3
Example of an End State Based Analysis of Technology Development Needs for the Hanford Tanks
The committee's reference scenario, emphasized in this report, is essentially the same as the scenario identified in the recent Hanford Tank Waste Remediation System (TWRS) Record of Decision [U.S. Department of Energy (DOE), 1997b] except for transportation to and internment in a national repository. This scenario calls for retrieving most (99 percent of the volume) of the waste from each of the tanks, separation of the waste into high-level waste (HLW) and low-activity waste (LAW) streams, closing the tanks subject to occasional surveillance, and leaving the tank farm area unsuitable for future unrestricted use. After separation, the HLW is vitrified for interim on-site storage, and the LAW is immobilized for permanent on-site disposal.
Two alternative scenarios were also defined by the committee. One, an in situ disposal scenario is defined to suggest a remediation approach that stabilizes tanks without waste retrieval. This scenario reflects the possibility that some of the tanks and their contents represent a relatively low risk and their contents might not require retrieval, or that budgets may alter the cost-risk-benefit balance to allow for retrieval of waste from only the higher-risk tanks. A second alternative scenario may be referred to as the extensive separations scenario to cover the case that could be driven by the need to reduce the HLW volume because of the cost of immobilizing and disposing of the waste in a repository. This scenario could also reduce the radionuclide content of the immobilized LAW should that be deemed necessary in the future.
Detailed information underpinning the example was obtained from the Hanford Site TWRS Environmental Impact Statement, previous reports of the National Research Council, and the DOE Environmental Management Office of Science and Technology (EM-OST) and its Tank Focus Area (TFA). In addition, as a framework for the identification, discussion, and recommendation of technologies, the committee reviewed selected risk studies (Colson et al., 1997; Franklin et al., 1996; Harper et al., 1996; Hesser et al., 1995; Johnson et al., 1993; MacFarlane et al., 1994, 1995 a, b) associated with the tanks to determine the role of risk assessment in identifying technology development needs. All these studies addressed the Hanford Site, but they were performed too recently for the committee to be able to judge conclusively the effects they may have had on actual decisions on the tank remediation or technology development programs. Although no impacts were observed up to this time, the committee, nevertheless, believed the studies to be important in understanding the overall risk presented by the tanks and has included a review of these risk studies in Appendix A.
Scope
The Hanford HLW tanks example will focus on remediation of buried tanks containing wastes in the form of alkaline nitrate supernatant, sludge, and saltcake, and the associated tank farms at the Hanford Site. This includes the tanks and their contents as well as the immediately surrounding soil and ground water contaminated with radionuclides or hazardous chemicals. The example does not consider remediation of other sites at Hanford [e.g., miscellaneous underground storage tanks (MUSTs), cesium/strontium capsules, reactors, canyons, cribs, low-level waste (LLW) burial grounds] or larger ground-water contamination issues.
Conditions Affecting Scenario Specification
Initial Conditions
The Hanford Site was established in 1943 as part of the Manhattan Project for the production of weapons-grade plutonium. The site (also known as the Hanford Reservation) occupies approximately 1,450 km 2 (560 square mi.) along the Columbia River in south-central Washington, north of the city of Richland. The site's primary mission remained production of weapons-grade plutonium until 1989. Its current mission is the management of waste generated by the weapons production program and the remediation of the site environment contaminated by that waste.
To produce plutonium, uranium metal was irradiated in graphite-moderated reactors. The irradiated uranium metal was allowed to partially decay, and plutonium was separated from the uranium and other radioactive waste by-products by chemical processing. Large amounts of uranium metal were processed to recover plutonium to make nuclear weapons, and the chemical separations processes resulted in large volumes of radioactive wastes that were ultimately stored in tanks.
From 1943 to 1989, the Hanford Site processed approximately 100,000 metric tons (110,000 short tons) of uranium metal and generated approximately 250,000 metric tons (280,000 short tons) of HLW. The waste was managed in compliance with the laws and regulations applicable at the time, but major changes in laws and regulations governing waste management and disposal have over time become more stringent and have resulted in changes in the waste management program.
Beginning in the 1940s and extending through the early 1960s, 149 single-shell carbon steel tanks with capacities of 210 m3 (55,000 gallons) to 3,800 m3 (1 million gallons) were built to store the HLW near the center of the Hanford Site in a region known as the 200 Areas. Management of the acidic HLW generated by the chemical separations plants consisted of neutralization by the addition of sodium hydroxide or calcium carbonate and storage in the large underground tanks until a long-term disposal solution could be found.
During the 1960s, uranium was extracted from some of the HLW stored in the single-shell tanks. This action introduced additional chemicals into the stored waste. To provide more tank space for plutonium production, efforts were made to concentrate the tank waste by separating radioactive solids from liquids. Chemicals (e.g., ferrocyanide) were added to the tanks to precipitate cesium-137, which had dissolved in the liquid phase, to the bottom of the tanks, thereby reducing the radioactivity of the liquid layer. Also, overflow piping connections built between several of the single-shell tanks allowed liquid waste to flow from one tank to another and radioactive solids to settle. The liquid waste resulting from these efforts was siphoned off
and sent to shallow subsurface drainfields, known as cribs, for disposal into the ground. These actions, while providing tank space for additional waste from plutonium production, resulted in higher concentrations of heat-generating radionuclides in the waste, which threatened the integrity of the tanks.
This heat generation problem was addressed during the 1960s and 1970s when much of the cesium and strontium was removed from the then-existing tank waste. The cesium and strontium were converted to salts, encapsulated in metal cylinders, and stored in a separate facility as waste by-product for commercial use, which never developed to any significant level.
Leakage of waste from single-shell tanks was first suspected in 1956 and confirmed in 1961. By the late 1980s, 67 of the single-shell tanks were known or suspected leakers (Hanlon, 1998). To address the issue of single-shell tank leakage, the Hanford Site adopted a new double-shell tank design that included an outer shell to contain any leakage from the liquid-containing inner shell. The double-shell tank design provided for leak detection and liquid recovery before any waste could reach the surrounding soil.
Between 1968 and 1986, 28 double-shell tanks with capacities ranging from 3,800 m3 (1 million gallons) to 4,400 m3 (1.16 million gallons) were constructed in the 200 Areas. Much of the free-standing liquid contained in the single-shell tanks was pumped into the double-shell tanks. However, the solids remaining in the single-shell tanks still contain some liquids in the interstitial void spaces. No leaks are known to have occurred from the inner shells of the double-shell tanks.
At the Hanford Site there are currently approximately 203,000 m3 (54 million gallons) of waste stored in 177 large tanks. Because the wastes were neutralized to permit storage in carbon steel tanks, most of the chemicals present in the waste precipitated or crystallized. In the liquid remaining in the tanks, the primary dissolved chemicals are non-radioactive sodium nitrate, nitrite, and hydroxide, with much smaller amounts of other chemicals.
To limit leakage and conserve tank space, as much liquid as possible, given other safety considerations, was evaporated from or pumped out of all single-shell tanks. This resulted in the precipitation of many soluble salts from the supersaturated liquid. There are now four distinct types of material—liquid, saltcake, sludge, and slurry—in most of the Hanford tanks (U.S. Department of Energy and Washington State Department of Ecology, 1996). All these materials contain radioactive components.
- Liquid includes the supernatant and drainable interstitial liquid in the tanks. It contains substantial amounts of dissolved chemicals, especially sodium salts such as hydroxide and nitrate/nitrite, often near or at their respective solubility limits. Major radionuclides include cesium-137, technetium-99, and a fraction of the strontium-90.
- Saltcake is a crystalline mixture of chemical salts that precipitated when neutralized liquids were concentrated to reduce storage volume or potential waste mobility. In general, it is composed of the same mix of chemicals and radionuclides as is in the liquid.
- Sludge is a generally viscous, amorphous mixture of relatively insoluble chemicals that precipitated in the tanks as a result of neutralization. Iron and aluminum hydrous oxide compounds are typically important components, but sludges are usually heterogeneous and contain a wide variety of cations and anions as well as interstitial saltcake or liquid. Phosphate ion forms a gelatinous precipitate in the sludge with a variety of cations. The sludge contains most of the radionuclides, with strontium-90 being a major constituent.
