1
Introduction
As advances in the biological and planetary sciences enable a shift from mere observation to active exploration of the solar system, space missions are increasingly likely to collect samples from planetary satellites and small solar system bodies and return them to Earth for study. NASA plans to return such samples not only during the MUSES-C mission but also during missions proposed for NASA's Discovery program and in possible joint work with the Department of Defense (i.e., Clementine II). Several such missions are possible early in the next century (see Table 1.1). This is an exciting development that offers the opportunity to search for extraterrestrial life forms and improve understanding of the origin and composition of the solar system.
However, sample return also involves potential risks that need to be understood and managed properly. Concerns about potential risks from returned extraterrestrial materials are not new, having been raised initially more than three decades ago with the return of lunar samples during the Apollo program. NASA's planetary protection policy seeks to preserve natural conditions on planets and other bodies in the solar system while also protecting Earth and its biosphere from potential extraterrestrial sources of contamination (Appendix D gives details on current planetary protection requirements).
As the primary advisor to NASA for planetary protection policy, the National Research Council's Space Studies Board recently produced Mars Sample Return: Issues and Recommendations (NRC, 1997), which assessed the potential for inclusion of a viable exogenous biological entity in a sample returned to Earth from Mars as well as the potential for large-scale effects if such an entity were inadvertently introduced into Earth's biosphere. The report addressed how to protect Earth from possible contamination by putative martian biota and provided justification for and recommendations on procedures for the quarantine of samples returned from Mars.
TABLE 1.1 Sample Return Missions Being Planned
|
Mission |
Destination |
Launch (year) |
Return (year) |
|
Stardust |
Comet Wild 2 and interstellar dust |
1999 |
2006 |
|
Genesis |
Solar wind |
2001 |
2003 |
|
MUSES-C |
Asteroid 4660 Nereus |
2002 |
2006 |
In a similar vein, this report of the Space Studies Board's Task Group on Sample Return from Small Solar System Bodies considers whether samples returned to Earth from small solar system bodies might harbor living entities that could harm terrestrial organisms or disrupt ecosystems.
SCOPE AND APPROACH OF THIS STUDY
Because only self-replicating entities could pose a significant danger to terrestrial organisms and ecosystems,1 the task group focused on the following topics:
-
The possibility that, at some time in the past, life originated on a body from which a sample might be taken, or that life was transported there from elsewhere in the solar system;
-
The possibility that life still exists on the body either in active or in reactivatable form; and
-
The potential hazard to terrestrial ecosystems from extraterrestrial life if it exists in a returned sample.
Assessing the potential for biological contamination of Earth by organisms present in samples returned from small solar system bodies requires identifying the range of conditions under which life can originate, as well as the environmental extremes that can be tolerated by metabolically active and inactive life forms. Life originates at the transition from a world of minerals and abiotically synthesized organic compounds to one of organic-based self-replicating systems capable of evolving by natural selection. This report recognizes forms of life that are composed of organic compounds and are dependent on organic chemistry; the task group had no relevant information for considering any other forms.
There is no direct evidence indicating that a living entity evolved or exists on small solar system bodies. Therefore, this report examines indirect evidence based on data from Earth, meteorites, other planets, the Sun, and the Moon and on astronomical observations of distant objects in an effort to assess whether NASA needs to treat samples returned from small solar system differently from samples returned from Mars. The quality of the available data varies for each type of small solar system body examined in this report. For example, far more data are available on the structure, composition, and history of the Moon than for any other body. The task group began by reviewing what is known about the origin of life on Earth, the conditions for the preservation of metabolically active organisms in a terrestrial environment, and the somewhat different conditions needed to preserve living organisms in an inactive form. It then attempted to generalize from terrestrial experience and to outline general requirements for the origin and survival of life that would apply on any solar body.
Based on this analysis, the task group identified six parameters (Box 1.1) as relevant to the assessment and arranged these parameters in a rough order of importance, while recognizing that the order might change somewhat depending on the solar system body being assessed.
A key concern of the task group was the identification of returned samples that do not require containment,2 because containment has important financial and scientific implications for mission planning. Based on the six parameters identified as relevant, the task group formulated a series of questions as a basis for identification of whether or not samples require containment. These questions were used to assess the potential for a biological entity to be present in or on samples returned from planetary satellites, asteroids, comets, and cosmic dust. Based on the answers to these questions, the task group derived findings that it then analyzed with respect to their implications for sample containment and handling. The task group considered only two possible containment and handling requirements: either (1) strict containment and handling as outlined in the Mars report (NRC, 1997) or (2) no special containment beyond what is needed for scientific purposes. (Sample handling requirements to support scientific investigations are currently under study by NASA.) The task group ruled out intermediate or compromise procedures involving partial containment. In certain cases (e.g., P- and D-type asteroids) the limitations of the available data led the task group to be less certain, and therefore more conservative, in its assessment of the need for containment.
|
BOX 1.1 Parameters Relevant to Assessment of the Potential for Presence of a Biological Entity in Returned Samples
|
CURRENT UNDERSTANDING OF ORIGINS, CONTINUANCE, AND SURVIVAL OF TERRESTRIAL LIFE FORMS–A SYNOPSIS
Early Earth as a Model for the Origins of Self-replicating Life Forms
The planet Earth is 4.6 billion years old. The first life form appeared more than 3.5 billion years ago (Schidlowski, 1988; Schopf; 1993; Mojzsis et al., 1996) and rapidly evolved to microscopic, relatively simple cells. Over the ensuing years, primitive cells evolved into at least 10 million different species, which represent Earth's existing biological diversity. All organisms, including animals, plants, fungi, and an untold collection of microbial species, have their common ancestral roots within these earliest life forms.
