This paper was presented at a colloquium entitled “Neuroimaging of Human Brain Function,” organized by Michael Posner and Marcus E.Raichle, held May 29–31, 1997, sponsored by the National Academy of Sciences at the Arnold and Mabel Beckman Center in Irvine, CA.
Frontoparietal cortical networks for directing attention and the eye to visual locations: Identical, independent, or overlapping neural systems?
MAURIZIO CORBETTA
Department of Neurology and Radiology, McDonnell Center for Studies of Higher Brain Functions, Washington University, 660 South Euclid Avenue, St. Louis. MO 63110
ABSTRACT Functional anatomical and single-unit recording studies indicate that a set of neural signals in parietal and frontal cortex mediates the covert allocation of attention to visual locations, as originally proposed by psychological studies. This frontoparietal network is the source of a location bias that interacts with extrastriate regions of the ventral visual system during object analysis to enhance visual processing. The frontoparietal network is not exclusively related to visual attention, but may coincide or overlap with regions involved in oculomotor processing. The relationship between attention and eye movement processes is discussed at the psychological, functional anatomical, and cellular level of analysis.
Attention defines the mental ability to select stimuli, responses, memories, or thoughts that are behaviorally relevant, among the many others that are behaviorally irrelevant. Selection is necessary because of computational limitations in the brain’s capacity to process information and to ensure that behavior is controlled by relevant information. Problems of selection are common throughout the brain, yet are different in terms of task demands, the computational strategy employed to solve them, and related neuronal implementation. Current research on attention therefore focuses on understanding different attentional mechanisms at all these levels of analysis: performance, computations, and neural systems.
This review describes progress in the field of visuospatial attention, or attention for visual location. This form of selection is important for a variety of visual behaviors. In species with a sophisticated color and object vision, like monkeys and humans, the identification of objects and the analysis of their spatial relations require the application of high-level visual routines at selected locations of the visual input array (1). In most animals, locations are also selected during the spatial localization of potentially interesting or dangerous stimuli, e.g., a prey or a predator. Finally, the visuomotor control of movements during reach and manipulation involves the continuous updating of spatial representations for the precise guidance of the limb onto the object of interest.
Visuospatial attention has been studied extensively in the context of visual orienting. In most natural situations, we explore a visual scene by means of saccadic eye movements that rapidly (in 50–70 msec) bring the fovea, the retinal region of highest acuity, and the neural machinery associated with it onto stimuli of interest. Stimuli are processed during interspersed periods of fixation that last up to 250 msec. This set of processes is defined as “overt visual orienting.” Behaviorally relevant stimuli, however, can be attended to in the absence of exploratory saccadic eye movements, i.e., the locus of attention is dissociable from eye fixation. Attention can be directed toward a location either voluntarily or reflexively when a stimulus abruptly appears in the visual field. This set of processes is defined as “covert visual orienting.”
This review first selectively covers some of the psychological evidence on the relationship between covert and overt orienting mechanisms. Next, functional neuroimaging studies in humans and single unit recording studies in the awake behaving monkey are reviewed to determine the neural basis of covert visual orienting and how they overlap with overt (saccadic) orienting mechanisms.
Psychological Mechanisms of Visual Orienting
Covert Orienting. Many researchers have found that advance knowledge of the position of an upcoming stimulus facilitates its detection even when eye movements are not allowed (2, 3). For example, Posner (3) presented a target at 7 degrees to the left or right of fixation. Before the presentation of the target, an arrow was presented at fixation pointing toward the correct target location on 80% of the trials. On 20% of the trials the arrow pointed toward the wrong location. Manual reaction times for the detection or discrimination of the target were faster when subjects were able to anticipate its location. The arrow (or central cue) provides advance location information that can be used to bias the processing of target stimuli. Because the cue has to be interpreted and voluntarily used, this form of cueing is called “endogenous” or “cognitive.” The cue per se is not necessary, however, because similar results are obtained when subjects are asked directly to “pay attention” to a certain location (4). A similar facilitation of stimulus processing is found when advance location information is provided as a sensory stimulus presented at the most likely stimulus location (5). This form of cueing is called “exogenous” or “sensory” to emphasize its dependence on sensory information.
The effects of spatial cueing on visual processing are not limited to the simple detection of suprathreshold visual stimuli, but extend to many other visual tasks, including threshold detection of luminance and discrimination of shape, size, color, and motion (6, 7). The widespread effect of spatial cueing on vision indicate that processes that mediate spatial selection have wide access to visual processes specialized for feature and object analysis.
The enhancement in stimulus processing produced by spatial cueing, in the absence of eye movements, is thought to reflect the activation of a mechanism that shifts attention (or the focus of processing) to the stimulus location before its appearance. This may facilitate stimulus analysis in two related ways. First, visual processes could complete stimulus analysis more rapidly at the
© 1998 by The National Academy of Sciences 0027–8424/98/95831–8$2.00/0
PNAS is available online at http://www.pnas.org.
attended location, because it takes time to reorient attention to the new (unattended) stimulus location. Second, attention could more directly influence visual processes, for instance, by enhancing their sensitivity at the attended location. This would explain how attention also improves sensory thresholds (6).
When recording brain activity either at the whole brain level or at the level of single neurons, different types of signals will correspond to the activation of the attentional mechanism (“source” signals) and its interaction with the visual system (“site” signals). For example, a source signal would be associated with a shift of attention to a location and would be recorded in areas that implement the attentional mechanism and/or in visual areas responsible for stimulus analysis. In visual areas, a source signal may prime visual processes to a more efficient response. Once a stimulus is presented, stimulus analysis may be enhanced by attention. This would produce a modulation of visual processing (“site” signal) that marks the site of the interaction between source attentional signals and visual processes. Whereas source signals provide information on the organization of attention systems, site signals provide information on how sensory (or motor or cognitive) systems are affected by attention.
