Page 33
2
CANNABINOIDS AND ANIMAL PHYSIOLOGY
Introduction
Much has been learned since the publication of the 1982 Institute of Medicine (IOM) report Marijuana and Health.* Although it was clear then that most of the effects of marijuana were due to its actions on the brain, there was little information about how THC acted on brain cells (neurons), which cells were affected by THC, or even what general areas of the brain were most affected by THC. Too little was known about cannabinoid physiology to offer any scientific insights into the harmful or therapeutic effects of marijuana. That is no longer true. During the past 16 years, there have been major advances in what basic science discloses about the potential medical benefits of cannabinoids, the group of compounds related to THC. Many variants are found in the marijuana plant, and other cannabinoids not found in the plant have been chemically synthesized. Sixteen years ago it was still a matter of debate as to whether THC acted nonspecifically by affecting the fluidity of cell membranes or whether a specific pathway of action was mediated by a receptor that responded selectively to THC (Table 2.1).
*The field of neuroscience has grown substantially since the publication of the 1982 IOM report. The number of members in the Society for Neuroscience provides a rough measure of the growth in research and knowledge about the brain: as of the middle of 1998, there were over 27,000 members, more than triple the number in 1982.
Page 34
| TABLE 2.1 Landmark Discoveries Since the 1982 IOM Report | ||
| Year | Discovery | Primary Investigators |
| 1986 | Potent cannabinoid agonists are developed; they are the key to discovering the receptor. | M. R. Johnson and L. S. Melvin75 |
| 1988 | First conclusive evidence of specific cannabinoid receptors. | A. Howlett and W. Devaneh36 |
| 1990 | The cannabinoid brain receptor (CB,) is cloned, its DNA sequence is identified, and its location in the brain is determined. | L. Matsuda107 and M. Herkenham |
| et al60 | ||
| 1992 | Anandamide is discovered—a naturally occurring substance in the brain that acts on cannabinoid receptors. | R. Mechoulam and W. Devane37 |
| 1993 | A cannabinoid receptor is discovered outside the brain; this receptor (CB2) is related to the brain receptor but is distinct. | S. Munro112 |
| 1994 | The first specific cannabinoid antagonist, SR 141716A, is developed. | M. Rinaldi-Carmonal32 |
| 1998 | The first cannabinoid antagonist, SR144528, that can distinguish between CB1 and CB2 receptors discovered. | M. Rinaldi-Carmona133 |
Basic science is the wellspring for developing new medications and is particularly important for understanding a drug that has as many effects as marijuana. Even committed advocates of the medical use of marijuana do not claim that all the effects of marijuana are desirable for every medical use. But they do claim that the combination of specific effects of marijuana enhances its medical value. An understanding of those specific effects is what basic science can provide. The multiple effects of marijuana can be singled out and studied with the goals of evaluating the medical value of marijuana and cannabinoids in specific medical conditions, as well as minimizing unwanted side effects. An understanding of the basic mechanisms through which cannabinoids affect physiology permits more strategic development of new drugs and designs for clinical trials that are most likely to yield conclusive results.
Research on cannabinoid biology offers new insights into clinical use, especially given the scarcity of clinical studies that adequately evaluate the medical value of marijuana. For example, despite the scarcity of sub-
Page 35
stantive clinical data, basic science has made it clear that cannabinoids can affect pain transmission and, specifically, that cannabinoids interact with the brain's endogenous opioid system, an important system for the medical treatment of pain (see chapter 4).
The cellular machinery that underlies the response of the body and brain to cannabinoids involves an intricate interplay of different systems. This chapter reviews the components of that machinery with enough detail to permit the reader to compare what is known about basic biology with the medical uses proposed for marijuana. For some readers that will be too much detail. Those readers who do not wish to read the entire chapter should, nonetheless, be mindful of the following key points in this chapter:
· The most far reaching of the recent advances in cannabinoid biology are the identification of two types of cannabinoid receptors (CB1 and CB2) and of anandamide, a substance naturally produced by the body that acts at the cannabinoid receptor and has effects similar to those of THC. The CB1 receptor is found primarily in the brain and mediates the psychological effects of THC. The CB2 receptor is associated with the immune system; its role remains unclear.
· The physiological roles of the brain cannabinoid system in humans are the subject of much active research and are not fully known; however, cannabinoids likely have a natural role in pain modulation, control of movement, and memory.
· Animal research has shown that the potential for cannabinoid dependence exists, and cannabinoid withdrawal symptoms can be observed. However, both appear to be mild compared to dependence and withdrawal seen with other drugs.
· Basic research in cannabinoid biology has revealed a variety of cellular pathways through which potentially therapeutic drugs could act on the cannabinoid system. In addition to the known cannabinoids, such drugs might include chemical derivatives of plantderived cannabinoids or of endogenous cannabinoids such as anandamide but would also include noncannabinoid drugs that act on the cannabinoid system.
This chapter summarizes the basics of cannabinoid biology—as known today. It thus provides a scientific basis for interpreting claims founded on anecdotes and for evaluating the clinical studies of marijuana presented in chapter 4.
Page 36
The Value of Animal Studies
Much of the research into the effects of cannabinoids on the brain is based on animal studies. Many speakers at the public workshops associated with this study argued that animal studies of marijuana are not relevant to humans. Animal studies are not a substitute for clinical trials, but they are a necessary complement. Ultimately, every biologically active substance exerts its effects at the cellular and molecular levels, and the evidence has shown that this is remarkably consistent among mammals, even those as different in body and mind as rats and humans. Animal studies typically provide information about how drugs work that would not be obtainable in clinical studies. At the same time, animal studies can never inform us completely about the full range of psychological and physiological effects of marijuana or cannabinoids on humans.
The Active Constituents of Marijuana
D9-THC and D8-THC are the only compounds in the marijuana plant that produce all the psychoactive effects of marijuana. Because D9-THC is much more abundant than D8-THC, the psychoactivity of marijuana has been attributed largely to the effects of D9-THC. 11-OH-D9-THC is the primary product of D9-THC metabolism by the liver and is about three times as potent as D9-THC.128
There have been considerably fewer experiments with cannabinoids other than A9-THC, although a few studies have been done to examine whether other cannabinoids modulate the effects of THC or mediate the nonpsychological effects of marijuana. Cannabidiol (CBD) does not have the same psychoactivity as THC, but it was initially reported to attenuate the psychological response to THC in humans;81,177 however, later studies reported that CBD did not attenuate the psychological effects of THC.11,69 One double-blind study of eight volunteers reported that CBD can block the anxiety induced by high doses of THC (0.5 mg/kg).177 There are numerous anecdotal reports claiming that marijuana with relatively higher ratios of THC:CBD is less likely to induce anxiety in the user than marijuana with low THC:CBD ratios; but, taken together, the results published thus far are inconclusive.
The most important effect of CBD seems to be its interference with drug metabolism, including D9-THC metabolism in the liver.14, 114 It exerts that effect by inactivating cytochrome P450s, which are the most important class of enzymes that metabolize drugs. Like many P450 inactivators, CBD can also induce P450s after repeated doses.13 Experiments in which mice were treated with CBD followed by THC showed that CBD treatment was associated with a substantial increase in brain concentrations of
Page 37
THC and its major metabolites, most likely because it decreased the rate of clearance of THC from the body.15
In mice, THC inhibits the release of luteinizing hormone, the pituitary hormone that triggers the release of testosterone from the testes; this effect is increased when THC is given with cannabinol or CBD.113
Cannabinol also lowers body temperature and increases sleep duration in mice.175 It is considerably less active than THC in the brain, but studies of immune cells have shown that it can modulate immune function (see ''Cannabinoids and the Immune System'' later in this chapter).
The Pharmacological Toolbox
A researcher needs certain key tools in order to understand how a drug acts on the brain. To appreciate the importance of these tools, one must first understand some basic principles of drug action. All recent studies have indicated that the behavioral effects of THC are receptor mediated.27 Neurons in the brain are activated when a compound binds to its receptor, which is a protein typically located on the cell surface. Thus, THC will exert its effects only after binding to its receptor. In general, a given receptor will accept only particular classes of compounds and will be unaffected by other compounds.
Compounds that activate receptors are called agonists. Binding to a receptor triggers an event or a series of events in the cell that results in a change in the cell's activity, its gene regulation, or the signals that it sends to neighboring cells (Figure 2.1). This agonist-induced process is called signal transduction.
Another set of tools for drug research, which became available only recently for cannabinoid research, are the receptor antagonists, so-called because they selectively bind to a receptor that would have otherwise been available for binding to some other compound or drug. Antagonists block the effects of agonists and are tools to identify the functions of a receptor by showing what happens when its normal functions are blocked. Agonists and antagonists are both ligands; that is, they bind to receptors. Hormones, neurotransmitters, and drugs can all act as ligands. Morphine and naloxone provide a good example of how agonists and antagonists interact. A large dose of morphine acts as an agonist at opioid receptors in the brain and interferes with, or even arrests, breathing. Naloxone, a powerful opioid antagonist, blocks morphine's effects on opiate receptors, thereby allowing an overdose victim to resume breathing normally. Naloxone itself has no effect on breathing.
Another key tool involves identifying the receptor protein and determining how it works. That makes it possible to locate where a drug activates its receptor in the brain—both the general region of the brain and
Page 38
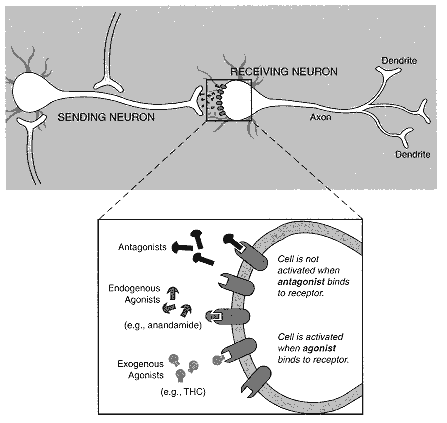
Figure 2.1
Diagram of neuron with synapse. Individual nerve cells, or neurons, both send and receive
cellular signals to and from neighboring neurons, but for the purposes of this diagram
only one activity is indicated for each cell. Neurotransmitter molecules are released from
the neuron terminal and move across the gap between the "sending" and "receiving"
neurons. A signal is transmitted to the receiving neuron when the neurotransmitters have
bound to the receptor on its surface. The effects of a transmitted signal include:
· Changing the cell's permeability to ions, such as calcium and potassium.
· Turning a particular gene on or off.
· Sending a signal to another neuron.
· Increasing or decreasing the responsiveness of the cell to other cellular signals.
Those effects can lead to cognitive, behavioral, or physiological changes, depending on which neuronal system is activated.
Continued on bottom of p. 39
Page 39
the cell type where the receptor is located. The way to find a receptor for a drug in the brain is to make the receptor "visible" by attaching a radioactive or fluorescent marker to the drug. Such markers show where in the brain a drug binds to the receptor, although this is not necessarily the part of the brain where the drug ultimately has its greatest effects.
Because drugs injected into animals must be dissolved in a waterbased solution, it is easier to deliver water-soluble molecules than to deliver fat-soluble (lipophilic) molecules such as THC. THC is so lipophilic that it can stick to glass and plastic syringes used for injection. Because it is lipophilic, it readily enters cell membranes and thus can cross the blood brain barrier easily. (This barrier insulates the brain from many bloodborne substances.) Early cannabinoid research was hindered by the lack of potent cannabinoid ligands (THC binds to its cannabinoid receptors rather weakly) and because they were not readily water soluble. The synthetic agonist CP 55,940, which is more water soluble than THC, was the first useful research tool for studying cannabinoid receptors because of its high potency and ability to be labeled with a radioactive molecule, which enabled researchers to trace its activity.
Cannabinoid Receptors
The cannabinoid receptor is a typical member of the largest known family of receptors: the G protein-coupled receptors with their distinctive pattern in which the receptor molecule spans the cell membrane seven times (Figure 2.2). For excellent recent reviews of cannabinoid receptor biology, see Childers and Breivogel,27 Abood and Martin,1 Felder and Glass,43 and Pertwee.124 Cannabinoid receptor ligands bind reversibly (they bind to the receptor briefly and then dissociate) and stereoselectively (when there are molecules that are mirror images of each other, only one
| The expanded view of the synapse illustrates a variety of ligands, that is, molecules that bind to receptors. Anandamide is a substance produced by the body that binds to and activates cannabinoid receptors; it is an endogenous agonist. THC can also bind to and activate cannabinoid receptors but is not naturally found in the body; it is an exogenous agonist. SR 141716A binds to but does not activate cannabinoid receptors. In this way it prevents agonists, such as anandamide and THC, from activating cannabinoid receptors by binding to the receptors without activating them; SR 141716A is an antagonist, but it is not normally produced in the body. Endogenous antagonists, that is, those normally produced in the body, might also exist, but none has been identified. |
Page 40
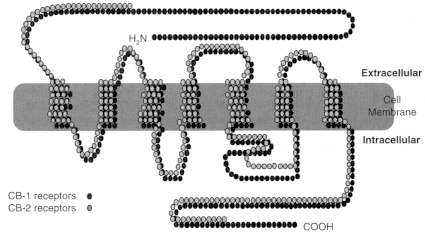
Figure 2.2
Cannabinoid receptors. Receptors are proteins, and proteins are
made up of strings of amino acids. Each circle in the diagram represents one amino
acid. The shaded bar represents the cell membrane, which like all cell membranes
in animals is composed largely of phospholipids. Like many receptors, the cannabinoid
receptors span the cell membrane; some sections of the receptor protein are outside
the cell membrane (extracellular); some are inside (intracellular). THC, anandamide,
and other known cannabinoid receptor agonists bind to the extracellular portion of the
receptor, thereby activating the signal pathway inside the cell. The CB1 molecule is
larger than CB2. The receptor molecules are most similar in four of the seven regions
where they are embedded in the cell membrane (known as the transmembrane regions).
