Page 137
4
THE MEDICAL VALUE OF MARIJUANA AND RELATED SUBSTANCES
During the course of drug development, a typical compound is found to have some medical benefit and then extensive tests are undertaken to determine its safety and proper dosage for medical use. In contrast, marijuana has been widely used in the United States for decades.162 In 1996, 68.6 million people— 32% of the U.S. population over 12 years old—had tried marijuana or hashish at least once; 5% were current users.162
The data on the adverse effects of marijuana are more extensive than the data on its effectiveness. Clinical studies of marijuana are difficult to conduct: researchers interested in clinical studies of marijuana face a series of barriers, research funds are limited, and there is a daunting thicket of regulations to be negotiated at the federal level (those of the Food and Drug Administration, FDA, and the Drug Enforcement Agency, DEA) and state levels (see chapter 5). Consequently, the rapid growth in basic research on cannabinoids contrasts with the paucity of substantial clinical studies on medical uses.
This chapter is devoted to an analysis of the therapeutic value of marijuana and cannabinoids for specific symptoms associated with various conditions. The risks associated with the medical use of marijuana are discussed in chapter 3. It should be noted that THC, the primary active ingredient in marijuana, is an FDA-approved drug referred to as dronabinol and marketed as Marinol. Marijuana is advocated primarily for relief from the symptoms of disease rather than as a cure.
For the most part, the logical categories for the medical use of mari-
Page 138
juana are not based on particular diseases but on symptoms—such as nausea, appetite loss, or chronic pain—each of which can be caused by various diseases or even by treatments for diseases. This chapter is therefore organized by symptoms rather than by diseases. There are eight sections. The first section explains clinical trials, the following five deal with specific symptoms and conditions, and the last two summarize the medical benefits of marijuana and cannabinoids. The five sections on symptoms and conditions are as follows: pain, nausea and vomiting, wasting syndrome and appetite stimulation, neurological symptoms (including muscle spasticity), and glaucoma.
The Institute of Medicine (IOM) study team received reports of more than 30 different medical uses of marijuana, more than could be carefully reviewed in a report of this length; even more uses are reported elsewhere.62,63 For most of the infrequently mentioned medical uses of marijuana there are only a few anecdotal reports. This report reviews only the most prominent symptoms that are reportedly relieved by marijuana. However, many of those diseases not reviewed here share common symptoms, such as pain, nausea and vomiting, and muscle spasms, which might be relieved by cannabinoid drugs.
Standards For Evaluating Clinical Trials
Before evaluating individual clinical trials concerning the efficacy and safety of medical uses of marijuana and cannabinoids, it is useful to review the general qualities of clinical trials. Clinical trials involve groups of individuals in which different treatments are compared among different groups. Such trials measure the efficacy of a medication and are required by the FDA for approval of any new drug or new use of a drug (discussed further in chapter 5).
The degree of assurance that the outcome of a clinical trial is due to the treatment being tested depends on how well the trial is designed. Three important factors to consider in evaluating the design of a clinical trial are sample selection, subjective effects, and effects that are independent of the treatment. For sample selection it is important to ensure that patients are allocated to different treatment groups in such a way that the groups are not biased toward a particular treatment outcome. For example, the health status, gender, and ages of different treatment groups should be equivalent. Subjective effects must be controlled because they influence experimental results in two important ways. First, a patient's expectation that a treatment will be effective can influence the degree of its effect (for example, in the control of nausea). Second, the investigator's expectation can influence his or her interpretation of the treatment effect (for example, when assessing the level of pain experienced by a patient).
Page 139
For these reasons, double blinding, in which neither the subject nor the person who assesses the drug's effect is aware of the subject's treatment group, is particularly important in cannabinoid drug studies. Another important control for subjective effects includes the use of placebo drugs, which are inert substances, or the use of comparison drugs that have effects similar to the experimental drug. Finally, the quality of the experimental design depends on controlling for factors that are unrelated to the test drug but that might nonetheless influence the treatment outcome. Sequencing effects are one example of such factors. For example, patients might react differently to the same medication depending on whether the medication was administered after an effective or an ineffective treatment. Likewise, a patient whose symptoms are initially mild might react differently to a drug than would a patient whose symptoms are initially severe. Because psychological effects are associated with cannabinoid drugs, it is important to consider how such side effects might influence the therapeutic value of the treatment. Conditions such as pain and nausea are especially susceptible to subjective influences. For example, depending on the person, THC can reduce or increase anxiety; it is important to determine to what extent this ''side effect'' contributes to the therapeutic effect.
While double-blind, randomized, controlled clinical trials offer the highest degree of assurance of drug efficacy, such trials are not always feasible. Vulnerable populations, such as children, older patients, and women of child-bearing age, are often excluded from experimental drug trials for safety reasons. Nonetheless, such patients are part of everyday clinical practice. The challenge of integrating the ideal of standardized and rigorous processes for treatment evaluation with everyday clinical practice has encouraged interest in single-patient trials.67 Methods for such trials have been established and tested in a variety of clinical settings, usually under everyday conditions.66,105,159 They are particularly valuable when physicians or patients are uncertain about the efficacy of treatment for symptomatic diseases. Controls can be incorporated even in this kind of trial. Such trials can be double blinded and can involve crossover designs in which the patient is treated with alternating treatments, such as placebo-drug-placebo or one drug followed by another drug. As with any other clinical trial, a single-patient trial should be designed to permit objective comparison between treatments.
Analgesia
Pain is the most common symptom for which patients seek medical assistance.5 Pain associated with structural or psychophysiological disorders can arise from somatic, visceral, or neural structures. Somatic pain results from activation of receptors outside the brain and is transmitted to
Page 140
the brain via peripheral nerves. Visceral pain results from activation of specific pain receptors in the intestine (visceral nociceptive receptors); it is characterized as a deep aching or cramping sensation, but its source is often experienced at sites remote from the site of receptor activation, a phenomenon known as referred pain. Neuropathic pain results from injury to peripheral receptors, nerves, or the central nervous system; it is typically burning, the skin feels abnormally unpleasant when gently touched (dysesthesia), and it often occurs in an area of sensory loss, as in the case of postherpetic neuralgia (shingles).
All of the currently available analgesic (pain-relieving) drugs have limited efficacy for some types of pain. Some are limited by dose-related side effects and some by the development of tolerance or dependence. A cannabinoid, or other analgesic, could potentially be useful under any of the following circumstances:
· There is a medical condition for which it is more effective than any currently available medication.
· It has a broad clinical spectrum of efficacy and a unique side effect profile.
· It has synergistic interactions with other analgesics.
· It exhibits "side effects" that are considered useful in some clinical situations.
· Its efficacy is enhanced in patients who have developed tolerance to opioids.
There have not been extensive clinical studies of the analgesic potency of cannabinoids, but the available data from animal studies indicate that cannabinoids could be useful analgesics. In general, cannabinoids seem to be mild to moderate analgesics. Opiates, such as morphine and codeine, are the most widely used drugs for the treatment of acute pain, but they are not consistently effective in chronic pain; they often induce nausea and sedation, and tolerance occurs in some patients. Recent research has made it clear that CB1 receptor agonists act on pathways that partially overlap with those activated by opioids but through pharmacologically distinct mechanisms (see chapter 2). Therefore, they would probably have a different side effect profile and perhaps additive or synergistic analgesic efficacy.
In light of the evidence that cannabinoids can reduce pain in animals, it is important to re-evaluate the evidence of analgesic efficacy in humans and to ask what clinical evidence is needed to decide whether cannabinoids have any use in the treatment of pain.
Page 141
Clinical Studies of Cannabinoids
There have been three kinds of studies of the effects of cannabinoids on pain in human volunteers: studies of experimentally induced acute pain, studies of postsurgical acute pain, and studies of chronic pain. Overall, there have been very few studies—only one since 1981—and they have been inconclusive.
Experimentally Induced Acute Pain
Early studies of cannabinoids on volunteers did not demonstrate consistent analgesia when experimental pain models were used. In fact, three early volunteer studies of THC and experimental pain caused by a variety of pain modalities—electrical stimulation, tourniquet pain, and thermal pain—resulted in an increase in pain sensitivity (hyperalgesia).22,84,108
Other studies also failed to show an analgesic effect of THC, but they were not well designed. Raft and co-workers found no evidence of THC effect on pain thresholds and pain tolerance following electrical stimulation and noxious pressure.150 But their study suffers from two major methodological problems. First, they measured only the extremes of pain sensation—threshold (the lowest intensity at which a particular stimulus is perceived as painful) and tolerance (the maximum intensity of pain that a subject can withstand). However, most pain is experienced in an intermediate range, where effects on pain suppression are most detectable. Modern methods of pain assessment in humans typically use ratings of the intensity of the sensation of pain; those methods are superior to assessing the effects of a drug on the extremes of pain.192 Second, Raft and coworkers did not include a positive control; that is, they did not demonstrate the adequacy of their method by showing that an established analgesic, such as an opiate or narcotic, was effective under their study conditions.
Clark and co-workers22 tested the effect of smoked marijuana on thermal pain in volunteers and failed to observe an analgesic effect. However, because of the study design, the results are inconclusive. First, there was no positive control to demonstrate the adequacy of their methods; second, the study subjects were habitual marijuana users. During the study, they were hospitalized and allowed free access to marijuana cigarettes for a period of four weeks, consuming an average of four to 17 marijuana cigarettes per day. Pain was tested "approximately every one to two weeks." Thus, it is quite likely that the subjects were tolerant to THC at the time of testing.
Page 142
Surgical Acute Pain
Raft and co-workers150 found no analgesic effect of THC on surgical pain induced by tooth extraction. However, that study suffered from several serious limitations: the tooth extraction included treatment with the local anesthetic lidocaine, the pain during the procedure was assessed 24 hours later, and there was no positive control. Levonantradol (a synthetic THC analogue) was tested in 56 patients who had moderate to severe postoperative or trauma pain.89 They were given intramuscular injections of levonantrodol or placebo 24 hours after surgery. To control for previous drug exposure, patients with a history of drug abuse or addiction and those who received an analgesic, antiinflammatory, tranquilizer, sedative, or anesthetic agent within 24 hours of the test drug were excluded from the study. On average, pain relief was significantly greater in the levonantradol-treated patients than in the placebo-treated patients. Because the authors did not report the number or percentage of people who responded, it is not clear whether the average represents consistent pain relief in all levonantradol-treated patients or whether some people experienced great relief and a few experienced none.
Chronic Pain
The most encouraging clinical data on the effects of cannabinoids on chronic pain are from three studies of cancer pain. Cancer pain can be due to inflammation, mechanical invasion of bone or other pain-sensitive structure, or nerve injury. It is severe, persistent, and often resistant to treatment with opioids. In one study, Noyes and co-workers found that oral doses of THC in the range of 5-20 mg produced analgesia in patients with cancer pain.139,140 The first experiment was a double-blind, placebo-controlled study of 10 subjects and measured both pain intensity and pain relief.140 Each subject received all drug treatments: placebo and 5, 10, 15, and 20 mg of THC in pill form; each pill was identical in appearance and given on successive days. The 15- and 20-mg doses of-THC produced significant analgesia. There were no reports of nausea or vomiting. In fact, at least half the patients reported increased appetite. With a 20-mg dose of THC, patients were heavily sedated and exhibited "depersonalization," characterized by a state of dreamy immobility, a sense of unreality, and disconnected thoughts. Five of 36 patients exhibited adverse reactions (extreme anxiety) and were eliminated from the study. Only one patient experienced this effect at the 10-mg dose of THC. The mean age of the patients was 51 years, and they were probably not experienced marijuana smokers. A limitation of this study is that there were no positive con-
Page 143
trols—that is, other analgesics that could provide a better measure of the degree of analgesia produced by THC.
In a later larger single-dose study, the same investigators reported that the analgesic effect of 10 mg of THC was equivalent to that of 60 mg of codeine; the effect of 20 mg of THC was equivalent to that of 120 mg of codeine.139 (Note that codeine is a relatively weak analgesic.) The side effect profiles were similar, though THC was more sedating than codeine. In a separate publication the same authors published data indicating that patients had improved mood, a sense of well-being, and less anxiety.139
The results of the studies mentioned above on cancer pain are consistent with the results of using a nitrogen analogue of THC. Two trials were reported: one compared this analogue with codeine in 30 patients, and a second compared it with placebo or secobarbital, a short-acting barbiturate.175 For mild, moderate, and severe pain, the THC analogue was equivalent to 50 mg of codeine and superior to placebo and to 50 mg of secobarbital.
Case Reports and Surveys
The few case reports of clinical analgesia trials of cannabinoids are not convincing.85,120 There are, however, anecdotal surveys that raise the possibility of a role for cannabinoids in some patients who have chronic pain with prominent spasticity. A recent survey of over 100 patients with multiple sclerosis reported that a large number obtained relief from spasticity and limb pain (discussed further under the section on multiple sclerosis).28 Several said that it relieved their phantom pain and headache.41
Migraine Headaches
There is clearly a need for improved migraine medications. Sumatriptan (Imitrex) is the best available medication for migraine headaches, but it fails to abolish migraine symptoms in about 30% of migraine patients.118,147 Marijuana has been proposed numerous times as a treatment for migraine headaches, but there are almost no clinical data on the use of marijuana or cannabinoids for migraine. Our search of the literature since 1975 yielded only one scientific publication on the subject. It presents three cases of cessation of daily marijuana smoking followed by migraine attacks—not convincing evidence that marijuana relieves migraine headaches.43 The same result could have been found if migraine headaches were a consequence of marijuana withdrawal. While there is no evidence that marijuana withdrawal is followed by migraines, when analyzing the strength of reports such as these it is important to consider
Page 144
all logical possibilities. Various people have claimed that marijuana relieves their migraine headaches, but at this stage there are no conclusive clinical data or published surveys about the effect of cannabinoids on migraine.
However, a possible link between cannabinoids and migraine is suggested by the abundance of cannabinoid receptors in the periaqueductal gray (PAG) region of the brain. The PAG region is part of the neural system that suppresses pain and is thought to be involved in the generation of migraine headaches.52 The link or lack thereof between cannabinoids and migraine might be elucidated by examining the effects of cannabinoids on the PAG region.110 Recent results indicating that both cannabinoid receptor subtypes are involved in controlling peripheral pain15 suggest that the link is possible. Further research is warranted.
Conclusions: Analgesia
A key question to address is whether there is any receptor selectivity for the analgesic efficacy of cannabinoids. Are the unwanted side effects (amnesia and sedation) caused by the same receptors in the same brain regions as those producing the analgesia? If the answer is yes, enhancing efficacy will not solve the problem of sedation. Similarly, are the pleasant side effects due to an action at the same receptor? Can the feelings of wellbeing and appetite stimulation be separated by molecular design? Recent results indicating that both cannabinoid receptor subtypes are independently involved in controlling peripheral pain15 (discussed in chapter 2) strongly suggest that this is possible and that further research is warranted.
Further research into the basic circuitry underlying cannabinoid analgesia should be valuable. The variety of neural pathways that underlie the control of pain suggests that a synergistic analgesia "cocktail" would be effective. For example, Lichtman and Martin have shown the involvement of an a2 adrenoreceptor in cannabinoid analgesia.111 Perhaps a combination of a CB1 agonist and an a2 agonist (such as clonidine) would provide enhanced analgesia with less severe side effects.
Clinical studies should be directed at pain patients for whom there is a demonstrated need for improved management and where the particular side effect profile of cannabinoids promises a clear benefit over current approaches. The following patient groups should be targeted for clinical studies of cannabinoids in the treatment of pain:
· Chemotherapy patients, especially those being treated for the mucositis, nausea, and anorexia.
Page 145
· Postoperative pain patients (using cannabinoids as an opioid adjunct to determine whether nausea and vomiting from opioids are reduced).
· Patients with spinal cord injury, peripheral neuropathic pain, or central poststroke pain.
· Patients with chronic pain and insomnia.
