4
Dosimetry of Ingested Radon and its Associated Risk
Estimates of dose to various tissues of the body from ingestion of radon dissolved in drinking water and the resulting health risks are developed in this chapter. A review of the literature indicated a wide range in the reported dose per unit intake (dose coefficient), and neither national nor international radiation-protection agencies have provided authoritative values. In particular, values reported for the dose to the stomach per unit radon activity ingested (a dose coefficient) vary widely, are often based on assumptions that are not documented, and often are not based on contemporary dosimetric methods. The central issues are: 1) the extent to which radon diffuses into the wall of the stomach, and 2) the behavior of radon and its decay products in the body. Studies of the behavior in the body of inhaled and ingested radon indicate that radon is readily absorbed by blood and is rapidly eliminated from the body in exhaled air. Because of the wide range in dose coefficients reported in the literature, the committee has undertaken an independent dosimetric analysis using the methods of contemporary radiation dosimetry.
The chapter begins with a brief review of the relevant physiochemical properties of radon, the consumption of drinking water, and estimates of dose and risk reported in the literature. Following these introductory discussions the committee's estimates of dose and risk are presented.
Intakes And Consumption Of Water
In regulating other drinking water contaminants (EPA 1994b) the US Environmental Protection Agency currently uses the quantity 2 L per day for adults
and 1 L per day for "infants" (individuals of 10 kg body mass or less) as default drinking water intakes (EPA 1994b). The combined mean value of 1.2 L d-1 consists of both direct use, such as tapwater ingestion as well as indirect use, such as juices and other beverages that contain tapwater, such as coffee. A National Research Council committee (1977) has suggested that daily consumption of water can vary with extent of physical activity and fluctuations in temperature and humidity and that people who live in warmer climates might have higher intakes of water.
Numerous studies have developed data on drinking-water intake. All the studies that are available were based on short-term survey data. One of the more commonly cited studies on water intake is the Ershow and Cantor (1989) study. They estimated water intake on the basis of data collected by the US Department of Agriculture 1977–1978 Nationwide Food Consumption Survey and calculated daily intake and total water intake by various age groups of males, females, and both sexes combined. They defined tapwater as "all water from the household tap consumed directly as a beverage or used to prepare foods and beverages" and defined total water intake as tapwater plus "water intrinsic to foods and beverages." Table 4.1 summarizes data from the Ershow and Cantor study.
The combined mean value of 1.2 L d-1 (table 4.1) is for all uses of tapwater, which consists of both direct use (i.e., direct ingestion) and indirect use, i.e. making coffee, tea, etc. As noted by the EPA (1994b), the concern about radon dissolved in water is largely for the water that is ingested directly. The EPA has estimated that slightly more than half of the tapwater use is directly ingested. The committee has adopted a value of 0.6 L d-1 for direct use. This value is similar to that used by the EPA in their Multimedia Risk Assessment (EPA 1994b). However, the committee has conservatively assumed for direct use, that all of the radon in the tapwater remains dissolved in the process of transferring the water from the tap to the stomach.
Physicochemical Properties Of Radon
Radon, a noble gas, is essentially chemically inert. Unlike the other noble gases, radon has no known stable isotope. Rather it has 36 radioactive isotopes and isomers, which range in mass number from 198 to 228. The radon isotope of interest here is 222Rn (physical half-life, 3.825 d), a member of the decay series beginning with the primordial radionuclide 238U. 222Rn emits alpha particles as it spontaneously decays to a series of short-lived radioactive decay products, which are followed by a longer-lived series headed by 210 Pb (half-life, 22.3 y), as shown earlier in figure 1.1. The cumulative energies of the radiation emitted by the members of the decay series (alpha particles, electrons, and photons) are shown in table 4.2. The tabulated values represent the average or expected energy of the indicated radiation emitted per atom of 222Rn initially present. The entry for a particular member includes the contribution of the member and its precursors
Table 4.1
Tapwater Intake by Sex and Agea
|
|
Daily Tapwater Intake (mL) |
|
|
|
||
|
Age Group (y) |
Mean |
SD |
Median |
90% UCLb |
95% UCLb |
99% UCLb |
|
Males |
||||||
|
<0.5 |
250 |
232 |
240 |
569 |
757 |
NA |
|
0.5 to 0.9 |
322 |
249 |
264 |
634 |
871 |
NA |
|
1 to 3 |
683 |
406 |
606 |
1228 |
1464 |
2061 |
|
4 to 6 |
773 |
414 |
693 |
1336 |
1530 |
1900 |
|
7 to 10 |
802 |
437 |
738 |
1391 |
1609 |
2055 |
|
11 to 14 |
970 |
547 |
877 |
1714 |
2019 |
2653 |
|
15 to 19 |
1120 |
644 |
1019 |
1974 |
2283 |
3090 |
|
20 tn 44 |
1354 |
788 |
1216 |
2309 |
2837 |
4065 |
|
45 to 64 |
1633 |
783 |
1510 |
2650 |
3094 |
4213 |
|
65 to 74 |
1594 |
719 |
1457 |
2502 |
2812 |
NA |
|
>75 |
1517 |
667 |
1443 |
2332 |
2696 |
NA |
|
All ages |
1250 |
759 |
1123 |
2205 |
2673 |
3760 |
|
Females |
||||||
|
<0.5 |
293 |
259 |
240 |
672 |
800 |
NA |
|
0.5 to 0.9 |
333 |
281 |
278 |
712 |
759 |
NA |
|
1 to 3 |
606 |
368 |
532 |
1114 |
1339 |
1806 |
|
4 to 6 |
709 |
395 |
622 |
1231 |
1491 |
1932 |
|
7 to 10 |
772 |
395 |
726 |
1299 |
1475 |
1888 |
|
11 to 14 |
881 |
490 |
797 |
1531 |
1814 |
2382 |
|
15 to 19 |
883 |
513 |
800 |
1565 |
1839 |
2452 |
|
20 to 44 |
1182 |
634 |
1089 |
1996 |
2323 |
3132 |
|
45 to 64 |
1483 |
670 |
1394 |
2303 |
2668 |
3666 |
|
65 to 74 |
1429 |
603 |
1360 |
2247 |
2561 |
3082 |
|
>75 |
1300 |
540 |
1250 |
1998 |
2242 |
2933 |
|
All ages |
1147 |
648 |
1049 |
1988 |
2316 |
3097 |
|
Males and Females Combined |
||||||
|
<0.5 |
272 |
247 |
240 |
640 |
800 |
NA |
|
0.5 to 0.9 |
328 |
265 |
268 |
688 |
764 |
NA |
|
1 to 3 |
646 |
390 |
567 |
1162 |
1419 |
1899 |
|
4 to 6 |
742 |
406 |
660 |
1302 |
1520 |
1932 |
|
7 to 10 |
787 |
417 |
731 |
1338 |
1556 |
1998 |
|
11 to 14 |
925 |
521 |
838 |
1621 |
1924 |
2503 |
|
15 to 19 |
999 |
593 |
897 |
1763 |
2134 |
2871 |
|
20 to 44 |
1255 |
709 |
1144 |
2121 |
2559 |
3634 |
|
45 to 64 |
1546 |
723 |
1439 |
2451 |
2870 |
3994 |
|
65 to 74 |
1500 |
660 |
1394 |
2333 |
2693 |
3479 |
|
>75 |
1381 |
600 |
1302 |
2170 |
2476 |
3087 |
|
All ages |
1193 |
702 |
1081 |
2092 |
2477 |
3415 |
|
a Data from Ershow and Cantor (1989). b UCL = upper confidence limit. |
||||||
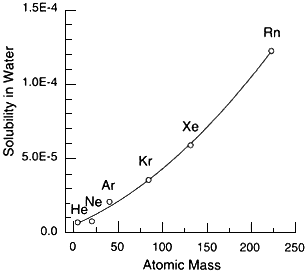
Figure 4.1
Solubility of the noble gas elements in water (CRC 1996) at body temperature, shown as a function of atomic mass. The solubility is expressed as the mole fraction of the gas in the mixture.
