This paper was presented at a colloquium entitled “Neuroimaging of Human Brain Function,” organized by Michael Posner and Marcus E.Raichle, held May 29–31, 1997, sponsored by the National Academy of Sciences at the Arnold and Mabel Beckman Center in Irvine, CA.
Event-related functional MRI: Past, present, and future
(single trial/echo planar imaging/functional neuroimaging/positron emission tomography/magnetoencephalography)
BRUCE R.ROSEN*†‡, RANDY L.BUCKNER*†§, AND ANDERS M.DALE*†
*Nuclear Magnetic Resonance Center, Massachusetts General Hospital, Charlestown, MA 02129; †Department of Radiology, Harvard University Medical School, Boston, MA 02115; and §Departments of Psychology, Radiology, and Anatomy and Neurobiology, Washington University, St. Louis, MO 63105
ABSTRACT The past two decades have seen an enormous growth in the field of human brain mapping. Investigators have extensively exploited techniques such as positron emission tomography and MRI to map patterns of brain activity based on changes in cerebral hemodynamics. However, until recently, most studies have investigated equilibrium changes in blood flow measured over time periods upward of 1 min. The advent of high-speed MRI methods, capable of imaging the entire brain with a temporal resolution of a few seconds, allows for brain mapping based on more transient aspects of the hemodynamic response. Today it is now possible to map changes in cerebrovascular parameters essentially in real time, conferring the ability to observe changes in brain state that occur over time periods of seconds. Furthermore, because robust hemodynamic alterations are detectable after neuronal stimuli lasting only a few tens of milliseconds, a new class of task paradigms designed to measure regional responses to single sensory or cognitive events can now be studied. Such “event related” functional MRI should provide for fundamentally new ways to interrogate brain function, and allow for the direct comparison and ultimately integration of data acquired by using more traditional behavioral and electrophysiological methods.
The field of human brain mapping has undergone enormous changes in the last several years. Newer-generation positron emission tomography imaging cameras now can dynamically scan the entire brain with high sensitivity, allowing for mapping of neurotransmitter and receptor uptake and regulation. Electrical and magnetic source, imaging have the ability to resolve patterns of brain activation on temporal scales measured in milliseconds, and users of these methods have made great strides in the challenging problem of source localization. However, perhaps none of these methods has done more to excite the community of systems-level neuroscientists, cognitive psychologists, and clinicians as the development over the last half-decade of functional brain imaging using magnetic resonance. Functional MRI (fMRI) combines at once the high spatial resolution anatomic imaging capabilities of conventional MRI with the hemodynamic specificity of nuclear tracer techniques (positron emission tomography), allowing spatially accurate mapping of human brain function to underlying anatomy. Combined with newer MRI methods capable of rapid sequential imaging at rates up to 20 images per sec (1), fMRI methods also allow for something completely new—the capability to tomographically image the second-by-second time course of the hemodynamic and metabolic responses underlying neuronal events.
This short report will review our current understanding of the physiology underpinning these rapid fMRI signal changes, and describe one important application of this high temporal resolution—the ability to design probes of cognitive function based on neuronal events evoked by single stimuli and task trials. Event-related fMRI should allow human brain imagers unprecedented flexibility in the design of task paradigms used to probe both primary sensory and higher-level neuronal function, and ultimately allow for the direct linkage of tomographic, hemodynamic-based brain imaging tools with electromagnetic source mapping techniques. This linkage could provide a wholly new “camera” capable of millimeter spatial and millisecond temporal resolution across the entire human brain.
Hemodynamics, Metabolism, and Brain Activity
The link between neuronal function and associated cerebrovascular hemodynamic changes has been studied for more than a century. Although the fundamental biochemical and electrical messengers linking increased local neuronal activity with increased regional cerebral blood flow (CBF), cerebral blood volume, and blood oxygen content remain incompletely understood, it has been clear from the earliest observations that electrical, hemodynamic, and metabolic parameters are coupled in both space and time. In fact, well before the spatial relationship between neuronal firing and CBF was directly measurable, it was known that the timing between neuronal and hemodynamic events was closely coordinated within seconds (2, 3).
Data resulting from a variety of techniques during the intervening century support this view, though more refined measurements raise important questions about the relationship between the various hemodynamic and metabolic parameters. For example, observations of cortical CBF after whisker barrel stimulation reveals that blood flow begins to increase within approximately 2 sec of the onset of stimulation, reaching its peak value within approximately 5–7 sec (4, 5). More recent data acquired by using equilibrium blood pool MRI agents suggests that cerebral blood volume changes appear to lag behind changes in CBF, suggesting a capacitance system within the capillary and postcapillary bed (6). Interestingly, however, recent optical data suggest that changes in total hemoglobin content may precede changes in CBF (Dov Malack, personnel communication). Reconciling these two perspectives remains an area of considerable interest in under-
© 1998 by The National Academy of Sciences 0027–8424/98/95773–8$2.00/0
PNAS is available online at http://www.pnas.org.
standing the dynamics of neuronal/vascular coupling. Similarly, although it has been known for some time that there is a rapid onset of increased tissue oxygen in both primary sensory (visual) and higher-order (language) cortex in humans after stimulation (7), the exact temporal ordering, indeed even the fundamental relationship, between changes in oxygen metabolism and the hemodynamic parameters of blood flow and blood volume is still to be fully defined.