Overt Orienting. The discovery of a mechanism for covertly (without eye movements) directing attention to locations raises the question of its relationship to mechanisms responsible for saccadic generation. In normal conditions, attention and eye movements move synchronously and select common targets in the visual field. Following Shepherd and colleagues (8), this relationship can take three forms. At one extreme attention and eye movement generation can involve entirely different mechanisms (independence hypothesis). For example, locations could be simultaneously computed in separate spatial maps by attentional and oculomotor systems. An implication of this view is that it should be possible to operate simultaneous shifts of attention and eye movements in opposite directions. At the other extreme, attention and eye movement generation involve the same mechanisms (identity hypothesis). A location is encoded by the attentional mechanism in a set of motor coordinates that specify direction and amplitude and that are also used for planning a saccadic eye movement (9). In dual-task conditions, in which different locations are selected respectively by attentional and eye movement mechanisms, one would predict that locations selected by eye movement mechanisms should control behavior. An intermediate position is that attention and eye movement processes share resources or computations at some stage (interdependence hypothesis). For example, both attention and eye movement systems may depend on an early sensory visual representation. When both systems select the same location on the representation, their performance is optimal; when different locations are to be selected by each system, their performance is impaired.
Early papers provided conflicting evidence on whether preparing an eye movement toward a location enhanced the visual processing of stimuli presented at the same location and, vice versa, whether a shift of attention facilitated oculomotor execution (10, 11). Furthermore, under certain conditions attention could move in the opposite direction of an eye movement (3). Altogether these results indicated that attention and eye movements were either independent processes (11) or separate but functionally related processes (3), such that they could be recruited in isolation or in concert depending on task demands.
More recent work, however, has established that attention and eye movements are more tightly related. Shepherd and colleagues (8) manipulated spatial attention by varying the probability that peripheral probe stimuli would appear in different positions, and eye movements by cueing saccades with a central arrow cue. They found that the preparation of a saccadic eye movement enhanced the manual detection of stimuli presented at the saccadic target location, irrespective of the direction of attention. That is, even when attention and eye movements were cued to opposite locations, stimuli at the location of the saccade were always detected more rapidly. The latency of the saccades was also uninfluenced by the direction of attention. Hoffman and Subramaniam (12) confirmed in a dual-task situation that target detection is superior at the saccade location regardless of the direction of attention. In this experiment, saccadic latencies were slowest when attention and saccades were directed toward opposite locations. Klein (11) suggested that processing facilitation at the saccade location is induced by saccadic execution, but not saccadic programming.
The current view is that attention and eye movement systems are tightly related. During the preparation of a saccade, the selection of a location is controlled by the oculomotor system, even when attention is directed elsewhere through cognitive manipulations. This supports an identity view in which attention shifts are organized in oculomotor coordinates. Because the direction of attention is dissociable from eye position during fixation, an additional veto-going signal has been postulated to prevent breakdowns of fixation (9). It is still under discussion whether attentional processes are separate when a saccade is planned but not performed, or when the eyes are fixated (13, 14). Finally, these findings are not inconsistent with the notion that attention and eye movement systems may be separate but share resources. For example, the slowing of saccadic latencies in Hoffman and Subramaniam (12) is consistent with some sharing of common resources. However, the prevalent control of saccades on location would suggest that the eye movement system has preferential access to those resources.
The next section considers functional anatomical data recorded in normal human volunteers with various methods including positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). These experiments indicate that during visual orienting a network of frontal and parietal regions is consistently activated in the human brain. These frontoparietal regions are the source of a location bias in ventral occipital regions involved in object analysis. Hence, ventral occipital regions are the site of the spatial modulation. Finally, the anatomical overlap between brain regions involved in covert and overt orienting is discussed to assess whether attention and eye movements share common or separate neural representations.
Functional Anatomy of Visual Orienting in the Human Brain
Covert Orienting. Functional neuroimaging methods like PET and fMRI record in the living human brain local changes in blood flow and oxygenation, respectively. These metabolic parameters are indirectly related to the level of neuronal activity. Functional neuroimaging methods are used to image brain regions active during sensory, motor, and cognitive processing (15). The greatest strength of neuroimaging is the capacity to visualize the whole brain with a spatial precision of about 1 cm for PET and 2–3 mm for fMRI. Further, the possibility to test human volunteers allows the use of sophisticated experimental protocols that can be compared directly with those employed in psychological studies. The greatest weakness of neuroimaging is the poor temporal resolution, about 40 sec for PET and 2–4 sec for fMRI, far above the millisecond scale of neuronal activity. This limitation prevents any meaningful analysis of the temporal sequence of task-related activations.
Several studies have investigated the functional anatomy of covert visual orienting to simple unstructured peripheral stimuli. These studies have shown that a specific set of frontal and parietal regions is consistently recruited during visual orienting. Corbetta et al. (16) asked subjects to voluntarily shift attention along a series of locations positioned in left or right visual field to detect brief visual stimuli with a speeded key-press response (shifting-attention task). This paradigm involves endogenous spatial cueing, and, as expected, stimuli at attended locations were detected faster than stimuli at unattended locations. Areas involved in covert orienting were localized by subtracting PET activity recorded during the shifting-attention task from activity recorded during a central-detection task. In the central-detection task subjects attended to and manually responded to stimuli in the
fovea while being presented with the same series of peripheral stimuli as in the shifting-attention task. The resulting subtraction image (shifting attention-central detection) is matched for peripheral sensory stimulation, arousal, and motor demands and should image processes specifically involved in shifting attention. Significant blood flow changes were visualized in superior parietal and frontal cortex (Fig. 1; see also Fig. 2, red foci). Areas of activation were bilateral but stronger in the hemisphere contralateral to the attended field. Other regions were less consistently active, including right inferior parietal and superior temporal cortex and anterior cingulate cortex.