(listed above it), including 222Rn. The kinetic energy of the emitted alpha particles for the 222Rn series (24.5 MeV) accounts for 89% of the total emitted energy (27.6 MeV). A substantial fraction (78%) of the alpha energy is associated with the short-lived radon decay products (19.2 of 24.5 MeV). If an atom of 222Rn entered the body, in the absence of any biologic removal mechanisms for it or its decay products, the energies listed in table 4.2 would be available for deposition within the tissues of the body. However, ingested and inhaled radon is known to be promptly removed from the body by exhalation. Biologic removal processes are also applicable to the decay products formed within the body, but the short half-life of some decay products limits the importance of these removal processes. The decay products formed within the body may enter their own metabolic pathways and routes of excretion from the body. Ingested radon is removed from the body through exhalation while the longer-lived decay products are eliminated by urinary and fecal excretion.
The extent to which radon is absorbed from the gastrointestinal (GI) tract and retained in the body is determined, in part, by its solubility in blood and in the tissues. The solubility of the various noble gases in water (CRC 1996) at body temperature is shown graphically as a function of atomic mass in figure 4.1. Radon is considerably more soluble in water than the lighter noble gases—about 15 times as soluble as helium and neon. Data on solubilities of the noble gases in body tissues exhibit a similar relationship although the data are more variable.
Table 4.2
Cumulative Energy of Radiations Emitted in the Decay of 222Rn and Members of Its Decay Series
|
|
Energy (MeV per 222Rn atom) |
|
|
|
|
|
Nuclide |
T1/2 |
Alpha |
Electron |
Photon |
Total |
|
Rn-222 |
3.8235 d |
5.49 |
— |
0.000399 |
5.49 |
|
Po-218 |
3.05 m |
11.5 |
— |
0.000408 |
11.5 |
|
Pb-214 |
26.8 m |
11.5 |
0.293 |
0.250 |
12.0 |
|
Bi-214 |
19.7 m |
11.5 |
0.952 |
1.76 |
14.2 |
|
Po-214 |
164.3 µs |
19.2 |
0.952 |
1.76 |
21.9 |
|
Pb-210 |
22.3 y |
19.2 |
0.990 |
1.76 |
22.0 |
|
Bi-210 |
5.01 d |
19.2 |
1.38 |
1.76 |
22.3 |
|
Po-210 |
138.38 d |
24.5 |
1.38 |
1.76 |
27.6 |
Radon is readily absorbed from the GI tract and distributed among the tissues, in part because of its relative solubility in blood and in tissue. The ratio of solubility in tissue to that in blood is referred to as the partition coefficient. Measurements of the solubilities and partition coefficients of argon, krypton, xenon, and radon have been reported. Considerable data are available on xenon because of its use in assessing blood flow. Data on radon are less plentiful; the work of Nussbaum (1957) is their major source. Data on the partition coefficients of krypton, xenon, and radon are summarized in table 4.3. Of particular note are the higher partition of radon in blood (7 times that of krypton) and its higher partition in adipose tissue. Adipose tissue is the major tissue of deposition of radon that has entered the systemic circulation.
Estimates of Dose from Ingested Radon
The inhalation hazard of radon and its short-lived decay products has long been of concern in occupational radiation protection and public health. Ingestion
Table 4.3
Partition Coefficients of Noble Gases
|
Organ |
Krypton |
Xenon |
Radon |
|
Blood/air |
0.06 |
0.18 |
0.43 |
|
Adipose tissue/blood |
5.50 |
8.00 |
11.2 |
|
Muscle/blood |
1.09 |
0.70 |
0.36 |
|
Brain/blood |
1.13 |
0.75 |
0.72 |
|
Kidney/blood |
— |
0.65 |
0.66 |
|
Testes/blood |
— |
— |
0.43 |
|
Liver/blood |
— |
0.70 |
0.71 |
|
Bone/blood |
— |
0.41 |
0.36 |
|
Lung/blood |
— |
0.70 |
0.70a |
|
GI-tract/blood |
— |
0.81 |
0.70a |
|
Other/blood |
— |
— |
0.70 |
|
a Default values used in analysis. |
|||
of radon has received considerably less attention from radiation protection agencies who are primarily concerned with occupational exposures, in part because ingestion intakes are readily avoided in the workplace. Thus, recommendations of the International Commission on Radiological Protection (ICRP) and the National Council on Radiation Protection and Measurements (NCRP) have not included guidance for the control of ingested radon. In the absence of such guidance a number of investigators have undertaken dosimetric and risk assessments of ingested radon.
The fate of radon in the body has been the subject of several investigations. In 1951, Harley and others (1994; 1958) examined the elimination of radon from the body in a series of measurements of radon in exhaled air after chronic inhalation. Hursh and others (1965) investigated the fate of ingested radon and proposed a concentration limit on radon dissolved in water. Von Doebeln and Lindell (1964) investigated the retention of ingested radon in the body. Brown and Hess (1992) investigated the transfer and kinetics of ingested radon. The retention in the body of the noble gases argon, krypton, and xenon were studied by Tobias and others (1949), Ellis and others (1977), Susskind and others (1977; 1976), and Bell and Leach (1982). The results of those investigations support a number of general observations:
- Ingested radon is absorbed from the gut.
- Exhalation is the major route of elimination from the body.
- Ingested radon is largely eliminated within an hour.
- Body adipose tissue is the major site of long-term retention.
The absorption and retention of inert gases in human body tissues have been extensively studied by several authors, including: Smith and Morales (1944), Morales and Smith (1944), Kety (1951), Bernard and Snyder (1975), Bell and Leach (1982), Palazzi and others (1983), Peterman and Perkins (1988), Harley and Robbins (1994), Sharma and others (1996). In vitro studies have provided data on the solubilities and partition coefficients of the noble gases and other chemically inert substances in human blood, adipose tissue, and individual tissues; the data have been summarized by Steward and others (1973).