· AIDS patients with cachexia, AIDS neuropathy, or any significant pain problem.
In any patient group an essential question to be addressed is whether the analgesic efficacy of opioids can be augmented. The strategy would be to find the ceiling analgesic effect with an opioid (as determined by pain intensity and tolerability of side effects) and then add a cannabinoid to determine whether additional pain relief can be obtained. That would begin the investigation of potential drug combinations. As with any clinical study on analgesic drugs, it will be important to investigate the development of tolerance and physical dependence; these are not themselves reasons to exclude the use of cannabinoids as analgesics, but such information is essential to the management of many drugs that are associated with tolerance or physical dependence.
A secondary question would be whether THC is the only or the best component of marijuana for analgesia. How does the analgesic effect of the plant extract compare with that of THC alone? If there is a difference, it will be important to identify the combinations of cannabinoids that are the most effective analgesics.
In conclusion, the available evidence from animal and human studies indicates that cannabinoids can have a substantial analgesic effect. One exception is the lack of analgesic effect in studies on experimentally induced acute pain, but because of limitations in the design of those studies they were inconclusive. Further clinical work is warranted to establish the magnitude of the effect in different clinical conditions and to determine whether the effect is sustained. Although the usefulness of cannabinoids appears to be limited by side effects, notably sedation, other effects such as anxiolysis, appetite stimulation, and perhaps antinausea and antispasticity effects should be studied in randomized, controlled clinical trials. These very "special" effects might warrant development of cannabinoid drugs for particular clinical populations.
Nausea and Vomiting
Nausea and vomiting (emesis) occur under a variety of conditions, such as acute viral illness, cancer, radiation exposure, cancer chemotherapy, postoperative recovery, pregnancy, motion, and poisoning. Both
Page 146
are produced by excitation of one or a combination of triggers in the gastrointestinal tract, brain stem, and higher brain centers (Figure 4.1, Emesis-stimulating pathways).127 There are numerous cannabinoid receptors in the nucleus of the solitary tract, a brain center that is important in the control of emesis.79,80 Although the same mechanisms appear to be involved in triggering both nausea and vomiting, either can occur without the other. Much more is known about the neural mechanisms that produce vomiting than about those that produce nausea, in large part because vomiting is a complex behavior involving coordinated changes in the gastrointestinal tract, respiratory muscles, and posture, whereas nausea is a sensation involving primarily higher brain centers and lacks a discrete observable action.104,128 Most reports on the antiemetic effects of marijuana or cannabinoids are based on chemotherapy-induced emesis; they are the subject of the following section.
Chemotherapy-Induced Nausea and Vomiting
The use of effective chemotherapeutic drugs has produced cures in some malignancies and retarded the growth of others, but nausea and
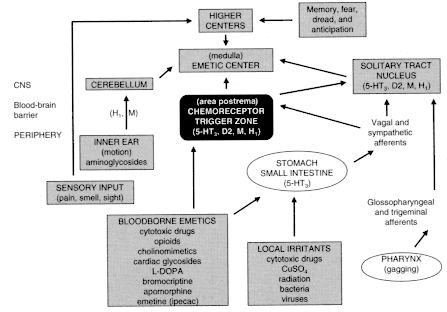
Figure 4.1
Emesis-stimulating pathways. Source: Bruton, L.L. 1996.
P. 929 in Hardman et al., eds., The Pharmacological Basis of Therapeutics,
9th edition. New York: McGraw-Hill. Reprinted with permission.
Page 147
vomiting are frequent side effects of these drugs. Nausea ranks behind only hair loss as a concern of patients on chemotherapy, and many patients experience it as the worst side effect of chemotherapy. The side effects can be so devastating that patients abandon therapy or suffer diminished quality of life. As a result, the development of effective strategies to control the emesis induced by many chemotherapeutic agents is a major goal in the supportive care of patients with malignancies.
The mechanism by which chemotherapy induces vomiting is not completely understood. Studies suggest that emesis is caused by stimulation of receptors in the central nervous system or the gastrointestinal tract. This stimulation appears to be caused by the drug itself, a metabolite of the drug, or a neurotransmitter.6,12,35 In contrast with an emetic like apomorphine, there is a delay between the administration of chemotherapy and the onset of emesis. This delay depends on the chemotherapeutic agent; emesis can begin anywhere from a few minutes after the administration of an agent like mustine to an hour for cisplatin.12
The most desirable effect of an antiemetic is to control emesis completely, which is currently the primary standard in testing new antiemetic agents (R. Gralla, IOM workshop). Patients recall the number of emetic episodes accurately, even if their antiemetics are sedating or affect memory;101 thus, the desired end point of complete control is also a highly reliable method of evaluation. The degree of nausea can be estimated through the use of established visual analogue scales.*21,55,101
Another consideration in using antiemetic drugs is that the frequency of emesis varies from one chemotherapeutic agent to another. For example, cisplatin causes vomiting in more than 99% of patients who are not taking an antiemetic (with about 10 vomiting episodes per dose), whereas methotrexate causes emesis in less than 10% of patients.55,82,83 Among chemotherapeutic agents, cisplatin is the most consistent emetic known and has become the benchmark for judging antiemetic efficacy. Antiemetics that are effective with cisplatin are at least as effective with other chemotherapeutic agents. Controlling for the influence of prior chemotherapy and balancing predisposing factors such as, sex, age, and prior heavy alcohol use among study groups are vital for reliability. Reliable randomization of patients and blinding techniques (easier when there are no psychoactive effects) are also necessary to evaluate the control of vomiting and nausea.
*The visual analogue scale is a continuous line representing all possible levels of a particular sensation. It is an estimation of a patient's subjective evaluation and not a true measurement. Patients select a point anywhere on the line to demonstrate the level of sensation they are experiencing, with one end representing one extreme, such as no sensations, and the other end representing the opposite extreme, such as a maximum level of that sensation.
Page 148
THC and Marijuana Therapy for Chemotherapy-Induced Nausea and Vomiting
Cannabinoids are mildly effective in preventing emesis in some patients who are receiving cancer chemotherapy. Several cannabinoids have been tested as antiemetics, including THC (both D9-THC and D8-THC) and the synthetic cannabinoids nabilone and levonantradol. Smoked marijuana has also been examined.
Antiemetic Properties of THC
The quality and usefulness of antiemetic studies depend on adherence to the methodological considerations outlined above. Many of the reported clinical experiences with cannabinoids are not based on definitive experimental methods. In studies that compared THC with a placebo, THC was usually found to possess antiemetic properties. However, the chemotherapeutic drug varied in most trials, and some studies included small numbers of patients. In one study THC was found to be superior to a placebo in patients receiving methotrexate, an agent that is not a strong emetic.18 When the same investigators studied THC in a small number of patients who were receiving a chemotherapeutic drug that is more likely to cause emesis than anthracycline, the antiemetic effect was poor.19
Other trials were designed to compare THC with that of Compazine (prochlorperazine).143,160( In the 1980s, prochlorperazine was one of the more effective antiemetics available, but it was not completely satisfactory, and the search for better agents continued. THC and prochlorperazine given orally showed similar degrees of efficacy, but the studies often used various chemotherapeutic agents. Even when administered in combination, THC and prochlorperazine failed to stop vomiting in two-thirds of patients.50
In a carefully controlled double-blind study comparing THC with the antiemetic drug metoclopramide, in which no patient had previously received chemotherapy and in which anticipatory emesis was therefore not a factor, all patients received the same dose of cisplatin and were randomly assigned to the THC group or the metoclopramide group. Complete control of emesis occurred in 47% of those treated with metoclopramide and 13% of those treated with THC.58 Major control (two or fewer episodes) occurred in 73% of the patients given metoclopramide compared to 27% of those given THC. There were many flaws in experimental methods, but those results suggest that THC has some, but not great, efficacy in reducing chemotherapy-induced emesis.18,19,50,161 The studies also indicate that the degree of efficacy is not high. In 1985, the
Page 149
FDA approved THC in the form of dronabinol for this treatment (discussed in chapter 5).
The THC metabolite, 11-OH-THC, is more psychoactive than THC but is a weaker antiemetic.121 Thus, it might be possible to design antiemetic cannabinoids without the psychological effects associated with marijuana or THC. D8-THC is less psychoactive than THC151 but was found to completely block both acute and delayed chemotherapy-induced emesis in a study of eight children, ages 3-13 years.* Two hours before the start of each cancer treatment and every six hours thereafter for 24 hours, the children were given D8-THC as oil drops on the tongue or in a bite of bread (18 mg/m2 body surface area). The children received a total of 480 treatments. The only side effects reported were slight irritability in two of the youngest children (3.5 and 4 years old). Based on the prediction that the THC-induced anxiety effects would be less in children than in adults, the authors used doses that were higher than those recommended for adults (5-10 mg/m2 body surface area).
Antiemetic Properties of Synthetic THC Analogues
Nabilone (Cesamet) and levonantradol were tested in various settings; the results were similar to those with THC. Efficacy was observed in several trials, but no advantage emerged for these agents.176,185 As in the THC trials, nabilone and levonantradol reduced emesis but not as well as other available agents in moderately to highly emetogenic settings. Neither is commercially available in the United States.
Antiemetic Properties of Marijuana
Among the efforts to study marijuana was a preliminary study conducted in New York state on 56 cancer patients who were unresponsive to conventional antiemetic agents.188 The patients were asked to rate the effectiveness of marijuana compared with results during prior chemotherapy cycles. In this survey, 34% of patients rated marijuana as moderately or highly effective. The authors concluded that marijuana had antiemetic efficacy, but its relative value was difficult to determine because no control group was used and the patients varied with respect to previous experiences, such as marijuana use and THC therapy.
*Note that the authors of this study chose to use D8-THC because it is more stable and easier to produce than D9-THC; it does not follow from this particular study that marijuana, with its mixture of cannabinoids, should be a more powerful antiemetic than D9-THC.
Page 150
A Canadian oncology group conducted a double-blind, cross-over, placebo-controlled study comparing smoked marijuana with THC in pill form in 20 patients who were receiving various chemotherapeutic drugs.107 The degree of emetic control was similar: only 25% of patients achieved complete control of emesis; 35% of the patients indicated a slight preference for the THC pills over marijuana, 20% preferred marijuana, and 45% expressed no preference. 107
Neither study showed a clear advantage for smoked marijuana over oral THC, but neither reported data on the time course of antiemetic control, possible advantages of self-titration with the smoked marijuana, or the degree to which patients were able to swallow the pills. Patients with severe vomiting would have been unlikely to be able to swallow or keep the pills down long enough for them to take effect. The onset of drug effect is much faster with inhaled or injected THC than it is for oral delivery.87,112,141 Although many marijuana users have claimed that smoked marijuana is a more effective antiemetic than oral THC, no controlled studies have yet been published that analyze this in sufficient detail to estimate the extent to which this is the case.
Side Effects Associated with THC and Marijuana in Antiemetic Therapy
Frequent side effects associated with THC or marijuana are dizziness, dry mouth, hypotension, moderate sedation, and euphoria or dysphoria.18,19,50,107,143,160,176,185 To patients, dry mouth and sedation are the least troubling side effects. Perhaps the most troubling side effects are orthostatic hypotension and dizziness, which could increase the patient's distress.
There is disagreement as to whether the psychoactive effects of THC correlate with its antiemetic activity. In the prospective double-blind trial comparing THC with metoclopramide, the authors reported no relationship between the occurrence of complete antiemetic control and euphoria or dysphoria.58 Other investigators believe that the occurrence of euphoria or dysphoria is often associated with improved antiemetic control.160 Nevertheless, there is a consensus among investigators that dysphoric effects are more common among patients who have had no prior experience with cannabinoids. An important and unexpected problem encountered in the New York state open trial with marijuana was the inability of nearly one-fourth of the patients to tolerate the administration of marijuana by smoking.188 The intolerance could have been due to inexperience with smoking marijuana and is an important consideration.
Page 151
Therapy for Chemotherapy-Induced Nausea and Vomiting
Present Therapy
New classes of antiemetics that have emerged over the past 10 years have dramatically reduced the nausea and vomiting associated with cancer chemotherapy and transformed the acceptance of cisplatin by cancer patients. The new antiemetics—including selective serotonin type 3 receptor antagonists, substituted benzamides, corticosteroids, butyrophenones, and phenothiazines—have few side effects when given over a short term and are convenient in various clinical settings.
The most effective commonly used antiemetics are serotonin receptor antagonists (ondansetron and granisetron) with or without corticosteroids.37,56,88,145,155 In a combination trial of dexamethasone (a corticosteroid) and a serotonin antagonist, complete control of acute cisplatin-induced emesis was observed in about 75% of patients. If the chemotherapy was only moderately emetogenic, up to 90% of the patients who received the combination achieved complete control of emesis. Side effects of those antiemetic agents include headache, constipation, and alterations in liver function, but they are generally well tolerated by most patients.13
Other commonly used antiemetics are phenothiazines—prochlorperazine (Compazine) and haloperidol—and metoclopramide. Metoclopramide is somewhat less effective than the serotonin antagonists and has more side effects, including acute dystonic reactions, drowsiness, diarrhea, and depression.13,37 Side effects associated with phenothiazines are severe or acute dystonic reactions, hypotension, blurred vision, drowsiness, dry mouth, urinary retention, allergic reactions, and occasional jaundice.13
The cost of effective antiemetic regimens can vary markedly, depending on the agent, dose, schedule, and route of administration. Overall, oral regimens cost less than intravenous regimens because of lower pharmacy and administration costs, as well as lower acquisition costs in many countries. Regimens with a cost to the pharmacy as low as about $30 to $35 per treatment session have been shown to be effective;57 these costs are for treatment of acute emesis and delayed emesis with generic agents where available.
Although it is generally not well known by the public, major progress in controlling chemotherapy-induced acute nausea and vomiting has been made since the 1970s. Patients receiving the most difficult to control emetic agents now have no more than about a 20-30% likelihood of experiencing acute emesis,155 whereas in the 1970s the likelihood was nearly 100% despite antiemetics.55,86 As has been seen, most antiemetic studies with
Page 152
| BOX 4.1 |
| Attitudes of Oncologists Toward Prescribing Marijuana |
| In the 1990s, two groups of investigators conducted three surveys on the attitudes of clinical oncologists toward prescribing marijuana as an antiemetic. These studies are arguably out of date in that the antiemetics available now are much more effective than those available when the studies were conducted. Nonetheless, the studies merit attention because they are still often cited as evidence for or against the use of marijuana as an antiemetic. |
| The two groups' results were contradictory. in 194, by which time serotonin receptor antagonists (5-HT receptors) had become available, Schwartz and Beveridge171 concluded that oncologists had little interest in prescribing marijuana to control emesis, whereas Doblin and Kleiman39 had concluded in 1991 that interest was great. Since 1994, the two groups have debated in the literature as to which study represents the rue sentiment among oncologists.38,72,177 in fact, numeous mthodological differences between the two studies might explain the different results,38,172 Ultimately, these studies are irrelevant. Both deal with perceptions rather than pharmacological realities based on well-designed outcome studies.177 |
cannabinoids had methodological difficulties and are inconclusive. The evidence from the well-conducted trials indicate that cannabinoids reduce emesis in about one-fourth of patients receiving cancer chemotherapy. Cannabinoids are not as effective as several other classes of agents, such as substituted benzamides, serotonin receptor antagonists, and corticosteroids. The side effects associated with cannabinoid use are generally tolerable. Like cannabinoids, smoked marijuana, was apparently effective, but the efficacy was no greater than that of available antiemetic agents now considered to be marginally satisfactory. At present, the most effective antiemetic regimens are combinations of oral serotonin receptor antagonists with dexamethasone in single-dose regimens given before chemotherapy. Neither multiple-dose regimens nor intravenous antiemetics provide better control, and both add unnecessary costs.59,81
Future Therapy
Advances in therapy for chemotherapy-induced nausea and vomiting will require discovery of agents that work through mechanisms dif-
Page 153
ferent from those of existing antiemetics, including the serotonin antagonists. Among the proposed new pathways, neurokinin-1 (NK-1) receptor antagonists appear to be the most promising. Neurokinin receptors are found in brain and intestine and are thought to be involved in motor activity, mood, pain and reinforcement. They might well be involved in mediating intestinal sensations, including nausea. In animal models, agents that block the NK-1 receptor prevent cisplatin-induced emesis. At the time of this writing, clinical trials with NK-1 receptor antagonists were under way (phase II or small phase III comparison studies). Preliminary results indicated that these agents have useful activity in both acute and delayed chemotherapy-induced emesis (that is, beginning or persisting 24 or more hours after chemotherapy) and are safe to administer orally.102,135
It is theoretically possible, considering that the mechanism of cannabinoid action appears to differ from that of the serotonin receptor antagonists and of corticosteroids, that THC added to more effective regimens might enhance control of emesis. Such combinations should aim to be as convenient as possible and have few additional side effects. The critical issue is not whether marijuana or cannabinoid drugs might be superior to the new drugs, but whether some group of patients might obtain added or better relief from marijuana or cannabinoid drugs.
