-4 U.S. Army Incapacitation Criteria for NO2
|
Concentration and Time |
Incapacitation |
|
1-50 ppm and 1-30 min |
Nonfatal reversible injury; no immediate incapacitation |
|
50-100 ppm and 20-30 min |
No immediate incapacitation, but some risk of nonfatal permanent injury |
|
150-200 ppm and 30 min |
No immediate incapacitation, but subsequent fatal injury |
|
300-750 ppm and few min |
100% incapacitation or fatal injury |
|
Source: Adapted from Mayorga 1994. |
|
EVALUATION OF TOXICITY INFORMATION
Available data indicate that exposure to NO2 can result in a wide variety of respiratory effects in humans and animals. Exposure to NO2 can cause reversible and irreversible effects, and the response depends on the concentration, the duration, and the specific pattern of exposure and the species. Although the severity of effects increases with increasing exposure concentration and duration, concentration has the more pronounced influence. Using data from accidental exposures, controlled human studies, and experimental animal studies, the goal of this chapter is to identify exposure levels associated with a threshold for effects in sensitive and normal populations and exposure levels that might result in moderate and severe effects. Given the large array of studies on NO2, it is important to judge the value of each study and try to reconcile divergent results.
Although the available human data indicate that NO2 can produce a variety of health effects with varying degrees of severity, depending on the concentration and duration of exposure and possibly on the sensitivity of the population being exposed, most studies have examined longterm exposures at concentrations that might be found in the environment or in the workplace. Reports of exposures at high concentrations for short durations were most often related to accidents, where exposure concentrations were poorly characterized.
Because high concentrations of NO2 cannot be administered to humans, health effects seen in animals must be extrapolated to humans. The effects seen in animals exposed to NO2 can reasonably be expected to occur in humans if the exposure concentrations were adequate to
induce the effect. However, quantitative extrapolation from animals to humans requires quantitative information on interspecies similarities and differences in dosimetry and sensitivity. Some of that type of information is available; however, it is not adequate for quantitative extrapolation. The relation between C × T and health effects for NO2 exposures is especially important for determining the importance of short-term peak concentrations compared with time-weighted-average (TWA) concentrations over longer exposure durations (e.g., 1 hr or more). Following a rocket launch, high concentrations of NO2 will most likely exist with other rocket-emission toxicants in the atmosphere. Exposure to NO2 together with other emission chemicals (although not HCl and HNO3 for normal launches) might result in additive effects.
Certain groups might be more sensitive to the effects of NO2 exposure than others; those groups are persons with pre-existing cardiopulmonary problems and children. Lower concentrations of NO2 might affect those groups more than healthy adults, or the severity of an effect at a given concentration might be greater. The data available to judge the potential impact of NO2 on particularly sensitive subgroups are not consistent, however. NO2 is emitted in the home environment by gas cooking and has been reported to increase susceptibility to respiratory tract infections in young children. However, that effect is believed to result from long-term exposures, which are not applicable to rocketlaunch situations. Short-term exposures of human volunteers to NO2 have generally provided conflicting results. The ATS (1996) compiled a list of nine controlled studies of asthmatic subjects published since 1980. For seven of the studies, no changes in pulmonary function or airway responsiveness were listed. One study (Bauer et al. 1986) showed that exposure of asthmatic subjects to NO2 at a concentration of 0.3 ppm potentiated exercise-induced bronchospasm and airway hyperactivity after cold-air provocation. By comparison, exposure of healthy humans at concentrations up to 4 ppm usually failed to affect pulmonary function (ATS 1996). However, in another study, no significant lung-function alterations could be found in asthmatic subjects exposed at 0.3 ppm NO2 for 1 hr (Morrow and Utell 1989). Mohsenin (1987) found heightened airway reactivity in asthmatic subjects exposed to NO2 at 0.5 ppm for 1 hr. However, Linn et al. (1985b) observed no effects from exposures to NO2 at concentrations up to 4 ppm for 1.25 hr in individuals with asthma or in healthy individuals; that observation was attributed to potential adaptation of the subjects who lived in an area
with frequent increases in common air pollutants. Responsiveness to a 4-hr exposure to NO2 at 0.3 ppm was slightly greater in patients with chronic obstructive pulmonary disease (COPD) than in elderly healthy subjects; interindividual variation in responsiveness also was substantially greater in elderly subjects than in the other groups (Morrow et al. 1992). Thus, some study comparisons suggest that individuals with asthma or COPD begin respond to NO2 at concentrations (0.3 ppm) approximately 10-fold lower than do healthy individuals (4 ppm), while other comparisons suggest no difference in the exposure concentration representing a threshold for effect in the two subgroups. Studies have not been conducted using high concentrations of NO2.
