5
Selecting Contaminants on the CCL for Future Action: Recommended Decision Process
The EPA faces a challenging task in determining which contaminants on the Drinking Water Contaminant Candidate List (CCL) warrant regulation. As explained in Chapter 2, existing algorithms for ranking environmental contaminants are of limited use for this purpose, because many of them were designed for priority setting, not necessarily for regulatory action, and because of data gaps and the need for policy judgments. This chapter presents a decision-making framework for selecting contaminants from a CCL for future action. It also discusses criteria for evaluating four categories of data—exposure, health effects, treatment, and analytical methods—that are needed for making this selection.
Decision-Making Framework
While a ranking algorithm may be appropriate for helping to determine contaminants to be listed on the CCL, this approach is not suitable for determining the appropriate disposition of contaminants on the CCL. Rather, the process requires considerable expert judgment to address uncertainties from the inevitable gaps in information about exposure potential and/or health effects; to evaluate, from a public health perspective, the many different effects that contaminants can cause; and to interpret available data in terms of statutory requirements. Therefore, such decisions necessarily involve subjective judgments, and the law designates EPA to make them.
For each contaminant on the CCL, there are three possible outcomes of EPA's decision process:
- Consider for immediate regulatory action, as required by the Safe Drinking Water Act (SDWA) Amendments of 1996, if information is sufficient to judge that a contaminant ''may adversely affect public health" and is known or is substantially likely to occur in public water systems with a frequency and at levels that pose a threat to public health.
- Drop from the CCL if information is sufficient to determine that the contaminant does not pose a risk to public health in drinking water.
- Conduct additional research on health effects and/or exposure if information is insufficient to determine whether the contaminant should be regulated.
These three outcomes are not mutually exclusive. For example, based on available evidence, EPA might choose to initiate regulatory action and issue a health advisory, while simultaneously pursuing research to fill information gaps that might result in subsequent further modifications of the regulatory level. The committee believes that public health will be served best by leaving EPA as much discretion as possible, within the limits of law.
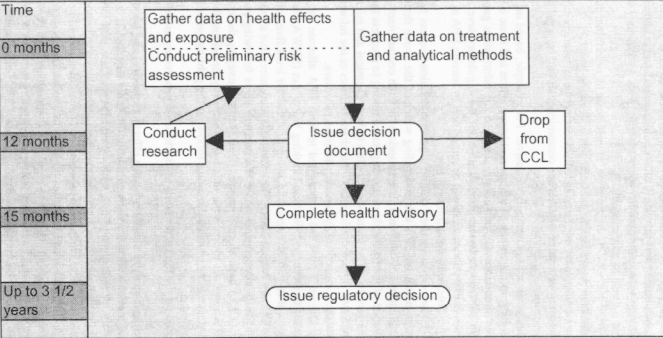
Figure 5-1 shows in simplified outline a general decision process that the committee recommends for EPA use in deciding which of the above three outcomes (or combinations of outcomes) is appropriate for each contaminant on the CCL. The left side of the figure shows the suggested timing to progress through each step of the process. The framework applies to both chemical and microbiological contaminants; differences in either their characteristics or the information available about them do not justify separate decision processes.
The time line on Figure 5-1 is provided to help EPA allocate time and resources in order to meet the 1996 Safe Drinking Water Act (SDWA) Amendments' requirement to publish regulatory determinations for least five contaminants from the CCL by August 2001. The committee recognizes that almost one year of the originally allotted time (three and one-half years following publication of the first CCL) have already passed. Thus, while conveying the urgency with which EPA must act to reach the mandated regulatory decisions, the suggested time line should be of more direct use following the publication of future CCLs.
As indicated on the figure, the steps in the decision process are as follows:
|
1. |
Gather and analyze available health effects, exposure, and treatment and analytical methods data for each contaminant. This step should be initiated immediately. It is a standard task with which EPA staff are well familiar. While data on the ability of drinking water treatment technologies to remove the contaminant and analytical methods to measure the contaminant should be gathered at this stage to avoid delays in future regulatory action, these data should not be part of the decision about whether to regulate a contaminant. Any contaminant that poses a health risk in drinking water, as defined in the SDWA Amendments of 1996, should be considered for regulation. The second half of this |
-
chapter describes factors to consider when gathering and assessing data on contaminant health effects, exposure, and treatment and analytical methods.
-
2.
Conduct a preliminary risk assessment for each contaminant based on the available data. The preliminary risk assessment integrates the hazard and exposure analyses to assess the public health implications of the contaminant. It should include consideration of possible effects of the contaminant on sensitive subpopulations, such as pregnant women, infants, the elderly, and those with compromised immune systems. It should be carried out even if there are data gaps and discrepancies in order to provide a basis for an initial decision on the disposition of the contaminant and, where there are such gaps, to guide research efforts. EPA's usual approaches to risk assessment are appropriate, and the committee does not see the need to create new procedures for this step. Although a critical step in the process, the preliminary risk assessment should not be overly detailed, time consuming, or resource intensive. It should resemble risk assessments conducted by EPA under the Toxic Substances Control Act to evaluate data on new chemicals, rather than the massive multi-year risk assessments (e.g., for dioxins) that EPA often performs.
-
3.
Issue a decision document for each contaminant describing the outcome of the preliminary risk assessment (i.e., whether the contaminant will be considered for regulation, dropped from the CCL, or retained on the CCL pending further research). This document should be issued within 12 months of compilation of the CCL. The document should describe information available to EPA at the time of the preliminary risk assessment, the weight EPA staff put on the available information and why, the reasons for EPA's decision, an action plan for implementing the decision (for example, indicating what research to conduct and how), and contacts for more information. It should be written in a language and format accessible by all interested parties.
-
4.
Issue a health advisory for each contaminant not dropped from the CCL after the preliminary risk assessment. The health advisory should be completed within three months of the decision (within 15 months of the CCL's completion). The purpose of such an advisory should be the same as for any drinking water advisory: to alert interested parties to the possibility of a threat to public health worthy of attention and to describe the nature of the available evidence, without committing EPA to any particular future action on the contaminant. Health advisories are currently used for drinking water contaminants when the occurrence of the contaminant is not deemed widespread enough to justify imposing monitoring requirements on all utilities and to advise, even in the presence of a promulgated regulation, those to whom the regulation would not apply yet who might be vulnerable to contaminated drinking water (for example, private well owners). The committee recommends that the purpose of health advisories be expanded beyond these current uses to promulgate information about all contaminants remaining on the CCL after the preliminary risk assessment.
|
5. |
Begin compiling a regulatory package or conducting research for each contaminant remaining on the CCL after the preliminary risk assessment. This step should begin in tandem with issuance of the health advisory and should not wait until the advisory is completed. For contaminants not slated for regulation, research results should be fed back into another preliminary risk assessment, and a new decision document should be issued based on the results of this second risk assessment. |
The committee's recommendation for a swift (12-month) initial decision on whether a contaminant should be put on a regulatory list is not intended to interfere with the agency's need to add and remove contaminants from such a list at any time within the five-year life of the CCL. This rapid initial action is intended to ensure that, to the extent that available information supports such an action, EPA begins as early as possible to develop a regulatory package that could support a decision to promulgate a regulation. Further, initial decisions should be made within 12 months to be sure that any information gaps (such as treatment availability and costs) standing in the way of issuing a regulation can be filled as quickly as possible.
In using this decision framework, EPA should keep in mind the importance of involving all interested parties (including regulated utilities, state and local regulators, public interest representatives, and consumers). The decision document for each contaminant should be disseminated for review by these parties, although consultation with these parties should not delay initiation of actions on the decisions that EPA has reached. Given the valid scientific disagreements noted in Chapter 1 and the way information and values are inevitably entwined, EPA would be wise to seek the insights of parties with a wide range of perspectives on contaminant priority setting during the entire decision process, not just in the period of formal regulatory procedures. Soliciting comments on the decision document will offer EPA independent perspectives and is an effective way to ensure that criteria developed after consideration of all the relevant issues have not been overlooked. In the long run, this will likely lead to a less contentious regulatory development process, if interested parties believe their views have been considered.
The EPA should also keep in mind that public health should be the guiding principle for making its decisions and that the decision to eliminate contaminants from the CCL should not be made lightly. However, there are cases when information initially used to include a contaminant on a CCL was faulty, and EPA should not be required to retain that contaminant on the list. Just as a decision to exclude a contaminant on the previous CCL from a new CCL would be explained and justified in the Federal Register announcing the draft CCL, a decision document would explain why EPA has decided to drop a contaminant from a CCL at other times. Conversely, if new information suggests that the contaminant is worthy of being included on the CCL after it has been eliminated
from the CCL, it should be returned to the CCL. Moreover, if important new information suggests that an unregulated contaminant not listed on the CCL is being found in many water systems and may pose health risks, EPA should consider adding it to the CCL immediately, or consider invoking its immediate regulatory authority under the "urgent threats to public health" provisions of the SDWA (1412[b][1][D]).
Uncertainty in the Decision Process
Under ideal circumstances, EPA would have a decision process that exactly selects only those contaminants whose regulation will reduce disease, disability, or death and dismisses those contaminants that have little or no effect on human health. Unfortunately, the true state of nature ("the truth") remains either unknown or shrouded in uncertainty for the majority of contaminants on the CCL. It is likely, therefore, that there will be some error in the decision process, allowing some contaminants that should be regulated to pass through while placing other, harmless contaminants on a regulatory track.