These issues have importance beyond their obvious physiological interest. For example, although a wide range of data support the notion that large-scale changes in hemodynamics occur within 2 to 3 sec of underlying neuronal events, several recent reports, including those using both optical (8) and MRI techniques (9, 51), note that there may be subtle, but observable, changes in these physiological parameters in times of a few hundred milliseconds after neuronal stimulation. The origin of such changes remains controversial, but is critical to understanding whether even more precise timing information may be obtainable by using these hemodynamic and metabolic measures, and to understanding the quantitative relationship between these physiological changes and the degree of underlying neural activity.
Dynamic Physiology and fMRI
Whatever its ultimate origin, the link between increased neuronal firing and changes in cerebral hemodynamics and metabolism is certainly a rapid one. Although early efforts at human functional brain imaging relied (and continue to rely) on the anatomic correspondence between neuronal activity and hemodynamics, nuclear tracer technologies, both positron emission tomography and single photon emission computed tomography, typically lacked the temporal resolution to take advantage of the temporal elements of this physiological linkage (10, 11). The earliest functional activation studies with MRI, which used exogenous paramagnetic tracers to map changes in cerebral blood volume, had similar limitations, allowing interrogation of changes in brain state with temporal sampling measured across several minutes (12).
This situation changed radically with the concurrent development of two advances in MRI. First, though initially described early in the history of MRI (13), very rapid MRI techniques were refined only to the point of human use at high field (1.5 Tesla) by the end of the 1980s (14). These techniques are capable of acquiring complete two-dimensional images after a single radio frequency excitation (by using echo planar imaging or other k-space trajectories such as spiral scanning). It was in fact this development that allowed for the initial studies of cerebral blood volume by using exogenous tracers (15), and thus was the key technical development for the first functional MR images of brain activity, by using contrast agents (12).
Second, two new “endogenous” contrast mechanisms were described. One of these mechanisms was based on changes to the net longitudinal magnetization within an organ produced by changing tissue perfusion. Originally described by Detre, Koretsky, and colleagues at Carnegie Mellon University (16), these first experiments now have spawned a whole host of quantitative methods, broadly named arterial spin labeling techniques, capable of measuring CBF with high spatial and temporal precision. The second of these mechanisms was based on changes in the transverse magnetization produced by local changes in the magnetic susceptibility induced by changing net tissue deoxyhemoglobin content. Labeled BOLD contrast for blood-oxygen-level-dependent changes by Seige Ogawa of Bell Labs (17), these early experiments demonstrated that, as had been observed in vitro by Thulborn et al. (18). changes in blood oxygenation induced changes in blood MR signal intensity in vivo.
It was the combination of these endogenous contrast mechanisms with rapid imaging technology that inspired a veritable revolution in human brain mapping. The first successful demonstrations of human brain activation were performed by combining high-speed imaging and both arterial spin labeling and BOLD contrast mechanisms (19–22). These initial demonstrations showed that the changes in MRI signal intensity within activated cortical regions could be reliably measured by using equipment designed for high signal sensitivity. Of greater importance for this discussion, these demonstrations revealed that the MR signal changes were truly dynamic, with MRI signal changes occurring only seconds after the neuronal activity onset (see Fig. 1; ref. 20). Thus these earliest experiments were often shown as cine (movie) images, allowing visualization of the temporal dimension to tomographic human brain functional imaging. Dynamic fMRI was born.
Despite the early recognition that these dynamic fMRI experiments were fundamentally different from previous hemodynamically based functional imaging methods, these early studies typically used experimental paradigms that could have been easily performed by using previous nuclear technologies. Specifically, most experiments were performed by using extended periods of “on” versus “off” activations—so-called block designs—which had been the stalwart of functional studies of sensory and higher cortical function using positron emission tomography and single photon emission computed tomography for more than a decade. Nevertheless, although such block designs are a necessity when imaging hemodynamics by using techniques that require a quasi-equilibrium physiological state for periods upward of 1 min, they were clearly not required for fMRI experiments, where activity was detectable within seconds of stimulus onset.
Movement away from blocked designs was gradual and aided by a number of studies exploring fMRI signal responses to brief stimulus events. Blamire et al. (23), for example, showed that a stimulus duration of as little as 2 sec could produce detectable signal changes. Under such a situation, the observed signal changes were actually after the stimulus offset, owing to the few-second rise time of the hemodynamic response. Bandettini and colleagues (24) demonstrated signal changes to even shorter task events. Subjects were instructed to make finger movements for durations ranging from 1/2 sec to 5 sec. In all situations, including the brief 1/2-sec movement duration, clear signal increases were detected. A number of similar studies of motor and visual stimulation followed (25– 28). The use of visual stimulation allowed the limits of duration
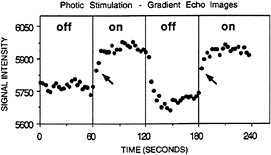
FIG. 1. Adapted from Kwong et al. (20). BOLD contrast signal change is shown for a region of visual cortex during stimulation (on) and during rest (off). These data originally were used to demonstrate the application of BOLD contrast fMRI in normal human subjects. As can be seen, the rise time of the signal (indicated with arrows) is very rapid and has occurred after just a few seconds of stimulation, indicating that shorter stimulus events should be detectable.
to be explored by permitting temporally precise visual stimulation. Savoy, O’Craven, and colleagues (25) showed that visual stimulation as brief as 34 msec in duration could elicit small, but clearly detectable, signal changes (see Fig. 2). Boynton et al. (29), in a detailed and important exploration of the linearity of the BOLD hemodynamic response, showed that not only could brief stimulus durations be detected but also that they integrated over time in a fashion approximating linear summation (see also ref. 30).
