This paper was presented at a colloquium entitled “Neuroimaging of Human Brain Function,” organized by Michael Posner and Marcus E.Raichle, held May 29–31, 1997, sponsored by the National Academy of Sciences at the Arnold and Mabel Beckman Center in Irvine, CA.
Components of verbal working memory: Evidence from neuroimaging
EDWARD E.SMITH*†, JOHN JONIDES*, CHRISTY MARSHUETZ*, AND ROBERT A.KOEPPE‡
*Department of Psychology, and ‡Division of Nuclear Medicine, University of Michigan, Ann Arbor, MI 48109–1109
ABSTRACT We review research on the neural bases of verbal working memory, focusing on human neuroimaging studies. We first consider experiments that indicate that verbal working memory is composed of multiple components. One component involves the subvocal rehearsal of phonological information and is neurally implemented by left-hemisphere speech areas, including Broca’s area, the premotor area, and the supplementary motor area. Other components of verbal working memory may be devoted to pure storage and to executive processing of the contents of memory. These studies rest on a subtraction logic, in which two tasks are imaged, differing only in that one task presumably has an extra process, and the difference image is taken to reflect that process. We then review studies that show that the previous results can be obtained with experimental methods other than subtraction. We focus on the method of parametric variation, in which a parameter that presumably reflects a single process is varied. In the last section, we consider the distinction between working memory tasks that require only storage of information vs. those that require that the stored items be processed in some way. These experiments provide some support for the hypothesis that, when a task requires processing the contents of working memory, the dorsolateral prefrontal cortex is disproportionately activated.
Working memory (WM) is the cognitive mechanism that allows us to keep a limited amount of information active for a brief period of time. Sometimes we need to keep information active solely for storage purposes, as when we maintain a just-looked-up phone number until we can dial it. Other times we need to keep information active so that we can use it in executing a complex task. For example, when we mentally multiply two digit numbers such as 29×19, we first may compute the partial product 9×9=81, and later use this partial product in further computations. Situations like this one are common. We frequently store the outcomes of intermediate computations temporarily when solving a problem, planning an activity, or understanding a complex sentence, and then use the outcomes in later operations necessary to achieve the desired final result. Indeed, computer simulations of these higher-cognitive processes rely extensively on WM (1, 2). For these reasons, an analysis of WM is critical not only for understanding memory systems, but for understanding thought itself.
Research on human WM has been carried out at both behavioral and biological levels. Both avenues of research indicate that there are different kinds of WMs for different kinds of information. In particular, behavioral, neuropsychological, and neuroimaging evidence converge in indicating that there are separate systems for verbal and spatial information (for recent reviews, see refs. 3 and 4). In this article, we focus entirely on verbal WM and its neural implementation, as revealed by neuroimaging studies using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). The verbal WM system is particularly important, given the role that linguistic processes seem to play in higher-cognitive processes. The study of a verbal system can be especially difficult, though, given that there can be no animal models to guide the effort.
Our concern in this article is with the neural bases of the various components of verbal WM. In the course of exploring this general issue, we will consider three specific issues. The first concerns whether verbal WM contains a component that is specialized for subvocal rehearsal. The neuroimaging evidence that we review makes the case that there is indeed an isolable subsystem that is specialized for the rehearsal of phonological information. The evidence also will provide a tentative hypothesis about the neural bases of other components of verbal WM.
Our second issue concerns the experimental logic used to obtain the relevant evidence. The evidence for a rehearsal component is based primarily on experiments that use “subtraction” logic; people are imaged while performing two tasks that differ only in that one task supposedly has an extra process, and the difference in images for the two tasks is taken to reflect the process of interest (5). This approach is somewhat controversial. Can the results of interest be obtained by an alternative experimental logic, that of “parametric variation,” in which a parameter that presumably reflects a single process (e.g., storage load) is varied quantitatively? Our evidence shows a convergence between results obtained by following both types of experimental logic, and supports our tentative hypothesis about the neural basis of verbal WM.
The third issue refers to the distinction between using WM solely for purposes of storage vs. using it for purposes of both storage and computation. There is considerable behavioral research on this distinction, with much of it showing that performance on complex reasoning tasks is more correlated with performance in WM tasks that require storage-plus-computation than with performance on pure storage tasks (e.g., refs. 6 and 7). As we will see, the neuroimaging data provide some support for this distinction as well.
