4
Implications for Clinical Practice and Public Health Policy
Efforts to measure and understand the implications of the exposure of Americans to radioactive fallout from the atomic bomb tests at the Nevada Test Site in the 1950s raise a variety of clinical and public health issues including directions for further research, strategies for informing the public about risk, and policies for disease screening. This chapter focuses on thyroid cancer screening for people thought to be at higher-than-average risk for thyroid cancer from exposure to iodine-131 (I-131) fallout. It also briefly considers screening for other thyroid disorders. The final sections consider clinical and public health policies based on a review of the scientific literature on the accuracy, benefits, and harms of screening.
In public health terms, screening for thyroid cancer is a form of secondary prevention.1 Like screening for other cancers, the goal is to detect disease in people without symptoms so that they can be treated early to reduce mortality and morbidity. Routine screening is directed at the population generally; targeted screening seeks people who have a higher-than-average risk of developing a disease. In either case, a screening program has value only when earlier detection of a disease results in earlier treatment that improves outcomes for the screened population.
The appeal of screening as a means of reducing the burden of disease is powerful, and much has been claimed for a large array of tests that screen for
diseases causing premature morbidity and mortality. The U.S. Preventive Services Task Force has, for example, examined and made recommendations about screening for 53 conditions, including heart disease, several kinds of cancer, infectious diseases, prenatal disorders, and sensory problems. As these and other recommendations and analyses make clear, clinical epidemiologic research confirms the value of some screening tests but does not support claims for others (USPSTF 1996; Russell 1994; Eddy 1991).
Efforts to evaluate screening strategies and to develop evidence-based recommendations for screening can generate considerable controversy. Science-based conclusions (especially when the conclusion is that the evidence for screening is negative, inconclusive, or lacking) can conflict with the understandable public desire to believe that a particular screening test will save lives. A case in point is the controversy over a recommendation from an NCI consensus panel that screening for breast cancer in women ages 40 to 49 should be a matter for women to decide with their clinicians rather than a routinely advised practice (Begley 1997; Eddy 1997; Ransohoff and Harris 1997). Following protests and criticism from some advocacy groups and some members of Congress, a different NCI panel (the National Cancer Advisory Board) recommended routine breast cancer screening for this age group (Taubes 1997) even though many scientists think that evidence is still inadequate to support general screening in this age group.
The discussion in this chapter builds on sections in other chapters of this report that have examined who is potentially at risk of thyroid cancer from I-131 exposure, how great the risk is, and how communication with the public should be structured. It also draws on the literature review and analyses presented in the background paper commissioned for this study (Appendix F). The discussion here also builds generally on the principles of evidence-based clinical practice and public health policy. It recapitulates some of the information on thyroid cancer presented earlier, so that this chapter can be read independently. This chapter reviews.
-
The concepts and principles for screening recommendations.
-
The burden of illness associated with thyroid cancer.
-
The benefits and harms of screening.
-
The tests used for screening.
-
The evidence about test accuracy and the benefits of early detection.
-
The screening recommendations of other groups.
-
This study's conclusions and recommendations.
PRINCIPLES FOR SCREENING RECOMMENDATIONS
The specific conclusions about thyroid cancer screening for exposed persons were developed by the Institute of Medicine (IOM) committee that was described in Chapter 1. In developing its recommendations, the committee examined the
scientific literature (including the literature review by Eden, Helfand, and Mahon in Appendix F), considered position statements and guidelines developed by other groups and individuals, and benefited from the discussion of diverse experts at a March 17-18, 1998, workshop convened by the committee (see Appendix A for the agenda and participants). In addition, the committee reviewed information from the public meetings, analyses, and deliberations of the National Research Council committee that considered the I-131 thyroid doses from the Nevada tests, the cancer risk posed by I-131 exposure, and the approaches to communicating with the public about exposure and risk.
Criteria for Clinical Recommendations
In developing guidelines for thyroid cancer screening for people potentially exposed to I-131 fallout from the Nevada tests, the IOM committee began with several broad principles. Consistent with the established and recognized mission of the IOM and the National Research Council, the guidelines would be based on careful review and assessment of the scientific evidence. They would be intended to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances and to inform policy decisions about public health strategies.
Recommendations set forth by the IOM and others (CDC 1996; USPSTF 1996; McCormick and others 1994; IOM 1992; Eddy 1991) have proposed a number of additional criteria for guidelines for clinical practice.
First, the disease targeted for screening should be
-
Important in terms of prevalence, incidence, and mortality or morbidity (disease burden).
-
Amenable to treatment that produces better outcomes (benefits balanced against harms) than observation alone or no treatment at all.
-
More successfully treatable (e.g., condition cured, progression slowed) if detected at an earlier stage than would be possible without screening (e.g., before symptoms are evident).
Second, screening recommendations should be based on evidence related to the technical characteristics of a test used for screening. A screening test should be
-
Reliable, accurate, and safe in detecting the disease earlier than would be possible under the conditions of usual care, as indicated by the test's sensitivity, specificity, and positive predictive value (see Addendum 4A for definitions and an illustration of the effect of disease prevalence on screening test performance).
-
Projected to have benefits (e.g., improved life expectancy or quality of life) that outweigh harms (e.g., possible unnecessary diagnostic work-ups or surgery for false-positive results or nonprogressive cancers).
Third, for screening to be implemented successfully, a screening strategy should be
-
Acceptable to the public (e.g., involve reasonable burdens or harms as perceived by those to be screened).
-
Acceptable to clinicians (e.g., not unreasonably burdensome to undertake).
-
Effective and feasible to implement in normal practice.
-
Cost-effective (e.g., have a cost per life saved or quality-adjusted life-year gained that is comparable to or less than that provided by other accepted interventions).
The last of these common criteria for screening—cost-effectiveness—was not considered by the committee in its analyses nor was such analysis specified in the charge to the IOM from the National Cancer Institute. Clinical evidence and considerations alone determined the committee's conclusions. The committee, however, noted that if evidence does not support claims that an intervention is clinically effective or that its benefits outweigh its harms, then the further step of cost-effectiveness analysis makes no sense.
The committee's criteria for making recommendations about thyroid cancer screening for those exposed to I-131 from the Nevada tests can be depicted as an evidence pyramid (Figure 4.1). The lowest tier involves evidence of a population health problem; next are the availability of effective treatment for the disease and of accurate and feasible screening tests; a yet higher tier involves evidence that early detection through screening improves outcomes; and at the top of the pyramid is evidence that benefits exceed harms. Recommendations for screening apparently health populations are generally held to a higher standard of effectiveness than recommendations for treatment in people with evident disease or injury.
To make a positive recommendation for screening for thyroid cancer in people exposed to I-131 from the Nevada nuclear tests, the committee determined in advance 1) that a chain of evidence was required that exposure to I-131 from fallout increases the risk of thyroid cancer; 2) that effective treatment is
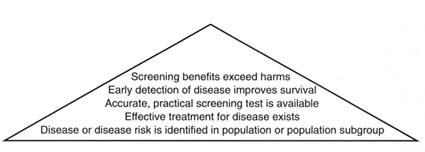
FIGURE 4.1 Pyramid of evidence for national screening policy.
available for the disease; 3) that screening tests for thyroid cancer are reliable, accurate, and practical in detecting disease earlier than would occur with usual care; 4) that early detection of thyroid cancer by screening improves treatment outcomes; and 5) that the potential benefits of screening outweigh its potential harms. The strength of either a positive or a negative recommendation would depend on the strength of the evidence on these four points. For example, if evidence suggested that screening was efficacious but that good results depended on the skill or experience of the clinicians performing the screening tests, then a recommendation might describe when referral to a more experienced clinician should be considered. A committee recommendation about an intensive public health program to encourage or pay for screening would have to note (as described above) that the committee did not analyze the cost-effectiveness of thyroid cancer screening compared with other screening strategies of demonstrated value.
BURDEN OF ILLNESS
General Burden of Mortality and Morbidity
Chapter 3 presented data for thyroid cancer in the United States. To recapitulate in the context of this discussion of screening policy, thyroid cancer is uncommon and rarely life-threatening. The overall age-adjusted incidence for all forms of thyroid cancer is 4.9 per 100,000 population (unless otherwise indicated, data are from NCI 1997b), and it accounts for about 1 percent of cancers diagnosed each year and about 0.2 percent of cancer deaths (an estimated 1,200 out of 564,800 in 1998) (ACS 1998a). The majority of these cancers are papillary carcinomas, which is also the form of thyroid cancer linked to I-131 exposure.
Naturally occurring thyroid cancer is considerably more common in women than it is in men, 6.9 cases per 100,000 for women versus 2.8 for men (NCI 1997b), and it is more frequent in whites than in blacks.2 For men, the incidence
of thyroid cancer increases fairly steadily until age 70; for women, incidence peaks in middle age. The median age at diagnosis for all forms of thyroid cancer is 43 years for women and 50 years for men. For those who die of all forms of thyroid cancer (a small proportion of those diagnosed), the median age at death is 75 years for women and 69 years for men.
Survival rates for this disease are high. The 10-year cancer-specific survival rate for persons with papillary carcinoma, the form linked to radiation exposure, is estimated at 95 percent and the 30-year survival rate is estimated at 90 percent (Wang and Crapo 1997; Mazzaferri and Jhiang 1994). Women's survival rates are somewhat higher than rates for men (96 percent for women versus 92 percent for men at 5 years for all thyroid cancers combined). Survival rates are somewhat lower for blacks than for whites (around 88 percent at 5 years for all thyroid cancers combined).
Age-adjusted mortality rates for all forms of thyroid cancer are 0.4 per 100,000 for women and 0.3 per 100,000 for men. (By way of comparison, the mortality rate for lung cancer is 32.8 and 73.2 per 100,000 for women and men respectively; for breast cancer in women, the mortality rate is 26.4 per 100,000 and for men, the mortality rate for prostate cancer is 26.9 per 100,000.) Although relatively low at all ages, mortality rates rise after about age 45, an age already reached or being approached by the cohort potentially at risk of thyroid cancer from exposure to radioactive fallout from the Nevada tests.
