3
Health Risks of I-131 Exposure
The major health risks associated with exposure to iodine-131 (I-131) involve the thyroid gland, which concentrates this radionuclide. Assessment of the magnitude of the public-health problem posed by exposure to I-131 estimated by the National Cancer Institute (NCI 1997a) entails understanding
-
The biology of the thyroid gland.
-
The relationship of exposure to ionizing radiation and the occurrence of thyroid cancer.
-
The effect of radiation on the frequency of nonmalignant thyroid disease.
-
Projections of the risk of thyroid cancer through the lifetime of exposed individuals.
-
The estimates of the proportion of cases of I-131 related thyroid cancer that have already occurred.
THYROID GLAND BIOLOGY
The thyroid gland (see Figure 3.1) is a butterfly-shaped, ductless gland astride the trachea on the anterior side of the throat.
The gland usually begins as an endodermal thickening and a pouch in the floor of the pharynx, visible about 3 weeks after conception. Thyroid follicular cells develop in the embryo, and by the 10th week of gestation, iodine is accumulated and colloid is present within the follicles. Thyroxine then becomes detectable and the gland is functional (O'Rahilly and Muller 1992). The thyroid gland is the source of several hormones in which iodine is an important constituent. The thyroid is the only organ in the body that greatly concentrates and retains iodine.
FIGURE 3.1 Anatomical drawing of thyroid location (courtesy of American Cancer Society).
Normal Thyroid Physiology
Concern about the carcinogenic effects of exposure to radioiodine on the thyroid gland is motivated by three major factors. First, evidence has accumulated that the thyroid gland is uniquely sensitive to the effects of radiation. There is some evidence that measurable increases in thyroid cancer can occur with external doses of radiation as low as 0.1 sievert (Sv) (10 rem). A finite risk at low doses of that magnitude is consistent with risks for other solid cancers reported for the Japanese atomic-bomb survivors (Pierce and others 1996). Second, the cow-milk-man pathway described in the NCI (1997a) report and discussed in Chapter 2 of this report provides a mechanism by which radioiodines in the environment can be greatly concentrated in the human food chain. Finally, because most of the radiation dose is from ingested or inhaled radioiodine, the radiation dose to the thyroid is 500-1,000 times greater than is the largest radiation dose to other organs in the body.
For several reasons, persons exposed to I-131 as children are uniquely at risk for carcinogenic effects. First, children drink more milk relative to their body size than do adults. Second, the same amount or a higher fraction of internalized iodine is concentrated in the smaller thyroid glands of children; therefore the radiation dose to the thyroid in children is higher than it is in adults. Finally, studies of children whose thyroid glands were exposed to external radiation suggest a strong inverse relationship between age at exposure and the carcinogenic effects of radiation on the thyroid. Over the age of 15, little increase in thyroid cancers has been observed. Below the age of 15, thyroid cancer increased by a factor of approximately 2 for every 5 years' decrease in age. Not only is the frequency of malignant nodules increased by thyroid irradiation, but benign nodules also occur with greater than usual frequency after irradiation (Wong and others 1996).
Stable iodine and its radioactive isotopes are water-soluble and readily absorbed, either from the gastrointestinal tract after ingestion or through the lungs
after inhalation. The first step in the synthesis and storage of thyroid hormone involves a mechanism for concentrating iodine from extracellular fluid, variably called the iodine pump, the transport mechanism, the iodide-concentrating mechanism, or the iodine trap. Transport of I- across the thyroid membrane is an energy-dependent process linked to the transport of sodium; this fact led to the concept of an Na+-I- cotransport (symport) system, with an ion gradient generated by Na+-K+ ATPase as the driving force. By this mechanism, the thyroid attains remarkably high concentrations of iodide; concentrations of 30 to 40 times that in blood are usual though values in excess of 400 fold over the level in the bloodstream have been recorded (Taurog 1996). Other tissues in humans contain sodium iodide symporters: the gastric mucosa, salivary glands, mammary glands, choroid plexus, ovaries, placenta, and skin (Smanik and others 1996). Breast tissue, which contains iodine symporters, can therefore pump iodine into breast milk.
Once iodine is concentrated in the thyroid follicular cell, it is incorporated into tyrosine molecules that form part of a larger protein, thyroglobulin. Thyroglobulin is the storage form of thyroid hormone that is kept, often for long periods, within the thyroid gland. Once iodine has been incorporated into proteins by the thyroid, the biologic half-life of iodine within the thyroid is typically 80-120 days; non-protein-bound iodine has a biologic half-life of several hours in the body. The long half-life of thyroid iodine results in nearly all of the energy from the I-131 being deposited in the thyroid. The liver inactivates thyroid hormone, breaking it into smaller, biologically inert components that are eventually excreted by the kidney. Thyroid hormone is essential to life. It regulates many metabolic processes, including the rate of cellular oxygen consumption, and it affects the performance of many body systems, including the heart and nervous systems.
Breaking down thyroglobulin within the thyroid produces two main forms of thyroid hormone, tetraiodothyronine and triiodothyronine, which are then secreted into the blood. Tetraiodothyronine (thyroxine) is secreted in much greater quantities than is triiodothyronine; it has 4 iodine molecules and a half-life of about 7 days in the circulation. Triiodothyronine, the most potent thyroid hormone, has 3 iodine molecules and a half-life of about 12 hours in serum. Most of the triiodothyronine in the blood comes from conversion of tetraiodothyronine to triiodothyronine by the body.
The unique ability of the thyroid gland to concentrate iodine has enabled the effective use of radioiodines in the diagnosis and treatment of thyroid disorders, including an overactive thyroid (Graves disease or toxic multinodular goiter), and differentiated (papillary and follicular) thyroid cancers (Mazzaferri and Jhiang 1994). Given for medical purposes in doses that range from 5 to 200 millicuries (mCi), I-131 efficiently destroys overactive and malignant thyroid tissues.
For many years, I-131 was used in very small amounts (50-100 mCi) for diagnostic studies. Typically, these were 24-hour thyroidal radioactive iodine uptake, which is a measurement of the amount of iodine taken up by the thyroid
from the blood and thyroid imaging studies that give some information about the configuration of the thyroid. (This is in contrast to larger doses of I-131, in the range of 10 to 200 mCi, that are given to ablate malignant thyroid tissue or to treat overactive thyroid glands. Large doses of I-131 ordinarily destroy the thyroid gland and thus do not induce thyroid cancer.) In addition to diagnostic and research exposure, children have also experienced therapeutic exposure to I-131 as described below.
Thyroid Cancer and Thyroid Nodules
Thyroid cancer is usually clinically manifested as a nodule on the gland. Most thyroid nodules are benign. Palpable thyroid nodules, both benign and malignant, increase in frequency with age and are more common among women than they are in men. Although studies vary, perhaps 5 percent of women over the age of 50 and about 1 percent of men over 50 have thyroid nodules that can be felt during physical examination.
The prevalence of thyroid nodules detected by ultrasonography is as much as 10-fold greater than the prevalence of palpable thyroid nodules, depending on the population (Tan and Gharib 1997; Ezzat and others 1994). Most thyroid nodules detected by ultrasound are small (<1 cm in diameter) and not palpable, whether or not the population being studied has received thyroid radiation (Schneider and others 1997; Tan and Gharib 1997; Ezzat and others 1994).
Larger thyroid nodules (1.5 cm or larger) are more likely to be associated with clinically significant thyroid cancer (Mazzaferri and Jhiang 1994). For several reasons, however, even these large nodules are not always palpable. First, to detect a nodule by palpation, its consistency must be recognizably different from the consistency of the normal thyroid gland. Second, some nodules are in areas that are difficult to palpate, such as on the back surface of the gland or behind the sternum. Third, the thickness of the neck of some patients makes examination of the thyroid difficult. Finally, the examiner's skill and the completeness of the examination will, in part, determine the palpability of the nodule. In one study of 54 individuals who had been exposed to therapeutic head and neck irradiation during childhood for benign conditions, ultrasound detected 157 nodules in 87 percent (47) of the subjects; 52 percent (28) had 40 nodules in all that were 1.0 cm or larger. Of the 11 nodules that were 1.5 cm or larger, palpation detected only 5, or 45 percent (Schneider and others 1997). Other studies of populations not exposed to I-131 have reported better results (Chapter 4).
Most thyroid nodules biopsied by fine-needle aspiration (FNA) are benign, even in patients who have a history of head and neck irradiation (Mazzaferri 1993a). The high prevalence of benign thyroid nodules in the general population and among persons with a history of head and neck irradiation increases the risk of false-positive test results. This is discussed in detail in Chapter 4 of this report.
Incidence of Clinically Manifest and Occult Disease
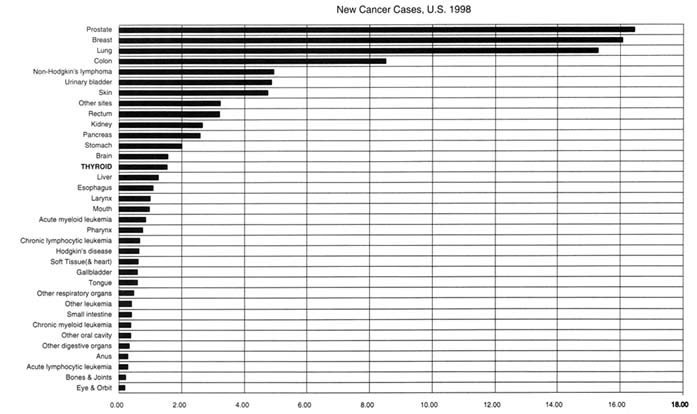
The two principal malignancies of the thyroid follicle cell are papillary and follicular thyroid cancer. Malignant tumors resulting from exposure to ionizing radiation are almost exclusively papillary cancers (Nikiforov and Gnepp 1994). Those tumors also account for more than 80 percent of the thyroid cancers occurring spontaneously among persons with no known history of thyroid radiation (Mazzaferri 1991). According to American Cancer Society estimates, 17,200 new cases of thyroid cancer will be diagnosed in 1998 in the United States, ranking thyroid cancer 14th in incidence among 35 categories (Figure 3.2). Its incidence varies with gender and age and is highest in women between the ages of 30 and 70 years; the peak incidence reaches 13.2 per 100,000 per year between the ages of 50 and 54 (see Table 3.1).
The incidence of thyroid cancer is lower in men. In men, thyroid cancer peaks between the ages of 60 and 70, when its annual incidence is 8.6 per 100,000 (NIH 1997). In the latest Surveillance, Epidemiology, and End Result report (SEER 1998), the average lifetime risk over a 95-year lifespan of being diagnosed with some form of thyroid cancer was 0.66 percent (6.6 per 1,000) for
TABLE 3.1 Thyroid Cancer (Invasive) Incidence Rates per 100,000 Persons, 1990+1994, by Age at Diagnosis
|
Age at Diagnosis |
Total |
Males |
Females |
|
All ages |
4.9 |
2.8 |
6.9 |
|
0-4 |
0.0 |
0.0 |
0.0 |
|
5-9 |
0.1 |
0.1 |
0.1 |
|
10-14 |
0.4 |
0.3 |
0.6 |
|
15-19 |
1.4 |
0.3 |
2.6 |
|
20-24 |
3.9 |
1.0 |
6.8 |
|
25-29 |
5.4 |
2.3 |
8.6 |
|
30-34 |
6.8 |
2.4 |
11.1 |
|
35-39 |
7.8 |
3.7 |
11.8 |
|
40-44 |
7.9 |
3.7 |
12.0 |
|
45-49 |
8.5 |
4.8 |
12.2 |
|
50-54 |
9.5 |
5.7 |
13.2 |
|
55-59 |
9.3 |
6.1 |
12.4 |
|
60-64 |
8.6 |
7.0 |
10.1 |
|
65-69 |
9.9 |
7.5 |
11.8 |
|
70-74 |
9.7 |
8.6 |
10.5 |
|
75-79 |
9.5 |
8.5 |
10.2 |
|
80-84 |
7.7 |
6.1 |
8.6 |
|
85+ |
7.9 |
7.2 |
8.2 |
|
SOURCE: (NCI 1997b). |
|||
women and 0.27 percent (2.7 per 1,000) for men. By way of comparison, the lifetime risks for women of developing invasive in situ breast cancer or invasive in situ colon cancer are 14.2 percent and 6 percent, respectively. For men, the lifetime risks of developing prostate or lung cancer are 18.8 percent and 8.4 percent, respectively. The lifetime risk of dying from some form of thyroid cancer was 0.07 percent for women and 0.04 percent for men. These figures compare to 3.46 percent and 2.53 percent lifetime risks of dying, respectively, from breast cancer and colon/rectal cancer for women and 3.64 percent and 2.57 percent for prostate and colon/rectal cancer for men.