These data all suggest that fMRI is sensitive to transient phenomena and can provide at least some degree of quantitative information on the underlying neuronal behavior. Together, these results thus suggest that it should be possible to interpret transient fMRI signal changes in ways directly analogous to electrophysiologic evoked potentials. A first step in this direction was made by Dale and Buckner (31), who recently showed that visual stimuli lateralized to one hemifield could be detected within intermixed trial paradigms. Although this result is unsurprising when trials are separated by long inter-trial intervals compared with the hemodynamic response function, they also showed that when left- or right-hemifield stimuli were randomly presented to the subjects much more rapidly than the hemodynamic response (in this case one stimulus every 2 sec) it was still possible to extract out the identical lateralization pattern to that seen with much longer interstimulus intervals. By using methods similar to those applied in the field of evoked response potential research, the trials were selectively averaged to reveal the predicted pattern of contralateral visual cortex activation. Taken together with the above observations, these collective data demonstrate convincingly that fMRI is capable of detecting changes related to single-task events and brief epochs of stimulation.
Although these early studies show significant promise in the ability to use fMRI data on a trial-by-trial basis, several fundamental questions remain. Specifically, considerably more work must be done to define the precise limits of these “linear modeling” approaches to fMRI data analysis. The difficulty in resolving this question lies in the fact that in many situations it is unknown whether the underlying neuronal activity is itself linearly additive across trials. Thus even when departures from linearity are observed, it remains undetermined whether this reflects an intrinsic nonlinear property of the hemodynamic or underlying neuronal response. To date, several direct tests of the linearity of the BOLD response have observed that, on first approximation, the response sums roughly linearly over trials and over time, at least for simple visual stimulation (29–31) (see Fig. 3). The description “roughly” should be taken seriously because subtle, but clear, departures from linearity have been observed in almost all of these studies. Auditory word stimuli also have been examined and were shown to exhibit roughly linear responses when stimuli were presented as frequently as one per 2 sec, though robust nonlinear
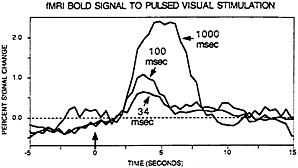
FIG. 2. Data from Robert Savoy and Kathleen O’Craven (25). BOLD contrast signal change are shown for visual stimuli of various brief durations. The three curves represent signal change for 34 msec, 100 msec, and 1,000 msec of stimuli, respectively. Importantly, clear signal change can be observed for events lasting as briefly as 34 msec.
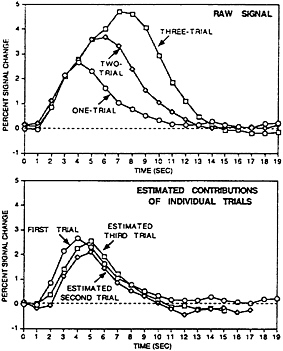
FIG. 3. Adapted from Dale and Buckner (31). (Upper) The raw BOLD fMRI signal evoked when either one, two, or three trials of visual checkerboard stimulation are presented. The trials were each 1 sec in duration and separated by 1 sec. The response increases and is prolonged with the addition of multiple trials, indicating it does not saturate going from one to three trials. (Lower) The explicit contribution of each individual trial by subtracting the one-trial condition from the two-trial condition (yielding the estimated response of the second trial) and the two-trial condition from the three-trial condition (yielding the estimated response of the third trial). The three estimated trials are roughly similar, although subtle but clear departures from linearity can be observed. This finding suggests the bold response can be shown to add linearly over trials, although the generalization of this finding to other brain regions and trial types is still an open question.
responses were present at higher presentation rates (32). The most parsimonious explanation for all of these findings is that the basic transformation between the summation of neuronal events and the BOLD response is approximately linear, at least with presentation rates typically used in fMRI studies. Instances where strong fMRI signal nonlinearities are observed, such as when very rapid stimuli are presented, may represent situations where there exist nonlinearities in either the hemodynamic response or, perhaps more likely, the summation of the underlying neuronal activity itself. Additional investigation clearly will be needed to resolve this issue as well as develop analysis methods to take into account the nonlinearities, regardless of their origin (32). Equally important, additional work must be performed to determine the presence of any heterogeneity in these response functions across functionally specialized regions within a sensory modality and across modalities.
An additional question left unresolved by these primary sensory system studies was whether the signal-to-noise properties of fMRI were capable of detecting individual trial events in higher-order cognitive paradigms, which were typically associated with smaller responses. Buckner et al. (33) answered this question by examining averaged individual trials of a word generation task and exploring the evoked hemodynamic response in sensory as well as higher-level prefrontal regions. Clear signal change was detected in both regions, even within individual subjects. Moreover, activation maps were produced solely from fMRI paradigms consisting of isolated task trials, rather than blocks of trials. The implication was that event-related fMRI could be used to explore cognitive functions. In fact, at the higher signal-to-noise levels achievable at higher
magnetic fields, it appears to be possible to detect activations from single events, without recourse to averaging of any kind (34). This point is addressed in the next section.