Even with the best antiemetic drugs, the control of nausea and vomiting that begins or persists 24 hours after chemotherapy remains imperfect. The pathophysiology of delayed emesis appears different from that of acute emesis, and it is more likely to occur with a strong emetic agent, but it varies from patient to patient. Treatment to prevent this emesis requires dosing both before and after chemotherapy.103
Conclusions: Chemotherapy-Induced Nausea
Most chemotherapy patients are unlikely to want to use marijuana or THC as an antiemetic. In 1999, there are more effective antiemetic agents available than were available earlier. By comparison, cannabinoids are only modest antiemetics. However, because modern antiemetics probably act through different mechanisms, cannabinoids might be effective in people who respond poorly to currently used antiemetic drugs, or cannabinoids might be more effective in combination with a new drug than is either alone. For both reasons, studies of the effects of adjunctive cannabinoids on chemotherapy-induced emesis are worth pursuing for patients whose emesis is not optimally controlled with other agents.
While some people who spoke to the IOM study team described the mood-enhancing and anxiety-reducing effects of marijuana as a positive contribution to the antiemetic effects of marijuana, one-fourth of the
Page 154
patients in the New York state study described earlier were unable to tolerate smoked marijuana. Overall, the effects of oral THC and smoked marijuana are similar, but there are differences. For example, in the residential studies of experienced marijuana users by Haney and co-workers, subjects reported that marijuana made them feel ''mellow,''71 whereas comparable doses of oral THC did not.70 Such differences might be due to the different routes of delivery of THC, as well as the different mixture of cannabinoids found in the marijuana plant. As of this writing, no studies had been published that weighed the relative contributions of those different factors.
The goal of antiemetic medications is to prevent nausea and vomiting. Hence, antiemetics are typically given before chemotherapy, in which case a pill is an effective from of drug delivery. However, in patients already experiencing severe nausea or vomiting, pills are generally ineffective because of the difficulty in swallowing or keeping a pill down and slow onset of the drug effect. Thus, an inhalation (but preferably not smoking) cannabinoid drug delivery system would be advantageous for treating chemotherapy-induced nausea.
Until the development of rapid-onset antiemetic drug delivery systems, there will likely remain a subpopulation of patients for whom standard antiemetic therapy is ineffective and who suffer from debilitating emesis. It is possible that the harmful effects of smoking marijuana for a limited period of time might be outweighed by the antiemetic benefits of marijuana, at least for patients for whom standard antiemetic therapy is ineffective and who suffer from debilitating emesis. Such patients should be evaluated on a case-by-case basis and treated under close medical supervision.
Wasting Syndrome and Appetite Stimulation
Wasting syndrome in acquired immune deficiency syndrome (AIDS) patients is defined by the Centers for Disease Control and Prevention as the involuntary loss of more than 10% of baseline average body weight in the presence of diarrhea or fever of more than 30 days that is not attributable to other disease processes.17 Anorexia (loss of appetite) can accelerate wasting by limiting the intake of nutrients. Wasting (cachexia) and anorexia are common end-stage features of some fatal diseases, such as AIDS, and of some types of metastatic cancers. In AIDS, weight loss of as little as 5% is associated with decreased survival, and a body weight about one-third below ideal body weight results in death.99,158
There are two forms of malnutrition: starvation and cachexia. Starvation, the deprivation of essential nutrients, results from famine or poverty, malabsorption, eating disorders such as anorexia nervosa, and so on. Star-
Page 155
vation leads to metabolic adaptations that deplete body fat before losses of lean tissue. Cachexia results from tissue injury, infection, or tumor and is characterized by a disproportionate loss of lean body mass, such as skeletal muscle. The effects of starvation regardless of the cause can usually be reversed by providing food, whereas the effects of cachexia can be reversed only through control of the underlying disease and—at least for some patients—drugs that stimulate metabolism, such as growth hormone or androgenic-anabolic hormones.
Malnutrition in HIV-Infected Patients
By 1997 more than 30 million people worldwide were infected with human immunodeficiency virus (HIV), and the number is predicted to increase to almost 40 million by the year 2000.126,186 Malnutrition is common among AIDS patients and plays an independent and important role in their prognosis.95,100,158 Because treatment for malnutrition depends on whether it is caused by starvation or cachexia, one needs to know the effects of HIV infection on metabolic processes. The answer depends on the clinical situation and can be either or both.94
The development of malnutrition in HIV infection has many facets. Malnutrition in HIV-infected patients results in a disproportionate depletion of body cell mass,* total body nitrogen, and skeletal muscle mass; all are consistent with cachexia.97,194 Body composition studies show that the depletion of body cell mass precedes the progression to AIDS (falling CD4 lymphocyte counts); this suggests that malnutrition is a consequence of the inflammatory response to the underlying viral infection, rather than a general complication of AIDS.144 In contrast, weight loss is often episodic and related to acute complications, such as febrile opportunistic infections.113 Mechanisms underlying wasting in HIV-infected patients depend on the stage of HIV infection and on specific associated complications.
The many reasons for decreased food intake among AIDS patients include mouth, throat, or esophageal infections or ulcers (oropharyngeal and esophageal pathology); adverse effects of medications;196 diarrhea; enteric infection; malabsorption; serious systemic infection; focal or diffuse neurological disease; HIV enteropathy; depression; fatigue; and poverty. Nutrient malabsorption is often the result of microorganism overgrowth or infection in the intestine, especially in the later stages of AIDS.95,157
*Body cell mass is the fat-free cellular mass. It is composed of the cells of the muscle and organs, plus circulating hematopoietic cells and the aqueous compartment of adipocytes. It is not fat, extracellular water, or extracellular solids (such as tendons).
Page 156
Marijuana and THC for Malnutrition in HIV-Infected Patients
Despite their frequency of use, little has been published about the effectiveness of marijuana or cannabinoids for the treatment of malnutrition and wasting syndrome in HIV-infected patients. The only cannabinoid evaluated in controlled clinical studies is THC, or dronabinol. Short-term (six-week) and long-term (one-year) therapy with dronabinol was associated with an increase in appetite and stable weight, and in a previous short-term (five-week) clinical trial in five patients, dronabinol was shown to increase body fat by 1%.8,9,179 In 1992, the FDA approved THC, under the trade name Marinol (dronabinol), as an appetite stimulant for the treatment of AIDS-related weight loss. Megestrol acetate (Megace) is a synthetic derivative of progesterone that can stimulate appetite and cause substantial weight gain when given in high doses (320640 mg/day) to AIDS patients. Megestrol acetate is more effective than dronabinol in stimulating weight gain, and dronabinol has no additive effect when used in combination with megestrol acetate.183 HIV/AIDS patients are the largest group of patients who use dronabinol. However, some reject it because of the intensity of neuropsychological effects, an inability to titrate the oral dose easily, and the delayed onset and prolonged duration of its action.3 There is evidence that cannabinoids modulate the immune system (see chapter 2, "Cannabinoids and the Immune System"), and this could be a problem in immunologically compromised patients. No published studies have formally evaluated use of any of the other cannabinoids for appetite stimulation in wasting.
Anecdotes abound that smoked marijuana is useful for the treatment of HIV-associated anorexia and weight loss.23,62 Some people report a preference for smoked marijuana over oral THC because it gives them the ability to titrate the effects, which depend on how much they inhale. In controlled laboratory studies of healthy adults, smoked marijuana was shown to increase body weight, appetite, and food intake.47,119 Unfortunately, there have been no controlled studies of the effect of smoked marijuana on appetite, weight gain, and body composition in AIDS patients. At the time of this writing, Donald Abrams, of the University of California, San Francisco, was conducting the first clinical trial to test the safety of smoked marijuana in AIDS patients, and the results were not yet available.
A major concern with marijuana smoking in HIV-infected patients is that they might be more vulnerable than other marijuana users to immunosuppressive effects of marijuana or to the exposure of infectious organisms associated marijuana plant material (see chapter 3, "Marijuana Smoke").
Page 157
Therapy for Wasting Syndrome in HIV-Infected Patients
Present Therapy
Generally, therapy for wasting in HIV-infected people focuses on appetite stimulation. Few therapies have proved successful in treatment of the AIDS wasting syndrome. The stimulant studied most is megestrol acetate, which has been shown to increase food intake by about 30% over baseline for reasons that remain unknown. Its effect in producing substantial weight gain is dose dependent, but most of the weight gained is in fat tissue, not lean body mass. Although the findings are still preliminary, anabolic compounds, such as testosterone or growth hormone, might be useful in preventing the loss of or in restoring lean body mass in AIDS patients.10,44,64,170 Enteral and parenteral nutrition have also been evaluated and shown to increase weight, but again the increase is due more to body fat than to lean body mass.96,98
Encouraging advances in the antiviral treatment of HIV infection and developments in the prophylaxis of and therapy for opportunistic infections have recently changed the outlook for the long-term health of HIVinfected people. Death rates have been halved, and the frequency of serious complications, including malnutrition, has fallen markedly.94,133
Future Therapy
The primary focus of future therapies for wasting in HIV-infected patients is to increase lean body mass as well as appetite. Active systemic infections are associated with profound anorexia, which is believed to be mediated by cytokines that stimulate inflammation through their actions in and outside the brain.132 Cytokine inhibitors, such as thalidomide, have been under investigation as potential treatments to increase lean body mass and reduce malnutrition. Even though cannabinoids do not appear to restore lean body mass, they might be useful as adjunctive therapy. For example, cannabinoids could be used as appetite stimulants, in patients with diminished appetite who are undergoing resistance exercises or anabolic therapy to increase lean body mass. They could also be beneficial for a variety of effects, such as increased appetite, while reducing the nausea and vomiting caused by protease inhibitors and the pain and anxiety associated with AIDS.
Considering current knowledge about malnutrition in HIV infection, cannabinoids, by themselves, will probably not constitute primary therapy for this condition but might be useful in combination with other therapies, such as anabolic agents. Specifically, the proposed mechanism of action of increasing food intake would most likely be ineffective in pro-
Page 158
moting an increase in skeletal muscle mass and functional capacity—the goal in the treatment of cachexia in AIDS patients.
Malnutrition in Cancer Patients
Malnutrition compromises the quality of life of many cancer patients and contributes to the progression of their disease. About 30% of Americans will develop cancer in their lifetimes, and two-thirds of those who get cancer will die as a result of it.5 Depending on the type of cancer, 5080% of patients will develop cachexia and up to 50% of them will die, in part, as a result of cachexia.11,40 The cachexia appears to result from the tumor itself, and cytokines (proteins secreted by the host during an immune response to tumor) are probably important factors in this development. Cachexia does not occur in all cancer patients, but generally occurs in the late stages of advanced cancer of the pancreas, lung, and prostate.
The only cannabinoid evaluated for treating cachexia in cancer patients is dronabinol, which has been shown to improve appetite and promote weight gain.54 Present treatments for cancer cachexia are similar to that for cachexia in AIDS patients. These treatments are usually indicated in late stages of advanced disease and include megestrol acetate and enteral and parenteral nutrition. Megestrol acetate stimulates appetite and promotes weight gain in cancer patients, although the gain is mostly in fat mass (reviewed by Bruera 199814). Both megestrol acetate and dronabinol have dose-related side effects that can be troublesome for patients: megestrol acetate can cause hyperglycemia and hypertension, and dronabinol can cause dizziness and lethargy. Cannabinoids have also been shown to modulate the immune system (see chapter 2, "Cannabinoids and the Immune System"), and this could be contraindicated in some cancer patients (both the chemotherapy and the cancer can be immunosuppressive).
Future treatments will probably depend on the development of methods that block cytokine actions and the use of selective b2-adrenergic receptor agonists to increase muscle mass.14,73 Treatments for cancer cachexia will also most likely need to identify individual patients' needs. Some patients might need only a cytokine inhibitor, whereas others could benefit from combined approaches, such as an appetite stimulant and b2-adrenergic receptor agonists. In this respect, such cannabinoids as THC might prove useful as part of a combination therapy as an appetite stimulant, antiemetic, analgesic, and anxiolytic, especially for patients in late stages of the disease.
Page 159
Anorexia Nervosa
Anorexia nervosa, a psychiatric disorder characterized by distorted body image and self-starvation, affects an estimated 0.6% of the U.S. population, with a greater prevalence in females than males.5 Its mortality is high, and response to standard treatments is poor.
THC appears to be ineffective in treating this disease. In one study it caused severe dysphoric reactions in three of 11 patients.65 One possible explanation of the dysphoria is that THC increases appetite and thus intensifies the mental conflict between hunger and food refusal.13 Furthermore, such patients might have underlying psychiatric disorders, such as schizophrenia and depression, in which cannabinoids might be hazardous (see chapter 3, "Psychological Harms").
Current treatments include psychological techniques to overcome emotional or behavioral problems and dietary intervention to reverse the malnutrition.195 Pharmacological treatments, such as antidepressants, have been used in addition to psychotherapy but tend to lack the desired level of efficacy.33 Recently, alterations in a gene for one of the serotonin receptors have been identified in some patients with anorexia nervosa.45 The possibility of a genetic component suggests a pathway for the development of new drugs to treat this disease.
Conclusions: Wasting Syndrome and Appetite Stimulation
The profile of cannabinoid drug effects suggests that they are promising for treating wasting syndrome in AIDS patients. Nausea, appetite loss, pain, and anxiety are all afflictions of wasting, and all can be mitigated by marijuana. Although some medications are more effective than marijuana for these problems, they are not equally effective in all patients. A rapid-onset (that is, acting within minutes) delivery system should be developed and tested in such patients. Smoking marijuana is not recommended. The long-term harm caused by smoking marijuana makes it a poor drug delivery system, particularly for patients with chronic illnesses.
Terminal cancer patients pose different issues. For those patients the medical harm associated with smoking is of little consequence. For terminal patients suffering debilitating pain or nausea and for whom all indicated medications have failed to provide relief, the medical benefits of smoked marijuana might outweigh the harm.
Neurological Disorders
Neurological disorders affect the brain, spinal cord, or peripheral nerves and muscles in the body. Marijuana has been proposed most often
Page 160
as a source of relief for three general types of neurological disorders: muscle spasticity, particularly in multiple sclerosis patients and spinal cord injury victims; movement disorders, such as Parkinson's disease, Huntington's disease, and Tourette's syndrome; and epilepsy. Marijuana is not proposed as a cure for such disorders, but it might relieve some associated symptoms.
Muscle Spasticity
Spasticity is the increased resistance to passive stretch of muscles and increased deep tendon reflexes. Muscles may also contract involuntarily (flexor and extensor spasms). In some cases these contractions are debilitating and painful and require therapy to relieve the spasms and associated pain.