The intracellular loops of the two receptor subtypes are quite different, which might
affect the cellular response to the ligand because these loops are known to mediate
G protein signaling, the next step in the cell signaling pathway after the receptor. Receptor
homology between the two receptor subtypes is 44% for the full-length protein
and 68% within the seven transmembrane regions. The ligand binding sites are typically
defined by the extracellular loops and the transmembrane regions.
version activates the receptor). Thus far, two cannabinoid receptor subtypes (CB1 and CB2) have been identified, of which only CB1 is found in the brain.
The cell responds in a variety of ways when a ligand binds to the cannabinoid receptor (Figure 2.3). The first step is activation of G proteins, the first components of the signal transduction pathway. That leads to changes in several intracellular components-such as cyclic AMP and calcium and potassium ions—which ultimately produce the changes in cell functions. The final result of cannabinoid receptor stimulation de-
Page 41
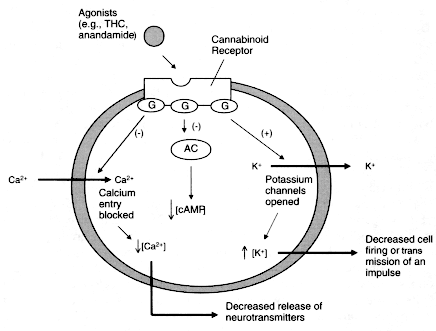
Figure 2.3
Cannabinoid agonists trigger a series of reactions within cells.
Cannabinoid receptors are embedded in the cell membrane, where they are
coupled to G proteins (G) and the enzyme adenylyl cyclase (AC). Receptors
are activated when they bind to ligands, such as anandamide or THC in this
case. This triggers a variety of reactions, including inhibition (-) of AC,
which decreases the production of cAMP and cellular activities dependent on
cAMP; opening of potassium (K+) channels, which decreases cell firing; and
closing of calcium (Ca2+) channels, which decreases the release of neurotransmitters.
Each of those changes can influence cellular communication.
pends on the particular type of cell, the particular ligand, and the other molecules that might be competing for receptor binding sites. Different agonists vary in binding potency, which determines the effective dose of the drug, and efficacy, which determines the maximal strength of the signal that they transmit to the cell. The potency and efficacy of THC are both relatively lower than those of some synthetic cannabinoids; in fact, synthetic compounds are generally more potent and efficacious than endogenous agonists.
CB1 receptors are extraordinarily abundant in the brain. They are more abundant than most other G protein-coupled receptors and 10 times more abundant than mu opioid receptors, the receptors responsible for the effects of morphine.148
Page 42
The cannabinoid receptor in the brain is a protein referred to as CB,. The peripheral receptor (outside the nervous system), CB2, is most abundant on cells of the immune system and is not generally found in the brain.43,124 Although no other receptor subtypes have been identified, there is a genetic variant known as CB1A (such variants are somewhat different proteins that have been produced by the same genes via alternative processing). In some cases, proteins produced via alternative splicing have different effects on cells. It is not yet known whether there are any functional differences between the two, but the structural differences raise the possibility.
CB1 and CB2 are similar, but not as similar as members of many other receptor families are to each other. On the basis of a comparison of the sequence of amino acids that make up the receptor protein, the similarity of the CB1 and CB2 receptors is 44% (Figure 2.2). The differences between the two receptors indicate that it should be possible to design therapeutic drugs that would act only on one or the other receptor and thus would activate or attenuate (block) the appropriate cannabinoid receptors. This offers a powerful method for producing biologically selective effects. In spite of the difference between the receptor subtypes, most cannabinoid compounds bind with similar affinity* to both CB1 and CB2 receptors. One exception is the plant-derived compound CBD, which appears to have greater binding affinity for CB2 than for CB1,112 although another research group has failed to substantiate that observation.129 Other exceptions include the synthetic compound WIN 55,212-2, which shows greater affinity for CB2 than CB,, and the endogenous ligands, anandamide and 2-AG, which show greater affinity for CB1, than CB2.43 The search for compounds that bind to only one or the other of the cannabinoid receptor types has been under way for several years and has yielded a number of compounds that are useful research tools and have potential for medical use.
Cannabinoid receptors have been studied most in vertebrates, such as rats and mice. However, they are also found in invertebrates, such as leeches and mollusks.156 The evolutionary history of vertebrates and invertebrates diverged more than 500 million years ago, so cannabinoid receptors appear to have been conserved throughout evolution at least this long. This suggests that they serve an important and basic function in animal physiology. In general, cannabinoid receptor molecules are similar among different species.124 Thus, cannabinoid receptors likely fill many similar functions in a broad range of animals, including humans.
*Affinity is a measure of how avidly a compound binds to a receptor. The higher the affinity of a compound, the higher its potency; that is, lower doses are needed to produce its effects.
Page 43
The Endogenous Cannabinoid System
For any drug for which there is a receptor, the logical question is, "Why does this receptor exist?" The short answer is that there is probably an endogenous agonist (that is, a compound that is naturally produced in the brain) that acts on that receptor. The long answer begins with a search for such compounds in the area of the body that produces the receptors and ends with a determination of the natural function of those compounds. So far, the search has yielded several endogenous compounds that bind selectively to cannabinoid receptors. The best studied of them are anandamide37 and arachidonyl glycerol (2-AG).108 However, their physiological roles are not yet known.
Initially, the search for an endogenous cannabinoid was based on the premise that its chemical structure would be similar to that of THC; that was reasonable, in that it was really a search for another "key" that would fit into the cannabinoid receptor "keyhole," thereby activating the cellular message system. One of the intriguing discoveries in cannabinoid biology was how chemically different THC and anandamide are. A similar search for endogenous opioids (endorphins) also revealed that their chemical structure is very different from the plant-derived opioids, opium and morphine.
Further research has uncovered a variety of compounds with quite different chemical structures that can activate cannabinoid receptors (Table 2.2 and Figure 2.4). It is not yet known exactly how anandamide and THC bind to cannabinoid receptors. Knowing this should permit more precise design of drugs that selectively activate the endogenous cannabinoid systems.
Anandamide
The first endogenous cannabinoid to be discovered was arachidonyl-ethanolamine, named anandamide from the Sanskrit word ananda, meaning "bliss."37 Compared with THC, anandamide has only moderate affinity for CB1 receptor and is rapidly metabolized by amidases (enzymes that remove amide groups). Despite its short duration of action, anand-amide shares most of the pharmacological effects of THC.37,152 Rapid degradation of active molecules is a feature of neurotransmitter systems that allows them control of signal timing by regulating the abundance of signaling molecules. It creates problems for interpreting the results of many experiments and might explain why in vivo studies with anandamide injected into the brain have yielded conflicting results.
Anandamide appears to have both central (in the brain) and peripheral (in the rest of the body) effects. The precise neuroanatomical localiza-
Page 44
| TABLE 2.2 Compounds That Bind to Cannabinoid Receptors | |
| Compound | Properties |
| Agonists (receptor activators) | |
| Plant-derived compounds | |
| D9-THC | Main psychoactive cannabinoid in marijuana plant; largely responsible for psychological and physiological effects (except in discussions of the different forms of THC, THC is used as a synonym for D9-THC). |
| D8-THC | Slightly less potent than D9-THC and much less abundant in marijuana plant but otherwise similar. |
| 11-OH-D9-THC | Bioactive compound formed when body breaks down D9-THC; presumed to be responsible for some effects of marijuana. |
| Cannabinoid agonists found in animals | |
| Anandamide | Found in animals ranging from mollusks to mammals; appears to be primary endogenous cannabinoid agonist in mammals; chemical structure very different from plant cannabinoids and related to prostaglandins. |
| (arachidonyl- | |
| ethanolamide) | |
| 2-AG (arachidonyl | Endogenous agonist; structurally similar to anandamide; more abundant but less potent than anandamide. |
| glycerol) | |
| THC analogues | |
| Dronabinol | Synthetic THC; marketed in the United States as Marinol for nausea associated with chemotherapy and for AIDS- related wasting. |
| Nabilone | THC analogue; marketed in the United Kingdom as Cesamet for same indications as dronabionol. |
| CP 55,940 | Synthetic cannabinoid; THC analogue; that is, it is structurally similar to THC. |
| Levonantradol | THC analogue. |
| HU-210 | THC analogue, 100- to 800-fold greater potency than THC97. |
| Chemical structure unlike THC or anandamide | |
| WIN-55,212-2 | Chemical structure different from known cannabinoids, but binds to both cannabinoid receptors; chemically related to cyclo-oxygenase inhibitors, which include antiinflammatory drugs. |
| Antagonists (receptor blockers) | |
| SR 141716A | Synthetic CB1 antagonist; developed in 1994132. |
| SR 144528 | Synthetic CB2 antagonist; developed in 1997133. |
| SOURCES: Mechoulam et al., 1998;109 Felder and Glass, 1998;43 and British Medical Association.17 | |
Page 45
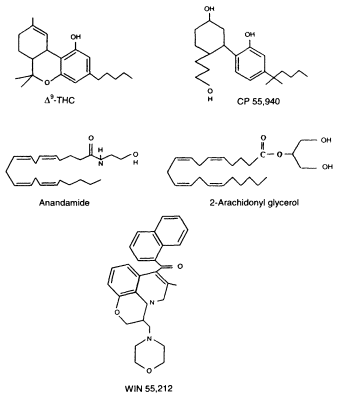
Figure 2.4
Chemical structures of selected cannabinoid agonists
or molecules that bind to and activate cannabinoid receptors.
THC is the primary psychoactive molecule found in marijuana.
CP 55,940 is a THC analogue; that is, its chemical structure is
related to THC. Anandamide and 2-arachidonyl glycerol (2-AG) are
endogenous molecules, meaning they are naturally produced
in the body. Although the chemical structure of WIN 55,212 is
very different from either THC or anandamide, it is also a canabinoid agonist.
tion of anandamide and the enzymes that synthesize it are not yet known. This information will provide essential clues to the natural role of anandamide and an understanding of the brain circuits in which it is a neurotransmitter. The importance of knowing specific brain circuits that involve anandamide (and other endogenous cannabinoid ligands) is that such circuits are the pivotal elements for regulating specific brain functions, such as mood, memory, and cognition. Anandamide has been found in numerous regions of the human brain: hippocampus (and
Page 46
| TABLE 2.3 Comparison of Cannabinoid Receptor Agonists | ||||
| Potency can be measured in a variety of ways, from behavioral to physiological to cellular. This table shows potency in terms of receptor binding, which is the most broadly applicable to the many possible actions of cannabinoids. For example, anandamide binds to the cannabinoid receptor only about half as avidly as does THC. Measures of potency might include effects on activity (behavior) or hypothermia (physiologic). The apparently low potency of 2-AG may, however, be misleading. A study published late in 1998 reports that 2-AG is found with two other closely related compounds that by themselves are biologically inactive; but in the presence of those two compounds, 2-AG is only three times less active than THC.9 Further, 2-AG is much more abundant than anandamide, although the biological significance of this remains to be determined. | ||||
| Receptor Binding in Brain Tissue124 | ||||
| Potency Relative | ||||
| Compound | to D9-THC | |||
| CP 55,940 | 59 | |||
| D9-THC | 1 | |||
| Anandamide | 0.47 | |||
| 2-AG | 0.08 | |||
parahippocampic cortex), striatum, and cerebellum; but it has not been precisely identified with specific neuronal circuits. CB1 receptors are abundant in these regions, and this further implies a physiological role for endogenous cannabinoids in the brain functions controlled by these areas. But substantial concentrations of anandamide are also found in the thalamus, an area of the brain that has relatively few CB1 receptors.124
Anandamide has also been found outside the brain. It has been found in spleen tissue, which also has high concentrations of CB2 receptors, and small amounts have been detected in heart tissue.44
In general, the affinity of anandamide for cannabinoid receptors is only one-fourth to one-half that of THC (see Table 2.3). The differences depend on the cells or tissue that are tested and on the experimental conditions, such as the binding assay used (reviewed by Pertwee124).
The molecular structure of anandamide is relatively simple, and it can be formed from arachidonic acid and ethanolamine. Arachidonic acid is a common precursor of a group of biologically active molecules known as eicosanoids, including prostaglandins.* Although anandamide can be synthesized in a variety of ways, the physiologically relevant pathway
*Eicosanoids all contain a chain of 20 carbon atoms and are named after eikosi, the Greek word for 20.
Page 47
seems to be through enzymatic cleavage of N-arachidonyl-phosphatidylethanolamine (NAPE), which yields anandamide and phosphatidic acid (reviewed by Childers and Breivogel27).
Anandamide can be inactivated in the brain via two mechanisms. In one it is enzymatically cleaved to yield arachidonic acid and ethanolamine-the reverse of what was initially proposed as its primary mode of synthesis. In the other it is inactivated through neuronal uptake—that is, by being transported into the neuron, which prevents its continuing activation of neighboring neurons.
Other Endogenous Agonists
Several other endogenous compounds that are chemically related to anandamide and that bind to cannabinoid receptors have been discovered, one of which is 2-AG.108 2-AG is closely related to anandamide and is even more abundant in the brain. At the time of this writing, all known endogenous cannabinoid receptor agonists (including anandamide) were eicosanoids, which are arachidonic acid metabolites. Arachidonic acid (a free fatty acid) is released via hydrolysis of membrane phospholipids.