Hursh and others (1965) derived a value for the maximum permissible concentration of 222Rn in water on the basis of limiting the dose to the stomach. They assumed that radon diffuses through the stomach wall and enters the splanchnic blood flowing to the liver. The concentration of radon in the stomach wall was taken, conservatively, to be equivalent to that in the stomach contents. Von Doebeln and Lindell (1964) used the data of Hursh and others to estimate the dose to the stomach. More recently, Crawford-Brown (1991; 1989) estimated the dose to the stomach using a linear radon concentration profile in the wall. The dose to other organs was based on kinetics inferred from measurements of retention of ingested 133Xe (Correia and others 1987). Harley and Robbins (1994) used
a compartment model (the parameter values are not given in the paper) for the distribution of radon in the body based on data from Hursh and others (1965), Harley and others (1994), and Harley and Robbins (1994). They assumed that a fraction of the ingested radon diffuses into the stomach wall but that the vascular structure in the mucosa intercepts the radon before it reaches a depth from which the alpha emissions could irradiate the stem cells (N. Harley personal communication, see appendix A). Sharma and others (1996) used the compartment model of Peterman and Perkins (1988) and the data of Brown and Hess (1992) to estimate the dose from dissolved radon. They make no statements regarding the diffusion of radon into the stomach wall and computed, in a most unusual manner, the stomach dose as the alpha energy emitted within the stomach contents divided by the mass of the stomach wall and contents (C.T. Hess personal communication). The estimates of the dose to the stomach obtained by the various investigators are listed in table 4.4. All authors assumed that the short-lived decay products of radon decayed at the site of the 222Rn decay; that is, the alpha energy of 19.2 MeV (see table 4.1) was associated with each 222Rn decay. Those specifically considering the diffusion of radon within the stomach wall generally associated the first two alpha emissions, 11.5 MeV, with the radon decay in the stomach wall.
The data in table 4.4 indicate that the estimated dose to the stomach depends on the extent to which the investigators considered diffusion as a mechanism by which radon comes into intimate contact with the stomach wall; the highest dose coefficient is about 200 times the lowest (Sharma and others 1996; Harley and Robbins 1994; Brown and Hess 1992; Crawford-Brown 1989; Suomela and Kahlos 1972; Hursh and others 1965; Von Doebeln and Lindell 1964). Except for Harley and Robbins (1994), none of the investigators identified any basis for their assumption regarding the movement or lack of movement of radon into the stomach wall. Harley and Robbins (1994) assumed that the absorption of radon follows that of water which is predominantly from the small intestine, and cited the large countercurrent flow of fluid (1500 mL per day) from the stomach wall as
Table 4.4
Summary of Estimates of Equivalent Dose to Stomach per Unit Activity of 222Rn Ingested
|
Authors |
Diffusion |
Dose Coefficient, Sv Bq-1 |
|
Hursh and others (1965) |
Yes |
1.1 × 10-7 |
|
Von Doebeln and Lindell (1964) |
Yes |
1.1 × l0-7 |
|
Suomela and Kahlos (1972) |
Yes |
1.3 × 10-7 |
|
Crawford-Brown (1989) |
Yes |
3.0 × 10-7 |
|
Brown and Hess (1992) |
See footnotea |
8.8 × 10-8 |
|
Sharma and others (1996) |
See footnotea |
8.2 × 10-8 |
|
Harley and Robbins (1994) |
Yes |
1.6 × 10-9 |
|
a Dose averaged over the mass of the stomach wall and contents. |
||
evidence for lack of significant wall transport. No experimental evidence was provided by any of the investigators as a basis for their assumptions concerning the movement or lack of movement of radon into the stomach wall.
Behavior of Radon in the Body
The investigations by Harley and colleagues (1994), Harly and Robbins (1994) and Hursh and others (1965) provide basic information on the behavior of radon in the body. Harley and coworkers fit their observations to a function involving five exponential terms and associated the terms with tissue compartments. Bernard and Snyder (1975) interpreted the Harley data using a mammillary model to derive their estimates of the distribution of radon among the tissues. It is possible to observe the tissue distribution of noble gases in medical studies using radioisotopes of krypton and xenon. Correia and others (1987) used nuclear-medicine instrumentation to observe the behavior of ingested 133Xe and then attempted to infer the fate of ingested radon. Presently, it is considered that compartment models describing the movement of a contaminant within the body which are consistent with anatomic and physiologic principles provide the best basis for interpretation of experimental observations. Models based on physiologic principles are referred to as physiologically based pharmacokinetic (PBPK) models. Details regarding this modeling practice are given in a review by Bischoff (1986).
The committee has used a PBPK model of ingested radon that was formulated using the blood-flow model of Leggett and Williams (1995) (see also Leggett and Williams 1991; Williams and Leggett 1989). The resulting model, shown in figure 4.2, is discussed in detail in appendix A. Briefly, radon is distributed by the blood flow to the organs, where its transfer depends on its solubility in tissue relative to that in blood—the partition coefficient. The blood volume of the body is apportioned among a number of compartments, which represent various blood pools. In figure 4.2, the compartment ''Large Veins'' represents the venous blood return from the systemic tissues, "Right Heart" and "Left Heart" the content of the heart chambers, "Pulmonary" the blood-exchanging gases in the lung, and "Large Arteries" represents the arterial blood flow to the systemic tissues. The compartment labeled "Gut Cont" in figure 4.2 is expanded in figure 4.3, where the GI tract is divided into four segments, the compartment "St Contents," representing the contents of the stomach, "SI Contents" that of the small intestine, "ULI Contents" that of the upper large intestine, and "LLI Contents" that of the lower large intestine. "St Wall," "SI Wall," "ULI Wall," and "LLI Wall" represent the walls of those segments. Ingested radon enters the stomach and is absorbed from the gut as indicated in the upper right of figure 4.3. In figures 4.2 and 4.3, dashed arrows denote the transfer of radon as a gas, and solid arrows correspond to the flow of radon dissolved in arterial (thicker arrows) and venous blood. As shown in figure 4.3, the gut is perfused by arterial blood,
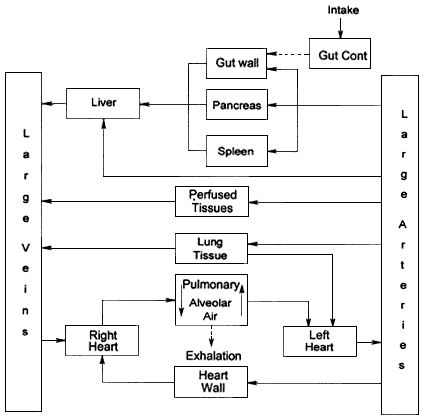
Figure 4.2
Diagram of PBPK model used for ingested radon (see Appendix A for details).
which, with blood flowing from the spleen and pancreas, enters the portal circulation to the liver as shown in figure 4.2. Venous blood is pumped by the right side of the heart to the pulmonary region of the lung, the "Pulmonary" compartment of figure 4.2, where radon dissolved in blood exchanges with alveolar air and is exhaled.