Blood flow changes during the shifting attention task may reflect a variety of neural signals. The visual responses to the peripheral stimuli may be enhanced by their behavioral relevance. This putative neural signal would reflect the interaction between attentional processes and visual processes dedicated to the spatial analysis of the peripheral visual stimuli. According to the distinction made in the introduction, this signal would localize the site of the attentional modulation. Alternatively or in addition, activity in parietal and frontal cortex may reflect source signals related to the covert allocation of attention to various locations. This latter interpretation was originally preferred because the task was specifically designed to stress attentional shifts and minimize sensory demands. The minimal activation of primary and associative visual areas found during the shifting task supports this interpretation.
Similar but not identical areas of activations were localized by Nobre et al. (17) during a visual detection task that emphasized exogenous or sensory-driven spatial cueing. Locations were cued by stimuli, and reaction times to probe stimuli were measured at cued and uncued locations. As expected, targets at cued locations were detected faster. When compared with a simple fixation condition, PET regions of activations were putatively localized, by using individual MR scans, to the intraparietal sulcus in parietal cortex and to the precentral region in frontal cortex. Other regions of activation were localized in right superior temporal and cingulate cortex.
To compare regions of brain activations during endogenous and exogenous spatial cueing, foci from Corbetta et al. (16) and Nobre et al. (17) were plotted on three-dimensional (3D) and flattened two-dimensional (2D) surface representation of a standardized atlas of the human brain, developed at Washington University by Heather Drury and David Van Essen (18, 19). Foci of activation from all studies on peripheral attention have been plotted onto the 3D and 2D brain atlas representation. Each focus [indicated by a small sphere whose center corresponds to x, y, z coordinates of the activation in Talairach and Torneaux (20)] is surrounded by a 10-mm radius, which accounts for the variability in the mean location estimate (see ref. 19). This variability is multifactorial, including: (i) imperfect registration of the functional data during the normalization to Talairach space; (ii) variability in the position of identified cortical areas in relation to nearby geographical landmarks; (iii) limited spatial resolution of the PET techniques; and (iv) variability in the average anatomy of different group of subjects.
Exogenous cueing (Fig. 2, yellow foci; ref. 17) and endogenous cueing (Fig. 2, red foci; ref. 16) activated similar but not identical regions in parietal and frontal cortex. The variability in some regions is higher than 10 mm, potentially indicating task differences or biological differences. Taken together these two studies indicate that a set of parietal and frontal regions coactivate when locations are cued endogenously and exogenously. Possible anatomical differences may reflect differences in the processes recruited by different cues.
Because both studies involved manual reaction times to peripheral visual stimuli, parietal and frontal activity may still reflect basic visuomotor processes related to the speeded manual response. We recently have studied a covert version of the shifting-attention task (21), in which subjects shifted attention voluntarily between peripheral locations but did not press a key
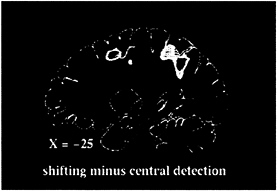
FIG. 1. Sagittal PET section, 25 mm left of midline, of group-averaged subtraction image between shifting-attention and central-detection tasks.
to signal stimulus detection. Shifts of attention were then entirely decoupled from manual activation. To have some measure of performance, manual reaction times were measured in a prior psychophysical session in which subjects were trained to covertly shift attention to different locations in the periphery of the visual field. This experiment was carried out by using fMRI, which allows a more precise localization of functional activity in relationship to the underlying anatomy in both single subjects and groups of subjects. Fig. 3 shows fMRI activity recorded in one subject during shifts of attention to left visual field locations. Individual data are presented to emphasize the relationship with the underlying anatomy, but similar results were obtained in a group of 12 subjects. Three regions are localized precisely near postcentral and intraparietal sulcus within the parietal lobe and precentral sulcus/gyrus within the frontal lobe (Fig. 3). This pattern of cortical activation is similar to the one obtained with PET when using a speeded manual response (16). Because the covert task further minimized visuomotor demands, i.e., no overt manual detection was required, the similarity between experiments shows that such pattern of cortical activation is unrelated to visuomotor manual processing per se but is related to the purely mental process of directing and shifting attention to different visual locations.
Other experiments have investigated the functional anatomy of visual orienting during peripheral shape/object discrimination tasks. In this paradigm, attention is endogenously directed throughout a block of trials to a single peripheral object location (tonic attention). Using the site/source distinction, tonic attention and spatial cueing paradigms should involve similar source signals because attention is endogenously directed to peripheral locations in both cases, but different site signals because task demands are so different. Although the spatial cueing paradigm imaged by other experiments (16, 17) requires the detection of stimuli that change in location, the tonic attention paradigm involves the detection of shape variations at a single location. If the frontoparietal network is the source of a selective location signal, there should be similar activations during spatial cueing and tonic attention. Conversely, if activity in the frontoparietal network reflects spatial processing of locations, one would predict weaker activations during tonic attention where only one location is relevant.