© 1998 by The National Academy of Sciences 0027–8424/98/95876–7$2.00/0
PNAS is available online at http://www.pnas.org.
|
|
Abbreviations: WM, working memory; PET, positron emission tomography; fMRI, functional magnetic resonance imaging; DLPFC, dorsolateral prefrontal cortex; BA, Broadmann area. |
|
† |
To whom reprint requests should be addressed at: Department of Psychology, University of Michigan, 525 East University, Ann Arbor, MI 48109–1109. e-mail: eesmith@umich.edu. |
The Rehearsal Component of Verbal WM
By the early 1990s, cognitive-behavioral studies had provided evidence that verbal WM contained a few distinct components, including a phonological rehearsal process (see refs. 8 and 9 for reviews). The experiments that follow provide converging evidence for the behavioral work, and, more importantly, tell us something about the neural bases of these components. Unless otherwise noted, these experiments involve visual presentation of the verbal materials.
A useful starting point is a PET experiment reported by Awh and colleagues (10). Subjects were imaged while performing an item-recognition task (11). The task includes a series of discrete trials, and the contents of a trial are presented schematically in the top of Fig. 1. Each trial consisted of four uppercase target letters, presented simultaneously, followed by a 3,000-ms blank delay period, during which subjects had to remember the letters. This delay was followed by a lowercase probe letter, to which subjects responded “yes” or “no” (by pressing one of two buttons), indicating whether or not the probe was identical in name to one of the targets. This task is of interest because of its verbal memory requirement. The task also includes other processes—e.g., perception of the letters, selection of a response, and execution of a response—and thus the corresponding PET image acquired during performance of the task will include activations caused by these processes as well. To eliminate these unwanted activations, subjects also participated in a control task, which presumably contained the irrelevant processes but not the memory process. This task was similar to the memory one except that the probe was presented immediately after the target letters, and the latter remained in view along with the probe. This task was primarily a perceptual one; hence, memory should not have been involved.
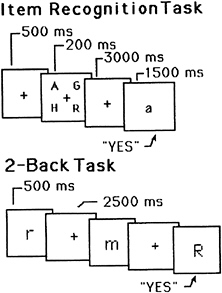
FIG. 1. Schematic representations of trials in two different WM tasks. (Upper) A sample trial for the item-recognition task. It includes the following events: (i) fixation point, (ii) four uppercase letters, (iii) blank delay interval, and (iv) a lowercase probe letter. The subject’s task was to decide whether the probe names one of the four target letters. (Lower) A sample trial for the 2-back task. Each letter is followed by a blank delay interval. The subject’s task was to decide whether each letter has the same name as the one that occurred two back in the sequence The durations for each trial event are shown.
When the image acquired during the control task was subtracted from the image acquired during the memory task, there were several activations, most of them in the left hemisphere. (There were also some deactivations, but in what follows we focus on activations.) These activated areas included: left posterior parietal cortex [Broadmann area (BA) 40], Broca’s area (BA 44), left premotor area (BA 6), and left supplementary motor area (BA 6). Given that the latter three areas are known to be involved in the planning and production of speech (12), implicit speech was very likely operative during the memory task. This finding is one piece of evidence for a rehearsal component.
This hypothesis is strengthened by the results of an earlier PET study of Paulesu and colleagues (13). They, too, contrasted an item-recognition task with a control task. When the control image was subtracted from the item-recognition image, there were activations in left posterior parietal cortex, Broca’s area, and bilateral supplementary motor area. These results are in good agreement with those described above. Paulesu et al. (13) included another condition, whose results provide further support for the claim that the frontal speech areas mediate a rehearsal of phonological information. In this additional condition, subjects were presented single letters; for each one, they had to decide whether it rhymed with the name of a target letter. When the image from a suitable control task was subtracted from the image from the rhyming task, there was again activation in Broca’s area, but not in left posterior parietal cortex. Paulesu et al. (13) interpret these results to mean that: (i) Broca’s area mediates phonological processes, including both rhyme judgments and subvocal rehearsal; and (ii) left posterior parietal cortex mediates the pure storage component of verbal WM, and hence was active in the memory, but not the rhyming, condition. Taken at face value, these results show that the rehearsal component can be dissociated from other components of verbal WM.