Without exception, life in Earth's biosphere is carbon based and is organized within a phase boundary or membrane that envelops reacting biomolecules. Every documented terrestrial cellular life form is a self-replicating entity that has genetic information in the form of nucleic acid polymers coding for proteins. Biologically active systems require at a minimum liquid water, carbon, nitrogen, phosphate, sulfur, various metals, and a source of energy either in the form of solar radiation or from chemosynthetic processes.
The conditions that nurtured early self-replicating systems and their transition into microbial cells are speculative. In contrast, it is much easier to model the early stages of prebiotic evolution. Origins-of-life experiments have outlined the synthesis of the basic building blocks of life, including amino acids, nucleotides, and simple polypeptides and polynucleotides (Miller, 1992). It is even possible to demonstrate the evolution of nucleic acids in vitro and to select for specific catalytic properties (Doudna and Szostak, 1989; Lohse and Szostak, 1996; Wright and Joyce, 1997). Yet creation of self-sustaining, self-replicating biological entities capable of evolution has not yet been achieved in the laboratory and even if successful would not necessarily mimic how life started on Earth or in other parts of the universe.
For life to originate, the presence of liquid water and a source of utilizable free energy are necessities. Furthermore, life as we know it could not have begun on Earth at temperatures much above 160 °C (Stetter et al., 1990) because of the limited thermal stability of macromolecules and other cell components. The synthesis and
polymerization of basic organic building blocks of life on Earth eventually led to self-replicating nucleic acids coding for proteins, but the earliest replicating systems were not necessarily composed of amino acids and nucleotides. If extraterrestrial biosystems exist, their modes of information storage, retrieval, and processing and their enzymatic activity may not be identical to those of biological entities on Earth.
Viability of Microorganisms
Microbes are far more likely than multicellular organisms to retain viability on small solar system bodies because they can adapt to a much wider range of environmental conditions. Single-cell organisms have infiltrated virtually every corner of Earth's biosphere and still constitute the bulk of Earth's biomass. They grow in temperate marine and terrestrial settings, within other microbial or multicellular organisms, in deep subsurface niches, and in extreme environments that would be lethal for other life forms. They often influence geochemical reactions within the biosphere and frequently play key roles in food webs and complex ecosystems.
The physiological state of microorganisms influences their ability to survive extreme environmental conditions (see Table 1.2). For example, organisms with active DNA repair mechanisms or a protective layer of material are more likely to endure exposure to ultraviolet (UV) or ionizing radiation than are cells without equivalent capabilities. Viable microorganisms are either metabolically active (vigorous) or dormant (quiescent). Communities of metabolically active microbes may increase in biomass or simply maintain a constant density by replacing dead cells with new ones. Sometimes metabolic activity is restricted to the repair of macromolecular machinery without cell division. Examples of metabolically active microorganisms include exponentially growing cells, cultures that have reached a stationary phase during which cell division occurs either very slowly or at a rate equivalent to that associated with cell death, and organisms that have shifted from exogenous to endogenous metabolism to survive starvation conditions. Dormant cells are metabolically inactive but are capable of returning to an active state. Examples include spores that are metabolically "frozen," freeze-dried cells, and cells that remain viable at suboptimal or freezing temperatures and/or in a desiccated state.
The distinguishing feature of a dormant versus a dead microorganism is the ability to recover from a quiescent to a metabolically active state. Differentiating dead cells from dormant or nonculturable microorganisms can be difficult. Only as few as 1 to 10 percent of the various kinds of microorganisms from most habitats such as the open ocean have been cultured successfully in the laboratory (Amann et al., 1995); the nutritional and physiological requirements of the vast number of viable but not yet cultured organisms are virtually unknown. Furthermore there is increasing evidence for the existence of consortia of microorganisms composed of taxa that are incapable of independent growth (Mar, 1982; Wolin, 1982; Warikoo et al., 1996; Zhang and Young, 1997), such as the symbionts of marine animals (Haygood and Davidson, 1997).
The inability to culture many of the microorganisms known to exist on Earth is of profound importance in estimating the possibility of microorganisms existing and/or surviving on small solar system bodies. Extremophiles not yet cultured certainly exist on Earth, and the ability of those that have been cultured to survive hostile conditions has already established new limits for the range of environmental conditions that can support viable organisms. Lack of knowledge about extremophiles on Earth is a significant source of uncertainty when assessing the probability of biological contamination of Earth by organisms that may be present in samples returned from small solar system bodies.
Factors That Influence the Survival of Metabolically Active Cells
The most important determinants for long-term survival of metabolically active organisms are the physical and chemical features of their environments, sources of available energy, chemical constituents of nutrients, and cellular processes associated with specific stages of an organism's life cycle.