Several laboratories also have developed methods for analyzing event-related fMRI paradigms. Initially, analyses based on assigning fMRI images occurring with a certain delay after a trial to the “on” task state and temporally separate images to the “off” state were used (28, 33). However, this approach is limited in that it does not take advantage of the transition information in the hemodynamic response and makes limited fixed assumptions about the shape of the hemodynamic response. More recently, several analyses have been proposed that model the hemodynamic response or a range of possible responses (e.g., a basis set of hemodynamic responses). Voxels best predicted by the models then are identified by using statistical procedures (e.g., refs. 31, 35, and 36). These methods and others are becoming increasingly used for fMRI studies as evidenced by the several examples described in the next section.
Applications to Cognitive Neuroscience
These developments in methodology have led to a number of applications. Perhaps most relevant to this review are those applications that could not have been easily done with conventional blocked task paradigms. We describe several examples to both demonstrate the impact event-related fMRI is having on the field and also to illustrate the range of task paradigms that have benefited from event-related procedures. Fig. 4 shows selected results from the studies discussed below.
One of the earliest examples of an application for event-related fMRI was reported by Susan Courtney and colleagues at the National Institute of Mental Health (37). They explored a working memory paradigm and organized intermixed trials such that the event of encoding a stimulus could be temporally separated from the act of maintaining the stimulus in working memory. By using analysis procedures that separated the within-trial components related to encoding from those related to maintenance, they were able to show a pathway of brain areas that were activated during both encoding and maintenance but showed differential participation in the two kinds of processes. Posterior visual areas contributed proportionately more to perceptual encoding operations, whereas prefrontal areas made their greatest contribution to maintenance operations. Cohen et al. (38) reported a similar finding by examining the within-trial time courses for a letter working memory task (called the n-back task). They looked for effects of time within the trials and its relation to a second factor, memory load. The logic was that sustained activity across the trial would indicate areas involved in maintenance or other sustained processes whereas transient activity (showing an effect of time) could not be related to sustained processes. Consistent with the work of Courtney et al. (37) they found activity within certain prefrontal areas was maintained across the delay, whereas activity within posterior visual areas was not.
Another notable example of event-related fMRI comes from work of McCarthy and colleagues at Yale. McCarthy et al. (39) explored how infrequent target events are processed by the brain. They presented continuous strings of characters with a target string appearing unpredictably once every 20 or so trials—a procedure that can be accomplished only within randomly intermixed trial designs. This paradigm yields the well-documented P300 when examined with evoked response potential. Exploring this effect with fMRI by averaging data in relation to the onsets of the infrequent events demonstrated prefrontal and parietal activation perhaps contributing to, or being evoked by, the P300.
Konishi et al. (40) also have exploited the ability to intermix and resolve signal from distinct trial types. They explored a go/no-go paradigm where a cue appears across trials and randomly indicates whether a response should be made or withheld. Of critical importance is that by having the trials randomly intermixed and encouraging a fast response criteria (350 msec) requires subjects to prepare a response on any given trial. The no-go cue, under such circumstances, signals subjects to withhold (inhibit) their prepared response. Konishi et al., using event-related fMRI procedures, found activation in right inferior prefrontal cortex that discriminated no-go from go trials and might indicate a component of the neural mechanisms underlying response inhibition. This example and that of McCarthy et al. (39) are particularly noteworthy because they both rely on stimuli that are unpredictable on trial-by-trial basis, a paradigm design that can be analyzed only by using event-related procedures.
Recent work by D’Esposito and colleagues (36) has expanded the information obtainable by event-related fMRI to include within-trial time courses explicitly. They had subjects make comparisons about the locations of pairs of objects either presented simultaneously (no-delay condition) or separated by 12-sec delays (delay condition). Comparison of activation time courses across the two conditions was able to clearly show temporal separation of the fMRI signal. The no-delay condition exhibited sensory-motor activation well before similar activation in the delay condition. Within a blocked task paradigm, the two temporally separate events likely would have been blurred together.
Another example of event-related fMRI comes out of the field of memory research. During certain memory paradigms subjects tell you, on a trial-by-trial basis, whether they remember a stimulus or not by pressing a key or recalling an item. For this reason it is highly desirable to be able to sort trials based on subject response. This information is most critical when considering highly unpredictable events such as the false memory effect where subjects sometimes report that they remember events that never happened (41). Schacter and colleagues (42) explored the false memory effect by using fMRI and sorted individual trial responses based on subject performance. They were able to show that many of the same regions activated by true recognition also were activated by false recognition, suggesting both kinds of recognition share a highly similar anatomy.
The manner and limits to how event-related paradigms can be applied are still being explored. Clark et al. (43), for example, have examined event-related procedures where different face or face-like stimuli are presented in random order with 2 sec between stimulus onsets. By using rapid display and analysis with multiple regression, they showed robust activation maps that differentiated between face and scrambled faces consistent with similar work using blocked-task paradigms (44) and also between new faces and target faces learned before scanning. Thus, there is every reason to believe that rapid presentation of stimuli in cognitive task paradigms is feasible.