Ingested 222Rn is readily absorbed and appears promptly in exhaled air. The studies of ingested radon have indicated that retention in the body is somewhat greater when radon is ingested with food (Brown and Hess 1992; Hursh and others 1965). The increased retention is presumably a result of the slower transfer of foodstuffs from the stomach to the small intestine. Whereas the small intestine is the major site of absorption of most nutrients, fractional amounts of some
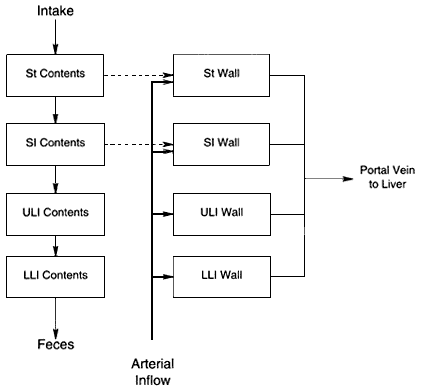
Figure 4.3
Expansion of the gut compartments of figure 4.2 to show the four segments of the gastrointestinal tract.
materials, such as water, aspirin, and alcohol, are known to be absorbed from the stomach.
Further details regarding the radon biokinetic model, including the numerical values of the transfer coefficients of the resulting differential equations, are given in appendix A. The equations are solved by assuming that a unit activity (1 Bq) of 222Rn is present in the stomach contents at time zero. The fractions of ingested radon that remain in the body (in the contents of GI tract and in systemic tissues) at various times after intake are shown in figure 4.4. The fraction of the initial activity residing in various tissues as a function of time is shown in figure 4.5. The high radon uptake in the liver shown in figure 4.5 is a direct reflection of the fact that all radon absorbed from the GI tract flows in blood from the GI tract walls to the liver. The importance of adipose tissue as a site of deposition and retention can be seen at later times; beyond about 30 min it is the major site of radon deposition in the body.
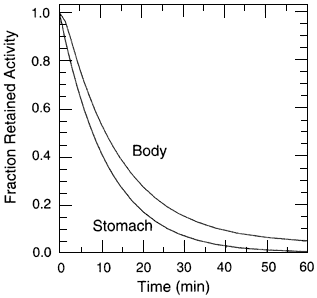
Figure 4.4
The fraction of ingested radon remaining in the body (with the contents of the gut and in systemic tissues) at various times following an intake by ingestion.
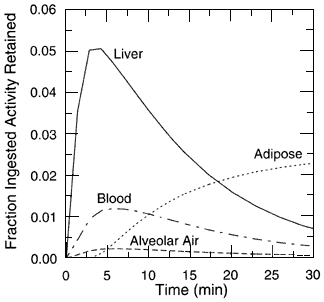
Figure 4.5
The fraction of the initial activity ingested residing in various tissues of the body as a function of time following the intake.
The PBPK model's predictions of radon retention in the body are compared with the observations of Hursh and others in figure 4.6. The solid line in figure 4.6 is the PBPK prediction, and the dashed lines are the fits of Hursh and others to their data on two male subjects who ingested radon on two occasions. The upper dashed line represents the retention observed shortly after a subject had ingested a breakfast that included "heavy whipping cream." The other observations were at least 2 h after a "normal light" breakfast. The comparisons in figure 4.6 were limited to the time period of the observations. The higher retention after the high-fat meal is consistent with the general observation noted above and presumably reflects a slower emptying of the stomach.
Figure 4.7 compares the PBPK model's prediction of radon retention with that of the mammillary model of Bernard and Snyder (1975) based on the Harley and others (1994; 1958) data on washout of inhaled radon. Also shown in the figure is the retention indicated by Crawford-Brown (1989) based on his analysis of the Correia and others (1987) data. The differences between the model predictions appear to be much as expected; for example, the Bernard and Snyder model might be expected to underestimate the retention because it was derived

Figure 4.6
The PBPK model's predictions of radon retention in the body are compared to the observations of Hursh and others (1965). The solid line of figure 4.6 is the PBPK predictions and the dashed lines are the fits of Hursh et al. to their data for two male subjects who ingested radon on two different occasions.
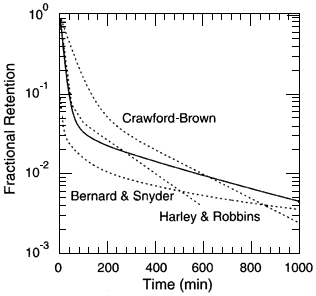
Figure 4.7
A comparison of the PBPK model predictions for the retention of radon with that indicated by the mammillary model of Bernard and Snyder (1975) based on the Harley and others (1994) data on the washout of inhaled radon. Also shown in the figure is the retention indicated by Crawford-Brown (1989) based on his analysis of the Correia and others data (1987) and the results of Harley and Robbins (1994).
from inhaled radon. The PBPK model appears to provide reasonable predictions of retained radon, and its physiological basis provides the basis of the distribution of radon among the organs of the body.
Diffusion of Radon in the Stomach
As seen from table 4.4, the dose to the stomach depends strongly on whether radon is considered to move into the stomach wall, presumably by diffusion. Alpha particles emitted within the contents of the stomach cannot penetrate the mucus layer lining the epithelium and cannot reach the stem cells at risk (the range of alpha particles in tissue is about 50–60 µm). This mucus layer is thought to be important in minimizing the exposure of the stomach epithelium to the acidic environment of the gastric lumen (Livingston and Engel 1995) and possibly acts as a barrier to the gastric absorption of drugs (Larhed and others 1997). The layer is composed primarily of mucin mol-
ecules that are continuously secreted and degraded by the gastric acids; although it is about 95% water, it substantially slows the diffusion of acid relative to water alone. It has been hypothesized that the continual movement of water and new mucin molecules away from the epithelial surface also carries acid away from the epithelium by convection (Livingston and Engel 1995). The interplay of these factors has been mathematically modeled (Engel and others 1984), but many of the physiological parameters of the model have not been measured.
