C Water-Mitigation Techniques
Aeration
A wide array of aeration methods can be used to remove radon from drinking water (table C.1). Some are already being used in the United States to treat radon in municipal drinking water (table 8.1); others have been used only in point-of-entry applications or are still being developed.
Packed-Tower Aeration
The most common technology currently used for treatment of large flows of water with high radon concentrations is packed-tower aeration (PTA). PTA is efficient because it has a high surface area available where mass transfer can occur. Usually, raw water is sprayed into the top of the tower (3–9 m high) and trickles down over plastic packing (for example, rings and saddles) that has a high ratio of surface area to volume. Simultaneously, a flow of air is pumped through the packing. Typically, this is a countercurrent flow of air, which enhances radon removal. The treated water is collected in a reservoir below the tower and pumped to a pressurized storage tank or directly into the distribution system. Air containing the radon is released from the top of the tower. One variant on PTA is cross-current technology in which the air flow is perpendicular to the water trickling down, so less energy is required to supply a given amount of air to the system.
Diffused-Bubble Aeration
Diffuser systems inject air (usually as bubbles) into water. The radon moves from the water to the bubbles as they rise through the liquid. Smaller bubbles,
Table C.1
Aeration Technologies Used for Removing Radon from Water
|
Common Name |
Other Name |
|
Packed tower aeration (PTA) |
— |
|
Diffused bubble aeration |
— |
|
Spray aeration |
— |
|
Tray aeration |
Slat-tray aeration |
|
Jet aeration |
Venturi or ejector aeration |
|
Shallow-tray aeration |
Sieve tray aeration |
|
Cross-current packed-tower aeration |
— |
|
Cascade aeration |
— |
|
Pressure aeration |
Aeration in hydrophor degasification |
although more difficult and expensive to produce, provide a greater surface area per unit volume over which mass transfer can occur. In the most common systems, the water passes through a series of tanks (0.5–6 m deep), simulating a plug-flow reactor. The radon-contaminated air leaves the water when the bubbles reach the surface and is vented out of the unit. Diffused aeration systems cannot match the surface area available for radon transfer from water to air in PTA, but they can be easily retrofitted into basins and made as compact package units to treat small to medium flows. Shallow-tray (20–30 cm deep) aeration is a variant of this technology in which a thin layer of water passes across a series of plates perforated with holes. Air coming up through the holes causes the water to froth, and mass transfer occurs.
Spray Aeration
In spray aeration, water is formed into droplets (with a high ratio of surface area to volume) when it is forced through a nozzle. The droplets are sprayed upward, downward, or at an inclined angle into a large volume of air that is often flowing in a countercurrent direction. The simplicity of spray-aeration systems means that they can easily be retrofitted onto the inlet of an existing atmospheric storage tank to enhance radon removal from water. Their radon-removal efficiency is mainly a function of the size of the water droplets and the ratio of air to water (A:W ratio).
Tray Aeration
Tray-aeration systems are similar to countercurrent PTA except that the tower contains a series of slats (for example, made of redwood) or trays with perforated bottoms (for example, made of wire mesh). In some cases, a solid medium (such as, stone, ceramic spheres) is placed in the trays to promote transfer of radon to the air (Drago 1998). Water entering the top of the aerator is
distributed over the trays or slats. Natural, forced, or induced draft causes air to flow past the thin film of water formed.
Cascade Aeration
Cascade aeration, a very simple technology, involves construction of a series of steps over which water tumbles as in a waterfall. The system requires a hydraulic head to operate, but little else except a method of ventilation to remove the radon-contaminated air from the unit.
Jet Aeration
Use of jet-aeration systems is favored in Europe, where they can be retrofitted into existing small storage-tank systems. The water is pumped through a venturi-like device (such as a jet, eductor, or ejector) that aspirates air into the water. The radon-contaminated air is released, and the treated water falls into an atmospheric storage tank. The water must be recirculated through the system several times before high removal efficiencies (>75%) are obtained. A venturi system tested on two US water supplies achieved radon-removal efficiencies of 78–95%.
Pressure Aeration
In pressure aeration, air is injected into a pressurized chamber [for example, tank (hydrophor), pipe]. The gas is released when the water is allowed to come to atmospheric pressure. Although this technology uses lower A:W ratios (1:1) compared to other aeration methods (10:1 to > 100:1), it might be applicable only to special situations because the energy required to inject the air can be very high.