We recently have explored whether time-course information can be reconstructed from rapidly presented trials using overlap correction methods initially developed for evoked response potential (45–47). Trials of either novel or repeated visual objects were presented between 6 and 10 sec apart, within the range where the hemodynamic response from one trial overlaps with the response from preceding trials. The overall shapes of the hemodynamic response, after overlap correction, were similar to those observed for distantly spaced trials (e.g., ref. 33). For certain regions such as in right extrastriate cortex, the time course of activation began similarly for both trial types but dropped off more rapidly and did not reach the same peak if the objects were repeated, suggesting a neural correlate of priming (48).
Another limit being pushed is how many trials are needed to detect event-related effects. Typically, signals from many trials are averaged or relied on to generate stable time courses and
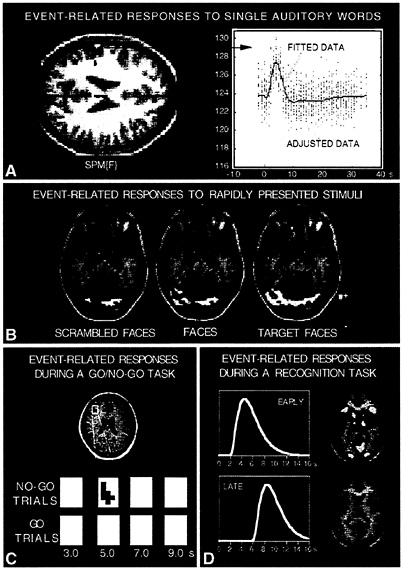
FIG. 4. Examples of applications for event-related fMRI procedures are shown from several different laboratories. (A) Provided by Karl Friston (Wellcome Department of Neurology). A statistical activation map (horizontal section through the brain) of a subject hearing single auditory words is shown on the left with individual trial data from one activated voxel shown on the right (labeled adjusted data). The data are fit with a modeled hemodynamic response function (labeled fitted data), which shows a clear increase in relation to the trial onset. (B) Adapted from Clark et al. (43). Statistical activation maps are shown for three different stimuli types (scrambled faces, faces, and a target face that subjects are matching to a learned template). These stimuli were presented randomly in rapid succession, just a few seconds separating their onsets (see text). Methods based on statistical regression were used to separate the contributions of each stimulus type and generate statistical activation maps. (C) Adapted from Konishi et al. (40). An activation map showing a right prefrontal area active in no-go trials (no-go vs. go trials) of a response inhibition task. Pixels significantly activated 5 sec after no-go stimulus presentation are coded red. The area containing no-go dominant brain activity foci are enlarged, and the brain activity at several time points after the stimulus onset is shown for no-go trials (upper panels) and go trials (lower panels). (D) Adapted from Schacter et al. (49). Brain areas activated by averaged individual trials of a recognition task (see text) are shown based on two separate hemodynamic models with varied delays. Most areas are active at a relatively short delay of about 4–6 sec to peak. However, anterior prefrontal regions were active with a delay of about 8–9 sec.
activation maps. However, Richter et al. (34) have shown the extreme instance: that a single trial of a mental rotation task is sufficient to detect parietal cortex activation. Although such a demonstration does not undermine the utility of averaging across event-related fMRI trials as is similarly done in nearly all behavioral and evoked response potential studies, it does provide confidence that signal-to-noise characteristics of event-related procedures are quite good and that correlation of behavioral responses with individual trial fMRI signals should be possible. Thus, averaging across trials will allow for central tendencies in the modal hemodynamic response to be observed, whereas examination of individual hemodynamic responses may provide information about the unique aspects of an isolated event. As our understanding of the characteristics and limitations of event-related fMRI studies improves, we anticipate that studies will increasingly take advantage of this property in the future.
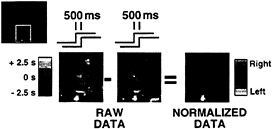
FIG. 5. Figure provided by Robert Savoy, Peter Bandettini, and Kathleen O’Craven (Massachusetts General Hospital). Activation within a region of visual cortex is shown for two separate conditions. In one condition (Left), the right visual hemifield stimulation proceeds the left by 500 msec. In the other condition (Middle), the left proceeds the right by 500 msec. Images from both of these conditions show signal changes with peaks spanning a considerable range in time (several seconds as indicated by scale on left). This intrinsic variance is too large to appreciate the 500-msec offset. However, once the data are normalized for this intrinsic variance by directly comparing the hemodynamic response from the two different lags within individual voxels, the offset between left and right hemifield stimulation can be appreciated (Right). Although not an explicit comparison across regions of brain, such a finding suggests that normalization of the hemodynamic lag within regions can allow small temporal offsets to be appreciated. These normalized offsets then can be compared across regions to make inferences about neuronal delay.