Heinze et al. (22) and Woldorff et al. (23) asked subjects to discriminate a subtle change in the shape of a left- or a right-sided object during bilateral presentation. The control condition involved the passive viewing of the same objects. Objects were displayed in the lower visual field in the latter study and in the upper visual field in the former study. Both groups reported blood flow increases above a passive viewing baseline (blood flow enhancement) in the occipital cortex contralateral to the at-
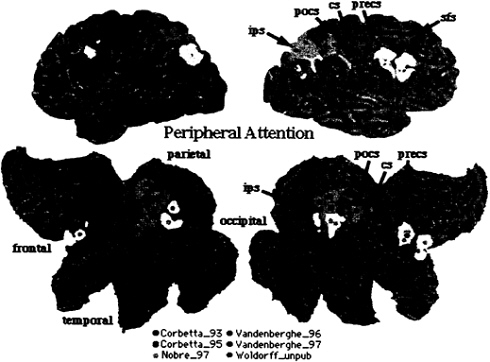
FIG. 2. 3D rendering and 2D flattened surface of the Visible Man Brain, with the left hemisphere on the left. Lobes are indicated in 2D surface. Sulci are indicated as follows: sfs, superior frontal sulcus (s.); precs, precentral s.; cs, central s.; pocs, postcentral s.; ips, intraparietal s. Foci of activation during shifting attention [red (16), yellow (17), and orange (53)] and tonic attention [pink (24), violet (25), and light orange (Woldorff et al., unpublished data)].
tended object. Only Woldorff et al. (23) reported parietal activations nearby the intraparietal sulcus (Fig. 2, light-orange foci).
Vandenberghe et al. (24) asked subjects to attend to and discriminate small changes in the orientation of a grating stimulus positioned either foveally or 10 degrees peripherally. The control condition involved the detection of the onset of the same stimuli. Again, blood flow enhancement above detection was recorded in ventral occipital cortex. Right superior parietal and frontal cortex were also active (Fig. 2, pink foci), with parietal activity significantly stronger when attention was directed onto the peripheral than when directed onto the foveal object. In a separate experiment, the same group (25) examined the effect of discriminating one vs. two features (orientation and displacement) in the same or in different peripheral objects. Again, in both conditions, a network of superior parietal and frontal regions was active above a detection baseline (Fig. 2, violet foci).
Fig. 2 directly compares the pattern of activation for spatial cueing with that of tonic attention. Overall, this analysis indicates a very strong overlap in the pattern of cortical activation for spatial cueing and tonic attention. In the right hemisphere, activity localizes along postcentral and intraparietal sulcus in parietal cortex, possibly defining the same two regions localized by fMRI in single subjects (Fig. 3). In the left hemisphere, activity straddles across postcentral and intraparietal sulcus. In frontal cortex two distinct foci of activations are evident: one near the precentral sulcus/gyrus, and the other near the posterior tip of the superior frontal sulcus. In summary, similarity in the functional anatomy of tonic and shifting-attention paradigms supports the idea that a frontoparietal network is the source of a selective location signal, and not the site of the attentional modulation.
The occipital regions active during object discriminations correspond to extrastriate visual regions of the “ventral visual system” (26). Their modulation by attention in the form of a blood flow enhancement reflects their interaction with the selective location signal. Correspondingly, powerful neuronal enhance-
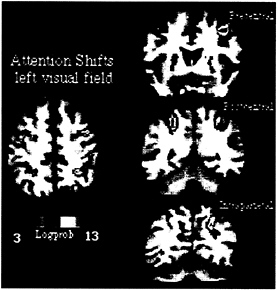
FIG. 3. MPrage anatomical and fMRI activity in single subject during shifting attention in left visual field. Transverse section, z=52. Coronal sections along precentral (precs), postcentral (pocs), and intraparietal sulcus (ips).
ment of visual responses for the attended object have been recorded in occipital visual areas with scalp and single-unit recordings virtually in the same paradigm (27, 28). A possible neuronal correlate of the selective location signal has been reported recently by Luck et al. (28). By recording activity with single unit in macaque areas V2 and V4 of the ventral visual system, they reported an increase in the baseline firing rate of a neuron when attention was directed toward the neuron’s receptive field before stimulus presentation. This baseline shift reflects the build-up of a location bias that originates, I would propose, in the frontoparietal network described above.
Overt Orienting. The psychological work reviewed earlier indicates a strong functional relationship between processes mediating eye movements and attention. In the previous section, functional imaging studies have defined a frontoparietal cortical network that is active when attention is directed to visual locations. Hereafter, the degree of anatomical overlap between this frontoparietal network and regions active during oculomotor processing is considered to evaluate further the linkage between attention and eye movement mechanisms.
The functional anatomy of the oculomotor system has been investigated extensively with a variety of oculomotor tasks, including voluntary, visually guided, memory-guided, and conditional saccades (29–33). Fig. 4 summarizes all studies in the literature, plus those from our own laboratory, that involved visually guided and memory-guided saccades. All foci are plotted onto the Visible Man Brain Atlas by using the same criteria of Fig. 2. Preliminary analysis showed no consistent difference in the pattern of activation between different types of saccades. The only exception was the presence of prefrontal activity (not plotted) in some experiments that involved memory-guided saccades. In the frontal lobe, activity centers onto
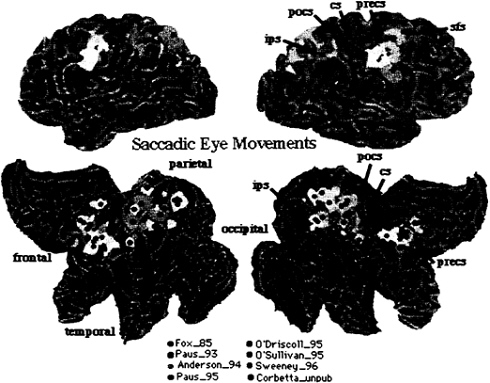
FIG. 4. Visible Man Brain as in Fig. 2. Foci for saccadic eye movements.
the precentral gyrus, extending from the central sulcus to the precentral sulcus. This region is considered to be the human homologue of the monkey’s frontal eye field (FEF). Lesions in the FEF cause acutely an eye deviation toward the side of the lesion, and chronically the inability to suppress reflexive saccades. A second cluster is evident nearby the posterior tip of the superior frontal sulcus. In parietal cortex, activity is again distributed near intraparietal and postcentral sulcus and adjacent gyri, but extends also toward the precuneus.