- Slurry is a tank waste comprising solid, generally crystalline particles suspended in a liquid. Most of the solids are alkaline nitrate salts that crystallized in the tanks when liquid wastes were concentrated, but some materials similar to sludges are also present. Slurry is found
- only in double-shell tanks at Hanford. Radionuclide constituents are similar to those in the liquid and saltcake.
The volumes of the various waste types for single-shell and double-shell tanks are summarized in Table 2. The estimated amount of non-radioactive chemical components of the tank wastes and the estimated amount of radioactive constituents are found in the Hanford TWRS Final Environmental Impact Statement (U.S. Department of Energy and Washington State Department of Ecology, 1996, Tables A.2.1.2 and A.2.1.3).
In addition to the chemicals and radionuclides contained within the tanks, an estimated one million curies of radionuclides and associated chemicals were released or leaked to the soil beneath the tanks due to an estimated 3,800 m3 (1 million gallons) of leaked liquid (U.S. Department of Energy and Washington State Department of Ecology, 1996, pp. 1-5 and 1-8; Hanlon, 1998) plus an estimated 3 million m3 (800 million gallons) of liquid effluents discharged to surface and subsurface drain fields (U.S. Department of Energy, 1996a, p. 2A-13). The close proximity to the tanks of a portion of the released contamination requires that it be considered as part of the tank closure problem (National Research Council, 1996a).
Management Strategies
Plans and strategies for the long-term management and disposal of Hanford high-level tank waste have been developed and modified over the last 25 years as national policy and regulations have evolved. A comprehensive discussion of these management strategies and their evolution during the past years is given in Appendix B.
The current DOE strategy for remediation of the Hanford HLW tanks is reliant on phased implementation of privatization. DOE's basic objective in implementing privatization is the significant cost and technology benefits that would accrue to the government. DOE expects privatization to result in reduced life cycle costs, access to innovative state-of-the-art technology, and reduced financial risk to the government. However, considerable skepticism exists that such benefits will actually be achieved (Weida, 1997). DOE's own alternative cost comparison in the TWRS environmental impact statement shows the phased implementation alternative to be potentially more costly than most other alternatives. In addition, privatization contractors are not likely to take large financial risks in implementing new untried technology without substantial risk premiums that would increase costs. Thus, the likelihood of accessing truly innovative state-of-the-art technology appears low. In May 1998, DOE down-selected from two privatization contractors to one. This action would appear to put successful implementation of privatization even more at risk and to make the need for DOE to conduct technology development on the privatized processing functions even greater. If the selected private contractor is unsuccessful in deploying its selected technology or is unsuccessful for any other reason, the responsibility for performing tank remediation will rest solely with DOE.
Since the potential for failure of the privatization approach exists and the impact on the schedule and cost is high, the committee concludes that it is important that OST maintain an orderly and comprehensive technology development program regardless of the status of the privatization program. The current DOE policy with regard to technology development diverts attention from critical needs (e.g., vitrification offgas cleanup) and rather focuses attention on
Table 2 Waste Volumes for the Hanford Tanks, as of May 31, 1998a
|
Waste Form |
Single-Shell Tanks, m3 (1000 gal) |
Double-Shell Tanks, m3 (1000 gal) |
Total, m3 (1000 gal) |
|
Liquid |
|||
|
Supernatant |
2,112 (558) |
54,305 (14,346) |
56,417 (14,904) |
|
Solidsb |
|||
|
Slurry |
0 |
1,552 (410) |
1,552 (410) |
|
Sludge |
44,914 (11,865) |
13,317 (3,518) |
58,231 (15,383) |
|
Saltcake |
86,784 (22,926) |
299 (79) |
87,083 (23,005) |
|
Total Waste |
133,810 (35,349) |
69,473 (18,353) |
203,283 (53,702) |
|
NOTES: a The accuracy of the data (reported in 1000 gal) is limited to two or three significant figures. b Solids contain interstitial liquid within the interstitial spaces of the sludge and saltcake that is not added to the total waste volume. For single-shell tanks the volume of interstitial liquid is 8,438 m3 (2,229,000 gal), for double-shell tanks the volume of interstitial liquid is 17,600 m3 (4,650,000 gal). The single-shell tanks' interstitial liquid remains in the tanks following interim stabilization. SOURCE: Hanlon (1998), Tables E-5 and E-6. |
|||
only those items that support providing a waste feed to the contractors; that is, addressing DOE commitments from the privatization contract. This in turn causes considerable confusion in the technology development prioritization process. Identified technology needs that directly support providing a waste feed to the contractors receive preference over those needs that are associated with a processing function belonging to the privatization contractors. The greater need may, in fact, be the latter.
End State Analysis
This chapter has, so far, provided the scope of the Hanford tanks example and background on the initial state of the waste. The next step in the end state based approach is to define possible end states for the waste products. Three potential end states for the committee's reference and alternative scenarios are identified for the example:
- Closed Tank Farms—the general end state for a closed tank farm will be remediation to the point that it is acceptable for stewardship (long-term institutional control), defined as not being acceptable for public use and requiring periodic surveillance to prevent intrusion. The definition of acceptable will vary with the scenario being considered.
- Immobilized HLW—the general end state for the retrieved HLW is standard borosilicate glass logs that are certified for transport and acceptance in a deep geologic repository but that are temporarily residing in a passively cooled on-site storage facility. Beyond this, it is assumed that there are no limits on radionuclide and chemical contents.
- Disposed LAW—the general end state for the LAW stream is an immobilized form containing most of the chemicals in the tanks and a very limited amount of radionuclides. The
- waste is assumed to be emplaced in on-site near-surface disposal facilities that meet long-term performance requirements imposed by regulations as demonstrated by a performance assessment.
The remediation operations could also produce a number of processed liquid and gaseous effluents and solid waste that must be managed in accordance with applicable regulations. There are end states for each of these streams that must also be considered in an actual implementation of the end state based approach. In general, existing technology is adequate to achieve these end states. However, the committee finds that one stream, the vitrifier offgas, may be particularly troublesome, and the technology needs for this stream are addressed later in this report.
In addition to a qualitative description, complete definition of an end state will involve specifications related to the physical and chemical characteristics of the waste products. Initially, the characteristics of a product such as a waste form in its disposal environment may be stated in terms of maximum allowable cost or impacts on a population. Through the process of cost and performance assessment and interactions with stakeholders, these are translated into specifications more directly relevant to the design of a tank remediation system. Examples are the maximum allowable cost per unit of waste, concentration of radionuclides in wastes, and the allowable release rate of radionuclides from wastes.
In the case of the Hanford TWRS program, the translation and allocation of general risk and cost requirements into end state specifications appropriate for use in tank remediation have been partially accomplished and documented (Acree, 1998). Although many aspects of this might be viewed as incomplete and interim, the committee believes that sufficient information on the end states is available to plan and conduct a prudently contingent technology development program for the Hanford baseline flowsheet.
For other site applications of the end state based process, it may be necessary for a group organized by the technology development program to specify the plausible range of end state characteristics without a substantial base of information such as that available on the Hanford tanks and the potential disposal and storage environment. This should generally be possible in a straightforward manner using existing information and experience.
The Hanford tank remediation scenario example has been specifically designed to accommodate uncertainties by requiring the technology development program to address a plausible range of end states, not a single set of end states. It is highly likely that the ultimate approach will fall within the plausible range if the alternatives are properly defined.
High-Level Waste
The present criteria that the vitrified HLW from Hanford tanks are found in several documents; TRW Environmental Safety Systems Inc. (1997) and U.S. Department of Energy (1996d, 1998b). This document is a primary source that consolidates engineering limits and requirements from other sources established as a part of the repository design and licensing process. Criteria resulting from the repository design establish limits on the physical dimensions, shape, weight, heat generation, criticality, etc., to ensure that the HLW canister can be handled within the anticipated design envelope of the repository. In the case of Hanford tank remediation, accommodating the physical (e.g., package size and shape) requirements for repository design is relatively straightforward, having negligible implications for technology needs.