Future Developments
The ultimate limits of temporal resolution achievable from hemodynamic-based brain mapping techniques is not yet de
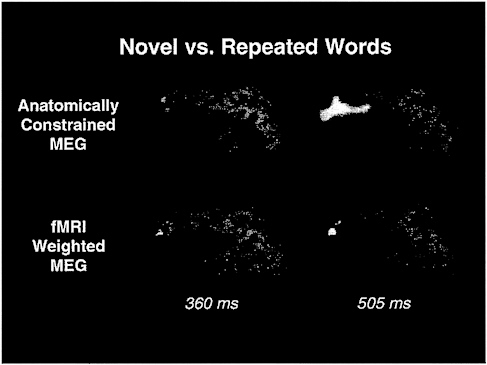
FIG. 6. Snapshots of activity evoked by novel versus repeated words in a semantic judgment task at two different latencies are displayed on an “inflated” (54) left hemisphere. These data represent MEG data constrained by structural MRI and fMRI providing an image of cortical activity that is sensitive to fine spatial as well as temporal changes (46). By combining separate methods it is possible to obtain information unavailable from data produced by either method alone. Event-related fMRI, which can allow task paradigms that randomly intermix trial types at rapid rates, will further allow fMRI, MEG, and EEG to be combined in paradigms that are identical across methods.
termined. Although delays of a few seconds are observed in the positive signal changes on BOLD and arterial spin labeling flow experiments, and upward of 1 sec for potential “negative” signal changes (9), the actual achievable temporal resolution is determined not by these delays, but by both the signal-to-noise of the imaging measurements and, ultimately, the underlying variance in these hemodynamic latencies. Empirically, variance in the delay and shape of the hemodynamic curve has been observed across brain regions and across subjects; it is the origin of this variance that is of greatest interest. For example, Buckner et al. (33, 42) noted delays between visual and prefrontal regions on the order of 1/2 to 1 sec during both stem completion and episodic memory tasks. Even more extreme delays between separate prefrontal regions (about 2–4 sec) were noted during the episodic memory task (ref. 49: see Fig. 4D).
Although the source of this variation is currently unknown, several possibilities exist. fMRI signal delays, at least as observed by BOLD contrast, are sensitive to vessel diameter, with larger vessels showing longer delays (50). Thus, one possible interpretation is that various delays result from differences in the underlying vasculature being sampled across regions. This difference likely accounts for much of the pixel-by-pixel variance seen within a single functionally specialized regions such as V1. However, this possibility seems unlikely to account for all of the broader, regional findings observed thus far because the longest delays have been noted in spatially averaged data and specifically in anterior prefrontal cortex, even in regions where large vasculature is minimal (49). A second, more intriguing, possibility is that the observed regional differences in delay reflect delayed neuronal processing. Although we cannot currently assert this second option as
a likely possibility, it should be fascinating to explore further the idea that there are classes of neuronal responses delayed and/or prolonged on the order of seconds after a task event.
Given what we know about the physiological and biophysical parameters that lead to variance in current fMRI signal change onset measurements, there are two complimentary approaches to reducing variance in regional onset delay measurements that might be used to measure neuronally evoked delays within or across brain regions with a temporal resolution down to fractions of a second. First, several MRI acquisition techniques are know to be selectively sensitive to physiological perturbations within the microvasculature.¶ Though at a loss of some sensitivity, these data acquisition methods should reduce the intrinsic temporal lags, and hence variation, created by imaging flow downstream into draining venous vessels. A similar reduction in sensitivity to downstream flow should be provided through use of some of the arterial spin labeling techniques recently developed, though again at a loss of sensitivity. The use of higher field strength imaging systems, and more advanced radio frequency receiver technology, should at least, in part, mitigate against these sensitivity losses and make such measurements practical. Second, at least under some circumstances it will be possible to make measurements relative to the baseline hemodynamic delay within regions or voxels. Savoy and colleagues (25) have performed such a normalization to reveal temporal lags on the order of 1/2 sec in visual cortex (Fig. 5). Their observation clearly demonstrates the potential of making temporal neuronal onset maps that have the underlying hemodynamic lag variance removed, even for time scales significantly below the apparent hemodynamic delays.
These data suggest that combining high-speed MRI techniques with event-related task paradigms can be used successfully to map quite rapid hemodynamic changes. “Rapid,” however, is a relative term—although changes on the order of seconds, or even several hundred milliseconds, is certainly fast enough to track changes in mental activity occurring with a broad range of tasks and state changes (for example, in studies of rapidly intermixed trials types, of sleep, and of learning), it is clearly not rapid enough to observe the transient coordination of neuronal activities known to occur across large segments of the cortex on time scales of tens to a few hundred milliseconds. The only noninvasive techniques capable of resolving brain activity at such short time scales are EEG and magnetoencephalography (MEG). Unfortunately, however, these techniques provide relatively coarse spatial resolution. To overcome this limitation, techniques recently have been developed for combining the temporal resolution of EEG and MEG with the spatial resolution of fMRI (52, 53). By using such techniques, it is now becoming possible to study the precise spatiotemporal orchestration of neuronal activity associated with perceptual and cognitive events. One example of MRI/MEG integration is shown in Fig. 6. Event-related fMRI allows a further refinement of such integration by affording the ability to study the same exact paradigms in both fMRI settings and during EEG and MEG sessions.
Conclusion
This paper has briefly reviewed several recent developments in fMRI that promise to significantly expand, in the temporal domain, the manner in which we map human brain function. Two developments should allow the field of fMRI to continue this dramatic growth. First, because of both the drive to ever higher magnetic field strengths and improvements in radio frequency receiver coil technology, MRI signal-to-noise shows no evidence of plateau. Second, new and more sophisticated data analytic methods offer greater understanding of our data’s true sensitivity and specificity and will continue to improve our ability to address increasingly sophisticated hypotheses about the underlying mechanisms of brain action. Thus there is every reason to expect that the next half-decade of activity in this field will show growth at least as fast as the last and carry us into new domains of spatial and temporal resolution in our efforts at human brain mapping.