Other, noneicosanoid, compounds that bind cannabinoid receptors have recently been isolated from brain tissue, but they have not been identified, and their biological effects are under investigation. This is a fastmoving field of research, and no review over six months old will be fully up to date.
The endogenous compounds that bind to cannabinoid receptors probably perform a broad range of natural functions in the brain. This neural signaling system is rich and complex and has many subtle variations, many of which await discovery. In the next few years much more will probably be known about these naturally occurring cannabinoids.
Some effects of cannabinoid agonists are receptor independent. For example, both THC and CBD can be neuroprotective through their antioxidative activity; that is, they can reduce the toxic forms of oxygen that are released when cells are under stress.54 Other likely examples of receptor-independent cannabinoid activity are modulation of activation of membrane-bound enzymes (such as ATPase), arachidonic acid release, and perturbation of membrane lipids. An important caution in interpreting those reports is that concentrations of THC or CBD used in cellular studies, such as these, are generally much higher than the concentrations of THC or CBD in the body that would likely be achieved by smoking marijuana.
Page 48
| TABLE 2.4 Cellular Processes That Can Be Targeted for Drug Development | ||
| Drug Action | Biological Result | |
| Block synthesis | Synthesis of bioactive compounds is a continuous process and is one means by which concentrations of that compound are regulated. | Weaker signal, due to decreased agonist concentration. |
| Inhibit degradation | Chemical breakdown is one method the body uses to inactivate endogenous substances. | Stronger signal, due to increased agonist concentration. |
| Facilitate neuronal uptake | Neuronal uptake is one of the natural ways in which a receptor agonist is inactivated. | Stronger signal, due to increased amount of time during which agonist is present in the synapse where it can stimulate the receptor. |
| NOTE: Endogenous cannabinoids are part of a cellular signaling system. This table lists categories of natural processes that regulate such systems and shows the results of altering those processes. | ||
Novel Targets for Therapeutic Drugs
Drugs that alter the natural biology of anandamide or other endogenous cannabinoids might have therapeutic uses (Table 2.4). For example, drugs that selectively inhibit neuronal uptake of anandamide would increase the brain's own natural cannabinoids, thereby mimicking some of the effects of THC. A number of important psychotherapeutic drugs act by inhibiting neurotransmitter uptake. For example, antidepressants like fluoxetine (Prozac) inhibit serotonin uptake and are known as selective serotonin reuptake inhibitors, or SSRIs. Another way to alter levels of endogenous cannabinoids would be to develop drugs that act on the enzymes involved in anandamide synthesis. Some antihypertensive drugs work by inhibiting enzymes involved in the synthesis of endogenous hypertensive agents. For example, anti-converting enzyme (ACE) inhibitors are used in hypertensive patients to interfere with the conversion of angiotensin I, which is inactive, to the active hormone, angiotensin II.
Sites Of Action
Cannabinoid receptors are particularly abundant in some areas of the brain. The normal biology and behavior associated with these brain areas
Page 49
| TABLE 2.5 Brain Regions in Which Cannabinoid Receptors Are Abundant | |
| Brain Region | Functions Associated with Region |
| Brain regions in which cannabinoid receptors are abundant | |
| Basal ganglia | Movement control |
| Substantia nigra pars reticulata | |
| Entopeduncular nucleus | |
| Globus pallidus | |
| Putamen | |
| Cerebellum | Body movement coordination |
| Hippocampus | Learning and memory, stress |
| Cerebral cortex, especially cingulate, | Higher cognitive functions |
| frontal, and parietal regions | |
| Nucleus accumbens | Reward center |
| Brain regions in which cannabinoid brain receptors are moderately concentrated | |
| Hypothalamus | Body housekeeping functions (body temperature regulation, salt and water balance, reproductive function) |
| Amygdala | Emotional response, fear |
| Spinal cord | Peripheral sensation, including pain |
| Brain stem | Sleep and arousal, temperature regulation, motor control |
| Central gray | Analgesia |
| Nucleus of the solitary tract | Visceral sensation, nausea and vomiting |
| SOURCES: Based on reviews by Pertwee (1997b)124 and Herkenham (1995).57 | |
are consistent with the behavioral effects produced by cannabinoids (Table 2.5 and Figure 2.5). The highest receptor density is found in cells of the basal ganglia that project locally and to other brain regions. These cells include the substantia nigra pars reticulata, entopeduncular nucleus, and globus pallidus, regions that are generally involved in coordinating body movements. Patients with Parkinson's or Huntington's disease tend to have impaired functions in these regions.
CB1 receptors are also abundant in the putamen, part of the relay system within the basal ganglia that regulates body movements; the cerebellum, which coordinates body movements; the hippocampus, which is involved in learning, memory, and response to stress; and the cerebral cortex, which is concerned with the integration of higher cognitive functions.
CB1 receptors are found on various parts of neurons, including the
Page 50
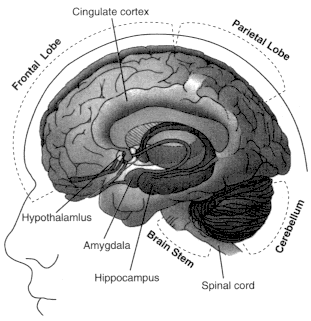
Figure 2.5
Locations of brain regions in which cannabinoid receptors are abundant.
See Table 2.5 for a summary of functions associated with those regions.
axon, cell bodies, terminals, and dendrites.57,165 Dendrites are generally the ''receiving'' part of a neuron, and receptors on axons or cell bodies generally modulate other signals. Axon terminals are the "sending" part of the neuron.
Cannabinoids tend to inhibit neurotransmission, although the results are somewhat variable. In some cases, cannabinoids diminish the effects of the inhibitory neurotransmitter, g-aminobutyric acid (GABA);144 in other cases, cannabinoids can augment the effects of GABA.120 The effect of activating a receptor depends on where it is found on the neuron: if cannabinoid receptors are presynaptic (on the "sending" side of the synapse) and inhibit the release of GABA, cannabinoids would diminish GABA effects; the net effect would be stimulation. However, if cannabinoid receptors are postsynaptic (on the "receiving" side of the synapse) and on the same cell as GABA receptors, they will probably mimic the effects of GABA; in that case, the net effect would be inhibition.120,144,160
CB1 is the predominant brain cannabinoid receptor. CB2 receptors have not generally been found in the brain, but there is one isolated report suggesting some in mouse cerebellum.150 CB2 is found primarily on cells
Page 51
| TABLE 2.6 Cannabinoid Receptors | ||
| CB1 | CB2 | |
| Effects of various cannabinoids | ||
| D9-THC | Agonist | Weak antagonist |
| Anandamide | Agonist | Agonist |
| Cannabinol | Weak agonist | Agonist; greater affinity for CB2 than |
| for CB1 | ||
| Cannabidiol | Does not bind to receptor | Does not bind to receptor |
| Receptor distribution | ||
| Areas of greatest | Brain | Immune system, especially B cells and |
| abundance | natural killer cells |
of the immune system. CB1 receptors are also found in immune cells, but CB2 is considerably more abundant there (Table 2.6) (reviewed by Kaminski80 in 1998).
As can be appreciated in the next section, the presence of cannabinoid systems in key brain regions is strongly tied to the functions and pathology associated with those regions. The clinical value of cannabinoid systems is best understood in the context of the biology of these brain regions.
Cannabinoid Receptors and Brain Functions
Motor Effects
Marijuana affects psychomotor performance in humans. The effects depend both on the nature of the task and the experience with marijuana. In general, effects are clearest in steadiness (body sway and hand steadiness) and in motor tasks that require attention. The results of testing cannabinoids in rodents are much clearer.
Cannabinoids clearly affect movement in rodents, but the effects depend on the dose: low doses stimulate and higher doses inhibit locomotion.111,159 Cannabinoids mainly inhibit the transmission of neural signals, and they inhibit movement through their actions on the basal ganglia and cerebellum, where cannabinoid receptors are particularly abundant (Figure 2.6). Cannabinoid receptors are also found in the neurons that project from the striatum and subthalamic nucleus, which inhibit and stimulate movement, respectively.58,101
Cannabinoids decrease both the inhibitory and stimulatory inputs to the substantia nigra and therefore might provide dual regulation of move-
Page 52
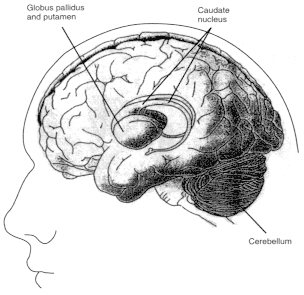
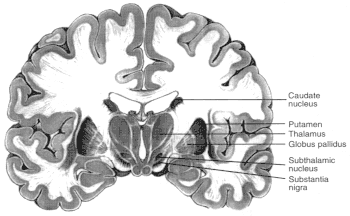
Figure 2.6
Diagrams showing motor regions of the brain. Basal ganglia
are a group of three brain regions, or nuclei —caudate, putamen,
and globus pallidus. Figure 2.6a is a three-dimensional view showing the
location of those nuclei in the brain. Figure 2.6b shows those structures
in a vertical cross-sectional view. The major output pathways of the
basal ganglia arise from the globus pallidus and pars reticulata of the
substantia nigra. Their main target is the thalamus. SOURCE: Figure
2.6a is reprinted from Principles of Neural Science, 2nd ed., 1985 (E.R.
Kandel and J.H. Schwartz, eds.), with permission from the copyright holder,
Appleton and Lange.
Page 53
ment at this nucleus. In the substantia nigra, cannabinoids decrease transmission from both the striatum and the subthalamic nucleus.141 The globus pallidus has been implicated in mediating the cataleptic effects of large doses of cannabinoids in rats.126 (Catalepsy is a condition of diminished responsiveness usually characterized by trancelike states and waxy rigidity of the muscles.) Several other brain regions—the cortex, the cerebellum, and the neural pathway from cortex to striatum—are also involved in the control of movement and contain abundant cannabinoid receptors.52, 59, 101 They are therefore possible additional sites that might underlie the effects of cannabinoids on movement.
Memory Effects
One of the primary effects of marijuana in humans is disruption of short-term memory.68 That is consistent with the abundance of CB1 receptors in the hippocampus, the brain region most closely associated with memory. The effects of THC resemble a temporary hippocampal lesion.63 Deadwyler and colleagues have demonstrated that cannabinoids decrease neuronal activity in the hippocampus and its, inputs.23,24, 83 In vitro, several cannabinoid ligands and endogenous cannabinoids can block the cellular processes associated with memory formation.29,30,116,157,163 Furthermore, cannabinoid agonists inhibit release of several neurotransmitters: acetylcholine from the hippocampus,49- 51 norepinephrine from human and guinea pig (but not rat or mouse) hippocampal slices,143 and glutamate in cultured hippocampal cells.144 Cholinergic and noradrenergic neurons project into the hippocampus, but circuits within the hippocampus are glutamatergic.* Thus, cannabinoids could block transmission both into and within the hippocampus by blocking presynaptic neurotransmitter release.
Pain
After nausea and vomiting, chronic pain was the condition cited most often to the IOM study team as a medical use for marijuana. Recent research presented below has shown intriguing parallels with anecdotal reports of the modulating effects of cannabinoids on pain—both the effects of cannabinoids acting alone and the effects of their interaction with opioids.
*Neurons are often defined by the primary neurotransmitter released at their terminals. Thus, cholinergic neurons release acetylcholine, noradrenergic neurons release noradrenalin (also known as norepinephrine), and glutamergic neurons release glutamate.
Page 54
Behavioral Studies
Cannabinoids reduce reactivity to acute painful stimuli in laboratory animals. In rodents, cannabinoids reduced the responsiveness to pain induced through various stimuli, including thermal, mechanical, and chemical stimuli.12, 19,46, 72, 96,154, 174 Cannabinoids were comparable with opiates in potency and efficacy in these experiments.12,72
Cannabinoids are also effective in rodent models of chronic pain. Herzberg and co-workers found that cannabinoids can block allodynia and hyperalgesia associated with neuropathic pain in rats.62 This is an important advance because chronic pain frequently results in a series of neural changes that increase suffering due to allodynia (pain elicited by stimuli that are normally innocuous), hyperalgesia (abnormally increased reactivity to pain), and spontaneous pain; furthermore, some chronic pain syndromes are not amenable to therapy, even with the most powerful narcotic analgesics.10
Pain perception is controlled mainly by neurotransmitter systems within the central nervous system, and cannabinoids clearly play a role in the control of pain in those systems.45 However, pain-relieving and pain-preventing mechanisms also occur in peripheral tissues, and endogenous cannabinoids appear to play a role in peripheral tissues. Thus, the different cannabinoid receptor subtypes might act synergistically. Experiments in which pain is induced by injecting dilute formalin into a mouse's paw have shown that anandamide and palmitylethanolamide (PEA) can block peripheral pain.22,73 Anandamide acts primarily at the CB1 receptor, whereas PEA has been proposed as a possible CB2 agonist; in short, there might be a biochemical basis for their independent effects. When injected together, the analgesic effect is stronger than that of either alone. That suggests an important strategy for the development of a new class of analgesic drug: a mixture of CB1 and CB2 agonists. Because there are few, if any, CB2 receptors in the brain, it might be possible to develop drugs that enhance the peripheral analgesic effect while minimizing the psychological effects.
Neural Sites of Altered Responsiveness to Painful Stimuli
The brain and spinal cord mediate cannabinoid analgesia. A number of brain areas participate in cannabinoid analgesia and support the role of descending pathways (neural pathways that project from the brain to the spinal cord).103,105 Although more work is needed to produce a comprehensive map of the sites of cannabinoid analgesia, it is clear that the effects are limited to particular areas, most of which have an established role in pain.