Chapter 3 noted that thyroid cancer is among the cancers that have increased in incidence but decreased in mortality rate over the past 30 years. The contrast between the incidence and mortality trends has been attributed to more sophisticated detection technologies (ultrasound for nodules and FNA biopsy for cancer) and more complete diagnostic reporting (Wang and Crapo 1997). The disease accounts for about 1 percent of all cancers and about 0.2 percent of cancer deaths (an estimated 1,200 of 564,800 in 1998, according to the American Cancer Society 1998a).
Mortality does not represent the only burden of thyroid cancer. Symptoms of the disease itself, the processes of diagnosis and treatment, and the consequences of and follow up after treatment also must be considered. Symptoms for treatable disease are relatively uncommon but can include hoarseness and difficulty swallowing or breathing. Diagnosis by fine-needle aspiration (FNA) biopsy can cause anxiety and minor physical discomfort, as described later in this chapter and can lead to overtreatment for small lesions.
Surgical treatment for clinically significant papillary thyroid cancer is generally considered to provide effective treatment and improve long-term survival (Mazzaferri and Jhiang 1994; Mazzaferri 1993b; Hay 1990). Some controversy, however, remains about specific surgical strategy.3 Surgical treatment involves
inpatient surgery that typically removes nearly all of the thyroid gland (leaving a small part of the gland near the entrance of the laryngeal nerve into the larynx) (Falk 1997). Surgical scars are generally visible on the neck above shirt or blouse collars, which may bother some people and not others. If a thyroid cancer, other than a very small one is found, surgery is often followed by radiation treatment with I-131 to destroy remnant thyroid tissue. After total or near-total thyroidectomy, patients require lifetime thyroid hormone replacement therapy, which if properly prescribed and monitored, does not present significant risk of adverse side effects.
In addition to a very small risk of complications from anesthesia, identified surgical risks include permanent damage to the laryngeal nerve (with resulting severe hoarseness) and inadvertent removal of the parathyroid glands (with medication required to treat subsequent problems related to hypocalcemia, which can include muscle tremors, cramps, and seizures). A recent review of seven studies, involving a total of 1,754 patients, reported permanent hypoparathyroidism rates of 0.8 to 5.4 percent (weighted mean, 2.6 percent) for those undergoing total thyroidectomy; for subtotal thyroidectomy, the weighted mean was 0.2 percent (Udelsman 1996). The review also reported permanent recurrent laryngeal nerve injury in 0 to 5.5 percent (weighted mean 3.0 percent) for total thyroidectomy patients and in 1.1 to 3.2 percent (weighted mean, 1.9 percent) of those undergoing the less extensive procedure. Although the committee is not aware of specific research on the relationship between thyroidectomy complication rates and surgical skill or experience, the technical difficulty of thyroid surgery, especially for total or near-total thyroidectomy, is such that lower complication rates might reasonably be expected from surgeons and centers that perform higher volumes of procedures.4 Patients who undergo surgery for large invasive tumors or who have extensive lateral lymph node dissections (i.e., standard radical neck dissection) combined with total thyroidectomy are at higher risk for complications, as are older patients who are likely to have comorbid conditions (Mazzaferri and others 1977).
Exposure to I-131 and Risk of Thyroid Problems
The NCI report links higher exposure to I-131 to a combination of geographic location, age at exposure, and milk consumption, especially milk from ''backyard" cows and, particularly, goats. As discussed in the NCI report and in Chapter 2 of this IOM/NRC report, the NCI's estimates of the American public's exposure to I-131 from the 1951-1962 nuclear tests in Nevada are characterized by
substantial uncertainty. This uncertainty, which is inevitable given data limitations related to geographic measurements of fallout and reconstruction of milk consumption information, applies to general characterizations of total population exposure but it creates the greatest problems for making individual estimates. Accurate estimates of a specific individual's exposure are usually not possible.
Chapter 3 concluded that evidence supports a link between exposure to radioactive iodine from fallout and papillary thyroid cancer. The major uncertainty of this relationship would be at the lower exposure levels that characterized most Americans' exposure from the Nevada weapons testing program. For the average American woman, the probability of being diagnosed with thyroid cancer by the age of 95 is roughly 1 chance in 150 without exposure and 1 in 90 with exposure to 0.1 Gy (10 rad). For the average man, the corresponding probabilities are 1 in 400 without exposure and 1 in 150 with exposure. As noted earlier, papillary thyroid cancer, the form related to radiation exposure, is rarely life threatening.
Nonmalignant Thyroid Disease Associated with I-131 Exposure
In addition to thyroid cancer, Chapter 3 investigates whether evidence links I-131 exposure to other thyroid disorders, which include thyroid nodules, hypothyroidism, hyperthyroidism, and autoimmune thyroiditis and goiter. The review in Chapter 3 cites considerable evidence of links at moderate and high exposures but little evidence suggesting a link between hypothyroidism or hyperthyroidism and I-131 doses in the range experienced by most of those exposed to fallout from the Nevada nuclear tests. For example, a study of Utah "downwinders" exposed to atomic weapons test fallout after birth (mean dose of 0.098 Gy [9.8 rad] with a maximum of 4.6 Gy [460 rad]) showed no excess of nonmalignant thyroid disease (Kerber and others 1993).
Given the conclusions in Chapter 3, the IOM committee did not investigate the disease burden of nonmalignant disease or the evidence base for screening for such disease in people exposed to I-131 from the Nevada nuclear tests. The results of the Hanford Thyroid Disease Study (see www.fhcrc.org/science/phs/htds) may clarify the extent to which childhood exposure to low doses of I-131 is linked to thyroid problems in adulthood. This clarification should, in turn, clarify whether it is warranted for DHHS to examine the evidence on screening for nonmalignant disease.5
THYROID CANCER SCREENING AND DIAGNOSTIC OPTIONS
Screening for thyroid cancer may involve two steps. For the first step, screening for thyroid nodules, the options reviewed by the committee are physical palpation
and ultrasound examinations of the neck (Charboneau and others 1998). If these tests detect a large nodule (1.5 cm or larger), the second step, FNA (fine needle aspirate) biopsy of the nodule, is usually performed. Observation (with periodic examinations) will be the approach for most of those who have small (less than <1.5 cm), otherwise unsuspicious lesions detected by palpation or ultrasound. Most such lesions, often discovered during ultrasound examinations for other conditions (e.g., carotid artery disease, parathyroid problems), are benign and even those that have cancer cells will usually not become life- or health-threatening. Some patients are referred directly for surgery following detection of a nodule, although this is not standard or recommended practice (AACE 1997a; Tan and Gharib 1997; Ezzat and others 1994; Mazzaferri 1993b).
Physical palpation of the thyroid6 is performed in conjunction with a visual examination of the neck and a patient history that documents possible risk factors such as age, sex, family history of thyroid disease (especially papillary or medullary cancer), personal history of previous head or neck irradiation or thyroid problems, or symptoms such as hoarseness or difficulty swallowing (Gharib 1997; Belfiore and others 1995; Mazzaferri 1993b). Palpation seeks to detect nodules in the thyroid, and if they are found, to assess their size, number, firmness, and adherence to adjacent structures. The size and firmness of nearby lymph nodes are also checked by palpation. For asymptomatic, average-risk people, routine screening for thyroid cancer through palpation or ultrasound examination of the thyroid gland is not recommended (USPSTF 1996).
Ultrasound examination of the thyroid involves noninvasive scanning of the thyroid gland with a machine that creates images by directing high-frequency sound—ultrasonic waves—to the gland and using computer algorithms to translate information about sound transmission or reflection (echoes) into two-dimensional images (Tan and others 1995; Ezzat and others 1994; Hsiao and Chang 1994). Ultrasound can detect many small nodules (less than 1 to 1.5 cm) that are not usually detectable by palpation and that will usually not progress to cause problems. Little is known about the natural history of these small nodules and the occasional cancers, including what causes a few of them to progress. Rather than further tests or therapy, such nodules generally warrant observation (Gharib and Mazzaferri 1998). Ultrasound examination also can provide additional information to further characterize nodules, including whether calcification is present and whether the nodule is solid or cystic (Watters and Ahuja 1992). Although such information—in combination with information from physical examination—can raise or lower the probability that a nodule is cancerous, ultrasound information is not by itself diagnostic.
To determine whether a detected thyroid nodule is benign or malignant, the current procedure of choice is FNA biopsy (Garcia-Mayor and others 1997; Gharib 1997; Lin and Huang 1997; Ashcraft and Van Herle 1981). Typically, a fine gauge needle is inserted into the nodule (or nodules) in several places and a sample drawn into the needle and an attached syringe. If the nodule is not readily palpated, the biopsy can be guided by ultrasound, although this technology is not available everywhere and can lead to the identification of additional, small nodules, few of which are likely to cause problems (Lin and Huang 1997; Taki and others 1997). Minor localized pain is common during and after the biopsy, and, rarely, a clinically apparent hematoma can occur at the FNA site (Gharib and Goellner 1993; Ashcraft and Van Herle 1981).
Following the biopsy, the aspirated material (tissue and blood) is placed on slides or filtered through mesh and applied to slides, stained with various dyes, and then microscopically examined. Pathologists evaluate the sample on the slides and classify the lesion. In addition to positive or negative results, FNA samples can be categorized as indeterminate or unsatisfactory. For results categorized as indeterminate, the pathologic features of the cells are ambiguous and cannot be readily categorized as benign or malignant. Patients with indeterminate biopsies are usually referred to surgery. Of satisfactory samples, about 10 percent are likely to be classified as indeterminate.
Unsatisfactory samples provide insufficient or inadequate material for evaluation and often prompt repeated biopsies aimed at securing an adequate sample for further analysis (LiVolsi 1997). Unsatisfactory FNA samples are obtained 10-15 percent of the time, even in the best-performing centers. The rate of unsatisfactory samples depends on the training and skill of the operator (e.g., endocrinologist, head-and-neck surgeon, radiologist, family practitioner), the number of sites sampled, preservation practices, the skill of the pathologist or cytopathologist, and the size of the nodule being biopsied (Merchant and Thomas 1995; Haas and Trujilla 1993; Piromalli and Martelli 1992). A screening approach that led to more FNA biopsies of smaller nodules (<1-1.5 cm) would likely generate more inadequate samples and, in turn, more repeat biopsies. Although no precise figures are available and recommendations vary, some patients, perhaps even a majority, with inadequate FNA samples will be referred for surgery. Addendum 4B considers the implications of indeterminate or unsatisfactory FNA biopsy results in more depth.