Temperature extremes and elevated levels of ionizing radiation dictate environmental limits in which organisms cannot survive. Other parameters modulate the response of microorganisms exposed to high temperatures and radiation. The documented range of temperatures at which microbial growth is possible is –10 °C to 113 °C (Blochl et al., 1997; Grossman and Gleitz, 1993; Helmke and Weyland, 1995; Nickerson and Sinskey, 1972; Stetter, 1996), although the absolute limits remain to be determined. Psychrophilic (cold-loving) microorganisms
TABLE 1.2 Microorganisms with Particular Physiological and Nutritional Characteristics
|
Physiological Characteristic |
Description |
|
Temperature |
|
|
Psychrophile/facultative psychrophile |
Optimal temperature for growth is 15 °C or lower, maximal temperature is approximately 20 °C, and minimal temperature is 0 °C or lower |
|
Psychrotroph |
Capable of growing at 5 °C or below, with maximal temperature generally above 25 °C to 30 °C; term in this case is a misnomer because it does not indicate nutritional characteristics |
|
Mesophile |
Generally defined by optimal temperature for growth, which is approximately 37 °C; frequently grows in the range from 8 °C to 10 °C and from 45 °C to 50 °C |
|
Thermophile |
Grows at 50 °C or above |
|
Hyperthermophile |
Grows at 90 °C or above, although optimal temperature for growth is generally above 80 °C; maximal growth of pure cultures occurs between 110 °C and 113 °C, although the maximum (113 °C) may well increase as further research is done |
|
Oxygen |
|
|
Aerobe |
Capable of using oxygen as a terminal electron acceptor; can tolerate a level of oxygen equivalent to or higher than the 21 percent oxygen present in an air atmosphere and has a strictly respiratory-type metabolism |
|
Anaerobe |
Grows in the absence of oxygen; some anaerobes have a fermentative-type metabolism; others may carry out anaerobic respiration in which a terminal electron acceptor other than oxygen is used |
|
Facultative anaerobe |
Can grow aerobically or anaerobically—characteristic of a large number of genera of bacteria including coliforms such as Escherichia coli |
|
Microaerophile |
Capable of oxygen-dependent growth but only at low oxygen levels; cannot grow in the presence of a level of oxygen equivalent to that present in an air atmosphere (21 percent oxygen) |
|
pH |
|
|
Acidophile |
Grows at pH values less than 2 |
|
Alkalophile |
Grows at pH values greater than 10 |
|
Neutrophile |
Grows best at pH values near 7 |
|
Salinity |
|
|
Halophile |
Requires salt for growth: extreme halophiles (all are archaea), 2.5 M to 5 M salt; moderate halophiles, usually low levels of NaCl as well as 15 to 20 percent NaCl |
|
Hydrostatic pressure (100 atmospheres per 1,000-m depth) |
|
|
Barophile |
Obligate barophiles, no growth at 1 atmosphere of pressure; barotolerant bacteria, growth at 1 atmosphere but also at higher pressures. A number of deep-sea bacteria are called barophilic if they grow optimally under pressure and particularly if they grow optimally at or near their in situ pressure (0.987 atm = 1 bar = 0.1 megapascal [Mpa]) |
|
Nutrition |
|
|
Autotroph |
Uses carbon dioxide as its sole source of carbon |
|
Heterotroph |
Unable to use carbon dioxide as its sole source of carbon and requires one or more organic compounds |
|
Chemoorganoheterotroph |
Derives energy from chemical compounds and uses organic compounds as a source of electrons |
|
Chemolithoautotroph |
Relies on chemical compounds for energy and uses inorganic compounds as a source of electrons Five classes: hydrogen bacteria, iron bacteria, sulfur bacteria, ammonia oxidizers, and nitrite oxidizers. Specific nutritional groups of bacteria that do not clearly fit in this category include obligate methane oxidizers and the carbon monoxide oxidizers. There are also photoorganoheterotrophs and photolithoautotrophs among the anoxigenic photosynthetic bacteria. |
|
Mixotroph |
Capable of growing both chemoorganoheterotrophically and chemolithoautotrophically; examples include some of the hydrogen bacteria and some species of Thiobacillus (sulfur-oxidizing bacteria) |
|
Oligotroph |
Can develop at first cultivation on media containing minimal organic material (1 to 15 micrograms carbon per liter) and grow on such media in subsequent cultivation |
|
Copiotroph |
Requires nutrients at levels 100 times those of oligotrophs |
are not metabolically active below eutectic freezing points where liquid water is no longer available. A theoretical 160 °C upper limit on growth (at 1 atm pressure) is dictated by the thermal instability of macromolecules, membranes, and other cellular structures, but growth at temperatures even higher cannot be discounted when all environmental parameters (such as high pressure) are considered. Because the upper temperature limit for growth will depend on pressure, and the environments likely to be encountered in sample return missions will be at low rather than high pressure, the theoretical 160 °C upper temperature limit for growth is reasonable.
Unless sequestered within a protective niche, microorganisms associated with small solar system bodies will also be exposed to UV and ionizing radiation. The regions of the electromagnetic spectrum known to cause light-induced damage to microorganisms are the far UV (200 to 290 nm), near UV (290 to 400 nm), and visible light from 400 to 750 nm. Radiation at all of these wavelengths can induce lethal or sublethal damage in microorganisms at various intensities and can inhibit photosynthesis in aquatic environments. Ionizing and UV radiation in the presence of oxygen at high levels can lead to the formation of destructive oxygen radicals. Exposure to electromagnetic radiation at high levels will have a profound effect on any microorganism. The dose at which radiation is lethal or sublethal will depend on intrinsic cellular characteristics such as ability to sporulate, the capacity to synthesize protective pigments, and the efficiency of DNA repair mechanisms, since DNA is the primary site of radiation damage. The mechanisms for repairing radiation damage appear to be similar in all metabolically active organisms, but there is considerable variation in the lethal dose for each taxon.
As documented in the literature to date, the microorganisms most resistant to ionizing radiation are mesophilic and thermophilic Deinococcus spp. (Minton, 1994; Mattimore and Battista, 1996; Battista, 1997). These gram-positive, aerobic bacteria are 100 percent resistant to gamma radiation at doses of 4 to 5 kGy,3 and a significant fraction can survive exposure at a level as high as 20 kGy—a level of ionizing radiation that is more than 100 times greater than the lethal dose for most organisms, including Escherichia coli and Staphylococcus aureus, and approximately 5 to 20 times the lethal dose for bacterial spores (Clark et al., 1998). Radiation resistance in Deinococcus is conferred by a very efficient repair system for double-stranded breaks in the DNA (Minton, 1994) and appears to be related to efficient physiological adaptation to desiccation (Mattimore and Battista, 1996; Battista, 1997). Little is known about the mechanisms of radiation resistance of other groups of microorganisms that live near natural sources of radiation, such as sulfide structures associated with hydrothermal vents (Cheery et al., 1992; Grasty et al., 1988) or anthropogenic reservoirs.