There are numerous anecdotal reports that marijuana can relieve the spasticity associated with multiple sclerosis or spinal cord injury, and animal studies have shown that cannabinoids affect motor areas in the brain—areas that might influence spasticity.51,78,130,168
Multiple Sclerosis
Multiple sclerosis (MS) is a condition in which multiple areas of the central nervous system (CNS) are affected. Many nerve fibers become demyelinated, some are destroyed, and scars (sclerosis) form, resulting in plaques scattered throughout the white matter of the CNS. (Myelin is the lipid covering that surrounds nerve cell fibers and facilitates the conduction of signals along nerve cells and ultimately between the brain, the spinal cord, and the rest of the body.) MS exacerbations appear to be caused by abnormal immune activity that causes inflammation and myelin destruction in the brain (primarily in the periventricular area), brain stem, or spinal cord. Demyelination slows or blocks transmission of nerve impulses and results in an array of symptoms such as fatigue, depression, spasticity, ataxia (inability to control voluntary muscular movements), vertigo, blindness, and incontinence. About 90% of MS patients eventually develop spasticity. There are an estimated 2.5 million MS patients worldwide, and spasticity is a major concern of many patients and physicians.134 Spasticity is variably experienced as muscle stiffness, muscle spasms, flexor spasms or cramps, muscle pain or ache. The tendency for the legs to spasm at night (flexor spasms) can interfere with sleep.
Marijuana is often reported to reduce the muscle spasticity associated with MS.62,123 In a mail survey of 112 MS patients who regularly use marijuana, patients reported that spasticity was improved and the associated pain and clonus decreased.287 However, a double-blind placebo-controlled
Page 161
study of postural responses in 10 MS patients and 10 healthy volunteers indicated that marijuana smoking impaired posture and balance in both MS patients and the volunteers.61 Nevertheless, the 10 MS patients felt that they were clinically improved. The subjective improvement, while intriguing, does not constitute unequivocal evidence that marijuana relieves spasticity. Survey data do not measure the degree of placebo effect, estimated to be as great as 30 percent in pain treatments.122,131 Furthermore, surveys do not separate the effects of marijuana or cannabinoids on mood and anxiety from the effects on spasticity.
The effects of THC on spasticity were evaluated in a series of three clinical trials testing a total of 30 patients.24,148,187 They were "open trials," meaning that the patients were informed before treatment that they would be receiving THC. Based on patient report or clinical exam by the investigator, spasticity was less severe after the THC treatment. However, THC was not effective in all patients and frequently caused unpleasant side effects. Spasticity was also reported to be less severe in a single case study after nabilone treatment (Figure 4.2).117
In general, the abundant anecdotal reports are not well supported by the clinical data summarized in Table 4.1. But this is due more to the limitation of the studies than to negative results. There are no supporting animal data to encourage clinical research in this area, but there also are no good animal models of the spasticity of MS. Without an appropriate model, studies to determine the physiological basis for how marijuana or THC might relieve spasticity cannot be conducted. Nonetheless, the survey results suggest that it would be useful to investigate the potential therapeutic value of cannabinoids in relieving symptoms associated with MS. Such research would require the use of objective measures of spasticity, such as the pendulum test.* Since THC is mildly sedating, it is also important to distinguish this effect from antispasticity effects in any such investigations. Mild sedatives, such as Benadryl or benzodiazepines, would be useful controls for studies on the ability of cannabinoids to relieve muscle spasticity. The regular use of smoked marijuana, however, would be contraindicated in a chronic condition like MS.
Spinal Cord Injury
In 1990, there were about 15 million patients worldwide with spinal cord injury, and an estimated 10,000 new cases are reported each year in
*The pendulum test is an objective and accurate measure of MS-induced spasticity. It is done by videotaping a patient who lies supine on a table with his or her leg extending off the edge. The leg is dropped and the resulting motion is mathematically analyzed by computer to provide a quantitative measure of spasticity.
Page 162
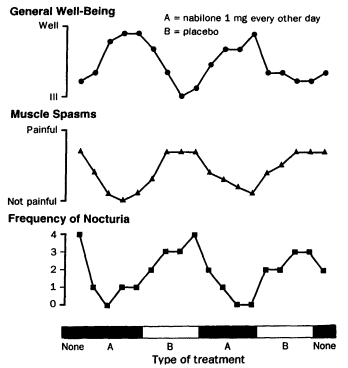
Figure 4.2
Effect of nabilone on multiple sclerosis symptoms. This figure shows the results of a trial in which a
45-year-old man with MS was given fourweek treatments alternately with placebo and nabilone.
The patient served as both experimental subject and control; his treatment sequence was nabilone-
placebonabilone-placebo. That pattern of alternating treatments reduces the possibility
that the observed changes are unrelated to the drug and are not simply due to other factors that
changed with time. The results of the trial are consistent with the possibility that THC might relieve spasticity, but
although more rigorous than many self-report studies of psychoactive substances, it has problems.
First, the patient could not distinguish the treatments at the time of taking them, but after the
nabilone treatment he felt sedated. Thus, it is not possible to know how much the expectation of
relief contributed to his perception of relief. Second, the study measured his perception of
pain, in which spasticity is an important factor but not the only factor. It is not possible
to know the extent to which the perception of pain was affected by nabilone and how much by the
stimulus that generated the pain—in this case, involuntary muscle contractions. Because it is unaffected by
conscious control, the frequency of nocturia is clearer evidence of the effect of THC, although it might also
represent how well the patient slept. This trial with a single person is intriguing but not definitive proof
that THC can reliably relieve spasticity. SOURCE: Martyn et al. (1995).117 Reprinted with permission.
Page 163
| TABLE 4.1 Studies on the Effects of Marijuana and Cannabinoids in Multiple Sclerosis | |||
| Drug and | Study Design | Results | Reference |
| Marijuana | Mail survey 112/233 MS patients | Survey was mailed to 233 MS | Consroe and |
| Marijuana | Clinical trial | Reduction in spasticity and | Meinck and |
| Marijuana | Double-blind, | MS patients felt they were | Greenberg |
| Oral THC | Open trial | 5 patients experienced subjective | Clifford |
| Oral THC | Double-blind, placebo controlled 9 MS patients | Spasticity was improved based | Petro and |
| Ellenberger | |||
| (1981)148 | |||
| Oral THC | Double-blind, | Patients reported subjective | Ungerleider |
| Nabilone | Placebo-controlled | The patient reported increased | Martyn and |
the United States alone.134,138 About 60% of spinal cord injuries occur in people younger than 35 years old. Most will need long-term care and some lifelong care.116
Many spinal cord injury patients report that marijuana reduces their muscle spasms.114 Twenty-two of 43 respondents to a 1982 survey of
Page 164
people with spinal cord injuries reported that marijuana reduced their spasticity.14 One double-blind study of a paraplegic patient with painful spasms in both legs suggested that oral THC was superior to codeine in reducing muscle spasms.72,120 Victims of spinal cord injury reporting at IOM workshops noted that smoking marijuana reduces their muscle spasms, their nausea, and the frequency of their sleepless nights. The caveats described for surveys of spasticity relief in MS patients also apply here.
Therapy for Muscle Spasticity
Present Therapy. Present therapy for spasticity includes the various medications listed in Table 4.2. Baclofen and tizanidine, the most commonly prescribed antispasticity drugs, relieve spasticity and spasms with various degrees of success. The benefit of these agents is generally only partial. Their use is complicated by the side effects of drowsiness, dry mouth, and increased weakness.
Future Therapy. The discovery of agents that work through mechanisms different from those of existing antispasticity drugs will be an important advance in the treatment of spasticity. The aim of new treatments will be to relieve muscle spasticity and pain without substantially increasing muscle weakness in conditions that result in spasticity. The treatment for MS itself will likely be directed at immunomodulation. Various immunomodulating agents, such as beta-interferon and glatiramer acetate, have been shown to reduce the frequency of symptomatic attacks, the progression of disability, and the rate of appearance of demyelinated lesions as detected by magnetic resonance imaging.5
Conclusion: Muscle Spasticity
Basic animal studies described in chapter 2 have shown that cannabinoid receptors are particularly abundant in areas of the brain that control
| TABLE 4.2 Classes of Antispasticity Drugs | |
| Drug Class | Drug |
| GABAB-receptor agonists | Baclofen |
| a-Receptor agonists | Tizanidine |
| Noncompetitive GABAA-receptor agonists | Benzodiazepines, including diazepam |
| Calcium blockers in skeletal muscle | Dantrolene |
Page 165
movement and that cannabinoids affect movement and posture in animals as well as humans. The observations are consistent with the possibility that cannabinoids have antispastic effects, but they do not offer any direct evidence that cannabinoids affect spasticity, even in animals. The available clinical data are too meager to either accept or dismiss the suggestion that marijuana or cannabinoids relieve muscle spasticity. But the few positive reports of the ability of THC and related compounds to reduce spasticity, together with the prevalence of anecdotal reports of the relief provided by marijuana, suggest that carefully designed clinical trials testing the effects of cannabinoids on muscle spasticity should be considered (see chapter 1).25,62 Such trials should be designed to assess the degree to which the anxiolytic effects of cannabinoids contribute to any observed antispastic effects.
Spasticity occurring at night can be very disruptive to sleep. Thus, a long—lasting medication would be especially useful for MS patients at bedtime—when drowsiness would be a beneficial rather than an unwanted side effect and mood—altering effects would be less of a problem. One caution is related to the effects of THC on the stages of sleep, which should be evaluated in MS patients who have sleep disturbances. If THC is proven to relieve spasticity, a pill might be the preferred route of delivery for nighttime use because of its long duration of action. Compared to the currently available therapies, the long half-life of THC might allow for a smoother drug effect throughout the day. The intensity of the symptoms resulting from spasticity, particularly in MS, can rapidly increase in an unpredictable fashion such that the patient develops an ''attack'' of intense muscle spasms lasting minutes to hours. An inhaled form of THC (if it were shown to be efficacious) might be appropriate for those patients.
Movement Disorders
Movement disorders are a group of neurological conditions caused by abnormalities in the basal ganglia and their subcortical connections through the thalamus with cortical motor areas. The brain dysfunctions ultimately result in abnormal skeletal muscle movements in the face, limbs, and trunk. The movement disorders most often considered for marijuana or cannabinoid therapy are dystonia, Huntington's disease, Parkinson's disease, and Tourette's syndrome. Movement disorders are often transiently exacerbated by stress and activity and improved by factors that reduce stress. This is of particular interest because for many people marijuana reduces anxiety.
Page 166
Dystonia
Dystonia can be a sign of other basal ganglion disorders, such as Huntington's disease and tardive dyskinesia (irreversible development of involuntary dyskinetic movements) and can be a primary basal ganglion disorder. Primary dystonias are a heterogeneous group of chronic slowly progressive neurological disorders characterized by dystonic movements—slow sustained involuntary muscle contractions that often result in abnormal postures of limbs, trunk, and neck. Dystonias can be confined to one part of the body, such as spasmodic torticollis (neck) or Meige's syndrome (facial muscles), or can affect many parts of the body, such as dystonia musculorum deformans.5 Dystonia can cause mild to severe disability and sometimes pain secondary to muscle aching or arthritis. Some dystonias are genetic; others are caused by drugs. The specific neuropathological changes in these diseases have not been determined.
No controlled study of marijuana in dystonic patients has been published, and the only study of cannabinoids was a preliminary open trial of cannabidiol (CBD) that suggested modest dose-related improvements in the five dystonic patients studied.30 In mutant dystonic hamsters, however, the cannabinoid receptor agonist, WIN 55,212-2, can produce antidystonic effects.153
Huntington's Disease
Huntington's disease is an inherited degenerative disease that usually appears in middle age and results in atrophy or loss of neurons in the caudate nucleus, putamen, and cerebral cortex. It is characterized by arrhythmic, rapid muscular contractions (chorea), emotional disturbance, and dementia (impairment in intellectual and social ability). Animal studies suggest that cannabinoids have antichoreic activity, presumably because of stimulation of CB1 receptors in the basal ganglia.129,168
On the basis of positive results in one of four Huntington's disease patients, CBD and a placebo were tested in a double-blind crossover study of 15 Huntington's disease patients who were not taking any antipsychotic drugs. Their symptoms neither improved nor worsened with CBD treatment.27,164
The effects of other cannabinoids on patients with Huntington's disease are largely unknown. THC and other CB1 agonists are more likely candidates than CBD, which does not bind to the CB1 receptor. Those receptors are densely distributed on the very neurons that perish in Huntington's disease.152 Thus far there is little evidence to encourage clinical studies of cannabinoids in Huntington's disease.
Page 167
Parkinson's Disease
Parkinson's disease, a degenerative disease, affects about 1 million Americans over the age of 50.5 It is characterized by bradykinesia (slowness in movement), akinesia (abrupt stoppage of movement), resting tremor, muscular rigidity, and postural instability.
Theoretically, cannabinoids could be useful for treating Parkinson's disease patients because cannabinoid agonists specifically inhibit the pathways between the subthalamic nucleus and substantia nigra and probably also the pathways between the subthalamic nucleus and globus pallidus (these structures shown in Figure 2.6).165,169 The latter effect was not directly tested but is consistent with what is known about these neural pathways. Hyperactivity of the subthalamic neurons, observed in both Parkinson's patients and animal models of Parkinson's disease, is hypothesized to be a major factor in the debilitating bradykinesia associated with the disease.36 Furthermore, although cannabinoids oppose the actions of dopamine in intact rats, they augment dopamine activation of movement in an animal model of Parkinson's disease. This suggests the potential for adjunctive therapy with cannabinoid agonists.165-167,169
At the time of this writing, we could find only one published clinical trial of marijuana involving five cases of idiopathic Parkinson's disease.48 That trial was prompted by a patient's report that smoking marijuana reduced tremor, but the investigators found no improvement in tremor after the five patients smoked marijuana—whereas all subjects benefited from the administration of standard medications for Parkinson's disease (levodopa and apomorphine).48 Although new animal data might someday indicate a use for cannabinoids in treating Parkinson's disease, current data do not recommend clinical trials of cannabinoids in patients with Parkinson's disease.
Tourette's Syndrome
Tourette's syndrome usually begins in childhood and is characterized by motor and vocal tics (involuntary rapid repetitive movements or vocalizations). It has been suggested that the symptoms might be mediated by a reduction in the activity of limbic-basal ganglia-thalamocortical circuits (shown in Figure 2.4).42 These circuits, while not well understood, appear to be responsible for translating a person's intentions to move into actual movements. Damage to these structures leads to either involuntary increases in movement (as in Huntington's disease) or the inability to make voluntary movements (as in Parkinson's disease). The nature of the deficit in Tourette's syndrome is unknown.
No clear link has been established between symptoms of Tourette's
Page 168
syndrome and cannabinoid sites or mechanism of action. Pimozide and haloperidol, two widely used treatments for Tourette's syndrome, inhibit effects mediated by the neurotransmitter dopamine, whereas cannabinoids can increase dopamine release.154 ,181 The physiological relevance, if any, of these two observations has not been established.
Clinical reports consist of four case histories indicating that marijuana use can reduce tics in Tourette's patients.75,163 In three of the four cases the investigators suggest that beneficial effects of marijuana might have been due to anxiety-reducing properties of marijuana rather than to a specific antitic effect.163
Therapy for Movement Disorders
Various drugs are available (Table 4.3) to treat the different movement disorders. Common side effects of many of these drugs are sedation, lethargy, school and work avoidance, social phobia, and increased risk of parkinsonism and tardive dyskinesia. With some of the medications, like those used for dystonia, efficacy is lacking in up to 50% of the patients. In addition to medications, surgical interventions, such as pallidotomy and neurosurgical transplantation of embryonic substantia nigra tissue into the patient's striatum, have been tried in Parkinson's disease patients. Surgery is generally palliative and is still considered to be in the developmental phase.
| TABLE 4.3 Drugs Used to Treat Movement Disorders | |
| Dystonia | Parkinson's disease |
| Benzodiazepines | Levodopa |
| Tetrabenazine | Carbidopa+levodopa combination |
| Intramuscular botulinum toxin | Amantadine |
| Anticholinergics | Bromocriptine |
| Baclofen | Pergolide |
| Pramipexole | |
| Huntington's disease | Ropinirole |
| Reserpine | Selegiline |
| Tetrabenazine | Trihexyphenidyl |
| Haloperidol | Benztropine |
| Tourette's syndrome tics | |
| Pimozide | |
| Clonidine | |
| Haloperidol |
Page 169
Conclusion: Movement Disorders
The abundance of CB1 receptors in basal ganglia and reports of animal studies showing the involvement of cannabinoids in the control of movement suggest that cannabinoids would be useful in treating movement disorders in humans. Marijuana or CB1 receptor agonists might provide symptomatic relief of chorea, dystonia, some aspects of parkinsonism, and tics. However, clinical evidence is largely anecdotal; there have been no well-controlled studies of adequate numbers of patients. Furthermore, nonspecific effects might confound interpretation of results of studies. For example, the anxiolytic effects of cannabinoids might make patients feel that their condition is improved, despite the absence of measurable change in their condition.