ACCURACY OF SCREENING AND FOLLOW-UP TESTS
Palpation and ultrasound examinations are widely accepted as safe, low-risk procedures (Gharib 1997; Mazzaferri 1993a; Ashcraft and Van Herle 1981). Concern about their use is not related to the direct risk of the procedures themselves but to their relative inaccuracy, particularly the probability of their producing false-positive results or identifying many very small nodules and cancers that are
not likely to cause harm. Because the likelihood of finding thyroid cancer (pretest prevalence) is low, even in most populations exposed to iodine-131, the great majority of positive test results for nodules will be false-positive test results for cancer when the nodule is biopsied. A detailed review of evidence about the accuracy of palpation and ultrasound in detecting thyroid nodules and of FNA in detecting thyroid cancer is presented in the "Screening for Thyroid Cancer" background paper (Appendix F).
Palpation
The standard for reporting sensitivity in the detection of thyroid nodules is ultrasound. Because ultrasound cannot definitively discriminate benign from malignant nodules, the test results in nodule—not cancer—detection. Research has not compared the accuracy of different approaches to palpation (e.g., whether it is best done from the patient's front or back) (Tan and Gharib 1997), but more experienced examiners are likely to produce more accurate results (Jarlov and others 1993; Jarlov and others 1991). Accuracy can be affected by the size, consistency, or location of nodules (e.g., on the front or back surface of the thyroid) and by a patient's physical characteristics (e.g., length and thickness of the neck).
The sensitivity results from three studies of palpation ranged from 0.10 to 0.31 in detecting nodules identifiable with ultrasound (Table 1 in Appendix F). The study that reported 0.31 overall sensitivity found sensitivity of 0.45 for detection of nodules larger than 0.5 cm and 0.84 for nodules larger than 1 cm (Ezzat and others 1994). A study of palpation in a radiation-exposed population reported sensitivity of 0.89 for detection of nodules larger than 2 cm and 0.83 for those between 1 and 2 cm (Mettler and Williamson 1992). Other studies of the sensitivity of palpation for detecting larger nodules gave poorer results. For example, one study reported sensitivity of only 0.42 for nodules larger than 2 cm (Brander and others 1992). The U.S. Preventive Services Task Force (USPSTF 1996) reports specificity levels for nodule detection of 0.93 to 1.0 from three studies that compare palpation with ultrasound.
Ultrasound
Ultrasound examination is the reference standard for nodule detection, so its sensitivity and specificity for this purpose are not reported. It very commonly reveals thyroid nodules in people without symptoms who are being examined for other purposes. For example, four U.S. studies of ultrasound examinations report nodules in 13 percent (Carroll 1982), 41 percent (Horlocker and Jay 1985), 50 percent (Stark and others 1983), and 67 percent (Ezzat and others 1994) of those examined. Current ultrasound technology does not allow reliable and accurate discrimination between cancerous and noncancerous nodules.
When ultrasound results are compared against FNA results for specificity in detecting cancer (rather than nodules), studies report a high rate of false positives,
that is, nodules without cancer. In patients examined by ultrasound for hyperparathyroidism, Horlocker and colleagues (Horlocker and Jay 1985) found thyroid nodules in 41 percent, but cancer was detected in only 2 percent of the 689 patients referred for surgical neck exploration. The trend of increasing nodule detection with age found by Horlocker and colleagues is fairly close to pathology findings at autopsy reported in a much earlier study (Mortensen and others 1955).
Fine-Needle Aspiration Biopsy
A recent set of guidelines on cytopathology for FNA specimens from thyroid nodules described FNA of the thyroid as "principally a triage procedure" to differentiate patients who need surgery from those who do not (Papanicolaou 1996). It noted, however, that "in experienced hands [FNA] may also be diagnostic for certain thyroid lesions" such as papillary and other carcinomas (p. 710).
The standard for reporting sensitivity and specificity of FNA is findings at surgery. Although most patients with negative or benign FNA results do not have surgery (and thus no comparison against this standard is possible), several longitudinal studies of patients with negative test results indicate that such results are reliable (Tan and Gharib 1997). For pathologists who are experienced at interpreting FNA biopsies of thyroid nodules, false-positive rates of 3 percent and false-negative rates of 2 percent have been reported (Gharib and Goellner 1993; Hall and others 1989; Boey and others 1986). As shown in Table 2a and 2b in Appendix F, however, sensitivity and specificity rates can vary depending on how analyses treat unsatisfactory or indeterminate FNA results. (See Addendum 4B for further discussion of different interpretations of indeterminate and unsatisfactory samples.) Not only will many patients referred for FNA turn out not to have thyroid cancer, but many will likely be referred for surgical resection of the thyroid because of unsatisfactory or indeterminate results.
BENEFITS AND HARMS OF SCREENING FOR THYROID CANCER
General Considerations
Most discussions of cancer screening emphasize potential benefits, but potential harms also must be considered and weighed. The primary benefit sought from screening is early detection of asymptomatic disease and treatment at a stage that permits improved outcomes including extended life (not just a longer period with a diagnosis), reduced morbidity, and better quality of life. Those whose screening results are negative (no disease detected) may feel comforted, particularly if they view themselves as being at special risk of the disease.7 Even
when early detection of a disease is not matched by effective treatment (e.g., as is now true for some genetic screens), some people might believe themselves better off with an early diagnosis because they could plan more realistically for the future. More generally, a screening program for a problem linked to past government actions may be viewed as a government admission of responsibility for harm, which may provide a psychological and financial benefit (in the form of compensation) for those who believe themselves harmed.
The potential harms of cancer screening as experienced and perceived by patients are not necessarily well defined or understood. For those who have false-positive screening results (i.e., no cancer is actually present), the harms can include anxiety, inconvenience, and physical discomfort attendant to follow-up diagnostic testing; unnecessary surgery or other treatment—and associated morbidity or mortality—either initially or when a follow-up diagnostic test is inaccurate or indeterminate; and self-labeling or external labeling (e.g., by insurers, employers, family) of a person as high risk or vulnerable. Even those with true-positive results can suffer if early detection and treatment do not affect outcomes. If they have a nonprogressing cancer, they may experience needless anxiety and treatment, and even if their cancer progresses, they may experience a longer period of anxiety about their condition or endure more years of treatment and adverse treatment-related side effects without any better outcomes than if they had not been screened. Those who have false-negative screening results can subsequently be less alert to early symptoms of disease and could delay seeking care.
As far as the committee is aware, there are very few studies of patient perceptions of quality of life for health states associated with different outcomes of screening, diagnosis, or treatment for specific cancers. It is possible that experts who assess screening tests either over- or underestimate the benefits and harms as perceived by patients who consider and undergo screening—especially patients who see themselves as being at higher risk of a disease. Some research suggests that the public may have a high tolerance for false-positives (Schwartz and Woloshin 1998). This research also suggests that people may be unaware that screening may identify many cancers that will not progress even without treatment and that people want to be informed and to factor this information into their decisionmaking process.
Evidence of Benefits from Early Detection through Thyroid Cancer Screening
A major difficulty faced by the committee in considering its recommendations on screening for thyroid cancer was the absence of sound clinical research evaluating whether early detection of the disease through screening of asymptomatic people provides benefits in the form of longer life, reduced morbidity, or improved quality of life and whether such benefits outweigh any harms generated by screening. Rigorous prospective, randomized clinical studies of screening benefits
are generally complicated, expensive, and time consuming. Ideally, they would randomly assign asymptomatic individuals to be screened or not screened and then track subsequent survival or other outcomes. No such studies of thyroid cancer screening have been published.
Thyroid cancer screening is a difficult subject for rigorous clinical research because the disease is uncommon, progresses slowly, and is rarely fatal. Because any change in survival would likely be quite small and not seen for many years, a clinical trial of screening would have to be both very large and very long. Indeed, by the time a trial was completed, the question of screening probably would be irrelevant for the population that is the focus of this report.
These same characteristics—along with the relative inaccuracy of the screening tests—make it unlikely that screening an asymptomatic population could improve already high survival rates. Screening test inaccuracy does, however, mean that a screening policy would produce many false-positive results (detection of noncancerous nodules), which would result in more surgical procedures than would occur absent a screening program.
The committee's review of the literature produced no randomized trials or case-control studies of thyroid cancer screening. It found only one study (Ishida and others 1988) that attempted any comparison of survival in screened and unscreened groups. That study compared Japanese women who participated in a mass screening program for breast and thyroid cancer with women who were being treated and followed for thyroid cancer in an outpatient clinic. The authors conclude that mass screening for thyroid cancer was useful because women who were screened were found to have disease that was in earlier stages than that found in the outpatient treatment group and that the screened group had a better 7-year survival rate (98 percent versus 90 percent).
Unfortunately, this study has such critical design flaws that it cannot provide a sound basis for a screening recommendation. First, the study is vulnerable to the problem of lead-time bias. Lead-time bias occurs when a screening program simply produces an earlier diagnosis and lengthens the measured period of survival after diagnosis without actually affecting true survival.
Second, people who volunteer for screening are often healthier and at lower risk than are those who do not. Compared with nonvolunteers, they would likely do better with or without screening.
Third, in this study, the screened group and the outpatient group differed in the kinds and prognosis of cancers detected, a common problem that is known as length bias. For the screened group, 99.5 percent of cancers detected were those with favorable prognosis (87.5 percent papillary, 12 percent follicular, 0 percent undifferentiated cancers). In contrast, among the cancer outpatients, 70 percent had papillary cancer, 25 percent had follicular cancer, and 4 percent had more lethal undifferentiated thyroid cancer. Seven of the 9 patients with the diagnosis of lethal undifferentiated cancer died within 5 months of the diagnosis. Thus, the
screened group would be expected to have higher survival rates because of more favorable tissue type alone.
Other research cited in support of thyroid cancer screening includes two studies that focus on higher risk people—those with a history of therapeutic head and neck irradiation (Schneider and others 1985; Shimaoka and Bakri 1982). These studies report more nodules and cancers than found in the general population. A recent follow-up report on one of the study populations (a group irradiated between 1939 and 1962) concludes that nodules continue to occur in the irradiated group, but it also cautions that "thyroid ultrasound is so sensitive that great caution is needed in interpreting the results" (Schneider and others 1997).