However, the requirements from the yet-to-be-completed licensing process could have significant technology development implications. In particular, the HLW product is a major
component of a system that maintains the hazard from the disposal facility within acceptable limits. The composition and resilience of the HLW package and contents are major factors in meeting these limits. The heterogeneous nature of the Hanford tank wastes could pose significant challenges to producing a waste form such as glass that consistently meets repository licensing requirements concerning composition and homogeneity. There are two interrelated primary documents containing criteria that have significant technology development implications, Title 40 of the Code of Federal Regulations (CFR) Part 191 and 10 CFR 60. Neither in its present form is applicable to HLW destined for a repository, currently being studied for siting at Yucca Mountain, Nevada. Following a complex series of legal, institutional, and legislative events (see National Research Council, 1995, pp. 15-18 for more details), 40 CFR 191 as it applies to the disposal of HLW has been remanded to the U.S. Environmental Protection Agency (USEPA) for revision and repromulgation. The USEPA is promulgating new standards, and as of this writing, the new draft standard that would be applicable to Hanford HLW has not been released. When the new USEPA standard is finally in place, the U.S. Nuclear Regulatory Commission (USNRC) draft regulation 10 CFR 63 [entitled "Disposal of High-Level Radioactive Wastes in a Proposed Geological Repository at Yucca Mountain, Nevada" (A. Campbell, USNRC, personal communication, February 1999)] will be revised to reflect the USEPA dose limits standard.
Low-Activity Waste
Most of the Hanford tank waste is classified as HLW because its source is the first solvent extraction cycle of the fuel processing that was conducted at Hanford. Reclassifying a portion of the separated and chemically treated waste so that it can be managed using the same approach as LLW (i.e., near-surface disposal) avoids having to meet much more demanding and costly requirements. Thus, the first step is to meet the criteria necessary to reclassify and manage this waste as non-HLW (i.e., LAW).
The framework used to reclassify the separated and treated material as LAW is to show that the radioactive constituent concentration in the waste is low enough so that the waste is incidental to the production of the HLW and, thus, is no longer HLW. After extensive discussion and analysis by the DOE and USNRC, the USNRC determined (Bernero, 1993) that the large volume of LAW separated from the tank contents would not be HLW if the following specifications were met:
|
An analysis of the Hanford LAW, including a preliminary risk assessment of on-site LAW disposal (Westinghouse Hanford Company, 1996), has been performed by the DOE and its contractors (Petersen, 1996). The analysis has been evaluated by the USNRC and its contractors (Mackin, et al., 1997). The USNRC's ". . . preliminary finding is a provisional agreement that the LAW portion of the Hanford tank waste planned for removal from the tanks and disposal on site
is incidental waste and is, therefore, not subject to NRC [USNRC] licensing authority" (Paperiello, 1997) if the tank waste is appropriately managed.
In quantitative terms relevant to identification of technology development needs, the requirements for removal of radionuclides are (Petersen, 1996):
- less than 3 percent solids carryover into the final waste product,
- greater than 97 percent removal of radiocesium from the LAW stream, and
- greater than 75 percent removal of transuranic nuclides from those tanks with chemical constituents that render these nuclides significantly soluble.
Determination of the extent of radionuclide removal is first based on meeting the less-than-Class-C waste criterion, but also reflects the maximum extent to which radionuclide removal is technically feasible and economically practical. Removal of other mobile radionuclides such as technetium-99, selenium-79, and uranium may be required, depending on the results of future site/design-specific performance assessments for the LAW. The need to have a solid form in specification (2) above would be met by using a waste form such as calcine, grout, or glass.
The final specification (3) is met by showing that the LAW meets other applicable acceptance criteria like any similar LLW from DOE operations. Primary among these criteria is the USEPA standard for dose from drinking water of less than 0.04mSv/y (4mrem/y), which has been adopted by DOE. After the waste is reclassified as non-HLW, it is subject to the LLW disposal requirements in DOE Order 5820.2A (U.S. Department of Energy, 1988b), although this does not appear to add any requirements that would define additional technology development needs. Finally, the material in the Hanford tanks is a chemically hazardous waste because of its characteristics and some USEPA-listed constituents that it contains. As a result, the waste is subject to the provisions of RCRA and the State of Washington Administrative Code Dangerous Waste Regulations 172-303, both of which require that the waste be processed to remove its hazardous characteristics (e.g., toxicity, corrosivity). This will impose certain requirements on the LAW form, such as being able to reduce the leachability of the toxic constituents to acceptable levels as defined by the standard Toxicity Characteristic Leaching Procedure.
Tank Farm Closure
After tank waste retrieval is completed, the committee assumed that the tanks and associated external contamination will not be physically removed. Consequently, it will be necessary to take actions to leave the tank farms in a suitable long-term disposal condition. As described above for the LAW, any residual waste heel in the tanks is initially classified as HLW. Thus, the first step is to reclassify the waste as being incidental to facilitate appropriate disposition. The approach would presumably be similar to that employed for the LAW. The tanks and their residual contents are assumed to be remediated to meet requirements that may include, but may not be limited to, the USNRC's incidental waste determination, DOE's regulations for near-surface disposal of radioactive waste, and the hazardous waste regulations in the state of Washington's Dangerous Waste regulations, Federal RCRA regulations, and the Federal Comprehensive Environmental Response, Compensation, and Liability Act of 1980, as amended (CERCLA).
In contrast to the relatively advanced state of criteria development for HLW and LAW, similar efforts for tank farm closure are just beginning. Closure considerations were specifically
excluded from the recent Hanford tank waste remediation environmental impact statement (U.S. Department of Energy and Washington State Department of Ecology, 1996). A plan to work through the many issues associated with closure of Hanford tank farms has been prepared (U.S. Department of Energy, 1996a). This leaves those attempting to define technology development needs to support Hanford tank closure in the uncertain position of having to establish provisional requirements in order to proceed. The committee believes that explicitly establishing provisional requirements with the operating organizations responsible for the tanks is the preferred course, as compared to doing nothing or allowing technology development to proceed with no consistent objectives. Since the process of retrieval of the residual heel will likely open leaks in the tanks and cause additional contamination of the surrounding environment (vadose zone), end state criteria for tank closure could include the requirement of no further significant vadose zone contamination.
Cross-Cutting Factors
There are a number of factors, termed cross-cutting factors by the committee, that can affect all end states of Hanford tank waste remediation and most other remediation programs. The first is technology limitations. Specifically, there are fundamental limits to what can be achieved by technology. Most noteworthy among these is that complete separation of two intermixed substances in waste is not possible. This means for example that waste retrieval cannot be absolutely complete, cesium separations from LAW streams cannot be absolutely complete. In addition, there are practical limits on what technology can achieve. These are most often expressed in economic terms, which are considered below.
A second cross-cutting factor that has had, and probably will continue to have, a major effect on end state criteria is stakeholder values. Stakeholder input can occur in a variety of forums, including public meetings, comments on draft documents, the legislative process, and through a variety of oversight committees. This input is highly variable and sometimes contradictory, and generally does not provide a suitable basis for defining end state criteria. When fully considered, integrated with other aspects of the issues, and transformed into a coherent result, information based on stakeholder values is an important part of establishing end state specifications. Such specifications are typically set forth in binding legal agreements, such as the Tri-Party Agreement (TPA) among the Washington State Department of Ecology, U.S. Environmental Protection Agency, and the U.S. Department of Energy (1996) and records of decision such as the recent environmental impact statement concerning remediation of Hanford tanks (U.S. Department of Energy and Washington State Department of Ecology, 1996). Of particular note in this regard are commitments to preclude grout as the LAW immobilization matrix and to establish a goal of retrieving 99 percent of the waste volume from each Hanford tank. Both commitments strongly reflect stakeholder values.