There is further evidence for such a dissociation in another PET experiment reported by Awh and colleagues (10). This study used a different memory paradigm. It is referred to as a “2-back” task (14, 15) and is presented schematically in the bottom of Fig. 1. Instead of viewing a series of discrete trials, subjects viewed a continuous stream of single letters, each presented for 500 ms, with a 2,500-ms interval between successive letters. For each letter, subjects had to decide whether it was identical in name to the letter two back in the sequence. Two different control conditions were used. One was a search task; subjects saw the same kind of sequence of letters as in the memory condition, but simply had to decide whether each letter matched a single target letter specified at the beginning of the experiment. This control should involve the same perceptual and response processes that are operative in the memory condition, and subtracting it from the memory condition should yield many of the same areas of activation that were obtained in item-recognition tasks. This was the case, as the subtraction image showed activations in left-hemisphere frontal speech regions and posterior parietal cortex. (There were new activations as well, including bilateral posterior and superior parietal cortex, BA 7, and right-hemisphere supplementary motor area; some of these new activations may reflect the greater processing demands of the 2-back task.)
What is of particular interest in this study, though, involves the second control condition. In this “rehearsal” control, subjects silently rehearsed each letter presented until the next one appeared, rehearsed the new one, and so on. Subtracting this control from the 2-back memory condition should have removed much of the rehearsal circuit. Indeed, in this subtraction image, neither Broca’a area nor the premotor area was significantly active. However, the supplementary area continued to be active. That we were unable to subtract out the entire rehearsal circuit may be because in our rehearsal control subjects rehearsed one letter at a time, whereas in our memory
condition they rehearsed two at a time. Still, the results provide additional evidence that a separable rehearsal component can be isolated from the rest of the neural circuitry for verbal WM.
In the preceding studies, we have assumed that the information rehearsed was phonological though the input was always visual. If rehearsal does indeed operate on phonological information (as opposed to visual information), then the pattern of memory results obtained above also should be present with auditory input. We tested this hypothesis in a 3-back memory task, with both visual and auditory presentation conditions (16). The visual condition was similar to the 2-back task we just considered, except in this case subjects decided whether each letter was identical to the one presented three earlier in the sequence. The auditory condition had the same structure as the visual one, except that the letters were presented auditorally and the only visual inputs were fixation crosses. Two different controls were used, a visual-search and an auditory-search condition; subtracting the visual-search condition from the visual 3-back memory condition, and the auditory search from the auditory 3-back condition, should have removed all input differences from the memory conditions.
Each subtraction image—visual 3-back minus visual search, and auditory 3-back minus auditory search—showed at least a dozen significant activation sites, and the patterns of activation were very similar for the two cases. When the auditory subtraction image was subtracted from the visual subtraction image (a double subtraction), there were no significant activations left. And when the visual subtraction image was subtracted from the auditory subtraction image, only a single area was left active (a site in Broca’s area). Another way to assess the similarity of activation patterns between the two conditions is to compare increases and decreases in activation in areas found to be activated or deactivated in previous WM studies. This comparison is presented in Fig. 2 for 10 activated and 10 deactivated regions, and it shows a similarity between the visual and auditory memory conditions. This is additional evidence that the underlying verbal-memory circuitry is the same, regardless of input modality.
Taken together, the preceding studies suggest a rough neural architecture of verbal WM. The system may involve at least three components: (i) a phonological rehearsal component mediated by left-hemisphere frontal speech regions (including BA 44 and the inferior and superior aspects of BA 6); (ii) a pure storage buffer mediated by left posterior parietal cortex (BA 40); and (iii) an executive component mediated by the dorsolateral prefrontal cortex (DLPFC) (BA 9/46), which was active in the 3-back memory conditions and which may reflect the need to temporally code the items in these conditions (see below). This network of regions is likely an underestimate of the true complexity involved. For example, the rehearsal component also may include parts of the right-hemisphere cerebellum, because this area often is activated in verbal memory studies, and it is known to project to the left frontal speech regions. In addition, WM tasks often activate attentional systems that may be involved in any difficult task and that appear to be mediated by posterior, superior parietal cortex (BA 7) and the anterior cingulate (BA 32), among other areas.
Subtraction Logic and Alternative Methods
Every result presented thus far is based on the subtraction method. As we have seen, the method assumes that one can isolate the neural mechanisms corresponding to a specific cognitive process by: (i) constructing a pair of behavioral tasks that supposedly differ in that one task recruits the process of interest (target task), whereas the other does not (control task); (ii) imaging during performance of both tasks, and (iii) subtracting the control image from the target image, and inferring that this difference image directly reflects the neural basis of the process of interest. This method has produced numerous systematic and useful results, but it rests on a problematic assumption.