Exposure to NO2 can produce a variety of clinical response depending on the intensity and duration of exposure. From the data presented in the preceding sections, hazards associated with short-term exposures can be classified into three general levels of response: (1) mild, (2) moderate, or (3) severe, as described in Chapter 4.1 Table E-5 outlines the subcommittee's assessment of mild, moderate, and severe adverse health effects associated with exposure to NO2 and the exposure concentrations at which such effects might begin to occur in healthy adults exposed for durations up to 1 hr. As indicated in Table E-1D, numerous studies have failed to identify measurable health effects in healthy subjects exposed at less than 1 ppm for up to 2 hr. At 4 to 5 ppm, Abe (1967) noted a 40% decrease in lung compliance and an increase in airway resistance in healthy humans exposed for 10 min, although Linn et al. (1985b) found no change in airway resistance for exposures at 4 ppm for 75 min. On the basis a review of case reports of individuals exposed to NO2 in silos, Lowry and Schuman (1956) concluded that exposure to NO2 at concentrations of 50 ppm for 30 to 60 min could cause bronchiolitis and focal pneumonia, although recovery could occur without medical intervention.
TABLE E-5 Subcommittee Assessment of Anticipated Health Effects Following NO2 Exposure in Healthy Humans for Up to 1 hra
|
Severity |
Concentration, ppm |
Effects |
|
Mild |
>1 |
No immediate incapacitation; symptomatic effects include: transient discomfort; unpleasant odor; respiratory and eye irritation; acute reversible respiratory function effects; minor changes in hematological indexes; reversible histological effects; changes in normal host defense mechanisms. |
|
Moderate |
>4 |
No immediate incapacitation; respiratory and eye irritation; reversible changes in respiratory function, pulmonary pathology and morphology; biochemical end points in lung. |
|
Severe |
> 50 |
Serious effects requiring medical treatment or hospitalization; progressive respiratory injury, including edema, permanent pulmonary injury, loss of consciousness, and possible death |
|
a The concentrations listed are the lowest estimated concentrations at which effects in the specified severity category might occur in some members of the healthy population. As exposure concentration increases, more individuals are expected to be affected, and the severity of response within an individual is expected to increase. For exposures for less than 1 hr, the concentrations at which mild, moderate, and severe effects might begin to occur in the healthy population could be somewhat higher than those listed. How much higher depends on the relationship between exposure duration and concentration to the effects seen. The concentrations shown in the table are based on available human data. Because of the limitations associated with these data, Haber's law should not be used for extrapolations, especially for those at the higher concentrations shown in the table. That relationship is described in Chapter 6. |
||
Although the effects listed in Table E-5 are classified on the basis of their expected severity, the subcommittee must emphasize that predicting severity is based on its best judgment given the available data. Individuals can vary greatly in their susceptibility to the harmful effects of exposure to any chemical such as NO2. That factor is especially critical with exposures to high concentrations, even for a short exposure time.
RESEARCH NEEDS
For purposes of the LATRA-ERF model, data that would be most useful to the Air Force would be those from controlled human exposures using
Experiments using the same exposure protocols on mice and rats as humans would be useful to identify the degree of difference in sensitivity to NO2 between laboratory animals and sensitive and healthy humans.
To evaluate the potential for additive effects among the rocket-emission toxicants, exposure of healthy humans to various combinations of HCl, NO2, and HNO3 at concentrations expected to occur following a catastrophic abort might be useful. To be safe, the tests could be limited to exposure concentrations expected to produce mild effects only.
Controlled human exposures at high NO2 exposure concentrations to determine the sensitivity of individuals with cardiopulmonary impairments and that of healthy individuals are not possible. Thus, to fill this data gap, an animal model that might be expected to react similarly to sensitive humans would be needed. The same animal model probably could be used to investigate this data gap for the other rocket-emission toxicants examined in this report.
REFERENCES
Abe, M. 1967. Effects of mixed NO2-SO2 gas on human pulmonary functions: Effects of air pollution on the human body. Bull. Tokyo Med. Dent. Univ. 14:415-433.
Abraham, W.M., M. Welker, W. Oliver, Jr., M. Mingle, A.J. Januszkiewicz, A. Wanner, and M.A. Sackner. 1980. Cardiopulmonary effects of short-term nitrogen dioxide exposure in conscious sheep. Environ. Res. 22:61-72.
ACGIH (American Conference of Governmental Industrial Hygienists). 1991. Documentation of the Threshold Limit Values and Biological Exposure Indices, 6th Ed. American Conference of Governmental Industrial Hygienists, Cincinnati, Ohio.
Acton, J.D., and Q.N. Myrvik. 1972. Nitrogen dioxide effects on alveolar macrophages. Arch. Environ. Health 24:48-52.
Adams, W.C., K.A. Brookes, and E.S. Schelegle. 1987. Effects of NO2 alone and in combination with 03 on young men and women. J. Appl. Physiol. 62:1698-1704.
Ahmed, T., R. Dougherty, and M.A. Sackner. 1983a. Effect of NO2 Exposure on Specific Bronchial Reactivity in Subjects with Allergic Bronchial Asthma. Final Report. Contract CR-83/07/BI. General Motors Research Laboratories, Warren, Mich.