All metabolically active cells require liquid water, but microorganisms vary widely in their ability to grow and survive at different levels of water activity (aw) and in their tolerance to desiccation, including that arising from increases in solute concentration. The maximum solute concentrations (w/v) permitting microbial growth vary with the specific solute. There are organisms that can grow in solutions that are 90 percent sucrose, 70 percent glycerol, and 30 percent NaCl (Kushner, 1978). The equivalent aw for each is 0.85, 0.75, and 0.80, respectively. On the other hand, many microorganisms can survive in higher concentrations of solute than will permit growth or can survive in the absence of water (desiccation) (Potts, 1994), and both freeze-drying and use of glycerol solutions have enabled long-term preservation of viable microorganisms. Survival times for prokaryotic microorganisms in the air-dried state range from less than 10 minutes for some gram-negative bacteria on glass slides or in petri dishes, to thousands of years for spores in dried soils and for endolithic bacteria, to possibly millions of years in permafrost (see Potts, 1994, and references therein).
Information on osmotic pressure owing to desiccation or to increases in solute concentrations, such as might occur in fluid inclusions or mineral crystals, is important in assessing the potential for small solar system bodies to harbor viable microorganisms.4 The osmotic pressure that limits the growth of extremophiles like the archaebacterium
Halobacterium, which grows in saturated NaCl (30 percent, and with an aw of 0.80), is 41 Mpa. Tychonema spp. can survive at 400 Mpa—virtually no water (Potts, 1994). Most gram-negative organisms are more fastidious since they cease to grow between 1.5 and 7 Mpa, and bacteria like Escherichia coli cannot survive at 51 Mpa (Potts, 1994).
Other environmental factors pose fewer problems, as evidenced by the growth of microorganisms over a range of pH from less than or equal to 1 to greater than or equal to 12 (Horikoshi, 1996; Schleper et al., 1995), at hydrostatic pressures exceeding those at the depth of the deepest ocean trenches (11,000 m) (Jannasch and Taylor, 1984; Yayanos, 1995), on the surfaces and in cracks of rocks, including deep subsurface basalts (Thorseth et al., 1995; Stevens, 1997; Pedersen, 1997), and in the presence of heavy metals at concentrations once believed to be toxic to all life (Wang et al., 1997).
Terrestrial organisms can use the energy of sunlight or the free energy associated with chemical disequilibrium for growth. Solar power for driving biological processes will also be available in many extraterrestrial environments; however, such a source of energy would require surface liquid water and perhaps atmospheres capable of filtering out harmful radiation without blocking beneficial wavelengths. It is highly probable that any metabolically active microorganisms on small solar system bodies would depend on geochemical energy sources, such as reduction by hydrogen, iron (Fe [III]), or ammonia, of various forms of sulfur (including elemental sulfur, polysulfides, and thiosulfate) or Fe (II). Other chemical systems capable of supporting metabolically active cells can be produced by reaction of basaltic rock with geothermally heated water (Baross and Deming, 1995; Liu et al., 1997).
As an alternative to photochemistry or volcanically driven chemistry, low-temperature abiotic reactions can provide an energy source. Recent reports describe microbial communities in deep basaltic rocks that utilize hydrogen formed from the oxidation of Fe (II) in fayalite using water as the electron acceptor (Stevens and McKinley, 1995). The energy from reduced sulfur compounds, Fe (II), and methane is derived from oxidation reactions in which O2 or nitrate is an electron acceptor. In anaerobic reducing environments, energy might be derived from reduction of CO2 by H2 to form methane or through the reduction of elemental sulfur, as is the case with the hyperthermophilic Thermoproteus spp. Both of these forms of anaerobic metabolism are present in archaea.
Based on available information, aerobic environments are considered not likely to be present on small solar system bodies. Therefore, the metabolically active microorganisms most likely to occur on small solar system bodies would be anaerobic chemotrophs. The closest terrestrial analogs would include the following:
-
Methanogens (CO2 + H2 → CH4);
-
Acetogens (H2, CO2, CO, HCOOH, CH3OH, etc. → CH3COOH);
-
Iron-reducers (acetate, fatty acids, aromatics + Fe [III] → CO2 + Fe [II]);
-
Anaerobic methane oxidizers;
-
Heterotrophic, CO2-fixing sulfur reducers (sulfur, sulfate and thiosulfate); and
-
Fermentors (assuming a source of appropriate carbon substrates formed abiotically or from decomposition of CO2-fixing microorganisms).
With the exception of acetogens, all of these metabolic groups have been isolated from volcanic or submarine hydrothermal vent environments, and most are either mesophiles or thermophiles.
Appropriate sources of carbon for extraterrestrial growth include low-molecular-weight organic compounds such as amino acids, hydrocarbons, and inorganic volatiles including carbon dioxide, methane, and carbon monoxide. On small solar system bodies with limited concentrations of organics (see, e.g., McCord et al., 1997), these compounds could be used by oligotrophs. In terrestrial settings, oligotrophic organisms typically grow on organic carbon at concentrations of 1 to 15 micrograms per liter (Morgan and Dow, 1986), and marine organisms have been shown to assimilate dissolved amino acids at concentrations of a few micrograms per liter or less (Wheeler et al., 1974). Most are sessile and divide very slowly. Chemolithoautotrophs (organisms that use inorganic forms of energy and carbon) could be the dominant microbial forms in some extraterrestrial environments. The recent description of subterranean lithoautotrophic microbial ecosystems (SLIMEs) in deep subsurface basalts points to
a microbial community that is not dependent on organic carbon or energy sources derived from photosynthesis (Stevens and McKinley, 1995). This community subsists on H2 that is formed from the abiotic interaction of basalt with water. It derives its energy from metals, sulfur, or hydrogen and fixes CO2. SLIME may be the closest analog for life in the subsurface lithology of Mars and similar solar system bodies.