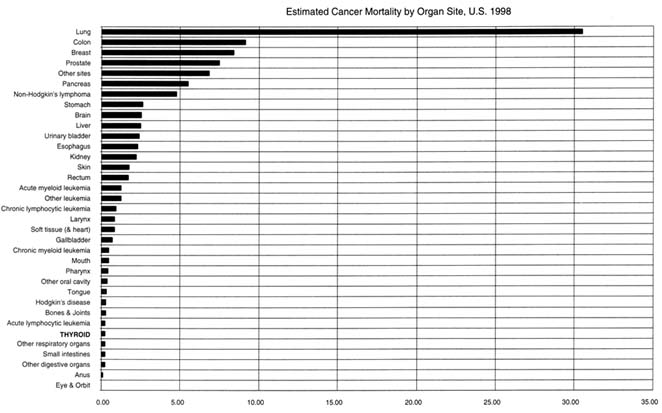
The risk of dying of thyroid cancer in countries with efficient medical care systems is low. The long-term mortality rates for papillary thyroid carcinoma are less than 10 percent at 30 years after diagnosis (Mazzaferri and Jhiang 1994). The American Cancer Society estimates that 1,200 people will die from thyroid cancer in 1998, accounting for about 0.2 percent of all cancer deaths. Unlike its incidence, which has been rising, the mortality rates for thyroid cancer have been falling. Between 1973 and 1994, the mortality rates for thyroid cancer dropped more than 23 percent, both for people younger than 65 years and for people older than 65 at the time of diagnosis (NIH 1997). See Figure 3.3.
Between 1973 and 1992, the incidence of thyroid cancer rose almost 28 percent (p < 0.05)—a change that has been observed in persons both under and over the age of 65 at the time of diagnosis. In the SEER reports, 14 of 23 cancer sites showed increasing incidence during this period; only 4 of the 14, including thyroid cancer, showed decreasing mortality. The contrast between the incidence and mortality trends has been attributed to more sophisticated detection technologies (ultrasound for nodules and FNA biopsy for cancer) and more complete diagnostic reporting (Wang and Crapo 1997).
A large number of thyroid cancers are small, occult tumors that are usually not detected during a person's lifetime and that rarely progress to cause problems. Clinically silent tumors are generally papillary microcancers smaller than 1.0 cm in diameter. They may be found unexpectedly during surgery for benign thyroid disease, at autopsy, or by FNA biopsy of a nodule discovered by ultrasonography. Their prevalence varies according to the geographic location and possibly ethnicity, the type of tumor, and the intensity of the pathologic examination (Moosa and Mazzaferri 1997). In autopsy studies of persons who died without known thyroid disease, the prevalence of occult thyroid cancer ranges from 5 to 13 percent among studies in the continental United States and 6 to 36 percent among studies in Europe (Moosa and Mazzaferri 1997; Thorvaldsson and others 1992; Harach and others 1985). Occult cancer is found in all age groups but is more frequent after the age of 40; there is no gender difference in frequency. Thus, there is good reason to suspect many healthy people harbor tiny thyroid cancers that will never harm them.
The problem of microcancers is not unique to the thyroid gland. Similar tumors are found even more commonly in the breast and prostate. The introduction
of sophisticated diagnostic tests has resulted in the discovery of many microcancers that are unlikely to harm the patient. A major challenge for medical research is to differentiate clinically significant microcancers from those that will never harm the patient. Failure to make this differentiation will result in some patients undergoing treatment for harmless diseases and others imprudently having their diseases ignored. Research cited in Chapter 4 suggests that people want to factor information about these usually nonprogressing cancers into their decisions about cancer screening and treatment.
Thyroid Cancer in Persons (All Ages) Not Exposed to Radiation
Most clinically apparent papillary thyroid cancers are first manifested as one or several palpable thyroid nodules, discovered in about half the cases by the patient (Mazzaferri 1993a). They are otherwise usually asymptomatic, although a small proportion of highly invasive tumors are very symptomatic. At the time of diagnosis, the primary tumor is typically 2.0-2.5 cm, but can range from a few millimeters to more than 5 cm in diameter.
With routine study of permanent histologic sections, about 20 percent of papillary cancers are multiple tumors thought to represent intrathyroidal metastases, but with meticulous study more small tumors (up to 80 percent in some studies) are usually apparent within the gland (Mazzaferri 1991). Some 5-10 percent of the primary tumors that occur without known exposure to radiation invade the thyroid capsule, growing directly into surrounding tissues, thus increasing both the morbidity and the mortality of papillary carcer (Mazzaferri and Jhiang 1994; Emerick and others 1993). The most commonly invaded structures are the neck muscles and vessels, recurrent laryngeal nerves, larynx, pharynx, and esophagus—but tumors can extend into the spinal cord and brachial plexus. At the time papillary cancer is diagnosed, about 40 percent of adult patients have metastases to regional lymph nodes and about 5 percent have distant metastases, usually to the lung (Mazzaferri 1991).
Mortality rates for adults with papillary thyroid cancer are generally less than 10 percent over several decades after initial therapy (Mazzaferri 1993b). Cancer-specific mortality rates in adults with papillary cancer are about 5 percent at 10 years and slightly less than 10 percent at 20-30 years after treatment; the 5-year survival rate is only about 50 percent for patients with distant metastases (Dinneen and others 1995; Mazzaferri and Jhiang 1994; Mazzaferri 1991; Hay 1990). As is characteristic of many cancers and other diseases, cancer-specific mortality rates are progressively higher for patients over age 40 (Figure 3.4) and among persons with more advanced tumor stages at the time of diagnosis.
Thyroid Cancer in Children Not Exposed to Radiation
Thyroid cancer that occurs spontaneously has somewhat different features in
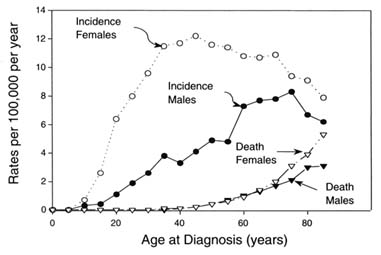
FIGURE 3.4 Incidence and cancer-specific mortality rates for thyroid carcinoma. Drawn from the data published by Kosary CL et al. 1995. SEER Cancer Statistic Review, 1973-1992: Tables and Graphs. National Cancer Institute. NIH Pub. No. 96-2789, Bethesda, MD.
young children than it has in adults. In children, it is almost always papillary and usually is at a more advanced stage at the time of diagnosis. Papillary cancer in children more frequently invades beyond the thyroid capsule, and it metastasizes to regional lymph nodes in almost all cases (Hung 1994; Robbins 1994; De Keyser and Van 1985). For example, in a study of 98 children with differentiated thyroid cancer (Travagli and others 1995), lymph node involvement was seen in 88 percent of children at the time of diagnosis, and invasion of the thyroid capsule had occurred in 59 percent.
Distant metastases also are more frequent in children than they are in adults with differentiated thyroid cancer. In some series, up to 20 percent of children have distant metastases at the time of diagnosis (about 4 times the rate that occurs in adults) and another 10-20 percent of children develop them during the course of the disease (Harness and others 1992; Schlumberger and others 1987; Goepfert and others 1984). In fact, distant metastases are most frequently observed in the youngest patients, especially those who are younger than 7 at initial treatment. There is a high recurrence rate in children after initial surgical removal of tumors.
Despite the aggressiveness of thyroid cancer in children, the long-term mortality rate is only about 2.5 percent, so survival is thus much better for children than it is for adults (Figure 3.2) (Robbins 1994). Because death from recurrent disease can occur many years later, however, the prognosis evolves over
decades. Moreover, the statistics are somewhat misleading. In the study of Travagli and others (1995), although relatively few deaths occurred in children, the standardized mortality rate (SMR) was 6.4; however, 95 percent confidence intervals (CIs) were not reported, and a few deaths might result in an unrealistically high SMR.
Ret Proto-Oncogene and Papillary Thyroid Cancer
Our understanding of the molecular genetics of thyroid cancer has grown substantially in recent years (Fagin 1994b; 1994a; Farid and others 1994). Of particular interest in patients with papillary thyroid carcinoma, and especially in children who have been irradiated, are the genes on chromosomes 10 and 17 involved in paracentric inversions or translocations that result in the activation of the tyrosine kinase domain of the ret proto-oncogene. This is the most common event in papillary thyroid cancers occurring naturally (PTC1) and among those in children after the Chernobyl accident (PTC3).
Normally, ret is not expressed in thyroid follicular cells and its promoter is thus inactive. In papillary thyroid cancer, but not in other thyroid neoplasms, the tyrosine kinase domain of ret is turned on and activated by a paracentric inversion on chromosome 10 involving ret and another gene, H4, producing PTC1 (papillary thyroid cancer 1) (Grieco and others 1990). Two other genes are similarly rearranged with ret: RI, which codes for a subunit of the receptor-associated Gs protein that forms PTC2 (Santoro and others 1994), and ELE1, to form PTC3 or PTC4 (Fugazzola and others 1996; Klugbauer and others 1996; Jhiang and others 1994).
Ret proto-oncogenes have been detected in 11-59 percent of naturally occurring human papillary thyroid cancers, depending on the means of detection and the population studied (Williams and Tronko 1996). The most common rearrangement among patients with sporadic tumors is PTC1 (Jhiang and Mazzaferri 1994), while PTC3 is the most common in children from the area around Chernobyl who developed thyroid cancer.
RADIATION AND THYROID CANCER
Thyroid Cancer from External Radiation Exposure
Studies evaluating the risk of thyroid cancer from radiation exposure have credence insofar as they use reasonably accurate dosimetry (calculation of radiation doses to the thyroid), have substantial numbers of persons in the dose range of interest (for this population, low to moderate doses), have a reasonably long follow-up period, and have a high follow-up rate. The statistical power and precision of such studies, which are important in weighing the study results, are positively related to the number of thyroid cancers observed and to the mean dose.
Summary results of the seven principal cohort studies of thyroid cancer incidence
among those irradiated externally before the age of 20 years are given in Table 3.2. The study of Japanese atomic-bomb survivors has an appreciable number of subjects in the low-to-moderate dose range, as do several medical irradiation studies. Some studies include people who received doses over a period of time rather than during a single episode.
This review concentrates on data concerning irradiation before age 20 and its long-term consequences. In the Japanese atomic bomb study (Thompson and others 1994), a strong effect of age at exposure on thyroid cancer incidence was seen, such that the excess relative risk (ERR) per Sv was 9.5, 3.0, 0.3, and -0.2 at ages 0-9, 10-19, 20-39, and 40+ y, respectively. (See Glossary for an explanation of ERR and other technical numbers in this chapter.) This provides compelling evidence that thyroid cancer risk is inversely related to age at irradiation and that there is little, if any, cancer risk from irradiation after age 20. The studies of radiotherapy for tinea capitis (ringworm of the scalp) in Israel (Ron and others 1989) and for enlarged tonsils in Chicago (Schneider and others 1993) also reported inverse relationship between age at exposure and cancer risk, although these studies had more restricted age ranges.