The assumption at issue is called “pure insertion,” and it consists of the claim that the addition of a particular processing stage to some task does not affect the operation of other stages in that task (17). In our “back” tasks, for example, the difference between the memory and search-control tasks is one of memory; according to the assumption of pure insertion, adding this memory requirement has no effect on other processes such as those concerned with perception and response. This assumption often has been challenged (e.g., ref. 18); for example, adding a memory requirement to a search task may lead to a different kind of perceptual analysis, and consequently the activations present in a memory-minus-search subtraction could reflect perceptual networks in addition to mnemonic ones.
To ameliorate this potential problem, we can augment the subtraction approach with the method of parametric variation.
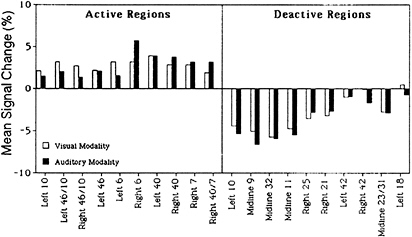
FIG. 2. Activation and deactivation in the memory-minus-control subtraction for 10 different regions of interest, separately for visual presentation (empty bars) and auditory presentation (filled bars). The regions of interest were based on activations and deactivations obtained in a previous 3-back verbal WM task. Values are mean changes in activation across spheres of 10-mm radius. Adapted from Schumacher et al. (16).
In this approach, one quantitatively varies an experimental factor that presumably affects the operation of a single processing stage, and determines whether this factor affects regional activation in a systematic way. For example, one might vary the number of items to be retained in a memory task, and determine whether this factor results in a monotonic or even linear increase in activation in regions of interest. Because the conditions differ quantitatively rather than qualitatively, there should be less chance of a qualitative change in processing between conditions. Also, that the conditions differ quantitatively allows one to examine the function relating memory load to regional activation, which can provide new information about the underlying processes.
This parametric approach has been used successfully in neuroimaging studies of long-term memory (19), and we have extended the method to verbal WM (20). Specifically, we used the back task in another PET experiment, and varied whether subjects had to look for memory matches 0-, 1-, 2-, or 3-back from the current item. In 0-back, subjects had to decide whether each letter matched a fixed target letter specified at the beginning of the letter sequence (a search task); in 1-back, subjects had to decide whether each letter matched the letter one back in the sequence; and so on for 2- and 3-back. In essence. WM load is being varied from 0 to 3, and the test of whether an area is part of the neural substrate of verbal WM is whether that area’s activation increases monotonically with memory load. [Because we know from a previous study (16) that there is more activation in the 3-back than the 0-back task, determining whether monotonicity holds amounts to determining the relation of the 1- and 2-back tasks to the more extreme tasks.]
Fig. 3 presents the results of the PET activations superimposed on a surface rendering of a brain, separately for each of the four tasks. There are many more areas of reliable activation in the 2- and 3-back tasks than in the 0- and 1-back tasks. But the rise in activation with memory load is actually more continuous than suggested by a count of significant areas. This result is shown by the data in Fig. 4, which plots the change in
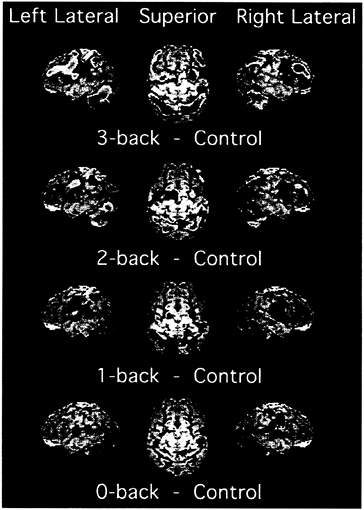
FIG. 3. PET activations for the four memory tasks in the 0-, 1-, 2-, 3-back experiment (20). In addition to the memory conditions, the experiment included a baseline condition, in which letters were presented in sequence and subjects pressed a key when a letter appeared. This baseline was subtracted from each memory condition to produce the images displayed. Shown are left and right lateral views as well as a superior view. The PET activations, shown in color, are superimposed on a surface rendering of a brain created from a standard MRI image. The color scale representing activations ranges from blue (lowest) to red (highest). The scale reflects the activation’s significance, with t values ranging from 1.65 to 7.00, with values above 7.00 displayed at the peak red color. Adapted from Smith and Jonides (21).