In making judgments about which contaminants to regulate, the committee recommends that EPA err on the side of public health protection. The CCL lists contaminants that are likely to pose greater risks to the public, compared to a list of randomly selected chemicals and microorganisms. For lists enriched in substances that pose risks, even a highly accurate decision process can result in many substances that need to be regulated remaining unregulated. Appendix A explains in mathematical terms why this is so. As shown in the appendix, a highly accurate decision process, when applied to such an enriched list, can still result in nearly a third of substances that need to be regulated going unregulated, while at the same time slating for regulation just three percent of substances that do not need to be regulated. Thus, for such a list, when the decision about whether or not to consider a contaminant for regulation is a close call, EPA should decide in favor of regulation.
Implementing the Decision Framework: Examples
Boxes 5-1, 5-2, 5-3, and 5-4 provide examples of how the initial data analysis step of the proposed decision framework might be implemented (or might have been implemented had the decision framework been available in the past) for four contaminants: trichloroethylene, a currently regulated contaminant; Cryptosporidium, which is monitored under the Information Collection Rule, but is not on the CCL; and aldicarbs and Rhodamine WT, which were both on an early draft of the CCL but were dropped before the final CCL was issued.
In presenting these examples, the committee does not seek to substitute its own judgment for EPA's. Rather, as these cases illustrate, implementing the decision framework requires a careful survey of available health effects and
|
BOX 5-1 Trichloroethylene: Decision Process for a Regulated Contaminant Trichloroethylene (TCE), a widely used organic solvent, is currently regulated in drinking water at a level of 5 micrograms per liter. The current regulation, however, was not developed as a direct result of an EPA contaminant selection and decision-making process but because Congress, in the 1986 Safe Drinking Water Act amendments, required that EPA develop a regulation for TCE and 82 other contaminants that had been slated for future regulation. At the time, Congress reacted to the belief that EPA had been too slow in developing drinking water standards following passage of the 1974 Safe Drinking Water Act and, in particular, that EPA had neglected to consider sufficiently the importance of regulating organic compounds such as TCE. If EPA had applied the decision framework recommended in this report in 1977, the decision would likely have been (1) issue a health advisory, (2) conduct additional research on exposure and health effects, and (3) consider possible regulation, based upon the partial data that were available. Exposure data: The primary exposure data on TCE available in 1977 were from the National Organics Monitoring Survey (NOMS), conducted in 1976-1977 (Westrick, 1990). NOMS involved the sampling of finished water (prior to distribution) from 113 water systems. The final phase of the survey found TCE above the reporting limit of 0.2-0.3 micrograms per liter in 2 of 17 ground water supplies and 17 of 88 surface water supplies, with a maximum reported concentration of 15 micrograms per liter. It is important to note that, at that time, this reported occurrence of TCE was not deemed significant. In addition, this survey was limited in that it covered mostly large water systems determined to be vulnerable to contamination. To obtain a more representative estimate of TCE occurrence EPA may have wanted to conduct additional surveys using random samples of water systems of various sizes. In fact, EPA conducted such a survey, the Community Water Supply Survey, in 1979 and found no TCE in 106 surface water systems and TCE at levels above 0.5 micrograms per liter in 14 of 330 ground water systems, with a maximum reported concentration of 210 micrograms per liter (Westrick, 1990). Thus, in 1977, additional research on exposure to TCE in drinking water likely would have been required before deciding whether to regulate TCE. Health effects data: Health effects data on TCE were also limited in 1977. Researchers knew that TCE was metabolized to trichloroacetic acid, trichlorethanol, and small amounts of chloroform and monochloroacetate in animals (NRC, 1977), but neither the kinetics of the pathways nor any possible species differences between various strains of mice, between mice and rats, and between rats and either mice or humans was known except in the most rudimentary way. A chronic bioassay had shown liver cancer in mice but not in rats. Epidemiological data were available essentially only for high-dose occupational accident type exposures (i.e., case studies), not for the low doses found in drinking water. There was even some discussion that TCE was found as a disinfection byproduct (NRC, 1977). Conclusions: In 1977, the existing health effects data likely would have been insufficient to drop TCE from the CCL. Therefore, TCE would warrant additional research and a health advisory. EPA would have had to decide whether or not the partial data available were sufficient to regulate the contaminant at precautionary levels. |
|
BOX 5-2 Cryptosporidium: Decision Process for an Unregulated Contaminant Cryptosporidium, an enteric protozoan, while monitored under the Information Collection Rule, is not one of the microbial contaminants listed on the current CCL. Using the framework in this report, the preliminary risk assessment of this contaminant will likely lead EPA to a decision that it should move forward with regulatory action, although additional data (for example, on removal of this organism in different treatment processes and development of reliable monitoring methods) are likely needed to complete the regulation. Exposure data: While other enteric protozoa have long been known to be transmitted by contaminated water, the potential for waterborne transmission of Cryptosporidium to humans was not recognized until the 1980s. The first documented waterborne outbreak, transmitted by well water in a small Texas community, occurred in 1984 (D'Antonio et al., 1985); a second documented outbreak occurred in Georgia in 1987 (Hayes et al., 1989). Several more outbreaks have been reported since then, with the largest occurring in Milwaukee in 1993 and affecting 400,000 individuals (MacKenzie et al., 1994; Smith and Rose, 1998). An increasing amount of research on the occurrence of Cryptosporidium has occurred since the first reported outbreaks. Surveys on the occurrence of oocysts were published by 1988 (Rose, 1988). Thus, the occurrence of Cryptosporidium in drinking water is known to be widespread enough to warrant concern. Health effects data: Early work on Cryptosporidium focused on its effects on animals. First described in 1907 in the intestinal tract of mice (Tyzzer, 1907), Cryptosporidium was later reported to cause diarrheal disease in young mammals, particularly calves (Barker and Carbonell, 1974; Anderson and Bulgin, 1981). Mammalian isolates were shown to cause infection in other mammals, and thus this protozoan was known to cross species barriers. The first identified case in humans occurred in 1976 (Meisel et al., 1976), but cryptosporidiosis was not thought to be a cause of severe disease until the AIDS epidemic struck; the disease leads to mortality in 50 percent of cases in the immunocompromised population (MMWR, 1982). By the early 1980s, Cryptosporidium was known to cause illness (five to seven days of diarrhea) in populations with normal immune functions (Tzipori, 1983). Conclusions: This organism has caused major public health concerns and is not limited to isolated water supplies. Therefore, EPA's preliminary risk assessment will likely lead EPA to decide to initiate regulatory action. |
exposure data on the contaminant followed by policy judgments about the significance of the risk as indicated by the available data and additional research to close essential data gaps. Treatment and analytical data are not described in these examples because they are not part of the initial decision about whether a contaminant should be moved forward to the list of contaminants to consider for regulation, although assessment of these data needs to begin in tandem with exposure and health effects assessments in order to avoid delaying regulatory action and to help set research priorities.
|
BOX 5-3 Aldicarbs: Decision Process for an Unregulated Contaminant Aldicarb, a highly toxic insecticide used on such crops as potatoes, peanuts, sugar bees, soybeans, sugarcane, and cotton, is not currently regulated. The exposure and health effects data summarized below are as they existed in 1984, when aldicarb was first considered for regulation. Using historical data, one possible conclusion that EPA might have reached in 1984 if the framework had been available then is that aldicarb should be considered for a health advisory and that EPA would need to decide whether the population potentially exposed to aldicarb is sufficiently large to warrant establishing a national drinking water standard. Exposure data (as of 1989): Aldicarb and its degradates (including aldicarb sulfoxide and aldicarb sulfone) have been found in ground water at levels that would be anticipated to be of health concern. Aldicarbs appear most frequently in agricultural areas with sandy soil, and public water supply wells in those areas are at risk of being contaminated. Water from wells near treated fields in eight states contained aldicarb at concentrations ranging from 10 to 200 micrograms per liter (EPA, 1984); these concentrations exceed health criteria suggested by the National Research Council in its 1977 report Drinking Water and Health (NRC, 1977). Higher levels (up to 500 micrograms per liter) have been found in New York. (EPA, 1984). Health effects data (as of 1984): Aldicarb is known to be toxic in animals and humans by the same mechanism. Mammals readily absorb aldicarb from their gastrointestinal tract. On an acute basis, aldicarb is one of the most potent, both orally and dermally, of the widely used insecticides (rat oral LD50: 0.8 mg/kg for males and 0.65 mg/kg for females; mouse oral LD50: 0.3 to 0.5 mg/kg). Aldicarb is also a potent toxin in humans, as was shown by a study in groups of four adult men (NRC, 1977). At the highest dose (0.1 mg/kg), those tested experienced mild cholinergic symptoms. Cholinesterase depression occurred at lower doses (0.05 mg/kg and 0.025 mg/kg), although the findings were not statistically significant. The subchronic and chronic effects of ingesting aldicarb were studied in a 93-day rat study; two two-year rat studies; a two-year dog study; a three-generation rat study; a rat teratology study; and a mouse carcinogenicity study. These studies did not identify a more sensitive endpoint than cholinesterase inhibition. The no-observed-adverse-effect level for cholinesterase inhibition is 0.1 mg/kg/day. Based on these data, a suggested no-adverse-effect level for drinking water is 7 micrograms/liter (NRC, 1977). Conclusions: Historical health effects data as of 1984 were sufficient to indicate that aldicarb posed a risk at concentrations found in drinking water. Therefore, according to the decision framework, EPA would have had to decide whether or not to regulate aldicarb based on its policy judgment as to whether exposure occurs with a frequency and at levels that pose a public health threat. |
|
BOX 5-4 Rhodamine WT: Dropping a Contaminant from the CCL A few chemicals will "come and go" from the CCL because consensus emerges quickly that they do not present a serious threat to drinking water quality. The decision framework proposed in this report is designed to accommodate such cases by allowing a contaminant to be dropped from the CCL after release of a decision document showing that the contaminant does not pose a significant risk in drinking water. Reasonable handling of contaminants that are judged to be of very low priority is illustrated by the case of Rhodamine WT. In the announcement of the first draft CCL (EPA, 1997), EPA included this fluorescent dye because the dye's use as a tracer in ground water flow studies apparently had resulted in detectable concentrations above the National Sanitation Foundation's (NSF's) standard of 0.1 mg/L. However, commenters on the draft list pointed out that the 0.1 mg/L standard was for drinking water and that the data that raised EPA's concern came from "ground water not associated with drinking water production," for which the NSF standard is 100 mg/L. In light of this clarification and because (1) there are no data indicating adverse health effects of Rhodamine WT and (2) the dye is used for very specific and limited purposes, EPA chose not to list Rhodamine WT on the final CCL. Conclusions: If these data had come to light after Rhodamine WT was included on a CCL, a decision document explaining EPA's reasoning would have allowed the contaminant to be dropped from the CCL. |
As noted by EPA, sufficient data are necessary to conduct analyses on extent of exposure and risk to populations via drinking water in order to determine appropriate regulatory action (EPA, 1998). If sufficient data are not available, additional data must be obtained before any meaningful assessment can be made for a specific contaminant. At the time of the final CCL's publication, the "regulatory determination priorities" category of the CCL included those contaminants for which EPA had sufficient data to conduct exposure and risk analyses. Therefore, the five or more contaminants considered for regulation by August 2001, as required by the SDWA amendments, would likely be selected from this category. However, EPA cautioned that the future regulatory action categories of the final CCL were based on current information, and some movement between categories could be expected as additional data are obtained and evaluated.