Ahmed, T., R. Dougherty, and M.A. Sackner. 1983b. Effect of 0.1 ppm NO2 on Pulmonary Functions and Non-specific Bronchial Reactivity of Normals and Asthmatics. Final Report. Contract CR-83/11/ BI. General Motors Research Laboratories, Warren, Mich.
AIHA (American Industrial Hygiene Association). 1964. Emergency Exposure Limits. Am. Ind. Hyg. Assoc. J. 25:578-586.
ATS (American Thoracic Society). 1996. Health effects of outdoor air pollution. Part 2. Am. J. Respir. Crit. Care Med. 153:477-498.
Avol, E.L., W.S. Linn, R.C. Peng, G. Valencia, D. Little, and J.D. Hackney. 1988. Laboratory study of asthmatic volunteers exposed to nitrogen dioxide and to ambient air pollution. Am. Ind. Hyg. Assoc. J. 49:143-149.
Bauer, M.A., M.J. Utell, P.E. Morrow, D.M. Speers, and F.R. Gibb. 1986. Inhalation of 0.30 ppm nitrogen dioxide potentiates exercise-induced bronchospasm in asthmatics. Am. Rev. Respir. Dis. 134:1203-1208.
Beil, M., and W.T. Ulmer. 1976. Effect of NO2 in workroom concentrations on respiratory mechanics and bronchial susceptibility to acetylcholine in normal persons [in German]. Int. Arch. Occup. Environ. Health 38:31-44.
Bondareva, E.N. 1963. Hygienic evaluation of low concentrations of nitrogen oxides present in acid. Pp. 98-101 in USSR Literature on Air Pollution and Related Occupational Diseases. A Survey, Vol. 8, B.S. Levine, ed. Publ. TT63-11570. U.S. Public Health Service, Washington, D.C.
Book, S.A. 1982. Scaling toxicity from laboratory animals to people: An example with nitrogen dioxide. J. Toxicol. Environ. Health 9:719-725.
Bylin, G., T. Lindvall, T. Rehn, and B. Sundin. 1985. Effects of short-term exposure to ambient nitrogen dioxide concentrations on human bronchial reactivity and lung function. Eur. J. Respir. Dis. 66:205-217.
Carson, T.R., M.S. Rosenholtz, F.T. Wilinski, and M.H. Weeks. 1962. The responses of animals inhaling nitrogen dioxide for single, short-term exposures. Am. Ind. Hyg. Assoc. J. 23:457-462.
Case, G.D., J.S. Dixon, and J.C. Schooley. 1979. Interactions of blood metalloproteins with nitrogen oxides and oxidant air pollutants. Environ. Res. 20:43-65.
Chaney, S., W. Blomquist, P. DeWitt, and K. Muller. 1981. Biochemical changes in humans upon exposure to nitrogen dioxide while at rest. Arch. Environ. Health 36:53-58.
Davis, J.K., M.K. Davidson, T.R. Schoeb, and J.R. Lindsey. 1992. Decreased
intrapulmonary killing of Mycoplasma pulmonis after short-term exposure to NO2 is associated with damaged alveolar macrophages. Am. Rev. Respir. Dis. 145(2 Part 1):406-411.
Devlin, R. D. Horstman, S. Becker, T. Gerrity, M. Madden, and H. Koren. 1992. Inflammatory response in humans exposed to 2.0 ppm NO 2 [abstract]. Am. Rev. Respir. Dis. 145:A456.
Dowell, A.R., K.H. Kilburn, and P.C. Pratt. 1971. Short-term exposure to nitrogen dioxide: Effects on pulmonary ultrastructure, compliance, and the surfactant system. Arch. Intern. Med. 128:74-80.
Drechsler-Parks, D.M. 1987. Effect of Nitrogen Dioxide, Ozone, and Peroxyacetyl Nitrate on Metabolic and Pulmonary Function . Res. Rep. 6. Cambridge, Mass.: Health Effects Institute.
Ehrlich, R. 1975. Interaction Between NO2 Exposure and Respiratory Infection. Scientific Seminar on Automotive Pollutants. EPA/600/9-75/003. U.S. Environmental Protection Agency, Office of Research and Development, Washington, D.C.
Ehrlich, R. 1980. Interaction between environmental pollutants and respiratory infections. Environ. Health Perspect. 35:89-100.
Elsayed, N.M., S. Smith, D. Ebel, N.V. Gorbounov, M.J. Topper, V.E. Eagon, and M.A. Mayorga. 1995. Pulmonary Alterations after Brief, Nose-Only Exposure of Rats to High Levels of Nitrogen Dioxide (Abstract 19-P-6). International Congress of Toxicology VII, July 2-6.
EPA (U.S. Environmental Protection Agency). 1971. National primary and secondary ambient air quality standards. Fed. Regist. 36(April 30):8186-8201.
EPA (U.S. Environmental Protection Agency). 1982. Air Quality Criteria for Oxides of Nitrogen. EPA/600/8-82-026. U.S. Environmental Protection Agency, Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office, Research Triangle Park, N.C. Available from NTIS, Springfield, Va., Doc. No. PB83-131011.