Page 55
Specific sites where cannabinoids act to affect pain processing include the periaqueductal gray,104 rostral ventral medulla,105, 110 thalamic nucleus submedius,102 thalamic ventroposterolateral nucleus,102 dorsal horn of the spinal cord,64,65 and peripheral sensory nerves.64,65,66,131 Those nuclei also participate in opiate analgesia. Although similar to opiate analgesia, cannabinoid analgesia is not mediated by opioid receptors; morphine and cannabinoids sometimes act synergistically, and opioid antagonists generally have no effect on cannabinoid-induced analgesia.171 However, a kappa-receptor antagonist has been shown to attenuate spinal, but not supraspinal, cannabinoid analgesia.153,170,171 (Kappa opioid receptors constitute one of the three major types of opioid receptors; the other two types are mu and delta receptors.)
Neurophysiology and Neurochemistry of Cannabinoid Analgesia
Because of the marked effects of cannabinoids on motor function, behavioral studies in animals alone cannot provide sufficient grounds for the conclusion that cannabinoids depress pain perception. Motor behavior is typically used to measure responses to pain, but this behavior is itself affected by cannabinoids. Thus, experimental results include an unmeasured combination of cannabinoid effects on motor and pain systems. The effects on specific neural systems, however, can be measured at the neurophysiological and neurochemical levels. Cannabinoids decrease the response of immediate-early genes (genes that are activated in the early or immediate stage of response to a broad range of cellular stimuli) to noxious stimuli in the spinal cord, decrease response of pain neurons in the spinal cord, and decrease the responsiveness of pain neurons in the ventral posterolateral nucleus of the thalamus.67,102 Those changes are mediated by cannabinoid receptors, are selective for pain neurons, and are unrelated to changes in skin temperature or depth of anesthesia, and they follow the time course of the changes in behavioral responses to painful stimuli but not the time course of motor changes.67 On-cells and off-cells in the rostral ventral medulla control pain transmission at the level of the spinal cord, and cannabinoids also modulate their responses in a manner that is very similar to that of morphine.110
Endogenous Cannabinoids Modulate Pain
Endogenous cannabinoids can modulate pain sensitivity through both central and peripheral mechanisms. For example, animal studies have shown that pain sensitivity can be increased when endogenous cannabinoids are blocked from acting at CB1 receptors.22,62,110,130,158 Administration of cannabinoid antagonists in either the spinal cord130 or paw22 in-
Page 56
crease the sensitivity of animals to pain. In addition, there is evidence that cannabinoids act at the site of injury to reduce peripheral inflammation.131
Current data suggest that the endogenous cannabinoid analgesic system might offer protection against the long-lasting central hyperalgesia and allodynia that sometimes follow skin or nerve injuries.130,158 These results raise the possibility that therapeutic interventions that alter the levels of endogenous cannabinoids might be useful for managing pain in humans.
Chronic Effects of THC
Most substances of abuse produce tolerance, physical dependence, and withdrawal symptoms. Tolerance is the most common response to repetitive use of a drug and is the condition in which, after repeated exposure to a drug, increasing doses are needed to achieve the same effect. Physical dependence develops as a result of a resetting of homeostatic mechanisms in response to repeated drug use. Tolerance, dependence, and withdrawal are not peculiar to drugs of abuse. Many medicines that are not addicting can produce these types of effects; examples of such medications include clonidine, propranolol, and tricyclic antidepressants. The following sections discuss what is known about the biological mechanisms that underlie tolerance, reward, and dependence; clinical studies about those topics are discussed in chapter 3.
Tolerance
Chronic administration of cannabinoids to animals results in tolerance to many of the acute effects of THC, including memory disruption,34 decreased locomotion,2,119 hypothermia,42,125 neuroendocrine effects,134 and analgesia.4 Tolerance also develops to the cardiovascular and psychological effects of THC and marijuana in humans (see also discussion in chapter 3).55,56,76
Tolerance to cannabinoids appears to result from both pharmacokinetic changes (how the drug is absorbed, distributed, metabolized, and excreted) and pharmacodynamic changes (how the drug interacts with target cells). Chronic treatment with the cannabinoid agonist, CP 55,940, increases the activity of the microsomal cytochrome P450 oxidative system,31 the system through which drugs are metabolized in the liver; this suggests pharmacokinetic tolerance. Chronic cannabinoid treatment also produces changes in brain cannabinoid receptors and cannabinoid receptor mRNA concentrations—an indication that pharmacodynamic effects are important as well.
Most studies have found that brain cannabinoid receptor concentra-
Page 57
tions usually decrease after prolonged exposure to agonists,42,119,136,138 although some studies have reported increases137 or no changes2 in receptor binding in brain. Differences among studies could be due to the particular agonist tested, the assay used, the brain region examined, or the treatment time. For example, the THC analogue, levonantradol, produces a greater desensitization of adenylyl cyclase inhibition than does THC in cultured neuroblastoma cells.40 This might be explained by differences in efficacy between these two agonists.18,147 A time course study revealed differences among brain regins in the rates and magnitudes of receptor down regulation.16 Those findings suggest that tolerance to different effects of cannabinoids develops at different rates.
Chronic treatment with THC also produces variable effects on cannabinoid-mediated signal transduction systems. It produces substantial desensitization of cannabinoid-activated G proteins in a number of rat brain regions.147 The time course of this desensitization varies across brain regions.16
It is difficult to extend the findings of short-term animal studies to human marijuana use. To simulate long-term use, higher doses are used in animal studies than are normally achieved by smoking marijuana. For example, the average human will feel "high" after injection of THC at a level of 0.06 mg/kg,118 compared with the 10-20 mg/kg per day used in many chronic rat studies. At the same time, doses of marijuana needed to observe behavioral changes in rats (usually changes in locomotor behavior) are substantially higher than doses at which people feel "high." The pharmacokinetics of THC distribution in the body are also dramatically different between rats and humans and depend heavily on whether it is inhaled, injected, or swallowed. It is likely that some of the same biochemical adaptations to chronic cannabinoid administration occur in laboratory animals and humans, but the magnitude of the effects in humans might be less than that in animals in proportion to the doses used.
Reward and Dependence
Experimental animals that are given the opportunity to self-administer cannabinoids generally do not choose to do so, which has led to the conclusion that they are not reinforcing and rewarding.38 However, behavioral95 and brain stimulation94 studies have shown that THC can be rewarding to animals. The behavioral study used a "place preference" test, in which an animal is given repeated doses of a drug in one place, and is then given a choice between a place where it received the drug and a place where it did not. The animals chose the place where they received the THC. These rewarding effects are highly dose dependent. In all models studied, cannabinoids are only rewarding at midrange; doses that are
Page 58
too low are not rewarding; doses that are too high can be aversive. Mice will self-administer the cannabinoid agonist WIN 55,212-2 but only at low doses.106 This effect is specifically mediated by CB1 receptors and indicates that stimulation of those receptors is rewarding to the mice. Antagonism of cannabinoid receptors is also rewarding in rats; in conditioned place preference tests, animals show a preference for the place they receive the cannabinoid antagonist SR 141716A at both low and high doses.140 Cannabinoids increase dopamine concentrations in the mesolimbic dopamine system of rats, a pathway associated with reinforcement.25,39,161 However, the mechanism by which THC increases dopamine concentrations appears to be different from that of other abused drugs51 (see chapter 3 for further discussion of reinforcement). THC-induced increases in dopamine are due to increases in the firing rate of dopamine cells in the ventral tegmental area by D9-THC.47 However, these increases in firing rate in the ventral tegmental area could not be explained by increases in the firing of the A10 dopamine cell group, where other abused drugs have been shown to act.51
Physical dependence on cannabinoids has been observed only under experimental conditions of "precipitated withdrawal" in which animals are first treated chronically with cannabinoids and then given the CB1 antagonist SR 141716A.3,166 The addition of the antagonist accentuates any withdrawal effect by competing with the agonist at receptor sites; that is, the antagonist helps to clear agonists off and keep them off receptor sites. This suggests that, under normal cannabis use, the long half-life and slow elimination from the body of THC and the residual bioactivity of its metabolite, 11-OH-THC, can prevent substantial abstinence symptoms. The precipitated withdrawal produced by SR 141716A has some of the characteristics of opiate withdrawal, but it is not affected by opioid antagonists, and it affects motor systems differently. An earlier study with monkeys also suggested that abrupt cessation of chronic THC is associated with withdrawal symptoms.8 Monkeys in that study were trained to work for food after which they were given THC on a daily basis; when the investigators stopped administering THC, the animals stopped working for food.
A study in rats indicated that the behavioral cannabinoid withdrawal syndrome is consistent with the consequences of withdrawal from other drugs of abuse in that it correlates with the effects of stimulation of central amygdaloid corticotropin-releasing hormone release.135 However, the withdrawal syndrome for cannabinoids and the corresponding increase in corticotropin-releasing hormone are observed only after administration of the CB1 antagonist SR 141716A to cannabinoid-tolerant animals.3,166 The implications of data based on precipitated withdrawal in animals for human cannabinoid abuse have not been established.166 Furthermore, acute administration of THC also produces increases in corticotropin-
Page 59
releasing hormone and adrenocorticotropin release; both are stress-related hormones.71 This set of withdrawal studies may explain the generally aversive effects of cannabinoids in animals and could indicate that the increase in corticotropin-releasing hormone is merely a rebound effect. Thus, cannabinoids appear to be conforming to some of the neurobiological effects of other drugs abused by humans, but the underlying mechanisms of these actions and their value for determining the reinforcement and dependence liability of cannabinoids in humans remain undetermined.
Cannabinoids and the Immune System
The human body protects itself from invaders, such as bacteria and viruses through the elaborate and dynamic network of organs and cells referred to as the immune system. Cannabinoids, especially THC, can modulate the function of immune cells in various ways-in some cases enhancing and in others diminishing the immune response85 (summarized in Table 2.7). However, the natural function of cannabinoids in the immune system is not known. Immune cells respond to cannabinoids in a variety of ways, depending on such factors as drug concentration, timing of drug delivery to leukocytes in relation to antigen stimulation, and type of cell function. Although the chronic effects of cannabinoids on the immune system have not been studied, based on acute exposure studies in experimental animals it appears that THC concentrations that modulate immunological responses are higher than those required for psychoactivity.
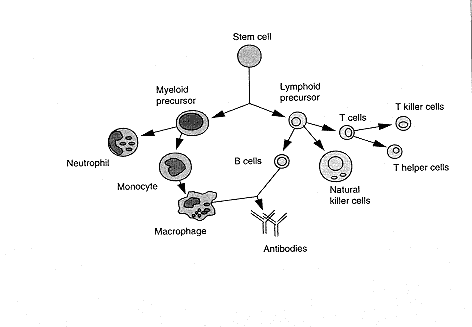
The CB2 receptor gene, which is not expressed in the brain, is particularly abundant in immune tissues, with an expression level 10-100 times higher than that of CB1. In spleen and tonsils the CB2 mRNA* content is equivalent to that of CB1 mRNA in the brain.48 The rank order, from high to low, of CB2 mRNA levels in immune cells is B-cells > natural killer cells >> monocytes > polymorphonuclear neutrophil cells > T8 cells > T4 cells. In tonsils the CB2 receptors appear to be restricted to B-lymphocyteenriched areas. In contrast, CB1 receptors are mainly expressed in the central nervous system and, to a lesser extent, in several peripheral tissues such as adrenal gland, heart, lung, prostate, uterus, ovary, testis, bone marrow, thymus, and tonsils.
*After a gene is transcribed, it is often spliced and modified into mRNA, or message RNA. The CB-2 mRNA is the gene "message" that moves from the cell nucleus into the cytoplasm where it will be translated into the receptor protein.