General Guidelines for Evaluating Contaminant-Related Data
Because of the variability in the types and quality of data available on different contaminants, defining precise criteria for placing contaminants in the three
decision categories (regulate, drop from CCL, or research) is not possible, as the examples presented in Boxes 5-1, 5-2, 5-3, and 5-4 illustrate. Nevertheless, establishing general guidelines is possible. Below, the committee recommends such guidelines for evaluating data on contaminant exposure, health effects, and treatment and analytical methods.
Assessing Exposure Data
Exposure data should be gathered from sources that will predict the dose of drinking water contaminants for individuals, whether it be through ingestion, inhalation or dermal absorption. Table 5-1 represents a hierarchy of data types for the assessment of exposure.
Ideally, the best estimate of an individual's exposure to drinking water contaminants would be determined from samples collected at the person's tap. Such samples reflect all of the changes that might occur in the distribution system, treatment plant, and source waters that precede it. By integrating the results of a tap-sampling program, it is possible to obtain a picture of population exposure to the contaminant of interest.
Rarely is a census of tap water quality available, however. Tap sampling information is more difficult to obtain because of potential problems with access and costs. It is also prohibitively expensive to determine the tap water quality of every customer. While some utilities use consumers' taps as sample points, utilities are converting to dedicated sampling stations located on distribution system mains to obtain representative samples of the water under their control.
The second most useful sampling locations to estimate contaminant exposure are in a drinking water distribution system. Distribution sampling locations must be carefully selected to represent the characteristics of the contaminants being monitored. For example, concentrations of trihalomethanes and a variety of other disinfection byproducts change during transport through distribution systems as a result of continued exposure to chlorine. Thus, the trihalomethane regulation requires that these compounds be sampled at three average and one
TABLE 5-1 Hierarchy of Data Needed for Exposure Assessment
|
Concentration at the tap |
|
Concentration in the distribution system |
|
Concentration in finished water of the water treatment plant |
|
Concentration in raw (source) water |
|
Concentration in watersheds and aquifers |
|
Concentration in historical contaminant release data |
|
Concentrations in production data |
|
Concentrations in biota and human tissue |
maximum detention time location for each treatment facility (EPA, 1979). Sampling of distribution systems, if properly designed, can be far less costly than sampling individual taps, but it provides less precise exposure information.
If distribution system water quality information is not available, samples collected from treatment plant-finished waters can be used to represent how consumers are typically exposed to contaminants. Collecting and analyzing samples from finished water locations is especially useful if no changes in contaminant concentration or composition are expected during transport through the distribution system (i.e., for ''conservative" contaminants). However, transport through the distribution system generally changes the concentration and characteristics of most contaminants. For example, the concentrations of microorganisms change between the treatment plant and the consumer's tap, because of continued action of the disinfectant or, where disinfection is inadequate, microbial regrowth in the distribution system. However, for substances (such as radon) that distribution transport might not affect, finished water sampling may be sufficient.
Similarly, determining exposure of consumers to contaminants by using data collected in a watershed for a surface water resource or samples collected from a groundwater aquifer has the potential for producing a misleading picture; many changes in contaminant concentration can occur during transport from the source to the treatment plant intake and during subsequent treatment. However, knowledge that a particular raw water source is or is not heavily polluted and the source(s) of the contaminants is always helpful.
Chemical release data or concentrations of microorganisms in discharges are examples of data that can be used for estimating how significant a contaminant in water sources could be. However, use of historical contaminant release data (or, even more removed, production data) to predict human exposure is problematic. As previously noted, a chemical's concentration and characteristics may change dramatically following production, release to the environment, and subsequent intake by humans from contaminated drinking water. Thus, only gross relationships between contaminants of vastly different release or production amounts may be possible, and even these may be misleading.
Lastly, data showing contaminant concentrations in human tissue or in plant or animal materials (i.e., biomarkers of exposure) are uncommon. This type of monitoring is expensive, is currently of unknown utility, and generally focuses on chemicals of known toxicity. Contaminant concentrations in tissues or biota are currently not a likely source for determining possible exposure to unknown chemicals, but the availability and utility of such data may increase in the future.
Criteria for Exposure Data Used in Risk Assessments
The available exposure data for a given contaminant may not be sufficient to support a defensible risk assessment. The EPA will need to determine specific,
contaminant-dependent criteria for which data are acceptable for this purpose. In general, exposure data for risk assessments should have sufficient spatial and temporal coverage, and exposure should be to a minimally defined number of people. As discussed in Chapter 1, EPA needs to define terms such as "sufficient" and "minimal number of people."
The quantity and quality of monitoring data for any one contaminant will depend on the contaminant's regulatory status and the primary purpose for which the analytical work was undertaken. Four cases can be distinguished: (1) monitoring of regulated compounds required under EPA's Information Collection Rule (ICR), (2) surveys of unregulated but targeted compounds required under the Unregulated Contaminant Monitoring Rule (UCMR), (3) information to be contained in the proposed National Drinking Water Contaminant Occurrence Database (NCOD), and (4) ad hoc studies focused on particular contaminants or surveys of particular families of compounds.
As discussed in Chapter 2, the ICR requires large public water systems to monitor for microbial contaminants and disinfection byproducts. In order to help ensure that monitoring data meet specific accuracy and precision requirements, EPA established a national laboratory approval process to identify laboratories qualified to perform analyses for the ICR. In general, occurrence data acquired by water utilities under the ICR should be adequate for an exposure assessment to evaluate compounds on the CCL. Occurrence data collected under the revised UCMR and stored in the NCOD should also be adequate to identify compounds from the CCL that may require regulation. Chapter 1 reviews the regulatory development, time line, and intended use of both the UCMR and the NCOD. However, additional occurrence data (presumably from detailed ad hoc studies of particular contaminants) may be required for compounds considered priority candidates for regulation. Raw (source) water data from federal surface water monitoring programs such as the National Water Quality Assessment program (run by the U.S. Geological Survey), the National Stream Quality Accounting Network (U.S. Geological Survey), and the Environmental Monitoring and Assessment Program (EPA) should also be of acceptable quality.
Considerations for Research and Monitoring
For contaminants that do not have sufficient exposure information to conduct a preliminary risk assessment, additional research and/or monitoring will be needed. To achieve this, sensitive analytical methodologies with sufficient spatial and temporal measurements are needed for each contaminant. The first step is to develop an analytical method if one does not currently exist. This method should be precise and accurate for the given contaminant. The greatest analytical challenges lie in the identification of new contaminants and the quantification of emerging contaminants that are intrinsically difficult to measure.
As previously noted, occurrence data are a high priority, but they require
considerable time and effort to collect. While designing and implementing such a monitoring program, the committee recommends that exposure concentrations be estimated from available data using models. A combination of models could be used to predict tap water concentrations of a contaminant from finished water data, environmental measurements, measurement of surrogates that are readily analyzed, or production/release data. For example, environmental measurement data for microbial contaminants could be used to model exposure from tap water using a fate-and-transport model coupled with a distribution system model. Analogous models could be used to translate production/release data for chemical contaminants into exposure concentrations.
The appropriate level of complexity of fate and transport modeling for this purpose depends on the spatial distribution of the input data. For very localized contaminant sources it may be appropriate to use a detailed, site-specific fate and transport model. For example, this type of modeling has been used to describe the migration of Cryptosporidium from known agricultural sources to the raw water intake in Milwaukee, Wisconsin. For widely distributed environmental contaminants, such as nonpoint-source pollutants, very simple fate-and-transport models may be used that include only parameters describing persistence and mobility in the environment. To estimate these properties, physical/chemical parameters such as the Henry's Law constant, octanol/water partition coefficient, aqueous solubility, and degradation rates are needed.