In the study of cancer incidence among Japanese following atomic-bomb
TABLE 3.2 Thyroid Cancer ERR and EAR for Cohort Studies with Acute External Irradiation before Age 20
|
Study (Reference) |
Mean Dose (Gy) |
Observed/Expected Cancers |
ERR per Gy (95% CI)a |
EAR per 104 person-year Gya |
|
A-bomb (<15 y at exposure) (Ron and others 1995; Thompson and others 1994) |
0.26 |
40/19.2 |
4.7 (1.7,11) |
2.7 |
|
Enlarged thymus (Shore and others 1993) |
1.36 |
37/2.8 |
9.1 (3.6,29) |
2.6 |
|
Tinea capitis (Ron and others 1989) |
0.09 |
44/11.2 |
32.5 (14,57)b |
7.6 |
|
Enlarged tonsils (Schneider and others 1993) |
0.59 |
309/125 |
2.5 (0.6,26) |
3.0 |
|
Lymphoid hyperplasia (Pottern and others 1990) |
0.24 |
13/~2.2c |
~20 (9.5,37) |
15.1 |
|
Skin hemangioma (Lundell and others 1994) |
0.26 |
17/7.5 |
4.9 (1.3,10) |
0.9 |
|
Skin hemangioma (Lindberg and others 1995) |
0.12 |
15/8.0 |
7.5 (0.4,18) |
1.6 |
|
a Both ERR and EAR estimates were based on dose-response analyses. b When an indicator variable for irradiated vs. control group was included, the ERR slope dropped to 6.6 per Gy. c Value estimated for this tabulation from the data available. |
||||
exposure (Thompson and others 1994), 132 thyroid cancers were observed among those with thyroid doses less than 10 mSv. There was a linear dose-response relationship (p < 0.001) with no significant nonlinearity (p = 0.17). As noted above, the excess risk caused by radiation was confined mainly to those under age 20 at the time of the bomb; the ERR was 0.10 (95 percent CI, -0.23 to 0.75) among those irradiated at age 20 or above. Among those under age 20 at irradiation, there were 59 thyroid cancers in the group receiving 10 mSv and 25 among those with <10 mSv. The excess risk was statistically significant for ages 0-9 and 10-19 at irradiation. The background incidence was about 3 times as high for females as for males and it was 2.5 times as high among those who received biennial examinations in the Adult Health Study (AHS) than it was among those who did not; but the radiation dose-response slopes were similar by gender (p > 0.5) and AHS status (p > 0.4).
A study in Israel of 10,834 children x-irradiated for tinea capitis found 43 thyroid cancers (RR, 4.0; 95 percent CI, 2.3-7.9) (Ron and others 1989). A dosimetric study for this group showed the average dose was about 0.09 Gy (9 rad) (Werner and others 1968), which has been supported by two other studies (Harley and others 1976; Lee and Youmans 1970). The 1968 study contributes strong evidence for an effect at a relatively low dose. A much smaller study of patients irradiated for scalp ringworm found no substantial excess of thyroid cancer (2 observed, 1.3 expected), but the two studies are marginally compatible statistically (p = 0.07 for the difference in risks after adjusting for gender and dose differences) (Shore 1992).
A Chicago study of 2,634 patients who received x-rays for enlarged tonsils showed a statistically significant excess of thyroid cancer after a mean dose of 0.6 Gy (60 rad) (based on 309 cancers) (Ron and others 1995; Schneider and others 1993). Follow-up in this study averaged 33 years. It is the only cohort study of radiogenic thyroid cancer that has included repeated thyroid screening over a period of years. Study limitations include the lack of an unexposed control group with a comparable intensity of screening and uncertainties in the thyroid doses.
A smaller study of x-irradiation for lymphoid hyperplasia (Pottern and others 1990) had a 29-year follow-up of 1,590 irradiated patients and a thyroid examination program. It showed an excess of thyroid cancer (13 cases) after an average thyroid dose of 0.24 Gy (24 rad).
A cohort of 2,657 infants x-irradiated for enlarged thymus gland has been followed an average of 37 years (Shore and others 1993). The mean dose was about 1.4 Gy (140 rad), but 56 percent had doses <0.5 Gy (50 rad). There was a strong dose-response association over the dose range. A statistically significant dose-response association was found even when the dose range was limited to <0.3 Gy (30 rad), but the limited statistical power precluded seeing a dose-response association at <0.2 Gy (20 rad). Thyroid cancer risk was elevated out to at least 45 years after exposure.
In Stockholm, Sweden, a cohort of 14,351 infants who were treated (mostly)
with radium-226 for skin hemangiomas has been followed up for an average of 39 years by the Swedish tumor registry (Lundell and others 1994). The mean age at treatment was 6 months. The dose ranged from <0.01 Gy (1 rad) to 4.3 Gy (430 rad) with a mean of 0.26 Gy (26 rad). There was an elevated risk of thyroid cancer (standardized incidence rate [SIR], 2.28; 95 percent CI, 1.3-3.7) based on 17 cancers. The thyroid cancer excess persisted at least 40 years after exposure.
A study was conducted in Gothenburg, Sweden, of 11,807 infants treated with Ra-226 for hemangiomas of the skin and followed up for an average of 31 years by the Swedish tumor registry (Lindberg and others 1995). The median age at treatment was 5 months. The mean estimated thyroid dose was 0.12 Gy (12 rad). An excess of thyroid cancer (SIR, 1.88; 95 percent CI, 1.05-3.1) was found based on 15 thyroid cancers. One limitation of that study and the study of Lundell and others (1994) is that thyroid cancers were not ascertained until 1958, when the Swedish tumor registry began, even though some of the patients were treated as early as the 1920s. Hence, some thyroid cancers were probably never included.
A case-control study of thyroid cancer nested within a cohort study of second malignant neoplasms among childhood cancer survivors has been reported by Tucker and others (1991). There was an excess of thyroid cancer, but many of the thyroid doses were greater than 10 Gy (1000 rad). The dose-response curve plateaued, apparently because of cell killing related to the high exposures; thus, the risk estimate is of questionable applicability to low-exposure studies.
Ron and others (1995) conducted a pooled analysis (based on the raw data) of 5 of the major cohort studies of thyroid cancer among those given external radiation at less than 15 years of age. The combined data included 436 thyroid cancers. The pooled ERR was 7.7 per Gy (95 percent CI, 2.1-28.7). The wide CI occurred because of heterogeneity among the studies in risk estimates, which necessitated a random-effects model; the corresponding fixed-effects model yielded 95 percent CI of 4.9-12.0. The pooled excess absolute risk (EAR) was 4.4 per 104 person-year Gy (95 percent CI, 1.9-10.1). The ERR was marginally (p = 0.07) higher among females than among males. it peaked about 15 years after exposure but was still elevated 40 or more years after exposure. The ERR after fractionated exposure was about 30 percent less than after a single exposure, but the difference was not statistically significant. Although the test for curvilinearity was not statistically significant, the data suggested that a linear fit somewhat underestimated the risk at lower doses and overestimated it at higher doses.
Several case-control studies have been performed to determine the effects of medical diagnostic irradiation on thyroid cancer rates (Hallquist and others 1994; Ron and others 1987; McTiernan and others 1984). Of these, only one (Inskip and others 1995) used objective information rather than patient reports of diagnostic irradiation, with their potential for recall bias. Inskip's group found no association between thyroid cancer and the number of x-ray examinations of
the head, neck, and upper spine (trend, p = 0.54) or the number of examinations of the chest, shoulders, and upper gastrointestinal tract (p = 0.50), nor was there an association for diagnostic x-ray examinations before 1960, when doses were probably much higher.
Thyroid Cancer from Exposure to Radioactive Iodine
The association between exposure of the thyroid gland to external ionizing radiation and the development of thyroid cancer is well documented for young children but not for older children or for adults (Ron and others 1995; Shore and others 1993). Whether internal radiation to the thyroid from radioiodine causes thyroid cancer in humans was, until recently, less certain, although it has been long recognized to induce thyroid cancer in animals (NCRP 1985; Lindsay and Chaikoff 1964). There is now strong evidence from Chernobyl that children exposed to radioiodine develop thyroid cancer at higher than usual rates.
Studies Other than Chernobyl
Table 3.3 summarizes information from studies of diagnostic, therapeutic, and fallout exposure to I-131. Hall and others (1995) have followed 34,104 patients who were administered I-131 for diagnostic purposes, but only a small
TABLE 3.3 Thyroid Cancer Excess Relative Risk (ERR) and Excess Absolute Risk (EAR) following Exposure to Iodine-131 before Age 20
|
Study (Reference) |
Mean Dose (Gy) |
Observed/Expected Cancers |
ERR per Gy (90% CI) |
EAR per 104 Person-Year Gy |
|
Swedish Diagnostic 131I (Hall and others 1995) |
1.5 |
2/1.4a |
0.3 (<0-2.7) |
0.2 |
|
FDA Diag. 131I (Hamilton and others 1989) |
~0.8 |
4/1.4b |
2.3 (<0-23) |
0.4 |
|
Utah 131I Fallout (Kerber and others 1993) |
0.098 |
8/5.4 |
7.9 (<0-16) |
2.1 |
|
Marshall Islandsc (Robbins and Adams 1989) |
12.4d |
6/1.2 |
0.3 (0.1-0.7) |
1.1 |
|
Juvenile Hyperthyroidisme |
~88 |
2/0.1 |
0.3 (0.0-0.9) |
0.1 |
|
a Based on patients whose 131I examination was not for suspicion of thyroid tumor. One additional case was detected among those who had been examined for possible thyroid tumor (total observed/expected = 3/1.8). b The result given is based on their relatively small control group. The expected value based on population rates was 3.7, and the ERR was 0.1 (<0-2.0) with EAR = 0.05. c Highly exposed group of 235 persons only. d Over 80% of this dose was from short-lived radioiodines and external radiation rather than 131I. e Composite of 9 studies. |
||||
percentage of those patients were under age 20 at the time of exposure. About 10,800 were being examined for suspicion of thyroid tumor, and they showed a subsequent excess of thyroid cancer. Among those not being examined for suspicion of thyroid tumor, the average thyroid dose was about 0.7 Gy (70 rad), and no excess thyroid cancer was subsequently found (standardized incidence rate [SIR], 0.75; 95 percent CI, 0.5-1.1). Of particular interest was the subset of 1,764 patients exposed before 20 years of age but not in evaluation for suspected tumors, for whom the mean dose was about 1.5 Gy (150 rad). Among this group there were 2 thyroid cancers (SIR, 1.38; 95 percent CI, 0.2-5.0). It should be noted that fewer than 400 of these subjects were exposed before age 10, whereas the external radiation studies included many subjects exposed in the first decade of life.
A study conducted by the U.S. Food and Drug Administration (Hamilton and others 1989) reported a small excess of thyroid cancer among 3,503 juveniles given diagnostic I-131. The thyroid doses ranged from <0.1 (10 rad) to >10 Gy (1000 rad), with a median dose of about 0.35 Gy (35 rad) and a mean dose of about 0.8 Gy (80 rad). When compared with the unirradiated control group of 2,594 patients under other diagnostic modalities, there appeared to be a small excess of thyroid cancer (observed/expected, 4/1.4, nonsignificant), but when compared with general population rates there was no excess (4/3.7). One uncertainty in the study is the question of whether some of the diagnostic I-131 procedures were performed because of a suspicion of thyroid tumor.
Results for the total number of juvenile patients from several studies of I-131 therapy for hyperthyroidism are shown in the last line of Table 3.3. The excess of subsequent thyroid cancer was not statistically significant in any of these studies, which suffer from having rather loosely defined cohorts and follow-ups of variable quality.
In addition to studies of medical exposure to I-131, several studies have looked at those exposed as a result of exposure to radioactive fallout from nuclear weapons tests. One early concern about I-131 exposure resulted from the BRAVO hydrogen bomb test in the Marshall Islands in March 1954. The inhabitants of the northernmost atolls were exposed when the wind direction changed unexpectedly. A total of 253 persons were on 3 heavily exposed atolls (Rongelap, Ailinginae, and Utirik). Recent reports (Cronkite and others 1997; Howard and others 1997) indicate that 10 clinically significant thyroid carcinomas (plus 7 occult carcinomas) have been detected in this population, in comparison with 2 thyroid cancers found in a group of 227 unexposed persons. However, this might not be a valid comparison because in the unexposed group ''some have not been seen for many years; others were added as recently as 1976" (Howard and others 1997). No thyroid surgery has been performed on this group since 1985 (the exposed group included surgery through 1990). Results in the unexposed group were not broken down by age so as to permit a comparison of those exposed before adulthood. The results from an earlier report (Robbins and Adams 1989) that did have an age breakdown therefore are given in Table 3.3. The Marshall
Islands data are of limited value in assessing the effects of I-131 exposure because more than 80 percent of the dose was from short-lived radioiodines and gamma radiation on these close-in atolls (Lessard and others 1985) rather than from I-131, and there were large uncertainties in the doses. In addition, many of the exposed subjects were put on thyroid suppression therapy beginning in 1965, but with variable compliance.