To directly compare eye movement- and attention-related activations, data from Fig. 2 and 4 have been rearranged in Fig. 5, such that foci for attention have been colored in red, foci for eye movements, in green, and areas of anatomical overlap, in yellow. Areas of large overlap occur bilaterally in intraparietal and postcentral regions and frontally in the precentral region and superior frontal sulcus region. Exclusive eye movement activity is evident dorsally in the right precuneus and left postcentral gyrus. Exclusive attention activity is evident ventrally in the intraparietal sulcus. In frontal cortex, attention foci tend to plot more anteriorly than eye movement foci.
This analysis shows both overlap and segregation in the spatial distribution of cortical activity when attention- and eye movement-related foci are compared across PET experiments. The biological interpretation of these findings must be cautious given the presence of nonbiological variability. Although the 10-mm radius of uncertainty associated with each focus should account for most of the methodological variability, differences in experimental variables across experiments (e.g., eccentricity of stimuli, rate of stimulus presentation) can increase the variability in Fig. 5. However, if one emphasizes anatomical overlap, all three major sites of activation for attention (intraparietal, postcentral, and precentral) show convergent activation during eye movement.
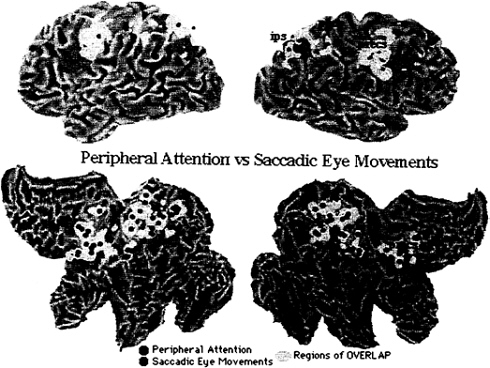
Furthermore, the presence of attentional activity in the human FEF indicates that signals related to attention can be recorded in an area that is strongly implicated in voluntary oculomotor planning. Vice versa, if one emphasizes anatomical segregation, there appears to be large sections of parietal and frontal cortex that are uniquely active for each condition. For example, atten

FIG. 6. Anatomical MRI and fMRI activity in single subject for shifting attention and saccadic eye movements in left (LVF) and right (RVF) hemispheres. Slices at z=52 mm.
tion foci are more anteriorly located in frontal cortex than eye movement foci.
In summary, this metaanalysis across PET experiments does not support the independence hypothesis, because of the anatomical overlap in the functional anatomy of attention and eye movements. The degree of overlap is most consistent with the interdependence hypothesis, according to which some neural substrates (processes, resources) are shared between systems. The variability in the data does not allow one to rule out the identity hypothesis. The accuracy of the metaanalysis would improve only if studies that are very similar in design were included. The variability in Fig. 5 provides an upper limit of what to expect in associative cortex when functional activations are dichotomized along two rather broad task variables.
A more precise test of the relationship between eye movements and attention is provided by functional mapping experiments in which the same subject undergoes testing for covert and overt visual orienting mechanisms. Fig. 6 provides preliminary data on such a comparison. A single subject (the same as in Fig. 3) was scanned in separate blocks during the covert shifting-attention task, in which attention was shifted sequentially along a series of locations, and during an overt shifting task, in which voluntary saccades were performed along the same series of locations as in the covert shifting attention task. The left and right visual field were independently tested. In this subject, activations for attention localized to identical brain regions as activations for saccadic eye movements, both for left and right visual field shifts. The frontal activation centers on the precentral region and extends to the posterior tip of the superior frontal sulcus. The precentral region corresponds to the FEF. The parietal activations may correspond to areas in macaque that contain both oculomotor and attentional signals [e.g., lateral intraparietal area (LIP) and 7a]. This pattern of overlapping activation, if confirmed, would
provide strong support for the notion that attention and eye movements colocalize to identical regions. It would not rule out the possibility that within the same area different neurons are devoted to attentional or eye movement processing, or that the same neurons share resources during different types of processing.
The next section considers neuronal mechanisms that are best investigated by recording neural activity in awake-behaving monkeys during conditioned behavior. These studies allow examination of the cellular correlates of covert and overt orienting and how they relate at the neuron level.
Neuronal Mechanisms of Visual Orienting in the Monkey Brain
Covert Orienting. The voluntary tonic allocation of attention onto a peripheral visual location produces two distinct types of visual modulations in posterior parietal cortex (34, 35). Before stimulus presentation, an increment in baseline firing occurs when the monkey can attend or anticipate the appearance of a visual stimulus within the neuron’s receptive field. The modulation is similar when the visual stimulus triggers a manual response or an eye movement response. After stimulus presentation, the visual response is enhanced when the stimulus within the receptive field is attended as compared with when it is unattended. Interestingly, the latency of neuronal response for attended and unattended stimuli is identical, suggesting that attention primes sensory pathways before stimulus presentation. Baseline shifts have been described in area LIP, whereas visual enhancement (independent of the type of motor response) has been found in areas LIP, 7a, and pulvinar nucleus of the thalamus (34–36). In terms of the site-source distinction, the increase in the baseline firing rate has the attributes of a source signal that primes receptive field activity before stimulus onset. Under the same conditions of tonic attention, baseline shifts also appear in ventral visual regions (V2, V4) before stimulus presentation (28). The source of baseline shifts in LIP, V2, and V4 is unknown but must derive from regions that encode selected spatial locations. These can include different parietal regions (e.g., 7a) or frontal regions that are active during covert orienting and working memory in humans. It would be interesting to produce functional lesions with chemicals that transiently disrupt neuronal processing at different stages of the dorsal visual system and verify which lesions disrupt the modulation of the baseline rate in the ventral or parietal areas, respectively. The visual enhancement effect instead has the attributes of a site signal, reflecting the modulation of a visual sensory response by attentional mechanisms.