EPA (U.S. Environmental Protection Agency). 1987. Technical Guidance for Hazards Analysis: Emergency Planning for Extremely Hazardous Substances . Prepared in cooperation with the Federal Emergency Management Agency, Washington, D.C., and U.S. Department of Transportation, Washington, D.C. U.S. Environmental Protection Agency, Office of Solid Waste and Emergency Response, Washington, D.C. Available from NTIS, Springfield, Va., Doc. No. PB93-206910.
EPA (U.S. Environmental Protection Agency). 1993. Air Quality Criteria for Oxides of Nitrogen, Vol. 3. EPA/600/8-91/049cF. U.S. Environmental Protection Agency, Office of Research and Development, Washington, D.C.
Feldman, Y.G. 1974. The combined action on a human body of a mixture of the main components of motor vehicle exhaust gases (carbon monoxide, nitrogen dioxide, formaldehyde, and hexane) [in Russian]. Gig. Sanit. 10:7-10.
Florey, C. du V., R.J.W. Melia, S. Chinn, B.D. Goldstein, A.G.F. Brooks, H.H.
John, I.B. Craighead, and X. Webster. 1979. The relation between respiratory illness in primary schoolchildren and the use of gas for cooking: III. Nitrogen dioxide, respiratory illness, and lung infection. Int. J. Epidemiol. 8:347-353.
Frampton, M.W., A.M. Smeglin, N.J. Roberts, Jr., J.N. Finkelstein, P.E. Morrow, and M.J. Utell. 1989. Nitrogen dioxide exposure in vivo and human alveolar macrophage inactivation of influenza virus in vitro. Environ. Res. 48:179-192.
Gardner, D.E., R.S. Holzman, and D.L. Coffin. 1969. Effects of nitrogen dioxide on pulmonary cell population. J. Bacteriol. 98:1041-1043.
Gardner, D.E., F.J. Miller, E.J. Blommer, and D.L. Coffin. 1977. Relationships between nitrogen dioxide concentration time and level of effect using an animal infectivity model. Pp. 513-525 in International Conference on Photochemical Oxidant Pollution and Its Control. Proceedings, Vol. 1, B. Dimitriades, ed. EPA-600/3-77-001a. U.S. Environmental Protection Agency, Environmental Sciences Research Laboratory, Research Triangle Park, N.C. Available from NTIS, Springfield, Va., Doc. No. PB-264232.
Gardner, D.E., F.J. Miller, E.J. Blommer, and D.L. Coffin. 1979. Influence of exposure mode on the toxicity of NO2. Environ. Health Perspect. 30:23-29.
Goldstein, E., M.C. Eagle, and P.D. Hoeprich. 1973. Effect of nitrogen dioxide on pulmonary bacterial defense mechanisms. Arch. Environ. Health 26:202-204.
Grayson, R.R. 1956. Silage gas poisoning: Nitrogen dioxide pneumonia, a new disease in agricultural workers. Ann. Intern. Med. 45:393-408.
Guidotti, T.L. 1980. Toxic inhalation of nitrogen dioxide: Morphologic and functional changes. Exp. Mol. Pathol. 33:90-103.
Guidotti, T.L., and A.A. Liebow. 1977. Toxic inhalation of NO2 in canines. In International Conference on Photochemical Oxidant Pollution and Its Control. Proceedings, Vol. 1, B. Dimitriades, ed. EPA-600/3-77-001a. U.S. Environmental Protection Agency, Environmental Sciences Research Laboratory, Research Triangle Park, N.C. Available from NTIS, Springfield, Va., Doc. No. PB-264232.
Hatton, D.V., C.S. Leach, E. Nicogossian, and N. Di Ferrante. 1977. Collagen breakdown and nitrogen dioxide inhalation. Arch. Environ. Health 32:33-36.
Hazucha, M.J., J.F. Ginsberg, W.F. McDonnell, E.D. Haak, Jr., R.L. Pimmel, D.E. House, and P.A. Bromberg. 1982. Changes in bronchial reactivity of asthmatics and normals following exposures to 0.1 ppm NO2. Pp. 387-400 in Air Pollution by Nitrogen Oxides. Proceedings of the US-Dutch International Symposium, T. Schneider and L. Grant, eds. Studies in Environmental Science 21. Amsterdam: Elsevier Scientific.
Hazucha, M.J., J.F. Ginsberg, W.F. McDonnell, E.D. Haak, Jr., R.L. Pimmel, S.A. Salaam, D.E. House, and P.A. Bromberg. 1983. Effects of 0.1 ppm nitrogen dioxide on airways of normal and asthmatic subjects. J. Appl. Physiol.
Respir. Environ. Exercise Physiol. 54:730-739.
Henry, M.C., R. Ehrlich, and W.H. Blair. 1969. Effect of nitrogen dioxide on resistance of squirrel monkeys to Klebsiella pneumoniae infection. Arch. Environ. Health 18:580-587.
Henschler, D., A. Stier, H. Beck, and W. Neumann. 1960. Olfactory threshold of some important irritant gases and effects in man at low concentrations. Arch. Gewerbepath. Gewerbehyg 17:547-570.