In view of the importance of the diffusion mechanism in estimating the dose to the stomach, the committee found it useful to formulate a model within which it could investigate this mechanism. The model consisted of a spherical representation of a stomach with a volume of 250 mL. A mucus layer 50 µm thick was assumed. This was followed by a layer of surface cells 50 µm thick. The stem cells were considered to be distributed throughout a layer of tissue 200 µm thick. Below this layer, diffusion into capillaries was assumed to remove radon and reduce the concentration to zero. The concentration of radon in the contents of the stomach, assumed to be well mixed, was taken to decline exponentially with a half-time of 20 min. The model and its results are discussed in detail in appendix B. When the above parameters were used with a radon diffusion coefficient for the gastric wall of 5 × 10-6 cm2 s-1, the time-integrated concentration of radon at the depth of the cells at risk (200 µm) was found to be 30% of the time-integrated radon concentration in the contents of the stomach. The time-integrated concentration was found to be insensitive to the value assumed for the diffusion coefficient and to depend somewhat on the depth to which radon was assumed to diffuse.
Although the diffusion model of the stomach does not permit definitive conclusions, it does suggest that both radon concentration and its time integral vary over a rather limited range for a wide range in the diffusion coefficient. If the mucus layer is a barrier to radon diffusion, concentration in the wall could be substantially reduced. The chemical composition of the layer (95% water, degraded mucin, and soluble polymeric mucin secreted by the mucosa) does not suggest a strong diffusion barrier to inert substances like radon. The concentration of radon reached in the wall is controlled by the blood flow through the gastric mucosa, and the depth of microvasculature may be of considerable importance. The influence of the microvasculature of the small intestine on absorption of gases has been investigated (Bond and others 1977), but little information is available on the stomach. Further studies clearly are needed to determine the influence of the mucus layer and the capillary structures on the concentration of radon in the stomach wall. It should be noted that because the PBPK model cannot fully adhere to the microvasculature which removes most radon directly to the blood before it can diffuse near stem cells, the model is a conservative model.
The calculations of dose and risk reported below assume that the time-integrated concentration of radon at the depth of the stem cells is 30% of that in
the lumen. The latter value, derived with the diffusion model of appendix B, is the basis of the committee's recommendation regarding the risks posed by radon dissolved in water. For comparative purposes, dose coefficients were calculated as bounding cases corresponding to the situations of table 4.4; that is, the assumptions that radon does not diffuse into the stomach wall and that concentration in the wall is the same as that in the stomach contents represent the two limiting cases.
Fate of Radon Decay Products in the Body
The members of the decay series through 214Po are referred to as the short-lived decay products relative to the long-lived series headed by 210Pb. The half-life of the "short-lived" decay product 214Pb (26.8 min) is not short relative to physiological processes, inasmuch as, for example, during this half-life, blood passes through the heart more than 30 times. Thus, it is reasonable to assume that the 214Pb has its own fate within the body, a fate that is distinct from that of radon. The calculations performed here include explicit consideration of the fate of each decay product in the manner of recent ICRP publications (1989; 1988); for further details, see appendix A.
Dose Coefficients for Ingestion of Dissolved Radon
The dosimetric analysis presented here is based on the current ICRP method (ICRP 1989), which is consistent with the schema of the Medical Internal Radiation Dose Committee (MIRD) of the US Society of Nuclear Medicine (Loevinger and others 1988). Both ICRP and MIRD consider the mean absorbed dose to a target region as the fundamental dosimetric quantity. The mean absorbed dose in the target region is relevant to cancer induction to the extent that it is representative of the dose to the cells at risk. If the cells at risk are not uniformly distributed within the target region or if the stochastic nature of the energy deposition is such that mean values are of questionable validity, it might be necessary to address the stochastic nature of irradiation.
The ICRP method considers two sets of anatomic regions. The set of "source regions" specifies the location of radionuclides in the body, and the set of "target regions" consists of organs and tissues for which the radiation doses are to be calculated. The source regions are those anatomical regions involved in the behavior of the radionuclide (and subsequent decay products) within the body. It is assumed that the radionuclide is uniformly distributed within the volume of the source region.
The mean energy absorbed in the target region depends on the types of the radiations (including their energies and intensities) emitted in the source regions, the spatial relationships between the source and target regions, and the nature of tissues between the regions. The details of these considerations are embodied in
a radionuclide-specific coefficient called specific energy, or SE. For any radionuclide, source region S, and target region T, the specific energy at age t is defined as
where Yi is the yield of radiation of type i per nuclear transformation, Ei is the average or unique energy of radiation type i, AFi (T![]() S;t) is the fraction of energy emitted in source region S that is absorbed within target region T at age t, and MT(t) is the mass of target region T at age t. Age dependence in SE arises from the changes with age in both the absorbed fraction and the mass of the target region. The quantity AFi (T
S;t) is the fraction of energy emitted in source region S that is absorbed within target region T at age t, and MT(t) is the mass of target region T at age t. Age dependence in SE arises from the changes with age in both the absorbed fraction and the mass of the target region. The quantity AFi (T![]() S;t) is called the absorbed fraction (AF) and, when divided by the mass of the target region, MT, is called the specific absorbed fraction (SAF). Information on the energies and intensities of the radiation emitted by the members of the radon series is tabulated in ICRP Publication 38 (1983).
S;t) is called the absorbed fraction (AF) and, when divided by the mass of the target region, MT, is called the specific absorbed fraction (SAF). Information on the energies and intensities of the radiation emitted by the members of the radon series is tabulated in ICRP Publication 38 (1983).
The SE values used here were. computed with the SEECAL code of Cristy and Eckerman (1993). The calculations use files (electronic libraries) of the nuclear-decay data, SAFs for the emitted radiation, and values for the masses of the organs in people of various ages. The nuclear-decay data files and SAFs are those now used by ICRP (Cristy and Eckerman 1993; 1987). Organ masses for adults are taken from ICRP Publication 23 (1975). For children, age-specific organ masses are taken from Cristy and Eckerman (1987).
The absorbed-dose rate in target region T includes contributions from each radionuclide in the body and from each region in which radionuclides are present. The absorbed-dose rate, DT(t, t0), at age t in region T of a person of age to at the time of intake, can be expressed as:
where qs,j(t) is the activity of radionuclide j present in source region S at age t, SE(T![]() S;t)j is the specific energy deposited in target region T per nuclear transformation of radionuclide j in source region S at age t, and c is any numerical constant required by the units of q and SE. The absorbed dose is the time integral of the absorbed-dose rate.
S;t)j is the specific energy deposited in target region T per nuclear transformation of radionuclide j in source region S at age t, and c is any numerical constant required by the units of q and SE. The absorbed dose is the time integral of the absorbed-dose rate.
The equivalent dose is the absorbed dose of the various kinds of radiation weighted by a factor that represents their relative contributions to the biologic insult. The weighting factor, referred to as the radiation weighting factor (earlier called the quality factor), represents a judgment of the relative biologic effectiveness of the different radiations (ICRP 1991). In the context of radon, the equivalent dose, H, is given as
where DLow-LET and DHigh-LET denote the absorbed doses due to electrons and photons of low linear energy transfer (LET) and alpha particles of high-LET, respectively, and 20 is the radiation weighting factor for alpha radiation (ICRP 1991). Equivalent dose is a dosimetric quantity of radiation protection and is of limited utility in health risk assessments because the radiation weighting factor embodies consideration of the relative biologic insults of the different kinds of radiation.