Limited information is available on the stress tolerance of microorganisms similar to those that might be associated with small solar system bodies. For example, there is no information about the response of chemolithoautotrophs to environmental stress, although there is some indication that anaerobic sulfate-reducing bacteria show high survival efficiency during starvation (Fukui et al., 1996). There is evidence for increased radiation resistance in halobacteria during starvation (Whitelam and Good, 1986). Most reports on the survival of metabolically active microorganisms describe chemoorganoheterotrophs (organisms that use preformed organic compounds). There are also accounts of starvation-survival strategies for aquatic and soil microorganisms that require high levels of organic material for growth. When exposed to conditions of starvation (e.g., for one or more required nutrients) or to low temperatures (e.g., below the minimum temperature for growth), some chemoorganoheterotrophs adapt by decreasing cell size to 0.2 to 0.5 microns, reducing genomes to a single copy, and diminishing concentrations of other macromolecules. At the same time these cells may increase their capacity for high-affinity binding and transport of organic compounds, which allows them to respond rapidly to low levels of organic substrates (Barcina et al., 1997; Kaprelyants et al., 1993; Roszak and Colwell, 1987). Another strategy involves a switch from a surface independent (i.e., suspended) to a sessile life form, which can require morphological changes ushered in by altered cell surface characteristics. A switch from a single polar flagellum for motility to multiple lateral flagella can aid gliding on surfaces prior to attachment. Because of long periods required to test the influence of environmental factors, there are few data on long-term survival of chemoorganotrophs. However, Morita (1986) reported that a marine psychrophilic vibrio was still viable after 2.5 years in the starvation state, and Munroe and Colwell (1996) reported viable but nonculturable cells able to survive for 3 years in synthetic seawater.
Factors That Affect the Survival of Dormant Microorganisms
Dormancy is an effective strategy that allows microorganisms to survive environmental conditions not conducive to active growth. The capacity of many organisms to form cysts or spores allows them to survive greater temperature excursions than those tolerated by vegetative cells, albeit in suspended states of metabolic activity. It is these forms that are likely to survive over extended periods of time, provided that they are protected from the detrimental effects of ionizing radiation. Other microorganisms become dormant in response to environmental change. For example, some species pathogenic to humans become metabolically inactive when exposed to low temperatures but may retain the ability to respond to added substrates. These organisms may be viable in natural settings (Weichart and Kjelleberg, 1996) but nonculturable in the laboratory (Xu et al., 1982; Oliver, 1993). Although metabolically inactive cells cannot correct defects caused by ionizing radiation, the molecular machinery required for such repair can sometimes be reactivated in response to environmental or physiological change.
There are several reports, some controversial, of quiescent eukaryotic and prokaryotic organisms having survived for thousands of years. For example, fungi and Streptomyces spp. were isolated from a well-preserved 5,300-year-old human corpse (Haselwandter and Ebner, 1994). According to one account, viable prokaryotic and eukaryotic microorganisms were preserved for 3 million years in arctic and antarctic permafrost (Gilichinsky, 1997). Another report describes cultivation of the spore-forming organism Bacillus sphaericus from the abdominal contents of a bee entombed in 25- to 40-million-year-old amber (Cano and Borucki, 1985). However, few microorganisms' survival capabilities have been studied rigorously. This is particularly true for microorganisms that can utilize very low concentrations of organic nutrients or inorganic energy sources and microbes that inhabit the most extreme environments on Earth.
Survival of cellular life forms in a dormant state is enhanced by very low levels of water activity and/or reduced temperatures. Cryo-preservation and freeze-drying are standard means for preserving microorganisms in culture collections. Both are forms of dehydration that suspend enzymatic activities, including the destructive activities of proteases, hydrolases, and nucleases. The survival of cells undergoing drying is dependent on the rate
of drying; in general, slow (24-hour) drying is associated with greater survival (Antheunisse et al., 1981). Cryo-preservation enhances the likelihood of survival because the Q10 effect5 of enzymatic hydrolysis is reduced and the rates of deleterious chemical reactions are slowed. The major constraint is eutectic freezing. Repeated freeze-thaw cycles are especially deleterious to cellular life because they lead to the rupture of critical membrane and cell wall structure. Once dehydrated, an organism can survive in a nearly total vacuum or, if frozen, can survive in an arrested metabolic state at temperatures below that required to liquefy nitrogen. However, when organisms are metabolically inactive, they no longer have the capacity to repair the devastating effects of exposure to relatively low levels of radiation or damage from the formation of free radicals.
QUESTIONS APPROPRIATE FOR ASSESSING THE BIOLOGICAL POTENTIAL OF SMALL BODIES
As indicated by the task group's review of current data and understanding, life on Earth and the survival of metabolically active cells absolutely require the presence of liquid water, a source of energy that can be tapped by metabolic processes, temperatures that do not exceed ca. 160 °C, carbonaceous material, and shielding from high-intensity or long-term exposure to ionizing radiation or UV flux. Conditions compatible with survival of dormant life forms are less stringent than those required for initiation of life or continuance of metabolically active cells. Some organisms can survive in nearly completely dehydrated states and tolerate higher levels of ionizing radiation even in the complete absence of organic compounds and sources of energy. The survival of microorganisms exposed to ionizing radiation will depend on their location in a solar system body, with those on the surface subject to higher levels of radiation than those within the interior (assuming no internal sources of radiation). Furthermore, metabolically active organisms are more likely to be capable of repairing radiation-induced damage. In most cases, based on the average background radiation levels in space of 10 to 30 rads per year, it is estimated that it would require from 50,000 to 1 million years to inactivate organisms on the surface of solar system bodies and up to 10 million years if the organisms were deep in regolith, e.g., up to 100 cm or more (Clark et al., 1998).