Hine, C.H., F.H. Meyers, and R.W. Wright. 1970. Pulmonary changes in animals exposed to nitrogen dioxide, effects of acute exposures. Toxicol. Appl. Pharmacol. 16:201-213.
Jakab, G.J. 1988. Modulation of Pulmonary Defense Mechanisms Against Viral and Bacterial Infections by Acute Exposures to Nitrogen Dioxide. Res. Rep. 20. Cambridge, Mass.: Health Effects Institute.
Januszkiewicz, A.J., and M.A. Mayorga. 1994. Nitrogen dioxide induced acute lung injury to sheep. Toxicology 89:279-300.
Januszkiewicz, A.J., J.R. Snapper, J.W. Sturgis, D.B. Rayburn, K.A. Dodd, Y.Y. Phillips, G.R. Ripple, D.D. Sharpnack, N.M. Coulson, and J.A. Bley. 1992. Pathophysiologic responses of sheep to brief high-level NO2 exposure. Inhal. Toxicol. 4:359-372.
Jones, G.R., A.T. Proudfoot, and J.I. Hall. 1973. Pulmonary effects of acute exposure to nitrous fumes. Thorax 28:61-65.
Kagawa, J. 1986. Experimental studies on human health effects of aerosol and gaseous pollutants. Pp. 683-697 in Aerosols: Research, Risk Assessment and Control Strategies, Proceedings of the Second U.S.-Dutch International Symposium, S.D. Lee, T. Schneider, L.D. Grant, and P.J. Verkerk, eds. Chelsea, Mich.: Lewis Publishers.
Kagawa, J., and K. Tsuru. 1979. Respiratory effects of 2-hour exposure to ozone and nitrogen dioxide alone and in combination in normal subjects performing intermittent exercise [in Japanese]. Nippon Kyobu Shikkan Gakkai Zasshi 17:765-774.
Keller, M.D., R.R. Lanese, R.I. Mitchell, and R.W. Cote. 1979. Respiratory illness in household using gas and electricity for cooking: II. Symptoms and objective findings. Environ. Res. 19:504-515.
Kerr, H.D., T.J. Kulle, M.L. McIlhany, and P. Swidersky. 1979. Effects of nitrogen dioxide on pulmonary function in human subjects: An environmental chamber study. Environ. Res. 19:392-404.
Kim, S.U., J.Q. Koenig, W.E. Pierson, and Q.S. Hanley. 1991. Acute pulmonary effects of nitrogen dioxide exposure during exercise in competitive athletes. Chest 99:815-819. Kleinman, M.T., and W.J. Mautz. 1991. The Effects of Exercise on Dose and Dose Distribution of Inhaled Automotive Pollutants. Res. Rep. 45. Cambridge, Mass.: Health Effects Institute. Koenig, J.Q., D.S. Covert, M.S. Morgan, M. Horike, N. Horike, S.G. Marshall,
and W.E. Pierson. 1985. Acute effects of 0.12 ppm ozone or 0.12 ppm nitrogen dioxide on pulmonary function in healthy and asthmatic adolescents. Am. Rev. Respir. Dis. 132:648-651.
Koenig, J.Q., D.S. Covert, S.G. Marshall, G. van Belle, and W.E. Pierson. 1987. The effects of ozone and nitrogen dioxide on pulmonary function in healthy and in asthmatic adolescents. Am. Rev. Respir. Dis. 136:1152-1157.
Koenig, J.Q., W.E. Pierson, D.S. Covert, S.G. Marshall, M.S. Morgan, and G. van Belle. 1988. The Effects of Ozone and Nitrogen Dioxide on Lung Function in Healthy and Asthmatic Adolescents. Res. Rep. 14. Cambridge, Mass.: Health Effects Institute.
Kulle, T.J. 1982. Effects of nitrogen dioxide on pulmonary function in normal healthy humans and subjects with asthma and chronic bronchitis. Pp. 477486 in Air Pollution by Nitrogen Oxides. Proceedings of the US-Dutch International Symposium, T. Schneider and L. Grant, eds. Studies in Environmental Science 21. Amsterdam: Elsevier Scientific.
Langloss, J.M., E.A. Hoover, and D.E. Kahn. 1977. Diffuse alveolar damage in cats induced by nitrogen dioxide or feline calicivirus. Am. J. Pathol. 89:637-644.
Lehnert, B.E., D.C. Archuleta, T. Ellis, W.S. Session, N.M. Lehnert, L.R. Gurley, and D.M. Stavert. 1994. Lung injury following exposure of rats to relatively high mass concentrations of nitrogen dioxide. Toxicology 89:239-277.
Linn, W.S., and J.D. Hackney. 1983. Short-term human respiratory effects of nitrogen dioxide: Determination of quantitative dose-response profiles, phase I. Exposure of healthy volunteers to 4 ppm NO2. Report CRCAPRAC-CAPM48-83. Atlanta, Ga.: Coordinating Research Council. Available from NTIS, Springfield, Va., Doc. No. PB84-132299.