Page 60
| TABLE 2.7 Effects of Cannabinoids on the Immune System | ||
| Drug Tested | Cell Types Tested or | Drug |
| THC, 2-AG, | Lymphocytes and splenocytes in vitro | 0.1-30 mM |
| THC, 2-AG | Lymphocytes and splenocytes | 0.1-25 mM |
| Anandamide | Splenocytes in vitro | 1-25 mM |
| THC, 11-OH-THC, 2-AG | Splenocytes in vitro | 3-30 mM |
| THC, CP 55,940, | Lymphocytes in vitro | 0.1-100 nM |
| THC | Drug injected into mice | >5 mg/kg |
| HU-210 | Drug injected into mice | >0.05 mg/kg |
| THC, 11-OH-THC, | Splenocytes in vitro | 1-30 mM |
| THC | Drug injected into rodent | 3 mg/kg per day for |
| THC, 11-OH-THC | Natural killer cells in vitro | 0.1-32 mM |
| THC | Peritoneal macrophages and | 3-30 mM |
| monocytes | ||
| THC, CBD | Drug injected into mice; in one case, | >5 mg/kg per day for |
| THC, CBD | Peripheral blood mononuclear cells | <0.1 mM 30 mM |
| THC, CBD | Splenocytes and T cells in vitro | 10 PM |
| THC | Phorbol myristate acetate- | 10-20 mM |
| THC | Endotoxin-activated macrophages | 10-30 mM |
| THC | Peritoneal macrophages in vitro | 10-30 mM |
(Table continued on next page)
Page 61
(Table continued from the previous page)
| TABLE 2.7 Effects of Cannabinoids on the Immune System | ||
| Drug Tested | Result | Reference |
| THC, 2-AG, | Higher doses suppressed T cell proliferation | Luo, 1992; Pross, 1992;'bKlein, 1985;c |
| THC, 2-AG | Lower doses increased T cell proliferation | Luo, 1992; Lee, 1995;a Pross, 1992'b |
| Anandamide | Little or no effect on T cell proliferation | Lee, 1995;a Devane, 1992 |
| THC, 11-OH-THC, 2-AG | Decreased B cell proliferation | Klein, 1985;c Lee, 1995c |
| THC, CP 55,940, | Increased B cell proliferation | Derocq, 1995 |
| THC | Antibody production suppressed | Baczynsky, 1983; Schatz, 1993 |
| HU-210 | Antibody production suppressed | Titishov, 1989 |
| HC, 11-OH-THC, | Antibody production suppressed | Klein, 1990; Baczynsky, 1983; Kaminski, |
| THC | Repeated low doses or a high dose of THC | Patel, 1985; Klein, 1987 |
| THC, 11-OH-THC | Doses of >10 }M suppressed natural killer | Klein, 1987; Luo, 1989 |
| THC | Variable doses of THC suppressed | Lopez-Cepero, 1986; Specter, 1991; |
| THC, CBD | THC suppressed normal immune response; | Cabral, 1986; Blanchard, 1986 |
| THC, CBD | Increased interferon production | Watzl, 1991 |
| Decreased interferon production | ||
| THC, CBD | Both THC and CBD suppressed | Condie, 1996 |
| THC | Increased tumor necrosis factor production | Shivers, 1994 |
| THC | Increased processing and release of | Zhu, 1994 |
| THC | Increased interleukin-1 bioactivity | Klein, 1990 |
(Table continued on next page)
Page 62
| TABLE 2.7 Continued | ||
| Drug Tested | Cell Types Tested or | Drug |
| THC | Drug and sublethal or lethal dose of | 8 mg/kg before and |
| <5 mg/kg doses | ||
| THC | Drug and herpes simplex virus | 100 mg/kg before |
| injected in immunodeficient mice | ||
| aCell density dependent. | ||
| bMitogen dependent. | ||
| cDependent on serum concentration in cell culture medium. | ||
| dDependent on timing of drug exposure relative to mitogen exposure. | ||
| *Drug concentrations are given in the standard format of molarity (M). A 1-M solution is the molecular weight of the compound (in grams) in 1 liter (L) of solution. The molecular | ||
| Box 2.1 Cells of the Immune system |
| The various organs of the immune system are positioned throughout the body and include b one marrow, thymus, lymph nodes, and spleen. the cells of the immune system consist of white blood cells, or leukocytes, which are formed in the bone marrow from stem cells—so called because a great variety of cells descend from them (see below). There are two kinds of leukocytes: lymphocytes and phagocytes. Lymphocytes consist of B cells, T cells (B and T refer to where the cells mature, either in the bone marrow [B] or thymus [T]), and natural killer (NK) cells; the major phagocytes are monocytes, macrophages, and neutrophils. Phagocytes have many important roles in the immune response; most important is that they initiate the response by engulfing and digesting foreign substances, or antigens (such as bacteria, viruses, and foreign proteins), that enter the body. Once digested, the antigens are exposed to specialized lymphocytes, some of which produce antibodies and effector T cells, which help destroy any antigens remaining in the body. Antibodies are proteins produced by B cells that bind to antigens and promote antigen destruction. Effector T cells include killer T cells, which attack and kill antigen laden cells, and helper T cells, which secrete special proteins called cytokines that promote antigen elimination. NK cells are specialized lymphocytes that are also activated by antigen to either kill infected targets or secrete immunoregulatory cytokines. |
Page 63
(Table continued from previous page)
| Drug Tested | Result | Reference |
| THC | Cytokine-mediated septic shock and death occurred with exposure to sublethal dose of bacteria | Klein, 1993, 1994; Newton, 1994 |
| Survival occurred, but with greater susceptiblity to infection when challenged with bacteria and death when challenged with a lethal dose of bacteria | ||
| THC | Two high doses of THC potentiated the effects of herpes simplex and enhanced the progression of death | Specter, 1991 |
| Single dose did not promote death | ||
| weight of THC is 314, so a 1-M solution would be 314 g of THC in 1 L of solution, and a 10-mM solution would be 3.14 mg THC/L. A 1- to 10-mM concentration will generally elicit a physiologically relevant response in immune cell cultures. Higher doses are often suspected of not being biologically meaningful because they are much larger than would ever be achieved in the body. The doses listed in this table are, for the most part, very high. See text for further discussion. | ||
|
|
Page 64
Cannabinoid Receptors and Intracellular Action in Immune Cells
CB2 appears to be the predominant gene expressed in resting leukocytes.78,112 The level of CB1 gene activity is normally low in resting cells but increases with cell activation.32 Thus the CB1 receptor might be important only when immune responses are stimulated, but the physiological relevance of this observation remains to be determined. Some of the cannabinoid effects observed in immune systems, especially at high drug concentrations, are likely mediated through nonreceptor mechanisms, but these have not yet been identified.4
Ligand binding to either CB1 or CB2 inhibits adenylate cyclase, an enzyme that is responsible for cAMP production and is, thus, an integral aspect of intracellular signal transduction (see Figure 2.3).53,79,91,122,139,151,167Increases in intracellular cAMP concentrations lead to immune enhancement, and decreases lead to an inhibition of the immune response.77 Cannabinoids inhibit the rise in intracellular cAMP that normally results from leukocyte activation, and this might be the pathway through which cannabinoids suppress immune cell functions.28,74,167 In addition, cannabinoids activate other molecular pathways such as the nuclear factor-kB pathway, and therefore these signals might be modified in drug-treated immune cells.33,74
T and B Cells
When stimulated by antigen, lymphocytes (see Box 2.1) first proliferate and then mature or differentiate to become potent effector cells, such as B cells that release antibodies or T cells that release cytokines. The normal T-cell proliferation that is seen when human lymphocytes and mouse splenocytes (spleen cells) are exposed to antigens and mitogens* can be inhibited by THC, 11-OH-THC, cannabinol, and 2-AG, as well as synthetic cannabinoid agonists such as CP 55,940; WIN 55,212-2; and HU210.61,89,93,99,127,155 In contrast, one study testing anandamide revealed little or no effect on T cell proliferation.93
However, these drug effects are variable and depend on experimental conditions, such as the experimental drug dose used, the mitogen used, the percentage of serum in the culture, and the timing of cannabinoid drug exposure. In general, lower doses of cannabinoids increase proliferation and higher doses suppress proliferation. Doses that are effective in suppressing immune function are typically greater than 10 mM in cell culture studies and greater than 5 mg/kg in whole-animal studies.85 By
*Mitogens are substances that stimulate cell division (mitosis) and cell transformation.
Page 65
comparison, at 0.05 mg/kg, people will experience the full psychoactive effects of THC; however, because of their high metabolic rates, small rodents frequently require drug doses that are 100-fold higher than doses needed for humans to achieve comparable drug effects. Thus, the immune effects of doses of cannabinoids higher than those ever experienced by humans should be interpreted with caution.93
As with T cells, B cell proliferation can be suppressed by various cannabinoids, such as THC, 11-OH-THC, and 2-AG, but B cell proliferation is more inhibited at lower drug concentrations than T cell proliferation.89,93 Conversely, low doses of THC, CP 55,940 and WIN 55,212-2 increase B cell proliferation in cultured human cells exposed to mitogen.35 This effect possibly involves the CB2 receptor, because the effect appears to be the same when the CB1 receptor was blocked by the antagonist SR 141716A (which does not block the CB2 receptor). The reason for the differences in B cell responsiveness to cannabinoids is probably due to differences in cell type and source; for example, B cells collected from mouse spleen might respond to cannabinoids somewhat differently than B cells from human tonsils.
Natural Killer Cells
Repeated injections of relatively low doses of THC (3 mg/kg/day121*) or two injections of a high dose (40 mg/kg86) suppress the ability of NK cells to destroy foreign cells in rats and mice. THC can also suppress cytolytic activity of the NK cells in cell cultures; 11-OH-THC is even more potent.86 In contrast, THC concentrations below 10 mM had no effect on NK cell activity in mouse cell cultures.98
Macrophages
Macrophages perform various functions, including phagocytosis (ingestion and destruction of foreign substances), cytolysis, antigen presentation to lymphocytes, and production of active proteins involved in destroying microorganisms, tissue repair, and modulation of immune cells. Those functions can be suppressed by THC doses similar to those capable of modulating lymphocyte functions (see above).88,109
*While 3 mg/kg would be a high dose for humans (see Table 3.1), in rodents, it is a low dose for immunological effects and a moderate dose for behavioral effects.
Page 66
Cytokines
Cytokines are proteins produced by immune cells. When released from the producing cell, they can alter the function of other cells they come in contact with. In a sense they are like hormones. Thus, cannabinoids can either increase or decrease cytokine production depending upon experimental conditions.
Some cytokines, such as interferon-y and interleukin-2 (IL-2), are produced by T helper-1 (Thl) cells. These cytokines help to activate cell-mediated immunity and the killer cells that eliminate microorganisms from the body (see Box 2.1). When injected into mice, THC suppresses the production of those cytokines that modulate the host response to infection (see below).115 Cannabinoids also modulate interferons induced by viral infection,21 as well as other interferon inducers.85 Furthermore, in human cell cultures, interferon production can be increased by low concentrations but decreased by high concentrations of either THC or CBD.168 In addition to Th1 cytokines, cannabinoids modulate the production of cytokines such as interleukin-1 (IL-1), tumor necrosis factor (TNF), and interleukin-6 (IL-6).145,176 At 8 mg/kg, THC can increase the in vivo mobilization of serum acute-phase cytokines, including IL-1, TNF, and IL-6.90 Finally, although these studies suggest that cannabinoids can induce an increase in cytokines, other studies suggest that they can also suppress cytokine production.85 The different results might be due to different cell culture conditions or because different cell lines were studied.
Antibody Production
Antibody production is an important measure of humoral immune function as contrasted with cellular (cell-mediated) immunity. Antibody production can be suppressed in mice injected with relatively low doses of THC (>5 mg/kg) or HU-210 (>0.05 mg/kg) and in mouse spleen cell cultures exposed to a variety of cannabinoids, including THC, 11-OHTHC, cannabinol, cannabidiol, CP 55,940, or HU-210.5,6,61,78,79,84,85,142,164 However, the inhibition of antibody response by cannabinoids was only observed when antibody-forming cells were exposed to T-cell-dependent antigens (the responses require functional T cells and macrophages as accessory cells). Conversely, antibody responses to several T-cell-independent antigens were not inhibited by THC; this suggests that B cells are relatively insensitive to inhibition by cannabinoids.142
Resistance to Infection in Animals Exposed to Cannabinoids
Evaluation of bacterial infections in mice that received injections of
Page 67
THC can suppress resistance to infection, although the effect depends on the dose and timing of drug administration. Mice pretreated with THC (8 mg/kg) one day before infection with a sublethal dose of the pneumoniacausing bacteria Legionella pneumophilia and then treated again one day after the infection with THC developed symptoms of cytokine-mediated septic shock and died; control mice that were not pretreated with THC became immune to repeated infection and survived the bacterial challenge.90 If only one injection of THC was given or doses less than 5 mg/kg were used, all the mice survived the initial infection but failed to survive later challenge with a lethal dose of the bacteria; hence, these mice failed to develop immune memory in response to the initial sublethal infection.87 Note that these are very high doses and are considerably higher than doses experienced by marijuana users (see Figure 3.1).115 In rats, doses of 4.0 mg/kg THC are aversive.95
Few studies have been done to evaluate the effect of THC on viral infections, and this subject needs further study.20 Compared to healthy animals, THC might have greater immunosuppressive effects in animals whose immune systems are severely weakened. For example, a very high dose of THC (100 mg/kg) given two days before and after herpes simplex virus infection was shown to be a cofactor with the virus in advancing the progression to death in an immunodeficient mouse model infected with a leukemia virus.85 However, THC given as a single dose (100 mg/kg) two days before herpes simplex virus infection did not promote the progression to death. Hence, whether THC is immunosuppressive probably depends on the timing of THC exposure relative to an infection.
Antiinflammatory Effects
As discussed above, cannabinoid drugs can modulate the production of cytokines, which are central to inflammatory processes in the body. In addition, several studies have shown directly that cannabinoids can be antiinflammatory. For example, in rats with autoimmune encephalomyelitis (an experimental model used to study multiple sclerosis), cannabinoids were shown to attenuate the signs and the symptoms of central nervous system damage.100, 172 (Some believe that nerve damage associated with multiple sclerosis is caused by an inflammatory reaction.) Likewise, the cannabinoid, HU-211, was shown to suppress brain inflammation that resulted from closed-head injury146 or infectious meningitis7 in studies on rats. HU-211 is a synthetic cannabinoid that does not bind to cannabinoid receptors and is not psychoactive;7 thus, without direct evidence, the effects of marijuana cannot be assumed to include those of HU211. CT-3, another atypical cannabinoid, suppresses acute and chronic joint inflammation in animals.178 It is a nonpsychoactive synthetic deriva-
Page 68
tive of 11-THC-oic acid (a breakdown product of THC) and does not appear to bind to cannabinoid receptors.129 Cannabichromene, a cannabinoid found in marijuana, has also been reported to have antiinflammatory properties.173 No mechanism of action for possible antiinflammatory effects of cannabinoids has been identified, and the effects of these atypical cannabinoids and effects of marijuana are not yet established.
It is interesting to note that two reports of cannabinoid-induced analgesia are based on the ability of the endogenous cannabinoids, anandamide and PEA, to reduce pain associated with local inflammation that was experimentally induced by subcutaneous injections of dilute formalin.22,73 Both THC and anandamide can increase serum levels of ACTH and corticosterone in animals.169 Those hormones are involved in regulating many responses in the body, including those to inflammation. The possible link between experimental cannabinoid-induced analgesia and reported antiinflammatory effects of cannabinoids is important for potential therapeutic uses of cannabinoid drugs but has not yet been established.