Granular Activated Carbon
Granular activated carbon (GAC) is made by subjecting materials such as bone, wood, or coconut shells to high heat and pressure. These processes increase the surface area of the material and activate it, improving its ability to adsorb substances, including organic chemicals, and dissolved gases. GAC has a finite number of sites where it can adsorb a specific substance. Hence, it normally becomes saturated with the contaminant that it is removing from water over the course of days to months and must be replaced or regenerated (for example, by steam-cleaning when used to remove volatile organic compounds) to sustain an adequate level of treatment.
Adsorption of radon from water does not follow the typical saturation model observed for many contaminants, but instead can be modeled with a steady-state first-order equation first proposed by Lowry and Brandow (1985). The relation-
ship can be used to calculate important design variables, such as the empty-bed contact time (EBCT) and the volume of GAC needed to reach a desired effluent quality.
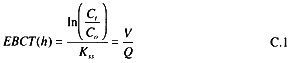
where
Ct = effluent radon concentration (kBq m-3),
Co = influent radon concentration (kBq m-3),
Kss = the adsorption/decay constant specific for the GAC and the water treated (h-1),
V = volume of GAC needed (m3), and
Q = flow of water treated (m3 h-1).
It has been suggested that saturation does not occur because the radon decays, allowing a new atom of the gas to be adsorbed to the same GAC site (Lowry and Brandow 1985). Others have suggested that very long run times occur before saturation because of the very low mass of radon being adsorbed (Kinner and others 1993; Cornwell and others 1999).
When used for small flows, the carbon is usually placed in a closed vessel (constructed of, for example, fiberglass or carbon steel), and the water is forced through the bed, using the pressure exerted by the well pump. Therefore, repumping is not required, because there is no break to atmospheric pressure. In large municipal facilities, operated at atmospheric pressure, the hydraulic head from the water above the GAC causes the water to flow past the GAC. In either system, head-loss problems resulting from accumulation of turbidity-causing substances or precipitates can be alleviated by backwashing. The effect of backwashing on radon removal is not clear; some studies have shown a decline in efficiency after backwashing (Lowry and Brandow 1985) (Lowry and others 1990), and others have not (Kinner and others 1990; Cornwell and others 1999). Lowry and others (1990) have observed desorption of radon during and immediately after backwashing, but the radon progeny remain sorbed.
When the efficiency of a GAC unit in removing radon from water begins to decline (Lowry and others 1991; Kinner and others 1989; Kinner and others 1990; Kinner and others 1993; Cornwell and others 1999), the GAC is usually not regenerated, although it is technically possible to remove accumulated 210Pb by using an acid pumped through the bed or by thermal desorption (Lowry and others 1990). Instead, it is usually easier to dispose of the old carbon before it accumulates a significant amount of radioactivity and add new GAC.
Vacuum Deaeration and Hollow-Fiber Membrane Technology
Vacuum deaeration (VD) exploits the high Henry's Law constant of radon by spraying the raw water into an enclosed tower that contains a packing material. A vacuum is applied to the top of the unit by an eductor or pump. The vapor is redissolved with an eductor into a continuously recirculating stream of water that passes through the GAC bed. Noncondensable gases (such as 02, N2, and CO2) are released from the sidestream via a constant-head tank and an oil trap. The efficiency of radon removal from the water is strongly linked to the strength of the vacuum. At high vacuums (under 0.1 atm absolute pressure), removals in the 70% range have been observed. Two difficulties with the process are the low efficiency of transfer of radon into the stream of recirculating water and the desorption of radon from the GAC (only 20–30% net radon removal observed). Implementation of this complex technology, which would be applicable only to medium and large flows, must await further testing.
The hollow-fiber membrane (HFM) technology is equally complex and differs from the VD process only in using a column of membranes, instead of a tower with packing, to remove radon from water initially. The raw water passes along one side of a series of microporous membranes. A stream of air induced by a vacuum passes along the other side. The radon and other dissolved gases are transferred to the air under vacuum. Again, the efficiency of transfer is a function of the strength of the vacuum applied. With a bench-scale system, a radon removal efficiency of 40–56% was obtained (Drago 1997). The problems with dissolving the radon in the sidestream and the removal efficiency of the GAC (40–80%) observed in evaluations of the HFM system are similar to those for the VD system. Applicability of HFM must be evaluated on pilot-and full-scales before it could be considered a best available technology for radon removal for medium and large communities, which need to remove radon from water to avoid discharging it to the atmosphere via the off-gas.