In 1987, a study was reported assessing whether there was an negative association between thyroid nodule prevalence on various Marshall Islands and the Bikini Atoll where shot BRAVO was detonated (Hamilton and others 1987). Residents on 14 atolls (n = 2,273) were given a thyroid screening, and information was obtained on where they lived at the time of the BRAVO shot. The researchers found a statistically significant association between distance and thyroid nodule prevalence, with additional accuracy of prediction if they added an assumed fallout cloud vector that initially went east, then southeast. A strength of the study is that the subjects had a second thyroid examination by a "blinded" examiner, and there was a good coefficient of agreement between the two examiners' results (kappa = 0.80). Weaknesses of the study include its reliance on palpation, the lack of dose information (risk estimates could not be computed), and the fact that the significant results were appreciably driven by the high nodule rates on the Rongelap and Utirik atolls, where much more thyroid screening occurred (nodules found in the past were included).
The prevalence of thyroid nodules has been investigated among residents of the island of Ebeye (Kwajalein Atoll); the island contains about one-quarter of the Marshall Islands population with former residents of many atolls now living there. Takahashi and others (1997) used palpation and ultrasound to examine 815 persons living in the Marshall Islands at the time of the BRAVO shot. Another 247 born after BRAVO but before the Bikini tests ended in 1958 and 260 born after 1958 also were examined. The researchers ascertained where each person in the first group resided at the time of the BRAVO shot. A marginal association (p = 0.08) was found for distance from Bikini and the prevalence of palpable thyroid nodules for the atolls that Hamilton and colleagues (Hamilton and others 1987) had used, and a similar association (p = 0.06) was found using all the atolls in their study. The results were slightly weaker for all nodular goiter (which apparently meant all nodules >2 mm in diameter detectable either by palpation or by ultrasound), with p-values of 0.07 and 0.12, respectively. Because there was thought to be reasonable similarity in the geographic distribution of cesium-137 and I-131, they correlated the mean Cs-137 measurements for the atolls in their study with thyroid nodule prevalence rates for those atolls. There was no association, calling into question the meaning of the Hamilton and others (1987) findings (although a limiting factor in the interpretation is the relatively low statistical power of this study because of the modest sample size). In addition, Takahashi and others (1997) found little association between the Cs-137 measurements and distance from Bikini, probably indicating that wind patterns, rainouts, and other
factors were important influences on dose. More information from that study will be forthcoming; an additional 2,000+ Marshall Islanders have been examined and data analysis is in progress (Trott and others 1998).
In Utah, there have been two rounds of thyroid examinations (1965-1967 and 1985-1986) of a cohort of schoolchildren exposed to radioactive fallout, including I-131, from the nuclear-bomb testing in Nevada (Kerber and others 1993). The first round of screening included 4,818 schoolchildren. After excluding those who lived in states other than Utah, Nevada, or Arizona in 1985-1986, those with nonwhite or Hispanic ethnicity, and those with a history of radiotherapy, 3,180 of the original group remained of those screened in the first round, 2,473 were examined in the second round. The mean thyroid dose for the cohort was estimated at 0.098 Gy (9.8 rad) (Stevens and others 1992); the mean for the Utah subset of the cohort was 0.17 Gy (17 rad). It could be worth noting that the Utah study applied modeling formalisms and parameter definitions similar to that of the NCI (NCI 1997a) study, except to the assessment of individual doses. A complete description of the dose reconstruction methods for the Utah study can be found in Simon and others (1990).
The composite of the two rounds of screening in Utah plus any interim thyroid diagnoses showed 8 thyroid cancers in the irradiated group; about 5.44 would have been expected (Kerber and others 1993). The dose-response analysis was not statistically significant, but the central risk estimate (ERR, 7.9 per Gy) was similar to the pooled estimate derived from studies of external radiation. The strengths of the study include its careful effort at dose reconstruction, the use of information on childhood milk consumption, and the sophisticated statistical analyses that incorporate the joint prevalence-incidence data and the uncertainties in dosimetry. Its limitations include the small number of thyroid cancers detected with the consequent low precision of the results. Palpation was used as ultrasonography was not routinely available for screening. The screening examiners and the physician who ordered further diagnostic tests were not totally blinded in that they knew whether the subject was from a high or low exposure area, though they did not know the subject's dose status.
Data from Studies of the Chernobyl Accident
Results from studies prior to the 1986 nuclear reactor accident in Chernobyl were suggestive of a link between I-131 exposure and thyroid cancer but studies following the accident are generally regarded as conclusive. Reports from Ukraine, Belarus, and, to a lesser extent, Russia indicate a substantial excess of thyroid cancer among children exposed to I-131 fallout from the Chernobyl nuclear reactor accident in 1986. The increased incidence of childhood cancers in the Chernobyl region began 4 years after the incident, which released very large amounts of I-131, other short-lived radioiodines, and other radioisotopes including cesium, xenon, krypton, and strontium (Becker and others 1996). The youngest
children diagnosed with thyroid cancer were only 4 years old at the time of diagnosis and were in utero at the time of the accident. The peak occurrence of thyroid cancer between 1990 and 1994 was for 8-year-old children in Ukraine and 9-year-old children in Belarus (Becker and others 1996). This is considerably younger than the usual age of onset (14 or older) for naturally occurring thyroid cancer elsewhere in Europe and the United States during the same period (Pacini and others 1997; Williams and Tronko 1996; Zimmerman and others 1994). Normally, thyroid cancer in young adults occurs predominantly in females, but after the Chernobyl accident the female-to-male ratios were below 2 (Pacini and others 1997; Becker and others 1996; Williams and Tronko 1996; Fuscoe and others 1992).
When the reports first began to appear about Chernobyl (Kazakov and others 1992), there was considerable skepticism that the cases represented a real radiation-induced increase. Five reasons for skepticism were cited including
-
Suspicions that additional cases were a function of additional screening and surveillance, a common problem.
-
Belief that I-131 was only weakly carcinogenic compared with externally generated x-rays.
-
Inconsistency with past epidemiological studies with the Chernobyl cases occurring earlier after exposure.
-
Questions about the accuracy of the thyroid dose estimates.
-
Concerns that thyroid cancer incidence could have been enhanced because some regions were borderline goitrogenic because of low iodine concentrations in drinking water and foods.
More recent studies of the aftermath of Chernobyl have addressed these sources of skepticism. Questions about dosimetry remain, but a remarkably coherent picture of cancer risk related to I-131 exposure has emerged from the Chernobyl studies.
Screening and Surveillance Effect One argument against a substantial surveillance effect is that the proportion of tumors of stage T4 at diagnosis (the tumor had broken through the thyorid capsule and invaded surrounding tissue) was unusually high, some 40 percent of cases in Belarus (Kazakov and others 1992). Diagnosis at such a late stage in the development of the tumor is consistent with the claim that most cases were ascertained as a result of a visit by the child to a doctor and not through screening programs. In addition, studies also show a very marked decline in incidence rates to preaccident levels in children born after the end of 1986, even though screening is still common (Stsjazhko and others 1995). This indicates that the increase seen in those who were young at the time of the accident is related to some short-lived initiation process operative at the time of the accident, which is consistent with the increase being caused by exposure
to the isotopes of iodine. In addition, because Belarus is an iodine-deficient area with a marked prevalence of goiter (Gembicki and others 1997; Nikiforova and others 1996), a system of surveillance for thyroid abnormalities was in place in schools at the time of the accident. This consisted of regular visits to schools by an endocrinologist to conduct palpation of the neck. There is no evidence that screening with ultrasound was widespread until 1991-1992. According to Williams and others (1996), 13,000 children had been screened in the Gomel region of Belarus by the end of 1994. Between 1986 and the end of 1994, 178 cases of childhood thyroid cancer were diagnosed in the same region. It is, therefore, clear that screening, even at that late date, was not the primary means of case ascertainment.
Relative Carcinogenic Effect of I-131 Among the reasons posited for the smaller carcinogenic potential of I-131, compared with externally generated x-rays, was the absence of an excess of thyroid cancer in the populations studied after the diagnostic administration of I-131. The results of a pooled analysis of 5 epidemiologic studies on infants, children, and adolescents irradiated with externally generated x-rays (Ron and others 1995), show that there is a steep decline in sensitivity, as measured by ERR, to cancer induction by x-rays with age at exposure. It was already known from the survivors of the atomic bombings in Japan that young adults were relatively insensitive and those over the age of 40 at small risk of thyroid cancer induced by the external gamma-rays from the bomb. Because I-131 is rarely given to children and only occasionally to adolescents, the lack of thyroid cancer associated with diagnostic administration of I-131 can be explained by the small number of children in the surveys and the insensitivity of the adult population (Ron 1996). As will be shown below, it is in fact the data accruing from the follow-up to the Chernobyl accident that are most likely to resolve this long-standing question.
Timing of Cases It has been claimed that too many cases occurred after Chernobyl too soon after exposure. In the pooled analysis of 5 non-Chernobyl studies cited earlier (Ron and others 1995) 2 cases in 81,000 person-years of follow-up were diagnosed within 5 years of exposure. In the Gomel region of Belarus 15 cases were diagnosed in the first 5 years in about 360,000 exposed (EP Demidchik, personal communication). The rates of appearance are therefore 25 and 8 per 1 million person-years, respectively, in the pooled studies and from Chernobyl. In view of the small numbers in the pooled study and the nonidentical dose distributions, there probably is no significance in the factor-of-3 difference.
Dosimetry Several measurements of activity in the thyroid were made in some settlements, but these were usually single measurements from which doses had to be derived using an assumed retention function. Attempts to relate I-131 activity to that of other longer lived nuclides, such as Cs-137, have not been
successful, probably because of the differential release of the nuclides over the 10 days of the incident. The authenticity of such dosimetric estimates as there are can best be judged by the coherence of the estimates of risk.
In an analysis of Chernobyl thyroid cancer risk that was more quantitative than earlier studies (Jacob and others 1998), average thyroid doses were estimated for children in nearly 6,000 settlements in Ukraine, Belarus, and Bryansk, Russia. The authors estimated that, at least in Ukraine, the 95 percent CI around the imputed settlement childhood thyroid doses was about a factor of 2 above and below the estimated doses. The mean settlement doses were then used to derive average thyroid doses for children in various regions, which in turn were regressed on thyroid cancer rates. The analysts reported a good fit to a linear dose-response association, with a slope indicating an EAR of 2.3 (95 percent CI, 1.4-3.8) per 104 person-year-Gy. The EAR is about half as large as that reported in the pooled analysis of thyroid cancer after childhood external irradiation (Ron and others 1995), but both estimates are statistically compatible. Nevertheless, if one considers that there may well be at least a modest surveillance effect, then the difference would be somewhat larger if numerical account could be taken of the fact that this population with high surveillance is being compared with populations from several external radiation studies in which there was little special surveillance. Because background thyroid cancer rates are very low for young people, the ERRs in this study were very high (22-90 per Gy) and are probably not a good basis for projecting risk as the group ages.
Measurements of iodine activity in the thyroid made in the few months after the accident in Belarus indicate doses ranging up to several Gy (OECD 1996). As in the case of the weapons testing in Nevada, the dominant contribution to dose from radioiodine is from I-131 transmitted from ground deposition on pasture through the food chain in milk. The shorter lived isotopes of iodine, relatively less abundant in fallout from a nuclear reactor than from an atomic weapon, are estimated to contribute less than 15 percent to the thyroid dose for those exposed after Chernobyl. I-131 thus seems overwhelmingly implicated in the cases arising after the Chernobyl accident.
A survey of activity in the thyroids of children living in the Gomel region of Belarus indicated that, for children aged 7 years or younger, the average absorbed dose was about 1 Gy (100 rad) (OECD 1996). About 162,000 such children were resident in Gomel at the time of the accident. The risk factor derived from the pooled study (Ron and others 1995) is 4.4 cases per 104 person-year-Gy. For 162,000 such children exposed to 1 Gy there should be about 71 cases per year averaged over the some 40 or more years during which the expression of the cancer takes place. Since the accident and up to 1995, there have been 122 cases in children aged 7 and younger, the greater proportion occurring since 1991. Thus, the average rate over this period is about half that expected in the longer term. This crude estimate is consistent with a recently published study (Jacob and other 1998) of children under 15 at the time of the accident in settlements in the
affected countries. The pooled data EAR is 2.3 (95 percent CI, 1.4-3.8) per 104 person-year Gy. This point estimate is about half the point estimate for the 5 pooled studies of children exposed to external radiation (Ron and others 1995). However, this estimate is influenced by a study from Israel; were this study excluded, the point estimates would be nearly coincident. In any event, the estimate from Chernobyl lies within the 95 percent CI for the x-ray studies (Ron and others 1995), which indicate a peak in incidence rate some 15-19 years after exposure. Estimates for thyroid cancer among the Chernobyl population could be expected to increase, rather than decrease, with time.