For contaminants with an existing, acceptable analytical method, monitoring data should also be collected if they are not already available. While concentrations at the tap are the ultimate goal, it would be most effective to design a monitoring plan that measures contaminants of interest in the raw water, finished water, or distribution system (depending on the best available analytical method) and then verifies exposure with selected tap monitoring. Such data are also useful for verifying the models described above. Finally, any data collected should provide representative spatial and temporal coverage needed for a defensible risk assessment.
Assessing Health Effects Data
The health effects-related information for the priority-setting process include (1) toxicological laboratory studies and data bases; (2) epidemiological studies, clinical studies, and case reports; and (3) predictive biological activity or effects models, commonly referred to as structure activity relationship (SAR) and/or quantitative structure activity relationship (QSAR) models.
The committee recommends the following general principles for assessing health effects-related criteria:
- Positive epidemiological studies should be considered of highest value
- for priority setting purposes even in the presence of negative toxicological studies.
- Although positive toxicological studies will take priority for regulation in most cases, negative or inconclusive epidemiological studies should be considered and an attempt should be made to explain their results when determining priority.
Human data offer several advantages over data from animal studies: (1) elimination of the uncertainty resulting from interspecies extrapolation; (2) reduction of the uncertainty caused by high-to-low-dose extrapolation, since, for example, the range between occupational exposures and likely environmental exposures is smaller than between the doses administered in animal studies and likely environmental exposures; (3) more accurate reflection of the relevant real-life exposure scenario; and (4) evaluation of the effects of the chemical on susceptible subgroups (Hertz-Picciotto, 1995; Federal Focus, 1996). However, primarily because of the bias introduced by exposure misclassification, as well as other biases, environmental and occupational epidemiologic studies may easily underestimate or miss a true adverse health effect. Therefore, it is important to evaluate all the evidence available, including animal studies and case reports, as well as epidemiologic studies (Shepard, 1994; Wartenberg and Simon, 1995).
The remainder of this section describes in more detail the nature of the information included in each health effects-related information category, the strengths and limitations of the type of information in each category, and guiding principles for using the information to evaluate CCL contaminants for further regulatory actions.
Toxicological Data
Information gained from studies in laboratory animals is commonly employed in estimating whether there might be potentially adverse human health risks associated with exposure to contaminants in drinking water. In preparing preliminary risk assessments of contaminants on the CCL, EPA should summarize in narrative form the available toxicological studies and highlight aspects relevant to the health effects the contaminant may cause.
In preparing the summaries, EPA should keep in mind that doses used to examine the toxicology of a chemical or mixture of chemicals are initially given at high levels to laboratory animals so that adverse effects can be observed, but that these high-dose studies may have limited relevance to drinking water. The primary goal of these high-dose experiments is to observe the qualitative nature of the toxicity, which includes organs and tissues involved, species differences, gender differences, time of onset, and permanence of the effects. High doses are also needed when the event of concern (for example, tumor formation) needs to be detected at a rate that would make the use of lower doses infeasible because of
the large number of animals needed. Although studies of chemical toxicity usually begin with high doses, exposure to contaminants in drinking water typically results in low and continued daily doses of substances. If these doses are not completely eliminated from the body on a daily basis, they may accumulate to levels that exceed a threshold for producing toxicity or that increase substantially the risk of contracting a disease. The situation in which acute toxicity results from a large dose of a drinking water contaminant is extremely rare. Chronic toxicity resulting from lower exposures presents a more likely scenario. Chronic toxicity tests also permit time for adaptive processes (e.g., induction of metabolizing enzymes) to affect the animal's response to the chemical. These adaptive processes may exacerbate or diminish the observed toxic response. Long-term exposures also allow for longer-latency diseases to develop (e.g., cancer) in the experimental animals. Thus, greater weight should be given to toxicity data obtained from laboratory animals given lower-dose, continual exposures than to acute toxicity tests using high doses.
The toxicity measurements made in laboratory animals should be as extensive as is practical and include lethality, organ damage, tissue and cell abnormalities at the microscopic level, and relevant biochemical parameters associated with physiological dysfunction in the animal. If possible and appropriate, it is also desirable to identify doses that produce no observable effects (the no observed effect level or NOEL and/or the no observed adverse effect level or NOAEL) and doses that produce changes that represent the first evidence of overt toxicity (the lowest observed adverse effect level or LOAEL). For some outcomes, such as cancer and reproductive effects, this might not be possible, because the outcome is of an "all-or-none" variety that occurs with a low probability that would still be of importance when large populations are exposed. The sensitivity with which a toxic effect is detected may be enhanced by using laboratory animal species that have high susceptibility to the toxic agent and by using measurements that detect a nontoxic physiological or biochemical change that represents a prelude to the toxic event (i.e., a biomarker of the early effect).
For interpretation of results obtained from chronic toxicity tests, it is useful to know the blood concentration of the chemical and its toxic metabolites in the animals several times during the tests. This information helps the decision maker judge the validity of the extrapolation to other animals species and to humans. In addition, blood concentrations often produce information to estimate whether species differences in toxicity are a result of toxicokinetic (absorption, distribution, metabolism and excretion effects of the organism on the chemical) or toxicodynamic (effects of the chemical on the organism) dissimilarities among species.
When evaluating the merits of different toxicological studies, in vivo studies with relevant endpoints and a range of dose-response data should be given greater weight than in vitro studies (EDSTAC, 1998). Further, studies that show a correlation between dose and effects, that have followed good laboratory prac-
tices, and that have been peer reviewed should be given greater weight than those that do not meet those criteria.
Knowledge of the biochemical pathways through which chemicals produce deleterious effects in laboratory animals can be used to improve the accuracy and validity of the prediction of human risk. Species differences in the qualitative and quantitative aspects of chemical-induced toxicity make extrapolation between species difficult. Predictions of chemical toxicity in humans from information obtained in laboratory animals could be greatly enhanced in the future by emerging knowledge of the human genome, as well as the genome of common laboratory animal species. At the moment, the use of mechanistic information remains limited because of very substantial data gaps and inconsistencies concerning the actual mechanisms at work in humans exposed under natural conditions and the extent of variability among individuals. The EPA should consider including studies of methods for incorporating mechanistic information into assessments of health risks from contaminants in drinking water and other exposure vectors as part of its research strategy for some contaminants on the CCL. Such studies will need to consider the possibility that a single contaminant may affect health through more than one mechanistic pathway and that interactive effects may occur when multiple contaminants are present.
In the absence of information from human epidemiology, data from toxicity experiments in several laboratory animal species is usually necessary (although mechanistic information that validates the use of a particular laboratory animal species as a model for the human may obviate the need for data in several species). The availability of information from well conducted human studies that indicate a sufficiently strong association between chemical exposure through drinking water and adverse health outcomes would require fewer (or no) supporting data from animal studies. A documented biological rationale (based on results from animal studies and other relevant information) for an association between human exposure to a drinking water contaminant and a particular adverse health effect enhances a conclusion of causality in an epidemiological study. However, a lack of supportive animal data for an association between contaminant exposure and a health outcome may indicate (among other possible explanations) that a causative association may not be present or that the particular animal models used were not appropriate (e.g., arsenic).
Given a lack of sensitivity for detection of health outcomes using epidemiology, or a lack of data because the problem may not have been studied or cannot be studied in human populations, animal toxicology data must still be used to provide a human risk evaluation. Only infrequently is it found that an agent known to produce human toxicity will not produce a similar effect in some laboratory animal species when given sufficiently high doses. While all possible scenarios describing the interaction of data derived from humans and from laboratory animals have not been addressed here, it should be apparent that the appli-
cation of both types of data represents the best approach to assessing the potential health risks from exposure to chemicals in drinking water.
Epidemiological Data
As for toxicological studies, in the evaluation of human data, EPA should systematically review each study or case report and summarize it in a narrative. In particular, aspects of each study/case report that might be relevant to the determination of heterogeneity of finding among the available studies/case reports should be highlighted in the narrative summaries. Such sources of heterogeneity (i.e., differences in study findings that cannot be accounted for by sampling variation) include differences in study design, in the distributions of susceptible subgroups in the study populations, and in the ability to adjust for potential confounders and the impact of other biases. In assessing available epidemiological studies, the findings are usually stratified by type of study design (case report, ecological study, individual-level "case-control" or cohort study) and the ability to adjust for important confounders. At this step of the evaluation, it might be tempting to discount or dismiss findings from case reports as being too subjective. In addition, findings from ecological studies (a type of epidemiological study in which health outcome and exposure information are known only for aggregate populations, not for individuals) might also be dismissed, because these studies are vulnerable to special "ecological biases." Nevertheless, this temptation should be resisted. Case reports and ecological studies have provided important evidence linking chemical exposures to diseases. In addition, a study that appears to use an exposure in an ecologic fashion may avoid the special ecologic biases if exposures are, in effect, imputed for each individual geographical unit (e.g., county, town, or region). This is commonly done in drinking water studies (e.g., Kramer et al., 1992; Bove et al., 1995; Munger et al., 1997).
Although differences in study design and ability to control important confounders may be sources of heterogeneity among studies, the most important and likely sources of heterogeneity in environmental (and occupational) studies are caused by differences in the characterizations of exposures and disease outcomes. Therefore, describing these sources of heterogeneity should be the major focus of the narrative.
