3
Ozone Reduction by HSCT-Emitted Nitrogen Oxides
Even without model calculations, it has been obvious since 1971 that the possible reduction of ozone by a fleet of HSCTs is a problem worthy of serious consideration. Nitrous oxide (N2O) is produced from soils and waters as a byproduct of biogeochemical nitrogen cycling. It is inert in the troposphere, is slowly transported up into the stratosphere by air motions, and reacts in the 30±5 km altitude range to form nitrogen oxides (Crutzen, 1971) at a rate of 1.8±0.5x109 kg as NO2 per year (Crutzen and Schmailzl, 1983).
The 500 supersonic transports (SSTs) planned in 1971, emitting nitrogen oxides in the exhaust at the same percentage by weight as the existing Concorde engines (see Fahey et al., 1995), would have injected into the stratosphere 1.5x109 kg NO2 per year, an amount approximately equal to the natural input (NRC, 1974). Although the rate of NOx emission from future HSCT engines is unknown, current estimates for a fleet of 500 Mach 2.4 HSCTs also give NOx injection into the stratosphere at values close to or slightly lower than the natural input noted above. Figure 5, showing calculations by a Lawrence Livermore National Laboratory (LLNL) model, indicates that natural NOx catalytically destroys ozone faster than the sum of all other ozone loss mechanisms at altitudes between 25 and 38 km, which is the production region supplying ozone to the region of its maximum concentration (see Figure 3). Thus, the possible near-doubling of the stratospheric input of NOx via HSCT emissions is a problem worthy of serious consideration. However, the HSCT injection of NOx into the stratosphere would occur at altitudes about 10 km lower than the altitude of natural NOx input. As AESA has confirmed, this difference in altitude makes a considerable difference in the action of added NOx on ozone (see Figure 5).
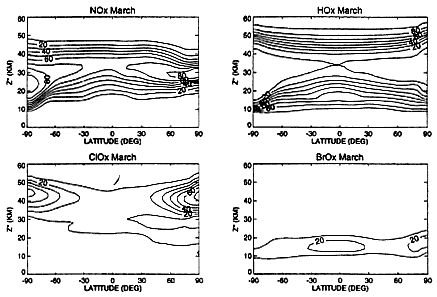
FIGURE 5
Calculated percentages of ozone loss caused by NOx, HOx, ClOx, and BrO x catalytic cycles, as a function of latitude and altitude, for the month of March. The LLNL model was used, with a background atmosphere and subsonic-aircraft emissions projected for 2015 (Stolarski et al., 1995) but with no HSCT emissions. The BrO x chemistry used did not include the hydrolysis of BrONO2 on aerosols (see reaction (11) in the text). The NOx percentages include reactions involving nitrogen and hydrogen; the ClO x chemistry includes reactions involving chlorine and nitrogen; the BrOx chemistry includes reactions involving bromine and chlorine. (Reprinted from Stolarski et al., 1995.)
A History
The Department of Transportation's Climatic Impact Assessment Program, inspired by Boeing's work on an SST and the advent of the Concorde, was devoted to exploring the potential effects of supersonic aviation. It did the pioneering work on stratospheric composition and chemistry, including the first measurements of NO and NO2 in the stratosphere; improved one-and two-dimensional atmospheric models; made systematic laboratory measurements of reaction rates applicable to the stratosphere; developed critical tables of chemical rate coefficients and light-absorption cross-sections; and sponsored studies of heterogeneous reactions in the atmosphere and of economic impacts and biological damage caused by ultraviolet radiation (CIAP, 1975a,b).
In 1976 Congress assigned NASA the role of primary agency for stratospheric research, and its Upper Atmospheric Research Program has been active since then. UARP has concentrated its efforts on the chlorofluorocarbon impact
on stratospheric ozone, has systematically built up a data base from laboratory photochemistry, has measured stratospheric trace species by satellite remote sensing and by in situ methods, and has developed two-dimensional and is developing three-dimensional radiative-photochemical-dynamic numerical models. No general study of the atmospheric effects of supersonic aviation was made between 1976 and 1988, but LLNL kept a year-by-year record of the calculated ozone effect of 500 SSTs flying at 20 km, consuming 7.7x1010 kg fuel per year, emitting 1.8 MT NOx (as NO2) per year, using each year the best available dynamical models and values of photochemical data. For two years (1978–1979) these one-dimensional model studies predicted that NOx from the standard CIAP fleet of supersonic transports would increase the ozone vertical column, but for all the other years between 1975 and 1988, the models predicted global ozone-column reductions of between 5 and 12 percent.