activation (blood flow) as a function of memory load, separately for 11 different regions of interest. These regions were defined by the significant activations in the subtraction-based, 3-back experiment of Schumacher et al. (16). Hence, to the extent that these regions show monotonic effects of memory load, we have evidence that they are part of the neural network for verbal WM, and a convergence between results based on parametric vs. subtraction methods. Every function but one in Fig. 4 is strictly monotonic (i.e., the function always increases), and every single function shows a significant linear trend (the lone exception to strict monotonicity is a function for the DLPFC—we will return to this exception later). Thus the parametric and subtraction methods show a good deal of convergence. This convergence does not arise because the monotonicity criterion is so lenient that any arbitrary brain area can meet it. When regions of interest are selected that a priori should not be part of a WM network (e.g., visual and motor areas), they show virtually no effect of memory load, let alone monotonic effects.
Recently, in conjunction with a neuroimaging group at Pittsburgh-Carnegie Mellon, we have replicated and extended these results by using fMRI, rather than PET, as the imaging modality (22). We used the four back tasks from the previous study, but extended the interval between letters (the delay interval) from 2.5 to 10 sec. Using echoplanar imaging, we were able to scan subjects performing the tasks at four time periods during the 10-sec delay period: 0–2.5 sec, 2.5–5.0 sec, 5.0–7.5 sec, and 7.5–10.0 sec. Again, we defined regions of interest around previously obtained verbal WM activations. We found that, for almost all of the WM regions, activation increased strictly monotonically with memory load. (This monotonic increase was often present at all four temporal points scanned during the 10-sec delay period.) Again, though, there was an exception to strict monotonicity in the function for the DLPFC. It showed a step function, with no increase at all between 0- and 1-back, a substantial increase from 1- to 2-back, and little increase after that. The relevant data are displayed in Fig. 5.
In the experiment just described, we measured a region’s activation at different temporal points of a delay interval. This method may provide another means for studying the neural bases of WM (in addition to subtraction and parametric variation). Given that we were scanning only during the retention interval, we have targeted memory processes with more precision than in the usual PET study, which relies on a comparison of conditions to isolate memory processes. We can refer to the fMRI method as the “temporal isolation” method. Though we scanned only the delay period, some of the active areas may reflect nonmemory processes, because the hemodynamic response that underlies our use of fMRI has a latency
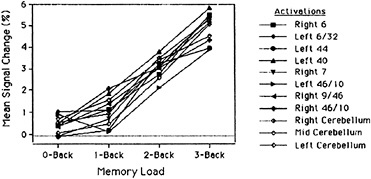
FIG. 4. Percentage change in activation in the Jonides et al. (20) 0-, 1-, 2-, 3-back PET study, as a function of memory load, separately for 11 regions of interest. The regions are ones that have been found active in previous studies of verbal WM using back tasks (10, 16). Values are the mean changes in activation across spheres of 5.4-mm radius. Adapted from Jonides et al. (20).
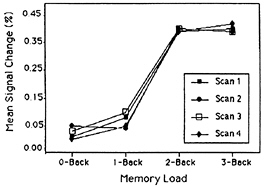
FIG. 5. Percentage fMRI signal change in activation in a DLPFC region as a function of WM load, with temporal interval as the parameter. Adapted from Cohen et al. (22).
of about 4 sec, thereby allowing perceptual and response processing of the letters to spill over into the delay period. In light of this complication, we need to adopt the following constraint in interpreting our results: an area contributes to verbal WM only if it is active throughout the entire delay period. A number of previously identified WM regions showed this pattern.
