1
Introduction
The Origins of Radon
Naturally occurring radioactivity can be found throughout the earth's crust. Some of these radionuclides decay into stable elements, such as 40K ![]() 40Ar, 14C
40Ar, 14C ![]() 14N and 87Rb
14N and 87Rb ![]() 87Sr. Others are members of sequences of radioactive decay in which one radionuclide decays into another radionuclide. The three principal such series found in nature originate with 238U, 235U, and 232Th (NCRP 1987a).
87Sr. Others are members of sequences of radioactive decay in which one radionuclide decays into another radionuclide. The three principal such series found in nature originate with 238U, 235U, and 232Th (NCRP 1987a).
The immediate disposition of an atom created in a radioactive series depends on physical and chemical properties of the element and on the surrounding soil or rocks. Many of the elements in the process are metals such as uranium, thorium, polonium, lead, and bismuth or alkaline earths such as radium. These elements vary greatly in solubility depending on ambient physical and chemical conditions and may go into solution or be absorbed onto organic particles or clay minerals. Uranium, radium, and radon are the most mobile, lead and bismuth are only moderately mobile, while thorium and polonium remain relatively immobile.
One of the most abundant sources of naturally occurring radioactivity is the series that begins with 238U, which is illustrated in figure 1.1. The first 14 members in this series collectively emit gamma, beta, and alpha radiation. Because of the arrangement of half-lives and chemical properties, the concentration of radioactivity of the early members of the series is proportional to the concentration of 238U in the earth.
An important deviation happens roughly midway through the 238U series: 226Ra decays by alpha emission, thereby creating 222Rn. In contrast with other

Figure 1.1
Decay scheme for natural occurring 238U chain.
members of the series, which are solids, radon is a chemically-inert noble gas and can migrate in the environment.
Migration of Radon
A radon atom that is created deep within a grain of rock usually remains there until it decays. However, when a radon atom is created near the surface of a grain, it can recoil into the pore between grains; such radon atoms do not attach or bind to the matrix that contains the immediate precursor, radium. The amount of radon that reaches the pores is described by the emanation fraction. For typical soils or bedrock, the emanation fraction can range from 5% to 50% (see the review in Nazaroff 1992).
In most situations, the pore between grains of material contains a mixture of air and water. Often, a recoil radon atom will come to rest in the water and remain
there (Tanner 1980). In addition to this direct process, a gas is partitioned between the air and water in the pore. This partitioning is described by Henry's law in terms of the Oswald coefficient, K:
where Cw and Ca are the radon concentrations by volume (Bq m-3) in the water and air, respectively. The Oswald coefficient varies inversely with temperature. At 10 °C, KRn = 0.3; it increases to about 0.5 near 0 °C (Lewis and others 1987). If the soil or bedrock is completely saturated with water, all the available radon will be dissolved in the water.
Migration of radon in soil gas is controlled by two processes: molecular diffusion and advective flow. Diffusion is the process whereby molecules migrate toward regions with lower concentrations. Radon concentrations in soil gas are typically 40,000 Bq m-3 and concentrations 10 to 100 times this value are not uncommon. The main reason for this is that the radon atoms are confined within a small volume defined by the pore space between the soil grains. Thus, radon will preferentially diffuse toward regions that have lower concentrations, such as caves, tunnels, buildings, and the atmosphere.
Advective flow is controlled by pressure differences. Air will flow toward locations with lower pressure, and changes in atmospheric pressure can force air into or out of the ground. Very often, the air inside a building is warmer than air in the soil that is in contact with the building. This temperature difference causes a pressure gradient that draws air containing high concentrations of radon into the structure. Wind—as well as airflow from a fan, furnace, or fireplace—can also reduce pressures inside a building, compared with the pressures in the soil adjacent to the building foundation. These processes constitute the primary reason that radon enters and may be present in buildings at higher concentrations than in ambient air.
The water supply can also contribute to indoor radon. When water leaves a faucet, dissolved gases are released. This process is increased by mechanical sprays during a shower or by the heating and agitation that occur during laundering, washing, and cooking. The increase in the indoor radon concentration due to radon release from indoor water use is described by the transfer coefficient:
where (![]() a) is the average increase of the indoor radon concentration that results from using water having an average radon concentration of
a) is the average increase of the indoor radon concentration that results from using water having an average radon concentration of ![]() w. The various sources of radon and the resulting radiation exposure pathways are shown in figure 1.2.
w. The various sources of radon and the resulting radiation exposure pathways are shown in figure 1.2.