A third cross-cutting factor that has a major effect on the need for technology development is cost. One aspect of cost is the adequacy of remediation and waste cleanup budgets, both near- and long-term. Inadequate near-term budgets can restrict the amount of technology development that can be performed irrespective of need and, as a consequence, can render certain otherwise desirable remediation scenarios infeasible. For example, if needed technology development for removal of radiocesium from LAW were not supported, the LAW might be too hazardous for near-surface disposal, thus requiring the LAW to remain with the HLW and be managed at significantly greater cost. Inadequate long-term budgets for remediation operations can have a feedback effect on the need for technology development. For
example, if budgets are substantially inadequate to support waste retrieval and processing from all tanks, as currently required by the TPA, this could lead to some of the low-risk tanks being closed without waste retrieval, resulting in a need for technology development to stabilize waste in place.
A second aspect of cost is its use as a driving force for optimization. As a standard practice, design engineers work to achieve the minimum life-cycle cost for a process, given constraints such as those mentioned in previous sections. This consideration frequently results in the need for technology development to lower the cost of performing a specific process or remediation function. For example, the cost and other problems resulting from the use of large volumes of water to sluice sludges from tanks resulted in the development of a new generation of sludge retrieval tools that send much less water to subsequent processes.
A very important use of cost in relation to Hanford tank remediation is to determine how much processing is enough. On one hand, it is possible to do very little processing, resulting in no separation of LAW from the HLW, and then to vitrify the entire composite waste stream. This approach would entail the need for technology development on high-throughput vitrifiers for HLW. While conceptually straightforward, the cost of disposing the HLW would be very high. On the other hand, it is possible to process extensively the tank waste to result in an extremely small volume of HLW, and the same or a modestly larger amount of LAW for less expensive onsite disposal. This approach would entail the need for technology development on a variety of tank waste processing techniques to separate more of the non-radioactive chemicals from the radioactive constituents that must be in the HLW. This would greatly reduce the disposal cost, but would result in higher processing costs. Technical staff at Hanford conducted such an evaluation (Westinghouse Hanford Company, 1993) and concluded that a middle course (what is now essentially the Hanford baseline tank remediation process) was probably the most cost-effective, but recommended further development of the alternative bounding scenarios. The committee also notes that optimizing cost may necessitate consideration of costs that are beyond the postulated end state (e.g., transportation and repository costs in optimizing HLW volumes).
Development of Functional Flowsheets
It is conceivable and likely desirable that not all the Hanford tanks and tank waste will be remediated using a single scenario. Instead, as recommended in an earlier study by the National Research Council (1996a), a phased decision-analysis approach based primarily on risk and cost should be considered. In this approach, some of the highest risk tanks would be subjected to extensive remediation, tanks involving moderate risks and costs could be addressed using current reference techniques, and lower risk tanks could be remediated using in situ techniques. Elements of this approach were recognized in the recent Hanford TWRS environmental impact statement (U.S. Department of Energy and Washington State Department of Ecology, 1996) in which two ex situ/in situ alternatives involving combinations of the in situ fill and cap and ex situ intermediate separations alternatives were considered (see Table 3), but not adopted.
The development and use of end state based scenarios for technology development supports a phased decision-making approach by identifying technology needs to support a
Table 3 Selected Impacts of Hanford Tank Waste Remediation Alternatives
|
Environmental Impact Statement Alternative |
Operational Impactsa (fatalities) |
Long-term (10,000-year) Impacts (fatalities) |
||||
|
|
|
On-Site Farmer |
Recreational User |
Cost ($ B) |
||
|
No action |
19 |
600 |
40 |
13 to 16 |
||
|
Long-term management |
19 |
600 |
40 |
19 to 23 |
||
|
In situ fill and cap |
3 |
300 |
20 |
7 to 9 |
||
|
In situ vitrification |
5 |
1 |
0 |
16 to 27 |
||
|
Ex situ/in situ combination 1 |
6 |
60 |
0 |
22 to 27 |
||
|
Ex situ/in situ combination 2 |
6 |
60 |
3 |
17 to 20 |
||
|
Ex situ no separations |
6 |
10 |
0 |
59 to 75 |
||
|
Ex situ intermediate separations |
7 |
10 |
0 |
29 to 35 |
||
|
Ex situ extensive separations |
7 |
10 |
0 |
27 to 38 |
||
|
Phased implementationb |
9 |
10 |
0 |
30 to 38 |
||
|
(preferred alternative) |
|
|
|
|
||
|
a Industrial accidents (including transportation) and radiological impacts, primarily occupational. b Essentially the ex situ intermediate separations alternative. SOURCE: U.S. Department of Energy and Washington State Department of Ecology (1996), Tables 5.7.1, 5.7.3, and 5.7.6. |
||||||
reference and plausible alternative scenarios. If end state based scenarios and technology identification are pursued to completion, the result would be demonstrated technology that could then be used to implement a reference scenario, either of the plausible alternative scenarios, or a decision-based combination of these using risk, cost, or any other considerations.
The risk studies summarized in Appendix A demonstrate that end state based approaches, including some consideration of alternative scenarios, have been pursued by EM. Moreover, the interest in risk-based analyses seems to have been increasing through at least 1994 (National Research Council, 1994b). However, the committee was unable to find any evidence that such risk studies had an effect on the choice of baseline or alternative remediation scenarios or strategies for the Hanford Site tank wastes. In particular, the only scenario currently being pursued for the Hanford tank remediation is a phased implementation, the estimated cost and risk of which are both higher than some other alternatives. The committee believes that such risk-based analyses could be advantageous to technology development decision making, and that the apparent trend toward decision making with the aid of end state based analyses should be continued.
Development of plausible alternative end state scenarios at Hanford should begin with the current Hanford baseline scenario (the most recent baseline available to the committee is defined in U.S. Department of Energy, 1997b). The committee's reference scenario embodies all the relevant factors in the present Hanford baseline. In the following section, the committee's reference and two alternative scenarios are identified and associated with the end states specified, based on the impacts of a range of plausible future events.
In the case of the Hanford tanks a useful perspective and basis for specifying the committee's reference scenario and plausible alternative scenarios is the wide spectrum of tank waste remediation alternatives in the recent TWRS environmental impact statement (U.S.
Department of Energy and Washington State Department of Ecology, 1996). A summary of the health and economic impacts of these alternatives is given in Table 3.
Committee's Reference Scenario
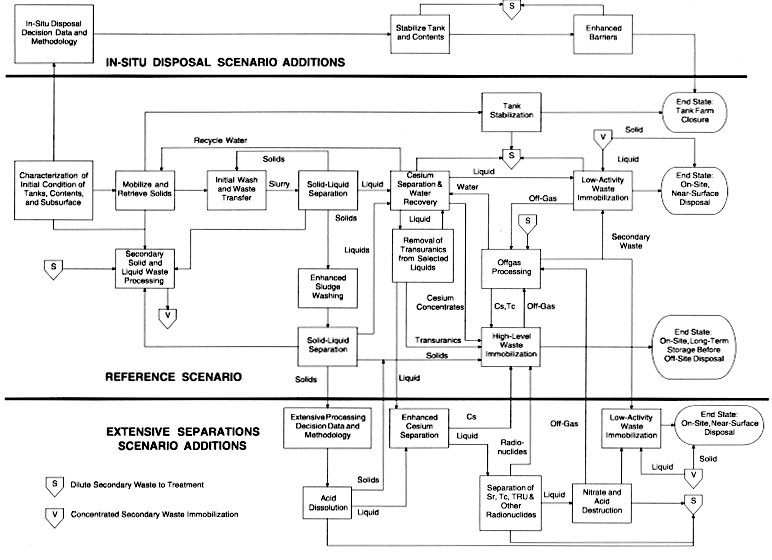
The committee's reference scenario is an adaptation of the Hanford tank remediation baseline scenario that was identified in the recent Record of Decision (U.S. Department of Energy, 1997b) and in Table 3 as the ex situ intermediate separations alternative. It is one of the more costly alternatives evaluated in the environmental impact statement and it entails a moderate level of operational impacts, but it results in one of the lowest levels of long-term impacts. The essence of this scenario is to retrieve most of the waste from all the tanks. The tanks would then be filled, closed, and subjected to occasional surveillance, resulting in the tank farm area being perpetually unsuitable for unrestricted use. The waste would be processed into a vitrified HLW fraction suitable for storage in a passively cooled on-site temporary storage facility and certified for transport to and acceptance in a deep geological repository, and a vitrified LAW fraction that is suitable for on-site near-surface disposal. The relative amounts of HLW and LAW to be produced appear to be consistent with the currently accepted balance of life-cycle cost and risk reduction. This scenario, shown as a schematic flowchart in the middle portion of Figure 4, is summarized below in terms of the operations (functions) in the committee's reference flowsheet.