The concordance between what is seen after Chernobyl and what is understood from epidemiologic studies of children treated with x rays is confirmed by another approach to determining the relationship between dose and effect. By comparing the cumulative incidence of thyroid cancer in those under the age of 15 at the time of the accident with the cumulative incidence in children irradiated for enlarged thymus gland in the United States (Shore and others 1993), it can be deduced that children in the Gomel region received from I-131 the equivalent of about 1 Gy (100 rad) of externally generated x rays. The average dose to children under the age of 8 is reported to be 1 Gy (OECD 1996), indicating an average dose to children under 15 of about 0.8 Gy (80 rad). Allowing for the early stage in the expected evolution of solid cancers after exposure to radiation, there is little room for a very markedly reduced efficiency of cancer induction by I-131 compared with that by externally generated x rays.
Contribution of Dietary and Other Factors Finally, the question arises whether the effects of radiation have combined with factors such as iodine deficiency and lifestyle to increase the extent to which cancer has been observed in the countries adjacent to Chernobyl. Dietary iodine deficiency is evident in the regions surrounding Chernobyl (Nikiforova and others 1996). It could increase the effectiveness of I-131 both by increasing the uptake of radioactive iodine and by accelerating the appearance of initiated disease through the increased expression of thyroid-stimulating hormone (TSH). The first of these effects seems unlikely to be important when iodine is taken into the body in small quantities over a prolonged period, as is the case with environmental exposure. Although Nikiforova and others (1996) failed to find a significant correlation between urinary iodine concentration and TSH concentration in some 5,000 children they studied, elevated concentrations of TSH have been associated with low urinary iodine and goiter prevalence.
About half of the people who live near Chernobyl lead a rural lifestyle and get their milk from private or backyard cows. The short cow-to-consumer interval and the increased possibility of being exposed to the short-lived isotopes of iodine and tellurium-132, the precursor of I-132, may increase the dose relative to people who live in towns. There is, however, no evidence that either of these
factors has had a dominant effect on the expression of thyroid cancer after Chernobyl.
Insofar as can be deduced from the early stages of the increase in thyroid cancer observed after Chernobyl, the risk is not less that 50 percent of the risk attributed to x-ray exposure of the child thyroid and, because of the early stage, could well prove equal. Thus, of the values commonly cited for relative biological effectiveness (RBE) (0.1, 0.33, 0.67, 1.0) or the dose and dose-rate effectiveness factor (DDREF) (10, 3, 1.5, 1.0), for irradiation of the thyroid by I-131, only the latter two (RBE, 0.67 or 1.0; DDREF, 1.5 or 1.0) receive support from the Chernobyl experience.
Histologic and Biologic Features of Thyroid Cancer in Children of Chernobyl Exposed to Radiation from Radioiodine
Most of the thyroid cancers (96-99 percent) that have occurred in children exposed to radioactive iodine from the Chernobyl accident are papillary thyroid cancers, but they seem to be more aggressive than usual. However, naturally occurring childhood thyroid cancers are also more aggressive, but not clearly more lethal, than those in adults (Pacini and others 1997; Becker and others 1996; Nikiforov and Gnepp 1994). There are certain histologic variants of papillary thyroid cancer that seem to occur with higher than usual frequency in the children of Chernobyl exposed to I-131 (Williams and Tronko 1996). In contrast, the histology of papillary cancers that occur after exposure to external radiation does not differ much from those that occur spontaneously, and the long-term outcome of the two is similar (see, for example, Viswanathan and others 1994).
Microscopically, some of the tumors in Chernobyl-exposed children seem to be especially aggressive, as evidenced by intraglandular tumor dissemination (92 percent), thyroid capsular and adjacent soft-tissue invasion (89 percent), and cervical lymph node metastases (88 percent) that occurred at a greater rate than usual. These observations, however, were not blinded as to the source of the tumor, and some bias could have been introduced (Nikiforov and Gnepp 1994). Another study compared post-Chernobyl thyroid cancers in Belarus children and adolescents with those occurring naturally among children in Italy and France (Pacini and others 1997). In the Belarus cases, extrathyroidal extension (49.1 percent, p = 0.001) and lymph node metastases (64.6 percent, p = 0.002) were more frequent than in the cases from Italy and France (24.9 percent and 53.9 percent, respectively). But distant metastases, potentially the more dangerous, were found in 7.8 percent of the Belarus group and in 17.3 percent of the Italy-France group.
The disease in children exposed as a result of the Chernobyl accident appears to have a short latency period, a higher proportion of tumors arising in young children (under age 5 to 8 years), and an almost equal sex ratio. Both young age at
disease onset, which could merely be a reflection of the short follow-up and the fact that young children are most sensitive to the effects of radiation,and radiation etiology appear to increase the aggressive growth of thyroid cancer (Pacini and others 1997; Robbins 1994). It is too soon to determine whether the long-term prognosis for survival in children exposed to radiation during the Chernobyl accident will be different from that for spontaneously occurring papillary cancer, although there are early indications these cancers are more aggressive. Future studies will indicate whether they are more life threatening.
At the molecular level, the radiation-induced tumors appear to be different from those that occur spontaneously. One study (Ito and others 1994) found ret activation in 57 percent (4/7) of thyroid cancer cases from Chernobyl. Another (Fugazzola and others 1995) found it in 66 percent (4/6), and a third study (Klugbauer and others 1995) reported it in 64 percent (9/14) of the cases from Chernobyl. Although there is an increased frequency of PTC rearrangements in young patients and in children unexposed to thyroid irradiation who develop thyroid cancer (Bongarzone and others 1996), it appears that ret activation is more frequent in the Chernobyl cases.
Moreover, chromosome rearrangements forming PTC3 and novel ret/PTC rearrangements seem to be more frequent in the cases from the Chernobyl accident. A larger controlled study (Nikiforov and others 1997) found 76 percent of 38 thyroid cancer cases from Belarus had ret rearrangements, 6 to form PTC1, 1 to form PTC2, and 22 to form PTC3. In comparison, 65 percent of 17 unexposed cases had ret rearrangements. Two variants of PTC3 also have been found. One variant (Klugbauer and others 1995) is a rearrangement lacking one exon of the ELE1 gene. The other variant (Fugazzola and others 1996) is a rearrangement with an additional 93 base pairs derived from the ret gene. A fifth novel ret rearrangement, PTC5, was detected in papillary thyroid cancers of 2 patients exposed to radioactive fallout after Chernobyl (Klugbauer and others 1998). PTC5 features a fusion of the ret tyrosine kinase domain with a sequence identical to that previously described as ret2, which is a transfection artifact in NIH3T3 cells not previously detected in human tumors. The ubiquitous ret-fused gene 5 was found in various normal tissues, including the thyroid gland.
NONMALIGNANT THYROID DISEASE ASSOCIATED WITH RADIOIODINE EXPOSURE
Data on the induction of nonmalignant thyroid disease are inconclusive in the I-131 dose range to which most people were exposed from Nevada Test Site fallout. Additional, possibly more conclusive information on the link between low to moderate doses of I-131 and thyroid diseases other than cancer should soon be available from the Hanford Thyroid Disease Study.
The thyroid is unusually susceptible to radiation exposure through its ability to concentrate radioiodine and because of its anatomic position, which is commonly
in the field of external radiation applied for therapeutic purposes. Acute radiation doses that cause potential cell cycle disturbances (short of immediate cell death) do not cause obvious injury in the short term until the irradiated cells attempt to divide—then they experience mitotic death. The thyroid follicular epithelium has a long turnover time (1-2 years), so evidence of direct radiation injury can be delayed. The degree of impairment of reproductive capability of the thyroid follicular epithelium is related to the radiation dose, with a possible lower threshold between 2 Gy (200 rad) and 4 Gy (400 rad) for this acute injury (Williams 1991). Beyond this, the pituitary axis can compensate for a considerable degree of thyroid injury with increases in TSH production.
It is well documented that I-131 therapy for thyrotoxicosis at radiation doses of 50 Gy (5,000 rad) and above is associated with destruction of the thyroid gland and hypothyroidism (Maxon and Saenger 1996; Cooper 1991). This treatment initially causes an inflammatory response, followed by long-term chronic inflammation, fibrosis, and atrophy, ultimately resulting in thyroid gland failure. According to Cooper (1991), hypothyroidism may be considered an inevitable consequence of radioiodine therapy. Up to 90 percent of patients are affected in the first year after therapy, with a continuing rate of 2-3 percent per year thereafter. The occurrence of hypothyroidism relates to radiation dose, but also to prior status of antithyroid antibodies in irradiated patients, there being a correlation between the occurrence of thyroid autoantibodies both before and after treatment and development of hypothyroidism (Cooper 1991; Lundell and Holm 1980).
High radiation doses clearly can cause direct follicular cell injury, but there is considerable evidence that this is not the only factor involved in radiation-related nonneoplastic thyroid disease. Various forms of primary thyroiditis are considered to have an autoimmune pathogenesis. Both hypothyroidism (including Hashimoto's and atrophic thyroiditis) and some forms of hyperthyroidism (Graves's disease) are caused by closely related genetic and immunologic disturbances, although they are considered distinct diseases with separate defects in immunoregulation. Autoimmune thyroiditis leading to hypothyroidism is related to the presence of autoantibodies directed against some component of the thyroid gland, such as thyroglobulin or thyroid cell microsomes, the latter being considered more significant (Volpé 1991a). In Graves's disease, there is diffusely hyperplastic goiter resulting from production of antibodies directed against the TSH receptors, which cause excessive stimulation of the follicular cells (Volpé 1991b). It is well known that both hypothyroidism and hyperthyroidism associated with autoimmune disease can be induced by exposure to ionizing radiation (De Groot 1988).
After I-131 therapy for hyperthyroidism, the occurrence of hypothyroidism depends on the dose, and as mentioned previously, on the nature of the autoimmune response. There is evidence of persistent thyroid-stimulating antibodies and other autoantibodies for several years after I-131 treatment in some patients (Williams 1991). Hypothyroidism also can occur after external exposure from
radiotherapy when the thyroid is in the field, for example, in patients treated for lymphomas, lymphoblastic leukemia, and various head and neck cancers (Tell and others 1997; Hancock and others 1991; Williams 1991; Fleming and others 1985). In a group of patients with prior irradiation for benign head and neck disorders, some sort of ''radiation thyroiditis" was found in resected thyroids from 10 to 75 years after exposure (Swelstad and others 1977). The foregoing cases were associated with therapeutic doses of radiation generally measured in the tens of Gy, but there is also ample evidence for hypothyroidism induced by somewhat lower doses. Thyroid hypofunction was reported in fallout-exposed Marshall Islanders, starting about 10 years after exposure and was most marked in those who were exposed as children under the age of 6 (Larsen and others 1982). Estimated doses to persons showing thyroid hypofunction ranged from 1.35 to 21 Gy (135 to 2,100 rad). Shorter-lived iodine isotopes (I-132, I-133, I-135) were responsible for a significant component of the dose in this population.
Data from the atomic bomb survivors are conflicting. Nagataki and others (1994) reported a significant, linear-quadratic, dose-response relationship for autoimmune hypothyroidism in 17.3 percent of 2,587 exposed subjects in Nagasaki. The response peaked at about 0.7 Sv (70 rem), but had an apparent increase in the 0-0.5 Sv (0-50 rem) dose range. It was also noted that the prevalence increased with each passing decade after exposure. Another study of atomic bomb survivors in Hiroshima reported a higher rate of hypothyroidism in both males and females in the 0.01 Gy (1 rad) to 0.99 Gy (99 rad) cohort, although paradoxically the antithyroid microsomal antibodies were decreased in this irradiated population (Ito and others 1987). Morimoto and others (1987) reported no association between radiation and hypothyroidism in Hiroshima and Nagasaki survivors who were less than 20 years old at exposure. Yoshimoto and others (1995) reported no statistical association between radiation exposure and chronic thyroiditis in Hiroshima atomic bomb survivors. Fujiwara and others (1994) found no effect of radiation on the prevalence of antithyroglobulin or antithyroid microsomal antibodies in atomic bomb survivors in Hiroshima and Nagasaki.