For reference purposes, table 4.5a gives the equivalent dose received by various tissues of adults, assuming an intake of a unit activity of 222Rn dissolved in water. Results are presented for the base case and the bounding cases describing the extent to which radon is assumed to diffuse into the blood vessels and tissue of the stomach wall. The dose to the stomach calculated for these cases can be compared with the values in table 4.4, which were extracted from the literature.
The equivalent doses received by individuals of various ages, assuming an intake of a unit activity of 222Rn in water, is given in table 4.5b for the base-case assumption regarding radon uptake in the stomach wall. These dose values reflect
Table 4.5a
Committed Equivalent Dose per Unit Activity of 222Rn Ingested (S.v Bq-1) in the Adult as a Function of Diffusion Into the Stomach Wall
|
|
Uptake in Stomach Wall |
||
|
Organ |
No Diffusion |
Base Case |
Saturated Diffusion |
|
Adrenals |
7.8E-10 |
2.0E-10 |
3.0E-10 |
|
Bladder |
3.4E-10 |
9.9E-11 |
1.4E-10 |
|
Endosteal Tissue |
7.0E-09 |
1.8E-09 |
2.8E-09 |
|
Brain |
7.7E-10 |
2.0E-10 |
3.0E-10 |
|
Breast Tissue |
2.8E-10 |
8.5E-11 |
1.2E-10 |
|
Stomach Wall |
8.9E-10 |
2.4E-08 |
3.1E-02 |
|
Small Intestine |
1.2E-09 |
1.6E-10 |
3.1E-09 |
|
Upper Large Intestine |
1.5E-09 |
1.3E-10 |
5.5E-10 |
|
Lower Large Intestine |
2.6E-09 |
1.7E-10 |
6.5E-10 |
|
Kidneys |
4.2E-09 |
1.2E-09 |
2.0E-09 |
|
Liver |
1.1E-09 |
1.7E-09 |
2.1E-09 |
|
Muscle |
5.0E-10 |
1.4E-10 |
2.0E-10 |
|
Ovaries |
3.1E-10 |
8.7E-11 |
1.3E-10 |
|
Pancreas |
8.8E-10 |
9.3E-11 |
3.4E-10 |
|
Red Marrow |
7.2E-09 |
1.8E-09 |
2.7E-09 |
|
Spleen |
6.8E-10 |
1.4E-10 |
3.0E-10 |
|
Testes |
5.8E-10 |
1.5E-10 |
2.2E-10 |
|
Esophagus |
2.8E-10 |
8.4E-11 |
1.2E-10 |
|
Thyroid |
7.6E-10 |
2.0E-10 |
2.9E-10 |
|
Uterus |
3.0E-10 |
8.6E-11 |
1.3E-10 |
|
Lung |
4.7E-10 |
1.36E-10 |
1.9E-10 |
|
Effectivea |
2.1E-09 |
3.5E-09 |
3.8E-08 |
|
a Sum of weighted equivalent doses (see ICRP 1991). |
|||
Table 4.5b
Committed Equivalent Dose per Unit Activity of 222Rn Ingested (Sv Bq-1) for Various Subjects (Base Case Assumption Regarding Diffusion of Radon into the Stomach Wall)
|
|
Age at Intake (yr) |
|||||
|
Organ |
Infant |
1-yr |
5-yr |
10-yr |
15-yr |
Adults |
|
Adrenals |
2.5E-09 |
1.0E-09 |
5.1E-10 |
3.0E-10 |
2.3E-10 |
2.0E-10 |
|
Bladder |
6.3E-10 |
4.6E-10 |
2.6E-10 |
1.4E-10 |
1.1E-10 |
9.9E-11 |
|
Endosteal Tissue |
1.5E-08 |
1.1E-08 |
5.0E-09 |
3.3E-09 |
2,7E-09 |
1.8E-09 |
|
Brain |
1.2E-09 |
9.9E-10 |
5.0E-10 |
2.9E-10 |
2.2E-10 |
2.0E-10 |
|
Breast |
5.9E-10 |
4.3E-10 |
2.4E-10 |
1.3E-10 |
9.7E-11 |
8.5E-11 |
|
Stomach Wall |
3.0E-07 |
1.6E-07 |
7.3E-08 |
4.2E-08 |
3.1E-08 |
2.4E-08 |
|
Small Intestine |
1.2E-09 |
7.9E-10 |
4.2E-10 |
2.5E-10 |
1.8E-10 |
1.6E-10 |
|
Upper Large Intestine |
9.8E-10 |
6.7E-10 |
3.8E-10 |
2.2E-10 |
1.5E-10 |
1.3E-10 |
|
Lower Large Intestine |
1.3E-09 |
8.7E-10 |
4.9E-10 |
2.8E-10 |
1.9E-10 |
1.7E-10 |
|
Kidneys |
6.1E-09 |
4.1E-09 |
2.1E-09 |
1.3E-09 |
9.3E-10 |
1.2E-09 |
|
Liver |
1.5E-08 |
1.2E-08 |
3.7E-09 |
2.4E-09 |
1.4E-09 |
1.7E-09 |
|
Muscle |
8.2E-10 |
6.6E-10 |
3.4E-10 |
1.9E-10 |
1.5E-10 |
1.4E-10 |
|
Ovaries |
6.1E-10 |
4.4E-10 |
2.5E-10 |
1.4E-10 |
1.0E-10 |
8.7E-11 |
|
Pancreas |
7.0E-10 |
4.4E-10 |
2.5E-10 |
1.5E-10 |
1.1E-10 |
9.3E-11 |
|
Red Marrow |
9.5E-09 |
8.5E-09 |
4.2E-09 |
2.5E-09 |
2.0E-09 |
1.8E-09 |
|
Spleen |
1.3E-09 |
4.5E-10 |
2.8E-10 |
1.8E-09 |
1.7E-10 |
1.4E-10 |
|
Testes |
2.2E-09 |
7.5E-10 |
3.9E-10 |
2.2E-10 |
1.7E-10 |
1.5E-10 |
|
Thymus |
5.9E-10 |
4.2E-10 |
2.4E-10 |
1.3E-10 |
9.7E-11 |
8.4E-11 |
|
Thyroid |
1.5E-09 |
9.7E-10 |
5.0E-10 |
2.9E-10 |
2.2E-10 |
2.0E-10 |
|
Uterus |
6.0E-10 |
4.3E-10 |
2.56E-10 |
1.3E-10 |
9.9E-11 |
8.6E-11 |
|
Lung |
9.1E-10 |
7.2E-10 |
3.8E-10 |
2.1E-10 |
1.6E-10 |
1.3E-10 |
|
Effectivea |
4.0E-08 |
2.3E-08 |
1.0E-08 |
5.9E-09 |
4.2E-09 |
3.5E-09 |
|
a Sum of weighted equivalent doses (see ICRP 1991). |
||||||
the age dependence in both the sizes of body organs and the behavior of radon and its decay products in the body. A decrease in the dose per unit intake with increasing age at intake is evident in table 4.5b. The lower consumption of tapwater during childhood results in an intake of dissolved radon during childhood that is a small fraction of the lifetime intake. Thus, despite the higher dose per unit intake at these ages, relative to that of the adult, the lower consumption rates result in intakes in the first 10 years that contribute about 30% to the lifetime risk.