Although it did not examine screening or screening benefits, another study (Mazzaferri and Jhiang 1994) suggests that once a thyroid cancer is manifested, delay in treatment lowers the survival rate. This study assessed a cohort of 1,355 patients with papillary or follicular cancer; the group was followed for about 25 years. Information was collected from patient records on when the patient or doctor first noticed a thyroid nodule and how long it took until surgery was undertaken. In some cases it took many years. The median time from the first recorded tumor manifestation to initial therapy was 4 months (range, <1 month to 20 years). The patients who died of cancer had a median delay of 18 months from the time a tumor was first clinically recognized (by palpation) compared with 4 months for those who survived (p < 0.0001, by Wilcoxon rank sum). Cancer mortality was 4 percent in patients who underwent initial therapy within a year compared with 10 percent in the others. Thirty-year cancer-specific mortality rates were 6 percent and 13 percent in the two groups, respectively. The reasons for the treatment delays are not clear and may have been related to diagnostic errors. The authors do not recommend screening for thyroid cancer but do argue for prompt assessment for larger nodules (1.5 cm or larger) and prompt treatment for those diagnosed as malignant.
Table 4.1 summarizes the committee's assessment of probabilities relevant to a screening program based on palpation and some of the consequences of such a program. It does not include probabilities or effects for thyroid cancer mortality and morbidity for lack of supporting data.
INFORMATION AND DECISIONMAKING
Explaining Potential Benefits and Harms to Patients
Whenever the issue of explaining screening risks and benefits arises, recommendations about screening strategies need to consider people's comprehension of information and explanations. Public health personnel and clinicians often rely on written materials to explain the pros and cons of screening, but literacy, more broadly, reading comprehension, limits the effectiveness of such materials. Language
TABLE 4.1 Summary of Evidence for Screening by Palpation for Nodules >1.5 cm
|
Probability of having a nodule (all age groups) |
|
|
Any size |
0.35 |
|
<0.5 cm |
0.13 |
|
0.5-0.9 cm |
0.10 |
|
1.0-1.4 cm |
0.07 |
|
1.5 cm or larger |
0.05 |
|
Probability given a nodule that cancer is present |
|
|
Nodules smaller than 1.5 cm |
0.03 |
|
Nodules 1.5 cm or larger |
0.10 |
|
Probability of having cancer |
|
|
All sizes |
0.0140 |
|
<0.5 cm |
0.0039 |
|
0.5-0.9 cm |
0.0030 |
|
1.0-1.4 cm |
0.0021 |
|
1.5 cm or larger |
0.0050 |
|
Sensitivity of palpation for nodules |
|
|
<1.0 cm |
0.00 |
|
1.0-1.4 cm |
0.55 |
|
1.5 cm or larger |
0.75 |
|
Specificity of palpation for nodules |
0.95 |
|
Fine needle aspiration (FNA) |
|
|
Sensitivity for cancer |
0.80 |
|
False positive rate including indeterminate and unsatisfactory samples with no cancer |
0.25 |
|
Complications of total thyroidectomy |
|
|
Recurrent laryngeal nerve injury |
0.030 |
|
Hypoparathyroidism |
0.026 |
|
Summary: Events per 10,000 patients screened |
|
|
Diagnosed to have nodules (true positives) |
760 |
|
Falsely diagnosed to have nodule (false positive) |
325 |
|
Undergo at least one FNA |
1085 |
|
Undergo at least one FNA and have cancer |
49 |
|
Undergo at least one FNA but do not have cancer |
1036 |
|
Undergo lobectomy after true and false positive FNA |
402 |
|
Cancers diagnosed |
|
|
Any size |
39 |
|
<0.5 cm |
0 |
|
0.5-0.9 cm |
0 |
|
1.0-1.4 cm |
9 |
|
1.5 cm or larger |
30 |
|
Cancers missed |
|
|
Any size |
101 |
|
<0.5 cm |
39 |
|
0.5-0.9 cm |
30 |
|
1.0-1.4 cm |
12 |
|
1.5 cm or larger |
20 |
|
Surgical complications |
23 |
and comprehension problems also limit the effectiveness of oral communication. For both written and oral communication about the benefits and harms of screening, ''numeracy" (a patient's ability to correctly interpret numerical information) is a particular concern.
Directly relevant research on numeracy and communication is limited. One recent study of women's interpretations of information about screening mammography concluded that "both accuracy in applying risk reduction information and numeracy were poor (one third of respondents thought that 1,000 flips of a fair coin would result in less than 300 heads)" (Schwartz and others 1997).
The same study concluded that the way information was framed was important for accurate understanding. Particularly important is information about baseline risk that allows people to look at information on screening outcomes in terms of change from baseline rather than in a vacuum. The study found best results when information was presented in terms of absolute risk compared to baseline (e.g., "reduction in risk to 4 in 1,000 with screening from 12 in 1,000 without screening"). Other research indicates that people find frequency information ("4 in 1,000") more understandable than probabilities ("0.4 percent") (Gigerenzer 1996). In addition, people tend to find a treatment more attractive when risk is expressed in terms of gain ("90 percent of patients with this treatment survive") rather than loss ("10 percent die") (Hux and Naylor 1995; Mazur and Hickam 1990b; O'Connor 1989; McNeil and others 1982). Choices about graphic displays of information likewise can affect assessments of benefits and risks (Sandman and others 1994; Mazur and Hickam 1990a; Huff 1954). Continued assessment of alternative graphic formats is important, particularly for people of limited numeracy.
Ransohoff and Harris (1997) suggest that the way mammography screening for breast cancer in younger women has been debated may reflect how numerical information is framed and perceived. For example, advocates for screening might describe 16-18 percent reductions in relative risk for death, whereas skeptics might refer to 1-2 fewer deaths per 1,000 women who have been screened annually for 10 years. Berry (cited in Ransohoff and Harris) has observed that the benefit sounds slight when it is expressed as an average of 3 days of life gained for women screened in their 40s, whereas the benefit sounds large when described as extending the lives of 800 American women in their 40s. The problems of presenting information appropriately and informing people adequately about risks and benefits are compounded because there is little direct evidence about the consequences of different strategies under different circumstances, and estimates of benefits and risks have been derived from indirect evidence of uncertain relevance.
Evidence, Decisionmaking, and Patient Preferences
Assessing the balance of benefits and harms of an intervention generally
involves objective and subjective elements alike. The objective component involves the probabilities and magnitudes of potential benefits and harms, which can be evaluated through clinical or epidemiologic research. The choice of whether the benefits outweigh the harms is a personal, subjective judgment about the relative importance of potential outcomes based on individual preferences, life plans, and priorities. For example, when carefully informed about the probabilities and nature of outcomes of different clinical management strategies for localized prostate cancer, some patients will prefer not to undergo surgery, which carries some risk of incontinence or impotence; others faced with the same information will prefer surgery (Flood and others 1996; Beck and others 1994; Litwin 1994).8
The ways of considering patient preferences and involving patients in decisionmaking range from what might be generally characterized as, simply, good communication to formal shared decisionmaking. The latter involves a structured process in which the clinician does not uniformly encourage or discourage a treatment but instead presents the patient with the available options, reviews the potential benefits and harms associated with each, discusses the probability and magnitude of those outcomes and the quality of the evidence on which the estimates are based, helps the patient evaluate the potential importance of those outcomes in his or her life, assesses patient comprehension of the information and options discussed, and, if necessary, provides information again in ways the patient can understand (Morgan and others 1997; Woolf 1997; Kaspar and others 1992). The choice preferred by the patient is prescribed. The physician's preference or recommendation is offered if the patient inquires.
When the evidence of screening benefits or harms is limited or weak, when patient perceptions of benefits and harms are variable or not well understood, or when patient preferences about outcomes are crucial to good decisionmaking, then the strong involvement of the patient in a process of shared decisionmaking or collaboration between clinician and patient becomes particularly important (Morgan and others 1997; Ransohoff and Harris 1997; Woolf 1997; Flood and others 1996; Barry and others 1995; Emanuel and Emanuel 1992; Kasper and others 1992). In decision theory, the most important factor in choosing a course of action for such "close call" situations can be the patient's views about possible benefits and harms (Pauker and Kassirer 1997; Kassirer and Pauker 1981).
As a structured and tested process, formal shared decisionmaking is still
evolving, and the effectiveness and feasibility of specific methods or techniques (e.g., computer programs, videotapes, printed materials, and formats for presenting quantitative information) for informing and deciding are still being evaluated. A formal process of shared decisionmaking is neither necessary nor appropriate in every clinical circumstance. In some medical emergencies, for example, it is not feasible. Moreover, clinicians' time with patients and patients' capacity to absorb health messages are finite, and time spent providing information about services of little or no demonstrated benefit could be time taken away from care or counseling with greater potential health benefit. Finally, many patients want a specific recommendation from their physician more than they want to play an active part in making decisions (Nease and Brooks 1995; Ende and others 1989). Still, even when formal shared decisionmaking is not appropriate and when a clinician actively encourages or discourages a particular choice, the clinician is obliged to provide a patient with information about benefits and harms and to listen to and address the patient's concerns.
RECOMMENDATIONS OF OTHERS
In addition to commissioning the literature review included in Appendix F, the committee investigated screening guidelines developed by others (Turkelson and Mitchell 1998). The review searched for screening guidelines for the general public and for people exposed to radiation. The number of thyroid-cancer guidelines located is fairly small, which is not surprising given the low prevalence and mortality of the disease.9
The U.S. Preventive Services Task Force guidelines (USPSTF 1996) recommend against screening for asymptomatic adults or children using either palpation or ultrasound. They characterize the evidence for this negative recommendation as fair. For asymptomatic persons exposed to upper-body radiation in infancy or childhood, they state that "recommendations for periodic palpation of the thyroid gland in such persons may be made on other grounds, including patient preference or anxiety regarding their increased risk of thyroid cancer" (USPSTF 1996). They grade this as a "C" or weak recommendation, which is defined to mean that the evidence is "poor" but that recommendations could still be made on other grounds, such as potential benefit being great and potential harm being minimal. Citing the lack of evidence, the Canadian Task Force on the Periodic
|
9 |
The committee did not consider research protocols to constitute screening recommendations. For example, in research to investigate the effects of radioactive releases from the Hanford Nuclear Facility, protocols for examining exposed individuals for thyroid nodules and other conditions have been developed for the Hanford Thyroid Diseases Study (www.fhcrc.org/science/phs/htds) being conducted by the Fred Hutchinson Cancer Research Center. Such research, of course, could clarify the risks of exposure to I-131 or the outcomes of screening. |
Health Examination (CTFPHE 1994) declined to make any recommendations on general or targeted screening.