Linn, W.S., D.A. Shamoo, C.E. Spier, L.M. Valencia, U.T. Anzar, T.G. Venet, E.L. Avol, and J.D. Hackney. 1985a. Controlled exposure of volunteers with chronic obstructive pulmonary disease to nitrogen dioxide. Arch. Environ. Health 40:313-317.
Linn, W.S., J.C. Solomon, S.C. Trim, C.E. Spier, D.A. Shamoo, T.G. Venet, E.L. Avol, and J.D. Hackney. 1985b. Effects of exposure to 4 ppm nitrogen dioxide in healthy and asthmatic volunteers . Arch. Environ. Health 40:234-239.
Linn, W.S., D.A. Shamoo, E.L. Avol, J.D. Whynot, K.R. Anderson, T.G. Venet, and J.D. Hackney. 1986. Dose-response study of asthmatic volunteers exposed to nitrogen dioxide during intermittent exercise. Arch. Environ. Health 41:292-296.
Love, G.J., S.P. Lan, C.M. Shy, and W.B. Riggan. 1982. Acute respiratory illness in families exposed to nitrogen dioxide ambient air pollution in Chattanooga, Tennessee. Arch. Environ. Health 37:75-80.
Lowry, T., and L.M. Schuman. 1956. Silo-filler's disease: A newly recognized syndrome caused by nitrogen dioxide inhalation with a report of six cases. Univ. Minn. Med. Bull. 27:234-238.
Man, S.F.P., D.J. Williams, R.A. Amy, G.C.W. Man, and D.C. Lein. 1990. Sequential changes in canine pulmonary epithelial and endothelial cell functions after nitrogen dioxide. Am. Rev. Respir. Dis. 142:199.
Mayorga, M.A. 1994. Overview of nitrogen dioxide effects on the lung with emphasis on military relevance . Toxicology 89:175-192.
Mayorga, M.A., A.J. Januszkiewicz, and B.E. Lenhert. 1995. Environmental nitrogen dioxide exposure hazards of concern to the U.S. Army. Pp. 323-343 in Fire and Polymers II: Materials and Tests for Hazard Prevention ACS Symposium Series No. 599. G.L. Nelson, ed. Washington, D.C.: American Chemical Society.
Meldrum, M. 1992. Toxicology of substances in relation to major hazards: Nitrogen dioxide [abstract]. British Library Document Center. London: HMSO.
Melia, R.J.W., C. du V. Florey, D.G. Altman, and A.V. Swan. 1977. Association between gas cooking and respiratory disease in children. Br. Med. J. 2:149-152.
Melia, R.J.W., C. du V. Florey, R.W. Morris, B.D. Goldstein, H.H. John, D. Clark, I.B. Craighead, and J.C. Mackinlay. 1982. Childhood respiratory illness and the home environment: II. Association between respiratory illness and nitrogen dioxide, temperature and relative humidity. Int. J. Epidemiol. 11:164-169.
Miller, F.J., J.A. Graham, J.W. Illing, and D.E. Gardner. 1980. Extrapulmonary effects of NO2 as reflected by pentobarbital-induced sleeping time in mice. Toxicol. Lett. 6:267-274.
Milne, J.E.H. 1969. Nitrogen dioxide inhalation and bronchitis obliterans. A review of the literature and report of a case. J. Occup. Med. 11:538-547.
Mohsenin, V. 1987. Airway responses to nitrogen dioxide in asthmatic subjects. J. Toxicol. Environ. Health 22:371-380.
Mohsenin, V. 1988. Airway responses to 2.0 ppm nitrogen dioxide in normal subjects. Arch. Environ. Health 43:242-246.
Mohsenin, V. 1991. Lipid peroxidation and antielastase activity in the lung under oxidant stress: Role of antioxidant defenses. J. Appl. Physiol. 70:1456-1462.
Mohsenin, V. 1994. Human exposure to oxides of nitrogen at ambient and supra-ambient concentrations. Toxicology 89:301-312.
Mohsenin, V., and J.B.L. Gee. 1987. Acute effect of nitrogen dioxide exposure on the functional activity of alpha-l-protease inhibitor in bronchoalveolar lavage fluid of normal subjects. Am. Rev. Respir. Dis. 136:646-650.
Morrow, P.E., and M.J. Utell. 1989. Responses of Susceptible Subpopulations to Nitrogen Dioxide. Res. Rep. 23. Cambridge, Mass.: Health Effects Institute.
Morrow, P.E., M.J. Utell, M.A. Bauer, A.M. Smeglin, M.W. Frampton, C. Cox, D.M. Speers, and F.R. Gibb. 1992. Pulmonary performance of elderly nor
mal subjects and subjects with chronic obstructive pulmonary disease exposed to 0.3 ppm nitrogen dioxide. Am. Rev. Respir. Dis. 145:291-300.
NIOSH (National Institute for Occupational Safety and Health). 1994. Documentation for Immediately Dangerous to Life or Health Concentrations (IDLHs). U.S. Department of Health and Human Services, National Institute for Occupational Safety and Health, Division of Standards Development and Technology Transfer, Cincinnati, Ohio. Available from NTIS, Springfield, Va., Doc. No. PB94-195047.