Cancer Risk Per Unit 222RN Concentration in Drinking Water
Estimates of the cancer mortality risk per unit concentration of 222Rn in drinking water were derived with the method of Federal Guidance Report 13 (EPA 1998). That method yields a risk estimate that applies to an average mem-
ber of the public, in the sense that the estimate is averaged over the age and sex distributions of a hypothetical closed ''stationary'' population whose survival functions and cancer mortality rates are based on recent data for the United States. Specifically, the total mortality rates in this population are defined by the 1989–1991 US decennial life tables (1989–91; 1997), and cancer mortality rates are defined by US cancer mortality data for the same period (NCHS 1993a). The hypothetical population is referred to as "stationary" because the sex-specific birth rates and survival functions are assumed to be invariant over time.
A schematic of the method of computation is shown in figure 4.8. The main steps in the computation are shown in the numbered boxes in the figure and summarized below.
1. Lifetime risk per unit absorbed dose at each age
For each of 14 cancer sites in the body, radiation-risk models are used to calculate sex-specific values for the lifetime risk per unit absorbed dose for each
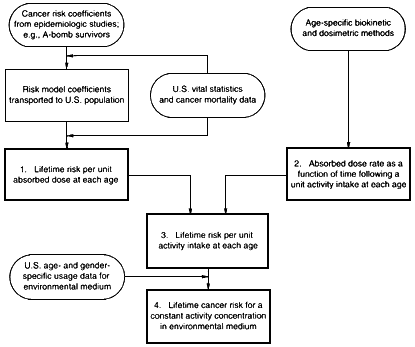
Figure 4.8
Schematic of method to estimate cancer mortality risk per unit concentration of Rn-222 in drinking water, derived using the methodology of Federal Guidance Report 13 (EPA 1998).
type of radiation received at each age. These models provide a method for calculation of radiogenic-cancer risks based on a critical review of data on the Japanese atomic-bomb survivors and other study groups and methods of applying radiation risk estimates across populations.
The cancer sites considered are the esophagus, stomach, colon, liver, lung, bone, skin, breast, ovary, bladder, kidney, thyroid, red marrow (leukemia), and residual (all remaining cancer sites combined). An absolute-risk model is applied to bone, skin, and thyroid; that is, it is assumed for these sites that the radiogenic cancer risk is independent of the baseline cancer mortality rate (cancer mortality death rate for a given site in an unexposed population). For the other cancer sites, a relative-risk model is used; it is assumed that the likelihood of a radiogenic cancer is proportional to its baseline cancer mortality rate. The baseline cancer mortality rates are calculated from US cancer mortality data for 1989–1991 (NCHS 1993a; 1993b; 1992).
The computation of sex-and site-specific values for the lifetime cancer risk per unit absorbed dose involves an integration over age, beginning at the age at which the dose is received, of the product of the age-specific risk-model coefficient (times the baseline mortality of the cancer in the case of a relative-risk model) and the survival function. The survival function is used to account for the possibility that the exposed person will die of a competing cause before a radiogenic cancer is expressed.
Estimates of the site-specific cancer mortality for a hypothetical low dose, low dose rate, uniform irradiation of the whole body by low-LET and high-LET radiations are given in table 4.6. Some organs such as male breast or brain are not explicitly included as these sites have not shown definitive dose responses (Pierce and others 1996). To the extent that these sites contribute to the total cancer response, they are included in the Residual category.
The risk estimates of table 4.6 are based on the risk-model coefficients in Federal Guidance Report 13 (EPA 1998). The estimates are age-averaged values for the hypothetical stationary population. For details regarding the method of computation, see Federal Guidance Report 13.
2. Absorbed-dose rates as a function of time after an acute intake
Age-specific biokinetic models for radon and its decay products are used to calculate the time-dependent inventories of activity in various regions of the body after acute intake of a unit activity of 222Rn. This calculation is performed for each of six "basic" ages at intake: infancy (100 d); 1, 5, 10, and 15 y; and maturity (20 y). The biokinetic model for radon used in the calculations was described above (see appendix A for further details). The biokinetic models for the radon decay products were taken from ICRP's recent series of documents on age-specific doses to members of the public from intake of radionuclides (ICRP 1996; ICRP 1995; ICRP 1993; ICRP 1989).
Table 4.6
Age-averaged Site-Specific Lifetime Cancer Mortality Risk Estimates (Deaths per Person-Gy) from Low-Dose, Low-and High-LET Uniform Irradiation of the Body
|
|
Low-LET |
High-LET |
||
|
Cancer Site |
Males |
Females |
Males |
Females |
|
Esophagus |
7.30 × 10-4 |
1.59 × 10-3 |
1.46 × 10-2 |
3.18 × 10-2 |
|
Stomach |
3.25 × 10-3 |
4.86 × 10-3 |
6.50 × 10-2 |
9.72 × 10-2 |
|
Colon |
8.38 × 10-3 |
1.24 × l0-2 |
1.68 × 10-1 |
2.48 × 10-1 |
|
Liver |
1.84 × 10-3 |
1.17 × 10-3 |
3.68 × 10-2 |
2.34 × 10-2 |
|
Lung |
7.71 × 10-3 |
1.19 × 10-2 |
1.54 × 10-1 |
2.38 × 10-1 |
|
Bone |
9.40 × 10-5 |
9.60 × 10-5 |
1.88 × 10-3 |
1.92 × 10-3 |
|
Skin |
9.51 × 10-5 |
1.05 × 10-4 |
1.90 × 10-3 |
2.10 × 10-3 |
|
Breast |
0.00 |
9.90 × 10-3 |
0.00 |
9.90 × 10-2 |
|
Ovary |
0.00 |
2.92 × 10-3 |
0.00 |
5.84 × l0-2 |
|
Bladder |
3.28 × 10-3 |
1.52 × 10-3 |
6.56 × 10-2 |
3.04 × 10-2 |
|
Kidney |
6.43 × 10-4 |
3.92 × 10-4 |
1.29 × 10-2 |
7.84 × 10-3 |
|
Thyroid |
2.05 × 10-4 |
4.38 × 10-4 |
4.10 × 10-3 |
8.76 × 10-3 |
|
Leukemia |
6.48 × 10-3 |
4.71 × 10-3 |
6.48 × 10-3 |
4.71 × 10-3 |
|
Residuala |
1.35 × 10-2 |
1.63 × 10-2 |
2.70 × 10-1 |
3.26 × 10-1 |
|
Total |
4.62 × 10-2 |
6.83 × 10-2 |
8.01 × 10-1 |
1.18 |
|
a Residual is a composite of all radiogenic cancers not explicitly identified in the table. |
||||
Age-specific dosimetric models are used to convert the calculated time-dependent regional activities in the body to absorbed dose rates for both the low-LET (photons and electrons) and high-LET (alpha) radiations to radiosensitive tissues as a function of age at intake and time after the intake. Absorbed-dose rates for intake ages intermediate to the six basic ages (infancy; 1, 5, 10, and 15 y; and maturity) are determined by interpolation.