We thank our many colleagues at the Nuclear Magnetic Resonance Center, Massachusetts General Hospital for discussion and support. Figures were generously provided by Ken Kwong, Robert Savoy, Kathy O’Craven, Peter Bandettini, Vince Clark, Karl Friston, Seiki Konishi, and Daniel Schacter. Nick Szumski provided help with preparation of the manuscript. R.L.B. was supported by National Institutes of Health Grant DC03245–01, the Charles A.Dana Foundation, and the Human Frontiers Science Project. A.M.D. was supported by the Human Frontiers Science Project.
1. Cohen, M.S. & Weisskoff, R.M. (1991) Magn. Reson. Imaging 9, 1–37.
2. Mosso, A. (1881) Ueber Den Kreislauf des Blutes in Menschlichen Gehirn (Verlag von View, Leipzig, Germany).
3. Posner, M.I. & Raichle, M.E. (1994) Images of Mind (Scientific American Books, New York).
4. Woolsey, T.A., Rovainen, C.M., Cox, S.B., Henegar, M.H., Liang, G.E., Liu, D., Moskalenko, Y.E., Sui, J. & Wei, L. (1996) Cereb. Cortex 6, 647–660.
5. Dalkara, T., Irikura, K., Huang, Z., Panahian, N. & Moskowitz, M.A. (1995) J. Cereb. Blood Flow Metab. 15, 631–638.
6. Mandeville, J.B., Marota, J., Keltner, J.R., Kosofsky, B., Burke, J., Hyman, S., LaPointe, L., Reese, T., Kwong, K., Rosen, B.R., Weissleder, R. & Weisskoff, R. (1996) Proc. Soc. Magn. Reson. Med. Fourth Sci. Meeting Exhib. 3, 292.
7. Cooper, R., Crow, H.J. & Papakostopoulos, D. (1975) J. Physiol. 245, 70–72.
8. Malonek, D. & Grinvald, A. (1996) Science 272, 551–554.
9. Hu, X., Le, T.H. & Ugurbil, K. (1997) Magn. Reson. Med. 37, 887–884.
10. Herscovitch, P. & Raichle, M.E. (1983) J. Cereb. Blood Flow Metab. 3, 407–415.
11. Raichle, M.E. (1987) in The Handbook of Physiology, eds. Plum, F. & Mountcastle, V. (Am. Physiol. Assoc., Bethesda, MD), Vol. V, Section 1, pp. 643–674.
12. Belliveau, J.W., Kennedy, D.N., McKinstry, R.C., Buchbinder, B.R., Weisskoff, R.M., Cohen, M.S., Vevea, J.M., Brady, T.J. & Rosen, B.R. (1991) Science 254, 716–719.
13. Mansfield, P. (1977) J. Physics C10, L55–L58.
14. Rzedzian, R.R. & Pykett, I.L. (1986) Radiology 161, 333.
15. Rosen, B.R., Belliveau, J.W., Vevea, J.M. & Brady, T.J. (1990) Magn. Reson. Med. 14, 249–265.
16. Detre, J.A., Leigh, J.S., Williams, D.S. & Koretsky, A.P. (1992) Magn. Reson. Med. 23, 37–45.
17. Ogawa, S., Lee, T., Nayak, A. & Glynn, P. (1990) Magn. Reson. Med. 14, 68–78.
18. Thulborn, K.R., Waterton, J.C., Matthews, P.M. & Radda, G.K. (1982) Biochim. Biophys. Acta 714, 265–270.
19. Hoppel, B.E., Weisskoff, R.M., Thulborn, K.R. & Moore, J.B.B.R. (1991) in Measurement of Regional Brain Oxygenation State Using Echo Planar Linewidth Mapping (Soc. Magnetic Resonance in Medicine, Berkeley, CA). Vol. 1, pp. 308.
20. Kwong, K.K., Belliveau, J.W., Chesler, D.A., Goldberg, I.E., Weisskoff, R.M., Poncelet, B.P., Kennedy, D.N., Hoppel, B.E., Cohen, M.S. & Turner, R. (1992) Proc. Natl. Acad. Sci. USA 89, 5675–5679.
21. Ogawa, S., Tank, D.W., Menon, R., Ellerman, J.M., Kim, S.G., Merkle, H. & Ugurbil, K. (1992) Proc. Natl. Acad. Sci. USA 89, 5951–5955.
22. Bandettini, P.A., Wong, E.C., Kinks, R.S., Tikofsky, R.S. & Hyde, J.S. (1992) Magn. Reson. Med. 25, 390–397.
23. Blamire, A.M., Ogawa, S., Ugurbil, K., Rothman, D., McCarthy, G., Ellerman, J.M., Hyder, F., Rattner, Z. & Shulman, R.G. (1992) Proc. Natl. Acad. Sci. USA 89, 11069–11073.
24. Bandettini, P.A. (1993) in Functional MRI of the Brain (Soc. Magnetic Resonance in Medicine, Berkeley, CA).
25. Savoy, R.L., Bandettini, P.A., O’Craven, K.M., Kwong, K.K., Davis, T.L., Baker, J.R., Weisskoff, R.M. & Rosen, B.R. (1995) Proc. Soc. Magn. Reson. Med. Third Sci. Meeting Exhib. 2, 450.