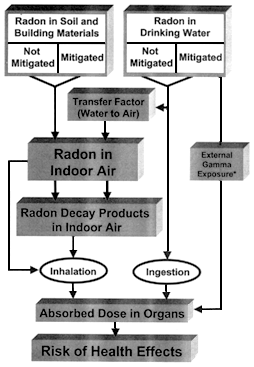
Figure 1.2
Sources of radon and related radiation exposure pathways.
*Gamma exposure from radon collected during some mitigation procedures (see Appendix E).
Exposure to Indoor Radon
The first four descendants of radon—218Po, 214Pb, 214Bi, and 214Po—are also radioactive and are collectively referred to as radon decay products. They are all metals and have half-lives ranging from a fraction of a second to 27 min (see figure 1.1). Indoors, some of these decay products come into contact with surfaces and are removed from the air by a process called plate-out. The rest of the decay products remain suspended in air as free atoms (unattached) or combined with other aerosols (attached). Although it is possible to measure the concentration of each radon decay product suspended in air, they are generally grouped. In addition, the concentration is not presented in terms of activity per unit volume (becquerel per cubic meter), but rather in terms of the total energy that would be released by alpha particles when all the short-lived atoms decayed completely. This quantity is called potential alpha energy (PAE), and the concentration in air
(PAEC), is measured in units of energy per unit volume of air (joules per cubic meter (J m-3).
The development of a PAEC from indoor radon concentration depends on air movement and aerosol conditions within a room. PAEC can depend on whether the radon entered a room from soil or from water during bathing. The relationship between indoor radon concentration and PAEC is expressed in terms of the equilibrium ratio (ER). For a room without any depletion of radon or plate-out of decay products, ER = 1.0. In domestic environments, ER ranges from 0.3 to 0.7 with a nominal value of 0.4 (Hopke and others 1995a).
The alpha-particle dose to lung tissues depends on PAEC and on the time that a person spends in a given location. A combination of PAEC and time is a measure of exposure expressed in joule-seconds per cubic meter (J s m-3).
Absorbed Dose from Indoor Radon
A person in a room will inhale radon decay products that are suspended in air. Some activity can deposit and accumulate in the respiratory airways, depending on breathing patterns and the aerodynamic size of the particles with which the decay products are associated. Because of the short half-lives, the radon decay products that are deposited in the lung will almost certainly decay completely in the lung. The radiations emitted within the lung during these decays can deposit energy in the body. However, this radioactivity is very near the lung epithelium, so alpha particles in particular can transfer copious amounts of energy to vulnerable cells. That is why radon decay products are characterized in terms of PAEC.
Radon gas itself is also inhaled. Most of it is exhaled immediately and therefore does not accumulate in the respiratory system, as do radon decay products. Because the radon does not get close to radiosensitive cells, the absorbed dose from alpha particles is small. However, some of the radon that reaches the interior region of the lung is transferred to blood and dispersed throughout the body. Radon and the decay products formed inside the body can deliver a radiation dose to tissues and organs.
On some occasions, water is consumed immediately after leaving the faucet before its radon is released into the air. This water goes directly to the stomach. Before the ingested water leaves the stomach, some of the dissolved radon can diffuse into and through the stomach wall. During that process, the radon passes next to stem or progenitor cells that are radiosensitive. These cells can receive a radiation dose from alpha particles emitted by radon and decay products that are created in the stomach wall. After passing through the wall, radon and decay products are absorbed in blood and transported throughout the body, where they can deliver a dose to other organs.
Ingested water eventually passes through the stomach into the small intestine, where the remaining radon and decay products are released from the water
and transferred to blood. They then circulate within the body; most are released from the blood into the lung and exhaled, but some remain in the blood and accumulate in organs and tissues, which receive an absorbed dose from alpha, beta, and gamma radiation.