Compared to the abundance of anecdotal reports concerning the beneficial effects of marijuana on muscle spasticity, there are relatively few claims that marijuana is useful for treating movement disorders. This might reflect a lack of effect or a lack of individuals with movement disorders who have tried marijuana. In any case, while there are a few isolated reports of individuals with movement disorders who report a benefit from marijuana, there are no published surveys indicating that a substantial percentage of patients with movement disorders find relief from marijuana. Existing studies involve too few patients from which to draw conclusions. The most promising reports involve symptomatic treatment of spasticity. If the reported neuroprotective effects of cannabinoids discussed in chapter 2 prove to be therapeutically useful, this could benefit patients with movement disorders, but without further data such a benefit is highly speculative. Since stress often transiently exacerbates movement disorders, it is reasonable to hypothesize that the anxiolytic effects of marijuana or cannabinoids might be beneficial to some patients with movement disorders. However, chronic marijuana smoking is a health risk that could increase the burden of chronic conditions, such as movement disorders.
Cannabinoids inhibit both major excitatory and inhibitory inputs to the basal ganglia. This suggests that a cannabinoid agonist could produce opposite effects on movement, depending on the type of transmission (excitatory or inhibitory) that is most active at the time of drug administration. This property could be used to design treatments in basal ganglia movement disorders, such as Parkinson's disease where either the excitatory subthalamic input becomes hyperactive or the inhibitory striatal input becomes hypoactive. The dose employed would be a major factor in the therapeutic uses of cannabinoids in movement disorders; low doses should be desirable, while higher doses could be expected to aggravate pathological conditions. Thus, there is a clear reason to recommend pre-
Page 170
clinical studies; that is, animal studies to test the hypothesis that cannabinoids play an important role in movement disorders.
With the possible exception of multiple sclerosis, the evidence to recommend clinical trials of cannabinoids in movement disorders is relatively weak. Ideally, clinical studies would follow animal research that provided stronger evidence than is currently available on the potential therapeutic value of cannabinoids in the treatment of movement disorders. Unfortunately, there are no good animal models for these disorders. Thus, double-blind, placebo-controlled clinical trials of isolated cannabinoids that include controls for relevant side effects should be conducted. Such effects include anxiolytic and sedative effects, which might either mask or contribute to the potential therapeutic effects of cannabinoids.
Epilepsy
Epilepsy is a chronic seizure disorder that affects about 2 million Americans and 30 million people worldwide.156 It is characterized by recurrent sudden attacks of altered consciousness, convulsions, or other motor activity. A seizure is the synchronized excitation of large groups of brain cells. These abnormal electrical events have a wide array of possible causes, including injury to the brain and chemical changes derived from metabolic faults of exposure to toxins.156
Seizures are classified as partial (focal) or generalized. Partial seizures are associated with specific sensory, motor, or psychic aberrations that reflect the function of the part of the cerebral cortex from which the seizures arise. Generalized seizures are usually the result of pathological conditions of brain sites that project to widespread regions of the brain. Such pathology can produce petit mal seizures or major grand mal convulsions.
Cannabinoids in Epilepsy
There are anecdotal and individual case reports that marijuana controls seizures in epileptics (reviewed in a 1997 British Medical Association report13), but there is no solid evidence. While there are no studies indicating that either marijuana or THC worsen seizures, there is no scientific basis to justify such studies.
In the only known case-controlled study that was designed to evaluate illicit drug use and the risk of first seizure, Ng and co-workers137 concluded that marijuana is a protective factor for first-time seizures in men but not women. Men who used marijuana reportedly had fewer first-time seizures than men who did not use marijuana. That report was based on a comparison of 308 patients who had been admitted to a hospital after
Page 171
their first seizure with a control group of 294 patients. The control group was made up of patients who had not had seizures and were admitted for emergency surgery, such as surgery for appendicitis, intestinal obstruction, or acute cholecystitis. Compared to men who did not use marijuana, the odds ratio of first seizure for men who had used marijuana within 90 days of hospital admission was 0.36 (95% confidence interval = 0.18-0.74). An odds ratio of less than one is consistent with the suggestion that marijuana users are less likely to have seizures. The results for women were not statistically significant. However, this was a weak study. It did not include measures of health status prior to hospital admissions for the patients' serious conditions, and differences in their health status might have influenced their drug use rather than—as suggested by the authors—that differences in their drug use influenced their health.
The potential antiepileptic activity of CBD has been investigated but is not promising. Three controlled trials were conducted in which CBD was given orally to patients who had had generalized grand mal seizures or focal seizures (Table 4.4). Two of these studies were never published, but information about one was published in a letter to the South African Medical Journal, and the other was presented at the 1990 Marijuana International Conference on Cannabis and Cannabinoids.184
| TABLE 4.4 Clinical Trials of Cannabidiol (CBD) in Epileptics | ||
| Study Design | Results | Reference |
| Double-blind placebo-controlled trial | 4 of 8 remained almost free | Cunha and |
| Double-blind placebo-controlled study | CBD had no effect on | Ames4 |
| Double-blind placebo-controlled, | CBD had no effect on | Trembly and |
| Open trial | Seizure frequency was | Trembly and |
Page 172
Even if CBD had antiepileptic properties, these studies were likely too small to demonstrate efficacy. Proving efficacy of anticonvulsants generally requires large numbers of patients followed for months because the frequency of seizures is highly variable and the response to therapy varies depending on seizure type.4,49
Therapy for Epilepsy
Present Therapy.
Standard pharmacotherapy for partial and generalized seizures, listed in Table 4.5, involves a variety of anticonvulsant drugs. These drugs suppress seizures completely in approximately 60% of patients who have chronic epilepsy and improve seizures in another 15% of patients. All of the anticonvulsants listed in Table 4.5 have side effects, some of the more common of which are drowsiness, mental slowing, ataxia, tremor, hair loss, increased appetite, headache, insomnia, and rash. Nevertheless, recurrent seizures are physically dangerous and emotionally devastating, and preventing them outweighs many undesirable side effects of anticonvulsant drugs.
Future Therapy.
The goal of epilepsy treatment is to halt the seizures with minimal or no side effects and then to eradicate the cause. Most of the anticonvulsant research on cannabinoids was conducted before 1986. Since then, many new anticonvulsants have been introduced and cannabinoid receptors have been discovered. At present, the only biological evidence of antiepileptic properties of cannabinoids is that CB1 receptors are abundant in the hippocampus and amygdala. Both regions are involved in partial seizures but are better known for their role in functions unrelated to seizures.26 Basic research might reveal stronger links between cannabinoids and seizure activity, but this is not likely to be as fruitful a
| TABLE 4.5 Anticonvulsant Drugs for Various Types of Seizures | |
| Generalized grand mal seizures | Partial (focal) seizures |
| Carbamazepine | Carbamazepine |
| Valproate | Phenytoin |
| Phenytoin | Valproate |
| Phenobarbital | Phenobarbital |
| Clonazepam | |
| Generalized petit mal seizures | Gabapentin |
| Ethosuximide | Lamotrigine |
| Clonazepam | Tiagabine (as adjunct therapy) |
| Valproate | |
| SOURCE: Adapted from Andreoli et al. (1997).5 | |
Page 173
subject of cannabinoid research as others. Given the present state of knowledge, clinical studies of cannabinoids in epileptics are not indicated.
Alzheimer's Disease
Food refusal is a common problem in patients who suffer from Alzheimer's type dementia. The causes of anorexia in demented people are not known but may be a symptom of depression. Antidepressants improve eating in some but not all patients with severe dementia. Eleven Alzheimer's patients were treated for 12 weeks on an alternating schedule of dronabinol and placebo (six weeks of each treatment). The dronabinol treatment resulted in substantial weight gains and declines in disturbed behavior.190 No serious side effects were observed. One patient had a seizure and was removed from the study, but the seizure was not necessarily caused by dronabinol. Recurrent seizures without any precipitating events occur in 20% of patients who have advanced dementia of Alzheimer's type.189 Nevertheless, these results are encouraging enough to recommend further clinical research with cannabinoids.
The patients in the study discussed above were in long-term institutional care, and most were severely demented with impaired memory. Although short-term memory loss is a common side effect of THC in healthy patients, it was not a concern in this study. However, the effect of dronabinol on memory in Alzheimer's patients who are not as severely disturbed as those in the above study would be an important consideration.
Glaucoma
After cataracts, glaucoma is the second-leading cause of blindness in the world; almost 67 million people are expected to be affected worldwide by the year 2000149 (for an excellent review, see Alward, 19982). The most common form of glaucoma, primary open-angle glaucoma (POAG), is a slowly progressive disorder that results in loss of retinal ganglion cells and degeneration of the optic nerve, causing deterioration of the visual fields and ultimately blindness. The mechanisms behind the disease are not understood, but three major risk factors are known: age, race, and high intraocular pressure (IOP). POAG is most prevalent among the elderly, with 1% affected in those over 60 years old and more than 9% in those over 80. In African Americans over 80, there is more than a 10% chance of having the disease, and older African Caribbeans (who are less racially mixed than African Americans) have a 20-25% chance of having the disease. 106
The eye's rigid shape is normally maintained in part by IOP, which is
Page 174
regulated by the circulation of a clear fluid, the aqueous humor,* between the front of the lens and the back of the cornea. Because of impaired outflow of aqueous humor from the anterior chamber of the eye, a high IOP is a risk factor for glaucoma, but the mechanism by which it damages the optic nerve and retinal ganglion cells remains unclear.174 The two leading possibilities are that high IOP interferes with nutrient blood flow to the region of the optic nerve or that it interferes with transport of nutrients, growth factors, and other compounds within the optic nerve axon (P. Kaufman, IOM workshop). If the interference continues, the retinal ganglion cells and optic nerve will permanently atrophy; the result is blindness.68 Because high IOP is the only known major risk factor that can be controlled, most treatments have been designed to reduce it. However, reducing it does not always arrest or slow the progression of visual loss.20,109
Marijuana and Cannabinoids in Glaucoma
Marijuana and THC have been shown to reduce IOP by an average of 24% in people with normal IOP who have visual-field changes. In a number of studies of healthy adults and glaucoma patients, IOP was reduced by an average of 25% after smoking a marijuana cigarette that contained approximately 2% THC—a reduction as good as that observed with most other medications available today.1,16,32,76,77,125,193 Similar responses have been observed when marijuana was eaten or THC was given in pill form (10-40 mg) to healthy adults or glaucoma patients.76,91 But the effect lasts only about three to four hours. Elevated IOP is a chronic condition and must be controlled continuously.
Intravenous administration of D9-THC, D8-THC, or 11-OH-THC to healthy adults substantially decreased IOP, whereas cannabinol, CBD, and b-OH-THC had little effect.31,146 The cause for the reduction in IOP remains unknown, but the effect appears to be independent of the frequently observed drop in arterial systolic blood pressure (Keith Green, Medical College of Georgia, personal communication).
Three synthetic cannabinoids were investigated; BW29Y, BW146Y, and nabilone. They were given orally to patients who had high IOP. BW146Y and nabilone were as effective as ingesting THC or smoking
*The cornea and lens must be optically clear, which means that there cannot be blood circulation in these tissues. The aqueous humor is a clear fluid that functions as alternative circulation across the rear of the cornea and to the lens, providing nutrients and removing waste from these tissues.
Page 175
marijuana but again with a very short duration of action; BW29Y was ineffective. 136,182
Topical treatments with cannabinoids have been ineffective in reducing IOP. When D9-THC was applied topically as eye drops, whether once or four times a day, there was no decrease in IOP.60,90 Suspensions of lipophilic THC tended to be irritating to the eye.
In summary, cannabinoids and marijuana can reduce IOP when administered orally, intravenously, or by inhalation but not when administered topically. Even though a reduction in IOP by standard medications or surgery clearly slows the rate of glaucoma symptom progression, there is no direct evidence of benefits of cannabinoids or marijuana in the natural progression of glaucoma, visual acuity, or optic nerve atrophy.92,115
In addition to lowering IOP, marijuana reduces blood pressure and has many psychological effects. Merritt and co-workers reported hypotension, palpitations, and psychotropic effects in glaucoma patients after inhalation of marijuana.125 Cooler and Gregg31 also reported increased anxiety and tachycardia after intravenous infusion of THC (1.5-3 mg). All those side effects are problematic, particularly for elderly glaucoma patients who have cardiovascular or cerebrovascular disease. The reduction in blood pressure can be substantial and might adversely affect blood flow to the optic nerve. 24 Many people with systemic hypertension have their blood pressure reduced to manageable and acceptable levels through medication, but this does not seem to affect their IOP. In contrast, there is evidence that reduction in blood pressure to considerably below-normal levels influences IOP and ocular blood flow.46,74,142 Hence, in the case of an eye with high IOP or an optic nerve in poor condition and susceptibility to high IOP, reduced blood flow to the optic nerve could compromise a functional retina and be a factor in the progression of glaucoma.
Because it is not known how these compounds work, it is also not known how they might interact with other drugs used to treat glaucoma. If the mechanism involves a final common pathway, the effects of cannabinoids might not be additive and might even interfere with effective drugs.
Therapy for Glaucoma
Present Therapy
Six classes of drugs are used to treat glaucoma; all reduce IOP (Table 4.6).93 In the late 1970s, when early reports of the effects of marijuana on IOP surfaced, only cholinomimetics, epinephrine, and oral carbonic anhydrase inhibitors were available. They are not popular today because of their side effects, such as pupil constriction or dilation, brow ache, tachycardia, and diuresis; all of them have been superseded by the other classes
Page 176
| TABLE 4.6 Classes of Drugs Used to Treat Glaucoma | |
| Cholinergic agonists | a2-Adrenergic agonists |
| Pilocarpine | Aproclonidine |
| Brimonidine | |
| b2-Adrenergic agonists | Carbonic anhydrase inhibitors |
| Epinephrine | Acetazolamide |
| Dipivefrin | Dorzolamide (Trusopt) |
| b2-Adrenergic antagonists | Prostaglandin-F2a analogues |
| Timolol | Latanoprost |
| Betaxolol (Betoptic) | Unoprostone |
of drugs.93 Surgical options are also available today to lower IOP, including laser trabeculoplasty, trabeculectomy/sclerostomy, drainage implantation, and cyclodestruction of fluid-forming tissues.73 Thus, there are now many effective options to slow the progression of glaucoma by reducing IOP.
One important factor in slowing the progression of glaucoma via medications that reduce IOP is patient compliance with dosing regimens. With respect to compliance, the ideal glaucoma drug is one that is applied at most twice a day (P. Kaufman, IOM workshop). If the dose must be repeated every three to four hours, patient compliance becomes a problem; for this reason, marijuana and the cannabinoids studied thus far would not be highly satisfactory treatments for glaucoma. Present therapies, especially combinations of approved topical drugs, can control IOP when administered once or twice a day, at a cost of about $60 per month.