3. Lifetime cancer risk per unit intake at each age
For each cancer site, the sex-specific values of lifetime risk per unit absorbed dose received at each age (derived in the first step) are used to convert the calculated absorbed-dose rates to lifetime cancer risks for the case of an acute intake of one unit of activity at each age xi. This calculation involves integration over age of the product of the absorbed-dose rate at age x for a unit intake at age xi, the lifetime risk per unit absorbed-dose received at age x, and the value of the survival function at age x divided by the value at age xi. The survival function is used to account for the probability that a person exposed at age xi is still alive at age x to receive the absorbed dose. It is assumed that the radiation dose is sufficiently low that the survival function is not seriously affected by the number of radiogenic-cancer deaths at any age.
4. Lifetime cancer risk for chronic intakes
For purposes of computing a risk coefficient, it is assumed that the radon concentration in the drinking water remains constant and that all persons in the population are exposed to that concentration throughout their lifetimes. It is assumed that the lifetime average drinking water consumption rate is 0.6 L d-1.
For each cancer site and each sex, the lifetime cancer risk for chronic intakes is obtained by integration over age x of the product of the lifetime cancer risk per unit intake at age x and the expected drinking water consumption at age x.
Except for the calculations of the time-dependent organ activities and absorbed-dose rates, each of the steps described above is performed separately for each sex and each cancer site. A total-risk coefficient is derived by first adding the risk estimates for the different cancer sites in each sex and then calculating a weighted mean of the coefficients for males and females. The weighted mean of coefficients for males and females involves the presumed sex ratio at birth, the sex-specific risk per unit intake at each age, and the sex-specific survival function at each age.
The cancer mortality risks associated with lifetime ingestion of 222Rn dissolved in drinking water at a concentration of 1 Bq m-3 are given in table 4.7; the total average over both sexes is 1.9 × 10-9. The uncertainty in this estimate is associated largely with the estimated dose to the stomach and with the epidemiologic data used to estimate the risk. Cancer of the stomach is a major late effect in
Table 4.7
Cancer Mortality Risk Associated with Lifetime Ingestion of 222Rn at a Concentration of 1 Bq m-3 in Drinking Watera
|
|
Cancer Mortality Risk (m3 Bq-1) |
||
|
Cancer Site |
Males |
Females |
Both Sexes |
|
Esophagus |
1.5E-12 |
3.3E-12 |
2.2E-12 |
|
Stomach |
1.3E-09 |
2.0E-09 |
1.6E-09 |
|
Colon |
4.6E-11 |
7.7E-11 |
5.9E-11 |
|
Liver |
6.8E-11 |
4.4E-11 |
5.8E-11 |
|
Lung |
2.6E-11 |
4.5E-11 |
3.4E-11 |
|
Bone |
5.4E-12 |
5.7E-12 |
5.5E-12 |
|
Breast |
— |
1.0E-11 |
4.5E-12 |
|
Ovary |
— |
6.1E-12 |
2.6E-12 |
|
Bladder |
7.8E-12 |
3.7E-12 |
6.0E-12 |
|
Kidney |
1.8E-11 |
1.1E-11 |
1.5E-11 |
|
Thyroid |
1.3E-12 |
3.1E-12 |
2.1E-12 |
|
Leukemia |
1.9E-11 |
1.5E-11 |
1.7E-11 |
|
Residualb |
6.9E-11 |
9.2E-11 |
7.9E-11 |
|
Total |
1.5E-09 |
2.3E-09 |
1.9E-09 |
|
a To express risk in the conventional units (L pCi-1), multiply the values by 37. b The average of the absorbed dose rates to muscle, pancreas, and adrenals is applied to this group of Cancers. |
|||
the survivors of the atomic bombings in Japan, where the high susceptibility appears to be related to the high baseline rate. The basic data used in the risk calculations for the present report were derived from the atomic-bomb survivors and applied to the US population with a relative-risk model in conjunction with the US stomach-cancer experience. It is noted that the high baseline stomach-cancer incidence among the Japanese and the declining incidence in the US contribute to the uncertainty in the risk estimate. An estimate of the uncertainty in the risk was derived on the basis of judgment that the absorbed dose to the stomach is probably not greater than three times the base case of table 4.4 (divide by 20 to obtain absorbed dose) and probably greater that one-fiftieth (2%) the base case. The asymmetric bounds reflect the judgment that the base case estimate is taken to be conservative; however, at this time sufficient information is not available to further refine the model and its parameter values. Similarly, it was judged that the stomach cancer mortality coefficients are probably not greater than three times the values of table 4.6 while they probably are greater than one-tenth the tabulated values. On the basis of those judgments, it is concluded that the risk posed by ingestion of water containing 222Rn at 1 Bq m-3 probably lies between 3.8 × 10-10 and 4.4 × 10-9, with 1.9 × 10-9 as the central value.
Special Populations At Risk
No information is available to identify the characteristics of individuals or to suggest that any population group that might be at increased carcinogenic risk because of the presence of radon in drinking water. A number of environmental factors have been associated with stomach cancer, although the incidence of gastric cancer has been declining during the last 50 y. Stomach cancer is essentially a disease of the poor, not only in developing countries, but also in the West, where there is an inverse correlation between stomach-cancer risk and socioeconomic status. A strong link appears to exist between the ubiquitous bacterium Helicobacter pylori and stomach cancer, but there is no known interaction between H. pylori and radiation or radon (McFarlane and Munro 1997).
Sikov and others (1992) investigated the developmental toxicology of radon exposures in the rat. They did not find any teratogenic or reproductive effects in pregnant rats exposed to airborne radon at high concentrations. Radon in the maternal blood would flow to the placenta and, depending on the relative solubilities of radon in maternal and fetal blood, could be absorbed by the fetus. However, the regional blood flow to the uteroplacental unit during the period when most teratogenic effects are possible (1st trimester) is very small (Thaler and others 1990). The same exchange is possible for either inhaled or ingested radon. Thus, it appears unlikely that radon in drinking water would have substantial teratologic or reproductive effects.