Reports on the populations exposed to radioiodine after the Chernobyl accident are also of interest. Children exposed to I-131 had increased autoantibodies to thyroglobulin, thyroid hormone, and TSH; antithyroid antibodies were increased in a dose-responsive fashion, including apparent increases at doses below 1 Gy (100 rad) (Vykhovanets and others 1997). It has been suggested that irradiation is associated with increased risk for chronic thyroiditis (Ito and others 1995). The Chernobyl effects are complicated by the presence of iodine deficiency in some affected areas, although Kasatkina and others (1997) report increased antithyroglobulin and antithyroid microsomal antibodies in children with both radiation exposure and poor iodine intake, suggesting a possible interaction.
A study of 297 children under the age of 16 exposed to diagnostic I-131 at an average dose of 3.8 Gy (380 rad) showed an increased risk for developing hypothyroidism up to 14 years after exposure; however, there were no cases of
hypothyroidism in 146 children with a mean thyroid dose of about 0.18 Gy (18 rad) (Maxon and Saenger 1996).
Other studies of relatively low dose irradiation have not confirmed increased nonmalignant thyroid disease risk. Thymic irradiation during infancy resulted in no observable thyroid disease, hormonal abnormalities, or antithyroid antibodies at exposures of 30-1,200 roentgen (R) × rays (Hildreth and others 1987). Irradiation for childhood hemangiomas with a variety of methods, including α, β, γ, and x-irradiation, resulted in no thyroid functional abnormalities at doses ranging from <0.01 to 2.74 Gy (1-274 rad), although thyroid nodules were increased (De Vathaire and others 1993). A study of the Utah "downwinders" exposed to atomic weapons test fallout after birth (mean dose of 0.098 Gy [9.8 rad] with a maximum of 4.6 Gy [460 rad]) showed no excess of nonmalignant thyroid disease (Kerber and others 1993).
There are reports of hyperthyroidism after irradiation of the thyroid gland for different conditions, including breast cancer (Williams 1991), gynecologic cancers (Katayama and others 1985), and Hodgkin's disease (Hancock and others 1991; Loeffler and others 1988). Hyperthyroidism after treatment of goiter with I-131 was associated with increased serum concentrations of TSH receptor antibodies (Huysmans and others 1997). It has been suggested that Graves's disease induced by radioiodine is associated with a release of antigen from the damaged thyroid and the subsequent production of antibodies that stimulate the TSH receptors (Kay and others 1987). It also has been suggested that suppressor lymphocytes in the thyroid might be more sensitive to radiation than are helper cells, increasing the autoimmune response (Williams 1991).
Overall, these data clearly indicate that there is a highly significant association between ionizing radiation exposure and the occurrence of nonmalignant thyroid disease at higher levels of exposure. It also is evident that this risk could extend down into the range of doses below 1 Gy (100 rad). For exposure to I-131, however, Maxon and Saenger (1996) indicate that hypothyroidism from I-131 would be unlikely at doses below 0.1-0.2 Gy (10 to 20 rad).
Thus, the data on nonmalignant disease induction are inconclusive in the dose range to which most people were exposed from fallout. As a result, the panel did not further consider the implications of nonmaligant disease. However, the current Hanford thyroid disease study is evaluating all thyroid diseases, including autoimmune thyroiditis and hypothyroidism and antithyroid antibody concentration, in association with radiation dose. The data from this study will be extremely valuable in analyzing risk for nonmalignant thyroid disease. Depending on the results, NCI should consider how to incorporate such information in any program to communicate about the risks from fallout from the Nevada nuclear weapons test and whether to undertake a formal evaluation of the screening for nonmalignant disease.
THYROID CANCER RISK BASED ON NCI ESTIMATES OF I-131 DOSES
The 1997 NCI report did not attempt to translate the thyroid dose estimates that it derived into risk of thyroid cancer or other thyroid diseases. However, simultaneous with the release of the report, a staff memorandum prepared by Dr. Charles Land presented calculations of lifetime thyroid cancer risk and corrected calculations were presented to the NRC committee in December 1997. (Appendix B presents this memo.) The corrected calculations yield an estimated 11,300 to 212,000 excess cases of thyroid cancer resulting from the Nevada weapons tests.
The Land estimates of lifetime thyroid cancer risk rely on a linear model for ERR of thyroid cancer as a function of two factors: thyroid dose and age at exposure. The statistical model for thyroid cancer (as well as the dose-response estimates used in the calculations) relies on meta-analysis of thyroid cancer caused by external radiation of the thyroid that was discussed earlier in this chapter (Ron and others 1995). Allowance for the possibly reduced RBE of beta-rays from internal exposure to I-131 compared with external doses also was considered in Land's calculations.
The basic features of the model used by Land to derive excess cancer estimates are (1) linearity (even the smallest dose to the thyroid results in some ERR for cancer), (2) additivity of the effects of multiple exposures (over the period of the Nevada atomic weapons tests) on risk, and (3) an assumption that irradiation of the thyroid in childhood leads to an elevated relative risk of thyroid cancer for a person's lifetime. These assumptions together mean that calculations of the excess fraction of thyroid cancer cases for a given age group caused by exposure are given by computing average doses for persons exposed at that age and multiplying the averages by age-specific excess risk estimates.
Table 3.4 gives ERR values per unit dose, according to age at exposure, from Ron and others (1995), and the estimated average dose for each age group from the NCI (1997a) report. The relative risk for thyroid cancer for a person exposed at that age, compared with an unexposed person of the same age and gender, is calculated using an RBE of 0.66, consistent with the concluding estimate of the Land analysis (which also presented estimates based on other RBE values). The committee believes that an RBE of 1.0 would be equally acceptable given the scientific information available at this time.
Table 3.4 also gives lifetime thyroid cancer risk for males and females for each age-at-exposure group and total excess cases within each age group. The lifetime risks are obtained by multiplying the SEER 1972-1992 baseline risk estimates (0.25 percent for males, 0.64 percent females, which are rates over 85 years for all forms of thyroid cancer including those not linked to radiation exposure) by the relative risk for each age-at-exposure group. According to this table, for a woman exposed to 0.1 Gy (10 rads) of I-131 at less than one year of age, the
TABLE 3.4 ERR per Unit Dose, Relative Risk, and Excess Cancer Cases, by Age at Exposure
|
Age at Exposure |
ERR at 1 rada |
Average Dose (rad) |
Relative Riskb |
Percentage Lifetime Risk Male |
Percentage Lifetime Risk Female |
Excess Cancer Cases |
|
<1 |
0.098 |
10.3 |
1.67 |
0.42 |
1.07 |
10,170 |
|
1-4 |
0.098 |
6.7 |
1.43 |
0.36 |
0.92 |
26,993 |
|
5-9 |
0.049 |
4.5 |
1.15 |
0.29 |
0.73 |
9,064 |
|
10-14 |
0.0245 |
2.8 |
1.05 |
0.26 |
0.67 |
2,451 |
|
15-19 |
0.01255 |
1.8 |
1.01 |
0.25 |
0.65 |
748 |
|
>20 |
Negligible |
1.8 |
1.00 |
0.25 |
0.65 |
0 |
|
a ERR values are taken from Ron and others (1995). b Relative risks are computed using an RBE of 0.66 consistent with the analysis of Land (see Appendix B). |
||||||
lifetime risk to age 85 of being diagnosed with thyroid cancer is estimated to increase from 0.64 percent to 1.07 percent, which would still make a diagnosis of thyroid cancer uncommon compared to a number of other cancers.
The period of aboveground testing in Nevada extended from 1951 to 1958, so exposure to I-131 would have occurred over a range of ages for most persons. However, the combination of the linearity and additivity assumptions implies that doses given at specific ages to different people can be combined in calculating excess cancer cases. An infant in 1951 moves to the 5-9-year category by 1958, but is replaced by other infants born in the intervening years, with these two effects essentially canceling out in the computation of excess cases for the <1-year age category. Thus, Land uses the U.S. population age distribution in 1952 and assumes that all exposures occurred in 1952 to approximate excess cases resulting from exposures that were actually distributed over a longer period to a larger number of people.
The Land analysis notes that there are substantial uncertainties in the estimate of ERR at 1 rad and the average dose for each age group. An uncertainty analysis, which allowed for the 95 percent confidence interval (0.021-0.287) reported by Ron and colleagues (1995) for the ERR estimate at 1 rad and a factor of 2 in the uncertainty of average thyroid dose, gave a range for the total excess cases due to exposure of 11,300-212,000. The central estimate implied (but not explicitly stated in that analysis) is 49,000 cases. An independent estimate of the uncertainty in the number of excess cases produced a 95 percent confidence range from 8,000 to 208,000. This latter analysis also included the uncertainty of the RBE for I-131 (F.O. Hoffman, communication to NAS, 20 December 1997). Beyond this uncertainty assessment, the validity of the assumptions of linearity,
constancy of ERR, and the additivity of effects of exposure are all critical to Land's estimate of excess cases. Both linearity and extended elevated risk are consistent with the publications reviewed by Ron and colleagues (1995). However, these data do not provide a clear test of these assumptions at either the range of doses given by NCI (1997a) or over the ranges of time since exposure that are important today. The highest average dose considered in Land's calculations (for infants) is just above the average thyroid dose (9 rad or 0.09 Gy) reported to have an effect on risk in one study. Although excess cases of thyroid cancer were found by the studies analyzed by Ron and colleagues (1995) to continue for long periods after exposure (up to 40 years) with little falling off in ERR, no study has followed exposed subjects over an entire lifetime. Today, the most heavily exposed subjects to I-131 fallout from the Nevada Test Site are nearly 45 years beyond their time of exposure, which is at the outer limits of follow-up of the subjects in the epidemiologic surveys.
Analyses of the thyroid cancer cohorts and case-control studies have shown important heterogeneity in the dose response for different groups of subjects. In reflecting this heterogeneity, Ron and colleagues (1995) were led to use a random-effects model when combining data from the 7 studies analyzed to form an overall estimate of ERR per unit dose. Only one of the studies supports a thyroid cancer effect at dose ranges consistent with average thyroid dose to large birth cohorts from Nevada weapons tests. This study of Israeli children treated with radiation for tinea capitis showed a much stronger dose response than was evidenced in other studies. Although causes of heterogeneous results between studies are difficult to determine, Shore has suggested (1992) that the subjects in the Israel study might have had a higher dose response because of a higher-than-normal prevalence of inherited ataxia telangiectasia (A-T) heterozygosity in the population. This disorder is associated with a defect in DNA repair that could increase sensitivity to cancers of many types in A-T patients and could do the same in the heterozygote. If this study were dropped from the analysis, the estimates in the Land memo would change.
For setting prospective dose limits for radiation protection, the assumption of linearity and the use of linear extrapolation is widespread. For example, in the absence of direct data on health effects at low levels of radiation exposure, major regulatory affiliated organizations (International Commission on Radiological Protection, National Commission on Radiological Protection, Environmental Protection Agency, National Research Council, and others) extrapolate all radiation effects through the zero effect-zero dose point in a linear fashion.
Although opinion leans heavily toward use of linear no-threshold methods of estimating risk for thyroid disease, this concept is not universally accepted. It clearly introduces another uncertainty factor into the calculation of risk. If the dose response were nonlinear, that could produce a lesser or greater response at low doses, and would reduce or increase the overall risk of health effects from exposure to I-131.
Uncertainty also applies both to the assumption of constancy of ERR and to the assumption of additivity of multiple exposures. This committee notes that natural background doses over the 10-year period of testing would present to each person in the United States an average dose that was approximately half of the average dose from the weapons testing (2 rad or 0.02 Gy). The amount of natural background radiation varies geographically, such that, in some areas, the 10-year average could have been lower or higher by 0.01 Gy (1 rad), when radon exposures are ignored, bringing the average dose to 0.03 Gy (3 rad) or 0.04 Gy (4 rad) to the thyroid over the 10 years of testing. Some areas would have been lower and some higher in the calculated fallout dose ranges. It would be informative, therefore, to determine how background radiation would alter total dose, and hence risk, to persons over this 10-year period. Calculations could be done to compare the total doses using the actual background dose for each county to add to the total estimated dose to the average individual in each county, but this would require substantial effort.
Analyses of the risk of thyroid cancer after exposure to ionizing radiation such as those analyzed by Ron and colleagues essentially assume similarity in naturally occurring and radiation related thyroid cancers. Although this chapter has cited histologic evidence suggesting that the differences in thyroid cancers in children exposed from the Chernobyl accident, there are no data yet relevant to effects in adults exposed as children to the I-131 levels associated with the Nevada weapons tests.