A suppressive type of modulation instead has been reported during tasks that emphasize exogenous cueing of a location. Robinson et al. (37) have recorded from areas 7a and LIP during the performance of a visual orienting task with exogenous cues. All neurons in parietal cortex gave a brisk response to the cue when it was flashed in the receptive field. Responses to subsequently presented probe stimuli at attended locations were either unaffected by the cue (48% of the neurons), depressed (42%), or enhanced (10%) as compared with probe stimuli at unattended locations. These suppressive effects for stimuli that appear at previously attended locations have been confirmed in a location match-to-sample paradigm by Steinmetz et al. (38) and have also been described in the ventral visual system (39). These findings would suggest that the sensitivity of parietal neurons decrement at one location after that location has been selected. The response to the cue may signal an initial shift of attention, but this cannot be differentiated by a sensory response. Parietal neurons may code for novel sensory events that appear at unattended locations. There have not been comparisons in the same monkey of facilitatory effects during tonic attention to a peripheral location and suppressive effects in the context of exogenous cueing.
How do these neuronal mechanisms relate to the hypothesis of a frontoparietal network for visuospatial attention in humans? First, this level of analysis provides putative neural correlates for the parietal activations recorded during covert visual orienting. For instance, during the shifting task, baseline activity may increase when attention is endogenously shifted to a new location, whereas the visual response may be enhanced when the probe is presented at the attended location. The temporal separation between endogenous cueing and probe presentation during a shifting-like paradigm in the monkey would allow the disentangling of the source from site effects that are confounded in the tonic attention paradigm in which the monkey’s attention is not tightly controlled on a trial-by-trial basis. Second, the presence of parietal modulations during visual orienting in both species suggest potential homologies between postcentral and intraparietal foci in humans and areas 7a and LIP in macaque. Further understanding of these anatomical homologies will require detailed mapping of human parietal cortex with multiple functional tests that are known to drive different regions in macaque and, vice versa, the application of attentional tests developed in humans to a larger sample of parietal areas in macaque. Third, the localization to FEF and adjacent superior frontal sulcus of attention-related activity in humans does not have a counterpart in the macaque neurophysiological literature. Neuronal modulations in FEF and dorsolateral prefrontal cortex (40, 41) have been detected only in relationship to saccadic responses, whereas enhancement in premotor cortex has been tested only with manual responses (42). Some shifts in baseline activity before stimulus presentation have been recorded in FEF during predictive saccades to visual targets (43). This discrepancy can be related to task differences or different degrees of practice between human subjects and monkeys. In monkeys, neurons in FEF and dorsolateral prefrontal cortex have been tested only with a limited set of spatial locations and only after extensive practice. It is possible that response-independent attentional modulations would be found in the monkey’s FEF if activity was recorded early during training. Vice versa, frontal activation might disappear in humans after extensive practice.
Overt Orienting. Oculomotor signals have been recorded in a large number of areas of the macaque brain. In most of these areas, including FEF, dorsolateral prefrontal cortex, caudate, and superficial layers of the superior colliculus, the neural response to visual stimuli is enhanced when the stimulus is the target of a saccadic eye movement (40, 41, 44, 45). This visual saccadic enhancement has to be distinguished from the visual enhancement recorded in posterior parietal cortex (areas LIP and 7a) and pulvinar, which instead occurs both for manual and saccadic responses (34, 36). This distinction was the strongest empirical evidence for a segregation of processes between visuospatial attention and eye movements.
More recent data, however, indicate that attention and eye movement signals are tightly related even at the neuronal level. For instance, neurons in area LIP respond to visual stimuli and show preparatory oculomotor activity. Visual responses are strongly modulated by tonic peripheral attention but also by eye-and head-position signals (46, 47). Furthermore, oculomotor preparatory activity can be maintained on-line during the memory period of a memory-guided saccade task (48) and produces changes in the position of visual receptive fields (49). These findings have led to the development of different views about the organization of space in parietal cortex and how these signals are combined. A discussion of these theories is beyond the purpose of this paper (see refs. 50 and 51). However, these data unequivocally indicate that attention and eye movement signals coexist in the same area of parietal cortex, even on the same neurons. Neural signals related to saccadic preparation are likely to underlie the psychophysical advantage produced by eye movements on visual processing. To evaluate the putative importance of oculomotor signals in the attentional modulation of ventral visual areas, it would be interesting to verify how shifts of the baseline activity recorded during peripheral attention task and central fixation vary during oculomotor programming.