In 1988 and 1989, NASA and the aircraft industry showed renewed interest in supersonic (Mach 2.4 to 3.2) and hypersonic (Mach 4 to 10) aircraft. The LLNL two-dimensional (2-D) model was used to calculate the effect on ozone of 500 SSTs with CIAP fuel-consumption rates and with three different NOx emission rates, corresponding to 1/3, 1, and 3 times the Crutzen and Schmailzl value of 1.8 x 109 kg NO2 per year. Flights were assumed to occur at each of seven altitudes between 16.5 and 34.5 km, and in various assumed flight corridors, such as between 37° and 49°N (Johnston et al., 1989). The 1989 LLNL model did not include heterogeneous reactions (nor did other models at that time), such as those important for the Antarctic ozone hole. For a wide range of input parameters in this sensitivity study, the calculated ozone reductions over the northern hemisphere varied from 0.9 to 28 percent. These calculated ozone reductions were extremely sensitive to flight altitude: For the CIAP value of NOx input, flight altitudes of 15–18 km, 18–21 km, and 21–24 km gave northern hemisphere ozone reductions of 0.9 percent, 10.4 percent, and 13.1 percent, respectively. These three altitude bands correspond approximately to the flight altitudes of aircraft with Mach numbers of 1.6, 2.4, and 3.2, suggesting that aircraft with a low, but still supersonic, Mach number might be able to operate under conditions such that their effect on ozone would be small. HSCTs with Mach numbers of 1.6, 2.0, and 2.4 were seriously discussed through 1993 in the various AESA reference publications.
A primary purpose of establishing AESA under the High-Speed Research Program in 1988 was to understand and if possible answer the question of the extent of potential ozone reduction by HSCTs, as indicated by model calculations. It was already known by that time that the ozone reduction by NOx from HSCT exhaust calculated for a flight altitude of 20 km decreased rapidly as the flight altitude was moved down to 15 km, at which point the ozone reduction changed to an ozone increase, as anticipated by Johnston and Quitevis (1975). It was obvious that the calculated reduction would be diminished if the aircraft were equipped with low-NOx engines. It was qualitatively recognized in 1990
that if the heterogeneous reaction (N2O5 + H2O ⇒ 2HNO3) was fast, it would decrease the calculated ozone reduction by HSCTs, but if the heterogeneous reaction ClONO2 + HCl ⇒ Cl2 + HNO3 was fast it would increase the calculated ozone reduction by HSCTs.
NASA took action on three fronts: First, shortly after the start of AESA, the HSRP dropped from consideration the high-altitude Mach 3.2 HSCT and selected the Mach 2.4 HSCT as the best candidate. HSRP also considered lower-flying Mach 1.6 and 2.0 models, partially in case the NOx-ozone problem could not be solved. Second, HSRP set the goal of developing the technology for new types of HSCT engine combustors that would have an EI(NOx) as low as 5, about a factor of 10 less NOx than a high-efficiency 1990-technology engine equipped with a conventional combustor design. The completion of this design process and initial construction of an actual engine is not expected until well after the year 2000, however. (See U.S. Supersonic Aircraft: Assessing NASA's High Speed Research Program (NRC, 1997c) for a discussion of HSRP progress in these areas.) Third, AESA launched a major program of studying heterogeneous reactions in the laboratory and making meaningful measurements in the stratosphere. By 1991, Weisenstein et al. had already made model calculations including heterogeneous reaction (6), and found almost an order of magnitude decrease in the model-calculated ozone reduction by HSCT exhaust. Other modelers' results soon confirmed the importance of including heterogeneous chemistry. Since then, AESA has made diagnostic measurements in the atmosphere, extensively studied heterogeneous chemistry as it applies to the atmosphere, and considered other problems such as the role of HSCT aircraft in contributing to possible global climate change.
Scenarios and Conditions
AESA's initial status and its progress through the summer of 1991 are described in Prather et al. (1992). The conditions AESA established for its model explorations of possible effects are summarized below.