Characterization. In this step, the wastes in the tanks, the tanks themselves, and the immediately surrounding soil and water are characterized to an extent adequate to resolve safety issues and to allow retrieval and treatment operations, including consideration of leakage during retrieval. This characterization may include measurement of the concentration of various waste constituents (chemicals or radionuclides), the physical properties, particularly rheological properties, of the heterogeneous waste, the integrity of the tanks, and the extent of soil and ground water contamination surrounding the tanks (for determining risk of residual waste left at the tank farms).
It is important to note that characterization will likely occur throughout the scenario. For example, retrieved waste liquids and solids may be characterized to obtain more precise information on the separate streams, the feed to a vitrifier would have to be characterized to determine feed adjustments, and tanks for which waste retrieval operations have been completed would have to be characterized with respect to their residual contents. For clarity, characterization is shown only once in Figure 4.
Mobilization and Retrieval. If substantial amounts of supernatant liquids remain in the tanks, these would first be retrieved by simple pumping. Then a dilute sodium hydroxide solution would be introduced to sluice the soft solids in the tank bottom into a pumpable slurry. This would simultaneously dissolve some of the highly soluble species, such as sodium nitrate and nitrite, that constitute a significant fraction of some of the solids. After the liquids and soft sludges are removed to the extent possible, more aggressive methods would be employed to recover residual soft solids and hard heels. These would include high-pressure water jets deployed on robotic arms or small in-tank vehicles to break up the hard heels, and small sluicer/vacuums to recover pockets of soft sludge and hard-heel fragments. Concrete removal technologies appear to be relevant to these tasks. The extent and type of application of such aggressive methods are strongly influenced by the end state requirements for the tanks.
The extent to which the removal of tank contents will be required is defined by an acceptable end state for the tank, which will be comprised of interrelated specifications on the allowable amount of residual tank contents, the methods used to stabilize the tanks, and ultimate stewardship requirements. Decisions on these specifications will be based on multi-attribute trade-offs involving stakeholders that include local and regional governments, Native Americans, the Department of Energy and its contractors, regulatory organizations, and the U.S. Congress. These deliberations are not yet complete and, as a result, finalized end state specifications are not available. However, an interim goal has been established in the TPA by the Washington State Department of Ecology, USEPA, and DOE (1996) to retrieve about 99 percent of the waste volume from each tank. The committee believes this is an appropriate scenario goal for planning and conducting a technology development program related to tank waste retrieval. The products of this operation are a solution with high concentrations of sodium salts in which solids are suspended, and almost-empty tanks. The former is transferred to a processing facility and the latter is subjected to stabilization, closure, and long-term institutional control (stewardship).
Initial Washes and Waste Transfers. Initial solids washing actually begins during mobilization and retrieval operations, as insoluble solids are mobilized and salts are dissolved and dispersed in the slurry. This washing action continues as the waste slurry is transferred through pipelines to a collection tank, where the output from several retrieval operations is accumulated and blended. The waste slurry is transferred through a double-walled pipeline (a primary pipe within a secondary pipe) that allows for detection of leaks in the primary pipe. These transfer pipes can extend for several miles to reach the processing facility, and careful operation is necessary to avoid conditions that result in plugging.
The retrieval of tank waste is not likely to proceed on a strictly sequential tank-by-tank basis. Instead, at some point, wastes from various tanks will be blended, to the extent possible within operational constraints such as available tank space and transfer lines and their integrity, to eliminate extremes in composition and adverse chemical reactions. Some of the tanks contain large amounts of particular species (radionuclides and chemicals) that, if not blended, could result in increased HLW volumes or the need for highly flexible (and therefore uneconomical) processes downstream. While this blending operation is likely and desirable, it is not shown in Figure 4 in the interests of keeping the diagram simple. Since processing of both blended and unblended wastes can be expected, subsequent steps and their technologies must be capable of handling both waste forms. The product of this operation is a slurry of cesium-contaminated salt-laden water containing insoluble solids, all of which proceed to solid-liquid separation operations.
Solid-Liquid Separation. In this operation the solids are separated from most of the water used for retrieval and initial solids washing operations using such techniques as decanting, filtration, and centrifugation. The operation is necessary because waste retrieval and transfer operations normally require much more water than is necessary or desirable in downstream operations, and the subsequent processing is different for the solid and liquid streams. The liquid stream proceeds to cesium separation and water recovery operations, and the solids to enhanced sludge washing.
The liquids do not have to be totally separated from the solids at this step because subsequent solids processing (i.e., enhanced sludge washing) will involve introducing additional liquids that will be separated and routed to cesium separations. However, the separation of solids from the liquid stream must be essentially complete for two reasons. First, solids can
compromise the cesium removal operation that immediately follows. The extent of the separation required to avoid this problem depends on the specific nature of the solids and the cesium removal process selected. Consequently, the extent of separation cannot be specified at the outset, but it must be taken into account during its execution by communication among various representatives of the technology development program. The second reason for the importance of the degree of solid-liquid separation is that, after cesium removal, this stream becomes LAW in near-surface disposal. The allowable concentration of a number of intermediate- to long-lived radionuclides (e.g., strontium and transuranic nuclides) in the LAW will be determined during preparation of the performance assessment, and may be further limited by waste classification requirements. Since many of the intermediate- to long-lived radionuclides occur in the solid phase, efficient solid-liquid separation is an important function. For this scenario, an appropriate basis for planning and conducting a technology development program would be that the solids in the liquid stream would result in the LAW having concentrations of radionuclides less than Class B concentrations for intermediate-lived radionuclides, such as radiocesium and radiostrontium, and less than Class C concentrations for long-lived radionuclides specified in 10 CFR 61 (U.S. Nuclear Regulatory Commission, 1982a) or the more detailed final environmental impact statement on 10 CFR 61 (U.S. Nuclear Regulatory Commission, 1982b). The resulting allowable concentrations of key radionuclides are listed in Table 4 and, where appropriate, are subject to reductions according to the sum of the fractions rule used by the USNRC as specified in 10 CFR 61.55(a)(7).
Cesium Removal and Water Recovery. In this operation the cesium dissolved in salt-laden water is removed by use of technologies such as ion exchange or solvent extraction to yield a liquid LAW. This step is required because cesium salts are generally very soluble and there is more radioactive cesium-135 and -137 in the waste than is tolerable in the LAW. For this scenario, it is assumed that the cesium must be removed so that its concentration in the immobilized LAW is less than the Class B level specified in 10 CFR 61 (see Table 4).
After the cesium has been removed, the resulting liquid stream contains mostly water with substantial amounts of dissolved soluble chemicals and traces of radionuclides. It is desirable to greatly reduce the amount of water in this stream that must be subsequently evaporated during vitrification and to provide essentially chemical-free water for reuse in tank retrieval operations. This is accomplished using techniques such as evaporation prior to transfer to vitrification.
The products of the cesium removal and water recovery are threefold: a concentrated cesium product (e.g., a liquid containing dissolved cesium, or a solid containing sorbed cesium) that will become part of the vitrified HLW; a concentrated, salt-laden liquid (evaporator bottoms) that will become part of the vitrified LAW; and a relatively pure water stream (evaporator overheads) that is recycled to retrieval operations.