Given some of the issues noted above, the committee suggests that DHHS consider some additional analyses to evaluate further the estimated confidence intervals for the risk projections and to improve understanding of the sensitivity of the projections to changes in key assumptions. Such analyses might include (1) use of alternative dose-response models, (2) choice of different average population doses, (3) use of a model of excess relative risk that declines as a function of years since exposure, and (4) exclusion of the tinea capitis study from the Ron analysis.
As indicated above, there are few data to show a statistically significant carcinogenic effect of radiation to an organ or whole body below a dose of 0.1 Gy (10 rad). Epidemiologic studies, which might be helpful, are complicated when estimated doses are low. For example, very large samples are needed to demonstrate an effect. Results of some epidemiologic analyses of the possible effects of I-131 fallout from the Nevada weapons tests are discussed below.
Epidemiologic Analyses Using Cancer Registries
Neither the NCI (1997a) report nor the analyses provided by Land (Appendix B, this report) consider whether there is any epidemiologic evidence of increases in thyroid cancer from exposure to Nevada Test Site fallout. Data from various tumor registries around the country could be useful in reducing the uncertainty in the estimate of the collective excess of thyroid cancer cases. In particular,
analysis of registry data allows for the comparison of thyroid cancer incidence between birth cohorts within the same general locality. The NRC panel had access to three such analyses—one undertaken by the committee, one for the state of Idaho, and one prepared by NCI analysts. In addition, it reviewed the Utah study (Kerber and others 1993) cited earlier in this chapter. That study, which was published in the Journal of the American Medical Association, found more thyroid cancers that would be expected for a nonexposed population and produced a risk coefficient of 7.9 per Gy, almost the same value as calculated by Ron (7.7 per Gy).
To get an idea of the power of epidemiologic studies, consider the use of tumor incidence data from registries reporting (as do the current SEER data) all thyroid cases occurring over the years 1973–1992. Note that, according to Land's analysis, there is a population of 17.6 × 106 who were 0-4 years in 1952, and thus were 21-44 between 1973 and 1992. Persons who were 0-4 years old in 1963 were born during the era of underground testing, and thus may be considered to have been largely unexposed. Their attained ages over the same 1973-1992 period will range from 10 to 33 years. Thus, we may compare, for example, the rates of thyroid cancer incidence among persons aged 25-29 between these two birth cohorts.
The average age of the earlier birth cohort over the time of atmospheric testing (1951–1958) is approximately 3.5 years. From Table 3.4, this corresponds to an average thyroid dose of approximately 0.067 Gy (6.7 rad), which, in turn, corresponds to an estimated excess risk of thyroid cancer of approximately 1.43 for this cohort compared with the later, unexposed birth cohort.
Assuming that the rate of thyroid cancer in the unexposed cohort is 4.5 per 100,000 person-years (SEER data), to have sufficient statistical power (90 percent) to detect such an excess incidence, the monitoring system would have to have covered approximately 617,000 people in each of the birth cohorts. This would yield an expected 199 cases of thyroid cancer in the exposed cohort and 139 in the unexposed cohort. If we use a risk factor 4.3 times smaller than median value of 7.7 per Gy (which gives the lower end of Land's CI), then approximately 9.9 million persons would have had to have been monitored from each birth cohort to have the same power to detect a 10 percent increase. The SEER registries cover about 10 percent of the entire U.S. population, or about 1.8 million persons from the 1948–1952 birth cohort born. Potentially, then SEER registry data could help assess whether past incidence in these birth cohorts is consistent with the higher part of the estimated range of excess risk provided by Land.
For this report, the committee examined thyroid cancer incidence rates by birth cohort using SEER data. It also reviewed an analysis of Idaho registry data and another analysis of SEER data (Gilbert and others, in press for the Journal of the National Cancer Institute ) that used incidence data from 1973–1994 and thyroid cancer mortality data from the 48 contiguous states from 1957 to 1994. The approach of Gilbert and colleagues was to relate county-level geographic variation in thyroid cancer incidence and mortality with the NCI's county-by-county
estimates of thyroid dose. This committee took a different approach. It compared "exposed" versus "unexposed" birth cohorts for differences in thyroid cancer rates. The analyses of Gilbert and colleagues are performed within each birth-cohort separately, so that no specific comparison of "unexposed" versus "exposed'' birth cohorts is given. The two approaches—variation by birth cohort in SEER and in Idaho, and variation by geographical region in SEER and in the mortality data—are complementary, but each is subject to certain limitations. For example, the Idaho analysis is limited because of the small population of the state, which, in turn, limits the numbers of thyroid cancers to relatively small numbers. The birth cohort approach to the SEER data ignores the geographical variation in estimated dose. Also, the interpretation of results may be complicated by external factors such as secular trends in thyroid incidence and by exposure to I-131 from other sources such as the Pacific or Siberian nuclear tests, which continued beyond the time of above ground testing at the NTS. The analysis of Gilbert and colleagues avoids the latter complexity, but introduces other, perhaps more important problems, because county level doses are known with far less certainty than are the average doses for the birth cohorts. Moreover, the analysis of Gilbert and colleagues is much more affected by the results of migration from county to county either at the time of exposure or in later life.
Publicly available thyroid cancer incidence data from 9 tumor registries that make up the SEER program are tabulated in Table 3.5 for the two birth cohorts described above, for the attained ages of 25-29 years, by region (far west and the rest of the country). The far-western registries are in San Francisco-Oakland, Hawaii, and Seattle; the other, not-far-western registries are in Connecticut, Detroit, Iowa, New Mexico, Utah, and Atlanta. The far-western areas were much less exposed to Nevada Test Site fallout than was the rest of the country, so their incidence data are tabulated separately, although considerable migration from eastern to western areas can be assumed to have taken place.
For the registries outside the far west, there is some evidence of about a 10 percent excess of thyroid cancers in the exposed birth cohort, but the estimate is not statistically significant. An evident excess for males in the far-western registries is unexpected, but the numbers of cases, 38 and 28, on which this observation is based are not large. Given the NCI dose estimates, such excess cases are not likely to be attributable to exposure to Nevada Test Site fallout.
Comparisons of thyroid cancer rates in an earlier birth cohort, 1938-1942, with rates among the 1948-1952 birth cohort also are of interest (Table 3.6). The 1938-1942 birth cohort was aged 9-20 during 1951-1958. According to Table 3.4, the estimated excess risk of thyroid cancer would have ranged from 5 to 15 percent for this cohort, compared with more than 40 percent for the 1948-1952 cohort. SEER data allow us to compare rates of cancer at ages 35-39 for these two cohorts (Table 3.6). Again, about a 10 percent excess risk for the 1948-1952 birth cohort is seen in the non-far-western registries, but this is not statistically significant. The data from the far-western registries are not consistent between males
and females; males show 32 percent fewer thyroid cancer cases in the 1948-1952 cohort, and females show a 23 percent increase. Because differences in thyroid cancer incidence in the western registries are less apt to be due to I-131 exposure from the Nevada Test Site than are differences in the other registries, they suggest some difficulties in attributing differences in the non-far-western cohorts to I-131.
Appendix D provides a detailed examination of the thyroid cancer rates in Idaho by birth cohort and county for the period 1970-1996. While several Idaho counties showed elevated rates of thyroid cancer, they did not correlate very closely with estimated thyroid dose. The birth cohort born from 1948 to 1958 showed a 5 percent excess of incident cases compared to earlier and later cohorts, but this increase was not significant with the 95 percent confidence interval as the excess among the counties ranges from -7 to +18 percent. Within the most sensitive of the three birth cohorts examined in the Idaho analysis, there was little evidence of association with county or the county-specific estimate of thyroid dose; however the power to detect a dose response, within the relatively small population of Idaho, is quite low.
The analysis of Gilbert and colleagues relates geographic variation in thyroid cancer incidence and mortality rates in counties from the 48 contiguous states to the NCI estimates of average dose within each county. Overall that analysis finds negative nonsignificant excess risk due to I-131 exposure. However, when the analysis is restricted to those subjects exposed in infancy (age <1 y), a positive association between dose and risk is marginally significant for both thyroid cancer incidence (p = 0.11) and mortality (p = 0.054). The findings for that very narrow age group (<1 y at exposure), however, contrast with those in the group aged 1-5 years at exposure, for which dose responses were estimated to be negative despite the fact that subjects aged 1-5 years ought to be nearly as radiosensitive as the infants (based on findings from the external radiation studies). When the analyses of Gilbert and colleagues were restricted to subjects who were aged 0-4 at any time during 1950-1954 (i.e. those born between 1950-59), a significant association between I-131 exposure and thyroid cancer mortality (ERR per Gy = 12.0, p = 0.005) was detected. No other birth cohort (including those born between 1955 and 1964) showed positive risk estimates for mortality based on the county level dose estimates. Moreover there was no increase in incidence detected in the 1950-1959 birth cohort (ERR per Gy = 0.3, p = 0.66) The fact that mortality but not incidence showed a dose response in this birth cohort is puzzling. There were almost 3 times as many incident cases as thyroid cancer deaths (12,657 vs. 4,602), which would imply that the power to detect increases in risk should be far greater in the thyroid cancer incidence data than in the mortality data.
The impression left by these three studies—birth cohort comparisons of incidence in Idaho, birth cohort comparisons in SEER, and the study of Gilbert and colleagues using geographical variation in SEER incidence and thyroid cancer mortality—is that there is little evidence of widespread increases in risk related to
TABLE 3.5 SEER Thyroid Cancer Incidence Data. Comparisons of Thyroid Cancer Rates at Ages 25-29 Between Birth Cohorts Born in 1948-1952 (Exposed) and 1959-1963 (Relatively Unexposed)
|
Birth Cohort |
Number of 25-29 Year Olds |
Number of Thyroid Cancers |
Approximate Rate per 100,000 Person-Years |
Percentage Excess in Birth Cohort |
p-Value for Excess |
|
Far western registries 1948-1952 (Monitored in 1977) |
|||||
|
Males |
317,724 |
38 |
2.39 |
65.6 |
0.10 |
|
Females |
310,404 |
136 |
8.76 |
8.1 |
0.50 |
|
1959-1963 (Monitored in 1988) |
|||||
|
Males |
387,623 |
28 |
1.44 |
— |
|
|
Females |
367,614 |
149 |
8.11 |
— |
|
|
Other registries 1948-1952 (Monitored in 1977) |
|||||
|
Males |
590,102 |
52 |
1.76 |
9.8 |
0.63 |
|
Females |
601,506 |
257 |
8.55 |
9.9 |
0.28 |
|
1959-1963 (Monitored in 1988) |
|||||
|
Males |
673,052 |
54 |
1.60 |
— |
|
|
Females |
683,896 |
266 |
7.78 |
— |
|
the pattern of exposure of I-131 as described in the NCI report. They suggest that the numbers of excess cases of thyroid cancer due to I-131 exposure from the Nevada weapons tests are likely to be in the lower part of the range estimated in the Land memo. Furthermore, the estimate of only a 10 percent increase in risk between the exposed and nonexposed birth cohorts as detected in SEER, lies in the lower portion of the range of excess cases that was estimated by Land. Translating the 10 percent increase in risk for these cohorts to excess lifetime risk (using the linear constant excess relative risk model) amounts to approximately 11,300 excess cases (using an RBE of 0.6). The range of uncertainty attached to this estimate, however, is itself large, so that the use of registry data beyond what is currently available in SEER is important to pursue and will allow more detailed comparisons of age-specific rates of thyroid cancer. Some registries, such as the one in Connecticut, predate the SEER data years by a considerable margin.
This discussion has emphasized the uncertainties inherent in using the NCI (NCI 1997a) report to determine the likely number of excess thyroid cancer cases caused by exposure of the American public to I-131 fallout from the Nevada Test Site. Nevertheless, based on the other data reviewed in this chapter, the committee finds it reasonable to conclude that some excess cases of thyroid cancer have occurred and will continue to occur as a result of the Nevada weapons tests.