Conclusions Regarding Effects on the Immune System
Cell culture and animal studies have established cannabinoids as immunomodulators—that is, they increase some immune responses and decrease others. The variable responses depend on such experimental factors as drug dose, timing of delivery, and type of immune cell examined. Cannabinoids affect multiple cellular targets in the immune system and a variety of effector functions. Many of the effects noted above appear to occur at concentrations over 5 mM in vitro and over 5 mg/kg in vivo.* By comparison, a 5-mg injection of THC into a person (about 0.06 mg/kg) is enough to produce strong psychoactive effects. It should be emphasized, however, that little is known about the immune effects of chronic lowdose exposure to cannabinoids.
Another issue in need of further clarification involves the potential usefulness of cannabinoids as therapeutic agents in inflammatory diseases. Glucocorticoids have historically been used for these diseases, but nonpsychotropic cannabinoids potentially have fewer side effects and might thus offer an improvement over glucocorticoids in treating inflammatory diseases.
*In vitro studies are those in which animal cells or tissue are removed and studied outside the animal; in vivo studies are those in which experiments are conducted in the whole animal.
Page 69
| TABLE 2.8 Historical Comparisons Between Cannabinoids and Opiates | ||
| Pharmacological | Cannabinoids | Opiates |
| Discovery of receptor existence | 1988 (Devane et al. and Dill and Howlett )36,40 | 1973(Pert and Snyder, Simon, and Terenius )123,149,162 |
| Identification of receptor antagonist | 1994SR 141716A (Rinaldi-Carmona et al.)132 | Before 1973: naloxone |
| Discovery of first endogenous ligand | 1992anandamide (Devane et al.)37 | 1975met- and leu-enkephalin (Hughes et al.)70 |
| First receptor cloned | 1990(Matsuda et al.)107 | 1992(Evans et al. and Kieffer et al.)41,82 |
| Natural functions | Unknown | Pain, reproduction, mood, movement, and others |
Conclusions and Recommendations
Given the progress of the past 15 years in understanding the effects of cannabinoids, research in the next decade is likely to reveal even more. It is interesting to compare how little we know about cannabinoids with how much we know about opiates. Table 2.8 suggests good reason for optimism about the future of cannabinoid drug development. Now that many of the basic tools of cannabinoid pharmacology and biology have been developed, one can expect to see rapid advances that can begin to match what is known of opiate systems in the brain.
Despite the tremendous progress in understanding the pharmacology and neurobiology of brain cannabinoid systems, this field is still in its early developmental stages. A key focus for future study is the neurobiology of endogenous cannabinoids; establishing the precise brain localization (in which cells and where) of cannabinoids, cellular storage and release mechanisms, and uptake mechanisms will be crucial in determining the biological role of this system. Technology needed to establish the biological significance of these systems will be broad based and include such research tools as the transgenic or gene knockout mice, as has already been accomplished for various opioid-receptor types.26 In 1997, both CB1 and CB2 knockout mice were generated by a team of scientists at the National Institutes of Health, and a group in France has developed another strain of CB1 knockout mice.92
Page 70
Several research tools will greatly aid such investigations, in particular a greater selection of agonists and antagonists that permit discrimination in activation between CB1 and CB2 and hydrophilic agonists that can be delivered to animals or cells more effectively than hydrophobic compounds. In the area of drug development, future progress should continue to provide more specific agonists and antagonists for CB1 and CB2 receptors, with varying potential for therapeutic uses.
There are certain areas that will provide keys to a better understanding of the potential therapeutic value of cannabinoids. For example, basic biology indicates a role for cannabinoids in pain and control of movement, which is consistent with a possible therapeutic role in these areas. The evidence is relatively strong for the treatment of pain and, intriguing although less well established, for movement disorders. The neuroprotective properties of cannabinoids might prove therapeutically useful, although it should be noted that this is a new area and other, better studied, neuroprotective drugs have not yet been shown to be therapeutically useful. Cannabinoid research is clearly relevant not only to drug abuse but also to understanding basic human biology. Further, it offers the potential for the discovery and development of new therapeutically useful drugs.
CONCLUSION: At this point, our knowledge about the biology of marijuana and cannabinoids allows us to make some general conclusions:
· Cannabinoids likely have a natural role in pain modulation, control of movement, and memory.
· The natural role of cannabinoids in immune systems is likely multi-faceted and remains unclear.
· The brain develops tolerance to cannabinoids.
· Animal research has demonstrated the potential for dependence, but this potential is observed under a narrower range of conditions than with benzodiazepines, opiates, cocaine, or nicotine.
· Withdrawal symptoms can be observed in animals but appear mild compared with those of withdrawal from opiates or benzodiazepines, such as diazepam (Valium).
CONCLUSION: The different cannabinoid receptor types found in the body appear to play different roles in normal physiology. In addition, some effects of cannabinoids appear to be independent of those receptors. The variety of mechanisms through which cannabinoids can influence human physiology underlies the variety
Page 71
of potential therapeutic uses for drugs that might act selectively on different cannabinoid systems.
RECOMMENDATION: Research should continue into the physiological effects of synthetic and plant-derived cannabinoids and the natural function of cannabinoids found in the body. Because different cannabinoids appear to have different effects, cannabinoid research should include, but not be restricted to, effects attributable to THC alone.
This chapter has summarized recent progress in understanding the basic biology of cannabinoids and provides a foundation for the next two chapters which review studies on the potential health risks (chapter 3) and benefits of marijuana use (chapter 4).
References
1. Abood ME, Martin BR. 1996. Molecular neurobiology of the cannabinoid receptor. International Review of Neurobiology 39:197-221.
2. Abood ME, Sauss C, Fan F, Tilton CL, Martin BR. 1993. Development of behavioral tolerance to delta 9-THC without alteration of cannabinoid receptor binding or mRNA levels in whole brain. Pharmacology, Biochemistry and Behavior 46:575-579.
3. Aceto MD, Scates SM, Lowe JA, Martin BR. 1995. Cannabinoid precipitated withdrawal by the selective cannabinoid receptor antagonist, SR 141716A. European Journal of Pharmacology 282:R1-R2.
4. Adams IB, Martin BR. 1996. Cannabis: Pharmacology and toxicology in animals and humans. Addiction 91:1585-1614.
5. Baczynsky WO, Zimmerman AM. 1983a. Effects of delta-9-tetrahydrocannabinol, cannabinol, and cannabidiol on the immune system in mice: I. In vivo investigation of the primary and secondary immune response. Pharmocology 26:1-11.
6. Baczynsky WO, Zimmerman AM. 1983b. Effects of delta 9-tetrahydrocannabinol, cannabinol and cannabidiol on the immune system in mice. II. In vitro investigation using cultured mouse splenocytes. Pharmacology 26:12-19.
7. Bass R, Engelhard D, Trembovler V, Shohami E. 1996. A novel nonpsychotropic cannabinoid, HU-211, in the treatment of experimental pneumococcal meningitis. Journal of Infectious Diseases 173:735-738.
8. Beardsley PM, Balster RL, Harris LS. 1986. Dependence on tetrahydrocannabinol in rhesus monkeys. Journal of Pharmacology and Experimental Therapeutics 239:311-319.
9. Ben-Shabat S, Fride E, Sheskin T, Tamiri T, Rhee MH, Vogel Z, Bisogno T, De Petrocellis L, Di Marzo V, Mechoulam R. 1998. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. European Journal of Pharmacology 353:23-31.
10. Bennett GJ. 1994. Neuropathic pain. In: Wall PD, Melzack R, Editors, Textbook of Pain. Edinburgh: Churchill Livingstone.
11. Bird KD, Boleyn T, Chesher GB, Jackson DM, Starmer GA, Teo RKC. 1980. Intercannabinoid and cannabinoid-ethanol interactions and their effects on human performance. Psychopharmacology 71:181-188.
Page 72
12. Bloom AS, Dewey WL, Harris LS, Brosius KK. 1977. 9-nor-9b-hydroxyhexahydrocannabinol, a cannabinoid with potent antinociceptive activity: Comparisons with morphine. Journal of Pharmacology and Experimental Therapeutics 200:263-270.
13. Bornheim LM, Everhart ET, Li J, Correia MA. 1994. Induction and genetic regulation of mouse hepatic cytochrome P450 by cannabidiol. Biochemical Pharmacology (England) 48:161-171.
14. Bomheim LM, Kim KY, Chen B, Correia MA. 1993. The effect of cannabidiol on mouse hepatic microsomal cytochrome P450-dependent anandamide metabolism. Biochenmical and Biophysical Research Communications (United States) 197:740-746.
15. Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ. 1995. Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metabolism and Disposition (United States) 23:825-831.
16. Breivogel CS, Sim LJ, Childers SR. 1997. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. Journal of Pharmacology and Experimental Therapeutics 282:1632-1642.
17. British Medical Association. 1997. Therapeutic Uses of Cannabis. Amsterdam, The Netherlands: Harwood Academic Publishers.
18. Burkey TH, Quock RM, Consroe P, Roeske WR, Yamamura HI. 1997. Delta-9-tetrahydrocannabinol is a partial agonist of cannabinoid receptors in mouse brain. European Journal of Pharmacology 323:R3-R4.
19. Buxbaum DM. 1972. Analgesic activity of A9-tetrahydrocannabinol in the rat and mouse. Psychopharmacology 25:275-280.
20. Cabral GA, Dove Pettit DA. 1998. Drugs and immunity: Cannabinoids and their role in decreased resistance to infectious disease. Journal of Neu roimmu n ology 83:116-123.
21. Cabral GA, Lockmuller JC, Mishkin EM. 1986. Delta-9-tetrahydrocannabinol decreases alpha/beta interferon response to herpes simplex virus type 2 in the B6C3F1 mouse. Proceedings of the Societyfor Experimental Biology and Medicine 181:305-311.
22. Calignano A, La Rana G, Giuffrida A, Piomelli D. 1998. Control of pain initiation by endogenous cannabinoids. Nature 394:277-281.
23. Campbell KA, Foster TC, Hampson RE, Deadwyler SA. 1986a. Delta-9-tetrahydrocannabinol differentially affects sensory-evoked potentials in the rat dentate gyrus. Journal of Pharmacology and Experimental Therapeutics 239:936-940.
24. Campbell KA, Foster TC, Hapson RE, Deadwyler SA. 1986b. Effects of delta-9-tetrahydrocannabinol on sensory-evoked discharges of granule cells in the dentate gyrus of behaving rats. Journal of Pharmacology and Experimental Therapeutics 239:941945.
25. Chen J, Marmur R, Pulles A, Paredes W, Gardner EL. 1993. Ventral tegmental microinjection of delta-9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: Evidence for local neural action by marijuana's psychoactive ingredient. Brain Research 621:65-70.
26. Childers SR. 1997. Opioid receptors: Pinning down the opiate targets. Current Biology 7:R695-R697.
27. Childers SR, Breivogel CS. 1998. Cannabis and endogenous cannabinoid systems. Drug and Alcohol Dependence 51:173-187.
28. Coffey RG, Yamamoto Y, Shella E, Pross S. 1996. Tetrahydrocannabinol inhibition of macrophage nitric oxide production. Biochemical Pharmacology 52:743-751.
29. Collins DR, Pertwee RG, Davies SN. 1994. The action of synthetic cannabinoids on the induction of long-term potentiation in the rat hippocampal slice. European Journal of Pharmacology 259:R7-R8.
Page 73
30. Collins DR, Pertwee RG, Davies SN. 1995. Prevention by the cannabinoid antagonist, SR141716A, of cannabinoid-mediated blockade of long-term potentiation in the rat hippocampal slice. British Journal of Pharmacology 115:869-870.
31. Costa B, Parolaro D, Colleoni M. 1996. Chronic cannabinoid, CP-55,940, administration alters biotransformation in the rat. European Journal of Pharmacology 313:17-24.
32. Daaka Y, Friedman H, Klein T. 1996. Cannabinoid receptor proteins are increased in Jurkat, human T-cell line after mitogen activation. Journal of Pharmacology and Experimental Therapeutics 276:776-783.
33. Daaka Y, Zhu W, Friedman H, Klein T. 1997. Induction of interleukin-2 receptor o gene by A9-tetrahydrocannabinol is mediated by nuclear factor KB and CBi cannabinoid receptor. DNA and Cell Biology 16:301-309.
34. Deadwyler SA, Heyser CJ, Hampson RE. 1995. Complete adaptation to the memory disruptive effects of delta-9-THC following 35 days of exposure. Neuroscience Research Communications 17:9-18.
35. Derocq JM, Segui M, Marchand J, Le Fur G, Casellas P. 1995. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Letters 369:177-182.
36. Devane WA, Dysarc FA, Johnson MR, Melvin LS, Howlett AC. 1988. Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmlacology 34:605-613.
37. Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffing F, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. 1992. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946-1949.
38. Dewey WL. 1986. Cannabinoid pharamacology. Pharmacology Review 38:151-178.
39. Di Chiara G, Imperato A. 1988. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences, USA 85:5274-5278.
40. Dill JA, Howlett AC. 1988. Regulation of adenylate cyclase by chronic exposure to cannabimimetic drugs. Journal of Pharmacology and Experimental Therapeutics 244:11571163.
41. Evans DJ, Keith DEJ, Morrison H, Magendzo K, Edwards RH. 1992. Cloning of a delta opioid receptor by functional expression. Science 258:1952-1955.