Removal of Transuranics from Selected Liquids. With the exception of compounds of cesium and technetium, most chemicals are quite insoluble under the highly alkaline conditions in the Hanford tanks. Some of the Hanford tanks contain chemical complexants that solubilize these ordinarily insoluble compounds to the extent that the resulting LAW will not meet the end state concentration goals shown in Table 4. This is particularly the case for the transuranics in at least three Hanford tanks containing organic chemicals such as ethylene diamine tetraacetic acid (EDTA). For these tanks it will be necessary either to destroy the remaining organic complexants and remove (e.g., extract, precipitate) the transuranic elements, or to scavenge a sufficient quantity from the liquid phase that will meet the concentration goals of the resulting LAW,
Table 4 Committee's Reference Hanford Tank Remediation Scenario—Concentration Goals for Key Radionuclides in Low-Activity Waste (LAW) for the Purposes of Planning Technology Development
|
Radionuclide |
Concentration Goals In LAW (Ci/m3) |
|
14C |
≤ 8 |
|
90Sr |
≤ 150 |
|
99Tc |
≤ 3 |
|
137Cs |
≤ 44 |
|
129I |
≤ 0.08 |
|
Transuranic elements |
≤ 100 nCi/g [<TRU (by definition)] |
|
SOURCE: U.S. Nuclear Regulatory Commission (1982a,b). |
|
estimated to require removal of about 75 percent of the transuranic elements (Petersen, 1996). The output of this operation is a solution of chemicals and trace radionuclides having a significantly reduced concentration of transuranic elements that proceeds to LAW immobilization, and a small stream of transuranic elements that goes to HLW vitrification.
Low-Activity Waste Immobilization. Although other immobilization processes are candidates for this function, only vitrification is discussed since it is the process currently accepted by the Hanford TPA signatories and stakeholders. In this operation the concentrated liquid product from cesium separation and water recovery is mixed with glass-making chemicals and heated to a temperature above 1000 °C. The result is first the evaporation of the residual water, then water of hydration, and then the decomposition of species such as nitrates, nitrites, carbonates, and sulfates to yield gaseous nitrogen oxides, carbon dioxide, and sulfur dioxide. What remains in the vitrifier is mostly oxides of various cations, which are incorporated into a glass matrix and poured into containers. The products of LAW vitrification are packages of LAW glass and a significant offgas stream discussed below. The end state of the vitrified LAW is on-site near-surface disposal. The performance requirement of the LAW disposal unit may dictate the maximum acceptable dissolution rate of certain radionuclides and limited concentrations of troublesome elements in the LAW glass.
On-Site Near-Surface Disposal. This type of disposal represents the physical end state for the vitrified, containerized LAW and secondary solid low-level wastes. As noted earlier in this chapter, the end state also imposes additional requirements on the long-term performance of the disposal site, very likely requiring the use of additional barriers beyond the waste form. For the purposes of the committee's reference scenario, an appropriate basis for planning and conducting a technology development program is to assume that the LAW disposal site will require a means to fill any void spaces in the waste emplacement horizon, a multicomponent cap designed to last for centuries, barriers to intruder access, monitoring wells, and occasional surveillance to detect and limit any intrusion.
Enhanced Sludge Washing. The solids from the solid-liquid separation operation still contain large amounts of non-radioactive process chemicals that are not highly soluble in near-neutral solutions. Examples of these are aluminum, chromium, and iron compounds. It is cost effective to remove some of these chemicals to reduce the volume of the HLW, which has much higher processing and disposal costs than the LAW. This is accomplished by enhanced sludge washing, which involves contacting the solids with a concentrated aqueous solution of caustic soda. Under these conditions it is thought that many of the HLW glass volume-limiting
constituents other than iron will be dissolved, leaving most of the radionuclides in the solid phase. The remaining solids are separated from the liquid by solid-liquid separation techniques similar to those described below.
The extent to which the solids should be solubilized is bounded by two competing goals. First, the extent and nature of enhanced sludge washing must not be such that too many radionuclides (except for cesium, which will be removed later) are dissolved or suspended in the liquid stream. If this occurs, the allowable concentration of radionuclides in the LAW would likely be exceeded. For planning and conducting a technology development program, the goals in Table 4 are again applicable. This consideration would indicate less solubilization. On the other hand, greater solubilization will result in fewer solids proceeding to the HLW vitrifier which, in turn, will reduce the volume of expensive HLW. Many uncertainties remain before a precise cost-effective balance can be specified. However, an appropriate goal for planning and conducting a technology development program would appear to be a vitrified HLW volume of about 15,000 m3, a value suggested in the TWRS final environmental impact statement (U.S. Department of Energy and Washington State Department of Ecology, 1996). The major product of enhanced sludge washing is a slurry composed of dissolved chemicals and insoluble suspended solids that are routed to the solid-liquid separators.
Solid-Liquid Separations. In this operation the solids are separated from most of the liquid used for enhanced sludge washing operations. The techniques and considerations involved in this are essentially the same as those described above for solid-liquid separation following the initial solids washing operation, and they are not repeated here. The liquid stream from this operation proceeds to cesium separation and water recovery operations, and the solids to HLW vitrification.
High-Level Waste Vitrifier. The HLW vitrifier will convert the insoluble solids to a glass product, essentially the same operation as the vitrification of LAW described above. The primary difference is the throughput of the HLW vitrifier, which is about 10 percent of that for the LAW vitrifier. However, HLW vitrification involves much greater radionuclide concentrations.
The general requirement placed on the HLW product is that it must meet specifications for sustained temporary on-site storage, transportation to a repository site, and emplacement at that site. As discussed earlier in this chapter under end state criteria, many of the regulations that might provide specifications for this product have not been finalized. However the previous requirements specified in 40 CFR 191, 10 CFR 60, and supporting documents from the DOE Office of Civilian Radioactive Waste Management (U.S. Department of Energy, 1993) provide an appropriate basis for planning and conducting a technology development program. In effect, this requires that careful attention be given to the formulation and manufacture of a substantially homogeneous glass to achieve a suitably low dissolution rate, to the type of package in which it is contained, and to the quality assurance of its production. In addition, for the purposes of this report, the vitrified HLW packages are required to withstand about 50 years of on-site dry storage without significant degradation. The products of this operation are vitrified HLW glass logs and a significant offgas stream discussed below.
On-Site Temporary Storage. This operation involves placing each HLW package in a separate cylindrical hole in a storage facility and sealing the hole with a shielding plug. The facility is typically of concrete construction and designed to remove through conduction and convection the relatively low levels of decay heat (i.e., no water cooling is used). Such a facility is in operation at the Savannah River Site.
This is the physical end state of the HLW for technology development planning purposes. Requirements for transportation and the repository are assumed to be outside the scope of the present effort. The design requirements for a facility of this type are straightforward, especially in an arid climate such as that at Hanford. The single exception is the design life. It is the view of the committee that a 50-year design life is an appropriate basis for planning and conducting a technology development program because of the uncertain schedules for approval, construction, and operation of a deep geologic repository.
Offgas Processing. Managing the offgases from the LAW and HLW vitrifiers is a complex and challenging undertaking because of the simultaneous presence of relatively large volumes of gas (air, steam, volatile oxides), corrosive species (nitric and sulfuric acids resulting from the volatilization of nitrates and sulfur oxides), semivolatile radionuclides (cesium, technetium, and ruthenium), and semivolatile chemicals (boron, sodium compounds), all at the very high temperatures typical of vitrifiers. The function of the offgas system is to remove the hazardous constituents to acceptable levels and recycle what is recovered. Processes used could include condensation, filtration, and liquid scrubbing.