Flight Routes and Fuel Usage
With detailed consideration of every major airport in the world and flight paths between pairs of them, realistic HSCT flight routes were identified, flight-altitude patterns for fleets with different Mach numbers were worked out, the economically justified number of HSCTs was tentatively set at 500, and amount of fuel expected to be burned along the various routes was derived. The estimated mass of fuel to be used was 6.6 x 1010 kg per year, of which the fractional consumption is 2.8 percent from 30°S to the south pole, 32.4 percent from 30°S to 30°N, and 64.8 percent from 30°N to the north pole (Prather et al., 1992). All
model calculations in the early years of AESA used these carefully derived flight patterns.
This 1992 plan has been modified somewhat (Baughcum and Henderson, 1995); the 1995 version is given by Figure 6, which shows at a glance the estimated latitudinal distribution of fuel consumption by HSCTs (top panel) and the vertical distribution of global fuel use by supersonic and subsonic aircraft as projected to year 2015 (bottom panel). The estimated mass of fuel to be used is 8.2x1010 kg per year, of which the fractional consumption is 3 percent from 30°S to the south pole, 38 percent from 30°S to 30°N, and 59 percent from 30°N to the north pole (Stolarski et al., 1995). While the most heavily traveled flight corridors are expected to continue to be north of 30°N, note that by 2015 more than one-third of the HSCT fuel may be expended in the tropics.
HSCT NOxEmissions
The definition of ''NOx emission index" is the number of grams of NOx (calculated as if it is all in the form of NO2) per kilogram of fuel consumed by an aircraft engine. It is properly written "EI(NO x) as NO2", though sometimes "as NO2" is omitted. Issues related to achieving a low NOx emission index were discussed at the AESA planning workshop held at NASA Ames Laboratory, October 17–19, 1990 (see Prather et al., 1992). It was noted that "With no in situ measurements, Concorde emission studies have been limited to test-stand studies of the engines . . . It will be extremely difficult to measure chemical products in the exhaust plume of an aircraft without a visible clue (e.g., contrail or colored exhaust) to help find the wake." Exhaust products for current engines, as well as for new technologies that might reduce the NO2 emission index (EI) from 40 to 5 g/kg of fuel, were described at that meeting.
A highlight of NASA's 1995 assessment is the report on the first measurements ever made in the plume region of a Concorde aircraft in flight (Fahey et al., 1995). The concentrations of NOx found in the wake yielded an EI(NOx) of 23 (±20 percent). In the 1970s, NOx emission levels of the Concorde's Olympus engine had been measured in altitude-simulation-chamber tests that included supersonic cruise operating conditions; the resulting EI values varied from 15.5 to 19.3, with a "consensus value" of 18 (CIAP, 1975a). These test-chamber values are somewhat below those for the 1995 in situ measurements, but are still in reasonable agreement.
The Concorde's Olympus engines embody 1960s technology; the engines being developed for the HSCT will have as much as 30 percent greater fuel efficiency. With conventional combustor technology, however, the changed combustor operating conditions that yield these efficiency improvements would result in at least a doubling of the NOx emission index. In 1989 NASA announced its intention of developing advanced low-emissions combustor technology that would permit HSCT engines with EI(NOx) levels as low as 5 to be built. These

FIGURE 6
Projected HSCT fuel consumption in 2015. Top panel: Latitudinal distribution of fuel used above 13 km altitude by a fleet of 500 Mach 2.4 aircraft. Bottom panel: Total estimated fuel burn as a function of altitude for an enhanced all-subsonic fleet, and for a smaller subsonic fleet plus 500 Mach 2.4 HSCTs. (Reprinted from Stolarski et al., 1995.)
advanced combustor concepts employ multiple burning stages. Combustor testing and further technology development are under way, and altitude-chamber tests of an engine equipped with an ultra-low-NOx combustor are expected to be made after the year 2000. Until such engines have been built and tested, the EI must be considered a large uncertainty in modeling HSCT NOx input into the stratosphere.