NRC (National Research Council). 1977. Nitrogen Oxides. Washington, D.C.: National Academy Press.
NRC (National Research Council). 1985. Pp. 83-95 in Emergency and Continuous Exposure Guidance Levels for Selected Airborne Contaminants; Vol. 4. Washington, D.C.: National Academy Press.
Oda, H., H. Tsubone, A. Suzuki, T. Ichinose, and K. Kubota. 1981. Alterations of nitrite and nitrate concentrations in the blood of mice exposed to nitrogen dioxide. Environ. Res. 25:294-301.
Orehek, J., J.P. Massari, P. Gayrard, C. Grimaud, and J. Charpin. 1976. Effect of short-term, low-level nitrogen dioxide exposure on bronchial sensitivity of asthmatic patients. J. Clin. Invest. 57:301-307.
Parker, R.F., J.K. Davis, G.H. Cassell, H. White, D. Dziedzic, D.K. Blalock R.B. Thorp, and J.W. Simecka. 1989. Short-term exposure to nitrogen dioxide enhances susceptibility to murine respiratory mycoplasmosis and decreases intrapulmonary killing of Mycoplasma pulmonis. Am. Rev. Respir. Dis. 140:502-512.
Patty, F.A. 1963. Inorganic compounds of oxygen, nitrogen, and carbon. Pp. 919-923 in Toxicology, D.W. Fassett and D.D. Irish, eds., Vol. 2 of Industrial Hygiene and Toxicology, 2nd Rev. Ed., F.A. Patty, ed. New York: Interscience.
Pearlman, M.E., J.F. Finklea, J.P. Creason, C.M. Shy, M.M. Young, and R.J. Horton. 1971. Nitrogen dioxide and lower respiratory illness. Pediatrics 47:391-398.
Rehn, T., M. Svartengren, K. Philipson, and P. Camner. 1982. Mucociliary transport in the lung and nose and air resistance after exposure to nitrogen dioxide [in Swedish]. Coal-Health Environment Project, Tech. Rep. 40. Vallingby, Sweden: Swedish State Power Board.
Roger, L.J., D.H. Horstman, W. McDonnell, H. Kehrl, P.J. Ives, E. Seal, R. Chapman, and E. Massaro. 1990. Pulmonary function, airway responsiveness, and respiratory symptoms in asthmatics following exercise in NO2. Toxicol. Ind. Health 6:155-171.
Rubenstein, I., B.G. Bigby, T.F. Resis, and H.A. Boushey, Jr. 1990. Short-term exposure to 0.3 ppm nitrogen dioxide does not potentiate airway responsiveness to sulfur dioxide in asthmatic subjects. Am. Rev. Respir. Dis. 141:381-385.
Samet, J.M., and M.J. Utell. 1990. The risk of nitrogen dioxide: What have we learned from epidemiological and clinical studies? Toxicol. Ind. Health 6:247-262.
Sandstroem, T., B. Kolmodin-Hedman, N. Stjernberg, and M.C. Andersson. 1989. Inflammatory cell response in bronchoalveolar fluid after nitrogen dioxide exposure of healthy subjects [abstract]. Am. Rev. Respir. Dis. 139(Suppl.):A124.
Sandstroem, T., M.C. Andersson, B. Kolmodin-Hedman, N. Stjernberg, and T. Angstrom. 1990. Bronchoalveolar mastocytosis and lymphocytosis after nitrogen dioxide exposure in man: A time-kinetic study. Eur. Respir. J. 3:138-143.
Schlesinger, R.B., K.E. Driscoll, B.D. Naumann, and T.A. Vollmuth. 1988. Particle clearance from the lungs: Assessment of effects due to inhaled irritants. Ann. Occup. Hyg. 32(Suppl. 1):113-123.
Schlesinger, R.B., K.E. Driscoll, A.F. Gunnison, and J.T. Zelikoff. 1990. Pulmonary arachidonic acid metabolism following acute exposures to ozone and nitrogen dioxide. J. Toxicol. Environ. Health 31:275-290.
Schlipköter, H.-W., and A. Brockhaus. 1963. Versuche über den Einfluss gasförmiger Luftverunreingungen auf die Deposition und Elimination inhalierter Stäube. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. Abt. 191:339-344.
Selgrade, M.K., M.L. Mole, F.J. Miller, G.E. Hatch, D.E. Gardner, and P.C. Hu. 1981. Effect of NO2 inhalation and vitamin C deficiency on protein and lipid accumulation in the lung. Environ. Res. 26:422-437.
Shalamberidze, O.P. 1967. Reflex effects of mixtures of sulfur and nitrogen dioxides. Hyg. Sanit. (USSR) 32:10-15.
Shy, C.M., J.P. Creason, M.E. Pearlman, K.E. McClain, F.B. Benson, and M.M. Young. 1970a. The Chattanooga school children study: Effects of community exposure to nitrogen dioxide. I. Methods, description of pollutant exposure, and results of ventilatory function testing. J. Air Pollut. Control Assoc. 20:539-545.