The end state of the offgas stream is that it is cleaned sufficiently to be acceptable for release to the atmosphere. Regulations on 'how clean is clean' are presently available for Hanford and are an appropriate basis for planning and conducting a technology development program. The applicable regulatory requirements for effluent discharges are in the TWRS Mission Analysis Report (Acree, 1998). Typically, products of offgas processing that lead to meeting the qualitative end state for the offgas are cleaned gases released to the environment, condensed steam (water) for recycle to retrieval operations or secondary waste treatment, recovered radionuclides recycled to the HLW vitrifier, LAW recycled to the LAW vitrifier, and other secondary LAW (e.g., contaminated nitric and sulfuric acids) sent to secondary waste processing.
Tank Stabilization. It is assumed that the tanks from which waste has been retrieved will not be exhumed because of the extensive cost and risk to workers. Tank stabilization is assumed to involve filling the tank with a material such as gravel or rocks that, at a minimum, prevents tank collapse and surface subsidence. Stabilization could be extended to involve the use of a grout matrix poured into the tank to completely fill it with a resulting strong monolith, eliminating voids and thus reducing subsequent water collection in the tank.
Establishment of end state requirements for the purpose of a tank stabilization and closure technology development program is in its fledgling stages. The committee believes that a prudent planning basis for its reference scenario is that the tank be filled with a pourable agent such as grout to eliminate voids completely where water might collect and leach the residual heel. The grout stabilizing agent could also be designed to retard leaching and to retain potentially troublesome waste products such as technetium.
Tank Farm Closure. This operation is essentially the same as the closure of the on-site, near-surface LAW disposal site discussed above, although additional characterization of the tank contents and adjacent contaminated soil before and after stabilization is likely to be required. The end state and attendant requirements are assumed to be the same for the committee's reference scenario.
Secondary Waste Processing. Many of the operations described above produce secondary wastes. These could be solid wastes ranging from failed equipment, protective
clothing, filters, etc., to a variety of liquids from the various processing operations. These wastes would be processed to yield a releasable liquid effluent, solid wastes suitably treated for on-site near-surface disposal, and a concentrated liquid that would be sent to the LAW vitrifier. Such treatment usually involves existing technology, although there can be exceptions such as disposition of used vitrifiers. The end state requirements for most of these have already been discussed. For the liquid effluent, the Hanford Site has already established criteria for release of liquid effluents (see Acree, 1998), which are an appropriate basis for planning and conducting a technology development program.
In Situ Disposal Scenario
It is the view of the committee that in situ disposal (i.e., tank waste left in place) constitutes another plausible scenario for planning and conducting a technology development program. This scenario could be driven by a combination of factors such as the need to accomplish remediation with a significantly reduced budget, or the recognition that many of the tanks represent a relatively low risk that may not warrant waste retrieval and treatment. As shown in Table 3, alternatives involving in situ techniques reduce the total cost of remediating the tanks by several billion dollars while somewhat decreasing operational impacts. The functions required to implement this scenario, which would replace essentially all of the functions in the committee's reference scenario, are shown in the upper portion of Figure 4 and are described below.
Decision Data and Methodology. The first step in proceeding with in situ tank remediation is to decide which tanks are acceptable for such disposition. A preliminary study (Nelson, 1995) to determine how many tanks could potentially be disposed in situ has been completed and is discussed in Appendix A. While such decisions have many non-technical aspects related to stakeholder values and risk management, there are also technical implications. It will first be necessary to know the contents and characteristics of the tanks to a greater precision than what might be required for retrieval, thus requiring additional characterization and potentially additional technology development. It will be necessary to use knowledge of the tank contents to design the stabilization process and thereby predict both the post-remediation performance of the waste-bearing tanks as an immobilization medium and the relevant geohydrology, both current and predictable future, to provide information on the potential future risks from in situ disposal. This then provides a partial basis for decisions on which tanks are suitable for in situ disposal.
Stabilize Tank and Contents. This operation is conceptually similar to the tank stabilization operation under the committee's reference scenario. However, there are two important differences. First, the tank would contain a significant amount of waste. Some of the waste is likely to be 'soft' and partially mixable with stabilization agents, while other portions are likely to be hard and not readily mixable. Further, many tanks are likely to have holes that would leak if substantial amounts of liquids were introduced. Achieving adequate in situ stabilization under these conditions will be a major challenge. Second, the stabilization agent, which should be appropriately reactive to retain selected waste components, must also be compatible with the waste, which is highly alkaline-again, a major technology challenge.
The performance requirements for this scenario have not been established. Potential performance of yet-to-be-developed stabilization and engineered barrier system technologies
(discussed below) have not been demonstrated. This fact, in turn, precludes determining the range of wastes that might be acceptable for in situ disposal and the trade-offs among cost, risk, and technical performance. The committee notes that the relationship between general performance requirements and the allowable concentration of radionuclides in LAW in on-site near-surface disposal has been established. The committee believes a prudent interim basis for planning and conducting technology development for an alternative scenario such as in situ disposal would be to assume that the impacts from radionuclides released from an in situ disposal site would be the same as those from the LAW disposal site, and to establish targets for site release limits for in situ disposal accordingly. After technology development has proceeded, the feasibility of the goal can be examined, and the technology development results will establish an initial basis for review of end state requirements with regulators.
The result of this operation is a tank with the contents mixed and immobilized to the extent practical and the tank completely filled with a solid material to prevent tank collapse.
Enhanced Barriers. Because of the increased inventory of radionuclides and toxic chemicals that would remain in the tanks in this scenario, it is assumed that enhanced barriers to water ingress and outward migration of toxic species would be employed. The most commonly identified forms of these barriers are impermeable surface caps and subsurface vertical and subhorizontal walls to reduce further the amount of water contacting the stabilized tank and the rate at which contaminated water can move into the biosphere. More advanced technologies involve barriers that chemically react with hazardous constituents (e.g., sorb radionuclides, precipitate toxic metals).
As with tank stabilization, the requirements for these barriers have not been established, but overall criteria for health risks exist. The approach to technology development is the same as that described for tank stabilization and, in fact, chemical adjustment, tank stabilization, and barrier technology must be developed in concert. The result of this operation is a tank farm in which the tank contents have been chemically adjusted and stabilized, and around which enhanced barriers have been installed. The tank farm is then capped as discussed earlier in this chapter under "Tank Farm Closure." At present it is not clear that permanent barriers can be established that will not require some long-term institutional control and maintenance over some of the more highly contaminated areas, including residual waste in remediated tanks and tank farms.
Extensive Separations Scenario
It is the opinion of the committee that an extensive separations scenario is another plausible alternative scenario for planning and conducting a technology development program for Hanford Site tank remediation. This scenario could be driven either by the need to further reduce radionuclide contents in LAW because of increased calculated risks or risk adversity, or the need to reduce vitrified HLW volume because of increases in the cost of disposing of waste in the repository, or both. As indicated in Table 3, the presently estimated operational and long-term impacts and total cost of this scenario may be about the same as for the committee's reference scenario. The drive to reduce the radionuclide contents of the LAW and the volume of HLW results in the need for significantly more intensive processing of the tank waste to remove additional radionuclides and volume-increasing chemicals. The additions to the reference scenario that would be required to implement this scenario are shown in the lower portion of Figure 4. Changes in the amount and nature of the HLW may be sufficiently large such that the
requirements on downstream operations involving HLW (e.g., vitrification, storage) could be altered.
Decision Data and Methodology. The first step in proceeding with this scenario is to decide which tank contents should be subjected to extensive processing. If the driving force is risk reduction, the information required would be related to the tank contents and potential releases of hazardous materials. If the driving force is HLW volume and cost, the information required would relate to chemicals (e.g., chromium) that significantly increase glass volume. In either case, there would be a need for more information on the contents of the tank wastes that will be treated using the extensive separations scenario, which has implications for characterization technology development requirements. These were described earlier in this chapter under the "In Situ Disposal Scenario" section on "Decision Data and Methodology."
Dissolution. The chemicals remaining in the radionuclide-laden solids resulting from enhanced sludge washing in the committee's reference scenario need to be removed if HLW volume reduction is desired. Instead of going to the HLW vitrifier, as in the committee's reference scenario, the solids selected for extensive separations would be dissolved. This is likely to require the use of one or more strong mineral acids and possibly alternative reagents. Since the solids and the radionuclides therein were already destined for the vitrifier in the committee's reference scenario, no additional risk reduction for the waste stream is achieved by dissolution and additional separations processing. The extent of dissolution required is a function of the degree to which cost analyses indicate volume reduction of the HLW is justified.