Modeling HSCT Ozone Reduction
To represent the three reference points discussed above—a new engine based on 1990 technology with no concern for low NOx, the existing Concorde Olympus engine, and the NASA goal for perhaps the year 2001—AESA modelers used 45, 15, and 5 as EI(NOx) to calculate the effect of NOx from HSCTs on ozone. These EIs cover the full range of reasonably expected final outcomes, and 15 (the geometric mean of 45 and 5) is reasonably close to the emission index of the Concorde. By about 1993, the favored HSCT was the Mach 2.4 type. To put this choice in perspective, AESA modelers calculated the ozone perturbation by HSCTs with Mach numbers 1.6, 2.4, and 3.2. The number of HSCTs was taken to be 500 unless otherwise noted, with a constant annual rate of fuel consumption.
Using Gas-Phase Chemistry Only
Seven models calculated the change of the ozone vertical column between 40°N and 50°N for various EIs and Mach numbers using gas-phase chemistry only. Their results (with the exception of one having limited runs), were presented in Stolarski and Wesoky (1993a) and are reproduced here in Table 1. They appear immediately below in a condensed form that gives an average ozone-column percentage change plus or minus two standard deviations:
|
Mach\EI(NOx) |
45 |
15 |
5 |
|
3.2 |
|
-8.6 ± 3.7 |
-2.6 ± 1.5 |
|
2.4 |
-14 ± 7 |
-3.9 ± 2.2 |
-1.3 ± 0.8 |
|
1.6 |
|
-0.9 ± 0.8 |
-0.16 ± 0.10 |
Relatively large ozone reductions, about 10 percent, are shown for (EI = 15, Mach = 3.2) and for (EI = 45, Mach = 2.4). At EI = 15, the calculated ozone reduction decreases a factor of ten in going from Mach 3.2 to Mach 1.6. At Mach = 2.4, the calculated ozone reduction decreases a factor of ten in going from EI = 45 to EI = 5. At Mach = 1.6 and EI of 15 or less, the calculated ozone reduction is less than 1 percent, and 0 percent reduction is barely outside the 95 percent confidence level. Results such as those given in Table 1A prompted aircraft
TABLE 1 Calculated percentage ozone-column changes between 40°N and 50°N by 500 HSCTs with various Mach numbers and various NOx emission indices. (After Stolarski and Wesoky, 1993a.)
|
Mach No. |
EI(NOx) |
AER |
DuPont |
GSFC |
Italy |
LLNL |
NCAR |
μ ± 2σ |
|
A. Gas-phase chemistry only |
||||||||
|
3.2 2.4 1.6 3.2 2.4 1.6 2.4 |
15 15 15 5 5 5 45 |
-8.8 -4.6 -0.91 -2.5 -1.3 -0.23 -14.0 |
-8.8 -5.6 -0.77 -2.9 -1.7 -0.12 -16.7 |
-10 -3.7 -1.4 |
-11 -4.1 -0.53 -3.3 1.47 -0.15 -17.2 |
-5.8 -2.7 -0.5 -1.5 -0.77 -0.14 -9.5 |
-7.3 -2.9 -1.3 |
-8.6 ± 3.7 -3.9 ± 2.2 -0.9 ± 0.8 -2.6 ± 1.5 -1.3 ± 0.8 -0.2 ± 0.1 -14 ± 7.0 |
|
B. Gas-phase chemistry and heterogeneous reactions |
||||||||
|
3.2 2.4 1.6 3.2 2.4 1.6 2.4 |
15 15 15 5 5 5 45 |
-3.3 -0.95 +0.09 -1.2 -0.42 +0.03 -4.0 |
-3.4 -1.3 +0.84 -1.1 -0.31 +0.53 -7.4 |
-4.1 -0.63 -0.01 -1.3 -0.23 -0.04 -3.3 |
-5.5 -0.42 +0.67 -1.9 +0.04 +0.39 -4.8 |
-2.2 -0.56 +0.08 |
-2.1 -0.27 -0.03 -0.20 -0.04 +0.04 -2.3 |
-3.4 ± 2.5 -0.7 ± 0.7 +0.3 ± 0.8 -1.1 ± 1.2 -0.2 ± 0.4 +0.2 ± 0.5 -4.4 ± 3.9 |
|
AER Atmospheric and Environmental Research, Inc. GSFC Goddard Space Flight Center LLNL Lawrence Livermore National Laboratory NCAR National Center for Atmospheric Research |
||||||||
manufacturers and AESA to give serious attention to HSCTs with Mach numbers 1.6 and 2.0 in the early stage of AESA.