Shy, C.M., J.P. Creason, M.E. Pearlman, K.E. McClain, F.B. Benson, and M.M. Young. 1970b. The Chattanooga school children study: Effects of community exposure to nitrogen dioxide. II. Incidence of acute respiratory illness. J. Air Pollut. Control Assoc. 20:582-588.
Silbaugh, S.A., J.L. Mauderly, and C.A. Macken. 1981. Effects of sulfuric acid and nitrogen dioxide on airway responsiveness of the guinea pig . J. Toxicol. Environ. Health 8:31-45.
Speizer, F.E., B. Ferris, Jr., Y.M.M. Bishop, and J. Spengler. 1980. Respiratory disease rates and pulmonary function in children associated with NO2 exposure. Am. Rev. Respir. Dis. 121:3-10.
Stavert, D.M., and B.E. Lehnert. 1990. Nitric oxide and nitrogen dioxide as
inducers of acute pulmonary injury when inhaled at relatively high concentrations for brief periods. Inhal. Toxicol. 2:53-67.
Stern, A.C., ed. 1968. Pp. 446-609 in Air Pollution, 2nd Ed. Vol. 1: Air Pollution and Its Effects. New York: Academic.
Suzuki, T., and K. Ishikawa. 1965. Pp. 199-221 in Research on the Effects of Smog on the Human Body: Report of the Specialized Study on Prevention of Air Pollution, No. 2 [in Japanese]. Research Coordination Bureau of the Science and Technology Agency, Tokyo, Japan.
Thomas, H.V., P.K. Mueller, and R. Wright. 1967. Response of rat lung mast cells to nitrogen dioxide inhalation. J. Air Pollut. Control Assoc. 17:33-35.
Toyama, T., T. Tsunoda, M. Nakaza, T. Higashi, and T. Nakadate. 1981. Airway response to short-term inhalation of NO2, 03 and their mixture in healthy men. Sangyo Igaku 23:285-293.
Tunnicliffe, W.S., P.S. Burge, and J.G. Ayres. 1994. Effect of domestic concentrations of nitrogen dioxide on airway responses to inhaled allergen in asthmatic patients. Lancet 344(8939-9840):1733-1736.
U.S. Department of Labor. 1998. Occupational Safety and Health Standards. Air Contaminants. Title 29, Code of Federal Regulations, Part 1910, Section 1910.1000. Washington, D.C.: U.S. Government Printing Office.
Utell, M., M.W. Frampton, N.J. Roberts, Jr., J.N. Finkelstein, and P.E. Morrow. 1991. Mechanisms of Nitrogen Dioxide Toxicity in Humans [abstract]. Investigators Report. Cambridge, Mass.: Health Effects Institute.
Valand, S.B., J.D. Acton, and Q.N. Myrvik. 1970. Nitrogen dioxide inhibition of viral-induced resistance in alveolar monocytes. Arch. Environ. Health 20:303-309.
Veninga, T., and W. Lemstra. 1975. Extrapulmonary effects of ozone whether in the presence of nitrogen dioxide or not. Int. Arch. Arbeitsmed. 34:209-220.
Vollmuth, T.A., K.E. Driscoll, and R.B. Schlesinger. 1986. Changes in early alveolar particle clearance due to single and repeated nitrogen dioxide exposures in rabbits. J. Toxicol. Environ. Health 19:255-266.
von Nieding, G., H.M. Wagner, H. Krekeler, U. Smidt, and K. Muysers. 1970. Absorption of NO2 in Low Concentrations in the Respiratory Tract and its Acute Effects on Lung Function and Circulation. Paper No. MB-15G presented at the Second International Clean Air Congress, Washington, D.C., December.
von Nieding, G., and H.M. Wagner. 1977. Experimental studies on the shortterm effect of air pollutants on pulmonary function in man: Two-hour exposure to NO2, O3, and SO2 alone and in combination. Pp. 5-8 in the Proceedings of the Fourth International Clean Air Congress, S. Kasuga, N. Suzuki, T. Yamada, G. Kimura, K. Inagaki, and K. Onoe, eds. Japanese Union of Air Pollution Prevention Associations, Tokyo, Japan.
von Nieding, G., H.M. Wagner, H. Krekeler, H. Loellgen, W. Fries, and A.
Beuthan. 1979. Controlled studies of human exposure to single and combined action of NO2, O3, and SO2. Int. Arch. Occup. Environ. Health 43:195-210.
WHO (World Health Organization). 1977. Environmental Health Criteria 4: Oxides of Nitrogen. Geneva, Switzerland: World Health Organization.
Yokoyama, E. 1972. The respiratory effects of exposure to SO2-NO2 mixtures on healthy subjects [in Japanese]. Jpn. J. Ind. Health 14:449-454.


















