The American Cancer Society (ACS 1998b) recommends thyroid palpation as part of a routine health check-up, every 3 years for those under 40 and every year thereafter. No scientific literature is cited in support of the recommendation.
A press release from the American Thyroid Association (ATA 1997) after publication of the NCI (1997a) report suggests that "people who feel that they have been exposed to significant amounts of fallout and feel that they are at particular risk might wish to see their physician for a neck examination" (p. 1). The American Association of Clinical Endocrinologists (AACE 1997b) recommends self-examination ("thyroid neck check") for thyroid nodules. It cites high prevalence of thyroid cancer as a rationale (but does not document or explain this assertion, which is not consistent with evidence cited here) but notes the disease's low mortality. It does not cite scientific evidence of efficacy or effectiveness.
The most direct recommendation for thyroid cancer screening in persons exposed to I-131 has been proposed (under the label "medical monitoring") by the Agency for Toxic Substances and Disease Registry (ATSDR 1997).10 ATSDR recommends screening for an estimated 14,000 people, most of whom live in a limited geographic area in Washington State around the Hanford Nuclear Reservation. That group is considered to be at risk of thyroid cancer and other thyroid disease from childhood exposure (estimated at 0.1 Gy [10 rad] and above) to I-131 releases from Hanford between 1945 and 1951. Medical monitoring is defined as "periodic medical testing to screen people at significant increased risk of disease" (ATSDR 1997) The protocol recommends palpation and blood tests for most of those screened, with follow-up visits and possibly FNA biopsy or ultrasound examination for people with detected nodules 1 cm or larger. For a higher exposure subpopulation (0.25 Gy or higher), it recommends that ultrasound be "offered" at the initial visit (p. 37). In making its recommendation for medical monitoring, ATSDR states "the evidence supports benefits associated with thyroid screening for populations exposed to radiation" (p. 63). In particular, as evidence of the survival benefits of early detection, it cites the study of Ishida and others (1988), which was critiqued earlier in this chapter. In response to critiques
from reviewers of a draft of its report, ATSDR stated that the Japanese study provided "important evidence that screening results in improved survival" (p. 113).
This committee paid careful attention to the ATSDR analysis and recommendation because it involves a population historically exposed to varying levels of I-131. This committee did not agree with ATSDR that systematic thyroid screening should be recommended for asymptomatic people whether or not they had exposure to I-131. In particular, this committee concluded that research did not support systematic screening and that the Ishida study cited by ATSDR was seriously flawed and did not provide valid, usable evidence of benefit. DHHS will need to establish some process for managing or resolving this conflict. If political pressure prompts DHHS to decide to recommend or encourage screening, it should make clear that scientific evidence does not support the recommendation.
In addition to considering specific recommendations about thyroid cancer screening from other groups, the committee also examined the approach to screening taken by one large managed-care organization, Group Health Cooperative (GHC, formerly Group Health Cooperative of Puget Sound, now part of Kaiser/Group Health) (Thompson 1996). GHC's preventive services program, which actively promotes preventive services of demonstrated value, does not explicitly include thyroid cancer screening. However, it is relevant here to examine the program's approach to other tests and conditions for which evidence to support screening is insufficient. In information for patients and clinicians, the organization will state when there is insufficient evidence to support routine screening for a problem. Rather than preclude screening, GHC may recommend shared patient-clinician decisionmaking about screening. In addition, GHC also may employ various means (including an educational program that uses opinion leaders, on-line guidelines, and supporting documentation and references) to inform its clinicians about the evidence on test accuracy and on the lack of research showing benefit from early detection through screening.
COMMITTEE FINDINGS AND RECOMMENDATIONS
Findings
When evaluated against the criteria for screening recommendations set forth earlier in this chapter, the evidence reviewed by the committee does not support a clinical recommendation for routine screening for thyroid cancer in asymptomatic persons exposed to radioactive iodine from nuclear weapons testing at the Nevada Test Site.
First, thyroid cancer is rare in the general population. By age 95, it is estimated that about 1 in 400 men and 1 in 150 women might be diagnosed with some form of thyroid cancer. In contrast, about 1 in 8 women might be diagnosed with breast cancer and 1 in 5 men with prostate cancer.
Second, exposure to I-131 in childhood does appear to increase the risk of thyroid cancer. Exposure to 0.1 Gy (10 rad) of I-131 at the age of less than 1 year has been estimated to yield a lifetime risk of thyroid cancer of about 1 in 240 for men and 1 in 90 for women.
Third, for most people, there will not be enough information to identify accurately the level of exposure to I-131 from the Nevada nuclear tests.
Fourth, papillary thyroid cancer, the most common form of naturally occurring thyroid cancer and the form linked to radiation exposures, can be effectively treated surgically and has a high survival rate, regardless of cause, when detected by routine clinical practice without screening. The 10-year cancer-specific survival rate for persons with papillary carcinoma is estimated at 95 percent and the 30-year survival rate is estimated at 90 percent.
Fifth, there is no evidence that early detection of thyroid cancer through systematic screening (rather than through routine clinical care) improves health outcomes or has benefits that outweigh harms. It is still possible—given the lack of directly relevant research—that early detection through routine screening might offer some net benefit.
Sixth, routine screening for thyroid cancer by palpation and, especially, by ultrasound will identify many nodules, most of which will not be malignant.
Seventh, FNA biopsies will find cancer in a few nodules, will not find cancer in most nodules, and will yield a significant proportion of indeterminate or unsatisfactory samples (20 to 30 percent or more) that may lead to unnecessary surgery for many people who do not have thyroid cancer or who have very small cancers that would never progress to cause health problems.
Overall, the committee found that routine screening for thyroid cancer does not meet the criteria set forth earlier in this chapter (Figure 4.1) related to prevalence, accuracy of screening tests, or evidence of improved survival or benefits that exceed harms.
Recommendations
Public Health and Clinical Policies
The committee recommends against public programs and clinical policies to promote or encourage routine screening for thyroid cancer in asymptomatic people possibly exposed to radioactive iodine from fallout as a consequence of the nuclear tests in Nevada during the 1950s.
The lack of evidence that early detection of thyroid cancer through routine screening of asymptomatic persons improves health precludes a positive recommendation to screen people routinely for a disease characterized by slow progression, high survival rates without screening, and high rates of false-positive test results that can lead to unnecessary surgery and other harms, including some,
such as anxiety and insurability problems, that are not well understood. The committee's recommendation is counter to ATSDR's recommendation in favor of medical monitoring for a subset of people exposed to releases of radioactive iodine from the Hanford Nuclear Reservation in Washington State in the 1940s. The committee recognizes that conflicting policy recommendations can be confusing to the public and distressing to some, but it firmly believes that the scientific evidence supports its position. DHHS will obviously require some process for managing or resolving this conflict.
It is appropriate for a clinician who sees a concerned patient to discuss that patient's concerns and history and decide jointly about screening.
Given popular fears of cancer and concern about radiation, the often modest reach of public information programs, and conflicting recommendations from other groups, clinicians will likely see some patients who express concern about possible exposure to radioactive fallout and who request screening for thyroid cancer.11 Although the committee recommends against policies that encourage or promote routine screening, it is essential that clinicians respond sensitively and constructively to concerned patients who come to them seeking advice. Such a response will involve listening to the patients' concerns; discussing their possible exposure to iodine-131 and other risk factors for thyroid cancer; explaining that thyroid cancer is uncommon even in people with some exposure to I-131 and that the thyroid cancer linked to I-131 exposure is rarely life threatening; describing the process, benefits, and harms of screening and the lack of evidence showing that people are better off with it than without it; checking patient understanding of the information presented; and jointly deciding how to proceed. If a nodule is palpated, FNA biopsy would normally be proposed, again with a discussion of what is known about its accuracy, benefits, and harms and about the patient's prospects without further testing.
Once the committee concluded that it could not recommend a routine program of screening, three points led the committee away from an explicit recommendation that physicians forego screening and toward the recommendation for patient-physician discussion and decisionmaking. The committee's recommendation that patients and physicians share in decisionmaking about thyroid cancer screening rests on several points. First, the evidence of the effects of systematic screening is not negative; it is absent. Neither positive nor negative effects have been documented from soundly designed clinical research. Second, understanding
of the benefits and harms of screening as perceived by patients—especially those at higher risk—is quite limited, although some committee members believe the potential harms exceeded the potential benefits. Certainly, the committee heard about the anxieties of those who had become aware of their possible exposure to I-131 from the Hanford facility and who were concerned about additional exposure from the Nevada tests. Third, small nodules (<1.5 cm) are much less likely to be detected by palpation than by ultrasound. Because small nodules seldom cause health problems, palpation has less potential than ultrasound to cause harm (in the form of unnecessary follow-up tests and surgery), which is important when the benefits of routinely screening asymptomatic people are uncertain (Sox 1998).
The communication and decision strategy described above is less formal than some strategies of shared decisionmaking described earlier in this chapter. The rationale is primarily practical. In the committee's view, it is feasible and desirable to listen to a patient's concerns about possible radiation exposure and its consequences and to discuss possible benefits and harms of different courses of care. It is less feasible for a clinician faced with a concerned patient to postpone a safe, noninvasive, personal examination while the patient reads a brochure, views a videotape, or otherwise is involved in a structured education and decisionmaking process. (If ATSDR proceeds with its medical monitoring program, it might nonetheless consider testing more and less formal ways of discussing benefits and harms with patients and reaching decisions about screening.) In addition to these practical considerations, a number of committee members also believed that formal shared decisionmaking is most appropriate for certain kinds of "close call" situations as explained above and that thyroid cancer screening does not qualify as such a situation.
Few physicians outside areas where radiation risk has been a prominent issue locally will be prepared to discuss radiation exposure and cancer risk. They will need easy access to information that will prepare them to counsel concerned patients. A sample information sheet for physicians is presented later in this chapter.
For asymptomatic patients concerned about exposure to I-131 and thyroid cancer, the committee recommends against using ultrasound examination for screening either initially or following a negative result from palpation.