Including Heterogeneous Chemical Reactions in the Models
Although a laboratory study in 1975 reported no reaction of N2O5 with sulfuric acid, Mozurkewich and Calvert (1988) found this to be a fast reaction on sulfuric acid/water solutions:
Shortly thereafter several groups studied this reaction using different techniques, and found that the reaction occurs rapidly regardless of water concentration in the sulfuric acid and regardless of temperature over its atmospheric range, as long as the solution is not frozen solid. AESA carried out atmospheric-measurement campaigns to explore the reaction in situ; the measurements and their interpretation ''proved beyond a reasonable doubt" that the heterogeneous reactions observed in the laboratory were indeed occurring in the stratosphere (Albritton et al., 1993).
Reaction (6) is of major importance to the HSCT-effect question. Homogeneous and heterogeneous chemistry relative to reaction (6) includes:
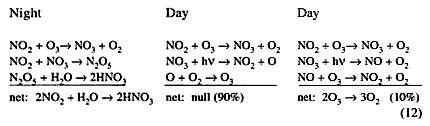
The moderately slow reaction of nitrogen dioxide with ozone leads to inactive nitric acid vapor at night, if the surface area of the aerosols is large enough. During the day the NO3 intermediate is almost instantly broken down by sunlight to NO2 or to NO, and no N2O5 is formed. The net effect is to convert active NO2 to non-reactive HNO3 at night. Thus removed, NO2 does not engage in reactions (4), which remove active OH, HOO, ClO, and BrO. In sunlight, NO2 and NO are in photochemical steady state; reduction of NO2 as the net effect of reaction (6) also reduces NO, and this loss of NO prevents ozone-destroying reactions (1) from occurring, replacing them with the null reactions (3).
Weisenstein et al. (1991) were the first to include a heterogeneous reaction, namely (6), in a 2-D model study concerning HSCTS. They found this single heterogeneous reaction greatly diminished the calculated ozone reduction, in
some cases reversing the sign of the net effect. Weisenstein et al.'s article, which was not published in time to be included in the initial AESA review (Prather et al., 1992), marks a "watershed" with respect to estimates of ozone reduction by HSCTS.
Typical calculated ozone-column changes from models including both gasphase and heterogeneous reactions are shown in Part B of Table 1. Those results are condensed here to give an average ozone-column percentage change plus or minus two standard deviations:
|
Mach\EI(NOx) |
45 |
15 |
5 |
|
3.2 |
|
-3.4 ± 2.5 |
-1.1 ± 1.2 |
|
2.4 |
-4.4 ± 3.9 |
-0.69 ± 0.75 |
-0.19 ± 0.38 |
|
1.6 |
|
+0.27 ± 0.76 |
+0.19 ± 0.50 |
The calculated ozone reductions in the models that included heterogeneous reactions (Part B of Table 1) are substantially less than those calculated using gasphase chemistry only (Part A). All calculated ozone changes are less than ±1 percent except for the cases of (EI = 15, Mach = 3.2), (EI = 5, Mach = 3.2), and (EI = 45, Mach = 2.4). For both EI = 5 and EI = 15, the calculated ozone-column changes for Mach 1.6 HSCTs are positive. The average result for all cases with Mach = 1.6 and for all cases with EI = 5 includes zero within the two-sigma error limits.
The factors by which the inclusion of heterogeneous reactions lowers the calculated ozone-column reductions—gas only/(gas + heterogeneous)—are as follows:
|
Mach\EI(NOx) |
45 |
15 |
5 |
|
3.2 |
|
2.6 |
2.1 |
|
2.4 |
3.1 |
6.8 |
indeterminate |
|
1.6 |
|
indeterminate |
indeterminate |
The ozone reductions calculated for the high-altitude HSCTs with Mach 3.2, which cruise at 21 to 24 km, are reduced by a factor of about 2 for an EI = 5; the reductions for the Mach 2.4 HSCTs, which cruise at 18 to 21 km, are reduced by about a factor of 7 for an EI = 15. For Mach 1.6 HSCTs, the calculated ozone changes indicate an increase of ozone column.