On the exposure side, studies may differ in exposure characterization (e.g., yes/no; low, medium, high; exposure based on modeling, sample data, residence, etc.); the level of exposure (a high exposure in one study could be a medium or low exposure in another study, and one study may average the sample data while another uses the maximum value); and the duration, frequency, and timing of exposure (Hertz-Picciotto and Neutra, 1994). Heterogeneity among studies could also be because each study is evaluating an effect at a different point in the exposure-effect curve. In addition, effects (e.g., a particular birth defect) seen at a relatively lower exposure level might differ from effects (e.g., spontaneous
abortion) seen at a higher level. While timing of exposure is an issue for adult cancers, it is especially important for outcomes associated with in utero exposures (birth defects, developmental disorders, and childhood cancers).
On the outcome side, studies may differ in the disease grouping evaluated (e.g., cancer grouping by organ system versus subgrouping by histology/grade, all leukemia versus childhood leukemia, etc.). The more etiologically homogeneous the grouping is, the less disease misclassification is introduced and the more likely a true effect will not be underestimated or missed.
To summarize, the narrative of each study/case report should fully discuss potential sources of heterogeneity, with an emphasis on exposure and disease characterization. Although study findings can be summarized in table form by grouping studies according to design, it is often more informative to group studies based on similarity of exposure characterization or exposure level and on similarity of disease characterization.
The narrative and summary tables are key to evaluating the available evidence. However, policymakers usually want some sort of classification framework with criteria in order to judge whether the chemical is likely to be toxic to humans (i.e., to determine whether evidence is sufficient), probably toxic (i.e., human evidence is limited, and in particular, biases cannot be ruled out as explanations for the association), possibly toxic (i.e., human evidence is limited, and there is a lack of supportive evidence from animal studies or case reports), unknown (i.e., no data are available), or possibly or probably not toxic at exposure levels encountered by humans. Candidate criteria that have been included in a proposed framework for the use of epidemiologic studies in risk assessment (Hertz-Picciotto, 1995), a framework used by the Institute of Medicine in its evaluation of herbicide exposure (Mosteller and Colditz, 1996), and a framework instituted by the Nordic Council of Ministers to evaluate the reproductive toxicity of chemicals (Taskinen, 1995) include the following:
- A positive association is present.
- Selection and information (exposure or disease misclassification) biases are reasonably judged as unlikely to account for the positive association (or failure to find a positive association).
- "Chance" is reasonably judged as unlikely to account for the positive association (or failure to find a positive association).
- Confounding bias has been controlled and/or is reasonably judged as unlikely to account for the positive association (or failure to find a positive association).
- Evidence of a (monotonic) dose-response relationship exists.
- The direction of the associations among the studies and with other evidence, including case reports and animal studies, is consistent.
A recent evaluation of studies of alcohol and breast cancer and vasectomy
and prostate cancer found that the criteria most often used to assess the evidence for carcinogenesis were (1) strength of the association (as measured, for example, by the risk ratio or the mean difference); (2) consistency across study designs and different populations; (3) existence of a dose-response gradient; (4) biological plausibility; and (5) the impact of biases on the strength of the association (Weed and Gorelic, 1996). While these could be the key criteria for a classification framework, and they correspond with the criteria listed above, the assessment of consistency among studies must take into account the many sources of heterogeneity among studies, especially differences in the characterization of exposures and outcomes.
Judging the impact of chance by referring to an arbitrary standard of statistical significance (e.g., p-value cutoff of 0.05 or the lower limit of the 95 percent confidence interval) is not useful for assessing a study, because it focuses attention on values for the parameter of interest (e.g., the risk ratio) that are not likely (i.e., have very low probability) given the actual results of the study. In addition, whether a study result is statistically significant will depend on the size of the study as well as on the magnitude of the effect. Although a larger study might appear to provide more convincing evidence than a smaller study, it is important to remember that there is often a tradeoff between size and validity. For example, a study may increase its size by diluting both its exposure and disease characterizations and thereby increase the impact of bias.
Predictive Biological Activity or Effect Models
Structure-activity relationship (SAR) and quantitative structure-activity relationship (QSAR) models are used to predict biological activity or effects through the identification of correlations between chemical structure or properties of molecules and biological activities, including those that can be identified through in vitro or in vivo screens and tests. They can be used to predict the biological activity of a number of chemicals, are relatively inexpensive tools, and are most useful when empirical toxicological or epidemiological data are unavailable for specific chemicals within a relatively well-characterized group of related chemicals, such as dioxins.
The SAR approach provides a qualitative means of predicting the hazards of a chemical by developing analogies between chemical substances for which there are few data and chemicals with well-documented health or environmental effects (Lavenhar and Maczka, 1985). The application of QSAR models requires the use of statistical techniques to quantify analogies based on numerical descriptors of physicochemical properties (e.g., lipophilicity, steric parameters, and electronic structure). Describing chemical structures numerically using physicochemical parameters allows the similarity or dissimilarity of a set of compounds to be objectively compared. EPA should systematically review all available SAR/QSAR data and summarize it for use, especially when epidemiological and
|
BOX 5-5 Guiding Principles for Using SAR/QSAR Data in Chemical Priority Setting Efforts (adapted from EDSTAC, 1998)
|
toxicological data are minimal or nonexistent for specific contaminants on the CCL.
The Endocrine Disruptor Screening and Testing Advisory Committee developed guiding principles for evaluating the application of SAR/QSAR models that may be useful to EPA in assessing contaminants on the CCL; Box 5-5 summarizes these principles.
Assessing Treatment Data
Before a final regulatory plan can be established for CCL contaminants, the Safe Drinking Water Act requires that available treatment methods be screened for each contaminant to determine which methods are technologically and economically feasible, which are affordable for small systems, and the degree of risk reduction that can be expected by each of the treatment technologies. The SDWA Amendments of 1986 require EPA to designate a best available technology (BAT) treatment for each contaminant to be regulated. (Designation of a BAT does not require the use of that particular technology to remove the contaminant, but it does require any treatment technique to perform at least as effectively as the BAT.) The SDWA Amendments of 1996 introduced the limited consideration of a cost-benefit analysis in the standard-setting process for certain contaminants. Hence, in establishing a regulation for a contaminant, EPA must determine that a meaningful risk reduction can be achieved by regulating and/or removing that particular contaminant, which means that the performance of treatment technologies must be quantifiable. Another important consideration with respect to regulation and treatment is the size of the public water supply system. The 1996 amendments focus particular attention on this issue. One of the major difficulties in developing and implementing new regulations has been the lack of acceptable and affordable approaches for meeting the needs of small water systems. Larger systems are more likely to have the resources to monitor for specific contaminants on a more frequent basis than do smaller systems, and certain treatment technologies that are feasible for large systems may not be feasible for small
systems. Thus, while EPA standards generally are to be set at levels feasible for large systems, the 1996 amendments require EPA to designate acceptable and affordable treatment technologies that can achieve these standards (if any) for small systems, with specific technologies for each of the following service population categories: 25-500; 500-3,300; 3,300-10,000; and greater than 10,000. If no feasible treatment technologies are available for these small systems, variances and exemptions are available.
As for health effects data, EPA should prepare a narrative summary of treatment data for each contaminant on the CCL, but treatment data should not be considered in the preliminary risk assessment recommended in this chapter. The key principle to keep in mind when assessing treatment options for contaminants on the CCL is that the effectiveness of a treatment technology depends on the physical and chemical characteristics of the contaminants in question, and the aquatic matrix in which the contaminants are found. For example, in connection with the existing CCL, contaminants that are only slightly soluble in water (e.g., aldrin, DDE) can be expected to be associated with particles in water and at relatively low dissolved aqueous concentrations. The particulate form of these contaminants should be removable by conventional solid-liquid separation processes, such as coagulation, sedimentation and filtration, and membrane filtration. The dissolved form of these slightly soluble contaminants should be readily removable by such adsorption processes as granular activated carbon adsorption. Contaminants whose solubilities are markedly influenced by pH (e.g., zinc, aluminum) can be removed by first adjusting the pH of the water in which they are found to a level at which they become insoluble, and then removing them by conventional solid-liquid separation processes. If the level of concern is below the solubility limit of the metal, even after pH adjustment, additional treatment processes may be required, such as ion exchange or other chemisorptive processes. For contaminants that are highly polar and have a high aqueous solubility (e.g., perchlorate and some of the substituted phenols), chemical oxidation or reduction, or photolytic or microbial degradation processes may be employed. Membrane processes (e.g., microfiltration, ultrafiltration, and reverse osmosis) if properly staged, are capable of removing both particulate and dissolved contaminants, including such conventional impurities as particulate material and hardness, but these processes can be relatively expensive. In all cases, the technologies that might be implemented to remove these candidate contaminants must do so without interfering with the other objectives of drinking water treatment (e.g., turbidity and color removal, elimination of objectionable tastes and odors) and the removal of other contaminants of health concern. In addition, the recommended processes must not introduce new contaminants to the water that may themselves have an adverse impact on public health.
Assessing Analytical Methods Data
To ensure that data on the occurrence of drinking water contaminants are adequate for exposure assessment, sampling and measurement methods must be reliable and well documented. Analytical methods for currently regulated contaminants in drinking water are well documented and should be adequate for most commonly recognized contaminants that comprise much of the CCL. The greatest analytical challenges lie in the identification of new contaminants and the quantification of emerging contaminants that are intrinsically difficult to measure. Along with written summaries of health effects, exposure, and treatment techniques data, EPA will need to summarize available analytical methods for contaminants on the CCL, focusing especially on newly recognized contaminants.