The committee's recommendation against screening by ultrasound examination for patients who come to their physicians concerned about I-131 exposure is based on the points noted earlier, in particular, the lack of evidence of screening benefit and the probability that ultrasound will reveal many small nodules that are unlikely to cause harm. It was also based on the additional point that if no nodule is detected by palpation, the likelihood is substantially diminished that an ultrasound will identify a nodule with microcancers that are unlikely to progress to
TABLE 4.2 Bayesian Analysis of Screening of Nodules >1.5 cm by Palpation
|
Test conditions for nodule detection: Sensitivity of test, 75% Specificity of test, 95% |
||||
|
Patient Status |
Prior Probability of a Nodule |
Probability of Negative Exam |
Product of Probabilities |
Revised Probability of a Nodule |
|
Nodule 1.5 cm or larger |
5% |
25% |
125 |
1.37% |
|
No nodule 1.5 cm |
95% |
95% |
9,025 |
98.63% |
|
|
|
|
Σ = 9,150 |
|
cause problems. The magnitude of this effect can be calculated by applying Bayes' rules (see Addendum 4A) (Pauker and Kopelman 1992; Pauker and Kassirer 1987). Table 4.2 illustrates the effect based on assumptions that the prevalence of nodules of 1.5 cm and larger is about 5 percent, that the sensitivity of physical examination in identifying nodules of this size is 75 percent (meaning that the chance of no such nodule being found when one is actually present is about 25 percent), and that the specificity of the exam is 95 percent (meaning that the chance of a clinician falsely feeling such a nodule when none exists is 5 percent). Application of Bayes' rules, as shown below, reveals that the likelihood of a nodule—given that none was palpated—is now less than 1.4 percent rather than 5 percent. The likelihood of no nodule of this size—given that none was palpated—is 98 percent rather than 95 percent. If 10 percent of nodules of this size are cancerous and if no nodule is detected through palpation, then the likelihood of a cancerous nodule larger than 1.5 cm is 0.137 percent (0.10 × 0.0137) meaning one such cancer would be detected for every 730 people screened by ultrasound after a negative physical examination. (Because clinical studies give varying estimates of the variables used in the table and because experts may disagree on the appropriate assumptions, the table can be recalculated using other numbers to see how probabilities change. See also the addendum to this chapter.)
Information and Communication
The committee recommends that DHHS develop a program of information and education for the concerned public and clinicians that builds on the analyses, conclusions, and information approaches described in this chapter and the experience the Department and others have gained in developing similar informational materials.
Patient and Public Information Although screening programs—whether or not they are supported by scientific evidence—can be a popular response to concerns
about cancer risk, the U. S. Department of Health and Human Services (DHHS) will best serve the public by declining to promote routine screening for those exposed to I-131 from the Nevada tests. The NCI has provided written and electronic information about its report and possible health problems, but this information should be reevaluated, refined, and expanded based on experience gained in the last year and on the experience of others in communicating similar kinds of information. Others with relevant experience in communicating about radiation risk include the Centers for Disease Control and Prevention, the Hanford Health Information Network, and the Hanford Thyroid Disease Study. Although managed care organizations are unlikely to have much experience with communicating about radiation risk, organizations such as Group Health Cooperative and Stanford's Center for Patient Preferences may have useful insights about developing effective public information about screening tests. Chapter 5 discusses the importance of early public involvement in developing such an information and communication program.
Also as discussed in the next chapter, the Department's information program should include several kinds of materials including brochures or the equivalent that clinicians, public health departments, managed care plans, and others could give to concerned individuals. Such materials should explain basic facts about the Nevada tests, I-131 and thyroid cancer (including symptoms) and provide general education about screening tests, including explanations of such concepts as false-positive and false-negative test results and their consequences. An Internet site is useful but should not be relied on to the near-exclusion of other kinds of information.
Particularly in materials designed for patients and the public, the limited research on the communication of information about health risks and benefits suggests that the risk of thyroid cancer related to exposure to I-131 from nuclear-bomb testing should be described in both quantitative and qualitative terms. The quantitative information for patients should include an estimate of baseline risk and should be expressed as frequencies (rather than probabilities) and in absolute rather than relative terms. Although some information should be written specifically for the lay public, it should include clear links to more extensive information aimed at clinicians. Box 4.1 presents a sample information page for physicians to provide patients. It is constructed to be consistent with the limited research cited in this report.
Information materials developed by NCI should be tested to assess whether likely audiences interpret the materials accurately. Professional groups and health care organizations may also want to develop and test both written information and sample scripts to guide face-to-face discussions between clinicians and patients. It is particularly important for such patient information to accompany any materials that provide people with a method or model for calculating their exposure to I-131 (e.g., like the method presented on the NCI Web site). Whatever information NCI develops, it should also be tested with advocacy groups and
|
BOX 4.1 Sample Information about Thyroid Cancer, Iodine-131, and Screening Thyroid cancer is rare and usually not life-threatening. Approximately one-third to one-half of Americans between 40 and 60 have small lumps or nodules in their thyroid glands, most of which are too small to be felt. Possibly 10 to 15 percent of people in this age group will have nodules that contain tiny cancers, most of which grow very slowly and will not progress to cause problems. For the most common kind of thyroid cancer, papillary cancer, about 99 out of 100 people with this diagnosis will be alive 5 years after diagnosis and about 90 of 100 will be alive 30 years later. Nuclear weapons tests in Nevada in the 1950s and 1960s exposed many Americans to small doses of radioactive iodine. Although radioactive iodine sometimes causes papillary thyroid cancer, the risk of thyroid problems for most potentially exposed people is still small. From birth to death, an average American woman has about 1 chance in 150 of being diagnosed with thyroid cancer without exposure to radioactive iodine and about 1 chance in 90 following exposure as a child under one year of age. For the average man, the probability of developing thyroid cancer is about 1 in 400 without exposure and 1 in 250 with exposure. Regardless of how they got thyroid cancer, most people will not die from the disease. It is usually not possible to identify accurately how much radiation someone received from the Nevada weapons tests. For this and other reasons, it is also difficult to estimate how likely it is that a particular person will develop radiation related thyroid cancer. In general, people are more at risk if they were under the age of 10 years and also drank milk (especially milk from "backyard" cows and goats) in the years 1951–1962. Routine screening for thyroid cancer or other thyroid disease in people who are unaware of any problems (e.g., a lump they can see or feel) is not recommended, whether or not people have been exposed to 1-131. This is because the available tests are often inaccurate and can lead to unnecessary testing and treatment and because screening has not been shown to improve health outcomes. People concerned about thyroid cancer should make a decision about screening in consultation with their doctor following discussion about the accuracy of the screening tests, the possible benefits and harms of screening, and the lack of evidence that thyroid cancer screening saves lives. |
with representative people in areas where information related to the NCI report was covered in the news media.
Information for Physicians For a decision about screening to be an informed one, clinicians will need to explain carefully the possible benefits and harms of screening for thyroid cancer. Although educational materials for physicians and materials for them to give their patients can help prepare physicians for discussions with patients, actual discussions between patient and physician cannot be governed by a single script but will need to be adapted to some degree to the characteristics and needs of specific patients. What is suitable for a high school physics teacher will likely differ from what is helpful for someone with less than a high school education. In any case, it is appropriate for clinicians to check patient comprehension of oral information and instructions.
In addition to patient and public information, NCI should provide information explicitly designed for clinicians. Except in some areas in the West, most clinicians probably have not seen and would not now expect to see patients concerned about I-131 exposure and cancer risk. This may change as a result of news coverage surrounding publication of this report. Clear, easily available information from NCI could be particularly helpful to busy, relatively uninformed physicians facing new inquiries about I-131 and thyroid cancer.
Although the National Cancer Institute Web site already includes information about its report on I-131 fallout and about thyroid cancer, it does not have information directed explicitly to physicians. When NCI reviews its Internet strategy, information for physicians should be one priority. A sample information sheet for physicians might read as follows:
IODINE-131 AND THYROID CANCER INFORMATION FOR PHYSICIANS [SAMPLE]
News coverage about thyroid cancer and fallout from the 1950s nuclear tests in Nevada may prompt people concerned about possible radiation exposure to seek advice from physicians. The points below briefly summarize information that may help physicians counsel their patients. Additional information is available from the report of the National Cancer Institute, ''Estimated Exposures and Thyroid Doses Received by the American People from Iodine-131 in Fallout Following Nevada Atmospheric Nuclear Bomb Tests" (NIH Pub. No. 97-4264, 1997), the Institute of Medicine/National Research Council report "Exposure of the American People to Iodine-131 From Nevada Nuclear-Bomb Tests: Review of the National Cancer Institute Report and Public Health Implications," and several Web pages at the National Cancer Institute site (http://rex.nci.nih.gov). In brief:
-
The risk of thyroid cancer may be higher than average in people with childhood exposure to iodine-131 (I-131) fallout from nuclear weapons testing in Nevada from 1951 to 1962. For most of this group, the risk of developing thyroid cancer is still small—from birth to death, less than 1 chance in 90 for women
-
and 1 in 240 for men. This is less than many other cancers in the general population.
-
Thyroid cancer is rarely life-threatening; 30-year survival is over 90 percent for papillary thyroid cancer, the form linked to radiation exposure.
-
Accurately identifying people's past exposure to I-131 is usually not possible because necessary data on the key risk factors from four decades ago are generally not available or are unreliable.
-
Routine screening for thyroid cancer is not recommended because the available tests produce a large number of false-positive results, there is no evidence that early detection by screening improves outcomes, and the benefits of screening may not outweigh the harms.
Why may my patients be concerned? News media have covered two recent reports on exposure to radioactive iodine in fallout from about 100 above-ground, nuclear weapons tested in Nevada in the 1950s and 1960s. These stories have reported that this exposure is linked to thyroid cancers.
The first report, mandated by Congress and published in 1997 by the National Cancer Institute, provided county-level estimates of the American people's exposure to I-131. A related memo estimated that an 11,000 to 212,000 excess cases of thyroid cancer were likely caused by the I-131 exposure but epidemiological analyses suggest the number is probably in the lower part of this range. An Internet site provides the public a complicated method to estimate individual exposure (http://rex.nci.nih.gov).
In 1999, the Institute of Medicine and the National Academy of Sciences, as requested by the U.S. Department of Health and Human Services, published a report assessing the health implications of I-131 exposure from the Nevada tests and advising the government on appropriate responses. The IOM/NRC report concluded that the available data from events four decades past were insufficient to permit either reliable or valid county-or individual-level assessments of exposure to I-131. It also concluded that there was no evidence to determine whether screening for thyroid cancer would improve survival or other health outcomes, taking possible harms of mass screening of the population into account.