We return to the exception to strict monotonicity that we have now observed in two studies: the DLPFC shows no increase in activation with memory load until moving to the 2-back task (see Fig. 5). An analysis of the back tasks suggests why the DLPFC might show this discontinuity. To begin, note that in the 3-back task, subjects must not only remember the last three items presented, but must also code the stored letters with respect to their temporal position (only a match 3-back counts), and inhibit responding to matches 1-back and 2-back. There is evidence from lesion studies with humans and other animals that both temporal coding and inhibition may be mediated by the DLPFC (e.g., refs. 12 and 23); consequently, we would expect the DLPFC to be activated in the 3-back task, as it in fact was. Similar remarks apply to the 2-back task. Again, there is no way to perform the task accurately without coding the temporal order of the stored items, and inhibiting responses to matches 1-back, and again we found that the DLPFC was activated. However, task requirements change at 1-back, as it is possible for subjects to accomplish this task accurately without coding the temporal order of the stored letters and inhibiting responses to spurious matches. Specifically, because the memory representation of the nth letter
should still be highly activated when the n+1st letter is presented, subjects can detect a match on trial n+1 simply by noting that the representation of the current letter is highly active; and there is no need to be concerned about spurious matches. Hence, there may be no need for temporal coding or inhibitory processes, and no reason to expect the DLPFC to be activated. A similar account applies to the 0-back task, as the single target letter may be kept in a highly activated state.
We should be cautious about accepting the preceding account. Along with the Pittsburgh-Carnegie Mellon group, we have performed an experiment similar to the last one described but in which DLPFC activation increased roughly linearly with memory load (24). Still, attributing DLPFC activation to temporal coding and/or inhibition has its positive aspects. It can help explain why some verbal WM tasks implicate the DLPFC and others do not (see below), and, more broadly, it offers a starting point for the analysis of neural differences between WM tasks requiring only storage and those requiring processing as well as storage. We turn now to this final topic.
Storage vs. Storage-Plus-Processing and the DLPFC
We have suggested that in 2- and 3-back tasks subjects must code the temporal order of the stored items, and that this coding, along with inhibitory processes, is mediated by the DLPFC. (The DLPFC also may mediate the maintenance of the temporal codes, given that it remains active during the entire retention interval—see Fig. 5.) Because such temporal coding amounts to performing an operation on the contents of WM, we hypothesize that the DLPFC is activated whenever people engage in a WM task that requires processing as well as storage. [A comparable hypothesis has been advanced for spatial WM (25), but in what follows, we maintain our verbal emphasis.] Evaluation of our hypothesis will lead us to consider some verbal WM tasks in addition to the item-recognition and back tasks. Before doing that, however, we focus on the difference between these two standard tasks from the perspective of the hypothesis of interest.
Unlike the 2- and 3- back tasks, the item-recognition task does not require keeping track of the temporal order of the stored items (nor does it require much in the way of inhibitory processes). Rather, in item recognition, all that subjects have to determine is that the probe item is included in the current memorized set (see Fig. 1). This task would be classified as a pure storage task (our use of the term “pure storage” here includes rehearsal as well as storage). Interestingly, this task did not produce any sign of DLPFC activation in the study we reported at the outset of this paper (10). This comparison of the results obtained in item-recognition and 2- and 3-back tasks is consistent with the hypothesis of interest—storage-plus-processing leads to DLPFC activation—but the comparison relies on contrasting results from different experiments.
To sharpen the comparison, we directly compared the item-recognition and 2-back tasks with the same subjects in a subtraction-based PET experiment (26). The memory tasks for item recognition and 2-back were virtually the same as those presented in the first section of this paper (Fig. 1), except that the presentation time for the letters was now 500 ms in both tasks. The control task for 2-back was again a search for a single target. The control for item recognition, however, differed from the one we used earlier, as the current control required subjects to remember a single letter for 200 ms (Thus, both the control and the memory tasks now required some memory, and consequently the differences between the control and the memory conditions were now quantitative rather than qualitative.) A comparison of the two subtraction images indicated a number of common regions, including many of the usual verbal WM areas: the left-hemisphere frontal speech regions (premotor area, supplementary motor area, and Broca’s area), and inferior and superior aspects of the posterior parietal cortex. There were also some areas of activation distinct to the 2-back task, most importantly, the right-hemisphere DLPFC. Additional tests established that there was significantly more DLPFC activation in 2-back than in item recognition. These results support the specific claim that the extra processes required in 2-back—temporal coding and inhibition—are partly mediated by the DLPFC, and are consistent with the more general hypothesis that performing operations on the contents of WM recruits DLPFC.