The conditions necessary for the origin of life and the survival of microorganisms on small solar system bodies can be inferred from the terrestrial experience, as outlined in the preceding section. Water may safely be regarded as a necessity for life on small solar system bodies because the chemistry on which life as we know it is based must take place in solution, and there is no other plausible solvent at this time. A source of energy to support the origin and continuation of life in any environment is a thermodynamic necessity. For extraterrestrial environments the energy sources are more likely to be geochemical than photosynthetic. The temperature limit of 160 °C for survival of metabolically active cells may not be exceeded by extraterrestrial organisms unless their biochemistry does not depend on the formation of amide, ester, or phosphodiester bonds or unless protective mechanisms exist that are not yet understood. Chemical building blocks for organic polymers must be available, and the ''biopolymers" are assumed not to differ greatly from terrestrial biopolymers in their sensitivity to radiation.
The microbial species composition of most anaerobic environments on Earth is not known, and so it is also not known how the species composition of these anaerobic microbial communities might change over time, what environmental factors might influence any changes, or how frequently and successfully these habitats might be colonized by new species of microorganisms. Accordingly, the task group concluded that although there is a low likelihood of a viable anaerobic microorganism surviving transport through space and finding a suitable anaerobic habitat on Earth, growth in a suitable habitat if found might be possible. This conclusion is necessary because of our current lack of information about anaerobic environments on Earth that may be analogous to environments on other solar bodies, and the likelihood that the metabolic properties of such an extraterrestrial anaerobe would resemble an Earth anaerobe from a similar environment.
To aid in identifying whether or not samples returned from a range of small solar system bodies require containment, the task group formulated the following series of questions:
-
Does the preponderance of scientific evidence indicate that there was never liquid water in or on the target body?
-
Does the preponderance of scientific evidence indicate that metabolically useful energy sources were never present?
-
Does the preponderance of scientific evidence indicate that there was never sufficient organic matter (or CO2 or carbonates and and appropriate source of reducing equivalents)6 in or on the target body to support life?
-
Does the preponderance of scientific evidence indicate that subsequent to the disappearance of liquid water, the target body has been subjected to extreme temperatures (i.e., >160 °C)?
-
Does the preponderance of scientific evidence indicate that there is or was sufficient radiation for biological sterilization of terrestrial life forms?
-
Does the preponderance of scientific evidence indicate that there has been a natural influx to Earth, e.g., via meteorites, of material equivalent to a sample returned from the target body?
For the purposes of this report, the term "preponderance of scientific evidence" is not used in a legal sense but rather is intended to connote a nonquantitative level of evidence compelling enough to research scientists in the field to support an informed judgment. In applying the questions, the task group drew on existing data on the origin, composition, and environmental conditions (past and present) of each small body or planetary satellite examined and then determined whether the quality and weight of the evidence were convincing enough to allow making judgments and deriving findings. The answers to the questions, taken together, were used by the task group to reach a considered conclusion that the potential for a living entity to be in or on a returned sample was either "negligible" or "not negligible." Because of the incomplete current state of knowledge about small solar system bodies, there are no definitive answers to the questions, and so all judgments regarding biological potential are qualitative (not quantitative).
Figure 1.1 shows the relationship of these questions and how they are answered to the task group's assessment of the need for sample containment. The questions allow a conservative, case-by-case approach, taking into account information about the different kinds of small solar system bodies, the natural influx to Earth of various relevant materials such as meteorites or interplanetary dust particles, and the possible nature of putative extraterrestrial life. An answer of "yes" to any question argues against the need for special containment beyond what is needed for scientific purposes. For containment procedures to be necessary, an answer of "no" must be returned to all the questions. For such samples, strict containment and handling as outlined in Chapter 7 are required.
CONTENT AND ORGANIZATION OF THIS REPORT
Chapter 2 discusses the natural influx to Earth of material from small solar system bodies and the exchange of material between solar system bodies other than Earth. Chapter 3 describes the origin, composition, and environmental conditions (both past and present) of planetary satellites, specifically those inside the orbit of Jupiter (Europa, Io, Ganymede, Callisto, and the Moon) as well as Phobos and Demos, the satellites of Mars. An assessment of the potential for a living entity to be present in returned samples and suggested scientific investigations to reduce the uncertainty in the assessment are presented. Chapters 4, 5, and 6 present similar analyses for asteroids (including a discussion of the connection between asteroids and meteorites), comets (large and small), and dust, respectively. Chapter 7 synthesizes relevant information from the NRC report Mars Sample Return: Issues and Recommendations (NRC, 1997) pertaining to potential risks and handling of returned samples and addresses biohazard and life detection concerns relative to small solar system bodies. Chapter 8 presents the task
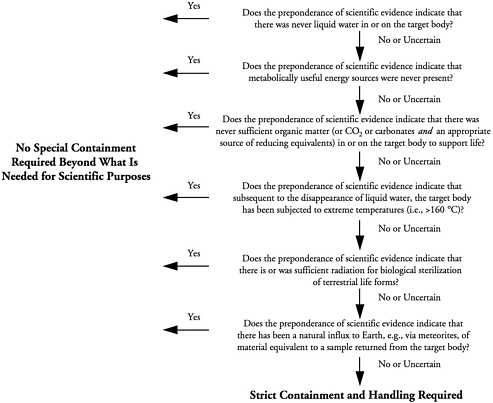
FIGURE 1.1 Relationship of the task group's criteria for assessing biological potential to its assessment of the need for sample containment. The terms "contained" and "containment" are used in this report to indicate physical and biological isolation and handling of returned samples as specified for samples returned from Mars (see NRC, 1997). For the purposes of this report, the term "preponderance of scientific evidence" is not used in a legal sense but rather is intended to connote a nonquantitative level of evidence compelling enough to research scientists in the field to support an informed judgment.
group's conclusions and recommendations. The appendixes include biographies of the task group members (Appendix A), the letter of request received from NASA (Appendix B), some references to related topics not discussed in the body of the report (Appendix C), information on planetary protection policies in NASA and the International Council of Scientific Unions' Committee on Space Research (COSPAR) (Appendix D), and a glossary (Appendix E).