26. Humberstone, M., Barlow, M., Clare, S., Coxon, R., Glover, P., Hykin, J., Macdonald, I.A., Morris, P.G. & Sawle, G.V. (1995) Proc. Soc. Magn. Reson. Third Sci. Meeting Exhib. 2, 858.
27. Boulanouar, K., Demonet, J.F., Berry, I., Chollet, F., Manelfe, C. & Celsis, P. (1996) Proc. Int. Soc. Magn. Reson. Med. Fourth Sci. Meeting Exhib. 3, 1764.
28. Konishi, S., Yoneyama, R., Itagaki, H., Uchida, I., Nakajima, K., Kato, H., Okajima, K., Koizumi, H. & Miyashita, Y. (1996) NeuroReport 8, 19–23.
29. Boynton, G.M., Engel, S.A., Glover, G.H. & Heeger, D.J. (1996) J. Neurosci. 16, 4207–4221.
30. Hykin, J., Bowtell, R., Glover, P., Coxon, R., Blumhardt, L.D. & Mansfield, P. (1995) Proc. Soc. Magn. Reson. Third Sci. Meeting Exhib. 2, 795.
31. Dale, A.M. & Buckner, R.L. (1997) Hum. Brain Map. 5, 329–340.
32. Friston, K.J., Josephs, O., Rees, G. & Turner, R. (1998) NeuroImage, in press.
33. Buckner, R.L., Bandettini, P.A., O’Craven, K.M., Savoy, R.L., Petersen, S.E., Raichle, M.E. & Rosen, B.R. (1996) Proc. Natl. Acad. Sci. USA 93, 14878–14883.
34. Richter, W., Georgopoulos, A.P., Ugurbil, K. & Kim, S.-G. (1997) NeuroImage 5, S49.
35. Josephs, O., Turner, R. & Friston, K. (1997) Hum. Brain Map. 5, 243–248.
36. Zarahn, E., Aguirre, G. & D’Esposito, M. (1998) NeuroImage, in press.
37. Courtney, S.M., Ungerleider, L.G., Keil, K. & Haxby, J.V. (1997) Nature (London) 386, 608–611.
38. Cohen, J.D., Perlstein, W.M., Braver, T.S., Nystrom, L.E., Noll, D.C., Jonides, J. & Smith, E.E. (1997) Nature (London) 386, 604–607.
39. McCarthy, G., Luby, M., Gore, J. & Goldman-Rakic, P. (1997) J. Neurophysiol. 77, 1630–1634.
40. Konishi, S., Nakajima, K., Uchida, I., Sekihara, K. & Miyashita, Y. (1997) NeuroImage 5, S120.
41. Roediger, H.L.I. & McDermott, K.B. (1995) J. Exp. Psychol. Learn. Mem. Cognit. 21, 803–814.
42. Schacter, D.L., Buckner, R.L., Koutstaal, W., Dale, A.M. & Rosen, B.R. (1998) NeuroImage, in press.
43. Clark, V.P., Maisog, J.M. & Haxby, J.V. (1997) NeuroImage 5, S50.
44. Haxby, J.V., Horwitz, B., Ungerleider, L.G., Maisog, J.M., Pietrini, P. & Grady, C.L. (1994) J. Neurosci. 14, 6336–6353.
45. Ganis, G., Kutas, M., Schendan, H.E. & Dale, A.M. (1997) Cog. Neurosci. Soc. 4th Annual Meeting 4, 73.
46. Dale, A.M., Halgren, E., Lewine, J.D., Buckner, R.L., Paulson, K., Marinkovic, K. & Rosen, B.R. (1997) NeuroImage 5, S592.
47. Woldorff, M.G. (1993) Psychophysiology 30, 98–119.
48. Buckner, R.L. & Koutstaal, W. (1998) Proc. Natl. Acad. Sci. USA 95, 891–898.
49. Schacter, D.L., Buckner, R.L., Koutstaal, W., Dale, A.M. & Rosen, B.R. (1997) Cog. Neurosci. Soc. 4th Annual Meeting 4, 68.
50. Lee, A.T., Glover, G.H. & Meyer, C.H. (1995) Magn. Reson. Med. 33, 745–754.
51. Hennig, E. (1994) Magn. Reson. Med. 32, 146–149.
52. Dale, A., Ahlfors, S.P., Aronen, H.J., Belliveau, J.W., Houtilainen, M., Ilmoniemi, R.J., Kennedy, W.A., Korvenoja, A., Liu, A.K., Reppas, J.B., Rosen, B.R., Sereno, M.I., Simpson, G.V., Standertskjold-Nordenstam, C.-G., Virtanen, J. & Tootell, R.B.H. (1995) Soc. Neurosci. Abstr. 21, 1275 (abstr).
53. Dale, A.M. & Sereno, M.I. (1993) J. Cognit. Neurosci. 5, 162– 176.
54. Sereno, M.I., Dale, A.M., Reppas, J.B., Kwong, K.K., Belliveau, J.W., Brady, T.J., Rosen, B.R. & Tootell, R.B.H. (1995) Science 268, 889–893.