Does exposure to I-131 cause cancer? Although there is still some disagreement, it is now generally accepted that exposure to I-131 at young ages—especially under age 10—can cause thyroid cancer. The strongest evidence comes from the studies of thyroid cancer in children exposed from the nuclear accident at Chernobyl in 1986. Other suggestive evidence links thyroid cancer to upper body external radiation during childhood.
The magnitude by which risk is increased is small, however—probably less than the normal variation in thyroid cancer in different populations around the world. In the general U.S. population, the chance of being diagnosed with thyroid cancer by the age of 95 is roughly 1 chance in 400 for men and 1 in 150 for women. For someone who received 0.1 Gy (10 rad) of I-131 as a child under age 1, the lifetime risk might be roughly 1 chance in 240 for men and 1 in 90 for women. As noted earlier, papillary thyroid cancer, the form related to radiation exposure, is rarely life threatening. By way of comparison, women have about a
1 in 8 lifetime risk to age 95 of being diagnosed with breast cancer, and men have about a 1 in 5 risk of being diagnosed with prostate cancer.
For those diagnosed with papillary carcinoma, the risk of dying from thyroid cancer is very small. Ten-year survival rates are about 95 percent and 30-year survival is about 90 percent. For the population in general, the lifetime risk over 95 years of dying of thyroid cancer (all forms) is considerably less than 1 in 1,000. In comparison, women have about a 1 in 30 risk of dying of breast cancer and men have about the same risk of dying of prostate cancer.
Is thyroid cancer linked to iodine-131 different from naturally occurring thyroid cancer? The cancer linked to I-131 exposure is papillary carcinoma, which has very high survival rates (95 percent), is a usually less aggressive type of thyroid cancer, and has a very good prognosis without screening (95 percent survival at 10 years, 90 percent at 30 years). For the population at risk, today's middle aged adults, there is no evidence that cancer related to childhood radiation exposure differs from naturally occurring cancer.
Can I identify whether a patient is at high risk of thyroid cancer from iodine-131 exposure? Unfortunately, in most cases the answer is no—except that there is virtually no risk for someone who was an adult during the testing period. For those who were children at the time of the testing, one problem in estimating exposure is that fallout data were collected in very few places in the United States. This information is not sufficient to make good estimates about fallout in places where data were not collected. Also, because the primary way that I-131 gets to the thyroid is through drinking milk, you would need to know how much milk a person drank as a young child, whether it came from a cow or a goat (because goats' milk concentrates I-131 more effectively than cows' milk), and whether the milk came from a backyard cow or other source that left little time for natural decay of radioactivity. Research indicates that dietary recall data are highly unreliable, especially four decades after the fact. In addition to the uncertainties in estimating I-131 exposure, there are also uncertainties involved in estimating the probability of thyroid cancer related to particular levels of exposure, especially at the low levels generally linked to the Nevada weapons tests.
Should people be screened for thyroid cancer? An Institute of Medicine committee made up of physicians (including specialists in endocrinology, radiology, pathology, surgery, and family practice) and others with expertise in public health and evidence-based medicine found that there is insufficient evidence to recommend routine screening for thyroid cancer or other thyroid disease in people who are asymptomatic, whether or not they have been exposed to I-131. For physicians who see patients concerned about thyroid cancer and interested in screening, a process of shared decisionmaking is appropriate. The U.S. Preventive Services Task Force reached similar conclusions about screening for thyroid cancer in the general population. The reasons for this policy are based on concerns about benefits and harms of screening, outlined below, and do not relate to the costs of a screening program.
The problem with screening for thyroid cancer is that palpation is not a very reliable test for thyroid nodules or thyroid cancer. Ultrasound is more accurate in finding nodules, but because it can detect very small lesions, it will find nodules
in perhaps 30 to 50 percent of older adults. The great majority will be benign, but ultrasound can't distinguish them. Many people will then be referred for FNA biopsy, the results of which are also not perfect. If the physical examination is normal and no symptoms are reported, ultrasound is not recommended.
One potential result of a screening program is that many people would have surgery when they did not have cancer or had cancers that would never cause problems. This extra surgery would be acceptable if the added vigilance from screening meant better survival and reduced morbidity from thyroid cancer. Survival rates for papillary cancer are already high, however, and no evidence exists to determine whether screening will produce better results. This combination of unproved benefits and clear possible harms is why a systematic screening for thyroid cancer is not recommended.
What should I tell patients interested in testing for thyroid cancer? Given the tradeoff of benefits and harms associated with screening, the choice of whether to proceed with screening is a personal decision that should be made, whenever possible, with the input of the patient. If you have a patient who is interested in testing, it is important to advise the patient of the potential benefits and harms so that he or she makes an informed decision about whether screening is worthwhile. Some of the quantiative information presented above may help in your discussions with patients, but people often don't interpret quantiative information correctly. It is appropriate to check their understanding.
You might start by explaining that thyroid cancer is uncommon and usually not life-threatening. Possibly 10 percent of the U.S. population may have tiny thyroid cancers, few of which will ever progress to cause problems. You can then explain that the primary screening test is palpation, which looks for thyroid nodules using a physical examination of the neck, combined with questions about possible symptoms (e.g., hoarseness) and risk factors (e.g., radiation therapy at a young age). Possibly one-third to one-half of American adults have thyroid nodules, most of which, however, are too small to be felt. If a lump is found, you may then recommend a biopsy, which uses a thin needle to draw tissue from the lump for examination under a microscope. Most of the time they will not find cancer.
Patients should know that screening has the potential to produce harm because the tests used are not always accurate. The tests often identify questionable lumps or cells that lead people to have major surgery either when they don't have cancer or when they have a cancer that will never progress and cause problems. The surgery has very low risk of death but it does cause discomfort and scarring, will require lifelong thyroid hormone replacement therapy, and for a small proportion of cases can disrupt calcium metabolism or cause serious, permanent hoarseness.
Patients should also know that there is no scientific evidence that they will enjoy better health as a result of screening or early treatment. If, after learning of these tradeoffs, the patient still wants to be screened, palpation of the neck combined with questions about possible symptoms (e.g., hoarseness) and risk factors (e.g., radiation therapy) may be reasonable and reassuring. Normally, if a nodule is palpated, FNA biopsy is recommended. If the physical examination is normal and no symptoms are reported, ultrasound is not recommended.
Consistent with the recommendations for patient and public information, information for physicians should be tested before distribution. DHHS should enlist physician organizations in the process of developing information, testing it with representative generalists and specialists, and disseminating it. The committee recognizes that the availability of information and recommendations does not translate automatically or easily into clinical practice. Physicians facing questions from their patients will, however, have some motivation to seek guidance on iodine-131 exposure and clinical practice. Following publication of this report, DHHS could organize briefings for relevant professional organizations in an effort to enlist their support and assistance in reaching their members, for example, through journal articles, stories in professional newsletters, and presentations at national and regional meetings. If DHHS pursues the regional information development and distribution strategy described in Chapter 5, physician leaders could be involved in the planning process.
Research and Surveillance
Given the lack of research on how people understand and perceive the risks of different cancers and the benefits and harms of cancer screening, the committee suggests that DHHS consider studies to develop more knowledge about perceptions of cancer risk and screening benefit and harms and about the ways perceptions may differ depending on disease characteristics (e.g., prevalence, risk factors, mortality and morbidity, screening test accuracy, treatment consequences). Such research would be helpful in developing strategies for informing and counseling people about risks and options, for example, in deciding whether and how strategies might need to vary depending on disease characteristics. Also helpful would be research on how perceptions are affected by different ways of presenting quantitative information and different ways of structuring clinician-patient communication.
With respect to thyroid cancer or radiation-exposed populations specifically, the committee suggests that the evaluation component of the ATSDR medical monitoring program might consider the feasibility of a controlled study to compare the responses to different information formats or different counseling strategies for eligible patients who come in for screening. Although the program might contribute to knowledge in this area, the ATSDR medical-monitoring program is not likely to produce useful information about mortality effects of screening for thyroid cancer because the population that requests screening and meets eligibility criteria will be self-selected and because high long-term survival rates can be expected without screening.
The committee already has noted that the results of the Hanford Thyroid Disease Study (which is a research effort rather than a screening program) may help clarify the extent to which childhood exposure to low doses of I-131 is linked to thyroid problems in adults. This clarification should, in turn, indicate whether
systematic examination of the benefits and harms of screening for nonmalignant thyroid disease in radiation-exposed persons is warranted.
In addition to supporting some additional research to inform clinical practice and public health policy, the committee also suggests that DHHS strengthen its surveillance capacity by considering ways to work closely with states to improve the quality and scope of SEER data collection and reporting. It should consider whether collection of additional patient information on residence and personal history is warranted and should review the information collected and the way information is coded to be sure that FNA and open biopsy results are being adequately captured, and that operative results and treatment of confirmed thyroid cancers are being documented.
CONCLUSIONS
This chapter has examined the potential benefits and harms of screening for people who could be at higher-than-general risk for thyroid cancer as a result of exposure to I-131 fallout. It briefly discussed screening for other thyroid disorders. The IOM committee that prepared this chapter recognized the significant uncertainties that surround the issues of I-131 exposure and cancer risk as illustrated in Figure 4.2, which summarizes the causal pathway from I-131 release to diagnosis of cancer and gives examples of the sources of variation and uncertainty associated with each step in the pathway. The IOM committee
-
accepted the analysis presented elsewhere in this report indicating that those most exposed to I-131 from fallout were at increased risk of thyroid cancer, although much uncertainty surrounds estimates of exposure for specific individuals;
-
concluded that the evidence did not support a positive recommendation for a program to promote systematic thyroid cancer screening for those potentially exposed to I-131 from the Nevada atomic bomb testing program;
-
described a simplified process of shared decisionmaking about screening by palpation as a reasonable approach for people who come to clinicians with requests for screening and with concerns about their risk of thyroid cancer due to I-131 exposure;
-
recommended against screening by ultrasound examination for those who choose screening;
-
suggested that DHHS develop materials for physicians and for physicians to give to concerned patients as part of its program of information and education for the concerned public and clinicians; and
-
proposed directions for research, including the development of more information about how people perceive the benefits and harms of screening and about how different ways of presenting risk information and structuring decisionmaking affect patient perceptions and understanding.