REFERENCES
Amann, R.I., W. Ludwig, and K.H. Scheliffer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143–169.
Antheunisse, J., J.W. de Bruin-Tol, and M.E. van der Pol-van Soest. 1981. Survival of microorganisms after drying and storage. Antonie Leeuwenhoek 47:539–545.
Barcina, I., P. Lebaron, and J. Vives-Rego. 1997. Survival of allochthonous bacteria in aquatic systems: A biological approach. FEMS Microbiol. Ecol. 23:1–9.
Baross, J.A., and J.W. Deming. 1995. Growth at high temperatures: Isolation and taxonomy, physiology and ecology. Pp. 169–217 in The Microbiology of Deep-Sea Hydrothermal Vents, D.M. Karl (ed.). Boca Raton, Florida : CRC Press, Inc.
Battista, J.R. 1997. Against all odds: The survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 51:203–224.
Blochl, E., R. Rachel, S. Burggraf, D. Hafenbradl, H.W. Jannasch, and K.O. Stetter. 1997. Pyrolobus fumarii, gen. and sp. nov., represents a novel group of Archaea, extending the upper temperature limit for life to 113 °C. Extremophiles 1:14–21.
Cano, R.J., and M.K. Borucki. 1985. Revival and identification of bacterial spores in 25- to 40-million-year-old Dominican amber. Science 268:1060–1064.
Cheery, R., D. Desbruyeres, M. Heyraud, and C. Nolan. 1992. High levels of natural radioactivity in hydrothermal vent polychaetes. C.R. Acad. Sci. Paris, 315, Serie III, pp. 21–26.
Clark, B.C., A.L. Baker, A.F. Cheng, S.J. Clemett, D. McKay, H.Y. McSween, C.M. Pieters, P. Thomas, and M. Zolensky. 1998. Survival of life on asteroids, comets and other small bodies . Origins of Life and Evolution of the Atmosphere, in press.
Doudna, J.A., and J.W. Szostak. 1989. RNA-catalyzed synthesis of complementary-strand RNA. Nature 339:519–522.
Fukui, M., Y. Suwa, and Y. Urushigawa. 1996. High survival efficiency and ribosomal RNA decaying pattern of Desulfobacter latus, a highly specific acetate-utilizing organism during starvation. FEMS Microbiol. Ecol. 19:17–25.
Gilichinsky, D.A. 1997. Permafrost as a microbial habitat: Extreme for the Earth, favorable in space. Pp. 472–480 in Instruments, Methods, and Missions for the Investigation of Extraterrestrial Microorganisms, R.B. Hoover (ed.). Bellingham, Washington: International Society for Optical Engineering.
Grasty, R.L., C.W. Smith, J.M. Franklin, and I.R. Jonasson. 1988. Radioactive orphans in barite-rich chimneys, Axial Caldera, Juan de Fuca Ridge. Can. Mineral. 26:627–636.
Grossman, S., and M. Gleitz. 1993. Microbial responses to experimental sea-ice formation: Implication for the establishment of Antarctic sea-ice communities. J. Exp. Mar. Biol. Ecol. 173:273–289.
Haselwandter, K., and M.R. Ebner. 1994. Microorganisms surviving for 5300 years . FEMS Microbiol. Lett. 116:189–194.
Haygood, M.G., and S.K. Davidson. 1997. Small-subunit RNA genes and in situ hybridization with oligonucleotides specific for the bacterial symbionts in the larvae of the bryozoan Bugula neritina and proposal of "Candidatus endobugula sertula." Appl. Environ. Microbiol. 63:4612–4616.
Helmke, E., and H. Weyland. 1995. Bacteria in sea ice and underlying water of the eastern Weddell Sea in midwinter. Mar. Ecol. Progr. Ser. 117:269–287.
Horikoshi, K. 1996. Alkaliphiles–from an industrial point of view. FEMS Microbiol. Rev. 18:259–270.
Jannasch, H.W., and C.D. Taylor. 1984. Deep-sea microbiology. Annu. Rev. Microbiol. 38:487–514.
Kaprelyants, A.S., J.C. Gottschal, and D.B. Kell. 1993. Dormancy in non-sporulating bacteria. FEMS Microbiol. Rev. 104:271–286.
Kushner, D.J. 1978. Life in high salt and solute concentrations: Halophilic bacteria. Pp. 318–368 in Microbial Life in Extreme Environments, D.J. Kushner (ed.). London: Academic Press.
Liu, S.V., J. Zhou, C. Zhang, D.R. Cole, M. Gajdarziska-Josifovska, and T.J. Phelps. 1997. Thermophilic Fe(III)-reducing bacteria from the deep subsurface: The evolutionary implications. Science 277:1106–1109.
Lohse, P.A., and J.W. Szostak. 1996. Ribozyme-catalyzed amino-acid transfer reactions. Nature 381:442–444.
Mar, R.A. 1982. Methanogenesis and methanogenic partnerships. Phil. Trans. R. Soc. Lond. B. 297:599–616.
Mattimore, V., and J.R. Battista. 1996. Radioresistance of Deinococcus radiodurans: Functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 178:633–637.
McCord, T.B., R.W. Carlson, W.D. Smythe, G.B. Hansen, R.N. Clark, C.A. Hibbitts, F.P. Fanale, J.C. Granahan, M. Sequra, D.L. Matson, T.V. Johnson, and P.D. Martin. 1997. Organics and other molecules in the surfaces of Callisto and Ganymede. Science 278:271–275.