42. Fan F, Tao Q, Abood ME, Martin BR. 1996. Cannabinoid receptor down-regulation without alteration of the inhibitory effect of CP 55,940 on adenylyl cyclase in the cerebellum of CP 55,940-tolerant mice. Brain Research 706:13-20.
43. Felder CC, Glass M. 1998. Cannabinoid receptors and their endogenous agonists. Ann ual Reviews of Pharmacology and Toxicology 38:179-200.
44. Felder CC, Nielsen A, Briley EM, Palkovits M, Priller J, Axelrod J, Nguyen DN, Richardson JM, Riggin RM, Koppel GA, Paul SM, Becker GW. 1996. Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Letters 393:231-235.
45. Fields HL. 1987. Pain. New York: McGraw-Hill.
46. Formukong EA, Evans AT, Evans FJ. 1988. Analgesic and antiinflammatory activity of constituents of Cannabis sativa L. Inflammation 12:361-371.
47. French ED. 1997. Delta-9-tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neuroscience Letters 226:159-162.
48. Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. 1995. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European Journal of Biochemistry 232:54-61.
Page 74
49. Gessa GL, Mascia MS, Casu MA, Carta G. 1997. Inhibition of hippocampal acetylcholine release by cannabinoids: Reversal by SR 141716A. European Journal of Pharmacology 327:R1-R2.
50. Gifford AN, Ashby Jr CR. 1996. Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid antagonist, SR 141716A. Journal of Pharmacology and Experimental Therapeutics 277:1431-1436.
51. Gifford AN, Gardner EL, Ashby CRJ. 1997. The effect of intravenous administration of delta-9-tetrahydrocannabinol on the activity of A10 dopamine neurons recorded in vivo in anesthetized rats. Neuropsychobiology 36:96-99.
52. Glass M, Dragunow M, Faull RLM. 1997. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77:299-318.
53. Hadden JW, Hadden EM, Haddox MK, Goldberg ND. 1972. Guanosine 3':5'-cyclic monophosphates: A possible intracellular mediator of mitogenic influences in lymphocytes. Proceedings of the National Academy of Sciences, USA 69:3024-3027.
54. Hampson AJ, Grimaldi M, Axelrod J, Wink D. 1998. Cannabidiol and (-)delta-9-tetrahydrocannabinol are neuroprotective antioxidants. Proceedings of the National Academy of Sciences, USA 95:8268-8273.
55. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. 1999. Abstinence symptoms following oral THC administration to humans. Psychopharmacology 141:385-394.
56. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. 1999. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology 141:395-404.
57. Herkenham M. 1995. Localization of cannabinoid receptors in brain and periphery. In: Pertwee RG, Editor, Cannabinoid Receptors. New York: Academic Press. Pp. 145166.
58. Herkenham M, Lynn AB, de Costa BR, Richfield EK. 1991a. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Research 547:267-274.
59. Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. 1991b. Characterization and localization of cannabinoid receptors in rat brain: A quantative in vitro autoradiographic study. Journal of Neuroscience 11:563-583.
60. Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. 1990. Cannabinoid receptor localization in the brain. Proceedings of the National Academy of Sciences, USA 87:1932-1936.
61. Herring AC, Koh WS, Kaminski NE. 1998. Inhibition of the cyclic AMP signaling cascade and nuclear factor binding to CRE and kappa B elements by cannabinol, a minimally CNS-active cannabinoid. Biochemical Pharmacology 55:1013-1023.
62. Herzberg U, Eliav E, Bennett GJ, Kopin IJ. 1997. The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neuroscience Letters 221:157-160.
63. Heyser CJ, Hampson RE, Deadwyler SA. 1993. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: Alterations in short-term memory associated with changes in task-specific firing of hippocampal cells. Journal of Pharmacology and Experimental Therapeutics 264:294-307.
64. Hohmann AG, Briley EM, Herkenham M. 1999. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Research 822:17-25.
65. Hohmann AG, Herkenham M. 1998. Regulation of cannabinoid and mu opioid receptor binding sites following neonatal capsaicin treatment. Neuroscience Letters 252:1316.
Page 75
66. Hohmann AG, Herkenham M. 1999. Localization of central cannabinoid CBI receptor mRNA in neuronal subpopulations of rat dorsal root ganglia: A double-label in situ hybridization study. Neuroscience 90:923-931.
67. Hohmann AG, Martin WJ, Tsou K, Walker JM. 1995. Inhibition of noxious stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid WIN 55,212-2. Life Sciences 56:2111-2119.
68. Hollister LE. 1986. Health aspects of cannabis. Pharmacological Reviews 38:1-20.
69. Hollister LE, Gillespie BA. 1975. Interactions in man of delta-9-THC. II. Cannabinol and cannabidiol. Clinical Pharmacology and Therapeutics 18:80-83.
70. Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. 1975. Identification of two related pentapeptides from the brain with potent opiate agonists activity. Nature 258:577-580.
71. Jackson AL, Murphy LL. 1997. Role of the hypothalamic-pituitary-adrenal axis in the suppression of luteinizing hormone release by delta-9-tetrahydrocannabinol. Neuroendocrinology 65:446-452.
72. Jacob J, Ramabadran K, Campos-Medeiros M. 1981. A pharmacological analysis of levonantradol antinociception in mice. Journal of Clinical Pharmacology 21:327S-333S.
73. Jaggar SI, Hasnie FS, Sellaturay S, Rice AS. 1998. The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain. Pain 76:189-199.
74. Jeon YJ, Yang K-H, Pulaski JT, Kaminski NE. 1996. Attenuation of inducible nitric oxide synthase gene expression by delta-9-tetrahydrocannabinol is mediated through the inhibition of nuclear factor-kB/Rel activation. Molecular Pharmacology 50:334-341.
75. Johnson MR, Melvin LS. 1986. The discovery of non-classical cannabinoid analgesics. In: Mechoulam R, Editor, Cannabinoids as Therapeutic Agents. Boca Raton, FL: CRC Press, Inc. Pp. 121-145.
76. Jones RT, Benowitz NL, Herning RI. 1981. Clinical relevance of cannabis tolerance and dependence. Journal of Clinical Pharmacology 21:143S-152S.
77. Kaminski NE. 1996. Immune regulation by cannabinoid compounds through the inhibition of the cyclic AMP signaling cascade and altered gene expression. Biochemical Pharmacology 52:1133-1140.
78. Kaminski NE, Abood ME, Kessler FK, Martin BR, Schatz AR. 1992. Identification of a functionally relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune modulation. Molecular Pharmacology 42:736-742.
79. Kaminski NE, Koh WS, Yang KH, Lee M, Kessler FK. 1994. Suppression of the humoral immune response by cannabinoids is partially mediated through inhibition of adenylate cyclase by a pertussis toxin-sensitive G-protein coupled mechanism. Biochemical Pharmacology 48:1899-1908.
80. Kaminski NE. 1998. Regulation of cAMP cascade, gene expression and immune function by cannabinoid receptors. Journal of Neuroimmunology 83:124-132.
81. Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. 1975. Cannabidiol interferes with the effects of delta-9-tetrahydrocannbinol in man. European Journal of Pharmacology 28:172-177.
82. Kieffer BL, Befort K, Gaveriaux-Ruff C, Hirth CG. 1992. The delta-opioid receptor: Isolation of a cDNA by expression cloning and pharmacological characterization. Proceedings of the National Academy of Sciences, USA 89:12048-12052.
83. Kirby MT, Hampson RE, Deadwyler SA. 1995. Cannabinoids selectively decrease paired-pulse perforant path synaptic potentials in the dentate gyrus in vitro. Brain Research 688:114-120.
Page 76
84. Klein TW, Friedman H. 1990. Modulation of murine immune cell function by marijuana components. In: Watson R, Editor, Drugs of Abuse and Immune Function. Boca Raton, FL: CRC Press.
85. Klein TW, Friedman H, Specter SC. 1998. Marijuana, immunity and infection. Journal of Neuroimmunology 83:102-115.
86. Klein TW, Newton C, Friedman H. 1987. Inhibition of natural killer cell function by marijuana components. Journal of Toxicology and Environmental Health 20:321-332.
87. Klein TW, Newton C, Friedman H. 1994. Resistance to Legionella pneumophila suppressed by the marijuana component, tetrahydrocannabinol. Journal of Infectious Diseases 169:1177-1179.
88. Klein TW, Newton C, Friedman H. 1998. Cannabinoid receptors and immunity. Immunology Today 19:373-381.
89. Klein TW, Newton C, Widen R, Friedman H. 1985. The effect of delta-9-tetrahydrocannabinol and 11-hydroxy-delta-9-tetrahydrocannabinol on T-lymphocyte and Blymphocyte mitogen responses. Journal of Immunopharmacology 7:451-466.
90. Klein TW, Newton C, Widen R, Friedman H. 1993. Delta-9-tetrahydrocannabinol injection induces cytokine-mediated mortality of mice infected with Legionella pneumophila. Journal of Pharmacology and Experimental Therapeutics 267:635-640.
91. Koh WS, Yang KH, Kaminski NE. 1995. Cyclic AMP is an essential factor in immune responses. Biochemical and Biophysical Research Communications 206:703-709.
92. Ledent C, Valverde 0, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. 1999. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283:401404.
93. Lee M, Yang KH, Kaminski NE. 1995. Effects of putative cannabinoid receptor ligands, anandamide and 2-arachidonyl-glycerol, on immune function in B6C3F1 mouse splenocytes. Journal of Pharmacology and Experimental Therapeutics 275:529-536.
94. Lepore M, Liu X, Savage V, Matalon D, Gardner EL. 1996. Genetic differences in delta 9-tetrahydrocannabinol-induced facilitation of brain stimulation reward as measured by a rate-frequency curve-shift electrical brain stimulation paradigm in three different rat strains. Life Sciences 58:365-372.
95. Lepore M, Vorel SR, Lowinson J, Gardner EL. 1995. Conditioned place preference induced by delta 9-tetrahydrocannabinol: Comparison with cocaine, morphine, and food reward. Life Sciences 56:2073-2080.
96. Lichtman AH, Martin BR. 1991a. Spinal and supraspinal components of cannabinoidinduced antinociception. Journal of Pharmacology and Experimental Therapeutics 258:517523.
97. Little PJ, Compton DR, Mechoulam R, Martin BR. 1989. Stereochemical effects of 11OH-delta-8-THC-dimethylheptyl in mice and dogs. Pharmacology, Biochemistry Behavior 32:661-666.
98. Lu F, Ou DW. 1989. Cocaine or delta-9-tetrahydrocannabinol does not affect cellular cytotoxicity in vitro. International Journal of Pharmacology 11:849-852.
99. Luo YD, Patel MK, Wiederhold MD, Ou DW. 1992. Effects of cannabinoids and cocaine on the mitogen-induced transformations of lymphocytes of human and mouse origins. International Journal of Immunopharmacology 14:49-56.
100. Lyman WD, Sonett JR, Brosnan CFER, Bornstein MB. 1989. Delta 9-tetrahydrocannabinol: A novel treatment for experimental autoimmune encephalomyelitis. Journal of Neuroimmunology 23:73-81.
101. Mailleux P, Vanderhaeghen JJ. 1992. Distribution of neuronal cannabinoid receptor in the adult rat brain: A comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience 48:655-668.
Page 77
102. Martin WJ, Hohmann AG, Walker JM. 1996. Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist: Correlation between electrophysiological and antinociceptive effects. The Journal of Neuroscience 16:6601-6611.
103. Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. 1995. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sciences 56:2103-2109.
104. Martin WJ, Patrick SL, Coffin PO, Tsou K, Walker JM. 1995. An examination of the central sites of action of cannabinoid-induced antinociception in the rat. Life Sciences 56:2103-2109.
105. Martin WJ, Tsou K, Walker JM. 1998. Cannabinoid receptor-mediated inhibition of the rat tail-flick reflex after microinjections into the rostral ventromedial medulla. Neuroscience Letters 242:33-36.
106. Martoletta MC, Cossu G, Fattore L, Gessa GL, Fratta W. 1998. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience 85:327-330.
107. Matsuda L, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561-564.
108. Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NSA, Gopher A, Almog S, Martin BR, Compton D, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. 1995. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical Pharmacology 50:83-90.
109. Mechoulam R, Hanus L, Fride E. 1998. Towards cannabinoid drugs—revisited. In: Ellis GP, Luscombe DK, Oxford AW, Editors, Progress in Medicinal Chemistry. v. 35. Amsterdam: Elsevier Science. Pp. 199-243.
110. Meng ID, Manning BH, Martin WJ, Fields HL. 1998. An analgesia circuit activated by cannabinoids. Nature 395:381-383.
111. Miller AS, Walker JM. 1996. Electrophysiological effects of a cannabinoid on neural activity in the globus pallidus. European Journal of Pharmacology 304:29-35.
112. Munro S, Thomas KL, Abu-Shaar M. 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61-65.
113. Murphy LL, Steger RW, Smith MS, Bartke A. 1990. Effects of delta-9-tetrahydrocannabinol, cannabinol and cannabidiol, alone and in combinations, on luteinizing hormone and prolactin release and on hypothalamic neurotransmitters in the male rat. Neuroendocrinology 52:316-321.
114. Narimatsu S, Watanabe K, Matsunaga T, Yamamoto I, Imaoka S, Funae Y, Yoshimura H. 1993. Suppression of liver microsomal drug-metabolizing enzyme activities in adult female rats pretreated with cannabidiol. Biological and Pharmaceutical Bulletin (Japan) 16:428-430.
115. Newton CA, Klein T, Friedman H. 1994. Secondary immunity to Legionella pneumophilia and Thl activity are suppressed by delta-9-tetrahydrocannabinol injection. Infection and Immunity 62:4015-4020.