Health Risks Posed by Indoor Radon
There is a direct implication between high doses of radiation and health effects in humans. For example, excess cancers have been observed in a cohort of survivors of the atomic-bomb blasts in Japan (National Research Council 1990a). A relationship between lung cancer and inhalation of radon decay products has been demonstrated in underground miners (Lubin and Boice 1997). Recent epidemiologic evidence suggests that inhalation of radon decay products in domestic environments could also be a cause of lung cancer (National Research Council 1999; Lubin and others 1995). Although the studies do not specifically identify health effects at low doses, there is compelling circumstantial evidence that they occur.
Under ambient conditions of low dose and low dose rate, any health effects associated with exposure to radon in air or water can be expected to occur from the passage of single alpha particles through individual cells. Any given cell is hit only once or not at all. An increase in exposure increases the number of cells that are hit, but it will not affect the primary damage experienced by each cell. Therefore, the initial events depend linearly on exposure or dose.
Exposed cells experience local damage in the form of DNA breaks and the products of reactive oxygen. The damage is metabolized by cellular-repair systems, and some fraction of it results in permanent genetic changes. Those changes can lead to the development of cancers; a cancer usually originates in a single transformed cell.
Risk projection models have been developed to predict the risk in situations where direct evidence is not available (National Research Council 1999; 1990a). The nature of the exposure to indoor radon, the kinds of DNA damage inflicted by alpha particles, and the extent of repair are consistent with the absence of a threshold for cancer induction. The preferred model is a straight line that reaches zero risk only when the dose or exposure is zero; it is referred to as the linear no-threshold (LNT) model.
Legislation and Regulations Regarding Indoor Radon
In 1988, Congress passed the Indoor Radon Abatement Act. Its stated goal was to reduce indoor radon concentrations to outdoor levels. The Environmental Protection Agency (EPA) was authorized to implement policies described in the law. In 1987 and again in 1992, EPA published A Citizen's Guide To Radon (EPA 1992a). The document summarized the risks associated with inhalation of
radon decay products in residential environments. It recommended that people measure indoor radon and consider taking action if the annual average concentration in their living areas exceeds 148 Bq m-3. EPA also developed programs in support of its recommendations for mitigation (Page 1993): public-information programs, a National Residential Radon Survey, Regional Radon Training Centers, the Radon Contractor Proficiency Program, the Radon Measurement Proficiency Program, Radon Reduction in New Construction, and support for the development of indoor-radon programs in individual states. As a result of those efforts, about 11 million of the approximate 100 million single family dwellings in the United States have been tested and about 300,000 (0.3%) mitigated in an effort to reduce indoor radon concentrations (CRCPD 1994). In addition, EPA estimates about 1.2 million new homes have been built with radon-resistant construction methods (A. Schmidt, personal communication), although the success of these methods is unknown.
It was recognized that water might also make a substantial contribution to and in some circumstances be the primary source of health risks associated with radon. In 1986, a revision to the Safe Drinking Water Act specifically required EPA to set a standard for 222Rn in drinking water (US Congress 1986). After litigation and a consent decree, EPA developed a criteria document that summarized the health effects of radon and its prevalence in drinking water (EPA 1991a). On the basis of the document and considerations of uncertainties in the analytic procedures for testing for radon in drinking water, a regulation was proposed in 1991 that established a maximum contaminant level (MCL) of 11,000 Bq m-3 (EPA 1991b). That MCL corresponded to an lifetime individual health risk of 10-4 posed largely by an increase in radon in indoor air.
During the period permitted for public response after the announcement of the proposed regulation, some groups supported reducing the MCL below 11,000 Bq m-3 because there is no known threshold for radiation-induced carcinogenesis. Others suggested raising the MCL because the increment in indoor-air radon from water radon at 11,000 Bq m-3 would be about 2% of the annual average residential radon concentration. There was also concern regarding the dosimetry model used to estimate the risk of stomach cancer associated with radon ingestion (Harley and Robbins 1994). As a result of those concerns, Congress intervened in 1992 and directed the administrator of EPA to prepare a multimedia risk assessment and cost estimates for compliance with regulations regarding radon in drinking water. The reanalysis resulted in EPA's revising its risk assessment for the ingestion of water containing radon. As a result, the ingestion risk and the inhalation risks (per unit of radon in drinking water) were estimated to be about equal (EPA 1994b). This document was reviewed by the Science Advisory Board (SAB) of EPA.