Chemical Contaminants
From an analytical perspective, it is useful to classify contaminants in drinking water as volatile, semivolatile, and nonvolatile. Volatile organic chemicals (VOCs) have relatively high vapor pressures (0.1 to 380 torr) (Mukund et al., 1995). Therefore, most VOCs are easily purged from the aqueous phase to the gas phase and are separated by gas chromatography. However, if not purged from finished water, remaining VOCs can lead to a large source of exposures by inhalation of indoor air, especially through showering. Variations on this approach have proven to be very robust and are routinely used for the analysis of VOCs in drinking water. In fact, six of the thirteen methods commonly used for determination of organic contaminants in drinking water (see Table 5-2) are for VOCs, and these methods were cited in the Federal Register of July 8, 1987, under the National Primary Drinking Water Regulations (EPA, 1987).
In contrast with VOCs, semivolatile organic compounds (SVOCs) have moderate vapor pressures (10-7 to 0.1 torr) and are not as amenable to routine analysis. Seven standard methods for non-VOC compounds were cited in proposed drinking water regulations in the Federal Register of May 22, 1989, and are also summarized in Table 5-2 (EPA, 1989). However, it will be necessary to develop and standardize new methods for SVOCs and nonvolatile organic compounds in order to obtain the occurrence data necessary to monitor and regulate some of the new and emerging contaminants that may appear on future CCLs. Analytical methods for detecting a wide range of chemical contaminants in drinking water are regularly published in the open research literature; and are not listed in Table 5-2. These methods are not generally validated by EPA, but they represent an important source of information on analytical methods for new and emerging chemical contaminants.
TABLE 5-2 EPA Methods for Determining Organic Compounds in Drinking Water
|
Number |
Method Name |
|
502.1 |
Volatile Halogenated Organic Compounds in Water by Purge and Trap Gas Chromatography |
|
502.2 |
Volatile Organic Compounds in Water By Purge and Trap Capillary Column Gas Chromatography with Photoionization and Electrolytic Conductivity Detectors in Series |
|
503.1 |
Volatile Aromatic and Unsaturated Organic Compounds in Water by Purge and Trap Gas Chromatography |
|
504 |
1,2-Dibromoethane (EDB) and 1,2-Dibromo-3-Chloropropane (DBCP) in Water by Microextraction and Gas Chromatography |
|
505 |
Analysis of Organohalide Pesticides and Commercial Polychlorinated Biphenyl Products in Water by Micro-Extraction and Gas Chromatography |
|
507 |
Determination of Nitrogen-and Phosphorus-Containing Pesticides in Water by Gas Chromatography with a Nitrogen-Phosphorus Detector |
|
508 |
Determination of Chlorinated Pesticides in Water by Gas Chromatography with an Electron Capture Detector |
|
508A |
Screening for Polychlorinated Biphenyls by Perchlorination and Gas Chromatography |
|
515.1 |
Determination of Chlorinated Acids in Water by Gas Chromatography with an Electron Capture Detector |
|
524.1 |
Measurement of Purgeable Organic Compounds in Water by Packed Column Gas Chromatography/Mass Spectrometry |
|
524.2 |
Measurement of Purgeable Organic Compounds in Water by Capillary Column Gas Chromatography/Mass Spectrometry |
|
525.1 |
Determination of Organic Compounds in Drinking Water by Liquid-Solid Extraction and Capillary Column Gas Chromatography/Mass Spectrometry |
|
531.1 |
Measurement of N-Methylcarbamoyloximes and N-Methylcarbamates in Water by Direct Aqueous Injection HPLC with Post Column Derivatization |
|
Source: EPA, 1988 |
|
Microbiological Contaminants
Methods are available for detecting the presence of almost any microorganism of concern, although difficulties can arise in collecting samples, determining frequency and sample sites, and interpreting the relationship between positive samples and public health (Hurst et al., 1997).
Bacteria
While cultivation techniques are well developed for enteric bacterial indicators, such as coliform and fecal coliform bacteria, little attention has been paid to the development of methods for analyzing enteric bacterial pathogens in water.
This is, in part, because of the historical success of using these indicators in preventing the occurrence of most enteric bacterial waterborne disease outbreaks.
In general, if pathogens are present in great enough concentrations, they can be assayed directly. However, as discussed in Chapter 1 (see Figure 1-1), the causative pathogens of more than half of reported waterborne disease outbreaks are then identified. Three basic methods are used for detection and enumeration of bacteria in environmental samples (Toranzos and McFeters, 1997): (1) most probable number (MPN), (2) membrane filter (MF), and (3) presence-absence (PA).
The MPN method measures the growth of organisms taken from a sample (or a serially diluted sample) on (usually) selective media through production of turbidity, acid, or gas. When the positive tubes have been identified and recorded, it is possible to estimate the number of organisms in the original sample by using an MPN table that gives the number of organisms per certain volume. MPN methods are very labor intensive and require large amounts of media and glassware, and, in the case of pathogens, may require several days to complete. In the MF test, a given volume of liquid is passed through a filter with a pore size less than the diameter of the bacteria, and then the filter is placed on the growth media. The bacteria then grow on the surface of the membrane as individual colonies. This method is more accurate, less time consuming, and more rapid than the MPN method. Lastly, PA tests, while not quantitative per se, can answer the simple question of whether the target organism is present in a sample. Since some standards require the absence of an indicator or pathogen in a certain volume (e.g., 0 coliforms per 100 ml of drinking water), the PA method can be used as a pass/fail screening test.
For the most part, culturable analytical methods have been used for bacteria, however, in some cases, only a small percentage of the total viable organisms present may be detected using these methods of bacterial detection (Colwell et al., 1996). Microscopic techniques, such as the use of antibodies, genetic probes, image analysis, and flow cytometry, have become highly sophisticated, specific, and rapid for the detection of bacteria (Lawrence et al., 1998). Staining with specific genetic probes can address not only total bacterial numbers but the genetic composition and taxonomic status of populations. Thus, the state of the microorganism, as well as its identification, can now be ascertained. Applications for digital microscopy include quantification, viability, metabolic condition, as well as the structure of the microenvironment. However, more emphasis needs to be placed on sample concentration and the use of more specific techniques for bacteria such as Helicobacter, which cannot be cultured.
Viruses
Methods for virus detection in water depend on their concentration in volumes ranging from 10 to 2,000 liters. This is accomplished by the adsorption of
the viruses to positively charged filters, which adsorb the negatively charged viruses from water (Sobsey and Glass, 1980). Adsorbed viruses are eluted from the filters with a protein solution and further concentrated to a final volume by precipitation of the proteins before assay. These concentrates are assayed in animal cell cultures of human or primate origin. The presence of the virus is indicated by production of cytopathogenic effects (CPE) (the destruction of the individual cells) or formation of plaques or clear zones produced by the destruction of cells under an agar overlay. The isolated viruses are identified by serological neutralization tests.
Currently, viral cultivation methods have largely been optimized for the detection of enteroviruses, and little information is available on other types of viruses that may be present at equal or greater concentrations in drinking water. The filters used to concentrate viruses from water do not concentrate all types of viruses with equal efficiency because of differences in charge on the different types of viruses (Gerba, 1984). Several studies have reported greater concentrations of adenoviruses than enteroviruses (e.g., Grohman et al., 1993) in sewage and sewage-polluted waters. An additional problem is that many viruses (e.g., hepatitis A) may grow in cell culture without the production of CPE. Further, it can take several days to many weeks before the virus produces CPE. A final problem is that sometimes substances are concentrated from the water that are toxic to the cell culture. Additional research is needed to overcome these problems and to develop better techniques for assessing all types of waterborne viruses, not just enteric viruses.
Protozoa
Protozoan parasites are sufficiently large that they can be observed under a normal light microscope, allowing for detection and quantification, and microscopy remains the traditional method for detecting protozoa.
Standard methods have been developed for collection, recovery, and detection of enteric protozoa. Typically, protozoan parasites are collected from large volumes of water by size exclusion through spun filters with a nominal pore size of one micron. These filters also collect suspended matter in the water and this makes visualization of the parasite cysts or oocyst difficult to observe (Rose et al., 1989; LeChevallier and Trok, 1990). To separate parasites from debris, the filters are cut apart and washed with an eluting solution of detergent. The eluate containing the cysts/oocysts and debris is further concentrated by centrifugation, where centrifugation separates the cysts/oocysts from much of the debris. The semi-purified sample is collected from the gradient and labeled with monoclonal antibodies specific to the cyst or oocyst cell wall using a specific immunofluorescent assay (IFA) procedure. The sample can be examined by epifluorescent microscopy for fluorescence, shape, and size, and by phase contrast or Nomarski
differential interference contrast microscopy for internal features (LeChevallier et al., 1991a,b).
The efficiency of recovery for cysts/oocysts for this process has been investigated in detail, with overall recovery rates varying from 28 percent to 86 percent for Giardia and from 5 percent to 68 percent for Cryptosporidium (LeChevallier et al., 1995; Nieminski et al., 1995). However, current methods for recovering and detecting parasites always underestimate the true concentration in environmental samples. While the use of IFA greatly aids the detection of cysts/oocysts, background fluorescence and nonspecific binding of the antibody may decrease their accurate identification. Another limitation is that no single antibody has been found to bind specifically only to species that cause infection in humans; thus, protozoa infecting only lower animals may also be detected. An added problem is that the viability of the cysts/oocysts cannot be assessed by IFA. LeChevallier et al. (1991a) reported that 10 percent to 30 percent of the organisms found in water samples were empty, without internal features, suggesting they were not viable. It is not clear whether this is an artifact of sample processing.