Future Therapy
In all likelihood the next generation of glaucoma therapies will deal with neural protection, neural rescue, neural regeneration, or blood flow, and the optic nerve and neural retina will be treated directly rather than just by lowering IOP (P. Kaufman, IOM workshop). There is some evidence that a synthetic cannabinoid, HU-211, might have neuroprotective effects in vitro; this presents a potential approach that has nothing to do with IOP.197 HU-211 is commonly referred to as a cannabinoid because its chemical structure is similar to THC; however, it does not bind to cannabinoid receptor.
It is known that cannabinoids lower IOP fairly substantially but not how. No one has tested whether the effect is receptor mediated (B. Martin, IOM workshop). To do so, one could test whether a receptor antagonist
Page 177
blocked the effects of THC or other cannabinoids. If the decrease were shown to be receptor mediated, it would be important to know whether it was through CB1, which mediates central nervous system effects, or CB2, which is not involved in CNS effects. If it were CB2, it might be possible to reduce IOP without the CNS side effects. Finally, it is not known whether the endogenous cannabinoid system is a natural regulator of IOP.
Conclusion: Glaucoma
Although glaucoma is one of the most frequently cited medical indications for marijuana, the data do not support this indication. High intraocular pressure (IOP) is a known risk factor for glaucoma and can, indeed, be reduced by cannabinoids and marijuana. However, the effect is too and short lived and requires too high doses, and there are too many side effects to recommend lifelong use in the treatment of glaucoma. The potential harmful effects of chronic marijuana smoking outweigh its modest benefits in the treatment of glaucoma. Clinical studies on the effects of smoked marijuana are unlikely to result in improved treatment for glaucoma.
Future research might reveal a therapeutic effect of isolated cannabinoids. For example, it might be possible to design a cannabinoid drug with longer-lasting effects on IOP and with less psychoactivity than THC.
Summary
Advances in cannabinoid science of the past 16 years have given rise to a wealth of new opportunities for the development of medically useful cannabinoid-based drugs. The accumulated data suggest a variety of indications, particularly for pain relief, antiemesis, and appetite stimulation. For patients such as those with AIDS or who are undergoing chemotherapy, and who suffer simultaneously from severe pain, nausea, and appetite loss, cannabinoid drugs might offer broad-spectrum relief not found in any other single medication. The data are weaker for muscle spasticity but moderately promising. The least promising categories are movement disorders, epilepsy, and glaucoma. Animal data are moderately supportive of a potential for cannabinoids in the treatment of movement disorders and might eventually yield stronger encouragement. The therapeutic effects of cannabinoids are most well established for THC, which is the primary psychoactive ingredient of marijuana. But it does not follow from this that smoking marijuana is good medicine.
Although marijuana smoke delivers THC and other cannabinoids to the body, it also delivers harmful substances, including most of those found in tobacco smoke. In addition, plants contain a variable mixture of
Page 178
biologically active compounds and cannot be expected to provide a precisely defined drug effect. For those reasons there is little future in smoked marijuana as a medically approved medication. If there is any future in cannabinoid drugs, it lies with agents of more certain, not less certain, composition. While clinical trials are the route to developing approved medications, they are also valuable for other reasons. For example, the personal medical use of smoked marijuana—regardless of whether or not it is approved—to treat certain symptoms is reason enough to advocate clinical trials to assess the degree to which the symptoms or course of diseases are affected. Trials testing the safety and efficacy of marijuana use are an important component to understanding the course of a disease, particularly diseases such as AIDS for which marijuana use is prevalent. The argument against the future of smoked marijuana for treating any condition is not that there is no reason to predict efficacy but that there is risk. That risk could be overcome by the development of a nonsmoked rapid-onset delivery system for cannabinoid drugs.
There are two caveats to following the traditional path of drug development for cannabinoids. The first is timing. Patients who are currently suffering from debilitating conditions unrelieved by legally available drugs, and who might find relief with smoked marijuana, will find little comfort in a promise of a better drug 10 years from now. In terms of good medicine, marijuana should rarely be recommended unless all reasonable options have been eliminated. But then what? It is conceivable that the medical and scientific opinion might find itself in conflict with drug regulations. This presents a policy issue that must weigh—at least temporarily—the needs of individual patients against broader social issues. Our assessment of the scientific data on the medical value of marijuana and its constituent cannabinoids is but one component of attaining that balance.
The second caveat is a practical one. Although most scientists who study cannabinoids would agree that the scientific pathways to cannabinoid drug development are clearly marked, there is no guarantee that the fruits of scientific research will be made available to the public. Cannabinoid-based drugs will become available only if there is either enough incentive for private enterprise to develop and market such drugs or sustained public investment in cannabinoid drug research and development. The perils along this pathway are discussed in chapter 5. Although marijuana is an abused drug, the logical focus of research on the therapeutic value of cannabinoid-based drugs is the treatment of specific symptoms or diseases, not substance abuse. Thus, the most logical research sponsors would be the several institutes within the National Institutes of Health or organizations whose primary expertise lies in the relevant symptoms or diseases.
Page 179
CONCLUSION: Scientific data indicate the potential therapeutic value of cannabinoid drugs, primarily THC, for pain relief, control of nausea and vomiting, and appetite stimulation; smoked marijuana, however, is a crude THC delivery system that also delivers harmful substances.
RECOMMENDATION: Clinical trials of cannabinoid drugs for symptom management should be conducted with the goal of developing rapid-onset, reliable, and safe delivery systems.
RECOMMENDATION: Clinical trials of marijuana use for medical purposes should be conducted under the following limited circumstances: trials should involve only short-term marijuana use (less than six months), should be conducted in patients with conditions for which there is reasonable expectation of efficacy, should be approved by institutional review boards, and should collect data about efficacy.
RECOMMENDATION: Short-term use of smoked marijuana (less than six months) for patients with debilitating symptoms (such as intractable pain or vomiting) must meet the following conditions:
· failure of all approved medications to provide relief has been documented,
· the symptoms can reasonably be expected to be relieved by rapid-onset cannabinoid drugs,
· such treatment is administered under medical supervision in a manner that allows for assessment of treatment effectiveness, and
· involves an oversight strategy comparable to an institutional review board process that could provide guidance within 24 hours of a submission by a physician to provide marijuana to a patient for a specified use.
Until a nonsmoked rapid-onset cannabinoid drug delivery system becomes available, we acknowledge that there is no clear alternative for people suffering from chronic conditions that might be relieved by smoking marijuana, such as pain or AIDS wasting. One possible approach is to treat patients as n-of-1 clinical trials, in which patients are fully informed of their status as experimental subjects using a harmful drug delivery system and in which their condition is closely monitored and documented under medical supervision, thereby increasing the knowledge base of the risks and benefits of marijuana use under such conditions. We recom-
Page 180
mend these n-of-1 clinical trials using the same oversight mechanism as that proposed in the above recommendations.
Other Reports On Marijuana As Medicine
Since 1996, five important reports pertaining to the medical uses of marijuana have been published, each prepared by deliberative groups of medical and scientific experts (Appendix E). They were written to address different facets of the medical marijuana debate, and each offers a somewhat different perspective. With the exception of the report by the Health Council of the Netherlands, each concluded that marijuana can be moderately effective in treating a variety of symptoms. They also agree that current scientific understanding is rudimentary; indeed, the sentiment most often stated is that more research is needed. And these reports record the same problem with herbal medications as noted here: the uncertain composition of plant material makes for an uncertain, and hence often undesirable, medicine.
The 1996 report by the Health Council of the Netherlands concluded that there is insufficient evidence to justify the medical use of marijuana or THC, despite the fact that the latter is an approved medication in the United States and Britain. However, that committee addressed only whether there was sufficient evidence to warrant the prescription of marijuana or cannabinoids, not whether the data are sufficient to justify clinical trials. Conclusions of the Health Council of the Netherlands contrast with that country's tolerance of marijuana use. The health council's report noted that marijuana use by patients in the terminal stages of illness is tolerated in hospitals. It also said that the council did ''not wish to judge patients who consume marihuana (in whatever form) because it makes them feel better....''
In contrast, the American Medical Association House of Delegates, National Institutes of Health (NIH), and the British Medical Association recommend clinical trials of smoked marijuana for a variety of symptoms. The NIH report, however, was alone in recommending clinical studies of marijuana for the treatment of glaucoma—and even then there was disagreement among the panel members (William T. Beaver, chair, NIH Ad Hoc Expert Panel on the Medical Use of Marijuana, personal communication, 1998).
Recent reviews that have received extensive attention from those who follow the medical marijuana debate have been written by strong advocate for (Grinspoon and Bakalar, 199362; Zimmer and Morgan, 1997198) or against (Voth and Schwartz, 1997191) the medical use of marijuana. Those reports represent the individual views of their authors, and they are not reviewed here but have been reviewed in major scientific jourals.7,69,178,180
Page 181
References
1. Aim A, Camras CB, Watson PG. 1997. Phase III latanoprost studies in Scandanavia, the United Kingdom and the United States. Survey of Ophthalnology 41:S105-S110.
2. Alward WL. 1998. Medical management of glaucoma. Tie New England Journal of Medicine 339:1298-1307.
3. AMA (American Medical Association Council on Scientific Affairs). 1997. Report to the AMA House of Delegates. Chicago: AMA.
4. Ames FR. 1986. Anticonvulsant effect of cannabidiol. South African Medical Journal 69:14.
5. Andreoli TE, Carpenter CC, Bennet CJ, Plum F, eds. 1997. Cecil Essentials of Medicine. Fourth Edition. Philadelphia: W.B. Saunders Co.
6. Andrews PL, Davis CJ. 1995. The physiology of emesis induced by anti-cancer therapy. In: Reynolds DJ, Andrews PL, Davis CJ, Editors, Serotonin and the Scientific Basis of Anti-Emetic Therapy. Oxford: Oxford Clinical Communications. Pp. 25-49.
7. Bayer R, O'Connell TJ, Lapey JD. 1997. Medicinal uses of marijuana (Letter to the Editor). Annals of Internal Medicine 127:1134-1135.
8. Beal JE, Olson RLL, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV. 1995. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. Journal of Pain and Symptom Management 10:89-97.
9. Beal JE, Olson R, Lefkowitz L, Laubenstein L, Bellman P, Yangco B, Morales JO, Murphy R, Powderly W, Plasse TF, Mosdell KW, Shepard KV. 1997. Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. Journal of Pain and Symptom Management 14:7-14.
10. Bhasin S, Storer TW, Asbel-Sethi N, Kilbourne A, Hays R, Sinha-Hikim I, Shen R, Arver S, Beall G. 1998. Effects of testosterone replacement with a nongenital, transdermal system, Androderm, in human immunodeficiency virus-infected men with low testosterone levels. Journal Clinical of Endocrinology and Metabolism 83:31553162.
11. Billingsley KG, Alexander HR. 1996. The pathophysiology of cachexia in advanced cancer and AIDS. In: Bruera E, Higginson I, Editors, Cachexia-Anorexia in Cancer Patients. New York: Oxford Universtiy Press. Pp. 1-22.
12. Borison HL, McCarthy LE. 1983. Neuropharmacology of chemotherapy-induced emesis. Drugs 25:8-17.
13. British Medical Association. 1997. Tllerapeutic Uses of Cannabis. Amsterdam, The Netherlands: Harwood Academic Publishers.
14. Bruera E. 1998. Pharmacological treatment of cachexia: Any progress? Supportive Care of Cancer 6:109-113.
15. Calignano A, La Rana G, Giuffrida A, Piomelli D. 1998. Control of pain initiation by endogenous cannabinoids. Nature 394:277-281.
16. Camras CB, Aim A, Watson P, Stjernschantz J. 1996. Latanoprost, a prostaglandin analog, for glaucoma therapy: Efficacy and safety after 1 year of treatment in 198 patients. Latanoprost Study Groups. Ophthalnology 103:1916-1924.
17. (CDC) Centers for Disease Control. 1992. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR (Morbidity Mortality Weekly Report) 41(RR-17):1-19.
18. Chang AE, Shiling DJ, Stillman RC, et al. 1979. Delta-9-tetrahydrocannabinol as an antiemetic in patients receiving high-dose methotrexate: A prospective, randomized evaluation. Annals of Internal Medicine 91:819-824.
19. Chang AE, Shiling DJ, Stillman RC, Goldberg NH, Seipp CA, Barofsky I, Rosenberg SA. 1981. A prospective evaluation of delta-9-tetrahydrocannabinol as an antiemetic in patients receiving adriamycin and cytoxan chemotherapy. Cancer 47:1746-1751.
Page 182
20. Chauhan BC, Drance SM. 1992. The relationship between intraocular pressure and visual field progression in glaucoma. Graefe's Archives for Clinical and Experinental Ophthalmology 230:521-526.
21. Clark RA, Tyson LB, Frisone M. 1985. A correlation of objective and subjective parameters in assessing antiemetic regimens. Proceedings of the Tenth Anniversary Congress of the Oncology Nursing Society 2:96.
22. Clark WC, Janal MN, Zeidenberg P, Nahas GG. 1981. Effects of moderate and high doses of marihuana on thermal pain: A sensory decision theory analysis. Journal of Clinical Pharmacology 21:299S-310S.
23. Clarke RC. 1995. Marijuana Botany-An Advanced Study: The Propagation and Breeding of Distinctive Cannabis. Berkeley, CA: Ronin Publishing.
24. Clifford DB. 1983. Tetrahydrocannabinol for tremor in multiple sclerosis. Annals of Neurology 13:669-671.
25. Consroe P. 1998a. Clinical and experimental reports of marijuana and cannabinoids in spastic disorders. In: Nahas GG, Sutin KM, Harvey DJ, Agurell S, Editors, Marijuana and Medicine. Totowa, NJ: Humana Press.
26. Consroe P. 1998b. Brain cannabinoid systems as target for the treatment of neurological disorders. Neurobiology of Disease 5:534-551.
27. Consroe P, Laguna J, Allender J, Snider S, Stern L, Sandyk R, Kennedy K, Schram K. 1991. Controlled clinical trial of cannabidiol in Huntington's disease. Pharmacology, Biochemistry and Behavior (New York) 40:701-708.
28. Consroe P, Musty R, Rein J, Tillery W, Pertwee RG. 1997. The perceived effects of smoked cannabis on patients with multiple sclerosis. European Neurology 38:44-48.
29. Consroe P, Sandyk R. 1992. Potential role of cannabinoids for therapy of neurological disorders. In: Bartke A, Murphy LL, Editors, Marijuana/Cannabinoids: Neurobiology and Neurophysiology. Boca Raton, FL: CRC Press. Pp. 459-524.
30. Consroe P, Sandyk R, Snider SR. 1986. Open label evaluation of cannabidiol in dystonic movement disorders. International Journal of Neuroscience 30:277-282.
31. Cooler P, Gregg JM. 1977. Effect of delta-9-tetrahydrocannabinol on intraocular pressure in humans. Southern Medical Journal 70:951-954.
32. Crawford WJ, Merritt JC. 1979. Effects of tetrahydrocannabinol on arterial andintraocular hypertension. International Journal of Clinical Pharmacology and Biopharmacy 17:191-196.
33. Crow S. 1997. Investigational drugs for eating disorders. Expert Opinion ol Investigational Drugs 6:427-436.
34. Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, Sanvito WL, Lander N, Mechoulam R. 1980. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 21:175-185.
35. Davis CJ. 1995. Emesis research: A concise history of the critical concepts and experiments. In: Reynolds DJ, Andrews PL, Davis CJ, Editors, Serotonin and the Scientific Basis of Anti-Emetic Therapy. Oxford : Oxford Clinical Communications. Pp. 9-24.
36. DeLong MR, Georgopoulos AP, Crutcher MD, Mitchell S J, Richardson RT, Alexander GE. 1984. Functional organization of the basal ganglia: Contributions of single-cell recording studies. CIBA Foundation Symposium 107:64-82.
37. DeMulder PH, Seynaeve C, Vermorker JB, et al. 1990. Ondansetron compared with high-dose metoclopramide in prophylaxis of acute and delayed cisplatin-induced nausea and vomiting: A multicenter, randomized, double-blind, crossover study. Annals of Internal Medicine 113:834-840.