The output from this operation is an acid solution of radionuclides and chemicals for subsequent processing and the undissolved solids that are sent to the HLW vitrifier as in the committee's reference scenario.
Enhanced Cesium Removal. The solids from enhanced sludge washing may contain significant amounts of cesium. The cesium will be released during dissolution and may require removal to assure adequate risk reduction before the liquid can proceed to LAW vitrification. Additionally, the cesium concentrations in the liquid stream in the committee's reference scenario may be sufficiently high to require enhanced cesium recovery using highly selective techniques such as specialized ion exchange media or solvent extraction. The output from this operation is recovered cesium, routed to the HLW vitrifier in the committee's reference scenario, and an acidic liquid stream, which is routed to additional separations.
The committee recommends that as a basis for planning and conducting a technology development program, the resulting LAW should contain no more than Class A concentrations of radionuclides (U.S. Nuclear Regulatory Commission, 1982a). The values for a few key radionuclides are given in Table 5.
Separation of Strontium, Technetium, Transuranic Elements, and Other Radionuclides. The acidic product from enhanced cesium recovery contains significant amounts of dissolved elements such as strontium, technetium, and transuranics. These will be recovered using such techniques as ion exchange and solvent extraction. As with cesium, the committee recommends that the basis for planning and conducting a technology development program for extensive separations should be that the resulting LAW contain Class A concentrations of strontium, technetium, transuranic elements, and any other radionuclides that might limit achieving equivalent levels of risk. The radionuclide concentrations for Class A waste are given in Table 5.
Table 5 Extensive Separations Hanford Tank Remediation Scenario—Concentration Goals for Key Radionuclides in Low-Activity Waste (LAW) for the Purposes of Planning Technology Development
|
Radionuclide |
Concentration Goals In LAW (Ci/m3) |
|
14C |
≤ 0.8 |
|
90Sr |
≤ 0.04 |
|
99Tc |
≤ 0.3 |
|
137Cs |
≤ 1 |
|
129I |
≤ 0.008 |
|
Transuranic elements |
≤ 10 nCi/g |
|
SOURCE: Based on Class A low-level waste radionuclide concentrations as found in U.S. Nuclear Regulatory Commission (1982a); 10 CFR 61.55, Table 1; and 10 CFR 61.55(a)(3)(i). |
|
The outputs from this operation are recovered radionuclides, which are routed to the HLW vitrifier, and an acidic liquid waste stream having smaller amounts of radionuclides than in the committee's reference scenario.
Nitrate and Acid Destruction. To make the acidic stream compatible with subsequent immobilization operations, the nitric acid must be destroyed. This is accomplished by adding non-volume-increasing chemicals such as formic acid or sugar, resulting in the evolution of nitrogen oxides and carbon dioxide gases, which may be scrubbed out. The extent to which the acid needs to be destroyed is defined by LAW operational considerations. The output from this operation is a near-neutral liquid waste stream which is routed to LAW immobilization operations.
LAW Immobilization. The lower radionuclide concentration in the LAW resulting from this scenario (as compared to the committee's reference scenario) should allow the use of less-expensive technology such as grout for the purposes of LAW immobilization. There is some debate about whether the life-cycle cost of technology such as grout is indeed less than that of vitrification. Some argue that the lower costs of grouting are outweighed by its larger volume, and thus the need for more costly disposal facilities. If this were the case, then the LAW might more properly be routed to the same type of LAW vitrification facility as that used in the committee's reference scenario. If grout were to be used, the LAW would be thoroughly mixed with grouting chemicals and formed into monoliths in retrievable containers (e.g., metal drums) or large near-surface vaults.
The performance of the engineered barriers should be such that, when in combination with the reduced radionuclide levels, the resulting risks are commensurate with Class A low-level wastes. The result of this operation is immobilized LAW for on-site disposal.
On-Site Near-Surface Disposal. This is the physical end state for the containerized LAW and secondary solid LLW. The considerations for this operation are essentially the same as for the committee's reference scenario and will not be repeated here.
Extreme Scenarios
It is possible to consider other scenarios that broaden the coverage of end states even further. For example, it is conceivable that the tank contents could be removed and processed
approximately as indicated in the committee's reference or extensive separations scenarios, and the tanks would be exhumed, sectioned, and sent for disposal elsewhere, leaving a greenfield site suitable for unrestricted access and use. On the other hand, one of the obligatory environmental impact statement alternatives is no action. In the committee's view both scenarios are too extreme and unlikely to constitute a plausible planning basis for a technology development program for Hanford tanks. Consequently, the technology development needs of these scenarios are not included in this analysis, and extreme scenarios should not be the basis of analyses in defining a technology development program.
Functional Flowsheet Consolidation
The functional flowsheets for the three scenarios described earlier in this chapter are diagrammed in Figure 4 to demonstrate the commonalties with the current Hanford baseline flowsheet. The purpose in doing so is to highlight the very important point that only a few additional processing functions over those required for the committee's reference flowsheet are necessary to implement any of the three scenarios. Specifically, by being able to immobilize tank waste in situ, install subsurface barriers, perform enhanced cesium recovery, dissolve residual sludge, and separate strontium, technetium, and transuranic elements in addition to the committee's reference scenario functions, it would be possible to remediate tanks and tank waste under a wide range of end state requirements. This provides a contingency in case less extensive technologies can or must be used, as well as more extensive separations technologies in case of HLW disposal cost considerations. It is beyond the scope of this study to estimate the cost of undertaking the incremental technology development to support all three scenarios, but such cost may be a fraction of the potential savings achieved by selectively deploying all three scenarios as opposed to pursuing only the committee's reference scenario. The following chapter will provide a summary technology assessment for selected functions of the flowsheets shown in Figure 4. The assessment identifies areas that could require technology development, and compares these requirements against ongoing technology development activities.
Summary
An examination of the alternatives proposed in the TWRS environmental impact statement (see Table 3) demonstrates that remediation of Hanford tanks using a combination of disposal end states associated with the committee's reference and alternative scenarios (i.e., in situ and ex situ, with varying degrees of radionuclide separations depending on the specific characteristics of the waste) offers the potential for substantial cost savings with little or no adverse impact on risks. The processing functions required to implement the alternative scenarios and reach the alternative end states, and the required performance of each function, can be determined by specifying a reference and two plausible bounding alternative scenarios. This can be done with modest effort even though uncertainty may exist in the completeness and formality of the specifications of all of the required end states.
The number of processing functions required to implement three scenarios (the committee's reference and two plausible alternatives) is substantially less than triple the number of operations for the committee's reference scenario alone. Thus, the committee believes that the incremental cost for a comprehensive and robust technology program would be reasonable when compared to the potential cost savings that would be experienced from employing an in situ
disposal scenario for some tank waste, and to the potential schedule and cost penalties that would be experienced if a more extensive separations scenario is needed in the future.
An end state based approach employing a reference scenario and plausible alternatives should be used in identifying technology needs for all DOE waste disposal and environmental remediation programs that do not have firmly defined short-term end states that can be achieved using demonstrated technology. Further, the greater the program uncertainties (technical, regulatory, institutional), the greater is the need for applying this approach to provide information and contingency options to decision makers.
Finally, the committee notes that, in some cases, critical TWRS program policy or requirements specification documents, which might provide the basis for the end state based approach, remain under review or in draft form for long periods of time. In many instances the Hanford program direction has changed before an applicable report could be completed, implying that major decisions and changes in program direction are sometimes undertaken by decision makers without the benefit of all pertinent information. While the committee does not recommend that documents be completed if they are already irrelevant, it is important that the relevant documents be completed promptly and made accessible so that all data and information, including risk-based decision analyses, are available to DOE decision makers in time to provide visible support for key decisions, especially those related to a sound technology development program.