More direct evidence for the idea that attention shifts are planned in oculomotor coordinates comes from stimulation experiments performed by Kustov and Robinson in the deep layers of the superior colliculus (52). They trained monkeys in a spatially cued oculomotor task, in which visual locations were cued peripherally by a sensory stimulus or symbolically by a change in color of the fixation point. Saccadic reaction times for cued locations were faster than for uncued locations both for sensory (exogenous) and symbolic (endogenous) cueing. During the task, electrical stimulation with microcurrents was applied to certain portions of the deep layers of the superior colliculus. Normally, this stimulation produces a saccadic eye movement of constant direction and amplitude. When the monkey was cued to a location, the stimulation produced a displacement of the constant saccadic vector in the direction of the cued location. This displacement occurred with both types of cues. Similar deviations of the eye movements evoked from the stimulation of the superior colliculus occurred during new symbolic and sensory cueing tasks, in which the monkey maintained central fixation and responded to the probe stimuli with a manual response only. Hence, attentional shifts that are independent of eye movements still lead to the modification of the evoked saccades. The simpler explanation for these findings is that attentional signals during spatial cueing are oculomotor in nature and coded in motor coordinates. Alternatively, they represent an epiphenomenon of no functional significance, i.e., a “spillover” of activity from cognitive circuits. This spillover, however, could be potentially detrimental to performance because the precision of an eye movement would be affected by the direction of attention. Unlikely, such an interference would have not been corrected during evolution.
Conclusions
This review highlights psychological, functional anatomical, and cellular levels of analysis of a relatively simple visual behavior such as visual orienting. There are two main conclusions that can be derived from this body of results. First, there appears to be a robust set of neural signals in parietal cortex (and frontal cortex) indexed both with neuroimaging and electrophysiological methods that clearly reflects spatial attentional processes during covert orienting. These signals can be reasonably linked to some of the psychological effects described when subjects (human or monkeys) reflexively or voluntarily allocate attention to a visual location/object. These neural signals are the source of a selective location signal that biases visual processing in ventral visual areas related to object analysis. Second, psychological, functional anatomical, and neuronal analyses indicate that attentional processes are tightly linked to oculomotor processes. An extreme view of this functional relationship is that attentional shifts are oculomotor in nature, i.e., being planned within oculomotor circuits in motor coordinates (amplitude, direction). This view is supported by the following results. (i) In dual-task conditions in which eye movement and attention are cued to opposite locations, the focus of processing is obligatorily linked to the eye movement. (ii) Neuronal signals related to covert (attentional) orienting have been recorded both in humans and monkeys in areas such as FEF and deep layers of the superior colliculus in monkeys that are essential nodes of the oculomotor system. (iii) The patterns of cortical activation for attention and eye movement overlap in our metaanalysis of functional imaging studies, (iv) In parietal cortex, the same neurons that show purely attentional modulations also code oculomotor parameters. A more moderate view of the relationship between attention and eye movement is that their processes are interdependent. This view is supported by: (i) a relative slowing of saccades when attention is oriented elsewhere: (ii) the partial segregation of regions for attention and eye movement in our metaanalysis; and (iii) some neural models of parietal function that emphasize sensory and attentional functions and deemphasize visuomotor functions. Finally, the hypothesis that attention and eye movements are segregated processes can be rejected on the basis of the current anatomical and physiological evidence.
I thank Gordon Shulman for discussion and careful reading of a draft of the manuscript, and Marc Raichle and Steve Petersen for enthusiastic support. This work was supported by National Institutes of Health Grant K08-EY00379–01, and the Charles A.Dana Foundation.
1. Ullman, S. (1984) Cognition 18, 97–159.
2. Eriksen, C.W. & Hoffman, J.E. (1972) Percept. Psychophys. 12, 201–204.
3. Posner, M.I. (1980) Q. J. Exp. Psychol. 32, 3–25.
4. Berlucchi, G., Tassinari, G., Marzi, C.A. & Di Stefano, M. (1989) Neuropsychologia 27, 201–221.
5. Jonides, J. (1981) in Voluntary vs. Automatic Control over the Mind’s Eve’s Movement, eds. Posner, M.I. & Marin, O. (Lawrence Erlbaum Associates, Hillsdale, NJ), pp. 187–205.
6. Bashinski, H.S. & Bachrach, V.R. (1984) Percept. Psychophys. 28, 241–248.
7. Downing, C.J. (1988) J. Exp. Psychol. Hum. Percept. Perform. 14, 188–197.
8. Shepherd, M., Findlay, J.M. & Hockey, R.J. (1986) Q. J. Exp. Psychol. 38, 475–491.
9. Rizzolatti, G., Riggio, L., Dascola, I. & Umiltá, C. (1987) Neuropsychologia 25, 31–40.
10. Remington, R. (1980) J. Exp. Psychol. Hum. Percept. Perform. 6, 726–744.
11. Klein, R. (1980) in Does Oculomotor Readiness Mediate Cognitive Control of Visual Attention?, ed. Nickerson, R.S. (Erlbaum, Hillsdale, NJ).
12. Hoffman, J.E. & Subramaniam, B. (1995) Percept. Psychophys. 57, 787–795.
13. Klein, R.M. (1994) Can. J. Exp. Psychol. 48, 167–181.
14. Rafal, R.D., Calabresi, P.A., Brennan, C.W. & Sciolto, T.K. (1989) J. Exp. Psychol. Hum. Percept. Perform. 15, 673–685.
15. Raichle, M.E. (1994) Sci. Am. 270, 58–64.
16. Corbetta, M., Miezin, F.M., Shulman, G.L. & Petersen, S.E. (1993) J. Neurosci. 13, 1202–1226.
17. Nobre, A.C., Sebestyen, G.N., Gitelman, D.R., Mesulam, M.M., Frackoviack, R.S.J. & Frith, C.D. (1997) Brain 120, 515–533.
18. Drury, H.A., Van Essen, D.C., Anderson, C.H., Lee, C.W., Coogan, T.A. & Lewis, J.W. (1996) J. Cognit. Neurosci. 8, 1–28.