TABLE 3.6 SEER Thyroid Cancer Incidence Data. Comparisons of Thyroid Cancer Rates at Age 35-39 Between Birth Cohorts Born in 1938-1942 (Relatively Unexposed) and 1948-1952 (Exposed)
|
Birth Cohort |
Number of 35-39 Year Olds |
Number of Thyroid Cancers |
Approximate Rate per 100,00 Person-Years |
Percentage Excess in Birth Cohort |
p-Value for Excess |
|
Far western registries 1938-1942 (Monitored in 1977) |
|||||
|
Males |
209,307 |
52 |
4.97 |
— |
|
|
Females |
203,509 |
95 |
9.34 |
— |
|
|
1948-1952 (Monitored in 1988) |
|||||
|
Males |
350,438 |
59 |
3.37 |
-32.2 |
0.05 |
|
Females |
344,993 |
198 |
11.48 |
22.9 |
0.09 |
|
Eastern registries 1938-1942 (Monitored in 1977) |
|||||
|
Males |
388,265 |
59 |
3.04 |
— |
|
|
Females |
407,757 |
182 |
8.97 |
— |
|
|
1948-1952 (Monitored in 1988) |
|||||
|
Males |
584,760 |
102 |
3.49 |
14.8 |
0.4 |
|
Females |
605,706 |
294 |
9.71 |
8.2 |
0.4 |
Estimate of the Number of Cases of Thyroid Cancer that Have Already Been Manifested
The population at excess risk of thyroid cancer from I-131 due to fallout from weapons testing at the Nevada Test Site consists of people born between approximately 1940 and 1957. These individuals are now in their early 40's to late 50's in age, and are more than 40 years past their initial exposures. Reported follow-up of the groups considered by Ron and colleagues (1995) is as yet insufficient to be certain that childhood exposure to thyroid radiation results in continued risk for the remainder of one's lifetime (that is, beyond 40 years from time of exposure). Assuming a constancy of excess relative risk obtains, then the distribution of excess thyroid cases would mirror the age specific rates of this cancer. Table 3.7 gives the fraction of thyroid cancer risk manifested by ages 40-60 for males, females, and the sexes combined, with the computations based upon the incidence rates published by SEER.
It should be noted that when the sexes are combined the expected values are closer to those for females than for males because females have a higher background risk of thyroid cancer. Since the birth cohort most at risk is presently between the ages of 45 and 50 years, a reasonable estimate of the fraction of excess cases that have already occurred is about 45 percent. This calculation, as
TABLE 3.7 Fraction of Expected Thyroid Cancer Cases Occurring by a Given Age.
|
Age (years) |
Females (%) |
Males (%) |
Sexes combined (%) |
|
40 |
33 |
20 |
30 |
|
45 |
42 |
27 |
38 |
|
50 |
52 |
35 |
48 |
|
55 |
61 |
45 |
57 |
|
60 |
69 |
54 |
65 |
are all of the risk calculations in this report, is strongly dependent upon the assumption of a constant excess relative risk throughout a lifetime after early childhood exposure to thyroid radiation. If the excess relative risk declines with attained age, the fraction of cases expressed to date would obviously be different, but in the absence of evidence of such a decline, the committee believes the number, 45 percent, is reasonable as said. Clearly, this value has important implications with regard to the steps that might be invoked to diminish the as yet unmanifested public health consequences of the exposures to I-131.
CONCLUSIONS
Exposure to I-131 as a by-product of nuclear reactions can cause thyroid cancer as shown conclusively by the 1986 nuclear accident in Chernobyl, which resulted in high level exposure for many people. The NCI dose reconstruction model indicates that the level of exposure to I-131 was sufficient to cause and continue to cause excess cases of thyroid cancer. Because of uncertainty about the doses and the estimates of cancer risk, the number of excess cases of thyroid cancer is impossible to predict except within a wide range.
Epidemiological analyses of past thyroid cancer incidence and mortality rates provide little evidence of widespread increases in thyroid cancer risk related to the pattern of exposure to I-131 described in the NCI report. They suggest that any increase in the number of thyroid cancer cases is likely to be in the lower part of the ranges estimated by NCI. The epidemiological analyses are, however, subject to considerable limitations and uncertainties. Given the uncertainties in both the dose reconstruction model and the epidemiological analyses, further epidemiological analyses will be necessary to clarify the extent to which the Nevada tests increased the incidence of thyroid cancer. Pending these studies, it is prudent for DHHS to plan its responses as if excess cases of thyroid cancer have occurred.
Individual-specific estimates of the probability of developing thyroid cancer from exposure to fallout from the Nevada testing program are uncertain to a greater degree than the dose estimates because of the additional uncertainty, in
particular, about the cancer-causing effect of low doses of I-131. Nonetheless, the committee concluded that the Nevada weapons-testing program resulted in I-131 exposure that has increased the usual risk of thyroid cancer for some members of the population, mainly those who were very young and drank milk from a backyard cow or, in particular, a backyard goat. The program of public information discussed in Chapter 5 will inform people about their possible exposure and the risk of thyroid cancer.
ADDENDUM 3: UNDERSTANDING RADIATION RISK FACTORS AND INDIVIDUAL RISK
Radiation cancer risk factors can be expressed in a variety of forms including Relative Risk (RR) and Excess Relative Risk (ERR). Values of these factors have been discussed in the text and appear in Table 3.4 but both are complex mathematical concepts. These factors can be used to derive a more useful figure for an individual exposed to radiation: that being the chance that the person will develop cancer in his or her remaining lifetime following a radiation exposure. This section is intended to elucidate the interpretation and use of these factors for that purpose.
Before proceeding with detailed explanations, it must be noted that there is considerable uncertainty associated with the cancer risk factors as derived from epidemiological studies. Thus, the risk1 of contracting cancer that one might determine from a calculation should be understood to be only an estimate and the true value of their risk may be several times higher or lower.2 In addition, the biological response to radiation exposure is different for every individual. Thus, some individuals will be more susceptible to radiation damage and its effects and others less so. Factors influencing individual sensitivity are presently not sufficiently well understood to be accounted for in individual risk calculations.
One useful expression of risk is the Percentage Lifetime Risk. Lifetime Risk expresses the chance of contracting a cancer within a lifetime (85+ years for a
newborn) or within the remainder of life following a radiation exposure. Because all types of cancers can occur in the absence of radiation, there is a background incidence rate even when no exposure has taken place. Thus, the Percentage Lifetime Risk is never zero, even in the absence of radiation exposure above that from natural background radiation.3 In the case of thyroid cancer, about 0.25 percent of males (1 in 400) and 0.65 percent of females (1 in 153) will have the disease within his or her lifetime for reasons unrelated to radiation exposure (above that from natural background radiation). Thus, without any radiation exposure above that from natural background radiation, the Percentage Lifetime Risk for males and females is 0.25 and 0.65, respectively (see line 6 of Table 3.4).
The chance of a single person developing a disease is a difficult concept for most people to understand because one either contracts the disease or does not. Lifetime Risk can perhaps be better explained by defining it to express the number of people out of each 100 similar people that would develop the disease. The same numerical value of Percentage Lifetime Risk can apply equally well to the chance for an individual to develop cancer, or to the proportion of people out of each 100 that will develop cancer. The interpretation used is largely a matter of individual preference.
In the case of thyroid cancer caused by radiation, adults of 20 years of age and greater at time of exposure are considered to be at negligible risk. Thus, their Lifetime Risk (see column 6 of Table 3.4) is equal to the background incidence of this disease in an unexposed American population (0.25 percent males, 0.65 percent females). The Percentage Lifetime Risk from the Nevada Tests for other age cohorts is shown in Table 3.4 based on the average radiation dose noted in column 3 as computed by NCI. The Percentage Lifetime Risk is, in general, higher for larger radiation doses, higher when exposure occurs at younger ages, and higher for females compared to males at the same age and the same radiation dose.
In Table 3.4, adults over 20 years of age (last line of the table) can serve as a reference group to which other age cohorts are compared. Using that simple idea, a Relative Risk figure (see column 6 of Table 3.4) can be calculated, which simply expresses a multiple of the risk to an unexposed person or the adult. In Table 3.4 for example, the Relative Risk for individuals of age 15-19 years at time of exposure is 1.01 or 1 percent higher than the adult groups for the same average dose as the adults received. This small increase in the Relative Risk does not significantly affect Lifetime Risk which is about 0.25 percent, the same as for older peers.
As mentioned earlier, the Relative Risk increases with increasing dose but is also greater for younger ages of exposure. That effect can also be seen in Table 3.4, which gives higher Relative Risks (always in relation to the adult cohort) for the younger groups who also had higher average doses from the Nevada tests. Higher Relative Risks result in proportionally higher Percentage Lifetime Risks.
For example, those less than 1 year of age at exposure (who received on average 10.3 rad) have a Relative Risk of 1.67. This is the same as saying the risk is 1.67 times the risk of adults (who received an average dose of 1.8 rad). Consequently the Percentage Lifetime Risk for the less than 1 year old group is 1.67 times that of adults, equal to 0.42 percent (males) or 1.07 percent (females).
In lieu of presenting further mathematical detail necessary to manipulate the risk factors, it appears more useful to further discuss only the Percentage Lifetime Risk. This value may be obtained directly from Table 3.4 if certain assumptions can be made. Specifically, the table presents the Percentage Lifetime Risks for individuals who received a radiation dose equal to the average for his or her age cohort as estimated by NCI. Column 5 of Table 3.4 gives those risk values given the assumptions used. The highest risk for a male is 0.42 percent, which is for an exposure of about 10 rad at one year of age. The highest risk for a female would be 1.1 percent.4 The risk of 0.42 percent (male) or 1.1 percent (females) equates to about 1 in 240 males of his age cohort or 1 in 92 females developing thyroid cancer sometime in her life if lived to age 85+.
The disease rates computed above for a 10 rad exposure to a 1 year old are 67 percent higher than the background incidence, which is 1 in 400 males or 1 in 173 females developing thyroid cancer sometime in his or her life. By way of comparison, women have about a 1 in 8 Lifetime Risk to age 85 of being diagnosed with breast cancer and men about a 1 in 5 Lifetime Risk of being diagnosed with prostate cancer.
For the Lifetime Risks presented in Table 3.4 to be applicable to any individual, that person must have received the national average dose for his or her age cohort. For a person to determine the dose with any confidence would require extensive calculations, even then the uncertainty would be great. For those individuals highly concerned about their individual risks, determining relevant doses specifically for themselves is a difficult undertaking. At present, there are few alternatives to completing complex calculations though an alternative method is suggested in Addendum 5.
One method for a person to estimate his or her personal risk would begin with obtaining an estimate of the individual total dose (from all tests) from the NCI Web site. That process is difficult and the concerns expressed in this report about the uncertainty of calculated doses should be borne in mind. Under the assumption that the computed dose accurately represents the exposure of the individual, one's Percentage Lifetime Risk could then be estimated by the following steps:
-
Obtain the Percentage Lifetime Risk from Table 3.4 for the age cohort.
-
Subtract 0.25 (males) or 0.65 (females) (the Lifetime Risk for the adult group) from the value found in step 1.
-
Calculate the ratio of the one's dose estimate to the average dose for the age cohort.
-
Multiply this ratio by the value from step 2 above.
-
Add the adult value for Percentage Lifetime Risk (0.25 for males or 0.65 for females) to the number from step 4 above.
The number calculated by the above five steps would be an estimate of one's individual Percentage Lifetime Risk. It could be interpreted as the percent chance of developing thyroid cancer during their lifetime to 85+ years of age, or the proportion of 100 people who would develop the disease. For example, if the person was less than 1 year old at time of exposure and his or her individual dose estimate was about 50 rad, he or she would calculate a Percentage Lifetime Risk of about 1.1 (males) or 2.9 (females), meaning that the chance of developing thyroid cancer by age 85 would be about 1 percent (males) or 3 percent (females). Equivalently, for this 50 rad dose, it would mean that 1 of every 100 males or 1 of every 35 females like themselves would contract the disease because of the radiation exposure. Relatively few individuals likely received doses as high as 50 rad, thus relatively few persons would have chances of developing thyroid cancer this great.5
Two final points are worthy of note in this discussion of risk estimates. First, for those who are diagnosed with radiation related thyroid cancer, the prognosis, as previously discussed, is good. Second, the individuals that are diagnosed with thyroid cancer can have a relatively high probability that the disease was caused by radiation exposure even if the likelihood that they would get the disease was low. For example, the probability that a cancer is a result of radiation exposure (this probability is termed "probability of causation" or PC) (NIH 1985) can be calculated as [Relative Risk - 1]/[Relative Risk].6 Thus, for the child having received an actual dose of 10.3 rad (see Table 3.4), the PC would equal (1.67 - 1)/1.67 = 0.40. This corresponds to a 40 percent chance the cancer was a result of radiation exposure. This can be compared with a chance of only 0.42 percent (male) or 1.07 percent (female) that they would have developed the disease during their lives following a 10.3 rad exposure at 1 year of age. The PC becomes increasingly higher for larger radiation doses.