New analytical methods are currently under development for improving both the recovery and detection of protozoa as well as interpretation of the results (Jakubowski et al., 1996). Methods using cell culture infectivity have been successfully applied to address the important question of Cryptosporidium viability (Slifko et al., 1997a,b). Immunomagnetic separation (IMS) techniques use antibodies tagged to iron beads and a magnetic system to pull the target oocysts and cysts from the suspension. These techniques have been applied in microscopic detection and polymerase chain reaction approaches (Johnson et al., 1995; Deng et al., 1997). Several IMS kits are now available for Cryptosporidium (e.g. Dynal in Lake Success, NY; Crypto-ScanTM in Portland, ME). The use of in-situ hybridization to identify Cryptosporidium (Lindquist, 1997) has widespread application for identification and detection efforts, because both microscopy and the specificity of the probe can be used. This also allows for such instrumentation as flow cytometry and digital microscopy to be used, which can greatly reduce the analytical time. To improve understanding of the relationship between potential exposure to waterborne oocysts and cysts and public health outcomes, the greatest research need may be in addressing the viability methods. In addition, when, where, and how often to sample for protozoa should be addressed, with corresponding development of guidance.
Molecular Techniques
Advances in molecular biology have allowed for the development of more rapid, sensitive, and lower-cost approaches to the detection of pathogens in the environment. These methods are designed to detect and analyze the genetic material of the organisms. Since each organism has a unique genetic code, this
can be used not only to identify specific species but also to ''fingerprint" the strain and clone it. Once a new pathogen has been isolated and its nucleic acid analyzed, methods can be rapidly developed for its detection. These methods also offer the potential to detect microorganisms without the need for cultivation.
The polymerase chain reaction (PCR) has offered the most promise for the rapid detection of pathogens in the environment and has been used for bacteria, protozoa, and viruses (Johnson et al., 1995; Toranzos, 1997). This method involves the specific amplification of the DNA in the genome of the microorganism with the aid of primers. Primers are fragments of DNA that are complementary to the DNA strain to be amplified (sequences specific to the region of the genome to be amplified). Within a few hours, millions of genome copies are produced. The principle of the method involves the repetitive enzymatic synthesis of DNA. Amplification only takes place if the specific nucleic acid of the target organism is present.
PCR has a number of advantages, including (1) specificity of the assay, (2) ability to detect non-cultivable microorganisms, (3) rapidity of the assay (24 hours), (4) ability to conduct multiple assays, and (5) use of automated instrumentation. PCR also has a number of limitations for use directly in environmental samples. First, the maximum volume that currently can be assayed is 0.1 ml. Extracts or concentrates from environmental samples for enteric viruses and protozoa range from 2 ml to 30 ml or more. Thus, further sample concentration is needed (Johnson et al., 1995). Second, environmental samples and concentrates usually contain substances that interfere with detection by masking the target DNA or inhibiting the enzyme reaction. This results in often laborious and time-consuming processing of samples (Abbaszadegan et al., 1993; Schwab et al., 1995; Toranzos, 1997), though it is possible to detect as little as one to two organisms when interfering substances are removed. Lastly, PCR will also detect dead or inactivated microorganisms (Reynolds et al., 1991; Kaucner and Stinear, 1998). Therefore without cultivation procedures it is not possible to assess viability. While PCR could not be used to assess the performance of disinfection processes, it is still useful for assessing occurrence where viability may not be an immediate need. This is the only method available for the detection of some currently uncultivable waterborne pathogens, such as the Norwalk virus.
Summary: Conclusions and Recommendations
In summary, the committee recommends that EPA use a phased process (see Figure 5-1) for determining which contaminants on the CCL are appropriate candidates for regulatory action and which will require research. The recommended process would proceed as follows:
- Within approximately one year of completion of the CCL, EPA should conduct a three-part assessment of each contaminant on the CCL. For each
- contaminant, the three parts consist of (1) a review of existing health effects data, (2) a review of existing exposure data, and (3) a review of existing data on treatment options and analytical methods. The first part of the assessment should consider data on the contaminant's effects on sensitive populations, such as pregnant women, infants, the elderly, and those with compromised immune systems. While general guidelines for reviewing existing data are possible and are presented in this chapter, an important component of the reviews will be policy judgments by EPA about the significance of the data.
- After completion of the three-part assessment, EPA should conduct a preliminary risk assessment based on available data identified in the three-part assessment. The risk assessment, which integrates hazard and exposure analyses to estimate the public health implications of the contaminant, should be carried out, even if there are data gaps, to provide a basis for an initial decision about the disposition of the contaminant and to guide research efforts, where needed. The preliminary risk assessment, while a critical step in the process, should not be overly detailed or resource intensive.
- After completing the preliminary risk assessment for each contaminant, EPA should prepare a separate decision document, that indicates whether the contaminant will be dropped from the CCL because it does not pose a risk, will be slated for additional research (on health effects, exposure, or risk reduction), or will be considered for regulation. The decision document should explain the reasoning for EPA's determination and should be publicly disseminated for comment. Decision documents for contaminants dropped from the CCL should specify the health and exposure data that EPA used to conclude that the contaminant poses little or no risk.
- When the three-part assessment or preliminary risk assessment identifies important information gaps, EPA should develop a research and monitoring plan to fill such gaps in time to serve as the basis for a revised assessment and decision document before the end of the three-and-a-half-year cycle required by Congress for evaluating contaminants on the CCL. In filling information gaps, EPA should solicit the voluntary participation of industry and others and should use its other authorities (such as those under the Toxic Substances Control Act) to help fill data gaps.
- Health advisories should be issued for all contaminants remaining on the CCL after completion of an initial set of decision documents. A health advisory is an informal technical guidance document that defines a nonregulatory (i.e., nonenforceable) concentration of a drinking water contaminant at which no adverse health effects are anticipated to occur over specific exposure durations. To provide the public with the best available information about the contaminant, EPA should develop a health advisory for any contaminant for which credible evidence of a risk in drinking water exists, even if existing data are insufficient to develop a full regulation. Contaminants subject to a health advisory may need
- additional research and monitoring even after completion of a revised assessment and decision document.
Decisions to drop a contaminant from the CCL, to issue a health advisory, or to proceed toward regulation should be based on health risk considerations only. However, EPA should fill data gaps in treatment technologies and analytical methods to avoid delaying regulatory action for contaminants for which current information on treatment and detection is inadequate.
In implementing this phased process, EPA should keep in mind that it should act immediately on all contaminants that meet the statutory tests of (1) adversely affecting public health, (2) being known or substantially likely to occur in public water systems with a frequency and at levels that pose a threat to public health, and (3) presenting a meaningful opportunity for health risk reduction. Development of regulations for contaminants that meet these three requirements (which are specified in the SDWA amendments) should not be delayed by implementation of the phased approach. The ability to act quickly and short-circuit the phased evaluation process is especially critical for protecting the public from newly discovered high-risk contaminants. EPA will need to remain flexible in order to be prepared to address such immediate risks.
References
Abbaszadegan, M., M. S. Huber, C. P. Gerba, and I. L. Pepper. 1993. Detection of enteroviruses in groundwater with the polymerase chain reaction. Applied Environmental Microbiology 59:1318-1324.
Anderson, B. C. and M. S. Bulgin. 1981. Enteritis caused by cryptosporidiosis in calves. Veterinary Medicine of Small Animal Clinics 76:865-868.
Barker, I. K., and P. L. Carbonell. 1974. Cryptosporidium agni sp.n. from lambs and Cryptosporidium bovis sp.n. from a calf with observations on the oocyst. Z. Parasitenkd. 44:289.
Bove, F. J., M. C. Fulcomer, J. B. Klotz, et al. 1995. Public drinking water contamination and birth outcomes. American Journal of Epidemiology 141:850-862.
Colwell, R. R., P. Brayton, A. Huq, B. Tall, P. Harrington, and M. Levine. 1996. Viable but nonculturable vibrio cholera 1 revert to a cultivable state in the human intestine. World Journal of Microbiology and Biotechnology 12:28-31.
D'Antonio, R. G., R. E. Winn, J. P. Taylor, T. L. Gustafson, W. L. Current, M. M. Rhodes, G. W. Gary, and R. A. Zajac. 1985. A waterborne outbreak of cryptosporidiosis in normal hosts. Annals of Internal Medicine 103:886-888.
Deng, M. Q., D. O. Cliver, and T. W. Mariam. 1997. Immunomagnetic capture PCR to detect viable Cryptosporidum parvum oocysts from environmental samples. Applied and Environmental Microbiology 63(8):3134-3138.
EDSTAC (Endocrine Disruptor Screening and Testing Advisory Committee). 1998. Final Report: Volume I. August 1998.
EPA (U.S. Environmental Protection Agency). 1979. National Interim Primary Drinking Water Regulations; Control of Trihalomethanes in Drinking Water; Final Rule. Federal Register 44(231):68624-68707.
EPA. 1984. Aldicarb; Special Review of Pesticide Products Containing Aldicarb. Federal Register 49(134):28320-28323.
EPA. 1987. National Primary and Secondary Drinking Water Regulations; Final Rule. Federal Register 52:25690.
EPA. 1988. Methods for the Determination of Organic Compounds in Drinking Water. EPA-600/4-88/039. Cincinnati, Ohio: EPA, Environmental Monitoring Systems Laboratory, Office of Research and Development.