19. Van Essen, D.C. & Drury, H.A. (1997) J. Neurosci. 17, 7079–7102.
20. Talairach, J. & Tournoux, P. (1988) Co-Planar Stereotaxic Atlas of the Human Brain (Thieme, New York).
21. Corbetta, M., Shulman, G.L., Conturo, T.E., Snyder, A.Z., Akbudak, E., Petersen, S.E. & Raichle, M.E. (1997) Int. Conf. Funct. Mapping Hum. Brain, 3rd.
22. Heinze, H.J., Mangun, G.R., Burchert, W., Hinrichs, H., Scholz, M., Munte, T.F., Gos, A., Scherg, M., Johannes, S., Hundeshagen, H., Gazzaniga, M.S. & Hillyard, S.A. (1994) Nature (London) 372, 543–546.
23. Woldorff, M.G., Fox, P.T., Matzke, M., Laucester, J.L., Veeraswamy, S., Zamerripa, F., Seabolt, M., Glass, T., Gao, J.H., Martin, C.C. & Jarabek, P. (1997) Hum. Brain Mapping 5, 280–286.
24. Vandenberghe, R., Dupont, P., Debruyn, B., Bormans, G., Michiels, J., Mortelmans, L. & Orban, G.A. (1996) Brain 119, 1263–1276.
25. Vandenberghe, R., Duncan, J., Dupont, P., Ward, R., Poline, J.-B., Bormans, G., Michiels, J., Mortelmans, L. & Orban, G.A. (1997) J. Neurosci. 17, 3739–3750.
26. Haxby, J.V., Horwitz, B., Ungerleider, L.G., Maisog, J.M., Pietrini, P. & Grady, C.L. (1994) J. Neurosci. 14, 6336–6353.
27. Hillyard, S.A. & Picton, T.W. (1987) in Electrophysiology of Cognition, eds. Plum, F., Mountcastle, V.B. & Geiger, S.T. (Am. Physiol. Soc., Bethesda, MD), Vol. 5, part 2, pp. 519–584.
28. Luck, S.J., Chelazzi, L., Hillyard, S.A. & Desimone, R. (1997) J. Neurophysiol. 77, 24–42.
29. Fox, P.T., Fox, J.M., Raichle, M.E. & Burde, R.M. (1985) J. Neurophysiol. 54, 348–369.
30. Anderson, T.J., Jenkins, I.K., Brooks, D.J., Hawken, M.B., Frackoviack, R.S.J. & Kennard, C. (1994) Brain 117, 1073–1084.
31. O’Driscoll, G.A., Alpert, N.M., Matthysse, S.W., Levy, D.L., Rauch, S.L. & Holzman, P.S. (1995) Proc. Natl. Acad. Sci. USA 92, 925–929.
32. O’Sullivan, B.T., Roland, P.E. & Kawashima, R. (1994) Eur. J. Neurosci. 6, 137–148.
33. Sweeney, J.A., Mintum, M.A., Kwee, S., Wiseman, M.B., Brown, D.L., Rosenberg, D.R. & Carl, J.R. (1996) J. Neurophysiol. 75, 454–468.
34. Bushnell, M.C., Goldberg, M.E. & Robinson, D.L. (1981) J. Neurophysiol. 46, 755–772.
35. Colby, C.L., Duhamel, J.R. & Goldberg, M.E. (1996) J. Neurophysiol. 76, 2841–2852.
36. Petersen, S.E., Robinson, D.L. & Morris, J.D. (1987) Neuropsychologia 25, 97–105.
37. Robinson, D.L., Bowman, E.M. & Kertzman, C. (1995) J. Neurophysiol. 74, 698–721.
38. Steinmetz, M.A., Connor, C.E., Constantinidis, C. & McLaughlin, J.R. (1994) J. Neurophysiol. 72, 1020–1023.
39. Miller, E.K. & Desimone, R. (1994) Science 263, 520–522.
40. Wurtz, R.H. & Mohler, C.W. (1976) J. Neurophysiol. 39, 766–772.
41. Boch, R.A. & Goldberg, M.E. (1989) J. Neurophysiol. 61, 1064–1084.
42. di Pellegrino, G. & Wise, S.P. (1993) Somatosens. Motor Res. 10, 245–262.
43. Bruce, C.J. & Goldberg, M.E. (1985) J. Neurophysiol. 53, 603–635.
44. Hikosaka, O., Sakamoto, M. & Usui, S. (1989) J. Neurophysiol. 61, 799–913.
45. Funahashi, S., Bruce, C.J. & Goldman-Rakic, P.S. (1991) J. Neurophysiol. 65, 1464–1483.
46. Andersen, R.A., Bracewell, R.M., Barash, S., Gnadt, J.W. & Fogassi, L. (1990) J. Neurosci. 10, 1176–1196.
47. Brotchie, P.R., Andersen, R.A., Snyder, L.A. & Goodman, S.J. (1995) Nature (London) 375, 232–235.
48. Gnadt, J.W. & Andersen, R.A. (1988) Exp. Brain Res. 70, 216–220.
49. Duhamel, J.-R., Colby, C. & Goldberg, M.E. (1992) Science 255, 90–92.
50. Colby, C., Duhamel, J. & Goldberg, M. (1995) Cereb. Cortex 5, 470–482.
51. Andersen, R.A. (1995) Cereb. Cortex 5, 457–469.
52. Kustov, A.A. & Robinson, D.L. (1996) Nature (London) 384, 74–77.
53. Corbetta, M., Shulman, G.L., Miezin, F.M. & Petersen, S.E. (1995) Science 270, 802–805.