38. Doblin R, Kleiman MA. 1995. The medical use of marijuana: The case for clinical trials [editorial; comment]. Journal of Addictive Diseases 14:5-14; Comment in Journal of Addictive Diseases 1994, 13(1):53-65.
Page 183
39. Doblin R, Kleiman M. 1991. Marijuana as antiemetic medicine: A survey of oncologists' experiences and attitudes. Journal of Clinical Oncology 9:1314-1319.
40. Dunlop R. 1996. Clinical epidemiology of cancer cachexia. In: Bruera E, Higginson I, Editors, Cachexia-Anorexia in Cancer Patients. Vol. 5. Oxford: Oxford University Press. Pp. 76-82.
41. Dunn M, Davis R. 1974. The perceived effects of marijuana on spinal cord injured males. Paraplegia 12:175.
42. Eidelberg D, Moeller JR, Antonini A, Kazumata K, Dhawan V, Budman C, Feigin A. 1997. The metabolic anatomy of Tourette's syndrome. Neurology 48:927-934.
43. El-Mallakh RS. 1987. Marijuana and migraine. Headache 27:442-443.
44. Engelson ES, Rabkin JG, Rabkin R, Kotler DP. 1996. Effects of testosterone upon body composition. Journal of Acquired Immune Deficiency Syndrome and Humnan Retrovirology 11:510-511.
45. Enoch M, Kaye WH, Rotondo A, Greenberg BD, Murphy DL, Goldman D. 1998. 5HT2A promoter polymorphism-1438G/A, anorexia nervosa, and obsessive-compulsive disorder. The Lancet 351:1785-1786.
46. Follmann P, Paltotas C, Suveges I, Petrovits A. 1996-1997. Nocturnal blood pressure and intraocular pressure measurement in glaucoma patients and healthy controls. International Ophthalmology 20:83-87.
47. Foltin RW, Fischman MW, Byrne MF. 1988. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite 11:1-14.
48. Frankel JP, Hughes A, Lees AJ, Stern GM. 1990. Marijuana for Parkinsonian tremor. Journal of Neurology, Neurosurgery and Psychiatry 53:436.
49. French J. 1998. The art of antiepileptic trial design. Advances in Neurology 76:113-123.
50. Frytak S, Moertel CG, O'Fallon J, et al. 1979. Delta-9-tetrahydrocannabinol as an antiemetic in patients treated with cancer chemotherapy: A double comparison with prochloperazine and a placebo. Annals of Internal Medicine 91:825-830.
51. Glass M, Dragunow M, Faull RLM. 1997. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77:299-318.
52. Goadsby PJ, Gundlach AL. 1991. Localization of [3H]-dihydroergotamine binding sites in the cat central nervous system: Relevance to migraine. Annals of Neurology 29:91-94.
53. Gonzalez EC, Brownlee HJ. 1998. Movement disorders. In: Taylor RB, Editor, Family Medicine: Principles and Practice. 5th Edition. New York: Springer-Verlag. Pp. 565-573.
54. Gorter R. 1991. Management of anorexia-cachexia associated with cancer and HIV infection. Oncology (Supplement) 5:13-17.
55. Gralla RJ, Itri LM, Pisko SE, et al. 1981. Antiemetic efficacy of high dose metoclopramide: Randomized trials with placebo and prochlorperazine in patients with chemotherapy-induced nausea and vomiting. New England Journal of Medicine 305:905-909.
56. Gralla RJ, Navari RM, Hesketh PJ, et al. 1998. Single-dose oral granisetron has equivalent antiemetic efficacy to intravenous ondansetron for highly emetogenic cisplatinbased chemotherapy. Journal of Clinical Oncology 16:1-7.
57. Gralla RJ, Rittenberg CN, Lettow LA, et al. 1995. A unique all-oral, single-dose, combination antiemetic regimen with high efficacy and marked cost saving potential. Proceedings of the American Societyfor Clinical Oncology 14:526.
58. Gralla RJ, Tyson LB, Borden LB, et al. 1984. Antiemetic therapy: A review of recent studies and a report of a random assignment trial comparing metoclopramide with delta-9-tetrahydrocannabinol. Cancer Treatment Reports 68:163-172.
Page 184
59. Grandara DR, Roila F, Warr D, Edelman MJ, Perez EA, Gralla RJ. 1998. Consensus proposal for 5HT3 antagonists in the prevention of acute emesis related to highly emetogenic chemotherapy: Dose, schedule, and route of administration. Supportive Care in Cancer 6:237-243.
60. Green K, Roth M. 1982. Ocular effects of topical administration of delta-9-tetrahydrocannabinol in man. Archives of Ophthalmology 100:265-267.
61. Greenberg HS, Werness SA, Pugh JE, Andrus RO, Anderson DJ, Domino EF. 1994. Short-term effects of smoking marijuana on balance in patients with multiple sclerosis and normal volunteers. Clinical Pharmacology and Therapeutics 55:324-328.
62. Grinspoon L, Bakalar JB. 1993. Marijuana: The Forbidden Medicine. New Haven: Yale University Press.
63. Grinspoon L, Bakalar JB, Zimmer L, Morgan JP. 1997. Marijuana addiction [Letter]. Science 277:749; discussion, 750-752.
64. Grinspoon S, Corcoran C, Askari H, Schoenfeld D, Wolf L, Burrows B, Walsh M, Hayden D, Parlman K, Anderson E, Basgoz N, Klibanski A. 1998. Effects of androgen administration in men with the AIDS wasting syndrome. Annals of Internal Medicine 129:18-26.
65. Gross H, Egbert MH, Faden VB, Godberg SC, Kaye WH, Caine ED, Hawks R, Zinberg NE. 1983. A double-blind trial of delta-9-THC in primary anorexia nervosa. Journal of Clinical Psychopharmacology 3:165-171.
66. Guyatt GH, Keller JL, Jaeschke R, Rosenbloom D, Adachi JD, Newhouse MT. 1990. The N-of-1 randomized controlled trial-clinical usefulness: Our three-year experience. Annals of Internal Medicine 112:293-299.
67. Guyatt GH, Sackett D, Taylor DW, Chong J, Roberts R, Pugsley S. 1986. Determining optimal therapy: Randomized trials in individual patients. New England Journal of Medicine 314:889-892.
68. Guyton AC. 1986. Textbook of Medical Physiology. 7th ed. Philadelphia: WB Saunders Company.
69. Hall W. 1997. An ongoing debate. Science 278:75.
70. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. 1999. Abstinence symptoms following oral THC administration to humans. Psychopharmacology 141:385-394.
71. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. 1999. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology 141:395-404.
72. Hanigan WC, Destree R, Truong XT. 1986. The effect of delta-9-THC on human spasticity. Clinical Pharmacology and Therapeutics 39:198.
73. Hardin TC. 1993. Cytokine mediators of malnutrition: Clinical implications. Nutrition in Clinical Practice 8:55-59.
74. Hayreh SS, Zimmerman MB, Podhajsky P, Alward W. 1994. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. American Journal of Ophthalmology 117:603-624.
75. Hemming M, Yellowlees PM. 1993. Effective treatment of Tourette's syndrome with marijuana. Journal of Psychopharmacology 7:389-391.
76. Hepler RS, Frank IM, Petrus R. 1976. Ocular effects of marijuana smoking. In: Braude MC, Szara S, Editors, The Pharmacology of Marijuana. New York: Raven Press. Pp. 815824.
77. Hepler RS, Frank IR. 1971. Marihuana smoking and intraocular pressure. Journal of the American Medical Association 217(10):1392.
78. Herkenham M, Lynn AB, de Costa BR, Richfield EK. 1991a. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Research 547:267-274.
Page 185
79. Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. 1991b. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. Journal of Neuroscience 11:563-583.
80. Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. 1990. Cannabinoid receptor localization in the brain. Proceedings of the National Academy of Sciences of the United States of America 87:1932-1936.
81. Herrstedt J, Aapro MS, Smyth JF, Del Favero A. 1998. Corticosteroids, dopamine antagonists and other drugs. Supportive Care in Cancer 6:204-214.
82. Hesketh PJ, Gralla RJ, duBois A, Tonato M. 1998. Methodology of antiemetic trials: Response assessment, evaluation of new agents and definition of chemotherapy emetogenicity. Supportive Care in Cancer 6:221-227.
83. Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, Aapro MS, Gandara D, Lindley CM. 1997. Proposal for classifying the acute emetogenicity of cancer chemotherapy. Journal of Clinical Oncology 15:103-109.
84. Hill SY, Schwin R, Goodwin DW, Powell BJ. 1974. Marihuana and pain. Journal of Pharmacology and Experimental Therapeutics 188:415-418.
85. Holdcroft A, Smith M, Jacklin A, Hodgson H, Smith B, Newton M, Evans F. 1997. Pain relief with oral cannabinoids in familial Mediterranean fever. Anaesthesia 5:483486.
86. Homesley HD, Gainey JM, Jobson VN, et al. 1982. Double-blind placebo-controlled study of metoclopramide in cisplatin-induced emesis. New England Journal of Medicine 307:250-251.
87. Huestis MA, Henningfield JE, Cone EJ. 1992. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. Journal of Analytical Toxicology 16:276-282.
88. Italian Group for Antiemetic Trials. 1995. Dexamethasone, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. New England Journal of Medicine 332:332-337.
89. Jain AK, Ryan JR, McMahon FG, Smith G. 1981. Evaluation of intramuscular levonantradol and placebo in acute postoperative pain. Journal of Clinical Pharmacology 21:320S-326S.
90. Jay WM, Green K. 1983. Multiple-drop study of topically applied 1%, A9-tetrahydrocannabinol in human eyes. Archives of Ophthalmology 101:591-593.
91. Jones RT, Benowitz NL, Heming RI. 1981. Clinical relevance of cannabis tolerance and dependence. Journal of Clinical Pharmacology 21:143S-152S.
92. Kass MA, Gordon MO, Hoff MR, Pardinson JM, Kolker AE, Hart WM, Becker B. 1989. Topical timolol administration reduces the incidence of glaucomatous damage in ocular hypertensive individuals: A randomized, double-masked, long-term clinical trial. Archives of Ophthalmology 107:1590-1598.
93. Kaufman P, Mittag TW. 1994. Medical therapy of glaucoma. In: Kaufman P, Mittag TW, Editors, Textbook of Ophthalmology. Volume 7. London: Mosby-Yearbook.
94. Kotler DP. 1997. Wasting Syndrome Pathogenesis and Clinical Markers. Institute of Medicine Workshop. Irvine, CA, December 15, 1997. Pp. 56-66. Washington, DC: Institute of Medicine.
95. Kotler DP, Gaetz HP, Klein EB, Lange M, Holt PR. 1984. Enteropathy associated with the acquired immunodeficiency syndrome. Annals of Internal Medicine 101:421-428.
96. Kotler DP, Tierney AR, Culpepper-Morgan JA, Wang J, Peirson RN. 1990. Effect of home total parental nutrition on body composition in patients with acquired immunodeficiency syndrome. Journal of Parenteral Nutrition 14:454-458.
Page 186
97. Kotler DP, Tierney AR, Dilmanian FA, Kamen Y, Wang J, Pierson Jr RN, Weber D. 1991. Correlation between total body potassium and total body nitrogen in patients with acquired immunodeficiency syndrome. Clinical Research 39:649A.
98. Kotler DP, Tierney AR, Ferraro R, et al. 1991. Enteral alimentation and repletion of body cell mass in malnourished patients with acquired immunodeficiency syndrome. American Journal of Clinical Nutrition 53:149-154.
99. Kotler DP, Tierney AR, Wang J, Pierson RN. 1989. Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. American Journal of Clinical Nutrition 50:444-447.
100. Kotler DP, Wang J, Pierson RN. 1985. Studies of body composition in patients with the acquired immunodeficiency syndrome. American Journal of Clinical Nutrition 42:1255-1265.
101. Kris MG, Gralla RJ, Clark RA, et al. 1987. Antiemetic control and prevention of side effects of anticancer therapy with lorazepam or diphenhydramine when used in combination with metoclopramide plus dexamethasone: A double-blind randomized trial. Cancer 60:2816-2822.
102. Kris MG, Radford JE, Pizzo BA, et al. 1997. Use of an NK-1 receptor antagonist to prevent delayed emesis following cisplatin. Journal of the National Cancer Institute 89:817-818.
103. Kris MG, Roila F, De Mulder PH, Marty M. 1998. Delayed emesis following anticancer chemotherapy. Supportive Care in Cancer 6:228-232.
104. Lang IM, Sarna SK. 1989. Motor and myoelectric activity associated with vomiting, regurgitation, and nausea. In: Wood JD, Editor, Handbook of Physiology: The Gastrointestinal System. 1, Motility and Circulation. Bethesda, MD: American Physiological Society. Pp. 1179-1198.
105. Larson EB, Ellsworth AJ, Oas J. 1993. Randomized clinical trials in single patients during a 2-year period. Journal of the American Medical Association 270:2708-2712.
106. Leske MC, Connell AM, Schachat AP, Hyman L. 1994. The Barbados Eye Study: Prevalence of open angle glaucoma. Archives of Oplitlhalmrology 112:821-829.
107. Levitt M, Faiman C, Hawks R, et al. 1984. Randomized double-blind comparison of delta-9-THC and marijuana as chemotherapy antiemetics. Proceedings of the American Societyfor Clinical Oncology 3:91.
108. Libman E, Stern MH. 1985. The effects of delta-9-tetrahydrocannabinol on cutaneous sensitivity and its relation to personality. Personality, Individuality and Difference 6:169174.
109. Lichter PR. 1988. A wolf in sheep's clothing. Ophthalmology 95:149-150.
110. Lichtman AH, Cook SA, Martin BR. 1996. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: Evidence supporting periaqueductal gray involvement. Journal of Pharmacology and Experimental Therapeutics 276:585-593.
111. Lichtman AH, Martin BR. 1991. Cannabinoid-induced antinociception is mediated by a spinal alpha-noradrenergic mechanism. Brain Research 559:309-314.
112. Lindgren JE, Ohlsson A, Agurell S, Hollister LE, Gillespie H. 1981. Clinical effects and plasma levels of delta 9-tetrahydrocannabinol (delta 9-THC) in heavy and light users of cannabis. Psychophlarmacology (Berlin) 74:208-212.
113. Macallan DC, Noble C, Baldwin C, Foskett M, McManus T, Griffin GE. 1993. Prospective analysis of patterns of weight change in stage IV human immunodeficiency virus infection. American Journal of Clinical Nutrition 58:417-424.
114. Malec J, Harvey RF, Cayner JJ. 1982. Cannabis effect on spasticity in spinal cord injury. Archives of Physical Medicine and Rehabilitation 63:116-118.
Page 187
115. Mao NK, Stewart WC, Shields M. 1991. Correlation between intraocular pressure control and progressive glaucomatous damage in primary open-angle glaucoma. American Journal of Ophthalmology 111:51-55.
116. Marotta JT. 1995. Spinal injury. In: Rowland LP, Editor, Merrit's Textbook of Neurology. 9th Edition. Philadelphia: Lea and Febiger. Pp. 440-447.
117. Martyn CN, Illis LS, Thom J. 1995. Nabilone in the treatment of multiple sclerosis [Letter]. Lancet 345:579.
118. Mathew NT. 1997. Serotonin 1D (5-HT 1D) agonists and other agents in acute migraine. Neurologic Clinics 15:61-83.
119. Mattes RD, Engelman K, Shaw LM, Elsohly MA. 1994. Cannabinoids and appetite stimulation. Pharmacology, Biochemistry and Behavior 49:187-195.
120. Maurer M, Henn V, Dittrich A, Hoffman A. 1990. Delta-9-tetrahydrocannabinol shows antispastic and analgesic effects in a single case double-blind trial. European Archives of Psychiatry and Clinical Neuroscience 240:1-4.