Model Results Reported in 1995
NASA's 1995 assessment gives a clear picture of the current status of model calculations of ozone change by a fleet of HSCTs. Figure 7 shows the calculated
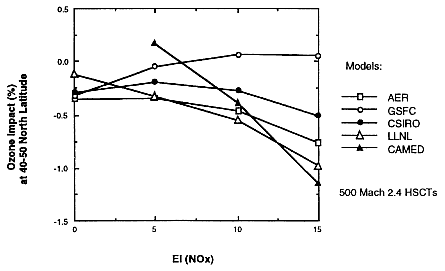
FIGURE 7
Five models' calculations of percentage changes in ozone column in the 40 to 50°N latitude band, as a function of NOx emission index, for a fleet of 500 Mach 2.4 HSCTs.* (Reprinted from Stolarski et al., 1995.)
ozone changes for 500 Mach 2.4 HSCTs as a function of NOx emission index for values of 0, 5, 10, and 15 as calculated by five 2-D assessment models. In general, the five models agree that there will be less than 1 percent ozone reduction for any NOx emission index up to 15. If one looks for fine structure, of the four models that reported results with zero NOx emissions, there is about 0.2 to 0.3 percent ozone-column reduction with no added NOx, which on average remains about the same for a NOx emission index of 5. One model shows essentially zero ozone reduction from EI = 5 to EI = 15, but the other four models give between 0.5 and 1.0 percent ozone reduction with EI = 15.
Figure 8 shows the calculated change of ozone concentration (molecules cm-3) as a function of altitude at 45°N latitude for the month of March. The calculation is for 500 Mach 2.4 HSCTs with NOx emission index of 5. Between 40 and 50°N (Figure 8), the five models all show very small changes in the ozone vertical column, from-0.3 percent to +0.2 percent (see Stolarski et al., 1995), which is excellent agreement. The striking feature of Figure 8, however, is the differences in the shapes of these five models' vertical profiles of ozone changes. The LLNL model results lie at one extreme, CAMED results at the other. At 20 km, two models show ozone increase, one model shows no change in ozone, and two models show an ozone decrease. The models differ from each other by large
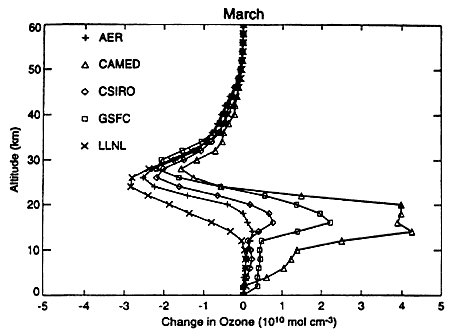
FIGURE 8
Five models' calculations of change in ozone concentration as a function of altitude for a fleet of 500 Mach 2.4 HSCTs with an EI (NOx) of 5. The results shown are for March at 45°N latitude. Units are 1010 molecules per cubic centimeter. (Reprinted from Stolarski et al., 1995.)
ratios for the troposphere and the lowermost stratosphere. In the troposphere nitric acid is rained out in a few days, and there are large sources of nitrogen oxides (subsonic aircraft and lightning). It is surprising that a relatively small increase in NOx in the overworld should increase ozone in the lower troposphere in two of the models; if it is correct, this is a matter of interest to current studies of the atmospheric effects of subsonic aircraft. These differences in model vertical profiles may represent uncertainty in formulating 2-D atmospheric models. On the other hand, they may be explicable in terms of some identifiable physical or chemical feature.
As new laboratory results, new atmospheric observations, or new theoretical insights are published, modelers may change their chemical coefficients, their boundary values, or their formulations of mixing. Model calculations of ozone changes therefore need to be updated almost every year. In recent years AESA has restricted its model calculations to HSCTs with Mach number 2.4 and its NOx emission indices to 5 and 15. During the model-development stage, it saves
computer time to omit other Mach numbers and emission indices. For a proper assessment, however, the surrounding space should be examined to see whether the results change rapidly or slowly outside the range of immediate interest. (Also, future practical considerations may call for information beyond the relatively narrow range of AESA's current scope.) For the final AESA reports, it would be useful (and require only trivial extra cost) if AESA modelers included in their calculations of ozone change Mach numbers 1.6 and 3.2, NOx emission indices of 0 and 45, and a wider range of sulfate number densities in the wake. This expansion would show the context within which the chosen model of HSCT lies, and make sure that no nearby opportunity or possible hazard will be missed.