There was continuing concern about the estimates of stomach cancer resulting from radon ingestion. In addition, the SAB committee questioned the prudence of regulating a small increase in indoor radon from water without consid-
ering the larger reductions in risk that might be obtained by reducing radon concentrations originating from soil gas (EPA-SAB 1993a).
The Safe Drinking Water Act was amended again in 1996 (US Congress 1996). The proposed national primary drinking-water regulation for radon was withdrawn. Before proposing a new regulation for radon in water, EPA was instructed to ask the National Academy of Sciences to prepare a risk assessment for radon in drinking water on the basis of the best science available. The assessment was to consider each of the pathways associated with exposure to radon from drinking water at concentrations and conditions likely to be experienced in residential environments. The Academy was also asked to prepare an assessment of health-risk reductions that have been realized from various methods used to reduce radon concentrations in indoor air to provide a basis for considering alternative or multi-media mitigation schemes as opposed to mitigation of water alone.
Charge to the Committee
The Committee on the Risk Assessment of Exposure to Drinking Water in the National Research Council's Board on Radiation Effects Research began deliberations in July 1997. The specific tasks assigned to the committee were:
- To examine the development of radon risk assessments for both inhalation of air and ingestion of water.
- To modify an existing risk model if it were deemed appropriate or develop a new one if necessary.
- To review the scientific data and technical methods used to arrive at risk coefficients for exposure to radon in water.
- To assess potential health-risk reductions associated with various measures to reduce radon concentrations in indoor air.
The final report was to include:
- Estimates of lung, stomach, and other potential cancer risks per unit concentration of radon in water.
- Assessment of whether health effects of radon in drinking water could be estimated for various sub-populations at risk, such as infants, children, pregnant women, smokers, elderly persons, and seriously ill persons.
- Examination of evidence for teratogenic and reproductive effects in men and women due to radon in water.
- Estimates of the transfer coefficient that relates radon in water to radon in indoor air.
- Population-weighted estimates of radon concentrations in ambient air.
- Estimates of increases in health risks that could result from methods used to comply with regulations for radon in drinking water.
- Discussion of health-risk reductions obtained by encouraging people to reduce radon concentrations in indoor air with methods already developed and comparison of them with the risk reductions associated with mitigation of radon in water.
Composition of the Report
Chapter 2 presents baseline data regarding concentrations of radon in water and indoor air. It includes a discussion of radon concentrations measured in outdoor air throughout the United States and an estimate of a national annual average concentration of ambient radon.
Chapter 3 describes the transfer coefficient that expresses the increase in indoor airborne radon in reference to the concentration of radon in water. It includes a survey of measurements and theoretical considerations.
Chapter 4 discusses the dosimetry of ingested radon. It describes patterns of consumption of water directly from the tap or faucet. The calculations make extensive use of physiologically-based pharmacokinetic (PBPK) models that have been developed for dosimetry of internal radioactivity. The chapter includes computations of equivalent dose and risk to individual tissues and organs. A special model was developed to estimate the concentration of radon and the alpha-particle radiation dose produced by decay of radon and its decay products occurring next to sensitive cells in the stomach wall.
Chapter 5 discusses the risk associated with inhalation of radon and radon decay products. It includes a summary of the methods used to form risk-projection models that were developed by the National Research Council's committees on Biological Effects of Ionizing Radiation (BEIR).
Chapter 6 discusses the basic mechanisms that are believed to be responsible for radiation-induced carcinogenesis.
Chapter 7 presents an analysis of the uncertainty and precision associated with the risk estimates obtained in the previous chapters.
Chapter 8 discusses the methods and efficiencies of radon mitigation in both indoor air and water. It includes an examination of techniques for reducing radon concentrations in existing buildings and procedures for reducing radon in new construction.
Chapter 9 analyzes the concepts associated with a multimedia approach to risk reduction. Several scenarios illustrate various ways to evaluate gains in risk reduction by using an alternative AMCL for water with other indirect approaches that encourage or even enforce mitigation in indoor air.
The committee's research recommendations are summarized in chapter 10.
A glossary and six appendixes present specific details and methods that were incorporated in the various chapters.