121. McCarthy LE, Flora KP, Vishnuvajjala BR. 1984. Antiemetic properties and plasma concentrations of delta-9-tetrahydrocannabinol against cisplatin vomiting in cats. In: Agurell S, Dewey WL, Willette RE, Editors, The Cannabinoids: Chemical, Pharmlacologic and Therapeutic Aspects. Orlando, FL: Academic Press. Pp. 859-870.
122. McQuay H, Carroll D, Moore A. 1996. Variation in the placebo effect in randomised controlled trials of analgesics: All is as blind as it seems. Pain 64:331-335.
123. Meinck HM, Schonle PW, Conrad B. 1989. Effect of cannabinoids on spasticity and ataxia in multiple sclerosis. Journal of Neurology 236:120-122.
124. Merritt JC, Cook CE, Davis KH. 1982. Orthostatic hypotension after delta 9-tetrahydrocannabinol marihuana inhalation. Ophthalmic Research 14:124-128.
125. Merritt JC, Crawford WJ, Alexander PC, Anduze AL, Gelbart SS. 1980. Effect of marihuana on intraocular and blood pressure in glaucoma. Ophthalnology 87:222-228.
126. Mertens TE, Low-Beer D. 1996. HIV and AIDS: Where is the epidemic going? Bulletin of the World Health Organization 74:121-129.
127. Miller AD. 1998. Nausea and vomiting: Underlying mechanisms and upcoming treatments. Journal of the Japanese Broncho-Esophagological Society 49:57-64.
128. Miller AD, Nonaka S, Siniaia MS, Jakus J. 1995. Multifunctional ventral respiratory group: Bulospinal expiratory neurons play a role in pudendal discharge during vomiting. Journal of the Autonomic Nervous System 54:253-260.
129. Miller AS, Walker JM. 1995. Effects of a cannabinoid on spontaneous and evoked neuronal activity in the substantia nigra pars reticulata. European Journal of Pharmacology 279:179-185.
130. Miller AS, Walker JM. 1996. Electrophysiological effects of a cannabinoid on neural activity in the globus pallidus. European Journal of Pharmacology 304:29-35.
131. Moertel CG, Taylor WF, Roth A, Tyce FA. 1976. Who responds to sugar pills? Mayo Clinic Proceedings 51:96-100.
132. Moldawer LL, Andersson C, Gelin J, Lundholm KG. 1988. Regulation of food intake and hepatic protein synthesis by recombinant-derived cytokines. American Journal of Physiology 254:G450-G456.
133. Mulligan K, Tai VW, Schambelan M. 1997. Cross-sectional and longitudinal evaluation of body composition in men with HIV infection. Journal of Acquired Inmmunodeficiency Syndrome 15:43-48.
134. Murray CJL, Lopez AD. 1996. Global Health Statistics: A Comwpenditum of Incidence, Prevalence, and Mortality Estimates for Over 200 Conditions. Global Burden of Disease and Injury Series, Volume II. Boston, MA: The Harvard School of Public Health.
Page 188
135. Navari RM, Reinhardt RR, Gralla RJ, Kris MG, Hesketh PJ, et al. 1999. Reduction of cisplatin-induced emesis by a selective neurokinin-l-receptor antagonist. The New England Journal of Medicine 340:190-195.
136. Newell FW, Stark P, Jay WM, Schanzlin DJ. 1979. Nabilone: A pressure-reducing synthetic benzopyran in open-angle glaucoma. Ophthalmology 86:156-160.
137. Ng SKC, Brust JCM, Hauser WA, Susser M. 1990. Illicit drug use and the risk of newonset seizures. American Journal of Epidemiology 132:47-57.
138. NIH. 1997. Spinal cord injury: Emerging concepts: An NIH workshop. Proceedings of an NIH Workshop on Spinal Cord Injury. Bethesda, MD, September 30-October 1, 1996. Bethesda, MD: National Institute of Neurological Disorders and Stroke.
139. Noyes R, Jr, Brunk SF, Avery DH, Canter A. 1975b. The analgesic properties of delta9-tetrahydrocannabinol and codeine. Clinical Pharmacology and Therapeutics 18:84-89.
140. Noyes Jr R, Brunk SF, Baram DA, Canter A. 1975a. Analgesic effect of delta-9-tetrahydrocannabinol. Journal of Clinical Pharmacology 15:139-143.
141. Ohlsson A, Lindgren J-E, Wahlen A, Agurell S, Hollister LE, Gillespie HK. 1980. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clinical Pharmacology and Therapeutics 28:409-416.
142. Orgul S, Kaiser HJ, Flammer J, Gasser P. 1995. Systemic blood pressure and capillary blood-cell velocity in glaucoma patients: A preliminary study. European Journal of Ophthalmology 5:88-91.
143. Orr LE, McKernan JF, Bloome B. 1980. Antiemetic effect of tetrahydrocannabinol. Compared with placebo and prochlorperazine in chemotherapy-associated nausea and emesis. Archives of Internal Medicine 140:1431-1433.
144. Ott M, Lambke B, Fischer H, Jagre R, Polat H, Geier H, Rech M, Staszeswki S, Helm EB, Caspary WF. 1993. Early changes of body composition in human immunodeficiency virus-infected patients: Tetrapolar body impedance analysis indicates significant malnutrition. American Journal of Clinical Nutrition 57:15-19.
145. Perez EA, Chawla SP, Kaywin PK, et al. 1997. Efficacy and safety of oral granisetron versus IV ondansetron in prevention of moderately emetogenic chemotherapyinduced nausea and vomiting. Proceedings of the American Societyfor Clinical Oncology 16:43.
146. Perez-Reyes M, Wagner D, Wall ME, Davis KH. 1976. Intravenous administration of cannabinoids and intraocular pressure. In: The Pharmacology of Marihuana, New York: Raven Press. Pp. 829-832.
147. Peroutka SJ. 1996. Drugs effective in the therapy of migraine. In: Hardman JG, Limbird LE, Editors, Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th Edition. New York: McGraw-Hill. Pp. 487-502.
148. Petro D, Ellenberger Jr C. 1981. Treatment of human spasticity with delta 9-tetrahydrocannabinol. Journal of Clinical Pharmacology 21:413S-416S.
149. Quigley HA. 1996. Number of people with glaucoma worldwide. British Journal of Oplhthalmology 80:389-393.
150. Raft D, Gregg J, Ghia J, Harris L. 1977. Effects of intravenous tetrahydrocannabinol on experimental and surgical pain: Psychological correlates of the analgesic response. Clinical Pharmacology and Therapeutics 21:26-33.
151. Razdan RK. 1986. Structure-activity relationships in cannabinoids. Pharmacology Review 38:75-149.
152. Richfield EK, Herkenham M. 1994. Selective vulnerability in Huntington's disease: Preferential loss of cannabinoid receptors in lateral globus pallidus. Annals of Neurology 36:577-584.
Page 189
153. Richter A, Loscher W. 1994. (+)-WIN55,212-2 A novel cannabinoid receptor agonist, exerts antidystonic effects in mutant dystonic hamsters. European Journal of Pharmacology 264:371-377.
154. Rodriguez de Fonseca F, Carrera MRA, Navarro M, Koob G, Weiss F. 1997. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal [see comments Science 1997., 276:1967-1968]. Science 276:2050-2054.
155. Roila F, Tonato M, Cognetti F, et al. 1991. Prevention of cisplatin-induced emesis: A double-blind multicenter randomized crossover study comparing ondansetron and ondansetron plus dexamethasone. Journal of Clinical Oncology 9:674-678.
156. Rosenzweig MR, Leiman AL, Breedlove SM. 1996. Biological Psychology. Sunderland, MA: Sinauer Associates, Inc.
157. Roth RI, Owen RL, Keren DF, Volberding PA. 1985. Intestinal infection with Mycobacterium avium in acquired immune deficiency syndrome (AIDS): Histological and clinical comparison with Whipple's disease. Digestive Disease Science 30:497-504.
158. Suttmann U, Ockenga J, Selberg 0, Hoogestraat L, Deicher H, Muller MJ. 1995. Incidence and prognostic value of malnutrition and wasting in human immunodeficiency virus-infected outpatients. Journal of Acquired Immune Deficiency Syndrome and Human Retrovirology 8:239-246.
159. Sackett D, Rosenberg W, Haynes B, Richardson S. 1997. Evidence-Based Medicine: How to Practice and Teach EBM. New York: Churchhill Livingston.
160. Sallan SE, Cronin CM, Zelen M, et al. 1980. Antiemetics in patients receiving chemotherapy for cancer: A randomized comparison of delta-9-tetrahydrocannabinol and prochlorperazine. New England Journal of Medicine 302:135-138.
161. Sallan SE, Zinberg NE, Frei E. 1975. Antiemetic effect of delta-9-THC in patients receiving cancer chemotherapy. New England Journal of Medicine 293:795-797.
162. SAMHSA (Substance Abuse and Mental Health Services Administration). 1998. National Household Survey on Drug Abuse: Population Estimates 1997. DHHS Pub. No. (SMA) 98-3250. Rockville, MD: SAMHSA, Office of Applied Studies.
163. Sandyk R, Awerbuch G. 1988. Marijuana and Tourette's syndrome. Journal of Clinical Psychopharmacology 8:444-445.
164. Sandyk R, Consroe P, Stern P, Biklen D. 1988. Preliminary trial of cannabidiol in Huntington's disease. Chesher G, Consroe P, Musty R., Editors, Marijuana: An International Research Report. Canberra: Australian Government Publishing Service.
165. Sanudo-Pena MC, Patrick SL, Patrick RL, Walker JM. 1996. Effects of intranigral cannabinoids on rotational behavior in rats: Interactions with the dopaminergic system. Neuroscience Letters 206:21-24.
166. Sanudo-Pena MC, Tsou K, and Walker JM. Cannabinoid dopamine interactions in the basal ganglia in an animal model of Parkinson disease. (in preparation).
167. Sanudo-Pena MC, Tsou K, and Walker JM. Superior colliculus and turning: Dopamine and cannabinoids. (in preparation).
168. Sanudo-Pena MC, Walker JM. 1997. Role of the subthalamic nucleus in cannabinoid actions in the substantia nigra of the rat. Journal of Neurophysiology 77:1635-1638.
169. Sanudo-Pena MC, Walker JM. 1998. Effects of intrastriatal cannabinoids on rotational behavior in rats: Interactions with the dopaminergic system. Synapse 30:221-226.
170. Schambelan M, Mulligan K, Grunfeld C, Daar ES, LaMarca A, Kotler DP. 1996. Recombinant human growth hormone in patients with HIV-associated wasting: A randomized, placebo-controlled trial: Serostim Study Group. Annals of Internal Medicine 125:873-882.
171. Schwartz RH, Beveridge RA. 1994. Marijuana as an antiemetic drug: How useful is it today? Opinions from clinical oncologists [see Comments]. Journal of Addictive Diseases 13:53-65.
Page 190
172. Schwartz RH, Voth EA. 1995. Marijuana as medicine: Making a silk purse out of a sow's ear. Journal of Addictive Diseases 14:15-21.
173. Shields MB. 1998. Textbook of Glaucoma. 4th Edition. Baltimore, MD: Williams & Wilkins.
174. Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt J, Singh K. 1991. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans: The Baltimore Eye Survey. Archives of Ophthanlology 109:1090-1095.
175. Staquet M, Gantt C, Machin D. 1978. Effect of a nitrogen analog of tetrahydrocannabinol on cancer pain. Clinical Pharmacology and Therapeutics 23:397-401.
176. Steele N, Gralla RJ, Braun Jr DW. 1980. Double-blind comparison of the antiemetic effects of nabilone and prochlorperazine on chemotherapy-induced emesis. Cancer Treatments Report 64:219-224.
177. Stimmel B. 1995. Medical marijuana: To prescribe or not to prescribe, that is the question [Editorial]. Journal of Addictive Diseases 14:1-3.
178. Strassman RJ. 1998. Marijuana: The Forbidden Medicine (book review). Journal of the American Medical Association 279:963-964.
179. Struwe M, Kaempfer SH, Geiger CJ, Pavia AT, Plasse TF, Shepard KV, Ries K, Evans TG. 1993. Effect of dronabinol on nutritional status in HIV infection. Annals of Pharmacotherapy 27:827-831.
180. Swift RM. 1994. Marijuana: The Forbidden Medicine (book review). The New England Journal of Medicine 331:749-750.
181. Tanda G, Pontieri FE, Di Chiara G. 1997. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common PL opioid receptor mechanism. Science 276:2048-2049.
182. Tiedeman JS, Shields MB, Weber PA, Crow JW, Cocchetto DM, Harris WA, Howes JF. 1981. Effect of synthetic cannabinoids on elevated intraocular pressure. Ophthalmology 88:270-277.
183. Timpone JG, Wright DJ, Li N, Egorin MJ, Enama ME, Mayers J, Galetto G, DATRI 004 Study Group. 1997. The safety and pharmacokinetics of single-agent and combination therapy with megestrol acetate and dronabinol for the treatment of HIV wasting syndrome: The DATRI 004 study group. AIDS Research and Human Retroviruses 13:305315.
184. Trembly B, Sherman M. 1990. Double-blind clinical study of cannabidiol as a secondary anticonvulsant. Unpublished manuscript presented at the Marijuana '90 International Conference on Cannabis and Cannabinoids. Kolympari, Crete, July 8-11.
185. Tyson LB, Gralla RJ, Clark RA, et al. 1985. Phase I trial of levonantradol in chemotherapy-induced emesis. American Journal of Clinical Oncology 8:528-532.
186. UNAIDS, WHO. 1998. Report on the Global HIV/AIDS Epidemic, June 1998.
187. Ungerleider JT, Andrysiak TA, Fairbanks L, Ellison GW, Myers LW. 1987. Delta-9-THC in the treatment of spasticity associated with multiple sclerosis. Advances in Alcohol and Substance Abuse 7:39-50.
188. Vinciguerra V, Moore T, Brennan E. 1988. Inhalation marijuana as an antiemetic for cancer chemotherapy. New York State Journal of Medicine 88:525-527.
189. Volicer L, Smith S, Volicer BJ. 1995. Effect of seizures on progression of dementia of the Alzheimer type. Dementia 6:258-263.
190. Volicer L, Stelly M, Morris J, McLaughlin J, Volicer BJ. 1997. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer's disease. International Journal of Geriatric Psychiatry 12:913-919.
191. Voth EA, Schwartz RH. 1997. Medicinal applications of delta-9-tetrahydrocannabinol and marijuana. Annals of Internal Medicine 126:791-798.
Page 191
192. Wall PD, Melzack R. 1994. Textbook of Pain. Edinburgh: Churchill Livingstone.
193. Walters TR. 1996. Development and use of brimonidine in treating acute and chronic elevations of intraocular pressure: A review of safety, efficacy, dose response, and dosing studies. Survey of Ophthalmology 41(Suppl. 1):S19-S26.
194. Wang ZM, Visser M, Ma R, Baumgartner RN, Kotler DP, Gallagher D, Heymsfield SB. 1996. Skeletal muscle mass: Validation of neutron activation and dual energy X-ray absorptiometry methods by computerized tomography. Journal of Applied Physiology 80:824-831.
195. Whitney EN, Cataldo CB, Rolfes SR. 1994. Understanditng Normal and Clinical Nutrition. 4th Edition. Minneapolis, MN: West Publishing Co.
196. Wood. 1998. HIV-protease inhibitors. Drug Therapy 338:1281-1292.
197. Yoles E, Belkin M, Schwartz M. 1996. HU-211, a nonpsychotropic cannabinoid, produces short- and long-term neuroprotection after optic nerve axotomy. Journal of Neurotrauma 13:49-57.
198. Zimmer L, Morgan JP. 1997. Marijuana Myths, Marijuana Facts. New York: The Lindesmith Center.
























