EPA. 1989. National Primary and Secondary Drinking Water Regulations; Proposed Rule. Federal Register 54(97):22082.
EPA. 1997. Announcement of the Draft Drinking Water Contaminant Candidate List. Federal Register 62(193):52194-52219.
Federal Focus, Inc. 1996. Principles for Evaluating Epidemiologic Data in Regulatory Risk Assessment. Washington, D.C.
Gerba, C. P. 1984. Recovering viruses from sewage, effluents, and water. In Methods for Recovering Viruses from the Environment, G. Berg, ed. Boca Raton, Fla: CRC Press.
Grohmann, G. S., N. J. Ashbolt, M. S. Genova, G. Logan, P. Cox, and C. S. W. Kueh. 1993. Detection of viruses in coastal and river water systems in Sydney, Australia. Water Science and Technology 27:457-461.
Hayes, E. B., T. D. Matte, T. R. O'Brien, T. W. McKinley, G. S. Logsdon, J. B. Rose, B. P. Ungar, D. M. Word, P. F. Pinsky, M. L. Cummings, M. A. Wilson, E. G. Long, and E. S. Hurwitz. 1989. Large community outbreak of cryptosporidiosis due to contamination of a filtered public water supply. New England Journal of Medicine 320:1372-1376
Hertz-Picciotto, I., and R. R. Neutra. 1994. Resolving discrepancies among studies: The influence of dose on effect size. Epidemiology 5:156-163.
Hertz-Picciotto, I. 1995. Epidemiology and quantitative risk assessment: A bridge from science to policy. American Journal of Public Health 85:484-491.
Hurst, C. J., G. R. Knudsen, M. J. McInerney, L. D. Stetzenbach, and M. V. Walter, eds. 1997. Manual of Environmental Microbiology. Washington, D.C.: ASM Press.
Jakubowski, W., S. Boutros, W. Faber, R. Fayer, W. Ghiorse, M. LeChevallier, J. Rose, S. Schaub, A. Singh, and M. Stewart. 1996. Environmental methods for Cryptosporidium. Journal of the American Water Works Association 88(9):107-121.
Johnson, D. W., N. J. Pieniazek, D. W. Griffin, L. Misener, and J. B. Rose. 1995. Development of a PCR protocol for sensitive detection of Cryptosporidium oocysts in water samples. Applied and Environmental Microbiology 61(11):3849-3855.
Kaucner, C., and T. Stinear. 1998. Sensitive and rapid detection of viable Giardia cysts and Cryptosporidium parvum oocysts in large-volume water samples with wound fiberglass cartridge filters and reverse transcription-PCR. Applied and Environmental Microbiology 64(5):1743-1749.
Kramer, M. D., C. F. Lynch, P. Isacson, et al. 1992. The association of waterborne chloroform with intrauterine growth retardation. Epidemiology 3:407-413.
Lavenhar, S. R., and C. A. Maczka. 1985. Structure-activity considerations in risk assessment: a simulation study. Journal of Toxicology and Industrial Health 1(4):249-259.
Lawrence, J. R., J. McInerney, and D. A. Stahl. 1998. Analytical imaging and microscopy techniques. Pp. 29-51 (ch. 5) in Manual of Environmental Microbiology, C. J. Hurst, G. R. Knudsen, M. J. McInerney, L. D. Stetzenbach, and M. V. Walter, eds. Washington, D.C.: ASM Press.
LeChevallier M. W., and T. M. Trok. 1990. Comparison of the zinc sulfate and immunofluorescence techniques for detecting Giardia and Cryptosporidium. Journal of the American Water Works Association 82:75-82.
LeChevallier M. W., W. D. Norton, and R. G. Lee. 1991a. Occurrence of Giardia and Cryptosporidium spp. in surface water supplies. Applied and Environmental Microbiology 57(9):2610-2616.
LeChevallier M. W., W. D. Norton, and R. G. Lee. 1991b. Giardia and Cryptosporidium spp. in filtered drinking water supplies. Applied and Environmental Microbiology 57(9): 2617-2621.
LeChevallier M. W., W. D. Norton, J. E. Siegel, and M. Abbaszadegan. 1995. Evaluation of the immunofluorescence procedure for detection of Giardia cysts and Cryptosporidium oocysts in water. Applied and Environmental Microbiology 61(2):690-697.
Lindquist, J. A. D. 1997. Probes for the specific detection of Cryptosporidium parvum. Water Resources 31(10):2668-2671.
MacKenzie, W. R., N. J. Hoxie, M. E. Proctor, S. Gradus, K. A. Blair, D. E. Peterson, J. J. Kazmierczak, K. Fox, D. G. Addiss, J. B. Rose, and J. P. Davis. 1994. Massive waterborne outbreak of Cryptosporidium infection associated with a filtered public water supply, Milwaukee, Wisconsin, March and April, 1993. New England Journal of Medicine 331(3):161-167.
Meisel, J. L., et al. 1976. Overwhelming water diarrhea associated with Cryptosporidium in an immunosuppressed patient. Gastroenterology 70:1156.
MMWR (Morbidity and Mortality Weekly Report). 1982 Cryptosporidiosis: An assessment of chemotherapy of males with acquired immune deficiency syndrome (AIDS). Morbidity and Mortality Weekly Report 31:589.
Mosteller, F., and G. A. Colditz. 1996. Understanding research synthesis (meta-analysis). Annual Review of Public Health 17:1-23.
Mukund, R., and T. J. Kelly, et al. 1995. Status of ambient measurement methods for hazardous air pollutants. Environmental Science and Technology 29(4):183A-187A.
Munger, R., P. Isacson, S. Hu, et al. 1997. Intrauterine growth retardation in Iowa communities with herbicide-contaminated drinking water supplies. Environmental Health Perspectives 105:308-314.
NRC (National Research Council). 1977. Drinking Water and Health. Washington, D.C.: National Academy Press.
Nieminski, E. C., F. W. Schaefer III, and J. E. Ongerth. 1995. Comparison of two methods for detection of Giardia cysts and Cryptosporidium oocysts in water. Applied and Environmental Microbiology 61:1714-1719.
Reynolds, K. A., C. P. Gerba, and I. L. Pepper. 1991. Detection of infectious enteroviruses by integrated cell culture-PCR procedure. Applied and Environmental Microbiology 62:1424-1427.
Rose, J. B. 1988. Occurrence and significance of Cryptosporidium in water. Journal of the American Water Works Association 80:53-58.
Rose, J. B., L. K. Landeen, R. K. Riley, and C. P. Gerba. 1989. Evaluation of immunofluorescence techniques for detection of Cryptosporidium oocysts and Giardia cysts from environmental samples. Applied and Environmental Microbiology 55:3189-3195.
Schwab, K. J., R. DeLeon, and M. D. Sobsey. 1995. Concentration and purification of beef extract mock eluates from water samples for the detection of enteroviruses, hepatitis A virus, and Norwalk virus by RT-PCR. Applied and Environmental Microbiology 61:531-537.
Shepard, T. 1994. "Proof" of human teratogenicity. Letter to the editor. Teratology 50:97-98.
Slifko, T. R., D. E. Friedman, J. B. Rose, S. J. Upton, and W. Jakubowski. 1997a. An in-vitro method for detection of infectious Cryptosporidium oocysts using cell culture. Applied and Environmental Microbiology 63(9):3669-3675.
Slifko, T. R., D. E. Friedman, J. B. Rose, S. J. Upton, W. Jakubowski. 1997b. Unique cultural methods used to detect viable Cryptosporidium parvum oocysts in environmental samples. Water Science and Technology 35(11-12):363-368.
Smith, H. V., and J. B. Rose. 1998. Waterborne cryptosporidiosis: Current status. Parasitology Today 14 (1):14-22.
Sobsey, M. D., and J. S. Glass. 1980. Poliovirus concentration from tap water with electropositive adsorbent filters. Applied and Environmental Microbiology 40:201-210.
Taskinen, H. K. 1995. Nordic criteria for reproductive toxicity. Journal of Occupational and Environmental Medicine 37:970-973.
Toranzos, G. A. 1997. Environmental Applications of Nucleic Acid Amplification Techniques. Lancaster, Pa.: Technomic Publishers.
Toranzos, G. A., and G. A. McFeters. 1997. Detection of indicator microorganisms in environmental freshwaters and drinking waters. Pp. 184-194 in Manual of Environmental Microbiology, C. J. Hurst, G. R. Knudsen, M. J. McInerney, L. D. Stetzenbach, and M. V. Water, eds. Washington, D.C.: ASM Press.
Tyzzer, E. E. 1907. A sporozoan found in the peptic glands of the common mouse. Proceedings of the Society of Experimental Biological Medicine 5:12.
Tzipori, S. 1983. Cryptosporidiosis in animals and humans. Microbiology Reviews 47:84.
Wartenberg, D., and R. Simon. 1995. Comment: Integrating epidemiologic data into risk assessment. American Journal of Public Health 85:491-493.
Weed, D. L., and L. S. Gorelic. 1996. The practice of causal inference in cancer epidemiology. Cancer Epidemiology, Biomarkers, and Prevention 5:303-311.
Westrick, J. J. 1990. National surveys of volatile organic compounds in ground and surface waters. Pp. 103-125 (ch.7) in Significance and Treatment of Volatile Organic Compounds in Water Supplies, N. M. Ram, R. F. Christman, and K. P. Cantor, eds. Chelsea, Mich: Lewis Publishers, Inc.