116. Norwicky AV, Teyler TJ, Vardaris RM. 1987. The modulation of long-term potentiation by delta-9-tetrahydrocannabinol in the rat hippocampus, in vitro. Brain Researcl Bulletin 19:663.
117. O'Leary D, Block RI, Flaum M, Boles Ponto LL, Watkins GL, Hichwa RD. 1998. Acute marijuana effects on rCBF and cognition: A PET study. Abstracts-Society for Neuroscience: 28th Annual Meeting. Los Angeles, November 7-12, 1998. Washington, DC: Society for Neuroscience.
Page 78
118. Ohlsson A, Lindgren J-E, Wahlen A, Agurell S, Hollister LE, Gillespie HK. 1980. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clinical Pharmacology and Therapeutics 28:409-416.
119. Oviedo A, Glowa J, Herkenham M. 1993. Chronic cannabinoid administration alters cannabinoid receptor binding in rat brain: A quantitative autoradiographic study. Brain Research 616:293-302.
120. Pacheco MA, Ward SJ, Childers SR. 1993. Identification of cannabinoid receptors in cultures of rat cerebellar granule cells. Brain Research 603:102-110.
121. Patel V, Borysenko M, Kumar MSA, Millard WJ. 1985. Effects of acute and subchronic delta-9-tetrahydrocannabinol administration on the plasma catecholamine, beta-endorphin, and corticosterone levels and splenic natural killer cell activity in rats. Proceedings of the Society for Experimental Biology and Medicine 180:400-404.
122. Pepe S, Ruggiero A, Tortora G, Ciaardiello F, Garbi C, Yokozaki H, Cho-Chung YS, Clair T, Skalhegg BS, Bianco AR. 1994. Flow cytometric detection of the RI alpha subunit of type-I cAMP-dependent protein kinase in human cells. Cytometry 15:7379.
123. Pert CB, Snyder SH. 1973. Opiate receptor: Demonstration in nervous tissue. Science 179:1011-1014.
124. Pertwee RG. 1997b. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacology and Therapeutics 74:129-180.
125. Pertwee RG, Stevenson LA, Griffin G. 1993. Cross-tolerance between delta-9-tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide [published erratum appears in British Journal of Pharmacology, 1994, 111(3):968]. British Journal of Pharmacology 110:1483-1490.
126. Pertwee RG, Wickens AP. 1991. Enhancement by chlordiazepoxide of catalepsy induced in rats by intravenous or intrapallidal injections of enantiomeric cannabinoids. Neuropharmacology 30:237-244.
127. Pross SH, Nakano Y, Widen R, McHugh S, Newton C, Klein TW, Friedman H. 1992. Differing effects of delta-9-tetrahydrocannabinol (THC) on murine spleen cell populations dependent upon stimulators. International Journal of Immunopharmacology 14:1019-1027.
128. Razdan RK. 1986. Structure-activity relationships in cannabinoids. Pharmacology Review 38:75-149.
129. Rhee MH, Vogel Z, Barg J, Bayewitch M, Levy R, Hanus L, Breuer A, Mechoulam R. 1997. Cannabinol derivatives: Binding to cannabinoid receptors and inhibition of adenyl-cyclase. Journal of Medicinal Chemistry 40:3228-3233.
130. Richardson JD, Aanonsen L, Hargreaves KM. 1998. Hypoactivity of the spinal cannabinoid system results in NMDA-dependent hyperalgesia. Journal of Neuroscience 18:451-457.
131. Richardson JD, Kilo S, Hargreaves KM. 1998. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CBI receptors. Pain 75:111-119.
132. Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, Ferrara P, Soubrie P, Breliere JC, Le Fur G. 1994. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Letters 350:240-244.
133. Rinaldi-Carmona M, Barth F, Millan J, Defrocq J, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere J, Le Fur G. 1998. SR144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. Journal of Pharmacology and Experimental Therapeutics 284:644-650.
Page 79
134. Rodriguez de Fonseca F, Fernandez-Ruiz JJ, Murphy LL, Eldridge JC, Steger RW, Bartke A. 1991. Effects of delta-9-tetrahydrocannabinol exposure on adrenal medullary function: Evidence of an acute effect and development of tolerance in chronic treatments. Pharmacology, Biochemistry and Behavior 40:593-598.
135 Rodriguez de Fonseca F, Carrera MRA, Navarro M, Koob G, Weiss F. 1997. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal [see comments Science 1997, 276:1967-1968]. Science 276:2050-2054.
136. Rodriguez de Fonseca F, Gorriti MA, Fernandez-Ruiz JJ, Palomo T, Ramos JA. 1994. Down-regulation of rat brain cannabinoid binding sites after chronic delta-9-tetrahydrocannabinol treatment. Pharmacology, Biochemistry and Behavior 47:33-40.
137. Romero J, Garcia L, Fernndez-Ruiz JJ, Cebeira M, Ramos JA. 1995. Changes in rat brain cannabinoid binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to delta-9-tetrahydrocannabinol. Pharmacology, Biochemistry and Behavior 51:731-737.
138. Romero J, Garcia-Palomero E, Castro JG, Garcia-Gil L, Ramos JA, Ferandez-Ruiz JJ. 1997. Effects of chronic exposure to delta-9-tetrahydrocannabinol on cannabinoid receptor binding and mRNA levels in several rat brain regions. Molecular Brain Research 46:100-108.
139. Russell DH. 1978. Type I cyclic AMP-dependent protein kinase as a positive effector of growth. Advances in Cyclic Nucleotide Research 9:493-506.
140. Sanudo-Pena MC, Tsou K, Delay ER, Hohmann AG, Force M, Walker JM. 1997. Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neuroscience Letters 223:125-128.
141. Sanudo-Pena MC, Walker JM. 1997. Role of the subthalamic nucleus in cannabinoid actions in the substantia nigra of the rat. Journal of Neurophysiology 77:1635-1638.
142. Schatz AR, Koh WS, Kaminski NE. 1993. Delta-9-tetrahydrocannabinol selectively inhibits T-cell dependent humoral immune responses through direct inhibition of accessory T-cell function. Immunopharmacology 26:129-137.
143. Schlicker E, Timm J, Zenter J, Goethert M. 1997. Cannabinoid CB1 receptor-mediated inhibition of noradrenaline release in the human and guinea-pig hippocampus. Naunyn-Schmiedeberg's Archives of Pharmacology 356:583-589.
144. Shen M, Piser TM, Seybold VS, Thayer SA. 1996. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. Journal of Neuroscience 16:4322-4334.
145. Shivers SC, Newton C, Friedman H, Klein TW. 1994. Delta 9-tetrahydrocannabinol (THC) modulates IL-1 bioactivity in human monocyte/macrophage cell lines. Life Sciences 54:1281-1289.
146. Shohami E, Gallily R, Mechoulam R, Bass R, Ben-Hur T. 1997. Cytokine production in the brain following closed head injury: Dexanabinol (HU-211) is a novel TNF-alpha inhibitor and an effective neuroprotectant. Journal of Neuroimmunology 72:169-177.
147. Sim LJ, Hampson RE, Deadwyler SA, Childers SR. 1996. Effects of chronic treatment with delta-9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPyS autoradiography in rat brain. Journal of Neuroscience 16:8057-8066.
148. Sim LJ, Xiao R, Selley DE, Childers SR. 1996. Differences in G-protein activation by mu- and delta-opioid, and cannabinoid, receptors in rat striatum. European Journal of Pharmacology 307:97-105.
149. Simon EJ. 1973. In search of the opiate receptor. American Journal of Medical Sciences 266:160-168.
Page 80
150. Skaper SD, Buriani A, Dal Toso R, Petrelli L, Romanello S, Facci L, Leon A. 1996. The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons. Proceedings of the National Academy of Sciences, USA 93:3984-3989.
151. Smith JW, Steiner AL, Newberry WM, Parker CW. 1971. Cyclic adenosine 3',5'-monophosphate in human lymphocytes: Alteration after phytohemagglutinin. Journal of Clinical Investigation 50:432-441.
152. Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. 1994. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. Journal of Pharmacology and Experimental Therapeutics 270:219-227.
153. Smith PB, Welch SP, Martin BR. 1994. Interactions between delta 9-tetrahydrocannabinol and kappa opioids in mice. Journal of Pharmacology and Experimental Therapeutics 268: 1381-1387.
154. Sofia RD, Nalepa SD, Harakal JJ, Vassar HB. 1973. Anti-edema and analgesic properties of delta-9-tetrahydrocannabinol (THC). Journal of Pharmacology and Experimental Therapeutics 186:646-655.
155. Specter S, Lancz G, Hazelden J. 1990. Marijuana and immunity: Tetrahydrocannabinol mediated inhibition of lymphocyte blastogenesis. International Journal of Immunophar-macology 12:261-267.
156. Stefano G, Salzet B, Salzet M. 1997. Identification and characterization of the leech CNS cannabinoid receptor: Coupling to nitric oxide release. Brain Research 753:219224.
157. Stella N, Schweitzer P, Piomelli D. 1997. A second endogenous cannabinoid that modulates long term potentiation. Nature 388:773-778.
158. Strangman NM, Patrick SL, Hohmann AG, Tsou K, Walker JM. 1998. Evidence for a role of endogenous cannabinoids in the modulation of acute and tonic pain sensitivity. Brain Research 813:323-328.
159. Sulcova E, Mechoulam R, Fride E. 1998. Biphasic effects of anandamide. Pharmacology, Biochemistry and Behavior 59:347-352.
160. Szabo B, Dorner L, Pfreundtner C, Norenberg W, Starke K. 1998. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience 85:395-403.
161. Tanda G, Pontieri FE, Di Chiara G. 1997. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common 1l opioid receptor mechanism. Science 276:2048-2049.
162. Terenius L. 1973. Characteristics of the ''receptor'' for narcotic analgesics in synaptic plasma membrane fraction from rat brain. Acta Pharmacologica Et Toxicologica 33:377384.
163. Terranova JP, Michaud JC, Le Fur G, Soubrie P. 1995. Inhibition of long-term potentiation in rat hippocampal slice by anandamide and WIN55212-2: Reversal by SR141716 A, a selective antagonist of CB] cannabinoid receptors. NaunynSchmiedeberg's Archives of Pharmacology 352:576-579.
164. Titishov N, Mechoulam R, Zimmerman AM. 1989. Stereospecific effects of (-) and (+)-7-hydroxy-delta-6-tetrahydrocannabinol-dimethylheptyl on the immune system of mice. Pharmacology 39:337-349.
165. Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. 1998. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83:393-411.
166. Tsou K, Patrick SL, Walker JM. 1995. Physical withdrawal in rats tolerant to delta-9-tetrahydrocannabinol precipitated by a cannabinoid receptor antagonist. European Journal of Pharmacology 280:R13-R15.
Page 81
167. Watson PF, Krupinski J, Kempinski A, Frankenfield C. 1994. Molecular cloning and characterization of the type VII isoform of mammalian adenylyl cyclase expressed widely in mouse tissues and in S49 mouse lymphoma cells. Journal of Biological Chemistry 269:28893-28898.
168. Watzl B, Scuder P, Watson RR. 1991. Marijuana components stimulate human peripheral blood mononuclear cell secretion of interferon-gamma and suppress interleukin-1 alpha in vitro. International Journal of Inmmnopharimology 13:1091-1097.
169. Weidenfeld J, Feldman S, Mechoulam R. 1994. Effect of the brain constituent anandamide, a cannabinoid receptor agonist, on the hypothalamo-pituitary-adrenal axis in the rat. Neuroendocrinology 59:110-112.
170. Welch SP. 1993. Blockade of cannabinoid-induced antinociception by norbinaltorphimine, but not N,N-diallyl-tyrosine-Aib-phenylalanine-leucine, ICI 174,864 or naloxone in mice. Journal of Pharmacology and Experimental Therapeutics 265:633-640.
171. Welch SP, Thomas C, Patrick GS. 1995. Modulation of cannabinoid-induced antinociception after intracerebroventricular versus intrathecal administration to mice: Possible mechanisms for interaction with morphine. Journal of Clinical and Experimental Therapeutics 272:310-321.
172. Wirguin I, Mechoulam R, Breuer A, Schezen E, Weidenfeld J, Brenner T. 1994. Suppression of experimental autoimmune encephalomyelitis by cannabinoids. lmmunopharma-cology 28:209-214.
173. Wirth PW, Watson ES, ElSohly M, Turner CE, Murphy JC. 1980. Anti-inflammatory properties of cannabichromene. Life Sciences 26:1991-1995.
174. Yaksh TL. 1981. The antinociceptive effects of intrathecally administered levonantradol and desacetyl-levonantradol in the rat. Journal of Clinical Pharmacology 21:334S-340S.
175. Yoshida H, Usami N, Ohishi Y, Watanabe K, Yamamoto I, Yoshimura H. 1995. Synthesis and pharmacological effects in mice of halogenated cannabinol derivatives. Chemical and Pharmlaceutical Bulletin 42:335-337.
176. Zhu W, Newton C, Daaka Y, Friedman H, Klein TW. 1994. Delta 9-tetrahydrocannabinol enhances the secretion of interleukin 1 from endotoxin-stimulated macrophages. Journal of Pharmacology and Experimental Therapeutics 270:1334-1339.
177. Zuardi AW, Shirakawa I, Finkelfarb E, Kariol IG. 1982. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychoplarlnacology (Berl) 76:245-250.
178. Zurier RB, Rossetti RG, Lane JH, Goldberg JM, Hunter SA, Burstein SH. 1998. Dimethylheptyl-THC-11 oic acid: A non-psychoactive antiinflammatory agent with a cannabinoid template structure. Arthritis and Rheumatism 41:163-170.