Two other tasks that require subjects to operate on the contents of WM were used in a PET study by Petrides et al. (27). In one condition, participants were required to say, at a rate of 1 per sec, the numbers from 1–10 in random order, without repeating any digits. The requirement that numbers not be repeated forces the subject to store the already produced numbers in WM and to continually monitor these stored items. Such monitoring could qualify as an operation on the contents of WM. The requirement of random production presumably induces the subject to check that the number(s) he or she is about to produce will not yield a regular sequence when combined with the numbers already produced (e.g., three consecutive numbers); the subject again needs to monitor the contents of WM, and also has to apply “checks for regularity” to the contents of WM, which is another kind of operation on its contents. When this “generate random” condition was compared with the control condition (subjects say the 10 numbers in a fixed order), the subtraction image showed some of the usual left-hemisphere WM areas, including the premotor area and inferior and superior aspects of the posterior parietal cortex. In addition, and most important for our present purposes, the DLPFC was activated bilaterally.
In another memory condition in this same experiment, subjects listened to a list of the numbers 1–10, presented randomly at a rate of 1 per sec, with one of the 10 numbers not presented; the subject’s task was to report the missing number. This task presumably induces the subject to store in WM a representation of what numbers have been presented, and to then search this representation for the missing number. Behavioral work suggests that subjects often adopt the strategy of representing all 10 numbers in WM at the start of a trial, and then checking off each number as it occurs so that they can easily find the missing number at the end of the trial (28). The checking and search processes presumably constitute operations on WM’s contents. When this “missing span” condition was compared with the control condition, the subtraction image again showed some of our usual WM areas plus bilateral DLPFC. Once more, when a verbal WM task requires some processing as well as storage, the DLPFC is activated.
Though we have discussed a relatively small number of tasks, already we have considerable diversity in the kind of processing involved—temporal coding, monitoring, searching, checking for regular sequences. Does the DLPFC mediate all of these different processes, or do all of these operations share a critical component that is really responsible for DLPFC activation? One plausible common component would be the need to divide one’s attention (or concentration) between two mental tasks. That is, whenever one has to store items and operate on them, one essentially has two tasks, maintaining the items via rehearsal, and performing the operations required; consequently, one has to divide one’s attention between the two tasks, and this division of attention may be what is mediated by DLPFC. This hypothesis receives some support from the fMRI findings of D’Esposito et al. (29) in which subjects performed a categorization task and a mental-rotation task, either one task at a time or concurrently. When either task was performed alone, there was no activation in DLPFC. However, when subjects had to do both tasks concurrently, and hence divide their attention, the DLPFC was activated.
Although we have suggestive evidence that storage-plus-processing activates the DLPFC, perhaps through the mechanism of divided attention, it is of interest to inquire into the status of the related hypothesis that pure storage tasks do not activate the DLPFC. The contrast we presented at the outset of this section between the item-recognition and 2-back tasks supports the related hypothesis in that the former task did not activate DLPFC. Also, neither of the other item-recognition experiments reported in this article (10, 13) showed significant activation in DLPFC.
In contrast, there are a couple of studies of verbal WM that on the face of it seem to require only pure storage, yet show activations of DLPFC. Activation of the left DLPFC was found in a recent fMRI experiment (30) in which subjects saw a series of letters and had to detect any X that had been preceded by an A. Although this paradigm seems to be a pure storage task, it is possible that subjects adopted the following strategy: search for an A, and if one is detected, check whether the next letter is an X. This strategy makes the paradigm a kind of dual task, requiring some attention switching whenever an A is detected, and the attention switching may have been the source of the DLPFC activation.§ In another relevant study (31), subjects were given five words or nonwords to remember for 60 sec, and then had to recall the items in any order they wanted. Subjects were scanned, by PET, only during the 60-sec interval they were remembering the items. Though this paradigm seems to be a pure storage task, there was activation in DLPFC. However, because the delay interval was very long for a WM study (far longer than the delay interval used in any other study mentioned in this paper), perhaps subjects engaged in some additional long-term processes, and the latter may have been the cause of the DLPFC activation.
The preceding accounts are speculative. We will need additional research to see how much credence they should be given. It may turn out that some pure storage tasks do activate the DLPFC, but that such activation increases disproportionately when a processing component is added to the storage component. This issue deserves further consideration because it will lead to a refinement of the neural circuitry of WM, a fundamental process in much of thought.
This work was supported by grants from the Department of Energy, McDonnel-Pew Program in Cognitive Neuroscience, National Institute of Aging, and Office of Naval Research.