Miller, S.L. 1992. The prebiotic synthesis of organic compounds as a step toward the origin of life. Pp. 1–28 in Major Events in the History of Life, J.W. Schopf (ed.). Boston, Massachusetts: Jones and Bartlett Publishers.
Minton, K.W. 1994. DNA repair in the extremely radioresistant bacterium Deinococcus radiodurans. Mol. Microbiol. 13:159–167.
Morgan P., and C.S. Dow. 1986. Bacterial adaptations for growth in low nutrient environments. Pp. 187–214 in Microbes in Extreme Environments, R.A. Herbert and G.A. Codd (eds.). Special Publications of the Society for General Microbiology, 17. London: Academic Press.
Mojzsis, S.J., G. Arrhenius, K.D. McKeegan, T.M. Harrison, A.P. Nutman, and C.R.L. Friend. 1996. Evidence for life on Earth before 3,800 million years ago. Nature 384:55–59.
Morita, R.Y. 1986. Autecological studies and marine ecosystems. Pp. 147–181 in Microbial Autecology, A Method for Environmental Studies, E.L. Tate III (ed.). New York: John Wiley & Sons.
Munroe, P.M., and R.R. Colwell. 1996. Fate of Vibrio cholerae O1 in seawater microcosms. Water Res. 30:47–50.
National Research Council (NRC), Space Studies Board. 1997. Mars Sample Return: Issues and Recommendations. Washington D.C.: National Academy Press.
Nickerson, J.T., and A.J. Sinskey. 1972. Microbiology of foods and food processing. New York: American Elsevier Publishing Co.
Oliver, J.D. 1993. Formation of viable but nonculturable cells. Pp. 239–272 in Starvation in Bacteria, S. Kjelleberg (ed.). New York: Plenum Press.
Pedersen, K. 1997. Microbial life in deep granitic rock. FEMS Microbiol. Rev. 20:399–414.
Potts, M. 1994. Desiccation tolerance of prokaryotes. Microbiol. Rev. 58:755–805.
Roszak, D.B., and R.R. Colwell. 1987. Survival strategies of bacteria in natural environments. Microbiol. Rev. 51:365–379.
Schidlowski, M. 1988. A 3,800-million-year isotopic record of life from carbon in sedimentary rocks. Nature 333:313–318.
Schopf, J.W. 1993. Microfossils of the early Archean Apex Chert: New evidence of the antiquity of life. Science 260:640–646.
Schleper, C., G. Puehler, T. Holz, A. Gambocorta, D. Janekovic, U. Santarius, H.P. Klank, and W. Zillig. 1995. Picrophilus gen. nov., fam. nov.: A novel aerobic, heterotrophic, thermoacidophilic genus and family comprising Archaea capable of growth around pH 0. J. Bacteriol. 177:7050–7059.
Stetter, K.O. 1996. Hyperthermophilic prokaryotes. FEMS Microbiol. Rev. 18:89–288.
Stetter, K.O., G. Fiala, G. Huber, R. Huber, and A. Sequerer. 1990. Hyperthermophilic microorganisms. FEMS Microbiol. Rev. 75:117–124.
Stevens, T.O. 1997. Lithoautotrophy in the subsurface. FEMS Microbiol. Rev. 20:327–337.
Stevens, T.O., and J.P. McKinley. 1995. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science 270:450–454.
Thorseth, I.H., T. Torsvik, H. Furnes, and K. Muehlenbachs. 1995. Microbes play an important role in the alteration of oceanic crust. Chem. Geol. 126:137–146.
Wang, C.L., P.C. Michels, S.C. Dawson, S. Kitisakkul, J.A. Baross, J.D. Keasling, and D.S. Clark. 1997. Cadmium removal by a new strain of Pseudomonas aeruginosa in aerobic culture. Appl. Environ. Microbiol. 63:4075–4078.
Warikoo, V., M.J. McInerney, J.A. Robinson, and J.M. Suflita. 1996. Interspecies acetate transfer influences the extent of anaerobic benzoate degradation by subtrophic consortia. Appl. Environ. Microbiol. 62:26–32.
Weichart, D., and S. Kjelleberg. 1996. Stress resistance and recovery potential of culturable and viable but nonculturable cells of Vibrio vulnificus. Microbiology 142:845–853.
Wheeler, P.A., B.B. North, and G.C. Stephens. 1974. Amino acid uptake by marine phytoplankters. Limnol. Oceanogr. 19:249–259.
Whitelam, G.C., and G.A. Good. 1986. Damaging effects of light on microorganisms. Pp. 129–169 in Microbes in Extreme Environments, R.A. Herbert and G.A. Codd (eds.). Special Publications of the Society for General Microbiology 17. London: Academic Press.
Wolin, M.J. 1982. Hydrogen transfer in microbial communities. Pp. 323–356 in Microbial Communities and Interactions, Vol. 1, A.T. Bull and J.H. Slater (eds.). London: Academic Press.
Wright, M.C., and G.F. Joyce. 1997. Continuous in vitro evolution of catalytic function. Science 276:614–617.
Yayanos, A.A. 1995. Microbiology to 10,500 meters in the deep sea. Annu. Rev. Microbiol. 49:777–805.
Xu, H.S., N. Roberts, F.L. Singleton, R.W. Atwell, D.J. Grimes, and R.R. Colwell. 1982. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microbial. Ecol. 8:313–323.
Zhang, X., and L.Y. Young. 1997. Carboxylation as an initial reaction in the anaerobic metabolism of naphthaline and phenanthrene by sulfidogenic consortia. Appl. Environ. Microbiol. 63:4759–4764.