FIGURE 4.2 Causal pathway: I-131 exposure and consequences with examples of sources of variation and uncertainty.
The committee was not charged with doing a cost-effectiveness analysis, and it based its conclusions solely on clinical and epidemiologic grounds. It would not, in any case, have proceeded from analysis of effectiveness to analysis of cost-effectiveness because it found no evidence showing that screening for thyroid cancer is effective.
Although screening for those at increased risk of thyroid cancer might seem an obvious strategy, screening asymptomatic persons has value only when earlier detection of disease results in earlier treatment that improves outcomes for the screened population and when the benefits of screening exceed the harms. For thyroid cancer screening, there is no evidence of improved outcomes or of benefits that exceed harms. The committee recognizes that it will be challenging to communicate these conclusions in ways that respond to understandable public concerns, beliefs, and values and that accommodate limits on popular comprehension of quantitative information and risk analyses. The next chapter discusses how DHHS might confront this challenge.
Figure 4.2 traces the steps involved in the causal pathway connecting atmospheric nuclear weapons tests to the detection and treatment of radiation-induced thyroid cancers. For each step of the pathway, the enclosed boxes contain information about factors likely to introduce uncertainty and variation. The cumulative effect is to dramatically reduce the ability to identify those individuals at highest risk for radiation-induced thyroid cancer and to assure an individual entering the screening portion of the pathway that benefits will exceed harms.
ADDENDUM 4A: INTERPRETING SENSITIVITY AND SPECIFICITY
Screening tests are accurate when they correctly identify disease in people who have the disease (true positive) or when they correctly identify no disease in people who have no disease (true negative). Tests are inaccurate when people without the disease have a positive test result (false positive) or when people with the disease have a negative result (false negative).
Measures of sensitivity, specificity, and positive predictive value are used to assess the accuracy and efficiency of screening tests in identifying people with and without disease (Table 4.3). These measures are defined for the straightforward screening situation when the alternatives are, first, that the disease is either present or absent and, second, that the test results are either positive or negative. When indeterminate test results and conditions are factored in, the computations of sensitivity and specificity are not defined, but grouping can be done. Treating indeterminate results as positive lowers the positive predictive value of the test.
Table 4.4 highlights the importance of disease prevalence in assessing the value of screening and shows a worked example using the concepts defined above. Given the same test accuracy, the positive predictive values of the test—or the probability of disease given a positive test result—goes from 8.3 percent to 0.9 percent when the prevalence of a disease drops from 1 percent to 0.1 percent. Even for a test that is reasonably sensitive and specific, the lower the prevalence of the disease, the more false-positive results will be generated relative to true-positive results.
Table 4.5 uses the same example but presents it as a Bayesian analysis. In the column headed "revised probability," the first number (8.3 percent) is the probability
TABLE 4.3 Definition of Terms
|
Term |
Definition |
Formulaa |
|
Sensitivity |
Proportion of persons with condition who test positive |
a/ (a + c) |
|
Specificity |
Proportion of persons without condition who test negative |
d/ (b + d) |
|
Positive predictive value |
Proportion of persons with positive test who have a condition |
a/ (a + b) |
|
Negative predictive value |
Proportion of persons with negative test who do not have a condition |
d/ (c + d) |
|
a Explanation of symbols: |
||
|
|
Condition present |
Condition absent |
|
Legend: |
|
Positive test |
a |
b |
a+b |
a = true positive |
|
Negative test |
c |
d |
c+d |
b = false positive |
|
|
a+c |
b+d |
|
c = false negative |
|
|
|
|
|
d = true negative |
|
SOURCE: USPSTF, 1996. |
||||
TABLE 4.4 Importance of Disease Prevalence
|
Testing Conditions: Size of Population = 100,000 Sensitivity of test = 90% Specificity of test = 90% If disease prevalence = 1% |
|||
|
|
Disease Present |
Disease Absent |
|
|
Positive Test |
900 (a) |
9,900 (b) |
10,800 (a+b) |
|
Negative Test |
100 (c) |
89,100 (d) |
89,200 (c+d) |
|
Probability of disease given a positive test result = 8.3% (a / a+b) × 100 |
1000 (a+c) |
99,000 (b+d) |
100,000 (a+b+c+d) |
|
If disease prevalence = 0.1% |
|||
|
|
Disease Present |
Disease Absent |
|
|
Positive Test |
90 (a) |
9,990 (b) |
10,080 (a+b) |
|
Negative Test |
10 (c) |
89,910 (d) |
89,920 (c+d) |
|
Probability of disease given a positive test result = 0.9% |
100 (a+c) |
99,900 (b+d) |
100,000 (a+b+c+d) |
TABLE 4.5 Importance of Disease Prevalence: Bayesian Probability Analysis
|
Test conditions: Sensitivity of test, 90% Specificity of test, 90% |
||||
|
Patient Status |
Prior probability |
Probability of positive exam |
Product of probabilities (col1 × col2) |
Revised probability col1/∑col1 |
|
If prevalence is 1% |
||||
|
Disease |
1% |
90% |
90 |
8.3% |
|
No Disease |
99% |
10% |
990 |
91.7% |
|
|
|
|
Σ = 1,080 |
|
|
If prevalence is 0.1% |
||||
|
Disease |
0.1% |
90% |
0.009 |
0.9% |
|
No Disease |
99.9% |
10% |
0.999 |
99.1% |
|
|
|
|
Σ = 1.008 |
|
of disease given a positive test and is the same as in the first part of Table 4.4. The first sum at the bottom of the column headed "product of probabilities" (10.80 percent) multiplied by 100,000 would give the number of positive tests as shown at the right side of the first part of Table 4.4 above.
ADDENDUM 4B: INTERPRETATION OF INDETERMINATE AND UNSATISFACTORY FNA SAMPLES
The terms sensitivity and specificity are clearly defined only when the test result is either positive or negative. In the case of FNA, the test results can also be indeterminate and unsatisfactory. As shown in Table 2a and 2b in Appendix F, the test performance of FNA will depend upon whether one treats indeterminate and unsatisfactory test results as positive or negative. The following analysis illustrates how treatment of these results affects the calculation of the probability of cancer. For these illustrative purposes, the analysis relies on experience from a major academic center as reported in the table below, which presents the approximate probability of positive, negative, indeterminate, and unsatisfactory results conditioned on the actual findings for the nodule aspirated.
|
|
Cancer |
No Cancer |
|
Positive |
85% |
3% |
|
Negative |
7% |
65% |
|
Indeterminate |
4% |
20% |
|
Unsatisfactory |
4% |
2% |
|
|
100% |
100% |
If it is assumed that the prevalence of cancer among nodules biopsied is 3 percent, then (based on the tabular algorithm for Bayes rule presented in Table 4.4) one can revise the probability of cancer based on a positive FNA result:
|
Positive |
||||
|
Diagnosis |
Prior |
Conditional |
Product |
Revised |
|
Cancer |
3% |
85% |
255 |
46.7% |
|
No cancer |
97% |
3% |
291 |
53.3% |
|
|
|
|
Σ = 546 |
|
|
The probability of a positive result increases from 3 percent to 46.7 percent. |
||||
Similarly, after a negative FNA result:
|
Negative |
||||
|
Diagnosis |
Prior |
Conditional |
Product |
Revised |
|
Cancer |
3% |
7% |
21 |
0.3% |
|
No cancer |
97% |
65% |
6,305 |
99.7% |
|
|
|
|
Σ = 6,326 |
|
|
The probability of cancer given a negative test decreases from 3 percent to 0.3 percent. |
||||
Now consider the information provided by an indeterminate or an unsatisfactory FNA result.
|
Indeterminate |
||||
|
Diagnosis |
Prior |
Conditional |
Product |
Revised |
|
Cancer |
3% |
4% |
12 |
0.6% |
|
No cancer |
97% |
20% |
1,940 |
99.4% |
|
|
|
|
Σ = 1,952 |
|
|
Unsatisfactory |
||||
|
Diagnosis |
Prior |
Conditional |
Product |
Revised |
|
Cancer |
3% |
4% |
12 |
1.0% |
|
No cancer |
97% |
12% |
1,164 |
99.0% |
|
|
|
|
Σ = 1,176 |
|
In both cases, the probability of cancer decreases (from 3 percent to 0.6 percent and 1 percent, respectively). This change occurs because both of these results carry information: The chance of either an indeterminate or an unsatisfactory result is only 4 percent if the nodule contains cancer but is higher (20 percent and 12 percent, respectively) if the nodule is free of cancer, because cancers tend to be more cellular and because criteria for the diagnosis of cancer are often more explicit than criteria for diagnosing benign disease.
How, then, should an indeterminate result be treated? The physician might treat the interdeterminate result in the same way he or she might treat a positive result, and proceed to surgery. In that case:
|
Positive (including indeterminate results) |
||||
|
Diagnosis |
Prior |
Conditional |
Product |
Revised |
|
Cancer |
3% |
89% |
267 |
10.7% |
|
No cancer |
97% |
23% |
2,231 |
89.3% |
|
|
|
|
Σ = 2,498 |
|
would be only 10.7 percent, but 24.98 percent of patients would proceed to surgery (compared to 5.46 percent, as calculated above for positive FNA results) whereas 75.02 percent would avoid surgery.
In contrast, if an indeterminate result were treated as negative (i.e., the work-up stops), then the revised probability of cancer would be 0.4 percent because of false negatives. In this case, 82.78 percent of patients would avoid surgery (compared to 75.02 percent above).
|
Negative (including indeterminate results) |
||||
|
Diagnosis |
Prior |
Conditional |
Product |
Revised |
|
Cancer |
3% |
11% |
33 |
0.4% |
|
No cancer |
97% |
85% |
8,245 |
99.6% |
|
|
|
|
Σ = 8,278 |
|
In a similar vein, consider the effect of an unsatisfactory FNA. Because cancers are more likely to provide satisfactory samples, an unsatisfactory result lowers the probability of cancer, as shown above, from 3 percent to 1 percent. Recall that a negative FNA only lowers the probability of cancer to 0.3 percent (because of the possibility of false-negative results).







