1. Newell, A. (1990) Unified Theories of Cognition (Harvard Univ. Press, Cambridge, MA).
2. Carpenter, P.A., Just, M.A. & Shell, P. (1990) Psychol. Rev. 97, 404–431.
3. Baddeley, A. (1996) Proc. Natl. Acad. Sci. USA 93, 13468–13472.
4. Jonides, J., Reuter-Lorenz, P., Smith, E.E., Awh, E., Barnes, L., Drain, M., Glass, J., Lauber, E., Patalano, A. & Schumacher, E.H. (1996) in The Psychology of Learning and Motivation, ed. Medin, D. (Academic, New York), pp. 43–88.
5. Posner, M.I., Peterson, S.E., Fox, P.T. & Raichle, M.E. (1988) Science 240, 1627–1631.
6. Just, M.A. & Carpenter, P.A. (1992) Psychol. Rev. 99, 122–149.
7. Daneman, M. & Merikle, P.M. (1996) Psychonomic Bull. Rev. 3, 422–433.
8. Baddeley, A.D. (1992) Science 225, 556–559.
9. Jonides, J. (1995) in An Invitation to Cognitive Science: Thinking, eds., Smith, E.E. & Osherson, D. (MIT Press, Cambridge, MA), Vol. 3, 2nd Ed., pp. 215–265.
10. Awh, E., Jonides, J., Smith, E.E., Schumacher, E.H., Koeppe, R.A. & Katz, S. (1996) Psychol. Sci. 7, 125–131.
11. Sternberg, S. (1966) Science 153, 652–654.
12. Fuster, J.M. (1995) Memory in the Cerebral Cortex (MIT Press, Cambridge, MA).
13. Paulesu, E., Frith, C.D. & Frackowiak, R.S.J. (1993) Nature (London) 362, 342–344.
14. Cohen, J.D., Forman, S.D., Braver, T.S., Casey, B.J., Servan-Schreiber, D. & Noll, D.C. (1994) Hum. Brain Mapp. 1, 293–304.
15. Gevins, A.S. & Cutillo, B.C. (1993) Electroencephalograph. Clin. Neurophysiol. 87, 128–143.
16. Schumacher, E.H., Lauber, E., Awh, E., Jonides, J., Smith, E.E. & Koeppe, R.A. (1996) NeuroImage 3, 79–88.
17. Sternberg, S. (1969) Acta Psychol. 30, 276–315.
18. Shulmam, R. (1996) J. Cognit. Neurosci. 8, 474–480.
19. Grasby, P.M., Frith, C.D., Friston, K.J., Bench, C., Frackowiak, R.S.J. & Dolan, R.J. (1993) Brain 116, 1–20.
20. Jonides, J., Schumacher, E.H., Smith, E.E., Lauber, E.J., Awh, E., Minoshima, S. & Koeppe, R.A. (1996) J. Cognit. Neurosci. 9, 462–475.
21. Smith, E.E. & Jonides, J. (1997) Cognit. Psych. 33, 5–42.
22. Cohen, J.D., Perlstein, W.M., Braver, T.S., Nystrom, L.E., Noll, D.C., Jonides, J. & Smith, E.E. (1997) Nature (London) 386, 604–608.
23. Diamond, A., Cruttenden, L. & Neiderman, D. (1994) Dev. Psychol. 30, 192–205.
24. Braver, T.S., Cohen, J.D., Jonides, J., Smith, E.E. & Noll, D.C. (1997) NeuroImage 5, 49–62.
25. Owen, A.M., Evans, A.C. & Petrides, M. (1996) Cereb. Cortex 6, 31–38.
26. Reuter-Lorenz, P., Jonides, J., Smith, E.E., Hartley, A.A., Cianciolo, A., Awh, E., Marshuetz, C. & Koeppe, R.A. (1996) Soc. Neurosci. Abstr. 22, 183.
27. Petrides, M., Alivisatos, B., Evans, A.C. & Meyer, E. (1993) Proc. Natl. Acad. Sci. USA 90, 873–877.
28. Buschke, H. (1963) Science 140, 56–57.
29. D’Esposito, M., Detre, J.A., Alsop, D.C., Shin, R.K., Atlas, S. & Grossman, M. (1995) Nature (London) 378, 279–281.
30. Barch, D.M., Braver, T.S., Nystrom, L.E., Forman, S.D, Noll, D.C. & Cohen, J.D. (1997) Neuropsychologia 35, 1373–1380.
31. Fiez, J.A., Raife, E.A., Balota, D.A., Schwarz, J.P., Raichle, M.E. & Petersen, S.E. (1996) J. Neurosci. 16, 808–822.







